Reaction and Flow Invariants in Chemical Reaction Systems with Inlet and Outlet Streams
|
|
|
- Aubrey Bryan
- 6 years ago
- Views:
Transcription
1 Reaction and Flow Invariants in Chemical Reaction Systems with Inlet and Outlet Streams B. Srinivasan, M. Amrhein, and D. Bonvin 1 Institut d Automatique, École Polytechnique Fédérale de Lausanne, CH 1015Lausanne, Switzerland The concept of reaction invariants is extended to include flow invariants of chemical reaction systems with inlet and outlet streams. A transformation to normal form is introduced that allows the separation of: (i) the evolution of the reactions, (ii) the influence of inlet streams, and (iii) the behavior of the reaction and flow invariants. Resulting from this transformation to normal form, model reduction, construction of the linearizing feedback and properties such as state accessibility, state reconstruction, and feedback linearizability are analyzed. Keywords: Chemical reaction systems, Reaction invariant, Flow invariant, Model reduction, State accessibility, State reconstruction, Feedback linearizability. 1 Introduction Models of chemical reaction systems include information regarding both the chemical reactions (stoichiometry and kinetics) and the operation mode of the reactor. For the analysis of such models, it is important to distinguish between the states that intrinsically vary with time and those which do not. A transformation has been proposed by Waller and Mäkilä (1981) to separate the reaction variant (same dimension as the number of reactions) from the reaction invariant states. For reaction systems with neither inlet nor outlet streams, the complete concentration vector can then be reconstructed by integrating only the reaction variants. However, for considerations of safety and productivity, the reactors used in the production of specialty chemicals are often of the semi-batch type (systems with inlets), or run continuously (systems with inlet and outlets). The aim here is to extend the concept of invariance to chemical reaction systems with inlet and outlet streams. This requires the concept of invariants to include flow invariants. A transformation to normal form is proposed herein that performs a three level decomposition of the states into: (i) the evolution of the reactions, (ii) the influence of inlet streams, and (iii) the behavior of the reaction and flow invariants. No kinetic information (except for the assumption of independent kinetics) is necessary for the analysis. The proposed transformation to normal form helps determine the minimal dimensionality of the model (i.e., the minimal number of differential equations) and analyze control-relevant properties 1 Corresponding author. dominique.bonvin@epfl.ch.
2 such as state accessibility and feedback linearizability. The important results regarding state accessibility proposed by Bastin and Lévine (1993) can be readily derived from the transformation to normal form. Under certain conditions, the system can be proven to be feedback linearizable, and the linearizing control can be obtained easily. For simplicity, reaction systems with constant temperature, density, and inlet molar concentrations are considered first. However, isothermal operation is sometimes difficult to achieve or simply undesirable because it is non-optimal. Furthermore, as there is a considerable effort to reduce the amount of solvent used in liquid-phase homogeneous reaction systems, the assumption of constant density is often not valid. Also, the inlet molar concentrations might vary due to variability in the raw materials or in the preprocessing steps. Thus, the assumptions of constant temperature, density, and inlet molar concentrations are relaxed, and indications for constructing the transformation to normal form are provided. Semi-batch reaction systems and continuous stirred-tank reaction systems (CSTRs) are considered as special cases. The paper is organized as follows: Section 2 describes the basic model used in this study. Section 3 develops the transformation to normal form, the implications of which are discussed in Section 4. Extensions to the basic model are provided in Section 5 and special cases are considered in Section 6. A simulated example is presented in Section 7, and Section 8 concludes the paper. 2 Model of a homogeneous, constant-density, isothermal reaction system Consider a homogeneous, constant-density, isothermal, semi-batch or continuous stirred-tank chemical reaction system comprising S species and R independent reactions, independent reactions being those which have both independent stoichiometries and independent kinetics (Amrhein et al., 1996). The material balance equations and the continuity equation are described by: ṅ = V Kr n (n,v)+c in q in (q out /V )n, V = ( ) 1 T pq in qout, n(0) = n 0, V (0) = V 0, (1) where n is the S-dimensional vector of the number of moles, q in the p-dimensional inlet volumetric flowrate vector, q out the outlet volumetric flowrate expressed as the sum of q outlet volumetric flowrates, V the reactor volume, K the S R stoichiometric matrix, r n the R-dimensional reaction rate vector, which is a function of n and V, C in =[c 1 in,, c p in], c i in the molar concentrations of the ith inlet stream, n 0 the initial number of moles, V 0 the initial volume, and 1 p a p-dimensional vector with all elements being 1. To completely describe (1), the R-dimensional vector function r n (n,v) must be specified. The terms V Kr n (n,v), C in q in, and (q out /V )n represent the effect of the reactions, the inlet streams, and outlet stream on the number of moles, respectively. The molar concentrations are given by c = n/v. A model similar to (1) can be derived for bioreactions by expressing the material balances and the continuity equation in mass units. K then represents the matrix of yield coefficients. It is assumed that the time profiles of the inlet flowrates q in are linearly independent and the inlet molar concentrations c i in (i =1,...,p) are constant. Finally, note that the outlet flowrate q out does not necessarily depend on q in. 2
3 3 Transformation to normal form 3.1 Reaction invariants in the absence of inlets and outlets The aim here is the extension of the concept of reaction invariants to obtain a normal form for chemical reaction systems. As a first step towards understanding reaction invariants, a chemical reaction system with neither inlet nor outlet streams is considered: ṅ = V Kr n (n,v), V =0, n(0) = n 0, V (0) = V 0. (2) Though there are (S + 1) differential equations in (2), it will be shown below that the reaction system has intrinsically only R states or directions in which n evolves with time since there are only R independent reactions. The other (S R) directions are unaffected by the reactions and are termed the invariants of the reaction system (Waller and Mäkilä, 1981). The state space will be separated into: (i) an R-dimensional vector space which evolves due to the R independent reactions (the reaction variants), and (ii) the (S R)-dimensional reaction invariants. The ideas of Moore Penrose pseudo-inverse (denoted by superscript +) and completion of space will be used for this purpose. Let P R S (S R) be a matrix with orthonormal columns which satisfies: (i) rank ([ KP]) = S, and (ii) PT K = 0 (S R) R. Then, the linear transformation which separates the R-dimensional reaction variants, z 1, and the (S R)-dimensional reaction invariants, z 2, is given by z 1 = K+ n. (3) P T n z 2 Substituting (3) in (2) and using r z (z,v) for the reaction rates in the new coordinates gives: ż 1 = V r z (z,v), ż 2 = 0 S R, V =0, z 1 (0) = K + n 0, z 2 (0) = P T n 0, V (0) = V 0. (4) Remark 1 (Construction of P) Note that the columns of P span the null space of K T. Therefore, one way to construct P is by Singular Value Decomposition (SVD) of K (Golub and Van Loan, 1983). P is the matrix of the right singular vectors corresponding to the zero singular values of K. 3.2 Reaction invariants in the presence of inlets and outlets Even when inlets and outlets are present, the reactions affect only an R-dimensional space. The reaction invariants can be separated using the transformation (3). Applying (3) to (1) gives, ż 1 = V r z (z,v)+k + C in q in (q out /V ) z 1, ż 2 = P T C in q in (q out /V ) z 2, V = ( ) 1 T pq in qout, z 1 (0) = K + n 0, z 2 (0) = P T n 0, V (0) = V 0. (5) 3
4 Note that, though z 2 and V are reaction invariants, they do not remain constant. Thus, the evolution of n is no longer restricted to an R-dimensional space as previously; it evolves in a larger space. A similar conclusion has been drawn by Fjeld et al. (1974). In the absence of inlets and outlets, both isolating the manifold in which n evolves and spotting the reaction invariants is the same problem. This is because that the variation in n is caused only through the R independent reactions. Alternatively, in the presence of inlets and outlets, the variation in n and V is not only due to reactions but also due to flows (inlets and outlets). Thus, isolating the manifold in which n and V evolve means finding and eliminating states which are invariant with respect to both reactions and flows. 3.3 Reaction and flow invariants The key aim of this paper is to provide a three-part decomposition of the system equations (1). The first part is that which is affected by the R independent reactions. Among the reaction invariants, the second part picks those states which evolve with the flows. The third part consists of those states which remain constant, i.e., reaction and flow invariants. Note that the transformation to normal form that will be proposed to this effect is nonlinear. The dimension of the reaction variant space is R. As will be seen below, the dimension of the manifold in which the transformed states evolve is given by σ + 1 where σ rank ([ KC in ]). This means that the dimension increases with every inlet whose molar concentrations are independent of K and the remaining inlet molar concentrations. First, it is assumed that σ = R + p. It will be shown that the dimension of the reaction variant space is R, that of the reaction invariant and flow variant space is p + 1, and that of the reaction and flow invariant space is S σ. Next, the transformation to normal form will be developed, and it will then be generalized to the case of σ<r+ p. In comparison with (3), the completion of the state space involving n and V takes a three-level structure. Let L R S (σ R) and N R S (S σ) be matrices with orthonormal columns which satisfy the following conditions: (i) rank ([ KLN]) = S, (ii) K, L and N are mutually orthogonal, (iii) N T C in = 0 S σ p, (iv) L T C in is invertible. For the application of the three-part decomposition, the transformation to normal form (3) has to be changed as follows: (i) instead of P, ans (σ R) matrix, M, has to be chosen which renders M T C in = I (σ R), achieved by choosing M L(C T in L) 1 ; (ii) K + C in q in has to be eliminated. For this, an additional projection matrix (I S C in M T ) is formulated which, by construction of M, satisfies (I S C in M T )K = K. With these modifications, the transformation to normal form reads: z 1 K + (I S C in M T ) n z 2 = M T n (6) z 3 N T n 4
