Quantum Orbits. Quantum Theory for the Computer Age Unit 9. Diving orbit. Caustic. for KE/PE =R=-3/8. for KE/PE =R=-3/8. p"...
|
|
|
- Hollie Crawford
- 5 years ago
- Views:
Transcription
1 W.G. Harter Coulomb Obits 6-1 Quantum Theory for the Computer Age Unit 9 Caustic for KE/PE =R=-3/8 F p' p g r p"... P F' F P Diving orbit T" T T' Contact Pt. for KE/PE =R=-3/8 Quantum Orbits
2 W.G. Harter Coulomb Obits 6- Unit 9 Quantum Orbits Quantum theory of particle orbits has a long history beginning with the Bohr atomic Coulomb orbits introduced in Chapter 5 and D and 3D oscillator orbits introduced in Chapter and shown to have U(3) and U(3) symmetry, respectively. Both are helped a lot by the R(3) rotational states and wavefunctions introduced in Chapter 3. The 3D Coulomb orbital problem has a higher symmetry R(4) that is something like the U(3) symmetry of the 3D isotropic oscillator. This connection will be explored. W. G. Harter Department of Physics University of Arkansas Fayetteville Hardware and Software by HARTER-Soft Elegant Educational Tools Since 001
3 W.G. Harter Coulomb Obits 6-3 QM for AMOP Ch. 6 Coulomb Orbits W. Harter The Bohr atomic Coulomb problem has nicely closed analytic solutions for both the classical and quantum versions. This is not an accident but due to symmetry. Like the 3D isotropic oscillator the 3D Coulomb orbital problem has a higher symmetry R(4) that is something like the U(3) symmetry of the 3D isotropic oscillator. This quantum symmetry connection will be explored and used to better understand the quantum orbitals and they will be compared with the classical orbits.
4 W.G. Harter Coulomb Obits 6-4 Unit 9 Quantum Orbits...5 Chapter 6 Coulomb Orbits Introduction to Radial Hamiltonians...5 (a) Kinetic energy operator in polar form...6 (b) Angular-radial separation...8 (c) Radial equations and solution Potential-free case Coulomb potential...10 (d) Radial wavefunctions...15
5 W.G. Harter Coulomb Obits 6-5 Unit 9 Quantum Orbits Chapter 6 Coulomb Orbits 6.1 Introduction to Radial Hamiltonians The ability to separate multi-dimensional equations into independent one-dimensional parts has generally been the prerequisite to finding exact analytic solutions for physics problems. This applies to classical equations of motion as well as the quantum mechanical equations of Schrodinger or Heisenberg. Further, such separation often depends on having some kind of symmetry which may or may not be obvious. Spherical rotational symmetry R(3) which has been exploited in the preceding sections is one of the more obvious ones. Closely related to R(3) is the U() symmetry of the isotropic two-dimensional oscillator. For systems with spherical symmetry, the separation of the angular parts from the radial parts is to be expected. In the case of a single orbiting particle, such as an electron around a nucleus, the wave function for eigenstates has the simple product form n,,m (r,, ) = R n (r) Y m (, ) where the angular part has already been given in closed analytic form in Chapter 3 by using the properties of rotational or R(3) symmetry operators. Now we consider examples of possible radial functions R n (r). The radial functions will depend on the form of the radial potential function V(r), and only for certain special potentials will symmetry analysis be helpful. Fortunately, two of the most common potentials, the harmonic oscillator V(r)=kr / and the Coulomb potential V(r)=k/r have high symmetries which allow symmetry analysis to aid in there analysis, as well. The three-dimensional oscillator has an obvious U(3) symmetry analogous to the U() symmetry of the two-dimensional oscillator. The Coulomb potential has a fairly non-obvious symmetry R(4) based on the eccentricity vector conservation. For the vast majority of possible radial potentials V(r), approximate solutions of their radial differential equations seem to be the only resort. Naturally, a modern approach offers numerical solution as a viable option in all cases. So far, our only treatment of radial equations has been a brief analysis of the radial equation for the isotopic two-dimensional oscillator in Sec. 1.(b). That technique will be used again here for some three-dimensional examples including that of the oscillator.
6 W.G. Harter Coulomb Obits 6-6 (a) Kinetic energy operator in polar form For an isotropic or spherical potential that is a function V(r) of radius alone, the separation of this degree of freedom from the angular ones is an obvious consequence of choosing polar coordinates. x = r cos sin, y = r sin sin, z = r cos (6.1.1) From this follows the Jacobian transformation matrix. x x x r y y y cos sin rcos cos rsin sin = sin sin rsin cos rcos sin (6.1.a) r z z z cos r sin 0 r The inverse (Kajobian) matrix is useful, too. It is inverse by chain rules: r x x x r y y y r cos sin sin sin cos z cos cos sin cos sin = z r r r sin cos 0 z rsin rsin x a r µ µ r µ x b = x a x b = b a, etc. (6.1.b) A transpose of the latter gives Cartesian momentum operators in terms of radial-polar angle derivatives. r cos cos sin x x x x r cos sin r rsin r r sin cos cos = = sin sin (6.1.3a) y y y y r rsin r sin z z z z cos 0 r The inverse of this is a Jacobian differential operator relation based on the transpose of (6.1.a). x y z r r r r x x y z cos sin sin sin cos x = = rcos cos rsin cos r sin (6.1.3b) y y x y z r sin sin rcos sin 0 z z The radial part of the above, multiplied by r, is worth noting. r r = rcos sin x + rsin sin y + rcos z = r r = r (6.1.3c)
7 W.G. Harter Coulomb Obits 6-7 Classical Lagrangian mechanics begins with the first differential vector. dx a = xa µ r µ drµ = xa a x xa dr + d + r d, where: xa = { x, y,z} Its square arc-length or metric has three terms (instead of nine) because polar coordinates are orthogonal. ( ds) = ( dx a ) = x a x a a a,µ, r µ r dr µ dr = ( dr ) + r ( d ) + r sin ( d ) This gives a velocity-squared which, multiplied by half the mass, is the non-relativistic kinetic energy. T = 1 M s = r + r + r sin Hamiltonian mechanics uses momenta p µ that use inverse (Kajobian) relations like (6.1.3). p a = M x a = rµ µ x a p µ = r x a p r + x a p + x a p, where: x a = dx dt, dy dt, dz (6.1.4) dt Again, this gives a three-term expression for classical kinetic energy using polar momenta. T = 1 p M ( a ) = r µ r a µ x a x a p µ p = pr M + p Mr + p Mr sin (6.1.5a) This is consistent with the fundamental Lagrangian definitions of momentum. p r = T r = M r, p = T = Mr, p = T = Mr sin, (6.1.5b) The quantum Hamiltonian is simplified by the square L orbital momentum in Cartesian form. L = L L = ( r p) ( r p) = abc r b p c ade r d p e a,b,c,d,e = b,c,d,e ( bd ce be cd )r b p c r d p e (6.1.6) = ( r b p c r b p c r b p c r c p b ) b,c The Levi-Civita identity a abc ade = bd ce be cd is used followed by commutation relations. r a p b = p b r a + i ab, p b r a = r a p b i ab. (6.1.7) This is rearranged to give r p = r b p b terms like (6.1.3c) wherever possible. ( ) ( ) L = r b r b p c p c ir b p c bc r b p c p b r c + ir b p c bc = r b r b p c p c r b p b p c r c b,c b,c = ( r b r b p c p c r b p b r c p c + ir b p b ) b,c (6.1.8)
8 W.G. Harter Coulomb Obits 6-8 The result is concise expression involving the radial coordinate and momentum only. L = ( r r)( p p) ( r p)( r p) + i r p ( ) = r p ( rp r )( rp r ) + i( rp r ) The quantum kinetic energy reduces to the following. The classical T emerges if we ignore the tiny 0. ( rp r )( rp r ) i rp r T = p M = ( ) Mr + L Mr = p r ( rp r ) ip r + L p Mr Mr r 0 M + L Mr The Jacobian relation (6.1.3c) gives a representation for rp r equal to (i/)r r. r p = rp r = i r r = i r r = i r This gives the complete quantum kinetic energy operator in polar coordinates. T = M = ( r r )( r r ) + r r M = r r + rr M r + L Mr ( ) + L ( ) r + L Mr (6.1.9) (6.1.10a) = 1 M r r r r Mr This may be compared with the polar coordinate Laplacian form arising from (6.1.3a). T = 1 M r r ( r r ) Mr sin sin ( ) Mr sin (6.1.10b) From this we infer the coordinate differential form for the square-angular momentum. L = sin ( sin ) + sin (6.1.10c) (b) Angular-radial separation The classical Hamiltonian for a radial potential V(r) separates nicely by assuming angular momentum conservation in the kinetic part (6.1.9). H = p M +V ( r) p r 0 M + L Mr +V ( r) (6.1.11) The result is a 1-D potential V(r) plus a 1/r effective potential known as the centrifugal barrier term. The quantum Hamiltonian Schrodinger energy eigenvalue equation has a purely radial form. H = M 1 +V (r) = E = M r r r r + L Mr (6.1.1) This separates nicely by using the eigenvalues (+1) from (3.1.10b)) for L acting on spherical harmonics m=y m(), for which we have analytic expressions (3.1.15) and (3.1.16).
