Water and sustainability
|
|
|
- Gyles Elwin Lawson
- 5 years ago
- Views:
Transcription
1 Water and sustainability Multiple Choice Test Answer key December, 5 th 2017
2 Answer key Biology questions Rate of flow of blood 1. D On average the total amount of blood running should stay the same (in fact slightly less in capillary vessels and vein due to run off of lymph through lymph vessels, but this influence can be neglected). So roughly the rate of flow will be a flipped version of the cross section area D is correct. Fermentation and Respiratory Quotient RQ 2. C Assume a mol is converted aerobically, so (0.5 a) mol glucose is converted anaerobically. This means that 6a mol CO 2 (aerobically) plus 2 (0.5 - a) mol CO 2 (anaerobically) is generated while 6a mol O 2 is used. Total amount generated mol CO 2 = 6a + 2 (0.5 - a) = 4a + 1 and this equals 1.8, so a = 0.2. In other words: 1.8 mol CO 2 is generated and 6 0.2=1.2 mol O 2 is used. Glucose concentration in blood 3. A Liver is essential as it releases glucose. So D is highest. From liver vein the blood flows into posterior (inferior) vena cava (C). There it is mixed with lower glucose containing blood stream coming from lower body parts. After right atrium and ventricle of the heart blood flows to lungs where some glucose is consumed. After that it flows back through pulmonary artery (B) to the heart. Next blood leaves the heart by aorta and flows partly to the brains (again some glucose consumption) and other body parts. From upper body finally blood returns to the heart by anterior (superior) vena cava (A). Sphagnum 4. C At some places (especially East part) Sphagnum squarrosum exist while ph<4.0, so conclusion I is wrong. Sphagnum recurvum and Sphagnum fibriatum are neighbors and do not mix. The boundary is sharp, but does not overlap with the ph boundaries. So competition must exist. Conclusion II is correct. The picture shows that S. squarrosum and S. recurvum exist in different ph ranges: S. squarrosum doesn t occur where ph<3, while S. recurvum doesn t exist where ph>4. So III is correct. answers page 2 of 8
3 5. A I Hay water II The beaker only contains heterotrophic organisms which are dissimilating and consuming organic materials. No organic compounds are produced by something like photosynthesis. So mass must decrease. The heterotrophic organisms are continuously using organic compounds, which will run out. Their number will gradually decrease to zero. A climax stadium will not be established. Determination of Caminalcules 6. D See table. Features present are indicated with for I up to VIII. With focusing only on long arms, long body and belly spots it is possible to identify all eight creatures. Fingers, only present in II and VII, are not needed. Feature I II III IV V VI VII VIII Long arms Long body Belly spots Fingers Water loss 7. B Diffusion of water through skin (do not mix up with sweating) is not influenced by body activity and must be constant. So this is II A and C are incorrect. Strenuous activity will result in extra air inhalation (breathing), causing extra water loss by lung ventilation. This must be process I. Meanwhile this extra water loss will result in reduction of urine production (process III). Temperature-sensitive alleles 8. A Genotype F1 is: ¼ EE + ½ Ee + ¼ ee, but EE will not develop at 19 C, so F1 offspring is ⅔ Ee and ⅓ ee. The combination EE again will not develop. The fraction of genotype EE in F2 will be: ½ ⅔ (Ee) ½ ⅔ (Ee) = 1/9. DNA and evolutionary relationship 9. C A & D and C & E are most related (short distance) and must be close together in the dendogram. D & E and D & C must be far from each other in the dendogram as they are least related (i.e. largest distance). The only dendogram matching with these statements is C. answers page 3 of 8
4 Legionella 10. A ( )/3 = 157, an integer result, so the T at position 671 is the first base of a new codon. This codon is T T C on the coding strand, which is transcribed into RNA as U U C, which is translated into Phe. The next codon is A G T, which is transcribed into A G U and translated into Ser, so answer A is correct. answers page 4 of 8
5 Chemistry questions Photosynthesis by algae 11. B In photosynthesis carbon dioxide and water are converted and oxygen is formed. 12. D On the right side only neutral molecules are written. 1 HPO 4 2 and 16 NO 3 are needed to supply the P and N atoms in C 106 H 263 O 110 N 16 P. 1 HPO 4 2 and 16 NO 3 have 18 negative charges, so 18 positive charges at the left side are needed to obtain neutrality. Green chemistry 13. B The atom economy represents how much of the starting materials gets into the products. The higher the better. The E-factor represents how much waste is obtained. The lower the better. Determination of oxygen 14. C If the burette is not rinsed with the solution of sodium thiosulfate, this solution is diluted with the water from the inside of the burette. Because of this V thio will be too high and consequently the result. If there is air in the tap aperture of the burette, in the beginning of the titration the level of the solution in the burette goes down while no solution leaves the burette. Because of this V thio will be too high and consequently the result. 15. B V thio ml 0,0100 M sodium thiosulphate solution contains V thio mmol S 2 O 3 2 ; this has reacted with V thio mmol I 2 and to form this amount of I 2, in the first reaction V thio mmol O 2 has reacted with iodide. V thio mmol O 2 is V thio mg. This was dissolved in ml surface water. So the O 2 concentration in the surface water is V thio = 8.00 V thio mg/l. Fertilizer from urine 16. D From figure 1 it can be seen that at ph = 8 the predominant phosphate species is HPO 4 2 and from figure 2 it can be seen that at ph = 8 the predominant ammonium species is NH 4 +. answers page 5 of 8
6 Hydrogen fuel cell 17. C In an electrochemical cell, the electrode where the oxidator, in this case oxygen, reacts is the positive electrode. The other electrode is the negative electrode. 18. C No CO 2 = ( 394) + ( 111) + ( 242) = +41 kj/mol. The reaction enthalpy is positive, so the reaction is endothermic. 19. A According to Le Chatelier s principle an increase in pressure leads to a decrease in the number of gas molecules. And an increase in temperature favors the endothermic reaction. Fertilizers 20. C All three fertilizers have the same amount of N atoms per mol. So the one with the least molar mass has the highest mass percentage of N. The molar masses are: (NH 4 ) 2 SO 4 : g/mol CaCN 2 : g/mol CO(NH 2 ) 2 : g/mol. answers page 6 of 8
