Biorenewable Polymers 1: The Isotactic Polymerisation of Lactide
|
|
|
- Tyrone Freeman
- 6 years ago
- Views:
Transcription
1 4A3 Advanced Polymer Synthesis Biorenewable Polymers 1: The Isotactic Polymerisation of Lactide Dr. Ed Marshall Rm: M220, RCS 1 e.marshall@imperial.ac.uk 4A3 - Slide 1
2 Learning objectives Over the next hour you should acquire the knowledge to: 1. Describe why the polymerisation of lactide is so intensely researched. 2. Explain how chiral and achiral (salen)-supported Al complexes may be used to prepare isotactic and syndiotactic polylactide. 4A3 - Slide 2
3 Polylactide or Poly(lactic acid) - PLA ( PLA is biorenewable, biocompatible and bioresorbable it is also readily degraded chemically for recycling, but it is not strictly biodegradable fermentation corn starch L-lactic acid step-growth condensation (-H 2 O) ring-opening polymerisation heat (chain growth) Poly(L-lactic acid), PLLA L-lactide oligomers We will focus on this step 4A3 - Slide 3
4 Initiators Typical initiators for lactide polymerisation are metal-alkoxides, e.g. Al(O i Pr) 3 : Acyl-oxygen bond breaks Metals used: Al, Mg, Zn, Fe, Y, Ti, Zr, Sn, Ca, La, Sm + others Industrially, the initiator used is a tin(ii) carboxylate - in the presence of alcohol, this is believed to form tin(ii) alkoxides: tin(ii) bis(2-ethylhexanoate), "SnOct 2 " 4A3 - Slide 4
5 Mechanism of propagation Coordinative-Insertion Mechanism - other lactones open in a similar way 2 1 NB: Every step is reversible Initiation involves nucleophilic attack of the alkoxide at the lactide carbonyl. This leaves the monomer heterocycle intact 2. In order to open the ring, the monomer rolls around 2 3 to place the acyl oxygen nearer the metal centre 3. This results in another metal alkoxide 4, which can now insert the next monomer unit. 4A3 - Slide 5
6 Lactide - three different stereoisomers (S,S)-lactide "L-lactide" (R,R)-lactide "D-lactide" (S,R)-lactide "meso-lactide" 50:50 mix = rac-lactide Since L-lactic acid is the naturally occurring form, (S,S)-lactide is much cheaper than (R,R)-lactide. Meso-lactide is not commercially available and must be separated from the (R,R) and (S,S) monomers by a steam distillation. Most commonly studied: L-lactide and rac-lactide 4A3 - Slide 6
7 PLA - stereoregular microstructures (tacticities) S,S isotactic -(SSSSSSSS)- "poly(l-lactide)" R,R isotactic -(RRRRRRRR)- "poly(d-lactide)" heterotactic -(SSRRSSRR)- rac meso syndiotactic -(SRSRSRSR)- 4A3 - Slide 7
8 PLA - physical properties depend on tacticity Polymer T m ( C) T g ( C) Modulus (GPa) Degradation time (months) isotactic PLA >24 syndiotactic PLA n/a n/a heterotactic PLA amorphous 49 n/a n/a atactic PLA amorphous There is one other important form of PLA known as an isotactic stereocomplex: isotactic poly(l-lactide) isotactic poly(d-lactide) mix and co-crystallise T m > 235 C 4A3 - Slide 8
9 Stereocomplex reason for increased thermal stability Hexagonal prismatic crystals: Crystalline domains of SSSSSS isotactic PLA S R R S S R Crystalline domains of RRRRRR isotactic PLA Crystalline domains of SSSSSS isotactic PLA Additional crystallinity arises from the interfaces between the trigonal prisms of all S and all R PLA 4A3 - Slide 9
10 Single-site catalysts L n M X e.g. [Cp 2 ZrMe] + sterically bulky ancillary ligand(s) substrate approaches vacant coordination site and may then react with X Polymer stereochemistry can potentially be controlled by the sterics / chirality of L n 4A3 - Slide 10
11 Early report on the stereoselective polymerisation of rac-lactide Spassky rac-la 60 C isotactic bias P m = 0.68 Stereoselectivity presumably arises from a chain-end control mechanism: [Al](OMe) (S,S)-LA [Al]-(S,S)-OMe k( S,S ) / k( R,R ) = 2.8 [Al](OMe) (R,R)-LA [Al]-(R,R)-OMe k( R,R ) / k( S,S ) = 2.8 Macromolecular Chemistry and Physics 1997, 198, A3 - Slide 11
12 Enantiomorphic site control with chiral salen Al initiators? Spassky rac-la 60 C k (R,R) / k (S,S) = 20 enantiopure The polymer produced was proposed to be a stereoblock copolymer, with a tapered junction: increasing R content -RRRRRRRRRRR- -RR SS- -SSSSSSSSSSSS- increasing S content T m = 185 C Macromolecular Chemistry and Physics 1996, 197, A3 - Slide 12
13 The stereocomplex myth Smith & Baker / Coates rac-la 60 C isotactic PLA T m = 191 C racemic initiator Smith and Baker: each enantiomer of the Al initiator preferentially consumes one monomer enantiomer and the isotactic chains of all... RRRRRR... and all... SSSSSS..., which then crystallise into a stereocomplex. Coates: each enantiomer of the initiator preferentially consumes one monomer enantiomer, giving short sequences of... RRRRRR... and... SSSSSS... but these can exchange between metal centres. J. Am. Chem. Soc. 2000, 122, WebCT Baker&Smith2000.pdf J. Polym. Sci. Polym. Chem. 2000, 38, WebCT Coates2000.pdf 4A3 - Slide 13
14 Formation of isotactic PLA from chiral salen Al initiators (S)-selective ligand (S)-selective ligand (R)-selective ligand (R)-selective ligand 4A3 - Slide 14
15 Formation of isotactic PLA from chiral salen Al initiators The PLA product is not a stereocomplex - it is a stereoblocky copolymer: -(RR RR)-(SS SS)-(RR RR)-(SS SS)- Elevated T m values arise from cocrystallisation of short isotactic domains In theory, if you could stop the exchange of polymer chains between the metal centres, then all R and all S chains would be obtained and these could then form the stereocomplex material 4A3 - Slide 15
