Practice of SAS Logistic Regression on Binary Pharmacodynamic Data Problems and Solutions. Alan J Xiao, Cognigen Corporation, Buffalo NY
|
|
|
- Lizbeth Meredith Douglas
- 6 years ago
- Views:
Transcription
1 Practice of SAS Logistic Regression on Binary Pharmacodynamic Data Problems and Solutions Alan J Xiao, Cognigen Corporation, Buffalo NY ABSTRACT Logistic regression has been widely applied to population pharmacodynamic analyses of dose-response (binary efficacy or safety endpoints). Limited by the model structure p(y) = exp(y)/(1+exp(y)), where p(y) is the response probability and y = logit(x) is a function of the explanatory variable vector x (usually drug exposure and other covariates), direct use of this procedure to some special data might yield misleading results. Although the logarithm (and other) transformations of explanatory variables can expand the use of logistic regression to those types of data, eligible explanatory variables for transformation cannot include those with zero values, such as dose or drug exposure in placebo subjects. This could make the analysis even more difficult, especially when all dosages lie near or at the plateau of the response. An alternative solution may be the use of PROC NLIN to model, as a continuous function, the outcome probabilities at each level of the explanatory variable. A dose-response case study with a limited number of treatment groups, including placebo, were illustrated, where alternative methods of modeling were better implemented in PROC NLIN. Utilization of SAS Logistic or alternative approaches requires thorough understanding of these procedures, the underlying methodology, data features, and the physiological meaning of variables. Keyword: Logistic regression, nonlinear, log transformation INTRODUCTION Exposure response relationship is very important in evaluating the efficacy and safety of a drug. The response, as a pharmacodynamic (PD) endpoint in both efficacy and safety studies for a drug, is frequently recorded as binary data. The exposure may refer to dose, drug or metabolite concentrations, or AUC (area under concentration-time profile) values. The exposure response relationship is frequently modeled using the method of logistic regression, such as SAS PROC LOGISTIC 1. In logistic regression, the basic model structure is illustrated as in Equation 1: p(y) = exp(y)/(1+exp(y)) (1) It assumes that the response probability (p) of a patient to y (a function of exposure, such as dose) is always from 0 to 1 in an S-shape pattern, as illustrated in Figure 1. This paper will discuss the application of the basic model defined by Equation 1 to pharmacodynamic data in different approaches. To simplify the description, a simulated case study was introduced in this paper. In this study, 400 patients were evenly grouped to take a daily dose of 0 (placebo), 2, 4 and 8 mg of a hypothetical drug. At the end of treatment, 12, 41, 41 and 54 patients (out of 100) in each group were observed to have response (yes) to a PD endpoint. That is to say, the population response probability was 0.12, 0.41, 0.41 and 0.54, respectively. Response probability p(y) Figure 1. The shape of the curve of p(y) versus y in Equation 1. ORDINARY LOGISTIC REGRESSION y When Equation 1 is actually applied to pharamcodynamic endpoints of a drug, y is usually a function of exposure variables such as dose or concentrations or AUC values. In the case of ordinary logistic regression, y is assumed to be a linear function of exposure (x), as expressed in Equation 2. y = intercept + coeff*x (2)
2 Substitution of y in Equation 1 with Equation 2 leads to: p(x) = B*exp(coeff*x)/(1+B*exp(coeff*x)) (3) where B = exp(intercept). Ordinary logistic regression is therefore to obtain the estimates of intercept and coeff in Equation 2 (expressed as the logit function in SAS ) by fitting Equations 3 to the exposure response data. Obviously, Equation 1 represents a special case of Equation 3 with intercept=0 (thus B=1) and coeff=1. When coeff = 1 while intercept 0 (then B 1), the curve of p(x) vs. x is equivalent to shift the curve of p(y) vs. y with a distance of intercept to the right (if intercept<0) or to the left (if intercept>0) in Figure 1. Therefore, the curves of p(x) vs. x and p(y) vs. y in this case have exactly the same shape: S-shape, in a regular coordinate system. Generally, the curve of p(x) vs. x has a different steepness (determined by coeff) and x 50 (defined as the x value at which the response probability is 0.5 and determined by B and coeff) than the curve of p(y) vs. y even though both have the same S-shape. Note that, in practice where ordinary logistic regression applies, the S- shape does not necessarily appear in the exposureresponse graph if the measured exposure (x) range is beyond the inflection point (x i = -intercept/coeff), i.e., x x i, where response probability p(x i ) 0.5. of odds ratios from logistic regression with log transformation were investigated by Keen 2 and Elswick Jr. et al 3, respectively. Assuming: y = a + b*log(x) (4) where a, b, x are intercept, coefficient and exposure, e.g., dose, respectively. The probability function (Equation 1) becomes: p(x) = A*x b /(1+A*x b ) (5) where A = exp(a). Note that when x=c, b=γ and A=1/C 50 γ, this equation becomes the same equation as that for the concentration-response probability relationship used by Bailey and Gregg 4 for investigating the inter-patient variability (Probit regression). When ordinary logistic regression is applied to the case study, the parameters (as in Equation 2) are estimated as (mean±standard deviation): intercept = ±0.178 and coeff = 0.200± The model predictions and measurements are illustrated in Figure 2. As is shown, the model-predictions of the response probability at placebo and the dosage of 2 mg are at least 0.1 off from the measurements (with a relative standard deviation up to 50% or 25%). In addition, the predicted S-shape dose-response relationship seems not to sufficiently agree with the measured C-shape relationship where measurements at all dose levels seem to be near or at the plateau of the curve. Since only four dose levels including placebo are available to develop a dose-response relationship and all three dose levels appear to be near or at the plateau of the response curve, it is worthwhile to explore other methods of developing the dose-response relationship. LOGISTIC REGRESSION WITH LOGARITHM TRANSFORMATION Log transformation expands logistic regression analysis from S-shape curves to C-shape curves and the interpretation of parameter estimates is different Characteristics of log transformation and interpretation Figure 2. Response probability versus dose for the case study. Pred_logo in the legend represents model predictions from ordinary logistic regression. In a regular coordinate system, the curve of p(x) vs. x expressed by Equation 5 is a C-shape curve when b 1 or an S-shape curve when b > 1. The maximum probability is 1 (when x infinity). Also note that, the S-shape of the curve may not appear in a graph of response probability vs. exposure if the exposure range (x) is beyond the inflection point (x i = [exp(-a)(b- 1)/(b+1)] 1/b ), i.e., x x i where the response probability p(x) (b-1)/(2b). 2
