TECHNICAL REPORT # 58 APRIL Design resampling for interim sample size recalculation
|
|
|
- Brice Shaw
- 5 years ago
- Views:
Transcription
1 TECHNICAL REPORT # 58 APRIL 2012 Design resampling for interim sample size recalculation Sergey Tarima, Peng He, Tao Wang, Aniko Szabo Division of Biostatistics, Medical College of Wisconsin Abstract Internal pilot designs allow re-estimation of the sample size at the interim analysis using available information on nuisance parameters. In general, this affects the Type I and II error rates. We propose a method based on resampling the whole design at the interim analysis, starting with sample size recalculation at the observed interim analysis values of nuisance parameters, and finishing with the decision to accept or reject the null hypothesis. This internal resampling is performed under both the null and under the alternative hypotheses allowing the estimation of the bias of the type I error and power. Finally, the bias corrected error rates are used in the original sample size calculation procedure to obtain an updated sample size. We explore the proposed resampling approach under a set of simulation scenarios and compare it with several others previously published internal pilot designs. KEYWORDS: Internal Pilot; Sample Size; Power Calculation; Hypothesis Testing; Study Design. 1 Introduction Ethical, financial, and recruitment constraints prevent researchers from enrolling arbitrarily many patients for a study to achieve statistically significant results. Pilot studies are used to provide information on parameters needed to determine an appropriate sample size for a larger confirmatory 1
2 study for which external funding is sought. The error variance and baseline rates are common examples of such parameters. In observational studies, there are often additional nuisance parameters involved in the sample size estimation, such as the proportion of patients belonging to each group, or the distribution and effect of other demographic variables which will be adjusted for in the analysis. These parameters can be estimated from the pilot data and incorporated into the sample size estimation. The effect size is also needed for sample size calculation, but it is usually defined as the smallest clinically relevant value. Having spent substantial effort and resources on the pilot study, investigators often would wish to include the pilot data in the final data analysis. However the use of this pilot data in both the design of subsequent data collection estimation of the sample size) as well as in the final analysis requires an adjustment in order to control the type I error rate and power appropriately. The methodology of internal pilot studies provides the tools for such adjustments. Multiple authors have discussed procedures for sample size re-estimation based on estimates of nuisance parameters obtained at an interim analysis. Proschan [7] and Friede and Kieser [2] review interim pilot designs. The majority of work in this area has been focused on twoarm prospective randomized studies with normally distributed or binomial outcomes. Sample size re-estimation for a general linear hypothesis tested through the general linear model was explored by Coffey and Muller [1]; an interesting extension toward linear mixed models was suggested by Glueck and Muller [4]. However neither of these papers accounts for randomness in the distribution of the covariate of interest. An interim pilot design for the analysis of covariance model ANCOVA) was explored via simulations by Friede and Kieser [3], while Gurka et al [5] analyzed the effect of unknown group size proportions. In this manuscript we propose a general methodology of constructing interim pilot designs for any situation for which a sample size calculation formula exists. Such a formula may include nuisance parameters that are not available at the beginning of the study, but could be estimated if data were available. The method is based on resampling the entire design, starting with sample size recalculation at the interim analysis using the values of nuisance parameters observed in the internal pilot data see Section 2). This resampling is performed under both the null and alternative hypotheses allowing the estimation of the bias of the type I error and power. We correct for this bias at the interim sample size recalculation to obtain a final 2
3 sample size. In Section 3 we explore the proposed approach under a set of simulation scenarios and compare its performance with several others previously published internal pilot designs. An illustrative example is presented in Section 4. The article is concluded with a short summary in Section 5. 2 Methodology Study design We explore an internal pilot design when n 1 < n max ) subjects are enrolled in the study as an internal pilot and the second stage sample size is recalculated at the interim analysis to secure the desired type I error, α, and power, 1 β. We assume that there always exists an upper bound for the total sample size, n max, chosen for example, from budgetary or time constraints. Consider the problem of independent sampling, where each observation Y i is generated from a known p.d.f. or p.m.f.) f Y y θ, η) with unknown parameters θ and η. We plan to test the null hypothesis H 0 : θ = θ 0 versus the alternative hypothesis H 1 : θ θ 0 with power 1001 β)% achieved at θ = θ 1. The parameters η are not in the research focus and will be treated as nuisance. Instead of focusing on the behavior of test statistics we direct our attention to the properties of decision functions, δ D), where δ ) is a binary function 1 to reject H 0, 0 otherwise) associated with a study design D. In this manuscript, we define a study design as 1) a set of data collection rules including the sample size calculation/recalculation procedure, 2) a definition of a test statistic, and 3) a definition of the decision rule itself. These definitions should cover all possible situations including rules for exceptions, such as having no observations in a certain category, or zero variance, etc. It is convenient to consider parameterized study designs, D α, β, θ 0, θ 1, other parameters), where in addition to the previously described α, β, θ 0, and θ 1 other parameters may be present, for example n 1 and n max. Fixed sample size and internal pilot designs To clarify the introduced concept of study design and notations, we show how several common designs fit into the described framework. 3
4 Designs with fixed sample size calculation D 1 ): These designs are associated with a rule for calculating a fixed sample size v) at the start of the study based on α, β, θ 0 and θ 1, and an assumed value of η. The functional form of a test statistic, T v = T v Y 1,..., Y v ) with a corresponding critical value calculation, is also defined in this design. Let D 1t α, β, θ0, θ 1, η 0)) D 1 ) denote the study design for the one sample t-test. The sample size is calculated for given α and β under an assumed value of the standard deviation, η 0). Thus, η 0) is the only nuisance parameter of D 1t. The statistical properties of D 1t are described by the power function P θ D 1t ) = Pr ) Tvα,β,θ0,θ 1,η 0) ) > kv) θ,d1t, 1) where vα, β, θ 0, θ 1, η 0) ) is the sample size formula, T v is the t-statistic, kv) = t v 1,α/2 is the critical value. A two sample t-test with a fixed allocation ratio r) always keeps r100% units allocated to group 1 and 1 r)100% to group 2. We denote this design as D 2t α, β, θ0, θ 1, η 0), r ) ). The nuisance parameters η 0) = η 0) 1, η0) 2 denote guesses on the standard deviation η 1 ) and the mean of group 1 η 2 ). We do not include r among the nuisance parameters since we assumed that the allocation ratio is controlled by the investigator via, say, a randomized block design. If the data are normally distributed, the distribution of the two sample t-test does not depend on η 2 under either the null or the alternative hypotheses. Only η 0) 1 affects the sample size calculations. If the allocation ratio is not fixed, then r describes the long term allocation ratio i.e., the probability that an observational unit belongs to group 1). Then, the distribution of the test statistic is a binomial mixture of two t-statistics with varying degrees of freedom. This design is denoted as D 2tr α, β, θ0, θ 1, η 0)), where η 0) = η 0) 1, η 0) 2, η 0) 3 ). Here we added η 0) a guessed value of the true allocation ratio η 3. The use of η 3 instead of r differentiates parameters responsible for random and fixed allocation ratios. Internal pilot designs D 2 ): Fixed sample designs can be augmented to become internal pilot designs by adding rules/parameters describing the interim sample size re-estimation procedure. A naive approach to interim sample size recalculation uses ˆη, the interim analysis estimate of the nuisance parameter without further adjustments. Then, the naive internal pilot design 3, 4
5 for the one sample t-test, D 1t,IPN α, β, θ 0, θ 1, n 1, n max ) D 2 ), is an alternative to D 1t, which does not use η 0) but depends on n 1 and n max. Its power function is P θ D 1t,IPN ) = Pr ) T vα,β,θ0,θ 1,ˆη θ ) > kv) θ,d 1t,IPN, 2) where ˆη θ depends on η, n 1, n max and possibly θ. In this manuscript we assume that ˆη θ is independent of θ, that is ˆη = ˆη θ. A naive internal pilot-based sample size recalculation for a two sample t-test will be denoted by D 2t,IPN. This design was first analyzed by Wittes and Brittain [9]. We also consider the internal pilot design D 2t,IPS suggested by Stein [8], which slightly modifies the functional form of the two-sample t-statistic, whereas D 2t,IPN uses the classical two sample t-statistic for T v. Internal sample size recalculation makes the final sample size a random variable, which makes the distribution of the test statistic T v and therefore the critical value of the test difficult to calculate. Exact control of the type I error is achieved by D 2t,IPS, but this is rather an exception than a rule for internal pilot designs. In general, the true type I error rate is rarely controlled, Eδ D 2t,IPN α, β, θ 0, θ 1, n 1, n max ) H 0 ) = a α, β D 2t,IPN ) α. The desired power is not controlled in either Stein s or the naive internal pilot designs, Eδ D α, β, θ 0, θ 1, n 1, n max ) H 1 ) = 1 b α, β D) 1 β. Sample size recalculation via resampling We propose a new approach to sample size re-estimation after the internal pilot that maintains both the type I and type II error rates. This approach is applicable to any internal pilot design. Key idea: For a design D D 2 we find α new and β new to control the desired type I error and power, Eδ D α new, β new, θ 0, θ 1, n 1, n max ) H 0 ) = α 5
