B: Discussion and Results 25
|
|
|
- Annice Shelton
- 5 years ago
- Views:
Transcription
1 B: Discussion and Results SYTESIS 2.1 Synthesis design A working and efficient synthesis design is crucial for the success of any chemical synthesis. In this specific case, given the synthetic targets outlined in the preceding section, it was required: - To identify the most convenient -nucleoamino acid, C -tetrasubstitued -amino acid and C -trisubstitued analogue for the preparation of rigid and flexible sequential helical nucleopeptides; - To design synthetic targets both for obtaining model compounds, for conformational characterization and synthetic protocol evaluation, and for obtaining nucleotide analogues soluble in physiological conditions; - To identify useful functionalisations for pairing and biological tests; - To devise efficient synthetic strategies for the synthesis of both classes of nucleopeptides and for their functionalisation, in particular concerning to the choice of the solution or solid phase, of suitable protecting groups, and of the activation protocols. Since the decisions taken about each point were conditioning the following steps, the discussion will address one point a time Choice of the amino acids n the basis of the literature [51], -alanyl -nucleoamino acids (AlaB) were identified as the most convenient for interactions studies of complementary functionalized nucleopeptides. DA nucleobases rather than RA nucleobases were chosen as pendants, since uracil poses more synthetic problems than thymine. [97] Aib, or -aminoisobutyric acid, the simplest C -tetrasubstituted -amino acid, was chosen for the synthesis of the rigid nucleopeptides, both because of its strong helical promoting properties and because the decrease in reactivity due to the sterical hindrance of the second methyl at the C is not so dramatic as in C -tetrasubstituted -amino acids carrying bulkier substituents. [98] Consequently, the protein amino acid alanine was chosen as the C - trisubstituted analogue for the synthesis of the flexible nucleopeptides. The tripeptide units
2 2. Synthesis 26 composing the two classes of nucleopeptides were therefore Aib-AlaB-Aib and Ala-AlaB- Ala for the rigid and flexible nucleopeptides, respectively. To favour water solubility for biological and pairing measurements, the incorporation of one or two lysine residue at the termini was planned, as already seen several times in the case of peptide nucleic acids and nucleopeptides. Concerning the amino-acid configuration, L-amino acids were employed, since they can form right-handed helices [99], with the correct spiralization sense in order to interact with nucleotides. As mentioned in the introduction, this holds true also for mixed peptides of the achiral Aib and of chiral C -trisubstituted amino acids Choice of the synthetic strategy For the synthesis of the rigid nucleopeptides, rich in Aib residues, the solution phase synthesis seemed the most appropriate: indeed, the reduced reactivity of this hindered amino acids generally makes couplings on solid-phase sluggish. Beside that, solution phase synthesis offers the possibility of isolation, purification and characterization of all synthetic intermediates. This is valuable for the conformational studies, as well as the production of larger amounts of products by solution-phase synthesis techniques. The sequential structure of the target nucleopeptides, terminated by an achiral residue, suggested the adoption of the fragment condensation approach for the synthesis, using the repeating tripeptide units as the fragments to be condensed. This approach generally allows to obtain larger quantities of final products, while speeding up the synthesis, and it can be used for parallel synthesis. This last advantage was particularly appealing, since, starting from four suitably protected nucleo-tripeptides containing each one of the DA bases, all possible 4 2 = 16 nucleohexapeptides or 4 3 = 64 nucleo-nonapeptides could potentially be prepared. For the synthesis of the flexible nucleopeptides, composed only of C - trisubstituted residues, the solid phase synthesis was the option of choice, since it allows the synthesis of longer sequences in short times. Because pairing and biological tests are generally performed on submicromolar scale, the reduced amount of product which can be obtained by solid-phase synthesis does not represent a problem, provided that efficient synthesis protocols are adopted.
3 B: Discussion and Results Choice of suitable protecting groups Generally speaking, the functions that require protection in peptide synthesis are the - terminal amino and C-terminal carboxyl group, as well as functional groups at the side chains. The ideal conditions are satisfied when orthogonal protecting groups are employed: [100] two protecting groups are fully orthogonal if either group can be removed under conditions which do not compromise the stability of the other; partial orthogonality refers to the case that only one can be removed without causing removal of the other. Concerning the terminal functionalities, in case of the solid-phase synthesis of the flexible nucleopeptides, the C-terminal carboxyl group is bound to the resin until the cleavage at the end of the synthesis; therefore only partial orthogonality with the -amino protecting group is required. n the contrary, the solution-phase synthesis of the rigid nucleopeptides by fragment condensation requires full orthogonality between - and C-terminal protecting groups, while the synthesis of the single fragments can be accomplished also in the case of partial orthogonality. Considering the side-chains of the target nucleopeptides, it appears that Aib and Ala are not an issue, whereas the nucleobase pendants of the nucleoamino acid and the - amino group of lysine (where present) must be taken into account. Indeed, three out of four bases (adenine, cytosine, guanine) require protection: cytosine for chemoselectivity reasons, the purine bases for solubility reasons. After their incorporation in the nucleoamino acids, nucleobase deprotection is not required until the very end of the synthesis, therefore partial orthogonality is sufficient for their protecting groups. The same is true for the -amino group of lysine if it is introduced simply for providing a positively charged ammonium moiety after complete deprotection; however, if this group is meant for specific derivatization, full orthogonality must be ensured ucleobase protecting groups ucleobases are difficult to manipulate, being highly hydrophilic, highly insoluble in the most common organic solvents, and sparingly soluble and aggregation prone even in the most polar solvents, such as MeC, DMF or DMS. [97] The protecting groups chosen for the nucleobases, in particular for the purines, should therefore make them less hydrophilic and increase their solubility in polar organic solvents. This means that not all protecting
4 2. Synthesis 28 groups generally adopted in peptide synthesis can be successfully employed for the protection of nucleobases, either because of the different reaction conditions required for introduction or removal, or because of the insufficient increase in lipophilicity offered by the protection. Feasible options are thus significantly limited, meaning that also the choice of protecting groups that can be used for the peptide functions is limited by their compatibility with the few groups that can be efficiently used for the nucleobases. For the protection of the 6 -amino group of adenine both the tert-butyloxycarbonyl () protecting group, [20,97] which is removed under acidic conditions, and the benzyloxycarbonyl () protecting group, [58b,97] which is removed by catalytic hydrogenolysis, can be used (Fig. 2.1, top). For the protection of the 4 -amino group of cytosine the same groups can be used [20,58b] (Fig. 2.1, middle). Moreover the use of the benzoyl (Bz) group has been reported. [101] The conditions for removal of the benzoyl moiety from -amino groups are so harsh that it is generally considered a blocking, rather than protecting, group, but, in the case of the exocyclic amino groups of the nucleobases, this can be displaced under mild conditions by nucleophilic attack from hydrazine. [101b] 2 2, py a, Cl DMF, 1h 80 C,18h rt DMF, 5h rt 2, DMAP DMS, 24h rt, 2 Cl, py, DMAP DMF, 3d rt Cl TFA/ 2 3:1 2 3d rt 2 Fig Conditions of introduction of protecting groups employed in the present work for adenine (top) and for cytosine (middle); formation of guanine from 2-amino-6-chloro-purine (bottom).
