Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry Periodic Trends in Atomic Properties Learning Objective
|
|
|
- Dwain Horn
- 5 years ago
- Views:
Transcription
1 Chapter 11 Chemical Bonds: The Formation of Compounds from Atoms Advanced Chemistry 11.1 Periodic Trends in Atomic Properties Discuss the atomic trends Metals are located on the left side of the periodic table, nonmetals for metals and nonmetals, on the right. atomic radius, and ionization energy. Atomic radius increases down a group and decreases across a period (from left to right).
2 Ionization energy decreases down a group and increases across a period (from left to right). Key Term Ionization energy The energy required to remove an electron from an atom, an ion, or a molecule. A + ionization energy à A + + e Lewis Structures of Atoms Draw the Lewis structure To determine a Lewis structure for representative elements, use for a given atom the group number as the number of electrons to place around the symbol for the element. Key Term Lewis structure A method of indicating the covalent bonds between atoms in a molecule or an ion such that a pair of electrons (:) represents the valence electrons forming the covalent bond.
3 11.3 The Ionic Bond: Transfer of Electrons From One Atom to Another Discuss the formation of The goal of bonding is to achieve stability. For representative an ionic bond and the elements, this stability can be achieved by attaining a valence chemical change that electron structure of a noble gas. results from the bond. In an ionic bond stability is attained by transferring an electron from one atom to another: o The atom that loses an electron becomes a cation. Positive ions are smaller than their parent atoms. Metals tend to form cations. o The atom gaining an electron becomes an anion. Negative ions are larger than their parent atoms. Nonmetals tend to form anions. o Ionic compounds do not exist as molecules. Ions are attracted by multiple ions of the opposite charge to form a crystalline structure. Key Term Ionic bond The chemical bond between a positively charged ion and a negatively charged ion.
4 11.4 Predicting Formulas of Ionic Compounds Predict the formulas of Chemical compounds are always electrically neutral. ionic compounds from Metals lose electrons and nonmetals gain electrons to form their position on the compounds. periodic table. Stability is achieved (for representative elements) by attaining a noble gas electron configuration The Covalent Bond: Sharing Electrons Draw the electron structure of a covalent bond. Key Terms Covalent bond Polar covalent bond Covalent bonds are formed when two atoms share a pair of electrons between them. This is the predominate type of bonding in compounds. True molecules exist in covalent compounds. Overlap of orbitals forms a covalent bond. Unequal sharing of electrons results in a polar covalent bond. A chemical bond formed between two atoms by sharing a pair of electrons. A covalent bond between two atoms with differing electronegativity values, resulting in unequal sharing of bonding electrons.
5 11.6 Electronegativity Explain how electronegativities of component atoms in a molecule determine the polarity of the molecule. Electrons spend more time closer to the more electronegative atom in a bond forming a polar bond. The polarity of a bond is determined by the electronegativity difference between the atoms involved in the bond. o The greater the difference, the more polar the bond is. o At the extremes, large differences result in ionic bonds and tiny differences (or no difference) result(s) in a nonpolar covalent bond. o A molecule that is electrically asymmetrical has a dipole, resulting in charged areas within the molecules. o If the electronegativity difference between two bonded atoms is greater than , then the bond will be more ionic than covalent. o Polar bonds do not always result in polar molecules.
6 Key Terms Electronegativity The relative attraction that an atom has for a pair of shared electrons in a covalent bond. Nonpolar covalent bond Dipole A covalent bond between two atoms with the same electronegativity value; thus, the electrons are shared equally between the two atoms. A molecule that is electrically asymmetrical, causing it to be oppositely charged at two points. For example:
7 11.7 Lewis Structures of Compounds Draw the Lewis structure PROBLEM-SOLVING STRATEGY: Writing a Lewis Structure of a covalent compound. 1. Obtain the total number of valence electrons to be used in the structure by adding the number of valence electrons in all the atoms in the molecule or ion. If you are writing the structure of an ion, add one electron for each negative charge or subtract one electron for each positive charge on the ion. 2. Write the skeletal arrangement of the atoms and connect hem with a single covalent bond (two dots or one dash). Hydrogen, which contains only one bonding electron, can form only one covalent bond. Oxygen atoms are not normally bonded to each other, except in compounds know to be peroxides. Oxygen atoms normally have a maximum of two covalent bonds (two single bonds or one double bond). 3. Satisfy the octet rule. Distribute pairs of electrons (pairs of dots) around each atom (except hydrogen) to give each atom a noble gas structure. 4. Count the total number of electrons used and compare it to the total number of valence electrons available as calculated in Step 1. If there are too many used, change single bonds between atoms to double or triple bonds by shifting the unbounded pairs of electrons as needed. Check to see that each atom has a noble gas electrons structure (two electrons for hydrogen and eight for the others). A double bond counts as four electrons for each atom to which it is bonded, and triple as six Complex Lewis Structures Draw the resonance When a single Lewis structure cannot be drawn for a molecule, structures for a resonance structures (multiple Lewis structures) are used to polyatomic ion. represent the molecule. For example, the nitrate ion NO3 - Key Term Resonance structure A molecule or ion that has multiple Lewis structures.
8 11.9 Compounds Containing Polyatomic Ions Describe a compound that Polyatomic ions behave like a single unit in many chemical contains both ionic and reactions. covalent bonds. The bonds within a polyatomic ion are covalent. For example, Na2CO3(s)!"#$% 2 Na + (aq) + CO3 2- (aq) Molecular Shape Determine the shape of a compound by using VSEPER method. Key Terms Linear structure Trigonal planar structure Tetrahedral structure Bent structure Lewis structures do not indicate the shape of a molecule. PROBLEM-SOLVING STRATEGY: Determining Molecular Shape Using VSEPR Draw the Lewis structure for the molecule. Count the electron pairs around the central atom and arrange them to minimize repulsions (as far apart as possible). This determines the electron pair arrangement. Determine the positions of the atoms. Name the molecular structure from the position of the atoms. In the VSEPR model, an arrangement where the pairs of electrons are arranged 180 apart for maximum separation. An arrangement of atoms in the VSEPR model where the three pairs of electrons are placed 120 apart on a flat plane. An arrangement of the VSEPR model where four pairs of electrons are placed degrees apart to form a tetrahedron. Bent structure in which the molecule looks like a V. The electron structure is tetrahedral but there are 2 pairs of nonbonding electrons.