9 W.G. Harter Coulomb Obits 6-9 L m = L Y m ( ) = ( ) sin sin Y m + Y m sin ( ) = +1 ( )Y m ( ) (6.1.13a) Then the equation for the radial part R n (r) of the product wave function n,,m (r,, ) = R n (r) Y m (, ) (6.1.13b) is as follows. 1 d M r dr r dr dr + ( +1) Mr + V (r) E R = 0 (6.1.13c) Before proceeding with solutions for various radial potentials of this equation, it is instructive to note that a polar coordinate separation is possible in some cases even if the potential has angular dependence as well as radial, provide the angular dependency has the following form. 1 r r r r + 1 r sin sin + 1 r sin + u vr ( ) ( ) w ( ) r r sin = 0 (6.1.14a) The energy and potential functions are in natural atomic units here. = M M E, v(r) = V (r), etc. (6.1.14b) Substituting (6.1.13b) and multiplying through by r /RY gives the following. 1 R r r R r + r 1 Y ( vr ( )) + sin Y sin u ( )Y + 1 Y sin w ( ) r sin Y = 0 The radial part must equal a first separation constant k 1 since it is independent of the angular part. An ordinary radial differential equation like (6.1.13c) results. The separation constant is k 1 =(+1). 1 d r dr r dr dr + vr ( ) k 1 r R = 0 (6.1.15a) Also the angular part is a constant, too. It is minus the separation constant. A product wave function Y(, )= P()Q() (6.15.5b) is assumed in order to separate the rest. Q P k 1 + sin POsin u ( )PQ + P Q sin w ( ) r sin PQ = 0 Divide by PQ/sin and rearrange so that a second separation constant k is warranted. sin P sin P ( u ( ) k 1 )sin + 1 Q Q w ( ) = 0 The result is two more ordinary differential equations. sin d dp sin d d (( u( ) k1 )sin + k ) P = 0 (6.15.5c) (6.1.15d)
10 W.G. Harter Coulomb Obits 6-10 The Q equation is a 1-D wave equation. For w=0 it reduces to Bohr orbital plane-wave exponentials. Q=Q m () = A e im + A e -im (6.1.16) The separation constant is k = m according to (6.1.15d). It may be substituted in the P=P(q) equation (6.1.15c).to give the following. sin d dp sin d d (( u( ) k1 )sin m ) P = 0 (6.1.17a) For u=0 this reduces to Legendre's equation. We use separation constant is k 1 =(+1). sin d dp sin d d + ( ( +1 )sin m ) P = b) Compare this to angular eigenfunction equation (6.1.13c) which has not yet separated and angles. (c) Radial equations and solution We now consider some special cases of the radial differential equation (6.1.13c) when wellknown potentials are used. The simplest cases involve no potential at all. 1. Potential-free case With V(r)=0 the radial equation (6.1.13c) becomes the following in atomic energy units. 1 d r dr r dr +1 dr ( ) r R = 0 (6.1.18) If the energy also happens to be zero (E=0) then this is the radial part of the Laplace equation =0. d dr r dr dr ( +1)R = 0 (6.1.19a) The solutions for the latter are the any combination of the following powers. R = R ( r) = Ar + B 1 r +1 (6.1.19b) This is consistent with the multipole expansions (3.4.10), all of which are solutions to the Laplace equation for electrostatic potentials in a vacuum around charges. However, the radial functions for quantum particles in free space are quite different. In general the solutions of (6.1.18) can be given in terms of spherical Bessel functions. R = R ( r) = Aj ( r) + Bh ( r) These important functions will be discussed in connection with scattering theory.. Coulomb potential With v(r)=k/r the radial equation (6.1.13c) becomes the following in atomic energy units. 1 d r dr r dr +1 dr ( ) r + k r R = 0 (6.1.0a)
11 W.G. Harter Coulomb Obits 6-11 We shall here consider just the bound state case which requires a negative value for both the Coulomb constant (k=- k makes the potential attractive) and the energy (=- makes the wave bounded in space.) 1 d r dr r dr +1 dr ( ) r k r + R = 0 (6.1.0b) For the Coulomb potential, several re-scaling factors are conventional and convenient. First, a radial scale R(r) = X (r) r, dr dr = 1 dx r dr - X(r) r, d R dr = 1 d X r dr dx X (r) r - dr r 3 makes the radial integral r R dr become simply X dr and gives the standard form derived below. From d R dr + dr ( +1 )R r dr r + k R R = 0 r we get an X-wave equation which has no first-derivative term. 1 d X r dr ( +1) X r r + k X r r X r = 0 or: d X +1 dr ( ) r X + k X X = 0 (6.1.0c) r Finally, most Coulomb treatments use atomic units of distance and energy for radius r=a and E =E R. d X +1 d ( ) X + X X = 0 (6.1.0d) where /a = M k / and /a = M E / are given in terms of Rydberg energy E R and Bohr radius a. r = a = M k = 4 0 Me Z (6.1.0e) E = E R = Ma (6.1.0f) One should pause and realize that this equation has been solved on the average of at least once a day for over 75 years; that is, roughly 7,375 times. (And, here goes 7,376!) So, by now everyone thinks they have a pretty good scheme of units. The Bohr radius a= 0.58 Å was discussed in (5.6.3). The Rydberg energy for atomic number Z=1 (Hydrogen) is E R = 13.6eV. To begin solving such an equation it helps to check out how it behaves for large and small radii. ( for ): d X d X = 0 implies: X ~ e (6.1.1a) ( for 0): d X +1 d ( ) X +* 0 = 0 implies:x ~ +1 (6.1.1b)
12 W.G. Harter Coulomb Obits 6-1 This helps decide what factors to take out of the trial solution in order to possibly get a residual polynomial solution. Recall that the oscillator has an approximate e -ax solution at large distances, and that turns out to ride on every exact solution including the ground state (for which it is the whole story!). The same happens here as we will see; an e - factor will grace every bound-state Coulomb wavefunction. So the trial solution taken here will be the following expansion around =0. X = e µ f = e f +µ (6.1.) =0 =0 With the factor µ we are pretending we don't know that (6.1.1b) suggests a factor +1, and derive this later. Now we have the unenviable job of organizing the derivatives of the trial X. dx d = e +µ + e ( + µ )f f =0 =0 +µ1 d X d = e f +µ e ( + µ )f +µ1 +e ( + µ 1) ( + µ ) f +µ =0 =0 =0 To save space let us label (+µ+a) by a and write out enough terms to bring the sum power up to 0. d X d = e f 0 0 f f where: a = + µ + a =0 =0 =0 d X d = e f 0 =0 +e µ f 0 µ1 1 f e + µ µ1 ( ) f 0 µ + µ +1 =0 ( )µ f 1 µ1 + 1 f + 0 =0 In this way all the terms of the radial equation (6.1.0d) are assembled power-by-power. Let L=(+1).
