PIN6 is required for nectary auxin response and short stamen development
|
|
|
- Aubrie Hampton
- 6 years ago
- Views:
Transcription
1 The Plant Journal (2013) doi: /tpj PIN6 is required for nectary auxin response and short stamen development Ricci L. Bender 1,, Megan L. Fekete 1,,, Peter M. Klinkenberg 1, Marshall Hampton 2, Brittany Bauer 1, Matthew Malecha 1, Khrystyne Lindgren 3, Jennifer A. Maki 3, M. Ann D. N. Perera 4,5, Basil J. Nikolau 4,5 and Clay J. Carter 1, * 1 Department of Biology, University of Minnesota Duluth, Duluth, MN 55812, USA, 2 Department of Mathematics & Statistics, University of Minnesota Duluth, Duluth, MN 55812, USA, 3 Department of Chemistry, The College of St Scholastica, Duluth, MN 55811, USA, 4 Department of Biochemistry, Biophysics & Molecular Biology, Iowa State University, Ames, IA 50011, USA, and 5 W.M. Keck Metabolomics Research Laboratory, Iowa State University, Ames, IA 50011, USA Received 17 July 2012; revised 15 March 2013; accepted 20 March *For correspondence ( cjcarter@d.umn.edu). These authors contributed equally to this work. Present address: Department of Molecular, Cellular, and Developmental Biology, University of Michigan, Ann Arbor, MI, USA. SUMMARY The PIN family of proteins is best known for its involvement in polar auxin transport and tropic responses. PIN6 (At1g77110) is one of the remaining PIN family members in Arabidopsis thaliana to which a biological function has not yet been ascribed. Here we report that PIN6 is a nectary-enriched gene whose expression level is positively correlated with total nectar production in Arabidopsis, and whose function is required for the proper development of short stamens. PIN6 accumulates in internal membranes consistent with the ER, and multiple lines of evidence demonstrate that PIN6 is required for auxin-dependent responses in nectaries. Wild-type plants expressing auxin-responsive DR5:GFP or DR5:GUS reporters displayed intense signal in lateral nectaries, but pin6 lateral nectaries showed little or no signal for these reporters. Further, exogenous auxin treatment increased nectar production more than tenfold in wild-type plants, but nectar production was not increased in pin6 mutants when treated with auxin. Conversely, the auxin transport inhibitor N 1 naphthylphthalamic acid (NPA) reduced nectar production in wild-type plants by more than twofold, but had no significant effect on pin6 lines. Interestingly, a MYB57 transcription factor mutant, myb57 2, closely phenocopied the loss-of-function mutant pin6 2. However, PIN6 expression was not dependent on MYB57, and RNA-seq analyses of pin6 2 and myb57 2 mutant nectaries showed little overlap in terms of differentially expressed genes. Cumulatively, these results demonstrate that PIN6 is required for proper auxin response and nectary function in Arabidopsis. These results also identify auxin as an important factor in the regulation of nectar production, and implicate short stamens in the maturation of lateral nectaries. Keywords: nectar, nectaries, nectary, PIN6, MYB57, Arabidopsis. INTRODUCTION Despite its central importance in plant animal interactions, the molecular and genetic basis of nectar synthesis and secretion is largely unknown. Floral nectar is offered to increase pollinator visitation, while extra-floral nectar is used to attract mutualistic insects that provide protection from herbivory (Heil, 2011). Interestingly, although Arabidopsis thaliana is highly self-fertile, it has maintained functional nectaries, which have been implicated in facilitating out-crossing events (Chen et al., 2003; Hoffmann et al., 2003; Tholl et al., 2005; Kram and Carter, 2009). Thus, Arabidopsis may be used as a model for functional nectary analysis (Kram and Carter, 2009). The Plant Journal 2013 Blackwell Publishing Ltd Arabidopsis flowers produce two types of nectaries: median and lateral. Lateral nectaries are located at the base of short stamens and produce >99% of total nectar, whereas median nectaries occur at the base of long stamens and petals and produce little or no nectar (Davis et al., 1998; Kram and Carter, 2009). Nectar production by both median and lateral nectaries is developmentally regulated. Immature lateral nectaries (to stage 12) accumulate starch, which is broken down at anthesis and the released sugars are secreted in mature flowers (stages 13 15; pollen shed and nectar secretion coincide) (Ren et al., 2007; Kram and Carter, 2009). In Arabidopsis and most Brassicaceae 1
2 2 Ricci L. Bender et al. species, the sugars in the secreted nectar are nearly all hexoses (glucose and fructose) and accumulate in approximately equal concentrations (Davis et al., 1998). In recent years, several genes and molecular processes have been implicated in nectar production in multiple plant species. For example, Arabidopsis CELL WALL INVERT- ASE 4 is required for maintaining sink status and ultimately nectar secretion (Ruhlmann et al., 2010), and the transcription factor MYB305 regulates starch accumulation and hydrolysis in tobacco nectaries (Liu et al., 2009; Liu and Thornburg, 2012). The role of invertases in generating hexose-rich nectars via post-secretory action has also been reported (Heil et al., 2005; Nepi et al., 2012; Shenoy et al., 2012). In addition, the transcription factors AtMYB21 and AtMYB24 are required for nectary maturation via jasmonic acid-dependent pathways (Reeves et al., 2012). Jasmonic acid also induces nectar secretion in the extra-floral nectaries of lima bean (Phaseolus lunatus) (Heil, 2004) and the floral nectaries of Brassica sp. (Radhika et al., 2010). Other aspects of nectary development and function have also been reviewed (Kram and Carter, 2009; Heil, 2011). Recent transcriptomic analyses identified a large number of genes whose expression is enriched in the nectaries of Arabidopsis and Brassica rapa (Kram et al., 2009; Hampton et al., 2010). One such nectary-enriched gene was PIN6 (At1g77110), a member of a family of auxin-efflux carriers. Here we demonstrate a role for PIN6 and auxin, as well as the transcription factor MYB57, in the regulation of nectar synthesis and secretion in Arabidopsis thaliana. RESULTS PIN6 and MYB57 have nectary-enriched expression profiles Previous Affymetrix ATH1 microarray analyses identified a large number of genes with enriched expression in Arabidopsis nectaries (Kram et al., 2009). One gene displaying extreme up-regulation in nectaries is PIN6 (At1g77110) (Figure 1a). Use of PIN6pro:GUS lines demonstrated enrichment in both median and lateral nectaries (Figure 1a), and also showed that PIN6 is expressed in immature (stage 8) stamen (Figure 1b,c,d). PIN6 expression was not observed in other floral organs or at other developmental time points. The expression profiles of all PIN family genes in mature lateral nectaries and other reference tissues were also examined. Analysis of previous microarray data indicated that PIN6 was the only PIN family member displaying significant expression in nectaries (Table S1); this result was later supported by RNA-seq analyses of mature lateral nectaries (Table 1 and Table S2). Another gene examined in this study, MYB57 (At3g01530), displayed nectary enrichment, as demonstrated by microarray and RT PCR analysis, although it is also expressed at lower levels in other floral tissues (Figure 1e). pin6 and myb57 mutants have altered nectar and nectary phenotypes To identify biological roles for PIN6 and MYB57, multiple T DNA mutant alleles were identified (Figure 2a). Three independent homozygous pin6 mutants (all with T DNA insertions in introns) were examined for altered expression level via quantitative RT PCR, whereas myb57 2 expression was examined by end-point RT PCR and Illuminabased RNA-seq (Figure S1 and Table S2). pin6 1, pin6 2 and pin6 3 were identified as knock-up, knock-out and knock-down mutants, respectively (Figure 2b, black bars), whereas myb57 2 was identified as a strong knock-down mutant due to a T DNA insertion near the transcriptional start site (Figure S1 and Table S2). The reason for the observed increase in PIN6 expression in pin6 1 is not clear; however, the quantitative RT PCR primers used for PIN6 spanned the T DNA insertion site for pin6 1, and were located 3 to the insertion sites for pin6 2 and pin6 3. Further, 3 RACE demonstrated that fulllength transcript is produced in pin6 1 (Figure S2). The presence of a truncated PIN6 transcript in pin6 2 was confirmed by RNA-seq in pin6 2 lateral nectaries (Figure S3). To demonstrate a role for PIN6 and MYB57 in nectary function, mutant lines were analyzed for total nectar glucose, as the vast majority (>99%) of sugars in Arabidopsis nectar are glucose and fructose in an approximately equal amounts (Davis et al., 1998). Significantly, PIN6 expression level was positively correlated with total nectar glucose (Figure 2b, gray bars), with nectar production being increased approximately 30% in the pin6 1 knock-up mutant, but significantly reduced in pin6 2 (knock-out) and pin6 3 (knock-down). Metabolite profiling of pin6 2 nectar (Table S3) confirmed a significant reduction in nectar sugar, with total glucose being approximately 75% lower than in wild-type Col 0, consistent with the results obtained from the enzymatic assays shown in Figure 2. Other nectar metabolites displaying significant differences between pin6 2 and Col 0 are highlighted in Table S3. Although not fully penetrant, pin6 1 flowers also often displayed large nectar droplets, which were not observed in wild-type Col 0 (Figure 2b). Total nectar glucose was also significantly reduced in myb57 2 (Figure 2). Finally, the putative involvement of PIN6 in auxin-dependent processes led us to examine nectar production in the auxin co-receptor mutant tir1 1 (Ruegger et al., 1998; Dharmasiri et al., 2005). Total nectar glucose was significantly increased in tir1 1, phenocopying the pin6 1 knock-up mutant. pin6 and myb57 mutants have altered floral morphology To identify potential reasons for the altered nectar secretion observed in pin6 and myb57 lines, mutant flowers were subjected to gross morphological analyses. pin6 1 and pin6 3 showed no observable differences in overall
3 PIN6 and auxin control nectary function 3 Figure 1. PIN6 and MYB57 have nectaryenriched expression patterns. (a) Normalized mean ATH1 GeneChip probe set signal intensity for Arabidopsis PIN6. Original array data for all tissues were presented by Kram et al. (2009). ILN, immature lateral nectaries; MLN, mature lateral nectaries; MMN, mature median nectaries. (b d) Staining of GUS activity in the nectaries of stage PIN6:GUS flowers; staining was not observed in other floral tissues or developmental stages. (b) GUS staining in nectaries. (c, d) GUS staining in a stage 8 stamen; side and top views, respectively. (e) Normalized mean ATH1 GeneChip probe set signal intensity for MYB57. Inset: RT PCR validation of MYB57 expression patterns; the nectary lane comprised pooled median and lateral nectaries. (a) (b) (c) (d) (e) Table 1 Normalized RNAseq counts for PIN-family gene expression in mature lateral nectaries a Col 0 myb57 2 pin6 2 PIN PIN PIN PIN PIN PIN b PIN PIN a Full read counts are presented in Table S2. b No reads were identified after the 3 end of the T DNA insertion site in pin6 2 (see Figure S3). floral morphology or nectary size compared with wild-type (Figure 3); however, pin6 2 flowers had petals that failed to fully expand, often had reduced nectary size, and lacked one or both short stamens significantly more frequently than wild-type (Figure 3 and Table 2). A wild-type Arabidopsis flower usually has four long stamens and two short stamens. The abnormal developmental phenotype of pin6 2 was complemented using a PIN6pro:PIN6-GFP construct (Figure S4), producing plants with petals that fully expanded, showed restored nectary size, and for which % of flowers had both short stamens present, compared to % of wild-type. myb57 2 partially phenocopied the pin6 2 knockout mutant, having smaller lateral nectaries and missing one or both short stamens significantly more often than Col 0 (Figure 3 and Table 2). Further, when present, the short stamens of myb57 2 displayed a petaloid phenotype (Figure 4b,c). Interestingly, myb57 2 in the DR5:GUS background displayed extensive staining in the anther portion of the petal/anther fusion. RT PCR on RNA isolated from myb57 2 anther/petal fusions also demonstrated a large
