Variable Activation Energy to Model Oil Shale Pyrolysis Kinetics. Jordan
|
|
|
- Julian Pitts
- 6 years ago
- Views:
Transcription
1 Variable Activation Energy to Model Oil Shale Pyrolysis Kinetics Omar S. Al-Ayed Ayed and Sulieman Q. Abu Ein Faculty of Engineering Technology Al-Balqa Applied University P.O. Box Marka Jordan
2 Introduction Most of the reported modeling studies are based on TGA, DGA, DSC and Rock-Eval Analyzer results. Integral and differential methods, the method of maximum rate have been used. These models have assumed a first-order depletion of kerogen to form oil and gas products.
3 Allred (1966) reported that the process of oil evolution is the sum of two separate steps. The first involves degradation and The second is the evaporation of the products, Each has different activation energies. As the temperature increases, this latter process is claimed to be the rate-determining step Braun and Rothman (1975), reported 44.6 kjmol -1 activation energy below 490 o C reaction Temperature. Previous Work Decomposition of bitumen involves breaking of relatively weak chemical bonds, Higher activation energy, kjmol - 1 involves breaking of much stronger chemical bonds in kerogen.
4 Previous Work (cont.) Weight loss in low temperature pyrolysis region attributed to loss of moisture, interlayer water and decomposition of nahcolite (NaHCO 3 ) which takes place up to 120 o C. Physical changes, such as softening and molecular rearrangement associated with the release of gases and structural water are attributed to the rapid evaporation of organic material not chemically bonded to the kerogen network. Campbell and co-workers (1980), studied rate of evolution of CH 4, H 2, CO, CO 2, and C 2, C 3 hydrocarbons during pyrolysis of Colorado Oil Shale at linear heating rates varying from 0.5 to 4.0 o Cmin -1. Dawsonite, NaAl(OH) 2 CO 3 decomposes to Na 2 CO 3, Al 2 O 3, H 2 O, CO 2 between o C
5 Additional studies Decomposition of oil shale involves large number of reactions in parallel and in series, TGA measures overall weight loss due to these reactions. Therefore activation energies derived from TGA data are apparent activation energies. Li and Yue 2003, studied pyrolysis kinetics of different Chinese oil shale samples at a constant heating rate of 5 o Cmin -1. The TGA data obtained used to develop a kinetic model which assumes 11 first order parallel reactions with changed activation energies es and frequency factors. They reported apparent activation energies in kjmol - 1 range and apparent frequency factor in the range 1.3*10 4 to 1.4* The calculated fractional conversion of each reaction is a complex function of activation energy.
6 Chemical Reactions and Variable Activation Energy Idea Low activation energies, rupture of weak cross linked bonds, such as, C-O, C C-S, C (17,18) etc. Also, rupture of branched functional groups in kerogen long molecular structure. These bonds have low rupture energy that produces gases such as H 2 O, CO 2, H 2 S, H 2 and light hydrocarbons Medium activation energy values are associated with break up of the side chains in β-site of aromatics, decomposition of normal alkane with large molecular weight, Diels-Alder cyclation reaction and the rupture of alicyclic hydrocarbon. This corresponds to pyrolysis temperature between o C
7 Chemical Reactions and Variable Activation Energy Idea High apparent activation energies are mainly the aromatization of alicyclic compounds, dehydrogenation and combination of aromatic rings, rupture of heterocyclic compounds coke formation reactions.
8 DTA Studies on Jordanian Oil Shale DTA thermal tests with a heating rate 10 o Cmin -1. conducted and the following heat effects recorded: o C endothermic release of the absorbed water o C exothermic reaction of first organic complex o C roasting of the pyrites o C dehydration of the phosphate complex o C reaction of the second organic complex o C endothermic dissociation of dolomite endothermic dissociation of calcite
9 Experimental Conditions 400 g sample Particle size less than 3 mm N 2 gas and o C temperature range Stainless Steel cylindrical retort Digital Balance Circulating coolant with anti freeze at 2 o C Glass condenser Electrical heater. Heating rates varied between o Cmin -1
10 Experimental Setup (1)
11 Experimental Setup(2)
12 Oil Shale Characteristics Component Raw Shale Moisture Content 1.21 Total Water 3.0 Total Oil Gas Loss 3.4 Spent Shale 86.3 Total Sulfur 2.29 Total Carbon Total Organic Carbon Hydrogen 1.76 CaCO Calorific Value, kj/kg 5467
13 Sample Losses, Total, Liquids, Gases Percent Lo Component gas total oil & water Pyrolysis Temperature, o C
14 Rate of liquid condensation (gmin -1 )with temperature, at different heating rates ( o Cmin -1 ) Rate of weight loss, g heating rate,h, o Cmin Poly. ( ) Pyrolysis Temperature, o C
15 Rate of liquid Accumu Final Pyrolysis Temperature o C,, with o Cmin -1, heating rate gmin-1 Final Pyrolysis Temp. o C Pyrolysis Temperature, ο C
16 Final Pyrolysis Temperature o C,, with o Cmin -1, heating rate Rate of Liquid Accumu gmin Final Temp.oC Pyrolysis Temperature, oc
17 Rate of Liquid Accumu Final Pyrolysis Temperature o C,, with o Cmin -1, heating rate gmin Final Pyrolysis temp. o C Pyrolysis Temperature, o C
18 Kinetic Equations dx dt k = k exp o h = = k 1 dt dt ( x ) E RT ln k RT 2 2 = ln o 1 [ ln1 ( x) ] he RT E E RT
19 Calculated ln[-ln(1 ln(1-x)] Vs. Predicted Theoretical ln[-ln(1-x)] Constant Activation Energy Experimental ln[-
20 ( x) E = Eo 1+ Modified Kinetic Equation ln [ ( )] k RT 2RT ln 1 x = ln o 1 he( x) E( x) 2 E ( x) RT E( x) = E ( 1+ x) o
21 Variable Activation Energy with Const. k o experimental ydata = 1.051x R2 = y =x line Theoretic
22 Activation Energy and k o are function of x Experimental ln(-ln(1-x)) ydata = x R2 = Exp. Vs. Theo. rates Theo ln(-ln(1
23 Rate of Liquid Production dw dt l = k ol exp E RT ( 1 x) 2
24 Liquid modeling for h o Cmin -1
25 Liquid modeling for h ocmin-1
26 Liquid modeling for (h) o Cmin -1
27 Calculated parameters Low and intermediate heating rate calculated parameters: k ol = 1.15*10 6 E = 77 kjmol -1 High heating rate calculated parameters: K ol =3.0*10 6 E = 96.5 kjmol -1
28 Conclusions and Recommendations 1. Total oil shale weight loss data have been fitted to a standard first order equation with new modifications for Activation Energy and Frequency factor. The complexity of reactions during pyrolysis dictate such thinking Excellent agreement is obtained between developed model and experimental data. 2. Rate of liquid accumulation has been modeled with a standard second order reaction model. 77 kjmol -1 activation energy calculated for heating rate less than 5 o Cmin kJmol -1 calculated for heating rate in 9 14 o Cmin -1 range. 3. Gaseous production rate has not been modeled yet. 4. Mathematical treatment of the model is required.