7 Physics Solar Shower 21. C The amount of heat the water takes up is: Q = mcδt = (35 18) = J. The heating time can be calculated as: = s and that is = 1.5 h. Liquid and vapor 22. C Because ρ = m/v, the density of the vapor becomes 1000 times lower than the density of the liquid. It is easy to see that statement II is also correct. Hydro pneumatic suspension 23. B p = = Pa Heating paraffin 24. B The specific heat capacity of the solid and liquid paraffin is indicated by the slopes of the first and the last part of the graph, respectively. If the slope is steeper, the heat capacity is lower, so the heat capacity of liquid paraffin is higher than the heat capacity of solid paraffin, so statement I is false. During melting, the temperature is constant, so the kinetic energy of the molecules is constant, so only the potential energy of the molecules increases. N.B. The work done on the environment is neglected here. A little boat and a bottle in the river 25. B Because we are considering relative motions here, we can omit the motion of the river for the time being. In that case, the boat travels for 10 minutes in one direction followed by 10 minutes in the opposite direction after which it overtakes the bottle. In truth, the bottle has covered a distance of 3 km in 20 minutes, so the speed of the river is 3 km per 1/3 hour, so 9 km/h. Electric circuit 26. B When the slide is displaced towards X, the total resistance in the circuit increases. As a result, both the current in, and the voltage over Q decrease. The voltage across P must increase, because the sum of the voltages is equal to the voltage of the source. This means that the current in P will also increase. answers page 7 of 8
8 Super tanker 27. A The density of salt water is higher than the density of fresh water, so the buoyancy of a volume of salt water is higher than the buoyancy of the same volume of fresh water. Hence, the tanker will displace a larger volume of fresh water and will have a deeper draft in the river, which contains fresh water. 28. C Electricity storage E rot = Iω 2, with I = mr 2 Thus E = mr 2 ω 2 = = J. Sky crane 29. D The thrust force is pointed diagonally upwards and is larger than its vertical component. Together, the vertical components must equal the gravitational force F g. So F thrust > ¼F g Properties of water 30. C Because of its high heat capacity, a lot of heat is needed to increase the temperature of a volume of water and, the other way round, a lot of heat is emitted when it cools down. Thus, water limits temperature changes in its surroundings: statement I is correct. Because the density of water of C is greater than the density of water between 0 0 C and 4 0 C, the bottom of the ditch will contain water of C: statement 2 is also correct. answers page 8 of 8
Water and sustainability
 Water and sustainability Multiple hoice Test ecember, 5 th 2017 arefully read the EXMINTION RULES and EXM INSTRUTIONS EXMINTION RULES 1. You are NOT allowed to bring any personal items into the examination
Water and sustainability Multiple hoice Test ecember, 5 th 2017 arefully read the EXMINTION RULES and EXM INSTRUTIONS EXMINTION RULES 1. You are NOT allowed to bring any personal items into the examination
5 Energy from chemicals
 5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
1. A 2.48 g sample of a noble gas is stored in a 3.50 L vessel at 157 torr and 25 ºC. What is the identity of the gas?
 hemistry 11 Spring 2008 Examination #2 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
hemistry 11 Spring 2008 Examination #2 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Main Topic Sub-topics Students should be able to R O G
 Second Year Science Syllabus Biology Revision Check-list Where is your learning at? Green: I know it all. Orange: I have some idea check the answers. Red: I need to start studying this section. Main Topic
Second Year Science Syllabus Biology Revision Check-list Where is your learning at? Green: I know it all. Orange: I have some idea check the answers. Red: I need to start studying this section. Main Topic
IB Chemistry Solutions Gasses and Energy
 Solutions A solution is a homogeneous mixture it looks like one substance. An aqueous solution will be a clear mixture with only one visible phase. Be careful with the definitions of clear and colourless.
Solutions A solution is a homogeneous mixture it looks like one substance. An aqueous solution will be a clear mixture with only one visible phase. Be careful with the definitions of clear and colourless.
Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT?
 1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
1 Based on the kinetic molecular theory of gases, which one of the following statements is INCORRECT? A) The collisions between gas molecules are perfectly elastic. B) At absolute zero, the average kinetic
Buffer Titrations Lab
 Buffer Titrations Lab The Buffers of the Oceans We ve discussed the ability of a buffer to resist changes in ph. The efficacy of a buffer is dependent upon the ph of the solution different buffers are
Buffer Titrations Lab The Buffers of the Oceans We ve discussed the ability of a buffer to resist changes in ph. The efficacy of a buffer is dependent upon the ph of the solution different buffers are
Full file at Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
3.2 Calorimetry and Enthalpy
 3.2 Calorimetry and Enthalpy Heat Capacity Specific heat capacity (c) is the quantity of thermal energy required to raise the temperature of 1 g of a substance by 1 C. The SI units for specific heat capacity
3.2 Calorimetry and Enthalpy Heat Capacity Specific heat capacity (c) is the quantity of thermal energy required to raise the temperature of 1 g of a substance by 1 C. The SI units for specific heat capacity
Selected Questions on Chapter 5 Thermochemistry
 Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
11/4/2017. General Chemistry CHEM 101 (3+1+0) Dr. Mohamed El-Newehy. Chapter 4 Physical Properties of Solutions
 General Chemistry CHEM 11 (3+1+) Dr. Mohamed El-Newehy http://fac.ksu.edu.sa/melnewehy Chapter 4 Physical Properties of Solutions 1 Types of Solutions A solution is a homogenous mixture of 2 or more substances.
General Chemistry CHEM 11 (3+1+) Dr. Mohamed El-Newehy http://fac.ksu.edu.sa/melnewehy Chapter 4 Physical Properties of Solutions 1 Types of Solutions A solution is a homogenous mixture of 2 or more substances.
Science Year 10 Unit 1 Biology
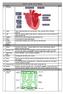 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
MOCK FINALS APPCHEN QUESTIONS
 MOCK FINALS APPCHEN QUESTIONS For questions 1-3 Aluminum dissolves in an aqueous solution of NaOH according to the following reaction: 2 NaOH + 2 Al + 2 H2O 2 NaAlO2 + 3 H2 If 84.1 g of NaOH and 51.0 g
MOCK FINALS APPCHEN QUESTIONS For questions 1-3 Aluminum dissolves in an aqueous solution of NaOH according to the following reaction: 2 NaOH + 2 Al + 2 H2O 2 NaAlO2 + 3 H2 If 84.1 g of NaOH and 51.0 g
CHEMISTRY HIGHER LEVEL
 *P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2007 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
*P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2007 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