3 For the case study, since only four dose levels including placebo are used in the clinical trial, direct log transformation will result in discarding of the data point for placebo. Thus, only three dose levels were available for regression. The model inference based on these three dose levels would be misleading the predicted response probability is constant across the dose range (not shown in Figure 3), as indicated by the parameter estimates in the last row in Table 1. At least, the model-predictions of constant probability (0.45) should not be extrapolated to placebo. closer to the measurement the model-predicted probability at placebo. When the perturbation is less than 10-6, the model-predicted dose-response curves are visually indistinguishable, although their parameter estimates in logistic model are different. Table 1 lists parameter estimates of the logistic regression model after log transformation with different perturbation levels to dose values. Visually, the fitting by logistic regression with log transformation and perturbation is slightly better than that by ordinary logistic regression. However, the approximation for placebo using perturbation approach is tentative and might not be readily accepted. As a matter of fact, this approximation can actually be avoided if Equation 5 is directly used to fit the data, instead of utilizing log transformation in logistic regression (Equation 1 plus 4). This nonlinear modeling can be implemented with SAS PROC NLIN, which is extensively applied in engineering. Table 1. The Parameter Estimates for A Logistic Model after Log Transformation (refer to Equation 4) Figure 3. Dose-response relationship fit by logistic regression with log transformation of dose. Pred_log2, Pred_log4 and Pred_log6 are model-predictions under 3 different perturbation levels: dose = dose+0.01, dose , or dose , respectively, in order to implement the log transformation on placebo. Perturbation level to doses a (0.115) Placebo excluded (0.116) b (0.053) (0.031) (0.022) 0 Therefore, when all response measurements are near or at plateau of the exposure-response curve, the data point that represents placebo is critical for analysis and should be included. To do this, a small perturbation to doses for log transformation might be helpful. For example, can be added to all dose levels, including placebo. Thus, the value of log transformation of placebo will be 13.8 (natural logarithm) while the values of log transformation of dose 2, 4, and 8 are actually not changed. When this strategy is used, the model predictions are reasonably consistent with measurements. Figure 3 demonstrates the fit of the model (Equation 5) with logistic regression after log transformation (Equation 4) with different levels of perturbation on doses. Stepwise selection of covariates was used with entry criterion of p=0.05 and elimination criterion of p=0.01. Generally, the smaller the perturbation, the PROC NLIN PROCEDURE When the regression model or the shape of the exposureresponse curve is known, SAS PROC NLIN is a good option for nonlinear models, including those for PD data. Actually, the dose-response curve shape for the case study can be described with the following general model structure: p(x) = α + β*x γ /(δ+ x γ ) (6) Equation 5 represents just a special case of Equation 6 when α=0, β=1, γ=b and δ=a -1. Similar to Equation 5, Equation 6 also represents 2 different shapes of curves: C-shape when γ 1 and S-shape otherwise, with the inflection point at x i = [β(γ-1)/(1+γ)] 1/γ. Therefore, 3
4 exposure-response models, which can be developed through PROC LOGISTIC with log transformation, can be potentially developed through PROC NLIN without any transformation. In addition, PROC NLIN can work on more types of datasets. One advantage of using PROC NLIN is that placebo data can be directly included for analysis without any approximation treatment. model. However, this model means that the maximum probability is 0.6. Theoretically, it does not make sense because for whatever drug, when the drug dose goes to infinity, the response probability should approach 1 (for either efficacy or safety). In practice, it could be true that in a certain range of dosages the response probability keeps under certain value. Whether this is really true or not is confirmable from measurements of additional expanded dose levels or prior information from fundamental studies. When the maximum probability is constrained to 1, the fitting is slightly worse, but it is at least comparable to, if not better than, that through logistic regression with log transformation. As a comparison, Equation 5 was directly used to fit the data using PROC NLIN and the model-prediction is shown in Figure 4 by the legend Pred_nlin4. The parameter estimates are (mean±standard deviation): A=0.486±0.162 and b=0.384±0.220 (refer to Equation 5). Obviously, this fit is much better than that using logistic regression with log transformation although the models are equivalent. Table 2. Parameter Estimates of the Model Expressed by Equation 6 via SAS PROC NLIN Procedure Figure 4. Model predictions of exposure-response relationship (Equation 6) with γ=1 under different conditions: Pred_nlin1 all three parameters α, β and δ are estimated from fitting; Pred_nlin2 α and δ are estimated from fitting while β is fixed as 1; Pred_nlin3 only δ is estimated from fitting while α is fixed as 0.12, referring to the measured response probability for the placebo, and β is fixed as 1. Pred_nlin4 model (Equation 5) fitting using PROC NLIN. Modeling α, β Nlin (0.046) Nlin α (0.070) fixed Nlin fixed 1 - α fixed δ (1.272) 7.95 (3.54) 9.02 (1.75) For the case study, a reduced function of Equation 6 with γ=1was tried. Different from logistic regression which directly uses raw binary data (0 or 1) for the response variable (dichotomous), the response variable in nonlinear models are continuous whose values are calculated subpopulation probabilities (from 0 to 1) at each exposure level and/or covariate group. Since no covariate is identified significant for this particular case study during logistic regression, the response probability is therefore simply calculated from the subpopulation at each dose level. The fittings by the model via PROC NLIN under 3 conditions are illustrated in Figure 4 while their parameter estimates are listed in Table 2. As expected, when all parameters are obtained from regression, the fitting is the best (refer to Pred_nlin1 in Figure 4) since more parameters are included in the MODEL/METHOD SELECTION As discussed previously, for the case study in this paper, there are at least three potential approaches to model the limited data: ordinary logistic regression, logistic regression with log transformation, and nonlinear model fitting. Each of them has advantages and disadvantages. The quality of fitting is also different, as demonstrated in Figure 5. If the measurements are representative and reliable, i.e. close to the true values, the model with the best prediction is the best. For this particular case study, general nonlinear model through PROC NLIN is superior to logistic regressions since the model (refer to Prednlin1 in Figure 5) is best fitting to the data. 4
5 REFERENCE 1. SAS Institute Inc. SAS/STAT User s Guide, Volume 2. Version 6, 4 th edition. Cary, NC: SAS Institute Inc., N Keene. The Log Transformation Is Special. Statistics in Medicine, Vol. 14, (1995). 3. R K Elswick, Jr, P F Schwartz and J A Welsh. Interpretation of the Odds Ratio from Logistic Regression after A Transformation of the Covariate Vector. Statistics in Medicine, Vol. 16, (1997). 4. J M Bailey and K M Gregg. A Technique for Population Pharmacodynamic Analysis of Concentration-Binary Response Data. Anaesthesiology1997; 86: Figure 5. Comparison of the quality of fitting for three different approaches: Pred_nlin1 totally free nonlinear model (Equation 6 with γ=1) fitting using PROC NLIN; Pred_nlin4 nonlinear model (Equation 5) fitting via PROC NLIN; Pred_logo ordinary logistic regression via PROC LOGISTIC; and Pred_log6 logistic regression after log transformation on doses plus approximating placebo with a dosage of SUMMARY Logistic regression is a powerful tool widely used to perform PD data analysis. However, its applicability is limited by its strict assumptions inherited in the model structure. Although log transformation can expand the application of logistic regression, the transformation process itself might restrict this expansion when placebo data has to be included for analysis. Nonlinear modeling through PROC NLIN is generally a more flexible and powerful approach. However, prior information about the model structure is required and whether PROC NLIN is successful or not sometime depends on the model structure and appropriateness of the initial guesses for parameter estimates. Exploratory graphs of exposure response probability should be helpful to select the