Materials and Methods
|
|
|
- Quentin Noel Blair
- 5 years ago
- Views:
Transcription
1 INVESTIGATION The Kinesin-, Unc-104 Regulates Dendrite Morphogenesis and Synaptic Development in Drosophila Jeannine V. Kern,*, Yao V. Zhang,*,, Stella Kramer, Jay E. Brenman, and Tobias M. Rasse*, *Junior Research Group Synaptic Plasticity and Department of Cellular Neurology, Hertie Institute for Clinical Brain Research, University of Tübingen, Tübingen, Germany, Graduate School of Cellular and Molecular Neuroscience, University of Tübingen, Tübingen, Germany, and Neuroscience Center, University of North Carolina School of Medicine, Chapel Hill, North Carolina ABSTRACT Kinesin-based transport is important for synaptogenesis, neuroplasticity, and maintaining synaptic function. In an anatomical screen of neurodevelopmental mutants, we identified the exchange of a conserved residue (R561H) in the forkhead-associated domain of the kinesin- family member Unc-104/KIF1A as the genetic cause for defects in synaptic terminal- and dendrite morphogenesis. Previous structure-based analysis suggested that the corresponding residue in KIF1A might be involved in stabilizing the activated state of kinesin- dimers. Herein we provide the first in vivo evidence for the functional importance of R561. The R561H allele (unc-104 bris ) is not embryonic lethal, which allowed us to investigate consequences of disturbed Unc-104 function on postembryonic synapse development and larval behavior. We demonstrate that Unc-104 regulates the reliable apposition of active zones and postsynaptic densities, possibly by controlling site-specific delivery of its cargo. Next, we identified a role for Unc-104 in restraining neuromuscular junction growth and coordinating dendrite branch morphogenesis, suggesting that Unc-104 is also involved in dendritic transport. Mutations in KIF1A/unc-104 have been associated with hereditary spastic paraplegia and hereditary sensory and autonomic neuropathy type 2. However, we did not observe synapse retraction or dystonic posterior paralysis. Overall, our study demonstrates the specificity of defects caused by selective impairments of distinct molecular motors and highlights the critical importance of Unc-104 for the maturation of neuronal structures during embryonic development, larval synaptic terminal outgrowth, and dendrite morphogenesis. Copyright 201 by the Genetics Society of America doi: /genetics Manuscript received March 26, 201; accepted for publication June 6, 201 Available freely online through the author-supported open access option. Supporting information is available online at doi:10.154/genetics /-/dc1. Corresponding author: Junior Research Group Synaptic Plasticity, Hertie Institute for Clinical Brain Research, University of Tübingen, Otfried Müller Strasse 25, Tübingen, Germany. tobias.rasse@uni-tuebingen.de SYNAPSE formation and maturation depend on kinesinbased fast anterograde transport (Hirokawa et al. 2010; Kondo et al. 2012; van den Berg and Hoogenraad 2012). Key synaptic components such as synaptic vesicle precursors (Hall and Hedgecock 1991), mitochondria (Nangaku et al. 1994), piccolo-bassoon transport vesicles (Cai et al. 2007), and RNA protein complexes (Ohashi et al. 2002) are transported by members of the kinesin-1 or kinesin- families (for review see Hirokawa and Noda 2008). To date, only a limited understanding of the mechanisms underlying cargo specificity and the dynamic regulation of cargo motor complexes has emerged. In particular, factors that mediate motor activation and cargo binding and release remain to be elucidated (for review see Verhey and Hammond 2009; Verhey et al. 2011). In an anatomical screen examining mutants that might be involved in synaptogenesis, we identified a weak hypomorphic mutation (R561H) in the kinesin- family member Unc KIF1A/unc-104 has been implicated in hereditary spastic paraplegia (HSP) (Erlich et al. 2011; Klebe et al. 2012) and hereditary sensory and autonomic neuropathy type 2 (Riviere et al. 2011). The R561H allele (unc-104 bris )isnotembryonic lethal allowing for detailed larval behavioral analysis. No synapse retraction or dystonic posterior paralysis, as described for the HSP type 10 Drosophila model, in which kinesin-1-based axonal transport is disturbed (Fuger et al. 2012), were observed. Rather, the observed defects were mostly neurodevelopmental. The affected arginine residue (R561) localizes to the b11- loop of the forkhead-associated (FHA) domain of Unc-104. Genetics, Vol. 195, September
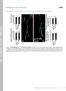
2 Figure 1 Bristly (bris) mutants are characterized by defects in synaptogenesis and dendritic filopodia formation. (A) Confocal immunofluorescence images showing several synaptic boutons at NMJ 4 of midthird instar Drosophila larvae. Wild-type and bris larvae were stained for glutamate receptor III (GluRIII, green) and Bruchpilot (Brp, gray and magenta). Many PSDs are unapposed by a presynaptic Brp puncta (arrowheads) in bris larvae. Bar, 1 mm. (B) While dendritic branching of the neuron ddaa (cell body, blue arrowhead) is unaffected, greatly increased filopodia formation at distal secondary branches is detected in bris third-instar larvae. Bar, 20 mm. *For visualization of secondary branches, brightness and contrast were adjusted in both enlargements. Bar, 10 mm. Structure-based analysis suggests that interaction of the corresponding residue (R58) in human KIF1A with the E499 residue located in the b1/b2-loop might stabilize kinesin- dimers (Huo et al. 2012). The functional relevance of the interaction has not yet been elucidated. Here, we employed larvae as a model to investigate the functional importance of Unc-104 during larval development and to characterize the effects of disrupting the proposed electrostatic interaction between the b11- and the b1/b2-loop in vivo. We show that R561 is important for kinesin- function and provide evidence that Unc-104 critically restricts neuromuscular junction (NMJ) growth during larval development. Moreover, Unc-104 is essential for the reliable apposition of active zones (AZs) and postsynaptic densities (PSDs) and controls dendrite branch morphogenesis. In addition to previously described roles (Pack-Chung et al. 2007; Barkus et al. 2008), these novel functions demonstrate that Unc-104 orchestrates synapse formation and maturation at the NMJ during embryonic and larval development and also regulates dendrite morphogenesis. Materials and Methods Fly stocks Flies were raised on standard medium at 25 unless otherwise noted. The following fly strains were used in this study: unc-104 d11204 (Thibault et al. 2004), Df(2R)Exel 7145 (O Farrell et al. 2008), Df(2R)Exel 6064 (Parks et al. 2004), Df(2R)ED 181, elav C155 -Gal4, D42-Gal4, and UAS-actin GFP. UAS-unc-104 mcherry wasobtainedfromthomasschwarz(harvard University,Cambridge,MA).TransgenicRNAistockswere obtained from the Vienna Drosophila RNAi Center (Dietzl et al. 2007). UAS-Brp and UAS-Brp-RNAi stocks were obtained from Stephan Sigrist (Freie Universität Berlin, Germany). Fly strains were obtained from the Bloomington Stock Center unless otherwise noted. Immunohistochemistry For preparation of larval fillets, third instar larvae were dissected essentially as previously described (Qin et al. 2005). In brief, larvae were gently stretched and immobilized on a dissection plate with the dorsal midline of the larvae facing upward. Insect pins were positioned at the anterior and posterior end of the larvae. After applying a drop of ice-cold hemolymph-like saline solution, the larvae were cut open along the dorsal midline. The epidermis was stretched and fixed using insect pins. Internal organs were removed using forceps. Care was taken to avoid damaging the body wall muscles and the central nervous system. Next, larvae were fixed (4% paraformaldehyde in phosphate buffered saline, PBS), blocked (PBS, 5% normal goat serum, 0.05% Triton X-100), and stained with primary (4, overnight) and secondary (2 h at room temperature) antibodies essentially as previously described (Qin et al. 2005). The peptide (PRRSLDKSLDRTPKS) was used to generate and affinity purify the rabbit anti-gluriii antibody. The purified antibody was used at a 1:1000 dilution. The following primary antibodies, obtained from the Developmental Studies Hybridoma Bank, were used at the concentrations indicated: Bruchpilot (nc82) 1:100 and Dlg (4F) 1:50. VGlut (1:1000) was obtained from Hermann Aberle (Mahr and Aberle 2006), and Unc-104/Imac (1:1000) from Thomas Schwarz (Pack-Chung et al. 2007). Horseradish peroxidase (HRP)-Cy (1:500) and HRP-Cy5 (1:500) were obtained from Dianova. The fluorescence-labeled secondary antibodies were from Invitrogen, MolecularProbes,orfromFluka/Sigma. Embryo collection and fixation Embryos were collected on apple juice plates at 25. At 16.5 h after egg laying (AEL), embryos were prepared for confocal microscopy as previously described (Mahr and Aberle 2006). Analysis of sensory neurons To analyze the dendritic branches in body-wall sensory neurons, larvae were killed by immersing them for 2 4 s in 65 water. Next, larvae were mounted in a chamber originally designed for in vivo imaging and imaged within 15 min on a Zeiss LSM 710 confocal microscope. Details on the construction of the chamber and larvae mounting have been previously described (Fuger et al. 2007; Zhang et al. 2010). Imaging and analysis Wholelarvaeorlarvalfillets were imaged on a Zeiss LSM 710 confocal microscope, equipped with 405, 440, 488, 514, 561, 60 J. V. Kern et al.