6 and Eδ D α new, β new, θ 0, θ 1, n 1, n max ) H 1 ) = 1 β. This definition leads to a fully defined internal pilot procedure D a α, β, θ 0, θ 1, n 1, n max ), since all the details about sample size re-estimation, final hypothesis testing, etc are already defined in D. Implementation: At the interim analysis we estimate ˆη and perform the following resampling procedure ) with M iterations. For each i = 1,..., M, we generate Y i) 1,..., Y n i) 1 from f Y y θ 0, ˆη), estimate v i [n 1, n max ] based on ) these n 1 observations, generate additional v i n 1 ) observations Y i) i) n 1 +1,..., Y v i from f Y y θ 0, ˆη), and calculate T v i) i on this i th sample. We add the subscript i to highlight dependence on iteration. The estimated type I error rate is â α, β D) = 1 M M i=1 I T i) v i > k i ) α, where k i is the critical value for an originally assumed distribution of T i) v i. On the logit scale logitx) = ln x/1 x))) the bias-corrected α new can be expressed as or logitα new ) = logitα) [logitâ) logitα)] α new = α 2 1 â) 1 α) 2 â + α 2 1 â). 3) Then, we perform a similar resampling ) procedure to find β new. For i = 1,..., M, we generate Y i) 1,..., Y n i) 1 from f Y y θ 1, ˆη), estimate v i [n 1, n max ] on these n 1 observations using α new and β in the sample ) size formula, generate additional v i n 1 ) observations Y i) i) n 1 +1,..., Y v i from f Y y θ 1, ˆη), and calculate T i) v i on this i th sample. The estimated power 1 ˆb α new, β D) = 1 M M i=1 I T i) v i > k i ) 1 β 6
7 leads to the bias-corrected value β 1 2 ˆb ) β new = 1 β) 2 ˆb + β 2 1 ˆb ). 4) This internal resampling scheme assumes that the functional form of the data distribution, including the distribution of covariates, is known. For example, we can assume that the covariates follow a normal distribution. Alternatively, a nonparametric bootstrap resampling of the internal pilot data can be used. The illustrative example in Section 4 uses a combination of nonparametric the distribution of covariates) and parametric the distribution of outcome conditional on covariates) bootstraps for internal resampling. 3 Simulation Studies Here we consider a few scenarios showing how the proposed general methodology works and compare it with other applicable approaches. Internal pilot for a one sample t-test Let Y 1,..., Y n1,... be an i.i.d. sample from Nθ, η 2 ) and the one sample t-test is planned for testing H 0 : θ = 0 against H 1 : θ 0, with target power 1001 β)% achieved at θ = θ 1. The test statistic on v observations is T v = v Ȳ v /S v, where Ȳv = v 1 v i=1 Y i and Sv 2 = v 1) 1 v i=1 Yi Ȳv) 2. Under H 1, T v has a noncentral t-distribution with v 1 degrees of freedom and noncentrality parameter ω 1 = vθ 1 θ 0 )/η. The cumulative distribution function will be denoted as P T v > x v 1, ω 1 ). Then, the sample size at a known η is a solution to P T v > k v 1, ω 1 ) = 1 β, P T v > k v 1, 0) = α, 5) where the critical value k is also found from these equations. Given an assumed value η 0), one can proceed with fixed sample size calculation for the design D 1t α, β, θ0, θ 1, η 0)). 7
8 Design D 1t,IPN α, β, θ 0, θ 1, n 1, n max ) does not formally depend on η and uses the internally estimated ˆη = 1 n 1 Yi n 1 1 Ȳ ) 2 when solving Equations 5). We define D a 1t,IPN α, β, θ 0, θ 1, n 1, n max ) as D 1t,IPN α, β, θ 0, θ 1, n 1, n max ) augmented with internal recalculation of α and β using Equations 3) and 4). Then, v is found by resolving i=1 P T v > k v 1, ˆω 1 ) = 1 β new, P T v > k v 1, 0) = α new, 6) where ˆω 1 = vθ 1 θ 0 )/ˆη. Tables 1 and 2 summarize the results of this simulation study with internal pilot size of n 1 = 5 and 10. For the fixed sample design D 1t we consider the unrealistic case of η = η 0), that is perfect knowledge of the nuisance parameter. Thus for this example the goal is to be as close as possible to this ideal case. Both tables show the expected inflation of the type I error rate by the naive design D 1t,IPN accompanied by loss of power with increasing values of the standard deviation. The resampling-adjusted design D a 1t,IPN eliminates most of the type I error inflation and power deflation, especially for n 1 = 10. The price for this correction is a higher expected sample size with more variability. Internal pilot for a two sample t-test with a fixed group allocation In this section we consider the randomized block design. The randomization is performed within blocks to ensure the desired allocation ratio r. The size of the block b) determines the magnitude of the sample size increments. Then, the internal pilot of n 1 experimental or observational) units consists of m 1 = n 1 /b blocks separating n 1 units to two treatment groups with n 10 and n 11 observations respectively, n 1 = n 10 + n 11. Mathematically, we consider two data generating processes Y 10,..., Y n10 0,..., Y v0 0,... Nη 2, η 2 1 ) 8
9 Table 1: Monte-Carlo Type I error, Power, and Sample Sizes; 100, 000 simulations; one sample t-test designs, n 1 = 10, n max = 300. D 1t D 1t,IPN D a 1t,IPN η Type I error Power ENSD) ) ) ) ) ) ) ) ) 9
10 Table 2: Monte-Carlo Type I error, Power, and Sample Sizes; 100, 000 simulations; one sample t-test designs, n 1 = 5, n max = 300. D 1t D 1t,IPN D a 1t,IPN η Type I error Power ENSD) ) ) ) ) ) ) ) ) ) ) 10
11 and where n 10, n 11, v 0 and v 1 satisfy Y 11,..., Y n11 1,..., Y v1 1,... Nη 2 + θ, η 2 1 ), n 10 n 10 + n 11 = n 10 n 1 = v 1 v 1 + v 2 = v 1 v = r. For testing H 0 : θ = θ 0 versus H 1 : θ θ 0 on v observations without internal sample size recalculation and known r and η 1 ) the two-sample t-test statistic v2 v 1 v 2) T v = v = Ȳ1 Ȳ0) η 1 1 v 1 v 2 = Ȳ 1 Ȳ0 v2 1) S 1 + v 1 1)S v1 1 ) 1/2 v2 1)S 1 η1 2 + v 1 1)S 0 η1 2 Z + ω 2 v 2) 1 χ 2 v 2 7) has a noncentral t-distribution with v 2 degrees of freedom and noncentrality parameter θ 1 θ 0 ω 2 =, η 1 v v1 1 where Ȳj = v 1 vj j i=1 Y ij, Sj 2 = v j 1) 1 v j i=1 Yij Ȳj) 2, j = 0, 1, Z is a standard normal random variable, and χ 2 v 2 is a χ 2 random variable with v 2 degrees of freedom. Then, v and k are found from P T v > k v 2, ω 2 ) = 1 β, P T v > k v 2, 0) = α. 8) Design D ) 2t α, β, θ0, θ 1, η 0)) uses a two dimensional nuisance guess η 0) = η 0) 1, η 0) 2. Its internal pilot counterpart D 2t,IPN α, β, θ 0, θ 1, n 1, n max ) uses ˆη 0) = ˆη 1, ˆη 2 ) based on the interim pilot for sample size recalculation. We also consider a restricted naive internal pilot, D 2t,IPNR, which is a variation of D 2t,IPN, which sets 2n 1 as the lower bound for the total sample size. This design approximates the Wittes-Brittain[9] idea of performing the interim analysis at half the originally planned sample size, and allowing only 11
12 Table 3: Monte-Carlo Type I error, Power, and Sample Sizes; 100, 000 simulations; two sample t-test designs; n 1 = per group); fixed allocation, r = 0.5 η 1 D 2t D 2t,IPS D 2t,IPN D 2t,IPNR D a 2t,IPN Type I error Power ENSD) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) increase in the targeted study size. The use of internal resampling to find α new and β new defines D a 2t,IPN. Simulations in Tables 3 and 4 consider only r = 0.5 for different sizes of internal pilot. This simplification allows us to compare the performance of several designs D 1t,IPN fixed sample size, under the unrealistic assumption of a correctly guessed η = η 0) ), D 2t,IPN naive internal pilot), D 2t,IPNR restricted naive internal pilot), D 2t,IPS naive internal pilot with Stein s approach to modify the test statistic, see [8]) and D a 2t,IPN internal resampling adjusted naive approach). Internal pilot for a two sample t-test with a random group allocation We saw that for a fixed sample size calculation with a fixed allocation ratio the test statistic 7) has a central under H 0 ) or noncentral under H 1 ) 12
13 Table 4: Monte-Carlo Type I error, Power, and Sample Sizes; 100, 000 simulations; two sample t-test designs; n 1 = 10 5 per group); fixed allocation, r = 0.5 η 1 D 2t D 2t,IPS D 2t,IPN D 2t,IPNR D a 2t,IPN Type I error Power ENSD) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) 13
14 t-distribution. However random allocation of subjects to groups leads to a different distribution. Since only the noncentrality parameter dependens on v 1 and v 2, the distribution under H 0 does not change, but under H 1 it becomes a mixture with P T v > k v 2, θ 1, θ 0, η 3 ) = v v 1 =0 v! v 1!v 2! ηv η 3) v 2 P T v > k v 2, ω 2 v 1, v 2 )). 9) Moreover, the test statistic is not defined if minv 1, v 2 ) 1 and has to be extended to these possible situations. For example, at v 1 = 1 or v 2 = 1 one can estimate the pooled standard deviation on one sample only; for the case v 1 = v 2 = 0 one can set T v = 0. Thus, even a fixed sample size calculation faces substantial complications in deriving the distribution of the two sample t-test statistic under H 1. In practice, the random aspect of the allocation is usually ignored in the sample size estimation formulas and the formula for a fixed allocation is used instead. Fixed allocation sample size calculation leads to two numbers v 1 and v 2, but real life recruitment rarely allows to enroll exactly v 1 and v 2 subjects in groups 1 and 2 respectively. Then, the investigator faces a dilemma: stop after recruiting v 1 + v 2 subjects even if there are not enough subjects in one of the groups, or continue recruiting until at least v 1 and v 2 subjects are enrolled in the groups 1 and 2 respectively. Our simulation study in Table 5 considers a fixed sample size calculation design D 2tr ) with a recruitment until both numbers are met. By analogy with previous simulation examples we consider D 2tr,IPN naive sample size recalculation), D 2tr,IPNR restricted naive sample size recalculation, the total sample size is at least twice as large as the size of the internal pilot) and the adjusted by interim resampling design D a 2tr,IPN. The results show that while type I error rate is only slightly inflated for the internal pilot designs, all the designs except our proposed method have above targeted power. 4 Example The examples described in the simulation studies were relatively simple to allow comparisons with existing internal pilot methodology. In this section we show a more realistic example of a hypothetical observational study. 14
15 Table 5: Monte-Carlo Type I error, Power, and Sample Sizes; 100, 000 simulations; two sample t-test designs; n 1 = 20; random allocation η 1 η 3 D 2tr D 2tr,IPN D 2tr,IPNR D a 2tr,IPN Type I error Power ENSD) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) ) 15
