Study of factors governing negative molecular ion yields of amino acid and peptide in FAB, MALDI and ESI mass spectrometry
|
|
|
- Poppy Brooks
- 5 years ago
- Views:
Transcription
1 Available online at International Journal of Mass Spectrometry 268 (2007) Study of factors governing negative molecular ion yields of amino acid and peptide in FAB, MALDI and ESI mass spectrometry Takashi Nishikaze, Mitsuo Takayama International Graduate School of Arts and Sciences, Yokohama City University, Yokohama , Japan Received 19 May 2007; received in revised form 5 August 2007; accepted 11 August 2007 Available online 17 August 2007 Abstract The factors governing negative and/or positive molecular ion yields of amino acids and peptides in the soft ionization methods of fast atom bombardment (FAB), matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI) mass spectrometry have been examined. The results obtained have been interpreted from the standpoint of two different components, namely desorption and ionization, by relating the ion yields to physicochemical properties of amino acid and peptides. The negative-ion yields of amino acids in FAB were found to be dependent on gas-phase acidity and aliphatic/hydrophobicity. In MALDI, a linear correlation was observed between negative-ion yields and gas-phase acidity of amino acids. In both FAB and MALDI mass spectra of peptides, the presence of aspartic acid residues tended to enhance the negative-ion yields. The presence of aliphatic/hydrophobic residues in FAB or aromatic amino acid residues in MALDI enhanced the negative-ion yields of peptides. In ESI, the charge state distributions of multiply-protonated peptides were dependent on the number of basic sites and their position in the sequence, while those of multiply-deprotonated peptides were dependent on the number of acidic sites. The total ion yields in ESI were governed by hydrophobicity of peptides and efficiency of acquiring the charge(s). The results suggest that the ion yields were governed by two independent processes, namely ionization and desorption, regardless of the ionization methods used Elsevier B.V. All rights reserved. Keywords: Negative-ion yield; FAB; MALDI; ESI; Peptide 1. Introduction Mass spectrometry (MS) has been used as a powerful analytical method for investigating various kinds of analytes such as organic and inorganic compounds. The advent of soft ionization methods such as matrix-assisted laser desorption/ionization (MALDI) [1 3] and electrospray ionization (ESI) [4,5] has allowed the development of MS techniques into more sensitive analytical tools allowing analysis without decomposition of large and fragile molecules such as peptides, proteins and other biological polymers. Mass spectrometers equipped with these soft and sensitive ionization sources have became indispensable tools for identification of proteins expressed by genomes [6]. In practice, peptide-mass fingerprinting [7,8] and amino acid Corresponding author. Present address: International Graduate School of Arts and Sciences, Yokohama City University, 22-2 Seto, Kanazawa-ku, Yokohama, Kanagawa , Japan. Tel.: ; fax: address: takayama@yokohama-cu.ac.jp (M. Takayama). sequencing by tandem mass spectrometry [9,10] have become common approaches for the characterization of proteins and determination of post-translational modifications. Although protein and peptide analysis using soft ionization has commonly been performed in positive-ion mode because of its superior ion yields compared to the negative-ion mode, simultaneous use of positive- and negative-ion mode often provides useful information in protein identification and in screening for phosphorylated sequences in protein digests. Bigwarfe and Wood [11] have reported that using negative-ion MALDI and ESI of protein digests results in the detection of a number of peaks in the mass spectra that are not observed in positiveion mode, and that greater sequence coverage and improved database search scores are obtained by combining the positiveand negative-ion data. Janek et al. [12] have reported that phosphorylated peptides, which give small ion yields in positiveion mode, exhibit improved signal intensities in negative-ion mode, and that comparative measurements using positive- and negative-ion MALDI-MS provide a simple and rapid method for screening for phosphorylated sequences in protein digests /$ see front matter 2007 Elsevier B.V. All rights reserved. doi: /j.ijms
2 48 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) These reports indicate that the use of negative-ion mode provides important and valuable information in protein identification. Regardless of whether positive- or negative-ion mode has been used, if all peaks from protein digests are observed as the molecular-related ion peaks [M +H] + or [M H] in mass spectra, accurate protein identification would be possible. However, the molecular ion yields of peptides are strongly dependent on their intrinsic physicochemical properties which are a result of the individual properties of the side chains of the constituent amino acids. Amino acids have a wide variety of physicochemical properties such as acidity, basicity, hydrophobicity associated with aliphatic or aromatic character and hydrophilicity associated with the acidic or basic nature and with the presence of other functional groups such as hydroxyl and thiol groups. Recent reports have suggested that several intrinsic properties of peptides influence their molecular ion yields in MALDI-MS. It has been reported that the presence of Arg, aliphatic, and aromatic amino acids residues enhance the ion yields of peptides in positive-ion mode in MALDI-MS [13 16], while little information is available concerning negative-ion mode. Bigwarfe and Wood [11] have pointed out that peptides from digests rich in Asp and Glu residues tend to give more prominent signals in negative-ion mode. Despite a good deal of work in the area [11,12], the relationship between the ion yields and physicochemical properties of analyte remains unclear for negative ion experiments. The molecular ion yields are of practical importance, as well as being of fundamental interests in MS. We recently reported [16] that physicochemical factors of individual amino acids cooperatively affected the ion yields of protonated amino acids and peptides [M +H] + in the desorption/ionization methods MALDI and fast atom bombardment (FAB). In order to relate the ion yields to the physicochemical factors such as basicity and hydrophobicity, the total ionization processes were divided into two different components, desorption and ionization. These two processes are quite different with desorption being a physical process which forms gaseous species from the condensed-phase, while ionization is a thermochemical process which forms molecular-related ions such as M +,[M +H] +, and [M H]. In conventional methods such as electron ionization and chemical ionization, the total ionization processes can be clearly divided into physical vaporization and thermochemical ionization components. For MS ionization techniques, it is also useful to divide the total ionization processes into two processes, namely gaseous species formation and ionization. In order to understand the ion yields in soft ionization MS, here we use a constitutional form where we have divided the total ionization processes J i into ionization efficiency of molecules I and the rate of desorption or vaporization of neutral molecules J v as follows: J i = IJ v (1) The efficiency I can be related to thermochemical quantities such as proton affinity, gas-phase basicity, ionization energy or electron affinity of a given analyte. The quantity J v represents the ability of analyte molecules to desorb or vaporize from condensed- to gas-phase. The aim of this paper is to investigate factors governing the molecular ion yields in negative-ion mode in the soft ionization methods of FAB, MALDI and ESI. 2. Experimental 2.1. Materials The angiotensin- and bradykinin-related peptides were purchased from Peptide Institute (Osaka, Japan). The sequences are summarized in Table 1, with the hydrophobicity index which has been derived using the data of Bull and Breeze Table 1 Angiotensin- and bradykinin-related peptides used Analyte peptide Monoisotopic mass (Mm) Sequence Hydrophobicity index a Angiotensin1 (A1) D R V Y I H P F H L 429 [Val5]-Angiotensin1 (A2) D R V Y V H P F H L 359 Angiotensin2 (A3) D R V Y I H P F 416 [Val5]-Angiotensin2 (A4) D R V Y V H P F 329 [Asn1,Val5]-Angiotensin2 (A5) N R V Y V H P F 294 Angiotensin (human1-7) (A6) D R V Y I H P 259 Des-Asp1-[Ile8]-Angiotensin2 (A7) R V Y I H P I 553 Angiotensin3 (A8) R V Y I H P F 563 Angiotensin4 (A9) V Y I H P F 772 Bradykinin (B1) R P P G F S P F R 104 [Tyr8]-Bradykinin (B2) R P P G F S P Y R 94 Des-Pro2-Bradykinin (B3) R P G F S P F R 96 Des-Arg9-Bradykinin (B4) R P P G F S P F 204 Des-Arg9-[Leu8]-Bradykinin (B5) R P P G F S P L 220 Lysyl-Bradykinin (Kallidin) (B6) K R P P G F S P F R 48 Tyrosyl-Bradykinin (B7) Y R P P G F S P F R 238 Des-Arg10-Kallidin (B8) K R P P G F S P F 130 Methionyl-Lysyl-Bradykinin (B9) M K R P P G F S P F R 104 Isoleucyl-Seryl-Bradykinin (B10) I S R P P G F S P F R 179 a Obtained by addition of F values for individual amino acids taken from Ref. [17], each sum being divided by the number of amino acids in the peptide.
3 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) [17]. All amino acids except for glutamic acid were purchased from Sigma Aldrich (Steinheim, Germany). Glutamic acid was purchased from Wako Pure Chemical (Osaka, Japan). The FAB matrix chemicals m-nitrobenzyl alcohol (mnba), dithiothreitol (DTT) and diethanolamine (DEA) were purchased from Tokyo Kasei (Tokyo, Japan). The MALDI matrix chemical -cyano-4-hydroxycinnamic acid (CHCA) was purchased from Sigma Aldrich (Steinheim, Germany). Trifluoroacetic acid (TFA), acetonitrile (HPLC Grade) and distilled water (HPLC Grade) were purchased from Wako Pure Chemical (Osaka, Japan). All reagents were used without further purification FAB-MS FAB mass spectra were acquired on a JEOL JMS-700 MStation reverse geometry double-focusing mass spectrometer (JEOL Ltd., Tokyo, Japan). The FAB gun was operated at 5 kv and 10.4 ma of current using Xe as the fast atom. The spectra were obtained in negative ion mode. The acceleration potential and mass resolution were set to 10 kv and 2000 (10% valley definition), respectively. Each analyte (amino acid or peptide) was dissolved in water at a concentration of 10 nmol/ L and 2 L of analyte solution was mixed with 8 L of matrix consisting of a 1:1 (v/v) mixture of mnba and DTT (mnba/dtt) or DEA. A volume of 2 L of the solution was deposited onto a FAB stainless probe tip. The reproducibility of spectral patterns was confirmed over scans 1 10, and the spectra were averaged over scans 3 8. The standard deviation of the peak intensity for molecular-related ions was estimated from the spectra obtained. The ion yields (Y)of[M H] ions of amino acids and peptides were estimated as the ratio of [M H] intensity to that for the matrix ions. For mnba/dtt matrix, the negative molecular ions m/z 153, B 1 for mnba and m/z 153, [B 2 H] for DTT were used as follows: Y(%) = 100 I[M H] I[B 1 ] + I[B 2 H] In DEA matrix, deprotonated DEA dimer (m/z 209, [2B H] ) was used in the ratio as follows: Y(%) = MALDI-TOFMS I[M H] I[2B H] MALDI-TOF mass spectra were acquired using an AXIMA- CFR instrument (Shimadzu Corp., Kyoto, Japan). A nitrogen laser (337 nm) was used to irradiate the sample for ionization. The spectra were obtained in negative-ion reflectron mode for amino acids and linear mode for peptides. The acceleration potential was set to 20 kv using a gridless-type electrode. Each amino acid or peptide analyte was dissolved in water at a concentration of 1 nmol/ L and 1 pmol/ L, respectively. Analyte solution (5 L) was mixed with 5 L of saturated solution of matrix in water/acetonitrile (1:1, v/v) with 0.1% TFA. A volume of 1 L of sample solution was deposited onto a MALDI sample plate and the solvents were removed by allowing evaporation in air at room temperature. Spectra were the sum of 100 profiles automatically acquired rastering the sample spot. Each profile was the result of 12 consecutive single laser pulses for peptides, and five consecutive laser pulses for amino acids. The ion yields (Y)of[M H] ions of amino acids and peptides were estimated as the ratio of [M H] intensity to that of the fragment ion of CHCA (m/z 93, [B frag ] ) as follows: Y(%) = ESI-MS I[M H] I[B frag ] Each peptide was dissolved in 20% acetonitrile at a concentration of 2 pmol/ L. Five microliters of the solutions were injected into a Finnigan Surveyor LC system (Thermo Electron, Bremen, Germany), and the eluate was directed to the ESI interface of a Finnigan LCQ Advantage MAX ion trap mass spectrometer (Thermo Electron, Bremen, Germany). For all the ESI experiments described here, the total combined flow rate provided by two LC pumps was 100 L/min. Pump A was used to deliver water, while pump B delivered acetonitrile. All fullscan mass spectra of peptides were obtained under isocratic conditions (acetonitrile/water ratio used was 20/80), without column and flow injection analysis of single components. Four replicate analyses were performed at room temperature. The positive- and negative-ion yields of peptides were calculated using the following formula: [M + nh] n+ (n = 1 4), [M nh] n (n = 1 2) where is the sum of the areas under the curves of all charge states of the peptides. The ion yields of peptides were calculated as the mean of four measurements. 3. Results and discussion 3.1. Negative-ion yields of amino acids in FAB with mnba/dtt matrix The molecular ion yields obtained from FAB of amino acids are summarized in Table 2, together with values of gas-phase acidity (GA) [18,19] and Bull and Breeze (B&B) indices ( F) [17] of hydrophobicity. The B&B index has been widely used to represent hydrophobicity of amino acids and peptides, because the index reflects the surface concentration of amino acid solution. A higher concentration of amino acid on the surface would be expected to have higher capability to desorb or vaporize from the surface. In negative-ion FAB with mnba/dtt matrix, acidic amino acids, Asp (0.92%) and Glu (0.82%), were detected with relatively high intensity. Medium ion yields were obtained for Asn (0.25%), Gln (0.34%), Leu (0.35%), Ile (0.22%) and Met (0.20%), while Trp, Thr, Cys and His gave yields lower than 0.13%. The peaks of Ser and His overlapped with the
4 50 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) Table 2 Ion yields Y(%) of [M H] measured for free amino acids in negative-ion FAB and MALDI, values of gas phase acidity [18], B&B hydrophobicity F [17] and monoisotopic mass (Mm) of amino acids Amino acids Mm (u) FAB (%) mnba/dtt FAB (%) DEA MALDI (%) GA (kcal/mol) F (kcal/mol) Glu = a 0.51 Asp b 0.61 Arg His = 1.17 = c 0.69 Gln = Asn = Thr Ser = = Cys Met = Trp = Tyr Lys = Val Ile Leu Phe Ala Gly Pro = : not detected. =: not estimated owing to overlap with intense matrix peaks. a Not determined. b Theoretical value [18]. c Value taken from Ref. [19]. intense background peaks originating from matrix ions. The other amino acids Phe, Tyr, Val, Pro, Lys, Ala and Gly did not show any molecular-related negative ions. The yields of deprotonated molecules [M H] observed in negative-ion FAB were plotted against the GA and B&B index of each amino acid as shown in Fig. 1(a) and (b), respectively. Fig. 1(a) does not include the acidic amino acid Glu, because gas-phase acidity of this amino acid has not been determined [17]. The higher ion yields of Asp and Glu can be attributed to their acidic nature. Although the value of GA for Glu has not been determined, it is expected that Glu has a large GA value in the region of that of Asp. This result suggests that GA is the dominant factor in the production of gas-phase negative ions [M H] under FAB conditions. Although Fig. 1(a) shows that increasing GA tends to increase ion yields for most amino acids, the data for Leu, Ile and Arg (enclosed within the left ellipse and center circle in Fig. 1(a)) deviates significantly from this tendency. Leu and Ile have relatively large hydrophobicity, while their GA values are relatively small. This indicates that the negative-ion yields are also governed by hydrophobicity to some extent. Note that Fig. 1. Yields of deprotonated molecules [M H] of amino acids in negative-ion FAB with mnba/dtt matrix. (a) Ion yields vs. gas-phase acidity and (b) ion yields vs. B&B hydrophobicity. The standard deviations for the yields were within 2 9% for all amino acids.