9
Ionic and Covalent Bonding
 1. Define the following terms: a) valence electrons Ionic and Covalent Bonding the electrons in the highest occupied energy level always electrons in the s and p orbitals maximum of 8 valence electrons
1. Define the following terms: a) valence electrons Ionic and Covalent Bonding the electrons in the highest occupied energy level always electrons in the s and p orbitals maximum of 8 valence electrons
Chapter 6. The Chemical Bond
 Chapter 6 The Chemical Bond Some questions Why do noble gases rarely bond to other elements? How does this relate to why the atoms of other elements do form bonds? Why do certain elements combine to form
Chapter 6 The Chemical Bond Some questions Why do noble gases rarely bond to other elements? How does this relate to why the atoms of other elements do form bonds? Why do certain elements combine to form
Chapter 12. Chemical Bonding
 Chapter 12 Chemical Bonding Chemical Bond Concept Recall that an atom has core and valence electrons. Core electrons are found close to the nucleus. Valence electrons are found in the most distant s and
Chapter 12 Chemical Bonding Chemical Bond Concept Recall that an atom has core and valence electrons. Core electrons are found close to the nucleus. Valence electrons are found in the most distant s and
CHAPTER 12 CHEMICAL BONDING
 CHAPTER 12 CHEMICAL BONDING Core electrons are found close to the nucleus, whereas valence electrons are found in the most distant s and p energy subshells. The valence electrons are responsible for holding
CHAPTER 12 CHEMICAL BONDING Core electrons are found close to the nucleus, whereas valence electrons are found in the most distant s and p energy subshells. The valence electrons are responsible for holding
Chapter 6 Chemical Bonding
 Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
Chapter 6 Chemical Bonding Section 6-1 Introduction to Chemical Bonding Chemical Bonds Valence electrons are attracted to other atoms, and that determines the kind of chemical bonding that occurs between
CHEM 101: CHAPTER 11: CHEMICAL BONDS: THE FORMATION OF COMPOUNDS FROM ATOMS
 1 CHEM 101: CHAPTER 11: CHEMICAL BONDS: THE FORMATION OF COMPOUNDS FROM ATOMS PERIODIC TRENDS: See pages 214-216, 221 Table 11.3, and 227 + 228 of text. Lewis Structures of Atoms: The Lewis Dot Diagram
1 CHEM 101: CHAPTER 11: CHEMICAL BONDS: THE FORMATION OF COMPOUNDS FROM ATOMS PERIODIC TRENDS: See pages 214-216, 221 Table 11.3, and 227 + 228 of text. Lewis Structures of Atoms: The Lewis Dot Diagram
Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction
 Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent
Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE INSTR : FİLİZ ALSHANABLEH
 C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE The Ionic Bond Formation of Ions The
C H E M 1 CHEM 101-GENERAL CHEMISTRY CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE 0 1 INSTR : FİLİZ ALSHANABLEH CHAPTER 7 CHEMICAL BONDING & MOLECULAR STRUCTURE The Ionic Bond Formation of Ions The
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking
 INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Sixth Edition by Charles H. Corwin Chapter 12 Chemical Bonding by Christopher Hamaker 2011 Pearson Education, Inc. Chapter 12 1 Chemical Bond Concept
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Sixth Edition by Charles H. Corwin Chapter 12 Chemical Bonding by Christopher Hamaker 2011 Pearson Education, Inc. Chapter 12 1 Chemical Bond Concept
Chapter 7. Ionic & Covalent Bonds
 Chapter 7 Ionic & Covalent Bonds Ionic Compounds Covalent Compounds 7.1 EN difference and bond character >1.7 = ionic 0.4 1.7 = polar covalent 1.7 Electrons not shared at
Chapter 7 Ionic & Covalent Bonds Ionic Compounds Covalent Compounds 7.1 EN difference and bond character >1.7 = ionic 0.4 1.7 = polar covalent 1.7 Electrons not shared at
Chapter 10. Valence Electrons. Lewis dot symbols. Chemical Bonding
 Chapter 10 Chemical Bonding Valence Electrons Recall: the outer electrons in an atom are valence electrons. Valence electrons are related to stability Valence electrons can be represented with dots in
Chapter 10 Chemical Bonding Valence Electrons Recall: the outer electrons in an atom are valence electrons. Valence electrons are related to stability Valence electrons can be represented with dots in
Unit 3 - Chemical Bonding and Molecular Structure
 Unit 3 - Chemical Bonding and Molecular Structure Chemical bond - A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together 6-1 Introduction
Unit 3 - Chemical Bonding and Molecular Structure Chemical bond - A mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together 6-1 Introduction
Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed.
 Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl & Donald J. DeCoste University of Illinois Chapter 12 Chemical Bonding Structure
Introductory Chemistry: A Foundation, 6 th Ed. Introductory Chemistry, 6 th Ed. Basic Chemistry, 6 th Ed. by Steven S. Zumdahl & Donald J. DeCoste University of Illinois Chapter 12 Chemical Bonding Structure
Bonding Chapter 7. Bond an attractive force that holds two atoms together. Atoms bond to obtain a more stable electronic configuration.
 Bonding Chapter 7 Bond an attractive force that holds two atoms together. Atoms bond to obtain a more stable electronic configuration. Ionic bonds attraction between oppositely charged atoms/molecules
Bonding Chapter 7 Bond an attractive force that holds two atoms together. Atoms bond to obtain a more stable electronic configuration. Ionic bonds attraction between oppositely charged atoms/molecules
RESONANCE STRUCTURE When a molecule has more than one possible structure. Draw all possible structures and place a double end arrow ( ) in between.
