Chapter 4. It s all about e -
|
|
|
- Leo Ethan Cook
- 6 years ago
- Views:
Transcription
1 Chatr 4 Major Goal of Chatr 4:. Fining th xact location (hom) for th lctron in an atom 2. Dicu hyical an chmical xrimntal vinc which uort a) lctronic tructur & b) th rioic trn in th rorti of atom. Bfor viwing thi owroint, ra th Chatr 4 Rviw: 4. Elctromagntic Raiation 4.2 Atomic Sctra & Enrgy Lvl 4. Enrgy Lvl (hll), Sublvl(ubhll) & Orbital 4.4. Writing Orbital Diagram & Elctron Configuration 4.5 Elctron Configuration & th Prioic Tabl 4.6 Prioic Trn of th Elmnt Look ovr ag 0 of your txtbook an th hanout nt to your -mail It all about - Prorti for an Elctron in an Atom. light wight articl; /2000th an atomic ma unit (amu) 2. (-) ngativly charg articl. looly boun; Amrican Hritag Dictionary fin loo a not fatn; unboun 4. attract to (+) oitivly charg articl 5. rll by othr ngativly charg articl 6. ynamic not tatic; I woul lik to mov about or jum aroun 7. at hom within an lctron hll hown by Bohr mol 8. a travlr an woul lov to travl but nvr far from hom 9. aily xcitabl Point 7 i in r bcau your txtbook o not icu Bohr mol irctly, only inirctly, on ag 0.
2 Sulmntal ackt ag 44 Sction Atomic Sctra & Enrgy Lvl Point 7 i in r bcau your txtbook o not icu Bohr mol irctly, only inirctly, on ag 0. Th lar an th concntric circl blow ar viual for Bohr mol. incraing nrgy n=4 n= n=2 n= "Elctron Shll" () nuclu Bohr icovr that Whr Sulmntal ackt ag 44 Elctron hav a hom in a icrt quantiz hll Th maximum numbr of lctron r hll i givn quantiz = icrt Bohr Mol for atom by 2(n) 2 "Elctron Shll" n = 4 n = n = 2 n = 2(4) 2 = 2 2() 2 = 8 2(2) 2 = 8 2() 2 = 2 For hi nw icovry, H wa awar th Nobl Priz in hyic 922 You on t hav to b mart to b awar a Nobl Priz. You jut hav to icovr omthing nw which rvolutioniz th way ocity viw th worl aroun u. 2
3 Sulmntal ackt ag 44 Bohr ba hi icovry on th miion ctrum for hyrogn F. 9 Nil Bohr. Th HYDROGEN atom ha lay a major rol in th vlomnt of mol of lctronic tructur. 2. In a hyrogn icharg tub, iniviual atom of hyrogn mit viibl light.. Whn th light i a through a rim, rfraction occur, an a quantiz miion ctrum aar. quantiz = icrt (color lin of cific nrgy) + nrgy + H 2 -> 2H viibl light aar a whit light H rim icrt color of light quantiz miion ctrum violt blu grn r n=4 n= n=2 n= Whr of th four color lit, violt color (viibl light) i hight in nrgy R color (viibl light) i lowt in nrgy Viibl light i an xaml of lctromagntic raiation Hi thought about th atom: quantiz miion ctrum giv quantiz nrgy lvl of fining an lctron. Txtbook 0 Emiion Sctra that uort Bohr icrt nrgy lvl quantiz = icrt quantiz = icrt (color lin of cific nrgy) violt blu grn r Bohr Mol n=4 n= n=2 n= continuou miion ctrum Hi thought about th atom: quantiz miion ctrum giv quantiz nrgy lvl of fining an lctron. quantiz = icrt quantiz = icrt (color lin of cific nrgy)
4 Sction 4. Elctromagntic Raiation Sulmntal ackt ag 45& 46 Stuy all th baic information on thi ag low numbr low frquncy Larg numbr HIGH frquncy HIGH nrgy i of lctromagntic ctrum WAVE *(hort wavlngth) A ingl wavlngth itanc i crt to crt A ingl wavlngth itanc i trough to trough Sction 4. Elctromagntic Raiation (Viibl Light) Comlmntary color ar on ooit i of th whl. Lowr nrgy, long wavlngth 750 nm Highr nrgy Short wavlngth 400 nm Wavlngth in mtr *(long wavlngth) V I B R G O Y 625 nm ROY G BIV 575 nm 460 nm 520 nm 4
5 Rcor into your not ooit ag 48 Grou Grou 2 Grou Grou 4 Grou 5 Grou 6 Grou 7 Grou 8 Lwi Dot Structur only how outrmot lctron (valnc lctron) th grou numbr qual th numbr of valnc lctron for rrntativ lmnt only how th valnc lctron a ot about th atom in a Lwi ot Summary: Row numbr = numbr of hll in Bohr Mol Grou numbr = numbr of valnc lctron in Lwi ot Sction 4. Enrgy Lvl (hll), Sublvl(ubhll), Orbital In toay worl, quantum hyic giv a bttr thortical mol for whr an lctron i locat. Elctron ri in an orbital within a ubhll of an lctron hll lctron hll ubhll f "Elctron Shll" n = 4 n = n = 2 n = 2 2 orbital What i th xact ar for th location of a hyrogn lctron? 5
6 On all about, know th orring, location, ha, & an atial orintation of orbital Shll ubhll orbital lvl ublvl on 2 two,, thr,, 4 four,,, f "Elctron Shll" n = 2 n = numbr of orbital "room" r ublvl Prha a goo illutration for fining an lctron ar woul b n = Numbr of Strt n = 4 n =,, f = ty of track hom f 2 2 Orbital = Room in hom A orbital f Sction 4.4 Writing Orbital Diagram & Elctron Configuration Our currnt mol: th location (th hom ar) for an lctron What i th xact ar for th location of a hyrogn lctron? Th xcact For th hyrogn atom location i: (ar) "Elctron Shll" Total numbr of lctron in ublvl hll ubhll n = lctron hll ubhll orbital 6
7 Sction 4.4 Writing Orbital Diagram & Elctron Configuration Our currnt mol: th location (th hom ar) for an lctron For th hlium atom "Elctron Shll" 2 Total numbr of lctron in ublvl hll ubhll n = lctron hll ubhll orbital 7
8 Elmnt Atomic Numbr Bohr Mol nuclu n hll Sulmntal ag 48 & 5 H + H 2 Li 2+ + Wav Mchanical Mol Elctron Configuration rincial hll & ubhll numbr firt row lmnt B 4 B 5 C 6 N 7 O valnc hll con row lmnt F 9 N Na + thir row lmnt Elmnt Atomic Numbr Bohr Mol nuclu n hll Sulmntal ag 48 & 5 H + H 2 Li B 4 B 5 C 6 N 7 O 8 F 9 N 0 Na Wav Mchanical Mol Elctron Configuration rincial hll & ubhll numbr firt row lmnt valnc hll con row lmnt thir row lmnt 8