5 and ż 1 = V r z (z,v) (q out /V ) z 1, ż 2 = q in (q out /V ) z 2, ż 3 = (q out /V ) z 3, V = ( ) 1 T pq in (qout /V ) V, z 1 (0) = K + (I S C in M T ) n 0, z 2 (0) = M T n 0, z 3 (0) = N T n 0, V (0) = V 0, (7) where z 1, z 2, and z 3 are vectors of dimension R, p, and (S σ), respectively. For the elimination of the term (q out /V ), a nonlinear transformation can be used: x = z/(v 1T p z 2 ), (8) ν V 1 T p z 2 where x and ν are the transformed states of dimension (R + S + p σ) and 1, respectively. By introducing a non-zero scaling factor η, the following theorem is proposed which formulates the so-called normal form of (1). Theorem 2 Let σ = rank ([ KC in ]) =(R + p). Then, a diffeomorphism T :[ n V ] [ x ν ] exists which transforms model (1) into: ẋ 1 = h(x 2 ) r(x), ẋ 2 = q in /ν, ẋ 3 = 0 S σ, ν = q out /h(x 2 ), x 1 (0) = g 0 K + (I S C in M T )n 0, x 2 (0) = g 0 M T n 0, x 3 (0) = g 0 N T n 0, ν(0)=1/g 0, (9) where h(x 2 )=η + 1 T px 2, g 0 = η/(v 0 1 T pm T n 0 ), (10) and x 1, x 2, and x 3 are vectors of dimension R, p, and (S σ), respectively, η a non-zero arbitrary constant, r the R-dimensional reaction rate vector expressed in terms of x, M = L(C T in L) 1, L R S (σ R), and N R S (S σ) matrices with orthonormal columns which satisfy: (i) rank ([ KLN]) = S, (ii) K, L and N are mutually orthogonal, (iii) N T C in = 0 S σ p, (iv) L T C in is invertible. The transformation to normal form is one-to-one and can be written as follows using g(n,v)= η/(v 1 T pm T n): n x : V ν x n : ν V x 1 g(n,v) K + (I S C in M T )n x 2 g(n,v) M T n = (11) x 3 g(n,v) N T n ν 1/g(n,V) n = ν (Kx 1 + C in x 2 + Nx 3 ). (12) V νh(x 2 ) (see Appendix A for proof) 5
6 Table 1 Summary of the transformation to normal form. Original Reaction Reaction invariants Reaction and Reaction invariants space variants and flow variants flow invariants and flow variants (Inlets) (Outlet) x 1 x 2 x 3 ν ẋ 1 = h(x 2 ) r(x) ẋ 2 = q in /ν ẋ 3 = 0 ν = q out /h(x 2 ) n = (Kx 1 + C in x 2 + Nx 3 ) ν V = h(x 2 ) ν c = (Kx 1 + C in x 2 + Nx 3 ) h(x 2 ) R S+1 R R R p R (S σ) R 1 The matrices N and L required in Theorem 2 can be constructed similarly as in Remark 1 by noting that the columns of N and L span the null spaces of [K, C in ] T and [K, N] T, respectively. Theorem 2 can be easily extended to the case where σ < R + p. For this, the matrix M is redefined using the pseudo-inverse of (C T in L) instead of the inverse such that M := L (C T in L) +.It will be shown in Subsection 3.4 that the transformation to normal form in this case is no longer one-to-one. Corollary 3 For σ<r+ p, the transformed model (9) represents (1), where M := L (C T in L) +. The relation [ x ν ] [ n V ] is defined by (12) (see Appendix B for proof). 3.4 Discussion The results obtained in Theorem 2 and Corollary 3 are summarized in Table 1. Though a rigorous interpretation of the results is difficult due to the nonlinear term in the transformation to normal form, an intuitive meaning for the various parts of x can be given. x 1 describes the R-dimensional reaction variants, while x 2 captures the p-dimensional flow part. x 3 are the (S σ)-dimensional reaction and flow invariants with ν being related to the outflow. The third row depicts the differential equations which explain why the interpretation of the states outlined above is possible. The fourth and fifth rows summarize the reconstruction of the original states n and V. With this transformation to normal form, c (sixth row) and n are decomposed along the directions determined by the columns of: (i) the stoichiometric matrix K, (ii) the inlet concentration matrix C in, and (iii) the reaction and flow invariant matrix N. Note that, in contrast to n, c is not directly influenced by the outflow through ν. In the last row, the dimensions of the various spaces are given. The transformed system is of 6
7 dimension (S + R + p σ + 1), whereas the original system has dimension (S + 1). Thus, if σ<(r + p), there is an increase in dimension as a result of the transformation to normal form. This is why the transformation to normal form is not one-to-one when σ<(r + p). An example is now given as illustration. Example 4 Consider a reaction system with two independent reactions (R =2): A B, B + C D. When A and B are fed as two different inlets (p =2), then [ KC in ] = 1 0 c in,a c in,b and σ =3< 4=R + p. This is due to the fact that feeding A along with the reaction A B is equivalent to feeding B. This redundancy is reflected in the loss of rank in [K, C in ]. Owing to redundancy, a clear separation of the influence of reactions and flows is no longer possible. One way to handle this is to explain the same effect by more than one state, thus, artificially increasing the dimension of the state space (see Table 1). Note that x 2 and ν form the flow variant space. However, since ν enters nonlinearly in the reconstruction of n and V, it is given a special symbol. Owing to the redundancy described above, amongst the (p + 1) states present in [ x 2 ν ] only (σ R + 1) states constitute the reaction invariant and flow variant space. 4 Implications of the transformation to normal form The transformation to normal form for chemical reaction systems with independent inlet and outlet streams proposed in the previous section clearly shows the evolution of n in three different spaces: the stoichiometric space (K), the inlet space (C in ), and the invariant space (N). Such a separation has immediate implications for model reduction, state accessibility, state reconstruction, feedback linearizability, and the construction of the linearizing feedback. 4.1 Model reduction From (9), it is clear that only (R+p+1) differential equations need to be integrated to compute the trajectories. In addition, a set of (S σ) constants must be computed from the initial conditions. In the case of S>(R + p), analysis and design of controllers, observers and optimizers can be achieved with the reduced model of (R + p + 1) states if the initial conditions n 0 and V 0 are assumed to be known. 7
8 4.2 State accessibility It is obvious from the transformation to normal form in Theorem 2 that: (i) x 3 is inaccessible, and (ii) x 2 and ν are accessible from the inlet and outlet streams. Moreover, a part of x 1 can be inaccessible depending on the kinetics. Thus, the dimension of the inaccessible part is at least (S σ) which leads to the following corollary. Corollary 5 The maximum dimension of the accessible part is (σ+1), where σ = rank ([ KC in ]). Therefore, if full state accessibility is required, the dimension of x 3 should be zero (i.e., σ = S) implying that the number of inlet streams should be at least (S R) (i.e., p S R). Corollary 6 σ = S is a necessary condition for full state accessibility. These results have been reported previously by Bastin and Lévine (1993). However, deriving them from the transformation to normal form in Theorem 2 provides an alternate proof. Another interesting aspect of the transformation to normal form is that the dynamics of the inaccessible part can be transformed to ẋ 3 = 0 S σ, which is marginally stable. 4.3 State reconstruction The reconstruction of n as in (12) can be used to reconstruct the complete state information from a subset of measured molar concentrations. The key idea is that x 2, x 3 and ν can be calculated at any point in time from the initial conditions and the flows without knowledge of the kinetics since they are reaction invariants. Then, if the molar concentrations, c s,ofs s R species are measured, x 1 can be calculated under certain minor assumptions. When x 1 is available, the number of moles n, and also, the molar concentration vector c = n/v can be reconstructed from (12). The idea is similar to that of the asymptotic observer proposed by Bastin and Dochain (1990). Proposition 7 Let the molar concentrations of S s R species be measured and K s be the S s R submatrix of K corresponding to these S s species. Given K, C in, q in, q out, c 0, and V 0,ifK s has a unique left pseudo-inverse, the molar concentrations, c t, of the remaining (S S s ) species can be reconstructed without knowledge of reaction kinetics using c t = K t K + s c s + ( C in,t K t K + s C in,s ) x2 /h(x 2 )+ ( N t K t K + s N s ) x3 /h(x 2 ), (13) where the subscript s represents a quantity corresponding to the measured S s molar concentrations and t to that of (S S s ) unmeasured molar concentrations. Let only an estimate of the initial molar concentrations of the S t species be available. Then the estimation error e ĉ t c t, with ĉ t being the estimated molar concentrations, is given by e = h(x 2(0)) h(x 2 ) e(0). (14) If at least one of the inlet flowrates is always non-zero, then the estimation error asymptotically converges to zero. (see Appendix C for proof). 8
9 The asymptotic convergence can intuitively be explained from (14). The numerator term is constant while the denominator monotonically increases if at least one of the inlet flowrates is always non-zero. h(x 2 ) tends to infinity, thereby pushing the error to zero. Note that since the estimation errors of the S t species are independent of each other, an initial molar concentration error of any species does not propagate on the molar concentration estimates of the remaining species. Note that, if molar concentrations of fewer than R species are measured, then observer techniques relying on the knowledge of reaction kinetics should be used (Soroush, 1997). 4.4 Feedback linearizability Consider a reduced system that does not include the inaccessible part x 3. The system is affine in the inputs and is given by: ẋ 1 f 1 (x) 0 R m ẋ = ẋ 2 = 0 p + (1/ν)I p 0 p u f(x)+ m g j u j, u = q in, (15) ν 0 0 T j=1 q out p 1/h(x 2 ) where f and g j are n-dimensional vector fields, f 1 an R-dimensional vector field, and u the m- dimensional input vector. m and n are the dimensions of u and x, respectively, with, in this case, m =(p + 1) and n =(R + m). Owing to the special structure of the vector fields, important results regarding the feedback linearizability of system (15) can be obtained. A system is feedback linearizable if a state feedback and a diffeomorphism exist which renders the closed-loop system linear (see Appendix D for conditions of feedback linearizability). Proposition 8 Given system (15) with σ =(R + p) and m R inputs. If the R m matrix J = [(1/ν) f 1 x 2, ( 1/h(x 2 )) f 1 ] is of rank R, then (15) is feedback linearizable. (see Appendix D ν for proof) An advantage of model (1) transformed into (15) is that the linearizing feedback can be calculated easily. With this feedback, the system is linear no further diffeomorphism is required. x 1 and a part of x 2 form the flat outputs (Fliess et al., 1995), linearization of (15) to ẍ 1 = v u and ẋ 2,v = v v with the new external inputs v u R R and v v R m R can be achieved, where x 2 = [ x 2,u x 2,v ]. Differentiating ẋ1 with respect to time, v u = ẍ 1 = f 1 x 1 f 1 + Ju. (16) Partitioning J =[J s, J t ] such that J s R R R is invertible, the following feedback is computed from (16): u = J 1 f 1 s x 1 f 1 J + 1 s J 1 s J t v u. (17) 0 m R 0 (m R) R I m R v v Note that (m R) inputs are superfluous and, thus, not necessary to control the R reactions. The assumption of full rank for J is not very restrictive, especially when only independent reactions are considered. If only non-reacting species are added, it is clear that they will not affect the 9
