the electrons. Expanding the exponential and neglecting the constant term Ze 2 λ, we have
|
|
|
- Jordan Warren
- 5 years ago
- Views:
Transcription
1 LECTURE.8 Prof.R.Parthasarathy Atomic Structur - Elmntary Tratmnt Th ground stat of hydrogn atom has bn solvd xactly in th nonrlativistic tratmnt. Th ground stat of hlium atom has bn handld in variational schm. What about havir atoms? Hr w assum that ach of th atomic lctrons movs in a sphrically symmtric potntial V (r) producd by th nuclus and all th othr lctrons. This is cntral fild approximation. W hav sn in hydrogn-lik atom, th potntial Z r producd infinit numbr of nrgy lvls. W xpct similar situation for V (r) as wll. Howvr, thr is going to b a diffrnc! W hav sn in hydrogn-lik atoms, th nrgy is proportional to n and indpndnt of l, so that for givn n, th stats having diffrnt l s ar dgnrat. For xampl, th stats m and 00 hav th sam nrgy. This dgnracy is rmovd in a non-coulomb cntral fild. How? Whn many lctrons ar prsnt, th nuclus is scrnd by lctrons. W want to show that for givn n, th stats of lowr l hav lowr nrgy. Lt us introduc th scrning by th scrnd Coulomb potntial V (r) = Z λr, whr λ is a positiv r constant. Thn, H = p Z λr. This is th hamiltonian flt by on of m r th lctrons. Expanding th xponntial and nglcting th constant trm Z λ, w hav whr H = p m Z r Z λ r + 6 Z λ 3 r = H 0 + H + H +, () H = Z λ r ; H = 6 Z λ 3 r. () In (), H 0 is th hamiltonian for hydrogn-lik atom whos Schrödingr quation has bn solvd xactly. Th nrgy ignvalus ar E n = Z a 0 and n th ignfunctions ar ψ nlm (r, θ, φ). For small λ, th trm H is rlvant and can b considrd as a prturbation. Th first ordr corrction to th nrgy is < H > nlm. For a givn
2 n, this prturbation cannot connct diffrnt l stats. So non-dgnrat prturbation thory is a good approximation! W hav calculatd th xpctation valu of r in our tratmnt of hydrogn atom. So, From (3), w gt < H > nlm = Z λ < r > nlm, = λ a 0 n ( 3 l(l + ) ). (3) n E 00 = E 0 3 λ a 0, E m = E 0.5 λ a 0, (4) whr E 0 is th unprturbd nrgy in n = stat Z 8a 0. Clarly th 00 stat has lowr nrgy that m stat. Thus, th first ordr corrction not only rmovs th l dgnracy but also givs th rsult that lowr angular momntum stats hav lowr nrgy. Idntical Particls W hav sn th quantum mchanics of a singl particl in a potntial. Whn w hav mor than on particl, say lctron, bcaus of th absns of dtrminism in quantum mchanics, it is impossibl to labl th lctrons. Thy ar indistinguishabl, whn both th lctrons ar having sam quantum numbrs. So, physical obsrvation must b invariant undr thir xchang. As an xampl, considr hlium atom. Thr ar two lctrons. Lt us nglct thir mutual intraction. What is th ground stat? Lt us dnot th lowst singl particl stat by s >. Thn th amplitud for finding lctron (labl it tmporarily!) and in this stat is < s > and < s >. Knowing th lctron has spin, w hav < s + >, < s > ; < s + >, < s >. Thn th possibl two-particl amplituds ar: < s + >< s + >, < s + >< s >, < s >< s + >, < s >< s >,(5) or thir linar combinations. Exprimntally, only on linar combination appars! i.., < s + >< s > < s >< s + > (6)
3 which changs sign whn. This is a rsult of th xclusion principl of Pauli- th amplitud for a systm containing svral lctrons must b antisymmtric undr th xchang of any two lctrons. Equivalntly, no two lctrons ar in th sam singl particl stat. For if any two lctrons ar in th sam stat, th totally antisymmtric amplitud vanishs. Givn no two lctrons can b in th sam singl particl stat, can w gt th antisymmtry? Lt us procd furthr to a gnral situation. Lt Φ I = < a >< b >: Φ II = < b >< a >. (7) Sinc and ar indistigushabl, w hav two linar combinations; Φ S = Φ I + Φ II, Bosons, Φ AS = Φ I Φ II. F rmions. (8) Considr a systm of two idntical particls and govrnd by a hamiltonian H(). Lt thr b a stationary stat I >, which can b charactrizd by lablling by an indx α and by β, whr α, β rfr to position, spin projction tc. Thn I >= α β > will b in th Hilbrt spac H, = H H. Sinc and ar idntical, th stat II >= α, β > will also b a stationary stat with sam nrgy as I > sinc H() = H(). Introducing P th prmutation oprator, P I >= II >, P II >= I >, [P, H] = 0. (9) So P and H hav simulatanous ignfunctions. Lt P ψ >= η ψ >. Thn P P ψ >= η ψ >= ψ > and so η = ±. Thn, th cas, P ψ >= ψ > corrsponds to symmtric stat rprsnting Bosons whil th cas with P ψ >= ψ > corrsponds to antisymmtric stat rprsnting Frmions. For a two lctron systm, th totally antisymmtric wav function will b α β > α β > = ψ α ()ψ β () ψ α ()ψ β (), = ψ α() ψ α () ψ β () ψ β () For a thr lctron systm, th totally antisymmtric wav function is ψ α () ψ α () ψ α (3) ψ β () ψ β () ψ β (3) ψ γ () ψ γ () ψ γ (3) (0) () 3
4 Antisymmtry in () undr, 3, 3 is vidnt using th proprty of dtrminants! Symmtric wav functions will rprsnt Bosons, particls of intrinsic spin 0,, tc and oby Bos-Einstin statistics. Antisymmtric wavfunctions rprsnt Frmions, particls of intrinsic spin, 3, tc and oby Frmi-Dirac statistics. This connction spin-statistics thorm has bn vrifid. But th sourc or proof is not wll undrstood. Th abov wav function is not normalizd. A normalizd antisymmtric wav function is givn by Slatr dtrminant. For th thr-frmion systm, ψ αβγ (,, 3) = ψ α () ψ α () ψ α (3) ψ β () ψ β () ψ β (3) 3! ψ γ () ψ γ () ψ γ (3) () Is this procdur always corrct? No. Whn th lctrons ar non-intracting, it is a corrct mthod. Whn th intraction among thm is wak, th abov stat can b usd for prturbativ calculations. Ground Stat Configurations Th quantum numbrs ar: n =,, 3, principal quantum numbr l = 0,,, (n ), orbital ang.momntum qtm numbr m = l to + l in stps of, z componnt of ang.mom qtm numbr s =, m s =,, Hydrogn Z =. Ground stat: n =, l = 0, m = 0, m s = ± Th singl lctron configuration is (s). (notation: (nl) occupants. Th chmical valncy is just th numbr of lctrons in th last shll which hr is incomplt and so th valncy is. Hlium 4
5 Z =. Th two lctrons occupy n =, l = 0 shll and hav m s = ± so as to rspct Pauli principl. Th lctronic configuration is (s). With two lctrons in s shll, it is compltly occupid. Th valncy is zro and this lmnt is chmically inrt. Hlium is an inrt gas. Lithium Z = 3. First th shll s is compltly filld by two of th thr lctrons. Th third lctron can go to n =, l = 0 with m s = or. Th lctronic configuration is (s) (s). Th valncy is du to th lctron in th s shll and so it is. This lmnt is chmically activ. Brylliium Z = 4. Th lctronic configuration is (s) (s). It has a closd sub-shll sinc th p stat is mpty. Th normal valncy is. W giv th lctronic configurations of som light lmnts. Elmnt(Z) ElctronicConf iguration Hydrogn() (s) Hlium() (s) Lithium(3) (s) (s) Bryllium(4) (s) (s) Boron(5) (s) (s) (p) Carbon(6) (s) (s) (p) Nitrogn(7) (s) (s) (p) 3 Oxygn(8) (s) (s) (p) 4 F luorin(9) (s) (s) (p) 5 Non(0) (s) (s) (p) 6 Sodium() (s) (s) (p) 6 (3s) Magnsium() (s) (s) (p) 6 (3s) Aluminum(3) (s) (s) (p) 6 (3s) (3p) Silicon(4) (s) (s) (p) 6 (3s)(3p) P hosphrous(5) (s) (s) (p) 6 (3s) (3p) 3 Sulphur(6) (s) (s) (p) 6 (3s) (3p) 4 Chlorin(7) (s) (s) (p) 6 (3s) (3p) 5 Argon(8) (s) (s) (p) 6 (3s) (3p) 8. 5
