q = F If we integrate this equation over all the mass in a star, we have q dm = F (M) F (0)
|
|
|
- Shanna Long
- 6 years ago
- Views:
Transcription
1 Astronomy 112: The Physics of Stars Class 4 Notes: Energy an Chemical Balance in Stars In the last class we introuce the iea of hyrostatic balance in stars, an showe that we coul use this concept to erive crue limits on their internal properties even without constructing a etaile moel. In this class we will apply the same sort of analysis to the energy an chemical balance in stars in effect examining the principles governing the energy an chemical content of stars rather than their mechanical equilibrium. We will see that this leas to similar non-obvious conclusions. It also sets us up to begin making etaile moels in the next few weeks. I. The Energy Equation A. Static Stars We ene last class by writing own the first law of thermoynamics for a given shell within a star. This enables us to make some interesting statements about the total energy content of a star. First consier the simplest case of a star in equilibrium, so that each shell s volume an specific internal energy are constant in time. In this case the left-han sie of the first law of thermoynamics is exactly zero because nothing is changing with time, an we have q = F m. If we integrate this equation over all the mass in a star, we have q m = F m m q m = F (M) F () Consier the physical meaning of this equation. The left-han sie is the total rate of nuclear energy generation in the star, summing over all the star s mass. We call this quantity the nuclear luminosity L nuc a luminosity because it has units of energy per time; i.e., it is the total rate at which nuclear reactions in the star release energy. When we integrate the right-han sie, we win up with the ifference between the flux passing through the last mass shell, F (M), an the flux entering the star at m =, F (). The latter is obviously zero, unless there is a magical energy source at the center of the star. The former, F (M) is just the energy per unit time leaving the stellar surface. Thus, F (M) must be the star s total luminous output, which we see as light, an enote L.
2 Thus the equation we have erive simply states L nuc = L, i.e. for a state in equilibrium, the total energy leaving the stellar surface must be equal to the total rate at which nuclear reactions within the star release energy. This isn t exactly a shocking conclusion, but the machinery we use to erive it will be prove extremely useful when we consier stars that are not exactly in equilibrium. B. Time-Variable Stars The calculation we just performe can be generalize to the case of a star that is not exactly in equilibrium, so that the time erivatives are not zero. If we retain these terms an integrate over mass again, we have u M t m + P t ( ) 1 M m = q m F (M) + F () = L nuc L. ρ For the first term on the left-han sie, since m oes not epen on t, we can interchange the integral an the time erivative. Thus, we have u t m = u m = t t U, where U is the total internal energy of the star. For the secon term, it is convenient to rewrite the time erivative of 1/ρ in a slightly ifferent form: ( ) 1 = ( ) V = ( ) V, t ρ t m m t where we have use the fact that m oes not epen on t to interchange the orer of the erivatives. Here V is the volume of the material insie mass shell m. If this shell is at raius r, then V = (4/3)πr 3, an V t = 4πr2 r t. In other wors, the rate at which the volume occupie by a given mass of gas changes is equal to the surface area of its outer bounary (4πr 2 ) multiplie by the rate at which it expans or contracts (r/t). Putting this into the integral, we have P ( ) 1 M m = P ( 4πr 2 r ) m. t ρ m t
3 We can evaluate this integral by parts. Doing so gives P ( ) [ 1 m = 4πr 2 P r ] M 4πr 2 r P t ρ t t m m. For the first term, note that at m =, r/t =. This is because the innermost shell must stay at the center, unless a vacuum appears aroun the origin. Similarly, P = at m = M, because the pressure rops to zero at the ege of the star. (Strictly speaking it is not exactly zero, but it is negligibly small.) Thus, the term in square brackets must be zero. For the secon term, we can evaluate using the equation of motion that we erive in the last class. To remin you, we erive the equation If we re-arrange this we get r = Gm r 2 1 P ρ r. P r = GMρ ρ r. r 2 Converting to Lagrangian coorinates, we have P m = 1 P 4πr 2 ρ r = Gm 4πr r 4 4πr 2 If we substitute this into our integral, we have P ( ) 1 M Gm m = t ρ r ṙ m + 2 ṙ r m The meanings of the terms are a bit clearer if we rewrite them a bit. In particular, we can write Gm r ṙ = ( ) Gm 2 t r an so that we have P t ( ) 1 m = ρ t ṙ r = 1 2 t (ṙ2 ), Gm r m t ṙ 2 m = Ω + T. As earlier, we have use the fact that mass oes not epen on time to interchange the integral with the time erivative. The first term, which I have written Ω, is clearly just minus the time erivative of the gravitational potential energy; Gm/r is the gravitational potential experience by the shell at mass m, so the integral of Gm m/r is just the total
4 gravitational potential energy over the entire star. Similarly, the term I have labelle T is just the time erivative of the total kinetic energy of the clou; ṙ 2 /2 is the kinetic energy per unit mass of a shell, so the integral of (1/2)ṙ 2 m is just the total kinetic energy of the star. Putting it all together, we arrive at the total energy equation for the star: U + Ω + T = L nuc L. This just represents the total energy equation for the star, an it is fairly intuitively obvious. It just says that the time rate of change of internal energy plus gravitational potential energy plus kinetic energy is equal to the rate at which nuclear reactions a energy minus the rate at which energy is raiate away from the stellar surface. We can get something slightly more interesting if we consier a star that is expaning or contracting extremely slowly, so that its very close to hyrostatic balance. In this case we can make two simplifications: (1) we can rop the term T, because the star s kinetic energy is tiny compare to its internal energy or gravitational potential energy; (2) we can use the virial theorem for hyrostatic objects, which says that U = Ω/2. In this case the energy equation reas 1 2 Ω = L nuc L. This equation is eciely non-obvious. It tells us how quickly the gravitational potential energy of the star changes in response to energy generation by nuclear reactions an energy loss by raiation. C. The Kelvin-Helmholtz Timescale It is useful to consier an orer-of-magnitue version of the energy equation, because it tells us something important about the nature of stars. Consier how long will it take the gravitational potential energy (an thus the stellar raius) to change by a significant amount in a star without any nuclear energy generation (i.e. where L nuc = ). The gravitational potential energy is Ω = α GM 2 R, where α is a number of orer unity that epens on the ensity istribution within the star, an R is the star s raius. If we efine t as the time require to alter the gravitational potential energy significantly, then at the orer-of-magnitue level the equation we have written reas 1 ( ) GM 2 L t R t GM 2 RL.
5 We efine the quantity t KH = GM 2 /(RL) as the Kelvin-Helmholtz timescale, name after the 19th century physicists Kelvin (of the Kelvin temperature scale) an Helmholtz, who first pointe out its importance. The meaning of t KH is that it is the time for which a star coul be powere by gravity alone without its raius changing very much. Similarly, if we have a star that is not in energy balance for some reason, so that L nuc L, then t KH tells us about the time that will be require for the star to reach energy equilibrium. If we plug in numerical values for the Sun, we fin t KH = GM /(R 2 L ) = 3 Myr. This number is the answer to the Star Trek problem of what woul happen if you somehow shut off all nuclear reactions insie a star. The answer is: absolutely nothing for about 3 Myr. You coul turn off all the nuclear reactions in the Sun, an unless you were observing neutrinos (which get out immeiately), you wouln t notice anything change for tens of millions of years. In fact, before the iscovery of nuclear energy, it was believe that gravity was the main process causing the Sun to shine. This playe an important role in the history of science, because, if gravity really i power the Sun, it woul imply that the Sun s properties coul only have been constant for 3 Myr. This woul be a natural limit for the age of the Earth, or at least for the time for which life as we know it coul have existe on Earth. This was an important argument against the Darwinian theory of evolution, which require billions of years to explain the evelopment of life. Of course, as we ll iscuss in a few minutes, in this case the biologists were right an the astronomers were wrong. In reality L nuc is very close to L, so the real timescale for the Sun s evolution is vastly longer than 3 Myr. II. Nuclear Energy an the Nuclear Timescale There is one more important timescale for star s, which comes from consiering how long L nuc L can be maintaine. This epens a bit on nuclear chemistry, which will be our topic in a few weeks. For now, we ll simply take on faith that the main nuclear reaction that occurs in the Sun is burning hyrogen into helium. You can figure out how much energy this yiels by comparing the masses of hyrogen an helium. The starting point of the reaction is 4 hyrogen nuclei an the final point is 1 helium nucleus. The mass of 1 hyrogen nucleus is g, while the mass of a helium nucleus is The ifference in mass is m = 4m H m He = g. If we phrase this as the change in mass per hyrogen atom we starte with, this is ɛ = m 4m H =.66. In other wors, this reaction converts.66% of the mass of each proton into energy. Einstein s relativity then tell us that the excess energy release per hyrogen atom by this reaction is E = ɛm H c 2 = erg = 6.2 MeV.