5 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) Arg has outstanding proton affinity (PA), while its GA is relatively large. The outstanding PA of Arg is advantageous for producing the gas-phase protonated form, but is disadvantageous in the production of the gas-phase deprotonated form. This is clearly shown in Fig. 2 which displays the comparison between positive- and negative-ion yields of amino acids in FAB with mnba/dtt matrix. In desorption/ionization methods, positive- and negative-ion formation occurs simultaneously and competitively. Although hydrophobicity was expected to be one of the factors governing molecular ion yields in FAB, Fig. 1(b) does not demonstrate a reasonable positive correlation. Excluding the ellipse and circle areas, however, the negative-ion yields roughly increase with increasing hydrophobicity. The left ellipse area contains the acidic amino acids Asp and Glu and amidic amino acids Asn and Gln. These amino acids possess large GA values, while their hydrophobicities are small. The right circle area includes the aromatic amino acids Phe and Tyr. These amino acids possess relatively high hydrophobicity as indicated by the B&B index (Table 2), and did not give any molecular-related negative ions (Fig. 1(b)) These results indicate that aliphatic hydrophobicity of Leu and Ile is advantageous for producing gas-phase ions, but aromatic hydrophobicity of Phe and Tyr is not. As seen in Fig. 2, the positive effect of aliphatic hydrophobicity and the negative effect of aromatic hydrophobicity on the ion yields are in accord with the data obtained in positive-ion FAB [16]. It is expected that in general the surface concentration of these hydrophobic amino acids would be high, and that these amino acids are favorable for FAB-induced vaporization and thus give higher ion yields. This has been suggested by the reports of positive-ion FAB [20 24], and is in consistent with the bubble chamber model for FAB events [25]. The results obtained here can be simply interpreted on the basis of the hypothesis [16] that total ionization process is governed by two processes, desorption and ionization and each can be considered independently. Thus, aliphatic hydrophobicity of Leu and Ile may increase the desorption term J v in Eq. (1), and therefore enhance the ion yields in both positive- and negative-ion mode. The negative contribution of aromatic hydrophobicity of Phe and Tyr to the ion yields might be rationalized in terms of intermolecular interactions between aromatic -electrons in the amino acids and in the matrix mnba molecules, as well as in positive-ion mode [16]. However, this is not the case. These aromatic/hydrophobic amino acids also gave low ion yields using DEA matrix, where aromatic -electrons are not involved, as described below Negative-ion yields of amino acids in FAB with basic DEA matrix Fig. 2. Yields of molecular-related ions of amino acids in FAB. (a) Yields of protonated molecules [M +H] + in positive-ion FAB [16]. (b) Yields of deprotonated molecules [M H] in negative-ion FAB. Asterisks indicate that the ion yields could not be estimated owing to overlap within intense peaks originating from matrix. It is widely accepted that use of a basic matrix such as DEA is suitable for negative-ion FAB. Using DEA matrix, 17 amino acids could be detected at significant intensity (Table 2). The peaks of Ser and Asn could not be estimated owing to overlap with the intense background peaks originating from the DEA matrix. Only the thiol-containing amino acid Cys did not show any signals. The yields of deprotonated amino acids [M H] observed in negative-ion FAB using DEA matrix are plotted against the GA and B&B index of each amino acid in Fig. 3(a) and (b), respectively. Based on the results of negativeion FAB using mnba/dtt, aliphatic/hydrophobic amino acids and acidic amino acids were expected to give higher ion yields than others. As expected, aliphatic/hydrophobic amino acids Leu, Ile and Val gave higher ion yields than other relatively hydrophilic amino acids. In contrast to the measurements using mnba/dtt, however, acidic Asp and Glu did not give higher ion yields. These unexpected results may be due to the basic nature of the DEA matrix. Basic DEA matrix can effectively extract protons from amino acids regardless of the acidity of analytes. Thus, DEA matrix may equalize the ionization efficiencies (I in Eq. (1)) of analyte amino acids. Thus, ion yields of amino acids using DEA matrix were only dependent on the desorption term J v, which corresponds to the ability of analytes to vaporize from matrix surface to gas-phase. With the exception of Met and aromatic amino acids, the ion yields of amino acids in negativeion FAB with DEA matrix showed reasonable dependence on hydrophobicity, as shown in Fig. 3(b). In particular, the ion yields of aliphatic amino acids (Gly, Ala, Pro, Val, Ile, and Leu; black triangle in Fig. 3(b)) increased linearly with their increasing hydrophobicity. This indicates that an aliphatic/hydrophobicity is the factor facilitating vaporization from the liquid matrix surface to gas-phase in negative-ion FAB using DEA matrix, as well as positive- and negative-ion FAB using mnba/dtt matrix. The reason for the extremely high ion yield of Met is not immediately apparent. As discussed above, the aromatic/hydrophobic amino acids Phe, Tyr, and Trp provided lower ion yields than that expected from their hydrophobicity. These amino acids also gave low ion yields using DEA matrix, as seen enclosed within the circle in Fig. 3(b). The negative contribution of aromaticity to the ion yields may be explained by intermolecular interactions between aromatic -electrons in the amino acids and and/or -electrons in the matrix molecules. These intermolec-
6 52 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) Fig. 3. Yields of deprotonated molecules [M H] of amino acids in negative-ion FAB with DEA matrix. (a) Ion yields vs. gas-phase acidity and (b) ion yields vs. B&B hydrophobicity. The standard deviations for the yields were within 4 9% for all amino acids. ular interactions can contribute to the solubility leading to a decrease in surface concentrations of aromatic amino acids. The remarkable deviation of Met and aromatic amino acids from the positive correlation in Fig. 3(b) should be further investigated. The results obtained using two different matrices, mnba/dtt and DEA, indicate that the negative-ion yields of amino acids are governed not only by the physicochemical properties of analyte, but to some extent also by the characteristics of matrix used. The specific nature of matrix such as the basic properties of DEA can obscure the effects of acidity of analyte amino acids on negative-ion yields, as described above. While details of matrix effect on the ion yields are beyond the scope of this paper, it is important to take into account that the nature of matrix also affects the desorption and ionization process independently. The basic nature of DEA affects the ionization process, and may equalize the ionization efficiency (I in Eq. (1)) of analyte amino acids. In this sense, mnba/dtt matrix is universal matrix, and is useful for study of factors governing molecular ion yields Negative-ion yields of amino acids in MALDI In this study, we used CHCA matrix because CHCA is a conventional and widely used MALDI matrix which gives decreased formation of sweet spots compared to other matrices such as 2,5-dihydroxybenzoic acid, allowing comparison of quantitative results. Unfortunately, the ion yields of seven deprotonated amino acids Tyr, Met, Pro, Lys, Glu, His and Gln could not be estimated owing to overlap with the strong peaks originating from the CHCA matrix in negative-ion MALDI. Of those amino acids not overlapping with matrix ions the peak of deprotonated Arg could not be detected. The highest ion yield was obtained for Asp (64.5%). Thr (15.6%), Cys (23.4%), Ser (12.2%), and Asn (20.5%) could be detected in moderate abundance. Other amino acids gave ion yields of less than 10%. The high ion yield of Asp can be attributed to its acidic nature. Moderate ion yields of Thr, Cys, Ser and Asn are due to the presence of hydroxyl, thiol and amide groups that possess relatively high gas-phase acidity compared to other amino acids (see Table 2). Although the aliphatic/hydrophobic amino acids such as Val, Ile and Leu gave high ion yields under negative-ion FAB, these amino acids did not give high ion yields under negative-ion MALDI conditions. The lack of dependence of ion yields on aliphatic/hydrophobicity is presumably due to the fact that in MALDI the analyte molecules are incorporated into the solid matrix, while in FAB the analytes are dissolved in liquid matrix. The yields Y of deprotonated amino acids [M H] observed in negative-ion MALDI are plotted separately against the gasphase acidity and B&B hydrophobicity of each amino acid in Fig. 4(a) and (b), respectively. Fig. 4(a) shows a reasonably simple linear correlation between Y values and GA, with the exception of Arg. Arg has a relatively large GA value, but is the most basic amino acid of the 20 naturally occurring amino acids. The high basicity of Arg preferentially leads to the protonation reaction, so that the formation of deprotonated Arg is completely suppressed under MALDI conditions. In fact, Arg gave the highest ion yield in positive-ion MALDI [16], as shown in Fig. 5 that shows a comparison between positive- and negative-ion yields of amino acids. In contrast, the plot of Y against B&B hydrophobicity does not show any correlations, as seen in Fig. 4(b). This indicates that in negative-ion MALDI the ion yield of amino acids depends on their ionization efficiency I which directly relates to GA. The results obtained from the negative-ion FAB and MALDI experiments with amino acids can be used to help understand the ion yields of peptides described below Negative-ion yields of peptides in FAB The negative-ion yields Y of angiotensin- and bradykininrelated peptides in FAB were estimated using the ratio I[M H] /(I[B 1 ]+I[B 2 H] ), and the yields were com-
7 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) Fig. 4. Yields of deprotonated molecules [M H] of amino acids in negative-ion MALDI. (a) Ion yields vs. gas-phase acidity and (b) ion yields vs. B&B hydrophobicity. pared with the positive-ion yields (Fig. 6). In negative-ion FAB with mnba/dtt matrix, angiotensin1 (A1, 3.45%), [Val 5 ]- angiotensin1 (A2, 2.80%), des-asp 1 -[Ile 8 ]-angiotensin2 (A7, 2.18%), and angiotensin4 (A9, 2.77%) gave higher ion yields than seen for other angiotensin-related peptides. Angiotensin2 (A3, 0.93%) and angiotensin3 (A8, 1.09%) gave medium ion yields, and other peptides gave ion yields of less than 0.5%. Among the bradykinin-related peptides, the highest ion yield was obtained for des-arg 9 -[Leu 8 ]-bradykinin (B5, 2.46%). Medium ion yield was obtained with des-arg 9 -bradykinin (B4, 1.17%). Other peptides gave ion yields of less than 0.5%. Comparison of the sequence of A1 and A2 indicates that the conversion of Val5 into Ile5 increased the ion yield from 2.80% to 3.45%. The same trend was observed in a comparison of A3 Fig. 5. Yields of molecular-related ions of amino acids in MALDI. (a) Yields of protonated molecules [M +H] + in positive-ion MALDI [16]. (b) Yields of deprotonated molecules [M H] in negative-ion MALDI. Asterisks indicate that the ion yields could not be estimated owing to overlap with intense peaks originating from matrix. (Ile at position 5) which gave a yield of 0.93% and A4 (Val at position 5) which gave a yield of 0.46%. In addition, the conversion of Phe8 in A8 into Ile8 in A7 enhanced the Y value from 1.09% to 2.18%. Such a positive effect due to the presence of an aliphatic/hydrophobic residue such as Ile can also be observed in the conversion of Phe8 in B4 (1.2%) into Leu8 in B5 (2.5%). Whereas aliphatic/hydrophobic residues were advantageous for the production of negative ions of peptides under FAB conditions, in the comparison between A3 (0.9%) and A6 (0.6%), the aromatic/hydrophobic residue did not play an important role in generating gas-phase deprotonated peptides. The effects of aliphatic/hydrophobicity and aromatic/hydrophobicity on the ion yields can be seen in both positive- and negativeion yields (Fig. 6). This figure indicates that the FAB ion yields of peptides are governed by surface activity which is directly related to aliphatic/hydrophobicity. The signal enhancement effects resulting from the aliphatic/hydrophobic nature of peptides is consistent with the results obtained from amino acids as described above. The positive influence of aliphatic/hydrophobic residues in peptides on the yields may be interpreted via the term corresponding to the rate of desorption J v in Eq. (1). The influence of the ionization efficiency I which is related to acidic and basic nature will be described below. Fig. 6 demonstrates that in negative-ion FAB the yields of A7 (2.2%) and A8 (1.1%) are lower than that of A9 (2.77%), while the positive-ion data shows a trend in the opposite direction. The peptide A9 lacks an Arg residue compared to A7 and A8. There are two reasons why removal of the Arg residue enhances the yield of deprotonated peptides. First, the absence of the basic Arg residue decreases the formation of protonated peptides, so that a larger fraction of peptides would be shunted into the deprotonation reaction. The lack of the Arg residue would be advantageous for the formation of [M H] ions by means of the ionization efficiency I in Eq. (1). Second, the removal of the hydrophilic Arg residue contributes to an increase in hydrophobicity of peptides. It is likely that the higher ion yield obtained with A9 over
