Organometallic Chemistry and Homogeneous Catalysis
|
|
|
- Darlene Jefferson
- 5 years ago
- Views:
Transcription
1 Organometallic hemistry and omogeneous atalysis Dr. Alexey Zazybin Lecture N2 Kashiwa ampus, October 16, 2009 Properties I. Nature of II. Nature of L II.a. Ligands with -atom attached to II.b. Ligands with other atoms attached to metal
2 Alkylidene (carbene) ligand A free carben R 2 has two spin states (not resonance forms): singlet ( ) and triplet ( ) 2 R R sp 2 R sp 2 R pz pz Two types of coordinated carbene can be distinguished: Fischer type carbene and Schrock type carbene R R sp 2 R sp 2 R pz pz
3 Fischer s group, 1964 (O) 5 W O 3 W(O) Li 3 ( 3 ) 3 O + (O) 5 W O 3 (O) 5 W O 3 3 Structural features of Fischer type carbenes: (O) 5 r=(oet)[n(i-pr) 2 ] Å (r-r single bond distances are Å) r OEt N(iPr) Å (normal distance should be 1.41 Å, a 0.06 Å shortening) 1.33 Å (normal distance should be 1.45 Å, a 0.12 Å shortening) r OEt r OEt N(iPr) 2 N(iPr) 2
4 Fischer type carbene: 4 - middle to late transition metals - low oxidation state - π acceptor ligands at (O, NO) - π donor substituents (Oe, Ne 2 ) δ δ + R R Schrock, 1973 (t-butyl- 2 ) 3 Ta 2 + 2Li( 2 -t-butyl) Ta( 2 -t-butyl) 5 unstable intermediate α-elimination (t-butyl- 2 ) 3 Ta e e e + neopentane
5 Structural features of Schrock type carbenes: 5 p 2 Ta(= 2 )( 3 ) 138 Ta Å The Ta= 2 bond is distinctly shorter than the Ta- 3 single bond! 2.03 Å Schrock type carbene: - early transition metals - high oxidation state - ligands with strong σ, π donor capacity - substituents with no π acceptor capacity (, R 3, SiR 3 ) δ + δ R R
6 Let s compare carbenes of Fischer and Schrock type: 6 Fischer arbenes Nucleophillic attacks at carbon atom of carbene (carbon is electron deficient) Electrophillic attacks on metal center (metal is more electron-rich, often d 6 18 e - system) Schrock Alkylidenes Electrophillic attacks at carbon atom of alkylidene (carbon is electron-rich) Nucleophillic attacks on metal center (metal is electron-deficient, usually d 2 or d 0 16 or 14 e - count) arbene is stabilized by heteroatom groups that can π- bond to it. Likes NR 2, SR, OR, or Ph groups. Later transition metals favored, especially with d 6 counts (carbene as neutral 2e - donor ligand) Alkylidene is destabilized by heteroatom groups that can π- bond to it. Strongly prefers or simple alkyl groups. Early transition metals favored, especially with d 0 centers (alkylidene as dianionic 4e - donor)
7 NR data for carbenes: 13 resonance for carbenes occurs to low field: ppm, 7 1 resonance for =R occurs at ppm ompound 13, ass δ (ppm) p 2 Ta(= 2 )(e) 224 Schrock (t-bu 2 ) 3 Ta(=(t-Bu) 250 Schrock (O) 5 r(=(ne 2 )) 246 Fischer (O) 5 r(=ph(oe)) 351 Fischer (O) 5 r(=ph 2 ) 399 Fischer IR data: carbene complexes are characterized usually only through ν IR absorption (ν = is seldom unambiguously identified) o= and 2945 cm 1
8 Olefinic ligands 8 First organometallic compound Zeise salt, 1831 Pt K[Pt 3 ( 2 = 2 )]. 2 O Alkenes act as neutral 2e - donors (per = double bond). Due to the presence of empty π* antibonding orbitals, there is the possibility of some π-backbonding: d c-c, nm Dewar-hatt- Duncanson bonding model (1953) 2 2 K[Pt 3 ( 2 = 2 )] p* 2 Ti( 2 = 2 ) σ-donation via the filled alkene π-system π-back donation via the empty alkene π -system
9 IR-spectroscopy in analysis of olefinic complexes Electronic effects with alkenes can often be s o m e what e a s i l y m o n i t o r e d using I R - spectroscopy. The more π-backbonding, the weaker the = double bond and the lower the = stretching frequency in the IR-spectrum Ethylene omplex ν = (cm 1 ) Free Ethylene 1623 [Ag(2=2)2] Fe(O)4(2=2) 1551 [Re(O)4(2=2)2] [pfe(o)2(2=2)] Pd24(2=2) The table shows a series of alkene compounds with differing amounts of σ-donation and π - b a c k b o n d i n g. [Pt3(2=2)] 1516 pn(o)2(2=2) 1508 Pt24(2=2) prh(2=2) Factors influence thermodynamic stability of olefinic complexes: 1) Electron-withdrawing groups on the alkene generally increase the strength of the metal-alkene bonding 2) In cases where cis-trans isomerism is possible, the more stable complex is almost always formed by the cis-alkene (steric factor) 3) Third-row metals form the strongest bonds and most stable complexes
10 Arene ligands 0 Arenes typically coordinate in an η 6 fashion and as such are neutral 6 e- donors, although they can adopt lower coordination modes (η 4 and η 2 ). An interesting aspect of metal-arene complexes is that π-back donation plays a relatively important role in the bonding and chemistry.
11 Other types of coordination: 1 The reactivity of arenes in metals complexes differs from the free state: + 3 O long time heating + 3 O r(o) 3 reflux, 1-2 h - O 3 r(o) 3 Electron-withdrawing r(o) 3 or n(o) 3 fragments facilitate nucleophilic addition and substitution and other processes.
