A White Paper Prepared and Reviewed by Pharmaceutical Issues Committee
|
|
|
- Barbara Nelson
- 5 years ago
- Views:
Transcription
1 A White Paper Prepared and Reviewed by Pharmaceutical Issues Committee LEAD CMPUD AALYSIS PST KSR Contributing Authors: Ted Ebersole, Pharmaceutical Issues Committee Hilary Lang, Pharmaceutical Issues Committee Patricia Folkins, Pharmaceutical Issues Committee This paper has been prepared by the Intellectual Property wners Association Pharmaceutical Issues Committee to provide background to IP members regarding the doctrine of lead compound analysis. It should not be construed as providing legal advice or as representing the views of IP Intellectual Property wners Association.
2 Introduction Following KSR, which rejected the rigid framework approach by courts to finding motivation in an obviousness inquiry, a question arose whether or not the existing lead compound analysis framework for determining whether a compound per se claim is obvious is still applicable. Based on our findings presented below, courts after KSR continue to apply this framework to compound claims directed toward new chemical entities finding such compound claims to be valid where these claims have been fully litigated. We analyze in detail the status of the lead compound analysis in several pharmaceutical cases that were decided by the courts post KSR, including four panel decisions by the Federal Circuit in Eisai, Takeda, Altana and Proctor & Gamble and two district court decisions in Daiichi Sankyo and ovartis. Pre KSR Courts generally adopted the so called lead compound analysis when addressing the question of obviousness of a chemical compound. The term lead compound for example refers to a compound in the prior art that would be most promising to modify in order to improve upon its [] activity and a compound with a better activity so as to perform further research, development and investigation. Takeda Chemical Industries v. Alphapharm Party, 492 F.3d 1350 (Fed. Cir. 2007). The Federal Circuit stressed that a prima facie case of obvious requires structural similarity between claimed and prior art subject matter... where the prior art gives reason or motivation to make the claim compositions. Eli Lilly v. Zenith Goldline Pharmaceuticals Inc. 471 F. 3d 1369, 1377 (Fed. Cir. 2006) (quoting In re Dillon 919 F.2d 688, 692 (Fed. Cir. 1990)(en banc). Specifically, this analysis required motivation to select a lead compound followed by motivation to modify that compound in a particular way in order to arrive at the claimed compound. Id. at Generally, the Federal Circuit required this motivation also referred to as the teaching suggestion motivation (TSM) to be explicit in the prior art. KSR The Supreme Court in KSR rejected the long term application by the Federal Circuit of a rigid TSM test as part of an obviousness determination. KSR International Co. v. Teleflex, Inc., 550 U.S. 398 (2007). The Court stated: The analysis need not seek out precise teachings directed to specific subject matter of the challenged claim, for a court can take account of the inferences and creative steps that a person of ordinary skill in the art would employ. 1
3 Id. at 418. As part of its more flexible approach, the Court also reversed the long standing rule that obvious to try could not constitute obviousness. Id. at 421. See In re Deuel, 51 F.3d 1552, 1559 (Fed. Cir. 1995)( [o]bvious to try has long been held not to constitute obviousness ). The Court explained its rationale for adopting, in certain circumstances, an obvious to try standard: When there is a design need or market pressure to solve a problem and there are a finite number of indentified, predictable solutions, a person of ordinary skill has good reasons to pursue the known options within his or her technical grasp. If this leads to the anticipated success, it is likely the product not of innovation but of ordinary skill and common sense. In that instance the fact that a combination was obvious to try might show that it was obvious under 103. Id. at 421. The application of this new flexible approach to obvious to try by the courts and its consequences on inventive activity in the pharmaceutical industry is yet to be fully understood, although precedence is starting to evolve in one particular area of subject matter lead compounds that may give the practitioner guidance for a post KSR analysis. Post KSR Eisai In Eisai, the patent at issue claimed the chemical compound rabeprazole and its salts, of which the sodium salt is the active ingredient in Aciphex. Eisai Co. Ltd., v. Dr. Reddy s Laboratories, Ltd., 533 F.3d 1353 (Fed. Cir. 2008). Aciphex, approved in 1991, is a proton pump inhibitor that treats duodenal ulcers and heartburn. The patent challenger identified lansoprazole as its lead candidate from a prior art reference that disclosed a broad class of gastric acid inhibiting compounds, including omeprazole (Prilosec). The panel pointed out that it is not necessary for the challenger to rigidly limit its obviousness arguments to only one lead compound. In this case, the challenger however anchored its obviousness theory to a single compound lansoprazole. Id. at Lansoprazole differs structurally from rabeprazole only at the 4 position on the pyridine ring, whereby lansoprazole has a trifluoroethoxy substituent and rabeprazole has a methoxy propoxy substituent. Id. at
4 Rabeprazole Lansoprazole H S H S CF 3 CH 3 The Federal Circuit panel consisting of Judges Rader, Linn, and Prost, with Judge Rader authoring the opinion, in its discussion of obviousness of rabeprazole applied the holding in KSR and, in doing so, advanced a three part framework comprised of several assumptions about the prior art relied upon. First, KSR assumes a starting reference point or points in the art, prior to the time of invention, from which a skilled artisan might identify a problem and pursue potential solutions. Second, KSR presupposes that the record up to the time of invention would give some reasons, available within the knowledge of one of skill in the art, to make particular modifications to achieve the claimed compound. ( Thus, in cases involving new chemical compounds, it remains necessary to identify some reason that would have led a chemist to modify a known compound in a particular manner to establish prima facie obviousness of a new claimed compound. )(Takeda, 492 F.3d at 1357). Third, the Supreme Court s analysis in KSR presumes that the record before the time of the invention would supply some reasons for narrowing the prior art universe to a finite number of identified, predictable solutions.to the extent an art is unpredictable, as the chemical arts often are, KSR s focus on these identified, predictable solutions may present a difficult hurdle because potential solutions are less likely to be genuinely predictable. Id. at Based on this framework, the Federal Circuit noted that post KSR, a prima facie case of obviousness for a chemical compound still, in general, begins 3
5 with the reasoned identification of a lead compound. Id. at Having acknowledged the identification of a lead compound, the court in Eisai considered the second step, i.e., modification of the lead compound, by replacing the trifluoroethoxy substituent at the 4 position with a methoxypropoxy substituent in order to arrive at rabeprazole. But, the court noted that the reference describing lansoprazole also explained that the fluorinated substituent resulted an increased ability to cross lipid membranes, which was considered a desirable physical property. Thus, the court concluded the record contains no reasons a skilled artisan would have considered modification of lansoprazole by removing the lipophilicity conferring fluorinated substituent as an identifiable, predictable solution. Id. at Accordingly, it affirmed the lower court s summary judgment finding of validity. Takeda The patent at issue claims the chemical compound pioglitazone, a thiazolidinedione, which is the active ingredient in the drug known as ACTS, a Type 2 diabetes treatment launched in Takeda Chemical Industries, Ltd. V. Alphapharm Pty. Ltd., 492 F.3d 1350 (Fed. Cir. 2007). The challenger identified prior art compound b as its lead compound. The portion of the molecules of interest is the right moiety having the substituted pyridyl ring. H S Pioglitazone 5 H Compound b S CH 3 6 CH 2 CH 3 The patent challenger argued that it would have been obvious to try homologation (changing the methyl substituent to an ethyl substituent) and ringwalking (moving the ethyl substituent from the 6 position to the 5 position). Id. at The Federal Circuit panel consisting of Judges Lourie, Bryson and Dyk disagreed and rejected Alphapharm s assertion that this would have been obvious to try. Based on the prior art as a whole, including a scientific article the court identified as exhaustive and reliable which disclosed undesirable side effects associated with compound b, the court held that one skilled in the art would not have selected compound b as a lead compound for antidiabetic research, stating: 4
