Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 31, April 12, 2006
|
|
|
- Kelly Bryant
- 5 years ago
- Views:
Transcription
1 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 31, April 12, 2006 (Some material in this lecture has been adapted from Cramer, C. J. Essentials of Computational Chemistry, Wiley, Chichester: 2002; pp ) Solved omework An extra electron would be expected to localize in the lowest-energy unoccupied molecular orbital (LUMO). In water, that is orbital 6. The appearance of orbital 6 is This orbital is antibonding between oxygen and the two hydrogen atoms, so filling it would be expected to lengthen the O bonds. A good way to think about the quantum mechanical rationale behind the lengthening of the bonds is that this decreases the curvature of the orbital wave function associated with the nodes (by moving the nodes further from one another) and thereby lowers the kinetic energy. The same argument (the decrease in kinetic energy associated with moving the nodes further from one another) suggests that one would expect the bond angle at oxygen to open up to be wider. The geometry of the water radical anion (from an independent computation) has bond lengths of Å and an O angle of deg. This agrees with our analysis based on the LUMO.
2 31-2 Other Computed Properties Partial Atomic Charges An enormous amount of chemical reactivity can ultimately be rationalized by a rather simple observation: positively charged things like to associate with negatively charged things and vice versa, but charges of like sign repel one another. This simple precept explains most acid/base reactions, bimolecular nucleophilic substitution (as well see in more detail below), ester hydrolysis, and many other reactions at a mechanistic level. As such, a key property about which chemists like to think is the so-called partial charge associated with an atom. Thus, for example, in formaldehyde, 2 C=O, we know that the C=O double bond is polarized in a way that makes the oxygen end more negative and the carbon end more positive, and thats why nucleophilic reagents add to the carbon atom of carbonyl groups. In principle, we might try to quantify that polarization by assigning partial charges to each atom (typically being fractional in magnitude, e.g., for C and 0.25 for O, ignoring the atoms for the moment). Part of the driving force for this conceit is that it allows one to conveniently ignore the wave character of the electrons and deal only with the pleasantly more particulate atoms, these atoms reflecting electronic distribution by the degree to which they carry positive or negative charge. From quantum mechanics, at least as associated with artree-fock calculations, we have a natural way to come up with quantitative charges on atoms because we have occupied orbitals that are made up of basis functions on different atoms. So, if basis functions on oxygen are used more than basis functions on carbon for the occupied orbitals, wed see more charge on the oxygen than the carbon. Lets look at this in a bit more detail. Dividing the electrons up and assigning them to specific atoms is called "population analysis". One of the first such schemes for such a partitioning was proposed by R. S. Mulliken in 1955, and this method of population analysis now bears his name. Conceptually, it is very simple, with the electrons being divided up amongst the atoms according to the degree to which different AO basis functions contribute to the overall wave function. Note that in restricted artree-fock theory we may compute the total number of electrons N as N = 2 occupied *! # " j ( rj )" j ( r j )dr j (31-1) j since each normalized, occupied molecular orbital ψ contains two electrons. If we now replace each ψ by its linear expansion in AO basis function we have
3 31-3 occupied # &# & N = 2! ) %! c jr " r ( r j )(%! c js " s ( r j )( dr j j r s occupied = 2!! ) c jr " r ( r j ) c js " s ( r j )dr j j r,s occupied# = 2! c 2 jr + c jr c js S & %!! rs ( j r r*s (31-2) From the last line of eq. 31-2, we see that we may divide the total number of electrons up into two sums, one including only squares of single AO basis functions, the other including products of two different AO basis functions. Clearly, electrons associated with only a single basis function (i.e., terms in the first sum in parentheses on the r.h.s. of the last line of eq. 31-2) should be thought of as belonging entirely to the atom on which that basis function resides. As for the second term, which represents the electrons shared between basis functions, Mulliken suggested that one might as well divide these up evenly between the two atoms on which basis functions r and s reside. If we follow this prescription and furthermore divide the basis functions up over atoms k so as to compute the atomic population N k, eq becomes occupied% ( 2 N k = 2!! c jr +! c jr c js S rs +! c jr c js S rs * (31-3) j & r"k r,s"k,r#s r"k,sk ) Note from the definition of the density matrix (eq. 29-3) that this can also be written N k = P rr + P rs S rs + P rs S rs (31-4) r"k r,s"k,r#s r"k,s%k Now, lets recall the density and overlap matrices for water. The overlap matrix weve already seen. The final density matrix is " % S = ! (31-5) # !0.313! &
4 31-4 " 2.106! % P = ! !0.015! ! (31-6) #!0.015! !0.539!0.470! & Lets compute the partial atomic charge for hydrogen atom a. The only basis function entirely on a is #6, so P 66 contributes electrons as belonging to a. There is only one basis function on a, so we dont need to worry about the second summation in eq As for the last term, we need P 61 S 61 + P 62 S 62 + P 63 S 63 + P 64 S 64 + P 65 S 65 + P 67 S 67 = = Thus, the total number of electrons on a is or We compute partial atomic charge q as q k = Z k! N k (31-7) For, Z = 1, so the partial atomic charge is = By symmetry it is clear that this will also be the charge on b, and, since the water molecule has a net charge of zero, it must be the case that the partial atomic charge on O is equal to the opposite of the sum of the charges on the atoms, or These charges are qualitatively as we expect them to be: oxygen is partially negative and the hydrogen atoms are partially positive. Mulliken population analysis is very simple and continues to be used extensively for assessing molecular polarity in quantum calculations. Other Computed Properties Multipole Moments In cartesian coordinates, the expectation values of multipole moment operators are computed as x k y l z m atoms electrons k l m =! Z i x i yi z i " # * ( r) x k l m * &! j yj z j )#( r)dr (31-8) i % j ( where the sum of k, l, and m determines the type of moment (0 = monopole, 1 = dipole, 2 = quadrupole, etc.), Z i is the nuclear charge on atom i, and the integration variable r contains the x, y, and z coordinates of all of the electrons j. When Ψ is expressed as a single Slater determinant, we may write
5 31-5 atoms electrons ( ) x k y l z m k l m =! Z i x i yi z i " * k l! # j ( rj ) x j yjz m j # j ( r j )dr j (31-9) i j where ψ j and r j are the molecular orbital occupied by electron j and its cartesian coordinate system, respectively. The simplest moment to evaluate is the monopole moment, which has only the component k=l=m=0, so that the operator becomes 1 and, independent of coordinate system, we have atoms electrons 1 =! Z i "! # * j ( r j )# j ( r j )dr j i j (31-10) atoms =! Z i " N i where N is the total number of electrons (the simplification of the second term on the r.h.s. follows from the normalization of the MOs). The monopole moment is thus the difference between the sum of the nuclear charges and the number of electrons, i.e., it is simply the molecular charge. For the dipole moment, there are three possible components: x, y, or z depending on which of k, l, or m is one (with the others set equal to zero). These are written µ x, µ y, and µ z. Experimentally, however, one rarely measures the separate components of the dipole moment, but rather the total magnitude, µ, which can be determined as µ = µ x 2 + µy 2 + µz 2 (31-11) The dipole moment measures the degree to which positive and negative charges are differentially distributed relative to one another, i.e., the overall molecular polarity. Thus, for instance, if the electronic wave function has a large amplitude at some positive x value while the nuclear charge is concentrated at some negative x value, inspection of eq indicates that the dipole moment in the x direction will be negative. If they are both concentrated at the same position and the total electronic charge is equal to the total nuclear charge, the first and second terms on the r.h.s. of eq cancel, and the dipole moment is zero. The figure below illustrates these concepts for the case of the water molecule.
