Direct Catalytic Aldol Reactions
|
|
|
- Giles Warner
- 5 years ago
- Views:
Transcription
1 Direct Catalytic Aldol eactions Shibasakiʼs and Trostʼs contributions toward the development of catalytic enolate reactions. Li Li Ln Li ʻThe time has come,ʼ the Walrus said, ʻTo talk of many things: f shoes and ships and sealing wax f cabbages and kings And why the sea is boiling hot And whether pigs have wings.ʼ Zn Zn N N
2 The Definition of a Direct Catalytic Enolate Addition A large number of enantioselective aldol reactions exist: Si 3 CuF 2 Tol-Binap SnBu 3 BINAP AgTf TBS Et Carriera, 1998 Yamamoto, 1997 Chen, 1997 Preconversion to the ketone is a prerequisite for the reactions. These are not direct aldol reactions. 1 1 A route to this reaction without stoichiometric amounts of base and/or adjunct reagents was desirable
3 Aldolases as Inspiration fructose-1,6-bisphosphate and dihydroxyacetone phosphate (DAP) aldolases are found in E. coli. P 3 2 enzyme P 3 2 L-fuculose P 3 2 Glu C 2 is 2 Is Zn is P 3 2 deprotonation Key Points facilitates both electrophilic activation and proton abstraction C-C bond formation Zn P 3 2 Tyr Glu C 2 Zn Glu C 2 Zn P 3 2 Lewis acid and Brønsted base Multifunctional P 3 2 Fessner, W.-D.; Schneider, A.; eld,.; Sinerius, G.; Walter, C.; ixon, M.; Schloss, J. V. ACIE 1996, 35,
4 Professor Masakatsu Shibasaki. D, University of Tokyo, 1974 (Yamada) Post-doc, arvard, , (Corey) Associate Prof, Teikyo, (Ikegama) Professor, okkaido, Professor, University of Tokyo, 1991-present To date, 465 publications The 1970ʼs Corey Group Shibasaki Nicolau Boger Tius Fuchs Seebach Noyori. Yamamoto B. Snider Takeda Lipshutz Mulzer Danheiser Keck Enders
5 Professor Masakatsu Shibasaki The : Li Li Ln Li N 2 N 2 91% yield 90% ee The lithium is important, Na gave much lower selectivities and yields small amounts of 2 are needed Uncertain of success in aldol reaction due to low basicity of alkoxide Sasai,.; Suzuki, T.; ai, S.; ai, T.; Shibasaki, M. JACS 1992, 114, Sasai,.; Suzuki, T.; Ito, N.; Shibasaki, M. Tetrahedron Lett. 1993, 34, Sasai,.; Suzuki, T.; Itoh, N.; Tanaka, K.; Date, T.; kamura, K.; Shibasaki, M. JACS 1993, 115,
6 S-U-C-C-E-S-S thatʼs the way you spell Success TF 81% yield 91% ee In this case, the rxn worked better without the addition of water. Positives A variety of ketones are compatible A variety of aldehydes are compatible selectivity is generally high Negatives Long reaction times (up to 253 h) Large excess of ketone (up to 50 equiv) igh loading (20 mol %) chanistic Insight: Employment of the Pr related resulted upfield shift of the aldehyde proton Using dilithium salt of ()-binapthol resulted in no chemical shift, afforded racemate Yamada, Y. M. A.; Yoshikawa, N.; Sasai,.; Shibasaki, M. ACIE 1997, 36,
7 Improvement to the eaction TF trace amount Bn Bn LiMDS, 2 C 2 Bn 12 h 99% yield 97% ee Bn 2 C ai, T.; Yamada, Y. M. A.; Yamamoto, N.; Sasai,.; Shibasaki, M. Chem. Eur. J. 1996, 2, Yoshikawa, N.; Yamada, Y. M. A.; Das, J.; Sasai,.; Shibasaki, M. JACS 1999, 121,
8 Improvement to the eaction TF with KMDS and 2 as an additive with only 8 mol % 5 h 74% yield 84% ee Bn Bn LiMDS, 2 C 2 Bn 12 h 99% yield 97% ee Bn 2 C ai, T.; Yamada, Y. M. A.; Yamamoto, N.; Sasai,.; Shibasaki, M. Chem. Eur. J. 1996, 2, Yoshikawa, N.; Yamada, Y. M. A.; Das, J.; Sasai,.; Shibasaki, M. JACS 1999, 121,
9 Improvement to the eaction TF with KMDS and 2 as an additive with only 8 mol % 5 h 74% yield 84% ee N 2 Bn 75%, 88% ee 68%, 70% ee 70%, 93% ee 72%, 88% ee TBS N 2 48 h 73% yield 99% ee no racemization of pre-existing stereocenter Self-condensation of the aldehyde was not observed. Yoshikawa, N.; Yamada, Y. M. A.; Das, J.; Sasai,.; Shibasaki, M. JACS 1999, 121,
10 New Player in the Game Zn Zn N N This uses Zn, which mimics aldolases. It is easily prepared from 2,6-(bromomethyl)-p-cresol. Similarly, phenoxide should assist in both deprotonation and then protonation of alkoxide in product. ne Zn is used for formation of enolate, the other for aldehyde coordination
