THE UNIVERSITY OF MICHIGAN
|
|
|
- Camron Harper
- 5 years ago
- Views:
Transcription
1 M!CHGAN MEMORIAL PHOENIX PROJECT PHOENIX MEMORIAL LABORATORY THE UNIVERSITY OF MICHIGAN ABSOLUTE POWER MEASUREMENTS OF THE FORD NUCLEAR REACTOR J. B. Bullock July 1965 Prepared for presentation at the ANS Conference on Reactor Operating Experience at Jackson Lake Lodge, Wyoming, July 28 3, 1965.
2 1. Introduction Measuring the absolute reactor power level is a problem common to many reactor operations groups. The most obvious need for an accurate determination of the reactor power level is that of satisfying the safety criteria established for a given system. Research reactors, however, are frequently involved with experiments requiring an accurate measure of the reactor power. Furthermore, the statistical significance of experimental measurements that are normalized to reactor power level cannot be mean ingfully stated unless the statistical variation of the reactor power level measurement is known. The more conventional (flow rate) x (core At) method for determining reactor power was investigated with the existing instrumentation at The University of Michigan Ford Nuclear Reactor, The uncertainties in the flow meter calibration accuracy and the errors associated with measuring a 14 F temperature difference prompted consider ation of another technique for power determination, The method investigated is a calorimetric method in which the pool water system is treated as a calorimeter and measurements are made to determine the rate at which energy is being added to the system. The errors inherent in this method appear to be reasonably small and the method has been adopted for routine reactor power measurements by both experimenters and the operations group, II. Description of Experiment The Ford Nuclear Reactor (FNR) is a 2 megawatt pool-type reactor containing approximately 48, gallons of light water in the pool and primary cooling system. An isometric view of the reactor is shown in Figure 1. The reactor pool is approximately 26 feet deep by 1 feet wide by 2 feet long, one end being a semi-cylinder having
3 -2- b Figure 1 Isometric View of the FNR
4 a 5 foot radius, The primary coolant water is pumped down through the core at approximately 1 gallons per minute through the heat exchanger and back to the pool The primary return is at the bottom of the pool where a deflector plate is used to spread the returned water over the pool floor, The calorimeter experiment consists of randomly locating 12 thermocouples in the reactor pooi and operating the reactor at full power without the use of the secondary cooling pump. In this mode of operation, it is observed that the return water to the pool is approximately 14 F warmer than the bulk pool temperature. The combination of the deflector dispersion and the buoyant forces due to the 14 F temperature difference result in a thorough mixing of the reactor pooi water during the calorimeter experiment. This postulated mixing is confirmed by the observation that the thermocouples measure the same rate of increase of the pool temperature regardless of their location, The thermocouple data are subsequently analyzed with the aid of an IBM 79 computer to determine the mean rate of increase of the bulk pool temperature and the statistical variation of the measurement, For the case of a perfect calorimeter it may be recalled that the rate of energy input to the system is simply the product of the mass of the pool water, the specific heat of the water and the average rate at which the pool temperature increases, Two obvious bias errors which must be evaluated for the experiment are 1) heat losses from the system during the experiment, and 2) the accuracy of the pool water mass.
5 -4- IllS Bias Errors Calculations for the heat losses from the reactor and primary system were made by using conservative heat transfer models which tend to overestimate the energy loss and thus to establish an upper limit estimate for the error in neglecting the losses. The loss factors considered were 1) conduction from the pool water to the ceramic tile lined reactor shield tank, 2) evaporation and radiation losses from the ft. pool surface, and 3) convection and radiation losses from the heat exchanger and primary piping. The details of the calculations are shown in Appendix A and a summary of the results is presented in Table I. It should be emphasized that all of these estimates are upper limit estimates and that the actual losses are expected to be lower than the 3.6% indicated by these calculations, TABLE I Summary of Heat Loss Calculations Loss Mechanism Thermal radiation from pool surface Max Energy Loss During Experiment 11.1 x STU Thermal radiation from primary piping 17,6x BTU Evaporation from pool surface 39,2x BTU Convection from primary piping components 12.2x BTU Conduction to pool walls 167.Ox BTU Total Losses 2471
6 -5- To determine the possible error in the pool water mass-, accurate measurements were taken of the pool dimensions, These measurements indicate that the pool volume is known to within ± 1 %. Corrections cre made for the primary coolant system and any large oblects in the pool at the time of the experiment. IV. Data Analysis An essential part of the data analysis procedure was in determining the copper consta ntan thermocouple calibration constants. The entire temperature measuring system, including the thermocouple switch box and potentiometer readout device, was calibrated by making repeated thermocouple measurements in a water filled Dewar flask. The Dewar temperature was determined with a mercury thermometer which was graduated in,1 degrees centigrade and read to the nearest 5 C. The calibration experiment consisted of taking measurements at various temperatures with the potentiometer readout dial being changed and readjusted for each measurement. Approximately 1 points were taken onach thermocouple and the data was reduced by a least squares analysis to determine the 95% confidence interval and the mean value of the calibration constant over the temperature range of interest, Recall that the object of the calorimeter experiment is to accurately determine the mean rate of increase of the pool water temperature. This determination is made by fitting each set of thermocouple data too straight line by the least squares method. The details of the statistical method used are given in Appendix B. From this analysis the value of the slope and its standard deviation are determined. The results of several experiments are plotted in Figure 2 along with the thermocouple calibration data. The error bar shown for each thermocouple represents the 95% confidence interval for the
7 RUN RUN RUN FIG.2 EXPERIMENTAL DATA Figure 2 Experimental Data
8 - 7.- slope as determined from each set of raw thermocouple data. After determining the average value of the slope from each thermocouple, a mean value for the average of all thermocouple slopes is determined and is taken to be the mean rate of the pool temperature increase, The t distribution is used to calculate the 95% confidence interval for this average. The dashed line shown in Figure 2 represents the 95% confidence interval for each of the calorimeter experiments. The t+iermocouples having a large variation were located at positions in the pool where the flow conditions caused a continuous movement of the T C. during the experiment. The removal of these data from the calculations would result in a marked reduction of the confidence intervals, During the routine calorimeter experiment data is also collected on the reactor control system temperature chart, By visually fitting a straight line to the slope on the temperature chart, it is also possible to get an estimate of the rate of increase of the pool temperature. Analysis of the temperature charts for several calorimeter experiments clearly indicates that the mean reactor power level can be determined from the strip chart recorder with reasonable accuracy. A typical trace is shown on Figure 3. The strip chart data technique is now frequently used for experiments and reactor operations requiring a quick reference to the absolute power, V. Conclusions The calorimetric power determination technique can be used to determine the reactor power level for systems having low heat losses, to within a few per cent, The method can be used to calibrate relative power measuring devices and to determine the confidence level for reference power meters,
9 :zj : _ T I [LZD ZEEEiEE EZE +1 H ii IL) W -- SI th ----z- -- za o iw Z
10 V Acknowledgements The author wishes to gratefully acknowledge the efforts oft. W. Craig, L A Feldkarnp and F P Petraits for their assistance in collecting and evaluating the experimental data, and the capable assistance of Mrs. Donna Zeeb for typing and preparing the final report.