8 54 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) Fig. 6. Yields of molecular-related ions of angiotensin-related peptides (A1 A9) and bradykinin-related peptides (B1 B10) in FAB. (a) Yields of protonated molecules [M +H] + in positive-ion FAB [16]. (b) Yields of deprotonated molecules [M H] in negative-ion FAB. that of A7 and A8 is due to a cooperative effect resulting from the lack of the Arg residue. This is supported by the positive-ion yields of A7, A8 and A9 as seen in Fig. 6. It is interesting to compare the negative-ion yield of A8 (1.1%) with that of A3 (0.9%) as it demonstrates that the presence of an Asp residue at the N-terminus of A3 did not affect the ion yield. It seems that the acidic nature of the Asp residue does not contribute to an enhancement in the negative-ion yields. According to the results obtained for the corresponding free amino acids under FAB conditions using mnba/dtt matrix, the acidic Asp residue should enhance the negative-ion yields. It has been reported that peptides rich in acidic residues tend to show increased signal intensity in negative-ion FAB [26]. It is important to recognize that Asp possesses both acidic and hydrophilic characteristics, because the two characteristics contribute to increase and decrease negative-ion yields, respectively. Although the acidic Asp residue probably contributes to the formation of the [M H] ion of A3, this effect is completely countered by their hydrophilic nature that interferes with analyte desorption from the liquid matrix. Eq. (1) is helpful to understand this complex observation. It maybe interpreted such that the acidic nature of the Asp residue enhances the ionization efficiency I, whereas the hydrophilic nature of Asp decreases the rate of desorption J v. These two factors independently and cooperatively affect the negative-ion yields. Among the bradykinin-related peptides, the peptides which possess an Arg residue in B4 and B5 gave higher ion yields than other peptides containing two Arg residues. In particular, the ion yield of B4 (1.2%) is significantly higher than that of B1 (0.4%). The only difference between these two peptides is the further addition of an Arg residue to the C-terminus in B4. The negative effect caused by further addition of Arg is also observed upon a comparison of B8 (0.5%) and B6 (0.1%). As described above, the negative contribution of an Arg residue on negative-ion yield is due to the preferential protonation reaction and hydrophilic nature. However, this is not the case with two Arg residues. We have reported that the presence of two Arg residues reduces the hydrophobicity of peptides, resulting in decreased ion yields in positive-ion FAB [16]. Therefore, it is likely that the negative contribution of two or more Arg residues on negative-ion yields is due to the hydrophilic nature. The peptide ion yields in negative-ion FAB are ultimately governed by the physicochemical nature of the constituent amino acids. The presence of aliphatic/hydrophobic Leu or Ile residues seems to be advantageous for the desorption process, which may be interpreted by means of the term J v in Eq. (1), corresponding to the rate of desorption. In contrast, aromatic/hydrophobic Phe does not play an important role in the desorption process. Although the presence of an Arg residue was effective in generation of higher ion yields in positive-ion FAB, its basic and hydrophilic nature seem to hinder the deprotonation reaction and the desorption, respectively, in negative-ion FAB.
9 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) Negative-ion yields of peptides in MALDI Although none of the protonated angiotensin- and bradykinin-related peptides generated any fragment ions in positive-ion reflectron MALDI, some deprotonated peptides (A1, A2, A3, A4, A6, B1, B2, B3, B6, B7, B9 and B10) gave considerable abundant metastable peaks in the negativeion reflectron MALDI mass spectra. To avoid reduction of ion yields by metastable decay, the analysis of peptides in negativeion MALDI was performed using linear mode. The ion yields of deprotonated angiotensin- and bradykinin-related peptides in negative-ion linear MALDI are shown in Fig. 7, together with the positive-ion yields of these peptides. Among the angiotensinrelated peptides, A3 (16.3%), A4 (22.1%) and A5 (22.7%) gave the highest ion yields. In contrast, A1 (7.5%), A2 (10.5%), A6 (7.3%), A7 (3.5%) and A8 (5.0%) gave relatively low ion yields. The A9 peptide did not show any molecular-related negativeions. Among the bradykinin-related peptides, B1 (25.7%), B2 (25.5%), B3 (23.0%), B6 (21.0%), B7 (24.6%), B9 (20.6%) and B10 (22.2%) gave the highest ion yields (>20%). Other peptides gave ion yields of less than 10%. Unlike the results obtained under FAB conditions, the positive effect of the presence of an acidic Asp residue on the yields is clearly observed under MALDI conditions. The presence of an Asp residue at the N-terminus of A3 enhanced the formation of deprotonated peptide compared to A8. Such positive influence of the Asp residue can be attributed to its acidic nature. It is likely that the presence of the Asp residue facilitates the deprotonation reaction and thus enhances the negative-ion yields. This is supported by the high negative-ion yield of acidic amino acid Asp in Fig. 5. The positive influence of Asp residues in peptides on the negative-ion yields may be interpreted via the term corresponding to the ionization efficiency I in Eq. (1). Comparison of the sequence of A4 and A5 indicates that conversion of Asp1 into Asn1 does not affect the ion yields (from 22.1% to 22.7%). This suggests that the role of the Asn residue on negative-ion yield of peptides is similar to the role of the Asp residue. Asn can thus also be regarded as a signal enhancing residue. It is highly unlikely that an amidic group of an Asn residue contributes to the deprotonation reaction effectively. In fact, the ion yield of Asn was lower than that of Asp (see Table 2). It is therefore likely that the amidic group of the Asn residue is advantageous for the desorption process from crystalline matrix to gas-phase. It has been reported that Phe is advantageous in the production of gas-phase protonated peptides in positive-ion MALDI [15,16]. Here we found that Phe also enhances the negative-ion yields of peptide. Comparison of the sequences of A6 and A3 indicates that the addition of a Phe residue increased the ion yields from 7.3% to 16.3%, as shown in Fig. 7(b). Furthermore, the conversion of an aliphatic residue into an aromatic residue (Ile8 of A7 into Phe 8 of A8, Leu11 of B5 into Phe11 of B4) resulted in an enhancement of the ion yields in negative-ion MALDI. It is likely that in MALDI the yields of both protonated and deprotonated peptides are enhanced by the presence of an aromatic residue over an aliphatic residue. The trend with these results is opposite to that seen in the results obtained by Fig. 7. Yields of molecular related ions of angiotensin-related peptides (A1 A9) and bradykinin-related peptides (B1 B10) in MALDI. (a) Yields of protonated molecules [M +H] + in positive-ion MALDI [16]. (b) Yields of deprotonated molecules [M H] in negative-ion MALDI.
10 56 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) FAB. The positive effect of aromatic residues on the yields in MALDI may be explained by the aromatic/hydrophobic residue enhancing the rate of desorption from crystalline matrix to gasphase, and thereby increasing the ion yields in both positiveand negative-ion mode. Thus, the positive contribution of aromatic residues to the ion yields can be interpreted in terms of desorption J v in Eq. (1). Based on the fact that the lack of an Arg residue produced signal enhancement in negative-ion FAB, it maybe expected that the lack of an Arg residue would enhance the ion yields in negative-ion MALDI. However, the opposite results were obtained. The removal of the N-terminal Arg residue from A8 to give A9 decreased the ion yield from 5% to zero (Fig. 7(b)). The same signal decrease upon removal of the Arg residue was observed in the comparison of B1 (25.7%) and B4 (7.0%), and the comparison of B6 (21%) and B8 (8.4%). These results indicate that in negative-ion MALDI the presence of an Arg residue contributes to enhancement of the yields of deprotonated peptides. It is unlikely that the basic Arg residue is effectively deprotonated. We thus believe that the presence of an Arg residue is advantageous for desorption from crystalline matrix to gas-phase in MALDI, though a plausible mechanism is not immediately apparent. This is currently under investigation Molecular ion yields of peptide in ESI Positive- and negative-ion ESI mass spectra of the peptides showed multiply protonated or deprotonated forms, [M + nh] n+ (n = 1 4) and [M nh] n (n = 1 2), respectively. The ion yields in ESI were estimated as the sum of the peak areas of all charge states of the peptides. It should be noted that in LCQ ion trap instruments the detector gain is dependent on charge state and therefore the charge state distribution observed in mass spectra is not exactly the same as the charge state distribution seen with peptide ions in the ion trap [27]. The gain of the detector system increases with charge state. Since multiply protonated peptides [M + nh] n+ have detector gains approximately n-times higher than the singly protonated peptide [M +H] +, the areas of the multiply protonated peptide were divided by their charge number in order to estimate the exact charge state distribution. Likewise, this is approximately true in negative-ion ESI. Fig. 8 shows the corrected total ion yields and charge state distributions of peptides in positive- and negative-ion ESI. Although we did not use acetic acid as an additive to mobile phases in the LC system, several peptides (B2, B6, and B7) were partially detected as acetate adducts [M +CH 3 COO] in negative-ion ESI. The charge state distributions drastically varied with sequence. Fig. 9 shows normalized charge state distributions Fig. 8. Corrected total ion yields and charge state distributions of molecular related ions of angiotensin-related peptides (A1 A9) and bradykinin-related peptides (B1 B10) in ESI. (a) Yields and their charge state distributions of multiply protonated molecules [M + nh] n+ (n = 1 4) in positive-ion ESI. (b) Yields and charge state distributions of multiply deprotonated molecules [M nh] n (n = 1, 2) and acetate ion adduct [M +CH 3 COO] in negative-ion ESI.
