Synthesis of UV-Curable Hyperbranched Urethane Acrylates
|
|
|
- Charles Walton
- 5 years ago
- Views:
Transcription
1 Synthesis of UV-Curable yperbranched Urethane Acrylates Ahmet ebioglu, James. Aerykssen, R. David Zopf, Igor V. Khudyakov, Bomar Specialties, Torrington, USA 1. Introduction yperbranched, highly branched, dendritic, arborescent polymers are synonyms or almost synonyms. These polymers/oligomers attract much attention in the last decades due to the interesting properties resulting from their architecture and a high number of functional groups. They are usually prepared in one pot which leads to a one or another molecular weight distribution (MWD). 1 Monodisperse (MWD=1.0) hyperbranched polymers with a defined architecture are dendrimers. Usually dendrimers are prepared in a rather complex multistage synthesis and cost ~$100/g. UV-curable urethane acrylates (UA) are widely used in the coating industry. We expect dendritic UA and UA dendrimers of acceptable cost to be promising new oligomers for coatings formulations. The present work is devoted to synthesis of such UA oligomers and their properties. 2. Synthesis of urethane acrylates Common UA oligomers are prepared usually in two stages: the first is a reaction of polyol (P) with diisocyanate (D) taken in an excess. The residual isocyanate groups of a prepolymer are capped with hydroxyl substituted acrylate or methacrylate. The most common in the industry are the known EA and EMA. 2 This synthesis is called direct addition. 3 A synthesis which starts with a reaction between D with hydroxy acrylate followed by a reaction with P as a second stage is called reverse addition. 3 Perstorp manufactures multifunctional polyols Boltorn which find a wide application in the coatings industry. It seems obvious to run a direct synthesis with Boltorns, get a multifunctional prepolymer and cap it with acrylate. owever, in our experience a gelation occurs in the case of a reaction of D (functionality f C = 2) with hyperbranched polyol P of f 4 due to branching and chain extension. It is known that the probability of gelation p is proportional to product of functionalities of monomers (oligomers) and inversely proportional to a ratio r of a number of equivalents of a reagent in excess to a deficient reagent: 4 p ~ f *f C /r (1) ur goal was to find ways to get hyperbranched UA oligomers. 3. Extreme reverse addition It is apparent that using monofunctional isocyanates (f C = 1) avoids gelation via chain extension and branching. Taking the reverse addition scheme described above to its extreme, we prepared a monofunctional isocyanate (f C =1.0) by reacting EA with an unsymmetrical D having different reactivity of -C groups. 5 The reaction conditions were structured making all attempts to get predominately monofunctional isocyanate acrylate of a generic structure EA-D. As a byproduct we got the D-EA-D adduct which often has beneficial properties in formulations or at least does not have an evident negative impact.
2 We used the known in the industry D = IPDI. Surprisingly, a reduction of temperature from ambient to 0 o C did not lead to an increase in the relative concentration of IPDI-EA. owever, the addition of the known catalyst DBTDL led to a yield of IPDI-EA of ~75-80% with the remainder being the EA-IPDI-EA byproduct. In this way a formal C functionality in the system becomes f C = 1. We made all efforts to get the maximum possible yield of IPDI-EA in a reaction of EA with IPDI and leave negligible amount of non-reacted IPDI. Capping polyols of high functionality with such isocyanate/adduct mixture allowed us to avoid gelation and get UVcurable oligomers useful for industrial applications. This method we named the extreme reverse addition. The properties of an oligomer prepared by this method and named XDR-1406 are presented in the Table 1: Table 1 Properties of the oligomer XDR-1406 prepared by the extreme reverse addition* Acrylate Functionaliy strength, Elongation to break, % ardness MEK Double rubs η,** P D * Determination error of values presented in the Table is 10-15% ** η is the viscosity of the formulation with diluent at 25 o C 4. Reactions with monoisocyanate-acrylates 6. f course, using a pure monofunctional isocyanate-acrylate would be better suited for capping high functionality polyols than an EA-D/EA-D-EA mixture. Such monofunctional isocyanates-acrylates are produced by Showa Denko, and their names are abbreviated as AI and MI, cf. below: C 2 C C C 2 C 2 C AI C 3 C 2 C C C 2 C 2 MI C The one-stage capping of any hyperbranched P by AI/MI will lead to a hyperbranched acrylate of the same functionality as that of P. owever, notice that such UA oligomers will have twice less urethane (carbamate) links than UA capped by a standard way by D and by EA/EMA. It is well-known that hard segments provided by D in polyurethanes are important. In combination with soft segments of P, hard segments provide the remarkable properties of polyurethanes (resilience, toughness, high elongation, abrasion resistance, etc. 5 ) Before moving directly to high functionality polyols, we explored the effect of using AI/MI by reacting them with common difunctional polyols (f = 2) of varying molecular weights (M W ). We then compared these products to the corresponding UAs prepared in the standard way. As seen in the Table 2, regardless of the M W of P, AI/MI-capped oligomers have much lower viscosities than standard UAs of similar structure. This property is believed to be attributable,
3 first, to the absence of any chain extension. AI/MI-capped UAs have only one P molecule in their structure, and thus lower molecular weights than standard UAs. Second, as mentioned above, AI/MI-capped UAs have one-half the number of links that are present in standard UAs. The reduced number of these links leads to reduced hydrogen bonding between UA molecules and therefore reduced viscosity. Surprisingly, these same factors contribute to marked differences in final properties of the cured oligomers depending on the M W of P. As depicted in Figure 1, the tensile strength of the cured oligomer is drastically reduced when the M W of P is increased to above g/mol. The elongation to break of AI/MI-capped oligomers is also lower than the standard UAs, especially with the higher molecular weight polyols. Both differences are likely due to the altered combination of hard and soft segments within the oligomer chain. Properties of cured formulations of oligomers named 4-1 thru 4-5 * Strength, Elongation to break, % Table 2 M w of P D ** / Methacrylate ***, P ligomer 4-1A 250 MI ligomer 4-1B 250 DesW/EMA 1, ligomer 4-2A 650 MI ligomer 4-2B 650 DesW/EMA ligomer 4-3A 1000 MI ligomer 4-3B 1000 DesW/EMA ligomer 4-4A 2000 MI ligomer 4-4B 2000 DesW/EMA ligomer 4-5A 2900 MI ligomer 4-5B, 2900 DesW/EMA * Determination error of the presented values is 15% ** DesW stands for the known in the industry D Desmodur W, a.k.a. 12 MDI 5 *** is the viscosity of an uncured formulation with diluent Strength () AI capped UA Standard UA M w, g/mol Figure 1. Dependence of a tensile strength of cured oligomers on M w of diol
4 5. Products of reactions of high functionality polyols with MI 6 With some understanding of AI/MI based UAs, we moved on to the capping of hyperbranched polyols with f 4. Table 3 below presents properties of the multifunctional polyols used in the present examples: Table 3 Properties of high f polyols * Boltorn P1000 Boltorn P500 Boltorn 2004 CAPA 4101 f M W M n MWD * Determination error of M W, M n and of MWD is 15% From these starting polyols, the following oligomers were produced: Boltorn P1000 capped with MI, wherein 80% of the -groups, on an equivalent basis, were capped (designated ligomer 5-1); Boltorn P500 capped with MI, wherein 70% of the -groups, on an equivalent basis, were capped (designated ligomer 5-2); Boltorn 2004 capped with MI, wherein 95% of the -groups, on an equivalent basis, were capped (designated ligomer 5-3), and CAPA 4101 capped with MI, wherein 100% of the -groups, on an equivalent basis, were capped (designated ligomer 5-4). Properties of the liquid and cured oligomers are summarized in the Tables 4 and 5: Table 4 Properties of oligomers prepared with high functionality polyols and MI * ligomer 1-1 ligomer 1-2 ligomer 1-3 ligomer 1-4 M W M n MWD C, P** Color (APA) * Determination error of M W, M n, MWD and of is 15% ** η is the viscosity of undiluted, neat oligomer 10 0 Light yellow, slight haze 0 Table 5 Properties of cured formulations prepared with high functionality polyols and MI * strength, Elongation to break, % ardness MEK double 25 C, P ligomer 5-1, , D ligomer 5-2, , D ligomer 5-3, D 8 13 ligomer 5-4, D * Determination error of the values presented is 15% ** is the viscosity of a formulation with diluent In a sense, these UAs combine the best properties of the oligomers described in Sections 3, 4 above. They are not diluted by any unintended byproducts and there is little to no chain extension evidenced by the near identical MWDs of the starting P and the final UA. Like the oligomers prepared with diols, these oligomers are of quite low viscosity. Yet despite having a reduced number of urethane links, the higher functionality, and therefore expected high crosslink
5 density of the cured films, imparts exceptional tensile and hardness properties to these oligomers. These properties are quite similar to the oligomer XDR-1406 (Section 3) which contains the typical number of two urethane links per capped hydroxyl of the polyol. 6. Dendrimer [G1]-ene 6 6 ur work presentation thus far has focused on hyperbranched UAs with poorly defined structure and relatively high MWD. In this section we describe the preparation of a true urethane acrylate dendrimer almost monodispersed (MWD = 1.07) and with acrylate functionality f =6. We managed to get it in two simple stages. First we reacted 1,3,5-tris(2- hydroxyethyl)isocyanurate triacrylate (SR-368 of Sartomer) with 1-thioglycerol of Evans- Chemetics. We found that unsaturated compounds and especially acrylates participate in dark reactions of addition with different primary thiols. 7 MR of this Michael addition product confirmed that it occurs in a manner identical to a free-radical mechanism, that is in an anti- Markovnikov way. 7 In a second stage we reacted the obtained hexol with MI to produce UA of the following structure: S S S Figure 2. exafunctional urethane acrylate dendrimer [G1]-ene 6 The dendrimer was diluted with 50% TRPGDA and cured the usual way. The properties reproduced in the Table 6 below show the material to be hard, strong, and highly temperature resistant. A photochemistry approach to synthesis of a similar dendrimer was used in ref. 8.
