Single-Cell Analysis of Drosophila Larval Neuromuscular Synapses
|
|
|
- Nicholas Lawson
- 6 years ago
- Views:
Transcription
1 Developmental Biology 229, (2001) doi: /dbio , available online at on Single-Cell Analysis of Drosophila Larval Neuromuscular Synapses Bao Hoang 1 and Akira Chiba 2 Department of Cell and Structural Biology, University of Illinois, Urbana, Illinois The neuromuscular system of Drosophila has been widely used in studies on synaptic development. In the embryo, the cellular components of this model system are well established, with uniquely identified motoneurons displaying specific connectivity with distinct muscles. Such knowledge is essential to analyzing axon guidance and synaptic matching mechanisms with single-cell resolution. In contrast, to date the cellular identities of the larval neuromuscular synapses are hardly established. It is not known whether synaptic connections seen in the embryo persist, nor is it known how individual motor endings may differentiate through the larval stages. In this study, we combine single-cell dye labeling of individual synaptic boutons and counterstaining of the entire nervous system to characterize the synaptic partners and bouton differentiation of the 30 motoneuron axons from four nerve branches (ISN, SNa, SNb, and SNd). We also show the cell body locations of 4 larval motoneurons (RP3, RP5, V, and MN13-Ib) and the types of innervation they develop. Our observations support the following: (1) Only 1 motoneuron axon of a given bouton type innervates a single muscle, while up to 4 motoneuron axons of different bouton types can innervate the same muscle. (2) The type of boutons which each motoneuron axon forms is likely influenced by cell-autonomous factors. The data offer a basis for studying the properties of synaptic differentiation, maintenance, and plasticity with a high cellular resolution Academic Press Key Words: bouton; Drosophila; larva; motoneuron; neuromuscular; single cell; synapse. INTRODUCTION The Drosophila nervous system, with its simplicity and easy access to genetics, has helped developmental neurobiologists in various studies. In the embryo, the overall body organization follows that of segmented animals. The second through seventh abdominal segments develop according to a bilaterally symmetric and essentially reiterated molecular and genetic program (Keshishian et al., 1996; Keshishian and Chiba, 1993). This means that one can observe the same sequence of events repeated multiple times within a single animal and also within a population of isogenic animals, facilitating quantitative analysis of the development of a single neuron and its synapse. In each embryonic halfsegment, there are 30 skeletal muscles with various orientations and positions, each of which is a multinucleated cell resulting from the fusion of about a dozen 1 Current address: Department of Medicine, UCLA School of Medicine, Los Angeles, CA To whom correspondence should be addressed at B605 CLS Laboratory, 601 South Goodwin Avenue, Urbana, IL Fax: (217) a-chiba@uiuc.edu. myoblasts (Fig. 1B). Recent studies reveal 32 motoneurons that innervate specific muscles in late stage embryos (Landgraf et al., 1997; Schmid et al., 1999). The motoneuron axons choose from six different nerve branches before reaching their specific target muscles (Fig. 1B). The advantage of the embryonic neuromuscular system is that the neurons and muscles are each uniquely identifiable and that their total number is vastly smaller than that in the vertebrate brain and musculature. Therefore, one can discuss, for example, how a particular motoneuron called the RP3 motoneuron interacts with particular muscles known as muscules 6 and 7 (Chiba and Rose, 1998; Halpern et al., 1991; Sink and Whitington, 1991b). This situation is entirely different from in vitro neuronal culture and many other in vivo nervous systems, in which one can discuss cellular interactions between a group of neurons and a group of muscles in only more or less generic terms. In studies in which one must deal with how the network specificity of neurons in the brain emerges, the ability to distinguish the unique molecular personalities of individual cellular components becomes essential. The embryonic neuromuscular system of Drosophila has proven invaluable for advancing our under /01 $35.00 Copyright 2001 by Academic Press All rights of reproduction in any form reserved. 55
2 56 Hoang and Chiba standing of such topics as axon guidance and synaptic target recognition (Chiba, 1999; Chiba and Keshishian, 1996). The subsequent 4-day period of larval development has also proven to be a convenient model to study synaptic function, differentiation, and plasticity (Budnik, 1996; Davis and Goodman, 1998; Keshishian et al., 1994). The same set of 30 muscles persists through all larval stages, during which their volume increases over a thousandfold (Budnik, 1996; Johansen et al., 1989a). However, except for one recent study by Lnenicka and Keshishian (2000) which focuses on two specific larval motoneuron terminals, the major drawback of larval studies so far has been that the axon terminals have been identified only collectively by their synaptic bouton differentiation as scored with immunocytochemistry, instead of relying on single-cell labeling methods as has been done repeatedly with embryos (e.g., Fig. 2A). The axon terminals that form predominantly large synaptic boutons, or the presynaptic swellings, are named type I, while those that form small boutons are called type II (Johansen et al., 1989a). Type I axon terminals are further subdivided into type Ib ( big type I) and type Is ( small type I) (Atwood et al., 1993; Budnik, 1996). Additionally, a type III innervation, found only on a single muscle, is named according to the presence of insulin in the boutons (Gorczyca et al., 1993; Jia et al., 1993). Based on physiological differences, it has been proposed that type Ib synapses resemble the crustacean tonic nerve endings while type Is synapses may correspond to the phasic terminals (Atwood et al., 1993; Kidokoro and Nishikawa, 1994; Kurdyak et al., 1994; Steward et al., 1996). Despite such an obvious heterogeneity among the larval neuromuscular synapses, analyses have routinely relied on visualization that utilizes panneural antibodies which label motoneurons collectively. For example, the motoneuron axon that innervates muscle 12 or 13 in the larva, instead of being given a specific name, is simply known as a type I axon or a type II axon (Fig. 2A). As a result, researchers have a limited ability to appreciate the genetic diversity that defines the type of synapse into which a particular motoneuron ending matures, test whether some synaptic boutons form on a muscle at the expense of excluding others, determine the extent to which the programming for axon terminal differentiation operates cell autonomously, or ask why certain manipulations affect a specific synapse more adversely than others. This study begins to unravel the synaptic connectivity and differentiation patterns of individual motoneuron axons that exist in the larval neuromuscular system of Drosophila. It combines a retrograde single-cell dye labeling technique with the additional use of panneuronal antibodies. The intracellular dye which, except for rare cases, fails to travel back to the cell body nevertheless provides a powerful means to visualize the peripheral innervation of individual motoneurons in the Drosophila larva. When combined with panneuronal immunocytochemistry (Jan and Jan, 1982), this offers an accurate comparison between the injected neuron and the other neurons that co-innervate the same target muscle. The results shown here represent the first systematic attempt to characterize the synaptic terminals of individual motoneurons in the larval Drosophila neuromuscular system. They illuminate the specificity of axon terminal differentiation that is achieved during the larval development. MATERIALS AND METHODS Dye injection. Each wild-type (Canton S) wandering thirdinstar larva, approximately 4 days from the time of hatching, was dissected and filleted under calcium-free insect saline (Johansen et al., 1989a,b). To access the boutons of the external muscles, some internal muscles were surgically removed with a glass micropipette and a pair of microtweezers. The larva was then fixed for 90 s with 4% paraformaldehyde in phosphate-buffered saline, rinsed with saline, and reacted overnight with FITC-conjugated anti-hrp antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) at 4 C. After being rinsed once again with saline, the fluorescently labeled synaptic boutons were viewed with epifluorescence microscopy (Fig. 2B) and then a single bouton was impaled and injected with 5 10% Lucifer yellow with a 0.2- to 3.0-nA negative current for min (Fig. 2C) according to a standard iontophoretic method (Cash et al., 1992). Immunocytochemistry. After dye injection, the larva was fixed again for 30 min with 4% paraformaldehyde and reacted with anti-lucifer yellow antibodies (dilution 1:100; Molecular Probes, Eugene, OR) followed by TRITC-conjugated secondary antibodies (for enhanced visualization of the injected Lucifer yellow) and FITC-conjugated anti-hrp (for visualization of the entire nervous system; Molecular Probes). Images were digitally acquired with the IPLab software (Scanalytics, Fairfax, VA) using a cooled CCD camera (Hamatsu C5985) and processed with Adobe PhotoShop. RESULTS The Drosophila embryonic/larval neuromuscular system exhibits bilaterally symmetric and segmentally reiterated organization (Fig. 1). Thirty distinct muscles persist throughout the embryonic and larval stages (Fig. 1B). These muscles are innervated by motoneuron axons that are grouped into six major nerve branches: ISN (intersegmental nerve branch), SNa (segmental nerve branch a), SNb (segmental nerve branch b, also known as ISNb ), SNc (segmental nerve branch c), SNd (segmental nerve branch d), and TN (transverse nerve) (Fig. 1B). While a total of 32 embryonic motoneurons establish neuromuscular synapses at the end of embryogenesis (Landgraf et al., 1997; Schmid et al., 1999), there has been no direct study on the individual motoneurons in the subsequent larval stages when both the synaptic terminals and the target muscles undergo extensive growth and differentiation. To establish a basis for interpreting the effects of various experimental manipulations on individual motoneurons and their synapses within the larval neuromuscular system, we visualized individual motoneuron axons by systematically impaling single synaptic boutons and then ionto-