11 -1- APPENDIX A Conduction t.osses from Pool to the Shield Assume the walls of the pool to be an infinite slab extending from.x = to +, and the pool water as slab extending from to - oo Assume on infinite heat transfer coefficient between pool water and pool walls (i.e. no film drop). Since the temperature of the pool increases with time, the heat losses from the pool will be a function of time, H2 Concrete U = F(t) T = initial temperature -x +X Using the notation and method of Churchill 1, the heat diffusion equation is written (1) U (x, t) = K U (x, t) (x> o, t> o) with boundary conditions: (a) U (x, o) = T (x> o) (b) U (o, t) = gt (t> o) (c) lim U (x, t) = T (t> o) x +
12 11 Transforming equation (1) and = applying B.. C. (a): (2) - T + $ U (x,. S) K u (x, s) (x> o) and B. C. (b) becomes u (o, s.) The real solution of (2) is: 2 S (3) u(x,s)= Ae (4) U (x, s) Using B. C. (b): A - =( - The heat flux per = unit area is: q(t) KU (o,t), A thus from (4) and after inverting: _ s 5 - Kr = (5) p(t) A w = L where F = U (x, a) (x < o) or, F F(ot). 2gf +(F.jt -T) To estimate the total heat lost during operating time T, (5) is integrated as follows: T (6) Q = AK L g T3/2 + 2 (F - T ) T 1/2
13 12- The thermal properties for barytes concrete are given by Rockwell 2 Os; K =.884BTU/hr ft, 2 F 2/hr. =.25ft. The total area of concrete in contact withpool water is: 2 A = 227 fl, From a calibration experiment at 2.MW, g was found to be approximately constant and equal to 18 F/hr. Whenever the initial wall temperature is the same as the initial pool temperature, F = T, and equation (6) becomes: (7) T = 4A K g T3/2 During the calibration experiment the pool tempertture increases for one hour. Therefore, the maximum possible heat loss to the shield is T = 4x 27x,884x 18 3xiy25O rr = L67x BTU
14 -13- II. Radiation Heat Losses The conventional equation 3 for heat losses by radiation from a surface of constant temperature T to an environment at temperature T is =A) ()4j it may be seen that the time dependent: case can be written as: = A /T \41 (a 1 The total heat loss by radiation during an experiment in which T increases linearly at g F/hr. from T to T in time t s obtained by integrating 5 T During a standard calorimeter experiment at 2 MW, the pool temperature increases at 18 F/hr, too maximum of 116 F. The initial temperatures are T = 98 F and Ta = 8 F. The use of these constants in the above equation with 1, gives the maximum total heat loss from the pool surface OS: = l.llx 1 BTU
15 -14- The same equat.ion isusedo.estrnite the maximum losses by radiation from the 88 feet of 12 inch primary pipe and heat exchange. For this case however, T is 13 F and T is 112 F. The calculation yields: = l.76x BTU Ill. Convection Losses from Horizontal Piping A simplified equation for the convection coefficient from the horizontal primary piping at constant temperature is given on page 177 of reference 3 as: (..t\.25 h = 27 c This equation can be written for the time dependent temperature case as: hc(t) D T. ai 1/4 or, in terms of the heat loss as a function of time: (t) = hc(t) (T(t) - T. ) air ird o L After substituting for h, c (t) the time dependent heat loss relation is: (t) = 27irD D L T/4 (TP(t) - T ) air 5/4
16 The total heat loss in operating time t is obtained by integrating, thusly: = 34Lf.27D (TP(t) - Tjdt, and, since TP(t) T +gt Q =.27 D 3/4 L (g t + T - T. 9/4 t T o o air 99 For the standard one hour experiment (T T..) 32 F and g t 18 F, therefore, the convection power loss from the 88 feet of 12 inch primqry piping can be calculated to be: = 1,22x 14 BTIJ IV. Evaporation Losses The water evaporation rate is calculated by using the equation from 4: 8rown W = 24+37T -15- (p- P). where: W = H2 evaporated in grains/hr ft, 1 T = poo water temperature - F = Vapor pressure of H2 P = Partial pressure of H2 at T - in. Hg vapor in air in. Hg. The equation is written for the variable temperature case as: (p* w (t) = T (t) P).
17 -16- A simplification which results in a maximum estimate for the evaporation rate is obtained by assuming: P a (T(t) - T), where, a = 1.B2and 13 =.72. The equation for the evaporation rate as a function of time is therefore: (t) = 24 + a.7 T(t) + 13 (T(t) - T) - P] For the case T(t) = g t + T, the equation is written: w(t) (gt + T) (a + f3 gt - P). Integrating from t = a to t = 1 hr. gives: W= L +T ap)9 +T (a-p) 3 L This equation has been evaluated for the maximum conditions of a 2% relative humidity in 7 F building air and fort of the pool = 116 F... max.. The equation predicts a maximum of 45 lbs. of pooi water will evaporate during the experiment. The total energy lost due to the heat of vaporization of the pool water is, therefore: = 3.92x BTU
18 -17- APPENDIX B Statistical Methods The thermocouple data is recorded with the aid of a stopwatch so that time errors or variations may be neglected in the data analysis. Imposing this condition, the data approximates a straight line so that deviations in y can be written: o y. = y. - (a + b x.) To minimize the deviations, the leost squares method imposes: a (oj and, ab The solution of the equations which result from the application of these conditions can be written in terms of the mean slope as: nx.y.-dy.dx. nx. II ( x.) I I The variance forl has been derived as: 5 U 2 b 2-2ay.-2bZx.y.+Na 2Ex. 2+2abEx.+b 2 Ny. N-2 N 2 -(x.) Zx. 2
19 -18- The mean of all slopes k calculated by: b* Zk kk The variance for b* is calculated by the variance of sums theorem: 1 [2 + (1. - b*)2]
20 -19- References 1. Churchill, R. V., Operational Mathematics, 2nd Ed., McGraw Hill Co., Inc., p 132, Rockwell Ill, T., Reactor Shielding Manual, 1st Ed., D. Van Nostrand Co.,.. Inc., p , McAdams, W. H., Heat Transmission, 3rd Ed., McGraw Hill Co., Inc. Eqn. 4-9, Brown, G. G., Unit Operation, John Wileyand Sons,p557, Parrat, L. G., Probability and Experimental Errors in Science, John Wiley and Sons, p 131, 1961.
If there is convective heat transfer from outer surface to fluid maintained at T W.
 Heat Transfer 1. What are the different modes of heat transfer? Explain with examples. 2. State Fourier s Law of heat conduction? Write some of their applications. 3. State the effect of variation of temperature
Heat Transfer 1. What are the different modes of heat transfer? Explain with examples. 2. State Fourier s Law of heat conduction? Write some of their applications. 3. State the effect of variation of temperature
Figure 1.1. Relation between Celsius and Fahrenheit scales. From Figure 1.1. (1.1)
 CHAPTER I ELEMENTS OF APPLIED THERMODYNAMICS 1.1. INTRODUCTION. The Air Conditioning systems extract heat from some closed location and deliver it to other places. To better understanding the principles
CHAPTER I ELEMENTS OF APPLIED THERMODYNAMICS 1.1. INTRODUCTION. The Air Conditioning systems extract heat from some closed location and deliver it to other places. To better understanding the principles
PowerPoint Presentation by: Associated Technical Authors. Publisher The Goodheart-Willcox Company, Inc. Tinley Park, Illinois
 Althouse Turnquist Bracciano PowerPoint Presentation by: Associated Technical Authors Publisher The Goodheart-Willcox Company, Inc. Tinley Park, Illinois Chapter 1 History and Fundamentals of Refrigeration
Althouse Turnquist Bracciano PowerPoint Presentation by: Associated Technical Authors Publisher The Goodheart-Willcox Company, Inc. Tinley Park, Illinois Chapter 1 History and Fundamentals of Refrigeration
Department of Mechanical Engineering ME 96. Free and Forced Convection Experiment. Revised: 25 April Introduction
 CALIFORNIA INSTITUTE OF TECHNOLOGY Department of Mechanical Engineering ME 96 Free and Forced Convection Experiment Revised: 25 April 1994 1. Introduction The term forced convection refers to heat transport
CALIFORNIA INSTITUTE OF TECHNOLOGY Department of Mechanical Engineering ME 96 Free and Forced Convection Experiment Revised: 25 April 1994 1. Introduction The term forced convection refers to heat transport
v ( ) x (b) Using Navier-Stokes equations, derive an expression
 Problem 1: Microscopic Momentum Balance In sweep flow filtration (see Figure below), a pressure drop forces fluid containing particles between two porous plates and there is a transverse (cross) flow that
Problem 1: Microscopic Momentum Balance In sweep flow filtration (see Figure below), a pressure drop forces fluid containing particles between two porous plates and there is a transverse (cross) flow that
Radiant Heating Panel Thermal Analysis. Prepared by Tim Fleury Harvard Thermal, Inc. October 7, 2003
 Radiant Heating Panel Thermal Analysis Prepared by Tim Fleury Harvard Thermal, Inc. October 7, 2003 Analysis Objective Perform a thermal test on a small sample of the concrete to determine the Thermal
Radiant Heating Panel Thermal Analysis Prepared by Tim Fleury Harvard Thermal, Inc. October 7, 2003 Analysis Objective Perform a thermal test on a small sample of the concrete to determine the Thermal
Thermal Power Calibration of the TRIGA Mark II Reactor
 ABSTRACT Thermal Power Calibration of the TRIGA Mark II Reactor Žiga Štancar Jožef Stefan Institute Jamova cesta 39 1000, Ljubljana, Slovenia ziga.stancar@gmail.com Luka Snoj Jožef Stefan Institute Jamova
ABSTRACT Thermal Power Calibration of the TRIGA Mark II Reactor Žiga Štancar Jožef Stefan Institute Jamova cesta 39 1000, Ljubljana, Slovenia ziga.stancar@gmail.com Luka Snoj Jožef Stefan Institute Jamova
Related Rates In each related rate problem there can be variations in the details. The problems, however, have the same general structure.