6 Table 6* 2% wt. loss, C strength, Properties of the cured and liquid [G1]-ene 6 Elongation Durometer to break, % ardness MEK double rubs 25 C, P T g, C D and 159 * Determination error of the presented values is 15% ** is the viscosity of the liquid formulation with diluent 7. Conclusions The goal of this work was capping of high functionality (f 4) dendritic polyols P with AI (MI) and getting UA oligomers. Syntheses were done a way which avoided the almost inevitable gelation typical of reactions of dendritic polyols with common diisocyanates. This enabled valuable hyperbranched urethane acrylate oligomers to be produced in one stage. In addition to common applications of dendritic UA as UV-curable protective coatings, the unique dendritic structure opens up opportunities for nanotechnology applications based on specific confinement of functional units and the formation of pores and cavities. We expect that a new dendrimer of modest cost will be useful for such purpose. 8. References 1. Voit, B.I.; Lederer, A., Chem. Rev. 2009, 109, The examples given below are valid for both acrylates and methacrylates. We specify what is what when necessary. Also, we will use known in the industry abbreviations of other common P and D. 3. Swiderski, K.W.; Khudyakov, I.V. Ind. Eng. Chem. Res. 2004, 43, iemenz, P.C.; Lodge, T.P. Polymer Chemistry, CRC Press, Boca Raton, 2007, ch Szycher, M. Szycher s andbook of Polyurethanes; CRC Press: Boca Raton, 1999, ch. 4,6. 6. Leon, J. A., ebioglu, A., Aerykssen, J.., Khudyakov, I.V.; Zopf, R. D. Patent is pending. 7. Aerykssen, J..; Leon, J.A.; Zopf R.D.; Khudyakov, I.V. Proc. RadTech Europe Conference, ice, Killops, K.L.; Campos, L.M.; awker, C.J. J. Amer. Chem. Soc. 2008, 130, 5062.
Introduction. Techniques
 New Developments in UV-Curable Urethane Acrylate Coatings by Igor V. Khudyakov, Bomar Specialties; Todd W. Gantt, Harmony Labs; Michael B. Purvis, Reichhold; and Bob J. verton, Alcatel NA Cable Systems
New Developments in UV-Curable Urethane Acrylate Coatings by Igor V. Khudyakov, Bomar Specialties; Todd W. Gantt, Harmony Labs; Michael B. Purvis, Reichhold; and Bob J. verton, Alcatel NA Cable Systems
COMPARATIVE STUDY OF EB AND UV CURED POLYMER FILMS
 COMPARATIVE STUDY OF EB AND UV CURED POLYMER FILMS M. Azam Ali, F. Akhtar and K. M. Idriss Ali Bangladesh Atomic Energy Commission P.O. Box 3787, Dhaka-1000, Bangladesh Fax: 8-2-81; e-mail:inst@bangla.net
COMPARATIVE STUDY OF EB AND UV CURED POLYMER FILMS M. Azam Ali, F. Akhtar and K. M. Idriss Ali Bangladesh Atomic Energy Commission P.O. Box 3787, Dhaka-1000, Bangladesh Fax: 8-2-81; e-mail:inst@bangla.net
Hyperbranched urethane-acrylates
 Hyperbranched urethane-acrylates Hyperbranched urethane-acrylates based on alkoxylated hydroxy acrylates combine high molecular weight, excellent reactivity and good coating properties, such as hardness,
Hyperbranched urethane-acrylates Hyperbranched urethane-acrylates based on alkoxylated hydroxy acrylates combine high molecular weight, excellent reactivity and good coating properties, such as hardness,
Novel UV Curable Polyurethanes from Glycidyl Carbamate (GC) Resins
 ovel UV Curable Polyurethanes from Glycidyl Carbamate (GC) Resins Umesh D. arka 1, Dusty Pfundhelle 1, Andrew J. Muehlber 1 and Dean C. Webste 1* Department of Coatings and Polymeric Materials orth Dakota
ovel UV Curable Polyurethanes from Glycidyl Carbamate (GC) Resins Umesh D. arka 1, Dusty Pfundhelle 1, Andrew J. Muehlber 1 and Dean C. Webste 1* Department of Coatings and Polymeric Materials orth Dakota
A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector
 A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector Abrasion resistance The ability of the coating membrane to resist mechanical action such as foot traffic and particles, which
A Glossary of Terms Used in the Adhesives, Coatings and Elastomers (ACE) Sector Abrasion resistance The ability of the coating membrane to resist mechanical action such as foot traffic and particles, which
RadTech e Technical Proceedings
 igh Performance on-traditional Acrylated ligomers Joseph D. DeSousa, ichard W. Finch, Igor V. Khudyakov, Joseph A. Leon, Kenneth W. Swiderski,. David Zopf Bomar Specialties, Winsted, T 06098 Abstract.
igh Performance on-traditional Acrylated ligomers Joseph D. DeSousa, ichard W. Finch, Igor V. Khudyakov, Joseph A. Leon, Kenneth W. Swiderski,. David Zopf Bomar Specialties, Winsted, T 06098 Abstract.