6 Rather than identify predictable solutions for antidiabetic treatment, the prior art disclosed a broad selection of compounds any one of which could have been selected as a lead compound for further investigation. Significantly, the closest prior art compound (compound b, the 6 methyl) exhibited negative properties that would have directed one of ordinary skill in the art away from that compound. Thus, this case fails to present the type of situation contemplated by the Court when it stated that an invention may be deemed obvious if it was obvious to try. The evidence showed that it was not obvious to try. Id. The court further stated: Here the court found nothing in the prior art to narrow the possibilities of a lead compound to compound b. In contrast, the court found that one of ordinary skill in the art would have chosen one of the many compounds disclosed in [the prior art reference], of which there were over ninety, that did not disclose the existence of toxicity or side effects, and to engage in research to increase the efficacy and confirm the absence of toxicity of those compounds, rather than to choose as a starting point a compound with identified adverse effects. Id. at The court nevertheless continued its analysis assuming that compound b was a valid lead, and considered whether there would have been motivation to modify compound b to form pioglitazone. Alphapharm argued that one skilled in the art would have made the two obvious chemical changes: homologation and ring walking. Id. at Regarding the allegedly obvious step of homologation, the district court found nothing in the prior art that would have given a reasonable expectation of success for improving compound b s side effects by replacing a methyl with an ethyl. Thus, researchers would have been inclined to focus research efforts elsewhere. Id. The district court similarly concluded that nothing in the prior art provided a reasonable expectation that ring walking would improve the properties of compound b. Id. at The Federal Circuit found no error in the district court s analysis. Id. at Rather, the court concluded that Alphapharm not only failed to identify a valid lead compound but also failed to show a reason that existed at the time of the invention to chemically modify the assumed lead compound to achieve 5
7 pioglitazone. Id. at Accordingly, the court affirmed the lower court s bench ruling that the patent was not invalid. Altana The challenged patent was directed toward the compound pantoprazole, which is the active ingredient in the anti ulcer drug Protonix, a proton pump inhibitor. Altana Pharma AG v. Teva Pharmaceuticals USA, Inc., 566 F.3d 999 (Fed. Cir. 2009). Altana filed a motion for preliminary injunction. Teva and Sun conceded infringement, but alleged that the patent was obvious over four prior art references. Specifically, they identified compound 12 from the prior art as a lead compound. Pantoprazole Compound 12 C H 3 CH 3 F F C H 3 CH 3 F F S H S H The Federal Circuit panel of Circuit Judges ewman and Gajarsa, and District Judge Ward, who authored the opinion, agreed that Teva had demonstrated a substantial question of invalidity. The court first noted that [o]bviousness based on structural similarity may be proven by the identification of some motivation that would have led one of ordinary skill in the art to select and modify a known compound in a particular way to achieve the claimed compound. Id. at 1006 (citing Eisai 533 F.3d at 1357). Consistent with KSR, the court stressed that [t]he requisite motivation can come from any number of sources and need not necessarily be explicit in the art. Id. Specifically, with regard to the first step, i.e., identification of a lead compound, the court found that Teva had raised a substantial argument that compound 12 was a natural choice for further development. Id. at The court identified several factors that led to this holding including: the prior art patent claimed that its compounds, including compound 12, were improved proton pump inhibitors compared to the prior art compounds; compound 12 was one of the more potent compounds disclosed in the prior art patent; expert testimony; and Altana s own selection of compound 12 for further development, even though pantaprazole was ultimately developed by other means. Id. at The Federal Circuit in consideration of the recent admonition by KSR against using a rigid test rejected the notion that identification of a single lead compound is required, and 6
8 instead adopted the flexible approach that was used by the district court to conclude that one of skill in the art would have used the more potent compounds of the 518 patent, including compound 12, as a starting point from which to pursue further development efforts. Id With regard to the next step modification of the lead compound, the Federal Circuit affirmed the district court s finding that Teva raised a substantial question that the combination of prior art references was at the very least obvious to try and that such would lead to a predictable variation of compound 12, i.e., a compound with better ph [sic] stability. Specifically, the court found that the prior art taught that better stability for a compound in this class of drugs could be achieved with a lower pka, and that this could be achieved by replacing a methyl with a methoxy. Id. at The court held that Altana failed to meet the burden of proof for granting a preliminary injunction. Judge ewman in her concurrence nonetheless found that the evidence presented to the district court does not, in [her] view, establish invalidity of the patent on the pharmaceutical product pantoprazole. Id. at Procter & Gamble In the fourth decision by the Federal Circuit, the patent at issue was directed toward the compound risedronate, the active ingredient in the osteoporosis drug Actonel. The Procter & Gamble Co. v. Teva Pharmaceuticals USA, Inc., 566 F.3d 989 (Fed. Cir. 2009). Teva identified as its lead compound 2 pyr EHDP, a structural (or positional) isomer of risedronate. In risedronate (3 pyr EHDP), the hydroxy ethane diphosphonate group is connected at the third position of a pyridine ring, whereas it is connected to the second position of a pyridine ring in 2 pyr EHDP. Risedronate 2 pyr EHDP 3 H P 3 H 2 P 3 H 2 2 H P 3 H 2 P 3 H 2 The Federal Circuit panel consisting of Circuit Judges Mayer and Dyk, and District Judge Huff, who wrote the opinion, in its obviousness analysis reasserted that the first step in an obviousness analysis based on structural similarity between claimed and prior art compounds is the preliminary finding that one of ordinary skill in the art would have selected [the prior art compound] as a lead compound. Id. at 994 (citing Takeda and Eisai). The district court found 7
9 that the skilled artisan would not have identified 2 pyr EHDP as the lead compound. evertheless, the Federal Circuit never addressed the appropriateness of 2 pyr EHDPas a lead compound. Instead, the court concluded that defendants failed to establish that it would have been obvious at the time of the invention to modify 2 pyr EHDP to create risedronate. Id. at 995. Addressing the second step in the lead compound analysis, i.e., motivation to modify the lead compound, the court found based on the prior art and expert testimony that the structural modification from a 2 pyr EHDP to a 3 pyr EHDP was not routine. Further, the court found that the nature of the class of compounds to which risedronate belongs, i.e., bisphosphonates, was extremely unpredictable at the time of the invention. Id. at Accordingly, modification of the claimed compound would not have been obvious to try at the time of the invention. Thus, the court affirmed the lower court s ruling that the patent was not invalid. Daiichi Sankyo The district court after a bench trial upheld the validity of a claim to a prodrug compound known as olmesartan medoximil, an ester, which is the active ingredient in several drugs that are angiotensin II receptor blockers (ARBs) used to treat hypertension, including Benicar and combinations Benicar HCT and Azor. Daiichi Sankyo Company, Ltd., v. Mylan Pharmaceuticals Inc., 2009 WL (D..J.). Mylan identified several compounds as potential lead compounds from a prior art 902 patent to Dupont. Mylan suggested these compounds were a continuation of Dupont s earlier research efforts that produced the first generation of ARBs, specifically losartan. In particular, one of the compounds is Example 1, which is shown below in comparison to olmesartan medoximil. lmesartan medoxomil Example 1 of the 902 Patent H H H 8
