Iridium concentration and noble gas composition of Cretaceous Tertiary boundary clay from Stevns Klint, Denmark
|
|
|
- Felicity Skinner
- 6 years ago
- Views:
Transcription
1 Geochemical Journal, Vol. 43, pp. 415 to 422, 2009 Iridium concentration and noble gas composition of Cretaceous Tertiary boundary clay from Stevns Klint, Denmark TAKAHITO OSAWA, 1 * YUICHI HATSUKAWA, 1 KEISUKE NAGAO, 2 MITSUO KOIZUMI, 3 MASUMI OSHIMA, 3 YOSUKE TOH, 3 ATSUSHI KIMURA 3 and KAZUYOSHI FURUTAKA 3 1 Neutron Imaging and Activation Analysis Group, Quantum Beam Science Directorate, Japan Atomic Energy Agency (JAEA), Tokai-mura, Naka-gun, Ibaraki , Japan 2 Laboratory for Earthquake Chemistry, Graduate School of Science, The University of Tokyo, Hongo, Tokyo , Japan 3 Innovative Nuclear Science Research Group, Nuclear Science and Engineering Directorate, Japan Atomic Energy Agency (JAEA), Tokai-mura, Naka-gun, Ibaraki , Japan (Received January 12, 2009; Accepted May 13, 2009) The Cretaceous Tertiary (K T) boundary about 65 million years ago records a mass extinction event caused by a bolide impact. K T boundary clay collected from Stevns Klint, Denmark was investigated in this work. Iridium concentrations of eight clays across the K T boundary were determined using a multiple gamma-ray analysis system after neutron activation. Anomalously high Ir concentrations were detected in five marl samples, with the highest concentration being 29.9 ppb. Four samples were analyzed for all noble gases. No extraterrestrial Ar, Kr, and Xe were discovered in any of the samples, although most of the 3 He which was detected was extraterrestrial. Solar-like Ne was observed only in the sample SK4, which had an Ir concentration of 14.3 ppb, indicating the presence of micrometeorites. The solar-like Ne clearly did not originate from an asteroid/comet associated with the bolide impact, as that asteroid is thought to have been extremely large. Also, because there was no sign of a high accretion rate of micrometeorites at the boundary it could not be ascertained whether the solar-like Ne was related to a catastrophic event that led to the extinction of the dinosaurs. Keywords: K T boundary, iridium, noble gas INTRODUCTION It is well known that the concentration of Ir in Cretaceous Tertiary (K T) boundary clay is anomalously high, over 30 ppb (Alvarez et al., 1980; Kyte et al., 1980; Kastner et al., 1984). The Ir-anomaly clearly indicates that a bolide impact occurred about 65 million years ago, corresponding to the Chicxulub crater buried beneath the Yucatán Peninsula in Mexico (e.g., Sharpton et al., 1992). Although noble gas is one of the most sensitive indicators for extraterrestrial material, no extraterrestrial noble gases associated with a bolide impact have been detected in the K T boundary layer (Eugster et al., 1985; Wolbach et al., 1985; Lewis and Wolbach, 1986; Amari et al., 1988; Takaoka and Miura, 1989). A study by Eugster et al. (1985) mainly attempted to find if there was any enrichment of extraterrestrial He in residue treated with HCl and HF. Although they did discover excessive amounts of 3 He in some Stevns Klint residue, they attributed it to the outgassing of primordial 3 He from the man- *Corresponding author ( osawa.takahito@jaea.go.jp) Copyright 2009 by The Geochemical Society of Japan. tle of the earth or 6 Li(n, αβ) 3 He reactions. Lewis and Wolbach (1986) attempted to detect Carbonaceous Chondrite Fission (CCF)-Xe in samples from Stevns Klint and Woodside Creek in New Zealand. Excess fission 136 Xe was discovered in all the samples after being treated with acid, but most or all of that excess was considered to have resulted from the n-induced fission of U. Amari et al. (1988) measured the 3 He/ 4 He ratios of clay from Hokkaido in Japan. However, they could only determine the upper limit of the 3 He/ 4 He ratio (< ), which is not a positive sign of it being extraterrestrial He. Takaoka and Miura (1989) also measured K T boundary clay from Stevns Klint and discovered spallogenic He and Ne in a fraction treated with HCl/HF. However, although this is a result positively indicating the presence of extraterrestrial noble gases, it is not demonstrative proof, because spallogenic He and Ne have also been reported in summit lava on Maui (Marti and Craig, 1987). In addition, the concentration of spallogenic He and Ne from a massive object that fell to the earth 65 million years ago (10 ± 4 km in diameter, Alvarez et al., 1980) would be very low. The lack of extraterrestrial noble gases associated with a bolide impact in the K T boundary can be explained in 415
2 Table 1. Ir concentration of fish clay Sample Weight (mg) Detection time (sec) Ir Noble gas measurement Color ppb error (%) SK1a gray SK1b light gray SK brown SK light gray SK gray SK gray SK light gray SK brown JP1* *Geochemical reference sample (Horoman peridotite). four ways. The first is that primordial noble gas components trapped in a huge meteorite were completely degassed during projection so that no extraterrestrial noble gases remained in the K T boundary clay. The second is that extraterrestrial noble gases were left there but their concentrations are very low. The third is that the noble gases were lost in the acid treatment. The fourth is that the K T mass extinction and Ir anomaly have no extraterrestrial cause. Even if one of these hypotheses is correct, previous studies clearly show that the concentration of extraterrestrial noble gas is low or that carrier phases of noble gases are easily decomposed by acids. The latter finding contradicts the already-known property of chondrites that the trapped primordial noble gas component of chondrites is concentrated in an acid-resistant phase (e.g., Lewis et al., 1975). However, the type of the impact asteroid cannot be completely identified, and the influence of diagenesis of the phase is not understood well. In our study, the noble gas composition of K T boundary clays without acid treatment was determined by a highly refined noble gas analytical system, and iridium concentrations of the clays were determined using a multiple gamma-ray analysis system. SAMPLE The K T boundary clay tested in the present study was collected by Nagao K. from Stevns Klint in Denmark in The specimens were removed from a thin layer of marl, which is known as fish clay. Danish fish clay was subdivided into 4 beds from bed II at the bottom to bed V at the top (Christensen et al., 1973). Under bed II was Maastrichtian white chalk (bed I) and over bed V was Cerithium limestone (bed VI). The samples used in this report were taken from beds II, III, or IV. Bed III was a dark brown color due to weathering of the pyrite concretion. The samples were stored at room temperature and were not dried. The samples are listed in Table 1. Four of eight samples were subjected to noble gas analysis. EXPERIMENTAL PROCEDURES Noble gas measurement The samples were not chemically or physically separated to determine their bulk noble gas composition. The samples were preheated at 150 C for a day in a high vacuum to remove any contaminating atmospheric gases adsorbed to their surfaces. Noble gases were extracted by heating in a tantalum oven, in which the samples were pyrolysed at 900 to 1800 C. All the temperature steps were held for 15 minutes. The extracted gases were then purified using three Ti Zr getters (getters 1, 2, and 3), and Ar, Kr, and Xe were trapped in a liquid-nitrogen-cooled charcoal trap. Since sedimentary material generally has a large variety of volatile species which can cause problems in the purification process, the released gas was purified for 30 minutes using getter 1. Ne was trapped in a cryogenically cooled trap at 15 K, He was introduced into the mass spectrometer, and the isotopic composition was then analyzed. Ne was released at 45 K from the cryogenically cooled trap and measured using the spectrometer. After Ar, Kr, and Xe were released from the charcoal trap, the Kr and Xe were captured in the cryogenic trap at 100 K and the Ar was then measured. Kr and Xe were released at 135 K and 200 K, respectively, from the trap and measured. The noble gas isotopes were analyzed using a highly sensitive mass spectrometer (modified VG-5400/MS-III). A Daly collector was used to measure the light noble gases (He, Ne and Ar) while the Kr and Xe isotopes were measured using a secondary electron multiplier with an ion counting system. The sensitivities to all the noble gases and mass discrimination effects were calibrated by measuring atmospheric noble 416 T. Osawa et al.