10 reaction rates, and J will loose rank. In contrast, if different reacting species in at least R inlets are fed to the reaction system, the assumption of full rank for J can be met easily. In the above proposition, only sufficient conditions for full-state feedback linearizability have been provided. An example illustrating the conservatism of these conditions is given next. Consider the semi-batch reaction system A + B C and A + C D with second-order kinetics and B being fed (m = p =1,R = 2). Although the conditions of Proposition 8 are not verified, it can easily be verified that the system is feedback linearizable. 5 Extensions to other types of reaction systems The transformation to normal form was stated in Section 3 for reaction systems with temperature, constant density, and inlet molar concentrations. Below, these results will be extended to cover practical situations commonly found in chemical reaction systems, in particular those dealing with varying temperature, density, and inlet molar concentrations. 5.1 Non-isothermal reaction systems In a non-isothermal scenario, the temperature, T, should be modeled as an additional state. Under the assumptions that: (i) the pressure remains constant, (ii) the mixing enthalpies are negligible, and (iii) the temperatures, T in,ofthep inlet streams remain constant, the heat balance equation is: d dt (Vρc p T )=V ( h T R) r n (n,v,t)+t T in S in Φ in q in (q out /V )(VρsT)+Q ext, (18) where h R is the R-dimensional vector of reaction enthalpies, c p the specific heat capacity, ρ the density of the mixture, S in and Φ in p-dimensional diagonal matrices with elements being the specific heat capacities and densities of the inlet streams, respectively, and Q ext the external heat energy such as external heating/cooling energy or heat dissipation. Using T = ρst, the model for non-isothermal constant-density reaction systems is given by: ṅ = V Kr n (n,v,t )+C in q in (q out /V ) n, d dt (VT )=V ( h T R) r n (n,v,t )+T,T in q in (q out /V )(VT )+Q ext, V =(1 T pq in ) q out, n(0) = n 0, T (0) = T0, V (0) = V 0. (19) Furthermore, it is assumed that S in and Φ in are constant. For the formulation of the transformation of (19) into normal form, the heat balance equation is not transformed when the external energy is non-negligible (Q ext 0). However, the remaining equations are transformed using (9) since the transformation to normal form proposed in Theorem 2 is independent of reaction kinetics and temperature. Note that the dimension of the inaccessible part x 3 does not decrease even when the temperature is used as an additional input for control. 10
11 When Q ext = 0, then (V T ) can be combined with n leading to, d n = V K r dt (V T n (n,v,t )+ C in q in (q out /V ) n. (20) ) h T R T,T in (V T ) From (20) the state (VT ) can be modeled as an additional species and, hence, S T S +1.If σ = rank ([K, C in ]) = R + p, then rank ([ ]) K C in h = R + p. Since T (ST σ) =(S σ) +1, R T,T in the number of reaction and flow invariants (inaccessible states) is increased by 1. This means that the additional state (V T ) is inaccessible in the absence of external energy. Intuitively, the temperature cannot vary independently of the reactions and the flows without external heating since the evolution of the reactions is already captured by n. 5.2 Varying-density reaction systems In reaction systems with constant density ρ, the conservation equation for total mass, m, translates directly into a conservation equation for the volume V, as was the case in equation (1). This is due to the relationship V = m/ρ with ρ being constant. However, in the case of varying density, the mass conservation leads to, d dt (ρv)=1t p Φ in q in ρq out, (ρv)(0) = ρ 0 V 0. (21) In the case of varying density, ṅ in (1) can still be transformed as in Theorem 2 if h is redefined d using the following algebra. Substituting V = hν in (21) and using (9), dt (ρh)=1t p Φ in (q in /ν). After integrating both sides, the following redefinition of h results: h(x,ν):=(1 T p Φ in x 2 + η )/ρ(x,ν), (22) where η is an arbitrary integration constant. An important difference between the original definition (10) and the redefinition (22) is that, since ρ depends on both x and ν, the redefined h depends not only on x 2 but also on ν and the entire x vector. Note that since g = h(x,ν)/v,it gets redefined as g(n,v):=η /(ρv 1 T pφ in M T n). Since Φ in is assumed to be constant and is usually available, availability of the inlet volumetric flowrates q in and the inlet mass flowrates u in = Φ in q in is equivalent. However, since the varying density ρ is usually unknown, the outlet mass flowrate, u out = ρq out, cannot be calculated from q out and vice versa. If u out is available, then ν in the transformed system (9) has to be replaced by: ν = q out /h(x,ν)= q out ρ/(1 T p Φ in x 2 + η ) = u out /(1 T p Φ in x 2 + η ). (23) In this case, ν, and hence x 2 (see 9), are independent of ρ which implies that ν and x 2 are reaction invariants. Note that h for ẋ 1 in (9) and for V in (12) is as defined in (22). In summary, the structure of the transformed system (9) is retained for varying-density reaction systems except for the redefinition of h. With volumetric flowrates, the interpretation of the threepart decomposition discussed in Section 3.4 is not possible due to coupling through the density. 11
12 Alternatively, if the mass flowrates are available, the interpretation of the three-part decomposition is still valid since ν and x 2 are independent of the density. 5.3 Varying inlet concentrations In the case of varying inlet concentrations, the reaction system is modeled by: ṅ = V Kr(c)+C in q in (q out /V ) n, V = 1 T p q in q out, n(0) = n 0 V (0) = V 0, (24) where C in and q in are the varying inlet molar concentrations and flowrates of the p physical inlets, respectively. The transformation to normal form is carried out in two steps. In the first step, (24), a system with p inlet streams of varying inlet molar concentrations, is transformed into (1), a system with p streams of constant inlet molar concentrations. The second step is the transformation of (1) to normal form, which has been discussed in earlier sections. For the first step, let the S K matrix F in, and the p K matrix Q in be defined as: F in [C in(0) q in(0),...,c in(k 1) q in(k 1)], Q in [q in(0),...,q in(k 1)], (25) where K is the number of discretization points. Let f rank (F in ). F in can be decomposed using, for example, SVD such that F in = C in Qin, where C in and Q in are the S f matrix of constant inlet molar concentrations and the f K matrix of corresponding inlet volumetric flowrates for K discretization points, respectively. Redefining p := f + 1, the S p inlet molar concentration matrix C in and the p K matrix of flowrates, Q in, are obtained: C in := [ Cin 0 S ], Qin Q in 1 T p Q in 1 T Q. (26) f in The p-dimensional inlet volumetric flowrate vector q in (k) atthekth discretization instant is the (k + 1)th column of Q in (k =0,...,K 1). With this redefinition: C in(k) q in(k) = C in q in (k) =C in q in (k), 1 T p q in(k) =1 T pq in (k). (27) Substituting (27) in (24) leads to (1). Note that the structure of the reaction system in normal form (9) is retained even for reaction systems with varying inlet concentrations except for redefinition of the inlet streams. Also, the interpretation of the three-part decomposition is valid. 6 Special cases In the development of the transformation to normal form in Section 3, it was assumed that the inlets and outlets are independent. However, in certain important special cases, q out is not independent, for example, for (i) semi-batch reaction systems where q out = 0, and (ii) CSTRs, where q out = 1 T pq in. That this dependence leads to inaccessibility of the volume since the outflow looses its direct influence on the volume will be shown next. 12
13 Semi-batch reaction systems. Owing to the absence of an outlet stream, ν = 0 implying ν(t) =ν 0. Thus, V = ν 0 h(x 2 ). The transformed set of equations is given below. ẋ 1 = h(x 2 ) r(x), ẋ 2 = q in /ν 0, ẋ 3 = 0 S σ, ν =0. (28) Note that the transformation given by (11) (12) is linear since g(n,v) is a constant with g(n,v)= 1/ν 0. Continuous stirred-tank reaction systems. In a CSTR, q out = 1 T pq in, V = 0 and V (t) =V0. Since the nonlinear transformation (8) of V to ν is not required to get to the normal form, a similar transformation as in (28) can be used to obtain the transformed system: ẋ 1 = h(x 2 ) r(x), ẋ 2 = h(x 2 ) q in /V 0, ẋ 3 = 0 S σ, V =0. (29) As discussed in Section 3.4, the reaction invariant and flow variant space has a maximum dimension of (σ R + 1). In the two special cases shown above, the same quantity is limited to a maximum of p since there are only p independent flows. Combining these two limits, the dimension of the reaction invariant and flow variant space is determined as min(σ R + 1,p). The number of reaction invariant and flow variants min(σ R +1,p) equals p for either of the following two cases: (i) σ =(R+p), and (ii) σ = R+p 1. This means that the number of accessible states is not reduced even when the molar concentrations of one inlet stream are dependent on the stoichiometry and the molar concentrations of the remaining inlet streams. Intuitively, this is due to the volume becoming accessible in the case of σ = R + p 1 as discussed below. Addition of solvent. The addition of solvent is considered as an example for σ = R + p 1. The inlet molar concentration matrix has the form C in := [c 1 in,, c (p 1) in, 0 S ]. If it is assumed that rank ([ Kc 1 in... c (p 1) ]) ([ ]) in = R+p 1, then rank KC in = R+p 1. Intuitively, when the outlet is dependent on the inlets, the addition of solvent makes the volume accessible, since adding the solvent changes the volume, thereby diluting other species and affecting n and V. Note that, for the tangent-linearized model with no outlet stream and rank ([ Kc 1 in... c (p 1) ]) in = R+p 1, local controllability of the volume by solvent addition has been proven by Dochain and Chen (1992). However, the work presented herein proposes global results for nonlinear chemical reaction systems. 7 Example The concept of model reduction, state reconstruction, state accessibility and feedback linearizability will now be illustrated on a homogeneous, constant-density, non-isothermal semi-batch reaction system (R = 3 and S = 7), namely the ethanolysis of phthalyl chloride (A) (de Vallière, 1989). In two successive irreversible ethanolysis reactions, phthalyl chloride monoethyl ester (C), the desired product, and phthalic diethylester (E) are produced from ethanol (B). Both reactions also produce hydrochloric acid (D). It is assumed that B also reacts with D in a reversible side reaction to produce ethyl chloride (F ) and water (G). The reaction system can be described by the following reaction scheme: 13
14 Table 2 Numerical values for the simulated reaction systems. Parameter Value Unit [ ] {κ i,0 } M 1 h 1 [ ] {Ei } K A + B r 1 C + D C + B r 2 with E + D K = D + B r 3 Ω F + G The molar concentration vector reads c =[c A,c B,c C,c D,c E,c F,c G ] T. Since a known temperature profile T (t) is imposed (see Fig. 1a), the differential equation for T can be neglected. Hence, the model is of order 8. The reaction rates are assumed to follow the so-called mass-action principle: r 1 = κ 1 (T )c A c B, r 2 = κ 2 (T )c B c C, r 3 = κ 3 (T )c B c D κ 4 (T )c F c G with κ i following the Arrhenius law κ i (T )=κ i,0 exp( E i /T ). The numerical values of the parameters are given in Table 2. Semi-batch operation with p = 2 inlet streams and no outlet will now be studied with C in chosen such that rank ([ ]) KC in = R+p. In this way, the transformation to normal form (diffeomorphism) of Theorem 2 can be illustrated. Ethanol (V 0 = 1.5 l with 2 w-% water) in 80 w-% solvent (dimethyl sulfoxide) is initially placed in a vessel, i.e. c 0 =[0, 4.2, 0, 0, 0, 0, 0.22] T M. The first inlet stream contains phthaloyl dichloride and the second an ethanol/water mixture, i.e. c 1 in = [4.9, 0, 0, 0, 0, 0, 0] T M and c 2 in =[0, 20, 0, 0, 0, 0, 1.5] T M. The inlet volumetric flowrate profiles are given in Fig. 1c, the volume profile in Fig. 1b, and the molar concentration profiles in Fig. 2. Model (1) can be reduced to (R + p) = 5 differential equations as given in Theorem 2. With the choice η V 0 1 T pm T n 0 =1.200 l, ν(t) =ν 0 = 1. From (28) and (12), the reduced model and the reconstructed molar concentrations c read: ẋ1 = ẋ 2 h(x ) r(x), x 1 (0) = q, x 2(0) = 0.007, in c =(Kx 1 + C in x 2 + Nx 3 ) /h(x 2 ), V = h(x 2 ), (30) where N = T 14