11 Professor Barry M. Trost.D- MIT, 1965 (ouse) Assistant Prof.- University of Wisconsin, Associate Prof.- University of Wisconsin, Professor- University of Wisconsin, Professor- Stanford, 1987-present ver 720 publications Best known for p-allyl palladium chemistry. Former Students/Post-docs: Curran Ferreira Frontier Molander Toste Krische Stambuli Taber Lautens McIntosh Parquette
12 Substrate Scope 5 mol % ligand 10 mol % ZnEt 2 N 15 mol % 3 PS 4Å MS Notice two equiv of ZnEt2 and use of triphenylphosphine sulfide Zn Zn N 49%, 68% ee 60%, 98% ee 79%, 99% ee 67%, 2:1 dr, 94% ee TBS 61%, 93% ee 66%, 97% ee 48%, 97% ee Trost, B. M.; Ito,. JACS 2000, 122,
13 Trostʼs Explanation of Pathway Zn Zn N N Zn Zn N N Zn Zn N N 3 active ʼs suggest that 2 Zn atoms may be involved 2 equiv of ZnEt2 per 1 equiv of ligand liberates 3 equiv of ethane Addition of 2, liberates 4 equiv of ethane
14 Application to ther Manifolds enry eaction: N 2 N 2 90% yield 92% ee Trost, B. M.; Yeh, V. S. C. ACIE 2002, 41, Mannich eaction: N Et 2 C N Et 2 C Trost, B. M.; Terrell, L.. JACS 2003, 125, Diol Desymmetrization: 70% yield 15:1 dr 99% ee C 93% yield 99% ee Trost, B. M.; Mino, T. JACS 2003, 125,
15 Incorporation of thyl Vinyl Ketone MVK is extremely unstable in both acidic and basic conditions Main problems associated with elimination of b-hydroxy ketone product There is a profound negative nonlinear effect Bn 53%, 92% ee 74%, 86% ee 64%, 83% ee 49%, 98% ee Trost, B. M.; Shin, S.; Sclafani. JACS 2005, 127,
16 ecent Advances Secondary Amine Catalysis: (S)-proline 68% yield 76% ee N 2 N 2 Palladium Catalysis: List, B.; Lerner,. A.; Barbas, C. F. III. J. Am. Chem. Soc. 2000, 122, Tf 2 P Pd 2 P 2 84% yield 90% ee amashima, Y.; otta, D.; Sodeoka, M. J. Am. Chem. Soc. 2002, 124, Nickel Catalysis: S S N () 3 C Ni(II) tol-binap BF 3 Et 2 S S N 73% yield 97% ee Evans, D. A.; Thomson,. J. J. Am. Chem. Soc. 2005, 127, 10506
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Stereoselective reactions of enolates
 1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
Chiral Bronsted Acids as Catalysts
 Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Hydrogen-Mediated C-C Bond Formation
 EPFL - ISIC - LSPN Hydrogen-Mediated C-C Bond Formation History and selected examples The Research of Prof. Michael Krische (University of Texas at Austin) LSPN Group Seminar Mathias Mamboury Table of
EPFL - ISIC - LSPN Hydrogen-Mediated C-C Bond Formation History and selected examples The Research of Prof. Michael Krische (University of Texas at Austin) LSPN Group Seminar Mathias Mamboury Table of
A Highly Efficient Organocatalyst for Direct Aldol Reactions of Ketones and Aldehydes
 A ighly Efficient rganocatalyst for Direct Aldol Reactions of Ketones and Aldehydes Zhuo Tang, Zhi-ua Yang, Xiao-ua Chen, Lin-Feng Cun, Ai-Qiao Mi, Yao-Zhong Jiang, and Liu-Zhu Gong Contribution from the
A ighly Efficient rganocatalyst for Direct Aldol Reactions of Ketones and Aldehydes Zhuo Tang, Zhi-ua Yang, Xiao-ua Chen, Lin-Feng Cun, Ai-Qiao Mi, Yao-Zhong Jiang, and Liu-Zhu Gong Contribution from the
Stereoselective reactions of the carbonyl group
 1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
Direct Organocatalytic Enantioselective Mannich Reactions of Ketimines: An Approach to Optically Active Quaternary α-amino Acid Derivatives
 Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
Strategies for Catalytic Asymmetric Electrophilic a Halogenation of Carbonyl Compounds
 Strategies for Catalytic Asymmetric Electrophilic a alogenation of Carbonyl Compounds 1 2 Y Catalyst [X + ] 1 X! 2 Y intermann, L. ; Togni, A. Angew. Chem. Int. Ed. 2000, 39, 4359 4362 amashima, Y.; Sodeoka,
Strategies for Catalytic Asymmetric Electrophilic a alogenation of Carbonyl Compounds 1 2 Y Catalyst [X + ] 1 X! 2 Y intermann, L. ; Togni, A. Angew. Chem. Int. Ed. 2000, 39, 4359 4362 amashima, Y.; Sodeoka,
ISCHIA ADVANCED SCHOOL OF ORGANIC CHEMISTRY
