CHEMISTRY OF THE HUMAN BODY
|
|
|
- Alexina McDowell
- 6 years ago
- Views:
Transcription
1 CHEMISTRY OF THE HUMAN BODY (Sample Questions) WUCT 2018 The three questions below are meant to give a sense of the kinds of questions that might be asked on the exam in April The actual exam is 60 minutes long, and it may differ in topic areas covered and difficulty. Necessary constants, equations, and conversions may be found on the last page of this document. Unless otherwise stated, explanations must be written in complete sentences, diagrams must be labeled, and units must be shown in calculations to receive full credit. Please answer the questions in the space provided. No work on the back of the sheets or attached sheets will be graded. The amount of points each question is worth will be indicated on the actual exam. Note that not all lone pairs are necessarily shown on every chemical structure. Team Members: High School: Page 1 of 9
2 1. This question concerns a tetrameric (consisting of four subunits) protein, hemoglobin (abbreviated as Hb), that binds O 2(g) a nd CO 2(g) in our body. Each subunit of the hemoglobin protein contains a single heme B group (shown below). We will examine various properties of gases, and of the hemoglobin protein itself. Note that there is a ferrous iron (Fe 2 + ) at the center of each of the heme B group. a. What is the oxidation state of free, molecular oxygen (not shown)? b. What is the ground-state electron configuration of Fe(II)? Noble gas abbreviations may not be used. Page 2 of 9
3 c. The equilibrium of dissociation with equilibrium constant K d of oxygenated Hb, Hb(O 2 ) 4(&'), is given by the following chemical equation: Hb O ( ) &' Hb &' + 4O (. Where the equilibrium constant K d is: K 0 = po ( ) [Hb] [Hb(O ( ) ) ] i. While exercising, oxygen is consumed via a process called aerobic respiration. Assuming that equilibrium is established, and that the person is at rest, explain how the equilibrium would shift after the person runs ten miles. ii. Carbon monoxide gas (CO (g) ) exposure can lead to CO poisoning in humans, which can result in hypoxia and/or death. Draw Lewis structures below for both CO (g) and O 2 (g). Circle the Lewis structure of the molecule that you predict to have a stronger affinity for the Fe 2+ of the heme B group shown on the first page. Page 3 of 9
4 iii. Given the following reactions and dissociation constants: Hb(CO) 4(&') Hb (&') + 4 CO (.) with K :'1 = 10 ;4.5 Hb(O 2 ) 4(&') Hb (&') + 4 O 2(.) with K :'2 = 10 ;2 Calculate the K eq. of the reaction: Hb(CO) 4(&') + 4O 2(.) Hb(O 2 ) 4(&') +4 CO (.) Page 4 of 9
5 2. Proteins serve many essential roles in the body including cell signaling, cell structure, and cellular transport. This question will explore some ways in which proteins interact with themselves and others in order to perform their respective functions. a. This question part will concern the intermolecular forces that are involved in some protein structures. One of the common structural aspect of proteins is the βsheet. Shown below are protein strand 1 and protein strand 2, which are part of a β- sheet. This diagram will be referenced parts i and ii of this question. i. Name the dominant intermolecular interaction that occurs between Strand 1 and Strand 2. ii. On the figure above, draw straight, dashed lines ( ) between the pairs of atoms participating in each interaction you indicated in part i. Page 5 of 9
6 b. While most amino acids interact through intermolecular forces, cysteine (shown below) is unique in that is can form a covalent bond with another cysteine. This disulfide bond is essential in many protein structures. For the purposes of this question, consider the R group (circled below) of cysteine to be polar. i. Is the reaction below to form a disulfide bond a reduction or oxidation? Justify your reasoning. Page 6 of 9
7 c. Consider peptide strand N which is the normal structure of this protein, and strand M which is a mutant because the two outermost circled groups have been changed. The full structures are shown below. i. Strand N is typically found within the interior of the cellular membrane, in between the lipid bilayer, where there is no water. Why might it be more favorable for strand M to be in the aqueous environment of the cytoplasm? Complete answers will analyze the circled groups of each strand. Page 7 of 9
8 ii. Suppose strand M is able to flex and fold about its bonds. Propose a model for how this peptide might bond in order to minimize the hydrophilic interactions so that it is more favorable to remain in a hydrophobic environment. Models may be a diagram (not all atoms need to be included) or written description, and they should include relevant interactions and bonds. iii. Consider the orientations of the middle two circled groups, -CH(CH 3 ) 2. How will these two groups be oriented so that the bonding interaction in part 2cii (previous question part) will occur? Diagrams and/or written explanations may be used. Page 8 of 9
9 3. The body must be able to maintain a wide range of ph values in various areas of the body. For example, the ph of blood is 7.35, the ph of the stomach ranges from 1.5 to 3.5. a. Many body systems use buffer systems to maintain their optimal ph. Using the equation of a weak acid dissociation show below, derive the Henderson- Hasselbalch equation as it is shown below. Include all intermediate steps. HA H? + A ; ph = pk & + log [A; ] [HA] b. The Phosphate Buffer system plays an important role in buffering renal tubular fluid and other intracellular fluids to additions of strong acids and bases. At physiological ph, the predominant species of this system are H 2 PO 4 - and its conjugate base, HPO The other species of this polyprotic system, such as H 3 PO 4 and PO 4 3-, can be ignored. If a solution consists of 35.3 mm HPO 4 2- and 2.3mM H 2 PO 4 -, what is the ph? (Hint: The pka of H 2 PO 4 - is 7.21). END OF EXAM Page 9 of 9
CHEMISTRY OF THE HUMAN BODY
 CHEMISTRY OF THE HUMAN BODY (Sample Questions Key) WUCT 2018 The three questions below are meant to give a sense of the kinds of questions that might be asked on the exam in April 2018. The actual exam
CHEMISTRY OF THE HUMAN BODY (Sample Questions Key) WUCT 2018 The three questions below are meant to give a sense of the kinds of questions that might be asked on the exam in April 2018. The actual exam
Problem Set 1
 2006 7.012 Problem Set 1 Due before 5 PM on FRIDAY, September 15, 2006. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. For each of the following parts, pick
2006 7.012 Problem Set 1 Due before 5 PM on FRIDAY, September 15, 2006. Turn answers in to the box outside of 68-120. PLEASE WRITE YOUR ANSWERS ON THIS PRINTOUT. 1. For each of the following parts, pick
Full file at Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Summary Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens.