2 Copyright Pearson Education, Inc., or its affiliates. All Rights Reserved.
 CHEMISTRY & YOU Chapter 17 Thermochemistry 17.1 The Flow of Energy 17. Measuring and Expressing Enthalpy Changes 17.3 Heat in Changes of State 17.4 Calculating Heats of Reaction Why does sweating help
CHEMISTRY & YOU Chapter 17 Thermochemistry 17.1 The Flow of Energy 17. Measuring and Expressing Enthalpy Changes 17.3 Heat in Changes of State 17.4 Calculating Heats of Reaction Why does sweating help
Chem 130 Name Exam 2 October 11, Points Part I: Complete all of problems 1-9
 Chem 130 Name Exam October 11, 017 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units and significant
Chem 130 Name Exam October 11, 017 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units and significant
2014 Academic Challenge Sectional Chemistry Exam Solution Set
 2014 Academic hallenge Sectional hemistry Exam Solution Set 1. E. A V-shaped molecule is possible in either the trigonal planar or the tetrahedral electron group geometry (A or B). 2. B. The fact that
2014 Academic hallenge Sectional hemistry Exam Solution Set 1. E. A V-shaped molecule is possible in either the trigonal planar or the tetrahedral electron group geometry (A or B). 2. B. The fact that
Name: Score: /100. Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each
 Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens. There are
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens. There are
4. Magnesium has three natural isotopes with the following masses and natural abundances:
 Exercise #1. Determination of Weighted Average Mass 1. The average mass of pennies minted after 1982 is 2.50 g and the average mass of pennies minted before 1982 is 3.00 g. Suppose that a bag of pennies
Exercise #1. Determination of Weighted Average Mass 1. The average mass of pennies minted after 1982 is 2.50 g and the average mass of pennies minted before 1982 is 3.00 g. Suppose that a bag of pennies
Slide 1 / Objects can possess energy as: (a) endothermic energy (b) potential energy (c) kinetic energy. a only b only c only a and c b and c
 Slide 1 / 84 1 Objects can possess energy as: (a) endothermic energy (b) potential energy (c) kinetic energy A B C D E a only b only c only a and c b and c Slide 2 / 84 2 The internal energy of a system
Slide 1 / 84 1 Objects can possess energy as: (a) endothermic energy (b) potential energy (c) kinetic energy A B C D E a only b only c only a and c b and c Slide 2 / 84 2 The internal energy of a system
Atoms, Elements, Atoms, Elements, Compounds and Mixtures. Compounds and Mixtures. Atoms and the Periodic Table. Atoms and the.
 Atoms, Elements, Compounds and Mixtures Explain how fractional distillation can be used to separate a mixture. 1 Atoms, Elements, Compounds and Mixtures Fractional distillation is used to separate components
Atoms, Elements, Compounds and Mixtures Explain how fractional distillation can be used to separate a mixture. 1 Atoms, Elements, Compounds and Mixtures Fractional distillation is used to separate components
Science 9-1 Grades Guidance
 U1 U2 U3 Science 9-1 Grades Guidance This guide is designed to help explain the level that a student is working at using examples of the curriculum that a student should be able to access. This is not
U1 U2 U3 Science 9-1 Grades Guidance This guide is designed to help explain the level that a student is working at using examples of the curriculum that a student should be able to access. This is not
Science Years 9 to 10
 Boardworks Contents Guide Boardworks Presentations: Acids and metal oxides 10 slides Reactions of metal oxides with acids. Adapting to changes 9 slides Ways that animals adapt to their habitats. Air pollution
Boardworks Contents Guide Boardworks Presentations: Acids and metal oxides 10 slides Reactions of metal oxides with acids. Adapting to changes 9 slides Ways that animals adapt to their habitats. Air pollution
CHEMISTRY 225 SEMESTER REACTION KINETICS
 CHEMISTRY 225 SEMESTER 01-2007 REACTION KINETICS 1) Dinitrogen pentoxide (N 2 O 5 ) decomposes slowly when in solution in tetrachloromethane to form nitrogen dioxide and oxygen. The reaction may be represented
CHEMISTRY 225 SEMESTER 01-2007 REACTION KINETICS 1) Dinitrogen pentoxide (N 2 O 5 ) decomposes slowly when in solution in tetrachloromethane to form nitrogen dioxide and oxygen. The reaction may be represented
General Chemistry I Final Exam 100 pts Fall 2010
 General Chemistry I Final Exam 100 pts Fall 2010 Name This is a closed-book exam: the only reference materials you may use are a periodic table of the elements, a table of enthalpies of formation, and
General Chemistry I Final Exam 100 pts Fall 2010 Name This is a closed-book exam: the only reference materials you may use are a periodic table of the elements, a table of enthalpies of formation, and
Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions 11 SUMMARY Section 2.1 Section 2.2 Section 2.3 Section 2.4 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive
Chapter 2 Water: The Solvent for Biochemical Reactions 11 SUMMARY Section 2.1 Section 2.2 Section 2.3 Section 2.4 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive
Standard enthalpies and standard Gibbs energies of formation 4. Calculating enthalpy changes and Gibbs energy changes for reactions 5
 Chemical Reactions as Sources of Energy Part 1: Thermodynamics Contents Standard enthalpies and standard Gibbs energies of formation 4 Calculating enthalpy changes and Gibbs energy changes for reactions
Chemical Reactions as Sources of Energy Part 1: Thermodynamics Contents Standard enthalpies and standard Gibbs energies of formation 4 Calculating enthalpy changes and Gibbs energy changes for reactions
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes
 Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Mr Chiasson Advanced Chemistry 12 / Chemistry 12 1 Unit B: Thermochemical Changes Students will be expected to: Compare the molar enthalpies of several combustion reactions involving organic compounds.
Buffer solutions Strong acids and bases dissociate completely and change the ph of a solution drastically. Buffers are solutions that resist changes i
 18.3 ph Curves Buffer solutions Strong acids and bases dissociate completely and change the ph of a solution drastically. Buffers are solutions that resist changes in ph even when acids and bases are added
18.3 ph Curves Buffer solutions Strong acids and bases dissociate completely and change the ph of a solution drastically. Buffers are solutions that resist changes in ph even when acids and bases are added
What is the volume of the unit cell of Ni in ml?
 P a g e 1 Chem 123 Practice Questions for EXAM II Fall 2014 Exam II on Mon 10/13/14 This HAS BEEN updated after Wed s lecture (10/8/14) JUST studying these questions is not sufficient preparation. There
P a g e 1 Chem 123 Practice Questions for EXAM II Fall 2014 Exam II on Mon 10/13/14 This HAS BEEN updated after Wed s lecture (10/8/14) JUST studying these questions is not sufficient preparation. There
Q1. (a) State what is meant by the term activation energy of a reaction. (1)
 Q1. (a) State what is meant by the term activation energy of a reaction. (c) State in general terms how a catalyst increases the rate of a chemical reaction. The curve below shows the Maxwell Boltzmann
Q1. (a) State what is meant by the term activation energy of a reaction. (c) State in general terms how a catalyst increases the rate of a chemical reaction. The curve below shows the Maxwell Boltzmann
Hazel Grove High School Year 10 Homework Book Term 2 Spring Focus Biology topic 8, Chemistry topic 7 and Physics topics 12/13.
