University of Bristol - Explore Bristol Research. Early version, also known as pre-print
|
|
|
- Edmund Randall
- 5 years ago
- Views:
Transcription
1 Chizhov, Anto, V., Rodrigues, S., & Terry, JR. (6). A comparative analysis of an EEG model and a conductance-based. Early version, also known as pre-print Link to published version (if available): 1.116/j.physleta Link to publication record in Explore Bristol Research PDF-document University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full ter of use are available:
2 A comparative analysis of an EEG model and a conductance-based neural population model Anton V. Chizhov, Serafim Rodrigues + and John R. Terry (*) A.F.Ioffe Technical Institute, 26 Politekhnicheskaya str., 19421, St.-Petersburg, Russia (+) School of Mathematics, Loughborough University, Leicestershire, LE11 3TU, UK ( ) Department of Engineering Mathematics, University of Bristol, BS8 1TR, UK August 1, 6 Abstract We consider a firing-rate based approach to modelling EEG, justifying its use by comparison with a conductance-based refractory density population model and a set of individual neurons. It is shown that stimulation of the system by applying a step-wise current, results in a sharp peak in the population activity that can be reproduced by the EEG-model. In addition the steady-state activity may also be reproduced. Similar comparisons are obtained for stimulation via oscillatory inputs. Keywords: neuron ensemble; population model; refractory density equation; conductance-based neuron; Fokker-Planck equation; firing-rate model; EEG 1 Introduction The highest system level of brain activity modelling occurs at the level of EEG, corresponding to the aggregated activity of large populations of excitatory cortical neurons. Many clinical studies of humans, during cognitive tasks or in the study of neurological disorders, such as epilepsy, rely on EEG and consequently the study of models that can replicate the observed phenomena is an important step towards an understanding of the brain [1], [2], [3], [4], [5]. A mathematical analysis of these models is a promising tool for understanding the dynamics, that may then be related to those obtained from an EEG of the human brain. However, a common complaint about EEG models is that they are detached from physiology and consequently bifurcation analysis of a mean-field model does not necessarily elucidate any useful information about the functioning of the human brain. The purpose of the present work is to make the first steps towards linking mean-field approaches to modelling EEG and conductance type models based upon physiology. To this end we wish to construct a hierarchy of models describing the dynamics of a neural ensemble. 1
3 This hierarchy should be developed from a set of equations for a conductance-based description of a single neuron and ultimately give rise to a firing-rate-based EEG model. At the intermediate level of the hierarchy a continuum population model may be considered, describing the evolution of a neural ensemble in ter of neural density in a phase space of neuron state parameters. The equation for the neural density could be described using a Fokker-Planck-Kolmogorov approach. However, since several variables are needed to describe the state of a neuron, this approach would result in multi-dimensional equation involving partial derivatives, which would prove too difficult to solve and analyze. A numerical treatment of such an approach would provide only limited advancement of existing knowledge. It is however possible to assume that the state variables of a neuron may be paramaterized by a single variable, resulting in a one-dimensional density equation. One such choice is based on the membrane potential [6] and is suitable for modelling a neuron assuming a monotonic profile of the membrane potential between spikes. Another technique is the so-called refractory density approach which is based upon the time elapsed since the last spike (t ) [7]. This approach has been considered in the case of simple neuron models such as integrate-and-fire or the so-called spike-response model [8]. The advantage of this technique is that it results in a low dimensional system that is amenable to mathematical analysis. Advancing these techniques, Chizhov and co-workers proposed an application of the refractory density approach in the case of conductance-based models [9], [1], [11]. The model considered in [9] is a reduced version of a hippocampal pyramidal cell model, considering a one-compartment neuron with voltage-dependent sodium and potassium currents (I DR, I A, I H, I M ) [Graham (1999)] as well as with an AHP-current, I AHP, which described the cumulative activity of calcium-dependent currents [12], ρ t + ρ t C ( U t + U t x t + x t y t + y t = ρh, ) = I DR I A I M I H I L I AHP + I a, = x (U) x τ x(u), = y (U) y τ y(u), (1) where C is the membrane capacity, I L is the leakage current, I i are the external or synaptic currents; the approximations for the ionic currents being given by I DR, I A, I H, I M, I AHP, with corresponding synaptic conductances g DR, g A, g H, g M, g AHP. The functions x (U), y (U), τ x (U), τ y (U) are derived from the equations for gating variables x, y and may be found in [Graham (1999)] or [9]. This model qualitatively reproduces the activity of a neural population, i.e., an infinite number of similar adaptive neurons receiving a common input and dispersed by noise. Exempler voltage traces calculated from (1) are shown in Fig.1. The currents I M and I AHP are particularly significant, contributing to spike frequency adaptation. We may reduce this conductance-based model to a threshold version, by omitting the fast sodium current and instead inserting an explicit threshold criterion, for the sub-threshold voltage U, which is reset at 2
4 5 pa Adaptive neuron Not-adaptive neuron mv Figure 1: Voltage traces calculated by the full conductance-based model for adaptive (gray) and nonadaptive (black) neurons in the case of stimulation by step-wise current I a = 5pA. The membrane area is implied to be cm 2. the threshold U T, to a reset value, U reset. Consideration of the sodium channel kinetics indicates that this threshold should be considered as dynamic rather than static. This may be approximated by the dependence: U T = U T (du/dt), (2) which may be calculated with the help of the full neuron model and shown in Fig.2. This reduced threshold model reproduces well both the spike timing and the voltage between spikes [5,7]. 15 U T, mv du/ dt, mv/ Figure 2: The threshold potential as a function of the potential slope. The approximation for the spike-release probability density, H, is defined to be: H(U(t, t )) = 1 τ m (A(U) + B(U, du/dt)), (3) where A(U) is given by the curve A = A(T ) in Fig.3 with T = (U T U)/ 2σ V ; τ m = C/(g DR (t, t )+ g A (t, t ) + g M (t, t ) + g H (t, t ) + g L + g AHP (t, t )) and B(U, du/dt) = 2 τ m dt dt F (T ), F (T ) = 2 π exp( T 2 ) 1 + erf(t ). (4) This hazard function (H) defines the expected firing activity of a neuron, given the expected state of the neuron as characterized by U and du/dt. The formula for H was derived as a result of the 3