4 4 Ricci L. Bender et al. complemented with a full-length genomic clone of MYB57 (Figure S4); however, the total number of short stamens present in complemented lines was only partially restored, with % of flowers having both short stamens present, compared to % in myb57 2 and % in wild-type. The total amount of nectar in complemented myb57 2 lines was also partially restored, to 72 19% of wild-type levels, whereas the amount of nectar in myb57 2 was 45 9% of wild-type levels. The reason for the partial complementation phenotype of myb57 2 is unknown, although it is possible that MYB57 is mis-expressed in the myb57 2 mutant background due to the T DNA insertion being located in the promoter region, and is thus not a true null allele. RNA profiling of lateral nectaries from pin6 2 and myb57 2 Figure 2. pin6 and myb57 T DNA mutant allele series and associated nectar secretion phenotypes. (a) Three independent pin6 T DNA mutants with altered gene expression levels were identified; the relative position of each mutation is indicated by arrowheads. A single mutant, myb57 2, resulted in a strong knockdown for MYB57. (b) Quantitative RT PCR (black bars) was used to examine changes in PIN6 expression in whole flowers of each mutant line (n = 3, P < for each mutant line versus wild-type). The mean PIN6 expression in each mutant relative to wild-type is shown; the mean wild-type expression level is indicated by a dashed line. Total nectar glucose (gray bars) was also evaluated in pin6 mutants, as well as myb57 2 and tir1 1, and is also shown relative to wildtype nectar glucose (n = 10, P < versus wild-type for each mutant line). Inset: the arrowhead indicates a large nectar droplet that is often present in pin6 1 (knock-up mutant) but is not observed in Col 0 flowers; LN, lateral nectary. n.a. indicates that quantitative RT PCR assays were not applicable. Table 2 Number of short stamens present in pin6 2 and myb57 2 flowers Plant line Mean percentages of plants with 0, 1 or 2 short stamens per flower a Wild-type (Col 0) pin (P = ) (P = ) (P = ) myb (P = 0.11) (P = ) (P = ) a n = 3 biological replicates of 100 flowers each. P values versus Col 0 are indicated (paired Student s t test); significant changes are shown in bold. Wild-type Arabidopsis flowers usually have two short stamens and four long stamens. increase in PIN6 expression compared with RNA isolated from Col 0 short stamen anthers. It should be noted that the petaloid stamen phenotype reverted to wild-type when pin6 2 and myb57 2 flowers partially phenocopied each other, displaying smaller lateral nectaries and reduced nectar production (Figures 2 and 3), as well as often lacking one or both short stamens (Figure 3 and Table 2). Further, PIN6 was mis-expressed in myb57 2 petaloid stamens (Figure 4). These results suggest that expression of these two genes may be dependent on the other for expression. RT-PCR analysis of gene expression in stage (postanthesis) flowers indicated that PIN6 is expressed at normal levels in myb57 2, and that MYB57 is also expressed normally in pin6 2 (Figure S1). Expression of a strong nectary-specific gene required for nectar production, AtSWEET9 (Lin IW, Chen L-Q, Sosso D, Gase K, Kim S-G, Kessler D, Klinkenberg P, Qu X-Q, Hou B-H, Carter C, Baldwin IT, Frommer WB, submitted), was also not altered in either myb57 2 or pin6 2. These results were later confirmed by transcriptome analysis of RNA isolated from the mature lateral nectaries of pin6 2 and myb57 2, as counts for both of these genes in each other s backgrounds were similar to those of the wild-type (Table S2, first column). Comparative analyses of the pin6 2 and myb57 2 mature lateral nectary transcriptomes identified many genes that were differentially expressed compared with wild-type in one or the other mutant background, but few that were differentially expressed in both pin6 2 and myb57 2 (Table S2). Genes with counts twofold higher or lower in both pin6 2 and myb57 2 versus wild-type are listed in the second and third columns of Table S2, respectively, with the vast majority being expressed at relatively low levels. No significant enrichment in gene ontologies was identified for genes that are differentially expressed in both pin6 2 and myb57 2 versus wild-type. It should be noted that we also examined the previously described myb57 1 mutant (SALK_065776), and did not observe any noticeable floral phenotype, consistent with previous findings (Cheng et al., 2009). MYB57 activity was previously implicated in stamen development through
5 PIN6 and auxin control nectary function 5 (a) (b) (c) Figure 3. Gross morphology of pin6 and myb57 mutant flowers. pin6 1 and pin6 3 displayed normal floral morphologies; however, pin6 2 had petals that failed to expand (a,b), smaller nectaries and missing short stamens (c) (Table 1). In addition, myb57 2 partially phenocopied pin6 2 in having reduced nectary size (c), missing short stamens at a significantly increased rate versus Col 0 (c) (Table 1), and less total nectar (see Figure 2). Scale bars = 500 lm (a,b), 50 lm (c). SS, short stamen; P, petal; LN, lateral nectary. redundant action with MYB21 and MYB24; however, only in higher-order mutants of these genes did stamens fail to properly elongate (Cheng et al., 2009). Cheng et al. (2009) did not report missing short stamens or petaloid stamen phenotypes in the myb57 1 or higher-order myb mutants. The T DNA insertion in myb57 1 occurs toward the 3 end of the third and final exon of this mutant, thus it is possible some functional MYB57 is produced, resulting in no visible change in phenotype. Auxin responses are altered in pin6 nectaries To further examine a role for PIN6 and auxin in nectar production, pin6 alleles were crossed into the auxin-responsive DR5:GFP reporter line and examined by confocal laser scanning microscopy. In the wild-type background, the DR5: GFP reporter displayed extensive signal in the distal portion of both lateral and median nectaries (Figure 5a), which co-localized with expression of a PIN6pro:PIN6 GFP reporter gene (Figure 5d f). However, the DR5:GFP signal was greatly reduced in the lateral nectaries of both the pin6 1 (knock-up) and pin6 2 (knock-out) backgrounds, even when the nectary morphology was normal (Figure 5b,c). Interestingly, DR5-dependent signal did not appear to be reduced in the median nectaries of pin6 1 or pin6 2. Similar observations were made for DR5:GUS for both the pin6 1 and pin6 2 alleles (Figure S5). Significantly, plant lines expressing PIN6pro:PIN6 GFP demonstrated that PIN6 expression overlaps with that of auxin response in Arabidopsis nectaries. As DR5 reporter genes were expressed at a lower level in both knock-out (pin6 2) and knock-up (pin6 1) mutants in the lateral nectaries, the mutants responses to exogenous synthetic auxin [a naphthaleneacetic acid (NAA)] and auxin transport inhibitor N 1 naphthylphthalamic acid (NPA) were examined. We observed a positive correlation between NAA concentration and nectar production in feeding experiments with up to 100 lm NAA in 10% sucrose solutions, with a sharp decline at higher concentrations (Peter M. Klinkenberg and Clay J. Carter, unpublished data); therefore 100 lm NAA and NPA were used in these studies. Exogenous NAA significantly increased nectar production in wild-type Col 0 (>10-fold), whereas NPA caused a > 2-fold reduction (Figure 6). Conversely, in pin6 1, NAA significantly reduced nectar production and NPA had no significant effect (Figure 6). No differences in nectar production were observed when pin6 2 was treated with NAA or NPA. While not statistically significant, nectar production in pin6 3 (knockdown) displayed responses to NAA and NPA that were similar to those for Col 0, but with smaller magnitude. These results may be due to pin6 3 being a knock-down rather than a knock-out mutant. DISCUSSION We previously identified a large number of genes in Arabidopsis with nectary-enriched expression profiles (Kram et al., 2009), and have subsequently used a large-scale reverse genetics approach to identify factors
6 6 Ricci L. Bender et al. (a) (c) (d) (e) Figure 4. MYB57 is required for proper development of short stamens. (a) Wild-type Col 0 flower with one sepal removed to reveal the location of a lateral nectary (LN) and a short stamen (SS). (b,c) myb57 2 flowers. When present, the short stamen in myb57 2 flowers displayed a petaloid phenotype. (d) myb57 2 in the DR5:GUS background stained intensely in the anther portion of the short stamen, and RNA isolated from the myb57 2 short stamen showed a significant increase in PIN6 expression compared with wild-type short stamens. controlling nectar production in the Brassicaceae. This report describes initial efforts to characterize the involvement of PIN6 and MYB57 in Arabidopsis nectary function. The canonical function of the PIN family of proteins is polar auxin transport, which controls differential growth and cellular response via establishment of auxin gradients (Feraru and Friml, 2008; Kleine-Vehn and Friml, 2008; Krecek et al., 2009; Petrasek and Friml, 2009; Robert and Friml, 2009; Vanneste and Friml, 2009; Friml, 2010; Grunewald and Friml, 2010; Wabnik et al., 2011a). PIN6 has been characterized as an irregular PIN protein that does not follow the typical intron exon motif of most characterized PINs. Specifically, PIN6 has a shorter hydrophilic loop in the middle of its protein structure; however, like PIN1 and PIN4, it has ten transmembrane domains (Paponov et al. 2005) and demonstrates in vitro auxin transporter activity (Petrasek et al., 2006). Nonetheless, a biological function has not yet been ascribed to PIN6. The role of PIN6 in the nectary auxin response Microarray and reporter assays demonstrated that PIN6 is highly up-regulated in nectaries (Figures 1 and 5), with (b) other PIN genes being expressed at very low levels (Table 1 and Table S1). This specificity of expression suggested a very particular role for PIN6 and auxin in nectary function, which was supported by several findings in this study as discussed below. Three independent pin6 T DNA mutant alleles with differing PIN6 expression levels were examined here. Interestingly, the level of PIN6 expression positively correlated with nectar production (Figure 2). To obtain an explanation for this phenotype, we first examined floral and nectary morphology. An attractive initial hypothesis was that PIN6 activity may affect