ROMP Reactivity of endo- and exo-dicyclopentadiene
|
|
|
- Brianna Stokes
- 6 years ago
- Views:
Transcription
1 7878 Macromolecules 2002, 35, ROMP Reactivity of endo- and exo-dicyclopentadiene Joseph D. Rule and Jeffrey S. Moore* The Beckman Institute for Advanced Science and Technology and Department of Chemistry, University of Illinois, Urbana, Illinois Received June 17, 2002; Revised Manuscript Received August 6, 2002 ABSTRACT: The activation parameters for the ring-opening metathesis polymerization (ROMP) of endo- (1) and exo-dicyclopentadiene (2), endo-1,2-dihydrodicylopentadiene (3), and norbornene (4) in the presence of Grubbs catalyst were determined using in situ NMR. The exo isomer of DCP was found to be more than an order of magnitude more reactive than the endo isomer. endo-dcp was found to have reactivity similar to its partially saturated counterpart 3, suggesting that the cause of the rate difference between the two isomers of DCP is primarily steric in nature. This interaction is shown to be predominantly entropic and is suspected to originate from an interaction of the penultimate repeat unit and the incoming monomer. Additionally, the alkylidene generated during the polymerization of endo-dcp was found to form an intramolecular complex, but this complex only affects the rate slightly. Introduction In the past few decades, dicyclopentadiene (DCP) has received significant attention as a monomer for ring opening metathesis polymerization (ROMP). Its low cost, high reactivity, and tendency to form tough, highly cross-linked materials have made it a good choice for reaction injection molding (RIM). 1 In the RIM process, the kinetics of the polymerization are critical and, in general, must be very fast. ROMP of DCP was also used in our recently developed self-healing composite material. 2 In this application, as in RIM, cure kinetics play a key role and fast cure times are desirable. DCP can exist as an endo (1) or an exo (2) isomer. Figure 1. (a) Chelating interaction corresponding to mechanism I. (b) Intramolecular complex corresponding to mechanism II. (c) Steric interaction corresponding to mechanism III. (d) Steric interaction corresponding to mechanism IV. Because commercially available DCP is >95% endo, most of the applications of DCP involve the endo isomer. Previous studies on the ROMP of other norbornene derivatives have shown that exo isomers often react faster than the corresponding endo isomers. 3-8 No direct examinations of the difference in ROMP kinetics of endo- and exo-dcp have previously been reported with a Ru-based catalyst, but a few studies suggest that exo- DCP reacts differently than endo-dcp Consequently, it seemed likely that exo-dcp would have higher ROMP rates than endo-dcp using Grubbs catalyst, and a deeper understanding of this behavior could prove valuable in both the RIM process and the generation of new self-healing materials. We report the use of in situ NMR to quantify the ROMP kinetics for exo- and endo-dcp using Grubbs catalyst (RuCl 2 (CHPh)(PCy 3 ) 2 ). Previous reports have provided speculative reasons for the differences in reactivity of exo and endo isomers of norbornene derivatives. 6,7,11 In this work, we examine this issue more deeply by systematically studying reactivity differences between exo- and endo-dcp and analyze this in the context of mechanistic models. This study involves adapting previously proposed mechanisms to the ROMP of DCP and proposing modifications that could potentially account for the different ROMP rates of the two isomers of DCP. In proposing these mechanisms, it is assumed that the strained norbornene-derived double bond in the bicyclic ring is much more reactive than the cyclopentene-derived double bond in the DCP monomers. 11 It is also assumed that the catalyst attacks the less hindered exo face of the monomers. Mechanism I. The bent shape of endo-dcp could facilitate a chelating interaction to form an intermolecular complex between free monomer and the alkylidene. This could inhibit the catalyst and slow the rate (Figure 1a). 7,11 Mechanism II. A related intramolecular effect could be imagined involving the propagating alkylidene. The geometry of the alkylidene formed from endo-dcp could permit coordination between the ruthenium atom and the double bond in the neighboring substituted cyclo /ma CCC: $ American Chemical Society Published on Web 09/06/2002
2 Macromolecules, Vol. 35, No. 21, 2002 ROMP Reactivity of Dicyclopentadienes 7879 pentene ring (Figure 1b). 6 This intramolecular complex could prevent free monomer from coordinating with the catalyst and thus slow the polymerization. Mechanism III. It has been suggested that the formation of the metallacyclobutane ring may be the rate-determining step in metathesis with first generation Grubbs catalysts. 12,13 In this case, as the metallacyclobutane is formed, an unfavorable steric interaction may exist between the protons on the newly formed sp 3 center and the cyclopentene ring (Figure 1c). 7 Mechanism IV. To give the catalyst s bulky tricyclohexylphosphine (PCy 3 ) group as much space as possible, the propagating species may favor a conformation that places the available coordination site of the ruthenium center over the terminal unit s cyclopentane ring. In this case, there is an unfavorable interaction between the polymer chain s penultimate unit and the endo ring of an approaching monomer (Figure 1d). Mechanism V. The alkylidene formed during the polymerization of endo-dcp has all the substituents of the cyclopentane ring in a cis relationship. This creates a rather congested area that could hinder the approach of free monomer. 7 By gaining a more complete understanding of factors that affect ROMP kinetics in the DCP system, the foundation is laid for being able to rationally design new monomers to have desired kinetic properties. Figure 2. Eyring plots for norbornene ([), exo-dcp (b), 3 (0), and endo-dcp (9). The reactions were performed in toluene-d 8. [Ru] ) 4 mm and [PCy 3] ) 4 mm. monomer Table 1. ROMP Activation Parameters k obs a (s -1 ) H (kj mol -1 ) S (J mol -1 K -1 ) G b (kj mol -1 ) endo-dcp exo-dcp norbornene a 20 C in d-8 toluene with 4 mm catalyst and 4 mm Pcy 3. b At 20 C. form of the catalyst and the active four-coordinate form (eq 1). Experimental Section 1 H NMR experiments were performed on a Varian UNITY INOVA 500NB instrument, and 31 P experiments were performed on a Varian UNITY 500 instrument. Grubbs catalyst (Strem), mesitylene (Aldrich), PCy 3 (Aldrich), and toluene-d 8 (Aldrich) were used as received from the suppliers. exo-dcp was synthesized according to the procedure described by Nelson and Kuo. 14 GC showed it to contain 1% endo-dcp. 3 was prepared through a Diels-Alder reaction of cyclopentene and cyclopentadiene via the literature procedure. 