12 Sandwich bis-arene complexes 2 Bis-arene complexes are close to bis-cyclopentadiene complexes, but arene ligand is less tightly bonded to the metal center. r- 6 6 bond energy is 40 kcal/mol Fe- 5 5 bond energy is 52 kcal/mol Ansa-metallocenes and metallocenophanes: When two rings (p or arene) are bridged together we have new class of organometallic compounds ansametallocenes. They have an application in the synthesis of stereoregular polymers, treating cancer and so on. r
13 arbonyl ligand Ludwig ond, Examples of complexes: Fe(O) 5, Ni(O) 4, o 2 (O) 8, n 2 (O) 10, r(o) 6 empty π*-acceptor orbitals on carbonyl powerful π-acceptor ligand! O excellent ligand, therefore, for stabilizing electron-rich low-valent metal centers Standard Bonding odes: O terminal mode 2e neutral donor O µ 2 bridging mode 2e neutral donor O µ 3 bridging mode 3e neutral donor
14 arbonyl IR Stretching Frequencies 4 The position of the carbonyl bands in the IR depends mainly on the bonding mode of the O (terminal, bridging) and the amount of electron density on the metal being π-backbonded to the O. The number (and intensity) of the carbonyl bands one observes depends on the number of O ligands present and the symmetry of the metal complex. Bonding odes: As one goes from a terminal O-bonding mode to µ 2 -bridging and finally µ 3 -bridging, there is a relatively dramatic drop in the O stretching frequency seen in the IR. O free O O terminal mode O O µ 2 bridging µ 3 bridging ν O IR (cm -1 ) (for neutral metal complexes)
15 The carbonyl region in the IR spectrum can be very distinctive and useful for help in assigning structures and for indicating the relative amount of electron density present on the metal: 5 Ni 2( µ -O)(O) 2(dppm) 2 O Ni O Ni O -O +O P P P P Ni (O) (dppm) O O Ni P P P P Ni O O -O +O 1 2Ni(O) ( η -dppm) 3 O O Ni P P O Wavenumbers (cm-1) 1700
16 arbonyl NR Shifts 6 Terminal carbonyl ligands generally gives rise to 13 NR resonances in the region ppm, the chemical shift (δ) being most significantly dependent on the metal center. In general, heavier metals cause shifts to the higher field. Bridging carbonyls typically resonate to lower field. O O O O 13 NR shifts: = Fe δ = 211 ppm = Ru = Os δ = 200 ppm δ = 183 ppm O Bonding -O could be drawn as a set of resonance forms: - O + O + O - X-Ray: d Ir- = 2.02 Å d Ir- = 1.87 Å [Ir(O) 5 ] 2+ d -O = 1.08 Å [(O) 2 Ir(S)(PPh 3 ) 2 ] + d -S = 1.51 Å
17 II.b. Ligands with other atoms attached to metal (, halogens, O, phosphines) 7 Phosphorus(III) ligands Examples: Phosphines: Pe 3, PPh 3 Phosphites: P(Oe) 3, P(OPh) Ph 2 P PPh 2 -P bond formation is close to -(O): R R R P Phosphine ligands excellent soft-donor ligands with a wide variety of easily adjusted steric and electronic factors neutral 2e donor But in compare with O phosphines have more donor ability: Pe 3 > PPh 3 > P(Oe) 3 > P(OPh) 3 > P(NR 2 ) 3 > O ~ PF 3
18 Tolman, cone angle θ Phosphine/Phosphite one angel Ph 3 P PPh 3 Pd P PF P(Oe) PPh 3 Ph 3 P PPh 3 Pd Ph 3 P PPh 3 Pe PPh P Pd P P(mesytil)
19 Structural features of phospine metal complexes 9 Some typical first row -PR3 average bond distances: Ti-P V-P r-p Ni-P 2.6 Å 2.5 Å 2.4 Å 2.1 Å NR 31 P characteristics of phosphine complexes 31 P NR shifts: ppm Downfield Region Upfield Region PPh (Oe) 2 PO 3 4 Pe Et 2 P 3 Pe 2 Pe 2 PPh 3 P 3 P(Oe) 3 O=Pe 3 PEt 3 Pe 3 P(N) 3 P
20 ydride ligand Walter ieber,1931 O O O Fe O 0 ydride complexes quite a common case, many of hydride complexes are stable at ambient conditions - - hydride ligand anionic 2e donor Depending on the T, the nature of this ligand can vary from acidic (o(o) 4 is a strong acid) to hydride (like in Na): δ Μ Η δ+ δ+ Μ Η δ protic hydridic 1 -NR scale: δ, ppm
21 NR characterization of hydride complexes: ost hydride ligands show 1 NR signal in hydride region from -3 to -25 ppm, although it could be find in a wider range from +25 to -60 ppm. But it could not be a valid criteria of the acidity of metal-hydrogen bond 1 o(o)4 1 NR δ = 10.7 ppm p*2zr2 1 NR δ = ppm o(o) o(o) 4 - p * 2Zr I p * Zr NR scale: δ, ppm oupling constants hydrides can couple with the atom, where this has ½ spin: 1) with other hydride ligand (if they are not equivalent) 2 J =1-10 z 2) with phosphorus atom (phosphines, phosphites) P 2 J P =15-30 z for cis-, z for trans-orientation 3) with metal atom (Pt, Rh, W) P 1 J Pt = z
22 IR characterization and types of hydride complexes 2 Terminal hydrides : o(o) 4 2- Fe 4 2- Re 9 Bridged hydrides: (O) 5 r r(o) 5 µ 2 hydride o o o o o o µ 6 hydride in [o 6 (O) 15 ] - - IR Spectra: cm-1 can be weak or absent cm -1 broader (weak or absent) D-test: ν = 2 ν D Rep 2 ν Re =2000 cm -1 DRep 2 ν ReD =1460 cm -1
23 Structural Features (X-ray, neutron diffraction) 3 ydride is the smallest ligand and as a result, - distances are typically quite short: 1.8 to about 1.5 Å, depending on the metal. ydrides can be quite difficult to observe via X-ray diffraction (the most common technique used to determine structures) due to the very small number of electrons on the hydride vs. adjacent atoms, especially the metal. Since X-rays are scattered by electron density, not by atomic nuclei it is the - bonding electrons that are detected, so that X-ray methods systematically underestimate the true - internuclear distance by approximately 0.1 Å Therefore, neutron diffraction studies are considered best for accurately locating and identifying hydrides on metal centers (proton itself scatters neutrons relatively efficiently), even so much larger crystals are usually needed for neutron work (1 mm 3 vs m 3 )
24 Reactivity of hydride complexes 4
25 alogen ligands (halide donors) 5 The halides are anionic donors that generally only donate 2e - to a metal center. Due to their relatively high electronegativity they are not especially good σ-donor ligands. Although they can theoretically act as π-donor ligands, once again, the higher electronegativity limits them to simple 2e - donor ligands. Fluoride is generally NOT a good ligand except for very high oxidation state metal centers. It is too electronegative to donate much of its electron density. Bonding modes:
26 When coordinating to a single metal center usually we regard halides as 2e - donors. 4e - can be more easily donated by I - then by any other halide, even so exceptions are possible: 6 Bridging: oordinatively unsaturated metal halides often exist as insoluble polymers connected by bridging halides. Pd 2 is a classic example. Ligands such as 3 N can displace the bridging halide to give the soluble square planar complex ( 3 N) 2 Pd 2.
27 Oxygen donors: 7 Alkoxides commonly act as bridging ligands for electropositive metals. To avoid formation of bridges, bulky alkoxides must be used. With high valent transition metals, π-donation becomes important.
28 Important information about bonding type gives -O- angle: 8 Usually in ethers (like Et2O) the -O- angle is about But in the metal-alkoxyde systems -O- angle is often approaching 120 or even 180 An angle near 120 suggests that the O is sp 2 hybridized and acting as a 4 electron donor. An angle near 180 suggests that the O is sp hybridized and acting as a 6 electron donor. In this case the alkoxide can be considered to be isoelectronic with the p ligand.
