Lithium Diisopropylamide-Mediated Enolizations: Solvent-Dependent Mixed Aggregation Effects
|
|
|
- Camron Garrison
- 6 years ago
- Views:
Transcription
1 J. Am. Chem. Soc. 2000, 122, Lithium Diisopropylamide-Mediated Enolizations: Solvent-Dependent Mixed Aggregation Effects Xiufeng Sun and David B. Collum* Contribution from the Department of Chemistry and Chemical Biology, Baker Laboratory, Cornell UniVersity, Ithaca, New York ReceiVed June 17, 1999 Abstract: 6 Li and 15 N NMR spectroscopic investigations of lithium diisopropylamide-mediated ester enolization in THF, t-buome, HMPA/THF, and DMPU/THF (HMPA ) hexamethylphosphoramide, DMPU ) 1,3- dimethyl-3,4,5,6-tetrahydro-2(1h)-pyrimidone) reveal substantial concentrations of mixed aggregates. While previous quantitative rate studies revealed that the metalations by lithium diisopropylamide (LDA) homonuclear dimers proceed at nearly solvent-independent rates, the reactivities of the intermediate mixed aggregates are markedly lower and quite sensitive to the choice of solvent. The autoinhibition correlates with the relative stabilities of the mixed aggregates. However, the relative stabilities do not correlate in a simple fashion with the ligating properties of the solvent. Introduction In the 1960s mixed aggregation effects on alkyllithiummediated anionic polymerizations were clearly documented. 1 Beginning with a seminal review in 1984 followed by a second in 1988, 2 Seebach mounted a campaign to alert synthetic organic chemists to the possible consequences of aggregation and mixed aggregation on organolithium reactions. 3a There is now ample evidence, for example, that mixed aggregates of lithium amides form and influence their chemistry. 3b However, there are very few instances in which insights have been gleaned as to how these mixed aggregates influence reactivity. 3-8 (1) Ions and Ion Pairs in Organic Reactions; Szwarc, M., Ed.; Wiley: New York, 1972; Vols. 1 and 2. Szwarc, M. Carbanions, LiVing Polymers, and Electron-Transfer Processes; Interscience: New York, Morton, M. Anionic Polymerization: Principles and Practice; Academic Press: New York, Anionic Polymerization: Kinetics, Mechanism, and Synthesis; McGrath, J. E., Ed.; American Chemical Society: Washington, Cubbon, R. C. P.; Margerison, D. Prog. React. Kinet. 1965, 3, 403. Roovers, J. E. L.; Bywater, S. Macromolecules 1968, 1, 328. (2) Seebach, D. Angew. Chem., Int. Ed. Engl. 1988, 27, Seebach, D. In Proceedings of the Robert A. Welch Foundation Conferences on Chemistry and Biochemistry; Wiley: New York, (3) (a) For leading references to mixed aggregation effects in organolithium chemistry, see: Jackman, L. M.; Rakiewicz, E. F. J. Am. Chem. Soc. 1991, 113, Kremer, T.; Harder, S.; Junge, M.; Schleyer, P. v. R. Organometallics 1996, 15, 585. Thompson, A.; Corley, E. G.; Huntington, M. F.; Grabowski, E. J. J.; Remenar, J. F.; Collum, D. B. J. Am. Chem. Soc. 1998, 120, (b) For leading references to mixed aggregation effects in lithium amides, see: Romesberg, F. E.; Collum, D. B. J. Am. Chem. Soc. 1994, 116, (c) Remenar, J. F.; Collum, D. B. J. Am. Chem. Soc. 1997, 119, (d) Leung, S. S. W.; Streitwieser, A. J. Org. Chem. 1999, 64, (4) Depue, J. S.; Collum, D. B. J. Am. Chem. Soc. 1988, 110, (5) Majewski, M.; Nowak, P. Tetrahedron Lett. 1998, 39, (6) Huisgen, R. In Organometallic Chemistry; Monograph Ser. No. 147 American Chemical Society: Washington, DC, 1960; pp (7) For crystal structures of lithium amide mixed aggregates, see: Williard, P. G.; Hintze, M. J. J. Am. Chem. Soc. 1987, 109, Mair, R. S.; Clegg, W.; O Neil, P. A. J. Am. Chem. Soc. 1993, 115, Engelhardt, L. M.; Jacobsen, G. E.; White, A. H.; Raston, C. L. Inorg. Chem. 1991, 30, Williard, P. G.; Hintz, M. J. J. Am. Chem. Soc. 1990, 112, Zerges, W.; Marsch, M.; Harmes, K.; Boche, G. Angew. Chem., Int. Ed. Engl. 1989, 28, Clegg, W.; Greer, J. C.; Hayes, J. M.; Mair, F. S.; Nolarn, P. M.; O Neil, P. A. Inorg. Chim. Acta 1997, 258, 1. The preceding paper describes quantitative rate studies of the lithium diisopropylamide-mediated ester enolization (eq 1). 9,10 By carrying out the reactions under pseudo-first-order conditions with the ester maintained at low concentrations so as to eliminate the effects of mixed aggregation, we showed that the nearly solvent-independent metalation rates in four very different solvents (t-buome, THF, HMPA/THF, and DMPU/THF) belie highly solvent-dependent changes in mechanism. However, under more standard conditions employed in preparative organic chemistry, the LDA and ester would be used in nearly equimolar proportions. During the course of such enolizations, lithium enolates are generated while the lithium diisopropylamide (LDA) is consumed; the conditions change continuously as a function of percent conversion. If LDA-lithium enolate mixed aggregates form to an appreciable extent along the reaction coordinate they will necessarily influence reactivity. This paper outlines investigations of the LDA-mediated enolization of ester 1 under conditions that optimize the influence of mixed aggregates. Using primarily 6 Li and 15 N NMR spectroscopy, we will show that intervening mixed aggregates retard the rates of enolization in all solvents. The onset and degree of autoinhibition correlates qualitatively with the formation and relative stability of intervening mixed (8) Collum, D. B. Acc. Chem. Res. 1993, 26, 227. For other reviews of lithium amide structural investigations, see: (a) Gregory, K.; Schleyer, P. v. R.; Snaith, R. AdV. Inorg. Chem. 1991, 37, 47. (b) Mulvey, R. E. Chem. Soc. ReV. 1991, 20, 167. Beswick, M. A.; Wright, D. S. In ComprehensiVe Organometallic Chemistry II; Abels, F. W., Stone, F. G. A., Wilkinson, G., Eds.; Pergamon: New York, 1994; Vol. 1, Chapter 1. (9) Sun, X.; Collum, D. B. J. Am. Chem. Soc. 2000, 122, (10) Sun, X.; Kenkre, S. L.; Remenar, J. F.; Gilchrist, J. H.; Collum, D. B. J. Am. Chem. Soc. 1997, 119, /ja992063r CCC: $ American Chemical Society Published on Web 03/01/2000
2 2460 J. Am. Chem. Soc., Vol. 122, No. 11, 2000 Sun and Collum Table 1. 6 Li and 15 N NMR Spectral Data a,b compd 6 Li, δ (m, J LiN) 15 N, δ (m, J LiN) 2a 0.38, 0.35, 0.28, 0.12, 0.01 c 3a 1.87 (t, 5.0) 70.2 (q, 5.0) 7a 1.36 (d, 5.3) 73.1 (q, 5.3) 8a d 2.55 (t, 4.8) 70.5 (br m) 1.65 (-) e 9a 2.08 (t, 4.5) 71.1 (q, 5.2) 1.34 (d, 6.4) 2b (s) c 3b 1.91 (t, 5.0) 74.7 (q, 5.0) 7b 0.83 (d, 5.1) 76.3 (q, 5.1) 2c -0.04, -0.25, -0.50, c 3c 1.60 (t, 5.0) 74.3 (q, 5.2) 7c 0.93 (d, 5.1) 76.9 (q, 4.7) 2d (s) c 3d 1.88 (t, 4.9) 73.3 (q, 4.7) 7d 0.90 (d, 5.1) 75.3 (q, 5.1) (t, 4.4) 71.1 (q, 4.9) 1.71 (t, 5.3) (t, 5.0), 79.2 (br q, 5.7) 1.19 (d, 5.4), 1.00 (d, 6.7) f 77.6 (q, 5.5) a Samples in t-buome were recorded at -60 C. All others were determined at -90 C. b Spectra were recorded on samples containing 0.13 M total lithium concentration (normality). Coupling constants were measured after resolution enhancement. Multiplicities are denoted as follows: s ) singlet, d ) doublet, t ) triplet, q ) quintet, br mult ) broad multiplet. The chemical shifts are reported relative to 0.3 M 6 LiCl/ MeOH at -100 C (0.0 ppm) and neat Me 2NEt (25.7 ppm). All J values are reported in hertz. c All signals attributed to homonuclear enolate aggregates (2a-d) are singlets. d Recorded at -125 C. e Obscured by another resonance. f This resonance is very sensitive to the [DMPU]. aggregates. However, the relative propensities to form mixed aggregates do not correlate with the coordinating power of the solvents. Results LDA Solution Structures. Previous investigations have shown that LDA is a disolvated dimer (3a and 3b) in ethereal solvents. 