5 B: Discussion and Results 29 Concerning guanine, both the 6 protection as an ether, [102a] or as a urethane, [102b] and the 2 protection as an amide [103] have been reported, but neither solution ensures both efficient and stable protection and removal under mild condition. In most cases dealing with nucleotide analogues, [54,104] guanine-based nucleotide analogues have been obtained starting from 2-amino-6-chloro-purine (-G Cl ). [105] The chlorine atom favours the solubility in organic media compared to guanine and it can be replaced by an hydroxyl under strongly acidic aqueous conditions (Fig. 2.1, bottom) amino- and carboxyl- protecting groups The protecting groups chosen for the preparation of the flexible nucleopeptides had to be compatible with solid-phase synthesis conditions. The two most common strategies for the synthesis of peptides on solid-phase are the /Bzl or the Fmoc/ t Bu. [106] In the first one, -amino groups are protected with the acid-labile tert-butyloxycarbonyl () [107] protecting group, in the second one the base labile fluorenylmethyloxycarbonyl (Fmoc) protecting group is used. [108] Both -protected [47] and Fmoc-protected [109] -alanyl - nucleoamino acids have been employed for the solid-phase synthesis of nucleopeptides. owever, two more steps are required in order to obtain the (non commercially available) Fmoc-protected nucleoamino acids compared to the -protected nucleoamino acids. This is because their synthesis relies on the nucleobase alkylation under strongly basic conditions (see section 2.2), which are not compatible with Fmoc-chemistry. Therefore nucleoamino acids must be prepared at first as - or - protected derivatives, then deprotected and protected again with Fmoc. Consequently the group has been chosen as -amino protecting group for the flexible nucleopeptides, while protecting groups stable to moderate acidic conditions were needed for the side-chains (e.g. for the adenine and cytosine the protecting was employed). Concerning the solution-phase synthesis of the rigid nucleopeptides, the situation was more complex, because of the need of an additional orthogonal protection for the carboxyl function. The most convenient urethane -protecting group for solution-phase synthesis is by far the group. [110] The three main advantages of the use of this group are: (i) the good physical and chemical stability of -protected peptides, (ii) the ease of deprotection by catalytic hydrogenolysis, forming volatile co-products, which are smoothly
6 2. Synthesis 30 eliminated by evaporation and (iii) the increased tendency to precipitation and even to crystallization of pure protected peptides. Using the group for -amino protection, the C-terminal carboxyl group is generally orthogonally protected as tert-butyl ester ( t Bu). [111] Among the advantages of this protecting group there are: (i) the increased solubility of the protected peptides in organic solvents; (ii) the reduced tendency to 2,5-dioxopiperazine formation of - deprotected peptides; (iii) the ease of deprotection by acidolysis, [111c] with formation of volatile side products. If a third orthogonal protecting group is necessary, base-labile groups like Fmoc are generally used. The latter protecting group was not suited for the protection of the nucleobases, because it would have been removed by the basic alkylation conditions (see section 2.2). f course simply exchanging the deprotection conditions for the - and carboxyl- functions (by the use of the acid-labile group and of the benzyl ester, Bzl, [112] removed by hydrogenolysis, [112c] respectively) would have not changed the situation. ne possibility was to use a base-labile ester, like the methyl ester (Me [113] ), for the carboxyl function, the only one not involved in the nucleobase alkylation step, thus allowing the use of and groups together for - and nucleobase protection. n the other side, care must be taken during the saponification of the methyl ester, since it can cause epimerization not only at the C-terminal but at other positions in the sequence. Moreover, the saponification of C -tetrasubstituted amino acid esters is particularly slow and it is generally performed only if the peptide contains exclusively achiral residues, since it requires harsher conditions compared to the saponification of protein amino acid methyl esters. Therefore, considering that no ideal set of protecting groups valid for all four nucleobases was available, a tailored strategy was employed for the synthesis of nucleopeptides containing each base. Since thymine does not require protection, the most convenient protecting groups ( and t Bu) were adopted for the - and C-terminal functions of its nucleopeptides. Adenine requires protection only for solubility reasons, since its exocyclic amino group is a quite poor nucleophile, therefore nucleobase deprotection during synthesis does not pose chemoselectivity problems. For this reason in a first time the same set of protecting groups was chosen for the -amino and carboxyl functions of adenine-based nucleopeptides, while the protecting group was used for the nucleobase just to increase its solubility and
7 B: Discussion and Results 31 facilitate its manipulation up to the synthesis of the protected nucleo-dipeptide, then simultaneous deprotection of both groups was planned. Unfortunately, the use of - protecting group for adenine-based nucleopeptides turned out to be not satisfactory (see section 2.3.2). Therefore, as completely orthogonal protecting groups the and Me moieties were employed for the backbone functions and the group was used for the nucleobase. Cytosine required an orthogonal set of protecting groups, its amino group being the most nucleophilic and basic (pka 4.2) [97] among nucleobases, therefore the same set of orthogonal protecting groups employed in the case of adenine was used. Concerning guanine-based nucleopeptides, it was chosen to use 2-amino-6-chloro-purine as the starting material. As it was not clear whether the 6-chloro group would have resisted or t Bu deprotection conditions (strong acid in aprotic solvent), the group was chosen for protection and the carboxyl group was protected as Me. In Fig. 2.2 nucleodipeptides containing the four bases are represented with the planned protecting groups. B B AlaT nucleopeptides AlaA nucleopeptides B B Cl AlaC, AlaA nucleopeptides AlaG Cl nucleopeptides Fig The planned set of protecting groups for protected nucleodipeptides containing the four DA nucleobases. Different colours are used for different deprotection conditions: hydrogenolysis (green), acidolysis (blue), saponification (red), acidic hydrolysis (purple). 2.2 ucleoamino acid synthesis The synthesis of the alanyl -nucleoamino acids relies on the nucleophilic attack of the suitably protected nucleobases on the -carbon of the -protected serine -lactone [44] in the presence of a strong, non-nucleophilic base. The lactone intermediate is not
8 2. Synthesis 32 commercially available in the -protected form and therefore its synthesis deserves some comments as a prerequisite for the nucleoamino acid synthesis Synthesis of -protected serine -lactone The synthesis of the -lactone of serine was performed by Mitsunobu reaction. [114] Briefly, a phosphonium electrophile is generated by attack of triphenylphosphine on a, -dialkyl azodicarboxylate and it binds to the carboxyl group of serine. This in turn activates the carboxylic carbon for the nucleophilic attack of the serine -hydroxyl group, facilitated by the ability of the azo-derivative of accepting the hydroxyl proton and by the formation of the good leaving group phosphine oxide, see Fig The reaction is performed in tetrahydrofurane (TF) at low temperature (-78 C), in order to minimise side reactions. The use of the dimethyl azodicarboxylate (DMAD) instead of the commercially available diethyl or diisopropyl derivatives was suggested [44] in order to facilitate its chromatographic separation from the -lactone product. owever this adds another step to the preparation of the nucleoamino acids, since DMAD can not be stored longer than one or two days, even at low temperature. PPh 3 + Ph 3 P + - Ph 3 P Y Ph 3 P + + Y Ph 3 P + + Y Y Ph 3 P Y Ph 3 P + Ph 3 P + Y Fig Mechanism of formation of the -protected serine -lactone by Mitsunobu reaction (Y=, ). The concerted attacks in the key step are highlighted in red.
9 B: Discussion and Results 33 The steric tension in the four member ring directs the selectivity of nucleophilic attacks by soft nucleophiles towards the -carbon instead of towards the carbonyl group, as expected for other esters, [44] thus allowing the following synthetic steps. This makes however the lactone extremely prone to decomposition. Indeed, the compound is not stable in the reaction mixture, as it can be attacked by unreacted phosphine or by the excess serine, and significant decomposition occurs even overnight at -20 C; therefore it must be immediately purified by flash-chromatography. Moreover, the compound is sensitive to nucleophilic impurities, particularly to moisture, so that the yield obtained in a humid summer was systematically lower than in winter. Even worse, the compound is extremely sensitive to radicals, even in traces, like the ones which are easily formed by TF or by remnants of the BS used for DMAD formation. In this cases no product was obtained. otwithstanding such serious problems, it was generally possible to reach yields close to literature values (55%). [44] ucleobase alkylation Performing the nucleobase alkylation with the serine -lactone in the presence of a strong, non-nucleophilic base exposes to the risk of loss of the -proton of the lactone with consequent racemisation. The reaction conditions must be carefully studied in order to avoid or minimise this insidious side reaction. The original paper of Eschenmoser [45] was not rich in details on reaction (nucleobase and non-nucleophilic base equivalents, addition order, etc.) and purification conditions, and the same details were missing in the relevant papers of Diederichsen. [47,48b,51] ptimization of the synthesis protocol was therefore necessary and thymine was chosen as the most convenient test nucleobase. owever, after completing the optimization process, a different, detailed alkylation protocol was found in the literature, [109] covering all nucleobases except guanine, even if using sometimes different protecting groups. Such protocols used exactly one equivalent of non-nucleophilic base (a for pyrimidine nucleobases, or DBU for the purines) and required the slow dropwise addition of the serine -lactone to a DMF solution containing a slight excess of the partially deprotonated nucleobase at -78 C. In order to compare the two methods, the alkylation was performed on a preparative scale with both and two series of protected nucleopeptides up to the hexamer were synthesised (see Section 2.3). By analyzing the
10 2. Synthesis 34 optical rotation of the nucleopeptides obtained with the two methods, it was found that the second method was more efficient in preserving the optical purity of the starting material. Therefore the latter method was adopted for the alkylation of protected cytosine and adenine and adapted to the alkylation of 2-amino-6-chloro-purine. After the alkylation the product must be purified from the excess nucleobase and from unwanted alkylation isomers formed in the case of thymine and of the purines (see Fig.2.5) Y -1-9 R Fig Possible alkylation sites for thymine (left) and for the purines (right). For adenine R=, Y= 2 ; for guanine R= 2, Y= ; for 2-amino-6-chloro-purine R= 2, Y= Cl. Straight arrows point to target alkylation sites, while curved arrows point to undesired alkylation sites. It results from the literature [47,115] that high regioselectivity can be obtained for thymine, whereas in the case of the unprotected purines the ratio of the (9)-alkylated product to the (7)-alkylated regioisomer does not exceed 3:1. In the case of adenine, protection does not change significantly the ratio; in the case of guanine, protection can even worsen the situation. Luckily enough, the use of 2-amino-6-chloro-purine allows a good selectivity towards the (9)-alkylated product. [51,104] Separation of most of the unreacted nucleobases from the nucleoamino acid products can be achieved either when the latter are extracted in organic phase from acidic aqueous solutions, or by careful precipitation from suitable solvent mixtures. This operation does not allow a complete elimination of the impurities, since the nucleoamino acids tend to coprecipitate, particularly when they are concentrated. nly in the case of the 2-amino-6-chloro-purine based nucleoamino acids (-AlaG Cl - and -AlaG Cl -) much of the product could be efficiently purified by precipitation and recrystallization. Chromatographic separation is therefore necessary both for the complete elimination of the excess nucleobases and for the separation of the unwanted regioisomers. The latter are particularly difficult to separate, because of their similar chromatographic behaviour compared to the products. The process is further complicated by solubility issues, and by the strong tailing in the column of both product and impurities, even in
11 B: Discussion and Results 35 highly polar eluents containing a significant amount of acetic acid. Generally, repeated flash-chromatographic purifications are necessary in order to obtain a satisfactory amount of acceptably pure product. Despite of the problems described, the synthesis and purification of the four -protected nucleoamino acids and of the -protected thymine-, adenine- and 2-amino-6-chloro-purine-based nucleoamino acids was achieved. Fig. 2.6 schematically presents the products obtained and the alkylation conditions, while Graph 2.1 gives the yields for the - and -protected derivatives containing the four nucleobases. -AlaC - Y Y -AlaT- -AlaT- DMF, -78 C to rt -T, a -C, a Y DMF, -78 C to rt -G Cl, DBU DMF, -78 C to rt 2 DMF, -78 C to rt -A, DBU Y Y Cl -AlaG Cl - -AlaG Cl - -AlaA - -AlaA - Fig Conditions for the synthesis of the -protected nucleoamino acids (Y= or ). Graph Yields of nucleoamino acid synthesis as a function of the nucleobase and of the - protecting group adopted. The synthesis of the --protected cytosine-based nucleoamino acid was not performed.