 Lab 6 Math 111 Spring 019 Related Rates In each related rate problem there can be variations in the details. The problems, however, have the same general structure. I. Relating Quantities: Independent
Lab 6 Math 111 Spring 019 Related Rates In each related rate problem there can be variations in the details. The problems, however, have the same general structure. I. Relating Quantities: Independent
MAC 2311 Chapter 5 Review Materials Topics Include Areas Between Curves, Volumes (disks, washers, and shells), Work, and Average Value of a Function
 MAC Chapter Review Materials Topics Include Areas Between Curves, Volumes (disks, washers, and shells), Work, and Average Value of a Function MULTIPLE CHOICE. Choose the one alternative that best completes
MAC Chapter Review Materials Topics Include Areas Between Curves, Volumes (disks, washers, and shells), Work, and Average Value of a Function MULTIPLE CHOICE. Choose the one alternative that best completes
Related Rates STEP 1 STEP 2:
 Related Rates You can use derivative analysis to determine how two related quantities also have rates of change which are related together. I ll lead off with this example. 3 Ex) A spherical ball is being
Related Rates You can use derivative analysis to determine how two related quantities also have rates of change which are related together. I ll lead off with this example. 3 Ex) A spherical ball is being
1. Thermal energy is transferred through the glass windows of a house mainly by. D. radiation and convection. (1)
 1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation. C. conduction and convection. D. radiation and convection. 2. The specific latent heat of vaporization
1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation. C. conduction and convection. D. radiation and convection. 2. The specific latent heat of vaporization
AE 3051, Lab #16. Investigation of the Ideal Gas State Equation. By: George P. Burdell. Group E3
 AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
PAPER 2 THEORY QUESTIONS
 PAPER 2 THEORY QUESTIONS 1 Fig. 1.1 shows the arrangement of atoms in a solid block. Fig. 1.1 (a) End X of the block is heated. Energy is conducted to end Y, which becomes warm. (i) Explain how heat is
PAPER 2 THEORY QUESTIONS 1 Fig. 1.1 shows the arrangement of atoms in a solid block. Fig. 1.1 (a) End X of the block is heated. Energy is conducted to end Y, which becomes warm. (i) Explain how heat is
Transient Heat Transfer Experiment. ME 331 Introduction to Heat Transfer. June 1 st, 2017
 Transient Heat Transfer Experiment ME 331 Introduction to Heat Transfer June 1 st, 2017 Abstract The lumped capacitance assumption for transient conduction was tested for three heated spheres; a gold plated
Transient Heat Transfer Experiment ME 331 Introduction to Heat Transfer June 1 st, 2017 Abstract The lumped capacitance assumption for transient conduction was tested for three heated spheres; a gold plated
Fluids Bernoulli s equation
 Chapter 11 Fluids Bernoulli s equation 11.9 Bernoulli s Equation W NC = ( P 2! P 1 )V W NC = E 1! E 2 = 1 mv 2 + mgy 2 1 1 ( )! ( 1 "v 2 + "gy 2 2 2 ) ( P 2! P 1 ) = 1 "v 2 + "gy 2 1 1 NC Work yields a
Chapter 11 Fluids Bernoulli s equation 11.9 Bernoulli s Equation W NC = ( P 2! P 1 )V W NC = E 1! E 2 = 1 mv 2 + mgy 2 1 1 ( )! ( 1 "v 2 + "gy 2 2 2 ) ( P 2! P 1 ) = 1 "v 2 + "gy 2 1 1 NC Work yields a
2.1. Accuracy, n- how close the indication of the thermometer is to the true value.
 AASHTO Accreditation Policy and Guidance on Thermometer Selection and Records Revised: January 25, 2018 Revision #: 0 1. Objective 1.1. The purpose of this document is to clearly define acceptance criteria
AASHTO Accreditation Policy and Guidance on Thermometer Selection and Records Revised: January 25, 2018 Revision #: 0 1. Objective 1.1. The purpose of this document is to clearly define acceptance criteria
Q ( q(m, t 0 ) n) S t.
 THE HEAT EQUATION The main equations that we will be dealing with are the heat equation, the wave equation, and the potential equation. We use simple physical principles to show how these equations are
THE HEAT EQUATION The main equations that we will be dealing with are the heat equation, the wave equation, and the potential equation. We use simple physical principles to show how these equations are
A Simple Analysis of Fuel Addition to the CWT of 747
 A Simple Analysis of Fuel Addition to the CWT of 747 Raza Akbar and Joseph E. Shepherd Graduate Aeronautical Laboratories California Institute of Technology Pasadena, CA 91125 USA 21 September 1998 Sponsored
A Simple Analysis of Fuel Addition to the CWT of 747 Raza Akbar and Joseph E. Shepherd Graduate Aeronautical Laboratories California Institute of Technology Pasadena, CA 91125 USA 21 September 1998 Sponsored
Practice Exam 1 Solutions
 Practice Exam 1 Solutions 1a. Let S be the region bounded by y = x 3, y = 1, and x. Find the area of S. What is the volume of the solid obtained by rotating S about the line y = 1? Area A = Volume 1 1
Practice Exam 1 Solutions 1a. Let S be the region bounded by y = x 3, y = 1, and x. Find the area of S. What is the volume of the solid obtained by rotating S about the line y = 1? Area A = Volume 1 1
Evaluate the following limit without using l Hopital s Rule. x x. = lim = (1)(1) = lim. = lim. = lim = (3 1) =
 5.4 1 Looking ahead. Example 1. Indeterminate Limits Evaluate the following limit without using l Hopital s Rule. Now try this one. lim x 0 sin3x tan4x lim x 3x x 2 +1 sin3x 4x = lim x 0 3x tan4x ( ) 3
5.4 1 Looking ahead. Example 1. Indeterminate Limits Evaluate the following limit without using l Hopital s Rule. Now try this one. lim x 0 sin3x tan4x lim x 3x x 2 +1 sin3x 4x = lim x 0 3x tan4x ( ) 3
COOLING TOWER MODEL IN MNR
 COOLING TOWER MODEL IN MNR 1 COOLING TOWER MODEL The cooling tower model used in Liu s study [2] was adapted from the model developed by Eric Weber (1988). One modification is the use of a counterflow
COOLING TOWER MODEL IN MNR 1 COOLING TOWER MODEL The cooling tower model used in Liu s study [2] was adapted from the model developed by Eric Weber (1988). One modification is the use of a counterflow
Basic Analysis of Data
 Basic Analysis of Data Department of Chemical Engineering Prof. Geoff Silcox Fall 008 1.0 Reporting the Uncertainty in a Measured Quantity At the request of your supervisor, you have ventured out into
Basic Analysis of Data Department of Chemical Engineering Prof. Geoff Silcox Fall 008 1.0 Reporting the Uncertainty in a Measured Quantity At the request of your supervisor, you have ventured out into
q x = k T 1 T 2 Q = k T 1 T / 12
