Question 1. Electron Configurations Noble Gases and The Rule of Eight. Chapter 1. What is the electronic configuration of carbon?
|
|
|
- Tracey Charles
- 6 years ago
- Views:
Transcription
1 hapter ~. nm Electronic Structure and Bonding Anders Jöns Ångström (84-874) Å = picometers =. nanometers = -4 microns = -8 centimeters Acids and Bases nm = Å An atom vs. a nucleus ~, x larger ucleus = /, of the atom Question What is the electronic configuration of carbon? A) s 2 2s 2 2p 2 x B) s 2 2s 2 2p x 2p y 2p z ) s 2 2s 2 2p x 2p y 2p z D) s 2 p x p y 2s 2 Electron onfigurations oble Gases and The Rule of Eight When two nonmetals react to form a covalent bond: They share electrons to achieve a oble gas electron configuration. When a nonmetal and a metal react to form an ionic compound: Valence electrons of the metal are lost and the nonmetal gains these electrons. G.. Lewis Photo Bancroft Library, University of alifornia/lbl Image Library Footnote: G.. Lewis, despite his insight and contributions to chemistry, was never awarded the obel prize. otes from Lewisʼs s notebook and his Lewis structure.
2 Ionic ompounds Ionic compounds are formed when electron(s) are transferred. Electrons go from less electronegative element to the more electronegative forming ionic bonds. ovalent ompounds Share electrons. pair = bond. ctet rule ( duet for hydrogen) Lewis structures: otice the charges: In one case they balance, can you name the compound? In the other they do not, can you name the polyatomic ion? More about formal charge to come. Question 2 Important Bond umbers (eutral Atoms / ormal electron distribution) Select the correct Lewis structure for methyl fluoride ( 3 F). one bond two bonds F l Br I ) D) three bonds four bonds Question 3 Question 4 What is the correct Lewis structure of formaldehyde ( 2 )? ) D) Which of the following contains a triple bond? A) S 2 B) ) 2 4 D) 3
3 Important Bond umbers (eutral Atoms / ormal electron distribution) one bond F l Br I Formal harge Formal charge is the charge of an atom in a Lewis structure which has a different than normal distribution of electrons. two bonds three bonds four bonds Important Bond umbers (eutral Atoms / ormal electron distribution) rganic hemistry # of Valence e - s Formal harge Formal charge = number of valence electrons (number of lone pair electrons /2 number of bonding electrons) Total # of Bonds (neutral atom) ombinations of bonds (neutral atom): # of single bonds # of double bonds # of triple bonds Total Bonds Equals the number of valence electrons (Group umber of the free atom) minus [the number of unshared valence electrons in the molecule /2 the number of shared valence electrons in the molecule]. Moving/Adding/Subtracting atoms and electrons. # of Free Pairs of electrons 2 2 omplete the following table. It summarizes the formal charge on a ( central ) atom for the most important species in organic chemistry. 3 itric Acid
4 Question 5 What is the formal charge of the carbon atom in the Lewis structure? A) - B) ) D) 2 Question 6 What is the formal charge of the oxygen atom in the Lewis structure? A) - B) ) D) 2 Resonance Resonance is a very important intellectual concept that was introduced by Linus Pauling in 928 to explain experimental observations. Resonance Eg. S 2 Bond order.5 Bond length > double bond; < single bond TUTRIAL Resonance Two or more Lewis structures may be legitimately written for certain compounds (or ions) that have double bonds and/or free pairs of non-bonded electrons It is a mental exercise in pushing or moving electrons. Refer to Table.6 Rules of Resonance Step : The atoms must stay in the same position. Atom connectivity is the same in all resonance structures. nly electrons move. -Example: The Lewis formulas below are not resonance forms. A hydrogen atom has changed position.
5 Rules of Resonance Step 2: Each contributing structure must have the same total number of electrons and the same net charge. Example: All structures have 8 electrons and a net charge of. Rules of Resonance Step 3: alculate formal charges for each atom in each structure. Example: one of the atoms possess a formal charge in this Lewis structure. Rules of Resonance Step 4: alculate formal charges for the second and third structures. Example: These structures have formal charges. TE: They are less favorable Lewis structures. Pushing Electrons same atomic positions differ in electron positions : : more stable less stable Lewis Lewis structure structure Pushing Electrons Why use Resonance Structures? same atomic positions differ in electron positions only : : more stable less stable Lewis Lewis structure structure Delocalization of electrons and charges between two or more atoms helps explain energetic stability and chemical reactivity. Electrons in a single Lewis structure are insufficient to show electron delocalization. A composite of all resonance forms more accurately depicts electron distribution. (YBRID) TE: Resonance forms are not always evenly weighted. Some forms are better than others.
