NARAYANA XII STD BATCHES [CF] (Hint & Solution) PART A : PHYSICS. When then material is removed, the force between them becomes
|
|
|
- Brittney Craig
- 5 years ago
- Views:
Transcription
1 NARAYANA I I T / N E E T A C A D E M Y Co m mo n Pr act i ce Te st Date: XII STD BATCHES [CF] ANSWER PHYSICS CHEMISTRY MATHEMATICS (Hint & Solution) PART A : PHYSICS.. Since q = ne n or n.5 08 The force between two point charges q and q placed in a material medium of dielectric constant r at a distance r is qq qq 4 0 r r r 4 0 r When then material is removed, the force between them becomes qq F' rf 4 0 r Charge q will follow circular path if electrostatic force between q and q is attractive, F 3. i.e., qq mv qq v 4 0 r r 4 0 mr Also, v r r T ()
2 6 3 0 mr 3 r v qq Net electrical force on Q is zero FB FA FC FD 0 T 4. Now FA FC FD qq. 4 0 a Q. 4 0 a Resultant of FA and FC, FAC qq. 4 0 a qq Q a 4 0 a Q Q Or q or q By symmetry, the components of force on charge q0 due to charges at A and B along X-axis will cancel each other while along Y-axis will add. The resultant force on charge q0 is FB 5. F F cos q0 q 4 0 a y y a y q0 qy 0 a y 3/ Force on charge q0 will be maximum, when df 0 dy 3 y y q0 q a y 3/ a y 5/ Or a y 3/ 3y a 5/ y 0 or = 3 y a y ()
3 a q or y= The situation is as shown in the figure Or y 6. The origin is given a small displacement along the y-axis then the situation is as shown in the figure. By symmetry, the components of forces on the particle of charge q0 due to charges at A and B along x-axis will cancel each other where along y-axis will add up. The net force acting on the particle is Fnet F cos q q / 4 0 y a qq0 4 0 y a y y a y y a q y 4 0 y a 3/ As y a Fnet 7. q y or Fnet y 4 0 a 3 Initially, F 6 C 9 C 4 0 d When a charge of 3 C is given to both the spheres, then charges on spheres are 6 C 3 C 3 C and 9 C 3 C 6 C F ' 3 C 6 C 4 0 d Dividing eqn. (ii) by eqn. (i) we get F' F F ' F Fr Nm C Nm 4 0 qq C 9. Here q q 3 C C (3)
4 r iˆ ˆj kˆ; r iˆ 3 ˆj 3kˆ then r iˆ 3 ˆj 3kˆ iˆ ˆj kˆ iˆ ˆj kˆ r 3 According to Coulomb s law F qq N 4 0 r 3 0. F ; so when r is halved the force becomes four times. r. F q q. and F a 4 0 a F F3. 3. Conceptual 4. From principle of super position F Net FA F B F C F D 5. The resultant force at the centre will be 0 due to symmetry 6. As net external force along on the system is zero m a m a hence a cm =0 = m m a 8 m m a 0 = gm 7. Charge at rest produces only electric field 8. By using Q = ne Q = = +.6C In the LHS of q, the fields due to (-q) and (q) are opposite.. (4)
5 . At the present position all the charges are in equilibrium. But when they displaced slightly from their present position, they do not return back. So, they are in unstable equilibrium position. a a 4q 4q -q v KE qe mv m at m t m C is the mid point of AB. The field is directed to the right (positive) in the region between A and C, and to the left (negative) between C and B. The magnitude of E will increase sharply for x 0 and x l 5. In the following fig, in equilibrium Fe = T sin 300, r = m 0 m m T cos T 0 C Fk 0 r Q T r T T sin 30 Fe 0 C mg T.8N. Q x x d df 0 dx 7. F 30o +Q A F -Q +Q B C The net force normal to F cos 30 F cos 30 = 0, since F = F = Q 4 0 a 8. The net electrostatic force due to Cs ions at the centre (Cl ion) is zero, due to symmetry. 9. Force on each charge is zero, But if any of the charge is displaced, the net force starts acting on all of them. (5)
6 PART B : CHEMISTRY 3. Pyridine CH 3CH CH OH SOCl CH 3CH CH Cl SO HCl 3. CCl4 PhCH COOAg PhCH Br CO AgBr reflux 33. CCl4 CH 3CH COOAg I CH 3CH COOC H 5 CO AgI Silver propanoate Ethyl propanoate Conceptual Being strained cyclopropane ring readily opens up to form only n-propyl bromide. In contrast, reaction (a) gives a mixture of n-propyl and isopropyl bromides, reaction (b) gives isopropyl bromide with reaction (c) does not occur at all O O C H 5 C OAg I C H 5 C OC H 5 CO AgI It is Birnbaum Simonini reaction 39. CH3 CH3 0 HI CH 3 C CH OH CH 3 C CH H O, I H H CH3 CH3 I CH 3 C CH CH 3 C CH I Allylic substitution It is allylic substitution (6)