15 endo-dcp (Acros), norbornene (Aldrich), and the other monomers were distilled and degassed (consecutive freeze-pump-thaw cycles) before use. For each series of kinetics experiments, a stock solution of Grubbs catalyst, PCy 3, and mesitylene (as an internal standard) in toluene-d 8 was prepared in a glovebox. Then, 0.70 g of this stock solution was transferred to each NMR tube. The tubes were capped with septa and wrapped with Parafilm. All samples were used within a few hours of preparation. For individual kinetics experiments, the instrument and the tube (with only the stock solution) were brought to the desired temperature. Monomer was then added to the tube via syringe (either 50 or 20 µl). The sample was shaken and immediately reinserted into the instrument. Spectra were taken at regular intervals, and the sample was left in the instrument at constant temperature until completion of the experiment. The monomer concentration was monitored by comparing the signal from the protons attached to the strained double bond in the monomers ( ppm) to the signal from the ring protons of the mesitylene internal standard (6.67 ppm). Results and Discussion The kinetics of ROMP of endo- and exo-dcp, the endo- 1,2-dihydro derivative 3, and norbornene were quantified using in situ 1 H NMR. Preliminary experiments gave complex saturation kinetics that were difficult to analyze. Grubbs and co-workers have shown 12,13 that adding PCy 3 to similar metathesis reactions forces a preequilibrium between the inactive five-coordinate The establishment of this preequilibrium produces much simpler kinetic behavior that can usually be approximated by eq 2. 12,13 - d[monomer] dt ) k [Ru] 0 [monomer] [PCy 3 ] 0 (2) Following this, 1 equiv of PCy 3 with respect to the catalyst was added to the samples, and the resulting kinetics showed a first-order dependence on monomer. The reaction rates were then measured at several temperatures, and an Eyring plot (Figure 2) of the results was used to determine the activation parameters (Table 1). It was observed that exo-dcp was nearly 20 times more reactive than endo-dcp at 20 C. As a comparison, the kinetics of neat ROMP (instead of solution ROMP) of the two isomers of DCP were qualitatively examined. In the presence of 0.2 wt % Grubbs catalyst, exo-dcp was found to gel in less than 1 min. When a similar experiment was performed with endo-dcp, the monomer required more than 2htogel. This shows that the large reactivity difference of endo- DCP and exo-dcp revealed in the solution polymerization is qualitatively observed in the neat polymerization as well. Also, the activation enthalpy we report for solution ROMP of endo-dcp is nearly identical to the activation energy reported for neat ROMP of endo-dcp using the same catalyst. 16 On the basis of this, it appears that many of the trends seen in solution ROMP of DCP also apply in neat ROMP. Insight into the reactivity difference between the two DCP isomers can be gained from the energies of activation listed in Table 1. exo-dcp and norbornene have very similar reactivities, suggesting that the cyclopen-
3 7880 Rule and Moore Macromolecules, Vol. 35, No. 21, 2002 Figure 3. Competition experiments in which 1 equiv of exo- DCP (b) was added to a solution of catalyst and (a) endo-dcp ([) or(b)3 (9) after 10 min. As a reference, a plot of the homopolymerization of exo-dcp (O) is included as well. The reactions were conducted in toluene-d 8 at 20 C. [Ru] ) 4 mm, and [PCy 3] ) 4 mm. tene ring of exo-dcp does not significantly affect ROMP. Also, the free energies of activation show that endo-dcp is only slightly less reactive than 3. Because 3 has only one double bond, mechanisms I and II (which involve coordination intermediates) cannot apply. If it is assumed that the steric effects in endo-dcp are essentially the same as those in 3, then the rate depression of endo- DCP must be primarily steric in nature. Because the difference in reactivity of endo-dcp and exo-dcp is due to steric interactions, the steric dominated mechanisms III, IV, and V were more closely examined. These mechanisms can be subdivided into two classes. Mechanisms III and IV depend on the shape of the monomer that is approaching the catalyst. Mechanism V does not depend on the monomer that approaches the catalyst, but it depends on the configuration of the ring to which the catalyst is attached. Because mechanism V depends on the ring to which the catalyst is attached instead of the approaching monomer, it would be expected that ROMP of a mixture of endo and exo isomers would consume both monomers indiscriminately. Therefore, the ROMP rates for both components of the reaction mixture would be almost equal (as opposed to the homopolymerizations that have very different rates). This effect has been observed previously in cases where the reactivity is governed by the propagating center rather than the approaching monomer. 17 In contrast, mechanisms III and IV depend principally on the geometry of the approaching monomer, so the unhindered monomer would be expected to react significantly faster than the hindered monomer. In this case, the rates for each isomer in a mixture of exo and endo monomers would be expected to be similar to the rates seen in the homopolymerization. On the basis of this reasoning, competition experiments (Figure 3) were performed to distinguish mechanisms III and IV from mechanism V. Polymerization of endo-dcp or 3 was initiated, and the initial reaction rate was monitored. After 10 min, exo-dcp was added to the solution and changes in rate were noted. Figure 3a shows that the polymerization of endo-dcp accelerated only slightly after the addition of exo-dcp. This small change in rate between the homopolymerization and the copolymerization of endo-dcp suggests that an interaction specific to the propagating catalyst s configuration (like mechanism V) may be contributing, but only to a small degree. A similar acceleration is seen in the competition experiment involving 3 (Figure 3b). The fact that endo-dcp and 3 behave similarly in these competition experiments suggests that this effect is due to sterics and indicates that the terminal unit s stereochemistry does contribute, at least in a minor way, to the reactivity (mechanism V). However, the fact that the endo isomers still react significantly slower than exo- DCP in the mixture suggests that the consequences of mechanism V are small. Rather, the predominant effect is monomer specific, as in mechanism III or IV. The activation parameters (Table 1) can be used to determine whether mechanism III or mechanism IV is more significant. Mechanism III generates strain in the developing bonds, so it is largely enthalpic in nature and would be expected to correspond to higher activation enthalpy for endo-dcp and 3. Clearly this is not the case, and in fact, the activation enthalpy for endo-dcp is lower than that of the other monomers. Mechanism IV is more consistent with the activation parameters. Mechanism IV allows a reaction to occur only when the alkylidene is in a conformation that maximizes the distance between the ruthenium center and the other substituents of the cyclopentane ring. Because mechanism IV requires a specific conformation for reaction to occur, its consequences are manifested in activation entropy rather than activation enthalpy. Thus, theoretically mechanism IV would involve lower activation entropy for both endo-dcp and 3, and Table 1 shows that this is the trend that is