5 sum of solutions of the Fokker-Planck equation in the two specific cases, the solution A, for the case of slow change of U, and the solution B for the case of rapidly increasing voltage. Interpreting this physiologically, the activity B arises as a result of threshold crossings by the mean membrane potential, U, whereas the activity A occurs due to threshold crossings as a result of noise-induced voltage fluctuations A T Figure 3: A component of hazard-function. According to the single-neuron threshold model, when the potential crosses the threshold U T, it is reset to the value U reset = 4mV under the assumption that this point of the repolarization phase gives I Na. The gating variables for the fast currents I DR, I A, I H are also reset to fixed values, whereas the gating variables corresponding to the slow adaptation currents I M, I AHP vary dynamically and are determined by their previous state. This gives rise to boundary conditions for the potential and gating variables of the ionic currents when t = of U(t, ) = U reset, x(t, ) = x reset, y(t, ) = y reset, where x reset and y reset are for I DR : x reset =.262, y reset =.473; (5) for I A : x reset =.743, y reset =.691; (6) for I H : y reset =.2; (7) for I M : x reset =.175 (1 x(t, t p )); (8) for I AHP : w reset =.18 (1 w(t, t p )); (9) These values were calculated by the full model at the descending phase of a spike, when U = U reset. For taking into account the duration of a spike the gating variables and the potential are kept constant during the time t AP = 1.5. The estimation of the characteristic interspike interval, t p, is used for the adaptation currents, namely, t p is such that ρ(t, t p ) H(U(t, t p )) = max ρ(t, < t <+ t ) H(U(t, t )). 4
6 The boundary condition for Eq.(1) implies that neurons return to the point t = when they spike, i.e. ν(t) ρ(t, ) = ρhdt, (1) where ρ(t, ) is the firing rate of the population. Assuming that ρ is normalized, the boundary condition (1) provides a conservation law for the number of neurons. The firing rate calculated by the described model compares well with the activity of an infinite number of individual neurons as illustrated in Fig pa Individual neurons Population model, HH Population model, adaptive HH Figure 4: The transient response of the population firing rate to a rapid change in input. Beginning at t =, the excitatory input current to the uncoupled non-adapting neurons in a single population is stepped up to 3 pa. Similar simulation but taking into account the slow adaptation current I M and both I M and I AHP is shown in dashed line. The firing rate transiently jumps up before returning to a new steady-state response. The population model firing rate (solid line) compares favourably with the averaged firing rate of individual neurons. The amplitude of noise, σ V, is set to 2 mv. We now proceed to consider successive reductions of the model resulting finally in a firing rate model reminiscent of the EEG-models [1], [3], [4], [5]. 2 Reduction 1: Population model for LIF-neurons. We now turn from conductance-based neurons to linear leak integrate-and-fire (LIF) neurons, C du dt = g L(U V L ) + I a, (11) if U > U T then U = V reset, with the reset fitted in order to provide reproduction of spike timing for a specific amplitude of the stimulating current (Fig.5). In this case the model provides an excellent reproduction of the spike timing for the specific amplitude of the current, however, discrepancies arise when considering different amplitudes. In addition, the model only roughly reproduces the voltage trace of a non-adaptive neuron across interspike intervals. 5
7 mv - -4 full non-adaptive neuron LIF V reset = -75 mv, g L = 25.4 ns, C =.37 nf, I a = 3 pa Figure 5: Voltage traces calculated by the full conductance-based model (gray) and LIF-model (black) in the case of stimulation by step-wise current I a = 3 pa. The parameters of the LIF-neuron were as follows: C =.37 nf, g L = 25.4 ns, V reset = 75 mv. The system of population model equations is now defined as follows: ρ ( t U C t + ρ = ρh. (12) t ) + U t = g L (U V L ) + I a, (13) H(U(t)) = 1 τ m (A(U) + B(U, du/dt)), (14) B(U, du/dt) = dt 2 τ m dt 2 exp( T F 2 ) (T ) = π 1 + erf(t ), U T = U T (du/dt) F (T ), A(U)is given by the curve A = A(T ) in Fig.2, T = (U T U)/ 2 σ V, τ m = C/g L. The boundary conditions are ν(t) ρ(t, ) = ρhdt, (15) + U(t, ) = U reset. (16) The rates calculated by the population model for the conductance-based neurons and for the LIF-neurons compare well (Fig.6) for an individual current, but differ more if the stimulating current differs from the value, at which the LIF model was fitted to the full neuron model. 6
8 8 6 3 pa Population model, HH Population model, LIF Figure 6: Population rate for the LIF-neurons (black) in the case of stimulation by step-wise current I a = 3pA. To compare, the population rate calculated by the full model is shown in gray, which repeats the curve from Fig.4. 3 Reduction 2: Population model for LIF-neurons, excluding sub-threshold firing. The ter in the approximation of the hazard-function have different physiological meanings. Recall, that the neuron voltage curve may cross the voltage threshold as a result of fluctuations due to noise, despite the mean voltage, U, being technically sub-threshold. This effect is described by the ter A in the eq.(3) or (14). The second possibility is for a genuine threshold crossing as a result of sufficient increase of the mean voltage, U. This effect was described by the term B. Typically, the term A dominates for low amplitude stimulation, whereas B dominates for strong stimulation. To demonstrate this, we consider the population firing rate omitting the term A. The result of this calculation is presented in Fig.7. Note that the model without A underestimates peaks of population rate and also it overestimates more and more the fraction of silent, sub-threshold neurons pa Population model, LIF Population model, LIF, A= Figure 7: Population rate for the LIF-neurons without taking into account sub-threshold firing term A in the hazard-function approximation by eq.(14) (black), compared to the population rate from the model with full hazard function, shown in gray, which repeats the curve from Fig.6. 7
9 4 Reduction 3: EEG-model not distinguishing neurons by their refractory states; for LIF-neurons with fixed thresholds Use of the partial derivative equations of the models described above is computationally expensive. Hence, to model very large scale processes, such as those recorded via EEG, we choose a very strong reduction. Specifically, we do not consider the distribution of neurons by their states between spikes. Using this assumption we now have only an ordinary differential equation for the mean potential, which describes a potential never crossing the threshold. The mean membrane potential provides the information about excitability of neurons, which is used by an analog of the hazard-function. The system of equations is defined to be C du dt = g L (U V L ) + I a, (17) ν(t) = max(a(u), B(U, du/dt)), (18) (U A(U) = (τ T U)/σ V 2 ) 1 m π u 2 (1 + erf(u)) du, (19) (V reset U)/σ V 2 e [ ] 1 du B(U, du/dt) = exp ( (U T U) 2 ) 2πσV dt + 2σV 2, () τ m = C/g L, U T = const, where steady and unsteady regimes of firing are considered separately and described by the ter A and B, correspondingly. The formula for the steady-state regime was derived in [13] and can be found in [8] (see eq.(5.14)). The formula for B was derived by considering the neurons dispersed by their voltages via a Gaussian distribution about the mean, U, f G (V ) = 1 (V U)2 exp 2πσ 2σ 2 Suppose that the mean voltage, U(t), is monotonically increasing, then the number of neurons that have not yet fired, when compared to the total number of neurons is given by ρ(t) = U T g G (V ) dv In this case the firing rate is defined to be B(U(t), du/dt) = dρ dt = dρ du du dt = 1 du ( 2πσV dt exp (U T U) 2 ) When considering only non-monotonic voltage changes in the voltage rate, du/dt, we obtain the Eq.(). The comparison of this firing-rate model, which we term an EEG-model, with the population model is shown in Fig.8, 9A. The EEG-model gives the correct steady state (due to the term A in eq.(18)) and also reproduces the first population rate peak reasonably well for rather strong stimulation (due to the term B in eq.(18)). The subsequent peaks in the population rate are not reproducible by the EEG-model. 2σ 2 V 8
