Technical Guidance Note (Monitoring) M16. Monitoring volatile organic compounds and methane in stack gas emissions
|
|
|
- David Fox
- 6 years ago
- Views:
Transcription
1 Technical Guidance Note (Monitoring) M16 Monitoring volatile organic compounds and methane in stack gas emissions Environment Agency Version 4 June 2012
2
3 Purpose This Technical Guidance Note (TGN) provides information about how we regulate emissions of volatile organic compounds (VOCs) to air from industrial processes. It defines what they are, why they are regulated, how we classify them, and provides an outline of the methods used to measure them. Although methane is not classed as a VOC, methods for its measurement are included in this TGN. Status of this document This TGN may be subject to review and amendment following publication. The latest version of this note is available from Record of changes Version Date Change Amended title to include methane. 4 June 2012 Updated text in sections Noted that FIDs measure both methane and VOCs. 6.2 deleted section on ECD Added guidance that PIDs are not suitable for stack emissions monitoring Deleted text on drying agents, permeation tube driers and cold traps. 7.5 updated section on mass spectrometry 9 - Added note about methane. 9 Added information about the revision of European TOC standards. Added Section 12 on the measurement of methane. Annex 1 - Added bullet point about limitation to using a FID to measure class B VOCs, if methane is present in the stack gas. Deleted Annex 2 on categorisation of VOCs. Deleted Annex 3 list of VOCs categorised according to harm rating. Feedback Any comments or suggested improvements to this TGN should be ed to Rupert Standring at rupert.standring@environment-agency.gov.uk.
4 Contents 1. What are volatile organic compounds? Why do we regulate VOCs? How do we regulate VOCs? How do we classify VOCs? How do we specify the concentration of VOCs? Techniques for monitoring TOC Techniques for monitoring individual VOCs Hierarchy of monitoring standards European periodic standard reference methods CEMs Measurement of methane...9 Annex 1: Guidance on approach to measuring highly harmful, class A and class B VOCs...10 References...12
5 1. What are volatile organic compounds? Volatile organic compounds (VOCs) are organic chemicals that have a significant vapour pressure at room temperature. If they are allowed to build up in the air, they can harm human health or damage the environment. VOCs come from many different classes of chemicals, including aliphatic, aromatic and halogenated hydrocarbons, aldehydes, ketones, esters, ethers, alcohols, acids and amines. There are various definitions of VOCs. We have adopted the United Nations Economic Council for Europe (UNECE) definition, which defines a VOC as any organic compound which is emitted from non-natural processes and has a photochemical ozone creation potential (POCP). We interpret this to mean any organic compound released to the atmosphere from an industrial plant or process, excluding methane. Many industrial processes emit VOCs. These include printing, surface coating, painting, manufacturing of chemicals, rubber fabrication, wood and plastic lamination or cleaning. However, besides industrial emissions of VOCs, households and road transport contribute a substantial fraction. 2. Why do we regulate VOCs? We regulate VOCs from industrial processes to minimise the harm that they do. They can cause harm in the following ways: harming human health directly promoting ozone pollution at ground level damaging the ozone layer in the stratosphere contributing to global climate change causing offensive smells Research has shown that certain VOCs are involved in the generation of photochemical oxidants, in particular ozone, in the lowest region of the atmosphere (the troposphere). VOCs are an important class of pollutants commonly found in the atmosphere at ground level in urban and industrial centres, and contribute to high levels of ozone there. Most industrial processes that can emit large quantities of VOCs require an Environmental Permit to operate. Before the Permit is granted, we assess the likely impact of all its emissions, including VOCs. We will not issue a Permit unless we are confident that the way that the process is operated will not put human health at risk and will minimise the environmental impact. 3. How do we regulate VOCs? VOCs from industrial processes are regulated under The Environmental Permitting (England and Wales) Regulations (EPR). The EPR contain a comprehensive list of industrial processes that can cause pollution. They place a duty on us to issue an Environmental Permit to every organisation that operates a listed process. The permit must contain conditions that require the process operator to prevent or minimise pollution by the use of Best Available Techniques (BAT). TGN M16, Version 4, June 2012 Page 1 of 12
6 Industrial processes release VOCs from a defined emission point (for example, an exhaust flue from a paint spray booth). These are known as point source emissions. Permits often control point source emissions by specifying emission limits. These may either state that the concentration of the pollutant must not exceed a certain value, or that the source must not release more than a certain mass of the pollutant over a defined period. If the potential for environmental harm from the process is substantial, the operator will be required to measure the concentration of the pollutant continuously and stop processing as soon as he safely can, if the concentration exceeds the permitted value. If the potential for harm is lower, the requirement to measure the concentration is reduced to an appropriate level. It becomes, in effect, a check that the process is not changing gradually over a period of time. There is also specific legislation that requires Environmental Permits for certain activities to set specific emission limits. These are the Waste Incineration Directive (WID) 1 and the Solvent Emissions Directive (SED) 2. The SED aims at reducing emissions of organic solvents by industry. It requires emission limits to be set for VOCs in a variety of processes. The value of the emission limit set depends upon the specific application and pollutant. 4. How do we classify VOCs? We classify VOCs according to their potential environmental harmfulness, so that an assessment can be made of the degree of control needed on their release from a process. We identify the following three classes of VOCs: Highly harmful such as benzene, vinyl chloride and 1,2 dichloroethane. They are substances that are known to be carcinogenic, mutagenic, toxic to reproduction, or very toxic by inhalation or ingestion. Emissions of these compounds must either be prevented or minimised by setting very low emission limits in permits. If a process could emit one or more highly harmful VOCs we would use the Environmental Permit to set an emission limit for each separate VOC. Class A compounds carry a lower, albeit significant risk. They are suspect carcinogens, or they contribute substantially to the creation of photochemical ozone, depletion of stratospheric ozone or global warming. They are considered as having a medium degree of harmfulness. Examples include acetaldehyde, aniline, benzyl chloride, carbon tetrachloride, chlorofluorocarbons (CFCs), ethyl acrylate, halons, maleic anhydride, 1,1,1-trichloroethane, trichloroethylene and trichlorotoluene. Class B compounds the remaining majority of VOCs are considered as having a lower degree of harmfulness. However, these VOCs are also regulated substances whose releases must be prevented or minimised. Examples include butane and ethyl acetate. We provide an index of VOCs 3, together with their classification. Industrial operators, regulators and monitoring contractors should use it to categorise VOCs. The Environmental Permit specifies the monitoring method that should be used. When an industrial operator uses a monitoring contractor to carry out the monitoring, they should provide the contractor with a copy of the relevant part of the Permit. TGN M16, Version 4, June 2012 Page 2 of 12
7 The industrial operator should tell the contractor, which VOCs are expected in the emission, so that they can plan the monitoring campaign accordingly. For class A and B VOCs it is important to know this in order to assess the emissions against the class A and B emission limit values. The operator should ensure that the contractor reports their results in the form required by the Permit. Further information is provided in Annex How do we specify the concentration of VOCs? There are three commonly used ways of reporting the concentrations of VOCs: the concentration of individual VOCs. the sum of the concentrations of specific, individual VOCs in a sample. as total organic carbon (TOC), which is the concentration of organic carbon in the gas stream. The concentrations are expressed in milligrams per cubic metre (mg/m 3 ). 6. Techniques for monitoring TOC 6.1 Flame ionisation detectors Flame ionisation detector (FID) techniques work by a gas being passed into a measurement chamber, which uses a flame to create ions from the VOCs. More specifically, FID analysers make use of the chemi-ionisation of organically bound carbon atoms in a hydrogen flame to provide measurements. The measurement cell contains a pair of electrodes, which the instrument applies a voltage between. If there are ions present in the cell, then a current can pass between the electrodes. The ionisation current measured by the FID depends upon the number of carbonhydrogen bonds of the organic compounds burning in the fuel gas flame and the ability with which these compounds ionise. The more ions present in the cell, the greater the current. As the abundance of ions within the cell depends on the concentration of the gas, then FID provides an effective means of measuring the concentrations of gases. FIDs do not differentiate between different compounds since they respond to carbonhydrogen bonds, rather than specific compounds. FIDs measure Total Organic Carbon (TOC), including methane. If methane is present in the gas stream, the measurement will be for both VOCs and methane. The main strength of the FID is that it is a useful instrument for measuring total carbon in a gas stream. As a general rule, the response of a FID is mostly influenced by the number of carbon atoms in a sample. Furthermore, FIDs only respond to gaseous or vapour phase molecules which contain carbon-hydrogen bonds. If the stack gas is relatively hot and wet, or the VOCs are concentrated, then there is a high probability of condensation when the gas sample touches a surface cooler than that in the stack. A FID for stack monitoring should, therefore, have a means of preventing condensation of either moisture or VOCs in the sample line (i.e. be equipped with a heated-line, heated detector and a heated by-pass). Where continuous or periodic sampling is carried out with an analyser close to the duct and the sampled gases are above ambient temperature, then the line (and its TGN M16, Version 4, June 2012 Page 3 of 12