seen experimentally. Therefore, because mechanism IV is more consistent with the measured activation parameters and the similarity in reactivity between endo-dcp and 3, it appears that interactions between the penultimate repeat unit and the incoming monomer are the primary cause of the reactivity difference between the two DCP isomers. However, the steric effects cannot completely account for the 14 kj mol -1 difference in activation enthalpy and the 50 J mol -1 K -1 difference in activation entropy between endo-dcp and 3 shown in Table 1. Assuming that endo-dcp and 3 are sterically equivalent, this difference in activation parameters could result from an electronic effect like mechanism I or II. Coincidentally, endo-dcp s activation enthalpy and activation entropy compensate one another to give a free energy of activation that is nearly equal to that of 3 at 20 C. Therefore, any electronic effect that changes the activation enthalpy and entropy of ROMP of endo-dcp does not significantly affect the overall reactivity of endo-dcp near ambient temperature. Although this electronic effect only slightly changes the rate, we investigated it further to determine whether it accounts for the unusual activation parameters of endo-dcp and whether it affected the reaction in other ways. It is clear that if an electronic coordination effect like mechanism I or II is present to a significant degree, the PCy 3 that is being displaced must accumulate in solu-
4 Macromolecules, Vol. 35, No. 21, 2002 ROMP Reactivity of Dicyclopentadienes 7881 different. To examine this interesting difference in dependence on PCy 3, the theoretical effects of mechanism II were compared to the observed dependences on phosphine and catalyst. With mechanism II, there are no longer only two forms of the catalyst as described by eq 1, but there are three forms, and each of the species should exist in equilibrium (eq 3). Only the fourcoordinate catalyst species is ROMP active, so the rate should depend on the concentration of this four coordinate species at equilibrium. On the basis of eq 3 and the fact that the total concentration of ruthenium and PCy 3 in the system must remain constant, the steadystate concentration of the active species at equilibrium can be calculated. This leads to the rate expression Figure 4. Rate dependence on (a) catalyst concentration and (b) added PCy 3. The observed data for exo-dcp ([) were taken at 15 C and are shown with a linear fit. The observed data for endo-dcp (9) were taken at 40 C and fit using eq 4. In part a, 8 mm of PCy 3 was added, and in part b, 8 mm of catalyst was added. tion. To test for this, 31 P NMR spectra were taken of endo-dcp and 3 during ROMP. The PCy 3 peak (10.5 ppm) was absent before the monomer was added, but clearly appeared during the polymerization of endo- DCP. Significantly, it was absent throughout the polymerization of 3. This confirms that some type of coordination is successfully competing with the coordination with PCy 3, but only in the case where a second double bond (as in endo-dcp) is present. Evidence for this type of interaction is also seen in the competition experiments discussed above. In the presence of endo-dcp, the polymerization of exo-dcp decelerates slightly (Figure 3a). However, the polymerization of exo-dcp does not decelerate noticeably in the presence of 3 (Figure 3b). This is consistent with interactions like mechanisms I and II that lower the concentration of active catalyst by generating a coordinated, inactive form of the catalyst. Because there is less active catalyst in solution, the polymerization of exo- DCP is slowed. There is also evidence to suggest a contribution from mechanism II, which appears to be more likely than mechanism I. Previous reports have confirmed the formation of intramolecular complexes involving the coordination of a ruthenium atom and a nearby double bond to form a five-membered ring. 18,19 These reported systems did not involve norbornene derivatives, but the proposed intramolecular complex in Figure 1b is very similar. The rates dependence on catalyst and PCy 3 (Figure 4) also suggest that mechanism II affects the reaction. As expected, the rate of ROMP of exo-dcp is directly proportional to both the catalyst concentration and the reciprocal of the phosphine concentration as predicted by eq 2. However, endo-dcp shows much more complex behavior, and its dependence on PCy 3 is dramatically -d[endo-dcpd] dt ) b(-[pcy 3 ] 0 - c + [PCy 3 ] c[PCy 3 ] 0 + 4c[Ru] 0 + c 2 )[endo-dcpd] (4) where b ) k 3 /[2(K 2-1)], c ) K 1 (K 2-1), and k 3 is the second-order rate constant describing the addition of monomer to the active catalyst. Stoichiometric concentrations [PCy 3 ] 0 and [Ru] 0 represent the amounts of PCy 3 and catalyst that were added to the solution, and they do not represent steady-state concentrations. This equation was used to fit the endo-dcp data in Figure 4, and it was found to correspond very well to the observed dependence on phosphine and catalyst. This provides even more support to the hypothesis that the intramolecular complex in Figure 1b is formed. Mechanism II is also consistent with the lower activation entropy of endo-dcp relative to the other monomers. The dissociation of PCy 3 shown in eq 1 generates two free molecules and would be expected to involve a significant increase in translational entropy. This step occurs before the rate-determining step of the reaction (which must be during or after the coordination of monomer), so it would translate to relatively high activation entropy. The complexes involved in mechanisms I and II would also have to dissociate for reaction to occur. Like the dissociation of phosphine, the dissociation of the complex involved in mechanism I (Figure 1a) would give two molecules and a large increase in entropy. However, because the complex involved in mechanism II (Figure 1b) is intramolecular, it dissociates into only one molecule, and no translational entropy would be gained. Consequently, this step results in a lower activation entropy. Thus, the 50 J mol -1 K -1 difference in activation entropy between endo-dcp and 3 is consistent with the intramolecular complex shown in Figure 1b, but it is not consistent with the interaction shown in Figure 1a. The 14 kj mol -1 difference in activation enthalpy for these two monomers could correspond to the bond energy between PCy 3 and ruthenium being greater than that of the pi-coordination of the olefin and ruthenium that is depicted in Figure 1b. Conclusion It has been shown that the ROMP of exo-dcp is much faster than that of endo-dcp both in bulk and in
