A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Engineering and Physical Science
|
|
|
- Junior Daniels
- 6 years ago
- Views:
Transcription
1 To Characterize the Black Carbon Using Single Particle Incandescence Technique: Instrumentation Development, Data Analysis Techniques and Quantitative Measurements A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Engineering and Physical Science 2009 Dantong Liu School of Earth, Atmospheric and Environmental Sciences
2 2
3 Contents List of figures....9 List of tables. 16 Abstract..17 Declaration Copyright Chapter 1: The Radiative Forcing of BC on Atmosphere- Earth System and Climate Response BC Sources and global distributions Regional Hotspots and Atmospheric Brown cloud (ABC) The radiative budget of the planet and radiative forcing The Direct Forcing of BC and other aerosols Global dimming BC deposited on snow and sea ice The Indirect Forcing of BC and other aerosols Indirect forcing of BC and non-bc aerosols Semi-direct forcing Glaciation indirect effect The importance of BC mixing state Climate system response and feedback
4 Chapter 2: The Characteristics of BC and Its Lifecycle in the Atmosphere-Current Knowledge and Limitations An introduction for the characteristics of BC (soot) The morphology of BC The size distribution The mixing of BC particles and related definitions The lifecycle of BC in the atmosphere The uncertainties and limitations of current models The objectives of this thesis Chapter 3: Overview of instruments for BC measurements Optical techniques Thermal techniques. 88 Chapter 4: The single particle soot photometer (SP2): instrument characterization, development and data analysis History and introduction Instrument configuration Quantification of BC The sensitivity to boiling point temperatures of different refractory compositions Size quantification of non-absorbing particles Determination of BC mixing state and the measurement of optical properties for absorbing particles
5 4.6.1 Evolution of scattering and incandescence signals in the laser beam for absorbing particles Determination of BC mixing state Perturbation of the scattered light signals from absorbing particles due to laser heating Determining the optical size of absorbing particles using the leading edge only (LEO) extrapolation technique Methodology of data analysis The instrument modification Data quality assurance and filtering Discrimination of scattering-only and absorbing particles Electronic Noise and Baseline Drift The fitting methodologies Saturation of channels Particle Coincidence Concentrations derived from SP2 measurements 170 Chapter 5: The investigations of BC close to the ground sources. 173 Overview Introduction Sites, instrumentations and data analysis techniques The experimental sites and sampling inlet BC physical properties Aerosol optical properties and BC mass loading Non-refractory aerosol compositions Aerosol hygroscopic properties
6 5.3 Results and discussions Diurnal variability in particle concentration Source tracking and attribution The mixing state of BC Influenced by different sources Relation to atmospheric processes Modification on hygroscopic properties of aerosols Summary of Chapter Chapter 6: The scavenging of BC and non-absorbing particles by water droplet of orographic cloud Overview Introduction Sampling inlets and instrumentations Data analysis The scavenged fraction of aerosol property Modelling on BC activation as influenced by coating contents Results and discussions Summary of Chapter Chapter 7: Characterizations of BC at an Alpine experimental site influenced by polluted boundary layer and free troposphere 235 Overview Site description and meteorological conditions
7 7.2 Instrumentation, sampling and data analysis methodology Sampling inlet Instrumentation and data analysis Results and discussions BC mass loading and absorption property Attributions of pollutant sources by air mass history and local wind The free tropospheric background under the influences of ground sources and wet removal The size distribution and mixing state of BC Summary of Chapter Chapter 8: The role of BC as an ice nuclei: a laboratory study and field measurements Introduction Laboratory chamber studies of the ice nucleating efficiency of BC The facilities The results The scavenging of BC and non-absorbing particles by mixed phase clouds Overview Experimental site, instrumentation and data analysis The experimental site Sampling inlets Aerosol measurements
8 Cloud microphysical measurements Aerosol scavenging by clouds Results and Discussions The BC and non-absorbing aerosol loadings on the total and interstitial inlets segregated by different phases of clouds The scavenged fraction (F) of aerosols influenced by cloud phases Summary of Chapter Chapter 9: Concluding remarks and future work The instrumentations The development of the instrument, calibrations and data analysis technique Ongoing and further technical development Summary of scientific findings, uncertainties and future work The aerosol properties and direct forcing The interactions of aerosol with cloud and indirect forcing..316 Acknowledgements 320 References. 321 Word count: 69,133 8
9 List of figures Figure A typical example of BC emission in South Africa, which is most responsible for reducing atmospheric visibility.. 24 Figure Summary of BC contributions by various sources for the year Figure the BC global distribution. 26 Figure A picture of Los Angeles ABC layer on 29 January Figure The annual and global mean energy balances of the earth Figure Summary of the radiative forcing by IPCC Assessment Figure The lower atmospheric solar heating (Wm -2 ) due to BC for the 2001 to 2003 period.. 34 Figure The direct radiative forcing of GHGs, BC and non-bc aerosols Figure A summary of the radiative forcing of different components Figure The surface dimming (Wm -2 ) due to ABCs Figure Schematic of non-bc aerosol indirect effects on warm clouds Figure Schematic of the BC indirect effects on warm and cirrus clouds..45 Figure A schematic illustration of BC mixing state. 50 Figure Absorption amplification calculated by the concentric core-shell model at 550 nm. 52 Figure Geographical distributions of estimated anthropogenic contribution to annual mean direct radiative forcing (W m -2 ) of BC at TOA Figure Electron microscope images of soot of different origins Figure Size classifications of atmospheric particles. 60 Figure Diagram of the BC lifecycle in the atmosphere.. 67 Figure A diversity plot of particle residence time in a range of global models in days
10 Figure A diagram to illustrate modelling steps in aerosol component modules of current global models. 74 Figure A summary of the main atmospheric processes during the lifecycle of BC in the atmosphere Figure the cross-section of the filter setup in the PSAP Figure The cross section of the MAAP instrument.. 86 Figure Schematic view of the prototype PAS.. 87 Figure Simplified block diagram for the Sunset Laboratory semi-continuous EC/OC field analyzer. 89 Figure Schematic of VTDMA and TD/SMPS volatility measurement systems...90 Figure the schematic diagram of the Manchester SP2 optical head. 94 Figure typical examples of single particle events detected by the SP Figure Calibration results using glassy carbon spheres from old and new versions of the SP Figure the correlation between the mass equivalent diameter measured by SP2 and the mobility diameter selected by DMA Figure The variations of peak incandescence signals for GCS Figure the temperature independence of P I, as indicated by a GCS calibration test under various levels of ambient temperatures Figure the blackbody irradiance as a function of emitted wavelength for materials with different absolute temperatures. 108 Figure Response of the SP2 to refractory materials with different compositions Figure Correlation between the incandescence broadband/narrowband ratio and boiling temperatures Figure the dots are the deduced boiling temperatures from measured P I(bb) /P I(nb) for a variety of BC mass equivalent diameters Figure the incandescence broadband/narrowband ratio distributions for ambient absorbing particles and CVI residues measured during CLACE6 project
11 Figure Peak scattering/incandescence intensity (P S /P I ) related to time delay (TD) for the absorbing particles measured from the atmosphere using a standard inlet and CVI residues during CLACE Figure Schematic show of the process for a scattering-only particle to pass through the laser beam Figure the scattering peak intensity varies with changed laser power for monodispersed PSLs Figure the digital points collected from the scattering channel are fitted with a Gaussian function to derive the peak amplitude Figure Calibration results using PSL solutions at a series of diameters. 122 Figure Mie calculations of the scattering cross section as a function of particle diameter collected by the SP Figure A schematic of FWHM and RT PO Figure A) Size dependence of RT PO distributions calibrated by mono-dispersed PSLs; B) the size dependence of RT PO for non-absorbing particles during the Holme Moss project Figure Schematic demonstrating the RT PO and FWHM for a large and small particle events Figure the FWHM distribution for non-absorbing particles collected from Holme Moss project Figure Results of the SP2 low temperature tests Figure P S as a function of SP2 chamber temperature calibrated by monodispersed PSL particles. 132 Figure A schematic representation of the time evolution of scattering and incandescence signals for an absorbing particle during its passage through the laser beam Figure Flame generated soot coated with sulfuric acid sampled during the AIDA experiment. 136 Figure schematic show of parameters TD and P S /P I for a BC coated with sulfuric acid Figure The relationship between TD and P S /P I for about 2000 BC particles measured during the Holme Moss project
12 Figure The refractory mass fraction in a single particle (MF) as a function of coating evaporation time (TD) Figure Comparison study measured at a semi-rural site and in an urban environment to validate the usage of coating evaporation time (TD)..142 Figure The lower and upper panel represents a single particle event for PSL and GCS respectively Figure The RT PO as a function of FWHM of scattering signals for monodispersed PSL and GCS particles. 146 Figure The P S distributions of monodispersed PSL and GCS particles at the sizes 300nm, 450nm and 600nm Figure The distributions of FWHM and RT PO for scattering signals of particles collected during the Holme Moss project. 148 Figure Schematic of the quadrant silicon APD configured as TEAPD Figure Schematic representation of the time evolution of a scattering signal Figure The scattering signal of a PSL particle imaged on the TEAPD and the normal APD Figure The scattered light imaged on the second quadrant of TEAPD is too low to detect the zero crossing point for absorbing particles Figure The distributions of FWHM and peak position as calibrated by monodispersed PSL particles at a range of sizes Figure Steps to apply the LEO technique to a PSL particle Figure The fitted peak amplitude of the scattering signal from the LEO technique correlates with the results from normal Gaussian fitting for PSL particles over a wide range of sizes. 156 Figure the fitting results using the LEO technique for soot particles generated with varying relative amounts of coating components during the AIDA project. 157 Figure The indications for LEO technique to reconstruct the original scattering signal for GCS 300nm Figure The P S derived using LEO technique for mono-dispersed PSL and GCS particles at a range of sizes
13 Figure Data analysis procedures for the Manchester SP2 instrument: A) the old version; B) the modified version Figure Typical solutions for digital saturation on scattering channel. 167 Figure the coincidence of two soot particles, fitting is only applied for the scattering signal of the leading particle. 169 Figure The number and mass based size distributions of BC, as measured by the old and modified SP Figure A) The map of the experimental site and surrounding area. B) Wind rose plot during the whole experiment period Figure Experimental set up of the aerosol instrumentation deployed during Holme Moss Figure The refractory mass fraction related to the coating evaporation time for continuously collected BC-containing particles. 184 Figure The relationship between BC mixing efficiency over the entire size range of the SP2 and at the median size ( nm) Figure Time series for the entire experiment: BC mass loading; the volume for the SP2 measured particles in the accumulation mode; HOA mass loading; CN number concentration. 189 Figure A) The correlation between BC mass and absorption coefficient. B) The probability distribution of observed mass absorption cross section. 190 Figure The diurnal variation in CN concentration; sub-micron/accumulation mode and the mobility diameter distribution at different times. 194 Figure The diurnal trends of: A) BC mass loading; B) BC number fraction; C) BC mass/non-refractory mass. 197 Figure The model spectra retrieved by a three-factor solution of PMF analysis on the AMS detected submicron organic aerosol in Holme Moss. 198 Figure The contributions to the total OA mass from different factors. 199 Figure A) The diurnal variation of BC mixing efficiency. B) A comparison of BC mass loading related to mixing efficiency for the whole dataset sampled from Holme Moss site and Manchester city centre Figure The correlation between the mass of BC and HOA when the sources are classified as a function of time
14 Figure A) Diurnal variations in non-refractory/bc mass ratio; B) the relationship between BC mixing efficiency and HOA/OA mass ratio. 206 Figure The diurnal variations of: A) the number fraction for particles with low growth factor (<1.2) in the different sizes; B) CCN activation fraction as a function of super-saturation. 208 Figure The influences on the CCN activation Figure Critical supersaturation for ammonium sulfate coated BC particles as a function of the refractory mass fraction in single particles Figure Time series of liquid water content, number concentration and mean diameter of cloud droplets. 221 Figure Time series of BC mass loading, non-absorbing particle volume, number concentration of CN and BC number fraction Figure The size distributions for: the volume of non-absorbing particles; the number of non-absorbing particles; C) mass of BC core and D) the number of BC core in the interstitial phase and cloud residues Figure A) The frequency distributions of coating evaporation time for interstitial aerosols and cloud residues. B) The BC scavenged fraction as a function of the refractory mass fraction of single BC particles (MF) Figure The experiment site was influenced by air mass history and local air transportation. 240 Figure Fire maps provided by the MODIS Rapid Response System Figure A) Two examples of detected BC in single particles; B) the refractory mass fraction related to the coating evaporation time Figure Time series of total BC mass loading from the SP2 and absorption coefficient measured by the Multi-Angle Absorption Photometer (MAAP) for the entire experiment. 248 Fiugure Figure A) Time series of aerosol properties during the entire experiment with the classified periods separated by dotted lines Figure Time series of aerosol properties during the entire experiment with the classified periods separated
15 Figure Frequency distributions of aerosol and trace gas properties under free tropospheric background conditions, under the influence of SE winds, and when influenced by precipitation Figure The mean BC mass size distribution measured under different conditions Figure The frequency distribution of BC mixing efficiency under different conditions Figure BC mixing efficiency as a function of the ratio between sub-micron and accumulation mode aerosol number Figure Statistical analysis of the BC mass loading at this site. 270 Figure Schematic view of the AIDA experimental facility. 276 Figure The mixing state of soot particles coated with sulphuric acid in the AIDA chamber before and after the expansions Figure The cloud microphysics for a typical expansion experiment Figure The distributions of coating evaporation time for the soot particles coated with sulphuric acid observed in the ice residues Figure The time series of ambient temperature, ice water content and liquid water content Figure The relationship between total water content and ice mass fraction Figure The time series of particle properties sampled from the total and interstitial inlets Figure Statistical analysis on BC mass loading measured downstream of total and interstitial inlet classified according to cloud phases Figure Statistical analysis on non-absorbing particle volume loading measured downstream of total and interstitial inlet classified by cloud phases Figure The frequency distributions of aerosol scavenged fraction for the whole dataset classified as cloud-free, liquid cloud and mixed phase cloud Figure The BC number fractions on the interstitial line during cloud free, liquid cloud and mixed phase cloud Figure A summary of BC mass loading as a function of mixing state for the three projects mentioned in this thesis
16 List of tables Table 3-1. A summary of experimental sites with SP2 measurements deployed Table 4-1. The hardware modification of the Manchester SP2 instrument. 97 Table 4-2. The functionality of the SP2 instrument with data assured quality before and after the modification Table6-1. Summary of the measured aerosol and cloud properties, expressed as the mean value ± σ Table A summary of meteorological conditions and aerosol properties categorized by five periods classified by back trajectory analysis Table A summary of the data presented in figure7.3-4, including the median value, mean value and 10%, 25%, 75%, 90% percentiles for each property
17 Abstract Black carbon aerosol (BC), also known as atmospheric soot, originating from the incomplete combustion, can efficiently absorb solar radiation in the visible and near infrared, significantly contributing to the heating of the lower troposphere and reducing radiation reaching the earth surface. BC has been recognized as the second main contributor to global warming following the Greenhouse Gases. This thesis reviews the importance of BC on modifying the solar-atmosphere-earth radiative budget via direct and indirect forcing, and identifies the current state of knowledge and important uncertainties in the characteristics of BC, including size distribution, mixing state, hygroscopic and optical properties, which are associated with its atmospheric processing and radiative forcing on the earth-atmospheric system. Much of the work presented in this thesis aims to calibrate and develop an instrument using a laser induced incandescence technique that detects single-particles to characterize BC - the single particle soot photometer (SP2). The SP2 laser can heat the absorbing BC particle until incandescence and can also optically size each particle passing through the laser. This instrument represents many advances compared to the traditional optical-thermal BC quantification techniques, including the high sensitivity to low aerosol concentration, the clear segregation of BC from other species of aerosols, the data availability for single particles and the capacity to detect the BC mixing state. The developed toolkit of data analysis in this work is capable of processing the raw data acquired from the field measurements or laboratory experiments, filtering the data to assure the quality, automatically applying calibration parameters and correction factors under a variety of conditions to derive useful and quantitative results. The SP2 instrument has been deployed at various types of field experimental sites and laboratory studies to investigate the BC characteristics in the atmosphere with varying focuses, including urban and rural sites close to emission sources, as well as the free troposphere remote from ground sources. The interactions between BC particle and cloud are investigated under various conditions at the experimental sites. The BC soon after emission can be mixed with some primary materials, accomplishing with the other co-products from incomplete combustion, such as carbon monoxide and primary organic components. During the transport, the BC mass loading is diluted and the BC is more strongly mixed: the BC mass loadings (mixing efficiency) measured at the urban, rural and free troposphere site range from ng/m 3 (0.05±0.03), ng/m 3 (0.21±0.07) and 1-20ng/m 3 (0.4±0.15) respectively. The atmospheric processing alters both physical properties and chemical compositions of BC. The largely coated BC may have exhibited some hygroscopicity to serve as cloud condensation nuclei (CCN). The BC transported to the free troposphere can also be sporadically influenced by fresher ground sources, and a significant fraction of BC is deposited on the snow when experiencing precipitations. In the free tropospheric background, the BC has been more efficiently incorporated into the ice clouds compared to the other species of aerosols. These combined observations build up a framework in improving the understanding of BC lifecycle in the atmosphere, addressing the climatic issues caused by the BC emission and will constrain the future modelling work importantly. 17
18 18
19 Declaration No portion of the work referred to in the thesis has been submitted in support of an application for another degree or qualification of this or any other university or other institute of learning. Dantong Liu 19
20 20
21 Copyright The author of this thesis (including any appendices and/or schedules to this thesis) owns any copyright in it (the Copyright ) and s/he has given The University of Manchester the right to use such Copyright for any administrative, promotional, educational and/or teaching purposes. Copies of this thesis, either in full or in extracts, may be made only in accordance with the regulations of the John Rylands University Library of Manchester. Details of these regulations may be obtained from the Librarian. This page must form part of any such copies made. The ownership of any patents, designs, trade marks and any and all other intellectual property rights except for the Copyright (the Intellectual Property Rights ) and any reproductions of copyright works, for example graphs and tables ( Reproductions ), which may be described in this thesis, may not be owned by the author and may be owned by third parties. Such Intellectual Property Rights and Reproductions cannot and must not be made available for use without the prior written permission of the owner(s) of the relevant Intellectual Property Rights and/or Reproductions. Further information on the conditions under which disclosure, publication and exploitation of this thesis, the Copyright and any Intellectual Property Rights and/or Reproductions described in it may take place is available from the Head of School of Earth, Atmospheric and Environmental Science (or the Vice-President). 21
22 22
23 Chapter 1 The Radiative Forcing of BC on Atmosphere-Earth System and Climate Response The effect of Greenhouse Gases (GHGs) on the radiative budget of the earth has long been known whereas the effect of aerosols is less well understood. A key component of atmospheric aerosol, black carbon (BC), which originates from incomplete combustion, has unique and efficient absorbing characteristics at visible and near-infrared wavelengths. It significantly contributes to solar absorption, resulting in heating the lower troposphere and reducing radiation reaching the earth surface. Combined with its capacity to form atmospheric brown clouds (ABCs) and mix with other aerosols or become incorporated into cloud particles, the importance of BC on climate change has been recognized as the second main contributor to global warming following the GHGs, as reviewed by Ramanathan and Carmichael [2008]. Its impact can be further enhanced when deposited onto snow and sea ice, as well as when incorporated into cloud particles where it can potentially act as ice nuclei in the upper troposphere and lower stratosphere (UT/LS). The strong absorption by BC within clouds could also modify cloud microphysics. The redistribution of energy between the earth and the atmosphere caused by elevated BC concentration are examples of the many feedbacks in the Earth s climate system. 23
24 1.1 BC Sources and global distributions Black carbon aerosol or BC is formed through the incomplete combustion of carboncontaining fuels and biomass, the latter through both anthropogenic activity and natural sources, and is also referred to as soot [Andreae et al. 1997]. Figure A typical example of BC emission in South Africa, which is most responsible for reducing atmospheric visibility [Seinfeld et al. 2008]. The sources of BC arise from the indoor and outdoor combustion. The BC in the indoor environment is largely due to cooking and domestic heating using biofuels such as wood, dung and crop residue. The fossil fuel (coal, diesel, petrol) combustion, e.g. power generation by coal burning, and anthropogenic use for transportation, industrial production etc, has been widespread in the industrialized regions like North America and Western Europe since the late 19th and early 20th century, but since about the 1950s, the developing nations in the tropics and East Asia have also become major source regions [Bond et al. 2007]. Open biomass burning in the outdoor environment is mainly associated with deforestation, crop residue burning and natural forest fire. 24
25 Figure Summary of BC contributions by various sources for the year 1996 from Bond et al. [2004]. In 1996, the global annual emissions of BC were about 8Tg/year according to Bond et al. [2004], as figure summarizes, 20±4% of BC was from residential biofuels burned with traditional technologies, 40% from fossil fuels, open biomass burning contributed 45±5%, largely from the forest and savanna burning. The BC emissions from transport use, like shipping [Lack et al. 2008], and aircraft engines [Hendricks et al. 2004] have been recently evaluated. BC, once emitted, can be transported and distributed on a global scale, but has a significant impact in highly-polluted regions. However, due to effective environmental policy and more advanced technology, from the late 20th century, the aerosol loading, including the anthropogenic BC, has been mitigated, displaying a weaker impact on climate relative to green house gases [Andreae et al. 2003]. 25
26 Figure the BC global distribution from Ramanathan and Carmichael [2008]. The unit is in tons per year applying the BC inventory study by Bond et al. [2004]. This includes BC sources from fuel combustion and open biomass burning for the year Particularly, the spatial and temporal uncertainty of regional emissions is about 100% or more. The regions with significant loadings of BC are indicated as red and yellow colour in figure BC in the tropics and the mid-latitude Northern Hemisphere is mainly emitted from fossil fuel combustion and biofuel cooking [Bond et al. 2004], largely contributed by south-eastern Asia [e.g. Menon et al. 2002, Ramanathan et al. 2001]; in the mid-latitude Southern Hemisphere, open biomass burning sources are more widespread, and the important BC source regions include Amazon forest burning [Andreae et al. 2004], Savanna burning [Po sfai et al. 2003] in southern Africa, as well as west Indonesia. BC is efficiently transported in the mid-high-latitude Northern Hemisphere. Sources originating in western Europe and north America have been demonstrated to be able to influence Arctic sea ice [McConnell et al. 2007]. The uncertainty of published estimates for BC emissions is a factor of two to five on regional scales and at least ±50% on global scales [Ramanathan and Carmichael 2008]. The uncertainties are not solely caused by the different emission inventories applied, the 26
27 predictions of BC lifecycle and evolution in the atmosphere can significantly alter its horizontal and vertical distributions [e.g. Chung and Seinfeld 2005, Jacobson 2002, Lauer and Hendricks 2006, and Holben et al. 2001], which rely on the availability of the field and satellite data. The limitations of the current models regarding BC are discussed in section Regional Hotspots and Atmospheric Brown cloud (ABC) Section 1.1 discussed the BC distribution at global scale, and revealed some significantly polluted regions with high loading of BC (figure1.1-3). The Intergovernmental Panel for Climate Change report (IPCC) and Ramanathan and Carmichael [2008] pointed out the regional impact of BC can be 2-5 times of the global average. The high concentrations of BC from these sources can be elevated in the lower troposphere, where it interacts and is mixed with other aerosols like sulfates, nitrates, as well as fly ash and water vapour, in some humidity conditions, to form haze. This aerosol mixture can form transcontinental plumes with vertical extents of 3 to 5 km. Previously, this phenomenon has been frequently observed above Asia and the Indian Ocean every year between January and March [e.g. Ramanathan et al. 2001]. Viewed from satellite photos, the cloud appears as a giant brown stain hanging in the air. These are thus named as Atmospheric Brown Clouds (ABC), which have been extensively documented by surface observatories, field observations and satellite data [e.g. Kaufman and Koren 2006, Haywood et al. 2003, Ramanathan et al. 2007a, Stone et al. 2007]. Recent work by Ramanathan et al. [2007b], who used a range of a range of satellite observations and surface measurements, combined with models simulating radiation and atmospheric transport, characterized brown clouds and their radiative effects worldwide, and identified the regional hotspots that have been highly influenced by ABC. These hotspots include most of Southeast Asia including Indonesia, Mexico, Central America, most of Brazil and Peru in South America. About 3 billion people are living under the influence of these regional ABC hotspots. 27
28 Figure shows a picture from a downward view above the ABC in Los Angeles [Seinfeld 2008], which clearly demonstrates that the atmospheric transmittance has been reduced by the ABC. The BC-containing mixture within ABC can dramatically enhance the solar absorption contributed by BC itself because BC is also absorbing the light reflected by other species of aerosols around them [Jacobson 2001]. The absorption by ABC heats atmospheric layers in which the ABC resides, usually the lower troposphere and reduces transmission of solar radiation downwards, leading to a surface cooling effect. The ABC therefore plays an important role in redistributing the energy between atmosphere and earth surface [e.g. Ramanathan et al. 2001]. Figure A picture of Los Angeles ABC layer on 29 January 2004 [Seinfeld 2008], this city was classified as a regional aerosol hotspot, and was one of 26 megacities evaluated by Ramananthan et al. [2007b]. The solar radiation is absorbed by ABC, leading to a heating of the lower atmosphere but dim the surface below by blocking more radiation downwards. 28
29 1.3. The radiative budget of the planet and radiative forcing Both the atmosphere and the Earth s surface receive energy from the Sun in the form of radiation. About half of the total solar radiation is emitted as visible light (λ at nm), and the other half is emitted mostly in the near-infrared part (the infrared part which is close to visible part: nm), with some in the ultraviolet part of the spectrum ( nm). A physical object will radiate some amount of energy at the wavelengths typical for the temperature of the object, and at higher temperatures more energy is radiated at shorter wavelengths. Due to the much lower temperature of the earth surface compared to the sun, thermal radiation emitted from the surface is largely at infrared longwave wavelengths (700nm-1mm). In the following discussions, longwave radiation is loosely associated with the low-temperature-radiation by earth (or atmosphere) in the infrared, whereas shortwave radiation decides the radiation by sun in the range of visible-near infrared. The global energy flow, defined as the radiative budget, can be quantified in terms of irradiance and radiant emittance with a unit of watts/m 2, which is firstly described by Kiehl and Trenberth [1997], as shown in figure About 30% of the incoming shortwave solar radiation at the top of the atmosphere is reflected by the surface, atmosphere and clouds. The remaining 70% is absorbed, warming the land, atmosphere and ocean. The heated earth surface and atmosphere emit infrared radiation, some of the infrared photons emitted by the atmosphere downwards can be returned to the earth surface, and the escaped energy must be very closely balanced by energy received, maintaining an average surface temperature of roughly +14 C. 29
30 Figure The annual and global mean energy balances of the earth in units of Watts/m 2, are shown as diagram by Kiehl and Trenberth [1997]. The yellow arrows on the left indicate about 50% of the incoming solar radiation (161 Wm -2 ) is absorbed by the earth surface, and the rest is reflected back to the outer space via cloud layer and surface. The heat absorbed by the surface is returned to the atmosphere as sensible heat in the form of evapo-transpiration and thermal infrared radiation. The atmosphere absorbs a large fraction of radiation, which in turn emits radiation both up and down. The pink arrows on the right demonstrate the impact by natural greenhouse gases, which mainly absorb the longwave infrared radiation from the surface and emit in turn infrared radiation in all directions including downward to the Earth's surface. The Greenhouse Gases (GHGs), which include water vapor, carbon dioxide and methane, efficiently absorb the thermal longwave radiation emitted by the Earth surface, the atmosphere itself, and clouds. If in the presence of additional GHGs, the heavily enhanced longwave absorption can dramatically warm the atmosphere, hereafter in turn 30
31 emit the radiation downward back to the earth. Thus, GHGs trap the heat within the surface-troposphere system. The remaining fraction of the heat budget is balanced by convection and conduction. This process by which energy is recycled in the atmosphere to warm the Earth's surface is known as the greenhouse effect and is an essential control of Earth's climate. To investigate the anthropogenic impact on climate change, the IPCC Assessment 2001 [Houghton et al. 2001] defined the radiative forcing as: the change in net (down minus up) irradiance (solar plus longwave; in W/m 2 ) at the tropopause AFTER allowing for stratospheric temperatures to readjust to radiative equilibrium, but with surface and tropospheric temperature and state held fixed at the unperturbed values. The radiative forcing of various components is reported in the IPCC Assessment 2007 [Forster et al. 2007], as figure shows. The statistical summaries with the uncertainties denoted by the error bars, indicate a better understanding on the forcing of GHGs than that of aerosols. Quite different from the GHGs, aerosols are also sensitive to the shortwave radiation, which means the aerosols can scatter and/or absorb the incoming solar radiation, referred as direct forcing [reviewed by Haywood and Boucher, 2000]. Aerosols can also be incorporated into clouds, modifying the cloud microphysical properties, thus altering the cloud impact on climate, termed as indirect forcing [e.g. a review by Lohmann and Feichter 2005]. Both aerosol effects are still subject to large uncertainties (figure 1.3-2). 31
32 Figure Summary of the radiative forcing by IPCC Assessment 2007 [Forster et al. 2007], which indicates a large uncertainty for the knowledge of aerosol impact on climate whereas the rate of GHGs is relatively well understood. As Chapter 2 will in detail discuss, the unique feature of BC compared with other species of aerosols is their capacity to absorb solar radiation, thus they are able to heat the atmosphere around them. Additionally, like other aerosols, they also reflect some of the incoming solar radiation back to space, cooling the Earth surface. However the magnitude of energy absorption by aerosols on the global scale and their contribution to global warming remains uncertain [Ramanathan and Carmichael 2008]. 32
33 1.4. The Direct Forcing of BC and other aerosols Aerosol interactions with sunlight have been widely investigated during the last decade. Most of the investigations have focused on sulfate [e.g. as early as Charlson et al. 1987], which decreases the globally averaged solar radiation absorbed by the Earth-Atmosphere system, due to reflection of incident solar radiation back to space. More recent global models including other anthropogenic aerosols, like nitrates [Adams et al. 2001] and organic carbon [Cooke et al.1999] have also been studied. All of these aerosols indicate a cooling effect at top of the atmosphere (TOA), as evidenced by satellite observation [e.g. Bellouin et al. 2005] and modelling work [e.g. Haywood and Shine 1995, Haywood and Boucher 2005]. BC differ profoundly from other aerosols in their interaction with solar radiation because of their efficient absorption, radiatively warming the surround atmosphere and cooling the earth surface below them by reducing transmission of solar radiation. Whereas other aerosols, such as sulfate, nitrate and OC, cool both the surface and atmosphere by significantly scattering the sunlight backwards into the space. Solar absorption by BC increases with inverse wavelength in the near-infrared to the ultraviolet, following a power law of between one and three depending on the sources [Bond and Bergstrom 2006a]. The efficient absorbing and scattering characteristics of BC are largely because of their morphology (see Chapter 2 for further discussions), with randomly oriented needles or disks being more absorbing than spheres of the same mass, by factors as much as two or more, and the voids can also proportionally increase the absorbing power [Liu and Mishchenko 2005]. The absorption behavior of BC is further amplified by mixing with other aerosols and incorporation into cloud droplets [e.g. Jacobson 2001, Chylek et al. 1996, the importance of BC mixing state is discussed in section 1.6]. Ambient aerosols or cloud particles strongly reflect light, increasing the chances of absorption by surrounding soot particles. Therefore, both positive forcing in the lower atmosphere and negative forcing at the earth surface will be enhanced in regional hotspots where the ABCs are prevalent 33
34 (section 1.2). Particularly, surface cooling is the combined effects of aerosol scattering/absorbing and cloud reflection, observed as global dimming. There is currently a matter of important understanding on whether this effect could offset or has masked greenhouse warming [e.g. Wild et al. 2005, Andreae et al. 2003]. Figure The lower atmospheric solar heating (Wm -2 ) due to BC for the 2001 to 2003 period by Ramanathan and Carmichael [2008]. The values presented here are calculated from the study by Chung et al. [2005], who integrated satellite aerosol data, surface network of aerosol remote sensing instruments and field observations with an aerosol-transport-chemical model and a radiative transfer model to obtain the forcing. Figure shows the lower atmosphere heating due to BC on a global scale, which clearly indicates the regional hotspots where BC is of high abundance, and the regional distribution is roughly a combination of solar irradiance as well as the capacity to form widespread atmospheric brown clouds (ABCs) in a mixture with other aerosols, hence Ramanathan and Carmichael [2008] claimed emissions of black carbon are the second strongest contribution to current global warming, after carbon dioxide emissions. A comparison of radiative forcing among GHGs, BC and non-bc aerosols in figure summarizes the different behaviours of these components on modifying the energy flux in the earth-atmosphere system. 34
35 Selected References: Kiehl and Trenberth 1997 Chung and Seinfeld 2005 Charlson et al Ramanathan et al Haywood and Ramaswamy 1998 Hansen and Nazarenko 2004 Figure The direct radiative forcing of GHGs, BC and non-bc aerosols. The green and red arrows indicate the shortwave and longwave radiation respectively (see the wavelength definitions in section 2.1). The thickness of the arrows is loosely associated with the light intensity. This diagram demonstrates the fundamental and major impacts on the energy flux but doesn t represent all of the atmospheric processes, and the unknown forcing trends are not shown here. The corresponding references are noted in the table below. 35
36 The fundamental difference between the absorption of BC and GHGs is that BC has the capacity to absorb the solar radiation mainly at the shortwave (visible and near-infrared light), whereas the efficient absorption of GHGs is mainly targeted at longwave radiation (infrared light) emitted by the earth surface. The BC thus reduces the transmission of incoming solar downwards towards the surface whereas GHGs recycle more heat back to the surface, however both BC and GHGs contribute to lower atmospheric heating. The cooling effect of non-bc aerosols are apparent because the scattering is a significant fraction of incoming solar radiation backwards and most of them are not absorbing, additionally, they can extend the cloud lifetime or cloud cover to enhance the cloud reflection, hereby increasing the indirect forcing effect (see section 1.5 on indirect forcing). If looking down on Earth from space, the anthropogenic GHGs are making the planet darker in the IR but the non-bc aerosols are making the planet brighter in the visible. The BC forces the earth to absorb more energy from the sun (a positive forcing at TOA) by absorbing the solar radiation reflected by the surface-atmosphere-cloud system and reducing the albedo of snow and sea ice when it is deposited onto them [e.g. Hansen and Nazarenko 2004]. Further enhanced positive forcing at the TOA is because soot inside cloud drops and ice crystals can decrease the albedo of clouds by enhancing absorption by droplets and ice crystals [e.g. Jacobson 2006, Mikhailov et al. 2006]. Figure also shows the high warming effect when BC is above the cloud, when it can additionally absorb the light strongly reflected by the cloud, absorbing more energy not allowing the transmission back to space [Haywood and Ramaswamy 1998, Chung and Seinfeld 2002]. Therefore BC absorption is highly sensitive to the vertical distribution of particles relative to clouds. When BC is deposited on snow and sea ice, decreased surface albedo leads to more surface absorption of solar radiation, playing a significant role on snow melting and glaciation retreat. Due to the high surface albedo from snow and ice, direct radiative forcing due to BC in the northern high latitudes is still high, although the BC loading in the northern high latitudes is relatively small 36
37 [Chung and Seinfeld 2005, Jacobson 2004]. All of these factors will contribute to a positive forcing at top of atmosphere (TOA) due to the absorption of BC. Ramanathan and Carmichael [2008] calculated the radiative forcing of these components in figure 1.4-3, BC s role on the lower atmosphere heating is emphasized. Figure A summary of the radiative forcing of different components, by Ramanathan and Carmichael [2008]. a) Forcing for all GHGs (CO 2, CH 4, N 2 O, halons and ozone); b) for CO 2, the forcing values represent the change in radiative forcing due to increases in gases from preindustrial times until the year 2005; c) BC forcing is valid for the period; d) Non-BC forcing includes the direct and the indirect forcing. The current debate on the issue of global warming is if the aerosol could offset the positive forcing caused by GHGs, reducing the aerosol loading might unmask significant increases in global warming. The following texts discuss more details about the global dimming and BC s influence on surface albedo in two individual sections due to their important roles on climate change. 37
38 1.4.1 Global dimming Systematic measurements of heat flux have been available since the 1950s, a phenomenon kept being observed that the amount of global direct irradiance emitted from the earth surface was gradually reduced for several decades, especially the case for the regional hotspots, where the surface cooling turns out to be almost in balance with heating of the atmosphere due to black carbon [Ramanathan et al. 2001]. This is known as global dimming, and this trend is further enhanced by the presence of increased anthropogenic aerosol emissions, including BC and the BC-non-BC mixture in brown clouds [Ramanathan and Carmichael 2008]. Figure from Ramanathan and Carmichael [2008] provides a surface dimming distribution at a global scale. Figure The surface dimming (Wm -2 ) due to ABCs by Ramanathan and Carmichael [2008]. This shows the reduction in absorbed solar radiation at the surface by all anthropogenic aerosols (BC and non-bc) in ABCs for the 2001 to 2003 period. Compared with figure 1.4-1, the surface dimming almost coincides with the lower atmospheric heating, indicating the high abundance of atmospheric brown clouds (ABCs) that significantly redistribute the energy between the earth surface and lower atmosphere. The sum forcing of BC and non-bc at TOA is about -1W/m 2 according to the calculation by Ramanathan and Carmichael [2008], which can also compensate for the warming trend by GHGs. 38
39 The decline of solar radiation received by the earth surface has been widely reported for the period of about 1960 to 1990 [Andreae et al and references therein], has not continued since then. Solar dimming coupling with increased effect of GHGs arising from increased air pollutants continued until the 1980s, but not thereafter because the atmosphere started to clean [Wild et al. 2005] as a result of more effective clean-air regulations. Aerosols have a much shorter lifetime than the GHGs in the atmosphere and therefore can be more efficiently controlled. Andreae et al. [2003] claimed the masking of the greenhouse effect and related impacts may no longer have been effective during the 1990s, enabling the greenhouse signals to become more evident [also see Wild et al. 2007]. 39
40 1.4.2 BC deposited on snow and sea ice Snow and ice have the capacity to reflect solar radiation back into space because of their high reflectivity. Deposited snow on Himalayan Glaciers and on sea ice in the Arctic contributes to cooling the Earth surface. Soot can be deposited over the snow and ice via wet deposition (section 2.2, figure2.2-1). This darkens the snow and significantly reduces the light reflectance but enhances the solar absorption by snow and ice. Along with accelerated melting of the snow-ice cover, BC allows the earth to absorb more energy from the sun [Warren and Wiscombe 1985]. Furthermore, if BC is present within ice crystals, rather than externally mixed, the absorbing power of BC can be amplified by a factor 1.4 or more [Hansen and Nazarenko 2004, Jacobson 2006]. The mid-high latitude areas in the Northern Hemisphere are more sensitive to BC deposition because of the widespread presence of snow and ice. Hansen and Nazarenko [2004] have estimated that climate forcing due to snow ice albedo change is of the order of 1W/m 2 at middle and high latitude land areas in the Northern Hemisphere. Over the Arctic Ocean, the melting of the snow concentrates aerosol at the surface as a residue, darkening the surface more in the late winter and spring, thus increasing absorption and lengthening the melt season. Jacobson [2004] obtained similar modeling results, BC was calculated to reduce snow and sea ice albedo by about 0.4% in the global average and 1% in the Northern Hemisphere. The most recent simulation study by Flanner et al. [2007] pointed out the reduction of sea ice and snow albedo by BC is three times as effective as CO 2 forcing for global average surface warming. McConnell et al. [2007] examined the historical ice-core records of BC deposited over Greenland from the early nineteenth century onwards, revealing correlations between warming throughout Greenland and elevated levels of soot concentration. They found the sources of BC through ice core record changed from natural causes such as forest fires and volcanic eruptions to industrial pollution during the period of the late 19th and early 20th century. In a model study, they speculated the industrial areas of the United 40
41 States and Canada were the likely sources of the increased soot levels during the past century. Ramanathan et al. [2007a] applied a general circulation model (GCM) simulations, and suggested that advection of the warmer air heated by BC from South and East Asia over the Himalayas contributes to a warming of about 0.6ºC (annual mean) in the lower and mid troposphere of the Himalayan region. Thus BC forcing is as important as GHGs in the observed retreat of over two thirds of the Himalayan glaciers. These studies provided direct and historical evidence on the importance of BC on sea ice and glacier retreat. The melting ice and sea level rise define the level of dangerous anthropogenic interference with the climate system. Therefore reducing the soot emissions to restore snow albedos to pristine high values, would benefit both reducing global warming and raising the global temperature level at which dangerous anthropogenic interference occurs. 41
42 1.5. The Indirect Forcing of BC and other aerosols Indirect forcing of BC and non-bc aerosols Anthropogenic aerosols modify cloud microphysics in different ways: by influencing the cover; changing lifetime of clouds; and modifying precipitation rate. These processes can feedback on the wet deposition of aerosols, alter cloud reflectance and therefore rebalance the energy flux within the solar-atmosphere-earth system. The radiative forcing caused by the aerosol-cloud interaction is known as indirect forcing. Aerosol particles can act as seeds for cloud droplet formation if they are good cloud condensation nuclei (CCN). For a given amount of water content, the increased cloud particle concentration will decrease the average radius of the cloud droplets due to the sharing of liquid water by more particles. The increased concentration of smaller cloud droplets leads to an increase in the reflectivity of polluted clouds. This effect was firstly demonstrated by Twomey [1974], which is known as the first indirect effect or cloud albedo effect [IPCC 2001 report]. In addition, the aerosol can further increase the cloud optical depth by virtue of increasing the lifetime of clouds and the amount of clouds, as the time scale before droplets grow large enough to form precipitations is extended [Albrecht 1989]. Cloud thickness was also observed to be increased by aerosols [Pincus and Baker 1994]. The suppression of drizzle is part of the cloud lifetime effect as being shown most clearly from ship track studies [e.g., Ferek et al. 1998]. These effects may lead to a further enhancement of cloudiness, enhancing reflection of solar radiation, giving rise to the socalled second indirect radiative forcing or cloud lifetime effect. Indirect forcing of non-bc aerosols is schematically summarized in figure If BC is incorporated into clouds, the resulting responses will be quite different due to their absorption characteristics. The strong heating effect associated with BC reduces the relative humidity thus leading to a suppression of cloud formation or vaporization of the 42
43 existing clouds. This is called the semi-direct forcing. BC transported to higher altitudes can potentially act as ice nuclei (IN), accelerating the glaciation of high altitude cirrus clouds - the glaciation indirect effect. These two effects are outlined for BC schematically in figure 1.5-2, and are discussed in section and respectively. 43
44 Selected references Twomey 1974 Albrecht 1989 Pincus and Baker 1994 Ferek et al Figure Schematic of non-bc aerosol indirect effects on warm clouds, the thickness of arrows loosely indicates the intensity of reflected lights, and the dotted lines represent the precipitation rate. The corresponding references are noted in the table below. 44
45 Selected references Ackerman et al Conant et al Nenes et al Lohmann 2002 Figure Schematic of the BC indirect effects on warm and cirrus clouds, the arrows and lines have the same meanings as in figure
46 1.5.2 Semi-direct forcing As section 2.2 will discuss, the activity of BC as CCN are considerably enhanced when freshly emitted BC acquire hydrophilic coatings during ageing in the atmosphere. As BC particles become sufficiently hydrophilic, they are able to form cloud droplets upon activation in a supersaturated environment. The absorption capacity of BC can be amplified if the absorption of solar radiation by these aerosol particles occurs within cloud droplets [Chylek et al., 1996]. These effects may alter the dynamical and hydrological processes governing cloud formation. On regional scales where ABCs are prevalent, the lower atmospheric heating coupled with earth surface dimming can lead to increased static stability of the earth-atmosphere system, reducing convective activity and cloud coverage [Ackerman et al. 2000, Ramanathan et al. 2001, Menon et al. 2002]. By extended modeling of the Köhler theory, Conant et al. [2002] identified that the heat released by BC included within the CCN population can increase the local temperature, reducing the relative humidity, hereby hindering the particle ability to activate due to the elevated critical supersaturation. This effect raises the droplet vapour pressure, inhibits activation of the most absorptive CCN or potentially evaporates the existing clouds, resulting in the reduction of cloud water content. The reduced cloud cover and cloud optical depth will in turn further amplify warming of the Earth-atmosphere system. Another pathway contributing to warming is the involvement of BC in clouds decreasing the cloud albedo (figure 1.5-2), enhancing the energy absorption by clouds. A positive feedback could also be important, that is the increase in water vapour at the expense of precipitation contributing to warming in addition to that of the cloud BC absorption itself because the water vapour is one of the main GHGs. However, modelling work conducted by Nenes et al. [2002] indicated BC could slow down the growth of Giant Cloud Condensation Nuclei (GCCN), which are defined as 46
47 particles that have a diameter larger than 5µm enough to decrease their size and ability in initiating the formation of drizzle. Compared to a non-heated and drizzle-formed cloud, this effect will increase the cloud droplet number concentration and result in more persistent clouds. A simulation study by Johnson et al. [2004] obtained a positive semidirect effect only when the absorbing aerosol layer was situated within the boundary layer but obtained a negative semi-direct effect when the absorbing aerosol layer is situated above the cloud layer. A further uncertainty that can influence the resulting cloud droplet population and optical properties is the extent to which BC mixes with other particles, i.e., whether the BC is within the interstitial aerosol, or whether it is included within the cloud droplets [Nenes et al. 2002]. Therefore, the global magnitude of the semi-direct effect is highly uncertain [Ramanathan and Carmichael 2008]. 47