29 Thanks and Gratitude to: Chairman of Symposium and Organizers.
30
Isothermal and Nonisothermal Kinetic Analyses of Mahogany Oil Shale with TGA
 Isothermal and Nonisothermal Kinetic Analyses of Mahogany Oil Shale with TGA Pankaj Tiwari, Kyeongseok Oh and Milind Deo Chemical Engineering Dept, University of Utah 1 Table of contents Introduction Background
Isothermal and Nonisothermal Kinetic Analyses of Mahogany Oil Shale with TGA Pankaj Tiwari, Kyeongseok Oh and Milind Deo Chemical Engineering Dept, University of Utah 1 Table of contents Introduction Background
APPENDIX G. Detailed Study of Shale Pyrolysis for Oil Production. A Subpart of Project Oil Shale Pyrolysis and In Situ Modeling
 APPENDIX G Detailed Study of Shale Pyrolysis for Oil Production A Subpart of Project Oil Shale Pyrolysis and In Situ Modeling Final Project Report Reporting period: June 21, 2006 to October 21, 2009 Milind
APPENDIX G Detailed Study of Shale Pyrolysis for Oil Production A Subpart of Project Oil Shale Pyrolysis and In Situ Modeling Final Project Report Reporting period: June 21, 2006 to October 21, 2009 Milind
Mathematical Modeling of Oil Shale Pyrolysis
 October, 19 th, 2011 Mathematical Modeling of Oil Shale Pyrolysis Pankaj Tiwari Jacob Bauman Milind Deo Department of Chemical Engineering University of Utah, Salt Lake City, Utah http://from50000feet.wordpress.com
October, 19 th, 2011 Mathematical Modeling of Oil Shale Pyrolysis Pankaj Tiwari Jacob Bauman Milind Deo Department of Chemical Engineering University of Utah, Salt Lake City, Utah http://from50000feet.wordpress.com
THERMAL BEHAVIOR OF RAW OIL SHALE AND ITS COMPONENTS SUJEEWA S. PALAYANGODA, QUOC P. NGUYEN *
 Oil Shale, 2015, Vol. 32, No. 2, pp. 160 171 ISSN 0208-189X doi: 10.3176/oil.2015.2.06 2015 Estonian Academy Publishers THERMAL BEHAVIOR OF RAW OIL SHALE AND ITS COMPONENTS SUJEEWA S. PALAYANGODA, QUOC
Oil Shale, 2015, Vol. 32, No. 2, pp. 160 171 ISSN 0208-189X doi: 10.3176/oil.2015.2.06 2015 Estonian Academy Publishers THERMAL BEHAVIOR OF RAW OIL SHALE AND ITS COMPONENTS SUJEEWA S. PALAYANGODA, QUOC
Kinetic modelling of kerogen cracking during oil shale process : influence of organic matter source F. Behar and P. Allix
 Kinetic modelling of kerogen cracking during oil shale process : influence of organic matter source F. Behar and P. Allix 1 General outline! I Introduction! II Kerogen characterization Definition of the
Kinetic modelling of kerogen cracking during oil shale process : influence of organic matter source F. Behar and P. Allix 1 General outline! I Introduction! II Kerogen characterization Definition of the
Kinetic Analysis of the Oil Shale Pyrolysis using Thermogravimetry and Differential Scanning Calorimetry
 559 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 61, 2017 Guest Editors: Petar S Varbanov, Rongxin Su, Hon Loong Lam, Xia Liu, Jiří J Klemeš Copyright 2017, AIDIC Servizi S.r.l. ISBN 978-88-95608-51-8;
559 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 61, 2017 Guest Editors: Petar S Varbanov, Rongxin Su, Hon Loong Lam, Xia Liu, Jiří J Klemeš Copyright 2017, AIDIC Servizi S.r.l. ISBN 978-88-95608-51-8;
10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics
 Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 OFB Chap. 10 2 Thermite Reaction
Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 OFB Chap. 10 2 Thermite Reaction
10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics
 Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 Chapter 10 Thermochemistry Heat
Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 Chapter 10 Thermochemistry Heat
The School For Excellence 2018 Unit 3 & 4 Chemistry Topic Notes Page 1
 The term fractional distillation refers to a physical method used to separate various components of crude oil. Fractional distillation uses the different boiling temperatures of each component, or fraction,
The term fractional distillation refers to a physical method used to separate various components of crude oil. Fractional distillation uses the different boiling temperatures of each component, or fraction,
2. Enthalpy changes. N Goalby chemrevise.org
 2. Enthalpy changes In an exothermic change energy is transferred from the system (chemicals) to the surroundings. The have less energy than the If an enthalpy change occurs then energy is transferred
2. Enthalpy changes In an exothermic change energy is transferred from the system (chemicals) to the surroundings. The have less energy than the If an enthalpy change occurs then energy is transferred
Chapter 8. Thermochemistry
 Chapter 8 Thermochemistry Copyright 2001 by Harcourt, Inc. All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department,
Chapter 8 Thermochemistry Copyright 2001 by Harcourt, Inc. All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department,
QUESTION 1 The boiling temperature of hydrocarbons making up crude oil depends on the strength of intermolecular forces known as:
 QUESTION 1 The boiling temperature of hydrocarbons making up crude oil depends on the strength of intermolecular forces known as: B C D Hydrogen bonding. Dipole-dipole interactions. Dispersion forces.
QUESTION 1 The boiling temperature of hydrocarbons making up crude oil depends on the strength of intermolecular forces known as: B C D Hydrogen bonding. Dipole-dipole interactions. Dispersion forces.