primary methods and models and logistic regression could be a convenient primary option. However, when logistic regression cannot work very well, alternative methods should be explored. The selection of the final model should be based on the combined information about the characteristics of data, quality of fitting and physiological rationale. SAS and all other SAS Institute Inc. product r service names are registered trademarks or trade marks of SAS Institute Inc. in the USA and other countries. indicates USA registration. Other brand and product names are registered trademarks or trademarks of their respective companies. DISCLAIMER This presentation only reflects my current personal thinking on potential alternative approaches to PD data analysis when logistic regression as a primary option cannot work well, based on my experience in engineering area. So far, none of these approaches has yet been applied to any real projects. No material in this presentation is from Cognigen Corporation. Under no circumstances should this presentation be related to the position that Cognigen Corporation takes on PD data analysis. CONTACT INFORMATION The author can be contacted at: Alan J Xiao, Ph.D. Population PK/PD Scientist Cognigen Corporation 395 Youngs Road Buffalo, NY Phone: ext. 265 Fax: alan.xiao@cognigencorp.com Web: 5
Integration of SAS and NONMEM for Automation of Population Pharmacokinetic/Pharmacodynamic Modeling on UNIX systems
 Integration of SAS and NONMEM for Automation of Population Pharmacokinetic/Pharmacodynamic Modeling on UNIX systems Alan J Xiao, Cognigen Corporation, Buffalo NY Jill B Fiedler-Kelly, Cognigen Corporation,
Integration of SAS and NONMEM for Automation of Population Pharmacokinetic/Pharmacodynamic Modeling on UNIX systems Alan J Xiao, Cognigen Corporation, Buffalo NY Jill B Fiedler-Kelly, Cognigen Corporation,
Paper: ST-161. Techniques for Evidence-Based Decision Making Using SAS Ian Stockwell, The Hilltop UMBC, Baltimore, MD
 Paper: ST-161 Techniques for Evidence-Based Decision Making Using SAS Ian Stockwell, The Hilltop Institute @ UMBC, Baltimore, MD ABSTRACT SAS has many tools that can be used for data analysis. From Freqs
Paper: ST-161 Techniques for Evidence-Based Decision Making Using SAS Ian Stockwell, The Hilltop Institute @ UMBC, Baltimore, MD ABSTRACT SAS has many tools that can be used for data analysis. From Freqs
Fitting PK Models with SAS NLMIXED Procedure Halimu Haridona, PPD Inc., Beijing
 PharmaSUG China 1 st Conference, 2012 Fitting PK Models with SAS NLMIXED Procedure Halimu Haridona, PPD Inc., Beijing ABSTRACT Pharmacokinetic (PK) models are important for new drug development. Statistical
PharmaSUG China 1 st Conference, 2012 Fitting PK Models with SAS NLMIXED Procedure Halimu Haridona, PPD Inc., Beijing ABSTRACT Pharmacokinetic (PK) models are important for new drug development. Statistical
ONE MORE TIME ABOUT R 2 MEASURES OF FIT IN LOGISTIC REGRESSION
 ONE MORE TIME ABOUT R 2 MEASURES OF FIT IN LOGISTIC REGRESSION Ernest S. Shtatland, Ken Kleinman, Emily M. Cain Harvard Medical School, Harvard Pilgrim Health Care, Boston, MA ABSTRACT In logistic regression,
ONE MORE TIME ABOUT R 2 MEASURES OF FIT IN LOGISTIC REGRESSION Ernest S. Shtatland, Ken Kleinman, Emily M. Cain Harvard Medical School, Harvard Pilgrim Health Care, Boston, MA ABSTRACT In logistic regression,
The SEQDESIGN Procedure
 SAS/STAT 9.2 User s Guide, Second Edition The SEQDESIGN Procedure (Book Excerpt) This document is an individual chapter from the SAS/STAT 9.2 User s Guide, Second Edition. The correct bibliographic citation
SAS/STAT 9.2 User s Guide, Second Edition The SEQDESIGN Procedure (Book Excerpt) This document is an individual chapter from the SAS/STAT 9.2 User s Guide, Second Edition. The correct bibliographic citation
RANDOM and REPEATED statements - How to Use Them to Model the Covariance Structure in Proc Mixed. Charlie Liu, Dachuang Cao, Peiqi Chen, Tony Zagar
 Paper S02-2007 RANDOM and REPEATED statements - How to Use Them to Model the Covariance Structure in Proc Mixed Charlie Liu, Dachuang Cao, Peiqi Chen, Tony Zagar Eli Lilly & Company, Indianapolis, IN ABSTRACT
Paper S02-2007 RANDOM and REPEATED statements - How to Use Them to Model the Covariance Structure in Proc Mixed Charlie Liu, Dachuang Cao, Peiqi Chen, Tony Zagar Eli Lilly & Company, Indianapolis, IN ABSTRACT
Hierarchical expectation propagation for Bayesian aggregation of average data
 Hierarchical expectation propagation for Bayesian aggregation of average data Andrew Gelman, Columbia University Sebastian Weber, Novartis also Bob Carpenter, Daniel Lee, Frédéric Bois, Aki Vehtari, and
Hierarchical expectation propagation for Bayesian aggregation of average data Andrew Gelman, Columbia University Sebastian Weber, Novartis also Bob Carpenter, Daniel Lee, Frédéric Bois, Aki Vehtari, and
A SAS/AF Application For Sample Size And Power Determination
 A SAS/AF Application For Sample Size And Power Determination Fiona Portwood, Software Product Services Ltd. Abstract When planning a study, such as a clinical trial or toxicology experiment, the choice
A SAS/AF Application For Sample Size And Power Determination Fiona Portwood, Software Product Services Ltd. Abstract When planning a study, such as a clinical trial or toxicology experiment, the choice
Application of Ghosh, Grizzle and Sen s Nonparametric Methods in. Longitudinal Studies Using SAS PROC GLM
 Application of Ghosh, Grizzle and Sen s Nonparametric Methods in Longitudinal Studies Using SAS PROC GLM Chan Zeng and Gary O. Zerbe Department of Preventive Medicine and Biometrics University of Colorado
Application of Ghosh, Grizzle and Sen s Nonparametric Methods in Longitudinal Studies Using SAS PROC GLM Chan Zeng and Gary O. Zerbe Department of Preventive Medicine and Biometrics University of Colorado
PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH
 PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH The First Step: SAMPLE SIZE DETERMINATION THE ULTIMATE GOAL The most important, ultimate step of any of clinical research is to do draw inferences;
PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH The First Step: SAMPLE SIZE DETERMINATION THE ULTIMATE GOAL The most important, ultimate step of any of clinical research is to do draw inferences;
STAT 7030: Categorical Data Analysis
 STAT 7030: Categorical Data Analysis 5. Logistic Regression Peng Zeng Department of Mathematics and Statistics Auburn University Fall 2012 Peng Zeng (Auburn University) STAT 7030 Lecture Notes Fall 2012
STAT 7030: Categorical Data Analysis 5. Logistic Regression Peng Zeng Department of Mathematics and Statistics Auburn University Fall 2012 Peng Zeng (Auburn University) STAT 7030 Lecture Notes Fall 2012
PROC LOGISTIC: Traps for the unwary Peter L. Flom, Independent statistical consultant, New York, NY
 Paper SD174 PROC LOGISTIC: Traps for the unwary Peter L. Flom, Independent statistical consultant, New York, NY ABSTRACT Keywords: Logistic. INTRODUCTION This paper covers some gotchas in SAS R PROC LOGISTIC.
Paper SD174 PROC LOGISTIC: Traps for the unwary Peter L. Flom, Independent statistical consultant, New York, NY ABSTRACT Keywords: Logistic. INTRODUCTION This paper covers some gotchas in SAS R PROC LOGISTIC.
GMM Logistic Regression with Time-Dependent Covariates and Feedback Processes in SAS TM
 Paper 1025-2017 GMM Logistic Regression with Time-Dependent Covariates and Feedback Processes in SAS TM Kyle M. Irimata, Arizona State University; Jeffrey R. Wilson, Arizona State University ABSTRACT The
Paper 1025-2017 GMM Logistic Regression with Time-Dependent Covariates and Feedback Processes in SAS TM Kyle M. Irimata, Arizona State University; Jeffrey R. Wilson, Arizona State University ABSTRACT The
CTP-656 Tablet Confirmed Superiority Of Pharmacokinetic Profile Relative To Kalydeco in Phase I Clinical Studies
 Tablet Confirmed Superiority Of Pharmacokinetic Profile Relative To Kalydeco in Phase I Clinical Studies 39 th European Cystic Fibrosis Conference 8-11 June 2016, Basel, Switzerland 2014 Concert Pharmaceuticals,