10 The district court rejected these compounds as leads based on Mylan s failure to prove why a person skilled in the art would have selected them over numerous other second generation ARBs in the prior art that relied on the same chemical backbone and had comparable, if not greater, improvements from losartan. Id. at The court stated [s]ince a person of ordinary skill in the art could have selected a lead compound from a broad selection of compounds, Mylan has failed to establish its prima facie case of obviousness. Id. at 13. Despite its finding that Mylan failed the first step for proving obviousness of a compound, i.e, identification of a lead compound, the court continued its analysis and found that one of skill in the art would not have been motivated to modify the 902 prior art compounds to form the claimed compound. Specifically, olmesartan medoxomil differed structurally in several respects to the compounds of the 902 patent at the 4 and 5 position of the imidazole ring. For example, the 902 patent taught various alkyls at the 4 position, including an ethyl, methyl, t butyl, and isopropyl, while olmesartan medoximil has a hydroxyisopropyl group, which provides hydrophilic instead of lipophilic properties. The 902 patent also taught a carboxylic acid (Examples 2 and 6) or aldehyde (Examples 1, 3, 4 and 5) at the 5 position, while olmesartan medoximil possesses a carboxlic acid linked to a medoxomil acid. The court found the prior art taught away from modifying the 4 position, and further, that it would have been illogical for one skilled in the art to undo the 4 position of the prior art compounds because it was this position and its substituents that gave the prior art compounds their advantageous lipophilic properties. Id. at Regarding position 5, the court found that Mylan failed to show that one skilled in the art would have been motivated to transform the 902 compounds into a prodrug comprising medoxomil as a result of the unpredictable and difficult nature involved with prodrug pharmacology and reproducibility as well as the lack of motivation to select medoxomil. Id. at 16. Accordingly, the court found the claimed compound not invalid for obviousness. ovartis Finally, the patent at issue was directed to famciclovir, ovartisʹ antiviral treatment for herpes. ovartis v. Teva, o (D..J. 2007). The court denied ovartis motion for a preliminary injunction to restrain Teva from releasing their generic famciclovir product. The court held that ovartis had failed to show that Tevaʹs obviousness and inequitable conduct defenses lacked merit. Famicolovir is a prodrug that is converted into an active compound inside the body. The active compound in famciclovir is penciclovir, an acyclic 9
11 nucleoside. Since the late 1970s, acyclic nucleosides have been recognized as potential anti mitotic or anti viral agents. Famciclovir H 2 H 2 Penciclovir H CH 3 H H CH 3 The court held that it was arguable that it would have been obvious to select penciclovir as a lead compound from which to design famciclovir because it was ʺone of only five known acyclic nucleosides to have strong activity and low toxicityʺ. While Takeda held that when there are many potential lead compounds, the selection of one particular compound may not be an obvious choice, the court distinguished the present case on the basis that the prior art in Takeda disclosed ʺhundreds of millionsʺ of potential lead compounds. Even though one article taught away from penciclovir, several other patents and applications taught that penciclovir was a potent antiviral agent. Thus, the court concluded that ʺprior art as a wholeʺ did not ʺteach awayʺ from using penciclovir as a lead compound. The court also found that ovartis failed to make a persuasive argument to rebut Tevaʹs allegations that it was obvious to modify penciclovir to create famciclovir. The court opined that a person of ordinary skill in the art would have expected penciclovir to share the poor oral bioavailability of other acyclic nucleosides. Teva highlighted British patent application o. GB , which instructs how to improve the bioavailability of compounds similar to penciclovir. While ovartis argued that it was ʺunknownʺ whether the modifications made to acyclovirs as described in the British application would have a similar effect on penciclovir, Teva countered that the obviousness inquiry does not ask whether a result is ʺunknownʺ but rather whether there is a reasonable probability of success. The court rejected ovartisʹ argument, which was the development of famciclovir was not obvious because it had unexpected advantages over penciclovir, for a number of reasons: (1) based on the prior art, such results had previously been produced; (2) ovartisʹ studies regarding 10
12 ʺdosing advantageʺ were not persuasive because it did not do a head to head comparison of the various treatments; (3) there was no evidence supporting a latency advantage for famciclovir over other drugs; and (4) these advantages were not related to the invention because they were not benefits achieved through modifications made to obtain famciclovir, but instead flowed from the inherent properties of penciclovir. Accordingly, the court denied the preliminary injunction. The above cases strongly suggest that the lead compound framework for analyzing claims still applies to compounds per se despite KSR and that compound claims upon such analysis continue to be upheld by courts after being fully litigated. 11
PROTECTING PHARMACEUTICAL INVENTIONS IN A KSR WORLD
 1 PROTECTING PHARMACEUTICAL INVENTIONS IN A KSR WORLD SCOTT D. LOCKE AND WILLIAM D. SCHMIDT I. INTRODUCTION The pharmaceutical sciences and their related disciplines present unique problems for the patent
1 PROTECTING PHARMACEUTICAL INVENTIONS IN A KSR WORLD SCOTT D. LOCKE AND WILLIAM D. SCHMIDT I. INTRODUCTION The pharmaceutical sciences and their related disciplines present unique problems for the patent
Standard for Patents Strategies to Withstand Obviousness Rejections and Attacks on Patent Validity
 Presenting a live 90 minute webinar with interactive Q&A New USPTO Guidelines and the Obviousness Standard for Patents Strategies to Withstand Obviousness Rejections and Attacks on Patent Validity WEDNESDAY,
Presenting a live 90 minute webinar with interactive Q&A New USPTO Guidelines and the Obviousness Standard for Patents Strategies to Withstand Obviousness Rejections and Attacks on Patent Validity WEDNESDAY,
Lead Compound Analysis for Chemicals: Obvious or Nonobvious?
 Michigan State University College of Law Digital Commons at Michigan State University College of Law Student Scholarship 2017 Lead Compound Analysis for Chemicals: Obvious or onobvious? Li Gao Follow this
Michigan State University College of Law Digital Commons at Michigan State University College of Law Student Scholarship 2017 Lead Compound Analysis for Chemicals: Obvious or onobvious? Li Gao Follow this
The Court of Appeals for the Federal Circuit ruled that a claim must be limited to
 LEXICOGRAPHERS BEWARE AN EXPLICIT DEFINITION WILL LIMIT CLAIM SCOPE The Court of Appeals for the Federal Circuit ruled that a claim must be limited to an explicit definition in the specification. In Sinorgchem
LEXICOGRAPHERS BEWARE AN EXPLICIT DEFINITION WILL LIMIT CLAIM SCOPE The Court of Appeals for the Federal Circuit ruled that a claim must be limited to an explicit definition in the specification. In Sinorgchem
Enablement in the Chemical Arts. Carlyn A. Burton Osha Liang LLP October 21, 2009
 Enablement in the Chemical Arts Carlyn A. Burton Osha Liang LLP October 21, 2009 Overview The Patent Bargain An inventor must, through a patent specification, place the public in possession of his invention,
Enablement in the Chemical Arts Carlyn A. Burton Osha Liang LLP October 21, 2009 Overview The Patent Bargain An inventor must, through a patent specification, place the public in possession of his invention,
SYRACUSE SCIENCE & TECHNOLOGY LAW REPORTER
 SYRACUSE SCIENCE & TECHNOLOGY LAW REPORTER VOLUME 21 FALL 2009 ARTICLE 4, PAGE 87 OBVIOUS FALLACY: IMPROVING THE STANDARD OF OBVIOUSNESS FOR CHEMICAL COMPOUNDS TO MORE ACCURATELY REFLECT COMMON PRACTICE
SYRACUSE SCIENCE & TECHNOLOGY LAW REPORTER VOLUME 21 FALL 2009 ARTICLE 4, PAGE 87 OBVIOUS FALLACY: IMPROVING THE STANDARD OF OBVIOUSNESS FOR CHEMICAL COMPOUNDS TO MORE ACCURATELY REFLECT COMMON PRACTICE
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE BOARD OF PATENT APPEALS AND INTERFERENCES
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE BOARD OF PATENT APPEALS AND INTERFERENCES Ex parte TERRY W. BALKO, JOHN F. DAEUBLE, PAUL R. SCHMITZER, CARLA N. YERKES, and THOMAS L. SIDDALL Technology
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE BOARD OF PATENT APPEALS AND INTERFERENCES Ex parte TERRY W. BALKO, JOHN F. DAEUBLE, PAUL R. SCHMITZER, CARLA N. YERKES, and THOMAS L. SIDDALL Technology
Paper No Entered: October 19, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper No. 9 571.272.7822 Entered: October 19, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD INCYTE CORPORATION, Petitioner, v. CONCERT PHARMACEUTICALS,
Trials@uspto.gov Paper No. 9 571.272.7822 Entered: October 19, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD INCYTE CORPORATION, Petitioner, v. CONCERT PHARMACEUTICALS,
ARTICLES STRUCTURAL UNCERTAINTY: UNDERSTANDING THE FEDERAL CIRCUIT S LEAD COMPOUND ANALYSIS
 ARTICLES STRUCTURAL UNCERTAINTY: UNDERSTANDING THE FEDERAL CIRCUIT S LEAD COMPOUND ANALYSIS BRIANA BARRON* I. INTRODUCTION... 401 A. Obviousness and Chemical Compounds... 402 B. Development of Lead Compound
ARTICLES STRUCTURAL UNCERTAINTY: UNDERSTANDING THE FEDERAL CIRCUIT S LEAD COMPOUND ANALYSIS BRIANA BARRON* I. INTRODUCTION... 401 A. Obviousness and Chemical Compounds... 402 B. Development of Lead Compound
Paper Entered: February 8, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper 9 571-272-7822 Entered: February 8, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD FUSTIBAL LLC, Petitioner, v. BAYER HEALTHCARE LLC, Patent
Trials@uspto.gov Paper 9 571-272-7822 Entered: February 8, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD FUSTIBAL LLC, Petitioner, v. BAYER HEALTHCARE LLC, Patent
MEET THE PRESENTERS. Ryan Connell - J.D. M.S. Jim Nelson - J.D., Ph.D.
 MEET THE PRESENTERS Jim Nelson - J.D., Ph.D. Registered Patent Attorney and Principal Over 40 years of IP experience in chemical, polymer, pharmaceutical, biotechnology, cosmetics and medical device fields.
MEET THE PRESENTERS Jim Nelson - J.D., Ph.D. Registered Patent Attorney and Principal Over 40 years of IP experience in chemical, polymer, pharmaceutical, biotechnology, cosmetics and medical device fields.