Chemical Kinetics of HC Combustion
 Spark Ignition Engine Combustion MAK65E Chemical Kinetics of HC Combustion Prof.Dr. Cem Soruşbay Istanbul Technical University Chemical Kinetics of HC Combustion Introduction Elementary reactions Multi-step
Spark Ignition Engine Combustion MAK65E Chemical Kinetics of HC Combustion Prof.Dr. Cem Soruşbay Istanbul Technical University Chemical Kinetics of HC Combustion Introduction Elementary reactions Multi-step
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation
 Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation Philip Davies TA Instruments UK Thermogravimetric Analysis Change in a samples weight (increase or decrease) as a function
Thermogravimetric Analysis Advanced Techniques for Better Materials Characterisation Philip Davies TA Instruments UK Thermogravimetric Analysis Change in a samples weight (increase or decrease) as a function
Thermodynamics. Thermodynamically favored reactions ( spontaneous ) Enthalpy Entropy Free energy
 Thermodynamics Thermodynamically favored reactions ( spontaneous ) Enthalpy Entropy Free energy 1 Thermodynamically Favored Processes Water flows downhill. Sugar dissolves in coffee. Heat flows from hot
Thermodynamics Thermodynamically favored reactions ( spontaneous ) Enthalpy Entropy Free energy 1 Thermodynamically Favored Processes Water flows downhill. Sugar dissolves in coffee. Heat flows from hot
Geol Supplementary Notes 463-RWR-1,2 GEOL RWR-1 GENERAL INTRODUCTION TO PETROLEUM GEOLOGY: OUTLINE OF MATERIAL TO BE COVERED
 GEOL 463.3 RWR-1 GENERAL INTRODUCTION TO PETROLEUM GEOLOGY: OUTLINE OF MATERIAL TO BE COVERED Recommended sections to read in the textbook: Chapters 1 and 2 (p. 2-22): Background to development of petroleum
GEOL 463.3 RWR-1 GENERAL INTRODUCTION TO PETROLEUM GEOLOGY: OUTLINE OF MATERIAL TO BE COVERED Recommended sections to read in the textbook: Chapters 1 and 2 (p. 2-22): Background to development of petroleum
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 Chem 102--Exam #2 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. When water is measured in a plastic graduated cylinder, a reverse meniscus
Chem 102--Exam #2 Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. When water is measured in a plastic graduated cylinder, a reverse meniscus
Modeling In Situ Oil Shale Retorting. Jack Parker and Fan Zhang Oak Ridge National Laboratory
 Modeling In Situ Oil Shale Retorting Jack Parker and Fan Zhang Oak Ridge National Laboratory Overview Describe a model for in situ oil shale retorting with nonequilibrium fracture-matrix heat and mass
Modeling In Situ Oil Shale Retorting Jack Parker and Fan Zhang Oak Ridge National Laboratory Overview Describe a model for in situ oil shale retorting with nonequilibrium fracture-matrix heat and mass
Chemistry Review Unit 5 Physical Behavior of Matter
 Chemistry Review Phases of Matter, Changes of Phase, Substances, Mixtures, Solutions, Effect of Solute on Solution, Energy, Kinetics of Solids, Liquids and Gases Matter, Phases and Gas Laws 1. Matter is
Chemistry Review Phases of Matter, Changes of Phase, Substances, Mixtures, Solutions, Effect of Solute on Solution, Energy, Kinetics of Solids, Liquids and Gases Matter, Phases and Gas Laws 1. Matter is
Kinetic Molecular Theory, Gases, and Phase Changes (Answer Key)
 Kinetic Molecular Theory, Gases, and Phase Changes (Answer Key) solid liquid melting sublimation freezing evaporation condensation deposition gas 8. See above. 9. Endothermic: melting, evaporation, sublimation.
Kinetic Molecular Theory, Gases, and Phase Changes (Answer Key) solid liquid melting sublimation freezing evaporation condensation deposition gas 8. See above. 9. Endothermic: melting, evaporation, sublimation.
Chemistry 123: Physical and Organic Chemistry Topic 4: Gaseous Equilibrium
 Topic 4: Introduction, Topic 4: Gaseous Equilibrium Text: Chapter 6 & 15 4.0 Brief review of Kinetic theory of gasses (Chapter 6) 4.1 Concept of dynamic equilibrium 4.2 General form & properties of equilbrium
Topic 4: Introduction, Topic 4: Gaseous Equilibrium Text: Chapter 6 & 15 4.0 Brief review of Kinetic theory of gasses (Chapter 6) 4.1 Concept of dynamic equilibrium 4.2 General form & properties of equilbrium
Dawsonite breakdown reactions during pyrolysis in Green River Formation oil shale
 Dawsonite breakdown reactions during pyrolysis in Green River Formation oil shale Jeremy Boak, Colorado School of Mines Justin Birdwell, U. S. Geological Survey Outline Saline Minerals in Green River Formation
Dawsonite breakdown reactions during pyrolysis in Green River Formation oil shale Jeremy Boak, Colorado School of Mines Justin Birdwell, U. S. Geological Survey Outline Saline Minerals in Green River Formation
UNIT 9: KINETICS & EQUILIBRIUM. Essential Question: What mechanisms affect the rates of reactions and equilibrium?
 UNIT 9: KINETICS & EQUILIBRIUM Essential Question: What mechanisms affect the rates of reactions and equilibrium? What is Kinetics? Kinetics is the branch of chemistry that explains the rates of chemical
UNIT 9: KINETICS & EQUILIBRIUM Essential Question: What mechanisms affect the rates of reactions and equilibrium? What is Kinetics? Kinetics is the branch of chemistry that explains the rates of chemical
Oil Shale Project in Thailand
 Oil Shale Project in Thailand Progress of Oil Shale Exploration in Thailand (Phase 2) The cooperation of Thai agencies between Department of Mineral Fuels (DMF) and Electricity Generating Authority of
Oil Shale Project in Thailand Progress of Oil Shale Exploration in Thailand (Phase 2) The cooperation of Thai agencies between Department of Mineral Fuels (DMF) and Electricity Generating Authority of
States of matter Part 2
 Physical Pharmacy Lecture 2 States of matter Part 2 Assistant Lecturer in Pharmaceutics Overview The Liquid State General properties Liquefaction of gases Vapor pressure of liquids Boiling point The Solid
Physical Pharmacy Lecture 2 States of matter Part 2 Assistant Lecturer in Pharmaceutics Overview The Liquid State General properties Liquefaction of gases Vapor pressure of liquids Boiling point The Solid
8. Energetics I. N Goalby chemrevise.org 1
 8. Energetics I Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. In an exothermic change energy is transferred
8. Energetics I Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. In an exothermic change energy is transferred
Homework 5 Answer Key. There are two possibilities. First, the finger prints could have evaporated. Second, the car could have been wiped down.
