A comparison of the virtual reality of Greenhouse Theory and the physical reality of the Earth
|
|
|
- Hilary Caldwell
- 6 years ago
- Views:
Transcription
1
2 A comparison of the virtual reality of Greenhouse Theory and the physical reality of the Earth Or Why CO2 has nothing to do with temperatures By Dr Darko Butina Scientific literature and most science-based blog sites have been saturated with the hottest topic of them all, the greenhouse theory, GHT, and the greenhouse gases classification, GHGC. This report brings novelty to this discussion in two major ways it looks at our planet and atmosphere as a space full of molecules and it is written by a retired scientist who worked with molecules all his working life. All molecules are real, they all have very unique physico-chemical properties and all the numbers that you will see in this report are obtained by very accurate measurements. First we need to establish some basic definitions to avoid any confusion. The most concise description of GHT and GHGC that I found in literature comes from the recent report by John Christy, and I quote: Earth s atmosphere includes some gasses which have a distinctive trait: They let sunlight pass through to heat Earth s surface, but they capture energy that leaves this sun-warmed surface. These are called greenhouse gasses. The problem that one has when trying to argue existence and validity of GHT is that it is impossible to use valid scientific arguments to falsify the greenhouse theory because that theory is not based on science that deals with physical reality of our planet. It is based on a purely theoretical framework that cannot be validated by measurements and therefore cannot be falsified. My approach to this problem is to use all the facts that we know about our atmosphere and to explain the differences between Earth, the planet that has the atmosphere, and the Moon which does not, without the need to evoke GHT or to blame humans or CO2 for anything. If I am successful, I will validate this approach and falsify GHT. However, if I cannot explain the physical reality that controls our planet without evoking GHT and CO2, then I will validate GHT and falsify this report. Before I start with the report, some background to the whole concept of global warming and the origin of GHT is needed. On June 23, 1988, NASA scientist James Hansen announced the arrival of global warming on our planet to US politicians and the global media. Let s keep in mind the year 1988 and address the origin of GHT and GHGC by asking three simple questions: 1. Who is the inventor of GHT/GHGC? 2. When was the key paper first published, explaining all the details and experimental evidence about GHT/GHGC? 3. Where was it published? Let me explain to non-scientists the standard procedure that needs to be followed when publishing the key paper informing the rest of scientific community about the new theory or classification. If you are introducing for the first time a new theory or classification labelled greenhouse it would be expected that the term greenhouse should appear at every part of that paper, in the title, in the abstract, throughout the paper and in the summary and the conclusions. For example, if you try to trace the Theory of Relativity, you would use a search engine like Google Scholar and you would find its author the great Einstein quite quickly. However, if you do the same search using the same engine, but definitely not Wikipedia, for greenhouse theory/effect/gases all you will find are the papers going back to 1990s, and none of them give any details how the theory was developed and what its purpose is. But you are wrong, I could hear people shouting, that theory goes back to 1900 and its father is the Nobel Prize winner Svante Arrhenius. 1
3 The problem with linking GHT to Arrhenius is that any scientists who has been trained in chemistry knows that Arrhenius never published in his working life a paper that uses the term greenhouse and that he would never confuse physico-chemical properties of CO2 in its pure state with the CO2 s contribution towards the properties of the mixture called air. No, the truth is that the link between GHT/GHGC and Arrhenius is one of the greatest scams in the history of science and it works in the following way: Dr Nobody-1 publishes the paper say in 1990, entitled greenhouse-something in some obscure journal that has no recognized peer reviewer system and makes the first link to Arrhenius. Then his best mate, Dr Nobody-2 writes the second paper entitled greenhouse-something-else and references/cites Dr Nobody-1 paper emphasizing the link to Arrhenius made in the first paper. By the time Dr Nobody-3 publishes the third paper, the first line in the introduction section will read It is well established fact that the great Arrhenius is the father of GHT and GHGC. Ten years later when you type Greenhouse and Arrhenius you will find that there are, say 100 citations, or references linking Greenhouse and Arrhenius. And why is this scam so foolproof? Because it is almost impossible to get hold of chemistry papers from the late 1800s and early 1900s. If you want to repeat an experiment that is published, say in German chemical journals in 1900, you will find out that there are probably only 4 libraries across the globe that keep those journals. Since those journals are all paper bound and since Arrhenius never published a paper with the title containing word greenhouse it is impossible to check those artificially created links. The fact of the matter is, that the whole religion of man-blamed science started in 1988, and since then, the same group of scientists have declared alarming global warming without publishing a single paper about the temperature trends in thermometer data, the same group of scientist have invented global temperature as a proxy-thermometer without publishing a single paper about that invention and letting the scientific community know who the inventor is, the same scientists have invented the thermometer-hockey stick and then the CO2-hockey stick without letting the scientific community know where the data is coming from. The same group of scientists have invented GHT and GHGC and yet again never published the details of that great discovery. I guess that we should be describing the period between 1988 to the present time as the great enlightenment era in the climate sciences since so many inventions in such a short period of time is unheard of in the history of frontline sciences. But let us go back now, to the real science and declare the working NULL hypothesis of this paper: The same molecules that prevent the earth overheating during daytime are also preventing the earth overcooling during night time. BTW, the same NULL hypothesis existed long before invention of GHG and has never been falsified or proven wrong by instrumental data! Part 1: Atom/elements, Molecules and Mixtures The key part of this report is to explain the difference between the physico-chemical properties of molecules and the physico-chemical properties of mixtures. Once that difference is understood, it will become very clear why any physico-chemical property of CO2 in its pure state has absolutely nothing to do with the physico-chemical properties of the mixture called atmosphere or air. If we describe a universal language called Chemistry as the language based on an alphabet called the periodic table, i.e. atoms/elements, then each word in that language is called a molecule. What is so unique about that language is that you cannot have two different words having the same meaning, since each word or molecule has a unique set of properties that are absolute and unequivocally differentiate one molecule from any other molecule. For example, the molecule CO2 has the same properties during daytime and during night time; whether it is detected on our planet, a planet in another galaxy or an asteroid aimlessly floating in outer-space for the last billions of years. One of the key assumptions of GHT is that during daytime we have normal-co2 that allows heat energy from the sun to pass through our atmosphere free-of-charge and without the need to do any work, i.e. heating the molecules in our atmosphere, while during night time, it changes to the werewolf-co2 which plays the role of the night time-sun and back-heats the Earth completely free of charge and again, all that heat energy gets back to the Earth surface without doing any work. 2