3 Table 2. Concentration and isotopic ratio of helium and neon Sample Weight (g) 4 He (10 9 cm 3 STP/g) 3 He (10 15 cm 3 STP/g) 3 He/ 4 He (10 6 ) 20 Ne (10 12 cm 3 STP/g) 20 Ne/ 22 Ne 21 Ne/ 22 Ne 4 He/ 20 Ne SK1a 1800 C ± ± ± SK1b 900 C ± ± ± SK1b 1800 C ± ± ± Total ± ± ± SK3 600 C ± ± ± SK3 900 C ± ± ± SK C ± ± ± SK C n.d. n.d. n.d ± ± n.d. SK C n.d. n.d. n.d ± ± n.d. Total ± ± ± SK C ± ± ± SK C ± ± ± SK C n.d. n.d ± ± SK C n.d. n.d ± ± SK C n.d. n.d ± ± SK C n.d. n.d ± ± n.d. SK C n.d. n.d. n.d ± ± n.d. Total > ± ± ± Calculated from 1000 C and 1100 C fractions. gases and a standard helium gas with 3 He/ 4 He = prepared by mixing pure 3 He and 4 He gases at our laboratory. The mass interference of 40 Ar ++ and CO 2 ++ in the neon analysis was corrected using experimentally determined 40 Ar ++ / 40 Ar + and CO 2 ++ /CO 2 + ratios (Osawa, 2004). For the Ne analysis, the argon and carbon dioxide were removed using a liquid-nitrogen-cooled trap beside a source of ions. The background was determined by heating an empty Mo crucible. The background levels of 4 He, 20 Ne, 40 Ar, 84 Kr, and 132 Xe at 1800 C were cm 3 STP, cm 3 STP, cm 3 STP, cm 3 STP, and cm 3 STP, respectively. Neutron activation analysis Iridium concentration can be determined using Instrumental Neutron Activation Analysis (INAA) with the multiple gamma-ray detection method. The nuclide quantification method was developed by Oshima et al. (Toh et al., 2001; Hatsukawa et al., 2002; Oshima et al., 2002, 2008) and has been successfully applied in a variety of fields. The iridium in the geological samples was determined using the multi-parameter coincidence method with no chemical separation after neutron irradiation. Fifty to one hundred mg of ground homogenized fish clay sediment samples were sealed in pure quartz vials and then irradiated for 48 hours in the JRR-3 reactor at JAEA with a neutron flux of ncm 2 s ng standard iridium samples made from diluted Ir standard solution (ACROS Organic Co.) were also prepared, sealed in quartz vials, and then irradiated together with the sediment samples. After irradiation, the samples were cooled for about four weeks. The gamma ray decay was measured using GEMINI-II, an array of 16 high purity Ge detectors, with a typical measurement taking about a day. The γ-γ coincidence peak of kev from 192 Ir (T 1/2 = 73.8 d) produced by the 191 Ir(n,γ) 192 Ir reaction was used in quantitative analysis. All the samples were measured with a standard 133 Ba source to perform dead-time calibration of the detector system. The iridium content of the sediment samples was obtained by comparing the 192 Ir intensity of the sediment samples with the standard iridium after decay time corrections. RESULTS Ir concentration The measured Ir concentrations of the 8 samples are listed in Table 1. The one-sigma errors are lower than 6%. Anomalously high concentrations over 10 ppb are detected in 5 of the samples. The highest concentration, 29.9 ppb, is found in SK7, which is consistent with the previously reported values of 31.0 and 36.0 ppb in bed II or III (Kyte et al., 1980). The Ir concentrations of the four samples for which noble gas analysis was carried Ir concentration and noble gas composition of K T boundary clay 417
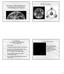
4 Table 3. Concentration and isotopic ratio of heavy noble gases Sample Weight (g) 40 Ar (10 9 cm 3 STP/g) 36 Ar (10 9 cm 3 STP/g) 40 Ar/ 36 Ar 38 Ar/ 36 Ar 84 Kr (10 12 cm 3 STP/g) 132 Xe (10 12 cm 3 STP/g) 36 Ar/ 132 Xe 84 Kr/ 132 Xe SK1a , ± ± SK1b 900 C , ± ± ,470 1, SK1b 1800 C ± ± Total 99, ± ± ,475 1, SK3 600 C ± ± SK3 900 C , ± ± SK C ± ± SK C ± ± SK C ± ± Total 2, ± ± SK C ± ± SK C ± ± SK C ± ± SK C ± ± SK C ± ± SK C ± ± SK C ± ± Total ± ± out varied. SK3 and SK1b had the relatively low Ir concentrations of 2.5 and 4.6 ppb, respectively. On the other hand, SK1a and SK4 have anomalously high Ir concentrations of 24.7 and 14.3 ppb, respectively, leading to the conclusion that extraterrestrial material is present. Noble gas composition The measured noble gas concentrations and isotopic ratios of the four clay samples are summarized in Tables 2 and 3. The error is one sigma. Helium 3 He is a very sensitive tracer of micrometeorites in sediments and is used to estimate the influx of micrometeorites onto the Earth (e.g., Farley, 1995; Mukhopadhyay et al., 2001). The extraterrestrial fraction of 3 He in the sediments can be calculated from 3 He/ 4 He ratio which is modeled as a simple two-component mixture of crustal and extraterrestrial He (Takayanagi and Ozima, 1987; Farley, 1995). 4 He/ 20 Ne ratios of the clay samples are more than 200 times the atmospheric ratio, 0.32 (Table 2), proving that the contribution of atmospheric 3 He is negligible. Assuming a 3 He/ 4 He ratio of for clay (Andrews, 1985), the calculated extraterrestrial 3 He accounts for at least 96% of the total 3 He. It is notable that there is no correlation between Ir and 3 He concentration. The highest 3 He concentration of cm 3 STP/g is found in SK1b, in which a low concentration of Ir (5.3 ppb) was detected. On the other hand, the lowest 3 He concentration of > cm 3 STP/g is observed in SK1b, which has a high Ir concentration of 14.3 ppb. SK1a has the highest Ir concentration (24.7 ppb) and high 3 He concentration ( cm 3 STP/g). The discrepancy between Ir and 3 He concentration can be explained by positing that 3 He concentration is affected by two factors: nonuniformity of the small number of micrometeorites stored in the clays and high variation of the accumulation rate. Since there is no correlation between Ir and 3 He concentration, it is clear that most of the extraterrestrial 3 He in the K T boundary clays is not due to a bolide hitting the earth, and originates from micrometeorites accreted on the Earth. Takaoka and Miura (1989) reported a definite excess of 3 He in a sample of Stevns Klint clay treated with HCl/ HF. They found a 3 He/ 4 He ratio of 97 ± , which is 69 times the atmospheric value. However, no such high isotopic ratios are found in our samples, which is presumably because there was no separation of the samples. Neon The Ne isotopic compositions are plotted in Fig. 1. The Ne released from SK1a, SK1b and SK3 is predominantly atmospheric Ne, their total Ne isotopic compositions corresponding to the results of Takaoka and Miura (1989), in which 20 Ne/ 22 Ne = 9.43 ± 0.08 and 21 Ne/ 22 Ne = ± However, the Ne concentration of SK4 is much lower than the other samples and its isotopic composition is exceptional. Both the 20 Ne/ 22 Ne and 21 Ne/ 22 Ne ratios of SK4 are clearly higher than those of the atmosphere, revealing the presence of a non-atmospheric source. The high ratio of 20 Ne/ 22 Ne can be explained by the contribution of the solar wind or mantle, but it is not easy to explain how mantle Ne can exist in marl. Micrometeorites are, therefore, a more plausible source 418 T. Osawa et al.
5 Fig. 2. Three isotope plot of Ar for all samples. Errors are one sigma. Q-Ar after Busemann et al. (2000) and Air-Ar after Nier (1950). Fig. 1. Three isotope plot of Ne for SK4 (a) and others (b). The data from all temperature fractions is given. Solar wind (SW)- Ne after Benkert et al. (1993) and Air-Ne after Eberhardt et al. (1965). Errors are one sigma. The numbers shown in the upper diagram give the temperature in centigrade. The shaded area indicates the range of micrometeorites (e.g., Osawa et al., 2003). In the lower diagram Takaoka and Miura (1989) give the data from SK2-4R, an HCl/HF treated residue. of the solar-like Ne because micrometeorites retain large amounts of solar noble gases (e.g., Osawa and Nagao, 2002; Osawa et al., 2003) and it is known that solarderived Ne gets concentrated in deep-sea sediment (Matsuda et al., 1990). The high ratio of 21 Ne/ 22 Ne in SK4 is probably due to nucleogenic 21 Ne produced in the 18 O(α,n) 21 Ne reaction, because cosmogenic 21 Ne is scarce in micrometeorites (e.g., Osawa and Nagao, 2002). Judging from the overall composition of the Ne in the sample ( 20 Ne/ 22 Ne = 11.6, 21 Ne/ 22 Ne = 0.062) the contribution of non-atmospheric Ne is high. This isotopic composition is also due to the low concentration of total Ne, only cm 3 STP/g. The isotopic composition of the Ne in SK1a is different from SK4, with no sign of solar-derived Ne, although the clay did have the highest Ir concentration of all the samples, thus revealing the presence of extraterrestrial material. The Ne trapped in SK1a is almost all terrestrial. Assuming that all 3 He is derived from micrometeorites and that the average 3 He/ 20 Ne ratio of micrometeorites is (Nakamura and Takaoka, 2000), the contribution of solar-ne can be roughly estimated from the concentrations of 3 He. The contributions of micrometeorite-derived 20 Ne in SK1a, SK1b, SK3 and SK4 are 5.1%, 3.1%, 0.5% and >5.3%, respectively. The low value of >5.3% in SK4 might indicate a preferential loss of 3 He, although the mechanism is not clear. Heavy noble gases Ar isotopic composition is the most sensitive indicator for extraterrestrial samples that have been heavily contaminated or degassed through heating or alteration. There are three reasons why Ar is a sensitive indicator for extraterrestrial material: 1) The sensitivity of the mass spectrometer to Ar is higher than to He or Ne. 2) Mass interferences of 36 Ar, 38 Ar, 40 Ar are not serious in a mass spectrometer with mass resolution of ) The shift of the 40 Ar/ 36 Ar ratio is very large, because most 40 Ar is produced from the electron-capture radioactive decay of 40 K. Osawa and Nagao (2003) revealed that about 40% of totally melted spherules contain extraterrestrial Ar because the Ar is well preserved compared to He and Ne. Ir concentration and noble gas composition of K T boundary clay 419
6 of terrestrial atmosphere, indicating that the high concentrations of heavy noble gases are mainly due to adsorbed air. However, the elemental composition of the clay is significantly fractionated ( 36 Ar/ 132 Xe = 243, 84 Kr/ 132 Xe = 10.3). The cause of the exceptionally high concentrations of heavy noble gases in SK1b may be large surface area due to the grain size. The Xe isotopic compositions, which are not specifically analyzed here, are closer to the terrestrial atmosphere than any other extraterrestrial source. DISCUSSION Fig. 3. Heavy noble gas elemental compositions for all fractions. Eugster et al. (1985) give the total amount of noble gases released from Stevns Klint 2 residue (run2) that had been treated with HCl/HF. Heavily fractionated terrestrial atmosphere is found in SK1a. Saturated noble gases in sea water at 25 C as calculated by Ozima and Podosek (2002). Since 40 Ar in primordial Ar in the solar system is scarce, the 40 Ar/ 36 Ar ratio can provide important information on extraterrestrial materials when the radiogenic 40 Ar and atmospheric contamination are not exceptionally large. A three isotope plot of the K T boundary clay is given in Fig. 2. No sample has a 40 Ar/ 36 Ar ratio lower than that of the terrestrial atmosphere, 296. SK1a and SK4, with their high Ir concentrations, have high 40 Ar/ 36 Ar ratios, reflecting the presence of radiogenic 40 Ar produced from in situ radioactive decay of 40 K. The Ar isotopic ratios of SK1b and SK3 are near that of the atmosphere. 38 Ar/ 36 Ar ratios higher than that of the atmosphere, 0.188, are found in all the samples, presumably indicating the presence of nucleogenic 38 Ar originating from the 35 Cl(α,p) 38 Ar reaction. As in the case of Eugster et al. (1985), there is no sign of extraterrestrial Ar isotopes in our samples. The elemental compositions of Ar, Kr, and Xe are given in Fig. 3. All the samples have 84 Kr/ 132 Xe and 36 Ar/ 132 Xe ratios lower than that of the terrestrial atmosphere, SK1a in particular being near the region of chondrite matrices. However, elemental compositions do not demonstrate the presence of extraterrestrial noble gases because adsorbed gases get heavily fractionated (Scherer et al., 1994). Eugster et al. (1985) also reported low 84 Kr/ 132 Xe and 36 Ar/ 132 Xe ratios. SK1b contains vary large amounts of Ar, Kr, and Xe compared to the other samples (Table 3) and its 40 Ar/ 36 Ar ratio of 312 ± 2 is close to that There is no evidence of extraterrestrial Ar, Kr, or Xe. However, the Ne in SK4 clearly indicates the presence of solar-derived Ne, which presumably originated in micrometeorites. In fact, when the nucleogenic (or cosmogenic) Ne is subtracted from the overall Ne, the corrected Ne composition corresponds to that of micrometeorites (Fig. 1). It is, however, very difficult to accept that a bolide hitting the earth would include a lot of solar-derived Ne, because solar winds can only penetrate several hundred angstroms into the surface of rock (Benkert et al., 1993). Further, since the diameter of the asteroid has been estimated to be 10 ± 4 kilometers (Alvarez et al., 1980) the amount of solar-ne is negligible. The solar-derived Ne therefore seems to be unrelated to the asteroid itself. If the source of the solar-derived Ne is micrometeorites, can it then be said that a flux of extraterrestrial dust rose at the K T boundary? Mukhopadhyay et al. (2001) measured the He concentration in marine carbonates throughout a time span of 63.9 to 65.4 million years ago and concluded that the bolide associated with the K T extinction event was not accompanied by enhanced solar system dustiness, due to the near-constant 3 He accretion rate across the boundary. The detected solar-like Ne might be merely due to low interference of atmospheric Ne. The 3 He/ 20 Ne ratio of SK4, , is significantly lower than that of average micrometeorites, (Nakamura and Takaoka, 2000), and the noble gas concentrations of SK4 are much lower than those of other samples. The result may reflect noble gas depletion by heating. In a stepwise heating experiment performed on micrometeorite-bearing particles separated from Antarctic ice, about 87% of He was extracted at 700 C but about 71% of Ne still remained (Nakamura and Takaoka, 2000). Indeed, severely heated extraterrestrial particles, such as cosmic spherules, have a low He/Ne ratio (Osawa and Nagao, 2003). In the age when SK4 cumulated, severely heated extraterrestrial particles, e.g., particles with very fast relative speed to the Earth, might have been predominant, and the event might be linked with some kind of 420 T. Osawa et al.