8 filter) carrying the sample to the analyser must be heated to prevent condensation and reduce adsorption losses. The lines within an analyser also need to be heated, while all gas chromatographs must have heated injection ports, ovens to heat the column and detectors in heated housings. Many simple, unheated, portable FIDs have been designed for applications in health and safety screening, landfill gas monitoring, contaminated land measurements, and fugitive emissions monitoring. The simpler FIDs also usually have a wider spectrum of response factors, so their accuracy and precision will never be as great as the highly engineered, complex, heated FIDs. 6.2 Catalytic oxidation An instrument called a catalytic oxidiser is an alternative way of measuring total carbon, although the technique is not used as widely as FID. A sample of VOCs enters a combustion chamber which contains a catalyst, and within this, the carbon in the VOCs is oxidised to carbon dioxide (CO 2 ). The instrument then measures the concentration of CO 2 using an infra-red gas analyser. This method has two disadvantages. First, the catalysts can be poisoned by some components of the gas stream and secondly, the conversion of carbon to CO 2 is not always completely efficient. 6.3 Photo Ionisation Detection (PID) These work on a similar principle to FIDs, in that the sample gas is ionised. The difference is that the source of ionisation is an intense UV light and not a flame, so there is no need for support gases. They are not as suitable as FIDs for total carbon counting, especially from combustion processes. The other major differences between FIDs and PIDs are the response factors are much more variable than in FIDs and they have much weaker responses for the small saturated hydrocarbons. They are not used as CEMs because of the problems caused by the high variability of response factors and difficulties with sample conditioning. 7. Techniques for monitoring individual VOCs 7.1 Sorbent tube followed by gas chromatography (GC) separation Solid adsorbents are versatile media for collecting hundreds of types of VOCs. They work by collecting the VOC on the surface of the medium, which is usually contained within a tube. Prior to analysis, the sampled VOCs are removed by either thermal desorption or by solvent extraction. There is no universal sampling sorbent, each tends to be suited for monitoring different categories of organic compound. For example, solid absorbents such as charcoal are typically used for VOCs, while polymeric sorbents are used for semi- VOCs. Organic polymeric sorbents include porous, polymeric resins such as 2,4-diphenyl-pphenylene oxide (e.g Tenax GC) and styrene di-vinylbenzene co-polymer resins (e.g. XAD). These materials are hydrophobic, so they usually collect minimal amounts of water during the sampling. This means they can be used to sample very large samples of air, which is important if the target analytes are present in very small TGN M16, Version 4, June 2012 Page 4 of 12
9 concentrations. On the other hand, these resins are not well suited to collecting highly volatile compounds, or certain polar materials, such as alcohols and ketones. Inorganic sorbents include magnesium aluminium silicate (e.g. Florosil), silica gel and molecular sieves. These are usually very polar adsorbents, so they are efficient for collecting high-polarity compounds. However, they are also efficient at collecting water, which tends to degrade the adsorbents, thus decreasing their efficiency. Carbon sorbents have a polarity in between the inorganic and organic, polymeric adsorbent, so water is less of a problem. However, collection of water can still inhibit some sampling programmes. Carbon based sorbents have a much stronger adsorption than organic polymers, so they are very efficient for collecting a vast array of VOCs. However, VOCs, which adhere strongly to carbon sorbents are also difficult to desorb. Apart from the versatility of the technique, its strength is that it effectively immobilises the sample on the adsorbent. However, there are caveats when using solid sorbents: the media often collect VOCs other than the target analytes, so the user must employ a means of separating the different VOCs once they have been removed from the medium; each medium has a saturation point, known as the breakthrough concentration. The user must therefore know when this occurs, which in turn means having some idea of the range of concentrations in the sampled gas. This can usually only be determined reliably by pre-surveys and some experimentation; there is no universal adsorbent for all VOCs; some VOCs cannot be entirely removed from the adsorbent medium once they have adsorbed onto it. The proportion of desorbed target analyte is known as the recovery rate, and the user must know this rate for the target VOCs and the media used to collect them; to apply this method correctly for compliance monitoring against an ELV, it is essential that the species of VOCs present in the stack gas is known beforehand. If they are not known, they may be determined by using this technique to carry out semi-quantitative screening. Once the species are known it is possible for the GC-FID analysis stage to be carried out quantitatively. Another technique is to use a canister, which is coated with an inert surface, such as PTFE, to collect a volume of sample gas. This can be done by using a pump, utilising positive pressure in a stack or drawing the sample into a previously evacuated canister. Once taken, the samples can then be analysed using methods, such as GC-FID or GC-MS. The technique is suitable for emissions where the VOCs are highly concentrated and relatively free of particulates and water vapour. However, samples obtained by this technique may only be stable for a limited period and analysis is usually required within 24 hours. Many VOCs may also be selectively trapped in liquid media. The VOC sample is passed through an impinger containing a solution, after which the target VOCs are analysed. The above techniques require a sample to be extracted from a stack or duct and transported to a laboratory for subsequent analysis. For sources at ambient TGN M16, Version 4, June 2012 Page 5 of 12
10 temperature this is relatively straightforward. If the stack contains water vapour and is at an elevated temperature, condensation in the sample container may cause problems. Condensation of water vapour can be dealt with by diluting the sample with dry air or nitrogen gas. In terms of analysis, GC is a straightforward and accurate means of separating the components of even quite complex mixtures. The FID is a very useful detector because of its cost, sensitivity, wide dynamic range, reliability, response to all carbon compounds, and linearity. 7.2 Non-dispersive infrared (NDIR) detection All VOCs absorb electromagnetic radiation, while different compounds absorb energy at different frequencies. This means that VOCs have an electromagnetic finger-print which is known as a spectrum. This feature can be exploited for measurements by targeting a peak or peaks in a compound's spectrum. The diatomic bonds in VOCs absorb infrared (IR) radiation and vibrate. This fact is exploited in the simplest and most widely used spectroscopic analysers. A source of radiation generates a beam which is then focused through a cell and a filter is placed in front of the beam in order to create a single wavelength beam of IR radiation. The wavelength is selected to coincide with an exclusive absorption peak in the spectrum of the target compound. The advantage of this technique is that most VOCs absorb strongly in the IR. However, if there is a mixture of VOCs, then the spectra can overlap. Therefore, the technique is suited best to single compounds or simple mixtures where there are no interferences. For some applications, infra-red analysers are well suited to long term monitoring of VOCs. Acetylene, ethylene, ethane, propane, propylene, butene-1, butane, pentane and hexane can all be measured at concentrations below ppm. 7.3 Fourier transform infrared (FTIR) FTIR uses the same basic principle as simple infra red (IR) analysers, but resolves interfering spectra by splitting the beam into two. One beam is then bounced off a fixed mirror while the other is bounced off a moving mirror. This causes the beams to be slightly out of phase. The beams are then directed by mirrors to collide, and the resulting new spectrum creates both constructive and destructive interference in such a way that software can carry out a Fourier transform calculation to identify distinct compounds. All VOCs absorb IR radiation and most can be detected by FTIR. A typical application may include measurement of methane, ethane, ethene, propane, hexane and formaldehyde. However, analysis of a matrix containing more compounds is possible. An assessment by a competent specialist of the feasibility of a speciated VOC measurement is required, particularly if there is a need to measure a low concentration of one VOC when another VOC is present at a high concentration. Many such applications are feasible but chemically similar compounds may have similar IR spectra and can be difficult to differentiate. A technical procedure for using FTIR to carry out periodic stack emissions monitoring is provided in TGN M22 4. TGN M16, Version 4, June 2012 Page 6 of 12
