DISTRIBUTION STATEMENT A
|
|
|
- Jonas Harrison
- 5 years ago
- Views:
Transcription
1 THIS REPORT HAS BEEN DELIMITED AND CLEARED FOR PUBLIC RELEASE UNDER DOD DIRECTIVE 5200,20 AND NO RESTRICTIONS ARE IMPOSED UPON ITS USE AND DISCLOSURE, DISTRIBUTION STATEMENT A APPROVED FOR PUBLIC RELEASEJ DISTRiBUTION UNLIMITED$
2 UNCLASSIFIED AD DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION. ALEXANDRIA. VIRGINIA w UNCLASS][FIED
3 NOTICE: When government or other dravings, specifications or other data are used for any purpose other than in connection with a definitely related government procurement operation, the U. S. Government thereby incurs no responsibility, nor any obligation whatsoever; and the fact that the Government may have formalated, furnished, or in any way supplied the said drawings, specifications, or other data is not to be regarded by implication or otherwise as in any manner licensing the holder or any other person or corporation, or conveying any rights or permission to manufacture, use or sell any patented invention that may in any way be related thereto.
4 Notre Dame Physical Electronics Group esearch - CL- Partially Suppo:--ed by The United States Navy j/, Office of Naval Research - 2n - REPRINT SERIES The Kinetics of Sr on W by DC Field Electron Microscopv by B. Barnaby, A. Petrauskas and E. Coomes \:R\\. A Technical Report, j Contract Ne. Nonr.162( 01,.NR 372/-731. MAY 2 "iq '! Presented in part at the Linfield Field Emission Symposium, McMinnville, Oregon, 31 August 1960 NO 0 1
5 The Kinetics of Sr on W ~4 by DC Field Electron Microscopy Stable states of Sr on W identified during deposition were also observed in migration and desorption experiments. These studies suggest that the adsorbate consists of a strongly bound layer next to the W substrate and a loosely bound outer layer. Experiments with SrO on W failed to show whether or not Sr influenced transition from one Sr-O-W complex to another for tip temperatures below K. Ap*5) _6 B, Barnaby A. Petrauskas E. Coomes Introduction The structure of adsorbed layers of SrO on W and Its effect on the electron field emission has been studied in detail. Cape and Coomes (1) examined the kinetics of thin SrO films on W by field electron microscopy (FEM). Previously, Moore, Allison and Morrison had examined the chemical reactions which occur when a W filament coated with SrO was heated in vacuum (2). They found that complexes of Sr-O-W were formed with an evolution of gaseous Sr and that the rate of reaction was in agreement with values computed from thermochemical data. In the FEM experiment, changes in micrograph patterns could be observed which were interpreted as the same chemical reactions studied by Moore et al. The results show reaction taking place on the{ 1 II} and the {f00} areas together with their environs. The high temperature modifications involving oxygen were identified from the work of other investigators (3, 4). (1) J. A. Cape and E. A. Coomes, J. Chem. Phys., (1960). (2) G. E. Moore, H. W. Allison, and J. Morrison, J. Chem. Phys., (1950). (3, 4) J. A. Becker and R. G. Brandes, J. Chem. Phys., (1955). E. W. Muiller, Elektrochem., (1955).
6 "2- Below I150 K there was possible evidence for the dissociation of SrO and the migration of Sr to the {llo} edges where clusters of crystalli tes form. Lack of FEM data on the behavior of Sr on W single crystals and inadequate temperature calibration made the interpretation of the results at K and lower temperatures difficult. This Is a report on FEM work carried out in the range 300 K to ll5o K. Moore and Allison had made a study of Sr on a polycrystalline W filament using thermionic eminsion technique (5). This earlier work measured the average work function decrease caused by the adsorbed strontium and the activation energy of desorption of this adsorbed layer. By repeating this same type of experiment on a single crystal using FEM techniques, details of the crystallographic distribution of adsorbent and the resulting crystallographic variation in work function could be observed. In the FEM technique 9 observations are made with the tip at room temperature so that the elect=n emission measurements are independent of the temperature selected for the kinetic process. The general approach in both the Sr and SrO experiments was the same. One looked for stable states of the absorbent-absorbate system and made measurements of the time and Fowler-Nordheim work function as the activation progressed to the end state. (5) G. E. Moore and HL W. Allison, J. Chem. Phys., (1955).
7 -3- Results A. Strontium Figure 1 shows the sequence of FEM patterns which were observed as strontium was deposited on a heated W tip of the FEM tube. Each row is a sequence of patterns observed at a particular temperature, Tr, of the tip. The first picture in each row was taken before the Sr source was turned on. The arrival rate of Sr at the tip, approximately 3. 8 x 10.7 gm/cm 2 hi is the same in all cases. The orientation of the source with respect to the tip is different in each of the sequences shown. In the second and fourth sequence, the axis of the source was parallel to the axis of the tip, while in the first and third, the two axes were perpendicular to each other. The first sequence of patterns shown in Figure 1 was observed at a temperature Tr equal to K. The times given in this figure are cumulative deposition times with the tip held at T r. In the early stages of the deposition the Sr coverage is small enough so that the clean tungsten pattern is still recognizable on the side away from the source. Thus, no detectable adsorbent was incident on this region of the tip. As the Sr accumulates on the side of the tip nearest to the source, the voltage required to draw a given current from this region decreases to the extent that the clean W pattern is no longer visible. Only a bright region on the side nearest the source appears as in pattern B. This bright region changes with increasing deposition time to a bright irregular line across the center of the pattern as in C. Later individual bright spots begin to appear behind the line as it becomes fainter and more irregular. These bright spots are interpreted as clusters of Sr. With the exception of the three planes, the bright line lies along a< IIb-- zone. Since a bright region always surrounds thef lane, it is suggested that the Sr incident on t, lo).ane migrates rapidly over the plane to its edges. Further deposition increases the
8 h I A B C D E F G H I h K L M N 0 P Q R S T
9 Figure I Deposition of Stront.umr Tr = 3000K Pattern A B C D E Time (min) S(ev) T = 350 K r Pattern F G H I J Time (min) * (ev) T K r Pattern K L M N 0 Time (min) * (ev) 4.5 T = 570&K r Pattern P Q R S T Time (min) (ev)
10 -4- size and brightness of the clusters. The second deposition sequence was made at Tr equal to K. At this temperature it appears that Sr was mobile and ourface migration proceeded at a rate comparable to the arrival rate. As a result of the geometry the initial accumulation occurred along a narrow band, Fig. IG. For a similar deposit at Tr equal to 300 K in the same geometry, clusters formed at approximately the same position as the bright region in pattern B, but the other portions of the tip remained dark to the end of the deposition time. In this case the migration proceeded with a sharp boundary, Fig. 1G to 1J Fig. 11 shows encirc!erment of thefll10regtons by nonuniform migration. The region behind the leading edge appears as a symmetric pattern. After 120 minutes, the deposition was stopped. and the temperature of the tip was held at K until migration was completed. The result is shown in pattern Jý Clustering did not occur in this sequence as in the first sequence when the Sr was not mobile. The third sequence of patterns shows a deposition at K. The Sr was again incident from the upper left. The axis of the source was perpendicular to the axis of the tip and the source was well back from the apex of the tip. Again migration with a sharp boundary occurred during deposition. In disttnction to the previous sequence clusters began to appear behind the migrating boundary. The symmetric pattern shown in Fig, 1O is dominated by clusters which are symmetrically located about thelloilanes. After depositing strontium for an additional hour beyond that required to obtain pattern 0, no changes in the pattern were observed indicating that a steady state condition has been reached by the strontium. The fourth deposition sequence was made at 570 K. The Sr was incident on the tip from the upper left in precisely the same geometry as used for the
11 "-5- second sequence. The patterns remained symmetrical throughout the entire sequence suggesting a migration rate higher than the arrival rate. The absence of boundary migration in this case may indicate that a large concentration gradient cannot be maintained due to the high mobility of Sr. Attention is called to the following details. In pattern Q, the general shape and form of the clean tungsten pattern was preserved with new dark regions appearing at thef03}planes which surrounded the(00}planes. The<l 1l-zones developed narrow dark bands and thefllplanes became dark. As the deposition continued, the high emission became restricted to a smaller region with little detail except at th42+}nd th++l}panes. The total emitting region then begin to expand again as in pattern S until finally after two hours of deposition a pattern similar to that for clean tungsten emerged, The main differences was that the 1 planes appeared brighter than the(10regions. The final pattern was stable and did not change with continued depocition. In Figure 2 it may be observed how the stable states are transformed into one another. The first sequence of patterns in Figure 2 shows the changes which occurred when deposition was continued at a lower T after the stable r state of Figure IT was reached. This pattern will be designated as stable state 1. Stahttag h tle lip in stable state 1, deposition was continued at Tr equal to 360 K. In Pattern C clusters appeared principally on thelu0+plane edges. After deposition for 1 hour the pattern was still symmetric with extensive clustering. A bright spot appeared on the side of the tip near the source, due to an accumulation of Sr. As the deposition continued, the accumulation became larger but no other changes occurred. It is evident in this sequence of patterns that migration occurred without a boundary. On the other hand, beginning with a clean tungsten tip, with Tr equal to 350&K, deposition was