7 A, through halogen exchange reaction 54. Gattermann reaction occurs in presence of Cu-Powder 55. CCl4 R CH COOAg Br R Br CO AgBr reflux Inversion of configuration takes place in SN reaction CH 3O is a negative nucleophile and a much stronger base than CH 3COO 59. Viny halides usually fail in nucleophile substitution due to resonance phenomena leading to increase in C X bond strength. 60. PART C : MATHEMATICS R is reflexive and transitive but not symmetric R is symmetric and transitive but not reflexive a a is not true. So, R is not reflexive a b and b c does not imply a c. So, R is not transitive But, a b b a is always true. (i) a a = 0 is always true (ii) a R b a b -(a b) b a b R a. (iii) R and R But, is not related to So, R is not transitive. R is reflexive (3, 3), (6,6), (9, 9), (, ) R again (6, ) R but (, 6) R R is not symmetric R is transitive [ (3, 6) R, (6, ) R, (3, ) R others are clear Clearly, (x, y)r(x, y), (x, y) A, since xy = yx. This shows that R is reflexive. Further, (x, y)r(u, v) xv = yu uy vx and hence (u, v)r(x, y). This shows that R is symmetric. Similarly, (x, y)r(u, v) and (u, v)r(a, b) xv = yu and ub = va xv 69. a a b a yu xv yu xb ya and hence u u v u (x, y)r(a, b). Thus, R is transitive. Thus, R is an equivalent relation. Given, r = {(a, b) a, b R and a b 3 as an irrational number} (7)
8 (i) Reflexive ara a a 3 3, which is irrational number. (ii) Symmetric Now, r 3 3 3, which is not an irrational. Also, 3 r , which is an irrational. Which is not symmetric. (iii) Transitive 3r and r 4 5, i.e., Now, r 4 5 It is not transitive. 70. Given, R = {(, 3), (4, ), (, 4), (, 3), (3, )} is a relation on the set A = {,, 3, 4}, then since (, 4) R and (, 3) R, so R is not a function. since (3, ) R and (, 3) R but (, ) R, so R is not transitive. since (, 3) R but (3, ) R, so R is not symmetric. since (a, a) R a =,, 3, 4. not reflexive 7. Let W = {CAT, TOY, YOU,..) Clearly, R is reflexive and symmetric but not transitive. Since, CATRTOY, TOYRYOU CATRYOU 7. Conceptual Conceptual mrm, as m is multiple of m R is reflexive mrn nrm R is not symmetric. mrn and nrp mrp R is transitive 75. l Rl l is not perpendicular to l l Rl l l l l l Rl, Hence, R is symmetric. l Rl and l Rl3 l Rl3. ( R is not transitive) Reflexive but not Symmetric as, R but, R Not transitive as (, ) and, 3 R but, 3 R arb bra Since, R. So, R is not reflexive. Now (, ) R but, R, (8)
9 R is not symmetric. Clearly R is transitive. xry, yrz xrz R, 4, 4,,, 3, 3, a + b = b + a = Let a R a.a a 0 a, a R R is reflexive on R. Let a, b R. ab 0 ba 0 b, a R R is symmetric on R Since, R and, R but, R R is not transitive on R. We have ()² =, ()² = 4, (3)² = 9, (4)² = 6, (5)² = 5, (6)² = 36 R = {(, ), (4, ), (9, 3), (6, 4), (5, 5), (36, 6)} Domain of R = {x; x, y R} {, 4,9,6, 5,36) Range of R = { y; x, y R} (,,3, 4,5, 6) x R y iff x < y. We have < 3, < 5, < 3, < 5, 3 < 5 R = {(,3), (,5), (, 3), (, 5), (3, 5)} Let n N, then n is a factor of n nrn R is reflexive 3 R 6 because 3 is factor of 6 arc R is transitive R {(, ), (, 4), ( 3, 9)( 4, 6), ( 5, 5)} {(, ), (3,5), (4,7), (6, 6)} Domain of R {x : ( x, y) R} {,3, 4, 5, 6} Range of R { y : ( x, y ) R} {,5,0,7, 6} We have R = {(, 3)}. Here,3 R but 3, R. R is not symmetric. The relation R is also transitive i i R 3 is wrong R ( 3) is wrong i i R is wrong i 0 ir is correct (9)
10 Since R A B and S B C, we have SoR A C. SoR is a relation from A to C. R is reflexive because a a for a A. We have 4, R because 4 but, 4 R. R is not symmetric. Let a, b b, c R a b, b c. a c a, c R R is transitive TOPIC/SUB TOPIC Q. NOS. PHYSICS,7,3,7,8,6,30,3,4,5,,8,9,0,,,4,5,6,0,,5,7,8,9 9,,3,4 CHEMISTRY (Preparation of alkyle and aryl halide) 3 to 60 Alkyle and Aryl Halide MATHEMATICS Relation 6 to 90 Charge and its properties Coulomb s Law Electric Field due to Point charge (0)
CPT XII-NEW (REG) Batches (Date: ) ANSWERS PHYSICS CHEMISTRY MATHEMATICS 1. (D) 31. (D) 61. (D) 2. (B) 32. (D) 62. (B) 3. (B) 33.
 CPT-0/XII-S (NEW)/09.05.16 NARAYANA I I T / P M T A C A D E M Y CPT - 0 XII-NEW (REG) Batches (Date: 09.05.16) ANSWERS PHYSICS CHEMISTRY MATHEMATICS 1. (D) 31. (D) 61. (D). (B) 3. (D) 6. (B) 3. (B) 33.
CPT-0/XII-S (NEW)/09.05.16 NARAYANA I I T / P M T A C A D E M Y CPT - 0 XII-NEW (REG) Batches (Date: 09.05.16) ANSWERS PHYSICS CHEMISTRY MATHEMATICS 1. (D) 31. (D) 61. (D). (B) 3. (D) 6. (B) 3. (B) 33.
NARAYANA. Co m m o n Pr act ice T e st 3 Date: XII STD BATCHES [CF] (Hint & Solution) PART A : PHYSICS
![NARAYANA. Co m m o n Pr act ice T e st 3 Date: XII STD BATCHES [CF] (Hint & Solution) PART A : PHYSICS NARAYANA. Co m m o n Pr act ice T e st 3 Date: XII STD BATCHES [CF] (Hint & Solution) PART A : PHYSICS](/thumbs/86/93679485.jpg) NARAYANA I I T / N E E T A C A D E M Y Co m m o n Pr act ice T e st Date: 0.04.7 XII STD BATCHES [CF] ANSWER PHYSICS... 4. 5. 6. 7. 8. 9. 0.... 4. 5. 6. 7. 8. 9. 0.... 4. 5. 6. 7. 8. 9. 0. CHEMISTRY...
NARAYANA I I T / N E E T A C A D E M Y Co m m o n Pr act ice T e st Date: 0.04.7 XII STD BATCHES [CF] ANSWER PHYSICS... 4. 5. 6. 7. 8. 9. 0.... 4. 5. 6. 7. 8. 9. 0.... 4. 5. 6. 7. 8. 9. 0. CHEMISTRY...
