Intent. a little geometry of eigenvectors, two-dimensional example. classical analog of QM, briefly
|
|
|
- Gordon Hopkins
- 5 years ago
- Views:
Transcription
1 Intent a little geometry of eigenvectors, two-dimensional example classical analog of QM, briefly review of the underpinnings of QM, emphasizing Hilbert space operators with some simplifying assumptions??? sketch of the QM of a particle moving on the x-axis, again emphasizing Hilbert space operators
2 "Eigenomety" T :V "V a linear transformation " an eigenvalue with eigenvector v " Tv)= "v L={ "v : " a complex number } " TL)=L L is an invariant line for T )
3 Example T = 5 "1 "1 5 $ " = 4 1 " = 6 eigenvalues; v 1= 1 $ $ 1 u 1= v 1 v 1 " 1 = $ 1 $ 1 u = v " v 1 1 = $ "1 v = 1 eigenvectors "1 $ unit eigenvectors L = k { " u k : " a complex number } are invariant lines
4 Diagonalization: change of basis/coordinate system W = 1 $ 1 "1 1 1 unitary W "1 =W * "1 W = 1 $ 1 1 "1 1 Wv = v W e k )= v k W "1 TW = 4 0 "1 = D or D=WTW 0 6 $ R= rotation through angle " = cos" sin" $ sin" cos" ) )
5 The Classical System 1 particle, 1 degree of freedom, v= dx dt <<<c interaction of particle with environment described by force Fx) or potential Vx) [Fx)" d dx Vx)] Examples 1. Fx) = -kx " Vx) = " kx dx = k x +C. Vx)= k x " Fx)= d dx " $ $ k x = k x 3. F moves particle in direction that decreases V 4. V minimum " stable equilibrium V maximum " unstable equilibrium
6 How does the state of the system evolve with time? state " xt),pt), where pt) = m vt) $ x p have precise, well-defined values at every instant, and measurement does not disturb the system Object: given F = m a, determine xt) pt). $ dx d t = Fx) m =" dv dx m Examples 1. Fx) = k or Vx) = k x " xt)= k m t + C 1 t+ C pt)= kt+mc. Fx) = kx with x = 0 v= v 0, when t = 0 " xt)= v 0 " sin k m t) pt)= v 0 " kmcos k m t)
7 How does energy enter the picture? Calculate work W = " x1 x Fx)dx in two different ways: x dv x dv = "Vx) x1 x = Vx 1 ) Vx ) 1. W = " x1 dx dx = W = " x 1. W = " x1 x m x m dv dt dx = " dv x 1 dx v dx dt dx = " v mvdv= mv = mv v 1 v1 Equating 1.. " mv +Vx ) mv 1 = +Vx 1) x 1 x arbitrary " E " mv mv 1 +Vx) is constant particle only moves in region where Vx) < E E Vx) "measures" speed slope of Vx) "measures" direction and magnitude of F Hamiltonian formulation: "H "p = p m "H "x = dv dx Hx,p)" p m +Vx) " dx dt = "p " Hx,p) dp dt =" x Hx,p)
8 The Quantum System Axiom 1. States = { unit vectors in Hilbert Space H } With c = 1 "x) c"x) represent the same state. embody all knowable information about the system x is just a dummy variable Axiom. Observables = { self adjoint operators on H } may be unbounded; may have no eigenvalues position: M x "x) = x "x) momentum: P"x) = ih d" dx h" x joule sec) product rule " M x P PM x = ihi Note Classical states and observables are "joint," and they both depend on time. Quantum states and observables are distinct entities, and observables do not depend on time.
9 Axiom 3. The strongest predictive statement about the measurement of observable A, when the system is in state ", is probalistic: Eϕ ) = expected value of measurement of A = A"," A has an eigenbasis { e i } with A e i = " i e i, i = 1,,3,.... " Ee i ) = Ae i,e i = " i " = " c i e i " E" ) = i=1 " " i c i c i = ",e i ) i=1 " Probmeasuring A yields specific " k ) = c k " Probmeasuring A yields some " i ) = 1 If A has no eigenvalues, it still has a spectral representation: "dr"), RS)s are projections in BH ) for all measurable subsets of, S, of R. f "H " RS)f = " S f. Probmeasure of A between a and b) = Ra,b)" Unpredictability is inherent in the measurement process. What does this say about "discreteness of possible measurement values.
10 An aside: Why is the momentum operator P"x) = ih d" dx? Bohr 1913) circular orbit model of hydrogen atom F = Q r xt),yt))=rcos"t),rsin"t)) a =" r Newtons nd Law " m" r = Q r " " = Q mr 3 " p= mq r " angular momentum = J = mq r J "quantized" " J = nh " r n = n h mq and electron can move from one state to another, emitting photon with frequency = 1 h E " E ) n m
11 de Broglie 194) electron represented by a wave p= hk k = angular frequency of wave)! orbit consists of integer periods " "r = n " k!! h r " "r = n" = "nh p mq! " r = Bohr radii!! momentum is encoded in the oscillations of the wave function????
12 Suppose state of system is "x)= eikx " L [0,"]), and that it is an eigenvector of the momentum operator, P. Then Pϕ) = " k ϕ and should be " k = p= hk with probability 1. P= ih d dx uniquely does the trick! prob p= hk ) = P"," $ = P" " dx 0 e ikx = $ "ih d ) e "ikx )dx 0 dx $ = "ihik eikx ) e "ikx )dx 0 = hk " " 1) dx 0 = hk " ") hk $ $
13 Axiom 4. "Collapse" of the wave function The act of measurement causes an uncontrollable, immediate change in the system. Suppose that the system is in state ". Measurement of A yields a value ". Immediately after measurement the system will be in a possibly new state, ". A has an eigenbasis { e i } with A e i = " i e i, i = 1,,3,... " " immediately after measurement system will be in state " with A " =$ "; i.e. " =e k and " = " k for some k. A has no eigenvalues and a<" <b " immediately after measurement system will be in state Ra,b)" Ra,b)" Following the measurement, the state of the system will evolve in the "usual" way. A significant time later, the system will probably no longer be in state ".
14 Another aside: uncertainty, commutativity simultaneity Some statistics O an event with possible outcomes {o k } and probo k ) = p k EVO) = expected value of O = p k "o k "O = rms deviation of O = " p k o k EVO)) easy calculation " "O= EVO ) [EVO)] For observable operator A and state vector ϕ, uncertainty is defined by "A) = A A, I), and EVA) = A",". calculation " "A= EVA ) [EVA)] ΔA is called the uncertainty in A in the state ϕ.
15 Uncertainty Principle: AB BA = ci " ΔA ΔB 0.5 c If uncertainty in measurements of A decrease, then uncertainty in measurements of B must increase.) Proof. 1) If A and B are self adjoint, then A = A EVA)I B = B EVB)I are self adjoint, also. ) AB BA = AB BA 3) A",A" =A) and B",B" =B) 4) [AB BA]"," = [AB BA]"," = AB"," BA"," = B",A" A",B" = B",A" B",A" = iim B",A" So [AB BA]"," = Im B",A" B",A". CBS " [AB BA]"," B" $ A" = B",B" $ A",A" 3) " [AB BA]"," $B$A " ci"," = c "," = c $B$A ΔA = 0 " ϕ must be eigenvector measurement certain.