 CHEMISTRY NOTES 6.1 COVALENT BONDS Objectives Explain the role and location of electrons in a covalent bond. Describe the change in energy and stability that takes place as a covalent bond forms. Distinguish
CHEMISTRY NOTES 6.1 COVALENT BONDS Objectives Explain the role and location of electrons in a covalent bond. Describe the change in energy and stability that takes place as a covalent bond forms. Distinguish
Chapter 12. Chemical Bonding
 Chapter 12 Chemical Bonding Chapter 12 Introduction to Chemical Bonding Chemical Bonding Valence electrons are the electrons in the outer shell (highest energy level) of an atom. A chemical bond is a mutual
Chapter 12 Chemical Bonding Chapter 12 Introduction to Chemical Bonding Chemical Bonding Valence electrons are the electrons in the outer shell (highest energy level) of an atom. A chemical bond is a mutual
What is Bonding? The Octet Rule. Getting an Octet. Chemical Bonding and Molecular Shapes. (Chapter Three, Part Two)
 Chemical Bonding and Molecular Shapes (Chapter Three, Part Two) What is Bonding? Bonding describes how atoms interact with each other in an attractive sense. There are three types of bonding: Ionic bonding
Chemical Bonding and Molecular Shapes (Chapter Three, Part Two) What is Bonding? Bonding describes how atoms interact with each other in an attractive sense. There are three types of bonding: Ionic bonding
Chapter 12 Structures and Characteristics of Bonds Objectives
 Objectives 1. To learn about ionic and covalent bonds and explain how they are formed - what holds compounds together? 2. To learn about the polar covalent bond are all covalent bonds equal? 3. To understand
Objectives 1. To learn about ionic and covalent bonds and explain how they are formed - what holds compounds together? 2. To learn about the polar covalent bond are all covalent bonds equal? 3. To understand
Chapter 4 Lecture Outline. Copyright McGraw-Hill Education. Permission required for reproduction or display.
 Chapter 4 Lecture Outline 1 Copyright McGraw-ill Education. Permission required for reproduction or display. 4.1 Introduction to Covalent Bonding Covalent bonds result from the sharing of electrons between
Chapter 4 Lecture Outline 1 Copyright McGraw-ill Education. Permission required for reproduction or display. 4.1 Introduction to Covalent Bonding Covalent bonds result from the sharing of electrons between
Chapter 7 Chemical Bonding
 Chapter 7 Chemical Bonding 7.1 Ionic Bonding Octet rule: In forming compounds atoms lose, gain or share electrons to attain a noble gas configuration with 8 electrons in their outer shell (s 2 p 6 ), except
Chapter 7 Chemical Bonding 7.1 Ionic Bonding Octet rule: In forming compounds atoms lose, gain or share electrons to attain a noble gas configuration with 8 electrons in their outer shell (s 2 p 6 ), except
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin
 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Chemical Bonding by Christopher G. Hamaker Illinois State University Chemical Bond Concept Recall that
Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin Chemical Bonding by Christopher G. Hamaker Illinois State University Chemical Bond Concept Recall that
Lewis Dot Formulas and Molecular Shapes
 Lewis Dot Formulas and Molecular Shapes Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent chemical bonds are formed by valence electrons
Lewis Dot Formulas and Molecular Shapes Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent chemical bonds are formed by valence electrons
Lewis Structures and Molecular Shapes
 Lewis Structures and Molecular Shapes Drawing Lewis Structures Determine from formula if ionic or covalent Count the electrons If ionic : add valence # to charge if (-), subtract if (+) - = 7+1 electrons;
Lewis Structures and Molecular Shapes Drawing Lewis Structures Determine from formula if ionic or covalent Count the electrons If ionic : add valence # to charge if (-), subtract if (+) - = 7+1 electrons;
Its Bonding Time. Chemical Bonds CH 12
 Its Bonding Time Chemical Bonds CH 12 What is a chemical bond? Octet Rule: Chemical compounds tend to form so that each atom, by gaining, losing, or sharing electrons, has an octet of electrons in its
Its Bonding Time Chemical Bonds CH 12 What is a chemical bond? Octet Rule: Chemical compounds tend to form so that each atom, by gaining, losing, or sharing electrons, has an octet of electrons in its
Bonding in Chemistry. Chemical Bonds All chemical reactions involve breaking of some bonds and formation of new ones where new products are formed.
