A time-dependent approach to the kinetics of homogeneous nucleation
|
|
|
- Buddy Moody
- 5 years ago
- Views:
Transcription
1 A time-epenent approach to the kinetics of homogeneous nucleation Terry Olson an Patrick Hamill Physics Department, San Jose State University, 1 Washington Square, San Jose, California Receive 29 August 1995; accepte 27 September 1995 We present a time-epenent moel for homogeneous nucleation an erive a time-epenent solution to the couple growth-rate equations for molecular cluster concentrations. A correction to the monomer concentration growth-rate equation is applie, which accounts for the gain an loss of molecules by all cluster populations. The conensation rate use consiers all embryos to be in motion, rather than assuming that only the monomers move. In orer to allow for size-epenent variations in a cluster s surface tension, the evaporation rate incorporates the revise parametrization of Laaksonen et al. Phys. Rev. E 49, of the homogeneous nucleation theory of Dillmann an Meier J. Chem. Phys Eigenvalue analysis illuminates how the steay-state is approache from the transient perio an allows us to estimate the accuracy of a steay-state approximation. It is shown that the istributions of clusters preicte by this theory generate nucleation currents that are more accurate than those prouce by other steay-state cluster istributions. More importantly, this moel can be use to calculate the time-epenent cluster behavior for any nucleation moel that has an expression for the change in ibbs free energy. The theory agrees well with the experimental ata of Miller et al. J. Chem. Phys. 78, an Viisanen et al. J. Chem. Phys , an with the theoretical preictions of Laaksonen et al American Institute of Physics. S I. INTRODUCTION Nucleation is the thermoynamic molecular process that initiates many phase transitions. We observe nucleation events on a ay-to-ay basis, some examples being clou creation the formation of liqui roplets within a vapor, making ice the formation of soli crystals within a liqui, an boiling water the formation of gaseous bubbles within a liqui. There is a strong motivation to stuy nucleation because phase transitions are pervasive in many important physical phenomena. In homogeneous nucleation, the nucleating substance an its environment are assume pure; there are no foreign particles, such as ust, wall surfaces, or ions, that can serve as sites for the onset of the phase change. Consequently, of all the nucleation theories, homogeneous nucleation is the simplest. Its simplicity oes not unermine its significance, however, as its concepts are applie by many theorists to explain more complicate processes. The classical approach to homogeneous nucleation was originate an evelope by Becker an Döring, 1 Farkas, 2 Frenkel, 3 Volmer an Weber, 4 an Zelovich; 5 an excellent two-part review is given by McDonal. 6,7 The theory focuses on the vapor-to-liqui phase transition an attempts to calculate the time-inepenent rate of roplet formation within a given volume of vapor; this rate is calle the steay-state nucleation current. Molecular clusters are allowe to change size only by gaining an losing single molecules monomers by conensation an evaporation. It is important to note that for a vapor-to-liqui transition, homogeneous nucleation will take place only if the vapor is supersaturate. In other wors, the ratio of the vapor pressure to the saturation vapor pressure must be greater than one; this ratio is calle the supersaturation ratio, an the saturate vapor pressure is efine as the pressure of a saturate vapor over a plane liqui surface. Thermoynamically, the growth of a roplet is assume to be governe by the change in ibbs free energy, which classically has two terms: the first is the bulk energy of liqui phase formation, which is negative an varies as the roplet s volume; the secon is the surface free energy, which is positive an varies as the roplet s surface area. For very small molecular clusters, the surface energy ominates the bulk energy, but for larger clusters the bulk energy becomes ominant. Therefore, the ibbs free energy curve is initially steep an increasing, but as a molecular cluster an embryo grows, the curve reaches a maximum an then ecreases, the rate of escent growing as the cluster gets larger Fig. 1 epicts a moifie version of this curve. Because a thermoynamic system will spontaneously ten to go to the lowest ibbs free energy state, the maximum in the curve serves as an activation barrier. Unless an embryo becomes large enough to overcome this barrier, it will ten to ecrease its ibbs free energy by losing molecules by evaporation. The cluster size at which the activation barrier occurs is calle the critical size. Because the ibbs curve is at a maximum, the critically size cluster is at an unstable equilibrium with respect to the supersaturate vapor. In other wors, the vapor is saturate with respect to the surface of the embryos at the critical size. Furthermore, the vapor is supersaturate with respect to the surfaces of supercritically size clusters an subsaturate with respect to the surfaces of subcritically size clusters. This explains the tenency of supercritical embryos to grow an subcritical embryos to shrink. The classical theory of homogeneous nucleation uses the ibbs free energy to calculate the time-inepenent istribution of embryos for a supersaturate vapor constraine to be in complete thermoynamic equilibrium; this is calle the balance steay state an is characterize by a steaystate nucleation current equal to zero. The constraine equi- 210 J. Chem. Phys. 104 (1), 1 January /96/104(1)/210/15/$ American Institute of Physics
2 T. Olson an P. Hamill: Kinetics of homogeneous nucleation 211 FI. 1. The ibbs free energy, as moifie by Laaksonen et al. Ref. 14, is compare with the rates of conensation an evaporation for water vapor having a supersaturation ratio of at K. librium istribution of embryos is a mathematical artifice. The balance steay state is nonphysical for a supersaturate vapor because a supersaturate vapor is not in complete thermoynamic equilibrium an, therefore, prouces a nonzero current. Simple kinetic arguments are employe by the classical theory to obtain a time-inepenent rate of the number of monomers conensing onto a motionless embryo. This conensation rate is combine with the constraine equilibrium istribution to erive the rate at which molecules evaporate from a given cluster. The classical theory then applies the conensation an evaporation rates to erive the sizeepenent currents, each of which is the net number of embryos becoming one molecule larger in a given time an volume. These currents represent the total flow of embryos between concentrations of molecular clusters. At this point in the classical theory two crucial assumptions are mae. The first assumption is that a steay-state perio exists uring nucleation in which the concentrations of equally size embryos remain constant. Consequently, the currents between the embryo concentrations must all have equal magnitues; a concentration of clusters coul not be constant if the rate of embryos flowing in were not equal to the rate flowing out. The istribution of cluster concentrations uring the steay-state perio is calle the steaystate istribution. Since the currents all have the same magnitue, they lose their size epenence an are consiere to be the steay-state current. This steay-state current is taken to be the nucleation rate. The secon assumption is that the time it takes for the embryo concentrations to initially grow an form the steay-state istribution is negligible when compare to the steay-state perio; this initial perio of time is calle the transient perio. Thus, the entire homogeneous nucleation process is moele with only the steaystate perio in consieration. Removing the time epenence of the cluster concentrations consierably simplifies the mathematics of the classical theory. When the previous two assumptions have been mae, it is not ifficult to obtain the value of the steay-state nucleation current. The classical theory usually conclues with this calculation. At first, experimentalists foun the agreement between the classical theory an experiment to be generally goo Unfortunately, they were not measuring nucleation rates; they were measuring the supersaturation ratios at which the steay-state current was equal to one roplet forme per cubic centimeter per secon. The classical theory agree with these measurements. In the last two ecaes, however, it has become possible to measure actual nucleation rates. New experimental ata has shown that in the case of water, the classical theory s agreement is limite to small currents. As the currents an supersaturation ratios become larger in magnitue, the theoretical agreement worsens, the preictions being consistently too high. Furthermore, the observe temperature epenence of nucleation rates is weaker than that which is preicte. There are many possible shortcomings in the classical theory of homogeneous nucleation, an the motivation to fin a more suitable approach is clear. One such efect is the omission of the transient perio. By assuming this time to be insignificant, an thereby immeiately placing the embryos into their steay-state istribution, one cannot obtain timeepenent expressions for either the cluster concentrations or the currents between them. Using a nonclassical semiphenomenological approach, Laaksonen et al. 14 evelope a revise version of the steay-state nucleation rate theory of Dillmann an Meier 15 that agrees with experimental ata for many ifferent substances over a wie range of temperatures. As with the classical theory, however, they have not prouce an expression that inclues the time epenence of the iniviual cluster concentrations. The utility of consiering this time evolution has been emonstrate by Abraham 16 an Wilcox an Bauer. 17 Abraham foun that uring the transient perio, the currents between concentrations of embryos smaller than the critical size were at times much larger than the steay-state current; these currents significantly change in magnitue even when the corresponing concentrations were almost steay. Neither he nor Wilcox an Bauer obtaine time-epenent expressions, however, but generate time evolution plots by numerically integrating the necessary growth-rate ifferential equations. In this paper, it is our goal to present a time-epenent expression for cluster concentrations within the context of homogeneous nucleation. The theoretical erivation is general an may be applie to ifferent substances. We incorporate the kinetic theory of ilute vapors to obtain a conensation rate that assumes all of the embryos are in motion; this is in contrast to the stanar technique of assuming that only the monomers move. The Laaksonen et al. reparametrization of the Dillmann an Meier homogeneous nucleation theory is then employe to erive the evaporation rate. Using the conservation of mass, we correct the stanar monomer concentration growth-rate equation, which can be shown to be erroneous. Finally, the entire system of cluster concentration