48 1.5.3 Glaciation indirect effect BC can be transported as high as the upper troposphere and lower stratosphere (UT/LS) [e.g. Cziczo et al., 2004, Schwarz et al. 2006]. Lohmann and Feichter [2001], Penner et al. [2003] concluded that the semi-direct effect is only marginally important at the top of the atmosphere in the global mean, whereas Jacobson [2002] pointed out that the climatic effect of black carbon is strongly positive. Lohmann [2002] showed that in addition to mineral dust, if a fraction of hydrophilic soot aerosol particles is assumed to act as contact ice nuclei at temperatures between 0ºC and 35ºC, then increases in aerosol concentration from pre-industrial times to present-day pose a new indirect effect, a glaciation indirect effect. The increased number of contact ice nuclei in the presentday climate will result in more frequent glaciation of supercooled clouds and increase the amount of precipitation via the ice phase. This process could occur at the expense of the surrounding water content because of the lower vapor pressure over ice compared to over liquid, called the Wegener-Bergeron-Findeisen (WBF) mechanism [Bergeron, 1935], and this glaciation effect may affect the mid- and high latitudes of the Northern Hemisphere more significantly. The reduction of cirrus cloudiness lessens the solar reflectivity in the UT/LS, exhibiting a positive radiative forcing. Aircraft emissions contain a considerable amount of BC and may contribute to the UT/LS BC loading in addition to BC transported from ground sources. The simulation studies by Hendricks et al. [2004] revisited the large-scale contribution of aircraft emissions to the UT/LS BC mass budget, suggesting even in the most frequent regions aviation impacts amount to only a few percent. However, the influence introduced by aircraft can cause large scale increases in the UT/LS BC particle number concentration in regions highly frequented by aircraft, larger than the corresponding perturbations of the BC mass loading. This is mainly caused by the small sizes of aircraft-generated BC particles. A general circulation modeling study [Hendricks et al. 2005] indicated the cloud modifications induced by aircraft BC particles could change the ice crystal number concentration at northern mid-latitudes significantly by up to 10 40%, provided the aviation induced BC particles serve as efficient ice nuclei. Significant cirrus 48
49 modifications by BC from aircraft cannot be excluded, and the potential indirect climatic impacts of BC from aviation may become of increasing importance in the future as air traffic increases. Kärcher et al. [2007] comprehensively investigated the role of soot on cirrus cloud formation, pointing out that understanding still need to be improved to access the behavior of soot on heterogeneous ice nucleation. Most of the current models are based on hypothetical scenarios with assumed ice nucleation efficiency of specified background. A critical discussion of laboratory experiments reveals that the ice nucleation efficiency of soot particles depends strongly on their source, and, by inference, on atmospheric aging processes. Particularly, the mass and chemistry of soluble surface coatings appear to be crucial factors. For example, a recent experimental study on soot heterogeneous ice nucleation was carried out in the AIDA (Aerosol Interaction and Dynamics in the Atmosphere) chamber Forschungszentrum, Karlsruhe, Germany. In this chamber, Möhler et al. [2005a] investigated graphite spark generated soot, observing that immersion in aqueous sulfuric acid slightly enhanced the ice nucleation onset of the soot, and Möhler et al. [2005b] found that increasing the OC content to 40% markedly suppressed the ice nucleation efficiency of flame soot. Furthermore, the modes of nucleation mechanism are not well understood. Most recent studies show little, or modest, ice nucleation activity of soot particles in the immersion mode [Kärcher et al. 2007]. The role of other ice nucleation modes such as contact freezing needs to be further clarified [Sastry, 2005]. 49
50 1.6. The importance of BC mixing state Purely or poorly mixed soot particle is quite water-insoluble due to its graphitic structure, which was observed to display a weak capacity for the water uptake [Weingartner et al. 1997, Mallet et al. 2004]. During transport after emission, the soot can acquire more water-soluble materials via condensation processes, when secondary components can be converted from gas phase to the aqueous/solid phase attaching inward/outward soot particle (see section for more details). Soot may also mix with other particles close to sources by coagulation but only occurs when number concentrations are high. To simplify the mixing state of a soot particle, the shell-and-core model is widely used [e.g. Jacobson 2000], which implies the particle composition is concentric with an absorbing core surrounded by a shell of negligibly absorbing material, as figure illustrates. This scenario defines the internal and external mixing sate of BC, and is applied widely in models to investigate the impact of BC mixing state on its hygroscopicity, optical properties and radiative forcing. Figure A schematic illustration of BC mixing state by Jacobson [2000], showing the possible core size relative to shell thickness. The configuration of an internally mixed particle is unphysical, since EC cannot be diluted through a particle, except in the limiting external-mixture case. 50
51 The initial hydrophobic soot can be transformed to be a more hydrophilic particle, especially when the internal mixing state is achieved. The conversion of soot to a hygroscopic particle is a crucial factor to determine its residence time in the atmosphere, because internal mixing can lead to an enhancement of cloud condensation nuclei (CCN) activation (see section 2.2 for detailed discussions), hereafter resulting in more efficient removal via wet deposition. The mixing state of BC also controls its dry deposition efficiency, since hydrophilic BC is deposited more efficiently over wetted surfaces. Hence, the model simulated ratio of hydrophilic to hydrophobic BC is a key parameter when determining the efficiency of both wet and dry BC removal processes, controlling its horizontal and vertical transport and distributions. The mixing state of BC also significantly influences its optical properties. The absorption enhancement of BC due to mixing has been long estimated by applying Mie theory [Mie 1908, and extended by Bohren and Huffman 1983]. The concentric coreshell scenario is the widely used modelling approximation [e.g. Fuller et al. 1999, Jacobson 2001]. Bond et al. [2006b] comprehensively reviewed the up-to-date studies on BC absorption enhancement, and presented the modelling results derived from the concentric core-shell model at incident light wavelength 550 nm in figure This plot indicates the absorption enhancement is extremely important for BC core smaller than 200nm, whereas the larger BC core is difficult to be encapsulated. The other used mixing rules are based on volume-weighting different properties of the components [e.g. Lesins et al. 2002] and also show similar results. In addition, absorption efficiency can be affected by the relative position of a BC core within the mixed particle. For example, the modeling work by Fuller et al. [1999] has shown that for small carbon inclusions with comparatively thick shells, the absorption was greatest when the inclusion is exactly positioned at the centre, and was strongly reduced when the inclusion moves away from the centre. 51
52 Figure Absorption amplification calculated by the concentric core-shell model at 550 nm from [Bond et al. 2006b] as presented by a contour plot of amplification. Assume the core refractive index (m) = i and shell m = e-6i. The modeling results were confirmed by several experimental studies. Mikhailov et al. [2006] observed that optical properties of hydrophobic soot (acetylene soot) showed very little dependence on relative humidity (RH) while the scattering and absorption coefficient of hydrophilic soot (mixture of acetylene soot and glutaric acid) increased markedly with RH, and the maximum enhancement of absorption for a soot water drop agglomerates could be as much as a factor of 3.5. Soot coated with secondary organics was investigated by Schnaiter et al. [2005], thick coatings of organic material showed a maximum amplification of These authors also calculated similar results using Mie theory combined with measured particle sizes. Under actual atmospheric conditions, the BC absorption can be further enhanced by optical focusing when it enters cloud drops or ice crystals during nucleation scavenging or aerosol-hydrometeor coagulation, and also by intense reflected light when BC is deposited on the snow and sea ice. The high reflectivity of surfaces over desert, fog, 52
53 snow, sea ice and cloud surfaces can increase the number of photons hitting a soot particle, providing more chances that it will be absorbed by nearby flecks of soot [Jacobson 2006]. The modeling results of anthropogenic BC s contribution on global annual radiative forcing by Chung and Seinfeld [2002] give a good example of the absorption enhancement. Figure Geographical distributions of estimated anthropogenic contribution to annual mean direct radiative forcing (W m -2 ) of BC at TOA for externally-mixed (left panel) and internally-mixed (right panel) BC, from Chung and Seinfeld [2002]. Results are averages of the last 75 years of simulation. Chung and Seinfeld [2002] emphasized the importance of BC mixing state contributing to the temperature response. Figure shows two extreme scenarios, the predicted globally and annually averaged radiative forcing increase of the internally-mixed case is almost twice that of the externally-mixed case. As Chung and Seinfeld modeled, the predicted water vapor increase is greater if BC is assumed to be internally mixed with sulfate instead of externally mixed. Additionally, the increase in temperature, changed precipitation pattern, and altered cloud coverage are all enhanced if BC is assumed to be internally mixed rather than externally mixed. 53
54 1.7. Climate system response and feedback The BC-contained aerosol layer can reduce the solar radiation reaching the earth surface, as well as heat the ambient air, this energy redistribution within the earth-atmosphere system changes the vertical and horizontal thermal structure of the atmosphere and surface. In turn this effects on the temperature profile, evaporation, latent heat fluxes, atmospheric stability, and the strength of aerodynamic convection governing cloud formation, which in turn, can modify the large-scale circulation, thus could be responsible for droughts and flood simultaneously [Menon et al. 2002, Prospero et al. 2003]. The destabilization of the atmosphere above the boundary layer as a result of BC heating within the boundary layer was obtained in a GCM climate model study by Menon et al. [2002]. Absorbing BC will reduce the atmospheric stability above the boundary layer, resulting in enhanced vertical motion. In the simulations, the large-scale circulation and precipitation pattern over China were affected, Menon et al. resembled these influence associated with climate events, like floods and droughts that China has experienced in recent years. More widely, this vertical heating gradient alters the latitudinal and interhemispheric gradients in solar heating and these gradients play a prominent role in driving the tropical circulation [Ramanathan et al., 2001], which can perturb the monsoon significantly. The stronger BC solar heating of the atmosphere over South Asia strengthens the monsoonal outflow with stronger rising motions over the subcontinent, accompanied by a stronger moisture flux into South Asia [Ramanathan and Carmichael 2008]. The latent heat flux from the surface to the atmosphere (e.g. evaporation) and sensible flux are balanced by the surface net radiation (figure 1.3-1). A major fraction of the reduction in surface solar radiation thus needs to be compensated by a reduction of one or all of these components. The resulting reduction in the evaporation will have to be balanced by a reduction in rainfall and effectively spin down the hydrological cycle. These changes in the hydrological cycle caused by aerosols are probably more important 54
55 than the mere temperature change because they have consequences for fresh water supply and food production among others [Smith 2005]. Chung and Seinfeld [2002] predicted the BC radiative forcing to increase precipitation between 0 and 20ºN and decrease precipitation between 0 and 20ºS, which is explained by the enhanced interhemispheric temperature difference, inducing a change in the zonal mean meridional circulation and convection in the tropics. The resulting change in the Hadley cell circulation leads to increased precipitation north of the equator and compensating reduction in precipitation south of the equator. Observational data show that precipitation trends during the last 50 years have been negative, particularly over Africa, South Asia and northern China, where the natural variability and anthropogenic aerosol forcing are emerging as major players in the observed trends [Chung and Ramanathan 2006]. The link between dimming, the north-south sea surface temperature (SST) gradient and a reduction in land rainfall has also been invoked to explain the Sahel drought occurring in the 1970s and 1980s [Rotstayn and Lohmann 2002]. 55
56 Chapter 2 The Characteristics of BC and Its Lifecycle in the Atmosphere-Current Knowledge and Limitations The climate effects of BC discussed in Chapter 1 result from its unique physic-chemical characteristics compared to other types of aerosols. This chapter aims to identify the current state of knowledge and important uncertainties in the fundamental properties of BC, including size distribution, mixing state, hygroscopic and optical properties, which are associated with its atmospheric processing and radiative forcing on the earth-atmospheric system. The lifecycle of BC in the atmosphere, from origin until sink, is discussed in detail. The current knowledge on the behaviour of BC in the atmosphere is still subject to large uncertainty due to the limitations of currently available measurements, but may be improved using the single-particle-based technique. The following chapters will give an overview on the current technologies of BC measurements, and present the advantages of the single particle technique to improve our understanding on BC in the atmosphere. 56
57 2.1. An introduction for the characteristics of BC (soot) The morphology of BC Figure Electron microscope images of soot of different origins: A) flamegenerated soot from propane fuel [Slowik et al. 2004]; B) soot sampled from an urban area in Mexico [Johnson et al. 2005], the arrow a indicates the BC component and arrow b shows sulfate condensed on the soot surface; C) aerosols observed during a Savanna burning event [Pósfai et al. 2003], the potassium is an indicator of biomass burning, this shows fractal soot and typical organic aerosol with an inorganic inclusion; D) sampled during the ACE-Asia project, outflow from China contains large amount of soot-dust mixtures [Clarke et al. 2004], this picture is from lower troposphere at an altitude about 700m, the middle solid circle is fractal soot, around which are tar balls and dust. 57
58 The formation process of BC is complex and varies with different types of combustion, normally, the particle formation process in flames begins with the creation of condensation nuclei such as polycyclic aromatic hydrocarbons (PAHs) that are subsequently pyrolized to form soot [Turns 1996]. More details on soot formation can be found in the books by Heywood [1988] and Turns [1996]. The initially formed soot particles are in the form of primary spherules with diameters typically in the range of about nm [Heywood 1988]. These small particles will grow after emission and eventually aggregate into larger chain clusters (picture A and also see Park et al. [2004], Slowik et al. [2004]). The irregular shape of soot raises the difficulty of defining the actual size of the soot particle. As the 2-D transmission electron microscopy (TEM) images show (figure1.2-1), the image projected area length and width can reflect the shape and size of the soot, this image method is widely used to discriminate soot with other species of aerosols [e.g. Chakrabarty et al. 2006, Po sfai et al. 2003, Clarke et al. 2004] because of its unique shape. Another important factor is the particle density, due to the branches and voids within the body of soot, the particle mass will be substantially reduced compared to a solid sphere of similar size. If a particle is charged and passes through a constant electric field, there will be a force balance between the electrical force on the net charges on the particle and the drag force experienced by particle itself. This force balance is used to size particles by instruments such as the differential mobility analyzer (DMA, see Williams [1999]). The electrical mobility diameter is defined as the diameter of a sphere with the same migration velocity in a constant electric field as the particle of interest [Flagan 2001]. The fractal shape and relatively large surface area of soot can amplify the drag force in the electric field to some extent [DeCarlo et al. 2004], leading to a shift in the measured size. The electrical mobility diameter of irregular soot particle has been characterized widely, by a combination of particle mass related instruments, like the aerosol mass spectrometer [Slowik et al. 2004], and the aerosol particle mass analyzer (APM, McMurry et al. 2002, Park et al. 2004). Typically, the AMS measures the so-called vacuum aerodynamic diameter, which is defined as the diameter of a sphere with a 58
59 standard density that travels through a gas at the same terminal velocity as the particle of interest under the free molecular regime [DeCarlo et al. 2004]. All of these experiments observed that the high drag force of the fractal soot leads to smaller masses or aerodynamic diameters being measured compared to the particle in a normal shape and without voids. Therefore, the authors point out the soot s effective density would be much lower than its actual density if derived from the mass divided by mobility diameter The size distribution In many cases it is not possible to define a single size of an aerosol, because in the real world aerosols have widely varying shapes and compositions and are not perfect spheres of uniform densities. The size distribution of a given population of particles is normally defined operationally. Atmospheric particles can range from the order of nanometers, e.g. formed by homogenous nucleation, to as large as several micrometers e.g. the cloud droplets. A general categorization of particle size, regardless of the different instrumentation characterization, is to classify the particle diameter into broad classes as: nucleation mode (3nm-10nm), Aitken mode (10nm-100nm), accumulation mode (100nm-1000nm) and coarse mode (>1000nm), although some other slightly different borders to divide the aerosol ranges appeared in some literatures [e.g. Kittelson et al. 2000, Hoppel and Frick 1990]. Raes et al. [2000] summarizes the physical and chemical processes affecting different sizes of particles that result in the formation and evolution of particles, as shown in figure A). With respect to atmospheric processes, the Aitken and accumulation mode particles are classified as the fine mode collectively. Particles with size smaller or larger than that are termed as ultrafine mode and coarse mode respectively. 59
60 Figure A) Size classifications of atmospheric particles, and the range of microphysical processes that influence the size distribution and chemical composition of the atmospheric aerosol from Raes et al. [2000]. B) Number weighted mobility diameter distributions from diesel engine emissions in which soot is the main component, measured by Harris and Maricq [2001], with the exhaust gas recirculation (EGR) varying from 0 to 37%, the lines represent lognormal fittings of measured data. The two vertical dot lines in grey in A) generally indicate the size range of soot. The chemical processes involved in aerosol transformation include the formation of condensable products from gas phase, heterogeneous reaction on/in particles and in- 60
61 cloud reactions. The main physical mechanisms for particle transformation are coagulation and condensation. The rate of coagulation depends on the background particle concentration [Raes et al. 2000], which is the result of small particles coming into contact due to Brownian diffusion on larger particles by gravitational settling. The nucleation and Aitken mode particles have relatively short residence time because of their ability of self-coagulate and undergo coagulation with larger particles. Condensation takes place in a supersaturated environment with respect to a gaseous vapour species, which condenses onto the particles, depending on the ambient pressure, temperature and the availability of condensable vapors. All of these processes can lead to particle growth and internal mixing, section 1.4 gives more detailed illustrations. Size distributions of aerosols can be idealized using a lognormal distribution, for the soot, this expectation was validated by measuring fossil fuel engine emissions at various combustion conditions. For example, Harris and Maricq [2001] studied the size distributions of diesel exhaust particulate matter from a number of vehicles and test engines. Regardless of the different operational conditions, the soot particles were distributed in lognormal functions, although the median size position varied with combustion conditions. If considering the definitions above, the size of soot generally peaked around the border of the Aitken and accumulation modes, as figure B indicates. The single lognormal distribution of ambient BC has been confirmed by many ground studies [e.g. Kondo et al. 2006, Rose et al. 2006], as well as in the free troposphere by Clarke et al. [2004]. All of these BC distributions are approximated by a single peak near µm diameter. However, one of the challenges to characterize the actual size or density of soot is that its morphology varies with distances away from sources, lifetime in the atmosphere, and different fuel and combustion processes [e.g. Chakrabarty et al studied several laboratory generated soot by burning different materials]. Flame generated soot was studied by Slowik et al. [2004], by a careful control of the fuel supply, they found a threshold of propane fuel/oxygen ratio above which the shape of generated soot altered significantly from fractal to compact, due to the varying amount of condensed organic 61
62 material at different operational conditions. The fractal shape of soot was known to be altered and reconstructed via atmospheric processes [e.g. Liousse et al. 1995], such as the deposition of coatings, and cloud/precipitation scavenging. The ageing time away from the originating sources makes the initial soot more compact, and this can be caused by mixing with other non-bc materials. 62
63 2.1.3 The mixing of BC particles and related definitions Soon after emission, soot particles are often mixed with other components simultaneously formed during combustion. For example, diesel generated soot are observed to be mixed with organic coating composed primarily of lubricating oil, unburned fuel, ash, and PAHs [e.g. Park et al. 2004, Kittelson 1998]. The sulfur in gasoline or diesel fuel may be converted to sulfate in the presence of water vapor and condense onto the fuel soot [figure2.1-1 B, and Kittelson 1998, Johnson et al. 2005], and aircraft-emitted soot particles were also observed to contain sulfate [Schneider et al. 2005]. The soot originating from biomass burning are highly mixed with species of primary organics, like some hydrocarbon like organics [Zhang et al. 2007, Kanakidou et al. 2005], quite frequently including some PAHs and Humic-Like Substances (HULIS) [e.g. Andreae et al. 1997, Clarke et al. 2007]. A recent study by Schwarz et al. [2008b] characterized the plumes from fossil fuel combustion and open biomass burning respectively, indicating a larger fraction of internal mixing for the BC from biomass burning closer to source. BC is quite absorbing at visible and near infrared wavelengths [as reviewed by Bond and Bergstrom 2006]. The details of the BC optical properties and the impact on radiative forcing have been discussed in Chapter 1. The term carbonaceous aerosols refers to any carbon-related aerosols, typically including the black carbon (BC) and organic carbon (OC). The term black carbon (BC) is commonly defined in an operational sense as the absorbing component of carbonaceous aerosols, thus this term is used when discussing the properties related to the absorption of visible radiation. Moreover, some organics, existing separately or mixed with BC, also contribute to solar absorption in addition to BC, whist BC is more efficient absorber in the visible. Organic materials absorb more efficiently at short wavelengths having a higher Angström coefficient than BC. These carbonaceous aerosols are termed brown carbon [Andreae and Geleneser 2006]. For example, the so-called Humic-Like Substances (HULIS) have been suggested as a major component of absorbing OC [Graber and Rudich, 2006], and 63
64 Schnaiter et al. [2006] observed the aromatic OC arising from long chained PAH had much greater shortwave absorption relative to the soot BC component. The term soot has been used by the Intergovernmental Panel on Climate Change to denote any light-absorbing, combustion generated aerosols [IPCC 1996]. This is a vague definition that may include any dark appearing, carbon-containing compound generated in combustion [Bond and Bergstrom 2006]. Therefore, soot can describe the BCcontaining particles when they are highly mixed with other non-bc components and/or BC soon after emission when it is externally mixed. The term elemental carbon also frequently appears in some literature. It is often used when thermal properties are considered, along with the term refractory carbon, as an operational definition based on the stability of carbon at elevated temperatures. Elemental carbon usually identifies carbon that does not volatilize below a certain temperature, usually about 550ºC [Bond and Bergstrom 2006]. Most of the definitions addressed here are developed from the operational technology of the traditional BC measurements, e.g. optical-based absorption or thermal-based instrument used to identify the refractory carbon (see Chapter 3 for details). The composition of coatings the soot can acquire can be sulfate, secondary organics and nitrate, although this depends on the circumstances and climatological conditions. Riemer et al. [2004] modeled aging of soot and calculated that condensation of sulfate (as sulfuric acid) dominated during the day whereas coagulation was favored at night, largely due to the daytime production of sulfate by OH oxidation of SO 2. Shiraiwa et al. [2007] applied Positive Matrix Factorization (PMF) analysis [Paterson et al., 1999] and demonstrated that sulfate and organics accounted for 90 (±7)% of the coating on BC during the daytime, while nitrate also contributed substantially to the coating during the nighttime in an urban environment. The soot can be driven to be more hydrophilic via surface oxidation by atmospheric ozone and oxidizing radicals [e.g. Zuberi et al., 2005]. 64
65 Soot particles can also be mixed with dust (figure D), which has an especially significant impact during the spring season dust storms from Asia and Africa transport across the Pacific Ocean [Clarke et al. 2004, Stith et al. 2007] and the Atlantic Ocean [Prospero and Lamb 2003]. The dust-soot mixture enhanced the solar absorption and earth surface dimming (Chapter 1). 65
66 2.2. The lifecycle of BC in the atmosphere The lifecycle of BC in the atmosphere includes emission, vertical or horizontal transport, during which the BC eventually evolves to an internal mixture through condensation, hydration, dissolution, dissociation, crystallization, aqueous chemistry, coagulation, and scavenging via cloud activation processes, by acting as cloud condensation nuclei (CCN) and ice nuclei (IN) or aerosol-cloud coagulation, cloud-cloud coagulation. Such processes lead to rainout or washout afterwards, and deposition onto the earth surface. Some particles are removed via dry deposition, landing on (in) snow and sea ice. These processes are summarized in figure
67 67
68 Soot particles experience a relatively lengthy atmospheric processing compared with other more hydrophilic aerosols, such as sulfate, nitrate and water-soluble organic [Kanakidou et al. 2005, Lauer and Hendricks 2006]. Textor et al. [2006] included sixteen global models into the intercomparison initiative AeroCom ( to evaluate aerosol life cycles in different global models by intercomparisons with each other and by comparisons to observations of aerosol properties and processes. The residence times of these aerosols among the models results are quantified in figure 2.2-2, from longest to shortest are: BC, POM, DU, SO 4 and SS, particularly, the residence time of BC is 5-10days. The main reason that BC has a longest atmospheric lifetime among these aerosols is that BC is the most insoluble compared to the others and the dominant removal process is via wet deposition [Jacobson 2004]. Figure A diversity plot of particle residence time in a range of global models in days compared as part of the aerosol model intercomparison initiative AeroCom ( [Textor et al. 2006]. The AeroCom system compared and investigated the parameters and processes that govern the simulated aerosol life cycles in sixteen global aerosol models. The abbreviations in this plot: SS means sea salt, POM (particulate organic matter), BC (black carbon), and AER (total dry aerosol). 68
69 The atmospheric residence time of the aerosol is defined as the time scale from emission at source until it is removed, e.g. deposited on the earth surface. Within the models particles can be classified as primary particles and secondary particles. Primary particles can be naturally generated by the wind: sea salt, soil and mineral dust, with most of their mass in the coarse mode [Raes et al. 2000]. This category also includes the emissions from biomass burning, which contributes a major source of carbonaceous material in tropical areas [Andreae et al. 2001]. Anthropogenic primary particles are mainly from fuel combustion, like the emissions from vehicle exhausts which often contain large concentrations of carbonaceous nucleation mode particles [Kittelson et al. 1998, 2004]. Secondary particles can be formed via the atmospheric oxidation of gas/radical phase species resulting in products of reduced vapor pressure which can partition into the aerosol phase. This can occur via condensation onto available solid/liquid surface area. Primary particles can grow or be mixed with secondary-formed composition via condensation and/or coagulation. Sulfate and nitrate aerosols are generally typical of secondary formed particles from a series of gas/radical reactions [e.g. as summarized in Atkinson et al. 2004], and the secondary organic aerosol is more complex [as reviewed by Kanakidou et al. 2005]. BC after emission will maintain a stable and unique chemical composition, but can be mixed with other non-bc species, by which its initial physical, hygroscopic and optical properties are altered when internally mixed with other components. Unlike BC, the organic aerosol (OC) has larger variability and has no fixed chemical composition. The primary organics can transform into secondary forms via various processes and the extent of the formation of secondary organic particle is a general parameter to identify the aerosol processing time scale. Due to these different stability between BC (also named as elemental carbon (EC) because of the operational techniques used for detection [Bae et al. 2004]) and OC in the atmosphere, the OC/EC ratio has been widely used to determine the amount of secondary organic aerosol formation [initialized by Turpin and Huntzicker, 1995]. The larger ratio generally indicates a stronger secondary formation of aerosols. 69
70 Aerosols are removed from the atmosphere via two pathways, dry deposition and wet deposition, both of which are highly dependent on particle size. The aerosol sink via dry deposition results from the combined effect of gravitational sedimentation, impaction and interception as a result of Brownian diffusion. Gravitational settling mainly controls the movement of high mass particles with large inertia, therefore the rate coefficients of dry deposition are large for the dust and sea salt [Textor et al. 2006]. The BC, as well as the sulfate, nitrate and organic aerosol, which are all of sub-micron size, are not strongly affected by dry deposition processes [Raes et al. 2000], but are mainly removed from the atmosphere via wet deposition. Wet deposition is the combined effect of nucleation scavenging and impaction scavenging, which leads to rainout or washout and deposits the aerosols on the earth surface. The effectiveness of particles being scavenged by cloud particles is dependent on their performance as cloud nuclei, which is termed nucleation scavenging. It is a complicated process related to the phases of water content within clouds. Cloud particles are classified as cloud droplets or ice particles if the water is in liquid and solid phase respectively, and the corresponding processes of nucleation by water droplet or ice particles follow different mechanisms. Water droplets in a warm cloud can be formed just above water saturation by condensation onto a hygroscopic particle a Cloud Condensation Nuclei (CCN). The CCN, provide a non-gaseous surface to transfer water from a vapor to a liquid [Charlson et al. 1987]. The performance of the aerosol as CCN has been widely studied [e.g. one of recent reviews by McFiggans et al. 2006]. This aerosol-cloud droplet interaction is a key factor in determining the aerosol lifetime, and can influence the cloud microphysics and so perturb the cloud s impact on the climate [e.g. Haywood and Boucher 2000, further discussions in section 1.5]. The CCN activity of aerosol is sensitive to particle size and chemical composition [McFiggans et al. 2006], but at various atmospheric conditions, these parameters can be of different importance. CCN activation is more efficient at larger dry particle sizes 70
71 [Dusek et al. 2006b] but will be mitigated if particles contain more water-insoluble content [e.g. Mochida et al. 2006]. The main reason that BC has relatively long residence time (figure 2.2-2) is that it is difficult to be activated because of its fairly smaller size (section 2.1) and water-insolubility, making it a relatively poor CCN. The CCN activity of a particle will determine the wet deposition efficiency. However, the BC-containing particle can still act as CCN if mixed with more watersoluble materials. It is expected that the water-soluble coating would enhance the soot CCN activation whereas the activation would be suppressed if the coating had weak capacity for water uptake. This hypothesis has been supported by a variety of experimental studies. For example, Dusek et al. [2006a] nebulized an aqueous suspension of carbon black in a collision atomizer, and they observed by adding 5% mass of NaCl to the carbon black suspension their CCN efficiency was greatly enhanced; Petzold et al. [2005] investigated the role of sulphuric acid coating on the CCN activation of the organic fraction of the combustion particles from a gas turbine engine, and found the sulfuric acid coating increased the potential CCN activation whereas the non-volatile OC partially compensated for the sulphuric acid-related enhancement of CCN activation of carbonaceous combustion aerosol particles; Kuwata et al. [2007] showed that in a urban environment BC particles mixed with higher volatile components exhibited more enhanced CCN activation than those with less faction of coatings. The atmospheric process to convert BC from hydrophobic to hydrophilic aerosol provides a crucial parameter in prediction of the vertical dispersion and meridional longrange transport of BC. In global models, this transformation is treated as an e-folding time but can vary from about 1 day [e.g. Park et al. 2005] to as long as 1.8 days [Koch et. al.1999]. However, there remains a lack of direct measurements of the mixing states of BC in the atmosphere to corroborate this (section2.3). Nucleation scavenging within ice clouds follows a different mechanism compared to scavenging by warm cloud droplets. Ice nucleation occurs in the atmosphere through homogeneous freezing of liquid solution droplets and/or through heterogeneous 71
72 interaction on a particulate nucleus (termed ice nuclei, IN). Homogeneous nucleation, or freezing, occurs at temperatures at or below about 35ºC and becomes more important with decreasing temperature, while heterogeneous freezing may occur at much warmer temperatures. Ice nucleating efficiency varies with temperature and supersaturation with respect to ice. The heterogeneous process is complex because the aerosol can interact with super cool water by different physical mechanisms, like immersion, condensation freezing, deposition and contact modes [firstly described by Vali 1985, a review by Cantrell and Heymsfield 2005]. The particles which act as IN are generally insoluble in order to provide a firm substrate for the ice initiation in the presence of water or water vapor, and they should be larger than the critical embryo size. Also favoured IN include materials which have a lattice structure similar to ice. Therefore, mineral dust [e.g. Sassen et al. 2003], some metals [Cziczo et al. 2004, Twohy and Poellot 2005], soot particles [DeMott 1990, Petzold et al. 1998], and some crystalline solids within partially soluble aerosols are suspected to act as ice nuclei [all of these are summarized by DeMott 2002]. The direct evidence of the chemical composition of IN has typically been obtained by evaporating the water content of ice particles with a counterflow virtual impactor (CVI). The non-volatile residual nuclei are then impacted onto electron microscope grids combined with X-ray spectrometry to determine the chemical compositions [e.g. Noone et al. 1993, Twohy and Poellot 2005]. The performance of soot as IN has been long studied, but mainly using laboratory surrogates, e.g. DeMott [1990] presented the capacity of soot to act as IN. However, soot s behavior is more complex than that of other heterogeneous ice nuclei, not only because the soot induced ice nucleation depends on a variety of environmental parameters (water saturation, pressure and temperature for instance), but is also sensitive to the coating materials [Möhler et al. 2005a, 2005b, Kärcher et al. 2007]. Research on the soot-in activity is of great importance because soot particles are ubiquitous, if they could act as IN, the cloud glaciation process would be significantly enhanced. BC particles that are nucleation scavenged can then be washed out when precipitation is initiated by a cloud. The BC particles that have not acted as cloud particle nuclei can 72
73 also enter the clouds by impaction scavenging (e.g. aerosol-hydrometeor coagulation, dissolving in precipitation particles). 2.3 The uncertainties and limitations of current models The common approach of current global models is to discriminate aerosols into at least five aerosol components: sulfate (SU), black carbon (BC), particulate organic matter (POM), dust (DU) and sea-salt (SS). Kinne et al. [2006] describes the necessary parameters ad processes in figure 2.3-1, to better characterize aerosol properties to predict their climatic impacts. The aerosol sizes that primarily impact radiative energy budgets of the atmosphere are those of the coarse mode dominated by DU, SS, and of the accumulation mode characterized by sulfate and carbonaceous aerosol. Regarding the optical properties, the common practice in global models is to stratify BC contributions into strongly absorbing, whereas most POM [McFiggans et al. 2005] and SU into predominantly scattering components. To separate the processing of these aerosol types adds complexity and diversity in a variety of ways. As summarized by Kinne et al. [2006], the model discrepancies for aerosol absorption, particularly associated with BC modelling, are significantly larger than that when determining aerosol optical thickness. 73
74 Figure A diagram by Kinne et al. [2006] to illustrate modelling steps in aerosol component modules of current global models: firstly the emission inventories of dust (DU), sulfate (SU), particulate organic matter (POM), sea salt (SS) and black carbon (BC) are applied, then their lifetimes are determined by the characteristic evolution and scavenging efficiency, the predictions for dry aerosol mass (m) and aerosol optical thickness (aot), which are based on satellite or field observations, are then incorporated into the modeling system to estimate their climatic impacts (radiative forcing). The current estimations of BC radiative forcing at top of atmosphere (TOA) range from to Wm -2 [Haywood and Shine 1995, Haywood and Ramaswamy 1998, Penner et al. 1998, Cooke et al. 1999, Jacobson 2000, Koch 2001, Chung and Seinfeld 2002, Wang 2004]. The differences in the estimates are due to the uncertainties in predicted global BC distributions. The challenges of modeling the BC atmospheric lifecycle result from the high sensitivity of their concentrations to emission and wet scavenging rates, the undetermined extent to which BC is mixed with other aerosols, the assumptions of surface properties, in particular surface albedo, and to the vertical distribution of BC with respect to cloud layers. Model predictions of global BC distribution are uncertain due to lack of long term data [Kinne et al. 2006]. The global burden and distribution of BC are strongly sensitive to the emission rate. Bond et al. [2004] suggest an uncertainty range for global BC 74
75 emissions of 4.3 to 22 Tg yr -1. The removal process, mainly via wet deposition for BC, is highly dependent on its hygroscopic properties. However, the knowledge about the hygroscopic properties of atmospheric BC particles currently is very sparse. Accurate modelling of the solubility of BC would require knowledge of the morphology of BC and the rate at which particles take up different coating components. Treating the degree of mixing properly is essential for aerosol processing in GCMs, including aerosol cloud interactions. For most of the global models, the hydrophobic fraction of emitted BC is assumed to be 80%. This ignores the variations due to origin, burning characteristics, or meteorological conditions [Textor et al. 2006]. Once emitted in the atmosphere, hydrophobic BC becomes hydrophilic with an approximately estimated e-folding lifetime of about 1-2 days (section 1.6). An improved understanding of these undetermined factors will put more constraints on the current BC models. The mixing state of BC is also crucial to determine its optical properties (as discussed in section 1.6). The large differences in compositional mixture for aerosol dry mass and water uptake can affect aerosol absorption significantly. Some of the models take into account the enhancement of BC absorption when included within cloud droplets [Jacobson 2006] or mixing with a sulfate coating [Chung and Seinfeld 2002], e.g. the model by Chung and Seinfeld [2002] provide upper and lower estimates for the climate impact of anthropogenic BC. However, the current knowledge of the total BC heating rate is not sufficient to fully characterize BC effects on climate. One of the reasons is the sulfate has been assumed as a surrogate of coating compositions of BC for most of the models [e.g. Chung and Seinfeld 2002], but in the real atmosphere, soot is emitted as a mixture of BC and OC such that all individual particles that contain BC also contain OC. These particles can be further mixed with other species after emission via coagulation and condensation. When the amount of scattering material, such as nitrates or organic carbon, that is mixed with BC is increased, the BC could have even greater climate impact. Additional uncertainty is introduced due to the observed BC concentrations reaching a maximum at about 2 km above the surface [Ramanathan et al. 2001, 2007a] whereas in 75
76 most models they are concentrated close to the surface [Textor et al. 2006]. The elevated BC would enhance solar absorption significantly because it can absorb the solar radiation reflected by low clouds [Ramanathan and Carmichael 2008, Haywood and Ramaswamy 1998, Chung and Seinfeld 2002]. The accurate size segregation of BC in current models is limited by current measurements, which adds more uncertainties on the global climate modeling because most of the models are based on the distributions of BC mass loading. However anthropogenic perturbations of the atmospheric BC particle number concentrations can be very different from the corresponding perturbations of the BC mass loading [Hendricks et al. 2004, Kärcher et al. 2007], and source differentiated BC can have different mass/number-based size distributions [Schwarz et al. 2008]. Therefore atmospheric models should include explicit predictions of BC number size distributions. The uncertainty of BC performance on ice nuclei results in the large problem of estimating the indirect effect of soot aerosols on cirrus cloud. This is because the lack of direct atmospheric observations in the UT/LS, although a variety of laboratory studies have been conducted, which employed idealized soot samples of unknown real atmospheric relevance. As summarized by Kärcher et al. [2007], the effects of coatings and particle size on the ice nucleation efficiency have not been fully explored. Real atmospheric soot particles might exhibit a wide range of ice nucleation activities, depending on their sources and atmospheric ageing processes, and these need to be further investigated via more direct observations and improved measurement techniques. 76
77 2.4 The objectives of this thesis The significant role of BC on influencing radiative budget of earth-atmosphere system, which is associated with climatic issues, has been emphasized in Chapter 1. To obtain a better knowledge of BC behaviour in the atmosphere is important to regulate human activities to make a more efficient control on BC emission, which can benefit both reducing global warming [Bond and Sun 2005] and raising the global temperature level at which dangerous anthropogenic interference occurs [Hansen and Nazarenko 2004]. The mixing state of BC particles is of great importance due to the control on particle s hygroscopic property hence determining the wet removal and its atmospheric residence time, as Chapter 2 discusses. However, the current knowledge on the hygroscopic property of BC is too sparse to understand its lifecycle in the atmosphere, and the mixing of BC is underestimated in the AeroCom suite of global aerosol models [Textor et al. 2006]. The time scale for the BC to be converted from hydrophobic to hydrophilic is subject to large uncertainty. The characterization of BC mixing state with more spatial-temporal coverage will improve the understanding on the lifecycle of BC in the atmosphere and reduce the uncertainties of assumptions in the aerosol models. Quantitative measurements of BC have been approached by its unique characteristics (section 2.1), however, as Chapter 3 will in detail discuss, the traditional thermaloptical techniques for the BC measurements are not satisfactory to obtain a full characterization of BC, because they are all based on a bulk collection method, leading to a reduced sensitivity when exposed on an environment of low concentrations, and the empirical conversion from the measured parameters to BC mass introduce large uncertainties. The information of BC size distribution and mixing state is not directly available from these instruments, although most of the current aerosol-climate models rely on these data. The input parameters in the models, such as the time scale for the conversion of BC from hydrophobic to hydrophilic, size distribution, tempo-spatially distributed mixing state of BC, are still under assumptions and lack of observational support (section 2.3). 77
78 Recently, a fairly novel method using laser induced incandescence (LII) technique has been introduced to detect BC mass in single-particles. This is available as a commercial instrument - the single particle soot photometer (SP2) by Droplet Measurement Technologies Inc. (DMT), Boulder, Colorado. This instrument delivers the refractory mass and optical size for each particle. The SP2 is selectively sensitive to the composition of BC thus the BC mass and size distribution can be directly measured. Additionally, the mixing state of BC can be determined. Chapter 4 fully characterizes the Manchester SP2 instrument, including the detailed description of SP2 techniques, instrument development (hardware and software), the functionality with related uncertainties, and data analysis methodology. The Manchester SP2 has been employed during several projects with a variety of scientific focuses. Table 3-1 summarizes the project date, the geographical position and the type of the experimental site classified by the pollutant sources and the extent to which the site has been polluted. 78
79 Project name Full name abbreviations CityFlux 2006 CityFlux summer HM2006 Holme Moss 2006 CLoud and Aerosol CLACE6 Characterization Experiments Aerosol Interaction AIDA2007 and Dynamics in the Atmosphere Experiment Experiment city and Area type duration country (month/year) Manchester, UK Urban 08/2006 Pennines, North West England Polluted rural 11/ /2006 Jungfraujoch, Switzerland Forschungszentr um Karlsruhe, Germany Free troposphere 02/ / m a.s.l. Soot generator in Sampling geographical coordinates: (if field work) 53.2 N 2.2 W 53.5 N 1.86 W 46.5 N 7.98 E conjunction with 11/2007 Laboratory study cloud chamber References Longley et al.[2003] Corris et al.[2008] Baltensperger et al.[1997] Möhler et al. [2001] Schnaiter et al. [2006] Table 3-1. A summary of experimental sites with SP2 measurements deployed. 79
80 The Greater Manchester is one of the major cities in the northwest of England, from which the pollutants originated can transport to a long distance, affecting the conurbation and nearby rural regions. Such as Holme Moss, this site routinely received the city emissions from Greater Manchester region, as well as the pollutants from localized residential heating during the winter season. The CityFlux project measured the urban fluxes directly emitted from Manchester city and the Holme Moss project characterized the semi-rural site which is under the influence of the urban outflow. Both of these projects focus on the measurements close to the ground sources where the ageing time of the BC is relatively young. During the experimental period, the experimental platform built at the Holme Moss site was frequently contacted by the orographic clouds, allowing the investigations on the scavenging of BC by water droplets. After emission from a variety of sources, the BC can be delivered more spatially as high as upper troposphere and lower stratosphere (UT/LS). During the CLACE6 project, the aerosol properties were characterized via a ground-based observation platform built on the Jungfraujoch, Switzerland, where is 3580m above sea level (a.s.l.). This site remotes from the ground sources, exposing to continental free tropospheric air over the Europe. The BC transported at this site had been well mixed by the atmospheric processing. The site also provides a platform to study the aerosol interaction with cloud particles as the research station was frequently engulfed by clouds. The clouds studied in CLACE6 project were different from the warm cloud in Holme Moss project, which were in mixed phase from liquid dominant to the state that contains a significant fraction of ice crystals. The importance of BC in incorporating into ice clouds is highlighted in the CLACE6 study, and this issue is further closely explored in the AIDA project, during which the soot particles are generated by laboratory facility and the conditions of ice formation is simulated in a cloud chamber. The soot particles coated with different compositions were injected into the chamber, and their performance on serving as ice nuclei was examined. 80
81 Figure summarizes the main atmospheric processes that the BC experience from the emission until the sink. The findings from the field experiments are presented in the following chapters focusing on different stages during the lifecycle of BC in the atmosphere. The Chapter 5 and Chapter 6 use the data measured from CityFlux and Holme Moss projects in the UK: the former investigated the characteristics of BC emitting from different ground sources and the property evolution during the transport; the latter studied the scavenging activity of aerosols, including the BC and other BC-free non-absorbing particles by water droplet of orographic cloud observed at the Holme Moss site. Chapter 7 and Chapter 8 use the data from CLACE6 project conducted in Jungfraujoch, Switzerland: the former focuses on the aerosol characterization, particularly investigating the BC particles that are transported to the free troposphere; the latter studies the aerosol-cloud interaction at this site, however unlike the clouds in Chapter 6, the clouds observed in Jungfraujoch featured in various phases, with the dominance of liquid droplet or ice crystal in cloud particles varying during different periods. These studies constrain the direct forcing (section 1.4) and indirect forcing (section 1.5) caused by the BC emission, building up a framework in understanding the atmospheric lifecycle of BC. 81
82 Figure A summary of the main atmospheric processes during the lifecycle of BC in the atmosphere. The four studies in this thesis are indicated in circled red text, positioned on this diagram according to the main objectives. Chapter 9 gives concluding remarks and the future work for the instrument development and scientific research. The single particle measurements of BC have improved and will further improve the understanding of BC behaviour in the atmosphere, and more constraints will be incorporated in the prospective global model to reduce the existing uncertainties. Due to the fairly early stage of the instrument usage in the scientific fields, the SP2 has large potential to be further developed and deployed more widely. 82
83 Chapter 3 Overview of instruments for BC measurements Historically, BC has been measured by optical and thermal techniques, which quantitatively characterize the absorbing and refractory components of particles respectively. The basic operation mechanisms of several versions of instruments are introduced in this chapter, as they represent one of these techniques or use some other supporting methods to improve instrumentation accuracy. The systematic limitations of these measurements are also discussed. 83
84 3.1 Optical techniques The method according to which the instruments are designed to measure light absorption by aerosols, which is then empirically related to BC content, is termed as optical technique. One of the early examples to optically quantify BC is the aethalometer instrument, whose measurement is based on the optical properties of BC deposited on a quartz fibre filter [e.g. Hansen et al. 1984]. Light absorption is quantified by the change in light transmission as a function of time. The further development of this instrument can be seen in Weingartner et al. [2003], who developed the measurements of absorption at multi-wavelengths. The most commonly used instrument is the particle soot absorption photometer (PSAP), which also performs a filter-based absorption measurement [Bond et al. 1999]. The aerosol extinction coefficient is obtained by measuring the time constant for light decay prior and after transmitting the filter, in a high finesse cavity containing the absorbing particles (figure 3.1-1). To derive the total extinction, a measurement of the scattering coefficient is often used in conjunction with the PSAP measurement, such as the nephelometer [Anderson and Ogren 1998]. Cavity ring-down techniques provide an insitu measurement of the aerosol absorption coefficient. 84
85 Figure the cross-section of the filter setup in the PSAP by Bond et al. [1999]. The sample is drawn through one of the holes, shown at the bottom, and the particles are deposited on the filter. Filtered air is drawn through the hole shown on the right for a reference measurement. The PSAP measurement could introduce the effects of light scattering from particles on the filter, as well as an interference of the particle transmission through the matrix of the quartz fibers, these potential factors could overestimate the absorbing coefficient. To overcome this, the improved version of the absorption measurement based method is the Multi-Angle Absorption Photometer (MAAP), which measures both reflectance at multiple angles and transmittance for particles on a filter [Petzold et al. 2004]. As figure shows, because the light can be scattered by both measured particles and the filter matrix, to derive the absorption coefficient from the decrease in the transmitted light, additional detectors are placed at selected angles, permitting a full characterization of the scattered light thus allowing the determination of the absorbance of the particle layer on top of the filter. 85
