CHEMICAL EQUILIBRIUM WITH APPLICATIONS X PROGRAM WITH USER INTERFACE AND VISUAL BASIC PROGRAMMING BY JAKE DUNCAN RUMEL THESIS
|
|
|
- Piers Sutton
- 5 years ago
- Views:
Transcription
1 CHEMICAL EQUILIBRIUM WITH APPLICATIONS X PROGRAM WITH USER INTERFACE AND VISUAL BASIC PROGRAMMING BY JAKE DUNCAN RUMEL THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Mechanical Engineering in the Graduate College of the University of Illinois at Urbana-Champaign, 2016 Urbana, Illinois Adviser: Professor M Quinn Brewster
2 ABSTRACT This thesis details the creation of a program named CEA X Program that calculates the chemical equilibrium compositions of complex mixtures using a user interface created in Visual Basic. The program uses the iterative method of minimizing the Gibbs energy or the Helmholtz energy first utilized in the NASA CEA program [1]. The original NASA CEA Program requires the use of FORTRAN compliers and knowledge of how to create program input files. The new CEA X Program uses Excel macros and user forms which improves the user s experience while retaining the accuracy of chemical equilibrium calculations and adds new functionality to provide feedback to the user earlier in the input process. This document includes an overview of how the program was developed, details on how to use the program, and provides examples of the CEA X Program output and testing. ii
3 TABLE OF CONTENTS Chapter 1: Introduction... 1 Chapter 2: Methodology for Migrating Code... 4 Chapter 3: Main Menu and Parameters... 7 Chapter 4: Product and Reactant Properties Chapter 5: Problem Input Screen Chapter 6: Problem Type Specific Screens Chapter 7: Output Document Chapter 8: Program Testing and Examples References Appendix A: Detailed Test Cases Appendix B: Date Codes Appendix C: Error, Warning, and Informational Messages Appendix D: Code Listing iii
4 Chapter 1: Introduction The CEA X Program described in this document is a reconceptualization of the NASA CEA 2 Program [1] for calculating chemical equilibrium compositions. The CEA X Program includes a user interface which calls the Excel macros containing the subroutines necessary for performing the chemical equilibrium equations and produces an output document for printing the results. This document will begin with references to the calculations methodology and an overview of how the CEA X Program recreated this functionality. The remainder of the document details how the user interacts with the user interface and can serve as a user manual for the program. The end of the document has several example problems and details the testing done to confirm the program produces consistent results with the NASA CEA 2 Program [1] Background on NASA CEA Program The NASA Lewis Research Center s computer programming department created a program called CEA (Chemical Equilibrium with Applications) to ascertain the chemical equilibrium compositions of complex mixtures. The program received an update to version CEA 2 with the last modifications made May 21 st, This document will refer to the CEA 2 version as the NASA CEA program [1]. The NASA CEA program [1] was written in ANSI standard FORTRAN 77 and requires the compiling of the cea2.f file with the cea.inc file and the thermodynamic data file (thermo.inp) and the optional thermal transport properties file (trans.inp). Once the program is compiled and run, it will take any (.inp) file and produce an (.out) file with the results of the chemical equilibrium calculations and an optional (.plt) file. The NASA CEA program [1] uses two thermodynamic state functions to obtain chemical equilibrium compositions for species within a user specified mixture. The chemical equilibrium compositions are calculated using a descent Newton-Raphson iteration method for minimizing the Gibbs energy or Helmholtz energy based on which state functions are used to describe the thermodynamic state. The program will use this iteration process until the convergence criteria are achieved. Gaseous products only are considered first for convergence, and then the program will check if condensed phases of the products need to be included in the system. To explain the NASA CEA program [1], the NASA Reference Publication 1311 [2,3] by Sanford Gordon and Bonnie J. McBride was created and is broken into two sections. Part I of the document details the approach and the calculations used for the chemical equilibrium analysis. Part II of the document serves as a user manual for the CEA Program and describes the various subroutines in the program. The CEA X 1
5 Program in this document uses the same calculations described in Part I of the NASA Reference Publication 1311 [2]. This document can be seen as a replacement of the Part II of the NASA Reference Publication 1311 [3] with the details specific to the CEA X Program User Interface Purpose and Enhancements The major difference between the CEA X Program and the NASA CEA program [1] is the addition of a user interface. The user interface was added to guide the user to specify the minimum necessary information required to perform the program calculations and removes the necessity for the user to understand how to create and format a problem (.inp) file. The new user interface also allows the user to quickly see all options for the input or output that are available from the program. Additionally, the program adds several checks of the input data to prevent information that will automatically result in an output error in the NASA CEA program [1] or CEA X Program. These checks include: Check that the minimum data input requirements are met Check that the reactant temperatures specified can retrieve the reactant enthalpy from the thermodynamic library Check that the fuel-to-oxidant ratios match the number of specified reactant fuels and oxidants Use dropdown menus for units to ensure the program recognizes unit input Add data type validations (numeric inputs for temperature, etc.) The user interface also includes the ability for the user to go in and view the thermodynamic properties that have been loaded into the reactant and product libraries of the program. The information was previously not available in the NASA CEA program [1] without the ability to read (.lib) files and could only be partially seen from the (thermo.inp) file. While the CEA X Program does not allow the user to edit these thermodynamic properties directly, it allows the user to update the reactants and products from the (thermo.inp) file or equivalent. Using a user interface for selecting reactants from a list during the problem phase input allows for additional performance enhancements for the program. The CEA X Program uses an integer to keep track of which row in the stored thermodynamic data to pull the properties for selected reactants. This allows the program to avoid searching the entire list of reactants using a name search (which is performed in the NASA CEA program [1] ). A similar integer approach is not used for searching for 2
6 available reactants since this search requires searching the library for available products based on the chemical formula instead of a set list. Finally, the user interface adds a simpler management of the program parameters. The NASA CEA program [1] required updating the parameters in the cea.inc file and recompiling the program. The CEA X Program allows the user to quickly change these parameters based on the user preferences Out of Scope Components The CEA X Program recreates much of the NASA CEA program [1] functionality for calculating the chemical equilibrium compositions and output capabilities, however the following features of the NASA CEA program [1] were considered out of scope for the CEA X Program: The Rocket Problem type The Shock Problem type The Chapman-Jouguet Detonation Problem type Calculating the thermal transport properties 3
7 Chapter 2: Methodology for Migrating Code The CEA X Program uses the same equations for calculating the chemical equilibrium compositions as the NASA CEA program [1]. For this reason, the subroutines in the program macros use a similar naming convention and the content is easily relatable. Additionally, names of specific code lines and section comment headers were kept consistent with the NASA CEA program [1] where appropriate to aide in the comparison of the code. However, the code language was updated from ANSI standard FORTRAN 77 to Visual Basic. In addition to the language update, the CEA X Program changed from using global variables to using cells in hidden Excel sheets to store problem values. The sheet names are consistent with the grouping of global variables in the cea.inc file. This allows for better debugging and visibility of the information stored within the program. The program macros contain many comments relating the cell names to the variables used in the NASA CEA program [1] Subroutine Relationships The CEA X Program subroutine differences from the NASA CEA program [1] are the most significant for those that relate to reading the problem input information or printing the results output. Many of the subroutines that read the input have been replaced by the user interface or parts have been integrated into the smaller subroutines that activate during specific screen interactions. The output subroutines have been modified to print the results in an Excel document instead of (.out) and (.plt) files. The CEA X Program uses the same thermo.inp file format for loading the thermodynamic data for the reactants and products. This allows for consistency of the data between both programs. While the CEA X Program performs the same calculations on the file as the NASA CEA program [1], the reactant and product data is stored in a hidden sheet within the Excel document instead of creating a separate (.lib) file. This allows the CEA X Program to be used on its own after the initial loading of reactants and products. The thermo.inp file is only required when this information needs to be updated. Table 1 below describes the relationship of each NASA CEA program [1] subroutine to the subroutines or screen in the CEA X Program. 4
8 Table 1 Subroutine Relationships NASA CEA [1] Subroutine Main Program BLOCKDATA CPHS and ALLCON DETON EFMT EQLBRM FROZEN GUASS HCALC INFREE INPUT MATRIX NEWOF OUT1 OUT2 OUT3 OUT4 REACT RKTOUT ROCKET SEARCH and READTR SETEN SHCK THERMP TRANIN TRANP UTHERM UTRAN VARFMT CEA X Program Relationship Separated into several subroutines with user interface Stored in Hidden sheets Updated for Visual Basic Unused Unused Updated for Visual Basic Unused Updated for Visual Basic Unused Replaced by user interface Split between Problem Input and Output screen and the Problem Type Specific screens Updated for Visual Basic Updated for Visual Basic Updated for Visual Basic and writing to Excel Updated for Visual Basic and writing to Excel Updated for Visual Basic and writing to Excel Unused Updated for Visual Basic Unused Unused Updated to search Excel instead of (.lib) file Updated for Visual Basic Unused Updated for Visual Basic Unused Unused Updated for Visual Basic and writing to Excel Unused Unused 2.2. Program Flow Diagram The CEA X Program uses several screens to replicate and add to the functionality offered by the NASA CEA program [1]. Figure 1 is a diagram which shows the flow of the CEA X Program screens which are detailed in the subsequent chapters. 5
9 Figure 1 Program Flow Diagram 6
10 Chapter 3: Main Menu and Parameters 3.1. Main Menu The program starts on the CEA tab which contains details about when the code was last updated and a button to start the main macro. Clicking on the Main Menu button brings up the screen CEA Program Main Menu (See Figure 2). This screen and all other screens generated by the program use a subroutine to center the starting position in the middle of the open Excel Document which allows for ease of use with multiple monitor displays. The Main Menu has five available options: Figure 2 Main Menu Table 2 Main Menu Options Button Description Input Begins user input for equilibrium problem (See Chapter 6) Displays the temperature ranges considered for the thermodynamic Thermo Ranges properties of the reactants and products (See Chapter 4) Displays and loads properties of the reactants and products View Products / Reactants (See Chapter 4) Parameters Allows the user to manage the CEA X Program settings (See Section 3.2) Exit Stops the program macro The Exit button does not clear any information that has been saved from the previous screens. 7
11 3.2. Parameters Selecting the Parameters button displays the Program Parameters screen seen in Figure 3. Figure 3 Program Parameters These parameters manage the number of data elements allowed for each species or input. Increasing these limits can allow for more complicated systems to be considered, but will decrease performance. The naming convention used in this screen matches PARAMETER definitions in the NASA CEA code [1]. Table 3 Parameter Descriptions Parameter MAXNGC MAXNC NCOL MAXMAT MAXTR MAXR MAXEL MAXNG MAXMIX MAXT MAXPV Description Maximum number of gaseous or condensed species that can be considered Maximum number of condensed species temperature intervals allowed Columns of data printed in the output document for formatting Maximum number of rows for the composition iteration matrix Number of gaseous products considered for thermal transport calculations (unused) Maximum number of reactants allowed to be specified Maximum number of elements allowed for consideration Maximum number of gaseous products allowed for consideration Maximum number of mixture values allowed Maximum number of temperature inputs allowed Maximum number of pressure or volume/density inputs allowed 8