 ewis acid activation ewis base activation ISCIA ADVACED SC F GAIC CEMISTY Dual in Enantioselective Synthesis of Cyanohydrins Me A A Me UM ewis base catalysis is the process by which an electronpair donor
ewis acid activation ewis base activation ISCIA ADVACED SC F GAIC CEMISTY Dual in Enantioselective Synthesis of Cyanohydrins Me A A Me UM ewis base catalysis is the process by which an electronpair donor
Denmark s Base Catalyzed Aldol/Allylation
 Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
Negishi Coupling of Secondary Alkylzinc Halides with Aryl Bromides and Chlorides
 Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
Negishi Coupling of Secondary Alkylzinc alides with Aryl Bromides and Chlorides X X = Br, Cl 2 1 ZnBr 1, 2 = Alkyl Cat. Pd(OAc) 2 Ligand TF/Toluene rt or 60 o C 1 2 J. Am. Chem. Soc. 2009, ASAP Article
Chiral Proton Catalysis in Organic Synthesis. Samantha M. Frawley Organic Seminar September 14 th, 2005
 Chiral Proton Catalysis in rganic Synthesis Samantha M. Frawley rganic Seminar September 14 th, 2005 Seminar utline Introduction Lewis Acid-assisted Chiral Brønsted Acids Enantioselective protonation for
Chiral Proton Catalysis in rganic Synthesis Samantha M. Frawley rganic Seminar September 14 th, 2005 Seminar utline Introduction Lewis Acid-assisted Chiral Brønsted Acids Enantioselective protonation for
Lewis Base Catalysis: the Aldol Reaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk
 Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
Stereoselective reactions of enolates: auxiliaries
 1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
CuI CuI eage lic R tal ome rgan gbr ommon
 Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Chiral Catalyst II. Palladium Catalysed Allylic Displacement ( -allyl complexes) 1. L n Pd(0) 2. Nuc
 Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
When something goes wrong. Goya: Mother showing her derformed child to two women Louvre, Paris
 1 ew Catalytic Asymmestric eactions Karl Anker Jørgensen Danish ational eserach Foundation: Center for Catalysis Department of Chemistry, Aarhus University Denmark kaj@chem.au.dk When something goes wrong
1 ew Catalytic Asymmestric eactions Karl Anker Jørgensen Danish ational eserach Foundation: Center for Catalysis Department of Chemistry, Aarhus University Denmark kaj@chem.au.dk When something goes wrong
Functionalization of C(sp 3 ) H Bonds Using a Transient Directing Group
 Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Back to Sugars: Enzymatic Synthesis
 Back to Sugars: Enzymatic Synthesis Zhensheng Ding ov. 04 orthrup, A. B.; M acm illan, D. W. C. Science 2004, 305, 1752 orthrup, A. B. and MacMillan, D. W. C. J. Am. Chem. Soc. 2002, 124, 6798-6799 orthrup,
Back to Sugars: Enzymatic Synthesis Zhensheng Ding ov. 04 orthrup, A. B.; M acm illan, D. W. C. Science 2004, 305, 1752 orthrup, A. B. and MacMillan, D. W. C. J. Am. Chem. Soc. 2002, 124, 6798-6799 orthrup,
Journal Club Presentation by Remond Moningka 04/17/2006
 β-alkyl-α-allylation of Michael Acceptors through the Palladium-Catalyzed Three-Component Coupling between Allylic Substrate, Trialkylboranes, and Activated lefins Yoshinori Yamamoto, et al. J. rg. Chem.
β-alkyl-α-allylation of Michael Acceptors through the Palladium-Catalyzed Three-Component Coupling between Allylic Substrate, Trialkylboranes, and Activated lefins Yoshinori Yamamoto, et al. J. rg. Chem.
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction Mechanisms. Group Meeting Aaron Bailey 12 May 2009
 Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction chanisms Group eting Aaron Bailey 12 May 2009 What is a Non-Linear Effect? In asymmetric catalysis, the ee (er) of the
Non-Linear Effects in Asymmetric Catalysis: A Useful Tool in Understanding Reaction chanisms Group eting Aaron Bailey 12 May 2009 What is a Non-Linear Effect? In asymmetric catalysis, the ee (er) of the
Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang!