9 Sulmntal ackt ag 49 Atomic Structur Atomic Numbr: Nam: Symbol: ma # 2 # # n # Elctronic Configuration: oium-2 2 Na Phyical Prorti: Atomic Numbr: 7 oft mtal, Nam: conuct - Symbol: Chmical Prorti: ract w/ H 2 O Lwi Dot: Na ma # 5 # # n # Elctronic Configuration: Phyical Prorti: Chmical Prorti: Lwi Dot: Atomic Numbr: Nam: Symbol: ma # 24 # # n # Elctronic magnium Mg Configuration: Phyical Prorti: Atomic Numbr: 8 uctil mtal, Nam: conuct - Symbol: Chmical Prorti: burn in O 2 Lwi Dot: Mg ma # 6 # # n # Elctronic Configuration: Phyical Prorti: Chmical Prorti: Lwi Dot: Sulmntal ackt ag 49 Atomic Structur Atomic Numbr: Nam: Symbol: ma # 2 # # n # Elctronic Configuration: oium-2 2 Na Phyical Prorti: Atomic Numbr: 7 oft mtal, Nam: chlorin-5 conuct - Symbol: 5 Cl Chmical 7 Prorti: ma # ract w/ H 2 O Lwi Dot: Na # # n # Elctronic Configuration: Phyical Prorti: yllow ga, nonconuctor Chmical Prorti: ract w/ Na() Lwi Dot: Cl Atomic Numbr: Nam: Symbol: ma # 24 # # n # Elctronic magnium Mg Configuration: Phyical Prorti: Atomic Numbr: 8 uctil mtal, Nam: oxygn-6 conuct - 6 Symbol: O 8 Chmical Prorti: burn in O 2 Lwi Dot: Mg ma # # # n # Elctronic Configuration: Phyical Prorti: colorl ga, nonconuctor Chmical Prorti: uort combution Lwi Dot: O 9
10 On all about, know th orring, location, ha, & atial orintation of orbital Shll ubhll orbital lvl ublvl on 2 two,, thr,, 4 four,,, f numbr of orbital "room" r ublvl Sulmnt ackt ag 56 Orbital hav ha ma out at 90 rcnt robability : f 2 x Hinburg Uncrtainty Princil - (Wrnr von Hinbrg) Nobl riz in hyic 92 Th Schröingr quation ma th orbital rgion mathmatically at 90% robability. (Erwin Schröingr) Nobl riz in hyic 99; rouctiv form of atomic thory y thr ovrlaying orbital gnrat th ublvl z ublvl Orbital ar rgion of gratt robability within a ubhll for fining an lctron; two lctron MAXIMUM r orbital. 0
11 Sction 4.4 Writing Orbital Diagram & Elctron Configuration Shll ubhll orbital lvl ublvl on 2 two,, thr,, 4 four,,, f numbr of orbital "room" r ublvl f What will b th arrangmnt of ubhll in an atom? Sulmntal ackt ag 5 Txtbook 7 Th ubhll ar arrang from lowt to hight nrgy valu outwar from th nuclu of th atom n (row) numbr ubhll lttr Thi lctron irctory i call an lctron configuration Sction 4.5 Elctron Configuration an th Prioic Tabl Th ubhll ar arrang from lowt to hight nrgy valu outwar from th nuclu of th atom Txtbook y q n (row) numbr y q ubhll lttr Thi lctron irctory i call an lctron configuration Bottom right cornr of ulmntal ackt ag 5 Th lctron configuration filling orr can b larn by looking at th rioic tabl arrang by incraing atomic # n= n=2 n= n=4 n=5 n=6 n=7 Draw thi imag ooit ag 5 ublvl rnt but not fill ublvl fill firt bfor 4 n=6 n=7 f
12 Sulmntal ackt ag 5 - Bohr Mol vru th currnt Schroingr Mol "Elctron Shll" Elctron hll Bohr Mol for Hyrogn n = 4 n = n = 2 n = Elctron hll, ubhll, orbital Quantum Wav Mchanical Mol (Schroingr) 4 ty of ubhll within n th lvl 2 5 f 7 5 orbital ar within ubhll rincial quantum n th hll two lctron r orbital Elctron configuration Whr i hyrogn on lctron locat?????? Total numbr of lctron in ublvl Quantum Wav Mchanical Mol (Schroingr) 4 ty of ubhll within n th lvl f hll ubhll 2 orbital ar within ubhll rincial quantum n th hll two lctron r orbital Elctron configuration
13 Whr ar th ix lctron for carbon locat??????? 6 C Maximum numbr in ublvl i 2 lctron ublvl i 6 lctron ublvl i 0 lctron f ublvl i 4 lctron Quantum Wav Mchanical Mol (Schroingr) 4 ty of ubhll within n th lvl rincial quantum n th hll 2 f orbital ar within ubhll two lctron r orbital An lctron irctory i Elctron configuration call lctron configuration It i bgin tarting from th lowt nrgy orbital, th Whr i a lctron for aluminum locat?????? Total numbr of lctron in ublvl ty of ubhll within n th lvl 4 f hll ubhll 2 orbital ar within ubhll rincial quantum n th hll two lctron r orbital Elctron configuration
14 Sulmntal ackt ag 5 Elctron Orbital Filling 9 0 Hun rul n to b ali: Fill ach orbital with on lctron ach bfor airing. 0 0 F - an Na + ar iolctronic (th am lctronically) with N All lmnt lo or gain lctron to achiv nobl ga - configuration Sulmntal ackt ag 52 & 57 2, 4,,,,,f (,,) 5 orbital 7 orbital
15 Whr woul a 2 lctron b locat? In ubhll locat within a hll. 2 Total numbr of lctron "Elctron Shll" n = 4 n = n = 2 n = Scon hll Th lttr rrnt A ublvl; Both th hll & ubhll ar crib mathmatically by Quantum Mchanic Th hll (rincial quantum lvl) I th mot imortant locator for an lctron Mor on orbital ha an volum 5
16 Txtbook 5 Th ubhll ar arrang from lowt to hight nrgy valu n (row) numbr Sulmntal ackt ag 52, 56 ublvl lttr 2 n (row) numbr ublvl lttr x y thr ovrlaying orbital gnrat th ublvl z ublvl Know th ha (volum) an atial orintation (itanc from th nuclu) for th ubhll an for th orbital lowr n valu man th lctron i clor to th nuclu ubhll incra in nrgy in th following orr < < < f lowr n valu man mallr iz an volum for th atom 6
September 23, Honors Chem Atomic structure.notebook. Atomic Structure
 Atomic Structur Topics covrd Atomic structur Subatomic particls Atomic numbr Mass numbr Charg Cations Anions Isotops Avrag atomic mass Practic qustions atomic structur Sp 27 8:16 PM 1 Powr Standards/ Larning
Atomic Structur Topics covrd Atomic structur Subatomic particls Atomic numbr Mass numbr Charg Cations Anions Isotops Avrag atomic mass Practic qustions atomic structur Sp 27 8:16 PM 1 Powr Standards/ Larning
Chapter 8: Electron Configurations and Periodicity
 Elctron Spin & th Pauli Exclusion Principl Chaptr 8: Elctron Configurations and Priodicity 3 quantum numbrs (n, l, ml) dfin th nrgy, siz, shap, and spatial orintation of ach atomic orbital. To xplain how
Elctron Spin & th Pauli Exclusion Principl Chaptr 8: Elctron Configurations and Priodicity 3 quantum numbrs (n, l, ml) dfin th nrgy, siz, shap, and spatial orintation of ach atomic orbital. To xplain how
Give the letter that represents an atom (6) (b) Atoms of A and D combine to form a compound containing covalent bonds.
 1 Th diagram shows th lctronic configurations of six diffrnt atoms. A B C D E F (a) You may us th Priodic Tabl on pag 2 to hlp you answr this qustion. Answr ach part by writing on of th lttrs A, B, C,
1 Th diagram shows th lctronic configurations of six diffrnt atoms. A B C D E F (a) You may us th Priodic Tabl on pag 2 to hlp you answr this qustion. Answr ach part by writing on of th lttrs A, B, C,
Exam 2 Thursday (7:30-9pm) It will cover material through HW 7, but no material that was on the 1 st exam.
