Solid: mg, g, kg; oz, lb. Numeric; From client inventory
|
|
|
- Alexis Watkins
- 5 years ago
- Views:
Transcription
1 Client Name INVENTORY INPUT SAMPLE JENSEN HUGHES CHI Team Building Name Sample Internal ID / Record # Chemical Name Mixture? (Multiple CAS) Components of Mixture Percent of Mixture Concentration (Single CAS) Physical State CAS # Manufacturer Container Size Size Units Container Material Container Relief Container State Container Quantity Inventory Owner Data Source Expiration Date MSDS Available Location Solid: mg, g, kg; oz, lb Numeric; From client inventory Liquid/aerosol: ml, L; fl oz, pint, quart, gallon Glass, Plastic, Metal, References program, if applicable Text Y/N Y/N Optional; Comma-delimited 0-100% Solid, liquid, gas, aerosol (2-6 digits)-##-#; w or w/o dashes Text Numeric Gas: L@NTP; SCF Fiber, Composite, Other Y/N Full, Empty, Waste Numeric Username Text Date: DD-MM-YYYY Y/N Location 1-BUTANOL N Liquid gallon BUTANOL N Liquid gallon BUTANOL N Liquid gallon PROPANOL N Liquid gallon PROPANOL N Liquid gallon PROPANOL N Liquid gallon 23 3 Acetic acid N Liquid fl oz 5 1 Acetone N Liquid fl oz 9 1 Benzoyl Peroxide N Solid 500 mg 12 1 Butane (gaseous) N Gas SCF 40 1 CALCIUM HYPOCHLORITE N Solid g 16 1 Chloroform N Liquid fl oz 3 2 Chloroform N Liquid gallon 2 3 Copper(II) Sulfate N Solid kg 3 2 Ethyl Acetate N Liquid gallon 1 2 Ethyl Acetate N Liquid gallon 8 1 Formic acid N Liquid gallon 10 1 Hexanes N Liquid gallon 4 1 Hydrochloric Acid 35-37% N 37 Liquid fl oz 2 1 Hydrochloric Acid 35-37% N 37 Liquid pint 3 2 Hydrogen N Gas SCF 3 2 HYDROGEN PEROXIDE 30% N 30 Liquid quart 5 3 MAGNESIUM SULFATE, 7-HYDRATE N Solid oz 5 2 Methyl Ethyl Ketone N Liquid gallon 1 1 Nitric Acid % N 90 Liquid gallon 10 1 ORTHOPHOSPHORIC ACID N Liquid gallon 2 2 Polyethylene Glycol N Liquid gallon 3 1 POTASSIUM BROMIDE N Solid lb 2 3 POTASSIUM FERRICYANIDE N Solid lb 6 1 Sodium Nitrate N Solid lb 7 3 SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE N Solid kg 3 2 SODIUM THIOSULFATE 0.025N N 0.62 Liquid gallon 1 1 SODIUM THIOSULFATE, 5-HYDRATE N Solid oz 10 1 SULFURIC ACID 98% N 98 Liquid gallon 3 2 Toluene N Liquid gallon 5 3 WD-40 Y Liquid 5 gallon 3 1 Zinc Standard Solution N Liquid 1 gallon 2 1
2 INVENTORY INPUT SAMPLE Client Name JENSEN HUGHES CHI Team Building Name Sample Sample Sample
3 INVENTORY INPUT SAMPLE Client Name Building Name JENSEN HUGHES CHI Team Sample Sample Y Y 1
4 CONTROL AREA 1 REPORT Physical Hazards Health Hazards Client Name Building Name Floor # Report Generated Reviewed By JENSEN HUGHES CHI Team Sample 1 10/30/2018 3:42 PM Jeremy Lebowitz Solid Liquid Gas Material Class Inventory Amount Regulatory Limit Inventory Amount Regulatory Limit Inventory Amount Regulatory Limit Combustible Liquid II 3.91 gal gal. IIIA 0.00 gal. 1, gal. IIIB gal. NL gal. Combustible Fiber Loose 0.00 cu. ft cu. ft. Baled 0.00 cu. ft. 4, cu. ft. Cryogenics Flammable 0.00 gal gal. Oxidizing 0.00 gal gal. Inert 0.00 gal. NL Explosives lb lb lb lb lb lb lb lb lb lb lb lb lb lb lb lb. 1.4G 0.00 lb lb. lb lb lb lb lb lb lb. lb. Flammable Gas Gaseous 4, cu. ft. 4, cu. ft. Liquified 0.00 lb lb. Flammable Liquid IA 0.00 gal gal. IB & IC gal gal. Combination IA, IB, IC gal gal. Flammable Solid 0.00 lb lb. Organic Peroxide U 0.00 lb lb lb lb. I 0.00 lb lb lb lb. II 0.01 lb lb lb lb. III 0.00 lb lb lb lb. IV 0.00 lb. NL 0.00 lb. NL V 0.00 lb. NL 0.00 lb. NL Oxidizer lb lb lb lb lb lb. 100 lb lb lb. 1, lb lb. 1, lb lb. NL lb lb. NL lb. Oxidizer - Gas Gaseous 0.00 cu. ft. 6, cu. ft. Liquified 0.00 lb lb. Pyrophoric 0.00 lb lb lb lb cu. ft cu. ft. Unstable Reactive lb lb lb lb cu. ft cu. ft lb lb lb lb cu. ft cu. ft lb lb lb lb cu. ft. 3, cu. ft lb. NL 0.00 lb. NL 0.00 cu. ft. NL Water Reactive lb lb lb lb lb lb lb lb lb. NL 0.00 lb. NL Corrosive 0.00 lb. 20, lb gal. 2, gal cu. ft. 3, cu. ft. Corrosive - Liq. Gas 0.00 lb lb. Highly Toxic 0.00 lb lb. 100 lb lb cu. ft cu. ft. Highly Toxic - Liq. Gas 0.00 lb lb. Toxic 0.00 lb. 2, lb lb. 2, lb cu. ft. 3, cu. ft. Toxic - Liq. Gas 0.00 lb lb. Permitted only in buildings equipped throughout with an automatic sprinkler system The permitted quantities are not limited in a building equipped throughout with an automatic sprinkler system Permitted only when stored in approved exhausted gas cabinets, exhausted enclosures or fume hoods
5 CONTROL AREA 2 REPORT Physical Hazards Health Hazards Client Name Building Name Floor # Report Generated Reviewed By JENSEN HUGHES CHI Team Sample 2 10/30/2018 3:42 PM Jeremy Lebowitz Solid Liquid Gas Material Class Inventory Amount Regulatory Limit Inventory Amount Regulatory Limit Inventory Amount Regulatory Limit Combustible Liquid II 0.00 gal gal. IIIA 0.00 gal gal. IIIB 0.00 gal. NL gal. Combustible Fiber Loose 0.00 cu. ft cu. ft. Baled 0.00 cu. ft. 3, cu. ft. Cryogenics Flammable 0.00 gal gal. Oxidizing 0.00 gal gal. Inert 0.00 gal. NL Explosives lb lb lb lb lb lb lb lb lb lb lb lb lb lb lb lb. 1.4G 0.00 lb lb. lb lb lb lb lb lb lb. lb. Flammable Gas Gaseous 3, cu. ft. 3, cu. ft. Liquified 0.00 lb lb. Flammable Liquid IA 0.00 gal gal. IB & IC gal gal. Combination IA, IB, IC gal gal. Flammable Solid 0.00 lb lb. Organic Peroxide U 0.00 lb lb lb lb. I 0.00 lb lb lb lb. II 0.00 lb lb lb lb. III 0.00 lb lb lb lb. IV 0.00 lb. NL 0.00 lb. NL V 0.00 lb. NL 0.00 lb. NL Oxidizer lb lb lb lb lb lb lb lb lb lb lb lb lb. NL lb lb. NL lb. Oxidizer - Gas Gaseous 0.00 cu. ft. 4, cu. ft. Liquified 0.00 lb lb. Pyrophoric 0.00 lb lb lb lb cu. ft cu. ft. Unstable Reactive lb lb lb lb cu. ft cu. ft lb lb lb lb cu. ft cu. ft lb lb lb lb cu. ft. 2, cu. ft lb. NL 0.00 lb. NL 0.00 cu. ft. NL Water Reactive lb lb lb lb lb lb lb lb lb. NL 0.00 lb. NL Corrosive 0.00 lb. 15, lb gal. 1, gal cu. ft. 2, cu. ft. Corrosive - Liq. Gas 0.00 lb lb. Highly Toxic 0.00 lb lb lb lb cu. ft cu. ft. Highly Toxic - Liq. Gas 0.00 lb lb. Toxic lb. 1, lb lb. 1, lb cu. ft. 2, cu. ft. Toxic - Liq. Gas 0.00 lb lb. Permitted only in buildings equipped throughout with an automatic sprinkler system The permitted quantities are not limited in a building equipped throughout with an automatic sprinkler system Permitted only when stored in approved exhausted gas cabinets, exhausted enclosures or fume hoods