12 A B C D E F G H I K L M N 0 P 0
13 Figure 2 Deposition of Strontium T = K r Pattern A B C D E Time (mln) (ev) T = 300 K r Pattern F G H Time (min) *(ev) Time = 60 minutes Pattern I J K L M Tr(0K) S(ev) T =.400 K r Pattern N 0 P Q Time (min) S
14 -6- accompanied by boundary migration. Therefore, one may assume that the observation of boundary migration depends on the surface conditions of the tip# as well as on the tip temperature, and concentration gradient. This deposition transforms etable state I to the end pattern of Figure l{) which will be labeled as stable state 2. The second se,4uence of patterns in Figure 2 shows what happens when Sr is deposited on the tip at room temperature beginning with stable state 1. After only one minute of deposition, clustering occurred which obscured the pattern in the region nearest the source. On further deposition, the clusters appeared to form a nearly symmetric ring about the{1 10plane nearest the source Just as in Figure 1D. Continued deposition resulted in nonsymmetrical brightemitting regions. There was no evidence of migration, and it appeared that the adsorbed Sr was immobile at room temperature regardless of whether the substrate was clean W or a symmetric di.stribution of Sr on W. Thus, an end pattern for a given temperature may be obtained from an end pattern corresponding to an elevated temperature merely by depositing additional Sr with the tip held at the lower temperature. At temperatures above K a series of steady end states may be obtained, The sequence I, J, K, L, M of Figure 2 shows 5 such states. In this sequence each pattern represents the end steady state observed when Sr was deposited on clean W at a particular temperature. Pattern I was the end pattern observed during deposition at K. Pattern J was that observed during deposition at 900KL In all cases, the end steady state pattern was not altered by continued deposition. The end pattern observed for any one temperature is an intermediate state observed during any lower temperature deposition. Clusters did not appear in any of these patterns. If deposition was continued in pattern $ at a L
15 -7- lower temperature clusters appeared as in the first and second sequences of Figure 2. Thus it appears that the final state was entirely a function of temperature provided that a certain minimum time of deposition had elapsed. Palterns N, 0, P, 0 illustrated in Figure 2 reveal how the exposure time may alter the appearance of the pattern. It shows the pattern observed after a two hour deposition at Tr equal to K. This pattern of stable state 2 was unmistakably identifiable on the microscope itself; however, the photographs can be made to emphasize predetermined aspects. If the negative is highly exposed the pattern shows a faint uniform structure similar to that of clean W underneath an intense symmetric structure apparently superimposed on the former. In some cases faint clusters can be observed on thell0plane edges. If the negative is briefly exposed, the faint structure cannot be seen and more detail can be observed in the previously intense structure. Clusters on the(21i}planes are a common feature of these additional details. The interpretation is that the Sr was adsorbed in two layers which differ considerably in their distribution and that the outer layer was less dense but exercised a greater control over the electron emission process. The clusters formed a part of the second layer. The similarity of the first layer to clean W indicates perhaps that this layer builds up on sites of the underlying W. The stable states of Sr on W identified during deposition can also be observed in migration and desorption experiments. FEM patterns for these experiments are illustrated in Figure 3. Strontium was deposited on the side of the tungsten tip from the upper left at the temperature Tr equal to 3000K for two hours. This was equivalent to about 3 layers if spread uniformly over the tungsten tip. Pattern A is that of clean tungsten before the deposition. Pattern B displays the emission immediately after the completion of the deposition.
16 A B C D E F G H I 4 K L M N 0 P1 p 0 QI R S TDT
17 Figure 3 Migration and Deposition of Strontium T = K a Pattern A B C D E Time R(La)V(kv) (ev) Ta = K Pattern F G H I J Time (min) 1/ (ILa)V(kv) , S(ev) ' T =570 0 K a Pattern K L M N 0 Time (mrin) l( 1 a)v(kv) (ev) Ta a K Pattern P Q R S T Time (mini) lria)v(kv) S(ev)
18 -8- Adsorbed Sr appears as a large cluster on the side of the tip. The remainder of the patterns in the first row show the progress of a boundary migration at K. The bright edge was visible together with the symmetric pattern behind that edge. In pattern D, the migration was almost complete after 240 minutes at this temperature. The symmetric end pattern E is a variation of stable state 2 previously identified in patterns N, 0, P, 0 of Figure 2. When the tip temperature was raised to 450K as in F, the clusters which were faint in pattern E quickly dominate the entire pattern especially on th+llo}plane edges. There was very little change in the pattern as well as the apparent work function with time. Immediately after the temperature was increased to 570K the regular array of clusters became "randomly distributed as seen in patterns K and L and the pattern lost its symmetry. Later a stable symmetric pattern developed as the clusters disappeared. This is illustrated in patterns M, N, and 0. Pattern 0 may be identified as the same as stable state 1. Activation at K caused a decrease in the apparent emitting area and a rapid increase in work function, followed by an increase in emitting area and a less rapid increase in work function. In time this process terminated in the clean W pattern and the work function of W. A study of deposition, migration, and desorption data suggests the following state of the adsorbate on the W tip. Stable state 2 consists of an inner strongly bound layer and an outer loosely bound layer. At low temperatures the loosely bound layer is held in a relatively uniform symmetric distribution. The Sr atoms are immobile on these uniform layers. At slightly higher temperatures, they move over the tightly bound layer until they are trapped at surface Imperfections such as the(lu0}plane edges, where they
19 are held as clusters of atoms. -9- Stable state I is the tightly bound layer of Sr on W. The total quantity of Sr which can be held in this condition depends primarily on the tip temperature. The concentration of immobile Sr following deposition at K spreads out, at elevated temperatures, to stable state 2 via a boundary migration. Surface migration proceeds by Sr atoms moving over the tightly bound layer until they reach the edge of that layer where they become bound to the clean W surface making it possible for other atoms to migrate over the freshly covered region. Thus the tightly bound layer is gradually extended by the momement of strontium over the tightly bound first layer. The sharp leading edge of the migration implies the lack of mobility of the inner layer at this temperature. The outer layer meanwhile forms on top of the inner layel as rapidly as the boundary proceeds. This type of migration has been reported for oxygen on W tips at 27 0 K (6) (The oxygen, however, increases the work function of the W tip ao that the FEM patterns show a dark region spreading out over the clean W), The transition from state 2 to state 1 i accomplished by desorption of the weakly bound layer. The transition from state 1 to clean W is one of desorption of the tightly bound inner layer. Transition 2 to I occurs at a lower temperature than does transition I to clean W. If the optimum quantity of Sr were deposited a tightly bound layer could be formed by deposition and migration without desorption. This is illustrated in Figure 4. With the tip held at K, strontium was deposited from the lower left, at the same arrival rate used above, for a period of 35 minutes. Pattern Bwhich can be identified with Figure lcwas observed after the source was turned off. By heating the tip to an activation temperature of K, (6) R. Gorner and J. K. Hulm, J. Chem. Phys., (1957).
20 00000 A B C D E 00000' F G H I J
21 Figure 4 Deposition of Strontium, at T = 3000(K for 35 minutes and migration at Ta = K. r Pattern A B C D E Time (min) I(g a) V(kv) * (ev) Pattern F G H Ij Time (min) so 57 I( I a) V(kv) (ev)
22 "-10- migration with a sharp leading edge was initiated. The progress of the migration was exhibited by the displacement of the bright region across patterns C "J, As above, the advancing boundary appeared to encircle thbe(1l0lanes and proceeded outward. Upon completion of the migration the symmetric pattern J showed characteristics only of a tightly bound layer. The work function for this state of the tip was 2. 5 ev as wa determined from the Fowler-Nordheim plot of Figure 5. For the purpose of determining the activation energy pattern H was selected as the end state. This was easily recognized and reproducible. Figure 6 shows the sequence of patterns observed during migration at four activation temperatures between K and K after identical depositions. The first pattern in each row represents the initial state of the migration procesp while the fourth pattern represents the final state. The last pattern exhibits the condition after the migration was complete. Measurements of this type can be extended up to temperatures of 750 K, however, the migration times are too short for accurate determination. In the temperature range between 5700K and 750 K desorption from the mobile loosely bound layer is competitive with the migration process. Figure 7 shows an Arrhenius plot from which activation energy of this mode of migration may be determined for the sequence presented in Figure 6. A least squares fit yields an activation energy of 16 kcal/mole or ev/atom to an estimated accuracy of * 3 kcal/mole. Figure 8 presents patterns of tightly bound layers formed by migration of smaller quantities of Sr. Strontium was deposited with the tip held at a receiver temperature of K. The deposition times were so short that optimum coverage for maximum emission was not achieved. Migration progressed without a boundary. In each sequence the activation temperature
23 Figure 5 Fowler-Nordheim plot (a) for clean W + assumed to be 4.5 ev, (b) Sr on W pattern J, figure 4-5 ev, (c) SrO on W pattern I, figure 11, ý = 2.0ev. The work function computed for (b) and (c) used the form factor detarrnined from (a).