6 31-6 y z O x The nuclear charges of the water molecule, here lying flat in the xy plane, are entirely at the nuclear positions. The electrons of the hydrogen atoms, however, are pulled to the positive x direction relative to the nuclei by bonding interactions with the more electronegative O atom (the polarization is exaggerated here by depicting the σ orbitals with surfaces that fail to encompass the nuclear positions). In addition, the oxygen atom contributes two electrons into its in-plane lone pair, the orbital for which is localized at large, positive values of x, while only contributing a single electron each to the σ orbitals, resulting in another net polarization of negative charge in the positive x direction. The sum of these and other effects is such that water has a dipole moment of 1.8 D in the direction indicated (parallel with the x axis by symmetry). Note that the out-of-plane p orbital may be thought of as canceling two protons in the oxygen nucleus when the dipole moment is computed, since it is circularly symmetric about the nucleus when projected into the xy plane (since it is also symmetric above and below the xy plane, as are all other orbitals, there is no z component to the dipole moment). Note that any movement of the origin will change the coordinates of all charges by the same amount; since the total amount of positive charge is equal to the total amount of negative charge in water, the dipole moment is unaffected by such an origin change, but this would not be true for a charged species. Our computed dipole moment for water at the F/STO-3G level (from evaluation of eq. 31-9, which we will leave to the digital computer) was 1.74 D, in outstanding agreement with the experimental moment of 1.8 D! Electrical moments are useful because at long distances from a molecule the total electronic distribution can be increasingly well represented as a truncated multipole expansion, and thus molecular interactions can be approximated as multipole-multipole interactions (charge-charge, charge-dipole, dipole-dipole, etc.), which are computationally particularly simple to evaluate. At short distances, however, the multipole expansion may be very slowly convergent, and the multipole approximation has less utility.
7 31-7 Other Computed Properties Molecular Electrostatic Potential A (truncated) multipole expansion is a computationally convenient single-center formalism that allows one to quantitatively compute the degree to which a positive or negative test charge is attracted to or repelled by the molecule that is being represented by the multipole expansion. This quantity, the molecular electrostatic potential (MEP), can be computed exactly for any position r as V MEP ( r) = nuclei % k Z k! " * r # r! r k ( ) 1 r! r # "( r #)d r # (31-12) Note that this assumes no polarization of the molecule in response to the test charge. The MEP is an observable, although in practice it is rather difficult to design appropriate experiments to measure it. The MEP is particularly useful when visualized on surfaces or in regions of space, since it provides information about local polarity. Typically, after having chosen some sort of region to be visualized, a color-coding convention is chosen to depict the MEP. For instance, the most negative potential is assigned to be red, the most positive potential is assigned to be blue, and the color spectrum is mapped to all other values by linear interpolation. If this is done on the molecular van der Waals surface, one can immediately discern regions of local negative and positive potential, which may be informative for purposes of predicting chemical reactivity. The below figure provides a particular example. N 2 Br NO 2 NO 2 Above is the MEP of the radical anion produced by one-electron reduction of the dinitroaromatic shown at left (an environmental contaminant from the dying of cloth). The spectrum is mapped so that red corresponds to maximum negative charge density and deep blue to minimum. This depiction indicates that the buildup of negative charge density is larger on the nitro group ortho to the amino group than on that para to N 2. Such polarization is consistent with the observed reactivity of the molecule under reducing conditions, where protonation of the more negative nitro group in water leads to
8 31-8 its ultimate reduction to an amino group but the para nitro group is not reduced. Quantum mechanics explains this selectivity. Other Computed Properties Frontier Molecular Orbital Reactivity A slight wrinkle on our above discussion vis a vis reactivity being explicable based on opposite-charge attraction is to consider how charge is most likely to flow from one molecule to another. If we think about this question for a moment, it should be clear that an electron is most easily removed from the highest occupied molecular orbital (OMO) of a nucleophile and added to the LUMO of an electrophile. Thus, in addition to recognizing that positively and negatively charged regions will tend to attract one another, we should recognize that that attraction will likely follow a spatial path mapped out by the OMO and LUMO of the reacting partners. Perhaps the most classic example of this analysis is the backside attack involved in nucleophilic substitution of alkyl halides via the S N 2 mechanism. For example F + Cl F + Cl From our analyses up to this point, and from our knowledge that halogen atoms are more electronegative than carbon, we would expect the carbon atom to bear a partial positive charge and the halogen atom a partial negative charge. This alone partly rationalizes why an incoming nucleophile like fluoride might prefer to attack the carbon atom from the back side. owever, if we consider that the alkyl halide is accepting a transfer of an electron across its framework to the chloride ion, we would expect that electron to move into the LUMO of the alkyl halide. That LUMO is depicted on the next page from a artree-fock calculation with a somewhat better basis set than STO-3G. Note that the LUMO has extensive amplitude behind the methyl group (in the backside attack region) so this trajectory is dictated in part by frontier orbital considerations. (Note also that the orbital is formally a σ* orbital between C and Cl, which is why the C Cl bond breaks when it becomes populated.) Such analysis is often helpful in understanding the reactivity of π systems, too. Thus, substituted aromatic rings have π-type OMOs and LUMOs that have larger amplitudes at some atoms than others, and their regioselective reactivity is dictated by these amplitudes and the nature of the other reagent either electrophile or nucleophile.