 Hazel Grove High School Year 10 Homework Book Term 2 Spring Focus Biology topic 8, Chemistry topic 7 and Physics topics 12/13 Homework Score on Quiz Introduction to Science Homework Science Homework will
Hazel Grove High School Year 10 Homework Book Term 2 Spring Focus Biology topic 8, Chemistry topic 7 and Physics topics 12/13 Homework Score on Quiz Introduction to Science Homework Science Homework will
TECHNICAL SCIENCE DAS12703 ROZAINITA BT. ROSLEY PUSAT PENGAJIAN DIPLOMA UNVERSITI TUN HUSSEIN ONN MALAYSIA
 TECHNICAL SCIENCE DAS12703 ROZAINITA BT. ROSLEY PUSAT PENGAJIAN DIPLOMA UNVERSITI TUN HUSSEIN ONN MALAYSIA ii TABLE OF CONTENTS TABLE OF CONTENTS... i LIST OF FIGURES... iii Chapter 1... 4 SOLUTIONS...
TECHNICAL SCIENCE DAS12703 ROZAINITA BT. ROSLEY PUSAT PENGAJIAN DIPLOMA UNVERSITI TUN HUSSEIN ONN MALAYSIA ii TABLE OF CONTENTS TABLE OF CONTENTS... i LIST OF FIGURES... iii Chapter 1... 4 SOLUTIONS...
Quantitative Chemistry
 Quantitative Chemistry When we do experiments to measure something in Chemistry, we: Repeat experiments (usually 3 times) to improve the reliability of the results, by calculating an average of our results.
Quantitative Chemistry When we do experiments to measure something in Chemistry, we: Repeat experiments (usually 3 times) to improve the reliability of the results, by calculating an average of our results.
1. How many grams of gas are present in 1.50 L of hydrogen peroxide, H 2 O 2 (g), at STP?
 Chemistry 11 Fall 2010 Examination #2 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
Chemistry 11 Fall 2010 Examination #2 ANSWER KEY For the first portion of this exam, select the best answer choice for the questions below and mark the answers on your scantron. Then answer the free response
 Unit 1 SOME BASIC CONCEPTS OF CHEMISTRY I. Multiple Choice Questions (Type-I) 1. Two students performed the same experiment separately and each one of them recorded two readings of mass which are given
Unit 1 SOME BASIC CONCEPTS OF CHEMISTRY I. Multiple Choice Questions (Type-I) 1. Two students performed the same experiment separately and each one of them recorded two readings of mass which are given
Chapter 14. The Ideal Gas Law and Kinetic Theory
 Chapter 14 The Ideal Gas Law and Kinetic Theory 14.1 Molecular Mass, the Mole, and Avogadro s Number To facilitate comparison of the mass of one atom with another, a mass scale know as the atomic mass
Chapter 14 The Ideal Gas Law and Kinetic Theory 14.1 Molecular Mass, the Mole, and Avogadro s Number To facilitate comparison of the mass of one atom with another, a mass scale know as the atomic mass
CHEMpossible. 101 Exam 2 Review
 CHEMpossible 1. Circle each statement that applies to thermal energy and heat: a. Thermal energy is the average kinetic energy of its molecules due to their motion b. High thermal energy is reflected in
CHEMpossible 1. Circle each statement that applies to thermal energy and heat: a. Thermal energy is the average kinetic energy of its molecules due to their motion b. High thermal energy is reflected in
KS3 Science Levelness Posters
 KS Science Levelness Posters Contents Year Year 8 Year 9 A. Cells 8A. Food and digestion 9A. Inheritance and selection B. Reproduction 8B. Respiration 9B. Fit and healthy C. Environment and feeding 8C.
KS Science Levelness Posters Contents Year Year 8 Year 9 A. Cells 8A. Food and digestion 9A. Inheritance and selection B. Reproduction 8B. Respiration 9B. Fit and healthy C. Environment and feeding 8C.
Alkalosis or alkalemia arterial blood ph rises above Acidosis or acidemia arterial ph drops below 7.35 (physiological acidosis)
 Acid-Base Balance Normal ph of body fluids Arterial blood is 7.4 Venous blood and interstitial fluid is 7.35 Intracellular fluid is 7.0 Alkalosis or alkalemia arterial blood ph rises above 7.45 Acidosis
Acid-Base Balance Normal ph of body fluids Arterial blood is 7.4 Venous blood and interstitial fluid is 7.35 Intracellular fluid is 7.0 Alkalosis or alkalemia arterial blood ph rises above 7.45 Acidosis
SAMPLE CHEMISTRY QUESTIONS MIXTURE OF UNIT 3 & 4 MATERIALS
 SAMPLE CHEMISTRY QUESTIONS MIXTURE OF UNIT 3 & 4 MATERIALS QUESTION 1 The equation describing the production of butyl ethanoate is given below. Catalyst C4H 9OH CH 3COOH CH 3COOC 4H 9 H 2O( l ) 0.0500
SAMPLE CHEMISTRY QUESTIONS MIXTURE OF UNIT 3 & 4 MATERIALS QUESTION 1 The equation describing the production of butyl ethanoate is given below. Catalyst C4H 9OH CH 3COOH CH 3COOC 4H 9 H 2O( l ) 0.0500
What makes water so special?
 What makes water so special? Water A water molecule (H 2 O), is made up of three atoms --- one oxygen and two hydrogen. H O H Atom review Bonding review Animations on atomic bonding So what makes water
What makes water so special? Water A water molecule (H 2 O), is made up of three atoms --- one oxygen and two hydrogen. H O H Atom review Bonding review Animations on atomic bonding So what makes water
CHEMISTRY HIGHER LEVEL
 *P15* Pre-Leaving Certificate Examination, 2012 Triailscrúdú na hardteistiméireachta, 2012 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
*P15* Pre-Leaving Certificate Examination, 2012 Triailscrúdú na hardteistiméireachta, 2012 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions
Chemical Energetics. First Law of thermodynamics: Energy can be neither created nor destroyed but It can be converted from one form to another.
 Chemical Energetics First Law of thermodynamics: Energy can be neither created nor destroyed but It can be converted from one form to another. All chemical reactions are accompanied by some form of energy
Chemical Energetics First Law of thermodynamics: Energy can be neither created nor destroyed but It can be converted from one form to another. All chemical reactions are accompanied by some form of energy
DE LA SALLE SCHOOL LEARNING PROGRAMME YEAR 8. Half Term 1a
 Half Term a What are chemical reactions? Atoms, elements and compounds. Word equations, symbol equations and balancing equations. Making compounds and recognising that a chemical reaction has occurred.