1) Which of the following represents the breaking of a noncovalent interaction? Topic: The Nature of Noncovalent Interactions
 Multiple Choice Questions 1) Which of the following represents the breaking of a noncovalent interaction? A) hydrolysis of an ester B) dissolving of salt crystals C) ionization of water D) decomposition
Multiple Choice Questions 1) Which of the following represents the breaking of a noncovalent interaction? A) hydrolysis of an ester B) dissolving of salt crystals C) ionization of water D) decomposition
Chapter 2 Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens. There are
Chapter 2 Water: The Solvent for Biochemical Reactions SUMMARY Section 2.1 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive charges on the hydrogens. There are
LS1a Midterm Exam 1 Review Session Problems
 LS1a Midterm Exam 1 Review Session Problems 1. n aqueous mixture of a weak acid and its conjugate base is often used in the laboratory to prepare solutions referred to as buffers. ne commonly used acid
LS1a Midterm Exam 1 Review Session Problems 1. n aqueous mixture of a weak acid and its conjugate base is often used in the laboratory to prepare solutions referred to as buffers. ne commonly used acid
Water, water everywhere,; not a drop to drink. Consumption resulting from how environment inhabited Deforestation disrupts water cycle
 Chapter 3 Water: The Matrix of Life Overview n n n Water, water everywhere,; not a drop to drink Only 3% of world s water is fresh How has this happened Consumption resulting from how environment inhabited
Chapter 3 Water: The Matrix of Life Overview n n n Water, water everywhere,; not a drop to drink Only 3% of world s water is fresh How has this happened Consumption resulting from how environment inhabited
BIOC 460 General Chemistry Review: Chemical Equilibrium, Ionization of H 2 O, ph, pk a
 BIOC 460 General Chemistry Review: Chemical Equilibrium, Ionization of H 2 O, ph, pk a General Equilibrium: What are the UNITS of K eq? Example reactions: A --> B units of K eq? A --> B + C units of K
BIOC 460 General Chemistry Review: Chemical Equilibrium, Ionization of H 2 O, ph, pk a General Equilibrium: What are the UNITS of K eq? Example reactions: A --> B units of K eq? A --> B + C units of K
Biochemistry I Fall 2013 Exam 1 Dr. Stone 100 points Name Ka for acetic acid = 1.74 x 10-5
 Biochemistry I Fall 2013 Exam 1 Dr. Stone 100 points Name Ka for acetic acid = 1.74 x 10-5 Ka = [H+][A-]/[HA] Ka for formic acid, CH 2 O 2 = 1.78 x 10-4 Ka for lactic acid, C 3 H 6 O 3 = 1.41 x 10-4 Kw
Biochemistry I Fall 2013 Exam 1 Dr. Stone 100 points Name Ka for acetic acid = 1.74 x 10-5 Ka = [H+][A-]/[HA] Ka for formic acid, CH 2 O 2 = 1.78 x 10-4 Ka for lactic acid, C 3 H 6 O 3 = 1.41 x 10-4 Kw
Sample Questions for the Chemistry of Life Topic Test
 Sample Questions for the Chemistry of Life Topic Test 1. Enzymes play a crucial role in biology by serving as biological catalysts, increasing the rates of biochemical reactions by decreasing their activation
Sample Questions for the Chemistry of Life Topic Test 1. Enzymes play a crucial role in biology by serving as biological catalysts, increasing the rates of biochemical reactions by decreasing their activation
Acid-Base Balance. Lecture # 5 Second class/ 2015
 Acid-Base Balance Lecture # 5 Second class/ 2015 Terms Acid Any substance that can yield a hydrogen ion (H + ) or hydronium ion when dissolved in water Release of proton or H + Base Substance that can
Acid-Base Balance Lecture # 5 Second class/ 2015 Terms Acid Any substance that can yield a hydrogen ion (H + ) or hydronium ion when dissolved in water Release of proton or H + Base Substance that can
Volume NaOH Delivered (ml)
 Chemistry Spring 011 Exam 3: Chapters 8-10 Name 80 Points Complete five (5) of the following problems. Each problem is worth 16 points. CLEARLY mark the problems you do not want graded. You must show your
Chemistry Spring 011 Exam 3: Chapters 8-10 Name 80 Points Complete five (5) of the following problems. Each problem is worth 16 points. CLEARLY mark the problems you do not want graded. You must show your
1. What is an ångstrom unit, and why is it used to describe molecular structures?
 1. What is an ångstrom unit, and why is it used to describe molecular structures? The ångstrom unit is a unit of distance suitable for measuring atomic scale objects. 1 ångstrom (Å) = 1 10-10 m. The diameter
1. What is an ångstrom unit, and why is it used to describe molecular structures? The ångstrom unit is a unit of distance suitable for measuring atomic scale objects. 1 ångstrom (Å) = 1 10-10 m. The diameter
Individual Exam. Individual. (Sample Questions) WUCT Team ID:
 Individual Exam (Sample Questions) WUCT 2018 The three questions below are meant to give a sense of the kinds of questions that might be asked on the exam in April 2018. The actual exam is 65 minutes long,
Individual Exam (Sample Questions) WUCT 2018 The three questions below are meant to give a sense of the kinds of questions that might be asked on the exam in April 2018. The actual exam is 65 minutes long,
Water: The Solvent for Biochemical Reactions
 Chapter 2 Water: The Solvent for Biochemical Reactions 11 SUMMARY Section 2.1 Section 2.2 Section 2.3 Section 2.4 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive
Chapter 2 Water: The Solvent for Biochemical Reactions 11 SUMMARY Section 2.1 Section 2.2 Section 2.3 Section 2.4 Water is a polar molecule, with a partial negative charge on the oxygen and partial positive
LS1a Midterm Exam 1 Review Session Problems
 LS1a Midterm Exam 1 Review Session Problems 1. n aqueous mixture of a weak acid and its conjugate base is often used in the laboratory to prepare solutions referred to as buffers. ne commonly used acid
LS1a Midterm Exam 1 Review Session Problems 1. n aqueous mixture of a weak acid and its conjugate base is often used in the laboratory to prepare solutions referred to as buffers. ne commonly used acid
BIOMEDICAL SCIENCE MIN WAN
 ACID-BASE LECTURE BIOMEDICAL SCIENCE MIN WAN (min.wan@ki.se) SEPT. 12-13, 2016 9/6/2016 1 Acid Base lecture 14-15 September 2015 Min Wan 1. Introduction to ph 2. Acid base concept -calculations 3. Buffer
ACID-BASE LECTURE BIOMEDICAL SCIENCE MIN WAN (min.wan@ki.se) SEPT. 12-13, 2016 9/6/2016 1 Acid Base lecture 14-15 September 2015 Min Wan 1. Introduction to ph 2. Acid base concept -calculations 3. Buffer
Prof. Jason D. Kahn Your Signature: Exams written in pencil or erasable ink will not be re-graded under any circumstances.