16 Position-Momentum Uncertainty Relation M x P PM x = ih " "M x "P$ h Compatibility Theorem A and B observables) A and B are simultaneously measurable compatible) " A and B have common eigenbasis " A and B commute Proof. Heuristically, why should this be true? If A measured, then B measured, then A measured again, all in rapid succession with no significant time evolution, the second measurement of A is certain to coincide with the first one.
17 Wave-Particle Duality " µ x)= 0 x µ $ x =µ eigenvectors of position M x "x)= x"x) spatially localized " PARTICLE " x)= e ix/h $ = cos x $ ) +i*sin x ) e.v.s of momentum P="ih d h h dx periodic with period "h " WAVE Cant be both wave and particle at the same time. Think about uncertainty relation and compatibility theorem. Problems: eigenvalues continuously distributed over the all reals. For all real a and b with a"µ"b, the integral of the Dirac delta b function is defined to be " µ x)dx = 1. a " = "," = $ e ix/h dx = $ 1dx = " needs to be normalized, but I dont know how.
18 Axiom 5. Time evolution/hamiltonian/schrödinger eq. d" dt = 1 ih H" where H" = momentum) " + potential)" m = m 1 P "+VM x )" = h m d " dx +Vx)" H behaves the way its supposed to. For example, " 0 =1 " " t =1 for all t > 0. Proof. Note that d dt "," " = t t t t," + " t t, " t write inner t product in its integral form, use product rule, and remember that integration wrt to x is independent of t). d" t dt = i h H" t " d dt " t = d dt " t," t = i h " t," t $ = i h H" t," t " t,h" t + " t, i h " t = 0, since H is self adjoint. ) Therefore, " t is constant.
19 Operator formulation doesnt make QM any easier. discover or invent appropriate H for given system solve Schrödinger eq. subject to " =" 0 when t = 0!!!!! How can we ever know " 0? "Prepare the system." Measure some observable A. System will be forced into eigenstate " k for A: " 0 = k. System will evolve according S.s eq. until another measurement is made. energy eigenvalue equation/time-independent S. equation: h m d dx " x) $ k +Vx)) " $ k x)= E k " k x) state time-evolution equation/time-dependent S. equation: h m d dx " x) $ t +Vx)) " x) $ t =ih *t * " x) t solve eigenvalue equation " can write down general sol. to time-evolution equation, given " t 0)
20 Example: Vx) = 0.5kx harmonic oscillator) eigenvalues of H = E k =k + 1 )h" k = 0, 1,, 3,... and " = k/m eigenvectors of H = " k x) = complicated exponential function of x
21 Time-evolution operators: Ut)" 0 x)=" t x) different Ut) for each " 0 Ut + s) = Ut)Us) U0) = I " {Ut)} is 1-param group think Stones Theorem) substitute into S.s eq. " ih "Ut) "t 0 x)= HUt) 0 x) want true for all " 0, so want ih "Ut) = HUt) with U0)= I "t solution of this operator d.e. is Ut)= e "iht/h Ut)s are linear operators, but not self adjoint, so not observables Why? Ut) = power series in H " e.v.s of Ut) are e "ie nt/h, where E n s are e.v.s of H, and these exps are not real.)
22 Time-Energy Uncertainty Relation The evolution time of observable A, T A, is defined by "A t T A deva) t dt T A is the time which must elapse before the average of the values measured for A in a series of repeated measurements, EVA) t, changes or evolves enough to be noticeable over the intrinsic spread in these values, "A t. T A "H t " h Proof. First, note that d dt EVA) t = i h " t,ha AH)" t. Then apply the Heisenberg Uncertainty Principle. The more precisely energy is defined the smaller "H t ) the slower any observable will change noticeably the larger T A ). If observable exhibits rapid variation, then system cannot have well-defined energy. If A commutes with H, then both EVA) t and "A t are constant in time. In particular, "H t and EVH) t are always constant in time.
23 Example: measure energy of electron in a hydrogen atom H has eigenvalues R n Suppose electron in state with energy R n1 Eventually decays into state with energy R n n <n 1 ) Photon emitted with energy E photon = R n R n1 = "E electron Frequency of photon " E photon which can be measured Can determine n 1 and n Do for many identically prepared electrons " get many frequencies/energies and probabilities look like Axiom 3. R = Rydberg constant = R= m eq 4 h
A few principles of classical and quantum mechanics
 A few principles of classical and quantum mechanics The classical approach: In classical mechanics, we usually (but not exclusively) solve Newton s nd law of motion relating the acceleration a of the system
A few principles of classical and quantum mechanics The classical approach: In classical mechanics, we usually (but not exclusively) solve Newton s nd law of motion relating the acceleration a of the system
Quantum Mechanics Solutions
 Quantum Mechanics Solutions (a (i f A and B are Hermitian, since (AB = B A = BA, operator AB is Hermitian if and only if A and B commute So, we know that [A,B] = 0, which means that the Hilbert space H
Quantum Mechanics Solutions (a (i f A and B are Hermitian, since (AB = B A = BA, operator AB is Hermitian if and only if A and B commute So, we know that [A,B] = 0, which means that the Hilbert space H
Chapter 1. From Classical to Quantum Mechanics
 Chapter 1. From Classical to Quantum Mechanics Classical Mechanics (Newton): It describes the motion of a classical particle (discrete object). dp F ma, p = m = dt dx m dt F: force (N) a: acceleration
Chapter 1. From Classical to Quantum Mechanics Classical Mechanics (Newton): It describes the motion of a classical particle (discrete object). dp F ma, p = m = dt dx m dt F: force (N) a: acceleration
CHAPTER 2: POSTULATES OF QUANTUM MECHANICS
 CHAPTER 2: POSTULATES OF QUANTUM MECHANICS Basics of Quantum Mechanics - Why Quantum Physics? - Classical mechanics (Newton's mechanics) and Maxwell's equations (electromagnetics theory) can explain MACROSCOPIC
CHAPTER 2: POSTULATES OF QUANTUM MECHANICS Basics of Quantum Mechanics - Why Quantum Physics? - Classical mechanics (Newton's mechanics) and Maxwell's equations (electromagnetics theory) can explain MACROSCOPIC
Simple Harmonic Oscillator
 Classical harmonic oscillator Linear force acting on a particle (Hooke s law): F =!kx From Newton s law: F = ma = m d x dt =!kx " d x dt + # x = 0, # = k / m Position and momentum solutions oscillate in
Classical harmonic oscillator Linear force acting on a particle (Hooke s law): F =!kx From Newton s law: F = ma = m d x dt =!kx " d x dt + # x = 0, # = k / m Position and momentum solutions oscillate in
Second quantization: where quantization and particles come from?
 110 Phys460.nb 7 Second quantization: where quantization and particles come from? 7.1. Lagrangian mechanics and canonical quantization Q: How do we quantize a general system? 7.1.1.Lagrangian Lagrangian
110 Phys460.nb 7 Second quantization: where quantization and particles come from? 7.1. Lagrangian mechanics and canonical quantization Q: How do we quantize a general system? 7.1.1.Lagrangian Lagrangian
Electron in a Box. A wave packet in a square well (an electron in a box) changing with time.
