Isotopes in Environmental and Health Studies
|
|
|
- Jesse Small
- 5 years ago
- Views:
Transcription
1 Isotopes in Environmental and Health Studies Continuous field measurements of δ 13 C-CO 2 and trace gases by FTIR spectroscopy J. Mohn *, M. J. Zeeman, R. A. Werner, W. Eugster and L. Emmenegger Empa, Swiss Federal Laboratories for Materials Testing and Research Laboratory for Air Pollution & Environmental Technology Ueberlandstrasse 129 CH-8600 Duebendorf Switzerland ETH Zurich Institute of Plant Sciences Universitaetsstrasse 2 CH-8092 Zurich Switzerland *Joachim Mohn Tel.: Fax: joachim.mohn@empa.ch Number of Pages: 21; Figures: 5; Tables: 0
2
3 1 Abstract Continuous analysis of the 13 C/ 12 C ratio of atmospheric CO 2 (δ 13 C-CO 2 ) is a powerful tool to quantify CO 2 flux strengths of the two major ecosystem processes assimilation and respiration. Traditional laboratory techniques such as isotope ratio mass spectrometry (IRMS) in combination with flask sampling are subject to technical limitations that do not allow to fully characterize variations of atmospheric δ 13 C-CO 2 at all relevant timescales. In our study we demonstrate the strength of Fourier transform infrared spectroscopy (FTIR) in combination with a PLS-based calibration strategy for online analysis of δ 13 C- CO 2 in ambient air. The ability of the instrument to measure δ 13 C-CO 2 was tested on a grassland field-site and compared to standard laboratory-based IRMS measurements made on field-collected flask samples. Both methods were in excellent agreement with an average difference of 0.4 (n = 81). Simultaneously, other important trace gases such as CO, N 2 O and CH 4 were analysed by FTIR spectroscopy. Keywords: Stable isotope; Carbon dioxide; FTIR; Infrared spectroscopy; Isotope ratio mass spectrometry
4 2 1. Introduction Precise and fast measurement of the 13 C/ 12 C ratio of CO 2 (δ 13 C-CO 2 ) finds application in several research fields, including atmospheric chemistry, biochemistry and geochemistry. The standard analytical technique for compound-specific isotope analysis is isotope ratio mass spectrometry (IRMS), which achieves an impressive level of precision of up to In spite of this, inter-laboratory studies with different IRMS reveal discrepancies in the order of Additionally, contamination during flask sampling and sample conservation may change δ 13 C-CO 2 before IRMS analysis. Moreover, flask sampling followed by laboratory analysis limits the number and frequency of measurements and, therefore, may be inadequate to fully characterize variations of atmospheric CO 2 at all relevant timescales. It is mainly for the perspective of field applicability that various spectroscopic methods are attracting a growing interest. Infrared absorption spectroscopy has the potential to overcome most of the above limitations, especially those related to data coverage. The mid-infrared region is particularly well suited for isotopic measurements, because carbon dioxide exhibits highly characteristic rotational-vibrational bands of strong fundamental vibrations in this region. Isotopic substitution affects the vibrational and rotational energy states of a molecule, and thus each isotopologue or even isotopomer has its own ro-vibrational spectrum. Various optical techniques have been published to measure all or a selection of the most abundant CO 2 isotopologues, including non-dispersive infrared (NDIR) spectroscopy, nearinfrared (NIR) diode lasers at 1.6 µm and 2 µm and mid-infrared (MIR) diode
5 3 lasers around 4.3 µm. The laser based techniques typically use short spectral regions containing one or more ro-vibrational transitions of the isotopologues of interest. To our knowledge, the best results are currently obtained using quantum cascade laser absorption spectroscopy (QCLAS). Using QCLAS, Tuzson et al. found a precision of 0.16 (1 σ of a 500 s average) and an agreement of 0.3 with IRMS [19], while Nelson et al. [21] reached an significantly better precision (0.03 after 400 s) and a comparable long term accuracy of These instruments are however very costly and limited to the measurement of CO 2 isotope ratios. Alternatively, a broader part of a ro-vibrational band can be employed to determine isotopologue concentrations using a more common, commercially available spectroscopic instrumentation, such as Fourier transform infrared (FTIR) spectrometers. In the most straightforward approach, 12 CO 2 and 13 CO 2 concentrations are calculated from the overlapping ν 3 ro-vibrational absorption bands at 4.3 µm using a classical least squares (CLS) algorithm. However, for the parameterization of the CLS quantification algorithm, exceedingly precise single component calibration spectra are required which cannot be collected experimentally with an uncertainty of less than several percent. Therefore, most publications on δ 13 C-CO 2 using FTIR spectrometry so far suffered from low accuracy and precision. Using synthetic calibration spectra for the individual isotopologues calculated from the HITRAN database including environmental (pressure and temperature) and instrumental (resolution, line shape, wave number shift and path length) parameters, Esler et al. obtained a precision of 0.15 for δ 13 C-CO 2 at ambient CO 2 concentrations in a laboratory study.
6 4 However, the precision of the follow-up field campaigns was lower, between 0.2 and 0.5, and the accuracy of the complete analytical method was not determined. To overcome problems with inaccurate single component calibrations spectra we developed a calibration and quantification procedure that is distinctly different from the concepts published so far using a set of multicomponent standards in combination with an optimized partial least-squares (PLS) algorithm. In this paper, we demonstrate the capability of our approach to measure δ 13 C-CO 2 in real-time in ambient CO 2. Optimisation included temperature and pressure stabilisation, selection of a suitable calibration and measurement strategy, as well as automation for continuous field operation. Excellent accuracy and precision were shown during one week of field measurements in Central Switzerland using flask sampling followed by IRMS laboratory analysis as an independent analytical method. Simultaneously, other important trace gases such as CO, N 2 O and CH 4 were analysed by FTIR spectroscopy. 2. Experimental 2.1 Site description The field experiments were conducted at the Chamau research station of the ETH Zurich, during May The research station is located near Lake Zug in Central Switzerland at 400 m asl (47 12'34'' N / 8 24'36'' E). The site is an intensively managed agricultural plot on which a mixed ryegrass-clover vegetation was raised for hay. The dominant plant species are Italian ryegrass (Lolium multiflorum) and white clover (Trifolium repens) with a canopy height of
7 5 20 ± 8 cm at the time of our measurements. Air temperature during the experiment ranged from 10.1 to 26.6 C. Air samples for concentration measurement and isotopic analysis were collected at 1.83 m intake height. FTIR spectroscopic analysis was performed on-site, and compared to flask sampling followed by laboratory IRMS measurement. 2.2 FTIR spectrometry Setup In order to minimize disturbances by outside temperature changes, the FTIR was placed in an air conditioned trailer (22.5 ± 1 C) located at the edge of the grassland site in lateral direction of the mean wind. The sample gas was drawn from the sample inlet to the FTIR through a 60 m PVC tubing (ID 14 mm) with an oil free vacuum pump (Model X, Gast Manufacturing Corp., USA) at a flow rate of 150 l min -1. In front of the vacuum pump, a gas flow of approx. 2 l min -1 was extracted with a membrane pump (KNF Neuberger, CH), filtered with a sintered metal filter (SS-6F-MM-2, Swagelok, USA), dried with a Nafion dryer (PD SS, Perma Pure, USA) to a dew point of about -40 C and connected to a custom manifold system (figure 1). The lag time for a sample entering the intake and reaching the FTIR gas cell was 10 s. The manifold system was controlled by a digital I/O module (National Instruments, USA) and a Lab View programme. The sampling system switched periodically between high-purity nitrogen ( %), pressurised air used as working standard (451.8 ppm CO 2, δ 13 CO , ppm CH 4, ppb CO, ppb N 2 O) and sample gas.
