Parkinson's disease (PD) is a chronic and progressive neurological. disorder that affects the motor system and is characterized by tremor, rigidity,
|
|
|
- Solomon Bradley
- 6 years ago
- Views:
Transcription
1 Chapter 2. Metabolism of Cyclic Tertiary Amines 2.1. MPTP - A Parkinsonian Inducing Agent Parkinson's disease (PD) is a chronic and progressive neurological disorder that affects the motor system and is characterized by tremor, rigidity, bradykinesia, or slowness of movement, and impaired balance and coordination. PD occurs when dopaminergic neurons of the substantia nigra die or become impaired. Loss of dopamine causes the nerve cells of the striatum to fire out of control, leaving patients unable to direct or control their movements in a normal manner. The cause of cell death is unknown but many researchers believe that a combination of oxidative damage, environmental toxins, genetic predisposition and accelerated aging may be shown to cause the disease. Motor deficits similar to those observed in Parkinson's disease were reported in young drug addicts following self-administration of a home-made street drug found to be contaminated with 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP, 44). 64 Langston has reported a striking similarity of the parkinsonian syndrome induced in human drug addicts with the syndrome observed in idiopathic Parkinson's disease. 65 MPTP has been effective in producing a non-human primate model of the disease in which selective nerve cell loss in the substantia nigra compacta, irreversible decreased dopamine levels in the striatum, and chronic impairment of extrapyramidal motor function are observed. 66,67 Many researchers have investigated the mechanism of action of this neurotoxin, its metabolism, and have exploited its potential as a model of Parkinson's disease. 35
2 Mechanism of Action of MPTP The mechanism of neurotoxicity of MPTP has been studied extensively. The toxication of MPTP is mediated by monoamine oxidase, principally type B, and to a lesser extent type A. 68 The enzyme catalyzes a two electron oxidation of MPTP to the unstable 2,3-dihydro-1-methyl-4-phenylpyridinium species MPDP + (Scheme 11, 45) MPDP + undergoes a further two electron oxidation by a pathway that may be enzyme mediated or by a disproportionation mechanism to result in the neurotoxic 1-methyl-4- phenylpyridinium species MPP + (46) MPP + is actively transported into the dopaminergic neurons by the dopamine uptake system. 75,76 It is concentrated in the matrix of the mitochondria 75 where it inhibits AD dehydrogenase 77,78 and mitochondrial electron transport leading to cessation of oxidative phosphorylation, ATP depletion and neuronal death. 79 Scheme 11. MAO Catalyzed Oxidation of MPTP MAO-B? The neurotoxicity of MPTP is blocked by (R)-deprenyl (2), a potent and selective inhibitor of MAO-B. MAO-B purified from beef liver catalyzes the oxidation of MPTP 38% as fast as benzylamine (the prefered MAO-B substrate) with a comparable K m value (Table 2). 80 MAO-A, isolated from human 36
3 placenta, catalyzes the oxidation of MPTP to the same dihydropyridinium product at about 12% the rate of kynuramine, again with a comparable K m value. This reaction is blocked by the MAO-A selective inhibitor clorgyline (1). This finding that MAO is responsible for the catalysis of MPTP oxidation was quite a surprise because no other cyclic tertiary amine had been reported to display substrate properties toward MAO. Table 2. Kinetic Parameters for the MAO Catalyzed Oxidation of Select Substrates MAO-A MAO-B k cat (min -1 ) K m (mm) k cat (min -1 ) K m (mm) Kynuramine (9) Benzylamine (8) MPTP (44) Metabolism of MPTP It was initially proposed that oxidation of MPTP to the dihydropyridinium metabolite (45) could result from direct attack at C-6 or from initial attack at C-2 followed by rearrangement of the isomeric 2,5-dihydropyridinium intermediate 44a to 45 via the free base 44b (Scheme 12)
4 Scheme 12. Pathway for MPDP + Formation a 44b owever, incubation of MPTP-6,6-d 2 (44-d 2 ) with MAO-B followed by ac treatment resulted in formation of 47-d 1 (Scheme 12a). Since no significant amounts of 48-d 2 were found, researchers ruled out a pathway proceeding via 44a. 81 They concluded that the formation of MPDP + occurs exclusively from oxidation of MPTP at the allylic C-6 position. Consequently, isotope effect measurements will not be complicated by metabolic switching to the C-2 position. 38
5 Scheme 12a. Treatment of MPDP + with ac D MAO-B ac C D D D 44-d 2 45-d 1 47-d 1 X MAO-B D ac C D D D 44a-d 2 48-d 2 It has been suggested that the α-carbon oxidation of MPTP is stereoselective. Previous studies showed that incubations of MPTP-6-d 1 with MAO-B gave a d 1 /d 0 product ratio that decreased with time. 81 These results suggest that the enantiomeric composition of the substrate changes with time due to the stereoselective loss of one of the enantiotopic hydrogen/deuterium atoms. Other MAO catalyzed reactions are known to be stereoselective such as the oxidation pathways of tyramine, 82 dopamine 83 and benzylamine
6 In addition to the discovery of MPTP's good MAO-B substrate properties, it was found that MPTP shows progressive and irreversible inactivation of both forms of MAO. 84,85 The inactivation process followed time-dependent, first order kinetics. Furthermore, the activity of MAO-B is not significantly regenerated following gel exclusion chromatography suggesting the formation of a covalent adduct with the enzyme. These results suggest that MPTP follows the criteria associated with a mechanism-based inactivator (see section ). It was reported by Singer et al. 86 that MPTP, MPDP + and MPP + are also reversible, competitive inhibitors of both MAO-A and MAO-B. The order of inhibition for the A form of the enzyme is MPDP + > MPP + > MPTP while for the B form the order shifts to MPTP > MPDP + > MPP +. From these studies the first order rate constants were determined for MPTP and its bioactivated products, MPDP + and MPP +, as regards to their time and concentration dependent inhibition of MAO-B (Table 3). Table 3. First Order Rate Constants for the Inhibition of MAO-B. Compound Concentration (mm) k (min -1 ) MPTP (44) x x x x x 10-2 MPDP + (45) x 10-2 MPP + (46)