86 Figure The cross section of the MAAP instrument, both backward and forward scattering light from particles and filter matrix are measured at selected angles, to correct some of the artefacts associated with the filter and perturbation by nonabsorbing particles. Another instrument using the optical technique is the Photo Acoustic Spectrometer (PAS), the advantage of this photoacoustic technique is to avoid the interference caused by the filter by detecting the light absorption from an increase in surrounding air pressure resulting from the absorbed laser light within particles, the heat is then transferred to the surrounding gas to produce a standing acoustic wave detected by a microphone [e.g. Arnott et al. 1999]. The structure of this instrument is shown in figure 3.1-3, the measured acoustic pressure is used in conjunction with instrument parameters to calculate the aerosol absorption coefficient. The new version of the PAS can be found on the DMT (Droplet Measurement Technologies, Boulder CO) company website ( This instrument extends the photoacoustic measurement to three wavelengths with improved sensitivity and time resolution. 86
87 Figure Schematic view of the prototype PAS by Arnott et al. [1999]. The drawback for all of these techniques is the need for an empirical conversion factor from optical response to BC mass, because BC often occurs in the form of highly fractal soot particles with complex morphology, and this task is further complicated due to the lack of an unambiguous chemical definition of BC composition with mixing state. All these factors can modify the absorptive properties of BC (section 1.6), therefore, the conversion factor relying on the optical response will be affected [Slowik et al. 2007], and the systematic limitations of the filter-based instruments can introduce further uncertainties. Moreover, the bulk-based sampling methodology limits the detectable sensitivity and instrument time resolution. Detailed analysis on the uncertainties of BC mass quantification using optical technique during field experiments can refer to section and section in this thesis. 87
88 3.2 Thermal techniques The high volatility temperature of BC is commonly used to segregate it from components that are more volatile. These methods are referred to as thermal techniques, which typically induce oxidation and/or evaporation of organic carbon, and define black carbon as the remaining refractory component. An example of such instruments is the semi-continuous EC/OC field analyzer designed by Sunset Laboratory [Turpin et al. 1990, Bae et al. 2004]. The sampled particles are accumulated on a quartz filter mounted within the instrument downstream of a gas phase denuder. The filter is then heated in an oxygen-free ultra high purity helium atmosphere in four increasing temperature steps to permit the detection of the organic carbon fractions with step-changed volatility (figure 3.2-1). One of the systematic uncertainties is the pyrolytic conversion of some organic compounds into the elemental carbon (EC) during these heating steps, which is continuously monitored by measuring the transmission of a laser beam through the filter. The organic compounds are vaporized and oxidized to carbon dioxide by a manganese dioxide catalyst held. The evolved CO 2 is measured to determine the OC composition whereas the EC quantity is obtained by heating the filter in two further temperature steps. 88
89 Figure Simplified block diagram for the Sunset Laboratory semi-continuous EC/OC field analyzer from Bae et al. [2004]. Another thermal technique is the Volatility Tandem DMA instrument (VTDMA) [initially by Orsini et al. 1998, a development by Philippin et al. 2003], which measures the size distributions of sampled particles before and after heating via two tandem positioned DMAs (see section 2.2 for the introduction of DMA instrument). A simplified diagram of this instrument is illustrated in figure by Wehner et al. [2004], the monodispersed aerosol selected by the first DMA is heated to remove the volatile material from the EC fraction, and then a second DMA is used to measure the size distributions of the non-volatile residuals. The soot with a thin or thick coating can be recognized and defined as less volatile or more volatile particles respectively [an example given by Rose et al. 2006], and the diameter change before and after heating can approximately estimate the mixing state of soot particles. 89
90 Figure Schematic of VTDMA and TD/SMPS volatility measurement systems from Wehner et al. [2004]. the abbreviations in this plot: N is neutralizer, AI is aerosol inlet, SDBam (ambient dry size distribution) and SDBnv (non-volatile size distribution). The largely classified steps are I: selection of a monodisperse aerosol fraction, II: conditioning (heating) unit, and III: DMPS-system for measuring SDBnv. However, some uncertainties can be introduced because the VTDMA system may not remove some of the less-volatile organics and sea salt like sodium chloride [Philippin et al. 2003], and some vaporized volatile gas can be recondensed on the non-volatile component when passing through the system [e.g. Wehner et al. 2004], furthermore, EClike products of OC pyrolysis can lead to an overestimate of the EC mass. Like the other usages of thermal techniques, the mass of measured EC is to a large extent operationally defined as different heating rates and thresholds, and the existence of multiple protocols for the heating/oxidation process leads to significant uncertainty in the distinction between OC and EC determined by different instruments, depending mainly on the temperature program and on the type of samples analyzed [Schmid et al. 2001]. 90
91 Chapter 4 The single particle soot photometer (SP2): instrument characterization, development and data analysis The laser induced incandescence technique has many advantages over the traditional methods of BC measurements. The single particle soot photometer (SP2) is a commercial instrument using this technique to characterize BC on a single particle basis. This chapter describes the instrument configuration, calibration and modification for the Manchester SP2. The raw data is quality assured, filtered and processed by the software as developed in the platform of LabVIEW (National Instruments Corporation, Austin, TX, USA) and IGOR pro (Wavemetrics Inc., Lake Oswego, OR, USA). The functionality, data outputs and uncertainties of this instrument are illustrated in specified sections. 91
92 4.1 History and introduction The measurement of particles with BC composition can be directly approached using laser induced incandescence (LII) techniques, which have been widely described [e.g. Michelsen et al and refs therein], however, the majority of previous LII usage to quantify BC focused on measurements in the vicinity of combustion sources, where pulsed lasers were mainly used and the high concentration of BC was required to access reasonable results. Stevens et al. [2003] developed the LII technique by mounting the laser mechanism in a solid-state laser cavity and captured the particle information from detectors with different collection angles. This was the prototype of the current commercial single particle soot photometer (SP2) manufactured by Droplet Measurement Technologies (DMT) incorporation in Boulder CO, USA. The basic theory of SP2 operation is that an aerosol sample passes through a jet to cross an intense continuum laser beam, particles exposed to the laser irradiation will scatter light at the wavelength of the incident laser beam (referred to as the scattering signal in the following discussions). If the particle contains an absorbing component, such as BC, it will also absorb the laser power and will be heated until it incandesces, emitting significant thermal visible radiation (denoted as the incandescence signal). The boiling temperature of the absorbing material corresponds to the composition of the incandescence material and the magnitude of the signal is proportional to the refractory mass. Both scattering and incandescence signals are detected and recorded by the instrument for each particle. The advantages of SP2 technology include the high sensitivity to very low concentration, the clear segregation of BC from other species of aerosols, and the availability of information on the mixing state on a single particle basis. The early applications of the commercial SP2 include the measurements of light absorbing particles in the lower stratosphere of Arctic vortex by Baumgardner et al. [2004], and atmospheric profiles sampled as high as the upper troposphere and lower stratosphere (UT/LS) over the mid-latitude US by Schwarz et al. [2006]. The 92
93 determination of the mass of BC by the SP2 and its independence from mixed coating materials, were validated in a dedicated intercomparison laboratory study using flamegenerated soot of different sizes and fractal dimensions [Slowik et al. 2007], Moteki and Kondo [2007] further revealed the high independence of the BC mass determination to the thick coating. These studies improved the understanding of the SP2 instrumentation and broadened its usage. Light absorbing particles in the laser beam undergo heating and vaporization, which leads to the evaporation of the mixed coating materials, and subsequent decrease in the scattering signal of absorbing particles (see section 4.6 for details). The peak shape is therefore no longer in Gaussian shape as is observed for absorbing particles. This issue was addressed by Gao et al. [2007], and a split detector was applied to determine the relative position of the expected peak occurrence, determine the peak shape of the scattering signal by an extrapolation from the leading edge of signal before the scattering signal is perturbed. The extrapolation methodology can also be achieved via statistical analysis based on the modelling of the evolution of scattering properties in the laser beam, as developed by Moteki and Kondo [2008]. The following sections describe the SP2 operation and the main analysis methods to deliver field data. Whilst the majority of the methods have been developed and are available in the literature, the data analysis tools are developed in the platform of LabVIEW and IGOR pro, which have been part of this work to process the collected SP2 raw data to produce useful results. The methodologies described in the following sections are from the basis of these analysis tools, and the laboratory and/or the data collected from field work are presented to illustrate the methods and to demonstrate the quantification methods used in this thesis. 93
94 4.2 Instrument configuration The central part of the SP2 instrument is the optical head, where the laser mechanism is implemented in a solid-state laser cavity. The aerosol jet controlled by a flow system passes through the laser beam with a direction that is perpendicular to the laser beam, and the sampling flow through the laser cavity is constrained by a sheath flow. Four complex lenses are positioned within the same plane as the laser beam to each capture a solid angle (about π/2 sr) of scattering or incandescence signals produced by particles in the laser beam, and image them onto either an avalanche photo-detector (APD) or photomultiplier tube (PMT). Figure schematically presents the configuration of the optical head. Figure the schematic diagram of the Manchester SP2 optical head, showing the laser mechanism, detecting system, injected aerosol jet into the chamber, the flow controlling system is not included in this diagram. The laser mechanism consists of a gain medium - the Nd:YAG crystal, which is optically pumped continuously with a diode laser at 808nm. The mode aperture on the cavity side of the gain medium is used to minimize pump light loss in the cavity and also 94
95 helps confine the laser, in addition to the two mode match lenses focusing the energy from the laser multimode fiber on to the gain medium. In the diagram, the aerosol jet is perpendicular to the plane of the page and sends the particles across the laser beam. The YAG laser operates continuously at 1064nm in TEM oo mode constrained by a single mode resonator, whose cross section has a Gaussian intensity distribution. At the centre of focus the laser intensity is approximately 1x10 6 Wcm -2 (about 1 mm over which the laser intensity is greater than 5% of its peak). In the laser cavity, which also acts as the detecting chamber, the sampling aerosol jet is injected via the perpendicular inlet controlled by a flow system, and experiences laser irradiation particle by particle. On the right part of figure 4.2-1, an output coupler is used to create a large flux of photons at the centre of focus, and is combined with coated laser power mirrors, which have a reflectivity of 99.97% or better at the 1064 nm wavelength of the YAG laser. The particles from the aerosol jet interact with the laser beam at the centre of the cavity. The continuous laser beam assures all of the passed particles can scatter light and those that contain material that absorb light at the wavelength of the laser will also emit light as they are heated and incandesce [Schwarz et al. 2006]. The focused signals from each particle are imaged at four optical detectors (figure 4.2-1), both the forward and backward scattering signals are sensed by two APDs collected over solid angles subtended from 13º to 77º and from 103º to 167º. These detectors are mounted with optical filters that reject light at wavelength <850nm to avoid interference with incandescence signal. The signals scattered by particles correspond to the intensity of incident laser power and thus exhibit a Gaussian distribution (section 4.5), and the peak intensity is used to derive the optical size. Particles which contain absorbing components will be heated and incandesce, emitting significant blackbody radiation in the visible region, which is captured by two PMTs filtered to pass broadband light (~ nm) and narrowband light (~ nm) respectively. The ratio between these two signals can be related to the blackbody irradiance property of the incandescence light and can therefore be used to identify the absorbing material (section 4.4). The absorbing particles also scatter the laser light but the scattering intensity will be perturbed due to the heat and vaporization process when transmitting the laser beam. 95
96 As a result, the scattering properties detected by the APD will be underestimated. To solve this problem, the original configuration of the SP2 was modified by installing a quadrant silicon APD-termed as two element APD (TEAPD) (as shown by the upper-left part of figure 4.2-1), replacing the normal APD with a low gain mounted in the old version of SP2 (table 4-1), to characterize the optical properties for absorbing particles. This has a notch to determine the relative position of the laser beam centre, allowing measurements of the leading edge of the scattering signal to be made (section 4.6). The modified SP2 instrument in addition has increased the detector sensitivity and improved measurement of sampling flow, as summarized in table 4-1. The datasets used in this thesis are all from the old version of SP2 except the specifically noted parts and the section using the data measured by TEAPD (section 4.6.4). 96
97 Hardware Ch0 Detectors Ch1 at different Channels Ch2 (Ch) Ch3 Measure of sample flow Old version of SP2 APD 76±18(*2.44mV) PMT in the broadband wavelength at ~ nm 164±25(*2.44mV) PMT in the narrowband wavelength at ~ nm 164±28(*2.44mV) APD in a low gain 4.76±1.5(*2.44mV) Derived from sheath and exhaust flow rate Modified SP2 APD 1298±195(*2.44mV) PMT Saturated PMT 385±60(*2.44mV) TEAPD, LEO fitted: 3060±480(*2.44mV) Direct measure by a laminar flow element Table 4-1. The hardware modification of the Manchester SP2 instrument. The gain of the Ch0 and Ch3 is illustrated in the peak voltage (mv) of the specific detector for DMA-selected PSL particle in 300nm; the Ch1 and Ch2 is calibrated by DMA-selected GCS particle in 300nm. The detector is saturated at the voltage of 2040*2.44mV. 97
98 Figure typical examples of single particle events detected by the SP2, the y axis shows the signal in units of adjusted digital voltages with Channel (Ch) 0, Ch1, Ch2 denoting the scattering signal, incandescence broadband and narrowband signals respectively. Note that Ch3 shown in the upper-left part of figure is used to collect the scattering signal with low gain in the old version of SP2, but has been replaced with the TEAPD in the modified version. The x axis indicates the relative elapsed time for a single particle event with a resolution of 0.2 µs. The amplified voltage signals are digitized by the A-D converter at a sampling rate of 5 MHz synchronously for all four detectors. This is equal to a time resolution of 0.2µs for each channel. The duration of the light pulse for one particle to traverse the width of the laser beam is typically about 30µs and the time resolution of 0.2µs for sampling is adequate to record the temporal evolution of incandescence and scattering intensities of single particles. Every single particle event is triggered by either the scattering or incandescence signal being above a specified threshold. The first 8µs of an event pulse are pre-triggered to diagnose the digital response and events with digital voltage below threshold are considered as non-particle events. Figure gives a typical example of single-particle events for a non-absorbing particle and an absorbing particle detected by the old version of SP2, which indicates the Gaussian pattern for the scattering signal of a 98
99 non-absorbing particle, and the two incandescence signals that can diagnose the existence of an absorbing particle. 4.3 Quantification of BC When a particle is incident with the laser beam and it contains absorbing components, like BC, the particle will absorb the laser light and be heated until incandescence occurs, emitting significant blackbody radiation at visible wavelengths. This incandescence signal will correspond to a specific refractory mass. To investigate the capacity of the SP2 to quantify the BC mass, a commercially available spherical carbon particle standard-glassy carbon spherical powder (GCS) (manufactured by Alpha Aesar, Inc., Ward Hill, Massachusetts, with tested density 1.42g/cm 3 ) is used in the routine calibration. The polydispersed particle sample is first put in an aqueous solution, nebulized, passed through a drier and then enters an electrostatic classifier where particles are size selected based upon their electrical mobility. The mass of size-selected GCS can be calculated because of the known density and their solid spherical shape. The carbon mass can then be used to relate the corresponding peak intensities of incandescence signals, to the mass loading of BC in single particles. Figure presents the calibration results from old and new versions of Manchester SP2, note the increased gains of incandescence detectors for the new version SP2 to increase the systematic sensitivity to small BC. 99
100 Figure Calibration results using glassy carbon spheres from old and new versions of the SP2. Each dot represents information collected from about 3000 particles, the results are linear for single particle mass below 120fg (on the left of the dotted line). The fitted lines suggest a good linear relationship between BC mass and peak amplitude of incandescence signals, but become non-linear relationship for BC mass above 170fg. The peak intensity of the incandescence signal (P I ) is linearly proportional to GCS mass below 120fg, in agreement with the results reported by Schwarz et al. [2006]. The scaling factors for incandescence signals (S I ) derived from the fits to the calibration data are applied to quantify BC mass (M) during measurements: M = P S I I This linear relationship can be theoretically explained through fundamental modeling work [Moteki and Kondo 2007]. Expressed as function 4.3-2, Moteki and Kondo [2007] point out for a particle with specified refractory composition, the P I is proportional to 100
101 black body emissivity (ε) multiplied by particle diameter square (D 2 p) within the wavelength range of the incident SP2 laser, by assuming the particle is spherical and adjusting filter transmission properties and detector sensitivities. P I ε D p Further modeling work of thermal radiation by Moteki and Kondo [2007] indicates the spectral emissivity of particles is proportional to D p when πd p /λ<1 (λ is the incident wavelength) and independent of D p when πd p /λ>1. Therefore according to 4.3-2, the P I will be proportional to D 3 p, and hence the particle mass, due to the linear dependence of emissivity on D p when πd p /λ<1. This explains the linear relationship with P I for small 2 particles. But P I would be proportional to D p rather than D 3 p when πd p /λ>1, because of the independence of ε to D p. This is indicated at the right part of figure 4.3-1, the P I for large particles (above 170fg) are apparently shifted away from the P I -mass (D 3 p ) line. The primary uncertainty during calibrations is the diameter of the particle selected by the classifier. The variation in diameter depends on the stability of the flow through the classifier and whether or not the particles are singly or multiply charged. Various studies have been done in the past to define this uncertainty and it is usually on the order of ±10% [Schwarz et al. 2006]. However, the idealized GCS samples do not fully represent the real atmospheric soot particles, which have complex morphology and mixing state. To test the sensitivity of P I to the soot morphology, Slowik et al. [2006] conducted a dedicated laboratory study using flame-generated soot of different sizes and fractal dimensions, validating the near linear P I -BC mass relationship for BC particles of fractal dimension in the range 1.6 to 3 and mass in the range of 2-30 fg/particle. The coating materials on BC have the potential to prevent heat conduction to the BC core when absorbing the laser light. During the experiments by Slowik et al. [2006], some liquid (oleic acid) and solid (anthracene) organic coatings up to 100-nm thick were added on the soot sample, and the coatings were shown to not affect the BC mass calibration of SP2. In addition, Moteki and Kondo [2007] coated the graphite particle 101
102 with glycerol and oleic acid in layers up to 650nm thick, observing little dependence on the amount of non-refractory components in the BC mass determination using P I. These experiments strongly indicate that the coating materials vaporized in the laser beam of the SP2 will not interfere with the detection of the BC core or undergo significant conversion into refractory carbon. Once the mass of BC is derived, if the density of atmospheric BC is known, a measure of the BC size, which is termed as mass equivalent diameter (D ME ), can then be obtained: 1/ 3 D ME = ( 6M / ρ BC π ) Where M is the particle refractory mass, derived from measured P I, and ρ BC is the average density of atmospheric BC recommended by Bond and Bergstrom [2006a]. If treating the coated BC particle as a core-shell-model (section 2.1.3), D ME can be considered to be the size of BC core based on a hypothesis that the irregular BC core is compacted to be a solid sphere. Therefore, due to the complex morphology of BC, the surface area or volume of single particle derived from D ME is largely underestimated compared to the real values. This discrepancy can be reduced when a thick coating restructures BC particles significantly and the soot aggregates become more compact [Schnaiter et al. 2005]. 102
103 The fractal shape and relatively large surface area of soot can amplify the drag force in electric fields to some extent (section 2.1.1), therefore a relatively small amount of soot mass can represent a large electronic mobility size. This assumption is supported by the graphite spark generated soot experiments conducted in the AIDA (Aerosol Interaction and Dynamics in the Atmosphere, see section for more details of this project) chamber, Forschungszentrum, Karlsruhe, Germany, where the mobility size-selected soot particle was further quantified by the SP2. The results shown by figure clearly demonstrate the strong influence of soot morphology on the relationship between its mobility size and mass. Figure the correlation between the mass equivalent diameter measured by SP2 and the mobility diameter selected by DMA, using the graphite spark generated soot sampled from AIDA chamber. 103
104 Calibrations have shown that the incandescence signals of BC spheres of sizes up to 1µm diameter shrink below the detection limit before exiting the laser beam, confirming that the laser power is sufficient to bring the detectable BC particles to their boiling point temperature and fully incandesce them. The laser power can be reduced from 3.5V to 1.3V, and the P I of the detectable carbon varies within ±10%, as figure shows. The slight decrease of P I introduced by elevated laser power is because of the significant vaporization of refractory carbon before the peak incandescence is reached, whereas the initial rise in signal with laser power is due to the lower power being insufficient to bring the particles to full incandescence. Figure The variations of peak incandescence signals for GCS 300nm, 450nm and 600nm corresponding to the laser power, the dots indicate the conditions under normal operation. 104
105 The instrument temperature can potentially modify the optical alignment resulting in mis-sizing. In addition a change in flow alignment, the laser power or the injected flow rate, can also result in unreliability of particle sizing. Low temperature tests were conducted because under some conditions, the SP2 instrument failed to be maintained at room temperature. Figure confirms the lack of dependence of incandescence signal at low instrument temperatures, during a GCS calibration. The multi-peaks in this figure correspond to the multi-charged GCS when selected by a DMA. This experiment simulated low temperature conditions by mounting the instrument in a freezer, the results validating the stability of carbon determination by the SP2. Figure the temperature independence of P I, as indicated by a GCS calibration test under various levels of ambient temperatures. 105
106 If considering particles with different refractory compositions, i.e. some metals, rather than carbon itself, more constraints should be added onto the formula 4.3.2, as expressed by formula 4.3-4: P I I 2 ( T, ) ε Dp λ πhc I( T ) T 2 4 = dλ = σ λ [exp( hc / hcλkbt ) 1] I(T, λ) is the irradiance (Wm 2 nm 1 ) at wavelength λ emitted from a blackbody surface at temperature T according to the Planck law (equation 4.3.5), where h, c, and k b are the Planck constant, speed of light, and the Boltzmann constant, respectively. The σ is the Stefan Boltzmann constant, according to the Stefan Boltzmann law, the blackbody irradiance, which determines the peak SP2 incandescence signal, is proportional to the fourth power of absolute temperature. Therefore, the calibration using GCS is only valid for aerosols with similar boiling point temperature, which might introduce some uncertainties when applying the GCS calibration results for the mass determination of atmospheric soot, due to their slightly different boiling temperatures. As is further discussed in section 4.4, the blackbody radiance captured in different wavelength bands can discriminate particles with different refractory compositions. 106
107 4.4 The sensitivity to boiling point temperatures of different refractory compositions For a refractory particle to incandesce it must have a boiling point temperature (T) high enough to emit measurable amounts of visible blackbody radiation and it must have the capacity, which is determined by its emissivity (ε), to absorb light efficiently to reach those high temperatures. The blackbody irradiance (I) follows the Planck law (equation 4.3.5), which is a function of its temperature, wavelength and emissivity. The incandescence signals captured by two PMTs (section 4.2) are the blackbody irradiances integrated over different wavelength bands. Functions and show the relationship between the peak of incandescence signals as measured by the PMTs and the intensity distributions of blackbody radiation I (T,λ) integrated over the broadband (bb, ~ µm) and narrowband (nb, ~ µm) wavelength ranges of the detectors respectively. P D ε I ( T, λ) dλ I ( bb) 2 p λ ( bb) P D ε I ( T, λ) dλ I ( nb) 2 p λ ( nb) 107
108 Figure the blackbody irradiance as a function of emitted wavelength for materials with different absolute temperatures, along with the dashed line indicating Wien's displacement law. The yellow and green areas denote the wavelength ranges of incandescence signals detected by the broadband and narrowband PMTs. Based on the Planck law, figure plots the blackbody irradiance as a function of emitted wavelength for materials with different absolute temperatures, along with the dashed line indicating Wien's displacement law - the radiation at higher temperature peaks at shorter wavelength. The incandescence signals collected in the different wavelength bands therefore have the potential capacity to discriminate materials with different refractory compositions. By adjusting the light collection angle, light collection efficiency, transmittances of the optical filter, detector responsivity, amplification factor and some other mathematical constants, the modelling study by Moteki and Kondo [2007] revealed the nearly linear relationship between boiling point (T BP ) and broadband/narrowband peak ratio (formula 4.4-3), and showed that the particle boiling temperature can be determined independently of the absolute value of emissivity (ε), if it is assumed that ε is independent of wavelength within the region of SP2 detecting wavelength. This simple 108
109 model of blackbody radiation may be used to scale the incandescence signal calibration to different materials. T P I ( bb) P I ( nb) To validate this method, a variety of materials were nebulized in water to test the response of the SP2 instrument to each one. Inorganic materials included aluminium, niobium, silicon and glassy carbon spheres [all supplied by Alpha Aesar, Inc. Ward Hill, MA]. The results (figure 4.4-2) didn t overlap extensively with each other, and the peak values of broadband/narrowband ratio distributions are distinct. This indicates the SP2 has the ability to discriminate at least four colour temperatures within acceptable uncertainty. The atmospheric soot particles measured in an urban environment, at a semi-rural site and in the free troposphere are also presented, combined with the GCS, demonstrate the broadband/narrowband ratios of various types of carbons are within 1.0±0.2, which are distinct from other detectable metallic materials, indicating a highly selective sensitivity to BC of SP2 incandescence signal. 109
110 Figure Response of the SP2 to refractory materials with different compositions. The maximum-normalized distributions of broadband/narrowband incandescence peak ratios are shown. These materials include laboratory generated Al, Ni, Si, GCS and atmospheric BC measured during CityFlux (urban environment), Holme Moss (semirural site) and CLACE6 projects (free troposphere) (section 5.1). The correlation between P I(bb) /P I(nb) and T BP is shown in figure The T value of materials are obtained from literature [e.g. Gale and Totemeier 2004], or from the specifications of manufacturers, and the corresponding measured P I(bb) /P I(nb) are linearly fitted as a function of T. This linear relationship has a high correlation coefficient and is in agreement with Schwarz et al. [2006], who report that the relationship between P I(bb) /P I(nb) and T is nearly linear for boiling temperatures below 5000K. The fitted line obtained from the materials with known compositions can be referenced to estimate the 110
111 boiling points of materials with unknown refractory compositions via measured P I(bb) /P I(nb). The black line in figure notes the reference line we used, along with the blue dots indicating the measured atmospheric aerosols. The boiling point distributions of atmospheric soot particles measured at different ambient conditions have little variations, but exhibit higher boiling temperatures than GCS. It can be speculated that crystal or amorphous structure, physical density or impurities play some role in determining the soot boiling points but this needs further investigation. Figure Correlation between the incandescence broadband/narrowband ratio and boiling temperatures. The linearly fitted line obtained from laboratory generated materials with known compositions is referenced to estimate the boiling temperatures of measured atmospheric aerosols with unknown refractory composition. The black and blue dots represent laboratory and atmospheric aerosols respectively, and the measured atmospheric soot in an urban environment, a semi-rural site and troposphere, as well as some other components measured downstream of CVI inlet are shown. 111
112 This simple model for boiling point determination is based on the assumption that the emissivity (ε) of a particle is independent of wavelength, however the ε of smaller particles has some dependence on wavelength according to Moteki and Kondo [2007], in addition to the systematic uncertainty of the instrument when lower levels of incandescence signals are measured due to relatively lower S/N ratios, these factors can lead to larger uncertainties of boiling temperature determination for smaller BC. As figure shows, the broader distributions of T BP primarily result from deviations at smaller sizes of BC but within ±350K. Figure the dots are the deduced boiling temperatures from measured P I(bb) /P I(nb) for a variety of BC mass equivalent diameters, the y axis on the right side denotes normalized probability for the boiling temperature distribution, as indicated in grey line. A successful application for the detection of non-bc refractory aerosols was demonstrated during the CLACE6 project (see section for greater details of this project) and shown in figure One of the scientific objectives was to characterize the aerosol which can potentially act as ice nuclei (IN, section 2.2), and this was approached via measurements downstream of a counterflow virtual impactor (CVI), by 112
113 which the water content of ice particles was evaporated and the residues were sampled by a series of instruments. Because of the high sensitivity to the very low concentrations, the SP2 was an ideal instrument to characterize the ice core. The SP2 instrument was calibrated several times during the entire project, and the variation of laser power was monitored to be within ±5%. As figure shows, the dominant compositions of ambient refractory particles are BC, as indicated by the boiling temperature (T BP ) distribution. However, another peak in the T BP distribution was observed continuously when running the SP2 downstream of the CVI to measure ice residues, as presented in figure The T BP prediction model shows this composition has a much lower T than carbon. The little presence of such materials in the ambient environment might support the suspicion that these materials are not primarily originated from the atmosphere but due to the ice abrading the inlet. Murphy et al. [2004] confirmed the ability of ice particles to abrade the inlet wall and re-suspend the remaining particles deposited on the walls of an aircraft inlet by observing some metal particles whose composition is similar to that from the inlet when measuring the ice residue after a CVI using a single particle mass spectrometer. Figure the incandescence broadband/narrowband ratio distributions for ambient absorbing particles and CVI residues measured during CLACE6 project. A significant fraction of particles having lower boiling point temperatures compared to BC were continuously observed when sampling CVI residues. 113
114 Due to the systematic uncertainties of the T BP prediction model, the real composition of these materials is difficult to deduce, the measured boiling points of carbon fall within a relatively narrow range (figure 4.4-2), and these materials almost certainly have noncarbon composition, but can also absorb the SP2 laser light and incandesce. A further validation of this conclusion is the observations of the scattering, incandescing properties, and the vaporization process within the SP2 laser beam, which behaved differently compared to the ambient BC when measuring these particles, as figure shows. In figure 4.4-6, the parameter P S /P I denotes the relationship between the peak scattering intensity and the peak incandescence intensity, which is an indicator of the relative coating content if considering the same composition of absorbing core (section 4.6.2), because the P I is proportional to the core size and increased coating thickness can enhance the scattering properties. The parameter TD describes the time delay between the occurrence of the peak scattering signal and the peak incandescence signal. This delay is interpreted as an approximate measure of the time required to vaporize a coating (see section 4.7 for greater detail). For a coated particle, the core is prevented from reaching its boiling point by heat transfer to the coating. The behaviour of the suspected non-carbon materials is different from the BC particles. As figure shows, the ambient BC (red dots) show a significant number of particles that have larger TD, which means the thicker coating requires longer time to be vaporized, and this corresponds to significantly elevated P S /P I, indicating enhanced scattering properties. The non-carbon particles observed from CVI residues are grouped within another region: the much larger P S /P I can result from the much weaker incandescence intensity due to their lower boiling point (formula 4.3.5), or the formation process introduced more mixing with other materials, causing stronger scattering. There is no relationship between increasing TD and an increase in P S /P I as seen with the ambient BC particles. 114
115 Figure Peak scattering/incandescence intensity (P S /P I ) related to time delay (TD) for the absorbing particles measured from the atmosphere using a standard inlet and CVI residues during CLACE6. The particles in CVI residues are coloured in blue, which contain a large fraction of suspected non-carbon particles, the BC-dominant ambient particles are coloured in red. The observation of some BC-like particles, which have similar optical properties and boiling temperature compared to BC, if these particles are originated from ambient atmosphere, that would be an important direct evidence that BC can be incorporated into ice particles, which is of great climatic importance as a result of the potential glaciation indirect effect (section 1.5.3). 115
116 4.5 Size quantification of non-absorbing particles When a non-absorbing particle transits the SP2 laser beam, it will not absorb the laser power but will elastically scatter the laser light. Consequently, the single particle event will only exhibit the pulse of scattering signal (figure 4.2-2). This scattering signal determines the optical size of a non-absorbing particle. The scattering signal of an absorbing particle, i.e. BC, will no longer represent the true optical size of the particle due to evaporation of the particle when the refractory core is heated to its boiling point. Instead, the perturbed scattering property needs to be interpreted by applying peak shape techniques, which will be further discussed in section 4.6. The TEM oo mode of the SP2 laser beam implies the radial distribution of laser intensity (L) follows a Gaussian distribution, which can be expressed by equation as a function of a radial distance (r) away from the laser beam centre. 2 r L( r) = PL exp( ) σ 2 r The second moment (σ 2 r ) can be loosely referred to as the width of laser beam, which depends on the instrument configuration, the shape of laser intensity distribution and the stability of laser power. Conceptually, the intensity of laser power (P L ) is peaked along the laser beam centre (r=0) and the distribution is concentrically symmetric, as schematically presented at the left part of figure
117 Figure The centrally darkened red circle represents the cross section of laser beam, and the Gaussian distribution of laser intensity is indicated. The blue dots denote the motion of a single particle at different positions as it transits the laser beam, during which it will scatter light as shown by red arrows. The light scattered by the particle is focused with a lens pair onto an APD. The image of the particle (red bars on APD surface) moves upward, as indicated by separate arrows, in the opposite direction to the real particle movement. The resulting Gaussian detector signal is shown as the curve on the far right-hand side, and the black arrow on this curve indicates the elapsed time recorded by SP2, in particular, t S and t 0 denote starting time for the triggered event and the time when peak scattering intensity (P S ) is reached respectively. The dotted line indicates the time when the particle is positioned at the centre of laser beam, where the maximum intensity of laser power and P S occurrence coincide. When a particle transits the laser beam, the light scattered by the particle is focused with a lens pair, filtered optically, imaged onto the APD with adjusted gain, and then digitally recorded. In consequence, the processed scattering signal will depend on the response of the APD, transmittance of the optical filter, the alignment of optics, light collection efficiency and amplification factor of the recording channel. For a given particle, assuming the stability of all of these factors, the scattering signal (S) at a given time (t) within the particle event, is proportional to the incident laser intensity (L) according to the Mie theory [Mie,1908], as expressed by formula 4.5-2, 117
118 S( t) L( r t ) Where the laser intensity is a function of the relative distance to laser beam centre (r), r t denotes the distance at the time when the particle is positioned at the corresponding position relative to the laser beam centre. According to formula 4.5-2, the intensity of scattered light will be proportional to the laser intensity at any time when the particle is positioned in the laser beam, which means the peak intensity of scattering signal (P S ) will occur when the particle is at the laser beam centre (figure 4.5-1), and for a given particle, the peak scattering intensity is proportional to the incident maximum laser intensity (formula 4.5-3). This can be shown by monitoring the P S of mono-dispersed polystyrene latex spheres (PSL) with varying laser power (figure 4.5-2). The intensity distribution of the scattering signal will also follow the distribution of laser intensity, exhibiting a Gaussian mode. P S P L Due to the dependence of P S on the laser power, frequent calibrations by PSL particles were usually carried out to monitor the variation of laser power and detector gain from time to time during field projects. 118
119 Figure the scattering peak intensity varies with changed laser power for monodispersed PSL 300nm, 450nm and 600nm, with the error bars denoting the standard deviations during each calibration particles were collected for each calibration. The time evolution of the scattering signal (S(t)) for a non-absorbing particle in the laser beam can be expressed as equation 4.5.4, as discussed above, which will follow a Gaussian behaviour due to the Gaussian shape of the laser beam intensity. v S t B P t t 2σ 2 ( ) = + p 2 S exp[ ( 2 0) ] Where B is the instrument background noise, which is dependent of the instrument configuration; t is the specific time for a particle to transit the laser beam if considering that t s is the starting time within a particle event (on the far right hand side of figure 4.5-1). t 0 is the time when the particle is located at the centre of laser beam; P S is the peak intensity of the scattering signal, for an ideal optical alignment, this will occur at the 119
120 relative time t 0, so t-t 0 denotes the time relative to the laser beam centre. If multiplied by the particle traversal speed v p, the distance relative to that at the laser beam centre can be obtained. v p is highly sensitive to the rate of sample flow, but independent of particle size within a large range (not showed laboratory results), the sample airflow through the jet is held constant to ensure a constant particle velocity through the laser beam. The square root of the second moment (σ) is loosely referred to as the Gaussian width, which can be used to estimate the width of the aerosol jet in the laser beam. The scattered light intensity from a particle can be deduced from any position inside a stable laser beam if the laser intensity at that position is known. However, in practice, it is difficult to determine the position of a particle accurately during a triggered event. The scattered light intensity from a particle can be practically determined with acceptable accuracy by examining the scattered light signals during a particle s complete traversal of the laser beam. Figure gives a typical example of the fitting methodology to derive the scattered light intensity of a non-absorbing particle. The collected digitalized signal from the scattering channel during a complete single particle event are fitted a Gaussian function, by which the high frequency noise of the scattering signal (primarily contributed by the signal at the beginning and end when low signal comparative to background noise) can be largely overcome. The Gaussian fitted peak amplitude of the scattering signal (P S ) necessarily occurs at the centre of laser beam, which corresponds to a specified particle size, can then be used to determine the optical size for a nonabsorbing particle. 120
121 Figure the digital points collected from the scattering channel are fitted with a Gaussian function to derive the peak amplitude (P S ), which corresponds to the particle size. The P S necessarily occurs at the centre of laser beam at relative time t 0. Two parameters FWHM and RT PO are introduced (see texts for explanations), and the upper panel shows the %difference of the fitted results relative to the raw digital points. To derive the optical diameter (D O ) from the scattering signal, a scaling factor (S S ) is needed to relate the scattering cross section of the particle to measured peak voltage (P S ). S S can be calculated using the Mie theory by consideration of the wavelength of the incident light, collection angles of the optical system, with an assumption of the sphericity of the particle and the knowledge of its refractive index (equation 4.5.5). DO = PS SS Although the various refractive indexes and complex morphologies of atmospheric aerosols vary considerably, optical size assumes the refractive index is that of the 121
122 reference particle that is used for calibration. The diameter of the particle can then be derived by assuming a refractive index for the particle and finding what diameter would correspond to the measured P S. The most commonly used material to calibrate the scattering signal of the SP2 is the commercially available polystyrene latex spheres (PSLs), which normally have narrow size distributions with diameter variation within ±3% as determined by the manufacturer. Figure shows an example of the calibration results using PSL solutions at a series of sizes. The calibration results may vary during different projects. Figure Calibration results using PSL solutions at a series of diameters, the P S value as a function of diameter can be fitted with two separate polynomial functions. The fitted lines can then be used to derive particle size from the measured P S values. Figure implies that the determination of the particle size is highly sensitive to the scattering signal, especially for the smaller particles. Additionally, the background noise can become non-negligible compared to the relatively weak signal from small particles, introducing more uncertainty when determining the optical size at the lower detection limit close to the triggering threshold. Increasing the laser power may extend the lower 122
123 detection limit to smaller sizes and increase the S/N ratio for small particles, but doing so will digitally saturate the detector at larger sizes, reducing the upper detection limit (section 4.9). Increasing the laser intensity may also reduce the laser power stability to some extent (figure 4.5-2). As discussed above, some uncertainties can be introduced if the characterized aerosols possess largely different refractive index rather than that of PSLs. A model study has shown that this uncertainty may not be large for SP2 measurements [Baumgardner et al. 2007], as shown in figure E-008 Scattering Cross Section (cm 2 ) 1E-009 1E-010 1E-011 1E-012 1E-013 λ = 1064 nm Sulfate (m = i) LAC (m = i) sulfate-lac mixture (50/50) 1E Diameter (µm) Figure Mie calculations of the scattering cross section as a function of particle diameter collected by the SP2, assuming the detected particle contains only sulfate, light absorbing carbon (LAC) or an equally partitioned composition of sulfate and LAC, from Baumgardner et al. [2007]. Sulfate and BC are ubiquitous in the atmosphere and so are representative of the optical properties of non-absorbing and absorbing aerosols respectively (section 1.4). An 123
124 estimate of the maximum possible error due to the variation of refractive index is to investigate the SP2 response to two extreme cases particles containing only BC or only sulfate. The modelling results generated from Mie calculation at the wavelength of incident SP2 laser, accounting for the SP2 optical alignment and collection angles, demonstrate that the maximum ensuing error for the determination of scattering cross section will only be on the order of 0.05 µm. The refractive index of PSLs ( i, according to Gao et al. [2007]) falls within the range between sulfate and BC, implying the uncertainty resulting from variance of assumed refractive index has a small effect. This is one of the advantages of the SP2 compared to many other optical counters because the optical alignment of the SP2 allows the scattered light to be collected from both the forward and backward directions, resulting in less sensitivity to refractive index. Figure introduces two parameters to characterize the shape of a single particle event, which are important for a better understanding of the time evolution of particle scattering in the laser beam. The relative time of peak occurrence (RT PO ), describes the relative time position when peak intensity occurs relative to the starting time point when that event is triggered (t s ), as equation expresses, RT = t t PO 0 S The full-width at half-maximum (FWHM) is the distance between the two times at which the signal value is equal to half of its maximum value. For a Gaussian distribution, FWHM = 2 2 ln 2σ σ These two parameters are further explained in figure which shows a cross section of the laser beam. 124
125 Figure A schematic of FWHM and RT PO, demonstrating the transmission of a scattering particle through the cross section of laser beam. The laser has a concentrically symmetric intensity and a Gaussian distribution. The effective laser beam is shown as the dark red circle and is the part of the laser beam that has sufficient intensity to cause scattered light from a particle to be detected, whereas the outer peripheral area of the laser beam (pink circle) is not intense enough to trigger a specified particle event. The particle event is triggered when the laser power is intense enough to cause the scattered light from a particle to be detectable above the noise level and to be recognized as a single particle event by the SP2. RT PO is size dependent as larger particles can cause increased scattering compared to smaller ones for the same laser intensity. The effective laser beam is defined as the central portion of the laser beam, which has the sufficient intensity to cause the scattered light of a particle to be detectable, whereas the outer peripheral area of the laser beam (non-effective) is not intense enough to trigger a specified particle event. By considering the peak occurrence time relative to the starting time when the particle event is triggered (RT PO ), the radius of the effective laser beam can be calculated by multiplying by the particle traversal velocity (v p ). r = v RT = v ( t t ) e p PO p 0 s 125
126 The size-dependence of RT PO was investigated by passing monodispersed particles of PSL from a DMA into the SP2, shown in figure A), and the narrow distributions of RT PO at different sizes imply a single-valued dependence of the effective laser beam width compared to the particle size. The larger particles exhibit a larger RT PO, the reason is that t 0 occurs at the centre of the laser beam which is the same location for all particle sizes, whereas t S occurs relatively earlier for larger particles as less of the laser beam is penetrated before the particle event is triggered. This positive relation between particle size and RT PO is also shown by the observations of non-absorbing particles in the semiurban environment (figure B)). 126
127 Figure A) Size dependence of RT PO distributions calibrated by mono-dispersed PSLs. The RT PO is recorded as digitalized units with time resolution of 0.2µs. B) the size dependence of RT PO for non-absorbing particles during the Holme Moss project. The slight discrepancy occurring at the diameter of 530nm is due to the detector saturation for which a different fitting methodology is applied (section 4.7.2). 127
128 Figure in further explains the influence of particle size on RT PO schematically. Assuming the constant particle traversal velocity in the laser beam, consequently the larger particle will have a wider effective laser beam (equation 4.5.8) because it can be triggered at a more peripheral position relative to the laser beam centre. Figure Schematic demonstrating the RT PO and FWHM for a large and small particle events. The shape of the Gaussian distributed scattering signal can be determined by the FWHM, according to equation This parameter is proportionally related to the second moment (σ), which is primarily determined by the stability of laser beam and sample flow rate, and so is expected to have no size dependence. This hypothesis is supported from a variety of field measurements, as figure shows. The slight increase of FWHM for larger particles is due to detector saturation but is a minor effect compared to the majority of the distribution. 128
129 Figure the black line (with y axis on the left hand side) shows the FWHM distribution for non-absorbing particles collected from Holme Moss project; the dots (y axis on the right) indicate the size dependence of the FWHM, about particles are presented in this plot. The narrow distribution of the FWHM for non-absorbing particles indicates a stable laser performance and constantly controlled sample flow. The FWHM, which is related to σ, is highly sensitive to the shape of the scattering signal, and a non-perfect Gaussian fitting can result in reduced FWHM compared to an ideal fitting (see section 4.6 for details). Thus FWHM can be applied to examine the validity of the Gaussian fitting procedure. There is a fraction of the scattered signals in figure with too low FWHMs because of non-perfect Gaussian fittings. The importance of FWHM is that it has a high independence on particle size for fixed instrument configurations. This enables FWHM to be used to extrapolate the perfect Gaussian shape from a non-perfect Gaussian signal to derive the scattering property for absorbing particles (section 4.6.1). The scattered light intensity from a particle is sensitive to the laser power and the relative position to the laser beam centre. Correct optical alignment is therefore a 129