nectary size, which in turn would affect nectar production. Indeed, pin6 2 often did have smaller nectaries and petals that failed to fully expand; however, no noticeable differences in nectary size or morphology were observed for pin6 1 (knock-up mutant) or pin6 3 (knock-down), even though they had contrasting nectar secretion phenotypes. These results suggest that PIN6 and auxin may play a direct role in the regulation of nectar production rather than singularly affecting nectary development, a conclusion supported by several findings in this study. For example, a very strong DR5:GFP signal was observed in post-anthesis nectaries of the Col 0 background, which co-localized with PIN6 GFP at nectary tips (Figure 5). This observation is consistent with previous studies suggesting that the floral nectaries of multiple plant species produce indoleacetic acid (IAA) immediately prior to anthesis (Endress, 1994; Aloni et al., 2006). Aloni et al. (2006) used DR5:GUS expression analyses as a proxy to follow the initiation and progression of free auxin production in floral organs throughout development, including nectaries. The authors suggested that free IAA has two primary functions in flower development: (i) promotion of growth within the organs that produce auxin, and (ii) repression of development in adjacent organs that do not produce auxin (Aloni et al., 2006). One conclusion of their study was that auxin production shifts from anthers to nectaries at anthesis. It was suggested that IAA derived from anthers in pre-anthesis Arabidopsis flowers prevents nectar secretion until anthesis, whereupon nectaries become the primary sites of auxin synthesis in flowers (Aloni et al., 2006). However, it is important to note that DR5- based reporters are only a proxy for the auxin response, not auxin synthesis itself. Thus, nectaries may sequester auxin from surrounding tissues instead of directly synthesizing auxin, thereby controlling auxin homeostasis and response. Indeed, an analysis of the RNA-seq data in Table S2 indicates that genes involved in IAA biosynthesis (Mano and Nemoto, 2012) are expressed at very low levels (Table S4, most of these genes are near or in the lowest quartile of all genes for total RNA-seq counts). This analysis suggests that nectaries may not produce large amounts of free IAA; however, more studies are required
7 PIN6 and auxin control nectary function 7 (a) (b) (c) (d) (e) (f) Figure 5. PIN6 is required for proper auxin responses in nectaries. (a) Confocal laser scanning microscopy of the DR5:GFP auxin-responsive reporter in the Col 0 background displayed strong signal in the distal portions of both lateral and median nectaries from stage 14 flowers (post-anthesis, secretory nectary). (b,c) The auxin-responsive signal was significantly reduced in the lateral nectaries of both pin6 2 (b) and pin6 1 (c). Interestingly, DR5:GFP signal was still observed in the median nectaries of both pin6 1 and pin6 2. (d f) Wild-type plants expressing a PIN6pro:PIN6-GFP fusion showed that PIN6 is also expressed in the distal nectary (d,e) (overlaps with DR5:GFP expression), and that it accumulates in internal membranes (f) (arrowheads indicate modified open stomata of a lateral nectary). LN, lateral nectary; MN, median nectary. Scale bars = 100 lm (a e) and 20 lm (f). DAPI was used as a counter-stain in (a) (c), (e) and (f); endogenous autofluorescence provided the background in (d). Figure 6. Exogenous auxin increases and auxin transport inhibitor (NPA) decreases total nectar sugar in wild-type Arabidopsis flowers. The peduncles of freshly cut inflorescences were placed in 10% buffered sucrose solutions containing either 100 lm NAA (synthetic auxin) or NPA (auxin transport inhibitor) in microcentrifuge tubes covered in Parafilm (Pechiney Plastic Packaging Company, Chicago, IL, USA). Peduncles were chosen based on the number of inflorescences nearing stage 13 (anthesis), which averaged between four and five for every three peduncles treated. Following 20 h incubation in a dark, humid environment, nectar was collected from the lateral nectaries of five stage 15 flowers (post-anthesis, secretory) using paper wicks, and assayed for total glucose. Data are presented as the percentage increase or decrease in total nectar glucose relative to mock treatments for each individual line (n = 3 biological replicates of nectar collected from five flowers each, **P < relative to mock treated flowers). to determine whether or not nectaries are active sites of auxin synthesis. Another piece of evidence for a role for PIN6 and auxin in nectary function was that the auxin-dependent DR5:GFP signal in both the pin6 1 (knock-up) and pin6 2 (knock-out) alleles was significantly decreased in mature lateral nectaries (even when the nectary morphology was normal in pin6 2), whereas median nectaries appeared unaffected (Figure 5). This result was unexpected because: (i) pin6 1 and pin6 2 have opposite expression levels and nectar secretion phenotypes relative to wild-type Col 0, and (ii) PIN6 is highly expressed in both median and lateral nectaries, so it is expected that both nectary types would be affected equally. PIN6 is most closely related to PIN5, which was previously localized to the ER membrane (Mravec et al., 2009), thus differing from the plasma membrane localization of other described PINs. Mravec et al. (2009) also showed via transient expression assays that PIN6 and PIN8 appear to be located in the ER of tobacco BY 2 cells. The observed localization patterns of PIN6pro:PIN6 GFP (Figure 5e,f) are consistent with the previously suggested ER localization; unfortunately, attempts to observe co-localization of PIN6 GFP with ER-specific dyes (e.g. ER-Tracker Red) and even diamidino-2-phenylindole (DAPI; nuclear stain) were unsuccessful because the thick cuticle covering the nectaries prevented staining in sub-epidermal cells. The presence of PIN6 on the ER membrane suggests that it may play a role in intracellular auxin homeostasis,
8 8 Ricci L. Bender et al. as demonstrated for PIN5 (Mravec et al., 2009) and later expanded upon from a mechanistic perspective by Wabnik et al. (2011b). Thus, the function of PIN6 may be to sequester auxin from the cytosol into the lumen of the ER, thereby modulating cytosolic auxin concentrations and its cellular availability for auxin-dependent processes such as the SCF TIR1/AFB signaling pathway (Gray et al., 2001; Dharmasiri et al., 2005; Kepinski and Leyser, 2005). This idea is supported by the finding that, in the pin6 1 mutant, which has nearly a twofold increase in PIN6 transcript (Figure 2), the DR5:GFP signal is significantly reduced in lateral nectaries (Figure 5), and did not respond to exogenously applied auxin (Figure 6). Thus, the increased PIN6 expression in pin6 1 may result in decreased cytosolically available auxin and a concomitant reduction in SCF TIR1/AFB signaling. This notion is further supported by the finding that the auxin co-receptor mutant tir1 1 (Ruegger et al., 1998) phenocopied pin6 1 with regard to nectar secretion, secreting significantly higher amounts of nectar than Col 0 (Figure 2). Additionally, one may expect auxin-responsive genes to be down-regulated in pin6 2 nectaries (due to decreased DR5:GFP signal in pin6 2 nectaries, Figure 5); indeed, seven of 271 genes with more than twofold higher counts in Col 0 versus pin6 2 nectaries were annotated as being auxin-responsive via GO annotation at org/tools/bulk/go/index.jsp (data from Table S2; only genes with counts in the top half of all genes expressed in nectaries were analyzed due to low counts in remaining genes). These genes included At4g30080 (auxin response factor 16), At4g36740 (homeobox protein 40), At3g11820 (syntaxin of plants 121), At4g23570 (phosphatase-related), At4g37390 (auxin-responsive GH3 family protein), At5g35735 (auxin-responsive family protein) and At2g33830 (dormancy/ auxin-associated family protein). However, enrichment of auxin-responsive genes was not statistically significant, which suggests that at least part of the auxin response in nectaries may be independent of transcriptional processes. As mentioned above, whereas exogenously applied auxin (via peduncles placed in 100 lm NAA in 10% sucrose solutions) resulted in a large increase in nectar production in Col 0, it caused a significant reduction in pin6 1, no change in pin6 2, and a small but statistically insignificant increase in pin6 3 (Figure 6). The results we observed with wild-type flowers were consistent with a previous study in which exogenous auxin and gibberellic acid (GA 3 ) (applied by spraying, not in cultured flowers) caused significant increases in nectar volume, nectar sugar concentration, dry nectar sugar mass, insect visitation abundance and seed yield in two species closely related to Arabidopsis (Brassica campestris and Brassica oleracea) (Mishra and Sharma, 1988). Other studies on excised flowers of snapdragon (Antirrhinum majus) supported a conflicting role for auxin in inhibiting nectar secretion (Shuel, 1959, 1964, 1978); however, under some conditions, exogenous IAA resulted in an increase in nectar production, suggesting a dual role for auxin in nectar production (Shuel, 1964). Whether these conflicting observations result from species differences (Brassicaceae versus snapdragon) or experimental design (the snapdragon studies used 500 lm IAA, whereas this study used 100 lm NAA) are unclear; however, we observed a positive correlation between NAA concentration and nectar production in our sucrose feeding experiments up to 100 lm NAA in 10% sucrose solutions, with a sharp decline at higher NAA concentrations (Peter M. Klinkenberg and Clay J. Carter, unpublished data). Significantly, through 14 C- labeled IAA and sucrose experiments, Shuel (1978) concluded that exogenously applied auxin affects the secretory process itself within nectaries, rather than the movement of sugars to nectaries. In contrast to NAA application, the auxin transport inhibitor NPA caused a significant decrease in nectar production in Col 0, but not in any of the pin6 mutants (Figure 6). The accumulation of PIN6 and auxin at nectary tips was also intriguing, and suggests that PIN6 may also play a role in defining nectary polarity. The reason for PIN6 expression in the distal nectary is uncertain; however, other nectaryenriched genes, such as CELL WALL INVERTASE 4 and a sesquiterpine synthase (At5g44630), appear to be expressed throughout the entire nectary (Tholl et al., 2005; Ruhlmann et al., 2010), suggesting that sub-domains exist within the Arabidopsis nectary. Cumulatively, these results indicate that fine-tuned control of auxin concentration in the sub-epidermal nectary parenchyma is essential for proper nectary function. As to why median nectaries still have a strong DR5:GFP signal in pin6 mutant backgrounds, it should be noted that lateral nectaries secrete >99% of nectar in Arabidopsis, and there are also clear differences in gene expression and development between median and lateral nectaries (Davis et al., 1998; Kram and Carter, 2009; Kram et al., 2009). For example, a cupin-family gene, At1g74820, was previously found to be highly up-regulated in median versus lateral nectaries (Kram et al., 2009). RNA-seq analysis in this study identified At1g74820 as being expressed at a level that was 24-fold higher in the mature lateral nectaries of pin6 2 compared with Col 0 (Table S2). The cupin family of genes includes the auxin receptor AUXIN BINDING PROTEIN 1 (Shi and Yang, 2011) and other auxin binding proteins (Ohmiya, 2002). Thus, it is possible that At1g74820 may play a role in regulating the auxin response in nectaries. The role of PIN6 and MYB57 in short stamen development We also found that the pin6 2 and myb57 2 mutants partially phenocopied one another in having significantly reduced nectar production, nectary size and short stamen presence; however, differences between these two lines
