trans-cyclooctene 4-fluoro-1-butanol 2-cyclohexenol 4. (5 pts) Provide structural formula for each of the following molecules: Br OH Cl OH
|
|
|
- Lenard Dawson
- 5 years ago
- Views:
Transcription
1 CEMISTRY MIDTERM # 2 answer key March 12, 2009 Statistics: Average: 72 pts (72%); ighest: 98 pts (98%); Lowest: 21 pts (21%) umber of students performing at or above average: 22 (50%) umber of students performing at or below 55%: 8 (18%) 1. (8 pts) Mark as true (T) or false (F) the following statements. Do not explain! (T) All E1 reactions involve formation of carbocations; (T) More stable carbocations are generated faster; (T) Carbocations are electrophiles; (T) Carbocations are electron deficient; (T) Free radicals are electron deficient; (T) Alcohols are ønsted bases; (F) The rate-determining step is always the fastest elementary step; (F) More stable alkenes have larger heats of hydrogenation; 2. Circle ALL that apply: A. (3 pts) Free-radical brominations: a. Are chain reactions; b. ccur via carbocations; c. Require an initiation step; d. Are selective for tertiary positions; B. (3 pts) E2 reactions: a. Require strong bases; b. ccur faster at lower temperatures; c. Are subject to stereoelectronic control; d. Generate alkyl halides; C. (3 pts) The following reactions can be used to prepare alkenes: a. Free-radical chlorination of alkanes; b. ydroboration oxidation of alkenes; c. Dehydration of alcohols; d. ydrogenation of alkenes; 3. (3 pts) Provide an acceptable name for each of the following molecules: F trans-cyclooctene 4-fluoro-1-butanol 2-cyclohexenol 4. (5 pts) Provide structural formula for each of the following molecules: I Vinyl chloride Methylenecyclopropane (E)-2-Penten-1-ol Allyl iodide Trans-2-bromocyclopentanol 5. (5 pts) Assign E or Z configuration to each of the following alkenes. Do not explain! F C C C 2 C C 2 3 C C C Z E E E C 2
2 6. (3 pts) For the species below, complete the Lewis structure and draw two more valid resonance structures. early show, using the curved arrow notation, the shift of electron pairs. Rank the resonance structures (4 pts) Arrange the following alkenes in order of increasing stability. A. Cis-2-pentene; B. Trans-2-pentene; C. 2-Methyl-2-butene; D < A < B < E < C 2 D. 1-Pentene; E. 2-Methyl-1-butene; 8. (2 pts) Select the alcohol which is expected to have the fastest reaction with hydrobromic acid. A. 1-Pentanol; B. eopentyl alcohol C. 3-Pentanol; D. 2-Methyl-2-butanol; 9. (20 pts) Predict the organic product in each of the following reactions. If more than one product is formed, indicate the component. 2 3 P 4 i ac 2 5 C 2 5 RR 1) B 3.TF 1) 3 2) 2 2, - 2) 2, Zn 1 eq. 2 hν 100% 2 S 4 2 P 3 S 3 2 hν, 3 C a C 2 S 2 pyridine ac 2 5 C 2 5
3 10. (4 pts) In each of the following cases, suggest the appropriate starting material for the indicated transformation. C 2 2 1) 3 2) ( ) 2 S 11. (4 pts) Each of the following carbocations can rearrange to a more stable one. Propose structures for the likely rearranged species. In each case clearly indicate the type of rearrangement (i.e. hydride shift, methyl shift, etc.). hydride shift methyl shift 12. (6 pts) Provide one example of each of the following: A. An anti-addition reaction to an alkene; 2 2 B. A concerted reaction; C. An E1 reaction; C P (2 pts) Provide the structure of a 5-carbon alkyl iodide, which yields a single alkene upon reaction with ac 2 5 /C 2 5. I 14. (4 pts) For each of the reactions below, select the appropriate starting alkyl bromide that would yield the indicated alkene as the exclusive product. a a 15. When trans-2-methylcyclohexanol is subjected to acid-catalyzed dehydration, the product is 1-methylcyclohexene. owever, when trans-1-bromo-2-methylcyclohexane is subjected to dehydrohalogenation with ac 2 / C 2, the only product is 3-methylcyclohexene. A. (4 pts) Provide equations for the above outlined reactions; 2 S 4 ac 2 5 heat C 2 5 only
4 B. (4 pts) Account for the difference in products in the two reactions (Show explicit structures; I will not accept a verbal only explanation!! More structures, fewer words!). Dehydration: It occurs via E1 mechanism. A carbocation is formed as an intermediate and it generates the alkene products according to the Zaitsev Rule, yielding predominantly the most substituted, 1-methylcyclohexene. C minor shift C2 minor Dehydrobromination: It occurs via E2 mechanism (i.e. a concerted process). The type and quantity of product(s) is subject to stereoelectronic control. Abstraction of proton can occur only from C β bonds that are coplanar to the C bond (best is anti-coplanar, or at least syn-coplanar). The starting material can exist in two conformations, of which only the diaxial has E C β bond, which is anti-coplanar to the C bond. nly this particular proton can be abstracted by the base, yielding a single product: 3-methylcyclohexene. 3 C C C no coplanar C - bonds E anti-coplanar C - bond 16. (2 pts) Provide the structure of an octane isomer, which yields only one monochlorinated product upon free-radical chlorination. 17. (4 pts) The addition of to 1-isopropylcyclohexene yields a rearranged product as shown below. ffer a detailed mechanism to account for the reaction outcome. -shift
