Resonance Energy of Benzene
|
|
|
- Sandra Lewis
- 6 years ago
- Views:
Transcription
1 Resonance Energy of Benzene Purpose The purpose of this experiment is to evaluate the resonance energy of benzene by calorimetry. The heat of combustion of a compound will be measured with a bomb calorimeter. That measurement, plus literature values of combustion and vaporization enthalpies, will lead to an experimental value of the resonance energy. Background The molecule trans,trans,cis-1,5,9-cyclododecatriene ("CDDT") is a 12-membered hydrocarbon. Labels "E,E,Z" are sometimes used in place of "trans, trans, cis". CDDT contains three double bonds in a ring. These bonds are not resonance stabilized. CDDT can split into benzene and cyclohexane according to the following hypothetical reaction: C 12 H 18 (gas) C 6 H 6 (gas) + C 6 H 12 (gas) (1) The heat of reaction (Δ rxn H) is related to the resonance energy (ΔU) of benzene because the number of bonds of each type (C-C, C=C, C-H) is the same on both sides of the reaction. 1 Notice that all three of the compounds in reaction 1 are liquids under standard conditions at 25 C. The resonance energy is determined from reaction 1 in the gas phase where intermolecular interactions are negligible. A bomb calorimeter will be used to measure the heat of combustion of liquid CDDT. Combustion in the bomb calorimeter occurs under constant volume, so heat equals energy. The ideal gas law suffices to correct energy, ΔU, to enthalpy, ΔH: ΔH = ΔU + Δ(PV) = U + ( n gas )RT (2) CDDTbomb.odt 1
2 Our bomb calorimeter is an isoperibol calorimeter. Combustion occurs in the bomb, which sits in a water-filled bucket. A water-filled calorimeter jacket surrounds the bucket. The calorimeter jacket is held at a constant temperature while heat from the burning sample causes the bomb and bucket temperature to rise. The temperature difference between the bucket and the jacket causes a small heat flow. The calorimeter automatically calculates and accounts for the heat lost to or gained from the calorimeter jacket. The reaction for combustion of CDDT under standard conditions is C 12 H 18 (l) + (33/2)O 2 (g) 12 CO 2 (g) + 9 H 2 O(l) (3) The change in PV, Δ(PV) = Δn gas RT = (12-33/2)RT = -4.5RT. Substitute R= J mol - 1 K -1 and T=298.15K to get a numerical value for Δ(PV) for combustion of CDDT. The enthalpy of vaporization of CDDT is kj/mol. 3,4 The resonance energy of benzene can be defined as the negative ΔU of reaction 1, with reactants and products in the gas phase. 5 After you calculate Δ 1 H, convert it to Δ 1 U and resonance energy. Reagents and Supplies benzoic acid pellets (approximately 1g each) for standardizing the bomb calorimeter. Run two times. trans,trans,cis-1,5,9-cyclododecatriene ("CDDT") weigh precisely about 0.5 g. Run twice. In the lab (or get from the stockroom if they are not in the lab): 2L volumetric flask 1 ml pipet and bulb gloves for handling CDDT CDDTbomb.odt 2
3 Bomb Calorimetry Procedure for the Parr 1261 calorimeter Use a pellet of benzoic acid to do a standardization run and determine the "energy equivalent" (EE) of the calorimeter. It should be about 2400 calories per degree. The EE value for a standardization is stored in the calorimeter's memory. By default, the calorimeter uses the last 10 values stored to calculate heats for determination runs. In this experiment, however, you will calculate the EE value yourself from the temperature rise and the known energy of combustion of benzoic acid. (Instructions are below.) Make two standardization runs with benzoic acid. Use liquid CDDT to determine the heat of combustion of CDDT. Because the bomb operates at constant volume, the heat observed equals ΔU. Make two runs with CDDT and average the results. Detailed instructions follow. I. Equilibrate the Calorimeter Operating the Bomb Calorimeter 1. Turn on calorimeter using the power switch on the back. Turn the water faucet on slightly; this supplies a slow flow of cooling water to the bomb. Press the F1 key on the calorimeter to start the water pump and heater. You will hear them and can also look at the back of the instrument to check that the heater light is on. Allow 20 minutes for the controller to warm up and the jacket temperature to stabilize. The STANDBY light will turn on when the jacket temperature has reached the setpoint, 25º C. 2. Fill the calorimeter bucket with exactly 2 liters of distilled water measured by a 2L volumetric flask. Close the top of the calorimeter. II. Weigh the Sample 1. Cut a 10 cm piece of fuse wire and weigh it. 2. For a "calibration", weigh a pre-made pellet of benzoic acid. 3. For each "determination" run, use g liquid CDDT. Record the mass precisely. III. Attaching the Fuse Set the bomb head on the support stand and fasten a 10 cm length of fuse wire between the two electrodes. To attach the fuse to quick-grip electrodes, insert the ends of the wire into the eyelet at the end of each stem and push the cap downward to pinch the wire into place. Figure 1. support stand and fuse wire. CDDTbomb.odt 3