Solutions and Ions. Pure Substances
 Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
MUNISH KAKAR s INSTITUTE OF CHEMISTRY
 REACTONS & PREPARATON OF HALOGENATED COMPDS. WS#3 Q1. Reagent for preparing a chloroalkane from an alcohol is (a) SOCl 2 (b) HCl/ZnCl 2 (c) PCl 3 (d) All of these Q2. n the addition of H to propene in
REACTONS & PREPARATON OF HALOGENATED COMPDS. WS#3 Q1. Reagent for preparing a chloroalkane from an alcohol is (a) SOCl 2 (b) HCl/ZnCl 2 (c) PCl 3 (d) All of these Q2. n the addition of H to propene in
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
M09/4/CHEMI/HPM/ENG/TZ2/XX+ CHEMISTRY. Monday 18 May 2009 (afternoon) 1 hour INSTRUCTIONS TO CANDIDATES
 M09/4/CEMI/PM/ENG/TZ/XX+ 096113 CEMISTRY igher level Paper 1 Monday 18 May 009 (afternoon) 1 hour INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer all the
M09/4/CEMI/PM/ENG/TZ/XX+ 096113 CEMISTRY igher level Paper 1 Monday 18 May 009 (afternoon) 1 hour INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer all the
Faculty of Natural and Agricultural Sciences Chemistry Department. Semester Test 1. Analytical Chemistry CMY 283. Time: 120 min Marks: 100 Pages: 6
 Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 120 min
Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 120 min
Conductors: External Electric Field 1/28/2018 1
 Conductors: External Electric Field 1/28/2018 1 Two Parallel Conducting Sheets Find the electric field to the left of the sheets, between the sheets and to the right of the sheets. 1/28/2018 2 Uniform
Conductors: External Electric Field 1/28/2018 1 Two Parallel Conducting Sheets Find the electric field to the left of the sheets, between the sheets and to the right of the sheets. 1/28/2018 2 Uniform
M09/4/CHEMI/SPM/ENG/TZ1/XX+ CHEMISTRY. Monday 18 May 2009 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M09/4/CHEMI/SPM/ENG/TZ1/XX+ 22096110 CHEMISTRY standard level Paper 1 Monday 18 May 2009 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so.
M09/4/CHEMI/SPM/ENG/TZ1/XX+ 22096110 CHEMISTRY standard level Paper 1 Monday 18 May 2009 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so.
NARAYANA I I T / P M T A C A D E M Y. C o m m o n P r a c t i c e T e s t 05 XII-IC SPARK Date:
 1. (A). (C). (B). (B) 5. (A) 6. (B) 7. (B) 8. (A) 9. (A) 1. (D) 11. (C) 1. (B) 1. (A) 1. (D) 15. (B) NARAYANA I I T / P M T A C A D E M Y C o m m o n P r a c t i c e T e s t 5 XII-IC SPARK Date: 9.1.17
1. (A). (C). (B). (B) 5. (A) 6. (B) 7. (B) 8. (A) 9. (A) 1. (D) 11. (C) 1. (B) 1. (A) 1. (D) 15. (B) NARAYANA I I T / P M T A C A D E M Y C o m m o n P r a c t i c e T e s t 5 XII-IC SPARK Date: 9.1.17
ANSWERS, HINTS & SOLUTIONS HALF COURSE TEST VII (Paper - 1) Q. No. PHYSICS CHEMISTRY MATHEMATICS. 1. p); (D q, r) p) (D s) 2.
 1 AIITS-HCT-VII (Paper-1)-PCM (Sol)-JEE(Advanced)/16 FIITJEE Students From All Programs have bagged in Top 100, 77 in Top 00 and 05 in Top 500 All India Ranks. FIITJEE Performance in JEE (Advanced), 015:
1 AIITS-HCT-VII (Paper-1)-PCM (Sol)-JEE(Advanced)/16 FIITJEE Students From All Programs have bagged in Top 100, 77 in Top 00 and 05 in Top 500 All India Ranks. FIITJEE Performance in JEE (Advanced), 015:
 lectures accompanying the book: Solid State Physics: An Introduction, by Philip ofmann (2nd edition 2015, ISBN-10: 3527412824, ISBN-13: 978-3527412822, Wiley-VC Berlin. www.philiphofmann.net 1 Bonds between
lectures accompanying the book: Solid State Physics: An Introduction, by Philip ofmann (2nd edition 2015, ISBN-10: 3527412824, ISBN-13: 978-3527412822, Wiley-VC Berlin. www.philiphofmann.net 1 Bonds between
Faculty of Natural and Agricultural Sciences Chemistry Department. Semester Test 1 MEMO. Analytical Chemistry CMY 283
 Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 MEMO Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 90
Faculty of Natural and Agricultural Sciences Chemistry Department Semester Test 1 MEMO Analytical Chemistry CMY 283 Date: 5 September 2016 Lecturers : Prof P Forbes, Dr Laurens, Mr SA Nsibande Time: 90
Fall 2011 CHEM Test 4, Form A
 Fall 2011 CHEM 1110.40413 Test 4, Form A Part I. Multiple Choice: Clearly circle the best answer. (60 pts) Name: 1. The common constituent in all acid solutions is A) H 2 SO 4 B) H 2 C) H + D) OH 2. Which
Fall 2011 CHEM 1110.40413 Test 4, Form A Part I. Multiple Choice: Clearly circle the best answer. (60 pts) Name: 1. The common constituent in all acid solutions is A) H 2 SO 4 B) H 2 C) H + D) OH 2. Which
8. Relax and do well.
 CHEM 1225 Exam I John I. Gelder February 4, 1999 Name KEY TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do
CHEM 1225 Exam I John I. Gelder February 4, 1999 Name KEY TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do
Last 4 Digits of USC ID:
 Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
The Periodic Table. Periodic Properties. Can you explain this graph? Valence Electrons. Valence Electrons. Paramagnetism
 Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
8. Relax and do well.
 CHEM 15 Exam II John II. Gelder March 4, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last two pages includes a periodic table, a solubility
CHEM 15 Exam II John II. Gelder March 4, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last two pages includes a periodic table, a solubility
Downloaded from
 Question 1.1: What is the force between two small charged spheres having charges of 2 10 7 C and 3 10 7 C placed 30 cm apart in air? Repulsive force of magnitude 6 10 3 N Charge on the first sphere, q
Question 1.1: What is the force between two small charged spheres having charges of 2 10 7 C and 3 10 7 C placed 30 cm apart in air? Repulsive force of magnitude 6 10 3 N Charge on the first sphere, q
Chapter 21 Coordination chemistry: reactions of complexes
 CHEM 511 chapter 21 page 1 of 7 Chapter 21 Coordination chemistry: reactions of complexes Reactions of Complexes Typically measure ligand substitution reactions in solution (usually water) Lability and
CHEM 511 chapter 21 page 1 of 7 Chapter 21 Coordination chemistry: reactions of complexes Reactions of Complexes Typically measure ligand substitution reactions in solution (usually water) Lability and
Bonding/Lewis Dots Lecture Page 1 of 12 Date. Bonding. What is Coulomb's Law? Energy Profile: Covalent Bonds. Electronegativity and Linus Pauling
 Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
E5 Lewis Acids and Bases: lab 2. Session two lab Parts 2B, 3, and 4. Session one lab Parts 1and 2A. Aquo Complex Ions. Aquo Complex Ion Reactions
 E5 Lewis Acids and Bases: lab 2 Session one lab Parts 1and 2A Part 2B. Complexation, Structure and Periodicity Compare the reactivity of aquo complex ions containing pre-transition, transition, and post-transition
E5 Lewis Acids and Bases: lab 2 Session one lab Parts 1and 2A Part 2B. Complexation, Structure and Periodicity Compare the reactivity of aquo complex ions containing pre-transition, transition, and post-transition
M11/4/CHEMI/SPM/ENG/TZ2/XX CHEMISTRY STANDARD LEVEL PAPER 1. Monday 9 May 2011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M11/4/CHEMI/SPM/ENG/TZ/XX 116116 CHEMISTRY STANDARD LEVEL PAPER 1 Monday 9 May 011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M11/4/CHEMI/SPM/ENG/TZ/XX 116116 CHEMISTRY STANDARD LEVEL PAPER 1 Monday 9 May 011 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
Chem 51, Spring 2015 Exam 8 (Chp 8) Use your Scantron to answer Questions There is only one answer for each Question. Questions are 2 pt each.
 Chem 51, Spring 2015 Exam 8 (Chp 8) Name 120 pt Use your Scantron to answer Questions 1-32. There is only one answer for each Question. Questions are 2 pt each. CHP 8.1 Solutions are Mixtures 1. A saturated
Chem 51, Spring 2015 Exam 8 (Chp 8) Name 120 pt Use your Scantron to answer Questions 1-32. There is only one answer for each Question. Questions are 2 pt each. CHP 8.1 Solutions are Mixtures 1. A saturated
Class XII Chapter 1 Electric Charges And Fields Physics
 Class XII Chapter 1 Electric Charges And Fields Physics Question 1.1: What is the force between two small charged spheres having charges of 2 10 7 C and 3 10 7 C placed 30 cm apart in air? Answer: Repulsive
Class XII Chapter 1 Electric Charges And Fields Physics Question 1.1: What is the force between two small charged spheres having charges of 2 10 7 C and 3 10 7 C placed 30 cm apart in air? Answer: Repulsive
Our country, our future 525/1 S6 CHEMISTRY PAPER 1 DURATION: 2 HOUR 45 MINUTES
 1 Our country, our future 525/1 S6 CHEMISTRY Exam 10 PAPER 1 DURATION: 2 HOUR 45 MINUTES For Marking guide contact and consultations: Dr. Bbosa Science 0776 802709, Instructions - This paper consists of
1 Our country, our future 525/1 S6 CHEMISTRY Exam 10 PAPER 1 DURATION: 2 HOUR 45 MINUTES For Marking guide contact and consultations: Dr. Bbosa Science 0776 802709, Instructions - This paper consists of
NAME: FIRST EXAMINATION
 1 Chemistry 64 Winter 1994 NAME: FIRST EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
1 Chemistry 64 Winter 1994 NAME: FIRST EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 READ ALL THE INSTRUCTIONS CAREFULLY
 WEDNESDAY MARCH 9th, 2016 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
WEDNESDAY MARCH 9th, 2016 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
8. Relax and do well.
 CHEM 1314 Name Exam IV TA Name John IV. Gelder December 14, 1992 Lab Section INSTRUCTIONS: 1. This examination consists of a total of 9 different pages. The last three pages includes a periodic table and
CHEM 1314 Name Exam IV TA Name John IV. Gelder December 14, 1992 Lab Section INSTRUCTIONS: 1. This examination consists of a total of 9 different pages. The last three pages includes a periodic table and
Discrete Structures: Sample Questions, Exam 2, SOLUTIONS
 Discrete Structures: Sample Questions, Exam 2, SOLUTIONS (This is longer than the actual test.) 1. Show that any postage of 8 cents or more can be achieved by using only -cent and 5-cent stamps. We proceed
Discrete Structures: Sample Questions, Exam 2, SOLUTIONS (This is longer than the actual test.) 1. Show that any postage of 8 cents or more can be achieved by using only -cent and 5-cent stamps. We proceed
r 4 r 2 q 2 r 3 V () r En dln (r) 1 q 1 Negative sign from integration of E field cancels the negative sign from the original equation.
 Question from last class Potential due to a Group of Point Charges rr V () r E dl r r 1 q 1 X rr N V () r E dl r n n N V(r) V n (r) 1 n1 4 o 1/30/2018 2 q 2 N r r N r r q n V () r En dln dr 2 n n r n r
Question from last class Potential due to a Group of Point Charges rr V () r E dl r r 1 q 1 X rr N V () r E dl r n n N V(r) V n (r) 1 n1 4 o 1/30/2018 2 q 2 N r r N r r q n V () r En dln dr 2 n n r n r
FIITJEE PET I (REG_2 ND YEAR)
 FIITJEE PET I (REG_ ND YEAR) MAINS_SET A DATE: 09.06.018 Time: 3 hours Maximum Marks: 360 INSTRUCTINS: Instructions to the Candidates 1. This Test Booklet consists of 90 questions. Use Blue/Black ball
FIITJEE PET I (REG_ ND YEAR) MAINS_SET A DATE: 09.06.018 Time: 3 hours Maximum Marks: 360 INSTRUCTINS: Instructions to the Candidates 1. This Test Booklet consists of 90 questions. Use Blue/Black ball
NARAYANA I I T / P M T A C A D E M Y. S. No. Ans. S. No. Ans. S. No. Ans.