8 Moreover, HMPA quantitatively displaces THF from dimer 3b to afford dimer 3c without detectable deaggregation. 11 To complete the structural foundations, we investigated LDA in DMPU/THF solutions using a combination of NMR and IR spectroscopies. The spectra are archived in the Supporting Information. IR spectra recorded on 0.1 M solutions of LDA in THF containing 2.0 equiv of DMPU per Li clearly show carbonyl absorbances at 1658 and 1636 cm -1 corresponding to free and bound DMPU (respectively). 9,12 The results are fully consistent with the formation of DMPU-solvated dimer 3d. 6 Li and 15 N NMR spectra recorded on 0.1 M solutions of [ 6 Li, 15 N]LDA 13 in DMPU/THF ( equiv) contain exclusively a 6 Li triplet and 15 N quintet anticipated for cyclic dimer 3d (Table 1). Addition of <1.0 equiv failed to show two discrete 6 Li resonances expected for mixed solvate at -120 C, indicating that the free and LDA-bound DMPU are in rapid exchange on the NMR time scales. While distinct free and lithium amidebound ligands have been observed on a number of occasions, 14 strongly coordinating ethereal cosolvents such as THF can catalyze the exchange of other ligands via an associative process. 14 Indeed, 6 Li and 15 N NMR spectra recorded on 0.1 M solutions of [ 6 Li, 15 N]LDA containing 0.5 equiv of DMPU in the poorly coordinating t-buome 11 reveal mixed solvate 4 along with dimers 3a and 3d (Table 1). Mixed solvate 4 displays two distinct 6 Li resonances coupled 15 to a single 15 N resonance. 16 As an aside, solutions of [ 6 Li, 15 N]LDA in DMPU/THF aged at 0 C slowly form a new species over several hours believed to be mixed aggregate 5 containing the dipole-stabilized 17 anion of DMPU. 18 The atomic connectivity of 5 was confirmed by single frequency decouplings 15 of the three 6 Li resonances (1: 1:1) and two 15 N resonances (1:1). Treatment of the solution containing putative mixed aggregate 5 with TMSCl affords the silylated DMPU derivative 6 as shown by comparison with a sample prepared independently using s-buli. 19 DMPU metalation does not occur detectably under the conditions of the ester enolizations. Structures of LDA/Lithium Enolate Mixed Aggregates. Analyses of [ 6 Li, 15 N]LDA (0.1 M) with 1.0 equiv of ester 1-d 1 20 at -90 C in THF, HMPA/THF, and DMPU/THF reveal exclusively LDA dimers 3b-d. 21 Warming the samples periodically to -50 C and cooling back down to -90 C reveals mixed dimers 7b-d, each displaying a characteristic 6 Li doublet and 15 N quintet (Table 1); spectra are archived in the Supporting Information. As the enolizations proceed to completion, uncharacterized enolates (2a-d) appear at the expense of the mixed dimers. 22 (11) Romesberg, F. E.; Gilchrist, J. H.; Harrison, A. T.; Fuller, D. J.; Collum, D. B. J. Am. Chem. Soc. 1991, 113, (12) Aitken, C. T.; Onyszchuk, M. J. Organomet. Chem. 1985, 295, 149. (13) Kim, Y.-J.; Bernstein, M. P.; Galiano-Roth, A. S.; Romesberg, F. E.; Williard, P. G.; Fuller, D. J.; Harrison, A. T.; Collum, D. B. J. Org. Chem. 1991, 56, t-buome affords somewhat different behavior. FT-IR studies 9 show that mixtures of LDA and ester 1 contain both the dimer (14) Hilmersson, G.; Davidsson, O. J. Org. Chem. 1995, 60, Hilmersson, G.; Ahlberg, P.; Davidsson, O. J. Am. Chem. Soc. 1996, 118, Hilmersson, G.; Arvidsson, P. I.; Davidsson, Ö. J. Am. Chem. Soc. 1996, 118, Lucht, B. L.; Collum, D. B. J. Am. Chem. Soc. 1996, 118, Lucht, B. L.; Collum, D. B. J. Am. Chem. Soc. 1994, 116, Lucht, B. L.; Bernstein, M. P.; Remenar, J. F.; Collum, D. B. J. Am. Chem. Soc. 1996, 118, Also, see ref 26. (15) Gilchrist, J. H.; Harrison, A. T.; Fuller, D. J.; Collum, D. B. J. Am. Chem. Soc. 1990, 112, (16) Addition of g1.0 equiv of DMPU to LDA in t-buome afforded a precipitate. (17) Beak, P. Chem. ReV. 1984, 84, 471. Beak, P.; Basu, D.; Gallagher, D. J.; Park, Y. S. Acc. Chem. Res. 1996, 29, 552. (18) The base-mediated decomposition of DMPU was noted previously: Mukhopadhyay, T.; Seebach, D. HelV. Chim. Acta 1982, 65, 385. (19) A sample of 6 was prepared independently by treatment of DMPU with s-buli at -78 C followed by Me 3SiCl.
3 Lithium Diisopropylamide-Mediated Enolizations J. Am. Chem. Soc., Vol. 122, No. 11, Figure 1. Concentrations of species vs time for the reaction of 0.1 M [ 6 Li]LDA with 0.1 M ester 1-d 1 at -50 C (as indicated by the percentage of integration in the 6 Li NMR spectra). Legend: b represents LDA dimers (3a-d); * represents LDA-enolate mixed dimers (7a-d); 0 represents homonuclear enolate aggregates (2a-d); represents mixed trimer (9a). (A) Neat t-buome; (B) neat THF; (C) 0.2 M HMPA in THF; and (D) 0.2 M DMPU in THF. 3a and an ester-lda complex (8a). NMR spectroscopic analysis on analogous solutions of ester 1 and [ 6 Li, 15 N]LDA in t-buome reveal LDA dimer 3a along with precomplex 8a. The appearance of two distinct 6 Li resonances and one 15 N resonance of 8a at -125 C confirms that the ligands are in the slow exchange limit. Warming of the samples to -50 C affords predominantly mixed trimer 9a at low conversion, mixed dimer 7a at intermediate conversion, and several homonuclear enolate aggregates (2a) at full conversion. Trimer 9a is characterized by a 6 Li doublet and 6 Li triplet (2:1) coupled to a single 15 N broad quintet (Table 1). Mixed dimer 7a displays a 6 Li doublet and 15 N quintet. 23 (20) The rate measurements using LDA/HMPA mixtures employed 1-d 1 to allow the rates to be monitored at temperatures that maximized the solubility of HMPA. (21) Traces of trimer 9 (9b; S) THF) could be detected. (22) Structural studies of enolates: Williard, P. G. In ComprehensiVe Organic Synthesis; Pergamon: New York, 1991; Vol 1, p 1. Setzer, W. N.; Schleyer, P. v. R. AdV. Organomet. Chem. 1985, 24, 353. Abbotto, A.; Leung, S. S.-W.; Streitwieser, A.; Kilway, K. V. J. Am. Chem. Soc. 1998, 120, Jackman, L. M.; Chen, X. J. Am. Chem. Soc. 1992, 114, 403. (23) As part of our rate studies, 9 we required the characterization of carboxamide precomplex i and mixed dimer ii. These spectra are archived in the Supporting Information. Solvent-Dependent Rates. Monitoring solutions of [ 6 Li]LDA and ester 1 at -50 ( 1 C in each of the four solvents provides insights into the degree of mixed aggregation and the consequences of mixed aggregation on the metalation rate. Figure 1 shows the time-dependent concentrations of LDA dimers (3a-d), mixed dimers (7a-d), mixed trimer (9a), and lithium enolates (2a-d). x-axes common to all plots (0-30 min) are employed to optimize direct comparisons; plots showing reaction to full conversion are included in the Supporting Information. In the discussion section, we will focus upon three facets of these plots: (1) the approach to the first few percent conversion in which the LDA is rapidly consumed and the mixed aggregates begin to appear, (2) the concentrations of all species as the mixed aggregates attain maximal concentrations, and (3) the timedependent loss of the mixed aggregates and formation of homonuclear enolate aggregates. We can crudely 24 quantitate the extent of mixed aggregation by measuring the concentrations of the homonuclear and mixed aggregates when mixed dimers reach maximum concentrations