 Exam 2 Thursday (7:30-9pm) It will covr matrial through HW 7, but no matrial that was on th 1 st xam. What happns if w bash atoms with lctrons? In atomic discharg lamps, lots of lctrons ar givn kintic
Exam 2 Thursday (7:30-9pm) It will covr matrial through HW 7, but no matrial that was on th 1 st xam. What happns if w bash atoms with lctrons? In atomic discharg lamps, lots of lctrons ar givn kintic
3.2.3 Molecules and Covalent Bonds
 Sav My Exams! Th Hom of Rvision For mor awsom GSE and lvl rsourcs, visit us at www.savmyxams.co.uk/ 3.2.3 Molculs and ovalnt onds Qustion Papr Lvl IGSE Subjct hmistry (0620) Exam oard ambridg Intrnational
Sav My Exams! Th Hom of Rvision For mor awsom GSE and lvl rsourcs, visit us at www.savmyxams.co.uk/ 3.2.3 Molculs and ovalnt onds Qustion Papr Lvl IGSE Subjct hmistry (0620) Exam oard ambridg Intrnational
Constants and Conversions:
 EXAM INFORMATION Radial Distribution Function: P 2 ( r) RDF( r) Br R( r ) 2, B is th normalization constant. Ordr of Orbital Enrgis: Homonuclar Diatomic Molculs * * * * g1s u1s g 2s u 2s u 2 p g 2 p g
EXAM INFORMATION Radial Distribution Function: P 2 ( r) RDF( r) Br R( r ) 2, B is th normalization constant. Ordr of Orbital Enrgis: Homonuclar Diatomic Molculs * * * * g1s u1s g 2s u 2s u 2 p g 2 p g
Alpha and beta decay equation practice
 Alpha and bta dcay quation practic Introduction Alpha and bta particls may b rprsntd in quations in svral diffrnt ways. Diffrnt xam boards hav thir own prfrnc. For xampl: Alpha Bta α β alpha bta Dspit
Alpha and bta dcay quation practic Introduction Alpha and bta particls may b rprsntd in quations in svral diffrnt ways. Diffrnt xam boards hav thir own prfrnc. For xampl: Alpha Bta α β alpha bta Dspit
REGISTER!!! The Farmer and the Seeds (a parable of scientific reasoning) Class Updates. The Farmer and the Seeds. The Farmer and the Seeds
 How dos light intract with mattr? And what dos (this say about) mattr? REGISTER!!! If Schrödingr s Cat walks into a forst, and no on is around to obsrv it, is h rally in th forst? sourc unknown Phys 1010
How dos light intract with mattr? And what dos (this say about) mattr? REGISTER!!! If Schrödingr s Cat walks into a forst, and no on is around to obsrv it, is h rally in th forst? sourc unknown Phys 1010
orbiting electron turns out to be wrong even though it Unfortunately, the classical visualization of the
 Lctur 22-1 Byond Bohr Modl Unfortunatly, th classical visualization of th orbiting lctron turns out to b wrong vn though it still givs us a simpl way to think of th atom. Quantum Mchanics is ndd to truly
Lctur 22-1 Byond Bohr Modl Unfortunatly, th classical visualization of th orbiting lctron turns out to b wrong vn though it still givs us a simpl way to think of th atom. Quantum Mchanics is ndd to truly
Atomic energy levels. Announcements:
 Atomic nrgy lvls Announcmnts: Exam solutions ar postd. Problm solving sssions ar M3-5 and Tusday 1-3 in G-140. Will nd arly and hand back your Midtrm Exam at nd of class. http://www.colorado.du/physics/phys2170/
Atomic nrgy lvls Announcmnts: Exam solutions ar postd. Problm solving sssions ar M3-5 and Tusday 1-3 in G-140. Will nd arly and hand back your Midtrm Exam at nd of class. http://www.colorado.du/physics/phys2170/
The van der Waals interaction 1 D. E. Soper 2 University of Oregon 20 April 2012
 Th van dr Waals intraction D. E. Sopr 2 Univrsity of Orgon 20 pril 202 Th van dr Waals intraction is discussd in Chaptr 5 of J. J. Sakurai, Modrn Quantum Mchanics. Hr I tak a look at it in a littl mor
Th van dr Waals intraction D. E. Sopr 2 Univrsity of Orgon 20 pril 202 Th van dr Waals intraction is discussd in Chaptr 5 of J. J. Sakurai, Modrn Quantum Mchanics. Hr I tak a look at it in a littl mor
The pn junction: 2 Current vs Voltage (IV) characteristics
 Th pn junction: Currnt vs Voltag (V) charactristics Considr a pn junction in quilibrium with no applid xtrnal voltag: o th V E F E F V p-typ Dpltion rgion n-typ Elctron movmnt across th junction: 1. n
Th pn junction: Currnt vs Voltag (V) charactristics Considr a pn junction in quilibrium with no applid xtrnal voltag: o th V E F E F V p-typ Dpltion rgion n-typ Elctron movmnt across th junction: 1. n
PH300 Modern Physics SP11 Final Essay. Up Next: Periodic Table Molecular Bonding
 PH Modrn Physics SP11 Final Essay Thr will b an ssay portion on th xam, but you don t nd to answr thos qustions if you submit a final ssay by th day of th final: Sat. 5/7 It dosnʼt mattr how smart you
PH Modrn Physics SP11 Final Essay Thr will b an ssay portion on th xam, but you don t nd to answr thos qustions if you submit a final ssay by th day of th final: Sat. 5/7 It dosnʼt mattr how smart you
Molecules and Covalent Bond
 Molculs and ovalnt ond Qustion Papr 1 Lvl IGSE Subjct hmistry (0620/0971) Exam oard ambridg Intrnational Examinations (IE) Topic toms, lmnts and compounds Sub-Topic Molculs and covalnt bonds ooklt Qustion
Molculs and ovalnt ond Qustion Papr 1 Lvl IGSE Subjct hmistry (0620/0971) Exam oard ambridg Intrnational Examinations (IE) Topic toms, lmnts and compounds Sub-Topic Molculs and covalnt bonds ooklt Qustion
Title: Vibrational structure of electronic transition
 Titl: Vibrational structur of lctronic transition Pag- Th band spctrum sn in th Ultra-Violt (UV) and visibl (VIS) rgions of th lctromagntic spctrum can not intrprtd as vibrational and rotational spctrum
Titl: Vibrational structur of lctronic transition Pag- Th band spctrum sn in th Ultra-Violt (UV) and visibl (VIS) rgions of th lctromagntic spctrum can not intrprtd as vibrational and rotational spctrum
Electrochemistry L E O
 Rmmbr from CHM151 A rdox raction in on in which lctrons ar transfrrd lctrochmistry L O Rduction os lctrons xidation G R ain lctrons duction W can dtrmin which lmnt is oxidizd or rducd by assigning oxidation
Rmmbr from CHM151 A rdox raction in on in which lctrons ar transfrrd lctrochmistry L O Rduction os lctrons xidation G R ain lctrons duction W can dtrmin which lmnt is oxidizd or rducd by assigning oxidation
Schrodinger Equation in 3-d
 Schrodingr Equation in 3-d ψ( xyz,, ) ψ( xyz,, ) ψ( xyz,, ) + + + Vxyz (,, ) ψ( xyz,, ) = Eψ( xyz,, ) m x y z p p p x y + + z m m m + V = E p m + V = E E + k V = E Infinit Wll in 3-d V = x > L, y > L,
Schrodingr Equation in 3-d ψ( xyz,, ) ψ( xyz,, ) ψ( xyz,, ) + + + Vxyz (,, ) ψ( xyz,, ) = Eψ( xyz,, ) m x y z p p p x y + + z m m m + V = E p m + V = E E + k V = E Infinit Wll in 3-d V = x > L, y > L,
A central nucleus. Protons have a positive charge Electrons have a negative charge
 Atomic Structur Lss than ninty yars ago scintists blivd that atoms wr tiny solid sphrs lik minut snookr balls. Sinc thn it has bn discovrd that atoms ar not compltly solid but hav innr and outr parts.
Atomic Structur Lss than ninty yars ago scintists blivd that atoms wr tiny solid sphrs lik minut snookr balls. Sinc thn it has bn discovrd that atoms ar not compltly solid but hav innr and outr parts.
Structure of the Atom. Thomson s Atomic Model. Knowledge of atoms in Experiments of Geiger and Marsden 2. Experiments of Geiger and Marsden
 CHAPTER 4 Structur of th Atom 4.1 Th Atomic Modls of Thomson and Ruthrford 4. Ruthrford Scattring 4.3 Th Classic Atomic Modl 4.4 Th Bohr Modl of th Hydrogn Atom 4.5 Succsss & Failurs of th Bohr Modl 4.6
CHAPTER 4 Structur of th Atom 4.1 Th Atomic Modls of Thomson and Ruthrford 4. Ruthrford Scattring 4.3 Th Classic Atomic Modl 4.4 Th Bohr Modl of th Hydrogn Atom 4.5 Succsss & Failurs of th Bohr Modl 4.6
Brief Introduction to Statistical Mechanics
 Brif Introduction to Statistical Mchanics. Purpos: Ths nots ar intndd to provid a vry quick introduction to Statistical Mchanics. Th fild is of cours far mor vast than could b containd in ths fw pags.
Brif Introduction to Statistical Mchanics. Purpos: Ths nots ar intndd to provid a vry quick introduction to Statistical Mchanics. Th fild is of cours far mor vast than could b containd in ths fw pags.