Half Term a What are chemical reactions? Atoms, elements and compounds. Word equations, symbol equations and balancing equations. Making compounds and recognising that a chemical reaction has occurred.
Name: Score: /100. Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each
 Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Extension 17: Haemoglobin, the transport of oxygen in the blood and ph buffers in the blood
 Extension 17: Haemoglobin, the transport of oxygen in the blood and ph buffers in the blood 1. Prerequisites The ideas which form the background to this case study are listed in the following table. Topic
Extension 17: Haemoglobin, the transport of oxygen in the blood and ph buffers in the blood 1. Prerequisites The ideas which form the background to this case study are listed in the following table. Topic
CHEMISTRY 2b SUMMARY
 CHEMISTRY 2b SUMMARY Items in ITALLICS are HIGHER TIER NLY C2.4.1 RATES F REACTIN Speeding up, or slowing down, chemical reactions is important in everyday life and in industry The rate of a chemical reaction
CHEMISTRY 2b SUMMARY Items in ITALLICS are HIGHER TIER NLY C2.4.1 RATES F REACTIN Speeding up, or slowing down, chemical reactions is important in everyday life and in industry The rate of a chemical reaction
CHEMISTRY 30 Assessment Enthalpy Change and Calorimetry Formative
 CHEMISTRY 30 Assessment Enthalpy Change and Calorimetry Formative Record all responses in this book. Keep this question book and the answer key as part of your notes. 1. Open and closed systems can be
CHEMISTRY 30 Assessment Enthalpy Change and Calorimetry Formative Record all responses in this book. Keep this question book and the answer key as part of your notes. 1. Open and closed systems can be
Chemistry 6/15/2015. Outline. Why study chemistry? Chemistry is the basis for studying much of biology.
 Chemistry Biology 105 Lecture 2 Reading: Chapter 2 (pages 20-29) Outline Why study chemistry??? Elements Atoms Periodic Table Electrons Bonding Bonds Covalent bonds Polarity Ionic bonds Hydrogen bonding
Chemistry Biology 105 Lecture 2 Reading: Chapter 2 (pages 20-29) Outline Why study chemistry??? Elements Atoms Periodic Table Electrons Bonding Bonds Covalent bonds Polarity Ionic bonds Hydrogen bonding
Secondary School Mathematics & Science Competition Chemistry. Time allowed : 11:45 am - 1:00 pm (1hour 15 minutes) Total marks : 75
 Secondary School Mathematics & Science Competition 2014 Chemistry Date : 11 th May 2014 Total no. of pages : 18 Time allowed : 11:45 am - 1:00 pm (1hour 15 minutes) Total marks : 75 1. Write your Candidate
Secondary School Mathematics & Science Competition 2014 Chemistry Date : 11 th May 2014 Total no. of pages : 18 Time allowed : 11:45 am - 1:00 pm (1hour 15 minutes) Total marks : 75 1. Write your Candidate
CHEMISTRY HIGHER LEVEL
 *P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2008 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
*P15* PRE-LEAVING CERTIFICATE EXAMINATION, 2008 CHEMISTRY HIGHER LEVEL TIME: 3 HOURS 400 MARKS Answer eight questions in all These must include at least two questions from Section A All questions carry
Vocabulary Polar Covalent Bonds Hydrogen Bonds Surface Tension Adhesion Cohesion Specific Heat Heat of Vaporation Hydrophilic Hydrophobic Diffusion Dy
 NOTES: 2.2 Water and Diffusion Vocabulary Polar Covalent Bonds Hydrogen Bonds Surface Tension Adhesion Cohesion Specific Heat Heat of Vaporation Hydrophilic Hydrophobic Diffusion Dynamic Equilibrium Water
NOTES: 2.2 Water and Diffusion Vocabulary Polar Covalent Bonds Hydrogen Bonds Surface Tension Adhesion Cohesion Specific Heat Heat of Vaporation Hydrophilic Hydrophobic Diffusion Dynamic Equilibrium Water
CHEMISTRY HIGHER LEVEL
 L.34 PRE-LEAVING CERTIFICATE EXAMINATION, 2012 CHEMISTRY HIGHER LEVEL TIME : 3 HOURS 400 MARKS Answer eight questions in all. These must include at least two questions from Section A. All questions carry
L.34 PRE-LEAVING CERTIFICATE EXAMINATION, 2012 CHEMISTRY HIGHER LEVEL TIME : 3 HOURS 400 MARKS Answer eight questions in all. These must include at least two questions from Section A. All questions carry
CHEM111 UNIT 1 MOLES, FORMULAE AND EQUATIONS QUESTIONS
 Lesson 1 1. (a) Deduce the number of protons, neutrons and electrons in the following species: (i) 37 Cl - (ii) 1 H + (iii) 45 Sc 3+ (b) Write symbols for the following species: (i) 8 protons, 8 neutrons,
Lesson 1 1. (a) Deduce the number of protons, neutrons and electrons in the following species: (i) 37 Cl - (ii) 1 H + (iii) 45 Sc 3+ (b) Write symbols for the following species: (i) 8 protons, 8 neutrons,
Chemistry Grade : 11 Term-3/Final Exam Revision Sheet
 Chemistry Grade : 11 Term-3/Final Exam Revision Sheet Exam Date: Tuesday 12/6/2018 CCS:Chem.6a,6b,6c,6d,6e,6f,7a,7b,7d,7c,7e,7f,1g Chapter(12):Solutions Sections:1,2,3 Textbook pages 378 to 408 Chapter(16):Reaction
Chemistry Grade : 11 Term-3/Final Exam Revision Sheet Exam Date: Tuesday 12/6/2018 CCS:Chem.6a,6b,6c,6d,6e,6f,7a,7b,7d,7c,7e,7f,1g Chapter(12):Solutions Sections:1,2,3 Textbook pages 378 to 408 Chapter(16):Reaction
SUPPLEMENTAL HOMEWORK SOLUTIONS WEEK 8
 SUPPLEMETAL MEWRK SLUTIS WEEK 8 Assignment for Tuesday, March 7 th 7.36 a) + + b) + + c) 6 14 4 + 6 14 4 + + d) 6 5 3 7 + 6 5 7 + Be sure to write the correct charges for the products. 7.4 a) l + l + b)
SUPPLEMETAL MEWRK SLUTIS WEEK 8 Assignment for Tuesday, March 7 th 7.36 a) + + b) + + c) 6 14 4 + 6 14 4 + + d) 6 5 3 7 + 6 5 7 + Be sure to write the correct charges for the products. 7.4 a) l + l + b)
AP Chemistry Summer Review Assignment
 Name: Period: Chem I Teacher/year: AP Chemistry Summer Review Assignment Due on the FIRST DAY OF SCHOOL! A. Chemical Foundations 1. The beakers shown below have different precisions. a. Label the amount
Name: Period: Chem I Teacher/year: AP Chemistry Summer Review Assignment Due on the FIRST DAY OF SCHOOL! A. Chemical Foundations 1. The beakers shown below have different precisions. a. Label the amount
SC 119 PRACTICE Assessment - ANSWERS:
 SC 119 PRACTICE Assessment - ANSWERS: 1. Outdoor grilling is a very popular method of cooking. Propane is the gas that is commonly used in grills. Three things are required for a gas grill to ignite: gas,
SC 119 PRACTICE Assessment - ANSWERS: 1. Outdoor grilling is a very popular method of cooking. Propane is the gas that is commonly used in grills. Three things are required for a gas grill to ignite: gas,
Biochemistry. The study of chemical processes in living organisms. Introduction to Chemistry Properties of Water Acids and Bases.