8 6 As shown by laboratory experiments, the temperature stability of the sample gas is of utmost importance for the precise determination of δ 13 C-CO 2. Therefore, a thermoelectric chiller (ThermoCube 300, Solid State Cooling Systems, USA) was used to thermostat the sample gas as well as the multipath cell to 25.0 ± 0.1 C (figure 1). A constant flow of coolant was first circulated through a copper tubing (ID 4 mm, length 8.8 m) curled around the cell. Afterwards, the same coolant was used to condition the purge air and the sample gas in a heat exchanger with a total volume of about 3 litres. Sample gas and purge air passed the heat exchanger in two coiled stainless steel tubing (ID 4 mm, length 5 m). Gas temperature in the cell was continuously measured with a highprecision Pt100 thermometer (GMH 3750, Greisinger Electronic, D) with a resolution of 10 mk. The pressure in the multipath cell was automatically adjusted to 1000 ± 0.2 hpa with a digital back pressure regulator (P-702C, Bronkhorst, CH) and monitored with a digital manometer (HM 35, Revue Thommen AG, CH) mounted on the multipath cell. Insert Figure 1 about here FTIR spectrometric analysis The spectroscopic measurements were performed using a commercially available FTIR spectrometer (Nicolet Avatar 370, Thermo Electron, USA) equipped with a 20 m multipath White cell (The Foxboro Company, USA). A automatically refilled cryogenically cooled InSb detector (InfraRed Associates,
9 7 USA), with a specific peak detectivity (D*) of 2.03 x cm Hz 1/2 W -1 was used, giving a root-mean-squared (rms) noise level of ~ 3 x 10-5 absorbance units between cm -1. A spectral resolution of 0.5 cm -1 was selected, and the Happ-Genzel apodization function was employed. The spectrometer was purged with thermostated dry and CO 2 free air to eliminate residual CO 2 and water vapour absorptions in the optical path lying outside the White cell. The concentrations of the two isotopologues 12 CO 2 and 13 CO 2 were calculated from the absorption spectra using an optimized partial least squares (PLS) algorithm (TQ Analyst 7.0, Thermo Electron, USA) in combination with a set of multi-component calibration gases, which span the range of naturally occurring δ 13 C-CO 2 values between -8.7 and -19.0, and CO 2 concentrations between 364 and 530 ppm. Quantification of both isotopologues was done in the wavelength region between and cm -1, where both species have strong absorption bands and the influence of other trace gases can be neglected. The concentrations of CH 4, CO and N 2 O were determined using a conventional classical least squares (CLS) algorithm (TQ Analyst 7.0) and single-component calibration spectra for every species, which cover ambient concentrations. The following wavelength regions were used for quantification; cm -1 for CH 4, cm -1 for CO, and cm -1 for N 2 O. Interferences of H 2 O and CO 2 were corrected. The following measuring sequence was applied: (i) background spectra collection using high-purity nitrogen gas every 24 hours integrating over 256 spectral scans (12 minutes); (ii) before and after automatic liquid nitrogen refill
10 8 of the InSb detector, every six hours, collection of working standard spectra averaged over 512 scans (24 minutes) to check for drifts; and (iii) continuous recording of sample gas spectra of ambient air averaged over 16 scans (1 minute) for the determination of δ 13 C-CO 2, CO 2, CH 4, CO and N 2 O. 13 C/ 12 C isotopic ratios were corrected for temperature drifts and averaged over 24 minutes for comparison with IRMS data. CO 2, CH 4, CO and N 2 O concentrations were corrected for slight drifts based on the measurement of the working standard. As shown before, the FTIR system is very stable, which is illustrated by the fact that the standard deviation of the mean decreases with 1 / sqrt(n) for at least 16 minutes (see Allan plot in [28]) reaching a maximum precision for δ 13 C- CO 2 of Consequently, the uncertainty of a 1 minute average can be expected to be about 5 times (sqrt 24) larger than for 24 minutes averages. 2.3 Flask sampling and IRMS analysis Simultaneously to the FTIR measurements, the atmospheric CO 2 mixing ratio was monitored at the sampling height by a photoacoustic gas-monitor (Model 1412, Innova AirTech Instruments, DK). Furthermore, computer-controlled mobile air samplers (ASA) were used to collect flask samples in the field in periodic time intervals. Inside the ASA, the CO 2 concentration was determined (LI-840, LI-COR, USA) and the sample gas was dried by Mg(ClO 4 ) 2. Sample volumes and connecting tubes were purged with 0.9 l min -1 for one or two minutes before sampling, depending on whether sample containers of 10 ml (metal tubes) or 300 ml (glass flasks) were used.
11 9 The air sampler was brought to the laboratory for δ 13 C-CO 2 analysis. All gases, including laboratory standards with known δ 13 C-CO 2 values, were measured with a gasbench II equipped with a home-built liquid nitrogen cold trap coupled to a DeltaPlus XP isotope ratio mass spectrometer (both Finnigan MAT, Germany). The δ 13 C-values of the laboratory air standards were determined at the Max-Planck-Institute for Biogeochemistry (Jena, Germany) according to Werner et al Isotope convention Our calibration procedure and data interpretation are referenced to the Vienna Peedee Belemnite (VPDB) scale maintained by the International Atomic Energy Agency. The isotope relative abundance (δ 13 C-CO 2 ) of our standard gases and atmospheric samples were calculated as R C R sample δ 13 standard (1) where R 13 Sample is the molar ratio of the abundances of 13 C and 12 C of the sample and 13 R Standard the standard molar ratio. δ 13 C is reported in parts per thousand (per mil or ).
12 10 Results and Discussion 2.5 Temperature effect on δ 13 C-CO 2 measurement by FTIR Temperature and pressure of the sample gas influence the absorption spectra by changing line strength, as well as width and shape of the rotational lines. For our experimental setup the temperature dependence was determined in the laboratory to be 14 K -1. This value was confirmed during our field campaign by regular analysis of pressurized air with known isotopic composition (data not shown). The temperature and pressure stability of the experimental setup was carefully optimized to enable a precise analysis of component concentrations. An increase in the outside temperature of approx. 10 K, led to a temperature rise in the trailer of around 1 K and of less than 0.1 K in the gas cell (figure 2). For the remaining temperature changes in the gas cell, a correction was applied to the δ 13 C-CO 2 values. Insert Figure 2 about here 2.6 Field measurement of δ 13 C-CO 2 and CO 2 Intercomparison Figure 3 shows a comparison between δ 13 C-CO 2 values determined by online FTIR spectroscopy and flask sampling followed by IRMS laboratory analysis. Very good agreement was generally observed between both methods, with few distinct outliers (figure 3). The average difference between online FTIR
13 11 spectroscopy (24 minutes averaging) and flask sampling / IRMS for corresponding points in time was 0.4 (n = 81), which is identical to the average accuracy determined during external validation of the FTIR quantification algorithm in the laboratory. Differences between both methods were smaller at night-time (10 pm to 6 am), around 0.3 (n = 36), although variations in the δ 13 C-CO 2 ratio were higher. One reason might be the lower fluctuations in the outside temperature. Figure 3 also shows a time series of the CO 2 concentration analysed by FTIR, a LI-COR NDIR analyser and an Innova photoacoustic gas monitor. CO 2 mixing ratios as well as trends were in good agreement for all three methods. Unfortunately, a software failure of the manifold system of the FTIR spectrometer and the mobile air sampler caused a one day data loss, from 25 th to 26 th May for FTIR, and from 27 th to 28 th May for flask sampling / IRMS. Insert Figure 3 about here Carbon dioxide concentrations as well as δ 13 C-CO 2 in the grassland air showed typical diurnal variations. The diurnal range was from approx. -7 to -18 in δ 13 C-CO 2 and from 370 to 670 ppm in the CO 2 concentration. The data in figure 3 clearly show the well-known diurnal cycle with substantial additions of CO 2 from the grassland after sunset, as an effect of respiration of plant, root and soil micro-organisms, which have a more negative δ 13 C-CO 2 than ambient air. During calm nights, that is 24 th /25 th and 27 th /28 th May, a shallow stable boundary layer (SBL) builds up over the ground surface. The reduced vertical
14 12 mixing in this SBL leads to a strong vertical CO 2 gradient. Since this extra CO 2 accumulating in the SBL during the night stems from respiration with a distinctly more negative δ 13 C-CO 2 isotopic signature than what is found in ambient air, the nocturnal δ 13 C-CO 2 values in the SBL also become very negative. In other nights (e.g. 26 th /27 th May) strong turbulent mixing due to cloudy and relatively windy weather conditions caused a dilution of the photosynthetic signature, and thus the minimum nocturnal δ 13 C-CO 2 values are less negative. At daytime, the isotopic values of CO 2 are strongly influenced by photosynthesis driven assimilation and less so by respiration. The isotope effects of the photosynthetic process are an interaction between CO 2 diffusion into the leaf stomata and the isotopic fractionation of the Rubisco catalyzed carbon fixation (e.g. ). However, due to strong mixing within the atmospheric boundary layer during the day, δ 13 C-CO 2 predominantly represented the atmospheric background values (δ 13 C-CO 2 of approx. -8 ). The good agreement suggested by Figure 3 is only partly reflected when plotting IRMS vs. FTIR data (not shown). A linear regression yields a slope of 0.83 or 0.86 and a R 2 of 0.87 or 0.94 for δ 13 C-CO 2 and CO 2, respectively. We believe that this is mainly due to the fact that the FTIR and the IRMS sampling system did not have the same inlet and temporal resolution. Therefore, a direct comparison of the values is a very conservative approach. Integration periods of both methods were different, because sampling flasks are rapidly purged within less than one minute, while an exchange of the FTIR gas cell volume takes almost 10 minutes (t 95 ). A much better comparison is obtained when comparing the Keeling Plots obtained by IRMS and FTIR (Figure 4) [34]. This implies a