 1! Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang! 2! utline! 1. Brief Introduction! 2. ucleophilic Dominoes! 3. Electrophilc Dominoes! 4. Radical
1! Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang! 2! utline! 1. Brief Introduction! 2. ucleophilic Dominoes! 3. Electrophilc Dominoes! 4. Radical
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols
 A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
Morita Baylis Hillman Reaction. Aaron C. Smith 11/10/04
 Morita Baylis Hillman Reaction Aaron C. Smith 11/10/04 Outline 1. Background 2. Development of Asymmetric Variants 3. Aza-Baylis Hillman Reaction 4. Applications of Baylis Hillman Adducts Outline 1. Background
Morita Baylis Hillman Reaction Aaron C. Smith 11/10/04 Outline 1. Background 2. Development of Asymmetric Variants 3. Aza-Baylis Hillman Reaction 4. Applications of Baylis Hillman Adducts Outline 1. Background
VI. Metal alkyls from oxidative addition / insertion
 V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
Keisuke Suzuki. Baran lab Group Meeting 6/11/16. Shigenobu Umemiya. Akira Suzuki. Takanori Suzuki (Hokkaido University)
 197.D., Teruaki Mukaiyama, University of Tokyo 193 Assistant Professor, Keio University 197 Lecturer, Keio University 199 Assocate Professor, Keio University 1990 Visiting Professor, ET 1994 ull Professor,
197.D., Teruaki Mukaiyama, University of Tokyo 193 Assistant Professor, Keio University 197 Lecturer, Keio University 199 Assocate Professor, Keio University 1990 Visiting Professor, ET 1994 ull Professor,
Chem 253 Problem Set 7 Due: Friday, December 3, 2004
 Chem 253 roblem Set 7 ue: Friday, ecember 3, 2004 Name TF. Starting with the provided starting material, provide a concise synthesis of. You may use any other reagents for your synthesis. It can be assumed
Chem 253 roblem Set 7 ue: Friday, ecember 3, 2004 Name TF. Starting with the provided starting material, provide a concise synthesis of. You may use any other reagents for your synthesis. It can be assumed
Disulfonimide-Catalyzed Asymmetric Vinylogous and Bisvinylogous Mukaiyama Aldol Reactions
 Disulfonimide-Catalyzed Asymmetric Vinylogous and Bisvinylogous Mukaiyama Aldol eactions atjen, L. Garcia-Garcia, P., Lay, F., Beck, M. E., List, B.; Angew. Chem. Int. Ed. 2010, ASAP. Convergent Total
Disulfonimide-Catalyzed Asymmetric Vinylogous and Bisvinylogous Mukaiyama Aldol eactions atjen, L. Garcia-Garcia, P., Lay, F., Beck, M. E., List, B.; Angew. Chem. Int. Ed. 2010, ASAP. Convergent Total
Parallel Kinetic Resolution. Group Meeting Timothy Chang
 Parallel Kinetic Resolution (PKR) Group Meeting 09 29 2009 Timothy Chang Vedejs, E.; Chen, X. J. Am. Chem. Soc. 1997, 119, 2584. Tanaka, K.; Fu, G. C. J. Am. Chem. Soc. 2003, 125, 8078. KR versus PKR The
Parallel Kinetic Resolution (PKR) Group Meeting 09 29 2009 Timothy Chang Vedejs, E.; Chen, X. J. Am. Chem. Soc. 1997, 119, 2584. Tanaka, K.; Fu, G. C. J. Am. Chem. Soc. 2003, 125, 8078. KR versus PKR The
Denmark Group Meeting. & Electrophilic rearrangement of amides
 Denmark Group Meeting Palladium catalyzed Dearomatizationeaction & Electrophilic rearrangement of amides 11 th Bo Peng th Feb. 2014 1 https://maps.google.com 2 Palladium catalyzed Dearomatization eaction
Denmark Group Meeting Palladium catalyzed Dearomatizationeaction & Electrophilic rearrangement of amides 11 th Bo Peng th Feb. 2014 1 https://maps.google.com 2 Palladium catalyzed Dearomatization eaction
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group.
 Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
PhD research with Prof. Lutz H. Gade at the Univ. of Strasbourg. Postdoc in 2003 with Andreas Pfaltz (Basel Switzerland)
 idium-catalyzed Asymmetric Isomerization of rimary Allylic Alcohols Mantilli, L., Gerard, D., Torche, S., Besnard, C., Mazet * C. Angew. Chem. Int. Ed., 2009, 48, 1-6 1,n-Glycols as Dialdehyde Equivalents
idium-catalyzed Asymmetric Isomerization of rimary Allylic Alcohols Mantilli, L., Gerard, D., Torche, S., Besnard, C., Mazet * C. Angew. Chem. Int. Ed., 2009, 48, 1-6 1,n-Glycols as Dialdehyde Equivalents
Graphical Abstract. Tandem Epoxysilane Rearrangement/Wittig-Type Reactions Using γ- Phosphinoyl- and γ-phosphonio-α β-epoxysilane
 Graphical Abstract Tandem Epoxysilane earrangement/wittig-type eactions Using γ- Phosphinoyl- and γ-phosphonio-α β-epoxysilane Michiko Sasaki, Mai orai, Kei Takeda * Tf t BuMe Si PPh. n-buli. C SiMe Bu
Graphical Abstract Tandem Epoxysilane earrangement/wittig-type eactions Using γ- Phosphinoyl- and γ-phosphonio-α β-epoxysilane Michiko Sasaki, Mai orai, Kei Takeda * Tf t BuMe Si PPh. n-buli. C SiMe Bu
Palladium-catalyzed sp 3 C H activation. Yan Xu Dong Group Meeting Apr. 2, 2014