130 cornerstone of the determination of the scattered light intensity. Ideally the particle should be detected to pass through the exact centre of the laser beam cross section. To achieve this, the aerosol jet is turned through many different radial positions whilst recording the P S of injected mono-dispersed PSL particles, so as to be positioned at the centre of the laser beam. The instrument needs to be calibrated routinely after adjustment as potential factors can influence the alignment of optics, and so affect the determination of particle optical size. For example, instrument performance under cold ambient temperature was examined by placing the SP2 in an environment where the chamber temperature was dropped from 21ºC to -8ºC, during which the laser power monitored by mono-dispersed PSL particles. The relative humidity (RH) of air flows was maintained under 15% to avoid humidity effects. This low temperature test was performed because under some conditions, the SP2 instrument failed to maintain a fixed temperature inside the laser housing. The results are presented in figure The stable performance above 7ºC is indicated by the narrow distributions, whereas below 5ºC, scattered light signals exhibited broader distributions with gradually increased peak values. 130
131 Figure results of the SP2 low temperature tests. The P S of mono-dispersed PSL particles of mobility diameter 450nm were measured under a variety of chamber temperatures. About 3000 particles were collected during each experiment. The tags mark the peak values of the P S distributions. Note the increasing P S and broader distributions when the SP2 chamber temperature was lowered. The broad fluctuations in the scattering signals under colder temperatures, as indicated by the broader distributions, imply that the instrument performance tends to be unstable under these conditions, but the reason for increased scattering amplitude under cold temperature is unknown. It may have resulted from increased laser power due to modified alignment of optics, or the relative position of aerosol jet had been moved by thermal changes. A correction factor needs to be incorporated if the operating temperature of the instrument is under 5ºC, as shown by the dark area in figure The calibration results from PSLs operated under corresponding chamber temperatures during Holme Moss and CLACE6 project are also included, indicating stable instrument performances. 131
132 Figure P S as a function of SP2 chamber temperature calibrated by monodispersed PSL particles. A nearly linear increasing trend is observed when operating the instrument below 5ºC, as the dark area shows. Stable performances during the Holme Moss and CLACE6 projects are also indicated. 132
133 4.6 Determination of BC mixing state and the measurement of optical properties for absorbing particles Evolution of scattering and incandescence signals in the laser beam for absorbing particles The time evolution of an absorbing particle in the laser beam, i.e. BC, will be different from that of a non-absorbing particle because it can absorb laser light until incandescence. During this time, heats transferred from the absorbing core to the particle surface, which results in evaporation of the surface material if it is composed of more volatile material. Such coating evaporation reduces the scattered light intensity from the particle. The BC core will also undergo vaporization when it reaches its boiling point, exhibiting an incandescence signal, and both the scattering and incandescence signal will decrease below the SP2 detection limits afterwards. 133
134 Figure A schematic representation of the time evolution (from top to bottom) of scattering and incandescence signals for an absorbing particle during its passage through the laser beam. Double ended arrows a and b denote the peak occurrence of P S and P I relative to the particle triggering time respectively; the time delay (TD) is indicated as the difference between them, which is an approximate estimation of a time required to evaporate the coating. The areas of the spheres represent the particle size during process (1)-the loss of materials from particle surface and process (2) absorbing laser power to reach the boiling point of the BC core, as indicated by the two blue arrows. The time evolution of scattering and incandescence signals is schematically explained in figure Once an absorbing particle, i.e. BC, enters the laser beam, it begins to scatter light, and if large enough, will be detected by the PMTs. The particle also absorbs the laser light, increasing its temperature. During this process, the heat transfer from BC core to surface evaporates the material on the particle surface, resulting in the reduction of particle size, perturbing the original scattering signal of the particle. This energy loss by heat conduction reduces the ability of the absorbed laser light from heating the absorbing core. If the particle contains a significant amount of coating, 134
135 evaporation could delay the peak occurrence of the incandescence signal (P I ) to a significant extent. This process is indicated as process 1) in figure 4.6-1, during which the scattering signal is reduced due to the lost particulate material by evaporation. However, as the particle moves into the region of higher photon intensity, the peak intensity of scattering (P S ) will be reached. Unlike a non-absorbing particle, the P S of an absorbing particle does not necessarily occur at the centre of the laser beam because the particle size and scattered light intensity change as the particle crosses the beam. The temperature of the particle body will keep increasing until the boiling point is reached when the heat transfer between the surface and core reaches equilibrium, shown as process 2) in figure During which P I is reached accompanying the vaporization of the absorbing core. The peak occurrence of P I is often later than that of P S if a considerable amount of coating material has been removed by heat conduction. Therefore, the time delay (TD) between P S and P I is expected to be quantitatively linked to the time required to evaporate the coating components. This is an important parameter indicating the mixing state of BC and will be discussed in greater detail. Normally both the scattering and incandescence signals will fall below the noise threshold of the detectors before the particle exits the beam, typically within 20µs, because the heating of the particle by light absorption is sufficient to bring it to P I at its boiling point when it is then vaporized. 135
136 4.6.2 Determination of BC mixing state If two particles with the same mass of BC cores are coated by varying amounts, the particle with an increased coating may be expected to enlarge the particle size provided no restructuring occurs. This will enhance the intensity of scattered light, elevating P S for that particle. However, mass determination of the absorbing core via the incandescence signal is independent of coating fraction (section 4.2), resulting in an invariant P I. This can be observed using the SP2 measurements conducted during the AIDA experiment (see section for details of this project), as presented in figure Figure Flame generated soot coated with sulfuric acid sampled during the AIDA experiment. BC cores at the diameter of around 150nm are coated with varying amount of coating. The coating thicknesses (CT) are calculated using the core-shell model (section2.1) via an extrapolation technique from the leading edge part of the scattering signals, as discussed in the following texts. 136
137 Figure demonstrates how the scattering and incandescence signals vary with coating thickness. P I remains invariant, indicating a BC core of similar mass. With increased coating thickness (CT), the P S values exhibit an increasing trend. Additionally, the peak occurrence of P I occurs later relative to that of P S in the presence of a thicker coating. Consequently, two parameters can be introduced to potentially characterize the mixing state of coated BC: the time delay (TD) and the peak ratio between P S and P I (P S /P I ), as schematically shown in figure Figure schematic show of parameters TD and P S /P I for a BC coated with sulfuric acid. TD and P S /P I are used to qualitatively assess the mixing state of BC. For an absorbing particle, the relative time of peak occurrence (RT PO ) for scattering or incandescence signal is defined as the time when peak intensity of the signal is reached relative to the time when the particle event is triggered. The TD is obtained from the time difference of RT PO between the incandescence and scattering signal, as defined by equation This is interpreted as a measure of the time required to evaporate the coating materials before the BC core reaches its boiling point temperature. P I frequently occurs later than 137
138 P S, and the particle reaches its boiling point when majority of coating has been evaporated. TD = RT RT PO( PO( S ) I) The parameter P S /P I is another indicator of the mixing state of BC because the scattering signal is associated with its optical property whereas the incandescence signal corresponds to the mass of BC core. The presence of coating can therefore only lead to an increasing P S /P I, and it can be used as a proxy of coating thickness [also see Schwarz et al. 2006]. The relationship between these two parameters is presented in figure 4.6-4, which contains about 2000 BC particles collected during the Holme Moss project. Figure The relationship between TD and P S /P I for about 2000 BC particles measured during the Holme Moss project, as coloured by BC mass in single particle. 138
139 For thinly coated BC, when P S /P I is less than 2, TD increases dramatically by a factor of about 2 due to the increasing coating material. When the particles are thickly coated (TD>5µs), TD shows only moderate further increase whereas P S /P I increases by over an order of magnitude. These results are in good agreement with the observations by Schwarz et al. [2006], who found the coating evaporation time (TD) was positively correlated with the coating thickness when TD was less than 10µs, but thicker coatings showed little increase in TD when TD was greater than 10µs. Schwarz et al. [2006] applied a critical point in TD of 10µs to discriminate the internally and externally mixed BC particles. Moteki and Kondo et al. [2007] also observed a steep change in TD (1-3µs) when the coating thickness was above a specified threshold, which was validated via a modelling study. The critical points of TD above which the BC is significantly coated, vary in different studies, because the coating evaporation time depends on both the volatility of coating materials and the size of absorbing core. More importantly, it relies on the instrument configuration, for example, the increased laser power may shorten the coating evaporation or this time can be also influenced by the particle velocity passing through the laser beam. The discontinuous change of TD has been widely used to examine the mixing state of BC in the SP2 community. Figure shows results from the SP2 used in this study measured during the Holme Moss project. More details related to the uncertainties for using TD to diagnose the mixing state of BC, can be found in Chapter
140 Figure the refractory mass fraction in a single particle (MF) as a function of coating evaporation time (TD), representative of about BC particles. The curves on the right and bottom express the histograms for MF and TD respectively. For a coated BC particle, the refractory mass fraction (MF) in a single particle is defined in equation M denotes the directly measured BC mass, D O is the optical diameter and ρ av is the average density of the particle. MF = π 6 M 3 D O ρ av As is shown in paper I, the complex morphology of BC particles, the undetermined particle density and mixing state, and particle perturbation by laser heating (which will be further discussed in section 4.6.3) result in the estimated uncertainty of refractory mass fraction (MF) in single particles to be ±28%. For an ideal pure BC particle MF=1, increased coating in a single particle leads to a reduction of MF. During this project, the particles with TD below about 3.5µs, corresponding to MF over 30%, are considered to 140
141 be thinly coated or non-coated BC, conversely, the MF over 30% are the particles with a thick coating. Figure demonstrates that longer times will be required to remove larger amounts of non-refractory coating components. The parameter TD can therefore be used as a proxy for the coating content relative to the mass of BC core within a single coated BC. The usage of TD to diagnose the mixing state of BC is further investigated via a comparison study, in which the results measured at a semi-rural site (the Holme Moss project) and urban environment (the CityFlux project) are compared, as shown in figure
142 Figure Comparison study measured at a semi-rural site and in an urban environment to validate the usage of coating evaporation time (TD). The coating thickness (CT) in figure is estimated from the difference between optical size of particle and the size of BC core, as defined by equation The CT relative to the size of the BC core is calculated as relative coating thickness (CT rela ) by equation The normalization to the size of BC core is because the mass of BC core has a potentially important influence on the coating evaporation efficiency. 142
143 CT D O D = ME CT rela D D O ME = D ME However, the optical size of an absorbing particle may be largely underestimated due to the loss of coating by evaporation. This can be overcome by extrapolating the leading unperturbed scattering signal (section 4.6.3). The complex morphology of soot further influences the reliability of the prediction of coating thickness due to the unevenly distributed coating layer on relatively fresh soot, whereas more aged soot particle can become more compact due to restructuring effects. Nevertheless, as the left panel of figure demonstrates, the positive correlation of TD with increased coating thickness validates the use of TD as a proxy for the mixing state of BC. This is further shown by comparing with the results measured at the urban site in Manchester city centre, where the freshly emitted soot particles from vehicle exhausts are dominant. The majority of points were taken at low TD, high MF and thin CT, and the atmospherically processed soot particles with thicker coatings were rarely observed. 143
144 4.6.3 Perturbation of the scattered light signals from absorbing particles due to laser heating Laser heating of an absorbing particle can lead a reduction in its size thus perturb the scattering signal from the particle, which is addressed by Gao et al. [2007]. As described previously, non-absorbing particles will produce a peak intensity of scattered light when they reach the most intense region of laser beam, which necessarily occurs at the laser beam centre and follows the profile of laser intensity producing a Gaussian pattern. An absorbing particle, like BC, however, may often show a maximum in the measured scattering signal before reaching the region of highest laser light intensity. This occurs because during the process of absorbing laser light and then being heated, the materials mixed with BC that have lower volatility will evaporate and the physical size of the particle will begin to decrease. Therefore, the optical diameter of absorbing particles derived from the peak intensity of the scattering signal will always be an underestimate of the real particle size due to this evaporative loss, and the scattering signal will no longer follow a perfect Gaussian shape. This is shown by comparing the scattering signals of PSL and GCS particles measured by the SP2 of the same physical size selected by DMA, as figure shows. 144
145 Figure The lower and upper panel represents a single particle event for PSL and GCS respectively. The Gaussian shape of the scattering signal of the GCS has been perturbed compared to that of non-perturbed PSL in figure The RT PO for the GCS has been reduced due to the significant particle loss before the onset of incandescence and has occurred before the particle reaches the laser beam centre. The perturbed Gaussian shape also reduces the FWHM if the perturbed scattering signal is fitted as a Gaussian function. The laboratory results of monodispersed PSL and GCS particles are shown in figure There is little variability in RT PO and FWHM for particles of a given type and composition. The Gaussian width of the GCS scattering signals have been considerably perturbed, as indicated by the reduced FWHM and relatively earlier peak occurrence. 145
146 Figure the RT PO as a function of FWHM of scattering signals for monodispersed PSL and GCS particles. Here the perturbed scattering signal of GCS particles have been forced to be fitted as Gaussian functions. As figure shows, the P S of monodispersed GCS particles exhibit a broader distribution. Compared to those of PSLs, the majority of GCS particles produce weaker scattering signals than those of PSL of the same size. Given GCS particles have larger refractive index than PSLs (Gao et al. [2007]), the dramatically decreased P S for GCS indicate a significant perturbation of the scattering signals from absorbing particles. 146
147 Figure The P S distributions of monodispersed PSL and GCS particles at the sizes 300nm, 450nm and 600nm, colored in blue and black histograms respectively. Figure shows data from the Holme Moss field experiment. The peak occurrence of the BC scattering signal occurs earlier for BC absorbing particles compared to the non-absorbing particles. The FWHM of the fitted Gaussian shape for BC was broadly distributed and reduced to a large extent, whereas the FWHM of the Gaussian shape fit for non-absorbing particles is narrowly distributed. The parameter FWHM is therefore an important indicator that whether the Gaussian fitting is an accurate representation of the scattered light from the original particle or whether this has been perturbed during evaporation. 147
148 Figure The distributions of FWHM and RT PO for scattering signals of particles collected during the Holme Moss project. BC absorbing and non-bc scattering particles are both shown. 148
149 4.6.4 Determining the optical size of absorbing particles using the leading edge only (LEO) extrapolation technique Due to the evaporation of particle coating by laser heating and the incandescence of the absorbing core, the scattering signal from an absorbing particle will not represent its original optical size [Gao et al. 2007]. However, for a short time period before the coating begins to evaporate, the scattering signal hasn t been perturbed until a significant fraction of the coating component has been removed. The hypothesis is that the perturbed Gaussian shape can be reconstructed by extrapolating the leading edge of the scattering signal if the relevant parameters are known to define the Gaussian function. The time evolution of the scattering signal for a non-absorbing particle in the laser beam is expressed by equation 4.5.4: v S t B P t t 2σ 2 ( ) = + p 2 S exp[ ( 2 0) ] Several parameters are needed to constrain a Gaussian function: the Gaussian width, described as FWHM, which is related to the first moment σ (equation 4.5.7); the second moment is the particle position as a function of time with respect to the centre of laser beam, expressed as V p *(t-t 0 ) in the above equation; the Gaussian baseline offset (B) is the background signal of the optical detector, which is readily determined using the signal measured without a particle in the laser beam; the scattering signal as a function of time, S(t), is recorded with time resolution of 0.2µs, which is sufficient to record the time evolution of a single particle. Hence P S can be derived. The width of the Gaussian (FWHM) is fixed a priori by the laser beam shape and sampling flow rate, both of which are held constant. The FWHM is determined experimentally by calibrating with monodispersed PSL particles of various sizes. The lack of variability in FWHM and its size independence is confirmed in figure , indicating a stable laser profile and constant sampling velocity. 149
150 To independently determine the absolute position of a particle with respect to the laser beam centre, a quadrant silicon avalanche photodiode was introduced as a position reference sensor. As schematically shown by figure , two of the quadrants of the APD are aligned along the image path of the detector, configuring it as a two-element APD (TEAPD). In practice this acts as a single APD with a gap. The signal from the leading quadrant (lower-right quadrant in figure ) is inverted before electronically combined with the signal from the second quadrant to reject the noise from the common mode. Figure Schematic of the quadrant silicon APD configured as TEAPD by Gao et al. [2007]. Two quadrants remain unused. The arrow indicates the movement of the image across the detector surface for a single particle. The amplifiers used to process the quadrant signals are shown. To reject common mode noise, the signal from the first quadrant is inverted before being added to the signal from the second quadrant. The gap in the TEAPD perpendicular to the particle motion results in a notch in the output scattering signal time series, schematically presented in figure The notch in the TEAPD is fixed with respect to laser beam centre, thus provides a reference position for the entire signal time series of a single particle event. The stability of the optical alignment and constantly controlled sample flow ensures that the conversion from time to distance, hereafter the relative position, is of high reproducibility. In addition, the time reference established by the TEAPD is valid for the signals of the 150
151 other three detectors because of the simultaneous recording for all four detector channels by the SP2. Figure Schematic representation of the time evolution of a scattering signal (from Gao et al. [2007]) analogous to figure 4.5-1, but the signal is imaged on a TEAPD with a notch along the image pathway. The right hand side of this plot represents the raw scattering signal, the inverted signal from the leading quadrant is also shown. Figure illustrates the scattering signal imaged on the TEAPD and the normal APD in greater detail for a PSL particle, which represents a non-absorbing particle producing only scattering laser light but not incandescence, therefore the signal is not perturbed by laser heating. 151
152 Figure The scattering signal of a PSL particle imaged on the TEAPD and the normal APD, the parameters used to constrain the Gaussian function are illustrated in red. The peak scattering signal detected by the normal APD necessarily occurs at the laser beam centre as shown in figure If the optical alignment is stable and the particle velocity is constant, the distance between the leading edge of the notch on the TEAPD and the laser beam centre is expected to be constant (defined as peak position in the figure above). If so, the position of the laser beam centre can be predicted from the measured position of the leading edge of TEAPD notch. The difference of this approach from that of Gao et al. [2007] is that they used the zero crossing point of the TEAPD signal (as the arrow in figure shows) to reference the position of the TEAPD gap. However, unlike the optical alignment of the TEAPD by Gao et al., the gap position of the TEAPD in this study was behind or too close to the laser beam centre, resulting in a largely weakened signal on the second quadrant detector. This problem is more apparent for absorbing particles because a significant fraction of the particle scattering signal has 152
153 been removed by evaporation after or when the particle crosses the laser beam centre (shown by figure ), leading to the difficulty in detecting the zero crossing point. In more recent studies the position of the TEAPD relative to the laser beam is adjusted to ensure that the leading edge occurs earlier but this was not the case in this work. Figure The scattered light imaged on the second quadrant of TEAPD is too low to detect the zero crossing point for absorbing particles. The distance between the TEAPD gap edge and the laser beam centre (peak position) is investigated via calibrating with mono-dispersed PSL particles at a range of sizes. As the right panel of figure shows, this distance has a narrow distribution and lack of size dependence, the uncertainty when using the median value is within ±25%. 153
154 Figure The distributions of FWHM and peak position as calibrated by monodispersed PSL particles at a range of sizes. The entire Gaussian distribution can therefore be extrapolated from the partial Gaussian profile as the FWHM, the peak position, S(t) and B are all known. For an absorbing particle, the leading edge of the scattering signal of a particle will not have been significantly reduced by laser heating since the amount of light absorption is weak at low laser energy at the edge of the beam, and the entire time profile of the scattering signal can be obtained from this. This methodology is termed the leading edge only (LEO) extrapolation technique, and aims to reconstruct the unperturbed scattering signal of absorbing particles. This LEO technique has first been validated using non-absorbing PSL particles because their scattering signals haven't been perturbed by laser heating. 154
155 Figure Steps to apply the LEO technique to a PSL particle. Another key factor affecting the effectiveness of the LEO is the number of digital points used in the extrapolation, as the green line in figure shows. This is not of great importance for a non-absorbing particle, like a PSL. The results of the LEO technique are positively correlated with those of the normal Gaussian fitting for PSL particles in the size range between 150nm and 600nm, as given by figure , which validates the LEO method for characterizing the scattering properties of non-absorbing particles. The derived scattering signals from the LEO method for very large particles slightly shift away from the linear correlation because of the limited digital points used for the extrapolation when the detector is saturated. 155
156 Figure the fitted peak amplitude of the scattering signal from the LEO technique correlates with the results from normal Gaussian fitting for PSL particles over a wide range of sizes. The usage of the LEO technique can be extended to reconstruct the scattering signal of unmodified absorbing particles. The median values of the parameters as calibrated by PSL particles are applied. In practice, double-mode peak shapes are frequently observed for coated BC, the leading peak resulting from the scattered light by the larger and coated particle which occurs earlier in the beam with lower laser intensity and the second peak occurring from the BC core towards the beam centre at a point when the laser intensity is higher. The relevant importance of both peaks compared to each other is determined by the amount of coating material relative to the mass of BC core, as figure demonstrates. Three flame soot particles were generated, with increased relative amounts of coating components during the AIDA project. Examples of these particles are shown in figure where it is seen that the second peak resulting from the scattering of BC core is becoming less important. The position of the expected Gaussian peak, as well as the digital points used for the LEO fitting, is adjusted by scanning the peak shape of the scattering signal for each particle. The representative 156
157 LEO-fitted results are also presented in figure with green line denoting the points of unperturbed scattering signal used for the extrapolation. Figure the fitting results using the LEO technique for soot particles generated with varying relative amounts of coating components during the AIDA project. The reconstruction of the scattering signal of the original particle can be investigated by comparing the consequent parameters with that from normal Gaussian fitting. The results tested by mono-dispersed uncoated 300nm GCS particle are given in figure The Gaussian scattering signals have been reconstructed to possess a similar Gaussian width to non-absorbing particles, and the occurrence of peak intensity is forced to coincide with the time when the particle transmits the laser beam centre. 157
158 Figure The indications for LEO technique to reconstruct the original scattering signal for GCS 300nm, as compared with the parameters from normal Gaussian fitting. The scattering properties as a function of particle size for PSL and GCS particles are presented in figure The GCS particle scatters light more intensively than PSL at the same size due to its larger refractive index. This is consistent with the laboratory results by Gao et al. [2007]. Comparing the reconstructed scattering signals with normal Gaussian fitting in figure 4.6-9, implies that the reconstructed scattering signals of GCS particles are significantly close to the real values. 158
159 Figure the P S derived using LEO technique for mono-dispersed PSL and GCS particles at a range of sizes. However, uncertainties of LEO technique can result from Rayleigh scattering at the quadrant detector, scattered light from the tip of the aerosol nozzle, the assumed constant velocity for a particle passing through the laser beam, and the ideality of the optical alignment with the assumption that the particle necessarily crosses the laser beam centre. 159
160 4.7 Methodology of data analysis The instrument modification The Manchester SP2 instrument has been under several modifications during the writing time of this thesis. As table 4-1 summarizes, the detector sensitivities have been increased for both scattering and incandescence signals to expand the lower detection limit. For the old version of SP2, the particle event is only triggered by the scattering channel, which means only when the scattered light by a particle is above a certain predetermined threshold, the particle event can be recognised. The modified SP2 uses both modes of scattering and incandescence triggering, for this case, if an absorbing particle that has not scattered sufficient light can also be triggered by the incandescence channel if the incandesced light of the particle is above a specific threshold. The incandescence triggering allows a significant fraction of small BC particles to be detected whose scattering properties have been largely perturbed in the laser beam (section 4.6.3). The detectors with increased sensitivity can collect the signal of small particles with a higher S/N ratio, thus for a given threshold, a particle can be triggered at a smaller size. The triggering capacity in the scattering channel is also dependent on the laser power, as a particle can scatter more light if the incident laser light is more intensive. However, the increase in the detector sensitivity constrains the instrument capacity of detecting the larger particles because the higher signal will saturate the circuit board of the detectors. The resultant signal of a single particle exhibits a plateau shape at the peak intensity, leading to a failure in particle sizing. The modified SP2 saturates channels at a smaller particle size compared to the old version of SP2. Before the modifications, the SP2 optically sizes a scattering particle using an APD with a lower gain installed at channel 3 (Ch3) if the other APD is saturated (table 4-1). However, the Ch3 has been occupied by the TEAPD in the modified SP2 configuration, thus the quantification using low gain detector is not applicable. An asymmetric fitting methodology is applied to resolve the detector saturation on the scattering channel, which will be discussed in the 160
161 following. The information on the optical size of BC particles is only available when the TEAPD is installed for the modified version of the SP2. The refractory compositions can be discriminated by the SP2 measurement by collecting the incandescence signals at different bands of wavelengths (section 4.4), and this method is only applicable when the signals from the two incandescence channels are comparable to obtain reasonable ratios between them. However, the modified SP2 has increased the sensitivity of both incandescence detectors but not equally on them, leading to a significant saturation on broadband channel (Ch1, table 4-1), thus the ratio between the broadband and narrowband channel (Ch2) can no longer be used to determine the refractory composition. The modified SP2 uses the Ch2 to size BC particle if the Ch1 is saturated as the Ch2 has a lower gain than Ch1, whereas the Ch1 is used to determine the presence of a BC particle as the Ch1 is more sensitive to the smaller particles. The functionality of the instrument with data assured quality before and after the modification is summarized in table 4-2. Figure summarises the data analysis procedures in flow diagrams for the different instrument configurations before and after the modifications. It is shown that the analysis procedures are classified into two main parts: the quality assurance and filtering on the raw data of single particles, and the bulk analysis to derive the concentrations in real time. The following section and section will in detail discuss the two parts of data analysis respectively. 161
162 Old version of SP2 Modified SP2 Software triggering Ch0 (scattering) triggering Both Ch0(scattering) and only Ch1(incandescence) triggering Detectable lower limit D O =200nm D O =140nm size ranges Ch0 Saturation limit D O =480nm D O =330nm* at different lower limit D ME =190nm D ME =70nm channels Ch1 Saturation limit D ME =650nm D ME =240nm** (illustrated as calibrated lower limit D ME =200nm D ME =150nm Ch2 particle Saturation limit D ME =650nm D ME =580nm*** diameter in lower limit D O =300nm D O =160nm(LEO applied) the shape of Ch3 solid sphere) Saturation limit D O =720nm D O =620nm**** Particle sizing Scattering particles D O = nm D O = nm range BC particles D ME = nm D ME =70-620nm Capacity to discriminate BC boiling point from other absorbing components YES NO Detection of original optical size for absorbing BC particles NO YES***** The detecting limits of scattering are sensitive to the laser power. * The saturated scattering signal can be extrapolated to achieve the Gaussian peak (figure4.7-1) up to D O =620nm. ** If Ch1 is saturated, the BC particle is sized by Ch2 signal. *** The BC particle is sized by the integrated area of Ch2 signal if the peak signal is saturated. **** The upper limit for applying the LEO fitting on Ch3 due to a large fraction of saturated signal. ***** The coating thickness (CT) detecting range depends on the size of BC core, with a preliminary estimation of CT=25-190nm. Due to the modified sensitivities of two incandescence detectors (table 4-1), the readings between Ch1 and Ch2 are not comparable. Table 4-2. The functionality of the SP2 instrument with data assured quality before and after the modification. 162
163 4.7.2 Data quality assurance and filtering To characterise particles using the scattering and incandescence signals measured by the SP2, the raw single particle signals recorded must be evaluated to determine if they are of sufficient quality to derive further information. The signals from the SP2 can be impacted by background electronic noise, baseline drift, the saturation of channels, particle coincidence, the particle trajectory not crossing the laser beam centre and some influences from optical filters. These interferences cannot be used to determine the properties of particles, and should be removed prior to the next procedures of data analysis Discrimination of scattering-only and absorbing particles The first step in identifying particles is to decide if the particle contains an absorbing core by the presence of an incandescence signal. As figure shows, any particle event that contains an incandescence signal above certain predetermined threshold value in a specified channel is considered to be an absorbing particle. If the signals from the two incandescence channels are comparable, the composition of BC can then be differentiated from other absorbing components by investigating the ratio between them for the old version of SP2 (section 4.4, figure4.7-1 A)). However, this information is not available for the modified SP2 due to the non-equally increased sensitivity at both incandescence channels (table 4-2). Other particle events, in which the peak amplitude of the scattering signal is above the threshold of the scattering channel but the incandescence signal exhibits inherent electronic noises, are grouped as scattering-only particles (or termed as non-absorbing particles). 163
164 A 164
165 Software triggering Ch0 Ch1 Ch2 Ch3 Above threshold? N Above threshold? Above noise level? Above noise level? Asymmetric fitting Y Scattering-only particle Y Saturated? N Gaussian fitting FWHM and RTPO filtered Y Absorbing particle Saturated? N Absorbing mass Y N Saturated? Y Y Ch2 peak area used Y Signal shape determination LEO fitting FWHM and RTPO filtered Optical size of absorbing particle Raw data quality assurance, filtering and single particle analysis Optical size Sampling volume from housekeeping data Number (volume) total concentration and size distribution for scattering-only particles Particle counting Coincidence recording Number (mass,volume) total concentration and size distribution for BC Mixing state of BC Bulk analysis according to sampling flow B Figure Data analysis procedures for the Manchester SP2 instrument: A) the old version; B) the modified version. 165
166 Electronic Noise and Baseline Drift A single particle event can be triggered by either scattering or incandescence signals. A scattering-only particle can only be triggered by the scattering signal (for old version of the SP2), whereas an absorbing particle can be triggered by scattering, and if the scattering signal is not above the threshold can also be triggered by the incandescence signal (for modified SP2). The threshold is set in digital units according to the electronic gain and offset of the detectors. The peak amplitudes of the scattering and incandescence signals can be determined from the magnitude of the signal above the threshold. During data collection, the thresholds can be variable, some inherent background electronic noise can also be triggered as a particle event, and occasionally, random spikes can be generated in the same amplitude ranges as real particle signals. The former noise can be largely removed via investigating the peak intensity of the signals or fitted Gaussian width (FWHM). If there is noise on the scattering signal, it can be extracted by filtering the particle events with abnormal relative time of peak occurrence (RT PO ). When sampling high concentrations of particles, the electronic baseline could begin to increase, because the detector responds more quickly than the restoration circuitry, resulting in a peak being superimposed on a DC signal. A positive offset can be introduced by this, which must be removed otherwise the subsequent derived size will be overestimated. This type of signal frequently exhibits an erroneous FWHM The fitting methodologies It should be noted that the Gaussian function can profile the scattering signal of a scattering-only particle to constrain its peak amplitude (section 4.5, figure 4.5-3), whereas the scattered signal from an absorbing particle is determined by the LEO fitting if the TEAPD is installed (section 4.6.4, available for the modified SP2, table 4-2). The fittings use least squares method by the analysis software, and a less interaction means a 166
167 higher reliability. The incandescence signals are not in a Gaussian shape thus the maximum intensity of the raw signal is used to size the mass of an absorbing particle Saturation of channels The upper limit for the SP2 to size a particle is whether the particle is large enough to scatter light or incandesce sufficiently to saturate the detectors. The saturated channel is not able to size particle because the signal exhibits a plateau shape at the peak intensity (figure 4.7-2). Figure A) A typical solution for digital saturation on scattering channel for a nonabsorbing particle. B) For the modified SP2, the Ch2 is used to size BC particle when Ch1 is saturated. The old version of the SP2 uses a scattering channel with low gain to optically size a particle if the normal scattering channel is saturated. This low gain scattering channel is not available for the modified SP2. The other solution for the saturated scattering signal of a non-absorbing particle is to reconstruct the expected Gaussian shape by 167
168 extrapolating the unsaturated leading part of the scattering signal, as figure A) shows. The fitted Gaussian peak position is chosen to be in the middle of the plateau edges according to the asymmetric shape of the saturated signal, thus this method is termed as asymmetric fitting to be differentiated from the LEO fitting. This method can considerably expand the upper limit of optically sizing, but the uncertainty of this becomes larger for increased particle size when scattering signal is significantly saturated and the number of leading digital points used for the extrapolation is limited. For the modified SP2, the signal on Ch1 is largely saturated as the increased sensitivity. For this case, the BC mass is quantified by the unsaturated signal from Ch2 due to the lower gain of this channel (figure B)) Particle Coincidence The event occurrence rate can be very high when sampling from an environment with a high loading of particles. More than one single particle will occasionally arrive in the laser beam whilst another particle is also in the beam. In this case, multiple peaks can appear in the same pulse. Figure shows an example of coincidence for two soot particles. The normal method of using the entire signal to fit this type of event will erroneously extend the width of the pulse, therefore only the partial signal that is generated by one of the particles can be used to extrapolate and determine the particle size, as shown by figure
169 Figure the coincidence of two soot particles, fitting is only applied for the scattering signal of the leading particle. Particle coincidences should not be removed because these types of events are real particles and the total particle counts are needed for calculating the total particle number concentration. By monitoring the peak shape of signals, which is defined by the number of peaks and peak positions within a particle event, the frequency of particle coincidence can be monitored, and a multiplication factor can then be incorporated to correct the particle counting. 169
170 4.7.3 Concentrations derived from SP2 measurements The total number concentration during a specified time interval is calculated by counting the total number of sampled particles, divided by the volume of the sampled air. The mass of BC and the volume of scattering-only particles, which are measured and recorded as single particles, are summed during a given sample period to calculate the corresponding total mass and volume concentration. The formulas used are summarized as follows: For both BC and scattering-only particles, C N = N i= 1 S n V t i For BC, C M = N i= 1 n M S i V t i For scattering-only and BC (only for the modified SP2) particles, C V N nivi i= = V t S C N, C M and C V are number, mass and volume concentrations respectively; i denotes a specified size channel in which the particles are of the same size that cannot be further differentiated, in the current analysis software, the binning interval can vary according to user s requirement, the suggested value is 5-15nm. The particle size is obtained from the 170
171 calibration results by PSL and GCS particle for the scattering-only (section 4.5) and BC particle (section 4.3) respectively. N is the total number of size channels; n i, M i and V i are the number, mass of BC, and volume of scattering-only particles within the corresponding size channels; V S represents the sampling volume rate, t is the time interval over which the concentration is calculated. The volume concentration for BC particles, which is determined by the optical size, is subjected to large uncertainty before the TEAPD is installed thus only calculated for the modified SP2. Figure shows typical average BC number and mass size distributions for both instrument configurations. The amount of BC number or mass that is outrange of SP2 detection limit is estimated by assuming that the size distribution of BC will follow a single lognormal function. The hypothesis that BC size distribution can be profiled in a lognormal mode has been shown by observations from the ground [e.g. Kondo et al. 2006, Rose et al. 2006] and in the free troposphere [Clarke et al. 2004]. Consequently, the entire BC mass size distribution can be created via extrapolating the frequency distribution of concentrations as a function of size. 171
172 Figure The number and mass based size distributions of BC, as measured by the old and modified SP2. The amount of BC existing at sizes smaller than the SP2 detection limit can be resolved by extrapolating the measurable concentration following a lognormal function. The shadows in the light blue indicate the size range used to perform the extrapolation. The sensitivity of the modified SP2 has been significantly improved. The extrapolations shown in figure are least squares fits of a lognormal function to the measurements. For the old version of SP2, the amount of BC outside the detection range of the SP2 is estimated to be about 55% and 70% on a mass and number basis respectively. The detection range of the modified SP2 has been expanded to a much lower size by using the high S/N level of a PMT with enhanced sensitivity (table 4-1, section 4.7.1), which confirms the single lognormal mode of BC size distribution by direct observation. 172
173 Chapter 5 The investigations of BC close to the ground sources 173
174 Overview Measurement were conducted at Holme Moss (53.53 N, 1.86 W, 525 m a.s.l.) in November-December 2006 for 28 days continuously. Holme Moss is positioned in the rural southern Pennines region in Northern England, approximately 30 km to the northeast of the city of Manchester. The exceptionally strong south-westerly flow typically experienced at the time of the year made this site an efficient receptor of anthropogenic pollutants from the Greater Manchester conurbation, in addition to the contribution from nearby residential home heating in that winter season. These contributing sources mean that the pollutants at this site contain substantial amounts of black carbon (BC). The aerosol optical properties, chemical composition and hygroscopic properties were characterized by a series of instruments. In particular, the single particle soot photometer (SP2) was deployed to characterize the physical properties of BC-containing particles. The evolution of aerosol characteristics was significantly controlled by the fraction of BC, and followed reproducible diurnal variability. The BC aerosols peaked both at noon and in the late afternoon with a mass loading of 150±30ng/m 3 and 270±50ng/m 3 respectively, which were considered to arise from different sources. About 27±6% of the BC was thickly coated when the source was influenced by solid fuel burning, which was higher than the vehicle emission dominant source (18±8%). The mixing state of BC was closely associated with the formation of secondary particulate matter, particularly the oxygenated fraction of organic aerosol (OA). The mixing state of BC coupled with the level of oxygenation of OA influenced the hygroscopic properties of aerosols. 174
175 5.1. Introduction Soot aerosol, known as black carbon (BC), is produced by incomplete combustion, and the sources can be from fossil fuel, biofuels and open biomass burning [Bond et al. 2004]. Metropolitan urban areas have been severely polluted by BC pollutants [e.g. Seinfeld 2008]. Due to its capacity to absorb solar radiation in the visible and infrared [Bond and Bergstrom 2006] and the capacity to form regional atmospheric brown cloud [e.g. Ramanathan et al. 2007], BC has been identified as the second main contributor of global warming besides the greenhouse gases (GHGs) [Ramanathan and Carmichael 2008]. Reducing the BC emission is a more effective way compared to controlling the GHGs to counteract the global warming [Bond and Sun 2005], which would benefit in raising the global temperature level at which dangerous anthropogenic interference occurs [Nenes et al. 2002], as well as reducing the health risk to human beings [Smith 2005]. Freshly emitted BC is primarily hydrophobic [e.g. Weingartner et al. 1997], but can acquire the other components soon after emission, such sulfate [Kittelson 1998], lubricating oil and ash [Johnson et al. 2005], and contains a considerable fraction of organic material particularly if the source is biomass burning [Pósfai et al. 2003]. The initial mixing state of BC can also be dependent on the type of sources. This was recently observed via a single particle approach [Schwarz et al. 2008]. BC can be further internally mixed with water soluble components, such as sulfate, secondary organic or nitrate [Shiraiwa et al. 2007] by condensation or coagulation processes [Riemer et al. 2004], or the surface can be oxidized by ozone and oxidizing radicals [Zuberi et al. 2005] to modify the originated hygroscopic property. The sink of BC is primarily governed by wet deposition [Textor et al. 2006], the time scale for BC to be converted from initial hydrophobic to hydrophilic is therefore of great importance to control the atmospheric lifetime of BC. Modelling work revealed the BC is more likely to be activated as CCN at much lower critical size if coated [Henson 2007], and it has been observed that a soluble coating on BC can significantly enhance 175
176 its CCN activity [Dusek et al. 2006], but might also depend on the compositions of coating material [Petzold et al. 2005]. However, due to the systematic limitations of most of the current bulk-based BC measurements [Schmid et al. 2001], the examining of BC mixing state has not been available, leading to most of the models assuming an e- folding time to estimate the hygroscopic evolution of BC [e.g. Koch et. al.2007, Park et al. 2005], which lacks observational support. To treat BC as internally or externally mixed can result in large difference in predicting the global radiative budget [Chung and Seinfeld 2002], because of the absorption enhancement by internal mixing [Bond et al and references therein]. The BC has long been considered as a combustion source tracer due to its relatively stable atmospheric composition, whereas the organic carbonaceous aerosol (OA) has larger variability without fixed chemical compositions, the primary organic aerosol (POA) can be transformed by secondary material (SOA) via various processes and the extent of the formation of secondary organic particle is a general parameter to identify the aerosol processing time scale [Kanakidou et al. 2005]. The ratio of organic and elemental carbon has been widely used to determine the amount of secondary organic aerosol formation [Turpin and Huntzicker, 1995], the larger ratio generally indicates a larger contribution from secondary aerosols. Recent investigations on the compositions of organic aerosol mass (OM) using the Aerodyne Aerosol Mass Spectrometer have shown that the organic mass can be split into Hydrocarbon-like Organic Aerosol (HOAlike) and Oxygenated Organic Aerosol (OOA-like), which are closely associated with POA and SOA respectively under certain conditions [e.g. Lanz et al. 2006, Zhang et al. 2007]. The results presented in this study were measured from Holme Moss (53.53 N, 1.86 W, 525m a.s.l.). Strong south-westerly flow is typically experienced during this time of the year in the UK, advecting air from the Greater Manchester conurbation to this site. The experiment was conducted during 07/Nov. to 04/Dec to characterize the aerosol properties via series of instruments. In addition to the influence of urban outflow, the localized sources of solid fuel burning due to the high usage of domestic heating in 176
177 winter season were significant. The influences of these combined sources establish this site to be a fairly unique environment to investigate the aerosol properties, in particular the differently originated BC and OA. The aerosols soon after emission in Manchester city centre were also characterized during the CityFlux project in Aug. 2006, compared with the data in Holme Moss to investigate the evolution of aerosol properties. This study aims to reveal the evolution of aerosol characteristics on the basis of diurnal variability, using BC as a tracer of anthropogenic emissions to study the atmospheric processing of aerosols, and linking this to the formation of secondary particles and the mixing state of BC, in turn modifying the optical and hygroscopic properties Sites, instrumentations and data analysis techniques The experimental sites and sampling inlet The experimental site of CityFlux was chosen alongside the Sackville Street building on the top roof (about 25m above the ground) of one of the buildings, in the University of Manchester. Manchester is one of the major cities in the northwest of England, which is surrounded by numerous satellite towns, making up the conurbation of Greater Manchester with a population of 2.5 million. This chosen site is close to the city centre and rail station, where the distinct peaks in weekday traffic flow occurred at 07:00 09:00 and 16:00 18:00h local time [Longley et al. 2003]. Manchester is subjected to frequent cyclonic conditions, thus the pollutants can be transported to influence the conurbation and nearby rural area. The SP2 was employed to act as one of the main instruments to characterize the relatively fresh urban emissions. The source of BC measured in this project is believed to be primarily from the direct emissions of vehicles, as indicated by the SP2 measurements to be high mass loading and thinly coated (figure 4.6-6). 177
178 A B Figure A) The map of the experimental site (marked as HM2) and surrounding area. B) Wind rose plot during the whole experiment period, individual wind direction is accumulated and coloured by probabilities of wind speeds. 178
179 The Holme Moss site is positioned in the rural southern Pennines of Northern England (geographical coordinate N, 1.86 W), approximately 30 km to the northeast of the city of Manchester (figure5.2-1 A)). The University of Manchester has used a research station on that site (525m a.s.l.) for more than a decade for both field campaigns and long term climatological measurements. The experimental period lasted from 07/11/2006 to 04/12/2006, during which the site continuously experienced a strong southwesterly air flow. Typically the locally monitored wind speed was over 10m/s, and the wind direction was from the southwest for 90% of the time (figure5.2-1 B)). Potentially, the polluted air mass from the Greater Manchester conurbation is efficiently advected to this site, but may undergo some atmospheric process during the transportation. The residents surrounding Holme Moss also act as localized pollution sources, which are suspected to be due to the usage of solid fuel for home heating. The weak incident solar radiation, the low temperature in the winter, as well as the frequent coverage of clouds, made photochemical processing less efficient during the experimental period. 179
180 Figure Experimental set up of the aerosol instrumentation deployed during Holme Moss The inlet system was comprised of a plastic stack with a height of ~10m, the inner diameter of which is about 21.4 cm, providing a sample flow of ~1000L/min. A 2.44 m long stainless steel tube with an inner diameter of approximately 4.7 cm is used to subsample the air isokinetically from the centreline of the flow inside the stack. The sampling system uses a flow of 150L/min, with the rest of the total flow being sheath air. This inlet system has been previously described by Sheridan et al. [2001], who gave a collection efficiency for particles of between 0.01 and 1 µm over 95% but the loss rises to 10% for particles larger than 10µm. A 5µm impactor is used to prevent sampling the larger aerosols and cloud droplets, thus the sampled aerosols are in the interstitial phase, which have not been scavenged by clouds. Another sampling line was connected after a counterflow virtual impactor (CVI) to collect the aerosols that have been incorporated into clouds by evaporating the cloud particles and sampling their residues. The sampled 180