6 We can use this to estimate how long the nuclear reaction that burns hyrogen into helium can keep a star going. The star raiates energy at a rate L, an thus the rate at which hyrogen atoms must be burne is L/ E = L/(ɛm H c 2 ). To estimate how long this can keep going, we simply ivie the total number of hyrogen atoms in the star, roughly M/m H, but the rate at which they are burne. This efines the nuclear timescale t nuc = M/m H L/(ɛm H c 2 ) = ɛmc2 L (In making this estimate we have implicitly neglecte the fact that not all of the Sun s mass is hyrogen, but that s a fairly small correction.) Evaluating this for the Sun gives 1 Gyr, a staggeringly long time almost a factor of 1 larger than the age of the universe. In fact, we ll see later in the course that the true time for which nuclear reactions can ol up the Sun is about a factor of 1 smaller, because the Sun can t actually use all of its hyrogen as fuel. Nonetheless, this result emonstrates that nuclear energy is able to hol up the Sun for much, much longer than the Kelvin-Helmholtz timescale. III. The Hierarchy of Timescales an Evolutionary Moels A. General Iea The three timescales we ve compute this week tell us a great eal about the ingreients we nee to make a moel of stars. Putting them in orer, we have t nuc t KH t yn, an this is true not just for the Sun, but for all stars. This has the important implication that, on timescales comparable to t nuc, we can assume that stars are in nearly perfect mechanical an thermal equilibrium. This enables a great simplification in making moels of stars. Our approach to making stellar moels for the rest of the course will therefore procee through a series of steps: 1. Assume that the star is in perfect mechanical equilibrium (since t yn t KH t nuc ), an compute the resulting luminosity. 2. Assume that the star is in perfect energy equilibrium (since t KH t nuc ), an compute the reaction rate require to provie this luminosity. 3. Evolve the chemical makeup of the star using the erive reaction rates. For a star that is powere by hyrogen burning into helium, steps 2 an 3 are extraorinarily simple. If we want to keep track of a star s evolution, we just nee to keep track of the mass of hyrogen an the mass of helium within it. Let M be the total stellar mass, an let M H an M He be the mass of hyrogen an helium. If we neglect the mass of other elements (a reasonable first approximation) an any change in mass ue to raiation (which is tiny) an ue to stellar wins (which is small), then we can write M = M H + M He = constant. Steps 2 an 3 therefore
7 are entirely emboie by the equation Ṁ He = ṀH = L ɛc 2. This is a bit of an oversimplification, since in reality we nee to o this on a shellby-shell basis, an to worry about chemical mixing between the shells. Nonetheless, it conveys the basic iea. Of course the har part of this is in step 1: compute the luminosity of a star that is in mechanical equilibrium. This luminosity, it turns out, will epen on nothing but the star s mass which is what observations have alreay tol us, if we recall the mass luminosity relation. Deriving that mass-luminosity relation from first principles is going to consume the next four weeks of the course. Once we ve one that, however, what this timescale analysis tells us is that we will essentially have a full theory for stellar evolution. B. The Chemical Evolution Equations To make this more precise, we nee to write own the equations governing change in the composition of the star. To o this, we nee to introuce some notation. A star is mae of many ifferent elements the vast majority are hyrogen an helium, but there are others, an it turns out that they can play important roles, particularly in evolve stars. If we examine some volume of gas in a star, we can iscuss its ensity ρ, but we coul also count only the hyrogen atoms, only the helium atoms, etc., an compute the ensity for that species only. For convenience we number these species, so that we might write the ensity of hyrogen as ρ 1, the ensity of helium as ρ 2., etc. Depening on the level of sophistication of our moel, we might also istinguish ifferent isotopes of the same atom, so that we woul count orinary hyrogen an euterium (which has an extra neutron) separately. It is often convenient to work with quantities other than mass ensities, so we efine some alternatives. The mass fraction of species i is written X i = ρ i ρ. We often also want to count the number of atoms, instea of measuring their mass. To o this, we have to take into account the ifferences in their atomic weights. For example, helium atoms have four times the mass of hyrogen atoms, so if there are four hyrogen atoms for every helium atom, then they both have the same mass fraction. We write the atomic mass number for species i as an integer A i, which means that each atom of that species has an atomic mass of approximately A i m p, where m p = is the proton mass. Hyrogen has A = 1, so m H = m p, an we frequently write masses in terms of m H rather than m p. Given this efinition, it is clear that the number ensity of atoms is relate to their mass ensity by n i = ρ i, A i m H
8 an substituting this into the mass fraction X i immeiately gives X i = n i A i ρ m H. We can escribe any species in terms of its atomic mass A i an also its atomic number, Z i. The atomic number gives the number of protons, an thus the charge of the nucleus. For example for the stable isotopes of the most common elements we have Element Name Z A 1 H Hyrogen H Deuterium He Helium He Helium C Carbon C Carbon Some nuclear reactions also involve electrons an positrons. This have Z = ±1 an A = (to goo approximation we can generally neglect the mass of electrons compare to protons an neutrons). The mass fractions X i can be altere by nuclear reactions, which leave the total mass ensity fixe (to very goo approximation), but convert atoms of one species into atoms of another. We can write one species as I(Z i, A i ), another as J(Z j, A j ), an so forth. In this notation, any chemical reaction is I(Z i, A i ) + J(Z j, A j ) K(Z k, A k ) + L(Z l, A l ), where this can obviously be extene to more elements as neee if the reaction involves more species. Chemical reactions always have to conserve mass an charge, so we have two conservation laws: Z i + Z j = Z k + Z l A i + A j = A k + A l. If we want to know how the star s composition changes in time, we nee to know the rate at which reactions between two species occur. Computing these reactions rates is a problem we ll efer for now, but we can begin to think about them by noting that the reaction rate will always be proportional to the rate at which atoms of the two reactant species run into one another. To see what this implies, consier a given volume of space within which a chemical reaction is taking place, for example 2 H + 1 H 3 He, one of the steps in the energy-generating reaction chain in the Sun. What woul happen to the rate at which this reaction occurre in that volume if I were to
9 remove half the euterium ( 2 H)? The remaining euterium atoms woul still encounter hyrogen atoms just as often, since their number woul be unchange, so the reaction rate woul just be reuce by a factor of 2. Similarly, the same argument shows that if I were, for example, ouble the number of hyrogen atoms, the reaction rate woul increase by a factor of 2. Clearly the reaction rate must be proportional to the number of members of each reactant species in the volume. Expressing this mathematically, the reaction rate per unit volume must be proportional to n i n j, where n i an n j are the number ensity of the species i an j involve in the reaction. If there are more species involve, then we just multiply by n k, n l, etc. We call the constant of proportionality the reaction rate, an write it R ijk, meaning the rate at which reactions between particles of species i an j occur, leaing to species k. If the reaction involves two members of the same species, the same argument applies, except that we have to be careful not to ouble-count. Thus rather than having the reaction rate be proportional to n i n j, it is proportional to n i (n i 1)/2 n 2 i /2, where the factor of 1/2 is to hanle the ouble-counting problem. You can convince yourself that this is right. Suppose there are 4 people in a room: Alice, Bob, Cathy, an Davi (A, B, C, an D). How many istinct couples can we make? Counting them is pretty easy, since we just pick one person, then pick another ifferent person. If we pick Alice, there are 3 possible partners: Bob, Cathy, an Davi. Similarly, if we pick Bob, there are three possible partners: Alice, Cathy, an Davi. We can write this out as AB AC AD BA BC BD CA CB CD DA DB DC The table has 3 4 = 12 entries. It s pretty clear, however, that half of the entries in this table are uplicates: we have both BA an AB. Thus to count the number of istinct couples, we have n i = 4 an n i (n i 1)/2 = 6. The argument is the same for chemical reactions: if we were to make a list of possible collisions, there woul be n i (n i 1)/2, which for large values of n i is approximately n 2 i /2. Thus the reaction rate is n 2 i R iik /2. With this notation out of the way, we are now prepare to write own the equations of chemical evolution. Suppose that we have a number ensity n i of species i, an that these atoms are estroye by a reaction with species j, which leas to species k. Clearly the rate of change in the number ensity of species i is just given by minus the rate at which reactions occur: t n i = n i n j R ijk. In this case we on t ivie by 2 when the reaction is species i with itself because each reaction estroys two atoms of species i, an the factors of 2 cancel. In
10 general there are multiple possible reactions with many possible partners, an we have to sum over all the reactions that estroy members of species i. Thus t n i = j,k n i n j R ijk. We must also take into account that reactions can create members of species i. Suppose we have a reaction between species l an species k that creates members of species i. In this case the rate at which members of species i are create is t n i = n l n k R lki if l an k are istinct, or t n i = 1 2 n ln k R lki if l an k are the same. We can unify the notation for these two cases by writing t n i = n ln k 1 + δ lk R lki, where δ lk is simply efine to be 1 if l an k are the same, an otherwise. This is nothing more than a notational convenience. Again, we nee to sum over all possible reactions that can create species i: t n i = l,k n l n k 1 + δ lk R lki, Finally, combining the rates of creation an estruction, we have t n i = l,k n l n k 1 + δ lk R lki j,k n i n j R ijk. If we prefer, we can also write this in terms of the composition fraction by substituting: ( ) t X i = ρ A i X l X k R lki X i X j R ijk m H A l A k 1 + δ lk A i A j l,k j,k C. The Evolution Equations We are now in a position to write own the basic equations governing a star s evolution. We envision a spherical star of fixe mass, whose chemical composition at some initial time is known. To figure out how its structure changes in time, our first step is to assume mechanical equilibrium, i.e. hyrostatic balance. We re alreay written own the equation for this: P m = Gm 4πr 4.