5 7882 Rule and Moore Macromolecules, Vol. 35, No. 21, 2002 solution. Steric interactions appear to be the primary cause of this reactivity difference. It was shown that this steric effect must result from an interaction specific to the approaching monomer, and it is mostly entropic in nature. This is most consistent with an interaction between the endo substituents of the approaching monomer and the substituents of the cyclopentane ring to which the propagating center is bound. The ROMP of endo-dcp also appears to involve the formation of an intramolecular complex between the ruthenium center and the adjacent cyclopentenyl double bond. While this interaction affects the rate only slightly at ambient temperatures, it significantly affects the reaction s dependence on temperature and added PCy 3.By understanding the origin of the rate differences in norbornene based systems like the one studied here, monomers or catalysts can be specifically designed to tune the rates. This, in turn, creates the possibility to refine applications like RIM in which kinetic control is critical. Acknowledgment. We are grateful to the Fannie and John Hertz Foundation and the Air Force Office of Scientific Research (Grant No. F ) for financial support. We are also grateful to Scott White, Josh Orlicki, Vera Mainz, Larry Markoski, and Stefan Kraft for technical support and fruitful discussions. Supporting Information Available: Figure containing 31 P NMR spectra showing the presence of free PCy 3 during the ROMP of endo-dcp. This material is available free of charge via the Internet at References and Notes (1) Matejka, L.; Houtman, C.; Macosko, C. W. J. Appl. Polym. Sci. 1985, 30, (2) White, S. R.; Sottos, N. R.; Geubelle, P. H.; Moore, J. S.; Kessler, M. R.; Sriram, S. R.; Brown, E. N.; Viswanathan, S. Nature (London) 2001, 409, (3) Fu, Q.; Seery, T. A. P. Polym. Prepr. 2001, 42, (4) Larroche, C.; Laval, J. P.; Lattes, A.; Basset, J. M. J. Org. Chem. 1984, 49, (5) Seehof, N.; Grutke, S.; Risse, W. Macromolecules 1993, 26, (6) Wolfe, P. S.; Wagener, K. B. Macromolecules 1999, 32, (7) Mathew, J. P.; Reinmuth, A.; Melia, J.; Swords, N.; Risse, W. Macromolecules 1996, 29, (8) Ivin, K. J.; Mol, J. C. Olefin Metathesis and Metathesis Polymerization; Academic Press: San Diego, CA, (9) Fisher, R. A.; Grubbs, R. H. Makromol. Chem., Macromol. Symp. 1992, 63, (10) Smirnova, N. N.; Lebedev, B. V.; Kiparisova, E. G.; Makovetskii, K. L.; Ostrovskaya, I. Y. Polym. Sci. U.S.S.R. 1997, 39, (11) Hamilton, J. G.; Ivin, K. J.; Rooney, J. J. J. Mol. Catal. 1986, 36, (12) Dias, E. L.; Nguyen, S. T.; Grubbs, R. H. J. Am. Chem. Soc. 1997, 119, (13) Sanford, M. S.; Love, J. A.; Grubbs, R. H. J. Am. Chem. Soc. 2001, 123, (14) Nelson, G. L.; Kuo, C.-L. Synthesis 1975, 105, (15) Cristol, S. J.; Seifert, W. K.; Soloway, S. B. J. Am. Chem. Soc. 1960, 82, (16) Kessler, M.; White, S. J. Polym. Sci., Part A: Polym. Chem. 2002, 40, (17) Patton, P. A.; McCarthy, T. J. Macromolecules 1987, 20, (18) Wu, Z.; Benedicto, A. D.; Grubbs, R. H. Macromolecules 1993, 26, (19) Tallarico, J. A.; Bonitatebus, P. J.; Snapper, M. L. J. Am. Chem. Soc. 1997, 119, MA
Cure Kinetics of Ring-Opening Metathesis Polymerization of Dicyclopentadiene *
 Cure Kinetics of Ring-Opening Metathesis Polymerization of Dicyclopentadiene * M. R. Kessler, S. R. White Department of Theoretical and Applied Mechanics Department of Aeronautical and Astronautical Engineering
Cure Kinetics of Ring-Opening Metathesis Polymerization of Dicyclopentadiene * M. R. Kessler, S. R. White Department of Theoretical and Applied Mechanics Department of Aeronautical and Astronautical Engineering
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Cis-Selective Ring-Opening Metathesis Polymerization with Ruthenium Catalysts Benjamin K. Keitz, Alexey Fedorov, Robert H. Grubbs* Arnold and Mabel Beckman Laboratories of Chemical
SUPPORTING INFORMATION Cis-Selective Ring-Opening Metathesis Polymerization with Ruthenium Catalysts Benjamin K. Keitz, Alexey Fedorov, Robert H. Grubbs* Arnold and Mabel Beckman Laboratories of Chemical
There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more single bonds between them)
 1 Chapter 15: Conjugation and Reactions of Dienes I. Introduction to Conjugation There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more
1 Chapter 15: Conjugation and Reactions of Dienes I. Introduction to Conjugation There are several possible arrangements for a molecule which contains two double bonds (diene): 1. Isolated: (two or more
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems
 Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Organic Chemistry II / CHEM 252 Chapter 13 Conjugated Unsaturated Systems Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction - Conjugated unsaturated systems
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
THE DIELS-ALDER REACTION
 22.6 TE DIELS-ALDER REATIN 977 2 Both overlaps are bonding. ± 2 ± 2 2 M of the diene LUM of the alkene ( 2 ) (*) The [ + 2] cycloaddition is allowed by a thermal pathway. Both overlaps are bonding, so
22.6 TE DIELS-ALDER REATIN 977 2 Both overlaps are bonding. ± 2 ± 2 2 M of the diene LUM of the alkene ( 2 ) (*) The [ + 2] cycloaddition is allowed by a thermal pathway. Both overlaps are bonding, so
We herein report the use of bidentate, chelating
 pubs.acs.org/macromolecules Bidentate Phenoxides as Ideal Activating Ligands for Living Ring- Opening Alkyne Metathesis Polymerization Danielle F. Sedbrook, Daniel W. Paley, Michael L. Steigerwald, Colin
pubs.acs.org/macromolecules Bidentate Phenoxides as Ideal Activating Ligands for Living Ring- Opening Alkyne Metathesis Polymerization Danielle F. Sedbrook, Daniel W. Paley, Michael L. Steigerwald, Colin
Chapter 1. Ring-Opening Cross-Metathesis of Low-Strain Cycloolefins
 1 Chapter 1. Ring-Opening Cross-Metathesis of Low-Strain Cycloolefins Abstract The ring-opening cross-metathesis (ROCM) of five- through eight-membered ring cycloolefins, catalyzed by a ruthenium alkylidene
1 Chapter 1. Ring-Opening Cross-Metathesis of Low-Strain Cycloolefins Abstract The ring-opening cross-metathesis (ROCM) of five- through eight-membered ring cycloolefins, catalyzed by a ruthenium alkylidene
Cure Kinetics of the Ring-Opening Metathesis Polymerization of Dicyclopentadiene
 Cure Kinetics of the Ring-Opening Metathesis Polymerization of Dicyclopentadiene M. R. KESSLER, 1 S.R. WHITE 2 1 Department of Mechanical Engineering, the University of Tulsa, Tulsa, Oklahoma 2 Department
Cure Kinetics of the Ring-Opening Metathesis Polymerization of Dicyclopentadiene M. R. KESSLER, 1 S.R. WHITE 2 1 Department of Mechanical Engineering, the University of Tulsa, Tulsa, Oklahoma 2 Department
Adhesion Promotion via Noncovalent Interactions in Self-Healing Polymers
 www.acsami.org Adhesion Promotion via Noncovalent Interactions in Self-Healing Polymers Gerald O. Wilson,, Mary M. Caruso,, Stuart R. Schelkopf, Nancy R. Sottos,, Scott R. White,,^ and Jeffrey S. Moore*,,,
www.acsami.org Adhesion Promotion via Noncovalent Interactions in Self-Healing Polymers Gerald O. Wilson,, Mary M. Caruso,, Stuart R. Schelkopf, Nancy R. Sottos,, Scott R. White,,^ and Jeffrey S. Moore*,,,
N-Heterocyclic Carbenes (NHCs)
 N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
Supporting Information for
 Supporting Information for Chelated Ruthenium Catalysts for Z-Selective Olefin Metathesis Koji Endo and Robert H. Grubbs* Arnold and Mabel Beckman Laboratory of Chemical Synthesis, Division of Chemistry
Supporting Information for Chelated Ruthenium Catalysts for Z-Selective Olefin Metathesis Koji Endo and Robert H. Grubbs* Arnold and Mabel Beckman Laboratory of Chemical Synthesis, Division of Chemistry