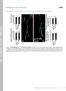
3 Synaptic Differentiation of Single Motoneurons 57 FIG. 1. Neuromuscular system of the Drosophila embryo and the larva. The bilaterally symmetrical and segmentally reiterated organization of the CNS (A) and the body wall muscles (B). The schematic shows the dorsal view of a fillet-dissected preparation after a dorsal midline incision. Anterior is to the top. The second abdominal right half-segment (A2) is highlighted. Each CNS segment has a dorsal midline nerve exit, from which the bilaterally symmetric TN nerve branches extend, and a pair of lateral nerves exit on each side, from which the ISN, SNa, SNb, SNc, and SNd nerve branches arise. Thirty muscles (1 30) in each half segment are innervated by the motoneurons through these six nerve branches. phoretically injecting fluorescent dye (Lucifer yellow) into the presynaptic axon terminals (Fig. 2C; see Materials and Methods). The dye diffuses through the axonal cytoplasm and the single motoneuron axon terminal can be unambiguously labeled in the background of the DIC optics and the panneural immunocytochemistry (see below). However, due to the very fragile nature of unfixed axonal plasma membrane, we rarely observed the dye to spread fully back to the cell body, typically being several hundred micrometers away from the axon terminal. Therefore, unless otherwise indicated, our data consist of the cases in which the dye revealed the axon terminal morphology but failed to reach the cell body. We followed each injection with immunocytochemical visualization of both the intracellular dye (with anti-lucifer yellow antibodies) and the panneuronal cell surface antigen (with anti-hrp antibodies) (see Materials and Methods). This double visualization permitted visual comparisons of the injected axons against all the axon terminals present in the system (Figs. 2B and 2C; also Figs. 3 5). In this way, we determined (1) the numbers of motoneuron axons that synapse on each muscle, (2) the types of boutons that each motoneuron axon develops, and (3) the specific muscle(s) with which each motor axon develops synaptic contacts. Our description, while adopting the conventional classification of synaptic differentiations into four synaptic bouton types (see below for our working definition), addresses the following specific issues which have been difficult to resolve previously. Type Ib Type Ib boutons are large (about 3 6 m) and the terminals bearing them tend to be short and minimally branching (Fig. 2A). They are glutamatergic and are found on all muscles of a mature larva (DiAntonio et al., 1999; Guan et al., 1996; Johansen et al., 1989a,b; Nishikawa and Kidokoro, 1995). We ask whether each type Ib motor axon innervates a single or multiple muscle(s) and whether each muscle receives innervation from one or multiple type Ib motoneuron axon(s). Type Is Type Is boutons are, on the average, slightly smaller (about 2 4 m) than type Ib boutons, and the terminals bearing them can often be longer and more elaborate than those with type Ib boutons (Fig. 2A). While generally thought to exist on all muscles (Budnik, 1996; Johansen et al., 1989a), there are controversies as to whether type Is boutons can be classified as Ib, Is, or even II (see below for our working definition). Type Is boutons are also glutamatergic (DiAntonio et al., 1999; Guan et al., 1996; Johansen et al., 1989a,b; Nishikawa and Kidokoro, 1995). We ask the same set of questions as applied to the type Ib boutons. Type II Type II boutons are small (about 1 2 m) and the terminals bearing them are very long and the most elaborate of all axon terminal types (Fig. 2A). Type II boutons are thought to be on most muscles (Johansen et al., 1989a; Monastirioti et al., 1995). Whether their choice of neurotransmitters includes glutamate and octopamine is not yet fully resolved (Johansen et al., 1989a; Monastirioti et al., 1995; Petersen et al., 1997). According to octopamine immunocytochemistry, there are two or three VUM (ventral medial unpaired) motoneurons arising at the CNS midline which bilaterally supply type II innervation for each bodywall segment (Monastirioti et al., 1995). We examine whether this notion is true and also compare any difference between type II and type Ib or Is axon terminals in their innervation patterns. Type III Type III boutons are of medium size (about 2 3 m) and the terminals bearing them are of medium length but are
4 58 Hoang and Chiba type III boutons contain insulin, a putative neural cotransmitter, and this immunoreactivity has been used for tracing the type III boutons (Gorczyca et al., 1993). We examine whether all type III boutons arise from a single motoneuron axon and determine if the axon that produces type III boutons exclusively innervates muscle 12 as proposed previously. Throughout our description, we use a simple naming scheme for the individual larval motoneurons which is based on the identities of their synaptic target muscles and their synaptic bouton types (e.g., MN1-Ib). It should be noted that, though we describe the motoneurons as containing only one bouton type, there exist cases in which a motoneuron axon possesses boutons that resemble the sizes of two types, being qualified for the size-based criteria of either types Ib and Is boutons or types Is and II boutons. This is entirely consistent with the previous descriptions of these synaptic boutons (e.g., Johansen et al., 1989a; Budnik et al., 1990). In these particular cases, we classify the motoneuron axon according to its most numerous type of bouton revealed through single-cell injections. This internal variability again emphasizes the usefulness of singlecell analyses over those relying on panneural observations. In the following sections, we show the innervation pattern of the 30 motor axons in the ISN, SNa, SNb, and SNd nerve branches in the late-stage (4-day-old, third-instar) larvae. These 30 motoneuron axons supply innervation to 26 of the 30 individual muscles found in every half-segment of the body wall. In each section, we separately describe the four different types of synaptic bouton differentiation. FIG. 2. Visualization of individual motoneuron axons. (A) The innervations on muscles 12 and 13 as labeled with panneuronal antibodies. The boutons are classified into types Ib, Is, II, and III, based on morphology and, in the case of type III, additionally with molecular markers (see text). (B, C) Single-cell dye injection. Before a bouton was impaled with a dye-filled micropipette (asterisk), all boutons were visualized with FITC-conjugated panneuronal (anti- HRP) antibodies (B). The dye-filled micropipette (asterisk) was then inserted into a bouton and Lucifer yellow dye was injected into the terminal (C). Note that only one of the several terminals on the muscle was labeled (compare C to B). Fluorescent images are overlaid onto corresponding DIC image in (B) and (C). Scale bars, 20 m. thought to occur only on muscle 12 (Fig. 2A). The morphological appearance of the type III axon resembles both type Is and type II axons in that it looks like type Is at the synaptic entry point but mimics type II as it arborizes its terminal farther on the muscle. In addition to glutamate, Motoneurons in the ISN Nerve Branch The dorsal (distal) region of the neuromuscular system includes 10 muscles (1, 2, 3, 4, 9, 10, 11, 18, 19, and 20) and a single ISN nerve branch that supplies all motoneuron axons that reach these muscles (Fig. 3, schematic inset). We show 12 distinct motoneuron axons providing the endings to these muscles and exhibiting specific innervation patterns: Type Ib. We find 9 distinct motoneuron axons and infer 1 additional motoneuron axon in the ISN nerve branch. Each of these motoneurons forms predominantly type Ib boutons (and thus is referred to as a type Ib axon here) and is matched to a specific ISN muscle. A single type Ib axon (MN1-Ib) enters the synaptic site of muscle 1 on the proximal edge and then arborizes both anteriorly and posteriorly (Fig. 3D). Similarly, muscles 2 and 3 receive their respective type Ib motoneuron axons (MN2-Ib and MN3-Ib) on their proximal edges (Figs. 3B and 3C). In the case of muscles 4, 9, 10, 11, 19, and 20, each muscle receives a different type Ib motoneuron axon (MN4-Ib, MN9-Ib, MN10-Ib, MN11-Ib, MN19-Ib, and MN20-Ib) that innervates on its internal surface (Figs. 3A and 3E 3I). Each type Ib motoneuron axon targets only 1 specific muscle, since injected dye fills the axons which do not form additional collaterals in the ISN nerve branch (e.g., Fig. 3J). They are
5 FIG. 3. Motoneurons in the ISN nerve branch. Single-cell Lucifer yellow labeling of individual motoneuron axons and panneuronal immunological counterlabeling. The red channel shows immunolabeling against Lucifer yellow dye, while the green channel depicts the panneuronal labeling with anti-hrp antibodies. The yellow is the result of the overlap between the red and the green channels. Asterisks indicate where the dye-labeled axons are cut off from the views. The double-fluorescent images are overlaid onto corresponding DIC images. Although dye injection was done in both right and left sides of the body, all images are shown as if they are in the right half-segments. Anterior is to the top. The schematic inset shows the relative locations of the 10 ISN muscles. The same scheme of image presentations applies to Figs. 4 through 6. Motoneurons that form type Ib boutons on single muscles are MN1-Ib (on muscle 1; D), MN2-Ib (on muscle 2; C), MN3-Ib (on muscle 3; B), MN4-Ib (on muscle 4; A), MN9-Ib (on muscle 9; H), MN10-Ib (on muscle 10; G), MN11-Ib (on muscle 11; F), MN19-Ib (on muscle 19; E), and MN20-Ib (on muscle 20; I). MN2-Ib axon extends through the ISN nerve branch and terminates only on a single muscle (muscle 2) (J, arrow). MNISN-Is, in contrast, is a multiple-muscle-innervating motoneuron and forms all type Is boutons found on ISN muscles (K). Scale bar, 20 m for (A I, K) and 10 m for (J). 59