 omework 5 Answer Key ingerprints ould she have been in the car? There are two possibilities. irst, the finger prints could have evaporated. Second, the car could have been wiped down. 1) Both the adult
omework 5 Answer Key ingerprints ould she have been in the car? There are two possibilities. irst, the finger prints could have evaporated. Second, the car could have been wiped down. 1) Both the adult
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
Section 1 - Thermochemistry
 Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Reaction Energy Section 1 - Thermochemistry Virtually every chemical reaction is accompanied by a change in energy. Chemical reactions usually absorb or release energy as heat. You learned in Chapter 12
Chapter 17. Free Energy and Thermodynamics. Chapter 17 Lecture Lecture Presentation. Sherril Soman Grand Valley State University
 Chapter 17 Lecture Lecture Presentation Chapter 17 Free Energy and Thermodynamics Sherril Soman Grand Valley State University First Law of Thermodynamics You can t win! The first law of thermodynamics
Chapter 17 Lecture Lecture Presentation Chapter 17 Free Energy and Thermodynamics Sherril Soman Grand Valley State University First Law of Thermodynamics You can t win! The first law of thermodynamics
Crude Oil, Fractional Distillation and Hydrocarbons
 Crude Oil, Fractional Distillation and ydrocarbons The formation of Crude Oil, how it is processed to produce a range of useful materials, including Plastics via Polymerisation. Crude Oil Crude oil is
Crude Oil, Fractional Distillation and ydrocarbons The formation of Crude Oil, how it is processed to produce a range of useful materials, including Plastics via Polymerisation. Crude Oil Crude oil is
Lattice Energy: The Born-Haber cycle. Introduction. Lattice Energy. Born-Haber Cycle
 Lattice Energy: The Born-Haber cycle Ionic solids tend to be very stable compounds. The enthalpies of formation of the ionic molecules cannot alone account for this stability. These compounds have an additional
Lattice Energy: The Born-Haber cycle Ionic solids tend to be very stable compounds. The enthalpies of formation of the ionic molecules cannot alone account for this stability. These compounds have an additional
Liquids and Solids Chapter 10
 Liquids and Solids Chapter 10 Nov 15 9:56 AM Types of Solids Crystalline solids: Solids with highly regular arrangement of their components Amorphous solids: Solids with considerable disorder in their
Liquids and Solids Chapter 10 Nov 15 9:56 AM Types of Solids Crystalline solids: Solids with highly regular arrangement of their components Amorphous solids: Solids with considerable disorder in their
Work hard. Be nice. 100% EVERYDAY.
 Name: Period: Date: UNIT 10: Energy Lesson 1: Entropy vs. Enthalpy By the end of today, you will have an answer to: What conditions are favored during reactions? Definition Enthalpy is another word for
Name: Period: Date: UNIT 10: Energy Lesson 1: Entropy vs. Enthalpy By the end of today, you will have an answer to: What conditions are favored during reactions? Definition Enthalpy is another word for
AP Chemistry A. Allan Chapter Six Notes - Thermochemistry
 AP Chemistry A. Allan Chapter Six Notes - Thermochemistry 6.1 The Nature of Energy A. Definition 1. Energy is the capacity to do work (or to produce heat*) a. Work is a force acting over a distance (moving
AP Chemistry A. Allan Chapter Six Notes - Thermochemistry 6.1 The Nature of Energy A. Definition 1. Energy is the capacity to do work (or to produce heat*) a. Work is a force acting over a distance (moving
CHAPTER 10: THERMOCHEMISTRY
 CHAPTER 10: THERMOCHEMISTRY Definition of heat Calorimetry and relationship between heat and temperature changes Enthalpy Hess s Law Bond enthalpies First Law of Thermodynamics The Zeroth Law Zeroth Law
CHAPTER 10: THERMOCHEMISTRY Definition of heat Calorimetry and relationship between heat and temperature changes Enthalpy Hess s Law Bond enthalpies First Law of Thermodynamics The Zeroth Law Zeroth Law
Thermal Methods of Analysis
 Thermal Methods of Analysis Calorie-something we know What is calorie? Can you see or touch a calorie? How is it measured? Working out in gym Change in weight Loss of calories-burning of fat? (10 km=500calories/9cal
Thermal Methods of Analysis Calorie-something we know What is calorie? Can you see or touch a calorie? How is it measured? Working out in gym Change in weight Loss of calories-burning of fat? (10 km=500calories/9cal
Notes: Balancing Chemical Equations
 Notes: Balancing Chemical Equations Effects of chemical reactions: Chemical reactions rearrange atoms in the reactants to form new products. The identities and properties of the products are completely
Notes: Balancing Chemical Equations Effects of chemical reactions: Chemical reactions rearrange atoms in the reactants to form new products. The identities and properties of the products are completely
CHEM*3440. Thermal Methods. Thermogravimetry. Instrumental Components. Chemical Instrumentation. Thermal Analysis. Topic 14
 Thermal Methods We will examine three thermal analytical techniques: Thermogravimetric Analysis (TGA) CHEM*3440 Chemical Instrumentation Topic 14 Thermal Analysis Differential Thermal Analysis (DTA) Differential
Thermal Methods We will examine three thermal analytical techniques: Thermogravimetric Analysis (TGA) CHEM*3440 Chemical Instrumentation Topic 14 Thermal Analysis Differential Thermal Analysis (DTA) Differential
Name: Class: Date: ID: A
 Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
Name: Class: _ Date: _ ID: A Chpter 17 review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of these phase changes is an endothermic process? a.