10 However, in the case of stronger noise, shown in Fig.9A, these subsequent peaks are decaying faster, thus the EEG-model being more precise in the case of a population with greater dispersion of neurons by their input currents or their intrinsic parameters. Concerning the implementation of the proposed kernel of EEG-model into a full model of interconnected populations, we note that the voltage for non-spiking neurons is known and thus synaptic currents could be introduced into the present model in a convenient way. That said, being based on a LIF-model, the EEG-model does not describe properties such as adaptation or refractoriness, as well as decreasing precision when varying stimulating current amplitudes different from the initial value at which the LIF-model was fitted to the conductance-based neuron. In the case of oscillating input current (Fig.9B), the EEG-model is also able to reproduce at every cycle the first population spike and subsequently the average of the following population spikes. 8 3 pa 15 5 pa 6 Population model, LIF, A= Population model, LIF EEG model, LIF 1 Population model, LIF, A= Population model, LIF EEG model, LIF Figure 8: The EEG-model based on Eqs.17- is compared to the population model based on Eqs (in grey) for two values of stimulating current amplitude. The gray curve repeats the black curve from Fig.6 and the dotted curve repeats the black curve from Fig.7. A 15 5 pa, σ V =4mV Population model, LIF EEG model, LIF B 15 8 pa, 8 Population model, LIF EEG model, LIF Figure 9: The EEG-model based on Eqs.17- is compared to the population model based on Eqs (in grey) for larger amplitude of noise, σ V current of 8pA, 8. = 4mV. A, step-wise input current; B, oscillating input 5 Conclusion When modelling neural activity at the level of EEG, particularly when considering epileptiform-like events, any model must be capable of reproducing at least two features. Firstly, the quasi-steady-state response 9
11 arising due to changes in incoming synaptic stimulation and secondly, the highly coherent responses observed when the stimulation rapidly excites large numbers of neurons. The proposed model is able to simulate these events, as is demonstrated by its comparison with the physiologically detailed population model, which in its turn is comparable with simulations of individual neurons. Consequently this model can be considered suitable for theoretical and clinical analysis of EEGs, as for example the study [2]. References [1] S.Rodrigues, J.R.Terry, M.Breakspear, On the genesis of spike-wave oscillations in a mean-field model of human thalamic and corticothalamic dynamics, Physics Letters A 355, (6). [2] M. Breakspear, J.A.Roberts, J.R.Terry, S.Rodrigues, N.Mahant and P.A.Robinson, A unifying explanation of primary generalized seizures through nonlinear brain modeling and bifurcation analysis, Cereb. Cortex 1.193/cercor/bhj75 (5). [3] P.A.Robinson, C.J.Rennie, J.J.Wright, Propagation and stability of waves of electrical activity in the cerebral cortex, Phys. Rev. E 56, (1997). [4] P.A. Robinson, C.J. Rennie, D.L.Rowe, S.C. O Connor, Estimation of multiscale neurophysiologic parameters by electroencephalographic means, Human Brain Mapping 23, (4). [5] M.N.Zhadin, Formation of rhythmic processes in the bioelectrical activity of the cerebral cortex, Biophysics 39, (1994). [6] B. Knight, Dynamics of Encoding in a Population of Neurons, Journal General Physiology (1972). [7] J. Eggert, J. L. van Hemmen, Modeling neuronal assemblies: Theory and implementation, Neural Computation 13, (1). [8] W. Gerstner, W.M. Kistler, Spiking Neuron Models Single Neurons, Populations, Plasticity, Cambridge University Press (2). [9] A.V. Chizhov, L.J. Graham, A population model of hippocampal pyramidal neurons (submitted to Phys. Rev.E 6). [1] A.V. Chizhov, L.J. Graham, A.A. Turbin, Simulation of neural population dynamics with a refractory density approach and a conductance-based threshold neuron model, Neurocomputing (in press 6). [11] A.V. Chizhov A.A. Turbin, From single-neuron models to models of neural populations, Neuroinformatics (in Russian) 1, (6). 1
12 [Graham (1999)] L. Borg-Graham, Interpretations of data and mechanis for hippocampal pyramidal cell models Cereb. Cortex 13, (1999). [12] N. Kopell, G.B. Ermentrout, M.A. Whittington, R.D. Traub, Gamma rhyth and beta rhyth have different synchronization properties, Neurobiology 97, (). [13] P.I.M. Johannesma, Diffusion models of the stochastic acticity of neurons, In Neural Networks, , Springer (Berlin), (1968). 11
Neural Modeling and Computational Neuroscience. Claudio Gallicchio
 Neural Modeling and Computational Neuroscience Claudio Gallicchio 1 Neuroscience modeling 2 Introduction to basic aspects of brain computation Introduction to neurophysiology Neural modeling: Elements
Neural Modeling and Computational Neuroscience Claudio Gallicchio 1 Neuroscience modeling 2 Introduction to basic aspects of brain computation Introduction to neurophysiology Neural modeling: Elements
Basic elements of neuroelectronics -- membranes -- ion channels -- wiring
 Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wires -- signal propagation -- processing
Consider the following spike trains from two different neurons N1 and N2:
 About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
Linearization of F-I Curves by Adaptation
 LETTER Communicated by Laurence Abbott Linearization of F-I Curves by Adaptation Bard Ermentrout Department of Mathematics, University of Pittsburgh, Pittsburgh, PA 15260, U.S.A. We show that negative
LETTER Communicated by Laurence Abbott Linearization of F-I Curves by Adaptation Bard Ermentrout Department of Mathematics, University of Pittsburgh, Pittsburgh, PA 15260, U.S.A. We show that negative
Fast neural network simulations with population density methods
 Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Biological Modeling of Neural Networks
 Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
Week 4 part 2: More Detail compartmental models Biological Modeling of Neural Networks Week 4 Reducing detail - Adding detail 4.2. Adding detail - apse -cable equat Wulfram Gerstner EPFL, Lausanne, Switzerland
University of Bristol - Explore Bristol Research. Peer reviewed version. Link to published version (if available): /j.physleta
 Rodrigues, S., Terry, JR., & Breakspear, M. (2006). On the genesis of spikewave oscillations in a mean-field model of human thalamic and corticothalamic dynamics. Physics letters A, 355, 352-357. DOI:
Rodrigues, S., Terry, JR., & Breakspear, M. (2006). On the genesis of spikewave oscillations in a mean-field model of human thalamic and corticothalamic dynamics. Physics letters A, 355, 352-357. DOI:
Neurophysiology of a VLSI spiking neural network: LANN21
 Neurophysiology of a VLSI spiking neural network: LANN21 Stefano Fusi INFN, Sezione Roma I Università di Roma La Sapienza Pza Aldo Moro 2, I-185, Roma fusi@jupiter.roma1.infn.it Paolo Del Giudice Physics