6 CONTROL AREA 3 REPORT Physical Hazards Health Hazards Client Name Building Name Floor # Report Generated Reviewed By JENSEN HUGHES CHI Team Sample 3 10/30/2018 3:42 PM Jeremy Lebowitz Solid Liquid Gas Material Class Inventory Amount Regulatory Limit Inventory Amount Regulatory Limit Inventory Amount Regulatory Limit Combustible Liquid II 0.00 gal gal. IIIA 0.00 gal gal. IIIB 0.00 gal. NL gal. Combustible Fiber Loose 0.00 cu. ft cu. ft. Baled 0.00 cu. ft. 2, cu. ft. Cryogenics Flammable 0.00 gal gal. Oxidizing 0.00 gal gal. Inert 0.00 gal. NL Explosives lb lb lb lb lb lb lb lb lb lb lb lb lb lb lb lb. 1.4G 0.00 lb lb. lb lb lb lb lb lb lb. lb. Flammable Gas Gaseous 0.00 cu. ft. 2, cu. ft. Liquified 0.00 lb lb. Flammable Liquid IA 0.00 gal gal. IB & IC gal gal. Combination IA, IB, IC gal gal. Flammable Solid 0.00 lb lb. Organic Peroxide U 0.00 lb lb lb lb. I 0.00 lb lb lb lb. II 0.00 lb lb lb lb. III 0.00 lb lb lb lb. IV 0.00 lb. NL 0.00 lb. NL V 0.00 lb. NL 0.00 lb. NL Oxidizer lb lb lb lb lb lb lb lb lb lb lb lb lb. NL lb lb. NL lb. Oxidizer - Gas Gaseous 0.00 cu. ft. 3, cu. ft. Liquified 0.00 lb lb. Pyrophoric 0.00 lb lb lb lb cu. ft cu. ft. Unstable Reactive lb lb lb lb cu. ft cu. ft lb lb lb lb cu. ft cu. ft lb lb lb lb cu. ft. 1, cu. ft lb. NL 0.00 lb. NL 0.00 cu. ft. NL Water Reactive lb lb lb lb lb lb lb lb lb. NL 0.00 lb. NL Corrosive 0.00 lb. 10, lb gal. 1, gal cu. ft. 1, cu. ft. Corrosive - Liq. Gas 0.00 lb lb. Highly Toxic 0.00 lb lb lb lb cu. ft cu. ft. Highly Toxic - Liq. Gas 0.00 lb lb. Toxic 0.00 lb. 1, lb lb. 1, lb cu. ft. 1, cu. ft. Toxic - Liq. Gas 0.00 lb lb. Permitted only in buildings equipped throughout with an automatic sprinkler system The permitted quantities are not limited in a building equipped throughout with an automatic sprinkler system Permitted only when stored in approved exhausted gas cabinets, exhausted enclosures or fume hoods
7 HMIS SAMPLE Client Name JENSEN HUGHES CHI Team Building Name Sample Owner Control Area Chemical Name CAS State Container Size Number of Containers Total Quantity Unit Density Normalized Quantity Manufacturer Customer ID Yes/No I II III II IIIA IIIB Dust 1 1-BUTANOL Li id Gallon 20 gal Unknown JENSEN HUGHES CHI Team Yes BUTANOL Liquid Gallon 30 gal Unknown JENSEN HUGHES CHI Team Yes BUTANOL Liquid Gallon 5 gal Unknown JENSEN HUGHES CHI Team Yes PROPANOL Li id Gallon 3 gal Unknown JENSEN HUGHES CHI Team Yes PROPANOL Li id Gallon 10 gal Unknown JENSEN HUGHES CHI Team Yes PROPANOL Liquid Gallon 23 gal Unknown JENSEN HUGHES CHI Team Yes 23 NON-HAZ Aerosol (lbs) COMB. LIQ. (gals) COMB. Solid (lbs) CRYOGENICS (gal) FLAMMABLE LIQ. (gal) EXPLOSIVES SOLID (lbs) EXPLOSIVES LIQUID (lbs) FLAM. SOLID (lbs) FLAM. GAS ORG. PEROX SOLID (lbs) ORG. PEROX LIQUID (lbs) OX. GAS OXIDIZER SOLID (lbs) OXIDIZER LIQUID (lbs) PYROPHORIC UNSTABLE SOLID (lbs) UNSTABLE LIQUID (lbs) UNSTABLE GAS (cu ft) W ATER REACT SOLID (lbs) W ATER REACT LIQUID (lbs) CORROSIVE SOLID (lbs) CORROSIVE LIQUID (gal) CORROSIVE GAS (cu ft) CORROSIVE AEROSOL (lbs) HIGHLY TOXIC SOLID (lbs) HIGHLY TOXIC LIQUID (lbs) Fiber - Loose Fiber - Baled Flammable Oxidizing Inert IA IB IC G Y/N Gaseous (cu ft) LG (lbs) UD I II III IV V UD I II III IV V Gaseous (cu ft) LG (lbs) Solid (lbs) Liquid (lbs) Gas (cu ft) Acetic acid Li id FluidOunce 3.9 gal Unknown JENSEN HUGHES CHI Team Yes Acetone Li id FluidOunce 35 gal Unknown JENSEN HUGHES CHI Team Yes 35 1 Benzoyl Peroxide Solid Milligram lbs Unknown JENSEN HUGHES CHI Team Yes Yes Butane (gaseous) Gas Scf 4000 cu ft Unknown JENSEN HUGHES CHI Team Yes CALCIUM HYPOCHLORITE Solid Gram 3.5 lbs Unknown JENSEN HUGHES CHI Team Yes No Chloroform Liquid FluidOunce 0.38 gal Unknown JENSEN HUGHES CHI Team Yes 3 Chloroform Li id Gallon 20 gal Unknown JENSEN HUGHES CHI Team Yes 2 Copper(II) Sulfate Solid Kilogram 26 lbs Unknown JENSEN HUGHES CHI Team Yes No 2 Ethyl Acetate Liquid Gallon 1 gal Unknown JENSEN HUGHES CHI Team Yes 1 1 Ethyl Acetate Li id Gallon 8 gal Unknown JENSEN HUGHES CHI Team Yes 8 1 Formic acid Li id Gallon 50 gal Unknown JENSEN HUGHES CHI Team Yes 50 1 HEXANES Liquid Gallon 40 gal Unknown JENSEN HUGHES CHI Team Yes 40 1 Hydrochloric Acid 35-37% Li id FluidOunce 1.6 gal Unknown JENSEN HUGHES CHI Team Yes Hydrochloric Acid 35-37% Li id Pint 1.5 gal Unknown JENSEN HUGHES CHI Team Yes Hydrogen Gas Scf 3000 cu ft Unknown JENSEN HUGHES CHI Team Yes HYDROGEN PEROXIDE 30% Li id Quart 2.5 gal Unknown JENSEN HUGHES CHI Team Yes MAGNESIUM SULFATE, 7-HYDRATE Solid Ounce 3.8 lbs Unknown JENSEN HUGHES CHI Team No 1 Methyl Ethyl Ketone Liquid Gallon 1 gal Unknown JENSEN HUGHES CHI Team Yes 1 1 Nitric Acid % Li id Gallon 1480 kg/m^3 10 gal Unknown JENSEN HUGHES CHI Team Yes ORTHOPHOSPHORIC ACID Li id Gallon 10 gal Unknown JENSEN HUGHES CHI Team Yes 10 1 Polyethylene Glycol Liquid Gallon 1130 kg/m^3 15 gal Unknown JENSEN HUGHES CHI Team Yes 15 3 POTASSIUM BROMIDE Solid Pound 2 lbs Unknown JENSEN HUGHES CHI Team Yes 1 POTASSIUM FERRICYANIDE Solid Pound 30 lbs Unknown JENSEN HUGHES CHI Team No 3 Sodium Nitrate Solid Pound 350 lbs Unknown JENSEN HUGHES CHI Team Yes No SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE Solid Kilogram 66 lbs Unknown JENSEN HUGHES CHI Team Yes 1 SODIUM THIOSULFATE 0.025N Li id Gallon 5 gal Unknown JENSEN HUGHES CHI Team No 1 SODIUM THIOSULFATE, 5-HYDRATE Solid Ounce 40 lbs Unknown JENSEN HUGHES CHI Team No 2 SULFURIC ACID 98% Li id Gallon 1840 kg/m^3 3 gal Unknown JENSEN HUGHES CHI Team Yes Toluene Liquid Gallon 870 kg/m^3 5 gal Unknown JENSEN HUGHES CHI Team Yes 5 1 WD-40 Liquid Gallon 15 gal Unknown JENSEN HUGHES CHI Team Yes 1 Zinc Standard Solution Liquid Gallon 2 gal Unknown JENSEN HUGHES CHI Team Yes