4 2462 J. Am. Chem. Soc., Vol. 122, No. 11, 2000 Sun and Collum Table 2. Proportions of Homonuclear and Mixed Aggregates (Eq 2) a b solvent 2:3:7 K eq(obsd) t-buome <1:<1:>99 c >10 4 THF 19:13:68 18 DMPU/THF 8:4: HMPA/THF 7:10:83 98 a Ratios derive by monitoring ester 1 (0.1 M) and [ 6 Li]LDA (0.1 M) at -50 C by 6 Li NMR spectroscopy. The ratios are measured at the maximum concentration of mixed dimer ([7] max). At these temperatures the resonances of the enolate aggregates (cf. Table 1) appear as only one singlet, except for t-buome in which several resonances are summed. b K eq(obsd) is loosely 24 defined by eq 2. c Although trimer 9a complicates the analysis, at no point do homonuclear aggregates 2a and 3a coexist. ([7] max ) and calculating K eq(obsd) (eq 2, Table 2). Thus, mixed aggregates form to a limited extent in THF as evidenced by the coexistence of considerable concentrations of all three structural forms. They form to a substantially greater degree in DMPU/ THF and in THF containing 1.0 equiv of HMPA. Metalations using LDA in t-buome are somewhat more complex due to the existence of both mixed dimers and trimers. However, it is quite clear that the homonuclear lithium enolate aggregate and LDA dimer do not measurably coexist, indicating an exceptional relative stabilization of mixed aggregates. Overall, the promotion of mixed aggregates and the accompanying reduction of the LDA dimer concentrations maintains the following order: t-buome > DMPU/THF > HMPA/THF > THF. This is central to the points made in the Discussion. Discussion Previous studies of LDA and LiTMP revealed that enolates readily form mixed dimers and trimers with lithium amides. 3a While one could imagine that strongly coordinating solvents might retard mixed aggregate formation, the relationship of solvation and mixed aggregation is not that simple. Strong ligands such as HMPA seem to either promote or retard mixed aggregation of LDA or LiTMP, depending upon the precise choice of LiX salt. 3a In contrast, lithium hexamethyldisilazide (LiHMDS) forms mixed dimers and trimers with enolates only in poorly coordinating solvents such as t-buome or hydrocarbons. 25 The observation of mixed dimers (7b-d) in THF, HMPA/THF, and DMPU/THF and a mixture of mixed dimer 7a and mixed trimer 9a in t-buome are congruent with previous investigations. Figure 1 offers insights into both the extent and consequences of mixed aggregation. It is instructive to focus upon three regions of these plots as follows: (1) The rates for the loss of LDA dimers and formation of mixed aggregates throughout the first 50% conversion are relatively high and nearly equivalent for all solvents. (LDA dimers 3a-d disappear quickly due to concurrent consumption (24) The values of K eq(obsd) listed in Table 2 are crudely approximated by integrating the absorbances of the LDA dimers (3), the mixed dimers (7), and the sum of all resonances corresponding to the homonuclear enolates (2). In the case of t-buome, the concentration of mixed aggregates was determined from the sum of the dimer and trimer resonances. (25) Lucht, B. L.; Collum, D. B., unpublished results. Figure 2. by the metalation as well as by the formation of mixed dimers 7a-d.) The earliest portion of the reaction coordinate was investigated under pseudo-first-order conditions as described in the preceding paper. A discussion of the numerous underlying mechanisms will not be repeated here. (2) The mixed dimers reach maximal concentrations (i.e., [7] max ) at approximately 50% conversion (t-buome excepted). Semiquantitative analysis of the data depicted in Figure 1 reveals that the promotion of mixed aggregation (eq 2) maintains the following order: t-buome > DMPU/THF > HMPA/THF > THF. This appears to correlate neither directly nor inversely with solvent binding constants. 26,27 (3) Comparisons of the loss of LDA dimers (3a-d) with the loss of the mixed aggregates (7a-d and 9a) in Figure 1 reveal autoinhibitions accompanying mixed aggregate formation. Moreover, these solvent-dependent inhibitions correlate crudely with the solvent-dependent mixed aggregate stabilities (eq 2, Table 2). For example, in THF mixed aggregates form to only a limited extent and the rates slow only modestly in the latter half-lives. At the other extreme, the exceptional stabilization of the mixed aggregates in t-buome is accompanied by almost a complete stalling of the metalation at partial conversion. The autoinhibition can be understood from either of two equivalent perspectives: (a) mixed aggregation depletes the concentration of LDA dimer 3 and, by inference, all species in equilibrium with 3 including the transition structure, or (b) mixed aggregation stabilizes the reactant without stabilizing the transition structure (Figure 2). Regardless of perspective, if mixed aggregation is an unproductive side equilibrium in which no new (mixed aggregate-based) reaction pathways emerge, then the rate of the metalation will be inhibited as appears to be the case. Summary and Conclusions In the previous paper, we described detailed rate studies of enolizations under conditions that precluded significant effects from mixed aggregation by maintaining pseudo-first-order conditions. These studies revealed that, while the mechanism of enolization proved highly solvent-dependent, the rates were nearly solvent-independent. In this paper we described qualitative NMR spectroscopic investigations of those same enolizations carried to full conversion, conditions that maximize the influence of mixed aggregation. Mixed aggregates do indeed intervene along the reaction coordinate and their influence on the reaction rates is highly solvent-dependent. In some sense, a very simple picture emerges: the solvents that promote the formation of mixed aggregates also cause the reaction to slow considerably as the mixed aggregates appear. The strong (26) Lucht, B. L.; Collum, D. B. J. Am. Chem. Soc. 1995, 117, (27) Reich, H. J.; Kulicke, K. J. J. Am. Chem. Soc. 1996, 118, 273.
5 Lithium Diisopropylamide-Mediated Enolizations J. Am. Chem. Soc., Vol. 122, No. 11, correlation of the autoinhibition with mixed aggregate stabilities is fully consistent with a model in which contributions from mixed aggregate-based enolization pathways are not significant. If so, the mechanisms, more precisely the rate-limiting transition structures, discussed in the preceding paper 9 are still operative. As is often the case, however, there are a number of interesting mechanistic subtleties. The most poorly coordinating solvent (t-buome) and strongly coordinating solvent (HMPA) both promote mixed aggregation and resulting autoinhibition when compared to solvents of intermediate solvating capacity (THF and DMPU). The solvent-independent reactivities of the homonuclear lithium amide dimers and the solvent-dependent mixed aggregate stabilities combine to produce overall solventdependent metalation rates. As discussed previously, 28 solventdependent reaction rates as measured by k rel or (even worse) by isolated yield provide little mechanistic insight when unsupported by detailed structural and rate studies. (28) Bernstein, M. P.; Collum, D. B. J. Am. Chem. Soc. 1993, 115, Collum, D. B. Acc. Chem. Res. 1992, 25, 448. Experimental Section Detailed discussions of closely related NMR spectroscopic analyses have been described in detail. 29 A brief description of these protocols as well as extensive spectroscopic data are archived in the Supporting Information. Acknowledgment. We acknowledge the National Science Foundation Instrumentation Program (CHE and PCM ), the National Institutes of Health (RR02002), and IBM for support of the Cornell Nuclear Magnetic Resonance Facility. We thank the National Institutes of Health for direct support of this work. Supporting Information Available: 6 Li, 13 C, and 15 N NMR spectra (PDF). This material is available free of charge via the Internet at JA992063R (29) Hall, P.; Gilchrist, J. H.; Harrison, A. T.; Fuller, D. J.; Collum, D. B. J. Am. Chem. Soc. 1991, 113, 9575.