11 7.4 Differential optical absorption spectrometry (DOAS) This technique was originally developed as an open-path monitor for atmospheric research. Most DOAS instruments use either UV or IR absorption to distinguish between different species. The technique can measure a selected handful of VOCs, such as benzene, toluene, ethylbenzene, xylene and formaldehyde. 7.5 Mass Spectroscopy In electron impact mass Spectrometry (MS), organic molecules are bombarded with electrons and converted to energetic, positively charged ions, which can break up into smaller ions. The charged ions are deflected by a series of either electric or magnetic fields to allow the selection of specific mass to charge species. Data is recorded in terms of either a full mass spectrum or by selected ion recording techniques. Developments in this technique have resulted in on-line instruments, which can take direct readings from stacks. The benefits of the technique are that it has very rapid response times (typically in milliseconds) and a wide dynamic range, from ppb levels to 100% concentrations. The technique can also monitor several compounds at once, although there is also the possibility of interference. Mass spectrometry is very useful for identifying unknown compounds in a mixture but is less suited to long-term routine monitoring of emissions - largely on grounds of capital cost and maintenance. 8. Hierarchy of monitoring standards A list of relevant standards for monitoring VOCs is given in the index of methods in TGN M2 5. The standards in this index are selected from the following hierarchy: Comité Européen de Normalisation ( International Standardisation Organisation ( National standards, such as US EPA ( If the VOC cannot be monitored using standards covered by the above, then a method from the following may be specified in TGN M2: Method for the Determination of Hazardous Substances ( series published by the Health and Safety Executive (HSE) National Institute of Occupational Safety and Health ( Occupational Safety and Health Administration ( TGN M16, Version 4, June 2012 Page 7 of 12
12 9. European periodic standard reference methods BS EN FID method for low concentrations of TOC CEN developed BS EN as a standard reference method (SRM) for monitoring TOC from incinerators. It has been validated as suitable for measuring low level gaseous or vapour phase TOC emissions over a range of 0-20 mg/m 3. It specifies a set of minimum performance requirements for an instrument using a FID, together with procedures for its calibration and operation. The system comprises a probe with a filter connected to a heated sample line, which is in turn connected to a heated FID. The method measures total organic carbon (TOC), which includes both VOCs and methane. BS EN FID method for high concentrations of TOC BS EN is similar to BS EN 12619, except that it has been validated for higher concentrations of TOC found in emissions from processes which use solvents. BS EN was developed for processes covered by the Solvent Directive. The standard has been validated as suitable for measuring emissions up to 500 mg/m 3, although FIDs can be used to measure higher concentrations. The method measures TOC, including methane, which includes both VOCs and methane. Combined FID standard A revised European standard that combines the above two standards, is due for publication in December The combined standard will have the reference number BS EN (the other standard will be withdrawn). BS EN charcoal tube method for individual VOCs BS EN was developed for monitoring individual VOC emissions from solvent using processes. It specifies procedures for the sampling, preparation and analysis. The VOCs are expressed as concentrations of individual species, averaged over a sampling period. The standard has been validated as suitable for use in the range from 0.5 mg/m 3 up to 2000 mg/m 3. The standard is based on the principles of sampling onto adsorption media followed by desorption and analysis by gas chromatography. However, although the standard specifies solvent desorption, thermal desorption may be acceptable if validated. There are several analytical methods which have been developed for monitoring concentrations of VOCs in workplace atmospheres (MDHS, NIOSH, OSHA). These analytical methods can be adapted for use in stack emissions monitoring. However, it is important that the sampling part of the method follows the framework given in BS EN TGN M16, Version 4, June 2012 Page 8 of 12
13 10. CEMs If a process operator s permit requires TOC to be measured continuously, the CEMs used must be MCERTS certified at the appropriate range. Information on our monitoring certification scheme is available from Measurement of methane Although methane is excluded from the definition of VOCs, it is classed as a greenhouse gas because of its potential contribution to climate change. There are two published CEN standards for measuring methane. EN ISO manual method for methane EN ISO is a manual method based on the collection of samples in an inert bag or canister, followed by analysis using gas chromatography in a laboratory. EN ISO automated method for methane EN ISO is an automated method that uses an FID fitted with a catalytic converter that removes all organic compounds in the sample gas, except methane. TGN M16, Version 4, June 2012 Page 9 of 12
14 Annex 1: Guidance on approach to measuring highly harmful, class A and class B VOCs Permits often categorise monitoring of VOCs based on three categories highly harmful, class A and B. A1.1 Highly harmful If a VOC is categorised as highly harmful it should be listed in the Permit with its emission limit value (ELV), alongside the method that is used for its measurement. The monitoring method used must measure the individual VOC. A1.2 Class A and B Permits may also require an operator to measure class A and B VOCs. Class A VOCs are medium harm VOCs, which should be listed in the Permit. These VOCs are usually but not always measured individually. Class B VOCs are low harm VOCs, which are not usually listed in the Permit. These VOCs can be measured as a total mass of organic carbon. There is no unique physical or chemical difference to distinguish class A VOCs from class B, which means there is no monitoring method available that can do this. To distinguish between class A and B VOCs, it is necessary to know the different species of VOCs that could be in the stack gas emissions, so that an appropriate monitoring approach can be applied. Firstly, an operator should assess the possible class A VOCs that could be present in the stack gas emissions. Class A VOCs are usually measured using the sampling framework provided by EN 13649, followed by analysis in an analytical laboratory. However, it is possible to measure class A VOCs using an instrumental analyser, such as FTIR, which can speciate each VOC. Typically, class B VOCs make up the majority of the VOCs in a mixed VOC stack gas emission. As class B ELVs are expressed as mass concentration of TOC, they should be measured using an FID. The FID will not distinguish between highly harmful, class A or class B VOCs but will give the combined TOC mass concentration of all three. However, this is usually acceptable because the concentrations of highly harmful and class A VOCs should be significantly lower than for class B. There are some exceptions to the above approach, for example: if there are a limited number of class B VOCs, it may be simpler to speciate them rather than use an FID. if there are a limited number of class B VOCs, which are known to have a poor response factor to an FID, it would be better to speciate them. TGN M16, Version 4, June 2012 Page 10 of 12
15 if the mass concentration of class A VOCs is significant, when compared to the mass of class B, it is better, if possible, to speciate both the class A and B VOCs, so that the class B result does not contain class A VOCs. If there is a significant proportion of methane present in the stack gas the use of FID to measure class B VOCs will not be suitable. If all or the majority of the VOCs are class A, it may be simpler to measure them using a FID, rather than speciating each individual VOC. In summary, it is important that the monitoring requirements in an Operator s Permit are discussed with the organisation responsible for the monitoring, so that a suitable measurement plan can be developed. TGN M16, Version 4, June 2012 Page 11 of 12
16 References /76/EC. Directive on the incineration of waste. Official Journal L332/91, Directive 1999/13/EC. On the limitation of emissions of volatile organic compounds due to the use of organic solvents in certain activities and installations. Official Journal L85/1, The categorisation of Volatile Organic Compounds, HMIP, Available on request from the Environment Agency (please 4. Guidance Note M22, Measuring stack gas emissions using FTIR instruments, Environment Agency (available from 5. Technical Guidance Note M2, Monitoring stack emissions to air, Environment Agency (available from 6. BS EN (1999) - Stationary source emissions - Determination of the mass concentration of total organic carbon at low concentrations in flue gases - continuous flame ionisation detector method. 7. BS EN (2001) - Stationary source emissions - Determination of the mass concentration of total gaseous organic carbon at high concentrations in flue gases - Continuous flame ionisation detector method. 8. BS EN (2002) Stationary source emissions Determination of the mass concentration of individual gaseous organic compounds Activated carbon and solvent desorption method. 9. EN ISO 25139:2011 Stationary source emissions - Manual method for the determination of the methane concentration using gas chromatography 10. EN ISO 25140:2010 Stationary source emissions - Automatic method for the determination of the methane concentration using flame ionisation detection TGN M16, Version 4, June 2012 Page 12 of 12