12 Each of these values can be changed and saved using the Save or Save and Close buttons. The program has two default settings that can be loading using the Default Large or Default Small buttons. The default values follow closely to those suggested in the NASA Reference Publication [3] and are as follows: Table 4 Parameter Defaults Parameter Default - Large Default Small MAXNGC MAXNC NCOL MAXMAT MAXTR MAXR MAXEL MAXNG MAXMIX MAXT MAXPV All sample outputs in this document were produced using the Default Large parameter settings. 9
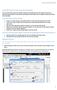
13 Chapter 4: Product and Reactant Properties 4.1. Thermodynamic Temperature Ranges The Thermodynamic Temperature Ranges screen is accessed through the Main Menu screen s Thermo Ranges button. This screen functions as information only for the temperature ranges used for gaseous species properties. The range values are loaded during the load process for the products and reactant properties (see Section 4.2.2). Since these temperature ranges are used to set up the thermodynamic properties, and extrapolate some thermodynamic properties during the load process, they cannot be changed unless the input file is modified and all products and reactants are re-loaded. The date listed in the Thermodynamic Temperature Ranges screen is also set from the product and reactant load process and describes when the thermodynamic properties were last modified in the file used for loading. The values seen in Figure 4 below are the values that were used for all sample problems in this document. Figure 4 Thermodynamic Temperature Ranges 4.2. Thermodynamic Product and Reactant Data The Chemical Equilibrium Program uses a set list of products and reactants which can be considered for inputs and outputs of problem sets. The products and reactants must be loaded prior to being able to run a program, but only need to be loaded once (unless a species needs to be added or modified) as the properties will be saved to a hidden sheet within the Excel document. The products and reactants can be viewed and managed through the Thermodynamic Data screen accessed through the View Products / Reactants button on the Main Menu screen. 10
14 Figure 5 Thermodynamic Data The Thermodynamic Data screen shown in Figure 5 above displays the Name and Notes for each species that have been loaded and are available for use by the program. The Name displayed is the name that will be presented to the User for selection in any selection screen and will appear in the output results. Names which begin with an * are gases which used explicitly defined thermodynamic properties for the third temperature interval (see Section 4.2.2). The Notes for each species are loaded from the input file and describe the source of the thermodynamic properties and the date of the source. The dropdown menu in the upper left corner of the screen allows the user to switch between the products and reactants. The default for the screen is to display the products, but changing the dropdown allows the User to view the reactants with the same Name and Notes conventions. The products and reactants will have only one instance of each gaseous species that is considered. However, condensed species can have multiple rows in this screen where each instance corresponds to a different temperature interval for the thermodynamic properties. This multi-instance convention is a result of the loading process (See Section 4.2.2) and is consistent with the NASA CEA program [1] View Individual Species Properties Selecting a species in the Figure 5 screen enables the View Button. Activating the View Button brings up a screen which displays the thermodynamic properties that have been loaded for the selected species. 11
15 Figure 6 below displays the properties for the ALCL 2 species. The screen title will display Thermodynamic Properties - and the name of the species selected from the previous screen. Figure 6 Species Thermodynamic Properties The information in the Thermodynamic Properties screen for each species is informational only and cannot be modified. These values can only be updated by modifying the thermodynamic properties input file and re-loading through the Section process. Table 5 below describes the information displayed in the Thermodynamic Properties screen: Table 5 Thermodynamic Property Descriptions Field Name Date Notes Molecular Formula NTL Molecular Weight Hform Temperature Range Description Name displayed in selection screens and Thermodynamic Data screen Six-character reference date code from input file. See Appendix B Specific details about source of thermodynamic properties Exploded form of the molecular formula. E used to identify ions Number of temperature intervals that were supplied in the input file Molecular weight of the species in g/mol Enthalpy of the species in (kg-mol)*k/kg Reactants Only Temperature range that species thermodynamic properties are defined for. If only one temperature is given, then temperature must be within +/- 10K from that temperature for thermodynamic properties to be considered. Clicking on the THERMO button displays the thermodynamic values used by the program for the number of temperature intervals (NTL). Figure 7 below shows the THERMO Details screen for the ALCL 2 12
16 product seen in Figure 6. The THERMO button will be disabled for all species that do not have thermodynamic properties defined over a temperature interval and only have a temperature and enthalpy specified. The thermodynamic values are calculated during the Section load process only and displayed as reference information in this screen. Figure 7 Species THERMO details Loading Product and Reactant Properties Updating or loading the products and reactants requires loading from an input file (.inp). The process is started by selecting the Update From File button on the Thermodynamic Data screen seen in Figure 5. Loading from the file removes all product and reactant information prior to loading in new data to avoid issues with duplicating species. Since this process will delete information, the User is prompted with a warning message in Figure 8 below prior to proceeding. Selecting OK will open up a browser for the User to select which Input File to load the products and reactants from. Figure 8 thermo.inp Load Warning The products and reactants with their respective thermodynamic properties are loaded using the same thermo.inp file that is used to create the thermo.lib file in the NASA CEA program [1]. Figure 9 below shows the format of the thermo.inp files. The information under the thermo header line has the 13
17 temperature ranges and date which get loaded into the screen seen in Figure 4. Following that line is the information for the electron product with thermodynamic properties for the three thermodynamic ranges. The full description of the format required for the thermo.inp file can be found in NASA Reference Publication 1311 Appendix A [3]. Figure 9 thermo.inp Format The program reads in the data from the input file into a temporary hidden sheet then processes the thermodynamic properties with a process that mimics the UTHERM subroutines from the NASA CEA program [1]. For gases, if coefficients are not given for the third temperature interval, then the program uses a straight line extrapolation for Cp/R. Gases that have been defined for all three intervals without extrapolation are designated with an *. Condensed species are loaded with only one temperature range and no extrapolations are performed. If the condensed species in the input file has multiple temperature ranges specified, these are broken into multiple species lines. The calculations performed during loading of the thermodynamic data is detailed in NASA Reference Publication 1311 [2] Section 4. 14
18 Chapter 5: Problem Input Screen The majority of interaction with the program for the User is done in the Problem Input and Output screen. This screen is accessed by clicking the Input Button on the Main Menu screen and is used for specifying the various input parameters and options for the output. The problem type specific state properties are the only parameters not specified prior to advancing beyond this screen. These state properties are described in Chapter 6. Figure 10 Problem Input and Output Clicking on the Input Button will reset all variables to their default values and clear any input information. So, any information entered in the Problem Input and Output Screen is not deleted after hitting Cancel which can be used for debugging but is deleted with subsequent openings of the Problem Input and Output screen. 15
19 The Problem Input and Output screen seen in Figure 10 above is broken into various sections based on the what problem information is impacted by the User selections. Some sections require a minimum amount of information to be supplied before the User is allowed to advance the screen and contains some validation to prevent the user from entering values that are outside of the program requirements. However, the program cannot guarantee problem convergence for all user scenarios inputted Problem Information The general problem information is located at the top of the screen just below the screen title. The component of this section is the Problem Type dropdown box. Table 6 below shows the different problem types as the following: Table 6 Problem Types Problem Types Temperature and Pressure Enthalpy and Pressure Entropy and Pressure Temperature and Volume Internal-Energy and Volume Entropy and Volume Changing the Problem Type influences which Problem Type Specific Screens will display after advancing from the Problem Input and Output screen and what minimum input information is required. The Problem Type Specific Screens are described in Chapter 6. The default option for the Problem Type is Temperature and Pressure. The problem information section allows the User to enter a name for the Case. This allows the User to provide a name that will be displayed in the problem output for reference. In the top right corner of the Problem Input and Output screen is an input status indicator which is the text surrounded by a frame. This contains various messages that help guide the user to what additional information is required before the screen can advance with the Next Button. The following Table 7 details the possible messages in the input status indicator and the required actions (See Section 5.2 for completing actions required). 16
20 Table 7 Input Status Messages Message Insert Reactants Need Reactant Amounts Need Temperatures for Library Reactants Oxidant Not Permitted When 100% Fuel Fuel Not Permitted When 100% Oxidant Must Specify a Fuel and Oxidant Ready Action Required Enter at least one reactant. Enter either reactant amounts or fuel-oxidant ratios. For Enthalpy or Internal-Energy problems, temperatures for each noncustom reactant have to be entered in the Properties screen. If 100% Fuel is specified in the Fuel-Oxidant screen, then no reactant can be designated as an oxidant in the Properties screen. If 100% Oxidant is specified in the Fuel-Oxidant screen, then no reactant can be designated as a fuel in the Properties screen. If 100% Fuel or Oxidant is not specified in the Fuel-Oxidant screen, then there must be at least one fuel reactant and oxidant reactant designated. Minimum data requirements met and Next button is enabled Reactants The reactants section of the Problem Input and Output screen contains the majority of the minimum required information and heavily influences the input status indicator (See Section 5.1). This section allows the User to specify which reactants are supplied for the chemical equilibrium problem and any necessary initial parameters needed for the reactants. Figure 11 Selected Reactants 17
21 The list of available reactants is a multi-selectable list that is generated from the reactants that have been loaded into the program (See Section 4.2). Scrolling through the long list of reactants can be time consuming when the user already knows which reactants are desired for selection. The program has a search filter to help reduce the number of reactants that display in the multi-select list. The list of reactants will be filtered to all reactants that begin with the text displayed in the search text box after the user clicks on the button. Figure 12 below shows the result of typing O2 as the search filter and activating the filter. Clearing all text from the search filter and clicking on the button will return all reactants to the multi-select list. Figure 12 Search Filtered Reactants Selecting one or more reactants in the available reactants list and clicking the Add button will place all highlighted reactants in the Selected list. Selecting a reactant will not remove that reactant from the available list, but the Add button checks the selected reactants list prior to adding an available reactant to avoid duplication. Clicking on a reactant in the selected list and activating the Remove button in the reactants section will delete that reactant from the selected list. Only one selected reactant can be removed per use of the remove button. The User will be prompted with an error message if the User tries to add more reactants than are allowed from the defined parameter (See Section 3.2) Custom Reactants If the user wants to supply a reactant that is not found in the list of available reactants, then the user may click on the Create button to add a custom reactant to the selected reactants list. The Create button will bring up the Create Temporary Reactant screen seen in Figure 13 below. This screen is used whenever the user adds a new custom reactant or chooses to edit a previously created reactant. 18