Tablet Confirmed Superiority Of Pharmacokinetic Profile Relative To Kalydeco in Phase I Clinical Studies 39 th European Cystic Fibrosis Conference 8-11 June 2016, Basel, Switzerland 2014 Concert Pharmaceuticals,
Marginal versus conditional effects: does it make a difference? Mireille Schnitzer, PhD Université de Montréal
 Marginal versus conditional effects: does it make a difference? Mireille Schnitzer, PhD Université de Montréal Overview In observational and experimental studies, the goal may be to estimate the effect
Marginal versus conditional effects: does it make a difference? Mireille Schnitzer, PhD Université de Montréal Overview In observational and experimental studies, the goal may be to estimate the effect
Chapter 1 Statistical Inference
 Chapter 1 Statistical Inference causal inference To infer causality, you need a randomized experiment (or a huge observational study and lots of outside information). inference to populations Generalizations
Chapter 1 Statistical Inference causal inference To infer causality, you need a randomized experiment (or a huge observational study and lots of outside information). inference to populations Generalizations
Estimating terminal half life by non-compartmental methods with some data below the limit of quantification
 Paper SP08 Estimating terminal half life by non-compartmental methods with some data below the limit of quantification Jochen Müller-Cohrs, CSL Behring, Marburg, Germany ABSTRACT In pharmacokinetic studies
Paper SP08 Estimating terminal half life by non-compartmental methods with some data below the limit of quantification Jochen Müller-Cohrs, CSL Behring, Marburg, Germany ABSTRACT In pharmacokinetic studies
STAT 5500/6500 Conditional Logistic Regression for Matched Pairs
 STAT 5500/6500 Conditional Logistic Regression for Matched Pairs The data for the tutorial came from support.sas.com, The LOGISTIC Procedure: Conditional Logistic Regression for Matched Pairs Data :: SAS/STAT(R)
STAT 5500/6500 Conditional Logistic Regression for Matched Pairs The data for the tutorial came from support.sas.com, The LOGISTIC Procedure: Conditional Logistic Regression for Matched Pairs Data :: SAS/STAT(R)
Generalized linear models for binary data. A better graphical exploratory data analysis. The simple linear logistic regression model
 Stat 3302 (Spring 2017) Peter F. Craigmile Simple linear logistic regression (part 1) [Dobson and Barnett, 2008, Sections 7.1 7.3] Generalized linear models for binary data Beetles dose-response example
Stat 3302 (Spring 2017) Peter F. Craigmile Simple linear logistic regression (part 1) [Dobson and Barnett, 2008, Sections 7.1 7.3] Generalized linear models for binary data Beetles dose-response example
Leverage Sparse Information in Predictive Modeling
 Leverage Sparse Information in Predictive Modeling Liang Xie Countrywide Home Loans, Countrywide Bank, FSB August 29, 2008 Abstract This paper examines an innovative method to leverage information from
Leverage Sparse Information in Predictive Modeling Liang Xie Countrywide Home Loans, Countrywide Bank, FSB August 29, 2008 Abstract This paper examines an innovative method to leverage information from
Qinlei Huang, St. Jude Children s Research Hospital, Memphis, TN Liang Zhu, St. Jude Children s Research Hospital, Memphis, TN
 PharmaSUG 2014 - Paper SP04 %IC_LOGISTIC: A SAS Macro to Produce Sorted Information Criteria (AIC/BIC) List for PROC LOGISTIC for Model Selection ABSTRACT Qinlei Huang, St. Jude Children s Research Hospital,
PharmaSUG 2014 - Paper SP04 %IC_LOGISTIC: A SAS Macro to Produce Sorted Information Criteria (AIC/BIC) List for PROC LOGISTIC for Model Selection ABSTRACT Qinlei Huang, St. Jude Children s Research Hospital,
Chapter 1. Modeling Basics
 Chapter 1. Modeling Basics What is a model? Model equation and probability distribution Types of model effects Writing models in matrix form Summary 1 What is a statistical model? A model is a mathematical
Chapter 1. Modeling Basics What is a model? Model equation and probability distribution Types of model effects Writing models in matrix form Summary 1 What is a statistical model? A model is a mathematical
Generating Half-normal Plot for Zero-inflated Binomial Regression
 Paper SP05 Generating Half-normal Plot for Zero-inflated Binomial Regression Zhao Yang, Xuezheng Sun Department of Epidemiology & Biostatistics University of South Carolina, Columbia, SC 29208 SUMMARY
Paper SP05 Generating Half-normal Plot for Zero-inflated Binomial Regression Zhao Yang, Xuezheng Sun Department of Epidemiology & Biostatistics University of South Carolina, Columbia, SC 29208 SUMMARY
Model Based Statistics in Biology. Part V. The Generalized Linear Model. Chapter 18.1 Logistic Regression (Dose - Response)
 Model Based Statistics in Biology. Part V. The Generalized Linear Model. Logistic Regression ( - Response) ReCap. Part I (Chapters 1,2,3,4), Part II (Ch 5, 6, 7) ReCap Part III (Ch 9, 10, 11), Part IV
Model Based Statistics in Biology. Part V. The Generalized Linear Model. Logistic Regression ( - Response) ReCap. Part I (Chapters 1,2,3,4), Part II (Ch 5, 6, 7) ReCap Part III (Ch 9, 10, 11), Part IV
Performing response surface analysis using the SAS RSREG procedure
 Paper DV02-2012 Performing response surface analysis using the SAS RSREG procedure Zhiwu Li, National Database Nursing Quality Indicator and the Department of Biostatistics, University of Kansas Medical
Paper DV02-2012 Performing response surface analysis using the SAS RSREG procedure Zhiwu Li, National Database Nursing Quality Indicator and the Department of Biostatistics, University of Kansas Medical
INFORMATION AS A UNIFYING MEASURE OF FIT IN SAS STATISTICAL MODELING PROCEDURES
 INFORMATION AS A UNIFYING MEASURE OF FIT IN SAS STATISTICAL MODELING PROCEDURES Ernest S. Shtatland, PhD Mary B. Barton, MD, MPP Harvard Medical School, Harvard Pilgrim Health Care, Boston, MA ABSTRACT
INFORMATION AS A UNIFYING MEASURE OF FIT IN SAS STATISTICAL MODELING PROCEDURES Ernest S. Shtatland, PhD Mary B. Barton, MD, MPP Harvard Medical School, Harvard Pilgrim Health Care, Boston, MA ABSTRACT
Statistics and Data Analysis
 Redesigning Experiments With Polychotomous Logistic Regression: A Power Computation Application Charles Vaughan, Xoma US, LLC, Berkeley, CA Serge Guzy, Xoma US, LLC, Berkeley, CA ABSTRACT Power and sample
Redesigning Experiments With Polychotomous Logistic Regression: A Power Computation Application Charles Vaughan, Xoma US, LLC, Berkeley, CA Serge Guzy, Xoma US, LLC, Berkeley, CA ABSTRACT Power and sample
Paper CD Erika Larsen and Timothy E. O Brien Loyola University Chicago
 Abstract: Paper CD-02 2015 SAS Software as an Essential Tool in Statistical Consulting and Research Erika Larsen and Timothy E. O Brien Loyola University Chicago Modelling in bioassay often uses linear,
Abstract: Paper CD-02 2015 SAS Software as an Essential Tool in Statistical Consulting and Research Erika Larsen and Timothy E. O Brien Loyola University Chicago Modelling in bioassay often uses linear,
Analysing categorical data using logit models
 Analysing categorical data using logit models Graeme Hutcheson, University of Manchester The lecture notes, exercises and data sets associated with this course are available for download from: www.research-training.net/manchester
Analysing categorical data using logit models Graeme Hutcheson, University of Manchester The lecture notes, exercises and data sets associated with this course are available for download from: www.research-training.net/manchester
Chapter 6. Logistic Regression. 6.1 A linear model for the log odds
 Chapter 6 Logistic Regression In logistic regression, there is a categorical response variables, often coded 1=Yes and 0=No. Many important phenomena fit this framework. The patient survives the operation,
Chapter 6 Logistic Regression In logistic regression, there is a categorical response variables, often coded 1=Yes and 0=No. Many important phenomena fit this framework. The patient survives the operation,
SAS macro to obtain reference values based on estimation of the lower and upper percentiles via quantile regression.
