102 高點建國學士後中醫 全套詳解 有機化學 (A) I (B) II (C) III (D) IV (E) V
|
|
|
- Pierce Thomas
- 5 years ago
- Views:
Transcription
1 有機化學 I. Choose one correct answer for the following questions 單選題單選題 每題 1 分, 共計 60 分, 答錯 1 題倒扣 0.25 分, 倒扣至本大題零分為止, 未作答, 不給分亦不扣分 (C) 1. What is the major product obtained from the following reaction sequence? (A) 2. The hydroboration-oxidation procedure can be successfully employed for synthesis of deuterated derivatives, by using BD 3 instead of BH 3. What major product would you expect from the following reaction? (C) 3. Predict the major product for the following reaction. (A) I (B) II (C) III (D) IV (E) Both I and III (B) 4. The following atoms are commonly encountered in organic molecules. For which is it not possible to isolate enantiomers due to rapid inversion? (A) trivalent phosphorus (B) trivalent nitrogen (C) divalent sulfur (D) trivalent sulfur (E) both B and C 3-1
2 (E) 5. Which of these alkyl halides can NOT be used to prepare amines using Gabriel synthesis? (A) 1-bromopentane (B) 1-bromo-3-methylbutane (C) 2-bromo-3-methylpentane (D) 1-bromo-2,3-dimethylbutane (E) 2-bromo-2,3-dimethylbutane (A) 6. Which of the following structures is a Fischer projection of (2S,3S,4R)-hexane-2,3,4-triol. (B) 7. Treatment of (S)-6-chloro-1-methyl-1-cyclohexene with H 3 O is expected to produce which of the following product(s)? (A) I and III (B) II (C) II and V (D) IV (E) I, III and V. (A) 8. Which of the following substituent has the highest priority according to the Cahn-Ingold-Prelog system? (A) COOH (B) CHO (C) CH 2 OH (D) CH 3 (E) H (C) 9. What is the IUPAC name of the expected major product formed upon reaction of HCl with 3-methyl-1-butene? (A) 1-Chloro-2-methylbutane (B) 1-Chloro-3-methylbutane (C) 2-Chloro-2-methylbutane (D) 2-Chloro-3-methylbutane (E) 1-Chloropentane (D) 10. Which sequence correctly ranks the following substrates in order of increasing reactivity in an S N 1 reaction? (A) 3 < 2 < 1 (B) 2 < 3 < 1 (C) 2 < 1 < 3 (D) 1 < 3 < 2 (E) 1 < 2 < 3 (D) 11. A pure sample of (S)-phenylalanine has a specific rotation of +70 o. A mixture of the two enantiomers of phenylalanine gives a specific rotation of -7.0 o. What are the percentages of the S and R enantiomers in the mixture? (A) 75 % S, 25 % R (B) 65 % S, 35 % R (C) 55 % S, 45 % R (D) 45 % S, 55 % R (E) 35 % S, 65 % R (B) 12. Predict the major product when pyridine is treated with a mixture of nitric acid and sulfuric acid (A) 2-nitropyridine (B) 3-nitropyridine (C) 4-nitropyridine (D) 2,3-dinitropyridine (E) 2,4-dinitropyridine (A) 13. Predict the product(s) for the following reaction. 3-2
3 Diethylheptanedioate (B) 14. Which molecular formula is consistent with the following mass spectrum data? at m/z= 84, relative height=10.0% at m/z= 85, relative height=0.56% (A) C 5 H 10 O (B) C 5 H 8 O (C) C 5 H 24 (D) C 6 H 12 (E) C 4 H 6 O 2 (D) 15. Which sequence correctly ranks the following dienes in order of increasing reactivity in the Diels-Alder reaction? (A) 3 < 2 < 1 (B) 2 < 3 < 1 (C) 2 < 1 < 3 (D) 1 < 3 < 2 (E) 1 < 2 < 3 (D) 16. Rank the following compounds in increasing order of reactivity in the intramolecular displacement of p-bromophenolate to form a cyclic anhydride. (A) A < B < C (B) B < A < C (C) C < A < B (D) B < C < A (E) None of above (B) 17. Which of the following undergoes solvolysis in water more rapidly? (A) I (B) II (C) III (D) I and II (E) all of the above (C) 18. Grignard reagents react with oxirane (ethylene oxide) to form 1 o -alcohols but can be prepared in tetrahydrofuran solvent. Why is this difference in behavior observed? (A) Steric hindrance in the case of tetrahydrofuran precludes reaction with the Grignard. (B) There is a better leaving group in the oxirane molecule. (C) The oxirane ring is the more highly strained. (D) It is easier to obtain tetrahydrofuran in anhydrous condition. (E) Oxirane is a cyclic ether, while tetrahydrofuran is a hydrocarbon. (B) 19. The regioselectivity and stereospecificity in the oxymercuration-demercuration of an alkene is best described as: (A) Markovnikov orientation with syn-addition (B) Markovnikov orientation with anti-addition (C) anti-markovnikov orientation with syn-addition (D) anti-markovnikov orientation with anti-addition 3-3