 Biochemistry The study of chemical processes in living organisms. Introduction to Chemistry Properties of Water Acids and Bases Chemistry Of Life Matter Everything living AND non living is made up of matter.
Biochemistry The study of chemical processes in living organisms. Introduction to Chemistry Properties of Water Acids and Bases Chemistry Of Life Matter Everything living AND non living is made up of matter.
Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 CP Chem Review 2 Matching Match each item with the correct statement below. a. activated complex d. activation energy b. reaction rate e. free energy c. inhibitor 1. the minimum energy colliding particles
CP Chem Review 2 Matching Match each item with the correct statement below. a. activated complex d. activation energy b. reaction rate e. free energy c. inhibitor 1. the minimum energy colliding particles
Summary Chapter One 1. Scientific Method (know definitions). 2. Quantitative versus Qualitative measurements. 3. Accuracy versus Precision.
 Summary Chapter One 1. Scientific Method (know definitions). 2. Quantitative versus Qualitative measurements. 3. Accuracy versus Precision. 4. Systematic versus Random errors. 5. Significant Figures. 6.
Summary Chapter One 1. Scientific Method (know definitions). 2. Quantitative versus Qualitative measurements. 3. Accuracy versus Precision. 4. Systematic versus Random errors. 5. Significant Figures. 6.
Preparation of a Coordination Compound. Step 1 Copy the balanced equation for the preparation of FeC 2 O 4.. 3H2 O from FeC 2 O 4. Mass of watch glass
 Student Name Lab Partner Demonstrator Lab Section DATA SHEET Marking scheme Prelab exercise Lab performance Sig figs, units Calculations Crystals Preparation of a Coordination Compound Step 1 Copy the
Student Name Lab Partner Demonstrator Lab Section DATA SHEET Marking scheme Prelab exercise Lab performance Sig figs, units Calculations Crystals Preparation of a Coordination Compound Step 1 Copy the
To use calorimetry results to calculate the specific heat of an unknown metal. To determine heat of reaction ( H) from calorimetry measurements.
 Calorimetry PURPOSE To determine if a Styrofoam cup calorimeter provides adequate insulation for heat transfer measurements, to identify an unknown metal by means of its heat capacity and to determine
Calorimetry PURPOSE To determine if a Styrofoam cup calorimeter provides adequate insulation for heat transfer measurements, to identify an unknown metal by means of its heat capacity and to determine
Quantitative Chemistry. AQA Chemistry topic 3
 Quantitative Chemistry AQA Chemistry topic 3 3.1 Conservation of Mass and Balanced Equations Chemical Reactions A chemical reaction is when atoms are basically rearranged into something different. For
Quantitative Chemistry AQA Chemistry topic 3 3.1 Conservation of Mass and Balanced Equations Chemical Reactions A chemical reaction is when atoms are basically rearranged into something different. For
Acid-Base Titration Acetic Acid Content of Vinegar
 Acid-Base Titration Acetic Acid Content of Vinegar Prelab Assignment Read the entire lab. Write an objective and any hazards associated with this lab in your laboratory notebook. On a separate sheet of
Acid-Base Titration Acetic Acid Content of Vinegar Prelab Assignment Read the entire lab. Write an objective and any hazards associated with this lab in your laboratory notebook. On a separate sheet of
Discovering Design With Chemistry
 Discovering Design With Chemistry Preliminary Table of Contents Chapter 1: Measuring Up... 1 Introduction... 1 Measuring Distance... 1 Using Different Units... 2 Significant Figures... 4 Using Significant
Discovering Design With Chemistry Preliminary Table of Contents Chapter 1: Measuring Up... 1 Introduction... 1 Measuring Distance... 1 Using Different Units... 2 Significant Figures... 4 Using Significant
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
The student s results are shown in the table below. Time / minutes Time / minutes
 Q1.(a) Anhydrous calcium chloride is not used as a commercial de-icer because it reacts with water. The reaction with water is exothermic and causes handling problems. A student weighed out 1.00 g of anhydrous
Q1.(a) Anhydrous calcium chloride is not used as a commercial de-icer because it reacts with water. The reaction with water is exothermic and causes handling problems. A student weighed out 1.00 g of anhydrous
compared to gases. They are incompressible. Their density doesn t change with temperature. These similarities are due
 Liquids and solids They are similar compared to gases. They are incompressible. Their density doesn t change with temperature. These similarities are due to the molecules being close together in solids
Liquids and solids They are similar compared to gases. They are incompressible. Their density doesn t change with temperature. These similarities are due to the molecules being close together in solids
Chemistry 2 nd Semester Final Exam Review
 Chemistry 2 nd Semester Final Exam Review Chemical Bonds 1. Give a physical description of how the atoms and molecules are arranged in solids, liquids, and gases. A: In a liquid, the forces between the
Chemistry 2 nd Semester Final Exam Review Chemical Bonds 1. Give a physical description of how the atoms and molecules are arranged in solids, liquids, and gases. A: In a liquid, the forces between the
KWANTLEN UNIVERSITY COLLEGE DEPARTMENT OF CHEMISTRY
 KWANTLEN UNIVERSITY COLLEGE DEPARTMENT OF CHEMISTRY NAME: CHEM. 1210 FINAL EXAMINATION December 13, 2001 Time: 3 hours INSTRUCTIONS: 1. Read all questions thoroughly and answer each question completely.
KWANTLEN UNIVERSITY COLLEGE DEPARTMENT OF CHEMISTRY NAME: CHEM. 1210 FINAL EXAMINATION December 13, 2001 Time: 3 hours INSTRUCTIONS: 1. Read all questions thoroughly and answer each question completely.