12 2. Synthesis 36 As it can be seen from the graphic, good yields were achieved with -AlaC - and - AlaG Cl -, moderate yields were obtained with the thymine-based nucleoamino acids and with -AlaG Cl -, and poor yields were obtained with the adenine-based nucleoamino acids. In general there is little difference between - and - protected nucleoamino acids, except for the two 2-amino-6-chloro-purine derivatives. In this case the very low solubility of the nucleobase-containing compounds in organic solvents cooperates with the general stronger ease of precipitation of the -protected derivatives as pure compounds compared to -protected ones to allow a more efficient purification process. 2.3 Synthesis of rigid nucleopeptides After the synthesis and purification of the nucleoamino acids, the building blocks for the solution-phase synthesis of the rigid nucleopeptides were available. The synthetic strategy chosen was to form nucleo-tripeptides by step-by-step synthesis, attaching one residue at time, before condensing suitably deprotected tripeptide units to obtain longer nucleopeptides. In order to perform the synthesis of the nucleo-tripeptides, each containing two hindered Aib residues, efficient methods of amino acid activation for the formation of the peptide bond (coupling) were required Coupling methods Three decades of research on C -tetrasubstituted amino acid containing peptides have provided the laboratory of Padova with a strong know-how about the activation of hindered, C -tetrasubstituted residues. [73] The two most common and powerful activation methods are the in situ formation of active esters (via EDC/At [116], Fig. 2.7, left and middle) and the use of preformed symmetric anhydrides (Fig. 2.7, right). Cl + c EDC. Cl X At (-Aib) 2 Fig Coupling reagents (EDC Cl, left; At, X=, Bt, X=C, middle) for the formation of active esters and symmetric anhydride derived from -Aib- (right).
13 B: Discussion and Results 37 Active esters are easily formed, the use of At efficiently suppresses racemisation due to carbodiimide overactivation, [116] and the side products deriving from the coupling reagents can be eliminated by acidic or basic washing. owever, the unreacted active ester is not completely hydrolyzed or eliminated by washing and often chromatographic purification is necessary. n the contrary, couplings via symmetric anhydride form a smaller number of side products, resulting in a simpler work-up, but they require more steps (anhydride synthesis and purification, coupling, sometimes -protected amino acid recovery). Indeed, 2 equivalent of -protected amino acid are required in order to generate 1 equivalent of acylating agent, and 1 equivalent of amino acid salt is formed after the coupling, thus making the amino acid recovery necessary when working with large amounts or valuable compounds. The active ester coupling method was therefore always used for the activation of the nucleoamino acids, while the activation as symmetric anhydride was often employed for Aib activation. Large scale couplings involving Aib activation and couplings between protein amino acids were performed via active esters, using the less expensive Bt [117] (Fig. 2.7, middle) instead of At Synthesis of protected nucleo-tripeptides The step-by-step synthesis of the thymine-based protected nucleo-tripeptide -Aib- AlaT-Aib- t Bu was smoothly achieved by coupling the activated nucleoamino acid with -Aib- t Bu to form the protected nucleo-dipeptide -AlaT-Aib- t Bu, which was then hydrogenated and acylated with the symmetric anhydride (-Aib) 2 -, as shown in Scheme 2.1. The same strategy was not efficient in the case of the adenine-based nucleopeptides. Indeed, during the step of dipeptide deprotection, while the 6 - protecting group was rapidly removed by catalytic hydrogenation, the removal of the - group was extremely slow, so that the reaction was not complete even after 7 hours (Fig. 2.8), and the coupling yield was negatively affected. Indeed slow hydrogenolysis of protecting groups next to the two most aromatic bases (adenine and cytosine) have been reported, [109] probably due to stacking interactions between the nucleobase and the phenyl ring hindering the removal of the latter.
14 2. Synthesis 38 2, Pd/C Me, fast 2, Pd/C Me, slow Fig Removal of the two protecting groups from -AlaA -Aib- t Bu by catalytic hydrogenolysis with undesired chemoselectivity. The - protecting group was therefore abandoned for the adenine-based nucleopeptides and not even tried for the cytosine-based ones. n the other side, the synthesis of both nucleotripeptides using the orthogonal / protecting groups was performed without problems (Scheme 2.1b). (a) (b) Aib AlaT Aib Aib AlaB Aib ( iii ) 2 v iv ii i t Bu t Bu t Bu t Bu t Bu ( iii ) 2 v vii Cl. vi ii Me Me Me Me Me Scheme 2.1. Reaction conditions for the synthesis of the protected nucleo-tripeptides -Aib- AlaT-Aib- t Bu (a), -Aib-AlaA -Aib-Me and -Aib-AlaC -Aib-Me (b, AlaB= AlaA and AlaC, respectively). (i): 2, Pd/C in DCM; (ii): EDC/At, MM, p 8, DMF; (iii): EDC in DCM; (iv): 2, Pd/C in Me; (v): MM, p 8, MeC; (vi): 1 eq MM; (vii) 20% TFA in DCM. Protected nucleopeptides are much more lipophilic than the corresponding nucleoamino acids, therefore they are easier to handle and purify. The partial purification of the thymine-based nucleopeptides by precipitation was possible, particularly in the case of the tripeptide. Accordingly, the coupling yields were quite satisfactory (>60%) for the three groups of nucleopeptides. Small amounts of the protected nucleo-tripeptides were used to obtain nucleopeptides with unprotected nucleobases and without other aromatic chromophores, in order to facilitate conformational analyses and pairing tests by CD. This was easily
15 B: Discussion and Results 39 achieved by catalytic hydrogenolysis of the group on cytosine and adenine, as well as on the of the thymine-based nucleopeptide (which was replaced by a blocking acetyl moiety). Similarly, small amounts of the thymine- and cytosine-based nucleoamino acids were employed to synthesise the corresponding methyl esters using EDC/DMAP in Me, in order to obtain nucleoamino acid derivatives soluble in chloroform, useful for IR conformational analyses. The synthesis of the protected guanine-based nucleo-dipeptide lead to an undesired result, since the Cl 6 on the nucleobase was replaced by At used for the nucleoamino acid activation (Fig. 2.9). This was not completely a surprise, since chlorine replacement by nucleophiles during the activation of nucleotide analogues containing 2-amino-6-chloro-purine had been reported before. [118] owever, since the synthesis of the guanine-based nucleoamino acid methyl ester was performed smoothly, following the same protocol adopted for the other nucleobases, it was hoped that the replacement would not have happened during the coupling. It seemed appealing to use the aromatic ether formed as an useful derivatization in order to increase the lipophilicity of the guanine-based nucleopeptides. A small scale - hydrogenolysis trial showed however that a complex mixture was formed, where together with traces of the desired derivative -AlaG At -Aib-Me, dipeptides containing unprotected guanine and even 2-amino-purine were formed (Fig. 2.9) EDC, At, MM DMF 2 -AlaG At -Aib-Me Cl -AlaG Cl - 2, Pd/C TFA/ 2 3:1 -AlaG -Aib-Me 2 Fig Replacement of the Cl 6 -atom by an At derived group and methods of removal of the aromatic moiety. 2 -AlaG -Aib-Me
16 2. Synthesis 40 Considering that 6 -Bzl guanine is easily deprotected under acidic treatment, [97] the complete removal of the aromatic moiety by acidic hydrolysis was decided, following the protocol reported for the deprotection of 2-amino-6-chloro-guanine to guanine. [104] The deprotection, although slow, occurred without side reactions, and the nucleodipeptide with free guanine -AlaG -Aib-Me was obtained. This is a extremely hydrophilic derivative, therefore it was not possible to eliminate the At coproduct by acidic wash as usual, and flash-chromatographic purification with a highly polar eluent mixture was necessary. Both to obtain a proof of concept of the viability of the - deprotection of this derivative and to prepare a nucleo-tripeptide with free guanine and without other aromatic moieties for CD characterization, like the ones prepared for the other nucleobases, a small amount of -AlaG -Aib-Me was hydrogenated and coupled with excess activated -Aib- (Scheme 2.2). The purification of the nucleotripeptide formed was not possible by standard work-up based on acidic and basic washes, given the high polarity, and even flash-chromatography proved not sufficient to separate it completely from some of the At used for Aib activation owever, by preparative PLC an efficient purification was achieved, although with a rather poor yield. Probably the choice of the symmetric anhydride coupling method instead of the active ester formation for this step could have allowed an easier purification and consequently a higher yield. Aib AlaG Aib ii iv Cl. Cl i At ii iii Me Me Me Me Me Me Scheme 2.2. Reaction conditions for the synthesis of the guanine-based nucleotripeptide - Aib-AlaG -Aib-Me. (i): 1 eq MM; (ii) EDC/At, MM, p 8, DMF; (iii) TFA/ 2 3:1, 3 d; (iv): 2, Pd/C, Me Synthesis of nucleopeptides containing a C-terminal lysine
17 B: Discussion and Results 41 To enhance the water-solubility of deprotected rigid nucleopeptides, the incorporation of a C-terminal lysine amide was planned. This was performed on the pyrimidyl based derivatives, which had proven easier to handle. Since the C-terminal carboxyl group is blocked as an amide, orthogonal protecting groups are necessary only for the -amino and for the side chain functional groups. In the case of the thymine-based nucleopeptides, the group was conserved for the, while the acid labile group was used for the -amino group of lysine; in the case of cytosine, the same - and side-chain protecting groups adopted for the synthesis of its protected nucleo-tripeptides were used. Suitably protected lysine amides were thus synthesised, -deprotected and coupled with activated -protected Aib residues, forming protected Aib-Lys dipeptides. The synthesis of protected C-terminal lysine-amide nucleo-tetrapeptides was then performed by using the -deprotected dipeptides as done for the Aib esters in the case of the synthesis of the nucleotripeptides (refer to Scheme 2.1). The presence of lysine hindered the purification by precipitation and yields were generally lower, in particular in the case of the cytosine containing nucleopeptides (40-45%) Synthesis of nucleopeptides bearing two nucleobases The synthesis of such nucleopeptides by fragment condensation was performed on the thymine-based nucleopeptides for several reasons: firstly, it was used as a way to check the preservation of the optical purity of the serine -lactone in the alkylations performed with different protocols, then large quantities of the protected thymine containing nucleo-tripeptide were available, finally the ideal set of protecting groups was present. n the other side, in the case of the nucleo-tripeptide methyl esters, the issue of racemisation-free saponification is raised. To devise a safe protocol, the saponification of the model peptide -Ala-Aib-Me to -Ala-Aib- was studied: as the protected nucleotripeptides, this compound has a chiral residue in the middle of the sequence, while the methyl ester is bound to the hindered Aib residue. The optical purity of the acid obtained was evaluated by comparison of its specific optical rotation with the value of the same derivative obtained by acidolysis of -Ala-Aib- t Bu, which is considered a racemisation-free procedure. It was found that the use of 2 equivalent of Li 0.05 M
18 2. Synthesis 42 in a 1:1 2 /Me mixture at 0 C lead to the quantitative saponification of the dipeptide without loss of optical purity (Fig. 2.10). 2 eq Li 0.05 M 3 + P C* R 1:1 Me/ 2, 0 C 2 P C* R Fig Racemisation-free protocol for Aib methyl ester saponification in peptides containing C -trisubstituted residues. With this result in mind, it was chosen to perform first the optimization of the fragment condensation conditions for the thymine-based nucleopeptides, and to defer the synthesis of peptides containing the other nucleobases. top). [107] When a peptide is activated, generally a 5-(4)-oxazolone is formed (Fig. 2.11, This species is less reactive than an active ester or an anhydride, therefore generally couplings involving peptide segments are performed under refluxing conditions. xazolones of C -trisubstituted amino acids racemise easily by loss of the C -proton and generation of a delocalized anion, but this is not possible for the C -tetrasubstituted Aib. In this case, however, racemisation of the residue next to the oxazolone can occur by a similar mechanism (Fig. 2.11, bottom). This side reaction can be favoured by basic conditions (p> 8.5) and by heating. R R' R'' Y R R' + R'' + Y - B R R' R'' + B. -Y R 1 R 2 C 3 C 3 R 1 B R 2 - C 3 C 3 Fig Top: formation of the oxazolone (or (4)-oxazolidin-5-one) by activation of a peptide (Y= activating group); bottom: racemisation of the residue next to the oxazolone, favoured by the generation of a delocalized anion.