13 W.G. Harter Coulomb Obits 6-13 X = e f +µ = e [ f ] =0 =0 +µ X = e f +µ1 = e f 0 µ1 +µ + [ f +1 ] =0 =0 LX = Le f +µ = e -Lf 0 µ - Lf 1 µ1 + [ Lf + ] +µ =0 =0 d X d = e µ ( µ1) f 0 µ + ( µ +1)µ f 1 µ1 + +µ [ f - 1 f f + ] =0 The sum of coefficients for each power must sum to zero. For the lowest power f 0 µ the sum gives µ(µ-1) = L = (+1) implies: µ = +1 or: µ =- (6.1.3) This is known as an index or indicial equation. Here we only accept the first solution (µ=+1) which also came from (6.1.1b) since the other possibility (µ =-) would cause µ to blow up at =0. A formula for coefficients f follows from zeroing the bracketed coefficients of +µ. ( 1 )f +1 = ( 1 L)f + where: L=(+1), or: 1 ( ( + µ +1) )f +1 = (( + µ +1) ( + µ + ) ( +1 ) f + (6.1.4) This is called a recursion relation. Let us replace by 1 and use indicial solution (µ=+1). ( ( + µ )1) f +1 = ( + µ )( + µ +1) ( +1) f = ( ( + +1)1 ) ( + +1) ( + + ) ( +1) f ( + +1 = ( )1) (6.1.5a) ( + +1) ( + + ) f Demanding that this recursion terminate for some integer = n, that is, the radial wave is a polynomial of order is n, leads to the Coulomb quantization condition on the energy parameter. 1 f n +1 = 0 implies: ( n + +1)1 = 0 or: = (6.1.5b) ( n + +1) From the energy definition (6.1.0f) follows the famous Rydberg energy level formula. 1 E = ( n + +1) E R = 1 ( n + +1) Ma = 1 N E R (6.1.5c) The energy is a function of only a single principle quantum number N. N = n (6.1.5d) N is a sum of radial quantum number n and the total angular quantum number. Since neither n nor may be negative the two quantum numbers are limited by each other in a given energy level belonging to a particular principle N, that is n is between zero and N--1 and is between zero to N-n- 1. The n-level structure for n(=0)=ns, n(=1)=np, n(=)=nd, n(=3)=nf, and n(=4)=ng levels are sketched in Fig below. Recall that each orbital level has a +1 degeneracy due to magnetic
14 W.G. Harter Coulomb Obits 6-14 or z-momentum m between and -. Together, this makes each (N = n + + 1)-level have a degeneracy of N. Fig Array of Coulomb states with their quantum numbers and degeneracy. The recursion relation (6.1.5a) may be expressed in terms of the principle N-number using the quantization condition (6.1.5b) N f +1 = ( + +1) ( + + ) f = ( + +1 N 1) N ( + +1) ( + + ) f (6.1.5e) ( N ) = N ( + +1) ( + + ) f Radial wave functions have the polynomial following coefficients. f 1 = (0 n) N ( + ) f 0, f = (1 n) N ( + 3) f 1, f 3 = ( n) N 3 ( + 4) f, = (0 n)(1 n) N ( + ) ( + 3) f 0, = 3 (0 n)(1 n)( n) N 3 ( + ) ( + 3) ( + 4 ) f 0, The resulting wavefunction is an N-degree polynomial with a factor f 0 µ e - =f 0 e -/N R N ( r ) = X N a = f 0 e /N a 1 + (0 n) + ( ) where: r = a (0 n)(1 n) + N! + ( )( + 3) N (0 n)(1 n)( n) + 3! + ( )( + 3) ( + 4 ) N (6.1.6) 3
15 W.G. Harter Coulomb Obits 6-15 (d) Radial wavefunctions Some examples of these waves are plotted in Fig The number and type of nodal surfaces for the complete Coulomb wave function R n (r) Y m (, ) are listed in the following table. n=n--1 concentric r-spheres -m - cones ("magic" angles) m -planes (azimuthal nodes) N-1 nodal surfaces of some kind (6.1.7) The principle or total quantum number N determines the number of nodal surfaces while the radial quantum number n=n--1 determines the number of radial nodes. The total angular quantum number determines the total number of angular modes and m determines the number of azimuthal nodes. Fig Lowest Coulomb atomic radial wavefunctions
16 W.G. Harter Coulomb Obits 6-16
The Hydrogen Atom. Dr. Sabry El-Taher 1. e 4. U U r
 The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
Time part of the equation can be separated by substituting independent equation
 Lecture 9 Schrödinger Equation in 3D and Angular Momentum Operator In this section we will construct 3D Schrödinger equation and we give some simple examples. In this course we will consider problems where
Lecture 9 Schrödinger Equation in 3D and Angular Momentum Operator In this section we will construct 3D Schrödinger equation and we give some simple examples. In this course we will consider problems where
The Hydrogen Atom. Nucleus charge +Ze mass m 1 coordinates x 1, y 1, z 1. Electron charge e mass m 2 coordinates x 2, y 2, z 2
 The Hydrogen Ato The only ato that can be solved exactly. The results becoe the basis for understanding all other atos and olecules. Orbital Angular Moentu Spherical Haronics Nucleus charge +Ze ass coordinates
The Hydrogen Ato The only ato that can be solved exactly. The results becoe the basis for understanding all other atos and olecules. Orbital Angular Moentu Spherical Haronics Nucleus charge +Ze ass coordinates
Physics 342 Lecture 23. Radial Separation. Lecture 23. Physics 342 Quantum Mechanics I
 Physics 342 Lecture 23 Radial Separation Lecture 23 Physics 342 Quantum Mechanics I Friday, March 26th, 2010 We begin our spherical solutions with the simplest possible case zero potential. Aside from
Physics 342 Lecture 23 Radial Separation Lecture 23 Physics 342 Quantum Mechanics I Friday, March 26th, 2010 We begin our spherical solutions with the simplest possible case zero potential. Aside from
The 3 dimensional Schrödinger Equation
 Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
1 Reduced Mass Coordinates
 Coulomb Potential Radial Wavefunctions R. M. Suter April 4, 205 Reduced Mass Coordinates In classical mechanics (and quantum) problems involving several particles, it is convenient to separate the motion
Coulomb Potential Radial Wavefunctions R. M. Suter April 4, 205 Reduced Mass Coordinates In classical mechanics (and quantum) problems involving several particles, it is convenient to separate the motion
PHYS 3313 Section 001 Lecture # 22
 PHYS 3313 Section 001 Lecture # 22 Dr. Barry Spurlock Simple Harmonic Oscillator Barriers and Tunneling Alpha Particle Decay Schrodinger Equation on Hydrogen Atom Solutions for Schrodinger Equation for
PHYS 3313 Section 001 Lecture # 22 Dr. Barry Spurlock Simple Harmonic Oscillator Barriers and Tunneling Alpha Particle Decay Schrodinger Equation on Hydrogen Atom Solutions for Schrodinger Equation for
which implies that we can take solutions which are simultaneous eigen functions of
 Module 1 : Quantum Mechanics Chapter 6 : Quantum mechanics in 3-D Quantum mechanics in 3-D For most physical systems, the dynamics is in 3-D. The solutions to the general 3-d problem are quite complicated,
Module 1 : Quantum Mechanics Chapter 6 : Quantum mechanics in 3-D Quantum mechanics in 3-D For most physical systems, the dynamics is in 3-D. The solutions to the general 3-d problem are quite complicated,
20 The Hydrogen Atom. Ze2 r R (20.1) H( r, R) = h2 2m 2 r h2 2M 2 R
 20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
Quantum Theory of Angular Momentum and Atomic Structure
 Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
Quantum Theory of Angular Momentum and Atomic Structure VBS/MRC Angular Momentum 0 Motivation...the questions Whence the periodic table? Concepts in Materials Science I VBS/MRC Angular Momentum 1 Motivation...the
Quantum Mechanics in Three Dimensions
 Physics 342 Lecture 21 Quantum Mechanics in Three Dimensions Lecture 21 Physics 342 Quantum Mechanics I Monday, March 22nd, 21 We are used to the temporal separation that gives, for example, the timeindependent
Physics 342 Lecture 21 Quantum Mechanics in Three Dimensions Lecture 21 Physics 342 Quantum Mechanics I Monday, March 22nd, 21 We are used to the temporal separation that gives, for example, the timeindependent
(3.1) Module 1 : Atomic Structure Lecture 3 : Angular Momentum. Objectives In this Lecture you will learn the following
 Module 1 : Atomic Structure Lecture 3 : Angular Momentum Objectives In this Lecture you will learn the following Define angular momentum and obtain the operators for angular momentum. Solve the problem
Module 1 : Atomic Structure Lecture 3 : Angular Momentum Objectives In this Lecture you will learn the following Define angular momentum and obtain the operators for angular momentum. Solve the problem
Chem 467 Supplement to Lecture 19 Hydrogen Atom, Atomic Orbitals
 Chem 467 Supplement to Lecture 19 Hydrogen Atom, Atomic Orbitals Pre-Quantum Atomic Structure The existence of atoms and molecules had long been theorized, but never rigorously proven until the late 19
Chem 467 Supplement to Lecture 19 Hydrogen Atom, Atomic Orbitals Pre-Quantum Atomic Structure The existence of atoms and molecules had long been theorized, but never rigorously proven until the late 19
4 Power Series Solutions: Frobenius Method
 4 Power Series Solutions: Frobenius Method Now the ODE adventure takes us to series solutions for ODEs, a technique A & W, that is often viable, valuable and informative. These can be readily applied Sec.