Introduction to the Basics of UV/EB Chemistry and Formulations
 Introduction to the Basics of UV/EB Chemistry and Formulations SUNY ESF Radiation Curing Program RadTech NA Dr. Mike J. Idacavage Colorado Photopolymer Solutions September 9, 2015 Agenda Introduction to
Introduction to the Basics of UV/EB Chemistry and Formulations SUNY ESF Radiation Curing Program RadTech NA Dr. Mike J. Idacavage Colorado Photopolymer Solutions September 9, 2015 Agenda Introduction to
Scratch and Abrasion Resistant UV Coatings: Various Approaches
 Scratch and Abrasion Resistant UV Coatings: Various Approaches Y. Du, J. Aerykssen, J. Seger, A. Nebioglu Dymax Corporation 51 Greenwoods Rd., Torrington, CT Abstract UV-curable nanocomposite coatings
Scratch and Abrasion Resistant UV Coatings: Various Approaches Y. Du, J. Aerykssen, J. Seger, A. Nebioglu Dymax Corporation 51 Greenwoods Rd., Torrington, CT Abstract UV-curable nanocomposite coatings
Post-Polymerization. . macromolecule (1) While dying, macro-radicals react with the monomer: k p R n + M Rn+1 (2)
 Post-Polymerization Technical Paper By Igor V Khudyakov, PhD, DSc UV-curing technology is often selected because of the highspeed nature of the process Although curing is initiated by UV irradiation, polymerization
Post-Polymerization Technical Paper By Igor V Khudyakov, PhD, DSc UV-curing technology is often selected because of the highspeed nature of the process Although curing is initiated by UV irradiation, polymerization
The present chapter deals with synthesis of Polyurethane acrylate Oligomers.
 The present chapter deals with synthesis of Polyurethane acrylate Oligomers. 2.1 Principle reactions in preparation of UV-curable polyurethane acrylates oligomers If polymer chain carries -NCO group as
The present chapter deals with synthesis of Polyurethane acrylate Oligomers. 2.1 Principle reactions in preparation of UV-curable polyurethane acrylates oligomers If polymer chain carries -NCO group as
Cationic UV Curing Speeding up reactivity 15x with Curalite
 Cationic UV Curing Speeding up reactivity 15x with Curalite European Coatings Show, April 5 th 2017 Presented by David Engberg Introduction Background MSc. In Chemical Engineering at the Faculty of Engineering
Cationic UV Curing Speeding up reactivity 15x with Curalite European Coatings Show, April 5 th 2017 Presented by David Engberg Introduction Background MSc. In Chemical Engineering at the Faculty of Engineering
The main purpose of this work
 Structure-Property Investigation of Functional Resins for UV-Curable Gaskets By Joel D. Schall and Eric Edo-Hernandez This paper was selected as the Best Paper at the RadTech 2012 Technology Conference
Structure-Property Investigation of Functional Resins for UV-Curable Gaskets By Joel D. Schall and Eric Edo-Hernandez This paper was selected as the Best Paper at the RadTech 2012 Technology Conference
Metal Containing Acrylic Oligomers. Gary W. Ceska, Catherine Leroy, Bill Schaeffer, Sartomer, USA
 Metal Containing Acrylic Oligomers Gary W. Ceska, Catherine Leroy, Bill Schaeffer, Sartomer, USA Introduction Many papers have been written describing metal containing oligomers. (1, 2, 3) Little has been
Metal Containing Acrylic Oligomers Gary W. Ceska, Catherine Leroy, Bill Schaeffer, Sartomer, USA Introduction Many papers have been written describing metal containing oligomers. (1, 2, 3) Little has been
Formulating for Reactivity
 Formulating for Reactivity Kurt Willard Scientist III TS&D Industrial Coatings-Radcure Cytec Smyrna, Georgia USA Introduction UV curable coatings are typically formulated to meet a variety of performance
Formulating for Reactivity Kurt Willard Scientist III TS&D Industrial Coatings-Radcure Cytec Smyrna, Georgia USA Introduction UV curable coatings are typically formulated to meet a variety of performance
TEPZZ A T EP A2 (19) (11) EP A2. (12) EUROPEAN PATENT APPLICATION published in accordance with Art.
 (19) TEPZZ 644668A T (11) EP 2 644 668 A2 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 13(4) EPC (43) Date of publication: 02..13 Bulletin 13/ (21) Application number: 1274694.2 (22)
(19) TEPZZ 644668A T (11) EP 2 644 668 A2 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 13(4) EPC (43) Date of publication: 02..13 Bulletin 13/ (21) Application number: 1274694.2 (22)
Rheological Characterization of Medical Thermoplastic Polyurethanes
 Rheological Characterization of Medical Thermoplastic Polyurethanes Ian Pierson, Emily Chen, Ajay D Padsalgikar Abbott, Rogers, MN Abstract The use of thermoplastic polyurethanes (TPUs) in the medical
Rheological Characterization of Medical Thermoplastic Polyurethanes Ian Pierson, Emily Chen, Ajay D Padsalgikar Abbott, Rogers, MN Abstract The use of thermoplastic polyurethanes (TPUs) in the medical
DEVELOPMENT OF A NOVEL COATING SYSTEM USING PHOTO-LATENT BASE TECHNOLOGY
 DEVELOPMENT OF A NOVEL COATING SYSTEM USING PHOTO-LATENT BASE TECHNOLOGY Himanshu Manchanda and Vijay Mannari, PhD Coatings Research Institute, Eastern Michigan University, Ypsilanti, MI GOALS AND OBJECTIVES
DEVELOPMENT OF A NOVEL COATING SYSTEM USING PHOTO-LATENT BASE TECHNOLOGY Himanshu Manchanda and Vijay Mannari, PhD Coatings Research Institute, Eastern Michigan University, Ypsilanti, MI GOALS AND OBJECTIVES
Tolonate X FLO 100. Bio-based & low viscosity aliphatic isocyanate polymer
 Tolonate X FLO 100 Bio-based & low viscosity aliphatic isocyanate polymer Discover the of Tolonate TM X FLO 100 Tolonate X FLO 100 is a new, partially bio-based, solvent-free and low viscosity aliphatic
Tolonate X FLO 100 Bio-based & low viscosity aliphatic isocyanate polymer Discover the of Tolonate TM X FLO 100 Tolonate X FLO 100 is a new, partially bio-based, solvent-free and low viscosity aliphatic
Structure-Property Investigation of Functional Resins for UV-Curable Gaskets
 Structure-Property Investigation of Functional Resins for UV-Curable Gaskets Joel D. Schall and Eric Edo-Hernandez Henkel Corporation Rocky Hill, CT USA Introduction The main purpose of this work was to
Structure-Property Investigation of Functional Resins for UV-Curable Gaskets Joel D. Schall and Eric Edo-Hernandez Henkel Corporation Rocky Hill, CT USA Introduction The main purpose of this work was to
Improving the Performance of UV Curable Coatings with Carbon Nanomaterials
 Improving the Performance of UV Curable Coatings with Carbon Nanomaterials Yun JIANG 1, Andy ZHANG 2, 3, Steven YU 3, Nick YIN 3, Willy DU 3, Tao ZHANG 1,* 1. College of Engineering and Applied Sciences,
Improving the Performance of UV Curable Coatings with Carbon Nanomaterials Yun JIANG 1, Andy ZHANG 2, 3, Steven YU 3, Nick YIN 3, Willy DU 3, Tao ZHANG 1,* 1. College of Engineering and Applied Sciences,
Flexible Packaging Adhesives The Basics. Larry Jopko Rohm and Haas Company. Abstract
 Flexible Packaging Adhesives The Basics Larry Jopko Rohm and Haas Company Abstract Flexible packaging adhesives are predominately based on urethane and acrylic chemistry. Backbone options create unique
Flexible Packaging Adhesives The Basics Larry Jopko Rohm and Haas Company Abstract Flexible packaging adhesives are predominately based on urethane and acrylic chemistry. Backbone options create unique
Safety of the UV/EB Curing Process
 Safety of the UV/EB Curing Process 2 Abstract The coatings and paint industries strive to provide high technology coatings while reducing volatile organic compounds (V.O.C.s) and energy consumption to
Safety of the UV/EB Curing Process 2 Abstract The coatings and paint industries strive to provide high technology coatings while reducing volatile organic compounds (V.O.C.s) and energy consumption to
Curing behavior of a UV-curable coating based on urethane acrylate oligomer: the influence of reactive monomers
 Songklanakarin J. Sci. Technol. 33 (2), 201-207, Mar. - Apr. 2011 http://www.sjst.psu.ac.th Original Article Curing behavior of a UV-curable coating based on urethane acrylate oligomer: the influence of