3 Figure 2 bristly is an allele of the neuronal kinesin- family member unc-104/kif1a. (A) Schematic of the unc-104 gene locus. Numbers indicate the chromosomal base pair position. Three overlapping deficiencies denoted as black boxes (from top to bottom Df (2R): Exel7145, Exel 6064, ED 181) failed to complement the bris locus. The three deficiencies overlap at position (2R) (orange) of the Drosophila genome. The XP insertion unc-104 d11204 (red arrowhead) failed to complement bris. Unc-104 is denoted as a green box; other genes in the identified region are shown in blue or pink. (B) Five alterations from the unc-104 reference sequence deposited at Flybase (Entrez GeneID: 6876, FBgn004155) could be identified. Four of these mutations (V722L, D107E, V1170M, and A1450V) represent likely polymorphisms because they were present in the parental strain used to perform the mutagenesis (left panel, unc-104 bris and right panel, parental strain). The original sequencing reads are shown. Arrow indicates the sequenced DNA strand. (C) Predicted domain structure of unc-104 and locations of selected mutations. R561H localizes to a conserved residue in the FHA domain of unc-104. Motor domain, purple; coiled coil-rich region, gray; FHA domain, orange; liprin binding domain (LBD), green; and PH domain, blue. (D) Alignment of Unc-104 in various species [Drosophila melanogaster (D.m.), Tribolium castaneum (T.c.), Caenorhabditis elegans (C. e.), Danio rerio (D.r.), and Homo sapiens (H.s.)]. The mutated R561 residue (red arrowhead) is conserved across all species examined. (E) Unc-104 abundance is not significantly reduced in ventral nerve cords of unc-104 bris larvae. (F) Percentage of PSDs unapposed by Brp were quantified in NMJ 4, segment A2 of mid-third instar Drosophila larvae of the following genotypes: elav-gal4/+;;uas-unc- 104 mcherry /+ (control), unc-104 bris /2; UAS-unc-104 mcherry /+ (unc-104 bris /2), and elav-gal4/+;unc-104 bris /2;UASunc-104 mcherry /+ (rescue). The standard error of the mean (SEM) is shown as a box, the standard deviation (SD) as a black line. ***P, (G) Synaptic boutons at NMJ 4 of mid-third instar Drosophila larvae stained for GluRIII (green) and Brp (gray and magenta). Panneuronal expression of unc-104 mcherry rescues defects in the apposition of AZs and PSDs. Arrowheads point at PSDs unapposed by a Brp-positive AZ. Bar, 1 mm. Unc-104 Regulates Synaptogenesis 61
4 Figure Dendrite morphology is unchanged in unc-104 bris /2 embryos at 16.5 h AEL. (A and B) Confocal images of sensory neurons in embryos that express UAS-actin GFP panneuronally (elav- Gal4/+;;UAS-actin GFP /+ [control] and unc- 104 bris /2;UAS-actin GFP /+ [unc-104 bris /2]). (A and B) No morphological alterations were observed in unc-104 bris /2 embryos. (A9 and B9) The axonal or dendritic morphology of bipolar (arrowheads) or dendritic arborization neurons (A99,A999,B99, and B999; arrows) is indistinguishable between unc-104 bris /2 and control embryos. Bars: A, 20 mm; A9 and A99, 10 mm; A999, 1mm. and 6 laser lines and a ConfoCor scanhead using a 40 Plan-Apochromat 1. N.A. or a 6 Plan-Apochromat 1.4 N.A. objective. Unless otherwise specified, the images were obtained using photomultiplier tube (PMT) detection. If fluorescence intensity was low, the sample was imaged using avalanche photodiode (APD) detection. Unless otherwise specified, the voxel dimensions (x/y/z) for NMJs were nm and for ventral nerve cords, nm. Embryonic or larval sensory neurons were recorded with the following voxel sizes: embryonic sensory neurons shown in Figure, A, A9, B,andB9: nm; embryonic sensory neurons shown in Figure, A99, A999, B99, B999: nm; and larval sensory neurons: nm. Images were scaled by a factor of 2, and Gaussian blur filtering was applied (pixel radius = 2). Gamma values were adjusted for illustrational purposes to For qualitative comparison of morphology, each genotype was imaged at the maximum level of brightness while avoiding saturation. For quantitative comparisons of intensities, common settings were chosen to avoid oversaturation in any of the genotypes. Image processing was performed using ImageJ v. 1.40g. The length of NMJs is the sum of the length of all branches. NMJ area was determined by measuring the HRP-positive area. The HRP signal was first rescaled by a factor of 2 and then filtered (Gaussian blur, pixel radius: 2) and z projected (maximum intensity projection). Next, a dynamic threshold was applied to the HRP signal to create a mask that defines the NMJ size. This dynamic threshold is constant within a given genotype, but might differ between genotypes. It was set to 40% of the mean HRP intensity measured in a given genotype. Bouton numbers were counted manually. NMJ size and bouton number were normalized to the square of the muscle length, and NMJ length was normalized to the muscle length. Only muscles within the size range: mm were included in the normalization. Nonnormalized NMJ length and size, as well as boutons per NMJ and muscle length are shown for comparison in Supporting Information, Figure S1, A D. Locomotion analysis All solutions used to wash larvae were at room temperature. Behavioral assays were performed at 25 at 70% humidity. For qualitative analysis of larval behavior, single larvae were placed on a thin slice of apple juice agar. Dystonic posterior paralysis behavior was scored visually. The animals were recorded at a frame rate of 0 fps for 5 min using a DCM510 camera (ScopeTek, P. R. China) integrated in a custom-built stereomicroscope. Videos were converted using Prism Video Converter, v (NCH Software, Canberra, Australia) and further processed using VirtualDub and ImageJ v. 1.40g. Size is the main determinant of larval locomotion speed (Fuger et al. 2012). We thus analyzed size-matched, rather than stageor age-matched larvae that might be slower due to delayed development and smaller size. For quantitative analysis, up to 200 larvae were recovered from food, dispersed in 15% sucrose solution, rinsed with tap water, and stored on a temperature- and humidity-controlled agar plate for 45 min. Next, the larvae were recorded on a cm agar plate for 10 min. Locomotion speed and size of larvae were analyzed with the custom-built software Animaltracer (Fuger et al. 2012). Larvae that touched each other were excluded from analysis. Larvae whose velocity was,10% of average velocity of the respective genotype were excluded from analysis. This selection criterion prevented the erroneous inclusion of dead larvae or debris in the dataset. Average locomotion speed was separately calculated for each size group. A minimum of six experiments per genotype were analyzed. 62 J. V. Kern et al.
5 generations. Next, we identified three overlapping deficiencies (Thibault et al. 2004; Ryder et al. 2007) (Df(2R): Exel 7145, Exel 6064, and ED 181) that failed to complement the bristly locus. These deficiencies overlap at position (2R) of the Drosophila genome. The same area was identified utilizing recombination-based mapping with molecularly defined P-element insertions (Zhai et al. 200). Next, we tested mutations that affect candidate genes in the identified region. Results bristly is an allele of the neuronal kinesin- family member unc-104/kif1a Figure 4 Impaired larval locomotor activity in unc-104 bris larvae. (A) unc- 104 bris larvae are slower than wild-type larvae. unc-104 bris /2 larvae are largely immobile and die in the late third instar stage. (B) Larvae in the act of crawling. No dystonic posterior paralysis is observed. unc-104 bris /2 larvae display a body posture defect that can be rescued by panneuronal expression of unc-104 mcherry. The SEM is shown as a box, the SD as a black line. ***P, For all further statistical analysis, n was defined as the number of experiments. Statistical analysis Statistical tests were performed with PAST software ( folk.uio.no/ohammer/past/index.html) unless otherwise noted. Sample errors are given as standard deviation (SD) and standard error of the mean (SEM). Statistical tests used in this study are documented in Table S1. The following alpha levels were used for all tests: *P, 0.05, **P, 0.01, and ***P, Genetic screen To score for defects in synapse formation and maintenance, immunostainings of type I boutons of Drosophila larvae were analyzed. At the investigated NMJ (NMJ 4, segment A) typically.95% of all PSDs are apposed by mature AZs. We used the cytomatrix of the AZ protein Bruchpilot (Brp) (Kittel et al. 2006) and the glutamate receptor subunit III (GluRIII) (Marrus et al. 2004) as molecular marker for AZs and PSDs, respectively. Mutant strains in which a significant number of unapposed PSDs or AZs were detected upon visual inspection were isolated for further analysis. Mapping of bristly To map the bris gene mutation, the original stock was outcrossed to a control Drosophila strain (white 1118 ) for three Although cytoskeletal structure, membrane protein sorting, and protein targeting mechanisms can be distinct between dendrites and axons, there are a considerable number of proteins important for both axonal and dendritic morphogenesis (Gao et al. 1999). We therefore decided to systematically screen mutants with abnormal dendrite morphogenesis for defects in synapse formation and maintenance. We selected bristly (bris) larvae, in which 17% of all PSDs at NMJs 4 are not apposed by a Brp-positive AZ for further characterization (Figure 1A). bris was originally isolated due to enhanced filopodia formation at the tip of the neuron ddaa (Figure 1B and Medina et al. 2006) but did not show obvious defects in dendritic branching (Figure 1B and Medina et al. 2006). The XP insertion unc-104 d11204 in the kinesin- unc-104 failed to complement the bristly mutation (Figure 2A, red arrowhead). Sequencing revealed five predicted amino acid changes compared to the referral sequence (Entrez Gene ID: 6876, FBgn004155): R561H, V722L, D107E, V1170M, and A1450V (Figure 2B). In contrast to the other four amino acid substitutions, the R561H mutation is absent in the parental strain used to perform the mutagenesis. Except for R561H, the other amino acid substitutions are predicted conservative substitutions. Hence, we concluded that four of the discrepancies from the database sequence (V722L, D107E, V1170M, and A1450V) are naturally occurring polymorphisms (Figure 2B), while R561H is responsible for the observed phenotype. The original sequences are available in File S1. R561 is a highly conserved residue in the FHA domain (Figure 2, C and D), which is considered potentially important for dimerization and activation of kinesin- motors (Huo et al. 2012). Previously described unc-104 null mutants (unc / Df(2R)DAlk21) are embryonic lethal (Pack-Chung et al. 2007); however, homozygous unc-104 bris larvae or transheterozygous (unc-104 bris /unc-104 d11204 and unc-104 bris /unc ) larvae die in the third instar larval stage. This new hypomorphic mutation therefore provides a powerful tool to investigate later neuronal processes that might depend on Unc-104/KIF1A. R561H does not adversely affect the stability of Unc-104 bris as quantified by immunostaining in ventral nerve cords (Figure 2E). Panneuronal expression of an mcherry-tagged unc-104 cdna construct is sufficient to Unc-104 Regulates Synaptogenesis 6
6 Figure 5 No synapse retraction observed in unc- 104 bris larvae. (A) Proximal and distal synaptic boutons at NMJ 4 of mid-third instar Drosophila larvae stained for GluRIII (green) and Brp (gray and magenta). Unapposed PSDs (arrowheads) localize to proximal and distal boutons in unc-104 bris larvae. Bar, 1 mm. (B E) NMJ 4, segment A2 or A of mid-third instar Drosophila wild-type (B and D) and unc-104 bris (C and E) larvae were stained for the membrane marker HRP (B and C; magenta), the postsynaptic scaffolding protein Discslarge (Dlg) (B E, green), and the vesicular glutamate transporter (VGlut) (D and E, gray and magenta). The presence of organized postsynaptic Dlg staining unapposed by presynaptic structures is indicative of synapse retraction, which typically occurs at distal boutons (arrowheads). No synapse retraction was observed in unc-104 bris larvae. Bars, 10 mm and 2 mm (magnified panel). rescue transheterozygous unc-104 bris /unc-104 d11204 larvae (from now on referred to as: unc-104 bris /2) to full viability. Panneuronal expression of unc-104 mcherry using elav-gal4 is also sufficient to rescue defects in the reliable apposition of AZs and PSDs in unc-104 bris /2 larvae (Figure 2, F and G). We thus concluded that observed phenotypes are specifically caused by the loss of Unc-104 function. Dendrite morphogenesis We next sought to address the cellular basis for the observed dendritic morphology alterations. Dendritic morphology is continuously refined by multiple interrelated pathways controlling outgrowth, branching, guidance, and pruning (Corty et al. 2009). For simplicity, we only differentiated between initial neurite outgrowth in the developing embryo and dendrite maturation, which includes morphological changes during later outgrowth, branching, guidance, and pruning. unc-104 expression is first detectable in stage 11 embryos (Pack-Chung et al. 2007). At 14 h AEL, homozygous unc null mutant embryos displayed abnormal axonal growth cone morphology (Pack-Chung et al. 2007). We scored defects in initial sensory neurite outgrowth in midstage 16 embryos (16.5 h AEL), in which unc-104 bris has been expressed for roughly 10 h to allow for the manifestation of defects. Sensory neurons were visualized by panneuronal expression of UAS-actin GFP. No gross morphological alterations of the peripheral sensory neuron system were observed in unc-104 bris /2 embryos (Figure, A and B). Dorsal dendriteextensionwasnearlycompletedat16.5haelbothin unc-104 bris /2 and control embryos. No obvious alterations in axonal or dendritic morphology of bipolar neurons (Figure, A9 and B9; arrowheads) or dendritic arborization neurons (Figure, A99, A999, B99, and B999; arrows) were observed. Hence, we concluded that the morphological defects observed in L larvae (Figure 1B and Medina et al. 2006) were caused by impairments in dendrite maturation. unc-104 bris larvae are characterized by impaired larval locomotion Mutations in the human unc-104 homolog KIF1A cause HSP (Erlich et al. 2011; Klebe et al. 2012) or hereditary sensory and autonomic neuropathy type 2 (Riviere et al. 2011). We 64 J. V. Kern et al.