6 Upto Z = 8, th filling up of th nrgy lvls with th undrstanding that for a givn n, lowr l lvls ar filld first works. Th rmoval of th l dgnrcay bing xplaind by th scrning of th nuclar charg by th rmaining lctrons. This squnc changs now! Aftr Argon, w hav Potassium Z = 9. Th nintnth lctron should go to th 3d stat. But it gos to 4s stat! Aftr filling th 4s subshll, 3d starts gtting lctrons. For xampl, th lctronic configuration of potassium (K,Z=9) is (Ar) + (4s), that of Calcium (Ca, Z=0) is (Ar) + (4s), that of Scandium (Sc, Z=) is (Ar) + (4s) (3d) and so on till, for Zinc (Zn, Z=30), it is (Ar) + (4s) (3d) 0. Now that th 3d subshll is compltly filld, th nxt lctron in Galium gos to 4p stat, namly, Ga(Z=3) has (Ar) + (4s) (3d) 0 (4p). Can w undrstand this qualitativly? From th first ordr corrction (3), it follows E 30 = Z E 400 = Z 8a 0 5.5λ a 0, 3a 0 λ a 0. (3) If th scrning is strong, thn it is plausibl to undrstand that th 4s lvl has lowr nrgy than 3d lvl. Alkali atoms Th alkali atoms ar Li, Na, K tc. From th lctronic configuration tabl, w not that th ground stat configuration of an alkali atom consists of filld shlls followd by a singl s lctron. Th filld shll configuration corrsponds to inrt gas configuration and so stabl. Thn, th xcitd stats of th alkali atom involv only th valnc s lctron. Thus, th alkali atom can b tratd to a good approximation in trms of a modl in which a singl lctron movs in a sphrically symmtric non-coulomb potntial nrgy V (r), non-coulomb du to scrning. In th absns of xtrnal filds, th hamiltonian could b + V (r). To this w add th spin-orbit intraction. m First of all w considr hydrogn atom. Th nrgy lvls of hydrogn atom obtaind using non-rlativistic Schrödingr quation dpnd on th principal quantum numbr n and thr is dgnracy for l. Classically, a particl of mass m, charg moving with vlocity v in an lctric fild E 6
7 xprincs a magntic fild B E v = and if th lctric fild E is drivabl c from a potntial V (r), E = r dv so that r dr dv dr B = c r = c rm r v, dv dr l, whr l = r p. Th nrgy of th intraction btwn B and a magntic dipol m is m B so that H s.o = m B = dv m l. For an lctron, mc r dr m = s so that m H s.o = m c r A corrct calculation (Dirac) lads to dv dr l s. H s.o = m c r For an lctron in hydrogn-lik atom, V = Z r H s.o = dv dr l s. (4) and so Z m c r 3 l s. (5) W know from hydrogn atom wav functions, < nlm r nlm > = Z 3 3 n 3 a 3 0l(l + (6) )(l + ). From th tim-indpndnt prturbation thory, th first ordr corrction to th nrgy lvls of hydrogn-lik atom du to th spin-orbit trm is < nlm H s.o nlm > = Z 4 m c a 3 0l(l + )(l + ) < l s >. (7) Th xprssion < l s > can b asily valuatd in th coupld basis sinc l s = {j l s }. 7
8 Thus th first ordr corrction is E s.o = Z 4 m c a 3 0n 3 l(l + )(l + ) h {j(j + ) l(l + ) s(s + )}. (8) Th abov rsult can b xprssd also as E s.o = Z E 0 n α n(l + ) l+ for j = l + l for j = l (9) whr E 0 n is th unprturbd nrgy. This can b asily gnralizd to alkali atoms. With this, lt us valuat th nrgis for 3p 3 and 3p. so that E 3p 3 E 3p = E3 0 + Z α E3 0 8, = E3 0 + Z α E3 0 9, (0) E 3p 3 E 3p = Z E 0 3 α 6. () Lt us considr sodium atom. Exprimntally, thr ar two dominant yllow doublt. Thir wavlngths ar: 5890Å and 5896Å. Thy corrspond to th atomic transitions 3p 3 3s and 3p 3s. For Coulomb potntials, such transitions cannot occur as th nrgy dpnds only on n. For non-coulombic potntials, such transitions can occur. As th non-coulombic potntial is not known (at this lvl), w cannot calculat th abov transition nrgis. Howvr, w adopt a modl: Th spctrum of sodium atom is dtrmind by th valnc lctron in 3s stat. Th closd shll configuration upto 0 lctrons corrsponds to Argon th inrt gas. Hr th nuclar charg (+ ) is compltly shildd by th 0 atomic lctrons, so that th valnc lctron ss th nuclar charg of on unit. In this modl, th diffrnc of th nrgis of 3p 3 and 3p is givn by (6) with E0 3 bing 8
9 simply 3.6 V. Using th valu of th fin structur constant α as 9 Z =, this nrgy diffrnc (6) bcoms, E 3p 3 E 3p 37 and = E = V. () For th transition E 3p 3 E 3s, th wavlngth of th radiation is λ = hc hc and for th othr transition, λ E 3p 3 E 3s =. Hnc, E 3p E 3s λ = λ λ E hc. (3) Using th valus of h = J.s and c = cm/s, w find, λ = 4.54Å whil th xprimntal valu is 6Å. Th simpl modl is abl to xplain th sodium doublt. It is possibl to calculat th intnsitis of ths lins using tim-dpndnt prturbation thory. Doublt of Sodium Yllow Lins 5890Å 5896Å Zman Effct W ar considring an atom with on valnc lctron placd in a magntic fild. Th rsult is that th nrgy lvls ar split into svral componnts, Zman splitting. Th intraction nrgy rsponsibl for ths splittings consists of two parts: () du to th spin of th lctron and () du to th orbital motion. Th lctron spin angular momntum is h and has projctions ± h. So th ltron has a componnt of magntic momnt h in th dirction in mc which th componnt of spin angular momntum is ± h. As th nrgy 9
10 of a particl of magntic momnt m in a fild H (xtrnal) is m H, th intraction nrgy of th lctron is mc s H = mc H s. (4) (In 95 Uhlnbck and Goudsmit (Natur 7 (96) 64) discovrd lctron possssing an intrinsic angular momntum whos componnt is rstrictd to ± h. Pauli in 97, dscribd it by his matrics!) Th ffct of xtrnal magntic fild on th orbital motion of th lctron is found now. Th hamiltonian for a particl of charg in th.m vctor potntial A ( H = A) is H = m ( p + c A) = m p + In hr, p, A ar oprators. Thn, ( p A)ψ = i h ( Aψ), mc ( p A + A p) + = i h( A)ψ i h A ( ψ), mc A. (5) = ( A p)ψ, (6) whr th gaug A = 0 (Coulomb gaug) is usd in th pnultimat stp. So th nrgy du to th magntic fild is A p + A. For a mc mc uniform magntic fild, w know A = ( H r). Show this! Thn, w s A = ( H r ( H r) ). W first nglct A trm. Thn, th intraction 4 can b writtn as: mc A p = = = = = mc ( H r) p, mc ɛ ijk H j r k p i, mc H j ɛ jki r k p i, H mc ( r p), H mc L, L = r p. (7) 0
11 Combining with th spin contribution, th total ffct of th xtrnal magntic fild on th atomic lctron is H = = = H mc s + H mc L, H mc ( L + s), H mc ( J + S), J = L + s. (8) If th applid magntic fild is along th z-axis, thn th intraction is mc H(J z + s z ). In (8), w hav combind th orbital and spin angular momnta. So, th wav functions should b appropriatly constructd. From th angular momntum wav functions lm > and spin wav functions m s > w hav th following: jm > = m s C(l j; mm sm) lm > m s >, This for j = l +, bcoms = C(l j; m m + ) lm > > + C(l j; m,, m ) lm > >. (9) l + l + M + M > = l + l M + + l + lm >, > lm + >, >, (30) and for j = l l l M + M > = l + l + M + + l + Now it is asy to find < l + H, M mc (J z + s z ) l +, M > = hh mc l, M > > l, M + >, >. (3) M(l + + ), l +
12 < l H, M mc (J z + s z ) l, M > = hh mc < l ± H, M mc (J z + s z ) l ±, M > = hh mc M(l + ), so that l + M(l ± + ) δ MM.(3) l + Th factor l±+ l+ is known as Landé g-factor. Wak Fild Cas Whn th xtrnal magntic fild is wak, th spin-orbit intractrion cannot b nglctd. This intraction ncssitats th j-j coupling. In this stat, w hav valuatd th matrix lmnts of th magntic intraction in (3). To this w add th spin-orbit coupling. Dnoting th radial matrix lmnt as ζ nl, w hav this as ζ nl{j(j + ) l(l + ) s(s + )}. Strong Fild Cas Whn th xtrnal magntic fild is strong, th spin-orbit intraction is small and in this cas, w us (n, l, m l, m s ) functions (without angular momntum coupling). Thn, < nlm l m s H mc (L z + s z ) nlm l m s > = hh mc (m l + m s ) = hh mc (M + m s). (33) Each lvl is split symmtrically into l + 3, as m l + m s taks l +, l, (l + ) which totals to l + 3. This cas is known as Paschn-Back ffct. W now includ th spin-orbit intraction. Th stats ar lablld by n, l, m l, m s. Th diagonal matrix lmnt < nlm l m s ξ l s nlm l m s > cannot connct x, y componnts of angular momnta and so this is ζ nl m l m s. Transition Cas W will us th fundamntal systm of ign functions lablld by nlm l m s following E.U.Condon and G.H.Shortly, Th Thory of Atomic Spctra. In this schm, th matrix of ɛ(l z + s z ) is diagonal, whr ɛ = H. W shall mc considr th matrix for a givn nl, say p and s prtaining to alkali atom sodium. W shall dnot th radial intgral of ξ by ζ.