6 Resonance Example Resonance Example zone ( 3 ) zone ( 3 ) Lewis structure of ozone shows one double bond and one single bond Lewis structure of ozone shows one double bond and one single bond Expect: one short bond and one long bond Reality: bonds are of equal length (28 pm) Resonance: zone ( 3 ) Resonance Example Detailed Resonance Examples Electrostatic potential map shows both end carbons are equivalent with respect to negative charge. Middle carbon is positive. Question 7 Which resonance structure contributes more to the hybrid? VSEPR Model Valence Shell Electron Pair Repulsion
7 VSEPR Model The molecular structure of a given atom is determined principally by minimizing electron pair (bonded &free) repulsions through maximizing separations. Some examples of minimizing interactions. Predicting a VSEPR Structure. Draw Lewis structure. 2. Put pairs as far apart as possible. 3. Determine positions of atoms from the way electron pairs are shared. 4. Determine the name of molecular structure from positions of the atoms. rbital Molecular Geometry Geometry Bond Angle # of lone pairs Lewis Structures / VSEPR / hem 226 Linear Linear Trigonal Planar Trigonal Planar Tetrahedral Tetrahedral Trigonal Planar Bent Tetrahedral Trigonal Pyramidal Molecular Models omputer Generated Models Tetrahedral Bent 2 ctahedral Seesaw T-shape Linear ctahedral 2 3 Ball and stick models of ammonia, water and methane. ctahedral ctahedral Square Pyramidal Square Planar 2 Worksheet : Bonds, Formulas, Structures & Shapes ovalent ompounds Equal sharing of electrons: nonpolar covalent bond, same electronegativity (e.g., 2 ) Unequal sharing of electrons between atoms of different electronegativities: polar covalent bond (e.g., F)
8 Question 8 Which of the following bonds is the most polar? ) D) Bond Dipole & Dipole Moment Question 9 Dipole moments are experimentally measured. Polar bonds have dipole moments. dipole moment (D) = µ = e x d (e) : magnitude of the charge on the atom (d) : distance between the two charges Which of the following bonds have the greatest dipole moment (µ)? ) D) Bond Polarity A molecule, such as F, that has a center of positive charge and a center of negative charge is polar, and has a dipole moment. The partial charge is represented by δ and the polarity with a vector arrow. F!!" Question In which of the compounds below is the δ for the greatest? A) 4 B) 3 ) Si 4 D) 2
9 Question In which of the following is oxygen the positive end of the bond dipole? A) -F B) - ) -S D) -
Electronic Structure and Anders Jöns Ångström ( ) Bonding 1 Å = 10 picometers = 0.1 nanometers = 10-4 microns = 10-8 centimeters Molecular
 Chapters 1 & 2 ~ 0.1 nm General Chemistry Review Electronic Structure and Bonding Anders Jöns Ångström (1814-1874) 1 Å = 10 picometers = 0.1 nanometers = 10-4 microns = 10-8 centimeters Molecular Representations
Chapters 1 & 2 ~ 0.1 nm General Chemistry Review Electronic Structure and Bonding Anders Jöns Ångström (1814-1874) 1 Å = 10 picometers = 0.1 nanometers = 10-4 microns = 10-8 centimeters Molecular Representations
Question 1.1. Electron Configurations Noble Gases and The Rule of Eight. Chapter 1
 ~ 0.1 nm Chapter 1 Structure and Bonding Anders Jöns Ångström (1814-1874) 1 Å = 10 picometers = 0.1 nanometers = 10-4 microns = 10-8 centimeters Acids and Bases Nucleus = 1/10,000 of the atom 1 nm = 10
~ 0.1 nm Chapter 1 Structure and Bonding Anders Jöns Ångström (1814-1874) 1 Å = 10 picometers = 0.1 nanometers = 10-4 microns = 10-8 centimeters Acids and Bases Nucleus = 1/10,000 of the atom 1 nm = 10
Organic Chemistry. Review Information for Unit 1. VSEPR Hybrid Orbitals Polar Molecules
 rganic hemistry Review Information for Unit 1 VSEPR ybrid rbitals Polar Molecules VSEPR The valence shell electron pair repulsion model (VSEPR) can be used to predict the geometry around a particular atom
rganic hemistry Review Information for Unit 1 VSEPR ybrid rbitals Polar Molecules VSEPR The valence shell electron pair repulsion model (VSEPR) can be used to predict the geometry around a particular atom
Chapter 13: Phenomena
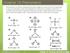 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
H-H bond length Two e s shared by two Hs: covalent bonding. Coulomb attraction: Stronger attraction for e Fractional charge A dipole
 8 Bonding: General Concepts Types of chemical bonds Covalent bonding Ex. 2 E (kj/mol) Repulsions of nucleus and e s r 0 458 0.074 r (nm) Zero interaction at long distance - bond length Two e s shared by
8 Bonding: General Concepts Types of chemical bonds Covalent bonding Ex. 2 E (kj/mol) Repulsions of nucleus and e s r 0 458 0.074 r (nm) Zero interaction at long distance - bond length Two e s shared by
Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry Periodic Trends in Atomic Properties Learning Objective
 Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry 11.1 Periodic Trends in Atomic Properties Discuss the atomic trends Metals are located on the left side of the periodic
Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry 11.1 Periodic Trends in Atomic Properties Discuss the atomic trends Metals are located on the left side of the periodic
Section 12: Lewis Structures
 Section 12: Lewis Structures The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 12.01 Electronegativity Chemistry (5)(C) 12.02 Electron
Section 12: Lewis Structures The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 12.01 Electronegativity Chemistry (5)(C) 12.02 Electron
Chapter 12 Structures and Characteristics of Bonds Objectives
 Objectives 1. To learn about ionic and covalent bonds and explain how they are formed - what holds compounds together? 2. To learn about the polar covalent bond are all covalent bonds equal? 3. To understand
Objectives 1. To learn about ionic and covalent bonds and explain how they are formed - what holds compounds together? 2. To learn about the polar covalent bond are all covalent bonds equal? 3. To understand
; (c) [Li] [: O :] [Li]. 5a. The electrostatic potential map that corresponds to IF is the one with the most red in it. ... C C H
![; (c) [Li] [: O :] [Li]. 5a. The electrostatic potential map that corresponds to IF is the one with the most red in it. ... C C H ; (c) [Li] [: O :] [Li]. 5a. The electrostatic potential map that corresponds to IF is the one with the most red in it. ... C C H](/thumbs/87/96516624.jpg) hapter 10 Answers ractice Examples 1a Mg 1b n, Ge, [: Br :], K, : e: + 2 : : +, [Tl ] +, 2 : : [] 2a (a) [a] [ ] [a] ; (b) [Mg] [: :] [Mg] [: :] [Mg] 2+ 3 2+ 3 2+ 2+ 2b (a) [: I :] [a] [: I :] 2+ 2 ; (b)
hapter 10 Answers ractice Examples 1a Mg 1b n, Ge, [: Br :], K, : e: + 2 : : +, [Tl ] +, 2 : : [] 2a (a) [a] [ ] [a] ; (b) [Mg] [: :] [Mg] [: :] [Mg] 2+ 3 2+ 3 2+ 2+ 2b (a) [: I :] [a] [: I :] 2+ 2 ; (b)
Chapter 7. Chemical Bonding I: Basic Concepts
 Chapter 7. Chemical Bonding I: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Chapter 7. Chemical Bonding I: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction
 Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent
Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent
Chemical Bonding II. Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds Hybridization MO theory
 Chemical Bonding II Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds ybridization MO theory 1 Molecular Geometry 3-D arrangement of atoms 2 VSEPR Valence-shell
Chemical Bonding II Molecular Geometry Valence Bond Theory Phys./Chem. Properties Quantum Mechanics Sigma & Pi bonds ybridization MO theory 1 Molecular Geometry 3-D arrangement of atoms 2 VSEPR Valence-shell
Hey, Baby. You and I Have a Bond...Ch. 8
 I. IONIC BONDING FUNDAMENTALS A. They form between... 1. A and a a. A to become b. A to become B. How it happens (Let s first focus on two atoms): 1. When a metal and a nonmetal meet, electrons get transferred
I. IONIC BONDING FUNDAMENTALS A. They form between... 1. A and a a. A to become b. A to become B. How it happens (Let s first focus on two atoms): 1. When a metal and a nonmetal meet, electrons get transferred
Orbital Shapes Carbon: Electron configuration Carbon: Full. Short form. Orbital energy diagram. Orbital energy levels diagram
 rganic hemistry involves mostly NPS and the halogens. rganic compounds use valence shell electrons to bond. Usually only in the s and p orbitals. rbital Shapes arbon: z y z y z y z y z y x x x x x 1s n=1
rganic hemistry involves mostly NPS and the halogens. rganic compounds use valence shell electrons to bond. Usually only in the s and p orbitals. rbital Shapes arbon: z y z y z y z y z y x x x x x 1s n=1
VSEPR. Valence Shell Electron Pair Repulsion Theory
 VSEPR Valence Shell Electron Pair Repulsion Theory Vocabulary: domain = any electron pair or bond (single, double or triple) is considered one domain. bonding pair = shared pair = any electron pair that
VSEPR Valence Shell Electron Pair Repulsion Theory Vocabulary: domain = any electron pair or bond (single, double or triple) is considered one domain. bonding pair = shared pair = any electron pair that
REVIEW: VALENCE ELECTRONS CHEMICAL BONDS: LEWIS SYMBOLS: CHEMICAL BONDING. What are valence electrons?
 REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
Chapter 8 Basic Concepts of Chemical Bonding
 hapter 8 Basic oncepts of hemical Bonding An Important Principle in hemistry The microscopic structure defines the properties of matter at our mesoscopic level. Ex. Graphite and Diamond (both are pure
hapter 8 Basic oncepts of hemical Bonding An Important Principle in hemistry The microscopic structure defines the properties of matter at our mesoscopic level. Ex. Graphite and Diamond (both are pure
Covalent Bonding. Chapter 8. Diatomic elements. Covalent bonding. Molecular compounds. 1 and 7
 hapter 8 ovalent bonding ovalent Bonding A metal and a nonmetal transfer An ionic bond Two metals just mix and don t react An alloy What do two nonmetals do? Neither one will give away an electron So they
hapter 8 ovalent bonding ovalent Bonding A metal and a nonmetal transfer An ionic bond Two metals just mix and don t react An alloy What do two nonmetals do? Neither one will give away an electron So they
CHAPTER 12 CHEMICAL BONDING
 CHAPTER 12 CHEMICAL BONDING Core electrons are found close to the nucleus, whereas valence electrons are found in the most distant s and p energy subshells. The valence electrons are responsible for holding
CHAPTER 12 CHEMICAL BONDING Core electrons are found close to the nucleus, whereas valence electrons are found in the most distant s and p energy subshells. The valence electrons are responsible for holding
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts
 AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
LEWIS STRUCTURES - The Geometry of Covalent Molecules
 LEWIS STRUTURES - The Geometry of ovalent Molecules The formulas of many covalent compounds, especially those involving only the elements of the first few periods of the periodic table, were brought within
LEWIS STRUTURES - The Geometry of ovalent Molecules The formulas of many covalent compounds, especially those involving only the elements of the first few periods of the periodic table, were brought within
Structure and Bonding of Organic Molecules
 Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Covalent Bonding 10/29/2013
 Bond Energies or Bond Dissociation Energies Tables 8.4 and 8.5 on page 72 gives a list of the energy required to dissociate or break bonds. This value is used to determine whether covalent bonds will form
Bond Energies or Bond Dissociation Energies Tables 8.4 and 8.5 on page 72 gives a list of the energy required to dissociate or break bonds. This value is used to determine whether covalent bonds will form
OFB Chapter 3 Chemical Periodicity and the Formation of Simple Compounds
 OFB hapter 3 hemical Periodicity and the Formation of Simple ompounds 3-1 Groups of Elements 3-2 The Periodic Table 3-3 Ions and Ionic ompounds 3-4 ovalent Bonding and Lewis Structures 3-5 3-6 Naming ompounds
OFB hapter 3 hemical Periodicity and the Formation of Simple ompounds 3-1 Groups of Elements 3-2 The Periodic Table 3-3 Ions and Ionic ompounds 3-4 ovalent Bonding and Lewis Structures 3-5 3-6 Naming ompounds
Lewis Theory of Shapes and Polarities of Molecules
 Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Chapter 8. Bonding: General Concepts
 Chapter 8 Bonding: General Concepts Chapter 8 Table of Contents 8.1 Types of Chemical Bonds 8.3 Bond Polarity and Dipole Moments 8.5 Energy Effects in Binary Ionic Compounds 8.6 Partial Ionic Character
Chapter 8 Bonding: General Concepts Chapter 8 Table of Contents 8.1 Types of Chemical Bonds 8.3 Bond Polarity and Dipole Moments 8.5 Energy Effects in Binary Ionic Compounds 8.6 Partial Ionic Character
Chemical Bonding Chapter 8
 Chemical Bonding Chapter 8 Get your Clicker, 2 magnets, goggles and your handouts Nov 15 6:15 PM Recall that: Ionic-Involves the transfer of electrons - forms between a metal and a nonmetal Covalent-Involves
Chemical Bonding Chapter 8 Get your Clicker, 2 magnets, goggles and your handouts Nov 15 6:15 PM Recall that: Ionic-Involves the transfer of electrons - forms between a metal and a nonmetal Covalent-Involves
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
18. Ionic solids are held together by strong electrostatic forces that are omnidirectional.
 APTER 8 BDIG: GEERAL EPTS Questions 15. In 2 and, the bonding is covalent in nature, with the bonding electrons pair shared between the atoms. In 2, the two atoms are identical, so the sharing is equal;
APTER 8 BDIG: GEERAL EPTS Questions 15. In 2 and, the bonding is covalent in nature, with the bonding electrons pair shared between the atoms. In 2, the two atoms are identical, so the sharing is equal;
Chapter 10: Molecular Structure and Bonding Theories
 hapter 10: Molecular Structure and Bonding Theories 10.1 See Section 10.1. The main premise of the VSEPR model is that the electron pairs within the valence shell of an atom repel each other and determine
hapter 10: Molecular Structure and Bonding Theories 10.1 See Section 10.1. The main premise of the VSEPR model is that the electron pairs within the valence shell of an atom repel each other and determine
Chapter 4 Lecture Outline. Copyright McGraw-Hill Education. Permission required for reproduction or display.