3 212 T. Olson an P. Hamill: Kinetics of homogeneous nucleation growth-rate equations is solve using matrix methos. The avantages of this time-epenent approach are many. This moel allows us to calculate the time-epenent behavior of embryos for almost any homogeneous nucleation theory; an expression for the change in ibbs free energy is all that is require. We are able to preict how many embryos of a particular size are present at a given time for any supersaturation ratio an temperature. It then becomes clear how the thermoynamic system approaches the steay-state from its transient perio of growth. Furthermore, the timeepenent expressions allow us to etermine the relative accuracy of the steay-state approximation, an to calculate when each iniviual embryo number concentration slows its growth enough to be consiere steay. The preicte nucleation currents are in excellent agreement with experimental ata an the results preicte by Laaksonen et al. 14 II. DERIVIN THE RATES OF CONDENSATION AND EVAPORATION We begin our analysis of the nucleation of roplets by restricting the ways which a molecular cluster may increase or ecrease in size. During nucleation the number of monomers in the supersaturate vapor is significantly larger than the total number of clusters imers, trimers, etc.. It is reasonable then to assume that the only way a cluster becomes larger or smaller is by gaining or losing single molecules. Let x be a iscrete variable representing the number of molecules in an embryo. An embryo consisting of x molecules an xmer is enote A x. It increases its size by gaining a single molecule A 1, A x A 1 A x1, an ecreases its size by losing a molecule, A x A x1 A 1. Of course, it is possible for an x-mer to absorb a imer, trimer, or any other embryo; the frequency of such an event, however, is negligible compare to monomer absorption. Assuming that only binary collisions take place is essentially the same as assuming that the supersaturate vapor is ilute. Even for a supersaturation of an a temperature of K, which correspons to a nucleation rate of 100 million roplets/cc/sec, 13 the maximum cluster iameter is only 6.5% of the mean molecular spacing of monomers within the vapor. Reactions 1 an 2 are starting points for many theoretical approaches to homogeneous nucleation. It has been suggeste that the formation of imers involves three molecules, as oppose to two; 18 the involvement of the thir molecule is a consequence of the conservation of energy an momentum. This use of an aitional reactant might also be necessary for slightly larger clusters; 17 however, it is most likely to be a neutral component, such as a carrier gas molecule. For the sake of simplicity, we will maintain the assumption that clusters grow an shrink only by gaining an losing monomers. 1 2 An x-mer gains an loses molecules by conensation an evaporation. The rates at which molecules conense onto an evaporate off of an x-mer in a given time interval are enote c x an e x, respectively. We use four funamental assumptions to obtain a numerical expression for c x. First, we assume that all x-mers are spherical; this approximation naturally loses accuracy as the embryos get smaller. Secon, we attribute the ensity of the liqui phase,, to each of the x-mers. These two assumptions can be summarize by the equation 4 3 r 3 x m 1 x, 3 where m 1 represents the mass of one molecule an r x represents the raius of the x-mer. The thir an fourth assumptions are that the number of monomers per unit volume, n 1, an the temperature of the vapor, T, are constant uring the nucleation perio. Of course, the monomer concentration must be eplete for molecular clustering to take place, but we assume that this epletion is insignificant. We obtain the conensation rate by evaluating the rate of molecular collisions using the kinetic theory of ilute vapors. This approach is avantageous because all embryos are consiere to be in motion. The classical treatment of conensation assumes that the embryos are suspene at rest in the vapor, with the monomers being the only moving boies. We treat the vapor as a gas mixture, each gas containing all of the embryos which have the same number of molecules. If a collision between a monomer an an x-mer occurs, the istance between the centers of the two, r x1, must be r x1 r x r 1. Using Eq. 3, r x1 3m 1/3 1x 4 3m 1/ m 1/3 1 x 4 1/ Envision a shell with raius r x1 surrouning the x-mer. Whenever the center of a monomer intersects this shell, the x-mer an monomer collie. The cross-sectional area of the shell is the total collision cross section an is enote by x1, where x1 r 2 x1. 6 Consier an x-mer moving with velocity x through a vapor of ranomly moving monomers. iven the monomer number ensity at time t, n 1 (t), a small number n 1 (t) will have velocities within the interval,. We choose a reference frame in which these monomers are at rest an the x-mer has the relative velocity rx1, where rx1 x. In time t, the x-mer, moving with velocity rx1 an having the total collision cross section x1, sweeps out a cyliner of volume x1 rx1 t. The chance that an x-mer moving with velocity x collies with a monomer having a velocity in the range, is n 1 (t) x1 rx1 t. This correspons to a collision frequency of f (t), 4 7
4 T. Olson an P. Hamill: Kinetics of homogeneous nucleation 213 f tn 1 t x1 rx1. The total collision frequency, which is the mean rate of collisions between the x-mer an the monomers, is obtaine by summing f (t) over all of the possible monomer spees: f x1 t f t n 1 t x1 rx1 n 1 t n 1 t x1 n 1 t rx1. 9 Note that n 1 (t)/n 1 (t) is the fraction of monomers having a velocity in the range,, so the summation in the last term of Eq. 9 is equal to the mean value of rx1. Thus, f x1 tn 1 t x1 rx One of our funamental assumptions was that the number ensity of monomers was time inepenent, n 1 tn This assumption removes the time epenence from the total collision frequency, hence f x1 n 1 0 x1 rx1. 12 From basic kinetic theory for a har-sphere molecule gas at equilibrium, 19 rx1 8 1/2 m r, 13 where m r is the reuce mass, m r m 1m x m 1xm 1 xm 1 m 1 m x m 1 xm 1 1x. 14 Using Eqs. 5, 6, 12, 13, an 14, we obtain a useful expression of the total collision frequency, f x1 n 1 0x 1/3 1 where 3m 1 4 2/3 8 m 1 2 x1 x 1/2, 15 1/2. 16 Let us efine the sticking coefficient x as the probability that a monomer hitting an x-mer sticks to it. The prouct of x an f x1 yiel a rate at which monomers hit an stick to an x-mer; this is the conensation rate c x, c x x n 1 0x 1/3 1 2 x1 x 1/2. 17 We will assume that x is equal to unity, so all molecules that collie with an embryo stick to it. One might be oppose to treating the supersaturate vapor as being at equilibrium in the erivation of Eq. 13. When a vapor is in an equilibrium state the number of molecules in any velocity class is time inepenent, so uring the steay-state perio, when fluctuations of the embryo concentrations are small, the vapor is at a quasiequilibrium. In fact, we have alreay assume a quasiequilibrium by assigning a single temperature to the vapor. In the spirit of Katz an Wieersich, 20 we erive the evaporation rate e x by consiering the equilibrium number ensity of x-mers, n x E,inasaturate vapor. This approach iffers from the classical theory, which invokes a supersaturate vapor constraine to be in equilibrium. The equilibrium concentration for x-mers is usually written in the form n E x n E 1 exp x, 18 where x is the change in the ibbs free energy of formation of an x-mer. The net flow of embryos per unit time per unit volume from the x-mer concentration, n x (t), to the (x 1)-mer concentration, n x1 (t), is efine as the nucleation current, I x (t), I x tc x n x te x1 n x1 t. 19 Homogeneous nucleation cannot occur in a saturate vapor, which is in equilibrium with a plane surface of the liqui phase; this means essentially that the critically size cluster within a saturate vapor must be infinitely large. Hence, the number ensities of x-mers are time inepenent an the nucleation currents between the concentrations of embryos are equal to zero. Using Eq. 19, 0c S1 x n E E x e x1 n x1, 20 where c (S1) x correspons to the conensation rate of a saturate vapor. The evaporation rate is then simply expresse as e x c n E S1 x1 x1 E. 21 n x Using Eq. 18, e x c S1 x1 exp x x1. 22 It remains to obtain formulas for the ibbs free energy terms in Eq. 22. There have been many theoretical approaches to etermining the ibbs free energy of a homogeneously nucleating embryo, 21 however, an expression that works for all substances uner all possible conitions has not yet been foun. We choose to use an approach evelope by For et al. 22 an Laaksonen et al. 14 in which the Dillmann an Meier theory is revise; this metho agrees with experimental ata for n-nonane, water, an the lower alcohols. In brief, we will erive the For et al. expression for steaystate cluster populations, impose a conition pose by Laaksonen et al., an apply this moifie expression to a saturate vapor. The result will be combine with a conensation rate in orer to obtain the evaporation rate. We begin by using an expression for the equilibrium population of clusters within a supersaturate vapor, 15
5 214 T. Olson an P. Hamill: Kinetics of homogeneous nucleation N x E exp K x x 2/3 ln xlnq 0 Vx. 23 The function K x escribes the surface energy eviations of an x-mer from the surface energy of a macroscopic liqui roplet; an q 0 are parameters relate to the configurational effects of the embryo as well as its rotational, vibrational, an translational egrees of freeom; the term is given by 6m 1 1/2 2/3, 24 in which is the surface tension of a macroscopic liqui roplet at temperature T, V is the volume occupie by the vapor, an is the ifference between the chemical potentials of a supersaturate an saturate vapor. Assuming that the partial pressure of the monomers is approximately equal to the total vapor pressure, we can express this ifference in chemical potentials as 23 lnsbpp OS 2, 25 where the last term enotes truncation error an B is the secon virial coefficient from the virial equation of state, in the form pv in E i i1 B j p j1. 26 j1 Using Eqs. 23 an 25, we obtain an expression for the equilibrium cluster populations, N x E S x exp K x x 2/3 ln xlnq 0 V x Bpp. 27 In orer to obtain an expression for K x, the surface energy moification, we will use a truncate form of the virial equation of state, pv N E E Bp N 2 This may be rewritten as pv pv Bp N E 1 12N E 2 /N E E 12N E 1 N 2 /N E Substituting Eq. 27 into Eq. 29, an using the fact that Bp 1, 30 we reexpress the virial equation of state as Bp p q 0 exp K 1 Bp 1 Bp S exp K 1 K 2 2 2/3 1ln 2 Bp. 31 Compare terms to obtain the values of K 1 an K 2 : 14 K 1 1 p ln q 0 Bp 32 an K ln Bp 2/3 2 exp K 1 Bp. 33 K x is expane in powers of x 1/3 which correspons to 1/r x K x 1a 1 x 1/3 a 2 x 2/3, 34 where a 1 an a 2 are etermine by Eq. 34 for x equal to 1 an 2, a 1 K 2K 1 2 2/3 2 2/ /3 2 2/3, 35 an a 2 K 1 1a Note that a 1 is inepenent of the quantity q 0, since K 2 K 1 2 2/3 1 2 ln Bp 2/3 2 Bp 1 2 ln Bp 2/ This q 0 inepenence was first note by For et al. 22 Neglecting the secon virial coefficient term in Eq. 27, asitis numerically insignificant Eq. 30, we obtain For et al. s expression for the equilibrium population of x-mers, N x E N 1 E expx 2/3 a 1 x 1/3 a 1 1 ln xx1ln S. 38 Laaksonen et al. 14 consiere in Eq. 38 to be a theoretical free parameter, removing its original epenence 15 on experimentally measurable values; they etermine that setting equal to zero prouce the best fit with nucleation ata for n-nonane, water, an the lower alcohols. Thus we arrive at their expression for the change in an x-mer s ibbs free energy by consiering Eq. 18, L x x 2/3 a 1 x 1/3 a 1 1x1ln S. 39 We now obtain the evaporation rate by combining Eqs. 39 an 22 with the saturation ratio equal to one, e x c S1 x1 expx 2/3 x1 2/3 a 1 x 1/3 a 1 x1 1/3, 40 where, using Eq. 17, 2 1/2 x c S1 x1 x1 n S1 1 0x1 1/3 1. x1 41 Equations 41 an 17 require formulas for the initial monomer concentrations of a saturate an supersaturate vapor; these can be obtaine using Eqs. 27 an 32,
