Chapter 3 Answers to Problems
|
|
|
- Allyson Fitzgerald
- 6 years ago
- Views:
Transcription
1 Chapter 3 Answers to Problems 3.1 (a) a = A 1 + B 2 + E (b) b = 3A 1 + A 2 + 4E (c) c = 2A 1' + E' + A 2" (d) d = 4A 1 + A 2 + 2B 1 + B 2 + 5E (e) e = A 1g + A 2g + B 2g + E 1g + 2E 2g + A 2u + B 1u + B 2u + 2E 1u + E2u (f) f = A 1 + E + T 1 + 3T2 (g) g = A 1g + E g + T 1g + T 2g + 3T 1u + T2u (h) h = 2A 1' + E 1' + E 2' + A 2" 3.2 (a) a = 4A + 3B + 4{E} * (b) b = 4A' + 5{E'} + 3A" + 2{E"} [Note: + = 2 cos 2 /3 = 2(-0.5) = -1] * 2 *2 (c) c = 3A + {E 1} + {E 2} [Note: + = 2 cos 2 /5 = , and + = 2 cos 4 /5 = ] 3.3 (a) C 4v C4 C4v C z, x +y, z A A z, x +y, z, R z Rz A x -y B1 B x -y, xy xy B 2 (x,y), (R x,r), y (xz, yz) E {E} (x,y), (R x,r), y (xz, yz) 17
2 (b) D 3h D3 D3h D x +y, z A 1' A1 x +y, z, ( ) Rz A 2' 2 2 (x,y), (x -y, xy) E' A2 z, Rz A" z A 2" E (x,y), (x -y, xy), (R,R), x y (xz, yz) (R,R), (xz, yz) E" x y (c) D C 5d 5v D 5d C 5v x +y, z A1g A1 z, x +y, z R z A 2g (xz, yz), (R x,r) y E1g A2 R, z ( ) 2 2 (x -y,xy) E 2g A1u E 1 (xz, yz), (R x,r) y (x,y) z A 2u 2 2 (x,y) E1u E 2 (x -y,xy), ( ) E 2u 18
3 3.4 D 4h D 2d, where 2C 2' of D 4h 2C 2' of D 2d, and 2 d of D 4h 2 d of D 2d. (Columns for operations of D 4h that are not shared by this definition of D 2d have been omitted in the table below.) D4h E 2S4 C2 2C 2' 2 d E 2S4 C2 2C 2' 2 d D2d A1g A1 A2g A2 B1g B1 B2g B2 Eg E A1u B1 A2u B2 B1u A1 B2u A2 Eu E 19
4 D 4h D 2d, where 2C 2" of D 4h 2C 2' of D 2d, and 2 v of D 4h 2 d of D 2d. (Columns for operations of D 4h that are not shared by this definition of D 2d have been omitted in the table below.) D4h E 2S4 C2 2C 2" 2 v E 2S4 C2 2C 2' 2 d D2d A1g A1 A2g A2 B1g B2 B2g B1 Eg E A1u B1 A2u B2 B1u A2 B2u A1 Eu E (a) In C 2v, a = 5A 1 + 5B 1 + 5B 2, from which it follows in C v, a = (b) In D, = A + B + B + 2B + 2B + 2B, from which it follows in D, = h b g 2g 3g 1u 2u 3u h b g + g u u 3.6 (a) In D 4d, B 2 B 2 = A 1 (totally symmetric representation) [Rule 5, p. 83] (b) In T d, for T 2 T 2, Rules 4 and 5 apply. (c) In D 6d, A 1 E 5 = E 5 by Rules 3 and 6. Td E 8C3 3C2 6S4 6 d T T = A 1 + E + T 1 + T2 20
5 (d) In D 2d, for B 1 B 2, Rules 1 and 6 apply. D2d E 2S4 C2 2C 2' 2C 2" B B = A (e) In C 4h, for B g A u, Rules 1 and 6 apply. Also, note g u = u. 3 3 C2h E C4 C2 C4 i S4 h S4 Bg Au = Bu (f) In D 3h, for A 1" A 2", Rules 1 and 6 apply. Also note " " = '. D3h E 2C3 3C2 h 2S3 3 v A 1" A 2" = A 2' (g) In C 4h, for A u E u, Rules 2 and 6 apply. Also note u u = g. 3 3 C4h E C4 C2 C4 i S4 h S4 Au {E u} = {E g}
6 (h) In D 3d, for E g E u, Rules 4 and 6 apply. Also note g u = u. D3d E 2C3 3C2 i 2S6 3 d E g Eu = A 1u + A 2u + Eu 3.7 (a) (i) D 3h, C 2v, C s (ii) I II: lost C 3, 2C 2, S 3, 2 of 3 v (the retained v becomes v', and the becomes in C ); II III: lost C, (or ') (iii) descent (iv) yes (v) yes h v 2v 2 v v (b) (i) T d, C 3v, C 2v (ii) I II: lost 3 of 4C 3, 3C 2, 3S 4, 3 d (the other 3 d are retained in C 3v); II III: lost C 3 and 2 of 3 v (one is retained as either v or v' in C 2v), and gained C 2 and another (iii) descent (iv) no (v) yes v (c) (i) D 3d, C s, C 3v (ii) I II: lost C 3, 3C 2, i, S 6, and 2 of 3 d (one d is retained as h of C s); II III: regained C and 2 (one other is from C ) (iii) ascent (iv) yes (v) yes 3 v v h s (d) (i) D 3d, C s, C 2v (ii) I II: lost C 3, 3C 2, i, S 6, and 2 of 3 d (one d is retained as h of C s); II III: gained C and another (other is of C ) (iii) ascent (iv) yes (v) no 2 v v h s 3d 3 3h 6 d h 3 v (e) (i) D, D, D (ii) I II: lost i, S, and 3 ; II III: gained, S, and 3 (iii) ascent (iv) yes (v) no 22
7 3.8 (a) D 3h C 2v C s. Use correlation tables in Appendix B to construct the following correlations. D3h C2v Cs A' 1 A' 2 A1 A' E' A 2 A" 1 B1 A" 2 B2 A" E" The E' and E" degeneracies are lifted in the descent D C. (b) T d C 3v. Use correlation tables in Appendix B to construct the following correlations. Note that there is no correlation between C 3v and C 2v, because they do not have a groupsubgroup relationship. 3h 2v T d C 3v A1 A1 A 2 E T1 A 2 E T 2 The T 1 and T 2 triple degeneracies are partially lifted to become a nondegenerate and doubly degenerate species in C. The double degeneracy of E is retained. 3v 23
8 (c) D 3d C s C 3v. The C s C 3v correlations can be obtained from the C 3v correlation table. The D 3d C s correlations can be deduced by matching vector transformations in the two groups. However, the axes orientations change on the descent D 3d C s. the z axis of C s is either the x or y axis of D 3d. If we assume that it is x of D 3d, then the descent causes the following axis transformations: z x, x z, y y. Thus A 2g (R z) correlates with A" (R x), etc. A 1u connects to A", because A" must connect to A 2 in C 3v to establish the overall correlation A 1u A 2 of D 3d C 3v, as listed in the correlation table. The check of the D 3d C s correlations is that they all provide a continuous path that makes the listed D 3d C3v correlations. D3d Cs C3v A 1g A 1 A 2g A' E g A 2 A 1u A" A 2u E E u The E g and E u degeneracies are lost in the D 3d C s descent, but A' and A" become degenerate as E in the ascent C C. s 3v (d) D 3d C s C 2v. As in (c), there is an axis shift with D 3d C s. The correlations shown for D 3d C s are the same as shown in (c) (see above for explanation). Assuming z x, x z, y y in the D 3d C s descent, the h plane of C s is in the yz plane of the D 3d coordinate system. The conventional orientation of the coordinate system in C 2v is the same as in D 3d. Therefore, on the ascent C s C 2v, h yz. The correlations shown below are based on h, taken from the C correlation table. yz 2v 24
9 D3d Cs C2v A1g A1 A 2g E g A' A 2 A 1u 1 A" A 2u B 2 E u B The E g and E u degeneracies are lost in the D 3d C s descent. Note that there is no direct correlation between D 3d and C 2v, because they do not have a group-subgroup relationship. Nonetheless, they can be linked through their shared subgroup, C. (e) D 3d D 3 D 3h. Use correlation tables in Appendix B to construct the following correlations. D 3d D3 D3h A A A ' 1g 1 A 1 2g 2 A ' s Eg E' A 2 A A " 1u 1 A2u A 2" E E E" u All degeneracies are retained, and no new degeneracies are established through the transformations. Note that there is no direct correlation between D 3d and D 3h, because they do not have a group-subgroup relationship. Nonetheless, they can be linked through their shared subgroup, D 3 25