6 60 Hoang and Chiba the only type Ib axons each muscle receives as evidenced by the fact that we never observe other type Ib boutons that are labeled with panneuronal antibodies and not by the injected dye (Figs. 3A 3J). We could not impale the type Ib boutons on muscle 18, a muscle that lies underneath layers of others. However, counterstaining clearly revealed that dyelabeled type Ib boutons overlap perfectly with all the type Ib boutons present on each of all the other 9 muscles (not shown). We suggest that a total of 10 motoneuron axons are present in the ISN nerve branch, that they develop type Ib boutons, and that they exhibit specific one-to-one matching with the 10 distinct muscles in the dorsal region (Table 1). Type Is. We observe that only one motoneuron axon supplies type Is innervation onto the ISN muscles. This motoneuron axon (MNISN-Is) innervates muscles 1 and 2 at their proximal edges and muscles 3 and 4 at their distal edges (Fig. 3K). In addition, it innervates muscles 9, 10, 19, and 20 on their internal surfaces (Fig. 3K). This synaptic pattern is repeatable in the sense that the dye fill of a type Is bouton on muscle 1, 2, or 4 produced the same innervation pattern of this motoneuron. Dye fills did not reach muscle 11. Contrasting the dye injection with all the synapses that exist on each muscle demonstrates that only one type Is motoneuron forms this particular bouton on each muscle of the ISN nerve branch (Fig. 3K). We suggest that a single motoneuron forms all type Is boutons for the ISN muscles (Table 1). Type II. It is likely that a single motoneuron provides all of the type II endings for the ISN muscles. Although each injection confirmed that the type II motoneuron axon polyinnervates many target muscles, each dye injection has failed to label all type II boutons from the ISN nerve branch at once. Nevertheless, a combination of dye injections into the type II boutons on various muscles showed overlaps that suggest that only a single type II motoneuron exists within the ISN nerve branch. For example, injection into a type II bouton on muscle 1, the most distal of the ISN muscles, allows diffusion of the dye onto muscles 2, 9, and 10 (not shown). Injection into a type II bouton of muscle 4, the most proximal of the ISN muscles, on the other hand, displays a dye-illuminated pattern of an axon forming type II boutons on muscles 19 and 20 and occasionally 2, 3, and 10. In all cases, the comparison with panneuronal immunofluorescence revealed that only one motoneuron forms all of the type II boutons on these muscles (Table 1). Motoneurons in the SNa Nerve Branch The lateral neuromuscular region contains six muscles (5, 8, 21, 22, 23, and 24). A single SNa nerve branch supplies all motoneuron axons innervating these muscles (Fig. 4, schematic inset). The general innervation pattern for the motoneurons of types Ib, Is, and II in the SNa nerve branch is similar to that described for the ISN motoneuron axons in that type Ib motoneurons tend to innervate only one synaptic location, whereas type Is and II motoneurons synapse at multiple sites. We show seven distinct motoneurons of the SNa nerve branch. Type Ib. Five motoneuron axons with type Ib boutons are found to innervate the SNa muscles. Each type Ib motoneuron innervates only one synaptic site. MN5-Ib innervates muscle 5 at the distal edge of the muscle, while MN8-Ib apparently synapses only with muscle 8 on its anterior edge (Figs. 4A and 4B). Three additional type Ib motoneurons project onto muscles 21 24, with each innervating at the 21/22 muscle cleft (MN21/22-Ib), 22/23 muscle cleft (MN22/23-Ib), and 23/24 muscle cleft (MN23/ 24-Ib), respectively (Figs. 4C 4E). Compared with anti-hrp immunofluorescence, the dye injection shows that only a single motoneuron forms all type Ib boutons on each of these synaptic sites (Figs. 4A 4E). We suggest that a total of five distinct type Ib motoneurons innervate the SNa muscles in specifically matched manners (Table 1). Type Is. We find a single type Is motoneuron axon (MNSNa-Is) that innervates the SNa muscles (Fig. 4F). Lucifer yellow backfill traced a neuron which innervated the external surface of muscle 8 and appears to branch into the muscle region, although we could not determine the exact muscle targets in the latter region as the dye ceased to diffuse before reaching the synaptic field (Fig. 4F, arrows). In addition, MNSNa-Is forms boutons inside the glial tissue located at the distal edge of muscle 5 (Fig. 4F, arrowheads). We suggest that at least one motoneuron develops the type Is boutons for the SNa muscles (Table 1). Type II. We observe a single motoneuron axon (MNSNa-II) that supplies type II boutons to SNa muscles. Injection of a type II bouton on muscle 8 labeled small boutons of this motoneuron wrapping around muscle 8 and also the axon extension reaching the distal edge of muscle 5 where it arborizes onto its surface (Fig. 4G). In addition, the same axon extended toward muscles (Fig. 4G, arrows). Because of stoppage of dye diffusion, we could not show whether muscles are also innervated by this motoneuron. We suggest that at least one motoneuron accounts for the type II boutons on the SNa muscles (Table 1). Motoneurons in the SNb and SNd Nerve Branches The motoneurons in the SNb and SNd nerve branches innervate the 10 ventral (proximal) muscles (6, 7, 12, 13, 14, 15, 16, 17, 28, and 30) (Fig. 5, schematic inset). We combine these two nerve branches here since both type Is and type II boutons arise from common motor axons projecting into both SNb and SNd simultaneously (see below). Overall, the pattern of types Ib, Is, and II innervation observed for the ISN and SNa motoneuron axons is generally repeated by the SNb/d motoneuron axons. However, there are notable exceptions. We describe a total of 11 distinct motoneurons in the SNb and SNd nerve branches. Type Ib. We find eight type Ib motoneuron axons that innervate the SNb and SNd muscles. Injection of dye into Ib of muscle 12 unveils a motoneuron axon (MN12-Ib) that synapses only at the proximal edge of muscle 12 (Fig. 5D).
7 Synaptic Differentiation of Single Motoneurons 61 TABLE 1 Motoneurons in an Abdominal Half-Segment of the Drosophila Larva No. Nerve Motoneuron a (aka) b Target muscles Type c Only d n e 1 ISN MN1-Ib 1 Ib Yes 4 2 MN2-Ib 2 Ib Yes 5 3 MN3-Ib 3 Ib Yes 3 4 MN4-Ib 4 Ib Yes 4 5 MN9-Ib 9 Ib Yes 3 6 MN10-Ib 10 Ib Yes 1 7 MN11-Ib 11 Ib Yes 2 8 MN18-Ib 18 Ib f 9 MN19-Ib 19 Ib Yes 1 10 MN20-Ib 20 Ib Yes 2 11 MNISN-Is 1, 2, 3, 4, 9, 10, 18, 19, 20 g Is Yes 7 12 MNISN-II 1, 2, 3, 4, 9, 10, 11, 18, 19, 20 g II Yes 5 13 SNa MN5-Ib 5 Ib Yes 3 14 MN8-Ib 8 Ib Yes 1 15 MN21/22-Ib 21, 22 Ib Yes 2 16 MN22/23-Ib 22, 23 Ib Yes 2 17 MN23/24-Ib 23, 24 Ib Yes 2 18 MNSNa-Is 8, 21, 22, 23, 24 g Is Yes 1 19 MNSNa-II 5, 8, 21, 22, 23, 24 g II Yes 1 20 SNb/SNd MN6/7-Ib (RP3?) 6, 7 Ib Yes MN12-Ib (RP5?) 12 Ib Yes MN13-Ib (Novel?) 13 Ib Yes MN14-Ib 14 Ib Yes 4 24 MN15/16/17-Ib 15, 16, 17 Ib Yes 7 25 MN28-Ib 28 Ib Yes 1 26 MN30-Ib 30 Ib Yes 1 27 MN15/16-I 15, 16 I Yes 6 28 MNSNb/d-Is 6, 7, 12, 13, 14, 15, 16, 30 Is Yes MNSNb/d-II 12, 13, 14, 15, 16, 17, 30 h II Yes 6 30 MN12-III (V?) 12 III Yes 9 SNc TN (No individual motoneurons known; both types I and II boutons present) i (No individual motoneurons known; only type I boutons present) i a Larval motoneuron names are based on their target muscle identities and synaptic bouton types. b Embryonic motoneurons that correspond to specific larval motoneurons (see text). c Types of synaptic boutons formed by motoneurons. d Comparison with panneuronal immunocytochemistry reveals whether the particular motoneurons are the only ones that form boutons of a particular type on a given muscle. e Sample sizes. The entire data set consists of a total of 142 dye fills and corresponding immunological counterlabelings. f Although we lacked direct evidence, the existence of a single motoneuron innervating exclusively muscle 18 and forming type Ib boutons there is inferred from circumstantial evidence (see text). g Parts of the lists are based on inferences, although dye has failed to diffuse into muscles 11 and 18 for MNISN-Is, muscles 11 and 18 for MNISN-II, and muscles for both MNSNa-Is and MNSNa-II. h MNSNb/d-II rarely (1 of 6 other cases) innervates muscles 6 and 7. Although 1 case of dye injection revealed this motoneuron to fail innervating muscle 17 (Fig. 5I), panneuronal immunocytochemistry suggests that it represents a rather exceptional case and that muscle cleft 16/17 usually contains type II boutons. i Observations based on panneuronal immunocytochemistry only. Contrasted against the panneuronal immunocytochemistry, this motoneuron is responsible for all of the type Ib boutons on this muscle. The same one-to-one matching is also seen for the type I motoneuron axons on muscle 13 (MN13-Ib, with proximal innervation) and also seems to hold for muscles 14 (MN14-Ib, with distal innervation), 28 (MN28-Ib, with proximal innervation), and 30 (MN30-Ib, with proximal innervation) (Figs. 5C, 5F, and 5G). With muscles 6 and 7, a single motoneuron axon (MN6/7-Ib) innervates the cleft between two muscles (Fig. 5B). Similar
8 62 Hoang and Chiba FIG. 4. Motoneurons in the SNa nerve branch. Single-cell Lucifer yellow labeling of individual motoneuron axons and panneuronal immunological counterlabeling. The schematic inset shows the relative locations of the six SNa muscles. Motoneurons that form type Ib boutons are MN5-Ib (on muscle 5; A), MN8-Ib (on muscle 8; B), MN21/22-Ib (at muscle cleft 21/22; C), MN22/23-Ib (at muscle cleft 22/23; D), and MN23/24-Ib (at muscle cleft 23/24; E). MNSNa-Is is a multiple-muscle-innervating motoneuron that forms type Is boutons on muscle 8 and likely also muscles 21/24 (F). Boutons formed by MNSNa-Is near muscle 5 (arrowheads) do not occur on this muscle but within the glia that associate with the SNa nerve branch. MNSNa-II is also a multiple-muscle-innervating motoneuron which forms type II boutons on muscles 5 and 8 and likely also muscles 21/24 (G). Note that the axons of MNSNa-Is and MNSNa-II extend toward muscles at the major SNa branch point (arrows in F and G), while those of the type Ib motoneurons do not make such a bifurcation at the same branch point (e.g., arrow in A). Scale bar, 20 m. to the other type Ib motoneurons, this motoneuron also forms all of the type Ib boutons at its innervation site. The MN6/7-Ib is called axon 1 by Lnenicka and Keshishian (2000). In contrast to these type Ib motoneuron axons, we found a single motoneuron axon (MN15/16/17-Ib) that forms type Ib boutons at the cleft between muscles 15 and 16 as well as the cleft between muscles 16 and 17 (Fig. 5A). Therefore, with the exception of MN15/16/17-Ib, each type Ib motoneuron projects only to a single muscle or a muscle cleft. In addition, another motoneuron axon (MN15/16-I) forms boutons only between muscles 15 and 16 (Fig. 5E). The bouton size is somewhere between types Ib and Is (and here we include it in the type Ib section). We suggest that eight motoneurons supply all of the type Ib (or I) innervation and exhibit specific matching to the SNb/SNd muscles (Table 1).