Lithium Dialkylamide Mixed Aggregation: An NMR Spectroscopic Study of the Influence of Hexamethylphosphoramide (HMPA)
 9198 J. Am. Chem. SOC. 1994,1, 9198-9202 Lithium Dialkylamide Mixed Aggregation: An NMR Spectroscopic Study of the Influence of Hexamethylphosphoramide () Floyd E. Romesberg and David B. Collum' Contribution
9198 J. Am. Chem. SOC. 1994,1, 9198-9202 Lithium Dialkylamide Mixed Aggregation: An NMR Spectroscopic Study of the Influence of Hexamethylphosphoramide () Floyd E. Romesberg and David B. Collum' Contribution
Alexander C. Hoepker, Lekha Gupta, Yun Ma, Marc F. Faggin, and David B. Collum*
 Regioselective thium Diisopropylamide-Mediated Ortholithiation of 1-Chloro-3-(trifluoromethyl)benzene: Role of Autocatalysis, thium Chloride Catalysis, and Reversibility Alexander C. Hoepker, Lekha Gupta,
Regioselective thium Diisopropylamide-Mediated Ortholithiation of 1-Chloro-3-(trifluoromethyl)benzene: Role of Autocatalysis, thium Chloride Catalysis, and Reversibility Alexander C. Hoepker, Lekha Gupta,
Structure and Reactivity of Lithium Diisopropylamide Solvated by Polyamines: Evidence of Monomer- and Dimer-Based Dehydrohalogenations
 J. Am. Chem. Soc. 1998, 120, 4081-4086 4081 Structure and Reactivity of Lithium Diisopropylamide Solvated by Polyamines: Evidence of Monomer- and Dimer-Based Dehydrohalogenations Julius F. Remenar and
J. Am. Chem. Soc. 1998, 120, 4081-4086 4081 Structure and Reactivity of Lithium Diisopropylamide Solvated by Polyamines: Evidence of Monomer- and Dimer-Based Dehydrohalogenations Julius F. Remenar and
Reversible Enolization of!-amino Carboxamides by Lithium Hexamethyldisilazide. Anne J. McNeil and David B. Collum*
 Reversible Enolization of!-amino Carboxamides by Lithium Hexamethyldisilazide Anne J. McNeil and David B. Collum* Baker Laboratory, Department of Chemistry and Chemical Biology, Cornell University, Ithaca,
Reversible Enolization of!-amino Carboxamides by Lithium Hexamethyldisilazide Anne J. McNeil and David B. Collum* Baker Laboratory, Department of Chemistry and Chemical Biology, Cornell University, Ithaca,
Lithium Diisopropylamide Solvated by Monodentate and Bidentate Ligands: Solution Structures and Ligand Binding Constants
 J. Am. Chem. Soc. 1997, 119, 5567-5572 5567 Lithium Diisopropylamide Solvated by Monodentate and Bidentate Ligands: Solution Structures and Ligand Binding Constants Julius F. Remenar, Brett L. Lucht, and
J. Am. Chem. Soc. 1997, 119, 5567-5572 5567 Lithium Diisopropylamide Solvated by Monodentate and Bidentate Ligands: Solution Structures and Ligand Binding Constants Julius F. Remenar, Brett L. Lucht, and
Lithium Diisopropylamide Solvated by Hexamethylphosphoramide: Substrate-Dependent Mechanisms for Dehydrobrominations
 Published on Web 11/11/2006 Lithium Diisopropylamide Solvated by Hexamethylphosphoramide: Substrate-Dependent Mechanisms for Dehydrobrominations Yun Ma, Antonio Ramirez, Kanwal Jit Singh, Ivan Keresztes,
Published on Web 11/11/2006 Lithium Diisopropylamide Solvated by Hexamethylphosphoramide: Substrate-Dependent Mechanisms for Dehydrobrominations Yun Ma, Antonio Ramirez, Kanwal Jit Singh, Ivan Keresztes,
SUPPORTING INFORMATION
 SUPPORTING INFORMATION thium Hexamethyldisilazide-Mediated Enolization of Highly Substituted yl Ketones: Structural and Mechanistic Basis of the E/Z Selectivities Kyle A. Mack, Andrew McClory, Haiming
SUPPORTING INFORMATION thium Hexamethyldisilazide-Mediated Enolization of Highly Substituted yl Ketones: Structural and Mechanistic Basis of the E/Z Selectivities Kyle A. Mack, Andrew McClory, Haiming
Mechanism of Acylation of Lithium Phenylacetylide with a Weinreb Amide
 Mechanism of Acylation of Lithium Phenylacetylide with a Weinreb Amide Bo Qu and David B. Collum* Department of Chemistry and Chemical Biology Baker Laboratory, Cornell University Ithaca, New York 14853-1301
Mechanism of Acylation of Lithium Phenylacetylide with a Weinreb Amide Bo Qu and David B. Collum* Department of Chemistry and Chemical Biology Baker Laboratory, Cornell University Ithaca, New York 14853-1301
Pinjing Zhao, Brett L. Lucht, Sarita L. Kenkre, and David B. Collum*
 Lithium Hexamethyldisilazide-Mediated Ketone Enolization: The Influence of Hindered Dialkyl Ethers and Isostructural Dialkylamines on Reaction Rates and Mechanisms Pinjing Zhao, Brett L. Lucht, Sarita
Lithium Hexamethyldisilazide-Mediated Ketone Enolization: The Influence of Hindered Dialkyl Ethers and Isostructural Dialkylamines on Reaction Rates and Mechanisms Pinjing Zhao, Brett L. Lucht, Sarita
Formation of Benzynes from 2,6-Dihaloaryllithiums: Mechanistic Basis of the Regioselectivity
 Formation of Benzynes from 2,6-Dihaloaryllithiums: Mechanistic Basis of the Regioselectivity Antonio Ramírez a, John Candler b, Crystal G. Bashore b, Michael C. Wirtz b, Jotham W. Coe a *, and David B.
Formation of Benzynes from 2,6-Dihaloaryllithiums: Mechanistic Basis of the Regioselectivity Antonio Ramírez a, John Candler b, Crystal G. Bashore b, Michael C. Wirtz b, Jotham W. Coe a *, and David B.
Organolithium Compounds *
 OpenStax-CNX module: m32444 1 Organolithium Compounds * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 One of the major uses of lithium
OpenStax-CNX module: m32444 1 Organolithium Compounds * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 One of the major uses of lithium
Published on Web 12/19/2001
 Published on Web 12/19/2001 Consequences of Correlated Solvation on the Structures and Reactivities of RLi-Diamine Complexes: 1,2-Addition and r-lithiation Reactions of Imines by TMEDA-Solvated n-butyllithium
Published on Web 12/19/2001 Consequences of Correlated Solvation on the Structures and Reactivities of RLi-Diamine Complexes: 1,2-Addition and r-lithiation Reactions of Imines by TMEDA-Solvated n-butyllithium
Angewandte. Lithium Diisopropylamide: Solution Kinetics and Implications for Organic Synthesis. Reviews. Chemie
 Reviews D. B. Collum et al. Lithium Reagents DOI: 10.1002/anie.200603038 Lithium Diisopropylamide: Solution Kinetics and Implications for Organic Synthesis David B. Collum,* Anne J. McNeil, and Antonio
Reviews D. B. Collum et al. Lithium Reagents DOI: 10.1002/anie.200603038 Lithium Diisopropylamide: Solution Kinetics and Implications for Organic Synthesis David B. Collum,* Anne J. McNeil, and Antonio
Published on Web 11/07/2007
 Published on Web 11/07/2007 Lithium Diisopropylamide-Mediated Reactions of Imines, Unsaturated Esters, Epoxides, and Aryl Carbamates: Influence of Hexamethylphosphoramide and Ethereal Cosolvents on Reaction
Published on Web 11/07/2007 Lithium Diisopropylamide-Mediated Reactions of Imines, Unsaturated Esters, Epoxides, and Aryl Carbamates: Influence of Hexamethylphosphoramide and Ethereal Cosolvents on Reaction
Reaction of Ketones with Lithium Hexamethyldisilazide: Competitive Enolizations and 1,2-Additions
 Published on Web 02/20/2004 Reaction of Ketones with Lithium Hexamethyldisilazide: Competitive Enolizations and 1,2-Additions Pinjing Zhao, Anthony Condo, Ivan Keresztes, and David B. Collum* Contribution
Published on Web 02/20/2004 Reaction of Ketones with Lithium Hexamethyldisilazide: Competitive Enolizations and 1,2-Additions Pinjing Zhao, Anthony Condo, Ivan Keresztes, and David B. Collum* Contribution
Lithium Dialkylamide Mixed Aggregation: MNDO Computational Study of Salt and Solvent Dependencies
 J. Am. Chem. SOC. 1994,116, 9187-9197 9187 Lithium Dialkylamide Mixed Aggregation: MNDO Computational Study of Salt and Solvent Dependencies Floyd E. Romesberg and David B. Collum' Contribution from the
J. Am. Chem. SOC. 1994,116, 9187-9197 9187 Lithium Dialkylamide Mixed Aggregation: MNDO Computational Study of Salt and Solvent Dependencies Floyd E. Romesberg and David B. Collum' Contribution from the
Brett L. Lucht, Max P. Bernstein, Julius F. Remenar, and David B. Collum* J. Am. Chem. Soc. 1996, 118,
 J. Am. Chem. Soc. 1996, 118, 10707-10718 10707 Polydentate Amine and Ether Solvates of Lithium Hexamethyldisilazide (LiHMDS): Relationship of Ligand Structure, Relative Solvation Energy, and Aggregation
J. Am. Chem. Soc. 1996, 118, 10707-10718 10707 Polydentate Amine and Ether Solvates of Lithium Hexamethyldisilazide (LiHMDS): Relationship of Ligand Structure, Relative Solvation Energy, and Aggregation
Aggregation and Reactivity of Phenyllithium Solutions
 J. Am. Chem. Soc. 1998, 120, 7201-7210 7201 Aggregation and Reactivity of Phenyllithium Solutions Hans J. Reich,* D. Patrick Green, Marco A. Medina, Wayne S. Goldenberg, Birgir O2. Gudmundsson, Robert
J. Am. Chem. Soc. 1998, 120, 7201-7210 7201 Aggregation and Reactivity of Phenyllithium Solutions Hans J. Reich,* D. Patrick Green, Marco A. Medina, Wayne S. Goldenberg, Birgir O2. Gudmundsson, Robert
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I
 Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
Hemi-Labile Ligands in Organolithium Chemistry: Rate Studies of the LDA-Mediated R- and β-metalations of Epoxides
 11114 J. Am. Chem. Soc. 1999, 121, 11114-11121 Hemi-Labile Ligands in Organolithium Chemistry: Rate Studies of the LDA-Mediated R- and β-metalations of Epoxides Antonio Ramírez and David B. Collum* Contribution
11114 J. Am. Chem. Soc. 1999, 121, 11114-11121 Hemi-Labile Ligands in Organolithium Chemistry: Rate Studies of the LDA-Mediated R- and β-metalations of Epoxides Antonio Ramírez and David B. Collum* Contribution