AQA C1 Atomic Structure
 AQA C1 Atomic Structure What s in an atom? Elements in the periodic have different sizes of atoms. The size of the atoms depends on the number of protons, electrons and neutrons they have. Each element
AQA C1 Atomic Structure What s in an atom? Elements in the periodic have different sizes of atoms. The size of the atoms depends on the number of protons, electrons and neutrons they have. Each element
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a
 Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Aliphatic Hydrocarbons Anthracite alkanes arene alkenes aromatic compounds alkyl group asymmetric carbon Alkynes benzene 1a Hard coal, which is high in carbon content any straight-chain or branched-chain
Ch 10 Practice Problems
 Ch 10 Practice Problems 1. Which of the following result(s) in an increase in the entropy of the system? I. (See diagram.) II. Br 2(g) Br 2(l) III. NaBr(s) Na + (aq) + Br (aq) IV. O 2(298 K) O 2(373 K)
Ch 10 Practice Problems 1. Which of the following result(s) in an increase in the entropy of the system? I. (See diagram.) II. Br 2(g) Br 2(l) III. NaBr(s) Na + (aq) + Br (aq) IV. O 2(298 K) O 2(373 K)
Solid to liquid. Liquid to gas. Gas to solid. Liquid to solid. Gas to liquid. +energy. -energy
 33 PHASE CHANGES - To understand solids and liquids at the molecular level, it will help to examine PHASE CHANGES in a little more detail. A quick review of the phase changes... Phase change Description
33 PHASE CHANGES - To understand solids and liquids at the molecular level, it will help to examine PHASE CHANGES in a little more detail. A quick review of the phase changes... Phase change Description
Chapter 7 An Introduction to Chemical Reactions. An Introduction to Chemistry by Mark Bishop
 Chapter 7 An Introduction to Chemical Reactions An Introduction to Chemistry by Mark Bishop Chapter Map Chemical Reaction A chemical change or chemical reaction is a process in which one or more pure substances
Chapter 7 An Introduction to Chemical Reactions An Introduction to Chemistry by Mark Bishop Chapter Map Chemical Reaction A chemical change or chemical reaction is a process in which one or more pure substances
Chapter Introduction Lesson 1 Understanding Chemical Reactions Lesson 2 Types of Chemical Reactions Lesson 3 Energy Changes and Chemical Reactions
 Chapter Introduction Lesson 1 Understanding Chemical Reactions Lesson 2 Types of Chemical Reactions Lesson 3 Energy Changes and Chemical Reactions Chapter Wrap-Up Changes in Matter A physical change does
Chapter Introduction Lesson 1 Understanding Chemical Reactions Lesson 2 Types of Chemical Reactions Lesson 3 Energy Changes and Chemical Reactions Chapter Wrap-Up Changes in Matter A physical change does
Chapter 9 Practice Test
 Chapter 9 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which of the following describes a chemical reaction? a) A gas is given off when
Chapter 9 Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1) Which of the following describes a chemical reaction? a) A gas is given off when
Section 16.3 Phase Changes
 Section 16.3 Phase Changes Solid Liquid Gas 3 Phases of Matter Density of Matter How packed matter is (The amount of matter in a given space) Solid: Liquid: Gas: High Density Medium Density Low Density
Section 16.3 Phase Changes Solid Liquid Gas 3 Phases of Matter Density of Matter How packed matter is (The amount of matter in a given space) Solid: Liquid: Gas: High Density Medium Density Low Density
 HEMISTRY 110 EXAM 3 April 6, 2011 FORM A When the path is blocked, back up and see more of the way. 1. A 250 L vessel is evacuated and then connected to a 50.0 L bulb with compressed nitrogen. The pressure
HEMISTRY 110 EXAM 3 April 6, 2011 FORM A When the path is blocked, back up and see more of the way. 1. A 250 L vessel is evacuated and then connected to a 50.0 L bulb with compressed nitrogen. The pressure
Environmental Aspects of Oil Shale Development: A Review
 Environmental Aspects of Oil Shale Development: A Review Tom Wildeman, Ron Klusman, & Jim Ranville Dept. of Chemistry & Geochemistry Colorado School of Mines Background During the last oil shale development
Environmental Aspects of Oil Shale Development: A Review Tom Wildeman, Ron Klusman, & Jim Ranville Dept. of Chemistry & Geochemistry Colorado School of Mines Background During the last oil shale development
COMPARATIVE STUDY OF PYROLYSIS AND THERMAL DISSOLUTION OF ESTONIAN AND MONGOLIAN KHOOT OIL SHALES
 Oil Shale, 2016, Vol. 33, No. 4, pp. 329 339 ISSN 0208-189X doi: 10.3176/oil.2016.4.02 2016 Estonian Academy Publishers COMPARATIVE STUDY OF PYROLYSIS AND THERMAL DISSOLUTION OF ESTONIAN AND MONGOLIAN
Oil Shale, 2016, Vol. 33, No. 4, pp. 329 339 ISSN 0208-189X doi: 10.3176/oil.2016.4.02 2016 Estonian Academy Publishers COMPARATIVE STUDY OF PYROLYSIS AND THERMAL DISSOLUTION OF ESTONIAN AND MONGOLIAN
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes
 Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
UNIQUE MINERALOGY OF OIL SHALE FROM THE PICEANCE BASIN, COLORADO
 UNIQUE MINERALOGY OF OIL SHALE FROM THE PICEANCE BASIN, COLORADO 27th Oil Shale Symposium Golden, Colorado Marcus Wigand (Presenter) Steve Chipera Giday Woldegabriel J. William Carey John Kaszuba Doug
UNIQUE MINERALOGY OF OIL SHALE FROM THE PICEANCE BASIN, COLORADO 27th Oil Shale Symposium Golden, Colorado Marcus Wigand (Presenter) Steve Chipera Giday Woldegabriel J. William Carey John Kaszuba Doug
Influence of Substituent on Thermal Decomposition of 1H-1,2,4-Triazole
 01 th International Conference on Biology, Environment and Chemistry IPCBEE vol.58 (01) (01) IACSIT Press, Singapore DOI: 10.776/IPCBEE. 01. V58. 1 Influence of Substituent on Thermal Decomposition of
01 th International Conference on Biology, Environment and Chemistry IPCBEE vol.58 (01) (01) IACSIT Press, Singapore DOI: 10.776/IPCBEE. 01. V58. 1 Influence of Substituent on Thermal Decomposition of
Name Chemistry / / SOL Questions Chapter 9 For each of the following, fill in the correct answer on the BLUE side of the scantron.
