Supplement of Hydrogen dynamics in soil organic matter as determined by
|
|
|
- Alannah Kennedy
- 6 years ago
- Views:
Transcription
1 Supplement of Biogeosciences, 13, , doi: /bg supplement Author(s) CC Attribution 3.0 License. Supplement of Hydrogen dynamics in soil organic matter as determined by 13 C and 2 H labeling experiments Alexia Paul et al. Correspondence to: Alexia Paul (alexia.paul@inra.fr) and Jérôme Balesdent (jerome.balesdent@inra.fr) The copyright of individual parts of the supplement might differ from the CC-BY 3.0 licence.
2 Supplementary material S1. Incubation characteristics The isotopic characteristics of the labeling experiment are summarized in the table S1. Table S1 : Incubation characteristics and the initial isotopic compositions of the labeling experiments. 13 A m, A m and A w are respectively the 13 C and 2 H abundance of the added labeled source (molecule or water) and 13 A tot_i, A tot_i are respectively the 13 C and 2 H abundance of the total soil at the initial conditions (time 0). 13 A m % 13 A tot_i % ± 0.02 A m or A w % A tot_i % ± Experience Added molecule mg 2 H 2 O Cambisol Podzol Leptosol Cambisol Podzol Leptosol Cambisol Podzol Leptosol Cambisol Podzol Leptosol Experiment C 2 H + 1 H 2 O yes yes Experiment yes C 1 H + 2 H 2 O 25 yes yes yes Experiment yes Control
3 S2. Mass balance calculations All the variables are summarized in the table S2. Carbon: C tot = C dfm + C dfs (SI1) 13 A tot *C tot = 13 A m *C dfm + 13 A tot_0 *C dfs (SI2.a) 13 A tot *C tot = 13 A m *C dfm + 13 A tot_0 *(C tot - C dfm ) (SI2.b) from SI1 and SI2 we derive: C dfm = ( 13 A tot - 13 A tot_0 )/ ( 13 A m - 13 A tot_0 )* C tot (1) Hydrogen: After freeze-drying, hydrogen is considered in three compartments: (i) NEH derived from labeled molecule atoms, (ii) NEH from unlabeled sources, and (iii) exchangeable hydrogen, which is by definition equilibrated with the atmosphere with a given fractionation ε 1 H tot = H dfm + H dfs + H e A tot *H tot = A m *H dfm + A dfs *H dfs + (A atm + ε 1 )*H e (SI3) (SI4) from SI3 and SI4 we derive: A tot *H tot = A m *H dfm + [A dfs *H dfs + (A atm + ε 1 )*H e ]/(H dfs +H e )*(H tot - H dfm ) (SI5) The term [A dfs *H dfs + (A atm + ε 1 )*H e ]/ (H dfs +H e ) is the average composition of unlabeled hydrogen. It is derived from the analysis of the unlabeled sample: H tot_0 = H dfs + H e A tot_0 = [A dfs *H dfs + (A atm + ε 1 )*H e ]/(H dfs +H e ) (SI6) (SI7) from SI5 and SI7 we derive: A tot *H tot = A m *H dfm + A tot_0 *(H tot - H dfm ) (SI8) H dfm = [(A tot - A tot_0 )/(A m - A tot_0 )]*H tot (2) By similarity, for Experiment 2 (labeled water), we write: 2
4 H dfw = (A tot - A tot_0 )/(A w - A tot_0 ]*H tot (3) These equations are based on approximations: the isotope fractionation term between atmosphere and exchangeable H is supposed to be equal in labeled and : this is supported by the fact that the amount of exchangeable hydrogen is considered unaffected by the (small) amount of introduced labeled material. The amount of unlabeled HNE (H dfs ) and its abundance (A dfs ) are considered equal in the labeled and. Inequality would affect the denominator in equations (2) and (3). Equality is a numerical approximation without consequence, owing to the very high enrichment of labeled material (A m, A w, see table S1). Table S2: Definition of the variables used for mass balance calculation Quantity (mg g -1 dry soil) Abundance Variables C tot H tot C m H m H w H e C dfs C dfm H dfs H dfw H dfm 13 A tot A tot 13 A tot_0 A tot_0 Definition Total amount of carbon in the soil Total amount of hydrogen in the dry soil Initial amount of carbon in the added molecule Initial amount of non-exchangeable hydrogen in the added molecule Total amount of water hydrogen in the soil Amount of exchangeable hydrogen in the dry soil Amount of unlabeled carbon already present in the soil Amount of carbon derived from molecule Amount of unlabeled non-exchangeable hydrogen present in the soil Amount of non-exchangeable hydrogen derived from water Amount of non-exchangeable hydrogen derived from molecule 13 C abundance of the total bulk soil 2 H abundance of the total bulk soil 13 C abundance of the unlabeled experiment (control) 2 H abundance of the unlabeled experiment (control) 13 A m Initial 13 C abundance of the labeled molecule A m A w A atm Initial 2 H abundance of the labeled molecule Initial 2 H abundance of the labeled water 2 H abundance of the atmosphere 3
5 To calculate the incorporation of the water hydrogen coming from the mineralisation of the added molecule (recycling), we assume that the labelled molecule is completely mineralised in water. The resulting isotopic composition of water in experiment 1(A w2 ) can be calculated from the isotopic composition of the labelled molecule as follow: A w2 = (A m *H m )/H w (SI9) Then, the amount of non-exchangeable hydrogen that can be derived from this water (H dfw2 ) can be calculated using the value H dfw calculated in equation (3) : H dfw2 = (A w2 A tot_0 )/ (A m - A tot_0 )*H dfw (4) The proportion of deuterium derived from the molecule but incorporated in the soil by the water is given by (H dfw2 /H dfm )*100 where H dfm is calculated in equation (2). S3. Propagation error calculation Uncertainties on the element and isotope ratio measurements affect the estimate of the amount of labeled-source derived carbon or hydrogen atoms. To assess the uncertainty σ Cdfm (err7), σ Hdfm (err8), σ 2 Hdfw (err9) or on the calculated values C dfm (Eq. 6), H dfm (Eq. 7), and H dfw (Eq.8) we calculated the statistical error propagation of the uncertainties on measured isotopic compositions and element content of replicated samples presenting in supporting information. σ 2 Cdfm = (σ 2 13Atot + σ 2 13Atot_0)*[C tot /( 13 A m - 13 A tot_0 )] 2 + (σ 2 13Am - σ 2 13Atot_0)*( 13 A tot 13 A tot_0 ) ²*C tot ²/( 13 A m - 13 A tot_0 )² + σ 2 Ctot*[( 13 A tot 13 A tot_0 )/( 13 A m - 13 A tot_0 )]² (err6) σ 2 Hdfm = (σ 2 Atot +σ 2 Atot_0)*[H tot /(A m A tot_0 )] 2 + (σ 2 Am - σ 2 Atot_0)*(A tot A tot_0 )² * H tot ²/(A m A tot_0 )² + σ 2 Htot*[(A tot A tot_0 )/(A m A tot_0 )]² (err7) σ 2 Hdfw = (σ 2 Atot +σ 2 Atot_0)*[H tot /(A w A w_0 )] 2 + (σ 2 Aw - σ 2 Aw_0)*(A tot A tot_0 )² * H tot ²/(A w A w_0 )² + σ 2 Htot*[(A tot A tot_0 )/(A w A w_0 )]² (err8) where the indices of the terms σ X stand for the standard deviation of the respective variable. In this calculation, the uncertainties σ 2 Am, σ 2 Aw and σ 2 13Am were considered as negligible. 4
6 S4. δ 13 C and δ 2 H results of the incubation samples δ 2 H δ 2 H 2920 a acide palmitique b 370 labeled sample δ13c c unlabeled sample Figure S4.1: Cambisol 13 C and 2 H isotopic variation a. δ 2 H variation through time of the bulk soil that received labeled,, and and. b. δ 2 H variation through time of bulk soil that received labeled water and. c. δ 13 C variation through time of the bulk soil that received labeled,, and and. Standard deviations are less than 3 for. 5