15 320 (a) T [K] (b) V [l] (c) q in [l h 1 ] 5 q 1 in q 2 in time [h] Fig. 1. Time profiles of: (a) the temperature T, (b) the volume V, and (c) the inlet volumetric flowrates q in. c [M] A B C D E F G time [h] Fig. 2. Time profiles of the molar concentrations c (right axis for c B, and left axis for the other species). 15
16 20 (a) x x 1,1 x 1,2 x 1, x (b) x 2,1 x 2, time [h] Fig. 3. Time profiles of: (a) x 1, and (b) x 2. and x 3 =[ 0.028, 0.094] T. The time profiles of x 1 and x 2 are shown in Fig. 3. If the molar concentrations of S s = R = 3 species are measured, e.g. c s =[c A,c D,c G ] T, the molar concentrations of the remaining species can be estimated using (13) and (30) with K s = From Corollary 6 it can be seen that p = 2 inlet streams are not sufficient to have full state accessibility since at least (S R) = 4 inlet streams would be necessary. Furthermore, in the absence of an outlet stream (q out = 0) as is the case here at least p = R = 3 inlet streams would be needed to guarantee feedback linearizability using Proposition 8. 8 Conclusions A generic nonlinear transformation to normal form for reaction systems with inlet and outlet streams has been presented. It leads to a normal form where the reaction and flow invariants can be easily identified. Such a transformation was used to prove results on state accessibility. Implications of this transformation to normal form in the fields of model reduction, state reconstruction and 16
17 feedback linearizability were discussed. Using this transformation for feedback linearization has the advantage that the linearizing control can be obtained in a straightforward manner. The results obtained for constant-density, isothermal reaction systems were extended to varying density and non-isothermal situations, and special cases such as semi-batch reaction systems and CSTRs were considered. Note that the results obtained are global in nature and deal with the full-fledged nonlinear system, also for extensions and special cases. In summary, major insight is gained by using the normal form for reaction systems which can help, in turn, to perform simulation, analysis, control, and optimization more efficiently. Acknowledgments The authors acknowledge the financial support of the Swiss Commission for Technology and Innovation through the Project No Dr. C. Benthack of BASF Ltd. and Prof. J. Lévine of École des Mines, Paris, are also thanked for fruitful discussions. Appendices A Proof of Theorem 2 The construction of the transformation to normal form was shown in the text. However, the reconstruction of the original states n and V from x and ν remains to be proven. The relationship [ V z ] [ x ν ] is given in (8). The inverse can be obtained after a few algebraic manipulations as: z = x ν. (A.1) V h(x 2 ) ν According to (6), the number of moles n satisfies: K + (I S C in M T ) z = M T n. N T (A.2) Multiplying both sides of (A.2) by [ KC in N] and noting that KK + + LL T + NN T = I S, [ KC in N] z = ( KK + (I S C in M T )+C in M T + NN T ) n = ( KK + +(LL T + NN T ) C in M T + NN T ) n = ( KK + + LL T + NN T ) n = n. (A.3) 17
18 Substituting z = x ν into (A.3) leads to n = ν (Kx 1 + C in x 2 + Nx 3 ) and, hence, the theorem follows. B Proof of Corollary 3 The only difference between Corollary 3 and Theorem 2 is that M = L(C T in L) +. However, since LL T C in M T = L(L T C in )(L T C in ) + L T = LL T, the equation KK + + LL T + NN T = I S in the proof of Theorem 2 still holds and, hence, the corollary follows. C Proof of Proposition 7 Note that c = n/v. The measured and unmeasured molar concentrations induce the following partition in (12): n ν = h(x 2) c = h(x 2 ) c s = K s x 1 + C in,s x 2 + N s x 3. (C.1) c t K t The states x 2, x 3, and ν can be reconstructed from the inlet and outlet streams, c 0, and V 0 without knowledge of the kinetics. Owing to the existence of K + s, by assumption x 1 can be reconstructed from c s as: C in,t N t x 1 = K + s (h(x 2 ) c s C in,s x 2 N s x 3 ). (C.2) Having reconstructed x 1, (13) follows. Adding and subtracting the initial conditions of x 2 and noting that x 3 stay at their initial value, the estimate can be written as ĉ t = K t K + s c s + ( C in,t K t K + s C in,s ) (ˆx2 ˆx 2 (0))/ĥ ( C in,t K t K + s C in,s ) ˆx2 (0)/ĥ + ( N t K t K + s N s ) ˆx3 (0)/ĥ. (C.3) By assumption, there is no uncertainity in the initial volume, and material exchange terms. Using this fact and noting that h 0 ν 0 = ĥ0ˆν 0 = V 0, and, thus, ˆνν 0 = ν ˆν 0, (x 2 x 2 (0))ν 0 =(ˆx 2 ˆx 2 (0))ˆν 0, hν 0 = ĥ ˆν 0 (x 2 x 2 (0))/h =(ˆx 2 ˆx 2 (0))/ĥ. (C.4) This means that there is no error in the estimation of (x 2 x 2 (0))/h. Using (C.4) and also the fact that c s is measured without uncertainty, the estimation error e ĉ t c t becomes: e = (C in,t K t K + s C in,s )(ˆx 2 (0) ν 0 /ˆν 0 x 2 (0)) + (N t K t K + s N s )(ˆx 3 (0) ν 0 /ˆν 0 x 3 (0)). h (C.5) 18
19 Note that the numerator in (C.5) is constant, which implies d dt (h e) =0 S t. Thus, the numerator is given by e(0) h(x 2 (0)), leading to (14). The error dynamics are given by ė = d e, (C.6) where d =(1 T pq in )/V is the so-called dilution term. If at least one of the inlet flowrates is non-zero for t, then d>0. Hence, the error dynamics (C.6) are stable, and e converges asymptotically to 0 St. Consequently, the result proposed by Bastin and Dochain (1990) is obtained: lim t ĉt = c t, d > 0. (C.7) D Proof of Proposition 8 A system is feedback linearizable iff (FL1) the distributions i = span{[f k g j ]: 0 k i, 1 j m} 2 have constant rank everywhere and are closed under Lie-bracketing (involutive) for all i 0, and (FL2) rank( n 1 )=n. Consider the distributions 0 = span{g j,j=1,...,m}, 1 = span{g j, [f, g j ],j=1,...,m}. (D.1) It can be verified that arranging the vectors of the distribution 1 leads to [ ] g 1... g m [f, g 1 ]... [f, g m ] = 0 R m J. I m 0 m m (D.2) Since by assumption J is of rank R, it follows that rank ( 1 )=(R + m) which is the maximum possible rank. Thus, 1 is involutive. Furthermore, 0 has rank m and is involutive since [g i, g j ]= 0, i, j =1,...,p, and [g i, g m ]=(g m g i )/(νh(x 2 )), i =1,...,m. Thus, (15) is feedback linearizable. References Amrhein, M., B. Srinivasan, D. Bonvin, and M. M. Schumacher, On the rank deficiency and rank augmentation of the spectral measurement matrix, Chemometrics Intellig. Lab. Sys. 33(1), (1996). Bastin, G. and D. Dochain, On-line Estimation and Adaptive Control of Bioreactors, Elsevier, Amsterdam (1990). Bastin, G. and J. Lévine, On state accessibility in reaction systems, IEEE Trans. on Automatic Control 38(5), (1993). 2 [, ] denotes the Lie-bracket and [f k g j ] the iterated Lie-bracket [f, f (k 1) g j ]. 19
20 de Vallière, P. R. L., State and Parameter Estimation in Batch Reactors for the Purpose of Inferring On-line the Rate of Heat Production, doctoral thesis No. 8847, ETH Zürich (1989). Dochain, D. and L. Chen, Local observability and controllability of stirred tank reactors, J. Process Control 2(3), (1992). Fjeld, M., O. A. Asbjørnsen, and K. J. Åström, Reaction invariants and their importance in the analysis of eigenvectors, state observability and controllability of the continuous stirred tank reactor, Chem. Eng. Sci. 29, (1974). Fliess, M., J. Lévine, P. Martin, and P. Rouchon, Flatness and defect of non-linear systems: introductory theory and examples, Int. J. Control 6, (1995). Golub, G. H. and C. F. Van Loan Matrix Computations, North Oxford Academic, Oxford (1983). Soroush, M., Nonlinear state-observer design with application to reactors, Chem. Eng. Sci. 52(3), (1997). Waller, K. V. and P. M. Mäkilä, Chemical reaction invariants and variants and their use in reactor modeling, simulation, and control, Ind. Eng. Chem. Process Des. Dev. 20(1), 1 11 (1981). 20
Development of Dynamic Models. Chapter 2. Illustrative Example: A Blending Process
 Development of Dynamic Models Illustrative Example: A Blending Process An unsteady-state mass balance for the blending system: rate of accumulation rate of rate of = of mass in the tank mass in mass out
Development of Dynamic Models Illustrative Example: A Blending Process An unsteady-state mass balance for the blending system: rate of accumulation rate of rate of = of mass in the tank mass in mass out
Development of Dynamic Models. Chapter 2. Illustrative Example: A Blending Process
 Development of Dynamic Models Illustrative Example: A Blending Process An unsteady-state mass balance for the blending system: rate of accumulation rate of rate of = of mass in the tank mass in mass out
Development of Dynamic Models Illustrative Example: A Blending Process An unsteady-state mass balance for the blending system: rate of accumulation rate of rate of = of mass in the tank mass in mass out
Chemical reactors. H has thermal contribution, pressure contribution (often negligible) and reaction contribution ( source - like)
 Chemical reactors - chemical transformation of reactants into products Classification: a) according to the type of equipment o batch stirred tanks small-scale production, mostly liquids o continuous stirred
Chemical reactors - chemical transformation of reactants into products Classification: a) according to the type of equipment o batch stirred tanks small-scale production, mostly liquids o continuous stirred
Chap. 3. Controlled Systems, Controllability
 Chap. 3. Controlled Systems, Controllability 1. Controllability of Linear Systems 1.1. Kalman s Criterion Consider the linear system ẋ = Ax + Bu where x R n : state vector and u R m : input vector. A :
Chap. 3. Controlled Systems, Controllability 1. Controllability of Linear Systems 1.1. Kalman s Criterion Consider the linear system ẋ = Ax + Bu where x R n : state vector and u R m : input vector. A :
ECE 275A Homework #3 Solutions
 ECE 75A Homework #3 Solutions. Proof of (a). Obviously Ax = 0 y, Ax = 0 for all y. To show sufficiency, note that if y, Ax = 0 for all y, then it must certainly be true for the particular value of y =
ECE 75A Homework #3 Solutions. Proof of (a). Obviously Ax = 0 y, Ax = 0 for all y. To show sufficiency, note that if y, Ax = 0 for all y, then it must certainly be true for the particular value of y =
Analytica Chimica Acta
 Analytica Chimica Acta 767 (213) 21 34 Contents lists available at SciVerse ScienceDirect Analytica Chimica Acta j ourna l ho me page: www.elsevier.com/locate/aca Extent-based kinetic identification using
Analytica Chimica Acta 767 (213) 21 34 Contents lists available at SciVerse ScienceDirect Analytica Chimica Acta j ourna l ho me page: www.elsevier.com/locate/aca Extent-based kinetic identification using
DESIGN OF PROBABILISTIC OBSERVERS FOR MASS-BALANCE BASED BIOPROCESS MODELS. Benoît Chachuat and Olivier Bernard
 DESIGN OF PROBABILISTIC OBSERVERS FOR MASS-BALANCE BASED BIOPROCESS MODELS Benoît Chachuat and Olivier Bernard INRIA Comore, BP 93, 692 Sophia-Antipolis, France fax: +33 492 387 858 email: Olivier.Bernard@inria.fr
DESIGN OF PROBABILISTIC OBSERVERS FOR MASS-BALANCE BASED BIOPROCESS MODELS Benoît Chachuat and Olivier Bernard INRIA Comore, BP 93, 692 Sophia-Antipolis, France fax: +33 492 387 858 email: Olivier.Bernard@inria.fr
MATRIX ALGEBRA AND SYSTEMS OF EQUATIONS. + + x 1 x 2. x n 8 (4) 3 4 2
 MATRIX ALGEBRA AND SYSTEMS OF EQUATIONS SYSTEMS OF EQUATIONS AND MATRICES Representation of a linear system The general system of m equations in n unknowns can be written a x + a 2 x 2 + + a n x n b a
MATRIX ALGEBRA AND SYSTEMS OF EQUATIONS SYSTEMS OF EQUATIONS AND MATRICES Representation of a linear system The general system of m equations in n unknowns can be written a x + a 2 x 2 + + a n x n b a
APPENDIX A. Background Mathematics. A.1 Linear Algebra. Vector algebra. Let x denote the n-dimensional column vector with components x 1 x 2.