Date December 24, 2014 Court Tokyo District Court, Case number 2013 (Wa) 4040
 Date December 24, 2014 Court Tokyo District Court, Case number 2013 (Wa) 4040 29th Civil Division A case in which the court recognized infringement under the doctrine of equivalents with regard to a patent
Date December 24, 2014 Court Tokyo District Court, Case number 2013 (Wa) 4040 29th Civil Division A case in which the court recognized infringement under the doctrine of equivalents with regard to a patent
MEMORANDUM OPINION AND ORDER I. BACKGROUND
 United States District Court, N.D. Illinois. QUANTUM GROUP, INC, Plaintiff. v. AMERICAN SENSOR, INC., American Sensor Electronics, Inc., and Klesman and Associates, Defendants. AMERICAN SENSOR ELECTRONICS
United States District Court, N.D. Illinois. QUANTUM GROUP, INC, Plaintiff. v. AMERICAN SENSOR, INC., American Sensor Electronics, Inc., and Klesman and Associates, Defendants. AMERICAN SENSOR ELECTRONICS
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION
 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION CYWEE GROUP LTD., Plaintiff, v. SAMSUNG ELECTRONICS CO. LTD. and SAMSUNG ELECTRONICS AMERICA, INC., Defendants. Case
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION CYWEE GROUP LTD., Plaintiff, v. SAMSUNG ELECTRONICS CO. LTD. and SAMSUNG ELECTRONICS AMERICA, INC., Defendants. Case
Paper No Entered: July 5, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper No. 42 571-272-7822 Entered: July 5, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DAIKIN INDUSTRIES, LTD. and DAIKIN AMERICA, INC., Petitioner,
Trials@uspto.gov Paper No. 42 571-272-7822 Entered: July 5, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DAIKIN INDUSTRIES, LTD. and DAIKIN AMERICA, INC., Petitioner,
THE PATENTS ACT, 1970 (39 of 1970) as amended by THE PATENTS (AMENDMENT) ACT, 2005 (15 of 2005) (with effect from )
 THE PATENTS ACT, 1970 (39 of 1970) as amended by THE PATENTS (AMENDMENT) ACT, 2005 (15 of 2005) (with effect from 1-1-2005) & THE PATENTS RULES, 2003 as amended by THE PATENTS (AMENDMENT) RULES, 2006 (with
THE PATENTS ACT, 1970 (39 of 1970) as amended by THE PATENTS (AMENDMENT) ACT, 2005 (15 of 2005) (with effect from 1-1-2005) & THE PATENTS RULES, 2003 as amended by THE PATENTS (AMENDMENT) RULES, 2006 (with
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE PATENT NO.: 5,922,695 ISSUED: Jul. 13, 1999 TO: FOR: Arimilli et al. ANTIVIRAL PHOSPHONOMETHYOXY NUCLEOTIDE ANALOGS HAVING INCREASED ORAL BIOAVAILABILITY
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE PATENT NO.: 5,922,695 ISSUED: Jul. 13, 1999 TO: FOR: Arimilli et al. ANTIVIRAL PHOSPHONOMETHYOXY NUCLEOTIDE ANALOGS HAVING INCREASED ORAL BIOAVAILABILITY
Paper 9 Tel: Entered: January 11, 2018 UNITED STATES PATENT AND TRADEMARK OFFICE
 Trials@uspto.gov Paper 9 Tel: 571-272-7822 Entered: January 11, 2018 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD CONCERT PHARMACEUTICALS, INC., Petitioner, v. INCYTE
Trials@uspto.gov Paper 9 Tel: 571-272-7822 Entered: January 11, 2018 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD CONCERT PHARMACEUTICALS, INC., Petitioner, v. INCYTE
Paper Entered: August 16, 2016 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper 10 571-272-7822 Entered: August 16, 2016 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD TEXAS ASSOCIATION OF REALTORS, Petitioner, v. POI SEARCH
Trials@uspto.gov Paper 10 571-272-7822 Entered: August 16, 2016 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD TEXAS ASSOCIATION OF REALTORS, Petitioner, v. POI SEARCH
FRANCESCA AMOS OPINION BY JUSTICE CYNTHIA D. KINSER v. Record No September 18, 1998
 Present: All the Justices FRANCESCA AMOS OPINION BY JUSTICE CYNTHIA D. KINSER v. Record No. 972018 September 18, 1998 NATIONSBANK, N.A. FROM THE CIRCUIT COURT OF THE CITY OF NORFOLK Charles E. Poston,
Present: All the Justices FRANCESCA AMOS OPINION BY JUSTICE CYNTHIA D. KINSER v. Record No. 972018 September 18, 1998 NATIONSBANK, N.A. FROM THE CIRCUIT COURT OF THE CITY OF NORFOLK Charles E. Poston,
Paper No Entered: August 3, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper No. 39 571-272-7822 Entered: August 3, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD GENERAL ELECTRIC COMPANY, Petitioner, v. UNITED TECHNOLOGIES
Trials@uspto.gov Paper No. 39 571-272-7822 Entered: August 3, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD GENERAL ELECTRIC COMPANY, Petitioner, v. UNITED TECHNOLOGIES
Paper Filed: January 9, 2019 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper 47 571.272.7822 Filed: January 9, 2019 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD NUEVOLUTION A/S, Petitioner, v. CHEMGENE HOLDINGS APS, Patent
Trials@uspto.gov Paper 47 571.272.7822 Filed: January 9, 2019 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD NUEVOLUTION A/S, Petitioner, v. CHEMGENE HOLDINGS APS, Patent
Writing Patent Specifications
 Writing Patent Specifications Japan Patent Office Asia-Pacific Industrial Property Center, JIPII 2013 Collaborator: Shoji HADATE, Patent Attorney, Intellectual Property Office NEXPAT CONTENTS Page 1. Patent
Writing Patent Specifications Japan Patent Office Asia-Pacific Industrial Property Center, JIPII 2013 Collaborator: Shoji HADATE, Patent Attorney, Intellectual Property Office NEXPAT CONTENTS Page 1. Patent
Paper No Entered: December 9, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE
 Trials@uspto.gov Paper No. 25 571.272.7822 Entered: December 9, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD NEPTUNE GENERICS, LLC., Petitioner, v. AUSPEX PHARMACEUTICALS,
Trials@uspto.gov Paper No. 25 571.272.7822 Entered: December 9, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD NEPTUNE GENERICS, LLC., Petitioner, v. AUSPEX PHARMACEUTICALS,
APPLICATION NO. FILING DATE FIRST NAMED INVENTOR ATTORNEY DOCKET NO. CONFIRMATION NO. 90/007,118 07/12/ US 8284
 UNITED STATES PATENT AND TRADEMARK OFFICE UNITED STATES DEPARTMENT OF COMMERCE United States Patent and Trademark Office Address: COMMISSIONER FOR PATENTS P.O. Box 1450 Alexandria, Virginia 22313-1450
UNITED STATES PATENT AND TRADEMARK OFFICE UNITED STATES DEPARTMENT OF COMMERCE United States Patent and Trademark Office Address: COMMISSIONER FOR PATENTS P.O. Box 1450 Alexandria, Virginia 22313-1450
SAINT PAUL HOLMES OPINION BY v. Record No JUSTICE LAWRENCE L. KOONTZ, JR. April 16, 1999 JOHN DOE
 Present: All the Justices SAINT PAUL HOLMES OPINION BY v. Record No. 981428 JUSTICE LAWRENCE L. KOONTZ, JR. April 16, 1999 JOHN DOE FROM THE CIRCUIT COURT OF MECKLENBURG COUNTY Charles L. McCormick, III,
Present: All the Justices SAINT PAUL HOLMES OPINION BY v. Record No. 981428 JUSTICE LAWRENCE L. KOONTZ, JR. April 16, 1999 JOHN DOE FROM THE CIRCUIT COURT OF MECKLENBURG COUNTY Charles L. McCormick, III,
Draft agreed by the QWP June Draft endorsed by the CMD(v) June Draft adopted by the CVMP for release for consultation July 2016
 13 July 2017 EMA/CVMP/QWP/3629/2016 Committee for Medicinal Products for Veterinary Use (CVMP) Reflection paper on the chemical structure and properties criteria to be considered for the evaluation of
13 July 2017 EMA/CVMP/QWP/3629/2016 Committee for Medicinal Products for Veterinary Use (CVMP) Reflection paper on the chemical structure and properties criteria to be considered for the evaluation of
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE PATENT NO.: 5,935,946 ISSUED: Aug. 10, 1999 TO: FOR: Munger, Jr. et al. NUCLEOTIDE ANALOG COMPOSITION AND SYNTHESIS METHOD ATTACHMENT TO FORM PTO 1465,
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE PATENT NO.: 5,935,946 ISSUED: Aug. 10, 1999 TO: FOR: Munger, Jr. et al. NUCLEOTIDE ANALOG COMPOSITION AND SYNTHESIS METHOD ATTACHMENT TO FORM PTO 1465,
Guidelines for Examining Pharmaceutical Patents
 Guidelines for Examining Pharmaceutical Patents Tahir Amin Director and Intellectual Property Solicitor I-MAK tahirmamin@gmail.com Manila, March 2007 Pharmaceutical Patenting Trends The number of patents
Guidelines for Examining Pharmaceutical Patents Tahir Amin Director and Intellectual Property Solicitor I-MAK tahirmamin@gmail.com Manila, March 2007 Pharmaceutical Patenting Trends The number of patents
United States District Court, E.D. Texas, Tyler Division. LONESTAR INVENTIONS LP, Plaintiff. v. NINTENDO OF AMERICA, INC, Defendant. No.
 United States District Court, E.D. Texas, Tyler Division. LONESTAR INVENTIONS LP, Plaintiff. v. NINTENDO OF AMERICA, INC, Defendant. No. 6:07CV261 April 14, 2009. Aaron James Pickell, Kurt Matthew Sauer,
United States District Court, E.D. Texas, Tyler Division. LONESTAR INVENTIONS LP, Plaintiff. v. NINTENDO OF AMERICA, INC, Defendant. No. 6:07CV261 April 14, 2009. Aaron James Pickell, Kurt Matthew Sauer,
No. C EDL UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF CALIFORNIA. August 15, 2002,
 Abstract In a dispute involving claim construction, Plaintiff's attempt at supporting their interpretation of a (claim) limitation via a PCT Authority's written opinion (receiving office, U.S.) was unsuccessful.
Abstract In a dispute involving claim construction, Plaintiff's attempt at supporting their interpretation of a (claim) limitation via a PCT Authority's written opinion (receiving office, U.S.) was unsuccessful.
United States Court of Appeals for the Federal Circuit
 United States Court of Appeals for the Federal Circuit 00-1310 LOCKHEED MARTIN CORPORATION, Plaintiff-Appellant, v. SPACE SYSTEMS/LORAL, INC., Defendant-Appellee, Edward V. Filardi, Skadden, Arps, Slate,
United States Court of Appeals for the Federal Circuit 00-1310 LOCKHEED MARTIN CORPORATION, Plaintiff-Appellant, v. SPACE SYSTEMS/LORAL, INC., Defendant-Appellee, Edward V. Filardi, Skadden, Arps, Slate,
An extended summary of the NCGR/Berkeley Double-Blind Test of Astrology undertaken by Shawn Carlson and published in 1985
 From: http://www.astrodivination.com/moa/ncgrberk.htm An extended summary of the NCGR/Berkeley Double-Blind Test of Astrology undertaken by Shawn Carlson and published in 1985 Introduction Under the heading
From: http://www.astrodivination.com/moa/ncgrberk.htm An extended summary of the NCGR/Berkeley Double-Blind Test of Astrology undertaken by Shawn Carlson and published in 1985 Introduction Under the heading
A Note on Bayesian Inference After Multiple Imputation
 A Note on Bayesian Inference After Multiple Imputation Xiang Zhou and Jerome P. Reiter Abstract This article is aimed at practitioners who plan to use Bayesian inference on multiplyimputed datasets in
A Note on Bayesian Inference After Multiple Imputation Xiang Zhou and Jerome P. Reiter Abstract This article is aimed at practitioners who plan to use Bayesian inference on multiplyimputed datasets in
Solid Form Patents Data in Claims
 Solid Form Patents Data in Claims June 7, 2016 PPXRD-14 2016 AMRI SSCI, LLC Eyal H Barash, Chief Patent Counsel eyal.barash@ebarashlaw.com This document was presented at PPXRD - Pharmaceutical Powder X-ray
Solid Form Patents Data in Claims June 7, 2016 PPXRD-14 2016 AMRI SSCI, LLC Eyal H Barash, Chief Patent Counsel eyal.barash@ebarashlaw.com This document was presented at PPXRD - Pharmaceutical Powder X-ray
Note: When any ambiguity of interpretation is found in this provisional translation, the Japanese text shall prevail.
 Note: When any ambiguity of interpretation is found in this provisional translation, the Japanese text shall prevail. 8. Court precedents relating to Special Application Classification Content No. Date
Note: When any ambiguity of interpretation is found in this provisional translation, the Japanese text shall prevail. 8. Court precedents relating to Special Application Classification Content No. Date
Statistical Practice
 Statistical Practice A Note on Bayesian Inference After Multiple Imputation Xiang ZHOU and Jerome P. REITER This article is aimed at practitioners who plan to use Bayesian inference on multiply-imputed
Statistical Practice A Note on Bayesian Inference After Multiple Imputation Xiang ZHOU and Jerome P. REITER This article is aimed at practitioners who plan to use Bayesian inference on multiply-imputed
For Excellence in Organic Chemistry
 Organix Inc. Your Contract Research and Custom Synthesis Company www.organixinc.com For Excellence in Organic Chemistry Organix Inc. A Contract Research and Custom Synthesis Company At the discovery end
Organix Inc. Your Contract Research and Custom Synthesis Company www.organixinc.com For Excellence in Organic Chemistry Organix Inc. A Contract Research and Custom Synthesis Company At the discovery end
WOODWARD V. MORRISON ET AL. [Holmes, 124; 5 Fish. Pat. Cas. 357; 2 O. G. 120; Merw. Pat Inv. 126.] 1 Circuit Court, D. Massachusetts. March 22, 1872.
![WOODWARD V. MORRISON ET AL. [Holmes, 124; 5 Fish. Pat. Cas. 357; 2 O. G. 120; Merw. Pat Inv. 126.] 1 Circuit Court, D. Massachusetts. March 22, 1872. WOODWARD V. MORRISON ET AL. [Holmes, 124; 5 Fish. Pat. Cas. 357; 2 O. G. 120; Merw. Pat Inv. 126.] 1 Circuit Court, D. Massachusetts. March 22, 1872.](/thumbs/93/112074861.jpg) YesWeScan: The FEDERAL CASES WOODWARD V. MORRISON ET AL. Case No. 18,008. [Holmes, 124; 5 Fish. Pat. Cas. 357; 2 O. G. 120; Merw. Pat Inv. 126.] 1 Circuit Court, D. Massachusetts. March 22, 1872. PATENTS
YesWeScan: The FEDERAL CASES WOODWARD V. MORRISON ET AL. Case No. 18,008. [Holmes, 124; 5 Fish. Pat. Cas. 357; 2 O. G. 120; Merw. Pat Inv. 126.] 1 Circuit Court, D. Massachusetts. March 22, 1872. PATENTS
All instruction should be three-dimensional. Page 1 of 12
 High School Conceptual Progressions Model Course 1 - Bundle 2 Electrical Forces and Matter or Interactions Between Particles This is the second bundle of the High School Conceptual Progressions Model Course
High School Conceptual Progressions Model Course 1 - Bundle 2 Electrical Forces and Matter or Interactions Between Particles This is the second bundle of the High School Conceptual Progressions Model Course
Brett A. Schatz, Gregory F. Ahrens, Wood, Herron and Evans, Cincinnati, OH, Miles D. Grant, Grant and Zeko, San Diego, CA, for Plaintiff.
 United States District Court, S.D. California. PRESIDIO COMPONENTS, INC, Plaintiff. v. AMERICAN TECHNICAL CERAMICS CORPORATION, Defendant. Civil Action No. 07CV893 IEG (NLS) June 11, 2008. Brett A. Schatz,
United States District Court, S.D. California. PRESIDIO COMPONENTS, INC, Plaintiff. v. AMERICAN TECHNICAL CERAMICS CORPORATION, Defendant. Civil Action No. 07CV893 IEG (NLS) June 11, 2008. Brett A. Schatz,
Case: 2:17-cv ALM-KAJ Doc #: 1 Filed: 10/26/17 Page: 1 of 10 PAGEID #: 1 UNITED STATES DISTRICT COURT SOUTHERN DISTRICT OF OHIO EASTERN DIVISION
 Case 217-cv-00946-ALM-KAJ Doc # 1 Filed 10/26/17 Page 1 of 10 PAGEID # 1 UNITED STATES DISTRICT COURT SOUTHERN DISTRICT OF OHIO EASTERN DIVISION Madeleine Entine c/o Cooper & Elliott LLC 2175 Riverside
Case 217-cv-00946-ALM-KAJ Doc # 1 Filed 10/26/17 Page 1 of 10 PAGEID # 1 UNITED STATES DISTRICT COURT SOUTHERN DISTRICT OF OHIO EASTERN DIVISION Madeleine Entine c/o Cooper & Elliott LLC 2175 Riverside
MEDICINAL CHEMISTRY Homework 2 - ANSWERS
 MEDICIAL CEMISTY omework 2 - ASWES 1. eceptor binding curves. Make a plot of %drug-bound versus -log[drug] for a drug whose K D is 5 x 10-6 M. Make a plot of %drug-bound vs [drug] for this same system.
MEDICIAL CEMISTY omework 2 - ASWES 1. eceptor binding curves. Make a plot of %drug-bound versus -log[drug] for a drug whose K D is 5 x 10-6 M. Make a plot of %drug-bound vs [drug] for this same system.
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD. DR. MICHAEL FARMWALD and RPX CORPORATION Petitioners,
 NO: 433133US IN THE UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DR. MICHAEL FARMWALD and RPX CORPORATION Petitioners, v. PARKERVISION, INC., Patent Owner. Patent
NO: 433133US IN THE UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DR. MICHAEL FARMWALD and RPX CORPORATION Petitioners, v. PARKERVISION, INC., Patent Owner. Patent
Chapter Three. Hypothesis Testing
 3.1 Introduction The final phase of analyzing data is to make a decision concerning a set of choices or options. Should I invest in stocks or bonds? Should a new product be marketed? Are my products being
3.1 Introduction The final phase of analyzing data is to make a decision concerning a set of choices or options. Should I invest in stocks or bonds? Should a new product be marketed? Are my products being
smb Doc 155 Filed 06/21/16 Entered 06/21/16 14:09:24 Main Document Pg 1 of 5
 Pg 1 of 5 Howard W. Kingsley, Esq. ROSENBERG & ESTIS, P.C. 733 Third Avenue New York, New York 10017 Telephone: (212) 687-6000 Facsimile: (212) 551-8484 Attorneys for Landlord-Creditor 900 Third Avenue,
Pg 1 of 5 Howard W. Kingsley, Esq. ROSENBERG & ESTIS, P.C. 733 Third Avenue New York, New York 10017 Telephone: (212) 687-6000 Facsimile: (212) 551-8484 Attorneys for Landlord-Creditor 900 Third Avenue,
Exhibit A Proposed Order
 Case 15-11755-CSS Doc 857-2 Filed 09/13/16 Page 1 of 94 Exhibit A Proposed Order DOCS_DE:209531.1 88601/001 Case 15-11755-CSS Doc 857-2 Filed 09/13/16 Page 2 of 94 UNITED STATES BANKRUPTCY COURT DISTRICT
Case 15-11755-CSS Doc 857-2 Filed 09/13/16 Page 1 of 94 Exhibit A Proposed Order DOCS_DE:209531.1 88601/001 Case 15-11755-CSS Doc 857-2 Filed 09/13/16 Page 2 of 94 UNITED STATES BANKRUPTCY COURT DISTRICT
Universalism Entails Extensionalism
 Universalism Entails Extensionalism Achille C. Varzi Department of Philosophy, Columbia University, New York [Final version published in Analysis, 69 (2009), 599 604] 1. Universalism (also known as Conjunctivism,
Universalism Entails Extensionalism Achille C. Varzi Department of Philosophy, Columbia University, New York [Final version published in Analysis, 69 (2009), 599 604] 1. Universalism (also known as Conjunctivism,
Conceivability and Modal Knowledge
 1 3 Conceivability and Modal Knowledge Christopher Hill ( 2006 ) provides an account of modal knowledge that is set in a broader context of arguing against the view that conceivability provides epistemic
1 3 Conceivability and Modal Knowledge Christopher Hill ( 2006 ) provides an account of modal knowledge that is set in a broader context of arguing against the view that conceivability provides epistemic
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD. ENERGETIQ TECHNOLOGY, INC., Patent Owner. Case IPR
 DOCKET NO.: 0107945.00235US1 Filed By: Donald R. Steinberg, Reg. No. 37,241 David L. Cavanaugh, Reg. No. 36,476 Michael H. Smith, Reg. No. 71,190 60 State Street, Boston, Massachusetts 02109 Tel: (617)
DOCKET NO.: 0107945.00235US1 Filed By: Donald R. Steinberg, Reg. No. 37,241 David L. Cavanaugh, Reg. No. 36,476 Michael H. Smith, Reg. No. 71,190 60 State Street, Boston, Massachusetts 02109 Tel: (617)
Words to avoid in proposals
 Crutch words used when writers don t understand what to say We understand Leverage our experience Thank you for the opportunity We look forward to state-of-the-art the right choice Never use the word understand
Crutch words used when writers don t understand what to say We understand Leverage our experience Thank you for the opportunity We look forward to state-of-the-art the right choice Never use the word understand
Chimica Farmaceutica (Insegnamento Integrato di Chimica e Biotecnologie Farmaceutiche) Drug design (2)
 (Insegnamento Integrato di Chimica e Biotecnologie Farmaceutiche) Drug design (2) Molecular Variations in Homologous Series: Vinylogues and Benzologues HOMOLOGOUS SERIES. Definition and classification
(Insegnamento Integrato di Chimica e Biotecnologie Farmaceutiche) Drug design (2) Molecular Variations in Homologous Series: Vinylogues and Benzologues HOMOLOGOUS SERIES. Definition and classification
PATENTING CRYSTALLINE FORMS OF
 1 PATENTING CRYSTALLINE FORMS OF PHARMACEUTICALS Using Solid State Science to Claim Form & Substance PPXRD-9 The Pharmaceutical Powder X-ray Diffraction Symposium Sponsored by the International Centre
1 PATENTING CRYSTALLINE FORMS OF PHARMACEUTICALS Using Solid State Science to Claim Form & Substance PPXRD-9 The Pharmaceutical Powder X-ray Diffraction Symposium Sponsored by the International Centre
Organic Chemistry. A. Introduction
 Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
Organic Chemistry A. Introduction 1. Organic chemistry is defined as the chemistry of CARBON compounds. There are a huge number of organic compounds. This results from the fact that carbon forms chains
Reading Passage. Darwin's Theory of Evolution - The Premise
 Darwin's Theory of Evolution - The Premise Reading Passage Darwin's Theory of Evolution is the widely held notion that all life is related and has descended from a common ancestor: the birds and the bananas,
Darwin's Theory of Evolution - The Premise Reading Passage Darwin's Theory of Evolution is the widely held notion that all life is related and has descended from a common ancestor: the birds and the bananas,
Data Mining in the Chemical Industry. Overview of presentation
 Data Mining in the Chemical Industry Glenn J. Myatt, Ph.D. Partner, Myatt & Johnson, Inc. glenn.myatt@gmail.com verview of presentation verview of the chemical industry Example of the pharmaceutical industry
Data Mining in the Chemical Industry Glenn J. Myatt, Ph.D. Partner, Myatt & Johnson, Inc. glenn.myatt@gmail.com verview of presentation verview of the chemical industry Example of the pharmaceutical industry
Lecture 12: Arguments for the absolutist and relationist views of space
 12.1 432018 PHILOSOPHY OF PHYSICS (Spring 2002) Lecture 12: Arguments for the absolutist and relationist views of space Preliminary reading: Sklar, pp. 19-25. Now that we have seen Newton s and Leibniz
12.1 432018 PHILOSOPHY OF PHYSICS (Spring 2002) Lecture 12: Arguments for the absolutist and relationist views of space Preliminary reading: Sklar, pp. 19-25. Now that we have seen Newton s and Leibniz
Chemists Do you have the Bayer Spirit?
 www.mybayerjob.de Chemists Do you have the Bayer Spirit? Research and Development, Synthesis, Analytics, In-house Consulting, Patents, Information Technology, Production, Marketing Chemists in Bayer s
www.mybayerjob.de Chemists Do you have the Bayer Spirit? Research and Development, Synthesis, Analytics, In-house Consulting, Patents, Information Technology, Production, Marketing Chemists in Bayer s
The Case for Use Cases
 The Case for Use Cases The integration of internal and external chemical information is a vital and complex activity for the pharmaceutical industry. David Walsh, Grail Entropix Ltd Costs of Integrating
The Case for Use Cases The integration of internal and external chemical information is a vital and complex activity for the pharmaceutical industry. David Walsh, Grail Entropix Ltd Costs of Integrating
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE. Ashish Shah, Christina Scheuer, Lauren Miller, and Barry Muffoletto
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE In Re: U.S. Patent No. 6,219,222 Filed: August 27, 1999 Issued: April 17, 2001 Inventor(s): Assignee: Title: Trial No: Panel: Attorney Docket No.: Ashish
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE In Re: U.S. Patent No. 6,219,222 Filed: August 27, 1999 Issued: April 17, 2001 Inventor(s): Assignee: Title: Trial No: Panel: Attorney Docket No.: Ashish
Surface Use in the Age of the Marcellus: Will Horizontal Wells Be Considered Reasonably Necessary to Develop the Marcellus Shale?
 Surface Use in the Age of the Marcellus: Will Horizontal Wells Be Considered Reasonably Necessary to Develop the Marcellus Shale? By Lori A. Dawkins and Allison J. Farrell, Steptoe & Johnson PLLC The Marcellus
Surface Use in the Age of the Marcellus: Will Horizontal Wells Be Considered Reasonably Necessary to Develop the Marcellus Shale? By Lori A. Dawkins and Allison J. Farrell, Steptoe & Johnson PLLC The Marcellus
Receptor Based Drug Design (1)
 Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
THE RELATIONSHIP BETWEEN NATURAL PRODUCTS AND SYNTHETIC. Poldhune Parc Owles, Carbis Bay, St. Ives, Cornwall TR26 2RE, UK.
 THE RELATIONSHIP BETWEEN NATURAL PRODUCTS AND SYNTHETIC CHEMISTRY IN THE DISCOVERY PROCESS by Dr. Stephen Brewer Consultant, Bioproducts Technology Poldhune Parc Owles, Carbis Bay, St. Ives, Cornwall TR26
THE RELATIONSHIP BETWEEN NATURAL PRODUCTS AND SYNTHETIC CHEMISTRY IN THE DISCOVERY PROCESS by Dr. Stephen Brewer Consultant, Bioproducts Technology Poldhune Parc Owles, Carbis Bay, St. Ives, Cornwall TR26
Page 1 of 13. Version 1 - published August 2016 View Creative Commons Attribution 3.0 Unported License at
 High School Conceptual Progressions Model Course II Bundle 3 Matter and Energy in Organisms This is the third bundle of the High School Conceptual Progressions Model Course II. Each bundle has connections
High School Conceptual Progressions Model Course II Bundle 3 Matter and Energy in Organisms This is the third bundle of the High School Conceptual Progressions Model Course II. Each bundle has connections
Characterization of Semantics for Argument Systems
 Characterization of Semantics for Argument Systems Philippe Besnard and Sylvie Doutre IRIT Université Paul Sabatier 118, route de Narbonne 31062 Toulouse Cedex 4 France besnard, doutre}@irit.fr Abstract
Characterization of Semantics for Argument Systems Philippe Besnard and Sylvie Doutre IRIT Université Paul Sabatier 118, route de Narbonne 31062 Toulouse Cedex 4 France besnard, doutre}@irit.fr Abstract
WIPO s Chemical Search Function
 WIPO s Chemical Search Function Empowering Ideas Towards the end of 2016 the World Intellectual Property Office (WIPO) launched its first ever chemical structure search function. We asked Emma Koncewicz,
WIPO s Chemical Search Function Empowering Ideas Towards the end of 2016 the World Intellectual Property Office (WIPO) launched its first ever chemical structure search function. We asked Emma Koncewicz,
EMMANUEL XAGORARAKIS
 EMMANUEL XAGORARAKIS (E-mail: exagorarakis@gmail.com) The singular connection between the Binary Code and the Decimal System and the algorithm which offers the Singularity of Human Vs. Computer And consequently
EMMANUEL XAGORARAKIS (E-mail: exagorarakis@gmail.com) The singular connection between the Binary Code and the Decimal System and the algorithm which offers the Singularity of Human Vs. Computer And consequently
EMPIRICAL VS. RATIONAL METHODS OF DISCOVERING NEW DRUGS
 EMPIRICAL VS. RATIONAL METHODS OF DISCOVERING NEW DRUGS PETER GUND Pharmacopeia Inc., CN 5350 Princeton, NJ 08543, USA pgund@pharmacop.com Empirical and theoretical approaches to drug discovery have often
EMPIRICAL VS. RATIONAL METHODS OF DISCOVERING NEW DRUGS PETER GUND Pharmacopeia Inc., CN 5350 Princeton, NJ 08543, USA pgund@pharmacop.com Empirical and theoretical approaches to drug discovery have often
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
STERIC ANALOGY PHARMACEUTICAL OBVIOUSNESS AT THE PTO* Harold C. Wegner**
 STERIC ANALOGY PHARMACEUTICAL OBVIOUSNESS AT THE PTO* DISCUSSION DRAFT FOR COMMENT - hwegner@gmail.com Harold C. Wegner** I. OVERVIEW 2 II. STERIC ANALOGY IN THE PHARMACEUTICAL LABORATORY 6 III. STERIC
STERIC ANALOGY PHARMACEUTICAL OBVIOUSNESS AT THE PTO* DISCUSSION DRAFT FOR COMMENT - hwegner@gmail.com Harold C. Wegner** I. OVERVIEW 2 II. STERIC ANALOGY IN THE PHARMACEUTICAL LABORATORY 6 III. STERIC
On Likelihoodism and Intelligent Design
 On Likelihoodism and Intelligent Design Sebastian Lutz Draft: 2011 02 14 Abstract Two common and plausible claims in the philosophy of science are that (i) a theory that makes no predictions is not testable
On Likelihoodism and Intelligent Design Sebastian Lutz Draft: 2011 02 14 Abstract Two common and plausible claims in the philosophy of science are that (i) a theory that makes no predictions is not testable
Lab 4: Stoichiometry and Green Chemistry
 Lab 4: Stoichiometry and Green Chemistry Goals: Learn about the philosophy of green chemistry Determine the composition of a mixture using stoichiometry Learn what is important in a good laboratory report
Lab 4: Stoichiometry and Green Chemistry Goals: Learn about the philosophy of green chemistry Determine the composition of a mixture using stoichiometry Learn what is important in a good laboratory report
Part II A Reexamination of Contemporary Utilitarianism
 Part II A Reexamination of Contemporary Utilitarianism In Part II of this book, we will turn to contemporary moral philosophers by this I mean twentieth-century philosophers who have reconstructed modern
Part II A Reexamination of Contemporary Utilitarianism In Part II of this book, we will turn to contemporary moral philosophers by this I mean twentieth-century philosophers who have reconstructed modern
EMMANUEL XAGORARAKIS. The singular connection between the Binary Code and the Decimal System. And by this
 EMMANUEL XAGORARAKIS (E-mail: exagorarakis@gmail.com) The singular connection between the Binary Code and the Decimal System And by this The Algorithm which offers the Singularity of Human Vs. Computer
EMMANUEL XAGORARAKIS (E-mail: exagorarakis@gmail.com) The singular connection between the Binary Code and the Decimal System And by this The Algorithm which offers the Singularity of Human Vs. Computer
Lecture Notes on Inductive Definitions
 Lecture Notes on Inductive Definitions 15-312: Foundations of Programming Languages Frank Pfenning Lecture 2 August 28, 2003 These supplementary notes review the notion of an inductive definition and give
Lecture Notes on Inductive Definitions 15-312: Foundations of Programming Languages Frank Pfenning Lecture 2 August 28, 2003 These supplementary notes review the notion of an inductive definition and give
Synthesis of highly pure esomeprazole sodium with polymorphic form J
 World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
World Journal of Pharmaceutical Sciences ISSN (Print): 2321-3310; ISSN (Online): 2321-3086 Published by Atom and Cell Publishers All Rights Reserved Available online at: http://www.wjpsonline.org/ Original
Paper Entered: August 14, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper 53 571-272-7822 Entered: August 14, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD FUJITSU SEMICONDUCTOR LIMITED, FUJITSU SEMICONDUCTOR AMERICA,
Trials@uspto.gov Paper 53 571-272-7822 Entered: August 14, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD FUJITSU SEMICONDUCTOR LIMITED, FUJITSU SEMICONDUCTOR AMERICA,
UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF CALIFORNIA SAN JOSE DIVISION
 UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF CALIFORNIA SAN JOSE DIVISION 0 DOMINION ASSETS LLC, Plaintiff, v. MASIMO CORPORATION, et al., Defendants. Case No. -cv-000-blf ORDER CONSTRUING CLAIMS
UNITED STATES DISTRICT COURT NORTHERN DISTRICT OF CALIFORNIA SAN JOSE DIVISION 0 DOMINION ASSETS LLC, Plaintiff, v. MASIMO CORPORATION, et al., Defendants. Case No. -cv-000-blf ORDER CONSTRUING CLAIMS
UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT
 FOR PUBLICATION UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT UNITED STATES OF AMERICA, Plaintiff, and LOWER ELWHA KLALLAM INDIAN TRIBE; JAMESTOWN S KLALLAM TRIBE; PORT GAMBLE S KLALLAM TRIBE, Petitioners-Appellees,
FOR PUBLICATION UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT UNITED STATES OF AMERICA, Plaintiff, and LOWER ELWHA KLALLAM INDIAN TRIBE; JAMESTOWN S KLALLAM TRIBE; PORT GAMBLE S KLALLAM TRIBE, Petitioners-Appellees,
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
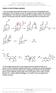 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Combining Forecasts: The End of the Beginning or the Beginning of the End? *
 Published in International Journal of Forecasting (1989), 5, 585-588 Combining Forecasts: The End of the Beginning or the Beginning of the End? * Abstract J. Scott Armstrong The Wharton School, University
Published in International Journal of Forecasting (1989), 5, 585-588 Combining Forecasts: The End of the Beginning or the Beginning of the End? * Abstract J. Scott Armstrong The Wharton School, University
EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY
 EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY Structure 7.1 Introduction Objectives 7.2 Principle 7.3 Requirements 7.4 Strategy for the Interpretation of IR Spectra 7.5 Practice Problems
EXPT. 7 CHARACTERISATION OF FUNCTIONAL GROUPS USING IR SPECTROSCOPY Structure 7.1 Introduction Objectives 7.2 Principle 7.3 Requirements 7.4 Strategy for the Interpretation of IR Spectra 7.5 Practice Problems
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE In re Patent of: Norikatsu Hattori et al. U.S. Patent No.: 8,728,590 Attorney Docket No.: 40959-0003IP1 Issue Date: May 20, 2014 Appl. Serial No.: 13/699,644
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE In re Patent of: Norikatsu Hattori et al. U.S. Patent No.: 8,728,590 Attorney Docket No.: 40959-0003IP1 Issue Date: May 20, 2014 Appl. Serial No.: 13/699,644
PRACTICE DIRECTION 3E - COSTS MANAGEMENT
 Coming into force 22 April 2014 subject to the approval of the Lord Chancellor PRACTICE DIRECTION 3E - COSTS MANAGEMENT This Practice Direction supplements Section II of CPR Part 3 Contents of this Practice
Coming into force 22 April 2014 subject to the approval of the Lord Chancellor PRACTICE DIRECTION 3E - COSTS MANAGEMENT This Practice Direction supplements Section II of CPR Part 3 Contents of this Practice
EUROPEAN PATENT APPLICATION
 @ Publication number: 0136 1 0 0 A2 EUROPEAN PATENT APPLICATION Application number: 84305891.8 Int. CI.*: A 61 K 31/70, A 61 K 47/00 @ Date of filing : 29.08.84 ( ) Priority: 02.09.83 US 528976 @ Applicant:
@ Publication number: 0136 1 0 0 A2 EUROPEAN PATENT APPLICATION Application number: 84305891.8 Int. CI.*: A 61 K 31/70, A 61 K 47/00 @ Date of filing : 29.08.84 ( ) Priority: 02.09.83 US 528976 @ Applicant:
COLLEGE OF PHYSICIANS & SURGEONS OF MANITOBA INQUIRY PANEL DECISION
 COLLEGE OF PHYSICIANS & SURGEONS OF MANITOBA INQUIRY PANEL DECISION INQUIRY: IC2459 DR. RANDY RAYMOND ALLAN On November 10, 2016, a hearing was convened before an Inquiry Panel (the Panel ) of the College
COLLEGE OF PHYSICIANS & SURGEONS OF MANITOBA INQUIRY PANEL DECISION INQUIRY: IC2459 DR. RANDY RAYMOND ALLAN On November 10, 2016, a hearing was convened before an Inquiry Panel (the Panel ) of the College
Digital Mapping License Agreement
 City of Des Moines, Iowa GIS Division, Information Technology Department Digital Mapping License Agreement PURPOSE AND NATURE OF THE AGREEMENT This Digital Mapping License Agreement ("Agreement") is executed
City of Des Moines, Iowa GIS Division, Information Technology Department Digital Mapping License Agreement PURPOSE AND NATURE OF THE AGREEMENT This Digital Mapping License Agreement ("Agreement") is executed
FUNCTIONALITY AFTER TRAFFIX
 FUNCTIONALITY AFTER TRAFFIX Presented by William J. Utermohlen 2004 OLIFF & BERRIDGE, PLC Inwood Labs., Inc. v. Ives Labs., 456 U.S. 844, 214 USPQ 1 (1982) a product feature is functional if it is essential
FUNCTIONALITY AFTER TRAFFIX Presented by William J. Utermohlen 2004 OLIFF & BERRIDGE, PLC Inwood Labs., Inc. v. Ives Labs., 456 U.S. 844, 214 USPQ 1 (1982) a product feature is functional if it is essential
Case 2:16-cv Document 1 Filed 12/15/16 Page 1 of 39 PageID #: 1
 Case 2:16-cv-01417 Document 1 Filed 12/15/16 Page 1 of 39 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION B/E AEROSPACE, INC., a Delaware Corporation;
Case 2:16-cv-01417 Document 1 Filed 12/15/16 Page 1 of 39 PageID #: 1 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION B/E AEROSPACE, INC., a Delaware Corporation;
Ping-Chiang Lyu. Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University.
 Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Announcements. Unit 3: Foundations for inference Lecture 3: Decision errors, significance levels, sample size, and power.
 Announcements Announcements Unit 3: Foundations for inference Lecture 3:, significance levels, sample size, and power Statistics 101 Mine Çetinkaya-Rundel October 1, 2013 Project proposal due 5pm on Friday,
Announcements Announcements Unit 3: Foundations for inference Lecture 3:, significance levels, sample size, and power Statistics 101 Mine Çetinkaya-Rundel October 1, 2013 Project proposal due 5pm on Friday,
*EP A1* EP A1 (19) (11) EP A1 (12) EUROPEAN PATENT APPLICATION. (43) Date of publication: Bulletin 2004/22
 (19) Europäisches Patentamt European Patent Office Office européen des brevets *EP0014222A1* (11) EP 1 422 2 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.0.04 Bulletin 04/22 (1) Int
(19) Europäisches Patentamt European Patent Office Office européen des brevets *EP0014222A1* (11) EP 1 422 2 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.0.04 Bulletin 04/22 (1) Int
Energetic Ionic Liquids
 Energetic Ionic Liquids The AMPAC-GaTech Partnership fosters research aimed at opening and developing new avenues and compounds that fit the American Pacific Corporation (AMPAC) s potential market. Under
Energetic Ionic Liquids The AMPAC-GaTech Partnership fosters research aimed at opening and developing new avenues and compounds that fit the American Pacific Corporation (AMPAC) s potential market. Under
IN THE MATTER OF THE SECURITIES ACT, R.S.O. 1990, c. S.5, AS AMENDED. - and -
 Ontario Commission des P.O. Box 55, 19 th Floor CP 55, 19e étage Securities valeurs mobilières 20 Queen Street West 20, rue queen ouest Commission de l Ontario Toronto ON M5H 3S8 Toronto ON M5H 3S8 IN
Ontario Commission des P.O. Box 55, 19 th Floor CP 55, 19e étage Securities valeurs mobilières 20 Queen Street West 20, rue queen ouest Commission de l Ontario Toronto ON M5H 3S8 Toronto ON M5H 3S8 IN
Temporal Extension. 1. Time as Quantity
 1 Boris Hennig IFOMIS Saarbrücken Draft version as of July 17, 2005 Temporal Extension Abstract. Space and time are continuous and extensive quantities. But according to Aristotle, time differs from space
1 Boris Hennig IFOMIS Saarbrücken Draft version as of July 17, 2005 Temporal Extension Abstract. Space and time are continuous and extensive quantities. But according to Aristotle, time differs from space
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD. DR. MICHAEL FARMWALD and RPX CORPORATION Petitioner
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DR. MICHAEL FARMWALD and RPX CORPORATION Petitioner v. PARKERVISION, INC. Patent Owner Case IPR2014-00948 U.S. Patent
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DR. MICHAEL FARMWALD and RPX CORPORATION Petitioner v. PARKERVISION, INC. Patent Owner Case IPR2014-00948 U.S. Patent
Services in Chemistry for a Sustainable World
 Table of Content 2 Services in Chemistry for a Sustainable World COMPANY PROFILE TECHNICAL PROFILE General Information... 3 Our Services... 4 Project Types & Pricing Options... 5 Reaction & Catalyst Portfolio...
Table of Content 2 Services in Chemistry for a Sustainable World COMPANY PROFILE TECHNICAL PROFILE General Information... 3 Our Services... 4 Project Types & Pricing Options... 5 Reaction & Catalyst Portfolio...
ISO Soil quality Determination of particle size distribution in mineral soil material Method by sieving and sedimentation
 INTERNATIONAL STANDARD ISO 11277 Second edition 2009-09-15 Soil quality Determination of particle size distribution in mineral soil material Method by sieving and sedimentation Qualité du sol Détermination
INTERNATIONAL STANDARD ISO 11277 Second edition 2009-09-15 Soil quality Determination of particle size distribution in mineral soil material Method by sieving and sedimentation Qualité du sol Détermination
Analog Technologies. ATE1-17 TEC Modules
 Figure 1.1. The Photo of Actual Figure 1.2. The Photo of Actual FEATURES Maximum Input Voltage: 2.2 V Low Cost Long Life Time 100 % Lead (Pb)-free and RoS Compliant APPLICATIONS Regulate the temperature
Figure 1.1. The Photo of Actual Figure 1.2. The Photo of Actual FEATURES Maximum Input Voltage: 2.2 V Low Cost Long Life Time 100 % Lead (Pb)-free and RoS Compliant APPLICATIONS Regulate the temperature
1. Justification of analogical reasoning 2. Representation
 1. Justification of analogical reasoning an argument that it is reasonable to give serious consideration to hypotheses supported by analogies that meet certain standards Serious consideration: non-negligible
1. Justification of analogical reasoning an argument that it is reasonable to give serious consideration to hypotheses supported by analogies that meet certain standards Serious consideration: non-negligible