 CHEMICAL BONDS Atoms or ions are held together in molecules or compounds by chemical bonds. The type and number of electrons in the outer electronic shells of atoms or ions are instrumental in how atoms
CHEMICAL BONDS Atoms or ions are held together in molecules or compounds by chemical bonds. The type and number of electrons in the outer electronic shells of atoms or ions are instrumental in how atoms
Covalent Bonding. In nature, only the noble gas elements exist as uncombined atoms. All other elements need to lose or gain electrons
 In nature, only the noble gas elements exist as uncombined atoms. They are monatomic - consist of single atoms. All other elements need to lose or gain electrons To form ionic compounds Some elements share
In nature, only the noble gas elements exist as uncombined atoms. They are monatomic - consist of single atoms. All other elements need to lose or gain electrons To form ionic compounds Some elements share
Chapter 8. Ions and the Noble Gas. Chapter Electron transfer leads to the formation of ionic compounds
 Chapter 8 Chemical Bonding: General Concepts 1 8.1 Electron transfer leads to the formation of ionic compounds Ionic compounds form when metals and nonmetals react The attraction between positive and negative
Chapter 8 Chemical Bonding: General Concepts 1 8.1 Electron transfer leads to the formation of ionic compounds Ionic compounds form when metals and nonmetals react The attraction between positive and negative
Hey, Baby. You and I Have a Bond...Ch. 8
 I. IONIC BONDING FUNDAMENTALS A. They form between... 1. A and a a. A to become b. A to become B. How it happens (Let s first focus on two atoms): 1. When a metal and a nonmetal meet, electrons get transferred
I. IONIC BONDING FUNDAMENTALS A. They form between... 1. A and a a. A to become b. A to become B. How it happens (Let s first focus on two atoms): 1. When a metal and a nonmetal meet, electrons get transferred
Bonding: Part Two. Three types of bonds: Ionic Bond. transfer valence e - Metallic bond. (NaCl) (Fe) mobile valence e - Covalent bond
 Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Chapter 8: Concepts of Chemical Bonding
 Chapter 8: Concepts of Chemical Bonding Learning Outcomes: Write Lewis symbols for atoms and ions. Define lattice energy and be able to arrange compounds in order of increasing lattice energy based on
Chapter 8: Concepts of Chemical Bonding Learning Outcomes: Write Lewis symbols for atoms and ions. Define lattice energy and be able to arrange compounds in order of increasing lattice energy based on
Bonding: Part Two. Three types of bonds: Ionic Bond. transfer valence e - Metallic bond. (NaCl) (Fe) mobile valence e - Covalent bond
 Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Bonding: Part Two Three types of bonds: Ionic Bond transfer valence e - Metallic bond mobile valence e - Covalent bond (NaCl) (Fe) shared valence e - (H 2 O) 1 Single Covalent Bond H + H H H H-atoms H
Chapter 6 PRETEST: Chemical Bonding
 Chapter 6 PRETEST: Chemical In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.The charge on an ion is a. always positive.
Chapter 6 PRETEST: Chemical In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.The charge on an ion is a. always positive.
4/25/2017. VSEPR Theory. Two Electron Groups. Shapes of Molecules. Two Electron Groups with Double Bonds. Three Electron Groups.
 Chapter 10 Lecture Chapter 10 Bonding and Properties of Solids and Liquids 10.3 Shapes of Molecules and Ions (VSEPR Theory) Learning Goal Predict the three-dimensional structure of a molecule or a polyatomic
Chapter 10 Lecture Chapter 10 Bonding and Properties of Solids and Liquids 10.3 Shapes of Molecules and Ions (VSEPR Theory) Learning Goal Predict the three-dimensional structure of a molecule or a polyatomic
Covalent Bonding. In nature, only the noble gas elements exist as uncombined atoms. All other elements need to lose or gain electrons
 In nature, only the noble gas elements exist as uncombined atoms. They are monatomic - consist of single atoms. All other elements need to lose or gain electrons To form ionic compounds Some elements share
In nature, only the noble gas elements exist as uncombined atoms. They are monatomic - consist of single atoms. All other elements need to lose or gain electrons To form ionic compounds Some elements share
Covalent Bonding bonding that results from the sharing of electron pairs.
 Unit 5 Notes Covalent Bonding, Covalent Compounds, and Intermolecular Forces Chemical Bond a mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms
Unit 5 Notes Covalent Bonding, Covalent Compounds, and Intermolecular Forces Chemical Bond a mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms
Section 12: Lewis Structures
 Section 12: Lewis Structures The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 12.01 Electronegativity Chemistry (5)(C) 12.02 Electron
Section 12: Lewis Structures The following maps the videos in this section to the Texas Essential Knowledge and Skills for Science TAC 112.35(c). 12.01 Electronegativity Chemistry (5)(C) 12.02 Electron
CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS
 CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS IONIC BONDING When an atom of a nonmetal takes one or more electrons from an atom of a metal so both atoms end up with eight valence
CHEMICAL BONDING IONIC BONDS COVALENT BONDS HYDROGEN BONDS METALLIC BONDS IONIC BONDING When an atom of a nonmetal takes one or more electrons from an atom of a metal so both atoms end up with eight valence
REVIEW: VALENCE ELECTRONS CHEMICAL BONDS: LEWIS SYMBOLS: CHEMICAL BONDING. What are valence electrons?
 REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
REVIEW: VALENCE ELECTRONS 13 CHEMICAL BONDING What are valence electrons? Which groups on the periodic table readily give up electrons? What group readily accepts electrons? CHEMICAL BONDS: What are chemical
CHAPTER 12: CHEMICAL BONDING
 CHAPTER 12: CHEMICAL BONDING Problems: 1-26, 27c, 28, 33-34, 35b, 36(a-c), 37(a,b,d), 38a, 39-40, 41-42(a,c), 43-58, 67-74 12.1 THE CHEMICAL BOND CONCEPT chemical bond: what holds atoms or ions together
CHAPTER 12: CHEMICAL BONDING Problems: 1-26, 27c, 28, 33-34, 35b, 36(a-c), 37(a,b,d), 38a, 39-40, 41-42(a,c), 43-58, 67-74 12.1 THE CHEMICAL BOND CONCEPT chemical bond: what holds atoms or ions together