24 -11.5 A A CL E U\, \ 2=2 -> 2 A \\A A t b " U ! (volts) V
25 B C D E D E AB C D E A B C D E
26 Figure 6 Migration of Strontium on Tungsten after Deposition at Room Temperature for 35 minutes. Ta K Pattern A B C D E Time (min) I a&) V(kv) *(ev) Ta = 4870K Pattern A B C D E Time (min) "I) t I (1 a) V(kv) 100 5,05 IOO 4.: , C48 S(ev) 27.6, 5 2, T = 4680K Pattern A ab C D E Time (min) ( 1.a) V(kv) (ev) * Ta K Pattern A B C D E Time (min) I( a)v(kv) *(ev)
27 Figure 7 * P toxf log a tvs. I/TI
28 / 3.0- SrO-W EE44+ 8 Kcal/mole ~ / 00 -J / ESr-W E 16 ± 3 Kcal/mole 0.5 o OD LO v~o o I o I I II a _.4 ( K) T (*K) TA
29 t A B C D E b F G H I J K L M
30 Figure K. The tip was at room temper- Migration of Sr on W at T a ature during deposition, a I Deposition time = W min. Pattern A B C D E Time (mln) (ev) Deposition time = Imin. Pattern F G H I Time (min) * (ev) Deposition time = 2. 5 min. Pattern J K L M Time (min) (ev)
31 -llfor migration was 63 'L The deposition time was one minute, two minutes, and two and a half minutes for the first, second, and third sequence respectively. In the first two sequences the center of the depos!tion was on a {100} plane while ta the last the deposition was on a {llo} plane. As migration proceeded the only observable change in the pattern is on the planes which are normally high emitting from clean tungsten. Larger deposition required a longer time to complete the migration. For these small coverages it was found that the work functions did vary although the final patterns are indistinguishable. Thus the work function was a more sensitive test of coverage than is the FEM pattern. B. Strontium Oxide When SrO is deposited on W and then heated to successively higher temperatures, a series of chemical reactions may occur terminating in an oxygen covered W surface. The FEM patterns observed during these processes are shown in Figure 9. A coverage equivalent to about three layers of SrO was deposited from the upper right on the clean W point for a period of two hours. Additional SrO would not change the appearance of the pattern at the end of the deposition not a.y stage de:--g the subsequent activations..this is considered a heavy coverage. Pattern A shows the emission from clean W, and pattern B shows the emission from the tip after the deposition was completed. Pattern B has no symmetry, but shows a bright grainy region on the side of the tip nearest the source which is distinctly different from the clusters observed for Sr on W. Upon activation at Ta equal to K, a series of patterns were observed that were symmetric except for the grainy bright regions which persisted on the side of the tip nearest the source. As seen in patterns C and D, the symmetric part changed with time, becoming stable only after the grainy region had disappeared as in pattern E. This stable pattern was characterized by high
32 I I 8@@@@ ABiDE F G H I J j 00. K L M N 0 9,., I
33 Figure 9 The FEM patterns observed for successive activation temperatures, Ta, after depositing a heavy coverage of SrO. Ta = K Pattern A B C D E Time (mrin) ( ja)v(kv) (ev) Ta = 10000K and 11850K Pattern F G H I J T ( K) W T -ne (min) I (ia) V(kv) * (ev) T = K a Pattern K L M N 0 Time (mrin) I( 1 a) V(kv) (ev) Ta = 1750 K Pattern P Q R S T Time (min) 1/ (v a) V(kv) , (ev)
34 "-12- emission from theulbdirection reducing the work function below the published values of bulk Sr(7). Two activation runs, the first at K, and the second at K are shown in the next row. The patterns are similar for the two runs with one exception. All patterns in the first run show emissfon a: thefll0odges, but none show this emission in the second. The work function inz-reased in both cases, apparently approaching a value near 4.1 ev. Further heating at K produced the sequence of patterns reported by Cape and Coomes (1). In this sequence there was a reversal in symmetry about the<411>direction accompanied by a sharp minimum in the work function. Heating to K cleaned up the W point, and patterns corresponding to oxygen on tungsten were observed during this process. The high temperature processes, represented by the last two rows of Figure 9, were Identified (1) with chemical reactions between SrO and W, the decomposition of a chemical compound to oxygen on W, and the final desorption of oxygen. Low temperature behavior of SrO on W was investigated by observing changes in the FEM pattern with time as SrO was deposited on W for a fixed tip temperature. Figure 10 shows the patterns observed during four separate depositions at successively higher temperatures. In all cases SrO was incident from the upper right with the same tip-source geometry. Pattern B in the sequence of patterns which was taken with the tip temperature at K, shows that the SrO was incident only on the side of the tip nearest the source. As the SrO accumulated on the tip, a bright region appeared on the corresponding side of the pattern. The clean W pattern, visible at first on the other side, seemingly vanished as a result of the difference in work functions. The bright leading edge and clusters characteristic of Sr did not appear, and instead a grainy bright region developed as shown in pattern C. This pattern maintains some of the (7) D. A. Wright, Proc. L E. E.,, (1953).
35 I A B C D E F G H I J K L M N 0 P 0 R S T
36 Figure 10 FEM patterns observed during deposition of SrO at several tip temperatures. Tr room temperature Pattern A B C D E Time (min) *(ev) Tr = 950 K Pattern F G H I J Time (min) * (ev) 4o T = K r Pattern K L M N 0 Time (min) * (ev) T = K r Pattern P Q R S T Time (mln) * (ev)
37 -13- symmetry of the W pattern. A sharp leading edge of the deposit could be observed after 60 minutes, and most of the symmetry was gone. Sixty minutes later the grainy bright region extended over most of the side cf the tip. Further deposition beyond 120 minutes caused no apparent char~ge in the patte.-n. These data show that SrOIike Sr,was irmnobile at K, bu4, unlike Sran optimumn quantity of SrO leading to maximum emission could not be found. The sharp line between bright and dark seen in pattern D merely delineated the edge of the deposit. The second depo3itton sequence was made at a temperature " r equal to K. The patterns observed during the initial part of this sequence were symmetric,indicating a migration more rapid than the arrival rate. Pattern G shows a marked recemblence to a light coverage of Sr such as pattern 0, row IV of Figure 1. The work function during this deposition decreased smoothly to a minimum for pattern I; thereafter, the measured work function was not reliable due to the formation of clusters. Continued deposition increased the size and number of the clusters. At T equal to K the pattern remained symmetric at all times r during deposition, and the work function went through a minimum. After the minimum in the work function the pattern consisted of clusters on the{lliplanes and the(i 10plane edges. Depositing more SrO caused no changes in the pattern. The final deposition was made at Tr equal to K. Here again the pattern remained symmetric at all times; the initial stages were similar to Sr on W or like the deposition of SrO at K. The work function decreased to a minimum, and then increased with equal speed. No clusters were observed on the 110 plane edges in this case. The final pattern was stable, and further deposition did not change its main features.
38 -14- The stable condition of the patterns reached when the tip temperatures were 9500K and above was probably due to the steady state attained between the rate of evaporation and the rate of deposition. SrO was deposited on the W tip held at room temperature and then the deposit was activated at some higher temperature. Patterns obtained in this way were compared with those obtained by deposition cn the tip held above room temperature. These are shown in Figure 11. Pattern B shows the distribution after a 25 minute deposit, which is the same as pattern C of Figure 10 row 1. When the tip was heated to an activation temperature of K the grainy bright region nearest the source become smaller, but maintained its grainy appearance as in patterns C to E. In pattern E, individual emitting centers could be distinguished in this region. No other emission could be observed even though very large electron currents were drawn from the tip. The emitting centers appeared to be in a continuous state of agitation similar to the clusters observed with Sr on W. In pattern F bright regions appeared in the center of the pattern at thef11}oplane edges. These regions increased in intensity with time until a symmetric distribution became visible in pattern H, however, the grainy region associated with the pile up of SrO was still evident in the upper right. A completely symmetric pattern I with no traces of the accumulation evolved from H only after a long time compared with the time required to arrive at H. This final pattern was stable, and the emitter had a work function of 2. 0 ev as determined from the Fowler-Nordhelm plot of Figure 5. This pattern and work function may be used to identify the final state of the activation process, Further heating at a temperature of K resulted in the stable patterns and characteristic work function minimum observed by Cape (1) in the temperature range K
39 I 1 0@@ iiii D E F' I
40 Figure 11 Sequence of patterns observed during the migration of SrO on W. Ta K. Tr =room temperature. Pattern A B C Time (min) 0 13 I( a) V(kv) S(ev) Pattern D E F Time (min) I (a) V(kv) ,(ev) Pattern G H I Time (min) I (a) V(kv) S(ev)
41 "-15- Figure 12 shows the sequence of patterns observed at four different activation temperatures in the range K after identical depositions at K. The second pattern in each sequence shows that the grainy region associated with the accumulation of SrO becomes smaller with time, and the third pattern shows that a symmetric pattern appears while this region is Still visible. An Arrhenius plot of these data is given in Figure 7. A least squares fit of the experimental points yields an activation energy of 44 kcal/mole, or 1.9 ev/molecule, to an estimated accuracy of 8 kcal/mole, Boundary free migration can account for the decrease in size of the grainy region and the appearance of the symmetric pattern. Another possibility is that SrO may d-losociate at these temperatures on the W surface and the components then mn.grate at different rates. The overall rate then should be determined by the slowest process. Cape and Coomes showed that the sequence of patterns observed for SrO on W, in the temperature range I could be reversed by the deposition of SrO at a temperature below 11500K. This sequence of patterns is shown in Figure 9 Row I11. The first pattern in this sequence was referred to as the X pattern and the last was called Z. After going through the sequence at Ta equal to 1435 K to the Z pattern, a deposit of SrO at Tr equal to 1050 K brought back the Z pattern and the sequence at Ti equal to K could be repeated. This procedure was used in an attempt to identify the role of Sr in this process. The FEM patterns are shown in Figure 13. Pattern A of Row I is the Z patterns, the terminal point of activation at Ta equal to K after deposition of SrO at Tr equal to 10750K. The tip temperature was reduced to K and Sr deposited from the lower left for a period of two hours at which time pattern B was observed. The tip was then heated to T equal to K a
42 I eec, A B C D A B C D A B C D
43 Figure 12 FEM Patterns observed during migration of SrO on W at different temperatures Ta. Tr, room temperature. Ta = 7OK Pattern A B C D Time (min) (A) V(kv) , (cv) Ta = 7500K Pattern A B D Time (min) I a)v(kv) *(ev) T = K a Pattern A B C D Time (min) ( pa) V(kv) * (ev) :2.3' T * K a Pattern A B C D Time (min) i ( pa)(v (kv) (ev)
44 "-16- and patterns C to E observed at the times indicated. Pattern E can be identified as the pattern Z but none of the other patterns resemble the X to Z sequence. The work function does not show the minimum, instead t.e work function starts at a value considerably less than that normally oboerved for X and gradually increases to that of the Z. The procedure was repeated at a number of different receiver temperatures from K to 1163 o K but the X pattern was never observed nor could the X to Z sequence be reproduced. Low work functions were always observed following the Sr deposition but the pattern depended on the receiver temperature. One point was significant; sufficient Sr could be adsorbed on the point to lower the work function considerably even at K, whereas clean W could not adsorb much Sr at this temperature and the work function decreased only slightly. The role of Sr in the formation of the X pattern was also investigated. The FEM patterns are shown in Figure 13, Row 11. SrO was deposited for 5 hours with Tr" equal to K giving pattern F, the end point pattern for deposition at this temperature. Activation for 35 minutes at Ta equal to K resulted in the X pattern as shown by G. At this point the temperature was reduced to K and Sr deposited for 160 minutes. The pattern is not symmetric after this deposition and the apparent work function is low. The part of the pattern which is bright is symmetric and not very different from the X pattern. Continued deposition at Tr equal to 300 K for one hour reverses the emitting part uf the pattern as in I but does not otherwise change the appearance, This second deposition was made to be sure that Sr was actually being adsorbed. The tip was heated to Ta equal to K in order to bring about a symmetric distribution of Sr. After 20 minutes pattern J was obtained with the general features of the X pattern but a slightly lower work function. The starting point
45 I A B C D E I F 6 H I 4
46 Figure 13 Sr - SrO on W Tr K, deposition time of Sr = 120 main., Ta = 1418 K Pattern A B C D E Time (mrin) * (ev) Pattern F G H I Temperature (OK) T T 4410 T =1155 T I30 T =525 Time (mini) A&) 16 id2, (ev)
47 "-17- of pattern FwaW never achieved but activation always terminated In what appears to be pattern X. TbeIe txperiments failed to show the specific role of Sr in transition from one Sr-O-W complex to another. They did show that Sr can be adsorbed on the Sr-O-W complex in larger quantities than it can on clean W in the temperature region between K.