Songklanakarin J. Sci. Technol. 33 (2), 201-207, Mar. - Apr. 2011 http://www.sjst.psu.ac.th Original Article Curing behavior of a UV-curable coating based on urethane acrylate oligomer: the influence of
Innovative. Technologies. Chemie des Klebens Chemistry of Adhesives. Dr. Jochen Stock, Laboratory Manager CRL Germany: Neuss, November 27 th, 2013
 Chemie des Klebens Chemistry of Adhesives Dr. Jochen Stock, Laboratory Manager CRL Germany: Neuss, November 27 th, 2013 Innovative Technologies 1 Overview Chemie des Klebens Chemistry of Adhesives Introduction
Chemie des Klebens Chemistry of Adhesives Dr. Jochen Stock, Laboratory Manager CRL Germany: Neuss, November 27 th, 2013 Innovative Technologies 1 Overview Chemie des Klebens Chemistry of Adhesives Introduction
SUPPORTING INFORMATION. Self-healable and Ultra-hydrophobic Polyurethane-POSS Hybrids by Diels-Alder. Click Reaction; A New Class of Coating Material
 SUPPORTING INFORMATION Self-healable and Ultra-hydrophobic Polyurethane-POSS Hybrids by Diels-Alder Click Reaction; A New Class of Coating Material Prasanta Kumar Behera, Prantik Mondal, Nikhil K. Singha*
SUPPORTING INFORMATION Self-healable and Ultra-hydrophobic Polyurethane-POSS Hybrids by Diels-Alder Click Reaction; A New Class of Coating Material Prasanta Kumar Behera, Prantik Mondal, Nikhil K. Singha*
Photopolymerization of Thermoplastic Polyurethane/Acrylate Blends
 Korean J. Chem. Eng., (5), 750-754 (005) Photopolymerization of Thermoplastic Polyurethane/Acrylate Blends Youngson Choe, Siyoung Park, Wonho Kim and Daewon Park Department of Chemical Engineering, Pusan
Korean J. Chem. Eng., (5), 750-754 (005) Photopolymerization of Thermoplastic Polyurethane/Acrylate Blends Youngson Choe, Siyoung Park, Wonho Kim and Daewon Park Department of Chemical Engineering, Pusan
Alkoxylates Promoting superior performance
 Our range of versatile liquid polyols Promote superior end-product performance in radiation curing Enhance properties of polyurethane elastomers and foams Tailor ideal additives for a wide variety of applications
Our range of versatile liquid polyols Promote superior end-product performance in radiation curing Enhance properties of polyurethane elastomers and foams Tailor ideal additives for a wide variety of applications
Segmented Copolymers. Segmented Polyurethanes (Prof. Hammond s thesis)
 0.569 Synthesis of Polymers Prof. Paula ammond Lecture 0: Introduction to Radical Polymerization Segmented opolymers Segmented Polyurethanes (Prof. ammond s thesis) soft segment ends in groups - oligomer
0.569 Synthesis of Polymers Prof. Paula ammond Lecture 0: Introduction to Radical Polymerization Segmented opolymers Segmented Polyurethanes (Prof. ammond s thesis) soft segment ends in groups - oligomer
Physical and Mechanical Properties of Polymers
 MATE 453/MSE 553 Physical and Mechanical Properties of Polymers Guided Lecture Notes for Fall 2012 Prof. Michael Kessler Department of Materials Science and Engineering Iowa State University PHYSICAL AND
MATE 453/MSE 553 Physical and Mechanical Properties of Polymers Guided Lecture Notes for Fall 2012 Prof. Michael Kessler Department of Materials Science and Engineering Iowa State University PHYSICAL AND
Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION Historical Perspective Some of the earliest useful polymeric
 Synthetics Polymers Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION 1.1.1 Historical Perspective Some of the earliest useful polymeric materials, the Bakelite resins formed from
Synthetics Polymers Introduction to Synthetic Methods in Step-Growth Polymers 1.1 INTRODUCTION 1.1.1 Historical Perspective Some of the earliest useful polymeric materials, the Bakelite resins formed from
Keeping the weight up
 Keeping the weight up Acrylic polymer dispersions give novel approach to waterborne coatings. Dirk Mestach, Derrick R. Twene. Waterborne UV-curing binders based on acrylic polymer dispersions have been
Keeping the weight up Acrylic polymer dispersions give novel approach to waterborne coatings. Dirk Mestach, Derrick R. Twene. Waterborne UV-curing binders based on acrylic polymer dispersions have been
race to the Second Edition Preface to the First Edition 'kno\t'ledgements Contents
 Contents race to the Second Edition Preface to the First Edition 'kno\t'ledgements Chemistry and Basic Intermediates. Introduction. Basic Chemistry Basic Structure of a Polyurethane Elastomer. Synthesis
Contents race to the Second Edition Preface to the First Edition 'kno\t'ledgements Chemistry and Basic Intermediates. Introduction. Basic Chemistry Basic Structure of a Polyurethane Elastomer. Synthesis
The effects of polymerizable
 Photopolymer-clay Nanocomposite Performance Technical Paper Utilizing Different Polymerizable Organoclays By Soon Ki Kim and C. Allan Guymon The effects of polymerizable organoclays on thermomechanical
Photopolymer-clay Nanocomposite Performance Technical Paper Utilizing Different Polymerizable Organoclays By Soon Ki Kim and C. Allan Guymon The effects of polymerizable organoclays on thermomechanical
Influences on Barrier Performance of UV/EB Cured Polymers
 Influences on Barrier Performance of UV/EB Cured Polymers Abstract: Joshua M. Oliver / Sartomer USA, LLC / Exton, PA, USA The use of UV/EB cured materials as barriers against moisture vapor and/or oxygen
Influences on Barrier Performance of UV/EB Cured Polymers Abstract: Joshua M. Oliver / Sartomer USA, LLC / Exton, PA, USA The use of UV/EB cured materials as barriers against moisture vapor and/or oxygen
UV curing of hyperbranched polyurethane acrylate polyurethane diacrylate/sio 2 dispersion and TGA/FTIR study of cured films
 J. Cent. South Univ. (2012) 19: 63 70 DOI: 10.1007/s11771 012 0973 x UV curing of hyperbranched polyurethane acrylate polyurethane diacrylate/sio 2 dispersion and TGA/FTIR study of cured films GAO Qiong
J. Cent. South Univ. (2012) 19: 63 70 DOI: 10.1007/s11771 012 0973 x UV curing of hyperbranched polyurethane acrylate polyurethane diacrylate/sio 2 dispersion and TGA/FTIR study of cured films GAO Qiong
Precursor provides selectivity
 Precursor provides selectivity Novel route to isocyanate-free blocked isocyanates with functional groups. Ton Loontjens, Bart Plum, Tim Kidd, Boudewijn Scholtens, Polyurethane coatings, prepared from polyols
Precursor provides selectivity Novel route to isocyanate-free blocked isocyanates with functional groups. Ton Loontjens, Bart Plum, Tim Kidd, Boudewijn Scholtens, Polyurethane coatings, prepared from polyols
Thiol-Enes: Fast Curing Systems with Exceptional Properties. Performance Materials, USA. Chemistry and Biochemistry, USA
 Thiol-Enes: Fast Curing Systems with Exceptional Properties Charles Hoyle, 1,2 Tolecial Clark, 2 Tai Yeon Lee, 1 Todd Roper, 1 Bo Pan, 1 Huanyu Wei, 1 Hui Zhou, 1 and Joe Lichtenhan 3 1 The University
Thiol-Enes: Fast Curing Systems with Exceptional Properties Charles Hoyle, 1,2 Tolecial Clark, 2 Tai Yeon Lee, 1 Todd Roper, 1 Bo Pan, 1 Huanyu Wei, 1 Hui Zhou, 1 and Joe Lichtenhan 3 1 The University
Supporting Information
 Supporting Information Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation Ning Zheng, Zizheng Fang, Weike Zou, Qian Zhao,* and Tao Xie* anie_201602847_sm_miscellaneous_information.pdf
Supporting Information Thermoset Shape-Memory Polyurethane with Intrinsic Plasticity Enabled by Transcarbamoylation Ning Zheng, Zizheng Fang, Weike Zou, Qian Zhao,* and Tao Xie* anie_201602847_sm_miscellaneous_information.pdf
Effects of Multifunctional Monomers on the Properties of UV Cured Films
 Effects of Multifunctional Monomers on the Properties of UV Cured Films Bentian Zhang, Lili Zhang, and Jianhua Liu/Nantong Fengtian Agent Factory Co., Ltd., 10, Xinjian West Road, Fengli Town, Rudong County,
Effects of Multifunctional Monomers on the Properties of UV Cured Films Bentian Zhang, Lili Zhang, and Jianhua Liu/Nantong Fengtian Agent Factory Co., Ltd., 10, Xinjian West Road, Fengli Town, Rudong County,
Chapter 5. Ionic Polymerization. Anionic.