6 T. Olson an P. Hamill: Kinetics of homogeneous nucleation 215 n 1 0 p 1 Bp 42. Note that e x is inepenent of the supersaturation ratio of the nucleating vapor, but is sensitive to the embryo size, vapor temperature, an surface tension see also Eq. 24 for surface tension an temperature terms within. e x is also unefine for x1, as it shoul be, since a monomer cannot evaporate. The relationship of c x an e x to the ibbs free energy is shown in Fig. 1. Note that the conensation an evaporation rates are equal when the free energy curve reaches its maximum; the cluster size at which this occurs is enote the critical size, x C. For x-mers smaller than the critical size, the slope of the ibbs free energy curve is positive; accoringly, the evaporation rate ominates over the conensation rate. On the other han, for x-mers larger than the critical size, the slope of the ibbs free energy curve is negative an the conensation rate ominates over the evaporation rate. This behavior is in agreement with the fact that thermoynamic systems ten to spontaneously assume the lowest possible state of ibbs free energy. Embryos smaller than the critical size ten to shrink by evaporation while those larger than the critical size ten to grow by conensation. III. DISCUSSION OF THE EMBRYO ROWTH-RATE EQUATIONS WITH A CORRECTION FOR THE MONOMERS Having euce the mechanisms by which a cluster increases an ecreases in size, we can erive the growth-rate equation for the concentration of x-mers at time t, n x (t). An x-mer is create when either an (x1)-mer grows by conensation or when an (x1)-mer shrinks by evaporation. However, an x-mer is estroye when it grows by conensation or shrinks by evaporation. Hence, t n xtc x1 n x1 tc x e x n x t e x1 n x1 t for x2, 43a where the first an thir terms on the right represent the inflow of clusters entering n x (t) x-mer creation while the secon term on the right represents the outflow x-mer estruction. Note that this is a simple inflow-outflow orinary ifferential equation for the time rate of change of each embryo concentration imers, trimers, etc.. It is convenient to reexpress this growth-rate equation using the efinition of the nucleation current, Eq. 19, t n xti x1 ti x t for x2. 43b The entire nucleating system except the monomers can be represente by a set of couple ifferential equations in the form of Eqs. 43a or 43b. This set has a lower boun, the imer concentration n 2 (t); the growth rate equation for the monomer concentration will be aresse later. Because of the forwar coupling in Eq. 43a, we cannot mathematically solve the couple set unless it has an upper boun, i.e., a maximum finite value for the cluster size x. A common technique is to choose some size,, that is about twice the critical size; clusters having this many molecules are assume to simply keep growing, assuming a negligible chance to shrink from evaporation. Some treatments effectively remove the -mers from the system by invoking the Szilar bounary conition, in which the -mer population is set to zero, ecompose into separate molecules, an then inserte back into the monomer population. 7 Another approach is to efine as an absorbing state, 24 in which case embryos are not allowe to leave the n (t) concentration once they have entere it, c e By setting c equal to zero, the absorption conition terminates the forwar coupling of the growth-rate equations, giving them an upper boun, t n tc 1 n 1 t. 45 The absorbing state is assume to be so large that its embryos will just keep growing, thus e is set equal to zero. This metho iffers from the Szilar metho because the -mers remain in the system, they are not remove. Note that both methos treat the n x (t) populations in the same manner. The growth-rate equation for n 1 (t) has no contribution from the -mers, t n 1tc 2 n 2 tc 1 e 1 n 1 t. 46 In this stuy we ecie to use the absorbing state metho for two reasons. First, by not setting the number of -mers to zero, we can check that our moel is conserving mass by confirming that the total number of molecules remains constant. Seconly, the absorbing state metho is avantageous because it allows us to etermine approximately when the first -mer is forme. The formation of the first -mer is important because it occurs at the maximum time for which the growth rate equations are completely accurate. As long as there are no -mers, Eq. 46 is physically realistic. However, as soon as the first cluster occupies the absorbing state there is a chance that it will lose a molecule by evaporation. This probability is smaller than the chance of growth by conensation, but it shoul not be neglecte, regarless of the absorption conition, Eq. 44. In fact, the ifference between e 2 an c 2 is usually much larger than that between c an e if they are not set to zero, yet we o not consier c 2 negligible because embryos woul be unable to grow beyon the imer state see Fig. 1. Therefore, once the absorbing state is first populate, Eq. 46 loses accuracy since it lacks the evaporation contribution to n 1 (t) from the -mers. Consequently, the calculate growth rate for n 1 (t) is smaller than its true value. This causes the calculate value of the (1)-mer concentration to also be smaller, an the error propagates to all of the embryo concentrations through the backwars coupling of the growth-rate equations 43a. It has been shown,
7 216 T. Olson an P. Hamill: Kinetics of homogeneous nucleation The time rate of change of the monomer concentration can be erive in a more quantitative manner by consiering the conservation of mass. Let Q be efine as the total number of molecules in the close nucleating system, QV in i t, i1 49 where V is the volume occupie by the system. Since the system is close, the total number of molecules, Q, is constant: FI. 2. The growth rate of the monomer concentration is affecte by the growth an ecay of all the embryo concentrations. For example, when c 2 n 2 (t) imers become trimers, n 1 (t) loses c 2 n 2 (t) monomers. t Q0V i1 i t n it. 50 however, that the nucleation rate is only slightly affecte by the value chosen for ; 7 therefore the inaccuracy in the growth-rate equations is small, an we will use the absorption conition, Eq. 44, regarless of the slight error. Note that the Szilar bounary conition also introuces this inaccuracy by setting n (t) to zero. One might be tempte to keep e equal to its calculate value, instea of setting it to zero, in orer to maintain the accuracy of the n 1 (t) concentration. This, however, prouces significant errors into the moel, resulting in negative currents which eter the concentrations from their steay state. In other wors, the unrealistically large -mer concentration causes e n (t) to ominate c 1 n 1 (t), an thus I 1 (t)0. From Eq. 28 we see that this causes n 1 (t) to grow, an the inaccuracy propagates to the other embryo concentrations. We mentione earlier that the growth-rate equations 43a an 43b o not apply to the monomer concentration, t n 1te 2 n 2 tc 1 n 1 t an 47a t n 1tI 1 t. 47b If a cluster can only change size by gaining or losing single molecules, then the time rate of change of the monomer concentration must epen on the concentrations of all the cluster sizes; if there are c 2 n 2 (t) imers becoming trimers every secon, then there are also c 2 n 2 (t) monomers eplete per secon. This is best illustrate by Fig. 2, which inicates that 1 t n 1te 2 n 2 tc 1 n 1 t i1 or, using Eq. 19, 1 t n 1tI 1 t I i t. i1 e i1 n i1 tc i n i t, 48a 48b We can solve this expression for the monomer growth rate, t n 1t i i2 an then use Eq. 43b, t n it, t n 1t2I 1 ti 2 t3i 2 ti 3 t 4I 3 ti 4 t I 1 t Combining terms, we obtain Eq. 48b. The correction to the monomer growth rate equation is significant for two reasons. First, because of the coupling in the growth-rate equations 33a, all of the embryo concentrations epen on the monomer concentration; an error like that suggeste by Eq. 47a is propagate to all of the embryos. Seconly, as the steay-state is approache, the currents between concentrations approach the same magnitue; the correct monomer growth-rate equation 48b will then have a epletion of monomers that is times as great as that preicte by Eq. 47b if it were an equality. IV. A TIME-DEPENDENT SOLUTION TO THE ROWTH-RATE EQUATIONS The set of couple ifferential equations escribing the nucleating system, Eqs. 48a an 43a, can be solve using matrix methos. We first efine a column matrix in which the xth row element is the concentration of x-mers, n x (t), ntn 1t n 2 t n t. 53 A matrix is built which contains the conensation an evaporation rates,
8 T. Olson an P. Hamill: Kinetics of homogeneous nucleation 217 2e2c2 e3c3 e1c1 0 c 1 c 2 e 2 e c 2 c 3 e 3 0 M2c1 0 0 c e 1 0 c 1 e c 1 0. This matrix, which we shall call the growth-rate matrix, is singular since the last column only contains zeros. Consier the time erivative of the matrix n(t), nt t n 1t 1 e i1 n i1 tc i n i t t n 2t c t 1 n 1 tc 2 e 2 n 2 te 3 n 3 t te2n2tc1n1ti1. 55 t n c 1 n 1 t The right-han sie comes from Eqs. 48a an 43a. But clearly, the right-han sie of Eq. 55 is just M n(t), so we thereby reuce the set of couple ifferential equations to one matrix ifferential equation representing the entire system, t ntm nt. 56 In orer to obtain the particular solution, we invoke the initial conition that at t0, the vapor is compose of only monomers. The concentration of monomers is given by Eq. 42, so 1 Bp n0p 0 0, 57 where p is the pressure of the supersaturate vapor. The particular solution of Eq. 56 with initial conition 57 is the exponential of the growth-rate matrix, M, times the initial conition column matrix, ntexpmt n0, 58 where the matrix exponential is given by 25 Mt expmt j. 59 j0 j! With Eq. 58 we have accomplishe our goal of obtaining an expression for the time-epenent istribution of each cluster concentration n x (t). Since computing the exponential of a matrix is a well stuie problem, 25,26 the solution 58 can be calculate. We must now etermine the maximum time for which the solution is vali, which we shall call t F. In the erivation of the conensation rates for the embryos, the number of monomers was assume to remain essentially constant. However, if anything is to nucleate, monomers must be eplete; the monomer growth-rate equation 48a escribes the mechanism for this to occur. Furthermore, monomer epletion causes a ecrease in the vapor pressure; this lowers the supersaturation ratio, an consequently lowers the nucleation rate. Therefore, we must choose a maximum time for the moel that is large enough for the embryo concentrations to fully grow, but small enough that the final concentration of monomers is still approximately equal to the initial concentration, such that the nucleation current is not rastically affecte. Miller et al. 12 use experimental ata to erive an empirical nucleation rate formula which is a function of only the vapor temperature an supersaturation ratio. We have use this formula to calculate the percentage ecrease in the nucleation current if, for a given temperature, the supersaturation ratio ecreases by 2%; Fig. 3 shows that for supersaturation ratios from 4 to 10, the current ecreases %, epening on the temperature. Since we assume that the partial pressure from the monomer concentration remaine approximately equal to the total vapor pressure p, n 1 (t) is approximately proportional to the supersaturation ratio S; using Eq. 42 we obtain this proportionality, n 1 tn 1 0 p S 1 Bp S p S. 60 Hence, if the monomer concentration ecreases by 2%, the supersaturation ratio ecreases by 2%, an the nucleation rate ecreases by %. Although such a ecrease in the current appears significant, it is of the same orer of magnitue. Since nucleation rates vary over many orers of magnitue for small changes in vapor pressure an temperature, a
9 218 T. Olson an P. Hamill: Kinetics of homogeneous nucleation FI. 3. The effects of a 2% ecrease in the supersaturation ratio are shown. Depening on the temperature an initial supersaturation ratio of the water vapor, the nucleation rate ecreases approximately %. FI. 5. The istribution of embryo concentrations for water vapor having a supersaturation ratio of at K evaluate at ifferent times. change of less than 1 orer of magnitue is acceptable. We therefore choose t F, the final time for which our moel is vali, to be the time when the monomer concentration has ecrease by 2% of its original value; thus, n 1 t F 0.98n Of course, the possibility exists that the effect of coagulation an embryo embryo interactions coul become greater than or equal to that of the reactions in Eqs. 1 an 2. Our theory alone coul not be applie in such an instance. However, it is beyon the scope of this paper to etermine when coagulation processes become ominant, so we will assume that t F is short enough that Eqs. 1 an 2 are the only significant reactions. Figures 4 an 5 show istributions of embryo concentrations calculate from Eq. 58 at particular times. The embryo concentrations seem to approach the steay state sequentially. Within 1 ns, the number ensities of the smallest clusters have stoppe growing significantly. As time progresses, successive concentrations appear to reach the steay state, an finally the concentrations with the largest embryos also appear to stop growing. In both cases it seems that the steay state is reache sometime between one hunre nanosecons an a microsecon; this marks the en of the transient perio. We can evaluate the en of the transient perio more precisely by using Figs. 6 an 7, which show the time evolution of embryo concentrations of particular sizes. The sequential approach to the steay state is also seen in these figures, more importantly, however, is the fact that the largest clusters require a microsecon before they appear FI. 4. The istribution of embryo concentrations for water vapor having a supersaturation ratio of 7.22 at K evaluate at ifferent times. FI. 6. The time-evolution of specific embryo concentrations is shown for water vapor having a supersaturation ratio of 7.22 at K. In escening orer from the top of the plot, we see the monomers, 12-mers, 25-mers, 49-mers the critical size, 74-mers, an 99-mers.