5 18. Suggest synthetic sequences for each of the following transformations: A. (3 pts) Conversion of 1-propanol to 2-propanol. 3 P B. (4 pts) Conversion of cyclopentane to. 2 hν a C (2 pts) BUS PRBLEM (In order to receive credit for this problem, it has to be solved entirely!!). There are other reagents, besides, that can add to alkenes following a free-radical mechanism, in the presence of peroxides. Suggest a plausible mechanism and predict the product of free-radical addition of methanethiol ( S) to propene. S RR?? Initiation: 1) R R 2 R 2) R S R S Propagation: 1) S S 2) S S S S verall: S RR S
B. (2 pts) The regiochemistry of alcohol dehydration is determined by the: a. Zaitsev rule; b. Hammond postulate;
 CEMISTRY 313-01 MIDTERM # answer key ctober 1, 008 Statistics: Average: 73 pts (73%); ighest: 98 pts (98%); Lowest: 3 pts (3%) Number of students performing at or above average: 36 (58%) Number of students
CEMISTRY 313-01 MIDTERM # answer key ctober 1, 008 Statistics: Average: 73 pts (73%); ighest: 98 pts (98%); Lowest: 3 pts (3%) Number of students performing at or above average: 36 (58%) Number of students
3. (6 pts) Provide an acceptable name for each of the following molecules: OH
 CEMISTRY 313-61 MIDTERM # 2 answer key June 07, 2005 Statistics: Average: 71 pts (71%); ighest: 99 pts (99%); Lowest: 45 pts (45%) Number of students performing at or above average: 15 (48%) 1. (11 pts)
CEMISTRY 313-61 MIDTERM # 2 answer key June 07, 2005 Statistics: Average: 71 pts (71%); ighest: 99 pts (99%); Lowest: 45 pts (45%) Number of students performing at or above average: 15 (48%) 1. (11 pts)
CHEMISTRY MIDTERM # 2 October 27, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 EMISTRY 313-01 MIDTERM # 2 ctober 27, 2005 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (7 pts) Mark as true (T) or false (F) the following
EMISTRY 313-01 MIDTERM # 2 ctober 27, 2005 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (7 pts) Mark as true (T) or false (F) the following
8. What is the slow, rate-determining step, in the acidcatalyzed dehydration of 2-methyl-2-propanol?
 CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
CHEMISTRY MIDTERM # 1 answer key October 05, 2010
 CEMISTRY 313-03 MIDTERM # 1 answer key ctober 05, 2010 Statistics: Average: 73 pts (73%); ighest: 99 pts (99%); Lowest: 31 pts (31%) Number of students performing at or above average: 61 (52%) Number of
CEMISTRY 313-03 MIDTERM # 1 answer key ctober 05, 2010 Statistics: Average: 73 pts (73%); ighest: 99 pts (99%); Lowest: 31 pts (31%) Number of students performing at or above average: 61 (52%) Number of
Structure and Preparation of Alkenes: Elimination Reactions
 Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
Structure and Preparation of Alkenes: Elimination Reactions Alkene Nomenclature First identify the longest continuous chain that includes the double bond. Replace the -ane ending of the corresponding unbranched
CHEMISTRY MIDTERM # 1 October 06, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 313-02 MIDTERM # 1 ctober 06, 2009 ame... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (12 pts) Mark as true (T) or false (F) the following
CEMISTRY 313-02 MIDTERM # 1 ctober 06, 2009 ame... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (12 pts) Mark as true (T) or false (F) the following
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
CHEMISTRY MIDTERM # 1 answer key September 29, 2005
 CEMISTRY 313-01 MIDTERM # 1 answer key September 29, 2005 Statistics: Average: 75 pts (75%); ighest: 99 pts (99%); Lowest: 31 pts (31%) Number of students performing at or above average: 28 (57%) Number
CEMISTRY 313-01 MIDTERM # 1 answer key September 29, 2005 Statistics: Average: 75 pts (75%); ighest: 99 pts (99%); Lowest: 31 pts (31%) Number of students performing at or above average: 28 (57%) Number
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Chapter 7 Alkenes; Elimination Reactions
 hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
hapter 7 Alkenes; Elimination Reactions Alkenes Alkenes contain a carbon-carbon double bond. They are named as derivatives of alkanes with the suffix -ane changed to -ene. The parent alkane is the longest
REVIEW PROBLEMS Key. 1. Draw a complete orbital picture for the molecule shown below. Is this molecule chiral? Explain. H H.