19 B: Discussion and Results 43 Initially, the thymine containing nucleo-hexapeptide was synthesised; both the -terminal and the C-terminal fragment were obtained by the protected thymine based nucleotripeptide, respectively by acidolysis of the tert-butyl ester and by catalytic hydrogenolysis of the protecting group. The nucleo-tripeptide acid thus formed was activated to the corresponding oxazolone with EDC and the coupling with the C-terminal fragment was performed at room temperature in DMF with the addition of the minimum amount of base to keep the p around 8, to reduce the risk of racemisation. The reaction was slow (taking about two weeks), but a good yield of the nucleo-hexapeptide -(Aib- AlaT-Aib) 2 - t Bu was obtained (around 60%). The process was repeated three times using nucleoamino acid obtained with three different alkylation protocols (the optimized protocol derived from the method of Eschenmoser, the same protocol with a different nonnucleophilic base and the detailed protocol found in the literature). Since in the nucleo-hexapeptide two chiral centres are present, in case of partial racemisation the four possible stereoisomers (the expected L,L, the epimerized L,D and D,L, as well as the D,D peptide) are formed. Two diastereoisomeric pairs can be therefore separated by analytical PLC and this allows the evaluation of the raceme fraction in the starting material under the assumption that no racemisation occurred during the fragment condensation. If racemisation did occur at that stage, the optical purity of the nucleoamino acid material would be underestimated. Setting x as the fraction of D-nucleoamino acid in the starting material (or 1-x as the optical purity) and Y as the fraction of the D,L and L,D diastereoisomers in the nucleo-hexapeptides, it is straightforward to write: Y= 2(1-x)x= 2x-2x 2 x= 1/2- (1/4-Y/2) (1-x)= ½+ (1/4-Y/2) In the case of trace racemisation (x<<1): Y= 2(1-x)x 2x x= Y/2 (1-x)= 1-Y/2 These equations were applied to the chromatograms of the nucleo-hexapeptides obtained using the nucleoamino acid synthesised with the three different protocols and it was found that the low-temperature protocol is the best for preserving the optical purity. Results are detailed in Table 2.1, while Fig shows the chromatograms for the original method by Eschenmoser and for the best method. This result was in good agreement with the polarimetric measurements performed on the protected di- and tripeptides obtained following the different protocols. It can be inferred that the control of the alkylation conditions is crucial for preserving the optical purity and that the low-temperature protocol can significantly reduce racemisation but not suppress it completely.
20 2. Synthesis 44 Table 2.1. Diastereoisomeric ratios, epimeric fraction and molar fractions of D and L nucleoamino acid obtained for the three methods of nucleobase alkylation tested in the case of thymine evaluated by PLC of the hexapeptides. The low-temperature protocol allowing the highest optical purity is in bold font. Alkylation Conditions (D,L+L,D)/ (L,L+D,D) Y xd xl=1-xd K 2 C 3, rt DBU, rt a, -78 C (a) (b) Fig Analytical chromatograms of the hexanucleopeptide synthesised using the nucleo amino acid obtained with the optimized protocol derived from Eschenmoser (a) and with the low temperature protocol (b). The peak at t r min in (a) and the shoulder at about t r min in (b) belong to the peptide epimers. PLC Agilent, % B in 30 min.
21 B: Discussion and Results 45 The synthesis of a lysine amide blocked nucleo-heptapeptide was then performed. In this case the same nucleo-tripeptide acid was used as -terminal fragment as for the last synthesis, whereas hydrogenolysis of -Aib-AlaT-Aib-Lys()- 2 afforded the C- terminal fragment (see Scheme 2.3). The coupling was performed in MeC under refluxing conditions because of the lower reactivity observed for the lysine containing nucleopeptides. After the flashchromatographic treatment, the PLC analysis evaluated the purity crude purity as around 70%, detecting minor quantities of several diastereoisomers, therefore it is likely that partial racemisation occurred. Unfortunately it was not possible to find elution conditions which allowed an efficient preparative PLC purification of the protected peptide, nor of the - or -deprotected derivatives. The crude peptide was therefore completely deprotected and at this stage preparative PLC was performed, obtaining the deprotected nucleo-heptapeptide -(Aib-AlaT-Aib) 2 -Lys()- 2 as its TFA salt at 95% purity (Fig. 2.13). -terminal fragment C-terminal fragment Aib AlaT Aib Aib AlaT Aib Lys i ( iv 2 ii t Bu 2 ii iii t Bu 2 iv v t Bu 2 vi v iv ) 2 t Bu 2 vii vi v t Bu viii ( ) 2 vii 2 ix 2 xl x xi 2 Scheme 2.3. Reaction conditions for the synthesis of 2 TFA -(Aib-AlaT-Aib) 2 -Lys()- 2. (i): EDC/Bt, 3(g) in DCM; (ii): 2, Pd/C, DCM; (iii) EDC/Bt, MM, p 8, DCM; (iv): EDC/At, MM, p 8, DMF; (v): 2, Pd/C, Me; (vi): EDC, DCM; (vii) MM, p 8, MeC; (viii) 50 % TFA in DCM; (ix) EDC in DMF; (x) MM, p 8 in MeC, reflux; (xi) 20 % TFA in DCM.
22 2. Synthesis 46 The viability of the fragment condensation approach for the synthesis of longer nucleopeptides had thus been demonstrated, even if optimization of the synthetic protocol might be necessary for application to the other nucleobases. Fig Analytical chromatogram of TFA -(Aib-AlaT-Aib) 2 -Lys()- 2 after preparative purification. PLC Agilent, % B in 30 min. 2.4 Synthesis of flexible nucleopeptides Model and functionalised flexible nucleopeptide libraries were designed and prepared. The term library refers to a set of compounds with similar structure synthesized by parallel synthesis. Firstly, a convenient solid support for the solid-phase peptide synthesis (SPPS) of the flexible nucleopeptides by /Bzl strategy was identified. MBA (4- methylbenzhydrylamine) resin was used, as the peptides are not cleaved under the reaction conditions used for - deprotection. Moreover, following cleavage from the resin, peptides are released as their C-terminal amides, thus avoiding the formation of a negative charge, which could decrease its affinity towards the polyanionic nucleotides. A semiautomatic peptide synthesizer conceived for /Bzl strategy was used for the synthesis of the nucleopeptide sequences, whereas cleavage and side-chain derivatization (when required) were performed manually Model nucleopeptide libraries
23 B: Discussion and Results 47 The synthesis started with the structurally simpler model libraries in order to assess an optimal synthetic protocol. It was decided to prepare model nucleopeptides based on one pyrimidine and on one purine base (thymine and adenine respectively), to obtain a representative and complementary nucleobase subset without wasting too much of the nucleoamino acids in the synthesis optimization process. The first library, used both as a proof of concept of the viability of the solid-phase synthesis and for the research of suitable activation methods and reaction condition, was made of two nucleo-hexapeptides, based on thymine and on adenine, each containing twice the same Ala-AlaB-Ala tripeptide units Synthetic protocol optimisation The synthesis was performed on a 10 mol scale by amino acid activation via active esters formed with BP/Bt [119] and DIEA was used as a base. This is a widespread protocol for solid-phase peptide synthesis using the /Bzl strategy. [120] Couplings were repeated twice and 5 equivalents of proteogenic amino acid and 3 equivalents of nucleoamino acid were used per coupling. Longer coupling times in the case of the nucleoamino acids (1 hour against minutes) were used to compensate the smaller excess added in order to spare the valuable building blocks. After completing the peptide sequences, the products were cleaved from the resin by treatment with a mixture of TFA, trimethylsilyl trifluoromethanesulfonate and para-chresol overnight. [121] The use of this cleavage cocktail allows simultaneous cleavage from the resin and removal of all benzyl-based protecting groups and it is much easier to handle than F, being liquid at room temperature and less aggressive. After recovering the peptides from the cleavage solutions by precipitation in cold, the PLC analysis showed acceptable purity of the crudes and purification through preparative PLC was not difficult. The yields, however, were modest (around 5 %). A second model nucleo-heptapeptide library was designed: its nucleopeptide components were made of two tripeptide units as before, but a lysine was added at the C- terminal (Fig. 2.14, top). This was not necessary for the water-solubility of the peptides themselves, as the peptides of the first library were well soluble in water, but it was meant as a test for the synthesis of longer, potentially less soluble, nucleopeptides, to be sure that the introduction of lysine would not have caused unexpected problems.