10 T. Olson an P. Hamill: Kinetics of homogeneous nucleation 219 etail later. Because the eigenvalues are istinct, the eigenvectors are linearly inepenent, which allows us to conclue that V iagonalizes M. This allows to write M as V 1 M VL MV L V The iagonalization of M allows us to use Eq. 59 to express the matrix exponential of Mt in terms of L an V, expmt j0 thus j0 Mt j j! V L V 1 j t j j! V j0 Lt j j! V 1, 65 FI. 7. The time evolution of specific embryo concentrations is shown for water vapor having a supersaturation ratio of at K. In escening orer from the top of the plot, we see the monomers, 9-mers, 18-mers, 35-mers the critical size, 53-mers, an 70-mers. steay. Hence, for the two systems moele, the transient perio lasts about a microsecon. V. REEXPRESSIN THE SOLUTION USIN EIENVALUE ANALYSIS Although Eq. 58 is an exact solution to the embryo growth-rate equations, we cannot preict the physical behavior of a particular embryo concentration by simple inspection; the matrix exponential must be numerically compute. However, we can reexpress this equation using the eigenvalues an eigenvectors of the growth-rate matrix. Eigenvalue analysis illuminates how the embryos approach the steaystate from the transient perio, an it allows us to estimate the accuracy of a steay-state approximation. The horizontal lines in Figs. 6 an 7 are eceptive, because they suggest that the embryo concentrations stop changing. This is not the case; the approach to the steaystate is asymptotic, an the concentrations never completely lose their time epenence. In orer to prove this, we must use some auxiliary matrices to reexpress the time epenence of the solution n(t), Eq. 58. Define a iagonal matrix L to contain the eigenvalues, j, of the growth-rate matrix M, L i, j j ij, i,j1,2,...,, 62 an efine another matrix V, the columns of which are the corresponing eigenvectors, M VV L. 63 In our calculations, we have observe three properties of these eigenvalues. First, the eigenvalues are istinct an real. Seconly, one has the value zero since M is singular an the rest are negative. Thirly, one of these negative eigenvalues is always significantly smaller in magnitue, i.e., less negative, than the others. This will be iscusse in greater expmtv explt V 1. Let us efine a iagonal matrix, E t, as follows 66 E t i, j ij exp j t, i, j1,2,...,. 67 Since L is a iagonal matrix, its matrix exponential is equivalent to taking the exponential of the iagonal elements, thus, explte t. 68 We can now use Eqs. 66 an 68 to rewrite the timeepenent number ensity solution, Eq. 58, in terms of the eigenvectors an eigenvalues of the growth-rate matrix, ntv E t V 1 n0. 69 It is convenient to express this matrix equation in terms of its elements, nt x,1 i i j l i j V x,i E t i, j V 1 j,l V x,i ij exp j tv 1 j,1 V x,i exp i tv 1 i,1 p 1 Bp l1 p 1 Bp. p 1 Bp We now have an expression for the single concentration of clusters with x molecules at time t: n x t p 1 Bp V x,i V 1 i,1 exp i t, i1 70 where x1,2,3,..., an 0tt F. The exponential term is rewritten as a reminer that the eigenvalues are negative. The steay-state behavior of the embryo concentrations in Figs. 4, 5, 6, an 7 result from the relative magnitues of the eigenvalues i in Eq. 70. As mentione earlier, one of these is equal to zero an the rest are negative. We choose to label the zero eigenvalue. Aitionally, we note that one
11 220 T. Olson an P. Hamill: Kinetics of homogeneous nucleation FI. 8. The absolute values of the subominant eigenvalue an the ominant eigenvalue line are shown for a supersaturate water vapor at K; the subominant eigenvalue is the least negative of the i group in Eq. 71. We note that there is a large ifference in magnitue between the two, an that the ominant eigenvalue approaches zero as the supersaturation ratio ecreases. The scale for D is on the right an the scale for min( i ) is on the left. of the negative values was much closer to zero than the rest; it will be referre to as the ominant eigenvalue, D. These relationships are summarize as follows, i D 0, id. 71 Figure 8 illustrates this relationship by comparing D to the least negative eigenvalue of the i group. The relation of the ominant eigenvalue to the steay state can be best unerstoo by consiering the time evolution of the cluster concentrations n x (t), as escribe by Eq. 70. Because of the relative magnitues of the eigenvalues, Eq. 71, there may come a time, enote by t x,d, when the i terms in Eq. 70 become negligible compare to the D term; note that the possibility exists for t x,d to be greater than t F, the maximum time for which our moel is vali. Furthermore, it coul have ifferent values for ifferent cluster sizes, which is the reason for the x subscript. If tt x,d, Eq. 70 will become simpler in form, n x t p 1 Bp V x, V 1,1 V x,d V 1 D,1 exp D t, 72 where t x,d tt F. Recalling the structure of the growth-rate matrix, Eq. 54, an noting that is the zero eigenvalue, it is easy to show that the eigenvector corresponing to must have zeros for all elements except the last, which must be nonzero, V x, 0, V, The -mers only absorb embryos; this results in a nonphysical behavior of growth. Therefore we ignore the -mers, an rewrite Eq. 72, n x t p 1 Bp V x,d V 1 D,1 exp D t, 74 where x1,2,...,1 an t x,d tt F. We see in Fig. 8 that the ominant eigenvalue rapily approaches zero as the supersaturation ratio ecreases. Thus we can approximate D to be zero, an the approximation becomes better as the supersaturation ratio ecreases. This removes the time epenence from Eq. 74, n x tn x p 1 Bp V x,d V 1 D,1, 75 where n x represents the steay-state concentration of x-mers, with x1,2,...,1 an t x,d tt F. Equation 75 is vali only if exp D t1, tt F. 76 We call this the steay-state conition; if it is satisfie, then after time t x,d the embryo concentrations will essentially lose their time epenence. The closer Eq. 76 is to equality, the more accurate a steay-state approximation will be. Using Eq. 75, we rewrite the x-mer concentration Eq. 70 by pulling the Dth term out of the sum an setting D equal to zero, n x tn x p 1 Bp 1 i1 V x,i V 1 i,1 exp i t, id 77 where x1,2,...,1 an 0tt F. Equation 77 is the final result of our eigenvalue analysis; its form which consists of a time-inepenent steaystate term ae to a time-epenent transient term allows us to etermine the general physical behavior of the embryos by simple inspection. iven the form of Eq. 77, it is clear that an x-mer concentration approaches the steay state asymptotically uring the nucleation process. Furthermore, knowing that the time-epenent transient portion becomes negligible for tt x,d, we can euce that t x,d is the uration of the transient perio for the x-mers. Finally, by evaluating the steay-state conition, Eq. 76, for a particular growthrate matrix, we can etermine the relative accuracy of a steay-state approximation. McDonal suggeste that evaluating the steay-state nucleation current is analogous to fining the steay-state heat flux through a slab which has a constant known temperature at each face. 7 Equation 77, having the same form as the heat-flux solution, supports his claim. VI. COMPARISONS BETWEEN THEORY AND EXPERIMENT Equations 58 an 70 are exact an equivalent solutions for the growth-rate equations 43a an 48a. For physically realistic problems the growth-rate matrix is large, e.g., for water vapor with a supersaturation ratio equal to 7.22 at K. Obtaining all of the eigenvalues an eigenvectors is certainly possible, but it is nontrivial.
12 T. Olson an P. Hamill: Kinetics of homogeneous nucleation 221 FI. 9. We compare the embryo concentration steay-state istributions of this work line, Abraham, an illespie for water vapor having a supersaturation ratio of 7.22 at K. FI. 11. The nucleation current at the critical size is ivie by the steaystate current, an the time evolution of this ratio is shown soli line. The ashe line marks the ratio s value at The time lag is then approximate as the moment when the soli an ashe lines intersect. The system consiere is water vapor having a supersaturation ratio of 4.91 at K. Furthermore, as the size of eigenvector matrix V increases, it becomes ill-conitione an approaches singularity; this complicates the calculation of V 1. Sometimes the aition of terms in Eq. 70 introuces significant roun-off problems. Therefore, although Eq. 70 is theoretically avantageous for eucing embryo concentration behavior, there are many chances for numerical error to ruin the results. We have foun that using Eq. 58, which involves calculating the exponential of the growth-rate matrix, is numerically more reliable an consistent; thus, we have use it for computing the results which we shall refer to. We have limite our calculations an comparisons to systems of supersaturate water vapor. Figures 9 an 10 compare the steay-state istributions evaluate using Eq. 58 at 1 ms to those of Abraham 16 an FI. 10. We compare the embryo concentration steay-state istributions of this work line, Abraham, an illespie for water vapor having a supersaturation ratio of at K. illespie. 24 Abraham s approach follows that of the classical theory, in which the classical free energy, the Szilar bounary conition, an the constraine equilibrium Boltzmann istribution are employe. He obtaine figures similar to 6 an 7 by using a nonlinear integration routine to numerically solve the growth-rate equations; it is interesting to note that Eq. 58 an the moel of Abraham preict the same general time evolution for the embryos. He i not obtain a close form expression for the time epenence of the cluster concentrations, but i erive a steay-state istribution that agrees with his numerical integration results; we have use this expression for our comparison. illespie also erive a steay-state istribution of embryos, which we have use. Although illespie i not explicitly present a solution for the time evolution of cluster concentrations, he erive a time-epenent matrix expression for the probability that an embryo, initially a monomer, woul be an x-mer at time t. He prove that one coul obtain the average number of x-mers at time t by simply multiplying this probability by the total number of embryos incluing monomers; unfortunately, he i not perform the necessary calculations to obtain the time-epenent istributions. We i the calculations, however, an foun that the embryo concentrations again exhibite the same general behavior as in Figs. 6 an 7, an that they relaxe into values that agree with illespie s steay-state expression. Figures 9 an 10 show us that Abraham s results preict more meium-size an large clusters than our moel, an that the illespie moel preicts the most clusters in the comparison. Many theories have been evelope to estimate the time it takes for the nucleating system to significantly approach the steay state. This time lag is usually efine as the moment when the current at the critical size reaches 11/e, approximately 63.2% of the steay-state current. Having a time-epenent expression for the embryo concentrations makes this estimation a trivial matter. Figure 11
13 222 T. Olson an P. Hamill: Kinetics of homogeneous nucleation shows that for water vapor having a supersaturation ratio of 4.91 an at a temperature of K, the time lag is approximately 0.28 ms. Abraham 18 Table 5.6 of Ref. 18 mae similar calculations for the same system using two ifferent efinitions of the time lag, his results were 0.46 an 0.43 ms. He also use the moels of Wakeshima, 27 which preicte 0.16 ms; Collins, 28 which preicte 0.49 ms; Feer et al., 29 which preicte 0.98 ms; an Anres an Bouart, 30 which preicte 0.55 ms. We can check the accuracy of our results by comparing our steay-state nucleation rates with experimental ata. This is convenient, since most experimentalists have been concerne with measuring nucleation rates, rather than istributions of embryo concentrations. Viisanen et al. 13 present ata that shows acceptable agreement with the work of Miller et al.; 12 we will use the expansion chamber results of both Viisanen et al. an Miller et al. for our subsequent comparisons. Our rates will be calculate by using the efinition of the nucleation current, Eq. 19, at the critical size an by evaluating Eq. 58 at 1 ms; 1 ms is chosen because it is enough time for the embryo concentrations to sufficiently approach their steay-state values an it is the general uration of a nucleation pulse in the expansion chamber experiments of Viisanen et al. Using the current at the critical size is purely an arbitrary choice, since the currents at each x value are approximately equal once they are steay. The thermoynamic parameters saturation vapor pressure, the secon virial coefficient, etc. were calculate using the functions presente by Dillmann an Meier. For the system having supersaturation ratio of 7.22 an a vapor temperature of K Fig. 9 Viisanen et al. measure the nucleation rate to be 1.9e05 embryos/cc/sec; the empirical fitting function of Miller et al. preicte a value of 5.47e05 embryos/cc/sec. Using Abraham s steay-state current equation which is essentially the classical Becker an Döring nucleation current, we calculate that Abraham s moel preicts 5.08e07 embryos/cc/sec. illespie s steay-state current expression yiels a nucleation rate of 1.38e11 embryos/cc/sec. Our moel preicts a value of 7.22e05 embryos/cc/sec. The system with a vapor temperature of K an a supersaturation ratio of Fig. 10 is beyon the range of the Miller et al. fitting function, so it will not be use here. Although Viisanen et al. measure the current to be 1e08 embryos/cc/sec, the moels of Abraham an illespie preict its value at 6.34e09 an 2.92e13 embryos/cc/sec, respectively. Our moel yiels a nucleation rate of 9.06e08 embryos/cc/sec. Note that in both systems, the nucleation current calculate from Eqs. 58 an 19 was significantly closer to the experimentally measure values than that preicte by Abraham or illespie. We will therefore assume that the istributions of cluster concentrations compute from Eq. 58 are also more accurate. Two cases of agreement are not enough to assume a general tren of accuracy, however. We now make a more general comparison with theory an experiment over a wie range of temperatures an supersaturations. In aition to FI. 12. The nucleation rates preicte by this work line are compare with the preictions of the Laaksonen et al. theory an the experimental measurements of Miller et al. an Viisanen et al.. The temperatures corresponing to the ata are given in Kelvin. A supersaturate water vapor is consiere. using the experimental ata of Miller et al. an Viisanen et al., we will compare our nucleation rates with those preicte by Laaksonen et al. 14 We choose Laaksonen et al. because their theory prouces currents that are in excellent agreement with experimental ata for many substances, incluing water, n-nonane, an the lower alchohols. Unfortunately, Laaksonen et al. i not present an expression for the nucleation rate within their stuy. Since they were moifying the theory of Dillmann an Meier, we have use Dillmann an Meier s general expression for the current, E N xc IC xc V x x 2 1/2, xx C 78 where C xc is Dillmann an Meier s conensation rate at the critical size. We have combine Eqs. 38 0an 39 with Eq. 78 to obtain the Laaksonen et al. nucleation currents. The results of this comparison are given in Fig. 12. We see that our preictions for the current agree quite well with the values obtaine from the Laaksonen et al. theory. The agreement with experiment is also very goo, remaining within 1 orer of magnitue. Note that the curves implie by our preictions are parallel to those implie by the ata of Viisanen et al., but not to those of Miller et al. Viisanen et al. foun that the Miller et al. fitting function ha slightly too much curvature, an consiere it vali only for rates between 1e02 an 1e05. This is a narrower range than we have use in Fig. 12, an our agreement with Miller et al. is improve if we only consier the ata within that range. In orer to make sure that one millisecon is less than t F for this ata set, we have calculate the monomer epletion ratio, which is the ratio of the time-epenent monomer concentration to its initial value; see Fig. 13. These ratios stay well above the limit of 0.98 set forth by Eq. 61. An interesting aspect of the monomer epletion ratios in Fig. 13 is
19 Eigenvalues, Eigenvectors, Ordinary Differential Equations, and Control
 19 Eigenvalues, Eigenvectors, Orinary Differential Equations, an Control This section introuces eigenvalues an eigenvectors of a matrix, an iscusses the role of the eigenvalues in etermining the behavior
19 Eigenvalues, Eigenvectors, Orinary Differential Equations, an Control This section introuces eigenvalues an eigenvectors of a matrix, an iscusses the role of the eigenvalues in etermining the behavior
Critical Size and Particle Growth
 Critical Size an article Growth rof. Sotiris E. ratsinis article Technology Laboratory Department of Mechanical an rocess Engineering, ETH Zürich, Switzerlan www.ptl.ethz.ch 1 Nucleation-Conensation A
Critical Size an article Growth rof. Sotiris E. ratsinis article Technology Laboratory Department of Mechanical an rocess Engineering, ETH Zürich, Switzerlan www.ptl.ethz.ch 1 Nucleation-Conensation A
Lectures - Week 10 Introduction to Ordinary Differential Equations (ODES) First Order Linear ODEs
 Lectures - Week 10 Introuction to Orinary Differential Equations (ODES) First Orer Linear ODEs When stuying ODEs we are consiering functions of one inepenent variable, e.g., f(x), where x is the inepenent
Lectures - Week 10 Introuction to Orinary Differential Equations (ODES) First Orer Linear ODEs When stuying ODEs we are consiering functions of one inepenent variable, e.g., f(x), where x is the inepenent
inflow outflow Part I. Regular tasks for MAE598/494 Task 1
 MAE 494/598, Fall 2016 Project #1 (Regular tasks = 20 points) Har copy of report is ue at the start of class on the ue ate. The rules on collaboration will be release separately. Please always follow the
MAE 494/598, Fall 2016 Project #1 (Regular tasks = 20 points) Har copy of report is ue at the start of class on the ue ate. The rules on collaboration will be release separately. Please always follow the
The Principle of Least Action
 Chapter 7. The Principle of Least Action 7.1 Force Methos vs. Energy Methos We have so far stuie two istinct ways of analyzing physics problems: force methos, basically consisting of the application of
Chapter 7. The Principle of Least Action 7.1 Force Methos vs. Energy Methos We have so far stuie two istinct ways of analyzing physics problems: force methos, basically consisting of the application of
1 dx. where is a large constant, i.e., 1, (7.6) and Px is of the order of unity. Indeed, if px is given by (7.5), the inequality (7.