 1 Biochemistry 461, Section I February 27, 1997 Exam #1 Prof. Jason D. Kahn Your Printed ame: Your SS#: Your Signature: You have 80 minutes for this exam. Exams written in pencil or erasable ink will not
1 Biochemistry 461, Section I February 27, 1997 Exam #1 Prof. Jason D. Kahn Your Printed ame: Your SS#: Your Signature: You have 80 minutes for this exam. Exams written in pencil or erasable ink will not
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor
 LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
LS1a Fall 2014 Problem Set #2 Due Monday 10/6 at 6 pm in the drop boxes on the Science Center 2 nd Floor Note: Adequate space is given for each answer. Questions that require a brief explanation should
Lec.1 Chemistry Of Water
 Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
Lec.1 Chemistry Of Water Biochemistry & Medicine Biochemistry can be defined as the science concerned with the chemical basis of life. Biochemistry can be described as the science concerned with the chemical
2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS
 2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS 2.1 Water and Polarity Both geometry and properties of molecule determine polarity Electronegativity - The tendency of an atom to attract electrons to itself
2. WATER : THE SOLVENT FOR BIOCHEMICAL REACTIONS 2.1 Water and Polarity Both geometry and properties of molecule determine polarity Electronegativity - The tendency of an atom to attract electrons to itself
Chemistry 222. Start mol End mol
 Chemistry Spring 019 Exam 3: Chapters 8-10 Name 80 Points Complete problem 1 and four of problems 6. CLEARLY mark the problem you do not want graded. You must show your work to receive credit for problems
Chemistry Spring 019 Exam 3: Chapters 8-10 Name 80 Points Complete problem 1 and four of problems 6. CLEARLY mark the problem you do not want graded. You must show your work to receive credit for problems
Prof. Jason Kahn Your Signature: Exams written in pencil or erasable ink will not be re-graded under any circumstances.
 1 Biochemistry 461 February 16, 1995 Exam #1 Prof. Jason Kahn Your Printed Name: Your SS#: Your Signature: You have 75 minutes for this exam. Exams written in pencil or erasable ink will not be re-graded
1 Biochemistry 461 February 16, 1995 Exam #1 Prof. Jason Kahn Your Printed Name: Your SS#: Your Signature: You have 75 minutes for this exam. Exams written in pencil or erasable ink will not be re-graded
Sample Question Solutions for the Chemistry of Life Topic Test
 Sample Question Solutions for the Chemistry of Life Topic Test 1. Enzymes play a crucial role in biology by serving as biological catalysts, increasing the rates of biochemical reactions by decreasing
Sample Question Solutions for the Chemistry of Life Topic Test 1. Enzymes play a crucial role in biology by serving as biological catalysts, increasing the rates of biochemical reactions by decreasing
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit. Lecture 2 (9/11/17)
 16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
Page 1 of Please choose the letter a as your answer for this question.
 CHEM 102 Winter 10 Exam 3 (a) On the answer sheet (scantron) write your Name, Student ID Number, and Recitation Section Number. Choose the best (most correct) answer for each question AND ENTER IT ON YOUR
CHEM 102 Winter 10 Exam 3 (a) On the answer sheet (scantron) write your Name, Student ID Number, and Recitation Section Number. Choose the best (most correct) answer for each question AND ENTER IT ON YOUR
Extension 17: Haemoglobin, the transport of oxygen in the blood and ph buffers in the blood
 Extension 17: Haemoglobin, the transport of oxygen in the blood and ph buffers in the blood 1. Prerequisites The ideas which form the background to this case study are listed in the following table. Topic
Extension 17: Haemoglobin, the transport of oxygen in the blood and ph buffers in the blood 1. Prerequisites The ideas which form the background to this case study are listed in the following table. Topic
Chapter 8 Lecture Notes: Acids, Bases, and ph
 Educational Goals Chapter 8 Lecture Notes: Acids, Bases, and ph 1. Given a chemical equation, write the law of mass action. 2. Given the equilibrium constant (K eq ) for a reaction, predict whether the
Educational Goals Chapter 8 Lecture Notes: Acids, Bases, and ph 1. Given a chemical equation, write the law of mass action. 2. Given the equilibrium constant (K eq ) for a reaction, predict whether the
Affinity labels for studying enzyme active sites. Irreversible Enzyme Inhibition. Inhibition of serine protease with DFP
 Irreversible Enzyme Inhibition Irreversible inhibitors form stable covalent bonds with the enzyme (e.g. alkylation or acylation of an active site side chain) There are many naturally-occurring and synthetic
Irreversible Enzyme Inhibition Irreversible inhibitors form stable covalent bonds with the enzyme (e.g. alkylation or acylation of an active site side chain) There are many naturally-occurring and synthetic
-log [H+][OH-] = - log [1 x ] Left hand side ( log H + ) + ( log OH - ) = ph + poh Right hand side = ( log 1) + ( log ) = 14 ph + poh = 14
![-log [H+][OH-] = - log [1 x ] Left hand side ( log H + ) + ( log OH - ) = ph + poh Right hand side = ( log 1) + ( log ) = 14 ph + poh = 14 -log [H+][OH-] = - log [1 x ] Left hand side ( log H + ) + ( log OH - ) = ph + poh Right hand side = ( log 1) + ( log ) = 14 ph + poh = 14](/thumbs/77/75028098.jpg) Autoionization of Water H 2 O H + + OH - K = [H + ][OH - ]/[H 2 O] = 1.802 x 10-16 Concentration of [H 2 O] is so HIGH autoionization is just a drop in the bucket, so [H 2 O] stays constant at 55.5 M,
Autoionization of Water H 2 O H + + OH - K = [H + ][OH - ]/[H 2 O] = 1.802 x 10-16 Concentration of [H 2 O] is so HIGH autoionization is just a drop in the bucket, so [H 2 O] stays constant at 55.5 M,
Now, the excess strong base will react: HA + OH - A - + H 2 O Start mol End mol
 Chemistry Spring 016 Exam 3: Chapters 8-10 Name 80 Points Complete problem 1 and four of problems -6. CLEARLY mark the problem you do not want graded. You must show your work to receive credit for problems
Chemistry Spring 016 Exam 3: Chapters 8-10 Name 80 Points Complete problem 1 and four of problems -6. CLEARLY mark the problem you do not want graded. You must show your work to receive credit for problems
Practice Midterm Exam 200 points total 75 minutes Multiple Choice (3 pts each 30 pts total) Mark your answers in the space to the left:
 MITES ame Practice Midterm Exam 200 points total 75 minutes Multiple hoice (3 pts each 30 pts total) Mark your answers in the space to the left: 1. Amphipathic molecules have regions that are: a) polar
MITES ame Practice Midterm Exam 200 points total 75 minutes Multiple hoice (3 pts each 30 pts total) Mark your answers in the space to the left: 1. Amphipathic molecules have regions that are: a) polar
Individual Exam. Individual. (Sample Questions) WUCT Team ID:
 Exam (Sample Questions) WUCT 2018 The three questions below are meant to give a sense of the kinds of questions that might be asked on the exam in April 2018. The actual exam is 65 minutes long, and it
Exam (Sample Questions) WUCT 2018 The three questions below are meant to give a sense of the kinds of questions that might be asked on the exam in April 2018. The actual exam is 65 minutes long, and it
Water, ph and pka. Lecture 2: Margaret A. Daugherty. Fall Water: What makes it so good for life? Solvent properties.