 Chapter 5. Ionic Polymerization. Anionic. Anionic Polymerization Dr. Houston S. Brown Lecturer of Chemistry UH-Downtown brownhs@uhd.edu What you should know: What is anionic polymerization? What is MWD,
Chapter 5. Ionic Polymerization. Anionic. Anionic Polymerization Dr. Houston S. Brown Lecturer of Chemistry UH-Downtown brownhs@uhd.edu What you should know: What is anionic polymerization? What is MWD,
This approach has been utilized in the preparation of many derivatives of dimethicone copolyol compounds 3.
 Reactive Silicones Editors Note: This edition of The Silicone Spectator will address silicone materials that can be used as reactants in the preparation of polymers in which silicone is merely one component.
Reactive Silicones Editors Note: This edition of The Silicone Spectator will address silicone materials that can be used as reactants in the preparation of polymers in which silicone is merely one component.
Chemical Engineering Seminar Series
 Effect of Reaction Conditions on Copolymer Properties Loretta Idowu Keywords: copolymer composition distribution; radical polymerization kinetics; semi-batch starved feed; hydroxyl-functionality Non-functional
Effect of Reaction Conditions on Copolymer Properties Loretta Idowu Keywords: copolymer composition distribution; radical polymerization kinetics; semi-batch starved feed; hydroxyl-functionality Non-functional
Photoinitiation, Photopolymerization, and Photocuring
 Jean-Pierre Fouassier Photoinitiation, Photopolymerization, and Photocuring Fundamentals and Applications Hanser Publishers, Munich Vienna New York Hanser/Gardner Publications, Inc., Cincinnati Contents
Jean-Pierre Fouassier Photoinitiation, Photopolymerization, and Photocuring Fundamentals and Applications Hanser Publishers, Munich Vienna New York Hanser/Gardner Publications, Inc., Cincinnati Contents
HELOXY Epoxy Functional Modifiers
 Product Selector HELXY Epoxy Functional Modifiers Momentive's epoxy functional product line of HELXY Modifiers offers formulators the ability to choose between monofunctional and polyfunctional glycidyl
Product Selector HELXY Epoxy Functional Modifiers Momentive's epoxy functional product line of HELXY Modifiers offers formulators the ability to choose between monofunctional and polyfunctional glycidyl
Compliant yet excellent
 Compliant yet excellent NMP- and critical-additive-free polyurethane dispersions for high performance parquet flooring applications. Waterborne polyurethane dispersions (PUDs) can produce coatings with
Compliant yet excellent NMP- and critical-additive-free polyurethane dispersions for high performance parquet flooring applications. Waterborne polyurethane dispersions (PUDs) can produce coatings with
Macromers and Monofunctional Silicones
 Macromers and Monofunctional Silicones Macromers are relatively high molecular weight species with a single functional polymerizeable group which, although used as monomers, have high enough molecular
Macromers and Monofunctional Silicones Macromers are relatively high molecular weight species with a single functional polymerizeable group which, although used as monomers, have high enough molecular
Polymer Systems and Film Formation Mechanisms in High Solids, Powder, and UV Cure Systems
 Polymer Systems and Film Formation Mechanisms in High Solids, Powder, and UV Cure Systems J. Baghdachi, Ph.D. Coatings Research Institute Eastern Michigan University (734) 487-3192 Freshpaint@aol.com jamil.baghdachi@emich.edu
Polymer Systems and Film Formation Mechanisms in High Solids, Powder, and UV Cure Systems J. Baghdachi, Ph.D. Coatings Research Institute Eastern Michigan University (734) 487-3192 Freshpaint@aol.com jamil.baghdachi@emich.edu
STUDIES ON ISOTHERMAL URETHANE POLY MERlZATlON
 POLY M.-PLAST. TECHNOL. ENG., 30(2&3), 227-238 (1 991) STUDIES ON ISOTHERMAL URETHANE POLY MERlZATlON REJI JOHN, E. T. THACHIL, P. MVINDRAN, N. R. NEELAKANTAN, AND N. SUBRAMAMAN Department of Chemical
POLY M.-PLAST. TECHNOL. ENG., 30(2&3), 227-238 (1 991) STUDIES ON ISOTHERMAL URETHANE POLY MERlZATlON REJI JOHN, E. T. THACHIL, P. MVINDRAN, N. R. NEELAKANTAN, AND N. SUBRAMAMAN Department of Chemical
Thermoset Resins and Their Composites
 ACCE 22, September 13-14, 14, 22 Bio-based Thermoset Resins and Their Composites M. Misra,, L. T. Drzal, A. K. Mohanty,, L. Belchler, G. Mehta,, J-P. J Latere Dwan sisa Michigan State University 21 Engineering
ACCE 22, September 13-14, 14, 22 Bio-based Thermoset Resins and Their Composites M. Misra,, L. T. Drzal, A. K. Mohanty,, L. Belchler, G. Mehta,, J-P. J Latere Dwan sisa Michigan State University 21 Engineering
POSS for Surface Modification and and Corrosion Prevention
 PSS for Surface Modification and and Corrosion Prevention Bill einerth Presented at the Nanostructured Chemicals Workshop September 7 th - 8 th, 2000 18237 Mount Baldy Circle Fountain Valley, CA 92708
PSS for Surface Modification and and Corrosion Prevention Bill einerth Presented at the Nanostructured Chemicals Workshop September 7 th - 8 th, 2000 18237 Mount Baldy Circle Fountain Valley, CA 92708
Urea urethane nanocomposites obtained from modified methylalumoxane oligomers
 Materials Science-Poland, Vol. 26, No. 2, 2008 Urea urethane nanocomposites obtained from modified methylalumoxane oligomers A. BOCZKOWSKA 1*, M. MARCZEWSKI 2, E. CIECIERSKA 1, B. SIENKIEWICZ 2, A. PIETRZYKOWSKI
Materials Science-Poland, Vol. 26, No. 2, 2008 Urea urethane nanocomposites obtained from modified methylalumoxane oligomers A. BOCZKOWSKA 1*, M. MARCZEWSKI 2, E. CIECIERSKA 1, B. SIENKIEWICZ 2, A. PIETRZYKOWSKI
Analysing monomer performance
 Analysing monomer performance Three types of reactive diluents were studied in detail regarding their performance in polyurethane acrylate based UV curing coating formulations. The results indicate that
Analysing monomer performance Three types of reactive diluents were studied in detail regarding their performance in polyurethane acrylate based UV curing coating formulations. The results indicate that
Polymerization shrinkage by investigation of uv curable dental restorative composites containing multifunctional methacrylates
 Polish Journal of Chemical Technology, 15, 2, 81 Pol. 85, J. 10.2478/pjct-2013-0027 Chem. Tech., Vol. 15, No. 2, 2013 81 Polymerization shrinkage by investigation of uv curable dental restorative composites
Polish Journal of Chemical Technology, 15, 2, 81 Pol. 85, J. 10.2478/pjct-2013-0027 Chem. Tech., Vol. 15, No. 2, 2013 81 Polymerization shrinkage by investigation of uv curable dental restorative composites
Cage Effect Dynamics under Photolysis of Photoinitiators
 Designed Monomers and Polymers 13 (2010) 487 496 brill.nl/dmp Cage Effect Dynamics under Photolysis of Photoinitiators Igor V. Khudyakov a and Nicholas J. Turro b, a Bomar Specialties, Torrington, CT 06790,
Designed Monomers and Polymers 13 (2010) 487 496 brill.nl/dmp Cage Effect Dynamics under Photolysis of Photoinitiators Igor V. Khudyakov a and Nicholas J. Turro b, a Bomar Specialties, Torrington, CT 06790,