 rganic hemistry II (E325) REVIEW PRBLEMS Key 1. Draw a complete orbital picture for the molecule shown below. Is this molecule chiral? Explain. 3 3 sp3 orbital p orbital sp2 orbital s orbital molecule
rganic hemistry II (E325) REVIEW PRBLEMS Key 1. Draw a complete orbital picture for the molecule shown below. Is this molecule chiral? Explain. 3 3 sp3 orbital p orbital sp2 orbital s orbital molecule
- NH2 or NH 3 H 2 O or CH 3 COO - BF 3 or F - I Br. or Br
 CEMSTRY 313-02 MDTERM # 3 answer key November 09, 2004 Statistics: Average: 71 pts (71%); ighest: 90 pts (90%); Lowest: 49 pts (49%) Number of students perfming at above average: 22 (51%) 1. (8 pts) Mark
CEMSTRY 313-02 MDTERM # 3 answer key November 09, 2004 Statistics: Average: 71 pts (71%); ighest: 90 pts (90%); Lowest: 49 pts (49%) Number of students perfming at above average: 22 (51%) 1. (8 pts) Mark
FOURTH EXAMINATION. (1)..20 pts... (2)..24 pts... (3)..18 pts... (4)..12 pts... (5)..12 pts... (6).. 6 pts... (7).. 8 pts... Bonus 4 pts...
 Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
CHEMISTRY MIDTERM # 1 answer key February 12, 2009
 CEMSTRY 313-01 MDTERM # 1 answer key February 12, 2009 Statistics: Average: 78 pts (78%); ighest: 97 pts (97%); Lowest: 43 pts (43%) umber of students performing at or above average: 28 (62%) umber of
CEMSTRY 313-01 MDTERM # 1 answer key February 12, 2009 Statistics: Average: 78 pts (78%); ighest: 97 pts (97%); Lowest: 43 pts (43%) umber of students performing at or above average: 28 (62%) umber of
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
Organic Chemistry The Functional Group Approach. Organic Chemistry The Functional Group Approach
 Organic Chemistry The Functional Group Approach OH Br alkane (no F.G.) alcohol halide alkene non-polar (grease, fats) O NH alkyne aromatic aldehyde/ketone imine linear flat Organic Chemistry The Functional
Organic Chemistry The Functional Group Approach OH Br alkane (no F.G.) alcohol halide alkene non-polar (grease, fats) O NH alkyne aromatic aldehyde/ketone imine linear flat Organic Chemistry The Functional
Classes of Alkenes. Alkenes and Alkynes. Saturated compounds (alkanes): Have the maximum number of hydrogen atoms attached to each carbon atom.
 Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
Alkenes and Alkynes Saturated compounds (alkanes): ave the maximum number of hydrogen atoms attached to each carbon atom. Unsaturated compounds: ave fewer hydrogen atoms attached to the carbon chain than
CHEM 2312 practice final. Version - II
 EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
EM 2312 practice final Version - II The following standardized final examination covers the entire introductory year of organic chemistry (EM 2311 and 2312 at Georgia Tech). The exam consists of 70 multiple
Solutions. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #2 - October 23, 2000
 Solutions Department of hemistry SUNY/Oneonta hem 221 - Organic hemistry I Examination #2 - October 23, 2000 INSTRUTIONS --- This examination has two parts. The first part is in multiple choice format;
Solutions Department of hemistry SUNY/Oneonta hem 221 - Organic hemistry I Examination #2 - October 23, 2000 INSTRUTIONS --- This examination has two parts. The first part is in multiple choice format;
CHEMISTRY FINAL EXAM June 25, 2005
 CEMISTRY 313-61 FINAL EXAM June 25, 2005 Name... The total number of points is 100. The total exam time is 120 min (2 h). Good luck! PART I: CNCEPTS 1. (10 pts) Mark as true (T) or false (F) the following
CEMISTRY 313-61 FINAL EXAM June 25, 2005 Name... The total number of points is 100. The total exam time is 120 min (2 h). Good luck! PART I: CNCEPTS 1. (10 pts) Mark as true (T) or false (F) the following
CHEMISTRY 341. Final Exam Tuesday, December 16, Problem 1 15 pts Problem 9 8 pts. Problem 2 5 pts Problem pts
 CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