7 astronomical phenomenon. Our results also show that noble gases trapped in the K T extraterrestrial projectile had been mostly depleted. If the Ir concentration of the projectile is assumed to have been 470 ppb, which is a typical concentration in CI chondrite and reflects the solar abundance, the extraterrestrial material in SK1a was diluted to 1/19. Further, if the 36 Ar concentration of the projectile is assumed to have been cm 3 STP/g, which is the average concentration in CI chondrite, the 36 Ar concentration in SK1a should be cm 3 STP/g, thirteen times higher than the 36 Ar concentration in SK1a found in this study. In SK4, only 0.45% of the extraterrestrial 36 Ar is estimated to have remained in the clay. This indicates that there was a rise in temperature to over 1000 C, which explains why the noble gases almost completely escaped from the noble gas carriers of the bolide even though the primordial noble gas component trapped in the chondrite was tightly captured. Although a highly sensitive mass spectrometer was used, no sign of chondritic noble gases was found in this study. Even if the sensitivity of mass spectrometers is greatly improved in the future, it will still be difficult to identify the noble gases associated with the projectile without the analysis of a large amount of the K T boundary clay. CONCLUSION The Ir concentrations of eight K T boundary samples collected from Stevns Klint were measured, and four of them were subjected to noble gas analysis. Five of the samples had Ir anomalies, with the highest value being 29.9 ppb, which is consistent with previously reported concentrations. As in the case of previous noble gas research conducted on K T boundary clay, there was no sign of extraterrestrial Ar, Kr, and Xe isotopic and elemental compositions in the Stevns Klint samples. The compositions of these three elements can be explained as being due to the contribution of adsorbed terrestrial atmosphere, radiogenic nuclides, and nucleogenic nuclides. On the other hand, most of the 3 He is extraterrestrial and can be traced to micrometeorites. Solar-like Ne was observed in SK4, in which the relatively high Ir concentration of 14.3 ppb was detected through multiple gammaray analysis. The source of the solar-like Ne was presumed to be micrometeorites accreted on the earth, because micrometeorites preserve solar noble gases. That said, though the projectile associated with the K T extinction was massive, the amount of solar-derived Ne it contained was negligible when compared with the overall amount of Ne. Further, no evidence of a dramatic increase in the accretion rate of extraterrestrial dust was discovered. It is, therefore, still unclear whether the solar-like Ne is related to the mass extinction event that led to the extinction of so many living things. Acknowledgments Des Patterson and an anonymous referee are thanked for their constructive comments and suggestions. REFERENCES Alvarez, L. W., Alvarez, W., Asaro, F. and Michel, H. V. (1980) Extraterretsrial cause for the Cretaceous Tertiary extinction. Science 208, Amari, S., Ozima, M. and Hamano, Y. (1988) The 3 He/ 4 He ratio in a clay from the K T boundary Hokaaido, Japan. Proc. NIPR Antarct. Meteorites 1, Andrews, J. N. (1985) The isotopic composition of radiogenic helium and its use to study groundwater movement in confined aquifers. Chem. Geol. 49, Benkert, J. P., Baur, H., Signer, P. and Wieler, R. (1993) He, Ne, and Ar from the solar wind and solar energetic particles in lunar ilmenites and pyroxenes. J. Geophys. Res. 98, E7, Busemann, H., Baur, H. and Wieler, R. (2000) Primordial noble gases in phase Q in carbonaceous and ordinary chondrites studied by closed-system stepped etching. Meteorit. Planet. Sci. 35, Christensen, L., Fregerslev, S., Simonsen, A. and Thiede, J. (1973) Sedimentology and depositional environment of lower Danian fish clay from Stevns Klint, Denmark. Bull. Geol. Soc. Den. 22, Eberhardt, P., Eugster, O. and Marti, K. (1965) A redetermination of the isotopic composition of atmospheric neon. Z. Naturforsch. 20a, Eugster, O., Geiss, J. and Krähenbühl, U. (1985) Noble gas isotopic abundances and noble metal concentrations in sediments from the Cretaceous Tertiary boundary. Earth Planet. Sci. Lett. 74, Farley, K. A. (1995) Cenozoic variations in the flux of interplanetary dust recorded by 3 He in a deep-sea sediment. Nature 376, Hatsukawa, Y., Oshima, M., Hayakawa, T., Toh, Y. and Shinohara, N. (2002) Application of multiparameter coincidence spectrometry using a Ge detectors array to neutron activation analysis. Nucl. Instrum, Methods Phys. Res. A 482, Kastner, M., Asaro, F., Michel, H. V., Alvarez, W. and Alvarez, L. W. (1984) The precursor of the Cretaceous Tertiary boundary clays at Stevns Klint, Denmark, and DSDP Hole 465A. Science 226, Kyte, F. T., Zhou, Z. and Wasson, T. (1980) Siderophileenriched sediments from the Cretaceous Tertiary boundary. Science 288, Lewis, R. S. and Wolbach, W. S. (1986) Search for noble gases at the K T boundary. Meteoritics 21, Lewis, R. S., Srinivasan, B. and Anders, E. (1975) Host phase of a strange xenon component in Allende. Science 190, Marti, K. and Craig, H. (1987) Cosmic-ray-produced neon and helium in the summit lavas of Maui. Nature 325, Ir concentration and noble gas composition of K T boundary clay 421
8 Matsuda, J., Murota, M. and Nagao, K. (1990) He and Ne isotopic studies on the extraterrestrial material in deep-sea sediments. J. Geophys. Res. 95, Mukhopadhyay, S., Farley, K. A. and Montanari, A. (2001) A short duration of the Cretaceous Tertiary boundary event: evidence from extraterrestrial helium-3. Science 291, Nakamura, T. and Takaoka, N. (2000) Solar-wind derived light noble gases in micrometeorites collected at the Dome Fuji Station: Characterization by trapped pyrolysis. Antarct. Meteorite Res. 13, Nier, A. O. (1950) A redetermination of the relative abundances of the isotopes of carbon, nitrogen, oxygen, argon and potassium. Phys. Rev. 77, Osawa, T. (2004) A new correction technique for mass interferences by 40 Ar ++ and CO 2 ++ during isotope analysis of a small amount of Ne. J. mass spectrum. Soc. Jpn. 52, Osawa, T. and Nagao, K. (2002) Noble gas compositions of Antarctic micrometeorites collected at the Dome Fuji Station in 1996 and Meteorit. Planet. Sci. 37, Osawa, T. and Nagao, K. (2003) Remnant extraterrestrial noble gases in Antarctic cosmic spherules. Antarct. Meteorite Res. 16, Osawa, T., Nakamura, T. and Nagao, K. (2003) Noble gas isotopes and mineral assemblages of Antarctic micrometeorites collected at the meteorite ice field around the Yamato Mountains. Meteorit. Planet. Sci. 38, Oshima, M., Toh, Y., Hatsukawa, Y., Hayakawa, T. and Shinohara, N. (2002) A high-sensitivity and non-destructive trace element analysis based on multiple gamma-ray detection. J. Nucl. Sci. Technol. 39, Oshima, M., Toh, Y., Hatsukawa, Y., Koizumi, M., Kimura, A., Haraga, A., Ebihara, M. and Sushida, K. (2008) Multiple gamma-ray detection method and its application to nuclear chemistry. J. Radioanal. Nucl. Chem. 278, Ozima, M. and Podosek, F. A. (2002) Noble Gas Geochemistry Second Edition. Cambridge University Press, Cambridge, U.K. Scherer, P., Schultz, L. and Loeken, T. (1994) Weathering and atmospheric noble gases in chondrites. Noble Gas Geochemistry and Cosmochemistry (Matsuda, J., ed.), 43 53, Terra Scientific Publishing Company, Japan. Sharpton, V. L., Dalrymple, G. B., Marín, L. E., Ryder, G., Schuraytz, B. C. and Urrutia-Fucugauchi, J. (1992) New links between the Chicxulub impact structure and the Cretaceous/Tertiary boundary. Nature 359, Takaoka, N. and Miura, Y. (1989) Noble gases in the K T boundary clay from Stevns Klint, Denmark. Proc. NIPR Antarct. Meteorites 2, Takayanagi, M. and Ozima, M. (1987) Temporal variation of 3 He/ 4 He ratio recorded in deep-sea sediment cores. J. Geophys. Res. 92, Toh, Y., Oshima, M., Hatsukawa, Y., Hayakawa, T. and Shinohara, N. (2001) Comparison method for neutron activation analysis with γ-γ matrix. J. Radioanal. Nucl. Chem. 250, Wolbach, W. S., Lewis, R. S. and Anders, E. (1985) Cretaceous extinctions: evidence for wildfires and search for meteoritic material. Science 230, T. Osawa et al.