11 This equation gives us a relationship between the position of each shell of mass, r, an the graient in the pressure of the gas P. The solution tells us how the shells of gas must arrange themselves to maintain mechanical equilibrium. The secon step is to require thermal equilibrium, which means that we balance the energy being generate in the star against the energy it is raiating. We ve alreay written own the integrate version of this as L = L nuc, but if we re going to moel the shells iniviually, we want to use the non-integrate version we wrote own earlier: F m = q, where F is the flux passing through a mass shell, an q is the heat generate within it by nuclear burning. The final step is to figure out how it is changing chemically, which is escribe by the equations we have just written out: ( ) t X i = ρ A i X l X k R lki X i X j R ijk m H A l A k 1 + δ lk A i A j for each species i. l,k Of course these equations are all couple. The rates of nuclear reactions in the final step etermine the rate of heat generation q in the secon one, or vice versa. Similarly, the ensities, which come from r(m), also affect the chemical evolution rates. Thus we have a set of couple non-linear ifferential equations to solve. Moreover, our set of equations is not yet complete. Most obviously, we haven t yet written own the reaction rates R ijk or the way that nuclear energy generation rate q epens on them. Also, we have not specifie yet how the pressure epens on ensity, temperature, or anything else. To solve for the evolution of a star, we will nee to fill in these gaps. j,k
Linear First-Order Equations
 5 Linear First-Orer Equations Linear first-orer ifferential equations make up another important class of ifferential equations that commonly arise in applications an are relatively easy to solve (in theory)
5 Linear First-Orer Equations Linear first-orer ifferential equations make up another important class of ifferential equations that commonly arise in applications an are relatively easy to solve (in theory)
MA 2232 Lecture 08 - Review of Log and Exponential Functions and Exponential Growth
 MA 2232 Lecture 08 - Review of Log an Exponential Functions an Exponential Growth Friay, February 2, 2018. Objectives: Review log an exponential functions, their erivative an integration formulas. Exponential
MA 2232 Lecture 08 - Review of Log an Exponential Functions an Exponential Growth Friay, February 2, 2018. Objectives: Review log an exponential functions, their erivative an integration formulas. Exponential
Assignment 1. g i (x 1,..., x n ) dx i = 0. i=1
 Assignment 1 Golstein 1.4 The equations of motion for the rolling isk are special cases of general linear ifferential equations of constraint of the form g i (x 1,..., x n x i = 0. i=1 A constraint conition
Assignment 1 Golstein 1.4 The equations of motion for the rolling isk are special cases of general linear ifferential equations of constraint of the form g i (x 1,..., x n x i = 0. i=1 A constraint conition
Lecture XII. where Φ is called the potential function. Let us introduce spherical coordinates defined through the relations
 Lecture XII Abstract We introuce the Laplace equation in spherical coorinates an apply the metho of separation of variables to solve it. This will generate three linear orinary secon orer ifferential equations:
Lecture XII Abstract We introuce the Laplace equation in spherical coorinates an apply the metho of separation of variables to solve it. This will generate three linear orinary secon orer ifferential equations:
Separation of Variables
 Physics 342 Lecture 1 Separation of Variables Lecture 1 Physics 342 Quantum Mechanics I Monay, January 25th, 2010 There are three basic mathematical tools we nee, an then we can begin working on the physical
Physics 342 Lecture 1 Separation of Variables Lecture 1 Physics 342 Quantum Mechanics I Monay, January 25th, 2010 There are three basic mathematical tools we nee, an then we can begin working on the physical
Calculus of Variations
 Calculus of Variations Lagrangian formalism is the main tool of theoretical classical mechanics. Calculus of Variations is a part of Mathematics which Lagrangian formalism is base on. In this section,
Calculus of Variations Lagrangian formalism is the main tool of theoretical classical mechanics. Calculus of Variations is a part of Mathematics which Lagrangian formalism is base on. In this section,
Quantum Mechanics in Three Dimensions
 Physics 342 Lecture 20 Quantum Mechanics in Three Dimensions Lecture 20 Physics 342 Quantum Mechanics I Monay, March 24th, 2008 We begin our spherical solutions with the simplest possible case zero potential.
Physics 342 Lecture 20 Quantum Mechanics in Three Dimensions Lecture 20 Physics 342 Quantum Mechanics I Monay, March 24th, 2008 We begin our spherical solutions with the simplest possible case zero potential.
The Exact Form and General Integrating Factors
 7 The Exact Form an General Integrating Factors In the previous chapters, we ve seen how separable an linear ifferential equations can be solve using methos for converting them to forms that can be easily
7 The Exact Form an General Integrating Factors In the previous chapters, we ve seen how separable an linear ifferential equations can be solve using methos for converting them to forms that can be easily
Vectors in two dimensions
 Vectors in two imensions Until now, we have been working in one imension only The main reason for this is to become familiar with the main physical ieas like Newton s secon law, without the aitional complication
Vectors in two imensions Until now, we have been working in one imension only The main reason for this is to become familiar with the main physical ieas like Newton s secon law, without the aitional complication
The Press-Schechter mass function
 The Press-Schechter mass function To state the obvious: It is important to relate our theories to what we can observe. We have looke at linear perturbation theory, an we have consiere a simple moel for
The Press-Schechter mass function To state the obvious: It is important to relate our theories to what we can observe. We have looke at linear perturbation theory, an we have consiere a simple moel for
Schrödinger s equation.