7 δ 2 H a δ 13 C c 280 b labeled samples δ 2 H Figure S4.2: Podzol 13 C and 2 H isotopic variation a. δ 2 H variation through time of the bulk soil that received labeled,, and and. b. δ 2 H variation through time of bulk soil that received labeled water and. c. δ 13 C variation through time of the bulk soil that received labeled,, and and. Standard deviations are less than 3 for. 6
8 δ 2 H a isolueucine δ 2 H b labeled samples δ 13 C c Figure S4.3: Leptosol 13 C and 2 H isotopic variation a. δ 2 H variation through time of the bulk soil that received labeled,, and and. b. δ 2 H variation through time of bulk soil that received labeled water and. c. δ 13 C variation through time of the bulk soil that received labeled,, and and. Standard deviations are less than 3 for. 7
Supplementary Figure 1. Example used to demonstrate the additive. sources, soil and PyOM, are conclusively partitioned. In Experiment 2, the
 Supplementary Figure 1. Example used to demonstrate the additive approach. Arrows represent CO2 flux, in µmol m -2 s -1. In Experiment 1, the two sources, soil and PyOM, are conclusively partitioned. In
Supplementary Figure 1. Example used to demonstrate the additive approach. Arrows represent CO2 flux, in µmol m -2 s -1. In Experiment 1, the two sources, soil and PyOM, are conclusively partitioned. In
Stable Isotopes OUTLINE
 Stable Isotopes OUTLINE Reading: White Ch 9.1 to 9.7.1 (or digital p370-400) Exercise answer? What does the salt do? Today 1. 2 leftovers 2. Stable Isotopes for hydrologic and climate applications 1 CaCO
Stable Isotopes OUTLINE Reading: White Ch 9.1 to 9.7.1 (or digital p370-400) Exercise answer? What does the salt do? Today 1. 2 leftovers 2. Stable Isotopes for hydrologic and climate applications 1 CaCO
1 BASIC CONCEPTS AND MODELS
 1 BASIC CONCEPTS AND ODELS 1.1 INTRODUCTION This Volume III in the series of textbooks is focused on applications of environmental isotopes in surface water hydrology. The term environmental means that
1 BASIC CONCEPTS AND ODELS 1.1 INTRODUCTION This Volume III in the series of textbooks is focused on applications of environmental isotopes in surface water hydrology. The term environmental means that
Average Atomic Mass: How are the masses on the periodic table determined?
 Chemistry Ms. Ye Name Date Block Average Atomic Mass: How are the masses on the periodic table determined? Most elements have more than one naturally occurring isotope. As you learned previously, the atoms
Chemistry Ms. Ye Name Date Block Average Atomic Mass: How are the masses on the periodic table determined? Most elements have more than one naturally occurring isotope. As you learned previously, the atoms
Last Name: First Name ID
 Last Name: First Name ID This is a set of practice problems for the final exam. It is not meant to represent every topic and is not meant to be equivalent to a 2-hour exam. These problems have not been
Last Name: First Name ID This is a set of practice problems for the final exam. It is not meant to represent every topic and is not meant to be equivalent to a 2-hour exam. These problems have not been
Midterm Exam III. CHEM 181: Introduction to Chemical Principles November 25, 2014 Answer Key
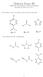 Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Scattering Lecture. February 24, 2014
 Scattering Lecture February 24, 2014 Structure Determination by Scattering Waves of radiation scattered by different objects interfere to give rise to an observable pattern! The wavelength needs to close
Scattering Lecture February 24, 2014 Structure Determination by Scattering Waves of radiation scattered by different objects interfere to give rise to an observable pattern! The wavelength needs to close
SIC CONCEPTS TS OF CHEMISTRY. Unit. I. Multiple Choice Questions (Type-I)
 Unit 1 SOME BASIC B SIC CONCEPTS CONCEP TS OF CHEMISTRY CHEMIS I. Multiple Choice Questions (Type-I) 1. Two students performed the same experiment separately and each one of them recorded two readings
Unit 1 SOME BASIC B SIC CONCEPTS CONCEP TS OF CHEMISTRY CHEMIS I. Multiple Choice Questions (Type-I) 1. Two students performed the same experiment separately and each one of them recorded two readings
Introduction. Chemistry the science of matter and the changes it can undergo.
 Introduction Chemistry the science of matter and the changes it can undergo. Physical Chemistry concerned with the physical principles that underlie chemistry. Seeks to account for the properties of matter
Introduction Chemistry the science of matter and the changes it can undergo. Physical Chemistry concerned with the physical principles that underlie chemistry. Seeks to account for the properties of matter
Supplementary Information
 Supplementary Information Supplementary Tables Major element compositions of host and daughter crystals: Table 1 reports composition for host-olivine. No daughter olivine has been found. The number for
Supplementary Information Supplementary Tables Major element compositions of host and daughter crystals: Table 1 reports composition for host-olivine. No daughter olivine has been found. The number for
1 Some Basic Concepts of Chemistry
 1 Some Basic Concepts of Chemistry Multiple Choice Questions (MCQs) Q. 1 Two students performed the same experiment separately and each one of them recorded two readings of mass which are given below.
1 Some Basic Concepts of Chemistry Multiple Choice Questions (MCQs) Q. 1 Two students performed the same experiment separately and each one of them recorded two readings of mass which are given below.
Chapter 2 Atoms and Elements. 2.4 The Atom
 Chapter 2 Atoms and Elements 2.4 The Atom Atoms Dalton s Atomic Theory Are tiny particles of matter. Of an element are similar and different from other elements. Of two or more different elements combine
Chapter 2 Atoms and Elements 2.4 The Atom Atoms Dalton s Atomic Theory Are tiny particles of matter. Of an element are similar and different from other elements. Of two or more different elements combine
Supplement of A new source of methylglyoxal in the aqueous phase
 Supplement of Atmos. Chem. Phys., 16, 2689 2702, 2016 http://www.atmos-chem-phys.net/16/2689/2016/ doi:10.5194/acp-16-2689-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of A new
Supplement of Atmos. Chem. Phys., 16, 2689 2702, 2016 http://www.atmos-chem-phys.net/16/2689/2016/ doi:10.5194/acp-16-2689-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of A new
CP/Honors Chemistry Unit 3: Atomic Theory Chapter 4, Sections 1, 2, and 3
 CP/Honors Chemistry Unit 3: Atomic Theory Chapter 4, Sections 1, 2, and 3 Subatomic Particles Warm-Up Quiz 1. What are the three subatomic particles? 2. Where are the particles located in the atom? 3.