Dating methods Stragraphic or geologic methods. Thomas INGICCO
 Dating methods Stragraphic or geologic methods Thomas INGICCO Electro-spin resonance (ESR) An electron can be represented by a negatively charged sphere animated of a rotational movement on itself. This
Dating methods Stragraphic or geologic methods Thomas INGICCO Electro-spin resonance (ESR) An electron can be represented by a negatively charged sphere animated of a rotational movement on itself. This
Noble gas isotopes and mineral assemblages of Antarctic micrometeorites collected at the meteorite ice field around the Yamato Mountains
 Meteoritics & Planetary Science 38, Nr 11, 1627 1640 (2003) Abstract available online at http://meteoritics.org Noble gas isotopes and mineral assemblages of Antarctic micrometeorites collected at the
Meteoritics & Planetary Science 38, Nr 11, 1627 1640 (2003) Abstract available online at http://meteoritics.org Noble gas isotopes and mineral assemblages of Antarctic micrometeorites collected at the
For thought: Excess volatiles
 For thought: Excess volatiles Term coined by William Rubey (circa 1955) Definition: Compounds present at Earth s surface that were not derived from converting igneous rock to sedimentary rock Rubey and
For thought: Excess volatiles Term coined by William Rubey (circa 1955) Definition: Compounds present at Earth s surface that were not derived from converting igneous rock to sedimentary rock Rubey and
Noble gases in solar-gas-rich and solar-gas-free polymict breccias
 Antarct. Meteorite Res., +3, /2 12,,**0,**0 National Institute of Polar Research Noble gases in solar-gas-rich and solar-gas-free polymict breccias Takahito Osawa +,, and Keisuke Nagao, + Research Group
Antarct. Meteorite Res., +3, /2 12,,**0,**0 National Institute of Polar Research Noble gases in solar-gas-rich and solar-gas-free polymict breccias Takahito Osawa +,, and Keisuke Nagao, + Research Group
Acid-susceptive material as a host phase of argon-rich noble gas in the carbonaceous chondrite Ningqiang
 Meteoritics & Planetary Science 38, Nr 2, 243 250 (2003) Abstract available online at http://meteoritics.org Acid-susceptive material as a host phase of argon-rich noble gas in the carbonaceous chondrite
Meteoritics & Planetary Science 38, Nr 2, 243 250 (2003) Abstract available online at http://meteoritics.org Acid-susceptive material as a host phase of argon-rich noble gas in the carbonaceous chondrite
Phys 214. Planets and Life
 Phys 214. Planets and Life Dr. Cristina Buzea Department of Physics Room 259 E-mail: cristi@physics.queensu.ca (Please use PHYS214 in e-mail subject) Lecture 10. Geology and life. Part 1 (Page 99-123)
Phys 214. Planets and Life Dr. Cristina Buzea Department of Physics Room 259 E-mail: cristi@physics.queensu.ca (Please use PHYS214 in e-mail subject) Lecture 10. Geology and life. Part 1 (Page 99-123)
Isotopic record of the atmosphere and hydrosphere
 Isotopic record of the atmosphere and hydrosphere W. F. McDonough 1 1 Department of Earth Sciences and Research Center for Neutrino Science, Tohoku University, Sendai 980-8578, Japan (Dated: March 7, 2018)
Isotopic record of the atmosphere and hydrosphere W. F. McDonough 1 1 Department of Earth Sciences and Research Center for Neutrino Science, Tohoku University, Sendai 980-8578, Japan (Dated: March 7, 2018)
Noble gases of the Yamato and Yamato lherzolitic shergottites from Mars
 Available online at www.sciencedirect.com Polar Science 2 (2008) 195e2 http://ees.elsevier.com/polar/ Noble gases of the Yamato 000027 and Yamato 000097 lherzolitic shergottites from Mars Keisuke Nagao
Available online at www.sciencedirect.com Polar Science 2 (2008) 195e2 http://ees.elsevier.com/polar/ Noble gases of the Yamato 000027 and Yamato 000097 lherzolitic shergottites from Mars Keisuke Nagao
Evidence for a Large Anomalous Nuclear Explosions in Mars Past. J.E. Brandenburg LPSC 2015
 Evidence for a Large Anomalous Nuclear Explosions in Mars Past J.E. Brandenburg LPSC 2015 Mars Isotopes: Baseline We can compare Mars isotopes with those from meteorites Earth and other Planets which sampled
Evidence for a Large Anomalous Nuclear Explosions in Mars Past J.E. Brandenburg LPSC 2015 Mars Isotopes: Baseline We can compare Mars isotopes with those from meteorites Earth and other Planets which sampled
Biodiversity Through Earth History
 Chapter 13 Biodiversity Through Earth History Underlying assumption is that the process of evolution is occurring evolution: creation of new species random mutation: genetic changes natural selection:
Chapter 13 Biodiversity Through Earth History Underlying assumption is that the process of evolution is occurring evolution: creation of new species random mutation: genetic changes natural selection:
Dating. AST111 Lecture 8a. Isotopic composition Radioactive dating
 Dating Martian Lafayette Asteroid with patterns caused by the passaged through the atmosphere. Line on the fusion crust were caused by beads of molten rock. AST111 Lecture 8a Isotopic composition Radioactive
Dating Martian Lafayette Asteroid with patterns caused by the passaged through the atmosphere. Line on the fusion crust were caused by beads of molten rock. AST111 Lecture 8a Isotopic composition Radioactive
Composition and the Early History of the Earth
 Composition and the Early History of the Earth Sujoy Mukhopadhyay CIDER 2006 What we will cover in this lecture Composition of Earth Short lived nuclides and differentiation of the Earth Atmosphere and
Composition and the Early History of the Earth Sujoy Mukhopadhyay CIDER 2006 What we will cover in this lecture Composition of Earth Short lived nuclides and differentiation of the Earth Atmosphere and
a clue to origin and histories
 Noble gas and oxygen isotope studies of aubrites: a clue to origin and histories Yayoi N. MIURA 1*, Hiroshi HIDAKA 2, Kunihiko NISHIIZUMI 3 and Minoru KUSAKABE 4 1 Earthquake Research Institute, University
Noble gas and oxygen isotope studies of aubrites: a clue to origin and histories Yayoi N. MIURA 1*, Hiroshi HIDAKA 2, Kunihiko NISHIIZUMI 3 and Minoru KUSAKABE 4 1 Earthquake Research Institute, University
For thought: Excess volatiles
 For thought: Excess volatiles Term coined by William Rubey (circa 1955) Definition: Compounds present at Earth s surface that were not derived from converting igneous rock to sedimentary rock Rubey and
For thought: Excess volatiles Term coined by William Rubey (circa 1955) Definition: Compounds present at Earth s surface that were not derived from converting igneous rock to sedimentary rock Rubey and
Vertical Variation of Rhenium, Cadmium, Silver and Platinum-group Elements within a Transitional Bed of Danish Cretaceous-Tertiary Boundary Layer
 No. 8] Proc. Japan Acad., 72, Ser. B (1996) 163 Vertical Variation of Rhenium, Cadmium, Silver and Platinum-group Elements within a Transitional Bed of Danish Cretaceous-Tertiary Boundary Layer By Takahiro
No. 8] Proc. Japan Acad., 72, Ser. B (1996) 163 Vertical Variation of Rhenium, Cadmium, Silver and Platinum-group Elements within a Transitional Bed of Danish Cretaceous-Tertiary Boundary Layer By Takahiro
Comet Science Goals II
 Comet Science Goals II {questions for goals} Don Brownlee Did the events postulated by the Nice Hypothesis really happen? Were there wide-spread solar system wide impact events that were coeval with the
Comet Science Goals II {questions for goals} Don Brownlee Did the events postulated by the Nice Hypothesis really happen? Were there wide-spread solar system wide impact events that were coeval with the
Meteorites. A Variety of Meteorite Types. Ages and Compositions of Meteorites. Meteorite Classification
 Meteorites A meteor that survives its fall through the atmosphere is called a meteorite Hundreds fall on the Earth every year Meteorites do not come from comets First documented case in modern times was
Meteorites A meteor that survives its fall through the atmosphere is called a meteorite Hundreds fall on the Earth every year Meteorites do not come from comets First documented case in modern times was
What we ll learn today:!