UNCLASSIFIED AD NUMBER LIMITATION CHANGES
 TO: UNCLASSIFIED AD NUMBER AD237455 LIMITATION CHANGES Approved for public release; distribution is unlimited. FROM: Distribution authorized to U.S. Gov't. agencies and their contractors; Administrative/Operational
TO: UNCLASSIFIED AD NUMBER AD237455 LIMITATION CHANGES Approved for public release; distribution is unlimited. FROM: Distribution authorized to U.S. Gov't. agencies and their contractors; Administrative/Operational
UNCLASSIFIED AD DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TCHNICAL INFORMATION CAMERON STATION, ALEXANDRIA. VIRGINIA UNCLASSIFIED
 UNCLASSIFIED AD.4101 13 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TCHNICAL INFORMATION CAMERON STATION, ALEXANDRIA. VIRGINIA UNCLASSIFIED.i NOTICE: Nen government or other dravings, specifications
UNCLASSIFIED AD.4101 13 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TCHNICAL INFORMATION CAMERON STATION, ALEXANDRIA. VIRGINIA UNCLASSIFIED.i NOTICE: Nen government or other dravings, specifications
Conduction Theories in Gaseous Plasmas and Solids: Final Report, 1961
 University of New Hampshire University of New Hampshire Scholars' Repository Physics Scholarship Physics 10-31-1961 Conduction Theories in Gaseous Plasmas and Solids: Final Report, 1961 Lyman Mower University
University of New Hampshire University of New Hampshire Scholars' Repository Physics Scholarship Physics 10-31-1961 Conduction Theories in Gaseous Plasmas and Solids: Final Report, 1961 Lyman Mower University
PhysicsAndMathsTutor.com 1
 PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
PhysicsAndMathsTutor.com 1 1. Millikan determined the charge on individual oil droplets using an arrangement as represented in the diagram. The plate voltage necessary to hold a charged droplet stationary
UNCLASSIFED AD DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION ALEXANDRIA. VIRGINIA UNCLASSIFIED
 UNCLASSIFED AD 4 6470 1 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION ALEXANDRIA. VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications
UNCLASSIFED AD 4 6470 1 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION ALEXANDRIA. VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications
Army Air Forces Specification 7 December 1945 EQUIPMENT: GENERAL SPECIFICATION FOR ENVIRONMENTAL TEST OF
 Army Air Forces 41065 Specification 7 December 1945 EQUIPMENT: GENERAL SPECIFICATION FOR ENVIRONMENTAL TEST OF A. APPLICABLE SPECIFICATIONS A-1. The current issue of the following specifications, in effect
Army Air Forces 41065 Specification 7 December 1945 EQUIPMENT: GENERAL SPECIFICATION FOR ENVIRONMENTAL TEST OF A. APPLICABLE SPECIFICATIONS A-1. The current issue of the following specifications, in effect
TO Approved for public release, distribution unlimited
 UNCLASSIFIED AD NUMBER AD403008 NEW LIMITATION CHANGE TO Approved for public release, distribution unlimited FROM Distribution authorized to U.S. Gov't. agencies and their contractors; Administrative/Operational
UNCLASSIFIED AD NUMBER AD403008 NEW LIMITATION CHANGE TO Approved for public release, distribution unlimited FROM Distribution authorized to U.S. Gov't. agencies and their contractors; Administrative/Operational
UNCLASSIFIED AD DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION. ALEXANDRIA. VIRGINIA UNCLASSIFIED
 UNCLASSIFIED AD 424039 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION. ALEXANDRIA. VIRGINIA UNCLASSIFIED NOTICE: When goverment or other drawings, specifications
UNCLASSIFIED AD 424039 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION. ALEXANDRIA. VIRGINIA UNCLASSIFIED NOTICE: When goverment or other drawings, specifications
UNCLASSIFIED AD Mhe ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA U NCLASSI1[FIED
 UNCLASSIFIED AD26 8 046 Mhe ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA w U NCLASSI1[FIED NOTICE: When government or other drawings, specifications or other
UNCLASSIFIED AD26 8 046 Mhe ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA w U NCLASSI1[FIED NOTICE: When government or other drawings, specifications or other
The illumination source: the electron beam
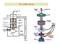 The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
The SEM Column The illumination source: the electron beam The probe of the electron microscope is an electron beam with very high and stable energy (10-100 kev) in order to get images with high resolution.
UNCLASSIFIED 'K AD ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED
 UNCLASSIFIED 'K AD2 8 3 4 4 1 ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications or other data
UNCLASSIFIED 'K AD2 8 3 4 4 1 ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications or other data
Auger Electron Spectroscopy (AES)
 1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
1. Introduction Auger Electron Spectroscopy (AES) Silvia Natividad, Gabriel Gonzalez and Arena Holguin Auger Electron Spectroscopy (Auger spectroscopy or AES) was developed in the late 1960's, deriving
UNCLASSIFIED AD ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTO 12, VIRGINIA UNCLASSIFIED
 UNCLASSIFIED AD273 591 ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTO 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications or other data are
UNCLASSIFIED AD273 591 ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTO 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications or other data are
The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado
 Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
Experiment 9 1 Introduction The Franck-Hertz Experiment Physics 2150 Experiment No. 9 University of Colorado During the late nineteenth century, a great deal of evidence accumulated indicating that radiation
UNCLASSIFIED Reptoduced. if ike ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED
 . UNCLASSIFIED. 273207 Reptoduced if ike ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications
. UNCLASSIFIED. 273207 Reptoduced if ike ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications
UNCLASSIFIED AD ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGT0 12, VIRGINIA UNCLASSIFIED
 UNCLASSIFIED AD 295 456 ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGT0 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications or other data are
UNCLASSIFIED AD 295 456 ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGT0 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications or other data are
M2 TP. Low-Energy Electron Diffraction (LEED)
 M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
M2 TP Low-Energy Electron Diffraction (LEED) Guide for report preparation I. Introduction: Elastic scattering or diffraction of electrons is the standard technique in surface science for obtaining structural
UNCLASSIFIED AD ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED
 UNCLASSIFIED AD 295 792 ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications or other data are
UNCLASSIFIED AD 295 792 ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications or other data are
Basic structure of SEM
 Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction
Table of contents Basis structure of SEM SEM imaging modes Comparison of ordinary SEM and FESEM Electron behavior Electron matter interaction o Elastic interaction o Inelastic interaction o Interaction
UNCLASSIFIED AD DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION, ALEXANDRIA, VIRGINIA UNCLASSIFIED
 UNCLASSIFIED AD 437890 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION, ALEXANDRIA, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications
UNCLASSIFIED AD 437890 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION, ALEXANDRIA, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications
X-RAY PRODUCTION. Prepared by:- EN KAMARUL AMIN BIN ABDULLAH
 X-RAY PRODUCTION Prepared by:- EN KAMARUL AMIN BIN ABDULLAH OBJECTIVES Discuss the process of x-ray being produced (conditions) Explain the principles of energy conversion in x-ray production (how energy
X-RAY PRODUCTION Prepared by:- EN KAMARUL AMIN BIN ABDULLAH OBJECTIVES Discuss the process of x-ray being produced (conditions) Explain the principles of energy conversion in x-ray production (how energy
"UNCLASSIFIED AD DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION, ALEXANDRIA. VIRGINIA UNCLASSIFIED.