16 Let s return to achiral salen Al initiators Numerous achiral salen ligands have been investigated (24 examples are reported in our recent paper: Nomura rac-la 60 C highly isotactic P m = 0.92 T m = 192 C J. Am. Chem. Soc. 2002, 124, Nomura2002.pdf Proc. Nat. Acad. Sci. 2006, 103, Gibson2006.pdf 4A3 - Slide 16
17 First report of syndiotactic PLA Coates Preferentially consumes (R,R)-LA instead of (S,S)-LA (see slide 12) enantiopure meso-la selectively cleaved at acyl bond adjacent to (R)-methyl J. Am. Chem. Soc. 2002, 124, WebCT Coates2002.pdf 4A3 - Slide 17
18 Lecture 1 Conclusions 1. Salen-supported Al-based initiators nearly always give highly isotactic PLA (from the racemic monomer). ligand-assisted chain end stereocontrol 2. The isotactic product is actually a stereoblocky material, not a stereocomplex. T m values are therefore higher than for isotactic poly(llactide), but lower than for the stereocomplex. 3. Syndiotactic PLA may be prepared from meso-lactide using a chiral salen ligand (with a 2,2'-diaminobinaphthyl backbone). 4A3 - Slide 18
Structure. Extremely interesting BPs (very high Tm ~ C) Excellent thermic and mechanical properties
 tructure Extremely interesting BPs (very high Tm ~ 220-230 C) Excellent thermic and mechanical properties Limitations: no straigthforward synthetic pathways available (Expensive material) Applications:
tructure Extremely interesting BPs (very high Tm ~ 220-230 C) Excellent thermic and mechanical properties Limitations: no straigthforward synthetic pathways available (Expensive material) Applications:
Polylactic acids produced from l- and dl-lactic acid anhydrosulfite: stereochemical aspects
 JPOL 3576 Polymer 40 (1999) 5073 5078 Polylactic acids produced from l- and dl-lactic acid anhydrosulfite: stereochemical aspects A.J. Amass a, *, K.L.R. N Goala a, B.J. Tighe a, F. Schué b a Speciality
JPOL 3576 Polymer 40 (1999) 5073 5078 Polylactic acids produced from l- and dl-lactic acid anhydrosulfite: stereochemical aspects A.J. Amass a, *, K.L.R. N Goala a, B.J. Tighe a, F. Schué b a Speciality
Polymers. Steep Slope = 3/5 : Self-Avoiding Walk (Polymer Solution) Shallow Slope = 1/2 : Gaussian Random Walk (Polymer Melt)
 Polymers 1 Polymers Steep Slope = 3/5 : Self-Avoiding Walk (Polymer Solution) Shallow Slope = 1/2 : Gaussian Random Walk (Polymer Melt) 2 If we consider a series of chains = 0 Except when i = j, and
Polymers 1 Polymers Steep Slope = 3/5 : Self-Avoiding Walk (Polymer Solution) Shallow Slope = 1/2 : Gaussian Random Walk (Polymer Melt) 2 If we consider a series of chains = 0 Except when i = j, and
Catalysis & Sustainable Processes
 Catalysis & Sustainable Processes The Polymers Story 8 lectures http://www.kcpc.usyd.edu.au/cem3113.html username: chem3 password: carbon12 Lecturer: Associate Professor Sébastien Perrier s.perrier@chem.usyd.edu.au;
Catalysis & Sustainable Processes The Polymers Story 8 lectures http://www.kcpc.usyd.edu.au/cem3113.html username: chem3 password: carbon12 Lecturer: Associate Professor Sébastien Perrier s.perrier@chem.usyd.edu.au;
Biodegradable Polymers
 Biodegradable Polymers The evolution of biomaterials materials for passive implants and devices Biofunctional materials 1 Design of biomaterials (Biocompatible) Processable Sterilizable Possibility to
Biodegradable Polymers The evolution of biomaterials materials for passive implants and devices Biofunctional materials 1 Design of biomaterials (Biocompatible) Processable Sterilizable Possibility to
Sommai Pivsa-Art *, Thikanda Tong-ngok, Supansa Junngam, Rutchaneekorn Wongpajan and Weraporn Pivsa-Art
 Available online at www.sciencedirect.com Energy Procedia 34 (2013 ) 604 609 10th Eco-Energy and Materials Science and Engineering (EMSES2012) Synthesis of Poly(D-lactic acid) Using a 2-Steps Direct Polycondensation
Available online at www.sciencedirect.com Energy Procedia 34 (2013 ) 604 609 10th Eco-Energy and Materials Science and Engineering (EMSES2012) Synthesis of Poly(D-lactic acid) Using a 2-Steps Direct Polycondensation
Repeated insertion. Multiple insertion leads to dimerization, oligomerization or polymerization. κ 1: mainly dimerization κ
 Repeated insertion ultiple insertion leads to dimerization, oligomerization or polymerization. k prop Et Key factor: k CT / k prop = κ κ 1: mainly dimerization κ 0.1-1.0: oligomerization (always mixtures)
Repeated insertion ultiple insertion leads to dimerization, oligomerization or polymerization. k prop Et Key factor: k CT / k prop = κ κ 1: mainly dimerization κ 0.1-1.0: oligomerization (always mixtures)
Biodegradable Solid Polymeric Materials (continued)
 Biodegradable Solid Polymeric Materials (continued) Last time: chemistry and physical chemistry of degrading polymeric solids for biomaterials Today: Factors controlling polymer degradation rates Theory
Biodegradable Solid Polymeric Materials (continued) Last time: chemistry and physical chemistry of degrading polymeric solids for biomaterials Today: Factors controlling polymer degradation rates Theory
The structures and common names of two amino acids are shown. Draw the structure of the zwitterion of proline.