7 Figure 6 Axonal transport of Brp is impaired in unc- 104 bris /2 larvae. (A and A9) Ventral nerve cords of midthird instar Drosophila larvae were stained for Brp, which is concentrated in the neuropil region (green N) of wildtype larvae, but is found in the cortex of unc-104 bris /2 larvae (green C). (A9) Panneuronal expression of Unc- 104 mcherry (rescue) restores Brp neuropil localization in unc-104 bris /2 larvae. Bar, 20 mm. (B) NMJ 4, segment A2 of mid-third instar Drosophila larvae of the following genotypes: elav-gal4/+;;uas-unc-104 mcherry /+ (control), unc-104 bris /2;UAS-unc-104 mcherry /+ (unc-104 bris /2), or elav-gal4/+;unc-104 bris /2;UAS-unc-104 mcherry /+ (rescue) were stained for Brp. Bar, 10 mm. (C) Brp density and (D) Brp abundance per NMJ are reduced in unc-104 bris /2 larvae compared to control larvae and unc-104 bris /2 larvae expressing unc-104 mcherry. The SEM is shown as a box, the SD as a black line. *P, 0.05, **P, 0.01, ***P, sought to use unc-104 bris larvae as a model for the cellular consequences of a moderate loss of unc-104 function that might be relevant in the context of HSP. HSP is characterized by progressive spasticity and paralysis of the lower extremities. The selective vulnerability of long motoneuron-axons in the corticospinal tract is an important cellular hallmark of HSP. For HSP type 10 (HSP10) caused by autosomal dominant mutations in KIF5A/khc, this axon lengthdependent vulnerability of motoneurons could be replicated in Drosophila larvae by ectopic expression of mutant khc in the wild-type background (HSP10-model larvae) (Fuger et al. 2012). A selective vulnerability of posterior segments was evident by exacerbated morphological defects at synaptic terminals and dystonic posterior paralysis (tail-flip phenotype) (Fuger et al. 2012). Neither homozygous unc-104 bris larvae nor transheterozygous unc-104 bris /2 larvae displayed dystonic posterior paralysis, indicating that these larvae suffered from impairments distinct from those caused by impaired Khc function (Hurd and Saxton 1996; Fuger et al. 2012). HSP10 model larvae displayed a size-dependent reduction in larval locomotion speed that is most pronounced in large larvae (Fuger et al. 2012). Semiautomated analysis using the custom-built algorithm Animaltracer (Fuger et al. 2012) revealed that both small and large homozygous unc- 104 bris larvae are slower than control larvae, whereby the degree of impairment in larval locomotion relative to control larvae is independent of larval size (for larval length mm, wt: mm/s, n =6;unc-104 bris : mm/s, n = 7 and for larval length mm, wt: mm/s, n =6;unc-104 bris : mm/s, n =8).The genetically more impaired transheterozygous unc-104 bris /2 larvae were almost paralyzed (Figure 4A) and displayed a body posture defect (Figure 4B). Comparative behavioral analysis revealed that impairments in anterograde molecular motor function caused by either ectopic overexpression of dominant-negative khc constructs or the hypomorphic unc-104 bris allele led to distinct behavioral defects. Those caused by the unc-104 bris allele were likely neurodevelopmental and not neurodegenerative in nature, possibly due to the impaired maturation of a subset of synapses. Synaptic terminals in unc-104 bris larvae are not subject to neurodegeneration KIF1A has been shown to be important for hippocampal synaptogenesis and synaptic bouton formation (Hirokawa et al. 2010). During Drosophila synaptogenesis, Brp is reliably recruited to nascent AZs within a few hours following new PSD formation (Rasse et al. 2005). This matching of PSDs and AZs is disturbed in unc-104 bris /2 larvae. Defects in apposition might occur due to (i) presynaptic nerve retraction, (ii) rate limiting abundance of Brp, (iii) delivery of Brp to extrasynaptic sites, or (iv) disturbances in the molecular mechanism that allocate AZ components such that all synapses receive at least the minimal amount necessary to assemble a fully functional AZ. First, we sought to address whether defects in the apposition of pre- and postsynaptic components are the result of degenerative processes. Distal NMJ regions are preferentially Unc-104 Regulates Synaptogenesis 65
8 Figure 7 Brp overexpression does not rescue the AZ and PSD apposition defect in unc-104 bris /2 larvae. (A) Confocal images of neuromuscular synapses immunostained with Brp (magenta and gray) and GluRIIC (green) in larvae of the following genotypes: elav- Gal4/+;; (control), elav-gal4/+;unc-104 bris /2 (unc- 104 bris /2), and elav-gal4/+;unc-104 bris /2;UAS-Brp/+ (unc-104 bris /2,Brp[). Arrows indicate PSDs unapposed by presynaptic Brp punctae. No Brp punctae without an apposed PSD were detected. Bar, 1 mm. Quantification of the abundance of Brp per NMJ (B), Brp quantity at a single puncta (C), and the percentage of PSDs unapposed by Brp (D). Experiments were performed at 29. The SEM is shown as a box, the SD as a black line. *P, 0.05, **P, affected by degenerative processes than proximal regions (Sherwood et al. 2004). In unc-104 bris larvae, however, unapposed PSDs were distributed in a salt-and-pepper pattern throughout the NMJ without obvious regional preference (Figure 5A, arrowheads), indicating that synaptic defects are likely caused by impaired synaptogenesis at the level of individual synapses. Postsynaptic membranes at the Drosophila NMJ are a complex series of membrane folds (subsynaptic reticulum, SSR) that develop only gradually upon presynaptic innervation and remain stable for hours after presynaptic retraction. Thus, terminal boutons positive for SSR marker but negative for the presynaptic membranes or synaptic vesicles (SV) are indicative of synapse retraction (Eaton et al. 2002). We visualized the presynaptic membrane, SSR, and SV with antibodies directed against HRP, the postsynaptic Discs-large protein (Dlg), and the vesicular glutamate transporter VGlut, respectively (Mahr and Aberle 2006). No signs of synapse retraction were observed in unc-104 bris larvae (Figure 5, B E). Immunostaining analyses suggested delayed SSR development, as more synaptic boutons positive for HRP and VGlut but negative for Dlg (Figure 5, C and E; arrowheads) were observed in homozygous unc-104 bris larvae (WT: , n =10;unc-104 bris : , n =10;P, 0.001, Student s two-tailed t-test). Second, the reliable apposition of PSDs and AZs might be disturbed by a rate-limited supply of Brp. The loss of Unc- 104 function has been shown to affect cargo transport as quantified by cargo redistribution in the ventral nerve cord (Pack-Chung et al. 2007; Barkus et al. 2008). In control larvae, Unc-104 cargos are scant in the cortex and enriched in the neuropil. As previously reported for unc-104 null embryos (Pack-Chung et al. 2007), this distribution is reversed in unc-104 bris /2 larvae (Figure 6A). Furthermore, Brp density and the amount of Brp per NMJ in unc-104 bris /2 larvae were reduced by 70 and 74%, respectively (Figure 6, B D). Panneuronal expression of unc-104 mcherry was sufficient to rescue impairments in Brp transport (Figure 6, A D). As insufficient supply of Brp is a potential cause of the observed defects in the apposition of AZ and PSDs at unc-104 bris mutant synaptic terminals, we next rescued overall Brp abundance at NMJs (Figure 7, A and B) by panneuronal overexpression of Brp. Although Brp overexpression was sufficient to restore the overall abundance of Brp at the NMJ and the normal size of individual Brp punctae (Figure 7, A and C), the percentage of presynaptically unapposed PSDs was still four times higher in Brp-overexpressing unc-104 bris /2 larvae compared to control larvae (Figure 7, A and D). Brp punctae are generally apposed by PSDs (Figure 7A), suggesting that the molecular mechanisms that prevent AZ formation in the perisynaptic regionsofthenmjareintactinunc-104 bris /2 larvae. However, PSDs are not reliably apposed by Brp punctae (Figure 7A, arrowheads), indicating that Unc-104 might orchestrate the site-specific delivery of AZ components to defined synapses, thereby ensuring the proper allocation of Brp to all AZs. Unc-104 is essential to restrict NMJ growth Impairments in kinesin-1- and kinesin--based anterograde transport cause a reduction in NMJ size and a decrease in the number of synaptic boutons (Hurd and Saxton 1996; Barkus et al. 2008; Fuger et al. 2012). However, loss of the HSP-associated genes atlastin, spastin, and spichthyin induce NMJ overgrowth (Bayat et al. 2011). To test whether impaired Unc-104 function leads to morphological changes at the synaptic terminal the following parameters were determined: NMJ length, NMJ size, PSDs per NMJ, boutons per NMJ, bouton size, and number of synapses per bouton. NMJ size is determined by the area of HRP staining. NMJ length is a measure of synaptic terminal outgrowth, while NMJsizeandthenumberofPSDsperNMJareameasureof both outgrowth and functional maturation. NMJs of unc- 104 bris /2 larvae were longer but not larger than NMJs in control larvae (Figure 8, A C). The number of receptor fields per NMJ was decreased in unc-104 bris /2 larvae (control: , n =9;unc-104 bris /2: , n = 9; rescue: , n =9; control/unc-104 bris /2; P, 0.001, Kruskal Wallis H-test). NMJs of mutant larvae consist of more but smaller synaptic boutons that contain fewer glutamate receptor fields than control boutons, suggesting that terminal outgrowth was not matched by proper functionalization (Figure 8, A and D G). The overgrowth of unc-104 bris /2 NMJs was unexpected because previously described null and hypomorphic alleles 66 J. V. Kern et al.