Liquid Crystal. Liquid Crystal. Liquid Crystal Polymers. Liquid Crystal. Orientation of molecules in the mesophase
 Liquid Crystal - Liquid crystals (LCs) are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. (Fourth state of matter) Liquid Crystal Orientation
Liquid Crystal - Liquid crystals (LCs) are a state of matter that have properties between those of a conventional liquid and those of a solid crystal. (Fourth state of matter) Liquid Crystal Orientation
Chapter 4 Acrylic Polyurethane Emulsion Polymers
 Chapter 4 Acrylic Polyurethane Emulsion Polymers In this chapter, isophorone diisocyanate (IPDI), a cycloaliphatic diisocyanate, has been selected to react with the different types of hydroxyl acrylic
Chapter 4 Acrylic Polyurethane Emulsion Polymers In this chapter, isophorone diisocyanate (IPDI), a cycloaliphatic diisocyanate, has been selected to react with the different types of hydroxyl acrylic
Curing Properties of Cycloaliphatic Epoxy Derivatives
 Curing Properties of Cycloaliphatic Epoxy Derivatives Hiroshi Sasaki Toagosei Co. Ltd. Nagoya, Japan Introduction UV-cationic-curing, based on the photo-generation of acid and consecutive cationic polymerization,
Curing Properties of Cycloaliphatic Epoxy Derivatives Hiroshi Sasaki Toagosei Co. Ltd. Nagoya, Japan Introduction UV-cationic-curing, based on the photo-generation of acid and consecutive cationic polymerization,
A Humidity Blocker Approach to Overcoming the Humidity Interference with Cationic Photopolymerization
 A Humidity Blocker Approach to vercoming the Humidity Interference with Cationic Photopolymerization Zhigang Chen, Ying Zhang, Bret. J. Chisholm, Dean C. Webster Department of Coatings and Polymeric Materials
A Humidity Blocker Approach to vercoming the Humidity Interference with Cationic Photopolymerization Zhigang Chen, Ying Zhang, Bret. J. Chisholm, Dean C. Webster Department of Coatings and Polymeric Materials
The Effects of Polymerizable Organoclays on Photopolymerization Behavior and Ultimate Properties of Thiol-ene Nanocomposite Systems.
 The Effects of Polymerizable Organoclays on Photopolymerization Behavior and Ultimate Properties of Thiol-ene Nanocomposite Systems. Soon Ki Kim and C. Allan Guymon, Department of Chemical & Biochemical
The Effects of Polymerizable Organoclays on Photopolymerization Behavior and Ultimate Properties of Thiol-ene Nanocomposite Systems. Soon Ki Kim and C. Allan Guymon, Department of Chemical & Biochemical
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
CARBON NANOTUBE-POLYMER COMPOSITES: AN OVERVIEW Brian Grady University of Oklahoma
 CARBON NANOTUBE-POLYMER COMPOSITES: AN OVERVIEW Brian Grady University of Oklahoma Abstract Carbon nanotubes are in many ways similar to polymers. Both molecules have contour lengths typically on the order
CARBON NANOTUBE-POLYMER COMPOSITES: AN OVERVIEW Brian Grady University of Oklahoma Abstract Carbon nanotubes are in many ways similar to polymers. Both molecules have contour lengths typically on the order
Effect of crystallinity on properties. Melting temperature. Melting temperature. Melting temperature. Why?
 Effect of crystallinity on properties The morphology of most polymers is semi-crystalline. That is, they form mixtures of small crystals and amorphous material and melt over a range of temperature instead
Effect of crystallinity on properties The morphology of most polymers is semi-crystalline. That is, they form mixtures of small crystals and amorphous material and melt over a range of temperature instead
Thermoplastic Polyurethane Elastomers: Synthesis, and Study of Effective Structural Parameters
 Iranian Polymer Journal ' Volume 5 Number 4 (29961 IO26-I265196 Thermoplastic Polyurethane Elastomers: Synthesis, and Study of Effective Structural Parameters Mehdi Barikani and Mohammad Barmar Polymer
Iranian Polymer Journal ' Volume 5 Number 4 (29961 IO26-I265196 Thermoplastic Polyurethane Elastomers: Synthesis, and Study of Effective Structural Parameters Mehdi Barikani and Mohammad Barmar Polymer
Polymers and Composite Materials
 Polymers and omposite Materials Shibu G. Pillai hemical Engineering Department shibu.pillai@nirmauni.ac.in ontents lassification of Polymers Types of polymerization Elastomers/ Rubber Advanced Polymeric
Polymers and omposite Materials Shibu G. Pillai hemical Engineering Department shibu.pillai@nirmauni.ac.in ontents lassification of Polymers Types of polymerization Elastomers/ Rubber Advanced Polymeric
New Classes of Photopolymerizable Monomers and Oligomers: Acrylamides, Urea Acrylamides and Carbonate Acrylates
 New Classes of Photopolymerizable Monomers and ligomers: Acrylamides, Urea Acrylamides and Carbonate Acrylates Adetoun Adebule, Idongesit Attang, Chace Craig, Garima Ghandir, Jason Henderson, Rebecca Herrington,
New Classes of Photopolymerizable Monomers and ligomers: Acrylamides, Urea Acrylamides and Carbonate Acrylates Adetoun Adebule, Idongesit Attang, Chace Craig, Garima Ghandir, Jason Henderson, Rebecca Herrington,
A Technical Whitepaper Polymer Technology in the Coating Industry. By Donald J. Keehan Advanced Polymer Coatings Avon, Ohio, USA
 A Technical Whitepaper Polymer Technology in the Coating Industry By Donald J. Keehan Advanced Polymer Coatings Avon, Ohio, USA INTRODUCTION Polymer Technology in the Coating Industry To properly understand
A Technical Whitepaper Polymer Technology in the Coating Industry By Donald J. Keehan Advanced Polymer Coatings Avon, Ohio, USA INTRODUCTION Polymer Technology in the Coating Industry To properly understand
Lecture No. (1) Introduction of Polymers
 Lecture No. (1) Introduction of Polymers Polymer Structure Polymers are found in nature as proteins, cellulose, silk or synthesized like polyethylene, polystyrene and nylon. Some natural polymers can also
Lecture No. (1) Introduction of Polymers Polymer Structure Polymers are found in nature as proteins, cellulose, silk or synthesized like polyethylene, polystyrene and nylon. Some natural polymers can also
Cationic UV Cure on Polyolefins. David James, Pia Appelkvist, Eva Gustavsson, Perstorp Specialty Chemicals AB, Sweden
 Cationic UV Cure on Polyolefins David James, Pia Appelkvist, Eva Gustavsson, Perstorp Specialty Chemicals AB, Sweden UV/EB cationic curing is an alternative radiation curing technology circumventing some
Cationic UV Cure on Polyolefins David James, Pia Appelkvist, Eva Gustavsson, Perstorp Specialty Chemicals AB, Sweden UV/EB cationic curing is an alternative radiation curing technology circumventing some
Novel Polymeric Photoinitiator and Sensitizer
 ovel Polymeric Photoinitiator and Sensitizer Dr. Qingjin YA a, Wenchao ZHA a, Shidian HA a, John HUIBERTS b (a: Insight High Technology Co., Ltd. Beijing P.R.China; b: IGM Resins B.V., the etherlands)