Neurophysiology of a VLSI spiking neural network: LANN21 Stefano Fusi INFN, Sezione Roma I Università di Roma La Sapienza Pza Aldo Moro 2, I-185, Roma fusi@jupiter.roma1.infn.it Paolo Del Giudice Physics
How Spike Generation Mechanisms Determine the Neuronal Response to Fluctuating Inputs
 628 The Journal of Neuroscience, December 7, 2003 23(37):628 640 Behavioral/Systems/Cognitive How Spike Generation Mechanisms Determine the Neuronal Response to Fluctuating Inputs Nicolas Fourcaud-Trocmé,
628 The Journal of Neuroscience, December 7, 2003 23(37):628 640 Behavioral/Systems/Cognitive How Spike Generation Mechanisms Determine the Neuronal Response to Fluctuating Inputs Nicolas Fourcaud-Trocmé,
6.3.4 Action potential
 I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
I ion C m C m dφ dt Figure 6.8: Electrical circuit model of the cell membrane. Normally, cells are net negative inside the cell which results in a non-zero resting membrane potential. The membrane potential
Lecture 11 : Simple Neuron Models. Dr Eileen Nugent
 Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Lecture 11 : Simple Neuron Models Dr Eileen Nugent Reading List Nelson, Biological Physics, Chapter 12 Phillips, PBoC, Chapter 17 Gerstner, Neuronal Dynamics: from single neurons to networks and models
Electrophysiology of the neuron
 School of Mathematical Sciences G4TNS Theoretical Neuroscience Electrophysiology of the neuron Electrophysiology is the study of ionic currents and electrical activity in cells and tissues. The work of
School of Mathematical Sciences G4TNS Theoretical Neuroscience Electrophysiology of the neuron Electrophysiology is the study of ionic currents and electrical activity in cells and tissues. The work of
The Spike Response Model: A Framework to Predict Neuronal Spike Trains
 The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
CORRELATION TRANSFER FROM BASAL GANGLIA TO THALAMUS IN PARKINSON S DISEASE. by Pamela Reitsma. B.S., University of Maine, 2007
 CORRELATION TRANSFER FROM BASAL GANGLIA TO THALAMUS IN PARKINSON S DISEASE by Pamela Reitsma B.S., University of Maine, 27 Submitted to the Graduate Faculty of the Department of Mathematics in partial
CORRELATION TRANSFER FROM BASAL GANGLIA TO THALAMUS IN PARKINSON S DISEASE by Pamela Reitsma B.S., University of Maine, 27 Submitted to the Graduate Faculty of the Department of Mathematics in partial
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best Koch, Chapters 6, 8, 9 See also Hodgkin and Huxley, J. Physiol. 117:500-544 (1952. (the source Clay, J. Neurophysiol. 80:903-913
All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model
 All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model S. A. Sadegh Zadeh, C. Kambhampati International Science Index, Mathematical and Computational Sciences waset.org/publication/10008281
All-or-None Principle and Weakness of Hodgkin-Huxley Mathematical Model S. A. Sadegh Zadeh, C. Kambhampati International Science Index, Mathematical and Computational Sciences waset.org/publication/10008281
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity
 Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Emergence of resonances in neural systems: the interplay between adaptive threshold and short-term synaptic plasticity Jorge F. Mejias 1,2 and Joaquín J. Torres 2 1 Department of Physics and Center for
Neuronal Dynamics: Computational Neuroscience of Single Neurons
 Week 4 part 5: Nonlinear Integrate-and-Fire Model 4.1 From Hodgkin-Huxley to 2D Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 4 Recing detail: Two-dimensional neuron models Wulfram
Week 4 part 5: Nonlinear Integrate-and-Fire Model 4.1 From Hodgkin-Huxley to 2D Neuronal Dynamics: Computational Neuroscience of Single Neurons Week 4 Recing detail: Two-dimensional neuron models Wulfram
5.4 Modelling ensembles of voltage-gated ion channels
 5.4 MODELLING ENSEMBLES 05 to as I g (Hille, 200). Gating currents tend to be much smaller than the ionic currents flowing through the membrane. In order to measure gating current, the ionic current is
5.4 MODELLING ENSEMBLES 05 to as I g (Hille, 200). Gating currents tend to be much smaller than the ionic currents flowing through the membrane. In order to measure gating current, the ionic current is
Voltage-clamp and Hodgkin-Huxley models
 Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best) Koch, Chapters 6, 8, 9 See also Clay, J. Neurophysiol. 80:903-913 (1998) (for a recent version of the HH squid axon model) Rothman
Voltage-clamp and Hodgkin-Huxley models Read: Hille, Chapters 2-5 (best) Koch, Chapters 6, 8, 9 See also Clay, J. Neurophysiol. 80:903-913 (1998) (for a recent version of the HH squid axon model) Rothman
arxiv: v1 [q-bio.nc] 13 Feb 2018
![arxiv: v1 [q-bio.nc] 13 Feb 2018 arxiv: v1 [q-bio.nc] 13 Feb 2018](/thumbs/76/73604465.jpg) Gain control with A-type potassium current: I A as a switch between divisive and subtractive inhibition Joshua H Goldwyn 1*, Bradley R Slabe 2, Joseph B Travers 3, David Terman 2 arxiv:182.4794v1 [q-bio.nc]
Gain control with A-type potassium current: I A as a switch between divisive and subtractive inhibition Joshua H Goldwyn 1*, Bradley R Slabe 2, Joseph B Travers 3, David Terman 2 arxiv:182.4794v1 [q-bio.nc]
Stochastic Oscillator Death in Globally Coupled Neural Systems
 Journal of the Korean Physical Society, Vol. 52, No. 6, June 2008, pp. 19131917 Stochastic Oscillator Death in Globally Coupled Neural Systems Woochang Lim and Sang-Yoon Kim y Department of Physics, Kangwon
Journal of the Korean Physical Society, Vol. 52, No. 6, June 2008, pp. 19131917 Stochastic Oscillator Death in Globally Coupled Neural Systems Woochang Lim and Sang-Yoon Kim y Department of Physics, Kangwon
Factors affecting phase synchronization in integrate-and-fire oscillators
 J Comput Neurosci (26) 2:9 2 DOI.7/s827-6-674-6 Factors affecting phase synchronization in integrate-and-fire oscillators Todd W. Troyer Received: 24 May 25 / Revised: 9 November 25 / Accepted: November
J Comput Neurosci (26) 2:9 2 DOI.7/s827-6-674-6 Factors affecting phase synchronization in integrate-and-fire oscillators Todd W. Troyer Received: 24 May 25 / Revised: 9 November 25 / Accepted: November
Rhythms in the gamma range (30 80 Hz) and the beta range
 Gamma rhythms and beta rhythms have different synchronization properties N. Kopell, G. B. Ermentrout, M. A. Whittington, and R. D. Traub Department of Mathematics and Center for BioDynamics, Boston University,
Gamma rhythms and beta rhythms have different synchronization properties N. Kopell, G. B. Ermentrout, M. A. Whittington, and R. D. Traub Department of Mathematics and Center for BioDynamics, Boston University,
When is an Integrate-and-fire Neuron like a Poisson Neuron?
 When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
When is an Integrate-and-fire Neuron like a Poisson Neuron? Charles F. Stevens Salk Institute MNL/S La Jolla, CA 92037 cfs@salk.edu Anthony Zador Salk Institute MNL/S La Jolla, CA 92037 zador@salk.edu
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
Synchronization in two neurons: Results for a two-component dynamical model with time-delayed inhibition
 Physica D 4 (998 47 7 Synchronization in two neurons: Results for a two-component dynamical model with time-delayed inhibition Gilles Renversez Centre de Physique Théorique, UPR 76 du Centre National de
Physica D 4 (998 47 7 Synchronization in two neurons: Results for a two-component dynamical model with time-delayed inhibition Gilles Renversez Centre de Physique Théorique, UPR 76 du Centre National de
Decoding. How well can we learn what the stimulus is by looking at the neural responses?