181 air is drawn isokinetically into the temperature maintained laboratory where the instruments are located. Figure5.2-2 shows both installed inlets during this project, and the aerosol instrumentations connected to each inlet are also indicated. This study presents the data from the interstitial inlet; the results using the CVI inlet are presented in Chapter BC physical properties The SP2 uses an intra-cavity Nd:YAG laser beam (1.064 µm, TEM oo mode, intensity ~1MW cm -2 ) to heat the refractory aerosol to incandescence on a single particle basis [Stephens et al. 2003, Schwarz et al. 2006]. The particles passing through the laser beam will scatter light at the wavelength of the incident laser. In particular, if any refractory components are present in the particles, such as BC, they can absorb the laser radiation and be heated eventually to reach the boiling point temperatures when they incandesce, emitting significant thermal radiation in visible wavelengths. The non-absorbing aerosols will not absorb the laser but only scatter light as a function of laser intensity and particle size. The detectors record the scattering and incandescence signals for each particle simultaneously with a time resolution of 0.2µs. The incandescence signal was calibrated via commercially available standard glassy carbon spheres (supplied by Alpha Aesar, Inc., Ward Hill, Massachusetts, with density 1.42g/cm 3 ), which were size-selected by introducing a differential mobility analyzer (DMA) upstream of the SP2. The mass determination for soot particles by the SP2 has been observed to be largely independent of particle shape [Slowik et al. 2007] and the coating components of BC [Moteki and Kondo 2007]. The scattering signal can be used to determine the optical size for each particle, which was calibrated by monodispersed polystyrene latex spheres (PSL). By this method, the laser power was monitored to be within 6% for the entire operation period, resulting in the variation of optical size of 3%. Additionally, Baumgardner et al. [2007] confirmed the lack of refractive index (RI) dependence on the optical sizing of particles by the SP2 using Mie calculations. Overall, 181
182 the optical diameter (D O ) of non-absorbing particles was determined from PSL calibrations with uncertainty about ±8% within size range nm for this study. The Do for absorbing particles can be underestimated due to the heating of the particle by laser light absorption. The particle size will begin to decrease as the coating material evaporates, which means the detected scattering signal cannot represent the original optical size. An extrapolation technique can overcome this problem by applying a detectable laser beam centre position and fixed signal distribution shape (section4.6.4). This needs a hardware modification that had not been carried out on the instrument at the time of the experiment. By comparing the scattering signal of BC and non-absorbing particles of the same size, the shape of BC scattering signals have been observed to be distorted, provided the scattering property of non-absorbing particles is not influenced by laser heating, the uncertainty of optical sizing of BC is estimated to be about ±10%, and becomes more significant for larger BC core and with lower volatility of coating materials. However, soot aggregation can enhance the scattering property significantly [Liu and Mishchenko 2005]. The mass fraction (MF) for a refractory BC core within a single particle is calculated by equation MF M = πd O ρ av 6 The average density (ρ av ) used in this study was 1.8g/cm 3, according to Cross et al. [2007], the uncertainty of density can vary with the coating compositions by approximately 41% from pure sulfate to organic, however, the coating can be of combined compositions in reality. The overall uncertainty for MF resulting from the ρ av is estimated to be ±33%. The onset of the peak intensity of the incandescence signal can be delayed if the BC core is significantly enclosed due to the time required to evaporate the coating, during which the heat absorbed by BC core will be conducted to the surrounding coating components. The gap between the peak intensity of scattering and incandescence is related to the 182
183 coating evaporation time (T), which has been observed to exhibit a step increase when BC is substantially coated [Schwarz et al. 2006, Moteki and Kondo 2007]. Therefore this parameter is widely used to diagnose the mixing state of BC. As the coating on a particle increases, for a fixed BC mass the MF will reduce and it is expected that T will be increased. This behaviour can be seen in figure Little variation in T is observed for particles with a MF above 0.25, indicating that little time is required to evaporate thin coatings on BC cores. These particles have such thin coatings that the definition of T is somewhat limited. On the other hand when particle MF<25%, coating thicknesses become substantial and more time is required to evaporate them before particle incandescence occurs. Figure5.2-3 shows a dramatic increase in T when the coating mass fraction is over 75% (MF<0.25). The BC particles within this region are likely to be entirely enclosed by relatively thick coatings, delaying the onset of incandescence. T = 4.6±0.2µs is observed to be a critical point above which T is more strongly dependent on MF. 183
184 Figure The refractory mass fraction related to the coating evaporation time for continuously collected BC-containing particles, the upper and right lines show the histograms of x-axis and y-axis values respectively. The definitions of thickly-coated and thinly-coated BC are illustrated. The coating thickness can be estimated assuming sphericity and will be in the range of 0-140nm and approaches 50±15nm when T approaches the critical point. The calculated thickness falls within the urban observations by Baumgardner et al. [2007] and Moteki and Kondo [2007] (0-150nm). However, the complex morphology of BC makes it difficult to define the coating thickness as the condensed coating layer may not be evenly distributed on the soot aggregates and compaction of the particle shape to a spheroid occurs as a result of atmospheric ageing [Weingartner et al. 1995]. The 184
185 advantage of using MF to characterize the coating abundance is this definition avoids the sphericity assumptions made when calculating a coating thickness. The mixing efficiency (ME) of BC is defined as the fraction of entire particle number concentration which have a thick coating (defined as MF<0.25), as figure5.2-3 illustrates. The size dependence of ME is also likely to be important as it will be difficult to condense sufficient material onto larger BC cores to obtain a thick coating, whereas the opposite tends to be true for the small core. Previously, these effects were reduced by investigating the mixing state over a fairly narrow range, i.e. close to the median D ME at nm [Schwarz et al. 2007, Moteki et al. 2007] and assuming that larger and smaller core sizes were nearly bare and had thick coatings respectively. In this experiment the ME for the total measured particulate was compared with that over the limited size range as conducted previously [Schwarz et al. 2007, Moteki et al. 2007]. Figure5.2-4 shows that the ME at the BC median size can represent the mixing trend of the entire group of BC particles during this experiment. The slightly lower ME derived from the total BC compared with that derived from particles close to the median size is due to the reduction in the collection efficiency of the SP2 at the lower detection limit, given small BC cores are more likely to be thickly coated. 185
186 Figure The relationship between BC mixing efficiency over the entire size range of the SP2 and at the median size ( nm) Aerosol optical properties and BC mass loading Scattering and absorbing coefficients of aerosols were quantified via classical filterbased techniques. An integrating nephelometer (Model 3563, TSI, 500 Cardigan Road Shoreview, MN, 55126, U.S.A.) was used to measure the total scattering coefficient simultaneously at three wavelengths (λ=450nm, 550nm and 700nm) [Anderson et al. 1996], from which the data was corrected for truncation errors following the procedures recommended by Anderson and Ogren [1998]. The absorption coefficient (σ abs ) was determined via a three-wavelength Particle Soot Absorption Photometer (PSAP) at λ=467, 530 and 660nm (Radiance Research, 535 NW 163 St, Seattle, WA, 98177, USA). The data filtering and error correction was performed according to Bond et al. [1999]. 186
187 Due to the indirect measurement of BC mass by PSAP, an empirical converter, which is defined as mass absorption cross section (MAC) [Bond and Bergstrom 2006], is needed to translate the measured absorption coefficient of aerosol bulks into the estimated BC mass. A variety of uncertainties can be propagated by this empirical conversion, because the MAC of BC is not a constant, but is dependent on different originating sources and combustion conditions, which are highly influenced by the mixing state of BC [Bond et al. 2006], i.e. the absorbing efficiency can be enhanced as much as 50% by internal coating. Because the SP2 directly measures BC mass concentration, the value of MAC can be derived by comparing the PSAP measured absorption coefficient (MAC=σ abs /Mass BC ). This approach is based on a strong positive correlation between the PSAP and SP2 measurement (figure5.2-5 and figure5.2-6 A)). The probability density distribution of the MAC values for the BC particles measured in this Chapter 5s presented in figure5.2-6 B). A median value 13.69m 2 /g of MAC is obtained, which falls within the reported MAC from biomass burning by Schwarz et al. [2008]. However, a fraction of particles have an unexpected large MAC as seen by the tail in figure5.2-6 B). This arises from either the overestimated absorption coefficient from the filter-based measurements or a possible underestimate of BC mass loading by SP2. The former not only results from the relatively low S/N ratio of the PSAP during periods of low particle concentration, but also occurs because of enhancement of absorption due to coatings. Furthermore, the presence of light absorbing organic aerosols may add increased absorption [Lack et al. 2008, Subramanian et al. 2007], and these organics are mainly composed of primary organic aerosols (POA) [Andreae and Geleneser 2006], which is prevalent at Holme Moss site that will be discussed in the following. The SP2 measurements systematically underestimate the total BC mass loading due to the undetectable BC at sizes smaller than its detection limit, which is about 190nm in this study. The total BC mass can be estimated by extrapolation assuming a lognormal distribution (section 4.7.2), which indicates that about 40-60% mass or 30% number of entire BC population has been detected by SP2 in this study. The BC mass reported in this work has been scaled using this fraction. However, this methodology may lead to an underestimate of total BC mass if a significant mass fraction of BC is present beyond the 187
188 SP2 detection limit [Johnson et al. 2005]. It is possible that smaller particles below the limit of SP2 detection have a more efficient absorbing activity but can influence the bulk-based absorption measurement, hence the MAC value derived from PSAP/SP2 can be amplified further. 188
189 Figure Time series for the entire experiment, from bottom to top: BC mass loading (on the right in grey color: absorption coefficient); the volume for the SP2 measured particles in the accumulation mode (on the right in grey color: scattering coefficient); HOA mass loading; CN number concentration. 189
190 A B Figure A) The correlation between BC mass and absorption coefficient. B) The probability distribution of observed mass absorption cross section. 190
191 Non-refractory aerosol compositions The time resolved chemical mass loadings and distributions of key submicron nonrefractory components of the aerosol were measured by an Aerodyne compact Time of Flight Aerosol Mass Spectrometer (ctof-ams) [Drewnick et al. 2005]. The ctof-ams is composed of an inlet that aerodynamically focuses a particle beam, a sizing chamber, and a chemical composition analysis section. The operational modes can be adjusted by a particle chopper that allows either short bursts of particles, a constant stream of particles or no particles to travel to the sizing chamber. The non-refractory component of particles is flash vaporised at~600 C and ionised by electron impact (70eV) under a high vacuum (~10-7 Torr), the positively charged ions are then analysed by the compact Tofwerk mass spectrometer. The measured non-refractory compositions measured by the AMS are typically classified as nitrate, sulfate, ammonium and organic compounds [Allan et al. 2004]. The AMS data in the mass spectrum (MS) mode delivers mass spectra with high resolution, allowing the chemical compositions of organic aerosol mass (OM) to be identified [e.g. Alfarra et al. 2004]. The category of OM is to relate the formation of OM to their atmospheric ageing time, different approaches consistently classify OM as Hydrocarbon-like Organic Aerosol (HOA) and Oxygenated Organic Aerosol (OOA) [Lanz et al. 2006, Ulbrich et al. 2008, Zhang et al. 2007], which are highly associated with primary OA (POA) and more aged secondary OA (SOA) respectively. The strong correlation of HOA with BC or CO was observed in these studies due to their coemissions from incomplete combustion, however the relationship among them depends on different sources and combustion conditions [Bond et al. 2004]. The positive matrix factorization analysis (PMF), as fully described by Paatero and Tapper [1993], has been widely used to apportion atmospheric sources multi-component of pollution and is proved to be a robust methodology [Hopke et al. 2005]. This technique has previously been used to characterize the AMS detected submicron OA 191
192 [e.g. Lanz et al. 2006, Ulbrich et al. 2008]. PMF seeks to represent the measured data by a linear superposition of a number of unchanging mass spectral profiles as factors, and the contribution from each profile to the measured data as a function of time can be determined. The profiles of factors represent mass spectra and the changing contributions capture the variation of mass loadings to determine the varying relative contributions from each type which may be linked to a source. A comprehensive analysis of diagnostic parameters was then performed and the retrieved model spectra were compared with the library references and laboratory AMS spectra Aerosol hygroscopic properties The ability of aerosols to take up water was investigated using a Hygroscopicity Tandem Differential Mobility Analyser (HTDMA) [Cubison et al. 2003; Gysel et al. 2007]. The principle of this instrument is to measure the size of a dry particle and detect the size enlargement due to water uptake at the conditions of high relative humidity (RH). The physical growth factor (g) of particles at a specified RH is defined by equation 4.1: D ( ) ( ) m RH g RH = 4.1 D (10%) m The numerator and denominator correspond to the mobility diameter operated at elevated RH (90% is applied for this experiment) and dry condition (RH=10%) respectively. The dry sizes of ambient aerosols at 45nm, 91nm, 138nm, 184nm, 231nm and 277nm are selected to investigate the size growth. The performance of aerosols as cloud condensation nuclei (CCN) was measured using a Condensation Nuclei counter (CCNc, Droplet Measurement Technologies model 100) [Roberts and Nenes 2005]. The supersaturation (SS) conditions were controlled by modifying the temperature gradient in the flow, in conjunction with an optical particle counter to detect the number of particles before entering and after exiting the 192
193 supersaturation chamber, constraining the fraction of the particle population that has the potential to act as CCN. The instrument was operated at SS of 0.07%, 0.15%, 0.37%, 0.75% and 1.25% during this experiment. In addition, condensation nuclei (CN) were measured using a Condensation Particle Counter (CPC, TSI model 3776, Shoreview, MN, USA) that can detect particles down to 2.5 nm. A Differential Mobility Particle Sizer (DMPS) was employed to determine the mobility size of aerosols between 3nm and 740nm. This DMPS system was developed by Williams [1999], which consists of two DMAs in parallel to size the mobility diameter at 3.4 to 34 nm and at nm respectively. 5.3 Results and discussions Diurnal variability in particle concentration The time series of aerosol properties shown in figure5.3-1 indicate aerosol properties have a strong diurnal variability at this site. The high correlation between the BC mass loading and the absorbing coefficient suggests a primary contribution of BC to light absorbing. The scattering coefficient correlated positively with the volume of particles in the accumulation mode but did not correlate with the total sub-micron aerosols (CN), indicating a more important role of the larger particles on light scattering. 193
194 A B C 194
195 Figure A) The diurnal variation in CN concentration. The width of box plot indicates the bin interval, the lower and upper edges of box are the 25% and 75% percentiles, the line in the middle of box and the cross marker denote the median and average values, with error bars denoting the 10% and 90% percentiles. The box pots in the following have the same meaning as explained here. B) The diurnal trend of submicron/accumulation mode as derived from CPC/SP2 measurements. C) The mobility diameter distribution at different times measured by DMAs. The diurnal variation in the CN concentration is analyzed in figure5.3-1 A). The enhancement of particle number can be clearly seen from 11:00 LST, maintaining a relatively high level but only decreasing after 19:00. This observation is different from some other urban studies such as Baumgardner et al. [2007] and Kondo et al. [2006], who consistently observed a decreased trend in the late morning because the increased ground temperature will lead to the growth of boundary layer, thus enhancing the vertical turbulence and dilution of aerosols, and inhibiting further concentration increase. The aerosol loading in the Manchester urban area has also been shown to follow this diurnal pattern, peaking in the early morning at about 7:00-9:00 [Martin et al. 2008]. The contrasting observation in this Chapter 5s that elevated pollutant concentrations lasted until early night. This site was continuously influenced by strong sources from late morning throughout the day. This occurred because the strong SE winds lead to mixing being efficient throughout the day, but a lack of turbulence prevented a deep convective boundary layer developing in the afternoon. The CPC counted sub-micron aerosols (>2.5nm) and the SP2 measured number concentration ( nm) (CPC/SP2) can be used to provide a micron/accumulation mode ratio. As figure5.3-1 B) shows, Aitken mode particles became dominant after 11:00. This is also supported by DMPS measurements shown in figure5.3-1 C), the population of smaller particles dramatically increased from late morning. This implies the existence of fresh particles close to the anthropogenic sources [e.g. Kittelson 1998]. The growth of particle size became dramatic after 20:00 when the concentration was significantly reduced, during which the particles were processed to be more aged. The 195
196 low concentration of aerosols in the mid-night corresponded to the size dominance in the accumulation mode, suggesting a large fraction of particles were aged and processed when the pollutants were stagnant at night Source tracking and attribution Given BC is an ideal tracer of primarily formed particles because of its lack of atmospheric reactivity [Kanakidou et al. 2005] and with well understood sources [Bond et al. 2004]. The total BC mass loading produced an apparent diurnal pattern as shown in figure5.2-5 and figure5.3-2 A). The increased amount of aerosol contains a significant fraction of BC, firstly peaking at about 12:00 with ng/m 3, and being further elevated at 20:00 with a higher peak of ng/m 3, representing 17% and 20% respectively of the accumulation mode aerosols as measured by the SP2 (figure5.3-2 B)). The non-refractory particulate is defined as the components that can be vaporized at or below 550ºC, being detectable by AMS, typically being present in the compositions of organic, sulfate, nitrate and ammonium. The refractory BC mass detected by the SP2 only contains the mass of the BC core if the particle is coated, therefore BC mass/nonrefractory mass ratio shown in figure5.3-2 C) is a parameter to determine the relative importance of BC core mass compared to the other non-refractory components. The dominant BC mass fraction increased at 11:00-13:00 and 18:00-21:00, implying the contributing sources increasing at both times from incomplete combustion, and the pollutant source tends to be stronger in the early evening. 196
197 A B C 197
198 Figure The diurnal trends of: A) BC mass loading; B) BC number fraction; C) BC mass/non-refractory mass, the latter is the sum of the mass of sulfate, nitrate, ammonium and organic measured by AMS. Figure5.3-3 shows the retrieved model spectra by applying for a three-factor solution of PMF analysis on the AMS detected organic mass spectra, from Corris [2008]. Figure The model spectra retrieved by a three-factor solution of PMF analysis on the AMS detected submicron organic aerosol in Holme Moss. Aged oxidized organics typically have a smaller m/z 43 than the m/z 44 mass fragment whereas HOA spectra typically have a higher m/z 43 peak than m/z 44 [Alfarra et al. 2004]. The m/z 44 signal arises from the molecular ionisation of CO 2 formed from the decarboxylation of poly-carboxylic acid and peroxide functionalities which occurs at the surface of the vaporiser in the ctof-ams. The urban primary emissions are also frequently associated with the m/z 55 and m/z 57 (butyl, C 4 H + 9 ). Factor1 shows a strong contribution from m/z 44 to its total mass, and is characteristics of highly aged secondary aerosol, thus Factor1 is interpreted as the aged oxygenated organic aerosol like component (OOA-like). m/z 55 and m/z 57 are clearly seen in Factor 2, which is highly correlated with the mass spectra of vehicle emissions, characteristics of the HOA-like component influenced by the urban outflow. Factor3 is similar to the spectra derived from fossil fuel use for small m/z and correlates with brush fire and wood smoke for 198
199 larger values of m/z, notably the m/z 60, a characteristic peak of levoglucosan, is present in the spectra. These may indicate the combined usage of solid fuels (e.g. wood and coal) for the home heating in the locality of the measurement site. A B Figure The contributions to the total OA mass from different factors: A) from urban vehicle emissions (Factor2); B) from burning of solid fuels of residential home heating (Factor3). Due to the weak photochemical activity and shortage of the possible precursors during the experimental period, the OA species were observed to be weakly oxygenated with 199
200 HOA (Factor2+Factor3) occupying about 50±15% of the total organic aerosol mass (OM), generally agreeing with the 60% mass fraction of HOA measured at Manchester in winter [Allan et al. 2003], and the HOA mass is positively associated with BC mass loading (figure5.2-5). The contributions to total OM by each type of HOA as contributed by different primary sources are explored in figure It can be clearly seen that the HOA from urban traffic emission became significant during late morning whereas the solid fuels were prevalent from early night. The latter is consistent with intensive activity of domestic home heating, which correspond to the BC peak loadings at 11:00-13:00 and 18:00-21:00 respectively. Therefore, it is speculated that the main contributor of the first peak of BC mass loading is the fossil fuel combustion of vehicles advected from the urban conurbation of Manchester. This is further evidenced by the frequent and strong southwest wind (>90%). If considering the average wind speed (10-13m/s) and the distance between Holme Moss and Manchester urban area (~30km), the approximate transport time scale is 1-2h, which in general explains the occurrence time of the first peak loading observed at this site when the pollutants were transported from Manchester (7:00-9:00) [Martin et al. 2008]. Due to the enhanced activity of surrounding domestic heating from early night, the detected highest BC mass loading at 18:00-21:00 is likely to have been significantly contributed by the solid fuel combustion (figure5.3-4 B)). However, the source from the vehicle emission cannot be entirely neglected due to the afternoon rush hour of traffic in Manchester [Martin et al. 2008]. The peak loading of BC mass from local sources at this time may have been further enhanced by weakened vertical turbulence as a result of reduced boundary layer. 200
201 The mixing state of BC Influenced by different sources The diurnal variation in the mixing efficiency of BC is shown in figure5.3-5 A). The lowest value of ME occurred at 10:00-12:00, suggesting the observed BC was from a relatively recent source, which coincided with the first peak of BC mass loading originating from urban outflow and the highest sub-micron/accumulation mode ratio observed at this time. The results for the whole dataset are further compared with the observation in the Manchester urban environment. As figure5.3-5 B) shows, the BC transported to the Holme Moss site has been significantly diluted and mixed compared to the measurements in the close vicinity of high-traffic Manchester urban area. The mixing efficiency of BC was 18±8% at 10:00-14:00 compared to 5±3% in Manchester city centre. The extent of BC mixing was steadily enhanced throughout the day, although some additional localized sources in the locality from solid fuel burning contributed in the early evening. The mixing efficiency was 27±6% for BC peak mass loading in the late afternoon higher than that during the noon peak (18±8%). This can be partially explained as the initial mixing state of BC from biofuel combustion is elevated near source, a finding that has been previously observed by Schwarz et al. [2008b]. At mid-night, BC appeared to have a higher ME and so had larger coatings, which corresponded to the most insensitively aged aerosols at that time (figure5.3-5 B)). 201
202 A B Figure A) The diurnal variation of BC mixing efficiency. B) A comparison of BC mass loading related to mixing efficiency for the whole dataset sampled from Holme Moss site and Manchester city centre. 202
203 The correlation between BC and HOA during these two peaks in BC is analyzed in figure HOA/BC is 1.54±0.04 when the source is primarily from vehicle emission at 10:00-14:00, which agrees with the results (1.41±0.22) reported by Zhang et al. [2005] from an urban area. The HOA/BC ratio is more widely distributed when influenced by combined sources at 18:00-22:00. Both the mass loadings of BC and HOA have been elevated compared to the source of vehicle emission, but the increase of mean BC mass loading is greater than that of HOA, leading to the average HOA/BC ratio being reduced to 1.11±0.02. The variation of HOA/BC ratio may result from the different characteristics of sources, for example, the aerosol products from burning of solid fuel contain a substantial amount of OM, a significant faction of which is of oxygenated characteristics [Weimer et al. 2008, Alfarra et al. 2007]. The observation presented in this Chapter indicates that for the solid fuel, the same amount of BC mass tends to contribute less HOA than from vehicle emission. 203
204 Figure The correlation between the mass of BC and HOA when the sources are classified as a function of time: A) 10:00-14:00; B) 18:00-22:
205 Relation to atmospheric processes BC particles can be mixed with primary organic materials soon after emission [Johnson et al. 2005]. During transport, volatile or semi-volatile components will condense on the fresh BC particles [Jacobson 2006], resulting in increased internal mixing between the BC and the other secondary materials. The strength of the mixing process of BC depends highly on the amount of condensable non-refractory components compared to the amount of BC. Thus the non-refractory/bc mass ratio indicates the relative abundance of the possible material that can mix with BC. Figure5.3-7 A) shows the diurnal trends of inorganic/bc and organic/bc mass ratio. The mass ratios are positively correlated to the BC mixing efficiency during the entire day apart from the period when the source was additionally contributed by the localized burning of solid fuel (18:00-21:00). The higher mixing efficiency compared to the mass ratios during 18:00-21:00 tends to result from the initial strong mixing state of BC from the afternoon source, when the BC was likely to be mixed with primarily formed or other co-emitted materials and the coating components only corresponded to a small amount of mass, which cannot raise the mass ratio largely enough to be indicated in this plot. At mid-night, the BC was mainly coated by secondary formed species. The formation of OOA is governed by the condensation process of the SOA or inorganic species onto the POA [Zhang et al. 2007], and the BC particles can also be involved in this process, mixing with secondary organic species. Figure5.3-7 B) shows the relationship between HOA/OM ratio and BC mixing efficiency for the whole data set: the higher BC mixing efficiency was linked to a lower HOA/OM. Given the HOA is mainly composed of POA, the decreasing dominance of HOA within the total mass of OM indicates an enhanced formation of SOA, during which the BC component can be mixed by the condensation of volatile or semi-volatile SOA. 205
206 A B Figure A) Diurnal variations in non-refractory/bc mass ratio, the mixing efficiency is also indicated in this plot as cross markers. B) the relationship between BC mixing efficiency and HOA/OA mass ratio. 206
207 Modification on hygroscopic properties of aerosols The growth factor (g) of particle size as measured by the HTDMA exhibited two modes, one with less growth (g<1.2) and one with larger growth (g>1.2). The secondary inorganic species were consistently observed to be dominant in larger growth mode [McFiggans et al and references therein]. In this study, the number fraction of particles in the low growth mode showed very similar diurnal variations over the whole particle size range (figure5.3-8 A)). The highest g at mid-night corresponds to the period when particles were more aged and without fresh sources contributing. The fraction of particles in the low growth mode follows the variation for the number fraction of BC particles until 15:00 (figure5.3-8 B)), which is expected because they are hydrophobic [Weingartner et al. 1997]. After 16:00 the fraction of particles in the low growth mode decreased, however at this time the fraction of BC particles were increased by the additional contribution from localized sources, and this is possibly because a considerable fraction of BC was well mixed around 16:00 (figure5.3-8 A)), showing some enhanced hygroscopicity. The CCN activated fraction of aerosols was substantially enhanced when supersaturation (SS) is above 0.37% (figure5.3-8 B)). In general, the particles with high g will exhibit more efficient CCN activation. 207
208 A B Figure The diurnal variations of: A) the number fraction for particles with low growth factor (<1.2) in the different sizes, as measured by HTDMA system; B) CCN activation fraction as a function of super-saturation, as measured by DMT CCNc. 208
209 A B C D E 209
210 Figure The influences on the CCN activation: A) total BC number fraction; B) thinly-coated BC number fraction; C) total OA mass fraction; D) HOA mass fraction; E) the mixing efficiency of BC. Given secondary inorganic species, such as ammonium nitrate and sulfate, are dominantly hydrophilic and act as efficient CCN, the fraction of BC and OM with less hygroscopicity will therefore significantly determine the hygroscopic properties for the overall population of aerosols, i.e. a larger fraction of BC/OM is expected to reduce the overall hygroscopicity. As figure5.3-9 A) and C) show, the higher CCN activity only occurs when BC/OM fraction is relatively low. However the BC/OM fraction and CCN activation are not necessarily negatively correlated for the whole dataset as expected, which means the total BC/OM fraction is not able to constrain the hygroscopicity and some of the BC/OM may act as CCN. If considering only the thinly-coated BC and primary HOA fraction instead of the total BC/OM fraction, the CCN activation will be more efficiently constrained, as figure5.3-9 B) and D) show. These comparisons indicate the BC(thinly coated)/hoa is less likely to be activated, and on the opposite suggest that some of the thickly coated BC or OOA had been activated. Furthermore, the mixing state of BC can represent a background implying on the ageing time of aerosols, where the atmospheric processes eventually allow BC to be more internally mixed and OM to be oxygenated, enlarging the particle size and converting their compositions from hydrophobic to hydrophilic, the overall CCN activity thus being enhanced (Figure5.3-9 E)). 210
211 5.4 Summary of Chapter 5 Holme Moss is downwind of the Greater Manchester conurbation in southwesterly wind conditions. An additional source of particulate arose from the home heating in the winter season. These sources contain a substantial amount of primary aerosols, such as BC, which serves as a tracer of pollution. The mass loading of BC peaked in the middle of the day between 11:00-13:00 at 150±30ng/m 3, and was further elevated at 18:00-21:00 up to 270±50ng/m 3. The mid-day peak was investigated to originate from urban emission by the analysis of the chemical composition for the hydrocarbon like organic (HOA) fraction of the total organic mass. The peak in mass loading in the late afternoon was observed to have a contribution from localized home heating [Corris 2008], however the meteorological conditions may have also influenced the level of pollutant concentration. The mixing state of BC measured by the SP2 defines thickly coated BC particles as particles whose coating is over 75% of the total mass, and the mixing efficiency (ME) is the fraction of thickly-coated BC. The mixing efficiency of BC was 18±8% at Holme Moss when the mass loading was significantly influenced by urban outflow, compared to the ME of 5±3% for a similar measurement conducted in Manchester city centre. Although the BC was contributed by some fresh sources in the late afternoon, the mixing efficiency of BC increased throughout the day, reaching 27±6% when the mass loading was at the maximum. The BC exhibited a thicker coating when influenced by the source of solid fuel burning. The different characteristics of different sources were also indicated as the HOA/BC ratio, which suggests a more oxygenated organic aerosol for the solid fuel burning source than the vehicle emission. The formation of secondary particles had a significant influence on the mixing state of BC. The mixing efficiency of BC reached a maximum at mid-night when central Manchester and local emissions were reduced, during which a relatively higher fraction of secondary inorganic aerosols were present and the particles were mostly aged. The oxygenation of OM was examined to be positively related to the BC mixing efficiency, 211
212 because the condensation of volatile or semi-volatile SOA is the main process for the formation of OOA, also coats the BC cores. The mixing state of BC has important influences on its hygroscopicity and CCN activation [Henson 2007]. This study shows that CCN activation fraction of aerosols is more substantially decreased by the increased fraction of thinly-coated BC compared to an increase of the total BC mass fraction. A similar comparison between the total OM and HOA fraction demonstrates that HOA tends to be more weakly activated. These comparisons indicate the coating or oxygenation process has allowed the BC or OA to exhibit some hygroscopicity. Given the secondary inorganic aerosols are efficient CCN, the fraction of primary BC and HOA in turn significantly constrain the CCN activation fraction for the overall aerosol population at this site. 212
213 Chapter 6 The scavenging of BC and non-absorbing particles by water droplet of orographic cloud 213
214 Overview An orographic cloud event lasting about 9 hours was observed at a field site on the Pennine hills of north-west England, Holme Moss, at 525m above sea level. During this event cloud droplets were sampled using a counterflow virtual impactor (CVI), by which the water content of cloud particles was evaporated, and the non-scavenged, interstitial aerosols were also collected by another inlet through an impactor which rejected particles larger than 5µm. Simultaneous comparisons between the cloud residues and particles in the interstitial phase were achieved by introducing measurements downstream of a valve switching between both inlets. Black carbon (BC) and BC-free non-absorbing particles in the accumulation size mode were characterized by a single particle soot photometer (SP2, Droplet Measurement Technologies, Boulder, USA), and Condensation Nuclei (CN) were counted by a condensation particle counter (CPC). Above the diameter of 200nm, BC had a lower scavenging efficiency (0.21±0.11 in mass; 0.18±0.09 in number) compared to the non-absorbing particles (0.72±0.18 in volume; 0.68±0.21 in number). The particles in the accumulation mode were more efficiently activated compared to the total CN, reaching 50% at the diameter of 285±10nm, above which the scavenged fraction was dramatically enhanced owing to increased size. The scavenging activity of BC was observed to be highly related to the mixing state, the fraction of thickly-coated BC in the residues was 60±10% compared to a less fraction of 15±6% in the interstitial phase. 214
215 6.1 Introduction Aerosols have the capacity to influence cloud properties through their role as cloud condensation nuclei (CCN) [McFiggans et al. 2006]. Indirect forcing of the climate system caused by such aerosol-cloud interaction is still subjected to large uncertainty [Lohmann and Feichter 2005]. Particles, such as sulfate, which only scatter radiation can extend the lifetime of cloud by decreasing the droplet size [Twomey 1974] hereinafter increasing the cloud reflectance. However, an ubiquitous product from incomplete combustion, black carbon aerosol (BC), can act in a different way because of its strong light absorption, which in certain conditions, can reduce cloud cover [e.g. Ackerman et al. 2000] or suppress the formation of cloud droplet [Conant et al. 2002], counteracting indirect forcing effects. In addition, the absorption of BC can be enhanced if incorporated into clouds [Jacobson 2006] through lensing effects. An adequate treatment of BC-cloud interaction in global climate models is therefore extremely important. About 98% of the BC in the atmosphere is removed by wet deposition on the global scale [Jacobson 2004]. However, little field evidence of the scavenging of BC currently exists [Myhre et al. 2009]. Mixing of BC with other species in single particles introduces complexity when studying its atmospheric lifetime. Most global models estimate the time scale for the transformation of BC from being initially hydrophobic [Weingartner et al. 1997] to being hygroscopic using an e-folding time that assumes a simplified condensation coating process [e.g. Park et al. 2005, Koch et al. 1999]. Previous studies of BC scavenging by cloud droplets have mainly relied on a comparison between the measured aerosol concentration in the collected cloud liquid and that in the interstitial line [Hitzenberger et al. 2000, 2001; Hallberg et al. 1992, 1994], however, the quantification of BC mass in bulk is influenced by potential artifacts of the instrumentation [Schmid et al. 2001] and the mixing state of BC is unknown. The potential importance of BC mixing state on influencing its scavenging efficiency has mostly been investigated experimentally. For example, Dusek et al. [2006] showed the CCN activation of BC is enhanced when BC is coated by NaCl; Petzold et al. [2005] 215
216 studied combustion emissions and showed that the coating of sulfuric acid increased CCN activation however organic coatings reduced the activation to some extent. The modification of the chemical composition of the surface through processes such as oxidation, can also allow an enhancement of water uptake for BC, as laboratory studies by Zuberi et al. [2005] have shown. Field evidence for the scavenging of BC as a function of its mixing state is sparse. Sellegri et al. [2003] observed a higher scavenged fraction (ε) of BC mass compared to organic species, leading the authors to hypothesize that the higher ε of BC may result from the internal mixing with other water-soluble species or the surface of BC had been modified. A volatility tandem differential mobility analyzer (VTDMA) has been used to size the particles before and after the nonrefractory compositions are vaporized, and by assuming the refractory component is principally composed of BC, the amount of volatile components associated with the refractory core can be quantified [Clarke et al. 2004]. A larger size shift after the particles have been vaporized potentially indicates a thicker coating around the BC core. Kuwata et al. [2007] observed a dramatic enhancement of CCN activity if the size shift was over 10nm. However, the current studies are still too limited to acquire an adequate knowledge on the mixing state of BC acting on its CCN activity. In this study, aerosols are characterized on a single-particle basis during an orographic cloud event lasting about 9 hours during the field experiment at Holme Moss (section5.2.1). Chapter 5 has investigated the sources of Holme Moss that are influenced by urban outflow and localized emissions, which contain a substantial amount of BC. Simultaneous measurements on a range of properties of cloud droplet residuals and interstitial, unactivated particles were made to derive scavenging efficiency. It is the first time that the mixing state of BC has been directly measured and can be related to its scavenging by cloud droplets. 216
217 6.2 Sampling inlets and instrumentations Holme Moss is located in the rural southern Pennines of Northern England (53.53 N, 1.86 W), approximately 30 km to the northeast of the city of Manchester (figure5.2-1). During the experimental period (07/11/ /12/2006), the experimental platform built at this site (525m a.s.l.) was frequently contacted by the orographic clouds. The site received pollutants from both the Greater Manchester urban outflow and local emissions, providing a combination of sources of BC with relatively young age (Chapter 5). A cloud event lasting about 9 hours provided sufficient time to examine the extent to which activation of aerosols by clouds at this site. To segregate the aerosols between those activated into cloud droplets and those remaining in the interstitial phase, two separate inlets were used as figure5.2-2 schematically shows. One inlet had a collection efficiency of over 95% for particles between 0.01 and 1 µm, as previously described by Sheridan et al. [2001]. This inlet was installed with an impactor with 5µm cut-off size to remove large particles and was used to sample interstitial aerosol particles. The other inlet was connected to a counterflow virtual impactor (CVI). This inlet sampled water droplets of diameters larger than 4-5µm while particles smaller than this size are excluded by the CVI counter flow; the sampled droplets are then evaporated in dry air to deliver cloud residues at temperatures lower than 15ºC to limit the volatilization of aerosol components [Ogren et al., 1985]. Given all particles larger than 5µm were assumed to be cloud droplets and all sub-micron particles were in the interstitial reservoir, a simultaneous comparison between the CCN activated and non-activated aerosols was achieved via introducing measurements downstream of a value switching between both inlets with a time interval of 10 minutes. The measurements of aerosols from both inlets were made at low RH to avoid the interference of water vapour. A Forward Scattering Spectrometer Probe (FSSP-100; modified Model SPP100) was employed to measure the size distribution of cloud hydrometeors with diameters 217
218 between 2 and 47µm. At the downstream of the switching valve, the condensation nuclei (CN) were measured using a Condensation Particle Counter (CPC, TSI model 3776, Shoreview, MN, USA) that can detect particle number concentrations from particles greater than 2.5 nm. The instrument calibration, data analysis methodology and uncertainty estimation for the SP2 instrument to quantify the BC physical properties have been illustrated in section The scattered light intensity is used to optically size the particles in the accumulation size mode above about 220nm in this study. The BC mass is quantified by the incandescence signal with over 95% collection efficiency above 200nm. The mass equivalent diameter (D ME ) is derived from the mass of BC core by assuming a density of 1.9g/cm 3 [Bond and Bergstrom 2006]. The mixing state of BC can also be quantified by utilizing the time delay between the scattering signal and the incandescence signal arising because of the evaporation of the coating on a BC core, and this parameter is correlated to the refractory mass fraction (MF) in the total mass of a single particle. A longer coating evaporation time generally indicates an increased coating thickness and a lower refractory mass fraction in a BC-containing particle, by which the BC particles are classified as thinly-coated and thickly-coated (figure5.2-3). In this study a non-absorbing particle is defined as a particle identified by its scattering signal that does not contain a measurable incandescence signal, and so can be differentiated from BC particle. 218
219 6.3 Data analysis The scavenged fraction of aerosol property The scavenged fraction (ε) of an aerosol property by cloud droplets is calculated as a fraction of the amount of that property found from the CVI residues (C CVI ) compared to the total amount of that property in both the aerosol and activated phase (C interstitial +C CVI ). α C ε = α C + C CVI CVI int erstitial The interstitial inlet has a collection efficiency of over 95%, whereas the windy conditions present at Holme Moss (wind speeds typically > 10m/s) reduced the CVI sampling efficiency. Based on wind tunnel studies by Noone et al. [1992], sampling efficiencies of CVI for winds > 10m/s are 45% for 8µm droplets and decrease significantly for larger drops, e.g. ~20% sampling efficiency for 10µm droplets at this wind speed. The mean droplet diameter of this cloud is around 8µm (figure6.3-2), thus a scaling factor (α) of 1/0.45 is applied to correct the sampling efficiency of CVI Modelling on BC activation as influenced by coating contents The mixing state of BC could greatly influence its nucleation activation. An extended Köhler equation aims to investigate the coating abundance relative to the BC core on influencing the critical supersaturation (SSc) of BC activation. Several assumptions are made to simplify the model: the coating composition is ammonium sulfate, which is water soluble, whereas the BC core is water insoluble, and both species are internally mixed. The calculated results are shown in figure The MF is defined as the mass fraction of BC core within single particle. The MF from 0 to 1 indicates a transition of composition from pure ammonium sulfate to pure BC in a specified particle size. With increased fraction of insoluble BC core, the activity of soluble molecules is weakened, 219
220 resulting in an increased SSc for the particle to be activated. At the same MF, the SSc is higher for smaller particles because the enhanced Kelvin effect increases the equilibrium vapour pressure of water due to the surface tension of the droplet. Figure1 shows a dramatically elevated SSc when the MF is above , and for a variety of sizes of BC core when MF<0.3, the SSc is below 0.2%. The modelled results are further compared with the field observation that will be discussed in the following. Figure Critical supersaturation for ammonium sulfate coated BC particles as a function of the refractory mass fraction in single particles (MF). A range of sizes of particles are shown, the MF from 0 to 1 denotes a pure ammonium sulfate particle to an uncoated BC particle at a specified size. 220
221 Figure Time series of liquid water content, number concentration and mean diameter of cloud droplets measured by a Forward Scattering Spectrometer Probe (FSSP-100). The blue bars indicate the time when cloud residues were sampled downstream of the CVI inlet. 221
222 6.4 Results and discussions The observed orographic cloud event lasted from 13:30 to 20:40 in 22 Nov./2006, whose microphysics was quantified by FSSP and is shown in figure6.3-2 and summarised in table1. The number concentration of cloud droplets (2-47µm) has little variation throughout the experiment at 360±195cm -3, while the mean droplet diameter and the liquid water content steadily increase until the extinction of the cloud event. The cloud particles scavenged a fraction of the background aerosols by nucleation or impaction processes as a result of the aerosol loadings measured downstream of the CVI inlet, repartitioning the aerosols between the cloud reservoir and in the interstitial phase. Figure6.4-1 shows the time evolution of aerosol properties during the cloud event, with the blue background colour marking the time periods when sampling the cloud residues (see table1 for the specific values). The aerosol loadings in the background, as indicated by CN, BC and BC-free non-absorbing particles, are steadily increased, reaching maximum in the early night. The pollution source is influenced by the outflow of the Greater Manchester conurbation, and the elevated particle concentration from late afternoon is largely contributed by the localized home heating as investigated in Chapter 5, thus the background aerosols are relatively fresh. It is shown that the aerosols exhibit distinct properties when partitioned by cloud droplets, e.g. the concentrations of CN and BC are lower in the cloud residues compared to those in the interstitial, whereas the scavenged non-absorbing accumulation mode particles have a higher abundance than the interstitial ones. About 3% of the CN are activated but most remain in the interstitial phase, whereas over 50% of the non-absorbing particles in the accumulation mode (>220nm) are observed as cloud residues. This indicates a higher scavenged fraction (ε) of aerosols in the accumulation mode than in the Aitken mode. A lot of previous studies support this observation: as McFiggans et al. [2006] reviewed, the accumulation mode particles are generally more hygroscopic than Aitken mode particles, and the minimum size of particles activated to form cloud droplets is often near the border between these two particle modes. A further analysis on the size dependence of ε in the accumulation mode 222
223 is shown in figure6.4-2 A) and B), indicating an almost identical ε calculated on both volume and number basis during the experimental period. A dramatic enhancement of ε occurs in the size range of nm, reaching 50% at the diameter of 285±10nm, and it is likely that the particles in the Aitken mode were not scavenged as efficiently as the particles in the accumulation mode, leading to a higher loading of CN in the interstitial phase compared to that in the cloud residues. The decreasing trend of ε observed for the larger particles, which is inconsistent with the previous observations [e.g. Sellegri et al. 2003], may result from the reduced CVI sampling efficiency for the larger cloud droplets. Given larger aerosols tend to be activated into larger droplets [McFiggans et al. 2006], the residues for the uncollected cloud droplets may not have been detected downstream of the CVI inlet. 223
224 Figure From bottom to top: time series of BC mass loading (BC number concentration shown in grey on the right), nonabsorbing particle volume (non-absorbing particle number concentration shown in grey on the right), number concentration of CN and BC number fraction. Blue bars indicate the sampling of cloud residues. 224
225 A B 225
226 C D Figure The size distributions for: A) the volume of non-absorbing particles; B) the number of non-absorbing particles; C) mass of BC core and D) the number of BC core in the interstitial phase and cloud residues. The upper plots are the respective size-segregated scavenged fractions. 226
227 Within the similar detectable size ranges between BC and other BC-free non-absorbing particles, the BC has a lower ε (0.21±0.11) compared to the non-absorbing particles in the accumulation mode (0.72±0.18). A higher fraction of BC thus exits in the interstitial phase rather than in the cloud residues (figure6.4-1 top). There is little field evidence that directly compares the scavenging of BC and non-bc particles [e.g. Gieray et al. 1997] though BC has been shown to be hydrophobic with a poor CCN activity [e.g. Hallberg et al. 1992, Weingartner et al. 1997]. BC at Holme Moss is relatively fresh, as the source is from the urban outflow of Greater Manchester and in the afternoon was largely composed of aerosols from localized burning of solid fuel (Chapter 5). Therefore it is not surprising to observe a lower ε for freshly emitted BC. The nonabsorbing particles in the accumulation size mode contain a substantial amount of oxygenated organic aerosol (OOA) and sulfate at Holme Moss during the experimental period, and the aerosol population with low CCN activation is mainly controlled by the primarily formed hydrocarbon-like organic aerosols (HOA) and the thinly-coated BC (Chapter 5). The size distributions of the BC cores in both activated and interstitial particles are shown in figure6.4-2 C) and D): the mass and number scavenged fraction show almost identical trends. The ε for small BC cores is generally lower, and a fraction of ε for larger cores is steadily enhanced. The elevated ε when BC core size is above 450nm could partly be explained as the reduced surface tension of larger particles to serve as more efficient CCN, however it may also result from the inertial scavenging of BC by cloud droplets. When more than one BC particle were in contact with the surface of a cloud droplet, after the droplet was evaporated by CVI, the aerosols on the surface or serving as a nuclei will coagulate into one lager particle measured in the form of residues. The importance of inertial scavenging for BC is also suggested by Baumgardner et al. [2008]. The impaction process with cloud hydrometeors potentially could contribute to the scavenging of BC significantly. 227
228 BC cores can be associated of coatings with varying thicknesses. The coating can enhance the hygroscopicity of BC by both enlarging the particle size and the attached coating species can modify the solubility [e.g. Dusek et al. 2006]. The mixing of BC has been considered to be a main process that increases its hygroscopicity hereafter being removed by wet deposition [Jacobson 2004]. The probability distribution for the mixing state of BC, as directly examined by the coating evaporation time (T) from the SP2, is shown in figure6.4-3 A). The particles are distributed into two distinct groups with a critical value of T of around 5µs. In interstitial particles, over 80% of the BC particles are present with a shorter T (<5µs), particles that are considered to be thinly-coated. There a much fewer thinly-coated BC particles in the cloud residues, where the fraction of thickly-coated BC (T>5µs) is as much as 68%. This observation clearly demonstrates that the BC particles that have been scavenged by cloud droplets are more significantly coated rather than the BC remaining in the interstitial phase. 228
229 A B Figure A) The frequency distributions of coating evaporation time for interstitial aerosols and cloud residues. B) The BC scavenged fraction as a function of the refractory mass fraction of single BC particles (MF). 229