Technical Guidance Note (Monitoring) M16. Monitoring volatile organic compounds in stack gas emissions. Environment Agency Version 2
 Technical Guidance Note (Monitoring) M16 Monitoring volatile organic compounds in stack gas emissions Environment Agency Version 2 September 2009 Foreword Purpose This Technical Guidance Note (TGN) is
Technical Guidance Note (Monitoring) M16 Monitoring volatile organic compounds in stack gas emissions Environment Agency Version 2 September 2009 Foreword Purpose This Technical Guidance Note (TGN) is
RS DYNAMICS ECOPROBE 5. Portable IR/PID Gas Analyzer PID. PID and IR Analyzers
 RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Vapor Intrusion Sampling Options: Performance Data for Canisters, Badges, and Sorbent Tubes for VOCs
 Vapor Intrusion Sampling Options: Performance Data for s, Badges, and Sorbent Tubes for VOCs Linda S. Coyne SKC Inc., 863 Valley View Road, Eighty Four, PA 1533 George Havalias, Maria C. Echarte American
Vapor Intrusion Sampling Options: Performance Data for s, Badges, and Sorbent Tubes for VOCs Linda S. Coyne SKC Inc., 863 Valley View Road, Eighty Four, PA 1533 George Havalias, Maria C. Echarte American
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Gaseous CEMS technology
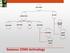 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
SERVOTOUGH SpectraScan
 SERVOTOUGH SpectraScan Continuous, real-time analysis of light hydrocarbons Jan Hordijk - Servomex Hydrocarbon stream analysis 5 major technologies used in HydroCarbon land for gas: IR (Infra Red) Laser
SERVOTOUGH SpectraScan Continuous, real-time analysis of light hydrocarbons Jan Hordijk - Servomex Hydrocarbon stream analysis 5 major technologies used in HydroCarbon land for gas: IR (Infra Red) Laser
Chromatography. Gas Chromatography
 Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
Chromatography Chromatography is essentially the separation of a mixture into its component parts for qualitative and quantitative analysis. The basis of separation is the partitioning of the analyte mixture
AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES
 JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
JPACSM 127 AUTOMATED ONLINE IDENTIFICATION AND MONITORING OF IMPURITIES IN GASES Trace Analytical Inc. Menlo Park, CA ABSTRACT GC based gas analyzers with Reduction Gas Detector (RGD) and Flame Ionization
Sampling for Gases and Vapors
 Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Sampling for Gases and Vapors EOH 466B Evaluating the Occupational Environment Spring 2008 Gases and Vapors Gases: don't exist in liquid state at STP e.g.: nitrous oxides, ozone, carbon monoxide Vapors:
Questions, Myths and Misconceptions about Using Photoionization Detectors
 Questions, Myths and Misconceptions about Using Photoionization Detectors Solvent, fuel and other VOC vapours are pervasively common in many workplace environments. Increased awareness of the toxicity
Questions, Myths and Misconceptions about Using Photoionization Detectors Solvent, fuel and other VOC vapours are pervasively common in many workplace environments. Increased awareness of the toxicity
Raw Material Finished product By product Exposure standards Length of shift Physical environment
 Measurement &Sampling Part 1 Introduction to Sampling What to sample? - Hazard Raw Material Finished product By product Exposure standards Length of shift Recognition Physical environment To Determine
Measurement &Sampling Part 1 Introduction to Sampling What to sample? - Hazard Raw Material Finished product By product Exposure standards Length of shift Recognition Physical environment To Determine
air protection technology
 Zeolite RC Zeolite rotoconcentrator Application field Ideal solution for the treatment of big air flows containing low s of polluting substances. In these project conditions, the traditional combustion
Zeolite RC Zeolite rotoconcentrator Application field Ideal solution for the treatment of big air flows containing low s of polluting substances. In these project conditions, the traditional combustion
Emission measurements according to, M1 (3 appendices)
 issued by an Accredited Testing Laboratory Contact person Ulrika Johansson 2017-01-30 6F024225-1 1 (6) Chemistry, Materials and Surfaces +46 10 516 53 22 ulrika.johansson@sp.se Accred. No. 1002 Testing
issued by an Accredited Testing Laboratory Contact person Ulrika Johansson 2017-01-30 6F024225-1 1 (6) Chemistry, Materials and Surfaces +46 10 516 53 22 ulrika.johansson@sp.se Accred. No. 1002 Testing
ANALYTICAL METHOD DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Air sampling and analysis
 DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
DETERMINATION OF VOLATILE ALDEHYDES IN AMBIENT AIR Page 1 of 11 Replaces: Dated: Author: Date: AM-No.: New New Nils Arne Jentoft 18.06.2014 0 CHANGES This procedure is new. 1 SCOPE This document describes
Chapter 3: Source Measurement Techniques
 Chapter 3 Source Measurement Techniques Measurement Methods Method 18, Measurement of Gaseous Organic Compound Emissions by Gas Chromatography Method 25, Determination of Total Gaseous Non-Methane Organic
Chapter 3 Source Measurement Techniques Measurement Methods Method 18, Measurement of Gaseous Organic Compound Emissions by Gas Chromatography Method 25, Determination of Total Gaseous Non-Methane Organic
Determination of Total Volatile Organic Compounds in Indoor Air Using Agilent 7667A mini TD and 7820A GC
 Determination of Total Volatile Organic Compounds in Indoor Air Using Agilent 77A mini TD and 70A GC Application Note Environmental Authors Tingting Bu, Xiaohua Li Agilent Technologies (Shanghai) Co.,
Determination of Total Volatile Organic Compounds in Indoor Air Using Agilent 77A mini TD and 70A GC Application Note Environmental Authors Tingting Bu, Xiaohua Li Agilent Technologies (Shanghai) Co.,
Real-Time Detection: From Gisclard et al.: A Simple Device for Air Analysis. AIHA Quarterly, 14(1):23-25 (1953)
 Real-Time Detection: 1953 From Gisclard et al.: A Simple Device for Air Analysis. AIHA Quarterly, 14(1):23-25 (1953) Sampling Gases and Vapors Gas: A state of matter characterized by very low density and
Real-Time Detection: 1953 From Gisclard et al.: A Simple Device for Air Analysis. AIHA Quarterly, 14(1):23-25 (1953) Sampling Gases and Vapors Gas: A state of matter characterized by very low density and
Sampling. Information is helpful in implementing control measures for reducing pollutant concentration to acceptable levels
 Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Chromatography & instrumentation in Organic Chemistry
 Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
Chromatography & instrumentation in Organic Chemistry What is Chromatography? Chromatography is a technique for separating mixtures into their components in order to analyze, identify, purify, and/or quantify
2B Technologies, Inc. An InDevR Company
 2B Technologies, Inc. An InDevR Company Technical Note No. 40 UV-Absorbing Interferences in Ozone Monitors Date: 22 April 2015 Author: John Birks Background Ozone measurements by absorbance of the 253.7-nm
2B Technologies, Inc. An InDevR Company Technical Note No. 40 UV-Absorbing Interferences in Ozone Monitors Date: 22 April 2015 Author: John Birks Background Ozone measurements by absorbance of the 253.7-nm
2401 Gas (liquid) Chromatography
 2401 Gas (liquid) Chromatography Chromatography Scheme Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic
2401 Gas (liquid) Chromatography Chromatography Scheme Gas chromatography - specifically gas-liquid chromatography - involves a sample being vaporized and injected onto the head of the chromatographic
VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR
 AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
AP-157 VALIDATION STUDY OF FTIR-BASED EMISSIONS MEASUREMENTS AT A MUNICIPAL WASTE COMBUSTOR Grant M. Plummer, Ph.D. Peter Zemek, Ph.D. Special Projects Manager Applications Manager Enthalpy Analytical,
MODULE 4.3 Atmospheric analysis of particulates
 MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
Exercise 9 - Petrochemicals and Climate
 113 Exercise 9 - Petrochemicals and Climate 1. The year of the first U.S. drilled oil well. c. 1859 2. Approximately, what percent of the world's remaining oil reserves are in the United States? a. 2%
113 Exercise 9 - Petrochemicals and Climate 1. The year of the first U.S. drilled oil well. c. 1859 2. Approximately, what percent of the world's remaining oil reserves are in the United States? a. 2%
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur
 VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
VALLIAMMAI ENGINEERING COLLEGE SRM Nagar, Kattankulathur 603 203 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING QUESTION BANK V SEMESTER EI6501 Analytical Instruments Regulation 2013 Academic
Application Note 116 Monitoring VOCs in Ambient Air Using Sorbent Tubes with Analysis by TD-GC/MS in Accordance with Chinese EPA Method HJ
 Application Note Monitoring VOCs in Ambient Air Using Sorbent Tubes with Analysis by TD-GC/MS in Accordance with Chinese EPA Method HJ -3 Application Note Abstract This application note demonstrates the
Application Note Monitoring VOCs in Ambient Air Using Sorbent Tubes with Analysis by TD-GC/MS in Accordance with Chinese EPA Method HJ -3 Application Note Abstract This application note demonstrates the
Monomer Analysis. Analysis by Gas Chromatography WASSON - ECE INSTRUMENTATION. Engineered Solutions, Guaranteed Results.