 Lecture 2: Water, ph and pka Margaret A. Daugherty Fall 2004 Water: What makes it so good for life? Structure ice vs. water or more technically solid vs. liquid Solvent properties High heat capacity High
Lecture 2: Water, ph and pka Margaret A. Daugherty Fall 2004 Water: What makes it so good for life? Structure ice vs. water or more technically solid vs. liquid Solvent properties High heat capacity High
Chem 204. Mid-Term Exam I. July 21, There are 3 sections to this exam: Answer ALL questions
 Chem 204 Mid-Term Exam I July 21, 2009 Name: Answer Key Student ID: There are 3 sections to this exam: Answer ALL questions Section I: Multiple-Choice 20 questions, 2 pts each Section II: Fill-in-the-Blank
Chem 204 Mid-Term Exam I July 21, 2009 Name: Answer Key Student ID: There are 3 sections to this exam: Answer ALL questions Section I: Multiple-Choice 20 questions, 2 pts each Section II: Fill-in-the-Blank
General Phenomena: Law of mass action, dissociation of water, ph, buffers
 General Phenomena: Law of mass action, dissociation of water, ph, buffers Ionization of Water, Weak Acids and Weak Bases Many properties of water can be explained in terms of uncharged H 2 O molecule Small
General Phenomena: Law of mass action, dissociation of water, ph, buffers Ionization of Water, Weak Acids and Weak Bases Many properties of water can be explained in terms of uncharged H 2 O molecule Small
Biochemistry 3100 Sample Problems Binding proteins, Kinetics & Catalysis
 (1) Draw an approximate denaturation curve for a typical blood protein (eg myoglobin) as a function of ph. (2) Myoglobin is a simple, single subunit binding protein that has an oxygen storage function
(1) Draw an approximate denaturation curve for a typical blood protein (eg myoglobin) as a function of ph. (2) Myoglobin is a simple, single subunit binding protein that has an oxygen storage function
Part I. Multiple choice. Circle the correct answer for each problem. 3 points each
 CEM 100 Name Exam 1 Summer 2010 Part I. Multiple choice. Circle the correct answer for each problem. 3 points each 1. The observation that 20 g of hydrogen gas always combines with 160 g of oxygen gas
CEM 100 Name Exam 1 Summer 2010 Part I. Multiple choice. Circle the correct answer for each problem. 3 points each 1. The observation that 20 g of hydrogen gas always combines with 160 g of oxygen gas
S H/T 0 ph = log([h + ]) E = mc 2 S = klnw G = H T S ph = pk a + log([a ]/[HA]) K a = [H + ][A ]/[HA] G = RTlnK eq e iπ + 1 = 0
![S H/T 0 ph = log([h + ]) E = mc 2 S = klnw G = H T S ph = pk a + log([a ]/[HA]) K a = [H + ][A ]/[HA] G = RTlnK eq e iπ + 1 = 0 S H/T 0 ph = log([h + ]) E = mc 2 S = klnw G = H T S ph = pk a + log([a ]/[HA]) K a = [H + ][A ]/[HA] G = RTlnK eq e iπ + 1 = 0](/thumbs/90/101592193.jpg) Biochemistry 463, Summer II Your Name: University of Maryland, College Park Your SID #: Biochemistry and Physiology Prof. Jason Kahn Exam I (100 points total) July 27, 2007 You have 80 minutes for this
Biochemistry 463, Summer II Your Name: University of Maryland, College Park Your SID #: Biochemistry and Physiology Prof. Jason Kahn Exam I (100 points total) July 27, 2007 You have 80 minutes for this
12U Biochemistry Unit Test
 1 12U Biology: Biochemistry Test 12U Biochemistry Unit Test Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true.
1 12U Biology: Biochemistry Test 12U Biochemistry Unit Test Modified True/False Indicate whether the statement is true or false. If false, change the identified word or phrase to make the statement true.
Kinetics & Equilibrium
 Kinetics & Equilibrium Name: Essential Questions How can one explain the structure, properties, and interactions of matter? Learning Objectives Explain Collision Theory Molecules must collide in order
Kinetics & Equilibrium Name: Essential Questions How can one explain the structure, properties, and interactions of matter? Learning Objectives Explain Collision Theory Molecules must collide in order
Chapter 1 1) Biological Molecules a) Only a small subset of the known elements are found in living systems i) Most abundant- C, N, O, and H ii) Less
 Chapter 1 1) Biological Molecules a) Only a small subset of the known elements are found in living systems i) Most abundant- C, N, O, and H ii) Less abundant- Ca, P, K, S, Cl, Na, and Mg b) Cells contain
Chapter 1 1) Biological Molecules a) Only a small subset of the known elements are found in living systems i) Most abundant- C, N, O, and H ii) Less abundant- Ca, P, K, S, Cl, Na, and Mg b) Cells contain
2: CHEMICAL COMPOSITION OF THE BODY
 1 2: CHEMICAL COMPOSITION OF THE BODY CHAPTER OVERVIEW This chapter provides an overview of basic chemical principles that are important to understanding human physiological function and ultimately homeostasis.
1 2: CHEMICAL COMPOSITION OF THE BODY CHAPTER OVERVIEW This chapter provides an overview of basic chemical principles that are important to understanding human physiological function and ultimately homeostasis.