 Physics 342 Lecture 5 Schröinger s Equation Lecture 5 Physics 342 Quantum Mechanics I Wenesay, February 3r, 2010 Toay we iscuss Schröinger s equation an show that it supports the basic interpretation of
Physics 342 Lecture 5 Schröinger s Equation Lecture 5 Physics 342 Quantum Mechanics I Wenesay, February 3r, 2010 Toay we iscuss Schröinger s equation an show that it supports the basic interpretation of
Experiment I Electric Force
 Experiment I Electric Force Twenty-five hunre years ago, the Greek philosopher Thales foun that amber, the harene sap from a tree, attracte light objects when rubbe. Only twenty-four hunre years later,
Experiment I Electric Force Twenty-five hunre years ago, the Greek philosopher Thales foun that amber, the harene sap from a tree, attracte light objects when rubbe. Only twenty-four hunre years later,
Math 1271 Solutions for Fall 2005 Final Exam
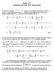 Math 7 Solutions for Fall 5 Final Eam ) Since the equation + y = e y cannot be rearrange algebraically in orer to write y as an eplicit function of, we must instea ifferentiate this relation implicitly
Math 7 Solutions for Fall 5 Final Eam ) Since the equation + y = e y cannot be rearrange algebraically in orer to write y as an eplicit function of, we must instea ifferentiate this relation implicitly
Lecture 2 Lagrangian formulation of classical mechanics Mechanics
 Lecture Lagrangian formulation of classical mechanics 70.00 Mechanics Principle of stationary action MATH-GA To specify a motion uniquely in classical mechanics, it suffices to give, at some time t 0,
Lecture Lagrangian formulation of classical mechanics 70.00 Mechanics Principle of stationary action MATH-GA To specify a motion uniquely in classical mechanics, it suffices to give, at some time t 0,
Implicit Differentiation
 Implicit Differentiation Thus far, the functions we have been concerne with have been efine explicitly. A function is efine explicitly if the output is given irectly in terms of the input. For instance,
Implicit Differentiation Thus far, the functions we have been concerne with have been efine explicitly. A function is efine explicitly if the output is given irectly in terms of the input. For instance,
d dx But have you ever seen a derivation of these results? We ll prove the first result below. cos h 1
 Lecture 5 Some ifferentiation rules Trigonometric functions (Relevant section from Stewart, Seventh Eition: Section 3.3) You all know that sin = cos cos = sin. () But have you ever seen a erivation of
Lecture 5 Some ifferentiation rules Trigonometric functions (Relevant section from Stewart, Seventh Eition: Section 3.3) You all know that sin = cos cos = sin. () But have you ever seen a erivation of
Kramers Relation. Douglas H. Laurence. Department of Physical Sciences, Broward College, Davie, FL 33314
 Kramers Relation Douglas H. Laurence Department of Physical Sciences, Browar College, Davie, FL 333 Introuction Kramers relation, name after the Dutch physicist Hans Kramers, is a relationship between
Kramers Relation Douglas H. Laurence Department of Physical Sciences, Browar College, Davie, FL 333 Introuction Kramers relation, name after the Dutch physicist Hans Kramers, is a relationship between
Astronomy 112: The Physics of Stars. Class 14 Notes: The Main Sequence
 Astronomy 112: The Physics of Stars Class 14 Notes: The Main Sequence In the last class we drew a diagram that summarized the basic evolutionary path of stars, as seen from their centers. In this class
Astronomy 112: The Physics of Stars Class 14 Notes: The Main Sequence In the last class we drew a diagram that summarized the basic evolutionary path of stars, as seen from their centers. In this class
Astronomy 112: The Physics of Stars. Class 13 Notes: Schematics of the Evolution of Stellar Cores
 Astronomy 112: The Physics of Stars Class 13 Notes: Schematics of the Evolution of Stellar Cores We re now done with our discussion of physical processes in stars, and we are ready to begin the last phase
Astronomy 112: The Physics of Stars Class 13 Notes: Schematics of the Evolution of Stellar Cores We re now done with our discussion of physical processes in stars, and we are ready to begin the last phase
The Principle of Least Action
 Chapter 7. The Principle of Least Action 7.1 Force Methos vs. Energy Methos We have so far stuie two istinct ways of analyzing physics problems: force methos, basically consisting of the application of
Chapter 7. The Principle of Least Action 7.1 Force Methos vs. Energy Methos We have so far stuie two istinct ways of analyzing physics problems: force methos, basically consisting of the application of
Euler equations for multiple integrals
 Euler equations for multiple integrals January 22, 2013 Contents 1 Reminer of multivariable calculus 2 1.1 Vector ifferentiation......................... 2 1.2 Matrix ifferentiation........................
Euler equations for multiple integrals January 22, 2013 Contents 1 Reminer of multivariable calculus 2 1.1 Vector ifferentiation......................... 2 1.2 Matrix ifferentiation........................
Introduction to the Vlasov-Poisson system
 Introuction to the Vlasov-Poisson system Simone Calogero 1 The Vlasov equation Consier a particle with mass m > 0. Let x(t) R 3 enote the position of the particle at time t R an v(t) = ẋ(t) = x(t)/t its
Introuction to the Vlasov-Poisson system Simone Calogero 1 The Vlasov equation Consier a particle with mass m > 0. Let x(t) R 3 enote the position of the particle at time t R an v(t) = ẋ(t) = x(t)/t its
The Virial Theorem for Stars
 The Virial Theorem for Stars Stars are excellent examples of systems in virial equilibrium. To see this, let us make two assumptions: 1) Stars are in hydrostatic equilibrium 2) Stars are made up of ideal
The Virial Theorem for Stars Stars are excellent examples of systems in virial equilibrium. To see this, let us make two assumptions: 1) Stars are in hydrostatic equilibrium 2) Stars are made up of ideal
Lecture 16: The chain rule
 Lecture 6: The chain rule Nathan Pflueger 6 October 03 Introuction Toay we will a one more rule to our toolbo. This rule concerns functions that are epresse as compositions of functions. The iea of a composition
Lecture 6: The chain rule Nathan Pflueger 6 October 03 Introuction Toay we will a one more rule to our toolbo. This rule concerns functions that are epresse as compositions of functions. The iea of a composition
Lectures - Week 10 Introduction to Ordinary Differential Equations (ODES) First Order Linear ODEs
 Lectures - Week 10 Introuction to Orinary Differential Equations (ODES) First Orer Linear ODEs When stuying ODEs we are consiering functions of one inepenent variable, e.g., f(x), where x is the inepenent
Lectures - Week 10 Introuction to Orinary Differential Equations (ODES) First Orer Linear ODEs When stuying ODEs we are consiering functions of one inepenent variable, e.g., f(x), where x is the inepenent
The derivative of a function f(x) is another function, defined in terms of a limiting expression: f(x + δx) f(x)
 Y. D. Chong (2016) MH2801: Complex Methos for the Sciences 1. Derivatives The erivative of a function f(x) is another function, efine in terms of a limiting expression: f (x) f (x) lim x δx 0 f(x + δx)
Y. D. Chong (2016) MH2801: Complex Methos for the Sciences 1. Derivatives The erivative of a function f(x) is another function, efine in terms of a limiting expression: f (x) f (x) lim x δx 0 f(x + δx)
Math Notes on differentials, the Chain Rule, gradients, directional derivative, and normal vectors
 Math 18.02 Notes on ifferentials, the Chain Rule, graients, irectional erivative, an normal vectors Tangent plane an linear approximation We efine the partial erivatives of f( xy, ) as follows: f f( x+
Math 18.02 Notes on ifferentials, the Chain Rule, graients, irectional erivative, an normal vectors Tangent plane an linear approximation We efine the partial erivatives of f( xy, ) as follows: f f( x+
Unit #6 - Families of Functions, Taylor Polynomials, l Hopital s Rule
 Unit # - Families of Functions, Taylor Polynomials, l Hopital s Rule Some problems an solutions selecte or aapte from Hughes-Hallett Calculus. Critical Points. Consier the function f) = 54 +. b) a) Fin
Unit # - Families of Functions, Taylor Polynomials, l Hopital s Rule Some problems an solutions selecte or aapte from Hughes-Hallett Calculus. Critical Points. Consier the function f) = 54 +. b) a) Fin
The total derivative. Chapter Lagrangian and Eulerian approaches
 Chapter 5 The total erivative 51 Lagrangian an Eulerian approaches The representation of a flui through scalar or vector fiels means that each physical quantity uner consieration is escribe as a function