CP/Honors Chemistry Unit 3: Atomic Theory Chapter 4, Sections 1, 2, and 3 Subatomic Particles Warm-Up Quiz 1. What are the three subatomic particles? 2. Where are the particles located in the atom? 3.
Supplement of Damage functions for climate-related hazards: unification and uncertainty analysis
 Supplement of Nat. Hazards Earth Syst. Sci., 6, 89 23, 26 http://www.nat-hazards-earth-syst-sci.net/6/89/26/ doi:.594/nhess-6-89-26-supplement Author(s) 26. CC Attribution 3. License. Supplement of Damage
Supplement of Nat. Hazards Earth Syst. Sci., 6, 89 23, 26 http://www.nat-hazards-earth-syst-sci.net/6/89/26/ doi:.594/nhess-6-89-26-supplement Author(s) 26. CC Attribution 3. License. Supplement of Damage
Chemistry 151 Spring Section 01 MWF 9:10-10:00 am - MWF 9:10-10:00 am. Course Name: Course Code: N/A
 Course Name: Chemistry 151 Spring 2018 - Section 01 MWF 9:10-10:00 am - MWF 9:10-10:00 am Course Code: N/A ALEKS Course: General Chemistry (First Semester) Instructor: Prof. Hascall Course Dates: Begin:
Course Name: Chemistry 151 Spring 2018 - Section 01 MWF 9:10-10:00 am - MWF 9:10-10:00 am Course Code: N/A ALEKS Course: General Chemistry (First Semester) Instructor: Prof. Hascall Course Dates: Begin:
CHEMISTRY 110 Final EXAM Dec 17, 2012 FORM A
 CEMISTRY 110 Final EXAM Dec 17, 2012 FORM A 1. Given the following reaction which of the following statements is true? Fe(s) + CuCl 2 (aq)! Cu(s) + FeCl 2 (aq) A. Iron is oxidized and copper is reduced.
CEMISTRY 110 Final EXAM Dec 17, 2012 FORM A 1. Given the following reaction which of the following statements is true? Fe(s) + CuCl 2 (aq)! Cu(s) + FeCl 2 (aq) A. Iron is oxidized and copper is reduced.
Chemical Alterations Occurring During Biomass Charring and their Impact on Char Recalcitrance
 Chemical Alterations ccurring During Biomass Charring and their Impact on Char Recalcitrance Heike Knicker Instituto de Recursos Naturales y Agrobiología, CSIC, Sevilla, Spain Impact of fire on soil organic
Chemical Alterations ccurring During Biomass Charring and their Impact on Char Recalcitrance Heike Knicker Instituto de Recursos Naturales y Agrobiología, CSIC, Sevilla, Spain Impact of fire on soil organic
 Unit 1 SOME BASIC CONCEPTS OF CHEMISTRY I. Multiple Choice Questions (Type-I) 1. Two students performed the same experiment separately and each one of them recorded two readings of mass which are given
Unit 1 SOME BASIC CONCEPTS OF CHEMISTRY I. Multiple Choice Questions (Type-I) 1. Two students performed the same experiment separately and each one of them recorded two readings of mass which are given
Ideal Gas Law. Name, Date, Partner Lab Section. Date, Partner and Lab Section
 Ideal Gas Law Jane Doe Physics 16, Tuesday Section Title Title Partner: Michelle Smith Sept. 12, 2006 Introduction: Name, Date, Partner and Name, Lab Section Date, Partner and Lab Section The purpose of
Ideal Gas Law Jane Doe Physics 16, Tuesday Section Title Title Partner: Michelle Smith Sept. 12, 2006 Introduction: Name, Date, Partner and Name, Lab Section Date, Partner and Lab Section The purpose of
Isotopes are different forms of the that have a. Isotopes of the same element have the but. Ions are atoms that have a. In an ion, the.
 Chemistry Ms. Ye Name Date Block Atomic Structure: 1. What is important about the atomic number? 2. How do you figure out the number of a. Protons in an atom? b. Electrons in an atom? c. Neutrons in an
Chemistry Ms. Ye Name Date Block Atomic Structure: 1. What is important about the atomic number? 2. How do you figure out the number of a. Protons in an atom? b. Electrons in an atom? c. Neutrons in an
NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY
 NMR Spectroscopy 1 NULEAR MAGNETI RESONANE SPETROSOPY Involves interaction of materials with the low-energy radiowave region of the electromagnetic spectrum Origin of Spectra Theory All nuclei possess
NMR Spectroscopy 1 NULEAR MAGNETI RESONANE SPETROSOPY Involves interaction of materials with the low-energy radiowave region of the electromagnetic spectrum Origin of Spectra Theory All nuclei possess
Prep for AP Chemistry
 Prep for AP Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence to meet curricular
Prep for AP Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence to meet curricular
Supporting Information. Electrochemical Reduction of Carbon Dioxide on Nitrogen-Doped Carbons: Insights from Isotopic Labeling Studies
 Supporting Information Electrochemical Reduction of Carbon Dioxide on Nitrogen-Doped Carbons: Insights from Isotopic Labeling Studies Dorottya Hursán 1,2 and Csaba Janáky 1,2* 1 Department of Physical
Supporting Information Electrochemical Reduction of Carbon Dioxide on Nitrogen-Doped Carbons: Insights from Isotopic Labeling Studies Dorottya Hursán 1,2 and Csaba Janáky 1,2* 1 Department of Physical
SI0 UV-vis absorption spectra and emission response of 6MQz and 6MQc
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 SI0 UV-vis absorption spectra and emission response of 6MQz and 6MQc The absorption and emission
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 SI0 UV-vis absorption spectra and emission response of 6MQz and 6MQc The absorption and emission
Topic 2.11 ANALYTICAL TECHNIQUES. High Resolution Mass Spectrometry Infra-red Spectroscopy
 Topic 2.11 ANALYTICAL TECHNIQUES High Resolution Mass Spectrometry Infra-red Spectroscopy HIGH RESOLUTION MASS SPECTROMETRY The technique of mass spectrometry was used in Unit 1 to: a) determine the relative
Topic 2.11 ANALYTICAL TECHNIQUES High Resolution Mass Spectrometry Infra-red Spectroscopy HIGH RESOLUTION MASS SPECTROMETRY The technique of mass spectrometry was used in Unit 1 to: a) determine the relative
Q1. The electronic structure of the atoms of five elements are shown in the figure below.
 Q1. The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements. Choose the element to answer the question. Each element can be
Q1. The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements. Choose the element to answer the question. Each element can be
Clumped isotopes of atmospheric trace gases
 Clumped isotopes of atmospheric trace gases Thomas Röckmann Institute for Marine and Atmospheric research Utrecht (IMAU) Utrecht University, The Netherlands Thanks to: Magdalena Hofmann, Dipayan Paul,
Clumped isotopes of atmospheric trace gases Thomas Röckmann Institute for Marine and Atmospheric research Utrecht (IMAU) Utrecht University, The Netherlands Thanks to: Magdalena Hofmann, Dipayan Paul,
c. The Grashof number is the ratio of buoyant forces to viscous forces acting on a fluid.