Chemistry Lab Fairfax High School Invitational January 7, Team Number: High School: Team Members Names:
 Chemistry Lab Fairfax High School Invitational January 7, 2017 Team Number: High School: Team Members Names: Reference Values: Gas Constant, R = 8.314 J mol -1 K -1 Gas Constant, R = 0.08206 L atm mol
Chemistry Lab Fairfax High School Invitational January 7, 2017 Team Number: High School: Team Members Names: Reference Values: Gas Constant, R = 8.314 J mol -1 K -1 Gas Constant, R = 0.08206 L atm mol
Chem 1411 Practice Exam 2
 Chem 1411 Practice Exam 2 Instructions 1. Write your name on your exam. 2. You may use only the scratch paper and periodic table provided with the exam. You may also use a calculator, provided it cannot
Chem 1411 Practice Exam 2 Instructions 1. Write your name on your exam. 2. You may use only the scratch paper and periodic table provided with the exam. You may also use a calculator, provided it cannot
1.Study the statement above. Which cell organelle manages the process by which proteins are sorted and packaged to be sent where they are needed?
 Cell organelles carry out specific metabolic processes. 1.Study the statement above. Which cell organelle manages the process by which proteins are sorted and packaged to be sent where they are needed?
Cell organelles carry out specific metabolic processes. 1.Study the statement above. Which cell organelle manages the process by which proteins are sorted and packaged to be sent where they are needed?
Hashem Al-Dujaily. Hala Al Suqi. Mamoun + Diala. Tamer Barakat + Hashem Al-Dujaily
 3 Hashem Al-Dujaily Tamer Barakat + Hashem Al-Dujaily Hala Al Suqi Mamoun + Diala Last time we talked about the Ionization of water and then started talking about kw (ion product for water) which is a
3 Hashem Al-Dujaily Tamer Barakat + Hashem Al-Dujaily Hala Al Suqi Mamoun + Diala Last time we talked about the Ionization of water and then started talking about kw (ion product for water) which is a
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY
 5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY SUB-TOPIC 1.2 METHODS OF PURIFICATION AND ANALYSIS LEARNING
5072 CHEMISTRY (NEW PAPERS WITH SPA) TOPIC 1: EXPERIMENTAL CHEMISTRY 5067 CHEMISTRY (NEW PAPERS WITH PRACTICAL EXAM) TOPIC 1: EXPERIMENTAL CHEMISTRY SUB-TOPIC 1.2 METHODS OF PURIFICATION AND ANALYSIS LEARNING
1.4 Enthalpy. What is chemical energy?
 1.4 Enthalpy What is chemical energy? Chemical energy is a form of potential energy which is stored in chemical bonds. Chemical bonds are the attractive forces that bind atoms together. As a reaction takes
1.4 Enthalpy What is chemical energy? Chemical energy is a form of potential energy which is stored in chemical bonds. Chemical bonds are the attractive forces that bind atoms together. As a reaction takes
Exam 2--PHYS 151--Spring 2017
 Name: Exam 2--PHYS 151--Spring 2017 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Consider this diagram of a human heart. Which section is the right
Name: Exam 2--PHYS 151--Spring 2017 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Consider this diagram of a human heart. Which section is the right
Name Final Exam Spring 2002 Page (16 points) Acetylsalicylic acid, the molecule pictured here, is better known as aspirin.
 Name Final Exam Spring 2002 Page 1 1. (16 points) Acetylsalicylic acid, the molecule pictured here, is better known as aspirin. 1 A D 2 B 3 4 Describing the bonding in aspirin, acetylsalicylic acid. (a)
Name Final Exam Spring 2002 Page 1 1. (16 points) Acetylsalicylic acid, the molecule pictured here, is better known as aspirin. 1 A D 2 B 3 4 Describing the bonding in aspirin, acetylsalicylic acid. (a)
2019 Enrolment The 1st. Japan University Examination. Advanced Chemistry
 2019 Enrolment The 1st Japan University Examination Advanced Chemistry Examination Date: November 2017 (60 min) Do not open the examination booklet until the starting signal for the exam is given. Please
2019 Enrolment The 1st Japan University Examination Advanced Chemistry Examination Date: November 2017 (60 min) Do not open the examination booklet until the starting signal for the exam is given. Please
Organic Molecules: (All contain carbon) Inorganic Molecules: (Do NOT contain carbon)
 Organic Molecules: (All contain carbon) 1.) Carbohydrates: Quick source of energy 2.) Lipids: Long-term energy storage 3.) Proteins: Raw materials and enzyme action (catalysts) Inorganic Molecules: (Do
Organic Molecules: (All contain carbon) 1.) Carbohydrates: Quick source of energy 2.) Lipids: Long-term energy storage 3.) Proteins: Raw materials and enzyme action (catalysts) Inorganic Molecules: (Do
General Chemistry I, Unit I: Study Guide
 General Chemistry I, Unit I: Study Guide General Chemistry I Unit I 1 CDS Chapter 1: Atomic Molecular Theory Law of Conservation of Mass the total mass of all products of a chemical reaction is equal to
General Chemistry I, Unit I: Study Guide General Chemistry I Unit I 1 CDS Chapter 1: Atomic Molecular Theory Law of Conservation of Mass the total mass of all products of a chemical reaction is equal to
GAS FORMULAE THE GENERAL GAS EQUATION. 1 dm = 1000 ml = 1 L. 1cm = 1 ml
 GAS FORMULAE THE GENERAL GAS EQUATION PV = nrt Note Useful Conversions P = Pressure ( kpa ) 760 mmhg = 1 atmosphere V = Volume ( L ) = 101,35Pa = 101.35 kpa n = Amount (in mol) of gas ( mol) 1 1 R = Gas
GAS FORMULAE THE GENERAL GAS EQUATION PV = nrt Note Useful Conversions P = Pressure ( kpa ) 760 mmhg = 1 atmosphere V = Volume ( L ) = 101,35Pa = 101.35 kpa n = Amount (in mol) of gas ( mol) 1 1 R = Gas
4-4 Bioenergetics Biology
 4-4 Bioenergetics Biology.0 Figure shows a plant cell. Figure. Draw one line from each part of the cell to its function. [3 marks] Part of the cell Nucleus Chloroplast Mitochondria Function Where most
4-4 Bioenergetics Biology.0 Figure shows a plant cell. Figure. Draw one line from each part of the cell to its function. [3 marks] Part of the cell Nucleus Chloroplast Mitochondria Function Where most
 CHEMISTRY 110 EXAM 3 April 2, 2012 FORM A 1. Which plot depicts the correct relationship between the volume and number of moles of an ideal gas at constant pressure and temperature? 2. The height of the