Contemporary, atomic, nuclear, and particle physics
 Contmporary, atomic, nuclar, and particl physics 1 Blackbody radiation as a thrmal quilibrium condition (in vacuum this is th only hat loss) Exampl-1 black plan surfac at a constant high tmpratur T h is
Contmporary, atomic, nuclar, and particl physics 1 Blackbody radiation as a thrmal quilibrium condition (in vacuum this is th only hat loss) Exampl-1 black plan surfac at a constant high tmpratur T h is
Y 0. Standing Wave Interference between the incident & reflected waves Standing wave. A string with one end fixed on a wall
 Staning Wav Intrfrnc btwn th incint & rflct wavs Staning wav A string with on n fix on a wall Incint: y, t) Y cos( t ) 1( Y 1 ( ) Y (St th incint wav s phas to b, i.., Y + ral & positiv.) Rflct: y, t)
Staning Wav Intrfrnc btwn th incint & rflct wavs Staning wav A string with on n fix on a wall Incint: y, t) Y cos( t ) 1( Y 1 ( ) Y (St th incint wav s phas to b, i.., Y + ral & positiv.) Rflct: y, t)
Ch. 24 Molecular Reaction Dynamics 1. Collision Theory
 Ch. 4 Molcular Raction Dynamics 1. Collision Thory Lctur 16. Diffusion-Controlld Raction 3. Th Matrial Balanc Equation 4. Transition Stat Thory: Th Eyring Equation 5. Transition Stat Thory: Thrmodynamic
Ch. 4 Molcular Raction Dynamics 1. Collision Thory Lctur 16. Diffusion-Controlld Raction 3. Th Matrial Balanc Equation 4. Transition Stat Thory: Th Eyring Equation 5. Transition Stat Thory: Thrmodynamic
Hydrogen Atom and One Electron Ions
 Hydrogn Atom and On Elctron Ions Th Schrödingr quation for this two-body problm starts out th sam as th gnral two-body Schrödingr quation. First w sparat out th motion of th cntr of mass. Th intrnal potntial
Hydrogn Atom and On Elctron Ions Th Schrödingr quation for this two-body problm starts out th sam as th gnral two-body Schrödingr quation. First w sparat out th motion of th cntr of mass. Th intrnal potntial
Mathematics 1110H Calculus I: Limits, derivatives, and Integrals Trent University, Summer 2018 Solutions to the Actual Final Examination
 Mathmatics H Calculus I: Limits, rivativs, an Intgrals Trnt Univrsity, Summr 8 Solutions to th Actual Final Eamination Tim-spac: 9:-: in FPHL 7. Brought to you by Stfan B lan k. Instructions: Do parts
Mathmatics H Calculus I: Limits, rivativs, an Intgrals Trnt Univrsity, Summr 8 Solutions to th Actual Final Eamination Tim-spac: 9:-: in FPHL 7. Brought to you by Stfan B lan k. Instructions: Do parts
Lecture 37 (Schrödinger Equation) Physics Spring 2018 Douglas Fields
 Lctur 37 (Schrödingr Equation) Physics 6-01 Spring 018 Douglas Filds Rducd Mass OK, so th Bohr modl of th atom givs nrgy lvls: E n 1 k m n 4 But, this has on problm it was dvlopd assuming th acclration
Lctur 37 (Schrödingr Equation) Physics 6-01 Spring 018 Douglas Filds Rducd Mass OK, so th Bohr modl of th atom givs nrgy lvls: E n 1 k m n 4 But, this has on problm it was dvlopd assuming th acclration
Unit II: Atoms Molecules & Ions
 Unit II: Atoms Molculs & Ions A. B. C. D. Th Priodic Tabl Why and how compounds form Ruls for writing ions Writing formulas and naming compounds 1. 2. 3. Binary compounds Non-binary compounds Formula writing
Unit II: Atoms Molculs & Ions A. B. C. D. Th Priodic Tabl Why and how compounds form Ruls for writing ions Writing formulas and naming compounds 1. 2. 3. Binary compounds Non-binary compounds Formula writing
High Energy Physics. Lecture 5 The Passage of Particles through Matter
 High Enrgy Physics Lctur 5 Th Passag of Particls through Mattr 1 Introduction In prvious lcturs w hav sn xampls of tracks lft by chargd particls in passing through mattr. Such tracks provid som of th most
High Enrgy Physics Lctur 5 Th Passag of Particls through Mattr 1 Introduction In prvious lcturs w hav sn xampls of tracks lft by chargd particls in passing through mattr. Such tracks provid som of th most
PHYS ,Fall 05, Term Exam #1, Oct., 12, 2005
 PHYS1444-,Fall 5, Trm Exam #1, Oct., 1, 5 Nam: Kys 1. circular ring of charg of raius an a total charg Q lis in th x-y plan with its cntr at th origin. small positiv tst charg q is plac at th origin. What
PHYS1444-,Fall 5, Trm Exam #1, Oct., 1, 5 Nam: Kys 1. circular ring of charg of raius an a total charg Q lis in th x-y plan with its cntr at th origin. small positiv tst charg q is plac at th origin. What
Multiple Short Term Infusion Homework # 5 PHA 5127
 Multipl Short rm Infusion Homwork # 5 PHA 527 A rug is aministr as a short trm infusion. h avrag pharmacokintic paramtrs for this rug ar: k 0.40 hr - V 28 L his rug follows a on-compartmnt boy mol. A 300
Multipl Short rm Infusion Homwork # 5 PHA 527 A rug is aministr as a short trm infusion. h avrag pharmacokintic paramtrs for this rug ar: k 0.40 hr - V 28 L his rug follows a on-compartmnt boy mol. A 300
Voltage, Current, Power, Series Resistance, Parallel Resistance, and Diodes
 Lctur 1. oltag, Currnt, Powr, Sris sistanc, Paralll sistanc, and Diods Whn you start to dal with lctronics thr ar thr main concpts to start with: Nam Symbol Unit oltag volt Currnt ampr Powr W watt oltag
Lctur 1. oltag, Currnt, Powr, Sris sistanc, Paralll sistanc, and Diods Whn you start to dal with lctronics thr ar thr main concpts to start with: Nam Symbol Unit oltag volt Currnt ampr Powr W watt oltag
The Death of Stars - I.
 Th Dath of Stars - I. Larning Objctivs! B abl to sktch th H-R diagram and includ stars by siz, sctral ty, liftim, color, mass, magnitud, tmratur and luminosity, rlativ to our Sun! Comar Rd Dwarfs to our
Th Dath of Stars - I. Larning Objctivs! B abl to sktch th H-R diagram and includ stars by siz, sctral ty, liftim, color, mass, magnitud, tmratur and luminosity, rlativ to our Sun! Comar Rd Dwarfs to our
How can I control light? (and rule the world?)
 How can I control light? (and rul th world?) "You know, I hav on simpl rqust. And that is to hav sharks with frickin' lasr bams attachd to thir hads! - Dr. Evil Phys 230, Day 35 Qustions? Spctra (colors
How can I control light? (and rul th world?) "You know, I hav on simpl rqust. And that is to hav sharks with frickin' lasr bams attachd to thir hads! - Dr. Evil Phys 230, Day 35 Qustions? Spctra (colors
2. Laser physics - basics
 . Lasr physics - basics Spontanous and stimulatd procsss Einstin A and B cofficints Rat quation analysis Gain saturation What is a lasr? LASER: Light Amplification by Stimulatd Emission of Radiation "light"
. Lasr physics - basics Spontanous and stimulatd procsss Einstin A and B cofficints Rat quation analysis Gain saturation What is a lasr? LASER: Light Amplification by Stimulatd Emission of Radiation "light"
ELECTRON-MUON SCATTERING
 ELECTRON-MUON SCATTERING ABSTRACT Th lctron charg is considrd to b distributd or xtndd in spac. Th diffrntial of th lctron charg is st qual to a function of lctron charg coordinats multiplid by a four-dimnsional
ELECTRON-MUON SCATTERING ABSTRACT Th lctron charg is considrd to b distributd or xtndd in spac. Th diffrntial of th lctron charg is st qual to a function of lctron charg coordinats multiplid by a four-dimnsional
Neutrinos are chargeless, nearly massless particles Most abundant particle in the Universe Interact with matter via weak nuclear force
 By Wndi Wamlr Nutrinos ar charglss, narly masslss articls Most abundant articl in th Univrs Intract with mattr via wak nuclar forc Narly transarnt to mattr Only known ty of articl that can sca from th
By Wndi Wamlr Nutrinos ar charglss, narly masslss articls Most abundant articl in th Univrs Intract with mattr via wak nuclar forc Narly transarnt to mattr Only known ty of articl that can sca from th
Physics 2D Lecture Slides Lecture 12: Jan 28 th 2004
 Brian Wcht, th TA, is away this wk. I will substitut for his offic hours (in my offic 3314 Mayr Hall, discussion and PS sssion. Pl. giv all rgrad rqusts to m this wk (only) Quiz 3 Will Covr Sctions.1-.5
Brian Wcht, th TA, is away this wk. I will substitut for his offic hours (in my offic 3314 Mayr Hall, discussion and PS sssion. Pl. giv all rgrad rqusts to m this wk (only) Quiz 3 Will Covr Sctions.1-.5
Exam 1. It is important that you clearly show your work and mark the final answer clearly, closed book, closed notes, no calculator.
 Exam N a m : _ S O L U T I O N P U I D : I n s t r u c t i o n s : It is important that you clarly show your work and mark th final answr clarly, closd book, closd nots, no calculator. T i m : h o u r
Exam N a m : _ S O L U T I O N P U I D : I n s t r u c t i o n s : It is important that you clarly show your work and mark th final answr clarly, closd book, closd nots, no calculator. T i m : h o u r
The Death of Stars - II.