24 2. Synthesis 48 Assuming that the not satisfactory yields obtained with the first library were due to insufficient nucleoamino acid activation, a more energetic activation protocol for the formation of the nucleoamino acid active esters was adopted. Thus ATU/At [122] were employed, while the activation of the proteogenic residues was performed with the usual method. The synthesis was performed on 25 mol scale, in order to obtain sufficient amounts of nucleopeptides for conformational analyses, not only by spectroscopic techniques, but also by 2D MR Incorporation of an Aib residue by SPPS As mentioned in the Introduction, generally short nucleopeptides made of C -trisubstitued residues are unfolded, while the presence of C -tetrasubstitued residues in a C -trisubstitued residue-based sequence can substantially improve the conformational stability of the peptides. To evaluate the ability of Aib to structure flexible nucleopeptides, two more nucleopeptides were added to the library, each one carrying an Aib residue in the middle of its sequence (Fig. 2.14, bottom). The central location was chosen in the hope that it would have exerted deeper effects on the peptide conformation than peripheric positions. + 3 T2A A2A T2U A2U Fig Flexible nucleo-heptapeptides based on thymine and adenine (A2A and T2A, top), and analogues containing an Aib residue (circled) at position 4 (A2U and T2U, bottom). Two positions were in principle available (3 and 4), both next to a nucleoamino acid (AlaB 2 or AlaB 5, respectively). Since the increased steric hindrance of Aib affects more the reactivity of the amino- than that of the carboxyl-group, it was thought that coupling the
25 B: Discussion and Results 49 activated Aib on a deprotected -terminal nucleoamino acid would have been less difficult than coupling an activated nucleoamino acid on the deprotected -terminal Aib. Position 4 rather than position 3 was therefore chosen. The incorporation of an Aib residue in a peptide sequence by SPPS techniques is not easy. [123] To achieve an efficient activation, allowing good coupling yields, the same ATU/At activation protocol used for the nucleoamino acids was applied to Aib, using a fivefold excess of the amino acid, and the coupling was repeated three times. Moreover, the coupling times for the following residue (Ala 3 ) were doubled. After cleavage, recovery and purification of the nucleopeptides, very inhomogeneous results were obtained. The thymine-based nucleopeptide without Aib was obtained and purified in good yield (29 %), while the purification of the adenine-based nucleopeptide without Aib was not successful, due to impurities very close to the product peak. n the other side, it was possible to purify the two Aib containing analogues, although with a low yield (2 %). The synthesis of the thymine-based Aib containg analogue was then repeated and this time two different protocols were used in parallel, each on a 15 mol scale. ne protocol was the same used the first time, the second used BP/Bt activation for all amino acids apart from Aib, activated with ATU/At. This was to test whether the ATU/At activation was too powerful for the nucleopeptides, particularly given the longer coupling times necessary. After cleavage, the PLC chromatograms of both crudes were quite similar (Fig. 2.15), however the crude yield obtained with the second protocol was significantly higher. Fig Analytical PLC chromatograms of the crude Aib containing thymine-based nucleoheptapeptide T2U obtained using ATU/At (a) or BP/Bt (b) for the nucleamino acid activation. PLC Varian, 0-50 % B in 20 min.
26 2. Synthesis 50 After purification, the yield obtained with the first protocol was half of the yield obtained with the second (13 % and 26 %, respectively), however even the lower yield was acceptable, given the presence of Aib in the sequence. n the other side, from this result, it is not possible to infer that the second protocol is positively better than the first one. In particular, it seemed strange that only the crude yields were different, while the crude purities were similar (about 45 % and 55 %, respectively) Functionalised nucleopeptide libraries In order to prepare guanine containing flexible nucleopeptides, it was decided to convert the 2-amino-6-chloro-purine-based nucleoamino acid -AlaG Cl - into its guaninebased derivative -AlaG - before use in the solid-phase synthesis, as reported in the literature for its superior homologues. [51] Since the reaction is performed under strongly acidic conditions, the - protecting group was also removed and it was necessary to reprotect the free nucleoamino acid thus formed (Fig.2.16). Cl 2 TFA/ 2 3:1 3d TFA , a 2 C 3 2 /diox, 3d 2 Fig Conversion of -AlaG Cl - to -AlaG - through acidic deprotection and reintroduction of the - protecting group. This was done by the use of 2, adapting standard procedures for -amino acid protection. [124] Given the extreme polarity of the derivative and the presence of the free 2 2 on guanine, the total yield for both the deprotection and the reprotection step was acceptable (63%). After gaining some experience on the behaviour of the nucleoamino acids on solidphase, the design of a functionalised nucleopeptide library containing all four DA nucleobases was addressed. The nucleopeptides of the library were constituted of four repeating tripeptide units Ala-AlaB-Ala and of two lysines set at the C- and -termini. While the C-terminal lysine
27 B: Discussion and Results 51 was introduced only for solubility reasons, the -terminal lysine was introduced also for furhter functionalisation of its side chain. -Lys(2Cl-)- was used for the introduction of the C-terminal lysine, whereas -Lys(Fmoc)- was used for the introduction of the -terminal lysine. It was therefore possible to selectively deprotect and derivatise the -amino group of the -terminal lysine after completion of the peptide sequence by treatment with a secondary amine (20 % piperidine in DCM). Thus, by splitting the resin beads containing to each nucleopeptide, deprotecting peptide -terminal lysine -amino groups and reacting them with different moieties, nucleopeptides with different functionalisations could be obtained from a single solid-phase synthesis run. The -terminal, rather the C-terminal, lysine was functionalised because it allowed the derivatization of the last, and therefore most accessible, residue of the peptide chain after the sequence was completed. The side-chain derivatization was performed with biotin and with a fluorophore, (fluoresceine isothiocyanate, FITC). The first functionalisation was meant for obtaining biotinylated nucleopeptides suited both for surface plasmon resonance pairing experiments and for biological testing, the second mainly for biological tests. The synthesis was planned on a 30 mol scale for each peptide sequence. By splitting, resin beads would have been divided in three equal portions, one not further derivatised, one for biotinylation, one allowed to react with FITC. Fig represents the general structure of the library members and the molecules used for their functionalisation. B4f (B= A, C, G, T; f=, Bt, FIT) 2 -Lys(f)-[Ala-AlaB-Ala] 4 -Lys()- 2 2 B B B B 2 S C f Fluoresceine isothiocyanate (f=fit) S D-biotin (f=bt) Fig Structure of the members of the functionalised library and of the molecules used for their functionalisation. ucleopeptides containing each four times one of the four nucleobases were derivatised with two different moieties or left underivatised, thus forming 4*(2+1)= 12 different nucleopeptides. A fifth group of nucleopeptides carrying three cytosines and one thymine in its sequence was also prepared.
28 2. Synthesis 52 BTU/Bt [125] were used for active ester formation for all amino acids, since side reactions with guanine derivatives were reported by using phosphonium-based coupling reagents, like BP. [118] This activation method has an intermediate reactivity between the ATU/At and BP, therefore it was considered a suited general purpose protocol for the synthesis. Biotin was coupled to the -amino group of lysine by activating its carboxyl group with BTU/Bt, like the carboxyl groups of the protected amino acids. FITC, being an electrophilic isocyanate, does not require activation to attack free amines, therefore only p adjustment to about 8 was necessary. In order to prepare a single-base mismatch model, a nucleopeptide containing two different nucleobases in its sequence (one thymine in position 5 and three cytosines in position 2, 8 and 11) was designed and derivatised in the same way as the homobase nucleopeptides. The crude mixtures were rather complex, making purification difficult. Therefore the yields of the 15 functionalised nucleopeptides after purification were modest, ranging between 3 % and 11 %. The (formal) average yields per step including the purification were however comparable with the yield obtained for the best nucleoheptapeptide prepared by SPPS (78-85 % against 84 %). nly the purification of the guanine-based biotinylated peptide was not possible, because the lyophilized crude product turned insoluble in any solvent mixture. A reason could be the well-known tendency to aggregation of guanine derivatives, [97,116] maybe increased by some serendipitously strong interaction with the biotin ring. Apart from that case, the water solubility of the underivatised nucleopeptides was high and even the more lipophilic FITC-derivatised peptides were well soluble up to 1 mm. In some cases the derivatised nucleopeptides were obtained in higher yields compared to the underivatised ones. This could be due to side reactions due to the free - amino group of the -terminal lysine in basic environment before the cleavage. Yields for all the derivatives are presented in Table 2.2. After this library, a novel library was designed, composed of thymine-based nucleopeptides containing five tripeptide units between two lysines (Fig. 2.18, top). Thymine was chosen for the preparation of this longer nucleopeptides because it had proven the less problematic nucleobase (indeed it had the highest average yield among the five nucleopeptide sequences tested, Table 2.2, last row) and because thymine-containing peptides had shown interesting pairing properties in the surface plasmon resonance experiments (see Section 4). If pairing is cooperative, a peptide with five nucleobases