 Electron in a Box A wave packet in a square well (an electron in a box) changing with time. Last Time: Light Wave model: Interference pattern is in terms of wave intensity Photon model: Interference in
Electron in a Box A wave packet in a square well (an electron in a box) changing with time. Last Time: Light Wave model: Interference pattern is in terms of wave intensity Photon model: Interference in
 Fundamental of Spectroscopy for Optical Remote Sensing Xinzhao Chu I 10 3.4. Principle of Uncertainty Indeterminacy 0. Expression of Heisenberg s Principle of Uncertainty It is worth to point out that
Fundamental of Spectroscopy for Optical Remote Sensing Xinzhao Chu I 10 3.4. Principle of Uncertainty Indeterminacy 0. Expression of Heisenberg s Principle of Uncertainty It is worth to point out that
A more comprehensive theory was needed. 1925, Schrödinger and Heisenberg separately worked out a new theory Quantum Mechanics.
 Ch28 Quantum Mechanics of Atoms Bohr s model was very successful to explain line spectra and the ionization energy for hydrogen. However, it also had many limitations: It was not able to predict the line
Ch28 Quantum Mechanics of Atoms Bohr s model was very successful to explain line spectra and the ionization energy for hydrogen. However, it also had many limitations: It was not able to predict the line
1 Mathematical preliminaries
 1 Mathematical preliminaries The mathematical language of quantum mechanics is that of vector spaces and linear algebra. In this preliminary section, we will collect the various definitions and mathematical
1 Mathematical preliminaries The mathematical language of quantum mechanics is that of vector spaces and linear algebra. In this preliminary section, we will collect the various definitions and mathematical
Complementi di Fisica Lectures 10-11
 Complementi di Fisica - Lectures 1-11 15/16-1-1 Complementi di Fisica Lectures 1-11 Livio Lanceri Università di Trieste Trieste, 15/16-1-1 Course Outline - Reminder Quantum Mechanics: an introduction Reminder
Complementi di Fisica - Lectures 1-11 15/16-1-1 Complementi di Fisica Lectures 1-11 Livio Lanceri Università di Trieste Trieste, 15/16-1-1 Course Outline - Reminder Quantum Mechanics: an introduction Reminder
( ) in the interaction picture arises only
 Physics 606, Quantum Mechanics, Final Exam NAME 1 Atomic transitions due to time-dependent electric field Consider a hydrogen atom which is in its ground state for t < 0 For t > 0 it is subjected to a
Physics 606, Quantum Mechanics, Final Exam NAME 1 Atomic transitions due to time-dependent electric field Consider a hydrogen atom which is in its ground state for t < 0 For t > 0 it is subjected to a
Supplementary information I Hilbert Space, Dirac Notation, and Matrix Mechanics. EE270 Fall 2017
 Supplementary information I Hilbert Space, Dirac Notation, and Matrix Mechanics Properties of Vector Spaces Unit vectors ~xi form a basis which spans the space and which are orthonormal ( if i = j ~xi
Supplementary information I Hilbert Space, Dirac Notation, and Matrix Mechanics Properties of Vector Spaces Unit vectors ~xi form a basis which spans the space and which are orthonormal ( if i = j ~xi
C/CS/Phys 191 Quantum Mechanics in a Nutshell I 10/04/05 Fall 2005 Lecture 11
 C/CS/Phys 191 Quantum Mechanics in a Nutshell I 10/04/05 Fall 2005 Lecture 11 In this and the next lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to
C/CS/Phys 191 Quantum Mechanics in a Nutshell I 10/04/05 Fall 2005 Lecture 11 In this and the next lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to
Quantum Mechanics of Atoms
 Quantum Mechanics of Atoms Your theory is crazy, but it's not crazy enough to be true N. Bohr to W. Pauli Quantum Mechanics of Atoms 2 Limitations of the Bohr Model The model was a great break-through,
Quantum Mechanics of Atoms Your theory is crazy, but it's not crazy enough to be true N. Bohr to W. Pauli Quantum Mechanics of Atoms 2 Limitations of the Bohr Model The model was a great break-through,
Mathematical Tripos Part IB Michaelmas Term Example Sheet 1. Values of some physical constants are given on the supplementary sheet
 Mathematical Tripos Part IB Michaelmas Term 2015 Quantum Mechanics Dr. J.M. Evans Example Sheet 1 Values of some physical constants are given on the supplementary sheet 1. Whenasampleofpotassiumisilluminatedwithlightofwavelength3
Mathematical Tripos Part IB Michaelmas Term 2015 Quantum Mechanics Dr. J.M. Evans Example Sheet 1 Values of some physical constants are given on the supplementary sheet 1. Whenasampleofpotassiumisilluminatedwithlightofwavelength3
Chemistry 3502/4502. Exam I Key. September 19, ) This is a multiple choice exam. Circle the correct answer.
 D Chemistry 350/450 Exam I Key September 19, 003 1) This is a multiple choice exam. Circle the correct answer. ) There is one correct answer to every problem. There is no partial credit. 3) A table of
D Chemistry 350/450 Exam I Key September 19, 003 1) This is a multiple choice exam. Circle the correct answer. ) There is one correct answer to every problem. There is no partial credit. 3) A table of
Quantum Physics 130A. April 1, 2006
 Quantum Physics 130A April 1, 2006 2 1 HOMEWORK 1: Due Friday, Apr. 14 1. A polished silver plate is hit by beams of photons of known energy. It is measured that the maximum electron energy is 3.1 ± 0.11
Quantum Physics 130A April 1, 2006 2 1 HOMEWORK 1: Due Friday, Apr. 14 1. A polished silver plate is hit by beams of photons of known energy. It is measured that the maximum electron energy is 3.1 ± 0.11
1 Fundamental physical postulates. C/CS/Phys C191 Quantum Mechanics in a Nutshell I 10/04/07 Fall 2007 Lecture 12
 C/CS/Phys C191 Quantum Mechanics in a Nutshell I 10/04/07 Fall 2007 Lecture 12 In this and the next lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to
C/CS/Phys C191 Quantum Mechanics in a Nutshell I 10/04/07 Fall 2007 Lecture 12 In this and the next lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to
Lecture 6. Four postulates of quantum mechanics. The eigenvalue equation. Momentum and energy operators. Dirac delta function. Expectation values
 Lecture 6 Four postulates of quantum mechanics The eigenvalue equation Momentum and energy operators Dirac delta function Expectation values Objectives Learn about eigenvalue equations and operators. Learn
Lecture 6 Four postulates of quantum mechanics The eigenvalue equation Momentum and energy operators Dirac delta function Expectation values Objectives Learn about eigenvalue equations and operators. Learn
Creation and Destruction Operators and Coherent States
 Creation and Destruction Operators and Coherent States WKB Method for Ground State Wave Function state harmonic oscillator wave function, We first rewrite the ground < x 0 >= ( π h )1/4 exp( x2 a 2 h )
Creation and Destruction Operators and Coherent States WKB Method for Ground State Wave Function state harmonic oscillator wave function, We first rewrite the ground < x 0 >= ( π h )1/4 exp( x2 a 2 h )
Quantum mechanics. Chapter The quantum mechanical formalism
 Chapter 5 Quantum mechanics 5.1 The quantum mechanical formalism The realisation, by Heisenberg, that the position and momentum of a quantum mechanical particle cannot be measured simultaneously renders
Chapter 5 Quantum mechanics 5.1 The quantum mechanical formalism The realisation, by Heisenberg, that the position and momentum of a quantum mechanical particle cannot be measured simultaneously renders
Physics 741 Graduate Quantum Mechanics 1 Solutions to Midterm Exam, Fall x i x dx i x i x x i x dx
 Physics 74 Graduate Quantum Mechanics Solutions to Midterm Exam, Fall 4. [ points] Consider the wave function x Nexp x ix (a) [6] What is the correct normaliation N? The normaliation condition is. exp,
Physics 74 Graduate Quantum Mechanics Solutions to Midterm Exam, Fall 4. [ points] Consider the wave function x Nexp x ix (a) [6] What is the correct normaliation N? The normaliation condition is. exp,
= ( Prove the nonexistence of electron in the nucleus on the basis of uncertainty principle.