7 The K i values for the competitive inhibition of both forms of the enzyme by MPTP, MPDP + and MPP + are shown in Table 4. Table 4. Inhibition of MAO-A and MAO-B by MPTP and its Metabolites K i (µm) at 30 C Enzyme Substrate MPTP MPDP + MPP + MAO-A Kynuramine MAO-B Benzylamine The data shown in Table 3 indicate that the rates of inactivation are slow compared to the rates of oxidation of MPTP (Table 2). This suggests a large partition coefficient in favor of substrate turnover and MPDP + formation. Of the two forms, MAO-B was more sensitive to irreversible inactivation. Interest in the interactions of MPTP with MAO-B has been intense since inhibition of this biotransformation blocks formation of 46, the putative ultimate nigrostriatal toxin, 72 and protects susceptible animals against MPTP's neurotoxic properties The possibility that the etiology of idiopathic Parkinson's disease may involve MPTP-type endogenous 90 or environmental substances 91 has also prompted studies to glean a better understanding of MAO. In addition, MAO-B is linked to Parkinson's disease as a result of its degradation of brain dopamine. 92 Consequently, compounds that selectively inhibit or inactivate MAO-B have been shown to be useful in the treatment of Parkinson's disease. 93 The elucidation of the mechanism of MAO may aid in the design of potential antiparkinsonian drugs. 41
8 2.2. MPTP Analogs - Structural Requirements A vast area of research has been directed toward gaining knowledge about the structural features that are required for MAO activity. A number of MPTP analogs have been prepared and examined for their MAO substrate properties. These findings include the following: 1) The 4-5 double bond of the tetrahydropyridine ring is essential for compounds to be MAO substrates. 94 2) Only substituents at the C-4 and -1 position are allowed. Placement of an alkyl group anywhere else in the tetrahydropyridine ring diminishes reactivity towards MAO. 95 3) Substitution at the -1 position is limited to small substituents. The -methyl group appears to be the ideal size while substituents such as -, -methyl, - ethyl and -α-hydroxy ethyl are less favorable. 96 4) The phenyl ring is not necessary for compounds to be MAO substrates; replacement of the phenyl ring by a 1-methyl-2-pyrrolyl, 97 a benzyl 96 or a phenoxy group 98 enhances MAO reactivity. The 4-cyclohexyl analog is as good a substrate as MPTP. 96 5) Para-substituents on the phenyl ring produce steric hindrance unfavorable to reactivity, while ortho- and meta-substituents may have stabilizing interactions within the active site increasing reactivity. 99 Because no X-ray crystal structure has been obtained for MAO, our knowledge of the active site and its interactions with potential substrates and inhibitors are dependent on structure-activity relationship studies (SAR). Quantitative SAR (QSAR) studies have identified lipophilicity, sterics and electronics as important determinants for MAO activity. 99 A recent study has 42
9 examined the MAO-B substrate properties of a variety of tetrahydropyridine derivatives and distance measurements along the 1 -C 4 axis to define the maximum size that can be accommodated by the MAO-B active site. 100 It was found that only those compounds measuring less than 14 Å displayed significant MAO-B substrate properties Cyclopropyl-4-phenyl-1,2,3,6-tetrahydropyridine Previous studies have shown that 1 and 2 amine substrates can be converted to good mechanism-based inactivators by replacement of a - or - group with a cyclopropyl group (see section ). The underlying concept is that good substrate properties, when properly exploited, may lead to good inactivator properties, i.e. the enzyme may catalyze the formation of a reactive intermediate which prior to dissociating to product (like a substrate) could covalently bind to the enzyme (like an inactivator). In an effort to gain better knowledge about the interactions of MAO with cyclic tertiary amines, the -cyclopropyl analog of MPTP was examined. 1-Cyclopropyl-4-phenyl-1,2,3,6-tetrahydropyridine (49) proved to be a good time and concentration dependent inhibitor of MAO-B (k inact /K I = 1.0 min - 1 mm -1 ). 101 The evidence that 49 is a mechanism-based inactivator of MAO implies that the enzyme processes the compound as a substrate but that an intermediate formed in the catalytic process inactivates the enzyme prior to escaping from the active site. A radical reaction pathway (Scheme 13) has been postulated analogous to that proposed by Silverman for - benzylcyclopropylamine (Scheme 5). Initial one-electron transfer from 49 to FAD would generate the aminium radical 50. This intermediate would have the option to ring open to the reactive primary radical 51 (pathway a) leading to 43
10 enzyme inactivation, or to lose an α-proton to give the secondary carbon radical 52, which subsequently undergoes a second one-electron loss to generate the dihydropyridinium intermediate 53 (pathway b). The absence of detectable levels of the dihydropyridinium metabolite 53 suggests that the deprotonation of 51 to form 52 (pathway b) does not compete effectively with the ring opening pathway (a). These results were not surprising since cyclopropyl groups attached to a radical bearing atom undergo very rapid ring opening. For example, the cyclopropylmethylcarbinyl radical 102 ring opens with a rate constant of 1.2 x 10 8 sec -1 while rates of ring opening of cyclopropylaminyl radicals have been reported at 7.2 x sec
11 Scheme 13. Proposed SET Pathway for the MAO-B Catalyzed Oxidation of 1-Cyclopropyl-4-phenyl-1,2,3,6-tetrahydropyridine e - a 51 e - b Benzyl-1-cyclopropyl-1,2,3,6-tetrahydropyridine As part of an ongoing study to characterize the catalytic mechanism of MAO and its potential role in the bioactivation of protoxins capable of causing neuronal degeneration, the interactions of 4-benzyl-1-cyclopropyl-1,2,3,6- tetrahydropyridine were examined with MAO-B. It was of interest to compare the inhibition properties of the 4-benzyl analog 54 with those of the 4-phenyl analog 49 because of the significantly improved substrate properties obtained when the phenyl group of MPTP is substituted with the benzyl group to give The k cat /K m value for the MAO-B catalyzed oxidation of MPTP at 30 C is 45