29 B: Discussion and Results 53 should display a much stronger binding than a peptide with four. Partial biotinylation of the peptide was therefore planned to carry on pairing tests. Moreover, a cysteine containing, thymine-based (see Fig. 2.18, middle) nucleopeptide was designed to prepare a nucleopeptide dimer linked by a disulphide bridge, as already reported in the literature with other nucleotide analogues of peptide-like structure. [58] Table 2.2. Yields of the various derivatised nucleopeptides obtained after purification. Yield (%) A4 C4 G4 T4 CTCC Bt FIT Average otes. Colums refer to the nucleobase sequence of the peptides (A4: adenine-based; C4: cytosinebased; G4: guanine-based; T4: thymine-based; CTCC: mixed base peptide), rows to the derivatization (: underivatised; Bt: biotinylated; FIT: reacted with fluoresceine isothiocyanate). The last row reports the average yield for the three derivatives. This dimer molecule would have carried ten bases (on two strands) and it could in principle have paired with complementary, adenine-based nucleopeptides or nucleotides both by independent binding of the two strands to form Watson-Crick bonds with 1:1 stoichiometry and by cooperative pairing of both strands, one by Watson-Crick, the other by ogsteen interactions, with the same complementary sequence (triplex formation). The latter possibility is represented in Fig. 2.18, bottom. The formation of a homodimer through a disulphide bridge can be achieved both on solid-phase and in solution. In the first case the protecting group for the thiol function is removed under oxidizing conditions when the peptides are still bound to the resin, so that a disulphide bridges are formed between next molecules. In the second case the peptide is synthesized, cleaved and purified as a monomer, then allowed to dimerize in solution. In this case, either the thiol protecting group is stable to the cleavage and removed after the purification stage, or the cleavage and the purification are performed under non-oxidizing conditions. The on-resin dimerization requires one purification step less than the dimerization in solution, on the other side the dimer is generally less pure and solubility problems can appear during the purification. Therefore, it was chosen to perform the dimerization in solution, using a protecting group that was removed during the cleavage,
30 2. Synthesis 54 and to purify the product under non-oxidizing conditions in order to allow the dimerization only of pure product. -Lys()-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Lys()- 2 T5 -Cys()-Lys()-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Lys()- 2 Caratterizzazione e prove biologiche sui T5S 1) air oxidation 2) A5 -Cys-Lys()-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Lys()- 2 S S -Lys()-Ala-AlaA-Ala-Ala-AlaA-Ala-Ala-AlaA-Ala-Ala-AlaA-Ala-Ala-AlaA-Ala-Lys()- 2 A5 -Cys-Lys()-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Ala-AlaT-Ala-Lys()- 2 T5S-S5T Fig Structure of the underivatised nucleopeptide containing five thymine bases (T5, top) and of its cysteine containing analogue (T5S, middle). Bottom: planned formation of a nucleopeptide dimer by disulphide bridge and triplex formation with a complementary adenine-based nucleopeptide (not succesfully synthesized). In the /Bzl strategy, cysteine is generally introduced as -Cys(Acm)-: the [126] acetamidomethyl protecting group (Acm) is removed by I 2 or other oxidising agents. Working in solution, the removal of the excess of oxidising reagent can be difficult, therefore this way was discarded. Since cysteine was introduced as the -terminal residue, it was possible to use Fmoc-Cys(Trt)-, whose thiol-protecting trityl group is easily cleaved during the cleavage, while its -protecting Fmoc group was deprotected manually prior to the cleavage from the resin. The presence of p-cresol as a scavenger in the cleavage cocktail protected the free thiol from oxidation. Since no derivatization of its -terminal lysine (Lys 2 ) was planned, the latter was introduced as -Lys(2Cl-)-, to avoid possible side-reactions due to the simultaneous formation of two free groups under basic conditions before the cleavage. The synthesis scale was set to 10 mol for the biotinylated derivative and to 22 mol for the underivatised and the cysteine-containing peptide. The activation protocol employed BP/Bt for -protected protein aminoacids and ATU/At for the nucleoamino acids and for Fmoc-Cys(Trt)-, because it is well known that -Fmocprotected amino acids are less reactive than the corresponding --protected derivatives. [107]
31 B: Discussion and Results 55 The crude mixtures were complex (Fig. 2.19a), as expected from the experience with the shorter nucleopeptides of analogous mixture. Purification of the three derivatives was successful (Fig. 2.19b), even if difficult, and yields were consequentially low (1-3 %). It was tried to obtain an adenine-based nucleopeptide with similar structure to perform pairing test on the adenine-thymine pair, but the synthesis failed completely (the PLC-MS analysis of the crude mixture showed no traces of the expected product). Fig Analytical PLC chromatograms of the crude (a) and purified (b) underivatised thymine based nucleopeptide T5. PLC Varian, 0-50 % B in 20 min Trials of cysteine-mediated nucleopeptide dimer formation trials After the synthesis and purification of the cysteine containing thymine-based nucleopeptide T5S, tests of its oxidative dimerization were carried on samples with purity % obtained during the purification process together with the best fraction (purity higher than 95 %). The monomer peptide was dissolved in 2 /DMS 9:1 at p 8-9 and the solution was stirred to favour oxygen saturation, while the reaction course was monitored by PLC. Soon after the dissolution, close to the monomer peak and with a slightly longer retention time, as expected, the dimer peak appeared. Its relative area increased with time until about 2 hours after dissolution, as it represented about 45 % of the total area, then the increase stopped and the relative area of the dimer compared to the monomer seemed to decrease (Graph. 2.2). A closer investigation showed that on the walls of the reaction vessel a precipitate was present, which proved completely insoluble both in aqueous solutions at different p and in every polar organic solvent mixture tried. It was assumed that when the dimer concentration passes a given threshold value, it starts to aggregate and precipitate in
Supporting Information For:
 Supporting Information For: Peptidic α-ketocarboxylic Acids and Sulfonamides as Inhibitors of Protein Tyrosine Phosphatases Yen Ting Chen, Jian Xie, and Christopher T. Seto* Department of Chemistry, Brown
Supporting Information For: Peptidic α-ketocarboxylic Acids and Sulfonamides as Inhibitors of Protein Tyrosine Phosphatases Yen Ting Chen, Jian Xie, and Christopher T. Seto* Department of Chemistry, Brown
Supporting Information
 Supporting Information Syntheses and characterizations: Compound 1 was synthesized according to Scheme S-1. Scheme S-1 2 N N 5 i N 4 P Et Et iii N 6 ii P Et Et iv v, vi N N i) Fmoc-Su, DIPEA, Acetone;
Supporting Information Syntheses and characterizations: Compound 1 was synthesized according to Scheme S-1. Scheme S-1 2 N N 5 i N 4 P Et Et iii N 6 ii P Et Et iv v, vi N N i) Fmoc-Su, DIPEA, Acetone;
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
1. Draw the structure of oxazolone formed upon activation of N-Acetylvaline
 Peptide quiz 1 (5 questions for 10 minutes) 10 points max 1. Draw the structure of oxazolone formed upon activation of -Acetylvaline 3 C 3 C 2. The following DKP (1) is prepared by cyclisation of dipeptide
Peptide quiz 1 (5 questions for 10 minutes) 10 points max 1. Draw the structure of oxazolone formed upon activation of -Acetylvaline 3 C 3 C 2. The following DKP (1) is prepared by cyclisation of dipeptide
26.7 Laboratory Synthesis of Peptides
 S Hornback_Ch26_1123-1161 12/15/04 8:18 PM Page 1148 1148 CHAPTER 26 AMI ACIDS, PEPTIDES, AD PRTEIS A chain B chain Gly Ile Val Glu Intramolecular disulfide bridge Gln Cys S S Cys Thr Ser Ile Cys Ser Leu
S Hornback_Ch26_1123-1161 12/15/04 8:18 PM Page 1148 1148 CHAPTER 26 AMI ACIDS, PEPTIDES, AD PRTEIS A chain B chain Gly Ile Val Glu Intramolecular disulfide bridge Gln Cys S S Cys Thr Ser Ile Cys Ser Leu
Under strongly acidic conditions at ph = 1 every functional group in phosphoserine that can pick up a proton, does.
 1. (48 pts) a. L-Phosphoserine is a modified amino acid that is generated in proteins by phosphorylation of serine residues. The amino acid side chain has two acidic protons, which exhibit different pk
1. (48 pts) a. L-Phosphoserine is a modified amino acid that is generated in proteins by phosphorylation of serine residues. The amino acid side chain has two acidic protons, which exhibit different pk
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
molecules ISSN
 Molecules 2003, 8, 467-471 Second Eurasian Meeting on eterocyclic Chemistry eterocycles in rganic and Combinatorial Chemistry molecules ISS 1420-3049 http://www.mdpi.org Solid-Phase Synthesis of Methyl
Molecules 2003, 8, 467-471 Second Eurasian Meeting on eterocyclic Chemistry eterocycles in rganic and Combinatorial Chemistry molecules ISS 1420-3049 http://www.mdpi.org Solid-Phase Synthesis of Methyl
Module No. 31: Peptide Synthesis: Definition, Methodology & applications
 PAPER 9: TECHNIQUES USED IN MOLECULAR BIOPHYSICS I Module No. 31: Peptide Synthesis: Definition, Methodology & applications Objectives: 1. Introduction 2. Synthesis of peptide 2.1. N-terminal protected
PAPER 9: TECHNIQUES USED IN MOLECULAR BIOPHYSICS I Module No. 31: Peptide Synthesis: Definition, Methodology & applications Objectives: 1. Introduction 2. Synthesis of peptide 2.1. N-terminal protected
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Solid phase peptide synthesis (SPPS)
 Tapio Nevalainen Drug synthesis II 2012 Solid phase peptide synthesis (SPPS) Solid phase peptide synthesis (SPPS) R. Bruce Merrifield, Professor at Rockefeller University, has been awarded the Nobel Prize
Tapio Nevalainen Drug synthesis II 2012 Solid phase peptide synthesis (SPPS) Solid phase peptide synthesis (SPPS) R. Bruce Merrifield, Professor at Rockefeller University, has been awarded the Nobel Prize
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Self-Assembly of Single Amino acid-pyrene Conjugates with Unique Structure-Morphology Relationship
 Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2017 Self-Assembly of Single Amino acid-pyrene Conjugates with Unique Structure-Morphology Relationship
Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 2017 Self-Assembly of Single Amino acid-pyrene Conjugates with Unique Structure-Morphology Relationship
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Conformational Analysis
 Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
Conformational Analysis C01 3 C C 3 is the most stable by 0.9 kcal/mole C02 K eq = K 1-1 * K 2 = 0.45-1 * 0.048 = 0.11 C04 The intermediate in the reaction of 2 has an unfavorable syn-pentane interaction,
به نام خدا. New topics in. organic chemistry. Dr Morteza Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran
 به نام خدا New topics in 2 organic chemistry Dr Morteza Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Combinatorial chemistry 1 http://www.combichemistry.com/
به نام خدا New topics in 2 organic chemistry Dr Morteza Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Combinatorial chemistry 1 http://www.combichemistry.com/
CHEM J-8 June Complete the following table. Make sure you give the name of the starting material where indicated. REAGENTS/ CONDITIONS
 CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
The Depsipeptide Methodology for Solid Phase Peptide Synthesis: Circumventing Side Reactions and Development of an Automated Technique via
 Supporting Information The Depsipeptide Methodology for Solid Phase Peptide Synthesis: Circumventing Side Reactions and Development of an Automated Technique via Depsidipeptide Units. Irene Coin, Rudolf
Supporting Information The Depsipeptide Methodology for Solid Phase Peptide Synthesis: Circumventing Side Reactions and Development of an Automated Technique via Depsidipeptide Units. Irene Coin, Rudolf
media), except those of aluminum and calcium
 1- Aspirin occurs as white crystals or as a white crystalline powder. 2- It is slightly soluble in water (1:300), soluble in alcohol (1 :5), chloroform (1:17) & ether (1:15). It dissolves easily in glycerin.