13 s Stats: Th ign functions ar Y0 0 χ ; Y 0 0χ. Th matrix lmnt of l s can b omittd sinc it dos not shift thm with rspct to ach othr. Th shift du to th magntic intraction ɛ(l z + s z ) is ±ɛ h for m l = 0; m s = ±. p Stats: Th ign functions Y χ ; Y additional nrgis ar χ hav m = ± 3. For thm th ζ {j(j + ) l(l + ) s(s + )} + ɛ < l z + s z >, (34) which for j = 3 and m = ± 3 givs ζ ± ɛ h., Y 0 χ combin in pairs accord- Th wav functions Y 0 χ, Y χ, Y ing to m l + m s = m = ±. Ths ar: {Y 0 χ, Y χ On such pair can b considrd. Lt χ ; Y χ, Y 0 ψ > = Y 0 χ, ψ > = Y χ χ }. (35), (36) corrsponding to m =. Now w us dgnrat prturbation thory, as ths stats ar dgnrat. W want to find th matrix lmnts of First, th asy on. It is sn that H = ξ(r) l s + ɛ(l z + s z ). (37) < ψ ɛ(l z + s z ) ψ > = ɛ h, < ψ ɛ(l z + s z ) ψ > = 0, < ψ ɛ(l z + s z ) ψ > = 0, < ψ ɛ(l z + s z ) ψ > = 0. (38) Nxt, w nd < ξ(r) l s > btwn ths stats. Writing l s = {l +s + l s + } + l z s z, (39) 3
14 and using w find l ± l, m l > = (l m l )(l ± m l + ) l, m l ± >, (40) < ψ ξ(r) l s ψ > = 0, < ψ ξ(r) l s ψ > = ζ h, < ψ ξ(r) l s ψ > = ζ h, < ψ ξ(r) l s ψ > = ζ h. (4) Thus in th spirit of dgnrat prturbation thory, w nd up with whos ignvalus ar M = ( ɛ h ζ h ζ h ζ h ), (4) λ = {ɛ h ζ h ± (ɛ h + ɛζ h ζ h 4 )}. (43) Hr w rcall that ζ rprsnts th spin-orbit trm contribution and ɛ th magntic prturbation. Thn, ζ h 0 corrsponds to th Strong Fild Cas. In this cas, you ɛ h can s th two ignvalus ar: Similarly, ɛ h ζ h λ + = ɛ h ; λ = ζ h. (44) 0 corrsponds to th Wak Fild Cas and thn in this cas, λ + = ζ h + 3 ɛ h ; λ = ζ h + ɛ h. (45) 3 W rcall in th Strong Fild Cas, w had th nrgy shift as ɛ h(m + m s ) + ζ h m l m s which for m =, m l = m m s, m s = ± givs: m s = ; ɛ h 4
15 and m s = ; ζ h agring with (44). In th Wak Fild Cas, w had th nrgy shift as: ɛ hm l±+ + l+ ζ h (j(j + ) l(l + ) s(s + )) which is ɛ hm l±+ + l+ ζ h {l or (l + )} for j = l + or j = l. This, for l = agrs with (45). 3 p 3 3 p s Wak Fild Zman splitting 5
16 p s 0 Strong Fild Paschn-Back splitting 6
Hydrogen Atom and One Electron Ions
 Hydrogn Atom and On Elctron Ions Th Schrödingr quation for this two-body problm starts out th sam as th gnral two-body Schrödingr quation. First w sparat out th motion of th cntr of mass. Th intrnal potntial
Hydrogn Atom and On Elctron Ions Th Schrödingr quation for this two-body problm starts out th sam as th gnral two-body Schrödingr quation. First w sparat out th motion of th cntr of mass. Th intrnal potntial
Classical Magnetic Dipole
 Lctur 18 1 Classical Magntic Dipol In gnral, a particl of mass m and charg q (not ncssarily a point charg), w hav q g L m whr g is calld th gyromagntic ratio, which accounts for th ffcts of non-point charg
Lctur 18 1 Classical Magntic Dipol In gnral, a particl of mass m and charg q (not ncssarily a point charg), w hav q g L m whr g is calld th gyromagntic ratio, which accounts for th ffcts of non-point charg
Schrodinger Equation in 3-d
 Schrodingr Equation in 3-d ψ( xyz,, ) ψ( xyz,, ) ψ( xyz,, ) + + + Vxyz (,, ) ψ( xyz,, ) = Eψ( xyz,, ) m x y z p p p x y + + z m m m + V = E p m + V = E E + k V = E Infinit Wll in 3-d V = x > L, y > L,
Schrodingr Equation in 3-d ψ( xyz,, ) ψ( xyz,, ) ψ( xyz,, ) + + + Vxyz (,, ) ψ( xyz,, ) = Eψ( xyz,, ) m x y z p p p x y + + z m m m + V = E p m + V = E E + k V = E Infinit Wll in 3-d V = x > L, y > L,
Background: We have discussed the PIB, HO, and the energy of the RR model. In this chapter, the H-atom, and atomic orbitals.
 Chaptr 7 Th Hydrogn Atom Background: W hav discussd th PIB HO and th nrgy of th RR modl. In this chaptr th H-atom and atomic orbitals. * A singl particl moving undr a cntral forc adoptd from Scott Kirby
Chaptr 7 Th Hydrogn Atom Background: W hav discussd th PIB HO and th nrgy of th RR modl. In this chaptr th H-atom and atomic orbitals. * A singl particl moving undr a cntral forc adoptd from Scott Kirby
Addition of angular momentum
 Addition of angular momntum April, 0 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat th
Addition of angular momntum April, 0 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat th
The van der Waals interaction 1 D. E. Soper 2 University of Oregon 20 April 2012
 Th van dr Waals intraction D. E. Sopr 2 Univrsity of Orgon 20 pril 202 Th van dr Waals intraction is discussd in Chaptr 5 of J. J. Sakurai, Modrn Quantum Mchanics. Hr I tak a look at it in a littl mor
Th van dr Waals intraction D. E. Sopr 2 Univrsity of Orgon 20 pril 202 Th van dr Waals intraction is discussd in Chaptr 5 of J. J. Sakurai, Modrn Quantum Mchanics. Hr I tak a look at it in a littl mor
Addition of angular momentum
 Addition of angular momntum April, 07 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat
Addition of angular momntum April, 07 Oftn w nd to combin diffrnt sourcs of angular momntum to charactriz th total angular momntum of a systm, or to divid th total angular momntum into parts to valuat
Chapter 8: Electron Configurations and Periodicity
 Elctron Spin & th Pauli Exclusion Principl Chaptr 8: Elctron Configurations and Priodicity 3 quantum numbrs (n, l, ml) dfin th nrgy, siz, shap, and spatial orintation of ach atomic orbital. To xplain how
Elctron Spin & th Pauli Exclusion Principl Chaptr 8: Elctron Configurations and Priodicity 3 quantum numbrs (n, l, ml) dfin th nrgy, siz, shap, and spatial orintation of ach atomic orbital. To xplain how
Chapter 7b Electron Spin and Spin- Orbit Coupling
 Wintr 3 Chm 356: Introductory Quantum Mchanics Chaptr 7b Elctron Spin and Spin- Orbit Coupling... 96 H- atom in a Magntic Fild: Elctron Spin... 96 Total Angular Momntum... 3 Chaptr 7b Elctron Spin and
Wintr 3 Chm 356: Introductory Quantum Mchanics Chaptr 7b Elctron Spin and Spin- Orbit Coupling... 96 H- atom in a Magntic Fild: Elctron Spin... 96 Total Angular Momntum... 3 Chaptr 7b Elctron Spin and
Intro to Nuclear and Particle Physics (5110)
 Intro to Nuclar and Particl Physics (5110) March 09, 009 Frmi s Thory of Bta Dcay (continud) Parity Violation, Nutrino Mass 3/9/009 1 Final Stat Phas Spac (Rviw) Th Final Stat lctron and nutrino wav functions
Intro to Nuclar and Particl Physics (5110) March 09, 009 Frmi s Thory of Bta Dcay (continud) Parity Violation, Nutrino Mass 3/9/009 1 Final Stat Phas Spac (Rviw) Th Final Stat lctron and nutrino wav functions
Derivation of Electron-Electron Interaction Terms in the Multi-Electron Hamiltonian
 Drivation of Elctron-Elctron Intraction Trms in th Multi-Elctron Hamiltonian Erica Smith Octobr 1, 010 1 Introduction Th Hamiltonian for a multi-lctron atom with n lctrons is drivd by Itoh (1965) by accounting
Drivation of Elctron-Elctron Intraction Trms in th Multi-Elctron Hamiltonian Erica Smith Octobr 1, 010 1 Introduction Th Hamiltonian for a multi-lctron atom with n lctrons is drivd by Itoh (1965) by accounting
Lecture 28 Title: Diatomic Molecule : Vibrational and Rotational spectra
 Lctur 8 Titl: Diatomic Molcul : Vibrational and otational spctra Pag- In this lctur w will undrstand th molcular vibrational and rotational spctra of diatomic molcul W will start with th Hamiltonian for
Lctur 8 Titl: Diatomic Molcul : Vibrational and otational spctra Pag- In this lctur w will undrstand th molcular vibrational and rotational spctra of diatomic molcul W will start with th Hamiltonian for
On the Hamiltonian of a Multi-Electron Atom
 On th Hamiltonian of a Multi-Elctron Atom Austn Gronr Drxl Univrsity Philadlphia, PA Octobr 29, 2010 1 Introduction In this papr, w will xhibit th procss of achiving th Hamiltonian for an lctron gas. Making
On th Hamiltonian of a Multi-Elctron Atom Austn Gronr Drxl Univrsity Philadlphia, PA Octobr 29, 2010 1 Introduction In this papr, w will xhibit th procss of achiving th Hamiltonian for an lctron gas. Making
Title: Vibrational structure of electronic transition
 Titl: Vibrational structur of lctronic transition Pag- Th band spctrum sn in th Ultra-Violt (UV) and visibl (VIS) rgions of th lctromagntic spctrum can not intrprtd as vibrational and rotational spctrum
Titl: Vibrational structur of lctronic transition Pag- Th band spctrum sn in th Ultra-Violt (UV) and visibl (VIS) rgions of th lctromagntic spctrum can not intrprtd as vibrational and rotational spctrum
Contemporary, atomic, nuclear, and particle physics
 Contmporary, atomic, nuclar, and particl physics 1 Blackbody radiation as a thrmal quilibrium condition (in vacuum this is th only hat loss) Exampl-1 black plan surfac at a constant high tmpratur T h is
Contmporary, atomic, nuclar, and particl physics 1 Blackbody radiation as a thrmal quilibrium condition (in vacuum this is th only hat loss) Exampl-1 black plan surfac at a constant high tmpratur T h is
Coupled Pendulums. Two normal modes.