Chemistry 4021/8021 Computational Chemistry 3/4 Credits Spring Semester 2013 Answer Key
 Chemistry 4021/8021 Computational Chemistry 3/4 Credits Spring Semester 2013 Answer Key 1. Let's return to our favorite natural products from the first problem set. In the templates subdirectory of my
Chemistry 4021/8021 Computational Chemistry 3/4 Credits Spring Semester 2013 Answer Key 1. Let's return to our favorite natural products from the first problem set. In the templates subdirectory of my
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Chapter 13. Conjugated Unsaturated Systems. +,., - Allyl. What is a conjugated system? AllylicChlorination (High Temperature)
 What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
Conjugated Dienes and Ultraviolet Spectroscopy
 Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Stereochemical Considerations in Planning Synthesis
 Chapter 2 Stereochemical Considerations in Planning Synthesis Chapter 2 Stereochemical Considerations in Planning Syntheses 2.1 Conformational Analysis Molecules that differ from each other by rotation
Chapter 2 Stereochemical Considerations in Planning Synthesis Chapter 2 Stereochemical Considerations in Planning Syntheses 2.1 Conformational Analysis Molecules that differ from each other by rotation
Lecture Notes Chem 51B S. King I. Conjugation
 Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
Lecture Notes Chem 51B S. King Chapter 16 Conjugation, Resonance, and Dienes I. Conjugation Conjugation occurs whenever p-orbitals can overlap on three or more adjacent atoms. Conjugated systems are more
B L U E V A L L E Y D I S T R I C T C U R R I C U L U M Science AP Chemistry
 B L U E V A L L E Y D I S T R I C T C U R R I C U L U M Science AP Chemistry ORGANIZING THEME/TOPIC UNIT 1: ATOMIC STRUCTURE Atomic Theory Electron configuration Periodic Trends Big Idea 1: The chemical
B L U E V A L L E Y D I S T R I C T C U R R I C U L U M Science AP Chemistry ORGANIZING THEME/TOPIC UNIT 1: ATOMIC STRUCTURE Atomic Theory Electron configuration Periodic Trends Big Idea 1: The chemical
CHEM 251 (4 credits): Description
 CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
CHEM 251 (4 credits): Intermediate Reactions of Nucleophiles and Electrophiles (Reactivity 2) Description: An understanding of chemical reactivity, initiated in Reactivity 1, is further developed based
sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito
 1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
1 sp 3 C-H insertion by α-oxo Gold Carbene B4 Kei Ito 2016. 1. 30 1. Introduction 2 About Carbene 3 Brief history of carbene (~2000) Carbene Neutral compounds featuring a divalent carbon atom with only
Electronic Supplementary Information
 Polydicyclopentadiene Aerogels Grafted with PMMA: I. Molecular and Interparticle Crosslinking Dhairyashil P. Mohite 1, Shruti Mahadik-Khanolkar 1, Huiyang Luo 2, Hongbing Lu 2,*, Chariklia Sotiriou-Leventis
Polydicyclopentadiene Aerogels Grafted with PMMA: I. Molecular and Interparticle Crosslinking Dhairyashil P. Mohite 1, Shruti Mahadik-Khanolkar 1, Huiyang Luo 2, Hongbing Lu 2,*, Chariklia Sotiriou-Leventis
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
Bulk Coordination Polymerization of Dicyclopentadiene (DCPD) by Pd Complexes Containing β-ketoiminate or β-diketiminate Ligands
 Bulk Coordination Polymerization of Dicyclopentadiene (DCPD) Bull. Korean Chem. Soc. 2012, Vol. 33, No. 12 4131 http://dx.doi.org/10.5012/bkcs.2012.33.12.4131 Bulk Coordination Polymerization of Dicyclopentadiene
Bulk Coordination Polymerization of Dicyclopentadiene (DCPD) Bull. Korean Chem. Soc. 2012, Vol. 33, No. 12 4131 http://dx.doi.org/10.5012/bkcs.2012.33.12.4131 Bulk Coordination Polymerization of Dicyclopentadiene
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Organic Chemistry: CHEM2322
 Conjugated Systems Organic Chemistry: We met in Chem 2321 unsaturated bonds as either a C=C bond or C C bond. If these unsaturated bonds are well separated then they react independently however if there
Conjugated Systems Organic Chemistry: We met in Chem 2321 unsaturated bonds as either a C=C bond or C C bond. If these unsaturated bonds are well separated then they react independently however if there
Development, Application and Understanding of Synthetic Tools!
 Homogene Katalyse Wie kann man schneller, effizienter und umweltfreundlicher organische Moleküle und Polymere synthetisieren? Wie funktionieren diese Synthesewerkzeuge? Prof. Dr., Organometallic Chemistry,
Homogene Katalyse Wie kann man schneller, effizienter und umweltfreundlicher organische Moleküle und Polymere synthetisieren? Wie funktionieren diese Synthesewerkzeuge? Prof. Dr., Organometallic Chemistry,
Supporting Information for On the relationship between structure and reaction rate in olefin ring-closing metathesis
 Supporting Information for On the relationship between structure and reaction rate in olefin ring-closing metathesis Ashworth, Carboni, Hillier, Nelson, Percy, Rinaudo and Vincent Contents T 1 Values for
Supporting Information for On the relationship between structure and reaction rate in olefin ring-closing metathesis Ashworth, Carboni, Hillier, Nelson, Percy, Rinaudo and Vincent Contents T 1 Values for
Olefin Metathesis ROMP. L n Ru= ROMP n RCM. dilute
 lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
lefin Metathesis MP: ing-opening metathesis polymerization Thermodynamically favored for 3,4, 8, larger ring systems Bridging groups (bicyclic olefins) make ΔG polymerization more favorable as a result
Combined metallocene catalysts: an efficient technique to manipulate long-chain branching frequency of polyethylene
 Macromol. Rapid Commun. 20, 541 545 (1999) 541 Combined metallocene catalysts: an efficient technique to manipulate long-chain branching frequency of polyethylene Daryoosh Beigzadeh, João B. P. Soares*,
Macromol. Rapid Commun. 20, 541 545 (1999) 541 Combined metallocene catalysts: an efficient technique to manipulate long-chain branching frequency of polyethylene Daryoosh Beigzadeh, João B. P. Soares*,
Real life example 1 Let s look at this series of chloroalcohols, and how fast the chloride gets displaced by an external nucleophile.
 Class 2 Carbocations Last time we talked about neighboring group participation in substitution reactions. I want to start today by talking about a few more real life examples. Real life example 1 Let s
Class 2 Carbocations Last time we talked about neighboring group participation in substitution reactions. I want to start today by talking about a few more real life examples. Real life example 1 Let s
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #4 - December 9, 2002 ANSWERS
 INSTRUCTINS Department of Chemistry SUNY/neonta Chem 221 - rganic Chemistry I Examination #4 - December 9, 2002 ANSWERS This examination is in multiple choice format; the questions are in this Exam Booklet
INSTRUCTINS Department of Chemistry SUNY/neonta Chem 221 - rganic Chemistry I Examination #4 - December 9, 2002 ANSWERS This examination is in multiple choice format; the questions are in this Exam Booklet
deactivation or decomposition is therefore quantified using the turnover number.