 APPENDIX A Background Mathematics A. Linear Algebra A.. Vector algebra Let x denote the n-dimensional column vector with components 0 x x 2 B C @. A x n Definition 6 (scalar product). The scalar product
APPENDIX A Background Mathematics A. Linear Algebra A.. Vector algebra Let x denote the n-dimensional column vector with components 0 x x 2 B C @. A x n Definition 6 (scalar product). The scalar product
Extent computation under rank-deficient conditions
 Extent computation under rank-deficient conditions Alma Mašić Julien Billeter Dominique Bonvin Kris illez Eawag: Swiss Federal Institute of Aquatic Science and Technology, Überlandstrasse 133, CH-86 Dübendorf,
Extent computation under rank-deficient conditions Alma Mašić Julien Billeter Dominique Bonvin Kris illez Eawag: Swiss Federal Institute of Aquatic Science and Technology, Überlandstrasse 133, CH-86 Dübendorf,
Chapter 6. Differentially Flat Systems
 Contents CAS, Mines-ParisTech 2008 Contents Contents 1, Linear Case Introductory Example: Linear Motor with Appended Mass General Solution (Linear Case) Contents Contents 1, Linear Case Introductory Example:
Contents CAS, Mines-ParisTech 2008 Contents Contents 1, Linear Case Introductory Example: Linear Motor with Appended Mass General Solution (Linear Case) Contents Contents 1, Linear Case Introductory Example:
Theoretical Models of Chemical Processes
 Theoretical Models of Chemical Processes Dr. M. A. A. Shoukat Choudhury 1 Rationale for Dynamic Models 1. Improve understanding of the process 2. Train Plant operating personnel 3. Develop control strategy
Theoretical Models of Chemical Processes Dr. M. A. A. Shoukat Choudhury 1 Rationale for Dynamic Models 1. Improve understanding of the process 2. Train Plant operating personnel 3. Develop control strategy
Linear Algebra and Robot Modeling
 Linear Algebra and Robot Modeling Nathan Ratliff Abstract Linear algebra is fundamental to robot modeling, control, and optimization. This document reviews some of the basic kinematic equations and uses
Linear Algebra and Robot Modeling Nathan Ratliff Abstract Linear algebra is fundamental to robot modeling, control, and optimization. This document reviews some of the basic kinematic equations and uses
SCALE-UP OF BATCH PROCESSES VIA DECENTRALIZED CONTROL. A. Marchetti, M. Amrhein, B. Chachuat and D. Bonvin
 SCALE-UP OF BATCH PROCESSES VIA DECENTRALIZED CONTROL A. Marchetti, M. Amrhein, B. Chachuat and D. Bonvin Laboratoire d Automatique Ecole Polytechnique Fédérale de Lausanne (EPFL) CH-1015 Lausanne, Switzerland
SCALE-UP OF BATCH PROCESSES VIA DECENTRALIZED CONTROL A. Marchetti, M. Amrhein, B. Chachuat and D. Bonvin Laboratoire d Automatique Ecole Polytechnique Fédérale de Lausanne (EPFL) CH-1015 Lausanne, Switzerland
Review of similarity transformation and Singular Value Decomposition
 Review of similarity transformation and Singular Value Decomposition Nasser M Abbasi Applied Mathematics Department, California State University, Fullerton July 8 7 page compiled on June 9, 5 at 9:5pm
Review of similarity transformation and Singular Value Decomposition Nasser M Abbasi Applied Mathematics Department, California State University, Fullerton July 8 7 page compiled on June 9, 5 at 9:5pm
Chapter 4 Copolymerization
 Chapter 4 Copolymerization 4.1 Kinetics of Copolymerization 4.1.1 Involved Chemical Reactions Initiation I 2 + M 2R 1 r = 2 fk d I 2 R I Propagation Chain Transfer Termination m,n + k p m+1,n m,n + B k
Chapter 4 Copolymerization 4.1 Kinetics of Copolymerization 4.1.1 Involved Chemical Reactions Initiation I 2 + M 2R 1 r = 2 fk d I 2 R I Propagation Chain Transfer Termination m,n + k p m+1,n m,n + B k
ROBUST STABLE NONLINEAR CONTROL AND DESIGN OF A CSTR IN A LARGE OPERATING RANGE. Johannes Gerhard, Martin Mönnigmann, Wolfgang Marquardt
 ROBUST STABLE NONLINEAR CONTROL AND DESIGN OF A CSTR IN A LARGE OPERATING RANGE Johannes Gerhard, Martin Mönnigmann, Wolfgang Marquardt Lehrstuhl für Prozesstechnik, RWTH Aachen Turmstr. 46, D-5264 Aachen,
ROBUST STABLE NONLINEAR CONTROL AND DESIGN OF A CSTR IN A LARGE OPERATING RANGE Johannes Gerhard, Martin Mönnigmann, Wolfgang Marquardt Lehrstuhl für Prozesstechnik, RWTH Aachen Turmstr. 46, D-5264 Aachen,
Solutions for Tutorial 3 Modelling of Dynamic Systems
 Solutions for Tutorial 3 Modelling of Dynamic Systems 3.1 Mixer: Dynamic model of a CSTR is derived in textbook Example 3.1. From the model, we know that the outlet concentration of, C, can be affected
Solutions for Tutorial 3 Modelling of Dynamic Systems 3.1 Mixer: Dynamic model of a CSTR is derived in textbook Example 3.1. From the model, we know that the outlet concentration of, C, can be affected
Plug flow Steady-state flow. Mixed flow
 1 IDEAL REACTOR TYPES Batch Plug flow Steady-state flow Mixed flow Ideal Batch Reactor It has neither inflow nor outflow of reactants or products when the reaction is being carried out. Uniform composition
1 IDEAL REACTOR TYPES Batch Plug flow Steady-state flow Mixed flow Ideal Batch Reactor It has neither inflow nor outflow of reactants or products when the reaction is being carried out. Uniform composition
Accuracy of Mathematical Model with Regard to Safety Analysis of Chemical Reactors*
 Accuracy of Mathematical Model with Regard to Safety Analysis of Chemical Reactors* A. MOLNÁR, J. MARKOŠ, and Ľ. JELEMENSKÝ Department of Chemical and Biochemical Engineering, Faculty of Chemical and Food
Accuracy of Mathematical Model with Regard to Safety Analysis of Chemical Reactors* A. MOLNÁR, J. MARKOŠ, and Ľ. JELEMENSKÝ Department of Chemical and Biochemical Engineering, Faculty of Chemical and Food
SPARSE signal representations have gained popularity in recent
 6958 IEEE TRANSACTIONS ON INFORMATION THEORY, VOL. 57, NO. 10, OCTOBER 2011 Blind Compressed Sensing Sivan Gleichman and Yonina C. Eldar, Senior Member, IEEE Abstract The fundamental principle underlying
6958 IEEE TRANSACTIONS ON INFORMATION THEORY, VOL. 57, NO. 10, OCTOBER 2011 Blind Compressed Sensing Sivan Gleichman and Yonina C. Eldar, Senior Member, IEEE Abstract The fundamental principle underlying
Module 6 : Solving Ordinary Differential Equations - Initial Value Problems (ODE-IVPs) Section 3 : Analytical Solutions of Linear ODE-IVPs
 Module 6 : Solving Ordinary Differential Equations - Initial Value Problems (ODE-IVPs) Section 3 : Analytical Solutions of Linear ODE-IVPs 3 Analytical Solutions of Linear ODE-IVPs Before developing numerical
Module 6 : Solving Ordinary Differential Equations - Initial Value Problems (ODE-IVPs) Section 3 : Analytical Solutions of Linear ODE-IVPs 3 Analytical Solutions of Linear ODE-IVPs Before developing numerical
Solutions for Tutorial 4 Modelling of Non-Linear Systems
 Solutions for Tutorial 4 Modelling of Non-Linear Systems 4.1 Isothermal CSTR: The chemical reactor shown in textbook igure 3.1 and repeated in the following is considered in this question. The reaction
Solutions for Tutorial 4 Modelling of Non-Linear Systems 4.1 Isothermal CSTR: The chemical reactor shown in textbook igure 3.1 and repeated in the following is considered in this question. The reaction
Linear Algebra. The analysis of many models in the social sciences reduces to the study of systems of equations.
 POLI 7 - Mathematical and Statistical Foundations Prof S Saiegh Fall Lecture Notes - Class 4 October 4, Linear Algebra The analysis of many models in the social sciences reduces to the study of systems
POLI 7 - Mathematical and Statistical Foundations Prof S Saiegh Fall Lecture Notes - Class 4 October 4, Linear Algebra The analysis of many models in the social sciences reduces to the study of systems
Linear Systems. Carlo Tomasi. June 12, r = rank(a) b range(a) n r solutions
 Linear Systems Carlo Tomasi June, 08 Section characterizes the existence and multiplicity of the solutions of a linear system in terms of the four fundamental spaces associated with the system s matrix
Linear Systems Carlo Tomasi June, 08 Section characterizes the existence and multiplicity of the solutions of a linear system in terms of the four fundamental spaces associated with the system s matrix
Chemical Reaction Engineering. Multiple Reactions. Dr.-Eng. Zayed Al-Hamamre
 Chemical Reaction Engineering Multiple Reactions Dr.-Eng. Zayed Al-Hamamre 1 Content Types of Reactions Selectivity Reaction Yield Parallel Reactions Series Reactions Net Rates of Reaction Complex Reactions
Chemical Reaction Engineering Multiple Reactions Dr.-Eng. Zayed Al-Hamamre 1 Content Types of Reactions Selectivity Reaction Yield Parallel Reactions Series Reactions Net Rates of Reaction Complex Reactions
Chemical Reaction Engineering - Part 14 - intro to CSTRs Richard K. Herz,
 Chemical Reaction Engineering - Part 4 - intro to CSTRs Richard K. Herz, rherz@ucsd.edu, www.reactorlab.net Continuous Stirred Tank Reactors - CSTRs Here are a couple screenshots from the ReactorLab, Division
Chemical Reaction Engineering - Part 4 - intro to CSTRs Richard K. Herz, rherz@ucsd.edu, www.reactorlab.net Continuous Stirred Tank Reactors - CSTRs Here are a couple screenshots from the ReactorLab, Division
APPENDIX A. Matrix Algebra. a n1 a n2 g a nk
 APPENDIX A Matrix Algebra A.1 TERMINOLOGY A matrix is a rectangular array of numbers, denoted a 11 a 12 g a 1K a A = [a ik ] = [A] ik = D 21 a 22 g a 2K T. g a n1 a n2 g a nk (A-1) The typical element
APPENDIX A Matrix Algebra A.1 TERMINOLOGY A matrix is a rectangular array of numbers, denoted a 11 a 12 g a 1K a A = [a ik ] = [A] ik = D 21 a 22 g a 2K T. g a n1 a n2 g a nk (A-1) The typical element
Mathematics for Control Theory
 Mathematics for Control Theory Geometric Concepts in Control Involutivity and Frobenius Theorem Exact Linearization Hanz Richter Mechanical Engineering Department Cleveland State University Reading materials
Mathematics for Control Theory Geometric Concepts in Control Involutivity and Frobenius Theorem Exact Linearization Hanz Richter Mechanical Engineering Department Cleveland State University Reading materials
Investigation of adiabatic batch reactor
 Investigation of adiabatic batch reactor Introduction The theory of chemical reactors is summarized in instructions to Investigation of chemical reactors. If a reactor operates adiabatically then no heat
Investigation of adiabatic batch reactor Introduction The theory of chemical reactors is summarized in instructions to Investigation of chemical reactors. If a reactor operates adiabatically then no heat
Measurement-based Run-to-run Optimization of a Batch Reaction-distillation System
 Measurement-based Run-to-run Optimization of a Batch Reaction-distillation System A. Marchetti a, B. Srinivasan a, D. Bonvin a*, S. Elgue b, L. Prat b and M. Cabassud b a Laboratoire d Automatique Ecole
Measurement-based Run-to-run Optimization of a Batch Reaction-distillation System A. Marchetti a, B. Srinivasan a, D. Bonvin a*, S. Elgue b, L. Prat b and M. Cabassud b a Laboratoire d Automatique Ecole
Solution of Linear Equations
 Solution of Linear Equations (Com S 477/577 Notes) Yan-Bin Jia Sep 7, 07 We have discussed general methods for solving arbitrary equations, and looked at the special class of polynomial equations A subclass
Solution of Linear Equations (Com S 477/577 Notes) Yan-Bin Jia Sep 7, 07 We have discussed general methods for solving arbitrary equations, and looked at the special class of polynomial equations A subclass
Chemical Reaction Engineering Lecture 5
 Chemical Reaction Engineering g Lecture 5 The Scope The im of the Course: To learn how to describe a system where a (bio)chemical reaction takes place (further called reactor) Reactors Pharmacokinetics
Chemical Reaction Engineering g Lecture 5 The Scope The im of the Course: To learn how to describe a system where a (bio)chemical reaction takes place (further called reactor) Reactors Pharmacokinetics