 Conductive oss through a Window Pane q T T 1 Examine the simple one-dimensional conduction problem as heat flow through a windowpane. The window glass thickness,, is 1/8 in. If this is the only window
Conductive oss through a Window Pane q T T 1 Examine the simple one-dimensional conduction problem as heat flow through a windowpane. The window glass thickness,, is 1/8 in. If this is the only window
Chapter: Heat and States
 Table of Contents Chapter: Heat and States of Matter Section 1: Temperature and Thermal Energy Section 2: States of Matter Section 3: Transferring Thermal Energy Section 4: Using Thermal Energy 1 Temperature
Table of Contents Chapter: Heat and States of Matter Section 1: Temperature and Thermal Energy Section 2: States of Matter Section 3: Transferring Thermal Energy Section 4: Using Thermal Energy 1 Temperature
Experiences in an Undergraduate Laboratory Using Uncertainty Analysis to Validate Engineering Models with Experimental Data
 Experiences in an Undergraduate Laboratory Using Analysis to Validate Engineering Models with Experimental Data W. G. Steele 1 and J. A. Schneider Abstract Traditionally, the goals of engineering laboratory
Experiences in an Undergraduate Laboratory Using Analysis to Validate Engineering Models with Experimental Data W. G. Steele 1 and J. A. Schneider Abstract Traditionally, the goals of engineering laboratory
S.E. (Chemical) (Second Semester) EXAMINATION, 2011 HEAT TRANSFER (2008 PATTERN) Time : Three Hours Maximum Marks : 100
 Total No. of Questions 12] [Total No. of Printed Pages 7 [4062]-186 S.E. (Chemical) (Second Semester) EXAMINATION, 2011 HEAT TRANSFER (2008 PATTERN) Time : Three Hours Maximum Marks : 100 N.B. : (i) Answers
Total No. of Questions 12] [Total No. of Printed Pages 7 [4062]-186 S.E. (Chemical) (Second Semester) EXAMINATION, 2011 HEAT TRANSFER (2008 PATTERN) Time : Three Hours Maximum Marks : 100 N.B. : (i) Answers
APPLICATIONS OF INTEGRATION
 6 APPLICATIONS OF INTEGRATION APPLICATIONS OF INTEGRATION 6.4 Work In this section, we will learn about: Applying integration to calculate the amount of work done in performing a certain physical task.
6 APPLICATIONS OF INTEGRATION APPLICATIONS OF INTEGRATION 6.4 Work In this section, we will learn about: Applying integration to calculate the amount of work done in performing a certain physical task.
Experiment 1. Measurement of Thermal Conductivity of a Metal (Brass) Bar
 Experiment 1 Measurement of Thermal Conductivity of a Metal (Brass) Bar Introduction: Thermal conductivity is a measure of the ability of a substance to conduct heat, determined by the rate of heat flow
Experiment 1 Measurement of Thermal Conductivity of a Metal (Brass) Bar Introduction: Thermal conductivity is a measure of the ability of a substance to conduct heat, determined by the rate of heat flow
Department of Energy Fundamentals Handbook
 Department of Energy Fundamentals Handbook THERMODYNAMICS, HEAT TRANSFER, AND FLUID FLOW, Module 2 TABLE OF CONTENTS TABLE OF CONTENTS LIST OF FIGURES... iii LIST OF TABLES... REFERENCES... OBJECTIVES...
Department of Energy Fundamentals Handbook THERMODYNAMICS, HEAT TRANSFER, AND FLUID FLOW, Module 2 TABLE OF CONTENTS TABLE OF CONTENTS LIST OF FIGURES... iii LIST OF TABLES... REFERENCES... OBJECTIVES...
Introduction to Mass Transfer
 Introduction to Mass Transfer Introduction Three fundamental transfer processes: i) Momentum transfer ii) iii) Heat transfer Mass transfer Mass transfer may occur in a gas mixture, a liquid solution or
Introduction to Mass Transfer Introduction Three fundamental transfer processes: i) Momentum transfer ii) iii) Heat transfer Mass transfer Mass transfer may occur in a gas mixture, a liquid solution or
Integration of Boiling Experiments in the Undergraduate Heat Transfer Laboratory
 Integration of Boiling Experiments in the Undergraduate Heat Transfer Laboratory Hosni I. Abu-Mulaweh, Josué Njock Libii Engineering Department Indiana University-Purdue University at Fort Wayne Fort Wayne,
Integration of Boiling Experiments in the Undergraduate Heat Transfer Laboratory Hosni I. Abu-Mulaweh, Josué Njock Libii Engineering Department Indiana University-Purdue University at Fort Wayne Fort Wayne,
C ONTENTS CHAPTER TWO HEAT CONDUCTION EQUATION 61 CHAPTER ONE BASICS OF HEAT TRANSFER 1 CHAPTER THREE STEADY HEAT CONDUCTION 127
 C ONTENTS Preface xviii Nomenclature xxvi CHAPTER ONE BASICS OF HEAT TRANSFER 1 1-1 Thermodynamics and Heat Transfer 2 Application Areas of Heat Transfer 3 Historical Background 3 1-2 Engineering Heat
C ONTENTS Preface xviii Nomenclature xxvi CHAPTER ONE BASICS OF HEAT TRANSFER 1 1-1 Thermodynamics and Heat Transfer 2 Application Areas of Heat Transfer 3 Historical Background 3 1-2 Engineering Heat
To become acquainted with simple laboratory measurements and calculations using common laboratory equipment.
 PURPOSE To become acquainted with simple laboratory measurements and calculations using common laboratory equipment. MATERIALS 250 beaker Piper and piper pump Hot plates Meter stick or ruler Balance Ice
PURPOSE To become acquainted with simple laboratory measurements and calculations using common laboratory equipment. MATERIALS 250 beaker Piper and piper pump Hot plates Meter stick or ruler Balance Ice
Pin Fin Lab Report Example. Names. ME331 Lab
 Pin Fin Lab Report Example Names ME331 Lab 04/12/2017 1. Abstract The purposes of this experiment are to determine pin fin effectiveness and convective heat transfer coefficients for free and forced convection
Pin Fin Lab Report Example Names ME331 Lab 04/12/2017 1. Abstract The purposes of this experiment are to determine pin fin effectiveness and convective heat transfer coefficients for free and forced convection
Math Exam 02 Review
 Math 10350 Exam 02 Review 1. A differentiable function g(t) is such that g(2) = 2, g (2) = 1, g (2) = 1/2. (a) If p(t) = g(t)e t2 find p (2) and p (2). (Ans: p (2) = 7e 4 ; p (2) = 28.5e 4 ) (b) If f(t)
Math 10350 Exam 02 Review 1. A differentiable function g(t) is such that g(2) = 2, g (2) = 1, g (2) = 1/2. (a) If p(t) = g(t)e t2 find p (2) and p (2). (Ans: p (2) = 7e 4 ; p (2) = 28.5e 4 ) (b) If f(t)
Universal Viscosity Curve Theory
 TM Universal Viscosity Curve Theory Turbine Flow Meters and Flow Viscosity Introduction Like any transducer, a turbine flow meter is sensitive to physical parameters other than the one which is of interest.
TM Universal Viscosity Curve Theory Turbine Flow Meters and Flow Viscosity Introduction Like any transducer, a turbine flow meter is sensitive to physical parameters other than the one which is of interest.