 SESUG 2012 Poster PO-12 SAS macro to obtain reference values based on estimation of the lower and upper percentiles via quantile regression. Neeta Shenvi Department of Biostatistics and Bioinformatics,
SESUG 2012 Poster PO-12 SAS macro to obtain reference values based on estimation of the lower and upper percentiles via quantile regression. Neeta Shenvi Department of Biostatistics and Bioinformatics,
2/26/2017. PSY 512: Advanced Statistics for Psychological and Behavioral Research 2
 PSY 512: Advanced Statistics for Psychological and Behavioral Research 2 When and why do we use logistic regression? Binary Multinomial Theory behind logistic regression Assessing the model Assessing predictors
PSY 512: Advanced Statistics for Psychological and Behavioral Research 2 When and why do we use logistic regression? Binary Multinomial Theory behind logistic regression Assessing the model Assessing predictors
Case Study in the Use of Bayesian Hierarchical Modeling and Simulation for Design and Analysis of a Clinical Trial
 Case Study in the Use of Bayesian Hierarchical Modeling and Simulation for Design and Analysis of a Clinical Trial William R. Gillespie Pharsight Corporation Cary, North Carolina, USA PAGE 2003 Verona,
Case Study in the Use of Bayesian Hierarchical Modeling and Simulation for Design and Analysis of a Clinical Trial William R. Gillespie Pharsight Corporation Cary, North Carolina, USA PAGE 2003 Verona,
Normalization of Peak Demand for an Electric Utility using PROC MODEL
 Normalization of Peak Demand for an Electric Utility using PROC MODEL Mark Harris, Jeff Brown, and Mark Gilbert* Central and South West Services, Inc. Tulsa, Oklahoma Abstract This paper discusses the
Normalization of Peak Demand for an Electric Utility using PROC MODEL Mark Harris, Jeff Brown, and Mark Gilbert* Central and South West Services, Inc. Tulsa, Oklahoma Abstract This paper discusses the
A Multistage Modeling Strategy for Demand Forecasting
 Paper SAS5260-2016 A Multistage Modeling Strategy for Demand Forecasting Pu Wang, Alex Chien, and Yue Li, SAS Institute Inc. ABSTRACT Although rapid development of information technologies in the past
Paper SAS5260-2016 A Multistage Modeling Strategy for Demand Forecasting Pu Wang, Alex Chien, and Yue Li, SAS Institute Inc. ABSTRACT Although rapid development of information technologies in the past
Lecture 13: More on Binary Data
 Lecture 1: More on Binary Data Link functions for Binomial models Link η = g(π) π = g 1 (η) identity π η logarithmic log π e η logistic log ( π 1 π probit Φ 1 (π) Φ(η) log-log log( log π) exp( e η ) complementary
Lecture 1: More on Binary Data Link functions for Binomial models Link η = g(π) π = g 1 (η) identity π η logarithmic log π e η logistic log ( π 1 π probit Φ 1 (π) Φ(η) log-log log( log π) exp( e η ) complementary
Optimal Design for Hill Model
 Optimal Design for Hill Moel DA 01 D-optimal; aaptive esign; personalize esign Russell Reeve, PhD Quintiles Durham, NC, USA Copyright 01 Quintiles Outline Motivating problem, from rheumatoi arthritis Hill
Optimal Design for Hill Moel DA 01 D-optimal; aaptive esign; personalize esign Russell Reeve, PhD Quintiles Durham, NC, USA Copyright 01 Quintiles Outline Motivating problem, from rheumatoi arthritis Hill
Binary Dependent Variables
 Binary Dependent Variables In some cases the outcome of interest rather than one of the right hand side variables - is discrete rather than continuous Binary Dependent Variables In some cases the outcome
Binary Dependent Variables In some cases the outcome of interest rather than one of the right hand side variables - is discrete rather than continuous Binary Dependent Variables In some cases the outcome
BAYESIAN ANALYSIS OF DOSE-RESPONSE CALIBRATION CURVES
 Libraries Annual Conference on Applied Statistics in Agriculture 2005-17th Annual Conference Proceedings BAYESIAN ANALYSIS OF DOSE-RESPONSE CALIBRATION CURVES William J. Price Bahman Shafii Follow this
Libraries Annual Conference on Applied Statistics in Agriculture 2005-17th Annual Conference Proceedings BAYESIAN ANALYSIS OF DOSE-RESPONSE CALIBRATION CURVES William J. Price Bahman Shafii Follow this
Multinomial Logistic Regression Models
 Stat 544, Lecture 19 1 Multinomial Logistic Regression Models Polytomous responses. Logistic regression can be extended to handle responses that are polytomous, i.e. taking r>2 categories. (Note: The word
Stat 544, Lecture 19 1 Multinomial Logistic Regression Models Polytomous responses. Logistic regression can be extended to handle responses that are polytomous, i.e. taking r>2 categories. (Note: The word
Modeling Effect Modification and Higher-Order Interactions: Novel Approach for Repeated Measures Design using the LSMESTIMATE Statement in SAS 9.
 Paper 400-015 Modeling Effect Modification and Higher-Order Interactions: Novel Approach for Repeated Measures Design using the LSMESTIMATE Statement in SAS 9.4 Pronabesh DasMahapatra, MD, MPH, PatientsLikeMe
Paper 400-015 Modeling Effect Modification and Higher-Order Interactions: Novel Approach for Repeated Measures Design using the LSMESTIMATE Statement in SAS 9.4 Pronabesh DasMahapatra, MD, MPH, PatientsLikeMe
Statistics in medicine
 Statistics in medicine Lecture 4: and multivariable regression Fatma Shebl, MD, MS, MPH, PhD Assistant Professor Chronic Disease Epidemiology Department Yale School of Public Health Fatma.shebl@yale.edu
Statistics in medicine Lecture 4: and multivariable regression Fatma Shebl, MD, MS, MPH, PhD Assistant Professor Chronic Disease Epidemiology Department Yale School of Public Health Fatma.shebl@yale.edu
More Statistics tutorial at Logistic Regression and the new:
 Logistic Regression and the new: Residual Logistic Regression 1 Outline 1. Logistic Regression 2. Confounding Variables 3. Controlling for Confounding Variables 4. Residual Linear Regression 5. Residual
Logistic Regression and the new: Residual Logistic Regression 1 Outline 1. Logistic Regression 2. Confounding Variables 3. Controlling for Confounding Variables 4. Residual Linear Regression 5. Residual
Chapter 10. Regression. Understandable Statistics Ninth Edition By Brase and Brase Prepared by Yixun Shi Bloomsburg University of Pennsylvania
 Chapter 10 Regression Understandable Statistics Ninth Edition By Brase and Brase Prepared by Yixun Shi Bloomsburg University of Pennsylvania Scatter Diagrams A graph in which pairs of points, (x, y), are
Chapter 10 Regression Understandable Statistics Ninth Edition By Brase and Brase Prepared by Yixun Shi Bloomsburg University of Pennsylvania Scatter Diagrams A graph in which pairs of points, (x, y), are
Dynamic Determination of Mixed Model Covariance Structures. in Double-blind Clinical Trials. Matthew Davis - Omnicare Clinical Research
 PharmaSUG2010 - Paper SP12 Dynamic Determination of Mixed Model Covariance Structures in Double-blind Clinical Trials Matthew Davis - Omnicare Clinical Research Abstract With the computing power of SAS
PharmaSUG2010 - Paper SP12 Dynamic Determination of Mixed Model Covariance Structures in Double-blind Clinical Trials Matthew Davis - Omnicare Clinical Research Abstract With the computing power of SAS
Binary Logistic Regression
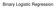 The coefficients of the multiple regression model are estimated using sample data with k independent variables Estimated (or predicted) value of Y Estimated intercept Estimated slope coefficients Ŷ = b
The coefficients of the multiple regression model are estimated using sample data with k independent variables Estimated (or predicted) value of Y Estimated intercept Estimated slope coefficients Ŷ = b
Chapter 9 Regression with a Binary Dependent Variable. Multiple Choice. 1) The binary dependent variable model is an example of a
 Chapter 9 Regression with a Binary Dependent Variable Multiple Choice ) The binary dependent variable model is an example of a a. regression model, which has as a regressor, among others, a binary variable.
Chapter 9 Regression with a Binary Dependent Variable Multiple Choice ) The binary dependent variable model is an example of a a. regression model, which has as a regressor, among others, a binary variable.