10 3.9 D 4 C4 D4 E 2C4 C2 2C 2' 2C 2" 3 E C 4, C4 C C4 A A A A B B B B E {E} D 4 C 2 (C 2 = C 2 4 ) D4 E 2C4 C2 2C 2' 2C 2" C2 A A A A B A B A E B D 4 C 2 (C 2 = C 2') D4 E 2C4 C2 2C 2' 2C 2" C2 A A A B B A B B E A + B 26
11 D 4 C 2 (C 2 = C 2") D4 E 2C4 C2 2C 2' 2C 2" C2 A A A B B B B A E A + B 3.10 There are several ways of doing this, including the following procedure. For the initial I T correlation, use matching of vector transformations in the two groups and also the method of examining characters of operations of I retained in T. Once the I T correlation has been established, use the tabulated correlations for T to make correlations to groups that are subgroups of both I and T. For example, for I C 3, use the I T correlations and then the tabulated T C 3 correlations to make the links. Likewise, for I D 2, use the I T correlations and then the tabulated T D 2 correlations to make the links. For I C 2, use the previous results for D 2 and make use of the tabulated D 2 C 2 correlations to make the links. For both I D 5 and I D 3, use matching of vector transformations in I and the subgroup and also the method of examining characters of operations of I retained in the subgroup. For I C 5, use the I D 5 results and the tabulated D 5 C 5 correlations to make the links. 27
Chapter 6 Answers to Problems
 Chapter 6 Answers to Problems 6.1 (a) NH 3 C3v E 2C3 3 v 4 1 2 3 0 1 12 0 2 3n = 3A 1 A 2 4E trans = A 1 E rot = A 2 E = 2A 2E = 4 frequencies 3n-6 1 Infrared 4 (2A 1 2E) Raman 4 (2A 1 2E) Polarized 2
Chapter 6 Answers to Problems 6.1 (a) NH 3 C3v E 2C3 3 v 4 1 2 3 0 1 12 0 2 3n = 3A 1 A 2 4E trans = A 1 E rot = A 2 E = 2A 2E = 4 frequencies 3n-6 1 Infrared 4 (2A 1 2E) Raman 4 (2A 1 2E) Polarized 2
CH 612 Advanced Inorganic Chemistry Structure and Reactivity. Exam # 2 10/25/2010. Print name:
 CH 612 dvanced Inorganic Chemistry Structure and Reactivity Print name: 1. (25 points) a) Given the set of operations C 4, h determine the other operations that must be present to form a complete point
CH 612 dvanced Inorganic Chemistry Structure and Reactivity Print name: 1. (25 points) a) Given the set of operations C 4, h determine the other operations that must be present to form a complete point
LECTURE 2 DEGENERACY AND DESCENT IN SYMMETRY: LIGAND FIELD SPLITTINGS AND RELATED MATTERS
 SYMMETRY II. J. M. GOICOECHEA. LECTURE 2. 1 LECTURE 2 DEGENERACY AND DESCENT IN SYMMETRY: LIGAND FIELD SPLITTINGS AND RELATED MATTERS 2.1 Degeneracy When dealing with non-degenerate symmetry adapted wavefunctions
SYMMETRY II. J. M. GOICOECHEA. LECTURE 2. 1 LECTURE 2 DEGENERACY AND DESCENT IN SYMMETRY: LIGAND FIELD SPLITTINGS AND RELATED MATTERS 2.1 Degeneracy When dealing with non-degenerate symmetry adapted wavefunctions
Other Crystal Fields
 Other Crystal Fields! We can deduce the CFT splitting of d orbitals in virtually any ligand field by " Noting the direct product listings in the appropriate character table to determine the ways in which
Other Crystal Fields! We can deduce the CFT splitting of d orbitals in virtually any ligand field by " Noting the direct product listings in the appropriate character table to determine the ways in which
Degrees of Freedom and Vibrational Modes
 Degrees of Freedom and Vibrational Modes 1. Every atom in a molecule can move in three possible directions relative to a Cartesian coordinate, so for a molecule of n atoms there are 3n degrees of freedom.
Degrees of Freedom and Vibrational Modes 1. Every atom in a molecule can move in three possible directions relative to a Cartesian coordinate, so for a molecule of n atoms there are 3n degrees of freedom.
LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES
 SYMMETRY II. J. M. GOICOECHEA. LECTURE 3 1 LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES 3.1 Direct products and many electron states Consider the problem of deciding upon the symmetry of
SYMMETRY II. J. M. GOICOECHEA. LECTURE 3 1 LECTURE 3 DIRECT PRODUCTS AND SPECTROSCOPIC SELECTION RULES 3.1 Direct products and many electron states Consider the problem of deciding upon the symmetry of
The heart of group theory
 The heart of group theory. We can represent a molecule in a mathematical way e.g. with the coordinates of its atoms. This mathematical description of the molecule forms a basis for symmetry operation.
The heart of group theory. We can represent a molecule in a mathematical way e.g. with the coordinates of its atoms. This mathematical description of the molecule forms a basis for symmetry operation.
Construction of the C 2v character table
 Construction of the C 2v character table The character table C 2v has the following form: C 2v E C 2 σ v (xz) σ v '(yz) Α 1 1 1 1 1 z x 2, y 2, z 2 Α 2 1 1-1 -1 R z xy Β 1 1-1 1-1 x, R y xz Β 2 1-1 -1
Construction of the C 2v character table The character table C 2v has the following form: C 2v E C 2 σ v (xz) σ v '(yz) Α 1 1 1 1 1 z x 2, y 2, z 2 Α 2 1 1-1 -1 R z xy Β 1 1-1 1-1 x, R y xz Β 2 1-1 -1
Review of Matrices. L A matrix is a rectangular array of numbers that combines with other such arrays according to specific rules.
 Review of Matrices L A matrix is a rectangular array of numbers that combines with other such arrays according to specific rules. T The dimension of a matrix is given as rows x columns; i.e., m x n. Matrix
Review of Matrices L A matrix is a rectangular array of numbers that combines with other such arrays according to specific rules. T The dimension of a matrix is given as rows x columns; i.e., m x n. Matrix
Symmetry. Chemistry 481(01) Spring 2017 Instructor: Dr. Upali Siriwardane Office: CTH 311 Phone Office Hours:
 Chemistry 481(01) Spring 2017 Instructor: Dr. Upali Siriwardane e-mail: upali@latech.edu Office: CT 311 Phone 257-4941 Office ours: M,W 8:00-9:00 & 11:00-12:00 am; Tu,Th, F 9:30-11:30 a.m. April 4, 2017:
Chemistry 481(01) Spring 2017 Instructor: Dr. Upali Siriwardane e-mail: upali@latech.edu Office: CT 311 Phone 257-4941 Office ours: M,W 8:00-9:00 & 11:00-12:00 am; Tu,Th, F 9:30-11:30 a.m. April 4, 2017:
b) For this ground state, obtain all possible J values and order them from lowest to highest in energy.
 Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible
Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible
b) For this ground state, obtain all possible J values and order them from lowest to highest in energy.
 Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible
Problem 1 (2 points) Part A Consider a free ion with a d 3 electronic configuration. a) By inspection, obtain the term symbol ( 2S+1 L) for the ground state. 4 F b) For this ground state, obtain all possible
2018 Ch112 problem set 6 Due: Thursday, Dec. 6th. Problem 1 (2 points)
 Problem 1 (2 points) a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions (υ1 and
Problem 1 (2 points) a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions (υ1 and
Chapter 3 Introduction to Molecular Symmetry
 CHEM 511 Chapter 3 page 1 of 12 Chapter 3 Introduction to Molecular Symmetry This chapter will deal with the symmetry characteristics of individual molecules, i.e., how molecules can be rotated or imaged
CHEM 511 Chapter 3 page 1 of 12 Chapter 3 Introduction to Molecular Symmetry This chapter will deal with the symmetry characteristics of individual molecules, i.e., how molecules can be rotated or imaged
Little Orthogonality Theorem (LOT)
 Little Orthogonality Theorem (LOT) Take diagonal elements of D matrices in RG * D R D R i j G ij mi N * D R D R N i j G G ij ij RG mi mi ( ) By definition, D j j j R TrD R ( R). Sum GOT over β: * * ( )
Little Orthogonality Theorem (LOT) Take diagonal elements of D matrices in RG * D R D R i j G ij mi N * D R D R N i j G G ij ij RG mi mi ( ) By definition, D j j j R TrD R ( R). Sum GOT over β: * * ( )
Crystal Field Theory History
 Crystal Field Theory History 1929 Hans Bethe - Crystal Field Theory (CFT) Developed to interpret color, spectra, magnetism in crystals 1932 J. H. Van Vleck - CFT of Transition Metal Complexes Champions
Crystal Field Theory History 1929 Hans Bethe - Crystal Field Theory (CFT) Developed to interpret color, spectra, magnetism in crystals 1932 J. H. Van Vleck - CFT of Transition Metal Complexes Champions
Mo 2+, Mo 2+, Cr electrons. Mo-Mo quadruple bond.
 Problem 1 (2 points) 1. Consider the MoMoCr heterotrimetallic complex shown below (Berry, et. al. Inorganica Chimica Acta 2015, p. 241). Metal-metal bonds are not drawn. The ligand framework distorts this
Problem 1 (2 points) 1. Consider the MoMoCr heterotrimetallic complex shown below (Berry, et. al. Inorganica Chimica Acta 2015, p. 241). Metal-metal bonds are not drawn. The ligand framework distorts this
Appendixes POINT GROUPS AND THEIR CHARACTER TABLES
 Appendixes APPENDIX I. POINT GROUPS AND THEIR CHARACTER TABLES The following are the character tables of the point groups that appear frequently in this book. The species (or the irreducible representations)
Appendixes APPENDIX I. POINT GROUPS AND THEIR CHARACTER TABLES The following are the character tables of the point groups that appear frequently in this book. The species (or the irreducible representations)
A COMPUTERIZED PROGRAM FOR FINDING THE SYMMETRIES OF THE MOLECULAR NORMAL MODES OF VIBRATION
 Journal of Optoelectronics and Advanced Materials Vol. 5, No. 2, June 23, p. 479-491 A COMPUTERIZED PROGRAM FOR FINDING THE SYMMETRIES OF THE MOLECULAR NORMAL MODES OF VIBRATION Ath. Trutia * University
Journal of Optoelectronics and Advanced Materials Vol. 5, No. 2, June 23, p. 479-491 A COMPUTERIZED PROGRAM FOR FINDING THE SYMMETRIES OF THE MOLECULAR NORMAL MODES OF VIBRATION Ath. Trutia * University
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Dr. Fred O. Garces Chemistry 201
 23.4 400! 500! 600! 800! The relationship between Colors, Metal Complexes and Gemstones Dr. Fred O. Garces Chemistry 201 Miramar College 1 Transition Metal Gems Gemstone owe their color from trace transition-metal
23.4 400! 500! 600! 800! The relationship between Colors, Metal Complexes and Gemstones Dr. Fred O. Garces Chemistry 201 Miramar College 1 Transition Metal Gems Gemstone owe their color from trace transition-metal
1. (4 pts) Give the electron configuration for these species. You may use core notation.
 EXAM ONE PART ONE CHM 451 (INORGANIC CHEMISTRY) DR. MATTSON 18 SEPTEMBER 2013 NAME: Instructions: This exam has two parts. In Part One, only a pencil and molecular models may be used. When you have completed
EXAM ONE PART ONE CHM 451 (INORGANIC CHEMISTRY) DR. MATTSON 18 SEPTEMBER 2013 NAME: Instructions: This exam has two parts. In Part One, only a pencil and molecular models may be used. When you have completed
Chem Spring, 2017 Assignment 5 - Solutions
 Page 1 of 10 Chem 370 - Spring, 2017 Assignment 5 - Solutions 5.1 Additional combinations are p z ± d z 2, p x ±d xz, and p y ±d yz. p z ± d z 2 p x ±d xz or p y ±d yz 5.2 a. Li 2 has the configuration
Page 1 of 10 Chem 370 - Spring, 2017 Assignment 5 - Solutions 5.1 Additional combinations are p z ± d z 2, p x ±d xz, and p y ±d yz. p z ± d z 2 p x ±d xz or p y ±d yz 5.2 a. Li 2 has the configuration
Free-Ion Terms to Ligand-field Terms
 Free-Ion Terms to Ligand-field Terms! Orbital term symbols for free atoms and ions are identical to symbols for irreducible representations in R 3. " The irreducible representations of R 3 include all
Free-Ion Terms to Ligand-field Terms! Orbital term symbols for free atoms and ions are identical to symbols for irreducible representations in R 3. " The irreducible representations of R 3 include all
Degrees of Freedom and Vibrational Modes
 Degrees of Freedom and Vibrational Modes 1. Every atom in a molecule can move in three possible directions relative to a Cartesian coordinate, so for a molecule of n atoms there are 3n degrees of freedom.
Degrees of Freedom and Vibrational Modes 1. Every atom in a molecule can move in three possible directions relative to a Cartesian coordinate, so for a molecule of n atoms there are 3n degrees of freedom.
Molecular Symmetry 10/25/2018
 Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy). Predict IR spectra or Interpret UV-Vis spectra Predict optical activity
Symmetry helps us understand molecular structure, some chemical properties, and characteristics of physical properties (spectroscopy). Predict IR spectra or Interpret UV-Vis spectra Predict optical activity
Absorption Spectra. ! Ti(H 2 O) 6 3+ appears purple (red + blue) because it absorbs green light at ~500 nm = ~20,000 cm 1.