9 31-9 Figure. The lowest unoccupied molecular orbital (LUMO) of chloromethane. omework To be solved in class: In eq. 31-9, the nuclei contribute to multipole moments in a classical way: they are point charges multiplied by their cartesian coordinates. The total multipole moment then considers the electrons "positions" which, because electrons are quantum mechanically "smeared out", requires integration. What if we were simply to replace the full nuclear charges in eq with the partial atomic charges from Mulliken analysis and ignore the electronic term what is the dipole moment in that case? (int: the atomic unit of dipole moment is electron charge bohr. So, you already have charges in atomic units, but youll need to convert the cartesian coordinates of the atoms in water given in Lecture 29 to bohr. Youll also need to convert your atomic units dipole moment to the more standard
10 31-10 units of "debye" (D). Youll find the relevant conversion factors in the notes to Lecture 15.) To be turned in for possible grading Apr. 14: Instead of thinking about the electrons that belong to a single atom, it is sometimes interesting to ask about the electrons belonging to each basis function. It should be reasonably clear from inspection of eq that the population of an AO basis function r, as opposed to an atom, is N r = P rr + # s"r P rs S rs where r and s are AO basis functions. Compute the population of each basis function for water using the P and S matrices given above. What chemical interpretation(s) can be associated with your computed values?
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester Christopher J. Cramer. Lecture 30, April 10, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 20056 Christopher J. Cramer Lecture 30, April 10, 2006 Solved Homework The guess MO occupied coefficients were Occupied
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 20056 Christopher J. Cramer Lecture 30, April 10, 2006 Solved Homework The guess MO occupied coefficients were Occupied
Molecular Orbital Theory. Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions between two bonded atoms
 Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
( ) + ( ) + ( ) = 0.00
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 32, April 14, 2006 (Some material in this lecture has been adapted from Cramer, C.
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 32, April 14, 2006 (Some material in this lecture has been adapted from Cramer, C.
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi. Lecture 28, December 08, 2014
 Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi Lecture 28, December 08, 2014 Solved Homework Water, H 2 O, involves 2 hydrogen atoms and an oxygen
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi Lecture 28, December 08, 2014 Solved Homework Water, H 2 O, involves 2 hydrogen atoms and an oxygen
Population Analysis. Mulliken Population Analysis APPENDIX S
 APPENDIX S Population Analysis On p. 665, electronic density ρ is defined. If the wave function is a Slater determinant p. 397) and assuming the double occupancy of orbitals ϕ i, we have see 11.7) ρ r
APPENDIX S Population Analysis On p. 665, electronic density ρ is defined. If the wave function is a Slater determinant p. 397) and assuming the double occupancy of orbitals ϕ i, we have see 11.7) ρ r
Carbon Compounds. Chemical Bonding Part 2
 Carbon Compounds Chemical Bonding Part 2 Introduction to Functional Groups: Alkanes! Alkanes Compounds that contain only carbons and hydrogens, with no double or triple bonds.! Alkyl Groups A part of a
Carbon Compounds Chemical Bonding Part 2 Introduction to Functional Groups: Alkanes! Alkanes Compounds that contain only carbons and hydrogens, with no double or triple bonds.! Alkyl Groups A part of a
Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi. Lecture 27, December 5, 2014
 Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi Lecture 27, December 5, 2014 (Some material in this lecture has been adapted from Cramer, C. J.
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi Lecture 27, December 5, 2014 (Some material in this lecture has been adapted from Cramer, C. J.
Chemistry 3502 Physical Chemistry II (Quantum Mechanics) 3 Credits Fall Semester 2003 Christopher J. Cramer. Lecture 25, November 5, 2003
 Chemistry 3502 Physical Chemistry II (Quantum Mechanics) 3 Credits Fall Semester 2003 Christopher J. Cramer Lecture 25, November 5, 2003 (Some material in this lecture has been adapted from Cramer, C.
Chemistry 3502 Physical Chemistry II (Quantum Mechanics) 3 Credits Fall Semester 2003 Christopher J. Cramer Lecture 25, November 5, 2003 (Some material in this lecture has been adapted from Cramer, C.
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 22, March 20, 2006
 Chem 350/450 Physical Chemistry II Quantum Mechanics 3 Credits Spring Semester 006 Christopher J. Cramer Lecture, March 0, 006 Some material in this lecture has been adapted from Cramer, C. J. Essentials
Chem 350/450 Physical Chemistry II Quantum Mechanics 3 Credits Spring Semester 006 Christopher J. Cramer Lecture, March 0, 006 Some material in this lecture has been adapted from Cramer, C. J. Essentials
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA
 CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
CHAPTER 9 THEORY OF RESONANCE BY, G.DEEPA Conjugation in Alkadienes and Allylic Systems conjugation a series of overlapping p orbitals The Allyl Group allylic position is the next to a double bond 1 allyl
Visualization in Chemistry. Giovanni Morelli
 Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
Lecture 1: Chemistry of the Carbonyl Group
 Lecture 1: Chemistry of the Carbonyl Group bjectives: By the end of this lecture you will be able to: 1. identify and name all major carbonyl functional groups; 2. use a molecular orbital approach to describe
Lecture 1: Chemistry of the Carbonyl Group bjectives: By the end of this lecture you will be able to: 1. identify and name all major carbonyl functional groups; 2. use a molecular orbital approach to describe
Practice Hour Examination # 1-1
 CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-1 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-1 Monday,
CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-1 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-1 Monday,
Structure and Bonding of Organic Molecules
 Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Atomic and Molecular Orbitals
 7 Atomic and Molecular Orbitals Chemists have developed a variety of methods for describing electrons in molecules. Lewis structures are the most familiar. These drawings assign pairs of electrons either
7 Atomic and Molecular Orbitals Chemists have developed a variety of methods for describing electrons in molecules. Lewis structures are the most familiar. These drawings assign pairs of electrons either
with the larger dimerization energy also exhibits the larger structural changes.