4 So what about mixtures? The terms atmosphere or air are abstract terms that can be described as a mixture of different molecules in their gas phase that are NOT connected by any type of chemical bond and they do not interact with each other. That mixture, i.e. atmosphere or air, does NOT have any physico-chemical property itself but it reflects properties of individual molecules that are part of that collection. Therefore, the property of any mixture can be calculated or derived from the contribution of each molecule towards that mixture: Figure 1. Contribution Index for molecules making-up the mixture called air : N2=0.79, O2=0.21, CO2= What we are seeing in Figure 1 is that every single property of our atmosphere can be explained by its two major contributors, the molecules of N2 and O2. It follows that CO2 s contribution cannot be detected by any standard instruments since every property of CO2 in its pure state has to be multiplied by to reflect its own contribution to the resulting property of that mixture! The set of measured properties, heat capacity Cp and Cv plus density, for molecules N2, O2 and CO2, and also for air, are listed in Table 1: The definition of heat capacity is the amount of heat energy in kj needed to warm 1kg of gas by 1 O K and since its importance in experimental sciences, very accurate measurements have been done for the most common molecules. Please note that contrary to the GHT, every molecule has a heat capacity with molecules N2 and O2 in their pure state absorbing more heat than CO2 in its pure state. If the law of mixtures works we should be able to derive the heat capacity for air from the measured heat capacity of its two major contributors, N2 and O2. Please note: Cp represents heat capacity at constant pressure while Cv is at constant volume. Exercise 1: Deriving the heat capacity of air at a constant pressure, Cp, from the measured heat capacities of N2+O2 alone: Contribution for N2: (78.0/100)*1.04(Cp) = 0.81 kj/kg Contribution for O2: (21.0/100)*0.92(Cp) = 0.19 kj/kg Total contribution (calculated) N2 +O2 = 1.00 kj/kg Measured (air): 1.01 kj/kg per 1K Exercise 2: The heat capacity of air at constant volume, Cv: Contribution for N2: (78.0/100)*0.74(Cv) = 0.58 kj/kg Contribution for O2: (21.0/100)*0.66(Cv) = 0.14 kj/kg 3
5 Total contribution (calculated) N2 +O2 = 0.72 kj/kg Measured (air): 0.72 kj/kg per 1K If we want to calculate the contribution of CO2 (used as 0.04% or 400 ppm for simplicity) towards any property of air all that is needed is to multiply that property with the CO2-contribution index (0.04/100) which is : The contribution of CO2 towards the heat capacity of air: (0.04/100)*0.84 (Cp) = kj/kg, or in terms of CO2 contribution towards 1 0 K of warming, *1 = K. Therefore, for air to warm by 1 O K, CO2 contributes exactly O K, or if we double the concentration of CO2, that amounts to massive O K of warming! I think that there is a quite clear message here don t estimate the contribution of CO2 towards warming, look it up. If we now look at the air mixture from N2+O2 vs. CO2 ratios: ppm of air belong to N2+O2 molecules, while 400 ppm to CO2 molecules (2500 : 1 ratio) From a single CO2 s molecule point of view, 1 molecule of CO2 is surrounded by 2500 molecules of N2+O2 The explanation that GHT offers, is that this mysterious blanket does not exist during daytime, but is acting as the proxy-sun during night time. The texture of the blanket consists of 2500 parts of nothing and 1 part of everything and while 1 part of everything is extra-warming the whole of the Earth, 2500 parts of nothing are just sitting there and doing nothing. A good analogy would be buying Swiss cheese where the cheese bit is CO2 while the holes bits are N2+O2. If you believe in GHT, you would buy an empty pack of Swiss cheese which would taste really great but only during night time when the cheese-bit miraculously appears out of nothing. However if you are the person who uses his/her brain you would see that as a great scam. Since everything in this report is connected with reality and measurements using real instruments, let us look at NASA s depiction of how the thermometer works: Figure 3. Thermometer in thermal equilibrium with the molecules surrounding it What the figure above is telling us is that the number recorded by the thermometer is not just another number, but the number that has physical meaning it reflects the kinetic energy of the molecules that are surrounding that thermometer. If we connect Figure 3 with the ratio of N2+O2 4
6 molecules to CO2 molecule of 2500:1 in the mixture called air, and if thermometer has an accuracy of +/- 0.5 C it becomes obvious that CO2 with its contribution index of will not influence the thermometer. Any experiment that involves air and standard thermometer will be detecting properties of only two molecules, N2 and O2. It is the same with a standard IR-spectrometer if we put, say 1g of air in spectrometer, all we will get is a flat line at 0% absorption indicating that no molecule in air is absorbing in IR. But how does that fit with IR-based CO2 detectors? They are specialised instruments that are not calibrated on the total mass that enters the detector but only take into account the amount of CO2 that can be detected, i.e % of molecules in the air sample are being ignored. Part2: Atmosphere and Water Mass The amount of overheating and overcooling could be seen in the table below: What makes our planet so unique is the fact that it retained water in its liquid state for millions of years and without that molecule we would not be discussing GHT today. What is important to realize is that everything on our planet is local and nothing is global, including temperatures. To estimate the number of molecules that make-up our atmosphere and the oceans, we need some accurate coordinate system where X-Y plane at Z=0 represents sea-levels, Z= +100km represents the top of the atmosphere and Z= -4km represents the average depth of oceans: Figure 2. Boundaries that define atmosphere and water heat capacity The total volume of our atmosphere is between Z=0 and Z = +100km, while the total volume of our main heat storage, i.e. the oceans, is between Z=0 and Z = -4km. In contrast, the Moon s atmosphere is between Z=0 and Z=0, while the Moon s water heat storage does not exist. 5
7 Estimating the number of molecules in atmosphere and the oceans 1 mol of any molecule contains molecules, known as Avogadro number. 1 mol of any gas molecule occupies volume of 22.4 litres; therefore 1 litre of any gas molecule contains 0.04 mols per litre (1/22.4 = 0.04). To estimate the total number of molecules contained within our atmosphere we need to multiply the total number of litres in atmosphere by 0.04 mols and by 6x10 23 molecules. On the other hand, water in its liquid state contains 1000g of H2O per 1 litre, and having a molecular weight of 18 grams it means 1000/18 = 55.6 mols per litre. Therefore, 1 litre of liquid water, i.e. the oceans, have 55.6/0.04 = 1390 more molecules per litre than 1 litre of our atmosphere. It is estimated that our atmosphere contains 4.2 billion cubic kilometres of air, while our oceans hold 1.3 billion cubic kilometres of water in its liquid state. Since every molecule has heat capacity, a huge amount of the sun s heat energy will be used during the daytime to keep warming this incomprehensibly large number of molecules that occupy the space between Z= +100km to Z= -4km. The heat capacity of Air and the Oceans The heat capacity of air (Cp) is 1.01 kj per 1kg, Table 1, and for simplicity of calculations I will approximate it to be 1.0 kj/kg. The sun will use 1kJ of its energy to warm each kg of air by 1 O C. There are quite a few estimates of the mass of our atmosphere but all of them come up with the same number, kg. To put that in perspective once the heat energy from the sun enters our atmosphere and by the time it reached Z=0 level it had to use 1.0x10 18 kj to warm-up air molecules by just 1 O C! And then, the water that covers 70% of our secondary surface at Z=0 comes into the equation! The heat capacity of water is 4.2 kj/kg with the best estimates that our hydro-mass is around kg which means 4.2x10 21 kj would be needed to warm-up our hydro mass by just 1 O C! Bearing in mind that water s heat capacity is 4 times larger than the heat capacity of our atmosphere and that 1 litre of water has 1390 times more molecules than 1 litre of air, it is estimated that the top 8 meters of oceans can store the same amount of heat as the whole of the atmosphere. Let me remind the reader that the two main assumptions that the GHT is built upon are: That the heat energy from the sun simply deposits itself at the Z=0 level during daytime without doing any work and all the work that GHG are doing is during night time That we should be able to balance all that energy along the lines that what comes in must come out, i.e. that the heat energy does not do any work and that it cannot be stored in our oceans for long periods of time However the physical reality that is controlling our planet tells us a completely different story. There are the same number of molecules in total, between our atmosphere and hydro mass and all of them have heat capacity and therefore will absorb the heat energy whether it is coming from the sun down or from Z=0 going up. There are only two types of molecules that count when it comes to atmosphere, N2 and O2 with total heat capacity at around kj. There is only one molecule that counts when it comes to our oceans, and that is H2O with the heat capacity of kj. What complicates the thermodynamics of top-water-layer-to-air exchange and top-water-to-bottomwater exchange is that the temperature at the bottom of the oceans is just above 0 O C so there is this great competition during night time between the surface water warming-up air above it, and warming the cold water below. The scientists who study the oceans tell us that to up-well the cold waters from the oceans floor to the surface might take up to 1000 years meaning that the sun s heat energy has to work very hard, very slowly and for long, long time to bring all those water molecules from the oceans floor back to the surface. 6