8 HIGHLY TOXIC GAS (cu ft) HIGHLY TOXIC AERSOL (lbs) TOXIC SOLID (lbs) TOXIC LIQUID (lbs) TOXIC GAS (cu ft) TOXIC AERSOL (cu ft) REQUEST ADDITIONAL INFORMATION 26 46
HAZARDOUS MATERIALS SURVEY. Note: List ALL virgin stock materials in current and projected inventories LAB NAME: PI: ADDRESS:
 HAZARDOUS MATERIALS SURVEY Note: List ALL virgin stock materials in current and projected inventories LAB NAME: PI: ADDRESS: Please read each definition of the following Hazardous Materials carefully and
HAZARDOUS MATERIALS SURVEY Note: List ALL virgin stock materials in current and projected inventories LAB NAME: PI: ADDRESS: Please read each definition of the following Hazardous Materials carefully and
Chemical Segregation and Storage Guide
 Chemical Segregation and Storage Guide This resource developed by LabCentral for the Pagliuca Harvard Life Lab Revision 1, Effective Dec 13, 2016 Table of Contents 1.0 PURPOSE AND SCOPE...3 2.0 CLASSES
Chemical Segregation and Storage Guide This resource developed by LabCentral for the Pagliuca Harvard Life Lab Revision 1, Effective Dec 13, 2016 Table of Contents 1.0 PURPOSE AND SCOPE...3 2.0 CLASSES
WESTERN CONNECTICUT STATE UNIVERSITY CHEMICAL STORAGE AND COMPATIBILITY GUIDELINES PROCEDURE S-118
 WESTERN CONNECTICUT STATE UNIVERSITY CHEMICAL STORAGE AND COMPATIBILITY GUIDELINES PROCEDURE S-118 Issued 6/4/02 Please direct any questions or comments about the applicability of this document to Luigi
WESTERN CONNECTICUT STATE UNIVERSITY CHEMICAL STORAGE AND COMPATIBILITY GUIDELINES PROCEDURE S-118 Issued 6/4/02 Please direct any questions or comments about the applicability of this document to Luigi
Flammable/Combustible Liquid Storage
 8.12.1 RESPONSIBILITY University personnel are responsible for using approved and safe methods for storing flammable/combustible liquids. Personnel must store flammable and combustible liquids in accordance
8.12.1 RESPONSIBILITY University personnel are responsible for using approved and safe methods for storing flammable/combustible liquids. Personnel must store flammable and combustible liquids in accordance
Chemical Storage Guidelines
 Office of Research Environmental Health & Safety Department 921 South 8th Avenue, Stop 8106 Pocatello, Idaho 83209-8106 Phone: (208) 282-2310 Fax: (208) 282-4649 www.isu.edu ISU is an Equal Opportunity
Office of Research Environmental Health & Safety Department 921 South 8th Avenue, Stop 8106 Pocatello, Idaho 83209-8106 Phone: (208) 282-2310 Fax: (208) 282-4649 www.isu.edu ISU is an Equal Opportunity
Proper Storage of Chemicals in Laboratories
 Proper Storage of Chemicals in Laboratories Dr. Ideisan I. Abu-Abdoun Department of Chemistry UOS abuabdoun@sharjah.ac.ae UOS 28 - Feb. 2013 Contents Introduction Requirements Storage Facilities and Practices
Proper Storage of Chemicals in Laboratories Dr. Ideisan I. Abu-Abdoun Department of Chemistry UOS abuabdoun@sharjah.ac.ae UOS 28 - Feb. 2013 Contents Introduction Requirements Storage Facilities and Practices
Principal Investigator: Department: Laboratory room #s: Updated/revised: Chemical Name Amount Physical state Location Hazard Class
 Chemical Inventory Principal Investigator: Department: Laboratory room #s: Updated/revised: Chemical Name Amount Physical state Location Hazard Class 1 SUGGESTED SHELF STORAGE PATTERN - ORGANIC Organic
Chemical Inventory Principal Investigator: Department: Laboratory room #s: Updated/revised: Chemical Name Amount Physical state Location Hazard Class 1 SUGGESTED SHELF STORAGE PATTERN - ORGANIC Organic
BGSU s Guide to Chemical Storage
 BGSU s Guide to Chemical Storage Environmental Health & Safety 372-2171 Chemical Storage Guidlines General Guidelines Store dry chemicals on shelves. Store flammables together. Use approved flammable storage
BGSU s Guide to Chemical Storage Environmental Health & Safety 372-2171 Chemical Storage Guidlines General Guidelines Store dry chemicals on shelves. Store flammables together. Use approved flammable storage
Chemical Storage Guidelines Page 1 of 10
 Guidelines Page 1 of 10 1.0 PURPOSE: Proper storage is needed to minimize the hazards associated with accidentally mixing incompatible chemicals. Due to the diverse individual properties of chemicals that
Guidelines Page 1 of 10 1.0 PURPOSE: Proper storage is needed to minimize the hazards associated with accidentally mixing incompatible chemicals. Due to the diverse individual properties of chemicals that
Chemical Storage According to Compatibility
 Chemical Storage According to Compatibility To lessen risk of exposure to hazardous chemicals, all chemicals should be separated and stored according to hazard category and compatibility. *Storage Groups
Chemical Storage According to Compatibility To lessen risk of exposure to hazardous chemicals, all chemicals should be separated and stored according to hazard category and compatibility. *Storage Groups
Chemical Storage Guidelines
 Storage Guidelines Do not store excess chemical containers on the benchtops, designate a storage location and return containers to that location after each use. Do not store chemicals in the fume hood.
Storage Guidelines Do not store excess chemical containers on the benchtops, designate a storage location and return containers to that location after each use. Do not store chemicals in the fume hood.
Physical and Chemical Changes. 3. One of the new materials was a precipitate that settled out of solution.
 One of the basic areas of interest for chemists is the study of the regrouping of atoms to form new substances. In order to determine if such a chemical change has occurred, there should be a change in
One of the basic areas of interest for chemists is the study of the regrouping of atoms to form new substances. In order to determine if such a chemical change has occurred, there should be a change in
SUGGESTED SEQUENCE OF STEPS TO MORE SAFELY ORGANIZE YOUR SCHOOL S CHEMICAL STORES AREA
 SUGGESTED SEQUENCE OF STEPS TO MORE SAFELY ORGANIZE YOUR SCHOOL S CHEMICAL STORES AREA 1 Take an inventory of all the chemicals in your school. You will never know the extent of your problem until you
SUGGESTED SEQUENCE OF STEPS TO MORE SAFELY ORGANIZE YOUR SCHOOL S CHEMICAL STORES AREA 1 Take an inventory of all the chemicals in your school. You will never know the extent of your problem until you
Completion of the Chemical Classification Packet
 Moreno Valley Fire Department Fire Prevention Bureau Completion of the Chemical Classification Packet Approved and Authorized By: Randall Metz, Fire Marshal Issued: March 16, 2009 Fire Prevention Bureau
Moreno Valley Fire Department Fire Prevention Bureau Completion of the Chemical Classification Packet Approved and Authorized By: Randall Metz, Fire Marshal Issued: March 16, 2009 Fire Prevention Bureau
Data Uploads 6: Instructions for Inventory
 Data Uploads 6: Instructions for Inventory Explanation of Required, Recommended, and Optional Fields Frequently Asked Questions about Uploading Chemical Data Once the other necessary data have been loaded
Data Uploads 6: Instructions for Inventory Explanation of Required, Recommended, and Optional Fields Frequently Asked Questions about Uploading Chemical Data Once the other necessary data have been loaded
This packet contains review material from Pre-AP Chemistry. Be prepared to take a quiz over this material during the first week of school.
 This packet contains review material from Pre-AP Chemistry. Be prepared to take a quiz over this material during the first week of school. How many significant figures (digits) are represented by each
This packet contains review material from Pre-AP Chemistry. Be prepared to take a quiz over this material during the first week of school. How many significant figures (digits) are represented by each
Chemical Health and Safety General Program
 Chemical Health and Safety General Program I. Objective To establish minimum requirements for storage, handling and use of chemicals. II. Scope This process applies to employees and operations involved
Chemical Health and Safety General Program I. Objective To establish minimum requirements for storage, handling and use of chemicals. II. Scope This process applies to employees and operations involved
Page No.: The Department of Environmental Health and Safety is responsible for;
 1 Introduction The have been developed in order to minimize the risk of accidental chemical reactions and exposure resulting from the improper storage of hazardous chemicals. The chemical storage procedures
1 Introduction The have been developed in order to minimize the risk of accidental chemical reactions and exposure resulting from the improper storage of hazardous chemicals. The chemical storage procedures
1 centimeter (cm) 5 10 millimeters (mm) 1 meter (m) centimeters. 1 kilometer (km) 5 1,000 meters. Set up equivalent ratios and cross multiply.
 Domain 2 Lesson 16 Convert Measurements Common Core State Standard: 6.RP.3.d Getting the Idea The tables below show some conversions for units of length in both the customary system and the metric system.
Domain 2 Lesson 16 Convert Measurements Common Core State Standard: 6.RP.3.d Getting the Idea The tables below show some conversions for units of length in both the customary system and the metric system.
Concentration of Solutions
 CHAPTER 4 Concentration of Solutions There are three principal ways to express solution concentration in chemistry percentage by mass, molarity, and molality. The following table compares these three ways
CHAPTER 4 Concentration of Solutions There are three principal ways to express solution concentration in chemistry percentage by mass, molarity, and molality. The following table compares these three ways
Classification of Mystery Substances
 Classification of Mystery Substances This document supports the safety activity Mystery Substance Identification: The Identification of Unlabeled Chemicals Found on School Premises from Flinn Scientific.
Classification of Mystery Substances This document supports the safety activity Mystery Substance Identification: The Identification of Unlabeled Chemicals Found on School Premises from Flinn Scientific.
Chemical Classification Package for Hazardous Materials
 Chemical Classification Package for Hazardous Materials Completion of the Chemical Classification Packet Chemical Classification Packet PURPOSE The classification of hazards for chemicals stored, used,
Chemical Classification Package for Hazardous Materials Completion of the Chemical Classification Packet Chemical Classification Packet PURPOSE The classification of hazards for chemicals stored, used,
Form S Chemical Reporting Challenges. Rich Bizzozero, Director Office of Technical Assistance May 2018
 Form S Chemical Reporting Challenges Rich Bizzozero, Director Office of Technical Assistance May 2018 Before You Get Started Review and know what chemicals are on the list Know what chemicals are used
Form S Chemical Reporting Challenges Rich Bizzozero, Director Office of Technical Assistance May 2018 Before You Get Started Review and know what chemicals are on the list Know what chemicals are used
Chemical Safety. Peter Yeung D.G. Manager. Safety Office The University of Hong Kong
 Chemical Safety Peter Yeung D.G. Manager Safety Office The University of Hong Kong Lesson Learned 1. Laboratory fire 2. Chemical spill 3. Incompatibles in waste disposal Lab. Fire Sodium/Solvent Chemical
Chemical Safety Peter Yeung D.G. Manager Safety Office The University of Hong Kong Lesson Learned 1. Laboratory fire 2. Chemical spill 3. Incompatibles in waste disposal Lab. Fire Sodium/Solvent Chemical
APPENDIX C CHEMICALS USED IN CLANDESTINE DRUG LABS
 APPENDIX C CHEMICALS USED IN CLANDESTINE DRUG LABS You must exercise extreme caution when ope at the scene of an illegal drug lab. Do not walk into, touch, or move chemicals or spilled material. Avoid
APPENDIX C CHEMICALS USED IN CLANDESTINE DRUG LABS You must exercise extreme caution when ope at the scene of an illegal drug lab. Do not walk into, touch, or move chemicals or spilled material. Avoid
Stoichiometry Chapter 9 Practice Assessment B
 NAME Hour Date Stoichiometry Chapter 9 Practice Assessment B Objective 1: Interpret balanced chemical equations in terms of interacting moles, representative particles, masses, and gas volume at STP. Directions:
NAME Hour Date Stoichiometry Chapter 9 Practice Assessment B Objective 1: Interpret balanced chemical equations in terms of interacting moles, representative particles, masses, and gas volume at STP. Directions:
Hazardous Materials Handling and Storage. Source:
 Hazardous Materials Handling and Storage This guidance section was quoted directly from the EPA s Small Laboratory Guide, with some modifications to the text to provide specific guidance for MSSM or to
Hazardous Materials Handling and Storage This guidance section was quoted directly from the EPA s Small Laboratory Guide, with some modifications to the text to provide specific guidance for MSSM or to
CHAPTER 9 CHEMICAL QUANTITIES
 Chemistry Name Hour Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 9 CHEMICAL QUANTITIES Day Plans for the day Assignment(s) for the day 1 Begin Chapter
Chemistry Name Hour Chemistry Approximate Timeline Students are expected to keep up with class work when absent. CHAPTER 9 CHEMICAL QUANTITIES Day Plans for the day Assignment(s) for the day 1 Begin Chapter
INCOMPATIBILITY BETWEEN CHEMICALS
 INCOMPATIBILITY BETWEEN S CODE SHR 301 Date: April 2009 Revision: 02 Page: 1 of 5 INCOMPATIBILITY BETWEEN S Products that could react violently with each other should not be stored together, particularly
INCOMPATIBILITY BETWEEN S CODE SHR 301 Date: April 2009 Revision: 02 Page: 1 of 5 INCOMPATIBILITY BETWEEN S Products that could react violently with each other should not be stored together, particularly
CTRNet Standard Operating Procedure Handling Hazardous Chemical Waste
 CTRNet Standard Operating Procedure SOP Number: 6.1.002 Version e1.0 Supersedes: SR 001.001 Effective Date 09 Jan 08 Subject: Handling Hazardous Chemical Waste Category Prepared By: Approved By: Approved
CTRNet Standard Operating Procedure SOP Number: 6.1.002 Version e1.0 Supersedes: SR 001.001 Effective Date 09 Jan 08 Subject: Handling Hazardous Chemical Waste Category Prepared By: Approved By: Approved
LABORATORY SAFETY PLAN APPENDIX
 LABORATORY SAFETY PLAN APPENDIX APPENDIX 1 REQUIRED CEE LAB ACCESS FORMS CEE Laboratory User Form As a laboratory user in the Department of Civil and Environmental Engineering at Northeastern University,
LABORATORY SAFETY PLAN APPENDIX APPENDIX 1 REQUIRED CEE LAB ACCESS FORMS CEE Laboratory User Form As a laboratory user in the Department of Civil and Environmental Engineering at Northeastern University,
Name Class Date. Symbol Meaning How to prepare Percentage % Moles solute per liter of solution. Moles solute per kilogram of solvent
 Skills Worksheet Problem Solving Concentration of Solutions There are three principal ways to express solution concentration in chemistry percentage by mass, molarity, and molality. The following table
Skills Worksheet Problem Solving Concentration of Solutions There are three principal ways to express solution concentration in chemistry percentage by mass, molarity, and molality. The following table
PREVENTING HAZARDOUS WASTE FINES
 University of Medicine and Dentistry of New Jersey EOHSS FACTSHEET PREVENTING HAZARDOUS WASTE FINES In the last few years institutions such as MIT, University of New Hampshire, Yale, Stanford, and Boston
University of Medicine and Dentistry of New Jersey EOHSS FACTSHEET PREVENTING HAZARDOUS WASTE FINES In the last few years institutions such as MIT, University of New Hampshire, Yale, Stanford, and Boston
NAMING IONIC COMPOUNDS. ammonium sulfide. iron(ii) carbonate. barium phosphate. titanium(iv) sulfide. Spelling matters!
 67 NAMING IONIC COMPOUNDS ammonium sulfide iron(ii) carbonate titanium(iv) sulfide barium phosphate Spelling matters! barium phosphide 68 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The
67 NAMING IONIC COMPOUNDS ammonium sulfide iron(ii) carbonate titanium(iv) sulfide barium phosphate Spelling matters! barium phosphide 68 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The
Chemical/Flammable Storage Management Program
 Chemical/Flammable Storage Management Program Inventory and Inspection Each laboratory is to maintain an inventory of the chemicals stored in the laboratory as part of the Laboratory Safety Manual. Designate
Chemical/Flammable Storage Management Program Inventory and Inspection Each laboratory is to maintain an inventory of the chemicals stored in the laboratory as part of the Laboratory Safety Manual. Designate
Advanced Placement Chemistry ~ Summer Assignment Part 2. Name
 Advanced Placement Chemistry ~ Summer Assignment Part 2 Name Topic 1: Measurements and Dimensional Analysis Appropriately read and report a measurement correctly with one uncertain digit. State the number
Advanced Placement Chemistry ~ Summer Assignment Part 2 Name Topic 1: Measurements and Dimensional Analysis Appropriately read and report a measurement correctly with one uncertain digit. State the number
Guideline for Completion of the Chemical Classification Disclosure Statement 2016 CODES PURPOSE SCOPE
 City of Perris Department of Development Service Office of the Fire Marshal 135 North D Street, Perris CA. 92570 Phone: (951) 443-1029 Fax: (951) 943-3293 Guideline for Completion of the Chemical Classification
City of Perris Department of Development Service Office of the Fire Marshal 135 North D Street, Perris CA. 92570 Phone: (951) 443-1029 Fax: (951) 943-3293 Guideline for Completion of the Chemical Classification
DesCartes Signs and Symbols... 2 Language Usage... 2 Mathematics... 3 Science... 5 Concepts and Processes... 5 General Science...
 DesCartes: A Continuum of Learning SIGNS AND SYMBOLS for the Web-based MAP system DesCartes Signs and Symbols... 2 Language Usage... 2 Mathematics... 3 Science... 5 Concepts and Processes... 5 General
DesCartes: A Continuum of Learning SIGNS AND SYMBOLS for the Web-based MAP system DesCartes Signs and Symbols... 2 Language Usage... 2 Mathematics... 3 Science... 5 Concepts and Processes... 5 General
Chapter 5: CHEMICAL STORAGE
 Chapter 5: CHEMICAL STORAGE Chemical storage areas in academic laboratory settings include central departmental stockrooms, storerooms, laboratory work areas, storage cabinets, refrigerators and freezers.
Chapter 5: CHEMICAL STORAGE Chemical storage areas in academic laboratory settings include central departmental stockrooms, storerooms, laboratory work areas, storage cabinets, refrigerators and freezers.
Useful Information to be provided on Exam 1:
 Chem 101A Study Questions, Chapters 1 & 2 Name: Review Tues 9/13 Due 9/15 (Exam 1 date) This is a homework assignment. You must show your work for full credit. If you do work on separate paper, attach
Chem 101A Study Questions, Chapters 1 & 2 Name: Review Tues 9/13 Due 9/15 (Exam 1 date) This is a homework assignment. You must show your work for full credit. If you do work on separate paper, attach
GUIDELINES FOR THE SAFE USE OF PYROPHORIC LIQUID REAGENTS
 Page 1 of 5 GUIDELINES FOR THE SAFE USE OF Pyrophoric liquid reagents are substances that spontaneously ignite when exposed to air and/or moisture. These reagents are commonly utilized in chemical synthesis
Page 1 of 5 GUIDELINES FOR THE SAFE USE OF Pyrophoric liquid reagents are substances that spontaneously ignite when exposed to air and/or moisture. These reagents are commonly utilized in chemical synthesis
NAMING IONIC COMPOUNDS
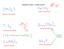 NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
NAMING IONIC COMPOUNDS iron(ii) carbonate ammonium sulfide titanium (IV) sulfide barium phosphate Spelling matters! calcium nitrate sodium phosphide DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME
 DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
DETERMINING THE FORMULA OF AN IONIC COMPOUND FROM THE NAME - The name of an ionic compound is made of the names of the CATION and ANION in the compound. - To get the FORMULA, you must figure out the SMALLEST
5 Focus Areas & Chemical Compatibility. (just a little, with a focus on REDOX)
 5 Focus Areas & Chemical Compatibility (just a little, with a focus on REDOX) RESEARCH LAB INCIDENT FREQUENCY 2014-8/2015 25 20 15 10 5 0 CLEANUP RESPONSE BY CHEMICAL/INCIDENT 2014-8/2015 16 14 12 10 8
5 Focus Areas & Chemical Compatibility (just a little, with a focus on REDOX) RESEARCH LAB INCIDENT FREQUENCY 2014-8/2015 25 20 15 10 5 0 CLEANUP RESPONSE BY CHEMICAL/INCIDENT 2014-8/2015 16 14 12 10 8
EXAMPLES. He VIA VIIA Li Be B C N O F Ne
 59 IA EXAMPLES VIIIA H IIA IIIA IVA VA He VIA VIIA Li Be B C N O F Ne Na Mg IIIB IVB VB Al Si P VIB VIIB VIIIB IB IIB S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru
59 IA EXAMPLES VIIIA H IIA IIIA IVA VA He VIA VIIA Li Be B C N O F Ne Na Mg IIIB IVB VB Al Si P VIB VIIB VIIIB IB IIB S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru
Which row correctly describes the type of reaction and the energy of the reactants and products? energy of the reactants and products
 1 The energy level diagram for the reaction between sodium hydrogen carbonate and dilute hydrochloric acid is shown. sodium chloride + water + carbon dioxide energy sodium hydrogen carbonate + dilute hydrochloric
1 The energy level diagram for the reaction between sodium hydrogen carbonate and dilute hydrochloric acid is shown. sodium chloride + water + carbon dioxide energy sodium hydrogen carbonate + dilute hydrochloric
Safety Manual > Incompatible Chemicals Partial Listing
 Safety Manual > Incompatible Chemicals Partial Listing C. Incompatible Chemicals Partial Listing Chemical Incompatible Chemicals Acetic acid Chromic acid, nitric acid, permanganates, and peroxides Acetic
Safety Manual > Incompatible Chemicals Partial Listing C. Incompatible Chemicals Partial Listing Chemical Incompatible Chemicals Acetic acid Chromic acid, nitric acid, permanganates, and peroxides Acetic
- Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS
 63 POLYATOMIC IONS - Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS - Polyatomic ions form ionic compounds in the same way that single-element ions
63 POLYATOMIC IONS - Some MOLECULES can gain or lose electrons to form CATIONS or ANIONS. These are called POLYATOMIC IONS - Polyatomic ions form ionic compounds in the same way that single-element ions
Exam 1 Worksheet Chemistry 102
 Exam 1 Worksheet Chemistry 102 Chapter 1 Introduction 1. Convert: a. 650 nm to inches b. 650 km to miles c. 345 mg to lb d. 145 lb to μg e. 4.50 10 10 km to μm f. 8.09 10 8 mg to Mg g. 9.00 10 5 ml to
Exam 1 Worksheet Chemistry 102 Chapter 1 Introduction 1. Convert: a. 650 nm to inches b. 650 km to miles c. 345 mg to lb d. 145 lb to μg e. 4.50 10 10 km to μm f. 8.09 10 8 mg to Mg g. 9.00 10 5 ml to
During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction:
 Example 4.1 Stoichiometry During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction: Suppose that a particular plant consumes 37.8 g of CO 2
Example 4.1 Stoichiometry During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction: Suppose that a particular plant consumes 37.8 g of CO 2
Arizona Division of Occupational Safety and Health Administration. 800 W. Washington Street, Phoenix, AZ Consultation:
 ADOSH Arizona Division of Occupational Safety and Health Administration 800 W. Washington Street, Phoenix, AZ 85007 Consultation: 602-542-1769 GLOBALLY HARMONIZED SYSTEM for HAZARD COMMUNICATION Steven
ADOSH Arizona Division of Occupational Safety and Health Administration 800 W. Washington Street, Phoenix, AZ 85007 Consultation: 602-542-1769 GLOBALLY HARMONIZED SYSTEM for HAZARD COMMUNICATION Steven
Formulas and Models 1
 Formulas and Models 1 A molecular formula shows the exact number of atoms of each element in the smallest unit of a substance An empirical formula shows the simplest whole-number ratio of the atoms in
Formulas and Models 1 A molecular formula shows the exact number of atoms of each element in the smallest unit of a substance An empirical formula shows the simplest whole-number ratio of the atoms in
1. How many moles of hydrogen are needed to completely react with 2.00 moles of nitrogen?
 Stoichiometry Mole-to-Mole 1. How many moles of hydrogen are needed to completely react with 2.00 moles of nitrogen? N 2 + H 2 NH 3 2. If 5.50 moles of calcium carbide (CaC 2 ) reacts with an excess of
Stoichiometry Mole-to-Mole 1. How many moles of hydrogen are needed to completely react with 2.00 moles of nitrogen? N 2 + H 2 NH 3 2. If 5.50 moles of calcium carbide (CaC 2 ) reacts with an excess of
Chemistry Lab Test 17-18
 Chemistry Lab Test 17-18 Name: Score: /185 pts Section I : Reaction Prediction Write net ionic equations for each of the reactions that follow. Use appropriate ionic and molecular formulas but omit formulas
Chemistry Lab Test 17-18 Name: Score: /185 pts Section I : Reaction Prediction Write net ionic equations for each of the reactions that follow. Use appropriate ionic and molecular formulas but omit formulas
4. Magnesium has three natural isotopes with the following masses and natural abundances:
 Exercise #1. Determination of Weighted Average Mass 1. The average mass of pennies minted after 1982 is 2.50 g and the average mass of pennies minted before 1982 is 3.00 g. Suppose that a bag of pennies
Exercise #1. Determination of Weighted Average Mass 1. The average mass of pennies minted after 1982 is 2.50 g and the average mass of pennies minted before 1982 is 3.00 g. Suppose that a bag of pennies
CLP Regulation (EC) No / 2008
 CLP Regulation (EC) No. 1272 / 2008 on the classification, labelling and packaging of substances and mixtures Class Explosives Unstable explosive Unst. Expl. H200 Unstable explosive Division 1.1 Expl.
CLP Regulation (EC) No. 1272 / 2008 on the classification, labelling and packaging of substances and mixtures Class Explosives Unstable explosive Unst. Expl. H200 Unstable explosive Division 1.1 Expl.
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which statement is incorrect? 1) A) The key to the scientific method is valid assumptions.
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which statement is incorrect? 1) A) The key to the scientific method is valid assumptions.
Hazard Communication. University of Southern Maine Environmental Health and Safety
 Hazard Communication University of Southern Maine Environmental Health and Safety Training Outline Federal Laboratory Standards Hazards at USM Material Safety Data Sheets (MSDS) Chemical Toxicology Personal
Hazard Communication University of Southern Maine Environmental Health and Safety Training Outline Federal Laboratory Standards Hazards at USM Material Safety Data Sheets (MSDS) Chemical Toxicology Personal
WKS 9.1 Calculating Molar Mass (1 page)
 WKS 9.1 Calculating Molar Mass (1 page) Find the molar mass (AKA the molecular or formula mass) for each of the following substances. Name of compound Formula of compound Work Molar Mass (g/mol) dichlorine
WKS 9.1 Calculating Molar Mass (1 page) Find the molar mass (AKA the molecular or formula mass) for each of the following substances. Name of compound Formula of compound Work Molar Mass (g/mol) dichlorine
The ions/polyatomic ions, solubility rules, and activity series will need to be memorized.
 AP Chemistry Summer Assignment 2012 The summer assignment is designed to help you practice: - writing chemical formulas, names, and chemical equations - reaction types - predicting reaction products -
AP Chemistry Summer Assignment 2012 The summer assignment is designed to help you practice: - writing chemical formulas, names, and chemical equations - reaction types - predicting reaction products -
Chemical Reactivity and Storage. Presented by QBE Loss Control Services
 Chemical Reactivity and Storage Presented by QBE Loss Control Services Objectives To understand basic terminology relating to chemical hazard classes and reactivity To be able to identify chemicals that
Chemical Reactivity and Storage Presented by QBE Loss Control Services Objectives To understand basic terminology relating to chemical hazard classes and reactivity To be able to identify chemicals that
Classifying Chemical Reactions Analyzing and Predicting Products
 Classifying Chemical Reactions Analyzing and Predicting Products Background A chemical reaction is defined as any process in which one or more substances are converted into new substances with different
Classifying Chemical Reactions Analyzing and Predicting Products Background A chemical reaction is defined as any process in which one or more substances are converted into new substances with different
AP CHEMISTRY. Summer Assignment
 AP CHEMISTRY Summer Assignment Welcome to AP Chemistry! In order to have a smooth transition, students are expected to come in with some strong background knowledge. These are some of the things you need
AP CHEMISTRY Summer Assignment Welcome to AP Chemistry! In order to have a smooth transition, students are expected to come in with some strong background knowledge. These are some of the things you need
8. Relax and do well.
 CHEM 1314.03 Exam I John I. Gelder September 25, 1997 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
CHEM 1314.03 Exam I John I. Gelder September 25, 1997 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
Globally Harmonized System of Classification and Labeling of Chemicals (GHS) LC-1033p Rev. 06/16
 Globally Harmonized System of Classification and Labeling of Chemicals (GHS) Rev. 06/16 Agenda Introduction to Globally Harmonized System of Classifying and Labeling Chemicals Hazard & Precautionary Statements
Globally Harmonized System of Classification and Labeling of Chemicals (GHS) Rev. 06/16 Agenda Introduction to Globally Harmonized System of Classifying and Labeling Chemicals Hazard & Precautionary Statements
CORROSIVES 1. Should be kept in acid resistant cabinets or on polyethylene trays. 2. Must never be stored on high shelves. 1
 Storing Chemicals (from Laboratory Safety Manual. June 1999) Proper chemical storage is extremely important in order to maximize personal safety with respect to chemical spills, chemical incompatibilities,
Storing Chemicals (from Laboratory Safety Manual. June 1999) Proper chemical storage is extremely important in order to maximize personal safety with respect to chemical spills, chemical incompatibilities,
Name: Thermochemistry. Practice Test C. General Chemistry Honors Chemistry
 Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
AP Chemistry Summer Assignment
 AP Chemistry Summer Assignment Due Date: Thursday, September 1 st, 2011 Directions: Show all of your work for full credit. Include units and labels. Record answers to the correct number of significant
AP Chemistry Summer Assignment Due Date: Thursday, September 1 st, 2011 Directions: Show all of your work for full credit. Include units and labels. Record answers to the correct number of significant
Chemical Analysis Instruction Book
 Chemical Analysis Instruction Book Part No. 500190001EA Rev. A TABLE OF CONTENTS SECTION PAGE NO. 1 Introduction...1 2 Safety Considerations...3 3 Alkalinity Determination (P-M Method)...5 4 Chloride
Chemical Analysis Instruction Book Part No. 500190001EA Rev. A TABLE OF CONTENTS SECTION PAGE NO. 1 Introduction...1 2 Safety Considerations...3 3 Alkalinity Determination (P-M Method)...5 4 Chloride
Name: Unit 9- Stoichiometry Day Page # Description IC/HW
 Name: Unit 9- Stoichiometry Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 Stoichiometry Notes IC 1 4 Mole Map IC X 1 5 Mole to Mole Practice IC 1 6 Mass to Mole Practice IC 1/2 X
Name: Unit 9- Stoichiometry Day Page # Description IC/HW Due Date Completed ALL 2 Warm-up IC 1 3 Stoichiometry Notes IC 1 4 Mole Map IC X 1 5 Mole to Mole Practice IC 1 6 Mass to Mole Practice IC 1/2 X
Summer Assignment Part 2
 Summer Assignment Part 2 Name: 1. Metric Conversions. Remember 1 cm 3 = 1 ml 1 L = 1 dm 3 ITEM GIVEN METRIC UNIT DESIRED METRIC UNIT A 8.43 cm mm B 2.41 x 10 2 cm m C 294.5 nm cm D 1.445 x 10 4 m km E
Summer Assignment Part 2 Name: 1. Metric Conversions. Remember 1 cm 3 = 1 ml 1 L = 1 dm 3 ITEM GIVEN METRIC UNIT DESIRED METRIC UNIT A 8.43 cm mm B 2.41 x 10 2 cm m C 294.5 nm cm D 1.445 x 10 4 m km E
STOICHIOMETRY CLASSWORK
 STOICHIOMETRY CLASSWORK Given the following equation: 2 C4H10 + 13 02 ---> 8 CO2 + 10 H20 Show what the following molar ratios should be. a, C4H10 / 02 b. 02 / CO2 o, 02 / H20 d, C4Hlo / CO2 e. C4Hlo /
STOICHIOMETRY CLASSWORK Given the following equation: 2 C4H10 + 13 02 ---> 8 CO2 + 10 H20 Show what the following molar ratios should be. a, C4H10 / 02 b. 02 / CO2 o, 02 / H20 d, C4Hlo / CO2 e. C4Hlo /
If Sally has 4.56 x atoms of oxygen in a sample of aluminum oxide, how many kilograms of aluminum does she have?
 If Sally has 4.56 x 10 34 atoms of oxygen in a sample of aluminum oxide, how many kilograms of aluminum does she have? Bertha has.025 milligrams of sodium that she got from a sample of Sodium phosphate,
If Sally has 4.56 x 10 34 atoms of oxygen in a sample of aluminum oxide, how many kilograms of aluminum does she have? Bertha has.025 milligrams of sodium that she got from a sample of Sodium phosphate,
Chemistry 143 Dr. Caddell Modesto Junior College. Chemical Reactions Key
 Chemical Reactions Key 1.) Combine about 2 ml of 0.1 M calcium chloride with about 2 ml of 0.1 M sodium phosphate. CATEGORY: Double Replacement 3CaCl 2 (aq) + 2Na 3 PO 4 (aq) 6NaCl(aq) + Ca3(PO 4 ) 2 (s)
Chemical Reactions Key 1.) Combine about 2 ml of 0.1 M calcium chloride with about 2 ml of 0.1 M sodium phosphate. CATEGORY: Double Replacement 3CaCl 2 (aq) + 2Na 3 PO 4 (aq) 6NaCl(aq) + Ca3(PO 4 ) 2 (s)
Silver nitrate solution is added to sodium dichromate solution
 Chem. 110 50 Points Final Exam Part 1 Practice Write the chemical names or formulas for the following a H 2 SO 4 b NiNO 2 c Aluminum thiosulfate d Plumbic acetate e Ag 2 C 2 O 4 f P 2 O 5 g Cyanic acid
Chem. 110 50 Points Final Exam Part 1 Practice Write the chemical names or formulas for the following a H 2 SO 4 b NiNO 2 c Aluminum thiosulfate d Plumbic acetate e Ag 2 C 2 O 4 f P 2 O 5 g Cyanic acid
The packing group(s) to which a substance is assigned is (are) indicated in Table A of Chapter 3.2.
 Chapter 2.1 2.1.1 Introduction General provisions 2.1.1.1 The classes of dangerous goods according to RID are the following: Class 1 Explosive substances and articles Class 2 Gases Class 3 Flammable liquids
Chapter 2.1 2.1.1 Introduction General provisions 2.1.1.1 The classes of dangerous goods according to RID are the following: Class 1 Explosive substances and articles Class 2 Gases Class 3 Flammable liquids
Full file at Scientific Measurements
 CHAPTER Scientific Measurements 2 Section 2.1 Uncertainty in Measurements 2. Unit Quantity Unit Quantity (a) meter length gram mass (c) liter volume (d) second time 4. (c) 15.50 cm and (d) 20.05 cm each
CHAPTER Scientific Measurements 2 Section 2.1 Uncertainty in Measurements 2. Unit Quantity Unit Quantity (a) meter length gram mass (c) liter volume (d) second time 4. (c) 15.50 cm and (d) 20.05 cm each
Chemical Reactions. Chemical changes are occurring around us all the time
 Chemical changes are occurring around us all the time Food cooking Fuel being burned in a car s engine Oxygen being used in the human body The starting materials are called reactants The ending materials
Chemical changes are occurring around us all the time Food cooking Fuel being burned in a car s engine Oxygen being used in the human body The starting materials are called reactants The ending materials
MSE Seminar Laboratory Safety Skills
 MSE Seminar Laboratory Safety Skills MSEGSA Safety Committee Cem Akatay John Koppes Kate Hess Pylin Sarobol Topics Personal Protective Equipment Proper glove selection and use When to use goggles and aprons
MSE Seminar Laboratory Safety Skills MSEGSA Safety Committee Cem Akatay John Koppes Kate Hess Pylin Sarobol Topics Personal Protective Equipment Proper glove selection and use When to use goggles and aprons
Website: Page 1. Page 14»Exercise» Page 15» Question 1:
 Page 14»Exercise» Question 1: Which of the statements about the reaction below are incorrect? (a) Lead is getting reduced. (b) Carbon dioxide is getting oxidised. (c) Carbon is getting oxidised. (d) Lead
Page 14»Exercise» Question 1: Which of the statements about the reaction below are incorrect? (a) Lead is getting reduced. (b) Carbon dioxide is getting oxidised. (c) Carbon is getting oxidised. (d) Lead
HAZARD COMMUNICATION PROGRAM
 HAZARD COMMUNICATION PROGRAM 5460 Created 1/30/10 Contents 1.0 SCOPE AND APPLICATION... 1 2.0 PURPOSE... 2 3.0 SAFETY DATA SHEETS (SDS)... 2 4.0 CONTAINER LABELS... 3 5.0 RECEIPT OF CHEMICALS... 6 6.0
HAZARD COMMUNICATION PROGRAM 5460 Created 1/30/10 Contents 1.0 SCOPE AND APPLICATION... 1 2.0 PURPOSE... 2 3.0 SAFETY DATA SHEETS (SDS)... 2 4.0 CONTAINER LABELS... 3 5.0 RECEIPT OF CHEMICALS... 6 6.0
Chemical Reactions and Equations
 Chemical Reactions and Equations 5-1 5.1 What is a Chemical Reaction? A chemical reaction is a chemical change. A chemical reaction occurs when one or more substances is converted into one or more new
Chemical Reactions and Equations 5-1 5.1 What is a Chemical Reaction? A chemical reaction is a chemical change. A chemical reaction occurs when one or more substances is converted into one or more new
Classifying Chemical Reactions: Lab Directions
 Classifying Chemical Reactions: Lab Directions Please Return Background: The power of chemical reactions to transform our lives is visible all around us in our homes, in our cars, even in our bodies. Chemists
Classifying Chemical Reactions: Lab Directions Please Return Background: The power of chemical reactions to transform our lives is visible all around us in our homes, in our cars, even in our bodies. Chemists
Due Friday, August 18 th, 2017 Mrs. Hockstok - AP Chemistry Class Olentangy Orange High School Summer Assignment
 Due Friday, August 18 th, 2017 Mrs. Hockstok - AP Chemistry Class Olentangy Orange High School Summer Assignment 2017-2018 You will have a quiz on the first day of school (August 16 th, 2017) on the polyatomic
Due Friday, August 18 th, 2017 Mrs. Hockstok - AP Chemistry Class Olentangy Orange High School Summer Assignment 2017-2018 You will have a quiz on the first day of school (August 16 th, 2017) on the polyatomic
Safe Operating Procedure (Revised 4/08)
 Safe Operating Procedure (Revised 4/08) HAZARDOUS/RADIOACTIVE MATERIAL COLLECTION PROCEDURES (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) Most used
Safe Operating Procedure (Revised 4/08) HAZARDOUS/RADIOACTIVE MATERIAL COLLECTION PROCEDURES (For assistance, please contact EHS at (402) 472-4925, or visit our web site at http://ehs.unl.edu/) Most used
5. [7 points] What is the mass of gallons (a fifth) of pure ethanol (density = g/cm 3 )? [1 gallon = Liters]
![5. [7 points] What is the mass of gallons (a fifth) of pure ethanol (density = g/cm 3 )? [1 gallon = Liters] 5. [7 points] What is the mass of gallons (a fifth) of pure ethanol (density = g/cm 3 )? [1 gallon = Liters]](/thumbs/71/66177002.jpg) 1 of 6 10/20/2009 3:55 AM Avogadro s Number, N A = 6.022 10 23 1. [7 points] Given the following mathematical expression: (15.11115.0)/(2.154 10 3 ) How many significant figures should the answer contain?
1 of 6 10/20/2009 3:55 AM Avogadro s Number, N A = 6.022 10 23 1. [7 points] Given the following mathematical expression: (15.11115.0)/(2.154 10 3 ) How many significant figures should the answer contain?
DETERMINING IONIC FORMULAS strontium oxide
 69 sodium sulfate DETERMINING IONIC FORMULAS strontium oxide tin(ii) phosphate chromium(iii) nitrate barium hydroxide titanium(iv) chloride Note: Be careful when adding subscripts to HYDROXIDE, CYANIDE,
69 sodium sulfate DETERMINING IONIC FORMULAS strontium oxide tin(ii) phosphate chromium(iii) nitrate barium hydroxide titanium(iv) chloride Note: Be careful when adding subscripts to HYDROXIDE, CYANIDE,
IDENTIFYING UNKNOWN CHEMICALS IN SCIENCE LABS
 IDENTIFYING UNKNOWN CHEMICALS IN SCIENCE LABS When a chemical or solution has not been labeled, was improperly labeled, or its label has deteriorated, become obscured or illegible, it becomes an unknown.
IDENTIFYING UNKNOWN CHEMICALS IN SCIENCE LABS When a chemical or solution has not been labeled, was improperly labeled, or its label has deteriorated, become obscured or illegible, it becomes an unknown.
Extra Questions. Chemical Formula IUPAC Name Ionic, Molecular, or Acid. ethanol. sulfurous acid. titanium (IV) oxide. gallium sulfate.
 Chemistry 30 Recap Chemistry 20 Complete the following chart: Extra Questions Name: Chemical Formula IUPAC Name Ionic, Molecular, or Acid PbI2 (s) ethanol NaHS (aq) sulfurous acid H2O2 (l) titanium (IV)
Chemistry 30 Recap Chemistry 20 Complete the following chart: Extra Questions Name: Chemical Formula IUPAC Name Ionic, Molecular, or Acid PbI2 (s) ethanol NaHS (aq) sulfurous acid H2O2 (l) titanium (IV)
Chemistry 20 Final Review Solutions Checklist Knowledge Key Terms Solutions
 Chemistry 20 Final Review Solutions Checklist Have you mastered the concepts, applications, and skills associated with the following items? Check them off when you are confident in your understanding.
Chemistry 20 Final Review Solutions Checklist Have you mastered the concepts, applications, and skills associated with the following items? Check them off when you are confident in your understanding.
- Use the STEM NAME of the element, then add "-ide" suffix
 65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
65 ANIONS 2 kinds Main-group nonmetals - Use the STEM NAME of the element, then add "-ide" suffix N : "nitride" ion P : "phosphide ion" O : "oxide ion" F : "fluoride ion" Polyatomic ions - Memorize list.(see
Unit 6 ~ Learning Guide Name:
 Unit 6 ~ Learning Guide Name: Instructions: Using a pencil, complete the following notes as you work through the related lessons. Show ALL work as is explained in the lessons. You are required to have
Unit 6 ~ Learning Guide Name: Instructions: Using a pencil, complete the following notes as you work through the related lessons. Show ALL work as is explained in the lessons. You are required to have
TA Wednesday, 3:20 PM Each student is responsible for following directions. Read this page carefully.
 Name Chemistry 111 Section FINAL EXAM Total Points = TA Wednesday, 3:20 PM 200 Directions: December 12, 2007 1. Each student is responsible for following directions. Read this page carefully. 2. Write
Name Chemistry 111 Section FINAL EXAM Total Points = TA Wednesday, 3:20 PM 200 Directions: December 12, 2007 1. Each student is responsible for following directions. Read this page carefully. 2. Write
CHEMISTRY 102B Practice Hour Exam I. Dr. D. DeCoste T.A (30 pts.) 16 (15 pts.) 17 (15 pts.) Total (60 pts)
 CHEMISTRY 102B Practice Hour Exam I Spring 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 5 numbered pages. Check now to make sure you have a complete exam. You have one hour
CHEMISTRY 102B Practice Hour Exam I Spring 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 17 questions on 5 numbered pages. Check now to make sure you have a complete exam. You have one hour
Chemicals Needed For Preparation of Chemical Solution(s):
 Name of Procedure: Merbromin Suggested Uses: Merbromin may be utilized in conjunction with a laser and/or alternate light source to develop latent impressions in blood. This technique may be used on porous
Name of Procedure: Merbromin Suggested Uses: Merbromin may be utilized in conjunction with a laser and/or alternate light source to develop latent impressions in blood. This technique may be used on porous
Class X. Exercises solution
 Exercises solution Question 1: Which of the statements about the reaction below are incorrect? Lead is getting reduced. Carbon dioxide is getting oxidised. Carbon is getting oxidised. Lead oxide is getting
Exercises solution Question 1: Which of the statements about the reaction below are incorrect? Lead is getting reduced. Carbon dioxide is getting oxidised. Carbon is getting oxidised. Lead oxide is getting
PRODUCT DATA SHEET PDS A88_E
 Plugs Suitable for A B C D Ref. Quantity Box/Bag (mm) (mm) (mm) (mm) TCP5 M12R + Pg7R M12R 4,5 8,5 10,8 4,5 3.000/100 TCP10 Pg9R Pg9R 6 12 12 4,5 2.000/100 TCP12 M12 + Pg7 M12 + Pg7 M16R + Pg11R M16R +
Plugs Suitable for A B C D Ref. Quantity Box/Bag (mm) (mm) (mm) (mm) TCP5 M12R + Pg7R M12R 4,5 8,5 10,8 4,5 3.000/100 TCP10 Pg9R Pg9R 6 12 12 4,5 2.000/100 TCP12 M12 + Pg7 M12 + Pg7 M16R + Pg11R M16R +
What do each of the hazard warning symbols below mean?
 Question 1 What do each of the hazard warning symbols below mean? Question 2 Draw a line to match the name of the separation technique to the type of mixture it is used to separate. filtration Used to
Question 1 What do each of the hazard warning symbols below mean? Question 2 Draw a line to match the name of the separation technique to the type of mixture it is used to separate. filtration Used to
DETERMINING IONIC FORMULAS strontium oxide
 69 sodium sulfate DETERMINING IONIC FORMULAS strontium oxide tin(ii) phosphate chromium(iii) nitrate barium hydroxide titanium(iv) chloride When adding a subscript to a polyatomic ion, put the polyatomic
69 sodium sulfate DETERMINING IONIC FORMULAS strontium oxide tin(ii) phosphate chromium(iii) nitrate barium hydroxide titanium(iv) chloride When adding a subscript to a polyatomic ion, put the polyatomic