 Lectures Nine an Ten The WKB Approximation The WKB metho is a powerful tool to obtain solutions for many physical problems It is generally applicable to problems of wave propagation in which the frequency
Lectures Nine an Ten The WKB Approximation The WKB metho is a powerful tool to obtain solutions for many physical problems It is generally applicable to problems of wave propagation in which the frequency
water adding dye partial mixing homogenization time
 iffusion iffusion is a process of mass transport that involves the movement of one atomic species into another. It occurs by ranom atomic jumps from one position to another an takes place in the gaseous,
iffusion iffusion is a process of mass transport that involves the movement of one atomic species into another. It occurs by ranom atomic jumps from one position to another an takes place in the gaseous,
'HVLJQ &RQVLGHUDWLRQ LQ 0DWHULDO 6HOHFWLRQ 'HVLJQ 6HQVLWLYLW\,1752'8&7,21
 Large amping in a structural material may be either esirable or unesirable, epening on the engineering application at han. For example, amping is a esirable property to the esigner concerne with limiting
Large amping in a structural material may be either esirable or unesirable, epening on the engineering application at han. For example, amping is a esirable property to the esigner concerne with limiting
The Press-Schechter mass function
 The Press-Schechter mass function To state the obvious: It is important to relate our theories to what we can observe. We have looke at linear perturbation theory, an we have consiere a simple moel for
The Press-Schechter mass function To state the obvious: It is important to relate our theories to what we can observe. We have looke at linear perturbation theory, an we have consiere a simple moel for
Math 342 Partial Differential Equations «Viktor Grigoryan
 Math 342 Partial Differential Equations «Viktor Grigoryan 6 Wave equation: solution In this lecture we will solve the wave equation on the entire real line x R. This correspons to a string of infinite
Math 342 Partial Differential Equations «Viktor Grigoryan 6 Wave equation: solution In this lecture we will solve the wave equation on the entire real line x R. This correspons to a string of infinite
Separation of Variables
 Physics 342 Lecture 1 Separation of Variables Lecture 1 Physics 342 Quantum Mechanics I Monay, January 25th, 2010 There are three basic mathematical tools we nee, an then we can begin working on the physical
Physics 342 Lecture 1 Separation of Variables Lecture 1 Physics 342 Quantum Mechanics I Monay, January 25th, 2010 There are three basic mathematical tools we nee, an then we can begin working on the physical
Applications of First Order Equations
 Applications of First Orer Equations Viscous Friction Consier a small mass that has been roppe into a thin vertical tube of viscous flui lie oil. The mass falls, ue to the force of gravity, but falls more
Applications of First Orer Equations Viscous Friction Consier a small mass that has been roppe into a thin vertical tube of viscous flui lie oil. The mass falls, ue to the force of gravity, but falls more
ensembles When working with density operators, we can use this connection to define a generalized Bloch vector: v x Tr x, v y Tr y
 Ph195a lecture notes, 1/3/01 Density operators for spin- 1 ensembles So far in our iscussion of spin- 1 systems, we have restricte our attention to the case of pure states an Hamiltonian evolution. Toay
Ph195a lecture notes, 1/3/01 Density operators for spin- 1 ensembles So far in our iscussion of spin- 1 systems, we have restricte our attention to the case of pure states an Hamiltonian evolution. Toay
Chapter 4. Electrostatics of Macroscopic Media
 Chapter 4. Electrostatics of Macroscopic Meia 4.1 Multipole Expansion Approximate potentials at large istances 3 x' x' (x') x x' x x Fig 4.1 We consier the potential in the far-fiel region (see Fig. 4.1
Chapter 4. Electrostatics of Macroscopic Meia 4.1 Multipole Expansion Approximate potentials at large istances 3 x' x' (x') x x' x x Fig 4.1 We consier the potential in the far-fiel region (see Fig. 4.1
APPROXIMATE SOLUTION FOR TRANSIENT HEAT TRANSFER IN STATIC TURBULENT HE II. B. Baudouy. CEA/Saclay, DSM/DAPNIA/STCM Gif-sur-Yvette Cedex, France
 APPROXIMAE SOLUION FOR RANSIEN HEA RANSFER IN SAIC URBULEN HE II B. Bauouy CEA/Saclay, DSM/DAPNIA/SCM 91191 Gif-sur-Yvette Ceex, France ABSRAC Analytical solution in one imension of the heat iffusion equation
APPROXIMAE SOLUION FOR RANSIEN HEA RANSFER IN SAIC URBULEN HE II B. Bauouy CEA/Saclay, DSM/DAPNIA/SCM 91191 Gif-sur-Yvette Ceex, France ABSRAC Analytical solution in one imension of the heat iffusion equation
Diagonalization of Matrices Dr. E. Jacobs
 Diagonalization of Matrices Dr. E. Jacobs One of the very interesting lessons in this course is how certain algebraic techniques can be use to solve ifferential equations. The purpose of these notes is
Diagonalization of Matrices Dr. E. Jacobs One of the very interesting lessons in this course is how certain algebraic techniques can be use to solve ifferential equations. The purpose of these notes is
ELECTRON DIFFRACTION
 ELECTRON DIFFRACTION Electrons : wave or quanta? Measurement of wavelength an momentum of electrons. Introuction Electrons isplay both wave an particle properties. What is the relationship between the
ELECTRON DIFFRACTION Electrons : wave or quanta? Measurement of wavelength an momentum of electrons. Introuction Electrons isplay both wave an particle properties. What is the relationship between the
Table of Common Derivatives By David Abraham
 Prouct an Quotient Rules: Table of Common Derivatives By Davi Abraham [ f ( g( ] = [ f ( ] g( + f ( [ g( ] f ( = g( [ f ( ] g( g( f ( [ g( ] Trigonometric Functions: sin( = cos( cos( = sin( tan( = sec
Prouct an Quotient Rules: Table of Common Derivatives By Davi Abraham [ f ( g( ] = [ f ( ] g( + f ( [ g( ] f ( = g( [ f ( ] g( g( f ( [ g( ] Trigonometric Functions: sin( = cos( cos( = sin( tan( = sec
Survey Sampling. 1 Design-based Inference. Kosuke Imai Department of Politics, Princeton University. February 19, 2013
 Survey Sampling Kosuke Imai Department of Politics, Princeton University February 19, 2013 Survey sampling is one of the most commonly use ata collection methos for social scientists. We begin by escribing
Survey Sampling Kosuke Imai Department of Politics, Princeton University February 19, 2013 Survey sampling is one of the most commonly use ata collection methos for social scientists. We begin by escribing
Math Notes on differentials, the Chain Rule, gradients, directional derivative, and normal vectors
 Math 18.02 Notes on ifferentials, the Chain Rule, graients, irectional erivative, an normal vectors Tangent plane an linear approximation We efine the partial erivatives of f( xy, ) as follows: f f( x+
Math 18.02 Notes on ifferentials, the Chain Rule, graients, irectional erivative, an normal vectors Tangent plane an linear approximation We efine the partial erivatives of f( xy, ) as follows: f f( x+
arxiv:physics/ v2 [physics.ed-ph] 23 Sep 2003
![arxiv:physics/ v2 [physics.ed-ph] 23 Sep 2003 arxiv:physics/ v2 [physics.ed-ph] 23 Sep 2003](/thumbs/79/79115961.jpg) Mass reistribution in variable mass systems Célia A. e Sousa an Vítor H. Rorigues Departamento e Física a Universiae e Coimbra, P-3004-516 Coimbra, Portugal arxiv:physics/0211075v2 [physics.e-ph] 23 Sep
Mass reistribution in variable mass systems Célia A. e Sousa an Vítor H. Rorigues Departamento e Física a Universiae e Coimbra, P-3004-516 Coimbra, Portugal arxiv:physics/0211075v2 [physics.e-ph] 23 Sep
THE VAN KAMPEN EXPANSION FOR LINKED DUFFING LINEAR OSCILLATORS EXCITED BY COLORED NOISE
 Journal of Soun an Vibration (1996) 191(3), 397 414 THE VAN KAMPEN EXPANSION FOR LINKED DUFFING LINEAR OSCILLATORS EXCITED BY COLORED NOISE E. M. WEINSTEIN Galaxy Scientific Corporation, 2500 English Creek
Journal of Soun an Vibration (1996) 191(3), 397 414 THE VAN KAMPEN EXPANSION FOR LINKED DUFFING LINEAR OSCILLATORS EXCITED BY COLORED NOISE E. M. WEINSTEIN Galaxy Scientific Corporation, 2500 English Creek
Linear First-Order Equations
 5 Linear First-Orer Equations Linear first-orer ifferential equations make up another important class of ifferential equations that commonly arise in applications an are relatively easy to solve (in theory)
5 Linear First-Orer Equations Linear first-orer ifferential equations make up another important class of ifferential equations that commonly arise in applications an are relatively easy to solve (in theory)
3.2 Shot peening - modeling 3 PROCEEDINGS
 3.2 Shot peening - moeling 3 PROCEEDINGS Computer assiste coverage simulation François-Xavier Abaie a, b a FROHN, Germany, fx.abaie@frohn.com. b PEENING ACCESSORIES, Switzerlan, info@peening.ch Keywors:
3.2 Shot peening - moeling 3 PROCEEDINGS Computer assiste coverage simulation François-Xavier Abaie a, b a FROHN, Germany, fx.abaie@frohn.com. b PEENING ACCESSORIES, Switzerlan, info@peening.ch Keywors:
Hyperbolic Systems of Equations Posed on Erroneous Curved Domains
 Hyperbolic Systems of Equations Pose on Erroneous Curve Domains Jan Norström a, Samira Nikkar b a Department of Mathematics, Computational Mathematics, Linköping University, SE-58 83 Linköping, Sween (
Hyperbolic Systems of Equations Pose on Erroneous Curve Domains Jan Norström a, Samira Nikkar b a Department of Mathematics, Computational Mathematics, Linköping University, SE-58 83 Linköping, Sween (
Model for Dopant and Impurity Segregation During Vapor Phase Growth
 Mat. Res. Soc. Symp. Proc. Vol. 648, P3.11.1-7 2001 Materials Research Society Moel for Dopant an Impurity Segregation During Vapor Phase Growth Craig B. Arnol an Michael J. Aziz Division of Engineering
Mat. Res. Soc. Symp. Proc. Vol. 648, P3.11.1-7 2001 Materials Research Society Moel for Dopant an Impurity Segregation During Vapor Phase Growth Craig B. Arnol an Michael J. Aziz Division of Engineering
Assignment 1. g i (x 1,..., x n ) dx i = 0. i=1
 Assignment 1 Golstein 1.4 The equations of motion for the rolling isk are special cases of general linear ifferential equations of constraint of the form g i (x 1,..., x n x i = 0. i=1 A constraint conition
Assignment 1 Golstein 1.4 The equations of motion for the rolling isk are special cases of general linear ifferential equations of constraint of the form g i (x 1,..., x n x i = 0. i=1 A constraint conition
Lecture XII. where Φ is called the potential function. Let us introduce spherical coordinates defined through the relations
 Lecture XII Abstract We introuce the Laplace equation in spherical coorinates an apply the metho of separation of variables to solve it. This will generate three linear orinary secon orer ifferential equations:
Lecture XII Abstract We introuce the Laplace equation in spherical coorinates an apply the metho of separation of variables to solve it. This will generate three linear orinary secon orer ifferential equations:
Unified kinetic model of dopant segregation during vapor-phase growth
 PHYSICAL REVIEW B 72, 195419 2005 Unifie kinetic moel of opant segregation uring vapor-phase growth Craig B. Arnol 1, * an Michael J. Aziz 2 1 Department of Mechanical an Aerospace Engineering an Princeton
PHYSICAL REVIEW B 72, 195419 2005 Unifie kinetic moel of opant segregation uring vapor-phase growth Craig B. Arnol 1, * an Michael J. Aziz 2 1 Department of Mechanical an Aerospace Engineering an Princeton
Math 1271 Solutions for Fall 2005 Final Exam
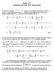 Math 7 Solutions for Fall 5 Final Eam ) Since the equation + y = e y cannot be rearrange algebraically in orer to write y as an eplicit function of, we must instea ifferentiate this relation implicitly
Math 7 Solutions for Fall 5 Final Eam ) Since the equation + y = e y cannot be rearrange algebraically in orer to write y as an eplicit function of, we must instea ifferentiate this relation implicitly
NOTES ON EULER-BOOLE SUMMATION (1) f (l 1) (n) f (l 1) (m) + ( 1)k 1 k! B k (y) f (k) (y) dy,
 NOTES ON EULER-BOOLE SUMMATION JONATHAN M BORWEIN, NEIL J CALKIN, AND DANTE MANNA Abstract We stuy a connection between Euler-MacLaurin Summation an Boole Summation suggeste in an AMM note from 196, which