 Decoding How well can we learn what the stimulus is by looking at the neural responses? Two approaches: devise explicit algorithms for extracting a stimulus estimate directly quantify the relationship
Decoding How well can we learn what the stimulus is by looking at the neural responses? Two approaches: devise explicit algorithms for extracting a stimulus estimate directly quantify the relationship
STUDENT PAPER. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters Education Program 736 S. Lombard Oak Park IL, 60304
 STUDENT PAPER Differences between Stochastic and Deterministic Modeling in Real World Systems using the Action Potential of Nerves. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters
STUDENT PAPER Differences between Stochastic and Deterministic Modeling in Real World Systems using the Action Potential of Nerves. Santiago Santana University of Illinois, Urbana-Champaign Blue Waters
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten. Lecture 2a. The Neuron - overview of structure. From Anderson (1995)
 Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Introduction to Neural Networks U. Minn. Psy 5038 Spring, 1999 Daniel Kersten Lecture 2a The Neuron - overview of structure From Anderson (1995) 2 Lect_2a_Mathematica.nb Basic Structure Information flow:
Bursting Oscillations of Neurons and Synchronization
 Bursting Oscillations of Neurons and Synchronization Milan Stork Applied Electronics and Telecommunications, Faculty of Electrical Engineering/RICE University of West Bohemia, CZ Univerzitni 8, 3064 Plzen
Bursting Oscillations of Neurons and Synchronization Milan Stork Applied Electronics and Telecommunications, Faculty of Electrical Engineering/RICE University of West Bohemia, CZ Univerzitni 8, 3064 Plzen
The homogeneous Poisson process
 The homogeneous Poisson process during very short time interval Δt there is a fixed probability of an event (spike) occurring independent of what happened previously if r is the rate of the Poisson process,
The homogeneous Poisson process during very short time interval Δt there is a fixed probability of an event (spike) occurring independent of what happened previously if r is the rate of the Poisson process,
Peripheral Nerve II. Amelyn Ramos Rafael, MD. Anatomical considerations
 Peripheral Nerve II Amelyn Ramos Rafael, MD Anatomical considerations 1 Physiologic properties of the nerve Irritability of the nerve A stimulus applied on the nerve causes the production of a nerve impulse,
Peripheral Nerve II Amelyn Ramos Rafael, MD Anatomical considerations 1 Physiologic properties of the nerve Irritability of the nerve A stimulus applied on the nerve causes the production of a nerve impulse,
Dynamical systems in neuroscience. Pacific Northwest Computational Neuroscience Connection October 1-2, 2010
 Dynamical systems in neuroscience Pacific Northwest Computational Neuroscience Connection October 1-2, 2010 What do I mean by a dynamical system? Set of state variables Law that governs evolution of state
Dynamical systems in neuroscience Pacific Northwest Computational Neuroscience Connection October 1-2, 2010 What do I mean by a dynamical system? Set of state variables Law that governs evolution of state
Causality and communities in neural networks
 Causality and communities in neural networks Leonardo Angelini, Daniele Marinazzo, Mario Pellicoro, Sebastiano Stramaglia TIRES-Center for Signal Detection and Processing - Università di Bari, Bari, Italy
Causality and communities in neural networks Leonardo Angelini, Daniele Marinazzo, Mario Pellicoro, Sebastiano Stramaglia TIRES-Center for Signal Detection and Processing - Università di Bari, Bari, Italy
A Universal Model for Spike-Frequency Adaptation
 A Universal Model for Spike-Frequency Adaptation Jan Benda 1 & Andreas V. M. Herz 2 1 Department of Physics, University of Ottawa, Ottawa, Ontario, K1N 6N5, Canada j.benda@biologie.hu-berlin.de 2 Institute
A Universal Model for Spike-Frequency Adaptation Jan Benda 1 & Andreas V. M. Herz 2 1 Department of Physics, University of Ottawa, Ottawa, Ontario, K1N 6N5, Canada j.benda@biologie.hu-berlin.de 2 Institute
3.3 Simulating action potentials
 6 THE HODGKIN HUXLEY MODEL OF THE ACTION POTENTIAL Fig. 3.1 Voltage dependence of rate coefficients and limiting values and time constants for the Hodgkin Huxley gating variables. (a) Graphs of forward
6 THE HODGKIN HUXLEY MODEL OF THE ACTION POTENTIAL Fig. 3.1 Voltage dependence of rate coefficients and limiting values and time constants for the Hodgkin Huxley gating variables. (a) Graphs of forward
EEG- Signal Processing
 Fatemeh Hadaeghi EEG- Signal Processing Lecture Notes for BSP, Chapter 5 Master Program Data Engineering 1 5 Introduction The complex patterns of neural activity, both in presence and absence of external
Fatemeh Hadaeghi EEG- Signal Processing Lecture Notes for BSP, Chapter 5 Master Program Data Engineering 1 5 Introduction The complex patterns of neural activity, both in presence and absence of external
Patterns of Synchrony in Neural Networks with Spike Adaptation
 Patterns of Synchrony in Neural Networks with Spike Adaptation C. van Vreeswijky and D. Hanselz y yracah Institute of Physics and Center for Neural Computation, Hebrew University, Jerusalem, 9194 Israel
Patterns of Synchrony in Neural Networks with Spike Adaptation C. van Vreeswijky and D. Hanselz y yracah Institute of Physics and Center for Neural Computation, Hebrew University, Jerusalem, 9194 Israel
Balance of Electric and Diffusion Forces
 Balance of Electric and Diffusion Forces Ions flow into and out of the neuron under the forces of electricity and concentration gradients (diffusion). The net result is a electric potential difference
Balance of Electric and Diffusion Forces Ions flow into and out of the neuron under the forces of electricity and concentration gradients (diffusion). The net result is a electric potential difference
ACTION POTENTIAL. Dr. Ayisha Qureshi Professor MBBS, MPhil
 ACTION POTENTIAL Dr. Ayisha Qureshi Professor MBBS, MPhil DEFINITIONS: Stimulus: A stimulus is an external force or event which when applied to an excitable tissue produces a characteristic response. Subthreshold
ACTION POTENTIAL Dr. Ayisha Qureshi Professor MBBS, MPhil DEFINITIONS: Stimulus: A stimulus is an external force or event which when applied to an excitable tissue produces a characteristic response. Subthreshold
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued
 Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Mathematical Foundations of Neuroscience - Lecture 3. Electrophysiology of neurons - continued Filip Piękniewski Faculty of Mathematics and Computer Science, Nicolaus Copernicus University, Toruń, Poland
Single neuron models. L. Pezard Aix-Marseille University
 Single neuron models L. Pezard Aix-Marseille University Biophysics Biological neuron Biophysics Ionic currents Passive properties Active properties Typology of models Compartmental models Differential
Single neuron models L. Pezard Aix-Marseille University Biophysics Biological neuron Biophysics Ionic currents Passive properties Active properties Typology of models Compartmental models Differential
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests
 Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
High-conductance states in a mean-eld cortical network model
 Neurocomputing 58 60 (2004) 935 940 www.elsevier.com/locate/neucom High-conductance states in a mean-eld cortical network model Alexander Lerchner a;, Mandana Ahmadi b, John Hertz b a Oersted-DTU, Technical
Neurocomputing 58 60 (2004) 935 940 www.elsevier.com/locate/neucom High-conductance states in a mean-eld cortical network model Alexander Lerchner a;, Mandana Ahmadi b, John Hertz b a Oersted-DTU, Technical
This script will produce a series of pulses of amplitude 40 na, duration 1ms, recurring every 50 ms.