Instructions: Closed book, notes, and no electronic devices. Points (out of 200) in parentheses
 ISQS 5349 Final Spring 2011 Instructions: Closed book, notes, and no electronic devices. Points (out of 200) in parentheses 1. (10) What is the definition of a regression model that we have used throughout
ISQS 5349 Final Spring 2011 Instructions: Closed book, notes, and no electronic devices. Points (out of 200) in parentheses 1. (10) What is the definition of a regression model that we have used throughout
Power calculation for non-inferiority trials comparing two Poisson distributions
 Paper PK01 Power calculation for non-inferiority trials comparing two Poisson distributions Corinna Miede, Accovion GmbH, Marburg, Germany Jochen Mueller-Cohrs, Accovion GmbH, Marburg, Germany Abstract
Paper PK01 Power calculation for non-inferiority trials comparing two Poisson distributions Corinna Miede, Accovion GmbH, Marburg, Germany Jochen Mueller-Cohrs, Accovion GmbH, Marburg, Germany Abstract
SAS/STAT 13.1 User s Guide. Introduction to Survey Sampling and Analysis Procedures
 SAS/STAT 13.1 User s Guide Introduction to Survey Sampling and Analysis Procedures This document is an individual chapter from SAS/STAT 13.1 User s Guide. The correct bibliographic citation for the complete
SAS/STAT 13.1 User s Guide Introduction to Survey Sampling and Analysis Procedures This document is an individual chapter from SAS/STAT 13.1 User s Guide. The correct bibliographic citation for the complete
2 >1. That is, a parallel study design will require
 Cross Over Design Cross over design is commonly used in various type of research for its unique feature of accounting for within subject variability. For studies with short length of treatment time, illness
Cross Over Design Cross over design is commonly used in various type of research for its unique feature of accounting for within subject variability. For studies with short length of treatment time, illness
SAS/STAT 15.1 User s Guide The SEQDESIGN Procedure
 SAS/STAT 15.1 User s Guide The SEQDESIGN Procedure This document is an individual chapter from SAS/STAT 15.1 User s Guide. The correct bibliographic citation for this manual is as follows: SAS Institute
SAS/STAT 15.1 User s Guide The SEQDESIGN Procedure This document is an individual chapter from SAS/STAT 15.1 User s Guide. The correct bibliographic citation for this manual is as follows: SAS Institute
Metabolite Identification and Characterization by Mining Mass Spectrometry Data with SAS and Python
 PharmaSUG 2018 - Paper AD34 Metabolite Identification and Characterization by Mining Mass Spectrometry Data with SAS and Python Kristen Cardinal, Colorado Springs, Colorado, United States Hao Sun, Sun
PharmaSUG 2018 - Paper AD34 Metabolite Identification and Characterization by Mining Mass Spectrometry Data with SAS and Python Kristen Cardinal, Colorado Springs, Colorado, United States Hao Sun, Sun
DEALING WITH MULTIVARIATE OUTCOMES IN STUDIES FOR CAUSAL EFFECTS
 DEALING WITH MULTIVARIATE OUTCOMES IN STUDIES FOR CAUSAL EFFECTS Donald B. Rubin Harvard University 1 Oxford Street, 7th Floor Cambridge, MA 02138 USA Tel: 617-495-5496; Fax: 617-496-8057 email: rubin@stat.harvard.edu
DEALING WITH MULTIVARIATE OUTCOMES IN STUDIES FOR CAUSAL EFFECTS Donald B. Rubin Harvard University 1 Oxford Street, 7th Floor Cambridge, MA 02138 USA Tel: 617-495-5496; Fax: 617-496-8057 email: rubin@stat.harvard.edu
Logistic regression. 11 Nov Logistic regression (EPFL) Applied Statistics 11 Nov / 20
 Logistic regression 11 Nov 2010 Logistic regression (EPFL) Applied Statistics 11 Nov 2010 1 / 20 Modeling overview Want to capture important features of the relationship between a (set of) variable(s)
Logistic regression 11 Nov 2010 Logistic regression (EPFL) Applied Statistics 11 Nov 2010 1 / 20 Modeling overview Want to capture important features of the relationship between a (set of) variable(s)
The general concept of pharmacokinetics
 The general concept of pharmacokinetics Hartmut Derendorf, PhD University of Florida Pharmacokinetics the time course of drug and metabolite concentrations in the body Pharmacokinetics helps to optimize
The general concept of pharmacokinetics Hartmut Derendorf, PhD University of Florida Pharmacokinetics the time course of drug and metabolite concentrations in the body Pharmacokinetics helps to optimize
Generalized Linear Models for Non-Normal Data
 Generalized Linear Models for Non-Normal Data Today s Class: 3 parts of a generalized model Models for binary outcomes Complications for generalized multivariate or multilevel models SPLH 861: Lecture
Generalized Linear Models for Non-Normal Data Today s Class: 3 parts of a generalized model Models for binary outcomes Complications for generalized multivariate or multilevel models SPLH 861: Lecture
SAS Macro for Generalized Method of Moments Estimation for Longitudinal Data with Time-Dependent Covariates
 Paper 10260-2016 SAS Macro for Generalized Method of Moments Estimation for Longitudinal Data with Time-Dependent Covariates Katherine Cai, Jeffrey Wilson, Arizona State University ABSTRACT Longitudinal
Paper 10260-2016 SAS Macro for Generalized Method of Moments Estimation for Longitudinal Data with Time-Dependent Covariates Katherine Cai, Jeffrey Wilson, Arizona State University ABSTRACT Longitudinal
Ninth ARTNeT Capacity Building Workshop for Trade Research "Trade Flows and Trade Policy Analysis"
 Ninth ARTNeT Capacity Building Workshop for Trade Research "Trade Flows and Trade Policy Analysis" June 2013 Bangkok, Thailand Cosimo Beverelli and Rainer Lanz (World Trade Organization) 1 Selected econometric
Ninth ARTNeT Capacity Building Workshop for Trade Research "Trade Flows and Trade Policy Analysis" June 2013 Bangkok, Thailand Cosimo Beverelli and Rainer Lanz (World Trade Organization) 1 Selected econometric
BIOL 51A - Biostatistics 1 1. Lecture 1: Intro to Biostatistics. Smoking: hazardous? FEV (l) Smoke
 BIOL 51A - Biostatistics 1 1 Lecture 1: Intro to Biostatistics Smoking: hazardous? FEV (l) 1 2 3 4 5 No Yes Smoke BIOL 51A - Biostatistics 1 2 Box Plot a.k.a box-and-whisker diagram or candlestick chart
BIOL 51A - Biostatistics 1 1 Lecture 1: Intro to Biostatistics Smoking: hazardous? FEV (l) 1 2 3 4 5 No Yes Smoke BIOL 51A - Biostatistics 1 2 Box Plot a.k.a box-and-whisker diagram or candlestick chart
Generalized Models: Part 1
 Generalized Models: Part 1 Topics: Introduction to generalized models Introduction to maximum likelihood estimation Models for binary outcomes Models for proportion outcomes Models for categorical outcomes
Generalized Models: Part 1 Topics: Introduction to generalized models Introduction to maximum likelihood estimation Models for binary outcomes Models for proportion outcomes Models for categorical outcomes
ABSTRACT INTRODUCTION. SESUG Paper
 SESUG Paper 140-2017 Backward Variable Selection for Logistic Regression Based on Percentage Change in Odds Ratio Evan Kwiatkowski, University of North Carolina at Chapel Hill; Hannah Crooke, PAREXEL International
SESUG Paper 140-2017 Backward Variable Selection for Logistic Regression Based on Percentage Change in Odds Ratio Evan Kwiatkowski, University of North Carolina at Chapel Hill; Hannah Crooke, PAREXEL International
Dose-response modeling with bivariate binary data under model uncertainty
 Dose-response modeling with bivariate binary data under model uncertainty Bernhard Klingenberg 1 1 Department of Mathematics and Statistics, Williams College, Williamstown, MA, 01267 and Institute of Statistics,
Dose-response modeling with bivariate binary data under model uncertainty Bernhard Klingenberg 1 1 Department of Mathematics and Statistics, Williams College, Williamstown, MA, 01267 and Institute of Statistics,
Dosing In NONMEM Data Sets an Enigma
 PharmaSUG 2018 - Paper BB-02 ABSTRACT Dosing In NONMEM Data Sets an Enigma Sree Harsha Sreerama Reddy, Certara, Clarksburg, MD; Vishak Subramoney, Certara, Toronto, ON, Canada; Dosing data plays an integral
PharmaSUG 2018 - Paper BB-02 ABSTRACT Dosing In NONMEM Data Sets an Enigma Sree Harsha Sreerama Reddy, Certara, Clarksburg, MD; Vishak Subramoney, Certara, Toronto, ON, Canada; Dosing data plays an integral
DEPARTMENT OF COMPUTER SCIENCE Autumn Semester MACHINE LEARNING AND ADAPTIVE INTELLIGENCE
 Data Provided: None DEPARTMENT OF COMPUTER SCIENCE Autumn Semester 203 204 MACHINE LEARNING AND ADAPTIVE INTELLIGENCE 2 hours Answer THREE of the four questions. All questions carry equal weight. Figures
Data Provided: None DEPARTMENT OF COMPUTER SCIENCE Autumn Semester 203 204 MACHINE LEARNING AND ADAPTIVE INTELLIGENCE 2 hours Answer THREE of the four questions. All questions carry equal weight. Figures
Model Selection in GLMs. (should be able to implement frequentist GLM analyses!) Today: standard frequentist methods for model selection
 Model Selection in GLMs Last class: estimability/identifiability, analysis of deviance, standard errors & confidence intervals (should be able to implement frequentist GLM analyses!) Today: standard frequentist
Model Selection in GLMs Last class: estimability/identifiability, analysis of deviance, standard errors & confidence intervals (should be able to implement frequentist GLM analyses!) Today: standard frequentist
Analyzing and Interpreting Continuous Data Using JMP
 Analyzing and Interpreting Continuous Data Using JMP A Step-by-Step Guide José G. Ramírez, Ph.D. Brenda S. Ramírez, M.S. Corrections to first printing. The correct bibliographic citation for this manual
Analyzing and Interpreting Continuous Data Using JMP A Step-by-Step Guide José G. Ramírez, Ph.D. Brenda S. Ramírez, M.S. Corrections to first printing. The correct bibliographic citation for this manual
Analysis of Longitudinal Data: Comparison between PROC GLM and PROC MIXED.