1- Aspirin occurs as white crystals or as a white crystalline powder. 2- It is slightly soluble in water (1:300), soluble in alcohol (1 :5), chloroform (1:17) & ether (1:15). It dissolves easily in glycerin.
Ethers. Synthesis of Ethers. Chemical Properties of Ethers
 Page 1 of 6 like alcohols are organic derivatives of water, but lack the labile -OH group. As a result, ethers, except for epoxides, are usually not very reactive and are often used as solvents for organic
Page 1 of 6 like alcohols are organic derivatives of water, but lack the labile -OH group. As a result, ethers, except for epoxides, are usually not very reactive and are often used as solvents for organic
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Practice Problems on Nucleic Acids
 A.1 ractice roblems on ucleic Acids 1. Which one of the following structures is not a purine? 2 A B 2 3 3 3 3 2. Which one of the following is not a pyrimidine? 2 A 3 B S Br 2 A.2 3. ow many of the following
A.1 ractice roblems on ucleic Acids 1. Which one of the following structures is not a purine? 2 A B 2 3 3 3 3 2. Which one of the following is not a pyrimidine? 2 A 3 B S Br 2 A.2 3. ow many of the following
Supporting Information: Regioselective esterification of vicinal diols on monosaccharide derivatives via
 Supporting Information: Regioselective esterification of vicinal diols on monosaccharide derivatives via Mitsunobu reactions. Guijun Wang,*Jean Rene Ella-Menye, Michael St. Martin, Hao Yang, Kristopher
Supporting Information: Regioselective esterification of vicinal diols on monosaccharide derivatives via Mitsunobu reactions. Guijun Wang,*Jean Rene Ella-Menye, Michael St. Martin, Hao Yang, Kristopher
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Dr. Mohamed El-Newehy
 By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Carboxylic acids and Their Derivatives 1 Structure of Carboxylic Acids -The functional
By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Carboxylic acids and Their Derivatives 1 Structure of Carboxylic Acids -The functional
Solution problem 22: Non-Benzoid Aromatic Sytems
 Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Solution problem 22: on-enzoid Aromatic Sytems 22.1 & 22.2 Each double bond and each heteroatom (, ) with lone pairs donates 2 π- electrons as well as a negative charge. oron or a positive charge does
Solutions In each case, the chirality center has the R configuration
 CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
CAPTER 25 669 Solutions 25.1. In each case, the chirality center has the R configuration. C C 2 2 C 3 C(C 3 ) 2 D-Alanine D-Valine 25.2. 2 2 S 2 d) 2 25.3. Pro,, Trp, Tyr, and is, Trp, Tyr, and is Arg,
Organic and Biochemical Molecules. 1. Compounds composed of carbon and hydrogen are called hydrocarbons.
 Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
Organic and Biochemical Molecules 1. Compounds composed of carbon and hydrogen are called hydrocarbons. 2. A compound is said to be saturated if it contains only singly bonded carbons. Such hydrocarbons
SUPPORTING INFORMATION
 SUPPRTIG IFRMATI Salicylaldehyde Ester-Induced Chemoselective Peptide Ligations Enabling Generation of atural Peptidic Linkages at the Serine/Threonine Sites Xuechen Li,* iu Yung Lam, Yinfeng Zhang, Chun
SUPPRTIG IFRMATI Salicylaldehyde Ester-Induced Chemoselective Peptide Ligations Enabling Generation of atural Peptidic Linkages at the Serine/Threonine Sites Xuechen Li,* iu Yung Lam, Yinfeng Zhang, Chun
Class XII: Chemistry Chapter 13: Amines Top concepts
 Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
Organic Chemistry FINAL EXAM A (250 points)
 UCSC, Binder ame Student ID # Section Day/Time rganic Chemistry FIAL EXAM A (250 points) D T BEGI TE EXAM R TUR TE PAGE UTIL ISTRUCTED T D S. In the meantime, please read the instructions below. In each
UCSC, Binder ame Student ID # Section Day/Time rganic Chemistry FIAL EXAM A (250 points) D T BEGI TE EXAM R TUR TE PAGE UTIL ISTRUCTED T D S. In the meantime, please read the instructions below. In each
2.222 Introductory Organic Chemistry II Midterm Exam
 NAME: STUDENT NUMBE: Page 1 of 7 University of Manitoba Department of hemistry 2.222 Introductory rganic hemistry II Midterm Exam Wednesday February 20, 2002 Put all answers in the spaces provided. If
NAME: STUDENT NUMBE: Page 1 of 7 University of Manitoba Department of hemistry 2.222 Introductory rganic hemistry II Midterm Exam Wednesday February 20, 2002 Put all answers in the spaces provided. If
The biomolecules of terrestrial life
 Functional groups in biomolecules Groups of atoms that are responsible for the chemical properties of biomolecules The biomolecules of terrestrial life Planets and Astrobiology (2017-2018) G. Vladilo 1
Functional groups in biomolecules Groups of atoms that are responsible for the chemical properties of biomolecules The biomolecules of terrestrial life Planets and Astrobiology (2017-2018) G. Vladilo 1
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
CHEM 345 Problem Set 07 Key
 CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
CHEM 345 Problem Set 07 Key 1.) Fill in the appropriate reaction arrow. The starting material is on the left, the product is on the right. Use. Simple Ring Size. 5 and 6 are favored. 3 is not. That s it.
CHAPTER 16 - CHEMISTRY OF BENZENE: ELECTROPHILIC AROMATIC SUBSTITUTION
 CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
CAPTR 16 - CMISTRY F BNZN: LCTRPILIC ARMATIC SUBSTITUTIN As stated in the previous chapter, benzene and other aromatic rings do not undergo electrophilic addition reactions of the simple alkenes but rather
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Chapter 23 Phenols CH. 23. Nomenclature. The OH group takes precedence as the parent phenol.
 CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
CH. 23 Chapter 23 Phenols Nomenclature The OH group takes precedence as the parent phenol. Carboxyl and acyl groups take precedence over the OH group. The OH group is a strong electron-donating group through
2: CHEMICAL COMPOSITION OF THE BODY
 1 2: CHEMICAL COMPOSITION OF THE BODY Although most students of human physiology have had at least some chemistry, this chapter serves very well as a review and as a glossary of chemical terms. In particular,
1 2: CHEMICAL COMPOSITION OF THE BODY Although most students of human physiology have had at least some chemistry, this chapter serves very well as a review and as a glossary of chemical terms. In particular,
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Review Experiments Formation of Polymers Reduction of Vanillin
 Review Experiments Formation of Polymers What is a polymer? What is polymerization? What is the difference between an addition polymerization and a condensation polymerization? Which type of polymerization
Review Experiments Formation of Polymers What is a polymer? What is polymerization? What is the difference between an addition polymerization and a condensation polymerization? Which type of polymerization
Chapter 20. Amines. Nomenclature for amines. Aryl amines
 Nomenclature for amines Chapter 20 Common names are widely used, named as alkylamines Systematic (IUPAC) nomenclature replaces the -e of the corresponding parent alkane with -amine Amines Simple secondary
Nomenclature for amines Chapter 20 Common names are widely used, named as alkylamines Systematic (IUPAC) nomenclature replaces the -e of the corresponding parent alkane with -amine Amines Simple secondary
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Ch 20 Carboxylic Acids and Nitriles
 Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Microwave-Enhanced Solid Phase Peptide Synthesis. Jonathan M. Collins Bioscience Division CEM Corporation
 Microwave-Enhanced Solid Phase Peptide Synthesis Jonathan M. Collins Bioscience Division CEM Corporation Microwave SPPS Reaction Vessel Standard Microwave Fmoc SPPS Parameters Deprotection Time Temperature
Microwave-Enhanced Solid Phase Peptide Synthesis Jonathan M. Collins Bioscience Division CEM Corporation Microwave SPPS Reaction Vessel Standard Microwave Fmoc SPPS Parameters Deprotection Time Temperature
Supporting Information
 Electronic upplementary Material (EI) for ChemComm. This journal is The Royal ociety of Chemistry 2014 upporting Information Materials and methods: Chemicals: Fmoc-amino acids were obtained from GL Biochem
Electronic upplementary Material (EI) for ChemComm. This journal is The Royal ociety of Chemistry 2014 upporting Information Materials and methods: Chemicals: Fmoc-amino acids were obtained from GL Biochem
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Topic 4.8 AMINO ACIDS. Structure Acid-Base Properties Condensation Reactions Proteins
 Topic 4.8 AMI AIDS Structure Acid-Base Properties ondensation eactions Proteins STUTUE F AMI AIDS Amino acids are molecules containing an amine group and a carboxylic acid group. aturally occurring amino
Topic 4.8 AMI AIDS Structure Acid-Base Properties ondensation eactions Proteins STUTUE F AMI AIDS Amino acids are molecules containing an amine group and a carboxylic acid group. aturally occurring amino
12. Aldehydes & Ketones (text )
 2009, Department of Chemistry, The University of Western ntario 12.1 12. Aldehydes & Ketones (text 13.1 13.11) A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is
2009, Department of Chemistry, The University of Western ntario 12.1 12. Aldehydes & Ketones (text 13.1 13.11) A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is
Structure at the isoelectric point: The predominant form of this tripeptide at ph 1 has a net charge of: (circle one)
 ame Key 21 F07-Final exam Page 2 I. (1 points) Using the pka information given on page 12, match the following isoelectric point (pi) values (3.08, 6.00, and 10.76) to their corresponding amino acids by
ame Key 21 F07-Final exam Page 2 I. (1 points) Using the pka information given on page 12, match the following isoelectric point (pi) values (3.08, 6.00, and 10.76) to their corresponding amino acids by
Here are a few examples that utilize Jeff Bode s chemistry: 1. Draw the product of the reaction shown below:
 Lecture 19 November 29, 2011 Today we will continue our discussion of peptide chemistry, and in particular, focus on two nontraditional ways to make amide bonds. 1. Jeff Bode chemistry: Until this point,
Lecture 19 November 29, 2011 Today we will continue our discussion of peptide chemistry, and in particular, focus on two nontraditional ways to make amide bonds. 1. Jeff Bode chemistry: Until this point,
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Chapter 25 Organic and Biological Chemistry
 Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Photo-switched self-assembly of Gemini -helical peptide into supramolecular architectures
 5 Electronic Supplementary Information of Photo-switched self-assembly of Gemini -helical peptide into supramolecular architectures Chang-Sheng Chen, Xiao-Ding Xu, Shi-Ying Li, Ren-Xi Zhuo, Xian-Zheng
5 Electronic Supplementary Information of Photo-switched self-assembly of Gemini -helical peptide into supramolecular architectures Chang-Sheng Chen, Xiao-Ding Xu, Shi-Ying Li, Ren-Xi Zhuo, Xian-Zheng
Rapid Synthesis and Purification of Sulfahydantion Library Using Microwave Assisted Organic Synthesis and Automated Flash Chromatography
 Rapid ynthesis and Purification of ulfahydantion Library Using Microwave Assisted rganic ynthesis and Automated Flash Chromatography hahnaz Ghassemi, Ph.D. March 17, 2005 Discovery Chemistry Group 1725
Rapid ynthesis and Purification of ulfahydantion Library Using Microwave Assisted rganic ynthesis and Automated Flash Chromatography hahnaz Ghassemi, Ph.D. March 17, 2005 Discovery Chemistry Group 1725
Chemistry 283g Experiment 4
 Chemistry 283g xperiment 4 XPRIMNT 4: lectrophilic Aromatic Substitution: A Friedel-Craft Acylation Reaction Relevant sections in the text: Fox & Whitesell, 3 rd d. Chapter 11, especially pg. 524-526,
Chemistry 283g xperiment 4 XPRIMNT 4: lectrophilic Aromatic Substitution: A Friedel-Craft Acylation Reaction Relevant sections in the text: Fox & Whitesell, 3 rd d. Chapter 11, especially pg. 524-526,
Affects pk a? Derivatization to Amides. Identify unknown. carboxylic acid
 sodium salt of unknown aromatic + soluble impurities + insoluble impurities total insoluble impurities dissolve in water, filter aq. sol n of unk. sodium salt + sol. impurities account for all masses;
sodium salt of unknown aromatic + soluble impurities + insoluble impurities total insoluble impurities dissolve in water, filter aq. sol n of unk. sodium salt + sol. impurities account for all masses;
 Top concepts Chapter: Amines 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification of amines: 3. Preparation
Top concepts Chapter: Amines 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification of amines: 3. Preparation
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
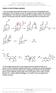 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Supporting Information for. PNA FRET Pair Miniprobes for Quantitative. Fluorescent in Situ Hybridization to Telomeric DNA in Cells and Tissue
 Supporting Information for PNA FRET Pair Miniprobes for Quantitative Fluorescent in Situ Hybridization to Telomeric DNA in Cells and Tissue Orenstein, et al Page S2... PNA synthesis procedure Page S3...