9 Synaptic Differentiation of Single Motoneurons 63
10 64 Hoang and Chiba Type Is. A single multi-innervating type Is motoneuron axon (MNSNb/d-Is) synapses with muscles of both SNb and SNd nerve branches (Fig. 5H). This MNSNb/d-Is is believed to be the same motoneuron axon called axon 2 by Lnenicka and Keshishian (2000). Injection of a type Is bouton on muscle 13 illuminated type Is boutons on muscles 6, 7, 12, 13, 14, 15, 16, and 30, thus reaching both the SNb and the SNd muscles. The part that contacts muscles 15 and 16 is short (Fig. 5H). Anti-HRP immunofluorescence does not label any type Is boutons on muscles 17 and 28 (Figs. 5A and 5F) and the injected dye did not spread onto this muscle. The synaptic entry point onto each muscle conforms with that of type Ib motoneurons. The same innervation pattern is seen when the dye is injected into type Is boutons from muscle 12 or muscle cleft 6/7. Comparison with the panneuronal antibody staining suggests that MNSNb/d-Is makes all of the type Is boutons present on the SNb and SNd muscles. We conclude that a single motoneuron forms all of the type Is boutons located on muscles of the SNb and SNd nerve branches (Table 1). Type II. There appears to be one motoneuron axon (MNSNb/d-II) that develops all of the type II boutons found on the SNb and SNd muscles. Dye injection of a type II ending on either muscle 12 or 13 labels type II boutons on muscles 12, 13, 14, and 30 and in the SNd nerve branch also at the muscle clefts of 15/16 and 16/17 (Fig. 5I; also see Fig. 5A for muscle cleft 16/17). The synaptic partners of this motoneuron are slightly variable. MNSNb/d-II on rare occasions fails to reach the muscle cleft 16/17 (e.g., Fig. 5I, arrow) and also occasionally innervates muscle cleft 6/7 (Table 1 footnotes). Compared to anti-hrp immunofluorescence, almost all type II boutons are typically labeled with dye injection. Boutons with no clear dye labeling tended to be on muscles located farthest from the impalement point. Furthermore, when one looks at a given muscle on which the dye was injected into a type II bouton, the dye consistently diffused throughout all of the type II boutons on that muscle. These observations support the notion that the failure to achieve a complete overlap of the dye-labeled and immunostained type II boutons on the SNb/SNd muscles reflects the inability of the dye to diffuse completely into the cytoplasm of the single motoneuron that is responsible for all of the type II boutons on this group of muscles. We suggest that a single type II motoneuron synapses with most of the muscles innervated by the SNb and SNd nerve branches (Table 1). Type III. The motoneuron axon of type III is said to be present only on muscle 12 based on experiments using antibodies against insulin (Gorczyca et al., 1993). Consistent with this, our dye injection into a type III bouton shows a single motoneuron axon (MN12-III) that projects only onto muscle 12 (Fig. 6C). Thus, despite its morphological resemblance to type Is and type II motoneurons, this type III motoneuron is similar to type Ib motoneurons in matching to only one specific muscle (Table 1). Motoneurons in the SNc and TN Nerve Branches The SNc and TN motoneurons innervate four ventral (proximal) muscles (25, 26, 27, and 29) in the superficial (close to cuticle) layers of the musculature (Fig. 1B). Due to the inaccessibility of their axon terminals to dye injection, we could not obtain data for the SNc and TN nerve branches. Correlating the Motoneurons Found in Larvae to Those Found in Embryos In the embryo, in which the identities of individual motoneurons are well established, one can interpret the results of various genetic and experimental manipulations at the level of individual neurons, providing vast information about the molecular mechanisms of synaptic target recognition and formation. However, as these synapses mature in the larva, it is unknown whether the same motoneurons persist through this period and, if so, which muscle(s) a given larval motoneuron innervates or what synaptic bouton type(s) it acquires. Unfortunately, there is no molecular marker known to distinguish among specific motoneurons in the larva. Using dye injection, we tried to identify wherever possible the cell body positions of the larval motoneurons and to match the larval motoneurons to those previously identified in the embryos. Here we describe four particular cases. MN6/7-Ib (embryonic motoneuron RP3?). Dye backfill linked the Ib boutons at the 6/7 muscle cleft to a contralateral and dorsal cell body situated proximal to the CNS midline (n 2) (Fig. 6A). This particular cell body position FIG. 5. Motoneurons in the SNb and SNd nerve branches. Single-cell Lucifer yellow labeling of individual motoneuron axons and panneuronal immunological counterlabeling. The schematic inset shows the relative locations of the 10 SNb/d muscles. Motoneurons that form type Ib boutons are MN12-Ib (on muscles 12; D), MN13-Ib (on muscle 13; C), MN14-Ib (on muscle 14; G), MN28-Ib (on muscle 28; F), MN6/7-Ib (at muscle cleft 6/7; B), and MN15/16/17-Ib (at muscle clefts 15/16 and 16/17; A). MN15/16-I forms boutons whose sizes fall between types Ib and Is and innervates only at muscle cleft 15/16 (E). MNSNb/d-Is is a multiple-muscle-innervating motoneuron and forms type Is boutons on all SNb/d muscles except for 17 and 28 (H). In the SNd nerve branch, this motoneuron targets only muscle cleft 15/16 (arrow) and does not bifurcate onto muscle 17 (dashed arrow). MNSNb/d-II is also a multiple-muscle-innervating motoneuron and forms type II boutons typically on muscles 12, 13, 14, 15, 16, 17, and 30 (I). In the example shown, however, muscle 17 (dashed arrow) is not innervated by this motoneuron. This motoneuron is also known to reach muscles 6 and 7 in rare cases (see Table 1 footnotes). Scale bar, 20 m.
11 Synaptic Differentiation of Single Motoneurons 65 and synaptic targets correspond well to the embryonic features of the RP3 motoneuron (Halpern et al., 1991; Landgraf et al., 1997; Sink and Whitington, 1991a,b). Thus, it is likely that the RP3 motoneuron, which forms its synapse at the cleft of muscles 6 and 7 during embryogenesis, maintains its connectivity while maturing into type Ib boutons (MN6/7-Ib) on these two muscles during the subsequent larvagenesis (Table 1). MN12-Ib (embryonic motoneuron RP5?). When the dye injected into a type Ib bouton on muscle 12 could be traced back into the CNS, we saw a cell body situated proximal but across the CNS midline, contralaterally to the muscle being innervated (n 2) (Fig. 6B). This cell body position closely resembles that of the embryonic RP5 motoneuron, whose axon establishes synaptic connection to muscle 12 during embryogenesis (Landgraf et al., 1997; Sink and Whitington, 1991a). We suggest that the RP5 motoneuron previously characterized in the embryo and the larval motoneuron MN12-Ib are the same and that this motoneuron maintains its connectivity with muscle 12 throughout larvagenesis while differentiating its terminal into type Ib boutons (Table 1). MN12-III (embryonic motoneuron V?). Dye fill into the boutons of MN12-III outlines a motoneuron with its axon also crossing the CNS midline and its cell body situated at the distal edge of the CNS several cell layers deep from the dorsal surface (n 2) (Fig. 6C). This conformed to the description of the embryonic motoneuron V (Landgraf et al., 1997) and agreed with previous speculations from embryonic analysis that type III boutons on muscle 12 arise from motoneuron V (Schmid et al., 1999) (Table 1). MN13-Ib (a novel motoneuron?). Injection into type Ib boutons of MN13-Ib leads to the retrograde diffusion of dye disclosing a cell body at the distal edge in the ipsilateral side of the CNS (n 4) (Fig. 6D). From the cell body, the axon was seen to extend first posteromedially but, without crossing the midline, makes a sharp posterolateral bend toward the lateral nerve exit (Fig. 6D 1 ). This clearly differs from the known cell body positions of embryonic motoneurons RP1 and RP4, the two motoneurons which in the embryo are known to extend axons onto muscle 13 of the contralateral side of the body (Landgraf et al., 1997; Sink and Whitington, 1991a). At this time, we cannot correlate any embryonic motoneuron to the larval motoneuron MN13-Ib and tentatively designate it a novel motoneuron previously unknown from the embryonic studies (Table 1). The cellular identities of other motoneurons were difficult to verify by our method, due either to the inaccessibility of the bouton or the distance between the cell body and the synapse being too great. Both Fig. 7 and Table 1 summarize our entire data set. DISCUSSION This study examined the innervation pattern of individual motoneuron axons in the ISN, SNa, SNb, and SNd nerve branches in the Drosophila larva. The results begin to reveal underlying principles for the neuromuscular synapse organization in this system. Here we highlight a few emerging points that are not obvious from previous studies. Synaptic Exclusion on a Multiply Innervated Postsynaptic Cell It has been proposed that a major difference exists between the vertebrate neuromuscular systems and the Drosophila counterpart in terms of their relative promiscuities to multimotoneuronal innervation. In the vertebrates that range from the mammals to chicks to frogs, an individual muscle fiber receives a transient innervation from multiple motoneuron axons, which subsequently undergo a period in which all but one is left to exclusively supply synapses to the muscle fiber (Sanes and Lichtman, 1999). This wellknown process of synaptic elimination has provided a paradigm in which the competitive nature of synaptic adjustments is studied. In Drosophila, electrophysiology supports the idea that at least two distinct axon terminals co-innervate a given muscle (Broadie and Bate, 1993; Jan and Jan, 1976; Kurdyak et al., 1994; Lnenicka and Keshishian, 2000; Nishikawa and Kidokoro, 1995). In the case of muscles 6 and 7, in which this idea has been most directly tested, the type Ib motoneuron (MN6/7-Ib) and the type Is motoneuron (MNSNb/d-Is) exhibit synaptic facilitation and depression, respectively, in