12 523 min -1 mm -1 while the corresponding value for 55 is 1250 min -1 mm -1. It was anticipated that substitution of the phenyl group present in 49 with the benzyl group present in 54 would lead to a more efficient inactivator k cat /K m = 523 min -1 mm -1 k cat /K m = 1250 min -1 mm -1 The 4-benzyl-1-cyclopropyl analog 54 proved to be a good time and concentration dependent inhibitor of MAO-B. 104 Attempts to determine the kinetic parameters k inact and K I were unsuccessful, however, because the inactivation process displayed nonlinear kinetic behavior. Unexpectedly compound 54 proved to be an excellent MAO-B substrate. Incubation of 54 with MAO-B showed a time and concentration dependent formation of the dihydropyridinium intermediate 59 (Scheme 14). The value for k cat /K m at 37 C (1500 min -1 mm -1 ) compares favorably with the corresponding values of the - methyl analog 55 (2700 min -1 mm -1 ) and MPTP (1400 min -1 mm -1 ). The exceptionally good substrate properties of 20 were a surprise since rates of ring opening of cyclopropylaminyl radical cations have been reported as too fast to measure. 29 The partition ratio, the ratio of product release to inactivation, for the MAO-B catalyzed oxidation of the 4-benzyl-1-cyclopropyl derivative 54 was estimated to be greater than Silverman has reported 46
13 partition ratios of 0 for some of the 1 and 2 cyclopropylamine inactivators, meaning every turnover of inactivator produces inactivated enzyme. 105 A partition ratio of 1000 clearly suggests that dihydropyridinium formation is the preferred pathway over enzyme inactivation. According to the SET mechanism, the intermediate cyclopropylaminyl radical cation 56 must partition between pathway a (Scheme 14), leading to the primary carbon centered radical 57 and enzyme inactivation, and pathway b, leading to the allylic radical intermediate 58 and product formation (59). The rates of ring opening of cyclopropylamine radical cations such as 56 have not been estimated experimentally, but based on the behavior of many α-radical bearing cyclopropyl systems, it was anticipated that the cyclopropylaminyl radical cation 56 would favor the ring opening pathway (a) over the proton loss pathway (b). owever, these considerations argue either that opening of the cyclopropyl ring of the radical cation 56 is slower than proton loss at C-6 or that dihydropyridinium (59) formation does not proceed via the radical cation
14 Scheme 14. Proposed SET Pathway for the MAO-B Catalyzed Oxidation of 4-Benzyl-1-cyclopropyl-1,2,3,6-tetrahydropyridine e - a b e - c It is possible that the favored partitioning of 54 to yield 59 rather than 57 may be explained by conformational constraints that would lead to poor overlap of the half-filled p-orbital on the nitrogen radical cation and the p-type orbitals of the cyclopropyl group. This restriction should retard the ring opening reaction leading to enzyme inactivation and therefore would favor the proton loss step leading to the dihydropyridinium product 59. R R bisected conformation ring opening occurs perpendicular conformation no ring opening 48
15 An alternative pathway that could account for the good substrate properties of 54 involves a direct hydrogen atom transfer reaction (Scheme 14, pathway c). This pathway relies on homolytic cleavage of the relatively weak allylic carbon-hydrogen bond which might be effected by an enzyme bound radical proposed to be present in MAO-B purified from beef liver Rational for Proposed Research Initial electron transfer vs hydrogen atom transfer in enzyme-catalyzed oxidations of amines has been a topic of debate. Although the SET pathway has enjoyed much favor, it also has been challenged. Results from rapid-scan stopped flow and magnetic field effect studies on the MAO-B catalyzed oxidation of [α,α- 2 ]benzylamine have led Edmondson to argue against the SET pathway since he could find no evidence of the formation of a flavin radical pair intermediate in this reaction. 106 Edmondson also has suggested that single electron transfer from an amine to the flavin is thermodynamically and kinetically improbable since the energy barrier for electron transfer is greater than the energy barrier for the reduction of FAD to FAD A similar debate concerns the cytochrome P450 catalyzed oxidations of tertiary amines. 51, Energy estimates derived from chemical models and the enzyme-catalyzed oxidation of,-dimethylaniline derivatives again suggest that, even in polar solvents that might stabilize the radical cation, hydrogen atom transfer is energetically preferred ever the SET pathway by several kcal/mol. 52 Similar conclusions based on isotope effects and stereochemical arguments have been reached in the case of the cytochrome P450 catalyzed α-carbon oxidation of (S)-nicotine
16 The unexpected substrate properties of 54 have prompted us to consider the possibility that the cyclopropylaminyl radical cation may not be an obligatory intermediate in the MAO-B catalytic pathway of 1,4-disubstituted tetrahydropyridines. This consideration has provoked new inquiry into the mechanism of catalysis of MAO-B. We would like to examine further the intermediacy of the aminium radical cation. In addition, we want to explore the possibility of another operative mechanism, specifically, a hydrogen atom transfer mechanism. The major effort of this research is to better define the mechanism of catalysis of MAO-B. We have elected to examine these questions using various 1,4- disubstituted-1,2,3,6-tetrahydropyridine derivatives as probes. In an attempt to gain additional insight into the interactions of the 4-benzyl-1-cyclopropyl derivative with MAO-B, we have examined the deuterium isotope effects on the rates of substrate turnover and enzyme inactivation. Kinetic isotope effect studies are often used in studying questions of mechanism in order to establish the rate determining step. In addition, because there is a partitioning between two pathways, one leading to enzyme inactivation and the other leading to product formation, we wanted to examine the influence of isotopic substitution on these pathways. In order to establish that the rate determining step does not change with various substrates, we have examined the deuterium isotope effects with additional 1,4-disubstituted tetrahydropyridines. We are particularly interested in the partitioning of substrates between product formation and enzyme inactivation. One possible explanation for the good substrate properties and poor inactivator properties of the 4-benzylcyclopropyl derivative (54) relative to the 4-phenyl analog (49) could be steric 50