15 13 normalization of the δ 13 C-CO 2 values for 1/CO 2 and thus also accounts for differences in the sampling interval. Only slight, insignificant differences were observed for δ 13 C r determined by FTIR and flask sampling / IRMS, combining daytime and night-time data of the entire measurement campaign. The correlation of δ 13 C-CO 2 and 1/CO 2 data (R 2 ) was even stronger for the FTIR measurements, indicating its excellent overall performance. Insert Figure 4 about here 2.7 Trace gas concentration measurement by FTIR spectroscopy Figure 4 shows the trace gas concentrations determined from 22 nd to 29 th May by FTIR spectroscopy with an averaging time of 12 minutes (256 coadded scans). Analytical precision determined during laboratory experiments using the Allan variance method was 1.5 ppb for CH 4, 0.3 ppb for CO, and 0.8 ppb for N 2 O using 10 to 15 minutes of spectral averaging. Thus, a single FTIR spectrometer can simultaneously analyse these components with the same level of precision that is usually reached using three individual single component analysers. During the nights between 24 th /25 th and 27 th /28 th of May the micrometeorological conditions were favourable to accumulate CH 4, CO and N 2 O in the SBL, resulting in elevated concentrations (Figure 5). Although turbulent mixing in the SBL is the same for all trace gases and isotope ratios, the time courses of the concentrations show significant differences that indicate
16 14 the source of the origin of the respective trace gas. For example, CO typically results from combustion processes, whereas CH 4 is rather related to cattle live stock activity and soil microbial production or consumption. CO peak concentrations are seen well before midnight in the calm nights, whereas CH 4 concentrations peak a couple of hours later. The daytime methane concentrations declined from day-to-day as the soil moisture content decreased since the last rain event on the evening of 22 nd of May (data not shown), which is consistent with the fact that methane fluxes and soil moisture content are closely linked. A detailed ecological interpretation is, however, beyond the scope of this study and will the topic of a subsequent paper. Insert Figure 5 about here
17 15 3. Conclusions We have developed a precise and accurate method for continuous on-line analysis of δ 13 C-CO 2 in ambient air using a commercially available FTIR spectrometer in combination with a robust partial least squares algorithm. Optimisation included temperature and pressure stabilisation, as well as the selection of a suitable calibration and measurement strategy for long-term monitoring. Independent operation during field studies was possible by synchronizing the gas-flow system and the spectrometer operation, and by using an automatic liquid nitrogen refill for the detector. Using our analytical method the 13 C/ 12 C isotopic ratio of atmospheric CO 2 can be determined with an accuracy better than 0.4 (n = 81) compared to standard flask sampling followed by IRMS laboratory analysis as an independent analytical approach. With our experimental setup it is possible to determine, besides δ 13 C-CO 2 and CO 2, other important trace gases such as CO, N 2 O and CH 4 which is a clear advantage over standard single component techniques. The developed FTIR methodology has the ability to be employed for long-term flux measurements with good spatial and temporal averaging capabilities. For these applications, FTIR is an excellent alternative to IRMS, which requires flask sampling followed by laboratory analysis. Our calibration method is in principle applicable to other stable isotope ratio analyses; however, these may require the use of higher resolution FTIR spectroscopy.
18 16 Acknowledgments Kerstin Zeyer and Christoph Zellweger are acknowledged for CO 2, N 2 O, CO and CH 4 analysis of the multi-component standard gases. We thank Bela Tuzson for helpful discussion. The authors are grateful to Willi Brand and Michael Rothe from the Max Planck Institute for Biochemistry for δ 13 C-CO 2 and analysis of the IRMS laboratory standards. We acknowledge E. Pieper from the Section Mechanical Engineering / Workshop and W. Knecht and R. Gross from the Section Electronics / Metrology for technical assistance. M. J. Zeeman, R. A. Werner and W. Eugster were supported by the Swiss National Science Foundation, grant
19 References 17
20 18 4. Figure captions Figure 1. Schematic drawing of the experimental set-up used during the field campaign. Gas inlets were mounted on a micrometerological tower. Figure 2. Effects of changes in the outside temperature (a) on the temperature in the air-conditioned trailer (b) and in the gas cell (c). Figure 3. Time series of (a) δ 13 C-CO 2 determined by on-line FTIR spectroscopy as well as by flask sampling with subsequent IRMS analysis; two distinct outliers are marked with a circle; (b) CO 2 mixing ratio as analysed by FTIR, a photoacoustic monitor (Innova) and a NDIR analyser (LI-840). Figure 4. δ 13 C-CO 2 ratios plotted against the inverse of the CO 2 concentration. Intercept and slope of the linear regression are given together with their standard deviation for FTIR and flask sampling / IRMS measurements. Figure 5. Time courses of trace gas concentrations determined by FTIR spectroscopy. Short gaps correspond with the calibration periods, whereas the large gap between 25 th and 26 th is due to a software failure of the manifold system.
Isotopic Ratio Measurements of Atmospheric Carbon Dioxide Using a 4.3 µm Pulsed QC Laser
 Isotopic Ratio Measurements of Atmospheric Carbon Dioxide Using a 4.3 µm Pulsed QC Laser David D. Nelson, J. Barry McManus, Scott C. Herndon and Mark S. Zahniser Aerodyne Research, Inc., 45 Manning Road,
Isotopic Ratio Measurements of Atmospheric Carbon Dioxide Using a 4.3 µm Pulsed QC Laser David D. Nelson, J. Barry McManus, Scott C. Herndon and Mark S. Zahniser Aerodyne Research, Inc., 45 Manning Road,
Frontiers in quantum cascade laser based analysis of greenhouse gas stable isotopes
 Isotope - Ecology Course at the ETHZ 2018 Frontiers in quantum cascade laser based analysis of greenhouse gas stable isotopes Joachim Mohn joachim.mohn@empa.ch Air Pollution / Environmental Technology
Isotope - Ecology Course at the ETHZ 2018 Frontiers in quantum cascade laser based analysis of greenhouse gas stable isotopes Joachim Mohn joachim.mohn@empa.ch Air Pollution / Environmental Technology
Compact Hydrogen Peroxide Sensor for Sterilization Cycle Monitoring
 Physical Sciences Inc. VG15-012 Compact Hydrogen Peroxide Sensor for Sterilization Cycle Monitoring January 26, 2015 Krishnan R. Parameswaran, Clinton J. Smith, Kristin L. Galbally-Kinney, William J. Kessler
Physical Sciences Inc. VG15-012 Compact Hydrogen Peroxide Sensor for Sterilization Cycle Monitoring January 26, 2015 Krishnan R. Parameswaran, Clinton J. Smith, Kristin L. Galbally-Kinney, William J. Kessler
Spectroscopic Applications of Quantum Cascade Lasers
 Spectroscopic Applications of Quantum Cascade Lasers F.K. Tittel, A. Kosterev, and R.F. Curl Rice University Houston, USA OUTLINE fkt@rice.edu http://www.ruf.rice.edu/~lasersci/ PQE 2000 Snowbird, UT Motivation
Spectroscopic Applications of Quantum Cascade Lasers F.K. Tittel, A. Kosterev, and R.F. Curl Rice University Houston, USA OUTLINE fkt@rice.edu http://www.ruf.rice.edu/~lasersci/ PQE 2000 Snowbird, UT Motivation
Atmospheric Analysis Gases. Sampling and analysis of gaseous compounds
 Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
Atmospheric Analysis Gases Sampling and analysis of gaseous compounds Introduction - External environment (ambient air) ; global warming, acid rain, introduction of pollutants, etc - Internal environment
The Claviature of Gas Analysis
 The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
The Claviature of Gas Analysis Mass spectrometric gas analysis for quality assurance of gases and gas mixtures High purity and special gases are becoming increasingly important in the development of modern
δ 13 C of DIC and carbonate samples: comparison of traditional mass spectrometry methods with an infrared spectrometry method
 APPLICATION NOTE 30486 δ 13 C of DIC and carbonate samples: comparison of traditional mass spectrometry methods with an infrared spectrometry method Authors Danijela Smajgl, 1 Magda Mandic, 1 Dirk Nürnberg,
APPLICATION NOTE 30486 δ 13 C of DIC and carbonate samples: comparison of traditional mass spectrometry methods with an infrared spectrometry method Authors Danijela Smajgl, 1 Magda Mandic, 1 Dirk Nürnberg,
Gaseous CEMS technology
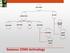 GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
GAS CEMS EXTRACTIVE IN-SITU COLD DRY HOT WET DRY DILUTION PROBE/ POINT IN-STACK CROSS STACK OUT OF STACK Gaseous CEMS technology Probe (at stack) Heated filter Heated sample line Condenser Pump Analyzers
Defining quality standards for the analysis of solid samples
 Defining quality standards for the analysis of solid samples Thermo Scientific Element GD Plus Glow Discharge Mass Spectrometer Redefine your quality standards for the elemental analysis of solid samples
Defining quality standards for the analysis of solid samples Thermo Scientific Element GD Plus Glow Discharge Mass Spectrometer Redefine your quality standards for the elemental analysis of solid samples
IRSENS. Méthodes optiques pour l analyse des gaz et des liquides, e.g. détection de cocaïne dans la salive. Nano Tera.