4 Power Series Solutions: Frobenius Method Now the ODE adventure takes us to series solutions for ODEs, a technique A & W, that is often viable, valuable and informative. These can be readily applied Sec.
The Hydrogen Atom Chapter 20
 4/4/17 Quantum mechanical treatment of the H atom: Model; The Hydrogen Atom Chapter 1 r -1 Electron moving aroundpositively charged nucleus in a Coulombic field from the nucleus. Potential energy term
4/4/17 Quantum mechanical treatment of the H atom: Model; The Hydrogen Atom Chapter 1 r -1 Electron moving aroundpositively charged nucleus in a Coulombic field from the nucleus. Potential energy term
ONE AND MANY ELECTRON ATOMS Chapter 15
 See Week 8 lecture notes. This is exactly the same as the Hamiltonian for nonrigid rotation. In Week 8 lecture notes it was shown that this is the operator for Lˆ 2, the square of the angular momentum.
See Week 8 lecture notes. This is exactly the same as the Hamiltonian for nonrigid rotation. In Week 8 lecture notes it was shown that this is the operator for Lˆ 2, the square of the angular momentum.
Outline Spherical symmetry Free particle Coulomb problem Keywords and References. Central potentials. Sourendu Gupta. TIFR, Mumbai, India
 Central potentials Sourendu Gupta TIFR, Mumbai, India Quantum Mechanics 1 2013 3 October, 2013 Outline 1 Outline 2 Rotationally invariant potentials 3 The free particle 4 The Coulomb problem 5 Keywords
Central potentials Sourendu Gupta TIFR, Mumbai, India Quantum Mechanics 1 2013 3 October, 2013 Outline 1 Outline 2 Rotationally invariant potentials 3 The free particle 4 The Coulomb problem 5 Keywords
C/CS/Phys C191 Particle-in-a-box, Spin 10/02/08 Fall 2008 Lecture 11
 C/CS/Phys C191 Particle-in-a-box, Spin 10/0/08 Fall 008 Lecture 11 Last time we saw that the time dependent Schr. eqn. can be decomposed into two equations, one in time (t) and one in space (x): space
C/CS/Phys C191 Particle-in-a-box, Spin 10/0/08 Fall 008 Lecture 11 Last time we saw that the time dependent Schr. eqn. can be decomposed into two equations, one in time (t) and one in space (x): space
Quantum Mechanics: The Hydrogen Atom
 Quantum Mechanics: The Hydrogen Atom 4th April 9 I. The Hydrogen Atom In this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen
Quantum Mechanics: The Hydrogen Atom 4th April 9 I. The Hydrogen Atom In this next section, we will tie together the elements of the last several sections to arrive at a complete description of the hydrogen
MITOCW watch?v=fxlzy2l1-4w
 MITOCW watch?v=fxlzy2l1-4w PROFESSOR: We spoke about the hydrogen atom. And in the hydrogen atom, we drew the spectrum, so the table, the data of spectrum of a quantum system. So this is a question that
MITOCW watch?v=fxlzy2l1-4w PROFESSOR: We spoke about the hydrogen atom. And in the hydrogen atom, we drew the spectrum, so the table, the data of spectrum of a quantum system. So this is a question that
8.1 The hydrogen atom solutions
 8.1 The hydrogen atom solutions Slides: Video 8.1.1 Separating for the radial equation Text reference: Quantum Mechanics for Scientists and Engineers Section 10.4 (up to Solution of the hydrogen radial
8.1 The hydrogen atom solutions Slides: Video 8.1.1 Separating for the radial equation Text reference: Quantum Mechanics for Scientists and Engineers Section 10.4 (up to Solution of the hydrogen radial
One-electron Atom. (in spherical coordinates), where Y lm. are spherical harmonics, we arrive at the following Schrödinger equation:
 One-electron Atom The atomic orbitals of hydrogen-like atoms are solutions to the Schrödinger equation in a spherically symmetric potential. In this case, the potential term is the potential given by Coulomb's
One-electron Atom The atomic orbitals of hydrogen-like atoms are solutions to the Schrödinger equation in a spherically symmetric potential. In this case, the potential term is the potential given by Coulomb's
The Central Force Problem: Hydrogen Atom
 The Central Force Problem: Hydrogen Atom B. Ramachandran Separation of Variables The Schrödinger equation for an atomic system with Z protons in the nucleus and one electron outside is h µ Ze ψ = Eψ, r
The Central Force Problem: Hydrogen Atom B. Ramachandran Separation of Variables The Schrödinger equation for an atomic system with Z protons in the nucleus and one electron outside is h µ Ze ψ = Eψ, r
IV. Electronic Spectroscopy, Angular Momentum, and Magnetic Resonance
 IV. Electronic Spectroscopy, Angular Momentum, and Magnetic Resonance The foundation of electronic spectroscopy is the exact solution of the time-independent Schrodinger equation for the hydrogen atom.
IV. Electronic Spectroscopy, Angular Momentum, and Magnetic Resonance The foundation of electronic spectroscopy is the exact solution of the time-independent Schrodinger equation for the hydrogen atom.
quantization condition.
 /8/016 PHYS 34 Modern Physics Atom II: Hydrogen Atom Roadmap for Exploring Hydrogen Atom Today Contents: a) Schrodinger Equation for Hydrogen Atom b) Angular Momentum in Quantum Mechanics c) Quantum Number
/8/016 PHYS 34 Modern Physics Atom II: Hydrogen Atom Roadmap for Exploring Hydrogen Atom Today Contents: a) Schrodinger Equation for Hydrogen Atom b) Angular Momentum in Quantum Mechanics c) Quantum Number
The Hydrogen Atom. Chapter 18. P. J. Grandinetti. Nov 6, Chem P. J. Grandinetti (Chem. 4300) The Hydrogen Atom Nov 6, / 41
 The Hydrogen Atom Chapter 18 P. J. Grandinetti Chem. 4300 Nov 6, 2017 P. J. Grandinetti (Chem. 4300) The Hydrogen Atom Nov 6, 2017 1 / 41 The Hydrogen Atom Hydrogen atom is simplest atomic system where
The Hydrogen Atom Chapter 18 P. J. Grandinetti Chem. 4300 Nov 6, 2017 P. J. Grandinetti (Chem. 4300) The Hydrogen Atom Nov 6, 2017 1 / 41 The Hydrogen Atom Hydrogen atom is simplest atomic system where
2m r2 (~r )+V (~r ) (~r )=E (~r )
 Review of the Hydrogen Atom The Schrodinger equation (for 1D, 2D, or 3D) can be expressed as: ~ 2 2m r2 (~r, t )+V (~r ) (~r, t )=i~ @ @t The Laplacian is the divergence of the gradient: r 2 =r r The time-independent
Review of the Hydrogen Atom The Schrodinger equation (for 1D, 2D, or 3D) can be expressed as: ~ 2 2m r2 (~r, t )+V (~r ) (~r, t )=i~ @ @t The Laplacian is the divergence of the gradient: r 2 =r r The time-independent
Lecture 4 Quantum mechanics in more than one-dimension
 Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Angular momentum. Quantum mechanics. Orbital angular momentum
 Angular momentum 1 Orbital angular momentum Consider a particle described by the Cartesian coordinates (x, y, z r and their conjugate momenta (p x, p y, p z p. The classical definition of the orbital angular
Angular momentum 1 Orbital angular momentum Consider a particle described by the Cartesian coordinates (x, y, z r and their conjugate momenta (p x, p y, p z p. The classical definition of the orbital angular
1 Commutators (10 pts)
 Final Exam Solutions 37A Fall 0 I. Siddiqi / E. Dodds Commutators 0 pts) ) Consider the operator  = Ĵx Ĵ y + ĴyĴx where J i represents the total angular momentum in the ith direction. a) Express both
Final Exam Solutions 37A Fall 0 I. Siddiqi / E. Dodds Commutators 0 pts) ) Consider the operator  = Ĵx Ĵ y + ĴyĴx where J i represents the total angular momentum in the ith direction. a) Express both
Schrödinger equation for central potentials
 Chapter 2 Schrödinger equation for central potentials In this chapter we will extend the concepts and methods introduced in the previous chapter for a one-dimensional problem to a specific and very important