 NARAYANA I I T / P M T A C A D E M Y C o o n P r a c t i c e T e s t XII STD BATCHES [CF] Date: 04.07.6 ANSWER PHYSICS CHEMISTRY MATHS S. No. Ans. S. No. Ans. S. No. Ans.. (A). (C) 6. (A). (B). (D) 6.
NARAYANA I I T / P M T A C A D E M Y C o o n P r a c t i c e T e s t XII STD BATCHES [CF] Date: 04.07.6 ANSWER PHYSICS CHEMISTRY MATHS S. No. Ans. S. No. Ans. S. No. Ans.. (A). (C) 6. (A). (B). (D) 6.
Experiment Three. Lab two: Parts 2B and 3. Halogens used in Parts 2 and 3. Lab one: Parts 1 and 2A. Halogens (Family VIIA) used in Parts 2 and 3
 Experiment Three Lab one: Parts 1 and 2A Lab two: Parts 2B and 3 1 1A 1 H 1s 1 2 IIA 3 Li 2s 1 1 1 Na 3s 1 1 9 K 4s 1 3 7 Rb 5s 1 5 5 Cs 6s 1 8 7 Fr 7s 1 4 Be 2s 2 1 2 Mg 3s 2 3 IIIB 4 IVB 5 VB 6 VIB 7
Experiment Three Lab one: Parts 1 and 2A Lab two: Parts 2B and 3 1 1A 1 H 1s 1 2 IIA 3 Li 2s 1 1 1 Na 3s 1 1 9 K 4s 1 3 7 Rb 5s 1 5 5 Cs 6s 1 8 7 Fr 7s 1 4 Be 2s 2 1 2 Mg 3s 2 3 IIIB 4 IVB 5 VB 6 VIB 7
REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2)
 REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2) 1.) Why do haloalkenes under go nucleophillic substitution whereas haloarenes under go electophillic substitution. Ans. Due to more electro negative
REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2) 1.) Why do haloalkenes under go nucleophillic substitution whereas haloarenes under go electophillic substitution. Ans. Due to more electro negative
M10/4/CHEMI/SPM/ENG/TZ2/XX+ CHEMISTRY. Wednesday 12 May 2010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M10/4/CHEMI/SPM/ENG/TZ/XX+ 106116 CHEMISTRY standard level Paper 1 Wednesday 1 May 010 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
INSTRUCTIONS: Exam III. November 10, 1999 Lab Section
 CHEM 1215 Exam III John III. Gelder November 10, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1215 Exam III John III. Gelder November 10, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
Practice Problem Solutions
 Chapter 14 Fields and Forces Practice Problem Solutions Student Textbook page 638 1. Conceptualize the Problem - Force, charge and distance are related by Coulomb s law. The electrostatic force, F, between
Chapter 14 Fields and Forces Practice Problem Solutions Student Textbook page 638 1. Conceptualize the Problem - Force, charge and distance are related by Coulomb s law. The electrostatic force, F, between
CHEM Come to the PASS workshop with your mock exam complete. During the workshop you can work with other students to review your work.
 It is most beneficial to you to write this mock midterm UNDER EXAM CONDITIONS. This means: Complete the midterm in 1.5 hours. Work on your own. Keep your notes and textbook closed. Attempt every question.
It is most beneficial to you to write this mock midterm UNDER EXAM CONDITIONS. This means: Complete the midterm in 1.5 hours. Work on your own. Keep your notes and textbook closed. Attempt every question.
CHEM 10123/10125, Exam 2
 CHEM 10123/10125, Exam 2 March 7, 2012 (50 minutes) Name (please print) Please box your answers, and remember that significant figures, phases (for chemical equations), and units do count! 1. (13 points)
CHEM 10123/10125, Exam 2 March 7, 2012 (50 minutes) Name (please print) Please box your answers, and remember that significant figures, phases (for chemical equations), and units do count! 1. (13 points)
Halogens HALOGENS. Parts 2A and 2B. Chem : Feb. 19, 20 and March 3. Compare the properties and reactivity of the halogens and halides
 Chem. 125-126: Feb. 19, 20 and March 3 Experiment 3 Session 2 (Three hour lab) Complete Experiment 3 Parts 2B and 3 Complete team report Complete discussion presentation Parts 2A and 2B Compare the properties
Chem. 125-126: Feb. 19, 20 and March 3 Experiment 3 Session 2 (Three hour lab) Complete Experiment 3 Parts 2B and 3 Complete team report Complete discussion presentation Parts 2A and 2B Compare the properties
E5 Lewis Acids and Bases: lab 2. Session two lab Parts 2B, 3, and 4. Session one lab Parts 1and 2A. Aquo Complex Ions
 E5 Lewis Acids and Bases: lab 2 Session one lab Parts 1and 2A Session two lab Parts 2B, 3, and 4 Part 2B. Complexation, Structure and Periodicity Compare the reactivity of aquo complex ions containing
E5 Lewis Acids and Bases: lab 2 Session one lab Parts 1and 2A Session two lab Parts 2B, 3, and 4 Part 2B. Complexation, Structure and Periodicity Compare the reactivity of aquo complex ions containing
ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom.
 178 (MAGNETIC) SPIN QUANTUM NUMBER: "spin down" or "spin up" - An ORBITAL (region with fixed "n", "l" and "ml" values) can hold TWO electrons. ORBITAL DIAGRAM - A graphical representation of the quantum
178 (MAGNETIC) SPIN QUANTUM NUMBER: "spin down" or "spin up" - An ORBITAL (region with fixed "n", "l" and "ml" values) can hold TWO electrons. ORBITAL DIAGRAM - A graphical representation of the quantum
Chem 102H Exam 2 - Spring 2005
 Name I.D. # Chem 102H Exam 2 - Spring 2005 PHYSICAL CNSTANTS/CNVERSIN FACTRS Speed of light = 3.00! 10 8 m/s Planck!s const. = 6.63! 10-34 J s Avagadro!s Number = 6.02! 10 23 Electron charge = 1.602! 10-19
Name I.D. # Chem 102H Exam 2 - Spring 2005 PHYSICAL CNSTANTS/CNVERSIN FACTRS Speed of light = 3.00! 10 8 m/s Planck!s const. = 6.63! 10-34 J s Avagadro!s Number = 6.02! 10 23 Electron charge = 1.602! 10-19
What is the major product of the following reaction?