4 (E) Markonikov orientation with both syn- and anti-addition (A) 20. What is the major product of the following reaction? (A) (B) (C) (D) (E) None of the above. (C) 21. What is the major product of the following reaction? (A) 22. Which of the following descriptions of the nucleoside uridine does NOT apply to the structure of the molecule? (A) The uracil base is directly bonded to the 1 position of ribofuranose in the α position. (B) The ribofuranose moiety is found in only the D configuration. (C) Nitrogen, at position 1 in the uracil base, is directly bonded to the ribofuranose moiety. (D) The 5 OH group is replaced with phosphate(s) in the nucleotide structure. (E) None of the above (C) 23. Which sequence ranks the following aromatic rings of this compound in order of increasing reactivity in an electrophilic aromatic substitution reaction (slowest to fastest reacting)? (A) 3 < 2 < 1 (B) 2 < 3 < 1 (C) 2 < 1 < 3 (D) 1 < 3 < 2 (E) 1 < 2 < 3 (A) 24. Which sequence ranks the indicated protons in order of increasing acidity? (A) 3 < 2 < 1 (B) 2 < 3 < 1 (C) 2 < 1 < 3 (D) 1 < 3 < 2 (E) 1 < 2 < 3 (B) 25. Which pair of structures represents the same compound? 3-4
5 (A) I and II (B) II and IV (C) III and IV (D) III and V (E) IV and V (D) 26. What is the major product of the following reaction? (C) 27. What is the major product of the following reaction? (E) 28. Which of the following is the strongest nucleophile? (A) NaOH (B) NaOMe (C) KOH (D) KOMe (E) KSMe ( ) 29. Which would be the major product of the reaction shown? 說明 : 答案應是, 故本題無解 (D) 30. Provide the major product for the following reaction (C) 31. Predict the major product for the following reaction. 3-5
6 (A) I (B) II (C) III (D) IV (E) I and II. (B) 32. Predict the major product for the following reaction. (B) 33. Which of the alkynes below, after undergoing an acid-catalyzed hydration, would be expected to produce two different ketones in nearly equivalent yields? (A) 1-hexyne (B) 2-hexyne (C) 3-hexyne (D) 3-methyl-1-pentyne (E) 4-methyl-1-pentyne (B) 34. Predict the products of the following reaction: (A) I, III, and IV (B) II, III, and IV (C) I, III, and V (D) II, IV, and VI (E) III and IV (D) 35. Which of the following is a correct prediction of the chemical shifts (ppm) for the signals in the 1 H NMR spectrum for the following compound? (A) a = 5.7, b = 5.2, c = 4.4, d = 1.9 (B) a = 5.2, b = 5.7, c = 1.9, d = 4.4 (C) a = 4.4, b = 5.2, c = 1.9, d = 1.9 (D) a = 5.2, b = 4.4, c = 5.7, d = 1.9 (E) a = 1.9, b = 5.7, c = 5.2, d = 4.4 (A) 36. Provide the name of the major alkene product that results when (2R,3R)-2,3-dibromopentane is treated with zinc in acetic acid. (A) (Z)-2-pentene (B) (E)-2-pentene (C) (R)-3-bromo-1-pentene (D) (S)-3-bromo-1-pentene (E) (R)-2-bromo-3-pentene 3-6
7 (D) 37. What is the major expected product(s) of the reaction shown below? (A) 2,2-Dichloropentane (B) 3,3-Dichloropentane (C) 2,3-Dichloropentane (D) A and B (E) B and C (B) 38. What is the expected major organic product from treatment of 4-methyl-2-pentyne with hydrogen in the presence of Lindlar s catalyst? (A) (E)-4-methyl-2-pentene (B) (Z)-4-methyl-2-pentene (C) (E)-2-methyl-2-pentene (D) (Z)-2-methyl-2-pentene (E) 2-methylpentane (B) 39. Refer to the equilibrium below, the correct name for the cyclic structure is. (A) α-l-ribofuranose (B) β-d-ribofuranose (C) α-l-ribopyranose (D) β-d-ribopyranose (E) None of the above (C) 40. Which of the following best describes the key mechanistic steps in the reaction of an acid chloride and an alcohol to form an ester? (A) elimination followed by addition (B) addition followed by decarboxylation (C) addition followed by elimination (D) substitution followed by addition (E) rearrangement (D) 41. Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base. Bases catalyze hydration by: (A) making the carbonyl group more electrophilic (B) shifting the equilibrium of the reaction (C) making the carbonyl group less electrophilic (D) converting the water to hydroxide ion, a much better nucleophile (E) None of the above (E) 42. Which of the following would produce a mixture of products when treated under appropriate conditions with N-bromosuccinimide? (A) oct-4-ene (B) hept-1-ene (C) 4,4-dimethylcyclopentene (D) 4,5-dimethylcyclohexene (E) all produce a mixture of products 說明 : 原題目因未說明反應條件, 因此產物可經由 radical 或 electrophilic 路徑產生 本題之正確答案應為 (E), 原公布之答案 (B) 有誤 (C) 43. Which of the following statements correctly characterizes the following compound? (A) contains 6π electrons and is aromatic (B) contains 6π electrons and is nonaromatic (C) contains 8π electrons and is antiaromatic (D) contains 8π electrons and is aromatic 3-7
8 (E) contains 8π electrons and is nonaromatic 說明 :Azepine 是平面且有 8 對電子, 屬 antiaromatic (A) 44. What is the major product of the following reaction? (A) (B) (C) (D) (E) (B) 45. The 1 H NMR spectrum of which of the compounds below, all of formula C 7 H 12 O 2, would consist of three singlets only? (C) 46. Arrange the following in order of increasing basicity: aniline, p-nitroaniline, p-toluidine, and p-methoxyaniline. (A) p-toluidine < p-methoxyaniline < aniline < p-nitroaniline (B) p-nitroaniline < p-toluidine < aniline < p-methoxyaniline (C) p-nitroaniline < aniline < p-toluidine < p-methoxyaniline (D) p-methoxyaniline < p-nitroaniline < p-toluidine < aniline (E) None of the above (D) 47. What is the thermodynamic product of the sulfonation of naphthalene? (A) (B) (C) (D) (E) (D) 48. For the reaction shown, which of the compounds listed below would be the expected major, and final, organic product? (D) 49. Predict the major product for the following Claisen rearrangement. 3-8
9 (A) I (B) II (C) III (D) IV (E) None of the above (A) 50. Predict the major product for the following reaction. (B) 51. Which of the following reagents can be used to cleave a tert-butoxycarbonyl (Boc) protecting group from a peptide? (A) H 2 /Pd (B) CF 3 CO 2 H (C) Na 2 CO 3, H 2 O (D) LiAlH 4 (E) None of the above (D) 52. The reaction shown below would be expected to produce as major products which of the following compounds? (A) (B) (C) (D) (E) (D) 53. What is the best method for the preparation of m-dibromobenzene from benzene? (A) 1). HNO 3 /H 2 SO 4 ; 2). Sn/HCl; 3). NaNO 2 /HCl, 0 C; 4). Br 2 /FeBr 3, twice. (B) 1). HNO 3 /H 2 SO 4 ; 2). Sn/HCl; 3). NaNO 2 /HCl, 0 C; 4). Br 2 /FeBr 3 ; 5). H 3 PO 2. (C) 1). HNO 3 /H 2 SO 4 ; 2). Sn/HCl; 3). NaNO 2 /HCl, 0 C; 4). H 3 PO 2 ; 5). Br 2 /FeBr 3, twice. (D) 1). HNO 3 /H 2 SO 4 ; 2). Br 2 /FeBr 3 ; 3). Sn/HCl; 4). NaNO 2 /HCl, 0 C; 5). CuBr. 3-9
10 (E) Br 2 /FeBr 3, twice. (B) 54. Which is the best method for the synthesis of tert-butyl methyl ether? (A) CH 3 ONa + (CH 3 ) 3 CBr (B) (CH 3 ) 3 CONa + CH 3 I (C) CH 3 OH + (CH 3 ) 3 COH + H 2 SO 4 at 140 C (D) (CH 3 ) 3 CONa + CH 3 OCH 3 (E) CH 3 ONa + (CH 3 ) 3 COH (E) 55. Which of the following will result in removal of a benzyl ester protecting group? (A) acid hydrolysis only (B) decarbonylation only (C) catalytic hydrogenation only (D) both acid hydrolysis and decarbonylation (E) both catalytic hydrogenation and acid hydrolysis (D) 56. Which of the following ketones will give a positive iodoform test? (A) 3-heptanone (B) 3-hexanone (C) cyclohexanone (D) 2-pentanone (E) 2-methyl-3-hexanone (B) 57. Which of the following compounds will display a singlet, a triplet and a quartet in the 1 H NMR spectrum? (A) 1-chloro-2,2-dimethylbutane (B) 3-chloro-3-methylpentane (C) 3-chloropentane (D) 2-chloro-4-methylpentane (E) 3-chloro-2-methylpentane (A) 58. Examining the infrared spectrum of a compound allows us to: (A) determine the types of functional groups present in the compound (B) determine the carbon-hydrogen framework of the compound (C) determine the molecular weight of the compound (D) determine the nature of the conjugated pi electron system in the compound (E) None of the above is correct (B) 59. Which of these is the least reactive type of organometallic compound? (A) RK (B) R 2 Hg (C) RLi (D) R 2 Zn (E) R 3 Al (C) 60. Which one of the following compounds will have the lowest wavenumber for carbonyl absorption? 