 Learning Objectives (LO) Lecture 17: Age Dating and Earth History Read: Chapter 12-13 Homework #14 What we ll learn today:! 1. 1. Define the concept of half-life and absolute age dating! 2. 2. List the
Learning Objectives (LO) Lecture 17: Age Dating and Earth History Read: Chapter 12-13 Homework #14 What we ll learn today:! 1. 1. Define the concept of half-life and absolute age dating! 2. 2. List the
NOBLE GASES AND 81 Kr-TERRESTRIAL AGE OF ASUKA LUNAR METEORITE. Keisuke NAGA0 1 and Y ayoi MIURA 1 *
 Proc. NIPR Symp. Antarct. Meteorites, 6, 76---87, 1993 NOBLE GASES AND 81 Kr-TERRESTRIAL AGE OF ASUKA-881757 LUNAR METEORITE Keisuke NAGA0 1 and Y ayoi MIURA 1 * 1 Institute for Study of the Earth's Interior,
Proc. NIPR Symp. Antarct. Meteorites, 6, 76---87, 1993 NOBLE GASES AND 81 Kr-TERRESTRIAL AGE OF ASUKA-881757 LUNAR METEORITE Keisuke NAGA0 1 and Y ayoi MIURA 1 * 1 Institute for Study of the Earth's Interior,
Geol. 656 Isotope Geochemistry
 ISOTOPE COSMOCHEMISTRY II ISOTOPIC ANOMALIES IN METEORITES In the previous lecture, we looked at the geochronology of meteorites, both from the perspective of conventional decay systems and from the perspective
ISOTOPE COSMOCHEMISTRY II ISOTOPIC ANOMALIES IN METEORITES In the previous lecture, we looked at the geochronology of meteorites, both from the perspective of conventional decay systems and from the perspective
Noble gases in ureilites released by crushing
 Meteoritics & Planetary Science 38, Nr 5, 767 781 (2003) Abstract available online at http://meteoritics.org Noble gases in ureilites released by crushing Ryuji OKAZAKI, 1, 2 * Tomoki NAKAMURA, 1 Nobuo
Meteoritics & Planetary Science 38, Nr 5, 767 781 (2003) Abstract available online at http://meteoritics.org Noble gases in ureilites released by crushing Ryuji OKAZAKI, 1, 2 * Tomoki NAKAMURA, 1 Nobuo
What can isotopes tell us about mantle dynamics? Sujoy Mukhopadhyay. Harvard University
 What can isotopes tell us about mantle dynamics? Sujoy Mukhopadhyay Harvard University The mantle zoo Hofmann, 1997 187 Os/ 188 Os 0.168 0.156 0.144 0.132 EM1 Hawaii Pitcairn DMM peridotites Shield Basalts
What can isotopes tell us about mantle dynamics? Sujoy Mukhopadhyay Harvard University The mantle zoo Hofmann, 1997 187 Os/ 188 Os 0.168 0.156 0.144 0.132 EM1 Hawaii Pitcairn DMM peridotites Shield Basalts
Primordial noble gases in a graphite-metal inclusion from the Canyon Diablo IAB iron meteorite and their implications
 Meteoritics & Planetary Science 40, Nr 3, 431 443 (2005) Abstract available online at http://meteoritics.org Primordial noble gases in a graphite-metal inclusion from the Canyon Diablo IAB iron meteorite
Meteoritics & Planetary Science 40, Nr 3, 431 443 (2005) Abstract available online at http://meteoritics.org Primordial noble gases in a graphite-metal inclusion from the Canyon Diablo IAB iron meteorite
Hypervelocity Impacts of Macroscopic Bodies
 154 Chapter 5 Hypervelocity Impacts of Macroscopic Bodies 5.1 Asteroid impacts on the Earth Although the Earth is constantly bombarded with small meteorites and micrometeorites, large impacts are (thankfully)
154 Chapter 5 Hypervelocity Impacts of Macroscopic Bodies 5.1 Asteroid impacts on the Earth Although the Earth is constantly bombarded with small meteorites and micrometeorites, large impacts are (thankfully)
Question 1 (1 point) Question 2 (1 point) Question 3 (1 point)
 Question 1 (1 point) If the Earth accreted relatively slowly, the heat obtained from the gravitational potential energy would have had time to escape during its accretion. We know that the Earth was already
Question 1 (1 point) If the Earth accreted relatively slowly, the heat obtained from the gravitational potential energy would have had time to escape during its accretion. We know that the Earth was already
INSTRUMENTAL NEUTRON ACTIVATION ANALYSIS (INAA)
 Instrumental neutron activation analysis (INAA) is used to determine the concentration of trace and major elements in a variety of matrices. A sample is subjected to a neutron flux and radioactive nuclides
Instrumental neutron activation analysis (INAA) is used to determine the concentration of trace and major elements in a variety of matrices. A sample is subjected to a neutron flux and radioactive nuclides
Noble gases in chondrules and associated metal-sulfide-rich samples: Clues on chondrule formation and the behavior of noble gas carrier phases
 Meteoritics & Planetary Science 39, Nr 1, 117 135 (2004) Abstract available online at http://meteoritics.org Noble gases in chondrules and associated metal-sulfide-rich samples: Clues on chondrule formation
Meteoritics & Planetary Science 39, Nr 1, 117 135 (2004) Abstract available online at http://meteoritics.org Noble gases in chondrules and associated metal-sulfide-rich samples: Clues on chondrule formation
Radiogenic Isotope Systematics and Noble Gases. Sujoy Mukhopadhyay CIDER 2006
 Radiogenic Isotope Systematics and Noble Gases Sujoy Mukhopadhyay CIDER 2006 What I will not cover.. U-Th-Pb sytematics 206 Pb 204 Pb 207 Pb 204 Pb 208 Pb 204 Pb = t = t = t 206 Pb 204 Pb 207 Pb 204 Pb
Radiogenic Isotope Systematics and Noble Gases Sujoy Mukhopadhyay CIDER 2006 What I will not cover.. U-Th-Pb sytematics 206 Pb 204 Pb 207 Pb 204 Pb 208 Pb 204 Pb = t = t = t 206 Pb 204 Pb 207 Pb 204 Pb
Noble gases in oxidized residue prepared from the Saratov L4 chondrite and Raman spectroscopic study of residues to characterize phase Q
 Meteoritics & Planetary Science 51, Nr 1, 70 79 (2016) doi: 10.1111/maps.12587 Noble gases in oxidized residue prepared from the Saratov L4 chondrite and Raman spectroscopic study of residues to characterize
Meteoritics & Planetary Science 51, Nr 1, 70 79 (2016) doi: 10.1111/maps.12587 Noble gases in oxidized residue prepared from the Saratov L4 chondrite and Raman spectroscopic study of residues to characterize
Measurements of Neutron Cross Section of the 243 Am(n,γ) 244 Am Reaction
 Measurements of Neutron Cross Section of the (n,γ) 44 Am Reaction Yuichi HATSUKAWA, Nobuo SHINOHARA, Kentaro HATA Nuclear Chemistry Laboratory Tokai-mura, Naka-gun, Ibaraki-ken 319-11 e-mail: hatsu@popsvr.tokai.jaeri.go.jp
Measurements of Neutron Cross Section of the (n,γ) 44 Am Reaction Yuichi HATSUKAWA, Nobuo SHINOHARA, Kentaro HATA Nuclear Chemistry Laboratory Tokai-mura, Naka-gun, Ibaraki-ken 319-11 e-mail: hatsu@popsvr.tokai.jaeri.go.jp
Noble gases in presolar diamonds III: Implications of ion implantation experiments with synthetic nanodiamonds
 Meteoritics & Planetary Science 43, Nr 11, 1811 1826 (2008) Abstract available online at http://meteoritics.org Noble gases in presolar diamonds III: Implications of ion implantation experiments with synthetic
Meteoritics & Planetary Science 43, Nr 11, 1811 1826 (2008) Abstract available online at http://meteoritics.org Noble gases in presolar diamonds III: Implications of ion implantation experiments with synthetic
Variation of Elements in Nature
 Variation of Elements in Nature by V.S. Venkatavaradan There are an infinite variety of things in an infinite variety of shapes, colours and states in nature. Despite varied properties, these multitudes
Variation of Elements in Nature by V.S. Venkatavaradan There are an infinite variety of things in an infinite variety of shapes, colours and states in nature. Despite varied properties, these multitudes
Geologic Time: Hutton s Outcrop at Siccar Point. How do we determine age (relative & absolute) What is the age of the earth? How do we know?
 Geologic Time: How do we determine age (relative & absolute) What is the age of the earth? How do we know? What is the age of the Earth? A. 4.44 million years B. 1 million years C. 4.55 billion years D.
Geologic Time: How do we determine age (relative & absolute) What is the age of the earth? How do we know? What is the age of the Earth? A. 4.44 million years B. 1 million years C. 4.55 billion years D.
Biodiversity Through Earth History. What does the fossil record tell us about past climates and past events?
 Biodiversity Through Earth History What does the fossil record tell us about past climates and past events? Useful terminology: Evolution Natural Selection Adaptation Extinction Taxonomy Logistic Growth
Biodiversity Through Earth History What does the fossil record tell us about past climates and past events? Useful terminology: Evolution Natural Selection Adaptation Extinction Taxonomy Logistic Growth
Since the synthesis, isolation, and characterization of
 Fullerenes: An extraterrestrial carbon carrier phase for noble gases Luann Becker*, Robert J. Poreda, and Ted E. Bunch *School of Ocean and Earth Science and Technology, University of Hawaii, Honolulu,
Fullerenes: An extraterrestrial carbon carrier phase for noble gases Luann Becker*, Robert J. Poreda, and Ted E. Bunch *School of Ocean and Earth Science and Technology, University of Hawaii, Honolulu,
Impact Events in Earth History: The Cretaceous-Paleogene Boundary Ejecta Layer and its Source Crater at Chicxulub
 Impact Events in Earth History: The Cretaceous-Paleogene Boundary Ejecta Layer and its Source Crater at Chicxulub Christian Koeberl Dept. of Lithospheric Research, Univ. Vienna, 1090 Vienna, Austria &
Impact Events in Earth History: The Cretaceous-Paleogene Boundary Ejecta Layer and its Source Crater at Chicxulub Christian Koeberl Dept. of Lithospheric Research, Univ. Vienna, 1090 Vienna, Austria &
Lab 5: An Investigation of Meteorites Geology 202: Earth s Interior
 Lab 5: An Investigation of Meteorites Geology 202: Earth s Interior Asteroids and Meteorites: What is the difference between asteroids and meteorites? Asteroids are rocky and metallic objects that orbit
Lab 5: An Investigation of Meteorites Geology 202: Earth s Interior Asteroids and Meteorites: What is the difference between asteroids and meteorites? Asteroids are rocky and metallic objects that orbit
ESS 312 Geochemistry Simulating Earth degassing using radionuclides
 ESS 312 Geochemistry Simulating Earth degassing using radionuclides CHEMICAL AND ISOTOPIC EVOLUTION OF A TWO-RESERVOIR SYSTEM In lectures we discussed radioactive decay and build-up of a daughter isotope
ESS 312 Geochemistry Simulating Earth degassing using radionuclides CHEMICAL AND ISOTOPIC EVOLUTION OF A TWO-RESERVOIR SYSTEM In lectures we discussed radioactive decay and build-up of a daughter isotope
Homework #3 is due Friday at 11:50am! Nighttime observing has 10 more nights. Check the webpage. 1 st exam is October 10 th 2 weeks from Friday.
 Homework #3 is due Friday at 11:50am! Nighttime observing has 10 more nights. Check the webpage. 1 st exam is October 10 th 2 weeks from Friday. Outline Back to Atoms for fun The Earth as a Planet. magnetic
Homework #3 is due Friday at 11:50am! Nighttime observing has 10 more nights. Check the webpage. 1 st exam is October 10 th 2 weeks from Friday. Outline Back to Atoms for fun The Earth as a Planet. magnetic
Outline. Atoms in the Solar System. Atoms in the Earth. Back to Atoms for fun The Earth as a Planet. Homework #3 is due Friday at 11:50am!