Non-Newtonian fluids is the fluids in which shear stress is not directly proportional to deformation rate, such as toothpaste,
 CHAPTER1: Basic Definitions, Zeroth, First, and Second Laws of Thermodynamics 1.1. Definitions What does thermodynamic mean? It is a Greeks word which means a motion of the heat. Water is a liquid substance
CHAPTER1: Basic Definitions, Zeroth, First, and Second Laws of Thermodynamics 1.1. Definitions What does thermodynamic mean? It is a Greeks word which means a motion of the heat. Water is a liquid substance
Measurement of Axial Thrust Number in Various Agitation Flow Regimes
 Measurement of Axial Thrust Number in Various Agitation Flow Regimes Matthew A. Reilly Abstract Structural design of agitators requires a detailed knowledge of the relationship between mixing strength
Measurement of Axial Thrust Number in Various Agitation Flow Regimes Matthew A. Reilly Abstract Structural design of agitators requires a detailed knowledge of the relationship between mixing strength
Laboratory 1: Calibration of a High-Volume Sampler
 Laboratory 1 Laboratory 1: Calibration of a High-Volume Sampler 1.1 To the User of this Manual This manual has been prepared to guide the students through a series of laboratory exercises developed to
Laboratory 1 Laboratory 1: Calibration of a High-Volume Sampler 1.1 To the User of this Manual This manual has been prepared to guide the students through a series of laboratory exercises developed to
CALCULUS AB SECTION II, Part A
 CALCULUS AB SECTION II, Part A Time 45 minutes Number of problems 3 A graphing calculator is required for some problems or parts of problems. pt 1. The rate at which raw sewage enters a treatment tank
CALCULUS AB SECTION II, Part A Time 45 minutes Number of problems 3 A graphing calculator is required for some problems or parts of problems. pt 1. The rate at which raw sewage enters a treatment tank
SECTION 1 - WHAT IS A BTU METER? BTU's = Flow x ΔT Any ISTEC BTU Meter System consists of the following main components:
 SECTION 1 - WHAT IS A BTU METER? ISTEC BTU Meters measure energy usage by multiplying flow rate and temperature difference. As the water (or other liquid) passes through these lines, the multi-wing turbine
SECTION 1 - WHAT IS A BTU METER? ISTEC BTU Meters measure energy usage by multiplying flow rate and temperature difference. As the water (or other liquid) passes through these lines, the multi-wing turbine
Heating, Ventilating, Air Conditioning and Refrigerating, Sixth Edition, First Printing ERRATA
 1 Heating, Ventilating, Air Conditioning and Refrigerating, Sixth Edition, First Printing ERRATA (Items in blue added August 14, 2007; previous revision was December 3, 2004) Inside Front Cover: Conversion
1 Heating, Ventilating, Air Conditioning and Refrigerating, Sixth Edition, First Printing ERRATA (Items in blue added August 14, 2007; previous revision was December 3, 2004) Inside Front Cover: Conversion
Introduction of Heat Transfer. Prepared by: Nimesh Gajjar GIT-MED
 Introduction of Heat Transfer Prepared by: Nimesh Gajjar GIT-MED Difference between heat and temperature Temperature is a measure of the amount of energy possessed by the molecules of a substance. It manifests
Introduction of Heat Transfer Prepared by: Nimesh Gajjar GIT-MED Difference between heat and temperature Temperature is a measure of the amount of energy possessed by the molecules of a substance. It manifests
Fluid Flow Analysis Penn State Chemical Engineering
 Fluid Flow Analysis Penn State Chemical Engineering Revised Spring 2015 Table of Contents LEARNING OBJECTIVES... 1 EXPERIMENTAL OBJECTIVES AND OVERVIEW... 1 PRE-LAB STUDY... 2 EXPERIMENTS IN THE LAB...
Fluid Flow Analysis Penn State Chemical Engineering Revised Spring 2015 Table of Contents LEARNING OBJECTIVES... 1 EXPERIMENTAL OBJECTIVES AND OVERVIEW... 1 PRE-LAB STUDY... 2 EXPERIMENTS IN THE LAB...
Standard Test Method for Thermal Conductivity of Refractories 1
 Designation: C 201 93 (Reapproved 1998) Standard Test Method for Thermal Conductivity of Refractories 1 This standard is issued under the fixed designation C 201; the number immediately following the designation
Designation: C 201 93 (Reapproved 1998) Standard Test Method for Thermal Conductivity of Refractories 1 This standard is issued under the fixed designation C 201; the number immediately following the designation
A simple calorimetric method to avoid artifacts in a controversial field: the ice calorimeter
 Dufour, J., et al. A simple calorimetric method to avoid artifacts in a controversial field: The ice calorimeter. in ICCF-14 International Conference on Condensed Matter Nuclear Science. 2008. Washington,
Dufour, J., et al. A simple calorimetric method to avoid artifacts in a controversial field: The ice calorimeter. in ICCF-14 International Conference on Condensed Matter Nuclear Science. 2008. Washington,
Experiment 1 - Mass, Volume and Graphing
 Experiment 1 - Mass, Volume and Graphing In chemistry, as in many other sciences, a major part of the laboratory experience involves taking measurements and then calculating quantities from the results
Experiment 1 - Mass, Volume and Graphing In chemistry, as in many other sciences, a major part of the laboratory experience involves taking measurements and then calculating quantities from the results
Chapter 2 HEAT CONDUCTION EQUATION
 Heat and Mass Transfer: Fundamentals & Applications 5th Edition in SI Units Yunus A. Çengel, Afshin J. Ghajar McGraw-Hill, 2015 Chapter 2 HEAT CONDUCTION EQUATION Mehmet Kanoglu University of Gaziantep
Heat and Mass Transfer: Fundamentals & Applications 5th Edition in SI Units Yunus A. Çengel, Afshin J. Ghajar McGraw-Hill, 2015 Chapter 2 HEAT CONDUCTION EQUATION Mehmet Kanoglu University of Gaziantep
Thermodynamics I Spring 1432/1433H (2011/2012H) Saturday, Wednesday 8:00am - 10:00am & Monday 8:00am - 9:00am MEP 261 Class ZA
 Thermodynamics I Spring 1432/1433H (2011/2012H) Saturday, Wednesday 8:00am - 10:00am & Monday 8:00am - 9:00am MEP 261 Class ZA Dr. Walid A. Aissa Associate Professor, Mech. Engg. Dept. Faculty of Engineering
Thermodynamics I Spring 1432/1433H (2011/2012H) Saturday, Wednesday 8:00am - 10:00am & Monday 8:00am - 9:00am MEP 261 Class ZA Dr. Walid A. Aissa Associate Professor, Mech. Engg. Dept. Faculty of Engineering
Heat Exchangers for Condensation and Evaporation Applications Operating in a Low Pressure Atmosphere
 Acta Polytechnica Vol. 52 No. 3/202 Heat Exchangers for Condensation and Evaporation Applications Operating in a Low Pressure Atmosphere Petr Kracík,JiříPospíšil, Ladislav Šnajdárek Brno University of
Acta Polytechnica Vol. 52 No. 3/202 Heat Exchangers for Condensation and Evaporation Applications Operating in a Low Pressure Atmosphere Petr Kracík,JiříPospíšil, Ladislav Šnajdárek Brno University of
Temperature and Heat. Chapter 10. Table of Contents. Chapter 10. Chapter 10. Bellringer. Objectives. Chapter 10. Chapter 10
 Heat and Heat Technology Table of Contents Temperature and Heat Section 3 Matter and Heat Bellringer Objectives The temperature of boiling water is 100 on the Celsius scale and 212 on the Fahrenheit scale.
Heat and Heat Technology Table of Contents Temperature and Heat Section 3 Matter and Heat Bellringer Objectives The temperature of boiling water is 100 on the Celsius scale and 212 on the Fahrenheit scale.
Chapter 4 TRANSIENT HEAT CONDUCTION
 Heat and Mass Transfer: Fundamentals & Applications Fourth Edition Yunus A. Cengel, Afshin J. Ghajar McGraw-Hill, 2011 Chapter 4 TRANSIENT HEAT CONDUCTION LUMPED SYSTEM ANALYSIS Interior temperature of
Heat and Mass Transfer: Fundamentals & Applications Fourth Edition Yunus A. Cengel, Afshin J. Ghajar McGraw-Hill, 2011 Chapter 4 TRANSIENT HEAT CONDUCTION LUMPED SYSTEM ANALYSIS Interior temperature of
On the Cooling and Evaporative Powers o f the, as Determined by the.