 Palladium-catalyzed sp 3 C H activation, Yan Xu Dong Group Meeting Apr. 2, 2014 Content 1 Allylic C H activation 2 Benzylic C H activation Palladiumcatalyzed sp 3 C H activation 3 4 Common sp 3 C H activation:
Palladium-catalyzed sp 3 C H activation, Yan Xu Dong Group Meeting Apr. 2, 2014 Content 1 Allylic C H activation 2 Benzylic C H activation Palladiumcatalyzed sp 3 C H activation 3 4 Common sp 3 C H activation:
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
Organocopper Reagents
 rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
Asymmetric Palladium Catalyzed Cross-Coupling Reactions. Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1
 Asymmetric Palladium Catalyzed Cross-Coupling Reactions Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1 Palladium Catalyzed Cross-Coupling Reactions 2 Kumada/Negishi Cross-Coupling Kumada:
Asymmetric Palladium Catalyzed Cross-Coupling Reactions Topic Talk September 4 th, 2014 Morken Lab Emma Edelstein 1 Palladium Catalyzed Cross-Coupling Reactions 2 Kumada/Negishi Cross-Coupling Kumada:
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Chapter 1. Michael Addition Reaction
 Chapter 1 Michael Addition Reaction 1.1 Introduction This chapter presents a brief overview of Michael addition 1 reaction in terms of recent developments and usefulness. Michael addition involves the
Chapter 1 Michael Addition Reaction 1.1 Introduction This chapter presents a brief overview of Michael addition 1 reaction in terms of recent developments and usefulness. Michael addition involves the
Bifunctional Asymmetric Catalysts: Design and Applications. Junqi Li CHEM Sep 2010
 Bifunctional Asymmetric Catalysts: Design and Applications Junqi Li CHEM 535 27 Sep 2010 Enzyme Catalysis vs Small-Molecule Catalysis Bronsted acid Lewis acid Lewis acid Bronsted base Activation of both
Bifunctional Asymmetric Catalysts: Design and Applications Junqi Li CHEM 535 27 Sep 2010 Enzyme Catalysis vs Small-Molecule Catalysis Bronsted acid Lewis acid Lewis acid Bronsted base Activation of both
B X A X. In this case the star denotes a chiral center.
 Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
O H HO H. !-D-galactopyranose
 ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
ame Key W06-Exam o. Page I. ( points) A disaccharide is cleaved by a β-glycosidase, an enzyme that specifically hydrolyzes a β- glycosidic linkage. When the disaccharide is treated with excess dimethyl
Intramolecular Ene Reactions Utilizing Oxazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129,
 Intramolecular Ene Reactions Utilizing xazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129, 3058-3059 - versus -Arylation of Aminoalcohols: rthogonal Selectivity in Copper-Based
Intramolecular Ene Reactions Utilizing xazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129, 3058-3059 - versus -Arylation of Aminoalcohols: rthogonal Selectivity in Copper-Based
CHEM 330. Final Exam December 11, This a closed-notes, closed-book exam. The use of molecular models is allowed. This exam contains 12 pages
 CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
CEM 330 Final Exam December 11, 2007 Your name: This a closed-notes, closed-book exam The use of molecular models is allowed This exam contains 12 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4. / 40
Mechanistic Studies of Allylsilane Rearrangement
 chanistic Studies of Allylsilane Rearrangement Thesis: The goal of our project is to determine the operating mechanism in the transformation of α-siloxy allylsilanes to vinyl silanes. Elucidating the mechanism
chanistic Studies of Allylsilane Rearrangement Thesis: The goal of our project is to determine the operating mechanism in the transformation of α-siloxy allylsilanes to vinyl silanes. Elucidating the mechanism
Zr-Catalyzed Carbometallation
 -Catalyzed Carbometallation C C C C ML n C C ML n ML n C C C C ML n ML n C C ML n Wipf Group esearch Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation
-Catalyzed Carbometallation C C C C ML n C C ML n ML n C C C C ML n ML n C C ML n Wipf Group esearch Topic Seminar Juan Arredondo November 13, 2004 Juan Arredondo @ Wipf Group 1 11/14/2004 Carbometallation
Additions to Metal-Alkene and -Alkyne Complexes
 Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Chapter 14. Principles of Catalysis
 Organometallics Study Meeting 2011/08/28 Kimura Chapter 14. Principles of Catalysis 14. 1. General Principles 14.1.1. Definition of a Catalyst 14.1.2. Energetics of Catalysis 14.1.3. Reaction Coordinate
Organometallics Study Meeting 2011/08/28 Kimura Chapter 14. Principles of Catalysis 14. 1. General Principles 14.1.1. Definition of a Catalyst 14.1.2. Energetics of Catalysis 14.1.3. Reaction Coordinate
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Chloroform and deuterated chloroform are cancer suspect agents and mutagens.