Ionization of acids and bases
 ionization equation Ionization of acids and bases Acid Base AH + H 2 O H 3 O + + A B + H 2 O OH + BH + simpler eq. AH H + + A B + H + BH + ionization K A = [H 3 O + ][A - ]/[AH] K B = [OH - ][BH + ]/[B]
ionization equation Ionization of acids and bases Acid Base AH + H 2 O H 3 O + + A B + H 2 O OH + BH + simpler eq. AH H + + A B + H + BH + ionization K A = [H 3 O + ][A - ]/[AH] K B = [OH - ][BH + ]/[B]
Chapter 10: Hemoglobin
 Chapter 10: Hemoglobin Voet & Voet: Pages 320-353 Slide 1 Hemoglobin Function Larger aerobic (oxygen utilizing) organism require an O 2 transport system to deliver sufficient O 2 to tissues Dissolved O
Chapter 10: Hemoglobin Voet & Voet: Pages 320-353 Slide 1 Hemoglobin Function Larger aerobic (oxygen utilizing) organism require an O 2 transport system to deliver sufficient O 2 to tissues Dissolved O
R = J/mole K = cal/mole K = N A k B K a K b = K w
 Chemistry 271 22XX Prof. Jason Kahn Your Name: University of Maryland, College Park Your SID #: General Chemistry and Energetics Exam II (100 points) You have 53 minutes for this exam. Your Section # or
Chemistry 271 22XX Prof. Jason Kahn Your Name: University of Maryland, College Park Your SID #: General Chemistry and Energetics Exam II (100 points) You have 53 minutes for this exam. Your Section # or
Part I. Multiple choice. Circle the correct answer for each problem. 3 points each
 CHEM 100 Name Exam 1 Summer 2010 Part I. Multiple choice. Circle the correct answer for each problem. 3 points each 1. The observation that 20 g of hydrogen gas always combines with 160 g of oxygen gas
CHEM 100 Name Exam 1 Summer 2010 Part I. Multiple choice. Circle the correct answer for each problem. 3 points each 1. The observation that 20 g of hydrogen gas always combines with 160 g of oxygen gas
Model Worksheet Teacher Key
 Introduction Despite the complexity of life on Earth, the most important large molecules found in all living things (biomolecules) can be classified into only four main categories: carbohydrates, lipids,
Introduction Despite the complexity of life on Earth, the most important large molecules found in all living things (biomolecules) can be classified into only four main categories: carbohydrates, lipids,
Multiple choice: 10 questions, worth 2 points each. Circle all of the correct answers. Some questions many have more than one correct answer.
 Biochemistry I Fall 2014 Exam 1 Dr. Stone Name Page 1 of 6 Some constants and equations that may be useful: K w = [H+][OH-]= 1 x 10 14 K a for H 2 CO 3 = 2.7 x 10-4 K eq for the formation of carbonic acid
Biochemistry I Fall 2014 Exam 1 Dr. Stone Name Page 1 of 6 Some constants and equations that may be useful: K w = [H+][OH-]= 1 x 10 14 K a for H 2 CO 3 = 2.7 x 10-4 K eq for the formation of carbonic acid
Water. Water participates in H-bonding with biomolecules.
 Water Most biochemical reactions occur in an aqueous environment. Water is highly polar because of its bent geometry. Water is highly cohesive because of intermolecular hydrogen bonding. Water participates
Water Most biochemical reactions occur in an aqueous environment. Water is highly polar because of its bent geometry. Water is highly cohesive because of intermolecular hydrogen bonding. Water participates
Chemistry 100 Final Exam April 21, 2012
 Chemistry 100 Final Exam April 21, 2012 G. Orlova, G. Marangoni, M. Aquino Name: ID: This exam has a three-hour (3 hour) time limit. Read over the entire exam before beginning. Do those questions that
Chemistry 100 Final Exam April 21, 2012 G. Orlova, G. Marangoni, M. Aquino Name: ID: This exam has a three-hour (3 hour) time limit. Read over the entire exam before beginning. Do those questions that
Chapter 8 Educational Goals
 Chapter 8 Educational Goals 1. Given a chemical equation, write the law of mass action. 2. Given the equilibrium constant (K eq ) for a reaction, predict whether the reactants or products are predominant.
Chapter 8 Educational Goals 1. Given a chemical equation, write the law of mass action. 2. Given the equilibrium constant (K eq ) for a reaction, predict whether the reactants or products are predominant.
CHAPTER 29 HW: AMINO ACIDS + PROTEINS
 CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
CAPTER 29 W: AMI ACIDS + PRTEIS For all problems, consult the table of 20 Amino Acids provided in lecture if an amino acid structure is needed; these will be given on exams. Use natural amino acids (L)
I. Acids & Bases. A. General ideas:
 Acid-Base Equilibria 1. Application of equilibrium concepts. 2. Not much else new in the way of theory is presented. 3. Specific focus on aqueous (H O is 2 solvent) systems. 4. Assume we are at equilibrium
Acid-Base Equilibria 1. Application of equilibrium concepts. 2. Not much else new in the way of theory is presented. 3. Specific focus on aqueous (H O is 2 solvent) systems. 4. Assume we are at equilibrium
Chemical and Physical Properties of Organic Molecules
 Chemical and Physical Properties of Organic Molecules I.Elements A. Chemical symbols: C H O P S N C=carbon, H=hydrogen, O=oxygen, P=phosphorus, S=sulfur, N=nitrogen B. Top 3 Earth s surface = O, Si, Al
Chemical and Physical Properties of Organic Molecules I.Elements A. Chemical symbols: C H O P S N C=carbon, H=hydrogen, O=oxygen, P=phosphorus, S=sulfur, N=nitrogen B. Top 3 Earth s surface = O, Si, Al
4) Chapter 1 includes heredity (i.e. DNA and genes) as well as evolution. Discuss the connection between heredity and evolution?
 Name- Chapters 1-5 Questions 1) Life is easy to recognize but difficult to define. The dictionary defines life as the state or quality that distinguishes living beings or organisms from dead ones and from
Name- Chapters 1-5 Questions 1) Life is easy to recognize but difficult to define. The dictionary defines life as the state or quality that distinguishes living beings or organisms from dead ones and from
Topic 1: The Chemical Context of Life, Holtzclaw and Holtzclaw, 2014
 Name Block Topic 1: The Chemical Context of Life, Holtzclaw and Holtzclaw, 2014 1. Complete the vocabulary on a separate piece of paper. 2. What are the elements that make up most of living matter? What
Name Block Topic 1: The Chemical Context of Life, Holtzclaw and Holtzclaw, 2014 1. Complete the vocabulary on a separate piece of paper. 2. What are the elements that make up most of living matter? What
Objective: Students will be able identify peptide bonds in proteins and describe the overall reaction between amino acids that create peptide bonds.
 Scott Seiple AP Biology Lesson Plan Lesson: Primary and Secondary Structure of Proteins Purpose:. To understand how amino acids can react to form peptides through peptide bonds.. Students will be able
Scott Seiple AP Biology Lesson Plan Lesson: Primary and Secondary Structure of Proteins Purpose:. To understand how amino acids can react to form peptides through peptide bonds.. Students will be able
Important - Please read this before you turn the page.