 438 On the Cooling and Evaporative Powers o f the, as Determined by the. B y L eonard H ill, F.R.S., and D. H argood-a sh. (Department of Applied Physiology, Medical Research Committee.) (Received December
438 On the Cooling and Evaporative Powers o f the, as Determined by the. B y L eonard H ill, F.R.S., and D. H argood-a sh. (Department of Applied Physiology, Medical Research Committee.) (Received December
Shielding. Principles. Effectiveness. Materials FM Appendix B
 Appendix B Shielding Shielding reduces the effects of gamma radiation on personnel and equipment. Metal, concrete, soil, water, and wood are good shielding materials. The denser the material, the better
Appendix B Shielding Shielding reduces the effects of gamma radiation on personnel and equipment. Metal, concrete, soil, water, and wood are good shielding materials. The denser the material, the better
A SIMPLE METHOD OF CALCULATING THE EFFECTIVENESS OF HIGH TEMPERATURE RADIATION HEAT SHIELDS, AND ITS
 SLAC-PUB-1124 WCC) September 1972 A SIMPLE METHOD OF CALCULATING THE EFFECTIVENESS OF HIGH TEMPERATURE RADIATION HEAT SHIELDS, AND ITS APPLICATION IN THE CONSTRUCTION OF A HIGH TEMPERATURE VACUUM FURNACE*
SLAC-PUB-1124 WCC) September 1972 A SIMPLE METHOD OF CALCULATING THE EFFECTIVENESS OF HIGH TEMPERATURE RADIATION HEAT SHIELDS, AND ITS APPLICATION IN THE CONSTRUCTION OF A HIGH TEMPERATURE VACUUM FURNACE*
Preview. Heat Section 1. Section 1 Temperature and Thermal Equilibrium. Section 2 Defining Heat. Section 3 Changes in Temperature and Phase
 Heat Section 1 Preview Section 1 Temperature and Thermal Equilibrium Section 2 Defining Heat Section 3 Changes in Temperature and Phase Heat Section 1 TEKS The student is expected to: 6E describe how the
Heat Section 1 Preview Section 1 Temperature and Thermal Equilibrium Section 2 Defining Heat Section 3 Changes in Temperature and Phase Heat Section 1 TEKS The student is expected to: 6E describe how the
ME332 FLUID MECHANICS LABORATORY (PART I)
 ME332 FLUID MECHANICS LABORATORY (PART I) Mihir Sen Department of Aerospace and Mechanical Engineering University of Notre Dame Notre Dame, IN 46556 Version: January 14, 2002 Contents Unit 1: Hydrostatics
ME332 FLUID MECHANICS LABORATORY (PART I) Mihir Sen Department of Aerospace and Mechanical Engineering University of Notre Dame Notre Dame, IN 46556 Version: January 14, 2002 Contents Unit 1: Hydrostatics
Contents. 1 Introduction 4. 2 Methods Results and Discussion 15
 Contents 1 Introduction 4 2 Methods 11 3 Results and Discussion 15 4 Appendices 21 4.1 Variable Definitions................................ 21 4.2 Sample Calculations............................... 22
Contents 1 Introduction 4 2 Methods 11 3 Results and Discussion 15 4 Appendices 21 4.1 Variable Definitions................................ 21 4.2 Sample Calculations............................... 22
(Refer Slide Time: 01:17)
 Heat and Mass Transfer Prof. S.P. Sukhatme Department of Mechanical Engineering Indian Institute of Technology, Bombay Lecture No. 7 Heat Conduction 4 Today we are going to look at some one dimensional
Heat and Mass Transfer Prof. S.P. Sukhatme Department of Mechanical Engineering Indian Institute of Technology, Bombay Lecture No. 7 Heat Conduction 4 Today we are going to look at some one dimensional
Introduction to Heat and Mass Transfer. Week 5
 Introduction to Heat and Mass Transfer Week 5 Critical Resistance Thermal resistances due to conduction and convection in radial systems behave differently Depending on application, we want to either maximize
Introduction to Heat and Mass Transfer Week 5 Critical Resistance Thermal resistances due to conduction and convection in radial systems behave differently Depending on application, we want to either maximize
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 --review Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Find the area of the shaded region. ) f() = + - ) 0 0 (, 8) 0 (0, 0) - - - - - - -0
--review Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Find the area of the shaded region. ) f() = + - ) 0 0 (, 8) 0 (0, 0) - - - - - - -0
University of New Mexico Mechanical Engineering Fall 2012 PhD qualifying examination Heat Transfer
 University of New Mexico Mechanical Engineering Fall 2012 PhD qualifying examination Heat Transfer Closed book. Formula sheet and calculator are allowed, but not cell phones, computers or any other wireless
University of New Mexico Mechanical Engineering Fall 2012 PhD qualifying examination Heat Transfer Closed book. Formula sheet and calculator are allowed, but not cell phones, computers or any other wireless
EXPERIMENT #3 A Beer's Law Study
 OBJECTVES: EXPERMENT #3 A Beer's Law Study To operate a Spectronic 20 To convert from percent transmission to absorbance units To plot absorbance versus wavelength and find max To plot absorbance versus
OBJECTVES: EXPERMENT #3 A Beer's Law Study To operate a Spectronic 20 To convert from percent transmission to absorbance units To plot absorbance versus wavelength and find max To plot absorbance versus
PURE PHYSICS THERMAL PHYSICS (PART I)
 PURE PHYSICS THERMAL PHYSICS (PART I) 1 The kinetic theory of matter states that all matters are made up of or, which are in and motion. forces hold the atoms or molecules together. The nature of these
PURE PHYSICS THERMAL PHYSICS (PART I) 1 The kinetic theory of matter states that all matters are made up of or, which are in and motion. forces hold the atoms or molecules together. The nature of these
TOTAL HEAD, N.P.S.H. AND OTHER CALCULATION EXAMPLES Jacques Chaurette p. eng., June 2003
 TOTAL HEAD, N.P.S.H. AND OTHER CALCULATION EXAMPLES Jacques Chaurette p. eng., www.lightmypump.com June 2003 Figure 1 Calculation example flow schematic. Situation Water at 150 F is to be pumped from a
TOTAL HEAD, N.P.S.H. AND OTHER CALCULATION EXAMPLES Jacques Chaurette p. eng., www.lightmypump.com June 2003 Figure 1 Calculation example flow schematic. Situation Water at 150 F is to be pumped from a
Math 122 Fall Solutions to Homework #5. ( ) 2 " ln x. y = x 2
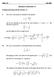 Math 1 Fall 8 Solutions to Homework #5 Problems from Pages 383-38 (Section 7.) 6. The curve in this problem is defined b the equation: = ( ) " ln and we are interested in the part of the curve between
Math 1 Fall 8 Solutions to Homework #5 Problems from Pages 383-38 (Section 7.) 6. The curve in this problem is defined b the equation: = ( ) " ln and we are interested in the part of the curve between
Measurement of Thermal Diffusivity by Lock-in Thermography
 Measurement of Thermal Diffusivity by Lock-in Thermography by P.G. Bison, S. Marinetti, E. Grinzato CNR-ITEF, C.so Stati Uniti 4, 35127 Padova, Italy, E-mail: paolo.bison@itef.pd.cnr.it Abstract Diffusivity
Measurement of Thermal Diffusivity by Lock-in Thermography by P.G. Bison, S. Marinetti, E. Grinzato CNR-ITEF, C.so Stati Uniti 4, 35127 Padova, Italy, E-mail: paolo.bison@itef.pd.cnr.it Abstract Diffusivity
Name Partner. Thermal Physics. Part I: Heat of Vaporization of Nitrogen. Introduction:
 Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
221 MATH REFRESHER session 3 SAT2015_P06.indd 221 4/23/14 11:39 AM