 UNCLASSIFIED AD 2 24385 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION, ALEXANDRIA. VIRGINIA "UNCLASSIFIED /,i NOTICE: When government or other drnwings, specifications
UNCLASSIFIED AD 2 24385 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION, ALEXANDRIA. VIRGINIA "UNCLASSIFIED /,i NOTICE: When government or other drnwings, specifications
UNCLASSIFIED ADL DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION ALEXANDRIA. VIRGINIA UNCLASSIFIED
 UNCLASSIFIED ADL 4 5 2981 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION ALEXANDRIA. VIRGINIA UNCLASSIFIED NOTICE: When goverment or other drawings, specifications
UNCLASSIFIED ADL 4 5 2981 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION ALEXANDRIA. VIRGINIA UNCLASSIFIED NOTICE: When goverment or other drawings, specifications
Chapter 4 Scintillation Detectors
 Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
Med Phys 4RA3, 4RB3/6R03 Radioisotopes and Radiation Methodology 4-1 4.1. Basic principle of the scintillator Chapter 4 Scintillation Detectors Scintillator Light sensor Ionizing radiation Light (visible,
THE FRANCK-HERTZ EXPERIMENT
 Rice University Physics 332 THE FRANCK-HERTZ EXPERIMENT I. INTRODUCTION... 2 II. THEORETICAL CONSIDERATIONS... 3 III. MEASUREMENT AND ANALYSIS... 5 Revised July 1991 I. Introduction By the early part of
Rice University Physics 332 THE FRANCK-HERTZ EXPERIMENT I. INTRODUCTION... 2 II. THEORETICAL CONSIDERATIONS... 3 III. MEASUREMENT AND ANALYSIS... 5 Revised July 1991 I. Introduction By the early part of
Dual Nature of Radiation and Matter-I
 Dual Nature of Radiation and Matter-I Physics Without Fear CONTENTS ELECTRON EMISSION PHOTOELECTRIC EFFECT; HERTZ S OBSERVATIONS HALLWACHS AND LENARD S OBSERVATIONS EXPERIMENTAL STUDY OF PHOTOELECTRIC
Dual Nature of Radiation and Matter-I Physics Without Fear CONTENTS ELECTRON EMISSION PHOTOELECTRIC EFFECT; HERTZ S OBSERVATIONS HALLWACHS AND LENARD S OBSERVATIONS EXPERIMENTAL STUDY OF PHOTOELECTRIC
= 6 (1/ nm) So what is probability of finding electron tunneled into a barrier 3 ev high?
 STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
STM STM With a scanning tunneling microscope, images of surfaces with atomic resolution can be readily obtained. An STM uses quantum tunneling of electrons to map the density of electrons on the surface
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0342021651* PHYSICS 0625/42 Paper 4 Theory (Extended) February/March 2017 1 hour 15 minutes Candidates
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *0342021651* PHYSICS 0625/42 Paper 4 Theory (Extended) February/March 2017 1 hour 15 minutes Candidates
THE INFRARED SPECTRA AND VIBRATIONAL ASSIGNMENTS FOR SOME ORGANO-METALLIC COMPOUNDS
 ' S C I- THE INFRARED SPECTRA AND VIBRATIONAL ASSIGNMENTS FOR SOME ORGANO-METALLIC COMPOUNDS FRED W. BEHNKE C. TAMBORSKI TECHNICAL DOCUMENTARY REPORT No. ASD-TDR-62-224 FEBRUARY 1962 DIRECTORATE OF MATERIALS
' S C I- THE INFRARED SPECTRA AND VIBRATIONAL ASSIGNMENTS FOR SOME ORGANO-METALLIC COMPOUNDS FRED W. BEHNKE C. TAMBORSKI TECHNICAL DOCUMENTARY REPORT No. ASD-TDR-62-224 FEBRUARY 1962 DIRECTORATE OF MATERIALS
Thermodynamic calculations on the catalytic growth of carbon nanotubes
 Thermodynamic calculations on the catalytic growth of carbon nanotubes Christian Klinke, Jean-Marc Bonard and Klaus Kern Ecole Polytechnique Federale de Lausanne, CH-05 Lausanne, Switzerland Max-Planck-Institut
Thermodynamic calculations on the catalytic growth of carbon nanotubes Christian Klinke, Jean-Marc Bonard and Klaus Kern Ecole Polytechnique Federale de Lausanne, CH-05 Lausanne, Switzerland Max-Planck-Institut
DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD
 Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
Life cycle of a tungsten cold field emitter
 JOURNAL OF APPLIED PHYSICS 99, 104903 2006 Life cycle of a tungsten cold field emitter K. S. Yeong and J. T. L. Thong a Department of Electrical and Computer Engineering, Faculty of Engineering, National
JOURNAL OF APPLIED PHYSICS 99, 104903 2006 Life cycle of a tungsten cold field emitter K. S. Yeong and J. T. L. Thong a Department of Electrical and Computer Engineering, Faculty of Engineering, National
Diffraction of Electrons
 Diffraction of Electrons Object: Apparatus: Verify that electrons are waves; i.e., that they diffract just like light waves. This lab is then used to measure their wavelength or, alternatively, measure
Diffraction of Electrons Object: Apparatus: Verify that electrons are waves; i.e., that they diffract just like light waves. This lab is then used to measure their wavelength or, alternatively, measure
Crystal Structure and Electron Diffraction
 Crystal Structure and Electron Diffraction References: Kittel C.: Introduction to Solid State Physics, 8 th ed. Wiley 005 University of Michigan, PHY441-44 (Advanced Physics Laboratory Experiments, Electron
Crystal Structure and Electron Diffraction References: Kittel C.: Introduction to Solid State Physics, 8 th ed. Wiley 005 University of Michigan, PHY441-44 (Advanced Physics Laboratory Experiments, Electron
TO Approved for public release, distribution unlimited
 UNCLASSIFIED AD NUMBER AD463752 NEW LIMITATION CHANGE TO Approved for public release, distribution unlimited FROM Distribution authorized to U.S. Gov't. agencies and their contractors; Administrative/Operational
UNCLASSIFIED AD NUMBER AD463752 NEW LIMITATION CHANGE TO Approved for public release, distribution unlimited FROM Distribution authorized to U.S. Gov't. agencies and their contractors; Administrative/Operational
UNCLASSIFIED. .n ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED
 UNCLASSIFIED.n 260610 ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications or other data are
UNCLASSIFIED.n 260610 ARMED SERVICES TECHNICAL INFORMATION AGENCY ARLINGTON HALL STATION ARLINGTON 12, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications or other data are
Supplementary Figure 1 Experimental setup for crystal growth. Schematic drawing of the experimental setup for C 8 -BTBT crystal growth.
 Supplementary Figure 1 Experimental setup for crystal growth. Schematic drawing of the experimental setup for C 8 -BTBT crystal growth. Supplementary Figure 2 AFM study of the C 8 -BTBT crystal growth
Supplementary Figure 1 Experimental setup for crystal growth. Schematic drawing of the experimental setup for C 8 -BTBT crystal growth. Supplementary Figure 2 AFM study of the C 8 -BTBT crystal growth
Name: (a) What core levels are responsible for the three photoelectron peaks in Fig. 1?
 Physics 243A--Surface Physics of Materials: Spectroscopy Final Examination December 16, 2014 (3 problems, 100 points total, open book, open notes and handouts) Name: [1] (50 points), including Figures
Physics 243A--Surface Physics of Materials: Spectroscopy Final Examination December 16, 2014 (3 problems, 100 points total, open book, open notes and handouts) Name: [1] (50 points), including Figures
UNCLASSI FILED L10160 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STAMTIOI, ALEXANDRIA, VIRGINIA UNCLASSIFIED
 UNCLASSI FILED AD L10160 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STAMTIOI, ALEXANDRIA, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications
UNCLASSI FILED AD L10160 DEFENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STAMTIOI, ALEXANDRIA, VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications
Chapter 16: Ionizing Radiation
 Chapter 6: Ionizing Radiation Goals of Period 6 Section 6.: To discuss unstable nuclei and their detection Section 6.2: To describe the sources of ionizing radiation Section 6.3: To introduce three types
Chapter 6: Ionizing Radiation Goals of Period 6 Section 6.: To discuss unstable nuclei and their detection Section 6.2: To describe the sources of ionizing radiation Section 6.3: To introduce three types
UJNCLASSI FIED A D:FENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION, AI.EXANDRIA. VIRGINIA UNCLASSIFIED
 UJNCLASSI FIED A2 24732 D:FENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION, AI.EXANDRIA. VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications
UJNCLASSI FIED A2 24732 D:FENSE DOCUMENTATION CENTER FOR SCIENTIFIC AND TECHNICAL INFORMATION CAMERON STATION, AI.EXANDRIA. VIRGINIA UNCLASSIFIED NOTICE: When government or other drawings, specifications
RED. BLUE Light. Light-Matter
 1 Light-Matter This experiment demonstrated that light behaves as a wave. Essentially Thomas Young passed a light of a single frequency ( colour) through a pair of closely spaced narrow slits and on the
1 Light-Matter This experiment demonstrated that light behaves as a wave. Essentially Thomas Young passed a light of a single frequency ( colour) through a pair of closely spaced narrow slits and on the
TWO COMPONENT (BINARY) PHASE DIAGRAMS. Experimental Determination of 2-Component Phase Diagrams
 Page 1 of 12 EENS 211 Earth Materials Tulane University Prof. Stephen A. Nelson TWO COMPONENT (BINARY) PHASE DIAGRAMS This document last updated on 08-Oct-2003 Experimental Determination of 2-Component
Page 1 of 12 EENS 211 Earth Materials Tulane University Prof. Stephen A. Nelson TWO COMPONENT (BINARY) PHASE DIAGRAMS This document last updated on 08-Oct-2003 Experimental Determination of 2-Component
Atomic Spectra. d sin θ = mλ (1)
 Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
Atomic Spectra Objectives: To measure the wavelengths of visible light emitted by atomic hydrogen and verify that the measured wavelengths obey the empirical Rydberg formula. To observe emission spectra
Astronomy 101 Lab: Spectra
 Name: Astronomy 101 Lab: Spectra You will access your textbook in this lab. Pre-Lab Assignment: In class, we've talked about different kinds of spectra and what kind of object produces each kind of spectrum.