4 Place the fuel capsule with its weighed sample in the electrode loop and bend the wire downward toward the surface of the charge. When using a sample that is a pellet, bend the wire so that the loop bears against the top of the pellet. The wire should not touch any liquid sample, however, but should be positioned just slightly above it. Pipet exactly 1 ml of distilled water into the bomb. This is to absorb and retain acidic byproducts that may form during combustion. IV Close the Bomb Care must be taken not to disturb the sample when moving the bomb head from the support stand to the bomb cylinder. Check the sealing ring to be sure that it is in good condition and moisten it with a bit of water so that it will slide freely into the cylinder; then slide the head into the cylinder and push it down as far as it will go. For easy insertion, push the head straight down without twisting and leave the gas release valve open during this operation. Set the screw cap on the cylinder and turn it down firmly by hand to a solid stop. When properly closed, no threads on the cylinder should be exposed. If the screw cap tends to bind to the cylinder at this point, indicating that it might be difficult to open the bomb after it has been fired, turn the screw cap back slightly - but only a few degrees - enough to release the binding, since the bottom thread must remain fully engaged. Hand tightening suffices. Do not use a wrench on the screw cap. V Fill the Bomb with Oxygen 1. Close the gas relief valve on the bomb. 2. Open main valve on oxygen tank. Gas pressure in tank should be at 2000 psi and the regulated gas pressure should be 450 psi. 3. Attach gas line to gas inlet valve 4. Push O 2 Fill key on the calorimeter. A pressure lower than 420 psi will cause a low pressure warning. VI Operate the Calorimeter 1. Set the bucket in the calorimeter, as follows. Attach the lifting handle to the two holes in the side of the screw cap and partially lower the bomb in the water. Handle the bomb carefully during this operation so that the sample will not be disturbed. Push the two ignition lead wires into the terminal sockets on the bomb head. Orient the wires away from the stirrer shaft so they do not become tangled in the stirring CDDTbomb.odt 4
5 mechanism. Lower the bomb completely into water with its feet spanning the circular boss in the bottom of the bucket. Remove the lifting handle from the bomb (not from the bucket) and shake any drops of water back into the bucket. 2. Close the calorimeter cover. This lowers the stirrer and thermistor probe into the bucket. 3. Check the LED display to see whether the calorimeter is prepared to do a standardization (i.e., benzoic acid) or a determination. In the former case, "STD" will be lit; in the latter, "DETR". Benzoic acid is for standardization but CDDT combustion is a determination, and it is important that the calorimeter display match the type of run you are doing. Use Shift-F2 if necessary to change the type of run. Push START to initiate the test. 4. The calorimeter may automatically supply a six-digit Sample Identification Number. If not, it will wait for you to enter a sample id: up to six digits of your choice. Press "ENTER." 5. The calorimeter will prompt for the sample weight by flashing the WEIGHT light. Type the mass, in grams, and press "ENTER." 6. The calorimeter will now take over and conduct the test. During the time it is establishing the initial equilibrium, it will light the PREPERIOD light. Just before it fires the bomb, it will sound a series of short beeps to warn the user to move away from the calorimeter. 7. CAUTION: Do not have the head, hands, or any part of the body over the calorimeter when the bomb is being fired. Continue to stand clear for 30 seconds after firing. 8. Once the bomb has been fired, the POST light will come on. The calorimeter will check to make certain that a temperature rise occurs and will then look for the final equilibrium conditions, or if it fails to detect a temperature rise within the allotted time, the calorimeter will terminate the test and advise the user of the error. 9. At the conclusion of the test, the calorimeter will signal the user with a long beep. Press the DONE key to store the test result in the memory of the calorimeter. The calorimeter will automatically print out a preliminary report. 10. Open the cover and remove the bomb and bucket. Remove the bomb from the bucket and open the knurled valve knob on the bomb head to release the residual gas pressure before attempting to remove the cap. This release should proceed slowly over a period of not less than one minute to avoid entrainment losses. After all pressure has been released, unscrew the cap; lift the head out of the cylinder and place it on the support stand. CDDTbomb.odt 5
6 Examine the interior of the bomb for soot or other evidence of incomplete combustion. If such evidence is found, the test will have to be discarded. 11. Wash all interior surfaces of the bomb with water and dry thoroughly for the next run. Pour the bucket of water back into the 2L volumetric flask and refill the flask to the mark. Dry the bucket. 12. Remove all unburned pieces of fuse wire from the bomb electrodes and weigh them to obtain the fuse wire correction in calories (1400 cal/g or kj/g). 13. Now that you have the fuse correction you can print out your final report. Press the RPT key and the calorimeter will prompt you for a sample ID. Key in the ID number of the sample and hit ENTER. You will be prompted for a fuse correction. Key in the number of calories calculated previously and hit ENTER. Now you will be prompted for an acid correction. Hit ENTER to accept the default. In the case of a determination, you will be prompted for a sulfur correction. Again, you can hit ENTER to accept the default. 14. When you are done with all of your runs and have printed all of your final reports you need to erase your information. Press SHIFT and then F1 and you will be prompted for a Sample ID. Key in your Sample ID and hit ENTER. Again, you will be prompted for a Sample ID and you can key in your second and press ENTER. Once you have erased all samples, press DONE. 15. If no one else is using the calorimeter, you should turn it off. Press F1, which will stop the water pump. Turn off the water faucet. Turn off the power switch on the back of the bomb. CDDTbomb.odt 6