 Math Refresher Session 3 1 Area, Perimeter, and Volume Problems Area, Perimeter, and Volume 301. Formula Problems. Here, you are given certain data about one or more geometric figures, and you are asked
Math Refresher Session 3 1 Area, Perimeter, and Volume Problems Area, Perimeter, and Volume 301. Formula Problems. Here, you are given certain data about one or more geometric figures, and you are asked
Chapter 17 Temperature and heat
 Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
HEAT TRANSFER 1 INTRODUCTION AND BASIC CONCEPTS 5 2 CONDUCTION
 HEAT TRANSFER 1 INTRODUCTION AND BASIC CONCEPTS 5 2 CONDUCTION 11 Fourier s Law of Heat Conduction, General Conduction Equation Based on Cartesian Coordinates, Heat Transfer Through a Wall, Composite Wall
HEAT TRANSFER 1 INTRODUCTION AND BASIC CONCEPTS 5 2 CONDUCTION 11 Fourier s Law of Heat Conduction, General Conduction Equation Based on Cartesian Coordinates, Heat Transfer Through a Wall, Composite Wall
Ministry of Higher Education And Scientific Research. University Of Technology Chemical Engineering Department. Heat Transfer
 Ministry of Higher Education And Scientific Research University Of Technology Heat Transfer Third Year By Dr.Jamal Al-Rubeai 2008-2009 Heat Transfer 1. Modes of Heat Transfer: Conduction, Convection and
Ministry of Higher Education And Scientific Research University Of Technology Heat Transfer Third Year By Dr.Jamal Al-Rubeai 2008-2009 Heat Transfer 1. Modes of Heat Transfer: Conduction, Convection and
Safety Analysis of Loss of Flow Transients in a Typical Research Reactor by RELAP5/MOD3.3
 International Conference Nuclear Energy for New Europe 23 Portorož, Slovenia, September 8-11, 23 http://www.drustvo-js.si/port23 Safety Analysis of Loss of Flow Transients in a Typical Research Reactor
International Conference Nuclear Energy for New Europe 23 Portorož, Slovenia, September 8-11, 23 http://www.drustvo-js.si/port23 Safety Analysis of Loss of Flow Transients in a Typical Research Reactor
Applications of Integration to Physics and Engineering
 Applications of Integration to Physics and Engineering MATH 211, Calculus II J Robert Buchanan Department of Mathematics Spring 2018 Mass and Weight mass: quantity of matter (units: kg or g (metric) or
Applications of Integration to Physics and Engineering MATH 211, Calculus II J Robert Buchanan Department of Mathematics Spring 2018 Mass and Weight mass: quantity of matter (units: kg or g (metric) or
PDHengineer.com Course M-3003
 PDHengineer.com Course M-3003 Heat Transfer Fundamentals To receive credit for this course This document is the course text. You may review this material at your leisure either before or after you purchase
PDHengineer.com Course M-3003 Heat Transfer Fundamentals To receive credit for this course This document is the course text. You may review this material at your leisure either before or after you purchase
PREDICTION OF MASS FLOW RATE AND PRESSURE DROP IN THE COOLANT CHANNEL OF THE TRIGA 2000 REACTOR CORE
 PREDICTION OF MASS FLOW RATE AND PRESSURE DROP IN THE COOLANT CHANNEL OF THE TRIGA 000 REACTOR CORE Efrizon Umar Center for Research and Development of Nuclear Techniques (P3TkN) ABSTRACT PREDICTION OF
PREDICTION OF MASS FLOW RATE AND PRESSURE DROP IN THE COOLANT CHANNEL OF THE TRIGA 000 REACTOR CORE Efrizon Umar Center for Research and Development of Nuclear Techniques (P3TkN) ABSTRACT PREDICTION OF
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Significant Figures: A Brief Tutorial
 Significant Figures: A Brief Tutorial 2013-2014 Mr. Berkin *Please note that some of the information contained within this guide has been reproduced for non-commercial, educational purposes under the Fair
Significant Figures: A Brief Tutorial 2013-2014 Mr. Berkin *Please note that some of the information contained within this guide has been reproduced for non-commercial, educational purposes under the Fair
Please read each question carefully. Each question worth s 1 point. For the following questions, please circle the correct answer.
 Please read each question carefully. Each question worth s 1 point. For the following questions, please circle the correct answer. Part 1. 1. An object maintains its state of motion because it has A) mass
Please read each question carefully. Each question worth s 1 point. For the following questions, please circle the correct answer. Part 1. 1. An object maintains its state of motion because it has A) mass
53:071 Principles of Hydraulics Laboratory Experiment #3 ANALYSIS OF OPEN-CHANNEL FLOW TRANSITIONS USING THE SPECIFIC ENERGY DIAGRAM
 53:071 Principles of Hydraulics Laboratory Experiment #3 ANALYSIS OF OPEN-CHANNEL FLOW TRANSITIONS USING THE SPECIFIC ENERGY DIAGRAM Principle Adaptation of the Bernoulli equation to open-channel flows
53:071 Principles of Hydraulics Laboratory Experiment #3 ANALYSIS OF OPEN-CHANNEL FLOW TRANSITIONS USING THE SPECIFIC ENERGY DIAGRAM Principle Adaptation of the Bernoulli equation to open-channel flows
Onset of Flow Instability in a Rectangular Channel Under Transversely Uniform and Non-uniform Heating
 Onset of Flow Instability in a Rectangular Channel Under Transversely Uniform and Non-uniform Heating Omar S. Al-Yahia, Taewoo Kim, Daeseong Jo School of Mechanical Engineering, Kyungpook National University
Onset of Flow Instability in a Rectangular Channel Under Transversely Uniform and Non-uniform Heating Omar S. Al-Yahia, Taewoo Kim, Daeseong Jo School of Mechanical Engineering, Kyungpook National University
2.8 Linear Approximation and Differentials
 2.8 Linear Approximation Contemporary Calculus 1 2.8 Linear Approximation and Differentials Newton's method used tangent lines to "point toward" a root of the function. In this section we examine and use
2.8 Linear Approximation Contemporary Calculus 1 2.8 Linear Approximation and Differentials Newton's method used tangent lines to "point toward" a root of the function. In this section we examine and use
Keller: Stats for Mgmt & Econ, 7th Ed July 17, 2006
 Chapter 17 Simple Linear Regression and Correlation 17.1 Regression Analysis Our problem objective is to analyze the relationship between interval variables; regression analysis is the first tool we will
Chapter 17 Simple Linear Regression and Correlation 17.1 Regression Analysis Our problem objective is to analyze the relationship between interval variables; regression analysis is the first tool we will
Protean Instrument Dutchtown Road, Knoxville, TN TEL/FAX:
 Application Note AN-0210-1 Tracking Instrument Behavior A frequently asked question is How can I be sure that my instrument is performing normally? Before we can answer this question, we must define what
Application Note AN-0210-1 Tracking Instrument Behavior A frequently asked question is How can I be sure that my instrument is performing normally? Before we can answer this question, we must define what
Open Channel Flow I - The Manning Equation and Uniform Flow COURSE CONTENT
 Open Channel Flow I - The Manning Equation and Uniform Flow Harlan H. Bengtson, PhD, P.E. COURSE CONTENT 1. Introduction Flow of a liquid may take place either as open channel flow or pressure flow. Pressure