 Absorption Spectra! Colors of transition metal complexes result from absorption of a small portion of the visible spectrum with transmission of the unabsorbed frequencies. Visible Spectra of [M(H 2 O)
Absorption Spectra! Colors of transition metal complexes result from absorption of a small portion of the visible spectrum with transmission of the unabsorbed frequencies. Visible Spectra of [M(H 2 O)
Chm December 2008
 Inorganic Exam 3 Chm 451 4 December 2008 Name: Instructions. Always show your work where required for full credit. 1. (15 pts) True/False a T F Ionization energy decreases as one moves down from Li to
Inorganic Exam 3 Chm 451 4 December 2008 Name: Instructions. Always show your work where required for full credit. 1. (15 pts) True/False a T F Ionization energy decreases as one moves down from Li to
13 Applications of molecular symmetry and group theory
 Subject Chemistry Paper No and Title Module No and Title Module Tag 13 Applications of molecular symmetry and 26 and and vibrational spectroscopy part-iii CHE_P13_M26 TABLE OF CONTENTS 1. Learning Outcomes
Subject Chemistry Paper No and Title Module No and Title Module Tag 13 Applications of molecular symmetry and 26 and and vibrational spectroscopy part-iii CHE_P13_M26 TABLE OF CONTENTS 1. Learning Outcomes
Created by T. Madas MIXED SURD QUESTIONS. Created by T. Madas
 MIXED SURD QUESTIONS Question 1 (**) Write each of the following expressions a single simplified surd a) 150 54 b) 21 7 C1A, 2 6, 3 7 Question 2 (**) Write each of the following surd expressions as simple
MIXED SURD QUESTIONS Question 1 (**) Write each of the following expressions a single simplified surd a) 150 54 b) 21 7 C1A, 2 6, 3 7 Question 2 (**) Write each of the following surd expressions as simple
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Ch120 - Study Guide 10
 Ch120 - Study Guide 10 Adam Griffith October 17, 2005 In this guide: Symmetry; Diatomic Term Symbols; Molecular Term Symbols Last updated October 27, 2005. 1 The Origin of m l States and Symmetry We are
Ch120 - Study Guide 10 Adam Griffith October 17, 2005 In this guide: Symmetry; Diatomic Term Symbols; Molecular Term Symbols Last updated October 27, 2005. 1 The Origin of m l States and Symmetry We are
B F N O. Chemistry 6330 Problem Set 4 Answers. (1) (a) BF 4. tetrahedral (T d )
 hemistry 6330 Problem Set 4 Answers (1) (a) B 4 - tetrahedral (T d ) B T d E 8 3 3 2 6S 4 6s d G xyz 3 0-1 -1 1 G unmoved atoms 5 2 1 1 3 G total 15 0-1 -1 3 If we reduce G total we find that: G total
hemistry 6330 Problem Set 4 Answers (1) (a) B 4 - tetrahedral (T d ) B T d E 8 3 3 2 6S 4 6s d G xyz 3 0-1 -1 1 G unmoved atoms 5 2 1 1 3 G total 15 0-1 -1 3 If we reduce G total we find that: G total
2x (x 2 + y 2 + 1) 2 2y. (x 2 + y 2 + 1) 4. 4xy. (1, 1)(x 1) + (1, 1)(y + 1) (1, 1)(x 1)(y + 1) 81 x y y + 7.
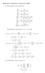 Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
EXAM INFORMATION. Radial Distribution Function: B is the normalization constant. d dx. p 2 Operator: Heisenberg Uncertainty Principle:
 EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
( ) ( ) SALCs as basis functions. LCAO-MO Theory. Rewriting the Schrödinger Eqn. Matrix form of Schrödinger Eqn. Hφ j. φ 1. φ 3. φ 2. φ j. φ i.
 LCAO-MO Theory In molecular orbital theory the MOs, { }, are usually expanded as combinations of atomic orbitals, {φ i }. For the µ th MO, we write this expansion as = c iµ φ i = c 1µ φ 1 + c µ φ + c 3µ
LCAO-MO Theory In molecular orbital theory the MOs, { }, are usually expanded as combinations of atomic orbitals, {φ i }. For the µ th MO, we write this expansion as = c iµ φ i = c 1µ φ 1 + c µ φ + c 3µ
In the fourth problem set, you derived the MO diagrams for two complexes containing Cr-Cr bonds:
 Problem 1 (2 points) Part 1 a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions
Problem 1 (2 points) Part 1 a. Consider the following V III complexes: V(H2O)6 3+, VF6 3-, and VCl6 3-. The table below contains the energies corresponding to the two lowest spin-allowed d-d transitions
Tables for Group Theory
 Tables for Group Theory By P. W. ATKINS, M. S. CHILD, and C. S. G. PHILLIPS This provides the essential tables (character tables, direct products, descent in symmetry and subgroups) required for those
Tables for Group Theory By P. W. ATKINS, M. S. CHILD, and C. S. G. PHILLIPS This provides the essential tables (character tables, direct products, descent in symmetry and subgroups) required for those
Diagonalization by a unitary similarity transformation
 Physics 116A Winter 2011 Diagonalization by a unitary similarity transformation In these notes, we will always assume that the vector space V is a complex n-dimensional space 1 Introduction A semi-simple
Physics 116A Winter 2011 Diagonalization by a unitary similarity transformation In these notes, we will always assume that the vector space V is a complex n-dimensional space 1 Introduction A semi-simple
Problem 1 (4 points) D2h. C2v. Part A.
 Problem 1 (4 points) In 2004, a bimetallic Zr compound exhibiting side-on N2 binding was reported by Chirik and coworkers (Nature, 2004, 427, pp. 527-530). The crystal structure of this compound was obtained,
Problem 1 (4 points) In 2004, a bimetallic Zr compound exhibiting side-on N2 binding was reported by Chirik and coworkers (Nature, 2004, 427, pp. 527-530). The crystal structure of this compound was obtained,
Notation. Irrep labels follow Mulliken s convention: A and B label nondegenerate E irreps, doubly T degenerate, triply degenerate
 Notation Irrep labels follow Mulliken s convention: A and B label nondegenerate E irreps, doubly T degenerate, triply degenerate Spectroscopists sometimes use F for triply degenerate; almost everyone G
Notation Irrep labels follow Mulliken s convention: A and B label nondegenerate E irreps, doubly T degenerate, triply degenerate Spectroscopists sometimes use F for triply degenerate; almost everyone G
Curvilinear Coordinates
 University of Alabama Department of Physics and Astronomy PH 106-4 / LeClair Fall 2008 Curvilinear Coordinates Note that we use the convention that the cartesian unit vectors are ˆx, ŷ, and ẑ, rather than
University of Alabama Department of Physics and Astronomy PH 106-4 / LeClair Fall 2008 Curvilinear Coordinates Note that we use the convention that the cartesian unit vectors are ˆx, ŷ, and ẑ, rather than
Tables for Group Theory
 Tables for Group Theory By P. W. ATKINS, M. S. CHILD, and C. S. G. PHILLIPS This provides the essential tables (character tables, direct products, descent in symmetry and subgroups) required for those
Tables for Group Theory By P. W. ATKINS, M. S. CHILD, and C. S. G. PHILLIPS This provides the essential tables (character tables, direct products, descent in symmetry and subgroups) required for those
Chapter 3. Second Order Linear PDEs