 Chapter 4 Lecture Outline 1 Copyright McGraw-ill Education. Permission required for reproduction or display. 4.1 Introduction to Covalent Bonding Covalent bonds result from the sharing of electrons between
Chapter 4 Lecture Outline 1 Copyright McGraw-ill Education. Permission required for reproduction or display. 4.1 Introduction to Covalent Bonding Covalent bonds result from the sharing of electrons between
Chapter 6 Chemical Bonding
 Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Molecular Geometry & Polarity
 Molecular Geometry & Polarity Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized
Molecular Geometry & Polarity Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized
OFB Chapter 3 Chemical Periodicity and the Formation of Simple Compounds
 OFB hapter 3 hemical Periodicity and the Formation of Simple ompounds 3-1 Groups of Elements 3-2 The Periodic Table 3-3 Ions and Ionic ompounds 3-4 ovalent Bonding and Lewis Structures 3-5 Drawing Lewis
OFB hapter 3 hemical Periodicity and the Formation of Simple ompounds 3-1 Groups of Elements 3-2 The Periodic Table 3-3 Ions and Ionic ompounds 3-4 ovalent Bonding and Lewis Structures 3-5 Drawing Lewis
4/25/2017. VSEPR Theory. Two Electron Groups. Shapes of Molecules. Two Electron Groups with Double Bonds. Three Electron Groups.
 Chapter 10 Lecture Chapter 10 Bonding and Properties of Solids and Liquids 10.3 Shapes of Molecules and Ions (VSEPR Theory) Learning Goal Predict the three-dimensional structure of a molecule or a polyatomic
Chapter 10 Lecture Chapter 10 Bonding and Properties of Solids and Liquids 10.3 Shapes of Molecules and Ions (VSEPR Theory) Learning Goal Predict the three-dimensional structure of a molecule or a polyatomic
Chapter 8. Bonding: General Concepts
 Chapter 8 Bonding: General Concepts Chapter 8 Table of Contents 8.1 Types of Chemical Bonds 8.2 Electronegativity 8.3 Bond Polarity and Dipole Moments 8.4 Ions: Electron Configurations and Sizes 8.5 Energy
Chapter 8 Bonding: General Concepts Chapter 8 Table of Contents 8.1 Types of Chemical Bonds 8.2 Electronegativity 8.3 Bond Polarity and Dipole Moments 8.4 Ions: Electron Configurations and Sizes 8.5 Energy
Covalent Bonding Introduction, 2. Chapter 7 Covalent Bonding. Figure 7.1 The Hydrogen Molecule. Outline. Covalent Bonding Introduction, 1. Figure 7.
 Covalent Bonding Introduction, 2 William L. Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 7 Covalent Bonding Electron density Electrons are located between nuclei Electrostatic
Covalent Bonding Introduction, 2 William L. Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 7 Covalent Bonding Electron density Electrons are located between nuclei Electrostatic
Lecture 17 - Covalent Bonding. Lecture 17 - VSEPR and Molecular Shape. Lecture 17 - Introduction. Lecture 17 - VSEPR and Molecular Shape
 Chem 103, Section F0F Unit VI - Compounds Part II: Covalent Compounds Lecture 17 Using the Valence-Shell Electron-Pair Repulsion (VSEPR) Theory to predict molecular shapes Molecular shape and polarity
Chem 103, Section F0F Unit VI - Compounds Part II: Covalent Compounds Lecture 17 Using the Valence-Shell Electron-Pair Repulsion (VSEPR) Theory to predict molecular shapes Molecular shape and polarity
Chapter 01 Covalent Bonding and Shapes of Molecules. Atomic Structure. Chapter 01 Topics. Structure. Atomic Structure. Subatomic Particles
 hapter 01 ovalent Bonding and Shapes of Molecules EM 240: Fall 2016 Prof. Greg ook hapter 01 Topics Mostly a review of general chemistry Atomic Structure Lewis Models and Bonding Bonding and Shapes of
hapter 01 ovalent Bonding and Shapes of Molecules EM 240: Fall 2016 Prof. Greg ook hapter 01 Topics Mostly a review of general chemistry Atomic Structure Lewis Models and Bonding Bonding and Shapes of
What is Bonding? The Octet Rule. Getting an Octet. Chemical Bonding and Molecular Shapes. (Chapter Three, Part Two)
 Chemical Bonding and Molecular Shapes (Chapter Three, Part Two) What is Bonding? Bonding describes how atoms interact with each other in an attractive sense. There are three types of bonding: Ionic bonding
Chemical Bonding and Molecular Shapes (Chapter Three, Part Two) What is Bonding? Bonding describes how atoms interact with each other in an attractive sense. There are three types of bonding: Ionic bonding
Unit 9: CHEMICAL BONDING
 Unit 9: CEMICAL BNDING Unit 9: Bonding: 1. Electronegativity 2. Intramolecular Bonding 3. Intermolecular Bonding 4. Drawing Lewis Structures 5. Lewis Structures for Polyatomic Ions 6. Exceptions to the
Unit 9: CEMICAL BNDING Unit 9: Bonding: 1. Electronegativity 2. Intramolecular Bonding 3. Intermolecular Bonding 4. Drawing Lewis Structures 5. Lewis Structures for Polyatomic Ions 6. Exceptions to the
Chapter 12 Structure and Shape
 Free Study Guide for Cracolice Peters Introductory Chemistry: An Active Learning Approach Second Edition www.brookscole.com/chemistry Chapter 12 Structure and Shape Chapter 12Assignment A: Lewis Diagrams
Free Study Guide for Cracolice Peters Introductory Chemistry: An Active Learning Approach Second Edition www.brookscole.com/chemistry Chapter 12 Structure and Shape Chapter 12Assignment A: Lewis Diagrams
Chapter 8. Bonding: General Concepts
 Chapter 8 Bonding: General Concepts Chapter 8 Questions to Consider What is meant by the term chemical bond? Why do atoms bond with each other to form compounds? How do atoms bond with each other to form
Chapter 8 Bonding: General Concepts Chapter 8 Questions to Consider What is meant by the term chemical bond? Why do atoms bond with each other to form compounds? How do atoms bond with each other to form
Chapter 8 H H H H. Molecular Compounds & Covalent Bonding. Why do covalent bonds form? 8.1 Molecular Compounds. Properties of Molecular Compounds
 Chapter 8 Molecular Compounds & Covalent Bonding Why do covalent bonds form? If only group 5A, 6A, 7A atoms existed, ionic bonds can t form. NNMETALS Each atom needs electrons so they are not willing to
Chapter 8 Molecular Compounds & Covalent Bonding Why do covalent bonds form? If only group 5A, 6A, 7A atoms existed, ionic bonds can t form. NNMETALS Each atom needs electrons so they are not willing to