 Tim Dpndnt Two Stat Problm Coupld Pndulums Wak spring Two normal mods. No friction. No air rsistanc. Prfct Spring Start Swinging Som tim latr - swings with full amplitud. stationary M +n L M +m Elctron
Tim Dpndnt Two Stat Problm Coupld Pndulums Wak spring Two normal mods. No friction. No air rsistanc. Prfct Spring Start Swinging Som tim latr - swings with full amplitud. stationary M +n L M +m Elctron
22/ Breakdown of the Born-Oppenheimer approximation. Selection rules for rotational-vibrational transitions. P, R branches.
 Subjct Chmistry Papr No and Titl Modul No and Titl Modul Tag 8/ Physical Spctroscopy / Brakdown of th Born-Oppnhimr approximation. Slction ruls for rotational-vibrational transitions. P, R branchs. CHE_P8_M
Subjct Chmistry Papr No and Titl Modul No and Titl Modul Tag 8/ Physical Spctroscopy / Brakdown of th Born-Oppnhimr approximation. Slction ruls for rotational-vibrational transitions. P, R branchs. CHE_P8_M
PH300 Modern Physics SP11 Final Essay. Up Next: Periodic Table Molecular Bonding
 PH Modrn Physics SP11 Final Essay Thr will b an ssay portion on th xam, but you don t nd to answr thos qustions if you submit a final ssay by th day of th final: Sat. 5/7 It dosnʼt mattr how smart you
PH Modrn Physics SP11 Final Essay Thr will b an ssay portion on th xam, but you don t nd to answr thos qustions if you submit a final ssay by th day of th final: Sat. 5/7 It dosnʼt mattr how smart you
Constants and Conversions:
 EXAM INFORMATION Radial Distribution Function: P 2 ( r) RDF( r) Br R( r ) 2, B is th normalization constant. Ordr of Orbital Enrgis: Homonuclar Diatomic Molculs * * * * g1s u1s g 2s u 2s u 2 p g 2 p g
EXAM INFORMATION Radial Distribution Function: P 2 ( r) RDF( r) Br R( r ) 2, B is th normalization constant. Ordr of Orbital Enrgis: Homonuclar Diatomic Molculs * * * * g1s u1s g 2s u 2s u 2 p g 2 p g
Now that we've developed our approximation methods, we can turn to solving the. 2m 4 r - 2e 4 r + e 2 0) 1
 11 Lctur 18 Now that w'v dvlopd our approximation mthods, w can turn to solving th hlium atom. As usual our Schrödingr quation is H ψ = E ψ, whr H = - ( + )- m 4 r - 4 r + 1 πε πε 4πε r 1 Lt s bgin by
11 Lctur 18 Now that w'v dvlopd our approximation mthods, w can turn to solving th hlium atom. As usual our Schrödingr quation is H ψ = E ψ, whr H = - ( + )- m 4 r - 4 r + 1 πε πε 4πε r 1 Lt s bgin by
Exam 1. It is important that you clearly show your work and mark the final answer clearly, closed book, closed notes, no calculator.
 Exam N a m : _ S O L U T I O N P U I D : I n s t r u c t i o n s : It is important that you clarly show your work and mark th final answr clarly, closd book, closd nots, no calculator. T i m : h o u r
Exam N a m : _ S O L U T I O N P U I D : I n s t r u c t i o n s : It is important that you clarly show your work and mark th final answr clarly, closd book, closd nots, no calculator. T i m : h o u r
Quasi-Classical States of the Simple Harmonic Oscillator
 Quasi-Classical Stats of th Simpl Harmonic Oscillator (Draft Vrsion) Introduction: Why Look for Eignstats of th Annihilation Oprator? Excpt for th ground stat, th corrspondnc btwn th quantum nrgy ignstats
Quasi-Classical Stats of th Simpl Harmonic Oscillator (Draft Vrsion) Introduction: Why Look for Eignstats of th Annihilation Oprator? Excpt for th ground stat, th corrspondnc btwn th quantum nrgy ignstats
PHYSICS 489/1489 LECTURE 7: QUANTUM ELECTRODYNAMICS
 PHYSICS 489/489 LECTURE 7: QUANTUM ELECTRODYNAMICS REMINDER Problm st du today 700 in Box F TODAY: W invstigatd th Dirac quation it dscribs a rlativistic spin /2 particl implis th xistnc of antiparticl
PHYSICS 489/489 LECTURE 7: QUANTUM ELECTRODYNAMICS REMINDER Problm st du today 700 in Box F TODAY: W invstigatd th Dirac quation it dscribs a rlativistic spin /2 particl implis th xistnc of antiparticl
September 23, Honors Chem Atomic structure.notebook. Atomic Structure
 Atomic Structur Topics covrd Atomic structur Subatomic particls Atomic numbr Mass numbr Charg Cations Anions Isotops Avrag atomic mass Practic qustions atomic structur Sp 27 8:16 PM 1 Powr Standards/ Larning
Atomic Structur Topics covrd Atomic structur Subatomic particls Atomic numbr Mass numbr Charg Cations Anions Isotops Avrag atomic mass Practic qustions atomic structur Sp 27 8:16 PM 1 Powr Standards/ Larning
As the matrix of operator B is Hermitian so its eigenvalues must be real. It only remains to diagonalize the minor M 11 of matrix B.
 7636S ADVANCED QUANTUM MECHANICS Solutions Spring. Considr a thr dimnsional kt spac. If a crtain st of orthonormal kts, say, and 3 ar usd as th bas kts, thn th oprators A and B ar rprsntd by a b A a and
7636S ADVANCED QUANTUM MECHANICS Solutions Spring. Considr a thr dimnsional kt spac. If a crtain st of orthonormal kts, say, and 3 ar usd as th bas kts, thn th oprators A and B ar rprsntd by a b A a and
Forces. Quantum ElectroDynamics. α = = We have now:
 W hav now: Forcs Considrd th gnral proprtis of forcs mdiatd by xchang (Yukawa potntial); Examind consrvation laws which ar obyd by (som) forcs. W will nxt look at thr forcs in mor dtail: Elctromagntic
W hav now: Forcs Considrd th gnral proprtis of forcs mdiatd by xchang (Yukawa potntial); Examind consrvation laws which ar obyd by (som) forcs. W will nxt look at thr forcs in mor dtail: Elctromagntic
ELECTRON-MUON SCATTERING
 ELECTRON-MUON SCATTERING ABSTRACT Th lctron charg is considrd to b distributd or xtndd in spac. Th diffrntial of th lctron charg is st qual to a function of lctron charg coordinats multiplid by a four-dimnsional
ELECTRON-MUON SCATTERING ABSTRACT Th lctron charg is considrd to b distributd or xtndd in spac. Th diffrntial of th lctron charg is st qual to a function of lctron charg coordinats multiplid by a four-dimnsional
Einstein Equations for Tetrad Fields
 Apiron, Vol 13, No, Octobr 006 6 Einstin Equations for Ttrad Filds Ali Rıza ŞAHİN, R T L Istanbul (Turky) Evry mtric tnsor can b xprssd by th innr product of ttrad filds W prov that Einstin quations for
Apiron, Vol 13, No, Octobr 006 6 Einstin Equations for Ttrad Filds Ali Rıza ŞAHİN, R T L Istanbul (Turky) Evry mtric tnsor can b xprssd by th innr product of ttrad filds W prov that Einstin quations for
Brief Introduction to Statistical Mechanics
 Brif Introduction to Statistical Mchanics. Purpos: Ths nots ar intndd to provid a vry quick introduction to Statistical Mchanics. Th fild is of cours far mor vast than could b containd in ths fw pags.
Brif Introduction to Statistical Mchanics. Purpos: Ths nots ar intndd to provid a vry quick introduction to Statistical Mchanics. Th fild is of cours far mor vast than could b containd in ths fw pags.