 Th Dath of Stars - II. Larning Objctivs! How can w us H-R diagrams to masur th ag of star clustrs (and hnc th ag of our Univrs)?! Why do high and low mass stars volv diffrntly? How ar havy lmnts such as
Th Dath of Stars - II. Larning Objctivs! How can w us H-R diagrams to masur th ag of star clustrs (and hnc th ag of our Univrs)?! Why do high and low mass stars volv diffrntly? How ar havy lmnts such as
ph People Grade Level: basic Duration: minutes Setting: classroom or field site
 ph Popl Adaptd from: Whr Ar th Frogs? in Projct WET: Curriculum & Activity Guid. Bozman: Th Watrcours and th Council for Environmntal Education, 1995. ph Grad Lvl: basic Duration: 10 15 minuts Stting:
ph Popl Adaptd from: Whr Ar th Frogs? in Projct WET: Curriculum & Activity Guid. Bozman: Th Watrcours and th Council for Environmntal Education, 1995. ph Grad Lvl: basic Duration: 10 15 minuts Stting:
What makes laser light special? Lasers. Lasers. Laser history. Lasers are everywhere! Review of atom discharge lamps. So how do we make laser light?
 Lasrs Lasrs What is diffrnt/spcial about lasr light. How dos a lasr work. - rviw atomic discharg lamps. - how light intracts with atoms - how ths idas ar usd to mak lasr. "You know, I hav on simpl rqust.
Lasrs Lasrs What is diffrnt/spcial about lasr light. How dos a lasr work. - rviw atomic discharg lamps. - how light intracts with atoms - how ths idas ar usd to mak lasr. "You know, I hav on simpl rqust.
Background: We have discussed the PIB, HO, and the energy of the RR model. In this chapter, the H-atom, and atomic orbitals.
 Chaptr 7 Th Hydrogn Atom Background: W hav discussd th PIB HO and th nrgy of th RR modl. In this chaptr th H-atom and atomic orbitals. * A singl particl moving undr a cntral forc adoptd from Scott Kirby
Chaptr 7 Th Hydrogn Atom Background: W hav discussd th PIB HO and th nrgy of th RR modl. In this chaptr th H-atom and atomic orbitals. * A singl particl moving undr a cntral forc adoptd from Scott Kirby
de/dx Effectively all charged particles except electrons
 de/dx Lt s nxt turn our attntion to how chargd particls los nrgy in mattr To start with w ll considr only havy chargd particls lik muons, pions, protons, alphas, havy ions, Effctivly all chargd particls
de/dx Lt s nxt turn our attntion to how chargd particls los nrgy in mattr To start with w ll considr only havy chargd particls lik muons, pions, protons, alphas, havy ions, Effctivly all chargd particls
On the Hamiltonian of a Multi-Electron Atom
 On th Hamiltonian of a Multi-Elctron Atom Austn Gronr Drxl Univrsity Philadlphia, PA Octobr 29, 2010 1 Introduction In this papr, w will xhibit th procss of achiving th Hamiltonian for an lctron gas. Making
On th Hamiltonian of a Multi-Elctron Atom Austn Gronr Drxl Univrsity Philadlphia, PA Octobr 29, 2010 1 Introduction In this papr, w will xhibit th procss of achiving th Hamiltonian for an lctron gas. Making
BETA DECAY VISUAL PHYSICS ONLINE
 VISUAL PHYSICS ONLINE BETA DECAY Suppos now that a nuclus xists which has ithr too many or too fw nutrons rlativ to th numbr of protons prsnt for stability. Stability can b achivd by th convrsion insid
VISUAL PHYSICS ONLINE BETA DECAY Suppos now that a nuclus xists which has ithr too many or too fw nutrons rlativ to th numbr of protons prsnt for stability. Stability can b achivd by th convrsion insid
Determination of Vibrational and Electronic Parameters From an Electronic Spectrum of I 2 and a Birge-Sponer Plot
 5 J. Phys. Chm G Dtrmination of Vibrational and Elctronic Paramtrs From an Elctronic Spctrum of I 2 and a Birg-Sponr Plot 1 15 2 25 3 35 4 45 Dpartmnt of Chmistry, Gustavus Adolphus Collg. 8 Wst Collg
5 J. Phys. Chm G Dtrmination of Vibrational and Elctronic Paramtrs From an Elctronic Spctrum of I 2 and a Birg-Sponr Plot 1 15 2 25 3 35 4 45 Dpartmnt of Chmistry, Gustavus Adolphus Collg. 8 Wst Collg
the electrons. Expanding the exponential and neglecting the constant term Ze 2 λ, we have
 LECTURE.8 Prof.R.Parthasarathy Atomic Structur - Elmntary Tratmnt Th ground stat of hydrogn atom has bn solvd xactly in th nonrlativistic tratmnt. Th ground stat of hlium atom has bn handld in variational
LECTURE.8 Prof.R.Parthasarathy Atomic Structur - Elmntary Tratmnt Th ground stat of hydrogn atom has bn solvd xactly in th nonrlativistic tratmnt. Th ground stat of hlium atom has bn handld in variational
Outline. Solar Photovoltaic Applications. Midterm Problem One. Midterm Results. Midterm Problem Two. Midterm Problem One II
 Photovoltaic Solar April 6, 010 Solar Photovoltaic Application Larry Cartto Mchanical Enginring 483 Altrnativ Enrgy Enginring II April 6, 010 Outlin Midtrm xam rult Mt on Wdnday at PPM Confrnc room for
Photovoltaic Solar April 6, 010 Solar Photovoltaic Application Larry Cartto Mchanical Enginring 483 Altrnativ Enrgy Enginring II April 6, 010 Outlin Midtrm xam rult Mt on Wdnday at PPM Confrnc room for
PH2200 Practice Final Exam Spring 2004
 PH2200 Practic Final Exam Spring 2004 Instructions 1. Writ your nam and studnt idntification numbr on th answr sht. 2. This a two-hour xam. 3. Plas covr your answr sht at all tims. 4. This is a closd book
PH2200 Practic Final Exam Spring 2004 Instructions 1. Writ your nam and studnt idntification numbr on th answr sht. 2. This a two-hour xam. 3. Plas covr your answr sht at all tims. 4. This is a closd book
PHYS-333: Problem set #2 Solutions
 PHYS-333: Problm st #2 Solutions Vrsion of March 5, 2016. 1. Visual binary 15 points): Ovr a priod of 10 yars, two stars sparatd by an angl of 1 arcsc ar obsrvd to mov through a full circl about a point
PHYS-333: Problm st #2 Solutions Vrsion of March 5, 2016. 1. Visual binary 15 points): Ovr a priod of 10 yars, two stars sparatd by an angl of 1 arcsc ar obsrvd to mov through a full circl about a point
(1) Then we could wave our hands over this and it would become:
 MAT* K285 Spring 28 Anthony Bnoit 4/17/28 Wk 12: Laplac Tranform Rading: Kohlr & Johnon, Chaptr 5 to p. 35 HW: 5.1: 3, 7, 1*, 19 5.2: 1, 5*, 13*, 19, 45* 5.3: 1, 11*, 19 * Pla writ-up th problm natly and
MAT* K285 Spring 28 Anthony Bnoit 4/17/28 Wk 12: Laplac Tranform Rading: Kohlr & Johnon, Chaptr 5 to p. 35 HW: 5.1: 3, 7, 1*, 19 5.2: 1, 5*, 13*, 19, 45* 5.3: 1, 11*, 19 * Pla writ-up th problm natly and
Why is a E&M nature of light not sufficient to explain experiments?
 1 Th wird world of photons Why is a E&M natur of light not sufficint to xplain xprimnts? Do photons xist? Som quantum proprtis of photons 2 Black body radiation Stfan s law: Enrgy/ ara/ tim = Win s displacmnt
1 Th wird world of photons Why is a E&M natur of light not sufficint to xplain xprimnts? Do photons xist? Som quantum proprtis of photons 2 Black body radiation Stfan s law: Enrgy/ ara/ tim = Win s displacmnt
COHORT MBA. Exponential function. MATH review (part2) by Lucian Mitroiu. The LOG and EXP functions. Properties: e e. lim.
 MTH rviw part b Lucian Mitroiu Th LOG and EXP functions Th ponntial function p : R, dfind as Proprtis: lim > lim p Eponntial function Y 8 6 - -8-6 - - X Th natural logarithm function ln in US- log: function
MTH rviw part b Lucian Mitroiu Th LOG and EXP functions Th ponntial function p : R, dfind as Proprtis: lim > lim p Eponntial function Y 8 6 - -8-6 - - X Th natural logarithm function ln in US- log: function
1. In the given figure PQRS is a parallelogram. Find the coordinates of R.
 Tst Assss Achiv Class : 9 CLASS : 9 Mathmatics 1. In th givn figur PQRS is a paralllogram. Find th coordinats of R. Y S(2, 3) R O P(1, 0) Q(5, 0) X (5, 2) (5, 3) (6, 2) (6, 3) 2. Th prpndicular distanc
Tst Assss Achiv Class : 9 CLASS : 9 Mathmatics 1. In th givn figur PQRS is a paralllogram. Find th coordinats of R. Y S(2, 3) R O P(1, 0) Q(5, 0) X (5, 2) (5, 3) (6, 2) (6, 3) 2. Th prpndicular distanc
Davisson Germer experiment
 Announcmnts: Davisson Grmr xprimnt Homwork st 5 is today. Homwork st 6 will b postd latr today. Mad a good guss about th Nobl Priz for 2013 Clinton Davisson and Lstr Grmr. Davisson won Nobl Priz in 1937.