7 Table 2. Sample Calorimeter Reports CALORIMETRY PRELIMINARY REPORT 04/01/09 12:34:55 STANDARDIZATION SAMPLE ID CAL ID 1 WEIGHT.9860 FUSE ACID 10 SULFUR 0.00 INIT. TEMP TEMP. RISE SPIKE WGHT. EE VALUE GROSS HEAT CAL/G DYNAMIC MODE CALORIMETRY FINAL REPORT 04/01/09 13:30:36 STANDARDIZATION SAMPLE ID CAL ID 1 WEIGHT (grams) FUSE (cal) 15.0 ACID 10 SULFUR 0.00 INIT. TEMP TEMP. RISE SPIKE WGHT. EE VALUE GROSS HEAT CAL/G DYNAMIC MODE CALORIMETRY PRELIMINARY REPORT 04/01/09 17:00:51 DETERMINATION SAMPLE ID CAL ID 1 WEIGHT.431 FUSE 14.8 ACID 10 SULFUR 0.00 INIT. TEMP TEMP. RISE SPIKE WGHT. EE VALUE GROSS HEAT CAL/G DYNAMIC MODE CALORIMETRY FINAL REPORT 04/01/09 17:37:52 DETERMINATION SAMPLE ID CAL ID 1 WEIGHT (grams).425 FUSE (cal) 13.4 ACID 10 SULFUR 0.00 INIT. TEMP TEMP. RISE SPIKE WGHT. EE VALUE GROSS HEAT CAL/G DYNAMIC MODE CDDTbomb.odt 7
8 Calculations In order to calculate the heat capacity, C, of the bomb calorimeter use results from the standardization run. The standardization run burns benzoic acid. C 6 H 5 COOH(s) + (15/2)O 2 (g) 7CO 2 (g) + 3H 2 O(l) The heat of combustion of the benzoic acid pellets used in this experiment is reported by the supplier, Parr Instrument Company, to be kj/g. That is equivalent to Δ c U = kj/mol. The heat released by combustion of the pellet, Q ba, may be calculated either as kj/g m ba or -Δ c U n ba, where m ba stands for the mass of benzoic acid, n ba for the moles. For a pellet of mass g, for example, Q ba = kj. Q ba = kj/g m ba Calculate the fuse correction. Fuse correction = 5.858kJ/g grams of fuse burned. For example, g kj/g = kj. Calculate the heat capacity of the bomb. C = [ Q ba + fuse correction] / (Temp. rise) where "Temp. rise" is the temperature rise, read from either the preliminary or final standardization report. For example, with the fuse correction Q ba given above, and with a temperature rise of K, C = kj/k. You will have preformed two standardization runs. Each gives a value of the heat capacity. Calculate the average and use the average C in all further calculations. For each of the two determination runs calculate the fuse correction. Convert the mass of cddt to moles. n cddt = (mass cddt) / ( molecular mass cddt) = (grams cddt)/ g/mol. For example, g / g/mol = mol. Calculate the enthalpy of combustion of liquid cddt. Δ c U(cddt,liquid) = - [(Temp rise) C - (grams fuse burned) 5.858kJ/g] / n cddt For example, using the data on the first of the two sample determination reports, and with an average C (just an example) of kj/k, CDDTbomb.odt 8
9 Δ c U(cddt,liquid) = -[( K) kj/k kj] / mol = kj/mol. Average the cddt combustion energies from the two runs. Convert from Δ c U(cddt,liquid) to Δ c H(cddt,liquid) as discussed earlier. Use T = 298K. Δ c H(cddt,liquid) = Δ c U(cddt,liquid) -4.5RT The enthalpy of vaporization of cddt is kj/mol. 3,4 The enthalpy of combustion of gaseous cddt can be calculated with the aid of the enthalpy of vaporization. Δ c H(cddt,gas) = Δ c H(cddt,liquid) - Δ vap H(cddt,liquid) Calculate the enthalpy of formation of gaseous cddt from its combustion enthalpy and the formation enthalpies of gaseous carbon dioxide and liquid water. From the stoichiometry of reaction 3, the combustion reaction, Δ f H o (cddt,gas) = 12Δ f H o (CO 2,gas) + 9Δ f H o (H 2 O,liquid) - Δ c H(cddt,gas) Look up literature values of the enthalpy of formation of gaseous benzene and gaseous cyclohexane. Using those and your Δ f H o (cddt,gas), calculate the standard enthalpy of reaction 1. Then convert ΔH for reaction 1 to ΔU, using Δ rxn1 U = ΔH - Δn gas RT, where Δn gas is the change in the number of moles of gas in reaction 1 (i.e., Δn gas =2-1) and T = 298K. Finally, Resonance Energy = U res = -Δ rxn1 U You may find a literature value of the resonance energy of benzene in books and on the web. Linus Pauling's early paper 6, and the textbook Organic Chemistry by McMurry 7, for example, discuss resonance energy. Yirong Mo and Paul von Ragué Schleyer 8 discuss the traditional value (their reaction 3) and more modern evaluations. CDDTbomb.odt 9
10 Experimental uncertainty in resonance energy The experimental uncertainty in resonance energy, U res, can be estimated based on uncertainties in the largest contributions to Δ rxn1 U. Assume that literature values of formation enthalpies are essentially exact. Fuse corrections and Δ(PV) corrections are small, so they contribute little to uncertainty in U res. The absolute uncertainty (i.e., not the percent uncertainty) in U res is approximately the same as the absolute uncertainty in the combustion enthalpy of liquid CDDT. Δ c H(CDDT) - ΔT C / n CDDT where n CDDT represents moles of CDDT in a sample. Uncertainties in ΔT, C and n CDDT may be estimated as follows. Moles is proportional to molecular mass (which is known essentially exactly) so relative uncertainty (i.e., percent uncertainty) in n CDDT is the same as relative uncertainty in mass of CDDT, which is determined primarily by the scale on which the mass was taken. σ ncddt /n CDDT = σ mcddt /m CDDT, where m CDDT is the mass of CDDT. The mass of CDDT will be measured to approximately 10-3 or 10-4 g, depending on the balance used. Suppose, for example, that σ mcddt = grams and m CDDT = 0.5 grams. Then σ ncddt /n CDDT = σ mass /m CDDT / 0.5 = The uncertainty in C, the heat capacity of the bomb, is approximately the difference between the two values of C that were measured. If, for example, the values of C measured were and kj/k, then σ C 0.04 kj/k and σ C /C = 0.04/10 = The uncertainty of ΔT may be taken to be ±1 in the last digit shown on the print-out, assuming that all printed digits are significant. The Parr calorimeter prints temperature rise to five decimal places, so σ ΔT /(ΔT) T / 2T = which is insignificant compared to uncertainties in moles and heat capacity. Combining uncertainties in C and n CDDT, neglecting σ ΔT, the uncertainty in Δ C H(CDDT) is σ ΔH / (Δ c H) [ (σ C /C) 2 + (σ mcddt /m CDDT ) 2 ] 1/2. The absolute uncertainty in U res is the same as the absolute uncertainty in Δ C H(CDDT). σ Ures = Δ C H(CDDT) [ (σ C /C) 2 + (σ mcddt /m CDDT ) 2 ] 1/2 Using the example uncertainties above suggests σ Ures = (7300kJ/mol) [ ( ) 2 +( ) 2 ] 1/2 = 30 kj/mol. Your experimental uncertainty may differ from that estimate. Calculate σ Ures based on your heat capacities and CDDT masses, as a basis for deciding whether the U res you calculate does or does not agree with a literature value within experimental uncertainty. CDDTbomb.odt 10