Chapter 2 Schrödinger equation for central potentials In this chapter we will extend the concepts and methods introduced in the previous chapter for a one-dimensional problem to a specific and very important
Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom
 Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Happy April Fools Day Example / Worked Problems What is the ratio of the
Physics 228 Today: Ch 41: 1-3: 3D quantum mechanics, hydrogen atom Website: Sakai 01:750:228 or www.physics.rutgers.edu/ugrad/228 Happy April Fools Day Example / Worked Problems What is the ratio of the
The Hydrogen atom. Chapter The Schrödinger Equation. 2.2 Angular momentum
 Chapter 2 The Hydrogen atom In the previous chapter we gave a quick overview of the Bohr model, which is only really valid in the semiclassical limit. cf. section 1.7.) We now begin our task in earnest
Chapter 2 The Hydrogen atom In the previous chapter we gave a quick overview of the Bohr model, which is only really valid in the semiclassical limit. cf. section 1.7.) We now begin our task in earnest
Lecture 4 Quantum mechanics in more than one-dimension
 Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
Lecture 4 Quantum mechanics in more than one-dimension Background Previously, we have addressed quantum mechanics of 1d systems and explored bound and unbound (scattering) states. Although general concepts
df(x) = h(x) dx Chemistry 4531 Mathematical Preliminaries Spring 2009 I. A Primer on Differential Equations Order of differential equation
 Chemistry 4531 Mathematical Preliminaries Spring 009 I. A Primer on Differential Equations Order of differential equation Linearity of differential equation Partial vs. Ordinary Differential Equations
Chemistry 4531 Mathematical Preliminaries Spring 009 I. A Primer on Differential Equations Order of differential equation Linearity of differential equation Partial vs. Ordinary Differential Equations
1.4 Solution of the Hydrogen Atom
 The phase of α can freely be chosen to be real so that α = h (l m)(l + m + 1). Then L + l m = h (l m)(l + m + 1) l m + 1 (1.24) L l m = h (l + m)(l m + 1) l m 1 (1.25) Since m is bounded, it follow that
The phase of α can freely be chosen to be real so that α = h (l m)(l + m + 1). Then L + l m = h (l m)(l + m + 1) l m + 1 (1.24) L l m = h (l + m)(l m + 1) l m 1 (1.25) Since m is bounded, it follow that
Chemistry 532 Practice Final Exam Fall 2012 Solutions
 Chemistry 53 Practice Final Exam Fall Solutions x e ax dx π a 3/ ; π sin 3 xdx 4 3 π cos nx dx π; sin θ cos θ + K x n e ax dx n! a n+ ; r r r r ˆL h r ˆL z h i φ ˆL x i hsin φ + cot θ cos φ θ φ ) ˆLy i
Chemistry 53 Practice Final Exam Fall Solutions x e ax dx π a 3/ ; π sin 3 xdx 4 3 π cos nx dx π; sin θ cos θ + K x n e ax dx n! a n+ ; r r r r ˆL h r ˆL z h i φ ˆL x i hsin φ + cot θ cos φ θ φ ) ˆLy i
Physics 606, Quantum Mechanics, Final Exam NAME ( ) ( ) + V ( x). ( ) and p( t) be the corresponding operators in ( ) and x( t) : ( ) / dt =...
 Physics 606, Quantum Mechanics, Final Exam NAME Please show all your work. (You are graded on your work, with partial credit where it is deserved.) All problems are, of course, nonrelativistic. 1. Consider
Physics 606, Quantum Mechanics, Final Exam NAME Please show all your work. (You are graded on your work, with partial credit where it is deserved.) All problems are, of course, nonrelativistic. 1. Consider
Introduction to Quantum Mechanics (Prelude to Nuclear Shell Model) Heisenberg Uncertainty Principle In the microscopic world,
 Introduction to Quantum Mechanics (Prelude to Nuclear Shell Model) Heisenberg Uncertainty Principle In the microscopic world, x p h π If you try to specify/measure the exact position of a particle you
Introduction to Quantum Mechanics (Prelude to Nuclear Shell Model) Heisenberg Uncertainty Principle In the microscopic world, x p h π If you try to specify/measure the exact position of a particle you
Quantum Mechanics Solutions
 Quantum Mechanics Solutions (a (i f A and B are Hermitian, since (AB = B A = BA, operator AB is Hermitian if and only if A and B commute So, we know that [A,B] = 0, which means that the Hilbert space H
Quantum Mechanics Solutions (a (i f A and B are Hermitian, since (AB = B A = BA, operator AB is Hermitian if and only if A and B commute So, we know that [A,B] = 0, which means that the Hilbert space H
4/21/2010. Schrödinger Equation For Hydrogen Atom. Spherical Coordinates CHAPTER 8
 CHAPTER 8 Hydrogen Atom 8.1 Spherical Coordinates 8.2 Schrödinger's Equation in Spherical Coordinate 8.3 Separation of Variables 8.4 Three Quantum Numbers 8.5 Hydrogen Atom Wave Function 8.6 Electron Spin
CHAPTER 8 Hydrogen Atom 8.1 Spherical Coordinates 8.2 Schrödinger's Equation in Spherical Coordinate 8.3 Separation of Variables 8.4 Three Quantum Numbers 8.5 Hydrogen Atom Wave Function 8.6 Electron Spin
Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras. Lecture - 21 Square-Integrable Functions
 Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 21 Square-Integrable Functions (Refer Slide Time: 00:06) (Refer Slide Time: 00:14) We
Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 21 Square-Integrable Functions (Refer Slide Time: 00:06) (Refer Slide Time: 00:14) We
Lecture 10. Central potential
 Lecture 10 Central potential 89 90 LECTURE 10. CENTRAL POTENTIAL 10.1 Introduction We are now ready to study a generic class of three-dimensional physical systems. They are the systems that have a central
Lecture 10 Central potential 89 90 LECTURE 10. CENTRAL POTENTIAL 10.1 Introduction We are now ready to study a generic class of three-dimensional physical systems. They are the systems that have a central
atoms and light. Chapter Goal: To understand the structure and properties of atoms.
 Quantum mechanics provides us with an understanding of atomic structure and atomic properties. Lasers are one of the most important applications of the quantummechanical properties of atoms and light.
Quantum mechanics provides us with an understanding of atomic structure and atomic properties. Lasers are one of the most important applications of the quantummechanical properties of atoms and light.
1 r 2 sin 2 θ. This must be the case as we can see by the following argument + L2
 PHYS 4 3. The momentum operator in three dimensions is p = i Therefore the momentum-squared operator is [ p 2 = 2 2 = 2 r 2 ) + r 2 r r r 2 sin θ We notice that this can be written as sin θ ) + θ θ r 2
PHYS 4 3. The momentum operator in three dimensions is p = i Therefore the momentum-squared operator is [ p 2 = 2 2 = 2 r 2 ) + r 2 r r r 2 sin θ We notice that this can be written as sin θ ) + θ θ r 2
Orbital Angular Momentum of the Hydrogen Atom
 Orbital Angular Momentum of the Hydrogen Atom Department of Physics and Astronomy School of Arts and Sciences Rutgers, The State University of New Jersey 136 Frelinghuysen Road Piscataway, NJ 08854-8019
Orbital Angular Momentum of the Hydrogen Atom Department of Physics and Astronomy School of Arts and Sciences Rutgers, The State University of New Jersey 136 Frelinghuysen Road Piscataway, NJ 08854-8019
H atom solution. 1 Introduction 2. 2 Coordinate system 2. 3 Variable separation 4
 H atom solution Contents 1 Introduction 2 2 Coordinate system 2 3 Variable separation 4 4 Wavefunction solutions 6 4.1 Solution for Φ........................... 6 4.2 Solution for Θ...........................
H atom solution Contents 1 Introduction 2 2 Coordinate system 2 3 Variable separation 4 4 Wavefunction solutions 6 4.1 Solution for Φ........................... 6 4.2 Solution for Θ...........................