response to repetitive stimulation (Lnenicka and Keshishian, 2000). Consistent with this, immunocytochemistry in the larva reveals axons with distinct types of bouton differentiation coexisting on individual muscles (Budnik, 1996; Keshishian et al., 1996). Different types of synaptic boutons may correspond to distinct synaptic transmission properties implied from the electrophysiological recordings, similar to other arthropod neuromuscular synapses (Atwood et al., 1993; Kurdyak et al., 1994; Lnenicka and Murphey, 1989). These observations have led to a view that, during the development of Drosophila and other arthropod neuromuscular synapses, the mechanisms for synaptic elimination are less important than in the vertebrates (Keshishian and Chiba, 1993). Our data reveal a rule that has not been apparent from previous work, that of bouton-type specific synaptic exclusion. The number of motoneurons that innervate a given muscle varies from 1 to 4 (Fig. 8). However, no more than one motoneuron axon of the given bouton type is present on each larval muscle (Fig. 8). This conclusion is based on our analysis using both single-cell retrograde labeling and panneuronal immunological counterlabeling (Table 1). Muscle 28 receives only one axon from a type Ib motoneuron and represents the simplest category (Fig. 8A). Muscles 6 and 7 are co-innervated by two distinct motoneurons, a single type Ib motoneuron and another type Is motoneuron (Fig. 8B), while muscles 5, 11, and 17 are each co-innervated also by two distinct motoneurons, a single type Ib motoneuron and a single type II motoneuron in this case (Fig. 8C). By far the most common situation is the triple innervation,
12 66 Hoang and Chiba FIG. 6. Cellular identities of larval motoneurons. Cell body labeling of individual larval motoneurons with Lucifer yellow retrograde injection. Four pairs of photos are from four cases of single axon tracing all the way back to the cell bodies within the CNS, often more than 500 m away. The axon terminals are in the right half-segments. The dash line represents the midline of the CNS. The cell bodies of the three motoneurons examined are located contralateral (across the midline) to the target muscles (A C), while that of the fourth motoneuron is located ipsilateral (D). The four larval motoneurons are MN6/7-Ib (or RP3) which forms a type Ib bouton on muscle cleft 6/7 (A 2 ) and whose cell body is found proximal to, but across from, the CNS midline (A 1 ), MN12-Ib (or RP5) which form type Ib boutons on muscle 12 (B 2 ) and whose cell body sits proximal and contralateral to the CNS midline (B 1 ), MN12-III (or V) which forms type III boutons on muscle 12 (C 2 ) and whose cell body is at the contralateral edge of the CNS several cell layers deep from the surface (C 1 ), and MN13-Ib which forms type Ib boutons on muscle 13 (D 2 ) and whose cell body is located ipsilateral and away from the CNS midline some cell layers deep (D 1 ). MN13-Ib s cell body position does not fit any of those known for embryonic motoneurons that innervate muscle 13. Scale bar, 20 m. with the first from a type Ib motoneuron, the second from a type Is motoneuron, and the third from a type II motoneuron (Fig. 8D). Most, if not all, ISN and SNa muscles, as well as muscles 13, 14, and 30 in the SNb group, fall into this category. Finally, three muscles receive innervation from a fourth motoneuron. In addition to the three types of motoneurons listed above, muscles 15 and 16 accept a type I motoneuron (i.e., the bouton size appears to be a hybrid between types Ib and Is) (Fig. 8E), while muscle 12 receives a type III motoneuron (Fig. 8F). Based on these observations, we propose that under normal conditions each muscle (postsynaptic cell) accommodates only one motoneuron of a given bouton type. The underlying molecular mechanisms for this boutonspecific synaptic exclusion on an individual muscle remain unclear. In wildtype, co-innervating axon terminals with distinct bouton differentiations intermingle and occupy more or less the same muscle membrane areas (e.g., Fig.
13 Synaptic Differentiation of Single Motoneurons 67 FIG. 7. study. Summary of the larval motoneurons. The target muscle specificities (black muscles) of all 30 larval motoneurons described in this 2A). Previous experiments, which rely on the panneuronal immunolabeling methods, hint at competition among axons of the same bouton type. Ectopic axon terminals, which result from experimental miswiring, rarely exhibit peaceful coexistence with the native axon terminals on that muscle (Cash et al., 1992; Kose et al., 1997; Shishido et al., 1998). Instead, the two sets of axon terminals seem to prefer nonoverlapping muscle surface areas. When the native axon terminals are eliminated, however, the foreign axon terminals sometimes invade the muscle surface areas normally occupied by the former (Chang and Keshishian, 1996). With the new knowledge gained regarding the individual larval motoneurons, it will be interesting to revisit these experimental situations and investigate how motoneurons of FIG. 8. Synaptic exclusion on a single muscle. A single muscle can receive innervation from up to four motoneurons. Muscle 28 receives one axon (A); muscles 6, 7, 11, and 17 each receive two axons (B, C); a total of 16 muscles each receive three axons (D); and muscles 15, 16, and 12 each receive four axons (E, F). In every case, no two axons of a particular bouton type coexist on a single muscle. While a type Ib motoneuron is always found on each muscle, additional innervation may come from any of the other types of motoneurons.
14 68 Hoang and Chiba different bouton types can coexist while those of the same bouton type exclude each other. Understanding how Drosophila orchestrates restricted co-innervation among diverse synapses on a single muscle may illuminate principles at play in a variety of central nervous systems in which a single postsynaptic cell is multiply innervated by functionally diverse presynaptic terminals. Cell Autonomy of Synaptic Bouton Differentiation Our single-axon visualization shows that a single motoneuron exhibits predominantly one type of bouton. This suggests that cell-autonomous factors have a strong influence over what bouton type into which each motoneuron axon differentiates. The axons forming type Ib boutons restrict their innervation to either single muscles or muscle clefts (MN1-Ib, MN2-Ib, MN3-Ib, MN4-Ib, MN5-Ib, MN6/ 7-Ib, MN8-Ib, MN9-Ib, MN10-Ib, MN11-Ib, MN12-Ib, MN13-Ib, MN14-Ib, MN19-Ib, MN20-Ib, MN21/22-Ib, MN22/23-Ib, MN23/24-Ib, MN28-Ib, and MN30-Ib). A notable exception is MN15/16/17-Ib, which innervates both 15/16 and 16/17 clefts and forms type Ib boutons in both sites. The motoneuron axons that innervate multiple muscles develop either type Is or type II boutons. All of these axons adopt only one type of bouton on all muscles with which each of them synapses. The target muscles, on the other hand, contain different combinations of bouton types. For example, muscles 12 has type Ib, Is, II, and III boutons, while muscle 13 has type Ib, Is and II boutons, and muscles 6 and 7 typically have type Ib and Is boutons. MNSNb/d-Is, the motoneuron that supplies innervation to all these muscles, forms type Is boutons on all of them regardless of what other motoneuron axons happen to coexist on a given muscle (Fig. 5H). Similar situations are thought to be true with other multiply innervating type Is and type II motoneurons, i.e., MNISN-Is, MNISN-II, MNSNa-Is, MNSNa-II, and MNSNb/d-II. This wild-type development, therefore, is consistent with a strong cellautonomous component to the bouton type specification of a given motoneuron. Various experiments implicate additional modulatory mechanisms. Mutations that either up- or downregulate the action potential conductance along the motoneuron axons widely influence the accuracy of synaptic targeting as well as the number and size of the synaptic boutons formed (Budnik et al., 1990; Jarecki and Keshishian, 1995; Zhong and Shanley, 1995). It is said that type II boutons are more sensitive to such activity changes than type I boutons. Mutations that alter the expression level of the synaptically enriched cell surface molecule Fasciclin II also result in abnormal bouton formations (Stewart et al., 1996). In the case of Fasciclin II, its postsynaptic expression level is crucial, supporting retrograde signaling mechanisms that also play a role in bouton specification (Davis et al., 1997; Thomas et al., 1997). The challenge we face when characterizing the molecular mechanisms of synaptic terminal differentiation is to determine how the cell-autonomous components interplay with environmental or targetdependent components in individual cases with specific neurons which may produce different outcomes. Embryo-to-Larva Transition and the Neuromuscular Synapses Our limited data on the cell body positions of several larval motoneurons begin to illuminate how the neuromuscular synapses continue their differentiation during the embryo-to-larva transition. In the embryo, muscles 6 and 7 are both targeted by two motoneurons, RP3 and 6/7b (Broadie, 1999; Keshishian and Chiba, 1993). The cell body of RP3 is contralateral (i.e., across the CNS midline) to its muscle target, while that of 6/7b is ipsilateral (Cash et al., 1992; Keshishian and Chiba, 1993). Whereas RP3 innervates the 6/7 muscle cleft exclusively (Chiba and Rose, 1998; Landgraf et al., 1997), whether 6/7b innervates only these two muscles or extends its axon terminals over other muscles has not been determined in embryos. In the larva, our study suggests that MN6/7-Ib is the larval form of RP3, which maintains its connection with both muscles 6 and 7, and develops type Ib boutons (Fig. 6A). One can then infer that the embryonic motoneuron 6/7b transforms itself into MNSNb/d-Is, the second larval motoneuron that innervates muscles 6 and 7, as well as six other muscles of the SNb and SNd groups (Fig. 5H). If this were the case, 6/7b (or MNSNb/ d-is) would be expanding the list of its synaptic partners during the larval period in a reproducible manner. Muscle 12 offers another opportunity for linking the embryonic and larval data. In the embryo, this muscle is targeted by two single-muscle-innervating motoneurons, RP5 and V, and also by the VUM-v motoneuron, which extends its bilateral axons toward many of the SNb and SNd muscles, e.g., 13, 14, 15, 16, 17, and 30 (Landgraf et al., 1997; Schmid et al., 1999). In the larva, four motoneuron axons (MN12-Ib, MN12-III, MNSNb/d-Is, and MNSNb/d-II) synapse with muscle 12. Based on the cell body positions revealed in this study, we infer two of these larval motoneurons to be RP5 (MN12-Ib) and V (MN12-III) (Figs. 6B and 6C). If this is correct, both RP5 and V are maintaining their exclusive connections with muscle 12 while developing type Ib and type II boutons, respectively, again in a reproducible manner. Of the two remaining motoneurons, it seems likely that MNSNb/d-Is (or 6/7b) is the one that reaches muscles 6 and 7 in the embryo and subsequently extends its type Is bouton-forming axon collaterals to muscle 12 and other SNb/d muscles. Based on octopaminergic immunocytochemistry, it has been proposed that the motoneuron which develops type II boutons on many of the SNb/d muscles is a bilaterally unpaired VUM (Monastirioti et al., 1995). We suspect that MNSNb/d-II found in the larva (Fig. 5L) is the mature state of the VUM-v motoneuron described in the embryo (Landgraf et al., 1997). These examples with muscles 6, 7, and 12, though representing a fraction of the motoneuron pool, suggest that the basic wiring accomplished through reproducible axon-to-muscle matchings