9 PIN6 and auxin control nectary function 9 included that pin6 2 had petals that failed to fully expand (Figure 3), whereas myb57 2 had petaloid short stamens (when short stamens were present). Since PIN6 is expressed at normal levels in both myb57 2 whole flowers and mature lateral nectaries, it is unlikely that MYB57 directly regulates PIN6 expression (Figure S1 and Table S2). However, the anther portion of petaloid stamens in myb57 2 stained strongly in the DR5:GUS background in mature flowers, and PIN6 was also found to be misexpressed in myb57 2 petaloid stamens instead of only being expressed in the nectaries of wild-type Col-0 flowers. As PIN6 is also expressed in immature stamens of wildtype plants (Figure 1), which coincides with auxin production or response in stamens at this stage (Aloni et al., 2006), these results are not necessarily surprising. It is currently unknown whether PIN6 activity is required for the petaloid short stamen phenotype observed in myb57 2, or whether PIN6 mis-expression in myb57 2 short stamens is a consequence of the petaloid phenotype. Nonetheless, the observed role of MYB57 in the proper development of short stamens is consistent with previous results, as MYB57 involvement in stamen elongation, through redundant action with MYB21 and MYB24, has been demonstrated previously (Cheng et al., 2009). Interestingly, MYB21 and MYB24 are also highly expressed in nectaries and are required for proper floral maturation (Reeves et al., 2012). Indeed, myb21 null mutants do not secrete nectar (Peter M. Klinkenberg and Clay J. Carter, unpublished data). Cumulatively, these observations suggest the existence of an indirect link between MYB57 and PIN6, as well as a general role for PIN6 in the auxin response in mature flowers. The fact that small under-developed nectaries occur when short stamens are absent is consistent with previous results. Most floral jasmonic acid appears to be produced in stamen filaments (Ishiguro et al., 2001), and is not only required for floral maturation as a whole, including nectaries (Cheng et al., 2009; Reeves et al., 2012), but may also induce floral nectar secretion (Radhika et al., 2010). Indeed, when short stamens are present in pin6 2 and myb57 2, nectary morphology occasionally appears normal (e.g. Figure 5b). The link between stamen presence and nectary development and maturation is unclear; however, one previously proposed model is that jasmonic acid is transported downwards from filaments to the rest of the flower for maturation and expansion of other organs, such as petals (Ishiguro et al., 2001). Regardless, PIN6 activity is clearly required for proper nectary function, even when nectary morpology is normal, as demonstrated by the reduced nectar secretion phenotype of pin6 3 (Figure 2). CONCLUSIONS To conclude, we have demonstrated a clear role for PIN6, auxin and MYB57 in nectary development and function. These results indicate a crucial role for auxin homeostasis in nectaries for proper nectar secretion and floral development. Future studies will focus on the involvement of endogenous nectary auxin production in flower and nectary maturation, the precise role of PIN6 in regulating the auxin response in nectaries, and how PIN6 expression is regulated. EXPERIMENTAL PROCEDURES Plant materials and growth The background for all plant materials was Arabidopsis thaliana cv Col 0. T DNA mutant lines for PIN6 (At1g77110: pin6 1, SALK_082098; pin6 2,SALK_046393; pin6 3, SALK_095142C) and MYB57 (At3g01530: myb57 2, SALK_030969) were obtained from the Arabidopsis Biological Resource Center (Columbus, OH) (Alonso et al., 2003), and genotyped to obtain homozygous mutants as described at PIN6pro:GUS seed was also obtained from the Arabidopsis Biological Resource Center (CS9371), and DR5:GUS, DR5:GFP and tir1-1 were previously described (Ulmasov et al., 1997; Ruegger et al., 1998; Benkova et al., 2003). PIN6pro:PIN6 GFP lines were created by amplifying a 6602 bp PIN6 genomic fragment from Col 0 DNA using the PIN6 FULL F and PIN6 GFP R primers (Table S5) and Phusion polymerase (New England BioLabs, and directly cloned into the XhoI and XmaI sites of the GFP-containing binary vector pore R4 (Coutu et al., 2007). This fragment contained 3051 bp of sequence upstream of the PIN6 start codon, together with the full PIN6 coding region, minus the stop codon, and was cloned in-frame with the GFP coding region found in pore R4. The sequence of the resulting construct, pcc15, was confirmed via dideoxy sequencing at the University of Minnesota DNA Sequencing and Analysis Facility (St Paul, MN), and transformed into Arabidopsis using Agrobacterium tumefaciens (GV3101) by the floral-dip method (Clough and Bent, 1998). Transformed seedlings were selected on solid Murashige and Skoog (MS) medium with kanamycin (50 lg ml). For myb57 2 complementation, the full-length gene (promoter and coding region of MYB57) was PCR-amplified using the primer pair MYB57 comp F and MYB57 comp R (Table S5), and then ligated into the EcoRI and SpeI sites of the plant transformation vector pore_o3 (Coutu et al., 2007), generating the construct ppmk23. myb57 2 was transformed with ppmk23 using Agrobacteriummediated transformation by the floral-dip method (Clough and Bent, 1998). Transformed plants were selected on half-strength Murashige & Skoog medium with 50 lm phosphinothricin. Healthy seedlings were transplanted into soil and genotyped for the presence of ppmk23. All plants were grown in individual pots on a peat-based growth medium with vermiculite and perlite (Pro-Mix BX; Premier Horticulture, in Percival AR66LX environmental chambers ( under standard conditions: 16 h day/8 h night cycle, with a photosynthetic flux of 150 lmol m 2 sec 1 and a temperature of 23 C. Chemicals and reagents Unless noted otherwise, all chemicals were obtained from Sigma- Aldrich Chemical Co. ( or Thermo Fisher Scientific ( Microscopic analyses Scanning electron microscopy and confocal analyses were performed as previously described (Ruhlmann et al., 2010), except
10 10 Ricci L. Bender et al. that DAPI, which is usually used as a nuclear stain, was found to extensively label the thick cuticle covering the nectaries. The cuticle prevented extensive staining of nectary parenchyma nuclei, even with extended periods of incubation. Thus, DAPI was used primarily as a counter-stain to observe the nectary surface. Flowers with sepals removed were briefly placed in 300 nm DAPI, and rinsed in ddh 2 O just prior to imaging. Gene expression analyses Promoter GUS assays were performed as previously described (Jefferson et al., 1987). For both end-point and quantitative RT PCR, RNA was isolated from tissues using an Absolutely RNA Miniprep kit according to the manufacturer s instructions (Agilent, RNA quality was confirmed by spectrophotometric analysis and agarose gel electrophoresis. For endpoint RT PCR analyses, total RNA (200 ng) was used as a template in the Promega reverse transcription system ( com/). The resulting cdna (1 ll) was PCR-amplified using GoTaq Green Master Mix (Promega) according to the manufacturer s instructions. Expression was analyzed on 1% agarose gels using SybrSafe (Invitrogen, A minimum of three biological replicates and corresponding PCR reactions were used to confirm results. The sequences of the primers designed for gene expression analyses are given in Table S5. For 3 RACE in pin6 mutants, total RNA was reverse-transcribed using the Promega reverse trancription system with the 3 adapter + the oligo (dt)oligonucleotide primer (Table S5), and subsequently subjected to PCR amplification using the PIN6 qpcr F and 3 RACE adapter oligonucleotide primers. RACE PCR products were subjected to DNA sequencing at the University of Minnesota DNA Sequencing and Analysis Facility (St Paul, MN). For quantitative real-time PCR analyses, total RNA (100 ng) from pin6 1, pin6 2, pin6 3 and wild-type Arabidopsis flowers was used as template for cdna synthesis via the QuantiTech reverse transcription kit (Qiagen, Synthesized cdna (50 ng) was added to the real-time PCR reaction set-up, which included 12.5 ll of 2 x Rotor-Gene SYBR Green PCR Master Mix (Qiagen), 2.5 lm forward primer and 2.5 lm reverse primer to a final reaction volume of 25 ll. Primers were designed based on the Roche Universal Probe Library ( com) using the accession numbers At1g77110 (PIN6) and At1g49240 (ACTIN8) (Table S1). The PIN6 quantitative RT PCR primers spanned the T DNA insertion site for pin6 1, and were located 3 to the insertion sites for pin6 2 and pin6 3. Standard curve reactions were prepared using serial dilutions of shoot cdna and ACTIN8 primers. The standard curve, pin6 and wildtype reactions were set up in triplicate and incubated at 95 C for 5 min, then underwent 40 cycles of 95 C for 5 sec followed by 60 C for 10 sec in a Corbett Rotor-Gene 3000 Light Cycler ( SYBR Green fluorescence was detected during the 60 C incubation step. Standard reaction analysis was performed using the ROTOR-GENE 6 software. PCR reactions were performed six times. The three most efficient trials were chosen for final comparison. Nectar collection and metabolite analysis Nectar collection was performed as previously described (Bender et al., 2012). Briefly, a single biological replicate for nectar glucose assays consisted of nectar collected from the lateral nectaries of ten stage flowers using small, uniform wicks cut from Whatman No. 1 filter paper ( Completed wicks were placed in 500 ll of nuclease-free water for elution, and stored at 20 C until further analysis. Relative glucose concentration was then analyzed using a modified glucose oxidase assay as previously described (Bethke and Busse, 2008; Ruhlmann et al., 2010; Bender et al., 2012). Nectar collection for full metabolite analyses was performed as for glucose assays, except that 20 flowers were used for a single biological replicate, and the nectar was eluted in 100 ll nuclease-free water. Nectar samples used for metabolomic analyses were flash-frozen and stored at 80 C until analyzed via GC9GC/MS (Agilent, Santa Clara, CA, USA, as previously described (Bender et al., 2012) at the W.M. Metabolomics Research Laboratory at Iowa State University (Ames, IA). Hormone treatment assays The peduncles of freshly cut inflorescences were placed in 10% w/v sucrose solutions in MES-buffered MS medium (ph 5.8) containing 100 lm of either the synthetic auxin a naphthaleneacetic acid (NAA; MP Biomedicals, or the auxin transport inhibitor N 1 naphthylphthalamic acid (NPA; Supelco, Peduncles were chosen based on the number of inflorescences nearing stage 13 (anthesis), which averaged between four and five for every three peduncles treated. Following a 20 h incubation in a dark, humid environment, nectar was collected from the lateral nectaries of five stage flowers (post-anthesis, secretory) using paper wicks and assayed for total glucose. Data are