29 9
30 For organic compounds: maximum 8 electrons on : 0 s p For metal complexes: maximum 18 electrons on : s d p omplexes with 18 e - counts are referred to as saturated, because there are no empty low-lying orbitals to which another incoming ligand can coordinate. omplexes with less than 18e - are called unsaturated and can electronically bind additional ligands
31 ationic 2e- donor: NO + (nitrosyl) 1 Neutral 2e- donors: PR3 (phosphines), O (carbonyl), R2=R2 (alkenes), R R (alkynes, can also donate 4 e - ), N R (nitriles) Anionic 2e - donors: - (chloride), Br - (bromide), I - (iodide), 3 - (methyl), R3 - (alkyl), Ph - (phenyl), - (hydride) The following can also donate 4 e - if needed, but initially count them as 2e - donors (unless they are acting as bridging ligands): OR - (alkoxide), SR - (thiolate), NR2 - (amide), PR2 - (phosphide) Anionic 4e - donors: 35 - (allyl), O 2- (oxide), S2 - (sulfide), NR2 - (imide), R2 2- (alkylidene) and from the previous list: OR - (alkoxide), SR - (thiolate), NR2 - (amide), PR2 - Anionic 6e - donors: p - (cyclopentadienyl), O2 - (oxide)
32 2 Electron ount Oxidation State oordination Number Basic tools for understanding structure and reactivity. Doing them should be automatic. Not always unambiguous don t just follow the rules, understand them!
33 3 The basis of counting electrons Every element has a certain number of valence orbitals: 1 (1s) for 4 (ns, 3 np) for main group elements 9 (ns, 3 np, 5 (n-1)d) for transition metals s px py pz dxy dxz dyz dx2-y2 dz2
34 4 The basis of counting electrons Every orbital wants to be used", i.e. contribute to binding an electron pair. Therefore, every element wants to be surrounded by 2/8/18 electrons. The strength of the preference for electron-precise structures depends on the position of the element in the periodic table.
35 5 The basis of counting electrons Too few electrons: An empty orbital makes the compound very electrophilic, i.e. susceptible to attack by nucleophiles. Too many electrons: There are fewer covalent bonds than one would think (not enough orbitals available). An ionic model is required to explain part of the bonding. The "extra" bonds are relatively weak. etal-centered (unshared) electron pairs: etal orbitals are fairly high in energy. A metal atom with a lone pair is a strong σ-donor (nucleophile) and susceptible to electrophilic attack.
36 Use a localized (valence-bond) model to count electrons 6 2 Every has 2 e. OK 4 has 2 e, 8. OK N 3 N has 8 e. Nucleophile! OK N
37 2 4 has 8 e. OK 7 singlet 2 has only 6 e, and an empty p z orbital: extremely reactive ("singlet carbene"). Unstable. Sensitive to nucleophiles and electrophiles. triplet 2 has only 6 e, is a "biradical" and extremely reactive ("triplet carbene"), but not especially for nucleophiles or electrophiles.
38 3 + has only 6 e, and an empty p z orbital: extremely reactive. Unstable. Sensitive to nucleophiles has 8 e, but a lone pair. Sensitive to electrophiles. - has 8 e, 4 lone pairs. OK Somewhat sensitive to electrophiles.
39 9 B 3 B has only 6 e, not stable as monomer, B forms B 2 6 : B 2 6 B has 8 e, all 's 2 (including the bridging Al 3!). 2-electron-3-center bonds! OK Al has only 6 e, not stable as monomer, forms Al 2 6 : B Al B Al 2 6 Al has 8 e, all 's too (including the bridging!). Regular 2-electron-2-center bonds! OK Al Al
40 2 eal 2 e 2 Al e e Al Al Al Al e 2-electron-3-center bonds are a stopgap! 3 B N 3 e B N N-B: donor-acceptor bond (nucleophile N 3 has attacked electrophile B 3 ). Organometallic chemists are "sloppy" and write: B B Writing or would be more correct (although the latter does not reflect the real charge distribution). N B N N
41 1 P 5 P would have 10 e, but only has 4 valence orbitals, so it cannot form more than 4 net P- bonds. You can describe the bonding using ionic structures (hyperconjugation). Easy dissociation in P 3 en 2. P P? F 2 - Write as F F -, mainly ion-dipole interaction. F F? F F
42 Examples of electron counting: 2 Pt2(PPh3)2 1. Nature of central atom (oxidation state, number of electrons) harge of the complex: 0 harge of the nonneutral ligands: 2 * (-1) = -2 etal oxidation state: +2 Number of electrons for Pt 2+ : for Pt 0 according to the periodic table 10, For Pt 2+ : 8e - 2. Nature of ligands (number of electrons donating to the metal) 4 * (2e - - ligands) = 8e - Final result: = 16 e - Try yourself: Ne 2 e 2 N o Ne 2 Ne 2 Ph 3 P Ph 3 P PPh 3 Ir F F Rh F F PhP P Ph 2 PPh Fe P Ph 2 Br +
43 3 Try yourself: Ne 2 e 2 N o Ne 2 Ne 2 Ph 3 P Ph 3 P PPh 3 Ir F F Rh F F PhP P Ph 2 PPh Fe P Ph 2 Br +
44 4 Exercises Give electron count and oxidation state for the following compounds. Draw conclusions about their (in)stability. e 2 g Pd(Pe 3 ) 4 ereo 3 Zn 4 Pd(Pe 3 ) 3 Zne 4 2- o(o) 4 - n(o) 5 - r(o) 6 V(O) 6 - V(O) 6 Zr(O) 6 4+ Pd(Pe 3 ) 3 Rh 2 (Pe 3 ) 2 OsO 4 (pyridine) Ni(Pe 3 ) 4 Ni(Pe 3 ) 3 Ni(Pe 3 ) 2 2
O CH 3. Mn CH 3 OC C. 16eelimination
 igratory Insertion igratory Insertion/Elimination 1 A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic
igratory Insertion igratory Insertion/Elimination 1 A migratory insertion reaction is when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic
Organometallic Chemistry and Homogeneous Catalysis
 rganometallic hemistry and omogeneous atalysis Dr. Alexey Zazybin Lecture N8 Kashiwa ampus, December 11, 2009 Types of reactions in the coordination sphere of T 3. Reductive elimination X-L n -Y L n +
rganometallic hemistry and omogeneous atalysis Dr. Alexey Zazybin Lecture N8 Kashiwa ampus, December 11, 2009 Types of reactions in the coordination sphere of T 3. Reductive elimination X-L n -Y L n +
π bonded ligands alkene complexes alkyne complexes allyl complexes diene complexes cyclopentadienyl complexes arene complexes metallacycles
 π bonded ligands alkene complexes alkyne complexes allyl complexes diene complexes cyclopentadienyl complexes arene complexes metallacycles M Transition metal alkene complexes The report in 1825 by William
π bonded ligands alkene complexes alkyne complexes allyl complexes diene complexes cyclopentadienyl complexes arene complexes metallacycles M Transition metal alkene complexes The report in 1825 by William
Course 201N 1 st Semester Inorganic Chemistry Instructor: Jitendra K. Bera
 andout-8 ourse 201N 1 st Semester 2006-2007 Inorganic hemistry Instructor: Jitendra K. Bera ontents 3. Organometallic hemistry yclopentadienyl, Alkyl and Alkene yclopentadienyl p The cyclopentadienyl ligand
andout-8 ourse 201N 1 st Semester 2006-2007 Inorganic hemistry Instructor: Jitendra K. Bera ontents 3. Organometallic hemistry yclopentadienyl, Alkyl and Alkene yclopentadienyl p The cyclopentadienyl ligand
Organometallic Chemistry and Homogeneous Catalysis
 Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N1 Kashiwa Campus, October 9, 2009 What compounds we can call organometallic compounds? Compounds containing direct metal-carbon
Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N1 Kashiwa Campus, October 9, 2009 What compounds we can call organometallic compounds? Compounds containing direct metal-carbon
Reaction chemistry of complexes Three general forms: 1. Reactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b.
 eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
eaction chemistry of complexes Three general forms: 1. eactions involving the gain and loss of ligands a. Ligand Dissoc. and Assoc. (Bala) b. Oxidative Addition c. eductive Elimination d. Nucleophillic
Course 201N 1 st Semester Inorganic Chemistry Instructor: Jitendra K. Bera
 andout-9 ourse 201N 1 st Semester 2006-2007 Inorganic hemistry Instructor: Jitendra K. Bera ontents 3. rganometallic hemistry xidative Addition, Reductive Elimination, Migratory Insertion, Elimination
andout-9 ourse 201N 1 st Semester 2006-2007 Inorganic hemistry Instructor: Jitendra K. Bera ontents 3. rganometallic hemistry xidative Addition, Reductive Elimination, Migratory Insertion, Elimination
PART 2. -BONDED ORGANOMETALLICS
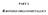 PART 2. -BODED ORGAOETALLIS V() 6 r() 6 o() 6 W() 6 Fe() 5 Ru() 5 Os() 5 i() 4 Figure 1. Binary, ononuclear etal arbonyls n n O Fe O O Fe O o O o n 2 () 10 Fe 2 () 9 o 2 () 8 Fe Fe Fe Ir() 3 Os Os Os o
PART 2. -BODED ORGAOETALLIS V() 6 r() 6 o() 6 W() 6 Fe() 5 Ru() 5 Os() 5 i() 4 Figure 1. Binary, ononuclear etal arbonyls n n O Fe O O Fe O o O o n 2 () 10 Fe 2 () 9 o 2 () 8 Fe Fe Fe Ir() 3 Os Os Os o
Introduction to Organometallic Chemistry. Stability of organometallic reagents
 Introduction to rganometallic hemistry. rganometallic chemistry is the chemistry of compounds that contain M- bonds, where M is either a main group metal or a transition metal.. ne common feature of all
Introduction to rganometallic hemistry. rganometallic chemistry is the chemistry of compounds that contain M- bonds, where M is either a main group metal or a transition metal.. ne common feature of all
Oxidative Addition/Reductive Elimination 1. Oxidative Addition
 Oxidative Addition Oxidative Addition/Reductive Elimination 1 An oxidative addition reaction is one in which (usually) a neutral ligand adds to a metal center and in doing so oxidizes the metal, typically
Oxidative Addition Oxidative Addition/Reductive Elimination 1 An oxidative addition reaction is one in which (usually) a neutral ligand adds to a metal center and in doing so oxidizes the metal, typically
Metal Hydrides, Alkyls, Aryls, and their Reactions
 Metal Hydrides, Alkyls, Aryls, and their Reactions A Primer on MO Theory σ-bonding in Organotransition Metal Complexes M-C Bond Energies in Organotransition Metal Complexes Thermodynamic Predictions
Metal Hydrides, Alkyls, Aryls, and their Reactions A Primer on MO Theory σ-bonding in Organotransition Metal Complexes M-C Bond Energies in Organotransition Metal Complexes Thermodynamic Predictions
Reaction Mechanisms - Ligand Substitutions. ML n-x P x + xl
 Reaction chanisms - igand Substitutions igand Substitutions 1 A substitution reaction is one in which an existing ligand on a metal center is replaced by another ligand. Exactly how this occurs depends
Reaction chanisms - igand Substitutions igand Substitutions 1 A substitution reaction is one in which an existing ligand on a metal center is replaced by another ligand. Exactly how this occurs depends
Chem 634. Introduction to Transition Metal Catalysis. Reading: Heg Ch 1 2 CS-B 7.1, , 11.3 Grossman Ch 6
 Chem 634 Introduction to Transition etal Catalysis eading: eg Ch 1 2 CS-B 7.1, 8.2 8.3, 11.3 Grossman Ch 6 Announcements Problem Set 1 due Thurs, 9/24 at beginning of class ffice our: Wed, 10:30-12, 220
Chem 634 Introduction to Transition etal Catalysis eading: eg Ch 1 2 CS-B 7.1, 8.2 8.3, 11.3 Grossman Ch 6 Announcements Problem Set 1 due Thurs, 9/24 at beginning of class ffice our: Wed, 10:30-12, 220
Carbenes and Olefin Metathesis
 arbenes and Olefin etathesis Peter H.. Budzelaar etal-carbon multiple bonds any transition metals form not only - single bonds but also = and (more rare) even bonds. omplexes containing an = bond are called
arbenes and Olefin etathesis Peter H.. Budzelaar etal-carbon multiple bonds any transition metals form not only - single bonds but also = and (more rare) even bonds. omplexes containing an = bond are called
Organometallics Study Meeting
 rganometallics Study eeting 04/21/2011.itsunuma 1. rystal field theory(ft) and ligand field theory(lft) FT: interaction between positively charged metal cation and negative charge on the non-bonding electrons
rganometallics Study eeting 04/21/2011.itsunuma 1. rystal field theory(ft) and ligand field theory(lft) FT: interaction between positively charged metal cation and negative charge on the non-bonding electrons
Organometallic Chemistry and Homogeneous Catalysis
 Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N6 Kashiwa Campus, November 27, 2009 Group VIB: Cr, Mo, W -Oxidation states from -2 to +6 -While +2 and +3 for Cr are quite
Organometallic Chemistry and Homogeneous Catalysis Dr. Alexey Zazybin Lecture N6 Kashiwa Campus, November 27, 2009 Group VIB: Cr, Mo, W -Oxidation states from -2 to +6 -While +2 and +3 for Cr are quite
Organometallic Chemistry
 Organometallic Chemistry Organometallic compounds combine an organic moiety with a metal in a molecule that has direct metal-carbon bonds. Ferrocene, first prepared in 1951, ushered in the modern era of
Organometallic Chemistry Organometallic compounds combine an organic moiety with a metal in a molecule that has direct metal-carbon bonds. Ferrocene, first prepared in 1951, ushered in the modern era of
EPIC LIGAND SURVEY: CARBON MONOXIDE
 EPIC LIGAND SURVEY: CARBON MONOXIDE As a young, growing field, organometallic chemistry may be taught in many ways. Some professors (e.g., Shaugnessy) spend a significant chunk of time discussing ligands,
EPIC LIGAND SURVEY: CARBON MONOXIDE As a young, growing field, organometallic chemistry may be taught in many ways. Some professors (e.g., Shaugnessy) spend a significant chunk of time discussing ligands,
Introduction to Alkenes and Alkynes
 Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene,
Insertion Reactions. 1) 1,1 insertion in which the metal and the X ligand end up bound to the same (1,1) atom
 Insertion Reactions xidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. With insertion, and its reverse reaction, elimination, we can now combine and transform
Insertion Reactions xidative addition and substitution allow us to assemble 1e and 2e ligands on the metal, respectively. With insertion, and its reverse reaction, elimination, we can now combine and transform
LIGAND DESIGN CARBENES. Fischer carbenes (B) have a heteroatom substituent on the alpha carbon atom.