ovel Polymeric Photoinitiator and Sensitizer Dr. Qingjin YA a, Wenchao ZHA a, Shidian HA a, John HUIBERTS b (a: Insight High Technology Co., Ltd. Beijing P.R.China; b: IGM Resins B.V., the etherlands)
Synthesis, Characterization and Properties of Polyisobutylene-Based Polyurethanes
 The University of Akron From the SelectedWorks of Joseph P. Kennedy June 15, 1985 Synthesis, Characterization and Properties of Polyisobutylene-Based Polyurethanes Joseph P. Kennedy, University of Akron
The University of Akron From the SelectedWorks of Joseph P. Kennedy June 15, 1985 Synthesis, Characterization and Properties of Polyisobutylene-Based Polyurethanes Joseph P. Kennedy, University of Akron
Molecular Weight Averages and Degree of Branching in Self-Condensing Vinyl Copolymerization in the Presence of Multifunctional Initiators
 Molecular Weight Averages and Degree of Branching in Self-Condensing Vinyl Copolymerization in the Presence of Multifunctional Initiators Galina I. Litvineno, and Axel H. E. Mu1 ller*, Karpov Institute
Molecular Weight Averages and Degree of Branching in Self-Condensing Vinyl Copolymerization in the Presence of Multifunctional Initiators Galina I. Litvineno, and Axel H. E. Mu1 ller*, Karpov Institute
2K water-borne PU for furniture coatings
 2K water-borne PU for furniture coatings Water-borne 2K polyurethane systems are a new technology which is already successfully used in many applications also for wood coatings and more specifically furniture
2K water-borne PU for furniture coatings Water-borne 2K polyurethane systems are a new technology which is already successfully used in many applications also for wood coatings and more specifically furniture
EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (43) Date of publication: Bulletin 2012/28
 (19) (12) EUROPEAN PATENT APPLICATION (11) EP 2 474 63 A1 (43) Date of publication: 11.07.12 Bulletin 12/28 (21) Application number: 12162484. (1) Int Cl.: C08F /00 (06.01) C08F 1/00 (06.01) C08G 18/62
(19) (12) EUROPEAN PATENT APPLICATION (11) EP 2 474 63 A1 (43) Date of publication: 11.07.12 Bulletin 12/28 (21) Application number: 12162484. (1) Int Cl.: C08F /00 (06.01) C08F 1/00 (06.01) C08G 18/62
Preparation of Water-borne Polyurethane-acrylate (PUA) and Application to UV-curing Coatings on the Package of Paper
 Preparation of Water-borne Polyurethane-acrylate (PUA) and Application to UV-curing Coatings on the Package of Paper Liu Xiaoxuan *, Hong Peng, Duan Lunyong and Li Yuanpei Department of Polymeric Materials
Preparation of Water-borne Polyurethane-acrylate (PUA) and Application to UV-curing Coatings on the Package of Paper Liu Xiaoxuan *, Hong Peng, Duan Lunyong and Li Yuanpei Department of Polymeric Materials
Operating Characteristics of Diene-Urethane Elastomers on the base of NISSOG 3000 Oligodienediol
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (5): Pg. 2363-2370 Operating Characteristics
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (5): Pg. 2363-2370 Operating Characteristics
University of Groningen
 University of Groningen Synthesis of α-hydroxy-ω-amino poly(ethylene oxide) and its use in reaction injection moulding (RIM) Loontjens, Ton J.A.; Scholtens, Boudewijn J.R.; Belt, Wil J.W.; Frisch, Kurt
University of Groningen Synthesis of α-hydroxy-ω-amino poly(ethylene oxide) and its use in reaction injection moulding (RIM) Loontjens, Ton J.A.; Scholtens, Boudewijn J.R.; Belt, Wil J.W.; Frisch, Kurt
Packaging design and cationic curing materials
 Packaging design and cationic curing materials 22 nd March 2016 Presented by Paul Kelly Introduction Background 35 years in Radiation Cured coatings and Inks since 1981 Speaker, Paul Kelly Technical Development
Packaging design and cationic curing materials 22 nd March 2016 Presented by Paul Kelly Introduction Background 35 years in Radiation Cured coatings and Inks since 1981 Speaker, Paul Kelly Technical Development
Organic Chemistry. REACTIONS Grade 12 Physical Science Mrs KL Faling
 Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Interaction of Photoexcited Photoinitiators with Nitroxyl Radicals. Igor V. Khudyakov. Department of Chemistry, Columbia University, New York, NY
 Interaction of Photoexcited Photoinitiators with Nitroxyl Radicals Igor V. Khudyakov Department of Chemistry, Columbia University, New York, NY Introduction. Formulations which undergo photopolymerization
Interaction of Photoexcited Photoinitiators with Nitroxyl Radicals Igor V. Khudyakov Department of Chemistry, Columbia University, New York, NY Introduction. Formulations which undergo photopolymerization
T. Buruiana a*, V. Melinte a, F. Jitaru a, E. C. Buruiana a, H. Aldea b. Keywords: carboxylic/phosphate monomer, photopolymerization, dental material.
 PRPERTIES F SME YBRID PLYMER CMPSITES CTAIIG CARBXYLIC/PSPATE DIMETACRYLATES AD YDRXYAPATITE R CALCIUM PSPATE WIT APPLICATIS I DETISTRY T. Buruiana a*, V. Melinte a, F. Jitaru a, E. C. Buruiana a,. Aldea
PRPERTIES F SME YBRID PLYMER CMPSITES CTAIIG CARBXYLIC/PSPATE DIMETACRYLATES AD YDRXYAPATITE R CALCIUM PSPATE WIT APPLICATIS I DETISTRY T. Buruiana a*, V. Melinte a, F. Jitaru a, E. C. Buruiana a,. Aldea
Chemistry 2.5 AS WORKBOOK. Working to Excellence Working to Excellence
 Chemistry 2.5 AS 91165 Demonstrate understanding of the properties of selected organic compounds WORKBOOK Working to Excellence Working to Excellence CONTENTS 1. Writing Excellence answers to Cis-Trans
Chemistry 2.5 AS 91165 Demonstrate understanding of the properties of selected organic compounds WORKBOOK Working to Excellence Working to Excellence CONTENTS 1. Writing Excellence answers to Cis-Trans
Photogenerated Amines as Novel Crosslinking Agents. D. Scott Bull Corporate Research National Starch and Chemical Company
 Photogenerated Amines as ovel rosslinking Agents D. Scott Bull orporate Research ational Starch and hemical ompany Introduction Reactive polyurethane-based coatings and adhesives are typically prepared
Photogenerated Amines as ovel rosslinking Agents D. Scott Bull orporate Research ational Starch and hemical ompany Introduction Reactive polyurethane-based coatings and adhesives are typically prepared
Chapter 3: Nomenclature, Experimental Materials & Procedures
 Chapter 3: Nomenclature, Experimental Materials & Procedures The linear block PUUs consist of hard and soft segments. The compositional variables of interest are therefore the type, or chemical composition,
Chapter 3: Nomenclature, Experimental Materials & Procedures The linear block PUUs consist of hard and soft segments. The compositional variables of interest are therefore the type, or chemical composition,
Ethers can be symmetrical or not:
 Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Organic Chemistry is the chemistry of compounds containing.
 Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Chapter 21 Lecture Notes Organic Chemistry Intro Organic Chemistry is the chemistry of compounds containing. The Bonding of Carbon Because carbon has four valence electrons, it can form covalent bonds.