22 No edits are allowed for library reactants using this screen (See Section for editing library reactants). Figure 13 Custom Reactant The reactants require a name to be supplied for the reactant (maximum length of fifteen characters). The name cannot match the name of a current library reactant and the user will be prompted with an error message if a matching name is supplied. The reactant name will be displayed in the selected reactants list after successfully creating the reactant and will be displayed in the output document (See Chapter 7) wherever the reactant name is displayed. Additionally, each custom reactant requires a chemical formula to be supplied in exploded form. The chemical formula allows for a maximum of twelve elements and coefficients to be supplied. The element symbols are cross-referenced against the list of atomic symbols and prevent the user from creating the custom reactant unless all supplied symbols are valid. The coefficients must be numeric, but do not need to be integers. The program uses a coefficient of one if the user supplies an element symbol with no coefficient. Finally, the custom reactant will require an enthalpy (H/R) or internal-energy (U/R) for each custom reactant. The enthalpy can be either set in the Create Temporary Reactant screen or the Reactant Properties screen (See Section 5.2.2). Leaving the value blank in the Figure 13 screen will set the enthalpy or internal-energy equal to zero. The zero value will have no impact on problems such as the Temperature and Pressure problem type in which the reactant enthalpy or internal-energy is not used. The enthalpy or internal-energy is supplied in j/mol, kj/mol, cal/mol, or kcal/mol units. Custom reactants are not added to the program s internal library of reactants. If they are removed from the selected reactants list using the Remove button, then all information about the custom reactant is deleted. The user will have to recreate the custom reactant using the Create Temporary Reactant screen again. Additionally, custom reactants must be created every time the program is restarted. This is done for stronger data quality governance and prevents duplication of reactant species. 19
23 Reactant Properties Once reactants have been added to the selected reactants list, the Properties button becomes available as seen in Figure 11. Figure 14 Reactant Properties The Reactants Properties screen shown above in Figure 14 is a dynamically created screen that allows for the input of various properties for each reactant that were in the selected reactants list at the time the Properties button was activated. A row is created for each reactant and the name of the reactant becomes the label for each row. The Fuel/Oxid column provides the choice to specify whether the reactant is to be the fuel or the oxidant for the reaction. There are no restrictions for which reactants need to be a fuel or which need to be an oxidant. However, there are rules for the number of fuels or oxidants specified if fuel/oxidant ratios have been specified (See Section 5.2.3). The relative amount can be specified for each reactant. The dropdown menu at the bottom of the relative amount column allows the user to specify the amount in either Weight % or Moles. If the relative amounts are not supplied for all reactants, then fuel/oxidant ratios must be specified (See Section 5.2.3). The temperature for each library reactant must be specified if the problem is an enthalpy or internalenergy problem. The temperatures can be specified in Kelvin (K), Fahrenheit (F), Rankine (R), or Celsius (C) with the use of the units dropdown menu at the bottom of the temperature column. The temperature for each reactant must be specified such that it s thermodynamic properties have been defined for that temperature. For a gas, this is in the temperature range specified in the Section 3.2 load process or within 10K of the specified temperature for condensed species. 20
24 When the User actives the Save or Save and Close button with an invalid temperature, the program will display an error message similar to Figure 15 below to aid the User in specifying a correct value. Figure 15 Temperature Property Error Message If the User wants the output to calculate the density of the total reactant, then the User may specify the density for each reactant. The dropdown menu for the units at the bottom of the density column allow the units for g/cm 3 or kg/m 3. For problems with specific volume, densities can be supplied instead of specific volume. However, these densities are entered in the Problem Type Specific Screens (See Chapter 6) instead of this reactants properties screen. Finally, if the reactant is a custom reactant and the problem is an enthalpy or internal-energy problem then an enthalpy (H/R) or internal energy (U/R) must be supplied. An enthalpy or internal-energy cannot be supplied for a library reactant since those will be calculated using the reactant temperature. If the user supplied an enthalpy or internal energy in Section 5.2.1, then this value will display in the Reactant Properties screen. The enthalpy or internal-energy must be supplied in either j/mol, kj/mol, cal/mol, or kcal/mol units Fuel-Oxidant Ratios As an alternate to supplying relative amounts for each reactant, the User may enter in fuel/oxidant ratios using the Fuel-Oxidant Mixture Values screen shown in Figure 16 below after activating the Fuel-Oxidant button. The dropdown menu in the upper left corner of the screen allows the User to specify different fuel to oxidant ratios. The User may supply: Percent Fuel by Weight Fuel-to-Oxidant Weight Ratios Oxidant-to-Fuel Ratios Equivalence Ratios Chemical Equivalence Ratios 21
25 Figure 16 Fuel / Oxidant Ratios The User has the option of supplying a maximum and minimum ratio with a number of equally spaced intervals using the Fuel Oxidant frame on the left. An error message will display if the maximum ratio is less than the minimum ratio. Alternatively, the user can supply a number of specific ratios using the Fuel Oxidant frame on the right. If both frames are filled out, the program will take the values from the maximum and minimum frames only. If fuel/oxidant ratios are used, then the number of reactants labeled as a fuel or oxidant must abide by the following restrictions in Table 8 below: Table 8 Fuel to Oxidant Constraints Fuel Oxidant Ratio Reactant Constraints 100% Fuel All reactants must be labeled as Fuel. 100% Oxidant All reactants must be labeled as Oxidant. Fuel and Oxidant Mixture There must be at least one fuel and one oxidant in the reactants. These constraints on the reactants prevent the User from running the problem with more than one of the above scenarios Products The products section of the Problem Input and Output screen allows the User to control the products that are considered in the problem. The available products list is a multi-selectable list that is generated from the products that have been loaded into the program (See Section 4.2). With one or more 22
26 products selected, the User may select one of the three buttons Only, Omit, or Insert to add all selected products to the corresponding list. The products will not be removed from the available products list after being added to another list, and the program will not add duplicates to any list. The Remove buttons under each of the lists will remove highlighted products in the Only, Omit, and Insert lists one at a time when activated. The following table describes the use case for each products list. Table 9 Product List Types Product List Only Omit Insert Use Case If products are given in the Only list, the program will only consider these as the possible products for the reaction. If products are given in the Omit list, the program will exclude these products from consideration in the reaction. If products are given in the Insert list, the program will start the iterations with these species as condensed (instead of starting with only gaseous products in the iterations) Due to the use cases, if the User adds a product to the omit list, then the program will remove that product from the only and insert lists (if necessary). Similarly, adding a product to either the only or insert lists will remove that product from the omit list if it has previously been added to the omit list. To help the user select the products from the long list of products, the CEA X Program has two tools to filter the list of available reactants. The first is the same type of search filter that exists for the reactants (See beginning of Section 5.2). The second tool is the Custom Filter button. This pulls a list of the products from the Filter sheet which is editable by the user. This helps when running multiple problems with similar long lists of Only, Omit, or Insert products repeatedly. The program will check to determine if each product supplied in the custom list matches a product in the program library. If there is no match, then an error message is displayed to the user. Clicking on the button with no text in the search bar will bring back the full list of products no matter which filter was used previously Output The output section of the Problem Input and Output screen provides several options for the problem output. The description of each output option is summarized in the following Table 10: 23
27 Table 10 Output Options Output Option SI units Massf Short Ions Debug Trace Description Changes the units used in the output document. Output values are given in mass fraction instead of mole fraction. Prints only error messages and final tables to the output file. Sets whether ionic products are considered. Creates a separate tab in the output document that prints the intermediate output of the program iterations for the selected parameter numbers. (See Section 7.2) Changes the value of the trace threshold for printing product compositions Plot Parameters The program is able to print several parameters in a format that is readily available for plotting. The plot parameters section of the Problem Input and Output screen is a multi-selection list of the available parameters for plot output. When one or more parameters have been selected the output document will create a tab with the parameters listed in table format (See Chapter 7). Figure 17 Plotting Products Selecting the product mole/mass fraction plot parameter, prompts the user with an additional screen after the Problem Type Specific Screens. The Plotting Products screen shown in Figure 17 above provides a multi-select list of the products that are being considered for the problem. The User can select as many products as desired to add the mole/mass faction of that product to the plot output. 24
28 Chapter 6: Problem Type Specific Screens Once the User has completed entering in all desired parameters into the Problem Input and Output screen, the program will advance to a Problem Type Specific Screen when the Next button is activated. The screen that displays depends on the selection in the Problem Type dropdown box from Section 5.1. The title of each screen will contain the selection from the dropdown for reference Temperature and Pressure Figure 18 Temperature and Pressure Input Temperature and Pressure problems require at least one value for each state properties, but the program also allows for a range of values to be supplied. If a range of values have been selected, the program will use a cross join method for the two ranges of parameters. This means that if two temperatures are given and one pressure, then two sets of results will be produced. Then, if two temperatures are given and two pressures are supplied, then four sets of results will be produced from the program. The user may supply each individual temperature or pressure desired using the frames on the left of the Problem Temperature and Pressure screen shown in Figure 18 above with a maximum number of elements set by the parameters (See Section 3.2). Alternatively, the user can use one or more of the 25
29 frames on the right of the screen to supply a maximum, minimum, and number of intervals that will create a range of equally spaced values for the user. The program uses the maximum and minimum range values in the event that the User enters values in both the left and right frames. Additionally, the program will check to make sure the maximum value supplied is larger than the minimum value supplied when the range frames on the right are being utilized. The lower left section of the Problem Temperature and Pressure screen controls the units for all temperature and pressure frames. The temperature may be supplied in Kelvin (K), Rankine (R), Celsius (C), or Fahrenheit (F). The pressure can be supplied in BAR, atmospheres (ATM), pounds per square inch (PSI), or millimeters Mercury (mmhg) Enthalpy and Pressure Figure 19 Enthalpy and Pressure Input Enthalpy and Pressure problems require at least one value to be supplied for the pressure. The program will use the enthalpies of the reactants at the temperatures supplied for each reactant (See Section 5.2.2). Alternatively, the user may choose to supply one enthalpy (H/R) for the mixture that will override the enthalpies from the individual reactant temperatures. This enthalpy is supplied in the 26