單選題單選題 每題 2 分, 共計 40 分, 答錯 1 題倒扣 0.5 分, 倒扣至本大題零分為止, 未作答, 不給分亦不扣分 (D) 61. A student measured the optical activity of an unknown sugar at two different concentrations. The results of his measurements are shown below. Given that the sample cell had a path length of 10.0 cm, calculate the specific rotation for the unknown sugar. concentration observed rotation 2.00 g sugar in 10.0 ml water g sugar in 10.0 ml water
11 (A) (B) (C) (D) (E) (B) 62. Which of the following series of synthetic steps could be used to carry out the transformation shown below? (I) H 2, Pt, (II) B 2 H 6, (III) NaNO 2, H 3 O, (IV) NaCN, HCl, (V) H 2 O 2, NaOH (VI) PCC (A) I II V (B) IV I III (C) III VI V (D) II V III (E) None of the above (A) 63. What is the major product of the following reaction? (D) 64. Which of the following sequences efficiently converts 2-methylpropene into 3-methylbutanal? (A) 1) HBr; 2) NaCCH; 3) O 3 ; 4) H 2 O (B) 1) HBr; 2) NaCCH; 3) O 3 ; 4) DMS (C) 1) HBr, ROOR; 2) NaCCH; 3) O 3 ; 4) H 2 O (D) 1) HBr, ROOR; 2) NaCCH; 3) H 2 /Ni 2 B; 4) O 3 ; (E) 1) NaCCH; 2) H 2 /Ni 2 B; 3) O 3 ; 4) DMS 5) DMS (B) 65. How many positive and negative peaks appear in the DEPT-135 and in the DEPT-90 spectrum of 2-methylpentane? (A) DEPT-135: two positive and one negative, DEPT-90: one positive (B) DEPT-135: three positive and two negative, DEPT-90: one positive (C) DEPT-135: three positive and two negative, DEPT-90: no signals (D) DEPT-135: two positive and three negative, DEPT-90: two positive (E) None of the above is correct (A) 66. The Fischer indole synthesis is the reaction of phenylhydrazine with a carbonyl compound to give the corresponding indole. For the preparation of the following indole, what carbonyl compound is needed? 3-11
12 (B) 67. What is the major product obtained from the following reaction sequence? (C) 68. Which of the following compounds can adopt a chair conformation in which there are no axial methyl groups? (A) 1,1-dimethylcyclohexane (B) cis-1,2-dimethylcyclohexane (C) trans-1,2-dimethylcyclohexane (D) trans-1,3-dimethylcyclohexane (E) everyone above have no axial methyl group (A) 69. For the following reaction sequence, which molecule is an expected major product? (A) (B ) (C) (D) (E) (B) 70. Which of these is NOT a useful method for the synthesis of 1,3-pentadiene? (A) 1,4-pentanediol + H 2 SO 4 at 180 (B) 2,4-dibromopentane + (CH 3 ) 3 COK, (CH 3 ) 3 COH at 75 (C) 2,4-pentanediol + H 2 SO 4 at 180 (D) HC CCH=CHCH 3 + H 2, Ni 2 B (P-2) (E) 1,4-dibromopentane + CH 3 CH 2 ONa, CH 3 CH 2 OH at 75 (C) 71. Which of the following series of synthetic steps could be used to carry out the transformation shown below? (I) NaOH, (II) heat, (III) CH 3 COCH 2 COOEt, EtONa, (IV) NaOH, heat, then HCl, H 2 O (A) I II III IV (B) IV II I III (C) III IV II I (D) II IV III I (E) None of the above (B) 72. What is the name of the following reaction? (A) Mixed Aldol condensation (B) Mixed Claisen condensation 3-12