 Chapter 3. Second Order Linear PDEs 3.1 Introduction The general class of second order linear PDEs are of the form: ax, y)u xx + bx, y)u xy + cx, y)u yy + dx, y)u x + ex, y)u y + f x, y)u = gx, y). 3.1)
Chapter 3. Second Order Linear PDEs 3.1 Introduction The general class of second order linear PDEs are of the form: ax, y)u xx + bx, y)u xy + cx, y)u yy + dx, y)u x + ex, y)u y + f x, y)u = gx, y). 3.1)
REFLECTIONS IN A EUCLIDEAN SPACE
 REFLECTIONS IN A EUCLIDEAN SPACE PHILIP BROCOUM Let V be a finite dimensional real linear space. Definition 1. A function, : V V R is a bilinear form in V if for all x 1, x, x, y 1, y, y V and all k R,
REFLECTIONS IN A EUCLIDEAN SPACE PHILIP BROCOUM Let V be a finite dimensional real linear space. Definition 1. A function, : V V R is a bilinear form in V if for all x 1, x, x, y 1, y, y V and all k R,
Little Orthogonality Theorem (LOT)
 Little Orthogonality Theorem (LOT) Take diagonal elements of D matrices in R G i * j G αµ βν = δδ ij µνδαβ mi D R D R N N i * j G G Dµµ R Dββ R = δijδ µ β δ µβ = δijδµβ R G mi mi j j ( j) By definition,
Little Orthogonality Theorem (LOT) Take diagonal elements of D matrices in R G i * j G αµ βν = δδ ij µνδαβ mi D R D R N N i * j G G Dµµ R Dββ R = δijδ µ β δ µβ = δijδµβ R G mi mi j j ( j) By definition,
Question: 1. Use suitable identities to find the following products:
 CH-2 Polynomial Question: 1. Use suitable identities to find the following products: (i) (x + 4) (x + 10) Solution:- (x+4)(x+10) = x 2 +10x+4x+4 x 10 = x 2 +14x+40 (ii) (x + 8) (x 10) Solution: x 2-10x+8x-80
CH-2 Polynomial Question: 1. Use suitable identities to find the following products: (i) (x + 4) (x + 10) Solution:- (x+4)(x+10) = x 2 +10x+4x+4 x 10 = x 2 +14x+40 (ii) (x + 8) (x 10) Solution: x 2-10x+8x-80
5.4. Electronic structure of water
 5.4. Electronic structure of water Water belongs to C 2v point group, we have discussed the corresponding character table. Here it is again: C 2v E C 2 σ v (yz) σ v (xz) A 1 1 1 1 1 A 2 1 1-1 -1 B 1 1-1
5.4. Electronic structure of water Water belongs to C 2v point group, we have discussed the corresponding character table. Here it is again: C 2v E C 2 σ v (yz) σ v (xz) A 1 1 1 1 1 A 2 1 1-1 -1 B 1 1-1
TRIPLE FACTORIZATION OF NON-ABELIAN GROUPS BY TWO MAXIMAL SUBGROUPS
 Journal of Algebra and Related Topics Vol. 2, No 2, (2014), pp 1-9 TRIPLE FACTORIZATION OF NON-ABELIAN GROUPS BY TWO MAXIMAL SUBGROUPS A. GHARIBKHAJEH AND H. DOOSTIE Abstract. The triple factorization
Journal of Algebra and Related Topics Vol. 2, No 2, (2014), pp 1-9 TRIPLE FACTORIZATION OF NON-ABELIAN GROUPS BY TWO MAXIMAL SUBGROUPS A. GHARIBKHAJEH AND H. DOOSTIE Abstract. The triple factorization
Molecular Symmetry PROBLEMS
 4 Molecular Symmetry PROBLEMS 4. A student sets up the coordinates for NH3 rather unconventionally, as shown on the right. In this coordinate system, the x-axis is the three-fold axis. One of the σ -planes,
4 Molecular Symmetry PROBLEMS 4. A student sets up the coordinates for NH3 rather unconventionally, as shown on the right. In this coordinate system, the x-axis is the three-fold axis. One of the σ -planes,
3. Minimization with constraints Problem III. Minimize f(x) in R n given that x satisfies the equality constraints. g j (x) = c j, j = 1,...
 3. Minimization with constraints Problem III. Minimize f(x) in R n given that x satisfies the equality constraints g j (x) = c j, j = 1,..., m < n, where c 1,..., c m are given numbers. Theorem 3.1. Let
3. Minimization with constraints Problem III. Minimize f(x) in R n given that x satisfies the equality constraints g j (x) = c j, j = 1,..., m < n, where c 1,..., c m are given numbers. Theorem 3.1. Let
Sect Least Common Denominator
 4 Sect.3 - Least Common Denominator Concept #1 Writing Equivalent Rational Expressions Two fractions are equivalent if they are equal. In other words, they are equivalent if they both reduce to the same
4 Sect.3 - Least Common Denominator Concept #1 Writing Equivalent Rational Expressions Two fractions are equivalent if they are equal. In other words, they are equivalent if they both reduce to the same
Chem 673, Problem Set 5 Due Thursday, November 29, 2007
 Chem 673, Problem Set 5 Due Thursday, November 29, 2007 (1) Trigonal prismatic coordination is fairly common in solid-state inorganic chemistry. In most cases the geometry of the trigonal prism is such
Chem 673, Problem Set 5 Due Thursday, November 29, 2007 (1) Trigonal prismatic coordination is fairly common in solid-state inorganic chemistry. In most cases the geometry of the trigonal prism is such
THE TRANSLATION PLANES OF ORDER 49 AND THEIR AUTOMORPHISM GROUPS
 MATHEMATICS OF COMPUTATION Volume 67, Number 223, July 1998, Pages 1207 1224 S 0025-5718(98)00961-2 THE TRANSLATION PLANES OF ORDER 49 AND THEIR AUTOMORPHISM GROUPS C. CHARNES AND U. DEMPWOLFF Abstract.
MATHEMATICS OF COMPUTATION Volume 67, Number 223, July 1998, Pages 1207 1224 S 0025-5718(98)00961-2 THE TRANSLATION PLANES OF ORDER 49 AND THEIR AUTOMORPHISM GROUPS C. CHARNES AND U. DEMPWOLFF Abstract.
FIG consists of 25 configurations. These configurations are divided into three main classes: 1. Nondegenerate Configurations (9 members)
 SEC. 10.4 CATALOG OF K-CONFIGURATION 201 10.4 Catalog of K Configurations for Shallow Depths In order to facilitate further theoretical studies of hypothesis III in Sec. 10.3 and,to anticipate alternate
SEC. 10.4 CATALOG OF K-CONFIGURATION 201 10.4 Catalog of K Configurations for Shallow Depths In order to facilitate further theoretical studies of hypothesis III in Sec. 10.3 and,to anticipate alternate
Lecture 16: Computation Tree Logic (CTL)
 Lecture 16: Computation Tree Logic (CTL) 1 Programme for the upcoming lectures Introducing CTL Basic Algorithms for CTL CTL and Fairness; computing strongly connected components Basic Decision Diagrams
Lecture 16: Computation Tree Logic (CTL) 1 Programme for the upcoming lectures Introducing CTL Basic Algorithms for CTL CTL and Fairness; computing strongly connected components Basic Decision Diagrams
Lecture 15 February 15, 2013 Transition metals
 Lecture 15 February 15, 2013 Transition metals Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course
Lecture 15 February 15, 2013 Transition metals Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course
PAPER No.7 : Inorganic Chemistry-II MODULE No.1 : Crystal Field Theory
 Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic Chemistry II 1, Crystal Field Theory CHE_P7_M1 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Crystal Field Theory
Subject Chemistry Paper No and Title Module No and Title Module Tag 7, Inorganic Chemistry II 1, Crystal Field Theory CHE_P7_M1 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction to Crystal Field Theory
First order wave equations. Transport equation is conservation law with J = cu, u t + cu x = 0, < x <.