 Monomer Analysis Analysis by Gas Chromatography Engineered Solutions, Guaranteed Results. WASSON - ECE INSTRUMENTATION Polymer Grade Monomer Analysis Monomer Analysis Impurities in feedstocks can adversely
Monomer Analysis Analysis by Gas Chromatography Engineered Solutions, Guaranteed Results. WASSON - ECE INSTRUMENTATION Polymer Grade Monomer Analysis Monomer Analysis Impurities in feedstocks can adversely
Detection of Volatile Organic Compounds in polluted air by an Agilent mini Thermal Desorber and an Agilent 5975T LTM GC/MS
 Detection of Volatile Organic Compounds in polluted air by an Agilent mini Thermal Desorber and an Agilent 5975T LTM GC/MS Application Note Environmental Author Xiaohua Li Agilent Technologies (Shanghai)
Detection of Volatile Organic Compounds in polluted air by an Agilent mini Thermal Desorber and an Agilent 5975T LTM GC/MS Application Note Environmental Author Xiaohua Li Agilent Technologies (Shanghai)
Combustible Gas Catalytic Bead (0-100 %LEL) Part No FM Performance Certified 1,4 FM Performance Certified 1
 Sensor Data Sheet Document No. 365-2211-31 (Rev D) Combustible Gas Catalytic Bead (0-100 %LEL) Part No. 823-0211-31 FM Performance Certified 1,4 FM Performance Certified 1 Minimum Indicated Concentration...
Sensor Data Sheet Document No. 365-2211-31 (Rev D) Combustible Gas Catalytic Bead (0-100 %LEL) Part No. 823-0211-31 FM Performance Certified 1,4 FM Performance Certified 1 Minimum Indicated Concentration...
Robustness of the QAL2 calibration (EN 14181) Uncertainty on the results given by a calibrated AMS
 Robustness of the 2 calibration (EN 14181) Uncertainty on the results given by a calibrated Jean.Poulleau*¹, Cécile.Raventos 1, Philippe.Fayolle², Emmanuel.Fiani 3 ¹ INERIS, Parc technologique ALATA BP
Robustness of the 2 calibration (EN 14181) Uncertainty on the results given by a calibrated Jean.Poulleau*¹, Cécile.Raventos 1, Philippe.Fayolle², Emmanuel.Fiani 3 ¹ INERIS, Parc technologique ALATA BP
method for monitoring exposure to gasoline vapour in air revision 2002
 method for monitoring exposure to gasoline vapour in air revision 2002 Prepared by: P. E. Tindle Reviewed for CONCAWE by: A. Bianchi E. Loader C. Money G. Pizzella J. Urbanus (Technical Co-ordinator) Reproduction
method for monitoring exposure to gasoline vapour in air revision 2002 Prepared by: P. E. Tindle Reviewed for CONCAWE by: A. Bianchi E. Loader C. Money G. Pizzella J. Urbanus (Technical Co-ordinator) Reproduction
Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS
 Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS 1. List two advantages of temperature programming in GC. a) Allows separation of solutes with widely varying retention factors in a reasonable
Chem 230, Fall, 2014 Homework Set # 3 Short Answer SOLUTIONS 1. List two advantages of temperature programming in GC. a) Allows separation of solutes with widely varying retention factors in a reasonable
Test Report. Glava AS. Emission test of Ecophon Venus according to M1 classification. October / November Smedeskovvej 38, DK-8464 Galten
 Test Report Glava AS Emission test of Ecophon Venus according to M1 classification October / November 2006 Client: Glava AS Nybråtveien 2 1832 Askim Norge Date: 8 th of November 2006 Testing Laboratory:
Test Report Glava AS Emission test of Ecophon Venus according to M1 classification October / November 2006 Client: Glava AS Nybråtveien 2 1832 Askim Norge Date: 8 th of November 2006 Testing Laboratory:
Cherry Hill Tuition A Level Chemistry OCR (A) Paper 12
 herry ill Tuition A Level hemistry OR (A) Paper 12 ADVANED SUBSIDIARY GE EMISTRY A hains, Energy and Resources F322 *F318530611* andidates answer on the question paper. OR Supplied Materials: Data Sheet
herry ill Tuition A Level hemistry OR (A) Paper 12 ADVANED SUBSIDIARY GE EMISTRY A hains, Energy and Resources F322 *F318530611* andidates answer on the question paper. OR Supplied Materials: Data Sheet
Instrumental Analysis
 Instrumental Analysis Bonds and Infrared Energy Covalent bonds can absorb infrared energy, which causes the bonds to vibrate. Depending on the masses of the bonded atoms, different frequencies of radiation
Instrumental Analysis Bonds and Infrared Energy Covalent bonds can absorb infrared energy, which causes the bonds to vibrate. Depending on the masses of the bonded atoms, different frequencies of radiation
The Claviature of Gas Analysis
 The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
Chemistry Instrumental Analysis Lecture 27. Chem 4631
 Chemistry 4631 Instrumental Analysis Lecture 27 Gas Chromatography Introduction GC covers all chromatographic methods in which the mobile phase is gas. It may involve either a solid stationary phase (GSC)
Chemistry 4631 Instrumental Analysis Lecture 27 Gas Chromatography Introduction GC covers all chromatographic methods in which the mobile phase is gas. It may involve either a solid stationary phase (GSC)
Method for the determination of 1,3-butadiene
 Federation of the Employment Accidents Insurance Institutions of Germany (Hauptverband der Berufsgenossenschaften) Centre for Accident Prevention and Occupational Medicine Alte Heerstraße 111, 53757 Sankt
Federation of the Employment Accidents Insurance Institutions of Germany (Hauptverband der Berufsgenossenschaften) Centre for Accident Prevention and Occupational Medicine Alte Heerstraße 111, 53757 Sankt
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY
 Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Harris: Quantitative Chemical Analysis, Eight Edition CHAPTER 23: GAS CHROMATOGRAPHY Chapter 23. Gas Chromatography What did they eat in the year 1,000? GC of Cholesterol and other lipids extracted from
Cherry Hill Tuition A Level Chemistry OCR (A) Paper 9 THIS IS A NEW SPECIFICATION
 THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE CHEMISTRY A Chains, Energy and Resources F322 * OCE / 1 9 2 3 4* Candidates answer on the Question Paper OCR Supplied Materials: Data Sheet for Chemistry
THIS IS A NEW SPECIFICATION ADVANCED SUBSIDIARY GCE CHEMISTRY A Chains, Energy and Resources F322 * OCE / 1 9 2 3 4* Candidates answer on the Question Paper OCR Supplied Materials: Data Sheet for Chemistry
Mercury Stack Monitor SM-4
 Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV Hound Series. Dependable High Quality Air Monitoring. Accurate Readings Within Seconds. Portable UVDOAS Multi-gas Analyzers. Multi-Gas Capability
 UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
UV Hound Series Portable UVDOAS Multi-gas Analyzers Multi-Gas Capability Dependable High Quality Air Monitoring Individual VOCs PPB Sensitivity Non-Contact Optical Measurement Automated Reporting Configurable
Gas Chromatography (GC)
 Gas Chromatography (GC) Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11541 Saudi Arabia Office: AA53
Gas Chromatography (GC) Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11541 Saudi Arabia Office: AA53
AIR. Ambient, Indoor, Workplace Air and Stack Emissions Proficiency Testing Scheme. Sample Preparation Instructions Round 1
 General Instructions Ambient, Indoor, Workplace Air and Sample Instructions Round 1 Sample Storage All samples should be stored in accordance with the instructions provided on the sample labels from the
General Instructions Ambient, Indoor, Workplace Air and Sample Instructions Round 1 Sample Storage All samples should be stored in accordance with the instructions provided on the sample labels from the
DETERMINATION OF UPTAKE RATES FOR VOCs IN AMBIENT AIR BY USING AXIAL TYPE THERMAL DESORPTION PASSIVE TUBES
 DETERMINATION OF UPTAKE RATES FOR VOCs IN AMBIENT AIR BY USING AXIAL TYPE THERMAL DESORPTION PASSIVE TUBES Mihriban Yılmaz Civan, Öznur Kuntasal and Gürdal Tuncel 1 1 OUTLINE Introduction Importance and
DETERMINATION OF UPTAKE RATES FOR VOCs IN AMBIENT AIR BY USING AXIAL TYPE THERMAL DESORPTION PASSIVE TUBES Mihriban Yılmaz Civan, Öznur Kuntasal and Gürdal Tuncel 1 1 OUTLINE Introduction Importance and
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
Ozone: Earth s shield from UV radiation
 Outline Ozone: Earth s shield from UV radiation Review electromagnetic radiation absorptivity by selective gases temperature vs. height in atmosphere Ozone production and destruction natural balance anthropogenic
Outline Ozone: Earth s shield from UV radiation Review electromagnetic radiation absorptivity by selective gases temperature vs. height in atmosphere Ozone production and destruction natural balance anthropogenic
Test Report ROMA USA LLC. Product Emissions of Furniture in accordance with. Cradle to Cradle section 5.8 ECODOMUS SATIN BASE TR.