Solution Structures of Lithium Dialkylamides and Related N-Lithiated Species: Results from 6Li-15N Double Labeling Experiments
 VOLUME 26 NUMBER 5 MAY 1993 Registered in U.S. Patent and Trademark Office; Copyright 1993 by the American Chemical Society Solution Structures of Lithium Dialkylamides and Related N-Lithiated Species:
VOLUME 26 NUMBER 5 MAY 1993 Registered in U.S. Patent and Trademark Office; Copyright 1993 by the American Chemical Society Solution Structures of Lithium Dialkylamides and Related N-Lithiated Species:
Addition of n-butyllithium to an Aldimine: On the Role of Chelation, Aggregation, and Cooperative Solvation
 Addition of n-butyllithium to an Aldimine: On the Role of Chelation, Aggregation, and Cooperative Solvation Bo Qu and David B. Collum* Department of Chemistry and Chemical Biology Baker Laboratory, Cornell
Addition of n-butyllithium to an Aldimine: On the Role of Chelation, Aggregation, and Cooperative Solvation Bo Qu and David B. Collum* Department of Chemistry and Chemical Biology Baker Laboratory, Cornell
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Effects of Solvent Acidity on the Free-Radical-Initiated Synthesis of Methanesulfonic Acid from CH 4 and SO 3
 Ind. Eng. Chem. Res. 2002, 41, 5901-5905 5901 APPLIED CHEMISTRY Effects of Solvent Acidity on the Free-Radical-Initiated Synthesis of Methanesulfonic Acid from CH 4 and SO 3 Sudip Mukhopadhyay and Alexis
Ind. Eng. Chem. Res. 2002, 41, 5901-5905 5901 APPLIED CHEMISTRY Effects of Solvent Acidity on the Free-Radical-Initiated Synthesis of Methanesulfonic Acid from CH 4 and SO 3 Sudip Mukhopadhyay and Alexis
ummary Manipulating Radicals
 Manipulating Radicals ummary Modern catalysis research tries to address issues such as material scarcity, sustainability or process costs. One solution is to replace expensive and scarce noble metal catalysts
Manipulating Radicals ummary Modern catalysis research tries to address issues such as material scarcity, sustainability or process costs. One solution is to replace expensive and scarce noble metal catalysts
Tautomerism and Keto Enol Equilibrium
 Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Tautomerism and Keto Enol Equilibrium Enols & enolates are important nucleophiles in organic & biochemistry. Keto-Enol Equilibrium: Tautomerisation can be catalyzed by either acids or bases. Relative stability
Direct Catalytic Cross-Coupling of Organolithium
 Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Organometallic Compounds of Magnesium *
 OpenStax-CNX module: m32494 1 Organometallic Compounds of Magnesium * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 While beryllium
OpenStax-CNX module: m32494 1 Organometallic Compounds of Magnesium * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 While beryllium
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
D. A. Evans, G. Lalic Chem 530A Chemistry 530A Advanced Organic Chemistry Lecture notes part 8 Carbanions Organolithium and Grignard reagents Organocopper reagents 1. Direct metalation 2. From radical
An unusual dianion equivalent from acylsilanes for the synthesis of substituted β-keto esters
 S1 An unusual dianion equivalent from acylsilanes for the synthesis of substituted β-keto esters Chris V. Galliford and Karl A. Scheidt* Department of Chemistry, Northwestern University, 2145 Sheridan
S1 An unusual dianion equivalent from acylsilanes for the synthesis of substituted β-keto esters Chris V. Galliford and Karl A. Scheidt* Department of Chemistry, Northwestern University, 2145 Sheridan
Aggregation and Alkylation of the Cesium Enolate of 2-p-Biphenylylcyclohexanone 1
 4860 J. Org. Chem. 1999, 64, 4860-4864 Aggregation and Alkylation of the Cesium Enolate of 2-p-Biphenylylcyclohexanone 1 Andrew Streitwieser,* Daniel Zerong Wang, and Manolis Stratakis Department of Chemistry,
4860 J. Org. Chem. 1999, 64, 4860-4864 Aggregation and Alkylation of the Cesium Enolate of 2-p-Biphenylylcyclohexanone 1 Andrew Streitwieser,* Daniel Zerong Wang, and Manolis Stratakis Department of Chemistry,
C H activation of aliphatic amines without unnecessary mask M2 Takaya Togo
 C H activation of aliphatic amines without unnecessary mask 2017.11.25 M2 Takaya Togo 1 Outline 1.Introduction 2.Free amines as DG Discovery of new activation mode Mechanistic studies Application of the
C H activation of aliphatic amines without unnecessary mask 2017.11.25 M2 Takaya Togo 1 Outline 1.Introduction 2.Free amines as DG Discovery of new activation mode Mechanistic studies Application of the
(2) After dissolving a solid in a solvent at high temperature, the solution is not filtered.
 Name Key 216 W13-Exam No. 1 Page 2 I. (10 points) The goal of recrystallization is to obtain purified material with a maximized recovery. For each of the following cases, indicate as to which of the two
Name Key 216 W13-Exam No. 1 Page 2 I. (10 points) The goal of recrystallization is to obtain purified material with a maximized recovery. For each of the following cases, indicate as to which of the two
Controlled synthesis of carbons-adjacent and -apart nido- and arachno-carborane anions and their metal complexes*
 Pure Appl. Chem., Vol. 75, No. 9, pp. 1335 1341, 2003. 2003 IUPAC Controlled synthesis of carbons-adjacent and -apart nido- and arachno-carborane anions and their metal complexes* Zuowei Xie Department
Pure Appl. Chem., Vol. 75, No. 9, pp. 1335 1341, 2003. 2003 IUPAC Controlled synthesis of carbons-adjacent and -apart nido- and arachno-carborane anions and their metal complexes* Zuowei Xie Department
Electronic Supplementary Material (ESI) for Chemical Communications This journal is The Royal Society of Chemistry 2012
 Ring Expansion of Alkynyl Cyclopropanes to Highly substituted Cyclobutenes via a N-Sulfonyl-1,2,3-Triazole Intermediate Renhe Liu, Min Zhang, Gabrielle Winston-Mcerson, and Weiping Tang* School of armacy,
Ring Expansion of Alkynyl Cyclopropanes to Highly substituted Cyclobutenes via a N-Sulfonyl-1,2,3-Triazole Intermediate Renhe Liu, Min Zhang, Gabrielle Winston-Mcerson, and Weiping Tang* School of armacy,
Homogeneous vs Heterogeneous Catalysts
 Homogeneous vs Heterogeneous Catalysts Homogeneous Liquid phase Low temperature High selectivity High diffusivity Easy heat transfer Difficult catalyst separation Expensive recycling Well-defined active
Homogeneous vs Heterogeneous Catalysts Homogeneous Liquid phase Low temperature High selectivity High diffusivity Easy heat transfer Difficult catalyst separation Expensive recycling Well-defined active
Unraveling the Mechanism of Water Oxidation by Ruthenium-Oxo Complexes
 Unraveling the Mechanism of Water xidation by thenium-xo Complexes Casseday Richers Literature Seminar ovember 20, 2007 The oxidation of water to dioxygen and protons represents one half the watersplitting
Unraveling the Mechanism of Water xidation by thenium-xo Complexes Casseday Richers Literature Seminar ovember 20, 2007 The oxidation of water to dioxygen and protons represents one half the watersplitting
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CHEM Chapter 13. Nuclear Magnetic Spectroscopy (Homework) W
 CHEM 2423. Chapter 13. Nuclear Magnetic Spectroscopy (Homework) W Short Answer 1. For a nucleus to exhibit the nuclear magnetic resonance phenomenon, it must be magnetic. Magnetic nuclei include: a. all
CHEM 2423. Chapter 13. Nuclear Magnetic Spectroscopy (Homework) W Short Answer 1. For a nucleus to exhibit the nuclear magnetic resonance phenomenon, it must be magnetic. Magnetic nuclei include: a. all
Synthesis of Tethered Chromium Carbene Complexes
 SYNTHESIS OF TETHERED CHROMIUM CARBENE COMPLEXES 375 Synthesis of Tethered Chromium Carbene Complexes Nicole S. Lueck Faculty Sponsor: Curtis Czerwinski, Department of Chemistry ABSTRACT Hydroxycarbene
SYNTHESIS OF TETHERED CHROMIUM CARBENE COMPLEXES 375 Synthesis of Tethered Chromium Carbene Complexes Nicole S. Lueck Faculty Sponsor: Curtis Czerwinski, Department of Chemistry ABSTRACT Hydroxycarbene
Supramolecular Control over Diels-Alder Reactivity by Encapsulation and Competitive Displacement
 Supporting information for: Supramolecular Control over Diels-Alder Reactivity by Encapsulation and Competitive Displacement Maarten M. J. Smulders and Jonathan R. Nitschke Department of Chemistry University
Supporting information for: Supramolecular Control over Diels-Alder Reactivity by Encapsulation and Competitive Displacement Maarten M. J. Smulders and Jonathan R. Nitschke Department of Chemistry University
Issue in Honor of Academician Michael G. Voronkov ARKIVOC 2001 (ix) 7-11
 Preparation of trialkylgermyl and -stannyl derivatives of methyl isothiocyanate by in situ trapping of anionic intermediates with chloro(trialkyl)germane and stannanes Lambert Brandsma a and Nina A. Nedolya
Preparation of trialkylgermyl and -stannyl derivatives of methyl isothiocyanate by in situ trapping of anionic intermediates with chloro(trialkyl)germane and stannanes Lambert Brandsma a and Nina A. Nedolya
Anionic Snieckus#Fries Rearrangement: Solvent Effects and Role of Mixed Aggregates
 Article Anionic Snieckus#Fries Rearrangement: Solvent Effects and Role of Mixed Aggregates Jason C. Riggs, Kanwal J. Singh, Ma Yun, and David B. Collum Subscriber access provided by CORNELL UNIV J. Am.