Chapter 9 Bonding 2 Polar Covalent Bond, Electronegativity, Formal Charge, Resonance. Dr. Sapna Gupta
 Chapter 9 Bonding 2 Polar Covalent Bond, Electronegativity, Formal Charge, Resonance Dr. Sapna Gupta Writing Lewis Structures 1. Draw the skeleton structure of the molecule or ion by placing the lowest
Chapter 9 Bonding 2 Polar Covalent Bond, Electronegativity, Formal Charge, Resonance Dr. Sapna Gupta Writing Lewis Structures 1. Draw the skeleton structure of the molecule or ion by placing the lowest
Chapter 6 Chemistry Review
 Chapter 6 Chemistry Review Multiple Choice Identify the choice that best completes the statement or answers the question. Put the LETTER of the correct answer in the blank. 1. The electrons involved in
Chapter 6 Chemistry Review Multiple Choice Identify the choice that best completes the statement or answers the question. Put the LETTER of the correct answer in the blank. 1. The electrons involved in
Covalent bonding occurs in nonmetal compounds. Use the highlighter to select the compounds that are covalently bonded. HCl
 Covalent bonding occurs in nonmetal compounds. Use the highlighter to select the compounds that are covalently bonded. 2 C 2 Cl Li NaF Mg C 4 N NaCl 3 Drag this to the target to reveal the answers. Properties
Covalent bonding occurs in nonmetal compounds. Use the highlighter to select the compounds that are covalently bonded. 2 C 2 Cl Li NaF Mg C 4 N NaCl 3 Drag this to the target to reveal the answers. Properties
CHEMISTRY Matter and Change Section 8.1 The Covalent Bond
 CHEMISTRY Matter and Change Section Chapter 8: Covalent Bonding CHAPTER 8 Table Of Contents Section 8.2 Section 8.3 Section 8.4 Section 8.5 Naming Molecules Molecular Structures Molecular Shapes Electronegativity
CHEMISTRY Matter and Change Section Chapter 8: Covalent Bonding CHAPTER 8 Table Of Contents Section 8.2 Section 8.3 Section 8.4 Section 8.5 Naming Molecules Molecular Structures Molecular Shapes Electronegativity
Chapter 9 Bonding. Dr. Sapna Gupta
 Chapter 9 Bonding Dr. Sapna Gupta Lewis Dot Symbol Lewis dot symbols is a notation where valence electrons are shown as dots. Draw the electrons symmetrically around the sides (top, bottom, left and right)
Chapter 9 Bonding Dr. Sapna Gupta Lewis Dot Symbol Lewis dot symbols is a notation where valence electrons are shown as dots. Draw the electrons symmetrically around the sides (top, bottom, left and right)
Lewis Theory of Shapes and Polarities of Molecules
 Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Molecular Geometry & Polarity
 Molecular Geometry & Polarity Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized
Molecular Geometry & Polarity Learn Shapes you will Because the physical and chemical properties of compounds are tied to their structures, the importance of molecular geometry can not be overstated. Localized
Ch 6 Chemical Bonding
 Ch 6 Chemical Bonding What you should learn in this section (objectives): Define chemical bond Explain why most atoms form chemical bonds Describe ionic and covalent bonding Explain why most chemical bonding
Ch 6 Chemical Bonding What you should learn in this section (objectives): Define chemical bond Explain why most atoms form chemical bonds Describe ionic and covalent bonding Explain why most chemical bonding
Chapter 8: Covalent Bonding. Chapter 8
 : Covalent Bonding Bonding Ionic Bonding - attracted to each other, but not fully committed Covalent Bonding - fully committed, and shares everything Two methods to gain or lose valence electrons: Transfer
: Covalent Bonding Bonding Ionic Bonding - attracted to each other, but not fully committed Covalent Bonding - fully committed, and shares everything Two methods to gain or lose valence electrons: Transfer
Chapter 6. Table of Contents. Section 1 Covalent Bonds. Section 2 Drawing and Naming Molecules. Section 3 Molecular Shapes. Covalent Compounds
 Covalent Compounds Table of Contents Section 1 Covalent Bonds Section 2 Drawing and Naming Molecules Section 3 Molecular Shapes Section 1 Covalent Bonds Bellringer Make a list of the elements that form
Covalent Compounds Table of Contents Section 1 Covalent Bonds Section 2 Drawing and Naming Molecules Section 3 Molecular Shapes Section 1 Covalent Bonds Bellringer Make a list of the elements that form
Bonding. Computer Lab: Ionic Bonds. Important Notes 3/22/18
 Bonding What are ionic bonds, and how are they formed? Computer Lab: Ionic Bonds Go to http://www.pbslearningmedia.org/asset/ lsps07_int_ionicbonding/ Read each screen and follow the directions where appropriate.
Bonding What are ionic bonds, and how are they formed? Computer Lab: Ionic Bonds Go to http://www.pbslearningmedia.org/asset/ lsps07_int_ionicbonding/ Read each screen and follow the directions where appropriate.
Chemical Bonds. Chapter 6
 Chemical Bonds Chapter 6 1 Ch. 6 Chemical Bonding I. How and Why Atoms Bond A. Vocabulary B. Chemical Bonds - Basics C. Chemical Bonds Types D. Chemical Bonds Covalent E. Drawing Lewis Diagrams F. Bond
Chemical Bonds Chapter 6 1 Ch. 6 Chemical Bonding I. How and Why Atoms Bond A. Vocabulary B. Chemical Bonds - Basics C. Chemical Bonds Types D. Chemical Bonds Covalent E. Drawing Lewis Diagrams F. Bond
Chapter 7: Chemical Bonding and Molecular Structure
 Chapter 7: Chemical Bonding and Molecular Structure Ionic Bond Covalent Bond Electronegativity and Bond Polarity Lewis Structures Orbital Overlap Hybrid Orbitals The Shapes of Molecules (VSEPR Model) Molecular
Chapter 7: Chemical Bonding and Molecular Structure Ionic Bond Covalent Bond Electronegativity and Bond Polarity Lewis Structures Orbital Overlap Hybrid Orbitals The Shapes of Molecules (VSEPR Model) Molecular
EXPERIMENT 15: MOLECULAR MODELS
 EXPERIMENT 15: MLEULAR MDELS Introduction: Given formulas of some molecules and ions, you will use the periodic table, valence electron count, and electronegativities to deduce their geometry and polarities.
EXPERIMENT 15: MLEULAR MDELS Introduction: Given formulas of some molecules and ions, you will use the periodic table, valence electron count, and electronegativities to deduce their geometry and polarities.
Carbon-based molecules are held together by covalent bonds between atoms
 hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
Unit 5: Bonding. Place a checkmark next to each item that you can do. If a sample problem is given, complete it as evidence.