 A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Chapter 4 Symmetry and Chemical Bonding
 Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 4 Symmetry and Chemical Bonding 4.1 Orbital Symmetries and Overlap 4.2 Valence Bond Theory and Hybrid Orbitals 4.3 Localized and Delocalized Molecular Orbitals 4.4 MX n Molecules with Pi-Bonding
Chapter 2 Structure and Properties of Organic Molecules. Advanced Bonding: Review
 hapter 2 Structure and Properties of Organic Molecules hemistry 231 Organic hemistry I Fall 2007 Advanced Bonding: Review Atomic Quantum Mechanics cannot explain how molecules like 4 form: Valence Bond
hapter 2 Structure and Properties of Organic Molecules hemistry 231 Organic hemistry I Fall 2007 Advanced Bonding: Review Atomic Quantum Mechanics cannot explain how molecules like 4 form: Valence Bond
Chem Spring, 2017 Assignment 5 - Solutions
 Page 1 of 10 Chem 370 - Spring, 2017 Assignment 5 - Solutions 5.1 Additional combinations are p z ± d z 2, p x ±d xz, and p y ±d yz. p z ± d z 2 p x ±d xz or p y ±d yz 5.2 a. Li 2 has the configuration
Page 1 of 10 Chem 370 - Spring, 2017 Assignment 5 - Solutions 5.1 Additional combinations are p z ± d z 2, p x ±d xz, and p y ±d yz. p z ± d z 2 p x ±d xz or p y ±d yz 5.2 a. Li 2 has the configuration
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory
 Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
LECTURE 2 STRUCTURE AND PROPERTIES OF ORGANIC MOLECULES
 LECTURE 2 STRUCTURE AND PROPERTIES OF ORGANIC MOLECULES 1. Atomic wave functions and orbitals. LCAO. The important thing to know is that atomic orbitals are represented by wave functions, and they have
LECTURE 2 STRUCTURE AND PROPERTIES OF ORGANIC MOLECULES 1. Atomic wave functions and orbitals. LCAO. The important thing to know is that atomic orbitals are represented by wave functions, and they have
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 17, March 1, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 17, March 1, 2006 (Some material in this lecture has been adapted from Cramer, C. J.
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 17, March 1, 2006 (Some material in this lecture has been adapted from Cramer, C. J.
The wavefunction that describes a bonding pair of electrons:
 4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2)
 REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2) 1.) Why do haloalkenes under go nucleophillic substitution whereas haloarenes under go electophillic substitution. Ans. Due to more electro negative
REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2) 1.) Why do haloalkenes under go nucleophillic substitution whereas haloarenes under go electophillic substitution. Ans. Due to more electro negative
General Physical Chemistry II
 General Physical Chemistry II Lecture 13 Aleksey Kocherzhenko October 16, 2014" Last time " The Hückel method" Ø Used to study π systems of conjugated molecules" Ø π orbitals are treated separately from
General Physical Chemistry II Lecture 13 Aleksey Kocherzhenko October 16, 2014" Last time " The Hückel method" Ø Used to study π systems of conjugated molecules" Ø π orbitals are treated separately from
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Organic Chemistry 6 th Edition Paula Yurkanis Bruice. Chapter 1. Electronic Structure and Bonding. Acids and Bases Pearson Education, Inc.
 Organic Chemistry 6 th Edition Paula Yurkanis Bruice Chapter 1 Electronic Structure and Bonding Acids and Bases 2011 Pearson Education, Inc. 1 Organic Chemistry Carbon-containing compounds were once considered
Organic Chemistry 6 th Edition Paula Yurkanis Bruice Chapter 1 Electronic Structure and Bonding Acids and Bases 2011 Pearson Education, Inc. 1 Organic Chemistry Carbon-containing compounds were once considered
Chem 232. Representation of Reaction Mechanisms. A Simple Guide to "Arrow Pushing"
 Chem 232 D. J. Wardrop wardropd@uic.edu Representation of Reaction Mechanisms A Simple Guide to "Arrow Pushing" 1. For a given reaction, draw out the structure of the reactants and reagents. Check that
Chem 232 D. J. Wardrop wardropd@uic.edu Representation of Reaction Mechanisms A Simple Guide to "Arrow Pushing" 1. For a given reaction, draw out the structure of the reactants and reagents. Check that
Constructing a MO of NH 3. Nitrogen AO symmetries are
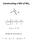 Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
Constructing a MO of NH 3 Nitrogen AO symmetries are To develop a MO scheme for NH 3 assume that only the 2s and2p orbitals of nitrogen interact with the hydrogen 1s orbitals (i.e., the nitrogen 1s orbital
11/30/ Substituent Effects in Electrophilic Substitutions. Substituent Effects in Electrophilic Substitutions
 Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Chapter 9 Problems: 9.1-29, 32-34, 36-37, 39-45, 48-56, 58-59, 61-69, 71-72. 9.8 Substituent effects in the electrophilic substitution of an aromatic ring Substituents affect the reactivity of the aromatic
Pauli Deformation APPENDIX Y
 APPENDIX Y Two molecules, when isolated say at infinite distance, are independent and the wave function of the total system might be taken as a product of the wave functions for the individual molecules.
APPENDIX Y Two molecules, when isolated say at infinite distance, are independent and the wave function of the total system might be taken as a product of the wave functions for the individual molecules.