5.80 Small-Molecule Spectroscopy and Dynamics
 MIT OpnCoursWar http://ocw.mit.du 5.80 Small-Molcul Spctroscopy and Dynamics Fall 008 For information about citing ths matrials or our Trms of Us, visit: http://ocw.mit.du/trms. Lctur # 3 Supplmnt Contnts
MIT OpnCoursWar http://ocw.mit.du 5.80 Small-Molcul Spctroscopy and Dynamics Fall 008 For information about citing ths matrials or our Trms of Us, visit: http://ocw.mit.du/trms. Lctur # 3 Supplmnt Contnts
The Relativistic Stern-Gerlach Force C. Tschalär 1. Introduction
 Th Rlativistic Strn-Grlach Forc C. Tschalär. Introduction For ovr a dcad, various formulations of th Strn-Grlach (SG) forc acting on a particl with spin moving at a rlativistic vlocity in an lctromagntic
Th Rlativistic Strn-Grlach Forc C. Tschalär. Introduction For ovr a dcad, various formulations of th Strn-Grlach (SG) forc acting on a particl with spin moving at a rlativistic vlocity in an lctromagntic
Modern Physics. Unit 5: Schrödinger s Equation and the Hydrogen Atom Lecture 5.6: Energy Eigenvalues of Schrödinger s Equation for the Hydrogen Atom
 Mdrn Physics Unit 5: Schrödingr s Equatin and th Hydrgn Atm Lctur 5.6: Enrgy Eignvalus f Schrödingr s Equatin fr th Hydrgn Atm Rn Rifnbrgr Prfssr f Physics Purdu Univrsity 1 Th allwd nrgis E cm frm th
Mdrn Physics Unit 5: Schrödingr s Equatin and th Hydrgn Atm Lctur 5.6: Enrgy Eignvalus f Schrödingr s Equatin fr th Hydrgn Atm Rn Rifnbrgr Prfssr f Physics Purdu Univrsity 1 Th allwd nrgis E cm frm th
Numerical Problem Set for Atomic and Molecular Spectroscopy. Yr 2 HT SRM
 Numrical Problm St for Atomic and Molcular Spctroscopy Yr HT SRM Sction 1: Atomic Spctra 1. For ach of th atomic trm symbols 1 S, P, 3 P, 3 D, 4 D, writ down: a) Th associatd valus of th total spin and
Numrical Problm St for Atomic and Molcular Spctroscopy Yr HT SRM Sction 1: Atomic Spctra 1. For ach of th atomic trm symbols 1 S, P, 3 P, 3 D, 4 D, writ down: a) Th associatd valus of th total spin and
Elements of Statistical Thermodynamics
 24 Elmnts of Statistical Thrmodynamics Statistical thrmodynamics is a branch of knowldg that has its own postulats and tchniqus. W do not attmpt to giv hr vn an introduction to th fild. In this chaptr,
24 Elmnts of Statistical Thrmodynamics Statistical thrmodynamics is a branch of knowldg that has its own postulats and tchniqus. W do not attmpt to giv hr vn an introduction to th fild. In this chaptr,
A central nucleus. Protons have a positive charge Electrons have a negative charge
 Atomic Structur Lss than ninty yars ago scintists blivd that atoms wr tiny solid sphrs lik minut snookr balls. Sinc thn it has bn discovrd that atoms ar not compltly solid but hav innr and outr parts.
Atomic Structur Lss than ninty yars ago scintists blivd that atoms wr tiny solid sphrs lik minut snookr balls. Sinc thn it has bn discovrd that atoms ar not compltly solid but hav innr and outr parts.
2. Laser physics - basics
 . Lasr physics - basics Spontanous and stimulatd procsss Einstin A and B cofficints Rat quation analysis Gain saturation What is a lasr? LASER: Light Amplification by Stimulatd Emission of Radiation "light"
. Lasr physics - basics Spontanous and stimulatd procsss Einstin A and B cofficints Rat quation analysis Gain saturation What is a lasr? LASER: Light Amplification by Stimulatd Emission of Radiation "light"
A Propagating Wave Packet Group Velocity Dispersion
 Lctur 8 Phys 375 A Propagating Wav Packt Group Vlocity Disprsion Ovrviw and Motivation: In th last lctur w lookd at a localizd solution t) to th 1D fr-particl Schrödingr quation (SE) that corrsponds to
Lctur 8 Phys 375 A Propagating Wav Packt Group Vlocity Disprsion Ovrviw and Motivation: In th last lctur w lookd at a localizd solution t) to th 1D fr-particl Schrödingr quation (SE) that corrsponds to
Lecture 37 (Schrödinger Equation) Physics Spring 2018 Douglas Fields
 Lctur 37 (Schrödingr Equation) Physics 6-01 Spring 018 Douglas Filds Rducd Mass OK, so th Bohr modl of th atom givs nrgy lvls: E n 1 k m n 4 But, this has on problm it was dvlopd assuming th acclration
Lctur 37 (Schrödingr Equation) Physics 6-01 Spring 018 Douglas Filds Rducd Mass OK, so th Bohr modl of th atom givs nrgy lvls: E n 1 k m n 4 But, this has on problm it was dvlopd assuming th acclration
The pn junction: 2 Current vs Voltage (IV) characteristics
 Th pn junction: Currnt vs Voltag (V) charactristics Considr a pn junction in quilibrium with no applid xtrnal voltag: o th V E F E F V p-typ Dpltion rgion n-typ Elctron movmnt across th junction: 1. n
Th pn junction: Currnt vs Voltag (V) charactristics Considr a pn junction in quilibrium with no applid xtrnal voltag: o th V E F E F V p-typ Dpltion rgion n-typ Elctron movmnt across th junction: 1. n
Collisions between electrons and ions
 DRAFT 1 Collisions btwn lctrons and ions Flix I. Parra Rudolf Pirls Cntr for Thortical Physics, Unirsity of Oxford, Oxford OX1 NP, UK This rsion is of 8 May 217 1. Introduction Th Fokkr-Planck collision
DRAFT 1 Collisions btwn lctrons and ions Flix I. Parra Rudolf Pirls Cntr for Thortical Physics, Unirsity of Oxford, Oxford OX1 NP, UK This rsion is of 8 May 217 1. Introduction Th Fokkr-Planck collision
Pair (and Triplet) Production Effect:
 Pair (and riplt Production Effct: In both Pair and riplt production, a positron (anti-lctron and an lctron (or ngatron ar producd spontanously as a photon intracts with a strong lctric fild from ithr a
Pair (and riplt Production Effct: In both Pair and riplt production, a positron (anti-lctron and an lctron (or ngatron ar producd spontanously as a photon intracts with a strong lctric fild from ithr a
Why is a E&M nature of light not sufficient to explain experiments?
 1 Th wird world of photons Why is a E&M natur of light not sufficint to xplain xprimnts? Do photons xist? Som quantum proprtis of photons 2 Black body radiation Stfan s law: Enrgy/ ara/ tim = Win s displacmnt
1 Th wird world of photons Why is a E&M natur of light not sufficint to xplain xprimnts? Do photons xist? Som quantum proprtis of photons 2 Black body radiation Stfan s law: Enrgy/ ara/ tim = Win s displacmnt
Brief Notes on the Fermi-Dirac and Bose-Einstein Distributions, Bose-Einstein Condensates and Degenerate Fermi Gases Last Update: 28 th December 2008
 Brif ots on th Frmi-Dirac and Bos-Einstin Distributions, Bos-Einstin Condnsats and Dgnrat Frmi Gass Last Updat: 8 th Dcmbr 8 (A)Basics of Statistical Thrmodynamics Th Gibbs Factor A systm is assumd to
Brif ots on th Frmi-Dirac and Bos-Einstin Distributions, Bos-Einstin Condnsats and Dgnrat Frmi Gass Last Updat: 8 th Dcmbr 8 (A)Basics of Statistical Thrmodynamics Th Gibbs Factor A systm is assumd to
Chemical Physics II. More Stat. Thermo Kinetics Protein Folding...
 Chmical Physics II Mor Stat. Thrmo Kintics Protin Folding... http://www.nmc.ctc.com/imags/projct/proj15thumb.jpg http://nuclarwaponarchiv.org/usa/tsts/ukgrabl2.jpg http://www.photolib.noaa.gov/corps/imags/big/corp1417.jpg
Chmical Physics II Mor Stat. Thrmo Kintics Protin Folding... http://www.nmc.ctc.com/imags/projct/proj15thumb.jpg http://nuclarwaponarchiv.org/usa/tsts/ukgrabl2.jpg http://www.photolib.noaa.gov/corps/imags/big/corp1417.jpg
Molecular Orbitals in Inorganic Chemistry
 Outlin olcular Orbitals in Inorganic Chmistry Dr. P. Hunt p.hunt@imprial.ac.uk Rm 167 (Chmistry) http://www.ch.ic.ac.uk/hunt/ octahdral complxs forming th O diagram for Oh colour, slction ruls Δoct, spctrochmical
Outlin olcular Orbitals in Inorganic Chmistry Dr. P. Hunt p.hunt@imprial.ac.uk Rm 167 (Chmistry) http://www.ch.ic.ac.uk/hunt/ octahdral complxs forming th O diagram for Oh colour, slction ruls Δoct, spctrochmical
Introduction to Condensed Matter Physics
 Introduction to Condnsd Mattr Physics pcific hat M.P. Vaughan Ovrviw Ovrviw of spcific hat Hat capacity Dulong-Ptit Law Einstin modl Dby modl h Hat Capacity Hat capacity h hat capacity of a systm hld at
Introduction to Condnsd Mattr Physics pcific hat M.P. Vaughan Ovrviw Ovrviw of spcific hat Hat capacity Dulong-Ptit Law Einstin modl Dby modl h Hat Capacity Hat capacity h hat capacity of a systm hld at
Ch. 24 Molecular Reaction Dynamics 1. Collision Theory
 Ch. 4 Molcular Raction Dynamics 1. Collision Thory Lctur 16. Diffusion-Controlld Raction 3. Th Matrial Balanc Equation 4. Transition Stat Thory: Th Eyring Equation 5. Transition Stat Thory: Thrmodynamic
Ch. 4 Molcular Raction Dynamics 1. Collision Thory Lctur 16. Diffusion-Controlld Raction 3. Th Matrial Balanc Equation 4. Transition Stat Thory: Th Eyring Equation 5. Transition Stat Thory: Thrmodynamic
2.3 Matrix Formulation
 23 Matrix Formulation 43 A mor complicatd xampl ariss for a nonlinar systm of diffrntial quations Considr th following xampl Exampl 23 x y + x( x 2 y 2 y x + y( x 2 y 2 (233 Transforming to polar coordinats,
23 Matrix Formulation 43 A mor complicatd xampl ariss for a nonlinar systm of diffrntial quations Considr th following xampl Exampl 23 x y + x( x 2 y 2 y x + y( x 2 y 2 (233 Transforming to polar coordinats,
1.2 Faraday s law A changing magnetic field induces an electric field. Their relation is given by:
 Elctromagntic Induction. Lorntz forc on moving charg Point charg moving at vlocity v, F qv B () For a sction of lctric currnt I in a thin wir dl is Idl, th forc is df Idl B () Elctromotiv forc f s any
Elctromagntic Induction. Lorntz forc on moving charg Point charg moving at vlocity v, F qv B () For a sction of lctric currnt I in a thin wir dl is Idl, th forc is df Idl B () Elctromotiv forc f s any
There is an arbitrary overall complex phase that could be added to A, but since this makes no difference we set it to zero and choose A real.