11 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) Fig. 9. Normalized charge state distributions of multiply-protonated peptides in positive-ion ESI and their amino acid sequences. (a) Angiotensin-related peptides and (b) bradykinin-related peptides. of multiply-protonated peptides and their sequences. The peptides which contain a large number of basic sites tend to give higher charge states in positive-ion ESI. With angiotensinrelated peptides, the highest charge state observed was the quadruply-protonated form [M + 4H] 4+ for A1 (1.7%) and A2 (1.9%). A1 and A2 contain four basic sites at Arg2, His6, His9 and the N-terminus. The triply-protonated form [M + 3H] 3+ was observed to be the dominant charge state for A1 (60.9%) and A2 (61.3%). The removal of the His-Leu portion from A1 and A2 to form A3 and A4 (Table 1) suppressed the generation of [M + 4H] 4+ and [M + 3H] 3+, and resulted in predominant detection of the doubly-protonated form [M + 2H] 2+ (A3; 87.8% and A4; 87.1%). It is of interest to compare the charge state distribution of A8 with that of A3. Although both A8 and A3 contain three basic sites at Arg2, His6 and the N-terminus, A8 showed a lower charge state distribution than A3. The only difference between the two peptides is the removal of the Asp residue from the N-terminus of A3. The lower charge state of A8 compared to A3 can be explained by coulomb repulsion between the protonated N-terminus and the guanidinium moiety of Arg1. It is likely that it is difficult to protonate both basic sites at the same time due to coulomb repulsion. This indicates that the charge state distribution is dependent on not only the number of basic sites, but also their position within the sequence. Furthermore, A9 had a lower charge state distribution (26.2% for [M +H] +, 73.8% for [M + 2H] 2+ ) than A8 (5.1% for [M +H] +, 94.9% for [M + 2H] 2+ ). Obviously, the lack of the Arg residue from A8 is disadvantageous to multiple protonation. Similar results were obtained with bradykinin-related peptides. The highest charge state was observed as [M + 4H] 4+ for B9 (1.8%). B9 contains four basic sites at Lys2, Arg3, Arg11 and the N-terminus. The [M + 4H] 4+ ion disappeared after removal of Met1 from B7 to give B6. The conversion of Lys1 in B6 to Tyr1 in B7 caused increased abundance of [M + 2H] 2+ (B7; 42.7%), and removal of Lys1 or Tyr1 to form B2 resulted in a lower charge state distribution of [M + 2H] 2+ (59.7%) and [M +H] + (2.5%). From comparison of B1 and B4, it was found that removal of the C-terminal Arg9 in B1 drastically affected the charge state distribution. The formation of [M + 3H] 3+ in B1 was completely suppressed by removal of Arg9 and B4 lacking Arg9 mainly gave [M + 2H] 2+ (72.5%) and [M +H] + (27.5%). Similarly, the removal of Arg10 from B6 to form B8 altered the charge
12 58 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) Fig. 10. Corrected total ion yields of peptides in ESI. (a) Positive-ion yields vs. hydrophobicity index and (b) negative-ion yields vs. hydrophobicity index. state distribution. B8 gave a lower charge state distribution of [M + 3H] 3+ (1.1%), [M + 2H] 2+ (93.8%), and [M +H] + (5.0%) than B6 (72.7% for [M + 3H] 3+ and 27.2% for [M + 2H] 2+ ). In negative-ion ESI, most peptides gave the singlydeprotonated form [M H], while some peptides (A1, A2, A3, and A4) gave abundant doubly-deprotonated form [M 2H] 2 due to the presence of an acidic Asp residue. The corrected total positive- and negative-ion yields of peptides were dependent on their hydrophobicity, which is directly related to surface activity, with some exceptions. The corrected total positive- and negative-ion yields of peptides as a function of their hydrophobicity indices calculated from B&B hydrophobicity [17] are shown in Fig. 10(a) and (b), respectively. Although the positive-ion yields of peptides increased with increasing hydrophobicity, the data for A7, A8, and A9 (enclosed within the ellipse in Fig. 10(a)) deviated significantly from the positive correlation. In the same way, the negative-ion yields of peptides also increased with increasing hydrophobicity, except for the data from A7, A8, and A9 (enclosed within the ellipse in Fig. 10(b)). These results can be explained as described below. It is well known that hydrophobicity, which is directly related to the surface activity, plays an important role in migration from the droplet interior to the surface and in desolvation during the electrospray process. Hydrophobic peptides therefore are advantageous for evaporation or desorption from the droplets to gas-phase, resulting in increased total positive- and negative-ion yields compared to less hydrophobic peptides. A plausible explanation for the significant deviation of the three peptides, A7, A8, and A9 from a linear correlation in Fig. 10(a) and (b) may be due to lower efficiency of the protonation or deprotonation reaction. Note that these peptides have relatively large hydrophobicities, while their charge state distributions and efficiencies of protonation or deprotonation are relatively low compared to other angiotensin-related peptides. As shown in Fig. 8, these three peptides showed lower charge state distributions than other angiotensin-related peptides in both positive- and negative-ion ESI. This indicates that the total positive- and negative-ion yields are governed not only by hydrophobicity, but also by efficiency of acquiring the charge(s). It seems that the positive contribution of hydrophobicity on the total ion yields can be interpreted in terms of desorption (J v in Eq. (1)). In contrast, it seems reasonable to assume that the term corresponding to ionization efficiency I is related to efficiency of protonation or deprotonation, which depends upon the number of basic sites and their position in positive-ion ESI or the number of acidic sites in negative-ion ESI. As described above, the efficiency of protonation or deprotonation greatly affects the charge state distributions in ESI. The corrected total ion yields in ESI were qualitatively explicable using the concept that gas-phase ion formation requires the ionization and vaporization process just as in FAB and MALDI. In order to enhance the peptide hydrophobicity and ionization efficiency, derivatization of peptides with a reagent containing a quaternary amine with an adjacent n-octyl chain has been investigated [28]. 4. Conclusions In the present work, we measured the negative molecular ion yields of amino acids and peptides using FAB-MS and MALDI- MS in order to discern the factors governing the ion yields. The positive- and negative-ion yields of peptides were investigated using ESI-MS. In FAB with mnba/dtt matrix, the ion yields of amino acids were found to be dependent on gas-phase acidity and hydrophobicity. In particular, aliphatic/hydrophobicity was more efficient for producing abundant gas-phase negative-ions than aromatic/hydrophobicity. Thus, acidic amino acids (Asp and Glu) and aliphatic amino acids (Leu and Ile) gave higher negative-ion yields, while aromatic amino acids (Phe and Tyr) did not show any molecular-related negative ions. In contrast, in FAB with DEA matrix, the negative-ion yields of amino acids showed reasonable dependence on their hydrophobicity. However, aromatic amino acids gave lower ion yields than would be expected from their hydrophobicities. In MALDI, a linear correlation was observed between negative-ion yields and gas-phase acidity of amino acids. However, Arg failed to ionize despite its medium GA value, because of its outstanding basicity. This indicates that positive- and negative-ion formation occurs simultaneously and competitively. No significant correlations were observed between negative-ion yields and any other physicochemical properties including hydrophobicity.
13 T. Nishikaze, M. Takayama / International Journal of Mass Spectrometry 268 (2007) In the analysis of peptides by both FAB and MALDI, the presence of an Asp residue contributed to enhanced negativeion yields. In FAB, the presence of an aliphatic hydrophobic Leu or Ile residue, or alteration to Leu or Ile from other amino acid resides, tends to enhance the negative-ion yields. Similar findings have been previously reported [16], where an aliphatic/hydrophobic residue enhanced the positive-ion yields in FAB. The aliphatic/hydrophobic nature of peptides is an enhancing factor on the yields in both positive- and negative-ion FAB. In MALDI, the presence of aromatic/hydrophobic amino acid residues in peptides enhanced the negative-ion yields. It is well known that aromatic residues contribute to increases in the positive-ion yield of peptides [14 16]. Hence the aromatic/hydrophobic nature of peptides is a positive factor in production of both protonated and deprotonated peptides. In ESI-MS, the charge state distributions of multiplyprotonated peptides were dependent on the number of basic sites and their position in the sequence, while that of multiplydeprotonated peptides were also dependent on the number of acidic sites. The total ion yields of peptides were governed by hydrophobicity and efficiency of acquiring the charge(s). Ion yields of peptides under ESI conditions were also cooperatively affected by two processes, ionization and desorption, in a similar way as seen in desorption/ionization methods. Taking these results together with those from our previous report [16], we can conclude that the ion yields of peptides are cooperatively affected by two processes, namely ionization and desorption, regardless of the ionization methods used. The ionization efficiency I in Eq. (1) can be related to thermochemical quantities such as proton affinity and gas-phase acidity, depending on the molecular-related ions such as the protonated and deprotonated form. In contrast, the rate of desorption J v which represents the capability of analyte molecules to desorb or vaporize from condensed- to gas-phase can be related to the aliphatic/hydrophobicity in FAB, aromaticity in MALDI, and hydrophobicity in ESI, regardless of the molecular-related ions generated. References [1] M. Karas, D. Bachmann, U. Bahr, F. Hillenkamp, Int. J. Mass Spectrom. Ion Processes 78 (1987) 53. [2] K. Tanaka, H. Waki, Y. Ido, S. Akita, T. Yoshida, Rapid Commun. Mass Spectrom. 2 (1988) 151. [3] M. Karas, F. Hillenkamp, Anal. Chem. 60 (1988) [4] C.M. Whitehouse, R.N. Dreyer, M. Yamashita, J.B. Fenn, Anal. Chem. 57 (1985) 675. [5] J.B. Fenn, M. Mann, C.K. Meng, S.F. Wong, C.M. Whitehouse, Science 246 (1989) 60. [6] P. Derrick, S. Patterson, Proteomics 1 (2001) 919. [7] W.J. Henzel, T.M. Billesci, J.T. Stults, S.C. Wong, C. Grimley, C. Watanabe, Proc. Natl. Acad. Sci. U.S.A. 90 (1993) [8] D.J.C. Pappin, P. Hojrup, A.J. Bleasby, Curr. Biol. 3 (1993) 327. [9] M. Mann, M. Wilm, Anal. Chem. 66 (1994) [10] J.K. Eng, A.L. McCormack, J.R. Yates, J. Am. Soc. Mass Spectrom. 5 (1994) 976. [11] P.M. Bigwarfe Jr., T.D. Wood, Int. J. Mass Spectrom. 234 (2004) 185. [12] K. Janek, H. Wenschuh, M. Bienert, E. Krause, Rapid Commun. Mass Spectrom. 15 (2001) [13] E. Krause, H. Wenschuh, P.R. Jungblut, Anal. Chem. 71 (1999) [14] M.L. Valero, E. Giralt, D. Andreu, Lett. Peptide Sci. 6 (1999) 109. [15] S. Baumgart, Y. Lindner, R. Kühne, A. Oberemm, H. Wenschuh, E. Krause, Rapid Commun. Mass Spectrom. 18 (2004) 863. [16] T. Nishikaze, M. Takayama, Rapid Commun. Mass Spectrom. 20 (2006) 376. [17] H.B. Bull, K. Breeze, Arch. Biochem. Biophys (1974) 665. [18] K.E. Colyer, C.M. Jones, R.A. Metz, A.K. Pawlow, E.D. Wischow, J.C. Poutsma, Proceedings of the 54th ASMS Conference on Mass Spectrometry and Allied Topics, Seattle, 2006, TP 145. [19] R.A.J. O Hair, J.H. Bowie, S. Gronert, Int. J. Mass Spectrom. 117 (1992) 23. [20] S. Naylor, A.F. Findeis, B.W. Gibson, D.H. Williams, J. Am. Chem. Soc. 108 (1986) [21] R.M. Caprioli, W.T. Moore, G. Petrie, K. Wilson, Int. J. Mass Spectrom. Ion Processes 86 (1988) 187. [22] G.M. Allmaier, C.E. Costello, Rapid Commun. Mass Spectrom. 2 (1988) 74. [23] R. Wang, L. Chen, R.J. Cotter, Anal. Chem. 62 (1990) [24] P. Pucci, C. Sepe, G. Marino, Biol. Mass Spectrom. 21 (1992) 22. [25] M.V. Kosevich, V.S. Shelkovsky, O.A. Boryak, V.V. Orlov, Rapid Commun. Mass Spectrom. 17 (2003) [26] A.M. Buko, L.R. Phillips, B.A. Fraser, Biomed. Mass Spectrom. 10 (1983) 387. [27] J.C. Schwartz, S.T. Quarmby, A.E. Schoen, Proceedings of the 46th ASMS Conference on Mass Spectrometry and Allied Topics, Orlando, 1998, p [28] H. Mirzaei, F. Regnier, Anal. Chem. 78 (2006) 4175.