16 Measurements of prostate-specific antigen PSA) levels are widely used for screening and diagnosing prostate cancer. PSA levels are known to be associated with measures of disease aggressiveness such as tumor stage as well as demographic characteristics predictive of screening behavior such as race/ethnicity, marital status, etc. A hypothetical) investigator in Atlanta, GA wishes to conduct a study to evaluate whether the effect of Black versus White race on PSA levels is the same for localized versus regionally or distantly extended tumors. In practice he or she would turn to the SEER cancer registry, as we will for the source of data, but for the sake of the example let s assume that the information of interest is not available in the registry. In fact, PSA levels were not available in SEER until recently. The specific goal of the study is to test the interaction effect of race White vs Black) and tumor stage localized vs others) on lnp SA) values controlling for the effect of marital status married vs others) and ethnicity Hispanic vs others). We use the linear regression model lnpsa i ) = β 0 + β 1 W i + β 2 L i + β 3 W i L i + β 4 M i + β 5 H i + ǫ i, 10) where W i, L i, M i, and H i are, respectively, indicators of White race, localized tumor, married status, and Hispanic ethnicity of the i th subject. The random noise ǫ i is assumed to follow a normal model with the zero mean and a finite unknown variance σ 2. We formulate the research question about the interaction via H 0 : β 3 = 0 and wish to design a study that would have 80% power to detect a 1.5-fold difference in the race effect among the localized versus non-localized tumors, corresponding to β 3 = ln1.5). To calculate the study sample size we use the formula proposed by Hsieh et al [6]. If X represents the predictor of interest and Z stands the other predictors, then the sample size required to detect an effect with a partial regression coefficient of δ with power 1001 β)% at a two-sided significance level α is ) 2 zα/2 + z β φ 2 r) + 3 N =, 11) 1 RX.Z 2 where r = δσ X /σ, RX.Z 2 is the multiple correlation coefficient of X and Z, and φr) = 1 ln1 + r)/1 r)) is Fisher s z-transform. As typical, this 2 expression includes multiple nuisance parameters that are difficult to obtain a priori. 16
17 Table 6: Linear regression on internal pilot data, n 1 = 100. Estimate Std.Error t value p value Intercept ˆβ 0 ) < White ˆβ 1 ) Localized ˆβ 2 ) Hispanic ˆβ 4 ) Married ˆβ 5 ) White Localized ˆβ 3 ) To simulate the conduct of the study we extracted a sample of 8142 prostate cancer cases from the Atlanta Metropolitan area SEER registry. Our inclusion criteria limited our scope to records with black or white races, year of diagnosis , and observed PSA values. The data were sorted by year and month of diagnosis to mimic a prospective study. The results of internal pilot based on first n 1 = 100 observations are reported in Table 6. To update α we performed 30, 000 internal resamplings. At the k th iteration of this resampling, we performed the following steps: 1. used nonparametric bootstrap to) resample the joint distribution of the predictors B k) i, R k) i, M k) i, H k) i, i = 1,..., n 1 ; 2. generated simulated lnpsa k) i ) values under the null hypothesis β 3 = 0) from the conditional normal distribution N ˆβ0 + ˆβ 1 B k) i + ˆβ 2 R k) i + ˆβ 4 M k) i where ˆσ 2 is the internal pilot estimate of σ 2 ; + ˆβ 5 H k) i, ˆσ 2 ), 12) 3. fitted the regression model 10) on the k th internal pilot resample; 4. estimated the total sample size N k) using 11); 5. generated additional N k) n 1 observations from the conditional distribution 12); 6. refitted the model 10) to the k th resample with N k) elements and tested H 0 with the P-value of the estimate of β 3. 17
18 Table 7: Regression model for the total sample, N = Estimate Std.Error t value p value Intercept ˆβ 0 ) < White ˆβ 1 ) Localized ˆβ 2 ) < Hispanic ˆβ 4 ) Married ˆβ 5 ) White Localized ˆβ 3 ) Note that in Step 3 it is possible that some of the regression parameters are inestimable due to singularity of the design matrix. In our example, nonlocalized disease is rare, with only a few cases present in the pilot sample, the resampling Step 1 occasionally leads to a zero vector W L in the resampled design matrix. The treatment of such exceptions should be specified in the design of the study, which should include provisions for the situation that the internal pilot sample results in a non-full rank design matrix. The interim resampling procedure should follow the same rules. In this example, we assume that the investigator pre-planned to resolve this exception by setting the total sample size to the prespecified maximal value of n max = 3, 000. The above 30, 000 iterations allow us to estimate ˆα with a standard error of and calculate α new. In this example, α new = A similar resampling scheme emulating the design behavior under H 1 leads to a new power 1 β new = and the final total sample size of The results of model fit on 1837 observations are reported in Table 7. We fail to reject the null hypothesis that there is no interaction > ). While it is unknown whether it is a correct decision, an analysis of all 8142 cases leads to a similar conclusion. 5 Discussion In this manuscript we suggested considering the interim sample size recalculation from the prospective of internal design resampling. The proposed approach is very flexible, and can be applied for a wide variety of situations, including prospective clinical trials, observational, and retrospective studies. We defined a study design as a set of rules for sample size cal- 18
19 culation/recalculation, the chosen test statistic, and a course of actions for dealing with rare but possible situations such as singularity of a design matrix, etc. Ultimately the design is the ability to make a decision for all possible realizations of a random variable. The use of internal design-resampling allows elimination of the formal dependence of a design on nuisance parameters and lowers their notorious effect on type I and II errors. We adjusted the type I error α and the power 1 β using information from the internal pilot data. The adjusted type I error, α new, and the adjusted power, 1 β new, are used for sample size re-estimation at the interim analysis. We emphasize that it is critical to have a clearly defined per-protocol design for all possible samples. Otherwise, internal design resampling may generate impossible sampling situations bringing additional uncertainty into the adjustment. The internal resampling can be computationally challenging especially when a Monte-Carlo study is used. We implemented and performed three designs for internal sample size recalculation: the paired data t-test, the independent samples t-test with a pre-defined allocation ratio, and the independent sample t-test with random allocation. To speed up the Monte-Carlo simulations we used C code pre-compiled into a shared object for internal resampling part, and R code for the rest of the program. Our simulation studies clearly show that our resampling methodology leads to a generally better control of type I and II error than the naive internal pilot design across all considered scenarios. For the simulation scenarios for the two-sample t-tests, the comparisons with Stein s, the naive, and the restricted Wittes and Brittain approaches, the internal design resampling also often keeps a better control for type I and II errors and has a smaller mean sample size. We deliberately considered small internal pilot sample sizes 5 or 10 per group) since moderate to large sample sizes generate relatively accurate estimates of nuisance parameters and all considered methods would show similar performance. However our regression example shows that in situations with a larger number of nuisance parameters even 100 internal pilot samples might not be sufficient to assume no inflation of error rates. The internal design resampling requires the defined in the study protocol internal pilot sample size n 1 ) and the largest possible n max ) sample sizes. If the tails of the distribution of the sample size obtained from the internal pilot heavily spill over these two lower and upper bounds for the sample size, 19
20 then the control for type I and II error becomes more problematic. Overall, we strongly recommend researchers to clearly define their designs for all possible situation and incorporate internal sample size recalculation in their study designs. This may substantially improve the use of their resources, better control type I and II errors, and protect against nuisance parameters misspecification. References [1] Christopher S Coffey and Keith E Muller. Exact test size and power of a Gaussian error linear model for an internal pilot study. Statistics in Medicine, 1810): , May [2] Tim Friede and Meinhard Kieser. Sample Size Recalculation in Internal Pilot Study Designs: A Review. Biometrical Journal, 484): , August [3] Tim Friede and Meinhard Kieser. Blinded sample size recalculation for clinical trials with normal data and baseline adjusted analysis. Pharmaceutical statistics, 101):8 13, [4] Deborah H Glueck and Keith E Muller. Adjusting power for a baseline covariate in linear models. Statistics in Medicine, 2216): , August [5] Matthew J Gurka, Christopher S Coffey, and Kelly K Gurka. Internal pilots for observational studies. Biometrical journal. Biometrische Zeitschrift, 525): , October [6] F. Y. Hsieh, Daniel A. Bloch, and Michael D. Larsen. A simple method of sample size calculation for linear and logistic regression. Statistics in Medicine, 1714): , [7] Michael A Proschan. Two-stage sample size re-estimation based on a nuisance parameter: a review. Journal of Biopharmaceutical Statistics, 154):559 74, January [8] Charles Stein. A two-sample test for a linear hypothesis whose power is independent of the variance. The Annals of Mathematical Statistics, 163): ,
21 [9] Janet T Wittes and Erica Brittain. The role of internal pilot studies in increasing the efficiency of clinical trials. Statistics in Medicine, 91-2):65 71; discussion 71 2,
TECHNICAL REPORT # 59 MAY Interim sample size recalculation for linear and logistic regression models: a comprehensive Monte-Carlo study
 TECHNICAL REPORT # 59 MAY 2013 Interim sample size recalculation for linear and logistic regression models: a comprehensive Monte-Carlo study Sergey Tarima, Peng He, Tao Wang, Aniko Szabo Division of Biostatistics,
TECHNICAL REPORT # 59 MAY 2013 Interim sample size recalculation for linear and logistic regression models: a comprehensive Monte-Carlo study Sergey Tarima, Peng He, Tao Wang, Aniko Szabo Division of Biostatistics,
A Type of Sample Size Planning for Mean Comparison in Clinical Trials
 Journal of Data Science 13(2015), 115-126 A Type of Sample Size Planning for Mean Comparison in Clinical Trials Junfeng Liu 1 and Dipak K. Dey 2 1 GCE Solutions, Inc. 2 Department of Statistics, University
Journal of Data Science 13(2015), 115-126 A Type of Sample Size Planning for Mean Comparison in Clinical Trials Junfeng Liu 1 and Dipak K. Dey 2 1 GCE Solutions, Inc. 2 Department of Statistics, University
Adaptive Designs: Why, How and When?