 Worked out examples (Quantum mechanics). A microscope, using photons, is employed to locate an electron in an atom within a distance of. Å. What is the uncertainty in the momentum of the electron located
Worked out examples (Quantum mechanics). A microscope, using photons, is employed to locate an electron in an atom within a distance of. Å. What is the uncertainty in the momentum of the electron located
PLEASE LET ME KNOW IF YOU FIND TYPOS (send to
 Teoretisk Fysik KTH Advanced QM (SI2380), Lecture 2 (Summary of concepts) 1 PLEASE LET ME KNOW IF YOU FIND TYPOS (send email to langmann@kth.se) The laws of QM 1. I now discuss the laws of QM and their
Teoretisk Fysik KTH Advanced QM (SI2380), Lecture 2 (Summary of concepts) 1 PLEASE LET ME KNOW IF YOU FIND TYPOS (send email to langmann@kth.se) The laws of QM 1. I now discuss the laws of QM and their
Short Course in Quantum Information Lecture 2
 Short Course in Quantum Information Lecture Formal Structure of Quantum Mechanics Course Info All materials downloadable @ website http://info.phys.unm.edu/~deutschgroup/deutschclasses.html Syllabus Lecture
Short Course in Quantum Information Lecture Formal Structure of Quantum Mechanics Course Info All materials downloadable @ website http://info.phys.unm.edu/~deutschgroup/deutschclasses.html Syllabus Lecture
Chapter 1 The Bohr Atom
 Chapter 1 The Bohr Atom 1 Introduction Niels Bohr was a Danish physicist who made a fundamental contribution to our understanding of atomic structure and quantum mechanics. He made the first successful
Chapter 1 The Bohr Atom 1 Introduction Niels Bohr was a Danish physicist who made a fundamental contribution to our understanding of atomic structure and quantum mechanics. He made the first successful
C/CS/Phys C191 Quantum Mechanics in a Nutshell 10/06/07 Fall 2009 Lecture 12
 C/CS/Phys C191 Quantum Mechanics in a Nutshell 10/06/07 Fall 2009 Lecture 12 In this lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to this course. Topics
C/CS/Phys C191 Quantum Mechanics in a Nutshell 10/06/07 Fall 2009 Lecture 12 In this lecture we summarize the essential physical and mathematical aspects of quantum mechanics relevant to this course. Topics
Lecture 3 Dynamics 29
 Lecture 3 Dynamics 29 30 LECTURE 3. DYNAMICS 3.1 Introduction Having described the states and the observables of a quantum system, we shall now introduce the rules that determine their time evolution.
Lecture 3 Dynamics 29 30 LECTURE 3. DYNAMICS 3.1 Introduction Having described the states and the observables of a quantum system, we shall now introduce the rules that determine their time evolution.
Lecture 4 (Sep. 18, 2017)
 Lecture 4 8.3 Quantum Theory I, Fall 07 Lecture 4 (Sep. 8, 07) 4. Measurement 4.. Spin- Systems Last time, we said that a general state in a spin- system can be written as ψ = c + + + c, (4.) where +,
Lecture 4 8.3 Quantum Theory I, Fall 07 Lecture 4 (Sep. 8, 07) 4. Measurement 4.. Spin- Systems Last time, we said that a general state in a spin- system can be written as ψ = c + + + c, (4.) where +,
Chemistry 3502/4502. Exam I. September 19, ) This is a multiple choice exam. Circle the correct answer.
 D Chemistry 350/450 Exam I September 9, 003 ) This is a multiple choice exam. Circle the correct answer. ) There is one correct answer to every problem. There is no partial credit. 3) A table of useful
D Chemistry 350/450 Exam I September 9, 003 ) This is a multiple choice exam. Circle the correct answer. ) There is one correct answer to every problem. There is no partial credit. 3) A table of useful
We also find the development of famous Schrodinger equation to describe the quantization of energy levels of atoms.
 Lecture 4 TITLE: Quantization of radiation and matter: Wave-Particle duality Objectives In this lecture, we will discuss the development of quantization of matter and light. We will understand the need
Lecture 4 TITLE: Quantization of radiation and matter: Wave-Particle duality Objectives In this lecture, we will discuss the development of quantization of matter and light. We will understand the need
Chapter 7. Bound Systems are perhaps the most interesting cases for us to consider. We see much of the interesting features of quantum mechanics.
 Chapter 7 In chapter 6 we learned about a set of rules for quantum mechanics. Now we want to apply them to various cases and see what they predict for the behavior of quanta under different conditions.
Chapter 7 In chapter 6 we learned about a set of rules for quantum mechanics. Now we want to apply them to various cases and see what they predict for the behavior of quanta under different conditions.
Review of paradigms QM. Read McIntyre Ch. 1, 2, 3.1, , , 7, 8
 Review of paradigms QM Read McIntyre Ch. 1, 2, 3.1, 5.1-5.7, 6.1-6.5, 7, 8 QM Postulates 1 The state of a quantum mechanical system, including all the informaion you can know about it, is represented mathemaically
Review of paradigms QM Read McIntyre Ch. 1, 2, 3.1, 5.1-5.7, 6.1-6.5, 7, 8 QM Postulates 1 The state of a quantum mechanical system, including all the informaion you can know about it, is represented mathemaically
1 Planck-Einstein Relation E = hν
 C/CS/Phys C191 Representations and Wavefunctions 09/30/08 Fall 2008 Lecture 8 1 Planck-Einstein Relation E = hν This is the equation relating energy to frequency. It was the earliest equation of quantum
C/CS/Phys C191 Representations and Wavefunctions 09/30/08 Fall 2008 Lecture 8 1 Planck-Einstein Relation E = hν This is the equation relating energy to frequency. It was the earliest equation of quantum
QM1 - Tutorial 1 The Bohr Atom and Mathematical Introduction
 QM - Tutorial The Bohr Atom and Mathematical Introduction 26 October 207 Contents Bohr Atom. Energy of a Photon - The Photo Electric Eect.................................2 The Discrete Energy Spectrum
QM - Tutorial The Bohr Atom and Mathematical Introduction 26 October 207 Contents Bohr Atom. Energy of a Photon - The Photo Electric Eect.................................2 The Discrete Energy Spectrum
Lecture #1. Review. Postulates of quantum mechanics (1-3) Postulate 1
 L1.P1 Lecture #1 Review Postulates of quantum mechanics (1-3) Postulate 1 The state of a system at any instant of time may be represented by a wave function which is continuous and differentiable. Specifically,
L1.P1 Lecture #1 Review Postulates of quantum mechanics (1-3) Postulate 1 The state of a system at any instant of time may be represented by a wave function which is continuous and differentiable. Specifically,
Quantum Mechanics Solutions. λ i λ j v j v j v i v i.