Announcmnts: Davisson Grmr xprimnt Homwork st 5 is today. Homwork st 6 will b postd latr today. Mad a good guss about th Nobl Priz for 2013 Clinton Davisson and Lstr Grmr. Davisson won Nobl Priz in 1937.
Classical Magnetic Dipole
 Lctur 18 1 Classical Magntic Dipol In gnral, a particl of mass m and charg q (not ncssarily a point charg), w hav q g L m whr g is calld th gyromagntic ratio, which accounts for th ffcts of non-point charg
Lctur 18 1 Classical Magntic Dipol In gnral, a particl of mass m and charg q (not ncssarily a point charg), w hav q g L m whr g is calld th gyromagntic ratio, which accounts for th ffcts of non-point charg
Derivation of Electron-Electron Interaction Terms in the Multi-Electron Hamiltonian
 Drivation of Elctron-Elctron Intraction Trms in th Multi-Elctron Hamiltonian Erica Smith Octobr 1, 010 1 Introduction Th Hamiltonian for a multi-lctron atom with n lctrons is drivd by Itoh (1965) by accounting
Drivation of Elctron-Elctron Intraction Trms in th Multi-Elctron Hamiltonian Erica Smith Octobr 1, 010 1 Introduction Th Hamiltonian for a multi-lctron atom with n lctrons is drivd by Itoh (1965) by accounting
0 +1e Radionuclides - can spontaneously emit particles and radiation which can be expressed by a nuclear equation.
 Radioactivity Radionuclids - can spontanously mit particls and radiation which can b xprssd by a nuclar quation. Spontanous Emission: Mass and charg ar consrvd. 4 2α -β Alpha mission Bta mission 238 92U
Radioactivity Radionuclids - can spontanously mit particls and radiation which can b xprssd by a nuclar quation. Spontanous Emission: Mass and charg ar consrvd. 4 2α -β Alpha mission Bta mission 238 92U
Pair (and Triplet) Production Effect:
 Pair (and riplt Production Effct: In both Pair and riplt production, a positron (anti-lctron and an lctron (or ngatron ar producd spontanously as a photon intracts with a strong lctric fild from ithr a
Pair (and riplt Production Effct: In both Pair and riplt production, a positron (anti-lctron and an lctron (or ngatron ar producd spontanously as a photon intracts with a strong lctric fild from ithr a
Higher order derivatives
 Robrto s Nots on Diffrntial Calculus Chaptr 4: Basic diffrntiation ruls Sction 7 Highr ordr drivativs What you nd to know alrady: Basic diffrntiation ruls. What you can larn hr: How to rpat th procss of
Robrto s Nots on Diffrntial Calculus Chaptr 4: Basic diffrntiation ruls Sction 7 Highr ordr drivativs What you nd to know alrady: Basic diffrntiation ruls. What you can larn hr: How to rpat th procss of
Chapter. 3 Wave & Particles I
 Announcmnt Cours wbpag http://highnrgy.phys.ttu.du/~sl/2402/ Txtbook PHYS-2402 Lctur 8 Quiz 1 Class avrag: 14.2 (out of 20) ~ 70% Fb. 10, 2015 HW2 (du 2/19) 13, 17, 23, 25, 28, 31, 37, 38, 41, 44 Chaptr.
Announcmnt Cours wbpag http://highnrgy.phys.ttu.du/~sl/2402/ Txtbook PHYS-2402 Lctur 8 Quiz 1 Class avrag: 14.2 (out of 20) ~ 70% Fb. 10, 2015 HW2 (du 2/19) 13, 17, 23, 25, 28, 31, 37, 38, 41, 44 Chaptr.
Davisson Germer experiment Announcements:
 Davisson Grmr xprimnt Announcmnts: Homwork st 7 is du Wdnsday. Problm solving sssions M3-5, T3-5. Th 2 nd midtrm will b April 7 in MUEN E0046 at 7:30pm. BFFs: Davisson and Grmr. Today w will go ovr th
Davisson Grmr xprimnt Announcmnts: Homwork st 7 is du Wdnsday. Problm solving sssions M3-5, T3-5. Th 2 nd midtrm will b April 7 in MUEN E0046 at 7:30pm. BFFs: Davisson and Grmr. Today w will go ovr th
MSLC Math 151 WI09 Exam 2 Review Solutions
 Eam Rviw Solutions. Comput th following rivativs using th iffrntiation ruls: a.) cot cot cot csc cot cos 5 cos 5 cos 5 cos 5 sin 5 5 b.) c.) sin( ) sin( ) y sin( ) ln( y) ln( ) ln( y) sin( ) ln( ) y y
Eam Rviw Solutions. Comput th following rivativs using th iffrntiation ruls: a.) cot cot cot csc cot cos 5 cos 5 cos 5 cos 5 sin 5 5 b.) c.) sin( ) sin( ) y sin( ) ln( y) ln( ) ln( y) sin( ) ln( ) y y
Elements of Statistical Thermodynamics
 24 Elmnts of Statistical Thrmodynamics Statistical thrmodynamics is a branch of knowldg that has its own postulats and tchniqus. W do not attmpt to giv hr vn an introduction to th fild. In this chaptr,
24 Elmnts of Statistical Thrmodynamics Statistical thrmodynamics is a branch of knowldg that has its own postulats and tchniqus. W do not attmpt to giv hr vn an introduction to th fild. In this chaptr,
Finite Element Analysis
 Finit Elmnt Analysis L4 D Shap Functions, an Gauss Quaratur FEA Formulation Dr. Wiong Wu EGR 54 Finit Elmnt Analysis Roamap for Dvlopmnt of FE Strong form: govrning DE an BCs EGR 54 Finit Elmnt Analysis
Finit Elmnt Analysis L4 D Shap Functions, an Gauss Quaratur FEA Formulation Dr. Wiong Wu EGR 54 Finit Elmnt Analysis Roamap for Dvlopmnt of FE Strong form: govrning DE an BCs EGR 54 Finit Elmnt Analysis
PRINCIPLES OF PLASMA PROCESSING Course Notes: Prof. J. P. Chang Part B3: ATOMIC COLLISIONS AND SPECTRA
 Atomic Collisions and Spctra 125 PRINCIPLES OF PLASMA PROCESSING Cours Nots: Prof. J. P. Chang Part B3: ATOMIC COLLISIONS AND SPECTRA I. ATOMIC ENERGY LEVELS Atoms and molculs mit lctromagntic radiation
Atomic Collisions and Spctra 125 PRINCIPLES OF PLASMA PROCESSING Cours Nots: Prof. J. P. Chang Part B3: ATOMIC COLLISIONS AND SPECTRA I. ATOMIC ENERGY LEVELS Atoms and molculs mit lctromagntic radiation
Deepak Rajput
 Q Prov: (a than an infinit point lattic is only capabl of showing,, 4, or 6-fold typ rotational symmtry; (b th Wiss zon law, i.. if [uvw] is a zon axis and (hkl is a fac in th zon, thn hu + kv + lw ; (c
Q Prov: (a than an infinit point lattic is only capabl of showing,, 4, or 6-fold typ rotational symmtry; (b th Wiss zon law, i.. if [uvw] is a zon axis and (hkl is a fac in th zon, thn hu + kv + lw ; (c
The failure of the classical mechanics
 h failur of th classical mchanics W rviw som xprimntal vidncs showing that svral concpts of classical mchanics cannot b applid. - h blac-body radiation. - Atomic and molcular spctra. - h particl-li charactr
h failur of th classical mchanics W rviw som xprimntal vidncs showing that svral concpts of classical mchanics cannot b applid. - h blac-body radiation. - Atomic and molcular spctra. - h particl-li charactr
fiziks Institute for NET/JRF, GATE, IIT JAM, M.Sc. Entrance, JEST, TIFR and GRE in Physics
 Atoic and olcular Physics JEST Q. Th binding nrgy of th hydrogn ato (lctron bound to proton) is.6 V. Th binding nrgy of positroniu (lctron bound to positron) is (a).6 / V (b).6 / 8 V (c).6 8 V (d).6 V.6
Atoic and olcular Physics JEST Q. Th binding nrgy of th hydrogn ato (lctron bound to proton) is.6 V. Th binding nrgy of positroniu (lctron bound to positron) is (a).6 / V (b).6 / 8 V (c).6 8 V (d).6 V.6
SCHUR S THEOREM REU SUMMER 2005
 SCHUR S THEOREM REU SUMMER 2005 1. Combinatorial aroach Prhas th first rsult in th subjct blongs to I. Schur and dats back to 1916. On of his motivation was to study th local vrsion of th famous quation
SCHUR S THEOREM REU SUMMER 2005 1. Combinatorial aroach Prhas th first rsult in th subjct blongs to I. Schur and dats back to 1916. On of his motivation was to study th local vrsion of th famous quation
Electrons and Conductors
 Elctrons and Conductors Atoms consist primarily of lctrons, protons, and nutrons. A modifid Bohr modl of th atom is shown blow. nuclus composd of a clustr of protons and nutrons orbital lctrons lctron