8 Let us summarise the facts presented so far and compare the assumptions used to postulate greenhouse theory and greenhouse gases classification against the well-known and well understood physico-chemical properties of molecules that make up our atmosphere and the oceans: 1. It assumes that the surface of the earth starts at Z=0 while in reality it starts at Z= +100 km. Error=100km and 4.2 billion cubic kilometres of air 2. It assumes that the oceans do not have any depth. Error=4km and 1.3 billion cubic kilometres of water 3. It assumes that the atmosphere does not have heat capacity during day time. Error=10 18 kj 4. It assumes that the oceans do not have heat capacity during daytime. Error=10 21 kj 5. Same assumptions as in 3. and 4. have been made for night time, with the same errors 6. It assumes that N2 and O2 do not have heat capacity. Error: 99% of kj 7. It assumes that molecules have different properties depending whether it is daytime or night time. This is not an error but either ignorance or fraudulent science 8. It assumes that the properties of molecules in their pure state is the same thing as their properties when part of a mixture. This is not an error but either ignorance fraudulent science 9. It assumes that the CO2 molecule functions as the night-time-sun. Error: kj kj 10. It assumes that 1,000,000ppm=400ppm. Error: 2500 The above list could be also called 10 reasons for mankind to be cheerful, since whatever is happening with the temperature patterns on our planet, it has nothing to do with burning fossil fuels, with CO2 or us. We are all protected by 3 molecules, N2 and O2 in the atmosphere and H2O in our oceans. Numbers: Virtual Reality vs. Reality The IPCC s report in 2007 quotes the increase of global temperature by 0.7 O C in the last 100 years which translates to O C per year. So let us come back to real Earth, look at some real numbers and see how sensitive, in terms of temperatures our planet really is. But to appreciate this part we must view the earth as a very dynamic system without any equilibrium and where each fixed-to-ground thermometer has its own and unique history. The dataset comes from one of the oldest archive of daily thermometer data that is in the public domain, the Armagh Observatory, UK, and covers the years between 1844 and All the details about this dataset can be found in my recent publication at The graph on the left compares daily Tmin averages against the corresponding Tmax averages for each of 365 days over 161 years (1844 to 2004). The graph on the right represents a difference between Tmin and Tmax for each day. So, the average difference between Tmax1 and Tmin1 (January 1) is around 5 O C, while the largest difference of 9.4C were observed on May 10, May 18 and June 3. So let us look at one of those days, May 10, and see what information in terms of heat energy from the sun we can extract from the daily data: 7
9 Since we are now looking at a single day, the X-axis represent the year when the difference between Tmax130 and Tmin130 (May 10) was observed. Four largest differences were observed in 1892(20.4C), 1909(18.9C), 1949(16.6C) and 1961(17.0), while the lowest differences observed in 1917(2.6C) and 1981(2.6C). The importance of the above graph cannot be overstated, since it gives us the key information about the maximum amount of the heat energy from the sun that is available to molecules surrounding the Armagh s thermometer on each day and in each year. For example, the largest amount of energy, 20.4 kj/kg (remember that the heat capacity of air is 1kJ per 1C, i.e. 1kJ=1C), was available on May 10 in 1892 enabling temperature increase of 20.4C from the night time temperature, Tmin, to the Tmax on May 10, In order for us to understand Tmax temperatures we must understand the corresponding Tmin temperature. When we take the difference between Tmax and Tmin on any given day and on any given year, we are detecting the maximum amount of heat energy available to the molecules (N2+O2) surrounding that thermometer and it follows that in order to understand the trends in Tmax space, we must first understand the trends in Tmin space, since the former is the result of the energy available to the latter. And to understand those trends we need the right type of data, yes, that must-not-be-mentioned, the daily thermometer data. Also note that the variations in daily energy/temperature ranges are in tens of O C in the real world of our planet, while in tenths of 1C in the virtual world of some single point in the space called global temperatures. When it comes to temperatures or CO2, the future research should be to treat the two as a completely different and not connected topics. If one wants to understand the temperature patterns on our planet, then the answer can be found in the understanding of the source of that heat, the sun, and the movements of the physical objects that make-up our atmosphere and stand between the sun and the thermometer at Z=0, i.e. the molecules of N2 and O2 at different weather stations. If however, one wants to understand different observed concentrations of CO2 at the ground level, one has to look at the water-to-air dynamics, driven solely by solubility of CO2 in water and therefore by temperature, and at the biomass-to-air dynamics driven solely by photosynthesis and therefore by CO2, water and sunlight. A report dedicated to CO2 along the lines: Everything you wanted to know about CO2 but afraid to ask will appear on the CO2-page by the end of this month, May
International Journal of Chemical Modeling ISSN: Darko Butina ABSTRACT
 International Journal of Chemical Modeling ISSN: 1941-3955 Volume 9, Number 1 Nova Science Publishers, Inc. LOOKING FOR PATTERNS IN EXTREME COLD AND HOT PERIODS ACROSS CANADA AND EURASIA IN DAILY TMAX
International Journal of Chemical Modeling ISSN: 1941-3955 Volume 9, Number 1 Nova Science Publishers, Inc. LOOKING FOR PATTERNS IN EXTREME COLD AND HOT PERIODS ACROSS CANADA AND EURASIA IN DAILY TMAX
International Journal of Chemical Modeling ISSN: Darko Butina ABSTRACT
 International Journal of Chemical Modeling ISSN: 1941-3955 Volume 9, Number 1 Nova Science Publishers, Inc. LOOKING FOR PATTERNS IN EXTREME COLD AND HOT PERIODS ACROSS THE USA IN DAILY TMAX TEMPERATURES
International Journal of Chemical Modeling ISSN: 1941-3955 Volume 9, Number 1 Nova Science Publishers, Inc. LOOKING FOR PATTERNS IN EXTREME COLD AND HOT PERIODS ACROSS THE USA IN DAILY TMAX TEMPERATURES
Lecture Presentation. Chapter 6. Thermochemistry. Sherril Soman Grand Valley State University Pearson Education, Inc.
 Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
Lecture Presentation Chapter 6 Thermochemistry Sherril Soman Grand Valley State University Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron 4 Fe(s)
Most hand warmers work by using the heat released from the slow oxidation of iron: The amount your hand temperature rises depends on several factors:
 Lecture Presentation Chapter 6 Thermochemistry Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron: Exothermic reaction 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O
Lecture Presentation Chapter 6 Thermochemistry Chemical Hand Warmers Most hand warmers work by using the heat released from the slow oxidation of iron: Exothermic reaction 4 Fe(s) + 3 O 2 (g) 2 Fe 2 O
IS THE ARCTIC MELTING? THEORY VS. OBSERVATIONS
 International Journal of Chemical Modeling ISSN: 1941-3955 Volume 7, Number 1 Nova Science Publishers, Inc. IS THE ARCTIC MELTING? THEORY VS. OBSERVATIONS Darko Butina Chemomine Consultancy Limited, Letchworth
International Journal of Chemical Modeling ISSN: 1941-3955 Volume 7, Number 1 Nova Science Publishers, Inc. IS THE ARCTIC MELTING? THEORY VS. OBSERVATIONS Darko Butina Chemomine Consultancy Limited, Letchworth
Heat Transfer. Thermal energy
 Thermal energy Heat Transfer Thermal energy is the total kinetic energy of the molecules of a substance. It is the energy needed to raise the temperature of a substance to its actual temperature from absolute
Thermal energy Heat Transfer Thermal energy is the total kinetic energy of the molecules of a substance. It is the energy needed to raise the temperature of a substance to its actual temperature from absolute
8.5 GREENHOUSE EFFECT 8.6 GLOBAL WARMING HW/Study Packet
 8.5 GREENHOUSE EFFECT 8.6 GLOBAL WARMING HW/Study Packet Required: READ Tsokos, pp 434-450 Hamper pp 294-307 SL/HL Supplemental: none REMEMBER TO. Work through all of the example problems in the texts
8.5 GREENHOUSE EFFECT 8.6 GLOBAL WARMING HW/Study Packet Required: READ Tsokos, pp 434-450 Hamper pp 294-307 SL/HL Supplemental: none REMEMBER TO. Work through all of the example problems in the texts
Investigating Planets Name: Block: E1:R6
 FYI: Planetary Temperatures and Atmospheres Read FYI: A Planet s Temperature, The Importance of an Atmosphere, and The Greenhouse Effect As you read answer the following questions about the readings: Word/Term
FYI: Planetary Temperatures and Atmospheres Read FYI: A Planet s Temperature, The Importance of an Atmosphere, and The Greenhouse Effect As you read answer the following questions about the readings: Word/Term
10/31/2017. Calculating the temperature of earth (The greenhouse effect) IR radiation. The electromagnetic spectrum
 Calculating the temperature of earth (The greenhouse effect) EM radiation so far Spectrum of EM radiation emitted by many objects may be approximated by the blackbody spectrum Blackbody spectrum (plot
Calculating the temperature of earth (The greenhouse effect) EM radiation so far Spectrum of EM radiation emitted by many objects may be approximated by the blackbody spectrum Blackbody spectrum (plot
A few final review points to emphasize on: Topic #6 Atmospheric Structure & Composition...