30 labeled frame seen in the Problem Enthalpy and Pressure screen shown in Figure 19 above and must be in units g*mole*k/(g of mixture). The pressure may be supplied in BAR, atmospheres (ATM), pounds per square inch (PSI), or millimeters Mercury (mmhg) which is controlled with the dropdown menu in the lower left section of the screen. While only one value is allowed for the enthalpy, the program allows for a range of pressures to be supplied. The range of values can be individually specified in the frame on the left of the screen with a maximum number of elements set by the parameters (See Section 3.2). The frame on the right of the Problem Enthalpy and Pressure screen allows the program to create an equally spaced range of values from a supplied maximum pressure, minimum pressure, and number of intervals. The program uses the maximum and minimum range values in the event that the user enters values in both the left and right frames. Additionally, the program will check to make sure the maximum value supplied is larger than the minimum value supplied when the range frames on the right are being utilized Entropy and Pressure Figure 20 Entropy and Pressure input 27
31 Entropy and Pressure problems require at least one value to be supplied for the pressure and exactly one value for the entropy (S/R). The entropy is supplied in the labeled frame on the right side of the screen above the Next and Back buttons seen in the Problem Entropy and Pressure screen shown in Figure 20 above and must be in units g*mole/(g of mixture). The pressure may be supplied in BAR, atmospheres (ATM), pounds per square inch (PSI), or millimeters Mercury (mmhg) which is controlled with the dropdown menu in the middle right section of the screen. While only one value is allowed for the entropy, the program allows for a range of pressures to be supplied. The range of values can be individually specified in the frame on the left of the screen with a maximum number of elements set by the parameters (See Section 3.2). The frame on the right of the Problem Entropy and Pressure screen allows the program to create an equally spaced range of values from a supplied maximum pressure, minimum pressure, and number of intervals. The program uses the maximum and minimum range values in the event that the user enters values in both the left and right frames. Additionally, the program will check to make sure the maximum value supplied is larger than the minimum value supplied when the range frames on the right are being utilized Temperature and Volume Figure 21 Temperature and Volume Input 28
32 Temperature and Volume problems require at least one value for temperature and at least one value for the volume or density. Similar to other problem types, the program also allows for a range of values to be supplied for each property. If a range of values have been selected, the program will use a cross join method for the two ranges of parameters. This means that if two temperatures are given and one pressure, then two sets of results will be produced. Then, if two temperatures are given and two pressures are supplied, then four sets of results will be produced from the program. The user may supply each individual temperature, volume, or density desired using the frames on the left of the Problem Temperature and Volume screen shown in Figure 21 above with a maximum number of elements set by the parameters (See Section 3.2). Alternatively, the user can use one or more of the frames on the right of the screen to supply a maximum, minimum, and number of intervals that will create a range of equally spaced values for the user. The program will check to make sure the maximum value supplied is larger than the minimum value supplied when the range frames on the right are being utilized. The program uses the maximum and minimum range values in the event that the User enters values in both the left and right frames. The program will also take preference to values entered for the volume over density specified values. The user has no restrictions on whether to use either the volume or the density even if individual reactant densities were supplied (See Section 5.2.2). The lower section of the Problem Temperature and Volume screen controls the units for all temperature, volume, and density frames. The temperature may be supplied in Kelvin (K), Rankine (R), Celsius (C), or Fahrenheit (F). The specific volume uses cm 3 /g or m 3 /kg while the density uses grams per cubic centimeter (g/cm 3 ) or kilograms per cubic meter (kg/m 3 ) units. 29
33 6.5. Internal-Energy and Volume Figure 22 Internal Energy and Volume Input Internal-Energy and Volume problems require that at least one value must be supplied for either the volume or density. The program will use the internal-energies of the reactants at the temperatures supplied for each reactant (See Section 5.2.2). Alternatively, the user may choose to supply one internal-energy (U/R) for the mixture that will override the internal-energies from the individual reactant temperatures. This internal-energy is supplied in the labeled frame seen in the Problem Internal-Energy and Volume screen shown in Figure 22 above and must be in units g*mole*k/(g of mixture). The specific volume uses cm 3 /g or m 3 /kg while the density uses grams per cubic centimeter (g/cm 3 ) or kilograms per cubic meter (kg/m 3 ) units which is controlled with the dropdown menu in the lower left section of the screen. The user may supply each individual volume or density desired using the frames on the left of the Problem Internal-Energy and Volume screen with a maximum number of elements set by the parameters (See Section 3.2). Alternatively, the user may use one or more of the frames on the right of the screen to supply a maximum, minimum, and number of intervals that will create a range of equally 30
34 spaced values for the user. The program will check to make sure the maximum value supplied is larger than the minimum value supplied when the range frames on the right are being utilized. The program uses the maximum and minimum range values in the event that the User enters values in both the left and right frames. The program will also take preference to values entered for the volume over density specified values. The user has no restrictions to use either the volume or the density even if individual reactant densities were supplied (See Section 5.2.2) Entropy and Volume Figure 23 Entropy and Volume Entropy and Volume problems require that at least one value must be supplied for either the volume or density and exactly one value for the entropy (S/R). The entropy is supplied in the labeled frame seen in the Problem Entropy and Volume screen shown in Figure 23 above and must be in units g*mole/(g of mixture). The specific volume uses cm 3 /g or m 3 /kg while the density uses grams per cubic centimeter (g/cm 3 ) or kilograms per cubic meter (kg/m 3 ) units which is controlled with the dropdown menu in the lower left section of the screen. The user may supply each individual volume or density desired using the frames on the left of the Problem Entropy and Volume screen with a maximum number of elements set by the parameters 31
35 (See Section 3.2). Alternatively, the user can use one or more of the frames on the right of the screen to supply a maximum, minimum, and number of intervals that will create a range of equally spaced values for the user. The program will check to make sure the maximum value supplied is larger than the minimum value supplied when the range frames on the right are being utilized. The program uses the maximum and minimum range values in the event that the User enters values in both the left and right frames. The program will also take preference to values entered for the volume over density specified values. The user has no restrictions to use either the volume or the density even if individual reactant densities were supplied (See Section 5.2.2). 32
36 Chapter 7: Output Document Upon completion of entering in all problem input information from Chapters 5 and 6, the program will run the chemical equilibrium iterations until convergence has been achieved or the program encounters a fatal error due to problems with a lack of supplied information. In both cases, the program will produce a new Excel document with the results of the calculations and exit all program screens. The information contained in the output document depends on the selection during the problem input (See Section 5.4 and 5.5) OUTPUT Tab The OUTPUT Tab will always be created for the output document and is defaulted as the active tab. The first five rows of the page will contain the title which includes: Program Name and Date Modified The Author Reference to the NASA CEA program [1] version the program is based upon The seventh row will contain the case name if one was given during the problem input. The eight row will have the problem type that was selected in dropdown menu on the Problem Input and Output screen (See Section 5.1). The remainder of the output is broken into sections which are printed or withheld depending on the user s selection of a short output. During the calculations, the program can experience errors with the specified input properties which prevent the problem from converging. The program also may require some warnings be printed to the user to highlight potential inaccuracies. These warnings and error messages will be printed to the OUTUT Tab. See Appendix C for a full list of possible errors and warnings Problem Inputs If the user has not selected the short output option, the OUTPUT Tab will contain information about the parameters that were selected by the user. If the user selected the short option, the output will skip to Section This information may be used as either a check of the inputs or a record of the selections for future review. The first part of this section displays the logical selections that the user specified for the values in the following Table
Getting started with BatchReactor Example : Simulation of the Chlorotoluene chlorination
 Getting started with BatchReactor Example : Simulation of the Chlorotoluene chlorination 2011 ProSim S.A. All rights reserved. Introduction This document presents the different steps to follow in order
Getting started with BatchReactor Example : Simulation of the Chlorotoluene chlorination 2011 ProSim S.A. All rights reserved. Introduction This document presents the different steps to follow in order
M E R C E R W I N WA L K T H R O U G H
 H E A L T H W E A L T H C A R E E R WA L K T H R O U G H C L I E N T S O L U T I O N S T E A M T A B L E O F C O N T E N T 1. Login to the Tool 2 2. Published reports... 7 3. Select Results Criteria...
H E A L T H W E A L T H C A R E E R WA L K T H R O U G H C L I E N T S O L U T I O N S T E A M T A B L E O F C O N T E N T 1. Login to the Tool 2 2. Published reports... 7 3. Select Results Criteria...
The View Data module
 The module Use to examine stored compound data (H, S, C p (T), G, etc.) in Compound type databases and list solutions and their constituents in Solution type databases. Table of contents Section 1 Section
The module Use to examine stored compound data (H, S, C p (T), G, etc.) in Compound type databases and list solutions and their constituents in Solution type databases. Table of contents Section 1 Section
Athena Visual Software, Inc. 1
 Athena Visual Studio Visual Kinetics Tutorial VisualKinetics is an integrated tool within the Athena Visual Studio software environment, which allows scientists and engineers to simulate the dynamic behavior
Athena Visual Studio Visual Kinetics Tutorial VisualKinetics is an integrated tool within the Athena Visual Studio software environment, which allows scientists and engineers to simulate the dynamic behavior
Planning Softproviding Meat User Documentation
 Great ideas are always simple Softproviding simply makes them happen. Planning Softproviding Meat User Documentation Version: 1.00 Date: 24 August 2017 Release: v5.50 Softproviding AG Riehenring 175 CH-4058
Great ideas are always simple Softproviding simply makes them happen. Planning Softproviding Meat User Documentation Version: 1.00 Date: 24 August 2017 Release: v5.50 Softproviding AG Riehenring 175 CH-4058
15. Exergy Balance Module
 14010-ORC-J 1 (6) 15. Exergy Balance Module 15.1. Introduction This module allows the user to calculate exergy, mass and heat balance for a system where there can be multiple input and output streams with
14010-ORC-J 1 (6) 15. Exergy Balance Module 15.1. Introduction This module allows the user to calculate exergy, mass and heat balance for a system where there can be multiple input and output streams with
An area chart emphasizes the trend of each value over time. An area chart also shows the relationship of parts to a whole.