 Name Chemistry / / SOL Questions Chapter 9 For each of the following, fill in the correct answer on the BLUE side of the scantron. 1. Which number on the graph to the right represents the effect of the
Name Chemistry / / SOL Questions Chapter 9 For each of the following, fill in the correct answer on the BLUE side of the scantron. 1. Which number on the graph to the right represents the effect of the
Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR)
 Technical articles Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR) Tadashi Arii* 1. Introduction Simultaneous
Technical articles Evolved gas analysis by simultaneous thermogravimetric differential thermal analysis-fourier transformation infrared spectroscopy (TG-DTA-FTIR) Tadashi Arii* 1. Introduction Simultaneous
15.1 The Concept of Equilibrium
 Lecture Presentation Chapter 15 Chemical Yonsei University 15.1 The Concept of N 2 O 4 (g) 2NO 2 (g) 2 Chemical equilibrium occurs when a reaction and its reverse reaction proceed at the same rate. The
Lecture Presentation Chapter 15 Chemical Yonsei University 15.1 The Concept of N 2 O 4 (g) 2NO 2 (g) 2 Chemical equilibrium occurs when a reaction and its reverse reaction proceed at the same rate. The
Chemistry Final Exam Study Guide June 2017
 Chemistry Final Exam Study Guide June 2017 Kinetic Molecular Theory, Gases, Phase Diagrams Phase Diagram for H 2 O 1. Label the above phase diagram for H 2 O, include all 6 possible transitions between
Chemistry Final Exam Study Guide June 2017 Kinetic Molecular Theory, Gases, Phase Diagrams Phase Diagram for H 2 O 1. Label the above phase diagram for H 2 O, include all 6 possible transitions between
StudyHub: AP Chemistry
 StudyHub+ 1 StudyHub: AP Chemistry Solution Composition and Energies, Boiling Point, Freezing Point, and Vapor Pressure StudyHub+ 2 Solution Composition: Mole Fraction: Formula: Mole Fraction of Component
StudyHub+ 1 StudyHub: AP Chemistry Solution Composition and Energies, Boiling Point, Freezing Point, and Vapor Pressure StudyHub+ 2 Solution Composition: Mole Fraction: Formula: Mole Fraction of Component
Name: Score: /100. Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each
 Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Chapter 12 Intermolecular Forces and Liquids
 Chapter 12 Intermolecular Forces and Liquids Jeffrey Mack California State University, Sacramento Why? Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature
Chapter 12 Intermolecular Forces and Liquids Jeffrey Mack California State University, Sacramento Why? Why is water usually a liquid and not a gas? Why does liquid water boil at such a high temperature
Thermodynamics Spontaneity. 150/151 Thermochemistry Review. Spontaneity. Ch. 16: Thermodynamics 12/14/2017
 Ch. 16: Thermodynamics Geysers are a dramatic display of thermodynamic principles in nature. As water inside the earth heats up, it rises to the surface through small channels. Pressure builds up until
Ch. 16: Thermodynamics Geysers are a dramatic display of thermodynamic principles in nature. As water inside the earth heats up, it rises to the surface through small channels. Pressure builds up until
Gummy Bear Demonstration:
 Name: Unit 8: Chemical Kinetics Date: Regents Chemistry Aim: _ Do Now: a) Using your glossary, define chemical kinetics: b) Sort the phrases on the SmartBoard into the two columns below. Endothermic Rxns
Name: Unit 8: Chemical Kinetics Date: Regents Chemistry Aim: _ Do Now: a) Using your glossary, define chemical kinetics: b) Sort the phrases on the SmartBoard into the two columns below. Endothermic Rxns
OCR Chemistry A H432
 All the energy changes we have considered so far have been in terms of enthalpy, and we have been able to predict whether a reaction is likely to occur on the basis of the enthalpy change associated with
All the energy changes we have considered so far have been in terms of enthalpy, and we have been able to predict whether a reaction is likely to occur on the basis of the enthalpy change associated with
Chapter 11 part 2: Properties of Liquids
 Chapter 11 part 2: Properties of Liquids Read: BLB 5.5; 11.4 HW: BLB 5:48, 49, 51; 11:33, 37, 39 Supplemental 11:5-10 Know: viscosity, surface tension cohesive & adhesive forces phase changes heat capacity
Chapter 11 part 2: Properties of Liquids Read: BLB 5.5; 11.4 HW: BLB 5:48, 49, 51; 11:33, 37, 39 Supplemental 11:5-10 Know: viscosity, surface tension cohesive & adhesive forces phase changes heat capacity
Ch. 11 States of matter
 Ch. 11 States of matter States of Matter Solid Definite volume Definite shape Liquid Definite volume Indefinite shape (conforms to container) Gas Indefinite volume (fills any container) Indefinite shape
Ch. 11 States of matter States of Matter Solid Definite volume Definite shape Liquid Definite volume Indefinite shape (conforms to container) Gas Indefinite volume (fills any container) Indefinite shape
AP CHEMISTRY 2009 SCORING GUIDELINES
 2009 SCORING GUIDELINES Question 1 (10 points) Answer the following questions that relate to the chemistry of halogen oxoacids. (a) Use the information in the table below to answer part (a)(i). Acid HOCl
2009 SCORING GUIDELINES Question 1 (10 points) Answer the following questions that relate to the chemistry of halogen oxoacids. (a) Use the information in the table below to answer part (a)(i). Acid HOCl
Chemical reactions. C2- Topic 5
 Chemical reactions C2- Topic 5 What is a chemical reaction? A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products)
Chemical reactions C2- Topic 5 What is a chemical reaction? A chemical reaction is a change that takes place when one or more substances (called reactants) form one or more new substances (called products)
Sensible Heat and Enthalpy Calculations
 Sensible Heat and Enthalpy Calculations Sensible Heat - The amount of heat that must be added when a substance undergoes a change in temperature from 298 K to an elevated temperature without a change in
Sensible Heat and Enthalpy Calculations Sensible Heat - The amount of heat that must be added when a substance undergoes a change in temperature from 298 K to an elevated temperature without a change in
Chapter 11 SOLIDS, LIQUIDS AND GASES Pearson Education, Inc.
 Chapter 11 SOLIDS, LIQUIDS AND GASES States of Matter Because in the solid and liquid states particles are closer together, we refer to them as. The States of Matter The state of matter a substance is
Chapter 11 SOLIDS, LIQUIDS AND GASES States of Matter Because in the solid and liquid states particles are closer together, we refer to them as. The States of Matter The state of matter a substance is
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
General Chemistry I. Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University. Module 4: Chemical Thermodynamics
 General Chemistry I Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University Module 4: Chemical Thermodynamics Zeroth Law of Thermodynamics. First Law of Thermodynamics (state quantities:
General Chemistry I Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University Module 4: Chemical Thermodynamics Zeroth Law of Thermodynamics. First Law of Thermodynamics (state quantities:
Matter. Gas. Solid Liquid. Both shape and volume are not fixed. It has a fixed shape and a fixed volume.