 Unit 5: Bonding Place a checkmark next to each item that you can do. If a sample problem is given, complete it as evidence. Intramolecular Forces: forces of attraction within the same molecule. Examples:
Unit 5: Bonding Place a checkmark next to each item that you can do. If a sample problem is given, complete it as evidence. Intramolecular Forces: forces of attraction within the same molecule. Examples:
Experiment 21 Lewis structures and VSEPR Theory
 Experiment 21 Lewis structures and VSEPR Theory Introduction 1. Lewis Structures and Formal Charge LG.N. Lewis, at the University of California at Berkeley devised a simple way to understand the nature
Experiment 21 Lewis structures and VSEPR Theory Introduction 1. Lewis Structures and Formal Charge LG.N. Lewis, at the University of California at Berkeley devised a simple way to understand the nature
8.1 Types of Chemical Bonds List and define three types of bonding. chapter 8 Bonding General Concepts.notebook. September 10, 2015
 chapter 8 Bonding General Concepts.notebook Chapter 8: Bonding: General Concepts Mar 13 11:15 AM 8.1 Types of Chemical Bonds List and define three types of bonding. Bonds are forces that hold groups of
chapter 8 Bonding General Concepts.notebook Chapter 8: Bonding: General Concepts Mar 13 11:15 AM 8.1 Types of Chemical Bonds List and define three types of bonding. Bonds are forces that hold groups of
Chemical Bonding I: Covalent Bonding. How are atoms held together in compounds?
 I: Covalent Bonding How are atoms held together in compounds? IONIC or COVALENT bonds or forces For most atoms, a filled outer shell contains 8 electrons ----- an octet Atoms want to form octets when they
I: Covalent Bonding How are atoms held together in compounds? IONIC or COVALENT bonds or forces For most atoms, a filled outer shell contains 8 electrons ----- an octet Atoms want to form octets when they
Chapter 8 Chemical Bonding
 Chapter 8 Chemical Bonding Types of Bonds Ionic Bonding Covalent Bonding Shapes of Molecules 8-1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Table 8.1 Two
Chapter 8 Chemical Bonding Types of Bonds Ionic Bonding Covalent Bonding Shapes of Molecules 8-1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Table 8.1 Two
of its physical and chemical properties.
 8.4 Molecular Shapes VSEPR Model The shape of a molecule determines many of its physical and chemical properties. Molecular l geometry (shape) can be determined with the Valence Shell Electron Pair Repulsion
8.4 Molecular Shapes VSEPR Model The shape of a molecule determines many of its physical and chemical properties. Molecular l geometry (shape) can be determined with the Valence Shell Electron Pair Repulsion
Chapter 8 The Concept of the Chemical Bond
 Chapter 8 The Concept of the Chemical Bond Three basic types of bonds: Ionic - Electrostatic attraction between ions (NaCl) Metallic - Metal atoms bonded to each other Covalent - Sharing of electrons Ionic
Chapter 8 The Concept of the Chemical Bond Three basic types of bonds: Ionic - Electrostatic attraction between ions (NaCl) Metallic - Metal atoms bonded to each other Covalent - Sharing of electrons Ionic
Chem 1075 Chapter 12 Chemical Bonding Lecture Outline. Chemical Bond Concept
 Chem 1075 Chapter 12 Chemical Bonding Lecture Outline Slide 2 Chemical Bond Concept Recall that an atom has and electrons. Core electrons are found to the nucleus. Valence electrons are found in the s
Chem 1075 Chapter 12 Chemical Bonding Lecture Outline Slide 2 Chemical Bond Concept Recall that an atom has and electrons. Core electrons are found to the nucleus. Valence electrons are found in the s
CHEMICAL BONDING. Chemical Bonds. Ionic Bonding. Lewis Symbols
 CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
CHEMICAL BONDING COVALENT BONDS IONIC BONDS METALLIC BONDS
 CHEMICAL BONDING COVALENT BONDS IONIC BONDS METALLIC BONDS Metallic Bonds How atoms are held together in solid metals. Metals hold onto their valence electrons very weakly. Think of them as positive ions
CHEMICAL BONDING COVALENT BONDS IONIC BONDS METALLIC BONDS Metallic Bonds How atoms are held together in solid metals. Metals hold onto their valence electrons very weakly. Think of them as positive ions
NOTES: UNIT 6: Bonding
 Name: Regents Chemistry: Mr. Palermo NOTES: UNIT 6: Bonding www.mrpalermo.com Name: Key Ideas Compounds can be differentiated by their chemical and physical properties. (3.1dd) Two major categories of
Name: Regents Chemistry: Mr. Palermo NOTES: UNIT 6: Bonding www.mrpalermo.com Name: Key Ideas Compounds can be differentiated by their chemical and physical properties. (3.1dd) Two major categories of
Chapter 8. Chemical Bonding: Basic Concepts
 Chapter 8. Chemical Bonding: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Chapter 8. Chemical Bonding: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Chapter 8 Covalent Boding
 Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
Chapter 8 Covalent Boding Molecules & Molecular Compounds In nature, matter takes many forms. The noble gases exist as atoms. They are monatomic; monatomic they consist of single atoms. Hydrogen chloride
For a quick and enjoyable introduction to Covalent vs Ionic Bonding watch this video:
 Covalent Bonding Covalent Bonding is the result of sharing of electron pairs between 2 nonmetal atoms Caution: sharing can be complicated Recall the Octet Rule: Atoms tend to gain, lose or share valence
Covalent Bonding Covalent Bonding is the result of sharing of electron pairs between 2 nonmetal atoms Caution: sharing can be complicated Recall the Octet Rule: Atoms tend to gain, lose or share valence
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts
 AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
AP Chemistry A. Allan Chapter 8 Notes - Bonding: General Concepts 8.1 Types of Chemical Bonds A. Ionic Bonding 1. Electrons are transferred 2. Metals react with nonmetals 3. Ions paired have lower energy
Chemistry: The Central Science
 Chemistry: The Central Science Fourteenth Edition Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds Three basic types of bonds Ionic Electrostatic attraction between ions Covalent Sharing of
Chemistry: The Central Science Fourteenth Edition Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds Three basic types of bonds Ionic Electrostatic attraction between ions Covalent Sharing of