 A catalyst may be defined by two important criteria related to its stability and efficiency. Name both of these criteria and describe how they are defined with respect to stability or efficiency. A catalyst
A catalyst may be defined by two important criteria related to its stability and efficiency. Name both of these criteria and describe how they are defined with respect to stability or efficiency. A catalyst
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Effect of Molecular Structure of Side Chain Polymers on "Click" Synthesis of Thermosensitive Molecular Brushes
 University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange University of Tennessee Honors Thesis Projects University of Tennessee Honors Program 5-2017 Effect of Molecular Structure
University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange University of Tennessee Honors Thesis Projects University of Tennessee Honors Program 5-2017 Effect of Molecular Structure
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Ultrasonic Spectroscopic Evaluation of the Ring-Opening Metathesis Polymerization of Dicyclopentadiene
 Ultrasonic Spectroscopic Evaluation of the Ring-Opening Metathesis Polymerization of Dicyclopentadiene GREGORY S. CONSTABLE, ALAN J. LESSER, E. BRYAN COUGHLIN Polymer Science and Engineering Department,
Ultrasonic Spectroscopic Evaluation of the Ring-Opening Metathesis Polymerization of Dicyclopentadiene GREGORY S. CONSTABLE, ALAN J. LESSER, E. BRYAN COUGHLIN Polymer Science and Engineering Department,
17.3 REACTIONS INVOLVING ALLYLIC AND BENZYLIC ANIONS
 798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
798 HAPTER 17 ALLYLI AND BENZYLI REATIVITY Because the unpaired electron is shared by two different carbons, this radical can react in the final propagation step to give two different products. Reaction
Big Idea 1: Structure of Matter Learning Objective Check List
 Big Idea 1: Structure of Matter Learning Objective Check List Structure of Matter Mole Concept: Empirical Formula, Percent Composition, Stoichiometry Learning objective 1.1 The student can justify the
Big Idea 1: Structure of Matter Learning Objective Check List Structure of Matter Mole Concept: Empirical Formula, Percent Composition, Stoichiometry Learning objective 1.1 The student can justify the
Synthesis of cyclic olefin polymers with high glass transition temperature and high transparency using tungsten-based catalyst system
 Polyolefins Journal, Vol. 5, No. 1 (218) DOI: 1.2263/poj.217.1476 ORIGINAL PAPER Synthesis of cyclic olefin polymers with high glass transition temperature and high transparency using tungsten-based catalyst
Polyolefins Journal, Vol. 5, No. 1 (218) DOI: 1.2263/poj.217.1476 ORIGINAL PAPER Synthesis of cyclic olefin polymers with high glass transition temperature and high transparency using tungsten-based catalyst
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Loudon Chapter 15 Review: Dienes and Aromaticity Jacquie Richardson, CU Boulder Last updated 1/28/2019
 This chapter looks at the behavior of carbon-carbon double bonds when several of them are in the same molecule. There are several possible ways they can be grouped. Conjugated dienes have a continuous
This chapter looks at the behavior of carbon-carbon double bonds when several of them are in the same molecule. There are several possible ways they can be grouped. Conjugated dienes have a continuous
Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras
 Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras Module No. #02 Lecture No. #09 Pericyclic Reactions Cycloaddition Reactions
Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras Module No. #02 Lecture No. #09 Pericyclic Reactions Cycloaddition Reactions
ADDITION OF HYDROGEN HALIDES TO CONJUGATED DIENES A. 1,2- and 1,4-Additions 700 CHAPTER 15 DIENES, RESONANCE, AND AROMATICITY
 700 CAPTER 15 DIENES, RESONANCE, AND AROMATICITY 15.18 Give the structures of the starting materials that would yield each of the compounds below in Diels Alder reactions. Pay careful attention to stereochemistry,
700 CAPTER 15 DIENES, RESONANCE, AND AROMATICITY 15.18 Give the structures of the starting materials that would yield each of the compounds below in Diels Alder reactions. Pay careful attention to stereochemistry,
Supporting Information
 Supporting Information 1. Microcapsules characterization 1.1. Thermogravimetric analysis (TGA) Thermal degradation in air and nitrogen of the synthesized microcapsules is shown in figure S1. 100 Weight
Supporting Information 1. Microcapsules characterization 1.1. Thermogravimetric analysis (TGA) Thermal degradation in air and nitrogen of the synthesized microcapsules is shown in figure S1. 100 Weight
Supramolecular Control over Diels-Alder Reactivity by Encapsulation and Competitive Displacement
 Supporting information for: Supramolecular Control over Diels-Alder Reactivity by Encapsulation and Competitive Displacement Maarten M. J. Smulders and Jonathan R. Nitschke Department of Chemistry University
Supporting information for: Supramolecular Control over Diels-Alder Reactivity by Encapsulation and Competitive Displacement Maarten M. J. Smulders and Jonathan R. Nitschke Department of Chemistry University
Alcohols, Ethers, & Epoxides
 Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
Alcohols, Ethers, & Epoxides Alcohols Structure and Bonding Enols and Phenols Compounds having a hydroxy group on a sp 2 hybridized carbon enols and phenols undergo different reactions than alcohols. Chapter
PAPER No. : 5; Organic Chemistry-II MODULE No. : 13; Mixed S N 1 and S N 2 Reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 13; Mixed S N 1 and S N 2 Reactions CHE_P5_M13 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. Nature
Basic Chemistry 2014 Timberlake
 A Correlation of Basic Chemistry Timberlake Advanced Placement Chemistry Topics AP is a trademark registered and/or owned by the College Board, which was not involved in the production of, and does not
A Correlation of Basic Chemistry Timberlake Advanced Placement Chemistry Topics AP is a trademark registered and/or owned by the College Board, which was not involved in the production of, and does not
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
N-Heterocyclic Carbenes (NHCs)
 N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
Exam. Name. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements is incorrect about benzene? 1) A) All of the carbon
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements is incorrect about benzene? 1) A) All of the carbon
Reactivity in Organic Chemistry. Mid term test October 31 st 2011, 9:30-12:30. Problem 1 (25p)
 eactivity in rganic Chemistry Mid term test ctober 31 st 2011, 9:30-12:30 Problem 1 (25p) - (+)- Limonone can be epoxidized (using peracetic acid) to give an inseparable mixture of two diastereomeric limonene
eactivity in rganic Chemistry Mid term test ctober 31 st 2011, 9:30-12:30 Problem 1 (25p) - (+)- Limonone can be epoxidized (using peracetic acid) to give an inseparable mixture of two diastereomeric limonene
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2008
 Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Name: CHEM 633: Advanced Organic Chem: Physical Problem Set 5 Due 11/10/17
 ame: CEM 633: Advanced rganic Chem: ysical Problem Set 5 Due 11/10/17 Please do not look up references until after you turn in the problem set unless otherwise noted. For the following problems, please
ame: CEM 633: Advanced rganic Chem: ysical Problem Set 5 Due 11/10/17 Please do not look up references until after you turn in the problem set unless otherwise noted. For the following problems, please
Classification of Reactions by:
 lassification of Reactions by: 1) Functional group 2) Kind a) Addition: A + B > b) Elimination: A > B + c) Substitution: A-B + -D > A- + B-D d) Rearrangement: A > B, where B is a constitutional isomer
lassification of Reactions by: 1) Functional group 2) Kind a) Addition: A + B > b) Elimination: A > B + c) Substitution: A-B + -D > A- + B-D d) Rearrangement: A > B, where B is a constitutional isomer
SYNTHESIS AND PROPERTIES OF CROSS-LINKED POLYMERS CONTAINING DIARYLBIBENZOFURANONE BY ADMET POLYMERIZATION