CHEMISTRY 110 EXAM 3 April 2, 2012 FORM A 1. Which plot depicts the correct relationship between the volume and number of moles of an ideal gas at constant pressure and temperature? 2. The height of the
Take Home Semester 2 Practice Test for Acc Chem MM 15-16
 Take Home Semester 2 Practice Test for Acc Chem MM 15-16 Thermochemistry 1. Determine ΔHrxn. 2SO2(g) + O2(g) 2SO3(g) a) 98.9 b) 98.9 c) 197.8 d) 197.8 ΔHf o SO2(g) 296.8 kj/mol SO3(g) 395.7 kj/mol O2(g)
Take Home Semester 2 Practice Test for Acc Chem MM 15-16 Thermochemistry 1. Determine ΔHrxn. 2SO2(g) + O2(g) 2SO3(g) a) 98.9 b) 98.9 c) 197.8 d) 197.8 ΔHf o SO2(g) 296.8 kj/mol SO3(g) 395.7 kj/mol O2(g)
Lesson Plans Chapter 15: Solutions & Solution Chemistry
 Lesson Plans Chapter 15: Solutions & Solution Chemistry I. Solutions a. A solution is simply a homogeneous mixture i. Homogeneous: same throughout (it does not mean one ) ex: water + sugar, air, alloys,
Lesson Plans Chapter 15: Solutions & Solution Chemistry I. Solutions a. A solution is simply a homogeneous mixture i. Homogeneous: same throughout (it does not mean one ) ex: water + sugar, air, alloys,
June Which is a closed system? (A) burning candle (B) halogen lightbulb (C) hot water in a sink (D) ripening banana
 June 2005 28. Which is a closed system? burning candle halogen lightbulb hot water in a sink ripening banana 29. Which involves the greatest energy change? chemical reaction nuclear reaction phase change
June 2005 28. Which is a closed system? burning candle halogen lightbulb hot water in a sink ripening banana 29. Which involves the greatest energy change? chemical reaction nuclear reaction phase change
1. Draw a graph showing what happens to water when we take ice in a beaker and heat it up until it boils.
 i Name: Date: Block: This is a basic guide and does not include everything that we covered; do not use this as your only study tool. Use your notebook, worksheets, tests, notes, and other materials to
i Name: Date: Block: This is a basic guide and does not include everything that we covered; do not use this as your only study tool. Use your notebook, worksheets, tests, notes, and other materials to
Name Page 1. N a m e F o rm u l a Soluble in Wa t e r? BaSO 4. Li 2 CO 3. Cu(OH) 2
 Name Page 1 AQUEOUS SOLUTIONS 1. (10 points) Names, formulas, and solubility of compounds. N a m e F o rm u l a Soluble in Wa t e r? Ammonium chloride NH 4 Cl Yes BaS Barium phosphate Manganese(IV) oxide
Name Page 1 AQUEOUS SOLUTIONS 1. (10 points) Names, formulas, and solubility of compounds. N a m e F o rm u l a Soluble in Wa t e r? Ammonium chloride NH 4 Cl Yes BaS Barium phosphate Manganese(IV) oxide
Practice I: Chemistry IGCSE
 Practice I: Chemistry IGCSE cristian.obiol@gmail.com 1) Explain the following processes related to changes of states of matter. -Melting:... -Vaporization:... -Freezing:... -Condensation:... -Sublimation:...
Practice I: Chemistry IGCSE cristian.obiol@gmail.com 1) Explain the following processes related to changes of states of matter. -Melting:... -Vaporization:... -Freezing:... -Condensation:... -Sublimation:...
CHEMISTRY 101 EXAM 1 FORM 1N
 CHEMISTRY 101 EXAM 1 SECTIONS 572-580 Dr. Joy Heising FORM 1N September 20, 2001 Directions: 1. Fill out your scantron sheet. a. Do not forget to include your SIGNATURE and ID number. b. Dept = CHEM, Course
CHEMISTRY 101 EXAM 1 SECTIONS 572-580 Dr. Joy Heising FORM 1N September 20, 2001 Directions: 1. Fill out your scantron sheet. a. Do not forget to include your SIGNATURE and ID number. b. Dept = CHEM, Course
Chem 1411 Practice Exam 2
 Chem 1411 Practice Exam 2 Instructions 1. Write your name on your exam. 2. You may use only the scratch paper and periodic table provided with the exam. You may also use a calculator, provided it cannot
Chem 1411 Practice Exam 2 Instructions 1. Write your name on your exam. 2. You may use only the scratch paper and periodic table provided with the exam. You may also use a calculator, provided it cannot
Chemical Reaction Defn: Chemical Reaction: when starting chemical species form different chemicals.
 Chemical Reaction Defn: Chemical Reaction: when starting chemical species form different chemicals. Evidence to indicate that a chemical reaction has occurred: Temperature change Different coloured materials
Chemical Reaction Defn: Chemical Reaction: when starting chemical species form different chemicals. Evidence to indicate that a chemical reaction has occurred: Temperature change Different coloured materials
Redox Reactions. CH102, Lab 11 Goals : Safety : Waste :
 Redox Reactions CH102, Lab 11 Goals : Safety : Waste : To explore the relative oxidizing and reducing strength of metals. To measure electrochemical potentials and use the reduction potentials to rank
Redox Reactions CH102, Lab 11 Goals : Safety : Waste : To explore the relative oxidizing and reducing strength of metals. To measure electrochemical potentials and use the reduction potentials to rank
Name Class Date. As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings.
 Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Set 1 Structure of the atoms & Chemical Equation Perfect Score F Matter is anything that. and has.
 STRUCTURE OF THE ATOMS 1. Matter is anything that. and has. 2. The particle theory of matter state that matter is.. 3. Type of particle Example 4. Property Solid Liquid Gas Diagrammatic representation
STRUCTURE OF THE ATOMS 1. Matter is anything that. and has. 2. The particle theory of matter state that matter is.. 3. Type of particle Example 4. Property Solid Liquid Gas Diagrammatic representation
Chem 112 Exam 1 Version A Spring /16/ :00am/Odago, M. O.
 Chem 112 Exam 1 Version A Spring 2011 02/16/2011 10:00am/Odago, M. O. 1. The pressure of a certain gas is measured to be 25.1 mmhg. What is this pressure expressed in units of pascals? (1 atm=1.0125 x10
Chem 112 Exam 1 Version A Spring 2011 02/16/2011 10:00am/Odago, M. O. 1. The pressure of a certain gas is measured to be 25.1 mmhg. What is this pressure expressed in units of pascals? (1 atm=1.0125 x10