Observability for deterministic systems and high-gain observers
 Observability for deterministic systems and high-gain observers design. Part 1. March 29, 2011 Introduction and problem description Definition of observability Consequences of instantaneous observability
Observability for deterministic systems and high-gain observers design. Part 1. March 29, 2011 Introduction and problem description Definition of observability Consequences of instantaneous observability
EE731 Lecture Notes: Matrix Computations for Signal Processing
 EE731 Lecture Notes: Matrix Computations for Signal Processing James P. Reilly c Department of Electrical and Computer Engineering McMaster University September 22, 2005 0 Preface This collection of ten
EE731 Lecture Notes: Matrix Computations for Signal Processing James P. Reilly c Department of Electrical and Computer Engineering McMaster University September 22, 2005 0 Preface This collection of ten
BASIC NOTIONS. x + y = 1 3, 3x 5y + z = A + 3B,C + 2D, DC are not defined. A + C =
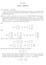 CHAPTER I BASIC NOTIONS (a) 8666 and 8833 (b) a =6,a =4 will work in the first case, but there are no possible such weightings to produce the second case, since Student and Student 3 have to end up with
CHAPTER I BASIC NOTIONS (a) 8666 and 8833 (b) a =6,a =4 will work in the first case, but there are no possible such weightings to produce the second case, since Student and Student 3 have to end up with
EXERCISE SET 5.1. = (kx + kx + k, ky + ky + k ) = (kx + kx + 1, ky + ky + 1) = ((k + )x + 1, (k + )y + 1)
 EXERCISE SET 5. 6. The pair (, 2) is in the set but the pair ( )(, 2) = (, 2) is not because the first component is negative; hence Axiom 6 fails. Axiom 5 also fails. 8. Axioms, 2, 3, 6, 9, and are easily
EXERCISE SET 5. 6. The pair (, 2) is in the set but the pair ( )(, 2) = (, 2) is not because the first component is negative; hence Axiom 6 fails. Axiom 5 also fails. 8. Axioms, 2, 3, 6, 9, and are easily
Dynamics and Control of Reactor - Feed Effluent Heat Exchanger Networks
 2008 American Control Conference Westin Seattle Hotel Seattle Washington USA June 11-13 2008 WeC08.2 Dynamics and Control of Reactor - Feed Effluent Heat Exchanger Networks Sujit S. Jogwar Michael Baldea
2008 American Control Conference Westin Seattle Hotel Seattle Washington USA June 11-13 2008 WeC08.2 Dynamics and Control of Reactor - Feed Effluent Heat Exchanger Networks Sujit S. Jogwar Michael Baldea
Chapter 7. Linear Algebra: Matrices, Vectors,
 Chapter 7. Linear Algebra: Matrices, Vectors, Determinants. Linear Systems Linear algebra includes the theory and application of linear systems of equations, linear transformations, and eigenvalue problems.
Chapter 7. Linear Algebra: Matrices, Vectors, Determinants. Linear Systems Linear algebra includes the theory and application of linear systems of equations, linear transformations, and eigenvalue problems.
Nonlinear Control Lecture 9: Feedback Linearization
 Nonlinear Control Lecture 9: Feedback Linearization Farzaneh Abdollahi Department of Electrical Engineering Amirkabir University of Technology Fall 2011 Farzaneh Abdollahi Nonlinear Control Lecture 9 1/75
Nonlinear Control Lecture 9: Feedback Linearization Farzaneh Abdollahi Department of Electrical Engineering Amirkabir University of Technology Fall 2011 Farzaneh Abdollahi Nonlinear Control Lecture 9 1/75
Econ Slides from Lecture 7
 Econ 205 Sobel Econ 205 - Slides from Lecture 7 Joel Sobel August 31, 2010 Linear Algebra: Main Theory A linear combination of a collection of vectors {x 1,..., x k } is a vector of the form k λ ix i for
Econ 205 Sobel Econ 205 - Slides from Lecture 7 Joel Sobel August 31, 2010 Linear Algebra: Main Theory A linear combination of a collection of vectors {x 1,..., x k } is a vector of the form k λ ix i for
Hierarchically Consistent Control Systems
 1144 IEEE TRANSACTIONS ON AUTOMATIC CONTROL, VOL. 45, NO. 6, JUNE 2000 Hierarchically Consistent Control Systems George J. Pappas, Member, IEEE, Gerardo Lafferriere, Shankar Sastry, Fellow, IEEE Abstract
1144 IEEE TRANSACTIONS ON AUTOMATIC CONTROL, VOL. 45, NO. 6, JUNE 2000 Hierarchically Consistent Control Systems George J. Pappas, Member, IEEE, Gerardo Lafferriere, Shankar Sastry, Fellow, IEEE Abstract
BACKSTEPPING CONTROL DESIGN FOR A CONTINUOUS-STIRRED TANK. Saleh Alshamali and Mohamed Zribi. Received July 2011; revised March 2012
 International Journal of Innovative Computing, Information and Control ICIC International c 202 ISSN 349-498 Volume 8, Number, November 202 pp. 7747 7760 BACKSTEPPING CONTROL DESIGN FOR A CONTINUOUS-STIRRED
International Journal of Innovative Computing, Information and Control ICIC International c 202 ISSN 349-498 Volume 8, Number, November 202 pp. 7747 7760 BACKSTEPPING CONTROL DESIGN FOR A CONTINUOUS-STIRRED
Feedback Linearization
 Feedback Linearization Peter Al Hokayem and Eduardo Gallestey May 14, 2015 1 Introduction Consider a class o single-input-single-output (SISO) nonlinear systems o the orm ẋ = (x) + g(x)u (1) y = h(x) (2)
Feedback Linearization Peter Al Hokayem and Eduardo Gallestey May 14, 2015 1 Introduction Consider a class o single-input-single-output (SISO) nonlinear systems o the orm ẋ = (x) + g(x)u (1) y = h(x) (2)
1. Introductory Material
 CHEE 321: Chemical Reaction Engineering 1. Introductory Material 1b. The General Mole Balance Equation (GMBE) and Ideal Reactors (Fogler Chapter 1) Recap: Module 1a System with Rxn: use mole balances Input
CHEE 321: Chemical Reaction Engineering 1. Introductory Material 1b. The General Mole Balance Equation (GMBE) and Ideal Reactors (Fogler Chapter 1) Recap: Module 1a System with Rxn: use mole balances Input
Full-State Feedback Design for a Multi-Input System
 Full-State Feedback Design for a Multi-Input System A. Introduction The open-loop system is described by the following state space model. x(t) = Ax(t)+Bu(t), y(t) =Cx(t)+Du(t) () 4 8.5 A =, B =.5.5, C
Full-State Feedback Design for a Multi-Input System A. Introduction The open-loop system is described by the following state space model. x(t) = Ax(t)+Bu(t), y(t) =Cx(t)+Du(t) () 4 8.5 A =, B =.5.5, C
The dual simplex method with bounds
 The dual simplex method with bounds Linear programming basis. Let a linear programming problem be given by min s.t. c T x Ax = b x R n, (P) where we assume A R m n to be full row rank (we will see in the
The dual simplex method with bounds Linear programming basis. Let a linear programming problem be given by min s.t. c T x Ax = b x R n, (P) where we assume A R m n to be full row rank (we will see in the
ChE 344 Winter 2013 Mid Term Exam I Tuesday, February 26, Closed Book, Web, and Notes. Honor Code
 ChE 344 Winter 2013 Mid Term Exam I Tuesday, February 26, 2013 Closed Book, Web, and Notes Name Honor Code (Sign at the end of exam period) 1) / 5 pts 2) / 5 pts 3) / 5 pts 4) / 5 pts 5) / 5 pts 6) / 5
ChE 344 Winter 2013 Mid Term Exam I Tuesday, February 26, 2013 Closed Book, Web, and Notes Name Honor Code (Sign at the end of exam period) 1) / 5 pts 2) / 5 pts 3) / 5 pts 4) / 5 pts 5) / 5 pts 6) / 5
Vector and Matrix Norms. Vector and Matrix Norms
 Vector and Matrix Norms Vector Space Algebra Matrix Algebra: We let x x and A A, where, if x is an element of an abstract vector space n, and A = A: n m, then x is a complex column vector of length n whose
Vector and Matrix Norms Vector Space Algebra Matrix Algebra: We let x x and A A, where, if x is an element of an abstract vector space n, and A = A: n m, then x is a complex column vector of length n whose
MATH 1120 (LINEAR ALGEBRA 1), FINAL EXAM FALL 2011 SOLUTIONS TO PRACTICE VERSION
 MATH (LINEAR ALGEBRA ) FINAL EXAM FALL SOLUTIONS TO PRACTICE VERSION Problem (a) For each matrix below (i) find a basis for its column space (ii) find a basis for its row space (iii) determine whether
MATH (LINEAR ALGEBRA ) FINAL EXAM FALL SOLUTIONS TO PRACTICE VERSION Problem (a) For each matrix below (i) find a basis for its column space (ii) find a basis for its row space (iii) determine whether
Linear Systems. Carlo Tomasi
 Linear Systems Carlo Tomasi Section 1 characterizes the existence and multiplicity of the solutions of a linear system in terms of the four fundamental spaces associated with the system s matrix and of
Linear Systems Carlo Tomasi Section 1 characterizes the existence and multiplicity of the solutions of a linear system in terms of the four fundamental spaces associated with the system s matrix and of
Lecture 2: Linear Algebra Review
 EE 227A: Convex Optimization and Applications January 19 Lecture 2: Linear Algebra Review Lecturer: Mert Pilanci Reading assignment: Appendix C of BV. Sections 2-6 of the web textbook 1 2.1 Vectors 2.1.1
EE 227A: Convex Optimization and Applications January 19 Lecture 2: Linear Algebra Review Lecturer: Mert Pilanci Reading assignment: Appendix C of BV. Sections 2-6 of the web textbook 1 2.1 Vectors 2.1.1
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Chemistry 5.76 Revised February, 1982 NOTES ON MATRIX METHODS
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY Chemistry 5.76 Revised February, 198 NOTES ON MATRIX METHODS 1. Matrix Algebra Margenau and Murphy, The Mathematics of Physics and Chemistry, Chapter 10, give almost
MASSACHUSETTS INSTITUTE OF TECHNOLOGY Chemistry 5.76 Revised February, 198 NOTES ON MATRIX METHODS 1. Matrix Algebra Margenau and Murphy, The Mathematics of Physics and Chemistry, Chapter 10, give almost
Queen s University at Kingston. CHEE Winter Process Dynamics and Control. M. Guay. Quiz 1
 Queen s University at Kingston CHEE 319 - Winter 2011 Process Dynamics and Control M. Guay Quiz 1 Instructions: 1. This is a 50 minute examination. 2. Please write your student number instead of your name
Queen s University at Kingston CHEE 319 - Winter 2011 Process Dynamics and Control M. Guay Quiz 1 Instructions: 1. This is a 50 minute examination. 2. Please write your student number instead of your name
Empirical Gramians and Balanced Truncation for Model Reduction of Nonlinear Systems
 Empirical Gramians and Balanced Truncation for Model Reduction of Nonlinear Systems Antoni Ras Departament de Matemàtica Aplicada 4 Universitat Politècnica de Catalunya Lecture goals To review the basic
Empirical Gramians and Balanced Truncation for Model Reduction of Nonlinear Systems Antoni Ras Departament de Matemàtica Aplicada 4 Universitat Politècnica de Catalunya Lecture goals To review the basic
Chemical Reaction Engineering - Part 12 - multiple reactions Richard K. Herz,
 Chemical Reaction Engineering - Part 12 - multiple reactions Richard K. Herz, rherz@ucsd.edu, www.reactorlab.net Multiple reactions are usually present So far we have considered reactors in which only
Chemical Reaction Engineering - Part 12 - multiple reactions Richard K. Herz, rherz@ucsd.edu, www.reactorlab.net Multiple reactions are usually present So far we have considered reactors in which only
Chemical Reaction Engineering. Dr. Yahia Alhamed
 Chemical Reaction Engineering Dr. Yahia Alhamed 1 Kinetics and Reaction Rate What is reaction rate? It is the rate at which a species looses its chemical identity per unit volume. The rate of a reaction
Chemical Reaction Engineering Dr. Yahia Alhamed 1 Kinetics and Reaction Rate What is reaction rate? It is the rate at which a species looses its chemical identity per unit volume. The rate of a reaction
Linear Algebra, Summer 2011, pt. 2
 Linear Algebra, Summer 2, pt. 2 June 8, 2 Contents Inverses. 2 Vector Spaces. 3 2. Examples of vector spaces..................... 3 2.2 The column space......................... 6 2.3 The null space...........................