 Quantum Mechanics Solutions 1. (a) If H has an orthonormal basis consisting of the eigenvectors { v i } of A with eigenvalues λ i C, then A can be written in terms of its spectral decomposition as A =
Quantum Mechanics Solutions 1. (a) If H has an orthonormal basis consisting of the eigenvectors { v i } of A with eigenvalues λ i C, then A can be written in terms of its spectral decomposition as A =
Complementi di Fisica Lectures 5, 6
 Complementi di Fisica - Lectures 5, 6 9/3-9-15 Complementi di Fisica Lectures 5, 6 Livio Lanceri Università di Trieste Trieste, 9/3-9-15 Course Outline - Reminder Quantum Mechanics: an introduction Reminder
Complementi di Fisica - Lectures 5, 6 9/3-9-15 Complementi di Fisica Lectures 5, 6 Livio Lanceri Università di Trieste Trieste, 9/3-9-15 Course Outline - Reminder Quantum Mechanics: an introduction Reminder
Quantum Mechanics. p " The Uncertainty Principle places fundamental limits on our measurements :
 Student Selected Module 2005/2006 (SSM-0032) 17 th November 2005 Quantum Mechanics Outline : Review of Previous Lecture. Single Particle Wavefunctions. Time-Independent Schrödinger equation. Particle in
Student Selected Module 2005/2006 (SSM-0032) 17 th November 2005 Quantum Mechanics Outline : Review of Previous Lecture. Single Particle Wavefunctions. Time-Independent Schrödinger equation. Particle in
CHAPTER 28 Quantum Mechanics of Atoms Units
 CHAPTER 28 Quantum Mechanics of Atoms Units Quantum Mechanics A New Theory The Wave Function and Its Interpretation; the Double-Slit Experiment The Heisenberg Uncertainty Principle Philosophic Implications;
CHAPTER 28 Quantum Mechanics of Atoms Units Quantum Mechanics A New Theory The Wave Function and Its Interpretation; the Double-Slit Experiment The Heisenberg Uncertainty Principle Philosophic Implications;
Quantum Theory and Group Representations
 Quantum Theory and Group Representations Peter Woit Columbia University LaGuardia Community College, November 1, 2017 Queensborough Community College, November 15, 2017 Peter Woit (Columbia University)
Quantum Theory and Group Representations Peter Woit Columbia University LaGuardia Community College, November 1, 2017 Queensborough Community College, November 15, 2017 Peter Woit (Columbia University)
Midterm Examination 1
 CHEM 332 Physical Chemistry Spring 2014 Name: Answer Key Midterm Examination 1 1. Match the individual with their accomplishment: A. Millikan G Proposed a probabilistic interpretation for *. B. Planck
CHEM 332 Physical Chemistry Spring 2014 Name: Answer Key Midterm Examination 1 1. Match the individual with their accomplishment: A. Millikan G Proposed a probabilistic interpretation for *. B. Planck
Theoretical Biophysics. Quantum Theory and Molecular Dynamics. Pawel Romanczuk WS 2017/18
 Theoretical Biophysics Quantum Theory and Molecular Dynamics Pawel Romanczuk WS 2017/18 http://lab.romanczuk.de/teaching/ 1 Introduction Two pillars of classical theoretical physics at the begin of 20th
Theoretical Biophysics Quantum Theory and Molecular Dynamics Pawel Romanczuk WS 2017/18 http://lab.romanczuk.de/teaching/ 1 Introduction Two pillars of classical theoretical physics at the begin of 20th
An operator is a transformation that takes a function as an input and produces another function (usually).
 Formalism of Quantum Mechanics Operators Engel 3.2 An operator is a transformation that takes a function as an input and produces another function (usually). Example: In QM, most operators are linear:
Formalism of Quantum Mechanics Operators Engel 3.2 An operator is a transformation that takes a function as an input and produces another function (usually). Example: In QM, most operators are linear:
Physics 43 Exam 2 Spring 2018
 Physics 43 Exam 2 Spring 2018 Print Name: Conceptual Circle the best answer. (2 points each) 1. Quantum physics agrees with the classical physics limit when a. the total angular momentum is a small multiple
Physics 43 Exam 2 Spring 2018 Print Name: Conceptual Circle the best answer. (2 points each) 1. Quantum physics agrees with the classical physics limit when a. the total angular momentum is a small multiple
Quantum Theory. Thornton and Rex, Ch. 6
 Quantum Theory Thornton and Rex, Ch. 6 Matter can behave like waves. 1) What is the wave equation? 2) How do we interpret the wave function y(x,t)? Light Waves Plane wave: y(x,t) = A cos(kx-wt) wave (w,k)
Quantum Theory Thornton and Rex, Ch. 6 Matter can behave like waves. 1) What is the wave equation? 2) How do we interpret the wave function y(x,t)? Light Waves Plane wave: y(x,t) = A cos(kx-wt) wave (w,k)
Problems and Multiple Choice Questions
 Problems and Multiple Choice Questions 1. A momentum operator in one dimension is 2. A position operator in 3 dimensions is 3. A kinetic energy operator in 1 dimension is 4. If two operator commute, a)
Problems and Multiple Choice Questions 1. A momentum operator in one dimension is 2. A position operator in 3 dimensions is 3. A kinetic energy operator in 1 dimension is 4. If two operator commute, a)
df(x) = h(x) dx Chemistry 4531 Mathematical Preliminaries Spring 2009 I. A Primer on Differential Equations Order of differential equation
 Chemistry 4531 Mathematical Preliminaries Spring 009 I. A Primer on Differential Equations Order of differential equation Linearity of differential equation Partial vs. Ordinary Differential Equations
Chemistry 4531 Mathematical Preliminaries Spring 009 I. A Primer on Differential Equations Order of differential equation Linearity of differential equation Partial vs. Ordinary Differential Equations
to the potential V to get V + V 0 0Ψ. Let Ψ ( x,t ) =ψ x dx 2
 Physics 0 Homework # Spring 017 Due Wednesday, 4/1/17 1. Griffith s 1.8 We start with by adding V 0 to the potential V to get V + V 0. The Schrödinger equation reads: i! dψ dt =! d Ψ m dx + VΨ + V 0Ψ.
Physics 0 Homework # Spring 017 Due Wednesday, 4/1/17 1. Griffith s 1.8 We start with by adding V 0 to the potential V to get V + V 0. The Schrödinger equation reads: i! dψ dt =! d Ψ m dx + VΨ + V 0Ψ.
The Birth of Quantum Mechanics. New Wave Rock Stars
 The Birth of Quantum Mechanics Louis de Broglie 1892-1987 Erwin Schrödinger 1887-1961 Paul Dirac 1902-1984 Werner Heisenberg 1901-1976 New Wave Rock Stars Blackbody radiation: Light energy is quantized.
The Birth of Quantum Mechanics Louis de Broglie 1892-1987 Erwin Schrödinger 1887-1961 Paul Dirac 1902-1984 Werner Heisenberg 1901-1976 New Wave Rock Stars Blackbody radiation: Light energy is quantized.