 QUESTION 1. (0 pts) With respect to free convection: a. What is an extensive, quiescent fluid? (4 points) b. What are the two major physical considerations or forces for free convection? (4 points) c.
QUESTION 1. (0 pts) With respect to free convection: a. What is an extensive, quiescent fluid? (4 points) b. What are the two major physical considerations or forces for free convection? (4 points) c.
Business. Business. Multiphase Systems Ch. 6. P vs T Diagram: Water (pure component) P vs T Diagram: CO 2 LYNN ORR
 Business LYNN ORR Izatt-Christensen Lecturer Former Assistant Secretary of Energy Public lecture Thursday 11 am, JSB Auditorium Technical lecture, Friday, 11 am, Varsity Theater Courtesy Corbin Critchfield
Business LYNN ORR Izatt-Christensen Lecturer Former Assistant Secretary of Energy Public lecture Thursday 11 am, JSB Auditorium Technical lecture, Friday, 11 am, Varsity Theater Courtesy Corbin Critchfield
Name: Date: Chemistry ~ Ms. Hart Class: Anions or Cations. Station Review Midterm January 2014 STATION 1: Chemical/physical properties and change
 Name: Date: STATION 1: Chemical/physical properties and change Physical changes are changes in matter in which the appearance of a substance changes but the identity of the compound remains the same Chemical
Name: Date: STATION 1: Chemical/physical properties and change Physical changes are changes in matter in which the appearance of a substance changes but the identity of the compound remains the same Chemical
Introductory College Chemistry
 Introductory College Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence to
Introductory College Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence to
Measurement of the 30 Si mole fraction in the new Avogadro silicon material by Neutron Activation and high resolution spectrometry
 Measurement of the 30 Si mole fraction in the new Avogadro silicon material by Neutron Activation and high resolution spectrometry Marco Di Luzio 1,2, Attila Stopic 3, Giancarlo D Agostino 1, John W Bennett
Measurement of the 30 Si mole fraction in the new Avogadro silicon material by Neutron Activation and high resolution spectrometry Marco Di Luzio 1,2, Attila Stopic 3, Giancarlo D Agostino 1, John W Bennett
Kinetic Isotope Effects
 1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
1 Experiment 31 Kinetic Isotope Effects Isotopic substitution is a useful technique for the probing of reaction mechanisms. The change of an isotope may affect the reaction rate in a number of ways, providing
Magnetic Nuclei other than 1 H
 Magnetic Nuclei other than 1 H 2 H (Deuterium): I = 1 H,D-Exchange might be used to simplify 1 H-NMR spectra since H-D couplings are generally small; - - - -O- - - -D 2 -O- triplet of triplets slightly
Magnetic Nuclei other than 1 H 2 H (Deuterium): I = 1 H,D-Exchange might be used to simplify 1 H-NMR spectra since H-D couplings are generally small; - - - -O- - - -D 2 -O- triplet of triplets slightly
Chapter 2 Notes The Chemistry of Life
 Name: Chapter 2 Notes The Chemistry of Life Section 2-1 The Nature of Matter Date: Atoms (p. 35) The study of chemistry begins with the basic unit of matter, the. Comes from the Greek word atomos, meaning
Name: Chapter 2 Notes The Chemistry of Life Section 2-1 The Nature of Matter Date: Atoms (p. 35) The study of chemistry begins with the basic unit of matter, the. Comes from the Greek word atomos, meaning
JRP SIB09 Elements GOOD PRACTICE GUIDE ON DEALING WITH INTERFERENCES IN ICP-MS FOR INVESTIGATING HIGH PURITY MATERIALS. Deliverable 1.2.
 JRP SIB09 Elements GOOD PRACTICE GUIDE ON DEALING WITH INTERFERENCES IN ICP-MS FOR INVESTIGATING HIGH PURITY MATERIALS Deliverable 1.2.32 (June 2015) Author : Guillaume Labarraque 1 Introduction High purity
JRP SIB09 Elements GOOD PRACTICE GUIDE ON DEALING WITH INTERFERENCES IN ICP-MS FOR INVESTIGATING HIGH PURITY MATERIALS Deliverable 1.2.32 (June 2015) Author : Guillaume Labarraque 1 Introduction High purity
Lab O2. Variation of Atmospheric Pressure with Altitude. We all live near the bottom of an ocean of air. At sea level, the weight of the air
 O2.1 Lab O2. Variation of Atmospheric Pressure with Altitude Introduction In this lab you will investigate the ideal gas law. By moving a plunger in a sealed container of air, you will find the variation
O2.1 Lab O2. Variation of Atmospheric Pressure with Altitude Introduction In this lab you will investigate the ideal gas law. By moving a plunger in a sealed container of air, you will find the variation
Chapter 10: Thermal Physics
 Chapter 10: hermal Physics hermal physics is the study of emperature, Heat, and how these affect matter. hermal equilibrium eists when two objects in thermal contact with each other cease to echange energy.
Chapter 10: hermal Physics hermal physics is the study of emperature, Heat, and how these affect matter. hermal equilibrium eists when two objects in thermal contact with each other cease to echange energy.
Identical Particles in Quantum Mechanics
 Identical Particles in Quantum Mechanics Chapter 20 P. J. Grandinetti Chem. 4300 Nov 17, 2017 P. J. Grandinetti (Chem. 4300) Identical Particles in Quantum Mechanics Nov 17, 2017 1 / 20 Wolfgang Pauli
Identical Particles in Quantum Mechanics Chapter 20 P. J. Grandinetti Chem. 4300 Nov 17, 2017 P. J. Grandinetti (Chem. 4300) Identical Particles in Quantum Mechanics Nov 17, 2017 1 / 20 Wolfgang Pauli
Objective #1 (46 topics, due on 09/04/ :59 PM) Section 0.1 (15 topics) Course Name: Chem Hybrid Fall 2016 Course Code: PVPTL-XH6CF
 Course Name: Chem 113.4 Hybrid Fall 2016 Course Code: PVPTL-XH6CF ALEKS Course: General Chemistry (First Semester) Instructor: Ms. D'Costa Course Dates: Begin: 08/25/2016 End: 12/23/2016 Course Content:
Course Name: Chem 113.4 Hybrid Fall 2016 Course Code: PVPTL-XH6CF ALEKS Course: General Chemistry (First Semester) Instructor: Ms. D'Costa Course Dates: Begin: 08/25/2016 End: 12/23/2016 Course Content:
Supplement of Observation of isoprene hydroxynitrates in the southeastern United States and implications for the fate of NO x
 Supplement of Atmos. Chem. Phys., 15, 11257 11272, 2015 http://www.atmos-chem-phys.net/15/11257/2015/ doi:10.5194/acp-15-11257-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of
Supplement of Atmos. Chem. Phys., 15, 11257 11272, 2015 http://www.atmos-chem-phys.net/15/11257/2015/ doi:10.5194/acp-15-11257-2015-supplement Author(s) 2015. CC Attribution 3.0 License. Supplement of
Course Name: CHEM 1311 Fall 2015 Course Code: N/A. ALEKS Course: General Chemistry (First Semester) Instructor: Master Templates
 Course Name: CHEM 1311 Fall 2015 Course Code: N/A ALEKS Course: General Chemistry (First Semester) Instructor: Master Templates Course Dates: Begin: 08/21/2015 End: 12/31/2015 Course Content: 229 topics
Course Name: CHEM 1311 Fall 2015 Course Code: N/A ALEKS Course: General Chemistry (First Semester) Instructor: Master Templates Course Dates: Begin: 08/21/2015 End: 12/31/2015 Course Content: 229 topics
Which of the following chemical elements corresponds to the symbol Cu?