Chapter 5 The total erivative 51 Lagrangian an Eulerian approaches The representation of a flui through scalar or vector fiels means that each physical quantity uner consieration is escribe as a function
Center of Gravity and Center of Mass
 Center of Gravity an Center of Mass 1 Introuction. Center of mass an center of gravity closely parallel each other: they both work the same way. Center of mass is the more important, but center of gravity
Center of Gravity an Center of Mass 1 Introuction. Center of mass an center of gravity closely parallel each other: they both work the same way. Center of mass is the more important, but center of gravity
Problem Set 2: Solutions
 UNIVERSITY OF ALABAMA Department of Physics an Astronomy PH 102 / LeClair Summer II 2010 Problem Set 2: Solutions 1. The en of a charge rubber ro will attract small pellets of Styrofoam that, having mae
UNIVERSITY OF ALABAMA Department of Physics an Astronomy PH 102 / LeClair Summer II 2010 Problem Set 2: Solutions 1. The en of a charge rubber ro will attract small pellets of Styrofoam that, having mae
Derivatives and the Product Rule
 Derivatives an the Prouct Rule James K. Peterson Department of Biological Sciences an Department of Mathematical Sciences Clemson University January 28, 2014 Outline Differentiability Simple Derivatives
Derivatives an the Prouct Rule James K. Peterson Department of Biological Sciences an Department of Mathematical Sciences Clemson University January 28, 2014 Outline Differentiability Simple Derivatives
Table of Common Derivatives By David Abraham
 Prouct an Quotient Rules: Table of Common Derivatives By Davi Abraham [ f ( g( ] = [ f ( ] g( + f ( [ g( ] f ( = g( [ f ( ] g( g( f ( [ g( ] Trigonometric Functions: sin( = cos( cos( = sin( tan( = sec
Prouct an Quotient Rules: Table of Common Derivatives By Davi Abraham [ f ( g( ] = [ f ( ] g( + f ( [ g( ] f ( = g( [ f ( ] g( g( f ( [ g( ] Trigonometric Functions: sin( = cos( cos( = sin( tan( = sec
Construction of the Electronic Radial Wave Functions and Probability Distributions of Hydrogen-like Systems
 Construction of the Electronic Raial Wave Functions an Probability Distributions of Hyrogen-like Systems Thomas S. Kuntzleman, Department of Chemistry Spring Arbor University, Spring Arbor MI 498 tkuntzle@arbor.eu
Construction of the Electronic Raial Wave Functions an Probability Distributions of Hyrogen-like Systems Thomas S. Kuntzleman, Department of Chemistry Spring Arbor University, Spring Arbor MI 498 tkuntzle@arbor.eu
Nuclear Physics and Astrophysics
 Nuclear Physics an Astrophysics PHY-302 Dr. E. Rizvi Lecture 2 - Introuction Notation Nuclies A Nuclie is a particular an is esignate by the following notation: A CN = Atomic Number (no. of Protons) A
Nuclear Physics an Astrophysics PHY-302 Dr. E. Rizvi Lecture 2 - Introuction Notation Nuclies A Nuclie is a particular an is esignate by the following notation: A CN = Atomic Number (no. of Protons) A
ensembles When working with density operators, we can use this connection to define a generalized Bloch vector: v x Tr x, v y Tr y
 Ph195a lecture notes, 1/3/01 Density operators for spin- 1 ensembles So far in our iscussion of spin- 1 systems, we have restricte our attention to the case of pure states an Hamiltonian evolution. Toay
Ph195a lecture notes, 1/3/01 Density operators for spin- 1 ensembles So far in our iscussion of spin- 1 systems, we have restricte our attention to the case of pure states an Hamiltonian evolution. Toay
Further Differentiation and Applications
 Avance Higher Notes (Unit ) Prerequisites: Inverse function property; prouct, quotient an chain rules; inflexion points. Maths Applications: Concavity; ifferentiability. Real-Worl Applications: Particle
Avance Higher Notes (Unit ) Prerequisites: Inverse function property; prouct, quotient an chain rules; inflexion points. Maths Applications: Concavity; ifferentiability. Real-Worl Applications: Particle
Survey Sampling. 1 Design-based Inference. Kosuke Imai Department of Politics, Princeton University. February 19, 2013
 Survey Sampling Kosuke Imai Department of Politics, Princeton University February 19, 2013 Survey sampling is one of the most commonly use ata collection methos for social scientists. We begin by escribing
Survey Sampling Kosuke Imai Department of Politics, Princeton University February 19, 2013 Survey sampling is one of the most commonly use ata collection methos for social scientists. We begin by escribing
Objective: To introduce the equations of motion and describe the forces that act upon the Atmosphere
 Objective: To introuce the equations of motion an escribe the forces that act upon the Atmosphere Reaing: Rea pp 18 6 in Chapter 1 of Houghton & Hakim Problems: Work 1.1, 1.8, an 1.9 on pp. 6 & 7 at the
Objective: To introuce the equations of motion an escribe the forces that act upon the Atmosphere Reaing: Rea pp 18 6 in Chapter 1 of Houghton & Hakim Problems: Work 1.1, 1.8, an 1.9 on pp. 6 & 7 at the
18 EVEN MORE CALCULUS
 8 EVEN MORE CALCULUS Chapter 8 Even More Calculus Objectives After stuing this chapter you shoul be able to ifferentiate an integrate basic trigonometric functions; unerstan how to calculate rates of change;
8 EVEN MORE CALCULUS Chapter 8 Even More Calculus Objectives After stuing this chapter you shoul be able to ifferentiate an integrate basic trigonometric functions; unerstan how to calculate rates of change;
Solving the Schrödinger Equation for the 1 Electron Atom (Hydrogen-Like)
 Stockton Univeristy Chemistry Program, School of Natural Sciences an Mathematics 101 Vera King Farris Dr, Galloway, NJ CHEM 340: Physical Chemistry II Solving the Schröinger Equation for the 1 Electron
Stockton Univeristy Chemistry Program, School of Natural Sciences an Mathematics 101 Vera King Farris Dr, Galloway, NJ CHEM 340: Physical Chemistry II Solving the Schröinger Equation for the 1 Electron
Section 2.7 Derivatives of powers of functions
 Section 2.7 Derivatives of powers of functions (3/19/08) Overview: In this section we iscuss the Chain Rule formula for the erivatives of composite functions that are forme by taking powers of other functions.
Section 2.7 Derivatives of powers of functions (3/19/08) Overview: In this section we iscuss the Chain Rule formula for the erivatives of composite functions that are forme by taking powers of other functions.
Prep 1. Oregon State University PH 213 Spring Term Suggested finish date: Monday, April 9
 Oregon State University PH 213 Spring Term 2018 Prep 1 Suggeste finish ate: Monay, April 9 The formats (type, length, scope) of these Prep problems have been purposely create to closely parallel those
Oregon State University PH 213 Spring Term 2018 Prep 1 Suggeste finish ate: Monay, April 9 The formats (type, length, scope) of these Prep problems have been purposely create to closely parallel those
Lecture 6: Calculus. In Song Kim. September 7, 2011
 Lecture 6: Calculus In Song Kim September 7, 20 Introuction to Differential Calculus In our previous lecture we came up with several ways to analyze functions. We saw previously that the slope of a linear
Lecture 6: Calculus In Song Kim September 7, 20 Introuction to Differential Calculus In our previous lecture we came up with several ways to analyze functions. We saw previously that the slope of a linear
TMA 4195 Matematisk modellering Exam Tuesday December 16, :00 13:00 Problems and solution with additional comments
 Problem F U L W D g m 3 2 s 2 0 0 0 0 2 kg 0 0 0 0 0 0 Table : Dimension matrix TMA 495 Matematisk moellering Exam Tuesay December 6, 2008 09:00 3:00 Problems an solution with aitional comments The necessary
Problem F U L W D g m 3 2 s 2 0 0 0 0 2 kg 0 0 0 0 0 0 Table : Dimension matrix TMA 495 Matematisk moellering Exam Tuesay December 6, 2008 09:00 3:00 Problems an solution with aitional comments The necessary
Integration Review. May 11, 2013
 Integration Review May 11, 2013 Goals: Review the funamental theorem of calculus. Review u-substitution. Review integration by parts. Do lots of integration eamples. 1 Funamental Theorem of Calculus In
Integration Review May 11, 2013 Goals: Review the funamental theorem of calculus. Review u-substitution. Review integration by parts. Do lots of integration eamples. 1 Funamental Theorem of Calculus In
Chapter 6: Energy-Momentum Tensors
 49 Chapter 6: Energy-Momentum Tensors This chapter outlines the general theory of energy an momentum conservation in terms of energy-momentum tensors, then applies these ieas to the case of Bohm's moel.
49 Chapter 6: Energy-Momentum Tensors This chapter outlines the general theory of energy an momentum conservation in terms of energy-momentum tensors, then applies these ieas to the case of Bohm's moel.