Chemical Bonding. Section 1 Introduction to Chemical Bonding. Section 2 Covalent Bonding and Molecular Compounds
 Chemical Bonding Table of Contents Section 1 Introduction to Chemical Bonding Section 2 Covalent Bonding and Molecular Compounds Section 3 Ionic Bonding and Ionic Compounds Section 4 Metallic Bonding Section
Chemical Bonding Table of Contents Section 1 Introduction to Chemical Bonding Section 2 Covalent Bonding and Molecular Compounds Section 3 Ionic Bonding and Ionic Compounds Section 4 Metallic Bonding Section
Chemistry Chapter 6 Test Review
 Chemistry Chapter 6 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A mutual electrical attraction between the nuclei and valence electrons
Chemistry Chapter 6 Test Review Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A mutual electrical attraction between the nuclei and valence electrons
Chemistry Review Unit 4 Chemical Bonding
 Chemistry Review The Nature of Chemical Bonding, Directional Nature of Covalent Bonds, Intermolecular Forces Bonding 1. Chemical compounds are formed when atoms are bonded together. Breaking a chemical
Chemistry Review The Nature of Chemical Bonding, Directional Nature of Covalent Bonds, Intermolecular Forces Bonding 1. Chemical compounds are formed when atoms are bonded together. Breaking a chemical
Chapter 6. Preview. Objectives. Molecular Compounds
 Section 2 Covalent Bonding and Molecular Compounds Preview Objectives Molecular Compounds Formation of a Covalent Bond Characteristics of the Covalent Bond The Octet Rule Electron-Dot Notation Lewis Structures
Section 2 Covalent Bonding and Molecular Compounds Preview Objectives Molecular Compounds Formation of a Covalent Bond Characteristics of the Covalent Bond The Octet Rule Electron-Dot Notation Lewis Structures
Chapter 7 Chemical Bonding and Molecular Structure
 Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Chapter 7 Chemical Bonding and Molecular Structure Three Types of Chemical Bonding (1) Ionic: formed by electron transfer (2) Covalent: formed by electron sharing (3) Metallic: attraction between metal
Chapters 9&10 Structure and Bonding Theories
 Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
Chapters 9&10 Structure and Bonding Theories Ionic Radii Ions, just like atoms, follow a periodic trend in their radii. The metal ions in a given period are smaller than the non-metal ions in the same
Introduction to Chemical Bonding
 Chemical Bonding Introduction to Chemical Bonding Chemical bond! is a mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together Why are most
Chemical Bonding Introduction to Chemical Bonding Chemical bond! is a mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together Why are most
Wold of Chemistry Notes for Students [Chapter 12, page 1] Chapter 12 Chemical Bonding
![Wold of Chemistry Notes for Students [Chapter 12, page 1] Chapter 12 Chemical Bonding Wold of Chemistry Notes for Students [Chapter 12, page 1] Chapter 12 Chemical Bonding](/thumbs/77/75442347.jpg) Wold of Chemistry Notes for Students [Chapter 12, page 1] Chapter 12 Chemical Bonding 1) The History of the Development of the Period Table (Not in the book!) Similarities between the chemical and physical
Wold of Chemistry Notes for Students [Chapter 12, page 1] Chapter 12 Chemical Bonding 1) The History of the Development of the Period Table (Not in the book!) Similarities between the chemical and physical
Intramolecular Bonding. Chapters 4, 12 Chemistry Mr. McKenzie
 Intramolecular Bonding Chapters 4, 12 Chemistry Mr. McKenzie What determines the type of intramolecular bond? An intramolecular bond is any force that holds two atoms together to form a compound; 3 types
Intramolecular Bonding Chapters 4, 12 Chemistry Mr. McKenzie What determines the type of intramolecular bond? An intramolecular bond is any force that holds two atoms together to form a compound; 3 types
CHAPTER 12 CHEMICAL BONDING
 Chemistry Name Hour Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 12 CHEMICAL BONDING Day Plans for the day Assignment(s) for the day 1 Begin Chapter
Chemistry Name Hour Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 12 CHEMICAL BONDING Day Plans for the day Assignment(s) for the day 1 Begin Chapter
Notes: Covalent Bonding
 Name Chemistry Pre-AP Notes: Covalent Bonding Period The main focus of this unit is on the covalent bond; however, we will briefly treat the ionic and metallic bond as well. I. Chemical Bonding Overview
Name Chemistry Pre-AP Notes: Covalent Bonding Period The main focus of this unit is on the covalent bond; however, we will briefly treat the ionic and metallic bond as well. I. Chemical Bonding Overview
Lewis Dot Structures and Molecular Geometry
 Experiment 11 Lewis Dot Structures and Molecular Geometry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose
Experiment 11 Lewis Dot Structures and Molecular Geometry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose
Name: Practice Packet. Regents Chemistry: Dr. Shanzer. Chapter 9: Chemical Bonding.
 Name: Regents Chemistry: Dr. Shanzer Practice Packet Chapter 9: Chemical Bonding http://drshanzerchemistry.weebly.com 1 Chemical Bonding Objectives Describe the 2 major types of chemical bonds in terms
Name: Regents Chemistry: Dr. Shanzer Practice Packet Chapter 9: Chemical Bonding http://drshanzerchemistry.weebly.com 1 Chemical Bonding Objectives Describe the 2 major types of chemical bonds in terms
Section 8.1 The Covalent Bond
 Section 8.1 The Covalent Bond Apply the octet rule to atoms that form covalent bonds. Describe the formation of single, double, and triple covalent bonds. Contrast sigma and pi bonds. Relate the strength
Section 8.1 The Covalent Bond Apply the octet rule to atoms that form covalent bonds. Describe the formation of single, double, and triple covalent bonds. Contrast sigma and pi bonds. Relate the strength
Carbon and Its Compounds
 Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
Chapter 1 Carbon and Its Compounds Copyright 2018 by Nelson Education Limited 1 1.2 Organic Molecules from the Inside Out I: The Modelling of Atoms Copyright 2018 by Nelson Education Limited 2 s orbitals:
Test Review # 4. Chemistry: Form TR4.11A
 Chemistry: Form TR4.11 REVIEW Name Date Period Test Review # 4 Bonding. The electrons of one atom are attracted to the protons of another. When atoms combine, there is a tug of war over the valence electrons.