 Excel 2003 Creating a Chart Introduction Page 1 By the end of this lesson, learners should be able to: Identify the parts of a chart Identify different types of charts Create an Embedded Chart Create a
Excel 2003 Creating a Chart Introduction Page 1 By the end of this lesson, learners should be able to: Identify the parts of a chart Identify different types of charts Create an Embedded Chart Create a
41. Sim Reactions Example
 HSC Chemistry 7.0 41-1(6) 41. Sim Reactions Example Figure 1: Sim Reactions Example, Run mode view after calculations. General This example contains instruction how to create a simple model. The example
HSC Chemistry 7.0 41-1(6) 41. Sim Reactions Example Figure 1: Sim Reactions Example, Run mode view after calculations. General This example contains instruction how to create a simple model. The example
Calculating Bond Enthalpies of the Hydrides
 Proposed Exercise for the General Chemistry Section of the Teaching with Cache Workbook: Calculating Bond Enthalpies of the Hydrides Contributed by James Foresman, Rachel Fogle, and Jeremy Beck, York College
Proposed Exercise for the General Chemistry Section of the Teaching with Cache Workbook: Calculating Bond Enthalpies of the Hydrides Contributed by James Foresman, Rachel Fogle, and Jeremy Beck, York College
The OptiSage module. Use the OptiSage module for the assessment of Gibbs energy data. Table of contents
 The module Use the module for the assessment of Gibbs energy data. Various types of experimental data can be utilized in order to generate optimized parameters for the Gibbs energies of stoichiometric
The module Use the module for the assessment of Gibbs energy data. Various types of experimental data can be utilized in order to generate optimized parameters for the Gibbs energies of stoichiometric
Stoichiometric Reactor Simulation Robert P. Hesketh and Concetta LaMarca Chemical Engineering, Rowan University (Revised 4/8/09)
 Stoichiometric Reactor Simulation Robert P. Hesketh and Concetta LaMarca Chemical Engineering, Rowan University (Revised 4/8/09) In this session you will learn how to create a stoichiometric reactor model
Stoichiometric Reactor Simulation Robert P. Hesketh and Concetta LaMarca Chemical Engineering, Rowan University (Revised 4/8/09) In this session you will learn how to create a stoichiometric reactor model
Appendix B Microsoft Office Specialist exam objectives maps
 B 1 Appendix B Microsoft Office Specialist exam objectives maps This appendix covers these additional topics: A Excel 2003 Specialist exam objectives with references to corresponding material in Course
B 1 Appendix B Microsoft Office Specialist exam objectives maps This appendix covers these additional topics: A Excel 2003 Specialist exam objectives with references to corresponding material in Course
Using SkyTools to log Texas 45 list objects
 Houston Astronomical Society Using SkyTools to log Texas 45 list objects You can use SkyTools to keep track of objects observed in Columbus and copy the output into the Texas 45 observation log. Preliminary
Houston Astronomical Society Using SkyTools to log Texas 45 list objects You can use SkyTools to keep track of objects observed in Columbus and copy the output into the Texas 45 observation log. Preliminary
Introduction to Computer Tools and Uncertainties
 Experiment 1 Introduction to Computer Tools and Uncertainties 1.1 Objectives To become familiar with the computer programs and utilities that will be used throughout the semester. To become familiar with
Experiment 1 Introduction to Computer Tools and Uncertainties 1.1 Objectives To become familiar with the computer programs and utilities that will be used throughout the semester. To become familiar with
SuperCELL Data Programmer and ACTiSys IR Programmer User s Guide
 SuperCELL Data Programmer and ACTiSys IR Programmer User s Guide This page is intentionally left blank. SuperCELL Data Programmer and ACTiSys IR Programmer User s Guide The ACTiSys IR Programmer and SuperCELL
SuperCELL Data Programmer and ACTiSys IR Programmer User s Guide This page is intentionally left blank. SuperCELL Data Programmer and ACTiSys IR Programmer User s Guide The ACTiSys IR Programmer and SuperCELL
v Prerequisite Tutorials GSSHA WMS Basics Watershed Delineation using DEMs and 2D Grid Generation Time minutes
 v. 10.1 WMS 10.1 Tutorial GSSHA WMS Basics Creating Feature Objects and Mapping Attributes to the 2D Grid Populate hydrologic parameters in a GSSHA model using land use and soil data Objectives This tutorial
v. 10.1 WMS 10.1 Tutorial GSSHA WMS Basics Creating Feature Objects and Mapping Attributes to the 2D Grid Populate hydrologic parameters in a GSSHA model using land use and soil data Objectives This tutorial
Create Satellite Image, Draw Maps
 Create Satellite Image, Draw Maps 1. The goal Using Google Earth, we want to create and import a background file into our Adviser program. From there, we will be creating paddock boundaries. The accuracy
Create Satellite Image, Draw Maps 1. The goal Using Google Earth, we want to create and import a background file into our Adviser program. From there, we will be creating paddock boundaries. The accuracy
ON SITE SYSTEMS Chemical Safety Assistant
 ON SITE SYSTEMS Chemical Safety Assistant CS ASSISTANT WEB USERS MANUAL On Site Systems 23 N. Gore Ave. Suite 200 St. Louis, MO 63119 Phone 314-963-9934 Fax 314-963-9281 Table of Contents INTRODUCTION
ON SITE SYSTEMS Chemical Safety Assistant CS ASSISTANT WEB USERS MANUAL On Site Systems 23 N. Gore Ave. Suite 200 St. Louis, MO 63119 Phone 314-963-9934 Fax 314-963-9281 Table of Contents INTRODUCTION
Cantera / Stancan Primer
 Cantera / Stancan Primer Matthew Campbell; A.J. Simon; Chris Edwards Introduction to Cantera and Stancan Cantera is an open-source, object-oriented software package which performs chemical and thermodynamic
Cantera / Stancan Primer Matthew Campbell; A.J. Simon; Chris Edwards Introduction to Cantera and Stancan Cantera is an open-source, object-oriented software package which performs chemical and thermodynamic
Tutorial: Premixed Flow in a Conical Chamber using the Finite-Rate Chemistry Model
 Tutorial: Premixed Flow in a Conical Chamber using the Finite-Rate Chemistry Model Introduction The purpose of this tutorial is to provide guidelines and recommendations for setting up and solving the
Tutorial: Premixed Flow in a Conical Chamber using the Finite-Rate Chemistry Model Introduction The purpose of this tutorial is to provide guidelines and recommendations for setting up and solving the
15. Studio ScaleChem Getting Started
 15. Studio ScaleChem Getting Started Terminology Analysis Brines Gases Oils Reconciliation Before we can discuss how to use Studio ScaleChem we must first discuss some terms. This will help us define some
15. Studio ScaleChem Getting Started Terminology Analysis Brines Gases Oils Reconciliation Before we can discuss how to use Studio ScaleChem we must first discuss some terms. This will help us define some
Copy the rules into MathLook for a better view. Close MathLook after observing the equations.
 Sample : Torsion on a Sha The Sha Design example is found the Sample Applications, Engeerg and Science section of the TK Solver Library. When it loads, the Variable and Rule Sheets appear as shown below.
Sample : Torsion on a Sha The Sha Design example is found the Sample Applications, Engeerg and Science section of the TK Solver Library. When it loads, the Variable and Rule Sheets appear as shown below.
OECD QSAR Toolbox v.4.1. Tutorial illustrating new options for grouping with metabolism
 OECD QSAR Toolbox v.4.1 Tutorial illustrating new options for grouping with metabolism Outlook Background Objectives Specific Aims The exercise Workflow 2 Background Grouping with metabolism is a procedure
OECD QSAR Toolbox v.4.1 Tutorial illustrating new options for grouping with metabolism Outlook Background Objectives Specific Aims The exercise Workflow 2 Background Grouping with metabolism is a procedure
REPLACE DAMAGED OR MISSING TEXTBOOK BARCODE LABEL
 Destiny Textbook Manager allows users to create and print replacement barcode labels for textbooks. In this tutorial you will learn how to: Replace damaged textbook barcode label(s) Replace missing textbook
Destiny Textbook Manager allows users to create and print replacement barcode labels for textbooks. In this tutorial you will learn how to: Replace damaged textbook barcode label(s) Replace missing textbook
MAGNETITE OXIDATION EXAMPLE
 HSC Chemistry 7.0 1 MAGNETITE OXIDATION EXAMPLE Pelletized magnetite (Fe 3 O 4 ) ore may be oxidized to hematite (Fe 2 O 3 ) in shaft furnace. Typical magnetite content in ore is some 95%. Oxidation is
HSC Chemistry 7.0 1 MAGNETITE OXIDATION EXAMPLE Pelletized magnetite (Fe 3 O 4 ) ore may be oxidized to hematite (Fe 2 O 3 ) in shaft furnace. Typical magnetite content in ore is some 95%. Oxidation is
Experiment 0 ~ Introduction to Statistics and Excel Tutorial. Introduction to Statistics, Error and Measurement
 Experiment 0 ~ Introduction to Statistics and Excel Tutorial Many of you already went through the introduction to laboratory practice and excel tutorial in Physics 1011. For that reason, we aren t going
Experiment 0 ~ Introduction to Statistics and Excel Tutorial Many of you already went through the introduction to laboratory practice and excel tutorial in Physics 1011. For that reason, we aren t going
Cerno Application Note Extending the Limits of Mass Spectrometry
 Creation of Accurate Mass Library for NIST Database Search Novel MS calibration has been shown to enable accurate mass and elemental composition determination on quadrupole GC/MS systems for either molecular
Creation of Accurate Mass Library for NIST Database Search Novel MS calibration has been shown to enable accurate mass and elemental composition determination on quadrupole GC/MS systems for either molecular
PDF-4+ Tools and Searches
 PDF-4+ Tools and Searches PDF-4+ 2018 The PDF-4+ 2018 database is powered by our integrated search display software. PDF-4+ 2018 boasts 72 search selections coupled with 125 display fields resulting in
PDF-4+ Tools and Searches PDF-4+ 2018 The PDF-4+ 2018 database is powered by our integrated search display software. PDF-4+ 2018 boasts 72 search selections coupled with 125 display fields resulting in
Ligand Scout Tutorials
 Ligand Scout Tutorials Step : Creating a pharmacophore from a protein-ligand complex. Type ke6 in the upper right area of the screen and press the button Download *+. The protein will be downloaded and
Ligand Scout Tutorials Step : Creating a pharmacophore from a protein-ligand complex. Type ke6 in the upper right area of the screen and press the button Download *+. The protein will be downloaded and
PDF-4+ Tools and Searches
 PDF-4+ Tools and Searches PDF-4+ 2019 The PDF-4+ 2019 database is powered by our integrated search display software. PDF-4+ 2019 boasts 74 search selections coupled with 126 display fields resulting in
PDF-4+ Tools and Searches PDF-4+ 2019 The PDF-4+ 2019 database is powered by our integrated search display software. PDF-4+ 2019 boasts 74 search selections coupled with 126 display fields resulting in
Newton's 2 nd Law. . Your end results should only be interms of m
 Newton's nd Law Introduction: In today's lab you will demonstrate the validity of Newton's Laws in predicting the motion of a simple mechanical system. The system that you will investigate consists of
Newton's nd Law Introduction: In today's lab you will demonstrate the validity of Newton's Laws in predicting the motion of a simple mechanical system. The system that you will investigate consists of
Two problems to be solved. Example Use of SITATION. Here is the main menu. First step. Now. To load the data.