Diagonalization of Matrices Dr. E. Jacobs
 Diagonalization of Matrices Dr. E. Jacobs One of the very interesting lessons in this course is how certain algebraic techniques can be use to solve ifferential equations. The purpose of these notes is
Diagonalization of Matrices Dr. E. Jacobs One of the very interesting lessons in this course is how certain algebraic techniques can be use to solve ifferential equations. The purpose of these notes is
Math 1B, lecture 8: Integration by parts
 Math B, lecture 8: Integration by parts Nathan Pflueger 23 September 2 Introuction Integration by parts, similarly to integration by substitution, reverses a well-known technique of ifferentiation an explores
Math B, lecture 8: Integration by parts Nathan Pflueger 23 September 2 Introuction Integration by parts, similarly to integration by substitution, reverses a well-known technique of ifferentiation an explores
Integration by Parts
 Integration by Parts 6-3-207 If u an v are functions of, the Prouct Rule says that (uv) = uv +vu Integrate both sies: (uv) = uv = uv + u v + uv = uv vu, vu v u, I ve written u an v as shorthan for u an
Integration by Parts 6-3-207 If u an v are functions of, the Prouct Rule says that (uv) = uv +vu Integrate both sies: (uv) = uv = uv + u v + uv = uv vu, vu v u, I ve written u an v as shorthan for u an
Lagrangian and Hamiltonian Mechanics
 Lagrangian an Hamiltonian Mechanics.G. Simpson, Ph.. epartment of Physical Sciences an Engineering Prince George s Community College ecember 5, 007 Introuction In this course we have been stuying classical
Lagrangian an Hamiltonian Mechanics.G. Simpson, Ph.. epartment of Physical Sciences an Engineering Prince George s Community College ecember 5, 007 Introuction In this course we have been stuying classical
and from it produce the action integral whose variation we set to zero:
 Lagrange Multipliers Monay, 6 September 01 Sometimes it is convenient to use reunant coorinates, an to effect the variation of the action consistent with the constraints via the metho of Lagrange unetermine
Lagrange Multipliers Monay, 6 September 01 Sometimes it is convenient to use reunant coorinates, an to effect the variation of the action consistent with the constraints via the metho of Lagrange unetermine
12.11 Laplace s Equation in Cylindrical and
 SEC. 2. Laplace s Equation in Cylinrical an Spherical Coorinates. Potential 593 2. Laplace s Equation in Cylinrical an Spherical Coorinates. Potential One of the most important PDEs in physics an engineering
SEC. 2. Laplace s Equation in Cylinrical an Spherical Coorinates. Potential 593 2. Laplace s Equation in Cylinrical an Spherical Coorinates. Potential One of the most important PDEs in physics an engineering
fv = ikφ n (11.1) + fu n = y v n iσ iku n + gh n. (11.3) n
 Chapter 11 Rossby waves Supplemental reaing: Pelosky 1 (1979), sections 3.1 3 11.1 Shallow water equations When consiering the general problem of linearize oscillations in a static, arbitrarily stratifie
Chapter 11 Rossby waves Supplemental reaing: Pelosky 1 (1979), sections 3.1 3 11.1 Shallow water equations When consiering the general problem of linearize oscillations in a static, arbitrarily stratifie
Markov Chains in Continuous Time
 Chapter 23 Markov Chains in Continuous Time Previously we looke at Markov chains, where the transitions betweenstatesoccurreatspecifietime- steps. That it, we mae time (a continuous variable) avance in
Chapter 23 Markov Chains in Continuous Time Previously we looke at Markov chains, where the transitions betweenstatesoccurreatspecifietime- steps. That it, we mae time (a continuous variable) avance in
Calculus of Variations
 16.323 Lecture 5 Calculus of Variations Calculus of Variations Most books cover this material well, but Kirk Chapter 4 oes a particularly nice job. x(t) x* x*+ αδx (1) x*- αδx (1) αδx (1) αδx (1) t f t
16.323 Lecture 5 Calculus of Variations Calculus of Variations Most books cover this material well, but Kirk Chapter 4 oes a particularly nice job. x(t) x* x*+ αδx (1) x*- αδx (1) αδx (1) αδx (1) t f t
Short Intro to Coordinate Transformation
 Short Intro to Coorinate Transformation 1 A Vector A vector can basically be seen as an arrow in space pointing in a specific irection with a specific length. The following problem arises: How o we represent
Short Intro to Coorinate Transformation 1 A Vector A vector can basically be seen as an arrow in space pointing in a specific irection with a specific length. The following problem arises: How o we represent
Transmission Line Matrix (TLM) network analogues of reversible trapping processes Part B: scaling and consistency
 Transmission Line Matrix (TLM network analogues of reversible trapping processes Part B: scaling an consistency Donar e Cogan * ANC Eucation, 308-310.A. De Mel Mawatha, Colombo 3, Sri Lanka * onarecogan@gmail.com
Transmission Line Matrix (TLM network analogues of reversible trapping processes Part B: scaling an consistency Donar e Cogan * ANC Eucation, 308-310.A. De Mel Mawatha, Colombo 3, Sri Lanka * onarecogan@gmail.com
arxiv: v1 [physics.flu-dyn] 8 May 2014
![arxiv: v1 [physics.flu-dyn] 8 May 2014 arxiv: v1 [physics.flu-dyn] 8 May 2014](/thumbs/87/97199974.jpg) Energetics of a flui uner the Boussinesq approximation arxiv:1405.1921v1 [physics.flu-yn] 8 May 2014 Kiyoshi Maruyama Department of Earth an Ocean Sciences, National Defense Acaemy, Yokosuka, Kanagawa
Energetics of a flui uner the Boussinesq approximation arxiv:1405.1921v1 [physics.flu-yn] 8 May 2014 Kiyoshi Maruyama Department of Earth an Ocean Sciences, National Defense Acaemy, Yokosuka, Kanagawa
A Second Time Dimension, Hidden in Plain Sight
 A Secon Time Dimension, Hien in Plain Sight Brett A Collins. In this paper I postulate the existence of a secon time imension, making five imensions, three space imensions an two time imensions. I will
A Secon Time Dimension, Hien in Plain Sight Brett A Collins. In this paper I postulate the existence of a secon time imension, making five imensions, three space imensions an two time imensions. I will
1 The Derivative of ln(x)
 Monay, December 3, 2007 The Derivative of ln() 1 The Derivative of ln() The first term or semester of most calculus courses will inclue the it efinition of the erivative an will work out, long han, a number
Monay, December 3, 2007 The Derivative of ln() 1 The Derivative of ln() The first term or semester of most calculus courses will inclue the it efinition of the erivative an will work out, long han, a number
ELECTRON DIFFRACTION
 ELECTRON DIFFRACTION Electrons : wave or quanta? Measurement of wavelength an momentum of electrons. Introuction Electrons isplay both wave an particle properties. What is the relationship between the
ELECTRON DIFFRACTION Electrons : wave or quanta? Measurement of wavelength an momentum of electrons. Introuction Electrons isplay both wave an particle properties. What is the relationship between the
2. Equations of Stellar Structure
 2. Equations of Stellar Structure We already discussed that the structure of stars is basically governed by three simple laws, namely hyostatic equilibrium, energy transport and energy generation. In this
2. Equations of Stellar Structure We already discussed that the structure of stars is basically governed by three simple laws, namely hyostatic equilibrium, energy transport and energy generation. In this
MATHEMATICS BONUS FILES for faculty and students
 MATHMATI BONU FIL for faculty an stuents http://www.onu.eu/~mcaragiu1/bonus_files.html RIVD: May 15, 9 PUBLIHD: May 5, 9 toffel 1 Maxwell s quations through the Major Vector Theorems Joshua toffel Department
MATHMATI BONU FIL for faculty an stuents http://www.onu.eu/~mcaragiu1/bonus_files.html RIVD: May 15, 9 PUBLIHD: May 5, 9 toffel 1 Maxwell s quations through the Major Vector Theorems Joshua toffel Department
RFSS: Lecture 4 Alpha Decay
 RFSS: Lecture 4 Alpha Decay Reaings Nuclear an Raiochemistry: Chapter 3 Moern Nuclear Chemistry: Chapter 7 Energetics of Alpha Decay Geiger Nuttall base theory Theory of Alpha Decay Hinrance Factors Different
RFSS: Lecture 4 Alpha Decay Reaings Nuclear an Raiochemistry: Chapter 3 Moern Nuclear Chemistry: Chapter 7 Energetics of Alpha Decay Geiger Nuttall base theory Theory of Alpha Decay Hinrance Factors Different
UNDERSTANDING INTEGRATION
 UNDERSTANDING INTEGRATION Dear Reaer The concept of Integration, mathematically speaking, is the "Inverse" of the concept of result, the integration of, woul give us back the function f(). This, in a way,
UNDERSTANDING INTEGRATION Dear Reaer The concept of Integration, mathematically speaking, is the "Inverse" of the concept of result, the integration of, woul give us back the function f(). This, in a way,
23 Implicit differentiation
 23 Implicit ifferentiation 23.1 Statement The equation y = x 2 + 3x + 1 expresses a relationship between the quantities x an y. If a value of x is given, then a corresponing value of y is etermine. For
23 Implicit ifferentiation 23.1 Statement The equation y = x 2 + 3x + 1 expresses a relationship between the quantities x an y. If a value of x is given, then a corresponing value of y is etermine. For
Calculus and optimization
 Calculus an optimization These notes essentially correspon to mathematical appenix 2 in the text. 1 Functions of a single variable Now that we have e ne functions we turn our attention to calculus. A function
Calculus an optimization These notes essentially correspon to mathematical appenix 2 in the text. 1 Functions of a single variable Now that we have e ne functions we turn our attention to calculus. A function
Chapter 2 Lagrangian Modeling
 Chapter 2 Lagrangian Moeling The basic laws of physics are use to moel every system whether it is electrical, mechanical, hyraulic, or any other energy omain. In mechanics, Newton s laws of motion provie
Chapter 2 Lagrangian Moeling The basic laws of physics are use to moel every system whether it is electrical, mechanical, hyraulic, or any other energy omain. In mechanics, Newton s laws of motion provie
It's often useful to find all the points in a diagram that have the same voltage. E.g., consider a capacitor again.