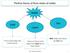 Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
Matter Solid Liquid Gas It has a fixed shape and a fixed volume. It does not have a fixed shape, but it does have a fixed volume. Both shape and volume are not fixed. Felix Yung and John Polias 1 Matter
5/14/14. How can you measure the amount of heat released when a match burns?
 CHEMISTRY & YOU Chapter 7 Thermochemistry How can you measure the amount of heat released when a match burns? 7. The Flow of Energy 7.3 Heat in Changes of State 7.4 Calculating Heats of Reaction Remember:
CHEMISTRY & YOU Chapter 7 Thermochemistry How can you measure the amount of heat released when a match burns? 7. The Flow of Energy 7.3 Heat in Changes of State 7.4 Calculating Heats of Reaction Remember:
Chapter 11 part 2: Properties of Liquids
 Chapter 11 part 2: Properties of Liquids Read: BLB 5.5; 11.4 HW: BLB 5:48, 49, 51; 11:33, 37, 39 Packet 11:5-10 Know: viscosity, surface tension cohesive & adhesive forces phase changes heat capacity calorimetry
Chapter 11 part 2: Properties of Liquids Read: BLB 5.5; 11.4 HW: BLB 5:48, 49, 51; 11:33, 37, 39 Packet 11:5-10 Know: viscosity, surface tension cohesive & adhesive forces phase changes heat capacity calorimetry
Hydrocarbons. Chapter 22-23
 Chapter 22-23 Hydrocarbons Organic Compounds All Carbon containing compounds Except carbon oxides, carbides, and carbonates which are inorganic. CO & CO2 Na4C CaCO3 +8 oxidation change CH 4 + O 2 CO 2
Chapter 22-23 Hydrocarbons Organic Compounds All Carbon containing compounds Except carbon oxides, carbides, and carbonates which are inorganic. CO & CO2 Na4C CaCO3 +8 oxidation change CH 4 + O 2 CO 2
Energy in Chemical Reaction Reaction Rates Chemical Equilibrium. Chapter Outline. Energy 6/29/2013
 Energy in Chemical Reaction Reaction Rates Chemical Equilibrium Chapter Outline Energy change in chemical reactions Bond dissociation energy Reaction rate Chemical equilibrium, Le Châtelier s principle
Energy in Chemical Reaction Reaction Rates Chemical Equilibrium Chapter Outline Energy change in chemical reactions Bond dissociation energy Reaction rate Chemical equilibrium, Le Châtelier s principle
Sensible Heat and Enthalpy Calculations
 * Sensible Heat and Enthalpy Calculations Sensible Heat - The amount of heat that must be added when a substance undergoes a change in temperature from 298 K to an elevated temperature without a change
* Sensible Heat and Enthalpy Calculations Sensible Heat - The amount of heat that must be added when a substance undergoes a change in temperature from 298 K to an elevated temperature without a change
Name: Score: /100. Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each
 Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Chapter 11 part 2. Properties of Liquids Viscosity Surface Tension Capillary Action. Phase Changes (energy of phase changes)
 Chapter 11 part 2 Properties of Liquids Viscosity Surface Tension Capillary Action Phase Changes (energy of phase changes) Dynamic Equilibrium Vapor pressure Phase diagram 1 Structure Affects Function
Chapter 11 part 2 Properties of Liquids Viscosity Surface Tension Capillary Action Phase Changes (energy of phase changes) Dynamic Equilibrium Vapor pressure Phase diagram 1 Structure Affects Function
Science 1206 Unit 3: Chemical Reactions Page 1 of 15
 Science 1206 Unit 3: Chemical Reactions Page 1 of 15 Introduction to Chemical Reactions Notes Part II TEXT p. 218-219 (word equations) There are many chemical reactions too many to count in fact! Like
Science 1206 Unit 3: Chemical Reactions Page 1 of 15 Introduction to Chemical Reactions Notes Part II TEXT p. 218-219 (word equations) There are many chemical reactions too many to count in fact! Like
SHALE HOLD TIME FOR OPTIMUM OIL SHALE RETORTING INSIDE A BATCH-LOADED FLUIDIZED- BED REACTOR
 Oil Shale, 2013, Vol. 30, No. 2, pp. 173 183 ISSN 0208-189X doi: 10.3176/oil.2013.2.07 2013 Estonian Academy Publishers SHALE HOLD TIME FOR OPTIMUM OIL SHALE RETORTING INSIDE A BATCH-LOADED FLUIDIZED-
Oil Shale, 2013, Vol. 30, No. 2, pp. 173 183 ISSN 0208-189X doi: 10.3176/oil.2013.2.07 2013 Estonian Academy Publishers SHALE HOLD TIME FOR OPTIMUM OIL SHALE RETORTING INSIDE A BATCH-LOADED FLUIDIZED-
1.4 Enthalpy. What is chemical energy?
 1.4 Enthalpy What is chemical energy? Chemical energy is a form of potential energy which is stored in chemical bonds. Chemical bonds are the attractive forces that bind atoms together. As a reaction takes
1.4 Enthalpy What is chemical energy? Chemical energy is a form of potential energy which is stored in chemical bonds. Chemical bonds are the attractive forces that bind atoms together. As a reaction takes
Chapter 9. Section #3! Stoichiometry and Cars!