 Test Report ROMA USA LLC Product Emissions of Furniture in accordance with Cradle to Cradle section 5.8 ECODOMUS SATIN BASE TR March 2015 Client: ROMA USA LLC 554 North Avenue NW, Suite B Atlanta, GA 30318
Test Report ROMA USA LLC Product Emissions of Furniture in accordance with Cradle to Cradle section 5.8 ECODOMUS SATIN BASE TR March 2015 Client: ROMA USA LLC 554 North Avenue NW, Suite B Atlanta, GA 30318
PETE 203: Properties of oil
 PETE 203: Properties of oil Prepared by: Mr. Brosk Frya Ali Koya University, Faculty of Engineering, Petroleum Engineering Department 2013 2014 Lecture no. (2): Crude oil chemistry and composition 5. Crude
PETE 203: Properties of oil Prepared by: Mr. Brosk Frya Ali Koya University, Faculty of Engineering, Petroleum Engineering Department 2013 2014 Lecture no. (2): Crude oil chemistry and composition 5. Crude
THEORETICAL DETERMINATION OF THE SAMPLING RATES OF DIFFUSION SAMPLERS FOR VOCS AND ALDEHYDES
 THEORETICAL DETERMINATION OF THE SAMPLING RATES OF DIFFUSION SAMPLERS FOR VOCS AND ALDEHYDES J Kouzaki 1*, S Sato 1, S Nakai 1, Y Shirasuna 2, K Hirano 2 1 Graduate School of Environmental and Information
THEORETICAL DETERMINATION OF THE SAMPLING RATES OF DIFFUSION SAMPLERS FOR VOCS AND ALDEHYDES J Kouzaki 1*, S Sato 1, S Nakai 1, Y Shirasuna 2, K Hirano 2 1 Graduate School of Environmental and Information
MASS and INFRA RED SPECTROSCOPY
 MASS and INFRA RED SPECTRSCPY Mass Spectroscopy The mass spectrometer was looked at in Unit 1. It was noted there that compounds produce fragmentation patterns when passes through a mass spectrometer.
MASS and INFRA RED SPECTRSCPY Mass Spectroscopy The mass spectrometer was looked at in Unit 1. It was noted there that compounds produce fragmentation patterns when passes through a mass spectrometer.
VOCs Emissions and Structural Changes of Polypropylene During Multiple Melt Processing
 VOCs Emissions and Structural Changes of Polypropylene During Multiple Melt Processing Q. Xiang, M. Xanthos*, S. Mitra and S. H. Patel* Department of Chemical Engineering, Chemistry and Environmental Science
VOCs Emissions and Structural Changes of Polypropylene During Multiple Melt Processing Q. Xiang, M. Xanthos*, S. Mitra and S. H. Patel* Department of Chemical Engineering, Chemistry and Environmental Science
Prima PRO Process Mass Spectrometer
 APPLICATION NOTE Prima PRO Process Mass Spectrometer No. xxxx Controlling emissions by multi-component analysis of Volatile Organic Compounds (VOC) Authors: Peter Traynor, Thermo Fisher Scientific, Franklin,
APPLICATION NOTE Prima PRO Process Mass Spectrometer No. xxxx Controlling emissions by multi-component analysis of Volatile Organic Compounds (VOC) Authors: Peter Traynor, Thermo Fisher Scientific, Franklin,
Experiment 5 Reactions of Hydrocarbons
 Experiment 5 Reactions of ydrocarbons ydrocarbons are compounds that only contain carbon and hydrogen. ydrocarbons can be classified further by the type of bonds they contain. If a hydrocarbon contains
Experiment 5 Reactions of ydrocarbons ydrocarbons are compounds that only contain carbon and hydrogen. ydrocarbons can be classified further by the type of bonds they contain. If a hydrocarbon contains
Application Note 032
 Application Note 3 Analysis of Sulfur Compounds Using On-line and Off line TD GC Application Note Abstract This application note shows how Markes TD technology is compatible with trace level sulfur compounds,
Application Note 3 Analysis of Sulfur Compounds Using On-line and Off line TD GC Application Note Abstract This application note shows how Markes TD technology is compatible with trace level sulfur compounds,
High-Speed Gas and Headspace Analysis for the Process-Line and Laboratory: SIFT- MS IFPAC 2017
 High-Speed Gas and Headspace Analysis for the Process-Line and Laboratory: SIFT- MS IFPAC 2017 Y.J. Mange D.B. Milligan V.S. Langford B.J. Prince M. Perkins C. Anderson T. Wilks Who is using Syft Technologies
High-Speed Gas and Headspace Analysis for the Process-Line and Laboratory: SIFT- MS IFPAC 2017 Y.J. Mange D.B. Milligan V.S. Langford B.J. Prince M. Perkins C. Anderson T. Wilks Who is using Syft Technologies
Wednesday 16 January 2013 Morning
 Wednesday 16 January 2013 Morning AS GCE CEMISTRY A F322/01 Chains, Energy and Resources *F314440113* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A (inserted)
Wednesday 16 January 2013 Morning AS GCE CEMISTRY A F322/01 Chains, Energy and Resources *F314440113* Candidates answer on the Question Paper. OCR supplied materials: Data Sheet for Chemistry A (inserted)
ORGANIC REACTIONS 14 APRIL 2015 Section A: Summary Notes
 ORGANIC REACTIONS 14 APRIL 2015 Section A: Summary Notes 1. Combustion Alkanes are very important fossil fuels. The combustion of alkanes is very exothermic and carbon dioxide and water are produced. General
ORGANIC REACTIONS 14 APRIL 2015 Section A: Summary Notes 1. Combustion Alkanes are very important fossil fuels. The combustion of alkanes is very exothermic and carbon dioxide and water are produced. General
NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25)
 Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Gas Chromatography NON-METHANE ORGANIC CARBON ANALYZER (NMOC Method 25) The Non-Methane Organic Compounds (NMOC) Analyzer is a gas chromatograph configured for analyzing gaseous samples for total organic
Copyright 2009 PerkinElmer LAS and CARO Analytical Services, Inc.