13 (C) Mixed Dieckmann condensation (D) Mixed Michael reaction (E) Mixed Knoevenagel reaction 說明 :Dieckmann condensation 為分子內的 Claisen condensation, 最後形成環狀產物. 而題目是由 cyclohexanone 先轉變成 enolate 接著進行 nucleophilic acyl substitution, 本題之正確答案應為 (B) Crossed Claisen condensation (or Mixed Claisen condensation) (B) 73. Which of the following represents the HOMO of pentadienyl anion? (B (A) (C) (D) (E) ) (D) 74. Predict the major product for the following reaction sequence. (E) 75. The H-bonds formed in the tertiary structure of proteins can be differentiated from those formed in secondary structures. What is the major distinguishing factor? (A) The H-bonds in 3 structures are significantly stronger than those found in 2 structures. (B) The H-bonds in 3 structures are more random than those formed in 2 structures. (C) The H-bonds in 3 structures are formed by predictable interactions among the peptide backbone α-amine and α-carboxylate groups. (D) The H-bonds in 3 structures are formed by interactions involving the side chain R-groups. (E) Both B and D are correct. (C) 76. Predict the outcome of the following sequence of reactions. (B) 77. Which of the following compounds exhibits the pattern of m/z values: 41, 43, 57, 87, 101, 116? (A) n-butyl n-propyl ether (B) sec-butyl iso-propyl ether (C) 2-heptanol (D) hexanoic acid (E) None of the above (B) 78. How many different β-hydroxyaldehydes and β-hydroxyketones, including constitutional isomers and stereoisomers, are formed upon treatment of a mixture of acetone and acetophenone with base? 3-13
14 (A) 4 (B) 6 (C) 9 (D) 10 (E) 12 說明 : 本題因限定生成產物為 β-hydroxyaldehydes and β-hydroxyketones, 無進一步脫水 反應, 故生成產物應只有 6 種 (D) 79. Deduce the identity of the compound from the data provided. C 5 H 10 O 2 : IR (cm -1 ): 3380 (br, s). 1 H-NMR (ppm): 1.30 (s, 3H), 3.50 (t, 1H), 3.64 (d, 2H), 4.38 (d, 2H), 4.52 (d, 2H). 13 C-NMR (ppm): (q), (t), (t), (t). (A) (2,3-dimethyloxiran-2-yl)methanol (B) 1-(2-methyloxiran-2-yl)ethanol (C) 1-(oxetan-3-yl)ethanol (D) (3-methyloxetan-3-yl)methanol (E) (tetrahydrofuran-2-yl)methanol (C) 80. Predict the major product for the following reaction. (A) I (B) II (C) III (D) IV (E) None of the above 3-14
高雄醫學大學 102 學年度學士後醫學系招生考試試題科目 : 有機化學考試時間 : 80 分鐘說明 : 一 選擇題用 2B 鉛筆在 答案卡 上作答, 修正時應以橡皮擦擦拭, 不得使用修正液 ( 帶 ), 未遵照正確作答方法而致電腦無法判讀者, 考生自行負責 二 試題及答案卡必須繳回, 不得攜出試場
 高雄醫學大學 102 學年度學士後醫學系招生考試試題科目 : 有機化學考試時間 : 80 分鐘說明 : 一 選擇題用 2B 鉛筆在 答案卡 上作答, 修正時應以橡皮擦擦拭, 不得使用修正液 ( 帶 ), 未遵照正確作答方法而致電腦無法判讀者, 考生自行負責 二 試題及答案卡必須繳回, 不得攜出試場. Choose one correct answer for the following questions
高雄醫學大學 102 學年度學士後醫學系招生考試試題科目 : 有機化學考試時間 : 80 分鐘說明 : 一 選擇題用 2B 鉛筆在 答案卡 上作答, 修正時應以橡皮擦擦拭, 不得使用修正液 ( 帶 ), 未遵照正確作答方法而致電腦無法判讀者, 考生自行負責 二 試題及答案卡必須繳回, 不得攜出試場. Choose one correct answer for the following questions
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
1. Radical Substitution on Alkanes. 2. Radical Substitution with Alkenes. 3. Electrophilic Addition
 1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH 320/328 N Summer II 2018
 CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CHEM 2312 practice final. Version - II
 EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D. III > IV > I > II
 Practice Final Exam, Chemistry 2220, Organic Chemistry II 1. Rank the designated protons by 1 H NMR chemical shift ( ), highest first. A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D.
Practice Final Exam, Chemistry 2220, Organic Chemistry II 1. Rank the designated protons by 1 H NMR chemical shift ( ), highest first. A. IV > III > II > I B. IV > II > III > I C. III > IV > II > I D.
1. Which of the following compounds is the weakest base?
 I. Multiple-choice Questions Fall 2018 1. Which of the following compounds is the weakest base? a. C3C2 b. C3C2 c. N3 d. C3 e. N2 2. Which of the following functional groups is indicated by a strong and
I. Multiple-choice Questions Fall 2018 1. Which of the following compounds is the weakest base? a. C3C2 b. C3C2 c. N3 d. C3 e. N2 2. Which of the following functional groups is indicated by a strong and
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
8. What is the slow, rate-determining step, in the acidcatalyzed dehydration of 2-methyl-2-propanol?
 CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
A. B. C. D. 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal?
 Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water.
 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
1) (100 pts) 5) (20 pts) 3) (35 pts) 4) (25pts. Total (200 pts) More Tutorial at
 Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds.