11 References 1. Pickering, M. A novel bomb calorimetric determination of the resonance energy of benzene. Journal of Chemical Education 1982, 59(4), Engel, T.; Reid, P. Physical Chemistry, 3rd edition; Pearson Education: Boston; See section 8.6 for the Clausius-Clapeyron equation. 3. Rauh, Von H.-J.; Geyer, W.; Schmidt, H.; Geiseler, G. Bildungsenthalpien und Mesomerieenergien von π-bindungssystemen. 5. Mitteilung: Bildungsenthalpien von Oligomeren des Butadiens. Zeitschrift fur Physikalische Chemie 1973, 253, NIST web book. Data downloaded 3 December 2012 from 5. Halpern, A.M. Experimental Physical Chemistry, 2nd ed; Prentice-Hall: Upper Saddle River, NJ, 1997; Experiment Pauling, Linus; Sherman, J. The Nature of the Chemical Bond. VI. The calculation from thermochemical data of the energy of resonance of molecules among several electronic structures, Journal of Chemical Physics, 1(8), , McMurry, John, Organic Chemistry, Sixth edition; Brooks/Cole - Thomson Learning: Belmont, California; 2004; pages Mo, Yirong; von Ragué Schleyer, Paul, An Energetic Measure of Aromaticity and Antiaromaticity Based on the Pauling Wheland Resonance Energies, Chemistry A European Journal 2006, 12(7), CDDTbomb.odt 11
Determination of Resonance Stabilization Energy of Benzene Jonathan Smith (With Small Adaptations by Amanda Nienow)
 Determination of Resonance Stabilization Energy of Benzene Jonathan Smith (With Small Adaptations by Amanda Nienow) Abstract In this investigation we will measure the heat evolved during the combustion
Determination of Resonance Stabilization Energy of Benzene Jonathan Smith (With Small Adaptations by Amanda Nienow) Abstract In this investigation we will measure the heat evolved during the combustion
Experiment 1: Adiabatic Bomb Calorimeter (Dated: August 25, 2009)
 Experiment 1: Adiabatic Bomb Calorimeter (Dated: August 25, 2009) I. INTRODUCTION Heat released in a chemical reaction can be determined experimentally by using an adiabatic calorimeter. The reaction must
Experiment 1: Adiabatic Bomb Calorimeter (Dated: August 25, 2009) I. INTRODUCTION Heat released in a chemical reaction can be determined experimentally by using an adiabatic calorimeter. The reaction must
Bomb Calorimetry: Heat of Combustion of Naphthalene
 Bomb Calorimetry: Heat of Combustion of Naphthalene Most tabulated H values of highly exothermic reactions come from bomb calorimeter experiments. Heats of combustion are most common, in which the combustible
Bomb Calorimetry: Heat of Combustion of Naphthalene Most tabulated H values of highly exothermic reactions come from bomb calorimeter experiments. Heats of combustion are most common, in which the combustible
Thermochemistry/Calorimetry. Determination of the enthalpy of combustion with a calorimetric bomb LEC 02. What you need:
 LEC 02 Thermochemistry/Calorimetry with a calorimetric bomb What you can learn about 1st law of thermodynamics Hess law Enthalpy of combustion Enthalpy of formation Heat capacity Principle and tasks The
LEC 02 Thermochemistry/Calorimetry with a calorimetric bomb What you can learn about 1st law of thermodynamics Hess law Enthalpy of combustion Enthalpy of formation Heat capacity Principle and tasks The
Heat of Combustion. Parr oxygen bomb calorimeter (or equivalent), pellet press, thermometer (0.01 C), fuse wire.
 Heat of Combustion PURPOSE The purposes of this experiment are to determine the heats of combustion of several related substances using a bomb calorimeter and relate differences in heats of combustion
Heat of Combustion PURPOSE The purposes of this experiment are to determine the heats of combustion of several related substances using a bomb calorimeter and relate differences in heats of combustion
The Enthalpy of Formation of Camphor by Bomb Calorimetry 1
 The Enthalpy of Formation of Camphor by Bomb Calorimetry 1 Purpose: The enthalpy of combustion of camphor will be determined in a bomb calorimeter. The enthalpy of formation of camphor will be calculated
The Enthalpy of Formation of Camphor by Bomb Calorimetry 1 Purpose: The enthalpy of combustion of camphor will be determined in a bomb calorimeter. The enthalpy of formation of camphor will be calculated
PROPULSION LAB MANUAL
 PROPULSION LAB MANUAL Measurement of Calorific Value of a Solid Fuel Sample using a Bomb Calorimeter DEPARTMENT OF AEROSPACE ENGINEERING Indian Institute of Technology Kharagpur CONTENTS 1. Introduction
PROPULSION LAB MANUAL Measurement of Calorific Value of a Solid Fuel Sample using a Bomb Calorimeter DEPARTMENT OF AEROSPACE ENGINEERING Indian Institute of Technology Kharagpur CONTENTS 1. Introduction
Bomb Calorimetry. Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
 Experiment 12 Bomb Calorimetry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose A bomb calorimeter will
Experiment 12 Bomb Calorimetry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose A bomb calorimeter will
4 BOMB CALORIMETRY. Chapter 4. Bomb Calorimetry 24 I. PREPARATION
 Chapter 4. Bomb Calorimetry 24 4 BOMB CALORIMETRY I. PREPARATION Read "Principles of Calorimetry" in Ch. VI of Shoemaker, Garland, and Nibler (SGN, pp. 145-151), as well as Experiment 6 (pp. 152-158).