CEMISTRY 341 Final Exam Tuesday, December 16, 1997 Name NAID Problem 1 15 pts Problem 9 8 pts Problem 2 5 pts Problem 10 21 pts Problem 3 26 pts Problem 11 15 pts Problem 4 10 pts Problem 12 6 pts Problem
(CH 3 ) 3 COH. CH 3 ONa
 1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1. Rank the following compounds in the trend requested. (15 points each) a. Rank by nucleophilicity. The strongest nucleophile is 1, while the weakest nucleophile is 5. C 3 PNa (C 3 ) 3 C C 3 Na C 3 C
1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors
 1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors I, Cl, N 3, 3 P 4 pka 10 to 5 Super strong acids 3 + pka 1.7 RC 2 pka ~ 5 acids Ph pka ~ 10 get 2, R pka ~ 16 weaker RCC (alkynes) pka ~ 26 RN 2 pka
1.13 Acid-Base Reactions: Lone-Pair Donors & Acceptors I, Cl, N 3, 3 P 4 pka 10 to 5 Super strong acids 3 + pka 1.7 RC 2 pka ~ 5 acids Ph pka ~ 10 get 2, R pka ~ 16 weaker RCC (alkynes) pka ~ 26 RN 2 pka
CHEMISTRY MIDTERM # 2 ANSWER KEY July 10, 2002
 EMISTRY 314-81 MIDTERM # 2 ANSWER KEY July 10, 2002 Statistics: Average: 47 pts (67%); ighest: 69 pts (99%); Lowest: 26 pts (37%) Number of students performing at or above average: 12 (46%) 1. (7 pts)
EMISTRY 314-81 MIDTERM # 2 ANSWER KEY July 10, 2002 Statistics: Average: 47 pts (67%); ighest: 69 pts (99%); Lowest: 26 pts (37%) Number of students performing at or above average: 12 (46%) 1. (7 pts)
CHEMISTRY MIDTERM # 3 March 31, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 Name: CEMISTRY 1-01 MIDTERM # March 1, 2009 The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (11 pts) Mark as true (T) false (F) the following statements.
Name: CEMISTRY 1-01 MIDTERM # March 1, 2009 The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (11 pts) Mark as true (T) false (F) the following statements.
ORGANIC CHEMISTRY- 1
 ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
Synthesis and Retrosynthesis
 Synthesis and Retrosynthesis Putting Reactions Together A large part of ganic chemistry involves building me complex molecules from smaller ones using a designed sequence of reactions, i.e. chemical synthesis.
Synthesis and Retrosynthesis Putting Reactions Together A large part of ganic chemistry involves building me complex molecules from smaller ones using a designed sequence of reactions, i.e. chemical synthesis.
CHE 275 REACTIONS OF ALKENES: ADDITION REACTIONS CHAP 8 ASSIGN
 HE 275 REATINS F ALKENES: ADDITIN REATINS HAP 8 ASSIGN 1. Which reagent or test could be used to distinguish between 2-methyl-2-pentene and 2-methylpentane? A. Br 2/l 4 B. KMn 4,. oncd. H 2S 4 D. Two of
HE 275 REATINS F ALKENES: ADDITIN REATINS HAP 8 ASSIGN 1. Which reagent or test could be used to distinguish between 2-methyl-2-pentene and 2-methylpentane? A. Br 2/l 4 B. KMn 4,. oncd. H 2S 4 D. Two of
Chem 3719 Example Exams. Chemistry 3719 Practice Exams
 Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
OChem1 Old Exams. Chemistry 3719 Practice Exams
 OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
OChem1 Old Exams Chemistry 3719 Practice Exams Fall 2017 Chemistry 3719 Practice Exam A1 This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
EM 331: hapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N 2 N 2 N 1 2 3 4 2. What hybrid orbitals are used to
Organic Chemistry 1 CHM 2210 Exam 3 (November 9, 2001)
 Organic Chemistry 1 CM 2210 Exam 3 (November 9, 2001) Name (print): Signature: Student ID Number: There are 14 multiple choice problems (5 points each) on this exam, however, you will only be graded out
Organic Chemistry 1 CM 2210 Exam 3 (November 9, 2001) Name (print): Signature: Student ID Number: There are 14 multiple choice problems (5 points each) on this exam, however, you will only be graded out
Chemistry 210 Organic Chemistry I Summer Semester 1999 Dr. Somnath Sarkar
 Chemistry 210 Organic Chemistry I Summer Semester 1999 Dr. Somnath Sarkar Examination #3 Elimination Reactions Structure, Synthesis and Reactions of Alkenes and alkynes. Friday, July 23, 1999, 8:40 9:40