 IRSENS Méthodes optiques pour l analyse des gaz et des liquides, e.g. détection de cocaïne dans la salive J. Faist, P. Jouy ETH Zurich Nano Tera.ch Info Day 2014 Sensing needs Gases fluids Environment
IRSENS Méthodes optiques pour l analyse des gaz et des liquides, e.g. détection de cocaïne dans la salive J. Faist, P. Jouy ETH Zurich Nano Tera.ch Info Day 2014 Sensing needs Gases fluids Environment
Cantilever enhanced tunable diode laser photoacoustic spectroscopy in gas purity measurement case study: acetylene in ethylene
 Cantilever enhanced tunable diode laser photoacoustic spectroscopy in gas purity measurement case study: acetylene in ethylene Juho Uotila, Jussi Raittila, Ismo Kauppinen ¹Gasera Ltd., Tykistökatu 4, 20520
Cantilever enhanced tunable diode laser photoacoustic spectroscopy in gas purity measurement case study: acetylene in ethylene Juho Uotila, Jussi Raittila, Ismo Kauppinen ¹Gasera Ltd., Tykistökatu 4, 20520
II. WAVELENGTH SELECTION
 Ozone detection by DFB QCL absorption technique using Multipass cell S.S. Yenaganti 1, S.S.Kulkarni 2, A.J.Verma 3, N.Sreevalsen 4, V.Rathore 5 1 Department of Electronics and Telecommunications Engineering,
Ozone detection by DFB QCL absorption technique using Multipass cell S.S. Yenaganti 1, S.S.Kulkarni 2, A.J.Verma 3, N.Sreevalsen 4, V.Rathore 5 1 Department of Electronics and Telecommunications Engineering,
Characterization of high temperature solar thermal selective absorber coatings at operation temperature
 Available online at www.sciencedirect.com Energy Procedia 00 (2013) 000 000 www.elsevier.com/locate/procedia SolarPACES 2013 Characterization of high temperature solar thermal selective absorber coatings
Available online at www.sciencedirect.com Energy Procedia 00 (2013) 000 000 www.elsevier.com/locate/procedia SolarPACES 2013 Characterization of high temperature solar thermal selective absorber coatings
Agilent Oil Analyzer: customizing analysis methods
 Agilent Oil Analyzer: customizing analysis methods Application Note Author Alexander Rzhevskii & Mustafa Kansiz Agilent Technologies, Inc. Introduction Traditionally, the analysis of used oils has been
Agilent Oil Analyzer: customizing analysis methods Application Note Author Alexander Rzhevskii & Mustafa Kansiz Agilent Technologies, Inc. Introduction Traditionally, the analysis of used oils has been
10.12 IN-SITU MEASUREMENT OF WATER VAPOR ISOTOPES FOR ATMOSPHERIC AND ECOLOGICAL APPLICATIONS
 10.12 IN-SITU MEASUREMENT OF WATER VAPOR ISOTOPES FOR ATMOSPHERIC AND ECOLOGICAL APPLICATIONS Xuhui Lee*, Steve Sargent, Ronald Smith, Bert Tanner School of Forestry and Environmental Studies, Yale University,
10.12 IN-SITU MEASUREMENT OF WATER VAPOR ISOTOPES FOR ATMOSPHERIC AND ECOLOGICAL APPLICATIONS Xuhui Lee*, Steve Sargent, Ronald Smith, Bert Tanner School of Forestry and Environmental Studies, Yale University,
A new lidar for water vapor and temperature measurements in the Atmospheric Boundary Layer
 A new lidar for water vapor and temperature measurements in the Atmospheric Boundary Layer M. Froidevaux 1, I. Serikov 2, S. Burgos 3, P. Ristori 1, V. Simeonov 1, H. Van den Bergh 1, and M.B. Parlange
A new lidar for water vapor and temperature measurements in the Atmospheric Boundary Layer M. Froidevaux 1, I. Serikov 2, S. Burgos 3, P. Ristori 1, V. Simeonov 1, H. Van den Bergh 1, and M.B. Parlange
atomic absorption spectroscopy general can be portable and used in-situ preserves sample simpler and less expensive
 Chapter 9: End-of-Chapter Solutions 1. The following comparison provides general trends, but both atomic absorption spectroscopy (AAS) and atomic absorption spectroscopy (AES) will have analyte-specific
Chapter 9: End-of-Chapter Solutions 1. The following comparison provides general trends, but both atomic absorption spectroscopy (AAS) and atomic absorption spectroscopy (AES) will have analyte-specific
Fourier Transform Infrared. Spectrometry
 Fourier Transform Infrared. Spectrometry Second Editio n PETER R. GRIFFITH S JAMES A. de HASETH PREFACE x v CHAPTER 1 INTRODUCTION TO VIBRATIONAL SPECTROSCOPY 1 1.1. Introduction 1 1.2. Molecular Vibrations
Fourier Transform Infrared. Spectrometry Second Editio n PETER R. GRIFFITH S JAMES A. de HASETH PREFACE x v CHAPTER 1 INTRODUCTION TO VIBRATIONAL SPECTROSCOPY 1 1.1. Introduction 1 1.2. Molecular Vibrations
EVALUATING LAND SURFACE FLUX OF METHANE AND NITROUS OXIDE IN AN AGRICULTURAL LANDSCAPE WITH TALL TOWER MEASUREMENTS AND A TRAJECTORY MODEL
 8.3 EVALUATING LAND SURFACE FLUX OF METHANE AND NITROUS OXIDE IN AN AGRICULTURAL LANDSCAPE WITH TALL TOWER MEASUREMENTS AND A TRAJECTORY MODEL Xin Zhang*, Xuhui Lee Yale University, New Haven, CT, USA
8.3 EVALUATING LAND SURFACE FLUX OF METHANE AND NITROUS OXIDE IN AN AGRICULTURAL LANDSCAPE WITH TALL TOWER MEASUREMENTS AND A TRAJECTORY MODEL Xin Zhang*, Xuhui Lee Yale University, New Haven, CT, USA
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION
 JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
JPACSM 5 Process Analytical Chemistry THE APPLICATION OF PROCESS MASS SPECTROMETRY TO FUMED SILICA PRODUCTION Peter Traynor and Steven Trimuar Thermo Electron Corporation Houston, TX Robert G. Wright Thermo
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth
 FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
FTIR measurement of NH 3, HCN, SO 2, H 2 S and COS in pulverized lignite oxy-fuel flames Daniel Fleig, Stefan Hjärtstam and Daniel Kühnemuth Abstract Nitrogen and sulphur compounds are investigated in
Introduction to Fourier Transform Infrared Spectroscopy
 Introduction to Fourier Transform Infrared Spectroscopy Introduction What is FTIR? FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared spectroscopy, IR
Introduction to Fourier Transform Infrared Spectroscopy Introduction What is FTIR? FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared spectroscopy, IR
White Paper. Overview: NDIR Definition:
 Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Title: NDIR Technology Overview, Compliance, and Comparison to Other Generally Available Gas Measurement Technologies TSN Number: 06 File:\\MII- SRV1\Metron\Bridge_Analyzers\Customer_Service_Documentation\White_Papers\06
Near infrared reflectance spectroscopy
 OIV-MA-BS-08 1. INTRODUCTION This method of determining the real alcoholic strength by volume of alcoholic beverages and distillates is based on the physical principle of the spectral analysis of materials
OIV-MA-BS-08 1. INTRODUCTION This method of determining the real alcoholic strength by volume of alcoholic beverages and distillates is based on the physical principle of the spectral analysis of materials
TO-15 Checklist Determination of VOCs in Air by GC-MS
 LAB ID: DATE: LAB NAME: ASSESSOR NAME: Method Number: TO-15 Checklist Determination of VOCs in Air by GC-MS SOP Number: Revision Number: SOP Date: Personnel records observed: Data records observed: Revision
LAB ID: DATE: LAB NAME: ASSESSOR NAME: Method Number: TO-15 Checklist Determination of VOCs in Air by GC-MS SOP Number: Revision Number: SOP Date: Personnel records observed: Data records observed: Revision
Commercialization of autonomous sensor systems for quantifying pco 2 and total inorganic carbon
 Commercialization of autonomous sensor systems for quantifying pco 2 and total inorganic carbon PI: Michael DeGrandpre, PhD Dept. of Chemistry, University of Montana, Missoula, MT 59812 Phone: (406) 243-4227
Commercialization of autonomous sensor systems for quantifying pco 2 and total inorganic carbon PI: Michael DeGrandpre, PhD Dept. of Chemistry, University of Montana, Missoula, MT 59812 Phone: (406) 243-4227
Reprinted from February Hydrocarbon