 Homework #3 is due Friday at 11:50am! Nighttime observing has more nights. Check the webpage. 1 st exam is October th 2 weeks from Friday. Outline Back to Atoms for fun The Earth as a Planet. magnetic
Homework #3 is due Friday at 11:50am! Nighttime observing has more nights. Check the webpage. 1 st exam is October th 2 weeks from Friday. Outline Back to Atoms for fun The Earth as a Planet. magnetic
The Terrestrial Planets
 The Terrestrial Planets Large Bodies: Earth (1 R E, 1 M E ) Venus (0.95 R E, 0.82 M E ) Small Bodies: Mars (0.53 R E, 0.11 M E ) Mercury (0.38 R E, 0.055 M E ) Moon (0.27 R E, 0.012 M E ) The surfaces
The Terrestrial Planets Large Bodies: Earth (1 R E, 1 M E ) Venus (0.95 R E, 0.82 M E ) Small Bodies: Mars (0.53 R E, 0.11 M E ) Mercury (0.38 R E, 0.055 M E ) Moon (0.27 R E, 0.012 M E ) The surfaces
General Introduction. The Earth as an evolving geologic body
 General Introduction The Earth as an evolving geologic body Unique/important attributes of Planet Earth 1. Rocky planet w/ strong magnetic field Mercury has a weak field, Mars has a dead field 1 Unique/important
General Introduction The Earth as an evolving geologic body Unique/important attributes of Planet Earth 1. Rocky planet w/ strong magnetic field Mercury has a weak field, Mars has a dead field 1 Unique/important
Where did all the dinosaurs go?
 Where did all the dinosaurs go? Mass extinctions Why are we asking about the dinosaurs in this book? The reason is that (as we examine in Ch. 17, due to global warming, and in Ch. 26), humanity is causing
Where did all the dinosaurs go? Mass extinctions Why are we asking about the dinosaurs in this book? The reason is that (as we examine in Ch. 17, due to global warming, and in Ch. 26), humanity is causing
Terrestrial Planets: The Earth as a Planet
 Terrestrial Planets: The Earth as a Planet In today s class, we want to look at those characteristics of the Earth that are also important in our understanding of the other terrestrial planets. This is
Terrestrial Planets: The Earth as a Planet In today s class, we want to look at those characteristics of the Earth that are also important in our understanding of the other terrestrial planets. This is
Volatiles in the terrestrial planets. Sujoy Mukhopadhyay University of California, Davis CIDER, 2014
 Volatiles in the terrestrial planets Sujoy Mukhopadhyay University of California, Davis CIDER, 2014 Atmophiles: Elements I will talk about rock-loving iron-loving sulfur-loving Temperatures in Protoplanetary
Volatiles in the terrestrial planets Sujoy Mukhopadhyay University of California, Davis CIDER, 2014 Atmophiles: Elements I will talk about rock-loving iron-loving sulfur-loving Temperatures in Protoplanetary
PRELIMINARY REPORT ON THE YAMAT LUNAR METEORITE: III. AGES, NOBLE GAS ISOTOPES, OXYGEN ISOTOPES AND CHEMICAL ABUNDANCES
 Proc. NIPR Symp. Antarct. Meteorites, 2, 25-35,. 19-S.9 PRELIMINARY REPORT ON THE YAMAT0-86032 LUNAR METEORITE: III. AGES, NOBLE GAS ISOTOPES, OXYGEN ISOTOPES AND CHEMICAL ABUNDANCES Otto EUGSTER 1, Samuel
Proc. NIPR Symp. Antarct. Meteorites, 2, 25-35,. 19-S.9 PRELIMINARY REPORT ON THE YAMAT0-86032 LUNAR METEORITE: III. AGES, NOBLE GAS ISOTOPES, OXYGEN ISOTOPES AND CHEMICAL ABUNDANCES Otto EUGSTER 1, Samuel
Neutron activation analysis. Contents. Introduction
 Neutron activation analysis Contents Neutron activation analysis... 1 Introduction... 1 Principle of method... 2 Detection of radionuclides... 3 Kinetics of activation... 4 Choosing the appropriate procedure...
Neutron activation analysis Contents Neutron activation analysis... 1 Introduction... 1 Principle of method... 2 Detection of radionuclides... 3 Kinetics of activation... 4 Choosing the appropriate procedure...
Origin of noble gases in the insoluble organic compounds of primitive meteorites. Yves Marrocchi
 Origin of noble gases in the insoluble organic compounds of primitive meteorites Yves Marrocchi Centre de Recherches Pétrographiques et Géochimiques CRPG-CNRS - UPR 2300 - Nancy Institut de Planétologie
Origin of noble gases in the insoluble organic compounds of primitive meteorites Yves Marrocchi Centre de Recherches Pétrographiques et Géochimiques CRPG-CNRS - UPR 2300 - Nancy Institut de Planétologie
AN INTRODUCTION TO COSMOCHEMISTRY
 AN INTRODUCTION TO COSMOCHEMISTRY CHARLES R. COWLEY Professor of Astronomy, University of Michigan CAMBRIDGE UNIVERSITY PRESS Foreword V a % e x i 1 Overview 1 1.1 The Scope of Cosmochemistry 1 1.2 Cosmochemistry
AN INTRODUCTION TO COSMOCHEMISTRY CHARLES R. COWLEY Professor of Astronomy, University of Michigan CAMBRIDGE UNIVERSITY PRESS Foreword V a % e x i 1 Overview 1 1.1 The Scope of Cosmochemistry 1 1.2 Cosmochemistry
Distillation purification and radon assay of liquid xenon
 Distillation purification and radon assay of liquid xenon Yasuo Takeuchi Kamioka Observatory, ICRR, Univ. of Tokyo, Kamioka-cho, Hida-shi, Gifu 56-125, Japan Abstract. We succeeded to reduce the Kr contamination
Distillation purification and radon assay of liquid xenon Yasuo Takeuchi Kamioka Observatory, ICRR, Univ. of Tokyo, Kamioka-cho, Hida-shi, Gifu 56-125, Japan Abstract. We succeeded to reduce the Kr contamination
Evidence for a Large Anomalous Nuclear Explosions in Mars Past. J.E. Brandenburg Mars Society Meeting
 Evidence for a Large Anomalous Nuclear Explosions in Mars Past J.E. Brandenburg Mars Society Meeting The following paper was presented at the STAIF II Conference in Albuquerque In April 2014 Agenda Introduction:
Evidence for a Large Anomalous Nuclear Explosions in Mars Past J.E. Brandenburg Mars Society Meeting The following paper was presented at the STAIF II Conference in Albuquerque In April 2014 Agenda Introduction:
Volatiles on Venus: A missing link in understanding terrestrial planet evolution
 Volatiles on Venus: A missing link in understanding terrestrial planet evolution Melissa G. Trainer Planetary Environments Laboratory NASA Goddard Space Flight Center 12 July 2017 Trainer - DS Mid-Term
Volatiles on Venus: A missing link in understanding terrestrial planet evolution Melissa G. Trainer Planetary Environments Laboratory NASA Goddard Space Flight Center 12 July 2017 Trainer - DS Mid-Term
Measurement of Iridium in the P/T Boundary Layers in Gifu by Neutron Activation Method
 1/6 2005/10/2613:58 Measurement of Iridium in the P/T Boundary Layers in Gifu by Neutron Activation Method テクニカルノート :Shin Y abushita, K. W ada, T. Fujii, S. K aw akam i,2003 年 5 月 29 日掲載 Abstract Measurement
1/6 2005/10/2613:58 Measurement of Iridium in the P/T Boundary Layers in Gifu by Neutron Activation Method テクニカルノート :Shin Y abushita, K. W ada, T. Fujii, S. K aw akam i,2003 年 5 月 29 日掲載 Abstract Measurement
Geologic History Unit Notes. Relative age - general age statement like older, younger more recent
 Geologic History Unit Notes Relative age - general age statement like older, younger more recent Absolute age - specific age like 4,600 million years old Fundamental Principles of Relative Dating 1. Uniformitarianism
Geologic History Unit Notes Relative age - general age statement like older, younger more recent Absolute age - specific age like 4,600 million years old Fundamental Principles of Relative Dating 1. Uniformitarianism
Earth s History. The principle of states that geologic processes that happened in the past can be explained by current geologic processes.
 Earth s History Date: Been There, Done That What is the principle of uniformitarianism? The principle of states that geologic processes that happened in the past can be explained by current geologic processes.
Earth s History Date: Been There, Done That What is the principle of uniformitarianism? The principle of states that geologic processes that happened in the past can be explained by current geologic processes.
The Moon. Tidal Coupling Surface Features Impact Cratering Moon Rocks History and Origin of the Moon
 The Moon Tidal Coupling Surface Features Impact Cratering Moon Rocks History and Origin of the Moon Earth Moon Semi-major Axis 1 A.U. 384 x 10 3 km Inclination 0 Orbital period 1.000 tropical year 27.32
The Moon Tidal Coupling Surface Features Impact Cratering Moon Rocks History and Origin of the Moon Earth Moon Semi-major Axis 1 A.U. 384 x 10 3 km Inclination 0 Orbital period 1.000 tropical year 27.32
Chapter 3 Time and Geology
 Chapter 3 Time and Geology Finding the age of rocks: Relative versus Actual Dating The science that deals with determining the ages of rocks is called geochronology. Methods of Dating Rocks 1. Relative
Chapter 3 Time and Geology Finding the age of rocks: Relative versus Actual Dating The science that deals with determining the ages of rocks is called geochronology. Methods of Dating Rocks 1. Relative
Radioactivity is the spontaneous disintegration of nuclei. The first radioactive. elements discovered were the heavy atoms thorium and uranium.
 Chapter 16 What is radioactivity? Radioactivity is the spontaneous disintegration of nuclei. The first radioactive elements discovered were the heavy atoms thorium and uranium. These heavy atoms and others
Chapter 16 What is radioactivity? Radioactivity is the spontaneous disintegration of nuclei. The first radioactive elements discovered were the heavy atoms thorium and uranium. These heavy atoms and others
IX Life on Earth.