Name: Astronomy 101 Lab: Spectra You will access your textbook in this lab. Pre-Lab Assignment: In class, we've talked about different kinds of spectra and what kind of object produces each kind of spectrum.
Chapter Six: X-Rays. 6.1 Discovery of X-rays
 Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
Chapter Six: X-Rays 6.1 Discovery of X-rays In late 1895, a German physicist, W. C. Roentgen was working with a cathode ray tube in his laboratory. He was working with tubes similar to our fluorescent
S 3 j ESD-TR W OS VL, t-i 1 TRADE-OFFS BETWEEN PARTS OF THE OBJECTIVE FUNCTION OF A LINEAR PROGRAM
 I >> I 00 OH I vo Q CO O I I I S 3 j ESD-TR-65-363 W-07454 OS VL, t-i 1 P H I CO CO I LU U4 I TRADE-OFFS BETWEEN PARTS OF THE OBECTIVE FUNCTION OF A LINEAR PROGRAM ESD RECORD COPY ESD ACCESSION LIST ESTI
I >> I 00 OH I vo Q CO O I I I S 3 j ESD-TR-65-363 W-07454 OS VL, t-i 1 P H I CO CO I LU U4 I TRADE-OFFS BETWEEN PARTS OF THE OBECTIVE FUNCTION OF A LINEAR PROGRAM ESD RECORD COPY ESD ACCESSION LIST ESTI
In-vessel Tritium Inventory in ITER Evaluated by Deuterium Retention of Carbon Dust
 FT/P1-19 In-vessel Tritium Inventory in ITER Evaluated by Deuterium Retention of Carbon Dust T. Hino 1), H. Yoshida 1), M. Akiba 2), S. Suzuki 2), Y. Hirohata 1) and Y. Yamauchi 1) 1) Laboratory of Plasma
FT/P1-19 In-vessel Tritium Inventory in ITER Evaluated by Deuterium Retention of Carbon Dust T. Hino 1), H. Yoshida 1), M. Akiba 2), S. Suzuki 2), Y. Hirohata 1) and Y. Yamauchi 1) 1) Laboratory of Plasma
Sponsored by. Contract No. N K-0073: Modification P00006 DARPA Order 5674 NR
 OTIC FILE COP Study of Interfacial Chemistry between Metals and Their Effects on Electronic Systems q. o Sponsored by 00 Defense Advanced Research Projects Agency (DOD) and The Office of Naval Research
OTIC FILE COP Study of Interfacial Chemistry between Metals and Their Effects on Electronic Systems q. o Sponsored by 00 Defense Advanced Research Projects Agency (DOD) and The Office of Naval Research
TM April 1963 ELECTRONIC SYSTEMS DIVISION AIR FORCE SYSTEMS COMMAND UNITED STATES AIR FORCE. L. G. Hanscom Field, Bedford, Massachusetts
 402 953 TM-3435 O REDUCTON OF SDE LOBES AND PONTNG ERRORS N PHASED ARRAY RADARS BY RANDOMZNG QUANTZATON STEPS TECHNCAL DOCUMENTARY REPORT NO. ESD-TDR-63-155 April 1963 C- C.1 DRECTORATE OF APPLED RESEARCH
402 953 TM-3435 O REDUCTON OF SDE LOBES AND PONTNG ERRORS N PHASED ARRAY RADARS BY RANDOMZNG QUANTZATON STEPS TECHNCAL DOCUMENTARY REPORT NO. ESD-TDR-63-155 April 1963 C- C.1 DRECTORATE OF APPLED RESEARCH
Secondary ion mass spectrometry (SIMS)
 Secondary ion mass spectrometry (SIMS) ELEC-L3211 Postgraduate Course in Micro and Nanosciences Department of Micro and Nanosciences Personal motivation and experience on SIMS Offers the possibility to
Secondary ion mass spectrometry (SIMS) ELEC-L3211 Postgraduate Course in Micro and Nanosciences Department of Micro and Nanosciences Personal motivation and experience on SIMS Offers the possibility to
SPECTROSCOPIC STUDY OF LUMINESCENCE PATTERNS IN DIAMOND BY ANNA MANI. Received August 21, 1944 (Communicated by Sir C. V. Raman, Kt., F.R.S., N.L.
 SPECTROSCOPIC STUDY OF LUMINESCENCE PATTERNS IN DIAMOND BY ANNA MANI (From the Department of Physics, Indian Institute of Science, Bangalore) Received August 21, 1944 (Communicated by Sir C. V. Raman,
SPECTROSCOPIC STUDY OF LUMINESCENCE PATTERNS IN DIAMOND BY ANNA MANI (From the Department of Physics, Indian Institute of Science, Bangalore) Received August 21, 1944 (Communicated by Sir C. V. Raman,
Chemical Reactions Induced by Ionizing and Electron-beam Irradiation in Freon/Water (Ice) Films
 Chemical Reactions Induced by Ionizing and Electron-beam Irradiation in Freon/Water (Ice) Films Johns Hopkins University (founded in 1876) Dr. C.C. Perry Prof. D.H. Fairborther School of Arts & Sciences
Chemical Reactions Induced by Ionizing and Electron-beam Irradiation in Freon/Water (Ice) Films Johns Hopkins University (founded in 1876) Dr. C.C. Perry Prof. D.H. Fairborther School of Arts & Sciences
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.2491 Experimental Realization of Two-dimensional Boron Sheets Baojie Feng 1, Jin Zhang 1, Qing Zhong 1, Wenbin Li 1, Shuai Li 1, Hui Li 1, Peng Cheng 1, Sheng Meng 1,2, Lan Chen 1 and
DOI: 10.1038/NCHEM.2491 Experimental Realization of Two-dimensional Boron Sheets Baojie Feng 1, Jin Zhang 1, Qing Zhong 1, Wenbin Li 1, Shuai Li 1, Hui Li 1, Peng Cheng 1, Sheng Meng 1,2, Lan Chen 1 and
UNCLASSIFIED AD AIMED SERVICES TECHNICAL INFORIATION MGWY ARLINGTON HALL STAION AILINGIM 12, VIRGINIA UNCLASSIFIED
 UNCLASSIFIED AD 274205. AIMED SERVICES TECHNICAL INFORIATION MGWY ARLINGTON HALL STAION AILINGIM 12, VIRGINIA UNCLASSIFIED NOTICE: When goverment or other dravings, specifications or other data are used
UNCLASSIFIED AD 274205. AIMED SERVICES TECHNICAL INFORIATION MGWY ARLINGTON HALL STAION AILINGIM 12, VIRGINIA UNCLASSIFIED NOTICE: When goverment or other dravings, specifications or other data are used
Structure of Surfaces
 Structure of Surfaces C Stepped surface Interference of two waves Bragg s law Path difference = AB+BC =2dsin ( =glancing angle) If, n =2dsin, constructive interference Ex) in a cubic lattice of unit cell
Structure of Surfaces C Stepped surface Interference of two waves Bragg s law Path difference = AB+BC =2dsin ( =glancing angle) If, n =2dsin, constructive interference Ex) in a cubic lattice of unit cell
Radiation Heat Transfer Prof. J. Srinivasan Centre for Atmospheric and Oceanic Sciences Indian Institute of Science, Bangalore
 Radiation Heat Transfer Prof. J. Srinivasan Centre for Atmospheric and Oceanic Sciences Indian Institute of Science, Bangalore Lecture - 10 Applications In the last lecture, we looked at radiative transfer
Radiation Heat Transfer Prof. J. Srinivasan Centre for Atmospheric and Oceanic Sciences Indian Institute of Science, Bangalore Lecture - 10 Applications In the last lecture, we looked at radiative transfer
Electrostatic charging e ects in fast H interactions with thin Ar
 Nuclear Instruments and Methods in Physics Research B 157 (1999) 116±120 www.elsevier.nl/locate/nimb Electrostatic charging e ects in fast H interactions with thin Ar lms D.E. Grosjean a, R.A. Baragiola
Nuclear Instruments and Methods in Physics Research B 157 (1999) 116±120 www.elsevier.nl/locate/nimb Electrostatic charging e ects in fast H interactions with thin Ar lms D.E. Grosjean a, R.A. Baragiola
Lab 5 - ELECTRON CHARGE-TO-MASS RATIO
 79 Name Date Partners OBJECTIVES OVERVIEW Lab 5 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
79 Name Date Partners OBJECTIVES OVERVIEW Lab 5 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
Lab 5 - ELECTRON CHARGE-TO-MASS RATIO
 81 Name Date Partners Lab 5 - ELECTRON CHARGE-TO-MASS RATIO OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass ratio
81 Name Date Partners Lab 5 - ELECTRON CHARGE-TO-MASS RATIO OBJECTIVES To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass ratio
Beyond the Solar System 2006 Oct 17 Page 1 of 5
 I. Stars have color, brightness, mass, temperature and size. II. Distances to stars are measured using stellar parallax a. The further away, the less offset b. Parallax angles are extremely small c. Measured
I. Stars have color, brightness, mass, temperature and size. II. Distances to stars are measured using stellar parallax a. The further away, the less offset b. Parallax angles are extremely small c. Measured
Lecture 7: Electron Emission
 Lecture 7: Electron Emission Solid state physics of metals E_f > E_c --> Many conduction carriers E_f - Fermi level E_c - minimum conduction band energy A realistic potential well in a metal crystal An