 There are two main classes of carbene ligands Alkylidene (or Schrock carbene) ligands (A) have one or two alkyl or aryl substituents on the alpha carbon atom. Fischer carbenes (B) have a heteroatom substituent
There are two main classes of carbene ligands Alkylidene (or Schrock carbene) ligands (A) have one or two alkyl or aryl substituents on the alpha carbon atom. Fischer carbenes (B) have a heteroatom substituent
Repeated insertion. Multiple insertion leads to dimerization, oligomerization or polymerization. κ 1: mainly dimerization κ
 Repeated insertion ultiple insertion leads to dimerization, oligomerization or polymerization. k prop Et Key factor: k CT / k prop = κ κ 1: mainly dimerization κ 0.1-1.0: oligomerization (always mixtures)
Repeated insertion ultiple insertion leads to dimerization, oligomerization or polymerization. k prop Et Key factor: k CT / k prop = κ κ 1: mainly dimerization κ 0.1-1.0: oligomerization (always mixtures)
Explain below in one sentence each (a) how we deal with overall charges on the complex in the two methods and (b) how this can be rationalized.
 An 18 Electron Guideline Worksheet Use what you learned in the primmer and the hints below to count electrons by both the losed Shell (S) and Neutral Ligand (NL) Methods. 1. First let s figure out how
An 18 Electron Guideline Worksheet Use what you learned in the primmer and the hints below to count electrons by both the losed Shell (S) and Neutral Ligand (NL) Methods. 1. First let s figure out how
A Summary of Organometallic Chemistry
 A Summary of Organometallic Chemistry Counting valence electrons (v.e.) with the ionic model 1. Look at the total charge of the complex Ph 3 P Cl Rh Ph 3 P PPh 3 OC CO 2 Fe OC CO Co + charge:0 charge:
A Summary of Organometallic Chemistry Counting valence electrons (v.e.) with the ionic model 1. Look at the total charge of the complex Ph 3 P Cl Rh Ph 3 P PPh 3 OC CO 2 Fe OC CO Co + charge:0 charge:
Oxidative Addition and Reductive Elimination
 xidative Addition and Reductive Elimination red elim coord 2 ox add ins Peter.. Budzelaar xidative Addition Basic reaction: n + X Y n X Y The new -X and -Y bonds are formed using: the electron pair of
xidative Addition and Reductive Elimination red elim coord 2 ox add ins Peter.. Budzelaar xidative Addition Basic reaction: n + X Y n X Y The new -X and -Y bonds are formed using: the electron pair of
5.03, Inorganic Chemistry Prof. Daniel G. Nocera Lecture 4 Apr 11: Bent Metallocenes and Ziegler Natta Catalysis
 5.03, Inorganic hemistry Prof. Daniel G. Nocera Lecture 4 Apr 11: Bent Metallocenes and Ziegler Natta atalysis The electronic structure of organometallic complexes follows directly from the sandwich compounds
5.03, Inorganic hemistry Prof. Daniel G. Nocera Lecture 4 Apr 11: Bent Metallocenes and Ziegler Natta atalysis The electronic structure of organometallic complexes follows directly from the sandwich compounds
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY
 4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
4 - BENZENE: AROMATICITY, CONJUGATION AND ASSOCIATED REACTIVITY During the early 1800's, a group of compounds of natural origin became collectively known as aromatic compounds. As several of these compounds
Answers to Assignment #5
 Answers to Assignment #5 A. 9 8 l 2 5 DBE (benzene + 1 DBE) ( 9 2(9)+2-9 8+1+1 = 10 ˆ 5 DBE) nmr pattern of two doublets of equal integration at δ7.4 and 7.9 ppm means the group (the δ7.9 shift) IR band
Answers to Assignment #5 A. 9 8 l 2 5 DBE (benzene + 1 DBE) ( 9 2(9)+2-9 8+1+1 = 10 ˆ 5 DBE) nmr pattern of two doublets of equal integration at δ7.4 and 7.9 ppm means the group (the δ7.9 shift) IR band
7. Haloalkanes (text )
 2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
deactivation or decomposition is therefore quantified using the turnover number.
 A catalyst may be defined by two important criteria related to its stability and efficiency. Name both of these criteria and describe how they are defined with respect to stability or efficiency. A catalyst
A catalyst may be defined by two important criteria related to its stability and efficiency. Name both of these criteria and describe how they are defined with respect to stability or efficiency. A catalyst
Chapter 2 Acids and Bases. Arrhenius Acid and Base Theory. Brønsted-Lowry Acid and Base Theory
 hapter 2 Acids and Bases A significant amount of chemistry can be described using different theories of acids and bases. We ll consider three different acid-base theories (listed in order of increasing
hapter 2 Acids and Bases A significant amount of chemistry can be described using different theories of acids and bases. We ll consider three different acid-base theories (listed in order of increasing
σ Bonded ligands: Transition Metal Alkyls and Hydrides
 σ Bonded ligands: Transition Metal Alkyls and Hydrides Simplest of organo-transitionmetal species Rare until and understanding of their stability in the 60 s and 70 s Metal alkyls can be considered as
σ Bonded ligands: Transition Metal Alkyls and Hydrides Simplest of organo-transitionmetal species Rare until and understanding of their stability in the 60 s and 70 s Metal alkyls can be considered as
MOLECULAR REPRESENTATIONS AND INFRARED SPECTROSCOPY
 MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
MOLEULAR REPRESENTATIONS AND INFRARED SPETROSOPY A STUDENT SOULD BE ABLE TO: 1. Given a Lewis (dash or dot), condensed, bond-line, or wedge formula of a compound draw the other representations. 2. Give
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
Loudon Chapter 18 Review: Vinyl/Aryl Reactivity Jacquie Richardson, CU Boulder Last updated 2/21/2016
 Chapter 18 covers leaving groups that are directly attached to double-bonded sp 2 carbons. These molecules don t do most of the regular alkyl halide chemistry from Ch. 9 (S N1/ S N2/E1), but they can do
Chapter 18 covers leaving groups that are directly attached to double-bonded sp 2 carbons. These molecules don t do most of the regular alkyl halide chemistry from Ch. 9 (S N1/ S N2/E1), but they can do
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320
 Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Nuggets of Knowledge for Chapter 17 Dienes and Aromaticity Chem 2320 I. Isolated, cumulated, and conjugated dienes A diene is any compound with two or C=C's is a diene. Compounds containing more than two
Introduction to Organic Chemistry
 Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
Introduction to rganic hemistry 59 Introduction to rganic hemistry andout 3 - chanism u u http://burton.chem.ox.ac.uk/teaching.html rganic hemistry J. layden,. Greeves, S. Warren Stereochemistry at a Glance
Chapter 8. Acidity, Basicity and pk a
 Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
Chapter 8 Acidity, Basicity and pk a p182 In this reaction water is acting as a base, according to our definition above, by accepting a proton from HCl which in turn is acting as an acid by donating a
N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES
 N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES Zachery Matesich 24 February 2015 Roadmap 2 Introduction Synthetic Methods History of NHCs Properties of NHCs Nature of the carbene Structural properties
N-HETEROCYCLIC CARBENES: STRUCTURE AND PROPERTIES Zachery Matesich 24 February 2015 Roadmap 2 Introduction Synthetic Methods History of NHCs Properties of NHCs Nature of the carbene Structural properties
Chapter 5. Nucleophilic aliphatic substitution mechanism. by G.DEEPA
 Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Chapter 5 Nucleophilic aliphatic substitution mechanism by G.DEEPA 1 Introduction The polarity of a carbon halogen bond leads to the carbon having a partial positive charge In alkyl halides this polarity