 FYI: Test #2 will cover: Topic 5: Radiation Law #6 (absorption curves) Topic 6 : Atmo Structure & Composition (all) Topic 7: Thermodynamics (content covered today & next Monday) A few final review points
FYI: Test #2 will cover: Topic 5: Radiation Law #6 (absorption curves) Topic 6 : Atmo Structure & Composition (all) Topic 7: Thermodynamics (content covered today & next Monday) A few final review points
Simple machines. ( Fxd) input. = (Fxd) output
 Announcements l LON-CAPA #5 and Mastering Physics Chapters 15 and 18 due Tuesday Feb. 18 l Average for exam 1 is 28/40 l The course will be graded on a curve with the average about 3.0, so if you received
Announcements l LON-CAPA #5 and Mastering Physics Chapters 15 and 18 due Tuesday Feb. 18 l Average for exam 1 is 28/40 l The course will be graded on a curve with the average about 3.0, so if you received
Section 1: The Science of Energy¹
 SECTION1: THE SCIENCE OF ENERGY Section 1: The Science of Energy¹ What Is Energy? Energy is the ability to do work or the ability to make a change. Everything that happens in the world involves the exchange
SECTION1: THE SCIENCE OF ENERGY Section 1: The Science of Energy¹ What Is Energy? Energy is the ability to do work or the ability to make a change. Everything that happens in the world involves the exchange
Ready for some more SCIENCE Homer?
 Ready for some more SCIENCE Homer? Alright brain, you don t like me and I don t like you; but let s get through this and I can get back to killing you with beer! Homer gives his brain a pep talk Disclaimer:
Ready for some more SCIENCE Homer? Alright brain, you don t like me and I don t like you; but let s get through this and I can get back to killing you with beer! Homer gives his brain a pep talk Disclaimer:
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
The Chemistry of Global Warming
 The Chemistry of Global Warming Venus Atmospheric pressure is 90x that of Earth 96% CO 2 and sulfuric acid clouds Average temperature = 450 C Expected temperature based on solar radiation and distance
The Chemistry of Global Warming Venus Atmospheric pressure is 90x that of Earth 96% CO 2 and sulfuric acid clouds Average temperature = 450 C Expected temperature based on solar radiation and distance
Introduction to Weather: Moisture in the Air Vapor Pressure and Dew Point
 IDS 102 Winter 2008 Introduction to Weather: Moisture in the Air Vapor Pressure and Dew Point During fall quarter we covered the topic of pressure and it has been a while since the, so let s review a couple
IDS 102 Winter 2008 Introduction to Weather: Moisture in the Air Vapor Pressure and Dew Point During fall quarter we covered the topic of pressure and it has been a while since the, so let s review a couple
Exploring Comets and Modeling for Mission Success National Science Education Standards Alignment Created for Deep Impact, A NASA Discovery mission
 Exploring Comets and Modeling for Mission Success National Science Education Standards Alignment Created for Deep Impact, A NASA Discovery mission Maura Rountree-Brown and Art Hammon Educator-Enrichment
Exploring Comets and Modeling for Mission Success National Science Education Standards Alignment Created for Deep Impact, A NASA Discovery mission Maura Rountree-Brown and Art Hammon Educator-Enrichment
An Intuitive Introduction to Motivic Homotopy Theory Vladimir Voevodsky
 What follows is Vladimir Voevodsky s snapshot of his Fields Medal work on motivic homotopy, plus a little philosophy and from my point of view the main fun of doing mathematics Voevodsky (2002). Voevodsky
What follows is Vladimir Voevodsky s snapshot of his Fields Medal work on motivic homotopy, plus a little philosophy and from my point of view the main fun of doing mathematics Voevodsky (2002). Voevodsky
The Methods of Science
 1 The Methods of Science What is Science? Science is a method for studying the natural world. It is a process that uses observation and investigation to gain knowledge about events in nature. 1 The Methods
1 The Methods of Science What is Science? Science is a method for studying the natural world. It is a process that uses observation and investigation to gain knowledge about events in nature. 1 The Methods
Prof. Dr. Anders Levermann Junior Professor for climate modelling on long timescales, Potsdam Institute for Climate Impact Research, Potsdam, Germany
 Prof. Dr. Anders Levermann Junior Professor for climate modelling on long timescales, Potsdam Institute for Climate Impact Research, Potsdam, Germany Points for discussion: The state of global climate;
Prof. Dr. Anders Levermann Junior Professor for climate modelling on long timescales, Potsdam Institute for Climate Impact Research, Potsdam, Germany Points for discussion: The state of global climate;
3 Newton s First Law of Motion Inertia. Forces cause changes in motion.
 Forces cause changes in motion. A ball at rest in the middle of a flat field is in equilibrium. No net force acts on it. If you saw it begin to move across the ground, you d look for forces that don t
Forces cause changes in motion. A ball at rest in the middle of a flat field is in equilibrium. No net force acts on it. If you saw it begin to move across the ground, you d look for forces that don t
Ready for some more SCIENCE Homer? ( Homer gives his brain a pep talk )
 Ready for some more SCIENCE Homer? ( Homer gives his brain a pep talk ) REVIEW: THE TWO LAWS OF THERMODYNAMICS #1 First Law (2 simple ways of understanding it) Energy can be transformed (changed from one
Ready for some more SCIENCE Homer? ( Homer gives his brain a pep talk ) REVIEW: THE TWO LAWS OF THERMODYNAMICS #1 First Law (2 simple ways of understanding it) Energy can be transformed (changed from one
Performance script for sixth graders By Thomas Kuo and Kimberly Kline LEAPS Fellows, University of California, Santa Barbara
 Performance script for sixth graders By Thomas Kuo and Kimberly Kline LEAPS Fellows, 2007-08 University of California, Santa Barbara [Remember to get answers from a wide variety of students in the audience.
Performance script for sixth graders By Thomas Kuo and Kimberly Kline LEAPS Fellows, 2007-08 University of California, Santa Barbara [Remember to get answers from a wide variety of students in the audience.
Notes: Matter & Change (text Ch. 1 &10)
 Name Per. Notes: Matter & Change (text Ch. 1 &10) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to get missing
Name Per. Notes: Matter & Change (text Ch. 1 &10) NOTE: This set of class notes is not complete. We will be filling in information in class. If you are absent, it is your responsibility to get missing
Amount of Substance and Its Unit Mole- Connecting the Invisible Micro World to the Observable Macro World Part 2 (English, mp4)
 Amount of Substance and Its Unit Mole- Connecting the Invisible Micro World to the Observable Macro World Part 2 (English, mp4) [MUSIC PLAYING] Instructor: Hi, everyone. Welcome back. I hope you had some
Amount of Substance and Its Unit Mole- Connecting the Invisible Micro World to the Observable Macro World Part 2 (English, mp4) [MUSIC PLAYING] Instructor: Hi, everyone. Welcome back. I hope you had some
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur
 Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture 2 First Law of Thermodynamics (Closed System) In the last
Applied Thermodynamics for Marine Systems Prof. P. K. Das Department of Mechanical Engineering Indian Institute of Technology, Kharagpur Lecture 2 First Law of Thermodynamics (Closed System) In the last
Lesson 2: Put a Label on That Number!
 Lesson 2: Put a Label on That Number! What would you do if your mother approached you, and, in an earnest tone, said, Honey. Yes, you replied. One million. Excuse me? One million, she affirmed. One million
Lesson 2: Put a Label on That Number! What would you do if your mother approached you, and, in an earnest tone, said, Honey. Yes, you replied. One million. Excuse me? One million, she affirmed. One million
Science. Earth Science. New Jersey Quality Single Accountability Continuum (NJQSAC) Department: Course Title. Textbook(s): Focus on Earth Science
 New Jersey Quality Single Accountability Continuum (NJQSAC) Textbook(s): Focus on Unit 1, September What is science? What is the Scientific Method? What is matter? How do atoms combine to form compounds?
New Jersey Quality Single Accountability Continuum (NJQSAC) Textbook(s): Focus on Unit 1, September What is science? What is the Scientific Method? What is matter? How do atoms combine to form compounds?