Chapter 4. Bomb Calorimetry 24 4 BOMB CALORIMETRY I. PREPARATION Read "Principles of Calorimetry" in Ch. VI of Shoemaker, Garland, and Nibler (SGN, pp. 145-151), as well as Experiment 6 (pp. 152-158).
COPYRIGHT FOUNTAINHEAD PRESS
 Calorimetry: Heats of Solution Objective: Use calorimetric measurements to determine heats of solution of two ionic compounds. Materials: Solid ammonium nitrate (NH 4 NO 3 ) and anhydrous calcium chloride
Calorimetry: Heats of Solution Objective: Use calorimetric measurements to determine heats of solution of two ionic compounds. Materials: Solid ammonium nitrate (NH 4 NO 3 ) and anhydrous calcium chloride
Thermochemistry/Calorimetry. Determination of the enthalpy of vaporization of liquids LEC 02. What you need: What you can learn about
 LEC 02 Thermochemistry/Calorimetry Determination of the enthalpy of vaporization of liquids What you can learn about Enthalpy of vaporisation Entropy of vaporisation Trouton s rule Calorimetry Heat capacity
LEC 02 Thermochemistry/Calorimetry Determination of the enthalpy of vaporization of liquids What you can learn about Enthalpy of vaporisation Entropy of vaporisation Trouton s rule Calorimetry Heat capacity
EXPERIMENT 5. THE ENTHALPY OF FORMATION OF CAMPHOR BY BOMB CALORIMETRY 1
 EXPERIMENT 5. THE ENTHALPY OF FORMATION OF CAMPHOR BY BOMB CALORIMETRY 1 The enthalpies of combustion and formation for camphor will be determined using a bomb calorimeter and tabulated values for the
EXPERIMENT 5. THE ENTHALPY OF FORMATION OF CAMPHOR BY BOMB CALORIMETRY 1 The enthalpies of combustion and formation for camphor will be determined using a bomb calorimeter and tabulated values for the
Heat of Neutralization
 Heat of Neutralization Purpose The purpose of this experiment is to determine the heat of reaction for a neutralization reaction. Problem to be Investigated HA + NAOH NaA + HOH For which acid, HCL, H 2
Heat of Neutralization Purpose The purpose of this experiment is to determine the heat of reaction for a neutralization reaction. Problem to be Investigated HA + NAOH NaA + HOH For which acid, HCL, H 2
Lab 2. Molar enthalpy of combustion
 Lab 2. Molar enthalpy of combustion Introduction Chemical reactions like burning an organic compound typically produce heat. In this experiment you will be measuring the amount of heat produced by burning
Lab 2. Molar enthalpy of combustion Introduction Chemical reactions like burning an organic compound typically produce heat. In this experiment you will be measuring the amount of heat produced by burning
Experiment 2 Heat of Combustion: Magnesium
 Experiment 2 Heat of Combustion: Magnesium Purpose Hess s Law states that when are going from a particular set of reactants to a particular set of products, the heat of reaction is the same whether the
Experiment 2 Heat of Combustion: Magnesium Purpose Hess s Law states that when are going from a particular set of reactants to a particular set of products, the heat of reaction is the same whether the
ENTHALPY OF FORMATION OF MgO
 ENTHALPY OF FORMATION OF MgO ELECTRONIC LABORATORY NOTEBOOK (ELN) INSTRUCTIONS All work for this experiment must be recorded, attached, or answered in the ELN. Create a pre & inlab page in the Experiment
ENTHALPY OF FORMATION OF MgO ELECTRONIC LABORATORY NOTEBOOK (ELN) INSTRUCTIONS All work for this experiment must be recorded, attached, or answered in the ELN. Create a pre & inlab page in the Experiment
Conductometric Titration & Gravimetric Determination of a Precipitate
 Conductometric Titration & Gravimetric Determination of a Precipitate Experiment 9 In this experiment, you will monitor conductivity during the reaction between sulfuric acid, H2SO4, and barium hydroxide,
Conductometric Titration & Gravimetric Determination of a Precipitate Experiment 9 In this experiment, you will monitor conductivity during the reaction between sulfuric acid, H2SO4, and barium hydroxide,
ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions
 Name Chem 161, Section: Group Number: ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a
Name Chem 161, Section: Group Number: ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
Calorimetry. Chapter 5. Week 2 Unit 1. Calorimetry. Since we cannot know the of the reactants and products, we measure H through, the of.
 Chapter 5 Week 2 Unit 1 Calorimetry John D. Bookstaver St. Charles Community College Cottleville, MO Calorimetry Since we cannot know the of the reactants and products, we measure H through, the of. 1
Chapter 5 Week 2 Unit 1 Calorimetry John D. Bookstaver St. Charles Community College Cottleville, MO Calorimetry Since we cannot know the of the reactants and products, we measure H through, the of. 1
Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio
![Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio](/thumbs/87/96015679.jpg) Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio ABSTRACT In this experiment a Parr 1341 Plain Jacket Oxygen Bomb Calorimeter was employed to determine the enthalpy
Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio ABSTRACT In this experiment a Parr 1341 Plain Jacket Oxygen Bomb Calorimeter was employed to determine the enthalpy
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
MEEN 3242 MEE LAB II
 MEEN 3242 MEE LAB II Experiment Report Lab # 2 Bomb Calorimeter Group Members: Hugo Diaz Reed Greenwood Juan Orona Alan Sanoja Micheal Shackelford Date: September 27, 2013 DEPARTMENT OF MECHANICAL AND
MEEN 3242 MEE LAB II Experiment Report Lab # 2 Bomb Calorimeter Group Members: Hugo Diaz Reed Greenwood Juan Orona Alan Sanoja Micheal Shackelford Date: September 27, 2013 DEPARTMENT OF MECHANICAL AND
1.4 Energetics. N Goalby chemrevise.org 1. Standard Enthalpy Change of Formation. Standard Enthalpy Change of Combustion
 1.4 Energetics Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. In an exothermic change energy is transferred
1.4 Energetics Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. In an exothermic change energy is transferred
Thermochemistry. Using Heats of Reaction - Hess s Law - Standard Enthalpies of Formation - Fuels Foods, Commercial Fuels, and Rocket Fuels
 Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy.
 Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Enthalpy changes
 3.2.1. Enthalpy changes In an exothermic change energy is transferred from the system (chemicals) to the surroundings. The products have less energy than the If an enthalpy change occurs then energy is
3.2.1. Enthalpy changes In an exothermic change energy is transferred from the system (chemicals) to the surroundings. The products have less energy than the If an enthalpy change occurs then energy is
Enthalpies of Reaction
 Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
EXPERIMENT 14 SPECIFIC HEAT OF WATER. q = m s T
 EXPERIMENT 14 SPECIFIC HEAT OF WATER INTRODUCTION: Heat is a form of energy which can pass from an object of relatively high temperature to an object of relatively low temperature. One physical property
EXPERIMENT 14 SPECIFIC HEAT OF WATER INTRODUCTION: Heat is a form of energy which can pass from an object of relatively high temperature to an object of relatively low temperature. One physical property
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat
 PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat Objective: The purpose of this activity is to determine whether the energy dissipated by a heating resistor
PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat Objective: The purpose of this activity is to determine whether the energy dissipated by a heating resistor
Name: Section: Score: /10 PRE LABORATORY ASSIGNMENT EXPERIMENT 7
 Name: Section: Score: /10 PRE LABORATORY ASSIGNMENT EXPERIMENT 7 1. Is the sign of Δ r H for an exothermic reaction positive or negative? Why? 2. When 4.21 grams of potassium hydroxide are added to 250.
Name: Section: Score: /10 PRE LABORATORY ASSIGNMENT EXPERIMENT 7 1. Is the sign of Δ r H for an exothermic reaction positive or negative? Why? 2. When 4.21 grams of potassium hydroxide are added to 250.
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School
 Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
Brown, LeMay Ch 5 AP Chemistry Monta Vista High School 1 From Greek therme (heat); study of energy changes in chemical reactions Energy: capacity do work or transfer heat Joules (J), kilo joules (kj) or
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
THER Mo CHEMISTRY: HEAT OF Ne UTRALIZATION
 Experiment 11 Name: 42 THER Mo CHEMISTRY: HEAT OF Ne UTRALIZATION In this experiment, you will use calorimetry to experimentally determine the heat of neutralization of NaOH-HCl, or the enthalpy of the
Experiment 11 Name: 42 THER Mo CHEMISTRY: HEAT OF Ne UTRALIZATION In this experiment, you will use calorimetry to experimentally determine the heat of neutralization of NaOH-HCl, or the enthalpy of the
How bad is that snack anyway?
 Physical Sciences 11 Experiment 1 How bad is that snack anyway? Monday, 2/10 Wednesday, 2/12 Science Center Room 117 Please read this entire document and complete the attached prelab before your lab. This
Physical Sciences 11 Experiment 1 How bad is that snack anyway? Monday, 2/10 Wednesday, 2/12 Science Center Room 117 Please read this entire document and complete the attached prelab before your lab. This
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 6 Energy and Chemical Change. Brady and Senese 5th Edition
 Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
8 Enthalpy of Reaction
 E x p e r i m e n t Enthalpy of Reaction Lecture and Lab Skills Emphasized Calculating the heat and enthalpy of reactions. Writing net ionic equations. Using Hess s law to determine the enthalpy of a reaction.
E x p e r i m e n t Enthalpy of Reaction Lecture and Lab Skills Emphasized Calculating the heat and enthalpy of reactions. Writing net ionic equations. Using Hess s law to determine the enthalpy of a reaction.
AC : PC-BASED MEASUREMENT OF THE HEAT OF COMBUSTION OF A SOLID FUEL USING OXYGEN BOMB CALORIMETER
 AC 2007-2839: PC-BASED MEASUREMENT OF THE HEAT OF COMBUSTION OF A SOLID FUEL USING OXYGEN BOMB CALORIMETER Ramesh Prasad, University of New Brunswick-St. John Ramesh C. Prasad, Professor of Mechanical
AC 2007-2839: PC-BASED MEASUREMENT OF THE HEAT OF COMBUSTION OF A SOLID FUEL USING OXYGEN BOMB CALORIMETER Ramesh Prasad, University of New Brunswick-St. John Ramesh C. Prasad, Professor of Mechanical
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
8. Energetics I. N Goalby chemrevise.org 1
 8. Energetics I Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. In an exothermic change energy is transferred
8. Energetics I Definition: Enthalpy change is the amount of heat energy taken in or given out during any change in a system provided the pressure is constant. In an exothermic change energy is transferred
Thermochemistry. Chapter 6. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
Name Partner. Thermal Physics. Part I: Heat of Vaporization of Nitrogen. Introduction:
 Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
Ch 6. Energy and Chemical Change. Brady & Senese, 5th Ed.
 Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
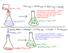 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 3. Thermochemistry: Energy Flow and Chemical Change. 5.1 Forms of Energy and Their Interconversion
 Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
Thermodynamics. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
Thermodynamics Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Thermodynamics is the scientific study of the interconversion of heat and other kinds of energy.