 February2012 When speed matters Loek van Eijck, Yokogawa, The Netherlands, questions whether rapid analysis of gases and liquids can be better achieved through use of a gas chromatograph or near infrared
February2012 When speed matters Loek van Eijck, Yokogawa, The Netherlands, questions whether rapid analysis of gases and liquids can be better achieved through use of a gas chromatograph or near infrared
Introduction to Fourier Transform Infrared Spectroscopy
 molecular spectroscopy Introduction to Fourier Transform Infrared Spectroscopy Part of Thermo Fisher Scientific Introduction What is FT-IR? FT-IR stands for Fourier Transform InfraRed, the preferred method
molecular spectroscopy Introduction to Fourier Transform Infrared Spectroscopy Part of Thermo Fisher Scientific Introduction What is FT-IR? FT-IR stands for Fourier Transform InfraRed, the preferred method
Tunable diode laser absorption spectroscopy for stable isotope studies of ecosystem atmosphere CO 2 exchange
 Agricultural and Forest Meteorology 118 (2003) 1 19 Tunable diode laser absorption spectroscopy for stable isotope studies of ecosystem atmosphere CO 2 exchange David R. Bowling a,, Steve D. Sargent b,
Agricultural and Forest Meteorology 118 (2003) 1 19 Tunable diode laser absorption spectroscopy for stable isotope studies of ecosystem atmosphere CO 2 exchange David R. Bowling a,, Steve D. Sargent b,
Mercury Stack Monitor SM-4
 Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
Member of the envea Group Mercury Stack Monitor SM-4 Automatic continuous emission monitor (CEM) for mercury Continuous operation Sample dilution directly at stack - works with virtually any sample matrix
UV Quality Assurance. Dark Current Correction
 UV Quality Assurance This document presents the Quality Assurance protocol used for the measurement of broad spectrum UV spectra according to the guidelines given by the United States Environmental Protection
UV Quality Assurance This document presents the Quality Assurance protocol used for the measurement of broad spectrum UV spectra according to the guidelines given by the United States Environmental Protection
Thermo Scientific ELEMENT GD PLUS Glow Discharge Mass Spectrometer. Defining quality standards for the analysis of solid samples
 Thermo Scientific ELEMENT GD PLUS Glow Discharge Mass Spectrometer Defining quality standards for the analysis of solid samples Redefine your quality standards for the elemental analysis of solid samples
Thermo Scientific ELEMENT GD PLUS Glow Discharge Mass Spectrometer Defining quality standards for the analysis of solid samples Redefine your quality standards for the elemental analysis of solid samples
ABB Remote Sensing Atmospheric Emitted Radiance Interferometer AERI system overview. Applications
 The ABB Atmospheric Emitted Radiance Interferometer AERI provides thermodynamic profiling, trace gas detection, atmospheric cloud aerosol study, air quality monitoring, and more. AERI high level overview
The ABB Atmospheric Emitted Radiance Interferometer AERI provides thermodynamic profiling, trace gas detection, atmospheric cloud aerosol study, air quality monitoring, and more. AERI high level overview
In-Situ 13 C/ 12 C Ratio Analysis in Water Carbonates using FTIR
 Authors In-Situ C/ 12 C Ratio Analysis in Water Carbonates using FTIR Application Note Environmental Ira Litvak 1, 3, Yaakov Anker 1, 3, Jan Wülfken 4 and Haim Cohen 2 1. Department of Chemical Engineering,
Authors In-Situ C/ 12 C Ratio Analysis in Water Carbonates using FTIR Application Note Environmental Ira Litvak 1, 3, Yaakov Anker 1, 3, Jan Wülfken 4 and Haim Cohen 2 1. Department of Chemical Engineering,
DEVELOPMENT OF MEASUREMENT AND
 DEVELOPMENT OF MEASUREMENT AND CHEMOMETRIC METHODS IN THE FOURIER TRANSFORM INFRARED AND RAMAN SPECTROSCOPY PhD Theses Written by: Brunó Hren Chemical engineer Supervisor: Prof. Dr. János Mink Made in
DEVELOPMENT OF MEASUREMENT AND CHEMOMETRIC METHODS IN THE FOURIER TRANSFORM INFRARED AND RAMAN SPECTROSCOPY PhD Theses Written by: Brunó Hren Chemical engineer Supervisor: Prof. Dr. János Mink Made in
10/8/2013. Alternatives to Mass Spectrometry and Some Real-time Monitoring Measurements. Project Driven Tasks -- Process Gas Measurements
 Alternatives to Mass Spectrometry and Some Real-time Monitoring Measurements Co-worker Acknowledgement Laura Tovo, Ronald Hooper, Louis Boone, Jack Zamecnik William A. Spencer SRNL Science and Technology
Alternatives to Mass Spectrometry and Some Real-time Monitoring Measurements Co-worker Acknowledgement Laura Tovo, Ronald Hooper, Louis Boone, Jack Zamecnik William A. Spencer SRNL Science and Technology
Calibration strategies for FTIR and other IRIS instruments for accurate δ 13 C and δ 18 O measurements of CO 2 in air
 Calibration strategies for FTIR and other IRIS instruments for accurate δ 13 C and δ 18 O measurements of CO 2 in air E. Flores 19th WMO/IAEA Meeting on Carbon Dioxide, Other Greenhouse Gases, and Related
Calibration strategies for FTIR and other IRIS instruments for accurate δ 13 C and δ 18 O measurements of CO 2 in air E. Flores 19th WMO/IAEA Meeting on Carbon Dioxide, Other Greenhouse Gases, and Related
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications
 Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
Design and Development of a Smartphone Based Visible Spectrophotometer for Analytical Applications Bedanta Kr. Deka, D. Thakuria, H. Bora and S. Banerjee # Department of Physicis, B. Borooah College, Ulubari,
Whole Tablet Measurements Using the Frontier Tablet Autosampler System
 a p p l i c a t i o n N O T E Whole Tablet Measurements Using the Frontier Tablet Autosampler System FT-NIR Spectroscopy Introduction Recent advances in NIR technology have changed the ways in which both
a p p l i c a t i o n N O T E Whole Tablet Measurements Using the Frontier Tablet Autosampler System FT-NIR Spectroscopy Introduction Recent advances in NIR technology have changed the ways in which both
Spectroscopy in Transmission
 Spectroscopy in Transmission + Reflectance UV/VIS - NIR Absorption spectra of solids and liquids can be measured with the desktop spectrograph Lambda 9. Extinctions up to in a wavelength range from UV
Spectroscopy in Transmission + Reflectance UV/VIS - NIR Absorption spectra of solids and liquids can be measured with the desktop spectrograph Lambda 9. Extinctions up to in a wavelength range from UV
S2 PICOFOX. Innovation with Integrity. Spectrometry Solutions TXRF
 S2 PICOFOX Spectrometry Solutions Innovation with Integrity TXRF S2 PICOFOX True Trace Analysis with XRF for the First Time! You need to know the concentration of trace elements in environmental samples?
S2 PICOFOX Spectrometry Solutions Innovation with Integrity TXRF S2 PICOFOX True Trace Analysis with XRF for the First Time! You need to know the concentration of trace elements in environmental samples?
Workshop on optical gas sensing
 Workshop on optical gas sensing 15.01.2015 1 Intensity (a.u.) The QCL frequency comb A laser emitting many wavelengths at the same time. Covering a broad spectral range of tens to a few 100 wavenumbers.
Workshop on optical gas sensing 15.01.2015 1 Intensity (a.u.) The QCL frequency comb A laser emitting many wavelengths at the same time. Covering a broad spectral range of tens to a few 100 wavenumbers.
FIRST HIGH-RESOLUTION ANALYSIS OF PHOSGENE 35 Cl 2. CO AND 35 Cl 37 ClCO FUNDAMENTALS IN THE CM -1 SPECTRAL REGION
 FIRST HIGH-RESOLUTION ANALYSIS OF PHOSGENE 35 Cl 2 CO AND 35 Cl 37 ClCO FUNDAMENTALS IN THE 250-480 CM -1 SPECTRAL REGION F. Kwabia Tchana 1, M. Ndao 1, L. Manceron 2, A. Perrin 1, J. M. Flaud 1, W.J.
FIRST HIGH-RESOLUTION ANALYSIS OF PHOSGENE 35 Cl 2 CO AND 35 Cl 37 ClCO FUNDAMENTALS IN THE 250-480 CM -1 SPECTRAL REGION F. Kwabia Tchana 1, M. Ndao 1, L. Manceron 2, A. Perrin 1, J. M. Flaud 1, W.J.