 This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
This reactivity makes alkenes an important class of organic compounds because they can be used to synthesize a wide variety of other compounds. Mechanism for the addition of a hydrogen halide What happens
ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES
 ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name when given the structure, and draw the structure given the name of open-chain and monocyclic
ALCOHOLS AND PHENOLS; ETHERS AND EPOXIDES; THIOLS AND SULFIDES A STUDENT SHOULD BE ABLE TO: 1. Give the IUPAC name when given the structure, and draw the structure given the name of open-chain and monocyclic
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm
 - 1 - Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm Name: (please print, 1 pt) Page Possible Points Score 1 1 2 9 3 10 4 12 5 14 6 14 7 10 8 15 9 10 10 10 11 10 12
- 1 - Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm Name: (please print, 1 pt) Page Possible Points Score 1 1 2 9 3 10 4 12 5 14 6 14 7 10 8 15 9 10 10 10 11 10 12
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Organic Chemistry 1 CHM 2210 Exam 4 (December 10, 2001)
 Exam 4 (December 10, 2001) Name (print): Signature: Student ID Number: There are 12 multiple choice problems (4 points each) on this exam. Record the answers to the multiple choice questions on THIS PAGE.
Exam 4 (December 10, 2001) Name (print): Signature: Student ID Number: There are 12 multiple choice problems (4 points each) on this exam. Record the answers to the multiple choice questions on THIS PAGE.
Double and Triple Bonds. The addition of an electrophile and a
 Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
Unit 5: Organic Chemistry
 Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Unit 5: Organic Chemistry Organic chemistry: discipline in chemistry focussing strictly on the study of hydrocarbons compounds made up of carbon & hydrogen Organic compounds can contain other elements
Chem 1120 Midterm points Dr. Luther Giddings
 Chem 1120 Midterm 1 100 points Dr. Luther Giddings Name Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam, until it has
Chem 1120 Midterm 1 100 points Dr. Luther Giddings Name Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam, until it has
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
CHEM 203 Exam 1. Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
Chem 251 Fall Learning Objectives
 Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Organic Chemistry I: Reactions and Overview
 Organic Chemistry I: Reactions and Overview Andrew Rosen Editor: Raghav Malik January 13, 2013 Contents I Library of Synthetic Reactions 3 II Organic Trends and Essentials 4 1 The Basics: Bonding and Molecular
Organic Chemistry I: Reactions and Overview Andrew Rosen Editor: Raghav Malik January 13, 2013 Contents I Library of Synthetic Reactions 3 II Organic Trends and Essentials 4 1 The Basics: Bonding and Molecular
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
CHEMISTRY 341. Final Exam Tuesday, December 16, Problem 1 15 pts Problem 9 8 pts. Problem 2 5 pts Problem pts
 CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
CHEM Review for test 1 (ch12-15). F17. Stafford
 CHEM Multiple Choice Identify the choice that best completes the statement or answers the question. 1. What range of wavelengths corresponds to the infrared region of the electromagnetic spectrum? a. 200-400
CHEM Multiple Choice Identify the choice that best completes the statement or answers the question. 1. What range of wavelengths corresponds to the infrared region of the electromagnetic spectrum? a. 200-400
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See
 Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Exam. Name. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements is incorrect about benzene? 1) A) All of the carbon
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements is incorrect about benzene? 1) A) All of the carbon
Chapter 8 - Alkenes and Alkynes II Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles
 Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
CHAPTER 24 Organic Chemistry
 CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
Organic Chemistry Curriculum Content Outline
 Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
Organic Chemistry 2014-15 Curriculum Content Outline CHEM 0203: Organic Structure and Reactivity 1. Structure & Bonding (Brief Review from General Chemistry) a. Ionic & Covalent Bonding b. Lewis Structures
Conjugated Systems & Pericyclic Reactions
 onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
onjugated Systems & Pericyclic Reactions 1 onjugated Dienes from heats of hydrogenation-relative stabilities of conjugated vs unconjugated dienes can be studied: Name 1-Butene 1-Pentene Structural Formula