 Q1.(a) The structures and common names of two amino acids are shown. (i) Draw the structure of the zwitterion of proline. Draw the structure of the tripeptide formed when a proline molecule bonds to two
Q1.(a) The structures and common names of two amino acids are shown. (i) Draw the structure of the zwitterion of proline. Draw the structure of the tripeptide formed when a proline molecule bonds to two
1.2.5 Initiator systems for the ROP of cyclic esters Synthesis and Characterization of Complexes... 34
 I INDEX INDEX... I LIST OF ABBREVIATIONS... V ABSTRACT... VII 1 INTRODUCTION... 1 1.1 BIOPLASTICS... 1 1.2 POLYESTERS... 4 1.2.1 Polylactide... 4 1.2.2 Polycaprolactone... 11 1.2.3 Copolymers of Lactide
I INDEX INDEX... I LIST OF ABBREVIATIONS... V ABSTRACT... VII 1 INTRODUCTION... 1 1.1 BIOPLASTICS... 1 1.2 POLYESTERS... 4 1.2.1 Polylactide... 4 1.2.2 Polycaprolactone... 11 1.2.3 Copolymers of Lactide
STRUCTURE OF MACROMOLECULES
 STUTUE F MMLEULES Introduction Life is polymeric in its essence: the most important component of living cell (proteins, carbohydrates and nucleic acids) are all polymers. Nature uses polymers both for
STUTUE F MMLEULES Introduction Life is polymeric in its essence: the most important component of living cell (proteins, carbohydrates and nucleic acids) are all polymers. Nature uses polymers both for
- Overview of polymeriza1on catalysis
 - verview of polymeriza1on catalysis Different coordina-on polymeriza-on mechanisms: RP, RMP, (meth)acrylate polymeriza6on, olefin polymeriza6on. Different catalysts: Metal- based catalysts, organic catalysts
- verview of polymeriza1on catalysis Different coordina-on polymeriza-on mechanisms: RP, RMP, (meth)acrylate polymeriza6on, olefin polymeriza6on. Different catalysts: Metal- based catalysts, organic catalysts
11 Homogeneous Catalyst Design for the Synthesis of Aliphatic Polycarbonates and Polyesters
 j343 11 Homogeneous Catalyst Design for the Synthesis of Aliphatic Polycarbonates and Polyesters Geoffrey W. Coates and Ryan C. Jeske 11.1 Introduction Synthetic polymers are more important now than at
j343 11 Homogeneous Catalyst Design for the Synthesis of Aliphatic Polycarbonates and Polyesters Geoffrey W. Coates and Ryan C. Jeske 11.1 Introduction Synthetic polymers are more important now than at
Issued: 02/07/06 Spring 2006 Due: 02/16/06 10 points/problem
 Problem Set 1 solutions 20.462J/3.962J Issued: 02/07/06 Spring 2006 Due: 02/16/06 10 points/problem 1. Listed in the table below are chemical and schematic structures of poly(l-lactide) and a number of
Problem Set 1 solutions 20.462J/3.962J Issued: 02/07/06 Spring 2006 Due: 02/16/06 10 points/problem 1. Listed in the table below are chemical and schematic structures of poly(l-lactide) and a number of
Stereochemistry. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects.
 Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Stereochemistry This is study of the 3 dimensional arrangement in space of molecules. In organic chemistry, subtle differences in spatial arrangements can give rise to prominent effects. E.g. the isomers
Isomerism and Carbonyl Compounds
 Isomerism and Carbonyl Compounds 18 Section B Answer all questions in the spaces provided. 7 Esters have many important commercial uses such as solvents and artificial flavourings in foods. Esters can
Isomerism and Carbonyl Compounds 18 Section B Answer all questions in the spaces provided. 7 Esters have many important commercial uses such as solvents and artificial flavourings in foods. Esters can
Synthesis of Lactide from Oligomeric PLA: Effects of Temperature, Pressure, and Catalyst
 Macromolecular Research, Vol. 14, No. 5, pp 510-516 (2006) Synthesis of Lactide from Oligomeric PLA: Effects of Temperature, Pressure, and Catalyst Dong Keun Yoo and Dukjoon Kim* Department of Chemical
Macromolecular Research, Vol. 14, No. 5, pp 510-516 (2006) Synthesis of Lactide from Oligomeric PLA: Effects of Temperature, Pressure, and Catalyst Dong Keun Yoo and Dukjoon Kim* Department of Chemical
In recent years, Al(salen) complexes [where salen is N,N bis(salicylaldimine)-1,2-ethylenediamine]
![In recent years, Al(salen) complexes [where salen is N,N bis(salicylaldimine)-1,2-ethylenediamine] In recent years, Al(salen) complexes [where salen is N,N bis(salicylaldimine)-1,2-ethylenediamine]](/thumbs/94/118482257.jpg) Study of ligand substituent effects on the rate and stereoselectivity of lactide polymerization using aluminum salen-type initiators Pimpa Hormnirun, Edward L. Marshall, Vernon C. Gibson*, Robert I. Pugh,
Study of ligand substituent effects on the rate and stereoselectivity of lactide polymerization using aluminum salen-type initiators Pimpa Hormnirun, Edward L. Marshall, Vernon C. Gibson*, Robert I. Pugh,
Chemistry 123: Physical and Organic Chemistry Topic 1: Organic Chemistry
 Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
Concept Check: Topic 1: Conformation Winter 2009 Page 112 Concept Check: Topic 1: Conformation Winter 2009 Page 113 1 STEREOCHEMISTRY Winter 2009 Page 114 We have already covered two kinds of isomerism:
CH 2 = CH - CH =CH 2
 MULTIPLE CHOICE QUESTIONS 1. Styrene is almost a unique monomer, in that it can be polymerized by practically all methods of chain polymerization. A. Free radical B. Anionic C. Cationic D. Co-ordination
MULTIPLE CHOICE QUESTIONS 1. Styrene is almost a unique monomer, in that it can be polymerized by practically all methods of chain polymerization. A. Free radical B. Anionic C. Cationic D. Co-ordination
Chiral Catalyst II. Palladium Catalysed Allylic Displacement ( -allyl complexes) 1. L n Pd(0) 2. Nuc
 Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Chapter 11. Polymer Structures. Natural vs man-made
 . Polymer Structures Polymers: materials consisting of long molecules - the word polymer comes from the Greek Polys = many Meros = parts Macromolecules (long size of the chains) many parts - typically,
. Polymer Structures Polymers: materials consisting of long molecules - the word polymer comes from the Greek Polys = many Meros = parts Macromolecules (long size of the chains) many parts - typically,
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
CO 2 and CO activation
 2 and activation Most organic chemicals are currently made commercially from ethylene, a product of oil refining. It is possible that in the next several decades we may have to shift toward other carbon
2 and activation Most organic chemicals are currently made commercially from ethylene, a product of oil refining. It is possible that in the next several decades we may have to shift toward other carbon
PHYSICS OF SOLID POLYMERS
 PYSIS OF SOLID POLYMERS Professor Goran Ungar WU E, Department of hemical and Biological Engineering Recommended texts: G. Strobl, The Physics of Polymers, Springer 996 (emphasis on physics) U. Gedde,
PYSIS OF SOLID POLYMERS Professor Goran Ungar WU E, Department of hemical and Biological Engineering Recommended texts: G. Strobl, The Physics of Polymers, Springer 996 (emphasis on physics) U. Gedde,
Exam 2 Chem 109a Fall 2004
 Exam 2 Chem 109a Fall 2004 Please put your name and perm number on both the exam and the scantron sheet. Next, answer the following 34 multiple-choice questions on the scantron sheet. Then choose one A-type
Exam 2 Chem 109a Fall 2004 Please put your name and perm number on both the exam and the scantron sheet. Next, answer the following 34 multiple-choice questions on the scantron sheet. Then choose one A-type
CH 3 C 2 H 5. Tetrahedral Stereochemistry
 Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Ch 5 Tetrahedral Stereochemistry Enantiomers - Two non-superimposable mirror image molecules - They are stereoisomers with the same atoms and bonds, but different spatial geometries. - The two molecules
Inorganic Chemistry Year 3
 Inorganic Chemistry Year 3 Transition Metal Catalysis Eighteen Electron Rule 1.Get the number of the group that the metal is in (this will be the number of d electrons) 2.Add to this the charge 1.Negative
Inorganic Chemistry Year 3 Transition Metal Catalysis Eighteen Electron Rule 1.Get the number of the group that the metal is in (this will be the number of d electrons) 2.Add to this the charge 1.Negative
Chapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution
 hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
hapter 8: Nucleophilic Substitution 8.1: Functional Group Transformation By Nucleophilic Substitution Nu: = l,, I Nu - Nucleophiles are Lewis bases (electron-pair donor) Nucleophiles are often negatively