Elctrons and Conductors Atoms consist primarily of lctrons, protons, and nutrons. A modifid Bohr modl of th atom is shown blow. nuclus composd of a clustr of protons and nutrons orbital lctrons lctron
Gradebook & Midterm & Office Hours
 Your commnts So what do w do whn on of th r's is 0 in th quation GmM(1/r-1/r)? Do w nd to driv all of ths potntial nrgy formulas? I don't undrstand springs This was th first lctur I actually larnd somthing
Your commnts So what do w do whn on of th r's is 0 in th quation GmM(1/r-1/r)? Do w nd to driv all of ths potntial nrgy formulas? I don't undrstand springs This was th first lctur I actually larnd somthing
Electric (Rocket) Propulsion. EP Overview
 Elctric (Rockt) Propulsion EP Ovrviw Elctric Propulsion-1 Basics Rockt Propulsion Elmnts Propllant Enrgy Sourc Storag Fd Systm sam in chmical rockts Storag Convrsion Acclrator Elctric Propulsion- 1 Elctric
Elctric (Rockt) Propulsion EP Ovrviw Elctric Propulsion-1 Basics Rockt Propulsion Elmnts Propllant Enrgy Sourc Storag Fd Systm sam in chmical rockts Storag Convrsion Acclrator Elctric Propulsion- 1 Elctric
Math 34A. Final Review
 Math A Final Rviw 1) Us th graph of y10 to find approimat valus: a) 50 0. b) y (0.65) solution for part a) first writ an quation: 50 0. now tak th logarithm of both sids: log() log(50 0. ) pand th right
Math A Final Rviw 1) Us th graph of y10 to find approimat valus: a) 50 0. b) y (0.65) solution for part a) first writ an quation: 50 0. now tak th logarithm of both sids: log() log(50 0. ) pand th right
Lecture 28 Title: Diatomic Molecule : Vibrational and Rotational spectra
 Lctur 8 Titl: Diatomic Molcul : Vibrational and otational spctra Pag- In this lctur w will undrstand th molcular vibrational and rotational spctra of diatomic molcul W will start with th Hamiltonian for
Lctur 8 Titl: Diatomic Molcul : Vibrational and otational spctra Pag- In this lctur w will undrstand th molcular vibrational and rotational spctra of diatomic molcul W will start with th Hamiltonian for
The Matrix Exponential
 Th Matrix Exponntial (with xrciss) by Dan Klain Vrsion 28928 Corrctions and commnts ar wlcom Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix () A A k I + A + k!
Th Matrix Exponntial (with xrciss) by Dan Klain Vrsion 28928 Corrctions and commnts ar wlcom Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix () A A k I + A + k!
Chemistry 342 Spring, The Hydrogen Atom.
 Th Hyrogn Ato. Th quation. Th first quation w want to sov is φ This quation is of faiiar for; rca that for th fr partic, w ha ψ x for which th soution is Sinc k ψ ψ(x) a cos kx a / k sin kx ± ix cos x
Th Hyrogn Ato. Th quation. Th first quation w want to sov is φ This quation is of faiiar for; rca that for th fr partic, w ha ψ x for which th soution is Sinc k ψ ψ(x) a cos kx a / k sin kx ± ix cos x
Unit 3 Periodic Table and Quantum HW Packet Name Date. Periodic Table Concepts. 1. In what family are the most active metals located?
 Directions: Answer the following questions. Periodic Table Concepts 1. In what family are the most active metals located? 2. In what family are the most active non-metals located? 3. What family on the
Directions: Answer the following questions. Periodic Table Concepts 1. In what family are the most active metals located? 2. In what family are the most active non-metals located? 3. What family on the
1.2 Faraday s law A changing magnetic field induces an electric field. Their relation is given by:
 Elctromagntic Induction. Lorntz forc on moving charg Point charg moving at vlocity v, F qv B () For a sction of lctric currnt I in a thin wir dl is Idl, th forc is df Idl B () Elctromotiv forc f s any
Elctromagntic Induction. Lorntz forc on moving charg Point charg moving at vlocity v, F qv B () For a sction of lctric currnt I in a thin wir dl is Idl, th forc is df Idl B () Elctromotiv forc f s any
Precise Masses of particles
 /1/15 Physics 1 April 1, 15 Ovrviw of topic Th constitunts and structur of nucli Radioactivity Half-lif and Radioactiv dating Nuclar Binding Enrgy Nuclar Fission Nuclar Fusion Practical Applications of
/1/15 Physics 1 April 1, 15 Ovrviw of topic Th constitunts and structur of nucli Radioactivity Half-lif and Radioactiv dating Nuclar Binding Enrgy Nuclar Fission Nuclar Fusion Practical Applications of
E hf. hf c. 2 2 h 2 2 m v f ' f 2f ' f cos c
 EXPERIMENT 9: COMPTON EFFECT Rlatd Topics Intractions of photons with lctrons, consrvation of momntum and nrgy, inlastic and lastic scattring, intraction cross sction, Compton wavlngth. Principl Whn photons
EXPERIMENT 9: COMPTON EFFECT Rlatd Topics Intractions of photons with lctrons, consrvation of momntum and nrgy, inlastic and lastic scattring, intraction cross sction, Compton wavlngth. Principl Whn photons
Unit 6: Solving Exponential Equations and More
 Habrman MTH 111 Sction II: Eonntial and Logarithmic Functions Unit 6: Solving Eonntial Equations and Mor EXAMPLE: Solv th quation 10 100 for. Obtain an act solution. This quation is so asy to solv that
Habrman MTH 111 Sction II: Eonntial and Logarithmic Functions Unit 6: Solving Eonntial Equations and Mor EXAMPLE: Solv th quation 10 100 for. Obtain an act solution. This quation is so asy to solv that
22/ Breakdown of the Born-Oppenheimer approximation. Selection rules for rotational-vibrational transitions. P, R branches.
 Subjct Chmistry Papr No and Titl Modul No and Titl Modul Tag 8/ Physical Spctroscopy / Brakdown of th Born-Oppnhimr approximation. Slction ruls for rotational-vibrational transitions. P, R branchs. CHE_P8_M
Subjct Chmistry Papr No and Titl Modul No and Titl Modul Tag 8/ Physical Spctroscopy / Brakdown of th Born-Oppnhimr approximation. Slction ruls for rotational-vibrational transitions. P, R branchs. CHE_P8_M
Unit 7 Charge-to-mass ratio of the electron
 Unit 7 Charg-to-ass ratio of th lctron Kywords: J. J. Thoson, Lorntz Forc, Magntic Filds Objctiv: Obsrv th rsults of lctron ba influncd by th agntic fild and calculat th charg-to-ass ratio of th lctron.
Unit 7 Charg-to-ass ratio of th lctron Kywords: J. J. Thoson, Lorntz Forc, Magntic Filds Objctiv: Obsrv th rsults of lctron ba influncd by th agntic fild and calculat th charg-to-ass ratio of th lctron.
The Matrix Exponential
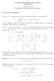 Th Matrix Exponntial (with xrciss) by D. Klain Vrsion 207.0.05 Corrctions and commnts ar wlcom. Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix A A k I + A + k!
Th Matrix Exponntial (with xrciss) by D. Klain Vrsion 207.0.05 Corrctions and commnts ar wlcom. Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix A A k I + A + k!
fiziks Institute for NET/JRF, GATE, IIT JAM, M.Sc. Entrance, JEST, TIFR and GRE in Physics NUCLEAR AND PARTICLE PHYSICS NET/JRF (JUNE-2011)
 NUCLEAR AND PARTICLE PHYSICS NET/JRF (JUNE-) 64 Q. Th radius of a 9Cu nuclus is masurd to b 4.8 - cm. (A). Th radius of a 7 Mg nuclus can b stimatd to b.86 - cm (b) 5. - cm (c).6 - cm (d) 8.6 - cm (c)
NUCLEAR AND PARTICLE PHYSICS NET/JRF (JUNE-) 64 Q. Th radius of a 9Cu nuclus is masurd to b 4.8 - cm. (A). Th radius of a 7 Mg nuclus can b stimatd to b.86 - cm (b) 5. - cm (c).6 - cm (d) 8.6 - cm (c)
Partial Derivatives: Suppose that z = f(x, y) is a function of two variables.