Open Channel Flow I - The Manning Equation and Uniform Flow Harlan H. Bengtson, PhD, P.E. COURSE CONTENT 1. Introduction Flow of a liquid may take place either as open channel flow or pressure flow. Pressure
NEBB Fundamental Formulas
 Approved NEBB - May 1, 17 Page 1 of 8 Version 1.3 A = Area (ft²) IP, (m²) SI M = Mass (lb) IP, (kg) SI ACH = Air Changes per Hour ma = Mixed Air Ak = Effective Area m = meter (metre) AV = Average m³/s
Approved NEBB - May 1, 17 Page 1 of 8 Version 1.3 A = Area (ft²) IP, (m²) SI M = Mass (lb) IP, (kg) SI ACH = Air Changes per Hour ma = Mixed Air Ak = Effective Area m = meter (metre) AV = Average m³/s
NATURAL CONVECTION HEAT TRANSFER CHARACTERISTICS OF KUR FUEL ASSEMBLY DURING LOSS OF COOLANT ACCIDENT
 NATURAL CONVECTION HEAT TRANSFER CHARACTERISTICS OF KUR FUEL ASSEMBLY DURING LOSS OF COOLANT ACCIDENT Ito D*, and Saito Y Research Reactor Institute Kyoto University 2-1010 Asashiro-nishi, Kumatori, Sennan,
NATURAL CONVECTION HEAT TRANSFER CHARACTERISTICS OF KUR FUEL ASSEMBLY DURING LOSS OF COOLANT ACCIDENT Ito D*, and Saito Y Research Reactor Institute Kyoto University 2-1010 Asashiro-nishi, Kumatori, Sennan,
123MEAN thermal properties KATEDRA MATERIÁLOVÉHO INŽENÝRSTVÍ A CHEMIE
 123MEAN thermal properties KATEDRA MATERIÁLOVÉHO INŽENÝRSTVÍ A CHEMIE Heat transport in substances: conduction transfer of kinetic energy on the bases of disorded movement of molecules. Own heat transfer
123MEAN thermal properties KATEDRA MATERIÁLOVÉHO INŽENÝRSTVÍ A CHEMIE Heat transport in substances: conduction transfer of kinetic energy on the bases of disorded movement of molecules. Own heat transfer
AR/IA 241 LN 231 Lecture 4: Fundamental of Energy
 Faculty of Architecture and Planning Thammasat University A/IA 24 LN 23 Lecture 4: Fundamental of Energy Author: Asst. Prof. Chalermwat Tantasavasdi. Heat For a specific substance, the heat given to the
Faculty of Architecture and Planning Thammasat University A/IA 24 LN 23 Lecture 4: Fundamental of Energy Author: Asst. Prof. Chalermwat Tantasavasdi. Heat For a specific substance, the heat given to the
MATHEMATICAL METHODS (CAS) PILOT STUDY
 Victorian CertiÞcate of Education 2005 SUPERVISOR TO ATTACH PROCESSING LABEL HERE STUDENT NUMBER Letter Figures Words MATHEMATICAL METHODS (CAS) PILOT STUDY Written examination 2 (Analysis task) Monday
Victorian CertiÞcate of Education 2005 SUPERVISOR TO ATTACH PROCESSING LABEL HERE STUDENT NUMBER Letter Figures Words MATHEMATICAL METHODS (CAS) PILOT STUDY Written examination 2 (Analysis task) Monday
Thermal Fluid System Design. Team Design #1
 Thermal Fluid System Design Team Design #1 Table of Contents Nomenclature Listing.3 Executive Summary.6 Introduction.7 Analysis.8 Results/Discussion..18 Conclusion..29 References..29 Appendix A: Detailed
Thermal Fluid System Design Team Design #1 Table of Contents Nomenclature Listing.3 Executive Summary.6 Introduction.7 Analysis.8 Results/Discussion..18 Conclusion..29 References..29 Appendix A: Detailed
Final Exam Review / AP Calculus AB
 Chapter : Final Eam Review / AP Calculus AB Use the graph to find each limit. 1) lim f(), lim f(), and lim π - π + π f 5 4 1 y - - -1 - - -4-5 ) lim f(), - lim f(), and + lim f 8 6 4 y -4 - - -1-1 4 5-4
Chapter : Final Eam Review / AP Calculus AB Use the graph to find each limit. 1) lim f(), lim f(), and lim π - π + π f 5 4 1 y - - -1 - - -4-5 ) lim f(), - lim f(), and + lim f 8 6 4 y -4 - - -1-1 4 5-4
Figure 1: Diagram of the top of the bloomcaster.
 85 HEAT TRANSFER N A CONTNUOUS BLOOMCASTER Four approaches to detecting disruptions to the primary cooling process in the continuous bloomcaster at BHP's Rod and Bar Division in Newcastle are investigated.
85 HEAT TRANSFER N A CONTNUOUS BLOOMCASTER Four approaches to detecting disruptions to the primary cooling process in the continuous bloomcaster at BHP's Rod and Bar Division in Newcastle are investigated.
Shell-and-Tube Heat Exchangers Unit Operations Laboratory - Sarkeys E111 February 11 th & 18 th, 2015 ChE Section 3
 Shell-and-Tube Heat Exchangers Unit Operations Laboratory - Sarkeys E111 February 11 th & 18 th, 2015 ChE 3432 - Section 3 Eric Henderson Eddie Rich Xiaorong Zhang Mikey Zhou 1 ABSTRACT Shell-and-tube
Shell-and-Tube Heat Exchangers Unit Operations Laboratory - Sarkeys E111 February 11 th & 18 th, 2015 ChE 3432 - Section 3 Eric Henderson Eddie Rich Xiaorong Zhang Mikey Zhou 1 ABSTRACT Shell-and-tube
Find the perimeter of the figure named and shown. Express the perimeter in the same unit of measure that appears on the given side or sides.
 Mth101 Chapter 8 HW Name Find the perimeter of the figure named and shown. Express the perimeter in the same unit of measure that appears on the given side or sides. 1) 1) Rectangle 6 in. 12 in. 12 in.
Mth101 Chapter 8 HW Name Find the perimeter of the figure named and shown. Express the perimeter in the same unit of measure that appears on the given side or sides. 1) 1) Rectangle 6 in. 12 in. 12 in.
Building Envelope Requirements Overview Page 3-4
 Building Envelope Requirements Overview Page 3-4 The benefit of a high reflectance surface is obvious: while dark surfaces absorb the sun s energy (visible light, invisible infrared. and ultraviolet radiation)
Building Envelope Requirements Overview Page 3-4 The benefit of a high reflectance surface is obvious: while dark surfaces absorb the sun s energy (visible light, invisible infrared. and ultraviolet radiation)
ANOTHER FIVE QUESTIONS:
 No peaking!!!!! See if you can do the following: f 5 tan 6 sin 7 cos 8 sin 9 cos 5 e e ln ln @ @ Epress sin Power Series Epansion: d as a Power Series: Estimate sin Estimate MACLAURIN SERIES ANOTHER FIVE
No peaking!!!!! See if you can do the following: f 5 tan 6 sin 7 cos 8 sin 9 cos 5 e e ln ln @ @ Epress sin Power Series Epansion: d as a Power Series: Estimate sin Estimate MACLAURIN SERIES ANOTHER FIVE
Chapter 16. Copyright 2010 Pearson Education, Inc.
 Chapter 16 Temperature and Heat Units of Chapter 16 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work Specific Heats Conduction, Convection,
Chapter 16 Temperature and Heat Units of Chapter 16 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work Specific Heats Conduction, Convection,
Reactivity Power and Temperature Coefficients Determination of the TRR
 Reactivity and Temperature Coefficients Determination of the TRR ABSTRACT Ahmad Lashkari Nuclear Science and Technology Research Institute (NSTRI), Atomic Energy Organization of Iran Tehran 14399-51113,
Reactivity and Temperature Coefficients Determination of the TRR ABSTRACT Ahmad Lashkari Nuclear Science and Technology Research Institute (NSTRI), Atomic Energy Organization of Iran Tehran 14399-51113,
6.5 Work and Fluid Forces
 6.5 Work and Fluid Forces Work Work=Force Distance Work Work=Force Distance Units Force Distance Work Newton meter Joule (J) pound foot foot-pound (ft lb) Work Work=Force Distance Units Force Distance
6.5 Work and Fluid Forces Work Work=Force Distance Work Work=Force Distance Units Force Distance Work Newton meter Joule (J) pound foot foot-pound (ft lb) Work Work=Force Distance Units Force Distance