Chemistry 210 Organic Chemistry I Summer Semester 1999 Dr. Somnath Sarkar Examination #3 Elimination Reactions Structure, Synthesis and Reactions of Alkenes and alkynes. Friday, July 23, 1999, 8:40 9:40
CHEMISTRY Statistics: 74 pts (74%) 94 pts (94%) 34 pts (34%) 26 (55%) 5 (11%)
 CEMISTRY 314-01 MITERM # 1 answer key bruary 06, 2007 Statistics: Average: 74 pts (74%) ighest: 94 pts (94%) Lowest: 34 pts (34%) Number of students performing at or above average: 26 (55%) Number of students
CEMISTRY 314-01 MITERM # 1 answer key bruary 06, 2007 Statistics: Average: 74 pts (74%) ighest: 94 pts (94%) Lowest: 34 pts (34%) Number of students performing at or above average: 26 (55%) Number of students
NAME: SPRING 2015 MIDTERM
 page 1 pts NAME: SPRING 2015 MIDTERM hemistry 231 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 4/6) Do your best on this take-home portion of your mid-term. I may grade
page 1 pts NAME: SPRING 2015 MIDTERM hemistry 231 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 4/6) Do your best on this take-home portion of your mid-term. I may grade
CHEM Lecture 7
 CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
CEM 494 Special Topics in Chemistry Illinois at Chicago CEM 494 - Lecture 7 Prof. Duncan Wardrop ctober 22, 2012 CEM 494 Special Topics in Chemistry Illinois at Chicago Preparation of Alkenes Elimination
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides"
 Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
Chapter 7 Alkenes and Alkynes I: Properties and Synthesis Elimination Reactions of Alkyl Halides The (E)-(Z) System for Designating Alkene Diastereomers The Cahn-Ingold-Prelog convention is used to assign
CHEMISTRY 231 GENERAL ORGANIC CHEMISTRY I FALL 2014 List of Topics / Examination Schedule
 Page 1 of 5 CHEMISTRY 231 FALL 2014 List of Topics / Examination Schedule Unit Starts Topic of Study 20 Aug 2014 STRUCTURE AND BONDING Suggested Reading: Chapter 1 29 Aug 2014 ALKANES & CYCLOALKANES Suggested
Page 1 of 5 CHEMISTRY 231 FALL 2014 List of Topics / Examination Schedule Unit Starts Topic of Study 20 Aug 2014 STRUCTURE AND BONDING Suggested Reading: Chapter 1 29 Aug 2014 ALKANES & CYCLOALKANES Suggested
CHEM 2423 Unit 8 Homework Answers
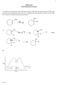 1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
1 CEM 2423 Unit 8 omework Answers 1. Show the mechanism of the following reaction. Label the rate-limiting step. Draw the potential energy diagram for this reaction. (c) Explain the regiochemistry of this
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
Part C- section 1 p-bonds as nucleophiles
 Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
H C C C C H C H C C H H. Cl H CH 3 O. Br CH 3 OH H 3 C. H Br H
 CEMISTRY 313-61 MITERM # 3 answer key June 17, 2005 Statistics: Average: 69 pts (69%); ighest: 96 pts (96%); Lowest: 46 pts (46%) Number of students perfming at above average: 16 (53%) 1. (10 pts) Mark
CEMISTRY 313-61 MITERM # 3 answer key June 17, 2005 Statistics: Average: 69 pts (69%); ighest: 96 pts (96%); Lowest: 46 pts (46%) Number of students perfming at above average: 16 (53%) 1. (10 pts) Mark
Lecture 11 Organic Chemistry 1
 EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
CHEMISTRY 241 Section 004 EXAMINATION I TUESDAY, October 11, :30-11:50 AM Professor William P. Dailey NAME: QUESTIONS POINTS SCORE
 CEMISTRY 241 Section 004 EXAMIATI I TUESDAY, ctober 11, 2005 10:30-11:50 AM Professor William P. Dailey AME: Student ID number : QUESTIS PITS SCRE 1. 16 2. 10 3. 12 4. 12 5. 12 6. 8 7. 9 8. 9 9. 15 10.
CEMISTRY 241 Section 004 EXAMIATI I TUESDAY, ctober 11, 2005 10:30-11:50 AM Professor William P. Dailey AME: Student ID number : QUESTIS PITS SCRE 1. 16 2. 10 3. 12 4. 12 5. 12 6. 8 7. 9 8. 9 9. 15 10.
What is the major product of the following reaction?