CH 3 CHCH 3 CH 3 CHCH 3 Isopropyl cation. Oxomium ion intermediate. intermediate (an electrophile)
 Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
 Organic Chemistry I Dr Alex Roche Organic chemistry is the chemistry of Carbon and its compounds. Organic molecules constitute the essence of life (fats, sugars, proteins, DNA), and also permeate our everyday
Organic Chemistry I Dr Alex Roche Organic chemistry is the chemistry of Carbon and its compounds. Organic molecules constitute the essence of life (fats, sugars, proteins, DNA), and also permeate our everyday
Essential Organic Chemistry. Chapter 1
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 1 Electronic Structure and Covalent Bonding Periodic Table of the Elements 1.1 The Structure of an Atom Atoms have an internal structure consisting
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 1 Electronic Structure and Covalent Bonding Periodic Table of the Elements 1.1 The Structure of an Atom Atoms have an internal structure consisting
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Nucleophilic Addition Reactions of Carboxylic Acid Derivatives
 Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
ANNOUNCEMENTS. If you have questions about your exam 2 grade, write to me or Chapter 8 homework due April. 13 th.
 ANNOUNCEMENTS If you have questions about your exam 2 grade, write to me or Chem200@mail.sdsu.edu. Chapter 8 homework due April. 13 th. Chapter 9 home work due April. 20th. Exam 3 is 4/14 at 2 pm. LECTURE
ANNOUNCEMENTS If you have questions about your exam 2 grade, write to me or Chem200@mail.sdsu.edu. Chapter 8 homework due April. 13 th. Chapter 9 home work due April. 20th. Exam 3 is 4/14 at 2 pm. LECTURE
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Chapter 6 Chemical Reactivity and Mechanisms
 Chapter 6 Chemical Reactivity and Mechanisms 6.1 Enthalpy Enthalpy (ΔH or q) is the heat energy exchange between the reaction and its surroundings at constant pressure Breaking a bond requires the system
Chapter 6 Chemical Reactivity and Mechanisms 6.1 Enthalpy Enthalpy (ΔH or q) is the heat energy exchange between the reaction and its surroundings at constant pressure Breaking a bond requires the system
Problem Set #3 Solutions
 Problem Set #3 Solutions 1. a) C 3 (methyl group) Since carbon (E = 2.5) is slightly more electronegative than hydrogen (E = 2.2), there will be a small dipole moment pulling electron density away from
Problem Set #3 Solutions 1. a) C 3 (methyl group) Since carbon (E = 2.5) is slightly more electronegative than hydrogen (E = 2.2), there will be a small dipole moment pulling electron density away from
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 2014 Laura Gagliardi. Lecture 21, November 12, 2014
 Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 204 Laura Gagliardi Lecture 2, November 2, 204 (Some material in this lecture has been adapted from Cramer, C. J. Essentials
Chem 4502 Introduction to Quantum Mechanics and Spectroscopy 3 Credits Fall Semester 204 Laura Gagliardi Lecture 2, November 2, 204 (Some material in this lecture has been adapted from Cramer, C. J. Essentials
1.1 The Fundamental Chemistry of life
 1.1 The Fundamental Chemistry of life Matter makes up everything in the universe, including all living organisms. Matter is composed of elements, a pure substance that cannot be broken down into simpler
1.1 The Fundamental Chemistry of life Matter makes up everything in the universe, including all living organisms. Matter is composed of elements, a pure substance that cannot be broken down into simpler
Introduction to Alkenes and Alkynes
 Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Organic Chemistry. Review Information for Unit 1. Atomic Structure MO Theory Chemical Bonds
 Organic Chemistry Review Information for Unit 1 Atomic Structure MO Theory Chemical Bonds Atomic Structure Atoms are the smallest representative particle of an element. Three subatomic particles: protons
Organic Chemistry Review Information for Unit 1 Atomic Structure MO Theory Chemical Bonds Atomic Structure Atoms are the smallest representative particle of an element. Three subatomic particles: protons
and Ultraviolet Spectroscopy
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Chapter 2 Polar Covalent Bonds; Acids and Bases SAMPLE. Chapter Outline
 Chapter 2 Polar Covalent Bonds; Acids and Bases Chapter utline I. Polar covalent bonds (Sections 2.1 2.3). A. Electronegativity (Section 2.1). 1. Although some bonds are totally ionic and some are totally
Chapter 2 Polar Covalent Bonds; Acids and Bases Chapter utline I. Polar covalent bonds (Sections 2.1 2.3). A. Electronegativity (Section 2.1). 1. Although some bonds are totally ionic and some are totally
Lecture 21 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
Reaction mechanisms offer us insights into how reactions work / how molecules react with one another.
 Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6)
Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6)
Bonding and Physical Properties The Molecular Orbital Theory
 Bonding and Physical Properties The Molecular Orbital Theory Ø Developed by F. Hund and R. S. Mulliken in 1932 Ø Diagram of molecular energy levels Ø Magnetic and spectral properties Paramagnetic vs. Diamagnetic
Bonding and Physical Properties The Molecular Orbital Theory Ø Developed by F. Hund and R. S. Mulliken in 1932 Ø Diagram of molecular energy levels Ø Magnetic and spectral properties Paramagnetic vs. Diamagnetic
Chapter 2 Acids and Bases. Arrhenius Acid and Base Theory. Brønsted-Lowry Acid and Base Theory
 hapter 2 Acids and Bases A significant amount of chemistry can be described using different theories of acids and bases. We ll consider three different acid-base theories (listed in order of increasing
hapter 2 Acids and Bases A significant amount of chemistry can be described using different theories of acids and bases. We ll consider three different acid-base theories (listed in order of increasing
Valence Bond Theory Considers the interaction of separate atoms brought together as they form a molecule. Lewis structures Resonance considerations
 CHEM 511 chapter 2 page 1 of 11 Chapter 2 Molecular Structure and Bonding Read the section on Lewis dot structures, we will not cover this in class. If you have problems, seek out a general chemistry text.
CHEM 511 chapter 2 page 1 of 11 Chapter 2 Molecular Structure and Bonding Read the section on Lewis dot structures, we will not cover this in class. If you have problems, seek out a general chemistry text.
1. It can help us decide which of several Lewis dot structures is closest to representing the properties of the real compound.