 Adaptive Designs: Why, How and When? Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj ISBS Conference Shanghai, July 2008 1 Adaptive designs:
Adaptive Designs: Why, How and When? Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj ISBS Conference Shanghai, July 2008 1 Adaptive designs:
Sample size re-estimation in clinical trials. Dealing with those unknowns. Chris Jennison. University of Kyoto, January 2018
 Sample Size Re-estimation in Clinical Trials: Dealing with those unknowns Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj University of Kyoto,
Sample Size Re-estimation in Clinical Trials: Dealing with those unknowns Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj University of Kyoto,
Pubh 8482: Sequential Analysis
 Pubh 8482: Sequential Analysis Joseph S. Koopmeiners Division of Biostatistics University of Minnesota Week 12 Review So far... We have discussed the role of phase III clinical trials in drug development
Pubh 8482: Sequential Analysis Joseph S. Koopmeiners Division of Biostatistics University of Minnesota Week 12 Review So far... We have discussed the role of phase III clinical trials in drug development
Structural Nested Mean Models for Assessing Time-Varying Effect Moderation. Daniel Almirall
 1 Structural Nested Mean Models for Assessing Time-Varying Effect Moderation Daniel Almirall Center for Health Services Research, Durham VAMC & Dept. of Biostatistics, Duke University Medical Joint work
1 Structural Nested Mean Models for Assessing Time-Varying Effect Moderation Daniel Almirall Center for Health Services Research, Durham VAMC & Dept. of Biostatistics, Duke University Medical Joint work
Adaptive designs beyond p-value combination methods. Ekkehard Glimm, Novartis Pharma EAST user group meeting Basel, 31 May 2013
 Adaptive designs beyond p-value combination methods Ekkehard Glimm, Novartis Pharma EAST user group meeting Basel, 31 May 2013 Outline Introduction Combination-p-value method and conditional error function
Adaptive designs beyond p-value combination methods Ekkehard Glimm, Novartis Pharma EAST user group meeting Basel, 31 May 2013 Outline Introduction Combination-p-value method and conditional error function
Comparing Adaptive Designs and the. Classical Group Sequential Approach. to Clinical Trial Design
 Comparing Adaptive Designs and the Classical Group Sequential Approach to Clinical Trial Design Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj
Comparing Adaptive Designs and the Classical Group Sequential Approach to Clinical Trial Design Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj
Blinded sample size reestimation with count data
 Blinded sample size reestimation with count data Tim Friede 1 and Heinz Schmidli 2 1 Universtiy Medical Center Göttingen, Germany 2 Novartis Pharma AG, Basel, Switzerland BBS Early Spring Conference 2010
Blinded sample size reestimation with count data Tim Friede 1 and Heinz Schmidli 2 1 Universtiy Medical Center Göttingen, Germany 2 Novartis Pharma AG, Basel, Switzerland BBS Early Spring Conference 2010
Marginal Screening and Post-Selection Inference
 Marginal Screening and Post-Selection Inference Ian McKeague August 13, 2017 Ian McKeague (Columbia University) Marginal Screening August 13, 2017 1 / 29 Outline 1 Background on Marginal Screening 2 2
Marginal Screening and Post-Selection Inference Ian McKeague August 13, 2017 Ian McKeague (Columbia University) Marginal Screening August 13, 2017 1 / 29 Outline 1 Background on Marginal Screening 2 2
Duke University. Duke Biostatistics and Bioinformatics (B&B) Working Paper Series. Randomized Phase II Clinical Trials using Fisher s Exact Test
 Duke University Duke Biostatistics and Bioinformatics (B&B) Working Paper Series Year 2010 Paper 7 Randomized Phase II Clinical Trials using Fisher s Exact Test Sin-Ho Jung sinho.jung@duke.edu This working
Duke University Duke Biostatistics and Bioinformatics (B&B) Working Paper Series Year 2010 Paper 7 Randomized Phase II Clinical Trials using Fisher s Exact Test Sin-Ho Jung sinho.jung@duke.edu This working
22s:152 Applied Linear Regression. Take random samples from each of m populations.
 22s:152 Applied Linear Regression Chapter 8: ANOVA NOTE: We will meet in the lab on Monday October 10. One-way ANOVA Focuses on testing for differences among group means. Take random samples from each
22s:152 Applied Linear Regression Chapter 8: ANOVA NOTE: We will meet in the lab on Monday October 10. One-way ANOVA Focuses on testing for differences among group means. Take random samples from each
Some Notes on Blinded Sample Size Re-Estimation
 Some Notes on Blinded Sample Size Re-Estimation arxiv:1301.4167v1 [stat.me] 17 Jan 013 Ekkehard Glimm 1 and Jürgen Läuter Abstract This note investigates a number of scenarios in which unadjusted testing
Some Notes on Blinded Sample Size Re-Estimation arxiv:1301.4167v1 [stat.me] 17 Jan 013 Ekkehard Glimm 1 and Jürgen Läuter Abstract This note investigates a number of scenarios in which unadjusted testing
22s:152 Applied Linear Regression. There are a couple commonly used models for a one-way ANOVA with m groups. Chapter 8: ANOVA
 22s:152 Applied Linear Regression Chapter 8: ANOVA NOTE: We will meet in the lab on Monday October 10. One-way ANOVA Focuses on testing for differences among group means. Take random samples from each
22s:152 Applied Linear Regression Chapter 8: ANOVA NOTE: We will meet in the lab on Monday October 10. One-way ANOVA Focuses on testing for differences among group means. Take random samples from each
COMPOSITIONAL IDEAS IN THE BAYESIAN ANALYSIS OF CATEGORICAL DATA WITH APPLICATION TO DOSE FINDING CLINICAL TRIALS
 COMPOSITIONAL IDEAS IN THE BAYESIAN ANALYSIS OF CATEGORICAL DATA WITH APPLICATION TO DOSE FINDING CLINICAL TRIALS M. Gasparini and J. Eisele 2 Politecnico di Torino, Torino, Italy; mauro.gasparini@polito.it
COMPOSITIONAL IDEAS IN THE BAYESIAN ANALYSIS OF CATEGORICAL DATA WITH APPLICATION TO DOSE FINDING CLINICAL TRIALS M. Gasparini and J. Eisele 2 Politecnico di Torino, Torino, Italy; mauro.gasparini@polito.it
Sample size determination for logistic regression: A simulation study
 Sample size determination for logistic regression: A simulation study Stephen Bush School of Mathematical Sciences, University of Technology Sydney, PO Box 123 Broadway NSW 2007, Australia Abstract This
Sample size determination for logistic regression: A simulation study Stephen Bush School of Mathematical Sciences, University of Technology Sydney, PO Box 123 Broadway NSW 2007, Australia Abstract This
Fall 2017 STAT 532 Homework Peter Hoff. 1. Let P be a probability measure on a collection of sets A.