 JCE #2004-0802 LAB DCUMETATI Chemicals and CAS egistry umbers Acetone [67-64-1] Isobutyraldehyde [78-84-2] L-proline [147-85-3] D-proline [344-25-2] Diethyl ether [60-29-7] Magnesium sulfate [7487-88-9]
JCE #2004-0802 LAB DCUMETATI Chemicals and CAS egistry umbers Acetone [67-64-1] Isobutyraldehyde [78-84-2] L-proline [147-85-3] D-proline [344-25-2] Diethyl ether [60-29-7] Magnesium sulfate [7487-88-9]
Direct Catalytic Cross-Coupling of Organolithium
 Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Ready; Catalysis Conjugate Addition
 eady; Catalysis Conjugate Addition Topics covered 1. 1,4 addition involving copper a. stoichiometric reactions b. catalytic reactions c. allylic substitution. Conjugate addition without copper a. Ni-based
eady; Catalysis Conjugate Addition Topics covered 1. 1,4 addition involving copper a. stoichiometric reactions b. catalytic reactions c. allylic substitution. Conjugate addition without copper a. Ni-based
Copper-mediated asymmetric transformations*
 Pure Appl. Chem., Vol. 74, No. 1, pp. 37 42, 2002. 2002 IUPAC Copper-mediated asymmetric transformations* Alexandre Alexakis Department of Organic Chemistry, University of Geneva, 30 quai Ernest Ansermet,
Pure Appl. Chem., Vol. 74, No. 1, pp. 37 42, 2002. 2002 IUPAC Copper-mediated asymmetric transformations* Alexandre Alexakis Department of Organic Chemistry, University of Geneva, 30 quai Ernest Ansermet,
Chiral phosphine Lewis bases in catalytic, asymmetric aza-morita Baylis Hillman reaction*
 Pure Appl. Chem., Vol. 77, No. 12, pp. 2105 2110, 2005. DOI: 10.1351/pac200577122105 2005 IUPAC Chiral phosphine Lewis bases in catalytic, asymmetric aza-morita Baylis Hillman reaction* Min Shi and Lian-Hui
Pure Appl. Chem., Vol. 77, No. 12, pp. 2105 2110, 2005. DOI: 10.1351/pac200577122105 2005 IUPAC Chiral phosphine Lewis bases in catalytic, asymmetric aza-morita Baylis Hillman reaction* Min Shi and Lian-Hui
Short Literature Presentation 10/4/2010 Erika A. Crane
 Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
The synthesis of molecules containing quaternary stereogenic centers via the intramolecular asymmetric Heck reaction. Eric Gillis 4/19/07
 The synthesis of molecules containing quaternary stereogenic centers via the intramolecular asymmetric Heck reaction Eric Gillis 4/19/07 Quaternary stereocenters in complex small molecules Retrosynthetic
The synthesis of molecules containing quaternary stereogenic centers via the intramolecular asymmetric Heck reaction Eric Gillis 4/19/07 Quaternary stereocenters in complex small molecules Retrosynthetic
Reactivity Umpolung-1 Ready
 eactivity Umpolung-1 eady eactivity Umpolung: reversal of normal polarity electrophiles become nucleophiles nucleophiles become electrophiles complimentary disconnections ormal reactivity: x = heteroatom
eactivity Umpolung-1 eady eactivity Umpolung: reversal of normal polarity electrophiles become nucleophiles nucleophiles become electrophiles complimentary disconnections ormal reactivity: x = heteroatom
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Organocopper Chemistry
 rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
Epoxidation with Peroxy Acids
 Epoxidation with Peroxy Acids RC 3 R C more reactive more likely Freccero, M.; Gandolfi, R.; Sarzi-Amadè, M.; Rastelli, A. J. rg. Chem. 2000, 65, 2030. Singleton, D. A.; Merrigan, S. R.; Liu, J.; ouk,
Epoxidation with Peroxy Acids RC 3 R C more reactive more likely Freccero, M.; Gandolfi, R.; Sarzi-Amadè, M.; Rastelli, A. J. rg. Chem. 2000, 65, 2030. Singleton, D. A.; Merrigan, S. R.; Liu, J.; ouk,
Chiral Supramolecular Catalyst for Asymmetric Reaction
 Chiral Supramolecular Catalyst for Asymmetric Reaction 2017/1/21 (Sat.) Literature Seminar Taiki Fujita (B4) 1 Introduction Rational design of chiral ligands remains very difficult. Conventional chiral
Chiral Supramolecular Catalyst for Asymmetric Reaction 2017/1/21 (Sat.) Literature Seminar Taiki Fujita (B4) 1 Introduction Rational design of chiral ligands remains very difficult. Conventional chiral
Shi Asymmetric Epoxidation
 Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions
 Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions Reporter: Hong-Qiang Shen Checker: Cong Liu Date: 2016/07/12 Masahiro Miura et al. Angew. Chem. Int. Ed. 2016, 55, 6973. Masahiro Miura Osaka
Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions Reporter: Hong-Qiang Shen Checker: Cong Liu Date: 2016/07/12 Masahiro Miura et al. Angew. Chem. Int. Ed. 2016, 55, 6973. Masahiro Miura Osaka
O or R E + R. - Keto-Enol Tautomerization (enol form usually very minor for simple ketones)
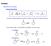 General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Synthesis of 1,3-Diols via Controlled, Radical-Mediated C-H Functionalization
 Synthesis of 1,3-Diols via Controlled, Radical-Mediated C- Functionalization Chen, K.; Richter, J. M.; Baran, P. S. J. Amer. Chem. Soc. 2008, 130, 7247-7249. Literature Group Presentation Wynter Gilson