The Origin, Location, and Projections of the Embryonic Abdominal Motorneurons of Drosophila
 The Journal of Neuroscience, December 15, 1997, 17(24):9642 9655 The Origin, Location, and Projections of the Embryonic Abdominal Motorneurons of Drosophila Matthias Landgraf, 1 Torsten Bossing, 2 Gerd
The Journal of Neuroscience, December 15, 1997, 17(24):9642 9655 The Origin, Location, and Projections of the Embryonic Abdominal Motorneurons of Drosophila Matthias Landgraf, 1 Torsten Bossing, 2 Gerd
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo
 Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling
 Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
MCN. Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases Regulate Axon Guidance in Drosophila
 MCN Molecular and Cellular Neuroscience 17, 274 291 (2001) doi:10.1006/mcne.2000.0939, available online at http://www.idealibrary.com on Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases
MCN Molecular and Cellular Neuroscience 17, 274 291 (2001) doi:10.1006/mcne.2000.0939, available online at http://www.idealibrary.com on Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases
Role of Neural Activity during Synaptogenesis in Drosophila
 The Journal of Neuroscience, December 1995, 15( 12): 8177-8190 Role of Neural Activity during Synaptogenesis in Drosophila Jill Jareckil and Haig Keshishian* Departments of Genetics and 2Biology, Yale
The Journal of Neuroscience, December 1995, 15( 12): 8177-8190 Role of Neural Activity during Synaptogenesis in Drosophila Jill Jareckil and Haig Keshishian* Departments of Genetics and 2Biology, Yale
Math in systems neuroscience. Quan Wen
 Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Math in systems neuroscience Quan Wen Human brain is perhaps the most complex subject in the universe 1 kg brain 10 11 neurons 180,000 km nerve fiber 10 15 synapses 10 18 synaptic proteins Multiscale
Connectin Mediates Adhesion in Drosophila
 Neuron, Vol. 18, 873 880, June, 1997, Copyright 1997 by Cell Press Connectin Mediates Adhesion in Drosophila Srikala Raghavan and Robert A. H. White in little observable perturbation of the normal axonal
Neuron, Vol. 18, 873 880, June, 1997, Copyright 1997 by Cell Press Connectin Mediates Adhesion in Drosophila Srikala Raghavan and Robert A. H. White in little observable perturbation of the normal axonal
Nervous Systems: Neuron Structure and Function
 Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Nervous Systems: Neuron Structure and Function Integration An animal needs to function like a coherent organism, not like a loose collection of cells. Integration = refers to processes such as summation
Toll, a muscle cell surface molecule, locally inhibits synaptic initiation of the RP3 motoneuron growth cone in Drosophila
 Development 124, 1561-1571 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV8399 1561 Toll, a muscle cell surface molecule, locally inhibits synaptic initiation of the RP3 motoneuron
Development 124, 1561-1571 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV8399 1561 Toll, a muscle cell surface molecule, locally inhibits synaptic initiation of the RP3 motoneuron
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae. BioNB4910 Cornell University.
 Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Control and Integration. Nervous System Organization: Bilateral Symmetric Animals. Nervous System Organization: Radial Symmetric Animals
 Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Pioneering and pathfinding by an identified neuron in the embryonic leech
 J. Embryol. exp. Morph. 86, 155-167, (1985) 155 Printed in Great Britain The Company of Biologists Limited 1985 Pioneering and pathfinding by an identified neuron in the embryonic leech JOHN Y. KUWADA
J. Embryol. exp. Morph. 86, 155-167, (1985) 155 Printed in Great Britain The Company of Biologists Limited 1985 Pioneering and pathfinding by an identified neuron in the embryonic leech JOHN Y. KUWADA
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Review. Wiring by Fly: The Neuromuscular System of the Drosophila Embryo. Neuron, Vol. 15, , September, 1995, Copyright 1995 by Ceil Press
 Neuron, Vol. 15, 513-525, September, 1995, Copyright 1995 by Ceil Press Wiring by Fly: The Neuromuscular System of the Drosophila Embryo Michael Bate and Kendal Broadie Department of Zoology University
Neuron, Vol. 15, 513-525, September, 1995, Copyright 1995 by Ceil Press Wiring by Fly: The Neuromuscular System of the Drosophila Embryo Michael Bate and Kendal Broadie Department of Zoology University
The Transmembrane Semaphorin Sema I Is Required in Drosophila for Embryonic Motor and CNS Axon Guidance
 Neuron, Vol. 20, 207 220, February, 1998, Copyright 1998 by Cell Press The Transmembrane Semaphorin Sema I Is Required in Drosophila for Embryonic Motor and CNS Axon Guidance Hung-Hsiang Yu, Houmam H.
Neuron, Vol. 20, 207 220, February, 1998, Copyright 1998 by Cell Press The Transmembrane Semaphorin Sema I Is Required in Drosophila for Embryonic Motor and CNS Axon Guidance Hung-Hsiang Yu, Houmam H.
Cellular Neuroanatomy II The Prototypical Neuron: Neurites. Reading: BCP Chapter 2
 Cellular Neuroanatomy II The Prototypical Neuron: Neurites Reading: BCP Chapter 2 Major Internal Features of a Neuron The neuron is the functional unit of the nervous system. A typical neuron has a soma
Cellular Neuroanatomy II The Prototypical Neuron: Neurites Reading: BCP Chapter 2 Major Internal Features of a Neuron The neuron is the functional unit of the nervous system. A typical neuron has a soma
Overview Organization: Central Nervous System (CNS) Peripheral Nervous System (PNS) innervate Divisions: a. Afferent
 Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Overview Organization: Central Nervous System (CNS) Brain and spinal cord receives and processes information. Peripheral Nervous System (PNS) Nerve cells that link CNS with organs throughout the body.
Nervous System Organization
 The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
Information processing. Divisions of nervous system. Neuron structure and function Synapse. Neurons, synapses, and signaling 11/3/2017
 Neurons, synapses, and signaling Chapter 48 Information processing Divisions of nervous system Central nervous system (CNS) Brain and a nerve cord Integration center Peripheral nervous system (PNS) Nerves
Neurons, synapses, and signaling Chapter 48 Information processing Divisions of nervous system Central nervous system (CNS) Brain and a nerve cord Integration center Peripheral nervous system (PNS) Nerves
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34
 NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
Nervous Tissue. Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation
 Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Nervous Tissue Neurons Electrochemical Gradient Propagation & Transduction Neurotransmitters Temporal & Spatial Summation What is the function of nervous tissue? Maintain homeostasis & respond to stimuli
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis
 Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila
 Development 126, 5833-5846 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8655 5833 A role for PS integrins in morphological growth and synaptic function at the postembryonic
Development 126, 5833-5846 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8655 5833 A role for PS integrins in morphological growth and synaptic function at the postembryonic
Systematic Screening of Drosophila Deficiency Mutations for Embryonic Phenotypes and Orphan Receptor Ligands
 Systematic Screening of Drosophila Deficiency Mutations for Embryonic Phenotypes and Orphan Receptor Ligands Ashley P. Wright 1, A. Nicole Fox 1, Karl G. Johnson 2, Kai Zinn 1 * 1 Division of Biology,
Systematic Screening of Drosophila Deficiency Mutations for Embryonic Phenotypes and Orphan Receptor Ligands Ashley P. Wright 1, A. Nicole Fox 1, Karl G. Johnson 2, Kai Zinn 1 * 1 Division of Biology,
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
MEMBRANE POTENTIALS AND ACTION POTENTIALS:
 University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
University of Jordan Faculty of Medicine Department of Physiology & Biochemistry Medical students, 2017/2018 +++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++ Review: Membrane physiology
This script will produce a series of pulses of amplitude 40 na, duration 1ms, recurring every 50 ms.
 9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Biosciences in the 21st century
 Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
NOTE: LOOK ON MY WEBSITE FOR THE MUSCLE LABELING POWER POINT/PDF Part I. Identify the parts of the neuron that are labeled below.