ICP-3000 Inductively Coupled Plasma Optical Emission Spectrometer
 Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer is powerful simultaneous full
Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer Inductively Coupled Plasma Optical Emission Spectrometer is powerful simultaneous full
What are the instrumentation requirements for measuring the isotopic composition of net ecosystem exchange of CO 2 using eddy covariance methods?
 Isotopes in Environmental and Health Studies Vol. 42, No. 2, June 2006, 115 133 What are the instrumentation requirements for measuring the isotopic composition of net ecosystem exchange of CO 2 using
Isotopes in Environmental and Health Studies Vol. 42, No. 2, June 2006, 115 133 What are the instrumentation requirements for measuring the isotopic composition of net ecosystem exchange of CO 2 using
costech instruments ECS 4010 Elemental Combustion System CHNS-O
 instruments ECS 4010 Elemental Combustion System CHNS-O costech Costech Analytical Tecnologies Inc. 26074 Avenue Hall, Suite 14 Valencia, CA 91355 - USA Phone: (661) 297-2395 Fax: (661) 297-5492 e-mail:
instruments ECS 4010 Elemental Combustion System CHNS-O costech Costech Analytical Tecnologies Inc. 26074 Avenue Hall, Suite 14 Valencia, CA 91355 - USA Phone: (661) 297-2395 Fax: (661) 297-5492 e-mail:
OCN 401. Photosynthesis
 OCN 401 Photosynthesis Photosynthesis Process by which carbon is reduced from CO 2 to organic carbon Provides all energy for the biosphere (except for chemosynthesis at hydrothermal vents) Affects composition
OCN 401 Photosynthesis Photosynthesis Process by which carbon is reduced from CO 2 to organic carbon Provides all energy for the biosphere (except for chemosynthesis at hydrothermal vents) Affects composition
Hiden CATLAB Systems Microreactor for Catalysis Studies & Thermal Analysis
 Hiden CATLAB Systems Microreactor for Catalysis Studies & Thermal Analysis vacuum analysis surface science gas analysis plasma diagnostics CATLAB overview The Hiden CATLAB is a catalyst characterisation
Hiden CATLAB Systems Microreactor for Catalysis Studies & Thermal Analysis vacuum analysis surface science gas analysis plasma diagnostics CATLAB overview The Hiden CATLAB is a catalyst characterisation
Thermochemistry/Calorimetry. Determination of the enthalpy of vaporization of liquids LEC 02. What you need: What you can learn about
 LEC 02 Thermochemistry/Calorimetry Determination of the enthalpy of vaporization of liquids What you can learn about Enthalpy of vaporisation Entropy of vaporisation Trouton s rule Calorimetry Heat capacity
LEC 02 Thermochemistry/Calorimetry Determination of the enthalpy of vaporization of liquids What you can learn about Enthalpy of vaporisation Entropy of vaporisation Trouton s rule Calorimetry Heat capacity
High Sensitivity Gas Sensor Based on IR Spectroscopy Technology and Application
 PHOTONIC SENSORS / Vol. 6, No. 2, 2016: 127 131 High Sensitivity Gas Sensor Based on IR Spectroscopy Technology and Application Hengyi LI Department of Electronic Information Engineering, Jincheng College
PHOTONIC SENSORS / Vol. 6, No. 2, 2016: 127 131 High Sensitivity Gas Sensor Based on IR Spectroscopy Technology and Application Hengyi LI Department of Electronic Information Engineering, Jincheng College
VOC measurements in ambient air using Selected Ion Flow Tube Mass Spectrometry-automation and calibration considerations
 VOC measurements in ambient air using Selected Ion Flow Tube Mass Spectrometry-automation and calibration considerations Environmental Chemistry group, water Science Forum and the Separation Science Group
VOC measurements in ambient air using Selected Ion Flow Tube Mass Spectrometry-automation and calibration considerations Environmental Chemistry group, water Science Forum and the Separation Science Group
Chem Homework Set Answers
 Chem 310 th 4 Homework Set Answers 1. Cyclohexanone has a strong infrared absorption peak at a wavelength of 5.86 µm. (a) Convert the wavelength to wavenumber.!6!1 8* = 1/8 = (1/5.86 µm)(1 µm/10 m)(1 m/100
Chem 310 th 4 Homework Set Answers 1. Cyclohexanone has a strong infrared absorption peak at a wavelength of 5.86 µm. (a) Convert the wavelength to wavenumber.!6!1 8* = 1/8 = (1/5.86 µm)(1 µm/10 m)(1 m/100
Sampling. Information is helpful in implementing control measures for reducing pollutant concentration to acceptable levels
 Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Types of pollutant sampling and measurement: Air quality monitoring: Sampling and measurement of air pollutants generally known, as air quality monitoring. It is an integral component of any air pollution
Whole Tablet Measurements Using the Spectrum One NTS Tablet Autosampler System
 Whole Tablet Measurements Using the Spectrum One NTS Tablet Autosampler System A P P L I C A T I O N N O T E Introduction Recent advances in NIR technology have changed the ways in which both the pharmaceutical
Whole Tablet Measurements Using the Spectrum One NTS Tablet Autosampler System A P P L I C A T I O N N O T E Introduction Recent advances in NIR technology have changed the ways in which both the pharmaceutical
Infrared Thermometer Calibration A Complete Guide
 Infrared Thermometer Calibration A Complete Guide Application note With proper setup and planning, infrared calibrations can be accurate. The steps outlined below should be followed to perform accurate
Infrared Thermometer Calibration A Complete Guide Application note With proper setup and planning, infrared calibrations can be accurate. The steps outlined below should be followed to perform accurate
Portable type TXRF analyzer: Ourstex 200TX
 Excerpted from Adv. X-Ray. Chem. Anal., Japan: 42, pp. 115-123 (2011) H. Nagai, Y. Nakajima, S. Kunimura, J. Kawai Improvement in Sensitivity and Quantification by Using a Portable Total Reflection X-Ray
Excerpted from Adv. X-Ray. Chem. Anal., Japan: 42, pp. 115-123 (2011) H. Nagai, Y. Nakajima, S. Kunimura, J. Kawai Improvement in Sensitivity and Quantification by Using a Portable Total Reflection X-Ray
Process Analytical Technology Diagnosis, Optimization and Monitoring of Chemical Processes
 FRAUNHOFER INSTITUTe FoR Chemical Technology ICT Process Analytical Technology Diagnosis, Optimization and Monitoring of Chemical Processes Process Analytical Technology Diagnosis, Optimization and Monitoring
FRAUNHOFER INSTITUTe FoR Chemical Technology ICT Process Analytical Technology Diagnosis, Optimization and Monitoring of Chemical Processes Process Analytical Technology Diagnosis, Optimization and Monitoring
473 Dew Point Hygrometer
 High Performance Chilled Mirror Hygrometer With Cable Mounted Measuring Heads Highly precise chilled mirror dew point technology Cable mounted dew point and temperature measurement Aspirated and direct
High Performance Chilled Mirror Hygrometer With Cable Mounted Measuring Heads Highly precise chilled mirror dew point technology Cable mounted dew point and temperature measurement Aspirated and direct
Quantitative Analysis of Caffeine in Energy Drinks by High Performance Liquid Chromatography
 Quantitative Analysis of Caffeine in Energy Drinks by High Performance Liquid Chromatography CHEM 329 Professor Vogt TA: Fahad Hasan Allison Poget Date Performed: April 5, 2016 Date Submitted: April 12,
Quantitative Analysis of Caffeine in Energy Drinks by High Performance Liquid Chromatography CHEM 329 Professor Vogt TA: Fahad Hasan Allison Poget Date Performed: April 5, 2016 Date Submitted: April 12,
Water Vapour Effects in Mass Measurement
 Water Vapour Effects in Mass Measurement N.-E. Khélifa To cite this version: N.-E. Khélifa. Water Vapour Effects in Mass Measurement. Measurement. Water Vapour Effects in Mass Measurement, May 2007, Smolenice,
Water Vapour Effects in Mass Measurement N.-E. Khélifa To cite this version: N.-E. Khélifa. Water Vapour Effects in Mass Measurement. Measurement. Water Vapour Effects in Mass Measurement, May 2007, Smolenice,
The Theory of HPLC. Quantitative and Qualitative HPLC
 The Theory of HPLC Quantitative and Qualitative HPLC i Wherever you see this symbol, it is important to access the on-line course as there is interactive material that cannot be fully shown in this reference
The Theory of HPLC Quantitative and Qualitative HPLC i Wherever you see this symbol, it is important to access the on-line course as there is interactive material that cannot be fully shown in this reference
Light emitting diode absorption spectroscopy for combustible gas monitoring
 IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Light emitting diode absorption spectroscopy for combustible gas monitoring Related content - Spectroscopy system based on a single
IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Light emitting diode absorption spectroscopy for combustible gas monitoring Related content - Spectroscopy system based on a single
 DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
DEPARTMENT OF ELECTRONICS AND INSTRUMENTATION ENGINEERING SRM NAGAR, KATTANKULATHUR-603203 EI 2302 ANALYTICAL INSTRUMENTS QUESTION BANK UNIT I COLORIMETRY AND SPECTROPHOTOMETRY Part A 1. State Lambert
A STUDY ON TRAPPING CO 2 USING MOLECULAR SIEVE FOR 14 C AMS SAMPLE PREPARATION
 A STUDY ON TRAPPING CO 2 USING MOLECULAR SIEVE FOR 14 C AMS SAMPLE PREPARATION Kyumin Choe 1,2 Sujin Song 1 Jang Hoon Lee 1 Young Mi Song 1 Jin Kang 1 Myoung-ho Yun 1 Jong Chan Kim 3 ABSTRACT. At the Seoul
A STUDY ON TRAPPING CO 2 USING MOLECULAR SIEVE FOR 14 C AMS SAMPLE PREPARATION Kyumin Choe 1,2 Sujin Song 1 Jang Hoon Lee 1 Young Mi Song 1 Jin Kang 1 Myoung-ho Yun 1 Jong Chan Kim 3 ABSTRACT. At the Seoul
Chemistry 524--Final Exam--Keiderling May 4, :30 -?? pm SES
 Chemistry 524--Final Exam--Keiderling May 4, 2011 3:30 -?? pm -- 4286 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted. No open books or
Chemistry 524--Final Exam--Keiderling May 4, 2011 3:30 -?? pm -- 4286 SES Please answer all questions in the answer book provided. Calculators, rulers, pens and pencils are permitted. No open books or
INTERNATIONAL JOURNAL OF PURE AND APPLIED RESEARCH IN ENGINEERING AND TECHNOLOGY
 INTERNATIONAL JOURNAL OF PURE AND APPLIED RESEARCH IN ENGINEERING AND TECHNOLOGY A PATH FOR HORIZING YOUR INNOVATIVE WORK SOIL NITROGEN DETECTION USING NEAR INFRARED SPECTROSCOPY SNEHA J. BANSOD Department
INTERNATIONAL JOURNAL OF PURE AND APPLIED RESEARCH IN ENGINEERING AND TECHNOLOGY A PATH FOR HORIZING YOUR INNOVATIVE WORK SOIL NITROGEN DETECTION USING NEAR INFRARED SPECTROSCOPY SNEHA J. BANSOD Department
Topic 2.11 ANALYTICAL TECHNIQUES. High Resolution Mass Spectrometry Infra-red Spectroscopy
 Topic 2.11 ANALYTICAL TECHNIQUES High Resolution Mass Spectrometry Infra-red Spectroscopy HIGH RESOLUTION MASS SPECTROMETRY The technique of mass spectrometry was used in Unit 1 to: a) determine the relative
Topic 2.11 ANALYTICAL TECHNIQUES High Resolution Mass Spectrometry Infra-red Spectroscopy HIGH RESOLUTION MASS SPECTROMETRY The technique of mass spectrometry was used in Unit 1 to: a) determine the relative
Student Projects for
 MINERALS RESOURCES Student Projects for 2016-17 The CSIRO On-line Analysis (OLA) Group offers opportunities for students to undertake applied physics research projects at our Lucas Heights laboratories.
MINERALS RESOURCES Student Projects for 2016-17 The CSIRO On-line Analysis (OLA) Group offers opportunities for students to undertake applied physics research projects at our Lucas Heights laboratories.
Dissolution Automation: Basic Questionnaire.
 Dissolution Automation: Basic Questionnaire. In order to quote the most suitable automated Dissolution System we would like you to answer this questionnaire: 1. Do you want to work : a) On line (on line
Dissolution Automation: Basic Questionnaire. In order to quote the most suitable automated Dissolution System we would like you to answer this questionnaire: 1. Do you want to work : a) On line (on line
Visible-near infrared spectroscopy to assess soil contaminated with cobalt
 Available online at www.sciencedirect.com Procedia Engineering 35 (2012 ) 245 253 International Meeting of Electrical Engineering Research ENIINVIE 2012 Visible-near infrared spectroscopy to assess soil
Available online at www.sciencedirect.com Procedia Engineering 35 (2012 ) 245 253 International Meeting of Electrical Engineering Research ENIINVIE 2012 Visible-near infrared spectroscopy to assess soil
AE 3051, Lab #16. Investigation of the Ideal Gas State Equation. By: George P. Burdell. Group E3
 AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
Regional methane emissions estimates in northern Pennsylvania gas fields using a mesoscale atmospheric inversion system
 Regional methane emissions estimates in northern Pennsylvania gas fields using a mesoscale atmospheric inversion system Thomas Lauvaux1, A. Deng1, B. Gaudet1, S. J. Richardson1, N. L. Miles1, J. N. Ciccarelli1,2,
Regional methane emissions estimates in northern Pennsylvania gas fields using a mesoscale atmospheric inversion system Thomas Lauvaux1, A. Deng1, B. Gaudet1, S. J. Richardson1, N. L. Miles1, J. N. Ciccarelli1,2,
High-pressure qnmr spectroscopy in condensed- and gas-phase towards determination of impurities and compositions of gas mixtures
 13.10.2016 High-pressure qnmr spectroscopy in condensed- and gas-phase towards determination of impurities and compositions of gas mixtures Klas Meyer, Heinrich Kipphardt, Michael Maiwald Online NMR Spectroscopy
13.10.2016 High-pressure qnmr spectroscopy in condensed- and gas-phase towards determination of impurities and compositions of gas mixtures Klas Meyer, Heinrich Kipphardt, Michael Maiwald Online NMR Spectroscopy
Atomic Emission Spectroscopy
 Atomic Emission Spectroscopy Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building:
Atomic Emission Spectroscopy Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451 Saudi Arabia Building:
2 SPECTROSCOPIC ANALYSIS
 2 SPECTROSCOPIC ANALYSIS 2.1 Introduction Chemical analysis falls into two basic categories: qualitative what is present quantitative how much is present Spectroscopy is capable of both types of analysis,
2 SPECTROSCOPIC ANALYSIS 2.1 Introduction Chemical analysis falls into two basic categories: qualitative what is present quantitative how much is present Spectroscopy is capable of both types of analysis,
Chem 310 rd. 3 Homework Set Answers
 -1- Chem 310 rd 3 Homework Set Answers 1. A double line labeled S 0 represents the _ground electronic_ state and the _ground vibrational_ state of a molecule in an excitation state diagram. Light absorption
-1- Chem 310 rd 3 Homework Set Answers 1. A double line labeled S 0 represents the _ground electronic_ state and the _ground vibrational_ state of a molecule in an excitation state diagram. Light absorption
Optical Properties of Thin Semiconductor Films
 Optical Properties of Thin Semiconductor Films Grolik Benno,KoppJoachim October, 31st 2003 1 Introduction Optical experiments provide a good way of examining the properties of semiconductors. Particulary
Optical Properties of Thin Semiconductor Films Grolik Benno,KoppJoachim October, 31st 2003 1 Introduction Optical experiments provide a good way of examining the properties of semiconductors. Particulary
Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer
 Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer Juergen Spielvogel, Lothar Keck, Xiaoai Guo, Markus Pesch Email: jsp@grimm-aerosol.com GRIMM Aerosol Technik GmbH
Comprehensive Measurement of Atmospheric Aerosols with a Wide Range Aerosol Spectrometer Juergen Spielvogel, Lothar Keck, Xiaoai Guo, Markus Pesch Email: jsp@grimm-aerosol.com GRIMM Aerosol Technik GmbH
Introduction to Gas Phase FTIR Spectroscopy
 Introduction to Gas Phase FTIR Spectroscopy Introduction to FTIR spectroscopy FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared (IR) spectroscopy, radiation
Introduction to Gas Phase FTIR Spectroscopy Introduction to FTIR spectroscopy FTIR stands for Fourier transform infrared, the preferred method of infrared spectroscopy. In infrared (IR) spectroscopy, radiation
Detection of trace contamination on metal surfaces using the handheld Agilent 4100 ExoScan FTIR
 Detection of trace contamination on metal surfaces using the handheld Agilent 4100 ExoScan FTIR Ensuring ultimate cleanliness for maximum adhesion Application Note Author John Seelenbinder Agilent Technologies,
Detection of trace contamination on metal surfaces using the handheld Agilent 4100 ExoScan FTIR Ensuring ultimate cleanliness for maximum adhesion Application Note Author John Seelenbinder Agilent Technologies,
Determination of Carbon d13c isotopic composition from DIC samples
 APPLICATION NOTE 30367 Determination of Carbon d13c isotopic composition from DIC samples Authors Introduction Magda Mandic, Nils Stoebener, Luka Mandic, Danijela Šmajgl Thermo Fisher Scientific, Bremen,
APPLICATION NOTE 30367 Determination of Carbon d13c isotopic composition from DIC samples Authors Introduction Magda Mandic, Nils Stoebener, Luka Mandic, Danijela Šmajgl Thermo Fisher Scientific, Bremen,
JRP SIB09 Elements GOOD PRACTICE GUIDE ON DEALING WITH INTERFERENCES IN ICP-MS FOR INVESTIGATING HIGH PURITY MATERIALS. Deliverable 1.2.