Chapter 1. The Basics Bonding and Molecular Structure. Table of Contents. 1. Life & the Chemistry of Carbon Compounds
 hapter 1 The Basics Bonding and Molecular Structure reated by Professor William Tam & Dr. Phillis hang Table of ontents 1. Life & the hemistry of arbon ompounds 2. Atomic Structure 3. hemical Bonds: The
hapter 1 The Basics Bonding and Molecular Structure reated by Professor William Tam & Dr. Phillis hang Table of ontents 1. Life & the hemistry of arbon ompounds 2. Atomic Structure 3. hemical Bonds: The
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE INSTR : FİLİZ ALSHANABLEH
 C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE The Ionic Bond Formation of Ions The
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE The Ionic Bond Formation of Ions The
Page III-7-1 / Chapter Seven Lecture Notes MAR. Ionic - complete transfer of. Covalent - electrons shared MAR. CH 221 Flashback:
 Page III-7-1 / hapter even Lecture otes hemical onding onding and Molecular tructure hapter 7 ocaine hemistry 222 Professor Michael Russell http://mhchem.org/222 Get the 222 ompanion and omposition Lab
Page III-7-1 / hapter even Lecture otes hemical onding onding and Molecular tructure hapter 7 ocaine hemistry 222 Professor Michael Russell http://mhchem.org/222 Get the 222 ompanion and omposition Lab
of its physical and chemical properties.
 8.4 Molecular Shapes VSEPR Model The shape of a molecule determines many of its physical and chemical properties. Molecular l geometry (shape) can be determined with the Valence Shell Electron Pair Repulsion
8.4 Molecular Shapes VSEPR Model The shape of a molecule determines many of its physical and chemical properties. Molecular l geometry (shape) can be determined with the Valence Shell Electron Pair Repulsion
A Simple Model for Chemical Bonds
 A Simple Model for hemical Bonds Multiple hoice 1. Modern organic chemistry a. is the study of carbon-containing compounds. b. is the study of compounds from living organisms. c. deals exclusively with
A Simple Model for hemical Bonds Multiple hoice 1. Modern organic chemistry a. is the study of carbon-containing compounds. b. is the study of compounds from living organisms. c. deals exclusively with
CHAPTER 8 BONDING: GENERAL CONCEPTS. Questions
 APTER 8 BDIG: GEERAL EPTS Questions 15. a. This diagram represents a polar covalent bond as in. In a polar covalent bond, there is an electron rich region (indicated by the red color) and an electron poor
APTER 8 BDIG: GEERAL EPTS Questions 15. a. This diagram represents a polar covalent bond as in. In a polar covalent bond, there is an electron rich region (indicated by the red color) and an electron poor
4 Molecules and Compounds
 4 Molecules and ompounds 4 Molecules and ompounds Atoms that have assembled into substances (new or old) are bonded (glued) together. The way the atoms are bonded together will create different properties
4 Molecules and ompounds 4 Molecules and ompounds Atoms that have assembled into substances (new or old) are bonded (glued) together. The way the atoms are bonded together will create different properties
Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Group 7 Group 8. Na Mg Al Si P S Cl Ar
 CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
RESONANCE STRUCTURE When a molecule has more than one possible structure. Draw all possible structures and place a double end arrow ( ) in between.
 CHEMISTRY NOTES 6.1 COVALENT BONDS Objectives Explain the role and location of electrons in a covalent bond. Describe the change in energy and stability that takes place as a covalent bond forms. Distinguish
CHEMISTRY NOTES 6.1 COVALENT BONDS Objectives Explain the role and location of electrons in a covalent bond. Describe the change in energy and stability that takes place as a covalent bond forms. Distinguish
6.1 Intro to Chemical Bonding Name:
 6.1 Intro to Chemical Bonding Name: A. Chemical bond Favored by nature because: 3 main types of bonds 1. 2. 3. B. Ionic Bonds C. Covalent Bonds D. Metallic Bond E. Bond Determination RECALL: Electronegativity
6.1 Intro to Chemical Bonding Name: A. Chemical bond Favored by nature because: 3 main types of bonds 1. 2. 3. B. Ionic Bonds C. Covalent Bonds D. Metallic Bond E. Bond Determination RECALL: Electronegativity
CHEMICAL BONDING. Chemical Bonds. Ionic Bonding. Lewis Symbols
 CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
Lewis Structures and Molecular Shapes
 Lewis Structures and Molecular Shapes Drawing Lewis Structures Determine from formula if ionic or covalent Count the electrons If ionic : add valence # to charge if (-), subtract if (+) - = 7+1 electrons;
Lewis Structures and Molecular Shapes Drawing Lewis Structures Determine from formula if ionic or covalent Count the electrons If ionic : add valence # to charge if (-), subtract if (+) - = 7+1 electrons;
Chemical Bonding polarity & Dipole Moments. Chapter 8 Part III
 Chemical Bonding polarity & Dipole Moments Chapter 8 Part III Exercise Arrange the following bonds from most to least polar: a) N F O F C F b) C F N O Si F c) Cl Cl B Cl S Cl Exercise a) C F, N F, O F
Chemical Bonding polarity & Dipole Moments Chapter 8 Part III Exercise Arrange the following bonds from most to least polar: a) N F O F C F b) C F N O Si F c) Cl Cl B Cl S Cl Exercise a) C F, N F, O F
Chem 105 Friday Review Lewis formulas and geometry Bond length qualitative Polarity Polarity and geometry 11/20/2009 1
 hem 105 riday 11-20-09 Review Lewis formulas and geometry Bond length qualitative Polarity Polarity and geometry 11/20/2009 1 Shown below is a partial Lewis formula for hydrogen carbonate ion ( 3- ). What
hem 105 riday 11-20-09 Review Lewis formulas and geometry Bond length qualitative Polarity Polarity and geometry 11/20/2009 1 Shown below is a partial Lewis formula for hydrogen carbonate ion ( 3- ). What
Covalent Bonds. Chapter 8 Chemical Bonds (+VSEPR from Chapter 9) Li Be B C N O F Ne delocalized electron sea. 3. Introduction. Types of Chemical Bonds
 hapter 8: hemical Bonds (+ VSEPR) hapter bjectives: hapter 8 hemical Bonds (+VSEPR from hapter 9) Understand the principal types of chemical bonds. Understand the properties of ionic and molecular compounds.