Chemistry: Form TR4.11 REVIEW Name Date Period Test Review # 4 Bonding. The electrons of one atom are attracted to the protons of another. When atoms combine, there is a tug of war over the valence electrons.
Chapter 13: Phenomena
 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Name AP CHEM / / Chapter 8 Outline Bonding: General Concepts
 Name AP CHEM / / Chapter 8 Outline Bonding: General Concepts Types of Chemical Bonds Information about the strength of a bonding interaction is obtained by measuring the bond energy, which is the energy
Name AP CHEM / / Chapter 8 Outline Bonding: General Concepts Types of Chemical Bonds Information about the strength of a bonding interaction is obtained by measuring the bond energy, which is the energy
bond energy- energy required to break a chemical bond -We can measure bond energy to determine strength of interaction
 bond energy- energy required to break a chemical bond -We can measure bond energy to determine strength of interaction ionic compound- a metal reacts with a nonmetal Ionic bonds form when an atom that
bond energy- energy required to break a chemical bond -We can measure bond energy to determine strength of interaction ionic compound- a metal reacts with a nonmetal Ionic bonds form when an atom that
Chapter 6. Chemical Bonding
 Chapter 6 Chemical Bonding Section 6.1 Intro to Chemical Bonding 6.1 Objectives Define chemical bond. Explain why most atoms form chemical bonds. Describe ionic and covalent bonding. Explain why most chemical
Chapter 6 Chemical Bonding Section 6.1 Intro to Chemical Bonding 6.1 Objectives Define chemical bond. Explain why most atoms form chemical bonds. Describe ionic and covalent bonding. Explain why most chemical
Unit 5: Bonding. Place a checkmark next to each item that you can do. If a sample problem is given, complete it as evidence.
 Unit 5: Bonding Place a checkmark next to each item that you can do. If a sample problem is given, complete it as evidence. Intramolecular Forces: 1. I can define intramolecular forces and intermolecular
Unit 5: Bonding Place a checkmark next to each item that you can do. If a sample problem is given, complete it as evidence. Intramolecular Forces: 1. I can define intramolecular forces and intermolecular
Unit Six --- Ionic and Covalent Bonds
 Unit Six --- Ionic and Covalent Bonds Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples
Unit Six --- Ionic and Covalent Bonds Electron Configuration in Ionic Bonding Ionic Bonds Bonding in Metals Valence Electrons Electrons in the highest occupied energy level of an element s atoms Examples
Chapter 6. Preview. Lesson Starter Objectives Chemical Bond
 Preview Lesson Starter Objectives Chemical Bond Section 1 Introduction to Chemical Bonding Lesson Starter Imagine getting onto a crowded elevator. As people squeeze into the confined space, they come in
Preview Lesson Starter Objectives Chemical Bond Section 1 Introduction to Chemical Bonding Lesson Starter Imagine getting onto a crowded elevator. As people squeeze into the confined space, they come in
Chapter 6. Preview. Lesson Starter Objectives Chemical Bond
 Preview Lesson Starter Objectives Chemical Bond Section 1 Introduction to Chemical Bonding Lesson Starter Imagine getting onto a crowded elevator. As people squeeze into the confined space, they come in
Preview Lesson Starter Objectives Chemical Bond Section 1 Introduction to Chemical Bonding Lesson Starter Imagine getting onto a crowded elevator. As people squeeze into the confined space, they come in
Chapter 8. Chemical Bonding: Basic Concepts
 Chapter 8. Chemical Bonding: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Chapter 8. Chemical Bonding: Basic Concepts Chemical bond: is an attractive force that holds 2 atoms together and forms as a result of interactions between electrons found in combining atoms We rarely
Chapter 8 : Covalent Bonding. Section 8.1: Molecular Compounds
 Chapter 8 : Covalent Bonding Section 8.1: Molecular Compounds What is a molecule? A molecular compound? A molecule is a neutral group of atoms joined together by covalent bonds A molecular compound is
Chapter 8 : Covalent Bonding Section 8.1: Molecular Compounds What is a molecule? A molecular compound? A molecule is a neutral group of atoms joined together by covalent bonds A molecular compound is
CP Covalent Bonds Ch. 8 &
 CP Covalent Bonds Ch. 8 & 9 2015-2016 Why do atoms bond? Atoms want stability- to achieve a noble gas configuration ( ) For bonds there is a transfer of electrons to get an octet of electrons For covalent
CP Covalent Bonds Ch. 8 & 9 2015-2016 Why do atoms bond? Atoms want stability- to achieve a noble gas configuration ( ) For bonds there is a transfer of electrons to get an octet of electrons For covalent
Chapter 13: Phenomena
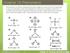 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 8. Basic Concepts of Chemical Bonding
 Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds An attractive force that holds two atoms together in a more complex unit Three basic types of bonds Ionic Electrons are transferred from one
Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds An attractive force that holds two atoms together in a more complex unit Three basic types of bonds Ionic Electrons are transferred from one
BONDING REVIEW. You need a Periodic Table, Electronegativity table & Polarity chart!
 BONDING REVIEW You need a Periodic Table, Electronegativity table & Polarity chart! What is the correct bond angle for Bent with 2 lone pairs on the central atom? 105 What is the predicted bond angle for
BONDING REVIEW You need a Periodic Table, Electronegativity table & Polarity chart! What is the correct bond angle for Bent with 2 lone pairs on the central atom? 105 What is the predicted bond angle for