Synthesis of 1,3-Diols via Controlled, Radical-Mediated C- Functionalization Chen, K.; Richter, J. M.; Baran, P. S. J. Amer. Chem. Soc. 2008, 130, 7247-7249. Literature Group Presentation Wynter Gilson
CATALYSIS MULTICATALYST SYSTEM IN ASYMMETRIC. Wiley. Department of Chemistry
 MULTICATALYST SYSTEM IN ASYMMETRIC CATALYSIS JIAN ZHOU Shanghai Key Laboratory of Green Chemistry and Chemical Processes Department of Chemistry East China Normal University Shanghai, China Wiley Preface
MULTICATALYST SYSTEM IN ASYMMETRIC CATALYSIS JIAN ZHOU Shanghai Key Laboratory of Green Chemistry and Chemical Processes Department of Chemistry East China Normal University Shanghai, China Wiley Preface
Career Review of Dean Toste I. 2015/9/9 Zhi Ren
 Career Review of Dean Toste I 2015/9/9 Zhi Ren Introduction F. Dean Toste, now in UC Berkeley Career: Full Professor since 2009-now Associate Professor 2006-2009 Assistant Professor 2002-2006 Faculty Scientist
Career Review of Dean Toste I 2015/9/9 Zhi Ren Introduction F. Dean Toste, now in UC Berkeley Career: Full Professor since 2009-now Associate Professor 2006-2009 Assistant Professor 2002-2006 Faculty Scientist
The Synthesis of Molecules Containing Quaternary Stereogenic Centers via the Intramolecular Asymmetric Heck Reaction
 The Synthesis of Molecules Containing Quaternary Stereogenic Centers via the Intramolecular Asymmetric Heck Reaction Reported by Eric P. Gillis April 19, 2007 INTRODUCTION The enantioselective synthesis
The Synthesis of Molecules Containing Quaternary Stereogenic Centers via the Intramolecular Asymmetric Heck Reaction Reported by Eric P. Gillis April 19, 2007 INTRODUCTION The enantioselective synthesis
Enantioselective Protonations
 Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Asymmetric Catalysis by Chiral Hydrogen-Bond Donors
 Asymmetric Catalysis by Chiral Hydrogen-Bond Donors Angew. Chem. Int. Ed., 2006, 45, 1520~1543 Mark S. Taylor and Eric N. Jacobsen* Current Literature Presentation Zhenglai Fang Wipf s Group at Pitt Zhenglai
Asymmetric Catalysis by Chiral Hydrogen-Bond Donors Angew. Chem. Int. Ed., 2006, 45, 1520~1543 Mark S. Taylor and Eric N. Jacobsen* Current Literature Presentation Zhenglai Fang Wipf s Group at Pitt Zhenglai
Nickel-Catalyzed Multicomponent Coupling of Alkynes. -Recent development in methodologies and applications. Zhenjie Lu. Department of Chemistry, MSU
 28 58.69 i ickel ickel-catalyzed Multicomponent Coupling of Alkynes -ecent development in methodologies and applications Zhenjie u Department of Chemistry, MSU January 28, 2004 Background Introduction
28 58.69 i ickel ickel-catalyzed Multicomponent Coupling of Alkynes -ecent development in methodologies and applications Zhenjie u Department of Chemistry, MSU January 28, 2004 Background Introduction
CHO. OMe. endo. xylene, 140 o C, 2 h 70% 1. CH 2 (OMe) 2, MeOH TsOH, rt 2. Bu 2 O, 1,2-dichloroethane 140 o C, 2 h 3. 6 M HCl, THF, rt 44%
 VII Abstracts 2010 p1 2.4.12 Arene rganometallic Complexes of Chromium, Molybdenum, and Tungsten M. Uemura This review is an update to Section 2.4 and covers the literature from 1999 to 2010. (h 6 -Arene)chromium
VII Abstracts 2010 p1 2.4.12 Arene rganometallic Complexes of Chromium, Molybdenum, and Tungsten M. Uemura This review is an update to Section 2.4 and covers the literature from 1999 to 2010. (h 6 -Arene)chromium
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
13.3A: The general mechanism for an aldol reaction
 Ashley Robison My Preferences Site Tools FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki BioWiki GeoWiki StatWiki
Ashley Robison My Preferences Site Tools FAQ Sign Out If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki BioWiki GeoWiki StatWiki
Molybdenum-Catalyzed Asymmetric Allylic Alkylation
 Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
C H activation of aliphatic amines without unnecessary mask M2 Takaya Togo
 C H activation of aliphatic amines without unnecessary mask 2017.11.25 M2 Takaya Togo 1 Outline 1.Introduction 2.Free amines as DG Discovery of new activation mode Mechanistic studies Application of the
C H activation of aliphatic amines without unnecessary mask 2017.11.25 M2 Takaya Togo 1 Outline 1.Introduction 2.Free amines as DG Discovery of new activation mode Mechanistic studies Application of the
Transition Metal-Catalyzed Carbon-Carbon Bond Cleavage (C-C Activation) Group Meeting Timothy Chang
 Transition Metal-Catalyzed Carbon-Carbon Bond Cleavage (C-C Activation) Group Meeting 01-15-2008 Timothy Chang Outlines - Fundamental considerations, C-H versus C-C activation - Orbital interactions -
Transition Metal-Catalyzed Carbon-Carbon Bond Cleavage (C-C Activation) Group Meeting 01-15-2008 Timothy Chang Outlines - Fundamental considerations, C-H versus C-C activation - Orbital interactions -