Lecture 7: Electron Emission Solid state physics of metals E_f > E_c --> Many conduction carriers E_f - Fermi level E_c - minimum conduction band energy A realistic potential well in a metal crystal An
Engineering Consultant, Lingnell Consulting Services, 1270 Shores Court, Rockwall, Texas 75087; phone:
 Needle in a Haystack: Identifying Failure Origin Pieces in Heat-Treated Glass Stewart M. Verhulst, M.S., P.E., M. ASCE 1 A. William Lingnell, M.S., P.E., M. ASCE 2 Brenden A. Adams, M.S., P.E., M. ASCE
Needle in a Haystack: Identifying Failure Origin Pieces in Heat-Treated Glass Stewart M. Verhulst, M.S., P.E., M. ASCE 1 A. William Lingnell, M.S., P.E., M. ASCE 2 Brenden A. Adams, M.S., P.E., M. ASCE
Backscattering enhancement of light by nanoparticles positioned in localized optical intensity peaks
 Backscattering enhancement of light by nanoparticles positioned in localized optical intensity peaks Zhigang Chen, Xu Li, Allen Taflove, and Vadim Backman We report what we believe to be a novel backscattering
Backscattering enhancement of light by nanoparticles positioned in localized optical intensity peaks Zhigang Chen, Xu Li, Allen Taflove, and Vadim Backman We report what we believe to be a novel backscattering
1 Written and composed by: Prof. Muhammad Ali Malik (M. Phil. Physics), Govt. Degree College, Naushera
 ELECTROMAGNETISM Q # 1. Describe the properties of magnetic field due to current in a long straight conductor. Ans. When the heavy current is passed through a straight conductor: i. A magnetic field is
ELECTROMAGNETISM Q # 1. Describe the properties of magnetic field due to current in a long straight conductor. Ans. When the heavy current is passed through a straight conductor: i. A magnetic field is
Scanning Tunneling Microscopy Studies of the Ge(111) Surface
 VC Scanning Tunneling Microscopy Studies of the Ge(111) Surface Anna Rosen University of California, Berkeley Advisor: Dr. Shirley Chiang University of California, Davis August 24, 2007 Abstract: This
VC Scanning Tunneling Microscopy Studies of the Ge(111) Surface Anna Rosen University of California, Berkeley Advisor: Dr. Shirley Chiang University of California, Davis August 24, 2007 Abstract: This
Robert A. Meger Richard F. Fernster Martin Lampe W. M. Manheimer NOTICE
 Serial Number Filing Date Inventor 917.963 27 August 1997 Robert A. Meger Richard F. Fernster Martin Lampe W. M. Manheimer NOTICE The above identified patent application is available for licensing. Requests
Serial Number Filing Date Inventor 917.963 27 August 1997 Robert A. Meger Richard F. Fernster Martin Lampe W. M. Manheimer NOTICE The above identified patent application is available for licensing. Requests
FXA UNIT G485 Module X-Rays. Candidates should be able to : I = I 0 e -μx
 1 Candidates should be able to : HISTORY Describe the nature of X-rays. Describe in simple terms how X-rays are produced. X-rays were discovered by Wilhelm Röntgen in 1865, when he found that a fluorescent
1 Candidates should be able to : HISTORY Describe the nature of X-rays. Describe in simple terms how X-rays are produced. X-rays were discovered by Wilhelm Röntgen in 1865, when he found that a fluorescent
ACTIVITY 2 Exploring Light Patterns
 Name: Class: SOLIDS & Visual Quantum Mechanics LIGHT ACTIVITY 2 Exploring Light Patterns Goal We will continue to investigate the properties of LEDs and the incandescent lamp by observing and exploring
Name: Class: SOLIDS & Visual Quantum Mechanics LIGHT ACTIVITY 2 Exploring Light Patterns Goal We will continue to investigate the properties of LEDs and the incandescent lamp by observing and exploring
QUESTIONS AND ANSWERS
 QUESTIONS AND ANSWERS (1) For a ground - state neutral atom with 13 protons, describe (a) Which element this is (b) The quantum numbers, n, and l of the inner two core electrons (c) The stationary state
QUESTIONS AND ANSWERS (1) For a ground - state neutral atom with 13 protons, describe (a) Which element this is (b) The quantum numbers, n, and l of the inner two core electrons (c) The stationary state
Reduced preferential sputtering of TiO 2 (and Ta 2 O 5 ) thin films through argon cluster ion bombardment.
 NATIOMEM Reduced preferential sputtering of TiO 2 (and Ta 2 O 5 ) thin films through argon cluster ion bombardment. R. Grilli *, P. Mack, M.A. Baker * * University of Surrey, UK ThermoFisher Scientific
NATIOMEM Reduced preferential sputtering of TiO 2 (and Ta 2 O 5 ) thin films through argon cluster ion bombardment. R. Grilli *, P. Mack, M.A. Baker * * University of Surrey, UK ThermoFisher Scientific
Supporting Information
 Temperature Effect on Transport, Charging and Binding of Low-Energy Electrons Interacting with Amorphous Solid Water Films Roey Sagi, Michelle Akerman, Sujith Ramakrishnan and Micha Asscher * Institute
Temperature Effect on Transport, Charging and Binding of Low-Energy Electrons Interacting with Amorphous Solid Water Films Roey Sagi, Michelle Akerman, Sujith Ramakrishnan and Micha Asscher * Institute
QPR No. 68 RADIO PHYSICS I. PHYSICAL ELECTRONICS. Prof. W. B. Nottingham J. L. Coggins B. L. Blackford L. E. Sprague RESEARCH OBJECTIVES
 RADIO PHYSICS I. PHYSICAL ELECTRONICS RESEARCH OBJECTIVES Prof. W. B. Nottingham J. L. Coggins B. L. Blackford L. E. Sprague 1. Theory of Energy Conversion Electronics The effectiveness of energy conversion
RADIO PHYSICS I. PHYSICAL ELECTRONICS RESEARCH OBJECTIVES Prof. W. B. Nottingham J. L. Coggins B. L. Blackford L. E. Sprague 1. Theory of Energy Conversion Electronics The effectiveness of energy conversion
HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS
 HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS J. Liu, T. Tsong To cite this version: J. Liu, T. Tsong. HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS. Journal de
HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS J. Liu, T. Tsong To cite this version: J. Liu, T. Tsong. HIGH RESOLUTION ION KINETIC ENERGY ANALYSIS OF FIELD EMITTED IONS. Journal de
MSE 321 Structural Characterization
 Optical Microscope Plan Lenses In an "ideal" single-element lens system all planar wave fronts are focused to a point at distance f from the lens; therefore: Image near the optical axis will be in perfect
Optical Microscope Plan Lenses In an "ideal" single-element lens system all planar wave fronts are focused to a point at distance f from the lens; therefore: Image near the optical axis will be in perfect
Reproduced DOCUMENT SERVICE CENTER ARMED SERVICES TECHNICAL INFORMATION AGENCY U. B. BUILDING, DAYTON, 2, OHIO
 r*' 1, -MrVM«^. * Ji, Reproduced by DOCUMENT SERVICE CENTER ARMED SERVICES TECHNICAL INFORMATION AGENCY U. B. BUILDING, DAYTON, 2, OHIO REEL C ^NOTICE: When Government or other drawings, specifications
r*' 1, -MrVM«^. * Ji, Reproduced by DOCUMENT SERVICE CENTER ARMED SERVICES TECHNICAL INFORMATION AGENCY U. B. BUILDING, DAYTON, 2, OHIO REEL C ^NOTICE: When Government or other drawings, specifications
FOR EXAMINER S USE There are four marks for the quality of written communication in Section Max. Mark
 ADVANCED SUBSIDIARY GCE 2861 PHYSICS B (ADVANCING PHYSICS) Understanding Processes THURSDAY 22 MAY 2008 Afternoon Time: 1 hour 30 minutes *CUP/T43053* Candidates answer on the question paper Additional
ADVANCED SUBSIDIARY GCE 2861 PHYSICS B (ADVANCING PHYSICS) Understanding Processes THURSDAY 22 MAY 2008 Afternoon Time: 1 hour 30 minutes *CUP/T43053* Candidates answer on the question paper Additional
Revision Guide. Chapter 7 Quantum Behaviour
 Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Revision Guide Chapter 7 Quantum Behaviour Contents CONTENTS... 2 REVISION CHECKLIST... 3 REVISION NOTES... 4 QUANTUM BEHAVIOUR... 4 Random arrival of photons... 4 Photoelectric effect... 5 PHASE AN PHASORS...