 Two problems to be solved Example Use of SITATION Mark S. Daskin Department of IE/MS Northwestern U. Evanston, IL 1. Minimize the demand weighted total distance (or average distance) Using 10 facilities
Two problems to be solved Example Use of SITATION Mark S. Daskin Department of IE/MS Northwestern U. Evanston, IL 1. Minimize the demand weighted total distance (or average distance) Using 10 facilities
Double Star Observations
 Double Star Observations Canopus now includes enhanced features for measurnig double stars. This includes easier setting of the reference position (the primary star) as well as recording the observations
Double Star Observations Canopus now includes enhanced features for measurnig double stars. This includes easier setting of the reference position (the primary star) as well as recording the observations
Experiment: Oscillations of a Mass on a Spring
 Physics NYC F17 Objective: Theory: Experiment: Oscillations of a Mass on a Spring A: to verify Hooke s law for a spring and measure its elasticity constant. B: to check the relationship between the period
Physics NYC F17 Objective: Theory: Experiment: Oscillations of a Mass on a Spring A: to verify Hooke s law for a spring and measure its elasticity constant. B: to check the relationship between the period
Creating Empirical Calibrations
 030.0023.01.0 Spreadsheet Manual Save Date: December 1, 2010 Table of Contents 1. Overview... 3 2. Enable S1 Calibration Macro... 4 3. Getting Ready... 4 4. Measuring the New Sample... 5 5. Adding New
030.0023.01.0 Spreadsheet Manual Save Date: December 1, 2010 Table of Contents 1. Overview... 3 2. Enable S1 Calibration Macro... 4 3. Getting Ready... 4 4. Measuring the New Sample... 5 5. Adding New
A GUI FOR EVOLVE ZAMS
 A GUI FOR EVOLVE ZAMS D. R. Schlegel Computer Science Department Here the early work on a new user interface for the Evolve ZAMS stellar evolution code is presented. The initial goal of this project is
A GUI FOR EVOLVE ZAMS D. R. Schlegel Computer Science Department Here the early work on a new user interface for the Evolve ZAMS stellar evolution code is presented. The initial goal of this project is
ISIS/Draw "Quick Start"
 ISIS/Draw "Quick Start" Click to print, or click Drawing Molecules * Basic Strategy 5.1 * Drawing Structures with Template tools and template pages 5.2 * Drawing bonds and chains 5.3 * Drawing atoms 5.4
ISIS/Draw "Quick Start" Click to print, or click Drawing Molecules * Basic Strategy 5.1 * Drawing Structures with Template tools and template pages 5.2 * Drawing bonds and chains 5.3 * Drawing atoms 5.4
Data Structures & Database Queries in GIS
 Data Structures & Database Queries in GIS Objective In this lab we will show you how to use ArcGIS for analysis of digital elevation models (DEM s), in relationship to Rocky Mountain bighorn sheep (Ovis
Data Structures & Database Queries in GIS Objective In this lab we will show you how to use ArcGIS for analysis of digital elevation models (DEM s), in relationship to Rocky Mountain bighorn sheep (Ovis
mylab: Chemical Safety Module Last Updated: January 19, 2018
 : Chemical Safety Module Contents Introduction... 1 Getting started... 1 Login... 1 Receiving Items from MMP Order... 3 Inventory... 4 Show me Chemicals where... 4 Items Received on... 5 All Items... 5
: Chemical Safety Module Contents Introduction... 1 Getting started... 1 Login... 1 Receiving Items from MMP Order... 3 Inventory... 4 Show me Chemicals where... 4 Items Received on... 5 All Items... 5
Mass Asset Additions. Overview. Effective mm/dd/yy Page 1 of 47 Rev 1. Copyright Oracle, All rights reserved.
 Overview Effective mm/dd/yy Page 1 of 47 Rev 1 System References None Distribution Oracle Assets Job Title * Ownership The Job Title [list@yourcompany.com?subject=eduxxxxx] is responsible for ensuring
Overview Effective mm/dd/yy Page 1 of 47 Rev 1 System References None Distribution Oracle Assets Job Title * Ownership The Job Title [list@yourcompany.com?subject=eduxxxxx] is responsible for ensuring
Stoichiometry Rockets
 Stoichiometry Rockets The objective of this lab is to to: calculate the needed volume of fuel to react with a given volume of gas and result in a productive explosion determine the heat of the reaction
Stoichiometry Rockets The objective of this lab is to to: calculate the needed volume of fuel to react with a given volume of gas and result in a productive explosion determine the heat of the reaction
Introduction to MoLEs Activities Computer Simulations *
 Introduction to MoLEs Activities Computer Simulations * Molecular Level Experiments (MoLEs) are chemistry laboratory experiments based on computer simulations. There are two types of simulation programs
Introduction to MoLEs Activities Computer Simulations * Molecular Level Experiments (MoLEs) are chemistry laboratory experiments based on computer simulations. There are two types of simulation programs
Aspen Dr. Ziad Abuelrub
 Aspen Plus Lab Pharmaceutical Plant Design Aspen Dr. Ziad Abuelrub OUTLINE 1. Introduction 2. Getting Started 3. Thermodynamic Models & Physical Properties 4. Pressure Changers 5. Heat Exchangers 6. Flowsheet
Aspen Plus Lab Pharmaceutical Plant Design Aspen Dr. Ziad Abuelrub OUTLINE 1. Introduction 2. Getting Started 3. Thermodynamic Models & Physical Properties 4. Pressure Changers 5. Heat Exchangers 6. Flowsheet
University of Colorado Denver Anschutz Medical Campus Online Chemical Inventory System User s Manual
 University of Colorado Denver Anschutz Medical Campus Online Chemical Inventory System User s Manual Hazardous Materials Division 303-724-0345 chemical.inventory@ucdenver.edu May, 2017 Table of Contents
University of Colorado Denver Anschutz Medical Campus Online Chemical Inventory System User s Manual Hazardous Materials Division 303-724-0345 chemical.inventory@ucdenver.edu May, 2017 Table of Contents
PDF-2 Tools and Searches
 PDF-2 Tools and Searches PDF-2 2019 The PDF-2 2019 database is powered by our integrated search display software. PDF-2 2019 boasts 69 search selections coupled with 53 display fields resulting in a nearly
PDF-2 Tools and Searches PDF-2 2019 The PDF-2 2019 database is powered by our integrated search display software. PDF-2 2019 boasts 69 search selections coupled with 53 display fields resulting in a nearly
Ammonia Synthesis with Aspen Plus V8.0
 Ammonia Synthesis with Aspen Plus V8.0 Part 1 Open Loop Simulation of Ammonia Synthesis 1. Lesson Objectives Become comfortable and familiar with the Aspen Plus graphical user interface Explore Aspen Plus
Ammonia Synthesis with Aspen Plus V8.0 Part 1 Open Loop Simulation of Ammonia Synthesis 1. Lesson Objectives Become comfortable and familiar with the Aspen Plus graphical user interface Explore Aspen Plus
HSC Chemistry 7.0 User's Guide
 HSC Chemistry 7.0 47-1 HSC Chemistry 7.0 User's Guide Sim Flowsheet Module Experimental Mode Pertti Lamberg Outotec Research Oy Information Service P.O. Box 69 FIN - 28101 PORI, FINLAND Fax: +358-20 -
HSC Chemistry 7.0 47-1 HSC Chemistry 7.0 User's Guide Sim Flowsheet Module Experimental Mode Pertti Lamberg Outotec Research Oy Information Service P.O. Box 69 FIN - 28101 PORI, FINLAND Fax: +358-20 -
User Manuel. EurotaxForecast. Version Latest changes ( )
 User Manuel EurotaxForecast Version 1.23.0771- Latest changes (19.07.2003) Contents Preface 5 Welcome to Eurotax Forecast...5 Using this manual 6 How to use this manual?...6 Program overview 7 General
User Manuel EurotaxForecast Version 1.23.0771- Latest changes (19.07.2003) Contents Preface 5 Welcome to Eurotax Forecast...5 Using this manual 6 How to use this manual?...6 Program overview 7 General
Electric Fields and Equipotentials
 OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
Table of content. Understanding workflow automation - Making the right choice Creating a workflow...05
 Marketers need to categorize their audience to maximize their r e a c h. Z o h o C a m p a i g n s a u t o m a t e s r e c i p i e n t c l a s s i fi c a t i o n a n d action performance to free up marketers
Marketers need to categorize their audience to maximize their r e a c h. Z o h o C a m p a i g n s a u t o m a t e s r e c i p i e n t c l a s s i fi c a t i o n a n d action performance to free up marketers
RADIATION PROCEDURES MANUAL Procedure Cover Sheet
 RADIATION PROCEDURES MANUAL Procedure Cover Sheet Procedure Title: Radioactive Material Inventory Procedure Number: TSO-09-16-REV 0 Effective Date: June 18, 2009 Approved By: Date: 11 August, 2009 Technical
RADIATION PROCEDURES MANUAL Procedure Cover Sheet Procedure Title: Radioactive Material Inventory Procedure Number: TSO-09-16-REV 0 Effective Date: June 18, 2009 Approved By: Date: 11 August, 2009 Technical
ncounter PlexSet Data Analysis Guidelines
 ncounter PlexSet Data Analysis Guidelines NanoString Technologies, Inc. 530 airview Ave North Seattle, Washington 98109 USA Telephone: 206.378.6266 888.358.6266 E-mail: info@nanostring.com Molecules That
ncounter PlexSet Data Analysis Guidelines NanoString Technologies, Inc. 530 airview Ave North Seattle, Washington 98109 USA Telephone: 206.378.6266 888.358.6266 E-mail: info@nanostring.com Molecules That
Using Microsoft Excel
 Using Microsoft Excel Objective: Students will gain familiarity with using Excel to record data, display data properly, use built-in formulae to do calculations, and plot and fit data with linear functions.
Using Microsoft Excel Objective: Students will gain familiarity with using Excel to record data, display data properly, use built-in formulae to do calculations, and plot and fit data with linear functions.