230 To avoid the sphericity assumptions made when calculating a coating thickness, the refractory mass fraction in single BC particles (MF) is used to characterize the coating abundance. Figure6.4-3 B) shows a step enhancement of ε when MF is lower than 0.3, which means that a coating mass fraction of over 70% significantly increases the BC scavenging efficiency. This result generally agrees with the modelling results in figure6.3-1, which shows a largely enhanced supersaturation for BC to be activated when MF is above 0.3. Some other studies also indirectly support this observation, for example, Sellegri et al. [2003] measured a dramatic enhancement of BC scavenging when the particle size was over 300nm, which probably resulted from the size enlargement owing to the coating; Kuwata et al. [2007] observed a step change of the CCN activation of BC when the volatile component mixed with BC has caused the diameter enlargement over 10nm; a modelling study by Henson [2007] also suggests the coating can largely allow the BC to be activated under a much lower supersaturation condition. However, the coating content as observed in the cloud residues may not represent the initial mixing state of BC before the onset of scavenging process, because the cloud processing may lead to additional condensable material once cloud has formed. After it is dried by the CVI such BC particles would then have a thick coating. BC particle could impact on an activated non-absorbing particle to form a cloud droplet, which can also be observed to be thickly coated after the water content is evaporated. These processes introduce uncertainties in relating the mixing state of BC to its scavenging activity in the real atmosphere. 230
231 6.5 Summary of Chapter 6 The aerosol scavenging by an orographic cloud at a polluted rural site has been investigated. The aerosols in the interstitial phase and cloud residues are simultaneously compared: above the diameter of 200nm, BC has a lower scavenging efficiency (0.21±0.11 in mass; 0.18±0.09 in number) compared to the BC-free non-absorbing particles in the accumulation mode (0.72±0.18 in volume; 0.68±0.21 in number), leading to a higher BC number fraction in the interstitial phase than in the cloud residues. The non-bc particles are preferentially scavenged in the accumulation size mode compared to the Aitken mode, and a dramatic scavenging enhancement occurs at the size nm. The scavenging of BC steadily enhances with increasing size of BC core. The mixing state of BC has great impact on the BC-cloud interaction, the fraction of thicklycoated BC is observed to be 15±6% for the interstitial aerosols but over 60±10% in the cloud residues. The scavenging efficiency of BC has been dramatically enhanced when the fraction of coating mass in a single BC particle is over 70%. The Holme Moss site is representative of a rural background that is downwind of urban outflow, the scavenged fraction of BC observed in this study generally falls within the previous reports that have been conducted in an urban/rural environment ( ) [e.g. Hallberg et al. 1992, 1994]. However field measurements especially from the urban/rural atmosphere are still too sparse to obtain a comprehensive understanding on the scavenging of BC related to various ground sources because of the complex mixing state of BC. A variety of experiments have been conducted where the pollutants were relatively far away from the originating sources [e.g. Sellegri et al. et al. 2003; Hitzenberger et al. 2000, 2001; Cozic et al. 2007] and the measured BC had been well mixed. Unlike the reports when the measurements are close to the sources, the BC particles were observed to have a much higher scavenged fraction in these studies ( ), because atmospheric processing can make the aerosol compositions internally mixed. 231
232 Cloud properties Droplet number concentration (cm -3 ) 360±195 Liquid water content(g/m 3 ) 0.35±0.12 Mean droplet size(µm) 8.2±1.3 Aerosol properties In the interstitial line Cloud residues CN concentration(cm -3 ) 2790±630 73±30 Non-absorbing particles in the accumulation mode ( nm) 0.06±0.04 5±3 0.19± ±7 (µm 3 /cm 3 ) (cm -3 ) BC mass loading (ng/m 3 ) (cm -3 ) 41.3± ± ± ±0.6 BC number fraction 0.38± ±0.04 Non-absorbing particles mean size(nm) 280±30 310±50 Refractory mass fraction of single BC particle 0.40± ±0.18 Scavenged fraction of BC mass number 0.21± ±0.09 Scavenged fraction of nonabsorbing particles 0.72± ±0.21 volume number Table 6-1. Summary of the measured aerosol and cloud properties, expressed as the mean value ± σ. 232
233 Appendix of Chapter 6: BC-containing particle CCN activation formula system The simplified Köhler equation can be expressed simply as: A B S 1 a.1 D 3 p D p Where S is the saturation ratio, S-1 denotes supersaturation ratio, D p is the wet particle size, where 4M wσ s A / ν 6υm sm w = ; B = a.2 RTρ πρ M w w s The subscripts s, w and υ relate to solute, water properties and solute species, respectively. M is molecular mass, ρ is density, and R is the universal gas constant, T is the droplet temperature; σ s/υ is the surface tension of the solution at the composition of the droplet, υ is the number of dissociated ions per solute molecule, m s is the solute mass. The term in A denotes the Kelvin or curvature term, and B is the Raoult or solute term. d( S 1) The analytical solution of S-1 at = 0 is defined as the critical supersaturation, dd p 4A 3 Sc = a.3 27B For internally mixed particles with insoluble content, B 6ευm M πρ M s w = a.4 w s 233
234 ε is the soluble mass fraction of the dry particle. By extension a particle containing an insoluble core by applying a core-shell model to treat particles with BC inclusions, and assuming that the insoluble BC core and the soluble shell part are internally mixed. 8 Sc = 1/ 2 3 / 2 1/ 2 2π M w M s σ s / υ 1 1 = w m 9 ρ s RT ευ msε m m s+ c s a.5 Where m s, m c, m s+c denote the shell mass, core mass, entire dry particle mass respectively. The definition of MF is the BC core mass fraction occupying the entire mass of dry particle. m mc MF s + c =, ms ms c mc = + a.6 By combining the formulas above, Sc = α m c MF ( 1 MF ) a.7 8 Where α = 2π M w 9 ρw( RT) ( σ s 3 / 2 3 / 2 / υ ) M s υ a.8 In the expression of α, σ 3/2 denotes the contribution of solution chemical species, the (M s /υ) 1/2 indicates dissociation activity of soluble molecules. 234
235 Chapter 7 Characterizations of BC at an Alpine experimental site influenced by polluted boundary layer and free troposphere 235
236 Overview Black carbon (BC) mass, size distribution and mixing state in sub-micron aerosols were characterized from late February to March 2007 using a single particle incandescence method at the high alpine research station Jungfraujoch (JFJ), Switzerland (46.33 o N, 7.59 o E, 3580m a.s.l.). JFJ is a ground based location exposed to continental free tropospheric air. The aerosols measured at the site were mostly well mixed and aged during transportation via the free troposphere. Pollutant sources were traced by air mass back trajectories, trace gases and the mass loading of BC. In southeasterly wind directions, katabatic flows provided the potential to vent the convective boundary layer that is polluted by the southern Alpine area and industrial northern Italy, identified by enhanced BC mass loading, condensation nuclei (CN) and CO concentrations. This free tropospheric background site was also significantly influenced by precipitation, which led to the removal of BC from the atmosphere. Overall, 40±15% of the observed BC particles were mixed with significant amounts of non-refractory materials present as a thick coating around the BC core. However, the presence of coating components showed slight dependency on the localized pollutants and precipitation removal. The growth of particle size into the accumulation mode was positively linked with the degree of BC mixing, suggesting the important role of condensable materials in increasing particle size as well as enhancing BC mixing state. It is the first time that BC mass, size distribution and mixing state are reported in the free troposphere over Europe. These ground based measurements also provide the first temporal study of BC in the European free troposphere by single particle methods. At the present time there is only limited information of BC and its mixing state in the free troposphere, especially above Europe. The results reported in this study provide an important constraint on modelled representation of BC over Europe. 236
237 7.1 Site description and meteorological conditions To address questions related to the size distribution and mixing state of BC in submicron aerosols, a study was conducted at the Sphinx Laboratory of the Jungfraujoch high-alpine research station (46.33 o N, 7.59 o E, 3580m a.s.l.), Switzerland. The research station is located at the northern side of the main central European Alpine area, and provides an ideal site to study aerosols which have been transported to the free troposphere from a large range of sources across Europe. The site also receives pollution from local valleys during the summer months. The transported particles contain considerable amounts of secondary material, as well as processed and/or relatively fresher primary particles. BC particles in the accumulation mode were characterized via a single particle soot photometer, which also measured the optical size of both black carbon and non absorbing particles. The instrument is highly sensitive to low concentration of aerosols, providing a clear segregation of BC from other types of aerosols, in addition to the derived information regarding the mixing state of BC on a single particle basis. This Chapter 5nvestigates the behaviour of BC at this site in a late winter season, considers the particle lifetime, and describes how removal processes relate to the size distribution of BC and its mixing state. It was the first time that the behaviours of BC have been investigated on a particle by particle basis in the lower free troposphere over Europe. The data presented in this study was sampled during an experiment within the CLoud and Aerosol Characterization Experiments (CLACE) programme conducted at the Jungfraujoch (JFJ) research station in the winter of This measurement site is surrounded by glaciers and rocks, and no local vegetation is present. The location and altitude make this site far remote from significant pollution sources and the local emissions from the station and the tourist facilities are negligible since all heating is electrical [Baltensperger et al., 1997]. During summer, the local meteorology and topography exert a predominant influence on the JFJ. The site is influenced by a combination of valley-mountain and mountain up- 237
238 slope circulations, giving a clear diurnal cycle of aerosol concentrations as a result of the injection of planetary boundary layer air into the free troposphere air during the afternoon. On occasion, the JFJ site can also be a receptor of pollutants from the relatively closer sources, for example from the Po Valley caused by ascent of air from the convective boundary layer, delivered to the site by katabatic venting of the valleys [Lugauer et al. 2000]. This tends to be more frequent during the summer months but has also been observed in wintertime. During the winter months, the convective processes are much milder less frequent and aerosol concentrations are mostly controlled by regional and long-range tropospheric circulation systems [Baltensperger et al., 1997; Lugauer et al., 1998]. The long-term aerosol measurements from this site have been thoroughly analyzed and reported by Baltensperger et al. [1997] and Collaud Coen et al. [2007]. Continuous aerosol sampling was conducted during CLACE6 from mid February 2007 to mid March For most of this time, the high-alpine site was receiving remote continental/marine air masses from the free troposphere, but under the influence of varying anticyclonic air systems, pollutants from different regions of Europe and Atlantic Ocean (figure7.1-1 A1-A5) were observed. On occasion, this site can also be a receptor of pollutants from the relatively closer sources, for example from the Po Valley caused by ascent of air from the convective boundary layer, delivered to the site by katabatic venting of the valleys [Lugauer et al. 2000]. The dominant local wind horizontal directions measured by sonic anemometer (Metek, manufactured by Lymington, UK) were either north (N) or southeast (SE) (figure B). This wind pattern has been observed in other studies and is due to the JFJ location between the Jungfrau (4158 m) and Mönch (4089 m) mountains, channelling the local flow in a north-western or south-eastern direction. In the northerly wind direction, air from the Swiss plateau is advected to the Jungfraujoch, while in south-easterly winds, the air comes from the southern Alpine area and industrial northern Italy. The satellite images from the MODIS Rapid Response System (available at show the dominant fire burning at southeastern Europe and north Italy during this winter season (figure7.1-2). In addition to this, Alfarra et al. [2007] have shown that many rural 238
239 regions in the Alps rely on wood burning for home heating during the winter and this provides a significant source of particulate in this season. However, the site was subjected to periods of heavy precipitation during the first half of the experiment, resulting in a considerable fraction of aerosols being washed out. Clouds were present at the site for approximately 60% of the experimental period. The phase of the cloud varied from periods when liquid-water clouds were dominant to periods when the clouds were almost entirely composed of ice particles. The aerosols sampled at this site experienced a combination of precipitation and cloud processing. 239
240 A1 A2 A3 A4 A5 B 240
241 Figure The experiment site was influenced by air mass history and local air transportation. A1-A5), back trajectories analysis calculated by NOAA Hybrid Single- Particle Lagrangian Integrated Trajectory model (HYSPLIT; Draxler 2003). Time periods are classified according to the history of the air mass over the previous three days. Back trajectories are indicated at 3400m, 3600m and 3800m above sea level over the Jungfraujoch, and are colored by air pressure. B) Wind rose plot for the whole experiment period, individual wind direction measurements are accumulated and the relative frequency is shown as a percentage. The plot is colored according to the probability of wind speed. A1 A2 A3 A4 241
242 A5 Figure Fire maps provided by the MODIS Rapid Response System (available at corresponding to the time periods of the back trajectories. 7.2 Instrumentation, sampling and data analysis methodology Sampling inlet Aerosol particles were sampled using an inlet system mounted on a platform above the Sphinx Laboratory. This sampling inlet was fitted with a heater (25ºC) at early stage, designed to evaporate cloud particles with an aerodynamic diameter smaller than 40 µm at wind speeds up to 20 m s -1 [Weingartner et al., 1999], with larger particles penetrating the inlet at lower wind speeds, thus both the aerosols incorporated into cloud particles and non-activated interstitial aerosols were sampled. The instruments were located behind the inlet system at laboratory room temperature to sample aerosols under dry conditions (relative humidity <20%). 242
243 Instrumentation and data analysis The physical properties of BC and other BC-free particles in the accumulation size mode were quantified by deploying SP2 measurements. The operation, calibration and data analysis of the SP2 instrument have been in detail described in section The particles are classified as BC or non-bc according to the presence of the incandescence signal. In this study, the non-bc particles can be optically sized within the diameter range of nm. For BC particle, the mixing state can be quantified by a delay time (T) arising from coating evaporation as well as a comparison between the scattering and incandescence signal. Figure7.2-1 A) gives two examples of single BC particles, both with the same size of BC core but with differing abundances of coating components. It can be seen that a thicker coating has increased the scattering signal and T. Figure7.2-1 B) shows a dramatic increase in T when the coating mass fraction is over 73% (MF<0.27). The BC particles within this region are likely to be entirely enclosed by relatively thick coatings, delaying the onset of incandescence. The mixing efficiency (ME) of BC is defined as the fraction of the entire particle number concentration of absorbing particles which have a thick coating (defined as MF<0.27), as Figure7.2-1 B) illustrates. In addition to the characterization by the SP2, a Multi-Angle Absorption Photometer (MAAP, Thermo ESM Andersen) was employed to quantify the aerosol light absorption coefficient σ abs. The MAAP measured the transmission and the back scattering of a light beam at a defined wavelength (λ=630 nm during this experiment) through a fibre filter, where the aerosol is sampled continuously and simultaneously. The light absorption coefficient is obtained from a radiative transfer scheme which corrects for artefacts caused by the interaction of the light with the filter material and off-axis detection at multiple angles is used to correct for the effect of scattering [Petzold and Schönlinner, 2004]. 243
244 A Condensation Particle Counter (CPC, TSI 3010 model) measured the condensation nucleus (CN) concentration of all sub-micron aerosols with a diameter greater than 10nm. Particles that can be optically sized by SP2 are in the diameter range 200nm- 720nm, so a measure of the sub-micron/accumulation mode ratio can be found from the ratio of the particle numbers measured by the CPC and the SP2. The type of precipitation was measured by the VPF-730 present weather sensor (BIRAL, 1 Beach Road West, Bristol, UK). The principal technique used for this instrument is to determine the precipitation type by the measured ratio of the back scatter atmospheric extinction coefficient to the forward scattering atmospheric extinction coefficient. Size and velocity distributions of the precipitation particles complement this primary measurement. The precipitation particles are counted by measuring the amplitude and duration of the light pulse created by each precipitation particle as its fall through the sample volume. Small numbers of particles with distributions not indicative of rain or snow are considered not to be precipitation and are rejected by false alarm algorithms. 244
245 A B Figure A) Two examples of detected BC in single particles, both having the same size of BC core but the left one is thinly coated compared to the thickly coated one on the right. B) the refractory mass fraction related to the coating evaporation time, the upper and right lines show the histograms of x-axis and y-axis values respectively. The definitions of thickly-coated and thinly-coated BC are illustrated. 245
246 7.3 Results and discussions BC mass loading and absorption property There are a significant number of BC particles that are smaller than the lower detection limit of the SP2, which is about 190nm in this study, leading to systematic underestimation of BC mass. To account for this, previous studies have estimated the total BC by extrapolation using a lognormal distribution [Schwarz et al. 2006]. A similar approach is applied to the dataset presented in this study, where about 40-60% mass or 30% number of entire BC population has been detected by the SP2. Figure7.3-1 presents the time evolution of total BC mass loading measured by the SP2 and the absorption coefficient determined by the MAAP during the entire experiment, indicating a general positive correlation between both instruments, as figure7.3-2 A) further reveals. Due to the indirect measurement of BC mass by the MAAP, an empirical converter, which is defined as mass absorption cross section (MAC) [Bond and Bergstrom 2006], is needed to translate the measured absorption coefficient (σ abs ) of bulk aerosols into the estimated BC mass. A variety of uncertainties can be propagated by this empirical conversion, because the MAC of BC is not a constant, rather it is associated with different sources and combustion conditions [Schwarz et al. 2008], and is highly influenced by the size and mixing state of BC [Bond et al. 2006], i.e. the absorbing efficiency can be enhanced as much as 50% by an internal coating. Because of the capacity of the SP2 to directly measure the BC mass concentration, the value of MAC can be derived by comparing the MAAP measured absorption coefficient (MAC=σ abs /Mass BC ). The distribution of the MAC values for the BC particles measured in this Chapter 5s presented in figure7.3-2 B). A median value 10.6m 2 /g of MAC at λ=630 nm is obtained, which is higher than the previously measured results at this site during winter (7.6m 2 /g) [Cozic et al. 2008a], but lower than that in the summer (11.1m 2 /g). This discrepancy largely resulted from the different measurements of BC mass, but can also be affected by different originating sources and varying mixing state of BC, i.e. the median MAC observed in this study falls within the reported MAC from 246
247 biomass burning by Schwarz et al. [2008b], whereas the MAC from urban emission is much lower. 247
248 Figure Time series of total BC mass loading from the SP2 and absorption coefficient measured by the Multi-Angle Absorption Photometer (MAAP) for the entire experiment. The five synoptic periods classified by back trajectory analysis (figure1 A)) are separated by dotted lines. Particularly, the time periods when the dominant horizontal wind direction is southeast (SE) are colored as yellow columns; the dark blue column marks the periods of heavy precipitation (heavy snow, precipitation particle concentration over 200cm -3 ) as recorded by a present weather sensor. 248
249 A B Fiugure A) The correlation between the MAAP measured absorption coefficient and the total BC mass loading measured using the SP2. The width of the box plot bars indicates the bin interval of averaged BC mass, the lower and upper edges of the box are the 25% and 75% percentiles of the absorption coefficient in the bin, the line in the middle of box and the cross markers denote the median and average values, with error bars denoting the 10% and 90% percentiles. B) The probability distribution of derived mass absorption cross section (MAC), with the tag marking the median value. 249
250 However, a large fraction of values in the frequency distribution show unexpected large MAC, exhibiting a broadly distributed tail in figure7.3-2 B). This discrepancy arises from the overestimated absorption coefficient from the filter-based measurements and a possible underestimate of BC mass loading by SP2. The former not only results from the relatively low S/N ratio of the MAAP at low particle concentrations, but also from the absorption enhancement due to the mixing state because the aerosols observed have experienced substantial transportation and intense cloud-precipitation scavenging. An inter-comparison study by Slowik et al. [2007] shows the MAAP measurement of optically-absorbing mass was higher by ~50% than the SP2 during laboratory tests, and this discrepancy could be further enhanced by ~20% when the soot was coated. Furthermore, the presence of light absorbing organic aerosols may also bias conversion between absorption coefficient and BC mass [Lack et al. 2008, Subramanian et al. 2007]. On the other hand, the extrapolation of the SP2 derived BC mass to determine the total BC mass may lead to an underestimation if a significant mass fraction of BC is present above the extrapolated lognormal distribution [Johnson et al. 2005]. The smaller particles display more efficient absorption [Bond and Bergstrom 2006], and such particles are not directly measurable by the SP2 but will be measured by the bulk-based absorption measurement. Figure7.3-2 B) thus provides a reference MAC value to translate the measured absorption coefficient to the BC mass at this site, and gives an estimate of the uncertainty for this conversion. 250
251 Attributions of pollutant sources by air mass history and local wind The entire experimental period was classified into five phases on the basis of three day back trajectory analysis, as figure7.1-1 A) shows, three arrival altitudes (3400m, 3600m and 3800m) were chosen for each trajectory calculation which were run every 6 hours using the NOAA Hybrid Single-Particle Lagrangian Integrated Trajectory model (HYSPLIT; Draxler 2003). Figure7.1-1 A) shows the Jungfraujoch (JFJ) sampling site was dominated by descending air for about 80% time of the experiment. The trajectories show that during each period, the air masses were transported along broadly consistent pathways. The back trajectories are named according to the directions of originating flows corresponding to each period in figure7.1-1 A). The classified periods are also identified in figure7.3-3, combined with the coloured columns to indicate the precipitation rate and wind direction shift. 251
252 A B 252
253 Figure A) Time series of aerosol properties during the entire experiment with the classified periods separated by dotted lines, from bottom to top: numerical ratio of submicron/accumulation mode particles as measured by the SP2; BC mixing efficiency; CN concentration measured by the CPC; BC number fraction within the population of submicron aerosols. B) Time series of trace gases, from bottom to top: NO x volume mixing ratio (ppbv); CO volume mixing ratio (ppbv); CO/BC (ppbv*m 3 /ng). The first two periods from 26/02-04/03 were relatively clean, and this is reflected by low concentrations of CN and absorbing BC (figure7.3-1, figure7.3-3 A) and table7.3-1). The air masses originated for the most part at altitude over the North Atlantic Ocean, with rapid transport across north Western Europe and descent to the Jungfraujoch. There is evidence of frontal passage early in this period. As MODIS satellite fire maps show (figure7.1-2), the transporting air mass over the previous three days hadn t passed over significant fire sources. Furthermore, the dominant local horizontal wind direction was northerly (N), and no significant fire sources were detected by MODIS to the north of sampling site during this phase, although home fire sources in local valleys may well be a source of BC. A sharp enhancement of BC concentration was observed as soon as the local wind changed to a southeasterly (SE) direction despite little change in the air mass history. These pollutants are consistent with the known regional sources in the densely populated and highly industrialized Po Valley, north Italy. As Seibert et al. [1998] revealed, there can be significant advection of the pollutants in the Po Valley to the northwest high alpine region. In addition, it should be also noted that during this period, the site received continuous heavy snow fall (figure7.3-1), when a large proportion of aerosols had been washed out by precipitation particles. From 05/03, the aerosol concentrations were elevated significantly. MODIS fire maps show that the prevailing fire burning existed in southeastern Europe and north Italy from in the period leading up to the 05/03 and declined thereafter. In the days proceeding 05/03 the air masses had passed over Spain and southeastern France prior to arrival at the Jungfraujoch. The pollutants were rarely removed by wet deposition during this phase, as figure7.3-1 demonstrates, during which BC mass loading reached the 253
254 maximum during the experiment. The consistent finding was the BC mass increase always coincided with SE local wind, and the mass loading was low when the local wind was from the N bringing air to the site from the high Alps. The measurements conducted during the 07/03 provided evidence that this site can also be a recipient of localized pollutants, as shown by figure7.3-3 B), both NO x and CO were elevated significantly, with NO x increasing by up to five times its mean value. Figure7.3-3 A) shows that during this period the observed BC was poorly mixed (low BC mixing efficiency) and large number of Aitken mode particles (high sub-micron/accumulation mode ratio) were present. The back trajectory analysis on 07/03 revealed the air mass that was ascending during advection. The presence of NO x suggested that the air mass was relatively fresh and the emissions were most likely to have occurred in the past day. For example, a fresh plume was clearly observed around 12:00 07/03 with low BC mixing efficiency, associated with a significant increase in the concentration of NO x and CO, and high loadings of smaller particles. The synoptic flow brought air to the site from the north during 09/03-11/03, with the flow mainly from mid-west Germany and above the Swiss plateau. The fire sources during this phase were much reduced and geographically much further east. It was therefore unlikely to influence the pollution at this site. SE local winds were rarely observed during this period. A few periods of enhanced BC were detected, but the overall contribution of BC was small. However, during this period a substantial number of sub-micron particles were observed, resulting in a decreased BC number fraction (figure7.3-3 A)). The sporadic peaks of BC loading coincided with pulses of NO x (figure7.3-3 B)). These periods were also associated with low BC mixing efficiency and enhanced Aitken mode particles, which implied more localized pollutants arising from sources on the Swiss plateau. The air mass history during the last period (12/03-13/03) essentially contrasted with the previous days, when the air was transported over southern-eastern Europe, some fires were detected by MODIS in this region at this time, though they were not intensive (figure7.1-2). The strong easterly flow persistently brought pollutants from eastern 254
255 Europe. The BC in this phase showed a high degree of internal mixing, NO x levels were low and only small proportion of submicron particles were observed to be in the Aitken mode, all of which indicated these BC had experienced a long distance transportation. 255
256 Periods classified by back Period I Period II Period III Period IV Period V trajectories Date 26/02-27/02 28/02-04/03 05/03-08/03 09/03-11/03 12/03-13/03 Meteorological conditions Relative humidity 96.7± ± ± ± ±22.8 (RH) Ambient temperature(ºc) -13.8± ± ± ± ±1.5 Precipitation type Heavy snow Heavy snow Weak snow Heavy snow+ medium snow Weak snow Local horizontal wind direction SE+N N SE N SE Local horizontal wind speed(m/s) 5.6± ± ± ± ±4.1 Aerosol based properties (median value) BC mass loading(ng/m 3 ) 3.48± ± ± ± ±20.49 BC number fraction 0.20± ± ± ± ±0.90 BC mixing efficiency 0.37± ± ± ± ±0.09 Sub-micron particles(cm -3 ) 300± ± ± ± ±581 Submicron/Accumulation mode ratio 2045± ± ± ± ±387 CO/BC(ppbv*m 3 /ng) 82.3±62.1(64.6) 69.4±65.8(42.2) 11.7±12.2(9.33) 44.0±46.8(27.5) 12.7±20.6(6.76) Table A summary of meteorological conditions and aerosol properties categorized by five periods classified by back trajectory analysis (figure1 A)). The mean and standard deviations are shown for each parameter and the number in brackets is median value. 256
257 The free tropospheric background under the influences of ground sources and wet removal The air mass history along with the aerosol and trace gas measurements indicates a typical free tropospheric background at this site for most of the experimental periods. The free troposphere background is defined as the periods when weak precipitation, without the influences of SE wind, NO x -CO concentrations were not enhanced, BC mass loadings showed little variation. The measured results at free tropospheric background as defined here are statistically analyzed and presented in the first row of figure However, pollutants in the background air can be influenced by valley sources as the SE winds can vent anthropogenic pollutants from the southern Alpine area and industrial northern Italy, defined as the periods influenced by SE wind. During transport, aerosols are processed, being scavenged, and subsequently removed by wet deposition in the form of precipitation, referring to the periods of influenced by precipitation. A significant fraction of aerosols may have been incorporated into clouds that have not precipitated, given these aerosols were also sampled by evaporating the cloud particles (section 7.2.1), the scavenging effect of aerosols by clouds was not indicated by the dataset presented in this study. The SE wind direction and precipitation rate therefore govern any perturbation to the tropospheric background at this site. These conditions have been identified and the frequency distributions of a range of aerosols and gas phase parameters under these situations are shown in figure Under the influence of SE wind, enhanced BC mass loadings were frequently observed to perturb the background (figure7.3-4 column A) by the order of 10±5ng/m 3 to 28±14ng/m 3. The BC mass in the tropospheric background is comparable with the value reported by Schwarz et al. [2006] in the Northern mid-latitude troposphere. Under SE local wind conditions, substantial amount of pollutants were from more localized pollution sources, like industrial North Italy. The BC could also originate from biomass burning as indicated by MODIS fire maps. First evidence on the importance of vertical transport for the Jungfraujoch aerosol was observed by Baltensperger et al. [1991], and has also been identified by Lugauer et al. [2000]. The precipitation had a significant 257
258 impact on the removal of BC, reducing the average BC mass loading to 4±2ng/m 3 when precipitation particles were over 200cm -3. On average, about 65% of BC mass was removed by precipitation compared with the tropospheric background when free of perturbations (figure7.3-4 column A, also see figure7.3-8 B)). 258
259 A B C Free tropospheric background Influenced by SE wind Influenced by precipitation 259
260 D E F Figure Frequency distributions of aerosol and trace gas properties under free tropospheric background conditions (top row), under the influence of SE winds (middle row), and when influenced by precipitation (bottom row): column A) BC mass loading; column B) CO concentration; C) CO/BC ratio; D) volume of non-bc particles ( nm); E) CN concentration; F) number ratio of submicron/accumulation mode. 260
261 BC mass loading(ng/m 3 ) CO(ppbv) CO/BC(ppbv/( ng/m 3 )) Percentiles 10% 25% 50% 75% 90% Mean 10% 25% 50% 75% 90% Mean 10% 25% 50% 75% 90% Mean Free tropospheric background Influenced by SE wind Influenced by precipitation Non-BC volume(µm 3 /cm 3 ) CN(cm -3 ) Sub-micron/Accumulation mode ratio Percentiles 10% 25% 50% 75% 90% Mean 10% 25% 50% 75% 90% Mean 10% 25% 50% 75% 90% Mean Free tropospheric background Influenced by SE wind Influenced by precipitation Table A summary of the data presented in figure7.3-4, including the median value, mean value and 10%, 25%, 75%, 90% percentiles for each property
262 Both CO and BC can act as tracers of primary pollutants, because their sources are similar, though their emission ratio is highly dependent of the sources and combustion conditions [Bond et al. 2004], as well as controlled by meteorological conditions. The tropospheric background maintained a median CO concentration of 150ppbv (figure7.3-4 column B), but was elevated frequently when influenced by SE wind, corresponding to the enhanced BC mass. The loading of CO was not intensively affected by precipitation removal compared to BC. The CO/BC ratio is further investigated in figure7.3-4 column C. The CO and BC is often correlated within ±30% for a given source, e.g. for biomass burning and fossil fuel combustion [Spackman et al. 2008] as they are both a result of incomplete combustion. This strong correlation has also been observed during ground based experiments conducted in urban environments [e.g. Baumgardner et al. 2002, Kondo et al. 2006]. However in the tropospheric background, there is a larger variation in CO for little change in background BC, leading to the CO/BC biased towards larger values, which agrees with the observation by Spackman et al. [2008]. The lifetime of CO in the atmosphere is principally controlled by oxidation via OH and is around 3 months [Holloway et al. 2000], whereas the atmospheric lifetime of BC is in the order of 5-10 days with the sink dominated by wet deposition [Textor et al. 2006]. A strong correlation between CO and BC was observed under the influence of SE winds, indicating that pollution from the relatively localized contributing sources of Alpine valleys did not experience significant wet removal. The loading of non-bc aerosols in the accumulation mode as optically sized by the SP2 ( nm) is shown in figure7.3-4 column D. This shows a significant enhancement of accumulation mode particles in SE wind direction, and about 78% of the total volume was removed by precipitation compared with the tropospheric background. Periods of SE wind and precipitation influenced the CN concentration (down to 10nm) in similar ways (figure7.3-4 column E). Accumulation mode aerosols principally contain secondary species via condensation and coagulation processes [Raes et al. 2000], whereas anthropogenic primary aerosols are produced predominantly as Aitken mode particles [e.g. Kittelson et al. 2004]. Therefore the relative fraction of accumulation 262
263 mode particles can be an indicator of particle ageing time. As figure7.3-4 column F shows, the relatively large fraction of accumulation mode particles were observed in SE wind direction, indicating that the aerosols transported to this site had been well aged. The remaining interstitial aerosols after wet deposition were biased towards smaller sizes compared to the overall particle distribution, suggesting the larger aerosols are preferentially removed by precipitation scavenging [Raes et al. 2000] The size distribution and mixing state of BC The size distributions of BC were of similar shape and had approximately the same modes of diameter in all three conditions (figure7.3-5). The mass distribution of BC was observed to peak at a diameter of nm, in agreement with the observations by Clarke et al. [2004] from the Asian free troposphere. This result suggests the overall wet removal of BC is highly independent of the core size, and the different sizes of BC could have been scavenged following different mechanisms, i.e. nucleation scavenging by cloud particles to form precipitation, or impaction scavenging by dissolving into the precipitation particle or aerosol-hydrometeor coagulation [Jacobson 2004]. 263
264 Figure The mean BC mass size distribution measured under different conditions, the dotted lines show the lognormal fittings on the size distributions to derive the total mass loadings. As discussed above, BC particles that contain an absorbing core with a mass fraction less than 27% are considered to be thickly coated, and the mixing efficiency (ME) indicates the number fraction of thickly coated BC. As figure7.3-6 A) shows, about 40±15% of the BC is thickly coated for the condition of the tropospheric background without significant perturbations, agreeing with data from the tropical troposphere [Schwarz et al. 2008a]. During SE winds, the ME increased to 48±17%, but also contained a considerable fraction of fresher BC with ME lower than The BC with low ME largely correlated to the enhancement of NO x concentration (figure7.3-6 B)), indicating that the lower ME values are associated with more recently formed BC. The BC remaining at the background after precipitation removal exhibited a slight lower ME 264
265 (0.36±0.14), which could be explained by the enhanced scavenging efficiency owing to higher degree of internal mixing. A B Figure A) The frequency distribution of BC mixing efficiency under different conditions as categorized in figure The range of values reported by Schwarz et al. [2008a] from the northern tropic troposphere is indicated. B) The BC mixing efficiency under the influence of SE wind, as categorized by different levels of NO x concentration. 265
266 Figure7.3-7 shows that throughout the experiment, increased numbers of accumulation mode particles are associated with periods when BC is more highly mixed. The conversion from Aitken mode to accumulation mode may be driven by self-coagulation of primary aerosols under high loadings as through condensation of volatile or semivolatile materials onto solid/liquid aerosol surfaces [Raes et al. 2000]. The coagulation process is of minor importance at ambient aerosol concentrations away from particle sources [Jacobson 2002], hence it is likely that the condensable materials drive the growth of Aitken mode aerosols. This includes the condensation onto BC surfaces, making them more internally mixed. Further evidence for condensation driving the transformation of BC in the free troposphere in the northern hemisphere is provided from the statistical analysis of Schröder et al. [2002], and also by Moteki et al. [2007]. Particles in the accumulation mode are more efficiently removed compared to particles in the Aitken mode. This can be seen in figure7.3-7, the large circles denoting increased precipitation are more frequent to the right hand side of the plot, indicating a high population of Aitken mode particles under these conditions. 266
267 Figure BC mixing efficiency as a function of the ratio between sub-micron and accumulation mode aerosol number, colored by BC mass loading. The size of the markers denotes the intensity of precipitation. 267
268 7.4 Summary of Chapter 7 This study presents one month of continuous measurements of single particles at the Jungfraujoch Research Station, a mountain site in the Swiss Alps, during the late winter season. This site provides an ideal platform to investigate the lower free tropospheric atmosphere. Air masses arriving at the JFJ were classified using back trajectory analysis, in addition to the measurements of BC, CN and trace gases. It is the first time that the physical properties of BC, including the particle size, total mass loading and mixing state, have been investigated in the free troposphere above Europe using a single particle approach. Southeasterly winds (SE) were observed to have the potential to katabatically vent the convective boundary layer that is impacted from pollution sources in the southern Alpine area and industrial northern Italy, and the pollutants at this site were also subjected to considerable influence by precipitation removal. A statistical analysis during the entire experimental period has been applied to classify the observation period into different types that are representative of: the free tropospheric background; periods of SE wind; and periods of significant precipitation scavenging. The free tropospheric background is categorized as free of significant precipitation, lack of NO x and CO enhancement, when the BC mass loading, accumulation mode particle concentration and CN displayed relatively low variation. The SE wind conditions were largely associated with enhanced concentrations of BC mass by the order of as much as 180% on average, CN and CO, during which BC mass was observed to be highly correlated with CO concentration. This suggests strong sources originating from incomplete combustion. This observation indicates the importance of surface sources to the burden in the free tropospheric background. Precipitation removed about 65% of the BC mass from the free tropospheric background reducing the mean loading from 10±5ng/m 3 to 4±2ng/m 3, and as CO concentration was not affected by wet removal, the CO/BC increased in these conditions. Particles in the accumulation mode were preferentially removed by precipitation compared to smaller particles in the Aitken mode. Figure7.3-8 shows the differences in BC loading in SE winds compared to the free tropospheric background and also compared to periods of increased precipitation. The background loading is of 268
269 the same order as has been reported from other tropospheric background stations in northern mid-latitudes [e.g. Schwarz et al. 2006]. The mass distribution of BC in the background free troposphere was observed to peak at a diameter of nm, in agreement with the observations by Clarke et al. [2004] from the Asian free troposphere. The size distribution during valley flows or during periods of wet removal did not vary significantly from the free tropospheric background, indicating that the ageing and sink of BC are highly independent of the core size. A method to examine the mixing state of BC has been demonstrated - particles with an absorbing core that have mass fractions less than 27% are considered to be thickly coated. About 40±15% of the observed BC was thickly coated during the periods when the site experienced free tropospheric background conditions. When SE winds vented air mass from southern Alpine valleys, the BC mixing state was more variable. Though the majority of BC particles were thickly coated (48±17%) a fraction of relatively fresh BC coexisted that largely correlated to the enhancement in NO x concentration. The relative abundance of accumulation mode particles is correlated with the degree of BC mixing, suggesting an important role for condensable materials in increasing particle size as well as enhancing BC mixing state. It is likely that these volatile or semi-volatile condensable materials could contain a considerable fraction of secondary organic and sulfate at this site [Choularton et al. 2008]. 269
270 A B Figure Statistical analysis of the BC mass loading at this site: A) contributed by ground sources delivered by SE wind; B) sink governed by precipitation. The upper and lower edges of box denote the 25% and 75% percentiles respectively. The lines in the middle of box and cross markers indicate the median and average values, with error bars explaining the 10% and 90% percentiles. 270
271 The BC transported in the tropospheric region is of great importance because of its potential ability to act as ice nuclei, hereafter reducing the lifetime of ice clouds by enhancing precipitation via ice phase [Lohmann 2002]. The CCN activation of BC internally mixed with other water soluble compositions could be enhanced due to the modified hygroscopicity as a result of a soluble coating. At temperatures below -35ºC such supercooled droplets will homogenously form ice particles. In addition BC has the potential to act as heterogeneous IN, though definitive evidence remains elusive [Kärcher et al. 2007]. Cozic et al. [2007] inferred that there was a high degree of BC internal mixing at the JFJ by observing a similar nucleation scavenging efficiency of BC compared to other species, however they did not provide directly measurable values of mixing state. During periods of precipitation, BC exhibited a slightly lower degree of mixing (36±14% thickly coated), possibly implying an enhanced scavenging due to the mixing with volatile/semi-volatile materials. However, rather than nucleation scavenging, the inertial scavenging of BC could play a more significant role when incorporated into ice particles of free tropospheric clouds [Cozic et al., 2008b] or cirrus [Baumgardner et al., 2008], and the impaction with hydrometeors may also be an important contributor to BC removal by precipitation [Jacobson 2004]. More work is necessary to investigate the role of inertial scavenging of BC and different mechanisms for BC to be nucleation activated as CCN or IN. The removed BC will add onto the snow along with the precipitation reaching the surface. This will address important climatic issues because the high absorbing property of BC will darken the snow, accelerating the snow melting, reducing the surface albedo, in turn resulting in the earth system receiving more absorbed solar radiation [Hansen and Nazarenko 2004, Ramanathan and Carmichael 2008]. 271
272 Chapter 8 The role of BC as an ice nuclei: a laboratory study and field measurements 272
273 8.1 Introduction Aerosols are believed to modify cloud microphysical properties, changing cloud droplet number and affecting precipitation, hence impacting on the climate system, these are known as indirect forcing effects. The influence of the aerosol population on warm cloud microphysics has been extensively studied over the last few years [McFiggans et al. 2006]. However the knowledge regarding aerosol processing on and feedback from the glaciated or mixed phases clouds is sparse [Lohmann and Feichter 2005]. An ice crystal can be more efficiently formed in the presence of an ice nucleus (IN) via heterogeneous freezing than through homogeneous freezing of a liquid droplet. Among the few candidates serving as IN, such as mineral dust [e.g. Sassen et al. 2003], metallic particles [Chen et al. 1998] and sulphur [Cziczo et al. 2004], BC can be a potentially efficient IN as evidenced from a variety of experimental studies [e.g. DeMott et al. 1990, 1999; Möhler et al. 2005], aircraft in-situ [e.g. Twohy and Poellot 2005] and ground measurements [e.g. Cozic et al. 2008]. The BC loading has been dramatically elevated from pre-industry to modern times due to enhanced anthropogenic activities, as shown by increased deposition rates on snow [Hansen and Nazarenko 2004] and incorporation into ice cores [McConnell et al. 2007]. If anthropogenic sources, such as BC, could increase the burden of IN, then the enhancement of ice formation would cause a glaciation indirect effect, whereby the lifetime and cloud cover would be reduced by increased precipitation via the ice phase. This would weaken the indirect cooling effect (section 1.5.3). This concern therefore stimulates the need to determine the behaviour of BC acting as IN. Ice nucleation laboratory studies were performed at the AIDA (Aerosol Interactions and Dynamics in the Atmosphere) aerosol chamber facility of Forschungszentrum Karlsruhe, Germany in 11/2007, during which the soot particles were generated by the laboratory facility and the conditions of ice formation were simulated in the AIDA cloud chamber. Soot particles coated with different compositions were injected into the chamber, and their performance as ice nuclei was examined under a variety of conditions. The preliminary results from this study are presented in section
274 The interactions of BC with ice particles in real cloud systems were investigated in the CLACE6 project (Chapter 7), as will be discussed in detail in section 8.3. The Sphinx research station on the Jungfraujoch (Switzerland, 3580m a.s.l.) is frequently engulfed by liquid and glaciated clouds. Aerosol-cloud interaction can be influenced by a variety of environmental factors: cloud particle activation is controlled by the amount of available water vapour, the cloud updraft velocity, temperature and the resulting supersaturation; the aerosol properties that influence cloud include the number concentration, particle size, chemical composition, mixing and hygroscopicity. The importance of these factors on influencing aerosol scavenging by cloud can vary under different conditions, however not all of these factors can be analyzed accurately due to the limitations of instrumentation. Previous studies at this site have suggested that it is sometimes possible to isolate individual effects on cloud activation: Verheggen et al. [2007] and Cozic et al. [2007] showed that the presence of ice phase of clouds at this site had important impacts on the aerosol partitioning into clouds, and the ice fraction of clouds could explain the observed effects to a larger extent compared to the other environmental factors. To date, this is the first time that the scavenging of BC has been investigated on a number basis as the previous measurements only quantified the BC mass based on bulk analysis. This study makes concurrent comparisons on the scavenging activities between BC and other non-absorbing particles to investigate the influences of cloud phase on different aerosol properties. 274
275 8.2. Laboratory chamber studies of the ice nucleating efficiency of BC The facilities Figure shows the schematic cross section of the AIDA chamber used in this study together with major technical components and scientific instrumentation used during the ice nucleation experiments. The cylindrical aerosol vessel positioned inside a large isolating containment is made of 2cm thick aluminium walls and has a height of 7m, a diameter of 4m, and a volume of 84m 3 [Möhler et al., 2001]. The aerosol vessel can be evacuated to a final pressure of about 0.01 hpa by two large mechanical pumps that can be operated at different pumping speeds. The interior of the containment, and thus the aluminium vessel, can be cooled to any temperature between ambient temperature and 183K by circulating air through heat exchangers located at the bottom of the containment. In atmospheric clouds, super-cooling is induced by expansion and adiabatic cooling of rising air parcels. The ice supersaturated conditions can be established in the aerosol vessel by expansion cooling with a mechanical pump. A wide range of atmospheric cooling rates can be simulated in the AIDA aerosol vessel by reducing the pressure with a mechanical pump which can be operated at variable pumping speeds. 275
276 Figure Schematic view of the AIDA experimental facility showing major technical components and instrumentation used for ice activation experiments by Möhler et al. [2001]. The sampled combustion aerosols were generated within a co-flow diffusion flame of propane and air (Combustion Aerosol Standard, CAST, Jing-CAST Technologies) [Schnaiter et al. 2006]. Apparatus to coat the soot used a flow of synthetic air saturated with the volatile or semi-volatile vapour of the compounds of interest at a temperature between 320 K and 370 K. The coating material condensed onto the soot particles upon cooling of the saturated mixture in a temperature gradient flow tube [Möhler et al. 2005a]. The soot particles from the CAST generator were then injected into the AIDA chamber for ice nucleation. The coating compounds used in this study were sulphuric 276
277 acid and succinic acid, aiming to access the different coating composition on influencing the ice nucleation of coated soot particles. The particulate emission from the CAST generator was characterized prior to and after the chamber expansion experiments. During the ice nucleation process, a counterflow virtual impactor (CVI) was introduced to remove the water content of ice particles, hereafter the residues were characterized to investigate the particles that had been activated to be ice nuclei. 277
278 8.2.2 The results Figure The mixing state of soot particles coated with sulphuric acid in the AIDA chamber before and after the expansions. The coating evaporation time measured by the SP2 can be approximately related to the abundance of coating materials. The distribution of coating evaporation time is in a bimodal mode as figure8.2-2 shows, and the mode with a longer time delay may be a consequence of the thicker coating that completely encloses the BC core. The black line in figure8.2-2 shows some fraction of soot particles generated from the CAST facility had been significantly coated by sulphuric acid, and when exposed in the AIDA chamber, the soot particles eventually acquired further coatings during the ice activation experiments (expansion). 278
279 Figure The cloud microphysics for a typical expansion experiment. The dashed lines indicate the period when the SP2 was in the operation to measure the ice residues downstream of a CVI. Figure shows a typical expansion experiment when the aerosols in the AIDA chamber were nucleated to form ice crystals. The size distributions of ice particles in real time were measured by a Cloud Particle Imager (refer to section for the details of this instrumentation). The ice crystals formed in the AIDA chamber were sampled through the CVI, by which the associated water was evaporated before the residues were characterized by the SP2. The expansion experiments were repeated developing a minimum temperature of -45 C on both occasions, in the middle of which the AIDA chamber was refilled and additional sulphuric acid was added. The collection of ice residues by the SP2 lasted for 20.5 minutes for both of the expansion experiments. 279