 Molecular Structure Properties The electron was discovered in the year of 1900, and it took about twenty years for the electronic nature of the chemical bond to come into wide acceptance. Particle-based
Molecular Structure Properties The electron was discovered in the year of 1900, and it took about twenty years for the electronic nature of the chemical bond to come into wide acceptance. Particle-based
Physical Chemistry II Recommended Problems Chapter 12( 23)
 Physical Chemistry II Recommended Problems Chapter 1( 3) Chapter 1(3) Problem. Overlap of two 1s orbitals recprobchap1.odt 1 Physical Chemistry II Recommended Problems, Chapter 1(3), continued Chapter
Physical Chemistry II Recommended Problems Chapter 1( 3) Chapter 1(3) Problem. Overlap of two 1s orbitals recprobchap1.odt 1 Physical Chemistry II Recommended Problems, Chapter 1(3), continued Chapter
An Introduction to Hyperfine Structure and Its G-factor
 An Introduction to Hyperfine Structure and Its G-factor Xiqiao Wang East Tennessee State University April 25, 2012 1 1. Introduction In a book chapter entitled Model Calculations of Radiation Induced Damage
An Introduction to Hyperfine Structure and Its G-factor Xiqiao Wang East Tennessee State University April 25, 2012 1 1. Introduction In a book chapter entitled Model Calculations of Radiation Induced Damage
BSc. II 3 rd Semester. Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1
 BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
BSc. II 3 rd Semester Submitted By Dr. Sangita Nohria Associate Professor PGGCG-11 Chandigarh 1 Introduction to Alkyl Halides Alkyl halides are organic molecules containing a halogen atom bonded to an
8.1 Types of Chemical Bonds List and define three types of bonding. chapter 8 Bonding General Concepts.notebook. September 10, 2015
 chapter 8 Bonding General Concepts.notebook Chapter 8: Bonding: General Concepts Mar 13 11:15 AM 8.1 Types of Chemical Bonds List and define three types of bonding. Bonds are forces that hold groups of
chapter 8 Bonding General Concepts.notebook Chapter 8: Bonding: General Concepts Mar 13 11:15 AM 8.1 Types of Chemical Bonds List and define three types of bonding. Bonds are forces that hold groups of
Name. Chapter 4 covers acid-base chemistry. That should help you get going.
 Name Chapter 4 covers acid-base chemistry. That should help you get going. 1 Use curved arrows to illustrate the transfer of a proton (i.e. an + ) from benzoic acid to phenoxide, and draw the products.
Name Chapter 4 covers acid-base chemistry. That should help you get going. 1 Use curved arrows to illustrate the transfer of a proton (i.e. an + ) from benzoic acid to phenoxide, and draw the products.
ORGANIC CHEMISTRY. Meaning of Organic?
 ORGANIC CHEMISTRY Meaning of Organic? Initially scientists believed there was a special force in living organisms -this was assumed the unique component of organic material In 1828 Wöhler synthesized urea
ORGANIC CHEMISTRY Meaning of Organic? Initially scientists believed there was a special force in living organisms -this was assumed the unique component of organic material In 1828 Wöhler synthesized urea
Bonding - Ch. 7. Types of Bonding
 Types of Bonding I. holds everything together! II. All bonding occurs because of III. Electronegativity difference and bond character A. A between two atoms results in a when those two atoms form a bond.
Types of Bonding I. holds everything together! II. All bonding occurs because of III. Electronegativity difference and bond character A. A between two atoms results in a when those two atoms form a bond.
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 9, February 8, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 9, February 8, 2006 The Harmonic Oscillator Consider a diatomic molecule. Such a molecule
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 9, February 8, 2006 The Harmonic Oscillator Consider a diatomic molecule. Such a molecule
We can model covalent bonding in molecules in essentially two ways:
 CHEM 2060 Lecture 22: VB Theory L22-1 PART FIVE: The Covalent Bond We can model covalent bonding in molecules in essentially two ways: 1. Localized Bonds (retains electron pair concept of Lewis Structures)
CHEM 2060 Lecture 22: VB Theory L22-1 PART FIVE: The Covalent Bond We can model covalent bonding in molecules in essentially two ways: 1. Localized Bonds (retains electron pair concept of Lewis Structures)
hand and delocalization on the other, can be instructively exemplified and extended
 Text Related to Segment 8.0 00 Claude E. Wintner The ideas developed up to this point, concerning stereochemistry on the one hand and delocalization on the other, can be instructively exemplified and extended
Text Related to Segment 8.0 00 Claude E. Wintner The ideas developed up to this point, concerning stereochemistry on the one hand and delocalization on the other, can be instructively exemplified and extended
Earth Solid Earth Rocks Minerals Atoms. How to make a mineral from the start of atoms?
 Earth Solid Earth Rocks Minerals Atoms How to make a mineral from the start of atoms? Formation of ions Ions excess or deficit of electrons relative to protons Anions net negative charge Cations net
Earth Solid Earth Rocks Minerals Atoms How to make a mineral from the start of atoms? Formation of ions Ions excess or deficit of electrons relative to protons Anions net negative charge Cations net
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms
 Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
Overview of Types of Organic Reactions and Basic Concepts of Organic Reaction Mechanisms Dr. Solomon Derese 1 A chemical reaction is the transformation of one chemical or collection of chemicals into another
Chapter 2 Polar Covalent Bonds; Acids and Bases. Chapter Outline
 rganic Chemistry 9th Edition McMurry SLUTINS MANUAL Full clear download at: https://testbankreal.com/download/organic-chemistry-9th-edition-mcmurrysolutions-manual/ rganic Chemistry 9th Edition McMurry
rganic Chemistry 9th Edition McMurry SLUTINS MANUAL Full clear download at: https://testbankreal.com/download/organic-chemistry-9th-edition-mcmurrysolutions-manual/ rganic Chemistry 9th Edition McMurry
Molecular Orbitals. Based on Inorganic Chemistry, Miessler and Tarr, 4 th edition, 2011, Pearson Prentice Hall
 Molecular Orbitals Based on Inorganic Chemistry, Miessler and Tarr, 4 th edition, 2011, Pearson Prentice Hall Images from Miessler and Tarr Inorganic Chemistry 2011 obtained from Pearson Education, Inc.