VCell Tutorial. Building a Rule-Based Model
 VCell Tutorial Building a Rule-Based Model We will demonstrate how to create a rule-based model of EGFR receptor interaction with two adapter proteins Grb2 and Shc. A Receptor-monomer reversibly binds
VCell Tutorial Building a Rule-Based Model We will demonstrate how to create a rule-based model of EGFR receptor interaction with two adapter proteins Grb2 and Shc. A Receptor-monomer reversibly binds
JOB REQUESTS C H A P T E R 3. Overview. Objectives
 C H A P T E R 3 JOB REQUESTS Overview Objectives Job Requests is one of the most critical areas of payroll processing. This is where the user can enter, update, and view information regarding an employee
C H A P T E R 3 JOB REQUESTS Overview Objectives Job Requests is one of the most critical areas of payroll processing. This is where the user can enter, update, and view information regarding an employee
Analyzing Data. Click a hyperlink or folder tab to view the corresponding slides. Exit
 Analyzing Data Section 2.1 Units and Measurements Section 2.2 Scientific Notation and Dimensional Analysis Section 2.3 Uncertainty in Data Section 2.4 Representing Data Click a hyperlink or folder tab
Analyzing Data Section 2.1 Units and Measurements Section 2.2 Scientific Notation and Dimensional Analysis Section 2.3 Uncertainty in Data Section 2.4 Representing Data Click a hyperlink or folder tab
Building Inflation Tables and CER Libraries
 Building Inflation Tables and CER Libraries January 2007 Presented by James K. Johnson Tecolote Research, Inc. Copyright Tecolote Research, Inc. September 2006 Abstract Building Inflation Tables and CER
Building Inflation Tables and CER Libraries January 2007 Presented by James K. Johnson Tecolote Research, Inc. Copyright Tecolote Research, Inc. September 2006 Abstract Building Inflation Tables and CER
Pressure Swing Distillation with Aspen Plus V8.0
 Pressure Swing Distillation with Aspen Plus V8.0 1. Lesson Objectives Aspen Plus property analysis RadFrac distillation modeling Design Specs NQ Curves Tear streams Understand and overcome azeotrope Select
Pressure Swing Distillation with Aspen Plus V8.0 1. Lesson Objectives Aspen Plus property analysis RadFrac distillation modeling Design Specs NQ Curves Tear streams Understand and overcome azeotrope Select
Chem 1 Kinetics. Objectives. Concepts
 Chem 1 Kinetics Objectives 1. Learn some basic ideas in chemical kinetics. 2. Understand how the computer visualizations can be used to benefit the learning process. 3. Understand how the computer models
Chem 1 Kinetics Objectives 1. Learn some basic ideas in chemical kinetics. 2. Understand how the computer visualizations can be used to benefit the learning process. 3. Understand how the computer models
How to Make or Plot a Graph or Chart in Excel
 This is a complete video tutorial on How to Make or Plot a Graph or Chart in Excel. To make complex chart like Gantt Chart, you have know the basic principles of making a chart. Though I have used Excel
This is a complete video tutorial on How to Make or Plot a Graph or Chart in Excel. To make complex chart like Gantt Chart, you have know the basic principles of making a chart. Though I have used Excel
MoLE Gas Laws Activities
 MoLE Gas Laws Activities To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser, go
MoLE Gas Laws Activities To begin this assignment you must be able to log on to the Internet using Internet Explorer (Microsoft) 4.5 or higher. If you do not have the current version of the browser, go
NINE CHOICE SERIAL REACTION TIME TASK
 instrumentation and software for research NINE CHOICE SERIAL REACTION TIME TASK MED-STATE NOTATION PROCEDURE SOF-700RA-8 USER S MANUAL DOC-025 Rev. 1.3 Copyright 2013 All Rights Reserved MED Associates
instrumentation and software for research NINE CHOICE SERIAL REACTION TIME TASK MED-STATE NOTATION PROCEDURE SOF-700RA-8 USER S MANUAL DOC-025 Rev. 1.3 Copyright 2013 All Rights Reserved MED Associates
Creation and modification of a geological model Program: Stratigraphy
 Engineering manual No. 39 Updated: 11/2018 Creation and modification of a geological model Program: Stratigraphy File: Demo_manual_39.gsg Introduction The aim of this engineering manual is to explain the
Engineering manual No. 39 Updated: 11/2018 Creation and modification of a geological model Program: Stratigraphy File: Demo_manual_39.gsg Introduction The aim of this engineering manual is to explain the
HSC Chemistry A. Roine June 28, ORC T
 HSC Chemistry 5.0 10 1 10. REACTION EQUATIONS Clicking the Reaction Equations button in the main menu shows the Reaction Equations Window, see Fig. 1. With this calculation option you can calculate the
HSC Chemistry 5.0 10 1 10. REACTION EQUATIONS Clicking the Reaction Equations button in the main menu shows the Reaction Equations Window, see Fig. 1. With this calculation option you can calculate the
The EpH module. Table of Contents. Section 3
 The module Use to calculate and plot isothermal (Pourbaix) diagrams. Note: accesses compound databases, i.e. treats the aqueous phase as an ideal solution. Table of Contents Section 1 Section 2 Section
The module Use to calculate and plot isothermal (Pourbaix) diagrams. Note: accesses compound databases, i.e. treats the aqueous phase as an ideal solution. Table of Contents Section 1 Section 2 Section
13. Equilibrium Module - Description of Menus and Options
 15008-ORC-J 1 (57) 13. Equilibrium Module - Description of Menus and Options 15008-ORC-J 2 (57) SUMMARY HSC Equilibrium module enables user to calculate multi-component equilibrium compositions in heterogeneous
15008-ORC-J 1 (57) 13. Equilibrium Module - Description of Menus and Options 15008-ORC-J 2 (57) SUMMARY HSC Equilibrium module enables user to calculate multi-component equilibrium compositions in heterogeneous
Sample Alignment Part
 Sample Alignment Part Contents Contents 1. How to set Part conditions...1 1.1 Setting conditions... 1 1.2 Customizing scan conditions and slit conditions... 6 2. Sample alignment sequence...13 2.1 Direct
Sample Alignment Part Contents Contents 1. How to set Part conditions...1 1.1 Setting conditions... 1 1.2 Customizing scan conditions and slit conditions... 6 2. Sample alignment sequence...13 2.1 Direct
Calculating Conflict Density and Change over Time in Uganda using Vector Techniques
 Calculating Conflict Density and Change over Time in Uganda using Vector Techniques Created by Patrick Florance and Kyle Monahan; revised by Patrick Florance April 2, 2018. OVERVIEW... 1 SET UP... 1 SET
Calculating Conflict Density and Change over Time in Uganda using Vector Techniques Created by Patrick Florance and Kyle Monahan; revised by Patrick Florance April 2, 2018. OVERVIEW... 1 SET UP... 1 SET
12. Petroleum Calculations
 12. Petroleum Calculations Overview Calculations with the OLI Software can be used to characterize crude oils. Here is a quote from the OLI Tricks of the Trade manual (AQSim) Crude oils are complex groups
12. Petroleum Calculations Overview Calculations with the OLI Software can be used to characterize crude oils. Here is a quote from the OLI Tricks of the Trade manual (AQSim) Crude oils are complex groups
Using the Budget Features in Quicken 2008
 Using the Budget Features in Quicken 2008 Quicken budgets can be used to summarize expected income and expenses for planning purposes. The budget can later be used in comparisons to actual income and expenses
Using the Budget Features in Quicken 2008 Quicken budgets can be used to summarize expected income and expenses for planning purposes. The budget can later be used in comparisons to actual income and expenses
Determination of Density 1
 Introduction Determination of Density 1 Authors: B. D. Lamp, D. L. McCurdy, V. M. Pultz and J. M. McCormick* Last Update: February 1, 2013 Not so long ago a statistical data analysis of any data set larger
Introduction Determination of Density 1 Authors: B. D. Lamp, D. L. McCurdy, V. M. Pultz and J. M. McCormick* Last Update: February 1, 2013 Not so long ago a statistical data analysis of any data set larger
NMR Predictor. Introduction
 NMR Predictor This manual gives a walk-through on how to use the NMR Predictor: Introduction NMR Predictor QuickHelp NMR Predictor Overview Chemical features GUI features Usage Menu system File menu Edit
NMR Predictor This manual gives a walk-through on how to use the NMR Predictor: Introduction NMR Predictor QuickHelp NMR Predictor Overview Chemical features GUI features Usage Menu system File menu Edit
13. EQUILIBRIUM MODULE
 HSC Chemistry 5.0 13 1 13. EQUILIBRIUM MODULE Fig. 1. Equilibrium Module Menu. This module enables you to calculate multi component equilibrium compositions in heterogeneous systems easily. The user simply
HSC Chemistry 5.0 13 1 13. EQUILIBRIUM MODULE Fig. 1. Equilibrium Module Menu. This module enables you to calculate multi component equilibrium compositions in heterogeneous systems easily. The user simply
ICM-Chemist How-To Guide. Version 3.6-1g Last Updated 12/01/2009
 ICM-Chemist How-To Guide Version 3.6-1g Last Updated 12/01/2009 ICM-Chemist HOW TO IMPORT, SKETCH AND EDIT CHEMICALS How to access the ICM Molecular Editor. 1. Click here 2. Start sketching How to sketch
ICM-Chemist How-To Guide Version 3.6-1g Last Updated 12/01/2009 ICM-Chemist HOW TO IMPORT, SKETCH AND EDIT CHEMICALS How to access the ICM Molecular Editor. 1. Click here 2. Start sketching How to sketch
Watershed Modeling Orange County Hydrology Using GIS Data
 v. 10.0 WMS 10.0 Tutorial Watershed Modeling Orange County Hydrology Using GIS Data Learn how to delineate sub-basins and compute soil losses for Orange County (California) hydrologic modeling Objectives
v. 10.0 WMS 10.0 Tutorial Watershed Modeling Orange County Hydrology Using GIS Data Learn how to delineate sub-basins and compute soil losses for Orange County (California) hydrologic modeling Objectives
LED Lighting Facts: Manufacturer Guide
 LED Lighting Facts: Manufacturer Guide 2018 1 P a g e L E D L i g h t i n g F a c t s : M a n u f a c t u r e r G u i d e TABLE OF CONTENTS Section 1) Accessing your account and managing your products...
LED Lighting Facts: Manufacturer Guide 2018 1 P a g e L E D L i g h t i n g F a c t s : M a n u f a c t u r e r G u i d e TABLE OF CONTENTS Section 1) Accessing your account and managing your products...