 IX Life on Earth http://sgoodwin.staff.shef.ac.uk/phy229.html 9.0 Introduction Life exists on the surface layers of the Earth. We cannot consider life and the planet separately: they interact with one
IX Life on Earth http://sgoodwin.staff.shef.ac.uk/phy229.html 9.0 Introduction Life exists on the surface layers of the Earth. We cannot consider life and the planet separately: they interact with one
SOURCES of RADIOACTIVITY
 Section 9: SOURCES of RADIOACTIVITY This section briefly describes various sources of radioactive nuclei, both naturally occurring and those produced artificially (man-made) in, for example, reactors or
Section 9: SOURCES of RADIOACTIVITY This section briefly describes various sources of radioactive nuclei, both naturally occurring and those produced artificially (man-made) in, for example, reactors or
Evolution of the Atmosphere: The Biological Connection
 Evolution of the Atmosphere: The Biological Connection The Earth s Four Spheres How It All Began Or At Least How We Think It Began O.k. it s a good guess Egg of energy The Big Bang splattered radiation
Evolution of the Atmosphere: The Biological Connection The Earth s Four Spheres How It All Began Or At Least How We Think It Began O.k. it s a good guess Egg of energy The Big Bang splattered radiation
Interactive comment on Noble gas signature of the late heavy bombardment in the Earth s atmosphere by B. Marty and A. Meibom
 eearth Discuss., 2, S86 S98, 2007 www.electronic-earth-discuss.net/2/s86/2007/ c Author(s) 2007. This work is licensed under a Creative Commons License. eearth Discussions Interactive comment on Noble
eearth Discuss., 2, S86 S98, 2007 www.electronic-earth-discuss.net/2/s86/2007/ c Author(s) 2007. This work is licensed under a Creative Commons License. eearth Discussions Interactive comment on Noble
Purification of N 2 and Ar. Hardy Simgen, MPIK Heidelberg
 Purification of N 2 and Ar Hardy Simgen, MPIK Heidelberg 1 Outline Requirements Gas purification by adsorption N 2 purification Ar purification Techniques for analysis Summary Page 2 Production N 2, Ar
Purification of N 2 and Ar Hardy Simgen, MPIK Heidelberg 1 Outline Requirements Gas purification by adsorption N 2 purification Ar purification Techniques for analysis Summary Page 2 Production N 2, Ar
NOBLE GASES IN GEOCHEMISTRY AND COSMOCHEMISTRY
 3m V IImm 41 NOBLE GASES IN GEOCHEMISTRY AND COSMOCHEMISTRY Editors:, '...., Donald Porcelli Chris J. Ballentine RainerWieler Department of Earth Sciences University of Oxford Oxford, United Kingdom Department
3m V IImm 41 NOBLE GASES IN GEOCHEMISTRY AND COSMOCHEMISTRY Editors:, '...., Donald Porcelli Chris J. Ballentine RainerWieler Department of Earth Sciences University of Oxford Oxford, United Kingdom Department
Comparative Planetology II: The Origin of Our Solar System. Chapter Eight
 Comparative Planetology II: The Origin of Our Solar System Chapter Eight ASTR 111 003 Fall 2007 Lecture 07 Oct. 15, 2007 Introduction To Modern Astronomy I: Solar System Introducing Astronomy (chap. 1-6)
Comparative Planetology II: The Origin of Our Solar System Chapter Eight ASTR 111 003 Fall 2007 Lecture 07 Oct. 15, 2007 Introduction To Modern Astronomy I: Solar System Introducing Astronomy (chap. 1-6)
National Science Standards Unit 1 Unit 2 Unit 3 Unit 4 Unit 5 Unit 6 Unit 7 Unit 8
 Unifying Concepts and Processes Geology Geologic Changes The Dynamic Earth Water and Water Systems National Science Standards Systems, order, and organization Evidence, models, and explanation Change,
Unifying Concepts and Processes Geology Geologic Changes The Dynamic Earth Water and Water Systems National Science Standards Systems, order, and organization Evidence, models, and explanation Change,
Analyzing the Chemical Composition and Classification of Miller Range 07273
 Alyssa Dolan Analyzing the Chemical Composition and Classification of Miller Range 07273 When small grains and debris that were present in the early solar system combine, they form meteoroids. Meteoroids
Alyssa Dolan Analyzing the Chemical Composition and Classification of Miller Range 07273 When small grains and debris that were present in the early solar system combine, they form meteoroids. Meteoroids
7) Applications of Nuclear Radiation in Science and Technique (1) Analytical applications (Radiometric titration)
 7) Applications of Nuclear Radiation in Science and Technique (1) (Radiometric titration) The radioactive material is indicator Precipitation reactions Complex formation reactions Principle of a precipitation
7) Applications of Nuclear Radiation in Science and Technique (1) (Radiometric titration) The radioactive material is indicator Precipitation reactions Complex formation reactions Principle of a precipitation
Planetary Atmospheres
 Planetary Atmospheres Structure Composition Clouds Meteorology Photochemistry Atmospheric Escape EAS 4803/8803 - CP 22:1 Where do planetary atmospheres come from? Three primary sources Primordial (solar
Planetary Atmospheres Structure Composition Clouds Meteorology Photochemistry Atmospheric Escape EAS 4803/8803 - CP 22:1 Where do planetary atmospheres come from? Three primary sources Primordial (solar
NOBLE GASES IN YAMAT AND LUNAR METEORITES
 Proc. NIPR Symp. Antarct. Meteorites, 5, 36-48, 1992 NOBLE GASES IN YAMAT0-793274 AND -86032 LUNAR METEORITES Nobuo TAKAOKA 1 and Yo-ichi YosHIDA '..! Department of Earth Sciences, Faculty of Science,
Proc. NIPR Symp. Antarct. Meteorites, 5, 36-48, 1992 NOBLE GASES IN YAMAT0-793274 AND -86032 LUNAR METEORITES Nobuo TAKAOKA 1 and Yo-ichi YosHIDA '..! Department of Earth Sciences, Faculty of Science,
Earth & Earthlike Planets. David Spergel
 Earth & Earthlike Planets David Spergel Course Logistics Life Everywhere and Rare Earths are now in the U-Store Each precept will be divided into two teams (at this week s s precept). Debate topic: Are
Earth & Earthlike Planets David Spergel Course Logistics Life Everywhere and Rare Earths are now in the U-Store Each precept will be divided into two teams (at this week s s precept). Debate topic: Are
What can noble gases really say about mantle. 2) Extent of mantle degassing
 What can noble gases really say about mantle convection and the deep Earth volatile cycles? 1) Constraints on mass flow 1) Constraints on mass flow 2) Extent of mantle degassing Outline: -Noble gas geochemistry
What can noble gases really say about mantle convection and the deep Earth volatile cycles? 1) Constraints on mass flow 1) Constraints on mass flow 2) Extent of mantle degassing Outline: -Noble gas geochemistry
Chapter 3 Time and Geology
 Chapter 3 Time and Geology Methods of Dating Rocks 1. Relative dating - Using fundamental principles of geology (Steno's Laws, Fossil Succession, etc.) to determine the relative ages of rocks (which rocks
Chapter 3 Time and Geology Methods of Dating Rocks 1. Relative dating - Using fundamental principles of geology (Steno's Laws, Fossil Succession, etc.) to determine the relative ages of rocks (which rocks
Isotopic measurements for the Mars Ice Cap -Matt Siegler PSI/SMU KISS workshop
 Isotopic measurements for the Mars Ice Cap -Matt Siegler PSI/SMU KISS workshop 1) Temperature dependent fractionation of stable isotopes ( 18 O/ 16 O, D/H ) 2) Cosmogenic Nuclides ( 10 Be, 1.4 Myr halflife)
Isotopic measurements for the Mars Ice Cap -Matt Siegler PSI/SMU KISS workshop 1) Temperature dependent fractionation of stable isotopes ( 18 O/ 16 O, D/H ) 2) Cosmogenic Nuclides ( 10 Be, 1.4 Myr halflife)
Earth as Planet. Earth s s Magnetic Field. The Earth s s Crust. Earth s s Interior
 Earth as Planet Earth s s Interior The Earth is a medium size planet with a diameter of 12,756 kilometers (7926 miles) Composed primarily of iron, silicon, and oxygen Nearly circular orbit and just the
Earth as Planet Earth s s Interior The Earth is a medium size planet with a diameter of 12,756 kilometers (7926 miles) Composed primarily of iron, silicon, and oxygen Nearly circular orbit and just the
Chapter 8 Lecture. The Cosmic Perspective Seventh Edition. Formation of the Solar System
 Chapter 8 Lecture The Cosmic Perspective Seventh Edition Formation of the Solar System Formation of the Solar System 8.1 The Search for Origins Our goals for learning: Develop a theory of solar system
Chapter 8 Lecture The Cosmic Perspective Seventh Edition Formation of the Solar System Formation of the Solar System 8.1 The Search for Origins Our goals for learning: Develop a theory of solar system
Dry Droplets of Fiery Rain Written by G. Jeffrey Taylor Hawai'i Institute of Geophysics and Planetology
 1 of 5 posted November 12, 1998 Dry Droplets of Fiery Rain Written by G. Jeffrey Taylor Hawai'i Institute of Geophysics and Planetology Chondrules are millimeter-sized spherical objects found in meteorites.
1 of 5 posted November 12, 1998 Dry Droplets of Fiery Rain Written by G. Jeffrey Taylor Hawai'i Institute of Geophysics and Planetology Chondrules are millimeter-sized spherical objects found in meteorites.
Neutron Activation Analysis of Ultrahigh-Purity Ti Al Alloys in Comparison with Glow-Discharge Mass Spectrometry
 Materials Transactions, Vol. 43, No. 2 (2002) pp. 116 to 120 Special Issue on Ultra-High Purity Metals (II) c 2002 The Japan Institute of Metals Neutron Activation Analysis of Ultrahigh-Purity Ti Al Alloys
Materials Transactions, Vol. 43, No. 2 (2002) pp. 116 to 120 Special Issue on Ultra-High Purity Metals (II) c 2002 The Japan Institute of Metals Neutron Activation Analysis of Ultrahigh-Purity Ti Al Alloys
What is the Moon? A natural satellite One of more than 96 moons in our Solar System The only moon of the planet Earth
 The Moon What is the Moon? A natural satellite One of more than 96 moons in our Solar System The only moon of the planet Earth Location, location, location! About 384,000 km (240,000 miles) from Earth
The Moon What is the Moon? A natural satellite One of more than 96 moons in our Solar System The only moon of the planet Earth Location, location, location! About 384,000 km (240,000 miles) from Earth
Lecture 9 : Meteorites and the Early Solar System
 Lecture 9 : Meteorites and the Early Solar System 1 Announcements Reminder: HW 3 handed out this week, due next week. HW 1 will be returned Wednesday and solutions posted. Midterm exam Monday Oct 22, in
Lecture 9 : Meteorites and the Early Solar System 1 Announcements Reminder: HW 3 handed out this week, due next week. HW 1 will be returned Wednesday and solutions posted. Midterm exam Monday Oct 22, in
The History of the Earth
 The History of the Earth We have talked about how the universe and sun formed, but what about the planets and moons? Review: Origin of the Universe The universe began about 13.7 billion years ago The Big
The History of the Earth We have talked about how the universe and sun formed, but what about the planets and moons? Review: Origin of the Universe The universe began about 13.7 billion years ago The Big
Dating the age of the Earth
 Dating the age of the Earth What is the age of the Earth? A. 4.44 million years B. 1 million years C. 4.55 billion years D. 10000 years Discuss this with your neighbor: How do we know the age of the Earth?