CHEM1902/ N-9 November 2014
 CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
CEM1902/4 2014-N-9 November 2014 The elimination of 2 O from alcohol A can form the isomeric alkenes B and C. Elimination of Br from the alkyl halide D can generate the same two alkenes. 7 Assign the absolute
Measuring enzyme (enantio)selectivity
 Measuring enzyme (enantio)selectivity Types of selectivity - review stereoisomers Stereoselective synthesis (create) vs. resolutions (separate) Enantioselectivity & enantiomeric purity Ways to measure
Measuring enzyme (enantio)selectivity Types of selectivity - review stereoisomers Stereoselective synthesis (create) vs. resolutions (separate) Enantioselectivity & enantiomeric purity Ways to measure
Chemistry 51 Exam #3. Name KEY November 20, 2001
 Chemistry 51 Exam #3 Name KEY November 20, 2001 This exam has nine (9) questions. Please check before beginning to make sure no questions are missing. All scratch work must be done on the attached blank
Chemistry 51 Exam #3 Name KEY November 20, 2001 This exam has nine (9) questions. Please check before beginning to make sure no questions are missing. All scratch work must be done on the attached blank
Oxidation of alcohols Chromic Acid KMnO4 PCC Swern 1 ROH RCOOH RCOOH RCHO RCHO 2 ROH Ketone Ketone Ketone Ketone 3 ROH NR NR NR NR
 Chapter 11 xidation in rganic terms xidation Reduction Neither Addition of, X2; loss of 2 Addition of 2; loss of or X2 Anytime you oxidize one carbon but reduce another (ex. Adding and to adjacent carbons)
Chapter 11 xidation in rganic terms xidation Reduction Neither Addition of, X2; loss of 2 Addition of 2; loss of or X2 Anytime you oxidize one carbon but reduce another (ex. Adding and to adjacent carbons)
STEREOCHEMISTRY AND STEREOELECTRONICS NOTES
 - 1 - STEREOCHEMISTRY AND STEREOELECTRONICS NOTES Stereochemistry in Organic Molecules Conventions used in drawing molecules Also, Fischer projections can sometimes be useful for acyclic molecules with
- 1 - STEREOCHEMISTRY AND STEREOELECTRONICS NOTES Stereochemistry in Organic Molecules Conventions used in drawing molecules Also, Fischer projections can sometimes be useful for acyclic molecules with
The catalytic microwave synthesis of biodegradable polyester polyols based on castor oil and l-lactide
 The catalytic microwave synthesis of biodegradable polyester polyols based on castor oil and l-lactide D Kojić 1, T Erceg 2, N Vukić 2, V Teofilović 2, I Ristić 2, J Budinski-Simendić 2 and V Aleksić 3
The catalytic microwave synthesis of biodegradable polyester polyols based on castor oil and l-lactide D Kojić 1, T Erceg 2, N Vukić 2, V Teofilović 2, I Ristić 2, J Budinski-Simendić 2 and V Aleksić 3
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture 6: Transition-Metal Catalysed C-C Bond Formation
 Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
Chem 112A: Final Exam
 Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Chem 112A: Final Exam December 14th, 2011 Please provide all answers in the spaces provided. You are not allowed to use a calculator for this exam, but you may use molecular model kits. nly cyclohexane
Midterm #2 Chem 3A - Fall 2013 Nov. 12, :00 8:45 pm. Name SID
 Midterm #2 Chem 3A - Fall 2013 Nov. 12, 2013 7:00 8:45 pm Name SID Including the title page, there should be 6 total questions spread over 8 pages (printed on both sides). Please provide all answers in
Midterm #2 Chem 3A - Fall 2013 Nov. 12, 2013 7:00 8:45 pm Name SID Including the title page, there should be 6 total questions spread over 8 pages (printed on both sides). Please provide all answers in
Introduction to Macromolecular Chemistry
 Introduction to Macromolecular Chemistry aka polymer chemistry Mondays, 8.15-9.45 am except for the following dates: 01.+29.05, 05.+12.06., 03.07. Dr. Christian Merten, Ruhr-Uni Bochum, 2017 www.ruhr-uni-bochum.de/chirality
Introduction to Macromolecular Chemistry aka polymer chemistry Mondays, 8.15-9.45 am except for the following dates: 01.+29.05, 05.+12.06., 03.07. Dr. Christian Merten, Ruhr-Uni Bochum, 2017 www.ruhr-uni-bochum.de/chirality
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
Chiral Amplification. Literature Talk Fabian Schneider Konstanz, Universität Konstanz
 Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
CHEM 263 Oct 25, stronger base stronger acid weaker acid weaker base
 CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Concerning the ring-opening polymerization of lactide and cyclic esters by coordination metal catalysts*
 Pure Appl. Chem., Vol. 82, No. 8, pp. 1647 1662, 2010. doi:10.1351/pac-con-09-09-24 2010 IUPAC, Publication date (Web): 19 June 2010 Concerning the ring-opening polymerization of lactide and cyclic esters
Pure Appl. Chem., Vol. 82, No. 8, pp. 1647 1662, 2010. doi:10.1351/pac-con-09-09-24 2010 IUPAC, Publication date (Web): 19 June 2010 Concerning the ring-opening polymerization of lactide and cyclic esters
Polymers and Composite Materials
 Polymers and omposite Materials Shibu G. Pillai hemical Engineering Department shibu.pillai@nirmauni.ac.in ontents lassification of Polymers Types of polymerization Elastomers/ Rubber Advanced Polymeric
Polymers and omposite Materials Shibu G. Pillai hemical Engineering Department shibu.pillai@nirmauni.ac.in ontents lassification of Polymers Types of polymerization Elastomers/ Rubber Advanced Polymeric
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Stereoselective reactions of the carbonyl group
 1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES.
 COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
COURSE OBJECTIVES / OUTCOMES / COMPETENCIES. By the end of the course, students should be able to do the following: See Test1-4 Objectives/Competencies as listed in the syllabus and on the main course
Anionic Polymerization - Initiation and Propagation
 Anionic Polymerization Initiation and Propagation As in free radical polymerization, there are initiation and propagation steps. NH 2 NaNH 2 Na + + NH 2 + H 2 N CH: Propagation proceeds in the usual manner,
Anionic Polymerization Initiation and Propagation As in free radical polymerization, there are initiation and propagation steps. NH 2 NaNH 2 Na + + NH 2 + H 2 N CH: Propagation proceeds in the usual manner,
CHEM 203. Midterm Exam 1 October 31, 2008 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
CEM 203 Midterm Exam 1 ctober 31, 2008 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This exam contains 8 pages Time: 1h 30 min 1. / 15 2. / 16 3. /
WARSAW UNIVERSITY OF TECHNOLOGY FACULTY OF CHEMISTRY. Major: CHEMICAL TECHNOLOGY. Maciej Damian Korzyński
 WARSAW UNIVERSITY OF TECHNOLOGY FACULTY OF CHEMISTRY Major: CHEMICAL TECHNOLOGY Maciej Damian Korzyński Design and synthesis optimization of bis(β-ketiminate) ligand alkyl zinc and alkoxy zinc derivatives
WARSAW UNIVERSITY OF TECHNOLOGY FACULTY OF CHEMISTRY Major: CHEMICAL TECHNOLOGY Maciej Damian Korzyński Design and synthesis optimization of bis(β-ketiminate) ligand alkyl zinc and alkoxy zinc derivatives
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Insertion and elimination. Peter H.M. Budzelaar
 Peter H.. Budzelaar Insertion reactions If at a metal centre you have a) a σ-bound group (hydride, alkyl, aryl) b) a ligand containing a π-system (olefin, alkyne, C) the σ-bound group can migrate to the
Peter H.. Budzelaar Insertion reactions If at a metal centre you have a) a σ-bound group (hydride, alkyl, aryl) b) a ligand containing a π-system (olefin, alkyne, C) the σ-bound group can migrate to the
2 Answer all the questions. 1 PLA is a biodegradable polymer made from corn starch. The structure of part of a PLA chain is shown below.
 2 Answer all the questions. 1 PLA is a biodegradable polymer made from corn starch. The structure of part of a PLA chain is shown below. CH 3 CH 3 CH 3 CH 3 PLA (a) (i) n the structure of PLA above, circle
2 Answer all the questions. 1 PLA is a biodegradable polymer made from corn starch. The structure of part of a PLA chain is shown below. CH 3 CH 3 CH 3 CH 3 PLA (a) (i) n the structure of PLA above, circle
CO 2 and CO activation
 2 and activation Most organic chemicals are currently made commercially from ethylene, a product of oil refining. Itispossiblethatinthenextseveraldecadeswemayhavetoshifttowardothercarbonsources for these
2 and activation Most organic chemicals are currently made commercially from ethylene, a product of oil refining. Itispossiblethatinthenextseveraldecadeswemayhavetoshifttowardothercarbonsources for these
Nitrogen Centered Radical Ligands Nagashima Nozomu
 1 Nitrogen Centered Radical Ligands 2015. 7. 4. Nagashima Nozomu 1. Introduction 2 3 Aminyl radical 1) D. E. Wiliams, JACS, 1966, 88, 5665 2) Y. Teki et al. JOC, 2000, 65, 7889 Sterically protected aminyl
1 Nitrogen Centered Radical Ligands 2015. 7. 4. Nagashima Nozomu 1. Introduction 2 3 Aminyl radical 1) D. E. Wiliams, JACS, 1966, 88, 5665 2) Y. Teki et al. JOC, 2000, 65, 7889 Sterically protected aminyl
Reaction Kinetics for the Synthesis of Oligomeric Poly(lactic acid)
 Macromolecular Research, Vol. 13, No. 1, pp 68-72 (2005) Reaction Kinetics for the Synthesis of Oligomeric Poly(lactic acid) Dong Keun Yoo and Dukjoon Kim* Department of Chemical Engineering, Polymer Technology
Macromolecular Research, Vol. 13, No. 1, pp 68-72 (2005) Reaction Kinetics for the Synthesis of Oligomeric Poly(lactic acid) Dong Keun Yoo and Dukjoon Kim* Department of Chemical Engineering, Polymer Technology
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Fisika Polimer Ariadne L Juwono. Sem /2007
 Chapter 4. Ionic and coordination (addition) polymerization 4.1. Similarities and contrast on ionic polymerization 4.2. Cationic polymerization 4.3. Anionic polymerization 4.4. Coordination polymerization
Chapter 4. Ionic and coordination (addition) polymerization 4.1. Similarities and contrast on ionic polymerization 4.2. Cationic polymerization 4.3. Anionic polymerization 4.4. Coordination polymerization
Elimination Reactions The E2 Mechanism
 Elimination Reactions The E2 Mechanism The E2 Mechanism X X- B: B- δ- B:- δ+ R 1 δ- R 2 δ+ X δ- The E2 Mechanism R 3 R 4 transition state Free energy (G) Eact B:- B R 1 R 2 X R 1 R 2 R 3 R 4 R 4 R 3 X:-
Elimination Reactions The E2 Mechanism The E2 Mechanism X X- B: B- δ- B:- δ+ R 1 δ- R 2 δ+ X δ- The E2 Mechanism R 3 R 4 transition state Free energy (G) Eact B:- B R 1 R 2 X R 1 R 2 R 3 R 4 R 4 R 3 X:-
Elimination Reactions. Chapter 6 1
 Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Insertion Reactions. 1) 1,1 insertion in which the metal and the X ligand end up bound to the same (1,1) atom
 Insertion Reactions xidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. With insertion, and its reverse reaction, elimination, we can now combine and transform
Insertion Reactions xidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. With insertion, and its reverse reaction, elimination, we can now combine and transform
Acceptable Answers Reject Mark. The sulfur trioxide can accept a pair of electrons An electron 1
 (a)(i sulfuric acid / fuming H SO 4 / oleum / H S O 7 Conc. (for fuming) Fuming dilute sulfuric acid Just sulfuric acid Just H SO 4 (a)(ii) Sulfur is δ+ and on at least one oxygen δ- Full + or charge(s)
(a)(i sulfuric acid / fuming H SO 4 / oleum / H S O 7 Conc. (for fuming) Fuming dilute sulfuric acid Just sulfuric acid Just H SO 4 (a)(ii) Sulfur is δ+ and on at least one oxygen δ- Full + or charge(s)
CHAPTER 5. Stereoisomers
 CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
CHAPTER 5 Stereoisomers We have already covered two kinds of isomerism: Constitutional Isomers (structural isomers) Stereoisomers Examples of Constitutional Isomers: Examples of Stereoisomers: Another
PHARMACEUTICAL CHEMISTRY EXAM #1 Februrary 21, 2008
 PHARMACEUTICAL CHEMISTRY EXAM #1 Februrary 21, 2008 1 Name SECTION B. Answer each question in this section by writing the letter corresponding to the best answer on the line provided (2 points each; 60
PHARMACEUTICAL CHEMISTRY EXAM #1 Februrary 21, 2008 1 Name SECTION B. Answer each question in this section by writing the letter corresponding to the best answer on the line provided (2 points each; 60
Oligolactate-Grafted Dextran Hydrogels: Detection of Stereocomplex Crosslinks by X-ray Diffraction
 Oligolactate-Grafted Dextran Hydrogels: Detection of Stereocomplex Crosslinks by X-ray Diffraction S. J. de Jong, 1 C.F. van Nostrum, 1 L.M. J. Kroon-Batenburg, 2 J.J. Kettenes-van den Bosch, 3 W. E. Hennink
Oligolactate-Grafted Dextran Hydrogels: Detection of Stereocomplex Crosslinks by X-ray Diffraction S. J. de Jong, 1 C.F. van Nostrum, 1 L.M. J. Kroon-Batenburg, 2 J.J. Kettenes-van den Bosch, 3 W. E. Hennink
Stereochemistry Terminology
 Stereochemistry Terminology Axis of symmetry: When an operation on an axis C n, where n = 360 /rotation, leads to a structure indistinguishable from the original. C 2 180 Plane of symmetry: (σ) A plane
Stereochemistry Terminology Axis of symmetry: When an operation on an axis C n, where n = 360 /rotation, leads to a structure indistinguishable from the original. C 2 180 Plane of symmetry: (σ) A plane
CHEM2077 HONORS ORGANIC CHEMISTRY SYLLABUS
 CHEM2077 HONORS ORGANIC CHEMISTRY SYLLABUS 1. STRUCTURE AND BONDING a] Atomic structure and bonding b] Hybridization and MO Theory c] Drawing chemical structures 2. POLAR COVALENT BONDS: ACIDS AND BASES
CHEM2077 HONORS ORGANIC CHEMISTRY SYLLABUS 1. STRUCTURE AND BONDING a] Atomic structure and bonding b] Hybridization and MO Theory c] Drawing chemical structures 2. POLAR COVALENT BONDS: ACIDS AND BASES
B X A X. In this case the star denotes a chiral center.
 Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
Lecture 13 Chirality III October 29, 2013 We can also access chiral molecules through the use of something called chiral auxiliaries, which basically is a chiral attachment that you add to your molecule
1.1 Basic Polymer Chemistry. 1.2 Polymer Nomenclature. 1.3 Polymer Synthesis. 1.4 Chain Growth Polymerization. Polymer =
 1.1 Basic Polymer hemistry Polymers are the largest class of soft materials: over 100 billion pounds of polymers made in US each year lassification systems 1.2 Polymer Nomenclature Polymer = Monomer =
1.1 Basic Polymer hemistry Polymers are the largest class of soft materials: over 100 billion pounds of polymers made in US each year lassification systems 1.2 Polymer Nomenclature Polymer = Monomer =
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
NH 2. 8 (c) (ii) This diamine is then reacted with benzene-l,4-dicarboxylic acid to form Kevlar. Draw the repeating unit of Kevlar.
 Polymers 8 (c) Isomer Y is used in the production of the polymer Kevlar. Y is first reduced to the diamine shown below. 20 Areas outside the will not be scanned for marking 2 N N 2 8 (c) (i) Identify a
Polymers 8 (c) Isomer Y is used in the production of the polymer Kevlar. Y is first reduced to the diamine shown below. 20 Areas outside the will not be scanned for marking 2 N N 2 8 (c) (i) Identify a
Chapter 10 Lecture Outline
 Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Chapter 7 Substitution Reactions 7.1 Introduction to Substitution Reactions Substitution Reactions: two reactants exchange parts to give new products
 hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
hapter 7 Substitution eactions 7.1 Introduction to Substitution eactions Substitution eactions: two reactants exchange parts to give new products A-B + -D A-D + B- 3 2 + Br 3 2 Br + Elimination eaction:
Chapter 5 Stereochemistry. Stereoisomers
 Chapter 5 Stereochemistry Stereoisomers Same bonding sequence Different arrangement in space Example: OOC-C=C-COO has two geometric (cis-trans) isomers: COO COO COO COO Stereochemistry Slide 5-2 1 Chirality
Chapter 5 Stereochemistry Stereoisomers Same bonding sequence Different arrangement in space Example: OOC-C=C-COO has two geometric (cis-trans) isomers: COO COO COO COO Stereochemistry Slide 5-2 1 Chirality
Reaction chemistry of complexes Three general forms: 1. Reactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b.
 eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Name/CG: 2012 Term 2 Organic Chemistry Revision (Session II) Deductive Question
 Name/G: 2012 Term 2 rganic hemistry Revision (Session II) Deductive Question 1(a) A yellow liquid A, 7 7 N 2, reacts with alkaline potassium manganate (VII) and on acidification gives a yellow solid B,
Name/G: 2012 Term 2 rganic hemistry Revision (Session II) Deductive Question 1(a) A yellow liquid A, 7 7 N 2, reacts with alkaline potassium manganate (VII) and on acidification gives a yellow solid B,
2/26/18. Practice Questions. Practice Questions B F. How many steps are there in this reaction?
 Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
STEREOCHEMISTRY A STUDENT SHOULD BE ABLE TO:
 A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
Homework Problem Set 8 Iverson CH320M/328M Due Friday, Nov. 9
 omework Problem Set 8 Iverson 320M/328M Due Friday, Nov. 9 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 8th omework November 2, 2018 Please print the first three letters of your last name
omework Problem Set 8 Iverson 320M/328M Due Friday, Nov. 9 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 8th omework November 2, 2018 Please print the first three letters of your last name
(1) Check to see if the two compounds are identical. (2) Recall the definitions of stereoisomers, conformational isomers, and constitutional isomers.
 MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
MCAT Organic Chemistry Problem Drill 04: Stereochemistry Question No. 1 of 10 Question 1. Determine the relationship of the molecules shown: O O Question #01 (A) Identical (B) Constitutional isomers (C)
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
10/4/2010. Sequence Rules for Specifying Configuration. Sequence Rules for Specifying Configuration. 5.5 Sequence Rules for Specifying.
 5.5 Sequence Rules for Specifying Configuration Configuration The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center:
5.5 Sequence Rules for Specifying Configuration Configuration The three-dimensional arrangement of substituents at a chirality center Sequence rules to specify the configuration of a chirality center:
(c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
 KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
KT121 Sem II 09/10 SETIN B: 1.(a) Name of mechanism: E1 elimination. 7 marks (b) (i) + 3 3 3 (ii) + 3 (iii) l 8 marks (c) The intermediate carbocation resulting from 3-bromobut-1-ene is resonance stabilized.
Chapter 8 Reactions of Alkenes
 Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
CHEM 203. Final Exam December 16, This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
CEM 203 Final Exam December 16, 2014 Your name: This a closed-notes, closed-book exam You may use your set of molecular models This test consists of 10 pages Time: 2h 30 min 1. / 20 2. / 20 3. / 30 4.
Paper No. 1: Organic Chemistry-I (Nature of bonding and
 Subject Chemistry Paper No and Title 1; Organic Chemistry-I (Nature of bonding and Module No and 31; Chirality due to helical shape, Cram s and Title Module Tag CHE_P1_M31 TABLE OF CONTENTS 1. Learning
Subject Chemistry Paper No and Title 1; Organic Chemistry-I (Nature of bonding and Module No and 31; Chirality due to helical shape, Cram s and Title Module Tag CHE_P1_M31 TABLE OF CONTENTS 1. Learning
Page 2. Q1.Repeating units of two polymers, P and Q, are shown in the figure below.
 Q1.Repeating units of two polymers, P and Q, are shown in the figure below. (a) Draw the structure of the monomer used to form polymer P. Name the type of polymerisation involved. Monomer Type of polymerisation...
Q1.Repeating units of two polymers, P and Q, are shown in the figure below. (a) Draw the structure of the monomer used to form polymer P. Name the type of polymerisation involved. Monomer Type of polymerisation...
O CH 3. Mn CH 3 OC C. 16eelimination
 igratory Insertion igratory Insertion/Elimination 1 A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic
igratory Insertion igratory Insertion/Elimination 1 A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic
Chemistry 234 (Organic I review) George A. O Doherty Please also refer to the Alkene Addition handout
 Chemistry 234 (Organic I review) George A. O Doherty Please also refer to the Alkene Addition handout So far we have built upon our understanding of the questions I and II, which deal with the number of
Chemistry 234 (Organic I review) George A. O Doherty Please also refer to the Alkene Addition handout So far we have built upon our understanding of the questions I and II, which deal with the number of
Chapter 11 Outline: Ethers, Epoxides & Sulfides
 Chapter 11 Outline: Ethers, Epoxides & Sulfides Review Nomenclature & Physical Properties on your own 1. Structure of Ethers & Epoxides 2. Preparation of Ethers & Thioethers 3. Reactions of Ethers 4. Preparation
Chapter 11 Outline: Ethers, Epoxides & Sulfides Review Nomenclature & Physical Properties on your own 1. Structure of Ethers & Epoxides 2. Preparation of Ethers & Thioethers 3. Reactions of Ethers 4. Preparation
CHEM 2423 Unit 8 Homework Answers
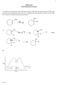 1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
Organic Chemistry: Structure and Reactivity Tutorial Six Question 1
 en Mills, University of istol, 4 March 2008 rganic Chemistry: Structure and Reactivity Tutorial Six Question 1 ydrobromination of styrene hydrogen adds to the terminal carbon, so that the carbocation can
en Mills, University of istol, 4 March 2008 rganic Chemistry: Structure and Reactivity Tutorial Six Question 1 ydrobromination of styrene hydrogen adds to the terminal carbon, so that the carbocation can