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Chem Selected Aspects of Main Group Chemistry
 Selected Aspects of Main Group Chemistry For the rest of the course, we will look at some aspects of the chemistry of main group compounds. The basic principles that you have learned concerning atoms,
Selected Aspects of Main Group Chemistry For the rest of the course, we will look at some aspects of the chemistry of main group compounds. The basic principles that you have learned concerning atoms,
N-Heterocyclic Carbenes (NHCs)
 N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
N-Heterocyclic Carbenes (NHCs) In contrast to Fischer and Schrock type carbenes NHCs are extremely stable, inert ligands when complexed to a metal centre. Similar to phosphine ligands they are electronically
Electronic structure / bonding in d-block complexes
 LN05-1 Electronic structure / bonding in d-block complexes Many, many properties of transition metal complexes (coordination number, structure, colour, magnetism, reactivity) are very sensitive to the
LN05-1 Electronic structure / bonding in d-block complexes Many, many properties of transition metal complexes (coordination number, structure, colour, magnetism, reactivity) are very sensitive to the
EPIC LIGAND SURVEY: METAL ALKYLS - I
 EPIC LIGAND SURVEY: METAL ALKYLS - I With this post we finally reach the defining ligands of organometallic chemistry, alkyls. Metal alkyls feature a metal-carbon σ bond and are usually actor ligands,
EPIC LIGAND SURVEY: METAL ALKYLS - I With this post we finally reach the defining ligands of organometallic chemistry, alkyls. Metal alkyls feature a metal-carbon σ bond and are usually actor ligands,
PAPER No.11 : Inorganic Chemistry-II MODULE No.1 : Π-acceptor ligand, metal carbonyls, bonding modes of CO, classification of metal carbonyls
 Subject Paper No and Title Module No and Title Module Tag 11: INORGANIC CHEMISTRY-III (METAL π- COMPLEXES AND METAL CLUSTERS) 1: π-acidity, Metal carbonyls, their classification and general features CHE_P11_M1
Subject Paper No and Title Module No and Title Module Tag 11: INORGANIC CHEMISTRY-III (METAL π- COMPLEXES AND METAL CLUSTERS) 1: π-acidity, Metal carbonyls, their classification and general features CHE_P11_M1
CHEM 203. Topics Discussed on Oct. 16
 EM 203 Topics Discussed on Oct. 16 ydrogenation (= saturation) of olefins in the presence of finely divided transition metal catalysts (Ni, Pd, Pt, Rh, Ru...): generic alkene R 1 finely divided Pd (or
EM 203 Topics Discussed on Oct. 16 ydrogenation (= saturation) of olefins in the presence of finely divided transition metal catalysts (Ni, Pd, Pt, Rh, Ru...): generic alkene R 1 finely divided Pd (or
5. Reactions of Alkenes (text )
 2009, Department of hemistry, The University of Western Ontario 5.1 5. Reactions of Alkenes (text 5.1 5.5) A. Addition Reactions In hapter 4, we saw that π bonds have electron density on two sides of the
2009, Department of hemistry, The University of Western Ontario 5.1 5. Reactions of Alkenes (text 5.1 5.5) A. Addition Reactions In hapter 4, we saw that π bonds have electron density on two sides of the
11, Inorganic Chemistry III (Metal π-complexes and Metal Clusters) Module 31: Preparation and reactions of metal clusters
 1 Subject Paper No and Title Module No and Title Module Tag Chemistry 11, Inorganic Chemistry III (Metal π-complexes and Module 31: Preparation and reactions of metal clusters CHE_P11_M31 2 TABLE OF CONTENTS
1 Subject Paper No and Title Module No and Title Module Tag Chemistry 11, Inorganic Chemistry III (Metal π-complexes and Module 31: Preparation and reactions of metal clusters CHE_P11_M31 2 TABLE OF CONTENTS
B. Electron Deficient (less than an octet) H-Be-H. Be does not need an octet Total of 4 valence electrons
 B. Electron Deficient (less than an octet) e.g. BeH 2 H-Be-H Be does not need an octet Total of 4 valence electrons Not the same as unsaturated systems that achieve the 8e - (octet) through the formation
B. Electron Deficient (less than an octet) e.g. BeH 2 H-Be-H Be does not need an octet Total of 4 valence electrons Not the same as unsaturated systems that achieve the 8e - (octet) through the formation
Introduction to Organometallic Compounds. Metal. R MgX. Wilkinson s catalyst is used for hydrogenation of alkene and alkyne. Wilkinson's catalyst
 Introduction to rganometallic mpounds 1 Chapter 1 Introduction to rganometallic mpounds Introduction : Edward Frankland was father of organometallic chemistry for a complex to be organometallic compound,
Introduction to rganometallic mpounds 1 Chapter 1 Introduction to rganometallic mpounds Introduction : Edward Frankland was father of organometallic chemistry for a complex to be organometallic compound,
Chap. 5 Reactive intermediates
 Energy surface hap. 5 Reactive intermediates The plot of energy (potential and kinetic) as a function of 3N- 6 coordinates of the chemical system (reactants, intermediates, products) Reaction oordinate
Energy surface hap. 5 Reactive intermediates The plot of energy (potential and kinetic) as a function of 3N- 6 coordinates of the chemical system (reactants, intermediates, products) Reaction oordinate
Basic Organic Chemistry
 Basic rganic hemistry ourse code: EM 12162 (Pre-requisites : EM 11122) hapter 06 hemistry of Aldehydes & Ketones Dr. Dinesh R. Pandithavidana ffice: B1 222/3 Phone: (+94)777-745-720 (Mobile) Email: dinesh@kln.ac.lk
Basic rganic hemistry ourse code: EM 12162 (Pre-requisites : EM 11122) hapter 06 hemistry of Aldehydes & Ketones Dr. Dinesh R. Pandithavidana ffice: B1 222/3 Phone: (+94)777-745-720 (Mobile) Email: dinesh@kln.ac.lk
THE ORGANOMETALLIC CHEMISTRY OF THE TRANSITION METALS
 THE ORGANOMETALLIC CHEMISTRY OF THE TRANSITION METALS Second Edition ROBERT H. CRABTREE Yale University New Haven, Connecticut A Wiley-Interscience Publication JOHN WILEY & SONS New York / Chichester /
THE ORGANOMETALLIC CHEMISTRY OF THE TRANSITION METALS Second Edition ROBERT H. CRABTREE Yale University New Haven, Connecticut A Wiley-Interscience Publication JOHN WILEY & SONS New York / Chichester /
Chapter 2 The Elementary Steps in TM Catalysis
 hapter 2 The Elementary Steps in TM atalysis + + ligand exchange A oxidative addition > n + A B n+2 reductive elimination < B n n+2 oxidative coupling + M' + M' transmetallation migratory insertion > (carbo-,
hapter 2 The Elementary Steps in TM atalysis + + ligand exchange A oxidative addition > n + A B n+2 reductive elimination < B n n+2 oxidative coupling + M' + M' transmetallation migratory insertion > (carbo-,
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
11/5/ Conjugated Dienes. Conjugated Dienes. Conjugated Dienes. Heats of Hydrogenation
 8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
8.12 Sites of unsaturation Many compounds have numerous sites of unsaturation If sites are well separated in molecule they react independently If sites are close together they may interact with one another
Week 6 notes CHEM
 Week 6 notes EM1002 2009 Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, hemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 1 Note
Week 6 notes EM1002 2009 Unless otherwise stated, all images in this file have been reproduced from: Blackman, Bottle, Schmid, Mocerino and Wille, hemistry, 2007 (John Wiley) ISBN: 9 78047081 0866 1 Note
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy
 Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Chapter 15: Conjugated Systems, Orbital Symmetry, and UV Spectroscopy Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double (e.g.