Influence of hydrophobicity on positive- and negative-ion yields of peptides in electrospray ionization mass spectrometry
 Research Article Received: 3 April 2014 Revised: 29 July 2014 Accepted: 31 July 2014 Published online in Wiley Online Library Rapid Commun. Mass Spectrom. 2014, 28, 2222 2226 (wileyonlinelibrary.com) DOI:
Research Article Received: 3 April 2014 Revised: 29 July 2014 Accepted: 31 July 2014 Published online in Wiley Online Library Rapid Commun. Mass Spectrom. 2014, 28, 2222 2226 (wileyonlinelibrary.com) DOI:
RESEARCH ARTICLE. Graduate School of Nanobioscience, Yokohama City University, 22-2 Seto, Kanazawa-ku, Yokohama, , Japan
 B American Society for Mass Spectrometry, 211 J. Am. Soc. Mass Spectrom. (212) 23:18Y115 DOI: 1.17/s13361-11-271- RESEARCH ARTICLE Influence of Amino Acid Composition and Phosphorylation on the Ion Yields
B American Society for Mass Spectrometry, 211 J. Am. Soc. Mass Spectrom. (212) 23:18Y115 DOI: 1.17/s13361-11-271- RESEARCH ARTICLE Influence of Amino Acid Composition and Phosphorylation on the Ion Yields
Desorption/Ionization Efficiency of Common Amino Acids in. Surface-assisted Laser Desorption/ionization Mass Spectrometry
 SUPPORTING INFORMATION Desorption/Ionization Efficiency of Common Amino Acids in Surface-assisted Laser Desorption/ionization Mass Spectrometry (SALDI-MS) with Nanostructured Platinum Syuhei Nitta, Hideya
SUPPORTING INFORMATION Desorption/Ionization Efficiency of Common Amino Acids in Surface-assisted Laser Desorption/ionization Mass Spectrometry (SALDI-MS) with Nanostructured Platinum Syuhei Nitta, Hideya
Other Methods for Generating Ions 1. MALDI matrix assisted laser desorption ionization MS 2. Spray ionization techniques 3. Fast atom bombardment 4.
 Other Methods for Generating Ions 1. MALDI matrix assisted laser desorption ionization MS 2. Spray ionization techniques 3. Fast atom bombardment 4. Field Desorption 5. MS MS techniques Matrix assisted
Other Methods for Generating Ions 1. MALDI matrix assisted laser desorption ionization MS 2. Spray ionization techniques 3. Fast atom bombardment 4. Field Desorption 5. MS MS techniques Matrix assisted
Chemistry Instrumental Analysis Lecture 37. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 37 Most analytes separated by HPLC are thermally stable and non-volatile (liquids) (unlike in GC) so not ionized easily by EI or CI techniques. MS must be at
Chemistry 4631 Instrumental Analysis Lecture 37 Most analytes separated by HPLC are thermally stable and non-volatile (liquids) (unlike in GC) so not ionized easily by EI or CI techniques. MS must be at
Lecture 15: Introduction to mass spectrometry-i
 Lecture 15: Introduction to mass spectrometry-i Mass spectrometry (MS) is an analytical technique that measures the mass/charge ratio of charged particles in vacuum. Mass spectrometry can determine masse/charge
Lecture 15: Introduction to mass spectrometry-i Mass spectrometry (MS) is an analytical technique that measures the mass/charge ratio of charged particles in vacuum. Mass spectrometry can determine masse/charge
M M e M M H M M H. Ion Sources
 Ion Sources Overview of Various Ion Sources After introducing samples into a mass spectrometer, the next important step is the conversion of neutral molecules or compounds to gas phase ions. The ions could
Ion Sources Overview of Various Ion Sources After introducing samples into a mass spectrometer, the next important step is the conversion of neutral molecules or compounds to gas phase ions. The ions could
MASS SPECTROMETRY. Topics
 MASS SPECTROMETRY MALDI-TOF AND ESI-MS Topics Principle of Mass Spectrometry MALDI-TOF Determination of Mw of Proteins Structural Information by MS: Primary Sequence of a Protein 1 A. Principles Ionization:
MASS SPECTROMETRY MALDI-TOF AND ESI-MS Topics Principle of Mass Spectrometry MALDI-TOF Determination of Mw of Proteins Structural Information by MS: Primary Sequence of a Protein 1 A. Principles Ionization:
Mass Spectrometry. General Principles
 General Principles Mass Spectrometer: Converts molecules to ions Separates ions (usually positively charged) on the basis of their mass/charge (m/z) ratio Quantifies how many units of each ion are formed
General Principles Mass Spectrometer: Converts molecules to ions Separates ions (usually positively charged) on the basis of their mass/charge (m/z) ratio Quantifies how many units of each ion are formed
Fundamentals of Mass Spectrometry. Fundamentals of Mass Spectrometry. Learning Objective. Proteomics
 Mass spectrometry (MS) is the technique for protein identification and analysis by production of charged molecular species in vacuum, and their separation by magnetic and electric fields based on mass
Mass spectrometry (MS) is the technique for protein identification and analysis by production of charged molecular species in vacuum, and their separation by magnetic and electric fields based on mass
Modeling Mass Spectrometry-Based Protein Analysis
 Chapter 8 Jan Eriksson and David Fenyö Abstract The success of mass spectrometry based proteomics depends on efficient methods for data analysis. These methods require a detailed understanding of the information
Chapter 8 Jan Eriksson and David Fenyö Abstract The success of mass spectrometry based proteomics depends on efficient methods for data analysis. These methods require a detailed understanding of the information
Olumide Adebolu. Chromatographic Fidelity and Matrix /Analyte Solubility in Complex Polymer Systems using HPLC-MALD/I TOF MS
 Chromatographic Fidelity and Matrix /Analyte Solubility in Complex Polymer Systems using HPLC-MALD/I TOF MS Olumide Adebolu CHEM 395 March 1 st, 2007 Instructor : Prof J.Rusling Overview 2 Introduction
Chromatographic Fidelity and Matrix /Analyte Solubility in Complex Polymer Systems using HPLC-MALD/I TOF MS Olumide Adebolu CHEM 395 March 1 st, 2007 Instructor : Prof J.Rusling Overview 2 Introduction
Identification of proteins by enzyme digestion, mass
 Method for Screening Peptide Fragment Ion Mass Spectra Prior to Database Searching Roger E. Moore, Mary K. Young, and Terry D. Lee Beckman Research Institute of the City of Hope, Duarte, California, USA
Method for Screening Peptide Fragment Ion Mass Spectra Prior to Database Searching Roger E. Moore, Mary K. Young, and Terry D. Lee Beckman Research Institute of the City of Hope, Duarte, California, USA
20.2 Ion Sources. ions electrospray uses evaporation of a charged liquid stream to transfer high molecular mass compounds into the gas phase as MH n
 20.2 Ion Sources electron ionization produces an M + ion and extensive fragmentation chemical ionization produces an M +, MH +, M +, or M - ion with minimal fragmentation MALDI uses laser ablation to transfer
20.2 Ion Sources electron ionization produces an M + ion and extensive fragmentation chemical ionization produces an M +, MH +, M +, or M - ion with minimal fragmentation MALDI uses laser ablation to transfer
Molecular Mass Spectrometry
 Molecular Mass Spectrometry Mass Spectrometry: capable of providing information about (1) Elemental composition of samples of matter: atomic mass (2) Structures of inorganic, organic, and biological molecules
Molecular Mass Spectrometry Mass Spectrometry: capable of providing information about (1) Elemental composition of samples of matter: atomic mass (2) Structures of inorganic, organic, and biological molecules
Ionization Methods in Mass Spectrometry at the SCS Mass Spectrometry Laboratory
 Ionization Methods in Mass Spectrometry at the SCS Mass Spectrometry Laboratory Steven L. Mullen, Ph.D. Associate Director SCS Mass Spectrometry Laboratory Contact Information 31 oyes Laboratory (8:00-5:00
Ionization Methods in Mass Spectrometry at the SCS Mass Spectrometry Laboratory Steven L. Mullen, Ph.D. Associate Director SCS Mass Spectrometry Laboratory Contact Information 31 oyes Laboratory (8:00-5:00
Chemistry Instrumental Analysis Lecture 34. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Chemistry 4631 Instrumental Analysis Lecture 34 From molecular to elemental analysis there are three major techniques used for elemental analysis: Optical spectrometry Mass spectrometry X-ray spectrometry
Mass Spectrometry. Electron Ionization and Chemical Ionization
 Mass Spectrometry Electron Ionization and Chemical Ionization Mass Spectrometer All Instruments Have: 1. Sample Inlet 2. Ion Source 3. Mass Analyzer 4. Detector 5. Data System http://www.asms.org Ionization
Mass Spectrometry Electron Ionization and Chemical Ionization Mass Spectrometer All Instruments Have: 1. Sample Inlet 2. Ion Source 3. Mass Analyzer 4. Detector 5. Data System http://www.asms.org Ionization
Identification of Human Hemoglobin Protein Variants Using Electrospray Ionization-Electron Transfer Dissociation Mass Spectrometry
 Identification of Human Hemoglobin Protein Variants Using Electrospray Ionization-Electron Transfer Dissociation Mass Spectrometry Jonathan Williams Waters Corporation, Milford, MA, USA A P P L I C AT
Identification of Human Hemoglobin Protein Variants Using Electrospray Ionization-Electron Transfer Dissociation Mass Spectrometry Jonathan Williams Waters Corporation, Milford, MA, USA A P P L I C AT
Two fascinating methods for analyzing the primary
 N C Bond Cleavage of the Peptide Backbone via Hydrogen Abstraction Mitsuo Takayama Graduate School of Sciences, Yokohama City University, Yokohama, 236-0027, Japan The specific cleavage of N C bonds on
N C Bond Cleavage of the Peptide Backbone via Hydrogen Abstraction Mitsuo Takayama Graduate School of Sciences, Yokohama City University, Yokohama, 236-0027, Japan The specific cleavage of N C bonds on
A Plausible Model Correlates Prebiotic Peptide Synthesis with. Primordial Genetic Code
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 A Plausible Model Correlates Prebiotic Peptide Synthesis with Primordial Genetic Code Jianxi Ying,
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 A Plausible Model Correlates Prebiotic Peptide Synthesis with Primordial Genetic Code Jianxi Ying,
TANDEM MASS SPECTROSCOPY
 TANDEM MASS SPECTROSCOPY 1 MASS SPECTROMETER TYPES OF MASS SPECTROMETER PRINCIPLE TANDEM MASS SPECTROMETER INSTRUMENTATION QUADRAPOLE MASS ANALYZER TRIPLE QUADRAPOLE MASS ANALYZER TIME OF FLIGHT MASS ANALYSER
TANDEM MASS SPECTROSCOPY 1 MASS SPECTROMETER TYPES OF MASS SPECTROMETER PRINCIPLE TANDEM MASS SPECTROMETER INSTRUMENTATION QUADRAPOLE MASS ANALYZER TRIPLE QUADRAPOLE MASS ANALYZER TIME OF FLIGHT MASS ANALYSER
Amino Acids and Peptides
 Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Amino Acids Amino Acids and Peptides Amino acid a compound that contains both an amino group and a carboxyl group α-amino acid an amino acid in which the amino group is on the carbon adjacent to the carboxyl
Chapter 4: Amino Acids
 Chapter 4: Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. lipid polysaccharide enzyme 1940s 1980s. Lipids membrane 1960s. Polysaccharide Are energy metabolites and many of
Chapter 4: Amino Acids All peptides and polypeptides are polymers of alpha-amino acids. lipid polysaccharide enzyme 1940s 1980s. Lipids membrane 1960s. Polysaccharide Are energy metabolites and many of
Auxiliary Techniques Soft ionization methods
 Auxiliary Techniques The limitations of the structural information in the normal mass spectrum can be partly offset by special mass-spectral techniques. Although a complete description of these is beyond
Auxiliary Techniques The limitations of the structural information in the normal mass spectrum can be partly offset by special mass-spectral techniques. Although a complete description of these is beyond
Mass spectrometry gas phase transfer and instrumentation
 Objectives of the Lecture spectrometry gas phase transfer and instrumentation Matt Renfrow January 15, 2014 1. Make ions 2. Separate/Analyze 3. Detect ions 4. What is mass resolution and mass accuracy?