 Analysis of Longitudinal Data: Comparison between PROC GLM and PROC MIXED. Maribeth Johnson, Medical College of Georgia, Augusta, GA ABSTRACT Longitudinal data refers to datasets with multiple measurements
Analysis of Longitudinal Data: Comparison between PROC GLM and PROC MIXED. Maribeth Johnson, Medical College of Georgia, Augusta, GA ABSTRACT Longitudinal data refers to datasets with multiple measurements
Modeling Land Use Change Using an Eigenvector Spatial Filtering Model Specification for Discrete Response
 Modeling Land Use Change Using an Eigenvector Spatial Filtering Model Specification for Discrete Response Parmanand Sinha The University of Tennessee, Knoxville 304 Burchfiel Geography Building 1000 Phillip
Modeling Land Use Change Using an Eigenvector Spatial Filtering Model Specification for Discrete Response Parmanand Sinha The University of Tennessee, Knoxville 304 Burchfiel Geography Building 1000 Phillip
SAS/STAT 13.2 User s Guide. Introduction to Survey Sampling and Analysis Procedures
 SAS/STAT 13.2 User s Guide Introduction to Survey Sampling and Analysis Procedures This document is an individual chapter from SAS/STAT 13.2 User s Guide. The correct bibliographic citation for the complete
SAS/STAT 13.2 User s Guide Introduction to Survey Sampling and Analysis Procedures This document is an individual chapter from SAS/STAT 13.2 User s Guide. The correct bibliographic citation for the complete
Data Analyses in Multivariate Regression Chii-Dean Joey Lin, SDSU, San Diego, CA
 Data Analyses in Multivariate Regression Chii-Dean Joey Lin, SDSU, San Diego, CA ABSTRACT Regression analysis is one of the most used statistical methodologies. It can be used to describe or predict causal
Data Analyses in Multivariate Regression Chii-Dean Joey Lin, SDSU, San Diego, CA ABSTRACT Regression analysis is one of the most used statistical methodologies. It can be used to describe or predict causal
DISPLAYING THE POISSON REGRESSION ANALYSIS
 Chapter 17 Poisson Regression Chapter Table of Contents DISPLAYING THE POISSON REGRESSION ANALYSIS...264 ModelInformation...269 SummaryofFit...269 AnalysisofDeviance...269 TypeIII(Wald)Tests...269 MODIFYING
Chapter 17 Poisson Regression Chapter Table of Contents DISPLAYING THE POISSON REGRESSION ANALYSIS...264 ModelInformation...269 SummaryofFit...269 AnalysisofDeviance...269 TypeIII(Wald)Tests...269 MODIFYING
Outline. The binary choice model. The multinomial choice model. Extensions of the basic choice model
 Outline The binary choice model Illustration Specification of the binary choice model Interpreting the results of binary choice models ME output The multinomial choice model Illustration Specification
Outline The binary choice model Illustration Specification of the binary choice model Interpreting the results of binary choice models ME output The multinomial choice model Illustration Specification
Models for Binary Outcomes
 Models for Binary Outcomes Introduction The simple or binary response (for example, success or failure) analysis models the relationship between a binary response variable and one or more explanatory variables.
Models for Binary Outcomes Introduction The simple or binary response (for example, success or failure) analysis models the relationship between a binary response variable and one or more explanatory variables.
Finansiell Statistik, GN, 15 hp, VT2008 Lecture 17-1: Regression with dichotomous outcome variable - Logistic Regression
 Finansiell Statistik, GN, 15 hp, VT2008 Lecture 17-1: Regression with dichotomous outcome variable - Logistic Regression Gebrenegus Ghilagaber, PhD, Associate Professor May 7, 2008 1 1 Introduction Let
Finansiell Statistik, GN, 15 hp, VT2008 Lecture 17-1: Regression with dichotomous outcome variable - Logistic Regression Gebrenegus Ghilagaber, PhD, Associate Professor May 7, 2008 1 1 Introduction Let
Investigating Models with Two or Three Categories
 Ronald H. Heck and Lynn N. Tabata 1 Investigating Models with Two or Three Categories For the past few weeks we have been working with discriminant analysis. Let s now see what the same sort of model might
Ronald H. Heck and Lynn N. Tabata 1 Investigating Models with Two or Three Categories For the past few weeks we have been working with discriminant analysis. Let s now see what the same sort of model might
PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH
 PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH From Basic to Translational: INDIRECT BIOASSAYS INDIRECT ASSAYS In indirect assays, the doses of the standard and test preparations are are applied
PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH From Basic to Translational: INDIRECT BIOASSAYS INDIRECT ASSAYS In indirect assays, the doses of the standard and test preparations are are applied
COMPLEMENTARY LOG-LOG MODEL
 COMPLEMENTARY LOG-LOG MODEL Under the assumption of binary response, there are two alternatives to logit model: probit model and complementary-log-log model. They all follow the same form π ( x) =Φ ( α
COMPLEMENTARY LOG-LOG MODEL Under the assumption of binary response, there are two alternatives to logit model: probit model and complementary-log-log model. They all follow the same form π ( x) =Φ ( α
Stepwise Gatekeeping Procedures in Clinical Trial Applications
 984 Biometrical Journal 48 (2006) 6, 984 991 DOI: 10.1002/bimj.200610274 Stepwise Gatekeeping Procedures in Clinical Trial Applications Alex Dmitrienko *,1, Ajit C. Tamhane 2, Xin Wang 2, and Xun Chen
984 Biometrical Journal 48 (2006) 6, 984 991 DOI: 10.1002/bimj.200610274 Stepwise Gatekeeping Procedures in Clinical Trial Applications Alex Dmitrienko *,1, Ajit C. Tamhane 2, Xin Wang 2, and Xun Chen
1 The problem of survival analysis
 1 The problem of survival analysis Survival analysis concerns analyzing the time to the occurrence of an event. For instance, we have a dataset in which the times are 1, 5, 9, 20, and 22. Perhaps those
1 The problem of survival analysis Survival analysis concerns analyzing the time to the occurrence of an event. For instance, we have a dataset in which the times are 1, 5, 9, 20, and 22. Perhaps those
Urban Transportation Planning Prof. Dr.V.Thamizh Arasan Department of Civil Engineering Indian Institute of Technology Madras
 Urban Transportation Planning Prof. Dr.V.Thamizh Arasan Department of Civil Engineering Indian Institute of Technology Madras Module #03 Lecture #12 Trip Generation Analysis Contd. This is lecture 12 on
Urban Transportation Planning Prof. Dr.V.Thamizh Arasan Department of Civil Engineering Indian Institute of Technology Madras Module #03 Lecture #12 Trip Generation Analysis Contd. This is lecture 12 on
A Clinical Trial Simulation System, Its Applications, and Future Challenges. Peter Westfall, Texas Tech University Kuenhi Tsai, Merck Research Lab
 A Clinical Trial Simulation System, Its Applications, and Future Challenges Peter Westfall, Texas Tech University Kuenhi Tsai, Merck Research Lab Acknowledgement Major collaborators Stephan Ogenstand,
A Clinical Trial Simulation System, Its Applications, and Future Challenges Peter Westfall, Texas Tech University Kuenhi Tsai, Merck Research Lab Acknowledgement Major collaborators Stephan Ogenstand,
McGill University. Faculty of Science. Department of Mathematics and Statistics. Statistics Part A Comprehensive Exam Methodology Paper
 Student Name: ID: McGill University Faculty of Science Department of Mathematics and Statistics Statistics Part A Comprehensive Exam Methodology Paper Date: Friday, May 13, 2016 Time: 13:00 17:00 Instructions
Student Name: ID: McGill University Faculty of Science Department of Mathematics and Statistics Statistics Part A Comprehensive Exam Methodology Paper Date: Friday, May 13, 2016 Time: 13:00 17:00 Instructions
Statistics in medicine
 Statistics in medicine Lecture 3: Bivariate association : Categorical variables Proportion in one group One group is measured one time: z test Use the z distribution as an approximation to the binomial
Statistics in medicine Lecture 3: Bivariate association : Categorical variables Proportion in one group One group is measured one time: z test Use the z distribution as an approximation to the binomial
Simple logistic regression
 Simple logistic regression Biometry 755 Spring 2009 Simple logistic regression p. 1/47 Model assumptions 1. The observed data are independent realizations of a binary response variable Y that follows a
Simple logistic regression Biometry 755 Spring 2009 Simple logistic regression p. 1/47 Model assumptions 1. The observed data are independent realizations of a binary response variable Y that follows a
Lecture 1 Introduction to Multi-level Models
 Lecture 1 Introduction to Multi-level Models Course Website: http://www.biostat.jhsph.edu/~ejohnson/multilevel.htm All lecture materials extracted and further developed from the Multilevel Model course
Lecture 1 Introduction to Multi-level Models Course Website: http://www.biostat.jhsph.edu/~ejohnson/multilevel.htm All lecture materials extracted and further developed from the Multilevel Model course
EPSY 905: Fundamentals of Multivariate Modeling Online Lecture #7
 Introduction to Generalized Univariate Models: Models for Binary Outcomes EPSY 905: Fundamentals of Multivariate Modeling Online Lecture #7 EPSY 905: Intro to Generalized In This Lecture A short review
Introduction to Generalized Univariate Models: Models for Binary Outcomes EPSY 905: Fundamentals of Multivariate Modeling Online Lecture #7 EPSY 905: Intro to Generalized In This Lecture A short review
Comparing Priors in Bayesian Logistic Regression for Sensorial Classification of Rice
 SAS 1018-2017 Comparing Priors in Bayesian Logistic Regression for Sensorial Classification of Rice Geiziane Oliveira, SAS Institute, Brazil; George von Borries, Universidade de Brasília, Brazil; Priscila
SAS 1018-2017 Comparing Priors in Bayesian Logistic Regression for Sensorial Classification of Rice Geiziane Oliveira, SAS Institute, Brazil; George von Borries, Universidade de Brasília, Brazil; Priscila
Whether to use MMRM as primary estimand.