Linear Algebra, Summer 2, pt. 2 June 8, 2 Contents Inverses. 2 Vector Spaces. 3 2. Examples of vector spaces..................... 3 2.2 The column space......................... 6 2.3 The null space...........................
SAMPLE OF THE STUDY MATERIAL PART OF CHAPTER 1 Introduction to Linear Algebra
 SAMPLE OF THE STUDY MATERIAL PART OF CHAPTER 1 Introduction to 1.1. Introduction Linear algebra is a specific branch of mathematics dealing with the study of vectors, vector spaces with functions that
SAMPLE OF THE STUDY MATERIAL PART OF CHAPTER 1 Introduction to 1.1. Introduction Linear algebra is a specific branch of mathematics dealing with the study of vectors, vector spaces with functions that
Stat 159/259: Linear Algebra Notes
 Stat 159/259: Linear Algebra Notes Jarrod Millman November 16, 2015 Abstract These notes assume you ve taken a semester of undergraduate linear algebra. In particular, I assume you are familiar with the
Stat 159/259: Linear Algebra Notes Jarrod Millman November 16, 2015 Abstract These notes assume you ve taken a semester of undergraduate linear algebra. In particular, I assume you are familiar with the
ROBUST CONSTRAINED ESTIMATION VIA UNSCENTED TRANSFORMATION. Pramod Vachhani a, Shankar Narasimhan b and Raghunathan Rengaswamy a 1
 ROUST CONSTRINED ESTIMTION VI UNSCENTED TRNSFORMTION Pramod Vachhani a, Shankar Narasimhan b and Raghunathan Rengaswamy a a Department of Chemical Engineering, Clarkson University, Potsdam, NY -3699, US.
ROUST CONSTRINED ESTIMTION VI UNSCENTED TRNSFORMTION Pramod Vachhani a, Shankar Narasimhan b and Raghunathan Rengaswamy a a Department of Chemical Engineering, Clarkson University, Potsdam, NY -3699, US.
A First Course on Kinetics and Reaction Engineering Unit 22. Analysis of Steady State CSTRs
 Unit 22. Analysis of Steady State CSRs Overview Reaction engineering involves constructing an accurate mathematical model of a real world reactor and then using that model to perform an engineering task
Unit 22. Analysis of Steady State CSRs Overview Reaction engineering involves constructing an accurate mathematical model of a real world reactor and then using that model to perform an engineering task
MATLAB Ordinary Differential Equation (ODE) solver for a simple example 1. Introduction
 MATLAB Ordinary Differential Equation (ODE) solver for a simple example 1. Introduction Differential equations are a convenient way to express mathematically a change of a dependent variable (e.g. concentration
MATLAB Ordinary Differential Equation (ODE) solver for a simple example 1. Introduction Differential equations are a convenient way to express mathematically a change of a dependent variable (e.g. concentration
Linear Algebra 2 Spectral Notes
 Linear Algebra 2 Spectral Notes In what follows, V is an inner product vector space over F, where F = R or C. We will use results seen so far; in particular that every linear operator T L(V ) has a complex
Linear Algebra 2 Spectral Notes In what follows, V is an inner product vector space over F, where F = R or C. We will use results seen so far; in particular that every linear operator T L(V ) has a complex
Parallel Singular Value Decomposition. Jiaxing Tan
 Parallel Singular Value Decomposition Jiaxing Tan Outline What is SVD? How to calculate SVD? How to parallelize SVD? Future Work What is SVD? Matrix Decomposition Eigen Decomposition A (non-zero) vector
Parallel Singular Value Decomposition Jiaxing Tan Outline What is SVD? How to calculate SVD? How to parallelize SVD? Future Work What is SVD? Matrix Decomposition Eigen Decomposition A (non-zero) vector
Image Registration Lecture 2: Vectors and Matrices
 Image Registration Lecture 2: Vectors and Matrices Prof. Charlene Tsai Lecture Overview Vectors Matrices Basics Orthogonal matrices Singular Value Decomposition (SVD) 2 1 Preliminary Comments Some of this
Image Registration Lecture 2: Vectors and Matrices Prof. Charlene Tsai Lecture Overview Vectors Matrices Basics Orthogonal matrices Singular Value Decomposition (SVD) 2 1 Preliminary Comments Some of this
Pseudoinverse & Moore-Penrose Conditions
 ECE 275AB Lecture 7 Fall 2008 V1.0 c K. Kreutz-Delgado, UC San Diego p. 1/1 Lecture 7 ECE 275A Pseudoinverse & Moore-Penrose Conditions ECE 275AB Lecture 7 Fall 2008 V1.0 c K. Kreutz-Delgado, UC San Diego
ECE 275AB Lecture 7 Fall 2008 V1.0 c K. Kreutz-Delgado, UC San Diego p. 1/1 Lecture 7 ECE 275A Pseudoinverse & Moore-Penrose Conditions ECE 275AB Lecture 7 Fall 2008 V1.0 c K. Kreutz-Delgado, UC San Diego
IDEAL REACTORS FOR HOMOGENOUS REACTION AND THEIR PERFORMANCE EQUATIONS
 IDEAL REACTORS FOR HOMOGENOUS REACTION AND THEIR PERFORMANCE EQUATIONS At the end of this week s lecture, students should be able to: Differentiate between the three ideal reactors Develop and apply the
IDEAL REACTORS FOR HOMOGENOUS REACTION AND THEIR PERFORMANCE EQUATIONS At the end of this week s lecture, students should be able to: Differentiate between the three ideal reactors Develop and apply the
Reactors. Reaction Classifications
 Reactors Reactions are usually the heart of the chemical processes in which relatively cheap raw materials are converted to more economically favorable products. In other cases, reactions play essential
Reactors Reactions are usually the heart of the chemical processes in which relatively cheap raw materials are converted to more economically favorable products. In other cases, reactions play essential
CONTROL OF MULTIVARIABLE PROCESSES
 Process plants ( or complex experiments) have many variables that must be controlled. The engineer must. Provide the needed sensors 2. Provide adequate manipulated variables 3. Decide how the CVs and MVs
Process plants ( or complex experiments) have many variables that must be controlled. The engineer must. Provide the needed sensors 2. Provide adequate manipulated variables 3. Decide how the CVs and MVs
SAMPLE OF THE STUDY MATERIAL PART OF CHAPTER 1 Introduction to Linear Algebra
 1.1. Introduction SAMPLE OF THE STUDY MATERIAL PART OF CHAPTER 1 Introduction to Linear algebra is a specific branch of mathematics dealing with the study of vectors, vector spaces with functions that
1.1. Introduction SAMPLE OF THE STUDY MATERIAL PART OF CHAPTER 1 Introduction to Linear algebra is a specific branch of mathematics dealing with the study of vectors, vector spaces with functions that
Degenerate Perturbation Theory. 1 General framework and strategy
 Physics G6037 Professor Christ 12/22/2015 Degenerate Perturbation Theory The treatment of degenerate perturbation theory presented in class is written out here in detail. The appendix presents the underlying
Physics G6037 Professor Christ 12/22/2015 Degenerate Perturbation Theory The treatment of degenerate perturbation theory presented in class is written out here in detail. The appendix presents the underlying
Singular Value Decomposition. 1 Singular Value Decomposition and the Four Fundamental Subspaces
 Singular Value Decomposition This handout is a review of some basic concepts in linear algebra For a detailed introduction, consult a linear algebra text Linear lgebra and its pplications by Gilbert Strang
Singular Value Decomposition This handout is a review of some basic concepts in linear algebra For a detailed introduction, consult a linear algebra text Linear lgebra and its pplications by Gilbert Strang
(a) If A is a 3 by 4 matrix, what does this tell us about its nullspace? Solution: dim N(A) 1, since rank(a) 3. Ax =
 . (5 points) (a) If A is a 3 by 4 matrix, what does this tell us about its nullspace? dim N(A), since rank(a) 3. (b) If we also know that Ax = has no solution, what do we know about the rank of A? C(A)
. (5 points) (a) If A is a 3 by 4 matrix, what does this tell us about its nullspace? dim N(A), since rank(a) 3. (b) If we also know that Ax = has no solution, what do we know about the rank of A? C(A)
ELA THE MINIMUM-NORM LEAST-SQUARES SOLUTION OF A LINEAR SYSTEM AND SYMMETRIC RANK-ONE UPDATES
 Volume 22, pp. 480-489, May 20 THE MINIMUM-NORM LEAST-SQUARES SOLUTION OF A LINEAR SYSTEM AND SYMMETRIC RANK-ONE UPDATES XUZHOU CHEN AND JUN JI Abstract. In this paper, we study the Moore-Penrose inverse
Volume 22, pp. 480-489, May 20 THE MINIMUM-NORM LEAST-SQUARES SOLUTION OF A LINEAR SYSTEM AND SYMMETRIC RANK-ONE UPDATES XUZHOU CHEN AND JUN JI Abstract. In this paper, we study the Moore-Penrose inverse
4 Matrix Diagonalization and Eigensystems
 14.102, Math for Economists Fall 2004 Lecture Notes, 9/21/2004 These notes are primarily based on those written by George Marios Angeletos for the Harvard Math Camp in 1999 and 2000, and updated by Stavros
14.102, Math for Economists Fall 2004 Lecture Notes, 9/21/2004 These notes are primarily based on those written by George Marios Angeletos for the Harvard Math Camp in 1999 and 2000, and updated by Stavros
Lecture: Face Recognition and Feature Reduction
 Lecture: Face Recognition and Feature Reduction Juan Carlos Niebles and Ranjay Krishna Stanford Vision and Learning Lab 1 Recap - Curse of dimensionality Assume 5000 points uniformly distributed in the
Lecture: Face Recognition and Feature Reduction Juan Carlos Niebles and Ranjay Krishna Stanford Vision and Learning Lab 1 Recap - Curse of dimensionality Assume 5000 points uniformly distributed in the
CONTROL DESIGN FOR SET POINT TRACKING
 Chapter 5 CONTROL DESIGN FOR SET POINT TRACKING In this chapter, we extend the pole placement, observer-based output feedback design to solve tracking problems. By tracking we mean that the output is commanded
Chapter 5 CONTROL DESIGN FOR SET POINT TRACKING In this chapter, we extend the pole placement, observer-based output feedback design to solve tracking problems. By tracking we mean that the output is commanded
Linear Algebra- Final Exam Review
 Linear Algebra- Final Exam Review. Let A be invertible. Show that, if v, v, v 3 are linearly independent vectors, so are Av, Av, Av 3. NOTE: It should be clear from your answer that you know the definition.