Objectives of the Lecture spectrometry gas phase transfer and instrumentation Matt Renfrow January 15, 2014 1. Make ions 2. Separate/Analyze 3. Detect ions 4. What is mass resolution and mass accuracy?
Rapid and Sensitive Fluorescent Peptide Quantification Using LavaPep
 Rapid and Sensitive Fluorescent Peptide Quantification Using LavaPep Purpose To develop a fast, sensitive and robust fluorescent-based assay, to quantify peptides that is compatible with downstream proteomics
Rapid and Sensitive Fluorescent Peptide Quantification Using LavaPep Purpose To develop a fast, sensitive and robust fluorescent-based assay, to quantify peptides that is compatible with downstream proteomics
LC-MS Based Metabolomics
 LC-MS Based Metabolomics Analysing the METABOLOME 1. Metabolite Extraction 2. Metabolite detection (with or without separation) 3. Data analysis Metabolite Detection GC-MS: Naturally volatile or made volatile
LC-MS Based Metabolomics Analysing the METABOLOME 1. Metabolite Extraction 2. Metabolite detection (with or without separation) 3. Data analysis Metabolite Detection GC-MS: Naturally volatile or made volatile
Mass spectrometry and elemental analysis
 Mass spectrometry and elemental analysis A schematic representation of a single-focusing mass spectrometer with an electron-impact (EI) ionization source. M: + e _ M +. + 2e _ Ionization and fragmentation
Mass spectrometry and elemental analysis A schematic representation of a single-focusing mass spectrometer with an electron-impact (EI) ionization source. M: + e _ M +. + 2e _ Ionization and fragmentation
Ionization Techniques Part IV
 Ionization Techniques Part IV CU- Boulder CHEM 5181 Mass Spectrometry & Chromatography Presented by Prof. Jose L. Jimenez High Vacuum MS Interpretation Lectures Sample Inlet Ion Source Mass Analyzer Detector
Ionization Techniques Part IV CU- Boulder CHEM 5181 Mass Spectrometry & Chromatography Presented by Prof. Jose L. Jimenez High Vacuum MS Interpretation Lectures Sample Inlet Ion Source Mass Analyzer Detector
Ultrasonics Sonochemistry
 Ultrasonics Sonochemistry xxx (2008) xxx xxx Contents lists available at ScienceDirect Ultrasonics Sonochemistry journal homepage: www.elsevier.com/locate/ultsonch Sonolytic hydrolysis of peptides in aqueous
Ultrasonics Sonochemistry xxx (2008) xxx xxx Contents lists available at ScienceDirect Ultrasonics Sonochemistry journal homepage: www.elsevier.com/locate/ultsonch Sonolytic hydrolysis of peptides in aqueous
BENG 183 Trey Ideker. Protein Sequencing
 BENG 183 Trey Ideker Protein Sequencing The following slides borrowed from Hong Li s Biochemistry Course: www.sb.fsu.edu/~hongli/4053notes Introduction to Proteins Proteins are of vital importance to biological
BENG 183 Trey Ideker Protein Sequencing The following slides borrowed from Hong Li s Biochemistry Course: www.sb.fsu.edu/~hongli/4053notes Introduction to Proteins Proteins are of vital importance to biological
Properties of amino acids in proteins
 Properties of amino acids in proteins one of the primary roles of DNA (but not the only one!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids repeated
Properties of amino acids in proteins one of the primary roles of DNA (but not the only one!) is to code for proteins A typical bacterium builds thousands types of proteins, all from ~20 amino acids repeated
LECTURE-13. Peptide Mass Fingerprinting HANDOUT. Mass spectrometry is an indispensable tool for qualitative and quantitative analysis of
 LECTURE-13 Peptide Mass Fingerprinting HANDOUT PREAMBLE Mass spectrometry is an indispensable tool for qualitative and quantitative analysis of proteins, drugs and many biological moieties to elucidate
LECTURE-13 Peptide Mass Fingerprinting HANDOUT PREAMBLE Mass spectrometry is an indispensable tool for qualitative and quantitative analysis of proteins, drugs and many biological moieties to elucidate
Fast Atom Bombardment (FAB)-MS
 Fast Atom Bombardment (FAB)-MS FAB-MS was developed by Barber and coworkers in the early 1980s and soon became a very powerful technique for desorption ionization of thermally labile molecules. In FAB
Fast Atom Bombardment (FAB)-MS FAB-MS was developed by Barber and coworkers in the early 1980s and soon became a very powerful technique for desorption ionization of thermally labile molecules. In FAB
Chemistry 311: Topic 3 - Mass Spectrometry
 Mass Spectroscopy: A technique used to measure the mass-to-charge ratio of molecules and atoms. Often characteristic ions produced by an induced unimolecular dissociation of a molecule are measured. These
Mass Spectroscopy: A technique used to measure the mass-to-charge ratio of molecules and atoms. Often characteristic ions produced by an induced unimolecular dissociation of a molecule are measured. These
Exam I Answer Key: Summer 2006, Semester C
 1. Which of the following tripeptides would migrate most rapidly towards the negative electrode if electrophoresis is carried out at ph 3.0? a. gly-gly-gly b. glu-glu-asp c. lys-glu-lys d. val-asn-lys
1. Which of the following tripeptides would migrate most rapidly towards the negative electrode if electrophoresis is carried out at ph 3.0? a. gly-gly-gly b. glu-glu-asp c. lys-glu-lys d. val-asn-lys
Lecture 15: Realities of Genome Assembly Protein Sequencing
 Lecture 15: Realities of Genome Assembly Protein Sequencing Study Chapter 8.10-8.15 1 Euler s Theorems A graph is balanced if for every vertex the number of incoming edges equals to the number of outgoing
Lecture 15: Realities of Genome Assembly Protein Sequencing Study Chapter 8.10-8.15 1 Euler s Theorems A graph is balanced if for every vertex the number of incoming edges equals to the number of outgoing
EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I Dr. Glen B Legge
 EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I 2009 Dr. Glen B Legge This is a Scantron exam. All answers should be transferred to the Scantron sheet using a #2 pencil. Write and bubble
EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I 2009 Dr. Glen B Legge This is a Scantron exam. All answers should be transferred to the Scantron sheet using a #2 pencil. Write and bubble
Diastereomeric resolution directed towards chirality. determination focussing on gas-phase energetics of coordinated. sodium dissociation
 Supplementary Information Diastereomeric resolution directed towards chirality determination focussing on gas-phase energetics of coordinated sodium dissociation Authors: samu Kanie*, Yuki Shioiri, Koji
Supplementary Information Diastereomeric resolution directed towards chirality determination focussing on gas-phase energetics of coordinated sodium dissociation Authors: samu Kanie*, Yuki Shioiri, Koji
Chapter 5. Complexation of Tholins by 18-crown-6:
 5-1 Chapter 5. Complexation of Tholins by 18-crown-6: Identification of Primary Amines 5.1. Introduction Electrospray ionization (ESI) is an excellent technique for the ionization of complex mixtures,
5-1 Chapter 5. Complexation of Tholins by 18-crown-6: Identification of Primary Amines 5.1. Introduction Electrospray ionization (ESI) is an excellent technique for the ionization of complex mixtures,
1) In what pressure range are mass spectrometers normally operated?
 Exercises Ionization 1) In what pressure range are mass spectrometers normally operated? Mass spectrometers are usually operated in the high vacuum regime to ensure mean free paths significantly longer
Exercises Ionization 1) In what pressure range are mass spectrometers normally operated? Mass spectrometers are usually operated in the high vacuum regime to ensure mean free paths significantly longer
Separation of Large and Small Peptides by Supercritical Fluid Chromatography and Detection by Mass Spectrometry
 Separation of Large and Small Peptides by Supercritical Fluid Chromatography and Detection by Mass Spectrometry Application Note Biologics and Biosimilars Author Edgar Naegele Agilent Technologies, Inc.
Separation of Large and Small Peptides by Supercritical Fluid Chromatography and Detection by Mass Spectrometry Application Note Biologics and Biosimilars Author Edgar Naegele Agilent Technologies, Inc.
SUPPORTING INFORMATION Antimicrobial activity of chlorinated amino acids and peptides
 SUPPORTING INFORMATION Antimicrobial activity of chlorinated amino acids and peptides Melanie S. A. Coker*, Wan-Ping Hu, Senti T Senthilmohan and Anthony J. Kettle Free Radical Research Group, University
SUPPORTING INFORMATION Antimicrobial activity of chlorinated amino acids and peptides Melanie S. A. Coker*, Wan-Ping Hu, Senti T Senthilmohan and Anthony J. Kettle Free Radical Research Group, University
Study of Non-Covalent Complexes by ESI-MS. By Quinn Tays
 Study of Non-Covalent Complexes by ESI-MS By Quinn Tays History Overview Background Electrospray Ionization How it is used in study of noncovalent interactions Uses of the Technique Types of molecules
Study of Non-Covalent Complexes by ESI-MS By Quinn Tays History Overview Background Electrospray Ionization How it is used in study of noncovalent interactions Uses of the Technique Types of molecules
Qualitative Organic Analysis CH 351 Mass Spectrometry
 Qualitative Organic Analysis CH 351 Mass Spectrometry Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA General Aspects Theoretical basis of mass spectrometry Basic Instrumentation
Qualitative Organic Analysis CH 351 Mass Spectrometry Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA General Aspects Theoretical basis of mass spectrometry Basic Instrumentation
Conformational Analysis
 Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
Secondary Structure. Bioch/BIMS 503 Lecture 2. Structure and Function of Proteins. Further Reading. Φ, Ψ angles alone determine protein structure
 Bioch/BIMS 503 Lecture 2 Structure and Function of Proteins August 28, 2008 Robert Nakamoto rkn3c@virginia.edu 2-0279 Secondary Structure Φ Ψ angles determine protein structure Φ Ψ angles are restricted
Bioch/BIMS 503 Lecture 2 Structure and Function of Proteins August 28, 2008 Robert Nakamoto rkn3c@virginia.edu 2-0279 Secondary Structure Φ Ψ angles determine protein structure Φ Ψ angles are restricted
Effective desalting and concentration of in-gel digest samples with Vivapure C18 Micro spin columns prior to MALDI-TOF analysis.