 CHEM-342 Introduction to Biochemistry Midterm Examination - Individual Part (75%) Wednesday, 21 March 2012 H. B. White Instructor Name Range 34-121/132 Average ± SD = 77.7 ± 30.9 N=27 Important - Please
CHEM-342 Introduction to Biochemistry Midterm Examination - Individual Part (75%) Wednesday, 21 March 2012 H. B. White Instructor Name Range 34-121/132 Average ± SD = 77.7 ± 30.9 N=27 Important - Please
Principles Of Acid-Base Balance
 Principles Of Acid-Base Balance I. Introduction A. For normal body function the free H+ concentration [H+] or ph must be kept within a narrow normal range. Some reasons why: 1. The proton "pump" within
Principles Of Acid-Base Balance I. Introduction A. For normal body function the free H+ concentration [H+] or ph must be kept within a narrow normal range. Some reasons why: 1. The proton "pump" within
Unit 1: Chemistry of Life Guided Reading Questions (80 pts total)
 Name: AP Biology Biology, Campbell and Reece, 7th Edition Adapted from chapter reading guides originally created by Lynn Miriello Chapter 1 Exploring Life Unit 1: Chemistry of Life Guided Reading Questions
Name: AP Biology Biology, Campbell and Reece, 7th Edition Adapted from chapter reading guides originally created by Lynn Miriello Chapter 1 Exploring Life Unit 1: Chemistry of Life Guided Reading Questions
Final Chem 4511/6501 Spring 2011 May 5, 2011 b Name
 Key 1) [10 points] In RNA, G commonly forms a wobble pair with U. a) Draw a G-U wobble base pair, include riboses and 5 phosphates. b) Label the major groove and the minor groove. c) Label the atoms of
Key 1) [10 points] In RNA, G commonly forms a wobble pair with U. a) Draw a G-U wobble base pair, include riboses and 5 phosphates. b) Label the major groove and the minor groove. c) Label the atoms of
2014 AP Biology Summer Homework Assignment
 2014 AP Biology Summer Homework Assignment Due the first day of class. Part 1 of 2: Complete the reading guides below by using the Mastering Biology site that has an ebook of our text Campbell's Biology
2014 AP Biology Summer Homework Assignment Due the first day of class. Part 1 of 2: Complete the reading guides below by using the Mastering Biology site that has an ebook of our text Campbell's Biology
AP Chemistry Chapter 14 Review Packet Multiple Choice Questions: 2. What is the equilibrium constant for the following reaction?
 AP Chemistry Chapter 14 Review Packet Multiple Choice Questions: Name 1. For the stepwise dissociation of aqueous H 3 PO 4, which of the following is NOT a conjugate acid-base pair? a. HPO 4 and PO 4 3
AP Chemistry Chapter 14 Review Packet Multiple Choice Questions: Name 1. For the stepwise dissociation of aqueous H 3 PO 4, which of the following is NOT a conjugate acid-base pair? a. HPO 4 and PO 4 3
2: CHEMICAL COMPOSITION OF THE BODY
 1 2: CHEMICAL COMPOSITION OF THE BODY Although most students of human physiology have had at least some chemistry, this chapter serves very well as a review and as a glossary of chemical terms. In particular,
1 2: CHEMICAL COMPOSITION OF THE BODY Although most students of human physiology have had at least some chemistry, this chapter serves very well as a review and as a glossary of chemical terms. In particular,
ph and buffers Dr. Mamoun Ahram Summer, 2018
 ph and buffers Dr. Mamoun Ahram Summer, 2018 Kw Kw is called the ion product for water What is ph? Example: Find the K a of a 0.04 M weak acid HA whose [H + ] is 1 x 10-4? HA H + + A - K a = [A - ] [H
ph and buffers Dr. Mamoun Ahram Summer, 2018 Kw Kw is called the ion product for water What is ph? Example: Find the K a of a 0.04 M weak acid HA whose [H + ] is 1 x 10-4? HA H + + A - K a = [A - ] [H
Big Idea 6 Equilibrium
 Big Idea 6 Equilibrium AP CHEMISTRY Course and Exam Description Effective Fall 2013 The College Board New York, NY AP Chemistry Curriculum Framework The Concept Outline The focus of Chapters 15-17 is on
Big Idea 6 Equilibrium AP CHEMISTRY Course and Exam Description Effective Fall 2013 The College Board New York, NY AP Chemistry Curriculum Framework The Concept Outline The focus of Chapters 15-17 is on
Placement Exam for Exemption from Chemistry 120 Fall 2006
 Placement Exam for Exemption from Chemistry 120 Fall 2006 Name: By submitting this exam you affirm that you have not consulted any person, book, website or any other source for assistance. You may use
Placement Exam for Exemption from Chemistry 120 Fall 2006 Name: By submitting this exam you affirm that you have not consulted any person, book, website or any other source for assistance. You may use
From Amino Acids to Proteins - in 4 Easy Steps
 From Amino Acids to Proteins - in 4 Easy Steps Although protein structure appears to be overwhelmingly complex, you can provide your students with a basic understanding of how proteins fold by focusing
From Amino Acids to Proteins - in 4 Easy Steps Although protein structure appears to be overwhelmingly complex, you can provide your students with a basic understanding of how proteins fold by focusing
[base] [acid] ph = pka + log
![[base] [acid] ph = pka + log [base] [acid] ph = pka + log](/thumbs/82/86175854.jpg) Fall 2013 CCBC-Catonsville (Wed 10/30/13) Use your time wisely. Do not get stuck on one question. NO CREDIT WILL BE GIVEN UNLESS WORK IS SHOWN CLEARLY. UNITS MUST BE INCLUDED IN YOUR SETUPS. Answers must
Fall 2013 CCBC-Catonsville (Wed 10/30/13) Use your time wisely. Do not get stuck on one question. NO CREDIT WILL BE GIVEN UNLESS WORK IS SHOWN CLEARLY. UNITS MUST BE INCLUDED IN YOUR SETUPS. Answers must
CHEM 109A Organic Chemistry
 CHEM 109A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter 2 Acids and Bases Central to Understanding Organic Chemistry Draw the conjugate acid of each of the following:
CHEM 109A Organic Chemistry https://labs.chem.ucsb.edu/zakarian/armen/courses.html Chapter 2 Acids and Bases Central to Understanding Organic Chemistry Draw the conjugate acid of each of the following:
Chemistry 14C Spring 2018 Final Exam Part A Page 1
 hemistry 14 Spring 2018 Final Exam Part A Page 1 The Deepwater orizon was a petroleum-drilling rig that exploded and sank in the Gulf of Mexico on April 22, 2010. This incident caused a massive crude oil
hemistry 14 Spring 2018 Final Exam Part A Page 1 The Deepwater orizon was a petroleum-drilling rig that exploded and sank in the Gulf of Mexico on April 22, 2010. This incident caused a massive crude oil
MODULE 2: BIOLOGICAL MOLECULES
 PEER-LED TEAM LEARNING INTRDUCTRY BILGY MDULE 2: BILGICAL MLECULES JSEP GRISWLD, + DEAN STETLER,* AND MICAEL GAINES, ( + City College of New York, *University of Kansas, Univ. of Miami;) I. Introduction
PEER-LED TEAM LEARNING INTRDUCTRY BILGY MDULE 2: BILGICAL MLECULES JSEP GRISWLD, + DEAN STETLER,* AND MICAEL GAINES, ( + City College of New York, *University of Kansas, Univ. of Miami;) I. Introduction
Biology Midterm Review
 Biology Midterm Review Unit 1 Keystone Objectives: A.1.1, A.1.2, B.4.1.1 1.1 Biology explores life from the global to the microscopic level. Put the levels of organization in order, starting with subatomic
Biology Midterm Review Unit 1 Keystone Objectives: A.1.1, A.1.2, B.4.1.1 1.1 Biology explores life from the global to the microscopic level. Put the levels of organization in order, starting with subatomic
When a person is stressed, their body will work in some way to alleviate the imposed stress.