Article Anionic Snieckus#Fries Rearrangement: Solvent Effects and Role of Mixed Aggregates Jason C. Riggs, Kanwal J. Singh, Ma Yun, and David B. Collum Subscriber access provided by CORNELL UNIV J. Am.
The Carbon-Lithium bond
 Andrea Ambrosi Denmark group meeting March 12, 2013 The Carbon-Lithium bond CH 3 Li 2.02 Å 80% ionic Tight ion pair with little covalent bonding BUT the covalent component cannot be neglected! ( 13 C 6
Andrea Ambrosi Denmark group meeting March 12, 2013 The Carbon-Lithium bond CH 3 Li 2.02 Å 80% ionic Tight ion pair with little covalent bonding BUT the covalent component cannot be neglected! ( 13 C 6
Calculate a rate given a species concentration change.
 Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Kinetics Define a rate for a given process. Change in concentration of a reagent with time. A rate is always positive, and is usually referred to with only magnitude (i.e. no sign) Reaction rates can be
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Supporting Information. Indole Synthesis via Cobalt(III)-Catalyzed Oxidative Coupling of N-Arylureas and Internal Alkynes
 Supporting Information Indole Synthesis via Cobalt(III)-Catalyzed Oxidative Coupling of N-Arylureas and Internal Alkynes Zhuo-Zhuo Zhang, Bin Liu, Jing-Wen Xu, Sheng-Yi Yan, Bing-Feng Shi * Department
Supporting Information Indole Synthesis via Cobalt(III)-Catalyzed Oxidative Coupling of N-Arylureas and Internal Alkynes Zhuo-Zhuo Zhang, Bin Liu, Jing-Wen Xu, Sheng-Yi Yan, Bing-Feng Shi * Department
A long-lived iridium(iii) chemosensor for the real-time
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 2017 Supporting Information A long-lived iridium(iii) chemosensor for the real-time
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 2017 Supporting Information A long-lived iridium(iii) chemosensor for the real-time
16.1 Introduction to NMR Spectroscopy. Spectroscopy. Spectroscopy. Spectroscopy. Spectroscopy. Spectroscopy 4/11/2013
 What is spectroscopy? NUCLEAR MAGNETIC RESONANCE (NMR) spectroscopy may be the most powerful method of gaining structural information about organic compounds. NMR involves an interaction between electromagnetic
What is spectroscopy? NUCLEAR MAGNETIC RESONANCE (NMR) spectroscopy may be the most powerful method of gaining structural information about organic compounds. NMR involves an interaction between electromagnetic
Lithium Diisopropylamide-Mediated Ortholithiation and Anionic Fries Rearrangement of Aryl Carbamates: Role of Aggregates and Mixed Aggregates
 Published on Web 09/29/2006 Lithium Diisopropylamide-Mediated Ortholithiation and Anionic Fries Rearrangement of Aryl Carbamates: Role of Aggregates and Mixed Aggregates Kanwal Jit Singh and David B. Collum*
Published on Web 09/29/2006 Lithium Diisopropylamide-Mediated Ortholithiation and Anionic Fries Rearrangement of Aryl Carbamates: Role of Aggregates and Mixed Aggregates Kanwal Jit Singh and David B. Collum*
Mechanism of Trifluoroborate Hydrolysis. Greg Morehouse Literature Review 16 June 2012
 Mechanism of Trifluoroborate Hydrolysis Greg Morehouse Literature Review 16 June 2012 History & Utility of Trifluoroborate Salts First example: Fowler & Krauss, 1940 Significant improvement: Vedejs, 1995
Mechanism of Trifluoroborate Hydrolysis Greg Morehouse Literature Review 16 June 2012 History & Utility of Trifluoroborate Salts First example: Fowler & Krauss, 1940 Significant improvement: Vedejs, 1995
Cole Curtis, Chemistry 213. Synthetic #1 FFR. Synthesis and Characterization of 4-methoxychalcone
 1 Cole Curtis, Chemistry 213 Synthetic #1 FFR Synthesis and Characterization of 4-methoxychalcone Introduction Recrystallization is a very effective technique commonly used by chemists to purify solids
1 Cole Curtis, Chemistry 213 Synthetic #1 FFR Synthesis and Characterization of 4-methoxychalcone Introduction Recrystallization is a very effective technique commonly used by chemists to purify solids
Determination of Structures of Solvated Lithium Dialkylamides by Semiempirical (MNDO) Methods. Comparison of Theory and Experiment
 21 12 offers efficient on-resonance decoupling over a large bandwidth and is well compensated for both radio-frequency inhomogeneity and flip angle errors because it is constructed with the same global
21 12 offers efficient on-resonance decoupling over a large bandwidth and is well compensated for both radio-frequency inhomogeneity and flip angle errors because it is constructed with the same global
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
SUPPORTING INFORMATION
 SUPPORTING INFORMATION Cooperative Phosphine and Titanocene Catalysis: Accelerated C X Activation for Efficient Generation of Reactive Organometallics Lauren M. Fleury, Andrew D. Kosal, James T. Masters,
SUPPORTING INFORMATION Cooperative Phosphine and Titanocene Catalysis: Accelerated C X Activation for Efficient Generation of Reactive Organometallics Lauren M. Fleury, Andrew D. Kosal, James T. Masters,
Supporting Information. Synthesis of Sulfur-Rich Polymers: Copolymerization of Episulfide with Carbon Disulfide
 upporting Information ynthesis of ulfur-rich Polymers: Copolymerization of Episulfide with Carbon Disulfide by Using [PPN]/(salph)(III) ystem Koji Nakano, Go Tatsumi and Kyoko Nozaki* Department of Chemistry
upporting Information ynthesis of ulfur-rich Polymers: Copolymerization of Episulfide with Carbon Disulfide by Using [PPN]/(salph)(III) ystem Koji Nakano, Go Tatsumi and Kyoko Nozaki* Department of Chemistry
Organomagnesium (Grignard) and organolithium reagents
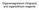 rganomagnesium (Grignard) and organolithium reagents Different polarization of non-metallic and organometallic reagents H CH 3 - I H - CH 3 + I H 3 H 3 C H 3 C H 3 + H 3 C H 3 C H 2 H 3 C H3C H I - CH
rganomagnesium (Grignard) and organolithium reagents Different polarization of non-metallic and organometallic reagents H CH 3 - I H - CH 3 + I H 3 H 3 C H 3 C H 3 + H 3 C H 3 C H 2 H 3 C H3C H I - CH
John H. MacMillan and Stephen S. Washburne. Dept of Chemistry, Temple University, Philadelphia, Pa 19122
 Further Studies of the Interaction of Carbonyl Compounds with Organometallic Azides, the Reaction of Napthoquinones with Trimethylsilyl Azide John H. MacMillan and Stephen S. Washburne Dept of Chemistry,
Further Studies of the Interaction of Carbonyl Compounds with Organometallic Azides, the Reaction of Napthoquinones with Trimethylsilyl Azide John H. MacMillan and Stephen S. Washburne Dept of Chemistry,
Advanced Organic Chemistry
 D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
D. A. Evans, G. Lalic Question of the day: Chemistry 530A TBS Me 2 C Me toluene, 130 C 70% TBS C 2 Me H H Advanced rganic Chemistry Me Lecture 16 Cycloaddition Reactions Diels _ Alder Reaction Photochemical
Theoretical Models for Chemical Kinetics
 Theoretical Models for Chemical Kinetics Thus far we have calculated rate laws, rate constants, reaction orders, etc. based on observations of macroscopic properties, but what is happening at the molecular
Theoretical Models for Chemical Kinetics Thus far we have calculated rate laws, rate constants, reaction orders, etc. based on observations of macroscopic properties, but what is happening at the molecular
CHEMISTRY (CHE) CHE 104 General Descriptive Chemistry II 3
 Chemistry (CHE) 1 CHEMISTRY (CHE) CHE 101 Introductory Chemistry 3 Survey of fundamentals of measurement, molecular structure, reactivity, and organic chemistry; applications to textiles, environmental,
Chemistry (CHE) 1 CHEMISTRY (CHE) CHE 101 Introductory Chemistry 3 Survey of fundamentals of measurement, molecular structure, reactivity, and organic chemistry; applications to textiles, environmental,
Course Syllabus. Department: Science & Technology. Date: April I. Course Prefix and Number: CHM 212. Course Name: Organic Chemistry II
 Department: Science & Technology Date: April 2012 I. Course Prefix and Number: CHM 212 Course Name: Organic Chemistry II Course Syllabus Credit Hours and Contact Hours: 5 credit hours and 7 (3:3:1) contact
Department: Science & Technology Date: April 2012 I. Course Prefix and Number: CHM 212 Course Name: Organic Chemistry II Course Syllabus Credit Hours and Contact Hours: 5 credit hours and 7 (3:3:1) contact