 Chaptr Functions o Two Variabls Applid Calculus 61 Sction : Calculus o Functions o Two Variabls Now that ou hav som amiliarit with unctions o two variabls it s tim to start appling calculus to hlp us solv
Chaptr Functions o Two Variabls Applid Calculus 61 Sction : Calculus o Functions o Two Variabls Now that ou hav som amiliarit with unctions o two variabls it s tim to start appling calculus to hlp us solv
First order differential equation Linear equation; Method of integrating factors
 First orr iffrntial quation Linar quation; Mtho of intgrating factors Exampl 1: Rwrit th lft han si as th rivativ of th prouct of y an som function by prouct rul irctly. Solving th first orr iffrntial
First orr iffrntial quation Linar quation; Mtho of intgrating factors Exampl 1: Rwrit th lft han si as th rivativ of th prouct of y an som function by prouct rul irctly. Solving th first orr iffrntial
3.1 Atomic Structure and The Periodic Table
 Sav My Exams! Th Hom of Rvisio For mor awsom GSE ad lvl rsourcs, visit us at www.savmyxams.co.uk/ 3. tomic Structur ad Th Priodic Tabl Qustio Par Lvl IGSE Subjct hmistry (060) Exam oard ambridg Itratioal
Sav My Exams! Th Hom of Rvisio For mor awsom GSE ad lvl rsourcs, visit us at www.savmyxams.co.uk/ 3. tomic Structur ad Th Priodic Tabl Qustio Par Lvl IGSE Subjct hmistry (060) Exam oard ambridg Itratioal
Principles of Humidity Dalton s law
 Principls of Humidity Dalton s law Air is a mixtur of diffrnt gass. Th main gas componnts ar: Gas componnt volum [%] wight [%] Nitrogn N 2 78,03 75,47 Oxygn O 2 20,99 23,20 Argon Ar 0,93 1,28 Carbon dioxid
Principls of Humidity Dalton s law Air is a mixtur of diffrnt gass. Th main gas componnts ar: Gas componnt volum [%] wight [%] Nitrogn N 2 78,03 75,47 Oxygn O 2 20,99 23,20 Argon Ar 0,93 1,28 Carbon dioxid
Coupled Pendulums. Two normal modes.
 Tim Dpndnt Two Stat Problm Coupld Pndulums Wak spring Two normal mods. No friction. No air rsistanc. Prfct Spring Start Swinging Som tim latr - swings with full amplitud. stationary M +n L M +m Elctron
Tim Dpndnt Two Stat Problm Coupld Pndulums Wak spring Two normal mods. No friction. No air rsistanc. Prfct Spring Start Swinging Som tim latr - swings with full amplitud. stationary M +n L M +m Elctron
Chapter 7b Electron Spin and Spin- Orbit Coupling
 Wintr 3 Chm 356: Introductory Quantum Mchanics Chaptr 7b Elctron Spin and Spin- Orbit Coupling... 96 H- atom in a Magntic Fild: Elctron Spin... 96 Total Angular Momntum... 3 Chaptr 7b Elctron Spin and
Wintr 3 Chm 356: Introductory Quantum Mchanics Chaptr 7b Elctron Spin and Spin- Orbit Coupling... 96 H- atom in a Magntic Fild: Elctron Spin... 96 Total Angular Momntum... 3 Chaptr 7b Elctron Spin and
Atomic Physics. Final Mon. May 12, 12:25-2:25, Ingraham B10 Get prepared for the Final!
 # SCORES 50 40 30 0 10 MTE 3 Rsults P08 Exam 3 0 30 40 50 60 70 80 90 100 SCORE Avrag 79.75/100 std 1.30/100 A 19.9% AB 0.8% B 6.3% BC 17.4% C 13.1% D.1% F 0.4% Final Mon. Ma 1, 1:5-:5, Ingraam B10 Gt
# SCORES 50 40 30 0 10 MTE 3 Rsults P08 Exam 3 0 30 40 50 60 70 80 90 100 SCORE Avrag 79.75/100 std 1.30/100 A 19.9% AB 0.8% B 6.3% BC 17.4% C 13.1% D.1% F 0.4% Final Mon. Ma 1, 1:5-:5, Ingraam B10 Gt
Molecular Orbitals in Inorganic Chemistry
 Outlin olcular Orbitals in Inorganic Chmistry Dr. P. Hunt p.hunt@imprial.ac.uk Rm 167 (Chmistry) http://www.ch.ic.ac.uk/hunt/ octahdral complxs forming th O diagram for Oh colour, slction ruls Δoct, spctrochmical
Outlin olcular Orbitals in Inorganic Chmistry Dr. P. Hunt p.hunt@imprial.ac.uk Rm 167 (Chmistry) http://www.ch.ic.ac.uk/hunt/ octahdral complxs forming th O diagram for Oh colour, slction ruls Δoct, spctrochmical
Physics 312 First Pledged Problem Set
 Physics 31 First Pldgd Problm St 1. Th ground stat of hydrogn is dscribd by th wavfunction whr a is th Bohr radius. (a) Comput th charg dnsity à (r) = 1 p ¼ µ 1 a 3 r=a ; ½ (r) = jã (r)j : and plot 4¼r
Physics 31 First Pldgd Problm St 1. Th ground stat of hydrogn is dscribd by th wavfunction whr a is th Bohr radius. (a) Comput th charg dnsity à (r) = 1 p ¼ µ 1 a 3 r=a ; ½ (r) = jã (r)j : and plot 4¼r
26-Sep-16. Nuclear energy production. Nuclear energy production. Nuclear energy production. Nuclear energy production
 Aim: valuat nrgy-gnration rat pr unit mass. Sun: (chck L /M, human ) nrgy-gnration rat producd from fusion of two nucli a + A: nrgy rlasd pr raction raction rat pr unit volum (includs cross sction and
Aim: valuat nrgy-gnration rat pr unit mass. Sun: (chck L /M, human ) nrgy-gnration rat producd from fusion of two nucli a + A: nrgy rlasd pr raction raction rat pr unit volum (includs cross sction and
How many neutrons does this aluminium atom contain? A 13 B 14 C 27 D 40
 alumiium atom has a uclo umbr of 7 ad a roto umbr of 3. How may utros dos this alumiium atom cotai? 3 4 7 40 atom of lmt Q cotais 9 lctros, 9 rotos ad 0 utros. What is Q? calcium otassium strotium yttrium
alumiium atom has a uclo umbr of 7 ad a roto umbr of 3. How may utros dos this alumiium atom cotai? 3 4 7 40 atom of lmt Q cotais 9 lctros, 9 rotos ad 0 utros. What is Q? calcium otassium strotium yttrium
Basics about radiative transfer
 aic about radiativ tranfr runo Carli Day Lctur aic about radiativ tranfr - runo Carli Tabl of Contnt Th radiativ tranfr quation. Th radiativ tranfr quation in a impl ca Analytical olution of th intgral
aic about radiativ tranfr runo Carli Day Lctur aic about radiativ tranfr - runo Carli Tabl of Contnt Th radiativ tranfr quation. Th radiativ tranfr quation in a impl ca Analytical olution of th intgral
Physics 2D Lecture Slides. Oct 21. UCSD Physics. Vivek Sharma
 Physics D Lctur Slids Oct 1 Vivk Sharma UCSD Physics Modrn Viw of Photolctric Effct E = hf = KE+ ϕ Is h sam in Photolctric Effct as in BBQ Radiation? Slop h = 6.66 x 10-34 JS Einstin Nobl Priz! No mattr
Physics D Lctur Slids Oct 1 Vivk Sharma UCSD Physics Modrn Viw of Photolctric Effct E = hf = KE+ ϕ Is h sam in Photolctric Effct as in BBQ Radiation? Slop h = 6.66 x 10-34 JS Einstin Nobl Priz! No mattr
Today. Wave-Matter Duality. Quantum Non-Locality. What is waving for matter waves?
 Today Wav-Mattr Duality HW 7 and Exam 2 du Thurs. 8pm 0 min rcap from last lctur on QM Finish QM odds and nds from ch.4 Th Standard Modl 4 forcs of Natur Fundamntal particls of Natur Fynman diagrams EM
Today Wav-Mattr Duality HW 7 and Exam 2 du Thurs. 8pm 0 min rcap from last lctur on QM Finish QM odds and nds from ch.4 Th Standard Modl 4 forcs of Natur Fundamntal particls of Natur Fynman diagrams EM