NOTES ON EULER-BOOLE SUMMATION JONATHAN M BORWEIN, NEIL J CALKIN, AND DANTE MANNA Abstract We stuy a connection between Euler-MacLaurin Summation an Boole Summation suggeste in an AMM note from 196, which
q = F If we integrate this equation over all the mass in a star, we have q dm = F (M) F (0)
 Astronomy 112: The Physics of Stars Class 4 Notes: Energy an Chemical Balance in Stars In the last class we introuce the iea of hyrostatic balance in stars, an showe that we coul use this concept to erive
Astronomy 112: The Physics of Stars Class 4 Notes: Energy an Chemical Balance in Stars In the last class we introuce the iea of hyrostatic balance in stars, an showe that we coul use this concept to erive
Thermal conductivity of graded composites: Numerical simulations and an effective medium approximation
 JOURNAL OF MATERIALS SCIENCE 34 (999)5497 5503 Thermal conuctivity of grae composites: Numerical simulations an an effective meium approximation P. M. HUI Department of Physics, The Chinese University
JOURNAL OF MATERIALS SCIENCE 34 (999)5497 5503 Thermal conuctivity of grae composites: Numerical simulations an an effective meium approximation P. M. HUI Department of Physics, The Chinese University
The derivative of a function f(x) is another function, defined in terms of a limiting expression: f(x + δx) f(x)
 Y. D. Chong (2016) MH2801: Complex Methos for the Sciences 1. Derivatives The erivative of a function f(x) is another function, efine in terms of a limiting expression: f (x) f (x) lim x δx 0 f(x + δx)
Y. D. Chong (2016) MH2801: Complex Methos for the Sciences 1. Derivatives The erivative of a function f(x) is another function, efine in terms of a limiting expression: f (x) f (x) lim x δx 0 f(x + δx)
To understand how scrubbers work, we must first define some terms.
 SRUBBERS FOR PARTIE OETION Backgroun To unerstan how scrubbers work, we must first efine some terms. Single roplet efficiency, η, is similar to single fiber efficiency. It is the fraction of particles
SRUBBERS FOR PARTIE OETION Backgroun To unerstan how scrubbers work, we must first efine some terms. Single roplet efficiency, η, is similar to single fiber efficiency. It is the fraction of particles
Calculus of Variations
 Calculus of Variations Lagrangian formalism is the main tool of theoretical classical mechanics. Calculus of Variations is a part of Mathematics which Lagrangian formalism is base on. In this section,
Calculus of Variations Lagrangian formalism is the main tool of theoretical classical mechanics. Calculus of Variations is a part of Mathematics which Lagrangian formalism is base on. In this section,
Introduction to the Vlasov-Poisson system
 Introuction to the Vlasov-Poisson system Simone Calogero 1 The Vlasov equation Consier a particle with mass m > 0. Let x(t) R 3 enote the position of the particle at time t R an v(t) = ẋ(t) = x(t)/t its
Introuction to the Vlasov-Poisson system Simone Calogero 1 The Vlasov equation Consier a particle with mass m > 0. Let x(t) R 3 enote the position of the particle at time t R an v(t) = ẋ(t) = x(t)/t its
ECE 422 Power System Operations & Planning 7 Transient Stability
 ECE 4 Power System Operations & Planning 7 Transient Stability Spring 5 Instructor: Kai Sun References Saaat s Chapter.5 ~. EPRI Tutorial s Chapter 7 Kunur s Chapter 3 Transient Stability The ability of
ECE 4 Power System Operations & Planning 7 Transient Stability Spring 5 Instructor: Kai Sun References Saaat s Chapter.5 ~. EPRI Tutorial s Chapter 7 Kunur s Chapter 3 Transient Stability The ability of
Qubit channels that achieve capacity with two states
 Qubit channels that achieve capacity with two states Dominic W. Berry Department of Physics, The University of Queenslan, Brisbane, Queenslan 4072, Australia Receive 22 December 2004; publishe 22 March
Qubit channels that achieve capacity with two states Dominic W. Berry Department of Physics, The University of Queenslan, Brisbane, Queenslan 4072, Australia Receive 22 December 2004; publishe 22 March
Influence of Radiation on Product Yields in a Film Boiling Reactor
 R&D NOTES Influence of Raiation on Prouct Yiels in a Film Boiling Reactor C. Thomas Aveisian, Wing Tsang, Terence Daviovits, an Jonah R. Allaben Sibley School of Mechanical an Aerospace Engineering, Cornell
R&D NOTES Influence of Raiation on Prouct Yiels in a Film Boiling Reactor C. Thomas Aveisian, Wing Tsang, Terence Daviovits, an Jonah R. Allaben Sibley School of Mechanical an Aerospace Engineering, Cornell
COUPLING REQUIREMENTS FOR WELL POSED AND STABLE MULTI-PHYSICS PROBLEMS
 VI International Conference on Computational Methos for Couple Problems in Science an Engineering COUPLED PROBLEMS 15 B. Schrefler, E. Oñate an M. Paparakakis(Es) COUPLING REQUIREMENTS FOR WELL POSED AND
VI International Conference on Computational Methos for Couple Problems in Science an Engineering COUPLED PROBLEMS 15 B. Schrefler, E. Oñate an M. Paparakakis(Es) COUPLING REQUIREMENTS FOR WELL POSED AND
EVOLUTION OF PARTICLE SIZE DISTRIBUTION IN AIR IN THE RAINFALL PROCESS VIA THE MOMENT METHOD
 137 THERMAL SCIENCE, Year 1, Vol. 16, No. 5, pp. 137-1376 EVOLUTION OF PARTICLE SIZE DISTRIBUTION IN AIR IN THE RAINFALL PROCESS VIA THE MOMENT METHOD by Fu-Jun GAN a an Jian-Zhong LIN a,b * a Department
137 THERMAL SCIENCE, Year 1, Vol. 16, No. 5, pp. 137-1376 EVOLUTION OF PARTICLE SIZE DISTRIBUTION IN AIR IN THE RAINFALL PROCESS VIA THE MOMENT METHOD by Fu-Jun GAN a an Jian-Zhong LIN a,b * a Department
Sources and Sinks of Available Potential Energy in a Moist Atmosphere. Olivier Pauluis 1. Courant Institute of Mathematical Sciences
 Sources an Sinks of Available Potential Energy in a Moist Atmosphere Olivier Pauluis 1 Courant Institute of Mathematical Sciences New York University Submitte to the Journal of the Atmospheric Sciences
Sources an Sinks of Available Potential Energy in a Moist Atmosphere Olivier Pauluis 1 Courant Institute of Mathematical Sciences New York University Submitte to the Journal of the Atmospheric Sciences
Sturm-Liouville Theory
 LECTURE 5 Sturm-Liouville Theory In the three preceing lectures I emonstrate the utility of Fourier series in solving PDE/BVPs. As we ll now see, Fourier series are just the tip of the iceberg of the theory
LECTURE 5 Sturm-Liouville Theory In the three preceing lectures I emonstrate the utility of Fourier series in solving PDE/BVPs. As we ll now see, Fourier series are just the tip of the iceberg of the theory
u t v t v t c a u t b a v t u t v t b a
 Nonlinear Dynamical Systems In orer to iscuss nonlinear ynamical systems, we must first consier linear ynamical systems. Linear ynamical systems are just systems of linear equations like we have been stuying
Nonlinear Dynamical Systems In orer to iscuss nonlinear ynamical systems, we must first consier linear ynamical systems. Linear ynamical systems are just systems of linear equations like we have been stuying
Lecture 2 Lagrangian formulation of classical mechanics Mechanics
 Lecture Lagrangian formulation of classical mechanics 70.00 Mechanics Principle of stationary action MATH-GA To specify a motion uniquely in classical mechanics, it suffices to give, at some time t 0,
Lecture Lagrangian formulation of classical mechanics 70.00 Mechanics Principle of stationary action MATH-GA To specify a motion uniquely in classical mechanics, it suffices to give, at some time t 0,
Generalization of the persistent random walk to dimensions greater than 1
 PHYSICAL REVIEW E VOLUME 58, NUMBER 6 DECEMBER 1998 Generalization of the persistent ranom walk to imensions greater than 1 Marián Boguñá, Josep M. Porrà, an Jaume Masoliver Departament e Física Fonamental,
PHYSICAL REVIEW E VOLUME 58, NUMBER 6 DECEMBER 1998 Generalization of the persistent ranom walk to imensions greater than 1 Marián Boguñá, Josep M. Porrà, an Jaume Masoliver Departament e Física Fonamental,
05 The Continuum Limit and the Wave Equation
 Utah State University DigitalCommons@USU Founations of Wave Phenomena Physics, Department of 1-1-2004 05 The Continuum Limit an the Wave Equation Charles G. Torre Department of Physics, Utah State University,
Utah State University DigitalCommons@USU Founations of Wave Phenomena Physics, Department of 1-1-2004 05 The Continuum Limit an the Wave Equation Charles G. Torre Department of Physics, Utah State University,
18 EVEN MORE CALCULUS
 8 EVEN MORE CALCULUS Chapter 8 Even More Calculus Objectives After stuing this chapter you shoul be able to ifferentiate an integrate basic trigonometric functions; unerstan how to calculate rates of change;
8 EVEN MORE CALCULUS Chapter 8 Even More Calculus Objectives After stuing this chapter you shoul be able to ifferentiate an integrate basic trigonometric functions; unerstan how to calculate rates of change;
Diophantine Approximations: Examining the Farey Process and its Method on Producing Best Approximations
 Diophantine Approximations: Examining the Farey Process an its Metho on Proucing Best Approximations Kelly Bowen Introuction When a person hears the phrase irrational number, one oes not think of anything
Diophantine Approximations: Examining the Farey Process an its Metho on Proucing Best Approximations Kelly Bowen Introuction When a person hears the phrase irrational number, one oes not think of anything
Math 1B, lecture 8: Integration by parts
 Math B, lecture 8: Integration by parts Nathan Pflueger 23 September 2 Introuction Integration by parts, similarly to integration by substitution, reverses a well-known technique of ifferentiation an explores
Math B, lecture 8: Integration by parts Nathan Pflueger 23 September 2 Introuction Integration by parts, similarly to integration by substitution, reverses a well-known technique of ifferentiation an explores
6. Friction and viscosity in gasses
 IR2 6. Friction an viscosity in gasses 6.1 Introuction Similar to fluis, also for laminar flowing gases Newtons s friction law hols true (see experiment IR1). Using Newton s law the viscosity of air uner
IR2 6. Friction an viscosity in gasses 6.1 Introuction Similar to fluis, also for laminar flowing gases Newtons s friction law hols true (see experiment IR1). Using Newton s law the viscosity of air uner
Analytic Scaling Formulas for Crossed Laser Acceleration in Vacuum
 October 6, 4 ARDB Note Analytic Scaling Formulas for Crosse Laser Acceleration in Vacuum Robert J. Noble Stanfor Linear Accelerator Center, Stanfor University 575 San Hill Roa, Menlo Park, California 945
October 6, 4 ARDB Note Analytic Scaling Formulas for Crosse Laser Acceleration in Vacuum Robert J. Noble Stanfor Linear Accelerator Center, Stanfor University 575 San Hill Roa, Menlo Park, California 945
θ x = f ( x,t) could be written as
 9. Higher orer PDEs as systems of first-orer PDEs. Hyperbolic systems. For PDEs, as for ODEs, we may reuce the orer by efining new epenent variables. For example, in the case of the wave equation, (1)
9. Higher orer PDEs as systems of first-orer PDEs. Hyperbolic systems. For PDEs, as for ODEs, we may reuce the orer by efining new epenent variables. For example, in the case of the wave equation, (1)
Differentiation ( , 9.5)
 Chapter 2 Differentiation (8.1 8.3, 9.5) 2.1 Rate of Change (8.2.1 5) Recall that the equation of a straight line can be written as y = mx + c, where m is the slope or graient of the line, an c is the
Chapter 2 Differentiation (8.1 8.3, 9.5) 2.1 Rate of Change (8.2.1 5) Recall that the equation of a straight line can be written as y = mx + c, where m is the slope or graient of the line, an c is the
Unit 5: Chemical Kinetics and Equilibrium UNIT 5: CHEMICAL KINETICS AND EQUILIBRIUM
 UNIT 5: CHEMICAL KINETICS AND EQUILIBRIUM Chapter 14: Chemical Kinetics 14.4 & 14.6: Activation Energy, Temperature Depenence on Reaction Rates & Catalysis Reaction Rates: - the spee of which the concentration
UNIT 5: CHEMICAL KINETICS AND EQUILIBRIUM Chapter 14: Chemical Kinetics 14.4 & 14.6: Activation Energy, Temperature Depenence on Reaction Rates & Catalysis Reaction Rates: - the spee of which the concentration
Planar sheath and presheath
 5/11/1 Flui-Poisson System Planar sheath an presheath 1 Planar sheath an presheath A plasma between plane parallel walls evelops a positive potential which equalizes the rate of loss of electrons an ions.