Linear Algebra- Final Exam Review. Let A be invertible. Show that, if v, v, v 3 are linearly independent vectors, so are Av, Av, Av 3. NOTE: It should be clear from your answer that you know the definition.
Analyzing Mass and Heat Transfer Equipment
 Analyzing Mass and Heat Transfer Equipment (MHE) Analyzing Mass and Heat Transfer Equipment Scaling up to solving problems using process equipment requires both continuum and macroscopic knowledge of transport,
Analyzing Mass and Heat Transfer Equipment (MHE) Analyzing Mass and Heat Transfer Equipment Scaling up to solving problems using process equipment requires both continuum and macroscopic knowledge of transport,
arxiv: v1 [cs.sc] 17 Apr 2013
![arxiv: v1 [cs.sc] 17 Apr 2013 arxiv: v1 [cs.sc] 17 Apr 2013](/thumbs/81/84216877.jpg) EFFICIENT CALCULATION OF DETERMINANTS OF SYMBOLIC MATRICES WITH MANY VARIABLES TANYA KHOVANOVA 1 AND ZIV SCULLY 2 arxiv:1304.4691v1 [cs.sc] 17 Apr 2013 Abstract. Efficient matrix determinant calculations
EFFICIENT CALCULATION OF DETERMINANTS OF SYMBOLIC MATRICES WITH MANY VARIABLES TANYA KHOVANOVA 1 AND ZIV SCULLY 2 arxiv:1304.4691v1 [cs.sc] 17 Apr 2013 Abstract. Efficient matrix determinant calculations
Nonlinear Observer Design for Fuel Processing Reactors in Fuel Cell Power Systems
 Nonlinear Observer Design for Fuel Processing Reactors in Fuel Cell Power Systems Haluk Görgün Murat Arcak Rensselaer Polytechnic Institute, roy, NY Subbarao Varigonda Scott A. Bortoff United echnologies
Nonlinear Observer Design for Fuel Processing Reactors in Fuel Cell Power Systems Haluk Görgün Murat Arcak Rensselaer Polytechnic Institute, roy, NY Subbarao Varigonda Scott A. Bortoff United echnologies
Foundations of Matrix Analysis
 1 Foundations of Matrix Analysis In this chapter we recall the basic elements of linear algebra which will be employed in the remainder of the text For most of the proofs as well as for the details, the
1 Foundations of Matrix Analysis In this chapter we recall the basic elements of linear algebra which will be employed in the remainder of the text For most of the proofs as well as for the details, the
A Study of Kruppa s Equation for Camera Self-calibration
 Proceedings of the International Conference of Machine Vision and Machine Learning Prague, Czech Republic, August 14-15, 2014 Paper No. 57 A Study of Kruppa s Equation for Camera Self-calibration Luh Prapitasari,
Proceedings of the International Conference of Machine Vision and Machine Learning Prague, Czech Republic, August 14-15, 2014 Paper No. 57 A Study of Kruppa s Equation for Camera Self-calibration Luh Prapitasari,
Feedback stabilisation with positive control of dissipative compartmental systems
 Feedback stabilisation with positive control of dissipative compartmental systems G. Bastin and A. Provost Centre for Systems Engineering and Applied Mechanics (CESAME Université Catholique de Louvain
Feedback stabilisation with positive control of dissipative compartmental systems G. Bastin and A. Provost Centre for Systems Engineering and Applied Mechanics (CESAME Université Catholique de Louvain
Chemical Kinetics and Reaction Engineering
 Chemical Kinetics and Reaction Engineering MIDTERM EXAMINATION II Friday, April 9, 2010 The exam is 100 points total and 20% of the course grade. Please read through the questions carefully before giving
Chemical Kinetics and Reaction Engineering MIDTERM EXAMINATION II Friday, April 9, 2010 The exam is 100 points total and 20% of the course grade. Please read through the questions carefully before giving
Lecture: Face Recognition and Feature Reduction
 Lecture: Face Recognition and Feature Reduction Juan Carlos Niebles and Ranjay Krishna Stanford Vision and Learning Lab Lecture 11-1 Recap - Curse of dimensionality Assume 5000 points uniformly distributed
Lecture: Face Recognition and Feature Reduction Juan Carlos Niebles and Ranjay Krishna Stanford Vision and Learning Lab Lecture 11-1 Recap - Curse of dimensionality Assume 5000 points uniformly distributed
Numerical Algorithms as Dynamical Systems
 A Study on Numerical Algorithms as Dynamical Systems Moody Chu North Carolina State University What This Study Is About? To recast many numerical algorithms as special dynamical systems, whence to derive
A Study on Numerical Algorithms as Dynamical Systems Moody Chu North Carolina State University What This Study Is About? To recast many numerical algorithms as special dynamical systems, whence to derive
EE731 Lecture Notes: Matrix Computations for Signal Processing
 EE731 Lecture Notes: Matrix Computations for Signal Processing James P. Reilly c Department of Electrical and Computer Engineering McMaster University October 17, 005 Lecture 3 3 he Singular Value Decomposition
EE731 Lecture Notes: Matrix Computations for Signal Processing James P. Reilly c Department of Electrical and Computer Engineering McMaster University October 17, 005 Lecture 3 3 he Singular Value Decomposition
FAULT-TOLERANT CONTROL OF CHEMICAL PROCESS SYSTEMS USING COMMUNICATION NETWORKS. Nael H. El-Farra, Adiwinata Gani & Panagiotis D.
 FAULT-TOLERANT CONTROL OF CHEMICAL PROCESS SYSTEMS USING COMMUNICATION NETWORKS Nael H. El-Farra, Adiwinata Gani & Panagiotis D. Christofides Department of Chemical Engineering University of California,
FAULT-TOLERANT CONTROL OF CHEMICAL PROCESS SYSTEMS USING COMMUNICATION NETWORKS Nael H. El-Farra, Adiwinata Gani & Panagiotis D. Christofides Department of Chemical Engineering University of California,
Web Solved Problems Web Example SP-8.1 Hydrodealkylation of Mesitylene in a PFR CH 3 H 2. m-xylene can also undergo hydrodealkylation to form toluene:
 Chapter 8 Multiple Reactions W8-1 Web Solved Problems Web Example SP-8.1 Hydrodealkylation of Mesitylene in a PFR The production of m-xylene by the hydrodealkylation of mesitylene over a Houdry Detrol
Chapter 8 Multiple Reactions W8-1 Web Solved Problems Web Example SP-8.1 Hydrodealkylation of Mesitylene in a PFR The production of m-xylene by the hydrodealkylation of mesitylene over a Houdry Detrol
Physics 202 Laboratory 5. Linear Algebra 1. Laboratory 5. Physics 202 Laboratory
 Physics 202 Laboratory 5 Linear Algebra Laboratory 5 Physics 202 Laboratory We close our whirlwind tour of numerical methods by advertising some elements of (numerical) linear algebra. There are three
Physics 202 Laboratory 5 Linear Algebra Laboratory 5 Physics 202 Laboratory We close our whirlwind tour of numerical methods by advertising some elements of (numerical) linear algebra. There are three
Run-to-Run MPC Tuning via Gradient Descent
 Ian David Lockhart Bogle and Michael Fairweather (Editors), Proceedings of the nd European Symposium on Computer Aided Process Engineering, 7 - June, London. c Elsevier B.V. All rights reserved. Run-to-Run
Ian David Lockhart Bogle and Michael Fairweather (Editors), Proceedings of the nd European Symposium on Computer Aided Process Engineering, 7 - June, London. c Elsevier B.V. All rights reserved. Run-to-Run
Lie Groups for 2D and 3D Transformations
 Lie Groups for 2D and 3D Transformations Ethan Eade Updated May 20, 2017 * 1 Introduction This document derives useful formulae for working with the Lie groups that represent transformations in 2D and
Lie Groups for 2D and 3D Transformations Ethan Eade Updated May 20, 2017 * 1 Introduction This document derives useful formulae for working with the Lie groups that represent transformations in 2D and
CHEE 222: PROCESS DYNAMICS AND NUMERICAL METHODS
 CHEE 222: PROCESS DYNAMICS AND NUMERICAL METHODS Winter 2017 Module 1: Introduction to Process Modeling Dr. Xiang Li 1 Module 1 Outline 1. Process and Model - Concepts of process and model - Classification
CHEE 222: PROCESS DYNAMICS AND NUMERICAL METHODS Winter 2017 Module 1: Introduction to Process Modeling Dr. Xiang Li 1 Module 1 Outline 1. Process and Model - Concepts of process and model - Classification
Data Mining and Analysis: Fundamental Concepts and Algorithms
 : Fundamental Concepts and Algorithms dataminingbook.info Mohammed J. Zaki 1 Wagner Meira Jr. 2 1 Department of Computer Science Rensselaer Polytechnic Institute, Troy, NY, USA 2 Department of Computer
: Fundamental Concepts and Algorithms dataminingbook.info Mohammed J. Zaki 1 Wagner Meira Jr. 2 1 Department of Computer Science Rensselaer Polytechnic Institute, Troy, NY, USA 2 Department of Computer
MCE693/793: Analysis and Control of Nonlinear Systems
 MCE693/793: Analysis and Control of Nonlinear Systems Input-Output and Input-State Linearization Zero Dynamics of Nonlinear Systems Hanz Richter Mechanical Engineering Department Cleveland State University
MCE693/793: Analysis and Control of Nonlinear Systems Input-Output and Input-State Linearization Zero Dynamics of Nonlinear Systems Hanz Richter Mechanical Engineering Department Cleveland State University
Thermodynamics revisited
 Thermodynamics revisited How can I do an energy balance for a reactor system? 1 st law of thermodynamics (differential form): de de = = dq dq--dw dw Energy: de = du + de kin + de pot + de other du = Work:
Thermodynamics revisited How can I do an energy balance for a reactor system? 1 st law of thermodynamics (differential form): de de = = dq dq--dw dw Energy: de = du + de kin + de pot + de other du = Work:
1 Differentiable manifolds and smooth maps. (Solutions)
 1 Differentiable manifolds and smooth maps Solutions Last updated: March 17 2011 Problem 1 The state of the planar pendulum is entirely defined by the position of its moving end in the plane R 2 Since
1 Differentiable manifolds and smooth maps Solutions Last updated: March 17 2011 Problem 1 The state of the planar pendulum is entirely defined by the position of its moving end in the plane R 2 Since
Modified Mathematical Model For Neutralization System In Stirred Tank Reactor
 Available online at BCREC Website: http://bcrec.undip.ac.id Bulletin of Chemical Reaction Engineering & Catalysis, (), 0, - Research Article Modified Mathematical Model For Neutralization System In Stirred
Available online at BCREC Website: http://bcrec.undip.ac.id Bulletin of Chemical Reaction Engineering & Catalysis, (), 0, - Research Article Modified Mathematical Model For Neutralization System In Stirred