 Section #3! Stoichiometry and Cars! CH 9 Section #3 An Air Bag Could Save Your Life q When inflated, air bags slow the motion of a person so that he or she does not strike the inside of a car with as much
Section #3! Stoichiometry and Cars! CH 9 Section #3 An Air Bag Could Save Your Life q When inflated, air bags slow the motion of a person so that he or she does not strike the inside of a car with as much
Equilibrium. What is equilibrium? Hebden Unit 2 (page 37 69) Dynamic Equilibrium
 Equilibrium What is equilibrium? Hebden Unit (page 37 69) Dynamic Equilibrium Hebden Unit (page 37 69) Experiments show that most reactions, when carried out in a closed system, do NOT undergo complete
Equilibrium What is equilibrium? Hebden Unit (page 37 69) Dynamic Equilibrium Hebden Unit (page 37 69) Experiments show that most reactions, when carried out in a closed system, do NOT undergo complete
Collision Theory. Collision theory: 1. atoms, ions, and molecules must collide in order to react. Only a small number of collisions produce reactions
 UNIT 16: Chemical Equilibrium collision theory activation energy activated complex reaction rate reversible reaction chemical equilibrium law of chemical equilibrium equilibrium constant homogeneous equilibrium
UNIT 16: Chemical Equilibrium collision theory activation energy activated complex reaction rate reversible reaction chemical equilibrium law of chemical equilibrium equilibrium constant homogeneous equilibrium
Enthalpy changes
 3.2.1. Enthalpy changes In an exothermic change energy is transferred from the system (chemicals) to the surroundings. The products have less energy than the If an enthalpy change occurs then energy is
3.2.1. Enthalpy changes In an exothermic change energy is transferred from the system (chemicals) to the surroundings. The products have less energy than the If an enthalpy change occurs then energy is
Section 3.0. The 1 st Law of Thermodynamics. (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities Definitions and Concepts
 Section 3.0. The 1 st Law of Thermodynamics (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities 3.2. Definitions and Concepts 3.3. The First Law of THERMODYNAMICS 3.4. Enthalpy 3.5. Adiabatic Expansion
Section 3.0. The 1 st Law of Thermodynamics (CHANG text Chapter 4) 3.1. Revisiting Heat Capacities 3.2. Definitions and Concepts 3.3. The First Law of THERMODYNAMICS 3.4. Enthalpy 3.5. Adiabatic Expansion
Chapter 14. Liquids and Solids
 Chapter 14 Liquids and Solids Review Solid - Has a definite (fixed) shape and volume (cannot flow). Liquid - Definite volume but takes the shape of its container (flows). Gas Has neither fixed shape nor
Chapter 14 Liquids and Solids Review Solid - Has a definite (fixed) shape and volume (cannot flow). Liquid - Definite volume but takes the shape of its container (flows). Gas Has neither fixed shape nor
Thermochemistry: Heat and Chemical Change
 Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
CHEMICAL REACTIONS & EQUATIONS
 CHEMICAL REACTIONS & EQUATIONS PHYSICAL AND CHEMICAL CHANGE In our daily life many processes occur around us. Some of them do not lead to formation of any new substance, while others may lead to formation
CHEMICAL REACTIONS & EQUATIONS PHYSICAL AND CHEMICAL CHANGE In our daily life many processes occur around us. Some of them do not lead to formation of any new substance, while others may lead to formation
Biology Unit 2 Chemistry of Life (Ch. 6) Guided Notes
 Name Biology Unit 2 Chemistry of Life (Ch. 6) Guided Notes Atoms, Elements, and Chemical Bonding I can draw atom models and identify the # protons, # neutrons, and # electrons in an atom. I can identify
Name Biology Unit 2 Chemistry of Life (Ch. 6) Guided Notes Atoms, Elements, and Chemical Bonding I can draw atom models and identify the # protons, # neutrons, and # electrons in an atom. I can identify
Changes in Matter. Introduction to Chemistry
 Changes in Matter Introduction to Chemistry Classifying Matter Matter: is anything that has mass and volume. Volume: the amount of space that something takes up Property: a characteristic of a material
Changes in Matter Introduction to Chemistry Classifying Matter Matter: is anything that has mass and volume. Volume: the amount of space that something takes up Property: a characteristic of a material
ENTHALPY CHANGE CHAPTER 4
 ENTHALPY CHANGE CHAPTER 4 ENTHALPY Is the total energy of a system. E k = Kinetic energy. Vibrational Rotational Translational E due to motion H = E k + E p E P = Potential energy Attractive force b/w
ENTHALPY CHANGE CHAPTER 4 ENTHALPY Is the total energy of a system. E k = Kinetic energy. Vibrational Rotational Translational E due to motion H = E k + E p E P = Potential energy Attractive force b/w
Chem 11 UNIT 3: STOICHIOMETRY Name:
 Chem 11 UNIT 3: STOICHIOMETRY Name: Ms. Pirvu Period: Writing & Balancing Equations Chemical reactions can be described by chemical equations. Recall Law of Conservation of Mass mass cannot be nor. This
Chem 11 UNIT 3: STOICHIOMETRY Name: Ms. Pirvu Period: Writing & Balancing Equations Chemical reactions can be described by chemical equations. Recall Law of Conservation of Mass mass cannot be nor. This
OFB Chapter 6 Condensed Phases and Phase Transitions
 OFB Chapter 6 Condensed Phases and Phase Transitions 6-1 Intermolecular Forces: Why Condensed Phases Exist 6- The Kinetic Theory of Liquids and Solids 6-3 Phase Equilibrium 6-4 Phase Transitions 6-5 Phase
OFB Chapter 6 Condensed Phases and Phase Transitions 6-1 Intermolecular Forces: Why Condensed Phases Exist 6- The Kinetic Theory of Liquids and Solids 6-3 Phase Equilibrium 6-4 Phase Transitions 6-5 Phase
WATER PROPERTIES. Supplemental Textbook Material Ch. 16, p
 WATER PROPERTIES Supplemental Textbook Material Ch. 16, p. 349-361 Be sure to attend lab this week Bring the lab manual Must pass lab to pass this class Instructors will give percent lab grade to one another
WATER PROPERTIES Supplemental Textbook Material Ch. 16, p. 349-361 Be sure to attend lab this week Bring the lab manual Must pass lab to pass this class Instructors will give percent lab grade to one another
Chemistry Lab Fairfax High School Invitational January 7, Team Number: High School: Team Members Names:
 Chemistry Lab Fairfax High School Invitational January 7, 2017 Team Number: High School: Team Members Names: Reference Values: Gas Constant, R = 8.314 J mol -1 K -1 Gas Constant, R = 0.08206 L atm mol
Chemistry Lab Fairfax High School Invitational January 7, 2017 Team Number: High School: Team Members Names: Reference Values: Gas Constant, R = 8.314 J mol -1 K -1 Gas Constant, R = 0.08206 L atm mol
Ch. 11: Liquids and Intermolecular Forces
 Ch. 11: Liquids and Intermolecular Forces Learning goals and key skills: Identify the intermolecular attractive interactions (dispersion, dipole-dipole, hydrogen bonding, ion-dipole) that exist between
Ch. 11: Liquids and Intermolecular Forces Learning goals and key skills: Identify the intermolecular attractive interactions (dispersion, dipole-dipole, hydrogen bonding, ion-dipole) that exist between