CHM201 General Chemistry and Laboratory I Laboratory 7 Thermochemistry and Hess s Law May 2, 2018
 Purpose: CHM201 General Chemistry and Laboratory I Laboratory 7 Thermochemistry and Hess s Law May 2, 2018 In this laboratory, you will measure heat changes arising from chemical reactions. You will use
Purpose: CHM201 General Chemistry and Laboratory I Laboratory 7 Thermochemistry and Hess s Law May 2, 2018 In this laboratory, you will measure heat changes arising from chemical reactions. You will use
CONSTANT PRESSURE CALORIMETRY: A STUDY OF GLYCINE PROTON-TRANSFER ENTHALPIES 1
 CONSTANT PRESSURE CALORIMETRY: A STUDY OF GLYCINE PROTON-TRANSFER ENTHALPIES 1 OBJECTIVES 1. To determine the reaction enthalpies for the proton transfer reactions of glycine. 2. To use a high-precision
CONSTANT PRESSURE CALORIMETRY: A STUDY OF GLYCINE PROTON-TRANSFER ENTHALPIES 1 OBJECTIVES 1. To determine the reaction enthalpies for the proton transfer reactions of glycine. 2. To use a high-precision
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
Workbook 5. Chem 1A Dr. White 1
 Chem 1A Dr. White 1 Workbook 5 5-1: Dalton s Law, KMT, Effusion/Diffusion/Real Gases 1. What is the total pressure and the partial pressure of each gas (in atm) in a mixture of 3.2 g of O 2, 1.6 g of CH
Chem 1A Dr. White 1 Workbook 5 5-1: Dalton s Law, KMT, Effusion/Diffusion/Real Gases 1. What is the total pressure and the partial pressure of each gas (in atm) in a mixture of 3.2 g of O 2, 1.6 g of CH
Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE LEARNING OBJECTIVES
 Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE The learning objectives of this experiment are LEARNING OBJECTIVES A simple coffee cup calorimeter will be used to determine the enthalpy of formation
Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE The learning objectives of this experiment are LEARNING OBJECTIVES A simple coffee cup calorimeter will be used to determine the enthalpy of formation
Using Conductivity to Find an Equivalence Point
 Experiment 25 PRE LAB DISCUSSION In this experiment, you will monitor conductivity during the reaction between sulfuric acid, and barium hydroxide in order to determine the equivalence point. From this
Experiment 25 PRE LAB DISCUSSION In this experiment, you will monitor conductivity during the reaction between sulfuric acid, and barium hydroxide in order to determine the equivalence point. From this
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
EXPERIMENT 9 ENTHALPY OF REACTION HESS S LAW
 EXPERIMENT 9 ENTHALPY OF REACTION HESS S LAW INTRODUCTION Chemical changes are generally accompanied by energy changes; energy is absorbed or evolved, usually as heat. Breaking chemical bonds in reactants
EXPERIMENT 9 ENTHALPY OF REACTION HESS S LAW INTRODUCTION Chemical changes are generally accompanied by energy changes; energy is absorbed or evolved, usually as heat. Breaking chemical bonds in reactants
In fact, we are going to be sneaky and use Hess s Law to determine the heat of magnesium combustion indirectly. Go to the website:
 Chemistry 161 Please prepare your notebook though the data tables before class on Wednesday, October 27. Write an abstract and place it at the front of your individual report. The abstract and the report
Chemistry 161 Please prepare your notebook though the data tables before class on Wednesday, October 27. Write an abstract and place it at the front of your individual report. The abstract and the report
Homework Problem Set 6 Solutions
 Chemistry 360 Dr. Jean M. Standard Homework Problem Set 6 Solutions 1. Determine the amount of pressure-volume work performed by 50.0 g of liquid water freezing to ice at 0 C and 1 atm pressure. The density
Chemistry 360 Dr. Jean M. Standard Homework Problem Set 6 Solutions 1. Determine the amount of pressure-volume work performed by 50.0 g of liquid water freezing to ice at 0 C and 1 atm pressure. The density
Conductimetric Titration and Gravimetric Determination of a Precipitate
 Conductimetric Titration and Gravimetric Determination of a Precipitate LabQuest 16 In this experiment, you will monitor conductivity during the reaction between sulfuric acid, H 2 SO 4, and barium hydroxide,
Conductimetric Titration and Gravimetric Determination of a Precipitate LabQuest 16 In this experiment, you will monitor conductivity during the reaction between sulfuric acid, H 2 SO 4, and barium hydroxide,
Chemical Thermodynamics. Chemical Thermodynamics. Changes of State. Chemical Thermodynamics. State Functions. State Functions 11/25/13
 Chemical Thermodynamics n Thermodynamics is the study of the energetics and order of a system. n A system is the thing we want to study it can be a chemical reaction, a solution, an automobile, or the
Chemical Thermodynamics n Thermodynamics is the study of the energetics and order of a system. n A system is the thing we want to study it can be a chemical reaction, a solution, an automobile, or the
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 6. Thermochemistry
 Chapter 6 Thermochemistry This chapter develops for the student the concepts of thermochemistry. Upon completion of Chapter 6, the student should be able to: 1. Define and explain the following terms:
Chapter 6 Thermochemistry This chapter develops for the student the concepts of thermochemistry. Upon completion of Chapter 6, the student should be able to: 1. Define and explain the following terms:
The Synthesis and Analysis of Aspirin
 The Synthesis and Analysis of Aspirin Computer 22 Aspirin, the ubiquitous pain reliever, goes by the chemical name acetylsalicylic acid. One of the compounds used in the synthesis of aspirin is salicylic
The Synthesis and Analysis of Aspirin Computer 22 Aspirin, the ubiquitous pain reliever, goes by the chemical name acetylsalicylic acid. One of the compounds used in the synthesis of aspirin is salicylic
Energy, Heat and Chemical Change
 Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
DETERMINING AND USING H
 DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
Name: Chemistry 103 Laboratory University of Massachusetts Boston HEATS OF REACTION PRELAB ASSIGNMENT
 Name: Chemistry 103 Laboratory University of Massachusetts Boston HEATS OF REACTION PRELAB ASSIGNMENT Chemical and physical changes usually involve the absorption or liberation of heat, given the symbol
Name: Chemistry 103 Laboratory University of Massachusetts Boston HEATS OF REACTION PRELAB ASSIGNMENT Chemical and physical changes usually involve the absorption or liberation of heat, given the symbol
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Calorimetry Measurements of Fusion, Hydration and Neutralization - Hess Law
 Calorimetry Measurements of Fusion, Hydration and Neutralization - Hess Law EXPERIMENT 9 Prepared by Edward L. Brown, Lee University and Verrill M. Norwood, Cleveland State Community College To become
Calorimetry Measurements of Fusion, Hydration and Neutralization - Hess Law EXPERIMENT 9 Prepared by Edward L. Brown, Lee University and Verrill M. Norwood, Cleveland State Community College To become
Apply the ideal gas law (PV = nrt) to experimentally determine the number of moles of carbon dioxide gas generated
 Teacher Information Ideal Gas Law Objectives Determine the number of moles of carbon dioxide gas generated during a reaction between hydrochloric acid and sodium bicarbonate. Through this investigation,
Teacher Information Ideal Gas Law Objectives Determine the number of moles of carbon dioxide gas generated during a reaction between hydrochloric acid and sodium bicarbonate. Through this investigation,
Energy and Chemical Change
 Energy and Chemical Change Reviewing Vocabulary Match the definition in Column A with the term in Column B. h e d p c f a r m t j i s l u k n q g o Column A 1. The ability to do work or produce heat 2.