 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
9 RELATIONS. 9.1 Reflexive, symmetric and transitive relations. MATH Foundations of Pure Mathematics
 MATH10111 - Foundations of Pure Mathematics 9 RELATIONS 9.1 Reflexive, symmetric and transitive relations Let A be a set with A. A relation R on A is a subset of A A. For convenience, for x, y A, write
MATH10111 - Foundations of Pure Mathematics 9 RELATIONS 9.1 Reflexive, symmetric and transitive relations Let A be a set with A. A relation R on A is a subset of A A. For convenience, for x, y A, write
Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas.
 Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas. On the other hand, aniline reacts with HNO2 at a low temperature to
Benzylamine reacts with nitrous acid to form unstable diazonium salt, which in turn gives alcohol with the evolution of nitrogen gas. On the other hand, aniline reacts with HNO2 at a low temperature to
Atomic Structure & Interatomic Bonding
 Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 READ ALL THE INSTRUCTIONS CAREFULLY
 TUESDAY MARCH 3rd, 2015 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
TUESDAY MARCH 3rd, 2015 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
Allyl radicals are especially stable due to resonance ( and double bond switch places):
 Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
Ch 10 Alkyl Halides Nomenclature Rules The parent is the longest alkyl chain or ring. The #1 C for a chain is at the end that is nearest to the first substituent. The #1 C for a ring possesses the first
AQA A2 CHEMISTRY TOPIC 5.3 REDOX EQUILIBRIA BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 5.3 REDOX EQUILIBRIA BOOKLET OF PAST EXAMINATION QUESTIONS 1. (a) Define the term oxidising agent in terms of electrons.... 2. Use the data in the table below, where appropriate,
AQA A2 CHEMISTRY TOPIC 5.3 REDOX EQUILIBRIA BOOKLET OF PAST EXAMINATION QUESTIONS 1. (a) Define the term oxidising agent in terms of electrons.... 2. Use the data in the table below, where appropriate,
Circle the letters only. NO ANSWERS in the Columns! (3 points each)
 Chemistry 1304.001 Name (please print) Exam 4 (100 points) April 12, 2017 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 1304.001 Name (please print) Exam 4 (100 points) April 12, 2017 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
-"l" also contributes ENERGY. Higher values for "l" mean the electron has higher energy.
 175 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
175 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
If anything confuses you or is not clear, raise your hand and ask!
 CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
CHEMICAL COMPOUNDS MOLECULAR COMPOUNDS
 48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
Chemistry Standard level Paper 1
 M15/4/CHEMI/SPM/ENG/TZ1/XX Chemistry Standard level Paper 1 Thursday 14 May 2015 (afternoon) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all
M15/4/CHEMI/SPM/ENG/TZ1/XX Chemistry Standard level Paper 1 Thursday 14 May 2015 (afternoon) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all
CHEM 167 FINAL EXAM MONDAY, MAY 2 9:45 11:45 A.M GILMAN HALL
 PROF. JOHN VERKADE SPRING 2005 THIS EXAM CONSISTS OF 12 QUESTIONS ON 9 PAGES CHEM 167 HOUR EXAM IV APRIL 20, 2005 SEAT NO. NAME RECIT. INSTR. RECIT. SECT. GRADING PAGE Page 2 Page 3 Page 4 Page 5 Page
PROF. JOHN VERKADE SPRING 2005 THIS EXAM CONSISTS OF 12 QUESTIONS ON 9 PAGES CHEM 167 HOUR EXAM IV APRIL 20, 2005 SEAT NO. NAME RECIT. INSTR. RECIT. SECT. GRADING PAGE Page 2 Page 3 Page 4 Page 5 Page
HALOALKANES AND HALOARENES
 Unit - 10 HALOALKANES AND HALOARENES 1. Write the IUPAC names of the following compounds. Br CH = CH C CH (vi) (vii) (ix) (CCl 3 ) 3 CCl 103 XII Chemistry 2. Write the structure of following halogen compounds
Unit - 10 HALOALKANES AND HALOARENES 1. Write the IUPAC names of the following compounds. Br CH = CH C CH (vi) (vii) (ix) (CCl 3 ) 3 CCl 103 XII Chemistry 2. Write the structure of following halogen compounds
Circle the letters only. NO ANSWERS in the Columns!
 Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
FIITJEE PET II (REG_2 ND YEAR)
 FIITJEE PET II (REG_ ND YEAR) MAINS DATE: 17.06.017 Time: hours Maximum Marks: 60 INSTRUCTIONS: 1. This Test Booklet consists of 90 questions. Instructions to the Candidates Use Blue/Black ball Point Pen
FIITJEE PET II (REG_ ND YEAR) MAINS DATE: 17.06.017 Time: hours Maximum Marks: 60 INSTRUCTIONS: 1. This Test Booklet consists of 90 questions. Instructions to the Candidates Use Blue/Black ball Point Pen
Physics 1202: Lecture 3 Today s Agenda
 Physics 1202: Lecture 3 Today s Agenda Announcements: Lectures posted on: www.phys.uconn.edu/~rcote/ HW assignments, solutions etc. Homework #1: On Masterphysics: due this coming Friday Go to the syllabus
Physics 1202: Lecture 3 Today s Agenda Announcements: Lectures posted on: www.phys.uconn.edu/~rcote/ HW assignments, solutions etc. Homework #1: On Masterphysics: due this coming Friday Go to the syllabus
Acids and Bases. Acids and Bases
 BrØnsted-Lowry A BrØnsted-Lowry acid is a proton donor. A BrØnsted-Lowry base is a proton acceptor. H + = proton BrØnsted-Lowry Some molecules contain both hydrogen atoms and lone pairs and thus, can act
BrØnsted-Lowry A BrØnsted-Lowry acid is a proton donor. A BrØnsted-Lowry base is a proton acceptor. H + = proton BrØnsted-Lowry Some molecules contain both hydrogen atoms and lone pairs and thus, can act
Chemistry Standard level Paper 1
 Chemistry Standard level Paper 1 Thursday 12 May 2016 (morning) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all the questions. For each question,
Chemistry Standard level Paper 1 Thursday 12 May 2016 (morning) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all the questions. For each question,
Chemistry 101 Chapter 9 CHEMICAL BONDING. Chemical bonds are strong attractive force that exists between the atoms of a substance
 CHEMICAL BONDING Chemical bonds are strong attractive force that exists between the atoms of a substance Chemical Bonds are commonly classified into 3 types: 1. IONIC BONDING Ionic bonds usually form between
CHEMICAL BONDING Chemical bonds are strong attractive force that exists between the atoms of a substance Chemical Bonds are commonly classified into 3 types: 1. IONIC BONDING Ionic bonds usually form between
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
PERIODIC TABLE OF THE ELEMENTS
 Useful Constants and equations: K = o C + 273 Avogadro's number = 6.022 x 10 23 d = density = mass/volume R H = 2.178 x 10-18 J c = E = h = hc/ h = 6.626 x 10-34 J s c = 2.998 x 10 8 m/s E n = -R H Z 2
Useful Constants and equations: K = o C + 273 Avogadro's number = 6.022 x 10 23 d = density = mass/volume R H = 2.178 x 10-18 J c = E = h = hc/ h = 6.626 x 10-34 J s c = 2.998 x 10 8 m/s E n = -R H Z 2
Electrochemistry Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry
 2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
2012 Pearson Education, Inc. Mr. Matthew Totaro Legacy High School AP Chemistry Electricity from Chemistry Many chemical reactions involve the transfer of electrons between atoms or ions electron transfer