presented as the percentage increase or decrease in total nectar glucose relative to mock treatments for each individual line (n = 3 biological replicates of nectar collected from five flowers each, **P < relative to mock treatments via Student s paired two-tailed t-test). Microarray data mining The mean probe set signal intensities for all PIN family genes expressed in Arabidopsis nectaries, as identified by an Affymetrix ATH1 GeneChip â microarray ( were compared to those in 13 tissues at multiple developmental stages, and are presented in Table S1. The raw normalized microarray data used for the analyses presented here were originally presented by Kram et al. (2009). RNA-seq analyses Collection of mature lateral nectaries from stage flowers (Col 0, pin6 2 and myb57 2), and subsequent RNA isolation and quality controls were performed as previously described (Kram et al., 2009). Sequencing libraries were created using TruSeq RNA Sample Prep Kits (Illumina, and sequenced as 100 bp single end reads via Illumina HiSeq 2000 at the University of Minnesota DNA Sequencing and Analysis Facility (St Paul, MN). The number of reads for the wild-type Col 0, myb57 2 and pin6 2 samples were , and , respectively. Sequences were compared to the TAIR10 reference (version updated 14 December 2010) using BLASTN version (National Center for Biotechnology Information; Altschul et al., 1997). Quantification of splice variants was performed using BOWTIE (version ) and TOPHAT (version 1.3.3) (Langmead et al., 2009; Trapnell et al., 2009). For blastn alignments, an E value cut-off of 10 5 and a bit score of at least 75 were required. Counts were normalized using upper-quartile normalization (Bullard et al., 2010). The correction multipliers were 1.05, 0.79 and 1.27 for the wild-type, myb57 2 and pin6 2 samples, respectively. Full RNA-seq count data are presented in Table S2, and corresponding data have been deposited in NCBI Sequence Read Archive as SRA
11 PIN6 and auxin control nectary function 11 ACKNOWLEDGEMENTS The authors thank Bryan Bandli (Research Instrumentation Laboratory, University of Minnesota Duluth) for assistance with scanning electron microscopy imaging, Molly Gorder and Mengyuan Jia for technical assistance, and Dr Marci Surpin (Valent Bio- Sciences Corporation, Long Grove, IL, USA) for helpful discussions. We also thank Dr Bill Gray (University of Minnesota) for helpful discussions and providing seed for the DR5 reporter lines and tir1-1. This work was funded by a grant from the US National Science Foundation (# ) to C.J.C. SUPPORTING INFORMATION Additional Supporting Information may be found in the online version of this article. Figure S1. PIN6 expression in mature flowers is not dependent on MYB57. Figure S2. 3 RACE analysis of PIN6 transcripts in pin6 1. Figure S3. RNA-seq count distribution for PIN6 in pin6 2 and wildtype mature lateral nectaries. Figure S4. Complementation phenotypes of pin6 2 and myb57 2. Figure S5. DR5:GUS expression in pin6 mutant backgrounds. Table S1. PIN family gene expression in Arabidopsis nectaries and reference tissues. Table S2. RNA-seq count data for pin6 2 and myb57 2 mature lateral nectaries. Table S3. Analysis of pin6 2 nectar metabolites. Table S4. Analysis of gene expression associated with IAA synthesis and inactivation/homeostasis. Table S5. Oligonucleotide primers used in this study. REFERENCES Aloni, R., Aloni, E., Langhans, M. and Ullrich, C.I. (2006) Role of auxin in regulating Arabidopsis flower development. Planta, 223, Alonso, J.M., Stepanova, A.N., Leisse, T.J. et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science, 301, Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W. and Lipman, D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, Bender, R., Klinkenberg, P., Jiang, Z., Bauer, B., Karypis, G., Nguyen, N., Perera, M.A.D.N., Nikolau, B.J. and Carter, C.J. (2012) Functional genomics of nectar production in the Brassicaceae. Flora, 207, Benkova, E., Michniewicz, M., Sauer, M., Teichmann, T., Seifertova, D., Jurgens, G. and Friml, J. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell, 115, Bethke, P.C. and Busse, J.C. (2008) Validation of a simple, colorimetric, microplate assay using Amplex Red for the determination of glucose and sucrose in potato tubers and other vegetables. Am. J. Potato Res. 85, Bullard, J.H., Purdom, E., Hansen, K.D. and Dudoit, S. (2010) Evaluation of statistical methods for normalization and differential expression in mrna-seq experiments. BMC Bioinformatics, 11, 94. Chen, F., Tholl, D., D Auria, J.C., Farooq, A., Pichersky, E. and Gershenzon, J. (2003) Biosynthesis and emission of terpenoid volatiles from Arabidopsis flowers. Plant Cell, 15, Cheng, H., Song, S.S., Xiao, L.T., Soo, H.M., Cheng, Z.W., Xie, D.X. and Peng, J.R. (2009) Gibberellin acts through jasmonate to control the expression of MYB21, MYB24, and MYB57 to promote stamen filament growth in Arabidopsis. PLoS Genet. 5, e Clough, S.J. and Bent, A.F. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, Coutu, C., Brandle, J., Brown, D., Brown, K., Miki, B., Simmonds, J. and Hegedus, D.D. (2007) pore: a modular binary vector series suited for both monocot and dicot plant transformation. Transgenic Res. 16, Davis, A.R., Pylatuik, J.D., Paradis, J.C. and Low, N.H. (1998) Nectar-carbohydrate production and composition vary in relation to nectary anatomy and location within individual flowers of several species of Brassicaceae. Planta, 205, Dharmasiri, N., Dharmasiri, S. and Estelle, M. (2005) The F box protein TIR1 is an auxin receptor. Nature, 435, Endress, P.K. (1994) Diversity and Evolutionary Biology of Tropical Flowers. Cambridge, UK: Cambridge University Press. Feraru, E. and Friml, J. (2008) PIN polar targeting. Plant Physiol. 147, Friml, J. (2010) Subcellular trafficking of PIN auxin efflux carriers in auxin transport. Eur. J. Cell Biol. 89, Gray, W.M., Kepinski, S., Rouse, D., Leyser, O. and Estelle, M. (2001) Auxin regulates SCF(TIR1)-dependent degradation of AUX/IAA proteins. Nature, 414, Grunewald, W. and Friml, J. (2010) The march of the PINs: developmental plasticity by dynamic polar targeting in plant cells. EMBO J. 29, Hampton, M., Xu, W.W., Kram, B.W., Chambers, E.M., Ehrnriter, J.S., Gralewski, J.H., Joyal, T. and Carter, C.J. (2010) Identification of differential gene expression in Brassica rapa nectaries through expressed sequence tag analysis. PLoS ONE, 5, e8782. Heil, M. (2004) Induction of two indirect defences benefits Lima bean (Phaseolus lunatus, Fabaceae) in nature. J. Ecol. 92, Heil, M. (2011) Nectar: generation, regulation and ecological functions. Trends Plant Sci. 16, Heil, M., Rattke, J. and Boland, W. (2005) Postsecretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science, 308, Hoffmann, M.H., Bremer, M., Schneider, K., Burger, F., Stolle, E. and Moritz, G. (2003) Flower visitors in a natural population of Arabidopsis thaliana. Plant Biol. 5, Ishiguro, S., Kawai-Oda, A., Ueda, J., Nishida, I. and Okada, K. (2001) The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell, 13, Jefferson, R.A., Kavanagh, T.A. and Bevan, M.W. (1987) GUS fusions: b glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, Kepinski, S. and Leyser, O. (2005) The Arabidopsis F box protein TIR1 is an auxin receptor. Nature, 435, Kleine-Vehn, J. and Friml, J. (2008) Polar targeting and endocytic recycling in auxin-dependent plant development. Annu. Rev. Cell Dev. Biol. 24, Kram, B.W. and Carter, C.J. (2009) Arabidopsis thaliana as a model for functional nectary analysis. Sex. Plant Reprod. 22, Kram, B.W., Xu, W.W. and Carter, C.J. (2009) Uncovering the Arabidopsis thaliana nectary transcriptome: investigation of differential gene expression in floral nectariferous tissues. BMC Plant Biol. 9, 92. Krecek, P., Skupa, P., Libus, J., Naramoto, S., Tejos, R., Friml, J. and Zazimalova, E. (2009) The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 10, 249. Langmead, B., Trapnell, C., Pop, M. and Salzberg, S.L. (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25. Liu, G. and Thornburg, R.W. (2012) Knockdown of MYB305 disrupts nectary starch metabolism and floral nectar production. Plant J. 70, Liu, G., Ren, G., Guirgis, A. and Thornburg, R.W. (2009) The MYB305 transcription factor regulates expression of nectarin genes in the ornamental tobacco floral nectary. Plant Cell, 21, Mano, Y. and Nemoto, K. (2012) The pathway of auxin biosynthesis in plants. J. Exp. Bot. 63, Mishra, R. and Sharma, S. (1988) Growth regulators affect nectar-pollen production and insect foraging in Brassica seed crops. Curr. Sci., 57, Mravec, J., Skupa, P., Bailly, A. et al. (2009) Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature, 459,
NECTAR COLLECTION AND ANALYSES. One significant consideration when performing nectar collection is timing, both
 NECTAR COLLECTION AND ANALYSES COLLECTION PROCEDURES Important Considerations One significant consideration when performing nectar collection is timing, both developmental and circadian. This is because
NECTAR COLLECTION AND ANALYSES COLLECTION PROCEDURES Important Considerations One significant consideration when performing nectar collection is timing, both developmental and circadian. This is because
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL
 GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated
 Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
www.sciencemag.org/cgi/content/full/1121356/dc1 Supporting Online Material for Polar PIN Localization Directs Auxin Flow in Plants Justyna Wiśniewska, Jian Xu, Daniela Seifertová, Philip B. Brewer, Kamil
Auxin Regulates Arabidopsis Anther Dehiscence, Pollen Maturation, and Filament Elongation W
 This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs,
This article is a Plant Cell Advance Online Publication. The date of its first appearance online is the official date of publication. The article has been edited and the authors have corrected proofs,
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family
 Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Supplemental Data. Perrella et al. (2013). Plant Cell /tpc
 Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Actions of auxin. Hormones: communicating with chemicals History: Discovery of a growth substance (hormone- auxin)
 Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Supplemental Data. Wang et al. (2014). Plant Cell /tpc
 Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Questions for Biology IIB (SS 2006) Wilhelm Gruissem
 Questions for Biology IIB (SS 2006) Plant biology Wilhelm Gruissem The questions for my part of Biology IIB, Plant Biology, are provided for self-study and as material for the exam. Please note that the
Questions for Biology IIB (SS 2006) Plant biology Wilhelm Gruissem The questions for my part of Biology IIB, Plant Biology, are provided for self-study and as material for the exam. Please note that the
Supplemental Data. Hou et al. (2016). Plant Cell /tpc
 Supplemental Data. Hou et al. (216). Plant Cell 1.115/tpc.16.295 A Distance to 1 st nt of start codon Distance to 1 st nt of stop codon B Normalized PARE abundance 8 14 nt 17 nt Frame1 Arabidopsis inflorescence
Supplemental Data. Hou et al. (216). Plant Cell 1.115/tpc.16.295 A Distance to 1 st nt of start codon Distance to 1 st nt of stop codon B Normalized PARE abundance 8 14 nt 17 nt Frame1 Arabidopsis inflorescence
Supplementary Figure 1. Phenotype of the HI strain.
 Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
GFP GAL bp 3964 bp
 Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Supplemental Data. Møller et al. (2009) Shoot Na + exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na + transport in Arabidopsis Supplemental Figure 1. Salt-sensitive
Supplemental Data. Chen and Thelen (2010). Plant Cell /tpc
 Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Supplemental Data. Chen and Thelen (2010). Plant Cell 10.1105/tpc.109.071837 1 C Total 5 kg 20 kg 100 kg Transmission Image 100 kg soluble pdtpi-gfp Plastid (PDH-alpha) Mito (PDH-alpha) GFP Image vector
Written Exam 15 December Course name: Introduction to Systems Biology Course no
 Technical University of Denmark Written Exam 15 December 2008 Course name: Introduction to Systems Biology Course no. 27041 Aids allowed: Open book exam Provide your answers and calculations on separate
Technical University of Denmark Written Exam 15 December 2008 Course name: Introduction to Systems Biology Course no. 27041 Aids allowed: Open book exam Provide your answers and calculations on separate
Identification of Differential Gene Expression in Brassica rapa Nectaries through Expressed Sequence Tag Analysis
 Identification of Differential Gene Expression in Brassica rapa Nectaries through Expressed Sequence Tag Analysis Marshall Hampton 1, Wayne W. Xu 2, Brian W. Kram 3, Emily M. Chambers 3, Jerad S. Ehrnriter
Identification of Differential Gene Expression in Brassica rapa Nectaries through Expressed Sequence Tag Analysis Marshall Hampton 1, Wayne W. Xu 2, Brian W. Kram 3, Emily M. Chambers 3, Jerad S. Ehrnriter
The Plant Cell, November. 2017, American Society of Plant Biologists. All rights reserved
 The Genetics of Floral Development Teaching Guide Overview The development of flowers in angiosperm plants provided a critical evolutionary advantage, allowing more options for pollen dispersal and seed
The Genetics of Floral Development Teaching Guide Overview The development of flowers in angiosperm plants provided a critical evolutionary advantage, allowing more options for pollen dispersal and seed
RNA Synthesis and Processing
 RNA Synthesis and Processing Introduction Regulation of gene expression allows cells to adapt to environmental changes and is responsible for the distinct activities of the differentiated cell types that
RNA Synthesis and Processing Introduction Regulation of gene expression allows cells to adapt to environmental changes and is responsible for the distinct activities of the differentiated cell types that
** * * * Col-0 cau1 CAU1. Actin2 CAS. Actin2. Supplemental Figure 1. CAU1 affects calcium accumulation.
 Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Ca 2+ ug g -1 DW Supplemental Data. Fu et al. Plant Cell. (213). 1.115/tpc.113.113886 A 5 4 3 * Col- cau1 B 4 3 2 Col- cau1 ** * * ** C 2 1 25 2 15 1 5 Shoots Roots *
AP Biology Summer 2017
 Directions: Questions 1 and 2 are long free response questions that require about 22 minutes to answer and are worth 10 points each. Questions 3-6 are short free- response questions that require about
Directions: Questions 1 and 2 are long free response questions that require about 22 minutes to answer and are worth 10 points each. Questions 3-6 are short free- response questions that require about
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach
 Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Characterisation of abiotic stress inducible plant promoters and bacterial genes for osmotolerance using transgenic approach ABSTRACT SUBMITTED TO JAMIA MILLIA ISLAMIA NEW DELHI IN PARTIAL FULFILMENT OF
Last time: Obtaining information from a cloned gene
 Last time: Obtaining information from a cloned gene Objectives: 1. What is the biochemical role of the gene? 2. Where and when is the gene expressed (transcribed)? 3. Where and when is the protein made?
Last time: Obtaining information from a cloned gene Objectives: 1. What is the biochemical role of the gene? 2. Where and when is the gene expressed (transcribed)? 3. Where and when is the protein made?
Supplemental Data. Fernández-Calvo et al. Plant Cell. (2011) /tpc
 Supplemental Data. Fernández-Calvo et al. Plant Cell. (2011). 10.1105/tpc.110.080788 Supplemental Figure S1. Phylogenetic tree of MYC2-related proteins from Arabidopsis and other plants. Phenogram representation
Supplemental Data. Fernández-Calvo et al. Plant Cell. (2011). 10.1105/tpc.110.080788 Supplemental Figure S1. Phylogenetic tree of MYC2-related proteins from Arabidopsis and other plants. Phenogram representation
Supplemental Data. Perea-Resa et al. Plant Cell. (2012) /tpc
 Supplemental Data. Perea-Resa et al. Plant Cell. (22)..5/tpc.2.3697 Sm Sm2 Supplemental Figure. Sequence alignment of Arabidopsis LSM proteins. Alignment of the eleven Arabidopsis LSM proteins. Sm and
Supplemental Data. Perea-Resa et al. Plant Cell. (22)..5/tpc.2.3697 Sm Sm2 Supplemental Figure. Sequence alignment of Arabidopsis LSM proteins. Alignment of the eleven Arabidopsis LSM proteins. Sm and
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/452/ra106/dc1 Supplementary Materials for Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots Hyo-Jun Lee, Jun-Ho
www.sciencesignaling.org/cgi/content/full/9/452/ra106/dc1 Supplementary Materials for Stem-piped light activates phytochrome B to trigger light responses in Arabidopsis thaliana roots Hyo-Jun Lee, Jun-Ho
Supplemental material
 Supplemental material Table 1- Segregation analysis of sgt1a sgt1b double mutant plants Parental genotype No. of lines Normal seeds Phenotype Abnormal seeds Ratio of abnormal seeds (%) WT 3 171 2 1.16
Supplemental material Table 1- Segregation analysis of sgt1a sgt1b double mutant plants Parental genotype No. of lines Normal seeds Phenotype Abnormal seeds Ratio of abnormal seeds (%) WT 3 171 2 1.16
Plant transformation
 Plant transformation Objectives: 1. What is plant transformation? 2. What is Agrobacterium? How and why does it transform plant cells? 3. How is Agrobacterium used as a tool in molecular genetics? References:
Plant transformation Objectives: 1. What is plant transformation? 2. What is Agrobacterium? How and why does it transform plant cells? 3. How is Agrobacterium used as a tool in molecular genetics? References:
Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport
 Ph.D. thesis Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport Zsigmond Laura Supervisor: Dr. Szabados László Arabidopsis Molecular Genetic Group Institute of Plant
Ph.D. thesis Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport Zsigmond Laura Supervisor: Dr. Szabados László Arabidopsis Molecular Genetic Group Institute of Plant
Figure 18.1 Blue-light stimulated phototropism Blue light Inhibits seedling hypocotyl elongation
 Blue Light and Photomorphogenesis Q: Figure 18.3 Blue light responses - phototropsim of growing Corn Coleoptile 1. How do we know plants respond to blue light? 2. What are the functions of multiple BL
Blue Light and Photomorphogenesis Q: Figure 18.3 Blue light responses - phototropsim of growing Corn Coleoptile 1. How do we know plants respond to blue light? 2. What are the functions of multiple BL
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA
 EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
Introduction. Gene expression is the combined process of :
 1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
1 To know and explain: Regulation of Bacterial Gene Expression Constitutive ( house keeping) vs. Controllable genes OPERON structure and its role in gene regulation Regulation of Eukaryotic Gene Expression
Genetic transformation of table grape via organogenesis and field evaluation of DefH9-iaaM transgenic plants
 Genetic transformation of table grape via organogenesis and field evaluation of DefH9-iaaM transgenic plants Mezzetti B., Silvestroni O., Costantini E. Dipartimento di Scienze Ambientali e delle Produzioni
Genetic transformation of table grape via organogenesis and field evaluation of DefH9-iaaM transgenic plants Mezzetti B., Silvestroni O., Costantini E. Dipartimento di Scienze Ambientali e delle Produzioni
Major Plant Hormones 1.Auxins 2.Cytokinins 3.Gibberelins 4.Ethylene 5.Abscisic acid
 Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
Plant Hormones Lecture 9: Control Systems in Plants What is a Plant Hormone? Compound produced by one part of an organism that is translocated to other parts where it triggers a response in target cells
Lipid transfer proteins confer resistance to trichothecenes
 Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Lipid transfer proteins confer resistance to trichothecenes John McLaughlin and Anwar Bin-Umer Tumer Laboratory National Fusarium Head Blight Forum December 6th, 2012 FY09-11: Identify trichothecene resistance
Supplementary Material. An analysis of the role of the ShSUT1 sucrose transporter in sugarcane using RNAi suppression
 10.1071/FP17073_AC CSIRO 2017 Supplementary Material: Functional Plant Biology, 2017, 44(8), 795 808. Supplementary Material An analysis of the role of the ShSUT1 sucrose transporter in sugarcane using
10.1071/FP17073_AC CSIRO 2017 Supplementary Material: Functional Plant Biology, 2017, 44(8), 795 808. Supplementary Material An analysis of the role of the ShSUT1 sucrose transporter in sugarcane using
Comparative RNA-seq analysis of transcriptome dynamics during petal development in Rosa chinensis
 Title Comparative RNA-seq analysis of transcriptome dynamics during petal development in Rosa chinensis Author list Yu Han 1, Huihua Wan 1, Tangren Cheng 1, Jia Wang 1, Weiru Yang 1, Huitang Pan 1* & Qixiang
Title Comparative RNA-seq analysis of transcriptome dynamics during petal development in Rosa chinensis Author list Yu Han 1, Huihua Wan 1, Tangren Cheng 1, Jia Wang 1, Weiru Yang 1, Huitang Pan 1* & Qixiang
SUPPLEMENTARY INFORMATION
 reverse 3175 3175 F L C 318 318 3185 3185 319 319 3195 3195 315 8 1 315 3155 315 317 Supplementary Figure 3. Stability of expression of the GFP sensor constructs return to warm conditions. Semi-quantitative
reverse 3175 3175 F L C 318 318 3185 3185 319 319 3195 3195 315 8 1 315 3155 315 317 Supplementary Figure 3. Stability of expression of the GFP sensor constructs return to warm conditions. Semi-quantitative
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice Supplementary Figure 2.
 Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Biological Roles of Cytokinins
 Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Multiple Choice Review- Eukaryotic Gene Expression
 Multiple Choice Review- Eukaryotic Gene Expression 1. Which of the following is the Central Dogma of cell biology? a. DNA Nucleic Acid Protein Amino Acid b. Prokaryote Bacteria - Eukaryote c. Atom Molecule
Multiple Choice Review- Eukaryotic Gene Expression 1. Which of the following is the Central Dogma of cell biology? a. DNA Nucleic Acid Protein Amino Acid b. Prokaryote Bacteria - Eukaryote c. Atom Molecule
SUPPLEMENTARY INFORMATION
 Supplementary Discussion Rationale for using maternal ythdf2 -/- mutants as study subject To study the genetic basis of the embryonic developmental delay that we observed, we crossed fish with different
Supplementary Discussion Rationale for using maternal ythdf2 -/- mutants as study subject To study the genetic basis of the embryonic developmental delay that we observed, we crossed fish with different
COMPETITIVE CANALIZATION OF AUXIN IN PEA CAN BE INVOLVED IN INITIATION OF AXILLARY BUD OUTGROWTH
 COMPETITIVE CANALIZATION OF AUXIN IN PEA CAN BE INVOLVED IN INITIATION OF AXILLARY BUD OUTGROWTH Medveďová Z. 1, Balla J. 1, 2, Procházka S. 1 1 CEITEC - Central European Institute of Technology, Mendel
COMPETITIVE CANALIZATION OF AUXIN IN PEA CAN BE INVOLVED IN INITIATION OF AXILLARY BUD OUTGROWTH Medveďová Z. 1, Balla J. 1, 2, Procházka S. 1 1 CEITEC - Central European Institute of Technology, Mendel
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles
 Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
Illegitimate translation causes unexpected gene expression from on-target out-of-frame alleles created by CRISPR-Cas9 Shigeru Makino, Ryutaro Fukumura, Yoichi Gondo* Mutagenesis and Genomics Team, RIKEN
SUPPLEMENTARY INFORMATION
 med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
Supplementary Figure 1 Characterization of wild type (WT) and abci8 mutant in the paddy field.