280 Figure The distributions of coating evaporation time for the soot particles coated with sulphuric acid observed in the ice residues during both of the expansion experiments. During the expansion II, a greater number of soot particles have nucleated to form ice crystals within the same sampling duration compared to expansion I, as shown by figure As discussed above, the soot particles in the expansion II are more significantly coated than that in the expansion I (figure8.2-2). Given the other experimental conditions are almost identical during both expansions, this indicates that the increased coating abundance of sulphuric acid has enhanced the activity of soot in serving as ice nuclei. Moreover, the fraction of thickly coated soot particles (in the longer time delay mode) was larger for the ice residues compared to the particles that had not been activated. 280
281 In addition, the soot particles coated by succinic acid were also examined in an identical way during this study, however such particles were observed to be rarely present in the ice residues. These results agree with the observations by Möhler et al. [2005a], who reported an enhanced ice nucleation of soot due to the coating of sulphuric acid; and Möhler et al. [2005b] reported an inhibited ice activation of soot by coated with some species of organic materials. The contrasting behaviours of soot particles in serving as ice nuclei when coated with different components suggest that the coating composition could have an important control on its ice nucleation activity. However, in reality, the components that can coat BC are likely to have been mostly internally mixed, leading to the overall composition dependence been difficult to ascertain directly. The comprehensive analysis on the impact of soot particles on the cloud glaciation is beyond the scope of this thesis. 8.3 The scavenging of BC and non-absorbing particles by mixed phase clouds Overview The aerosol scavenging by mixed phase clouds was investigated at the high alpine research station Jungfraujoch (3580m a.s.l., Switzerland) in winter The scavenged fraction (F) of aerosols that have been incorporated into clouds was determined from the measurements downstream of two different inlets that sampled the sum of activated and interstitial aerosols (total) and interstitial aerosols alone respectively. Cloud microphysical measurements were made using a range of probes. The black carbon (BC) and non-absorbing accumulation mode aerosols were discriminated by a single particle soot photometer (SP2), sampling from both the total and interstitial inlets. The aerosol loadings when encountering the cloud events resembled an environment of free tropospheric background without significant perturbation of pollution outbreak. Both BC 281
282 and non-absorbing particles (>200nm) exhibited a higher mass/volume F than the F in number, indicating a more efficient scavenging activity for larger particles. About 0.5±0.2 volume of the non-absorbing particles were scavenged by cloud which was predominately in the liquid phase (ice mass fraction<0.18), and the presence of a considerable fraction of ice reduced the cloud scavenging of these particles to 0.22±0.1 in volume. This could result from the formation of ice at the expense of liquid droplets, releasing the previously activated aerosols into interstitial phase the Wegener- Bergeron-Findeissen process, or through preventing a further CCN activation. However, the scavenging of BC was not observed to be highly influenced by this process, with a mass scavenged fraction of 0.50±0.23 when liquid-dominant cloud compared to a fraction of 0.48±0.25 when ice mass fraction >0.18. This study suggests a more significant incorporation of BC into ice clouds compared to the other bulk aerosols at this site during the period of this experiment Experimental site, instrumentation and data analysis The experimental site The data presented in this study was sampled during the Cloud and Aerosol Characterization Experiment (CLACE6) project at the Sphinx research station on the Jungfraujoch (46.33 N, 7.59 E, 3580m a.s.l.), Switzerland from 25 Feb. to 11 March The experimental site is located at the northern side of the main central European Alpine area, which is remote from significant pollution sources and local emissions [Baltensperger et al. 1997]. This site is representative of a European lower free troposphere and the air has experienced significant transport and so represents a large range of sources across Europe, in addition to the pollutants from local valleys (Chapter 7). Cloud was present at the site for approximately 60% of the experimental period. The phase of the cloud varied from periods when liquid-water clouds were dominant to periods when the clouds almost contain pure ice particles. The aerosols sampled at this site experienced a combination of precipitation and cloud processing. 282
283 Sampling inlets To differentiate between aerosols that have been incorporated into clouds from those remaining in the interstitial phase, two inlets were installed: one, the total inlet, was fitted with a heater (25ºC) that was used to evaporate cloud particles smaller than 40µm at wind speeds of up to 20m/s [Weingartner et al.1999], and so sampled both activated and non-activated interstitial aerosols. The other inlet was designed to sample only interstitial aerosol by removing cloud particles using an impactor with a 2µm upper size cut at a high flow rate of about 20lpm [Weingartner et al. 1999, Henning et al. 2002]. Thus this interstitial inlet sampled either non-scavenged particles or particles that had not yet grown to sizes greater than 2µm. The inlet was cleaned regularly to avoid riming and clogging by snow. An automated ball valve switched between these two inlets every 6 minutes to enable near simultaneous comparisons of measurements downstream of each inlet. Behind each inlet, the aerosols were sampled at laboratory room temperature under dry conditions (relative humidity <20%) Aerosol measurements The physical properties of BC and other BC-free particles in the accumulation size mode (>220nm) were quantified by deploying SP2 measurements. The operation, calibration and data analysis of the SP2 instrument have been in detail described in section The non-absorbing (BC-free) particles in this study are defined as particles that do not contain a measurable incandescence signal, to be differentiated from BC particles. 283
284 Cloud microphysical measurements The Liquid Water Content (LWC) of clouds was measured with a Particulate Volume Monitor (PVM-100, Gerber Scientific), which determines LWC by the scattered light of a laser beam. The instrument response was calibrated typically every cloud-free day. When the offset became higher than a certain threshold value (as recommended by the manufacturer) or when the detection unit was clogged with snow, the PVM was taken inside to defrost and the lens was cleaned. A Cloud Particle Imager (CPI, SPEC Inc. 230X) was used to record images of individual cloud particles in the size range 10 to 2300µm. A semi-empirical calibration was developed to correct the particle size and accurately define the probe sample volume [Connolly et al. 2006]. The method enables the quantitative measurements of cloud particle size distributions. The ice water content (IWC) of the cloud was computed from the volume of each 2D imaged particle from this instrument, based on the empirical sizemass relationships given by Heymsfield et al. [2004]. The ice mass fraction (IMF) is calculated as the fraction of IWC over the total water content (TWC) of clouds that is a sum of IWC and LWC, expressed as IMF=IWC/(IWC+LWC). Figure8.3-1 shows the time series of LWC and IWC measured by the PVM and CPI respectively. The periods when the average TWC is over 0.02g/m 3 are regarded as cloud events, to exclude as much as possible measurements made in patchy clouds, cloud edges and regions influenced by entrainment. The IMF is only calculated when experiencing cloud events and both PVM and CPI were functioned ideally. 284
285 Figure The time series of ambient temperature, ice water content and liquid water content as measured by CPI and PVM respectively, and calculated ice mass fraction for the recognized cloud events. The liquid cloud and mixed phase cloud is classified according to a critical point of ice mass fraction of 0.18, shown as columns in light blue and dark blue respectively. 285
286 Figure The relationship between total water content and ice mass fraction: the lower and upper edges of the boxes are the 25% and 75% percentiles of the water content corresponding to each value of IMF, the line in the middle of box and the cross marker denote the median and average values, with error bars identifying the 10% and 90% percentiles. The widths of the boxes indicate the width of the bins used to derive the average statistics. The definitions of cloud phase are also indicated. The accumulated hours for both cloud phases encountered during this experiment are shown on top of this plot. 286
287 It should be noted that the PVM can also respond to IWC in mixed phase clouds, as has been shown by Verheggen et al. [2007] the error in the LWC determination from the PVM increases with increasing LWC, therefore the LWC can be overestimated when measuring the supercooled liquid water with some contribution from the ice phase. Correspondingly, the IMF tends to be underestimated especially for glaciated clouds that contain a large fraction of ice. Based on the frequency distribution of IMF as measured during this study, a critical point of IMF=0.18 is chosen to segregate liquid and mixed phase clouds, above this value the cloud contains a substantial fraction of ice. The sampling duration for both types of clouds are almost identical (~25hours, figure8.3-2 upper panel), allowing the statistically analyzed results to be reasonably compared under both conditions. Though the existence of pure liquid or ice cloud is difficult to establish due to the uncertainty of IMF determination, the definitions here are simplified only to compare the cloud properties between periods when liquid phase is dominant and periods when the cloud is more significantly influenced by ice. Figure8.3-2 shows the statistics of the water content for both the defined liquiddominant cloud and mixed phase cloud. It is shown that the increased fraction of ice in clouds steadily decreases the total water content (TWC) of clouds. The water content ranges g/m 3 during periods of mixed phase cloud and g/m 3 when liquid clouds are dominant, which fell within the periods of water contents of clouds reported from long term studies at this site [Verheggen et al. 2007] Aerosol scavenging by clouds The scavenged fraction (F) of aerosols is derived from the measurements downstream of the switching valve between the total inlet and interstitial inlet, which measured the scavenged plus interstitial aerosols and interstitial-only aerosols respectively. The F is expressed as: 287
288 F = Aerosol Total Aerosol Aerosol Total Interstitial Where Aerosol Total and Aerosol Interstitial denote the abundances of the aerosol property under consideration as measured by the total and interstitial inlet respectively. Given the precipitation particles are not efficiently sampled by the total inlet, the sampling for the particles as scavenged by clouds is not affected by precipitation, but the background loadings of aerosols can be greatly reduced if a considerable amount of aerosols are washed out by precipitation Results and Discussions The BC and non-absorbing aerosol loadings on the total and interstitial inlets segregated by different phases of clouds Figure8.3-3 shows the temporal evolution of BC and non-absorbing particles during the experiment when the total and interstitial inlets functioned correctly. The number concentration for both BC and non-absorbing particles are presented, in particular, the BC is investigated as a function of the mass loading as determined by the incandescence signal and the optically sized non-absorbing particles are also calculated as volume concentration. For most of the periods, the aerosol loadings downstream of the total inlet were higher than that in the interstitial line, indicating a scavenging effect by cloud particles. 288
289 A B Figure The time series of particle properties sampled from the total and interstitial inlets: A) BC mass loading and number concentration; B) non-absorbing particle volume and number concentration. 289
290 The aerosol loadings are statistically analyzed in figure8.3-4 and figure8.3-5 for BC and non-absorbing particles respectively. At the first step the aerosol loadings are segregated by cloud phase, and the categories under the influence of liquid dominant cloud and mixed phase cloud are compared. The influence of cloud phase on the BC loading is negligible downstream of both the total and interstitial inlets (figure8.3-3 and figure8.3-4 A1, A2, B1, B2), however for the non-absorbing particles, the higher aerosol loadings more frequently occurred in the mixed phase clouds (figure8.3-5 A1, A2, B1, B2). Targino et al. [2009] observed that the conditions of elevated pollution (organic, inorganic and BC aerosols) were accompanied by an increase in occurrence of glaciated periods at this site during winters 2004 and 2005, which agrees with the result for the non-absorbing particles in this experiment, however an elevation of BC mass loading when coinciding with ice clouds was not observed in this study. This is largely because the cloud events studied during this experiment had not coincided with pollution outbreaks (figure8.3-1 and figure8.3-3), although the free tropospheric background at this site was sporadically injected by polluted boundary layer, in addition the aerosol loadings were significantly washed out by precipitations at times. The background BC mass loading ranges between 2-45ng/m 3 (figure8.3-4 A1, A2), which is much lower than the values reported from Targino et al. [2009] (20-200ng/m 3 ). Therefore the investigation of aerosol scavenging in this study mainly focuses on the environment of free tropospheric background without significant perturbations of additional aerosol burdens. The aerosols that have incorporated into clouds can be quantified by subtracting the measured aerosol abundance in the interstitial from the total inlet (section ). The aerosol loadings of BC and non-absorbing particles in the total inlet are statistically higher than in the interstitial inlet for both specified phase of clouds, however the differences between both inlets vary with aerosol types and cloud phases. The aerosol loadings incorporated into clouds are statistically analyzed in the right columns of figure8.3-4 and figure It is shown that the mass/number loadings of BC incorporated into clouds had not been apparently influenced by cloud phases, whereas the scavenged volume/number of non-absorbing particles are significantly reduced in the 290
291 presence of glaciated clouds, and this reduction is more obvious for the number concentration of non-absorbing particles (figure8.3-5 B3). 291
292 A1 A2 A3 B1 B2 B3 Figure Statistical analysis on BC mass loading measured downstream of total (A1) and interstitial inlet (A2) classified according to cloud phases, the scavenged amount of BC mass is shown in A3. B1-B3 have identical meanings but show the statistics of BC number concentration. The box and whiskers have the same meanings as in figure
293 A1 A2 A3 B1 B2 B3 Figure Statistical analysis on non-absorbing particle volume loading measured downstream of total (A1) and interstitial inlet (A2) classified by cloud phase, the scavenged amount of volume is shown in A3. B1-B3 have identical meanings but show the statistics of non-absorbing particle number concentration. 293
294 The scavenged fraction (F) of aerosols influenced by cloud phases The scavenged aerosol properties relative to the background aerosol abundance-the scavenged fraction (F), are analyzed in figure During cloud-free conditions, the response of the total and interstitial inlet should be identical, however a small systematic difference between both inlets was observed. The total inlet measured about 5% higher than the interstitial. This was to be expected as particle losses were expected to be higher as the interstitial inlet had a longer residence time and in addition had a cyclone installed on it [Weingartner et al.1999]. The grey histograms in figure8.3-6 show that when out of cloud, the frequency distribution of F for each aerosol property is skewed a little towards positive values as a result of the inlet efficiency differences. The out of cloud data is also broadly distributed as a result of the variability in ambient conditions during the 6 minute switching samples on the different inlets and inherent variability in the measurements. This uncertainty was consistent throughout the experiment. When cloud events occur, a greater frequency of elevated values of F above the noise level can be seen for each aerosol property. During liquid clouds, the median value of F for non-absorbing particles in volume concentration is 0.5±0.2 (figure8.3-6 C)), while the number scavenging efficiency is reduced to be 0.22±0.18 (figure8.3-6 D)). The mass based F BC in liquid cloud (0.50±0.23, figure8.3-6 A)) is also higher than that based on number (0.25±0.27, figure8.3-6 B)). This is caused by the size dependence of particles when activated into condensation nuclei of liquid cloud. It has been long established that a larger particle tends to be more efficiently activated due to the reduced equilibrium vapour pressure of water arising from the less surface tension. [e.g. McFiggans et al. 2006]. The trend that a larger size of particle will lead to a higher scavenging efficiency was also evidenced by the observations at this site, which was more obviously exhibited when the clouds were dominant in the liquid phase [Henning et al. 2002, 2004; Verheggen et al. 2007]. These studies also found the presence of glaciated clouds could mitigate this relationship, resulting in a broadly low scavenged fraction that showed a 294
295 lack of size dependence. In liquid cloud, as larger particles can be more efficiently scavenged with more volume/mass occupation but less numeric, the scavenged fraction on a volume/mass basis would be biased higher than that calculated on a number basis, which explains the observations in this study. Under the conditions of glaciated clouds, the number or volume based F non-absorbing had not been obviously affected compared to that in the liquid clouds, shown in figure8.3-6 C) and D), indicating the independence of F non-absorbing on particle size in ice clouds, and this is also consistent with the previous studies as discussed above. 295
296 A B C D Figure The frequency distributions of aerosol scavenged fraction for the whole dataset classified as cloud-free, liquid cloud and mixed phase cloud for: A) BC mass; B) BC number; C) the volume of non-absorbing particles; D) the number of non-absorbing particles. 296
297 In figure8.3-6 C) and D), it can be clearly seen that the F non-absorbing had been decreased by the presence of ice clouds, from 0.5±0.2 to 0.22±0.1 in volume and from 0.22±0.18 to 0.13±0.11 in number. Verheggen et al. [2007] also found a considerable amount of ice decreased the F from around 0.5 in the liquid water-dominant cloud to about 0.1, concluding that the Wegener-Bergeron-Findeisen (WBF) process [Bergeron 1935] triggered this trend more importantly than the other relevant environmental factors, because of the lower saturation vapour pressure over ice than water, a large presence of ice crystals can cause the existing water droplet to evaporate, consequently releasing the formerly activated particles (CCN) back into the interstitial phase, on the other hand this can also prevent further CCN-activation to form liquid droplets. As the ice particles are less numerous than liquid droplets, the particles serving as cloud nuclei are thus largely reduced, leading to reduced nucleation scavenging efficiency. The decrease of F nonabsorbing due to ice cloud was more obviously exhibited in number than in volume, and was also shown by a higher scavenged amount of non-absorbing aerosols in number (Figure8.3-5 B3)) than in volume (figure8.3-5 A3). This is because the presence of ice will cause the small droplets to be evaporated before the larger ones, preferentially releasing the smaller CCN, or if reducing the supersaturation conditions, the smaller aerosols are more likely to be prevented from CCN-activated. The F BC-mass of liquid clouds (0.50±0.23) was studied during the long term investigation by Cozic et al. [2007], who obtained a value of However, unlike the non-absorbing particles, the glaciation of clouds did not significantly affect the F BC in both number and mass as figure8.3-6 shows, which corresponds to a nearly invariable scavenged amount of BC mass or number under the influence of ice clouds (figure8.3-4 A3 and B3). The relative independence of F BC on the ice mass fraction of clouds observed in this Chapter 5s different from the reports by Cozic et al. [2007], which is mainly because of the different datasets used in both studies, as the latter study has a longer temporal resolution covering a broadly ranged temperature (-25-5 C) and BC mass loadings (0-400ng/m 3 ), while this study mainly focuses on the background BC mass loading (2-45ng/m 3 when experiencing the cloud events) without sampling a significant pollution
298 outbreak. Considering the conditions of BC mass loading and the ambient temperature during this study (-15 C to -8 C, figure8.3-1), the F BC-mass by mixed phase clouds observed in this study (0.48±0.25) agrees with the previous study at this site, as Cozic et al. [2007] showed a much higher F BC-mass ranging from when BC mass loading was below 40ng/m 3. Figure The BC number fractions on the interstitial line during cloud free, liquid cloud and mixed phase cloud. A combination of the investigations on the scavenged fraction for BC and other nonabsorbing aerosols demonstrate that the glaciation of clouds during this study had reduced the magnitude of non-absorbing aerosols to be incorporated into clouds however this effect had not affected the scavenging of BC particles. The speculation that BC particles had been more significantly incorporated into ice clouds than non-absorbing particles is supported by the analysis in figure8.3-7: the more efficient scavenging of BC rather than the non-absorbing particles by ice clouds leads to a lower BC number fraction measured on the interstitial line under the condition of mixed phase cloud than in the liquid cloud. The background BC number fraction in the interstitial phase when cloud free is much lower than that when liquid clouds scavenge on the aerosols, 298
299 indicating that the non-absorbing particles are more preferentially scavenged by liquid clouds. The enrichment of BC fraction in the ice residues compared to that in the cloud-free environment has been observed by several studies, such as at this site by Cozic et al. [2008], and the airborne measurements over the northern Pacific in the upper troposphere by Baumgardner et al. [2008]. These results are not directly comparable with this study as the ice residues were not characterized in this experiment, but support the conclusion that the BC had importantly incorporated into ice clouds. 299
300 8.4 Summary of Chapter 8 Scavenging of black carbon using a single particle method was studied at the Jungfraujoch site in liquid and mixed phase clouds during late winter 2007 and was compared with the scavenging of BC-free non-absorbing aerosols in the accumulation mode (>220nm) measured using the same technique. The aerosol loadings when encountering cloud events during this experiment resembled an environment of free tropospheric background without significant perturbation of pollution outbreak. The BC and non-absorbing particles were measured simultaneously and their scavenged fractions (F) were examined separately. The F BC on a mass basis or F non-absorbing in the volume is higher than the F calculated in number concentration, indicating a more efficient scavenging activity for larger particles. The size dependence of F non-absorbing is more obvious exhibited in liquid clouds than in mixed phase clouds, as it has been long established that larger particles have a more efficient CCN activity. The preferential F BC in the larger size was also observed by Baumgardner et al. [2008] by investigating the size of BC in ice residues compared to that at the cloud-free background, suggesting the inertial scavenging mechanism may partly contribute to this relationship. The presence of ice clouds significantly affected the F non-absorbing. The clouds with ice mass fraction >0.18 showed a reduced F non-absorbing in volume from 0.5±0.2 in liquid dominant clouds to 0.22±0.1 and from 0.22±0.18 to 0.13±0.11 in number. This effect agrees with the previous studies at this site [Verheggen et al. 2007], as a result of Wegener-Bergeron-Findeissen process where ice can grow at the expense of water due to its lower saturation vapour pressure, releasing the activated CCN back into the interstitial phase, or reducing the supersaturation to prevent aerosols from being CCNactivated. However, this process is observed to be moderate in influencing the F BC : the F BC-mass is 0.50±0.23 and 0.48±0.25 by liquid and mixed phase clouds respectively. The BC mass incorporated into mixed phase clouds is 5-60ng BC mass /g water, within the similar range reported by Baumgardner et al. [2008] (7-44ng/g) in the ice crystals over the northern Pacific. 300
301 The significant incorporation of BC into ice clouds leads to a lower BC number fraction measured downstream of the interstitial inlet during the presence of ice clouds than when influenced by liquid dominant clouds. This suggests a more efficient scavenging activity of BC than the other species of aerosols by ice particles. However, the liquid cloud had scavenged the non-absorbing particles more efficiently than BC, resulting in a higher BC number fraction in the interstitial phase rather than the cloud-free environment. The aerosols at this site in wintertime constitute of a significant fraction of sulfate and secondary organic aerosols [Choularton et al. 2008]. By analyzing the single particle mass spectrometry at this site during the same period with this study, Kamphus et al. [2009] found that the sulfate and nitrate containing particles were strongly depleted in the ice residues, and the sulfate was found to dominate the droplet residues more frequently than in the ice residues, which supports the observations in this study. The incorporation of BC into mixed phase clouds may be via several different possible mechanisms. Because the BC transported to this site had been significantly mixed with condensable material (Chapter 7), it is possible that it may act as an efficient CCN or be impaction scavenged by liquid droplets. At low temperatures (<-35 C), water droplets can glaciate via homogenous freezing [Cantrell and Heymsfield 2005], and BC will be transferred to the ice phase if it is included within homogeneously frozen droplets. More importantly, BC has been long been believed to potentially act as a heterogeneous ice nuclei [e.g. DeMott et al. 1990, 1999], and this ability can be enhanced if coated with sulfate [Möhler et al. 2005]. Considering the temperature range (-15 C to -8 C) in this study, heterogeneous ice nucleation may make an important contribution to the efficient scavenging of BC by ice clouds. In addition, ice particles may act as efficient scavengers of interstitial BC particles via inertial scavenging when several BC particles can impact on a single ice crystal, as suggested by Baumgardner et al. [2008], by which the F BC can be enhanced significantly. 301
302 Chapter 9 Concluding remarks and future work 302
303 9.1 The instrumentations The development of the instrument, calibrations and data analysis technique The single particle soot photometer (SP2) has many advantages for quantifying BC particles in the atmosphere. To date, it is the only instrument that can directly detect and differentiate the BC particles. The SP2 instrument used in this project has undergone several hardware modifications since initial receipt, including the increased S/N ratio on the gains of detectors, baseline adjustment of signals, optical alignments, the cleaning of optics, flow rate regulation and a new split detector installed. The instrument is also equipped for aircraft use. Each time a modification was conducted, the instrument was recalibrated to determine new parameters that need to be applied in further data analysis. Much of the work presented in this thesis focuses on the data analysis procedures that process the raw data acquired from the field measurements or laboratory experiments, filtering the data to assure the quality, automatically applying calibration parameters and correction factors under a variety of conditions to derive the useful and quantitative results. The software was developed to reject single particle events that represent noise but have been erroneously triggered as particle event by data acquisition. The real particle event was then recognised to be either a BC or a BC-free non-absorbing particle according to the presence or absence of the incandescence signal, thus allowing a simultaneous comparison between the properties of BC and other species of aerosols. The optical property of each particle is determined by the scattered laser light, for the non-absorbing particles, the scattering signal follows a Gaussian function, but for the absorbing particle, such as BC, the scattering signal is distorted due to the evaporation of surface materials when heating the refractory core. The scattering signal of absorbing particles therefore needs to be reconstructed by extrapolating the unperturbed leading edge part of the scattering signal. The incandescence signal is highly independent on the 303
304 coating abundance and the complex morphology, and can be directly related to the mass of a single BC particle. Previous modellers have assumed the BC particle to act as an inclusion that is surrounded by the coating when it is mixed the core-shell model, however lack of observational support. The capacity of SP2 to quantify both the shell size by optically sizing and the core size by the incandescence signal allows the coreshell model to be treated based on in-situ observations, consequently can relate the Mie calculation to the measurements in the real atmosphere. The mixing state of BC can also be quantified by a parameter known as the time delay that refers to a time scale for the coating materials to be evaporated, and this approach is unique for the SP2 measurement. A data analysis toolkit has been developed to analyse data from the SP2 that is capable of loading information from each single particle and producing the aerosol concentration and size distributions in real time. The high sensitivity of SP2 measurement to the low concentration of aerosols enables precise quantification of carbon mass even at high altitude. This makes the deployment of the SP2 measurements on aircraft throughout the troposphere and operation in the free troposphere possible. Additionally, the quantification of BC can be approached on both a number and mass basis. The traditional characterization of BC has only been by bulk mass analysis. The number concentration of BC provided by the SP2 is useful in many areas of research, for instance, the study on the nucleation scavenging of aerosol by cloud particles Ongoing and further technical development One of the challenges of the SP2 is to quantify small particles on a single particle basis at the small size limit when decreased S/N is not large enough to be triggered as a particle event. This systematic limitation leads to the main uncertainty in quantifying the total BC mass loading as small particles are not detected. For the old version of SP2, the lower detection limit of a single BC particle is about 190nm diameter, below which the information on the BC size distribution is not available. The previous approach to obtain the distribution over the whole BC size range is to apply a lognormal fitting routine to 304
305 the detected distribution curve, however this will introduce some uncertainties as whether there is another size mode below the SP2 detection limit. The modified SP2 increased the sensitivity of the detectors to capture the incandescence signals, extending the detection limits down to the BC diameter of 70nm with acceptable S/N ratio. This improves the data quality and expands the range of applications. The upper limit of particle sizing depends on the point when the signal saturates the circuit board. The upper detection limit will be reduced by the increased sensitivity of detectors because the signal will reach the saturation point at smaller particle sizes. Detector saturation occurs on both the larger BC and non-absorbing particles due to the maximum threshold digital voltages for both scattering and incandescence detectors set in the configuration. The old version of the SP2 uses a scattering channel with a low gain to quantify the optical size of particle when the signal in the high gain channel is saturated. However, for the modified SP2, the low gain channel is replaced to detect the signal from the newly installed TEAPD (section 4.2). Although the saturated scattering signal can be reconstructed by asymmetric fitting (section ), this method still introduces some uncertainty when the signal shape is extensively clipped and only a few digital points can be used for the fitting. Work is ongoing to use a data acquisition board with more channels to overcome the current board limitation which only uses four channels. As the incandescence signal is not a regular shape, asymmetric fitting cannot be applied, the current SP2 uses one incandescence channel to size the BC if the other incandescence channel is saturated. Refractory composition can be discriminated by the ratio of incandescence signals collected at different bands of wavelength (section 4.4). This technique is only applicable when the signals from the two incandescence channels are comparable and not saturated. The modified SP2 has increased the gain for both incandescence channels but, as the more sensitive channel is then largely saturated, it can no longer be compared with the lower gain channel to allow examination of different refractory compositions. The capacity of discriminating refractory compositions is important because under some conditions, the contaminations rather than the BC dominate (section 4.4). Therefore the 305
306 incandescence channel with comparable gain but different waveband collection needs to be recovered, and this channel could be added on the forth-coming SP2 data acquisition board. Another challenge is to determine the coating abundance associated with a BC core. The time delay between the scattering and incandescence signals, which can be approximated by the coating evaporation time (section 4.6.2), has been widely used to detect the mixing state of BC. However, this parameter can only generally indicate the extent to which the BC is coated, and cannot explicitly quantify the amount of the coating. The ideal approach is to optically size the coated BC however the scattering signal is distorted owing to the coating evaporation by the heat of BC core (section 4.6.3). Much of the work in section focuses on the reconstruction of the initial scattering property of coated BC particles. This technique needs the TEAPD with a fixed notch position to determine where the laser beam centre is and then extrapolate the leading edge only (LEO) signal that has not been significantly perturbed. The notch position relative to the laser beam centre is crucial to the accuracy of the LEO fitting, and this parameter is dependent on the alignment of the SP2 optics and flow rate. The current software developed is to apply for the notch position in real time to adjust the fluctuation of this parameter during the instrument operation. The optical alignment and the regulation of the injected sampling flow are important procedures for the instrument configuration. As the scattering signal is highly sensitive to the intensity of the incident laser light, the scattered light from a particle will depend on the position of that particle within the laser beam (section 4.5). Because the laser beam is in distributed as a concentric Gaussian with a central maximum intensity, the scattered light intensity will be decreased if the particle has not passed through the laser beam centre. For an extreme case, the particle passing through the edge of the laser beam will not exhibit sufficient scattering signal to be detected. This means that adjustment of the laser beam to let the sampling aerosol jet pass through the laser beam centre needs to be optimised, and hence data quality can be maximised and the sampling loss minimised. This work is delicate and needs to be achieved by iteratively adjusting 306
307 the components of the laser mechanism, during which the peak amplitudes of the monodispersed PSL particles are used to monitor the intensity of the laser light incident on the particle. The optics of the instrument could be contaminated occasionally, such as during the failure of the purge flow, or a sharp increase of the sampling flow rate which the sheath flow is not able to effectively constrain, resulting in the sampled materials being dispersed in the laser cavity. If this occurs the optics need to be cleaned. If not, a drop of laser power can be seen and this will largely reduce the data collection efficiency. Once cleaned, the optics needs to be realigned if necessary and then the calibrations need to be redone. The data analysis software is regularly upgraded to incorporate any correction factors once the instrument has been reconfigured. New parameters need to be applied each time the new calibrations have been performed. As the refractive index is one of the factors influencing the scattering property of particle and this is linked to the composition, current laboratory work is aimed at calibrating the SP2 instrument using different compositions of scattering particles. The analysis software will be correspondingly improved to account for the influences from the particle refractive index. Another uncertainty can result from the fluctuation of the sample flow when conducting the bulk data analysis by the software to derive the aerosol concentrations. A maintained flow rate is crucial to determine the time delay between the scattering and incandescence signal and to assure the data quality of the LEO fitting as the notch position relative to laser beam centre is a time-based parameter. The current version of the SP2 regulates the flow rate by using an automatically controlled value to adjust the pressure difference between the instrument chamber and ambient environment, and the sample flow rate is recorded just before the aerosol jet enters the detection chamber. On occasion, the sample flow is not ideally regulated or the reading of the sample flow rate does not 307
308 indicate the real value. In such circumstances unreliable flow readings are filtered by the post data analysis. It is greatly desirable to be able to make measurements from an airborne platform, because for the ground based measurements, the spatial or vertical distributions of the aerosols and particularly the study of specific pollution event is limited when the plumes pass directly over the measurement site. Recent efforts have been concentrating on deploying the SP2 instrument aboard the Facility for Airborne Atmospheric Research (FAAM). This is a NERC large research aircraft (BAe Systems model ), featuring instrumentation from several UK academic research institutes and the Met Office. At the time of writing, the FAAM SP2 has been successfully deployed in a series of science flights during the European Integrated Project on Aerosol Cloud Climate Air Quality Interactions (EUCAARI) - LONG Range Experiment (LONGREX) project, when the BAe-146 flew across western Europe during periods of anticyclonic circulation with clear sky conditions and pollution plumes in May 2008 to examine the effects of atmospheric aging on aerosol chemical, physical and optical properties. 308
309 9.2 Summary of scientific findings, uncertainties and future work The aerosol properties and direct forcing An SP2 has been employed in a series of experiments that represent a variety of environments, including urban and rural sites close to emission sources, as well as in the free troposphere remote from ground sources. The physico-chemical properties of aerosols and their spatio-temporal evolutions are characterized by comparing results within and between these projects. BC particles exhibit some different properties compared to other components of atmospheric aerosols, for example they are efficient light absorbers and have only weak hygroscopicity. Hence the influences of BC particles are highlighted in these studies and the BC properties are compared with the other aerosols to obtain a better understanding on their atmospheric lifecycles. The emission of BC can occur from a variety of sources such as vehicle exhaust, open biomass burning, indoor heating usage and industrial use, from which it is emitted with other co-products from incomplete combustion. These co-products vary with different sources, and can include some primary organic matter, inorganic aerosol, ash and various gases. These materials can externally exist or be internally mixed with BC particles. In the real atmosphere, the observed mixing state of BC, once emitted, will be further altered during transport. Correlations between particulate components arising from combustion and gas phase products with known emission can be used as indicators of different sources. Previous studies have used the mass spectra of organic particulate to attribute the possible pollution sources [e.g. Lanz et al. 2006, Ulbrich et al. 2008]. An investigation on the mass spectra of organic aerosols during the downwind urban study at Holme Moss (Chapter 5) showed that both urban outflow and locally emitted solid fuel burning are the dominant pollution sources at different times within a diurnal pattern. At this site, the characteristics of pollutants partitioned by different sources were also reflected by different microphysical properties of BC and different relationships among the primary components of combustion. The Hydrocarbon-like Organic Aerosol (HOA), which is largely associated with the primary organic aerosol, was observed to be 309
310 highly correlated with the BC mass throughout the whole experiment. By investigating the periods with different source dominance, it was found that for the burning of solid fuels, the same amount of BC mass tends to contribute less HOA than from the vehicle emission, which may result from the less oxygenated characteristics of organic matter from vehicle emission. When the pollutants are transported to the Holme Moss site from the Greater Manchester conurbation over approximately 30km, the relationship between the HOA and BC may have been altered compared to the pollutants directly from urban emission. HOA and BC are both main species of primary sub-micron aerosols in the atmosphere, which dominate Aitken mode particles. More work seeks to explore the evolution of HOA-BC relationship during their lifetimes in the atmosphere. The carbon monoxide (CO) is another main product of combustion, and its relationship with BC mass has been widely studied. Most of the previous experiments were conducted regionally in the close vicinity of high-traffic area in densely populated cities, and the studying environments were not significantly interrupted by the local meteorological conditions [e.g. Kondo et al. 2004, Baumgardner et al. 2002, Park et al. 2005]. CO and BC were mostly observed to have a strong correlation during these experiments. However, in the tropospheric background as investigated during the CLACE6 experiment in Chapter 7, there was a larger variation in CO for little change in background BC, leading to the CO/BC biased towards larger values. The relationship between CO and BC relationship was studied by Spackman et al. [2008] over a fairly large altitude range. Spackman et al. [2008] obtained similar results, showing that at high altitude, BC was more efficiently removed than CO, resulting in a lack of correlation between them. Chapter 7 also considered the conditions when the background experienced significant precipitation, during which the BC mass loading was significantly reduced by wash out, a process that does not affect the CO concentration. The results presented in this thesis suggest that the relationship between BC and the other co-products is affected by atmospheric processing and complex meteorological conditions during transport, and these relationships could be indicators of the time scale on which the plume from ground source has been atmospherically processed. 310
311 Another relationship that has been investigated in this thesis is that between BC mass and the aerosol absorption coefficient. The instruments used to quantify the absorbing coefficient in this thesis were Particle Soot Absorption Photometer (PSAP) during the Holme Moss project and the Multi-Angle Absorption Photometer (MAAP) in the CLACE6 project respectively. Both instruments use the filter-based bulk measurement, while the MAAP directly accounts for scattering from the filter and applies a correction. BC mass is often derived from these instruments by empirically assuming an absorption efficiency of per unit of BC mass that is defined as the mass absorption cross section (MAC). This conversion has some systematic bias because not only BC contributes to light absorption in the atmosphere but also some organic particulate contribute [e.g. Andreae and Geleneser 2006]. As the instrument quantifies the absorption coefficient, it is not able to segregate the BC component from the aerosol bulk. Several studies have addressed this concern that the presence of light absorbing organic aerosols may bias the conversion between absorption coefficient and BC mass [Lack et al 2008, Subramanian et al 2007]. In addition, the MAC value of BC could vary if originating from different sources, as Schwarz et al [2008b] observed. Modelling studies also suggest that for the same amount of BC mass, the coating can lead to an enhancement of the absorbing property by the BC core [Bond et al. 2006]. As the SP2 directly quantifies the mass of BC, the measured absorbing coefficient can be assessed by comparison with filter-based instruments. The average MAC value as obtained from either PSAP/SP2 or MAAP/SP2 is 13.7±6.9m 2 /g at the Holme Moss site and 10.6±7.8m 2 /g in the free troposphere during the CLACE6 project. The higher MAC at Holme Moss may result from the prevalence of primary organic matter that could contribute some light absorbing, in particular the contribution from solid fuel burning [Weimer et al. 2008, Alfarra et al. 2007]. These analyses suggest that the conversion method for the BC mass quantification from measured absorbing coefficient should be treated with caution in the future work. The results presented in this thesis establish a framework to investigate the evolution of BC in the atmosphere during transport. Figure synthesises all the datasets discussed in this thesis, representing the environments of urban, polluted rural and free 311
312 troposphere. The BC soon after emission, as measured from Manchester city centre, has a high mass loading ( ng/m 3 ). This value is lower than, but comparable to, the observation also quantified by the SP2 in Mexico urban area by Baumgardner et al. [2007] ( ng/m 3 ), indicating Manchester is not as large a source of BC as Mexico City. The emitted BC mass is diluted during transport. The BC mass loading observed at the Holme Moss site ranges from ng/m 3, which followed a diurnal pattern with the peak loading at noon mainly contributed by the urban outflow and the localised solid fuel burning significantly contributed the peak loading in the early evening (Chapter 5). The BC loadings in the lower atmosphere may have large spatio-temporal variability because the emission of BC in the urban area is dependent on the anthropogenic activity, which varies across different regions of the globe; BC from open biomass burning, such as the forest burning is largely seasonal. The BC mass loading transported in the free troposphere will be further diluted compared to the sites close to ground sources. Chapter 7 characterizes particulate BC at the Jungfraujoch site, which is representative of the free troposphere over the Europe. The BC mass loading in the background ranges from 1-20ng/m 3 at this site, within the same order that has been reported from other tropospheric background stations in northern mid-latitudes, for example 1-10 ng/m 3 [e.g. Schwarz et al 2006]. However, it has been observed that localised polluted air mass can be occasionally advected upon the Jungfraujoch site, leading to the enhancement of BC mass loading. The BC mass loading under each type of environment can be influenced by the local meteorological conditions, such as the precipitation washing out and air flow convections. Figure9.2-1 gives a general overview of the evolution of BC mass loading during transport over the regions of Europe. 312
313 Figure A summary of BC mass loading as a function of mixing state for the three projects mentioned in this thesis. The central dots and the edges of the boxes denote the median, 25% and 75% percentiles for the corresponding axis, and the outliers indicate the 10% and 90% percentiles. The constitutients of combustion particles vary with different sources, as is discussed above. The initial mixing state of BC emitted from different sources could also be different. It has been observed by Schwarz et al [2008b] that the internally mixing fraction for open biomass burning (0.7±0.09) and urban plume (0.09±0.06) are distinct, quantified by airborne measurements. The mixing efficiency of BC observed in the Manchester city centre is 0.05±0.03, agreeing with the value of fresh urban plume reported by Schwarz et al. [2008b]. The mixing efficiency of BC at the Holme Moss site when influenced by the Greater Manchester conurbation was observed to be 0.18±0.08, and was increased to be 0.27±0.06 when the sources were significantly contributed by 313
CHAPTER 8. AEROSOLS 8.1 SOURCES AND SINKS OF AEROSOLS
 1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
1 CHAPTER 8 AEROSOLS Aerosols in the atmosphere have several important environmental effects They are a respiratory health hazard at the high concentrations found in urban environments They scatter and
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle
 Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Implications of Sulfate Aerosols on Clouds, Precipitation and Hydrological Cycle Source: Sulfate aerosols are produced by chemical reactions in the atmosphere from gaseous precursors (with the exception
Climate Dynamics (PCC 587): Feedbacks & Clouds
 Climate Dynamics (PCC 587): Feedbacks & Clouds DARGAN M. W. FRIERSON UNIVERSITY OF WASHINGTON, DEPARTMENT OF ATMOSPHERIC SCIENCES DAY 6: 10-14-13 Feedbacks Climate forcings change global temperatures directly
Climate Dynamics (PCC 587): Feedbacks & Clouds DARGAN M. W. FRIERSON UNIVERSITY OF WASHINGTON, DEPARTMENT OF ATMOSPHERIC SCIENCES DAY 6: 10-14-13 Feedbacks Climate forcings change global temperatures directly
Weather Forecasts and Climate AOSC 200 Tim Canty. Class Web Site: Lecture 27 Dec
 Weather Forecasts and Climate AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Climate Natural Variations Feedback Mechanisms Lecture 27 Dec 4 2018 1 Climate
Weather Forecasts and Climate AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Climate Natural Variations Feedback Mechanisms Lecture 27 Dec 4 2018 1 Climate
The Structure and Motion of the Atmosphere OCEA 101
 The Structure and Motion of the Atmosphere OCEA 101 Why should you care? - the atmosphere is the primary driving force for the ocean circulation. - the atmosphere controls geographical variations in ocean
The Structure and Motion of the Atmosphere OCEA 101 Why should you care? - the atmosphere is the primary driving force for the ocean circulation. - the atmosphere controls geographical variations in ocean
Climate Dynamics (PCC 587): Clouds and Feedbacks
 Climate Dynamics (PCC 587): Clouds and Feedbacks D A R G A N M. W. F R I E R S O N U N I V E R S I T Y O F W A S H I N G T O N, D E P A R T M E N T O F A T M O S P H E R I C S C I E N C E S D A Y 7 : 1
Climate Dynamics (PCC 587): Clouds and Feedbacks D A R G A N M. W. F R I E R S O N U N I V E R S I T Y O F W A S H I N G T O N, D E P A R T M E N T O F A T M O S P H E R I C S C I E N C E S D A Y 7 : 1
CLIMATE AND CLIMATE CHANGE MIDTERM EXAM ATM S 211 FEB 9TH 2012 V1
 CLIMATE AND CLIMATE CHANGE MIDTERM EXAM ATM S 211 FEB 9TH 2012 V1 Name: Student ID: Please answer the following questions on your Scantron Multiple Choice [1 point each] (1) The gases that contribute to
CLIMATE AND CLIMATE CHANGE MIDTERM EXAM ATM S 211 FEB 9TH 2012 V1 Name: Student ID: Please answer the following questions on your Scantron Multiple Choice [1 point each] (1) The gases that contribute to
Atmospheric Aerosol in High Latitudes: Linkages to Radiative Energy Balance and Hydrological Cycle
 Atmospheric Aerosol in High Latitudes: Linkages to Radiative Energy Balance and Hydrological Cycle Irina N. Sokolik School of Earth and Atmospheric Sciences Georgia Institute of Technology Atlanta, GA,
Atmospheric Aerosol in High Latitudes: Linkages to Radiative Energy Balance and Hydrological Cycle Irina N. Sokolik School of Earth and Atmospheric Sciences Georgia Institute of Technology Atlanta, GA,
The Atmosphere and Atmospheric Energy Chapter 3 and 4
 The Atmosphere and Atmospheric Energy Chapter 3 and 4 Size of the Earth s Atmosphere Atmosphere produced over 4.6 billion years of development Protects us from radiation Completely surrounds the earth
The Atmosphere and Atmospheric Energy Chapter 3 and 4 Size of the Earth s Atmosphere Atmosphere produced over 4.6 billion years of development Protects us from radiation Completely surrounds the earth
Chapter 14: The Changing Climate
 Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
Chapter 14: The Changing Climate Detecting Climate Change Natural Causes of Climate Change Anthropogenic Causes of Climate Change Possible Consequences of Global Warming Climate Change? -Paleo studies
Climate 1: The Climate System
 Climate 1: The Climate System Prof. Franco Prodi Institute of Atmospheric Sciences and Climate National Research Council Via P. Gobetti, 101 40129 BOLOGNA SIF, School of Energy, Varenna, July 2014 CLIMATE
Climate 1: The Climate System Prof. Franco Prodi Institute of Atmospheric Sciences and Climate National Research Council Via P. Gobetti, 101 40129 BOLOGNA SIF, School of Energy, Varenna, July 2014 CLIMATE
1. The frequency of an electromagnetic wave is proportional to its wavelength. a. directly *b. inversely
 CHAPTER 3 SOLAR AND TERRESTRIAL RADIATION MULTIPLE CHOICE QUESTIONS 1. The frequency of an electromagnetic wave is proportional to its wavelength. a. directly *b. inversely 2. is the distance between successive
CHAPTER 3 SOLAR AND TERRESTRIAL RADIATION MULTIPLE CHOICE QUESTIONS 1. The frequency of an electromagnetic wave is proportional to its wavelength. a. directly *b. inversely 2. is the distance between successive
Lecture 3: Global Energy Cycle
 Lecture 3: Global Energy Cycle Planetary energy balance Greenhouse Effect Vertical energy balance Latitudinal energy balance Seasonal and diurnal cycles Solar Flux and Flux Density Solar Luminosity (L)
Lecture 3: Global Energy Cycle Planetary energy balance Greenhouse Effect Vertical energy balance Latitudinal energy balance Seasonal and diurnal cycles Solar Flux and Flux Density Solar Luminosity (L)
( 1 d 2 ) (Inverse Square law);
 ATMO 336 -- Exam 3 120 total points including take-home essay Name The following equations and relationships may prove useful. F d1 =F d2 d 2 2 ( 1 d 2 ) (Inverse Square law);! MAX = 0.29 " 104 µmk (Wien's
ATMO 336 -- Exam 3 120 total points including take-home essay Name The following equations and relationships may prove useful. F d1 =F d2 d 2 2 ( 1 d 2 ) (Inverse Square law);! MAX = 0.29 " 104 µmk (Wien's
Lecture 2: Global Energy Cycle
 Lecture 2: Global Energy Cycle Planetary energy balance Greenhouse Effect Vertical energy balance Solar Flux and Flux Density Solar Luminosity (L) the constant flux of energy put out by the sun L = 3.9
Lecture 2: Global Energy Cycle Planetary energy balance Greenhouse Effect Vertical energy balance Solar Flux and Flux Density Solar Luminosity (L) the constant flux of energy put out by the sun L = 3.9