5/11/1 Flui-Poisson System Planar sheath an presheath 1 Planar sheath an presheath A plasma between plane parallel walls evelops a positive potential which equalizes the rate of loss of electrons an ions.
LINEAR DIFFERENTIAL EQUATIONS OF ORDER 1. where a(x) and b(x) are functions. Observe that this class of equations includes equations of the form
 LINEAR DIFFERENTIAL EQUATIONS OF ORDER 1 We consier ifferential equations of the form y + a()y = b(), (1) y( 0 ) = y 0, where a() an b() are functions. Observe that this class of equations inclues equations
LINEAR DIFFERENTIAL EQUATIONS OF ORDER 1 We consier ifferential equations of the form y + a()y = b(), (1) y( 0 ) = y 0, where a() an b() are functions. Observe that this class of equations inclues equations
The effect of nonvertical shear on turbulence in a stably stratified medium
 The effect of nonvertical shear on turbulence in a stably stratifie meium Frank G. Jacobitz an Sutanu Sarkar Citation: Physics of Fluis (1994-present) 10, 1158 (1998); oi: 10.1063/1.869640 View online:
The effect of nonvertical shear on turbulence in a stably stratifie meium Frank G. Jacobitz an Sutanu Sarkar Citation: Physics of Fluis (1994-present) 10, 1158 (1998); oi: 10.1063/1.869640 View online:
A Course in Machine Learning
 A Course in Machine Learning Hal Daumé III 12 EFFICIENT LEARNING So far, our focus has been on moels of learning an basic algorithms for those moels. We have not place much emphasis on how to learn quickly.
A Course in Machine Learning Hal Daumé III 12 EFFICIENT LEARNING So far, our focus has been on moels of learning an basic algorithms for those moels. We have not place much emphasis on how to learn quickly.
Unit #6 - Families of Functions, Taylor Polynomials, l Hopital s Rule
 Unit # - Families of Functions, Taylor Polynomials, l Hopital s Rule Some problems an solutions selecte or aapte from Hughes-Hallett Calculus. Critical Points. Consier the function f) = 54 +. b) a) Fin
Unit # - Families of Functions, Taylor Polynomials, l Hopital s Rule Some problems an solutions selecte or aapte from Hughes-Hallett Calculus. Critical Points. Consier the function f) = 54 +. b) a) Fin
6 Wave equation in spherical polar coordinates
 6 Wave equation in spherical polar coorinates We now look at solving problems involving the Laplacian in spherical polar coorinates. The angular epenence of the solutions will be escribe by spherical harmonics.
6 Wave equation in spherical polar coorinates We now look at solving problems involving the Laplacian in spherical polar coorinates. The angular epenence of the solutions will be escribe by spherical harmonics.
The total derivative. Chapter Lagrangian and Eulerian approaches
 Chapter 5 The total erivative 51 Lagrangian an Eulerian approaches The representation of a flui through scalar or vector fiels means that each physical quantity uner consieration is escribe as a function
Chapter 5 The total erivative 51 Lagrangian an Eulerian approaches The representation of a flui through scalar or vector fiels means that each physical quantity uner consieration is escribe as a function
Laplace s Equation in Cylindrical Coordinates and Bessel s Equation (II)
 Laplace s Equation in Cylinrical Coorinates an Bessel s Equation (II Qualitative properties of Bessel functions of first an secon kin In the last lecture we foun the expression for the general solution
Laplace s Equation in Cylinrical Coorinates an Bessel s Equation (II Qualitative properties of Bessel functions of first an secon kin In the last lecture we foun the expression for the general solution
d dx But have you ever seen a derivation of these results? We ll prove the first result below. cos h 1
 Lecture 5 Some ifferentiation rules Trigonometric functions (Relevant section from Stewart, Seventh Eition: Section 3.3) You all know that sin = cos cos = sin. () But have you ever seen a erivation of
Lecture 5 Some ifferentiation rules Trigonometric functions (Relevant section from Stewart, Seventh Eition: Section 3.3) You all know that sin = cos cos = sin. () But have you ever seen a erivation of
Hyperbolic Moment Equations Using Quadrature-Based Projection Methods
 Hyperbolic Moment Equations Using Quarature-Base Projection Methos J. Koellermeier an M. Torrilhon Department of Mathematics, RWTH Aachen University, Aachen, Germany Abstract. Kinetic equations like the
Hyperbolic Moment Equations Using Quarature-Base Projection Methos J. Koellermeier an M. Torrilhon Department of Mathematics, RWTH Aachen University, Aachen, Germany Abstract. Kinetic equations like the
Applications of the Wronskian to ordinary linear differential equations
 Physics 116C Fall 2011 Applications of the Wronskian to orinary linear ifferential equations Consier a of n continuous functions y i (x) [i = 1,2,3,...,n], each of which is ifferentiable at least n times.
Physics 116C Fall 2011 Applications of the Wronskian to orinary linear ifferential equations Consier a of n continuous functions y i (x) [i = 1,2,3,...,n], each of which is ifferentiable at least n times.
Quantum Mechanics in Three Dimensions
 Physics 342 Lecture 20 Quantum Mechanics in Three Dimensions Lecture 20 Physics 342 Quantum Mechanics I Monay, March 24th, 2008 We begin our spherical solutions with the simplest possible case zero potential.
Physics 342 Lecture 20 Quantum Mechanics in Three Dimensions Lecture 20 Physics 342 Quantum Mechanics I Monay, March 24th, 2008 We begin our spherical solutions with the simplest possible case zero potential.
Parameter estimation: A new approach to weighting a priori information
 Parameter estimation: A new approach to weighting a priori information J.L. Mea Department of Mathematics, Boise State University, Boise, ID 83725-555 E-mail: jmea@boisestate.eu Abstract. We propose a
Parameter estimation: A new approach to weighting a priori information J.L. Mea Department of Mathematics, Boise State University, Boise, ID 83725-555 E-mail: jmea@boisestate.eu Abstract. We propose a
arxiv: v1 [physics.flu-dyn] 8 May 2014
![arxiv: v1 [physics.flu-dyn] 8 May 2014 arxiv: v1 [physics.flu-dyn] 8 May 2014](/thumbs/87/97199974.jpg) Energetics of a flui uner the Boussinesq approximation arxiv:1405.1921v1 [physics.flu-yn] 8 May 2014 Kiyoshi Maruyama Department of Earth an Ocean Sciences, National Defense Acaemy, Yokosuka, Kanagawa
Energetics of a flui uner the Boussinesq approximation arxiv:1405.1921v1 [physics.flu-yn] 8 May 2014 Kiyoshi Maruyama Department of Earth an Ocean Sciences, National Defense Acaemy, Yokosuka, Kanagawa
Calculus Class Notes for the Combined Calculus and Physics Course Semester I
 Calculus Class Notes for the Combine Calculus an Physics Course Semester I Kelly Black December 14, 2001 Support provie by the National Science Founation - NSF-DUE-9752485 1 Section 0 2 Contents 1 Average
Calculus Class Notes for the Combine Calculus an Physics Course Semester I Kelly Black December 14, 2001 Support provie by the National Science Founation - NSF-DUE-9752485 1 Section 0 2 Contents 1 Average
The Exact Form and General Integrating Factors
 7 The Exact Form an General Integrating Factors In the previous chapters, we ve seen how separable an linear ifferential equations can be solve using methos for converting them to forms that can be easily
7 The Exact Form an General Integrating Factors In the previous chapters, we ve seen how separable an linear ifferential equations can be solve using methos for converting them to forms that can be easily
Schrödinger s equation.
 Physics 342 Lecture 5 Schröinger s Equation Lecture 5 Physics 342 Quantum Mechanics I Wenesay, February 3r, 2010 Toay we iscuss Schröinger s equation an show that it supports the basic interpretation of
Physics 342 Lecture 5 Schröinger s Equation Lecture 5 Physics 342 Quantum Mechanics I Wenesay, February 3r, 2010 Toay we iscuss Schröinger s equation an show that it supports the basic interpretation of
The proper definition of the added mass for the water entry problem
 The proper efinition of the ae mass for the water entry problem Leonaro Casetta lecasetta@ig.com.br Celso P. Pesce ceppesce@usp.br LIE&MO lui-structure Interaction an Offshore Mechanics Laboratory Mechanical
The proper efinition of the ae mass for the water entry problem Leonaro Casetta lecasetta@ig.com.br Celso P. Pesce ceppesce@usp.br LIE&MO lui-structure Interaction an Offshore Mechanics Laboratory Mechanical
Lagrangian and Hamiltonian Mechanics
 Lagrangian an Hamiltonian Mechanics.G. Simpson, Ph.. epartment of Physical Sciences an Engineering Prince George s Community College ecember 5, 007 Introuction In this course we have been stuying classical
Lagrangian an Hamiltonian Mechanics.G. Simpson, Ph.. epartment of Physical Sciences an Engineering Prince George s Community College ecember 5, 007 Introuction In this course we have been stuying classical
and from it produce the action integral whose variation we set to zero:
 Lagrange Multipliers Monay, 6 September 01 Sometimes it is convenient to use reunant coorinates, an to effect the variation of the action consistent with the constraints via the metho of Lagrange unetermine
Lagrange Multipliers Monay, 6 September 01 Sometimes it is convenient to use reunant coorinates, an to effect the variation of the action consistent with the constraints via the metho of Lagrange unetermine
NON-ADIABATIC COMBUSTION WAVES FOR GENERAL LEWIS NUMBERS: WAVE SPEED AND EXTINCTION CONDITIONS
 ANZIAM J. 46(2004), 1 16 NON-ADIABATIC COMBUSTION WAVES FOR GENERAL LEWIS NUMBERS: WAVE SPEED AND EXTINCTION CONDITIONS A. C. MCINTOSH 1,R.O.WEBER 2 ang.n.mercer 2 (Receive 14 August, 2002) Abstract This
ANZIAM J. 46(2004), 1 16 NON-ADIABATIC COMBUSTION WAVES FOR GENERAL LEWIS NUMBERS: WAVE SPEED AND EXTINCTION CONDITIONS A. C. MCINTOSH 1,R.O.WEBER 2 ang.n.mercer 2 (Receive 14 August, 2002) Abstract This
Optimization of Geometries by Energy Minimization
 Optimization of Geometries by Energy Minimization by Tracy P. Hamilton Department of Chemistry University of Alabama at Birmingham Birmingham, AL 3594-140 hamilton@uab.eu Copyright Tracy P. Hamilton, 1997.