 Optimizing Sampling and Analytical Parameters for Soil Vapour Samples using Automated Thermal Desorption / Gas Chromatography / Mass Spectrometry (ATD/GC/MS) Stephen Varisco, Technical Manager, CARO Analytical
Optimizing Sampling and Analytical Parameters for Soil Vapour Samples using Automated Thermal Desorption / Gas Chromatography / Mass Spectrometry (ATD/GC/MS) Stephen Varisco, Technical Manager, CARO Analytical
Analysis of TO-15/TO-17 air toxics in urban air using TD GC/TOF MS and automated compound identification software
 Analysis of TO-15/TO-17 air toxics in urban air using TD GC/TOF MS and automated compound identification software NEMC, Washington D.C. August 6 th 2012 Nicola Watson Environmental Specialist Markes International
Analysis of TO-15/TO-17 air toxics in urban air using TD GC/TOF MS and automated compound identification software NEMC, Washington D.C. August 6 th 2012 Nicola Watson Environmental Specialist Markes International
M1 Test Report. 1 Sample Information. 2 Evaluation of the Results. Report No A_04
 Würth International AG Aspermontstrasse 1 CH-7000 Chur Switzerland M1 Test Report Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing Date 28
Würth International AG Aspermontstrasse 1 CH-7000 Chur Switzerland M1 Test Report Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing Date 28
Catalytic bead sensors are used primarily to detect
 Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
Chapter 3 Catalytic Combustible Gas Sensors Catalytic bead sensors are used primarily to detect combustible gases. They have been in use for more than 50 years. Initially, these sensors were used for monitoring
Assessment Schedule 2015 Chemistry: Demonstrate understanding of aspects of carbon chemistry (90932)
 NCEA Level 1 Chemistry (90932) 2015 page 1 of 6 Assessment Schedule 2015 Chemistry: Demonstrate understanding of aspects of carbon chemistry (90932) Evidence Statement Q Evidence Achievement Merit Excellence
NCEA Level 1 Chemistry (90932) 2015 page 1 of 6 Assessment Schedule 2015 Chemistry: Demonstrate understanding of aspects of carbon chemistry (90932) Evidence Statement Q Evidence Achievement Merit Excellence
Fourier Transform Infrared Spectrometry Prelab Last modified: June 17, 2014
 Fourier Transform Infrared Spectrometry Prelab Recommended reading: AirUCI Lab Manual: Environmental Chemistry Text: FTIR Lab Pages: 6, 7, 13 16 on Light Absorption Pages: 175 177, 184, 185 on Molecular
Fourier Transform Infrared Spectrometry Prelab Recommended reading: AirUCI Lab Manual: Environmental Chemistry Text: FTIR Lab Pages: 6, 7, 13 16 on Light Absorption Pages: 175 177, 184, 185 on Molecular
EMICODE Test Report. 1 Sample Information. 2 Evaluation of the Results. Report No A_04
 Sika Technology AG Tüffenwies 16 8048 Zürich SWITZERLAND EMICODE Test Report 1 Sample Information Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing
Sika Technology AG Tüffenwies 16 8048 Zürich SWITZERLAND EMICODE Test Report 1 Sample Information Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing
Use the three tables of spectral data on the Data Sheet where appropriate.
 Q1. Organic chemists use a variety of methods to identify unknown compounds. When the molecular formula of a compound is known, spectroscopic and other analytical techniques are used to distinguish between
Q1. Organic chemists use a variety of methods to identify unknown compounds. When the molecular formula of a compound is known, spectroscopic and other analytical techniques are used to distinguish between
Test Report- VOC emission regulations in Europe
 Protox Fabriksvej 19 6000 Kolding DENMARK Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing Date 13 August 2015 Test Report- VOC emission regulations
Protox Fabriksvej 19 6000 Kolding DENMARK Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing Date 13 August 2015 Test Report- VOC emission regulations
Advanced Pharmaceutical Analysis
 Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Lecture 2 Advanced Pharmaceutical Analysis IR spectroscopy Dr. Baraa Ramzi Infrared Spectroscopy It is a powerful tool for identifying pure organic and inorganic compounds. Every molecular compound has
Test Report- VOC emission regulations in Europe
 Protox Aps Fabriksvej 19 6000 Kolding Denmark Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing Date 14 November 2014 Test Report- VOC emission
Protox Aps Fabriksvej 19 6000 Kolding Denmark Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing Date 14 November 2014 Test Report- VOC emission
Introduction to Pharmaceutical Chemical Analysis
 Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
Introduction to Pharmaceutical Chemical Analysis Hansen, Steen ISBN-13: 9780470661222 Table of Contents Preface xv 1 Introduction to Pharmaceutical Analysis 1 1.1 Applications and Definitions 1 1.2 The
Test Report. Tremco-Illbruck GmbH & Co KG. Product Emissions Test (AgBB/DIBt Test Protocol) Butyl und Bitumenprimer. June 2011
 Test Report Tremco-Illbruck GmbH & Co KG Product Emissions Test (AgBB/DIBt Test Protocol) Butyl und Bitumenprimer June 2011 Client: Tremco-Illbruck GmbH & Co KG Von Der Wettern Str. 27 51149 Köln Germany
Test Report Tremco-Illbruck GmbH & Co KG Product Emissions Test (AgBB/DIBt Test Protocol) Butyl und Bitumenprimer June 2011 Client: Tremco-Illbruck GmbH & Co KG Von Der Wettern Str. 27 51149 Köln Germany
Choosing the best detection technologies for measuring combustible gas and VOC vapors
 AP 11: Choosing the best detection technologies for measuring combustible gas and VOC vapors gas an oxygen sensor responds to is oxygen. Electrochemical sensors designed to measure a particular gas may
AP 11: Choosing the best detection technologies for measuring combustible gas and VOC vapors gas an oxygen sensor responds to is oxygen. Electrochemical sensors designed to measure a particular gas may
C (s) + O 2 (g) CO 2 (g) S (s) + O 2 (g) SO 2 (g)
 Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
Combustion The rapid combination of oxygen with a substance. A major type of chemical reaction. When elemental carbon or carbon-containing compounds burn in air, oxygen combines with the carbon to form
GC Instruments. GC Instruments - Sample Introduction
 GC Instruments 1 Fairly simple instrumentation Maintaining constant average pressure is important! Pressure controls flow rate T influences retention (k ) Flow rate monitoring Changing flow rate changes
GC Instruments 1 Fairly simple instrumentation Maintaining constant average pressure is important! Pressure controls flow rate T influences retention (k ) Flow rate monitoring Changing flow rate changes
Test Report- VOC emission regulations in Europe
 L Isolante K-flex Srl Via Leonardi da Vinci 36 20877 Roncello (MB) Italy Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing Date 29 August 2013
L Isolante K-flex Srl Via Leonardi da Vinci 36 20877 Roncello (MB) Italy Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing Date 29 August 2013
Quality Assurance of Automated Measuring Systems: practical implementation of pren 14181:2001. Results of field experience at two Italian plants.
 Quality Assurance of Automated Measuring Systems: practical implementation of pren 14181:2001. Results of field experience at two Italian plants. Cipriano D. (1), Ferrari A. (2), Negri A. (1) (1) CESI
Quality Assurance of Automated Measuring Systems: practical implementation of pren 14181:2001. Results of field experience at two Italian plants. Cipriano D. (1), Ferrari A. (2), Negri A. (1) (1) CESI
Alternative Monitoring Technologies: A Brief Overview
 Alternative Monitoring Technologies: A Brief Overview a technical solution to meet every need Cemtek Environmental Inc. 3041 S. Orange Ave. Santa Ana, CA 92707 800-400-0200 www.cemteks.com 1 What s New?
Alternative Monitoring Technologies: A Brief Overview a technical solution to meet every need Cemtek Environmental Inc. 3041 S. Orange Ave. Santa Ana, CA 92707 800-400-0200 www.cemteks.com 1 What s New?
Analytical techniques: Environmental samples. Lecture 2 Universidade do Algarve
 Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
Analytical techniques: Environmental samples Lecture 2 Universidade do Algarve Terms, definitions & applications Difference between technique and method: Analytical technique: Fundamental scientific application
Reprinted from February Hydrocarbon
 February2012 When speed matters Loek van Eijck, Yokogawa, The Netherlands, questions whether rapid analysis of gases and liquids can be better achieved through use of a gas chromatograph or near infrared
February2012 When speed matters Loek van Eijck, Yokogawa, The Netherlands, questions whether rapid analysis of gases and liquids can be better achieved through use of a gas chromatograph or near infrared
Appendix: Laboratory Testing Methods
 Appendix: Laboratory Testing Methods A.1 Heavy Metals Testing Based on Method 200.8 (USEPA 1994), nitric and hydrochloric acid digestion was carried out to extract total recoverable heavy metals from the
Appendix: Laboratory Testing Methods A.1 Heavy Metals Testing Based on Method 200.8 (USEPA 1994), nitric and hydrochloric acid digestion was carried out to extract total recoverable heavy metals from the
Test Report- VOC emission regulations in Europe
 CONICA AG Industriestr. 26 CH-8207 Schauffhausen Switzerland Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing Date 31 July 2014 Test Report-
CONICA AG Industriestr. 26 CH-8207 Schauffhausen Switzerland Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark voc@eurofins.com www.eurofins.com/voc-testing Date 31 July 2014 Test Report-
Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each.
 Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each. 1. A. ammonia D. HFCs B. CFCs E. NONE of these
Part I Short Answer Choose a letter to fill in the blanks. Use choices as many times as you wish. Only one choice is needed per blank. All are 3 points each. 1. A. ammonia D. HFCs B. CFCs E. NONE of these
Balancing chemical reaction equations (stoichiometry)
 Balancing chemical reaction equations (stoichiometry) This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit
Balancing chemical reaction equations (stoichiometry) This worksheet and all related files are licensed under the Creative Commons Attribution License, version 1.0. To view a copy of this license, visit
Understanding catalytic LEL combustible gas sensor performance
 : Understanding catalytic LEL combustible gas sensor performance These four conditions are frequently diagrammed as the "Fire Tetrahedron". If any side of the tetrahedron is missing, incomplete or insubstantial;
: Understanding catalytic LEL combustible gas sensor performance These four conditions are frequently diagrammed as the "Fire Tetrahedron". If any side of the tetrahedron is missing, incomplete or insubstantial;
Rosemary extract liquid
 EUROPEAN COMMISSION DIRECTORATE-GENERAL JOINT RESEARCH CENTRE Institute for Reference Materials and Measurements Community Reference Laboratory - Feed Additives Authorisation Evaluation Report of the Community
EUROPEAN COMMISSION DIRECTORATE-GENERAL JOINT RESEARCH CENTRE Institute for Reference Materials and Measurements Community Reference Laboratory - Feed Additives Authorisation Evaluation Report of the Community
Organic Compounds - Formation Fate and Impact on Troposphere
 Organic Compounds - Formation Fate and Impact on Troposphere i.gensch@fz-juelich.de 2 / 20 Organic Compounds - Formation Fate and Impact on Troposphere i.gensch@fz-juelich.de 2 / 20 Definitions VOC: organic
Organic Compounds - Formation Fate and Impact on Troposphere i.gensch@fz-juelich.de 2 / 20 Organic Compounds - Formation Fate and Impact on Troposphere i.gensch@fz-juelich.de 2 / 20 Definitions VOC: organic
Methods of pollution control and waste management - laboratory. Adsorptive removal of volatile organic compounds from gases streams
 Methods of pollution control and waste management - laboratory Adsorptive removal of volatile organic compounds from gases streams Manual for experiment 17 dr Hanna Wilczura-Wachnik and dr inż. Jadwiga
Methods of pollution control and waste management - laboratory Adsorptive removal of volatile organic compounds from gases streams Manual for experiment 17 dr Hanna Wilczura-Wachnik and dr inż. Jadwiga
HYDROCARBONS. Section A
 MCQs Section A Q1The products obtained by cracking an alkane, X, are methane, ethene and propene. The mole fraction of ethene in the products is 0.5. What is the identity of X? A C6H14 B C8H18 C C9H20
MCQs Section A Q1The products obtained by cracking an alkane, X, are methane, ethene and propene. The mole fraction of ethene in the products is 0.5. What is the identity of X? A C6H14 B C8H18 C C9H20
Chromatography. writing in color
 Chromatography writing in color Outlines of Lecture Chromatographic analysis» Principles and theory.» Definition.» Mechanism.» Types of chromatography.» Uses of Chromatography. In 1906 Mikhail Tswett used
Chromatography writing in color Outlines of Lecture Chromatographic analysis» Principles and theory.» Definition.» Mechanism.» Types of chromatography.» Uses of Chromatography. In 1906 Mikhail Tswett used
The Analysis of Trace Contaminants in High Purity Ethylene and Propylene Using GC/MS. Application. Agilent Technologies/Wasson ECE Monomer Analyzer
 The Analysis of Trace Contaminants in High Purity Ethylene and Propylene Using GC/MS Agilent Technologies/Wasson ECE Monomer Analyzer Application Authors Fred Feyerherm 119 Forest Cove Dr. Kingwood, TX
The Analysis of Trace Contaminants in High Purity Ethylene and Propylene Using GC/MS Agilent Technologies/Wasson ECE Monomer Analyzer Application Authors Fred Feyerherm 119 Forest Cove Dr. Kingwood, TX
VOC TEST REPORT M1. 23 October Regulation or protocol Conclusion Version of regulation or protocol
 Würth Oy Würthintie 1 11710 Riihimäki Finland Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark VOC@eurofins.com www.eurofins.com/voc-testing VOC TEST REPORT M1 23 October 2017 1 Sample
Würth Oy Würthintie 1 11710 Riihimäki Finland Eurofins Product Testing A/S Smedeskovvej 38 8464 Galten Denmark VOC@eurofins.com www.eurofins.com/voc-testing VOC TEST REPORT M1 23 October 2017 1 Sample
Definition: A hydrocarbon is an organic compound which consists entirely of hydrogen and carbon.
 Hydrocarbons Definition: A hydrocarbon is an organic compound which consists entirely of hydrogen and carbon. It is important to note that carbon atoms have 4 free bonds and that hydrogen has 1 free bond.
Hydrocarbons Definition: A hydrocarbon is an organic compound which consists entirely of hydrogen and carbon. It is important to note that carbon atoms have 4 free bonds and that hydrogen has 1 free bond.
10/27/10. Chapter 27. Injector typically 50 C hotter than oven
 Sample and solvent are vaporized onto the head of a column Vaporized solvent and solute are carried through the column by an inert gas (mobile phase) The mobile phase does not interact with compounds of
Sample and solvent are vaporized onto the head of a column Vaporized solvent and solute are carried through the column by an inert gas (mobile phase) The mobile phase does not interact with compounds of
HYDROCARBONS, AROMATIC 1501
 HYDROCARBONS, AROMATIC 1501 FORMULA: Table 1 MW: Table 1 CAS: Table 1 RTECS: Table 1 METHOD: 1501, Issue 3 EVALUATION: Full Issue 1: 15 August 1990 Issue 3: 15 March 2003 OSHA : Table 2 NIOSH: Table 2
HYDROCARBONS, AROMATIC 1501 FORMULA: Table 1 MW: Table 1 CAS: Table 1 RTECS: Table 1 METHOD: 1501, Issue 3 EVALUATION: Full Issue 1: 15 August 1990 Issue 3: 15 March 2003 OSHA : Table 2 NIOSH: Table 2
Test Report. Muovihaka Oy. Product Emissions in accordance with the Swedish Building Material Assessment Ultima graphite.
 Test Report Muovihaka Oy Product Emissions in accordance with the Swedish Building Material Assessment July 2012 Client: Muovihaka Oy Kuusimäentie 12 01900 Nurmijärvi Finland Date: 26 July 2012 Testing
Test Report Muovihaka Oy Product Emissions in accordance with the Swedish Building Material Assessment July 2012 Client: Muovihaka Oy Kuusimäentie 12 01900 Nurmijärvi Finland Date: 26 July 2012 Testing
NIST gas standards containing volatile organic compounds in support of ambient air pollution measurements
 Air Pollution XVI 357 NIST gas standards containing volatile organic compounds in support of ambient air pollution measurements G. C. Rhoderick Analytical Chemistry Division, Chemical Science and Technology
Air Pollution XVI 357 NIST gas standards containing volatile organic compounds in support of ambient air pollution measurements G. C. Rhoderick Analytical Chemistry Division, Chemical Science and Technology
GAW Expert Workshop VOC, C. Plass-Dülmer et al., FEHp-B 1
 ACTRIS-GAW: Uncertainty Evaluation in VOC Analysis Draft VOC Measurement Guidelines C. Plass-Duelmer, A. Werner, S. Reimann, S. Sauvage, R. Steinbrecher and the ACTRIS VOC workshop participants comparable
ACTRIS-GAW: Uncertainty Evaluation in VOC Analysis Draft VOC Measurement Guidelines C. Plass-Duelmer, A. Werner, S. Reimann, S. Sauvage, R. Steinbrecher and the ACTRIS VOC workshop participants comparable