13. EQUILIBRIUM MODULE
 HSC Chemistry 7.0 13-1 13. EQUILIBRIUM MODULE Fig. 1. Equilibrium Module Menu. This module enables you to calculate multi-component equilibrium compositions in heterogeneous systems easily. The user simply
HSC Chemistry 7.0 13-1 13. EQUILIBRIUM MODULE Fig. 1. Equilibrium Module Menu. This module enables you to calculate multi-component equilibrium compositions in heterogeneous systems easily. The user simply
Quantification of JEOL XPS Spectra from SpecSurf
 Quantification of JEOL XPS Spectra from SpecSurf The quantification procedure used by the JEOL SpecSurf software involves modifying the Scofield cross-sections to account for both an energy dependency
Quantification of JEOL XPS Spectra from SpecSurf The quantification procedure used by the JEOL SpecSurf software involves modifying the Scofield cross-sections to account for both an energy dependency
CE-300 Mathcad Tutorial
 CE-00 Mathcad Tutorial The purposes of this tutorial are: (1) to get you acquainted with the Mathcad procedures and synta you will use to solve typical problems in CE-00 and (2) to demonstrate how to set
CE-00 Mathcad Tutorial The purposes of this tutorial are: (1) to get you acquainted with the Mathcad procedures and synta you will use to solve typical problems in CE-00 and (2) to demonstrate how to set
Downloading GPS Waypoints
 Downloading Data with DNR- GPS & Importing to ArcMap and Google Earth Written by Patrick Florance & Carolyn Talmadge, updated on 4/10/17 DOWNLOADING GPS WAYPOINTS... 1 VIEWING YOUR POINTS IN GOOGLE EARTH...
Downloading Data with DNR- GPS & Importing to ArcMap and Google Earth Written by Patrick Florance & Carolyn Talmadge, updated on 4/10/17 DOWNLOADING GPS WAYPOINTS... 1 VIEWING YOUR POINTS IN GOOGLE EARTH...
Sample Alignment (2D detector) Part
 Sample Alignment (2D detector) Part Contents Contents 1 How to set Part conditions...1 1.1 Setting conditions... 1 1.2 Customizing scan conditions and slit conditions... 6 2 Sample alignment sequence...13
Sample Alignment (2D detector) Part Contents Contents 1 How to set Part conditions...1 1.1 Setting conditions... 1 1.2 Customizing scan conditions and slit conditions... 6 2 Sample alignment sequence...13
Supporting Information Practical Prediction of Heteropolymer Composition and Drift
 Supporting Information Practical Prediction of Heteropolymer Composition and Drift Anton A. A. Smith,,, Aaron Hall, Vincent Wu, and Ting Xu,,, Department of Materials Science and Engineering, University
Supporting Information Practical Prediction of Heteropolymer Composition and Drift Anton A. A. Smith,,, Aaron Hall, Vincent Wu, and Ting Xu,,, Department of Materials Science and Engineering, University
Forces and Newton s Second Law
 Forces and Newton s Second Law Goals and Introduction Newton s laws of motion describe several possible effects of forces acting upon objects. In particular, Newton s second law of motion says that when
Forces and Newton s Second Law Goals and Introduction Newton s laws of motion describe several possible effects of forces acting upon objects. In particular, Newton s second law of motion says that when
TECDIS and TELchart ECS Weather Overlay Guide
 1 of 24 TECDIS and TELchart ECS provides a very advanced weather overlay feature, using top quality commercial maritime weather forecast data available as a subscription service from Jeppesen Marine. The
1 of 24 TECDIS and TELchart ECS provides a very advanced weather overlay feature, using top quality commercial maritime weather forecast data available as a subscription service from Jeppesen Marine. The
O P E R A T I N G M A N U A L
 OPERATING MANUAL WeatherJack OPERATING MANUAL 1-800-645-1061 The baud rate is 2400 ( 8 bits, 1 stop bit, no parity. Flow control = none) To make sure the unit is on line, send an X. the machine will respond
OPERATING MANUAL WeatherJack OPERATING MANUAL 1-800-645-1061 The baud rate is 2400 ( 8 bits, 1 stop bit, no parity. Flow control = none) To make sure the unit is on line, send an X. the machine will respond
Comparing whole genomes
 BioNumerics Tutorial: Comparing whole genomes 1 Aim The Chromosome Comparison window in BioNumerics has been designed for large-scale comparison of sequences of unlimited length. In this tutorial you will
BioNumerics Tutorial: Comparing whole genomes 1 Aim The Chromosome Comparison window in BioNumerics has been designed for large-scale comparison of sequences of unlimited length. In this tutorial you will
Computer simulation of radioactive decay
 Computer simulation of radioactive decay y now you should have worked your way through the introduction to Maple, as well as the introduction to data analysis using Excel Now we will explore radioactive
Computer simulation of radioactive decay y now you should have worked your way through the introduction to Maple, as well as the introduction to data analysis using Excel Now we will explore radioactive
Kinetics of Crystal Violet Bleaching
 Kinetics of Crystal Violet Bleaching Authors: V. C. Dew and J. M. McCormick* From Update March 12, 2013 with revisions Nov. 29, 2016 Introduction Chemists are always interested in whether a chemical reaction
Kinetics of Crystal Violet Bleaching Authors: V. C. Dew and J. M. McCormick* From Update March 12, 2013 with revisions Nov. 29, 2016 Introduction Chemists are always interested in whether a chemical reaction
Chapter 2 Introduction to Aqueous Speciation
 Chapter 2 Introduction to Aqueous Speciation Overview It is our belief that the predictive modeling of aqueous systems requires that the system be fully speciated. This allows for smoother extrapolation
Chapter 2 Introduction to Aqueous Speciation Overview It is our belief that the predictive modeling of aqueous systems requires that the system be fully speciated. This allows for smoother extrapolation
Lecture #19 MINEQL: Intro & Tutorial Benjamin; Chapter 6
 Updated: 6 October 2013 Print version Lecture #19 MINEQL: Intro & Tutorial Benjamin; Chapter 6 David Reckhow CEE 680 #19 1 MINEQL today MINEQL is available from Environmental Research Software: http://www.mineql.com/
Updated: 6 October 2013 Print version Lecture #19 MINEQL: Intro & Tutorial Benjamin; Chapter 6 David Reckhow CEE 680 #19 1 MINEQL today MINEQL is available from Environmental Research Software: http://www.mineql.com/
Rain Watch TM Set up Manual. IC System with Rain Bird IC CONNECT
 Rain Watch TM Set up Manual IC System with Rain Bird IC CONNECT December 2018 Table of Contents Required materials to configure Rain Watch TM... 3 Installation... 4 Location... 4 Field Installation...
Rain Watch TM Set up Manual IC System with Rain Bird IC CONNECT December 2018 Table of Contents Required materials to configure Rain Watch TM... 3 Installation... 4 Location... 4 Field Installation...
Determining the Conductivity of Standard Solutions
 Determining the Conductivity of Standard Solutions by Anna Cole and Shannon Clement Louisiana Curriculum Framework Content Strand: Science as Inquiry, Physical Science Grade Level 11-12 Objectives: 1.
Determining the Conductivity of Standard Solutions by Anna Cole and Shannon Clement Louisiana Curriculum Framework Content Strand: Science as Inquiry, Physical Science Grade Level 11-12 Objectives: 1.
Lab 1: Dynamic Simulation Using Simulink and Matlab
 Lab 1: Dynamic Simulation Using Simulink and Matlab Objectives In this lab you will learn how to use a program called Simulink to simulate dynamic systems. Simulink runs under Matlab and uses block diagrams
Lab 1: Dynamic Simulation Using Simulink and Matlab Objectives In this lab you will learn how to use a program called Simulink to simulate dynamic systems. Simulink runs under Matlab and uses block diagrams
Simulating Future Climate Change Using A Global Climate Model
 Simulating Future Climate Change Using A Global Climate Model Introduction: (EzGCM: Web-based Version) The objective of this abridged EzGCM exercise is for you to become familiar with the steps involved
Simulating Future Climate Change Using A Global Climate Model Introduction: (EzGCM: Web-based Version) The objective of this abridged EzGCM exercise is for you to become familiar with the steps involved
Advanced Forecast. For MAX TM. Users Manual
 Advanced Forecast For MAX TM Users Manual www.maxtoolkit.com Revised: June 24, 2014 Contents Purpose:... 3 Installation... 3 Requirements:... 3 Installer:... 3 Setup: spreadsheet... 4 Setup: External Forecast
Advanced Forecast For MAX TM Users Manual www.maxtoolkit.com Revised: June 24, 2014 Contents Purpose:... 3 Installation... 3 Requirements:... 3 Installer:... 3 Setup: spreadsheet... 4 Setup: External Forecast
SeeSAR 7.1 Beginners Guide. June 2017
 SeeSAR 7.1 Beginners Guide June 2017 Part 1: Basics 1 Type a pdb code and press return or Load your own protein or already existing project, or Just load molecules To begin, let s type 2zff and download
SeeSAR 7.1 Beginners Guide June 2017 Part 1: Basics 1 Type a pdb code and press return or Load your own protein or already existing project, or Just load molecules To begin, let s type 2zff and download
Investigation reply tool operation manual
 CITIZEN ELECTRONICS CO.,LTD GreenSurveyTool Ver.2.00 Investigation reply tool operation manual May, 2011 CITIZEN ELECTRONICS CO., LTD. Contents 1.Purpose... 2 2.Survey Scope... 2 3.Survey Items... 3 4.Notes
CITIZEN ELECTRONICS CO.,LTD GreenSurveyTool Ver.2.00 Investigation reply tool operation manual May, 2011 CITIZEN ELECTRONICS CO., LTD. Contents 1.Purpose... 2 2.Survey Scope... 2 3.Survey Items... 3 4.Notes
Remember that C is a constant and ë and n are variables. This equation now fits the template of a straight line:
 CONVERTING NON-LINEAR GRAPHS INTO LINEAR GRAPHS Linear graphs have several important attributes. First, it is easy to recognize a graph that is linear. It is much more difficult to identify if a curved
CONVERTING NON-LINEAR GRAPHS INTO LINEAR GRAPHS Linear graphs have several important attributes. First, it is easy to recognize a graph that is linear. It is much more difficult to identify if a curved
Structures in the Magnetosphere 2. Hover the curser over a point on the image. The coordinates and value at that point should appear.
 Investigating the Magnetosphere Introduction In this investigation, you will explore the properties of the regions of the magnetosphere using simulation results from the BATS-R-US model from the magnetosphere
Investigating the Magnetosphere Introduction In this investigation, you will explore the properties of the regions of the magnetosphere using simulation results from the BATS-R-US model from the magnetosphere
Version 1.2 October 2017 CSD v5.39
 Mogul Geometry Check Table of Contents Introduction... 2 Example 1. Using Mogul to assess intramolecular geometry... 3 Example 2. Using Mogul to explain activity data... 5 Conclusions... 8 Further Exercises...
Mogul Geometry Check Table of Contents Introduction... 2 Example 1. Using Mogul to assess intramolecular geometry... 3 Example 2. Using Mogul to explain activity data... 5 Conclusions... 8 Further Exercises...