Appendix A. Linear Relationships in the Real World Unit
 Appendix A The Earth is like a giant greenhouse. The sun s energy passes through the atmosphere and heats up the land. Some of the heat escapes back into space while some of it is reflected back towards
Appendix A The Earth is like a giant greenhouse. The sun s energy passes through the atmosphere and heats up the land. Some of the heat escapes back into space while some of it is reflected back towards
Sunlight and Temperature
 Sunlight and Temperature Name Purpose: Study microclimate differences due to sunlight exposure, location, and surface; practice environmental measurements; study natural energy flows; compare measurements;
Sunlight and Temperature Name Purpose: Study microclimate differences due to sunlight exposure, location, and surface; practice environmental measurements; study natural energy flows; compare measurements;
Today. Events. Terrestrial Planet Geology - Earth. Terrestrial Planet Atmospheres. Homework DUE next time
 Today Terrestrial Planet Geology - Earth Terrestrial Planet Atmospheres Events Homework DUE next time Ring of Fire Boundaries of plates traced by Earthquakes and Volcanos Plate Motions Measurements of
Today Terrestrial Planet Geology - Earth Terrestrial Planet Atmospheres Events Homework DUE next time Ring of Fire Boundaries of plates traced by Earthquakes and Volcanos Plate Motions Measurements of
International Conference on Climate Change natural greenhouse effect radiative transfer model
 One of the points that Dr. Richard Lindzen made during his keynote speech at the 2nd International Conference on Climate Change, held in New York City March 8-10 this year, is that we global warming skeptics
One of the points that Dr. Richard Lindzen made during his keynote speech at the 2nd International Conference on Climate Change, held in New York City March 8-10 this year, is that we global warming skeptics
Surveying Prof. Bharat Lohani Department of Civil Engineering Indian Institute of Technology, Kanpur. Module - 3 Lecture - 4 Linear Measurements
 Surveying Prof. Bharat Lohani Department of Civil Engineering Indian Institute of Technology, Kanpur Module - 3 Lecture - 4 Linear Measurements Welcome again to this another video lecture on basic surveying.
Surveying Prof. Bharat Lohani Department of Civil Engineering Indian Institute of Technology, Kanpur Module - 3 Lecture - 4 Linear Measurements Welcome again to this another video lecture on basic surveying.
Lungs of the Planet. 1. Based on the equations above, describe how the processes of photosynthesis and cellular respiration relate to each other.
 Lungs of the Planet Name: Date: Why do people call rain forests the lungs of the planet? Usually it is because people think that the rain forests produce most of the oxygen we breathe. But do they? To
Lungs of the Planet Name: Date: Why do people call rain forests the lungs of the planet? Usually it is because people think that the rain forests produce most of the oxygen we breathe. But do they? To
FOLLOW THE ENERGY! EARTH S DYNAMIC CLIMATE SYSTEM
 Investigation 1B FOLLOW THE ENERGY! EARTH S DYNAMIC CLIMATE SYSTEM Driving Question How does energy enter, flow through, and exit Earth s climate system? Educational Outcomes To consider Earth s climate
Investigation 1B FOLLOW THE ENERGY! EARTH S DYNAMIC CLIMATE SYSTEM Driving Question How does energy enter, flow through, and exit Earth s climate system? Educational Outcomes To consider Earth s climate
Special Theory Of Relativity Prof. Shiva Prasad Department of Physics Indian Institute of Technology, Bombay
 Special Theory Of Relativity Prof. Shiva Prasad Department of Physics Indian Institute of Technology, Bombay Lecture - 6 Length Contraction and Time Dilation (Refer Slide Time: 00:29) In our last lecture,
Special Theory Of Relativity Prof. Shiva Prasad Department of Physics Indian Institute of Technology, Bombay Lecture - 6 Length Contraction and Time Dilation (Refer Slide Time: 00:29) In our last lecture,
UNIT 2 PHYSICAL & CHEMICAL PROPERTIES
 UNIT 2 PHYSICAL & CHEMICAL PROPERTIES What Is Matter? How matter is made of Elements? What atoms make up? Theory Law and Hypothesis Physical and Chemical Changes Heterogenous and Homogenous Substances
UNIT 2 PHYSICAL & CHEMICAL PROPERTIES What Is Matter? How matter is made of Elements? What atoms make up? Theory Law and Hypothesis Physical and Chemical Changes Heterogenous and Homogenous Substances
Lecture 3: Light and Temperature
 Lecture 3: Light and Temperature terrestrial radiative cooling Solar radiative warming (Light) Global Temperature atmosphere ocean land Light Temperature Different forms of energy Energy conservation energy,
Lecture 3: Light and Temperature terrestrial radiative cooling Solar radiative warming (Light) Global Temperature atmosphere ocean land Light Temperature Different forms of energy Energy conservation energy,
Darko Butina * Chemomine Consultancy, Letchworth Garden City, Hertfordshire, UK. Abstract
 International Journal of Chemical Modeling Volume 4, Number 2/3 ISSN: 1941-3955 c 2012 Nova Science Publishers, Inc. SHOULD WE WORRY ABOUT THE EARTH CALCULATED WARMING AT 0.7 0 C OVER THE LAST 100 YEARS
International Journal of Chemical Modeling Volume 4, Number 2/3 ISSN: 1941-3955 c 2012 Nova Science Publishers, Inc. SHOULD WE WORRY ABOUT THE EARTH CALCULATED WARMING AT 0.7 0 C OVER THE LAST 100 YEARS
Exploring The Planets: Mercury
 Exploring The Planets: Mercury By NASA.gov, adapted by Newsela staff on 02.28.17 Word Count 861 Level MAX The planet Mercury, the nearest planet to the sun, as seen from the spacecraft Mariner 10. Photo
Exploring The Planets: Mercury By NASA.gov, adapted by Newsela staff on 02.28.17 Word Count 861 Level MAX The planet Mercury, the nearest planet to the sun, as seen from the spacecraft Mariner 10. Photo
Ratios, Proportions, Unit Conversions, and the Factor-Label Method
 Ratios, Proportions, Unit Conversions, and the Factor-Label Method Math 0, Littlefield I don t know why, but presentations about ratios and proportions are often confused and fragmented. The one in your
Ratios, Proportions, Unit Conversions, and the Factor-Label Method Math 0, Littlefield I don t know why, but presentations about ratios and proportions are often confused and fragmented. The one in your
Global Energy Balance Climate Model. Dr. Robert M. MacKay Clark College Physics & Meteorology
 Global Energy Balance Climate Model Dr. Robert M. MacKay Clark College Physics & Meteorology rmackay@clark.edu (note: the value of 342 W/m 2 given in this figure is the solar constant divided by 4.0 (1368/4.0).
Global Energy Balance Climate Model Dr. Robert M. MacKay Clark College Physics & Meteorology rmackay@clark.edu (note: the value of 342 W/m 2 given in this figure is the solar constant divided by 4.0 (1368/4.0).
Thursday Oct 2nd. A Study Session will be held from 4:30 5:30 pm Monday Oct 6th
 Thursday Oct 2nd SIT WITH YOUR GROUP TODAY! TOPIC # 8 Part II LAWS OF THERMODYNAMICS & MOTION TEST #2 is NEXT TUESDAY Oct 7th The Top 10 is now posted in D2L under Study Guides and Quick Links A Study
Thursday Oct 2nd SIT WITH YOUR GROUP TODAY! TOPIC # 8 Part II LAWS OF THERMODYNAMICS & MOTION TEST #2 is NEXT TUESDAY Oct 7th The Top 10 is now posted in D2L under Study Guides and Quick Links A Study
Unit 1: Introduction to Chemistry
 Unit 1: Introduction to Chemistry I. Observations vs. Inferences Observation: information you gather using your five senses ***You will NEVER use taste in class! o Describes facts Examples You see the
Unit 1: Introduction to Chemistry I. Observations vs. Inferences Observation: information you gather using your five senses ***You will NEVER use taste in class! o Describes facts Examples You see the
Mahopac Central School District Curriculum Introduction to Science 8
 Introduction to Science 8 A. The goal of science is to understand the natural world 1. As you make new observations and test new explanations your view of the natural world may change again and again 2.
Introduction to Science 8 A. The goal of science is to understand the natural world 1. As you make new observations and test new explanations your view of the natural world may change again and again 2.
Why I Am a Climate Realist. by Dr. Willem de Lange
 Why I Am a Climate Realist by Dr. Willem de Lange SPPI Commentary & Essay Series! May 27, 2009 Why I Am a Climate Realist by Dr. Willem de Lange May 23, 2009 In 1996 the United Nations Intergovernmental
Why I Am a Climate Realist by Dr. Willem de Lange SPPI Commentary & Essay Series! May 27, 2009 Why I Am a Climate Realist by Dr. Willem de Lange May 23, 2009 In 1996 the United Nations Intergovernmental
The Nature of Science
 Chapter 1 Earth Science Lesson 1 The Nature of Science Main idea: Earth science encompasses five areas of study: astronomy, meteorology, geology oceanography, and environmental science. Earth has four
Chapter 1 Earth Science Lesson 1 The Nature of Science Main idea: Earth science encompasses five areas of study: astronomy, meteorology, geology oceanography, and environmental science. Earth has four
Introductory Quantum Chemistry Prof. K. L. Sebastian Department of Inorganic and Physical Chemistry Indian Institute of Science, Bangalore
 Introductory Quantum Chemistry Prof. K. L. Sebastian Department of Inorganic and Physical Chemistry Indian Institute of Science, Bangalore Lecture - 4 Postulates Part 1 (Refer Slide Time: 00:59) So, I
Introductory Quantum Chemistry Prof. K. L. Sebastian Department of Inorganic and Physical Chemistry Indian Institute of Science, Bangalore Lecture - 4 Postulates Part 1 (Refer Slide Time: 00:59) So, I
Hypothesis: an informal idea that has not been thoroughly tested by the scientific community. Most are discarded.