 SYNTHESIS AND PROPERTIES OF CROSS-LINKED POLYMERS CONTAINING DIARYLBIBENZOFURANONE BY ADMET POLYMERIZATION T. Ohishi, 1 K. Imato, 2 T. Kanehara, 2 A. Takahara, 1,2 and H. Otsuka 1,2 1 Institute for Materials
SYNTHESIS AND PROPERTIES OF CROSS-LINKED POLYMERS CONTAINING DIARYLBIBENZOFURANONE BY ADMET POLYMERIZATION T. Ohishi, 1 K. Imato, 2 T. Kanehara, 2 A. Takahara, 1,2 and H. Otsuka 1,2 1 Institute for Materials
Chapter 1: Introduction to Olefin Metathesis
 Chapter 1: Introduction to lefin Metathesis 1 Brief History of lefin Metathesis lefin metathesis is a unique process undergoing C=C bond rearrangement as shown in Scheme 1. 1 The reaction is catalyzed
Chapter 1: Introduction to lefin Metathesis 1 Brief History of lefin Metathesis lefin metathesis is a unique process undergoing C=C bond rearrangement as shown in Scheme 1. 1 The reaction is catalyzed
Supporting Information
 Supporting Information Protonolysis of a Ruthenium-Carbene Bond and Applications in Olefin Metathesis Benjamin K. Keitz, Jean Bouffard, Guy Bertrand, Robert H. Grubbs*, Arnold and Mabel Beckman Laboratories
Supporting Information Protonolysis of a Ruthenium-Carbene Bond and Applications in Olefin Metathesis Benjamin K. Keitz, Jean Bouffard, Guy Bertrand, Robert H. Grubbs*, Arnold and Mabel Beckman Laboratories
Chapter 8 Alkyl Halides and Elimination Reactions
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 8 Alkyl Halides and Elimination Reactions Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 8 Alkyl Halides and Elimination Reactions Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Exam (6 pts) Show which starting materials are used to produce the following Diels-Alder products:
 Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
Exam 1 Name CHEM 212 1. (18 pts) Complete the following chemical reactions showing all major organic products; illustrate proper stereochemistry where appropriate. If no reaction occurs, indicate NR :
Multivalent interactions in human biology
 Cooperativity Multivalent interactions in human biology Multivalent interactions in supramolecular chemistry Additivity (?) Multivalent interactions in supramolecular chemistry In order to obtain a
Cooperativity Multivalent interactions in human biology Multivalent interactions in supramolecular chemistry Additivity (?) Multivalent interactions in supramolecular chemistry In order to obtain a
17.1 Classes of Dienes
 17.1 Classes of Dienes There are three categories for dienes: Cumulated: pi bonds are adjacent. Conjugated: pi bonds are separated by exactly ONE single bond. Isolated: pi bonds are separated by any distance
17.1 Classes of Dienes There are three categories for dienes: Cumulated: pi bonds are adjacent. Conjugated: pi bonds are separated by exactly ONE single bond. Isolated: pi bonds are separated by any distance
Chapter 6 Chemical Reactivity and Mechanisms
 Chapter 6 Chemical Reactivity and Mechanisms 6.1 Enthalpy Enthalpy (ΔH or q) is the heat energy exchange between the reaction and its surroundings at constant pressure Breaking a bond requires the system
Chapter 6 Chemical Reactivity and Mechanisms 6.1 Enthalpy Enthalpy (ΔH or q) is the heat energy exchange between the reaction and its surroundings at constant pressure Breaking a bond requires the system
F G C G H H C B B A A DD E E
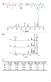 a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
6 Synthesis of Alcohols
 6 Synthesis of Alcohols 6.1 Synthesis of Saturated Alcohols Hydroboration-oxidation is now a standard method for anti-markovnikov s cishydration [1] of alkenes from the less hindered side of the double
6 Synthesis of Alcohols 6.1 Synthesis of Saturated Alcohols Hydroboration-oxidation is now a standard method for anti-markovnikov s cishydration [1] of alkenes from the less hindered side of the double
and Ultraviolet Spectroscopy
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Cationic Alkylaluminum-Complexed Zirconocene Hydrides as Participants in Olefin-Polymerization Catalysis. Supporting Information
 Cationic Alkylaluminum-Complexed Zirconocene Hydrides as Participants in Olefin-Polymerization Catalysis Steven M. Baldwin, John E. Bercaw, *, and Hans H. Brintzinger*, Contribution from the Arnold and
Cationic Alkylaluminum-Complexed Zirconocene Hydrides as Participants in Olefin-Polymerization Catalysis Steven M. Baldwin, John E. Bercaw, *, and Hans H. Brintzinger*, Contribution from the Arnold and
Diels-Alder Reaction
 Diels-Alder Reaction Method for synthesis of 6-membered ring ne-step, concerted reaction Termed [4+2] cycloaddition reaction where 4 and 2 electrons react. + Diels-Alder Reaction Discovered by. Diels and
Diels-Alder Reaction Method for synthesis of 6-membered ring ne-step, concerted reaction Termed [4+2] cycloaddition reaction where 4 and 2 electrons react. + Diels-Alder Reaction Discovered by. Diels and
Complex Promoted by Electron-Deficient Alkenes. Brian V. Popp and Shannon S. Stahl*
 Oxidatively-Induced Reductive Elimination of Dioxygen from an η 2 -Peroxopalladium(II) Complex Promoted by Electron-Deficient Alkenes Brian V. Popp and Shannon S. Stahl* Department of Chemistry, University
Oxidatively-Induced Reductive Elimination of Dioxygen from an η 2 -Peroxopalladium(II) Complex Promoted by Electron-Deficient Alkenes Brian V. Popp and Shannon S. Stahl* Department of Chemistry, University
N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES
 N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES Zachery Matesich 24 February 2015 Roadmap 2 Introduction Synthetic Methods History of NHCs Properties of NHCs Nature of the carbene Structural properties
N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES Zachery Matesich 24 February 2015 Roadmap 2 Introduction Synthetic Methods History of NHCs Properties of NHCs Nature of the carbene Structural properties
Supporting Information
 Supporting Information Z-Selective Homodimerization of Terminal Olefins with a Ruthenium Metathesis Catalyst Benjamin K. Keitz, Koji Endo, Myles B. Herbert, Robert H. Grubbs* Arnold and Mabel Beckman Laboratories
Supporting Information Z-Selective Homodimerization of Terminal Olefins with a Ruthenium Metathesis Catalyst Benjamin K. Keitz, Koji Endo, Myles B. Herbert, Robert H. Grubbs* Arnold and Mabel Beckman Laboratories
Catalytic Polymerization of Olefins and Cycloolefins with Transition Metal Complexes
 NATIONAL AND KAPODISTRIAN UNIVERSITY OF ATHENS SCHOOL OF SCIENCE DEPARTMENT OF CHEMISTRY PhD THESIS Catalytic Polymerization of Olefins and Cycloolefins with Transition Metal Complexes GRIGORIOS RAPTOPOULOS
NATIONAL AND KAPODISTRIAN UNIVERSITY OF ATHENS SCHOOL OF SCIENCE DEPARTMENT OF CHEMISTRY PhD THESIS Catalytic Polymerization of Olefins and Cycloolefins with Transition Metal Complexes GRIGORIOS RAPTOPOULOS
22. The Diels-Alder Cycloaddition Reaction
 22. The Diels-Alder Cycloaddition Reaction A. Introduction In 1928, tto Diels and his student Kurt Alder at the University of Kiel in Germany first reported their work in the reactions of electron poor
22. The Diels-Alder Cycloaddition Reaction A. Introduction In 1928, tto Diels and his student Kurt Alder at the University of Kiel in Germany first reported their work in the reactions of electron poor
Nucleophilic Substitution and Elimination
 Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Nucleophilic Substitution and Elimination Alkyl halides react with a nucleophile in one of two ways. Either they eliminate an X to form an alkene, or they undergo a substitution with the nucleophile, Nu,
Lecture 6: Transition-Metal Catalysed C-C Bond Formation
 Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Chapter 14: Conjugated Dienes
 Chapter 14: Conjugated Dienes Coverage: 1. Conjugated vs Nonconjugated dienes and Stability 2. MO picture of 1,3-butadiene 3. Electrophilic addition to Dienes 4. Kinetic vs Thermodynamic Control 5. Diels-Alder
Chapter 14: Conjugated Dienes Coverage: 1. Conjugated vs Nonconjugated dienes and Stability 2. MO picture of 1,3-butadiene 3. Electrophilic addition to Dienes 4. Kinetic vs Thermodynamic Control 5. Diels-Alder
Course Goals for CHEM 202
 Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Course Goals for CHEM 202 Students will use their understanding of chemical bonding and energetics to predict and explain changes in enthalpy, entropy, and free energy for a variety of processes and reactions.