1. Weather and climate.
 Lecture 31. Introduction to climate and climate change. Part 1. Objectives: 1. Weather and climate. 2. Earth s radiation budget. 3. Clouds and radiation field. Readings: Turco: p. 320-349; Brimblecombe:
Lecture 31. Introduction to climate and climate change. Part 1. Objectives: 1. Weather and climate. 2. Earth s radiation budget. 3. Clouds and radiation field. Readings: Turco: p. 320-349; Brimblecombe:
2018 Science Olympiad: Badger Invitational Meteorology Exam. Team Name: Team Motto:
 2018 Science Olympiad: Badger Invitational Meteorology Exam Team Name: Team Motto: This exam has 50 questions of various formats, plus 3 tie-breakers. Good luck! 1. On a globally-averaged basis, which
2018 Science Olympiad: Badger Invitational Meteorology Exam Team Name: Team Motto: This exam has 50 questions of various formats, plus 3 tie-breakers. Good luck! 1. On a globally-averaged basis, which
Introduction to Climate Change
 Ch 19 Climate Change Introduction to Climate Change Throughout time, the earth's climate has always been changing produced ice ages Hence, climate variations have been noted in the past what physical processes
Ch 19 Climate Change Introduction to Climate Change Throughout time, the earth's climate has always been changing produced ice ages Hence, climate variations have been noted in the past what physical processes
Recent Climate History - The Instrumental Era.
 2002 Recent Climate History - The Instrumental Era. Figure 1. Reconstructed surface temperature record. Strong warming in the first and late part of the century. El Ninos and major volcanic eruptions are
2002 Recent Climate History - The Instrumental Era. Figure 1. Reconstructed surface temperature record. Strong warming in the first and late part of the century. El Ninos and major volcanic eruptions are
AT 350 EXAM #1 February 21, 2008
 This exam covers Ahrens Chapters 1 and 2, plus related lecture notes Write the letter of the choice that best completes the statement or answers the question. b_ 1. The Earth s atmosphere is currently
This exam covers Ahrens Chapters 1 and 2, plus related lecture notes Write the letter of the choice that best completes the statement or answers the question. b_ 1. The Earth s atmosphere is currently
Science of Global Warming and Climate Change
 Science of Global Warming and Climate Change Part 1 Science Dr. David H. Manz, P. Eng. University of Calgary May 2015 Weather vs. Climate Weather happens day to day (moment to moment) best forecast is
Science of Global Warming and Climate Change Part 1 Science Dr. David H. Manz, P. Eng. University of Calgary May 2015 Weather vs. Climate Weather happens day to day (moment to moment) best forecast is
Atmospheric Radiation
 Atmospheric Radiation NASA photo gallery Introduction The major source of earth is the sun. The sun transfer energy through the earth by radiated electromagnetic wave. In vacuum, electromagnetic waves
Atmospheric Radiation NASA photo gallery Introduction The major source of earth is the sun. The sun transfer energy through the earth by radiated electromagnetic wave. In vacuum, electromagnetic waves
climate change Contents CO 2 (ppm)
 climate change CO 2 (ppm) 2007 Joachim Curtius Institut für Physik der Atmosphäre Universität Mainz Contents 1. Summary 2. Background 3. Climate change: observations 4. CO 2 5. OtherGreenhouse Gases (GHGs):
climate change CO 2 (ppm) 2007 Joachim Curtius Institut für Physik der Atmosphäre Universität Mainz Contents 1. Summary 2. Background 3. Climate change: observations 4. CO 2 5. OtherGreenhouse Gases (GHGs):
Radiation in the atmosphere
 Radiation in the atmosphere Flux and intensity Blackbody radiation in a nutshell Solar constant Interaction of radiation with matter Absorption of solar radiation Scattering Radiative transfer Irradiance
Radiation in the atmosphere Flux and intensity Blackbody radiation in a nutshell Solar constant Interaction of radiation with matter Absorption of solar radiation Scattering Radiative transfer Irradiance
Aerosol Effects on Water and Ice Clouds
 Aerosol Effects on Water and Ice Clouds Ulrike Lohmann Department of Physics and Atmospheric Science, Dalhousie University, Halifax, N. S., Canada Contributions from Johann Feichter, Johannes Hendricks,
Aerosol Effects on Water and Ice Clouds Ulrike Lohmann Department of Physics and Atmospheric Science, Dalhousie University, Halifax, N. S., Canada Contributions from Johann Feichter, Johannes Hendricks,
Climate Change: Global Warming Claims
 Climate Change: Global Warming Claims Background information (from Intergovernmental Panel on Climate Change): The climate system is a complex, interactive system consisting of the atmosphere, land surface,
Climate Change: Global Warming Claims Background information (from Intergovernmental Panel on Climate Change): The climate system is a complex, interactive system consisting of the atmosphere, land surface,
What are Aerosols? Suspension of very small solid particles or liquid droplets Radii typically in the range of 10nm to
 What are Aerosols? Suspension of very small solid particles or liquid droplets Radii typically in the range of 10nm to 10µm Concentrations decrease exponentially with height N(z) = N(0)exp(-z/H) Long-lived
What are Aerosols? Suspension of very small solid particles or liquid droplets Radii typically in the range of 10nm to 10µm Concentrations decrease exponentially with height N(z) = N(0)exp(-z/H) Long-lived
Extremes of Weather and the Latest Climate Change Science. Prof. Richard Allan, Department of Meteorology University of Reading
 Extremes of Weather and the Latest Climate Change Science Prof. Richard Allan, Department of Meteorology University of Reading Extreme weather climate change Recent extreme weather focusses debate on climate
Extremes of Weather and the Latest Climate Change Science Prof. Richard Allan, Department of Meteorology University of Reading Extreme weather climate change Recent extreme weather focusses debate on climate
Spectrum of Radiation. Importance of Radiation Transfer. Radiation Intensity and Wavelength. Lecture 3: Atmospheric Radiative Transfer and Climate
 Lecture 3: Atmospheric Radiative Transfer and Climate Radiation Intensity and Wavelength frequency Planck s constant Solar and infrared radiation selective absorption and emission Selective absorption
Lecture 3: Atmospheric Radiative Transfer and Climate Radiation Intensity and Wavelength frequency Planck s constant Solar and infrared radiation selective absorption and emission Selective absorption
Lecture 3: Atmospheric Radiative Transfer and Climate
 Lecture 3: Atmospheric Radiative Transfer and Climate Solar and infrared radiation selective absorption and emission Selective absorption and emission Cloud and radiation Radiative-convective equilibrium
Lecture 3: Atmospheric Radiative Transfer and Climate Solar and infrared radiation selective absorption and emission Selective absorption and emission Cloud and radiation Radiative-convective equilibrium
FOLLOW THE ENERGY! EARTH S DYNAMIC CLIMATE SYSTEM
 Investigation 1B FOLLOW THE ENERGY! EARTH S DYNAMIC CLIMATE SYSTEM Driving Question How does energy enter, flow through, and exit Earth s climate system? Educational Outcomes To consider Earth s climate
Investigation 1B FOLLOW THE ENERGY! EARTH S DYNAMIC CLIMATE SYSTEM Driving Question How does energy enter, flow through, and exit Earth s climate system? Educational Outcomes To consider Earth s climate
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds. What is an atmosphere? About 10 km thick
 Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds What is an atmosphere? Sources of Gas Losses of Gas Thermal Escape Earth s Atmosphere About 10 km thick Consists mostly of molecular
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds What is an atmosphere? Sources of Gas Losses of Gas Thermal Escape Earth s Atmosphere About 10 km thick Consists mostly of molecular
Aerosol. Challenge: Global Warming. Observed warming during 20 th century, Tapio. 1910s. 1950s. 1990s T [Kelvin]
![Aerosol. Challenge: Global Warming. Observed warming during 20 th century, Tapio. 1910s. 1950s. 1990s T [Kelvin] Aerosol. Challenge: Global Warming. Observed warming during 20 th century, Tapio. 1910s. 1950s. 1990s T [Kelvin]](/thumbs/86/93830655.jpg) Aerosol Challenge: Global Warming 1910s 1950s 1990s 2 1 0 +1 +2 T [Kelvin] Observed warming during 20 th century, Tapio Schneider, J. Climate, 2001 1 Aerosols are liquid or solid particles suspended in
Aerosol Challenge: Global Warming 1910s 1950s 1990s 2 1 0 +1 +2 T [Kelvin] Observed warming during 20 th century, Tapio Schneider, J. Climate, 2001 1 Aerosols are liquid or solid particles suspended in
Data and formulas at the end. Exam would be Weds. May 8, 2008
 ATMS 321: Science of Climate Practice Mid Term Exam - Spring 2008 page 1 Atmospheric Sciences 321 Science of Climate Practice Mid-Term Examination: Would be Closed Book Data and formulas at the end. Exam
ATMS 321: Science of Climate Practice Mid Term Exam - Spring 2008 page 1 Atmospheric Sciences 321 Science of Climate Practice Mid-Term Examination: Would be Closed Book Data and formulas at the end. Exam
Climate Change. April 21, 2009
 Climate Change Chapter 16 April 21, 2009 Reconstructing Past Climates Techniques Glacial landscapes (fossils) CLIMAP (ocean sediment) Ice cores (layering of precipitation) p Otoliths (CaCO 3 in fish sensory
Climate Change Chapter 16 April 21, 2009 Reconstructing Past Climates Techniques Glacial landscapes (fossils) CLIMAP (ocean sediment) Ice cores (layering of precipitation) p Otoliths (CaCO 3 in fish sensory
Arctic Climate Change. Glen Lesins Department of Physics and Atmospheric Science Dalhousie University Create Summer School, Alliston, July 2013
 Arctic Climate Change Glen Lesins Department of Physics and Atmospheric Science Dalhousie University Create Summer School, Alliston, July 2013 When was this published? Observational Evidence for Arctic
Arctic Climate Change Glen Lesins Department of Physics and Atmospheric Science Dalhousie University Create Summer School, Alliston, July 2013 When was this published? Observational Evidence for Arctic
Arctic climate: Unique vulnerability and complex response to aerosols
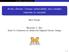 Arctic climate: Unique vulnerability and complex response to aerosols Mark Flanner November 2, 2011 Santa Fe Conference on Global and Regional Climate Change 1 / 18 Arctic: Unique vulnerability to positive
Arctic climate: Unique vulnerability and complex response to aerosols Mark Flanner November 2, 2011 Santa Fe Conference on Global and Regional Climate Change 1 / 18 Arctic: Unique vulnerability to positive
Impact of Atmoshpheric Brown Clouds (ABCs) on Agriculture. Dr.A.K.Gogoi, ADG(Agro) ICAR, New Delhi- 12
 Impact of Atmoshpheric Brown Clouds (ABCs) on Agriculture Dr.A.K.Gogoi, ADG(Agro) ICAR, New Delhi- 12 What are ABCs Atmospheric Brown Clouds (ABC s ) are regional scale slums of air pollution that consists
Impact of Atmoshpheric Brown Clouds (ABCs) on Agriculture Dr.A.K.Gogoi, ADG(Agro) ICAR, New Delhi- 12 What are ABCs Atmospheric Brown Clouds (ABC s ) are regional scale slums of air pollution that consists
Lecture Outlines PowerPoint. Chapter 16 Earth Science 11e Tarbuck/Lutgens
 Lecture Outlines PowerPoint Chapter 16 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
Lecture Outlines PowerPoint Chapter 16 Earth Science 11e Tarbuck/Lutgens 2006 Pearson Prentice Hall This work is protected by United States copyright laws and is provided solely for the use of instructors
Understanding the Greenhouse Effect
 EESC V2100 The Climate System spring 200 Understanding the Greenhouse Effect Yochanan Kushnir Lamont Doherty Earth Observatory of Columbia University Palisades, NY 1096, USA kushnir@ldeo.columbia.edu Equilibrium
EESC V2100 The Climate System spring 200 Understanding the Greenhouse Effect Yochanan Kushnir Lamont Doherty Earth Observatory of Columbia University Palisades, NY 1096, USA kushnir@ldeo.columbia.edu Equilibrium
ATMOSPHERIC ENERGY and GLOBAL TEMPERATURES. Physical Geography (Geog. 300) Prof. Hugh Howard American River College
 ATMOSPHERIC ENERGY and GLOBAL TEMPERATURES Physical Geography (Geog. 300) Prof. Hugh Howard American River College RADIATION FROM the SUN SOLAR RADIATION Primarily shortwave (UV-SIR) Insolation Incoming
ATMOSPHERIC ENERGY and GLOBAL TEMPERATURES Physical Geography (Geog. 300) Prof. Hugh Howard American River College RADIATION FROM the SUN SOLAR RADIATION Primarily shortwave (UV-SIR) Insolation Incoming
Aerosols AP sizes AP types Sources Sinks Amount and lifetime Aerosol radiative effects. Aerosols. Trude Storelvmo Aerosols 1 / 21
 Aerosols Trude Storelvmo Aerosols 1 / 21 Aerosols: Definition Definition of an aerosol: disperse system with air as carrier gas and a solid or liquid or a mixture of both as disperse phases. Aerosol particles
Aerosols Trude Storelvmo Aerosols 1 / 21 Aerosols: Definition Definition of an aerosol: disperse system with air as carrier gas and a solid or liquid or a mixture of both as disperse phases. Aerosol particles
GLOBAL WARMING AND THE GREENHOUSE EFFECT
 GLOBAL WARMING AND THE GREENHOUSE EFFECT Our planet temperature is warming significantly due to human activities? OR Last few years warming is part of a natural global cycle? 1998 : The warmest year on
GLOBAL WARMING AND THE GREENHOUSE EFFECT Our planet temperature is warming significantly due to human activities? OR Last few years warming is part of a natural global cycle? 1998 : The warmest year on
AT350 EXAM #1 September 23, 2003
 AT350 EXAM #1 September 23, 2003 Name and ID: Enter your name and student ID number on the answer sheet and on this exam. Record your answers to the questions by using a No. 2 pencil to completely fill
AT350 EXAM #1 September 23, 2003 Name and ID: Enter your name and student ID number on the answer sheet and on this exam. Record your answers to the questions by using a No. 2 pencil to completely fill
Prentice Hall EARTH SCIENCE. Tarbuck Lutgens
 Prentice Hall EARTH SCIENCE Tarbuck Lutgens Chapter 17 The Atmosphere: Structure and Temperature 17.1 Atmosphere Characteristics Composition of the Atmosphere Weather is constantly changing, and it refers
Prentice Hall EARTH SCIENCE Tarbuck Lutgens Chapter 17 The Atmosphere: Structure and Temperature 17.1 Atmosphere Characteristics Composition of the Atmosphere Weather is constantly changing, and it refers
Arctic Chemistry And Climate
 21 July 2016 Connaught Summer Institute 1 Arctic Chemistry And Climate Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
21 July 2016 Connaught Summer Institute 1 Arctic Chemistry And Climate Connaught Summer Institute 2016 William (Bill) Simpson Geophysical Institute and Department of Chemistry, University of Alaska Fairbanks
Torben Königk Rossby Centre/ SMHI
 Fundamentals of Climate Modelling Torben Königk Rossby Centre/ SMHI Outline Introduction Why do we need models? Basic processes Radiation Atmospheric/Oceanic circulation Model basics Resolution Parameterizations
Fundamentals of Climate Modelling Torben Königk Rossby Centre/ SMHI Outline Introduction Why do we need models? Basic processes Radiation Atmospheric/Oceanic circulation Model basics Resolution Parameterizations
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds. What is an atmosphere? Earth s Atmosphere. Atmospheric Pressure
 Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds
 Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric
Chapter 10 Planetary Atmospheres: Earth and the Other Terrestrial Worlds 10.1 Atmospheric Basics Our goals for learning What is an atmosphere? How does the greenhouse effect warm a planet? Why do atmospheric
Topic 6: Insolation and the Seasons
 Topic 6: Insolation and the Seasons Solar Radiation and Insolation Insolation: In Sol ation The Sun is the primary source of energy for the earth. The rate at which energy is radiated is called Intensity
Topic 6: Insolation and the Seasons Solar Radiation and Insolation Insolation: In Sol ation The Sun is the primary source of energy for the earth. The rate at which energy is radiated is called Intensity
Aerosol Basics: Definitions, size distributions, structure
 Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Aerosol Basics: Definitions, size distributions, structure Antti Lauri NetFAM Summer School Zelenogorsk, 9 July 2008 Department of Physics, Division of Atmospheric Sciences and Geophysics, University of
Satellite analysis of aerosol indirect effect on stratocumulus clouds over South-East Atlantic
 1/23 Remote sensing of atmospheric aerosol, clouds and aerosol-cloud interactions. Bremen, 16-19 December 2013 Satellite analysis of aerosol indirect effect on stratocumulus clouds over South-East Atlantic
1/23 Remote sensing of atmospheric aerosol, clouds and aerosol-cloud interactions. Bremen, 16-19 December 2013 Satellite analysis of aerosol indirect effect on stratocumulus clouds over South-East Atlantic
The inputs and outputs of energy within the earth-atmosphere system that determines the net energy available for surface processes is the Energy
 Energy Balance The inputs and outputs of energy within the earth-atmosphere system that determines the net energy available for surface processes is the Energy Balance Electromagnetic Radiation Electromagnetic
Energy Balance The inputs and outputs of energy within the earth-atmosphere system that determines the net energy available for surface processes is the Energy Balance Electromagnetic Radiation Electromagnetic
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NGEO1854 Anthropogenic aerosol forcing of Atlantic tropical storms N. J. Dunstone 1, D. S. Smith 1, B. B. B. Booth 1, L. Hermanson 1, R. Eade 1 Supplementary information
SUPPLEMENTARY INFORMATION DOI: 10.1038/NGEO1854 Anthropogenic aerosol forcing of Atlantic tropical storms N. J. Dunstone 1, D. S. Smith 1, B. B. B. Booth 1, L. Hermanson 1, R. Eade 1 Supplementary information
What is the IPCC? Intergovernmental Panel on Climate Change
 IPCC WG1 FAQ What is the IPCC? Intergovernmental Panel on Climate Change The IPCC is a scientific intergovernmental body set up by the World Meteorological Organization (WMO) and by the United Nations
IPCC WG1 FAQ What is the IPCC? Intergovernmental Panel on Climate Change The IPCC is a scientific intergovernmental body set up by the World Meteorological Organization (WMO) and by the United Nations
ATM S 111 Global Warming Exam Review. Jennifer Fletcher Day 31, August 3, 2010
 ATM S 111 Global Warming Exam Review Jennifer Fletcher Day 31, August 3, 2010 Earth gets most of its energy from the sun. Solar Radiation Solar radiation is mostly in visible, near infrared, and near UV
ATM S 111 Global Warming Exam Review Jennifer Fletcher Day 31, August 3, 2010 Earth gets most of its energy from the sun. Solar Radiation Solar radiation is mostly in visible, near infrared, and near UV
A) usually less B) dark colored and rough D) light colored with a smooth surface A) transparency of the atmosphere D) rough, black surface
 1. Base your answer to the following question on the diagram below which shows two identical houses, A and B, in a city in North Carolina. One house was built on the east side of a factory, and the other
1. Base your answer to the following question on the diagram below which shows two identical houses, A and B, in a city in North Carolina. One house was built on the east side of a factory, and the other
Day 1 of Global Warming. Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings
 Day 1 of Global Warming Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Atmosphere Atmosphere = the thin layer (1/100 th of Earth s diameter) of gases that surrounds
Day 1 of Global Warming Copyright 2008 Pearson Education, Inc., publishing as Pearson Benjamin Cummings The Atmosphere Atmosphere = the thin layer (1/100 th of Earth s diameter) of gases that surrounds
Remote Sensing C. Rank: Points: Science Olympiad North Regional Tournament at the University of Florida. Name(s): Team Name: School Name:
 Remote Sensing C Science Olympiad North Regional Tournament at the University of Florida Rank: Points: Name(s): Team Name: School Name: Team Number: Instructions: DO NOT BEGIN UNTIL GIVEN PERMISSION. DO
Remote Sensing C Science Olympiad North Regional Tournament at the University of Florida Rank: Points: Name(s): Team Name: School Name: Team Number: Instructions: DO NOT BEGIN UNTIL GIVEN PERMISSION. DO
ATM S 111: Global Warming Solar Radiation. Jennifer Fletcher Day 2: June
 ATM S 111: Global Warming Solar Radiation Jennifer Fletcher Day 2: June 22 2010 Yesterday We Asked What factors influence climate at a given place? Sunshine (and latitude) Topography/mountains Proximity
ATM S 111: Global Warming Solar Radiation Jennifer Fletcher Day 2: June 22 2010 Yesterday We Asked What factors influence climate at a given place? Sunshine (and latitude) Topography/mountains Proximity
HEATING THE ATMOSPHERE
 HEATING THE ATMOSPHERE Earth and Sun 99.9% of Earth s heat comes from Sun But
HEATING THE ATMOSPHERE Earth and Sun 99.9% of Earth s heat comes from Sun But
Lecture 4: Heat, and Radiation
 Lecture 4: Heat, and Radiation Heat Heat is a transfer of energy from one object to another. Heat makes things warmer. Heat is measured in units called calories. A calorie is the heat (energy) required
Lecture 4: Heat, and Radiation Heat Heat is a transfer of energy from one object to another. Heat makes things warmer. Heat is measured in units called calories. A calorie is the heat (energy) required
Class Web Site:
 Modeling Earth s Climate: Water Vapor, Cloud, Lapse Rate, & Surface Albedo Feedbacks as well as Effect of Aerosols on Clouds ACC 433/633 & CHEM 433 Ross Salawitch Class Web Site: http://www.atmos.umd.edu/~rjs/class/spr2017
Modeling Earth s Climate: Water Vapor, Cloud, Lapse Rate, & Surface Albedo Feedbacks as well as Effect of Aerosols on Clouds ACC 433/633 & CHEM 433 Ross Salawitch Class Web Site: http://www.atmos.umd.edu/~rjs/class/spr2017
Major climate change triggers
 Major climate change triggers Variations in solar output Milankovitch cycles Elevation & distribution of continents Ocean interactions Atmospheric composition change (CO 2 and other volcanic gasses) Biological
Major climate change triggers Variations in solar output Milankovitch cycles Elevation & distribution of continents Ocean interactions Atmospheric composition change (CO 2 and other volcanic gasses) Biological
Lecture 2: Global Energy Cycle
 Lecture 2: Global Energy Cycle Planetary energy balance Greenhouse Effect Selective absorption Vertical energy balance Solar Flux and Flux Density Solar Luminosity (L) the constant flux of energy put out
Lecture 2: Global Energy Cycle Planetary energy balance Greenhouse Effect Selective absorption Vertical energy balance Solar Flux and Flux Density Solar Luminosity (L) the constant flux of energy put out
Solar Flux and Flux Density. Lecture 2: Global Energy Cycle. Solar Energy Incident On the Earth. Solar Flux Density Reaching Earth
 Lecture 2: Global Energy Cycle Solar Flux and Flux Density Planetary energy balance Greenhouse Effect Selective absorption Vertical energy balance Solar Luminosity (L) the constant flux of energy put out
Lecture 2: Global Energy Cycle Solar Flux and Flux Density Planetary energy balance Greenhouse Effect Selective absorption Vertical energy balance Solar Luminosity (L) the constant flux of energy put out
Earth s Heat Budget. What causes the seasons? Seasons
 Earth s Heat Budget Solar energy and the global heat budget Transfer of heat drives weather and climate Ocean circulation A. Rotation of the Earth B. Distance from the Sun C. Variations of Earth s orbit
Earth s Heat Budget Solar energy and the global heat budget Transfer of heat drives weather and climate Ocean circulation A. Rotation of the Earth B. Distance from the Sun C. Variations of Earth s orbit
Features of Global Warming Review. GEOG/ENST 2331 Lecture 23 Ahrens: Chapter 16
 Features of Global Warming Review GEOG/ENST 2331 Lecture 23 Ahrens: Chapter 16 The Greenhouse Effect 255 K 288 K Ahrens, Fig. 2.12 What can change the global energy balance? Incoming energy Solar strength
Features of Global Warming Review GEOG/ENST 2331 Lecture 23 Ahrens: Chapter 16 The Greenhouse Effect 255 K 288 K Ahrens, Fig. 2.12 What can change the global energy balance? Incoming energy Solar strength
Climate Change 2007: The Physical Science Basis
 Climate Change 2007: The Physical Science Basis Working Group I Contribution to the IPCC Fourth Assessment Report Presented by R.K. Pachauri, IPCC Chair and Bubu Jallow, WG 1 Vice Chair Nairobi, 6 February
Climate Change 2007: The Physical Science Basis Working Group I Contribution to the IPCC Fourth Assessment Report Presented by R.K. Pachauri, IPCC Chair and Bubu Jallow, WG 1 Vice Chair Nairobi, 6 February
Climate Variability and Change Past, Present and Future An Overview
 Climate Variability and Change Past, Present and Future An Overview Dr Jim Salinger National Institute of Water and Atmospheric Research Auckland, New Zealand INTERNATIONAL WORKSHOP ON REDUCING VULNERABILITY
Climate Variability and Change Past, Present and Future An Overview Dr Jim Salinger National Institute of Water and Atmospheric Research Auckland, New Zealand INTERNATIONAL WORKSHOP ON REDUCING VULNERABILITY
The Atmosphere Made up of mainly two gases: Nitrogen 78% Oxygen 21% Trace Gases 1%
 The Atmosphere 18.1 The Atmosphere Made up of mainly two gases: Nitrogen 78% Oxygen 21% Trace Gases 1% Layers of the Atmosphere made made up of 5 layers: Troposphere Stratosphere Mesosphere Ionosphere
The Atmosphere 18.1 The Atmosphere Made up of mainly two gases: Nitrogen 78% Oxygen 21% Trace Gases 1% Layers of the Atmosphere made made up of 5 layers: Troposphere Stratosphere Mesosphere Ionosphere
Atmosphere Weather and Climate
 Atmosphere Weather and Climate Weather and Climate Weather Atmospheric conditions at a particular time and place Climate Long-term average of weather conditions Often over decades or centuries Coastal
Atmosphere Weather and Climate Weather and Climate Weather Atmospheric conditions at a particular time and place Climate Long-term average of weather conditions Often over decades or centuries Coastal
Balancing planetary energy budgets
 Balancing planetary energy budgets Energy transfer, by radiation and other processes, takes place in a planet s and at its surface, and drives its climate system. How do these processes differ between
Balancing planetary energy budgets Energy transfer, by radiation and other processes, takes place in a planet s and at its surface, and drives its climate system. How do these processes differ between
2/22/ Atmospheric Characteristics
 17.1 Atmospheric Characteristics Atmosphere: the gaseous layer that surrounds the Earth I. In the past, gases came from volcanic eruptions A. Water vapor was a major component of outgassing B. Other gases
17.1 Atmospheric Characteristics Atmosphere: the gaseous layer that surrounds the Earth I. In the past, gases came from volcanic eruptions A. Water vapor was a major component of outgassing B. Other gases
Effective radiative forcing in the aerosol climate model CAM5.3- MARC-ARG
 Effective radiative forcing in the aerosol climate model CAM5.3- MARC-ARG Benjamin S. Grandey 1, Daniel Rothenberg 2, Alexander Avramov 2,3, Qinjian Jin 2, Hsiang-He Lee 1, Xiaohong Liu 4, Zheng Lu 4,
Effective radiative forcing in the aerosol climate model CAM5.3- MARC-ARG Benjamin S. Grandey 1, Daniel Rothenberg 2, Alexander Avramov 2,3, Qinjian Jin 2, Hsiang-He Lee 1, Xiaohong Liu 4, Zheng Lu 4,
Thursday, November 1st.
 Thursday, November 1st. Announcements. Homework 7 - due Tuesday, Nov. 6 Homework 8 - paper 2 topics, questions and sources due Tuesday, Nov. 13 Midterm Paper 2 - due Tuesday, Nov. 20 I will hand out a
Thursday, November 1st. Announcements. Homework 7 - due Tuesday, Nov. 6 Homework 8 - paper 2 topics, questions and sources due Tuesday, Nov. 13 Midterm Paper 2 - due Tuesday, Nov. 20 I will hand out a
CLIMATE CHANGE Albedo Forcing ALBEDO FORCING
 ALBEDO FORCING Albedo forcing is the hypothesis that variations in the Earth s reflectance of solar radiation can bring about global climate change. This hypothesis is undeniable in principle; since virtually
ALBEDO FORCING Albedo forcing is the hypothesis that variations in the Earth s reflectance of solar radiation can bring about global climate change. This hypothesis is undeniable in principle; since virtually
3. Carbon Dioxide (CO 2 )
 3. Carbon Dioxide (CO 2 ) Basic information on CO 2 with regard to environmental issues Carbon dioxide (CO 2 ) is a significant greenhouse gas that has strong absorption bands in the infrared region and
3. Carbon Dioxide (CO 2 ) Basic information on CO 2 with regard to environmental issues Carbon dioxide (CO 2 ) is a significant greenhouse gas that has strong absorption bands in the infrared region and
Why is it difficult to predict climate? Understanding current scientific challenges
 Why is it difficult to predict climate? Understanding current scientific challenges Akua Asa-Awuku October 22, 2009 Global Climate Change (GCC) Workshop University of California - Riverside Bourns College
Why is it difficult to predict climate? Understanding current scientific challenges Akua Asa-Awuku October 22, 2009 Global Climate Change (GCC) Workshop University of California - Riverside Bourns College
T eff = [F s (1 - A)/(4σ)] ¼ = K.
![T eff = [F s (1 - A)/(4σ)] ¼ = K. T eff = [F s (1 - A)/(4σ)] ¼ = K.](/thumbs/82/85857260.jpg) Lectures 16-18. Sci A-30 8. 10 & 15 April 2008 The Planck function gives the energy emission rate for an object, which is a function of it's temperature. The earth exchanges energy with it's environment
Lectures 16-18. Sci A-30 8. 10 & 15 April 2008 The Planck function gives the energy emission rate for an object, which is a function of it's temperature. The earth exchanges energy with it's environment
Lecture 9: Climate Sensitivity and Feedback Mechanisms
 Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Lecture 9: Climate Sensitivity and Feedback Mechanisms Basic radiative feedbacks (Plank, Water Vapor, Lapse-Rate Feedbacks) Ice albedo & Vegetation-Climate feedback Cloud feedback Biogeochemical feedbacks
Mon April 17 Announcements: bring calculator to class from now on (in-class activities, tests) HW#2 due Thursday
 Mon April 17 Announcements: bring calculator to class from now on (in-class activities, tests) HW#2 due Thursday Today: Fundamentals of Planetary Energy Balance Incoming = Outgoing (at equilibrium) Incoming
Mon April 17 Announcements: bring calculator to class from now on (in-class activities, tests) HW#2 due Thursday Today: Fundamentals of Planetary Energy Balance Incoming = Outgoing (at equilibrium) Incoming
Effects of Black Carbon on Temperature Lapse Rates
 Effects of Black Carbon on Temperature Lapse Rates Joyce E. Penner 1 Minghuai Wang 1, Akshay Kumar 1, Leon Rotstayn 2, Ben Santer 1 University of Michigan, 2 CSIRO, 3 LLNL Thanks to Warren Washington and
Effects of Black Carbon on Temperature Lapse Rates Joyce E. Penner 1 Minghuai Wang 1, Akshay Kumar 1, Leon Rotstayn 2, Ben Santer 1 University of Michigan, 2 CSIRO, 3 LLNL Thanks to Warren Washington and
7/5/2018. Global Climate Change
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Global Climate Change Earth, Chapter 21 Chapter 21 Global Climate Change Climate and Geology The climate system is a multidimensional system of many interacting parts,
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Global Climate Change Earth, Chapter 21 Chapter 21 Global Climate Change Climate and Geology The climate system is a multidimensional system of many interacting parts,
Lecture 2 Global and Zonal-mean Energy Balance
 Lecture 2 Global and Zonal-mean Energy Balance A zero-dimensional view of the planet s energy balance RADIATIVE BALANCE Roughly 70% of the radiation received from the Sun at the top of Earth s atmosphere
Lecture 2 Global and Zonal-mean Energy Balance A zero-dimensional view of the planet s energy balance RADIATIVE BALANCE Roughly 70% of the radiation received from the Sun at the top of Earth s atmosphere
Science Challenges of a Changing Climate
 Science Challenges of a Changing Climate Julia Slingo Met Office Chief Scientist Global Warming Fact or Fiction? Future changes will be outside anything experienced over the last 1000 years Global Warming
Science Challenges of a Changing Climate Julia Slingo Met Office Chief Scientist Global Warming Fact or Fiction? Future changes will be outside anything experienced over the last 1000 years Global Warming
2. Fargo, North Dakota receives more snow than Charleston, South Carolina.
 2015 National Tournament Division B Meteorology Section 1: Weather versus Climate Chose the answer that best answers the question 1. The sky is partly cloudy this morning in Lincoln, Nebraska. 2. Fargo,
2015 National Tournament Division B Meteorology Section 1: Weather versus Climate Chose the answer that best answers the question 1. The sky is partly cloudy this morning in Lincoln, Nebraska. 2. Fargo,
Lecture # 04 January 27, 2010, Wednesday Energy & Radiation
 Lecture # 04 January 27, 2010, Wednesday Energy & Radiation Kinds of energy Energy transfer mechanisms Radiation: electromagnetic spectrum, properties & principles Solar constant Atmospheric influence
Lecture # 04 January 27, 2010, Wednesday Energy & Radiation Kinds of energy Energy transfer mechanisms Radiation: electromagnetic spectrum, properties & principles Solar constant Atmospheric influence
Chapter 3. Multiple Choice Questions
 Chapter 3 Multiple Choice Questions 1. In the case of electromagnetic energy, an object that is hot: a. radiates much more energy than a cool object b. radiates much less energy than a cool object c. radiates
Chapter 3 Multiple Choice Questions 1. In the case of electromagnetic energy, an object that is hot: a. radiates much more energy than a cool object b. radiates much less energy than a cool object c. radiates
Cloud Brightening and Climate Change
 Cloud Brightening and Climate Change 89 Hannele Korhonen and Antti-Ilari Partanen Contents Definitions... 778 Aerosols and Cloud Albedo... 778 Cloud Brightening with Sea-Salt Aerosol... 779 Climate Effects
Cloud Brightening and Climate Change 89 Hannele Korhonen and Antti-Ilari Partanen Contents Definitions... 778 Aerosols and Cloud Albedo... 778 Cloud Brightening with Sea-Salt Aerosol... 779 Climate Effects
Assessment Schedule 2017 Earth and Space Science: Demonstrate understanding of processes in the atmosphere system (91414)
 NCEA Level 3 Earth and Space Science (91414) 2017 page 1 of 6 Assessment Schedule 2017 Earth and Space Science: Demonstrate understanding of processes in the atmosphere system (91414) Evidence Statement
NCEA Level 3 Earth and Space Science (91414) 2017 page 1 of 6 Assessment Schedule 2017 Earth and Space Science: Demonstrate understanding of processes in the atmosphere system (91414) Evidence Statement
Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds. What is an atmosphere? Planetary Atmospheres
 Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds What is an atmosphere? Planetary Atmospheres Pressure Composition Greenhouse effect Atmospheric structure Color of the sky 1 Atmospheres
Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds What is an atmosphere? Planetary Atmospheres Pressure Composition Greenhouse effect Atmospheric structure Color of the sky 1 Atmospheres
All objects emit radiation. Radiation Energy that travels in the form of waves Waves release energy when absorbed by an object. Earth s energy budget
 Radiation Energy that travels in the form of waves Waves release energy when absorbed by an object Example: Sunlight warms your face without necessarily heating the air Shorter waves carry more energy
Radiation Energy that travels in the form of waves Waves release energy when absorbed by an object Example: Sunlight warms your face without necessarily heating the air Shorter waves carry more energy
Australian Meteorological and Oceanographic Society (AMOS) Statement on Climate Change
 Australian Meteorological and Oceanographic Society (AMOS) Statement on Climate Change This statement provides a summary of some aspects of climate change and its uncertainties, with particular focus on
Australian Meteorological and Oceanographic Society (AMOS) Statement on Climate Change This statement provides a summary of some aspects of climate change and its uncertainties, with particular focus on
Energy Systems, Structures and Processes Essential Standard: Analyze patterns of global climate change over time Learning Objective: Differentiate
 Energy Systems, Structures and Processes Essential Standard: Analyze patterns of global climate change over time Learning Objective: Differentiate between weather and climate Global Climate Focus Question
Energy Systems, Structures and Processes Essential Standard: Analyze patterns of global climate change over time Learning Objective: Differentiate between weather and climate Global Climate Focus Question
Chapter 11 Lecture Outline. Heating the Atmosphere
 Chapter 11 Lecture Outline Heating the Atmosphere They are still here! Focus on the Atmosphere Weather Occurs over a short period of time Constantly changing Climate Averaged over a long period of time
Chapter 11 Lecture Outline Heating the Atmosphere They are still here! Focus on the Atmosphere Weather Occurs over a short period of time Constantly changing Climate Averaged over a long period of time
Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds
 Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds What is an atmosphere? 10.1 Atmospheric Basics Our goals for learning:! What is an atmosphere?! How does the greenhouse effect warm
Chapter 10 Planetary Atmospheres Earth and the Other Terrestrial Worlds What is an atmosphere? 10.1 Atmospheric Basics Our goals for learning:! What is an atmosphere?! How does the greenhouse effect warm
The flux density of solar radiation at the Earth s surface, on a horizontal plane, is comprised of a fraction of direct beam and diffuse radiation
 Instructor: Dennis Baldocchi Professor of Biometeorology Ecosystem Science Division Department of Environmental Science, Policy and Management 35 Hilgard Hall University of California, Berkeley Berkeley,
Instructor: Dennis Baldocchi Professor of Biometeorology Ecosystem Science Division Department of Environmental Science, Policy and Management 35 Hilgard Hall University of California, Berkeley Berkeley,
Polar regions Temperate Regions Tropics High ( cirro ) 3-8 km 5-13 km 6-18 km Middle ( alto ) 2-4 km 2-7 km 2-8 km Low ( strato ) 0-2 km 0-2 km 0-2 km
 Clouds and Climate Clouds (along with rain, snow, fog, haze, etc.) are wet atmospheric aerosols. They are made up of tiny spheres of water from 2-100 m which fall with terminal velocities of a few cm/sec.
Clouds and Climate Clouds (along with rain, snow, fog, haze, etc.) are wet atmospheric aerosols. They are made up of tiny spheres of water from 2-100 m which fall with terminal velocities of a few cm/sec.
2/18/2013 Estimating Climate Sensitivity From Past Climates Outline
 Estimating Climate Sensitivity From Past Climates Outline Zero-dimensional model of climate system Climate sensitivity Climate feedbacks Forcings vs. feedbacks Paleocalibration vs. paleoclimate modeling
Estimating Climate Sensitivity From Past Climates Outline Zero-dimensional model of climate system Climate sensitivity Climate feedbacks Forcings vs. feedbacks Paleocalibration vs. paleoclimate modeling