 Supplementary Figure 1 Characterization of wild type (WT) and abci8 mutant in the paddy field. A, Phenotypes of 30-day old wild-type (WT) and abci8 mutant plants grown in a paddy field under normal sunny
Supplementary Figure 1 Characterization of wild type (WT) and abci8 mutant in the paddy field. A, Phenotypes of 30-day old wild-type (WT) and abci8 mutant plants grown in a paddy field under normal sunny
Plant Growth and Development
 Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Small RNA in rice genome
 Vol. 45 No. 5 SCIENCE IN CHINA (Series C) October 2002 Small RNA in rice genome WANG Kai ( 1, ZHU Xiaopeng ( 2, ZHONG Lan ( 1,3 & CHEN Runsheng ( 1,2 1. Beijing Genomics Institute/Center of Genomics and
Vol. 45 No. 5 SCIENCE IN CHINA (Series C) October 2002 Small RNA in rice genome WANG Kai ( 1, ZHU Xiaopeng ( 2, ZHONG Lan ( 1,3 & CHEN Runsheng ( 1,2 1. Beijing Genomics Institute/Center of Genomics and
Plants are sessile. 10d-17/giraffe-grazing.jpg
 Plants are sessile www.mccullagh.org/db9/ 10d-17/giraffe-grazing.jpg Plants have distinct requirements because of their sessile nature Organism-level requirements Must adjust to environment at given location
Plants are sessile www.mccullagh.org/db9/ 10d-17/giraffe-grazing.jpg Plants have distinct requirements because of their sessile nature Organism-level requirements Must adjust to environment at given location
CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON
 PROKARYOTE GENES: E. COLI LAC OPERON CHAPTER 13 CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON Figure 1. Electron micrograph of growing E. coli. Some show the constriction at the location where daughter
PROKARYOTE GENES: E. COLI LAC OPERON CHAPTER 13 CHAPTER 13 PROKARYOTE GENES: E. COLI LAC OPERON Figure 1. Electron micrograph of growing E. coli. Some show the constriction at the location where daughter
A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development
 A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development Xuemei Chen Waksman Institute, Rutgers University, Piscataway, NJ 08854, USA. E-mail: xuemei@waksman.rutgers.edu Plant
A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development Xuemei Chen Waksman Institute, Rutgers University, Piscataway, NJ 08854, USA. E-mail: xuemei@waksman.rutgers.edu Plant
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E
 CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
SMALL AUXIN UP RNA (SAUR) genes are the largest class of primary auxin (growth
 Abstract SMALL AUXIN UP RNA (SAUR) genes are the largest class of primary auxin (growth hormone) responsive genes in all land plants, many of which are expressed in actively growing plant tissues. This
Abstract SMALL AUXIN UP RNA (SAUR) genes are the largest class of primary auxin (growth hormone) responsive genes in all land plants, many of which are expressed in actively growing plant tissues. This
Chapter 18 Lecture. Concepts of Genetics. Tenth Edition. Developmental Genetics
 Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Chapter 18 Lecture Concepts of Genetics Tenth Edition Developmental Genetics Chapter Contents 18.1 Differentiated States Develop from Coordinated Programs of Gene Expression 18.2 Evolutionary Conservation
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Newly made RNA is called primary transcript and is modified in three ways before leaving the nucleus:
 m Eukaryotic mrna processing Newly made RNA is called primary transcript and is modified in three ways before leaving the nucleus: Cap structure a modified guanine base is added to the 5 end. Poly-A tail
m Eukaryotic mrna processing Newly made RNA is called primary transcript and is modified in three ways before leaving the nucleus: Cap structure a modified guanine base is added to the 5 end. Poly-A tail
Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang Chen, Qiang Dong, Jiangqi Wen, Kirankumar S. Mysore and Jian Zhao
 New Phytologist Supporting Information Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bhlh transcription factor MtTT8 Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang
New Phytologist Supporting Information Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bhlh transcription factor MtTT8 Penghui Li, Beibei Chen, Gaoyang Zhang, Longxiang
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering
 Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Supplemental material
 Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
Supplemental material THE JOURNAL OF CELL BIOLOGY Mourier et al., http://www.jcb.org/cgi/content/full/jcb.201411100/dc1 Figure S1. Size and mitochondrial content in Mfn1 and Mfn2 knockout hearts. (A) Body
The geneticist s questions. Deleting yeast genes. Functional genomics. From Wikipedia, the free encyclopedia
 From Wikipedia, the free encyclopedia Functional genomics..is a field of molecular biology that attempts to make use of the vast wealth of data produced by genomic projects (such as genome sequencing projects)
From Wikipedia, the free encyclopedia Functional genomics..is a field of molecular biology that attempts to make use of the vast wealth of data produced by genomic projects (such as genome sequencing projects)
Eukaryotic vs. Prokaryotic genes
 BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 18: Eukaryotic genes http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Eukaryotic vs. Prokaryotic genes Like in prokaryotes,
BIO 5099: Molecular Biology for Computer Scientists (et al) Lecture 18: Eukaryotic genes http://compbio.uchsc.edu/hunter/bio5099 Larry.Hunter@uchsc.edu Eukaryotic vs. Prokaryotic genes Like in prokaryotes,
HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds
 HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds Chongming Wu 1,2., Juanjuan Feng 1,2., Ran Wang 1,2, Hong Liu 1,2, Huixia Yang 1,2, Pedro
HRS1 Acts as a Negative Regulator of Abscisic Acid Signaling to Promote Timely Germination of Arabidopsis Seeds Chongming Wu 1,2., Juanjuan Feng 1,2., Ran Wang 1,2, Hong Liu 1,2, Huixia Yang 1,2, Pedro
Other funding Sources Agency Name: MSU Agricultural Experiment Station /Project GREEEN Amount requested or awarded: 30,000
 FINAL PROJECT REPORT Project Title: Functional genomics of flowering in apple PI: Herb Aldwinckle Co-PI(2): Steve VanNocker Organization: Cornell University Organization: Michigan State University Telephone/email:
FINAL PROJECT REPORT Project Title: Functional genomics of flowering in apple PI: Herb Aldwinckle Co-PI(2): Steve VanNocker Organization: Cornell University Organization: Michigan State University Telephone/email:
DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Other cellular processes (17%)
 Fig. 35-24 Other metabolism (18%) DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Unknown (24%) Energy pathways (3%) Cell division and organization (3%) Transport (4%) Transcription
Fig. 35-24 Other metabolism (18%) DNA or RNA metabolism (1%) Signal transduction (2%) Development (2%) Unknown (24%) Energy pathways (3%) Cell division and organization (3%) Transport (4%) Transcription
Ethylene is critical to the maintenance of primary root growth and Fe. homeostasis under Fe stress in Arabidopsis
 Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis Guangjie Li, Weifeng Xu, Herbert J. Kronzucker, Weiming Shi * Supplementary Data Supplementary
Ethylene is critical to the maintenance of primary root growth and Fe homeostasis under Fe stress in Arabidopsis Guangjie Li, Weifeng Xu, Herbert J. Kronzucker, Weiming Shi * Supplementary Data Supplementary
Anatomy of Plants Student Notes
 Directions: Fill in the blanks. Anatomy of Plants Student Notes Plant Cell Biology Segment 1. Plants Plants are organisms are incapable of movement produce food through 2. Animals Animals are multicellular
Directions: Fill in the blanks. Anatomy of Plants Student Notes Plant Cell Biology Segment 1. Plants Plants are organisms are incapable of movement produce food through 2. Animals Animals are multicellular
Introduction to Molecular and Cell Biology
 Introduction to Molecular and Cell Biology Molecular biology seeks to understand the physical and chemical basis of life. and helps us answer the following? What is the molecular basis of disease? What
Introduction to Molecular and Cell Biology Molecular biology seeks to understand the physical and chemical basis of life. and helps us answer the following? What is the molecular basis of disease? What
NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA
 NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA PŘÍRODNÍ VARIACE METABOLISMU CYTOKININŮ U ARABIDOPSIS THALIANA Samsonová Z. 1, 2, 3, Kuklová A. 1, 2, Mazura P. 1, 2, Rotková
NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA PŘÍRODNÍ VARIACE METABOLISMU CYTOKININŮ U ARABIDOPSIS THALIANA Samsonová Z. 1, 2, 3, Kuklová A. 1, 2, Mazura P. 1, 2, Rotková
AUXIN BINDING PROTEIN 1 (ABP1): A matter of fact. Chun-Ming Liu, Professor
 Commentary AUXIN BINDING PROTEIN 1 (ABP1): A matter of fact Chun-Ming Liu, Professor Editor-in-Chief Journal of Integrative Plant Biology (JIPB) Correspondence: cmliu@ibcas.ac.cn This article has been
Commentary AUXIN BINDING PROTEIN 1 (ABP1): A matter of fact Chun-Ming Liu, Professor Editor-in-Chief Journal of Integrative Plant Biology (JIPB) Correspondence: cmliu@ibcas.ac.cn This article has been
Regulation of Phosphate Homeostasis by microrna in Plants
 Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
Regulation of Phosphate Homeostasis by microrna in Plants Tzyy-Jen Chiou 1 *, Kyaw Aung 1,2, Shu-I Lin 1,3, Chia-Chune Wu 1, Su-Fen Chiang 1, and Chun-Lin Su 1 Abstract Upon phosphate (Pi) starvation,
2012 Univ Aguilera Lecture. Introduction to Molecular and Cell Biology
 2012 Univ. 1301 Aguilera Lecture Introduction to Molecular and Cell Biology Molecular biology seeks to understand the physical and chemical basis of life. and helps us answer the following? What is the
2012 Univ. 1301 Aguilera Lecture Introduction to Molecular and Cell Biology Molecular biology seeks to understand the physical and chemical basis of life. and helps us answer the following? What is the
Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Tuesday, December 27, 16
 Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Enduring understanding 3.B: Expression of genetic information involves cellular and molecular
Big Idea 3: Living systems store, retrieve, transmit and respond to information essential to life processes. Enduring understanding 3.B: Expression of genetic information involves cellular and molecular
Structures and Functions of Living Organisms
 Structures and Functions of Living Organisms 6.L.1 Understand the structures, processes and behaviors of plants that enable them to survive and reproduce. 6.L.1.1 Summarize the basic structures and functions
Structures and Functions of Living Organisms 6.L.1 Understand the structures, processes and behaviors of plants that enable them to survive and reproduce. 6.L.1.1 Summarize the basic structures and functions
The combined use of Arabidopsis thaliana and Lepidium sativum to find conserved mechanisms of seed germination within the Brassicaceae family
 www.seedbiology.de The combined use of Arabidopsis thaliana and Lepidium sativum to find conserved mechanisms of seed germination within the Brassicaceae family Linkies, A., Müller, K., Morris, K., Gräber,
www.seedbiology.de The combined use of Arabidopsis thaliana and Lepidium sativum to find conserved mechanisms of seed germination within the Brassicaceae family Linkies, A., Müller, K., Morris, K., Gräber,
Cytokinin. Fig Cytokinin needed for growth of shoot apical meristem. F Cytokinin stimulates chloroplast development in the dark
 Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Cytokinin Abundant in young, dividing cells Shoot apical meristem Root apical meristem Synthesized in root tip, developing embryos, young leaves, fruits Transported passively via xylem into shoots from
Cells: 3 Star. Which row in the chart below best explains the movement of some molecules between the model cell and the solution in the beaker?