 Midtrm #, Physics 37A, Spring 07. Writ your rsponss blow or on xtra pags. Show your work, and tak car to xplain what you ar doing; partial crdit will b givn for incomplt answrs that dmonstrat som concptual
Midtrm #, Physics 37A, Spring 07. Writ your rsponss blow or on xtra pags. Show your work, and tak car to xplain what you ar doing; partial crdit will b givn for incomplt answrs that dmonstrat som concptual
u 3 = u 3 (x 1, x 2, x 3 )
 Lctur 23: Curvilinar Coordinats (RHB 8.0 It is oftn convnint to work with variabls othr than th Cartsian coordinats x i ( = x, y, z. For xampl in Lctur 5 w mt sphrical polar and cylindrical polar coordinats.
Lctur 23: Curvilinar Coordinats (RHB 8.0 It is oftn convnint to work with variabls othr than th Cartsian coordinats x i ( = x, y, z. For xampl in Lctur 5 w mt sphrical polar and cylindrical polar coordinats.
High Energy Physics. Lecture 5 The Passage of Particles through Matter
 High Enrgy Physics Lctur 5 Th Passag of Particls through Mattr 1 Introduction In prvious lcturs w hav sn xampls of tracks lft by chargd particls in passing through mattr. Such tracks provid som of th most
High Enrgy Physics Lctur 5 Th Passag of Particls through Mattr 1 Introduction In prvious lcturs w hav sn xampls of tracks lft by chargd particls in passing through mattr. Such tracks provid som of th most
Introduction to the quantum theory of matter and Schrödinger s equation
 Introduction to th quantum thory of mattr and Schrödingr s quation Th quantum thory of mattr assums that mattr has two naturs: a particl natur and a wa natur. Th particl natur is dscribd by classical physics
Introduction to th quantum thory of mattr and Schrödingr s quation Th quantum thory of mattr assums that mattr has two naturs: a particl natur and a wa natur. Th particl natur is dscribd by classical physics
University of Illinois at Chicago Department of Physics. Thermodynamics & Statistical Mechanics Qualifying Examination
 Univrsity of Illinois at Chicago Dpartmnt of hysics hrmodynamics & tatistical Mchanics Qualifying Eamination January 9, 009 9.00 am 1:00 pm Full crdit can b achivd from compltly corrct answrs to 4 qustions.
Univrsity of Illinois at Chicago Dpartmnt of hysics hrmodynamics & tatistical Mchanics Qualifying Eamination January 9, 009 9.00 am 1:00 pm Full crdit can b achivd from compltly corrct answrs to 4 qustions.
Deepak Rajput
 Q Prov: (a than an infinit point lattic is only capabl of showing,, 4, or 6-fold typ rotational symmtry; (b th Wiss zon law, i.. if [uvw] is a zon axis and (hkl is a fac in th zon, thn hu + kv + lw ; (c
Q Prov: (a than an infinit point lattic is only capabl of showing,, 4, or 6-fold typ rotational symmtry; (b th Wiss zon law, i.. if [uvw] is a zon axis and (hkl is a fac in th zon, thn hu + kv + lw ; (c
VII. Quantum Entanglement
 VII. Quantum Entanglmnt Quantum ntanglmnt is a uniqu stat of quantum suprposition. It has bn studid mainly from a scintific intrst as an vidnc of quantum mchanics. Rcntly, it is also bing studid as a basic
VII. Quantum Entanglmnt Quantum ntanglmnt is a uniqu stat of quantum suprposition. It has bn studid mainly from a scintific intrst as an vidnc of quantum mchanics. Rcntly, it is also bing studid as a basic
E hf. hf c. 2 2 h 2 2 m v f ' f 2f ' f cos c
 EXPERIMENT 9: COMPTON EFFECT Rlatd Topics Intractions of photons with lctrons, consrvation of momntum and nrgy, inlastic and lastic scattring, intraction cross sction, Compton wavlngth. Principl Whn photons
EXPERIMENT 9: COMPTON EFFECT Rlatd Topics Intractions of photons with lctrons, consrvation of momntum and nrgy, inlastic and lastic scattring, intraction cross sction, Compton wavlngth. Principl Whn photons
2. Background Material
 S. Blair Sptmbr 3, 003 4. Background Matrial Th rst of this cours dals with th gnration, modulation, propagation, and ction of optical radiation. As such, bic background in lctromagntics and optics nds
S. Blair Sptmbr 3, 003 4. Background Matrial Th rst of this cours dals with th gnration, modulation, propagation, and ction of optical radiation. As such, bic background in lctromagntics and optics nds
1 Isoparametric Concept
 UNIVERSITY OF CALIFORNIA BERKELEY Dpartmnt of Civil Enginring Spring 06 Structural Enginring, Mchanics and Matrials Profssor: S. Govindj Nots on D isoparamtric lmnts Isoparamtric Concpt Th isoparamtric
UNIVERSITY OF CALIFORNIA BERKELEY Dpartmnt of Civil Enginring Spring 06 Structural Enginring, Mchanics and Matrials Profssor: S. Govindj Nots on D isoparamtric lmnts Isoparamtric Concpt Th isoparamtric
Atomic Physics. Final Mon. May 12, 12:25-2:25, Ingraham B10 Get prepared for the Final!
 # SCORES 50 40 30 0 10 MTE 3 Rsults P08 Exam 3 0 30 40 50 60 70 80 90 100 SCORE Avrag 79.75/100 std 1.30/100 A 19.9% AB 0.8% B 6.3% BC 17.4% C 13.1% D.1% F 0.4% Final Mon. Ma 1, 1:5-:5, Ingraam B10 Gt
# SCORES 50 40 30 0 10 MTE 3 Rsults P08 Exam 3 0 30 40 50 60 70 80 90 100 SCORE Avrag 79.75/100 std 1.30/100 A 19.9% AB 0.8% B 6.3% BC 17.4% C 13.1% D.1% F 0.4% Final Mon. Ma 1, 1:5-:5, Ingraam B10 Gt
AS 5850 Finite Element Analysis
 AS 5850 Finit Elmnt Analysis Two-Dimnsional Linar Elasticity Instructor Prof. IIT Madras Equations of Plan Elasticity - 1 displacmnt fild strain- displacmnt rlations (infinitsimal strain) in matrix form
AS 5850 Finit Elmnt Analysis Two-Dimnsional Linar Elasticity Instructor Prof. IIT Madras Equations of Plan Elasticity - 1 displacmnt fild strain- displacmnt rlations (infinitsimal strain) in matrix form
10. The Discrete-Time Fourier Transform (DTFT)
 Th Discrt-Tim Fourir Transform (DTFT Dfinition of th discrt-tim Fourir transform Th Fourir rprsntation of signals plays an important rol in both continuous and discrt signal procssing In this sction w
Th Discrt-Tim Fourir Transform (DTFT Dfinition of th discrt-tim Fourir transform Th Fourir rprsntation of signals plays an important rol in both continuous and discrt signal procssing In this sction w
surface of a dielectric-metal interface. It is commonly used today for discovering the ways in
 Surfac plasmon rsonanc is snsitiv mchanism for obsrving slight changs nar th surfac of a dilctric-mtal intrfac. It is commonl usd toda for discovring th was in which protins intract with thir nvironmnt,
Surfac plasmon rsonanc is snsitiv mchanism for obsrving slight changs nar th surfac of a dilctric-mtal intrfac. It is commonl usd toda for discovring th was in which protins intract with thir nvironmnt,
That is, we start with a general matrix: And end with a simpler matrix:
 DIAGON ALIZATION OF THE STR ESS TEN SOR INTRO DUCTIO N By th us of Cauchy s thorm w ar abl to rduc th numbr of strss componnts in th strss tnsor to only nin valus. An additional simplification of th strss
DIAGON ALIZATION OF THE STR ESS TEN SOR INTRO DUCTIO N By th us of Cauchy s thorm w ar abl to rduc th numbr of strss componnts in th strss tnsor to only nin valus. An additional simplification of th strss
GEOMETRICAL PHENOMENA IN THE PHYSICS OF SUBATOMIC PARTICLES. Eduard N. Klenov* Rostov-on-Don, Russia
 GEOMETRICAL PHENOMENA IN THE PHYSICS OF SUBATOMIC PARTICLES Eduard N. Klnov* Rostov-on-Don, Russia Th articl considrs phnomnal gomtry figurs bing th carrirs of valu spctra for th pairs of th rmaining additiv
GEOMETRICAL PHENOMENA IN THE PHYSICS OF SUBATOMIC PARTICLES Eduard N. Klnov* Rostov-on-Don, Russia Th articl considrs phnomnal gomtry figurs bing th carrirs of valu spctra for th pairs of th rmaining additiv
fiziks Institute for NET/JRF, GATE, IIT JAM, M.Sc. Entrance, JEST, TIFR and GRE in Physics NUCLEAR AND PARTICLE PHYSICS NET/JRF (JUNE-2011)
 NUCLEAR AND PARTICLE PHYSICS NET/JRF (JUNE-) 64 Q. Th radius of a 9Cu nuclus is masurd to b 4.8 - cm. (A). Th radius of a 7 Mg nuclus can b stimatd to b.86 - cm (b) 5. - cm (c).6 - cm (d) 8.6 - cm (c)
NUCLEAR AND PARTICLE PHYSICS NET/JRF (JUNE-) 64 Q. Th radius of a 9Cu nuclus is masurd to b 4.8 - cm. (A). Th radius of a 7 Mg nuclus can b stimatd to b.86 - cm (b) 5. - cm (c).6 - cm (d) 8.6 - cm (c)
The Matrix Exponential
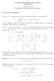 Th Matrix Exponntial (with xrciss) by D. Klain Vrsion 207.0.05 Corrctions and commnts ar wlcom. Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix A A k I + A + k!