 First order wave equations Transport equation is conservation law with J = cu, u t + cu x = 0, < x
First order wave equations Transport equation is conservation law with J = cu, u t + cu x = 0, < x
11.1 Three-Dimensional Coordinate System
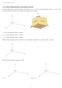 11.1 Three-Dimensional Coordinate System In three dimensions, a point has three coordinates: (x,y,z). The normal orientation of the x, y, and z-axes is shown below. The three axes divide the region into
11.1 Three-Dimensional Coordinate System In three dimensions, a point has three coordinates: (x,y,z). The normal orientation of the x, y, and z-axes is shown below. The three axes divide the region into
CRYSTALLOGRAPHIC POINT AND SPACE GROUPS. Andy Elvin June 10, 2013
 CRYSTALLOGRAPHIC POINT AND SPACE GROUPS Andy Elvin June 10, 2013 Contents Point and Space Groups Wallpaper Groups X-Ray Diffraction Electron Wavefunction Neumann s Principle Magnetic Point Groups Point
CRYSTALLOGRAPHIC POINT AND SPACE GROUPS Andy Elvin June 10, 2013 Contents Point and Space Groups Wallpaper Groups X-Ray Diffraction Electron Wavefunction Neumann s Principle Magnetic Point Groups Point
Chapter 11. Non-rigid Molecules
 Chapter. Non-rigid olecules Notes: ost of the material presented in this chapter is taken from Bunker and Jensen (998), Chap. 5, and Bunker and Jensen (2005), Chap. 3.. The Hamiltonian As was stated before,
Chapter. Non-rigid olecules Notes: ost of the material presented in this chapter is taken from Bunker and Jensen (998), Chap. 5, and Bunker and Jensen (2005), Chap. 3.. The Hamiltonian As was stated before,
Coordination Chemistry: Bonding Theories. Crystal Field Theory. Chapter 20
 Coordination Chemistry: Bonding Theories Crystal Field Theory Chapter 0 Review of the Previous Lecture 1. We discussed different types of isomerism in coordination chemistry Structural or constitutional
Coordination Chemistry: Bonding Theories Crystal Field Theory Chapter 0 Review of the Previous Lecture 1. We discussed different types of isomerism in coordination chemistry Structural or constitutional
LESSON 7.1 FACTORING POLYNOMIALS I
 LESSON 7.1 FACTORING POLYNOMIALS I LESSON 7.1 FACTORING POLYNOMIALS I 293 OVERVIEW Here s what you ll learn in this lesson: Greatest Common Factor a. Finding the greatest common factor (GCF) of a set of
LESSON 7.1 FACTORING POLYNOMIALS I LESSON 7.1 FACTORING POLYNOMIALS I 293 OVERVIEW Here s what you ll learn in this lesson: Greatest Common Factor a. Finding the greatest common factor (GCF) of a set of
Chem Symmetry and Introduction to Group Theory. Symmetry is all around us and is a fundamental property of nature.
 Chem 59-65 Symmetry and Introduction to Group Theory Symmetry is all around us and is a fundamental property of nature. Chem 59-65 Symmetry and Introduction to Group Theory The term symmetry is derived
Chem 59-65 Symmetry and Introduction to Group Theory Symmetry is all around us and is a fundamental property of nature. Chem 59-65 Symmetry and Introduction to Group Theory The term symmetry is derived
Types of Molecular Vibrations
 Important concepts in IR spectroscopy Vibrations that result in change of dipole moment give rise to IR absorptions. The oscillating electric field of the radiation couples with the molecular vibration
Important concepts in IR spectroscopy Vibrations that result in change of dipole moment give rise to IR absorptions. The oscillating electric field of the radiation couples with the molecular vibration
A. 1 Character tables for some chemically important symmetry groups. E = exp(21ti/3) T., R, x2 + yz, z2 x2 - yz, xy (T., Ty), (R.
 Appendix A Character and correlation tables and multiplication properties of irreducible representations A. 1 Character tables for some chemically important symmetry groups E (Th C; E A' 1 T., Ty, R, xz,
Appendix A Character and correlation tables and multiplication properties of irreducible representations A. 1 Character tables for some chemically important symmetry groups E (Th C; E A' 1 T., Ty, R, xz,
5 Symmetries and point group in a nut shell
 30 Phys520.nb 5 Symmetries and point group in a nut shell 5.1. Basic ideas: 5.1.1. Symmetry operations Symmetry: A system remains invariant under certain operation. These operations are called symmetry
30 Phys520.nb 5 Symmetries and point group in a nut shell 5.1. Basic ideas: 5.1.1. Symmetry operations Symmetry: A system remains invariant under certain operation. These operations are called symmetry
Linear Algebra. Chapter 8: Eigenvalues: Further Applications and Computations Section 8.2. Applications to Geometry Proofs of Theorems.
 Linear Algebra Chapter 8: Eigenvalues: Further Applications and Computations Section 8.2. Applications to Geometry Proofs of Theorems May 1, 2018 () Linear Algebra May 1, 2018 1 / 8 Table of contents 1
Linear Algebra Chapter 8: Eigenvalues: Further Applications and Computations Section 8.2. Applications to Geometry Proofs of Theorems May 1, 2018 () Linear Algebra May 1, 2018 1 / 8 Table of contents 1
Subrings and Ideals 2.1 INTRODUCTION 2.2 SUBRING
 Subrings and Ideals Chapter 2 2.1 INTRODUCTION In this chapter, we discuss, subrings, sub fields. Ideals and quotient ring. We begin our study by defining a subring. If (R, +, ) is a ring and S is a non-empty
Subrings and Ideals Chapter 2 2.1 INTRODUCTION In this chapter, we discuss, subrings, sub fields. Ideals and quotient ring. We begin our study by defining a subring. If (R, +, ) is a ring and S is a non-empty
Lecture 16 February 20 Transition metals, Pd and Pt
 Lecture 16 February 20 Transition metals, Pd and Pt Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course
Lecture 16 February 20 Transition metals, Pd and Pt Nature of the Chemical Bond with applications to catalysis, materials science, nanotechnology, surface science, bioinorganic chemistry, and energy Course
Chapter 5 Equations for Wave Function
 Chapter 5 Equations for Wave Function In very simple cases, the explicit expressions for the SALCs could be deduced by inspection, but not for complicated system. It would be useful for cases like these
Chapter 5 Equations for Wave Function In very simple cases, the explicit expressions for the SALCs could be deduced by inspection, but not for complicated system. It would be useful for cases like these
On the algebraic structure of Dyson s Circular Ensembles. Jonathan D.H. SMITH. January 29, 2012
 Scientiae Mathematicae Japonicae 101 On the algebraic structure of Dyson s Circular Ensembles Jonathan D.H. SMITH January 29, 2012 Abstract. Dyson s Circular Orthogonal, Unitary, and Symplectic Ensembles
Scientiae Mathematicae Japonicae 101 On the algebraic structure of Dyson s Circular Ensembles Jonathan D.H. SMITH January 29, 2012 Abstract. Dyson s Circular Orthogonal, Unitary, and Symplectic Ensembles
8. Find r a! r b. a) r a = [3, 2, 7], r b = [ 1, 4, 5] b) r a = [ 5, 6, 7], r b = [2, 7, 4]
![8. Find r a! r b. a) r a = [3, 2, 7], r b = [ 1, 4, 5] b) r a = [ 5, 6, 7], r b = [2, 7, 4] 8. Find r a! r b. a) r a = [3, 2, 7], r b = [ 1, 4, 5] b) r a = [ 5, 6, 7], r b = [2, 7, 4]](/thumbs/95/122742655.jpg) Chapter 8 Prerequisite Skills BLM 8-1.. Linear Relations 1. Make a table of values and graph each linear function a) y = 2x b) y = x + 5 c) 2x + 6y = 12 d) x + 7y = 21 2. Find the x- and y-intercepts of
Chapter 8 Prerequisite Skills BLM 8-1.. Linear Relations 1. Make a table of values and graph each linear function a) y = 2x b) y = x + 5 c) 2x + 6y = 12 d) x + 7y = 21 2. Find the x- and y-intercepts of
Exponents, Polynomials, and Polynomial Functions. Copyright 2014, 2010, 2006 Pearson Education, Inc. Section 5.1, 1
 5 Exponents, Polynomials, and Polynomial Functions Copyright 2014, 2010, 2006 Pearson Education, Inc. Section 5.1, 1 5.1 Integer Exponents R.1 Fractions and Scientific Notation Objectives 1. Use the product
5 Exponents, Polynomials, and Polynomial Functions Copyright 2014, 2010, 2006 Pearson Education, Inc. Section 5.1, 1 5.1 Integer Exponents R.1 Fractions and Scientific Notation Objectives 1. Use the product
Polynomial Functions
 Polynomial Functions NOTE: Some problems in this file are used with permission from the engageny.org website of the New York State Department of Education. Various files. Internet. Available from https://www.engageny.org/ccss-library.