 ells: 3 Star 1. ase your answer(s) to the following question(s) on the diagram below and on your knowledge of biology. The diagram represents a model cell setup. The locations of three different substances
ells: 3 Star 1. ase your answer(s) to the following question(s) on the diagram below and on your knowledge of biology. The diagram represents a model cell setup. The locations of three different substances
Introduction to Bioinformatics
 CSCI8980: Applied Machine Learning in Computational Biology Introduction to Bioinformatics Rui Kuang Department of Computer Science and Engineering University of Minnesota kuang@cs.umn.edu History of Bioinformatics
CSCI8980: Applied Machine Learning in Computational Biology Introduction to Bioinformatics Rui Kuang Department of Computer Science and Engineering University of Minnesota kuang@cs.umn.edu History of Bioinformatics
SUPPLEMENTARY INFORMATION
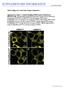 doi:10.1038/nature09606 Chen Li-Qing et al. A novel class of sugar transporters. Supplementary Figure 1. Confocal imaging of FRET sensor localization in HEK293T cells. The subcellular localization of the
doi:10.1038/nature09606 Chen Li-Qing et al. A novel class of sugar transporters. Supplementary Figure 1. Confocal imaging of FRET sensor localization in HEK293T cells. The subcellular localization of the
Heterosis and inbreeding depression of epigenetic Arabidopsis hybrids
 Heterosis and inbreeding depression of epigenetic Arabidopsis hybrids Plant growth conditions The soil was a 1:1 v/v mixture of loamy soil and organic compost. Initial soil water content was determined
Heterosis and inbreeding depression of epigenetic Arabidopsis hybrids Plant growth conditions The soil was a 1:1 v/v mixture of loamy soil and organic compost. Initial soil water content was determined
SUSTAINABLE AND INTEGRAL EXPLOITATION OF AGAVE
 SUSTAINABLE AND INTEGRAL EXPLOITATION OF AGAVE Editor Antonia Gutiérrez-Mora Compilers Benjamín Rodríguez-Garay Silvia Maribel Contreras-Ramos Manuel Reinhart Kirchmayr Marisela González-Ávila Index 1.
SUSTAINABLE AND INTEGRAL EXPLOITATION OF AGAVE Editor Antonia Gutiérrez-Mora Compilers Benjamín Rodríguez-Garay Silvia Maribel Contreras-Ramos Manuel Reinhart Kirchmayr Marisela González-Ávila Index 1.
Automated Illumina TruSeq Stranded mrna library construction with the epmotion 5075t/TMX
 SHORT PROTOCOL No. 02 I November 2014 Automated Illumina TruSeq Stranded mrna library construction with the epmotion 5075t/TMX Introduction For the MiSeq and HiSeq next generation sequencing (NGS) systems,
SHORT PROTOCOL No. 02 I November 2014 Automated Illumina TruSeq Stranded mrna library construction with the epmotion 5075t/TMX Introduction For the MiSeq and HiSeq next generation sequencing (NGS) systems,
Ph.D. thesis. Study of proline accumulation and transcriptional regulation of genes involved in this process in Arabidopsis thaliana
 Ph.D. thesis Study of proline accumulation and transcriptional regulation of genes involved in this process in Arabidopsis thaliana Written by: Edit Ábrahám Temesváriné Supervisors: Dr. László Szabados
Ph.D. thesis Study of proline accumulation and transcriptional regulation of genes involved in this process in Arabidopsis thaliana Written by: Edit Ábrahám Temesváriné Supervisors: Dr. László Szabados
Arabidopsis thaliana. Lucia Strader. Assistant Professor, Biology
 Arabidopsis thaliana Lucia Strader Assistant Professor, Biology Arabidopsis as a genetic model Easy to grow Small genome Short life cycle Self fertile Produces many progeny Easily transformed HIV E. coli
Arabidopsis thaliana Lucia Strader Assistant Professor, Biology Arabidopsis as a genetic model Easy to grow Small genome Short life cycle Self fertile Produces many progeny Easily transformed HIV E. coli
Useful Propagation Terms. Propagation The application of specific biological principles and concepts in the multiplication of plants.
 Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
Predicting Protein Functions and Domain Interactions from Protein Interactions
 Predicting Protein Functions and Domain Interactions from Protein Interactions Fengzhu Sun, PhD Center for Computational and Experimental Genomics University of Southern California Outline High-throughput
Predicting Protein Functions and Domain Interactions from Protein Interactions Fengzhu Sun, PhD Center for Computational and Experimental Genomics University of Southern California Outline High-throughput
Genetic controls of apple fruit-specific auxin metabolism. PI: Yanmin Zhu Co-PI(2): James Mattheis
 FINAL PROJECT REPORT Project Title: Genetic controls of apple fruit-specific auxin metabolism PI: Yanmin Zhu Co-PI(2): James Mattheis Organization: TFRL-ARS-USDA Organization: TFRL-ARS-USDA Telephone:
FINAL PROJECT REPORT Project Title: Genetic controls of apple fruit-specific auxin metabolism PI: Yanmin Zhu Co-PI(2): James Mattheis Organization: TFRL-ARS-USDA Organization: TFRL-ARS-USDA Telephone:
CONJOINT 541. Translating a Transcriptome at Specific Times and Places. David Morris. Department of Biochemistry
 CONJOINT 541 Translating a Transcriptome at Specific Times and Places David Morris Department of Biochemistry http://faculty.washington.edu/dmorris/ Lecture 1 The Biology and Experimental Analysis of mrna
CONJOINT 541 Translating a Transcriptome at Specific Times and Places David Morris Department of Biochemistry http://faculty.washington.edu/dmorris/ Lecture 1 The Biology and Experimental Analysis of mrna
Richik N. Ghosh, Linnette Grove, and Oleg Lapets ASSAY and Drug Development Technologies 2004, 2:
 1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
1 3/1/2005 A Quantitative Cell-Based High-Content Screening Assay for the Epidermal Growth Factor Receptor-Specific Activation of Mitogen-Activated Protein Kinase Richik N. Ghosh, Linnette Grove, and Oleg
Increasing Processing Tomato Fruit Soluble Solids
 Increasing Processing Tomato Fruit Soluble Solids Diane M Beckles Department of Plant Sciences dmbeckles@ucdavis.edu Processing Tomato Conference @ UC Davis December 13 th 2018 Soil Micronutrients Cultivar
Increasing Processing Tomato Fruit Soluble Solids Diane M Beckles Department of Plant Sciences dmbeckles@ucdavis.edu Processing Tomato Conference @ UC Davis December 13 th 2018 Soil Micronutrients Cultivar
Stress Effects on Myosin Mutant Root Length in Arabidopsis thaliana
 University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange University of Tennessee Honors Thesis Projects University of Tennessee Honors Program 5-2011 Stress Effects on Myosin
University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange University of Tennessee Honors Thesis Projects University of Tennessee Honors Program 5-2011 Stress Effects on Myosin
Supplementary Methods
 Supplementary Methods Microarray analysis Grains of 7 DAP of the wild-type and gif1 were harvested for RNA preparation. Microarray analysis was performed with the Affymetrix (Santa Clara, CA) GeneChip
Supplementary Methods Microarray analysis Grains of 7 DAP of the wild-type and gif1 were harvested for RNA preparation. Microarray analysis was performed with the Affymetrix (Santa Clara, CA) GeneChip
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
Regulation and signaling. Overview. Control of gene expression. Cells need to regulate the amounts of different proteins they express, depending on
 Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
Regulation and signaling Overview Cells need to regulate the amounts of different proteins they express, depending on cell development (skin vs liver cell) cell stage environmental conditions (food, temperature,
(17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON
 (17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON Jim Burton 1 and Marianne Pedersen Abstract. Cyclanilide [1-(2,4-dichlorophenylaminocarbonyl)-cyclopropane carboxylic
(17) CYCLANILIDE: MECHANISM OF ACTION AND USES AS A PLANT GROWTH REGULATOR IN COTTON Jim Burton 1 and Marianne Pedersen Abstract. Cyclanilide [1-(2,4-dichlorophenylaminocarbonyl)-cyclopropane carboxylic
Bio/Life: Cell Biology
 Bio/Life: Cell Biology 1a The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism's cells. As a basis for understanding
Bio/Life: Cell Biology 1a The fundamental life processes of plants and animals depend on a variety of chemical reactions that occur in specialized areas of the organism's cells. As a basis for understanding
Mole_Oce Lecture # 24: Introduction to genomics
 Mole_Oce Lecture # 24: Introduction to genomics DEFINITION: Genomics: the study of genomes or he study of genes and their function. Genomics (1980s):The systematic generation of information about genes
Mole_Oce Lecture # 24: Introduction to genomics DEFINITION: Genomics: the study of genomes or he study of genes and their function. Genomics (1980s):The systematic generation of information about genes
Analysis of regulatory function of circadian clock. on photoreceptor gene expression
 Thesis of Ph.D. dissertation Analysis of regulatory function of circadian clock on photoreceptor gene expression Tóth Réka Supervisor: Dr. Ferenc Nagy Biological Research Center of the Hungarian Academy
Thesis of Ph.D. dissertation Analysis of regulatory function of circadian clock on photoreceptor gene expression Tóth Réka Supervisor: Dr. Ferenc Nagy Biological Research Center of the Hungarian Academy
Reproduction, Seeds and Propagation
 Reproduction, Seeds and Propagation Diploid (2n) somatic cell Two diploid (2n) somatic cells Telophase Anaphase Metaphase Prophase I One pair of homologous chromosomes (homologues) II Homologues condense
Reproduction, Seeds and Propagation Diploid (2n) somatic cell Two diploid (2n) somatic cells Telophase Anaphase Metaphase Prophase I One pair of homologous chromosomes (homologues) II Homologues condense