 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
CH Organic Chemistry I (Katz) Practice Exam #3- Fall 2013
 C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
1. Root of name depends on longest chain of C containing the double bond; ends in "ene"
 Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
Alkenes (β-carotene, an antioxidant pigment) n 2n (acyclic) n 2n-2 (cyclic) R R R R Key features sp 2 -hybridized carbons, 120 o bond angles σ + π bonding between = planar geometry around = "unsaturated"
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2018
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2015
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry2050 IntroductiontoOrganicChemistry FallSemester2011 Dr.RainerGlaser Examination #1 Practice Edition Bonding, Alkanes, Alkenes & Alkynes Wednesday, September 14, 2011, 10 10:50 am Answer Key Question
Chemistry2050 IntroductiontoOrganicChemistry FallSemester2011 Dr.RainerGlaser Examination #1 Practice Edition Bonding, Alkanes, Alkenes & Alkynes Wednesday, September 14, 2011, 10 10:50 am Answer Key Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry2050 IntroductiontoOrganicChemistry FallSemester2011 Dr.RainerGlaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Wednesday, September 14, 2011, 10 10:50 am Answer Key Question 1. Atomic
Chemistry2050 IntroductiontoOrganicChemistry FallSemester2011 Dr.RainerGlaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Wednesday, September 14, 2011, 10 10:50 am Answer Key Question 1. Atomic
1) (100 pts) 5) (20 pts) 3) (35 pts) 4) (25pts. Total (200 pts) More Tutorial at
 Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
Name: Perm number: Question 1) (100 pts) 2) (20 pts) 3) (35 pts) 4) (25pts 5) (20 pts) Total (200 pts) Your score 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points)
 Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points) Important notes: - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
Chemistry 2541, Fall 2017 Midterm Exam 2 (100 points) Important notes: - Please use the provided Scantron form for your answers; you can keep the sheet with the questions and can use it as scratch paper
Introduction to Alkyl Halides, Alcohols, Ethers, Thiols, and Sulfides
 8 Introduction to Alkyl alides, Alcohols, Ethers, Thiols, and Sulfides Solutions to In-Text Problems 8.1 (b) exyl iodide is a primary alkyl halide. (d) Tert-butyl chloride is a tertiary alkyl halide. 8.2
8 Introduction to Alkyl alides, Alcohols, Ethers, Thiols, and Sulfides Solutions to In-Text Problems 8.1 (b) exyl iodide is a primary alkyl halide. (d) Tert-butyl chloride is a tertiary alkyl halide. 8.2
1. Which of the following reactions would have the smallest energy of activation?.
 Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
Name: Date: 1. Which of the following reactions would have the smallest energy of activation?. A) +. +. B) + +. C) +.. + D) +.. + E) +.. + 2. Which of the following reactions would have the smallest energy
Elimination Reactions Heating an alkyl halide with a strong base causes elimination of a. molecule of HX
 Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Elimination eactions eating an alkyl halide with a strong base causes elimination of a molecule of X 1. Potassium hydroxide dissolved in ethanol and the sodium salts of alcohols (such as sodium ethoxide)
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2
 C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
C h a p t e r S e v e n : Haloalkanes: Nucleophilc Substitution and Elimination Reactions S N 2 CHM 321: Summary of Important Concepts Concepts for Chapter 7: Substitution Reactions I. Nomenclature of
CONCERTED sp 2 H. HO Et
 Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
tbuo OtBu Br 1) B 2 H 6 /THF OH 2) OH - + H 2 O 2 NaNH 2 NH3 Na NH 3 /-35 C CH 3 CCH 2 CHCH 3
 EM 12A Name KEY EXAM II all 2003 1.(16 pts) Draw the structure of the major product expected from each of the following sets of reactants. Specify stereochemistry where appropriate. + 2 Pt + 2 2 2 + enantiomer
EM 12A Name KEY EXAM II all 2003 1.(16 pts) Draw the structure of the major product expected from each of the following sets of reactants. Specify stereochemistry where appropriate. + 2 Pt + 2 2 2 + enantiomer
Chapter 7: Alkene reactions conversion to new functional groups
 hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
Chapter 8 Reactions of Alkenes
 Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chemistry 250B Final Exam Answer Key December 19, 2008
 Name: NSWER KEY 1 hemistry 250 Final Exam nswer Key ecember 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) omplete the following reactions. Show
Name: NSWER KEY 1 hemistry 250 Final Exam nswer Key ecember 19, 2008 Show non-zero formal charges on all atoms for all structures. There are 11 pages. 1. (42 pts) omplete the following reactions. Show
1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below?