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Use of Cp 2 TiCl in Synthesis
 Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
David W.C. MacMillan: Career-in-Review. Yan Xu Dong Group Meeting Jan. 2, 2014
 David W.C. MacMillan: Career-in-Review Yan Xu Dong Group Meeting Jan. 2, 2014 David W.C. MacMillan: A Brief Introduction Career 1968 Born in Bellshill, Scotland. 1987-1991 Undergraduate degree in chemistry
David W.C. MacMillan: Career-in-Review Yan Xu Dong Group Meeting Jan. 2, 2014 David W.C. MacMillan: A Brief Introduction Career 1968 Born in Bellshill, Scotland. 1987-1991 Undergraduate degree in chemistry
Homogeneous Gold Catalysis - Unique Reactivity for Activation of C C Multiple Bonds
 1 rganic Seminar 2011.05.09 omogeneous Gold Catalysis - Unique Reactivity for Activation of C C Multiple Bonds ysical rganic Chemistry Laboratory (Nakamura Laboratory) D2 Masaki Sekine Development of Gold
1 rganic Seminar 2011.05.09 omogeneous Gold Catalysis - Unique Reactivity for Activation of C C Multiple Bonds ysical rganic Chemistry Laboratory (Nakamura Laboratory) D2 Masaki Sekine Development of Gold
π-alkyne metal complex and vinylidene metal complex in organic synthesis
 Literature Seminar 080220 Kenzo YAMATSUGU (D1) π-alkyne metal complex and vinylidene metal complex in organic synthesis 0. Introduction ' ' = π-alkyne metal complex vinylidene metal complex ecently, electrophilic
Literature Seminar 080220 Kenzo YAMATSUGU (D1) π-alkyne metal complex and vinylidene metal complex in organic synthesis 0. Introduction ' ' = π-alkyne metal complex vinylidene metal complex ecently, electrophilic
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
Total Synthesis of ( )-Himandrine
 Total Synthesis of ( )-imandrine M. Movassaghi,* M. Tjandra and J. Qi J. Am. hem. Soc. 2009, 131, 9648-9650 Bz Adam T. oye urrent Literature ctober 3, 2009 Adam oye @ Wipf Group Page 1 of 22 10/3/2009
Total Synthesis of ( )-imandrine M. Movassaghi,* M. Tjandra and J. Qi J. Am. hem. Soc. 2009, 131, 9648-9650 Bz Adam T. oye urrent Literature ctober 3, 2009 Adam oye @ Wipf Group Page 1 of 22 10/3/2009
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
Kinetic Resolutions. Some definitions and examples Resolution: A process leading to the separation of enantiomers, or derivatives thereof.
 Material outline: For the Scientist in you: Definitions Theoretical treatment Kinetic esolutions General eferences: Vedejs, ACIEE, 2005, 3974 Jacobsen, Adv. Syn. Cat. 2001, 5 Kagan, Topics in Stereochemistry,
Material outline: For the Scientist in you: Definitions Theoretical treatment Kinetic esolutions General eferences: Vedejs, ACIEE, 2005, 3974 Jacobsen, Adv. Syn. Cat. 2001, 5 Kagan, Topics in Stereochemistry,
Synthesis of Amphidinolide X and an Exploration of Key Reactions
 PJM 1/12/05 Synthesis of Amphidinolide X and an Exploration of Key eactions Lepage,.; Kattnig, E.; Furstner, A. JACS, 2004, 126, 15970-15971. 7 13 1 6 19 - Produced by marine dinoflagellates, Amphidinium
PJM 1/12/05 Synthesis of Amphidinolide X and an Exploration of Key eactions Lepage,.; Kattnig, E.; Furstner, A. JACS, 2004, 126, 15970-15971. 7 13 1 6 19 - Produced by marine dinoflagellates, Amphidinium
Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines
 Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
A Stille or Suzuki reaction is a good choice for this coupling O O because they are functional group tolerant, no radical chemistry F
 Chemistry 253 roblem et 3 Due: Friday, ctober 15th ame TF 1. For the following products of cross coupling reactions and indicated bond disconnections, please indicate a reasonable cross coupling protocol
Chemistry 253 roblem et 3 Due: Friday, ctober 15th ame TF 1. For the following products of cross coupling reactions and indicated bond disconnections, please indicate a reasonable cross coupling protocol
Chemistry of the Double Bond
 Chemistry of the Double ond. eactions of the Carbonyl Group. At Carbonyl.. eduction (hydride addition)..2 kylation..3 lylation/propargylation.2 At α-center (Enolate Chemistry).2. kylation.2.2 dol eaction.3
Chemistry of the Double ond. eactions of the Carbonyl Group. At Carbonyl.. eduction (hydride addition)..2 kylation..3 lylation/propargylation.2 At α-center (Enolate Chemistry).2. kylation.2.2 dol eaction.3
Tautomerism and Keto Enol Equilibrium
 Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Organic Tutorials 3 rd Year Michaelmas Transition Metals in Organic Synthesis: (General paper level) ! 1! Reading
 rganic Tutorials 3 rd Year Michaelmas 2010 Transition Metals in rganic Synthesis: (General paper level) Reading 1. Lecture Course, and suggested references from this. 2. Clayden, Greaves, Warren and Wothers.
rganic Tutorials 3 rd Year Michaelmas 2010 Transition Metals in rganic Synthesis: (General paper level) Reading 1. Lecture Course, and suggested references from this. 2. Clayden, Greaves, Warren and Wothers.