J. Am. Chem. Soc. 2001, 123,
 J. Am. Chem. Soc. 2001, 123, 9135-9143 9135 NMR Spectroscopic Investigations of Mixed Aggregates Underlying Highly Enantioselective 1,2-Additions of Lithium Cyclopropylacetylide to Quinazolinones Rodney
J. Am. Chem. Soc. 2001, 123, 9135-9143 9135 NMR Spectroscopic Investigations of Mixed Aggregates Underlying Highly Enantioselective 1,2-Additions of Lithium Cyclopropylacetylide to Quinazolinones Rodney
Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY Purpose: This is an exercise to introduce the use of nuclear magnetic resonance spectroscopy, in conjunction with infrared spectroscopy, to determine
Experiment 11: NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY Purpose: This is an exercise to introduce the use of nuclear magnetic resonance spectroscopy, in conjunction with infrared spectroscopy, to determine
NMR = Nuclear Magnetic Resonance
 NMR = Nuclear Magnetic Resonance NMR spectroscopy is the most powerful technique available to organic chemists for determining molecular structures. Looks at nuclei with odd mass numbers or odd number
NMR = Nuclear Magnetic Resonance NMR spectroscopy is the most powerful technique available to organic chemists for determining molecular structures. Looks at nuclei with odd mass numbers or odd number
Manganese-Catalyzed Late- Stage Aliphatic C H Azidation
 Wipf group current literature Manganese-Catalyzed Late- Stage Aliphatic C H Azidation J. Am. Chem. Soc. 2015, 137, 5300 5303 Xiongyi Huang, Tova M. Bergsten, and John T. Groves Department of Chemistry,
Wipf group current literature Manganese-Catalyzed Late- Stage Aliphatic C H Azidation J. Am. Chem. Soc. 2015, 137, 5300 5303 Xiongyi Huang, Tova M. Bergsten, and John T. Groves Department of Chemistry,
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds*
 Pure Appl. Chem., Vol. 72, No. 9, pp. 1715 1719, 2000. 2000 IUPAC Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds* O. G. Kulinkovich
Pure Appl. Chem., Vol. 72, No. 9, pp. 1715 1719, 2000. 2000 IUPAC Titanacyclopropanes as versatile intermediates for carbon carbon bond formation in reactions with unsaturated compounds* O. G. Kulinkovich
Organic Chemistry Laboratory Summer Lecture 6 Transition metal organometallic chemistry and catalysis July
 344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
344 Organic Chemistry Laboratory Summer 2013 Lecture 6 Transition metal organometallic chemistry and catalysis July 30 2013 Summary of Grignard lecture Organometallic chemistry - the chemistry of compounds
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #4 - December 7, 1998*
 Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #4 - December 7, 1998* INSTRUTINS --- This examination is in multiple choice format; the questions are in this Exam Booklet and
Department of hemistry SUNY/neonta hem 221 - rganic hemistry I Examination #4 - December 7, 1998* INSTRUTINS --- This examination is in multiple choice format; the questions are in this Exam Booklet and
The aza-baylis-hillman Reaction: Mechanism, Asymmetric Catalysis, & Abnormal Adducts. Larry Wolf SED Group Meeting
 The aza-baylis-hillman Reaction: Mechanism, Asymmetric Catalysis, & Abnormal Adducts Larry Wolf SED Group Meeting 04-10-07 Outline Brief historical account and Utility Mechanism Different methods for asymmetric
The aza-baylis-hillman Reaction: Mechanism, Asymmetric Catalysis, & Abnormal Adducts Larry Wolf SED Group Meeting 04-10-07 Outline Brief historical account and Utility Mechanism Different methods for asymmetric
Basics of Catalysis and Kinetics
 Basics of Catalysis and Kinetics Nobel laureates in catalysis: Haber (1918) Ziegler and Natta (1963) Wilkinson, Fischer (1973) Knowles, Noyori, Sharpless (2001) Grubbs, Schrock, Chauvin (2006) Ertl (2007)
Basics of Catalysis and Kinetics Nobel laureates in catalysis: Haber (1918) Ziegler and Natta (1963) Wilkinson, Fischer (1973) Knowles, Noyori, Sharpless (2001) Grubbs, Schrock, Chauvin (2006) Ertl (2007)
Palladium-catalyzed cross-addition of triisopropylsilylacetylene to unactivated alkynes*
 Pure Appl. Chem., Vol. 80, No. 5, pp. 1161 1166, 2008. doi:10.1351/pac200880051161 2008 IUPAC Palladium-catalyzed cross-addition of triisopropylsilylacetylene to unactivated alkynes* Naofumi Tsukada, Satoshi
Pure Appl. Chem., Vol. 80, No. 5, pp. 1161 1166, 2008. doi:10.1351/pac200880051161 2008 IUPAC Palladium-catalyzed cross-addition of triisopropylsilylacetylene to unactivated alkynes* Naofumi Tsukada, Satoshi
IR, MS, UV, NMR SPECTROSCOPY
 CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET All Sections CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET General Instructions for the 318 Spectroscopy Problem Set Consult the Lab Manual,
CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET All Sections CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET General Instructions for the 318 Spectroscopy Problem Set Consult the Lab Manual,
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Figure 4.10 HPLC Chromatogram of the Carbazole-Phenoxy Based Methacrylate
 The percent yield of the methacrylation was 85.2 %, with a purity of 98.2 % determined by HPLC (Figure 4.10). Elemental analysis gave excellent agreement to expected elemental ratios (Table 4.2). Disregarding
The percent yield of the methacrylation was 85.2 %, with a purity of 98.2 % determined by HPLC (Figure 4.10). Elemental analysis gave excellent agreement to expected elemental ratios (Table 4.2). Disregarding
به نام خدا روشهای سنتز مواد آلی
 به نام خدا روشهای سنتز مواد آلی 1 References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Reactions and Synthesis (Part B), 5th ed., Springer, 2007. 2. Carey, F. A.; Sundberg, R. J. Advanced
به نام خدا روشهای سنتز مواد آلی 1 References: 1. Carey, F. A.; Sundberg, R. J. Advanced Organic Chemistry: Reactions and Synthesis (Part B), 5th ed., Springer, 2007. 2. Carey, F. A.; Sundberg, R. J. Advanced
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Exam 3 Chem 3045x Friday, December 5, 1997
 Exam 3 Chem 3045x Friday, December 5, 1997 Instructions: This is a closed book examination. You may not use any notes, books or external materials during the course of the examination. Please print your
Exam 3 Chem 3045x Friday, December 5, 1997 Instructions: This is a closed book examination. You may not use any notes, books or external materials during the course of the examination. Please print your
Microwave-promoted synthesis in water
 Microwave-promoted synthesis in water icholas E. Leadbeater nicholas.leadbeater@uconn.edu Outline of what we do Synthesis / Methodology / ew techniques Pure organic synthesis eg. Baylis-Hillman eaction
Microwave-promoted synthesis in water icholas E. Leadbeater nicholas.leadbeater@uconn.edu Outline of what we do Synthesis / Methodology / ew techniques Pure organic synthesis eg. Baylis-Hillman eaction
Structural Studies on Lithium Phenolates in Ethereal Solvents Using NMR Spectroscopy and Method of Continuous Variation
 Structural Studies on thium enolates in Ethereal Solvents Using NMR Spectroscopy and thod of Continuous Variation nandarup Goswami, Tim De Vries and David B. Collum Research Summary Common Solution Structures
Structural Studies on thium enolates in Ethereal Solvents Using NMR Spectroscopy and thod of Continuous Variation nandarup Goswami, Tim De Vries and David B. Collum Research Summary Common Solution Structures
Electronic Supporting Information. for. Group 13 Complexes of Dipyridylmethane, a Forgotten Ligand in Coordination Chemistry
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 Electronic Supporting Information for Group 13 Complexes of Dipyridylmethane, a Forgotten
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2015 Electronic Supporting Information for Group 13 Complexes of Dipyridylmethane, a Forgotten
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry
 OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
OAT Organic Chemistry - Problem Drill 19: NMR Spectroscopy and Mass Spectrometry Question No. 1 of 10 Question 1. Which statement concerning NMR spectroscopy is incorrect? Question #01 (A) Only nuclei
You are advised to spend an equal amount of time on each question.