 1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below? 2. Which of the following does not have an octet of electrons surrounding the central atom? A. B 3 B. C
1. What is the letter designation given to dumbbell shaped orbitals like the one depicted below? 2. Which of the following does not have an octet of electrons surrounding the central atom? A. B 3 B. C
B. (3 pts) The following values are independent of the operating frequency of the NMR: a. Coupling constant; c. Chemical shift; b. Gyromagnetic ratio;
 CEMISTRY 314-01 MIDTERM # 1 answer key September 29, 2009 Statistics: Average: 70 pts (70%); ighest: 96 pts (96%); Lowest: 37 pts (37%) Number of students performing at or above average: 16 (57%) Number
CEMISTRY 314-01 MIDTERM # 1 answer key September 29, 2009 Statistics: Average: 70 pts (70%); ighest: 96 pts (96%); Lowest: 37 pts (37%) Number of students performing at or above average: 16 (57%) Number
1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound?
 CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CEM 331: Chapter 1/2: Structures (Atoms, Molecules, Bonding) 1. What are the respective hybridizations of the atoms numbered 1 to 4 in this compound? N C 2 C N C 2 C N 1 2 3 4 1: three sigma bonds and
CHAPTER 3 ALKENES, ALKYNES & CONJUGATE DIENES
 CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
Chapter 8 Alkenes and Alkynes II: Addition Reactions "
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
Chapter 5. 3-Chloro-2-methylpentane. Cl 2. 2-Chloro-2-methylpentane. 1-Chloro-2-methylpentane. Cl 2-Chloro-4-methylpentane. 1-Chloro-4-methylpentane
 hapter 5 5.1 lassify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) 3Br K 3 KBr (b) 3 2 2 2 2 (c) 2 2 2 3 3 a. substitution b. elimination c. addition
hapter 5 5.1 lassify each of the following reactions as an addition, elimination, substitution, or rearrangement: (a) 3Br K 3 KBr (b) 3 2 2 2 2 (c) 2 2 2 3 3 a. substitution b. elimination c. addition
Name the following compounds (include stereochemistry, cis/trans, E/Z when appropriate). Cl E- 6 chloro, 5 ethyl, 4 methyl 3-octene
 Problem Set 6 Name the following compounds (include stereochemistry, cis/trans, E/Z when appropriate). E- 6 chlo, 5 ethyl, 4 methyl 3-octene 5 methyl, 3 vinyl cyclohexene 7Z- 7 bromo 4 ethyl, 6 methyl
Problem Set 6 Name the following compounds (include stereochemistry, cis/trans, E/Z when appropriate). E- 6 chlo, 5 ethyl, 4 methyl 3-octene 5 methyl, 3 vinyl cyclohexene 7Z- 7 bromo 4 ethyl, 6 methyl
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
6.10 Acid-Catalyzed Hydration of Alkenes. Acid-Catalyzed Hydration of Alkenes
 Acid-atalyzed ydration of Alkenes 6.10 Acid-atalyzed ydration of Alkenes O O reaction is acid catalyzed; typical hydration medium is 50% 2 SO 4-50% 2 O Follows Markovnikov's Rule Follows Markovnikov's
Acid-atalyzed ydration of Alkenes 6.10 Acid-atalyzed ydration of Alkenes O O reaction is acid catalyzed; typical hydration medium is 50% 2 SO 4-50% 2 O Follows Markovnikov's Rule Follows Markovnikov's
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
As time allows, additional practice questions for Exam 2 follow. This is an incomplete collection. Content & emphasis will vary.
 Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
Chem 226 Exam 2 Fall 2005: will cover Bruice, Chaps 3 through 6; Note: review e-mail & inclass quizzes and Worksheets, suggest on-line practice questions and quizzes plus ACS rganic Chemistry Guide As