Th Matrix Exponntial (with xrciss) by D. Klain Vrsion 207.0.05 Corrctions and commnts ar wlcom. Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix A A k I + A + k!
doi: /PhysRevB
 doi: 10.1103/PhysRvB.53.8561 PHYSICAL REVIEW B VOLUME 53, NUMBER 13 1 APRIL 1996-I Exact rsults on suprconductivity du to intrband coupling Y. Morita* Institut for Solid Stat Physics, Univrsity of Tokyo,
doi: 10.1103/PhysRvB.53.8561 PHYSICAL REVIEW B VOLUME 53, NUMBER 13 1 APRIL 1996-I Exact rsults on suprconductivity du to intrband coupling Y. Morita* Institut for Solid Stat Physics, Univrsity of Tokyo,
DIELECTRIC AND MAGNETIC PROPERTIES OF MATERIALS
 DILCTRIC AD MAGTIC PROPRTIS OF MATRIALS Dilctric Proprtis: Dilctric matrial Dilctric constant Polarization of dilctric matrials, Typs of Polarization (Polarizability). quation of intrnal filds in liquid
DILCTRIC AD MAGTIC PROPRTIS OF MATRIALS Dilctric Proprtis: Dilctric matrial Dilctric constant Polarization of dilctric matrials, Typs of Polarization (Polarizability). quation of intrnal filds in liquid
Atomic and Laser Spectroscopy
 L-E B, OL, MOV 83 Atomic and Lasr Spctroscopy Th aim of this xrcis is to giv an ovrviw of th fild of lasr spctroscopy and to show modrn spctroscopic mthods usd in atomic, molcular and chmical physics.
L-E B, OL, MOV 83 Atomic and Lasr Spctroscopy Th aim of this xrcis is to giv an ovrviw of th fild of lasr spctroscopy and to show modrn spctroscopic mthods usd in atomic, molcular and chmical physics.
Basic Polyhedral theory
 Basic Polyhdral thory Th st P = { A b} is calld a polyhdron. Lmma 1. Eithr th systm A = b, b 0, 0 has a solution or thr is a vctorπ such that π A 0, πb < 0 Thr cass, if solution in top row dos not ist
Basic Polyhdral thory Th st P = { A b} is calld a polyhdron. Lmma 1. Eithr th systm A = b, b 0, 0 has a solution or thr is a vctorπ such that π A 0, πb < 0 Thr cass, if solution in top row dos not ist
The Matrix Exponential
 Th Matrix Exponntial (with xrciss) by Dan Klain Vrsion 28928 Corrctions and commnts ar wlcom Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix () A A k I + A + k!
Th Matrix Exponntial (with xrciss) by Dan Klain Vrsion 28928 Corrctions and commnts ar wlcom Th Matrix Exponntial For ach n n complx matrix A, dfin th xponntial of A to b th matrix () A A k I + A + k!
5. Equation of state for high densities
 5 1 5. Equation of stat for high dnsitis Equation of stat for high dnsitis 5 Vlocity distribution of lctrons Classical thrmodynamics: 6 dimnsional phas spac: (x,y,z,px,py,pz) momntum: p = p x+p y +p z
5 1 5. Equation of stat for high dnsitis Equation of stat for high dnsitis 5 Vlocity distribution of lctrons Classical thrmodynamics: 6 dimnsional phas spac: (x,y,z,px,py,pz) momntum: p = p x+p y +p z
MCB137: Physical Biology of the Cell Spring 2017 Homework 6: Ligand binding and the MWC model of allostery (Due 3/23/17)
 MCB37: Physical Biology of th Cll Spring 207 Homwork 6: Ligand binding and th MWC modl of allostry (Du 3/23/7) Hrnan G. Garcia March 2, 207 Simpl rprssion In class, w drivd a mathmatical modl of how simpl
MCB37: Physical Biology of th Cll Spring 207 Homwork 6: Ligand binding and th MWC modl of allostry (Du 3/23/7) Hrnan G. Garcia March 2, 207 Simpl rprssion In class, w drivd a mathmatical modl of how simpl
Fourier Transforms and the Wave Equation. Key Mathematics: More Fourier transform theory, especially as applied to solving the wave equation.
 Lur 7 Fourir Transforms and th Wav Euation Ovrviw and Motivation: W first discuss a fw faturs of th Fourir transform (FT), and thn w solv th initial-valu problm for th wav uation using th Fourir transform
Lur 7 Fourir Transforms and th Wav Euation Ovrviw and Motivation: W first discuss a fw faturs of th Fourir transform (FT), and thn w solv th initial-valu problm for th wav uation using th Fourir transform
linarly acclratd []. Motivatd by ths complications w latr xamind mor carfully th cts of quantum uctuations for an lctron moving in a circular orbit [2
![linarly acclratd []. Motivatd by ths complications w latr xamind mor carfully th cts of quantum uctuations for an lctron moving in a circular orbit [2 linarly acclratd []. Motivatd by ths complications w latr xamind mor carfully th cts of quantum uctuations for an lctron moving in a circular orbit [2](/thumbs/87/96360411.jpg) OSLO-TP 3-98 April-998 Acclratd Elctrons and th Unruh Ect Jon Magn Linaas Dpartmnt of Physics P.O. Box 048 Blindrn N-036 Oslo Norway Abstract Quantum cts for lctrons in a storag ring ar studid in a co-moving,
OSLO-TP 3-98 April-998 Acclratd Elctrons and th Unruh Ect Jon Magn Linaas Dpartmnt of Physics P.O. Box 048 Blindrn N-036 Oslo Norway Abstract Quantum cts for lctrons in a storag ring ar studid in a co-moving,
Magnetic Neutron Scattering and Spin-Polarized Neutrons
 agntic Nutron Scattring and Spin-Polarizd Nutrons Physical origin: potntial of magntic dipol momnt of th nutron in magntic fild gnratd by lctron spins and orbital momnts in th solid. µ n µ H Spcializ to
agntic Nutron Scattring and Spin-Polarizd Nutrons Physical origin: potntial of magntic dipol momnt of th nutron in magntic fild gnratd by lctron spins and orbital momnts in th solid. µ n µ H Spcializ to
Construction of asymmetric orthogonal arrays of strength three via a replacement method
 isid/ms/26/2 Fbruary, 26 http://www.isid.ac.in/ statmath/indx.php?modul=prprint Construction of asymmtric orthogonal arrays of strngth thr via a rplacmnt mthod Tian-fang Zhang, Qiaoling Dng and Alok Dy
isid/ms/26/2 Fbruary, 26 http://www.isid.ac.in/ statmath/indx.php?modul=prprint Construction of asymmtric orthogonal arrays of strngth thr via a rplacmnt mthod Tian-fang Zhang, Qiaoling Dng and Alok Dy
orbiting electron turns out to be wrong even though it Unfortunately, the classical visualization of the
 Lctur 22-1 Byond Bohr Modl Unfortunatly, th classical visualization of th orbiting lctron turns out to b wrong vn though it still givs us a simpl way to think of th atom. Quantum Mchanics is ndd to truly
Lctur 22-1 Byond Bohr Modl Unfortunatly, th classical visualization of th orbiting lctron turns out to b wrong vn though it still givs us a simpl way to think of th atom. Quantum Mchanics is ndd to truly
BETA DECAY VISUAL PHYSICS ONLINE
 VISUAL PHYSICS ONLINE BETA DECAY Suppos now that a nuclus xists which has ithr too many or too fw nutrons rlativ to th numbr of protons prsnt for stability. Stability can b achivd by th convrsion insid
VISUAL PHYSICS ONLINE BETA DECAY Suppos now that a nuclus xists which has ithr too many or too fw nutrons rlativ to th numbr of protons prsnt for stability. Stability can b achivd by th convrsion insid
8 Foldy-Wouthuysen Transformation
 8 Foldy-Wouthuysn Transformation W now hav th Dirac quation with intractions. For a givn problm w can solv for th spctrum and wavfunctions ignoring th ngativ nrgy solutions for a momnt, for instanc, th
8 Foldy-Wouthuysn Transformation W now hav th Dirac quation with intractions. For a givn problm w can solv for th spctrum and wavfunctions ignoring th ngativ nrgy solutions for a momnt, for instanc, th
Bifurcation Theory. , a stationary point, depends on the value of α. At certain values
 Dnamic Macroconomic Thor Prof. Thomas Lux Bifurcation Thor Bifurcation: qualitativ chang in th natur of th solution occurs if a paramtr passs through a critical point bifurcation or branch valu. Local
Dnamic Macroconomic Thor Prof. Thomas Lux Bifurcation Thor Bifurcation: qualitativ chang in th natur of th solution occurs if a paramtr passs through a critical point bifurcation or branch valu. Local
COHORT MBA. Exponential function. MATH review (part2) by Lucian Mitroiu. The LOG and EXP functions. Properties: e e. lim.