hapter 8: hemical Bonds (+ VSEPR) hapter bjectives: hapter 8 hemical Bonds (+VSEPR from hapter 9) Understand the principal types of chemical bonds. Understand the properties of ionic and molecular compounds.
Homework - Chapter 1 Chem 2310
 omework - hapter 1 hem 2310 ame I. Introduction to rganic hemistry 1. Explain in your own words what organic chemistry is, and what it is useful for. 2. Why do you think the field of study that you are
omework - hapter 1 hem 2310 ame I. Introduction to rganic hemistry 1. Explain in your own words what organic chemistry is, and what it is useful for. 2. Why do you think the field of study that you are
Problems and questions How is a molecule or polyatomic ion held together? Why are atoms distributed at strange angles? Why are molecules not flat?
 1 Cocaine 2 Problems and questions ow is a molecule or polyatomic ion held together? Why are atoms distributed at strange angles? Why are molecules not flat? Can we predict the structure? ow is structure
1 Cocaine 2 Problems and questions ow is a molecule or polyatomic ion held together? Why are atoms distributed at strange angles? Why are molecules not flat? Can we predict the structure? ow is structure
Lewis Dot Formulas and Molecular Shapes
 Lewis Dot Formulas and Molecular Shapes Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent chemical bonds are formed by valence electrons
Lewis Dot Formulas and Molecular Shapes Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent chemical bonds are formed by valence electrons
13 Bonding: General Concepts. Types of chemical bonds. Covalent bonding Ex. H 2. Repulsions of nuclei and e s. Zero interaction at long distance
 13 Bonding: General Concepts Types of chemical bonds Covalent bonding Ex. 2 E (kj/mol) epulsions of nuclei and e s r 0 458 0.074 r (nm) - bond length Two e s shared by two s: covalent bonding Zero interaction
13 Bonding: General Concepts Types of chemical bonds Covalent bonding Ex. 2 E (kj/mol) epulsions of nuclei and e s r 0 458 0.074 r (nm) - bond length Two e s shared by two s: covalent bonding Zero interaction
Molecular Models: The shape of simple molecules and ions
 Molecular Models: The shape of simple molecules and ions Background The shape of a molecule is very important when investigating its properties and reactivity. For example, compare CO 2 and SO 2. Carbon
Molecular Models: The shape of simple molecules and ions Background The shape of a molecule is very important when investigating its properties and reactivity. For example, compare CO 2 and SO 2. Carbon
BONDING REVIEW. You need a Periodic Table, Electronegativity table & Polarity chart!
 BONDING REVIEW You need a Periodic Table, Electronegativity table & Polarity chart! What is the correct bond angle for Bent with 2 lone pairs on the central atom? 105 What is the predicted bond angle for
BONDING REVIEW You need a Periodic Table, Electronegativity table & Polarity chart! What is the correct bond angle for Bent with 2 lone pairs on the central atom? 105 What is the predicted bond angle for
Chapter 8 : Covalent Bonding. Section 8.1: Molecular Compounds
 Chapter 8 : Covalent Bonding Section 8.1: Molecular Compounds What is a molecule? A molecular compound? A molecule is a neutral group of atoms joined together by covalent bonds A molecular compound is
Chapter 8 : Covalent Bonding Section 8.1: Molecular Compounds What is a molecule? A molecular compound? A molecule is a neutral group of atoms joined together by covalent bonds A molecular compound is
CHAPTER EIGHT BONDING: GENERAL CONCEPTS. For Review
 APTER EIGT BDIG: GEERAL EPTS or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the energy
APTER EIGT BDIG: GEERAL EPTS or Review 1. Electronegativity is the ability of an atom in a molecule to attract electrons to itself. Electronegativity is a bonding term. Electron affinity is the energy
Chemical Bonding. Burlingame High School
 Chemical Bonding Electronegativity Is a measure of the ability of an atom in a molecule to attract electrons to itself. Concept proposed by Linus Pauling 1901-1994 Electronegativity Trends Forms of Chemical
Chemical Bonding Electronegativity Is a measure of the ability of an atom in a molecule to attract electrons to itself. Concept proposed by Linus Pauling 1901-1994 Electronegativity Trends Forms of Chemical
Chapter 6 Chemistry Review
 Chapter 6 Chemistry Review Multiple Choice Identify the choice that best completes the statement or answers the question. Put the LETTER of the correct answer in the blank. 1. The electrons involved in
Chapter 6 Chemistry Review Multiple Choice Identify the choice that best completes the statement or answers the question. Put the LETTER of the correct answer in the blank. 1. The electrons involved in
Ionic and Covalent Bonding
 1. Define the following terms: a) valence electrons Ionic and Covalent Bonding the electrons in the highest occupied energy level always electrons in the s and p orbitals maximum of 8 valence electrons
1. Define the following terms: a) valence electrons Ionic and Covalent Bonding the electrons in the highest occupied energy level always electrons in the s and p orbitals maximum of 8 valence electrons
Chapter 7 Chemical Bonding
 Chapter 7 Chemical Bonding 7.1 Ionic Bonding Octet rule: In forming compounds atoms lose, gain or share electrons to attain a noble gas configuration with 8 electrons in their outer shell (s 2 p 6 ), except
Chapter 7 Chemical Bonding 7.1 Ionic Bonding Octet rule: In forming compounds atoms lose, gain or share electrons to attain a noble gas configuration with 8 electrons in their outer shell (s 2 p 6 ), except
CHEM 3013 ORGANIC CHEMISTRY I LECTURE NOTES CHAPTER 2
 EM 3013 RGANI EMISTRY I LETURE NTES 1 APTER 2 1. ormal harge The Lewis structures we have drawn thus far have all been neutral covalent molecules. owever, some covalently bonded molecules may contain charged
EM 3013 RGANI EMISTRY I LETURE NTES 1 APTER 2 1. ormal harge The Lewis structures we have drawn thus far have all been neutral covalent molecules. owever, some covalently bonded molecules may contain charged