 Chemistry 12 Equilibrium II Name: Date: Block: 1. Le Châtelier s Principle 2. Equilibrium Graphs Le Châtelier s Principle http://en.wikipedia.org/wiki/henry_louis_le_chatelier When a person is, their body
Chemistry 12 Equilibrium II Name: Date: Block: 1. Le Châtelier s Principle 2. Equilibrium Graphs Le Châtelier s Principle http://en.wikipedia.org/wiki/henry_louis_le_chatelier When a person is, their body
+3 for pka = 14 pkb = 9.25 [+1 for the idea that pka and pkb are related.]
![+3 for pka = 14 pkb = 9.25 [+1 for the idea that pka and pkb are related.] +3 for pka = 14 pkb = 9.25 [+1 for the idea that pka and pkb are related.]](/thumbs/82/86175751.jpg) Chemistry 271, Section 21xx Your Name: Key University of Maryland, College Park Your SID #: General Chemistry and Energetics Prof. Jason Kahn Exam I (100 points total) March 11, 2009 You have 50 minutes
Chemistry 271, Section 21xx Your Name: Key University of Maryland, College Park Your SID #: General Chemistry and Energetics Prof. Jason Kahn Exam I (100 points total) March 11, 2009 You have 50 minutes
Chem 150, Spring Unit 4 - Acids & Bases. Introduction
 Chem 150, Spring 2015 Unit 4 - Acids & Bases Introduction Patients with emphysema cannot expel CO2 from their lungs rapidly enough. This can lead to an increase of carbonic (H2CO3) levels in the blood
Chem 150, Spring 2015 Unit 4 - Acids & Bases Introduction Patients with emphysema cannot expel CO2 from their lungs rapidly enough. This can lead to an increase of carbonic (H2CO3) levels in the blood
BA, BSc, and MSc Degree Examinations
 Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes
Examination Candidate Number: Desk Number: BA, BSc, and MSc Degree Examinations 2017-8 Department : BIOLOGY Title of Exam: Molecular Biology and Biochemistry Part I Time Allowed: 1 hour and 30 minutes
2. In regards to the fluid mosaic model, which of the following is TRUE?
 General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
General Biology: Exam I Sample Questions 1. How many electrons are required to fill the valence shell of a neutral atom with an atomic number of 24? a. 0 the atom is inert b. 1 c. 2 d. 4 e. 6 2. In regards
Chemistry 25 (Spring term 2016) Final Examination for NON-SENIORS GOOD LUCK AND HAVE A GREAT SUMMER!!!
 Name ANSWER KEY Distributed Thursday, June 2 Due Chemistry 25 (Spring term 2016) Final Examination for NON-SENIORS GOOD LUCK AND HAVE A GREAT SUMMER!!! by Thursday, June 2 at 1 pm to 362 Broad a drop box
Name ANSWER KEY Distributed Thursday, June 2 Due Chemistry 25 (Spring term 2016) Final Examination for NON-SENIORS GOOD LUCK AND HAVE A GREAT SUMMER!!! by Thursday, June 2 at 1 pm to 362 Broad a drop box
Aqueous Equilibria, Part 1 AP Chemistry Lecture Outline
 Aqueous Equilibria, Part 1 AP Chemistry Lecture Outline Name: Acids and Bases Arrhenius...acids increase the when dissolved in H 2 O....bases increase the when dissolved in H 2 O. e.g., HCl and NaOH Bronsted-Lowry
Aqueous Equilibria, Part 1 AP Chemistry Lecture Outline Name: Acids and Bases Arrhenius...acids increase the when dissolved in H 2 O....bases increase the when dissolved in H 2 O. e.g., HCl and NaOH Bronsted-Lowry
Key Terms (1 point each). Fill in the blank with the proper term. Each term may be used only once.
 CHM60 Takehome Test 3 Form Student Page 1 of 6 Key Terms (1 point each). Fill in the blank with the proper term. Each term may be used only once. Acid Active transport Alpha helix Beta pleated sheet Buffer
CHM60 Takehome Test 3 Form Student Page 1 of 6 Key Terms (1 point each). Fill in the blank with the proper term. Each term may be used only once. Acid Active transport Alpha helix Beta pleated sheet Buffer
EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I Dr. Glen B Legge
 EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I 2009 Dr. Glen B Legge This is a Scantron exam. All answers should be transferred to the Scantron sheet using a #2 pencil. Write and bubble
EXAM 1 Fall 2009 BCHS3304, SECTION # 21734, GENERAL BIOCHEMISTRY I 2009 Dr. Glen B Legge This is a Scantron exam. All answers should be transferred to the Scantron sheet using a #2 pencil. Write and bubble
BI/CH421 Biochemistry I Exam 1 09/29/2014
 Part I: Multiple choice for each question, circle the choice that best answers the question. Do not write the letter in the margin to indicate your answer, circle it. 3 points each. 1. For a reaction with
Part I: Multiple choice for each question, circle the choice that best answers the question. Do not write the letter in the margin to indicate your answer, circle it. 3 points each. 1. For a reaction with
A) at equilibrium B) endergonic C) endothermic D) exergonic E) exothermic.
 CHEM 2770: Elements of Biochemistry Mid Term EXAMINATION VERSION A Date: October 29, 2014 Instructor: H. Perreault Location: 172 Schultz Time: 4 or 6 pm. Duration: 1 hour Instructions Please mark the Answer
CHEM 2770: Elements of Biochemistry Mid Term EXAMINATION VERSION A Date: October 29, 2014 Instructor: H. Perreault Location: 172 Schultz Time: 4 or 6 pm. Duration: 1 hour Instructions Please mark the Answer
What binds to Hb in addition to O 2?