Photoemission Spectroscopy
 FY13 Experimental Physics - Auger Electron Spectroscopy Photoemission Spectroscopy Supervisor: Per Morgen SDU, Institute of Physics Campusvej 55 DK - 5250 Odense S Ulrik Robenhagen,
FY13 Experimental Physics - Auger Electron Spectroscopy Photoemission Spectroscopy Supervisor: Per Morgen SDU, Institute of Physics Campusvej 55 DK - 5250 Odense S Ulrik Robenhagen,
TMT4320 Nanomaterials November 10 th, Thin films by physical/chemical methods (From chapter 24 and 25)
 1 TMT4320 Nanomaterials November 10 th, 2015 Thin films by physical/chemical methods (From chapter 24 and 25) 2 Thin films by physical/chemical methods Vapor-phase growth (compared to liquid-phase growth)
1 TMT4320 Nanomaterials November 10 th, 2015 Thin films by physical/chemical methods (From chapter 24 and 25) 2 Thin films by physical/chemical methods Vapor-phase growth (compared to liquid-phase growth)
CHARACTERIZATION of NANOMATERIALS KHP
 CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
CHARACTERIZATION of NANOMATERIALS Overview of the most common nanocharacterization techniques MAIN CHARACTERIZATION TECHNIQUES: 1.Transmission Electron Microscope (TEM) 2. Scanning Electron Microscope
PREFERRED RELIABILITY PRACTICES. Practice:
 PREFERRED RELIABILITY PRACTICES Practice No. PD-ED-1239 Page 1 of 6 October 1995 SPACECRAFT THERMAL CONTROL COATINGS DESIGN AND APPLICATION Practice: Select and apply thermal coatings for control of spacecraft
PREFERRED RELIABILITY PRACTICES Practice No. PD-ED-1239 Page 1 of 6 October 1995 SPACECRAFT THERMAL CONTROL COATINGS DESIGN AND APPLICATION Practice: Select and apply thermal coatings for control of spacecraft
Copyright 1965, by the author(s). All rights reserved.
 Copyright 1965, by the author(s). All rights reserved. Permission to make digital or hard copies of all or part of this work for personal or classroom use is granted without fee provided that copies are
Copyright 1965, by the author(s). All rights reserved. Permission to make digital or hard copies of all or part of this work for personal or classroom use is granted without fee provided that copies are
UNCLASSIFIED AD NUMBER LIMITATION CHANGES
 TO: UNCLASSIFIED AD NUMBER AD450636 LIMITATION CHANGES Approved for public release; distribution is unlimited. FROM: Distribution authorized to U.S. Gov't. agencies and their contractors; Administrative/Operational
TO: UNCLASSIFIED AD NUMBER AD450636 LIMITATION CHANGES Approved for public release; distribution is unlimited. FROM: Distribution authorized to U.S. Gov't. agencies and their contractors; Administrative/Operational
Electric Deflection of Electrons
 Electric Deflection of Electrons Objective The purpose of this experiment is to observe that the spacial deflection of an electron in a cathode ray tube is directly proportional to the deflection potential.
Electric Deflection of Electrons Objective The purpose of this experiment is to observe that the spacial deflection of an electron in a cathode ray tube is directly proportional to the deflection potential.
Brown University PHYS 0060 Physics Department LAB B -190
 Physics Department LAB B -190 THE FORCE OF A MAGNETIC FIELD ON A MOVING ELECTRIC CHARGE DETERMINATION OF THE RATIO OF CHARGE TO MASS, e/m, FOR ELECTRONS References: H.D. Young, University Physics, Eleventh
Physics Department LAB B -190 THE FORCE OF A MAGNETIC FIELD ON A MOVING ELECTRIC CHARGE DETERMINATION OF THE RATIO OF CHARGE TO MASS, e/m, FOR ELECTRONS References: H.D. Young, University Physics, Eleventh
APAS Laboratory { PAGE } Spectroscopy SPECTROSCOPY
 SPECTROSCOPY SYNOPSIS: In this lab you will eplore different types of emission spectra, calibrate a spectrometer using the spectrum of a known element, and use your calibration to identify an unknown element.
SPECTROSCOPY SYNOPSIS: In this lab you will eplore different types of emission spectra, calibrate a spectrometer using the spectrum of a known element, and use your calibration to identify an unknown element.
object objective lens eyepiece lens
 Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
Advancing Physics G495 June 2015 SET #1 ANSWERS Field and Particle Pictures Seeing with electrons The compound optical microscope Q1. Before attempting this question it may be helpful to review ray diagram
AP5301/ Name the major parts of an optical microscope and state their functions.
 Review Problems on Optical Microscopy AP5301/8301-2015 1. Name the major parts of an optical microscope and state their functions. 2. Compare the focal lengths of two glass converging lenses, one with
Review Problems on Optical Microscopy AP5301/8301-2015 1. Name the major parts of an optical microscope and state their functions. 2. Compare the focal lengths of two glass converging lenses, one with
Resistance (R) Temperature (T)
 CHAPTER 1 Physical Properties of Elements and Semiconductors 1.1 Introduction Semiconductors constitute a large class of substances which have resistivities lying between those of insulators and conductors.
CHAPTER 1 Physical Properties of Elements and Semiconductors 1.1 Introduction Semiconductors constitute a large class of substances which have resistivities lying between those of insulators and conductors.
Evidence for structure sensitivity in the high pressure CO NO reaction over Pd(111) and Pd(100)
 Evidence for structure sensitivity in the high pressure CO NO reaction over Pd(111) and Pd(100) Scott M. Vesecky, Peijun Chen, Xueping Xu, and D. Wayne Goodman a) Department of Chemistry, Texas A&M University,
Evidence for structure sensitivity in the high pressure CO NO reaction over Pd(111) and Pd(100) Scott M. Vesecky, Peijun Chen, Xueping Xu, and D. Wayne Goodman a) Department of Chemistry, Texas A&M University,
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons
 Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Chapter 1 The discovery of the electron 1.1 Thermionic emission of electrons Learning objectives: What are cathode rays and how were they discovered? Why does the gas in a discharge tube emit light of
Matter. Properties & Changes
 Matter Properties & Changes Properties of Matter Substances anything that has mass and takes up space - matter that has a uniform and unchanging composition also known as a pure substance Physical Properties
Matter Properties & Changes Properties of Matter Substances anything that has mass and takes up space - matter that has a uniform and unchanging composition also known as a pure substance Physical Properties
Lab 6 - ELECTRON CHARGE-TO-MASS RATIO
 101 Name Date Partners OBJECTIVES OVERVIEW Lab 6 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
101 Name Date Partners OBJECTIVES OVERVIEW Lab 6 - ELECTRON CHARGE-TO-MASS RATIO To understand how electric and magnetic fields impact an electron beam To experimentally determine the electron charge-to-mass
(12) Patent Application Publication (10) Pub. No.: US 2005/ A1
 US 2005.0068.047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0068047 A1 Claus (43) Pub. Date: Mar. 31, 2005 (54) METHOD AND DEVICE FOR Publication Classification DISTINGUISHING
US 2005.0068.047A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0068047 A1 Claus (43) Pub. Date: Mar. 31, 2005 (54) METHOD AND DEVICE FOR Publication Classification DISTINGUISHING
HIGH ENERGY DENSITY CAPACITOR CHARACTERIZATION
 GENERAL ATOMICS ENERGY PRODUCTS Engineering Bulletin HIGH ENERGY DENSITY CAPACITOR CHARACTERIZATION Joel Ennis, Xiao Hui Yang, Fred MacDougall, Ken Seal General Atomics Energy Products General Atomics
GENERAL ATOMICS ENERGY PRODUCTS Engineering Bulletin HIGH ENERGY DENSITY CAPACITOR CHARACTERIZATION Joel Ennis, Xiao Hui Yang, Fred MacDougall, Ken Seal General Atomics Energy Products General Atomics
Metal Deposition. Filament Evaporation E-beam Evaporation Sputter Deposition
 Metal Deposition Filament Evaporation E-beam Evaporation Sputter Deposition 1 Filament evaporation metals are raised to their melting point by resistive heating under vacuum metal pellets are placed on
Metal Deposition Filament Evaporation E-beam Evaporation Sputter Deposition 1 Filament evaporation metals are raised to their melting point by resistive heating under vacuum metal pellets are placed on
High Efficiency Collector for Laser Plasma EUV Source.
 University of Central Florida UCF Patents Patent High Efficiency Collector for Laser Plasma EUV Source. 7-11-2006 Jonathan Arenberg Northrop Grumman Corporation Find similar works at: http://stars.library.ucf.edu/patents
University of Central Florida UCF Patents Patent High Efficiency Collector for Laser Plasma EUV Source. 7-11-2006 Jonathan Arenberg Northrop Grumman Corporation Find similar works at: http://stars.library.ucf.edu/patents
Engineering Physics 1 Prof. G.D. Vermaa Department of Physics Indian Institute of Technology-Roorkee
 Engineering Physics 1 Prof. G.D. Vermaa Department of Physics Indian Institute of Technology-Roorkee Module-04 Lecture-02 Diffraction Part - 02 In the previous lecture I discussed single slit and double
Engineering Physics 1 Prof. G.D. Vermaa Department of Physics Indian Institute of Technology-Roorkee Module-04 Lecture-02 Diffraction Part - 02 In the previous lecture I discussed single slit and double
Electron Diffraction
 Exp-3-Electron Diffraction.doc (TJR) Physics Department, University of Windsor Introduction 64-311 Laboratory Experiment 3 Electron Diffraction In 1924 de Broglie predicted that the wavelength of matter
Exp-3-Electron Diffraction.doc (TJR) Physics Department, University of Windsor Introduction 64-311 Laboratory Experiment 3 Electron Diffraction In 1924 de Broglie predicted that the wavelength of matter