CHM320 EXAM #2 USEFUL INFORMATION
 CHM30 EXAM # USEFUL INFORMATION Constants mass of electron: m e = 9.11 10 31 kg. Rydberg constant: R H = 109737.35 cm 1 =.1798 10 18 J. speed of light: c = 3.00 10 8 m/s Planck constant: 6.66 10 34 Js
CHM30 EXAM # USEFUL INFORMATION Constants mass of electron: m e = 9.11 10 31 kg. Rydberg constant: R H = 109737.35 cm 1 =.1798 10 18 J. speed of light: c = 3.00 10 8 m/s Planck constant: 6.66 10 34 Js
IB Physics SL Y2 Option B (Quantum and Nuclear Physics) Exam Study Guide Practice Problem Solutions
 IB Physics SL Y2 Option B (Quantum and Nuclear Physics) Exam Study Guide Practice Problem Solutions Objectives: 1. Describe the photoelectric effect. (B.1.1) 2. Describe the concept of the photon and use
IB Physics SL Y2 Option B (Quantum and Nuclear Physics) Exam Study Guide Practice Problem Solutions Objectives: 1. Describe the photoelectric effect. (B.1.1) 2. Describe the concept of the photon and use
Bohr s Correspondence Principle
 Bohr s Correspondence Principle In limit that n, quantum mechanics must agree with classical physics E photon = 13.6 ev 1 n f n 1 i = hf photon In this limit, n i n f, and then f photon electron s frequency
Bohr s Correspondence Principle In limit that n, quantum mechanics must agree with classical physics E photon = 13.6 ev 1 n f n 1 i = hf photon In this limit, n i n f, and then f photon electron s frequency
Quantum Orbits. Quantum Theory for the Computer Age Unit 9. Diving orbit. Caustic. for KE/PE =R=-3/8. for KE/PE =R=-3/8. p"...
 W.G. Harter Coulomb Obits 6-1 Quantum Theory for the Computer Age Unit 9 Caustic for KE/PE =R=-3/8 F p' p g r p"... P F' F P Diving orbit T" T T' Contact Pt. for KE/PE =R=-3/8 Quantum Orbits W.G. Harter
W.G. Harter Coulomb Obits 6-1 Quantum Theory for the Computer Age Unit 9 Caustic for KE/PE =R=-3/8 F p' p g r p"... P F' F P Diving orbit T" T T' Contact Pt. for KE/PE =R=-3/8 Quantum Orbits W.G. Harter
Chapter 2 Heisenberg s Matrix Mechanics
 Chapter 2 Heisenberg s Matrix Mechanics Abstract The quantum selection rule and its generalizations are capable of predicting energies of the stationary orbits; however they should be obtained in a more
Chapter 2 Heisenberg s Matrix Mechanics Abstract The quantum selection rule and its generalizations are capable of predicting energies of the stationary orbits; however they should be obtained in a more
Basic concepts from quantum theory
 80 CHAPTER III. QUANTUM COMPUTATION Figure III.1: Probability density of first six hydrogen orbitals. The main quantum number (n =1, 2, 3) and the angular momentum quantum number (` =0, 1, 2=s,p,d)areshown.
80 CHAPTER III. QUANTUM COMPUTATION Figure III.1: Probability density of first six hydrogen orbitals. The main quantum number (n =1, 2, 3) and the angular momentum quantum number (` =0, 1, 2=s,p,d)areshown.
Final Exam. Tuesday, May 8, Starting at 8:30 a.m., Hoyt Hall.
 Final Exam Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall. Summary of Chapter 38 In Quantum Mechanics particles are represented by wave functions Ψ. The absolute square of the wave function Ψ 2
Final Exam Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall. Summary of Chapter 38 In Quantum Mechanics particles are represented by wave functions Ψ. The absolute square of the wave function Ψ 2
4.3 Lecture 18: Quantum Mechanics
 CHAPTER 4. QUANTUM SYSTEMS 73 4.3 Lecture 18: Quantum Mechanics 4.3.1 Basics Now that we have mathematical tools of linear algebra we are ready to develop a framework of quantum mechanics. The framework
CHAPTER 4. QUANTUM SYSTEMS 73 4.3 Lecture 18: Quantum Mechanics 4.3.1 Basics Now that we have mathematical tools of linear algebra we are ready to develop a framework of quantum mechanics. The framework
Semiconductor Physics and Devices
 Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
STRUCTURE OF MATTER, VIBRATIONS AND WAVES, AND QUANTUM PHYSICS
 Imperial College London BSc/MSci EXAMINATION June 2008 This paper is also taken for the relevant Examination for the Associateship STRUCTURE OF MATTER, VIBRATIONS AND WAVES, AND QUANTUM PHYSICS For 1st-Year
Imperial College London BSc/MSci EXAMINATION June 2008 This paper is also taken for the relevant Examination for the Associateship STRUCTURE OF MATTER, VIBRATIONS AND WAVES, AND QUANTUM PHYSICS For 1st-Year
A Minimal Uncertainty Product for One Dimensional Semiclassical Wave Packets
 A Minimal Uncertainty Product for One Dimensional Semiclassical Wave Packets George A. Hagedorn Happy 60 th birthday, Mr. Fritz! Abstract. Although real, normalized Gaussian wave packets minimize the product
A Minimal Uncertainty Product for One Dimensional Semiclassical Wave Packets George A. Hagedorn Happy 60 th birthday, Mr. Fritz! Abstract. Although real, normalized Gaussian wave packets minimize the product
Chapter 38 Quantum Mechanics
 Chapter 38 Quantum Mechanics Units of Chapter 38 38-1 Quantum Mechanics A New Theory 37-2 The Wave Function and Its Interpretation; the Double-Slit Experiment 38-3 The Heisenberg Uncertainty Principle
Chapter 38 Quantum Mechanics Units of Chapter 38 38-1 Quantum Mechanics A New Theory 37-2 The Wave Function and Its Interpretation; the Double-Slit Experiment 38-3 The Heisenberg Uncertainty Principle
Quantum Theory and Atomic Structure. Quantum Mechanics. Quantum Theory and Atomic Structure. 7.3 The Wave-Particle Duality of Matter and Energy
 Chapter 7 Quantum Theory and Atomic Structure Chap 7-1 Quantum Theory and Atomic Structure 7.1 The Nature of Light 7.2 Atomic Spectra 7.3 The Wave-Particle Duality of Matter and Energy 7.4 The Quantum-Mechanical
Chapter 7 Quantum Theory and Atomic Structure Chap 7-1 Quantum Theory and Atomic Structure 7.1 The Nature of Light 7.2 Atomic Spectra 7.3 The Wave-Particle Duality of Matter and Energy 7.4 The Quantum-Mechanical
Chapter 7. Quantum Theory and Atomic Structure. Quantum Mechanics. Chap 7-1
 Chapter 7 Quantum Theory and Atomic Structure Chap 7-1 Quantum Theory and Atomic Structure 7.1 The Nature of Light 7.2 Atomic Spectra 7.3 The Wave-Particle Duality of Matter and Energy 7.4 The Quantum-Mechanical
Chapter 7 Quantum Theory and Atomic Structure Chap 7-1 Quantum Theory and Atomic Structure 7.1 The Nature of Light 7.2 Atomic Spectra 7.3 The Wave-Particle Duality of Matter and Energy 7.4 The Quantum-Mechanical
20th Century Atomic Theory- Hydrogen Atom
 Background for (mostly) Chapter 12 of EDR 20th Century Atomic Theory- Hydrogen Atom EDR Section 12.7 Rutherford's scattering experiments (Raff 11.2.3) in 1910 lead to a "planetary" model of the atom where
Background for (mostly) Chapter 12 of EDR 20th Century Atomic Theory- Hydrogen Atom EDR Section 12.7 Rutherford's scattering experiments (Raff 11.2.3) in 1910 lead to a "planetary" model of the atom where
Quantum Theory. Thornton and Rex, Ch. 6