 Which of the following chemical elements corresponds to the symbol Cu? A) copper B) gold C) lead D) silver E) none of the above Which of the following chemical elements corresponds to the symbol Cu? A)
Which of the following chemical elements corresponds to the symbol Cu? A) copper B) gold C) lead D) silver E) none of the above Which of the following chemical elements corresponds to the symbol Cu? A)
Average Atomic Mass: How are the masses on the periodic table determined?
 Chemistry Ms. Ye Name Date Block Average Atomic Mass: How are the masses on the periodic table determined? Most elements have more than one naturally occurring isotope. As you learned previously, the atoms
Chemistry Ms. Ye Name Date Block Average Atomic Mass: How are the masses on the periodic table determined? Most elements have more than one naturally occurring isotope. As you learned previously, the atoms
Supplementary Information Supplementary Figures
 Supplementary Information Supplementary Figures Supplementary Fig. 1 Methanol-derived protons in methanediol in the formaldehyde production from methanol: The water formed in the oxidation is used to form
Supplementary Information Supplementary Figures Supplementary Fig. 1 Methanol-derived protons in methanediol in the formaldehyde production from methanol: The water formed in the oxidation is used to form
NSS Chemistry Part 2 The Microscopic World I HKCEE Past Paper Questions Structural Questions
 NSS Chemistry Part 2 The Microscopic World I HKCEE Past Paper Questions Structural Questions 1. HKCEE 1994 Q7b The table below lists some physical properties of lead, bromine and lead(ii) bromide. Lead
NSS Chemistry Part 2 The Microscopic World I HKCEE Past Paper Questions Structural Questions 1. HKCEE 1994 Q7b The table below lists some physical properties of lead, bromine and lead(ii) bromide. Lead
Worksheet 5 - Chemical Bonding
 Worksheet 5 - Chemical Bonding The concept of electron configurations allowed chemists to explain why chemical molecules are formed from the elements. In 1916 the American chemist Gilbert Lewis proposed
Worksheet 5 - Chemical Bonding The concept of electron configurations allowed chemists to explain why chemical molecules are formed from the elements. In 1916 the American chemist Gilbert Lewis proposed
10. 2 P R O B L E M S L I Q U I D S A N D G A S E S
 South Pasadena AP Chemistry Name 10 States of Matter Period Date 10. 2 P R B L E M S L I Q U I D S A N D G A S E S 1. Use the following table to answer these questions. Vapor Pressures of Various Liquids
South Pasadena AP Chemistry Name 10 States of Matter Period Date 10. 2 P R B L E M S L I Q U I D S A N D G A S E S 1. Use the following table to answer these questions. Vapor Pressures of Various Liquids
Preparation for General Chemistry
 Preparation for General Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence
Preparation for General Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence
Homework 4 Solutions. 1. Fun physics with mean molecular/atomic weight (µ).
 1 Homework 4 Solutions 1. Fun physics with mean molecular/atomic weight µ). Why does the pitch of your voice change if you inhale helium? Assume the size of your vocal cords stay the same this sets the
1 Homework 4 Solutions 1. Fun physics with mean molecular/atomic weight µ). Why does the pitch of your voice change if you inhale helium? Assume the size of your vocal cords stay the same this sets the
Long-Term Climate Regulation (Ch.12) The Faint Young Sun Paradox Revisited (P )
 Long-Term Climate Regulation (Ch.12) The Faint Young Sun Paradox Revisited (P. 230-236) Dating with radioactive elements Carbon 14 (read Ch.5, P97-98) Natural radioactivity Atomic nuclei are composed of
Long-Term Climate Regulation (Ch.12) The Faint Young Sun Paradox Revisited (P. 230-236) Dating with radioactive elements Carbon 14 (read Ch.5, P97-98) Natural radioactivity Atomic nuclei are composed of
CO + H 2. hydrocarbons oxygenates
 C + 2 hydrocarbons oxygenates Why? ydrocarbons and oxygenates as fuels and chemicals. Conversion of synthesis gas from diverse carbon sources (biomass, coal, natural gas, tar sands, ) Known chemistries,
C + 2 hydrocarbons oxygenates Why? ydrocarbons and oxygenates as fuels and chemicals. Conversion of synthesis gas from diverse carbon sources (biomass, coal, natural gas, tar sands, ) Known chemistries,
The Stoichiometry of Reactions Introduction
 The Stoichiometry of Reactions Introduction Copyright c 2015 by Nob ill Publishing, LLC Stoichiometry: the determination of the proportions in which chemical elements combine or are produced and the weight
The Stoichiometry of Reactions Introduction Copyright c 2015 by Nob ill Publishing, LLC Stoichiometry: the determination of the proportions in which chemical elements combine or are produced and the weight
Spitzer Infrared Spectrograph (IRS) Observations of Large Magellanic Cloud Planetary Nebula SMP 83
 Spitzer Infrared Spectrograph (IRS) Observations of Large Magellanic Cloud Planetary Nebula SMP 83 J. Bernard Salas, J. R. Houck, P. W. Morris, G. C. Sloan, S. R. Pottasch, & D. J. Barry ApJS, 154, 271
Spitzer Infrared Spectrograph (IRS) Observations of Large Magellanic Cloud Planetary Nebula SMP 83 J. Bernard Salas, J. R. Houck, P. W. Morris, G. C. Sloan, S. R. Pottasch, & D. J. Barry ApJS, 154, 271
If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out.
 Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Sign In Forgot Password Register username username password password Sign In If you like us, please share us on social media. The latest UCD Hyperlibrary newsletter is now complete, check it out. ChemWiki
Supplementary Figure 1. Extinction spectra of rhodium nanocubes. UV-vis spectra of the Rh nanocubes in ethanol solution (black) and on a porous Al2O3
 Supplementary Figure 1. Extinction spectra of rhodium nanocubes. UV-vis spectra of the Rh nanocubes in ethanol solution (black) and on a porous Al2O3 support (blue). The Rh nanocubes in ethanol solution
Supplementary Figure 1. Extinction spectra of rhodium nanocubes. UV-vis spectra of the Rh nanocubes in ethanol solution (black) and on a porous Al2O3 support (blue). The Rh nanocubes in ethanol solution
What we will learn about now
 Chapter 4: Gases What we will learn about now We will learn how volume, pressure, temperature are related. You probably know much of this qualitatively, but we ll learn it quantitatively as well with the
Chapter 4: Gases What we will learn about now We will learn how volume, pressure, temperature are related. You probably know much of this qualitatively, but we ll learn it quantitatively as well with the
TR ACE ORGANIC. lgcstandards.com/foodandenvironment
 U S E O F S TA B L E I S OTO P E I NTE R N A L S TA N D A R D S F O R TR ACE ORGANIC A N A LY S I S lgcstandards.com/foodandenvironment dr.ehrenstorfer@lgcgroup.com This paper explains why stable isotope
U S E O F S TA B L E I S OTO P E I NTE R N A L S TA N D A R D S F O R TR ACE ORGANIC A N A LY S I S lgcstandards.com/foodandenvironment dr.ehrenstorfer@lgcgroup.com This paper explains why stable isotope
Name Date Class CHEMICAL QUANTITIES. SECTION 10.1 THE MOLE: A MEASUREMENT OF MATTER (pages )
 10 CHEMICAL QUANTITIES SECTION 10.1 THE MOLE: A MEASUREMENT OF MATTER (pages 287 296) This section defines the mole and explains how the mole is used to measure matter. It also teaches you how to calculate
10 CHEMICAL QUANTITIES SECTION 10.1 THE MOLE: A MEASUREMENT OF MATTER (pages 287 296) This section defines the mole and explains how the mole is used to measure matter. It also teaches you how to calculate
Supplement of Constraining uncertainties in particle-wall deposition correction during SOA formation in chamber experiments
 Supplement of Atmos. Chem. Phys., 17, 2297 2310, 2017 http://www.atmos-chem-phys.net/17/2297/2017/ doi:10.5194/acp-17-2297-2017-supplement Author(s) 2017. CC Attribution 3.0 License. Supplement of Constraining
Supplement of Atmos. Chem. Phys., 17, 2297 2310, 2017 http://www.atmos-chem-phys.net/17/2297/2017/ doi:10.5194/acp-17-2297-2017-supplement Author(s) 2017. CC Attribution 3.0 License. Supplement of Constraining
Advanced Subsidiary Unit 1: The Core Principles of Chemistry
 Write your name here Surname Other names Pearson Edexcel International Advanced Level Centre Number Chemistry Advanced Subsidiary Unit 1: The Core Principles of Chemistry Candidate Number Thursday 14 January
Write your name here Surname Other names Pearson Edexcel International Advanced Level Centre Number Chemistry Advanced Subsidiary Unit 1: The Core Principles of Chemistry Candidate Number Thursday 14 January
Uncertainty in Measurement of Isotope Ratios by Multi-Collector Mass Spectrometry
 1 IAEA-CN-184/168 Uncertainty in Measurement of Isotope Ratios by Multi-Collector Mass Spectrometry R. Williams Lawrence Livermore National Laboratory Livermore, California U.S.A. williams141@llnl.gov
1 IAEA-CN-184/168 Uncertainty in Measurement of Isotope Ratios by Multi-Collector Mass Spectrometry R. Williams Lawrence Livermore National Laboratory Livermore, California U.S.A. williams141@llnl.gov
Environmental Isotopes in Hydrology. Woocay substituting for Walton
 Environmental Isotopes in Hydrology Oct 7, 2010 1 What is an Isotope? An element is defined by the number of protons (Z) in the nucleus The number of neutrons (N) defines the isotope(s) of that element
Environmental Isotopes in Hydrology Oct 7, 2010 1 What is an Isotope? An element is defined by the number of protons (Z) in the nucleus The number of neutrons (N) defines the isotope(s) of that element
Phase Change: solid to liquid. Melting
 Phase Change: solid to liquid Melting Most solids shrink in size when frozen. What substance is an exception and actually expands? water Use the phase diagram below to answer the following question. What
Phase Change: solid to liquid Melting Most solids shrink in size when frozen. What substance is an exception and actually expands? water Use the phase diagram below to answer the following question. What
FIRST RADIOCARBON MEASUREMENTS AT BINP AMS
 FIRST RADIOCARBON MEASUREMENTS AT BINP AMS A.R Frolov, A.D. Goncharov, V.F. Klyuev, S.G. Konstantinov, E.S. Konstantinov, L.A. Kutnykova, V. V. Parkhomchuk, M.V. Petrichenkov, A.V. Petrozhitskii, S.A.
FIRST RADIOCARBON MEASUREMENTS AT BINP AMS A.R Frolov, A.D. Goncharov, V.F. Klyuev, S.G. Konstantinov, E.S. Konstantinov, L.A. Kutnykova, V. V. Parkhomchuk, M.V. Petrichenkov, A.V. Petrozhitskii, S.A.
Using different flavours of oxygen to measure biological production from ship-based and autonomous platforms
 Using different flavours of oxygen to measure biological production from ship-based and autonomous platforms Jan Kaiser University of East Anglia Centre for Ocean and Atmospheric Sciences School of Environmental
Using different flavours of oxygen to measure biological production from ship-based and autonomous platforms Jan Kaiser University of East Anglia Centre for Ocean and Atmospheric Sciences School of Environmental
Isotopes: Climate, Sea Level, Ecology
 Isotopes: Climate, Sea Level, Ecology Definitions Isotopes Atoms of the same element (i.e., same number of protons and electrons) but different numbers of neutrons. Stable Isotope Do not undergo radioactive
Isotopes: Climate, Sea Level, Ecology Definitions Isotopes Atoms of the same element (i.e., same number of protons and electrons) but different numbers of neutrons. Stable Isotope Do not undergo radioactive
Equilibrium Practice Problems page 1
 Equilibrium Practice Problems page 1 1988 D NH 4 HS(s) NH 3 (g) + H 2 S(g) ΔHº = +93 kilojoules The equilibrium above is established by placing solid NH 4 HS in an evacuated container at 25ºC. At equilibrium,
Equilibrium Practice Problems page 1 1988 D NH 4 HS(s) NH 3 (g) + H 2 S(g) ΔHº = +93 kilojoules The equilibrium above is established by placing solid NH 4 HS in an evacuated container at 25ºC. At equilibrium,
Data Analysis and Modeling with Stable Isotope Ratios. Chun-Ta Lai San Diego State University June 2008
 Data Analysis and Modeling with Stable Isotope Ratios Chun-Ta Lai San Diego State University June 2008 Leaf water is 18 O-enriched via transpiration δ 18 O vapor : -12 H 2 16 O H 2 18 O δ 18 O leaf : +8
Data Analysis and Modeling with Stable Isotope Ratios Chun-Ta Lai San Diego State University June 2008 Leaf water is 18 O-enriched via transpiration δ 18 O vapor : -12 H 2 16 O H 2 18 O δ 18 O leaf : +8