 9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
9.16 Problem Set #4 In the final problem set you will combine the pieces of knowledge gained in the previous assignments to build a full-blown model of a plastic synapse. You will investigate the effects
Phase Response Properties and Phase-Locking in Neural Systems with Delayed Negative-Feedback. Carter L. Johnson
 Phase Response Properties and Phase-Locking in Neural Systems with Delayed Negative-Feedback Carter L. Johnson Faculty Mentor: Professor Timothy J. Lewis University of California, Davis Abstract Oscillatory
Phase Response Properties and Phase-Locking in Neural Systems with Delayed Negative-Feedback Carter L. Johnson Faculty Mentor: Professor Timothy J. Lewis University of California, Davis Abstract Oscillatory
Naseem Demeri. Mohammad Alfarra. Mohammad Khatatbeh
 7 Naseem Demeri Mohammad Alfarra Mohammad Khatatbeh In the previous lectures, we have talked about how the difference in permeability for ions across the cell membrane can generate a potential. The potential
7 Naseem Demeri Mohammad Alfarra Mohammad Khatatbeh In the previous lectures, we have talked about how the difference in permeability for ions across the cell membrane can generate a potential. The potential
Problem Set Number 02, j/2.036j MIT (Fall 2018)
 Problem Set Number 0, 18.385j/.036j MIT (Fall 018) Rodolfo R. Rosales (MIT, Math. Dept., room -337, Cambridge, MA 0139) September 6, 018 Due October 4, 018. Turn it in (by 3PM) at the Math. Problem Set
Problem Set Number 0, 18.385j/.036j MIT (Fall 018) Rodolfo R. Rosales (MIT, Math. Dept., room -337, Cambridge, MA 0139) September 6, 018 Due October 4, 018. Turn it in (by 3PM) at the Math. Problem Set
IN THIS turorial paper we exploit the relationship between
 508 IEEE TRANSACTIONS ON NEURAL NETWORKS, VOL. 10, NO. 3, MAY 1999 Weakly Pulse-Coupled Oscillators, FM Interactions, Synchronization, Oscillatory Associative Memory Eugene M. Izhikevich Abstract We study
508 IEEE TRANSACTIONS ON NEURAL NETWORKS, VOL. 10, NO. 3, MAY 1999 Weakly Pulse-Coupled Oscillators, FM Interactions, Synchronization, Oscillatory Associative Memory Eugene M. Izhikevich Abstract We study
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons
 Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Computing with Inter-spike Interval Codes in Networks of Integrate and Fire Neurons Dileep George a,b Friedrich T. Sommer b a Dept. of Electrical Engineering, Stanford University 350 Serra Mall, Stanford,
Reduction of Conductance Based Models with Slow Synapses to Neural Nets
 Reduction of Conductance Based Models with Slow Synapses to Neural Nets Bard Ermentrout October 1, 28 Abstract The method of averaging and a detailed bifurcation calculation are used to reduce a system
Reduction of Conductance Based Models with Slow Synapses to Neural Nets Bard Ermentrout October 1, 28 Abstract The method of averaging and a detailed bifurcation calculation are used to reduce a system
Fast and exact simulation methods applied on a broad range of neuron models
 Fast and exact simulation methods applied on a broad range of neuron models Michiel D Haene michiel.dhaene@ugent.be Benjamin Schrauwen benjamin.schrauwen@ugent.be Ghent University, Electronics and Information
Fast and exact simulation methods applied on a broad range of neuron models Michiel D Haene michiel.dhaene@ugent.be Benjamin Schrauwen benjamin.schrauwen@ugent.be Ghent University, Electronics and Information
Synchrony in Neural Systems: a very brief, biased, basic view
 Synchrony in Neural Systems: a very brief, biased, basic view Tim Lewis UC Davis NIMBIOS Workshop on Synchrony April 11, 2011 components of neuronal networks neurons synapses connectivity cell type - intrinsic
Synchrony in Neural Systems: a very brief, biased, basic view Tim Lewis UC Davis NIMBIOS Workshop on Synchrony April 11, 2011 components of neuronal networks neurons synapses connectivity cell type - intrinsic
Computational Explorations in Cognitive Neuroscience Chapter 2
 Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
Computational Explorations in Cognitive Neuroscience Chapter 2 2.4 The Electrophysiology of the Neuron Some basic principles of electricity are useful for understanding the function of neurons. This is
Nervous Lecture Test Questions Set 2
 Nervous Lecture Test Questions Set 2 1. The role of chloride in a resting membrane potential: a. creates resting potential b. indirectly causes repolarization c. stabilization of sodium d. it has none,
Nervous Lecture Test Questions Set 2 1. The role of chloride in a resting membrane potential: a. creates resting potential b. indirectly causes repolarization c. stabilization of sodium d. it has none,
Topics in Neurophysics
 Topics in Neurophysics Alex Loebel, Martin Stemmler and Anderas Herz Exercise 2 Solution (1) The Hodgkin Huxley Model The goal of this exercise is to simulate the action potential according to the model
Topics in Neurophysics Alex Loebel, Martin Stemmler and Anderas Herz Exercise 2 Solution (1) The Hodgkin Huxley Model The goal of this exercise is to simulate the action potential according to the model
Controllingthe spikingactivity in excitable membranes via poisoning
 Physica A 344 (2004) 665 670 www.elsevier.com/locate/physa Controllingthe spikingactivity in excitable membranes via poisoning Gerhard Schmid, Igor Goychuk, Peter Hanggi Universitat Augsburg, Institut
Physica A 344 (2004) 665 670 www.elsevier.com/locate/physa Controllingthe spikingactivity in excitable membranes via poisoning Gerhard Schmid, Igor Goychuk, Peter Hanggi Universitat Augsburg, Institut
Synaptic dynamics. John D. Murray. Synaptic currents. Simple model of the synaptic gating variable. First-order kinetics
 Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Synaptic dynamics John D. Murray A dynamical model for synaptic gating variables is presented. We use this to study the saturation of synaptic gating at high firing rate. Shunting inhibition and the voltage
Evaluating Bistability in a Mathematical Model of Circadian Pacemaker Neurons
 International Journal of Theoretical and Mathematical Physics 2016, 6(3): 99-103 DOI: 10.5923/j.ijtmp.20160603.02 Evaluating Bistability in a Mathematical Model of Circadian Pacemaker Neurons Takaaki Shirahata
International Journal of Theoretical and Mathematical Physics 2016, 6(3): 99-103 DOI: 10.5923/j.ijtmp.20160603.02 Evaluating Bistability in a Mathematical Model of Circadian Pacemaker Neurons Takaaki Shirahata
Action Potential Initiation in the Hodgkin-Huxley Model
 Action Potential Initiation in the Hodgkin-Huxley Model The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version
Action Potential Initiation in the Hodgkin-Huxley Model The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version
Dynamical Constraints on Computing with Spike Timing in the Cortex
 Appears in Advances in Neural Information Processing Systems, 15 (NIPS 00) Dynamical Constraints on Computing with Spike Timing in the Cortex Arunava Banerjee and Alexandre Pouget Department of Brain and
Appears in Advances in Neural Information Processing Systems, 15 (NIPS 00) Dynamical Constraints on Computing with Spike Timing in the Cortex Arunava Banerjee and Alexandre Pouget Department of Brain and
Activity Driven Adaptive Stochastic. Resonance. Gregor Wenning and Klaus Obermayer. Technical University of Berlin.
 Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Activity Driven Adaptive Stochastic Resonance Gregor Wenning and Klaus Obermayer Department of Electrical Engineering and Computer Science Technical University of Berlin Franklinstr. 8/9, 187 Berlin fgrewe,obyg@cs.tu-berlin.de
Topological target patterns and population oscillations in a network with random gap junctional coupling
 0 0 0 0 Neurocomputing }0 (00) } Topological target patterns and population oscillations in a network with random gap junctional coupling Timothy J. Lewis*, John Rinzel Center for Neural Science and Courant
0 0 0 0 Neurocomputing }0 (00) } Topological target patterns and population oscillations in a network with random gap junctional coupling Timothy J. Lewis*, John Rinzel Center for Neural Science and Courant
Dynamic Modeling of Brain Activity
 0a Dynamic Modeling of Brain Activity EIN IIN PC Thomas R. Knösche, Leipzig Generative Models for M/EEG 4a Generative Models for M/EEG states x (e.g. dipole strengths) parameters parameters (source positions,
0a Dynamic Modeling of Brain Activity EIN IIN PC Thomas R. Knösche, Leipzig Generative Models for M/EEG 4a Generative Models for M/EEG states x (e.g. dipole strengths) parameters parameters (source positions,
Model of Motor Neural Circuit of C. elegans
 Model of Motor Neural Circuit of C. elegans Different models for oscillators There are several different models to generate oscillations. Among these models, some have different values for the same parameters,