1. Which of the following compounds is the weakest base?
 I. Multiple-choice Questions Fall 2018 1. Which of the following compounds is the weakest base? a. C3C2 b. C3C2 c. N3 d. C3 e. N2 2. Which of the following functional groups is indicated by a strong and
I. Multiple-choice Questions Fall 2018 1. Which of the following compounds is the weakest base? a. C3C2 b. C3C2 c. N3 d. C3 e. N2 2. Which of the following functional groups is indicated by a strong and
CHEM Exam 2 October 25, 2007
 CEM 3311-200 Exam 2 October 25, 2007 By printing my name below, I pledge that "On my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized assistance on
CEM 3311-200 Exam 2 October 25, 2007 By printing my name below, I pledge that "On my honor, as a University of Colorado at Boulder student, I have neither given nor received unauthorized assistance on
Alkenes. Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula C n H 2n. Nomenclature
 Alkenes Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula n 2n. Nomenclature Alkenes are named in the same manner as alkanes with the following adjustments. 1. Find the longest
Alkenes Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula n 2n. Nomenclature Alkenes are named in the same manner as alkanes with the following adjustments. 1. Find the longest
CHEM 203. Topics Discussed on Oct. 16
 EM 203 Topics Discussed on Oct. 16 ydrogenation (= saturation) of olefins in the presence of finely divided transition metal catalysts (Ni, Pd, Pt, Rh, Ru...): generic alkene R 1 finely divided Pd (or
EM 203 Topics Discussed on Oct. 16 ydrogenation (= saturation) of olefins in the presence of finely divided transition metal catalysts (Ni, Pd, Pt, Rh, Ru...): generic alkene R 1 finely divided Pd (or
Electrophilic Addition
 . Reactivity of = Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. arbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the
. Reactivity of = Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. arbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
Chemistry 3719, Fall 2012 Exam 1 Name: Student number:
 Chemistry 3719, Fall 2012 Exam 1 Name: Student number: This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete the exam and you may use molecular
Chemistry 3719, Fall 2012 Exam 1 Name: Student number: This exam is worth 100 points out of a total of 600 points for Chemistry 3719/3719L. You have 50 minutes to complete the exam and you may use molecular
Homework - Chapter 9 Chem 2310
 Homework - Chapter 9 Chem 2310 me:. ntroduction to organic halides 1. Draw a line structure for a compound with the following description: an alkyl halide with the formula C 5 H 11 a vinyl halide with
Homework - Chapter 9 Chem 2310 me:. ntroduction to organic halides 1. Draw a line structure for a compound with the following description: an alkyl halide with the formula C 5 H 11 a vinyl halide with
HONORS ORGANIC CHEM. HAHS MRS. RICHARDS
 NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
Departmental Final Examination. Organic Chemistry I Caffein
 Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
Departmental Final Examination rganic Chemistry I 2423 Caffein Name CEMISTRY 2423 FINAL EXAM FALL, 2005 DIRECTINS: A periodic table is attached at the end of this exam. Please answer all questions as completely
NAME: SUMMER 2015 MIDTERM
 page 1 pts NAME: SUMMER 2015 MIDTERM hemistry 350 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 6/16) Do your best on this take-home portion of your midterm. I may grade
page 1 pts NAME: SUMMER 2015 MIDTERM hemistry 350 Professor: Dr. Gergens take-home portion (DUE at the beginning of the period, 6/16) Do your best on this take-home portion of your midterm. I may grade
H H C C. Alkenes C n H 2n unsaturated hydrocarbons. C 2 H 4 ethylene. Functional group = carbon-carbon double bond
 Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene H H C C H H Functional group = carbon-carbon double bond sp 2 hybridization => flat, 120 o bond angles σ bond & π bond => H 2 C=CH 2 No rotation
Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene H H C C H H Functional group = carbon-carbon double bond sp 2 hybridization => flat, 120 o bond angles σ bond & π bond => H 2 C=CH 2 No rotation
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2014 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2014 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 18, 2014, 8:00 8:50 am Question 1. Atomic Structure
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2014 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 18, 2014, 8:00 8:50 am Question 1. Atomic Structure
Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) Can act as weak nucleophiles
 Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
Alkenes - Structure, Stability, Nomenclature Also called an olefin but alkene is better General formula C n H 2n (if one alkene present) unsaturated - contain fewer than maximum H's possible per C Can
CHEM Fall Exam 2 Professor R. Hoenigman. Signature. Name (printed) Last four digits of your student ID number.
 Average = 68 I pledge to uphold the CU on Code: CEM 3311-200 Fall 2006 Exam 2 Profess R. oenigman igh = 98 Low = 18 Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation
Average = 68 I pledge to uphold the CU on Code: CEM 3311-200 Fall 2006 Exam 2 Profess R. oenigman igh = 98 Low = 18 Signature Name (printed) Last four digits of your student ID number Recitation TA Recitation
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 21 Organic Chemistry 1
 CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
CEM 232 Organic Chemistry I at Chicago Lecture 21 Organic Chemistry 1 Professor Duncan Wardrop March 30, 2010 1 Self Test Question Ethanolysis of alkyl halide 1 gives ether 2 as one product; however, several
2. Hydrohalogenation: Propylene reacts with HBr to form 2-bromopropane.
 Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
and Ultraviolet Spectroscopy
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy 2010, Prentice all Conjugated Systems Conjugated double bonds are separated
CHEM J-10 June The structure of ( )-linalool, a commonly occurring natural product, is shown below.
 CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
CEM1102 2014-J-10 June 2014 The structure of ( )-linalool, a commonly occurring natural product, is shown below. 4 What is the molecular formula of ( )-linalool? C 10 18 O Which of the following best describes