17 constraints imposed by the active site that prevent proper orbital alignment required for ring opening. We have examined the MAO-B substrate and inactivator properties of a series of 1-methyl- and 1- cyclopropyltetrahydropyridine derivatives bearing C-4 heteroaromatic substituents. Since all of these compounds are 4-aryltetrahydropyridine derivatives with the potential to assume similar conformations with the active site of the enzyme, we speculated that the inactivation properties of the - cyclopropyl derivatives would not be so influenced by differences in the extent to which the orbitals may align for ring opening as might be encountered with the benzyl analog. On the other hand, the putative allylic radical intermediates should be stabilized by electron rich heterocyclic aromatic groups at C-4, in which case the -methyl analogs might be expected to display a relatively wide range of substrate properties. We anticipated that the results from these types of comparative studies would help to assess the intermediacy of allylic radicals in these α-carbon oxidation reactions. QSAR studies using a series of MPTP analogs bearing various substituents on the phenyl ring have provided a more in depth analysis of the physicochemical parameters influencing the substrate properties. Lipophilicity was established as a primary determinant. In order to explore further the effects of polar substituents in the MAO-B catalyzed oxidation of MPTP analogs, we have carried out a study involving a variety of polar aromatic groups in which a nitrogen is directly attached to the C-4 position. The substrate/inactivation properties of the -cyclopropyl derivatives were also examined. In an attempt to gain additional information regarding the MAO-B catalytic pathway, we have exploited two chemical models that will allow us to induce a 51
18 single electron transfer reaction or a hydrogen atom transfer reaction on various tetrahydropyridine substrates. We have compared the rates of dihydropyridinium formation and rates of enzyme inactivation of the enzymatic reactions to the rates we obtain with the chemical model reactions. 52
Deuterium Isotope Effect Studies on the MAO-B Catalyzed Oxidation of 4-Benzyl-1-cyclopropyl-1,2,3,6-tetrahydropyridine
 Biochemistry 1996, 35, 3335-3340 3335 Deuterium Isotope Effect Studies on the MAO-B Catalyzed Oxidation of 4-Benzyl-1-cyclopropyl-1,2,3,6-tetrahydropyridine Andrea H. Anderson, Simon Kuttab, and Neal Castagnoli,
Biochemistry 1996, 35, 3335-3340 3335 Deuterium Isotope Effect Studies on the MAO-B Catalyzed Oxidation of 4-Benzyl-1-cyclopropyl-1,2,3,6-tetrahydropyridine Andrea H. Anderson, Simon Kuttab, and Neal Castagnoli,
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
2054, Chap. 8, page 1
 2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
2013 W. H. Freeman and Company. 6 Enzymes
 2013 W. H. Freeman and Company 6 Enzymes CHAPTER 6 Enzymes Key topics about enzyme function: Physiological significance of enzymes Origin of catalytic power of enzymes Chemical mechanisms of catalysis
2013 W. H. Freeman and Company 6 Enzymes CHAPTER 6 Enzymes Key topics about enzyme function: Physiological significance of enzymes Origin of catalytic power of enzymes Chemical mechanisms of catalysis
Overview of Kinetics
 Overview of Kinetics [P] t = ν = k[s] Velocity of reaction Conc. of reactant(s) Rate of reaction M/sec Rate constant sec -1, M -1 sec -1 1 st order reaction-rate depends on concentration of one reactant
Overview of Kinetics [P] t = ν = k[s] Velocity of reaction Conc. of reactant(s) Rate of reaction M/sec Rate constant sec -1, M -1 sec -1 1 st order reaction-rate depends on concentration of one reactant
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Biological Chemistry and Metabolic Pathways
 Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
Biological Chemistry and Metabolic Pathways 1. Reaction a. Thermodynamics b. Kinetics 2. Enzyme a. Structure and Function b. Regulation of Activity c. Kinetics d. Inhibition 3. Metabolic Pathways a. REDOX
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
PAPER No. 05: TITLE: ORGANIC CHEMISTRY-II MODULE No. 12: TITLE: S N 1 Reactions
 Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
Subject hemistry Paper o and Title Module o and Title Module Tag 05, ORGAI EMISTRY-II 12, S 1 Reactions E_P5_M12 EMISTRY PAPER o. 05: TITLE: ORGAI EMISTRY-II TABLE OF OTETS 1. Learning Outcomes 2. Introduction
Lecture 7: Enzymes and Energetics
 Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Lecture 7: Enzymes and Energetics I. Biological Background A. Biological work requires energy 1. Energy is the capacity to do work a. Energy is expressed in units of work (kilojoules) or heat energy (kilocalories)
Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems
 Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems Introduction Enzymes are Biological Catalysis A catalyst is a substance
Biochemistry 462a - Enzyme Kinetics Reading - Chapter 8 Practice problems - Chapter 8: (not yet assigned); Enzymes extra problems Introduction Enzymes are Biological Catalysis A catalyst is a substance
Chapter 15: Enyzmatic Catalysis
 Chapter 15: Enyzmatic Catalysis Voet & Voet: Pages 496-508 Slide 1 Catalytic Mechanisms Catalysis is a process that increases the rate at which a reaction approaches equilibrium Rate enhancement depends
Chapter 15: Enyzmatic Catalysis Voet & Voet: Pages 496-508 Slide 1 Catalytic Mechanisms Catalysis is a process that increases the rate at which a reaction approaches equilibrium Rate enhancement depends
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Citation for published version (APA): Nouri-Nigjeh, E. (2011). Electrochemistry in the mimicry of oxidative drug metabolism Groningen: s.n.