Lecture 1: Chemistry of the Carbonyl Group
 Lecture 1: Chemistry of the Carbonyl Group bjectives: By the end of this lecture you will be able to: 1. identify and name all major carbonyl functional groups; 2. use a molecular orbital approach to describe
Lecture 1: Chemistry of the Carbonyl Group bjectives: By the end of this lecture you will be able to: 1. identify and name all major carbonyl functional groups; 2. use a molecular orbital approach to describe
Learning Guide for Chapter 17 - Dienes
 Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Learning Guide for Chapter 17 - Dienes I. Isolated, conjugated, and cumulated dienes II. Reactions involving allylic cations or radicals III. Diels-Alder Reactions IV. Aromaticity I. Isolated, Conjugated,
Chemistry 424 Organometallic Chemistry
 hemistry 424 rganometallic hemistry 424 chem ourse Dr. Waed Z Al-Kayali First term, 1435-36 Department of hemistry King Saud University lass eeting: theory (Sun, Tues)(10-11) and (sun 8-9) Semester credit
hemistry 424 rganometallic hemistry 424 chem ourse Dr. Waed Z Al-Kayali First term, 1435-36 Department of hemistry King Saud University lass eeting: theory (Sun, Tues)(10-11) and (sun 8-9) Semester credit
The 18 Electron Rule. References: Gray: chapter 5 OGN: chapter 18
 The 18 Electron Rule References: Gray: chapter 5 OGN: chapter 18 Element Groups Alkali metals nert or Noble gases Alkali earths alogens e Li Na Be Mg Transition metals B Al Si N P O S F l Ne Ar K Rb s
The 18 Electron Rule References: Gray: chapter 5 OGN: chapter 18 Element Groups Alkali metals nert or Noble gases Alkali earths alogens e Li Na Be Mg Transition metals B Al Si N P O S F l Ne Ar K Rb s
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry
 OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
CHAPTER 2: Structure and Properties of Organic Molecules
 1 HAPTER 2: Structure and Properties of Organic Molecules Atomic Orbitals A. What are atomic orbitals? Atomic orbitals are defined by special mathematical functions called wavefunctions-- (x, y, z). Wavefunction,
1 HAPTER 2: Structure and Properties of Organic Molecules Atomic Orbitals A. What are atomic orbitals? Atomic orbitals are defined by special mathematical functions called wavefunctions-- (x, y, z). Wavefunction,
Classes of Organic Compounds
 Unit 1 Functional Groups Depicting Structures of rganic ompounds Lewis Structures ondensed structural formulas Line angle drawings 3-dimensional structures Resonance Structures Acid-Base Reactions urved
Unit 1 Functional Groups Depicting Structures of rganic ompounds Lewis Structures ondensed structural formulas Line angle drawings 3-dimensional structures Resonance Structures Acid-Base Reactions urved
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Topic 9. Aldehydes & Ketones
 Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Recommended Reading: 20, , 13 (3rd edition); 19, , 13 (4th edition)
 Recommended Reading: 20, 21.2-21.4, 13 (3rd edition); 19, 20.2-20.4, 13 (4th edition) h 102 Problem Set 5 Due: Thursday, May 17 Before lass Problem 1 (2 points) Part A Hard and soft acids and bases (HSAB)
Recommended Reading: 20, 21.2-21.4, 13 (3rd edition); 19, 20.2-20.4, 13 (4th edition) h 102 Problem Set 5 Due: Thursday, May 17 Before lass Problem 1 (2 points) Part A Hard and soft acids and bases (HSAB)
Amines Reading Study Problems Key Concepts and Skills Lecture Topics: Amines: structure and nomenclature
 Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Amines Reading: Wade chapter 19, sections 19-1-19-19 Study Problems: 19-37, 19-39, 19-40, 19-41, 19-44, 19-46, 19-47, 19-48, 19-51, 19-54 Key Concepts and Skills: Explain how the basicity of amines varies
Chemistry 2000 Lecture 18: Reactions of organic compounds
 hemistry 2000 Lecture 18: Reactions of organic compounds Marc R. Roussel March 6, 2018 Marc R. Roussel Reactions of organic compounds March 6, 2018 1 / 27 Reactions of organic compounds Organic chemists
hemistry 2000 Lecture 18: Reactions of organic compounds Marc R. Roussel March 6, 2018 Marc R. Roussel Reactions of organic compounds March 6, 2018 1 / 27 Reactions of organic compounds Organic chemists
5.03 In-Class Exam 3
 5.03 In-Class Exam 3 Christopher C. Cummins April 9, 2010 Instructions Clearly write your name at the top of this front page, but otherwise do not write on this front page as it will be used for scoring.
5.03 In-Class Exam 3 Christopher C. Cummins April 9, 2010 Instructions Clearly write your name at the top of this front page, but otherwise do not write on this front page as it will be used for scoring.