Polynomial Functions NOTE: Some problems in this file are used with permission from the engageny.org website of the New York State Department of Education. Various files. Internet. Available from https://www.engageny.org/ccss-library.
Power Systems Control Prof. Wonhee Kim. Modeling in the Frequency and Time Domains
 Power Systems Control Prof. Wonhee Kim Modeling in the Frequency and Time Domains Laplace Transform Review - Laplace transform - Inverse Laplace transform 2 Laplace Transform Review 3 Laplace Transform
Power Systems Control Prof. Wonhee Kim Modeling in the Frequency and Time Domains Laplace Transform Review - Laplace transform - Inverse Laplace transform 2 Laplace Transform Review 3 Laplace Transform
Where have we been? Lectures 1 and 2 Bohr s Model/ Wave Mechanics/ Radial and Angular Wavefunctions/ Radial Distribution Functions/ s and p orbitals
 Where have we been? Lectures 1 and 2 Bohr s Model/ Wave Mechanics/ Radial and Angular Wavefunctions/ Radial Distribution unctions/ s and p orbitals Where are we going? Lecture 3 Brief wavefunction considerations:
Where have we been? Lectures 1 and 2 Bohr s Model/ Wave Mechanics/ Radial and Angular Wavefunctions/ Radial Distribution unctions/ s and p orbitals Where are we going? Lecture 3 Brief wavefunction considerations:
Congruent Number Problem and Elliptic curves
 Congruent Number Problem and Elliptic curves December 12, 2010 Contents 1 Congruent Number problem 2 1.1 1 is not a congruent number.................................. 2 2 Certain Elliptic Curves 4 3 Using
Congruent Number Problem and Elliptic curves December 12, 2010 Contents 1 Congruent Number problem 2 1.1 1 is not a congruent number.................................. 2 2 Certain Elliptic Curves 4 3 Using
PYTHAGOREAN DESCENT KEITH CONRAD
 PYTHAGOREAN DESCENT KEITH CONRAD 1. Introduction A Pythagorean triple is a triple of positive integers (a, b, c) such that a 2 + b 2 = c 2. Examples include (3, 4, 5), (5, 12, 13), and (6, 8, 10). A Pythagorean
PYTHAGOREAN DESCENT KEITH CONRAD 1. Introduction A Pythagorean triple is a triple of positive integers (a, b, c) such that a 2 + b 2 = c 2. Examples include (3, 4, 5), (5, 12, 13), and (6, 8, 10). A Pythagorean
PAPER No. : 8 (PHYSICAL SPECTROSCOPY) MODULE NO. : 23 (NORMAL MODES AND IRREDUCIBLE REPRESENTATIONS FOR POLYATOMIC MOLECULES)
 Subject Chemistry Paper No and Title Module No and Title Module Tag 8/ Physical Spectroscopy 23/ Normal modes and irreducible representations for polyatomic molecules CHE_P8_M23 TABLE OF CONTENTS 1. Learning
Subject Chemistry Paper No and Title Module No and Title Module Tag 8/ Physical Spectroscopy 23/ Normal modes and irreducible representations for polyatomic molecules CHE_P8_M23 TABLE OF CONTENTS 1. Learning
Chapter 6 Vibrational Spectroscopy
 Chapter 6 Vibrational Spectroscopy As with other applications of symmetry and group theory, these techniques reach their greatest utility when applied to the analysis of relatively small molecules in either
Chapter 6 Vibrational Spectroscopy As with other applications of symmetry and group theory, these techniques reach their greatest utility when applied to the analysis of relatively small molecules in either
Group Theory: Math30038, Sheet 6
 Group Theory: Math30038, Sheet 6 Solutions GCS 1. Consider the group D ofrigidsymmetriesofaregularn-gon (which may be turned over). Prove that this group has order 2n, is non-abelian, can be generated
Group Theory: Math30038, Sheet 6 Solutions GCS 1. Consider the group D ofrigidsymmetriesofaregularn-gon (which may be turned over). Prove that this group has order 2n, is non-abelian, can be generated
PYTHAGOREAN DESCENT KEITH CONRAD
 PYTHAGOREAN DESCENT KEITH CONRAD 1. Introduction A Pythagorean triple is a triple of positive integers (a, b, c) such that a 2 + b 2 = c 2. Examples include (3, 4, 5), (5, 12, 13), and (6, 8, 10). A Pythagorean
PYTHAGOREAN DESCENT KEITH CONRAD 1. Introduction A Pythagorean triple is a triple of positive integers (a, b, c) such that a 2 + b 2 = c 2. Examples include (3, 4, 5), (5, 12, 13), and (6, 8, 10). A Pythagorean
Name: Class: IM8 Block:
 Name: Block: Class: IM8 Investigation 1: Mathematical Properties and Order of Operations Mathematical Properties 2 Practice Level 1: Write the name of the property shown in the examples below. 1. 4 + 5
Name: Block: Class: IM8 Investigation 1: Mathematical Properties and Order of Operations Mathematical Properties 2 Practice Level 1: Write the name of the property shown in the examples below. 1. 4 + 5
LESSON 7.2 FACTORING POLYNOMIALS II
 LESSON 7.2 FACTORING POLYNOMIALS II LESSON 7.2 FACTORING POLYNOMIALS II 305 OVERVIEW Here s what you ll learn in this lesson: Trinomials I a. Factoring trinomials of the form x 2 + bx + c; x 2 + bxy +
LESSON 7.2 FACTORING POLYNOMIALS II LESSON 7.2 FACTORING POLYNOMIALS II 305 OVERVIEW Here s what you ll learn in this lesson: Trinomials I a. Factoring trinomials of the form x 2 + bx + c; x 2 + bxy +
- parametric equations for the line, z z 0 td 3 or if d 1 0, d 2 0andd 3 0, - symmetric equations of the line.