9 Figure 8 Altered NMJ morphology in unc- 104 bris /2 larvae. (A) NMJ 4, segment A2 of mid-third instar Drosophila wild-type, unc- 104 bris /+, unc-104 bris, and unc-104 bris /2 larvae were stained with antibodies against HRP (A and E), GluRIII (E and L), and Brp (L). Bars: A, 10 mm and E and L, 1 mm. NMJ length (B), NMJ size (C), bouton number (D), bouton size (F), and PSD per boutons (G) were determined. The same genotypes were stained with Brp (H). Brp density (I), Brp per NMJ (J), and percentage of PSDs unapposed by Brp (K) were quantified. The SEM is shown as a box, the SD as a black line. *P, 0.05, **P, 0.01, ***P, (L) PSDs unapposed by a presynaptic Brp puncta (arrowheads) were detected in unc- 104 bris and unc-104 bris /2 larvae. cause defects in bouton formation and decreased number of boutons per NMJ, respectively (Pack-Chung et al. 2007; Barkus et al. 2008). We thus sought to (i) further investigate the nature of the putatively hypomorphic unc-104 bris mutation and (ii) verify that defects are solely caused by a presynaptic loss of Unc-104 function. The phenotype of hypomorphic alleles is less severe when homozygous than transheterozygous to a null allele or a deletion. unc-104 bris /2 larvae have stronger impairments in larval locomotion than homozygous unc-104 bris larvae. Generally, morphological defects were more pronounced in the former (Figure 8, A, B, D, E, and G L); however, this difference was only statistically significant for Brp density (Figure 8I). Defects in bouton size do not follow this general trend (Figure 8F). Despite a smaller bouton size and less PSDs per bouton, no NMJ morphogenesis defects were observed in heterozygous unc-104 bris /+ larvae (Figure 8, E G). RNAi-mediated knockdown of Unc-104 in neurons (elav- Gal4, D42-Gal4) but not in muscles (Mhc-Gal4, 24B-Gal4) is sufficient to induce lethality (data not shown). No Unc-104 signal was detectable in nonneuronal tissue (Pack-Chung et al. 2007). Panneuronal expression of unc-104 mcherry in the unc-104 mutant background was sufficient to rescue defects in NMJ morphogenesis (Figure 9, A G). Postsynaptic expression of unc-104 mcherry did not rescue neuromuscular morphological defects (Figure 10, A C). Overall, the morphological and behavioral analyses suggest that (i) unc-104 bris is a hypomorphic allele and (ii) defects are solely caused by loss of neuronal Unc-104 function. Unc-104 Regulates Synaptogenesis 67
10 Figure 9 Panneuronal expression of unc-104 rescues NMJ morphology defects in unc-104 bris /2 larvae. (A) NMJ 4, segment A2 of mid-third instar Drosophila larvae of the following genotypes elav-gal4/+;;uas-unc- 104 mcherry /+ (control), unc-104 bris /2;UAS-unc-104 mcherry /+ (unc-104 bris /2), and elav-gal4/+;unc-104 bris /2;UAS-unc- 104 mcherry /+ (rescue) were stained with antibodies against HRP (A and E) and GluRIII (E). Enlargements of A are shown in the lower panel. Bars: A, 10 mm and E, 1 mm. NMJ length (B), NMJ size (C), bouton number (D), bouton size (F), and PSDs per boutons (G) were determined. The SEM is shown as a box, the SD as a black line. **P, 0.01, ***P, NMJ overgrowth is independent of presynaptic Brp abundance NMJ overgrowth in unc-104 bris mutants, as quantified by NMJ length and the number of synaptic boutons, might be attributable to the loss of a specific function of Unc-104. Alternatively, defects in AZ assembly and maturation of synapses caused by the loss of Brp from some synapses might induce compensatory NMJ overgrowth. We favor the former possibility for the following reasons: first, reduction in synaptic Brp levels alone, using transgenic RNAi, do not cause changes in overall NMJ size (Wagh et al. 2006). Second, Brp clusters calcium channels at AZs (Kittel et al. 2006). Reduced expression of the voltage-dependent calcium channel subunit cacophony (cac) or a point mutation (cac NT27 ) that disrupts calcium entry and subsequent vesicle fusion limits NMJ growth rather than promoting NMJ overgrowth (Rieckhof et al. 200). If NMJ overgrowth is triggered as a compensatory response to the reduced level of synaptic Brp, further reduction or overexpression of Brp should exacerbate or rescue existing defects, respectively. To evaluate this hypothesis, we utilized previously validated UAS-Brp and Brp-RNAi constructs (Wagh et al. 2006). NMJ overgrowth was neither suppressed by the overexpression of Brp nor exacerbated by further reduction of Brp using RNAi (Figure 11, A F). Defects in the enlargement andmaturationofboutonspersistedbothafterdecreasingand increasing Brp abundance (Figure 11, E and F). We thus conclude that neither the overgrowth of NMJs nor the defects in bouton enlargement are secondary to reduced Brp abundance at the NMJ or its loss from a subset of AZs. Rather, they constitute novel functions of the Unc-104 FHA domain in limiting NMJ length and coordinating bouton enlargement. Discussion Dendrite morphogenesis Sensory neurons that normally bear actin-rich filopodia display increased filopodial density in unc-104 bris mutants with no obvious defects in dendritic branching (observed herein and previously Medina et al. 2006). Interestingly, alterations in the structure of actin-rich dendritic spines were among the first neuroanatomical changes associated with mental retardation 68 J. V. Kern et al.
11 Figure 10 Postsynaptic expression of unc-104 does not rescue morphological defects in unc-104 bris /2 larvae. (A C) NMJ 4, segment A2 of midthird instar Drosophila larvae of the following genotypes: wild-type, unc-104 bris /2, and unc-104 bris /2;24B-Gal4/UAS-unc-104 mcherry (postsynaptic rescue) were stained for the membrane marker HRP. No rescue was detectable following postsynaptic expression of unc-104 mcherry. Enlargements are shown in the lower panel. Bar, 10 mm. (Marin-Padilla 1972). More than 100 genes that coordinate dendrite arborization have been identified to date. Among these genes are two molecular motors: KIF5 (Hoogenraad et al. 2005) and dynein (Zhou et al. 2012). Our results suggest that Unc-104-based dendritic transport is important for maintaining correct dendrite morphology. Because no defects in dendrites were detected 16.5 h AEL, we concluded that impaired Unc-104 function during later outgrowth, branching, guidance, and pruning leads to the defects in larval dendrite morphology described here. Sensory neuron dendritic arbor morphology has been suggested to affect the ability of cells to perceive and transmit external stimuli (Hall and Treinin 2011). The resulting association between form and function might explain the divergence of dendritic morphologies. For example, class I, II, and III multidendritic sensory neurons, which respond to touch and serve as proprioceptors to coordinate larval contraction wave propagation, differ morphologically from class IV multidendritic neurons that sense noxious stimuli, such as heat, high-threshold mechanical stimuli, parasitoid wasps, and noxious light (Hall and Treinin 2011; Tsubouchi et al. 2012). unc-104 bris larvae are characterized by enhanced filopodia formation. As the number of dendritic sensory filopodia can correlate with the strength of the gentle touch response in class III multidendritic neurons (Tsubouchi et al. 2012), a detailed investigation of the behavioral responses to external stimuli in unc-104 bris larvae might enhance our understanding of the molecular pathways underlying sensory neuron dendrite structure and function. Synapse apposition unc-104 bris larvae are characterized by impairments in the reliable apposition of AZs and PSDs as quantified by staining for Brp (Wagh et al. 2006) and glutamate receptors. Brp clusters calcium channels at AZs and stabilizes T-bars, which are electron-dense presynaptic structures that have been shown to facilitate synaptic release (Kittel et al. 2006). In wild-type larvae, primarily very young (, h), immature synapses are Brp negative (Rasse et al. 2005). Because Brp-negative synapses have a low vesicle release probability, the accumulation of Brp at nascent AZs is an important step during synapse maturation (Rasse et al. 2005; Kittel et al. 2006; Schmid et al. 2008). The high percentage of Brpnegative synapses in the unc-104 bris mutant suggests that synapse maturation is impaired either by rate-limiting axonal transport of Brp, defective delivery of Brp to AZs, or the inability to stabilize synaptic Brp. Restoration of Brp abundance at NMJs in unc-104 bris larvae ameliorates but does not rescue defects in the apposition of AZs and PSDs. We thus propose that the R561H mutation in Unc-104 s FHA domain might disrupt a previously unknown function of Unc-104 in orchestrating the site-specific delivery of Brp to defined synapses. The specificity of defects caused by the R561H mutation is further supported by the analysis of unc /2 mutant embryos. The total amount of Brp per NMJ at 21 h AEL was more severely reduced than in unc-104 bris larvae (Pack- Chung et al. 2007). Nonetheless, no defects in the apposition of AZ and PSDs were reported; most PSDs were apposed by a weak Brp-positive signal (Pack-Chung et al. 2007). The precise coordination of synaptic cargo delivery to defined synapses is important to functionalize and facilitate these synapses (for review see Vitureira et al. 2011; Clarke et al. 2012). Intact Unc-104/KIF1A-based cargo trafficking might, reminiscent of kinesin-1-based long-range transport (Puthanveettil et al. 2008), be important for the late phase of long-term potentiation (Bliss and Lomo 197), which is highly relevant in the context of learning and memory. Indeed, environmental enrichment has been shown to be sufficient to induce brain-derived neurotrophic factor (BDNF)- dependent up-regulation of KIF1A (Kondo et al. 2012). The resulting hippocampal synaptogenesis and learning enhancement is absent in transheterozygous Bdnf +/2 and Kif1a +/2 mutant mice, highlighting that Unc-104/KIF1A is essential for experience-dependent neuroplasticity (Kondo et al. 2012). We propose that the presynaptic mechanism underlying neuroplasticity depends on both Unc-104-based long-range axonal transport and the site-specific delivery of synaptic cargo to defined synapses. Both processes might be perturbed by mutation of the R561 residue that localizes to the b11-loop of the FHA domain. The corresponding residue (R58) in KIF1A has been suggested to further stabilize kinesin- dimers by electrostatic interaction with the E499 residue (Huo et al. 2012). Further investigation of the effects of R561H on the activation and deactivation of Unc-104 upon cargo loading and release might provide novel insights into the biomechanics of kinesin--based cargo trafficking. Unc-104 Regulates Synaptogenesis 69
12 Figure 11 Altering Brp abundance does not overtly alter NMJ morphology. (A F) NMJ 4, segment A2 of mid-third instar Drosophila larvae of the following genotypes: elav- Gal4/+;unc-104 bris /2 (unc-104 bris /2), elav-gal4/+;unc- 104 bris /2,UAS-Brp-RNAi/+ (BrpY), and elav-gal4/+; unc-104 bris /2;UAS-Brp/+ (Brp[) were stained for the membrane marker HRP (A). The following characteristics of NMJs were determined: NMJ length (B), NMJ size (C), bouton number (D), bouton size (E), and PSDs per bouton (F). Bar, 10 mm. The SEM is shown as a box, the SD as a black line. Molecular pathways underlying structural defects at NMJs The herein described overgrowth of NMJs in unc-104 bris larvae was surprising as both impairments in kinesin-1- and kinesin- -based anterograde transport were reported to result in reductions in both NMJ size and synaptic bouton number (Hurd and Saxton 1996; Barkus et al. 2008; Fuger et al. 2012). Conversely, phenotypic comparison with other HSPassociated genes revealed that the NMJ overgrowth is a common phenotype in HSP fly models. Null alleles in atlastin, spastin, and spichthyin result in synaptic bouton number increases of 17, 60, and 100% respectively (Bayat et al. 2011). Interestingly, in unc-104 bris /2 larvae, NMJ overgrowth and defects in bouton enlargement and maturation seem to be independent of Unc-104 s role in fast axonal transport of AZ components and might thus represent novel functions of Unc-104. Defects in bidirectional communication between muscles and motoneurons might induce NMJ overgrowth. The absence of bone morphogenetic protein (BMP)-signaling suppressors, such as daughters against decapentaplegic and the HSP-related gene spichthyin, causes NMJ overgrowth (Sweeney and Davis 2002; Wang et al. 2007). Thus, the NMJ overgrowth observed in unc-104 bris larvae is consistent with potential overactivation of BMP signaling. Due to the importance of BMP signaling in synapse stabilization (Eaton and Davis 2005), its overactivation might prevent the elimination of functionally impaired synaptic terminals in unc-104 bris mutant larvae. Conclusions Our identification of a novel unc-104 allele highlights the importance of kinesin activity during later stages of neuronal development. The results described here shed light on three previously unknown roles of Unc-104. First, it is important for maintaining correct dendritic morphology. Second, Unc-104 is required for the reliable apposition of AZs and glutamate receptor fields. Third, Unc-104 is important to limit NMJ outgrowth as measured by NMJ length and bouton number. Alterations in dendritic and synaptic terminal maturation are independently associated with mental retardation and neurodegeneration. The fact that a single amino acid substitution of arginine for histidine in the FHA domain of Unc- 104 is sufficient to perturb all these processes presents an ideal tool to decipher the biophysical underpinnings of neurodevelopmental disorders. Further study is needed to decipher the exact role of Unc- 104 in orchestrating synaptic terminal outgrowth and functional maturation. Studies of the FHA domain in a biochemical setting may provide novel insights into (i) the biomechanics of kinesin--based cargo trafficking, (ii) Unc-104 s roles in synapse development and synaptic plasticity, and (iii) the molecular mechanism underlying HSP and neurodevelopmental diseases in which synapses might fail to mature. Acknowledgments We thank Christian Hünefeld, Roman Beck, Jörg Odenthal, Raphael S. Zinser, Shabab Hannan, Junyi Zhu, and Jutta Bloschies for help and/or advice. We thank Stephan Sigrist, Hermann Aberle, Ann Goldstein, and Thomas Schwarz for providing reagents. This work was supported by grants from the Deutsche Forschungsgemeinschaft (RA 1804/2-1 to T.M.R.) and National Institute of Neurological Disorders and 70 J. V. Kern et al.