Three Type Of Carbene Complexes
 Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Survey of Elements. 1s - very small atom All noble gases have very high first I.E. s which is in line with their chemical inertness
 Survey of Elements 354 Hydrogen 1s 1 Naturally, loss of one e - dominates 1s Helium 1s 2 - closed shell 1s - very small atom All noble gases have very high first I.E. s which is in line with their chemical
Survey of Elements 354 Hydrogen 1s 1 Naturally, loss of one e - dominates 1s Helium 1s 2 - closed shell 1s - very small atom All noble gases have very high first I.E. s which is in line with their chemical
CHE1502. Tutorial letter 203/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry
 E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
E1502/203/1/2016 Tutorial letter 203/1/2016 General hemistry 1B E1502 Semester 1 Department of hemistry This tutorial letter contains the answers to the questions in assignment 3. FIRST SEMESTER: KEY T
Name Date Class STUDY GUIDE FOR CONTENT MASTERY. covalent bond molecule sigma bond exothermic pi bond
 Covalent Bonding Section 9.1 The Covalent Bond In your textbook, read about the nature of covalent bonds. Use each of the terms below just once to complete the passage. covalent bond molecule sigma bond
Covalent Bonding Section 9.1 The Covalent Bond In your textbook, read about the nature of covalent bonds. Use each of the terms below just once to complete the passage. covalent bond molecule sigma bond
KOT 222 Organic Chemistry II
 KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
KOT 222 Organic Chemistry II Course Objectives: 1) To introduce the chemistry of alcohols and ethers. 2) To study the chemistry of functional groups. 3) To learn the chemistry of aromatic compounds and
Molecular Orbital Theory (MOT)
 Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
Molecular Orbital Theory (MOT) In this section, There are another approach to the bonding in metal complexes: the use of molecular orbital theory (MOT). In contrast to crystal field theory, the molecular
Chapter 7 - Alkenes and Alkynes I
 Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name
Andrew Rosen Chapter 7 - Alkenes and Alkynes I 7.1 - Introduction - The simplest member of the alkenes has the common name of ethylene while the simplest member of the alkyne family has the common name
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid
![Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid Acid-Base -Bronsted-Lowry model: -Lewis model: -The more equilibrium lies to the right = More [H 3 O + ] = Higher K a = Lower pk a = Stronger acid](/thumbs/95/123929992.jpg) Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Revision Hybridisation -The valence electrons of a Carbon atom sit in 1s 2 2s 2 2p 2 orbitals that are different in energy. It has 2 x 2s electrons + 2 x 2p electrons are available to form 4 covalent bonds.
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides"
 Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Chapter 6 Ionic Reactions-Nucleophilic Substitution and Elimination Reactions of Alkyl Halides" t Introduction" The polarity of a carbon-halogen bond leads to the carbon having a partial positive charge"
Hydrides and Dihydrogen as Ligands: Lessons from Organometallic Chemistry. Lecture 9
 ydrides and Dihydrogen as Ligands: Lessons from Organometallic Chemistry Lecture 9 Inorganic Chemistry Chapter 1: Figure 10.1 2009 W.. Freeman Synthesis of Organometallic Complex ydrides Reaction of MCO
ydrides and Dihydrogen as Ligands: Lessons from Organometallic Chemistry Lecture 9 Inorganic Chemistry Chapter 1: Figure 10.1 2009 W.. Freeman Synthesis of Organometallic Complex ydrides Reaction of MCO
FINAL EXAMINATION 12/17/93.
 INORGANIC CHEMISTRY 413/571 FINAL EXAMINATION 12/17/93. DR. J. SHERIDAN Write all answers in the answer book. WRITE NEATLY. This will help me to understand your answers and maybe get you a few more points!
INORGANIC CHEMISTRY 413/571 FINAL EXAMINATION 12/17/93. DR. J. SHERIDAN Write all answers in the answer book. WRITE NEATLY. This will help me to understand your answers and maybe get you a few more points!
Module 10 : Reaction mechanism. Lecture 1 : Oxidative addition and Reductive elimination. Objectives. In this lecture you will learn the following
 Module 10 : Reaction mechanism Lecture 1 : Oxidative addition and Reductive elimination Objectives In this lecture you will learn the following The oxidative addition reactions. The reductive elimination
Module 10 : Reaction mechanism Lecture 1 : Oxidative addition and Reductive elimination Objectives In this lecture you will learn the following The oxidative addition reactions. The reductive elimination
PART CHAPTER2. Atomic Bonding
 PART O N E APTER2 Atomic Bonding The scanning tunneling microscope (Section 4.7) allows the imaging of individual atoms bonded to a material surface. In this case, the microscope was also used to manipulate
PART O N E APTER2 Atomic Bonding The scanning tunneling microscope (Section 4.7) allows the imaging of individual atoms bonded to a material surface. In this case, the microscope was also used to manipulate
Exam Analysis: Organic Chemistry, Midterm 1
 Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
Exam Analysis: Organic Chemistry, Midterm 1 1) TEST BREAK DOWN: There are three independent topics covered in the first midterm, which are hybridization, structure and isomerism, and resonance. The test
Mechanical Approach to Drawing 2D, 3D and Resonance Structures from a condensed line formula.
 2D structure drawing hem 314 Beauchamp Mechanical Approach to Drawing 2D, 3D and esonance tructures from a condensed line formula. 1. Draw a 2D structure based on given arrangement shown in condensed line
2D structure drawing hem 314 Beauchamp Mechanical Approach to Drawing 2D, 3D and esonance tructures from a condensed line formula. 1. Draw a 2D structure based on given arrangement shown in condensed line
Benzene and Aromatic Compounds. Chapter 15 Organic Chemistry, 8 th Edition John McMurry
 Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Benzene and Aromatic Compounds Chapter 15 Organic Chemistry, 8 th Edition John McMurry 1 Background Benzene (C 6 H 6 ) is the simplest aromatic hydrocarbon (or arene). Four degrees of unsaturation. It
Module 6 : General properties of Transition Metal Organometallic Complexes. Lecture 2 : Synthesis and Stability. Objectives
 Module 6 : General properties of Transition Metal Organometallic Complexes Lecture 2 : Synthesis and Stability Objectives In this lecture you will learn the following Understand the role lead by ligands
Module 6 : General properties of Transition Metal Organometallic Complexes Lecture 2 : Synthesis and Stability Objectives In this lecture you will learn the following Understand the role lead by ligands
Acid/Base stuff Beauchamp 1
 cid/base stuff Beauchamp 1 Problems You should be able to match a pk a value with its acid in each group below and explain the differences. You should be able to draw an arrow-pushing mechanism with general
cid/base stuff Beauchamp 1 Problems You should be able to match a pk a value with its acid in each group below and explain the differences. You should be able to draw an arrow-pushing mechanism with general
Problem 1 (4 points) D2h. C2v. Part A.
 Problem 1 (4 points) In 2004, a bimetallic Zr compound exhibiting side-on N2 binding was reported by Chirik and coworkers (Nature, 2004, 427, pp. 527-530). The crystal structure of this compound was obtained,
Problem 1 (4 points) In 2004, a bimetallic Zr compound exhibiting side-on N2 binding was reported by Chirik and coworkers (Nature, 2004, 427, pp. 527-530). The crystal structure of this compound was obtained,
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Reaction mechanisms offer us insights into how reactions work / how molecules react with one another.
 Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6)
Introduction 1) Lewis Structures 2) Representing Organic Structures 3) Geometry and Hybridization 4) Electronegativities and Dipoles 5) Resonance Structures (a) Drawing Them (b) Rules for Resonance 6)
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