1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES. Vikasana - CET 2012
 CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
2. Which one of the following structures represents a different compound from the other three?
 1. Provide a Newman projection of the most stable conformation of 2-methylpentane, (CH 3 ) 2 CHCH 2 CH 2 CH 3, looking along the C2-C3 bond 2. Which one of the following structures represents a different
1. Provide a Newman projection of the most stable conformation of 2-methylpentane, (CH 3 ) 2 CHCH 2 CH 2 CH 3, looking along the C2-C3 bond 2. Which one of the following structures represents a different
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Physical Properties. Alcohols can be: CH CH 2 OH CH 2 CH 3 C OH CH 3. Secondary alcohol. Primary alcohol. Tertiary alcohol
 Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
Chapter 10: Structure and Synthesis of Alcohols 100 Physical Properties Alcohols can be: CH 3 CH 3 CH CH 2 OH * Primary alcohol CH 3 OH CH * CH 2 CH 3 Secondary alcohol CH 3 CH 3 * C OH CH 3 Tertiary alcohol
2011 建國學士後西醫 全套詳解 有機化學. I. Choose one correct answer for the following questions 單選題 每題 1 分, 共計 60 分, 答錯 1 題倒扣 0.25 分, 倒扣至本大題零分為止, 未作答, 不給分亦不扣分
 有機化學 I. Choose one correct answer for the following questions 單選題 每題 1 分, 共計 60 分, 答錯 1 題倒扣 0.25 分, 倒扣至本大題零分為止, 未作答, 不給分亦不扣分 (A1. Which of the following amines gives the correct order of base strengths?
有機化學 I. Choose one correct answer for the following questions 單選題 每題 1 分, 共計 60 分, 答錯 1 題倒扣 0.25 分, 倒扣至本大題零分為止, 未作答, 不給分亦不扣分 (A1. Which of the following amines gives the correct order of base strengths?
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
CH Organic Chemistry I (Katz) Practice Exam #3- Fall 2013
 C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008
 Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
Chem 1220 Midterm points Dr. Luther Giddings
 Chem 1220 Midterm 1 100 points r. Luther Giddings Name Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam, until it has
Chem 1220 Midterm 1 100 points r. Luther Giddings Name Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam, until it has
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
D) u.ltraviolet light. B) 2,3-pentadiene. B) a 1:l mixture of enantiomeric epoxides. D) a 1:l mixture of enantiomeric diols
 1) In the UV-visible spectrum of (El-1,3,5-hexatriene, the lowest energy absorption corresponds to: A) a o to n transition. B) a o to n* transition. C) a rc to o* transition. D) a o to a* transition. E)
1) In the UV-visible spectrum of (El-1,3,5-hexatriene, the lowest energy absorption corresponds to: A) a o to n transition. B) a o to n* transition. C) a rc to o* transition. D) a o to a* transition. E)
Chem ORGANIC CHEMISTRY I
 ORGANIC CHEMISTRY I CHEM 221 /2 01 Final Examination December 20, 2005 1400-1700 Dr. Cerrie ROGERS x x molecular model kits (without instructions) programmable calculators must be reset Chem 221 --- ORGANIC
ORGANIC CHEMISTRY I CHEM 221 /2 01 Final Examination December 20, 2005 1400-1700 Dr. Cerrie ROGERS x x molecular model kits (without instructions) programmable calculators must be reset Chem 221 --- ORGANIC
Chapter 7. dehydration
 hapter 7 7.1 ne problem with elimination reactions is that mixtures of products are often formed. For example, treatment of 2-bromo-2-methylbutane with K in ethanol yields a mixture of two alkene products.
hapter 7 7.1 ne problem with elimination reactions is that mixtures of products are often formed. For example, treatment of 2-bromo-2-methylbutane with K in ethanol yields a mixture of two alkene products.
ROADMAP FOR REACTIONS Chapter 6
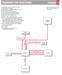 RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
RADMAP FR REACTINS Chapter 6 (A) (B) (C) (D) (E) (F) (G) (H) (I) (J) (K) (L) (M) (N) () Regiochemistry: Markovnikov addition to a bond Stereochemistry: anti-addition Regiochemistry: non-markovnikov addition
Study Union: CHM 2210 Final Exam Review 1. The following molecules are related in what manner?
 Study Union: CHM 2210 Final Exam Review 1. The following molecules are related in what manner? A) They are isotopes B) They are constitutional isomers C) They are the same structure D) They are composed
Study Union: CHM 2210 Final Exam Review 1. The following molecules are related in what manner? A) They are isotopes B) They are constitutional isomers C) They are the same structure D) They are composed
IB Topics 10, 20 & 21 MC Practice
 IB Topics 10, 20 & 21 MC Practice 1. What is the major product of the reaction between HCl and but-2-ene? 1,2-dichlorobutane 2,3-dichlorobutane 1-chlorobutane 2-chlorobutane 2. Which compound can be oxidized
IB Topics 10, 20 & 21 MC Practice 1. What is the major product of the reaction between HCl and but-2-ene? 1,2-dichlorobutane 2,3-dichlorobutane 1-chlorobutane 2-chlorobutane 2. Which compound can be oxidized
CHEMISTRY 231 GENERAL ORGANIC CHEMISTRY I FALL 2014 List of Topics / Examination Schedule
 Page 1 of 5 CHEMISTRY 231 FALL 2014 List of Topics / Examination Schedule Unit Starts Topic of Study 20 Aug 2014 STRUCTURE AND BONDING Suggested Reading: Chapter 1 29 Aug 2014 ALKANES & CYCLOALKANES Suggested
Page 1 of 5 CHEMISTRY 231 FALL 2014 List of Topics / Examination Schedule Unit Starts Topic of Study 20 Aug 2014 STRUCTURE AND BONDING Suggested Reading: Chapter 1 29 Aug 2014 ALKANES & CYCLOALKANES Suggested
Chapter 4. Reactions of alkenes. Addition reactions Carbocations Selectivity of reactions
 Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
CHE 275 REACTIONS OF ALKENES: ADDITION REACTIONS CHAP 8 ASSIGN
 HE 275 REATINS F ALKENES: ADDITIN REATINS HAP 8 ASSIGN 1. Which reagent or test could be used to distinguish between 2-methyl-2-pentene and 2-methylpentane? A. Br 2/l 4 B. KMn 4,. oncd. H 2S 4 D. Two of
HE 275 REATINS F ALKENES: ADDITIN REATINS HAP 8 ASSIGN 1. Which reagent or test could be used to distinguish between 2-methyl-2-pentene and 2-methylpentane? A. Br 2/l 4 B. KMn 4,. oncd. H 2S 4 D. Two of
1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below?