 AGS Productions (2009) Hypothesis: an informal idea that has not been thoroughly tested by the scientific community. Most are discarded. Theory: A hypothesis becomes a theory when it can explain and predict
AGS Productions (2009) Hypothesis: an informal idea that has not been thoroughly tested by the scientific community. Most are discarded. Theory: A hypothesis becomes a theory when it can explain and predict
INTRODUCTION TO SCIENCE CHAPTER 1
 INTRODUCTION TO SCIENCE CHAPTER 1 1 Science is the study of Everything!! A way of learning about the natural world. Scientist: a person who studies, or has expert WHAT IS SCIENCE? knowledge of a natural
INTRODUCTION TO SCIENCE CHAPTER 1 1 Science is the study of Everything!! A way of learning about the natural world. Scientist: a person who studies, or has expert WHAT IS SCIENCE? knowledge of a natural
CHAPTER 1. REVIEW: NUMBERS
 CHAPTER. REVIEW: NUMBERS Yes, mathematics deals with numbers. But doing math is not number crunching! Rather, it is a very complicated psychological process of learning and inventing. Just like listing
CHAPTER. REVIEW: NUMBERS Yes, mathematics deals with numbers. But doing math is not number crunching! Rather, it is a very complicated psychological process of learning and inventing. Just like listing
Modern Algebra Prof. Manindra Agrawal Department of Computer Science and Engineering Indian Institute of Technology, Kanpur
 Modern Algebra Prof. Manindra Agrawal Department of Computer Science and Engineering Indian Institute of Technology, Kanpur Lecture 02 Groups: Subgroups and homomorphism (Refer Slide Time: 00:13) We looked
Modern Algebra Prof. Manindra Agrawal Department of Computer Science and Engineering Indian Institute of Technology, Kanpur Lecture 02 Groups: Subgroups and homomorphism (Refer Slide Time: 00:13) We looked
Lecture Outline: Spectroscopy (Ch. 4)
 Lecture Outline: Spectroscopy (Ch. 4) NOTE: These are just an outline of the lectures and a guide to the textbook. The material will be covered in more detail in class. We will cover nearly all of the
Lecture Outline: Spectroscopy (Ch. 4) NOTE: These are just an outline of the lectures and a guide to the textbook. The material will be covered in more detail in class. We will cover nearly all of the
Sniffing out new laws... Question
 Sniffing out new laws... How can dimensional analysis help us figure out what new laws might be? (Why is math important not just for calculating, but even just for understanding?) (And a roundabout way
Sniffing out new laws... How can dimensional analysis help us figure out what new laws might be? (Why is math important not just for calculating, but even just for understanding?) (And a roundabout way
18 : ( ( ( ( ( ( ( ( ( ( ( (3-4. (1. (2. (3. (4-5» «. (4 (3 (2
 1 1389-18 25 1 25 20 50 26 25 17 75 51 25 20 100 76 25 75 : 100 : 135 : 175 : 18 : - - - (2 - - - (4 - - - (2 - - - (4 - - - (2 - - - (4 - - - (1 - - - (3-2 - - - (1 - - - (3-3 - - - (1 - - - (3-4. (1.
1 1389-18 25 1 25 20 50 26 25 17 75 51 25 20 100 76 25 75 : 100 : 135 : 175 : 18 : - - - (2 - - - (4 - - - (2 - - - (4 - - - (2 - - - (4 - - - (1 - - - (3-2 - - - (1 - - - (3-3 - - - (1 - - - (3-4. (1.
"Global Warming Has Stopped"? How to Fool People Using "Cherry-Picked" Climate Data
 Peter Gleick, Contributor CEO Pacific Institute, MacArthur Fellow, National Academy of Sciences ENER GY 2/05/2012 @ 12:39PM 25,696 views "Global Warming Has Stopped"? How to Fool People Using "Cherry-Picked"
Peter Gleick, Contributor CEO Pacific Institute, MacArthur Fellow, National Academy of Sciences ENER GY 2/05/2012 @ 12:39PM 25,696 views "Global Warming Has Stopped"? How to Fool People Using "Cherry-Picked"
NATIONAL 5 PHYSICS THERMODYNAMICS
 NATIONAL 5 PHYSICS THERMODYNAMICS HEAT AND TEMPERATURE Heat and temperature are not the same thing! Heat Heat is a type of energy. Like all types of energy it is measured in joules (J). The heat energy
NATIONAL 5 PHYSICS THERMODYNAMICS HEAT AND TEMPERATURE Heat and temperature are not the same thing! Heat Heat is a type of energy. Like all types of energy it is measured in joules (J). The heat energy
Lecture 2 - Length Contraction
 Lecture 2 - Length Contraction A Puzzle We are all aware that if you jump to the right, your reflection in the mirror will jump left. But if you raise your hand up, your reflection will also raise its
Lecture 2 - Length Contraction A Puzzle We are all aware that if you jump to the right, your reflection in the mirror will jump left. But if you raise your hand up, your reflection will also raise its
Life on a New Planet
 Life on a New Planet Your Assignment: A national magazine is sponsoring a short story contest. Your teacher has assigned your class the task of entering this contest. The first, second, and third place
Life on a New Planet Your Assignment: A national magazine is sponsoring a short story contest. Your teacher has assigned your class the task of entering this contest. The first, second, and third place
Earth s Layers Activity
 Earth s Layers Activity Purpose: To visualize the basic structure of the Earth and how we study it. Materials: Procedure: A piece of poster board shaped like a pizza slice with radius of about 16 cm Compass
Earth s Layers Activity Purpose: To visualize the basic structure of the Earth and how we study it. Materials: Procedure: A piece of poster board shaped like a pizza slice with radius of about 16 cm Compass
3 Newton s First Law of Motion Inertia. Forces cause changes in motion.
 Forces cause changes in motion. A ball at rest in the middle of a flat field is in equilibrium. No net force acts on it. If you saw it begin to move across the ground, you d look for forces that don t
Forces cause changes in motion. A ball at rest in the middle of a flat field is in equilibrium. No net force acts on it. If you saw it begin to move across the ground, you d look for forces that don t
Lecture 14 - Radiative equilibrium and the atmospheric greenhouse effect
 We now have most of the tools we will need to begin to study energy balance on the earth. It will be a balance between incoming sunlight energy and outgoing energy emitted by the earth. We will look at
We now have most of the tools we will need to begin to study energy balance on the earth. It will be a balance between incoming sunlight energy and outgoing energy emitted by the earth. We will look at
Interactive Chalkboard
 1 Interactive Chalkboard 1 Table of Contents Unit 1: Energy and Motion Chapter 1: The Nature of Science 1.1: The Methods of Science 1.2: Standards of Measurement 1.3: Communicating with Graphs 1.1 The
1 Interactive Chalkboard 1 Table of Contents Unit 1: Energy and Motion Chapter 1: The Nature of Science 1.1: The Methods of Science 1.2: Standards of Measurement 1.3: Communicating with Graphs 1.1 The
AST 301 Introduction to Astronomy
 AST 301 Introduction to Astronomy John Lacy RLM 16.332 471-1469 lacy@astro.as.utexas.edu Myoungwon Jeon RLM 16.216 471-0445 myjeon@astro.as.utexas.edu Bohua Li RLM 16.212 471-8443 bohuali@astro.as.utexas.edu
AST 301 Introduction to Astronomy John Lacy RLM 16.332 471-1469 lacy@astro.as.utexas.edu Myoungwon Jeon RLM 16.216 471-0445 myjeon@astro.as.utexas.edu Bohua Li RLM 16.212 471-8443 bohuali@astro.as.utexas.edu
(Refer Slide Time: 0:15)
 (Refer Slide Time: 0:15) Engineering Thermodynamics Professor Jayant K Singh Department of Chemical Engineering Indian Institute of Technology Kanpur Lecture 18 Internal energy, enthalpy, and specific
(Refer Slide Time: 0:15) Engineering Thermodynamics Professor Jayant K Singh Department of Chemical Engineering Indian Institute of Technology Kanpur Lecture 18 Internal energy, enthalpy, and specific
Fig. 3.2 on Page 101. Warming. Evidence for CO 2. History of Global Warming-2. Fig. 3.2 Page 101. Drilled cores from ocean floors
 Chemistry in Context: Chapter 3:The Chemistry of Global Warming Practice Problems: All Ch. 3 problems with the blue codes or answers on Page 521. Venus Atmospheric pressure is 90x that of Earth 96% CO
Chemistry in Context: Chapter 3:The Chemistry of Global Warming Practice Problems: All Ch. 3 problems with the blue codes or answers on Page 521. Venus Atmospheric pressure is 90x that of Earth 96% CO
Special Theory of Relativity Prof. Dr. Shiva Prasad Department of Physics Indian Institute of Technology, Bombay
 (Refer Slide Time: 00:36) Special Theory of Relativity Prof. Dr. Shiva Prasad Department of Physics Indian Institute of Technology, Bombay Lecture - 7 Examples of Length Contraction and Time Dilation Hello,
(Refer Slide Time: 00:36) Special Theory of Relativity Prof. Dr. Shiva Prasad Department of Physics Indian Institute of Technology, Bombay Lecture - 7 Examples of Length Contraction and Time Dilation Hello,
Solving Equations by Adding and Subtracting
 SECTION 2.1 Solving Equations by Adding and Subtracting 2.1 OBJECTIVES 1. Determine whether a given number is a solution for an equation 2. Use the addition property to solve equations 3. Determine whether
SECTION 2.1 Solving Equations by Adding and Subtracting 2.1 OBJECTIVES 1. Determine whether a given number is a solution for an equation 2. Use the addition property to solve equations 3. Determine whether
Please bring the task to your first physics lesson and hand it to the teacher.