 1. Let P be a probability measure on a collection of sets A. (a) For each n N, let H n be a set in A such that H n H n+1. Show that P (H n ) monotonically converges to P ( k=1 H k) as n. (b) For each n
1. Let P be a probability measure on a collection of sets A. (a) For each n N, let H n be a set in A such that H n H n+1. Show that P (H n ) monotonically converges to P ( k=1 H k) as n. (b) For each n
Group Sequential Tests for Delayed Responses. Christopher Jennison. Lisa Hampson. Workshop on Special Topics on Sequential Methodology
 Group Sequential Tests for Delayed Responses Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj Lisa Hampson Department of Mathematics and Statistics,
Group Sequential Tests for Delayed Responses Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj Lisa Hampson Department of Mathematics and Statistics,
Machine Learning Linear Classification. Prof. Matteo Matteucci
 Machine Learning Linear Classification Prof. Matteo Matteucci Recall from the first lecture 2 X R p Regression Y R Continuous Output X R p Y {Ω 0, Ω 1,, Ω K } Classification Discrete Output X R p Y (X)
Machine Learning Linear Classification Prof. Matteo Matteucci Recall from the first lecture 2 X R p Regression Y R Continuous Output X R p Y {Ω 0, Ω 1,, Ω K } Classification Discrete Output X R p Y (X)
Adaptive Extensions of a Two-Stage Group Sequential Procedure for Testing a Primary and a Secondary Endpoint (II): Sample Size Re-estimation
 Research Article Received XXXX (www.interscience.wiley.com) DOI: 10.100/sim.0000 Adaptive Extensions of a Two-Stage Group Sequential Procedure for Testing a Primary and a Secondary Endpoint (II): Sample
Research Article Received XXXX (www.interscience.wiley.com) DOI: 10.100/sim.0000 Adaptive Extensions of a Two-Stage Group Sequential Procedure for Testing a Primary and a Secondary Endpoint (II): Sample
The Design of a Survival Study
 The Design of a Survival Study The design of survival studies are usually based on the logrank test, and sometimes assumes the exponential distribution. As in standard designs, the power depends on The
The Design of a Survival Study The design of survival studies are usually based on the logrank test, and sometimes assumes the exponential distribution. As in standard designs, the power depends on The
Pubh 8482: Sequential Analysis
 Pubh 8482: Sequential Analysis Joseph S. Koopmeiners Division of Biostatistics University of Minnesota Week 10 Class Summary Last time... We began our discussion of adaptive clinical trials Specifically,
Pubh 8482: Sequential Analysis Joseph S. Koopmeiners Division of Biostatistics University of Minnesota Week 10 Class Summary Last time... We began our discussion of adaptive clinical trials Specifically,
Research Article Sample Size Calculation for Controlling False Discovery Proportion
 Probability and Statistics Volume 2012, Article ID 817948, 13 pages doi:10.1155/2012/817948 Research Article Sample Size Calculation for Controlling False Discovery Proportion Shulian Shang, 1 Qianhe Zhou,
Probability and Statistics Volume 2012, Article ID 817948, 13 pages doi:10.1155/2012/817948 Research Article Sample Size Calculation for Controlling False Discovery Proportion Shulian Shang, 1 Qianhe Zhou,
Power and Sample Size Calculations with the Additive Hazards Model
 Journal of Data Science 10(2012), 143-155 Power and Sample Size Calculations with the Additive Hazards Model Ling Chen, Chengjie Xiong, J. Philip Miller and Feng Gao Washington University School of Medicine
Journal of Data Science 10(2012), 143-155 Power and Sample Size Calculations with the Additive Hazards Model Ling Chen, Chengjie Xiong, J. Philip Miller and Feng Gao Washington University School of Medicine
Group Sequential Designs: Theory, Computation and Optimisation
 Group Sequential Designs: Theory, Computation and Optimisation Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj 8th International Conference
Group Sequential Designs: Theory, Computation and Optimisation Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj 8th International Conference
Type I error rate control in adaptive designs for confirmatory clinical trials with treatment selection at interim
 Type I error rate control in adaptive designs for confirmatory clinical trials with treatment selection at interim Frank Bretz Statistical Methodology, Novartis Joint work with Martin Posch (Medical University
Type I error rate control in adaptive designs for confirmatory clinical trials with treatment selection at interim Frank Bretz Statistical Methodology, Novartis Joint work with Martin Posch (Medical University
An Introduction to Causal Analysis on Observational Data using Propensity Scores
 An Introduction to Causal Analysis on Observational Data using Propensity Scores Margie Rosenberg*, PhD, FSA Brian Hartman**, PhD, ASA Shannon Lane* *University of Wisconsin Madison **University of Connecticut
An Introduction to Causal Analysis on Observational Data using Propensity Scores Margie Rosenberg*, PhD, FSA Brian Hartman**, PhD, ASA Shannon Lane* *University of Wisconsin Madison **University of Connecticut
Categorical Predictor Variables
 Categorical Predictor Variables We often wish to use categorical (or qualitative) variables as covariates in a regression model. For binary variables (taking on only 2 values, e.g. sex), it is relatively
Categorical Predictor Variables We often wish to use categorical (or qualitative) variables as covariates in a regression model. For binary variables (taking on only 2 values, e.g. sex), it is relatively
Extending causal inferences from a randomized trial to a target population
 Extending causal inferences from a randomized trial to a target population Issa Dahabreh Center for Evidence Synthesis in Health, Brown University issa dahabreh@brown.edu January 16, 2019 Issa Dahabreh
Extending causal inferences from a randomized trial to a target population Issa Dahabreh Center for Evidence Synthesis in Health, Brown University issa dahabreh@brown.edu January 16, 2019 Issa Dahabreh
Model Estimation Example
 Ronald H. Heck 1 EDEP 606: Multivariate Methods (S2013) April 7, 2013 Model Estimation Example As we have moved through the course this semester, we have encountered the concept of model estimation. Discussions
Ronald H. Heck 1 EDEP 606: Multivariate Methods (S2013) April 7, 2013 Model Estimation Example As we have moved through the course this semester, we have encountered the concept of model estimation. Discussions
SCHOOL OF MATHEMATICS AND STATISTICS. Linear and Generalised Linear Models
 SCHOOL OF MATHEMATICS AND STATISTICS Linear and Generalised Linear Models Autumn Semester 2017 18 2 hours Attempt all the questions. The allocation of marks is shown in brackets. RESTRICTED OPEN BOOK EXAMINATION
SCHOOL OF MATHEMATICS AND STATISTICS Linear and Generalised Linear Models Autumn Semester 2017 18 2 hours Attempt all the questions. The allocation of marks is shown in brackets. RESTRICTED OPEN BOOK EXAMINATION
Tutorial 5: Power and Sample Size for One-way Analysis of Variance (ANOVA) with Equal Variances Across Groups. Acknowledgements:
 Tutorial 5: Power and Sample Size for One-way Analysis of Variance (ANOVA) with Equal Variances Across Groups Anna E. Barón, Keith E. Muller, Sarah M. Kreidler, and Deborah H. Glueck Acknowledgements:
Tutorial 5: Power and Sample Size for One-way Analysis of Variance (ANOVA) with Equal Variances Across Groups Anna E. Barón, Keith E. Muller, Sarah M. Kreidler, and Deborah H. Glueck Acknowledgements:
Structural Nested Mean Models for Assessing Time-Varying Effect Moderation. Daniel Almirall
 1 Structural Nested Mean Models for Assessing Time-Varying Effect Moderation Daniel Almirall Center for Health Services Research, Durham VAMC & Duke University Medical, Dept. of Biostatistics Joint work
1 Structural Nested Mean Models for Assessing Time-Varying Effect Moderation Daniel Almirall Center for Health Services Research, Durham VAMC & Duke University Medical, Dept. of Biostatistics Joint work
Statistics Boot Camp. Dr. Stephanie Lane Institute for Defense Analyses DATAWorks 2018
 Statistics Boot Camp Dr. Stephanie Lane Institute for Defense Analyses DATAWorks 2018 March 21, 2018 Outline of boot camp Summarizing and simplifying data Point and interval estimation Foundations of statistical
Statistics Boot Camp Dr. Stephanie Lane Institute for Defense Analyses DATAWorks 2018 March 21, 2018 Outline of boot camp Summarizing and simplifying data Point and interval estimation Foundations of statistical
Personalized Treatment Selection Based on Randomized Clinical Trials. Tianxi Cai Department of Biostatistics Harvard School of Public Health
 Personalized Treatment Selection Based on Randomized Clinical Trials Tianxi Cai Department of Biostatistics Harvard School of Public Health Outline Motivation A systematic approach to separating subpopulations
Personalized Treatment Selection Based on Randomized Clinical Trials Tianxi Cai Department of Biostatistics Harvard School of Public Health Outline Motivation A systematic approach to separating subpopulations
Chapter 1 Statistical Inference
 Chapter 1 Statistical Inference causal inference To infer causality, you need a randomized experiment (or a huge observational study and lots of outside information). inference to populations Generalizations
Chapter 1 Statistical Inference causal inference To infer causality, you need a randomized experiment (or a huge observational study and lots of outside information). inference to populations Generalizations
Approximation of Survival Function by Taylor Series for General Partly Interval Censored Data
 Malaysian Journal of Mathematical Sciences 11(3): 33 315 (217) MALAYSIAN JOURNAL OF MATHEMATICAL SCIENCES Journal homepage: http://einspem.upm.edu.my/journal Approximation of Survival Function by Taylor
Malaysian Journal of Mathematical Sciences 11(3): 33 315 (217) MALAYSIAN JOURNAL OF MATHEMATICAL SCIENCES Journal homepage: http://einspem.upm.edu.my/journal Approximation of Survival Function by Taylor
Classification. Chapter Introduction. 6.2 The Bayes classifier
 Chapter 6 Classification 6.1 Introduction Often encountered in applications is the situation where the response variable Y takes values in a finite set of labels. For example, the response Y could encode
Chapter 6 Classification 6.1 Introduction Often encountered in applications is the situation where the response variable Y takes values in a finite set of labels. For example, the response Y could encode
3003 Cure. F. P. Treasure
 3003 Cure F. P. reasure November 8, 2000 Peter reasure / November 8, 2000/ Cure / 3003 1 Cure A Simple Cure Model he Concept of Cure A cure model is a survival model where a fraction of the population
3003 Cure F. P. reasure November 8, 2000 Peter reasure / November 8, 2000/ Cure / 3003 1 Cure A Simple Cure Model he Concept of Cure A cure model is a survival model where a fraction of the population
STAT 536: Genetic Statistics
 STAT 536: Genetic Statistics Tests for Hardy Weinberg Equilibrium Karin S. Dorman Department of Statistics Iowa State University September 7, 2006 Statistical Hypothesis Testing Identify a hypothesis,
STAT 536: Genetic Statistics Tests for Hardy Weinberg Equilibrium Karin S. Dorman Department of Statistics Iowa State University September 7, 2006 Statistical Hypothesis Testing Identify a hypothesis,
Today. HW 1: due February 4, pm. Aspects of Design CD Chapter 2. Continue with Chapter 2 of ELM. In the News:
 Today HW 1: due February 4, 11.59 pm. Aspects of Design CD Chapter 2 Continue with Chapter 2 of ELM In the News: STA 2201: Applied Statistics II January 14, 2015 1/35 Recap: data on proportions data: y