Chapter 10 Radical Reactions
 Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Gel growth in free radical crosslinking copolymerization: Effect of inactive gel radicals
 354 Macromol. Theory Simul. 9, 354 361 (2000) A kinetic model is presented for the post-gelation period of free-radical crosslinking copolymerization. The model takes into account the trapped radical centers
354 Macromol. Theory Simul. 9, 354 361 (2000) A kinetic model is presented for the post-gelation period of free-radical crosslinking copolymerization. The model takes into account the trapped radical centers
Polymerization of trans-2-butene with (-Diimine)Ni(II) Complex in Combination with Et 2 AlCl
 Polymer Journal, Vol. 38, No. 11, pp. 116 1164 (26) #26 The Society of Polymer Science, Japan Polymerization of trans-2-butene with (-Diimine)Ni(II) Complex in Combination with Et 2 AlCl Kiyoshi ENDO y
Polymer Journal, Vol. 38, No. 11, pp. 116 1164 (26) #26 The Society of Polymer Science, Japan Polymerization of trans-2-butene with (-Diimine)Ni(II) Complex in Combination with Et 2 AlCl Kiyoshi ENDO y
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Chem 263 Sept 22, Beta-carotene (depicted below) is the orange-red colour in carrots. β-carotene
 hem 263 Sept 22, 2009 onjugated Dienes and olour ontinued Beta-carotene (depicted below) is the orange-red colour in carrots. Xanthophylls β-carotene Xanthophylls are oxygenated carotene molecules. Zeaxanthin,
hem 263 Sept 22, 2009 onjugated Dienes and olour ontinued Beta-carotene (depicted below) is the orange-red colour in carrots. Xanthophylls β-carotene Xanthophylls are oxygenated carotene molecules. Zeaxanthin,
Supporting Information for: End-capping ROMP Polymers with Vinyl. Lactones
 Supporting Information for: End-capping RMP Polymers with Vinyl Lactones Stefan Hilf, Robert H. Grubbs and Andreas F.M. Kilbinger * Institut für rganische Chemie, Johannes Gutenberg-Universität Mainz,
Supporting Information for: End-capping RMP Polymers with Vinyl Lactones Stefan Hilf, Robert H. Grubbs and Andreas F.M. Kilbinger * Institut für rganische Chemie, Johannes Gutenberg-Universität Mainz,
FLOW INDUCED MECHANOCHEMICAL ACTIVATION
 FLOW INDUCED MECHANOCHEMICAL ACTIVATION H Magnus Andersson 1, Charles R Hickenboth 2, Stephanie L Potisek 2, Jeffrey S Moore 2, Nancy R Sottos 3 and Scott R White 4 1 Beckman Institute, 2 Department of
FLOW INDUCED MECHANOCHEMICAL ACTIVATION H Magnus Andersson 1, Charles R Hickenboth 2, Stephanie L Potisek 2, Jeffrey S Moore 2, Nancy R Sottos 3 and Scott R White 4 1 Beckman Institute, 2 Department of
17.1 Classes of Dienes
 W 2/1 Due: HW14, spec03 Due: n/a M 2/6 Lecture HW14 grading Lect17a Conjugated π systems Lecture quiz Lect17b Lab Lab02 Qual Analysis II (cont) 7-1 17.1 Classes of Dienes There are three categories for
W 2/1 Due: HW14, spec03 Due: n/a M 2/6 Lecture HW14 grading Lect17a Conjugated π systems Lecture quiz Lect17b Lab Lab02 Qual Analysis II (cont) 7-1 17.1 Classes of Dienes There are three categories for
NIH Public Access Author Manuscript J Am Chem Soc. Author manuscript; available in PMC 2014 July 10.
 NIH Public Access Author Manuscript Published in final edited form as: J Am Chem Soc. 2013 July 10; 135(27): 10032 10035. doi:10.1021/ja405559y. Synthesis of Highly Cis, Syndiotactic ROMP Polymers Using
NIH Public Access Author Manuscript Published in final edited form as: J Am Chem Soc. 2013 July 10; 135(27): 10032 10035. doi:10.1021/ja405559y. Synthesis of Highly Cis, Syndiotactic ROMP Polymers Using
Organic Chemistry I (Chem340), Spring Final Exam
 rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
rganic Chemistry I (Chem340), pring 2005 Final Exam This is a closed-book exam. No aid is to be given to or received from another person. Model set and calculator may be used, but cannot be shared. Please
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
Supporting Information
 Supporting Information Molecular Weight Dependence of Zero-Shear Viscosity in Atactic Polypropylene Bottlebrush Polymers Samuel J. Dalsin, Marc A. Hillmyer,*, and Frank S. Bates*, Department of Chemical
Supporting Information Molecular Weight Dependence of Zero-Shear Viscosity in Atactic Polypropylene Bottlebrush Polymers Samuel J. Dalsin, Marc A. Hillmyer,*, and Frank S. Bates*, Department of Chemical
Conformational Analysis
 Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
Chapter 15 Dienes, Resonance, and Aromaticity
 Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 15 Dienes, Resonance, and Aromaticity Solutions to In-Text Problems 15.2 The delocalization energy is the energy
Instructor Supplemental Solutions to Problems 2010 Roberts and Company Publishers Chapter 15 Dienes, Resonance, and Aromaticity Solutions to In-Text Problems 15.2 The delocalization energy is the energy
Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) Can act as weak nucleophiles
 Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Organometallic Catalysis
 Organometallic Catalysis The catalysts we will study are termed homogeneous catalysts as they are dissolved in th e same solvent as the substrate. In contrast, heterogeneous catalysts, such as palladium
Organometallic Catalysis The catalysts we will study are termed homogeneous catalysts as they are dissolved in th e same solvent as the substrate. In contrast, heterogeneous catalysts, such as palladium
Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras
 Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras Module No. #02 Lecture No. #08 Pericyclic Reactions -Cycloaddition Reactions
Pericyclic Reactions and Organic Photochemistry S. Sankararaman Department of Chemistry Indian Institute of Technology, Madras Module No. #02 Lecture No. #08 Pericyclic Reactions -Cycloaddition Reactions
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