Optimization of Geometries by Energy Minimization by Tracy P. Hamilton Department of Chemistry University of Alabama at Birmingham Birmingham, AL 3594-140 hamilton@uab.eu Copyright Tracy P. Hamilton, 1997.
A new proof of the sharpness of the phase transition for Bernoulli percolation on Z d
 A new proof of the sharpness of the phase transition for Bernoulli percolation on Z Hugo Duminil-Copin an Vincent Tassion October 8, 205 Abstract We provie a new proof of the sharpness of the phase transition
A new proof of the sharpness of the phase transition for Bernoulli percolation on Z Hugo Duminil-Copin an Vincent Tassion October 8, 205 Abstract We provie a new proof of the sharpness of the phase transition
Time-of-Arrival Estimation in Non-Line-Of-Sight Environments
 2 Conference on Information Sciences an Systems, The Johns Hopkins University, March 2, 2 Time-of-Arrival Estimation in Non-Line-Of-Sight Environments Sinan Gezici, Hisashi Kobayashi an H. Vincent Poor
2 Conference on Information Sciences an Systems, The Johns Hopkins University, March 2, 2 Time-of-Arrival Estimation in Non-Line-Of-Sight Environments Sinan Gezici, Hisashi Kobayashi an H. Vincent Poor
Calculus in the AP Physics C Course The Derivative
 Limits an Derivatives Calculus in the AP Physics C Course The Derivative In physics, the ieas of the rate change of a quantity (along with the slope of a tangent line) an the area uner a curve are essential.
Limits an Derivatives Calculus in the AP Physics C Course The Derivative In physics, the ieas of the rate change of a quantity (along with the slope of a tangent line) an the area uner a curve are essential.
G j dq i + G j. q i. = a jt. and
 Lagrange Multipliers Wenesay, 8 September 011 Sometimes it is convenient to use reunant coorinates, an to effect the variation of the action consistent with the constraints via the metho of Lagrange unetermine
Lagrange Multipliers Wenesay, 8 September 011 Sometimes it is convenient to use reunant coorinates, an to effect the variation of the action consistent with the constraints via the metho of Lagrange unetermine
Entanglement is not very useful for estimating multiple phases
 PHYSICAL REVIEW A 70, 032310 (2004) Entanglement is not very useful for estimating multiple phases Manuel A. Ballester* Department of Mathematics, University of Utrecht, Box 80010, 3508 TA Utrecht, The
PHYSICAL REVIEW A 70, 032310 (2004) Entanglement is not very useful for estimating multiple phases Manuel A. Ballester* Department of Mathematics, University of Utrecht, Box 80010, 3508 TA Utrecht, The
Computing Exact Confidence Coefficients of Simultaneous Confidence Intervals for Multinomial Proportions and their Functions
 Working Paper 2013:5 Department of Statistics Computing Exact Confience Coefficients of Simultaneous Confience Intervals for Multinomial Proportions an their Functions Shaobo Jin Working Paper 2013:5
Working Paper 2013:5 Department of Statistics Computing Exact Confience Coefficients of Simultaneous Confience Intervals for Multinomial Proportions an their Functions Shaobo Jin Working Paper 2013:5
RFSS: Lecture 4 Alpha Decay
 RFSS: Lecture 4 Alpha Decay Reaings Nuclear an Raiochemistry: Chapter 3 Moern Nuclear Chemistry: Chapter 7 Energetics of Alpha Decay Geiger Nuttall base theory Theory of Alpha Decay Hinrance Factors Different
RFSS: Lecture 4 Alpha Decay Reaings Nuclear an Raiochemistry: Chapter 3 Moern Nuclear Chemistry: Chapter 7 Energetics of Alpha Decay Geiger Nuttall base theory Theory of Alpha Decay Hinrance Factors Different
Prep 1. Oregon State University PH 213 Spring Term Suggested finish date: Monday, April 9
 Oregon State University PH 213 Spring Term 2018 Prep 1 Suggeste finish ate: Monay, April 9 The formats (type, length, scope) of these Prep problems have been purposely create to closely parallel those
Oregon State University PH 213 Spring Term 2018 Prep 1 Suggeste finish ate: Monay, April 9 The formats (type, length, scope) of these Prep problems have been purposely create to closely parallel those
Quantum optics of a Bose-Einstein condensate coupled to a quantized light field
 PHYSICAL REVIEW A VOLUME 60, NUMBER 2 AUGUST 1999 Quantum optics of a Bose-Einstein conensate couple to a quantize light fiel M. G. Moore, O. Zobay, an P. Meystre Optical Sciences Center an Department
PHYSICAL REVIEW A VOLUME 60, NUMBER 2 AUGUST 1999 Quantum optics of a Bose-Einstein conensate couple to a quantize light fiel M. G. Moore, O. Zobay, an P. Meystre Optical Sciences Center an Department
Outline. Calculus for the Life Sciences II. Introduction. Tides Introduction. Lecture Notes Differentiation of Trigonometric Functions
 Calculus for the Life Sciences II c Functions Joseph M. Mahaffy, mahaffy@math.ssu.eu Department of Mathematics an Statistics Dynamical Systems Group Computational Sciences Research Center San Diego State
Calculus for the Life Sciences II c Functions Joseph M. Mahaffy, mahaffy@math.ssu.eu Department of Mathematics an Statistics Dynamical Systems Group Computational Sciences Research Center San Diego State
Construction of the Electronic Radial Wave Functions and Probability Distributions of Hydrogen-like Systems
 Construction of the Electronic Raial Wave Functions an Probability Distributions of Hyrogen-like Systems Thomas S. Kuntzleman, Department of Chemistry Spring Arbor University, Spring Arbor MI 498 tkuntzle@arbor.eu
Construction of the Electronic Raial Wave Functions an Probability Distributions of Hyrogen-like Systems Thomas S. Kuntzleman, Department of Chemistry Spring Arbor University, Spring Arbor MI 498 tkuntzle@arbor.eu
Mathematical Review Problems
 Fall 6 Louis Scuiero Mathematical Review Problems I. Polynomial Equations an Graphs (Barrante--Chap. ). First egree equation an graph y f() x mx b where m is the slope of the line an b is the line's intercept
Fall 6 Louis Scuiero Mathematical Review Problems I. Polynomial Equations an Graphs (Barrante--Chap. ). First egree equation an graph y f() x mx b where m is the slope of the line an b is the line's intercept
JUST THE MATHS UNIT NUMBER DIFFERENTIATION 2 (Rates of change) A.J.Hobson
 JUST THE MATHS UNIT NUMBER 10.2 DIFFERENTIATION 2 (Rates of change) by A.J.Hobson 10.2.1 Introuction 10.2.2 Average rates of change 10.2.3 Instantaneous rates of change 10.2.4 Derivatives 10.2.5 Exercises
JUST THE MATHS UNIT NUMBER 10.2 DIFFERENTIATION 2 (Rates of change) by A.J.Hobson 10.2.1 Introuction 10.2.2 Average rates of change 10.2.3 Instantaneous rates of change 10.2.4 Derivatives 10.2.5 Exercises
Experiment 2, Physics 2BL
 Experiment 2, Physics 2BL Deuction of Mass Distributions. Last Upate: 2009-05-03 Preparation Before this experiment, we recommen you review or familiarize yourself with the following: Chapters 4-6 in Taylor
Experiment 2, Physics 2BL Deuction of Mass Distributions. Last Upate: 2009-05-03 Preparation Before this experiment, we recommen you review or familiarize yourself with the following: Chapters 4-6 in Taylor
A Model of Electron-Positron Pair Formation
 Volume PROGRESS IN PHYSICS January, 8 A Moel of Electron-Positron Pair Formation Bo Lehnert Alfvén Laboratory, Royal Institute of Technology, S-44 Stockholm, Sween E-mail: Bo.Lehnert@ee.kth.se The elementary
Volume PROGRESS IN PHYSICS January, 8 A Moel of Electron-Positron Pair Formation Bo Lehnert Alfvén Laboratory, Royal Institute of Technology, S-44 Stockholm, Sween E-mail: Bo.Lehnert@ee.kth.se The elementary
Prof. Dr. Ibraheem Nasser electric_charhe 9/22/2017 ELECTRIC CHARGE
 ELECTRIC CHARGE Introuction: Orinary matter consists of atoms. Each atom consists of a nucleus, consisting of protons an neutrons, surroune by a number of electrons. In electricity, the electric charge
ELECTRIC CHARGE Introuction: Orinary matter consists of atoms. Each atom consists of a nucleus, consisting of protons an neutrons, surroune by a number of electrons. In electricity, the electric charge
Integration Review. May 11, 2013
 Integration Review May 11, 2013 Goals: Review the funamental theorem of calculus. Review u-substitution. Review integration by parts. Do lots of integration eamples. 1 Funamental Theorem of Calculus In
Integration Review May 11, 2013 Goals: Review the funamental theorem of calculus. Review u-substitution. Review integration by parts. Do lots of integration eamples. 1 Funamental Theorem of Calculus In
Lecture Introduction. 2 Examples of Measure Concentration. 3 The Johnson-Lindenstrauss Lemma. CS-621 Theory Gems November 28, 2012
 CS-6 Theory Gems November 8, 0 Lecture Lecturer: Alesaner Mąry Scribes: Alhussein Fawzi, Dorina Thanou Introuction Toay, we will briefly iscuss an important technique in probability theory measure concentration
CS-6 Theory Gems November 8, 0 Lecture Lecturer: Alesaner Mąry Scribes: Alhussein Fawzi, Dorina Thanou Introuction Toay, we will briefly iscuss an important technique in probability theory measure concentration
Parametrization of non-convective condensation processes May 1987
 Parametrization of non-convective conensation processes May 987 By M. Tietke European Centre for Meium-Range Weather Forecasts Table of contents. Thermoynamics of moist air 2. Clou physical processes 2.
Parametrization of non-convective conensation processes May 987 By M. Tietke European Centre for Meium-Range Weather Forecasts Table of contents. Thermoynamics of moist air 2. Clou physical processes 2.
Equilibrium in Queues Under Unknown Service Times and Service Value
 University of Pennsylvania ScholarlyCommons Finance Papers Wharton Faculty Research 1-2014 Equilibrium in Queues Uner Unknown Service Times an Service Value Laurens Debo Senthil K. Veeraraghavan University
University of Pennsylvania ScholarlyCommons Finance Papers Wharton Faculty Research 1-2014 Equilibrium in Queues Uner Unknown Service Times an Service Value Laurens Debo Senthil K. Veeraraghavan University
Fundamental Laws of Motion for Particles, Material Volumes, and Control Volumes
 Funamental Laws of Motion for Particles, Material Volumes, an Control Volumes Ain A. Sonin Department of Mechanical Engineering Massachusetts Institute of Technology Cambrige, MA 02139, USA August 2001
Funamental Laws of Motion for Particles, Material Volumes, an Control Volumes Ain A. Sonin Department of Mechanical Engineering Massachusetts Institute of Technology Cambrige, MA 02139, USA August 2001
Section 2.7 Derivatives of powers of functions
 Section 2.7 Derivatives of powers of functions (3/19/08) Overview: In this section we iscuss the Chain Rule formula for the erivatives of composite functions that are forme by taking powers of other functions.
Section 2.7 Derivatives of powers of functions (3/19/08) Overview: In this section we iscuss the Chain Rule formula for the erivatives of composite functions that are forme by taking powers of other functions.