 Reading: Ch5; 158-169, 162-166, 169-174 Problems: Ch5 (text); 3,7,8,10 Ch5 (study guide-facts); 1,2,3,4,5,8 Ch5 (study guide-apply); 2,3 Remember Today at 5:30 in CAS-522 is the second chance for the MB
Reading: Ch5; 158-169, 162-166, 169-174 Problems: Ch5 (text); 3,7,8,10 Ch5 (study guide-facts); 1,2,3,4,5,8 Ch5 (study guide-apply); 2,3 Remember Today at 5:30 in CAS-522 is the second chance for the MB
Lecture Presentation. Chapter 16. Aqueous Ionic Equilibrium. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 16 Aqueous Ionic Equilibrium Sherril Soman Grand Valley State University The Danger of Antifreeze Each year, thousands of pets and wildlife species die from consuming antifreeze.
Lecture Presentation Chapter 16 Aqueous Ionic Equilibrium Sherril Soman Grand Valley State University The Danger of Antifreeze Each year, thousands of pets and wildlife species die from consuming antifreeze.
Basic Chemistry. Chemistry Review. Bio 250: Anatomy & Physiology
 Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Basic Chemistry Bio 250: Anatomy & Physiology Chemistry Review It is going to be your responsibility to review the basic principles of chemistry you learned in BIO 101 This basic set of notes will help
Denaturation and renaturation of proteins
 Denaturation and renaturation of proteins Higher levels of protein structure are formed without covalent bonds. Therefore, they are not as stable as peptide covalent bonds which make protein primary structure
Denaturation and renaturation of proteins Higher levels of protein structure are formed without covalent bonds. Therefore, they are not as stable as peptide covalent bonds which make protein primary structure
Chemistry 105 ~ Skills Summary for Laboratory Final Exam
 Chemistry 105 ~ Skills Summary for Laboratory Final Exam The Final Exam questions will have a variety of formats, including: true/false, multiple-choice, fill-in-the-blank, and problem-solving. Each student
Chemistry 105 ~ Skills Summary for Laboratory Final Exam The Final Exam questions will have a variety of formats, including: true/false, multiple-choice, fill-in-the-blank, and problem-solving. Each student
General Chemistry and Energetics Your Section #: Exam I (100 points total) October 6, 2010
 Chemistry 271, Section 23xx Your Name: Prof. Jason Kahn University of Maryland, College Park Your SID #: General Chemistry and Energetics Your Section #: Exam I (100 points total) October 6, 2010 You have
Chemistry 271, Section 23xx Your Name: Prof. Jason Kahn University of Maryland, College Park Your SID #: General Chemistry and Energetics Your Section #: Exam I (100 points total) October 6, 2010 You have
Solubility Equilibria
 Solubility Equilibria Heretofore, we have investigated gas pressure, solution, acidbase equilibriums. Another important equilibrium that is used in the chemistry lab is that of solubility equilibrium.
Solubility Equilibria Heretofore, we have investigated gas pressure, solution, acidbase equilibriums. Another important equilibrium that is used in the chemistry lab is that of solubility equilibrium.
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1
 Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1 1) Which compound in Figure 2.1 is an ester? 1) A) a b c d e Answer: D 2) A scientist
Exam Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. Figure 2.1 1) Which compound in Figure 2.1 is an ester? 1) A) a b c d e Answer: D 2) A scientist
ACIDS AND BASES. Note: For most of the acid-base reactions, we will be using the Bronsted-Lowry definitions.
 DEFINITIONS: ACIDS AND BASES Arrhenius Definition An acid in aqueous solution produces H + ions. A base in aqueous solution produces OH - ions. Bronsted Lowry Theory An acid is a proton donor A base is
DEFINITIONS: ACIDS AND BASES Arrhenius Definition An acid in aqueous solution produces H + ions. A base in aqueous solution produces OH - ions. Bronsted Lowry Theory An acid is a proton donor A base is
Chapter 1. Topic: Overview of basic principles
 Chapter 1 Topic: Overview of basic principles Four major themes of biochemistry I. What are living organism made from? II. How do organism acquire and use energy? III. How does an organism maintain its
Chapter 1 Topic: Overview of basic principles Four major themes of biochemistry I. What are living organism made from? II. How do organism acquire and use energy? III. How does an organism maintain its
The following question(s) were incorrectly answered.
 Name: Marcie Joseph Module: Cells & chemistry Test topic/animation: My animations/all animations Test type: Multiple choice Score: 48/50 Percent correct: 96% The following question(s) were incorrectly
Name: Marcie Joseph Module: Cells & chemistry Test topic/animation: My animations/all animations Test type: Multiple choice Score: 48/50 Percent correct: 96% The following question(s) were incorrectly
BCH 4053 Spring 2001 Chapter 2 Lecture Notes
 BCH 4053 Spring 001 Chapter Lecture Notes 1 Chapter Water, ph and Ionic Equilibria Physical Properties of Water High boiling point High melting point High heat of vaporization High heat of fusion 3 Physical
BCH 4053 Spring 001 Chapter Lecture Notes 1 Chapter Water, ph and Ionic Equilibria Physical Properties of Water High boiling point High melting point High heat of vaporization High heat of fusion 3 Physical
Midterm Exam III. CHEM 181: Introduction to Chemical Principles November 25, 2014 Answer Key
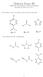 Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
ORGANIC - BROWN 8E CH AMINO ACIDS AND PROTEINS.
 !! www.clutchprep.com CONCEPT: INTRODUCTION TO PROTEINS Proteins are polypeptides that have some biological function. Peptides are composed of polymers of monomeric units called α-amino acids The 20 most
!! www.clutchprep.com CONCEPT: INTRODUCTION TO PROTEINS Proteins are polypeptides that have some biological function. Peptides are composed of polymers of monomeric units called α-amino acids The 20 most
K w. Acids and bases 8/24/2009. Acids and Bases 9 / 03 / Ionization of water. Proton Jumping Large proton and hydroxide mobility
 Chapter 2 Water Acids and Bases 9 / 03 / 2009 1. How is the molecular structure of water related to physical and chemical behavior? 2. What is a Hydrogen Bond? 3Wh 3. What are Acids Aid and db Bases? 4.
Chapter 2 Water Acids and Bases 9 / 03 / 2009 1. How is the molecular structure of water related to physical and chemical behavior? 2. What is a Hydrogen Bond? 3Wh 3. What are Acids Aid and db Bases? 4.