8. Relax and do well.
 CHEM 1225 Exam III John III. Gelder April 8, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last two pages includes a periodic table and
CHEM 1225 Exam III John III. Gelder April 8, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last two pages includes a periodic table and
CHEM 107 (Spring-2005) Exam 3 (100 pts)
 CHEM 107 (Spring-2005) Exam 3 (100 pts) Name: ------------------------------------------------------------------------, Clid # ------------------------------ LAST NAME, First (Circle the alphabet segment
CHEM 107 (Spring-2005) Exam 3 (100 pts) Name: ------------------------------------------------------------------------, Clid # ------------------------------ LAST NAME, First (Circle the alphabet segment
The exam must be written in ink. No calculators of any sort allowed. You have 2 hours to complete the exam. Periodic table 7 0
 Email: The exam must be written in ink. No calculators of any sort allowed. You have 2 hours to complete the exam. CEM 610B Exam 3 Spring 2002 Instructor: Dr. Brian Pagenkopf Page Points 2 6 3 7 4 9 5
Email: The exam must be written in ink. No calculators of any sort allowed. You have 2 hours to complete the exam. CEM 610B Exam 3 Spring 2002 Instructor: Dr. Brian Pagenkopf Page Points 2 6 3 7 4 9 5
7. Relax and do well.
 CHEM 1014 Exam III John III. Gelder November 18, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1014 Exam III John III. Gelder November 18, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
Class XII: Chemistry Chapter 13: Amines Top concepts
 Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr
 Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
8. Relax and do well.
 CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
Advanced Placement. Chemistry. Integrated Rates
 Advanced Placement Chemistry Integrated Rates 204 47.90 9.22 78.49 (26) 50.94 92.9 80.95 (262) 52.00 93.94 83.85 (263) 54.938 (98) 86.2 (262) 55.85 0. 90.2 (265) 58.93 02.9 92.2 (266) H Li Na K Rb Cs Fr
Advanced Placement Chemistry Integrated Rates 204 47.90 9.22 78.49 (26) 50.94 92.9 80.95 (262) 52.00 93.94 83.85 (263) 54.938 (98) 86.2 (262) 55.85 0. 90.2 (265) 58.93 02.9 92.2 (266) H Li Na K Rb Cs Fr
Organic Reactions Susbstitution S N. Dr. Sapna Gupta
 Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Organic Reactions Susbstitution S N 2 Dr. Sapna Gupta Kinetics of Nucleophilic Reaction Rate law is order of reaction 0 order is when rate of reaction is unaffected by change in concentration of the reactants
Real Analysis. Joe Patten August 12, 2018
 Real Analysis Joe Patten August 12, 2018 1 Relations and Functions 1.1 Relations A (binary) relation, R, from set A to set B is a subset of A B. Since R is a subset of A B, it is a set of ordered pairs.
Real Analysis Joe Patten August 12, 2018 1 Relations and Functions 1.1 Relations A (binary) relation, R, from set A to set B is a subset of A B. Since R is a subset of A B, it is a set of ordered pairs.
Chem 401 Unit 2 Exam Spr 2018 (Acids/ Bases/ General Equilibria /Acid-Base Equilibria)
 Name: Date: Exam #: _ Chem 401 Unit 2 Exam Spr 2018 (Acids/ Bases/ General Equilibria /Acid-Base Equilibria) Multiple Choice Identify the letter of the choice that best completes the statement or answers
Name: Date: Exam #: _ Chem 401 Unit 2 Exam Spr 2018 (Acids/ Bases/ General Equilibria /Acid-Base Equilibria) Multiple Choice Identify the letter of the choice that best completes the statement or answers
(please print) (1) (18) H IIA IIIA IVA VA VIA VIIA He (2) (13) (14) (15) (16) (17)
 CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEMICAL COMPOUNDS MOLECULAR COMPOUNDS
 48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
48 CHEMICAL COMPOUNDS - Dalton's theory does not mention this, but there is more than one way for atoms to come together to make chemical compounds! - There are TWO common kinds of chemical compound, classified
CBSE Class-12 Chemistry Quick Revision Notes Chapter-10: Haloalkanes and Haloarenes
 CBSE Class-12 Chemistry Quick Revision Notes Chapter-10: Haloalkanes and Haloarenes Nature of C-X bond in alkyl halides: X is more electronegative than carbon. So, the C-X bond is polarized with C having
CBSE Class-12 Chemistry Quick Revision Notes Chapter-10: Haloalkanes and Haloarenes Nature of C-X bond in alkyl halides: X is more electronegative than carbon. So, the C-X bond is polarized with C having
30 Zn(s) 45 Rh. Pd(s) Ag(s) Cd(s) In(s) Sn(s) white. 77 Ir. Pt(s) Au. Hg(l) Tl. 109 Mt. 111 Uuu. 112 Uub. 110 Uun. 65 Tb. 62 Sm. 64 Gd. 63 Eu.