Model of Motor Neural Circuit of C. elegans Different models for oscillators There are several different models to generate oscillations. Among these models, some have different values for the same parameters,
Neocortical Pyramidal Cells Can Control Signals to Post-Synaptic Cells Without Firing:
 Neocortical Pyramidal Cells Can Control Signals to Post-Synaptic Cells Without Firing: a model of the axonal plexus Erin Munro Department of Mathematics Boston University 4/14/2011 Gap junctions on pyramidal
Neocortical Pyramidal Cells Can Control Signals to Post-Synaptic Cells Without Firing: a model of the axonal plexus Erin Munro Department of Mathematics Boston University 4/14/2011 Gap junctions on pyramidal
Population dynamics of interacting spiking neurons
 Population dynamics of interacting spiking neurons Maurizio Mattia* Physics Laboratory, Istituto Superiore di Sanità, INFN - Gr. Coll. Roma I, V.le Regina Elena 299, 00161 Roma, Italy Paolo Del Giudice
Population dynamics of interacting spiking neurons Maurizio Mattia* Physics Laboratory, Istituto Superiore di Sanità, INFN - Gr. Coll. Roma I, V.le Regina Elena 299, 00161 Roma, Italy Paolo Del Giudice
1 Hodgkin-Huxley Theory of Nerve Membranes: The FitzHugh-Nagumo model
 1 Hodgkin-Huxley Theory of Nerve Membranes: The FitzHugh-Nagumo model Alan Hodgkin and Andrew Huxley developed the first quantitative model of the propagation of an electrical signal (the action potential)
1 Hodgkin-Huxley Theory of Nerve Membranes: The FitzHugh-Nagumo model Alan Hodgkin and Andrew Huxley developed the first quantitative model of the propagation of an electrical signal (the action potential)
DISCRETE EVENT SIMULATION IN THE NEURON ENVIRONMENT
 Hines and Carnevale: Discrete event simulation in the NEURON environment Page 1 Preprint of a manuscript that will be published in Neurocomputing. DISCRETE EVENT SIMULATION IN THE NEURON ENVIRONMENT Abstract
Hines and Carnevale: Discrete event simulation in the NEURON environment Page 1 Preprint of a manuscript that will be published in Neurocomputing. DISCRETE EVENT SIMULATION IN THE NEURON ENVIRONMENT Abstract
Comparing integrate-and-fire models estimated using intracellular and extracellular data 1
 Comparing integrate-and-fire models estimated using intracellular and extracellular data 1 Liam Paninski a,b,2 Jonathan Pillow b Eero Simoncelli b a Gatsby Computational Neuroscience Unit, University College
Comparing integrate-and-fire models estimated using intracellular and extracellular data 1 Liam Paninski a,b,2 Jonathan Pillow b Eero Simoncelli b a Gatsby Computational Neuroscience Unit, University College
Spike-Frequency Adaptation of a Generalized Leaky Integrate-and-Fire Model Neuron
 Journal of Computational Neuroscience 10, 25 45, 2001 c 2001 Kluwer Academic Publishers. Manufactured in The Netherlands. Spike-Frequency Adaptation of a Generalized Leaky Integrate-and-Fire Model Neuron
Journal of Computational Neuroscience 10, 25 45, 2001 c 2001 Kluwer Academic Publishers. Manufactured in The Netherlands. Spike-Frequency Adaptation of a Generalized Leaky Integrate-and-Fire Model Neuron
Physiology Unit 2. MEMBRANE POTENTIALS and SYNAPSES
 Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
Physiology Unit 2 MEMBRANE POTENTIALS and SYNAPSES Neuron Communication Neurons are stimulated by receptors on dendrites and cell bodies (soma) Ligand gated ion channels GPCR s Neurons stimulate cells
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS
 AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
AT2 Neuromodeling: Problem set #3 SPIKE TRAINS Younesse Kaddar PROBLEM 1: Poisson spike trains Link of the ipython notebook for the code Brain neuron emit spikes seemingly randomly: we will aim to model
Conductance-Based Integrate-and-Fire Models
 NOTE Communicated by Michael Hines Conductance-Based Integrate-and-Fire Models Alain Destexhe Department of Physiology, Laval University School of Medicine, Québec, G1K 7P4, Canada A conductance-based
NOTE Communicated by Michael Hines Conductance-Based Integrate-and-Fire Models Alain Destexhe Department of Physiology, Laval University School of Medicine, Québec, G1K 7P4, Canada A conductance-based
Bursting and Chaotic Activities in the Nonlinear Dynamics of FitzHugh-Rinzel Neuron Model
 Bursting and Chaotic Activities in the Nonlinear Dynamics of FitzHugh-Rinzel Neuron Model Abhishek Yadav *#, Anurag Kumar Swami *, Ajay Srivastava * * Department of Electrical Engineering, College of Technology,
Bursting and Chaotic Activities in the Nonlinear Dynamics of FitzHugh-Rinzel Neuron Model Abhishek Yadav *#, Anurag Kumar Swami *, Ajay Srivastava * * Department of Electrical Engineering, College of Technology,
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 2, W.W. Lytton, Hodgkin-Huxley Model) 9. Passive and active membrane models In the previous lecture we have considered a passive
Title. Author(s)Fujii, Hiroshi; Tsuda, Ichiro. CitationNeurocomputing, 58-60: Issue Date Doc URL. Type.
 Title Neocortical gap junction-coupled interneuron systems exhibiting transient synchrony Author(s)Fujii, Hiroshi; Tsuda, Ichiro CitationNeurocomputing, 58-60: 151-157 Issue Date 2004-06 Doc URL http://hdl.handle.net/2115/8488
Title Neocortical gap junction-coupled interneuron systems exhibiting transient synchrony Author(s)Fujii, Hiroshi; Tsuda, Ichiro CitationNeurocomputing, 58-60: 151-157 Issue Date 2004-06 Doc URL http://hdl.handle.net/2115/8488
Frequency Adaptation and Bursting
 BioE332A Lab 3, 2010 1 Lab 3 January 5, 2010 Frequency Adaptation and Bursting In the last lab, we explored spiking due to sodium channels. In this lab, we explore adaptation and bursting due to potassium
BioE332A Lab 3, 2010 1 Lab 3 January 5, 2010 Frequency Adaptation and Bursting In the last lab, we explored spiking due to sodium channels. In this lab, we explore adaptation and bursting due to potassium
Liquid Computing in a Simplified Model of Cortical Layer IV: Learning to Balance a Ball
 Liquid Computing in a Simplified Model of Cortical Layer IV: Learning to Balance a Ball Dimitri Probst 1,3, Wolfgang Maass 2, Henry Markram 1, and Marc-Oliver Gewaltig 1 1 Blue Brain Project, École Polytechnique
Liquid Computing in a Simplified Model of Cortical Layer IV: Learning to Balance a Ball Dimitri Probst 1,3, Wolfgang Maass 2, Henry Markram 1, and Marc-Oliver Gewaltig 1 1 Blue Brain Project, École Polytechnique
Event-driven simulations of nonlinear integrate-and-fire neurons
 Event-driven simulations of nonlinear integrate-and-fire neurons A. Tonnelier, H. Belmabrouk, D. Martinez Cortex Project, LORIA, Campus Scientifique, B.P. 239, 54 56 Vandoeuvre-lès-Nancy, France Abstract
Event-driven simulations of nonlinear integrate-and-fire neurons A. Tonnelier, H. Belmabrouk, D. Martinez Cortex Project, LORIA, Campus Scientifique, B.P. 239, 54 56 Vandoeuvre-lès-Nancy, France Abstract
Fokker-Planck Solution for a Neuronal Spiking Model Derek J. Daniel a a
 This article was downloaded by: [Montana State University] On: 31 March 2010 Access details: Access Details: [subscription number 790568798] Publisher Taylor & Francis Informa Ltd Registered in England
This article was downloaded by: [Montana State University] On: 31 March 2010 Access details: Access Details: [subscription number 790568798] Publisher Taylor & Francis Informa Ltd Registered in England
Membrane equation. VCl. dv dt + V = V Na G Na + V K G K + V Cl G Cl. G total. C m. G total = G Na + G K + G Cl
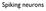 Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Spiking neurons Membrane equation V GNa GK GCl Cm VNa VK VCl dv dt + V = V Na G Na + V K G K + V Cl G Cl G total G total = G Na + G K + G Cl = C m G total Membrane with synaptic inputs V Gleak GNa GK
Approximate, not perfect synchrony maximizes the downstream effectiveness of excitatory neuronal ensembles
 Börgers et al. RESEARCH Approximate, not perfect synchrony maximizes the downstream effectiveness of excitatory neuronal ensembles Christoph Börgers *, Jie Li and Nancy Kopell 2 * Correspondence: cborgers@tufts.edu
Börgers et al. RESEARCH Approximate, not perfect synchrony maximizes the downstream effectiveness of excitatory neuronal ensembles Christoph Börgers *, Jie Li and Nancy Kopell 2 * Correspondence: cborgers@tufts.edu
Signal processing in nervous system - Hodgkin-Huxley model
 Signal processing in nervous system - Hodgkin-Huxley model Ulrike Haase 19.06.2007 Seminar "Gute Ideen in der theoretischen Biologie / Systembiologie" Signal processing in nervous system Nerve cell and
Signal processing in nervous system - Hodgkin-Huxley model Ulrike Haase 19.06.2007 Seminar "Gute Ideen in der theoretischen Biologie / Systembiologie" Signal processing in nervous system Nerve cell and
80% of all excitatory synapses - at the dendritic spines.
 Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Dendritic Modelling Dendrites (from Greek dendron, tree ) are the branched projections of a neuron that act to conduct the electrical stimulation received from other cells to and from the cell body, or
Basic elements of neuroelectronics -- membranes -- ion channels -- wiring. Elementary neuron models -- conductance based -- modelers alternatives
 Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wiring neurons together -- synapses
Computing in carbon Basic elements of neuroelectronics -- membranes -- ion channels -- wiring Elementary neuron models -- conductance based -- modelers alternatives Wiring neurons together -- synapses
! Depolarization continued. AP Biology. " The final phase of a local action
 ! Resting State Resting potential is maintained mainly by non-gated K channels which allow K to diffuse out! Voltage-gated ion K and channels along axon are closed! Depolarization A stimulus causes channels
! Resting State Resting potential is maintained mainly by non-gated K channels which allow K to diffuse out! Voltage-gated ion K and channels along axon are closed! Depolarization A stimulus causes channels
CSE/NB 528 Final Lecture: All Good Things Must. CSE/NB 528: Final Lecture
 CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
Chapter 24 BIFURCATIONS
 Chapter 24 BIFURCATIONS Abstract Keywords: Phase Portrait Fixed Point Saddle-Node Bifurcation Diagram Codimension-1 Hysteresis Hopf Bifurcation SNIC Page 1 24.1 Introduction In linear systems, responses
Chapter 24 BIFURCATIONS Abstract Keywords: Phase Portrait Fixed Point Saddle-Node Bifurcation Diagram Codimension-1 Hysteresis Hopf Bifurcation SNIC Page 1 24.1 Introduction In linear systems, responses
Modulation ofexcitatory synaptic coupling facilitates synchronization and complex dynamics in a nonlinear model ofneuronal dynamics
 Neurocomputing 52 54 (23) 151 158 www.elsevier.com/locate/neucom Modulation ofexcitatory synaptic coupling facilitates synchronization and complex dynamics in a nonlinear model ofneuronal dynamics Michael
Neurocomputing 52 54 (23) 151 158 www.elsevier.com/locate/neucom Modulation ofexcitatory synaptic coupling facilitates synchronization and complex dynamics in a nonlinear model ofneuronal dynamics Michael
9 Generation of Action Potential Hodgkin-Huxley Model
 9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
9 Generation of Action Potential Hodgkin-Huxley Model (based on chapter 12, W.W. Lytton, Hodgkin-Huxley Model) 9.1 Passive and active membrane models In the previous lecture we have considered a passive
COGNITIVE SCIENCE 107A
 COGNITIVE SCIENCE 107A Electrophysiology: Electrotonic Properties 2 Jaime A. Pineda, Ph.D. The Model Neuron Lab Your PC/CSB115 http://cogsci.ucsd.edu/~pineda/cogs107a/index.html Labs - Electrophysiology
COGNITIVE SCIENCE 107A Electrophysiology: Electrotonic Properties 2 Jaime A. Pineda, Ph.D. The Model Neuron Lab Your PC/CSB115 http://cogsci.ucsd.edu/~pineda/cogs107a/index.html Labs - Electrophysiology
A model of slow plateau-like oscillations based upon the fast Na current in a window mode
 Neurocomputing 38}40 (2001) 159}166 A model of slow plateau-like oscillations based upon the fast Na current in a window mode G.S. Cymbalyuk*, R.L. Calabrese Biology Department, Emory University, 1510
Neurocomputing 38}40 (2001) 159}166 A model of slow plateau-like oscillations based upon the fast Na current in a window mode G.S. Cymbalyuk*, R.L. Calabrese Biology Department, Emory University, 1510
Neuronal Dynamics: Computational Neuroscience of Single Neurons
 Nonlinear Integrate-and-Fire Model Neronal Dynamics: Comptational Neroscience of Single Nerons Nonlinear Integrate-and-fire (NLIF) - Definition - qadratic and expon. IF - Extracting NLIF model from data
Nonlinear Integrate-and-Fire Model Neronal Dynamics: Comptational Neroscience of Single Nerons Nonlinear Integrate-and-fire (NLIF) - Definition - qadratic and expon. IF - Extracting NLIF model from data
The Axonal Plexus. A description of the behavior of a network of neurons connected by gap junctions. Erin Munro, 4/4/2007
 The Axonal Plexus A description of the behavior of a network of neurons connected by gap junctions Erin Munro, 4/4/2007 Why are we interested in studying the axonal plexus? Gap junctions are indicated
The Axonal Plexus A description of the behavior of a network of neurons connected by gap junctions Erin Munro, 4/4/2007 Why are we interested in studying the axonal plexus? Gap junctions are indicated
MANY scientists believe that pulse-coupled neural networks
 IEEE TRANSACTIONS ON NEURAL NETWORKS, VOL. 10, NO. 3, MAY 1999 499 Class 1 Neural Excitability, Conventional Synapses, Weakly Connected Networks, and Mathematical Foundations of Pulse-Coupled Models Eugene
IEEE TRANSACTIONS ON NEURAL NETWORKS, VOL. 10, NO. 3, MAY 1999 499 Class 1 Neural Excitability, Conventional Synapses, Weakly Connected Networks, and Mathematical Foundations of Pulse-Coupled Models Eugene
Self-organized Criticality and Synchronization in a Pulse-coupled Integrate-and-Fire Neuron Model Based on Small World Networks
 Commun. Theor. Phys. (Beijing, China) 43 (2005) pp. 466 470 c International Academic Publishers Vol. 43, No. 3, March 15, 2005 Self-organized Criticality and Synchronization in a Pulse-coupled Integrate-and-Fire
Commun. Theor. Phys. (Beijing, China) 43 (2005) pp. 466 470 c International Academic Publishers Vol. 43, No. 3, March 15, 2005 Self-organized Criticality and Synchronization in a Pulse-coupled Integrate-and-Fire
Single-Compartment Neural Models
 Single-Compartment Neural Models BENG/BGGN 260 Neurodynamics University of California, San Diego Week 2 BENG/BGGN 260 Neurodynamics (UCSD) Single-Compartment Neural Models Week 2 1 / 18 Reading Materials
Single-Compartment Neural Models BENG/BGGN 260 Neurodynamics University of California, San Diego Week 2 BENG/BGGN 260 Neurodynamics (UCSD) Single-Compartment Neural Models Week 2 1 / 18 Reading Materials
Lecture IV: LTI models of physical systems
 BME 171: Signals and Systems Duke University September 5, 2008 This lecture Plan for the lecture: 1 Interconnections of linear systems 2 Differential equation models of LTI systems 3 eview of linear circuit
BME 171: Signals and Systems Duke University September 5, 2008 This lecture Plan for the lecture: 1 Interconnections of linear systems 2 Differential equation models of LTI systems 3 eview of linear circuit