Brief review of Quantum Mechanics (QM)
 Brief review of Quantum Mechanics (QM) Note: This is a collection of several formulae and facts that we will use throughout the course. It is by no means a complete discussion of QM, nor will I attempt
Brief review of Quantum Mechanics (QM) Note: This is a collection of several formulae and facts that we will use throughout the course. It is by no means a complete discussion of QM, nor will I attempt
Quantum Mechanics in 3-Dimensions
 Quantum Mechanics in 3-Dimensions Pavithran S Iyer, 2nd yr BSc Physics, Chennai Mathematical Institute Email: pavithra@cmi.ac.in August 28 th, 2009 1 Schrodinger equation in Spherical Coordinates 1.1 Transforming
Quantum Mechanics in 3-Dimensions Pavithran S Iyer, 2nd yr BSc Physics, Chennai Mathematical Institute Email: pavithra@cmi.ac.in August 28 th, 2009 1 Schrodinger equation in Spherical Coordinates 1.1 Transforming
Atomic Systems (PART I)
 Atomic Systems (PART I) Lecturer: Location: Recommended Text: Dr. D.J. Miller Room 535, Kelvin Building d.miller@physics.gla.ac.uk Joseph Black C407 (except 15/1/10 which is in Kelvin 312) Physics of Atoms
Atomic Systems (PART I) Lecturer: Location: Recommended Text: Dr. D.J. Miller Room 535, Kelvin Building d.miller@physics.gla.ac.uk Joseph Black C407 (except 15/1/10 which is in Kelvin 312) Physics of Atoms
Mathematical Tripos Part IB Michaelmas Term Example Sheet 1. Values of some physical constants are given on the supplementary sheet
 Mathematical Tripos Part IB Michaelmas Term 2015 Quantum Mechanics Dr. J.M. Evans Example Sheet 1 Values of some physical constants are given on the supplementary sheet 1. Whenasampleofpotassiumisilluminatedwithlightofwavelength3
Mathematical Tripos Part IB Michaelmas Term 2015 Quantum Mechanics Dr. J.M. Evans Example Sheet 1 Values of some physical constants are given on the supplementary sheet 1. Whenasampleofpotassiumisilluminatedwithlightofwavelength3
Solved radial equation: Last time For two simple cases: infinite and finite spherical wells Spherical analogs of 1D wells We introduced auxiliary func
 Quantum Mechanics and Atomic Physics Lecture 16: The Coulomb Potential http://www.physics.rutgers.edu/ugrad/361 h / d/361 Prof. Sean Oh Solved radial equation: Last time For two simple cases: infinite
Quantum Mechanics and Atomic Physics Lecture 16: The Coulomb Potential http://www.physics.rutgers.edu/ugrad/361 h / d/361 Prof. Sean Oh Solved radial equation: Last time For two simple cases: infinite
A few principles of classical and quantum mechanics
 A few principles of classical and quantum mechanics The classical approach: In classical mechanics, we usually (but not exclusively) solve Newton s nd law of motion relating the acceleration a of the system
A few principles of classical and quantum mechanics The classical approach: In classical mechanics, we usually (but not exclusively) solve Newton s nd law of motion relating the acceleration a of the system
Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours.
 Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours. There are 10 problems, totalling 180 points. Do all problems. Answer all problems in the white books provided.
Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours. There are 10 problems, totalling 180 points. Do all problems. Answer all problems in the white books provided.
Quantum Numbers. principal quantum number: n. angular momentum quantum number: l (azimuthal) magnetic quantum number: m l
 Quantum Numbers Quantum Numbers principal quantum number: n angular momentum quantum number: l (azimuthal) magnetic quantum number: m l Principal quantum number: n related to size and energy of orbital
Quantum Numbers Quantum Numbers principal quantum number: n angular momentum quantum number: l (azimuthal) magnetic quantum number: m l Principal quantum number: n related to size and energy of orbital
3. Quantum Mechanics in 3D
 3. Quantum Mechanics in 3D 3.1 Introduction Last time, we derived the time dependent Schrödinger equation, starting from three basic postulates: 1) The time evolution of a state can be expressed as a unitary
3. Quantum Mechanics in 3D 3.1 Introduction Last time, we derived the time dependent Schrödinger equation, starting from three basic postulates: 1) The time evolution of a state can be expressed as a unitary
We now turn to our first quantum mechanical problems that represent real, as
 84 Lectures 16-17 We now turn to our first quantum mechanical problems that represent real, as opposed to idealized, systems. These problems are the structures of atoms. We will begin first with hydrogen-like
84 Lectures 16-17 We now turn to our first quantum mechanical problems that represent real, as opposed to idealized, systems. These problems are the structures of atoms. We will begin first with hydrogen-like
Schrödinger equation for central potentials
 Chapter 2 Schrödinger equation for central potentials In this chapter we will extend the concepts and methods introduced in the previous chapter ifor a one-dimenional problem to a specific and very important
Chapter 2 Schrödinger equation for central potentials In this chapter we will extend the concepts and methods introduced in the previous chapter ifor a one-dimenional problem to a specific and very important
Lectures 21 and 22: Hydrogen Atom. 1 The Hydrogen Atom 1. 2 Hydrogen atom spectrum 4
 Lectures and : Hydrogen Atom B. Zwiebach May 4, 06 Contents The Hydrogen Atom Hydrogen atom spectrum 4 The Hydrogen Atom Our goal here is to show that the two-body quantum mechanical problem of the hydrogen
Lectures and : Hydrogen Atom B. Zwiebach May 4, 06 Contents The Hydrogen Atom Hydrogen atom spectrum 4 The Hydrogen Atom Our goal here is to show that the two-body quantum mechanical problem of the hydrogen
Lecture 11 Spin, orbital, and total angular momentum Mechanics. 1 Very brief background. 2 General properties of angular momentum operators
 Lecture Spin, orbital, and total angular momentum 70.00 Mechanics Very brief background MATH-GA In 9, a famous experiment conducted by Otto Stern and Walther Gerlach, involving particles subject to a nonuniform
Lecture Spin, orbital, and total angular momentum 70.00 Mechanics Very brief background MATH-GA In 9, a famous experiment conducted by Otto Stern and Walther Gerlach, involving particles subject to a nonuniform
Physics 6303 Lecture 11 September 24, LAST TIME: Cylindrical coordinates, spherical coordinates, and Legendre s equation
 Physics 6303 Lecture September 24, 208 LAST TIME: Cylindrical coordinates, spherical coordinates, and Legendre s equation, l l l l l l. Consider problems that are no axisymmetric; i.e., the potential depends
Physics 6303 Lecture September 24, 208 LAST TIME: Cylindrical coordinates, spherical coordinates, and Legendre s equation, l l l l l l. Consider problems that are no axisymmetric; i.e., the potential depends
ψ s a ˆn a s b ˆn b ψ Hint: Because the state is spherically symmetric the answer can depend only on the angle between the two directions.
 1. Quantum Mechanics (Fall 2004) Two spin-half particles are in a state with total spin zero. Let ˆn a and ˆn b be unit vectors in two arbitrary directions. Calculate the expectation value of the product
1. Quantum Mechanics (Fall 2004) Two spin-half particles are in a state with total spin zero. Let ˆn a and ˆn b be unit vectors in two arbitrary directions. Calculate the expectation value of the product
P3317 HW from Lecture and Recitation 7
 P3317 HW from Lecture 1+13 and Recitation 7 Due Oct 16, 018 Problem 1. Separation of variables Suppose we have two masses that can move in 1D. They are attached by a spring, yielding a Hamiltonian where
P3317 HW from Lecture 1+13 and Recitation 7 Due Oct 16, 018 Problem 1. Separation of variables Suppose we have two masses that can move in 1D. They are attached by a spring, yielding a Hamiltonian where
CHEM-UA 127: Advanced General Chemistry I
 1 CHEM-UA 127: Advanced General Chemistry I Notes for Lecture 11 Nowthatwehaveintroducedthebasicconceptsofquantummechanics, wecanstarttoapplythese conceptsto build up matter, starting from its most elementary
1 CHEM-UA 127: Advanced General Chemistry I Notes for Lecture 11 Nowthatwehaveintroducedthebasicconceptsofquantummechanics, wecanstarttoapplythese conceptsto build up matter, starting from its most elementary
d 1 µ 2 Θ = 0. (4.1) consider first the case of m = 0 where there is no azimuthal dependence on the angle φ.
 4 Legendre Functions In order to investigate the solutions of Legendre s differential equation d ( µ ) dθ ] ] + l(l + ) m dµ dµ µ Θ = 0. (4.) consider first the case of m = 0 where there is no azimuthal
4 Legendre Functions In order to investigate the solutions of Legendre s differential equation d ( µ ) dθ ] ] + l(l + ) m dµ dµ µ Θ = 0. (4.) consider first the case of m = 0 where there is no azimuthal
1 Schroenger s Equation for the Hydrogen Atom
 Schroenger s Equation for the Hydrogen Atom Here is the Schroedinger equation in D in spherical polar coordinates. Note that the definitions of θ and φ are the exact reverse of what they are in mathematics.