 JRP SIB09 Elements GOOD PRACTICE GUIDE ON DEALING WITH INTERFERENCES IN ICP-MS FOR INVESTIGATING HIGH PURITY MATERIALS Deliverable 1.2.32 (June 2015) Author : Guillaume Labarraque 1 Introduction High purity
JRP SIB09 Elements GOOD PRACTICE GUIDE ON DEALING WITH INTERFERENCES IN ICP-MS FOR INVESTIGATING HIGH PURITY MATERIALS Deliverable 1.2.32 (June 2015) Author : Guillaume Labarraque 1 Introduction High purity
Signal Processing Evaluated by Allan and Hadamard Variances
 Signal Processing Evaluated by Allan and Hadamard Variances J. Skřínský, M. Skřínská, and Zdeněk Zelinger Abstract Laser spectrometry offers high sensitivity and broad dynamic range, permitting monitoring
Signal Processing Evaluated by Allan and Hadamard Variances J. Skřínský, M. Skřínská, and Zdeněk Zelinger Abstract Laser spectrometry offers high sensitivity and broad dynamic range, permitting monitoring
Ultraviolet-Visible and Infrared Spectrophotometry
 Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
Ultraviolet-Visible and Infrared Spectrophotometry Ahmad Aqel Ifseisi Assistant Professor of Analytical Chemistry College of Science, Department of Chemistry King Saud University P.O. Box 2455 Riyadh 11451
Tips & Tricks GPC/SEC: Quantify and Get More Than Molar Mass Averages
 Tips & Tricks GPC/SEC: Quantify and Get More Than Molar Mass Averages Daniela Held, PSS Polymer Standards Service GmbH, Mainz, Germany Gel permeation chromatography/size-exclusion chromatography (GPC/SEC)
Tips & Tricks GPC/SEC: Quantify and Get More Than Molar Mass Averages Daniela Held, PSS Polymer Standards Service GmbH, Mainz, Germany Gel permeation chromatography/size-exclusion chromatography (GPC/SEC)
Luminescence transitions. Fluorescence spectroscopy
 Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Luminescence transitions Fluorescence spectroscopy Advantages: High sensitivity (single molecule detection!) Measuring increment in signal against a dark (zero) background Emission is proportional to excitation
Fourier transform infrared spectroscopy (FTIR) is a method used to obtain an infrared
 Fourier Transform Infrared Spectroscopy: Low Density Polyethylene, High Density Polyethylene, Polypropylene and Polystyrene Eman Mousa Alhajji North Carolina State University Department of Materials Science
Fourier Transform Infrared Spectroscopy: Low Density Polyethylene, High Density Polyethylene, Polypropylene and Polystyrene Eman Mousa Alhajji North Carolina State University Department of Materials Science
473-SHX Dew Point Mirror
 Humidity and Temperature Reference Hygrometer For Temperature up to 125 C Precise and stable chilled mirror dew point mirror technology High temperature optical components High temperature sample fan Cable
Humidity and Temperature Reference Hygrometer For Temperature up to 125 C Precise and stable chilled mirror dew point mirror technology High temperature optical components High temperature sample fan Cable
Live Webinar : How to be more Successful with your ACQUITY QDa Detector?
 Live Webinar : How to be more Successful with your ACQUITY QDa Detector? Q&A Transcript ---------------- Q - How do you generate multiple charges reproductively? A - If you use the same settings on the
Live Webinar : How to be more Successful with your ACQUITY QDa Detector? Q&A Transcript ---------------- Q - How do you generate multiple charges reproductively? A - If you use the same settings on the
Product Descriptions and Offerings
 Product Descriptions and Offerings Teledyne Leeman Labs is a leading innovator of analytical instrumentation for elemental analysis. Laboratories in industries ranging from environmental science, oil and
Product Descriptions and Offerings Teledyne Leeman Labs is a leading innovator of analytical instrumentation for elemental analysis. Laboratories in industries ranging from environmental science, oil and
Magnetic Property Measurement System
 Magnetic Property Measurement System Product Description Quantum Design's MPMS 3 represents the culmination of more than 3 years of development and design in the world of SQUID Magnetometry. Providing
Magnetic Property Measurement System Product Description Quantum Design's MPMS 3 represents the culmination of more than 3 years of development and design in the world of SQUID Magnetometry. Providing
IRMS. perspective. Isotope Ratio Mass Spectrometry.
 IRMS perspective Isotope Ratio Mass Spectrometry www.nu-ins.com Perspective The Nu Instruments Perspective geometry forms the basis for two state of the art IRMS instruments. Designed for flexibility,
IRMS perspective Isotope Ratio Mass Spectrometry www.nu-ins.com Perspective The Nu Instruments Perspective geometry forms the basis for two state of the art IRMS instruments. Designed for flexibility,
SmartNotes. What is meant by the term interference?
 Interference Removal on ICP-OES icap 7000 Plus Series ICP-OES SmartNotes What is meant by the term interference? In ICP-OES we speak of interference when a result is biased either by other components in
Interference Removal on ICP-OES icap 7000 Plus Series ICP-OES SmartNotes What is meant by the term interference? In ICP-OES we speak of interference when a result is biased either by other components in
MODULE 4.3 Atmospheric analysis of particulates
 MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
MODULE 4.3 Atmospheric analysis of particulates Measurement And Characterisation Of The Particulate Content 1 Total particulate concentration 1 Composition of the particulate 1 Determination of particle
Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System
 Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System Presented at the BevTech Conference, Albuquerque, NM 2018 by Charles M. Phillips Ph.D., Max Analytical Technologies Mark Taylor, Vice
Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System Presented at the BevTech Conference, Albuquerque, NM 2018 by Charles M. Phillips Ph.D., Max Analytical Technologies Mark Taylor, Vice
Nitrogen, ammonia, colorimetry, salicylate-hypochlorite, automated-segmented flow
 1. Application Nitrogen, ammonia, colorimetry, salicylate-hypochlorite, automated-segmented flow Parameters and Codes: Nitrogen, ammonia, dissolved, I-2522-90 (mg/l as N): 00608 Nitrogen, ammonia, total-in-bottom-material,
1. Application Nitrogen, ammonia, colorimetry, salicylate-hypochlorite, automated-segmented flow Parameters and Codes: Nitrogen, ammonia, dissolved, I-2522-90 (mg/l as N): 00608 Nitrogen, ammonia, total-in-bottom-material,
Advanced Spectroscopy Laboratory
 Advanced Spectroscopy Laboratory - Raman Spectroscopy - Emission Spectroscopy - Absorption Spectroscopy - Raman Microscopy - Hyperspectral Imaging Spectroscopy FERGIELAB TM Raman Spectroscopy Absorption
Advanced Spectroscopy Laboratory - Raman Spectroscopy - Emission Spectroscopy - Absorption Spectroscopy - Raman Microscopy - Hyperspectral Imaging Spectroscopy FERGIELAB TM Raman Spectroscopy Absorption
The Low-Temperature Evaporative Light-Scattering Detector (LT-ELSD)
 The Low-Temperature Evaporative Light-Scattering Detector (LT-ELSD) Basically all compounds which are less volatile than the mobile phase can be detected. Detection is based on a Universal property of
The Low-Temperature Evaporative Light-Scattering Detector (LT-ELSD) Basically all compounds which are less volatile than the mobile phase can be detected. Detection is based on a Universal property of
ESTIMATION OF UNCERTAINTY IN THE DETERMINATION OF NITROGEN OXIDES EMISSIONS
 Chemical Eng. Dept., ISEL From the SelectedWorks of João F Gomes 2006 ESTIMATION OF UNCERTAINTY IN THE DETERMINATION OF NITROGEN OXIDES EMISSIONS João F Gomes Available at: https://works.bepress.com/joao_gomes/9/
Chemical Eng. Dept., ISEL From the SelectedWorks of João F Gomes 2006 ESTIMATION OF UNCERTAINTY IN THE DETERMINATION OF NITROGEN OXIDES EMISSIONS João F Gomes Available at: https://works.bepress.com/joao_gomes/9/