Energy and Chemical Change Reviewing Vocabulary Match the definition in Column A with the term in Column B. h e d p c f a r m t j i s l u k n q g o Column A 1. The ability to do work or produce heat 2.
Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat.
 CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
Chapter 5 Practice Multiple Choice & Free
 Name Response 1. A system has an increase in internal energy, E, of 40 kj. If 20 kj of work, w, is done on the system, what is the heat change, q? a) +60 kj d) -20 kj b) +40 kj e) -60 kj c) +20 kj 2. Which
Name Response 1. A system has an increase in internal energy, E, of 40 kj. If 20 kj of work, w, is done on the system, what is the heat change, q? a) +60 kj d) -20 kj b) +40 kj e) -60 kj c) +20 kj 2. Which
Ch. 6 Enthalpy Changes
 Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
Ch. 6 Enthalpy Changes Energy: The capacity to do work. In Physics, there are 2 main types of energy Kinetic (energy of motion) = ½ mv 2 Potential (energy of position due to gravity)= mgh In Chemistry,
COMBUSTION OF FUEL 12:57:42
 COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
Chapter 6 Thermochemistry Thermochemistry Thermochemistry is a part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? - Will a reaction proceed
Thermochemistry: Calorimetry and Hess s Law
 Thermochemistry: Calorimetry and Hess s Law Some chemical reactions are endothermic and proceed with absorption of heat while others are exothermic and proceed with an evolution of heat. The magnitude
Thermochemistry: Calorimetry and Hess s Law Some chemical reactions are endothermic and proceed with absorption of heat while others are exothermic and proceed with an evolution of heat. The magnitude
Chem 105 Wed
 10/12/2011 1 hem 105 Wed 10-12-2011 FRIDAY s class will start with a demo of an exothermic reaction. Please assemble Friday 1:00 PM sharp on the West lawn of Reichardt. Be careful of vehicular traffic,
10/12/2011 1 hem 105 Wed 10-12-2011 FRIDAY s class will start with a demo of an exothermic reaction. Please assemble Friday 1:00 PM sharp on the West lawn of Reichardt. Be careful of vehicular traffic,
SPECIFIC HEAT CAPACITY
 SPECIFIC HEAT CAPACITY Apparatus: Thermometer, balance, two large double Styrofoam cups, lid, hooked metal cube, lifting tool, hot plate, boiling pot. Any material is capable of storing some heat or thermal
SPECIFIC HEAT CAPACITY Apparatus: Thermometer, balance, two large double Styrofoam cups, lid, hooked metal cube, lifting tool, hot plate, boiling pot. Any material is capable of storing some heat or thermal
Gravity is a force which keeps us stuck to the earth. The Electrostatic force attracts electrons to protons in an atom.
 Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Guided Notes and Practice- Topi 5.1: Calorimetry and Enthalpy Calculations
 Name: Date: Pd: Guided Notes and Practice- Topi 5.1: Calorimetry and Enthalpy Calculations Endothermic vs. Exothermic 1. Label each ΔH value as being exothermic or endothermic. Thermochemical Equations
Name: Date: Pd: Guided Notes and Practice- Topi 5.1: Calorimetry and Enthalpy Calculations Endothermic vs. Exothermic 1. Label each ΔH value as being exothermic or endothermic. Thermochemical Equations
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE
 Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
LECTURE 4 Variation of enthalpy with temperature
 LECTURE 4 Variation of enthalpy with temperature So far, we can only work at 25 C. Like c v we define a constant pressure heat capacity, c p, as the amount of heat energy needed to raise the temperature
LECTURE 4 Variation of enthalpy with temperature So far, we can only work at 25 C. Like c v we define a constant pressure heat capacity, c p, as the amount of heat energy needed to raise the temperature
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
CHAPTER 17: THERMOCHEMISTRY. Mrs. Brayfield
 CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
CHAPTER 17: THERMOCHEMISTRY Mrs. Brayfield REVIEW What is the law of conservation of energy? It states that energy cannot be created or destroyed So the energy of any process is the same THERMOCHEMISTRY
Acid-Base Titration. Sample
 Acid-Base Titration Computer 7 A titration is a process used to determine the volume of a solution that is needed to react with a given amount of another substance. In this experiment, your goal is to
Acid-Base Titration Computer 7 A titration is a process used to determine the volume of a solution that is needed to react with a given amount of another substance. In this experiment, your goal is to