Schroenger s Equation for the Hydrogen Atom Here is the Schroedinger equation in D in spherical polar coordinates. Note that the definitions of θ and φ are the exact reverse of what they are in mathematics.
Representation theory and quantum mechanics tutorial Spin and the hydrogen atom
 Representation theory and quantum mechanics tutorial Spin and the hydrogen atom Justin Campbell August 3, 2017 1 Representations of SU 2 and SO 3 (R) 1.1 The following observation is long overdue. Proposition
Representation theory and quantum mechanics tutorial Spin and the hydrogen atom Justin Campbell August 3, 2017 1 Representations of SU 2 and SO 3 (R) 1.1 The following observation is long overdue. Proposition
r R A 1 r R B + 1 ψ(r) = αψ A (r)+βψ B (r) (5) where we assume that ψ A and ψ B are ground states: ψ A (r) = π 1/2 e r R A ψ B (r) = π 1/2 e r R B.
 Molecules Initial questions: What are the new aspects of molecules compared to atoms? What part of the electromagnetic spectrum can we probe? What can we learn from molecular spectra? How large a molecule
Molecules Initial questions: What are the new aspects of molecules compared to atoms? What part of the electromagnetic spectrum can we probe? What can we learn from molecular spectra? How large a molecule
Second quantization: where quantization and particles come from?
 110 Phys460.nb 7 Second quantization: where quantization and particles come from? 7.1. Lagrangian mechanics and canonical quantization Q: How do we quantize a general system? 7.1.1.Lagrangian Lagrangian
110 Phys460.nb 7 Second quantization: where quantization and particles come from? 7.1. Lagrangian mechanics and canonical quantization Q: How do we quantize a general system? 7.1.1.Lagrangian Lagrangian
CHAPTER 6 Quantum Mechanics II
 CHAPTER 6 Quantum Mechanics II 6.1 6.2 6.3 6.4 6.5 6.6 6.7 The Schrödinger Wave Equation Expectation Values Infinite Square-Well Potential Finite Square-Well Potential Three-Dimensional Infinite-Potential
CHAPTER 6 Quantum Mechanics II 6.1 6.2 6.3 6.4 6.5 6.6 6.7 The Schrödinger Wave Equation Expectation Values Infinite Square-Well Potential Finite Square-Well Potential Three-Dimensional Infinite-Potential
Phys 622 Problems Chapter 5
 1 Phys 622 Problems Chapter 5 Problem 1 The correct basis set of perturbation theory Consider the relativistic correction to the electron-nucleus interaction H LS = α L S, also known as the spin-orbit
1 Phys 622 Problems Chapter 5 Problem 1 The correct basis set of perturbation theory Consider the relativistic correction to the electron-nucleus interaction H LS = α L S, also known as the spin-orbit
Physics 342 Lecture 26. Angular Momentum. Lecture 26. Physics 342 Quantum Mechanics I
 Physics 342 Lecture 26 Angular Momentum Lecture 26 Physics 342 Quantum Mechanics I Friday, April 2nd, 2010 We know how to obtain the energy of Hydrogen using the Hamiltonian operator but given a particular
Physics 342 Lecture 26 Angular Momentum Lecture 26 Physics 342 Quantum Mechanics I Friday, April 2nd, 2010 We know how to obtain the energy of Hydrogen using the Hamiltonian operator but given a particular
Legendre Polynomials and Angular Momentum
 University of Connecticut DigitalCommons@UConn Chemistry Education Materials Department of Chemistry August 006 Legendre Polynomials and Angular Momentum Carl W. David University of Connecticut, Carl.David@uconn.edu
University of Connecticut DigitalCommons@UConn Chemistry Education Materials Department of Chemistry August 006 Legendre Polynomials and Angular Momentum Carl W. David University of Connecticut, Carl.David@uconn.edu
Self-consistent Field
 Chapter 6 Self-consistent Field A way to solve a system of many electrons is to consider each electron under the electrostatic field generated by all other electrons. The many-body problem is thus reduced
Chapter 6 Self-consistent Field A way to solve a system of many electrons is to consider each electron under the electrostatic field generated by all other electrons. The many-body problem is thus reduced
Physics 227 Exam 2. Rutherford said that if you really understand something you should be able to explain it to your grandmother.
 Physics 227 Exam 2 Rutherford said that if you really understand something you should be able to explain it to your grandmother. For each of the topics on the next two pages, write clear, concise, physical
Physics 227 Exam 2 Rutherford said that if you really understand something you should be able to explain it to your grandmother. For each of the topics on the next two pages, write clear, concise, physical
Welcome back to PHY 3305
 Welcome back to PHY 3305 Today s Lecture: Hydrogen Atom Part I John von Neumann 1903-1957 One-Dimensional Atom To analyze the hydrogen atom, we must solve the Schrodinger equation for the Coulomb potential
Welcome back to PHY 3305 Today s Lecture: Hydrogen Atom Part I John von Neumann 1903-1957 One-Dimensional Atom To analyze the hydrogen atom, we must solve the Schrodinger equation for the Coulomb potential
eff (r) which contains the influence of angular momentum. On the left is
 1 Fig. 13.1. The radial eigenfunctions R nl (r) of bound states in a square-well potential for three angular-momentum values, l = 0, 1, 2, are shown as continuous lines in the left column. The form V (r)
1 Fig. 13.1. The radial eigenfunctions R nl (r) of bound states in a square-well potential for three angular-momentum values, l = 0, 1, 2, are shown as continuous lines in the left column. The form V (r)
Nuclear Shell Model. Experimental evidences for the existence of magic numbers;
 Nuclear Shell Model It has been found that the nuclei with proton number or neutron number equal to certain numbers 2,8,20,28,50,82 and 126 behave in a different manner when compared to other nuclei having
Nuclear Shell Model It has been found that the nuclei with proton number or neutron number equal to certain numbers 2,8,20,28,50,82 and 126 behave in a different manner when compared to other nuclei having
CHAPTER 8 The Quantum Theory of Motion
 I. Translational motion. CHAPTER 8 The Quantum Theory of Motion A. Single particle in free space, 1-D. 1. Schrodinger eqn H ψ = Eψ! 2 2m d 2 dx 2 ψ = Eψ ; no boundary conditions 2. General solution: ψ
I. Translational motion. CHAPTER 8 The Quantum Theory of Motion A. Single particle in free space, 1-D. 1. Schrodinger eqn H ψ = Eψ! 2 2m d 2 dx 2 ψ = Eψ ; no boundary conditions 2. General solution: ψ
Multielectron Atoms.