 Introduction The identification of proteins plays an important role in today s pharmaceutical and proteomics research. Commonly used methods for separating proteins from complex samples are 1D or 2D gels.
Introduction The identification of proteins plays an important role in today s pharmaceutical and proteomics research. Commonly used methods for separating proteins from complex samples are 1D or 2D gels.
CHEM J-9 June 2014
 CEM1611 2014-J-9 June 2014 Alanine (ala) and lysine (lys) are two amino acids with the structures given below as Fischer projections. The pk a values of the conjugate acid forms of the different functional
CEM1611 2014-J-9 June 2014 Alanine (ala) and lysine (lys) are two amino acids with the structures given below as Fischer projections. The pk a values of the conjugate acid forms of the different functional
Chapter 14. Molar Mass Distribution.
 Chapter 14. Molar Mass Distribution. Difficulty with M n and M w, etc. osome polymers are hard to describe from just M n, M w, etc. o Examples: Bimodal, multimodal, nonuniform, broad, etc. MWDs. oin early
Chapter 14. Molar Mass Distribution. Difficulty with M n and M w, etc. osome polymers are hard to describe from just M n, M w, etc. o Examples: Bimodal, multimodal, nonuniform, broad, etc. MWDs. oin early
Peptides And Proteins
 Kevin Burgess, May 3, 2017 1 Peptides And Proteins from chapter(s) in the recommended text A. Introduction B. omenclature And Conventions by amide bonds. on the left, right. 2 -terminal C-terminal triglycine
Kevin Burgess, May 3, 2017 1 Peptides And Proteins from chapter(s) in the recommended text A. Introduction B. omenclature And Conventions by amide bonds. on the left, right. 2 -terminal C-terminal triglycine
Molecular Mass Spectrometry
 Molecular Mass Spectrometry Mass Spectrometry: capable of providing information about (1) Elemental composition of samples of matter: atomic mass (2) Structures of inorganic, organic, and biological molecules
Molecular Mass Spectrometry Mass Spectrometry: capable of providing information about (1) Elemental composition of samples of matter: atomic mass (2) Structures of inorganic, organic, and biological molecules
Supporting Information
 Supporting Information Highly Efficient Ionization of Phosphopeptides at Low ph by Desorption Electrospray Ionization Mass Spectrometry Ning Pan, a, b Pengyuan Liu, b Weidong Cui, c Bo Tang, a Jingmin
Supporting Information Highly Efficient Ionization of Phosphopeptides at Low ph by Desorption Electrospray Ionization Mass Spectrometry Ning Pan, a, b Pengyuan Liu, b Weidong Cui, c Bo Tang, a Jingmin
The emergence of new soft ionization techniques
 High-Frequency AC Electrospray Ionization Source for Mass Spectrometry of Biomolecules Nishant Chetwani, a Catherine A. Cassou, b David B. Go, c and Hsueh-Chia Chang a a Department of Chemical and Biomolecular
High-Frequency AC Electrospray Ionization Source for Mass Spectrometry of Biomolecules Nishant Chetwani, a Catherine A. Cassou, b David B. Go, c and Hsueh-Chia Chang a a Department of Chemical and Biomolecular
Press Release: The Nobel Prize in Chemistry October 2002 The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in
 Press Release: The Nobel Prize in Chemistry 2002 9 October 2002 The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry for 2002 for the development of methods for identification
Press Release: The Nobel Prize in Chemistry 2002 9 October 2002 The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry for 2002 for the development of methods for identification
MS Goals and Applications. MS Goals and Applications
 MS Goals and Applications 3 Several variations on a theme, three common steps Form gas-phase ions choice of ionization method depends on sample identity and information required Separate ions on basis
MS Goals and Applications 3 Several variations on a theme, three common steps Form gas-phase ions choice of ionization method depends on sample identity and information required Separate ions on basis
Protein Fragment Search Program ver Overview: Contents:
 Protein Fragment Search Program ver 1.1.1 Developed by: BioPhysics Laboratory, Faculty of Life and Environmental Science, Shimane University 1060 Nishikawatsu-cho, Matsue-shi, Shimane, 690-8504, Japan
Protein Fragment Search Program ver 1.1.1 Developed by: BioPhysics Laboratory, Faculty of Life and Environmental Science, Shimane University 1060 Nishikawatsu-cho, Matsue-shi, Shimane, 690-8504, Japan
Laser desorption/ionization on the layer of graphene nanoparticles coupled with mass spectrometry for characterization of polymer
 Laser desorption/ionization on the layer of graphene nanoparticles coupled with mass spectrometry for characterization of polymer Minghua Lu, a Yongquan Lai, a Guonan Chen, b and Zongwei Cai* a a Department
Laser desorption/ionization on the layer of graphene nanoparticles coupled with mass spectrometry for characterization of polymer Minghua Lu, a Yongquan Lai, a Guonan Chen, b and Zongwei Cai* a a Department
Content : Properties of amino acids.. Separation and Analysis of Amino Acids
 قسم الكيمياء الحيوية.دولت على سالمه د. استاذ الكيمياء الحيوية ٢٠١٥-٢٠١٤ المحاضرة الثانية 1 Content : Properties of amino acids.. Separation and Analysis of Amino Acids 2 3 Physical Properties of Amino
قسم الكيمياء الحيوية.دولت على سالمه د. استاذ الكيمياء الحيوية ٢٠١٥-٢٠١٤ المحاضرة الثانية 1 Content : Properties of amino acids.. Separation and Analysis of Amino Acids 2 3 Physical Properties of Amino
CHMI 2227 EL. Biochemistry I. Test January Prof : Eric R. Gauthier, Ph.D.
 CHMI 2227 EL Biochemistry I Test 1 26 January 2007 Prof : Eric R. Gauthier, Ph.D. Guidelines: 1) Duration: 55 min 2) 14 questions, on 7 pages. For 70 marks (5 marks per question). Worth 15 % of the final
CHMI 2227 EL Biochemistry I Test 1 26 January 2007 Prof : Eric R. Gauthier, Ph.D. Guidelines: 1) Duration: 55 min 2) 14 questions, on 7 pages. For 70 marks (5 marks per question). Worth 15 % of the final
Proteins: Characteristics and Properties of Amino Acids
 SBI4U:Biochemistry Macromolecules Eachaminoacidhasatleastoneamineandoneacidfunctionalgroupasthe nameimplies.thedifferentpropertiesresultfromvariationsinthestructuresof differentrgroups.thergroupisoftenreferredtoastheaminoacidsidechain.
SBI4U:Biochemistry Macromolecules Eachaminoacidhasatleastoneamineandoneacidfunctionalgroupasthe nameimplies.thedifferentpropertiesresultfromvariationsinthestructuresof differentrgroups.thergroupisoftenreferredtoastheaminoacidsidechain.
Solutions In each case, the chirality center has the R configuration
 CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
Viewing and Analyzing Proteins, Ligands and their Complexes 2
 2 Viewing and Analyzing Proteins, Ligands and their Complexes 2 Overview Viewing the accessible surface Analyzing the properties of proteins containing thousands of atoms is best accomplished by representing
2 Viewing and Analyzing Proteins, Ligands and their Complexes 2 Overview Viewing the accessible surface Analyzing the properties of proteins containing thousands of atoms is best accomplished by representing
Chapter 1. Introduction
 1-1 Chapter 1. Introduction 1.1. Background Non covalent interactions are important for the structures and reactivity of biological molecules in the gas phase as well as in the solution phase. 1 It is
1-1 Chapter 1. Introduction 1.1. Background Non covalent interactions are important for the structures and reactivity of biological molecules in the gas phase as well as in the solution phase. 1 It is
Collision Cross Section: Ideal elastic hard sphere collision:
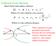 Collision Cross Section: Ideal elastic hard sphere collision: ( r r 1 ) Where is the collision cross-section r 1 r ) ( 1 Where is the collision distance r 1 r These equations negate potential interactions
Collision Cross Section: Ideal elastic hard sphere collision: ( r r 1 ) Where is the collision cross-section r 1 r ) ( 1 Where is the collision distance r 1 r These equations negate potential interactions
Electron Transfer Dissociation of N-linked Glycopeptides from a Recombinant mab Using SYNAPT G2-S HDMS
 Electron Transfer Dissociation of N-linked Glycopeptides from a Recombinant mab Using SYNAPT G2-S HDMS Jonathan P. Williams, Jeffery M. Brown, Stephane Houel, Ying Qing Yu, and Weibin Chen Waters Corporation,
Electron Transfer Dissociation of N-linked Glycopeptides from a Recombinant mab Using SYNAPT G2-S HDMS Jonathan P. Williams, Jeffery M. Brown, Stephane Houel, Ying Qing Yu, and Weibin Chen Waters Corporation,
Mass Spectrometry Course
 Mass Spectrometry Course Árpád Somogyi Mass Spectrometry Laboratory, Department of Chemistry and Biochemistry University of Arizona, Tucson, AZ Eötvös University, Budapest April 11-20, 2012 1 2 UA Chemistry
Mass Spectrometry Course Árpád Somogyi Mass Spectrometry Laboratory, Department of Chemistry and Biochemistry University of Arizona, Tucson, AZ Eötvös University, Budapest April 11-20, 2012 1 2 UA Chemistry
Analysis of Polar Metabolites using Mass Spectrometry
 Analysis of Polar Metabolites using Mass Spectrometry TransMed Course: Basics in Clinical Proteomics and Metabolomics. Oct 10-19, 2012 dd.mm.yyyy Vidya Velagapudi, Ph.D, Adjunct Professor Head of the Metabolomics
Analysis of Polar Metabolites using Mass Spectrometry TransMed Course: Basics in Clinical Proteomics and Metabolomics. Oct 10-19, 2012 dd.mm.yyyy Vidya Velagapudi, Ph.D, Adjunct Professor Head of the Metabolomics
Supporting information
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 Supporting information Influence
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 Supporting information Influence
A. Two of the common amino acids are analyzed. Amino acid X and amino acid Y both have an isoionic point in the range of
 Questions with Answers- Amino Acids & Peptides A. Two of the common amino acids are analyzed. Amino acid X and amino acid Y both have an isoionic point in the range of 5.0-6.5 (Questions 1-4) 1. Which
Questions with Answers- Amino Acids & Peptides A. Two of the common amino acids are analyzed. Amino acid X and amino acid Y both have an isoionic point in the range of 5.0-6.5 (Questions 1-4) 1. Which
What makes a good graphene-binding peptide? Adsorption of amino acids and peptides at aqueous graphene interfaces: Electronic Supplementary
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 21 What makes a good graphene-binding peptide? Adsorption of amino acids and
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 21 What makes a good graphene-binding peptide? Adsorption of amino acids and
Supplementary Figure 3 a. Structural comparison between the two determined structures for the IL 23:MA12 complex. The overall RMSD between the two
 Supplementary Figure 1. Biopanningg and clone enrichment of Alphabody binders against human IL 23. Positive clones in i phage ELISA with optical density (OD) 3 times higher than background are shown for
Supplementary Figure 1. Biopanningg and clone enrichment of Alphabody binders against human IL 23. Positive clones in i phage ELISA with optical density (OD) 3 times higher than background are shown for
Section Week 3. Junaid Malek, M.D.
 Section Week 3 Junaid Malek, M.D. Biological Polymers DA 4 monomers (building blocks), limited structure (double-helix) RA 4 monomers, greater flexibility, multiple structures Proteins 20 Amino Acids,
Section Week 3 Junaid Malek, M.D. Biological Polymers DA 4 monomers (building blocks), limited structure (double-helix) RA 4 monomers, greater flexibility, multiple structures Proteins 20 Amino Acids,
Determination of the Amino Acid Sequence of an Unknown Dipeptide
 Wilson 1 Determination of the Amino Acid Sequence of an Unknown Dipeptide Martin C. Wilson Department of Biology, University of North Carolina - Asheville, Asheville, North Carolina 28804, United States
Wilson 1 Determination of the Amino Acid Sequence of an Unknown Dipeptide Martin C. Wilson Department of Biology, University of North Carolina - Asheville, Asheville, North Carolina 28804, United States
Operating Instructions
 Sequazyme Peptide Mass Standards Kit Operating Instructions 1 Product Description The Sequazyme Peptide Mass Standards Kit includes reagents needed to test instrument function, optimize instrument parameters,
Sequazyme Peptide Mass Standards Kit Operating Instructions 1 Product Description The Sequazyme Peptide Mass Standards Kit includes reagents needed to test instrument function, optimize instrument parameters,
Selecting an LC/MS Interface Becky Wittrig, Ph.D.