 17-7 (SJP, Phys 22, Sp ') It's often useful to fin all the points in a iagram that have the same voltage. E.g., consier a capacitor again. V is high here V is in between, here V is low here Everywhere
17-7 (SJP, Phys 22, Sp ') It's often useful to fin all the points in a iagram that have the same voltage. E.g., consier a capacitor again. V is high here V is in between, here V is low here Everywhere
Introduction to Markov Processes
 Introuction to Markov Processes Connexions moule m44014 Zzis law Gustav) Meglicki, Jr Office of the VP for Information Technology Iniana University RCS: Section-2.tex,v 1.24 2012/12/21 18:03:08 gustav
Introuction to Markov Processes Connexions moule m44014 Zzis law Gustav) Meglicki, Jr Office of the VP for Information Technology Iniana University RCS: Section-2.tex,v 1.24 2012/12/21 18:03:08 gustav
0.1 The Chain Rule. db dt = db
 0. The Chain Rule A basic illustration of the chain rules comes in thinking about runners in a race. Suppose two brothers, Mark an Brian, hol an annual race to see who is the fastest. Last year Mark won
0. The Chain Rule A basic illustration of the chain rules comes in thinking about runners in a race. Suppose two brothers, Mark an Brian, hol an annual race to see who is the fastest. Last year Mark won
Conservation Laws. Chapter Conservation of Energy
 20 Chapter 3 Conservation Laws In orer to check the physical consistency of the above set of equations governing Maxwell-Lorentz electroynamics [(2.10) an (2.12) or (1.65) an (1.68)], we examine the action
20 Chapter 3 Conservation Laws In orer to check the physical consistency of the above set of equations governing Maxwell-Lorentz electroynamics [(2.10) an (2.12) or (1.65) an (1.68)], we examine the action
05 The Continuum Limit and the Wave Equation
 Utah State University DigitalCommons@USU Founations of Wave Phenomena Physics, Department of 1-1-2004 05 The Continuum Limit an the Wave Equation Charles G. Torre Department of Physics, Utah State University,
Utah State University DigitalCommons@USU Founations of Wave Phenomena Physics, Department of 1-1-2004 05 The Continuum Limit an the Wave Equation Charles G. Torre Department of Physics, Utah State University,
APPROXIMATE SOLUTION FOR TRANSIENT HEAT TRANSFER IN STATIC TURBULENT HE II. B. Baudouy. CEA/Saclay, DSM/DAPNIA/STCM Gif-sur-Yvette Cedex, France
 APPROXIMAE SOLUION FOR RANSIEN HEA RANSFER IN SAIC URBULEN HE II B. Bauouy CEA/Saclay, DSM/DAPNIA/SCM 91191 Gif-sur-Yvette Ceex, France ABSRAC Analytical solution in one imension of the heat iffusion equation
APPROXIMAE SOLUION FOR RANSIEN HEA RANSFER IN SAIC URBULEN HE II B. Bauouy CEA/Saclay, DSM/DAPNIA/SCM 91191 Gif-sur-Yvette Ceex, France ABSRAC Analytical solution in one imension of the heat iffusion equation
JUST THE MATHS UNIT NUMBER DIFFERENTIATION 2 (Rates of change) A.J.Hobson
 JUST THE MATHS UNIT NUMBER 10.2 DIFFERENTIATION 2 (Rates of change) by A.J.Hobson 10.2.1 Introuction 10.2.2 Average rates of change 10.2.3 Instantaneous rates of change 10.2.4 Derivatives 10.2.5 Exercises
JUST THE MATHS UNIT NUMBER 10.2 DIFFERENTIATION 2 (Rates of change) by A.J.Hobson 10.2.1 Introuction 10.2.2 Average rates of change 10.2.3 Instantaneous rates of change 10.2.4 Derivatives 10.2.5 Exercises
Free rotation of a rigid body 1 D. E. Soper 2 University of Oregon Physics 611, Theoretical Mechanics 5 November 2012
 Free rotation of a rigi boy 1 D. E. Soper 2 University of Oregon Physics 611, Theoretical Mechanics 5 November 2012 1 Introuction In this section, we escribe the motion of a rigi boy that is free to rotate
Free rotation of a rigi boy 1 D. E. Soper 2 University of Oregon Physics 611, Theoretical Mechanics 5 November 2012 1 Introuction In this section, we escribe the motion of a rigi boy that is free to rotate
G j dq i + G j. q i. = a jt. and
 Lagrange Multipliers Wenesay, 8 September 011 Sometimes it is convenient to use reunant coorinates, an to effect the variation of the action consistent with the constraints via the metho of Lagrange unetermine
Lagrange Multipliers Wenesay, 8 September 011 Sometimes it is convenient to use reunant coorinates, an to effect the variation of the action consistent with the constraints via the metho of Lagrange unetermine
Applications of the Wronskian to ordinary linear differential equations
 Physics 116C Fall 2011 Applications of the Wronskian to orinary linear ifferential equations Consier a of n continuous functions y i (x) [i = 1,2,3,...,n], each of which is ifferentiable at least n times.
Physics 116C Fall 2011 Applications of the Wronskian to orinary linear ifferential equations Consier a of n continuous functions y i (x) [i = 1,2,3,...,n], each of which is ifferentiable at least n times.