 Lines and Planes in Space -(105) Questions: What do we need to know to determine a line in space? What are the fms of a line? If two lines are not parallel in space, must they be intersect as two lines
Lines and Planes in Space -(105) Questions: What do we need to know to determine a line in space? What are the fms of a line? If two lines are not parallel in space, must they be intersect as two lines
Representation Theory
 Part II Year 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2018 Paper 1, Section II 19I 93 (a) Define the derived subgroup, G, of a finite group G. Show that if χ is a linear character
Part II Year 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2018 Paper 1, Section II 19I 93 (a) Define the derived subgroup, G, of a finite group G. Show that if χ is a linear character
EXAM INFORMATION. Harmonic Oscillator. Anharmonic Oscillator 1 ~ 1. Rigid Rotor
 EXAM INFORMATION Harmonc Oscllator Hamltonan: H d dx 1 kx Energy Levels: 1 k mm 1 En n n 0,1,, c m m 1 Anharmonc Oscllator Energy Levels: E n 1 ~ 1 n hc n hcx ~ e n 0,1,,... Rgd Rotor Quantum Numbers:
EXAM INFORMATION Harmonc Oscllator Hamltonan: H d dx 1 kx Energy Levels: 1 k mm 1 En n n 0,1,, c m m 1 Anharmonc Oscllator Energy Levels: E n 1 ~ 1 n hc n hcx ~ e n 0,1,,... Rgd Rotor Quantum Numbers:
we can assume without loss of generality that x and y are of the form x pabcq and y pbcdq,
 1. Symmetric and alternating groups. SOLUTIONS Math A4900 Homework 9 11/1/2017 (a) In class we showed that S n is generated by T tpi jq 1 ď i ă j ď nu, the set of transpositions in S n. Show by induction
1. Symmetric and alternating groups. SOLUTIONS Math A4900 Homework 9 11/1/2017 (a) In class we showed that S n is generated by T tpi jq 1 ď i ă j ď nu, the set of transpositions in S n. Show by induction
台灣大學開放式課程 化學鍵 本著作除另有註明, 所有內容皆採用創用 CC 姓名標示 - 非商業使用 - 相同方式分享 3.0 台灣授權條款釋出
 台灣大學開放式課程 化學鍵 本著作除另有註明, 所有內容皆採用創用 CC 姓名標示 - 非商業使用 - 相同方式分享 3.0 台灣授權條款釋出 Chemical Bonding Lecture 0: MO for AXn system, Multicenter Bonding, VSEPR, Hybridization 5//202 Pictorial Symmetry MO AXn n=3, 4,
台灣大學開放式課程 化學鍵 本著作除另有註明, 所有內容皆採用創用 CC 姓名標示 - 非商業使用 - 相同方式分享 3.0 台灣授權條款釋出 Chemical Bonding Lecture 0: MO for AXn system, Multicenter Bonding, VSEPR, Hybridization 5//202 Pictorial Symmetry MO AXn n=3, 4,
CS5371 Theory of Computation. Lecture 5: Automata Theory III (Non-regular Language, Pumping Lemma, Regular Expression)
 CS5371 Theory of Computation Lecture 5: Automata Theory III (Non-regular Language, Pumping Lemma, Regular Expression) Objectives Prove the Pumping Lemma, and use it to show that there are non-regular languages
CS5371 Theory of Computation Lecture 5: Automata Theory III (Non-regular Language, Pumping Lemma, Regular Expression) Objectives Prove the Pumping Lemma, and use it to show that there are non-regular languages
Quantum Number. i. Degeneracy is when orbitals have the same value of n.
 Quantum Number 1. Principal Quantum Number a. Is represented by n b. Has values from ranging from 1 c. Indicates the size and energy level in which the electron is housed. The bigger the n value, the further
Quantum Number 1. Principal Quantum Number a. Is represented by n b. Has values from ranging from 1 c. Indicates the size and energy level in which the electron is housed. The bigger the n value, the further
Demonstration of the Coupled Evolution Rules 163 APPENDIX F: DEMONSTRATION OF THE COUPLED EVOLUTION RULES
 Demonstration of the Coupled Evolution Rules 163 APPENDIX F: DEMONSTRATION OF THE COUPLED EVOLUTION RULES Before going into the demonstration we need to point out two limitations: a. It assumes I=1/2 for
Demonstration of the Coupled Evolution Rules 163 APPENDIX F: DEMONSTRATION OF THE COUPLED EVOLUTION RULES Before going into the demonstration we need to point out two limitations: a. It assumes I=1/2 for
EXAM INFORMATION. Radial Distribution Function: B is the normalization constant. d dx. p 2 Operator: Heisenberg Uncertainty Principle:
 EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
MTH5102 Spring 2017 HW Assignment 3: Sec. 1.5, #2(e), 9, 15, 20; Sec. 1.6, #7, 13, 29 The due date for this assignment is 2/01/17.
 MTH5102 Spring 2017 HW Assignment 3: Sec. 1.5, #2(e), 9, 15, 20; Sec. 1.6, #7, 13, 29 The due date for this assignment is 2/01/17. Sec. 1.5, #2(e). Determine whether the following sets are linearly dependent
MTH5102 Spring 2017 HW Assignment 3: Sec. 1.5, #2(e), 9, 15, 20; Sec. 1.6, #7, 13, 29 The due date for this assignment is 2/01/17. Sec. 1.5, #2(e). Determine whether the following sets are linearly dependent
Chimica Inorganica 3
 A symmetry operation carries the system into an equivalent configuration, which is, by definition physically indistinguishable from the original configuration. Clearly then, the energy of the system must
A symmetry operation carries the system into an equivalent configuration, which is, by definition physically indistinguishable from the original configuration. Clearly then, the energy of the system must
CO350 Linear Programming Chapter 8: Degeneracy and Finite Termination
 CO350 Linear Programming Chapter 8: Degeneracy and Finite Termination 22th June 2005 Chapter 8: Finite Termination Recap On Monday, we established In the absence of degeneracy, the simplex method will
CO350 Linear Programming Chapter 8: Degeneracy and Finite Termination 22th June 2005 Chapter 8: Finite Termination Recap On Monday, we established In the absence of degeneracy, the simplex method will
one-dimensional box with harmonic interaction
 On the symmetry of four particles in a arxiv:1607.00977v [quant-ph] 8 Jul 016 one-dimensional box with harmonic interaction Francisco M. Fernández INIFTA (CONICET, UNLP), División Química Teórica Blvd.
On the symmetry of four particles in a arxiv:1607.00977v [quant-ph] 8 Jul 016 one-dimensional box with harmonic interaction Francisco M. Fernández INIFTA (CONICET, UNLP), División Química Teórica Blvd.
Assignment 11 (C + C ) = (C + C ) = (C + C) i(c C ) ] = i(c C) (AB) = (AB) = B A = BA 0 = [A, B] = [A, B] = (AB BA) = (AB) AB
![Assignment 11 (C + C ) = (C + C ) = (C + C) i(c C ) ] = i(c C) (AB) = (AB) = B A = BA 0 = [A, B] = [A, B] = (AB BA) = (AB) AB Assignment 11 (C + C ) = (C + C ) = (C + C) i(c C ) ] = i(c C) (AB) = (AB) = B A = BA 0 = [A, B] = [A, B] = (AB BA) = (AB) AB](/thumbs/82/86038312.jpg) Arfken 3.4.6 Matrix C is not Hermition. But which is Hermitian. Likewise, Assignment 11 (C + C ) = (C + C ) = (C + C) [ i(c C ) ] = i(c C ) = i(c C) = i ( C C ) Arfken 3.4.9 The matrices A and B are both
Arfken 3.4.6 Matrix C is not Hermition. But which is Hermitian. Likewise, Assignment 11 (C + C ) = (C + C ) = (C + C) [ i(c C ) ] = i(c C ) = i(c C) = i ( C C ) Arfken 3.4.9 The matrices A and B are both