Molecular Orbitals Based on Inorganic Chemistry, Miessler and Tarr, 4 th edition, 2011, Pearson Prentice Hall Images from Miessler and Tarr Inorganic Chemistry 2011 obtained from Pearson Education, Inc.
p Bonds as Electrophiles
 Chapter 7 p Bonds as Electrophiles REACTIONS OF CARBONYLS AND RELATED FUNCTIONAL GROUPS Copyright 2018 by Nelson Education Limited 1 7.2.1 Orbital structure of the carbonyl group Because oxygen is more
Chapter 7 p Bonds as Electrophiles REACTIONS OF CARBONYLS AND RELATED FUNCTIONAL GROUPS Copyright 2018 by Nelson Education Limited 1 7.2.1 Orbital structure of the carbonyl group Because oxygen is more
Alcohols, protons α to ketones. Aromatics, Amides. Acids, Aldehydes. Aliphatic. Olefins. ppm TMS
 Interpretation of 1 spectra So far we have talked about different NMR techniques and pulse sequences, but we haven t focused seriously on how to analyze the data that we obtain from these experiments.
Interpretation of 1 spectra So far we have talked about different NMR techniques and pulse sequences, but we haven t focused seriously on how to analyze the data that we obtain from these experiments.
Molecular Structure and Bonding- 2. Assis.Prof.Dr.Mohammed Hassan Lecture 3
 Molecular Structure and Bonding- 2 Assis.Prof.Dr.Mohammed Hassan Lecture 3 Hybridization of atomic orbitals Orbital hybridization was proposed to explain the geometry of polyatomic molecules. Covalent
Molecular Structure and Bonding- 2 Assis.Prof.Dr.Mohammed Hassan Lecture 3 Hybridization of atomic orbitals Orbital hybridization was proposed to explain the geometry of polyatomic molecules. Covalent
2. Polar Covalent Bonds: Acids and Bases
 2. Polar Covalent Bonds: Acids and Bases Based on McMurry s Organic Chemistry, 6 th edition, Chapter 2 2003 Ronald Kluger Department of Chemistry University of Toronto 2.1 Polar Covalent Bonds: Electronegativity!
2. Polar Covalent Bonds: Acids and Bases Based on McMurry s Organic Chemistry, 6 th edition, Chapter 2 2003 Ronald Kluger Department of Chemistry University of Toronto 2.1 Polar Covalent Bonds: Electronegativity!
Ch 2 Polar Covalent Bonds
 h 2 Polar ovalent Bonds Two primary bond types: ovalent (shared e -1 s) and Ionic (transferred e -1 s) Ionic bonds can have covalent character, such as with Na:l. An e -1 pair on l -1 can fill the 3s orbital
h 2 Polar ovalent Bonds Two primary bond types: ovalent (shared e -1 s) and Ionic (transferred e -1 s) Ionic bonds can have covalent character, such as with Na:l. An e -1 pair on l -1 can fill the 3s orbital
Section Practice Exam II Solutions
 Paul Bracher Chem 30 Section 7 Section Practice Exam II s Whether problems old r problems new, You d better practice, r you ll fail exam II. -- Anonymous TF Problem 1 a) Rank the following series of electrophiles
Paul Bracher Chem 30 Section 7 Section Practice Exam II s Whether problems old r problems new, You d better practice, r you ll fail exam II. -- Anonymous TF Problem 1 a) Rank the following series of electrophiles
Loudon Ch. 1 Review: Chemical Structure & Bonds Jacquie Richardson, CU Boulder Last updated 2/8/2018
 Organic chemistry focuses most heavily on the top three rows of the periodic table, plus a few elements from lower rows: H (1) He (2) Li (3) Be (4) B (5) C (6) N (7) O (8) F (9) Ne (10) Na (11) Mg (12)
Organic chemistry focuses most heavily on the top three rows of the periodic table, plus a few elements from lower rows: H (1) He (2) Li (3) Be (4) B (5) C (6) N (7) O (8) F (9) Ne (10) Na (11) Mg (12)
Introduction to Alkenes. Structure and Reactivity
 4 4 Introduction to Alkenes. Structure and Reactivity Alkenes are hydrocarbons that contain one or more carbon carbon double bonds. Alkenes are sometimes called olefins, particularly in the chemical industry.
4 4 Introduction to Alkenes. Structure and Reactivity Alkenes are hydrocarbons that contain one or more carbon carbon double bonds. Alkenes are sometimes called olefins, particularly in the chemical industry.
ANNOUNCEMENTS. If you have questions about your exam 2 grade, write to me or Chapter 7 homework due Nov, 9 th.