 University of Groningen Electrochemistry in the mimicry of oxidative drug metabolism Nouri-Nigjeh, Eslam IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish
University of Groningen Electrochemistry in the mimicry of oxidative drug metabolism Nouri-Nigjeh, Eslam IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish
An Introduction to Metabolism
 An Introduction to Metabolism I. All of an organism=s chemical reactions taken together is called metabolism. A. Metabolic pathways begin with a specific molecule, which is then altered in a series of
An Introduction to Metabolism I. All of an organism=s chemical reactions taken together is called metabolism. A. Metabolic pathways begin with a specific molecule, which is then altered in a series of
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides"
 Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Elimination Reactions. Chapter 6 1
 Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Module No and Title. PAPER No: 5 ; TITLE : Organic Chemistry-II MODULE No: 25 ; TITLE: S E 1 reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Chapter 5 Ground Rules of Metabolism Sections 1-5
 Chapter 5 Ground Rules of Metabolism Sections 1-5 5.1 A Toast to Alcohol Dehydrogenase In the liver, the enzyme alcohol dehydrogenase breaks down toxic ethanol to acetaldehyde, an organic molecule even
Chapter 5 Ground Rules of Metabolism Sections 1-5 5.1 A Toast to Alcohol Dehydrogenase In the liver, the enzyme alcohol dehydrogenase breaks down toxic ethanol to acetaldehyde, an organic molecule even
Electronic Structure. Hammett Correlations. Linear Free Energy Relationships. Quantitative Structure Activity Relationships (QSAR)
 Electronic Structure ammett Correlations Quantitative Structure Activity Relationships (QSAR) Quantitative Structure Activity Relationships (QSAR) We have already seen that by changing groups in a drug
Electronic Structure ammett Correlations Quantitative Structure Activity Relationships (QSAR) Quantitative Structure Activity Relationships (QSAR) We have already seen that by changing groups in a drug
Reactivity in Organic Chemistry. Mid term test October 31 st 2011, 9:30-12:30. Problem 1 (25p)
 eactivity in rganic Chemistry Mid term test ctober 31 st 2011, 9:30-12:30 Problem 1 (25p) - (+)- Limonone can be epoxidized (using peracetic acid) to give an inseparable mixture of two diastereomeric limonene
eactivity in rganic Chemistry Mid term test ctober 31 st 2011, 9:30-12:30 Problem 1 (25p) - (+)- Limonone can be epoxidized (using peracetic acid) to give an inseparable mixture of two diastereomeric limonene
Catalysis. Instructor: Dr. Tsung-Lin Li Genomics Research Center Academia Sinica
 Catalysis Instructor: Dr. Tsung-Lin Li Genomics Research Center Academia Sinica References: Biochemistry" by Donald Voet and Judith G. Voet Biochemistry" by Christopher K. Mathews, K. E. Van Hold and Kevin
Catalysis Instructor: Dr. Tsung-Lin Li Genomics Research Center Academia Sinica References: Biochemistry" by Donald Voet and Judith G. Voet Biochemistry" by Christopher K. Mathews, K. E. Van Hold and Kevin
Chemical Reactions and the enzimes
 Chemical Reactions and the enzimes LESSON N. 6 - PSYCHOBIOLOGY Chemical reactions consist of interatomic interactions that take place at the level of their orbital, and therefore different from nuclear
Chemical Reactions and the enzimes LESSON N. 6 - PSYCHOBIOLOGY Chemical reactions consist of interatomic interactions that take place at the level of their orbital, and therefore different from nuclear
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Ground Rules of Metabolism CHAPTER 6
 Ground Rules of Metabolism CHAPTER 6 Antioxidants You ve heard the term. What s the big deal? Found naturally in many fruits and vegetables Added to many products What do they actually do? Antioxidants
Ground Rules of Metabolism CHAPTER 6 Antioxidants You ve heard the term. What s the big deal? Found naturally in many fruits and vegetables Added to many products What do they actually do? Antioxidants
Chapter 5. Aromatic Compounds
 Chapter 5. Aromatic Compounds 5.1 Structure of Benzene: The Kekule Proposal Mid-1800s, benzene was known to have the molecular formula C 6 6. Benzene reacts with 2 in the presence of iron to give substitution
Chapter 5. Aromatic Compounds 5.1 Structure of Benzene: The Kekule Proposal Mid-1800s, benzene was known to have the molecular formula C 6 6. Benzene reacts with 2 in the presence of iron to give substitution
CHEM 251 (4 credits): Description
 CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
10/26/2010. An Example of a Polar Reaction: Addition of H 2 O to Ethylene. to Ethylene
 6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
9/25/2011. Outline. Overview: The Energy of Life. I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V.
 Chapter 8 Introduction to Metabolism Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes Overview: The Energy of Life Figure 8.1 The living cell is a miniature
Chapter 8 Introduction to Metabolism Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes Overview: The Energy of Life Figure 8.1 The living cell is a miniature
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 14. Principles of Catalysis
 Organometallics Study Meeting 2011/08/28 Kimura Chapter 14. Principles of Catalysis 14. 1. General Principles 14.1.1. Definition of a Catalyst 14.1.2. Energetics of Catalysis 14.1.3. Reaction Coordinate
Organometallics Study Meeting 2011/08/28 Kimura Chapter 14. Principles of Catalysis 14. 1. General Principles 14.1.1. Definition of a Catalyst 14.1.2. Energetics of Catalysis 14.1.3. Reaction Coordinate
Elimination Reactions Heating an alkyl halide with a strong base causes elimination of a. molecule of HX
 Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations
 11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
11. Reactions of Alkyl Halides: Nucleophilic Substitutions and Eliminations Based on McMurry s Organic Chemistry, 6 th edition 2003 Ronald Kluger Department of Chemistry University of Toronto Alkyl Halides
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
240 Chem. Aromatic Compounds. Chapter 6
 240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
240 Chem Aromatic Compounds Chapter 6 1 The expressing aromatic compounds came to mean benzene and derivatives of benzene. Structure of Benzene: Resonance Description C 6 H 6 1.It contains a six-membered
An Introduction to Metabolism
 An Introduction to Metabolism PREFACE The living cell is a chemical factory with thousands of reactions taking place, many of them simultaneously This chapter is about matter and energy flow during life
An Introduction to Metabolism PREFACE The living cell is a chemical factory with thousands of reactions taking place, many of them simultaneously This chapter is about matter and energy flow during life
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
An Introduction to Metabolism. Chapter 8
 An Introduction to Metabolism Chapter 8 METABOLISM I. Introduction All of an organism s chemical reactions Thousands of reactions in a cell Example: digest starch use sugar for energy and to build new
An Introduction to Metabolism Chapter 8 METABOLISM I. Introduction All of an organism s chemical reactions Thousands of reactions in a cell Example: digest starch use sugar for energy and to build new
CHEMISTRY. Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para ipso attack, orientation in other ring systems.