 Enthalpy changes: experimentally it is much easier to measure heat flow at const pressure - this is enthalpy q p = )H : also nearly all chemical reactions are done at constant pressure. Enthalpy (heat)
Enthalpy changes: experimentally it is much easier to measure heat flow at const pressure - this is enthalpy q p = )H : also nearly all chemical reactions are done at constant pressure. Enthalpy (heat)
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Made the FIRST periodic table
 Made the FIRST periodic table 1869 Mendeleev organized the periodic table based on the similar properties and relativities of certain elements Later, Henri Moseley organized the elements by increasing
Made the FIRST periodic table 1869 Mendeleev organized the periodic table based on the similar properties and relativities of certain elements Later, Henri Moseley organized the elements by increasing
Guide to the Extended Step-Pyramid Periodic Table
 Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Electric Forces. For a field force, they do not need to touch and force can exist at large separation distances. Gravity is an example.
 Physics for Scientists and Engineers Foundations and Connections Advance Edition Volume 1st Edition Katz SOLUTIONS MANUAL Full clear download (no formatting errors) at: https://testbankreal.com/download/physics-scientists-engineersfoundations-connections-advance-edition-volume--1st-edition-katzsolutions-manual/
Physics for Scientists and Engineers Foundations and Connections Advance Edition Volume 1st Edition Katz SOLUTIONS MANUAL Full clear download (no formatting errors) at: https://testbankreal.com/download/physics-scientists-engineersfoundations-connections-advance-edition-volume--1st-edition-katzsolutions-manual/
Narayana IIT Academy INDIA Sec: Sr. IIT_IZ Jee-Advanced Date: Time: 02:00 PM to 05:00 PM 2013_P2 MODEL Max.Marks:180
 INDIA Sec: Sr. IIT_IZ Jee-Advanced Date: 9--7 Time: 0:00 PM to 05:00 PM 0_P MODEL Max.Marks:80 KEY SHEET PHYSICS B ABC BCD 4 CD 5 BD D 7 ABD 8 ABC 9 C 0 B C C A 4 B 5 A C 7 A 8 B 9 D 0 B CHEMISTRY ABCD
INDIA Sec: Sr. IIT_IZ Jee-Advanced Date: 9--7 Time: 0:00 PM to 05:00 PM 0_P MODEL Max.Marks:80 KEY SHEET PHYSICS B ABC BCD 4 CD 5 BD D 7 ABD 8 ABC 9 C 0 B C C A 4 B 5 A C 7 A 8 B 9 D 0 B CHEMISTRY ABCD
M14/4/CHEMI/SPM/ENG/TZ1/XX CHEMISTRY. Monday 19 May 2014 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M14/4/CEMI/SPM/ENG/TZ1/XX 22146110 CEMISTRY standard level Paper 1 Monday 19 May 2014 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
M14/4/CEMI/SPM/ENG/TZ1/XX 22146110 CEMISTRY standard level Paper 1 Monday 19 May 2014 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so. Answer
CHEM 303 Organic Chemistry II Problem Set III Chapter 14 Answers
 CHEM 303 rganic Chemistry II Problem Set III Chapter 14 Answers 1) Give the major products of each of the following reactions. If a mixture is expected, identify the major product. + H 3 CHC CHCH 3 H 2
CHEM 303 rganic Chemistry II Problem Set III Chapter 14 Answers 1) Give the major products of each of the following reactions. If a mixture is expected, identify the major product. + H 3 CHC CHCH 3 H 2
Speed of light c = m/s. x n e a x d x = 1. 2 n+1 a n π a. He Li Ne Na Ar K Ni 58.
 Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
Reaction chemistry of complexes Three general forms: 1. Reactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b.
 eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
RED. Fall 2016 Student Submitted Sample Questions
 RED Fall 2016 Student Submitted Sample Questions Name: Last Update: November 22, 2016 The questions are divided into three sections: True-false, Multiple Choice, and Written Answer. I will add questions
RED Fall 2016 Student Submitted Sample Questions Name: Last Update: November 22, 2016 The questions are divided into three sections: True-false, Multiple Choice, and Written Answer. I will add questions
ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom.
 160 ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom. 4p 3d 4s 3p 3s 2p 2s 1s Each blank represents an ORBITAL, and can hold two electrons. The 4s subshell
160 ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom. 4p 3d 4s 3p 3s 2p 2s 1s Each blank represents an ORBITAL, and can hold two electrons. The 4s subshell
Q1. A wave travelling along a string is described by
 Coordinator: Saleem Rao Wednesday, May 24, 2017 Page: 1 Q1. A wave travelling along a string is described by y( x, t) = 0.00327 sin(72.1x 2.72t) In which all numerical constants are in SI units. Find the
Coordinator: Saleem Rao Wednesday, May 24, 2017 Page: 1 Q1. A wave travelling along a string is described by y( x, t) = 0.00327 sin(72.1x 2.72t) In which all numerical constants are in SI units. Find the
HANDOUT SET GENERAL CHEMISTRY II
 HANDOUT SET GENERAL CHEMISTRY II Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
HANDOUT SET GENERAL CHEMISTRY II Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
SCH4U1 Atomic & Molecular Structure Test Review
 SCH4U1 Atomic & Molecular Structure Test Review 1. Which object(s) would you use to describe the shape of the 2p orbital? a. a dumb-bell b. a circle c. a sphere d. two perpendicular dumb-bells e. a doughnut
SCH4U1 Atomic & Molecular Structure Test Review 1. Which object(s) would you use to describe the shape of the 2p orbital? a. a dumb-bell b. a circle c. a sphere d. two perpendicular dumb-bells e. a doughnut
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Discrete Structures: Sample Questions, Exam 2, SOLUTIONS
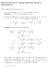 Discrete Structures: Sample Questions, Exam, SOLUTIONS This is longer than the actual test.) 1. List all the strings over X = {a, b, c} of length or less. λ, a, b, c, aa, ab, ac, ba, bb, bc, ca, cb, cc..
Discrete Structures: Sample Questions, Exam, SOLUTIONS This is longer than the actual test.) 1. List all the strings over X = {a, b, c} of length or less. λ, a, b, c, aa, ab, ac, ba, bb, bc, ca, cb, cc..
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals.
 2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