Covalent Bonding & Molecular Structure
 ovalent Bonding & Molecular Structure I. Electronic onfiguration and e! sharing. A. The Periodic Table s shape helps you understand outer- (and inner-) shell e! configuration. Which e! were of greatest
ovalent Bonding & Molecular Structure I. Electronic onfiguration and e! sharing. A. The Periodic Table s shape helps you understand outer- (and inner-) shell e! configuration. Which e! were of greatest
William H. Brown & Christopher S. Foote
 William. Brown & Christopher S. Foote Requests for permission to make copies of any part of the work should be mailed to:permissions Department, arcourt Brace & Company, 6277 Sea arbor Drive, rlando, Florida
William. Brown & Christopher S. Foote Requests for permission to make copies of any part of the work should be mailed to:permissions Department, arcourt Brace & Company, 6277 Sea arbor Drive, rlando, Florida
Chapter 10. Valence Electrons. Lewis dot symbols. Chemical Bonding
 Chapter 10 Chemical Bonding Valence Electrons Recall: the outer electrons in an atom are valence electrons. Valence electrons are related to stability Valence electrons can be represented with dots in
Chapter 10 Chemical Bonding Valence Electrons Recall: the outer electrons in an atom are valence electrons. Valence electrons are related to stability Valence electrons can be represented with dots in
Classes of Organic Compounds
 Unit 1 Functional Groups Depicting Structures of rganic ompounds Lewis Structures ondensed structural formulas Line angle drawings 3-dimensional structures Resonance Structures Acid-Base Reactions urved
Unit 1 Functional Groups Depicting Structures of rganic ompounds Lewis Structures ondensed structural formulas Line angle drawings 3-dimensional structures Resonance Structures Acid-Base Reactions urved
Chapter 7 Chemical Bonding and Molecular Structure
 Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
8.1 Types of Chemical Bonds List and define three types of bonding. chapter 8 Bonding General Concepts.notebook. September 10, 2015
 chapter 8 Bonding General Concepts.notebook Chapter 8: Bonding: General Concepts Mar 13 11:15 AM 8.1 Types of Chemical Bonds List and define three types of bonding. Bonds are forces that hold groups of
chapter 8 Bonding General Concepts.notebook Chapter 8: Bonding: General Concepts Mar 13 11:15 AM 8.1 Types of Chemical Bonds List and define three types of bonding. Bonds are forces that hold groups of
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model.
 Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
EXPERIMENT 15: MOLECULAR MODELS
 EXPERIMENT 15: MLEULAR MDELS Introduction: Given formulas of some molecules and ions, you will use the periodic table, valence electron count, and electronegativities to deduce their geometry and polarities.
EXPERIMENT 15: MLEULAR MDELS Introduction: Given formulas of some molecules and ions, you will use the periodic table, valence electron count, and electronegativities to deduce their geometry and polarities.
A DOT STRUCTURE FOR A LARGER MOLECULE ETHANOL! Count valence electrons
 212 A DOT STRUCTURE FOR A LARGER MOLECULE Count valence electrons Pick central atom and draw skeletal structure - central atom is usually the one that needs to gain the most electrons! - skeletal structure
212 A DOT STRUCTURE FOR A LARGER MOLECULE Count valence electrons Pick central atom and draw skeletal structure - central atom is usually the one that needs to gain the most electrons! - skeletal structure
Molecular Geometry. Objectives N H H. The objectives of this laboratory are to:
 Objectives The objectives of this laboratory are to: Molecular Geometry Write Lewis structure representations of the bonding and valence electrons in molecules. Use the VSEPR model to predict the molecular
Objectives The objectives of this laboratory are to: Molecular Geometry Write Lewis structure representations of the bonding and valence electrons in molecules. Use the VSEPR model to predict the molecular
Bonding in Organic Compounds
 Bonding in rganic ompounds hapter 1 1 Bonding in rganic ompounds APTER SUMMARY rganic chemistry is the study of compounds of carbon This is a separate branch of chemistry because of the large numbers of
Bonding in rganic ompounds hapter 1 1 Bonding in rganic ompounds APTER SUMMARY rganic chemistry is the study of compounds of carbon This is a separate branch of chemistry because of the large numbers of
Chapter 9: Chemical Bonding I: Lewis Theory. Lewis Theory: An Overview
 Chapter 9: Chemical Bonding I: Lewis Theory Dr. Chris Kozak Memorial University of ewfoundland, Canada Lewis Theory: An verview Valence e - play a fundamental role in chemical bonding. e - transfer leads
Chapter 9: Chemical Bonding I: Lewis Theory Dr. Chris Kozak Memorial University of ewfoundland, Canada Lewis Theory: An verview Valence e - play a fundamental role in chemical bonding. e - transfer leads
Molecular Compounds Compounds that are bonded covalently (like in water, or carbon dioxide) are called molecular compounds
 Chapter 8: Covalent Bonding Section 1: Molecular Compounds Bonds are Forces that hold groups of atoms together and make them function as a unit. Two types: Ionic bonds transfer of electrons (gained or
Chapter 8: Covalent Bonding Section 1: Molecular Compounds Bonds are Forces that hold groups of atoms together and make them function as a unit. Two types: Ionic bonds transfer of electrons (gained or
CHAPTER 12: CHEMICAL BONDING
 CHAPTER 12: CHEMICAL BONDING Problems: 1-26, 27c, 28, 33-34, 35b, 36(a-c), 37(a,b,d), 38a, 39-40, 41-42(a,c), 43-58, 67-74 12.1 THE CHEMICAL BOND CONCEPT chemical bond: what holds atoms or ions together
CHAPTER 12: CHEMICAL BONDING Problems: 1-26, 27c, 28, 33-34, 35b, 36(a-c), 37(a,b,d), 38a, 39-40, 41-42(a,c), 43-58, 67-74 12.1 THE CHEMICAL BOND CONCEPT chemical bond: what holds atoms or ions together
Molecular Geometries. Molecular Geometries. Remember that covalent bonds are formed when electrons in atomic orbitals are shared between two nuclei.