 Quantum Theory Thornton and Rex, Ch. 6 Matter can behave like waves. 1) What is the wave equation? 2) How do we interpret the wave function y(x,t)? Light Waves Plane wave: y(x,t) = A cos(kx-wt) wave (w,k)
Quantum Theory Thornton and Rex, Ch. 6 Matter can behave like waves. 1) What is the wave equation? 2) How do we interpret the wave function y(x,t)? Light Waves Plane wave: y(x,t) = A cos(kx-wt) wave (w,k)
Statistical Interpretation
 Physics 342 Lecture 15 Statistical Interpretation Lecture 15 Physics 342 Quantum Mechanics I Friday, February 29th, 2008 Quantum mechanics is a theory of probability densities given that we now have an
Physics 342 Lecture 15 Statistical Interpretation Lecture 15 Physics 342 Quantum Mechanics I Friday, February 29th, 2008 Quantum mechanics is a theory of probability densities given that we now have an
Wave nature of particles
 Wave nature of particles We have thus far developed a model of atomic structure based on the particle nature of matter: Atoms have a dense nucleus of positive charge with electrons orbiting the nucleus
Wave nature of particles We have thus far developed a model of atomic structure based on the particle nature of matter: Atoms have a dense nucleus of positive charge with electrons orbiting the nucleus
Quantum Mechanics for Mathematicians: Energy, Momentum, and the Quantum Free Particle
 Quantum Mechanics for Mathematicians: Energy, Momentum, and the Quantum Free Particle Peter Woit Department of Mathematics, Columbia University woit@math.columbia.edu November 28, 2012 We ll now turn to
Quantum Mechanics for Mathematicians: Energy, Momentum, and the Quantum Free Particle Peter Woit Department of Mathematics, Columbia University woit@math.columbia.edu November 28, 2012 We ll now turn to
10/17/11. Chapter 7. Quantum Theory and Atomic Structure. Amplitude (intensity) of a wave. Quantum Theory and Atomic Structure
 Quantum Theory and Atomic Structure Chapter 7 7. The Nature of Light Quantum Theory and Atomic Structure 7. Atomic Spectra 7. The Wave-Particle Duality of Matter and Energy 7.4 The Quantum-Mechanical Model
Quantum Theory and Atomic Structure Chapter 7 7. The Nature of Light Quantum Theory and Atomic Structure 7. Atomic Spectra 7. The Wave-Particle Duality of Matter and Energy 7.4 The Quantum-Mechanical Model
2 Canonical quantization
 Phys540.nb 7 Canonical quantization.1. Lagrangian mechanics and canonical quantization Q: How do we quantize a general system?.1.1.lagrangian Lagrangian mechanics is a reformulation of classical mechanics.
Phys540.nb 7 Canonical quantization.1. Lagrangian mechanics and canonical quantization Q: How do we quantize a general system?.1.1.lagrangian Lagrangian mechanics is a reformulation of classical mechanics.
5.1 Classical Harmonic Oscillator
 Chapter 5 Harmonic Oscillator 5.1 Classical Harmonic Oscillator m l o l Hooke s Law give the force exerting on the mass as: f = k(l l o ) where l o is the equilibrium length of the spring and k is the
Chapter 5 Harmonic Oscillator 5.1 Classical Harmonic Oscillator m l o l Hooke s Law give the force exerting on the mass as: f = k(l l o ) where l o is the equilibrium length of the spring and k is the
8 Wavefunctions - Schrödinger s Equation
 8 Wavefunctions - Schrödinger s Equation So far we have considered only free particles - i.e. particles whose energy consists entirely of its kinetic energy. In general, however, a particle moves under
8 Wavefunctions - Schrödinger s Equation So far we have considered only free particles - i.e. particles whose energy consists entirely of its kinetic energy. In general, however, a particle moves under
Quantum mechanics in one hour
 Chapter 2 Quantum mechanics in one hour 2.1 Introduction The purpose of this chapter is to refresh your knowledge of quantum mechanics and to establish notation. Depending on your background you might
Chapter 2 Quantum mechanics in one hour 2.1 Introduction The purpose of this chapter is to refresh your knowledge of quantum mechanics and to establish notation. Depending on your background you might
Basic concepts from quantum theory
 B. BASIC CONCEPTS FROM QUANTUM THEORY 59 Figure III.1: Probability density of first six hydrogen orbitals. The main quantum number (n =1, 2, 3) and the angular momentum quantum number (` =0, 1, 2=s,p,d)areshown.
B. BASIC CONCEPTS FROM QUANTUM THEORY 59 Figure III.1: Probability density of first six hydrogen orbitals. The main quantum number (n =1, 2, 3) and the angular momentum quantum number (` =0, 1, 2=s,p,d)areshown.
The Hydrogen Atom According to Bohr
 The Hydrogen Atom According to Bohr The atom We ve already talked about how tiny systems behave in strange ways. Now let s s talk about how a more complicated system behaves. The atom! Physics 9 4 Early
The Hydrogen Atom According to Bohr The atom We ve already talked about how tiny systems behave in strange ways. Now let s s talk about how a more complicated system behaves. The atom! Physics 9 4 Early
2. As we shall see, we choose to write in terms of σ x because ( X ) 2 = σ 2 x.
 Section 5.1 Simple One-Dimensional Problems: The Free Particle Page 9 The Free Particle Gaussian Wave Packets The Gaussian wave packet initial state is one of the few states for which both the { x } and
Section 5.1 Simple One-Dimensional Problems: The Free Particle Page 9 The Free Particle Gaussian Wave Packets The Gaussian wave packet initial state is one of the few states for which both the { x } and
MP463 QUANTUM MECHANICS
 MP463 QUANTUM MECHANICS Introduction Quantum theory of angular momentum Quantum theory of a particle in a central potential - Hydrogen atom - Three-dimensional isotropic harmonic oscillator (a model of
MP463 QUANTUM MECHANICS Introduction Quantum theory of angular momentum Quantum theory of a particle in a central potential - Hydrogen atom - Three-dimensional isotropic harmonic oscillator (a model of
Chapter 4 Section 2 Notes
 Chapter 4 Section 2 Notes Vocabulary Heisenberg Uncertainty Principle- states that it is impossible to determine simultaneously both the position and velocity of an electron or any other particle. Quantum
Chapter 4 Section 2 Notes Vocabulary Heisenberg Uncertainty Principle- states that it is impossible to determine simultaneously both the position and velocity of an electron or any other particle. Quantum
Quantum Mechanics. The Schrödinger equation. Erwin Schrödinger
 Quantum Mechanics The Schrödinger equation Erwin Schrödinger The Nobel Prize in Physics 1933 "for the discovery of new productive forms of atomic theory" The Schrödinger Equation in One Dimension Time-Independent
Quantum Mechanics The Schrödinger equation Erwin Schrödinger The Nobel Prize in Physics 1933 "for the discovery of new productive forms of atomic theory" The Schrödinger Equation in One Dimension Time-Independent
kg meter ii) Note the dimensions of ρ τ are kg 2 velocity 2 meter = 1 sec 2 We will interpret this velocity in upcoming slides.