13 Stroke (NS to J.E.B.). Y.V.Z. was supported by a fellowship of the China Scholarship Council. Literature Cited Barkus, R. V., O. Klyachko, D. Horiuchi, B. J. Dickson, and W. M. Saxton, 2008 Identification of an axonal kinesin- motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol. Biol. Cell 19: Bayat, V., M. Jaiswal, and H. J. Bellen, 2011 The BMP signaling pathway at the Drosophila neuromuscular junction and its links to neurodegenerative diseases. Curr. Opin. Neurobiol. 21: Bliss, T. V., and T. Lomo, 197 Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 22: Cai, Q., P. Y. Pan, and Z. H. Sheng, 2007 Syntabulin-kinesin-1 family member 5B-mediated axonal transport contributes to activity-dependent presynaptic assembly. J. Neurosci. 27: Clarke, G. L., J. Chen, and H. Nishimune, 2012 Presynaptic Active Zone Density during Development and Synaptic Plasticity. Front Mol Neurosci 5: 12. Corty, M. M., B. J. Matthews, and W. B. Grueber, 2009 Molecules and mechanisms of dendrite development in Drosophila. Development 16: Dietzl, G., D. Chen, F. Schnorrer, K. C. Su, Y. Barinova et al., 2007 A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: Eaton, B. A., and G. W. Davis, 2005 LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron 47: Eaton, B. A., R. D. Fetter, and G. W. Davis, 2002 Dynactin is necessary for synapse stabilization. Neuron 4: Erlich, Y., S. Edvardson, E. Hodges, S. Zenvirt, P. Thekkat et al., 2011 Exome sequencing and disease-network analysis of a single family implicate a mutation in KIF1A in hereditary spastic paraparesis. Genome Res. 21: Fuger, P., L. B. Behrends, S. Mertel, S. J. Sigrist, and T. M. Rasse, 2007 Live imaging of synapse development and measuring protein dynamics using two-color fluorescence recovery after photo-bleaching at Drosophila synapses. Nat. Protoc. 2: Fuger, P., V. Sreekumar, R. Schule, J. V. Kern, D. T. Stanchev et al., 2012 Spastic paraplegia mutation N256S in the neuronal microtubule motor KIF5A disrupts axonal transport in a Drosophila HSP model. PLoS Genet. 8: e Gao, F. B., J. E. Brenman, L. Y. Jan, and Y. N. Jan, 1999 Genes regulating dendritic outgrowth, branching, and routing in Drosophila. Genes Dev. 1: Hall, D. H., and E. M. Hedgecock, 1991 Kinesin-related gene unc- 104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65: Hall, D. H., and M. Treinin, 2011 How does morphology relate to function in sensory arbors? Trends Neurosci. 4: Hirokawa, N., and Y. Noda, 2008 Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol. Rev. 88: Hirokawa, N., S. Niwa, and Y. Tanaka, 2010 Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron 68: Hoogenraad, C. C., A. D. Milstein, I. M. Ethell, M. Henkemeyer, and M. Sheng, 2005 GRIP1 controls dendrite morphogenesis by regulating EphB receptor trafficking. Nat. Neurosci. 8: Huo, L., Y. Yue, J. Ren, J. Yu, J. Liu et al., 2012 The CC1-FHA tandem as a central hub for controlling the dimerization and activation of kinesin- KIF1A. Structure 20: Hurd, D. D., and W. M. Saxton, 1996 Kinesin Mutations Cause Motor Neuron Disease Phenotypes by Disrupting Fast Axonal Transport in Drosophila. Genetics 144: Kittel, R. J., C. Wichmann, T. M. Rasse, W. Fouquet, M. Schmidt et al., 2006 Bruchpilot promotes active zone assembly, Ca2 + channel clustering, and vesicle release. Science 12: Klebe, S., A. Lossos, H. Azzedine, E. Mundwiller, R. Sheffer et al., 2012 KIF1A missense mutations in SPG0, an autosomal recessive spastic paraplegia: distinct phenotypes according to the nature of the mutations. Eur. J. Hum. Genet. 20: Kondo, M., Y. Takei, and N. Hirokawa, 2012 Motor protein KIF1A is essential for hippocampal synaptogenesis and learning enhancement in an enriched environment. Neuron 7: Mahr, A., and H. Aberle, 2006 The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expr. Patterns 6: Marin-Padilla, M., 1972 Structural abnormalities of the cerebral cortex in human chromosomal aberrations: a Golgi study. Brain Res. 44: Marrus, S. B., S. L. Portman, M. J. Allen, K. G. Moffat, and A. DiAntonio, 2004 Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J. Neurosci. 24: Medina, P. M., L. L. Swick, R. Andersen, Z. Blalock, and J. E. Brenman, 2006 A novel forward genetic screen to identify mutations affecting larval neuronal dendrite development in Drosophila melanogaster. Genetics 172: Nangaku, M., R. Sato-Yoshitake, Y. Okada, Y. Noda, R. Takemura et al., 1994 KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell 79: O Farrell, F., S. S. Esfahani, Y. Engstrom, and P. Kylsten, 2008 Regulation of the Drosophila lin-41 homologue dappled by let-7 reveals conservation of a regulatory mechanism within the LIN-41 subclade. Dev. Dyn. 27: Ohashi, S., K. Koike, A. Omori, S. Ichinose, S. Ohara et al., 2002 Identification of mrna/protein (mrnp) complexes containing Puralpha, mstaufen, fragile X protein, and myosin Va and their association with rough endoplasmic reticulum equipped with a kinesin motor. J. Biol. Chem. 277: Pack-Chung, E., P. T. Kurshan, D. K. Dickman, and T. L. Schwarz, 2007 A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat. Neurosci. 10: Parks, A. L., K. R. Cook, M. Belvin, N. A. Dompe, R. Fawcett et al., 2004 Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat. Genet. 6: Puthanveettil, S. V., F. J. Monje, M. C. Miniaci, Y. B. Choi, K. A. Karl et al., 2008 A new component in synaptic plasticity: upregulation of kinesin in the neurons of the gill-withdrawal reflex. Cell 15: Qin, G., T. Schwarz, R. J. Kittel, A. Schmid, T. M. Rasse et al., 2005 Four different subunits are essential for expressing the synaptic glutamate receptor at neuromuscular junctions of Drosophila. J. Neurosci. 25: Rasse, T. M., W. Fouquet, A. Schmid, R. J. Kittel, S. Mertel et al., 2005 Glutamate receptor dynamics organizing synapse formation in vivo. Nat. Neurosci. 8: Rieckhof, G. E., M. Yoshihara, Z. Guan, and J. T. Littleton, 200 Presynaptic N-type calcium channels regulate synaptic growth. J. Biol. Chem. 278: Unc-104 Regulates Synaptogenesis 71
14 Riviere, J. B., S. Ramalingam, V. Lavastre, M. Shekarabi, S. Holbert et al., 2011 KIF1A, an axonal transporter of synaptic vesicles, is mutated in hereditary sensory and autonomic neuropathy type 2. Am. J. Hum. Genet. 89: Ryder,E.,M.Ashburner,R.Bautista-Llacer,J.Drummond,J.Webster et al., 2007 The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics 177: Schmid,A.,S.Hallermann,R.J.Kittel,O.Khorramshahi,A.M.Frolich et al., 2008 Activity-dependent site-specific changes of glutamate receptor composition in vivo. Nat. Neurosci. 11: Sherwood, N. T., Q. Sun, M. Xue, B. Zhang, and K. Zinn, 2004 Drosophila spastin regulates synaptic microtubule networks and is required for normal motor function. PLoS Biol. 2: e429. Sweeney, S. T., and G. W. Davis, 2002 Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron 6: Thibault, S. T., M. A. Singer, W. Y. Miyazaki, B. Milash, N. A. Dompe et al., 2004 A complementary transposon tool kit for Drosophila melanogaster using P and piggybac. Nat. Genet. 6: Tsubouchi, A., J. C. Caldwell, and W. D. Tracey, 2012 Dendritic filopodia, Ripped Pocket, NOMPC, and NMDARs contribute to the sense of touch in Drosophila larvae. Curr. Biol. 22: van den Berg, R., and C. C. Hoogenraad, 2012 Molecular motors in cargo trafficking and synapse assembly. Adv. Exp. Med. Biol. 970: Verhey, K. J., and J. W. Hammond, 2009 Traffic control: regulation of kinesin motors. Nat. Rev. Mol. Cell Biol. 10: Verhey, K. J., N. Kaul, and V. Soppina, 2011 Kinesin assembly and movement in cells. Annu Rev Biophys 40: Vitureira, N., M. Letellier, and Y. Goda, 2011 Homeostatic synaptic plasticity: from single synapses to neural circuits. Curr. Opin. Neurobiol. 22: Wagh, D. A., T. M. Rasse, E. Asan, A. Hofbauer, I. Schwenkert et al., 2006 Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron 49: Wang, X., W. R. Shaw, H. T. Tsang, E. Reid, and C. J. O Kane, 2007 Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat. Neurosci. 10: Zhai, R. G., P. R. Hiesinger, T. W. Koh, P. Verstreken, K. L. Schulze et al., 200 Mapping Drosophila mutations with molecularly defined P element insertions. Proc. Natl. Acad. Sci. USA 100: Zhang, Y., P. Fuger, S. B. Hannan, J. V. Kern, B. Lasky et al., 2010 In vivo imaging of intact Drosophila larvae at sub-cellular resolution. J. Vis. Exp. 10: pii: Zhou, B., Q. Cai, Y. Xie, and Z. H. Sheng, 2012 Snapin Recruits Dynein to BDNF-TrkB Signaling Endosomes for Retrograde Axonal Transport and Is Essential for Dendrite Growth of Cortical Neurons. Cell Rep 2: Communicating editor: P. K. Geyer 72 J. V. Kern et al.
15 GENETICS Supporting Information The Kinesin-, Unc-104 Regulates Dendrite Morphogenesis and Synaptic Development in Drosophila Jeannine V. Kern, Yao V. Zhang, Stella Kramer, Jay E. Brenman, and Tobias M. Rasse Copyright 201 by the Genetics Society of America DOI: /genetics
16 Figure S1 Altered NMJ morphology in unc 104 bris / larvae. Analysis of NMJ 4, Segment A2 of mid third instar Drosophila wild type, unc 104 bris /+, unc 104 bris, and unc 104 bris / larvae. Non normalized raw data of NMJ length (A), NMJ size (B), bouton number (C) is shown. Data normalized by (D) the muscle length or area is shown in Figure 8 B,C,D. The SEM is shown as a box, the SD as a black line. ** p< SI J. V. Kern et al.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
doi:10.1038/nature10923 Supplementary Figure 1 Ten-a and Ten-m antibody and cell type specificities. a c, Representative single confocal sections of a Drosophila NMJ stained with antibodies to Ten-a (red),
Chapter 4 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays.
 Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
Evaluating a potential interaction between deltex and git in Drosophila: genetic interaction, gene overexpression and cell biology assays. The data described in chapter 3 presented evidence that endogenous
The neuron as a secretory cell
 The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
The neuron as a secretory cell EXOCYTOSIS ENDOCYTOSIS The secretory pathway. Transport and sorting of proteins in the secretory pathway occur as they pass through the Golgi complex before reaching the
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
Supplemental material Kasprowicz et al., http://www.jcb.org/cgi/content/full/jcb.201310090/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. NMJ morphology of shi 12-12B ; shi-4c not treated
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
DOI: 10.1038/ncb3267 Supplementary Figure 1 A group of genes required for formation or orientation of annular F-actin bundles and aecm ridges: RNAi phenotypes and their validation by standard mutations.
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila
 Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Baz, Par-6 and apkc are not required for axon or dendrite specification in Drosophila Melissa M. Rolls and Chris Q. Doe, Inst. Neurosci and Inst. Mol. Biol., HHMI, Univ. Oregon, Eugene, Oregon 97403 Correspondence
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
www.sciencesignaling.org/cgi/content/full/6/301/ra98/dc1 Supplementary Materials for Regulation of Epithelial Morphogenesis by the G Protein Coupled Receptor Mist and Its Ligand Fog Alyssa J. Manning,
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Overexpression of YFP::GPR-1 in the germline.
 Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Supplementary Figure 1 Overexpression of YFP::GPR-1 in the germline. The pie-1 promoter and 3 utr were used to express yfp::gpr-1 in the germline. Expression levels from the yfp::gpr-1(cai 1.0)-expressing
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae. BioNB4910 Cornell University.
 Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Modulation of central pattern generator output by peripheral sensory cells in Drosophila larvae BioNB4910 Cornell University Goals 1) Observe the behavioral effects of remotely activating different populations
Nature Neuroscience: doi: /nn.2662
 Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Supplementary Figure 1 Atlastin phylogeny and homology. (a) Maximum likelihood phylogenetic tree based on 18 Atlastin-1 sequences using the program Quicktree. Numbers at internal nodes correspond to bootstrap
Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening
 APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
APPLICATION NOTE ArrayScan High Content Platform Monitoring neurite morphology and synapse formation in primary neurons for neurotoxicity assessments and drug screening Suk J. Hong and Richik N. Ghosh
Nature Neuroscience: doi: /nn.2717
 Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Supplementary Fig. 1. Dendrite length is not secondary to body length. Dendrite growth proceeds independently of the rate of body growth and decreases in rate in adults. n 20 on dendrite measurement, n
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo
 Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
Fig. S1. Expression pattern of moody-gal4 in third instar. Maximum projection illustrating a dissected moody-gal4>ngfp L3 larva stained for Repo (magenta), Fas2 (blue) and GFP (green) in overview (A) and
downstream (0.8 kb) homologous sequences to the genomic locus of DIC. A DIC mutant strain (ro- 6
 A B C D ts Figure S1 Generation of DIC- mcherry expressing N.crassa strain. A. N. crassa colony morphology. When a cot1 (top, left panel) strain is grown at permissive temperature (25 C), it exhibits straight
A B C D ts Figure S1 Generation of DIC- mcherry expressing N.crassa strain. A. N. crassa colony morphology. When a cot1 (top, left panel) strain is grown at permissive temperature (25 C), it exhibits straight
The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control
 Article The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control Carleton P. Goold 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics,
Article The BMP Ligand Gbb Gates the Expression of Synaptic Homeostasis Independent of Synaptic Growth Control Carleton P. Goold 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics,
Bypass and interaction suppressors; pathway analysis
 Bypass and interaction suppressors; pathway analysis The isolation of extragenic suppressors is a powerful tool for identifying genes that encode proteins that function in the same process as a gene of
Bypass and interaction suppressors; pathway analysis The isolation of extragenic suppressors is a powerful tool for identifying genes that encode proteins that function in the same process as a gene of
Anterograde Activin Signaling Regulates Postsynaptic Membrane Potential and GluRIIA/B Abundance at the Drosophila Neuromuscular Junction
 Anterograde Activin Signaling Regulates Postsynaptic Membrane Potential and GluRIIA/B Abundance at the Drosophila Neuromuscular Junction Myung-Jun Kim, Michael B. O Connor* Department of Genetics, Cell
Anterograde Activin Signaling Regulates Postsynaptic Membrane Potential and GluRIIA/B Abundance at the Drosophila Neuromuscular Junction Myung-Jun Kim, Michael B. O Connor* Department of Genetics, Cell
Supplementary Figure 1. Real time in vivo imaging of SG secretion. (a) SGs from Drosophila third instar larvae that express Sgs3-GFP (green) and
 Supplementary Figure 1. Real time in vivo imaging of SG secretion. (a) SGs from Drosophila third instar larvae that express Sgs3-GFP (green) and Lifeact-Ruby (red) were imaged in vivo to visualize secretion
Supplementary Figure 1. Real time in vivo imaging of SG secretion. (a) SGs from Drosophila third instar larvae that express Sgs3-GFP (green) and Lifeact-Ruby (red) were imaged in vivo to visualize secretion
Open Syntaxin Docks Synaptic Vesicles
 Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Marc Hammarlund 1,2[, Mark T. Palfreyman 1,2[, Shigeki Watanabe 1,2[, Shawn Olsen 1,2, Erik M. Jorgensen 1,2* PLoS BIOLOGY 1 Department of Biology, University of Utah, Salt Lake City, Utah, United States
Nervous System Organization
 The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
The Nervous System Nervous System Organization Receptors respond to stimuli Sensory receptors detect the stimulus Motor effectors respond to stimulus Nervous system divisions Central nervous system Command
Cell Death & Trophic Factors II. Steven McLoon Department of Neuroscience University of Minnesota
 Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Cell Death & Trophic Factors II Steven McLoon Department of Neuroscience University of Minnesota 1 Remember? Neurotrophins are cell survival factors that neurons get from their target cells! There is a
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis
 Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
Positional Cues in the Drosophila Nerve Cord: Semaphorins Pattern the Dorso-Ventral Axis Marta Zlatic 1,2,3 *, Feng Li 1, Maura Strigini 4, Wesley Grueber 2, Michael Bate 1 * 1 Department of Zoology, University
Drosophila Spastin Regulates Synaptic Microtubule Networks and Is Required for Normal Motor Function
 Drosophila Spastin Regulates Synaptic Microtubule Networks and Is Required for Normal Motor Function Nina Tang Sherwood 1, Qi Sun 1 1, Mingshan Xue 2 2, Bing Zhang 2, Kai Zinn 1 * Open access, freely available
Drosophila Spastin Regulates Synaptic Microtubule Networks and Is Required for Normal Motor Function Nina Tang Sherwood 1, Qi Sun 1 1, Mingshan Xue 2 2, Bing Zhang 2, Kai Zinn 1 * Open access, freely available
C. elegans L1 cell adhesion molecule functions in axon guidance
 C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
C. elegans L1 cell adhesion molecule functions in axon guidance Biorad Lihsia Chen Dept. of Genetics, Cell Biology & Development Developmental Biology Center C. elegans embryogenesis Goldstein lab, UNC-Chapel
Cells. Steven McLoon Department of Neuroscience University of Minnesota
 Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Cells Steven McLoon Department of Neuroscience University of Minnesota 1 Microscopy Methods of histology: Treat the tissue with a preservative (e.g. formaldehyde). Dissect the region of interest. Embed
Neurite initiation. Neurite formation begins with a bud that sprouts from the cell body. One or several neurites can sprout at a time.
 Neurite initiation. Neuronal maturation initiation f-actin polarization and maturation tubulin stage 1: "spherical" neuron stage 2: neurons extend several neurites stage 3: one neurite accelerates its
Neurite initiation. Neuronal maturation initiation f-actin polarization and maturation tubulin stage 1: "spherical" neuron stage 2: neurons extend several neurites stage 3: one neurite accelerates its
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY METHODS Mef2 primary screen. RNAi hairpins from the VDRC collection were crossed to Mef2-GAL4 at 27 C. After 2 weeks lethality rate and stage was scored, and if possible 20-30 males containing
SUPPLEMENTARY METHODS Mef2 primary screen. RNAi hairpins from the VDRC collection were crossed to Mef2-GAL4 at 27 C. After 2 weeks lethality rate and stage was scored, and if possible 20-30 males containing
Neurite formation & neuronal polarization. The cytoskeletal components of neurons have characteristic distributions and associations
 Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Mechanisms of neuronal migration & Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 The cytoskeletal
Neurite formation & neuronal polarization
 Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Neurite formation & neuronal polarization Paul Letourneau letou001@umn.edu Chapter 16; The Cytoskeleton; Molecular Biology of the Cell, Alberts et al. 1 An immature neuron in cell culture first sprouts
Cellular Neuroanatomy II The Prototypical Neuron: Neurites. Reading: BCP Chapter 2
 Cellular Neuroanatomy II The Prototypical Neuron: Neurites Reading: BCP Chapter 2 Major Internal Features of a Neuron The neuron is the functional unit of the nervous system. A typical neuron has a soma
Cellular Neuroanatomy II The Prototypical Neuron: Neurites Reading: BCP Chapter 2 Major Internal Features of a Neuron The neuron is the functional unit of the nervous system. A typical neuron has a soma
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p.
 Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Introduction Principles of Signaling and Organization p. 3 Signaling in Simple Neuronal Circuits p. 4 Organization of the Retina p. 5 Signaling in Nerve Cells p. 9 Cellular and Molecular Biology of Neurons
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03
 Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
Lecture 6: Non-Cortical Visual Pathways MCP 9.013/7.68, 03 Roger W. Sperry The problem of central nervous reorganization after nerve regeneration and muscle transposition. R.W. Sperry. Quart. Rev. Biol.
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
Reading: Chapter 5, pp ; Reference chapter D, pp Problem set F
 Mosaic Analysis Reading: Chapter 5, pp140-141; Reference chapter D, pp820-823 Problem set F Twin spots in Drosophila Although segregation and recombination in mitosis do not occur at the same frequency
Mosaic Analysis Reading: Chapter 5, pp140-141; Reference chapter D, pp820-823 Problem set F Twin spots in Drosophila Although segregation and recombination in mitosis do not occur at the same frequency
Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling
 Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
Name: AP Biology Mr. Croft Section 1 1. What is a neuron? Chapter 37 Active Reading Guide Neurons, Synapses, and Signaling 2. Neurons can be placed into three groups, based on their location and function.
RPM-1 is localized to distinct subcellular compartments and regulates axon length in GABAergic motor neurons
 Opperman and Grill Neural Development 14, 9:1 RESERCH RTICLE Open ccess RPM-1 is localized to distinct subcellular compartments and regulates axon length in Gergic motor neurons Karla J Opperman and rock
Opperman and Grill Neural Development 14, 9:1 RESERCH RTICLE Open ccess RPM-1 is localized to distinct subcellular compartments and regulates axon length in Gergic motor neurons Karla J Opperman and rock
A complementation test would be done by crossing the haploid strains and scoring the phenotype in the diploids.
 Problem set H answers 1. To study DNA repair mechanisms, geneticists isolated yeast mutants that were sensitive to various types of radiation; for example, mutants that were more sensitive to UV light.
Problem set H answers 1. To study DNA repair mechanisms, geneticists isolated yeast mutants that were sensitive to various types of radiation; for example, mutants that were more sensitive to UV light.