 ANNOUNCEMENTS If you have questions about your exam 2 grade, write to me or Chem200@sdsu.edu. Chapter 7 homework due Nov, 9 th. Chapter 8 homework due Nov. 16 th. Exam 3 is 11/17 at 2 pm. LECTURE OBJECTIVES
ANNOUNCEMENTS If you have questions about your exam 2 grade, write to me or Chem200@sdsu.edu. Chapter 7 homework due Nov, 9 th. Chapter 8 homework due Nov. 16 th. Exam 3 is 11/17 at 2 pm. LECTURE OBJECTIVES
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
362 Lecture 6 and 7. Spring 2017 Monday, Jan 30
 362 Lecture 6 and 7 Spring 2017 Monday, Jan 30 Quantum Numbers n is the principal quantum number, indicates the size of the orbital, has all positive integer values of 1 to (infinity) l is the angular
362 Lecture 6 and 7 Spring 2017 Monday, Jan 30 Quantum Numbers n is the principal quantum number, indicates the size of the orbital, has all positive integer values of 1 to (infinity) l is the angular
I5 ELECTROPHILIC SUBSTITUTIONS OF
 Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
AP Chemistry Chapter 7: Bonding
 AP Chemistry Chapter 7: Bonding Types of Bonding I. holds everything together! I All bonding occurs because of! Electronegativity difference and bond character A. A difference in electronegativity between
AP Chemistry Chapter 7: Bonding Types of Bonding I. holds everything together! I All bonding occurs because of! Electronegativity difference and bond character A. A difference in electronegativity between
Benzene a remarkable compound. Chapter 14 Aromatic Compounds. Some proposed structures for C 6 H 6. Dimethyl substituted benzenes are called xylenes
 Benzene a remarkable compound Chapter 14 Aromatic Compounds Discovered by Faraday 1825 Formula C 6 H 6 Highly unsaturated, but remarkably stable Whole new class of benzene derivatives called aromatic compounds
Benzene a remarkable compound Chapter 14 Aromatic Compounds Discovered by Faraday 1825 Formula C 6 H 6 Highly unsaturated, but remarkably stable Whole new class of benzene derivatives called aromatic compounds
4. Alkenes are nucleophiles and like to attack electrophiles, like protons, with positive charges:
 Mechanism Steps: Answer Key 1. Alcohols are nucleophilic and like to grab protons,, to form water molecules: 2. 3. 4. Alkenes are nucleophiles and like to attack electrophiles, like protons, with positive
Mechanism Steps: Answer Key 1. Alcohols are nucleophilic and like to grab protons,, to form water molecules: 2. 3. 4. Alkenes are nucleophiles and like to attack electrophiles, like protons, with positive
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chemical Bonding. The Octet Rule
 Chemical Bonding There are basically two types of chemical bonds: 1. Covalent bonds electrons are shared by more than one nucleus 2. Ionic bonds electrostatic attraction between ions creates chemical bond
Chemical Bonding There are basically two types of chemical bonds: 1. Covalent bonds electrons are shared by more than one nucleus 2. Ionic bonds electrostatic attraction between ions creates chemical bond
CHEMISTRY. Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para ipso attack, orientation in other ring systems.
 Subject Chemistry Paper No and Title Paper-5, Organic Chemistry-II Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para Module Tag CHE_P5_M29 TABLE OF CONTENTS 1. Learning Outcomes
Subject Chemistry Paper No and Title Paper-5, Organic Chemistry-II Module No and Title Module-, Electrophilic Aromatic Substitution: The ortho/para Module Tag CHE_P5_M29 TABLE OF CONTENTS 1. Learning Outcomes
Chemistry 2. Lecture 1 Quantum Mechanics in Chemistry
 Chemistry 2 Lecture 1 Quantum Mechanics in Chemistry Your lecturers 8am Assoc. Prof Timothy Schmidt Room 315 timothy.schmidt@sydney.edu.au 93512781 12pm Assoc. Prof. Adam J Bridgeman Room 222 adam.bridgeman@sydney.edu.au
Chemistry 2 Lecture 1 Quantum Mechanics in Chemistry Your lecturers 8am Assoc. Prof Timothy Schmidt Room 315 timothy.schmidt@sydney.edu.au 93512781 12pm Assoc. Prof. Adam J Bridgeman Room 222 adam.bridgeman@sydney.edu.au
Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals.
 Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
There are two main electronic effects that substituents can exert:
 Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
Substituent Effects There are two main electronic effects that substituents can exert: RESONANCE effects are those that occur through the π system and can be represented by resonance structures. These
6-2 This exercise is worked out on page 220 as "Working with Concepts".
 Copyright 2009 James K Whitesell 6-1 Although we can approach this exercise from a chemical perspective, one can also teach a non-chemist how to derive the answer once the name of the starting material
Copyright 2009 James K Whitesell 6-1 Although we can approach this exercise from a chemical perspective, one can also teach a non-chemist how to derive the answer once the name of the starting material
Lecture 26: Qualitative Molecular Orbital Theory: Hückel Theory
 MASSACHUSETTS INSTITUTE OF TECHNOLOGY 5.6 Physical Chemistry I Fall, 07 Professor Robert W. Field Lecture 6: Qualitative Molecular Orbital Theory: Hückel Theory Models in Physical Chemistry Our job is
MASSACHUSETTS INSTITUTE OF TECHNOLOGY 5.6 Physical Chemistry I Fall, 07 Professor Robert W. Field Lecture 6: Qualitative Molecular Orbital Theory: Hückel Theory Models in Physical Chemistry Our job is
Synthesis Using Aromatic Materials
 Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
Chapter 10 Synthesis Using Aromatic Materials ELECTROPHILIC AROMATIC SUBSTITUTION AND DIRECTED ORTHO METALATION Copyright 2018 by Nelson Education Limited 1 10.2 p Bonds Acting as Nucleophiles Copyright
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Writing a Correct Mechanism
 Chapter 2 1) Balancing Equations Writing a Correct Mechanism 2) Using Arrows to show Electron Movement 3) Mechanisms in Acidic and Basic Media 4) Electron rich Species: Nucleophile or Base? 5) Trimolecular
Chapter 2 1) Balancing Equations Writing a Correct Mechanism 2) Using Arrows to show Electron Movement 3) Mechanisms in Acidic and Basic Media 4) Electron rich Species: Nucleophile or Base? 5) Trimolecular
HOMEWORK PROBLEMS: POLAR BONDS, RESONANCE, ACIDS & BASES 1. Which of the following molecules is the most polar?
 CEM 31 MEWRK PRBLEMS: PLAR BDS, RESACE, ACIDS & BASES 1. Which of the following molecules is the most polar? 2. Trans-dichlorodifluoroethylene, C 2 Cl 2 2, has a number of polar bonds but no net dipole
CEM 31 MEWRK PRBLEMS: PLAR BDS, RESACE, ACIDS & BASES 1. Which of the following molecules is the most polar? 2. Trans-dichlorodifluoroethylene, C 2 Cl 2 2, has a number of polar bonds but no net dipole
CHEM3023: Spins, Atoms and Molecules
 CHEM3023: Spins, Atoms and Molecules Lecture 5 The Hartree-Fock method C.-K. Skylaris Learning outcomes Be able to use the variational principle in quantum calculations Be able to construct Fock operators
CHEM3023: Spins, Atoms and Molecules Lecture 5 The Hartree-Fock method C.-K. Skylaris Learning outcomes Be able to use the variational principle in quantum calculations Be able to construct Fock operators