Experiment 2, Physics 2BL
 Experiment 2, Physics 2BL Deuction of Mass Distributions. Last Upate: 2009-05-03 Preparation Before this experiment, we recommen you review or familiarize yourself with the following: Chapters 4-6 in Taylor
Experiment 2, Physics 2BL Deuction of Mass Distributions. Last Upate: 2009-05-03 Preparation Before this experiment, we recommen you review or familiarize yourself with the following: Chapters 4-6 in Taylor
Math 115 Section 018 Course Note
 Course Note 1 General Functions Definition 1.1. A function is a rule that takes certain numbers as inputs an assigns to each a efinite output number. The set of all input numbers is calle the omain of
Course Note 1 General Functions Definition 1.1. A function is a rule that takes certain numbers as inputs an assigns to each a efinite output number. The set of all input numbers is calle the omain of
PDE Notes, Lecture #11
 PDE Notes, Lecture # from Professor Jalal Shatah s Lectures Febuary 9th, 2009 Sobolev Spaces Recall that for u L loc we can efine the weak erivative Du by Du, φ := udφ φ C0 If v L loc such that Du, φ =
PDE Notes, Lecture # from Professor Jalal Shatah s Lectures Febuary 9th, 2009 Sobolev Spaces Recall that for u L loc we can efine the weak erivative Du by Du, φ := udφ φ C0 If v L loc such that Du, φ =
0.1 Differentiation Rules
 0.1 Differentiation Rules From our previous work we ve seen tat it can be quite a task to calculate te erivative of an arbitrary function. Just working wit a secon-orer polynomial tings get pretty complicate
0.1 Differentiation Rules From our previous work we ve seen tat it can be quite a task to calculate te erivative of an arbitrary function. Just working wit a secon-orer polynomial tings get pretty complicate
Many problems in physics, engineering, and chemistry fall in a general class of equations of the form. d dx. d dx
 Math 53 Notes on turm-liouville equations Many problems in physics, engineering, an chemistry fall in a general class of equations of the form w(x)p(x) u ] + (q(x) λ) u = w(x) on an interval a, b], plus
Math 53 Notes on turm-liouville equations Many problems in physics, engineering, an chemistry fall in a general class of equations of the form w(x)p(x) u ] + (q(x) λ) u = w(x) on an interval a, b], plus
3.7 Implicit Differentiation -- A Brief Introduction -- Student Notes
 Fin these erivatives of these functions: y.7 Implicit Differentiation -- A Brief Introuction -- Stuent Notes tan y sin tan = sin y e = e = Write the inverses of these functions: y tan y sin How woul we
Fin these erivatives of these functions: y.7 Implicit Differentiation -- A Brief Introuction -- Stuent Notes tan y sin tan = sin y e = e = Write the inverses of these functions: y tan y sin How woul we
SYNCHRONOUS SEQUENTIAL CIRCUITS
 CHAPTER SYNCHRONOUS SEUENTIAL CIRCUITS Registers an counters, two very common synchronous sequential circuits, are introuce in this chapter. Register is a igital circuit for storing information. Contents
CHAPTER SYNCHRONOUS SEUENTIAL CIRCUITS Registers an counters, two very common synchronous sequential circuits, are introuce in this chapter. Register is a igital circuit for storing information. Contents
A Course in Machine Learning
 A Course in Machine Learning Hal Daumé III 12 EFFICIENT LEARNING So far, our focus has been on moels of learning an basic algorithms for those moels. We have not place much emphasis on how to learn quickly.
A Course in Machine Learning Hal Daumé III 12 EFFICIENT LEARNING So far, our focus has been on moels of learning an basic algorithms for those moels. We have not place much emphasis on how to learn quickly.
1 dx. where is a large constant, i.e., 1, (7.6) and Px is of the order of unity. Indeed, if px is given by (7.5), the inequality (7.
 Lectures Nine an Ten The WKB Approximation The WKB metho is a powerful tool to obtain solutions for many physical problems It is generally applicable to problems of wave propagation in which the frequency
Lectures Nine an Ten The WKB Approximation The WKB metho is a powerful tool to obtain solutions for many physical problems It is generally applicable to problems of wave propagation in which the frequency
Math 342 Partial Differential Equations «Viktor Grigoryan
 Math 342 Partial Differential Equations «Viktor Grigoryan 6 Wave equation: solution In this lecture we will solve the wave equation on the entire real line x R. This correspons to a string of infinite
Math 342 Partial Differential Equations «Viktor Grigoryan 6 Wave equation: solution In this lecture we will solve the wave equation on the entire real line x R. This correspons to a string of infinite
SOLUTIONS for Homework #3
 SOLUTIONS for Hoework #3 1. In the potential of given for there is no unboun states. Boun states have positive energies E n labele by an integer n. For each energy level E, two syetrically locate classical
SOLUTIONS for Hoework #3 1. In the potential of given for there is no unboun states. Boun states have positive energies E n labele by an integer n. For each energy level E, two syetrically locate classical
Section 7.2. The Calculus of Complex Functions
 Section 7.2 The Calculus of Complex Functions In this section we will iscuss limits, continuity, ifferentiation, Taylor series in the context of functions which take on complex values. Moreover, we will
Section 7.2 The Calculus of Complex Functions In this section we will iscuss limits, continuity, ifferentiation, Taylor series in the context of functions which take on complex values. Moreover, we will
Chapter 4. Electrostatics of Macroscopic Media
 Chapter 4. Electrostatics of Macroscopic Meia 4.1 Multipole Expansion Approximate potentials at large istances 3 x' x' (x') x x' x x Fig 4.1 We consier the potential in the far-fiel region (see Fig. 4.1
Chapter 4. Electrostatics of Macroscopic Meia 4.1 Multipole Expansion Approximate potentials at large istances 3 x' x' (x') x x' x x Fig 4.1 We consier the potential in the far-fiel region (see Fig. 4.1
Section 7.1: Integration by Parts
 Section 7.1: Integration by Parts 1. Introuction to Integration Techniques Unlike ifferentiation where there are a large number of rules which allow you (in principle) to ifferentiate any function, the
Section 7.1: Integration by Parts 1. Introuction to Integration Techniques Unlike ifferentiation where there are a large number of rules which allow you (in principle) to ifferentiate any function, the
Computational Applications in Nuclear Astrophysics using JAVA
 Computational Applications in Nuclear Astrophysics using JAVA Lecture: Friday 10:15-11:45 Room NB 6/99 Jim Ritman and Elisabetta Prencipe j.ritman@fz-juelich.de e.prencipe@fz-juelich.de Computer Lab: Friday
Computational Applications in Nuclear Astrophysics using JAVA Lecture: Friday 10:15-11:45 Room NB 6/99 Jim Ritman and Elisabetta Prencipe j.ritman@fz-juelich.de e.prencipe@fz-juelich.de Computer Lab: Friday
Stellar energy generation on the main sequence
 Stellar energy generation on the main sequence Once fusion reactions begin at the center of a cloud of gas, we call the object a star. For the bulk of its lifetime, a star fuses hydrogen into helium in
Stellar energy generation on the main sequence Once fusion reactions begin at the center of a cloud of gas, we call the object a star. For the bulk of its lifetime, a star fuses hydrogen into helium in
inflow outflow Part I. Regular tasks for MAE598/494 Task 1
 MAE 494/598, Fall 2016 Project #1 (Regular tasks = 20 points) Har copy of report is ue at the start of class on the ue ate. The rules on collaboration will be release separately. Please always follow the
MAE 494/598, Fall 2016 Project #1 (Regular tasks = 20 points) Har copy of report is ue at the start of class on the ue ate. The rules on collaboration will be release separately. Please always follow the
Antiderivatives. Definition (Antiderivative) If F (x) = f (x) we call F an antiderivative of f. Alan H. SteinUniversity of Connecticut
 Antierivatives Definition (Antierivative) If F (x) = f (x) we call F an antierivative of f. Antierivatives Definition (Antierivative) If F (x) = f (x) we call F an antierivative of f. Definition (Inefinite
Antierivatives Definition (Antierivative) If F (x) = f (x) we call F an antierivative of f. Antierivatives Definition (Antierivative) If F (x) = f (x) we call F an antierivative of f. Definition (Inefinite
Fundamental Stellar Parameters. Radiative Transfer. Stellar Atmospheres
 Fundamental Stellar Parameters Radiative Transfer Stellar Atmospheres Equations of Stellar Structure Basic Principles Equations of Hydrostatic Equilibrium and Mass Conservation Central Pressure, Virial
Fundamental Stellar Parameters Radiative Transfer Stellar Atmospheres Equations of Stellar Structure Basic Principles Equations of Hydrostatic Equilibrium and Mass Conservation Central Pressure, Virial
6 Wave equation in spherical polar coordinates
 6 Wave equation in spherical polar coorinates We now look at solving problems involving the Laplacian in spherical polar coorinates. The angular epenence of the solutions will be escribe by spherical harmonics.
6 Wave equation in spherical polar coorinates We now look at solving problems involving the Laplacian in spherical polar coorinates. The angular epenence of the solutions will be escribe by spherical harmonics.
Optimization of Geometries by Energy Minimization
 Optimization of Geometries by Energy Minimization by Tracy P. Hamilton Department of Chemistry University of Alabama at Birmingham Birmingham, AL 3594-140 hamilton@uab.eu Copyright Tracy P. Hamilton, 1997.
Optimization of Geometries by Energy Minimization by Tracy P. Hamilton Department of Chemistry University of Alabama at Birmingham Birmingham, AL 3594-140 hamilton@uab.eu Copyright Tracy P. Hamilton, 1997.