Dating the age of the Earth What is the age of the Earth? A. 4.44 million years B. 1 million years C. 4.55 billion years D. 10000 years Discuss this with your neighbor: How do we know the age of the Earth?
Origin of the Solar System
 Origin of the Solar System Look for General Properties Dynamical Regularities Orbits in plane, nearly circular Orbit sun in same direction (CCW from N.P.) Rotation Axes to orbit plane (Sun & most planets;
Origin of the Solar System Look for General Properties Dynamical Regularities Orbits in plane, nearly circular Orbit sun in same direction (CCW from N.P.) Rotation Axes to orbit plane (Sun & most planets;
Guided Notes Geologic History
 Guided Notes Geologic History Relative Age Sequence of Events Correlation Techniques Volcanic Ash Markers Index Fossils Geologic Time Scale Evolution Radioactive Dating 9) How has Earth changed over time?
Guided Notes Geologic History Relative Age Sequence of Events Correlation Techniques Volcanic Ash Markers Index Fossils Geologic Time Scale Evolution Radioactive Dating 9) How has Earth changed over time?
Noble gases in mineral separates from three shergottites: Shergotty, Zagami, and EETA79001
 Meteoritics & Planetary Science 42, Nr 3, 387 412 (2007) Abstract available online at http://meteoritics.org Noble gases in mineral separates from three shergottites: Shergotty, Zagami, and EETA79001 Susanne
Meteoritics & Planetary Science 42, Nr 3, 387 412 (2007) Abstract available online at http://meteoritics.org Noble gases in mineral separates from three shergottites: Shergotty, Zagami, and EETA79001 Susanne
Climate Change. But first... Throughout the Solar System. Lecture 11: Earth as a Planet. Earth as a Planet. PDFs for Lectures 7, 8, and 11 are posted
 Climate Change Throughout the Solar System Lecture 11: Earth as a Planet Astro 202 Prof. Jim Bell (jfb8@cornell.edu) Spring 2008 But first... PDFs for Lectures 7, 8, and 11 are posted Paper 3 is due at
Climate Change Throughout the Solar System Lecture 11: Earth as a Planet Astro 202 Prof. Jim Bell (jfb8@cornell.edu) Spring 2008 But first... PDFs for Lectures 7, 8, and 11 are posted Paper 3 is due at
Rivelazione di neutrini solari - Borexino Lino Miramonti 6 Giugno 2006 Gran Sasso
 Rivelazione di neutrini solari - Borexino Lino Miramonti 6 Giugno 2006 Gran Sasso 1 RADIOCHEMICAL Integrated in energy and time CHERENKOV Less than 0.01% of the solar neutrino flux is been measured in
Rivelazione di neutrini solari - Borexino Lino Miramonti 6 Giugno 2006 Gran Sasso 1 RADIOCHEMICAL Integrated in energy and time CHERENKOV Less than 0.01% of the solar neutrino flux is been measured in
Table of Isotopic Masses and Natural Abudances
 Table of Isotopic Masses and Natural Abudances in amu, where 1amu = 1/12 mass 12 C Atomic weight element = M i (abun i )+M j (abun j ) + Four types of radioactive decay 1) alpha (α) decay - 4 He nucleus
Table of Isotopic Masses and Natural Abudances in amu, where 1amu = 1/12 mass 12 C Atomic weight element = M i (abun i )+M j (abun j ) + Four types of radioactive decay 1) alpha (α) decay - 4 He nucleus
Near-Earth Supernovae Probed by Deep-Sea Deposits of Radioactive 60 Fe
 Near-Earth Supernovae Probed by Deep-Sea Deposits of Radioactive 60 Fe Sara Ayoub PHY802-SP16 Final Presentation Abstract After decades of lack of precision in estimating the frequency of supernovae and
Near-Earth Supernovae Probed by Deep-Sea Deposits of Radioactive 60 Fe Sara Ayoub PHY802-SP16 Final Presentation Abstract After decades of lack of precision in estimating the frequency of supernovae and
ESR APPLICATIONS TO METEORITE SAMPLES. Chihiro y AMANAKA, Shin TOYODA and Motoji IKEY A
 Proc. NIPR Symp. Antarct. Meteorites, 6, 417-422, 1993 ESR APPLICATIONS TO METEORITE SAMPLES Chihiro y AMANAKA, Shin TOYODA and Motoji IKEY A Osaka University, Department of Earth and Space Science, J
Proc. NIPR Symp. Antarct. Meteorites, 6, 417-422, 1993 ESR APPLICATIONS TO METEORITE SAMPLES Chihiro y AMANAKA, Shin TOYODA and Motoji IKEY A Osaka University, Department of Earth and Space Science, J
Nitrogen speciation in upper mantle fluids and the origin of Earth s nitrogen-rich atmosphere
 Supporting Online Material for Nitrogen speciation in upper mantle fluids and the origin of Earth s nitrogen-rich atmosphere Sami Mikhail & Dimitri Sverjensky S1. Supplementary discussion S1.1 The selection
Supporting Online Material for Nitrogen speciation in upper mantle fluids and the origin of Earth s nitrogen-rich atmosphere Sami Mikhail & Dimitri Sverjensky S1. Supplementary discussion S1.1 The selection
An introduction to Neutron Resonance Densitometry (Short Summary)
 An introduction to Neutron Resonance Densitometry (Short Summary) H. Harada 1, M. Koizumi 1, H. Tsuchiya 1, F. Kitatani 1, M. Seya 1 B. Becker 2, J. Heyse 2, S. Kopecky 2, C. Paradela 2, P. Schillebeeckx
An introduction to Neutron Resonance Densitometry (Short Summary) H. Harada 1, M. Koizumi 1, H. Tsuchiya 1, F. Kitatani 1, M. Seya 1 B. Becker 2, J. Heyse 2, S. Kopecky 2, C. Paradela 2, P. Schillebeeckx
Comparative Planetology II: The Origin of Our Solar System. Chapter Eight
 Comparative Planetology II: The Origin of Our Solar System Chapter Eight ASTR 111 003 Fall 2007 Lecture 06 Oct. 09, 2007 Introduction To Modern Astronomy I: Solar System Introducing Astronomy (chap. 1-6)
Comparative Planetology II: The Origin of Our Solar System Chapter Eight ASTR 111 003 Fall 2007 Lecture 06 Oct. 09, 2007 Introduction To Modern Astronomy I: Solar System Introducing Astronomy (chap. 1-6)
Radioactivity. Lecture 11 The radioactive Earth
 Radioactivity Lecture 11 The radioactive Earth The Violent Beginning Most of the planet s radioactivity was generated in neutron driven nucleosynthesis processes in previous star generations and implemented
Radioactivity Lecture 11 The radioactive Earth The Violent Beginning Most of the planet s radioactivity was generated in neutron driven nucleosynthesis processes in previous star generations and implemented
Module 4: Astronomy The Solar System Topic 3 Content: The Terrestrial Planets Notes Introduction
 Introduction The four planets closest to the Sun are called "the terrestrial planets." These inner planets are considered to be small and rocky. Although they are all rocky, their varying distances from
Introduction The four planets closest to the Sun are called "the terrestrial planets." These inner planets are considered to be small and rocky. Although they are all rocky, their varying distances from
GEOL 562 Notes: U-Series and Th-series nuclides. Guide Questions: Reading: White, Lecture 10
 GEOL 562 Notes: U-Series and Th-series nuclides Reading: White, Lecture 10 Motivation: Up to now we have dealt with long half-life nuclides (all left over from the birth of the solar system). What are
GEOL 562 Notes: U-Series and Th-series nuclides Reading: White, Lecture 10 Motivation: Up to now we have dealt with long half-life nuclides (all left over from the birth of the solar system). What are
Measuring the Age of things (Astro 202 2/12/08) Nomenclature. Different Elements. Three Types of Nuclear Decay. Carbon 14 Decay.
 Measuring the Age of things (Astro 202 2/12/08) Nomenclature + Proton Different Elements Neutron Electron Element: Number of Protons Carbon 12 6 protons 6 neutrons 6 electrons Nitrogen 14 7 protons 7 neutrons
Measuring the Age of things (Astro 202 2/12/08) Nomenclature + Proton Different Elements Neutron Electron Element: Number of Protons Carbon 12 6 protons 6 neutrons 6 electrons Nitrogen 14 7 protons 7 neutrons
Radioactivity. Lecture 11 The radioactive Earth
 Radioactivity Lecture 11 The radioactive Earth The Violent Beginning Most of the planet s radioactivity was generated in neutron driven nucleosynthesis processes in previous star generations and implemented
Radioactivity Lecture 11 The radioactive Earth The Violent Beginning Most of the planet s radioactivity was generated in neutron driven nucleosynthesis processes in previous star generations and implemented
Question #1: What are some ways that you think the climate may have changed in the area where you live over the past million years?
 Reading 5.2 Environmental Change Think about the area where you live. You may see changes in the landscape in that area over a year. Some of those changes are weather related. Others are due to how the
Reading 5.2 Environmental Change Think about the area where you live. You may see changes in the landscape in that area over a year. Some of those changes are weather related. Others are due to how the
The noble gas are. Helium(H), neon (Ne),argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn)
 The noble gas are Helium(H), neon (Ne),argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) Uniqueness What makes my family unique? Their nobility. They act as though they are better than all the rest!
The noble gas are Helium(H), neon (Ne),argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) Uniqueness What makes my family unique? Their nobility. They act as though they are better than all the rest!