 Molecular Geometries Lewis dot structures are very useful in determining the types of bonds in a molecule, but they may not provide the best insight into the spatial geometry of a molecule, i.e., how the
Molecular Geometries Lewis dot structures are very useful in determining the types of bonds in a molecule, but they may not provide the best insight into the spatial geometry of a molecule, i.e., how the
Chapter 8. Basic Concepts of Chemical Bonding
 Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds An attractive force that holds two atoms together in a more complex unit Three basic types of bonds Ionic Electrons are transferred from one
Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds An attractive force that holds two atoms together in a more complex unit Three basic types of bonds Ionic Electrons are transferred from one
Wold of Chemistry Notes for Students [Chapter 12, page 1] Chapter 12 Chemical Bonding
![Wold of Chemistry Notes for Students [Chapter 12, page 1] Chapter 12 Chemical Bonding Wold of Chemistry Notes for Students [Chapter 12, page 1] Chapter 12 Chemical Bonding](/thumbs/77/75442347.jpg) Wold of Chemistry Notes for Students [Chapter 12, page 1] Chapter 12 Chemical Bonding 1) The History of the Development of the Period Table (Not in the book!) Similarities between the chemical and physical
Wold of Chemistry Notes for Students [Chapter 12, page 1] Chapter 12 Chemical Bonding 1) The History of the Development of the Period Table (Not in the book!) Similarities between the chemical and physical
Lecture Notes Chem 51A S. King Chapter 1 Structure and Bonding
 Lecture otes hem 51A S. King hapter 1 Structure and Bonding rganic hemistry: The chemistry of compounds that contain carbon >95% of all chemical compounds are organic. aturally occurring organic molecules:
Lecture otes hem 51A S. King hapter 1 Structure and Bonding rganic hemistry: The chemistry of compounds that contain carbon >95% of all chemical compounds are organic. aturally occurring organic molecules:
Test Review # 4. Chemistry: Form TR4.11A
 Chemistry: Form TR4.11 REVIEW Name Date Period Test Review # 4 Bonding. The electrons of one atom are attracted to the protons of another. When atoms combine, there is a tug of war over the valence electrons.
Chemistry: Form TR4.11 REVIEW Name Date Period Test Review # 4 Bonding. The electrons of one atom are attracted to the protons of another. When atoms combine, there is a tug of war over the valence electrons.
Chemical Bonding and Molecular Models
 25 Chemical Bonding and Molecular Models A chemical bond is a force that holds groups of two or more atoms together and makes them function as a unit. Bonding involves only the valence (outer shell) electrons
25 Chemical Bonding and Molecular Models A chemical bond is a force that holds groups of two or more atoms together and makes them function as a unit. Bonding involves only the valence (outer shell) electrons
Carbon and Its Compounds
 Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
***Occurs when atoms of elements combine together to form compounds.*****
 CHEMICAL BONDING ***Occurs when atoms of elements combine together to form compounds.***** Formation of compounds involve adjustments in the position of one or more valence electrons. PE is lower in bonded
CHEMICAL BONDING ***Occurs when atoms of elements combine together to form compounds.***** Formation of compounds involve adjustments in the position of one or more valence electrons. PE is lower in bonded
Organic Chemistry Lecture I. Dr. John D. Spence
 HEMISTRY 3 Organic hemistry Lecture I Dr. John D. Spence jdspence@scu.edu jspence@csus.eduedu http://www.csus.edu/indiv/s/spencej What is Organic hemistry? 780 s hemistry of compounds from living organisms
HEMISTRY 3 Organic hemistry Lecture I Dr. John D. Spence jdspence@scu.edu jspence@csus.eduedu http://www.csus.edu/indiv/s/spencej What is Organic hemistry? 780 s hemistry of compounds from living organisms
Carbon-based molecules are held together by covalent bonds between atoms
 hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
Chapter 12. Chemical Bonding
 Chapter 12 Chemical Bonding Chemical Bond Concept Recall that an atom has core and valence electrons. Core electrons are found close to the nucleus. Valence electrons are found in the most distant s and
Chapter 12 Chemical Bonding Chemical Bond Concept Recall that an atom has core and valence electrons. Core electrons are found close to the nucleus. Valence electrons are found in the most distant s and
Chemistry Chapter 6 Test Review
 Chemistry Chapter 6 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A mutual electrical attraction between the nuclei and valence electrons
Chemistry Chapter 6 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A mutual electrical attraction between the nuclei and valence electrons
CHAPTER 12 CHEMICAL BONDING
 Chemistry Name Hour Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 12 CHEMICAL BONDING Day Plans for the day Assignment(s) for the day 1 Begin Chapter
Chemistry Name Hour Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 12 CHEMICAL BONDING Day Plans for the day Assignment(s) for the day 1 Begin Chapter
Chemical Bonds, Molecular Models, and Molecular Shapes
 Chemical Bonds, Molecular Models, and Molecular Shapes PRELAB ASSINGMENT Read the entire laboratory write up and answer the following questions before coming to lab. Read the entire laboratory write up
Chemical Bonds, Molecular Models, and Molecular Shapes PRELAB ASSINGMENT Read the entire laboratory write up and answer the following questions before coming to lab. Read the entire laboratory write up