 Anatomy & Physiology Nervous System Part I 2/26/16 NOTE: LOOK ON MY WEBSITE FOR THE MUSCLE LABELING POWER POINT/PDF Part I. Identify the parts of the neuron that are labeled below. 1. 2. 3. 5. 4. 6. Part
Anatomy & Physiology Nervous System Part I 2/26/16 NOTE: LOOK ON MY WEBSITE FOR THE MUSCLE LABELING POWER POINT/PDF Part I. Identify the parts of the neuron that are labeled below. 1. 2. 3. 5. 4. 6. Part
BIOLOGY 11/10/2016. Neurons, Synapses, and Signaling. Concept 48.1: Neuron organization and structure reflect function in information transfer
 48 Neurons, Synapses, and Signaling CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Concept 48.1: Neuron organization
48 Neurons, Synapses, and Signaling CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Concept 48.1: Neuron organization
Open Syntaxin Docks Synaptic Vesicles
 Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
BIOLOGY. 1. Overview of Neurons 11/3/2014. Neurons, Synapses, and Signaling. Communication in Neurons
 CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
CAMPBELL BIOLOGY TENTH EDITION 48 Reece Urry Cain Wasserman Minorsky Jackson Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick 1. Overview of Neurons Communication
INTERSEGMENTAL TO INTRASEGMENTAL CONVERSION BY GANGLIONIC FUSION IN LATERAL GIANT INTERNEURONES OF CRAYFISH
 J. exp. Biol. 107, 515-519 (1983) 5 \ 5 Printed in Great Britain The Company of Biologists Limited 1983 INTERSEGMENTAL TO INTRASEGMENTAL CONVERSION BY GANGLIONIC FUSION IN LATERAL GIANT INTERNEURONES OF
J. exp. Biol. 107, 515-519 (1983) 5 \ 5 Printed in Great Britain The Company of Biologists Limited 1983 INTERSEGMENTAL TO INTRASEGMENTAL CONVERSION BY GANGLIONIC FUSION IN LATERAL GIANT INTERNEURONES OF
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03
 Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Neuronal cell death in grasshopper embryos: variable patterns in different species, clutches, and clones
 J. Embryol. exp. Morph. 78, 169-182 (1983) Printed in Great Britain The Company of Biologists Limited 1983 Neuronal cell death in grasshopper embryos: variable patterns in different species, clutches,
J. Embryol. exp. Morph. 78, 169-182 (1983) Printed in Great Britain The Company of Biologists Limited 1983 Neuronal cell death in grasshopper embryos: variable patterns in different species, clutches,
Conclusions. The experimental studies presented in this thesis provide the first molecular insights
 C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
Neurons, Synapses, and Signaling
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
Embryogenesis of an insect nervous system II: A second class of neuron precursor cells and the origin of the intersegmental connectives
 J. Embryol. exp. Morph. Vol. 61,pp. 317-330, 1981 3J7 Printed in Great Britain @ Company of Biologists Limited 1981 Embryogenesis of an insect nervous system II: A second class of neuron precursor cells
J. Embryol. exp. Morph. Vol. 61,pp. 317-330, 1981 3J7 Printed in Great Britain @ Company of Biologists Limited 1981 Embryogenesis of an insect nervous system II: A second class of neuron precursor cells
Chapter 9. Nerve Signals and Homeostasis
 Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
Chapter 9 Nerve Signals and Homeostasis A neuron is a specialized nerve cell that is the functional unit of the nervous system. Neural signaling communication by neurons is the process by which an animal
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons
 Development 114, 825-831 (1992) Printed in Great Britain The Company of Biologists Limited 1992 825 Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons SUSAN H. PIKE, ELOINE F.
Development 114, 825-831 (1992) Printed in Great Britain The Company of Biologists Limited 1992 825 Pathfinding by zebrafish motoneurons in the absence of normal pioneer axons SUSAN H. PIKE, ELOINE F.
Nerve Signal Conduction. Resting Potential Action Potential Conduction of Action Potentials
 Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
Nerve Signal Conduction Resting Potential Action Potential Conduction of Action Potentials Resting Potential Resting neurons are always prepared to send a nerve signal. Neuron possesses potential energy
Ch 8: Neurons: Cellular and Network Properties, Part 1
 Developed by John Gallagher, MS, DVM Ch 8: Neurons: Cellular and Network Properties, Part 1 Objectives: Describe the Cells of the NS Explain the creation and propagation of an electrical signal in a nerve
Developed by John Gallagher, MS, DVM Ch 8: Neurons: Cellular and Network Properties, Part 1 Objectives: Describe the Cells of the NS Explain the creation and propagation of an electrical signal in a nerve
NOTES: CH 48 Neurons, Synapses, and Signaling
 NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
NOTES: CH 48 Neurons, Synapses, and Signaling A nervous system has three overlapping functions: 1) SENSORY INPUT: signals from sensory receptors to integration centers 2) INTEGRATION: information from
Dendrites - receives information from other neuron cells - input receivers.
 The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
The Nerve Tissue Neuron - the nerve cell Dendrites - receives information from other neuron cells - input receivers. Cell body - includes usual parts of the organelles of a cell (nucleus, mitochondria)
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Overexpression of YFP::GPR-1 in the germline.
 Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Pathfinding in the central nervous system and periphery by identified embryonic Drosophila motor axons
 Development 112, 307-316 (1991) Pnnted in Great Britain The Company of Biologists Limited 1991 307 Pathfinding in the central nervous system and periphery by identified embryonic Drosophila motor axons
Development 112, 307-316 (1991) Pnnted in Great Britain The Company of Biologists Limited 1991 307 Pathfinding in the central nervous system and periphery by identified embryonic Drosophila motor axons
Peripheral Glia Direct Axon Guidance across the CNS/PNS Transition Zone
 Developmental Biology 238, 47 63 (2001) doi:10.1006/dbio.2001.0411, available online at http://www.idealibrary.com on Peripheral Glia Direct Axon Guidance across the CNS/PNS Transition Zone Katharine J.
Developmental Biology 238, 47 63 (2001) doi:10.1006/dbio.2001.0411, available online at http://www.idealibrary.com on Peripheral Glia Direct Axon Guidance across the CNS/PNS Transition Zone Katharine J.
Neuronal Differentiation: Synapse Formation NMJ
 Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
Cell Type-Specific Expression of Fasciclin II Isoforms Reveals Neuronal Glial Interactions during Peripheral Nerve Growth
 Developmental Biology 234, 24 41 (2001) doi:10.1006/dbio.2001.0247, available online at http://www.idealibrary.com on Cell Type-Specific Expression of Fasciclin II Isoforms Reveals Neuronal Glial Interactions
Developmental Biology 234, 24 41 (2001) doi:10.1006/dbio.2001.0247, available online at http://www.idealibrary.com on Cell Type-Specific Expression of Fasciclin II Isoforms Reveals Neuronal Glial Interactions
Chapter 48 Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Chapter 48 Neurons, Synapses, and Signaling Concept 48.1 Neuron organization and structure reflect function in information transfer Neurons are nerve cells that transfer information within the body Neurons
Developmental Biology
 Developmental Biology 366 (2012) 204 217 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology Developmental changes in
Developmental Biology 366 (2012) 204 217 Contents lists available at SciVerse ScienceDirect Developmental Biology journal homepage: www.elsevier.com/locate/developmentalbiology Developmental changes in
Neural development its all connected
 Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
The Nervous System. Nerve Impulses. Resting Membrane Potential. Overview. Nerve Impulses. Resting Membrane Potential
 The Nervous System Overview Nerve Impulses (completed12/03/04) (completed12/03/04) How do nerve impulses start? (completed 19/03/04) (completed 19/03/04) How Fast are Nerve Impulses? Nerve Impulses Nerve
The Nervous System Overview Nerve Impulses (completed12/03/04) (completed12/03/04) How do nerve impulses start? (completed 19/03/04) (completed 19/03/04) How Fast are Nerve Impulses? Nerve Impulses Nerve
PROPERTY OF ELSEVIER SAMPLE CONTENT - NOT FINAL. The Nervous System and Muscle
 The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
DEPARTMENT OF SCIENCE
 DEPARTMENT OF SCIENCE COURSE OUTLINE ZOOLOGY 2420 ANIMAL PHYSIOLOGY II: INTERCELLULAR COMMUNICATIONS INSTRUCTOR: Dr. Georgia Goth PHONE: 780-513-1041 OFFICE: J222 E-MAIL: ggoth@gprc.ab.ca OFFICE HOURS:
DEPARTMENT OF SCIENCE COURSE OUTLINE ZOOLOGY 2420 ANIMAL PHYSIOLOGY II: INTERCELLULAR COMMUNICATIONS INSTRUCTOR: Dr. Georgia Goth PHONE: 780-513-1041 OFFICE: J222 E-MAIL: ggoth@gprc.ab.ca OFFICE HOURS:
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016
 PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
and its origins G. E. Schneider 2009 Part 1: Introduction MIT 9.14 Class 1 Brain talk, and
 A sketch of the central nervous system and its origins G. E. Schneider 2009 Part 1: Introduction MIT 9.14 Class 1 Brain talk, and the ancient activities of brain cells 1. Introduction a) b) The plan for
A sketch of the central nervous system and its origins G. E. Schneider 2009 Part 1: Introduction MIT 9.14 Class 1 Brain talk, and the ancient activities of brain cells 1. Introduction a) b) The plan for
The shaking-b 2 Mutation Disrupts Electrical Synapses in a Flight Circuit in Adult Drosophila
 The Journal of Neuroscience, June 15, 1997, 17(12):4700 4710 The shaking-b 2 Mutation Disrupts Electrical Synapses in a Flight Circuit in Adult Drosophila James R. Trimarchi and R. K. Murphey Department
The Journal of Neuroscience, June 15, 1997, 17(12):4700 4710 The shaking-b 2 Mutation Disrupts Electrical Synapses in a Flight Circuit in Adult Drosophila James R. Trimarchi and R. K. Murphey Department
MEMBRANE STRUCTURE. Lecture 9. Biology Department Concordia University. Dr. S. Azam BIOL 266/
 MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
MEMBRANE STRUCTURE Lecture 9 BIOL 266/4 2014-15 Dr. S. Azam Biology Department Concordia University RED BLOOD CELL MEMBRANE PROTEINS The Dynamic Nature of the Plasma Membrane SEM of human erythrocytes
Homeostatic Control of Presynaptic Release Is Triggered by Postsynaptic Membrane Depolarization
 Neuron, Vol. 30, 737 749, June, 2001, Copyright 2001 by Cell Press Homeostatic Control of Presynaptic Release Is Triggered by Postsynaptic Membrane Depolarization Suzanne Paradis, Sean T. Sweeney, and
Neuron, Vol. 30, 737 749, June, 2001, Copyright 2001 by Cell Press Homeostatic Control of Presynaptic Release Is Triggered by Postsynaptic Membrane Depolarization Suzanne Paradis, Sean T. Sweeney, and
Dimorphism and Homeostasis of Neuromuscular Synaptic Function
 Dimorphism and Homeostasis of Neuromuscular Synaptic Function - Epp m ε Release + + φ - q Sensitivity The nature of the problem Size, Strength, Resistance.. The Power of Drosophila The nature of the problem
Dimorphism and Homeostasis of Neuromuscular Synaptic Function - Epp m ε Release + + φ - q Sensitivity The nature of the problem Size, Strength, Resistance.. The Power of Drosophila The nature of the problem
Membrane Potentials and Bioelectricity
 Membrane Potentials and Bioelectricity Hugh Purdy Honors University Physics II November 29, 2010 Most, if not all, cells in the human body have a net electric charge to some degree on either side of their
Membrane Potentials and Bioelectricity Hugh Purdy Honors University Physics II November 29, 2010 Most, if not all, cells in the human body have a net electric charge to some degree on either side of their
Neurons. The Molecular Basis of their Electrical Excitability
 Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
Neurons The Molecular Basis of their Electrical Excitability Viva La Complexity! Consider, The human brain contains >10 11 neurons! Each neuron makes 10 3 (average) synaptic contacts on up to 10 3 other
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals?
 LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
LESSON 2.2 WORKBOOK How do our axons transmit electrical signals? This lesson introduces you to the action potential, which is the process by which axons signal electrically. In this lesson you will learn
Quantitative Electrophysiology
 ECE 795: Quantitative Electrophysiology Notes for Lecture #4 Wednesday, October 4, 2006 7. CHEMICAL SYNAPSES AND GAP JUNCTIONS We will look at: Chemical synapses in the nervous system Gap junctions in
ECE 795: Quantitative Electrophysiology Notes for Lecture #4 Wednesday, October 4, 2006 7. CHEMICAL SYNAPSES AND GAP JUNCTIONS We will look at: Chemical synapses in the nervous system Gap junctions in
37 Neurons, Synapses, and Signaling
 CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: Lines of Communication
CAMPBELL BIOLOGY IN FOCUS Urry Cain Wasserman Minorsky Jackson Reece 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge Overview: Lines of Communication
Domain 6: Communication
 Domain 6: Communication 6.1: Cell communication processes share common features that reflect a shared evolutionary history. (EK3.D.1) 1. Introduction to Communication Communication requires the generation,
Domain 6: Communication 6.1: Cell communication processes share common features that reflect a shared evolutionary history. (EK3.D.1) 1. Introduction to Communication Communication requires the generation,
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Patterns of peanut agglutinin binding within the developing grasshopper central nervous system
 /. Embryol. exp. Morph. 90, 49-56 (1985) Printed in Great Britain The Company of Biologists Limited 1985 49 Patterns of peanut agglutinin binding within the developing grasshopper central nervous system
/. Embryol. exp. Morph. 90, 49-56 (1985) Printed in Great Britain The Company of Biologists Limited 1985 49 Patterns of peanut agglutinin binding within the developing grasshopper central nervous system
Introduction and summary of the chapters
 Introduction and summary of the chapters 1. Electroreception Electroreception is the ability of animal species to detect weak electric fields. It is mediated by a sensory system that occurs in some aquatic
Introduction and summary of the chapters 1. Electroreception Electroreception is the ability of animal species to detect weak electric fields. It is mediated by a sensory system that occurs in some aquatic
Genetic Specification of Axonal Arbors: atonal Regulates robo3 to Position Terminal Branches in the Drosophila Nervous System
 Neuron, Vol. 37, 41 51, January 9, 2003, Copyright 2003 by Cell Press Genetic Specification of Axonal Arbors: atonal Regulates robo3 to Position Terminal Branches in the Drosophila Nervous System Marta
Neuron, Vol. 37, 41 51, January 9, 2003, Copyright 2003 by Cell Press Genetic Specification of Axonal Arbors: atonal Regulates robo3 to Position Terminal Branches in the Drosophila Nervous System Marta
Neurons, Synapses, and Signaling
 Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 48 Neurons, Synapses, and Signaling PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 11. Development: Differentiation and Determination
 KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
Nature Neuroscience: doi: /nn.2717
 Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Chapter Chemical Uniqueness 1/23/2009. The Uses of Principles. Zoology: the Study of Animal Life. Fig. 1.1
 Fig. 1.1 Chapter 1 Life: Biological Principles and the Science of Zoology BIO 2402 General Zoology Copyright The McGraw Hill Companies, Inc. Permission required for reproduction or display. The Uses of
Fig. 1.1 Chapter 1 Life: Biological Principles and the Science of Zoology BIO 2402 General Zoology Copyright The McGraw Hill Companies, Inc. Permission required for reproduction or display. The Uses of
Neurochemistry 1. Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906
 Neurochemistry 1 Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906 How Many Neurons Do We Have? The human brain contains ~86 billion neurons and
Neurochemistry 1 Nervous system is made of neurons & glia, as well as other cells. Santiago Ramon y Cajal Nobel Prize 1906 How Many Neurons Do We Have? The human brain contains ~86 billion neurons and
Introduction to Animals
 Introduction to Animals Characteristics of Animals multicellular Except for sponges, animal cells are arranged into tissues. Tissues are necessary to produce organs and organ systems. Tissues, organs,
Introduction to Animals Characteristics of Animals multicellular Except for sponges, animal cells are arranged into tissues. Tissues are necessary to produce organs and organ systems. Tissues, organs,
Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions:
 Nervous System Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions: Sensory Input: Obtaining stimulation from the environment (light, heat, pressure, vibration, chemical,
Nervous System Purpose: Perception, Movement, Learning, Memory, Thinking, Communication Functions: Sensory Input: Obtaining stimulation from the environment (light, heat, pressure, vibration, chemical,
CELL BIOLOGY - CLUTCH CH. 9 - TRANSPORT ACROSS MEMBRANES.
 !! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
!! www.clutchprep.com K + K + K + K + CELL BIOLOGY - CLUTCH CONCEPT: PRINCIPLES OF TRANSMEMBRANE TRANSPORT Membranes and Gradients Cells must be able to communicate across their membrane barriers to materials
A Screen of Cell-Surface Molecules Identifies Leucine-Rich Repeat Proteins as Key Mediators of Synaptic Target Selection
 Article A Screen of Cell-Surface Molecules Identifies Leucine-Rich Repeat Proteins as Key Mediators of Synaptic Target Selection Mitsuhiko Kurusu, 1,2, * Amy Cording, 1 Misako Taniguchi, 2 Kaushiki Menon,
Article A Screen of Cell-Surface Molecules Identifies Leucine-Rich Repeat Proteins as Key Mediators of Synaptic Target Selection Mitsuhiko Kurusu, 1,2, * Amy Cording, 1 Misako Taniguchi, 2 Kaushiki Menon,
Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast
 Development 126, 4065-4076 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8625 4065 Development of the Drosophila mushroom bodies: sequential generation of three distinct types
Development 126, 4065-4076 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8625 4065 Development of the Drosophila mushroom bodies: sequential generation of three distinct types
BIOLOGY. Neurons, Synapses, and Signaling CAMPBELL. Reece Urry Cain Wasserman Minorsky Jackson
 CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 48 Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Lines of Communication The
CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 48 Neurons, Synapses, and Signaling Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick Lines of Communication The
Questions in developmental biology. Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration
 Questions in developmental biology Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration Representative cell types of a vertebrate zygote => embryo => adult differentiation
Questions in developmental biology Differentiation Morphogenesis Growth/apoptosis Reproduction Evolution Environmental integration Representative cell types of a vertebrate zygote => embryo => adult differentiation
Developmental Dynamics of Peripheral Glia in Drosophila melanogaster
 GLIA 30:122 133 (2000) Developmental Dynamics of Peripheral Glia in Drosophila melanogaster KATHARINE J. SEPP, JOOST SCHULTE, AND VANESSA J. AULD* Department of Zoology, University of British Columbia,
GLIA 30:122 133 (2000) Developmental Dynamics of Peripheral Glia in Drosophila melanogaster KATHARINE J. SEPP, JOOST SCHULTE, AND VANESSA J. AULD* Department of Zoology, University of British Columbia,
Drosophila Plexin B is a Sema-2a receptor required for axon guidance
 RESEARCH ARTICLE 2125 Development 133, 2125-2135 (2006) doi:10.1242/dev.02380 Drosophila Plexin B is a Sema-2a receptor required for axon guidance Joseph C. Ayoob 1, Jonathan R. Terman 1,2 and Alex L.
RESEARCH ARTICLE 2125 Development 133, 2125-2135 (2006) doi:10.1242/dev.02380 Drosophila Plexin B is a Sema-2a receptor required for axon guidance Joseph C. Ayoob 1, Jonathan R. Terman 1,2 and Alex L.
Action Potentials and Synaptic Transmission Physics 171/271
 Action Potentials and Synaptic Transmission Physics 171/271 Flavio Fröhlich (flavio@salk.edu) September 27, 2006 In this section, we consider two important aspects concerning the communication between
Action Potentials and Synaptic Transmission Physics 171/271 Flavio Fröhlich (flavio@salk.edu) September 27, 2006 In this section, we consider two important aspects concerning the communication between
According to the diagram, which of the following is NOT true?
 Instructions: Review Chapter 44 on muscular-skeletal systems and locomotion, and then complete the following Blackboard activity. This activity will introduce topics that will be covered in the next few
Instructions: Review Chapter 44 on muscular-skeletal systems and locomotion, and then complete the following Blackboard activity. This activity will introduce topics that will be covered in the next few
Nervous system. 3 Basic functions of the nervous system !!!! !!! 1-Sensory. 2-Integration. 3-Motor
 Nervous system 3 Basic functions of the nervous system 1-Sensory 2-Integration 3-Motor I. Central Nervous System (CNS) Brain Spinal Cord I. Peripheral Nervous System (PNS) 2) Afferent towards afferent
Nervous system 3 Basic functions of the nervous system 1-Sensory 2-Integration 3-Motor I. Central Nervous System (CNS) Brain Spinal Cord I. Peripheral Nervous System (PNS) 2) Afferent towards afferent
Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening
 APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
STUDENT PAPER. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters Education Program 736 S. Lombard Oak Park IL, 60304
 STUDENT PAPER Differences between Stochastic and Deterministic Modeling in Real World Systems using the Action Potential of Nerves. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters
STUDENT PAPER Differences between Stochastic and Deterministic Modeling in Real World Systems using the Action Potential of Nerves. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters
