Error Propagation 1. November 13, HMS, 2017, v1.0
|
|
|
- Brianne Cobb
- 5 years ago
- Views:
Transcription
1 Error Propagation 1 November 13, HMS, 2017, v1.0
2 Chapter References Diez: None Navidi, Chapter 3 Chapter References 2
3 Motivation Measured Value = True Value + Bias + Random Error The bias refers to the systematic error due to bias in the instrument or bias in the human operator or even software. Bias can be corrected. Random errors however are not easily reduced and propagate into any additional calculations we may do with the data. Errors 3
4 Motivation Let us measure the area of a circle: A = πr 2 In measuring the area we note that there is uncertainty in our measurement of the radius, r. For example r = 2.5 ± 0.2 Given this uncertainty what is the uncertainty the computed area: A ±? Error Propagation 4
5 Motivation The reaction rate for an enzyme catalyzed reaction is given by: v = V ms K m + S where V m is the maximal velocity and K m the substrate concentration, S at half the maximal rate. The V m and K m are estimated to be 15.8 ± 0.9 mm/s and 0.5 ± 0.05 mm respectively. At a substrate concentration of 5 mm, what is the reaction velocity and associated uncertainty? Error Propagation 5
6 Linear Combination of Measurements If c is a constant, then: σ c1x 1 + σ c2x = σ cx = c σ X c 2 1 σ2 X 1 + c 2 1 σ2 X Important caveat: X 1, X 2,... are independent measurements. Error Propagation 6
7 Example The radius of a circle, R, is measured to be 3.0 ± 0.1 cm. Estimate the circumference and find the uncertainty, σ c, in the estimate. Using: R = 2πR σ cx = c σ X σ c = 2π σ R = 0.63 cm The circumference is therefore: ± 0.63 cm Error Propagation 7
8 Example A surveyor is measuring the perimeter of a rectangular plot. Two adjacent sides are measured to be ± 0.05m and ± 0.08 cm. Estimate the perimeter of the lot and the uncertainty. P = 2X + 2Y = cm Using: σ c1x 1 + σ c2x = c 2 1 σ2 X 1 + c 2 1 σ2 X σ P = σ 2X + σ 2Y = The perimeter is ± 0.19 m 4σ 2 X + 4σ2 2 = 0.19 m You cannot use P = X + X + Y + Y and form σ P = σ X + σ X + σ Y + σ Y because X + X is not the sum of independent quantities. Error Propagation 8
9 Dependent Measurements For dependent measurements, one can compute an upper bound on the uncertainty in a linear combination: σ c1x 1 + σ c2x c 1 σ X1 + c 2 σ X Error Propagation 9
10 Uncertainties for Functions of One Measurement What happens if your function is nonlinear rather than a simple linear combination? Given a random variable X, with known standard deviation σ X and given U = U(X) how do we compute σ U? Error Propagation 10
11 Uncertainties for Functions of One Measurement σ U du dx σ X Note the equation gives an approximate estimate. Error Propagation 11
12 Uncertainties for Functions of One Measurement Going back to the first example of finding the area of a circle: A = πr 2. If R = 5.00 ± 0.01 cm then find the area and the uncertainty in the Area. σ A da dr σ R da dr = 2πR σ R = 10π 0.01 = 0.31 cm 2 The area is therefore given by 78.5 ± 0.3 cm 2 Error Propagation 12
13 Relative Uncertainties of One Measurement If U is a measurement with true mean µ and uncertainty σ U, then the relative uncertainty is: σ U U The relative uncertainty is also called the coefficient of variation Error Propagation 13
14 Relative Uncertainties of One Measurement Recall the area of the circle in the previous example was 78.5 ± 0.3 cm 2. The absolute uncertainty was 0.3 cm 2. The relative uncertainty is: σ A A = = = 0.4% Error Propagation 14
15 Uncertainties for Functions of Several Measurements If A, B,..., are independent measurements whose uncertainties are σ A, σ B, and if U = U(A, B,...) then σ U = ( U A ) 2 ( ) 2 U σa 2 + σb 2 B +... Error Propagation 15
16 Uncertainties for Functions of Several Measurements Assume we have a function: U = U(A, B) The total deviation as a result of uncertainty in the measurements, A or B is given by: du = U U A + A B B This assumes the deviations are small. Error Propagation 16
17 How to Propagate U = U U A + A B B The variance in U is given by: σ 2 U = 1 N N ( U i ) 2 i=1 The variances in the individual measurements, A and B are given by σ 2 A = 1 N N ( A i ) 2, i=1 σ 2 B = 1 N N ( B i ) 2, i=1 Error Propagation 17
18 How to Propagate Inserting U into σ 2 U σ 2 U = 1 N ( U A A i + U ) 2 B B i Expanding the squared term yields: σu 2 = 1 [ ( ) 2 ( ) ] 2 U U N A A i + B B i + 2 U U A B A i B i Let us assume that the errors are random and independent, this means that the cross-term will on average equal zero (positive and well as negative deviations are equally possible) Error Propagation 18
19 How to Propagate We are therefore left with: σu 2 = 1 [ ( ) 2 ( ) ] 2 U U N A A i + B B i We can rearrange this equation by pulling out the derivatives: σ 2 U = ( ) 2 U 1 A N ( ) 2 U ( Ai ) ( Bi ) 2 B N Do you recognized the terms: 1 N ( Ai ) 2 and 1 N ( Bi ) 2? They are the variances for A and B, therefore... Error Propagation 19
20 How to Propagate σ 2 U = ( ) 2 U σa 2 + A ( ) 2 U σb 2 B A similar proof can also be made if the σ s are standard errors. ( U ) 2 ( ) 2 U σ U = σa 2 A + σb 2 B +... Error Propagation 20
21 General Expression for product/quotient For the expression, U = abc/(xyz) the uncertainty in U can be shown to be: σ U U = (σa a ) 2 + ( σb b ) 2 + ( σc c ) 2 + ( σx x ) ( ) 2 2 σy + + y ( σz ) 2 z Error Propagation 21
22 General Rules Addition/Subtraction x = a + b c σ x = Mutiplication/Division x = a b/c σ x x = Exponential x = a k σ ( x x = k σa a σ 2 a + σ 2 b + σ2 c ( σa ) 2 ( σb + a b ) ) 2 ( σc ) 2 + c Error Propagation 22
23 Standard Error Consider the mean x: x = x 1 + x N In measuring an individual x i there will be uncertainty in the x i by an amount σ xi. Let us propagate the uncertainties in x i into the mean standard deviation: ( ) 2 ( ) 2 x x σ x = σx x σx 1 x Error Propagation 23
24 Standard Error σ x = ( x x 1 ) 2 ( ) 2 x σx σx x Let us assume that the uncertainties in each x i are the same, that is σ x1 = σ x2 =... = σ x Since x = x i /N we can evaluate the partial derivatives: x = x =... = 1 x 1 x 2 N Inserting these into the propagation relationship yields: Error Propagation 24
25 Standard Error σ x = ( ) 2 ( ) N σ x + N σ x +... Rearranging yields: And finally: σ x = N σ2 x N 2 σ x = σ x N The standard error simply expresses how uncertainty from the sampling propagates into the random variable that describes the means. Error Propagation 25
26 Example Assume the mass of a rock is measured to be m = ± 1 gm and the volume is measured to be V = ± 0.1 ml. Estimate the density of the rock and the uncertainty of the estate. Apply Density = m V = = g σ D = ( ) 2 D σm m 2 + ( ) 2 D σv 2 V +... D m = 1 = ml 1 m D V = m = g/ml2 V 2 Error Propagation 26
27 Example Assume the mass of a rock is measured to be m = ± 1 g and the volume is measured to be V = ± 0.1 ml. ( ) 2 ( ) 2 D D σ D = σm m 2 + σv 2 V σ D = g/ml The density of the rock is: ± g/ml Error Propagation 27
28 Example Suppose a concentration of a given solution is 13.7 ± 0.3 moles L 1. A UV spec is used to measure the absorbance using a cuvette with a path length of 1.0 ± 0.1 cm. The absorbance is found to be ± Estimate the molar absorptivity, ε, using Beer s law ε = A/(lc). Since the expression is of the form product/quotient, we can use: σ (σa ) 2 ( ε ε = σl ) 2 ( σc ) A l c Error Propagation 28
29 Example Inserting the values into the equation yields: ( ) 2 ( ) 2 ( ) 2 σ ε ε = σ ε ε = Using Beer s law we can compute ε: Therefore: ε = /(1 13.7) = L mol 1 cm 1 ε = ± L mol 1 cm 1 Error Propagation 29
30 Effect on Error Bars The following data was measured for an enzymatic reaction with standard error of 0.5 for each measurement Substrate Reaction Rate Standard Error Error Propagation 30
31 Effect on Error Bars The enzymatic reaction is governed by: Transforming to: v = V m s K m + s 1 v = K m V m 1 s + 1 V m That is a plot of 1/v versus 1/s will yield a straight line. Error Propagation 31
32 Effect on Error Bars 1 v = K m V m 1 s + 1 V m Error Propagation 32
33 Effect on Error Bars Plot of reaction velocity versus substrate concentration. Error Propagation 33
34 Effect on Error Bars Left hand-side plot is reaction velocity versus substrate concentration. Left plot is plot of 1/v versus 1/s Error Propagation 34
35 Effect on Error Bars How is the error propagated into 1/v? σ (σa ) 2 x x = +... a Therefore σ 1/v 1/v = (σv v σ 1/v = σ v v 2 ) 2 = σ v v Error Propagation 35
36 Effect on Error Bars σ 1/v = σ v v 2 As at low v (high 1/v), the uncertainty in 1/v significantly increases. Error Propagation 36
37 Effect on Error Bars High v Low v Error Propagation 37
38 Error Propagation using Python Use the uncertainties package: te.installpackage( uncertainties ) Other Pythons use: pip install --upgrade uncertainties Error Propagation 38
39 Error Propagation using Python >>> from uncertainties import ufloat >>> x = ufloat(1, 0.1) # x = 1+/-0.1 >>> print 2*x 2.00+/-0.20 >>> v = ufloat (4.5, 0.25) >>> print 1/v / Error Propagation 39
40 Error Propagation using Python >>> from uncertainties import unumpy >>>y = unumpy.uarray ([0.3636, 0.533, 0.631, 0.695, , 0.774,0.8,0.821, 0.837, 0.851], [0.5,0.5,0.5,0.5,0.5,0.5,0.5,0.5,0.5,0.5]) >>>print 1/y [ / / / / / / / / / / ] >>>print 0.5/(0.3636*0.3636) Error Propagation 40
41 Linear Regression This leads eventually on to Linear Regression. But first we must introduce Analysis of Variance. Error Propagation 41
Biochemical Kinetics: the science that studies rates of chemical reactions An example is the reaction (A P), The velocity, v, or rate, of the
 Biochemical Kinetics: the science that studies rates of chemical reactions An example is the reaction (A P), The velocity, v, or rate, of the reaction A P is the amount of P formed or the amount of A consumed
Biochemical Kinetics: the science that studies rates of chemical reactions An example is the reaction (A P), The velocity, v, or rate, of the reaction A P is the amount of P formed or the amount of A consumed
Error propagation. Alexander Khanov. October 4, PHYS6260: Experimental Methods is HEP Oklahoma State University
 Error propagation Alexander Khanov PHYS660: Experimental Methods is HEP Oklahoma State University October 4, 017 Why error propagation? In many cases we measure one thing and want to know something else
Error propagation Alexander Khanov PHYS660: Experimental Methods is HEP Oklahoma State University October 4, 017 Why error propagation? In many cases we measure one thing and want to know something else
Introduction. The amount of radiation absorbed may be measured in a number of ways: Transmittance, T = P / P 0 % Transmittance, %T = 100 T
 Introduction Many compounds absorb ultraviolet (UV) or visible (Vis.) light. The diagram below shows a beam of monochromatic radiation of radiant power P 0, directed at a sample solution. Absorption takes
Introduction Many compounds absorb ultraviolet (UV) or visible (Vis.) light. The diagram below shows a beam of monochromatic radiation of radiant power P 0, directed at a sample solution. Absorption takes
Appendix 4. Some Equations for Curve Fitting
 Excep for Scientists and Engineers: Numerical Methods by E. Joseph Billo Copyright 0 2007 John Wiley & Sons, Inc. Appendix 4 Some Equations for Curve Fitting This appendix describes a number of equation
Excep for Scientists and Engineers: Numerical Methods by E. Joseph Billo Copyright 0 2007 John Wiley & Sons, Inc. Appendix 4 Some Equations for Curve Fitting This appendix describes a number of equation
MORE LIGHTS, COLOR, ABSORPTION!
 Name Partner(s) Section Date MORE LIGHTS, COLOR, ABSORPTION! PRE-LAB QUERIES 1. The terms absorption and transmittance are often used when describing the interaction of light with matter. Explain what
Name Partner(s) Section Date MORE LIGHTS, COLOR, ABSORPTION! PRE-LAB QUERIES 1. The terms absorption and transmittance are often used when describing the interaction of light with matter. Explain what
A Quick Introduction to Data Analysis (for Physics)
 A Quick Introduction to Data Analysis for Physics Dr. Jeff A. Winger What is data analysis? Data analysis is the process by which experimental data is used to obtain a valid and quantifiable result. Part
A Quick Introduction to Data Analysis for Physics Dr. Jeff A. Winger What is data analysis? Data analysis is the process by which experimental data is used to obtain a valid and quantifiable result. Part
Lab Investigation 4 - How could you make more of this dye?
 Lab Investigation 4 - How could you make more of this dye? USING SPECTROSCOPY TO DETERMINE SOLUTION CON- CENTRATION Guiding Question How could you make more of this dye? INTRODUCTION A solution is a homogeneous
Lab Investigation 4 - How could you make more of this dye? USING SPECTROSCOPY TO DETERMINE SOLUTION CON- CENTRATION Guiding Question How could you make more of this dye? INTRODUCTION A solution is a homogeneous
Lecture 13: Data Analysis for the V versus [S] Experiment and Interpretation of the Michaelis-Menten Parameters
![Lecture 13: Data Analysis for the V versus [S] Experiment and Interpretation of the Michaelis-Menten Parameters Lecture 13: Data Analysis for the V versus [S] Experiment and Interpretation of the Michaelis-Menten Parameters](/thumbs/89/100978514.jpg) Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 13: Data Analysis for the V versus [S] Experiment and Interpretation of the Michaelis-Menten Parameters 20 February 2018
Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 13: Data Analysis for the V versus [S] Experiment and Interpretation of the Michaelis-Menten Parameters 20 February 2018
A First Course on Kinetics and Reaction Engineering Example 13.2
 Example 13. Problem Purpose This example illustrates the analysis of kinetics data from a CSTR where the rate expression must be linearized. Problem Statement A new enzyme has been found for the dehydration
Example 13. Problem Purpose This example illustrates the analysis of kinetics data from a CSTR where the rate expression must be linearized. Problem Statement A new enzyme has been found for the dehydration
Spectrophotometry. Introduction
 Spectrophotometry Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle
Spectrophotometry Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle
Bioengineering Laboratory I. Enzyme Assays. Part II: Determination of Kinetic Parameters Fall Semester
 Bioengineering Laboratory I Enzyme Assays Part II: Determination of Kinetic Parameters 2016-2017 Fall Semester 1. Theoretical background There are several mathematical models to determine the kinetic constants
Bioengineering Laboratory I Enzyme Assays Part II: Determination of Kinetic Parameters 2016-2017 Fall Semester 1. Theoretical background There are several mathematical models to determine the kinetic constants
MOLEBIO LAB #4: Using a Spectrophotometer
 Introduction: Spectrophotometry MOLEBIO LAB #4: Using a Spectrophotometer Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while
Introduction: Spectrophotometry MOLEBIO LAB #4: Using a Spectrophotometer Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while
Confidence Intervals 1
 Confidence Intervals 1 November 1, 2017 1 HMS, 2017, v1.1 Chapter References Diez: Chapter 4.2 Navidi, Chapter 5.0, 5.1, (Self read, 5.2), 5.3, 5.4, 5.6, not 5.7, 5.8 Chapter References 2 Terminology Point
Confidence Intervals 1 November 1, 2017 1 HMS, 2017, v1.1 Chapter References Diez: Chapter 4.2 Navidi, Chapter 5.0, 5.1, (Self read, 5.2), 5.3, 5.4, 5.6, not 5.7, 5.8 Chapter References 2 Terminology Point
Expt 4: Determination of Iron by Absorption Spectrophotometry Calibration solutions: Iron(II)ammonium sulfate = ferrous ammonium sulfate
 Expt 4: Determination of Iron by Absorption Spectrophotometry Calibration solutions: Iron(II)ammonium sulfate = ferrous ammonium sulfate FeSO 4 (NH 4 ) 2 SO 4 6H 2 O, M.W. = 392.14 g Stock 1 0.210 g in
Expt 4: Determination of Iron by Absorption Spectrophotometry Calibration solutions: Iron(II)ammonium sulfate = ferrous ammonium sulfate FeSO 4 (NH 4 ) 2 SO 4 6H 2 O, M.W. = 392.14 g Stock 1 0.210 g in
Cambridge International Examinations CambridgeOrdinaryLevel
 Cambridge International Examinations CambridgeOrdinaryLevel * 2 5 4 0 0 0 9 5 8 5 * ADDITIONAL MATHEMATICS 4037/12 Paper1 May/June 2015 2 hours CandidatesanswerontheQuestionPaper. NoAdditionalMaterialsarerequired.
Cambridge International Examinations CambridgeOrdinaryLevel * 2 5 4 0 0 0 9 5 8 5 * ADDITIONAL MATHEMATICS 4037/12 Paper1 May/June 2015 2 hours CandidatesanswerontheQuestionPaper. NoAdditionalMaterialsarerequired.
Correlation 1. December 4, HMS, 2017, v1.1
 Correlation 1 December 4, 2017 1 HMS, 2017, v1.1 Chapter References Diez: Chapter 7 Navidi, Chapter 7 I don t expect you to learn the proofs what will follow. Chapter References 2 Correlation The sample
Correlation 1 December 4, 2017 1 HMS, 2017, v1.1 Chapter References Diez: Chapter 7 Navidi, Chapter 7 I don t expect you to learn the proofs what will follow. Chapter References 2 Correlation The sample
Experiment 4 - BIOC 221 W2019. The Subject for the should be: BIOCHEM 221 EXP4
 EXCEL SHEET INSTRUCTIONS 1. a) On the web page there is a file called YOURNAME_Bioc221Exp4W2019.xlsx. Fill in the information colored RED with your own information. (Please do not readjust where the cells
EXCEL SHEET INSTRUCTIONS 1. a) On the web page there is a file called YOURNAME_Bioc221Exp4W2019.xlsx. Fill in the information colored RED with your own information. (Please do not readjust where the cells
Data Fitting and Uncertainty
 TiloStrutz Data Fitting and Uncertainty A practical introduction to weighted least squares and beyond With 124 figures, 23 tables and 71 test questions and examples VIEWEG+ TEUBNER IX Contents I Framework
TiloStrutz Data Fitting and Uncertainty A practical introduction to weighted least squares and beyond With 124 figures, 23 tables and 71 test questions and examples VIEWEG+ TEUBNER IX Contents I Framework
ELASTICITY (MDM 10203)
 ELASTICITY () Lecture Module 3: Fundamental Stress and Strain University Tun Hussein Onn Malaysia Normal Stress inconstant stress distribution σ= dp da P = da A dimensional Area of σ and A σ A 3 dimensional
ELASTICITY () Lecture Module 3: Fundamental Stress and Strain University Tun Hussein Onn Malaysia Normal Stress inconstant stress distribution σ= dp da P = da A dimensional Area of σ and A σ A 3 dimensional
Answers to test yourself questions
 nswers to test yourself questions Topic Measurement in physics 5 Taking the diameter of a proton to be order m 5 we find 3 8 3 = 3 = 3 s 7 The mass of the Earth is about kg and the mass of a hydrogen atom
nswers to test yourself questions Topic Measurement in physics 5 Taking the diameter of a proton to be order m 5 we find 3 8 3 = 3 = 3 s 7 The mass of the Earth is about kg and the mass of a hydrogen atom
Lambert s law. Beer s law. di x / I x = -kdx (-di x = k I x dx) = - a c dx. I/I 0 = e -kl T = A = - log (T) = - log (I/I 0 )
 di x / I x = -kdx (-di x = k I x dx) Integrating this equation from x=0 ~ l (I x =I 0 ~I) gives ; ln I ln I 0 = -kl ln I/I 0 = -kl Expressing the number of photons absorbed by the slab as di x, and the
di x / I x = -kdx (-di x = k I x dx) Integrating this equation from x=0 ~ l (I x =I 0 ~I) gives ; ln I ln I 0 = -kl ln I/I 0 = -kl Expressing the number of photons absorbed by the slab as di x, and the
CHEM Exam 1 February 11, 2016 Constants and Equations: R = 8.31 J/mol-K. Beer-Lambert Law: A log bc. Michaelis-Menten Equation: v0 M
 CHEM 1423 - Exam 1 February 11, 2016 Constants and Equations: R = 8.31 J/mol-K Io Beer-Lambert Law: A log bc I Vm[ S] Michaelis-Menten Equation: v0 K [ S] M CHEM 1423 - Exam 1 February 11, 2016 Name (60)
CHEM 1423 - Exam 1 February 11, 2016 Constants and Equations: R = 8.31 J/mol-K Io Beer-Lambert Law: A log bc I Vm[ S] Michaelis-Menten Equation: v0 K [ S] M CHEM 1423 - Exam 1 February 11, 2016 Name (60)
Regression and Nonlinear Axes
 Introduction to Chemical Engineering Calculations Lecture 2. What is regression analysis? A technique for modeling and analyzing the relationship between 2 or more variables. Usually, 1 variable is designated
Introduction to Chemical Engineering Calculations Lecture 2. What is regression analysis? A technique for modeling and analyzing the relationship between 2 or more variables. Usually, 1 variable is designated
University of Massachusetts Boston - Chemistry Department Physical Chemistry Laboratory Introduction to Maximum Probable Error
 University of Massachusetts Boston - Chemistry Department Physical Chemistry Laboratory Introduction to Maximum Probable Error Statistical methods describe random or indeterminate errors in experimental
University of Massachusetts Boston - Chemistry Department Physical Chemistry Laboratory Introduction to Maximum Probable Error Statistical methods describe random or indeterminate errors in experimental
Clinical Chemistry (CHE221) Professor Hicks Week 1. Statistics Made Slightly Less Boring and Introduction to Spectrophotometry. Accuracy vs Precision
 Clinical Chemistry (CHE221) Professor Hicks Week 1 Statistics Made Slightly Less Boring and Introduction to Spectrophotometry 3 Accuracy vs Precision Precision is the consistency of a measurement made
Clinical Chemistry (CHE221) Professor Hicks Week 1 Statistics Made Slightly Less Boring and Introduction to Spectrophotometry 3 Accuracy vs Precision Precision is the consistency of a measurement made
 IE 361 EXAM #3 FALL 2013 Show your work: Partial credit can only be given for incorrect answers if there is enough information to clearly see what you were trying to do. There are two additional blank
IE 361 EXAM #3 FALL 2013 Show your work: Partial credit can only be given for incorrect answers if there is enough information to clearly see what you were trying to do. There are two additional blank
Algebraic Expressions and Identities
 9 Algebraic Epressions and Identities introduction In previous classes, you have studied the fundamental concepts of algebra, algebraic epressions and their addition and subtraction. In this chapter, we
9 Algebraic Epressions and Identities introduction In previous classes, you have studied the fundamental concepts of algebra, algebraic epressions and their addition and subtraction. In this chapter, we
CHEM Lab 7: Determination of an Equilibrium Constant using Spectroscopy
 CHEM 0012 Lab 7: Determination of an Equilibrium Constant using Spectroscopy 1 Determination of the equilibrium constant of the following equilibrium system at room temperature. Fe 3+ (aq) + SCN- (aq)
CHEM 0012 Lab 7: Determination of an Equilibrium Constant using Spectroscopy 1 Determination of the equilibrium constant of the following equilibrium system at room temperature. Fe 3+ (aq) + SCN- (aq)
Homework Problem Set 8 Solutions
 Chemistry 360 Dr. Jean M. Standard Homework roblem Set 8 Solutions. Starting from G = H S, derive the fundamental equation for G. o begin, we take the differential of G, dg = dh d( S) = dh ds Sd. Next,
Chemistry 360 Dr. Jean M. Standard Homework roblem Set 8 Solutions. Starting from G = H S, derive the fundamental equation for G. o begin, we take the differential of G, dg = dh d( S) = dh ds Sd. Next,
An Introduction to Stochastic Calculus
 An Introduction to Stochastic Calculus Haijun Li lih@math.wsu.edu Department of Mathematics Washington State University Weeks 3-4 Haijun Li An Introduction to Stochastic Calculus Weeks 3-4 1 / 22 Outline
An Introduction to Stochastic Calculus Haijun Li lih@math.wsu.edu Department of Mathematics Washington State University Weeks 3-4 Haijun Li An Introduction to Stochastic Calculus Weeks 3-4 1 / 22 Outline
CN#5 Objectives 5/11/ I will be able to describe the effect on perimeter and area when one or more dimensions of a figure are changed.
 CN#5 Objectives I will be able to describe the effect on perimeter and area when one or more dimensions of a figure are changed. When the dimensions of a figure are changed proportionally, the figure will
CN#5 Objectives I will be able to describe the effect on perimeter and area when one or more dimensions of a figure are changed. When the dimensions of a figure are changed proportionally, the figure will
Mathematics 1 Lecture Notes Chapter 1 Algebra Review
 Mathematics 1 Lecture Notes Chapter 1 Algebra Review c Trinity College 1 A note to the students from the lecturer: This course will be moving rather quickly, and it will be in your own best interests to
Mathematics 1 Lecture Notes Chapter 1 Algebra Review c Trinity College 1 A note to the students from the lecturer: This course will be moving rather quickly, and it will be in your own best interests to
Enzymes. Lab Exercise 7. Introduction. Contents. Objectives
 Lab Exercise Enzymes Contents Objectives 1 Introduction 1 Activity.1 Optimal ph 3 Activity.2 Optimal Temperature 4 Activity.3 Reaction Rates 4 Resutls Section 5 Objectives - Appreciate the sensitivity
Lab Exercise Enzymes Contents Objectives 1 Introduction 1 Activity.1 Optimal ph 3 Activity.2 Optimal Temperature 4 Activity.3 Reaction Rates 4 Resutls Section 5 Objectives - Appreciate the sensitivity
Modulating Enzymatic Activity in the Presence of Gold Nanoparticles
 Modulating Enzymatic Activity in the Presence of Gold Nanoparticles Jashmini Deka, 1 Anumita Paul* 1 and Arun Chattopadhyay* 1,2 Department of Chemistry 1 and Centre for Nanotechnology 2, Indian Institute
Modulating Enzymatic Activity in the Presence of Gold Nanoparticles Jashmini Deka, 1 Anumita Paul* 1 and Arun Chattopadhyay* 1,2 Department of Chemistry 1 and Centre for Nanotechnology 2, Indian Institute
Introduction to PML in time domain
 Introduction to PML in time domain Alexander Thomann Introduction to PML in time domain - Alexander Thomann p.1 Overview 1 Introduction 2 PML in one dimension Classical absorbing layers One-dimensional
Introduction to PML in time domain Alexander Thomann Introduction to PML in time domain - Alexander Thomann p.1 Overview 1 Introduction 2 PML in one dimension Classical absorbing layers One-dimensional
Experimental Uncertainty (Error) and Data Analysis
 Experimental Uncertainty (Error) and Data Analysis Advance Study Assignment Please contact Dr. Reuven at yreuven@mhrd.org if you have any questions Read the Theory part of the experiment (pages 2-14) and
Experimental Uncertainty (Error) and Data Analysis Advance Study Assignment Please contact Dr. Reuven at yreuven@mhrd.org if you have any questions Read the Theory part of the experiment (pages 2-14) and
Calculus I Homework: Rates of Change in the Natural and Social Sciences Page 1
 Calculus I Homework: Rates of Change in the Natural and Social Sciences Page 1 Questions Example If a ball is thrown vertically upward with a velocity of 80 ft/s, then its height after t seconds is s 80t
Calculus I Homework: Rates of Change in the Natural and Social Sciences Page 1 Questions Example If a ball is thrown vertically upward with a velocity of 80 ft/s, then its height after t seconds is s 80t
Uncertainty sources of reference measurement procedures for enzymes
 Medical School Hannover Institute for Clinical Chemistry Director: Prof. Dr. K. Brand Uncertainty sources of reference measurement procedures for enzymes R. Klauke and G. Schumann Renate Strache Rainer
Medical School Hannover Institute for Clinical Chemistry Director: Prof. Dr. K. Brand Uncertainty sources of reference measurement procedures for enzymes R. Klauke and G. Schumann Renate Strache Rainer
Practice SAC. Unit 4 Further Mathematics: Geometry and Trigonometry Module
 Practice SAC Unit 4 Further Mathematics: Geometry and Trigonometry Module Student Name: Subject Teacher s Name: Equipment Permitted: Writing materials, 1 bound reference, CAS- Calculator Structure of book
Practice SAC Unit 4 Further Mathematics: Geometry and Trigonometry Module Student Name: Subject Teacher s Name: Equipment Permitted: Writing materials, 1 bound reference, CAS- Calculator Structure of book
Degree of labeling (DOL)
 Degree of labeling (DOL) A written explanation for the determination of the degree of labeling when using NuLink reagents. The DOL is the average number of reagent molecules that have been covalently attached
Degree of labeling (DOL) A written explanation for the determination of the degree of labeling when using NuLink reagents. The DOL is the average number of reagent molecules that have been covalently attached
Chapter - II. Isomorphism on Fuzzy Graphs
 Chapter - II Isomorphism on Fuzzy Graphs CHAPTER - II ISOMORPHISM ON FUZZY GRAPHS In this chapter, the order, size and degree of the nodes of the isomorphic fuzzy graphs are discussed. Isomorphism between
Chapter - II Isomorphism on Fuzzy Graphs CHAPTER - II ISOMORPHISM ON FUZZY GRAPHS In this chapter, the order, size and degree of the nodes of the isomorphic fuzzy graphs are discussed. Isomorphism between
Experiment 1 - Mass, Volume and Graphing
 Experiment 1 - Mass, Volume and Graphing In chemistry, as in many other sciences, a major part of the laboratory experience involves taking measurements and then calculating quantities from the results
Experiment 1 - Mass, Volume and Graphing In chemistry, as in many other sciences, a major part of the laboratory experience involves taking measurements and then calculating quantities from the results
Chemistry 213. A KINETIC STUDY: REACTION OF CRYSTAL VIOLET WITH NaOH LEARNING OBJECTIVES
 Chemistry 213 A KINETIC STUDY: REACTION OF CRYSTAL VIOLET WITH NaOH The objectives of this experiment are to... LEARNING OBJECTIVES study the reaction rate of crystal violet with NaOH using a Spectronic
Chemistry 213 A KINETIC STUDY: REACTION OF CRYSTAL VIOLET WITH NaOH The objectives of this experiment are to... LEARNING OBJECTIVES study the reaction rate of crystal violet with NaOH using a Spectronic
Learning Gaussian Process Models from Uncertain Data
 Learning Gaussian Process Models from Uncertain Data Patrick Dallaire, Camille Besse, and Brahim Chaib-draa DAMAS Laboratory, Computer Science & Software Engineering Department, Laval University, Canada
Learning Gaussian Process Models from Uncertain Data Patrick Dallaire, Camille Besse, and Brahim Chaib-draa DAMAS Laboratory, Computer Science & Software Engineering Department, Laval University, Canada
CHEM 334 Quantitative Analysis Laboratory
 The Methods of Calibration Curve and Standard Addition Introduction One of the principle activities in the Quantitative Analysis Laboratory is the measurement of the concentration or total quantity of
The Methods of Calibration Curve and Standard Addition Introduction One of the principle activities in the Quantitative Analysis Laboratory is the measurement of the concentration or total quantity of
Physics 403. Segev BenZvi. Propagation of Uncertainties. Department of Physics and Astronomy University of Rochester
 Physics 403 Propagation of Uncertainties Segev BenZvi Department of Physics and Astronomy University of Rochester Table of Contents 1 Maximum Likelihood and Minimum Least Squares Uncertainty Intervals
Physics 403 Propagation of Uncertainties Segev BenZvi Department of Physics and Astronomy University of Rochester Table of Contents 1 Maximum Likelihood and Minimum Least Squares Uncertainty Intervals
Introduction to Computational Finance and Financial Econometrics Matrix Algebra Review
 You can t see this text! Introduction to Computational Finance and Financial Econometrics Matrix Algebra Review Eric Zivot Spring 2015 Eric Zivot (Copyright 2015) Matrix Algebra Review 1 / 54 Outline 1
You can t see this text! Introduction to Computational Finance and Financial Econometrics Matrix Algebra Review Eric Zivot Spring 2015 Eric Zivot (Copyright 2015) Matrix Algebra Review 1 / 54 Outline 1
Measurements of a Table
 Measurements of a Table OBJECTIVES to practice the concepts of significant figures, the mean value, the standard deviation of the mean and the normal distribution by making multiple measurements of length
Measurements of a Table OBJECTIVES to practice the concepts of significant figures, the mean value, the standard deviation of the mean and the normal distribution by making multiple measurements of length
Basic Calibration of UV/ Visible Spectrophotometer
 International Journal of Science and Technology Volume 2 No. 3, March, 2013 Basic Calibration of UV/ Visible Spectrophotometer Adeeyinwo, C.E, 1 Okorie, N. N. 2, Idowu, G. O. 3 1,2 Department of Chemistry,
International Journal of Science and Technology Volume 2 No. 3, March, 2013 Basic Calibration of UV/ Visible Spectrophotometer Adeeyinwo, C.E, 1 Okorie, N. N. 2, Idowu, G. O. 3 1,2 Department of Chemistry,
Lecture 7: Curve Fitting, Part II, and Overlapping Spectra
 Biological Chemistry Laboratory Biology 355/Chemistry 355 Spring 28 Lecture 7: Curve Fitting, Part II, and Overlapping Spectra 3 January 28 c David P. Goldenberg University of Utah goldenberg@biology.utah.edu
Biological Chemistry Laboratory Biology 355/Chemistry 355 Spring 28 Lecture 7: Curve Fitting, Part II, and Overlapping Spectra 3 January 28 c David P. Goldenberg University of Utah goldenberg@biology.utah.edu
Beer-Lambert law Decomposition of the manganese oxalate ion
 A34 Beer-Lambert law Decomposition of the manganese oxalate ion Task: 1. Determine the wavelength of maximal absorbance λ max of a hydrated Cu(NH 3 ) 4 2+ complex in the wavelength region of 400 to 800
A34 Beer-Lambert law Decomposition of the manganese oxalate ion Task: 1. Determine the wavelength of maximal absorbance λ max of a hydrated Cu(NH 3 ) 4 2+ complex in the wavelength region of 400 to 800
Determination of an Equilibrium Constant
 Last updated 1/29/2014 - GES Learning Objectives Students will be able to: Determine the numerical value of an equilibrium constant from measured concentrations of all reaction species. Use an absorption
Last updated 1/29/2014 - GES Learning Objectives Students will be able to: Determine the numerical value of an equilibrium constant from measured concentrations of all reaction species. Use an absorption
One of the main objectives of this study is the estimation of parameters of
 C H A P T E R 3 M E T H O D O L O G Y 31 Introduction One of the main objectives of this study is the estimation of parameters of Makeham's law of mortality I believe that it is mandatory to explain briefly
C H A P T E R 3 M E T H O D O L O G Y 31 Introduction One of the main objectives of this study is the estimation of parameters of Makeham's law of mortality I believe that it is mandatory to explain briefly
Measurement. New Topics accuracy vs. precision rounding in chemistry significant figures determining uncertainty of a measurement % error moles - 1 -
 Measurement Unit Description In this unit we will focus on the mathematical tools we use in science, especially chemistry the metric system and moles. We will also talk about how to gauge the accuracy
Measurement Unit Description In this unit we will focus on the mathematical tools we use in science, especially chemistry the metric system and moles. We will also talk about how to gauge the accuracy
Baldragon Academy National 4 Maths Checklist
 Baldragon Academy National 4 Maths Checklist Contents: Page Numeracy Number..2 Measure.4 Statistics...6 Expressions and Formulae Algebra..8 Geometry.....9 Statistics..11 Relationships Linear Equations
Baldragon Academy National 4 Maths Checklist Contents: Page Numeracy Number..2 Measure.4 Statistics...6 Expressions and Formulae Algebra..8 Geometry.....9 Statistics..11 Relationships Linear Equations
Introduction to Maximum Likelihood Estimation
 Introduction to Maximum Likelihood Estimation Eric Zivot July 26, 2012 The Likelihood Function Let 1 be an iid sample with pdf ( ; ) where is a ( 1) vector of parameters that characterize ( ; ) Example:
Introduction to Maximum Likelihood Estimation Eric Zivot July 26, 2012 The Likelihood Function Let 1 be an iid sample with pdf ( ; ) where is a ( 1) vector of parameters that characterize ( ; ) Example:
Experiment 7A ANALYSIS OF BRASS
 Experiment 7A ANALYSIS OF BRASS FV 10/21/10 MATERIALS: Spectronic 20 spectrophotometers, 2 cuvettes, brass sample, 7 M HNO 3, 0.100 M CuSO 4, 2 M NH 3, two 50 ml beakers, 100 ml beaker, two 25 ml volumetric
Experiment 7A ANALYSIS OF BRASS FV 10/21/10 MATERIALS: Spectronic 20 spectrophotometers, 2 cuvettes, brass sample, 7 M HNO 3, 0.100 M CuSO 4, 2 M NH 3, two 50 ml beakers, 100 ml beaker, two 25 ml volumetric
An introduction to particle filters
 An introduction to particle filters Andreas Svensson Department of Information Technology Uppsala University June 10, 2014 June 10, 2014, 1 / 16 Andreas Svensson - An introduction to particle filters Outline
An introduction to particle filters Andreas Svensson Department of Information Technology Uppsala University June 10, 2014 June 10, 2014, 1 / 16 Andreas Svensson - An introduction to particle filters Outline
Biology 3B LABORATORY Quantitative determination of chlorophyll using spectroscopy
 Biology 3B LABORATORY Quantitative determination of chlorophyll using spectroscopy Objectives Gain an understanding of the Beer-Lambert Law Use this principle to quantify the concentration of total chlorophyll
Biology 3B LABORATORY Quantitative determination of chlorophyll using spectroscopy Objectives Gain an understanding of the Beer-Lambert Law Use this principle to quantify the concentration of total chlorophyll
Regression and correlation. Correlation & Regression, I. Regression & correlation. Regression vs. correlation. Involve bivariate, paired data, X & Y
 Regression and correlation Correlation & Regression, I 9.07 4/1/004 Involve bivariate, paired data, X & Y Height & weight measured for the same individual IQ & exam scores for each individual Height of
Regression and correlation Correlation & Regression, I 9.07 4/1/004 Involve bivariate, paired data, X & Y Height & weight measured for the same individual IQ & exam scores for each individual Height of
Enzymes II. Dr. Mamoun Ahram Summer, 2017
 Enzymes II Dr. Mamoun Ahram Summer, 2017 Kinetics Kinetics is deals with the rates of chemical reactions. Chemical kinetics is the study of the rates of chemical reactions. For the reaction (A P), The
Enzymes II Dr. Mamoun Ahram Summer, 2017 Kinetics Kinetics is deals with the rates of chemical reactions. Chemical kinetics is the study of the rates of chemical reactions. For the reaction (A P), The
CHEM Exam 2 March 3, 2016
 CHEM 123 - Exam 2 March 3, 2016 Constants and Conversion Factors R = 0.082 L-atm/mol-K R = 8.31 J/mol-K 1 atm. = 760 torr Molar Masses: C6H12O6-180. C12H22O11-32. C2H6O - 6. H2O - 18. Al(NO3)3-213. NaOH
CHEM 123 - Exam 2 March 3, 2016 Constants and Conversion Factors R = 0.082 L-atm/mol-K R = 8.31 J/mol-K 1 atm. = 760 torr Molar Masses: C6H12O6-180. C12H22O11-32. C2H6O - 6. H2O - 18. Al(NO3)3-213. NaOH
Sect Formulas and Applications of Geometry:
 72 Sect 2.6 - Formulas and Applications of Geometry: Concept # Solving Literal Equations for a particular variable. Now, we will examine solving formulas for a particular variable. Sometimes it is useful
72 Sect 2.6 - Formulas and Applications of Geometry: Concept # Solving Literal Equations for a particular variable. Now, we will examine solving formulas for a particular variable. Sometimes it is useful
(b) x = (d) x = (b) x = e (d) x = e4 2 ln(3) 2 x x. is. (b) 2 x, x 0. (d) x 2, x 0
 1. Solve the equation 3 4x+5 = 6 for x. ln(6)/ ln(3) 5 (a) x = 4 ln(3) ln(6)/ ln(3) 5 (c) x = 4 ln(3)/ ln(6) 5 (e) x = 4. Solve the equation e x 1 = 1 for x. (b) x = (d) x = ln(5)/ ln(3) 6 4 ln(6) 5/ ln(3)
1. Solve the equation 3 4x+5 = 6 for x. ln(6)/ ln(3) 5 (a) x = 4 ln(3) ln(6)/ ln(3) 5 (c) x = 4 ln(3)/ ln(6) 5 (e) x = 4. Solve the equation e x 1 = 1 for x. (b) x = (d) x = ln(5)/ ln(3) 6 4 ln(6) 5/ ln(3)
Supporting Information. for. Hexene. Clark R. Landis*,
 Supporting Information for Chromophore Quench-Labeling: An Approach to Quantifying Catalyst Speciation as Demonstrated for (EBI)ZrMe 2 /B(C 6 F 5 ) 3 -Catalyzed Polymerization of 1- Hexene D. Luke Nelsen,
Supporting Information for Chromophore Quench-Labeling: An Approach to Quantifying Catalyst Speciation as Demonstrated for (EBI)ZrMe 2 /B(C 6 F 5 ) 3 -Catalyzed Polymerization of 1- Hexene D. Luke Nelsen,
Bureau International des Poids et Mesures Comparison of ozone reference standards of the CHMI and the BIPM
 Rapport BIPM-2018/03 Bureau International des Poids et Mesures Comparison of ozone reference standards of the CHMI and the BIPM by J. Viallon, F. Idrees, P. Moussay and R.I. Wielgosz BIPM and J. Šilhavý
Rapport BIPM-2018/03 Bureau International des Poids et Mesures Comparison of ozone reference standards of the CHMI and the BIPM by J. Viallon, F. Idrees, P. Moussay and R.I. Wielgosz BIPM and J. Šilhavý
Stochastic optimization - how to improve computational efficiency?
 Stochastic optimization - how to improve computational efficiency? Christian Bucher Center of Mechanics and Structural Dynamics Vienna University of Technology & DYNARDO GmbH, Vienna Presentation at Czech
Stochastic optimization - how to improve computational efficiency? Christian Bucher Center of Mechanics and Structural Dynamics Vienna University of Technology & DYNARDO GmbH, Vienna Presentation at Czech
Cutting Elliptical Pizza into Equal Slices
 Cutting Elliptical Pizza into Equal Slices (12 November 2017) Jim Stevenson Having immersed myself in studying Kepler s discovery that the planetary orbits were ellipses ([1]), I was immediately aware
Cutting Elliptical Pizza into Equal Slices (12 November 2017) Jim Stevenson Having immersed myself in studying Kepler s discovery that the planetary orbits were ellipses ([1]), I was immediately aware
Chapters 0, 1, 3. Read Chapter 0, pages 1 8. Know definitions of terms in bold, glossary in back.
 1 Chapters 0, 1, 3 Analytical chemistry is chemical measurement science. Qualitative analysis what is it? Quantitative analysis how much of it is there? This class covers the following: 1. Measurement
1 Chapters 0, 1, 3 Analytical chemistry is chemical measurement science. Qualitative analysis what is it? Quantitative analysis how much of it is there? This class covers the following: 1. Measurement
(a) (3 points) Construct a 95% confidence interval for β 2 in Equation 1.
 Problem 1 (21 points) An economist runs the regression y i = β 0 + x 1i β 1 + x 2i β 2 + x 3i β 3 + ε i (1) The results are summarized in the following table: Equation 1. Variable Coefficient Std. Error
Problem 1 (21 points) An economist runs the regression y i = β 0 + x 1i β 1 + x 2i β 2 + x 3i β 3 + ε i (1) The results are summarized in the following table: Equation 1. Variable Coefficient Std. Error
Experiment 13. Dilutions and Data Handling in a Spreadsheet rev 1/2013
 Absorbance Experiment 13 Dilutions and Data Handling in a Spreadsheet rev 1/2013 GOAL: This lab experiment will provide practice in making dilutions using pipets and introduce basic spreadsheet skills
Absorbance Experiment 13 Dilutions and Data Handling in a Spreadsheet rev 1/2013 GOAL: This lab experiment will provide practice in making dilutions using pipets and introduce basic spreadsheet skills
MATH 12 CLASS 23 NOTES, NOV Contents 1. Change of variables: the Jacobian 1
 MATH 12 CLASS 23 NOTES, NOV 11 211 Contents 1. Change of variables: the Jacobian 1 1. Change of variables: the Jacobian So far, we have seen three examples of situations where we change variables to help
MATH 12 CLASS 23 NOTES, NOV 11 211 Contents 1. Change of variables: the Jacobian 1 1. Change of variables: the Jacobian So far, we have seen three examples of situations where we change variables to help
Regression. Oscar García
 Regression Oscar García Regression methods are fundamental in Forest Mensuration For a more concise and general presentation, we shall first review some matrix concepts 1 Matrices An order n m matrix is
Regression Oscar García Regression methods are fundamental in Forest Mensuration For a more concise and general presentation, we shall first review some matrix concepts 1 Matrices An order n m matrix is
Relevant self-assessment exercises: [LIST SELF-ASSESSMENT EXERCISES HERE]
![Relevant self-assessment exercises: [LIST SELF-ASSESSMENT EXERCISES HERE] Relevant self-assessment exercises: [LIST SELF-ASSESSMENT EXERCISES HERE]](/thumbs/90/102394766.jpg) Chapter 6 Finite Volume Methods In the previous chapter we have discussed finite difference methods for the discretization of PDEs. In developing finite difference methods we started from the differential
Chapter 6 Finite Volume Methods In the previous chapter we have discussed finite difference methods for the discretization of PDEs. In developing finite difference methods we started from the differential
Sample questions for Fundamentals of Machine Learning 2018
 Sample questions for Fundamentals of Machine Learning 2018 Teacher: Mohammad Emtiyaz Khan A few important informations: In the final exam, no electronic devices are allowed except a calculator. Make sure
Sample questions for Fundamentals of Machine Learning 2018 Teacher: Mohammad Emtiyaz Khan A few important informations: In the final exam, no electronic devices are allowed except a calculator. Make sure
DETERMINATION OF K c FOR AN EQUILIBRIUM SYSTEM
 DETERMINATION OF K c FOR AN EQUILIBRIUM SYSTEM 1 Purpose: To determine the equilibrium constant K c for an equilibrium system using spectrophotometry to measure the concentration of a colored complex ion.
DETERMINATION OF K c FOR AN EQUILIBRIUM SYSTEM 1 Purpose: To determine the equilibrium constant K c for an equilibrium system using spectrophotometry to measure the concentration of a colored complex ion.
BEST PROXIMITY POINT RESULTS VIA SIMULATION FUNCTIONS IN METRIC-LIKE SPACES
 Kragujevac Journal of Mathematics Volume 44(3) (2020), Pages 401 413. BEST PROXIMITY POINT RESULTS VIA SIMULATION FUNCTIONS IN METRIC-LIKE SPACES G. V. V. J. RAO 1, H. K. NASHINE 2, AND Z. KADELBURG 3
Kragujevac Journal of Mathematics Volume 44(3) (2020), Pages 401 413. BEST PROXIMITY POINT RESULTS VIA SIMULATION FUNCTIONS IN METRIC-LIKE SPACES G. V. V. J. RAO 1, H. K. NASHINE 2, AND Z. KADELBURG 3
8 Basics of Hypothesis Testing
 8 Basics of Hypothesis Testing 4 Problems Problem : The stochastic signal S is either 0 or E with equal probability, for a known value E > 0. Consider an observation X = x of the stochastic variable X
8 Basics of Hypothesis Testing 4 Problems Problem : The stochastic signal S is either 0 or E with equal probability, for a known value E > 0. Consider an observation X = x of the stochastic variable X
Credits: Four. School. Level 2 Mathematics and Statistics. Practice Assessment B (2.7) Apply calculus methods in solving problems.
 Name: Teacher: NZAMT 2 Credits: Four School Level 2 Mathematics and Statistics Practice Assessment B 91262 (2.7) Apply calculus methods in solving problems 5 credits You should answer ALL parts of ALL
Name: Teacher: NZAMT 2 Credits: Four School Level 2 Mathematics and Statistics Practice Assessment B 91262 (2.7) Apply calculus methods in solving problems 5 credits You should answer ALL parts of ALL
Experiment 13I THE REACTION OF RED FOOD COLOR WITH BLEACH 1
 Experiment 13I FV 1/11/16 THE REACTION OF RED FOOD COLOR WITH BLEACH 1 PROBLEM: Determine the rate law for the chemical reaction between FD&C Red Dye #3 and sodium hypochlorite. LEARNING OBJECTIVES: By
Experiment 13I FV 1/11/16 THE REACTION OF RED FOOD COLOR WITH BLEACH 1 PROBLEM: Determine the rate law for the chemical reaction between FD&C Red Dye #3 and sodium hypochlorite. LEARNING OBJECTIVES: By
Lecture 22: Variance and Covariance
 EE5110 : Probability Foundations for Electrical Engineers July-November 2015 Lecture 22: Variance and Covariance Lecturer: Dr. Krishna Jagannathan Scribes: R.Ravi Kiran In this lecture we will introduce
EE5110 : Probability Foundations for Electrical Engineers July-November 2015 Lecture 22: Variance and Covariance Lecturer: Dr. Krishna Jagannathan Scribes: R.Ravi Kiran In this lecture we will introduce
Understanding Organic Reactions
 Understanding Organic Reactions Energy Diagrams For the general reaction: The energy diagram would be shown as: Understanding Organic Reactions Energy Diagrams Energy Diagrams Understanding Organic Reactions
Understanding Organic Reactions Energy Diagrams For the general reaction: The energy diagram would be shown as: Understanding Organic Reactions Energy Diagrams Energy Diagrams Understanding Organic Reactions
Köhler Curve. Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics
 Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
Köhler Curve Covers Reading Material in Chapter 10.3 Atmospheric Sciences 5200 Physical Meteorology III: Cloud Physics Review of Kelvin Effect Gibbs Energy for formation of a drop G = G &'()*+, G ).'+
UNIT 13 Areas: CSEC Revision Test
 Area of square 64 cm (4 marks) MEP Jamaica: STRAND E : 1. The shaded square has sides of length 1 cm. It is enlarged a number of times as shown. Complete the table. Length of side of square 1 cm cm 3 cm
Area of square 64 cm (4 marks) MEP Jamaica: STRAND E : 1. The shaded square has sides of length 1 cm. It is enlarged a number of times as shown. Complete the table. Length of side of square 1 cm cm 3 cm
Variance. Standard deviation VAR = = value. Unbiased SD = SD = 10/23/2011. Functional Connectivity Correlation and Regression.
 10/3/011 Functional Connectivity Correlation and Regression Variance VAR = Standard deviation Standard deviation SD = Unbiased SD = 1 10/3/011 Standard error Confidence interval SE = CI = = t value for
10/3/011 Functional Connectivity Correlation and Regression Variance VAR = Standard deviation Standard deviation SD = Unbiased SD = 1 10/3/011 Standard error Confidence interval SE = CI = = t value for
Force and Acceleration in Circular Motion
 Force and Acceleration in Circular Motion INTRODUCTION Acceleration is the time rate of change of velocity. Since velocity is a vector, it can change in two ways: its magnitude can change and its direction
Force and Acceleration in Circular Motion INTRODUCTION Acceleration is the time rate of change of velocity. Since velocity is a vector, it can change in two ways: its magnitude can change and its direction
Typing Equations in MS Word 2010
 CM3215 Fundamentals of Chemical Engineering Laboratory Typing Equations in MS Word 2010 https://www.youtube.com/watch?v=cenp9mehtmy Professor Faith Morrison Department of Chemical Engineering Michigan
CM3215 Fundamentals of Chemical Engineering Laboratory Typing Equations in MS Word 2010 https://www.youtube.com/watch?v=cenp9mehtmy Professor Faith Morrison Department of Chemical Engineering Michigan
April 28, 2017 Geometry 11.1 Circumference and Arc Length
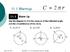 11.1 Warmup April 28, 2017 Geometry 11.1 Circumference and Arc Length 1 Geometry 11.1 Circumference and Arc Length mbhaub@mpsaz.org 11.1 Essential Question How can you find the length of a circular arc?
11.1 Warmup April 28, 2017 Geometry 11.1 Circumference and Arc Length 1 Geometry 11.1 Circumference and Arc Length mbhaub@mpsaz.org 11.1 Essential Question How can you find the length of a circular arc?
x n+1 = ( x n + ) converges, then it converges to α. [2]
![x n+1 = ( x n + ) converges, then it converges to α. [2] x n+1 = ( x n + ) converges, then it converges to α. [2]](/thumbs/92/110299841.jpg) 1 A Level - Mathematics P 3 ITERATION ( With references and answers) [ Numerical Solution of Equation] Q1. The equation x 3 - x 2 6 = 0 has one real root, denoted by α. i) Find by calculation the pair
1 A Level - Mathematics P 3 ITERATION ( With references and answers) [ Numerical Solution of Equation] Q1. The equation x 3 - x 2 6 = 0 has one real root, denoted by α. i) Find by calculation the pair
Modern Methods of Data Analysis - WS 07/08
 Modern Methods of Data Analysis Lecture VIc (19.11.07) Contents: Maximum Likelihood Fit Maximum Likelihood (I) Assume N measurements of a random variable Assume them to be independent and distributed according
Modern Methods of Data Analysis Lecture VIc (19.11.07) Contents: Maximum Likelihood Fit Maximum Likelihood (I) Assume N measurements of a random variable Assume them to be independent and distributed according
Techniques in Molecular Genetics Spectroscopy and Enzyme Assays
 Techniques in Molecular Genetics Spectroscopy and Enzyme Assays H.E. Schellhorn Spectroscopy Chromophore Molar Extinction Coefficient Absorbance Transmittance Spectroscopy Many biological materials have
Techniques in Molecular Genetics Spectroscopy and Enzyme Assays H.E. Schellhorn Spectroscopy Chromophore Molar Extinction Coefficient Absorbance Transmittance Spectroscopy Many biological materials have
371 Lab Rybolt Data Analysis Assignment Name
 Data Analysis Assignment 1 371 Lab Rybolt Data Analysis Assignment Name You wake up one morning and feel you may have a fever. You have an oral thermometer marked in Celsius degrees and find your temperature
Data Analysis Assignment 1 371 Lab Rybolt Data Analysis Assignment Name You wake up one morning and feel you may have a fever. You have an oral thermometer marked in Celsius degrees and find your temperature
Hydration of Propionaldehyde by Temperature-Jump Relaxation 1
 1 Hydration of Propionaldehyde by Temperature-Jump Relaxation 1 Purpose: Determine the rate constants for the reversible hydration of propionaldehyde using temperature-jump relaxation. Pre-lab Reading:
1 Hydration of Propionaldehyde by Temperature-Jump Relaxation 1 Purpose: Determine the rate constants for the reversible hydration of propionaldehyde using temperature-jump relaxation. Pre-lab Reading:
For simplicity, we ll represent BTB s ionization in a solution by the equilibrium: HBTB = H + + BTB -
 Chemistry 160 Please have the following pages ready before class on Wednesday, April 11. An abstract (see the end of this handout) is needed for this write-up. The abstract and photocopied pages of the
Chemistry 160 Please have the following pages ready before class on Wednesday, April 11. An abstract (see the end of this handout) is needed for this write-up. The abstract and photocopied pages of the
3. The Theorems of Green and Stokes
 3. The Theorems of Green and tokes Consider a 1-form ω = P (x, y)dx + Q(x, y)dy on the rectangle R = [a, b] [c, d], which consists of points (x, y) satisfying a x b and c y d. The boundary R of R have
3. The Theorems of Green and tokes Consider a 1-form ω = P (x, y)dx + Q(x, y)dy on the rectangle R = [a, b] [c, d], which consists of points (x, y) satisfying a x b and c y d. The boundary R of R have
Concentric Circles Puzzle
 In the image above, the inner circle has a circumference of 10 and the distance between the inner and outer circles is 3. If the circumference of the inner circle is increased to 11, and the distance between
In the image above, the inner circle has a circumference of 10 and the distance between the inner and outer circles is 3. If the circumference of the inner circle is increased to 11, and the distance between
Optimization: Other Applications
 Optimization: Other Applications MATH 151 Calculus for Management J. Robert Buchanan Department of Mathematics Fall 2018 Objectives After completing this section, we will be able to: use the concepts of
Optimization: Other Applications MATH 151 Calculus for Management J. Robert Buchanan Department of Mathematics Fall 2018 Objectives After completing this section, we will be able to: use the concepts of
Typical kinetics Time. 1. What happens to the concentration of A over time? a. Increases b. Decreases c.
 Cheryl Coolidge Guided Inquiry Activity Enzyme Kinetics, Part One Why? Understanding the rate behavior of enzyme catalyzed reactions can help to explain how these biologically important reactions are controlled
Cheryl Coolidge Guided Inquiry Activity Enzyme Kinetics, Part One Why? Understanding the rate behavior of enzyme catalyzed reactions can help to explain how these biologically important reactions are controlled