Nervous System Organization
 The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
The Nervous System Chapter 44 Nervous System Organization All animals must be able to respond to environmental stimuli -Sensory receptors = Detect stimulus -Motor effectors = Respond to it -The nervous
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins
 13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
13-3. Synthesis-Secretory pathway: Sort lumenal proteins, Secrete proteins, Sort membrane proteins Molecular sorting: specific budding, vesicular transport, fusion 1. Why is this important? A. Form and
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
www.sciencemag.org/cgi/content/full/science.1244624/dc1 Supplementary Materials for Cytoneme-Mediated Contact-Dependent Transport of the Drosophila Decapentaplegic Signaling Protein Sougata Roy, Hai Huang,
Chapter 11. Development: Differentiation and Determination
 KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
KAP Biology Dept Kenyon College Differential gene expression and development Mechanisms of cellular determination Induction Pattern formation Chapter 11. Development: Differentiation and Determination
Neural development its all connected
 Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Neural development its all connected How do you build a complex nervous system? How do you build a complex nervous system? 1. Learn how tissue is instructed to become nervous system. Neural induction 2.
Clicker Question. Discussion Question
 Connectomics Networks and Graph Theory A network is a set of nodes connected by edges The field of graph theory has developed a number of measures to analyze networks Mean shortest path length: the average
Connectomics Networks and Graph Theory A network is a set of nodes connected by edges The field of graph theory has developed a number of measures to analyze networks Mean shortest path length: the average
RACK-1 Acts with Rac GTPase Signaling and UNC-115/ ablim in Caenorhabditis elegans Axon Pathfinding and Cell Migration
 RACK-1 Acts with Rac GTPase Signaling and UNC-115/ ablim in Caenorhabditis elegans Axon Pathfinding and Cell Migration Rafael S. Demarco, Erik A. Lundquist* Programs in Genetics and Molecular, Cellular,
RACK-1 Acts with Rac GTPase Signaling and UNC-115/ ablim in Caenorhabditis elegans Axon Pathfinding and Cell Migration Rafael S. Demarco, Erik A. Lundquist* Programs in Genetics and Molecular, Cellular,
Mitochondria associate with the cytoskeleton. Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008)
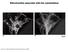 Mitochondria associate with the cytoskeleton Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008) Special arrangements of mitochondria Figure 14-6 Molecular Biology of the Cell ( Garland Science
Mitochondria associate with the cytoskeleton Figure 14-5 Molecular Biology of the Cell ( Garland Science 2008) Special arrangements of mitochondria Figure 14-6 Molecular Biology of the Cell ( Garland Science
A role for PS integrins in morphological growth and synaptic function at the postembryonic neuromuscular junction of Drosophila
 Development 126, 5833-5846 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8655 5833 A role for PS integrins in morphological growth and synaptic function at the postembryonic
Development 126, 5833-5846 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8655 5833 A role for PS integrins in morphological growth and synaptic function at the postembryonic
Neuronal Differentiation: Synapse Formation NMJ
 Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
Neuronal Differentiation: Synapse Formation NMJ Approaches to study synapse formation Rodent NMJ (cholinergic) Drosophila NMJ (glutamatergic) Rodent CNS synapse (glutamatergic or GABAergic) C. elegans
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
DOI: 10.1038/ncb2647 Figure S1 Other Rab GTPases do not co-localize with the ER. a, Cos-7 cells cotransfected with an ER luminal marker (either KDEL-venus or mch-kdel) and mch-tagged human Rab5 (mch-rab5,
Neurons and Nervous Systems
 34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
34 Neurons and Nervous Systems Concept 34.1 Nervous Systems Consist of Neurons and Glia Nervous systems have two categories of cells: Neurons, or nerve cells, are excitable they generate and transmit electrical
Synaptic Homeostasis Is Consolidated by the Cell Fate Gene gooseberry,adrosophila pax3/7 Homolog
 The Journal of Neuroscience, June 16, 2010 30(24):8071 8082 8071 Development/Plasticity/Repair Synaptic Homeostasis Is Consolidated by the Cell Fate Gene gooseberry,adrosophila pax3/7 Homolog Bruno Marie,
The Journal of Neuroscience, June 16, 2010 30(24):8071 8082 8071 Development/Plasticity/Repair Synaptic Homeostasis Is Consolidated by the Cell Fate Gene gooseberry,adrosophila pax3/7 Homolog Bruno Marie,
Supporting Information
 Supporting Information Cao et al. 10.1073/pnas.1306220110 Gram - bacteria Hemolymph Cytoplasm PGRP-LC TAK1 signalosome Imd dfadd Dredd Dnr1 Ikk signalosome P Relish Nucleus AMP and effector genes Fig.
Supporting Information Cao et al. 10.1073/pnas.1306220110 Gram - bacteria Hemolymph Cytoplasm PGRP-LC TAK1 signalosome Imd dfadd Dredd Dnr1 Ikk signalosome P Relish Nucleus AMP and effector genes Fig.
Supplementary Figures Supplementary Tables 1 and 2. Supplementary References
 The Nuclear Import of a Non-Canonical Frizzled2 Signal by Importins-β11 and α2 Promotes Postsynaptic Development Timothy J. Mosca and Thomas L. Schwarz * The F.M. Kirby Neurobiology Center, Children s
The Nuclear Import of a Non-Canonical Frizzled2 Signal by Importins-β11 and α2 Promotes Postsynaptic Development Timothy J. Mosca and Thomas L. Schwarz * The F.M. Kirby Neurobiology Center, Children s
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
Supplementary Figure 1 Supplementary Figure 1. HSP21 expression in 35S:HSP21 and hsp21 knockdown plants. (a) Since no T- DNA insertion line for HSP21 is available in the publicly available T-DNA collections,
purpose of this Chapter is to highlight some problems that will likely provide new
 119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
119 Chapter 6 Future Directions Besides our contributions discussed in previous chapters to the problem of developmental pattern formation, this work has also brought new questions that remain unanswered.
SUPPLEMENTARY INFORMATION
 GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
GP2 Type I-piliated bacteria FAE M cell M cell pocket idc T cell mdc Generation of antigenspecific T cells Induction of antigen-specific mucosal immune response Supplementary Figure 1 Schematic diagram
Conclusions. The experimental studies presented in this thesis provide the first molecular insights
 C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
C h a p t e r 5 Conclusions 5.1 Summary The experimental studies presented in this thesis provide the first molecular insights into the cellular processes of assembly, and aggregation of neural crest and
MCN. Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases Regulate Axon Guidance in Drosophila
 MCN Molecular and Cellular Neuroscience 17, 274 291 (2001) doi:10.1006/mcne.2000.0939, available online at http://www.idealibrary.com on Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases
MCN Molecular and Cellular Neuroscience 17, 274 291 (2001) doi:10.1006/mcne.2000.0939, available online at http://www.idealibrary.com on Complex Genetic Interactions among Four Receptor Tyrosine Phosphatases
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
doi:10.1038/nature11589 Supplementary Figure 1 Ciona intestinalis and Petromyzon marinus neural crest expression domain comparison. Cartoon shows dorsal views of Ciona mid gastrula (left) and Petromyzon
Solutions to Problem Set 4
 Question 1 Solutions to 7.014 Problem Set 4 Because you have not read much scientific literature, you decide to study the genetics of garden peas. You have two pure breeding pea strains. One that is tall
Question 1 Solutions to 7.014 Problem Set 4 Because you have not read much scientific literature, you decide to study the genetics of garden peas. You have two pure breeding pea strains. One that is tall
Physiology 2 nd year. Neuroscience Optional Lecture
 Academic year 2018/2019 Physiology 2 nd year Semester 1 Curricula Nervous system physiology Blood physiology Acid-base equilibrium Bibliography: Boron & Boulpaep Medical Physiology, 3 rd edition Physiology
Academic year 2018/2019 Physiology 2 nd year Semester 1 Curricula Nervous system physiology Blood physiology Acid-base equilibrium Bibliography: Boron & Boulpaep Medical Physiology, 3 rd edition Physiology
Control and Integration. Nervous System Organization: Bilateral Symmetric Animals. Nervous System Organization: Radial Symmetric Animals
 Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
Control and Integration Neurophysiology Chapters 10-12 Nervous system composed of nervous tissue cells designed to conduct electrical impulses rapid communication to specific cells or groups of cells Endocrine
REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR
 REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR Grade Requirement: All courses required for the Biochemistry major (CH, MATH, PHYS, BI courses) must be graded and passed with a grade of C- or better. Core Chemistry
REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR Grade Requirement: All courses required for the Biochemistry major (CH, MATH, PHYS, BI courses) must be graded and passed with a grade of C- or better. Core Chemistry
Cellular Neuroanatomy I The Prototypical Neuron: Soma. Reading: BCP Chapter 2
 Cellular Neuroanatomy I The Prototypical Neuron: Soma Reading: BCP Chapter 2 Functional Unit of the Nervous System The functional unit of the nervous system is the neuron. Neurons are cells specialized
Cellular Neuroanatomy I The Prototypical Neuron: Soma Reading: BCP Chapter 2 Functional Unit of the Nervous System The functional unit of the nervous system is the neuron. Neurons are cells specialized
Systematic Screening of Drosophila Deficiency Mutations for Embryonic Phenotypes and Orphan Receptor Ligands
 Systematic Screening of Drosophila Deficiency Mutations for Embryonic Phenotypes and Orphan Receptor Ligands Ashley P. Wright 1, A. Nicole Fox 1, Karl G. Johnson 2, Kai Zinn 1 * 1 Division of Biology,
Systematic Screening of Drosophila Deficiency Mutations for Embryonic Phenotypes and Orphan Receptor Ligands Ashley P. Wright 1, A. Nicole Fox 1, Karl G. Johnson 2, Kai Zinn 1 * 1 Division of Biology,
Segment boundary formation in Drosophila embryos
 Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
Segment boundary formation in Drosophila embryos Development 130, August 2003 Camilla W. Larsen, Elizabeth Hirst, Cyrille Alexandre and Jean Paul Vincent 1. Introduction: - Segment boundary formation:
"Small Brains, Big Ideas: The value of model organisms to science. Dr. Yuly Fuentes-Medel Executive Director Descience
 "Small Brains, Big Ideas: The value of model organisms to science Dr. Yuly Fuentes-Medel Executive Director Descience Outline 1. What is a model organism? 2. Big questions solved by small-brained organisms
"Small Brains, Big Ideas: The value of model organisms to science Dr. Yuly Fuentes-Medel Executive Director Descience Outline 1. What is a model organism? 2. Big questions solved by small-brained organisms
Heather Currinn, Benjamin Guscott, Zita Balklava, Alice Rothnie and Thomas Wassmer*
 Online Resources APP controls the formation of PI(3,5)P 2 vesicles through its binding of the PIKfyve complex. Cellular and Molecular Life Sciences Heather Currinn, Benjamin Guscott, Zita Balklava, Alice
Online Resources APP controls the formation of PI(3,5)P 2 vesicles through its binding of the PIKfyve complex. Cellular and Molecular Life Sciences Heather Currinn, Benjamin Guscott, Zita Balklava, Alice
REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR
 REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR Grade Requirement: All courses required for the Biochemistry major (CH, MATH, PHYS, BI courses) must be graded and passed with a grade of C- or better. Core Chemistry
REQUIREMENTS FOR THE BIOCHEMISTRY MAJOR Grade Requirement: All courses required for the Biochemistry major (CH, MATH, PHYS, BI courses) must be graded and passed with a grade of C- or better. Core Chemistry
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34
 NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
NEURONS, SENSE ORGANS, AND NERVOUS SYSTEMS CHAPTER 34 KEY CONCEPTS 34.1 Nervous Systems Are Composed of Neurons and Glial Cells 34.2 Neurons Generate Electric Signals by Controlling Ion Distributions 34.3
7.06 Problem Set #4, Spring 2005
 7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
7.06 Problem Set #4, Spring 2005 1. You re doing a mutant hunt in S. cerevisiae (budding yeast), looking for temperaturesensitive mutants that are defective in the cell cycle. You discover a mutant strain
Drosophila melanogaster and D. simulans, two fruit fly species that are nearly
 Comparative Genomics: Human versus chimpanzee 1. Introduction The chimpanzee is the closest living relative to humans. The two species are nearly identical in DNA sequence (>98% identity), yet vastly different
Comparative Genomics: Human versus chimpanzee 1. Introduction The chimpanzee is the closest living relative to humans. The two species are nearly identical in DNA sequence (>98% identity), yet vastly different
Supplementary Figure 1. Nature Genetics: doi: /ng.3848
 Supplementary Figure 1 Phenotypes and epigenetic properties of Fab2L flies. A- Phenotypic classification based on eye pigment levels in Fab2L male (orange bars) and female (yellow bars) flies (n>150).