 Multielectron Atoms. Chem 639. Spectroscopy. Spring 00 S.Smirnov Atomic Units Mass m e 1 9.109 10 31 kg Charge e 1.60 10 19 C Angular momentum 1 1.055 10 34 J s Permittivity 4 0 1 1.113 10 10 C J 1 m 1
Multielectron Atoms. Chem 639. Spectroscopy. Spring 00 S.Smirnov Atomic Units Mass m e 1 9.109 10 31 kg Charge e 1.60 10 19 C Angular momentum 1 1.055 10 34 J s Permittivity 4 0 1 1.113 10 10 C J 1 m 1
Total Angular Momentum for Hydrogen
 Physics 4 Lecture 7 Total Angular Momentum for Hydrogen Lecture 7 Physics 4 Quantum Mechanics I Friday, April th, 008 We have the Hydrogen Hamiltonian for central potential φ(r), we can write: H r = p
Physics 4 Lecture 7 Total Angular Momentum for Hydrogen Lecture 7 Physics 4 Quantum Mechanics I Friday, April th, 008 We have the Hydrogen Hamiltonian for central potential φ(r), we can write: H r = p
Physics 113!!!!! Spring 2009!! Quantum Theory Seminar #9
 Physics 113!!!!! Spring 2009!! Quantum Theory Seminar #9 Readings:!! Zettili - Chapter - 6!! Boccio - Chapter - 9 - Sections 9.6.1-9.6.13 Presentations: 3D Finite Well!!!!! _Sarah_ (Section 9.6.3)! 2D
Physics 113!!!!! Spring 2009!! Quantum Theory Seminar #9 Readings:!! Zettili - Chapter - 6!! Boccio - Chapter - 9 - Sections 9.6.1-9.6.13 Presentations: 3D Finite Well!!!!! _Sarah_ (Section 9.6.3)! 2D
1 Solutions in cylindrical coordinates: Bessel functions
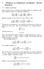 1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
Probability and Normalization
 Probability and Normalization Although we don t know exactly where the particle might be inside the box, we know that it has to be in the box. This means that, ψ ( x) dx = 1 (normalization condition) L
Probability and Normalization Although we don t know exactly where the particle might be inside the box, we know that it has to be in the box. This means that, ψ ( x) dx = 1 (normalization condition) L
Deformed (Nilsson) shell model
 Deformed (Nilsson) shell model Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 January 31, 2011 NUCS 342 (Lecture 9) January 31, 2011 1 / 35 Outline 1 Infinitely deep potential
Deformed (Nilsson) shell model Introduction to Nuclear Science Simon Fraser University Spring 2011 NUCS 342 January 31, 2011 NUCS 342 (Lecture 9) January 31, 2011 1 / 35 Outline 1 Infinitely deep potential
Sparks CH301. Quantum Mechanics. Waves? Particles? What and where are the electrons!? UNIT 2 Day 3. LM 14, 15 & 16 + HW due Friday, 8:45 am
 Sparks CH301 Quantum Mechanics Waves? Particles? What and where are the electrons!? UNIT 2 Day 3 LM 14, 15 & 16 + HW due Friday, 8:45 am What are we going to learn today? The Simplest Atom - Hydrogen Relate
Sparks CH301 Quantum Mechanics Waves? Particles? What and where are the electrons!? UNIT 2 Day 3 LM 14, 15 & 16 + HW due Friday, 8:45 am What are we going to learn today? The Simplest Atom - Hydrogen Relate
( ) = 9φ 1, ( ) = 4φ 2.
 Chemistry 46 Dr Jean M Standard Homework Problem Set 6 Solutions The Hermitian operator A ˆ is associated with the physical observable A Two of the eigenfunctions of A ˆ are and These eigenfunctions are
Chemistry 46 Dr Jean M Standard Homework Problem Set 6 Solutions The Hermitian operator A ˆ is associated with the physical observable A Two of the eigenfunctions of A ˆ are and These eigenfunctions are
MITOCW ocw lec8
 MITOCW ocw-5.112-lec8 The following content is provided by MIT OpenCourseWare under a Creative Commons license. Additional information about our license and MIT OpenCourseWare in general is available at
MITOCW ocw-5.112-lec8 The following content is provided by MIT OpenCourseWare under a Creative Commons license. Additional information about our license and MIT OpenCourseWare in general is available at
An Introduction to Hyperfine Structure and Its G-factor
 An Introduction to Hyperfine Structure and Its G-factor Xiqiao Wang East Tennessee State University April 25, 2012 1 1. Introduction In a book chapter entitled Model Calculations of Radiation Induced Damage
An Introduction to Hyperfine Structure and Its G-factor Xiqiao Wang East Tennessee State University April 25, 2012 1 1. Introduction In a book chapter entitled Model Calculations of Radiation Induced Damage
Atomic Structure and Atomic Spectra
 Atomic Structure and Atomic Spectra Atomic Structure: Hydrogenic Atom Reading: Atkins, Ch. 10 (7 판 Ch. 13) The principles of quantum mechanics internal structure of atoms 1. Hydrogenic atom: one electron
Atomic Structure and Atomic Spectra Atomic Structure: Hydrogenic Atom Reading: Atkins, Ch. 10 (7 판 Ch. 13) The principles of quantum mechanics internal structure of atoms 1. Hydrogenic atom: one electron
Chem 442 Review for Exam 2. Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative (3D) components.
 Chem 44 Review for Exam Hydrogenic atoms: The Coulomb energy between two point charges Ze and e: V r Ze r Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative
Chem 44 Review for Exam Hydrogenic atoms: The Coulomb energy between two point charges Ze and e: V r Ze r Exact separation of the Hamiltonian of a hydrogenic atom into center-of-mass (3D) and relative
Quantum Mechanics in Three Dimensions
 8 Quantum Mechanics in Three Dimensions Chapter Outline 8.1 Particle in a Three-Dimensional Box 8. Central Forces and Angular Momentum 8.3 Space Quantization 8.4 Quantization of Angular Momentum and Energy
8 Quantum Mechanics in Three Dimensions Chapter Outline 8.1 Particle in a Three-Dimensional Box 8. Central Forces and Angular Momentum 8.3 Space Quantization 8.4 Quantization of Angular Momentum and Energy
Angular momentum & spin
 Angular momentum & spin January 8, 2002 1 Angular momentum Angular momentum appears as a very important aspect of almost any quantum mechanical system, so we need to briefly review some basic properties
Angular momentum & spin January 8, 2002 1 Angular momentum Angular momentum appears as a very important aspect of almost any quantum mechanical system, so we need to briefly review some basic properties
PHYS 502 Lecture 8: Legendre Functions. Dr. Vasileios Lempesis
 PHYS 502 Lecture 8: Legendre Functions Dr. Vasileios Lempesis Introduction Legendre functions or Legendre polynomials are the solutions of Legendre s differential equation that appear when we separate
PHYS 502 Lecture 8: Legendre Functions Dr. Vasileios Lempesis Introduction Legendre functions or Legendre polynomials are the solutions of Legendre s differential equation that appear when we separate
Physics 342 Lecture 22. The Hydrogen Atom. Lecture 22. Physics 342 Quantum Mechanics I
 Physics 342 Lecture 22 The Hydrogen Atom Lecture 22 Physics 342 Quantum Mechanics I Friday, March 28th, 2008 We now begin our discussion of the Hydrogen atom. Operationally, this is just another choice
Physics 342 Lecture 22 The Hydrogen Atom Lecture 22 Physics 342 Quantum Mechanics I Friday, March 28th, 2008 We now begin our discussion of the Hydrogen atom. Operationally, this is just another choice
Single Electron Atoms
 Single Electron Atoms In this section we study the spectrum and wave functions of single electron atoms. These are hydrogen, singly ionized He, doubly ionized Li, etc. We will write the formulae for hydrogen
Single Electron Atoms In this section we study the spectrum and wave functions of single electron atoms. These are hydrogen, singly ionized He, doubly ionized Li, etc. We will write the formulae for hydrogen
Potential energy, from Coulomb's law. Potential is spherically symmetric. Therefore, solutions must have form
 Lecture 6 Page 1 Atoms L6.P1 Review of hydrogen atom Heavy proton (put at the origin), charge e and much lighter electron, charge -e. Potential energy, from Coulomb's law Potential is spherically symmetric.
Lecture 6 Page 1 Atoms L6.P1 Review of hydrogen atom Heavy proton (put at the origin), charge e and much lighter electron, charge -e. Potential energy, from Coulomb's law Potential is spherically symmetric.
Addition of Angular Momenta
 Addition of Angular Momenta What we have so far considered to be an exact solution for the many electron problem, should really be called exact non-relativistic solution. A relativistic treatment is needed
Addition of Angular Momenta What we have so far considered to be an exact solution for the many electron problem, should really be called exact non-relativistic solution. A relativistic treatment is needed
QUANTUM MECHANICS AND ATOMIC STRUCTURE
 5 CHAPTER QUANTUM MECHANICS AND ATOMIC STRUCTURE 5.1 The Hydrogen Atom 5.2 Shell Model for Many-Electron Atoms 5.3 Aufbau Principle and Electron Configurations 5.4 Shells and the Periodic Table: Photoelectron
5 CHAPTER QUANTUM MECHANICS AND ATOMIC STRUCTURE 5.1 The Hydrogen Atom 5.2 Shell Model for Many-Electron Atoms 5.3 Aufbau Principle and Electron Configurations 5.4 Shells and the Periodic Table: Photoelectron
QUANTUM MECHANICS Intro to Basic Features
 PCES 4.21 QUANTUM MECHANICS Intro to Basic Features 1. QUANTUM INTERFERENCE & QUANTUM PATHS Rather than explain the rules of quantum mechanics as they were devised, we first look at a more modern formulation
PCES 4.21 QUANTUM MECHANICS Intro to Basic Features 1. QUANTUM INTERFERENCE & QUANTUM PATHS Rather than explain the rules of quantum mechanics as they were devised, we first look at a more modern formulation