 MTH rviw part b Lucian Mitroiu Th LOG and EXP functions Th ponntial function p : R, dfind as Proprtis: lim > lim p Eponntial function Y 8 6 - -8-6 - - X Th natural logarithm function ln in US- log: function
MTH rviw part b Lucian Mitroiu Th LOG and EXP functions Th ponntial function p : R, dfind as Proprtis: lim > lim p Eponntial function Y 8 6 - -8-6 - - X Th natural logarithm function ln in US- log: function
Self-interaction mass formula that relates all leptons and quarks to the electron
 Slf-intraction mass formula that rlats all lptons and quarks to th lctron GERALD ROSEN (a) Dpartmnt of Physics, Drxl Univrsity Philadlphia, PA 19104, USA PACS. 12.15. Ff Quark and lpton modls spcific thoris
Slf-intraction mass formula that rlats all lptons and quarks to th lctron GERALD ROSEN (a) Dpartmnt of Physics, Drxl Univrsity Philadlphia, PA 19104, USA PACS. 12.15. Ff Quark and lpton modls spcific thoris
de/dx Effectively all charged particles except electrons
 de/dx Lt s nxt turn our attntion to how chargd particls los nrgy in mattr To start with w ll considr only havy chargd particls lik muons, pions, protons, alphas, havy ions, Effctivly all chargd particls
de/dx Lt s nxt turn our attntion to how chargd particls los nrgy in mattr To start with w ll considr only havy chargd particls lik muons, pions, protons, alphas, havy ions, Effctivly all chargd particls
Alpha and beta decay equation practice
 Alpha and bta dcay quation practic Introduction Alpha and bta particls may b rprsntd in quations in svral diffrnt ways. Diffrnt xam boards hav thir own prfrnc. For xampl: Alpha Bta α β alpha bta Dspit
Alpha and bta dcay quation practic Introduction Alpha and bta particls may b rprsntd in quations in svral diffrnt ways. Diffrnt xam boards hav thir own prfrnc. For xampl: Alpha Bta α β alpha bta Dspit
Solid State Theory Physics 545 Band Theory III
 Solid Stat Thory Physics 545 Band Thory III ach atomic orbital lads to a band of allowd stats in th solid Band of allowd stats Gap: no allowd stats Band of allowd stats Gap: no allowd stats Band of allowd
Solid Stat Thory Physics 545 Band Thory III ach atomic orbital lads to a band of allowd stats in th solid Band of allowd stats Gap: no allowd stats Band of allowd stats Gap: no allowd stats Band of allowd
Electrochemistry L E O
 Rmmbr from CHM151 A rdox raction in on in which lctrons ar transfrrd lctrochmistry L O Rduction os lctrons xidation G R ain lctrons duction W can dtrmin which lmnt is oxidizd or rducd by assigning oxidation
Rmmbr from CHM151 A rdox raction in on in which lctrons ar transfrrd lctrochmistry L O Rduction os lctrons xidation G R ain lctrons duction W can dtrmin which lmnt is oxidizd or rducd by assigning oxidation
Division of Mechanics Lund University MULTIBODY DYNAMICS. Examination Name (write in block letters):.
 Division of Mchanics Lund Univrsity MULTIBODY DYNMICS Examination 7033 Nam (writ in block lttrs):. Id.-numbr: Writtn xamination with fiv tasks. Plas chck that all tasks ar includd. clan copy of th solutions
Division of Mchanics Lund Univrsity MULTIBODY DYNMICS Examination 7033 Nam (writ in block lttrs):. Id.-numbr: Writtn xamination with fiv tasks. Plas chck that all tasks ar includd. clan copy of th solutions
REGISTER!!! The Farmer and the Seeds (a parable of scientific reasoning) Class Updates. The Farmer and the Seeds. The Farmer and the Seeds
 How dos light intract with mattr? And what dos (this say about) mattr? REGISTER!!! If Schrödingr s Cat walks into a forst, and no on is around to obsrv it, is h rally in th forst? sourc unknown Phys 1010
How dos light intract with mattr? And what dos (this say about) mattr? REGISTER!!! If Schrödingr s Cat walks into a forst, and no on is around to obsrv it, is h rally in th forst? sourc unknown Phys 1010
Finite element discretization of Laplace and Poisson equations
 Finit lmnt discrtization of Laplac and Poisson quations Yashwanth Tummala Tutor: Prof S.Mittal 1 Outlin Finit Elmnt Mthod for 1D Introduction to Poisson s and Laplac s Equations Finit Elmnt Mthod for 2D-Discrtization
Finit lmnt discrtization of Laplac and Poisson quations Yashwanth Tummala Tutor: Prof S.Mittal 1 Outlin Finit Elmnt Mthod for 1D Introduction to Poisson s and Laplac s Equations Finit Elmnt Mthod for 2D-Discrtization
Cosmology and particle physics
 Cosmology and particl physics Lctur nots Timm Wras Lctur 8 Th thrmal univrs - part IV In this lctur w discuss th Boltzmann quation that allows on to dscrib th volution of procsss in our univrs that ar
Cosmology and particl physics Lctur nots Timm Wras Lctur 8 Th thrmal univrs - part IV In this lctur w discuss th Boltzmann quation that allows on to dscrib th volution of procsss in our univrs that ar
ph People Grade Level: basic Duration: minutes Setting: classroom or field site
 ph Popl Adaptd from: Whr Ar th Frogs? in Projct WET: Curriculum & Activity Guid. Bozman: Th Watrcours and th Council for Environmntal Education, 1995. ph Grad Lvl: basic Duration: 10 15 minuts Stting:
ph Popl Adaptd from: Whr Ar th Frogs? in Projct WET: Curriculum & Activity Guid. Bozman: Th Watrcours and th Council for Environmntal Education, 1995. ph Grad Lvl: basic Duration: 10 15 minuts Stting:
Self-Adjointness and Its Relationship to Quantum Mechanics. Ronald I. Frank 2016
 Ronald I. Frank 06 Adjoint https://n.wikipdia.org/wiki/adjoint In gnral thr is an oprator and a procss that dfin its adjoint *. It is thn slf-adjoint if *. Innr product spac https://n.wikipdia.org/wiki/innr_product_spac
Ronald I. Frank 06 Adjoint https://n.wikipdia.org/wiki/adjoint In gnral thr is an oprator and a procss that dfin its adjoint *. It is thn slf-adjoint if *. Innr product spac https://n.wikipdia.org/wiki/innr_product_spac
MCE503: Modeling and Simulation of Mechatronic Systems Discussion on Bond Graph Sign Conventions for Electrical Systems
 MCE503: Modling and Simulation o Mchatronic Systms Discussion on Bond Graph Sign Convntions or Elctrical Systms Hanz ichtr, PhD Clvland Stat Univrsity, Dpt o Mchanical Enginring 1 Basic Assumption In a
MCE503: Modling and Simulation o Mchatronic Systms Discussion on Bond Graph Sign Convntions or Elctrical Systms Hanz ichtr, PhD Clvland Stat Univrsity, Dpt o Mchanical Enginring 1 Basic Assumption In a
Neutrino Mass and Forbidden Beta Decays
 NUCLEAR THEORY Vol. 35 016) ds. M. Gaidarov N. Minkov Hron Prss Sofia Nutrino Mass and Forbiddn Bta Dcays R. Dvornický 1 D. Štfánik F. Šimkovic 3 1 Dzhlpov Laboratory of Nuclar Problms JINR 141980 Dubna
NUCLEAR THEORY Vol. 35 016) ds. M. Gaidarov N. Minkov Hron Prss Sofia Nutrino Mass and Forbiddn Bta Dcays R. Dvornický 1 D. Štfánik F. Šimkovic 3 1 Dzhlpov Laboratory of Nuclar Problms JINR 141980 Dubna
The Standard Model Lagrangian
 Th Standard Modl aranian Elmntary Particl Physics Stron Intraction Fnomnoloy Dio Bttoni Acadmic ar - D. Bttoni Fnomnoloia Intrazioni Forti Dirac Formalism m i j Consrvd Currnt i i i 5 i m Gau Invarianc
Th Standard Modl aranian Elmntary Particl Physics Stron Intraction Fnomnoloy Dio Bttoni Acadmic ar - D. Bttoni Fnomnoloia Intrazioni Forti Dirac Formalism m i j Consrvd Currnt i i i 5 i m Gau Invarianc
Determination of Vibrational and Electronic Parameters From an Electronic Spectrum of I 2 and a Birge-Sponer Plot
 5 J. Phys. Chm G Dtrmination of Vibrational and Elctronic Paramtrs From an Elctronic Spctrum of I 2 and a Birg-Sponr Plot 1 15 2 25 3 35 4 45 Dpartmnt of Chmistry, Gustavus Adolphus Collg. 8 Wst Collg
5 J. Phys. Chm G Dtrmination of Vibrational and Elctronic Paramtrs From an Elctronic Spctrum of I 2 and a Birg-Sponr Plot 1 15 2 25 3 35 4 45 Dpartmnt of Chmistry, Gustavus Adolphus Collg. 8 Wst Collg
Today. Wave-Matter Duality. Quantum Non-Locality. What is waving for matter waves?
 Today Wav-Mattr Duality HW 7 and Exam 2 du Thurs. 8pm 0 min rcap from last lctur on QM Finish QM odds and nds from ch.4 Th Standard Modl 4 forcs of Natur Fundamntal particls of Natur Fynman diagrams EM
Today Wav-Mattr Duality HW 7 and Exam 2 du Thurs. 8pm 0 min rcap from last lctur on QM Finish QM odds and nds from ch.4 Th Standard Modl 4 forcs of Natur Fundamntal particls of Natur Fynman diagrams EM
1 Minimum Cut Problem
 CS 6 Lctur 6 Min Cut and argr s Algorithm Scribs: Png Hui How (05), Virginia Dat: May 4, 06 Minimum Cut Problm Today, w introduc th minimum cut problm. This problm has many motivations, on of which coms
CS 6 Lctur 6 Min Cut and argr s Algorithm Scribs: Png Hui How (05), Virginia Dat: May 4, 06 Minimum Cut Problm Today, w introduc th minimum cut problm. This problm has many motivations, on of which coms