 Whether to use MMRM as primary estimand. James Roger London School of Hygiene & Tropical Medicine, London. PSI/EFSPI European Statistical Meeting on Estimands. Stevenage, UK: 28 September 2015. 1 / 38
Whether to use MMRM as primary estimand. James Roger London School of Hygiene & Tropical Medicine, London. PSI/EFSPI European Statistical Meeting on Estimands. Stevenage, UK: 28 September 2015. 1 / 38
THE IMPORTANCE OF THE SIMULATION EXPECTATION AS A GOODNESS OF FIT DIAGNOSTIC FOR CATEGORICAL POPULATION PHARMACODYNAMIC MODELS
 THE IMPORTANCE OF THE SIMULATION EXPECTATION AS A GOODNESS OF FIT DIAGNOSTIC FOR CATEGORICAL POPULATION PHARMACODYNAMIC MODELS Marc R. Gastonguay, Jeffrey T. Hane Metrum Research Group LLC, Avon, CT 06001
THE IMPORTANCE OF THE SIMULATION EXPECTATION AS A GOODNESS OF FIT DIAGNOSTIC FOR CATEGORICAL POPULATION PHARMACODYNAMIC MODELS Marc R. Gastonguay, Jeffrey T. Hane Metrum Research Group LLC, Avon, CT 06001
Class Notes: Week 8. Probit versus Logit Link Functions and Count Data
 Ronald Heck Class Notes: Week 8 1 Class Notes: Week 8 Probit versus Logit Link Functions and Count Data This week we ll take up a couple of issues. The first is working with a probit link function. While
Ronald Heck Class Notes: Week 8 1 Class Notes: Week 8 Probit versus Logit Link Functions and Count Data This week we ll take up a couple of issues. The first is working with a probit link function. While
Clinical Trials. Olli Saarela. September 18, Dalla Lana School of Public Health University of Toronto.
 Introduction to Dalla Lana School of Public Health University of Toronto olli.saarela@utoronto.ca September 18, 2014 38-1 : a review 38-2 Evidence Ideal: to advance the knowledge-base of clinical medicine,
Introduction to Dalla Lana School of Public Health University of Toronto olli.saarela@utoronto.ca September 18, 2014 38-1 : a review 38-2 Evidence Ideal: to advance the knowledge-base of clinical medicine,
BIAS OF MAXIMUM-LIKELIHOOD ESTIMATES IN LOGISTIC AND COX REGRESSION MODELS: A COMPARATIVE SIMULATION STUDY
 BIAS OF MAXIMUM-LIKELIHOOD ESTIMATES IN LOGISTIC AND COX REGRESSION MODELS: A COMPARATIVE SIMULATION STUDY Ingo Langner 1, Ralf Bender 2, Rebecca Lenz-Tönjes 1, Helmut Küchenhoff 2, Maria Blettner 2 1
BIAS OF MAXIMUM-LIKELIHOOD ESTIMATES IN LOGISTIC AND COX REGRESSION MODELS: A COMPARATIVE SIMULATION STUDY Ingo Langner 1, Ralf Bender 2, Rebecca Lenz-Tönjes 1, Helmut Küchenhoff 2, Maria Blettner 2 1
Introduction to Generalized Models
 Introduction to Generalized Models Today s topics: The big picture of generalized models Review of maximum likelihood estimation Models for binary outcomes Models for proportion outcomes Models for categorical
Introduction to Generalized Models Today s topics: The big picture of generalized models Review of maximum likelihood estimation Models for binary outcomes Models for proportion outcomes Models for categorical
Growth Mixture Model
 Growth Mixture Model Latent Variable Modeling and Measurement Biostatistics Program Harvard Catalyst The Harvard Clinical & Translational Science Center Short course, October 28, 2016 Slides contributed
Growth Mixture Model Latent Variable Modeling and Measurement Biostatistics Program Harvard Catalyst The Harvard Clinical & Translational Science Center Short course, October 28, 2016 Slides contributed
Repeated ordinal measurements: a generalised estimating equation approach
 Repeated ordinal measurements: a generalised estimating equation approach David Clayton MRC Biostatistics Unit 5, Shaftesbury Road Cambridge CB2 2BW April 7, 1992 Abstract Cumulative logit and related
Repeated ordinal measurements: a generalised estimating equation approach David Clayton MRC Biostatistics Unit 5, Shaftesbury Road Cambridge CB2 2BW April 7, 1992 Abstract Cumulative logit and related
As mentioned in the introduction of the manuscript, isoboles are commonly used to analyze
 Appendix 1: Review of Common Drug Interaction Models As mentioned in the introduction of the manuscript, isoboles are commonly used to analyze anesthetic drug interactions. Isoboles show dose combinations
Appendix 1: Review of Common Drug Interaction Models As mentioned in the introduction of the manuscript, isoboles are commonly used to analyze anesthetic drug interactions. Isoboles show dose combinations
STA 303 H1S / 1002 HS Winter 2011 Test March 7, ab 1cde 2abcde 2fghij 3
 STA 303 H1S / 1002 HS Winter 2011 Test March 7, 2011 LAST NAME: FIRST NAME: STUDENT NUMBER: ENROLLED IN: (circle one) STA 303 STA 1002 INSTRUCTIONS: Time: 90 minutes Aids allowed: calculator. Some formulae
STA 303 H1S / 1002 HS Winter 2011 Test March 7, 2011 LAST NAME: FIRST NAME: STUDENT NUMBER: ENROLLED IN: (circle one) STA 303 STA 1002 INSTRUCTIONS: Time: 90 minutes Aids allowed: calculator. Some formulae
Mixed Models for Longitudinal Binary Outcomes. Don Hedeker Department of Public Health Sciences University of Chicago.
 Mixed Models for Longitudinal Binary Outcomes Don Hedeker Department of Public Health Sciences University of Chicago hedeker@uchicago.edu https://hedeker-sites.uchicago.edu/ Hedeker, D. (2005). Generalized
Mixed Models for Longitudinal Binary Outcomes Don Hedeker Department of Public Health Sciences University of Chicago hedeker@uchicago.edu https://hedeker-sites.uchicago.edu/ Hedeker, D. (2005). Generalized