Today HW 1: due February 4, 11.59 pm. Aspects of Design CD Chapter 2 Continue with Chapter 2 of ELM In the News: STA 2201: Applied Statistics II January 14, 2015 1/35 Recap: data on proportions data: y
Two-stage k-sample designs for the ordered alternative problem
 Two-stage k-sample designs for the ordered alternative problem Guogen Shan, Alan D. Hutson, and Gregory E. Wilding Department of Biostatistics,University at Buffalo, Buffalo, NY 14214, USA July 18, 2011
Two-stage k-sample designs for the ordered alternative problem Guogen Shan, Alan D. Hutson, and Gregory E. Wilding Department of Biostatistics,University at Buffalo, Buffalo, NY 14214, USA July 18, 2011
PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH
 PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH The First Step: SAMPLE SIZE DETERMINATION THE ULTIMATE GOAL The most important, ultimate step of any of clinical research is to do draw inferences;
PubH 7470: STATISTICS FOR TRANSLATIONAL & CLINICAL RESEARCH The First Step: SAMPLE SIZE DETERMINATION THE ULTIMATE GOAL The most important, ultimate step of any of clinical research is to do draw inferences;
Monitoring clinical trial outcomes with delayed response: incorporating pipeline data in group sequential designs. Christopher Jennison
 Monitoring clinical trial outcomes with delayed response: incorporating pipeline data in group sequential designs Christopher Jennison Department of Mathematical Sciences, University of Bath http://people.bath.ac.uk/mascj
Monitoring clinical trial outcomes with delayed response: incorporating pipeline data in group sequential designs Christopher Jennison Department of Mathematical Sciences, University of Bath http://people.bath.ac.uk/mascj
Power and sample size calculations
 Patrick Breheny October 20 Patrick Breheny University of Iowa Biostatistical Methods I (BIOS 5710) 1 / 26 Planning a study Introduction What is power? Why is it important? Setup One of the most important
Patrick Breheny October 20 Patrick Breheny University of Iowa Biostatistical Methods I (BIOS 5710) 1 / 26 Planning a study Introduction What is power? Why is it important? Setup One of the most important
McGill University. Faculty of Science MATH 204 PRINCIPLES OF STATISTICS II. Final Examination
 McGill University Faculty of Science MATH 204 PRINCIPLES OF STATISTICS II Final Examination Date: 20th April 2009 Time: 9am-2pm Examiner: Dr David A Stephens Associate Examiner: Dr Russell Steele Please
McGill University Faculty of Science MATH 204 PRINCIPLES OF STATISTICS II Final Examination Date: 20th April 2009 Time: 9am-2pm Examiner: Dr David A Stephens Associate Examiner: Dr Russell Steele Please
Pairwise rank based likelihood for estimating the relationship between two homogeneous populations and their mixture proportion
 Pairwise rank based likelihood for estimating the relationship between two homogeneous populations and their mixture proportion Glenn Heller and Jing Qin Department of Epidemiology and Biostatistics Memorial
Pairwise rank based likelihood for estimating the relationship between two homogeneous populations and their mixture proportion Glenn Heller and Jing Qin Department of Epidemiology and Biostatistics Memorial
Weighting. Homework 2. Regression. Regression. Decisions Matching: Weighting (0) W i. (1) -å l i. )Y i. (1-W i 3/5/2014. (1) = Y i.
 Weighting Unconfounded Homework 2 Describe imbalance direction matters STA 320 Design and Analysis of Causal Studies Dr. Kari Lock Morgan and Dr. Fan Li Department of Statistical Science Duke University
Weighting Unconfounded Homework 2 Describe imbalance direction matters STA 320 Design and Analysis of Causal Studies Dr. Kari Lock Morgan and Dr. Fan Li Department of Statistical Science Duke University
Optimising Group Sequential Designs. Decision Theory, Dynamic Programming. and Optimal Stopping
 : Decision Theory, Dynamic Programming and Optimal Stopping Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj InSPiRe Conference on Methodology
: Decision Theory, Dynamic Programming and Optimal Stopping Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj InSPiRe Conference on Methodology
Tutorial 3: Power and Sample Size for the Two-sample t-test with Equal Variances. Acknowledgements:
 Tutorial 3: Power and Sample Size for the Two-sample t-test with Equal Variances Anna E. Barón, Keith E. Muller, Sarah M. Kreidler, and Deborah H. Glueck Acknowledgements: The project was supported in
Tutorial 3: Power and Sample Size for the Two-sample t-test with Equal Variances Anna E. Barón, Keith E. Muller, Sarah M. Kreidler, and Deborah H. Glueck Acknowledgements: The project was supported in
Goodness-of-Fit Tests for the Ordinal Response Models with Misspecified Links
 Communications of the Korean Statistical Society 2009, Vol 16, No 4, 697 705 Goodness-of-Fit Tests for the Ordinal Response Models with Misspecified Links Kwang Mo Jeong a, Hyun Yung Lee 1, a a Department
Communications of the Korean Statistical Society 2009, Vol 16, No 4, 697 705 Goodness-of-Fit Tests for the Ordinal Response Models with Misspecified Links Kwang Mo Jeong a, Hyun Yung Lee 1, a a Department
Improving Efficiency of Inferences in Randomized Clinical Trials Using Auxiliary Covariates
 Improving Efficiency of Inferences in Randomized Clinical Trials Using Auxiliary Covariates Anastasios (Butch) Tsiatis Department of Statistics North Carolina State University http://www.stat.ncsu.edu/
Improving Efficiency of Inferences in Randomized Clinical Trials Using Auxiliary Covariates Anastasios (Butch) Tsiatis Department of Statistics North Carolina State University http://www.stat.ncsu.edu/
Guideline on adjustment for baseline covariates in clinical trials
 26 February 2015 EMA/CHMP/295050/2013 Committee for Medicinal Products for Human Use (CHMP) Guideline on adjustment for baseline covariates in clinical trials Draft Agreed by Biostatistics Working Party
26 February 2015 EMA/CHMP/295050/2013 Committee for Medicinal Products for Human Use (CHMP) Guideline on adjustment for baseline covariates in clinical trials Draft Agreed by Biostatistics Working Party
Discussion of Identifiability and Estimation of Causal Effects in Randomized. Trials with Noncompliance and Completely Non-ignorable Missing Data
 Biometrics 000, 000 000 DOI: 000 000 0000 Discussion of Identifiability and Estimation of Causal Effects in Randomized Trials with Noncompliance and Completely Non-ignorable Missing Data Dylan S. Small
Biometrics 000, 000 000 DOI: 000 000 0000 Discussion of Identifiability and Estimation of Causal Effects in Randomized Trials with Noncompliance and Completely Non-ignorable Missing Data Dylan S. Small
Sample Size Calculations for Group Randomized Trials with Unequal Sample Sizes through Monte Carlo Simulations
 Sample Size Calculations for Group Randomized Trials with Unequal Sample Sizes through Monte Carlo Simulations Ben Brewer Duke University March 10, 2017 Introduction Group randomized trials (GRTs) are
Sample Size Calculations for Group Randomized Trials with Unequal Sample Sizes through Monte Carlo Simulations Ben Brewer Duke University March 10, 2017 Introduction Group randomized trials (GRTs) are
Model Based Statistics in Biology. Part V. The Generalized Linear Model. Chapter 18.1 Logistic Regression (Dose - Response)
 Model Based Statistics in Biology. Part V. The Generalized Linear Model. Logistic Regression ( - Response) ReCap. Part I (Chapters 1,2,3,4), Part II (Ch 5, 6, 7) ReCap Part III (Ch 9, 10, 11), Part IV
Model Based Statistics in Biology. Part V. The Generalized Linear Model. Logistic Regression ( - Response) ReCap. Part I (Chapters 1,2,3,4), Part II (Ch 5, 6, 7) ReCap Part III (Ch 9, 10, 11), Part IV
A new strategy for meta-analysis of continuous covariates in observational studies with IPD. Willi Sauerbrei & Patrick Royston
 A new strategy for meta-analysis of continuous covariates in observational studies with IPD Willi Sauerbrei & Patrick Royston Overview Motivation Continuous variables functional form Fractional polynomials
A new strategy for meta-analysis of continuous covariates in observational studies with IPD Willi Sauerbrei & Patrick Royston Overview Motivation Continuous variables functional form Fractional polynomials
Introduction to Statistical Analysis
 Introduction to Statistical Analysis Changyu Shen Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology Beth Israel Deaconess Medical Center Harvard Medical School Objectives Descriptive
Introduction to Statistical Analysis Changyu Shen Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology Beth Israel Deaconess Medical Center Harvard Medical School Objectives Descriptive
SUPPLEMENTARY SIMULATIONS & FIGURES
 Supplementary Material: Supplementary Material for Mixed Effects Models for Resampled Network Statistics Improve Statistical Power to Find Differences in Multi-Subject Functional Connectivity Manjari Narayan,
Supplementary Material: Supplementary Material for Mixed Effects Models for Resampled Network Statistics Improve Statistical Power to Find Differences in Multi-Subject Functional Connectivity Manjari Narayan,
A Bayesian Nonparametric Approach to Monotone Missing Data in Longitudinal Studies with Informative Missingness
 A Bayesian Nonparametric Approach to Monotone Missing Data in Longitudinal Studies with Informative Missingness A. Linero and M. Daniels UF, UT-Austin SRC 2014, Galveston, TX 1 Background 2 Working model
A Bayesian Nonparametric Approach to Monotone Missing Data in Longitudinal Studies with Informative Missingness A. Linero and M. Daniels UF, UT-Austin SRC 2014, Galveston, TX 1 Background 2 Working model
BIOS 2083 Linear Models c Abdus S. Wahed
 Chapter 5 206 Chapter 6 General Linear Model: Statistical Inference 6.1 Introduction So far we have discussed formulation of linear models (Chapter 1), estimability of parameters in a linear model (Chapter
Chapter 5 206 Chapter 6 General Linear Model: Statistical Inference 6.1 Introduction So far we have discussed formulation of linear models (Chapter 1), estimability of parameters in a linear model (Chapter
Ron Heck, Fall Week 8: Introducing Generalized Linear Models: Logistic Regression 1 (Replaces prior revision dated October 20, 2011)
 Ron Heck, Fall 2011 1 EDEP 768E: Seminar in Multilevel Modeling rev. January 3, 2012 (see footnote) Week 8: Introducing Generalized Linear Models: Logistic Regression 1 (Replaces prior revision dated October
Ron Heck, Fall 2011 1 EDEP 768E: Seminar in Multilevel Modeling rev. January 3, 2012 (see footnote) Week 8: Introducing Generalized Linear Models: Logistic Regression 1 (Replaces prior revision dated October
22s:152 Applied Linear Regression
 22s:152 Applied Linear Regression Chapter 7: Dummy Variable Regression So far, we ve only considered quantitative variables in our models. We can integrate categorical predictors by constructing artificial
22s:152 Applied Linear Regression Chapter 7: Dummy Variable Regression So far, we ve only considered quantitative variables in our models. We can integrate categorical predictors by constructing artificial
arxiv: v1 [cs.ne] 9 Aug 2018
![arxiv: v1 [cs.ne] 9 Aug 2018 arxiv: v1 [cs.ne] 9 Aug 2018](/thumbs/83/87511607.jpg) Sample size estimation for power and accuracy in the experimental comparison of algorithms Felipe Campelo 1,2 and Fernanda Takahashi 1,3 arxiv:1808.02997v1 [cs.ne] 9 Aug 2018 1 Operations Research and
Sample size estimation for power and accuracy in the experimental comparison of algorithms Felipe Campelo 1,2 and Fernanda Takahashi 1,3 arxiv:1808.02997v1 [cs.ne] 9 Aug 2018 1 Operations Research and
401 Review. 6. Power analysis for one/two-sample hypothesis tests and for correlation analysis.
 401 Review Major topics of the course 1. Univariate analysis 2. Bivariate analysis 3. Simple linear regression 4. Linear algebra 5. Multiple regression analysis Major analysis methods 1. Graphical analysis
401 Review Major topics of the course 1. Univariate analysis 2. Bivariate analysis 3. Simple linear regression 4. Linear algebra 5. Multiple regression analysis Major analysis methods 1. Graphical analysis
Estimating Optimal Dynamic Treatment Regimes from Clustered Data
 Estimating Optimal Dynamic Treatment Regimes from Clustered Data Bibhas Chakraborty Department of Biostatistics, Columbia University bc2425@columbia.edu Society for Clinical Trials Annual Meetings Boston,
Estimating Optimal Dynamic Treatment Regimes from Clustered Data Bibhas Chakraborty Department of Biostatistics, Columbia University bc2425@columbia.edu Society for Clinical Trials Annual Meetings Boston,
Lecture 5: ANOVA and Correlation
 Lecture 5: ANOVA and Correlation Ani Manichaikul amanicha@jhsph.edu 23 April 2007 1 / 62 Comparing Multiple Groups Continous data: comparing means Analysis of variance Binary data: comparing proportions
Lecture 5: ANOVA and Correlation Ani Manichaikul amanicha@jhsph.edu 23 April 2007 1 / 62 Comparing Multiple Groups Continous data: comparing means Analysis of variance Binary data: comparing proportions
STAT 5500/6500 Conditional Logistic Regression for Matched Pairs
 STAT 5500/6500 Conditional Logistic Regression for Matched Pairs Motivating Example: The data we will be using comes from a subset of data taken from the Los Angeles Study of the Endometrial Cancer Data
STAT 5500/6500 Conditional Logistic Regression for Matched Pairs Motivating Example: The data we will be using comes from a subset of data taken from the Los Angeles Study of the Endometrial Cancer Data
Binary Logistic Regression
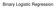 The coefficients of the multiple regression model are estimated using sample data with k independent variables Estimated (or predicted) value of Y Estimated intercept Estimated slope coefficients Ŷ = b
The coefficients of the multiple regression model are estimated using sample data with k independent variables Estimated (or predicted) value of Y Estimated intercept Estimated slope coefficients Ŷ = b
Power and sample size calculations for designing rare variant sequencing association studies.
 Power and sample size calculations for designing rare variant sequencing association studies. Seunggeun Lee 1, Michael C. Wu 2, Tianxi Cai 1, Yun Li 2,3, Michael Boehnke 4 and Xihong Lin 1 1 Department
Power and sample size calculations for designing rare variant sequencing association studies. Seunggeun Lee 1, Michael C. Wu 2, Tianxi Cai 1, Yun Li 2,3, Michael Boehnke 4 and Xihong Lin 1 1 Department
The t-distribution. Patrick Breheny. October 13. z tests The χ 2 -distribution The t-distribution Summary
 Patrick Breheny October 13 Patrick Breheny Biostatistical Methods I (BIOS 5710) 1/25 Introduction Introduction What s wrong with z-tests? So far we ve (thoroughly!) discussed how to carry out hypothesis
Patrick Breheny October 13 Patrick Breheny Biostatistical Methods I (BIOS 5710) 1/25 Introduction Introduction What s wrong with z-tests? So far we ve (thoroughly!) discussed how to carry out hypothesis
An Adaptive Futility Monitoring Method with Time-Varying Conditional Power Boundary
 An Adaptive Futility Monitoring Method with Time-Varying Conditional Power Boundary Ying Zhang and William R. Clarke Department of Biostatistics, University of Iowa 200 Hawkins Dr. C-22 GH, Iowa City,
An Adaptive Futility Monitoring Method with Time-Varying Conditional Power Boundary Ying Zhang and William R. Clarke Department of Biostatistics, University of Iowa 200 Hawkins Dr. C-22 GH, Iowa City,
Group sequential designs for negative binomial outcomes
 Group sequential designs for negative binomial outcomes Tobias Mütze a, Ekkehard Glimm b,c, Heinz Schmidli b, and Tim Friede a,d a Department of Medical Statistics, University Medical Center Göttingen,
Group sequential designs for negative binomial outcomes Tobias Mütze a, Ekkehard Glimm b,c, Heinz Schmidli b, and Tim Friede a,d a Department of Medical Statistics, University Medical Center Göttingen,
Statistical Methods for Handling Incomplete Data Chapter 2: Likelihood-based approach
 Statistical Methods for Handling Incomplete Data Chapter 2: Likelihood-based approach Jae-Kwang Kim Department of Statistics, Iowa State University Outline 1 Introduction 2 Observed likelihood 3 Mean Score
Statistical Methods for Handling Incomplete Data Chapter 2: Likelihood-based approach Jae-Kwang Kim Department of Statistics, Iowa State University Outline 1 Introduction 2 Observed likelihood 3 Mean Score
Sample size determination for a binary response in a superiority clinical trial using a hybrid classical and Bayesian procedure
 Ciarleglio and Arendt Trials (2017) 18:83 DOI 10.1186/s13063-017-1791-0 METHODOLOGY Open Access Sample size determination for a binary response in a superiority clinical trial using a hybrid classical
Ciarleglio and Arendt Trials (2017) 18:83 DOI 10.1186/s13063-017-1791-0 METHODOLOGY Open Access Sample size determination for a binary response in a superiority clinical trial using a hybrid classical
Chapter Fifteen. Frequency Distribution, Cross-Tabulation, and Hypothesis Testing
 Chapter Fifteen Frequency Distribution, Cross-Tabulation, and Hypothesis Testing Copyright 2010 Pearson Education, Inc. publishing as Prentice Hall 15-1 Internet Usage Data Table 15.1 Respondent Sex Familiarity
Chapter Fifteen Frequency Distribution, Cross-Tabulation, and Hypothesis Testing Copyright 2010 Pearson Education, Inc. publishing as Prentice Hall 15-1 Internet Usage Data Table 15.1 Respondent Sex Familiarity
Comparison of Different Methods of Sample Size Re-estimation for Therapeutic Equivalence (TE) Studies Protecting the Overall Type 1 Error
 Comparison of Different Methods of Sample Size Re-estimation for Therapeutic Equivalence (TE) Studies Protecting the Overall Type 1 Error by Diane Potvin Outline 1. Therapeutic Equivalence Designs 2. Objectives
Comparison of Different Methods of Sample Size Re-estimation for Therapeutic Equivalence (TE) Studies Protecting the Overall Type 1 Error by Diane Potvin Outline 1. Therapeutic Equivalence Designs 2. Objectives
Stat 642, Lecture notes for 04/12/05 96
 Stat 642, Lecture notes for 04/12/05 96 Hosmer-Lemeshow Statistic The Hosmer-Lemeshow Statistic is another measure of lack of fit. Hosmer and Lemeshow recommend partitioning the observations into 10 equal
Stat 642, Lecture notes for 04/12/05 96 Hosmer-Lemeshow Statistic The Hosmer-Lemeshow Statistic is another measure of lack of fit. Hosmer and Lemeshow recommend partitioning the observations into 10 equal
Review: General Approach to Hypothesis Testing. 1. Define the research question and formulate the appropriate null and alternative hypotheses.
 1 Review: Let X 1, X,..., X n denote n independent random variables sampled from some distribution might not be normal!) with mean µ) and standard deviation σ). Then X µ σ n In other words, X is approximately
1 Review: Let X 1, X,..., X n denote n independent random variables sampled from some distribution might not be normal!) with mean µ) and standard deviation σ). Then X µ σ n In other words, X is approximately
Testing a secondary endpoint after a group sequential test. Chris Jennison. 9th Annual Adaptive Designs in Clinical Trials
 Testing a secondary endpoint after a group sequential test Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj 9th Annual Adaptive Designs in
Testing a secondary endpoint after a group sequential test Christopher Jennison Department of Mathematical Sciences, University of Bath, UK http://people.bath.ac.uk/mascj 9th Annual Adaptive Designs in
EPSY 905: Fundamentals of Multivariate Modeling Online Lecture #7
 Introduction to Generalized Univariate Models: Models for Binary Outcomes EPSY 905: Fundamentals of Multivariate Modeling Online Lecture #7 EPSY 905: Intro to Generalized In This Lecture A short review
Introduction to Generalized Univariate Models: Models for Binary Outcomes EPSY 905: Fundamentals of Multivariate Modeling Online Lecture #7 EPSY 905: Intro to Generalized In This Lecture A short review
MONTE CARLO ANALYSIS OF CHANGE POINT ESTIMATORS
 MONTE CARLO ANALYSIS OF CHANGE POINT ESTIMATORS Gregory GUREVICH PhD, Industrial Engineering and Management Department, SCE - Shamoon College Engineering, Beer-Sheva, Israel E-mail: gregoryg@sce.ac.il
MONTE CARLO ANALYSIS OF CHANGE POINT ESTIMATORS Gregory GUREVICH PhD, Industrial Engineering and Management Department, SCE - Shamoon College Engineering, Beer-Sheva, Israel E-mail: gregoryg@sce.ac.il
Group sequential designs with negative binomial data
 Group sequential designs with negative binomial data Ekkehard Glimm 1 Tobias Mütze 2,3 1 Statistical Methodology, Novartis, Basel, Switzerland 2 Department of Medical Statistics, University Medical Center
Group sequential designs with negative binomial data Ekkehard Glimm 1 Tobias Mütze 2,3 1 Statistical Methodology, Novartis, Basel, Switzerland 2 Department of Medical Statistics, University Medical Center
General Linear Model: Statistical Inference
 Chapter 6 General Linear Model: Statistical Inference 6.1 Introduction So far we have discussed formulation of linear models (Chapter 1), estimability of parameters in a linear model (Chapter 4), least
Chapter 6 General Linear Model: Statistical Inference 6.1 Introduction So far we have discussed formulation of linear models (Chapter 1), estimability of parameters in a linear model (Chapter 4), least
Multiple linear regression S6
 Basic medical statistics for clinical and experimental research Multiple linear regression S6 Katarzyna Jóźwiak k.jozwiak@nki.nl November 15, 2017 1/42 Introduction Two main motivations for doing multiple
Basic medical statistics for clinical and experimental research Multiple linear regression S6 Katarzyna Jóźwiak k.jozwiak@nki.nl November 15, 2017 1/42 Introduction Two main motivations for doing multiple
STA 303 H1S / 1002 HS Winter 2011 Test March 7, ab 1cde 2abcde 2fghij 3
 STA 303 H1S / 1002 HS Winter 2011 Test March 7, 2011 LAST NAME: FIRST NAME: STUDENT NUMBER: ENROLLED IN: (circle one) STA 303 STA 1002 INSTRUCTIONS: Time: 90 minutes Aids allowed: calculator. Some formulae
STA 303 H1S / 1002 HS Winter 2011 Test March 7, 2011 LAST NAME: FIRST NAME: STUDENT NUMBER: ENROLLED IN: (circle one) STA 303 STA 1002 INSTRUCTIONS: Time: 90 minutes Aids allowed: calculator. Some formulae
Analysis of variance, multivariate (MANOVA)
 Analysis of variance, multivariate (MANOVA) Abstract: A designed experiment is set up in which the system studied is under the control of an investigator. The individuals, the treatments, the variables
Analysis of variance, multivariate (MANOVA) Abstract: A designed experiment is set up in which the system studied is under the control of an investigator. The individuals, the treatments, the variables
Normal distribution We have a random sample from N(m, υ). The sample mean is Ȳ and the corrected sum of squares is S yy. After some simplification,
 Likelihood Let P (D H) be the probability an experiment produces data D, given hypothesis H. Usually H is regarded as fixed and D variable. Before the experiment, the data D are unknown, and the probability
Likelihood Let P (D H) be the probability an experiment produces data D, given hypothesis H. Usually H is regarded as fixed and D variable. Before the experiment, the data D are unknown, and the probability
Clinical Trials. Olli Saarela. September 18, Dalla Lana School of Public Health University of Toronto.
 Introduction to Dalla Lana School of Public Health University of Toronto olli.saarela@utoronto.ca September 18, 2014 38-1 : a review 38-2 Evidence Ideal: to advance the knowledge-base of clinical medicine,
Introduction to Dalla Lana School of Public Health University of Toronto olli.saarela@utoronto.ca September 18, 2014 38-1 : a review 38-2 Evidence Ideal: to advance the knowledge-base of clinical medicine,
Tutorial 1: Power and Sample Size for the One-sample t-test. Acknowledgements:
 Tutorial 1: Power and Sample Size for the One-sample t-test Anna E. Barón, Keith E. Muller, Sarah M. Kreidler, and Deborah H. Glueck Acknowledgements: The project was supported in large part by the National
Tutorial 1: Power and Sample Size for the One-sample t-test Anna E. Barón, Keith E. Muller, Sarah M. Kreidler, and Deborah H. Glueck Acknowledgements: The project was supported in large part by the National
Math 494: Mathematical Statistics
 Math 494: Mathematical Statistics Instructor: Jimin Ding jmding@wustl.edu Department of Mathematics Washington University in St. Louis Class materials are available on course website (www.math.wustl.edu/
Math 494: Mathematical Statistics Instructor: Jimin Ding jmding@wustl.edu Department of Mathematics Washington University in St. Louis Class materials are available on course website (www.math.wustl.edu/
SEQUENTIAL MULTIPLE ASSIGNMENT RANDOMIZATION TRIALS WITH ENRICHMENT (SMARTER) DESIGN
 SEQUENTIAL MULTIPLE ASSIGNMENT RANDOMIZATION TRIALS WITH ENRICHMENT (SMARTER) DESIGN Ying Liu Division of Biostatistics, Medical College of Wisconsin Yuanjia Wang Department of Biostatistics & Psychiatry,
SEQUENTIAL MULTIPLE ASSIGNMENT RANDOMIZATION TRIALS WITH ENRICHMENT (SMARTER) DESIGN Ying Liu Division of Biostatistics, Medical College of Wisconsin Yuanjia Wang Department of Biostatistics & Psychiatry,
10. Time series regression and forecasting
 10. Time series regression and forecasting Key feature of this section: Analysis of data on a single entity observed at multiple points in time (time series data) Typical research questions: What is the
10. Time series regression and forecasting Key feature of this section: Analysis of data on a single entity observed at multiple points in time (time series data) Typical research questions: What is the
Introducing Generalized Linear Models: Logistic Regression
 Ron Heck, Summer 2012 Seminars 1 Multilevel Regression Models and Their Applications Seminar Introducing Generalized Linear Models: Logistic Regression The generalized linear model (GLM) represents and
Ron Heck, Summer 2012 Seminars 1 Multilevel Regression Models and Their Applications Seminar Introducing Generalized Linear Models: Logistic Regression The generalized linear model (GLM) represents and
Introduction to Empirical Processes and Semiparametric Inference Lecture 01: Introduction and Overview
 Introduction to Empirical Processes and Semiparametric Inference Lecture 01: Introduction and Overview Michael R. Kosorok, Ph.D. Professor and Chair of Biostatistics Professor of Statistics and Operations
Introduction to Empirical Processes and Semiparametric Inference Lecture 01: Introduction and Overview Michael R. Kosorok, Ph.D. Professor and Chair of Biostatistics Professor of Statistics and Operations
Master s Written Examination - Solution
 Master s Written Examination - Solution Spring 204 Problem Stat 40 Suppose X and X 2 have the joint pdf f X,X 2 (x, x 2 ) = 2e (x +x 2 ), 0 < x < x 2
Master s Written Examination - Solution Spring 204 Problem Stat 40 Suppose X and X 2 have the joint pdf f X,X 2 (x, x 2 ) = 2e (x +x 2 ), 0 < x < x 2
STA 114: Statistics. Notes 21. Linear Regression
 STA 114: Statistics Notes 1. Linear Regression Introduction A most widely used statistical analysis is regression, where one tries to explain a response variable Y by an explanatory variable X based on
STA 114: Statistics Notes 1. Linear Regression Introduction A most widely used statistical analysis is regression, where one tries to explain a response variable Y by an explanatory variable X based on
Precision of maximum likelihood estimation in adaptive designs
 Research Article Received 12 January 2015, Accepted 24 September 2015 Published online 12 October 2015 in Wiley Online Library (wileyonlinelibrary.com) DOI: 10.1002/sim.6761 Precision of maximum likelihood
Research Article Received 12 January 2015, Accepted 24 September 2015 Published online 12 October 2015 in Wiley Online Library (wileyonlinelibrary.com) DOI: 10.1002/sim.6761 Precision of maximum likelihood
Propensity Score Weighting with Multilevel Data
 Propensity Score Weighting with Multilevel Data Fan Li Department of Statistical Science Duke University October 25, 2012 Joint work with Alan Zaslavsky and Mary Beth Landrum Introduction In comparative
Propensity Score Weighting with Multilevel Data Fan Li Department of Statistical Science Duke University October 25, 2012 Joint work with Alan Zaslavsky and Mary Beth Landrum Introduction In comparative
Adaptive Prediction of Event Times in Clinical Trials
 Adaptive Prediction of Event Times in Clinical Trials Yu Lan Southern Methodist University Advisor: Daniel F. Heitjan May 8, 2017 Yu Lan (SMU) May 8, 2017 1 / 19 Clinical Trial Prediction Event-based trials:
Adaptive Prediction of Event Times in Clinical Trials Yu Lan Southern Methodist University Advisor: Daniel F. Heitjan May 8, 2017 Yu Lan (SMU) May 8, 2017 1 / 19 Clinical Trial Prediction Event-based trials:
