Paper No Entered: December 9, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE
|
|
|
- Grace Bradley
- 6 years ago
- Views:
Transcription
1 Paper No Entered: December 9, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD NEPTUNE GENERICS, LLC., Petitioner, v. AUSPEX PHARMACEUTICALS, INC., Patent Owner. Case IPR Before LORA M. GREEN, DEBORAH KATZ, and JACQUELINE WRIGHT BONILLA, Administrative Patent Judges. GREEN, Administrative Patent Judge. DECISION Denying Institution of Inter Partes Review 37 C.F.R
2 I. INTRODUCTION Neptune Generics, LLC ( Petitioner ) filed a Petition requesting an inter partes review of claims 1 10 of U.S. Patent No. 7,465,317 B2 (Ex. 1001, the 317 patent ). Paper 1 ( Pet. ). Auspex Pharmaceuticals, Inc. ( Patent Owner ) filed a Preliminary Response to the Petition. Paper 13 ( Prelim. Resp. ). An inter partes review may not be instituted unless... there is a reasonable likelihood that the petitioner would prevail with respect to at least 1 of the claims challenged in the petition. 35 U.S.C Upon considering the Petition and the Preliminary Response, we determine that Petitioner has not shown a reasonable likelihood that it would prevail in showing the unpatentability of claims Accordingly, we decline to institute an inter partes review of the challenged claims. A. Related Proceedings Petitioner states that it is unaware of any other matters related to the 317 patent. Pet. 6; see also Paper 7 (stating that there is no related litigation pending, and no related inter partes or other administrative review proceedings pending ). B. The 317 Patent (Ex. 1001) The 317 patent issued on November 25, 2008, with Thomas G. Gant and Sepehr Sarshar as the listed inventors. Ex The claims of the 317 patent are drawn to a deuterated form of venlafaxine ( VF ), an inhibitor of monoamine neurotransmitters. Id. at 69:30 70:38, 1: The 317 patent teaches that the human body expresses several enzymes that aid in breaking down or solubilizing nutrients and chemicals that have been absorbed into the blood, producing novel intermediates or 2
3 metabolites. Id. at 1: Those enzymes include cytochrome P-450 enzymes (also referred to as CYPs ), esterases, proteases, reductases, and others. Id. at 1: As taught by the 317 patent: Some of the most common metabolic reactions of pharmaceutical compounds involve the oxidation of a carbonhydrogen (C H) bond to either a carbon-oxygen (C O) or carbon-carbon (C C) π-bond. The resultant metabolites may be stable or unstable under physiological conditions, and can have substantially different pharmacokinetic, pharmacodynamic, acute and long-term toxicity profiles relative to the parent compounds. For most drugs, such oxidations are generally rapid and ultimately lead to administration of multiple or high daily doses. There is therefore an obvious and immediate need for improvements of such drugs. Id. at 1: The 317 patent teaches that the chemical bond between a carbon and deuterium (an isotope of hydrogen) is stronger than the bond between a carbon and hydrogen. Id. at 2: Thus, if breaking a carbon-hydrogen bond is a rate limiting step in a reaction, substituting the hydrogen with deuterium will decrease the reaction rate, slowing the reaction down. Id. That phenomenon is known as the deuterium kinetic isotope effect, and can range from a value of 1, with no isotope effect, to over 50, in which the substitution of deuterium with hydrogen makes the reaction proceed 50 times more slowly. Id. at 2: According to the 317 patent, [d]euteration of pharmaceuticals to improve pharmokinetics (PK), pharmacodynamics (PD), and toxicity profiles, has been demonstrated previously with some classes of drugs. Id. at 3: The 317 patent teaches that the method, however, may not be applicable to all classes of pharmaceuticals due to the phenomenon of metabolic switching, which can lead to different proportions of known 3
4 metabolites, as well as the production of new metabolites, which may impart more or less toxicity. Id. at 3: During prosecution of the 317 patent, in the statement of the reasons for allowance, the Examiner noted: The prior art neither teaches, nor suggests the limitations of Applicant s claims.... Nor would it have been obvious to modify the prior art s venlafaxine to arrive at the instantly claimed invention. There is no teaching in the prior art that correlates improvements in the pharmacological properties of venlafaxine with the instantly claimed D-9 deuterated derivatives of venlafaxine. In addition, venlafaxine has 27 positions that can be deuterated, along with 134,217,727 potential deuterated analogs of venlafaxine. Because of the large number of possible deuterated analogs of venlafaxine, it would not be obvious to try to synthesize the instantly claimed D-9 deuterated derivative of venlafaxine. Ex. 2032, 3. C. Illustrative Claim Petitioner challenges claims 1 10 of the 317 patent. Claim 1 is the only independent claim, is illustrative, and is reproduced below, with the symbol D in the structural formula indicating a deuterium: 1. A compound having the structural formula: or a pharmaceutically acceptable salt, solvate, or prodrug thereof. 4
5 C. The Asserted Grounds of Unpatentability Petitioner challenges the patentability of claims 1 10 of the 317 patent on the following grounds (Pet. 7 8): References Basis Claims Challenged Fogelman 1 and Miwa 2 103(a) 1 Fogelman, Miwa, and 103(a) 2 and 8 Platteeuw 3 Fogelman, Miwa, Platteeuw, and Jerussi 4 103(a) 3 7, 9, and 10 II. ANALYSIS A. Claim Construction In an inter partes review, claim terms in an unexpired patent are interpreted according to their broadest reasonable construction in light of the specification of the patent in which they appear. 37 C.F.R (b); In re Cuozzo Speed Techs., LLC, 793 F.3d 1268, (Fed. Cir. 2015); Office Patent Trial Practice Guide, 77 Fed. Reg. 48,756, 48,766 (Aug. 14, 2012). Claim terms also are given their ordinary and customary meaning, as would be understood by one of ordinary skill in the art in the context of the entire disclosure. In re Translogic Tech., Inc., 504 F.3d 1249, 1 Fogelman et al. ( Fogelman ), O- and N-demethylation of Venlafaxine In Vitro by Human Liver Microsomes and by Microsomes from cdna- Transfected Cells: Effet of Metabolic Inhibitors and SSRI Antidepressants, 20 NEUROPSYCHOPHARMACOLOGY (1999) (Ex. 1003). 2 GERALD T. MIWA AND ANTHONY Y. H. LU ( MIWA ), Kinetic Isotope Effects and Metabolic Switching in Cytochrome P450-Catalyzed Reactions, 7 BIOESSAYS (1987) (Ex. 1004). 3 Platteeuw et al. ( Platteeuw), US 2003/ AI, published Oct. 9, 2003 (Ex. 1005). 4 Jerussi et al., ( Jerussi ), US 6,197,828 B1, issued Mar. 6, 2001(Ex. 1006). 5
6 1257 (Fed. Cir. 2007). Only terms which are in controversy need to be construed, and then only to the extent necessary to resolve the controversy. Vivid Techs., Inc. v. Am. Sci. & Eng g, Inc., 200 F.3d 795, 803 (Fed. Cir. 1999). We determine that explicit construction of any specific claim term is not necessary to determine whether to institute a trial in this case. B. Obviousness of Claim 1 Over the Combination of Fogelman (Ex. 1003) and Miwa (Ex. 1004) Petitioner asserts that claim 1 is rendered obvious by the combination of Fogelman and Miwa. Pet i. Overview of Fogelman (Ex. 1003) Fogelman studied the transformation of VF into its two major metabolites O-desmethylvenlafaxine ( ODV ) and N- desmethylvenlafaxine ( NDV ). Ex. 1003, Abstract. Fogelman teaches that VF is transformed in the liver to ODV, NDV, and N,Odidesmethylvenlafaxine ( N,O-DV ). Id. at 481. According to Fogelman, ODV has a receptor affinity profile similar to its parent compound VF, while the latter two metabolites have little if any affinity for the... receptor sites. Id. 6
7 Table 4 of Fogelman is reproduced below. Id. at 486. Table 4 provides details of the formation of ODV and NDV by microsomes containing human cytochromes expressed by cdna-transfected human lymphoblastoid cells. Id. Fogelman found that [u]sing the V max //K m ratio as a quantity to estimate intrinsic clearance, approximately 90% of total intrinsic clearance was accounted for by the O-demethylation pathway. Id. at 483. ii. Overview of Miwa (Ex. 1004) Miwa is a review of kinetic isotope effect and metabolic switching in cytochrome P-450-catalyzed reactions. Ex. 1004, Summary. Miwa teaches that the carbon-deuterium bond is stronger than the carbon-hydrogen bond, and thus many reactions that involve cleavage of that bond are 7
8 retarded by deuterium substitution, a phenomenon known as kinetic isotope effect. Id. at 215. Miwa teaches: In most enzymic reactions involving a C-H bond cleavage step, the reduction in product formation for the deuterated substrate is accompanied by a concomitant decrease in substrate metabolism. The tight complimentary binding between the substrate and the enzyme active site places severe restrictions on alternative orientations of the substrate, thus limiting metabolism to only a single site. In contrast, an unexpected change in regional selectivity was observed when the products of cytochrome P-450-catalyzed reactions were carefully determined.... [T]he term metabolic switching [has been coined] to describe this change in selectivity during the metabolism of deuterated substrates. In this essay, metabolic switching is defined more generally and refers to the increase in substrate flux through alternative pathways, catalyzed by either cytochrome P-450 or alternate enzymes, when the primary pathway for metabolism is reduced by a hydrogen isotope or other substituents. Id. (reference omitted). Thus, Miwa teaches that for some drugs, isotope substitution (i.e., from hydrogen to deuterium) may reduce the rate of drug metabolism, and, therefore, may reduce the rate of clearance of the drug from the body. Id. at 218. Miwa teaches further, however, that for other drugs, isotope substitution may not give rise to significant isotope effects in metabolism or clearance, which may be due to the lack of contribution of C-H bond in the overall metabolism of the drug, or because of metabolic switching, which results in the potentiation of other metabolic pathways. Id. When metabolic switching occurs, the pharmacological and toxicological consequences depend on the new metabolic pathway, which may either enhance or inhibit the production of toxic metabolites. Id. 8
9 ii. Analysis Petitioner asserts that claim 1 is unpatentable as being rendered obvious by the combination of Fogelman and Miwa. Pet Petitioner relies on the Declaration of Dr. William Braunlin (Ex. 1002) to support its obviousness challenge. Patent Owner disagrees. Prelim. Resp Petitioner relies on Fogelman for teaching that the transformation of VF to ODV is the major pathway for clearance of VF. Pet. 24 (citing Ex ). Petitioner relies also on Fogelman for teaching the major metabolites of VF, the specific P-450 enzymes responsible for the production of the O-demethylation and N-demethylation metabolism products of VF, as well as the extent of which P-450 enzyme is responsible for the production of those products. Id. at Petitioner relies on Miwa for demonstrating that the use of strategic deuteration of the active sites of pharmaceutically active compounds to alter their preferred metabolic route and rate of clearance was known. Id. at 24. Miwa is also relied upon for its teaching that the possibility of metabolic switching was known, as well as the factors that may influence its occurrence. Id. (citing Ex ). Petitioner relies on Dr. Braunlin for his statement that an important aspect of the effectiveness of a particular drug is how long it remains in the body before being metabolized and cleared from the body. Id. at 28 (quoting Ex ). Fogelman teaches that the major pathway of clearance for VF is through the ODV pathway, and that approximately 90% of total intrinsic clearance is through that pathway. Id. at 29 (citing Ex ). Thus, Petitioner contends that one of ordinary skill in the art would have been motivated to deuterate the drug venlafaxine at the sites acted upon 9
10 by cytochrome P-450 enzymes in order to reduce the rate of venlafaxine metabolism, reduce the clearance of venlafaxine from the body and improve the duration of action and peak plasma levels of venlafaxine. Id. at (quoting Ex ). As for a reasonable expectation of success, Petitioner relies on the Declaration of Dr. Braunlin for the explanation that VF has only two cytochrome P-450 enzyme active sites that would be expected to be successful for deuteration, the carboxymethyl oxygen and the two methyl groups on the nitrogen. Id. at 30 (citing Ex ). Thus, Petitioner contends that a person of ordinary skill in the art would have had a reasonable expectation of success in determining the sites for deuteration that are recited in claim 1 of the 317 patent. Id. (citing Ex ). Patent Owner responds that it is not possible to predict whether any given kinetic isotope effect will provide a beneficial change in metabolism without extensive testing. Prelim. Resp. 22 (citing Ex. 2021, ). Specifically, Patent Owner argues that a primary deuterium kinetic isotope effect will only be observed if the breaking of the carbon-hydrogen bond is the rate-limiting step. Id. at 23 (reference omitted) (citing Ex. 2020, 6 102). According to Patent Owner, the evidence of record demonstrates that the ordinary artisan would not have known, nor been able to predict, the rate-limiting step of P-450 mediated metabolic oxidation of VF. Id. 5 Scott L. Harbeson and Roger D. Tung ( Harbeson ), Deuterium in Drug Discovery and Development, 46 ANNUAL REPORTS IN MEDICINAL CHEMISTRY (John E. Macor, Ed., 2011). 6 Fisher et al. ( Fisher ), The Complexities Inherent in Attempts to Decrease Drug Clearance by Blocking Sites of CYP-Mediated Metabolism, 9 CURRENT OPINION IN DRUG DISCOVERY & DEVELOPMENT (2006). 10
11 Patent Owner cites to evidence that shows that even if the breaking of the carbon-hydrogen bond is the rate-limiting step of P-450 mediated metabolic oxidation of VF, the evidence of record demonstrates further that the ordinary artisan would not have been able to predict the magnitude of the deuterium substitution effect. Id. at 24 (citing Ex. 2021, 104). If the effect is too low, there may be no impact on the metabolism of the drug, and if it is too high, metabolic switching may occur. Id. (citing Ex. 2020, ). Patent Owner cites to evidence that the ordinary artisan would not have been able to predict the metabolites that would be formed by metabolic switching, nor the impact of those metabolites. Id. at 26 (citing Ex. 1004, 218). Thus, Patent Owner contends that the ordinary artisan would not have had a reasonable expectation of success of deuterating VF to achieve a molecule with improved duration of action and peak plasma levels. Id. at 27. In fact, according to Patent Owner, Miwa relied upon by Petitioner teaches that isotope substitution may not give rise to significant isotope effects in metabolism or clearance. Id. (quoting Ex. 1004, 218). As noted above, Petitioner relies on Fogelman for teaching that the major pathway for clearance of VF is oxidation to its major metabolite, ODV. Id. at 28. In that pathway, the methoxy group is converted to a hydroxyl, and thus, according to Petitioner, it would have been obvious to replace the -OH 3 group with an -OD 3 group to replace the rate of clearance of VF. Id. Patent Owner argues, however, that the exact opposite effect occurred for two deuterated forms of paroxetine in which methylene hydrogens adjacent to an oxygen were substituted with deuterium. Id. (citing Ex. 2021, ). Patent Owner points out that deuterium 11
12 substitution of paroxetine, which is also a serotonin reuptake inhibitor, resulted in an increase in metabolism, not a decrease. Id. at Patent Owner argues further that the compound of claim 1 is not just the substitution of an -OH 3 group with an -OD 3 group, but also replaces the hydrogen with deuterium on two N-methyl groups, making for a total of nine replacements, leaving the other hydrogens on the compound unreplaced. Id. at 29. The deuteration of alkyl groups in an N-alkylated compound, Patent Owner contends, is also not predictable. Id. at 30. According to Patent Owner, when phentermine was tested, which has a N-di-(tri-deuteromethyl) group similar to VF, no deuterium kinetic isotope effect was observed. Id. (citing Ex. 2019, ). And even if decreased metabolism is observed, Patent Owner argues, there may be no net benefit in vivo. Id. Patent Owner cites studies of tramadol, which is also a serotonin reuptake inhibitor, and which, Patent Owner argues, shares many structural features with VF, and is also metabolized via O- and N-demethylation. Id. at 31. Patent Owner argues that although some of the deuterated versions of tramadol that were prepared, including a d 9 -tramadol, exhibited reduced in vitro metabolism, none of the prepared deuterated versions were superior to tramadol in terms of potency or duration of effect; clearance was not reduced and half-life was not increased. Id. (citing Ex. 2026, ). According to Patent Owner, the deuterated venlafaxine claimed in the 317 patent, however, has 7 Allan B. Foster ( Foster ), Deuterium Isotope Effects in the Metabolism of Drugs and Xenobiotics: Implications for Drug Design, 14 ADVANCES IN DRUG RESEARCH 1 40 (1985). 8 Shao et al. ( Shao ), Derivatives of Tramadol for Increased Duration of Effect, 16 BIOORGANIC & MEDICINAL CHEMISTRY LETTERS (2006). 12
13 superior pharmacokinetic properties compared to venlafaxine, including increased half-life, reduced Cmax, and reduced inter-patient variability. Id. (citing Ex ). Patent Owner contends also that Petitioner provides no credible motivation... to modify venlafaxine by deuteration. Id. at 31. Petitioner, Patent Owner asserts, relies on the unsupported assertion of its expert that the ordinary artisan would have been motivate to deuterated the sites acted upon by cytochrome P-450 to reduce the rate of metabolism and clearance of VF, thereby improving its peak plasma levels and its duration of action. Id. at 32. The prior art, however, teaches that ODV has the same pharmacological activity of VF, but that it has a longer duration of action and is more potent than VF. Id. According to Patent Owner, the Petition does not identify a reason why the ordinary artisan would want to deuterate VF to inhibit ODV production given its useful pharmacologic activity, arguing that [t]his flaw exemplifies the unfounded generalizations that characterize Petitioner s arguments. Id. We recognize that an obvious analysis requires us to consider whether an ordinary artisan would have had a reasonable expectation of success, not absolute predictability of success. In re O Farrell, 853 F.2d 894, (Fed. Cir. 1988). We agree with Patent Owner, however, that Petitioner has not demonstrated that the ordinary artisan would have combined Fogelman with Miwa with a reasonable expectation of arriving at the invention claimed by the 317 patent. Although Fogelman teaches that ODV may account for 90% of the pathway for intrinsic clearance of VF (Ex. 1003, 483), Fogelman 9 Amanda Yarnell, Heavy Hydrogen Drugs Turn Heads, Again, 87(25) Chem, Eng. News (2009). 13
14 teaches also that ODV has a receptor affinity profile similar to its parent compound VF (id. at 481). As noted by Patent Owner (Prelim. Resp. 32), Petitioner has not explained, through evidence or adequate scientific reasoning, why the ordinary artisan would have wanted to prevent the metabolism of VF to its pharmaceutically active metabolite, ODV. Stated differently, because ODV is a desired metabolite, as it has the same pharmacological activity as its parent compound, VF, Petitioner has not explained why the ordinary artisan would have had a reason to prevent VF from being metabolized to ODV, rather than only preventing metabolism to a non-active metabolite, or make a substitution that would prevent the further metabolism of ODV. In addition, Miwa teaches that isotope substitution may not give rise to significant isotope effects in metabolism or clearance, which may be due to the lack of contribution of C-H bond in the overall metabolism of the drug. Ex. 1004, 218. Fisher, relied upon by Patent Owner, also states that if cleavage of the C-H bond is the rate-limiting step, the turnover of the drug would depend on deuterium substitution. Ex. 2020, 102. Fisher notes, however, that other steps, such as product release, may be rate-limiting, and thus, suppress any isotope effect. Id. Thus, according to Fisher, deuterium isotope effect theory and the mechanism of CYP enzymes taken together suggest that the strategy will usually not result in significant alterations in overall metabolic clearance of the substrate. Id. at In addition, Harbeson, also cited by Patent Owner, teaches that [i]t is difficult to predict a priori which effect deuterium may have on a drug s metabolism. Ex. 2021, 404. Harbeson notes that while a deuterium isotope effect has the potential to affect the metabolism of drugs that are metabolized by the 14
15 cleavage of a C-H bond, in practice, the effect is often masked. Id. at 405. Thus, the evidence of record demonstrates that it was not predictable what would be the effect of replacing one or more hydrogens of VF with deuterium on the metabolism of VF by the P-450 enzymes. That is, although Petitioner argues that it would be obvious to substitute the two main sites of VF acted upon by the P-450 enzymes, that is, -OCH 3 and N(CH 3 ) 2 (Pet ), due to the presence of other possible rate-limiting steps, as well as the possibility of metabolic switching, the ordinary artisan would not have had a reasonable expectation that substitution of all of the nine hydrogens at those sites for deuterium would result in a deuterated VF derivative having enhanced bioavailability and which maintains its activity for a longer period of time. That conclusion is supported further by evidence relied upon by Patent Owner. For example, Harbeson, cited by Patent Owner, teaches that while one deuterated analog of paroxetine, whose structure is unknown, mitigated inactivation by a P-450 enzyme in a clinical setting, two other analogs, with structures 16 and 17 showed increased metabolism in vivo. Ex. 2021, 413. Structures 16 and 17 are reproduced below. 15
16 Patent Owner notes that methylene hydrogens adjacent to an oxygen were substituted with deuterium (Prelim. Resp. 28), which Petitioner argues would have been obvious to do with VF (Pet ). Patent Owner notes further that when phentermine was tested, which has a N-di-(tri-deuteromethyl) group similar to VF, no deuterium kinetic isotope effect was observed. Prelim. Resp. 30 (citing Ex. 2019, 22 23). The structures of N,N-dimethylphenteramine (Formula 87), and its deuterated derivatives (Formulas 88 and 89), are reproduced below: Ex. 2019, 23. Foster teaches that it was found that the C-H bond did not contribute to the rate-limiting step, and that the N-demethylation could involve a transition state different from that associated with O-demethylation. Id. Shao, also cited by Patent Owner (Prelim. Resp ), demonstrates that even if deuteration slows the formation of major metabolites, there may be no in vivo effect. Ex. 2026, Shao looked at deuteration of tramadol, which is used in the treatment of moderate to moderately severe pain. Id. at 691. The structures of tramadol (Formula 1), and its major metabolite, M1, are reproduced below: 16
17 Id. Shao attempted to slow CYP450-mediated metabolism by replacing hydrogen with deuterium at metabolically active sites, that is, by replacing the hydrogen atoms at the O-methyl and N-methyl groups, as demonstrated below: Id. As reported by Shao: In conclusion, the substitution of deuterium for hydrogen in the methyl groups of tramadol did not adversely affect the in vitro binding affinity. In vitro testing in human microsomes confirmed that replacing hydrogen with deuterium in the metabolically labile O-methyl position of 1 slowed the formation of the primary metabolite M1 by approximately 5- fold. The deuterated derivatives 7 (D6) and 9 (D9) were active 17
18 analgesics in the rat tail-flick model, but were not superior to tramadol in terms of potency or duration of effect. Deuterium for hydrogen replacement at metabolically active sites had no obvious deleterious effects in vivo but did not result in a longer duration of effect. In this case, deuteration at metabolically active sites produced a pharmacological agent equipotent in vivo with tramadol. Id. at Thus, the evidence of record demonstrates that the effect of the deuteration on the metabolic and pharmacologic properties of a drug are unpredictable, and thereby supports Patent Owner s contention that the ordinary artisan would not have had a reasonable expectation of success at arriving at the invention claimed by the 317 patent. We have considered the Declaration of Dr. Braunlin, relied upon by Petitioner to demonstrate a reasonable expectation of success (Pet. 30), but it does not convince us otherwise. Dr. Braunlin states: It is my opinion that a person of ordinary skill in the art would have had a reasonable expectation of success of achieving the claimed invention. As taught by Miwa, strategic deuteration of active sites on drugs to alter their preferred metabolic route and rate of clearance was known. And, in terms of functionality, there are only two cytochrome P-450 enzyme active sites on venlafaxine that would be expected to be successful sites for deuteration: -CD3 substitution of the carboxymethyl oxygen and -CD3 substitution of the two methyl groups on nitrogen. Ex Dr. Braunlin opines further that as the primary metabolic pathway involves O-demethylation, it makes sense to fully deuterate the single O-methyl group to enhance bioavailability and maintain activity for a longer prior to elimination. Id. 21. Relying on a reference to Foster (for 18
19 which an exhibit number was not provided nor appear does the reference appear to be made of record), Dr. Braunlin states that O-methyl deuteration has a very large isotope effect. Id. Because the second pathway involved N-demethylation, Dr. Braunlin concludes a person of ordinary skill in the art would have had a reasonable expectation of success in determining sites for deuteration that are recited in claim 1 of the 317 patent. Id. As discussed above, although Miwa teaches that the use of deuteration to alter drug clearance rate was known, Miwa teaches also that for some drugs, isotope substitution may not give rise to significant isotope effects in metabolism or clearance. Ex. 1004, 218. The lack of a kinetic isotope effect may be due to the lack of contribution of C-H bond in the overall metabolism of the drug, or because of metabolic switching, which results in the potentiation of other metabolic pathways. Id. Thus, Miwa supports the unpredictability of the effect of substituting deuterium for hydrogen on the pharmacological and toxicological effects of a drug. Moreover, the reference relied upon by Dr. Braunlin to support his statement that O-methyl deuteration has a very large isotope effect was not provided; whereas Shao, relied upon by Patent Owner demonstrates that deuteration of the O-methyl group of tramadol had no in vivo effect. Ex. 2026, Patent Owner argues further that the Petition does not demonstrate that it would have been obvious to try deuteration of VF to arrive at the claims of the 317 patent. Prelim. Resp. 32. In particular, Patent Owner notes that there were not a finite number of identified, predictable solutions, as there are approximately , or 124,217,727 deuterated forms of VF, minus some number of forms which are equivalent due to symmetry or rotatable bonds. Id. at 32 33, 35. According to Patent Owner, [t]o explore 19
20 all of these possibilities would be to vary all parameters in the bare hope of success. Id. at 35. We agree. In In re O Farrell, the Federal Circuit set forth two situations in which an obvious to try rationale was usually applied improperly. In re O Farrell, 853 F.2d at 903. The first improper situation was to vary all parameters or try each of numerous possible choices until one possibly arrived at a successful result, where the prior art gave either no indication of which parameters are critical or no direction as to which of many possible choices is likely to be successful. Id. The second improper situation was to explore a new technology or general approach that seemed to be a promising field of experimentation, where the prior art gave only general guidance as to the particular form of the claimed invention or how to achieve it. Id.; see also In re Kubin, 561 F.3d 1351, (Fed. Cir. 2009) (noting that the rationale in O Farrell was affirmed in the logical inverse in KSR, 550 U.S. at 417, in a statement that 103 bars patentability unless the improvement is more than the predictable use of prior art elements according to their established functions. ). Based on the above framework, we conclude that it would not have been obvious to try to achieve the invention of claim 1 of the 317 patent. As noted by Patent Owner (Prelim. Resp. 35), and not disputed by Petitioner (see Pet ), there are approximately , or 124,217,727 deuterated forms of VF, minus some number of forms which are equivalent due to symmetry or rotatable bonds. Although Petitioner contends that as P-450 enzymes act on two main sites of VF that is, -OCH 3 and N(CH 3 ) 2, arguing that it would have been obvious to deuterate only those sites (Pet ) as discussed above, the ordinary artisan would not have had a 20
21 reasonable expectation that deuteration of those sites would result in enhanced bioavailability and maintaining the activity of VF for a longer period of time. Thus, the ordinary artisan would be left with trying the over 100,000,000 possible deuterated forms of VF until possibly arriving at a successful result. iii. Conclusion For the reasons set forth above, we conclude that Petitioner has not established a reasonable likelihood that claim 1 is rendered obvious by the combination of Fogelman and Miwa. C. Obviousness over the Combination of Fogelman (Ex. 1003), Miwa (Ex. 1004), and Platteeuw (Ex. 1005), and the Combination of Fogelman, Miwa, Platteeuw, and Jerussi (Ex. 1006). Petitioner contends that claims 2 and 8 are unpatentable over the combination of Fogelman, Miwa, and Platteeuw (Pet ), and that claims 3 7, 9, and 10 are unpatentable as obvious over the combination of Fogelman, Miwa, Platteeuw, and Jerussi (Pet ). Petitioner relies on Platteeuw for teaching a therapeutically effective amount of VF (Pet. 31), and Jerussi for teaching oral, parental, and intravenous infusion (id. at 34). Thus, neither Platteeuw nor Jerussi remedy the deficiencies of the combination of Fogelman and Miwa, as discussed above in relation to claim 1, upon which claims 2 10 depend. As neither Platteeuw nor Jerussi remedy the deficiencies of the combination of Fogelman and Miwa, discussed above, Petitioner has not established a reasonable likelihood that claims 2 10 are rendered obvious by the combination of Fogelman, Miwa, and Platteeuw, or the combination of Fogelman, Miwa, Platteeuw, and Jerussi. 21
22 III. PENDING MOTIONS Patent Owner filed a Motion for Additional Discovery (Paper 9). In addition, Petitioner filed a Motion to Seal (Paper 18), as well as a Motion to Expunge Paper 17 (Paper 19). We grant Petitioner s Motion to Expunge. We deny Patent Owner s Motion for Additional Discovery as moot in view of our denial of the Petition. Finally, as we did not rely on the material Petitioner sought to seal, we decline to address the merits of the Motion to Seal. Petitioner is authorized to file a motion to expunge any material that it seeks to keep confidential within thirty (30) days of the date of this decision, or within thirty (30) days of a decision on rehearing, if rehearing is requested. IV. CONCLUSION For the foregoing reasons, we are not persuaded that the Petition establishes a reasonable likelihood that Petitioner would prevail in showing claims 1 10 of the 317 patent are unpatentable under 35 U.S.C. 103(a). V. ORDER In consideration of the foregoing, it is hereby: ORDERED that the Petition is denied as to all of the challenged claims of the 317 patent; FURTHER ORDERED that Paper 17 is expunged; and FURTHER ORDERED that Petitioner is authorized to file a motion to expunge any material that it seeks to keep confidential within thirty (30) days of the date of this decision, or within thirty (30) days of a decision on rehearing, if rehearing is requested. 22
23 PETITIONER: Dr. Gregory J. Gonsalves Dr. Cynthia Soumoff PATENT OWNER: Marta Gross Matthew Phillips Marta E. Delsignore Dennis Bennett 23
Paper No Entered: October 19, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper No. 9 571.272.7822 Entered: October 19, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD INCYTE CORPORATION, Petitioner, v. CONCERT PHARMACEUTICALS,
Trials@uspto.gov Paper No. 9 571.272.7822 Entered: October 19, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD INCYTE CORPORATION, Petitioner, v. CONCERT PHARMACEUTICALS,
Paper Entered: August 16, 2016 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper 10 571-272-7822 Entered: August 16, 2016 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD TEXAS ASSOCIATION OF REALTORS, Petitioner, v. POI SEARCH
Trials@uspto.gov Paper 10 571-272-7822 Entered: August 16, 2016 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD TEXAS ASSOCIATION OF REALTORS, Petitioner, v. POI SEARCH
Paper 9 Tel: Entered: January 11, 2018 UNITED STATES PATENT AND TRADEMARK OFFICE
 Trials@uspto.gov Paper 9 Tel: 571-272-7822 Entered: January 11, 2018 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD CONCERT PHARMACEUTICALS, INC., Petitioner, v. INCYTE
Trials@uspto.gov Paper 9 Tel: 571-272-7822 Entered: January 11, 2018 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD CONCERT PHARMACEUTICALS, INC., Petitioner, v. INCYTE
Paper Entered: February 8, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper 9 571-272-7822 Entered: February 8, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD FUSTIBAL LLC, Petitioner, v. BAYER HEALTHCARE LLC, Patent
Trials@uspto.gov Paper 9 571-272-7822 Entered: February 8, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD FUSTIBAL LLC, Petitioner, v. BAYER HEALTHCARE LLC, Patent
Paper No Entered: August 3, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper No. 39 571-272-7822 Entered: August 3, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD GENERAL ELECTRIC COMPANY, Petitioner, v. UNITED TECHNOLOGIES
Trials@uspto.gov Paper No. 39 571-272-7822 Entered: August 3, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD GENERAL ELECTRIC COMPANY, Petitioner, v. UNITED TECHNOLOGIES
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE BOARD OF PATENT APPEALS AND INTERFERENCES
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE BOARD OF PATENT APPEALS AND INTERFERENCES Ex parte TERRY W. BALKO, JOHN F. DAEUBLE, PAUL R. SCHMITZER, CARLA N. YERKES, and THOMAS L. SIDDALL Technology
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE BOARD OF PATENT APPEALS AND INTERFERENCES Ex parte TERRY W. BALKO, JOHN F. DAEUBLE, PAUL R. SCHMITZER, CARLA N. YERKES, and THOMAS L. SIDDALL Technology
Paper No Entered: July 5, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper No. 42 571-272-7822 Entered: July 5, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DAIKIN INDUSTRIES, LTD. and DAIKIN AMERICA, INC., Petitioner,
Trials@uspto.gov Paper No. 42 571-272-7822 Entered: July 5, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DAIKIN INDUSTRIES, LTD. and DAIKIN AMERICA, INC., Petitioner,
Paper Filed: January 9, 2019 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper 47 571.272.7822 Filed: January 9, 2019 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD NUEVOLUTION A/S, Petitioner, v. CHEMGENE HOLDINGS APS, Patent
Trials@uspto.gov Paper 47 571.272.7822 Filed: January 9, 2019 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD NUEVOLUTION A/S, Petitioner, v. CHEMGENE HOLDINGS APS, Patent
Enablement in the Chemical Arts. Carlyn A. Burton Osha Liang LLP October 21, 2009
 Enablement in the Chemical Arts Carlyn A. Burton Osha Liang LLP October 21, 2009 Overview The Patent Bargain An inventor must, through a patent specification, place the public in possession of his invention,
Enablement in the Chemical Arts Carlyn A. Burton Osha Liang LLP October 21, 2009 Overview The Patent Bargain An inventor must, through a patent specification, place the public in possession of his invention,
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE. Ashish Shah, Christina Scheuer, Lauren Miller, and Barry Muffoletto
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE In Re: U.S. Patent No. 6,219,222 Filed: August 27, 1999 Issued: April 17, 2001 Inventor(s): Assignee: Title: Trial No: Panel: Attorney Docket No.: Ashish
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE In Re: U.S. Patent No. 6,219,222 Filed: August 27, 1999 Issued: April 17, 2001 Inventor(s): Assignee: Title: Trial No: Panel: Attorney Docket No.: Ashish
Paper Entered: August 14, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper 53 571-272-7822 Entered: August 14, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD FUJITSU SEMICONDUCTOR LIMITED, FUJITSU SEMICONDUCTOR AMERICA,
Trials@uspto.gov Paper 53 571-272-7822 Entered: August 14, 2015 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD FUJITSU SEMICONDUCTOR LIMITED, FUJITSU SEMICONDUCTOR AMERICA,
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD. ENERGETIQ TECHNOLOGY, INC., Patent Owner. Case IPR
 DOCKET NO.: 0107945.00235US1 Filed By: Donald R. Steinberg, Reg. No. 37,241 David L. Cavanaugh, Reg. No. 36,476 Michael H. Smith, Reg. No. 71,190 60 State Street, Boston, Massachusetts 02109 Tel: (617)
DOCKET NO.: 0107945.00235US1 Filed By: Donald R. Steinberg, Reg. No. 37,241 David L. Cavanaugh, Reg. No. 36,476 Michael H. Smith, Reg. No. 71,190 60 State Street, Boston, Massachusetts 02109 Tel: (617)
IN THE COURT OF CRIMINAL APPEALS OF TENNESSEE AT JACKSON
 IN THE COURT OF CRIMINAL APPEALS OF TENNESSEE AT JACKSON PHILIP R. WORKMAN, ) Petitioner, ) ) v. ) No. W2001-00881-CCA-R9-PD ) Shelby County STATE OF TENNESSEE, ) Respondent. ) ANSWER IN OPPOSITION TO
IN THE COURT OF CRIMINAL APPEALS OF TENNESSEE AT JACKSON PHILIP R. WORKMAN, ) Petitioner, ) ) v. ) No. W2001-00881-CCA-R9-PD ) Shelby County STATE OF TENNESSEE, ) Respondent. ) ANSWER IN OPPOSITION TO
Paper Entered: June 20, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 Trials@uspto.gov Paper 37 571-272-7822 Entered: June 20, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD VALEO NORTH AMERICA, INC. AND VALEO EMBRAYAGES, Petitioner,
Trials@uspto.gov Paper 37 571-272-7822 Entered: June 20, 2017 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD VALEO NORTH AMERICA, INC. AND VALEO EMBRAYAGES, Petitioner,
The Court of Appeals for the Federal Circuit ruled that a claim must be limited to
 LEXICOGRAPHERS BEWARE AN EXPLICIT DEFINITION WILL LIMIT CLAIM SCOPE The Court of Appeals for the Federal Circuit ruled that a claim must be limited to an explicit definition in the specification. In Sinorgchem
LEXICOGRAPHERS BEWARE AN EXPLICIT DEFINITION WILL LIMIT CLAIM SCOPE The Court of Appeals for the Federal Circuit ruled that a claim must be limited to an explicit definition in the specification. In Sinorgchem
Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability
 Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability Optibrium Webinar 2015, June 17 2015 Jonathan Tyzack, Matthew Segall, Peter Hunt Optibrium, StarDrop, Auto-Modeller
Quantum Mechanical Models of P450 Metabolism to Guide Optimization of Metabolic Stability Optibrium Webinar 2015, June 17 2015 Jonathan Tyzack, Matthew Segall, Peter Hunt Optibrium, StarDrop, Auto-Modeller
MEDCHEM 562 Kent Kunze Lecture 2. Physicochemical Properties of Drugs and Drug Disposition. Stereochemistry (Double the trouble. or double the fun?
 15 MEDCHEM 562 Kent Kunze Lecture 2 Physicochemical Properties of Drugs and Drug Disposition Stereochemistry (Double the trouble. or double the fun?) The vast majority of drugs contain at least one stereo-center
15 MEDCHEM 562 Kent Kunze Lecture 2 Physicochemical Properties of Drugs and Drug Disposition Stereochemistry (Double the trouble. or double the fun?) The vast majority of drugs contain at least one stereo-center
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD. DR. MICHAEL FARMWALD and RPX CORPORATION Petitioners,
 NO: 433133US IN THE UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DR. MICHAEL FARMWALD and RPX CORPORATION Petitioners, v. PARKERVISION, INC., Patent Owner. Patent
NO: 433133US IN THE UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DR. MICHAEL FARMWALD and RPX CORPORATION Petitioners, v. PARKERVISION, INC., Patent Owner. Patent
CTP-656 Tablet Confirmed Superiority Of Pharmacokinetic Profile Relative To Kalydeco in Phase I Clinical Studies
 Tablet Confirmed Superiority Of Pharmacokinetic Profile Relative To Kalydeco in Phase I Clinical Studies 39 th European Cystic Fibrosis Conference 8-11 June 2016, Basel, Switzerland 2014 Concert Pharmaceuticals,
Tablet Confirmed Superiority Of Pharmacokinetic Profile Relative To Kalydeco in Phase I Clinical Studies 39 th European Cystic Fibrosis Conference 8-11 June 2016, Basel, Switzerland 2014 Concert Pharmaceuticals,
On drug transport after intravenous administration
 On drug transport after intravenous administration S.Piekarski (1), M.Rewekant (2) Institute of Fundamental Technological Research Polish Academy of Sciences (1), Medical University of Warsaw, Poland Abstract
On drug transport after intravenous administration S.Piekarski (1), M.Rewekant (2) Institute of Fundamental Technological Research Polish Academy of Sciences (1), Medical University of Warsaw, Poland Abstract
CH MEDICINAL CHEMISTRY
 CH 458 - MEDICINAL CHEMISTRY SPRING 2011 M: 5:15pm-8 pm Sci-1-089 Prerequisite: Organic Chemistry II (Chem 254 or Chem 252, or equivalent transfer course) Instructor: Dr. Bela Torok Room S-1-132, Science
CH 458 - MEDICINAL CHEMISTRY SPRING 2011 M: 5:15pm-8 pm Sci-1-089 Prerequisite: Organic Chemistry II (Chem 254 or Chem 252, or equivalent transfer course) Instructor: Dr. Bela Torok Room S-1-132, Science
PHARMACOKINETIC DERIVATION OF RATES AND ORDERS OF REACTIONS IN MULTI- COMPARTMENT MODEL USING MATLAB
 IJPSR (2016), Vol. 7, Issue 11 (Research Article) Received on 29 May, 2016; received in revised form, 07 July, 2016; accepted, 27 July, 2016; published 01 November, 2016 PHARMACOKINETIC DERIVATION OF RATES
IJPSR (2016), Vol. 7, Issue 11 (Research Article) Received on 29 May, 2016; received in revised form, 07 July, 2016; accepted, 27 July, 2016; published 01 November, 2016 PHARMACOKINETIC DERIVATION OF RATES
MEMORANDUM OPINION AND ORDER I. BACKGROUND
 United States District Court, N.D. Illinois. QUANTUM GROUP, INC, Plaintiff. v. AMERICAN SENSOR, INC., American Sensor Electronics, Inc., and Klesman and Associates, Defendants. AMERICAN SENSOR ELECTRONICS
United States District Court, N.D. Illinois. QUANTUM GROUP, INC, Plaintiff. v. AMERICAN SENSOR, INC., American Sensor Electronics, Inc., and Klesman and Associates, Defendants. AMERICAN SENSOR ELECTRONICS
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD UMICORE AG & CO. KG, Petitioner
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD UMICORE AG & CO. KG, Petitioner Patent No. 8,404,203 Issue Date: March 16, 2013 Title: PROCESS FOR REDUCING NITROGEN OXIDES
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD UMICORE AG & CO. KG, Petitioner Patent No. 8,404,203 Issue Date: March 16, 2013 Title: PROCESS FOR REDUCING NITROGEN OXIDES
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD. PRESIDIO COMPONENTS, INC. Petitioner v.
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD PRESIDIO COMPONENTS, INC. Petitioner v. AVX CORPORATION Patent Owner Patent 6,144,547 PRESIDIO COMPONENTS, INC. S PETITION
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD PRESIDIO COMPONENTS, INC. Petitioner v. AVX CORPORATION Patent Owner Patent 6,144,547 PRESIDIO COMPONENTS, INC. S PETITION
Nonlinear pharmacokinetics
 5 Nonlinear pharmacokinetics 5 Introduction 33 5 Capacity-limited metabolism 35 53 Estimation of Michaelis Menten parameters(v max andk m ) 37 55 Time to reach a given fraction of steady state 56 Example:
5 Nonlinear pharmacokinetics 5 Introduction 33 5 Capacity-limited metabolism 35 53 Estimation of Michaelis Menten parameters(v max andk m ) 37 55 Time to reach a given fraction of steady state 56 Example:
MEDCHEM 562 Kent Kunze Lecture 2. Physicochemical Properties of Drugs and Drug Disposition. Stereochemistry (Double the trouble. or double the fun?
 12 MEDCHEM 562 Kent Kunze Lecture 2 Physicochemical Properties of Drugs and Drug Disposition Stereochemistry (Double the trouble. or double the fun?) The vast majority of drugs contain at least one stereo-center
12 MEDCHEM 562 Kent Kunze Lecture 2 Physicochemical Properties of Drugs and Drug Disposition Stereochemistry (Double the trouble. or double the fun?) The vast majority of drugs contain at least one stereo-center
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD MEYER PRODUCTS, LLC. Petitioner v. DOUGLAS DYNAMICS, LLC. Patent Owner Case IPR2015- U.S. Patent No. 6,928,757 PETITION
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD MEYER PRODUCTS, LLC. Petitioner v. DOUGLAS DYNAMICS, LLC. Patent Owner Case IPR2015- U.S. Patent No. 6,928,757 PETITION
Early Stages of Drug Discovery in the Pharmaceutical Industry
 Early Stages of Drug Discovery in the Pharmaceutical Industry Daniel Seeliger / Jan Kriegl, Discovery Research, Boehringer Ingelheim September 29, 2016 Historical Drug Discovery From Accidential Discovery
Early Stages of Drug Discovery in the Pharmaceutical Industry Daniel Seeliger / Jan Kriegl, Discovery Research, Boehringer Ingelheim September 29, 2016 Historical Drug Discovery From Accidential Discovery
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION
 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION CYWEE GROUP LTD., Plaintiff, v. SAMSUNG ELECTRONICS CO. LTD. and SAMSUNG ELECTRONICS AMERICA, INC., Defendants. Case
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION CYWEE GROUP LTD., Plaintiff, v. SAMSUNG ELECTRONICS CO. LTD. and SAMSUNG ELECTRONICS AMERICA, INC., Defendants. Case
COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS
 COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.)
COMPUTER AIDED DRUG DESIGN (CADD) AND DEVELOPMENT METHODS DRUG DEVELOPMENT Drug development is a challenging path Today, the causes of many diseases (rheumatoid arthritis, cancer, mental diseases, etc.)
APPLICATION NO. FILING DATE FIRST NAMED INVENTOR ATTORNEY DOCKET NO. CONFIRMATION NO. 90/007,118 07/12/ US 8284
 UNITED STATES PATENT AND TRADEMARK OFFICE UNITED STATES DEPARTMENT OF COMMERCE United States Patent and Trademark Office Address: COMMISSIONER FOR PATENTS P.O. Box 1450 Alexandria, Virginia 22313-1450
UNITED STATES PATENT AND TRADEMARK OFFICE UNITED STATES DEPARTMENT OF COMMERCE United States Patent and Trademark Office Address: COMMISSIONER FOR PATENTS P.O. Box 1450 Alexandria, Virginia 22313-1450
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ARKEMA FRANCE, Petitioner,
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ARKEMA FRANCE, Petitioner, v. HONEYWELL INTERNATIONAL, INC., Patent Owner. IPR No. IPR2015-00915 U.S. Patent No. 8,710,282
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ARKEMA FRANCE, Petitioner, v. HONEYWELL INTERNATIONAL, INC., Patent Owner. IPR No. IPR2015-00915 U.S. Patent No. 8,710,282
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE In re Patent of: Norikatsu Hattori et al. U.S. Patent No.: 8,728,590 Attorney Docket No.: 40959-0003IP1 Issue Date: May 20, 2014 Appl. Serial No.: 13/699,644
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE In re Patent of: Norikatsu Hattori et al. U.S. Patent No.: 8,728,590 Attorney Docket No.: 40959-0003IP1 Issue Date: May 20, 2014 Appl. Serial No.: 13/699,644
Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette
 Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Chapter 18 Amines and Neurotransmitters Based on Material Prepared by Andrea D. Leonard University of Louisiana at Lafayette Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD. DR. MICHAEL FARMWALD and RPX CORPORATION Petitioner
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DR. MICHAEL FARMWALD and RPX CORPORATION Petitioner v. PARKERVISION, INC. Patent Owner Case IPR2014-00948 U.S. Patent
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD DR. MICHAEL FARMWALD and RPX CORPORATION Petitioner v. PARKERVISION, INC. Patent Owner Case IPR2014-00948 U.S. Patent
The general concept of pharmacokinetics
 The general concept of pharmacokinetics Hartmut Derendorf, PhD University of Florida Pharmacokinetics the time course of drug and metabolite concentrations in the body Pharmacokinetics helps to optimize
The general concept of pharmacokinetics Hartmut Derendorf, PhD University of Florida Pharmacokinetics the time course of drug and metabolite concentrations in the body Pharmacokinetics helps to optimize
MEET THE PRESENTERS. Ryan Connell - J.D. M.S. Jim Nelson - J.D., Ph.D.
 MEET THE PRESENTERS Jim Nelson - J.D., Ph.D. Registered Patent Attorney and Principal Over 40 years of IP experience in chemical, polymer, pharmaceutical, biotechnology, cosmetics and medical device fields.
MEET THE PRESENTERS Jim Nelson - J.D., Ph.D. Registered Patent Attorney and Principal Over 40 years of IP experience in chemical, polymer, pharmaceutical, biotechnology, cosmetics and medical device fields.
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD. KAZ USA, INC., Petitioner. EXERGEN CORPORATION, Patent Owner
 of U.S. Patent No. 9,194,749 UNITED STATES PATENT AND TRADEMARK OFFICE Paper No. 1 BEFORE THE PATENT TRIAL AND APPEAL BOARD KAZ USA, INC., Petitioner v. EXERGEN CORPORATION, Patent Owner Patent No. 9,194,749
of U.S. Patent No. 9,194,749 UNITED STATES PATENT AND TRADEMARK OFFICE Paper No. 1 BEFORE THE PATENT TRIAL AND APPEAL BOARD KAZ USA, INC., Petitioner v. EXERGEN CORPORATION, Patent Owner Patent No. 9,194,749
THE PATENTS ACT, 1970 (39 of 1970) as amended by THE PATENTS (AMENDMENT) ACT, 2005 (15 of 2005) (with effect from )
 THE PATENTS ACT, 1970 (39 of 1970) as amended by THE PATENTS (AMENDMENT) ACT, 2005 (15 of 2005) (with effect from 1-1-2005) & THE PATENTS RULES, 2003 as amended by THE PATENTS (AMENDMENT) RULES, 2006 (with
THE PATENTS ACT, 1970 (39 of 1970) as amended by THE PATENTS (AMENDMENT) ACT, 2005 (15 of 2005) (with effect from 1-1-2005) & THE PATENTS RULES, 2003 as amended by THE PATENTS (AMENDMENT) RULES, 2006 (with
From Friday s material
 5.111 Lecture 35 35.1 Kinetics Topic: Catalysis Chapter 13 (Section 13.14-13.15) From Friday s material Le Chatelier's Principle - when a stress is applied to a system in equilibrium, the equilibrium tends
5.111 Lecture 35 35.1 Kinetics Topic: Catalysis Chapter 13 (Section 13.14-13.15) From Friday s material Le Chatelier's Principle - when a stress is applied to a system in equilibrium, the equilibrium tends
G. GENERAL ACID-BASE CATALYSIS
 G. GENERAL ACID-BASE CATALYSIS Towards a Better Chemical Mechanism via Catalysis There are two types of mechanisms we ll be discussing this semester. Kinetic mechanisms are concerned with rate constants
G. GENERAL ACID-BASE CATALYSIS Towards a Better Chemical Mechanism via Catalysis There are two types of mechanisms we ll be discussing this semester. Kinetic mechanisms are concerned with rate constants
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE PATENT NO.: 5,922,695 ISSUED: Jul. 13, 1999 TO: FOR: Arimilli et al. ANTIVIRAL PHOSPHONOMETHYOXY NUCLEOTIDE ANALOGS HAVING INCREASED ORAL BIOAVAILABILITY
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE PATENT NO.: 5,922,695 ISSUED: Jul. 13, 1999 TO: FOR: Arimilli et al. ANTIVIRAL PHOSPHONOMETHYOXY NUCLEOTIDE ANALOGS HAVING INCREASED ORAL BIOAVAILABILITY
Citation for published version (APA): Nouri-Nigjeh, E. (2011). Electrochemistry in the mimicry of oxidative drug metabolism Groningen: s.n.
 University of Groningen Electrochemistry in the mimicry of oxidative drug metabolism Nouri-Nigjeh, Eslam IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish
University of Groningen Electrochemistry in the mimicry of oxidative drug metabolism Nouri-Nigjeh, Eslam IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
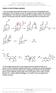 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Receptor Based Drug Design (1)
 Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Induced Fit Model For more than 100 years, the behaviour of enzymes had been explained by the "lock-and-key" mechanism developed by pioneering German chemist Emil Fischer. Fischer thought that the chemicals
Date December 24, 2014 Court Tokyo District Court, Case number 2013 (Wa) 4040
 Date December 24, 2014 Court Tokyo District Court, Case number 2013 (Wa) 4040 29th Civil Division A case in which the court recognized infringement under the doctrine of equivalents with regard to a patent
Date December 24, 2014 Court Tokyo District Court, Case number 2013 (Wa) 4040 29th Civil Division A case in which the court recognized infringement under the doctrine of equivalents with regard to a patent
A White Paper Prepared and Reviewed by Pharmaceutical Issues Committee
 A White Paper Prepared and Reviewed by Pharmaceutical Issues Committee LEAD CMPUD AALYSIS PST KSR Contributing Authors: Ted Ebersole, Pharmaceutical Issues Committee Hilary Lang, Pharmaceutical Issues
A White Paper Prepared and Reviewed by Pharmaceutical Issues Committee LEAD CMPUD AALYSIS PST KSR Contributing Authors: Ted Ebersole, Pharmaceutical Issues Committee Hilary Lang, Pharmaceutical Issues
SAINT PAUL HOLMES OPINION BY v. Record No JUSTICE LAWRENCE L. KOONTZ, JR. April 16, 1999 JOHN DOE
 Present: All the Justices SAINT PAUL HOLMES OPINION BY v. Record No. 981428 JUSTICE LAWRENCE L. KOONTZ, JR. April 16, 1999 JOHN DOE FROM THE CIRCUIT COURT OF MECKLENBURG COUNTY Charles L. McCormick, III,
Present: All the Justices SAINT PAUL HOLMES OPINION BY v. Record No. 981428 JUSTICE LAWRENCE L. KOONTZ, JR. April 16, 1999 JOHN DOE FROM THE CIRCUIT COURT OF MECKLENBURG COUNTY Charles L. McCormick, III,
2 4 Chemical Reactions and Enzymes Chemical Reactions
 Chemical Reactions A chemical reaction occurs when chemical bonds are broken and reformed. Rust forms very slowly, while rocket fuel combustion is explosive! The significance of this comparison is that
Chemical Reactions A chemical reaction occurs when chemical bonds are broken and reformed. Rust forms very slowly, while rocket fuel combustion is explosive! The significance of this comparison is that
Introduction to medicinal chemisry. Mohammed Nooraldeen (PhD)
 Introduction to medicinal chemisry Med Chem I Mohammed Nooraldeen (PhD) كيمياء دوائية )1 ) محمد نورالدين محمود Introduction to medicinal chemistry Medicinal Chemistry Biology Medicine Biochemistry Physics
Introduction to medicinal chemisry Med Chem I Mohammed Nooraldeen (PhD) كيمياء دوائية )1 ) محمد نورالدين محمود Introduction to medicinal chemistry Medicinal Chemistry Biology Medicine Biochemistry Physics
Saba Al Fayoumi. Tamer Barakat. Dr. Mamoun Ahram + Dr. Diala Abu-Hassan
 1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
1 Saba Al Fayoumi Tamer Barakat Dr. Mamoun Ahram + Dr. Diala Abu-Hassan What is BIOCHEMISTRY??? Biochemistry = understanding life Chemical reactions are what makes an organism (An organism is simply atoms
Ping-Chiang Lyu. Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University.
 Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
Pharmacophore-based Drug design Ping-Chiang Lyu Institute of Bioinformatics and Structural Biology, Department of Life Science, National Tsing Hua University 96/08/07 Outline Part I: Analysis The analytical
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE PATENT NO.: 5,935,946 ISSUED: Aug. 10, 1999 TO: FOR: Munger, Jr. et al. NUCLEOTIDE ANALOG COMPOSITION AND SYNTHESIS METHOD ATTACHMENT TO FORM PTO 1465,
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE PATENT NO.: 5,935,946 ISSUED: Aug. 10, 1999 TO: FOR: Munger, Jr. et al. NUCLEOTIDE ANALOG COMPOSITION AND SYNTHESIS METHOD ATTACHMENT TO FORM PTO 1465,
2 4 Chemical Reactions and Enzymes Slide 1 of 34
 2 4 Chemical Reactions and Enzymes 1 of 34 Chemical Reactions Chemical Reactions A chemical reaction is a process that changes one set of chemicals into another set of chemicals. Some chemical reactions
2 4 Chemical Reactions and Enzymes 1 of 34 Chemical Reactions Chemical Reactions A chemical reaction is a process that changes one set of chemicals into another set of chemicals. Some chemical reactions
Introduction to medicinal chemisry
 2017 1 st edition Introduction to medicinal chemisry Med Chem I Mohammed Nooraldeen (PhD) كيمياء دوائية )1 ) محمد نورالدين محمود Introduction to medicinal chemistry Medicinal Chemistry Biology Medicine
2017 1 st edition Introduction to medicinal chemisry Med Chem I Mohammed Nooraldeen (PhD) كيمياء دوائية )1 ) محمد نورالدين محمود Introduction to medicinal chemistry Medicinal Chemistry Biology Medicine
Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR
 Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction Structure-Activity Relationship (SAR) - similar
Medicinal Chemistry/ CHEM 458/658 Chapter 3- SAR and QSAR Bela Torok Department of Chemistry University of Massachusetts Boston Boston, MA 1 Introduction Structure-Activity Relationship (SAR) - similar
Stephen McDonald, Mark D. Wrona, Jeff Goshawk Waters Corporation, Milford, MA, USA INTRODUCTION
 Combined with a Deep Understanding of Complex Metabolic Routes to Increase Efficiency in the Metabolite Identification Search Stephen McDonald, Mark D. Wrona, Jeff Goshawk Waters Corporation, Milford,
Combined with a Deep Understanding of Complex Metabolic Routes to Increase Efficiency in the Metabolite Identification Search Stephen McDonald, Mark D. Wrona, Jeff Goshawk Waters Corporation, Milford,
COLEMAN SUDOL SAPONE P.C.
 COLEMAN SUDOL SAPONE P.C. The America Invents Act s To U.S. Patent Practice The America Invents Act which became law on September 16, 2011 will affect our patent practice in a number of ways. FEE INCREASE
COLEMAN SUDOL SAPONE P.C. The America Invents Act s To U.S. Patent Practice The America Invents Act which became law on September 16, 2011 will affect our patent practice in a number of ways. FEE INCREASE
Site-Specific Prodrug Release Using Visible Light
 Site-Specific Prodrug Release Using Visible Light Michael Y. Jiang and David Dolphin* J. Am. Chem. Soc., 2008, 130, 4236-4237 Li Zhang Current literature presentation 04-26-2008 Li Zhang @ Wipf Group Page
Site-Specific Prodrug Release Using Visible Light Michael Y. Jiang and David Dolphin* J. Am. Chem. Soc., 2008, 130, 4236-4237 Li Zhang Current literature presentation 04-26-2008 Li Zhang @ Wipf Group Page
Chapter 5 Ground Rules of Metabolism Sections 1-5
 Chapter 5 Ground Rules of Metabolism Sections 1-5 5.1 A Toast to Alcohol Dehydrogenase In the liver, the enzyme alcohol dehydrogenase breaks down toxic ethanol to acetaldehyde, an organic molecule even
Chapter 5 Ground Rules of Metabolism Sections 1-5 5.1 A Toast to Alcohol Dehydrogenase In the liver, the enzyme alcohol dehydrogenase breaks down toxic ethanol to acetaldehyde, an organic molecule even
Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp
![Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp](/thumbs/74/70848503.jpg) Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp. 216-225 Updated on: 2/4/07 at 9:00 pm Key Concepts Kinetics is the study of reaction rates. Study of enzyme kinetics
Lecture 11: Enzymes: Kinetics [PDF] Reading: Berg, Tymoczko & Stryer, Chapter 8, pp. 216-225 Updated on: 2/4/07 at 9:00 pm Key Concepts Kinetics is the study of reaction rates. Study of enzyme kinetics
Chapter 5. Energy Flow in the Life of a Cell
 Chapter 5 Energy Flow in the Life of a Cell Including some materials from lectures by Gregory Ahearn University of North Florida Ammended by John Crocker Copyright 2009 Pearson Education, Inc.. Review
Chapter 5 Energy Flow in the Life of a Cell Including some materials from lectures by Gregory Ahearn University of North Florida Ammended by John Crocker Copyright 2009 Pearson Education, Inc.. Review
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ARKEMA FRANCE, Petitioner,
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ARKEMA FRANCE, Petitioner, v. HONEYWELL INTERNATIONAL, INC., Patent Owner. IPR No. IPR2015-00916 U.S. Patent No. 8,710,282
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ARKEMA FRANCE, Petitioner, v. HONEYWELL INTERNATIONAL, INC., Patent Owner. IPR No. IPR2015-00916 U.S. Patent No. 8,710,282
Principles of Drug Design
 (16:663:502) Instructors: Longqin Hu and John Kerrigan Direct questions and enquiries to the Course Coordinator: Longqin Hu For more current information, please check WebCT at https://webct.rutgers.edu
(16:663:502) Instructors: Longqin Hu and John Kerrigan Direct questions and enquiries to the Course Coordinator: Longqin Hu For more current information, please check WebCT at https://webct.rutgers.edu
Confirmation of In Vitro Nefazodone Metabolites using the Superior Fragmentation of the QTRAP 5500 LC/MS/MS System
 Confirmation of In Vitro Nefazodone Metabolites using the Superior Fragmentation of the QTRAP 5500 LC/MS/MS System Claire Bramwell-German, Elliott Jones and Daniel Lebre AB SCIEX, Foster City, California
Confirmation of In Vitro Nefazodone Metabolites using the Superior Fragmentation of the QTRAP 5500 LC/MS/MS System Claire Bramwell-German, Elliott Jones and Daniel Lebre AB SCIEX, Foster City, California
Measurement of Metabolic Stability Using SIM and Identification of Metabolites by Data Dependent Full-Scan MS/MS and CNL Scanning
 Application Note: 349 Measurement of Metabolic Stability Using SIM and Identification of Metabolites by Data Dependent Full-Scan MS/MS and CNL Scanning Peter B Ehmer, Ethirajulu Kantharaj, Katie De Wagter,
Application Note: 349 Measurement of Metabolic Stability Using SIM and Identification of Metabolites by Data Dependent Full-Scan MS/MS and CNL Scanning Peter B Ehmer, Ethirajulu Kantharaj, Katie De Wagter,
Chapter Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow,
 Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Chapter 6 6.1 Cells and the Flow of Energy A. Forms of Energy 1. Energy is capacity to do work; cells continually use energy to develop, grow, repair, reproduce, etc. 2. Kinetic energy is energy of motion;
Principles of Drug Design
 Advanced Medicinal Chemistry II Principles of Drug Design Tentative Course Outline Instructors: Longqin Hu and John Kerrigan Direct questions and enquiries to the Course Coordinator: Longqin Hu I. Introduction
Advanced Medicinal Chemistry II Principles of Drug Design Tentative Course Outline Instructors: Longqin Hu and John Kerrigan Direct questions and enquiries to the Course Coordinator: Longqin Hu I. Introduction
Targeted Covalent Inhibitors: A Risk-Benefit Perspective
 Targeted Covalent Inhibitors: A Risk-Benefit Perspective 2014 AAPS Annual Meeting and Exposition San Diego, CA, November 4, 2014 Thomas A. Baillie School of Pharmacy University of Washington Seattle, WA
Targeted Covalent Inhibitors: A Risk-Benefit Perspective 2014 AAPS Annual Meeting and Exposition San Diego, CA, November 4, 2014 Thomas A. Baillie School of Pharmacy University of Washington Seattle, WA
MITOCW enzyme_kinetics
 MITOCW enzyme_kinetics In beer and wine production, enzymes in yeast aid the conversion of sugar into ethanol. Enzymes are used in cheese-making to degrade proteins in milk, changing their solubility,
MITOCW enzyme_kinetics In beer and wine production, enzymes in yeast aid the conversion of sugar into ethanol. Enzymes are used in cheese-making to degrade proteins in milk, changing their solubility,
Chemistry Problem Set #9 Due on Thursday 11/15/18 in class.
 Chemistry 391 - Problem Set #9 Due on Thursday 11/15/18 in class. Name 1. There is a real enzyme called cocaine esterase that is produced in bacteria that live at the base of the coca plant. The enzyme
Chemistry 391 - Problem Set #9 Due on Thursday 11/15/18 in class. Name 1. There is a real enzyme called cocaine esterase that is produced in bacteria that live at the base of the coca plant. The enzyme
ADME SCREENING OF NOVEL 1,4-DIHYDROPYRIDINE DERIVATIVES
 ITERATIAL JURAL F RESEARC I PARMACY AD CEMISTRY Available online at www.ijrpc.com Research Article ADME SCREEIG F VEL 1,4-DIYDRPYRIDIE DERIVATIVES A. Asma samaunnisa 1*, CS. Venkataramana 1 and V Madhavan
ITERATIAL JURAL F RESEARC I PARMACY AD CEMISTRY Available online at www.ijrpc.com Research Article ADME SCREEIG F VEL 1,4-DIYDRPYRIDIE DERIVATIVES A. Asma samaunnisa 1*, CS. Venkataramana 1 and V Madhavan
TRAINING REAXYS MEDICINAL CHEMISTRY
 TRAINING REAXYS MEDICINAL CHEMISTRY 1 SITUATION: DRUG DISCOVERY Knowledge survey Therapeutic target Known ligands Generate chemistry ideas Chemistry Check chemical feasibility ELN DBs In-house Analyze
TRAINING REAXYS MEDICINAL CHEMISTRY 1 SITUATION: DRUG DISCOVERY Knowledge survey Therapeutic target Known ligands Generate chemistry ideas Chemistry Check chemical feasibility ELN DBs In-house Analyze
UNITED STATES DEPARTMENT OF COMMERCE PATENT AND TRADEMARK OFFICE Address: Commissioner of Patents and Trademarks Washington, D.C.
 UNITED STATES DEPARTMENT OF COMMERCE PATENT AND TRADEMARK OFFICE Address: Commissioner of Patents and Trademarks Washington, D.C. 20231 Control Number: 90/005,307 Filing Date: 03/30/99 Patent Under Examination:
UNITED STATES DEPARTMENT OF COMMERCE PATENT AND TRADEMARK OFFICE Address: Commissioner of Patents and Trademarks Washington, D.C. 20231 Control Number: 90/005,307 Filing Date: 03/30/99 Patent Under Examination:
Evidence for the Existence of Non-monotonic Dose-response: Does it or Doesn t it?
 Evidence for the Existence of Non-monotonic Dose-response: Does it or Doesn t it? Scott M. Belcher, PhD University of Cincinnati Department of Pharmacology and Cell Biophysics Evidence for the Existence
Evidence for the Existence of Non-monotonic Dose-response: Does it or Doesn t it? Scott M. Belcher, PhD University of Cincinnati Department of Pharmacology and Cell Biophysics Evidence for the Existence
Kinetics. Chapter 14. Chemical Kinetics
 Lecture Presentation Chapter 14 Yonsei University In kinetics we study the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light
Lecture Presentation Chapter 14 Yonsei University In kinetics we study the rate at which a chemical process occurs. Besides information about the speed at which reactions occur, kinetics also sheds light
MA/ST 810 Mathematical-Statistical Modeling and Analysis of Complex Systems
 MA/ST 810 Mathematical-Statistical Modeling and Analysis of Complex Systems Integrating Mathematical and Statistical Models Recap of mathematical models Models and data Statistical models and sources of
MA/ST 810 Mathematical-Statistical Modeling and Analysis of Complex Systems Integrating Mathematical and Statistical Models Recap of mathematical models Models and data Statistical models and sources of
Biology Slide 1 of 34
 Biology 1 of 34 2 4 Chemical Reactions and Enzymes 2 of 34 2 4 Chemical Reactions and Enzymes Chemical Reactions Chemical Reactions A chemical reaction is a process that changes one set of chemicals into
Biology 1 of 34 2 4 Chemical Reactions and Enzymes 2 of 34 2 4 Chemical Reactions and Enzymes Chemical Reactions Chemical Reactions A chemical reaction is a process that changes one set of chemicals into
Energy, Enzymes, and Metabolism. Energy, Enzymes, and Metabolism. A. Energy and Energy Conversions. A. Energy and Energy Conversions
 Energy, Enzymes, and Metabolism Lecture Series 6 Energy, Enzymes, and Metabolism B. ATP: Transferring Energy in Cells D. Molecular Structure Determines Enzyme Fxn Energy is the capacity to do work (cause
Energy, Enzymes, and Metabolism Lecture Series 6 Energy, Enzymes, and Metabolism B. ATP: Transferring Energy in Cells D. Molecular Structure Determines Enzyme Fxn Energy is the capacity to do work (cause
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ARKEMA FRANCE, Petitioner,
 UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ARKEMA FRANCE, Petitioner, v. HONEYWELL INTERNATIONAL, INC., Patent Owner. IPR No. IPR2015-00917 U.S. Patent No. 8,710,282
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD ARKEMA FRANCE, Petitioner, v. HONEYWELL INTERNATIONAL, INC., Patent Owner. IPR No. IPR2015-00917 U.S. Patent No. 8,710,282
SAR. Structure - Activity Relationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny, uhľovodíky) 2/28/2016
 Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Structure Activity elationships (SA) identifies which functional groups are important for binding and activity SA Structure - Activity elationships (alkoholy, amíny, aldehydy, ketóny, estery, amidy, kyseliny,
Life Sciences 1a Lecture Slides Set 10 Fall Prof. David R. Liu. Lecture Readings. Required: Lecture Notes McMurray p , O NH
 Life ciences 1a Lecture lides et 10 Fall 2006-2007 Prof. David R. Liu Lectures 17-18: The molecular basis of drug-protein binding: IV protease inhibitors 1. Drug development and its impact on IV-infected
Life ciences 1a Lecture lides et 10 Fall 2006-2007 Prof. David R. Liu Lectures 17-18: The molecular basis of drug-protein binding: IV protease inhibitors 1. Drug development and its impact on IV-infected
UNITED STATES OF AMERICA NATIONAL TRANSPORTATION SAFETY BOARD WASHINGTON, D.C.