A brief summary of isotopes and fractionation
 A brief summary of isotopes and fractionation 1. Isotopes are chemically identical, but mechanically different. 2. Different masses lead to different bond frequencies... ν = 1 2π κ µ Hooke's Law 3....which
A brief summary of isotopes and fractionation 1. Isotopes are chemically identical, but mechanically different. 2. Different masses lead to different bond frequencies... ν = 1 2π κ µ Hooke's Law 3....which
22.56J Noninvasive Imaging in Biology and Medicine Instructor: Prof. Alan Jasanoff Fall 2005, TTh 1-2:30
 22.56J Noninvasive Imaging in Biology and Medicine Instructor: Prof. Alan Jasanoff Fall 2005, TTh 1-2:30 Sample problems HW1 1. Look up (e.g. in the CRC Manual of Chemistry and Physics www.hbcpnetbase.com)
22.56J Noninvasive Imaging in Biology and Medicine Instructor: Prof. Alan Jasanoff Fall 2005, TTh 1-2:30 Sample problems HW1 1. Look up (e.g. in the CRC Manual of Chemistry and Physics www.hbcpnetbase.com)
Stellar Interiors Nuclear Energy ASTR 2110 Sarazin. Fusion the Key to the Stars
 Stellar Interiors Nuclear Energy ASTR 2110 Sarazin Fusion the Key to the Stars Energy Source for Stars For Sun, need total energy E = L t Sun = L x (10 10 years) ~ 10 51 erg N atoms = / m p ~ 10 57 atoms
Stellar Interiors Nuclear Energy ASTR 2110 Sarazin Fusion the Key to the Stars Energy Source for Stars For Sun, need total energy E = L t Sun = L x (10 10 years) ~ 10 51 erg N atoms = / m p ~ 10 57 atoms
Chapter 1 Chemistry: The Central Science. CHEM 101 Dr. Geoff Sametz Fall 2009
 Chapter 1 Chemistry: The Central Science CHEM 101 Dr. Geoff Sametz Fall 2009 What IS Chemistry? Text: The study of matter and the changes that matter undergoes Focus: how matter interacts at the atomic/molecular
Chapter 1 Chemistry: The Central Science CHEM 101 Dr. Geoff Sametz Fall 2009 What IS Chemistry? Text: The study of matter and the changes that matter undergoes Focus: how matter interacts at the atomic/molecular
CS Communication Complexity: Applications and New Directions
 CS 2429 - Communication Complexity: Applications and New Directions Lecturer: Toniann Pitassi 1 Introduction In this course we will define the basic two-party model of communication, as introduced in the
CS 2429 - Communication Complexity: Applications and New Directions Lecturer: Toniann Pitassi 1 Introduction In this course we will define the basic two-party model of communication, as introduced in the
NMR Spectroscopy: A Quantum Phenomena
 NMR Spectroscopy: A Quantum Phenomena Pascale Legault Département de Biochimie Université de Montréal Outline 1) Energy Diagrams and Vector Diagrams 2) Simple 1D Spectra 3) Beyond Simple 1D Spectra 4)
NMR Spectroscopy: A Quantum Phenomena Pascale Legault Département de Biochimie Université de Montréal Outline 1) Energy Diagrams and Vector Diagrams 2) Simple 1D Spectra 3) Beyond Simple 1D Spectra 4)
Statistical mechanics of biological processes
 Statistical mechanics of biological processes 1 Modeling biological processes Describing biological processes requires models. If reaction occurs on timescales much faster than that of connected processes
Statistical mechanics of biological processes 1 Modeling biological processes Describing biological processes requires models. If reaction occurs on timescales much faster than that of connected processes
Atoms, Ions and Molecules Calculations
 Atoms, Ions and Molecules Calculations 1. How do you calculate the atomic mass of an element? Atomic Mass = (% abundance of isotope 1)(mass of isotope 1) + (% abundance of isotope2)(mass of isotope 2)
Atoms, Ions and Molecules Calculations 1. How do you calculate the atomic mass of an element? Atomic Mass = (% abundance of isotope 1)(mass of isotope 1) + (% abundance of isotope2)(mass of isotope 2)
Chem Hughbanks Final Exam, May 11, 2011
 Chem 107 - Hughbanks Final Exam, May 11, 2011 Name (Print) UIN # Section 503 Exam 3, Version # A On the last page of this exam, you ve been given a periodic table and some physical constants. You ll probably
Chem 107 - Hughbanks Final Exam, May 11, 2011 Name (Print) UIN # Section 503 Exam 3, Version # A On the last page of this exam, you ve been given a periodic table and some physical constants. You ll probably
Study Notes on the Latent Dirichlet Allocation
 Study Notes on the Latent Dirichlet Allocation Xugang Ye 1. Model Framework A word is an element of dictionary {1,,}. A document is represented by a sequence of words: =(,, ), {1,,}. A corpus is a collection
Study Notes on the Latent Dirichlet Allocation Xugang Ye 1. Model Framework A word is an element of dictionary {1,,}. A document is represented by a sequence of words: =(,, ), {1,,}. A corpus is a collection
Q1. The electronic structure of the atoms of five elements are shown in the figure below.
 Q. The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements. Choose the element to answer the question. Each element can be
Q. The electronic structure of the atoms of five elements are shown in the figure below. The letters are not the symbols of the elements. Choose the element to answer the question. Each element can be
CCT/03-21 Evaluation of the depression constants for D and 18 O isotopes for the triple-point temperature of water
 CCT/03- Evaluation of the depression constants for D and 8 O isotopes for the triple-point temperature of water D R White Measurement Standards Laboratory of New Zealand, rwhite@irlcrinz W L Tew National
CCT/03- Evaluation of the depression constants for D and 8 O isotopes for the triple-point temperature of water D R White Measurement Standards Laboratory of New Zealand, rwhite@irlcrinz W L Tew National
Fundamentals of Nuclear Science and Engineering
 PROBLEM SOLUTION MANUAL FOR Fundamentals of Nuclear Science and Engineering Third Edition by J. Kenneth Shultis and Richard E. Faw Dept. of Mechanical and Nuclear Engineering Kansas State University Manhattan,
PROBLEM SOLUTION MANUAL FOR Fundamentals of Nuclear Science and Engineering Third Edition by J. Kenneth Shultis and Richard E. Faw Dept. of Mechanical and Nuclear Engineering Kansas State University Manhattan,
Lesson 12: Atoms and Subatomic Particles
 NOTES Name: Date: Class: Lesson 12: Atoms and Subatomic Particles Element: fundamental substance that ; all matter consists of ~100 elements Atom: that can exist; smallest unit of an element that can enter
NOTES Name: Date: Class: Lesson 12: Atoms and Subatomic Particles Element: fundamental substance that ; all matter consists of ~100 elements Atom: that can exist; smallest unit of an element that can enter
Hirsch - Binary phase diagrams problems KEY
 Hirsch - Binary phase diagrams problems KEY Name Question 1. 1A. Using the phase rule, determine the degrees of freedom: a. at point e F = 2-3 + 1 = 0 b. within the field A + B F = 2-2 + 1 = 1 c. within
Hirsch - Binary phase diagrams problems KEY Name Question 1. 1A. Using the phase rule, determine the degrees of freedom: a. at point e F = 2-3 + 1 = 0 b. within the field A + B F = 2-2 + 1 = 1 c. within
WATER IN THE ATMOSPHERE