Title: Cesa-extend a User Friendly Technology to Enhance Reprocessing and Recycling of Condensation Plastics
 GPEC 24 Paper Abstract #52: Title: Cesa-extend a User Friendly Technology to Enhance Reprocessing and Recycling of Condensation Plastics Author(s): V. Karayan, Clariant Masterbatches, and M. Villalobos,
GPEC 24 Paper Abstract #52: Title: Cesa-extend a User Friendly Technology to Enhance Reprocessing and Recycling of Condensation Plastics Author(s): V. Karayan, Clariant Masterbatches, and M. Villalobos,
Release coatings: Defining Performance
 Release coatings: Defining Performance Igor V. Khudyakov, Michael M. Hawkins, Steven A. Barth, Lisa Y. Winckler Solutia Inc., Performance Films, Fieldale, VA, USA Abstract Films with release coatings are
Release coatings: Defining Performance Igor V. Khudyakov, Michael M. Hawkins, Steven A. Barth, Lisa Y. Winckler Solutia Inc., Performance Films, Fieldale, VA, USA Abstract Films with release coatings are
B7.6 9 th International Conference on Insulated Power Cables B7.6
 B76 9 th International onference on Insulated Power ables B76 Development of a LPE insulating with low peroxide by-products Jean-hristophe GARD, Isabelle DENIZET, Mohamed MAMMERI General able, Montereau,
B76 9 th International onference on Insulated Power ables B76 Development of a LPE insulating with low peroxide by-products Jean-hristophe GARD, Isabelle DENIZET, Mohamed MAMMERI General able, Montereau,
F G C G H H C B B A A DD E E
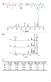 a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
a D E F G H D E I F G H F F I DD E E G H H b c [1] 0 (M) [2a] 0 (M) [1] (M) [2a] (M) [3a] (M) K (M -1 ) a) 0.160 0.139 0.049 0.028 0.111 81 b) 0.147 0.180 0.025 0.058 0.122 84 c) 0.157 0.270 0.013 0.126
Multiple Photoinitiators for Improved Performance Timothy E. Bishop DSM Desotech, Inc. Elgin, IL USA
 Multiple Photoinitiators for Improved Performance Timothy E. Bishop DSM Desotech, Inc. Elgin, IL USA Abstract Higher levels of photoinitiator can increase extractables, reduce weatherability, limit film
Multiple Photoinitiators for Improved Performance Timothy E. Bishop DSM Desotech, Inc. Elgin, IL USA Abstract Higher levels of photoinitiator can increase extractables, reduce weatherability, limit film
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Synergistic Effect of Hydroxyl-containing Acrylates in Epoxide-Acrylate Hybrid Photopolymerizations Abstract Introduction Experimental Materials
 Synergistic Effect of Hydroxyl-containing Acrylates in Epoxide-Acrylate Hybrid Photopolymerizations Gbenga I. Ajiboye, Julie L. P. Jessop* Department of Chemical & Biochemical Engineering, University of
Synergistic Effect of Hydroxyl-containing Acrylates in Epoxide-Acrylate Hybrid Photopolymerizations Gbenga I. Ajiboye, Julie L. P. Jessop* Department of Chemical & Biochemical Engineering, University of
Study on Military Polyurethane Adhesive
 Research Paper Study on Military Polyurethane Adhesive Changming Zhang 1 *, Zhanggen Huang 1, Jun Ping Li 2 and Xiao Juan Lu 2 1 State Key Lab. of Coal Conversion, Institute of Coal Chemistry, Chinese
Research Paper Study on Military Polyurethane Adhesive Changming Zhang 1 *, Zhanggen Huang 1, Jun Ping Li 2 and Xiao Juan Lu 2 1 State Key Lab. of Coal Conversion, Institute of Coal Chemistry, Chinese
THERMAL AND ACTIVATION ENERGY OF RENEWABLE POLYMER AFTER UV IRRADIATION
 THERMAL AND ACTIVATION ENERGY OF RENEWABLE POLYMER AFTER UV IRRADIATION Nik Normunira Mat Hassan and Anika Zafiah M. Rus Sustainable Polymer Engineering, Advanced Manufacturing and Material Center (AMMC),
THERMAL AND ACTIVATION ENERGY OF RENEWABLE POLYMER AFTER UV IRRADIATION Nik Normunira Mat Hassan and Anika Zafiah M. Rus Sustainable Polymer Engineering, Advanced Manufacturing and Material Center (AMMC),
Waterborne UV Curable Polyurethane Dispersions
 Waterborne Curable Polyurethane Dispersions Bo Lv, Zhongqun Xue Jiangsu Sanmu Group, Yixing 214258, Jiangsu, China Abstract Two high molecular weight polyurethane dispersions (PUDs) were synthesized by
Waterborne Curable Polyurethane Dispersions Bo Lv, Zhongqun Xue Jiangsu Sanmu Group, Yixing 214258, Jiangsu, China Abstract Two high molecular weight polyurethane dispersions (PUDs) were synthesized by
Silicone elastomers : from fast curing to biomedical applications
 Silicone elastomers : from fast curing to biomedical applications Khai D. Q. Nguyen, Dexu Kong, William V. Megone, Lihui Peng, Julien Gautrot RIEG afternoon meeting 23 rd March 2018 Biomaterials designs
Silicone elastomers : from fast curing to biomedical applications Khai D. Q. Nguyen, Dexu Kong, William V. Megone, Lihui Peng, Julien Gautrot RIEG afternoon meeting 23 rd March 2018 Biomaterials designs
GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR (preparation of carboxylic acid esters by telomerisation C07C 67/47; telomerisation C08F)
 CPC - C07B - 2017.08 C07B GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR (preparation of carboxylic acid esters by telomerisation C07C 67/47; telomerisation C08F) General methods for the preparation
CPC - C07B - 2017.08 C07B GENERAL METHODS OF ORGANIC CHEMISTRY; APPARATUS THEREFOR (preparation of carboxylic acid esters by telomerisation C07C 67/47; telomerisation C08F) General methods for the preparation
Figure 4.10 HPLC Chromatogram of the Carbazole-Phenoxy Based Methacrylate
 The percent yield of the methacrylation was 85.2 %, with a purity of 98.2 % determined by HPLC (Figure 4.10). Elemental analysis gave excellent agreement to expected elemental ratios (Table 4.2). Disregarding
The percent yield of the methacrylation was 85.2 %, with a purity of 98.2 % determined by HPLC (Figure 4.10). Elemental analysis gave excellent agreement to expected elemental ratios (Table 4.2). Disregarding
Dual UV-Curing System. Using a Dimethacrylate Containing a Chalcone Moiety
 Dual UV-Curing System Using a Dimethacrylate Containing a Chalcone Moiety Technical Paper By Haruyuki Okamura, Yuta Ueda, Masamitsu Shirai and Akikazu Matsumoto We have designed and synthesized a dimethacrylate
Dual UV-Curing System Using a Dimethacrylate Containing a Chalcone Moiety Technical Paper By Haruyuki Okamura, Yuta Ueda, Masamitsu Shirai and Akikazu Matsumoto We have designed and synthesized a dimethacrylate
United States Patent (19) 11 Patent Number: 5,571,297 Swei et al. (45) Date of Patent: Nov. 5, 1996
 III IIII USO071297A United States Patent (19) 11 Patent Number: Swei et al. () Date of Patent: Nov. 5, 1996 54 DUAL-CURE BINDER SYSTEM 5,5,711 5/1996 Helmin... 511298 75) Inventors: Gwo S. Swei, East Amherst,
III IIII USO071297A United States Patent (19) 11 Patent Number: Swei et al. () Date of Patent: Nov. 5, 1996 54 DUAL-CURE BINDER SYSTEM 5,5,711 5/1996 Helmin... 511298 75) Inventors: Gwo S. Swei, East Amherst,