Supporting Information for PNA FRET Pair Miniprobes for Quantitative Fluorescent in Situ Hybridization to Telomeric DNA in Cells and Tissue Orenstein, et al Page S2... PNA synthesis procedure Page S3...
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Suggested solutions for Chapter 29
 s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
Chem 263 Oct. 4, 2016
 Chem 263 ct. 4, 2016 ow to determine position and reactivity: Examples The strongest donating group wins: 2 3 2 S 4 + 3 2 2 S 4 2 2 + 2 2 3 2 S 4 2 2 2 2,4,6-trinitrophenol picric acid This reactivity
Chem 263 ct. 4, 2016 ow to determine position and reactivity: Examples The strongest donating group wins: 2 3 2 S 4 + 3 2 2 S 4 2 2 + 2 2 3 2 S 4 2 2 2 2,4,6-trinitrophenol picric acid This reactivity
Diastereomeric resolution directed towards chirality. determination focussing on gas-phase energetics of coordinated. sodium dissociation
 Supplementary Information Diastereomeric resolution directed towards chirality determination focussing on gas-phase energetics of coordinated sodium dissociation Authors: samu Kanie*, Yuki Shioiri, Koji
Supplementary Information Diastereomeric resolution directed towards chirality determination focussing on gas-phase energetics of coordinated sodium dissociation Authors: samu Kanie*, Yuki Shioiri, Koji
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
CHAPTER 23 HW: ENOLS + ENOLATES
 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione
 Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione The purpose of this experiment was to synthesize 5-isopropyl-1,3-cyclohexanedione from commercially available compounds. To do this, acetone and
Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione The purpose of this experiment was to synthesize 5-isopropyl-1,3-cyclohexanedione from commercially available compounds. To do this, acetone and
Chapter 8 Alkyl Halides and Elimination Reactions
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 8 Alkyl Halides and Elimination Reactions Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 8 Alkyl Halides and Elimination Reactions Prepared by Rabi Ann Musah State University of New York at Albany Copyright
Chem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below.
 hem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below. TP l Et step 8 4 5 eagents used in synthesis A B D E F a DMF (solvent) 1. (i-pr) 2 Li (LDA)/TF
hem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below. TP l Et step 8 4 5 eagents used in synthesis A B D E F a DMF (solvent) 1. (i-pr) 2 Li (LDA)/TF
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
ACETONE. PRODUCT IDENTIFICATION CAS NO EINECS NO MOL WT H.S. CODE Oral rat LD50: 5800 mg/kg
 ACETONE www.pawarchemicals.com PRODUCT IDENTIFICATION CAS NO 67-64-1 EINECS NO. 200-662-2 FORMULA (CH3)2C=O MOL WT. 58.08 H.S. CODE 2914.11 TOXICITY SYNONYMS Oral rat LD50: 5800 mg/kg Dimethyl ketone;
ACETONE www.pawarchemicals.com PRODUCT IDENTIFICATION CAS NO 67-64-1 EINECS NO. 200-662-2 FORMULA (CH3)2C=O MOL WT. 58.08 H.S. CODE 2914.11 TOXICITY SYNONYMS Oral rat LD50: 5800 mg/kg Dimethyl ketone;
CHAPTER 22 HW: CO 2 H DERIVATIVES
 APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
Chem 263 Oct. 10, The strongest donating group determines where new substituents are introduced.
 Chem 263 ct. 10, 2013 The strongest donating group determines where new substituents are introduced. N 2 N 3 2 S 4 + N 3 N 2 2 S 4 N 2 N 2 + 2 N N 2 N 3 2 S 4 N 2 2 N N 2 2,4,6-trinitrophenol picric acid
Chem 263 ct. 10, 2013 The strongest donating group determines where new substituents are introduced. N 2 N 3 2 S 4 + N 3 N 2 2 S 4 N 2 N 2 + 2 N N 2 N 3 2 S 4 N 2 2 N N 2 2,4,6-trinitrophenol picric acid
FINAL EXAM ANSWER KEY Winter Session 2011R
 ANSWE KEY Page 1 of 11 CEM 2220 rganic Chemistry II: eactivity and Synthesis Prof. P.G. ultin, Prof. T. egmann, Dr.. Luong FINAL EXAM ANSWE KEY Winter Session 2011 Monday April 25, 2011 9:00 am 12:00 pm
ANSWE KEY Page 1 of 11 CEM 2220 rganic Chemistry II: eactivity and Synthesis Prof. P.G. ultin, Prof. T. egmann, Dr.. Luong FINAL EXAM ANSWE KEY Winter Session 2011 Monday April 25, 2011 9:00 am 12:00 pm
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Chemistry Final Examinations Summer 2006 answers
 Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
Chemistry 235 - Final Examinations Summer 2006 answers A GEERAL CEMISTRY answers are given from 1-20: no reaction C 3 CC 3 Ph C C C C C 3 Et 2 2 2 B. REACTIS AD REAGETS [32 MARKS] 2. A single substance
A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility
 (P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
(P&S Ch 5; Fer Ch 2, 9; Palm Ch 10,11; Zub Ch 9) A. Reaction Mechanisms and Catalysis (1) proximity effect (2) acid-base catalysts (3) electrostatic (4) functional groups (5) structural flexibility B.
Chapter 25. Organic and Biological Chemistry. Organic and
 Chapter 25 Calculate grade: (Add exam1 - exam 4 scores)x1.5 Add 7 best quizzes (each quiz is worth 29) Gives you the # points you have so far. Final (worth 200 points) Grades: 800-4 750-3.5 700-3 650-2.5
Chapter 25 Calculate grade: (Add exam1 - exam 4 scores)x1.5 Add 7 best quizzes (each quiz is worth 29) Gives you the # points you have so far. Final (worth 200 points) Grades: 800-4 750-3.5 700-3 650-2.5
Loudon Chapter 20 & 21 Review: Carboxylic Acids & Derivatives CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
Figure 4.10 HPLC Chromatogram of the Carbazole-Phenoxy Based Methacrylate
 The percent yield of the methacrylation was 85.2 %, with a purity of 98.2 % determined by HPLC (Figure 4.10). Elemental analysis gave excellent agreement to expected elemental ratios (Table 4.2). Disregarding
The percent yield of the methacrylation was 85.2 %, with a purity of 98.2 % determined by HPLC (Figure 4.10). Elemental analysis gave excellent agreement to expected elemental ratios (Table 4.2). Disregarding
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
 Chapter No. 2 EXPERIMENTAL TECHNIQUES IN CHEMISTRY SHORT QUESTIONS WITH ANSWERS Q.1 Define analytical chemistry? The branch of chemistry which deals with the qualitative and quantitative analyses of sample
Chapter No. 2 EXPERIMENTAL TECHNIQUES IN CHEMISTRY SHORT QUESTIONS WITH ANSWERS Q.1 Define analytical chemistry? The branch of chemistry which deals with the qualitative and quantitative analyses of sample
Chapter 22 Amines. Nomenclature Amines are classified according to the degree of substitution at nitrogen.
 CH. 22 Chapter 22 Amines Amines are very important in biological chemistry. Most of the bases in biological acid-base reactions are amines. They are also very important nucleophiles in biochemical reactions.
CH. 22 Chapter 22 Amines Amines are very important in biological chemistry. Most of the bases in biological acid-base reactions are amines. They are also very important nucleophiles in biochemical reactions.