 Subject Chemistry Paper No and Title Paper-5, Organic Chemistry-II Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para Module Tag CHE_P5_M29 TABLE OF CONTENTS 1. Learning Outcomes
Subject Chemistry Paper No and Title Paper-5, Organic Chemistry-II Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para Module Tag CHE_P5_M29 TABLE OF CONTENTS 1. Learning Outcomes
Introduction and. Properties of Enzymes
 Unit-III Enzymes Contents 1. Introduction and Properties of enzymes 2. Nomenclature and Classification 3. Mechanism of enzyme-catalyzed reactions 4. Kinetics of enzyme-catalyzed reactions 5. Inhibition
Unit-III Enzymes Contents 1. Introduction and Properties of enzymes 2. Nomenclature and Classification 3. Mechanism of enzyme-catalyzed reactions 4. Kinetics of enzyme-catalyzed reactions 5. Inhibition
BIOLOGY 10/11/2014. An Introduction to Metabolism. Outline. Overview: The Energy of Life
 8 An Introduction to Metabolism CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes
8 An Introduction to Metabolism CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Forms of Energy II. Laws of Thermodynamics III. Energy and metabolism IV. ATP V. Enzymes
General Biology. The Energy of Life The living cell is a miniature factory where thousands of reactions occur; it converts energy in many ways
 Course No: BNG2003 Credits: 3.00 General Biology 5. An Introduction into Cell Metabolism The Energy of Life The living cell is a miniature factory where thousands of reactions occur; it converts energy
Course No: BNG2003 Credits: 3.00 General Biology 5. An Introduction into Cell Metabolism The Energy of Life The living cell is a miniature factory where thousands of reactions occur; it converts energy
What is an enzyme? Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics. Margaret A. Daugherty Fall General Properties
 Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics Margaret A. Daugherty Fall 2003 ENZYMES: Why, what, when, where, how? All but the who! What: proteins that exert kinetic control over
Lecture 12: Enzymes & Kinetics I Introduction to Enzymes and Kinetics Margaret A. Daugherty Fall 2003 ENZYMES: Why, what, when, where, how? All but the who! What: proteins that exert kinetic control over
Chapter 6. Ground Rules Of Metabolism
 Chapter 6 Ground Rules Of Metabolism Alcohol Dehydrogenase An enzyme Breaks down ethanol and other toxic alcohols Allows humans to drink Metabolism Is the totality of an organism s chemical reactions Arises
Chapter 6 Ground Rules Of Metabolism Alcohol Dehydrogenase An enzyme Breaks down ethanol and other toxic alcohols Allows humans to drink Metabolism Is the totality of an organism s chemical reactions Arises
Chapter Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow,
 Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
and Stereochemistry) PAPER 1: ORGANIC CHEMISTRY- I (Nature of Bonding and Stereochemistry) MODULE 4: Applications of Electronic Effects
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 1: ORGANIC - I (Nature of Bonding Module 4: Applications of Electronic Effects CHE_P1_M4 PAPER 1: ORGANIC - I (Nature of Bonding
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 1: ORGANIC - I (Nature of Bonding Module 4: Applications of Electronic Effects CHE_P1_M4 PAPER 1: ORGANIC - I (Nature of Bonding
Two requirements for life: Self-replication and appropriate catalysis. A. Most enzymes (def.: biological catalysts) are proteins
 Enzymes We must be able to enhance the rates of many physical and chemical processes to remain alive and healthy. Support for that assertion: Maladies of genetic origin. Examples: Sickle-cell anemia (physical)
Enzymes We must be able to enhance the rates of many physical and chemical processes to remain alive and healthy. Support for that assertion: Maladies of genetic origin. Examples: Sickle-cell anemia (physical)
Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras
 Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras Module No. #02 Lecture No. #09 Pericyclic Reactions Cycloaddition Reactions
Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras Module No. #02 Lecture No. #09 Pericyclic Reactions Cycloaddition Reactions
Biochemistry. Lecture 8 Enzyme Kinetics
 Biochemistry Lecture 8 Enzyme Kinetics Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase -
Biochemistry Lecture 8 Enzyme Kinetics Why Enzymes? igher reaction rates Greater reaction specificity Milder reaction conditions Capacity for regulation C - - C N 2 - C N 2 - C - C Chorismate mutase -
Some Answers to Hour Examination #1, Chemistry 302/302A, 2004
 Some Answers to our Examination #1, Chemistry 302/302A, 2004 1. In this variation of the Diels-Alder reaction, both the diene and the dienophile are cyclic compounds. The first complication is to decide
Some Answers to our Examination #1, Chemistry 302/302A, 2004 1. In this variation of the Diels-Alder reaction, both the diene and the dienophile are cyclic compounds. The first complication is to decide
Ch 16 Electrophilic Aromatic Substitution
 Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
Ch 16 Electrophilic Aromatic Substitution Mechanism - Aromatic rings typically undergo substitution, where an H is replaced with an electrophile (E+). - The rings do not typically undergo addition across
B X A X. In this case the star denotes a chiral center.
 Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
σ Bonded ligands: Transition Metal Alkyls and Hydrides
 σ Bonded ligands: Transition Metal Alkyls and Hydrides Simplest of organo-transitionmetal species Rare until and understanding of their stability in the 60 s and 70 s Metal alkyls can be considered as
σ Bonded ligands: Transition Metal Alkyls and Hydrides Simplest of organo-transitionmetal species Rare until and understanding of their stability in the 60 s and 70 s Metal alkyls can be considered as
An Introduction to Metabolism
 Chapter 8 An Introduction to Metabolism Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 8 1. An organism s metabolism transforms matter and energy, subject to the laws of
Chapter 8 An Introduction to Metabolism Dr. Wendy Sera Houston Community College Biology 1406 Key Concepts in Chapter 8 1. An organism s metabolism transforms matter and energy, subject to the laws of
Energy Transformation and Metabolism (Outline)
 Energy Transformation and Metabolism (Outline) - Definitions & Laws of Thermodynamics - Overview of energy flow ecosystem - Biochemical processes: Anabolic/endergonic & Catabolic/exergonic - Chemical reactions
Energy Transformation and Metabolism (Outline) - Definitions & Laws of Thermodynamics - Overview of energy flow ecosystem - Biochemical processes: Anabolic/endergonic & Catabolic/exergonic - Chemical reactions
Recommended Reading: 23, 29 (3rd edition); 22, 29 (4th edition) Ch 102 Problem Set 7 Due: Thursday, June 1 Before Class. Problem 1 (1 points) Part A
 Recommended Reading: 23, 29 (3rd edition); 22, 29 (4th edition) Ch 102 Problem Set 7 Due: Thursday, June 1 Before Class Problem 1 (1 points) Part A Kinetics experiments studying the above reaction determined
Recommended Reading: 23, 29 (3rd edition); 22, 29 (4th edition) Ch 102 Problem Set 7 Due: Thursday, June 1 Before Class Problem 1 (1 points) Part A Kinetics experiments studying the above reaction determined
An Introduction to Metabolism
 An Introduction to Metabolism The living cell is a microscopic factory where life s giant processes can be performed: -sugars to amino acids to proteins and vise versa -reactions to dismantle polymers
An Introduction to Metabolism The living cell is a microscopic factory where life s giant processes can be performed: -sugars to amino acids to proteins and vise versa -reactions to dismantle polymers
CHAPTER 2. Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules
 CHAPTER 2 Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules 2-1 Kinetics and Thermodynamics of Simple Chemical Processes Chemical thermodynamics: Is concerned with the extent that
CHAPTER 2 Structure and Reactivity: Acids and Bases, Polar and Nonpolar Molecules 2-1 Kinetics and Thermodynamics of Simple Chemical Processes Chemical thermodynamics: Is concerned with the extent that
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
f) Adding an enzyme does not change the Gibbs free energy. It only increases the rate of the reaction by lowering the activation energy.
 Problem Set 2-Answer Key BILD1 SP16 1) How does an enzyme catalyze a chemical reaction? Define the terms and substrate and active site. An enzyme lowers the energy of activation so the reaction proceeds
Problem Set 2-Answer Key BILD1 SP16 1) How does an enzyme catalyze a chemical reaction? Define the terms and substrate and active site. An enzyme lowers the energy of activation so the reaction proceeds
Hour Examination # 1
 CEM 347 rganic Chemistry II Spring 2015 Exam # 1 Solutions Key Page 1 of 11 CEM 347 rganic Chemistry II Spring 2015 Instructor: Paul Bracher our Examination # 1 Wednesday, February 11 th, 2015 6:00 8:00
CEM 347 rganic Chemistry II Spring 2015 Exam # 1 Solutions Key Page 1 of 11 CEM 347 rganic Chemistry II Spring 2015 Instructor: Paul Bracher our Examination # 1 Wednesday, February 11 th, 2015 6:00 8:00
Lecture 15: Enzymes & Kinetics. Mechanisms ROLE OF THE TRANSITION STATE. H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl. Margaret A. Daugherty.
 Lecture 15: Enzymes & Kinetics Mechanisms Margaret A. Daugherty Fall 2004 ROLE OF THE TRANSITION STATE Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products
Lecture 15: Enzymes & Kinetics Mechanisms Margaret A. Daugherty Fall 2004 ROLE OF THE TRANSITION STATE Consider the reaction: H-O-H + Cl - H-O δ- H Cl δ- HO - + H-Cl Reactants Transition state Products
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
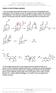 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution. Reactivity of Benzene
 hapter 16 hemistry of Benzene: Electrophilic Aromatic Substitution Reactivity of Benzene - stabilization due to aromaticity makes benzene significantly less reactive than isolated alkenes 2 no reaction
hapter 16 hemistry of Benzene: Electrophilic Aromatic Substitution Reactivity of Benzene - stabilization due to aromaticity makes benzene significantly less reactive than isolated alkenes 2 no reaction
An Introduction to Metabolism
 Chapter 8 An Introduction to Metabolism Edited by Shawn Lester PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley
Chapter 8 An Introduction to Metabolism Edited by Shawn Lester PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley
Organometallic Catalysis
 Organometallic Catalysis The catalysts we will study are termed homogeneous catalysts as they are dissolved in th e same solvent as the substrate. In contrast, heterogeneous catalysts, such as palladium
Organometallic Catalysis The catalysts we will study are termed homogeneous catalysts as they are dissolved in th e same solvent as the substrate. In contrast, heterogeneous catalysts, such as palladium
PAPER No. : 5; Organic Chemistry-II MODULE No. : 13; Mixed S N 1 and S N 2 Reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
Chapter 6- An Introduction to Metabolism*
 Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
Chapter 6- An Introduction to Metabolism* *Lecture notes are to be used as a study guide only and do not represent the comprehensive information you will need to know for the exams. The Energy of Life
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
S N 1 Displacement Reactions
 S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
S N 1 Displacement Reactions Tertiary alkyl halides cannot undergo S N 2 reactions because of the severe steric hindrance blocking a backside approach of the nucleophile. They can, however, react via an
An Introduction to Metabolism
 An Introduction to Metabolism PREFACE The living cell is a chemical factory with thousands of reactions taking place, many of them simultaneously This chapter is about matter and energy flow during life
An Introduction to Metabolism PREFACE The living cell is a chemical factory with thousands of reactions taking place, many of them simultaneously This chapter is about matter and energy flow during life
ENZYME KINETICS AND INHIBITION