Supplementary Figure 1 Phenotypes and epigenetic properties of Fab2L flies. A- Phenotypic classification based on eye pigment levels in Fab2L male (orange bars) and female (yellow bars) flies (n>150).
SUPPLEMENTARY INFORMATION
 Supplementary Notes Downregulation of atlastin does not affect secretory traffic We investigated whether Atlastin might play a role in secretory traffic. Traffic impairment results in disruption of Golgi
Supplementary Notes Downregulation of atlastin does not affect secretory traffic We investigated whether Atlastin might play a role in secretory traffic. Traffic impairment results in disruption of Golgi
Synaptotagmin 2 Mutations Cause Autosomal-Dominant Form of Lambert-Eaton Myasthenic Syndrome and Nonprogressive Motor Neuropathy
 The American Journal of Human Genetics, Volume 95 Supplemental Data Synaptotagmin 2 Mutations Cause AutosomalDominant Form of LambertEaton Myasthenic Syndrome and Nonprogressive Motor Neuropathy David
The American Journal of Human Genetics, Volume 95 Supplemental Data Synaptotagmin 2 Mutations Cause AutosomalDominant Form of LambertEaton Myasthenic Syndrome and Nonprogressive Motor Neuropathy David
Cells of the nervous system
 Cells of the nervous system There are approximately 100 billion neurons in the human brain There are about 100 times as many glial cells in the human brain Similar origin, different functions Other cells
Cells of the nervous system There are approximately 100 billion neurons in the human brain There are about 100 times as many glial cells in the human brain Similar origin, different functions Other cells
SUPPLEMENTARY INFORMATION
 med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
med!1,2 Wild-type (N2) end!3 elt!2 5 1 15 Time (minutes) 5 1 15 Time (minutes) med!1,2 end!3 5 1 15 Time (minutes) elt!2 5 1 15 Time (minutes) Supplementary Figure 1: Number of med-1,2, end-3, end-1 and
PROPERTY OF ELSEVIER SAMPLE CONTENT - NOT FINAL. The Nervous System and Muscle
 The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
The Nervous System and Muscle SECTION 2 2-1 Nernst Potential 2-2 Resting Membrane Potential 2-3 Axonal Action Potential 2-4 Neurons 2-5 Axonal Conduction 2-6 Morphology of Synapses 2-7 Chemical Synaptic
Neurons, Synapses, and Signaling
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 48 Neurons, Synapses, and Signaling
A Hierarchy of Cell Intrinsic and Target-Derived Homeostatic Signaling
 Article A Hierarchy of Cell Intrinsic and Target-Derived Homeostatic Signaling Sharon Bergquist, 1 Dion K. Dickman, 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics, Program in Neuroscience,
Article A Hierarchy of Cell Intrinsic and Target-Derived Homeostatic Signaling Sharon Bergquist, 1 Dion K. Dickman, 1 and Graeme W. Davis 1, * 1 Department of Biochemistry and Biophysics, Program in Neuroscience,
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016
 PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
PHYSIOLOGY CHAPTER 9 MUSCLE TISSUE Fall 2016 2 Chapter 9 Muscles and Muscle Tissue Overview of Muscle Tissue types of muscle: are all prefixes for muscle Contractility all muscles cells can Smooth & skeletal
SUPPLEMENTARY INFORMATION
 Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
Supplementary Figure 1 Sns and Duf co-localise in embryonic nephrocytes a-c, Wild-type stage 11 (a),14 (b) and 16 (c) embryos stained with anti-duf (green) and anti-sns (red). Higher magnification images
7.06 Spring 2004 PS 6 KEY 1 of 14
 7.06 Spring 2004 PS 6 KEY 1 of 14 Problem Set 6. Question 1. You are working in a lab that studies hormones and hormone receptors. You are tasked with the job of characterizing a potentially new hormone
7.06 Spring 2004 PS 6 KEY 1 of 14 Problem Set 6. Question 1. You are working in a lab that studies hormones and hormone receptors. You are tasked with the job of characterizing a potentially new hormone
Neurophysiology. Danil Hammoudi.MD
 Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Neurophysiology Danil Hammoudi.MD ACTION POTENTIAL An action potential is a wave of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal
Nervous Tissue. Neurons Neural communication Nervous Systems
 Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Nervous Tissue Neurons Neural communication Nervous Systems What is the function of nervous tissue? Maintain homeostasis & respond to stimuli Sense & transmit information rapidly, to specific cells and
Developmental genetics: finding the genes that regulate development
 Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Developmental Biology BY1101 P. Murphy Lecture 9 Developmental genetics: finding the genes that regulate development Introduction The application of genetic analysis and DNA technology to the study of
Biosciences in the 21st century
 Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
Biosciences in the 21st century Lecture 1: Neurons, Synapses, and Signaling Dr. Michael Burger Outline: 1. Why neuroscience? 2. The neuron 3. Action potentials 4. Synapses 5. Organization of the nervous
CIE Biology A-level Topic 15: Control and coordination
 CIE Biology A-level Topic 15: Control and coordination Notes Neuron structure The nerve cells called neurones play an important role in coordinating communication within the nervous system. The structure
CIE Biology A-level Topic 15: Control and coordination Notes Neuron structure The nerve cells called neurones play an important role in coordinating communication within the nervous system. The structure
Supplemental Information. The Mitochondrial Fission Receptor MiD51. Requires ADP as a Cofactor
 Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Structure, Volume 22 Supplemental Information The Mitochondrial Fission Receptor MiD51 Requires ADP as a Cofactor Oliver C. Losón, Raymond Liu, Michael E. Rome, Shuxia Meng, Jens T. Kaiser, Shu-ou Shan,
Neuronal Activity and CaMKII Regulate Kinesin- Mediated Transport of Synaptic AMPARs
 Article Neuronal Activity and CaMKII Regulate Kinesin- Mediated Transport of Synaptic AMPARs Highlights d AMPAR transport is regulated by synaptic transmission, VGCCs and CaMKII Authors Frédéric J. Hoerndli,
Article Neuronal Activity and CaMKII Regulate Kinesin- Mediated Transport of Synaptic AMPARs Highlights d AMPAR transport is regulated by synaptic transmission, VGCCs and CaMKII Authors Frédéric J. Hoerndli,
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Genes required for axon pathfinding and extension in the C. elegans nerve ring
 Development 126, 3679-3692 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8610 3679 Genes required for axon pathfinding and extension in the C. elegans nerve ring Jennifer A.
Development 126, 3679-3692 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV8610 3679 Genes required for axon pathfinding and extension in the C. elegans nerve ring Jennifer A.
Reference: Forscher, P., Kaczmarek, L.K., Buchanan, J. and Smith, S.J. (1987) Cyclic AMP induces changes in distribution and transport of organelles
 Reference: Forscher, P., Kaczmarek, L.K., Buchanan, J. and Smith, S.J. (1987) Cyclic AMP induces changes in distribution and transport of organelles within growth cones of Aplysia bag cell neurons. J.
Reference: Forscher, P., Kaczmarek, L.K., Buchanan, J. and Smith, S.J. (1987) Cyclic AMP induces changes in distribution and transport of organelles within growth cones of Aplysia bag cell neurons. J.
Profiling Synaptic Proteins Identifies Regulators of Insulin Secretion and Lifespan
 Profiling Synaptic Proteins Identifies Regulators of Insulin Secretion and Lifespan QueeLim Ch ng. a *, Derek Sieburth. b, Joshua M. Kaplan* Department of Molecular Biology, Massachusetts General Hospital,
Profiling Synaptic Proteins Identifies Regulators of Insulin Secretion and Lifespan QueeLim Ch ng. a *, Derek Sieburth. b, Joshua M. Kaplan* Department of Molecular Biology, Massachusetts General Hospital,
Neurons, Synapses, and Signaling
 CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
CAMPBELL BIOLOGY IN FOCUS URRY CAIN WASSERMAN MINORSKY REECE 37 Neurons, Synapses, and Signaling Lecture Presentations by Kathleen Fitzpatrick and Nicole Tunbridge, Simon Fraser University SECOND EDITION
Highwire Regulates Synaptic Growth in Drosophila
 Neuron, Vol. 26, 313 329, May, 2000, Copyright 2000 by Cell Press Highwire Regulates Synaptic Growth in Drosophila Hong I. Wan,* Aaron DiAntonio,* Richard D. Fetter,* Kendra Bergstrom,* Roland Strauss,
Neuron, Vol. 26, 313 329, May, 2000, Copyright 2000 by Cell Press Highwire Regulates Synaptic Growth in Drosophila Hong I. Wan,* Aaron DiAntonio,* Richard D. Fetter,* Kendra Bergstrom,* Roland Strauss,
The foraging locus: behavioral tests for normal muscle movement in rover and sitter Drosophila melanogaster larvae
 Genetica 85: 205-209, 1992. 0 1992 Kluwer Acadernic Publishers. Primed in the Nerhrrlands. The foraging locus: behavioral tests for normal muscle movement in rover and sitter Drosophila melanogaster larvae
Genetica 85: 205-209, 1992. 0 1992 Kluwer Acadernic Publishers. Primed in the Nerhrrlands. The foraging locus: behavioral tests for normal muscle movement in rover and sitter Drosophila melanogaster larvae
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice Supplementary Figure 2.
 Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Supplementary Figure 1. Markedly decreased numbers of marginal zone B cells in DOCK8 mutant mice. Percentage of marginal zone B cells in the spleen of wild-type mice (+/+), mice homozygous for cpm or pri
Supplementary Figure 1. Biochemical and sequence alignment analyses the
 Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Supplementary Figure 1. Biochemical and sequence alignment analyses the interaction of OPTN and TBK1. (a) Analytical gel filtration chromatography analysis of the interaction between TBK1 CTD and OPTN(1-119).
Report. Functional Diversity of Robo Receptor Immunoglobulin Domains Promotes Distinct Axon Guidance Decisions
 Current Biology 20, 567 572, March 23, 2010 ª2010 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2010.02.021 Functional Diversity of Robo Receptor Immunoglobulin Domains Promotes Distinct Axon Guidance
Current Biology 20, 567 572, March 23, 2010 ª2010 Elsevier Ltd All rights reserved DOI 10.1016/j.cub.2010.02.021 Functional Diversity of Robo Receptor Immunoglobulin Domains Promotes Distinct Axon Guidance
7.06 Cell Biology EXAM #3 KEY
 7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
7.06 Cell Biology EXAM #3 KEY May 2, 2006 This is an OPEN BOOK exam, and you are allowed access to books, a calculator, and notes BUT NOT computers or any other types of electronic devices. Please write