 1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below? 2. Which of the following does not have an octet of electrons surrounding the central atom? A. B 3 B. C
1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below? 2. Which of the following does not have an octet of electrons surrounding the central atom? A. B 3 B. C
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Drawing Hydrocarbons. Classifying Hydrocarbons. Four types of diagrams can be used to represent the structure of a hydrocarbon: e.g.
 Classifying Hydrocarbons alkanes- single C-C bonds, if all C s have H s attached, molecules are called hydrocarbons alkenes- have one or more C=C bonds alkynes- have one or more CΞC bonds alkenes & alkynes
Classifying Hydrocarbons alkanes- single C-C bonds, if all C s have H s attached, molecules are called hydrocarbons alkenes- have one or more C=C bonds alkynes- have one or more CΞC bonds alkenes & alkynes
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. A) I B) II C) III D) IV E) V
 Practice Questions : Chem 226 / Exam 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about benzene is correct?
Practice Questions : Chem 226 / Exam 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about benzene is correct?
Alcohol Synthesis. Dr. Sapna Gupta
 Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohols, Ethers and Epoxides. Chapter Organic Chemistry, 8th Edition John McMurry
 Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
Alcohols, Ethers and Epoxides Chapter 17-18 Organic Chemistry, 8th Edition John McMurry 1 Introduction Structure and Bonding Alcohols contain a hydroxy group (OH) bonded to an sp 3 hybridized carbon. 2
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22
 Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry
 Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Chapter 9 Aldehydes and Ketones
 Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 9 Aldehydes and Ketones 9.1 Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al The aldehyde functional group is always carbon
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
C h a p t e r E i g h t: Alkenes: Structure and Preparation via Elimination Reactions. 5-Androstene, the parent alkene for most anabolic steroids
 C h a p t e r E i g h t: Alkenes: Structure and Preparation via Elimination Reactions 5-Androstene, the parent alkene for most anabolic steroids CHM 321: Summary of Important Concepts YConcepts for Chapter
C h a p t e r E i g h t: Alkenes: Structure and Preparation via Elimination Reactions 5-Androstene, the parent alkene for most anabolic steroids CHM 321: Summary of Important Concepts YConcepts for Chapter
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Chapter 13 Alkenes and Alkynes & Aromatic Compounds
 Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
Chapter 13 Alkenes and Alkynes & Aromatic Compounds Chapter Outline 13.1 Alkenes and Alkynes 13.2 Nomenclature of Alkenes and Alkynes 13.3 Cis Trans Isomers 13.4 Alkenes in Food and Medicine 13.6 Reactions
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Chapter 9 Alkynes. Introduction
 hapter 9 Alkynes Introduction Alkynes contain a triple bond. General formula is n 2n-2. Two elements of unsaturation for each triple bond. MST reactions are like alkenes: addition and oxidation. Some reactions
hapter 9 Alkynes Introduction Alkynes contain a triple bond. General formula is n 2n-2. Two elements of unsaturation for each triple bond. MST reactions are like alkenes: addition and oxidation. Some reactions
OCH 2 CH 3. A) 2-chlorohexyl ethanoate C) ethyl 2-chlorohexanoate B) 1-chlorohexyl ethanoate D) ethyl 1-chlorohexanoate
 (2 points each) Write your answer in the box to the right of the question. 1. A mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous sodium bicarbonate solution. Which line
(2 points each) Write your answer in the box to the right of the question. 1. A mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous sodium bicarbonate solution. Which line
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
 EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
EM120 - RGANI EMISTRY WRKSEET 1 Some of the objectives To understand and know the hybridization concept Be able to distinguish different geometries, including basic bond lengths and angles within organic
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