 Selecting an LC/MS Interface Becky Wittrig, Ph.D. RESTEK CORPORATION LC/MS Interfaces I. Background of LC/MS I. Historical Perspective II. Reasons for use II. Interfaces I. Transport devices II. Particle
Selecting an LC/MS Interface Becky Wittrig, Ph.D. RESTEK CORPORATION LC/MS Interfaces I. Background of LC/MS I. Historical Perspective II. Reasons for use II. Interfaces I. Transport devices II. Particle
7.012 Problem Set 1 Solutions
 ame TA Section 7.012 Problem Set 1 Solutions Your answers to this problem set must be inserted into the large wooden box on wheels outside 68120 by 4:30 PM, Thursday, September 15. Problem sets will not
ame TA Section 7.012 Problem Set 1 Solutions Your answers to this problem set must be inserted into the large wooden box on wheels outside 68120 by 4:30 PM, Thursday, September 15. Problem sets will not
Mass Spectrometry. Hyphenated Techniques GC-MS LC-MS and MS-MS
 Mass Spectrometry Hyphenated Techniques GC-MS LC-MS and MS-MS Reasons for Using Chromatography with MS Mixture analysis by MS alone is difficult Fragmentation from ionization (EI or CI) Fragments from
Mass Spectrometry Hyphenated Techniques GC-MS LC-MS and MS-MS Reasons for Using Chromatography with MS Mixture analysis by MS alone is difficult Fragmentation from ionization (EI or CI) Fragments from
The Power of LC MALDI: Identification of Proteins by LC MALDI MS/MS Using the Applied Biosystems 4700 Proteomics Analyzer with TOF/TOF Optics
 APPLICATION NOTE TOF MS The Power of LC MALDI: Identification of Proteins by LC MALDI MS/MS Using the Applied Biosystems 4700 Proteomics Analyzer with TOF/TOF Optics Purpose The Applied Biosystems 4700
APPLICATION NOTE TOF MS The Power of LC MALDI: Identification of Proteins by LC MALDI MS/MS Using the Applied Biosystems 4700 Proteomics Analyzer with TOF/TOF Optics Purpose The Applied Biosystems 4700
Amino Acid Side Chain Induced Selectivity in the Hydrolysis of Peptides Catalyzed by a Zr(IV)-Substituted Wells-Dawson Type Polyoxometalate
 Amino Acid Side Chain Induced Selectivity in the Hydrolysis of Peptides Catalyzed by a Zr(IV)-Substituted Wells-Dawson Type Polyoxometalate Stef Vanhaecht, Gregory Absillis, Tatjana N. Parac-Vogt* Department
Amino Acid Side Chain Induced Selectivity in the Hydrolysis of Peptides Catalyzed by a Zr(IV)-Substituted Wells-Dawson Type Polyoxometalate Stef Vanhaecht, Gregory Absillis, Tatjana N. Parac-Vogt* Department
7.012 Problem Set 1. i) What are two main differences between prokaryotic cells and eukaryotic cells?
 ame 7.01 Problem Set 1 Section Question 1 a) What are the four major types of biological molecules discussed in lecture? Give one important function of each type of biological molecule in the cell? b)
ame 7.01 Problem Set 1 Section Question 1 a) What are the four major types of biological molecules discussed in lecture? Give one important function of each type of biological molecule in the cell? b)
Instrumental Analysis. Mass Spectrometry. Lecturer:! Somsak Sirichai
 303351 Instrumental Analysis Mass Spectrometry Lecturer:! Somsak Sirichai Mass Spectrometry What is Mass spectrometry (MS)? An analytic method that employs ionization and mass analysis of compounds in
303351 Instrumental Analysis Mass Spectrometry Lecturer:! Somsak Sirichai Mass Spectrometry What is Mass spectrometry (MS)? An analytic method that employs ionization and mass analysis of compounds in
Mass Spectrometry for Chemists and Biochemists
 Erasmus Intensive Program SYNAPS Univ. of Crete - Summer 2007 Mass Spectrometry for Chemists and Biochemists Spiros A. Pergantis Assistant Professor of Analytical Chemistry Department of Chemistry University
Erasmus Intensive Program SYNAPS Univ. of Crete - Summer 2007 Mass Spectrometry for Chemists and Biochemists Spiros A. Pergantis Assistant Professor of Analytical Chemistry Department of Chemistry University
Peptide Syntheses. Illustrative Protection: BOC/ t Bu. A. Introduction. do not acid
 Kevin Burgess, May 3, 2017 1 Peptide yntheses from chapter(s) in the recommended text A. Introduction do not acid -Met-e- -Met-Met- -e-e- -e-met- dipeptide dipeptide dipeptide dipeptide diketopiperazine
Kevin Burgess, May 3, 2017 1 Peptide yntheses from chapter(s) in the recommended text A. Introduction do not acid -Met-e- -Met-Met- -e-e- -e-met- dipeptide dipeptide dipeptide dipeptide diketopiperazine
Postsynthetic modification of unprotected peptides via S-tritylation reaction
 Supporting Information Postsynthetic modification of unprotected peptides via S-tritylation reaction Masayoshi Mochizuki, Hajime Hibino and Yuji Nishiuchi *,, SAITO Research Center, Peptide Institute,
Supporting Information Postsynthetic modification of unprotected peptides via S-tritylation reaction Masayoshi Mochizuki, Hajime Hibino and Yuji Nishiuchi *,, SAITO Research Center, Peptide Institute,
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor
 LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
Unraveling the degradation of artificial amide bonds in Nylon oligomer hydrolase: From induced-fit to acylation processes
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 Supporting Information for Unraveling the degradation of artificial amide bonds
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2015 Supporting Information for Unraveling the degradation of artificial amide bonds
Supporting Information. Experimental details
 Electronic Supporting Information Experimental details Chemicals and reagents Pseudoboehmite (78.4 wt% Al 2 O 3 ), phosphoric acid (85 wt%), triethylamine (TEA, 99%), tetrabutyl titanate (IV) (99%) and
Electronic Supporting Information Experimental details Chemicals and reagents Pseudoboehmite (78.4 wt% Al 2 O 3 ), phosphoric acid (85 wt%), triethylamine (TEA, 99%), tetrabutyl titanate (IV) (99%) and
CHROMATOGRAPHY AND MASS SPECTROMETER
 22 CHROMATOGRAPHY AND MASS SPECTROMETER 22.1 INTRODUCTION We know that the biochemistry or biological chemistry deals with the study of molecules present in organisms. These molecules are called as biomolecules
22 CHROMATOGRAPHY AND MASS SPECTROMETER 22.1 INTRODUCTION We know that the biochemistry or biological chemistry deals with the study of molecules present in organisms. These molecules are called as biomolecules
Biological Mass Spectrometry
 Biochemistry 412 Biological Mass Spectrometry February 13 th, 2007 Proteomics The study of the complete complement of proteins found in an organism Degrees of Freedom for Protein Variability Covalent Modifications
Biochemistry 412 Biological Mass Spectrometry February 13 th, 2007 Proteomics The study of the complete complement of proteins found in an organism Degrees of Freedom for Protein Variability Covalent Modifications
Electrospray Ion Trap Mass Spectrometry. Introduction
 Electrospray Ion Source Electrospray Ion Trap Mass Spectrometry Introduction The key to using MS for solutions is the ability to transfer your analytes into the vacuum of the mass spectrometer as ionic
Electrospray Ion Source Electrospray Ion Trap Mass Spectrometry Introduction The key to using MS for solutions is the ability to transfer your analytes into the vacuum of the mass spectrometer as ionic
(Refer Slide Time 00:09) (Refer Slide Time 00:13)
 (Refer Slide Time 00:09) Mass Spectrometry Based Proteomics Professor Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology, Bombay Mod 02 Lecture Number 09 (Refer
(Refer Slide Time 00:09) Mass Spectrometry Based Proteomics Professor Sanjeeva Srivastava Department of Biosciences and Bioengineering Indian Institute of Technology, Bombay Mod 02 Lecture Number 09 (Refer
BIOINF 4399B Computational Proteomics and Metabolomics
 BIOINF 4399B Computational Proteomics and Metabolomics Sven Nahnsen WS 13/14 3. Chromatography and mass spectrometry Overview Recall last lecture Basics of liquid chromatography Algorithms to predict and
BIOINF 4399B Computational Proteomics and Metabolomics Sven Nahnsen WS 13/14 3. Chromatography and mass spectrometry Overview Recall last lecture Basics of liquid chromatography Algorithms to predict and
Mass Spectrometry. A truly interdisciplinary and versatile analytical method
 Mass Spectrometry A truly interdisciplinary and versatile analytical method MS is used for the characterization of molecules ranging from small inorganic and organic molecules to polymers and proteins.
Mass Spectrometry A truly interdisciplinary and versatile analytical method MS is used for the characterization of molecules ranging from small inorganic and organic molecules to polymers and proteins.
Biochemistry Quiz Review 1I. 1. Of the 20 standard amino acids, only is not optically active. The reason is that its side chain.
 Biochemistry Quiz Review 1I A general note: Short answer questions are just that, short. Writing a paragraph filled with every term you can remember from class won t improve your answer just answer clearly,
Biochemistry Quiz Review 1I A general note: Short answer questions are just that, short. Writing a paragraph filled with every term you can remember from class won t improve your answer just answer clearly,
Analysis of chemically modified proteins by FT-ICR MS
 Analysis of chemically modified proteins by FT-ICR MS PETR NOVAK EU FT-ICR MS End User School 1 UEF Chemistry August 19-24 2018 Joensuu, Finland Structural Mass Spectrometry Disulfide bonds mapping Limited
Analysis of chemically modified proteins by FT-ICR MS PETR NOVAK EU FT-ICR MS End User School 1 UEF Chemistry August 19-24 2018 Joensuu, Finland Structural Mass Spectrometry Disulfide bonds mapping Limited
Electronic Supplementary Information
 Electronic Supplementary Information 1. Reagents All the L-amino acids and L-glutathione (reduced form) were purchased from Sangon Biotch. o. (Shanghai, hina); Methyl orange, potassium tetrachloropalladate(ii),
Electronic Supplementary Information 1. Reagents All the L-amino acids and L-glutathione (reduced form) were purchased from Sangon Biotch. o. (Shanghai, hina); Methyl orange, potassium tetrachloropalladate(ii),
4. How can fragmentation be useful in identifying compounds? Permits identification of branching not observed in soft ionization.
 Homework 9: Chapters 20-21 Assigned 12 April; Due 17 April 2006; Quiz on 19 April 2006 Chap. 20 (Molecular Mass Spectroscopy) Chap. 21 (Surface Analysis) 1. What are the types of ion sources in molecular
Homework 9: Chapters 20-21 Assigned 12 April; Due 17 April 2006; Quiz on 19 April 2006 Chap. 20 (Molecular Mass Spectroscopy) Chap. 21 (Surface Analysis) 1. What are the types of ion sources in molecular
Lecture 8: Mass Spectrometry
 intensity Lecture 8: Mass Spectrometry Relative abundance m/z 1 Ethylbenzene CH 2 CH 3 + m/z = 106 CH 2 + m/z = 91 C 8 H 10 MW = 106 CH + m/z = 77 + 2 2 What information can be obtained from a MS spectrum?
intensity Lecture 8: Mass Spectrometry Relative abundance m/z 1 Ethylbenzene CH 2 CH 3 + m/z = 106 CH 2 + m/z = 91 C 8 H 10 MW = 106 CH + m/z = 77 + 2 2 What information can be obtained from a MS spectrum?