 Pre-enrolment task for 2014 entry Physics Why do I need to complete a pre-enrolment task? This bridging pack serves a number of purposes. It gives you practice in some of the important skills you will
Pre-enrolment task for 2014 entry Physics Why do I need to complete a pre-enrolment task? This bridging pack serves a number of purposes. It gives you practice in some of the important skills you will
Ready for some more SCIENCE Homer?
 Ready for some more SCIENCE Homer? Alright brain, you don t like me and I don t like you; but let s get through this and I can get back to killing you with beer! Homer gives his brain a pep talk Disclaimer:
Ready for some more SCIENCE Homer? Alright brain, you don t like me and I don t like you; but let s get through this and I can get back to killing you with beer! Homer gives his brain a pep talk Disclaimer:
Rocket propulsion Prof. K. Ramamurthi Department of Mechanical Engineering Indian Institute of Technology, Madras. Lecture 09 Theory of Nozzles
 Rocket propulsion Prof. K. Ramamurthi Department of Mechanical Engineering Indian Institute of Technology, Madras Lecture 09 Theory of Nozzles (Refer Slide Time: 00:14) Good morning. We will develop the
Rocket propulsion Prof. K. Ramamurthi Department of Mechanical Engineering Indian Institute of Technology, Madras Lecture 09 Theory of Nozzles (Refer Slide Time: 00:14) Good morning. We will develop the
LECSS Physics 11 Introduction to Physics and Math Methods 1 Revised 8 September 2013 Don Bloomfield
 LECSS Physics 11 Introduction to Physics and Math Methods 1 Physics 11 Introduction to Physics and Math Methods In this introduction, you will get a more in-depth overview of what Physics is, as well as
LECSS Physics 11 Introduction to Physics and Math Methods 1 Physics 11 Introduction to Physics and Math Methods In this introduction, you will get a more in-depth overview of what Physics is, as well as
Our Created Solar System Video
 Our Created Solar System Video After the first segment of the video (0:00 8:54 min.) is played, the video will be stopped. Then, answer the following questions: 1) In short, what is the solar system? 2)
Our Created Solar System Video After the first segment of the video (0:00 8:54 min.) is played, the video will be stopped. Then, answer the following questions: 1) In short, what is the solar system? 2)
Chapter 11 Heat Engines and The Second Law of Thermodynamics
 Chapter 11 Heat Engines and The Second Law of Thermodynamics Heat Engines Heat engines use a temperature difference involving a high temperature (T H ) and a low temperature (T C ) to do mechanical work.
Chapter 11 Heat Engines and The Second Law of Thermodynamics Heat Engines Heat engines use a temperature difference involving a high temperature (T H ) and a low temperature (T C ) to do mechanical work.
Introduction to Algebra: The First Week
 Introduction to Algebra: The First Week Background: According to the thermostat on the wall, the temperature in the classroom right now is 72 degrees Fahrenheit. I want to write to my friend in Europe,
Introduction to Algebra: The First Week Background: According to the thermostat on the wall, the temperature in the classroom right now is 72 degrees Fahrenheit. I want to write to my friend in Europe,
1. The diagram below shows Earth, four different positions of the Moon, and the direction of incoming sunlight.
 G8 Semester I MCAS Pre-Test Please answer on Scantron Card; not on this test form Standard: 9 - Describe lunar and solar eclipses, the observed moon phases, and tides. Relate them to the relative positions
G8 Semester I MCAS Pre-Test Please answer on Scantron Card; not on this test form Standard: 9 - Describe lunar and solar eclipses, the observed moon phases, and tides. Relate them to the relative positions
SU230R Grades 4-8. Hayes FAST FACTS & DAZZLING DATA OUR SOLAR SYSTEM
 Hayes SU230R Grades 4-8 FAST FACTS & DAZZLING DATA OUR SOLAR SYSTEM Fast Facts & Dazzling Data Our Solar System This book was developed for Hayes School Publishing Co., Inc. by Good Neighbor Press, Inc.,
Hayes SU230R Grades 4-8 FAST FACTS & DAZZLING DATA OUR SOLAR SYSTEM Fast Facts & Dazzling Data Our Solar System This book was developed for Hayes School Publishing Co., Inc. by Good Neighbor Press, Inc.,
THE GREENHOUSE EFFECT
 ASTRONOMY READER THE GREENHOUSE EFFECT 35.1 THE GREENHOUSE EFFECT Overview Planets are heated by light from the Sun. Planets cool off by giving off an invisible kind of light, longwave infrared light.
ASTRONOMY READER THE GREENHOUSE EFFECT 35.1 THE GREENHOUSE EFFECT Overview Planets are heated by light from the Sun. Planets cool off by giving off an invisible kind of light, longwave infrared light.
(Refer Slide Time: 00:10)
 Chemical Reaction Engineering 1 (Homogeneous Reactors) Professor R. Krishnaiah Department of Chemical Engineering Indian Institute of Technology Madras Lecture No 10 Design of Batch Reactors Part 1 (Refer
Chemical Reaction Engineering 1 (Homogeneous Reactors) Professor R. Krishnaiah Department of Chemical Engineering Indian Institute of Technology Madras Lecture No 10 Design of Batch Reactors Part 1 (Refer
INTRODUCTIONS Part 1
 Weather and Climate Jim Keller & Paul Belanger Classroom assistant: Fritz Ihrig Week 1: January 15 th, 2019 1 INTRODUCTIONS Part 1 Fritz Ihrig; classroom assistant, liaison to OLLI: fgihrig@msn.com ; h.
Weather and Climate Jim Keller & Paul Belanger Classroom assistant: Fritz Ihrig Week 1: January 15 th, 2019 1 INTRODUCTIONS Part 1 Fritz Ihrig; classroom assistant, liaison to OLLI: fgihrig@msn.com ; h.
Table of Contents. Chapter: Atmosphere. Section 1: Earth's Atmosphere. Section 2: Energy Transfer in the Atmosphere. Section 3: Air Movement
 Table of Contents Chapter: Atmosphere Section 1: Earth's Atmosphere Section 2: Energy Transfer in the Atmosphere Section 3: Air Movement Table of Contents Chapter: Atmosphere Section 2: Energy Transfer
Table of Contents Chapter: Atmosphere Section 1: Earth's Atmosphere Section 2: Energy Transfer in the Atmosphere Section 3: Air Movement Table of Contents Chapter: Atmosphere Section 2: Energy Transfer
Climate Change Lecture Notes
 Climate Change Lecture Notes (Topic 12A) page 1 Climate Change Lecture Notes Learning Outcomes for the Climate Change Unit 1. Students can list observations which suggest that the world is warming, and
Climate Change Lecture Notes (Topic 12A) page 1 Climate Change Lecture Notes Learning Outcomes for the Climate Change Unit 1. Students can list observations which suggest that the world is warming, and
A supernova is the explosion of a star. It is the largest explosion that takes place in space.