 WATER IN THE ATMOSPHERE During a rainstorm, the air feels moist On a clear, cloudless day, the air may feel dry As the sun heats the land and oceans, the amount of water in the atmosphere changes Water
WATER IN THE ATMOSPHERE During a rainstorm, the air feels moist On a clear, cloudless day, the air may feel dry As the sun heats the land and oceans, the amount of water in the atmosphere changes Water
Chemical Kinetics and
 Chemical Kinetics and Equilibrium Part 2: Chemical Equilibrium David A. Katz Department of Chemistry Pima Community College Tucson, AZ USA The Concept of Equilibrium Kinetics applies to the speed of a
Chemical Kinetics and Equilibrium Part 2: Chemical Equilibrium David A. Katz Department of Chemistry Pima Community College Tucson, AZ USA The Concept of Equilibrium Kinetics applies to the speed of a
Inferred Ionic Charge States for Solar Energetic Particle Events from with ACE and STEREO
 Inferred Ionic Charge States for Solar Energetic Particle Events from 2012-2015 with ACE and STEREO A. W. Labrador 1,*, L. S. Sollitt 2, C. M. S. Cohen 1, A. C. Cummings 1, R. A. Leske 1, G. M. Mason 3,
Inferred Ionic Charge States for Solar Energetic Particle Events from 2012-2015 with ACE and STEREO A. W. Labrador 1,*, L. S. Sollitt 2, C. M. S. Cohen 1, A. C. Cummings 1, R. A. Leske 1, G. M. Mason 3,
SUPPLEMENTARY INFORMATION
 DOI:.38/NCHEM.1467 Prebiotic synthesis of simple sugars by photoredox systems chemistry Dougal Ritson & John D. Sutherland 1 MRC Laboratory of Molecular Biology, Hills Road, Cambridge CB2 QH, UK. The Supplementary
DOI:.38/NCHEM.1467 Prebiotic synthesis of simple sugars by photoredox systems chemistry Dougal Ritson & John D. Sutherland 1 MRC Laboratory of Molecular Biology, Hills Road, Cambridge CB2 QH, UK. The Supplementary
Spring 2004 O2.1. Lab O2. Variation of Atmospheric Pressure with Altitude
 Spring 2004 O2.1 Introduction Lab O2. Variation of Atmospheric Pressure with Altitude In this lab you will investigate the ideal gas law. By pulling and pushing a plunger you will find the variation of
Spring 2004 O2.1 Introduction Lab O2. Variation of Atmospheric Pressure with Altitude In this lab you will investigate the ideal gas law. By pulling and pushing a plunger you will find the variation of
Diffusion of silver in silicate glass and clustering in hydrogen atmosphere
 Defect and Diffusion Forum Vols. 7-4 (5) pp. 689-694 online at http://www.scientific.net 5 Trans Tech Publications, Switzerland Diffusion of silver in silicate glass and clustering in hydrogen atmosphere
Defect and Diffusion Forum Vols. 7-4 (5) pp. 689-694 online at http://www.scientific.net 5 Trans Tech Publications, Switzerland Diffusion of silver in silicate glass and clustering in hydrogen atmosphere
2.1. SYMBOLS AND FORMULAS A unique symbol is used to represent each element. Formulas are used to represent compounds.
 2.1. SYMBOLS AND FORMULAS A unique symbol is used to represent each element. Formulas are used to represent compounds. Symbols of Elements Atoms and Molecules ELEMENTAL SYMBOLS A symbol is assigned to
2.1. SYMBOLS AND FORMULAS A unique symbol is used to represent each element. Formulas are used to represent compounds. Symbols of Elements Atoms and Molecules ELEMENTAL SYMBOLS A symbol is assigned to
Atoms Section 2 Section 2: The Structure of Atoms
 Section 2: The Structure of Atoms Preview Key Ideas Bellringer What Is in an Atom? Atomic Number and Mass Number Isotopes Atomic Masses Math Skills Key Ideas What is the difference between protons, neutrons,
Section 2: The Structure of Atoms Preview Key Ideas Bellringer What Is in an Atom? Atomic Number and Mass Number Isotopes Atomic Masses Math Skills Key Ideas What is the difference between protons, neutrons,
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/4/10/eaas9319/dc1 Supplementary Materials for Transformation of alcohols to esters promoted by hydrogen bonds using oxygen as the oxidant under metal-free conditions
advances.sciencemag.org/cgi/content/full/4/10/eaas9319/dc1 Supplementary Materials for Transformation of alcohols to esters promoted by hydrogen bonds using oxygen as the oxidant under metal-free conditions
GASES (Chapter 5) Temperature and Pressure, that is, 273 K and 1.00 atm or 760 Torr ) will occupy
 I. Ideal gases. A. Ideal gas law review. GASES (Chapter 5) 1. PV = nrt Ideal gases obey this equation under all conditions. It is a combination ofa. Boyle's Law: P 1/V at constant n and T b. Charles's
I. Ideal gases. A. Ideal gas law review. GASES (Chapter 5) 1. PV = nrt Ideal gases obey this equation under all conditions. It is a combination ofa. Boyle's Law: P 1/V at constant n and T b. Charles's
Physics 213. Practice Final Exam Spring The next two questions pertain to the following situation:
 The next two questions pertain to the following situation: Consider the following two systems: A: three interacting harmonic oscillators with total energy 6ε. B: two interacting harmonic oscillators, with
The next two questions pertain to the following situation: Consider the following two systems: A: three interacting harmonic oscillators with total energy 6ε. B: two interacting harmonic oscillators, with
All atoms of an element must have the same number of protons but not neutrons.
 Counting Atoms Key Terms atomic number nuclide mole isotope unified atomic mass unit Avogadro s number mass number average atomic mass molar mass Consider neon, Ne, the gas used in many illuminated signs.
Counting Atoms Key Terms atomic number nuclide mole isotope unified atomic mass unit Avogadro s number mass number average atomic mass molar mass Consider neon, Ne, the gas used in many illuminated signs.
Electromagnetic Radiation. Radiation and the Planetary Energy Balance. Electromagnetic Spectrum of the Sun
 Radiation and the Planetary Energy Balance Electromagnetic Radiation Solar radiation warms the planet Conversion of solar energy at the surface Absorption and emission by the atmosphere The greenhouse
Radiation and the Planetary Energy Balance Electromagnetic Radiation Solar radiation warms the planet Conversion of solar energy at the surface Absorption and emission by the atmosphere The greenhouse
1. The most important aspects of the quantum theory.
 Lecture 5. Radiation and energy. Objectives: 1. The most important aspects of the quantum theory: atom, subatomic particles, atomic number, mass number, atomic mass, isotopes, simplified atomic diagrams,
Lecture 5. Radiation and energy. Objectives: 1. The most important aspects of the quantum theory: atom, subatomic particles, atomic number, mass number, atomic mass, isotopes, simplified atomic diagrams,
5 Stable and radioactive isotopes
 5 Stable and radioactive isotopes Outline 1 Stable isotopes Measuring stable isotopic abundances Equilibrium isotope effects Kinetic isotope effects Rayleigh distillation Isotopes: a mainstay of chemical
5 Stable and radioactive isotopes Outline 1 Stable isotopes Measuring stable isotopic abundances Equilibrium isotope effects Kinetic isotope effects Rayleigh distillation Isotopes: a mainstay of chemical