 SERVED: June 12, 1998 NTSB Order No. EA-4667 UNITED STATES OF AMERICA NATIONAL TRANSPORTATION SAFETY BOARD WASHINGTON, D.C. Adopted by the NATIONAL TRANSPORTATION SAFETY BOARD at its office in Washington,
SERVED: June 12, 1998 NTSB Order No. EA-4667 UNITED STATES OF AMERICA NATIONAL TRANSPORTATION SAFETY BOARD WASHINGTON, D.C. Adopted by the NATIONAL TRANSPORTATION SAFETY BOARD at its office in Washington,
smb Doc 155 Filed 06/21/16 Entered 06/21/16 14:09:24 Main Document Pg 1 of 5
 Pg 1 of 5 Howard W. Kingsley, Esq. ROSENBERG & ESTIS, P.C. 733 Third Avenue New York, New York 10017 Telephone: (212) 687-6000 Facsimile: (212) 551-8484 Attorneys for Landlord-Creditor 900 Third Avenue,
Pg 1 of 5 Howard W. Kingsley, Esq. ROSENBERG & ESTIS, P.C. 733 Third Avenue New York, New York 10017 Telephone: (212) 687-6000 Facsimile: (212) 551-8484 Attorneys for Landlord-Creditor 900 Third Avenue,
Enzyme Enzymes are proteins that act as biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of
 Enzyme Enzyme Enzymes are proteins that act as biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme
Enzyme Enzyme Enzymes are proteins that act as biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme
Integration of SAS and NONMEM for Automation of Population Pharmacokinetic/Pharmacodynamic Modeling on UNIX systems
 Integration of SAS and NONMEM for Automation of Population Pharmacokinetic/Pharmacodynamic Modeling on UNIX systems Alan J Xiao, Cognigen Corporation, Buffalo NY Jill B Fiedler-Kelly, Cognigen Corporation,
Integration of SAS and NONMEM for Automation of Population Pharmacokinetic/Pharmacodynamic Modeling on UNIX systems Alan J Xiao, Cognigen Corporation, Buffalo NY Jill B Fiedler-Kelly, Cognigen Corporation,
1. (18) Multiple choice questions. Please place your answer on the line preceding each question.
 CEM 5720 ame KEY Exam 2 ctober 21, 2015 Read all questions carefully and attempt those questions you are sure of first. Remember to proof your work; art work carries as much importance as written responses.
CEM 5720 ame KEY Exam 2 ctober 21, 2015 Read all questions carefully and attempt those questions you are sure of first. Remember to proof your work; art work carries as much importance as written responses.
Finding the Nucleoli of Large Cooperative Games: A Disproof with Counter-Example
 Finding the Nucleoli of Large Cooperative Games: A Disproof with Counter-Example Holger I. MEINHARDT arxiv:1603.00226v1 [cs.gt] 1 Mar 2016 March 6, 2016 Nguyen and Thomas (2016) claimed that they have
Finding the Nucleoli of Large Cooperative Games: A Disproof with Counter-Example Holger I. MEINHARDT arxiv:1603.00226v1 [cs.gt] 1 Mar 2016 March 6, 2016 Nguyen and Thomas (2016) claimed that they have
Program for the rest of the course
 Program for the rest of the course 16.4 Enzyme kinetics 17.4 Metabolic Control Analysis 19.4. Exercise session 5 23.4. Metabolic Control Analysis, cont. 24.4 Recap 27.4 Exercise session 6 etabolic Modelling
Program for the rest of the course 16.4 Enzyme kinetics 17.4 Metabolic Control Analysis 19.4. Exercise session 5 23.4. Metabolic Control Analysis, cont. 24.4 Recap 27.4 Exercise session 6 etabolic Modelling
Free Energy. because H is negative doesn't mean that G will be negative and just because S is positive doesn't mean that G will be negative.
 Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
Biochemistry 462a Bioenergetics Reading - Lehninger Principles, Chapter 14, pp. 485-512 Practice problems - Chapter 14: 2-8, 10, 12, 13; Physical Chemistry extra problems, free energy problems Free Energy
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD SFC CO. LTD. Petitioner IDEMITSU KOSAN CO., LTD.
 IN THE UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD SFC CO. LTD. Petitioner v. IDEMITSU KOSAN CO., LTD. Patent Owner U.S. Patent No.: 8,334,648 Filing Date: May 4,
IN THE UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD SFC CO. LTD. Petitioner v. IDEMITSU KOSAN CO., LTD. Patent Owner U.S. Patent No.: 8,334,648 Filing Date: May 4,
Noncompartmental vs. Compartmental Approaches to Pharmacokinetic Data Analysis Paolo Vicini, Ph.D. Pfizer Global Research and Development David M.
 Noncompartmental vs. Compartmental Approaches to Pharmacokinetic Data Analysis Paolo Vicini, Ph.D. Pfizer Global Research and Development David M. Foster., Ph.D. University of Washington October 28, 2010
Noncompartmental vs. Compartmental Approaches to Pharmacokinetic Data Analysis Paolo Vicini, Ph.D. Pfizer Global Research and Development David M. Foster., Ph.D. University of Washington October 28, 2010
Parkinson's disease (PD) is a chronic and progressive neurological. disorder that affects the motor system and is characterized by tremor, rigidity,
 Chapter 2. Metabolism of Cyclic Tertiary Amines 2.1. MPTP - A Parkinsonian Inducing Agent Parkinson's disease (PD) is a chronic and progressive neurological disorder that affects the motor system and is
Chapter 2. Metabolism of Cyclic Tertiary Amines 2.1. MPTP - A Parkinsonian Inducing Agent Parkinson's disease (PD) is a chronic and progressive neurological disorder that affects the motor system and is
2054, Chap. 8, page 1
 2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
2054, Chap. 8, page 1 I. Metabolism: Energetics, Enzymes, and Regulation (Chapter 8) A. Energetics and work 1. overview a. energy = ability to do work (1) chemical, transport, mechanical (2) ultimate source
Chimica Farmaceutica (Insegnamento Integrato di Chimica e Biotecnologie Farmaceutiche) Drug design (2)
 (Insegnamento Integrato di Chimica e Biotecnologie Farmaceutiche) Drug design (2) Molecular Variations in Homologous Series: Vinylogues and Benzologues HOMOLOGOUS SERIES. Definition and classification
(Insegnamento Integrato di Chimica e Biotecnologie Farmaceutiche) Drug design (2) Molecular Variations in Homologous Series: Vinylogues and Benzologues HOMOLOGOUS SERIES. Definition and classification
Noncompartmental vs. Compartmental Approaches to Pharmacokinetic Data Analysis Paolo Vicini, Ph.D. Pfizer Global Research and Development David M.
 Noncompartmental vs. Compartmental Approaches to Pharmacokinetic Data Analysis Paolo Vicini, Ph.D. Pfizer Global Research and Development David M. Foster., Ph.D. University of Washington October 18, 2012
Noncompartmental vs. Compartmental Approaches to Pharmacokinetic Data Analysis Paolo Vicini, Ph.D. Pfizer Global Research and Development David M. Foster., Ph.D. University of Washington October 18, 2012
Metabolism and enzymes
 Metabolism and enzymes 4-11-16 What is a chemical reaction? A chemical reaction is a process that forms or breaks the chemical bonds that hold atoms together Chemical reactions convert one set of chemical
Metabolism and enzymes 4-11-16 What is a chemical reaction? A chemical reaction is a process that forms or breaks the chemical bonds that hold atoms together Chemical reactions convert one set of chemical
UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD. AVX CORPORATION Petitioner v. GREATBATCH, LTD.
 Paper No. UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD AVX CORPORATION Petitioner v. GREATBATCH, LTD. Patent Owner Patent No. 7,035,077 Issue Date: April 25, 2006
Paper No. UNITED STATES PATENT AND TRADEMARK OFFICE BEFORE THE PATENT TRIAL AND APPEAL BOARD AVX CORPORATION Petitioner v. GREATBATCH, LTD. Patent Owner Patent No. 7,035,077 Issue Date: April 25, 2006
Validation of the GastroPlus TM Software Tool and Applications
 Validation of the GastroPlus TM Software Tool and Applications Fagen Zhang and Leah Luna The Dow Chemical Company FZ/MB 01.11.11 Acknowledgements Michael Bartels Barun Bhhatarai (Novartis) Tyler Auernhammer
Validation of the GastroPlus TM Software Tool and Applications Fagen Zhang and Leah Luna The Dow Chemical Company FZ/MB 01.11.11 Acknowledgements Michael Bartels Barun Bhhatarai (Novartis) Tyler Auernhammer