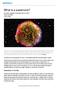 What is a supernova? By NASA, adapted by Newsela staff on 03.28.17 Word Count 974 Level 1110L TOP: A vivid view of a supernova remnant captured by NASA's Spitzer and Chandra space observatories and the
What is a supernova? By NASA, adapted by Newsela staff on 03.28.17 Word Count 974 Level 1110L TOP: A vivid view of a supernova remnant captured by NASA's Spitzer and Chandra space observatories and the
- ( ). 1. THE PLANETARY GAMBLING MACHINE.
 There is no dark side of the Moon really, as a matter of fact it is all dark, - Pink Floyd ( The Dark Side of the Moon ). 1. THE PLANETARY GAMBLING MACHINE. Everybody living on this planet must have heard
There is no dark side of the Moon really, as a matter of fact it is all dark, - Pink Floyd ( The Dark Side of the Moon ). 1. THE PLANETARY GAMBLING MACHINE. Everybody living on this planet must have heard
Section Objectives: Recognize some possible benefits from studying biology. Summarize the characteristics of living things.
 Section Objectives: Recognize some possible benefits from studying biology. Summarize the characteristics of living things. The Science of Biology The concepts, principles, and theories that allow people
Section Objectives: Recognize some possible benefits from studying biology. Summarize the characteristics of living things. The Science of Biology The concepts, principles, and theories that allow people
The sun, yellow dwarf star at the heart of the solar system NASA.gov, adapted by Newsela staff
 Name: Period: Date: Article of the Week Directions: Read the following article carefully and annotate. You need to include at least 1 annotation per paragraph. Be sure to include all of the following in
Name: Period: Date: Article of the Week Directions: Read the following article carefully and annotate. You need to include at least 1 annotation per paragraph. Be sure to include all of the following in
Climate Change. Model-Evidence Link Diagram (MEL) MEL Key Points Model A: Current climate. released from the Sun.
 A Content Secondary Science Newsletter from the Southern Nevada Regional Professional Development Program Science Dissected Climate Change Model-Evidence Link Diagram (MEL) Earth s climate is a complex
A Content Secondary Science Newsletter from the Southern Nevada Regional Professional Development Program Science Dissected Climate Change Model-Evidence Link Diagram (MEL) Earth s climate is a complex
Heat Transfer. Conduction, Convection, and Radiation. Review: Temperature
 Heat Transfer Conduction, Convection, and Radiation Review: Temperature! Temperature is:! The quantity that tells how hot or cold something is compared with a standard! A measure of the average kinetic
Heat Transfer Conduction, Convection, and Radiation Review: Temperature! Temperature is:! The quantity that tells how hot or cold something is compared with a standard! A measure of the average kinetic
LIVING IN THE ENVIRONMENT 17 TH
 MILLER/SPOOLMAN LIVING IN THE ENVIRONMENT 17 TH CHAPTER 2 Science, Matter, Energy, and Systems Core Case Study: A Story About a Forest Hubbard Brook Experimental Forest in New Hampshire Compared the loss
MILLER/SPOOLMAN LIVING IN THE ENVIRONMENT 17 TH CHAPTER 2 Science, Matter, Energy, and Systems Core Case Study: A Story About a Forest Hubbard Brook Experimental Forest in New Hampshire Compared the loss
Atomic Masses and Molecular Formulas *
 OpenStax-CNX module: m44278 1 Atomic Masses and Molecular Formulas * John S. Hutchinson This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 1 Introduction
OpenStax-CNX module: m44278 1 Atomic Masses and Molecular Formulas * John S. Hutchinson This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 1 Introduction
Semiconductor Optoelectronics Prof. M. R. Shenoy Department of physics Indian Institute of Technology, Delhi
 Semiconductor Optoelectronics Prof. M. R. Shenoy Department of physics Indian Institute of Technology, Delhi Lecture - 18 Optical Joint Density of States So, today we will discuss the concept of optical
Semiconductor Optoelectronics Prof. M. R. Shenoy Department of physics Indian Institute of Technology, Delhi Lecture - 18 Optical Joint Density of States So, today we will discuss the concept of optical
Second Grade: Unit 2: Properties of Matter. Matter solid liquid gas property
 Second Grade: Unit 2: Properties of Matter Matter solid liquid gas property Background: The universe is made of only two entities: matter and energy. Examples of energy are light, heat, and sound. Everything
Second Grade: Unit 2: Properties of Matter Matter solid liquid gas property Background: The universe is made of only two entities: matter and energy. Examples of energy are light, heat, and sound. Everything
Response to Unified Theory of Climate
 Response to Unified Theory of Climate Dr. Daniel M. Sweger National College Introduction During the last two years that I have been teaching Environmental Science and attempting to explain global warming
Response to Unified Theory of Climate Dr. Daniel M. Sweger National College Introduction During the last two years that I have been teaching Environmental Science and attempting to explain global warming
 Since ancient times, people have needed thermal energy (heat) to cook their food and to keep them warm. And since the beginning of time, uncontrolled heat has scorched and spoiled the taste of food and
Since ancient times, people have needed thermal energy (heat) to cook their food and to keep them warm. And since the beginning of time, uncontrolled heat has scorched and spoiled the taste of food and
Thursday, November 1st.
 Thursday, November 1st. Announcements. Homework 7 - due Tuesday, Nov. 6 Homework 8 - paper 2 topics, questions and sources due Tuesday, Nov. 13 Midterm Paper 2 - due Tuesday, Nov. 20 I will hand out a
Thursday, November 1st. Announcements. Homework 7 - due Tuesday, Nov. 6 Homework 8 - paper 2 topics, questions and sources due Tuesday, Nov. 13 Midterm Paper 2 - due Tuesday, Nov. 20 I will hand out a
Today. A little more scale... The Scientific Method. Naked Eye Observations: the Appearance of the Sky
 Today A little more scale... The Scientific Method Naked Eye Observations: the Appearance of the Sky The "student set" for Smartwork5 is 40700, which is labeled CWRU_ASTR201_Fall207. Add yourself to this
Today A little more scale... The Scientific Method Naked Eye Observations: the Appearance of the Sky The "student set" for Smartwork5 is 40700, which is labeled CWRU_ASTR201_Fall207. Add yourself to this
Earth Math. A Brief Mathematical Guide to Earth Science and Climate Change
 Earth Math A Brief Mathematical Guide to Earth Science and Climate Change Dear Student, It is hard to avoid the disturbing news that our environment and climate are both changing more rapidly now than
Earth Math A Brief Mathematical Guide to Earth Science and Climate Change Dear Student, It is hard to avoid the disturbing news that our environment and climate are both changing more rapidly now than
Solving with Absolute Value
 Solving with Absolute Value Who knew two little lines could cause so much trouble? Ask someone to solve the equation 3x 2 = 7 and they ll say No problem! Add just two little lines, and ask them to solve
Solving with Absolute Value Who knew two little lines could cause so much trouble? Ask someone to solve the equation 3x 2 = 7 and they ll say No problem! Add just two little lines, and ask them to solve
PRE-LAB FOR PLANETARY ATMOSPHERES
 PRE-LAB FOR PLANETARY ATMOSPHERES 1. Find pictures of Venus, Earth, and Mars in an astronomy textbook or other book or online at a website. Estimate, to the nearest 10%, the percentage of surface of each
PRE-LAB FOR PLANETARY ATMOSPHERES 1. Find pictures of Venus, Earth, and Mars in an astronomy textbook or other book or online at a website. Estimate, to the nearest 10%, the percentage of surface of each
Title: Thermodynamics I. Systems A system is a group of interacting parts, including energy and matter, forming a complex whole with a common
 Title: Thermodynamics I. Systems A system is a group of interacting parts, including energy and matter, forming a complex whole with a common purpose. 1. Open System Definition Allows energy and matter
Title: Thermodynamics I. Systems A system is a group of interacting parts, including energy and matter, forming a complex whole with a common purpose. 1. Open System Definition Allows energy and matter