 II. Generalizing the 1-dimensional wave equation First generalize the notation. i) "q" has meant transverse deflection of the string. Replace q Ψ, where Ψ may indicate other properties of the medium that
II. Generalizing the 1-dimensional wave equation First generalize the notation. i) "q" has meant transverse deflection of the string. Replace q Ψ, where Ψ may indicate other properties of the medium that
Properties of Commutators and Schroedinger Operators and Applications to Quantum Computing
 International Journal of Engineering and Advanced Research Technology (IJEART) Properties of Commutators and Schroedinger Operators and Applications to Quantum Computing N. B. Okelo Abstract In this paper
International Journal of Engineering and Advanced Research Technology (IJEART) Properties of Commutators and Schroedinger Operators and Applications to Quantum Computing N. B. Okelo Abstract In this paper
Physics 486 Discussion 5 Piecewise Potentials
 Physics 486 Discussion 5 Piecewise Potentials Problem 1 : Infinite Potential Well Checkpoints 1 Consider the infinite well potential V(x) = 0 for 0 < x < 1 elsewhere. (a) First, think classically. Potential
Physics 486 Discussion 5 Piecewise Potentials Problem 1 : Infinite Potential Well Checkpoints 1 Consider the infinite well potential V(x) = 0 for 0 < x < 1 elsewhere. (a) First, think classically. Potential
Physics 202 Laboratory 5. Linear Algebra 1. Laboratory 5. Physics 202 Laboratory
 Physics 202 Laboratory 5 Linear Algebra Laboratory 5 Physics 202 Laboratory We close our whirlwind tour of numerical methods by advertising some elements of (numerical) linear algebra. There are three
Physics 202 Laboratory 5 Linear Algebra Laboratory 5 Physics 202 Laboratory We close our whirlwind tour of numerical methods by advertising some elements of (numerical) linear algebra. There are three
PHY4604 Introduction to Quantum Mechanics Fall 2004 Final Exam SOLUTIONS December 17, 2004, 7:30 a.m.- 9:30 a.m.
 PHY464 Introduction to Quantum Mechanics Fall 4 Final Eam SOLUTIONS December 7, 4, 7:3 a.m.- 9:3 a.m. No other materials allowed. If you can t do one part of a problem, solve subsequent parts in terms
PHY464 Introduction to Quantum Mechanics Fall 4 Final Eam SOLUTIONS December 7, 4, 7:3 a.m.- 9:3 a.m. No other materials allowed. If you can t do one part of a problem, solve subsequent parts in terms
B. Physical Observables Physical observables are represented by linear, hermitian operators that act on the vectors of the Hilbert space. If A is such
 G25.2651: Statistical Mechanics Notes for Lecture 12 I. THE FUNDAMENTAL POSTULATES OF QUANTUM MECHANICS The fundamental postulates of quantum mechanics concern the following questions: 1. How is the physical
G25.2651: Statistical Mechanics Notes for Lecture 12 I. THE FUNDAMENTAL POSTULATES OF QUANTUM MECHANICS The fundamental postulates of quantum mechanics concern the following questions: 1. How is the physical
Lecture 6 Quantum Mechanical Systems and Measurements
 Lecture 6 Quantum Mechanical Systems and Measurements Today s Program: 1. Simple Harmonic Oscillator (SHO). Principle of spectral decomposition. 3. Predicting the results of measurements, fourth postulate
Lecture 6 Quantum Mechanical Systems and Measurements Today s Program: 1. Simple Harmonic Oscillator (SHO). Principle of spectral decomposition. 3. Predicting the results of measurements, fourth postulate
5.4 Given the basis e 1, e 2 write the matrices that represent the unitary transformations corresponding to the following changes of basis:
 5 Representations 5.3 Given a three-dimensional Hilbert space, consider the two observables ξ and η that, with respect to the basis 1, 2, 3, arerepresentedby the matrices: ξ ξ 1 0 0 0 ξ 1 0 0 0 ξ 3, ξ
5 Representations 5.3 Given a three-dimensional Hilbert space, consider the two observables ξ and η that, with respect to the basis 1, 2, 3, arerepresentedby the matrices: ξ ξ 1 0 0 0 ξ 1 0 0 0 ξ 3, ξ
1 Heisenberg Representation
 1 Heisenberg Representation What we have been ealing with so far is calle the Schröinger representation. In this representation, operators are constants an all the time epenence is carrie by the states.
1 Heisenberg Representation What we have been ealing with so far is calle the Schröinger representation. In this representation, operators are constants an all the time epenence is carrie by the states.
Energy levels and atomic structures lectures chapter one
 Structure of Atom An atom is the smallest constituent unit of ordinary matter that has the properties of a element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are
Structure of Atom An atom is the smallest constituent unit of ordinary matter that has the properties of a element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are
Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours.
 Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours. There are 10 problems, totalling 180 points. Do all problems. Answer all problems in the white books provided.
Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours. There are 10 problems, totalling 180 points. Do all problems. Answer all problems in the white books provided.
CHAPTER 6 Quantum Mechanics II
 CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6.2 Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite-Potential Well
CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6.2 Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite-Potential Well
The Schrödinger Equation
 Chapter 13 The Schrödinger Equation 13.1 Where we are so far We have focused primarily on electron spin so far because it s a simple quantum system (there are only two basis states!), and yet it still
Chapter 13 The Schrödinger Equation 13.1 Where we are so far We have focused primarily on electron spin so far because it s a simple quantum system (there are only two basis states!), and yet it still
The Hydrogen Atom. Dr. Sabry El-Taher 1. e 4. U U r
 The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
1 Position Representation of Quantum State Function
 C/CS/Phys C191 Quantum Mechanics in a Nutshell II 10/09/07 Fall 2007 Lecture 13 1 Position Representation of Quantum State Function We will motivate this using the framework of measurements. Consider first
C/CS/Phys C191 Quantum Mechanics in a Nutshell II 10/09/07 Fall 2007 Lecture 13 1 Position Representation of Quantum State Function We will motivate this using the framework of measurements. Consider first
Review of Quantum Mechanics, cont.
 Review of Quantum Mechanics, cont. 1 Probabilities In analogy to the development of a wave intensity from a wave amplitude, the probability of a particle with a wave packet amplitude, ψ, between x and
Review of Quantum Mechanics, cont. 1 Probabilities In analogy to the development of a wave intensity from a wave amplitude, the probability of a particle with a wave packet amplitude, ψ, between x and
This is the important completeness relation,
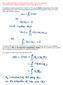 Observable quantities are represented by linear, hermitian operators! Compatible observables correspond to commuting operators! In addition to what was written in eqn 2.1.1, the vector corresponding to
Observable quantities are represented by linear, hermitian operators! Compatible observables correspond to commuting operators! In addition to what was written in eqn 2.1.1, the vector corresponding to
Announcements. Lecture 20 Chapter. 7 QM in 3-dims & Hydrogen Atom. The Radial Part of Schrodinger Equation for Hydrogen Atom
 Announcements! HW7 : Chap.7 18, 20, 23, 32, 37, 38, 45, 47, 53, 57, 60! Physics Colloquium: Development in Electron Nuclear Dynamics Theory on Thursday @ 3:40pm! Quiz 2 (average: 9), Quiz 3: 4/19 *** Course
Announcements! HW7 : Chap.7 18, 20, 23, 32, 37, 38, 45, 47, 53, 57, 60! Physics Colloquium: Development in Electron Nuclear Dynamics Theory on Thursday @ 3:40pm! Quiz 2 (average: 9), Quiz 3: 4/19 *** Course
One-electron Atom. (in spherical coordinates), where Y lm. are spherical harmonics, we arrive at the following Schrödinger equation:
 One-electron Atom The atomic orbitals of hydrogen-like atoms are solutions to the Schrödinger equation in a spherically symmetric potential. In this case, the potential term is the potential given by Coulomb's
One-electron Atom The atomic orbitals of hydrogen-like atoms are solutions to the Schrödinger equation in a spherically symmetric potential. In this case, the potential term is the potential given by Coulomb's