 UNIVERSITY OF EAST ANGLIA School of Chemistry Main Series UG Examination 2016-17 ADVANCED TOPICS IN INORGANIC CHEMISTRY CHE-7301Y Time allowed: 2 hours. Answer THREE of the following FOUR questions. You
UNIVERSITY OF EAST ANGLIA School of Chemistry Main Series UG Examination 2016-17 ADVANCED TOPICS IN INORGANIC CHEMISTRY CHE-7301Y Time allowed: 2 hours. Answer THREE of the following FOUR questions. You
Asymmetric synthesis mediated by chiral ligands
 Pure & Appl. Chem., Vol. 66, No. 7, pp. 1487-1492,1994. Printed in Great Britain. 0 1994 IUPAC Asymmetric synthesis mediated by chiral ligands Kenji Koga Faculty of Pharmaceutical Sciences, University
Pure & Appl. Chem., Vol. 66, No. 7, pp. 1487-1492,1994. Printed in Great Britain. 0 1994 IUPAC Asymmetric synthesis mediated by chiral ligands Kenji Koga Faculty of Pharmaceutical Sciences, University
Kinetic Isotope Effects The Study of Organometallic Reaction Mechanisms
 Kinetic Isotope Effects The Study of Organometallic Reaction Mechanisms Alexander J. Kendall D.R. Tyler Group Meeting 7/23/2014 Gómez-Gallego, M.; Sierra, M. A. Chem. Rev. 2011, 111, 4857-4963. Outline
Kinetic Isotope Effects The Study of Organometallic Reaction Mechanisms Alexander J. Kendall D.R. Tyler Group Meeting 7/23/2014 Gómez-Gallego, M.; Sierra, M. A. Chem. Rev. 2011, 111, 4857-4963. Outline
Synthesis of Secondary and Tertiary Amine- Containing MOFs: C-N Bond Cleavage during MOF Synthesis
 Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2015 Supporting Information Synthesis of Secondary and Tertiary Amine- Containing MFs: C-N Bond
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2015 Supporting Information Synthesis of Secondary and Tertiary Amine- Containing MFs: C-N Bond
Two Dimensional (2D) NMR Spectroscopy
 The two important parameters obtained from NMR spectra are; Two Dimensional (2D) NMR Spectroscopy py Correlation NMR a. Chemical shift b. Spin-spin coupling constant Large molecules with numerous atoms
The two important parameters obtained from NMR spectra are; Two Dimensional (2D) NMR Spectroscopy py Correlation NMR a. Chemical shift b. Spin-spin coupling constant Large molecules with numerous atoms
Lithium Diisopropylamide-Mediated Ortholithiation of 2 Fluoropyridines: Rates, Mechanisms, and the Role of Autocatalysis
 pubs.acs.org/joc Lithium Diisopropylamide-Mediated Ortholithiation of 2 Fluoropyridines: Rates, Mechanisms, and the Role of Autocatalysis Lekha Gupta, Alexander C. Hoepker, Yun Ma, Mihai S. Viciu, Marc
pubs.acs.org/joc Lithium Diisopropylamide-Mediated Ortholithiation of 2 Fluoropyridines: Rates, Mechanisms, and the Role of Autocatalysis Lekha Gupta, Alexander C. Hoepker, Yun Ma, Mihai S. Viciu, Marc
Autocatalysis in Lithium Diisopropylamide-Mediated Ortholithiations
 Published on Web 11/25/2008 Autocatalysis in Lithium Diisopropylamide-Mediated Ortholithiations Kanwal J. Singh, Alexander C. Hoepker, and David B. Collum* Department of Chemistry and Chemical Biology,
Published on Web 11/25/2008 Autocatalysis in Lithium Diisopropylamide-Mediated Ortholithiations Kanwal J. Singh, Alexander C. Hoepker, and David B. Collum* Department of Chemistry and Chemical Biology,
BIOCHEMICAL AND BIOPHYSICAL RESEARCH COMMUNICATIONS. HINDERED ROTATION IN N-METHYLFORMAMIDE. A PEPTIDE-BOND MODEL SYSTEM.*t
 HINDERED ROTATION IN N-METHYLFORMAMIDE. A PEPTIDE-BOND MODEL SYSTEM.*t Robert C. Neuman, Jr., Violet Jonas, Karen Anderson, and Ronald Barry Department of Chemistry University of California Riverside,
HINDERED ROTATION IN N-METHYLFORMAMIDE. A PEPTIDE-BOND MODEL SYSTEM.*t Robert C. Neuman, Jr., Violet Jonas, Karen Anderson, and Ronald Barry Department of Chemistry University of California Riverside,
Writing a Correct Mechanism
 Chapter 2 1) Balancing Equations Writing a Correct Mechanism 2) Using Arrows to show Electron Movement 3) Mechanisms in Acidic and Basic Media 4) Electron rich Species: Nucleophile or Base? 5) Trimolecular
Chapter 2 1) Balancing Equations Writing a Correct Mechanism 2) Using Arrows to show Electron Movement 3) Mechanisms in Acidic and Basic Media 4) Electron rich Species: Nucleophile or Base? 5) Trimolecular
Org. Commun. 2:2 (2009) 60-65
 ORIGINAL ARTICLE Org. Commun. 2:2 (2009) 60-65 Solvent and electronic effects on kinetics of cyclization of thermolabile aryllithium reagents. A comparison between 1-bromo-2-(2-bromoethyl)benzene and 4,5-dimethoxy-1-
ORIGINAL ARTICLE Org. Commun. 2:2 (2009) 60-65 Solvent and electronic effects on kinetics of cyclization of thermolabile aryllithium reagents. A comparison between 1-bromo-2-(2-bromoethyl)benzene and 4,5-dimethoxy-1-
Solution Structures of Lithium Enolates of Cyclopentanone, Cyclohexanone, Acetophenones, and Benzyl Ketones. Triple Ions and Higher Lithiate Complexes
 Published on Web 07/27/2009 Solution Structures of Lithium Enolates of Cyclopentanone, Cyclohexanone, Acetophenones, and Benzyl Ketones. Triple Ions and Higher Lithiate Complexes Kristopher J. Kolonko,
Published on Web 07/27/2009 Solution Structures of Lithium Enolates of Cyclopentanone, Cyclohexanone, Acetophenones, and Benzyl Ketones. Triple Ions and Higher Lithiate Complexes Kristopher J. Kolonko,
A. Loupy, B.Tchoubar. Salt Effects in Organic and Organometallic Chemistry
 A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
Supporting Information
 Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Palladium-catalyzed oxidative direct arylation polymerization (Oxi-DArP)
Electronic Supplementary Material (ESI) for Polymer Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Palladium-catalyzed oxidative direct arylation polymerization (Oxi-DArP)
Chapter 9. Nuclear Magnetic Resonance. Ch. 9-1
 Chapter 9 Nuclear Magnetic Resonance Ch. 9-1 1. Introduction Classic methods for organic structure determination Boiling point Refractive index Solubility tests Functional group tests Derivative preparation
Chapter 9 Nuclear Magnetic Resonance Ch. 9-1 1. Introduction Classic methods for organic structure determination Boiling point Refractive index Solubility tests Functional group tests Derivative preparation
Module 6 : Reaction Kinetics and Dynamics Lecture 28 : Elementary Reactions and Reaction Mechanisms
 Module 6 : Reaction Kinetics and Dynamics Lecture 28 : Elementary Reactions and Reaction Mechanisms Objectives In this Lecture you will learn to do the following Define what is an elementary reaction.
Module 6 : Reaction Kinetics and Dynamics Lecture 28 : Elementary Reactions and Reaction Mechanisms Objectives In this Lecture you will learn to do the following Define what is an elementary reaction.
3.15 Nuclear Magnetic Resonance Spectroscopy, NMR
 3.15 Nuclear Magnetic Resonance Spectroscopy, NMR What is Nuclear Magnetic Resonance - NMR Developed by chemists and physicists together it works by the interaction of magnetic properties of certain nuclei
3.15 Nuclear Magnetic Resonance Spectroscopy, NMR What is Nuclear Magnetic Resonance - NMR Developed by chemists and physicists together it works by the interaction of magnetic properties of certain nuclei
ORGANIC - EGE 5E CH UV AND INFRARED MASS SPECTROMETRY
 !! www.clutchprep.com CONCEPT: IR SPECTROSCOPY- FREQUENCIES There are specific absorption frequencies in the functional group region that we should be familiar with EXAMPLE: What are the major IR absorptions
!! www.clutchprep.com CONCEPT: IR SPECTROSCOPY- FREQUENCIES There are specific absorption frequencies in the functional group region that we should be familiar with EXAMPLE: What are the major IR absorptions