 ENZYME KINETICS AND INHIBITION The kinetics of reactions involving enzymes are a little bit different from other reactions. First of all, there are sometimes lots of steps involved. Also, the reaction
ENZYME KINETICS AND INHIBITION The kinetics of reactions involving enzymes are a little bit different from other reactions. First of all, there are sometimes lots of steps involved. Also, the reaction
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
An Introduction to Metabolism
 Chapter 8 An Introduction to Metabolism PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
Chapter 8 An Introduction to Metabolism PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from
An Introduction to Metabolism
 An Introduction to Metabolism Chapter 8 Objectives Distinguish between the following pairs of terms: catabolic and anabolic pathways; kinetic and potential energy; open and closed systems; exergonic and
An Introduction to Metabolism Chapter 8 Objectives Distinguish between the following pairs of terms: catabolic and anabolic pathways; kinetic and potential energy; open and closed systems; exergonic and
Energy Transformation, Cellular Energy & Enzymes (Outline)
 Energy Transformation, Cellular Energy & Enzymes (Outline) Energy conversions and recycling of matter in the ecosystem. Forms of energy: potential and kinetic energy The two laws of thermodynamic and definitions
Energy Transformation, Cellular Energy & Enzymes (Outline) Energy conversions and recycling of matter in the ecosystem. Forms of energy: potential and kinetic energy The two laws of thermodynamic and definitions
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
An Overview of Organic Reactions. Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants:
 An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
An Introduction to Metabolism
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 8 An Introduction to Metabolism
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 8 An Introduction to Metabolism
Chapter 6. Chemical Reactivity and Reaction Mechanisms
 hapter 6 hemical Reactivity and Reaction Mechanisms hemical Reactivity Enthalpy A simple chemical reaction can be broken down into bond creating and bond breaking components: A-B + Y-Z A-Y + B-Z A-B A
hapter 6 hemical Reactivity and Reaction Mechanisms hemical Reactivity Enthalpy A simple chemical reaction can be broken down into bond creating and bond breaking components: A-B + Y-Z A-Y + B-Z A-B A
Loudon Chapter 17 Review: Allylic/Benzylic Reactivity
 Chapter 17 is all about reactions that happen at the position one away from an aromatic ring, or one away from a double bond. These are called the benzylic and allylic positions respectively. Benzyl and
Chapter 17 is all about reactions that happen at the position one away from an aromatic ring, or one away from a double bond. These are called the benzylic and allylic positions respectively. Benzyl and
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
An Introduction to Metabolism
 CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 6 An Introduction to Metabolism Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: The Energy of Life The
CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 6 An Introduction to Metabolism Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: The Energy of Life The
Objectives INTRODUCTION TO METABOLISM. Metabolism. Catabolic Pathways. Anabolic Pathways 3/6/2011. How to Read a Chemical Equation
 Objectives INTRODUCTION TO METABOLISM. Chapter 8 Metabolism, Energy, and Life Explain the role of catabolic and anabolic pathways in cell metabolism Distinguish between kinetic and potential energy Distinguish
Objectives INTRODUCTION TO METABOLISM. Chapter 8 Metabolism, Energy, and Life Explain the role of catabolic and anabolic pathways in cell metabolism Distinguish between kinetic and potential energy Distinguish
It is generally believed that the catalytic reactions occur in at least two steps.
 Lecture 16 MECHANISM OF ENZYME ACTION A chemical reaction such as A ----> P takes place because a certain fraction of the substrate possesses enough energy to attain an activated condition called the transition
Lecture 16 MECHANISM OF ENZYME ACTION A chemical reaction such as A ----> P takes place because a certain fraction of the substrate possesses enough energy to attain an activated condition called the transition
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
Chapter 8: An Introduction to Metabolism
 Chapter 8: An Introduction to Metabolism Key Concepts 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 8.2 The free-energy change of a reaction tells us
Chapter 8: An Introduction to Metabolism Key Concepts 8.1 An organism s metabolism transforms matter and energy, subject to the laws of thermodynamics 8.2 The free-energy change of a reaction tells us
Amines. Amines are organic compounds containing a nitrogen functionality. primary secondary tertiary quaternary
 Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Amines Amines are organic compounds containing a nitrogen functionality Depending upon the number of alkyl, or aryl, groups attached to nitrogen determines its classification, or order 2 primary secondary
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 8: Energy and Metabolism
 Chapter 8: Energy and Metabolism Why do organisms need energy? How do organisms manage their energy needs? Defining terms and issues: energy and thermodynamics metabolic reactions and energy transfers
Chapter 8: Energy and Metabolism Why do organisms need energy? How do organisms manage their energy needs? Defining terms and issues: energy and thermodynamics metabolic reactions and energy transfers
Examples of Substituted Benzenes
 Organic Chemistry 5 th Edition Paula Yurkanis Bruice Examples of Substituted Benzenes Chapter 15 Reactions of Substituted Benzenes Irene Lee Case Western Reserve University Cleveland, OH 2007, Prentice
Organic Chemistry 5 th Edition Paula Yurkanis Bruice Examples of Substituted Benzenes Chapter 15 Reactions of Substituted Benzenes Irene Lee Case Western Reserve University Cleveland, OH 2007, Prentice
a) Write the mechanism of Friedel-Crafts alkylation of ethyl benzene to give 1,4- diethylbenzene. Show all arrow pushing.
 a) Write the mechanism of Friedel-Crafts alkylation of ethyl benzene to give 1,4- diethylbenzene. Show all arrow pushing. Br AlBr 3 b) Using resonance and inductive effects, explain why an ethyl group
a) Write the mechanism of Friedel-Crafts alkylation of ethyl benzene to give 1,4- diethylbenzene. Show all arrow pushing. Br AlBr 3 b) Using resonance and inductive effects, explain why an ethyl group
Metabolism and Enzymes
 Energy Basics Metabolism and Enzymes Chapter 5 Pgs. 77 86 Chapter 8 Pgs. 142 162 Energy is the capacity to cause change, and is required to do work. Very difficult to define quantity. Two types of energy:
Energy Basics Metabolism and Enzymes Chapter 5 Pgs. 77 86 Chapter 8 Pgs. 142 162 Energy is the capacity to cause change, and is required to do work. Very difficult to define quantity. Two types of energy:
