QUANTUM CHEMISTRY PROJECT 1
|
|
|
- Dora Melton
- 5 years ago
- Views:
Transcription
1 Chemistry 460 Fall 2017 Dr. Jean M. Standard September 11, 2017 QUANTUM CHEMISTRY PROJECT 1 OUTLINE This project focuses on applications and solutions of quantum mechanical problems involving one-dimensional piece-wise constant potentials. In the first part of the project, you will study a system called a quantum corral, which has been realized in the laboratory by arranging atoms in a circle on a metal surface. Using methods similar to those discussed in class, you will solve the quantum corral problem and investigate its wavefunctions and energy eigenvalues. In the second part of the project, you will apply a simple numerical method in order to solve the onedimensional Schrödinger equation for the finite well problem. You also will be able to compare the energies and wavefunctions of the infinite well with those of the half-infinite well problem discussed in class. DUE DATE The project is due on MONDAY, SEPTEMBER 25, GRADING Project 1 is worth 50 points and consists of two parts. Part A is worth 25 points and Part B is worth 25 points.
2 PART A (25 POINTS) THE QUANTUM CORRAL 2 This problem illustrates the use of a quantum mechanical model to represent the behavior of electrons in a quantum corral. The figure below shows a plot of the electron density on a Cu metal surface on which individual Fe atoms (shown in orange) have been placed in a ring (or corral). The Fe atoms were positioned on the Cu surface by using the tip of a scanning tunneling microscope. Figure 1. Quantum corral. Reference for quantum corral figure and information: IBM Corporation, See also Electrons inside the ring of Fe atoms are effectively trapped. This is like a two-dimensional particle in a circular box problem. The coordinates can be chosen to be r, the radius out from the origin at the center of the ring and the angle φ around the ring, such that the cartesian coordinates are x = r cosφ and y = r sinφ, and x 2 + y 2 = r 2. In our model of this behavior, the potential is defined to be V (r) = $ & 0, 0 r L '. (1) (, r > L ) Here, L is the radius of the corral. Note that the potential and the observed electron density in this case are independent of the angle φ and hence the problem reduces to a one-dimensional one that depends upon r only. The radial part of the kinetic energy operator in these coordinates can be defined as T ˆ =!2 2m # d 2 $ dr r d & dr (. (2) ' The Schrödinger equation for the quantum corral is therefore d 2 2m + dr 2!2 1 r d dr ψ(r) + V(r) ψ(r) = E ψ(r). (3)
3 3 Since the potential is infinite (our assumption) outside the corral, the wavefunction is zero outside, ψ out (r) = 0. Thus, we only have to worry about the solution inside the corral, where the potential is zero. The Schrödinger equation for the solution inside the quantum corral is given by d 2 2m dr d r dr ψ in (r) = E ψ in (r), (4)!2 where the potential V has been set equal to zero. In order to simplify the equation, we can scale the radial coordinate such that ρ = α r, with α = 2mE! 2. (5) The Schrödinger equation for the solution inside the quantum corral expressed in terms of the scaled radial coordinate ρ is d 2 dρ d ρ dρ ψ in (ρ) = ψ in (ρ). (6) When this equation is rearranged so that all the terms are moved to the left side, it becomes d 2 dρ d ρ dρ + 1 ψ in (ρ) = 0. (7) This is a well-known equation. The solutions are functions called Bessel functions. For this particular equation, the solution is a Bessel function of the first kind, denoted J 0 (ρ). This function looks something like a damped cosine function. Thus, we have that ψ in (ρ) = J 0 (ρ). (8) In this part of the project, you will explore the wavefunctions of the quantum corral and use techniques similar to those demonstrated in class for applying the boundary conditions to obtain the energy eigenvalues of the quantum corral. 1. First, you need to get a feel for what the Bessel function J 0 (ρ) looks like. Microsoft Excel includes the Bessel functions as built-in functions just like sine and cosine. In Excel, Bessel functions of the first kind are denoted BESSELJ(Z,N), where Z is the argument of the Bessel function (i.e., in our case it would represent the scaled radial coordinate ρ ) and N is the order of the Bessel function (in our case, N=0). Use Microsoft Excel to make a plot of the Bessel function J 0 (ρ) for ρ ranging from 0 to 20. Include this plot in your project. Select an appropriate step size in ρ such that your data generates a smooth plot. 2. Next, plot the probability density ψ in 2 (ρ) for ρ ranging from 0 to 20. How does the probability density compare with the quantum corral picture shown in Figure 1? Remember, you are plotting the radial part only since the solution is independent of the angular coordinate.
4 3. Recall the solution that we obtained in class for the particle in a one-dimensional box. The quantized energies came from applying the boundary conditions at x = 0 and x = L in order to require the wavefunction to be continuous. At x = L, the matching condition required that the wavefunction inside the box, which was proportional to sin kl, vanish. Therefore, we had to find the roots of the sine function; that is the locations where the sine function equals zero. This turned out to be the condition that kl = nπ, because the sine function has roots (or zeroes) at the values given by nπ. 4 Now, for the quantum corral, we need to do the same thing. Since the wavefunction outside the corral is zero, matching at r = L (or equivalently, ρ = αl) requires that the Bessel function vanish, J 0 (αl) = 0. So, we need to find the roots (or zeroes) of the Bessel function. Your plot from part #1 should give you some idea of the position of the roots; however, there is no simple formula for the roots like there is for the sine function. You will have to use the Solver in Microsoft Excel to find the roots. Using Solver, if you want the root of a function, you start with a guess. As an example, suppose you want to find the roots of the function f (x) = x 2 + x 2. This is a trivial example, since we can factor the quadratic function to obtain the roots analytically (they are x = 1 and x = 2). However, to find the roots numerically, a guess for the root is entered in one cell and the equation for the function is entered in another cell. For example, for this function, the following might be entered in the spreadsheet: 2 4 In the first cell, a guess for the root is entered this is the value "2". If there are multiple roots, then the guess will dictate which root is found. In the second cell, the value of the function evaluated for the guess is entered. In this case, if the guess is in cell A1, the equation entered in cell B1 would be "=A1^2+A1 2", so that the function f (x) = x 2 + x 2 is represented. The result that appears in cell B1 is the value f (2) = 4. You may need to add Solver to the Tools menu of Excel. To do this, select Tools Excel Add-ins. Check the box for Solver Add-in and click OK. Then, to use Solver to determine the roots, it must be selected from the Tools menu. Once selected, the Solver window appears:
5 5 Three settings must be entered in the Solver window to find the roots: (1) The Set Objective: box should be set to the cell location for the equation (in this case, $B$1 for cell B1). This may be typed in or the cell may be selected on the worksheet. (2) Since we want to find a root of the function, the To: setting should be set to Value of: 0. (3) And third, the By Changing Variable Cells: box should be set to the cell holding the guess (in this case, $A$1 for cell A1). Once these settings are entered, click Solve and the guess cell will be updated to hold the root x=1 (or something very close to 1), and the equation cell will be updated to hold value of the function (something very close to 0). For the example function, the results are shown below E-07 For the other root, a guess of 1 might be entered, for which Solver returns the second root, x= 2. Using this method, employ Microsoft Excel to find the first five roots of the Bessel function, such that J 0 (ρ) = 0. Report these values of ρ. 4. Use the definition of the scaled coordinate ρ, along with the roots that you found in part (3), to calculate the energy of the first five levels of the quantum corral if the corral radius is 71.3 Å (this is the radius of the circle of Fe atoms shown in the figure). Report your energies in electron volts.
6 PART B (25 POINTS) PARTICLE IN A FINITE WELL 6 In this problem, you will consider a particle in a finite potential well of width L and depth V 0 : V (x) = # $ & V 0 0 V 0 x < 0 0 x L x > L ' (. ) V=V 0 I II III x=0 x=l Suppose for this problem that L=5.0 bohr and V 0 = 4.5 hartrees. It will be helpful to employ atomic units in this problem, and you will consider only the case for which E < V 0. While this problem may be solved in a manner similar to that shown in class for the half-infinite well, in this case you will solve the Schrödinger equation numerically to obtain the quantized energies and wavefunctions. The approach that you will take to find the solution will be to construct a numerical approximation of the solution using Microsoft Excel. The numerical method that you will apply to this problem is called the Numerov method. It is based on Taylor series approximation of the derivatives that appear in the Schrödinger equation. For small increments, δ, the second derivative of the wavefunction may be approximated as ψ ""(x) = ( ) ψ " ( x δ /2) ψ " x + δ /2 δ. (9) Similarly, finite difference approximations of the first derivative of the wavefunction are given as ψ ʹ(x + δ / 2) = ψ ʹ(x δ / 2) = ( ) ψ ( x) ψ x + δ ψ x δ ( ) ψ ( x δ) δ, (10). (11) Substituting these equations into the expression for the second derivative gives ψ "" (x) = ψ x + δ ( ) 2ψ x The one-dimensional Schrödinger equation may be written as [ ( ) E] ψ "" (x) = 2m V x! 2 ψ x ( ) ( ) + ψ x δ δ 2. (12) ( ). (13)
7 7 Equating the second derivative on the left side of the Schrödinger equation to the numerical approximation of the second derivative given above yields ( ) 2ψ( x) + ψ( x δ) ψ x + δ δ 2 = 2m V x [ ( ) E ]! 2 ψ x ( ). (14) This equation may be solved for ψ( x +δ) to yield a form of the Numerov algorithm for the Schrödinger equation, ψ( x + δ) = 2ψ( x) ψ( x δ) + δ 2 2m [ V( x) E ]! 2 ψ x ( ). (15) The Numerov algorithm works because if the right side of the equation is known, then the wavefunction ψ( x +δ) can be calculated. The mass and potential are determined by the system that is being investigated. The parameter δ is selected to be a very small increment in x such as 0.01 or ( ) and ψ( x 0 δ), where x 0 is an initial value of x The Numerov algorithm is initiated by guessing values for ψ x 0 chosen to be in well into the classically forbidden region. Since the wavefunction is expected to be close to zero far into the classically forbidden region, it is usually acceptable to set ψ x 0 δ ( ) = 0 and ψ( x 0 ) = , for example. Once the initial values for the wavefunction are guessed, the right hand side of the Numerov algorithm may be evaluated if the energy E is known. Since the energy is not known, it must be guessed. Once the guess for the energy is substituted into the right side of the Numerov equation, it may be evaluated to yield the left side, the value of the wavefunction at the next point, ψ x +δ the complete range of x. The calculated value of ψ x +δ ( ) for the next iteration. ψ x δ ( ). This process then can be iterated to generate the wavefunction over ( ) becomes ψ( x) and the previous value of ψ( x) becomes If the guess for the energy E was correct, then when the numerical solution of the wavefunction reaches the classically forbidden region for large x, it will go to zero. If the energy guess was wrong, then the wavefunction will diverge. A new guess must then be entered. This process is repeated until the boundary conditions for large x are satisfied. The energy and wavefunction obtained are the correct solutions of the Schrödinger equation. 1.) To begin, you will use Microsoft Excel to construct a set of data points to represent the potential energy of the finite well as a function of x. Since the Numerov algorithm must begin its iterations far into the classically forbidden region, you will want to generate the potential about 3 bohr on either side of the well. So, for example, if the well is defined from x=0 to x=5.0 bohr, then you should generate the potential from about x= 3 to x=+8 bohr. The step size for the Numerov algorithm also must be selected. A good recommendation is for you to use a step size δ=0.02 bohr. With the range of the potential and the step size selected, you will first generate the array of x values and then the array of data points for the potential. Start with the x array. Enter the value 3 in one of the cells (this is the smallest value of x that you will use in the Numerov method). In the cell below, define the next value to be the previous value plus the step size (0.02). The second cell should contain the value Now, use the cursor to copy that calculation into the cells below and continue all the way down the column until you reach the value of x of There are about 550 points. You now have generated the array of x values.
8 Next, since the potential is piecewise constant, in the cell next to x= 3.0, enter the value of V 0 (in this case, 4.5 hartrees). Then, copy this value in the cells below until you reach x=0. At this point, change the value that you are entering to 0.0 hartrees and copy that value into the cells until you reach x=l (in this case, x=5.0 bohr). Finally, in the remaining cells from x> 5.0 bohr to x=8.0 bohr, enter the value of V 0. You have now generated the array of data points for the potential energy function. 8 2.) Next you will need to enter a guess for the first energy eigenvalue in a cell somewhere on the spreadsheet. Start with a guess of 0.1 hartrees. At this point you are ready to enter the first two data points for the wavefunction into the spreadsheet and implement the Numerov algorithm. To start this, enter the value 0.00 into a cell next to the first point in the potential array. This is ψ x 0 δ ( ). Now, in the cell below that, enter the value this is ψ( x 0 ). These are the two wavefunction initial conditions required to start the Numerov algorithm. Recall that the Numerov algorithm is defined as ψ( x + δ) = 2ψ( x) ψ( x δ) + δ 2 2m [ V( x) E ] In atomic units, since m =1 and! = 1, the equation becomes! 2 ψ x ( ). ψ( x + δ) = 2ψ( x) ψ( x δ) + 2δ 2 [ V( x) E] ψ( x). In the third cell corresponding to the wavefunction array, enter this equation. Use the guess for the energy (0.1 hartrees) for E and for V x ( ), select the corresponding point from the data array that you generated in step 1. For the fourth and subsequent points of the wavefunction array, copy the formula that you entered in the third cell. If you have done this correctly, the third cell of the wavefunction array should hold the value and the fourth cell should hold the value for an energy guess of 0.1 hartrees. If you need assistance getting this to agree, please see Dr. Standard. 3.) In order to see whether or not the energy guess corresponds to the eigenvalue, you will need to plot the wavefunction. In the same worksheet, create a chart with the wavefunction on the y-axis and the x data on the x-axis. With E guess =0.1 hartrees, the plot of the wavefunction should look similar to the figure below ψ(x) Trial wavefunction obtained for E guess =0.1 hartrees.
9 9 Notice that the trial wavefuntion blows up at large x. This means that the guessed energy did not correspond to an eigenvalue, and so the wavefunction does not satisfy the boundary conditions it is not a valid solution. You should be able to change the energy guess, and if you have done it correctly, the wavefunction and the graph should automatically be updated. If an energy guess of 0.2 hartrees now is entered, the wavefunction again blows up, but this time it gets very negative instead of very positive. When the trial wavefunction flips sign like this, it is an indication that the true energy eigenvalue lies between the two guesses (in this case, between E=0.1 and 0.2 hartrees) ψ(x) Trial wavefunction obtained for E guess =0.2 hartrees. To complete the solution, all you need to do is systematically vary the energy guess until the trial wavefunction goes to zero for large values of x. When you are searching for energy eigenvalues using the Numerov method, you will need to be patient and carefully zoom in on the energy. You will need from about 3-6 decimal places in order to find a converged energy. A larger number of decimal places is required for the lower energy levels. The converged ground state wavefunction should look like the one in the figure below (note that this function is not necessarily normalized) ψ(x) Converged ground state wavefunction for finite well potential. 4.) To complete this portion of the project, use your Numerov algorithm to determine the energies and wavefunctions for all the bound states of the finite well with L=5.0 bohr and V 0 = 4.5 hartrees. Note that for a state to be bound, its energy must be lower than V 0. Tabulate the energy eigenvalues that you found using the Numerov method.
10 10 5.) Compare the energy eigenvalues that you found for the finite well in part #4 with the energies of a particle in an infinite box with the same well width and with a particle in a half-infinite box with the same well width and depth. What are the effects of the finite depth on the energies? Hint: You should be able to calculate the energy eigenvalues of the particle in an infinite box analytically for comparison. The energy eigenvalues of the particle in a half-infinite box with α=15.0 are listed below for [ ] 1/ 2. convenience. These energies were obtained by solving the equation z cot z = α 2 z 2 Half-Infinite Well Eigenvalues Eigenvalue No. Energy (hartrees) ) Include plots of the wavefunctions of each of the states that you found with the Numerov method for the particle in a finite well. Compare the tunneling behavior of these wavefunctions to those of the particle in an infinite box and to those of the particle in a half-infinite box. You should be able to generate wavefunctions for the particle in an infinite box from the analytic solutions (or from the Excel spreadsheet used in class) if you want to compare them to the wavefunctions generated from your Numerov method for the particle in a finite well. For the particle in a half-infinite well, you can generate the wavefunctions using the Excel spreadsheet from class; however, they are shown below and on the next page for your convenience ψ 1 (x) ψ 2 (x) (a) (b) (a) Ground and (b) first excited state wavefunctions for particle in a half-infinite well with L=5.0 bohr and V 0 =4.5 hartrees. -0.8
11 ψ 3 (x) ψ 3 (x) (c) (d) (c) Second and (d) third excited state wavefunctions for particle in a half-infinite well with L=5.0 bohr and V 0 =4.5 hartrees ψ 3 (x) (e) (e) Fourth excited state wavefunction for particle in a half-infinite well with L=5.0 bohr and V 0 =4.5 hartrees. To compare the half-infinite well wavefunctions shown above to the wavefunctions generated from your Numerov method for the particle in a finite well, you might want to normalize the Numerov wavefunctions. To do this, in another column in your Excel spreadsheet, calculate ψ 2 at each value of x. Then, the integral over all space can be approximated by summing up these values and multiplying by the step size, ψ * ( x) ψ( x) dx ψ 2 ( x i ) δ = δ ψ 2 ( x i ), n i=1 where δ is the step size for the Numerov method and we have assumed that the wavefunction is real. If the wavefunction is normalized, the integral and therefore the approximate summation should give the result of 1. If the sum is not equal to 1, then the wavefunction must be normalized and the sum can be set equal to1/n 2, where N is the normalization constant. Then, the normalization constant N may be determined as N = 1/ SUM, n i=1 where SUM is the summation defined above. Note that SUM(A1:A551) will sum the values in the cells from A1 to A551 in Excel. Remember to include the factor δ when defining the SUM. Then, to scale the wavefunction, in a new column in your Excel spreadsheet multiply all the values of ψ by the normalization constant N.
Homework Problem Set 3 Solutions
 Chemistry 460 Dr. Jean M. Standard Homework Problem Set 3 Solutions 1. See Section 2-5 of Lowe and Peterson for more information about this example. When a particle experiences zero potential energy over
Chemistry 460 Dr. Jean M. Standard Homework Problem Set 3 Solutions 1. See Section 2-5 of Lowe and Peterson for more information about this example. When a particle experiences zero potential energy over
Sample Quantum Chemistry Exam 1 Solutions
 Chemistry 46 Fall 217 Dr Jean M Standard September 27, 217 Name SAMPE EXAM Sample Quantum Chemistry Exam 1 Solutions 1 (24 points Answer the following questions by selecting the correct answer from the
Chemistry 46 Fall 217 Dr Jean M Standard September 27, 217 Name SAMPE EXAM Sample Quantum Chemistry Exam 1 Solutions 1 (24 points Answer the following questions by selecting the correct answer from the
Introduction to Quantum Mechanics (Prelude to Nuclear Shell Model) Heisenberg Uncertainty Principle In the microscopic world,
 Introduction to Quantum Mechanics (Prelude to Nuclear Shell Model) Heisenberg Uncertainty Principle In the microscopic world, x p h π If you try to specify/measure the exact position of a particle you
Introduction to Quantum Mechanics (Prelude to Nuclear Shell Model) Heisenberg Uncertainty Principle In the microscopic world, x p h π If you try to specify/measure the exact position of a particle you
( ) = 9φ 1, ( ) = 4φ 2.
 Chemistry 46 Dr Jean M Standard Homework Problem Set 6 Solutions The Hermitian operator A ˆ is associated with the physical observable A Two of the eigenfunctions of A ˆ are and These eigenfunctions are
Chemistry 46 Dr Jean M Standard Homework Problem Set 6 Solutions The Hermitian operator A ˆ is associated with the physical observable A Two of the eigenfunctions of A ˆ are and These eigenfunctions are
Quantum Mechanical Tunneling
 Chemistry 460 all 07 Dr Jean M Standard September 8, 07 Quantum Mechanical Tunneling Definition of Tunneling Tunneling is defined to be penetration of the wavefunction into a classically forbidden region
Chemistry 460 all 07 Dr Jean M Standard September 8, 07 Quantum Mechanical Tunneling Definition of Tunneling Tunneling is defined to be penetration of the wavefunction into a classically forbidden region
Physics 342 Lecture 23. Radial Separation. Lecture 23. Physics 342 Quantum Mechanics I
 Physics 342 Lecture 23 Radial Separation Lecture 23 Physics 342 Quantum Mechanics I Friday, March 26th, 2010 We begin our spherical solutions with the simplest possible case zero potential. Aside from
Physics 342 Lecture 23 Radial Separation Lecture 23 Physics 342 Quantum Mechanics I Friday, March 26th, 2010 We begin our spherical solutions with the simplest possible case zero potential. Aside from
Time part of the equation can be separated by substituting independent equation
 Lecture 9 Schrödinger Equation in 3D and Angular Momentum Operator In this section we will construct 3D Schrödinger equation and we give some simple examples. In this course we will consider problems where
Lecture 9 Schrödinger Equation in 3D and Angular Momentum Operator In this section we will construct 3D Schrödinger equation and we give some simple examples. In this course we will consider problems where
August 7, 2007 NUMERICAL SOLUTION OF LAPLACE'S EQUATION
 August 7, 007 NUMERICAL SOLUTION OF LAPLACE'S EQUATION PURPOSE: This experiment illustrates the numerical solution of Laplace's Equation using a relaxation method. The results of the relaxation method
August 7, 007 NUMERICAL SOLUTION OF LAPLACE'S EQUATION PURPOSE: This experiment illustrates the numerical solution of Laplace's Equation using a relaxation method. The results of the relaxation method
QUANTUM CHEMISTRY PROJECT 2: THE FRANCK CONDON PRINCIPLE
 Chemistry 460 Fall 2017 Dr. Jean M. Standard October 4, 2017 OUTLINE QUANTUM CHEMISTRY PROJECT 2: THE FRANCK CONDON PRINCIPLE This project deals with the Franck-Condon Principle, electronic transitions
Chemistry 460 Fall 2017 Dr. Jean M. Standard October 4, 2017 OUTLINE QUANTUM CHEMISTRY PROJECT 2: THE FRANCK CONDON PRINCIPLE This project deals with the Franck-Condon Principle, electronic transitions
20 The Hydrogen Atom. Ze2 r R (20.1) H( r, R) = h2 2m 2 r h2 2M 2 R
 20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
20 The Hydrogen Atom 1. We want to solve the time independent Schrödinger Equation for the hydrogen atom. 2. There are two particles in the system, an electron and a nucleus, and so we can write the Hamiltonian
Numerical solution of the time-independent 1-D Schrödinger equation. Matthias E. Möbius. September 24, 2010
 Numerical solution of the time-independent 1-D Schrödinger equation Matthias E. Möbius September 24, 2010 1 Aim of the computational lab Numerical solution of the one-dimensional stationary Schrödinger
Numerical solution of the time-independent 1-D Schrödinger equation Matthias E. Möbius September 24, 2010 1 Aim of the computational lab Numerical solution of the one-dimensional stationary Schrödinger
CHAPTER 8 The Quantum Theory of Motion
 I. Translational motion. CHAPTER 8 The Quantum Theory of Motion A. Single particle in free space, 1-D. 1. Schrodinger eqn H ψ = Eψ! 2 2m d 2 dx 2 ψ = Eψ ; no boundary conditions 2. General solution: ψ
I. Translational motion. CHAPTER 8 The Quantum Theory of Motion A. Single particle in free space, 1-D. 1. Schrodinger eqn H ψ = Eψ! 2 2m d 2 dx 2 ψ = Eψ ; no boundary conditions 2. General solution: ψ
Sample Quantum Chemistry Exam 2 Solutions
 Chemistry 46 Fall 7 Dr. Jean M. Standard Name SAMPE EXAM Sample Quantum Chemistry Exam Solutions.) ( points) Answer the following questions by selecting the correct answer from the choices provided. a.)
Chemistry 46 Fall 7 Dr. Jean M. Standard Name SAMPE EXAM Sample Quantum Chemistry Exam Solutions.) ( points) Answer the following questions by selecting the correct answer from the choices provided. a.)
Created: 2/3/96 Modified: September 29, Author: Theresa Julia Zielinski Page 1
 Exploring Orthonormal Functions by Theresa Julia Zielinski Department of Chemistry, Medical Technology, and Physics Monmouth University West Long Branch, NJ 7764-898 tzielins@monmouth.edu Copyright 7 by
Exploring Orthonormal Functions by Theresa Julia Zielinski Department of Chemistry, Medical Technology, and Physics Monmouth University West Long Branch, NJ 7764-898 tzielins@monmouth.edu Copyright 7 by
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 9, February 8, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 9, February 8, 2006 The Harmonic Oscillator Consider a diatomic molecule. Such a molecule
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 9, February 8, 2006 The Harmonic Oscillator Consider a diatomic molecule. Such a molecule
Visualizing Particle-in-a-Box Wavefunctions
 Particle-in-a-box-5.nb 1 Visualizing Particle-in-a-Box Wavefunctions By Edmund L. Tisko Department of Chemistry University of Nebraska at Omaha Omaha, NE 68182 etisko@unomaha.edu Translated and modified
Particle-in-a-box-5.nb 1 Visualizing Particle-in-a-Box Wavefunctions By Edmund L. Tisko Department of Chemistry University of Nebraska at Omaha Omaha, NE 68182 etisko@unomaha.edu Translated and modified
REVIEW: The Matching Method Algorithm
 Lecture 26: Numerov Algorithm for Solving the Time-Independent Schrödinger Equation 1 REVIEW: The Matching Method Algorithm Need for a more general method The shooting method for solving the time-independent
Lecture 26: Numerov Algorithm for Solving the Time-Independent Schrödinger Equation 1 REVIEW: The Matching Method Algorithm Need for a more general method The shooting method for solving the time-independent
Quantum Physics II (8.05) Fall 2002 Assignment 11
 Quantum Physics II (8.05) Fall 00 Assignment 11 Readings Most of the reading needed for this problem set was already given on Problem Set 9. The new readings are: Phase shifts are discussed in Cohen-Tannoudji
Quantum Physics II (8.05) Fall 00 Assignment 11 Readings Most of the reading needed for this problem set was already given on Problem Set 9. The new readings are: Phase shifts are discussed in Cohen-Tannoudji
The Sommerfeld Polynomial Method: Harmonic Oscillator Example
 Chemistry 460 Fall 2017 Dr. Jean M. Standard October 2, 2017 The Sommerfeld Polynomial Method: Harmonic Oscillator Example Scaling the Harmonic Oscillator Equation Recall the basic definitions of the harmonic
Chemistry 460 Fall 2017 Dr. Jean M. Standard October 2, 2017 The Sommerfeld Polynomial Method: Harmonic Oscillator Example Scaling the Harmonic Oscillator Equation Recall the basic definitions of the harmonic
CHAPTER 6 Quantum Mechanics II
 CHAPTER 6 Quantum Mechanics II 6.1 6.2 6.3 6.4 6.5 6.6 6.7 The Schrödinger Wave Equation Expectation Values Infinite Square-Well Potential Finite Square-Well Potential Three-Dimensional Infinite-Potential
CHAPTER 6 Quantum Mechanics II 6.1 6.2 6.3 6.4 6.5 6.6 6.7 The Schrödinger Wave Equation Expectation Values Infinite Square-Well Potential Finite Square-Well Potential Three-Dimensional Infinite-Potential
Illustrating the Bohr Correspondence Principle
 Illustrating the Bohr Correspondence Principle Glenn V. Lo Department of Physical Sciences Nicholls State University Thibodaux, LA 70310 phsc-gl@nicholls.edu Copyright 2002 by the Division of Chemical
Illustrating the Bohr Correspondence Principle Glenn V. Lo Department of Physical Sciences Nicholls State University Thibodaux, LA 70310 phsc-gl@nicholls.edu Copyright 2002 by the Division of Chemical
CHAPTER 6 Quantum Mechanics II
 CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6.2 Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite-Potential Well
CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6.2 Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite-Potential Well
Topic 4: The Finite Potential Well
 Topic 4: The Finite Potential Well Outline: The quantum well The finite potential well (FPW) Even parity solutions of the TISE in the FPW Odd parity solutions of the TISE in the FPW Tunnelling into classically
Topic 4: The Finite Potential Well Outline: The quantum well The finite potential well (FPW) Even parity solutions of the TISE in the FPW Odd parity solutions of the TISE in the FPW Tunnelling into classically
Section 6.2 Notes Page Trigonometric Functions; Unit Circle Approach
 Section Notes Page Trigonometric Functions; Unit Circle Approach A unit circle is a circle centered at the origin with a radius of Its equation is x y = as shown in the drawing below Here the letter t
Section Notes Page Trigonometric Functions; Unit Circle Approach A unit circle is a circle centered at the origin with a radius of Its equation is x y = as shown in the drawing below Here the letter t
Opinions on quantum mechanics. CHAPTER 6 Quantum Mechanics II. 6.1: The Schrödinger Wave Equation. Normalization and Probability
 CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6. Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite- 6.6 Simple Harmonic
CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6. Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite- 6.6 Simple Harmonic
df(x) = h(x) dx Chemistry 4531 Mathematical Preliminaries Spring 2009 I. A Primer on Differential Equations Order of differential equation
 Chemistry 4531 Mathematical Preliminaries Spring 009 I. A Primer on Differential Equations Order of differential equation Linearity of differential equation Partial vs. Ordinary Differential Equations
Chemistry 4531 Mathematical Preliminaries Spring 009 I. A Primer on Differential Equations Order of differential equation Linearity of differential equation Partial vs. Ordinary Differential Equations
Using Microsoft Excel
 Using Microsoft Excel Objective: Students will gain familiarity with using Excel to record data, display data properly, use built-in formulae to do calculations, and plot and fit data with linear functions.
Using Microsoft Excel Objective: Students will gain familiarity with using Excel to record data, display data properly, use built-in formulae to do calculations, and plot and fit data with linear functions.
David J. Starling Penn State Hazleton PHYS 214
 Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in beer. -Dave Barry David J. Starling Penn State Hazleton
Not all chemicals are bad. Without chemicals such as hydrogen and oxygen, for example, there would be no way to make water, a vital ingredient in beer. -Dave Barry David J. Starling Penn State Hazleton
Final Exam. Tuesday, May 8, Starting at 8:30 a.m., Hoyt Hall.
 Final Exam Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall. Summary of Chapter 38 In Quantum Mechanics particles are represented by wave functions Ψ. The absolute square of the wave function Ψ 2
Final Exam Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall. Summary of Chapter 38 In Quantum Mechanics particles are represented by wave functions Ψ. The absolute square of the wave function Ψ 2
Lectures 21 and 22: Hydrogen Atom. 1 The Hydrogen Atom 1. 2 Hydrogen atom spectrum 4
 Lectures and : Hydrogen Atom B. Zwiebach May 4, 06 Contents The Hydrogen Atom Hydrogen atom spectrum 4 The Hydrogen Atom Our goal here is to show that the two-body quantum mechanical problem of the hydrogen
Lectures and : Hydrogen Atom B. Zwiebach May 4, 06 Contents The Hydrogen Atom Hydrogen atom spectrum 4 The Hydrogen Atom Our goal here is to show that the two-body quantum mechanical problem of the hydrogen
One-dimensional Schrödinger equation
 Chapter 1 One-dimensional Schrödinger equation In this chapter we will start from the harmonic oscillator to introduce a general numerical methodology to solve the one-dimensional, time-independent Schrödinger
Chapter 1 One-dimensional Schrödinger equation In this chapter we will start from the harmonic oscillator to introduce a general numerical methodology to solve the one-dimensional, time-independent Schrödinger
The Particle in a Box
 Page 324 Lecture 17: Relation of Particle in a Box Eigenstates to Position and Momentum Eigenstates General Considerations on Bound States and Quantization Continuity Equation for Probability Date Given:
Page 324 Lecture 17: Relation of Particle in a Box Eigenstates to Position and Momentum Eigenstates General Considerations on Bound States and Quantization Continuity Equation for Probability Date Given:
Particle in a 3 Dimensional Box just extending our model from 1D to 3D
 CHEM 2060 Lecture 20: Particle in a 3D Box; H atom L20-1 Particle in a 3 Dimensional Box just extending our model from 1D to 3D A 3D model is a step closer to reality than a 1D model. Let s increase the
CHEM 2060 Lecture 20: Particle in a 3D Box; H atom L20-1 Particle in a 3 Dimensional Box just extending our model from 1D to 3D A 3D model is a step closer to reality than a 1D model. Let s increase the
Continuum Limit and Fourier Series
 Chapter 6 Continuum Limit and Fourier Series Continuous is in the eye of the beholder Most systems that we think of as continuous are actually made up of discrete pieces In this chapter, we show that a
Chapter 6 Continuum Limit and Fourier Series Continuous is in the eye of the beholder Most systems that we think of as continuous are actually made up of discrete pieces In this chapter, we show that a
Quantum Mechanics in Three Dimensions
 Physics 342 Lecture 21 Quantum Mechanics in Three Dimensions Lecture 21 Physics 342 Quantum Mechanics I Monday, March 22nd, 21 We are used to the temporal separation that gives, for example, the timeindependent
Physics 342 Lecture 21 Quantum Mechanics in Three Dimensions Lecture 21 Physics 342 Quantum Mechanics I Monday, March 22nd, 21 We are used to the temporal separation that gives, for example, the timeindependent
PLC Papers. Created For:
 PLC Papers Created For: t followed by close scrutiny of the marking scheme followed by reassessing every 3 days to attain at least 8 out o Approximate solutions to equations using iteration 1 Grade 9 Objective:
PLC Papers Created For: t followed by close scrutiny of the marking scheme followed by reassessing every 3 days to attain at least 8 out o Approximate solutions to equations using iteration 1 Grade 9 Objective:
PHYS 7411 Spring 2015 Computational Physics Homework 4
 PHYS 7411 Spring 215 Computational Physics Homework 4 Due by 3:pm in Nicholson 447 on 3 March 215 Any late assignments will be penalized in the amount of 25% per day late. Any copying of computer programs
PHYS 7411 Spring 215 Computational Physics Homework 4 Due by 3:pm in Nicholson 447 on 3 March 215 Any late assignments will be penalized in the amount of 25% per day late. Any copying of computer programs
CHAPTER 6 Quantum Mechanics II
 CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6.2 Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite-Potential Well
CHAPTER 6 Quantum Mechanics II 6.1 The Schrödinger Wave Equation 6.2 Expectation Values 6.3 Infinite Square-Well Potential 6.4 Finite Square-Well Potential 6.5 Three-Dimensional Infinite-Potential Well
It illustrates quantum mechanical principals. It illustrates the use of differential eqns. & boundary conditions to solve for ψ
 MODEL SYSTEM: PARTICLE IN A BOX Important because: It illustrates quantum mechanical principals It illustrates the use of differential eqns. & boundary conditions to solve for ψ It shows how discrete energy
MODEL SYSTEM: PARTICLE IN A BOX Important because: It illustrates quantum mechanical principals It illustrates the use of differential eqns. & boundary conditions to solve for ψ It shows how discrete energy
Physics 201 Lab 2 Air Drag Simulation
 Physics 201 Lab 2 Air Drag Simulation Jan 28, 2013 Equipment Initial Set Up Type the data from Table 1 into the appropriate cells. By preceding the content of the cell with an equal sign (as in cell A6)
Physics 201 Lab 2 Air Drag Simulation Jan 28, 2013 Equipment Initial Set Up Type the data from Table 1 into the appropriate cells. By preceding the content of the cell with an equal sign (as in cell A6)
Physics 606, Quantum Mechanics, Final Exam NAME ( ) ( ) + V ( x). ( ) and p( t) be the corresponding operators in ( ) and x( t) : ( ) / dt =...
 Physics 606, Quantum Mechanics, Final Exam NAME Please show all your work. (You are graded on your work, with partial credit where it is deserved.) All problems are, of course, nonrelativistic. 1. Consider
Physics 606, Quantum Mechanics, Final Exam NAME Please show all your work. (You are graded on your work, with partial credit where it is deserved.) All problems are, of course, nonrelativistic. 1. Consider
TP computing lab - Integrating the 1D stationary Schrödinger eq
 TP computing lab - Integrating the 1D stationary Schrödinger equation September 21, 2010 The stationary 1D Schrödinger equation The time-independent (stationary) Schrödinger equation is given by Eψ(x)
TP computing lab - Integrating the 1D stationary Schrödinger equation September 21, 2010 The stationary 1D Schrödinger equation The time-independent (stationary) Schrödinger equation is given by Eψ(x)
Lecture 10 - Moment of Inertia
 Lecture 10 - oment of Inertia A Puzzle... Question For any object, there are typically many ways to calculate the moment of inertia I = r 2 dm, usually by doing the integration by considering different
Lecture 10 - oment of Inertia A Puzzle... Question For any object, there are typically many ways to calculate the moment of inertia I = r 2 dm, usually by doing the integration by considering different
Appendix F. + 1 Ma 1. 2 Ma Ma Ma ln + K = 0 (4-173)
 5:39p.m. Page:949 Trimsize:8.5in 11in Appendix F F.1 MICROSOFT EXCEL SOLVER FOR NON-LINEAR EQUATIONS The Solver is an optimization package that finds a maximum, minimum, or specified value of a target
5:39p.m. Page:949 Trimsize:8.5in 11in Appendix F F.1 MICROSOFT EXCEL SOLVER FOR NON-LINEAR EQUATIONS The Solver is an optimization package that finds a maximum, minimum, or specified value of a target
Harmonic Oscillator Eigenvalues and Eigenfunctions
 Chemistry 46 Fall 217 Dr. Jean M. Standard October 4, 217 Harmonic Oscillator Eigenvalues and Eigenfunctions The Quantum Mechanical Harmonic Oscillator The quantum mechanical harmonic oscillator in one
Chemistry 46 Fall 217 Dr. Jean M. Standard October 4, 217 Harmonic Oscillator Eigenvalues and Eigenfunctions The Quantum Mechanical Harmonic Oscillator The quantum mechanical harmonic oscillator in one
Physics 202 Laboratory 5. Linear Algebra 1. Laboratory 5. Physics 202 Laboratory
 Physics 202 Laboratory 5 Linear Algebra Laboratory 5 Physics 202 Laboratory We close our whirlwind tour of numerical methods by advertising some elements of (numerical) linear algebra. There are three
Physics 202 Laboratory 5 Linear Algebra Laboratory 5 Physics 202 Laboratory We close our whirlwind tour of numerical methods by advertising some elements of (numerical) linear algebra. There are three
6. Qualitative Solutions of the TISE
 6. Qualitative Solutions of the TISE Copyright c 2015 2016, Daniel V. Schroeder Our goal for the next few lessons is to solve the time-independent Schrödinger equation (TISE) for a variety of one-dimensional
6. Qualitative Solutions of the TISE Copyright c 2015 2016, Daniel V. Schroeder Our goal for the next few lessons is to solve the time-independent Schrödinger equation (TISE) for a variety of one-dimensional
PDEs in Spherical and Circular Coordinates
 Introduction to Partial Differential Equations part of EM, Scalar and Vector Fields module (PHY2064) This lecture Laplacian in spherical & circular polar coordinates Laplace s PDE in electrostatics Schrödinger
Introduction to Partial Differential Equations part of EM, Scalar and Vector Fields module (PHY2064) This lecture Laplacian in spherical & circular polar coordinates Laplace s PDE in electrostatics Schrödinger
7. Numerical Solutions of the TISE
 7. Numerical Solutions of the TISE Copyright c 2015 2016, Daniel V. Schroeder Besides the infinite square well, there aren t many potentials V (x) for which you can find the energies and eigenfunctions
7. Numerical Solutions of the TISE Copyright c 2015 2016, Daniel V. Schroeder Besides the infinite square well, there aren t many potentials V (x) for which you can find the energies and eigenfunctions
Quantum Mechanics & Atomic Structure (Chapter 11)
 Quantum Mechanics & Atomic Structure (Chapter 11) Quantum mechanics: Microscopic theory of light & matter at molecular scale and smaller. Atoms and radiation (light) have both wave-like and particlelike
Quantum Mechanics & Atomic Structure (Chapter 11) Quantum mechanics: Microscopic theory of light & matter at molecular scale and smaller. Atoms and radiation (light) have both wave-like and particlelike
If electrons moved in simple orbits, p and x could be determined, but this violates the Heisenberg Uncertainty Principle.
 CHEM 2060 Lecture 18: Particle in a Box L18-1 Atomic Orbitals If electrons moved in simple orbits, p and x could be determined, but this violates the Heisenberg Uncertainty Principle. We can only talk
CHEM 2060 Lecture 18: Particle in a Box L18-1 Atomic Orbitals If electrons moved in simple orbits, p and x could be determined, but this violates the Heisenberg Uncertainty Principle. We can only talk
QUANTUM MECHANICS A (SPA 5319) The Finite Square Well
 QUANTUM MECHANICS A (SPA 5319) The Finite Square Well We have already solved the problem of the infinite square well. Let us now solve the more realistic finite square well problem. Consider the potential
QUANTUM MECHANICS A (SPA 5319) The Finite Square Well We have already solved the problem of the infinite square well. Let us now solve the more realistic finite square well problem. Consider the potential
1 Solutions in cylindrical coordinates: Bessel functions
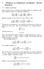 1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
1 Solutions in cylindrical coordinates: Bessel functions 1.1 Bessel functions Bessel functions arise as solutions of potential problems in cylindrical coordinates. Laplace s equation in cylindrical coordinates
The Central Force Problem: Hydrogen Atom
 The Central Force Problem: Hydrogen Atom B. Ramachandran Separation of Variables The Schrödinger equation for an atomic system with Z protons in the nucleus and one electron outside is h µ Ze ψ = Eψ, r
The Central Force Problem: Hydrogen Atom B. Ramachandran Separation of Variables The Schrödinger equation for an atomic system with Z protons in the nucleus and one electron outside is h µ Ze ψ = Eψ, r
Problems and Multiple Choice Questions
 Problems and Multiple Choice Questions 1. A momentum operator in one dimension is 2. A position operator in 3 dimensions is 3. A kinetic energy operator in 1 dimension is 4. If two operator commute, a)
Problems and Multiple Choice Questions 1. A momentum operator in one dimension is 2. A position operator in 3 dimensions is 3. A kinetic energy operator in 1 dimension is 4. If two operator commute, a)
(Refer Slide Time: 1:20) (Refer Slide Time: 1:24 min)
 Engineering Chemistry - 1 Prof. K. Mangala Sunder Department of Chemistry Indian Institute of Technology, Madras Lecture - 5 Module 1: Atoms and Molecules Harmonic Oscillator (Continued) (Refer Slide Time:
Engineering Chemistry - 1 Prof. K. Mangala Sunder Department of Chemistry Indian Institute of Technology, Madras Lecture - 5 Module 1: Atoms and Molecules Harmonic Oscillator (Continued) (Refer Slide Time:
AP Calculus AB Summer Assignment
 AP Calculus AB Summer Assignment Name: When you come back to school, it is my epectation that you will have this packet completed. You will be way behind at the beginning of the year if you haven t attempted
AP Calculus AB Summer Assignment Name: When you come back to school, it is my epectation that you will have this packet completed. You will be way behind at the beginning of the year if you haven t attempted
Class 21. Early Quantum Mechanics and the Wave Nature of Matter. Physics 106. Winter Press CTRL-L to view as a slide show. Class 21.
 Early and the Wave Nature of Matter Winter 2018 Press CTRL-L to view as a slide show. Last Time Last time we discussed: Optical systems Midterm 2 Today we will discuss: Quick of X-ray diffraction Compton
Early and the Wave Nature of Matter Winter 2018 Press CTRL-L to view as a slide show. Last Time Last time we discussed: Optical systems Midterm 2 Today we will discuss: Quick of X-ray diffraction Compton
Lab 1. EXCEL plus some basic concepts such as scientific notation, order of magnitude, logarithms, and unit conversions
 COMPUTER LAB 1 EARTH SYSTEMS SCIENCE I PG250 Fall 2010 Hunter College Lab 1. EXCEL plus some basic concepts such as scientific notation, order of magnitude, logarithms, and unit conversions Low Impact
COMPUTER LAB 1 EARTH SYSTEMS SCIENCE I PG250 Fall 2010 Hunter College Lab 1. EXCEL plus some basic concepts such as scientific notation, order of magnitude, logarithms, and unit conversions Low Impact
Physics 6303 Lecture 15 October 10, Reminder of general solution in 3-dimensional cylindrical coordinates. sinh. sin
 Physics 6303 Lecture 15 October 10, 2018 LAST TIME: Spherical harmonics and Bessel functions Reminder of general solution in 3-dimensional cylindrical coordinates,, sin sinh cos cosh, sin sin cos cos,
Physics 6303 Lecture 15 October 10, 2018 LAST TIME: Spherical harmonics and Bessel functions Reminder of general solution in 3-dimensional cylindrical coordinates,, sin sinh cos cosh, sin sin cos cos,
Applied Nuclear Physics (Fall 2006) Lecture 3 (9/13/06) Bound States in One Dimensional Systems Particle in a Square Well
 22.101 Applied Nuclear Physics (Fall 2006) Lecture 3 (9/13/06) Bound States in One Dimensional Systems Particle in a Square Well References - R. L. Liboff, Introductory Quantum Mechanics (Holden Day, New
22.101 Applied Nuclear Physics (Fall 2006) Lecture 3 (9/13/06) Bound States in One Dimensional Systems Particle in a Square Well References - R. L. Liboff, Introductory Quantum Mechanics (Holden Day, New
quantization condition.
 /8/016 PHYS 34 Modern Physics Atom II: Hydrogen Atom Roadmap for Exploring Hydrogen Atom Today Contents: a) Schrodinger Equation for Hydrogen Atom b) Angular Momentum in Quantum Mechanics c) Quantum Number
/8/016 PHYS 34 Modern Physics Atom II: Hydrogen Atom Roadmap for Exploring Hydrogen Atom Today Contents: a) Schrodinger Equation for Hydrogen Atom b) Angular Momentum in Quantum Mechanics c) Quantum Number
Quantum Orbits. Quantum Theory for the Computer Age Unit 9. Diving orbit. Caustic. for KE/PE =R=-3/8. for KE/PE =R=-3/8. p"...
 W.G. Harter Coulomb Obits 6-1 Quantum Theory for the Computer Age Unit 9 Caustic for KE/PE =R=-3/8 F p' p g r p"... P F' F P Diving orbit T" T T' Contact Pt. for KE/PE =R=-3/8 Quantum Orbits W.G. Harter
W.G. Harter Coulomb Obits 6-1 Quantum Theory for the Computer Age Unit 9 Caustic for KE/PE =R=-3/8 F p' p g r p"... P F' F P Diving orbit T" T T' Contact Pt. for KE/PE =R=-3/8 Quantum Orbits W.G. Harter
Dimensional analysis
 Dimensional analysis Calvin W. Johnson September 9, 04 Dimensional analysis Dimensional analysis is a powerful and important tool in calculations. In fact, you should make it a habit to automatically use
Dimensional analysis Calvin W. Johnson September 9, 04 Dimensional analysis Dimensional analysis is a powerful and important tool in calculations. In fact, you should make it a habit to automatically use
The Hydrogen Atom. Dr. Sabry El-Taher 1. e 4. U U r
 The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
The Hydrogen Atom Atom is a 3D object, and the electron motion is three-dimensional. We ll start with the simplest case - The hydrogen atom. An electron and a proton (nucleus) are bound by the central-symmetric
Problem Set 5: Solutions
 University of Alabama Department of Physics and Astronomy PH 53 / eclair Spring 1 Problem Set 5: Solutions 1. Solve one of the exam problems that you did not choose.. The Thompson model of the atom. Show
University of Alabama Department of Physics and Astronomy PH 53 / eclair Spring 1 Problem Set 5: Solutions 1. Solve one of the exam problems that you did not choose.. The Thompson model of the atom. Show
Properties of a Taylor Polynomial
 3.4.4: Still Better Approximations: Taylor Polynomials Properties of a Taylor Polynomial Constant: f (x) f (a) Linear: f (x) f (a) + f (a)(x a) Quadratic: f (x) f (a) + f (a)(x a) + 1 2 f (a)(x a) 2 3.4.4:
3.4.4: Still Better Approximations: Taylor Polynomials Properties of a Taylor Polynomial Constant: f (x) f (a) Linear: f (x) f (a) + f (a)(x a) Quadratic: f (x) f (a) + f (a)(x a) + 1 2 f (a)(x a) 2 3.4.4:
Illustrating the Bohr Correspondence Principle
 Illustrating the Bohr Correspondence Principle Glenn V. Lo Department of Physical Sciences Nicholls State University Thibodaux, LA 70310 phsc-gl@nicholls.edu Copyright 2002 by the Division of Chemical
Illustrating the Bohr Correspondence Principle Glenn V. Lo Department of Physical Sciences Nicholls State University Thibodaux, LA 70310 phsc-gl@nicholls.edu Copyright 2002 by the Division of Chemical
AP Calculus (BC) Chapter 9 Test No Calculator Section Name: Date: Period:
 WORKSHEET: Series, Taylor Series AP Calculus (BC) Chapter 9 Test No Calculator Section Name: Date: Period: 1 Part I. Multiple-Choice Questions (5 points each; please circle the correct answer.) 1. The
WORKSHEET: Series, Taylor Series AP Calculus (BC) Chapter 9 Test No Calculator Section Name: Date: Period: 1 Part I. Multiple-Choice Questions (5 points each; please circle the correct answer.) 1. The
Chapter 1 Review of Equations and Inequalities
 Chapter 1 Review of Equations and Inequalities Part I Review of Basic Equations Recall that an equation is an expression with an equal sign in the middle. Also recall that, if a question asks you to solve
Chapter 1 Review of Equations and Inequalities Part I Review of Basic Equations Recall that an equation is an expression with an equal sign in the middle. Also recall that, if a question asks you to solve
Final Exam: Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall.
 Final Exam: Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall. Chapter 38 Quantum Mechanics Units of Chapter 38 38-1 Quantum Mechanics A New Theory 37-2 The Wave Function and Its Interpretation; the
Final Exam: Tuesday, May 8, 2012 Starting at 8:30 a.m., Hoyt Hall. Chapter 38 Quantum Mechanics Units of Chapter 38 38-1 Quantum Mechanics A New Theory 37-2 The Wave Function and Its Interpretation; the
Physics 221A Fall 1996 Notes 16 Bloch s Theorem and Band Structure in One Dimension
 Physics 221A Fall 1996 Notes 16 Bloch s Theorem and Band Structure in One Dimension In these notes we examine Bloch s theorem and band structure in problems with periodic potentials, as a part of our survey
Physics 221A Fall 1996 Notes 16 Bloch s Theorem and Band Structure in One Dimension In these notes we examine Bloch s theorem and band structure in problems with periodic potentials, as a part of our survey
P3317 HW from Lecture 15 and Recitation 8
 P3317 HW from Lecture 15 and Recitation 8 Due Oct 23, 218 Problem 1. Variational Energy of Helium Here we will estimate the ground state energy of Helium. Helium has two electrons circling around a nucleus
P3317 HW from Lecture 15 and Recitation 8 Due Oct 23, 218 Problem 1. Variational Energy of Helium Here we will estimate the ground state energy of Helium. Helium has two electrons circling around a nucleus
A Computer Study of Molecular Electronic Structure
 A Computer Study of Molecular Electronic Structure The following exercises are designed to give you a brief introduction to some of the types of information that are now readily accessible from electronic
A Computer Study of Molecular Electronic Structure The following exercises are designed to give you a brief introduction to some of the types of information that are now readily accessible from electronic
Brief review of Quantum Mechanics (QM)
 Brief review of Quantum Mechanics (QM) Note: This is a collection of several formulae and facts that we will use throughout the course. It is by no means a complete discussion of QM, nor will I attempt
Brief review of Quantum Mechanics (QM) Note: This is a collection of several formulae and facts that we will use throughout the course. It is by no means a complete discussion of QM, nor will I attempt
2. How will we adjust our fitting procedure to compensate for fact that the acceleration differs depending on the direction of motion?
 The Coefficient of Kinetic Friction 1 Name: Lab Section Number: Pre-Lab Questions: 1. What type of data will we be using to determine the acceleration of the cart up and down the ramp this week? What type
The Coefficient of Kinetic Friction 1 Name: Lab Section Number: Pre-Lab Questions: 1. What type of data will we be using to determine the acceleration of the cart up and down the ramp this week? What type
The 3 dimensional Schrödinger Equation
 Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Chapter 6 The 3 dimensional Schrödinger Equation 6.1 Angular Momentum To study how angular momentum is represented in quantum mechanics we start by reviewing the classical vector of orbital angular momentum
Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours.
 Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours. There are 10 problems, totalling 180 points. Do all problems. Answer all problems in the white books provided.
Quantum Physics III (8.06) Spring 2007 FINAL EXAMINATION Monday May 21, 9:00 am You have 3 hours. There are 10 problems, totalling 180 points. Do all problems. Answer all problems in the white books provided.
QUANTUM CHEMISTRY PROJECT 3: PARTS B AND C
 Chemistry 460 Fall 2017 Dr. Jean M. Standard November 6, 2017 QUANTUM CHEMISTRY PROJECT 3: PARTS B AND C PART B: POTENTIAL CURVE, SPECTROSCOPIC CONSTANTS, AND DISSOCIATION ENERGY OF DIATOMIC HYDROGEN (20
Chemistry 460 Fall 2017 Dr. Jean M. Standard November 6, 2017 QUANTUM CHEMISTRY PROJECT 3: PARTS B AND C PART B: POTENTIAL CURVE, SPECTROSCOPIC CONSTANTS, AND DISSOCIATION ENERGY OF DIATOMIC HYDROGEN (20
Physics 220. Exam #2. May 23 May 30, 2014
 Physics 0 Exam # May 3 May 30, 014 Name Please read and follow these instructions carefully: Read all problems carefully before attempting to solve them. Your work must be legible, with clear organization,
Physics 0 Exam # May 3 May 30, 014 Name Please read and follow these instructions carefully: Read all problems carefully before attempting to solve them. Your work must be legible, with clear organization,
Chem 6 Practice Exam 2
 These problems are from past Chem 6 exams. Each exam contained a page of universal constant values and common equations; yours will, too, along with a Periodic Table! The answers follow after all the questions.
These problems are from past Chem 6 exams. Each exam contained a page of universal constant values and common equations; yours will, too, along with a Periodic Table! The answers follow after all the questions.
Electric Fields and Equipotentials
 OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
OBJECTIVE Electric Fields and Equipotentials To study and describe the two-dimensional electric field. To map the location of the equipotential surfaces around charged electrodes. To study the relationship
Example 2.1. Draw the points with polar coordinates: (i) (3, π) (ii) (2, π/4) (iii) (6, 2π/4) We illustrate all on the following graph:
 Section 10.3: Polar Coordinates The polar coordinate system is another way to coordinatize the Cartesian plane. It is particularly useful when examining regions which are circular. 1. Cartesian Coordinates
Section 10.3: Polar Coordinates The polar coordinate system is another way to coordinatize the Cartesian plane. It is particularly useful when examining regions which are circular. 1. Cartesian Coordinates
Chemistry 125: Instructions for Erwin Meets Goldilocks
 Chemistry 125: Instructions for Erwin Meets Goldilocks [Note the 5 problems for Monday s problem set are found at the end of this document. Many of the details on operating the program relate to an earlier
Chemistry 125: Instructions for Erwin Meets Goldilocks [Note the 5 problems for Monday s problem set are found at the end of this document. Many of the details on operating the program relate to an earlier
Probability and Normalization
 Probability and Normalization Although we don t know exactly where the particle might be inside the box, we know that it has to be in the box. This means that, ψ ( x) dx = 1 (normalization condition) L
Probability and Normalization Although we don t know exactly where the particle might be inside the box, we know that it has to be in the box. This means that, ψ ( x) dx = 1 (normalization condition) L
Chem 467 Supplement to Lecture 19 Hydrogen Atom, Atomic Orbitals
 Chem 467 Supplement to Lecture 19 Hydrogen Atom, Atomic Orbitals Pre-Quantum Atomic Structure The existence of atoms and molecules had long been theorized, but never rigorously proven until the late 19
Chem 467 Supplement to Lecture 19 Hydrogen Atom, Atomic Orbitals Pre-Quantum Atomic Structure The existence of atoms and molecules had long been theorized, but never rigorously proven until the late 19
Chapter 6: Quantum Theory of the Hydrogen Atom
 Chapter 6: Quantum Theory of the Hydrogen Atom The first problem that Schrödinger tackled with his new wave equation was that of the hydrogen atom. The discovery of how naturally quantization occurs in
Chapter 6: Quantum Theory of the Hydrogen Atom The first problem that Schrödinger tackled with his new wave equation was that of the hydrogen atom. The discovery of how naturally quantization occurs in
Ch 125a Problem Set 1
 Ch 5a Problem Set Due Monday, Oct 5, 05, am Problem : Bra-ket notation (Dirac notation) Bra-ket notation is a standard and convenient way to describe quantum state vectors For example, φ is an abstract
Ch 5a Problem Set Due Monday, Oct 5, 05, am Problem : Bra-ket notation (Dirac notation) Bra-ket notation is a standard and convenient way to describe quantum state vectors For example, φ is an abstract
Investigating Nano-Space
 Name Partners Date Visual Quantum Mechanics The Next Generation Investigating Nano-Space Goal You will apply your knowledge of tunneling to understand the operation of the scanning tunneling microscope.
Name Partners Date Visual Quantum Mechanics The Next Generation Investigating Nano-Space Goal You will apply your knowledge of tunneling to understand the operation of the scanning tunneling microscope.
3.23 Electrical, Optical, and Magnetic Properties of Materials
 MIT OpenCourseWare http://ocw.mit.edu 3.23 Electrical, Optical, and Magnetic Properties of Materials Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
MIT OpenCourseWare http://ocw.mit.edu 3.23 Electrical, Optical, and Magnetic Properties of Materials Fall 2007 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms.
Chemistry 3502/4502. Exam I. February 6, ) Circle the correct answer on multiple-choice problems.
 D Chemistry 3502/4502 Exam I February 6, 2006 1) Circle the correct answer on multiple-choice problems. 2) There is one correct answer to every multiple-choice problem. There is no partial credit. On the
D Chemistry 3502/4502 Exam I February 6, 2006 1) Circle the correct answer on multiple-choice problems. 2) There is one correct answer to every multiple-choice problem. There is no partial credit. On the
Chapter 7. Bound Systems are perhaps the most interesting cases for us to consider. We see much of the interesting features of quantum mechanics.
 Chapter 7 In chapter 6 we learned about a set of rules for quantum mechanics. Now we want to apply them to various cases and see what they predict for the behavior of quanta under different conditions.
Chapter 7 In chapter 6 we learned about a set of rules for quantum mechanics. Now we want to apply them to various cases and see what they predict for the behavior of quanta under different conditions.
To Create a Simple Formula using the Point and Click Method:
 To Create a Simple Formula that Adds Two Numbers: Click the cell where the formula will be defined (C5, for example). Type the equal sign (=) to let Excel know a formula is being defined. Type the first
To Create a Simple Formula that Adds Two Numbers: Click the cell where the formula will be defined (C5, for example). Type the equal sign (=) to let Excel know a formula is being defined. Type the first
Gravity: How fast do objects fall? Student Advanced Version
 Gravity: How fast do objects fall? Student Advanced Version Kinematics is the study of how things move their position, velocity, and acceleration. Acceleration is always due to some force acting on an
Gravity: How fast do objects fall? Student Advanced Version Kinematics is the study of how things move their position, velocity, and acceleration. Acceleration is always due to some force acting on an
GRAVITATION. F = GmM R 2
 GRAVITATION Name: Partner: Section: Date: PURPOSE: To explore the gravitational force and Kepler s Laws of Planetary motion. INTRODUCTION: Newton s law of Universal Gravitation tells us that the gravitational
GRAVITATION Name: Partner: Section: Date: PURPOSE: To explore the gravitational force and Kepler s Laws of Planetary motion. INTRODUCTION: Newton s law of Universal Gravitation tells us that the gravitational
3 Charged Particle Motion in a Magnetic Field
 3 Charged Particle Motion in a Magnetic Field When you have completed the Particle Annihilation section and read all the text (especially section 2.2), click the Next button in the Particle Annihilation
3 Charged Particle Motion in a Magnetic Field When you have completed the Particle Annihilation section and read all the text (especially section 2.2), click the Next button in the Particle Annihilation
Outline Spherical symmetry Free particle Coulomb problem Keywords and References. Central potentials. Sourendu Gupta. TIFR, Mumbai, India
 Central potentials Sourendu Gupta TIFR, Mumbai, India Quantum Mechanics 1 2013 3 October, 2013 Outline 1 Outline 2 Rotationally invariant potentials 3 The free particle 4 The Coulomb problem 5 Keywords
Central potentials Sourendu Gupta TIFR, Mumbai, India Quantum Mechanics 1 2013 3 October, 2013 Outline 1 Outline 2 Rotationally invariant potentials 3 The free particle 4 The Coulomb problem 5 Keywords
C/CS/Phys C191 Particle-in-a-box, Spin 10/02/08 Fall 2008 Lecture 11
 C/CS/Phys C191 Particle-in-a-box, Spin 10/0/08 Fall 008 Lecture 11 Last time we saw that the time dependent Schr. eqn. can be decomposed into two equations, one in time (t) and one in space (x): space
C/CS/Phys C191 Particle-in-a-box, Spin 10/0/08 Fall 008 Lecture 11 Last time we saw that the time dependent Schr. eqn. can be decomposed into two equations, one in time (t) and one in space (x): space
Gravity Measurements Making Corrections and Calculating Anomalies
 Gravity Measurements Making Corrections and Calculating Anomalies After completing this practical you should be able to: Use Excel to perform basic calculations using formulae. Use formulae to automatically
Gravity Measurements Making Corrections and Calculating Anomalies After completing this practical you should be able to: Use Excel to perform basic calculations using formulae. Use formulae to automatically
LAB Exercise #4 - Answers The Traction Vector and Stress Tensor. Introduction. Format of lab. Preparation reading
 LAB Exercise #4 - Answers The Traction Vector and Stress Tensor Due: Thursday, 26 February 2009 (Special Thanks to D.D. Pollard who pioneered this exercise in 1991) Introduction Stress concentrations in
LAB Exercise #4 - Answers The Traction Vector and Stress Tensor Due: Thursday, 26 February 2009 (Special Thanks to D.D. Pollard who pioneered this exercise in 1991) Introduction Stress concentrations in
