Studies towards Lightfast Automotive Dyes for Polyester
|
|
|
- Bridget Watson
- 6 years ago
- Views:
Transcription
1 Studies towards Lightfast Automotive Dyes for Polyester arold S. Freeman, atacha Berthelon, and Laura C. Edwards Fiber and Polymer Science Program orth Carolina State University, Box 8301 Raleigh, C 27695, USA arold_freeman@ncsu.edu ABSTRACT Volume 3, Issue 4, Winter 2004 This paper provides an overview of studies conducted in our dye chemistry laboratories that were directed towards the design, synthesis, and evaluation of dyes for polyester fibers for applications requiring high lightfastness. Presented are results from the early studies in our program that involved characterizing the chemistry associated with the light-induced (photo) degradation of azo, anthraquinone, and nitrodiphenylamine disperse dyes in an ester environment. This is followed by a summary of molecular design efforts based on results from dye photodegradation experiments. In this case, results pertaining to the utility of built-in photostabilizer groups for enhancing disperse dye lightfastness (resistance to light-induced fading) are discussed. Special attention is given in this paper to studies in which the effects of natural and artificial light on oriented polyester films following the application of azo, anthraquinone and nitrodiphenylamine dyes were assessed. This work was part of an investigation aimed at determining the contribution of polyester to the fading of disperse dyes. In this regard, dyed polyester films were exposed to sunlight from two regions in the United States and to the artificial light of an Atlas Weatherometer. The results of the different exposures indicate that artificial light exposures were far more damaging to the polymer host than natural light. It was also apparent that a significant level of dye fading could be attributed to substrate degradation. Keywords: dye, fading, lightfastness, polyester films, exposure 1. Background It is both ironic and unfortunate that the very types of processes responsible for the colors we see on textiles are also responsible for their demise when the adsorbed dyes are exposed to sunlight for prolonged periods. Color is seen whenever an object absorbs light of a given wavelength in the visible region ( nm) of the electromagnetic spectrum and reflects the remaining incident light to our eyes. The colors we perceive as a consequence of these processes are summarized in Figure 1 1. ote that the colors seen are complementary to the one absorbed. Invariably, organic compounds, including the colorants used to dye textile fibers, also absorb light in the ultraviolet (UV) region ( nm). See, for example, the Pisystem 2 calculated absorption spectrum for Disperse Blue 14 in Figure 2, where the absorption at 600 nm is responsible for color and those at 324 nm and 385 nm probably contribute most to fading since window glass filter systems typically give a 310 nm cut off. 1
2 Wavelength Absorbed (nm Color Absorbed Color Seen Violet Yellow-Green Blue Yellow Green-Blue range Blue-Green Red Green Purple Yellow-Green Violet Yellow Blue range Green-Blue Red Blue-Green Figure 1. Colors perceived following visible light absorption. Figure 2. Calculated absorption spectrum for Disperse Blue 14. Just as the absorption of UV light by our skin is harmful to the cells comprising human tissue, the absorption of this type light by organic dyes can modify the molecular structure of dyes to cause color changes/fading. Interestingly, the same types of compounds used to protect our skin against UV damage can be used to control the dye fading process. It has long been known that the fading of dyes on a polymer substrate is influenced by: the structure of the dye the structure of the substrate the presence of moisture and atmospheric contaminants temperature the properties of the light source the physical state of the adsorbed dye 2
3 It is also clear that studies in this area have focused primarily on the relationships between dye structure and lightfastness and the molecular transformations that take place when dyes undergo fading. The majority of these studies were conducted by using solvents as models for textile substrates, and in this regard, ethyl acetate, dimethyl formamide, and methanol have been used as models for polyester, polyamide, and cellulosic substrates, respectively. The details of such studies have been reviewed 3, and a somewhat older reference summarizes studies involving polymeric media 4. In a more recent review paper, the photochemistry of disperse dyes has been summarized 5. This is important in the present context, as disperse dyes are the only dyes suitable for dyeing polyester fibers. With regard to the influence of disperse dye structure on lightfastness, results of studies have shown that anthraquinone dyes are generally more lightfast than their azo counterparts. This is especially true for red and blue anthraquinone dyes that have an (hydroxyl) group in one or more of the α- positions of the anthraquinone ring system, examples of which are Disperse Red 60 and Blue 27 (Figure 3). The lightfastness of these dyes arises from their ability to undergo intra-molecular proton transfer from the group to the adjacent C= (carbonyl) group following light absorption in the UV region. This is an internal conversion process, as the absorbed UV light energy is dissipated as harmless heat. 2 2 C 2 C 2 Figure 3. Structures of two lightfast anthraquinone disperse dyes for polyester: Disperse Red 60 (left) and Disperse Blue 27 (right). The small number of lightfast azo disperse dyes include Disperse Red 167:1 (Figure 4), which takes advantage of intramolecular hydrogen bonding (bonding) between the azo (-=-) bond and adjacent group to stabilize the dye against UV light degradation. Similarly, lightfast yellow dyes for automotive fabric have proton transfer as an internal mechanism for UV stabilization. An example is Disperse Yellow 86 (Figure 4). 2 Cl Ac (C 2 C 2 Ac) 2 Et S 2 Me 2 Figure 4. Structures of two lightfast disperse dyes for polyester: Disperse Red 167:1 (left) and Disperse Yellow 86 (right). 3 UV absorbers are co-applied with disperse dyes to give lightfastness at levels essential to long-lived automotive body
4 fabric. These compounds enhance dye lightfastness by an energy transfer mechanism 4. In this regard, the energy taken on by dye molecules following UV light absorption is transferred to UV absorber molecules, and the transferred energy is subsequently dissipated via internal conversion. While a number of commercial photostabilizers can be used to protect disperse dyes on polyester fibers, commonly used agents are hydroxybenzotriazoles and hydroxybenzophenones (Figure 5), where R 1 is a linear alkyl group and R 2 is usually methyl or t-butyl. R 1 R 1 Cl CMe 3 R 2 Figure 5. Structures of benzophenone (left) and benzotriazole (right) type UV stabilizers for disperse dyes on polyester. n the other hand, it is also known that certain structural features lead to poor lightfastness in disperse dyes. In this case, the presence of 1) -hydroxethyl groups in azo or anthraquinone dyes 6, 2) ortho-nitro groups in azo dyes 3,7, and 3) groups leading to azoxy formation 3,8 cause reduced lightfastness. Examples of dyes that fit into this category are shown in Figure 6. Me 1 C 2 C Et C 2 C 2 Figure Et C 2 C 2 C 3 Examples of disperse dye structures having low lightfastness on polyester. The above types of background information were used as a springboard for our studies in this area that began in the mid-1980s. A summary of this work is presented in section Dye Considerations The initial studies in our program focused on two areas: 1) determination of the reactions associated with the photodegradation of automotive disperse dyes on polyester fabric and 2) design and synthesis of lightfast disperse dye analogs of commercial prototypes. In both cases, dyes such as 4-7 were used as prototypes, and
5 light exposures were conducted according to a published method for automotive dyes 9. With regard to dye degradation, it was determined that Red 91 produced products arising from cleavage of the alkyl group, oxidation of the substituted ring to an iminoketone system, and substitution of the amino group by a hydroxyl group. Red 167:1 underwent reductive cleavage of the azo bond to give the corresponding aromatic amines. Like Red 91, Blue 56 produced products arising from substitution of the amino groups by a hydroxyl group. Dye 7a (Yellow 42) underwent cleavage of the sulfonamide bond, producing aniline, which in turn was oxidized to give the fabric a darker hue Cl (C 2 ) 6 Ac 2 (C 2 C 2 Ac) 2 4 (C.I. Disperse Red 91) 5 (C.I. Disperse Red 167:1) 2 2 Br X S 2 Y 2 6 (C.I. Disperse Blue 56) 7 (X/Y = a: /Ph; b: Et/Me 2 ) In view of the aforementioned results and the commercial practice of using UV absorbers to enhance the lightstability of disperse dyes on polyester, we elected to develop some disperse dye analogs containing built-in photostabilizers This led to the synthesis of experimental disperse dyes such as 8-14, in which benzophenone, benzotriazole, hindered amine and oxalanilide groups were employed to improve the stability of the parent dye. We found that the lightfastness of Red 167:1 (5) on polyester was best improved by incorporating an oxalanilide stabilizer (cf. dye 8), with X = ethyl (Et) giving the best results 14. In this case, the azo ( = ) group was stabilized by intramolecular -bonding with the adjacent group (Figure 7). This critical feature was lost when experimental dye 9a was produced, as x-ray crystallography showed that the hydroxybenzoyl group does not lie in the same plane as the azo group 12 (cf. Figure 7). This pre-emptied -bonding between the azo group and the anticipated UV (hν) light-induced enol form (9b) of the ortho-hydroxybenzophenone moiety. While intramolecular -bonding was restored when dye 10 was produced, this dye had to be applied by thermosol dyeing, as it decomposed in the dyebath during exhaust dyeing at 130 o C (i.e. under pressure). Forming the methyl ether of the phenolic ( ) group (cf. dye 11) solved the thermal stability problem but did not enhance lightfastness. 5
6 X Me 2 Cl 8 2 (C 2 C 2 Ac) 2 Cl (C 2 C 2 Ac) 2 9a hv Me 2 Cl (C 2 C 2 Ac) 2 9b Figure 7. Me Geometry optimized structures showing the molecular conformations for dyes 8 (left) and 9a (right). Me Me Ac Ac 2 (C 2 C 2 Ac) 2 2 (C 2 C 2 Ac)
7 The lightfastness of nitrodiphenylamine dye 7a (Yellow 42) was improved by forming dye 12, which contains a built-in benzophenone stabilizer moiety. n the other hand, incorporating a benzotriazole moiety (e.g. 13) or hindered amine moiety (e.g. 14) did not have a beneficial effect. It was anticipated that the latter structural variation would prevent the oxidation of aniline that accompanied the fading of Yellow 42. With regard to the inferiority of dye 13, it is possible that the absence of a bulky group (e.g. t-butyl) ortho to the group reduced the efficiency of intramolecular proton transfer in the excited state. The synthesis of a structure containing such a group proved impractical. 2 S 2 12 Me 2 S 2 Cl 2 13 Me Me Me S 2 C 14 Me Me It is clear from our studies in this area, that the design of lightfast disperse dyes containing a built-in UV absorber group is a viable approach to enhancing the lightfastness of existing commercial azo disperse red and nitrophenylamine yellow dyes. It should be noted that the choice and location of the stabilizer group are critical to success. While it was also possible to synthesize anthraquinone disperse blue dyes with a built-in stabilizer group (cf. Figure 8) the existing high dye lightfastness was not further enhanced. Me 2 2 Figure 8. Structure of the automotive dye Disperse Blue 77 (left) and its experimental analog (right). Article Designation: ITMA 7
8 3. Substrate Considerations Although much is known about the ability of certain dyes to cause phototendering of fabrics to which they are applied 4, very few studies have been devoted to assessing the contribution(s) of the polymer-based substrate to the photodegradation of adsorbed dyes. ur dye chemistry group became interested in this subject following studies involving the irradiation of several commercial disperse dyes on polyester films using natural (South Florida and Arizona sunlight) and artificial light (xenon arc weatherometer) 15. utdoor studies were conducted at 98 o C (Arizona) and 99 o C (South Florida) without humidity control, and the dark/light cycle was environmentally dependent. For xenon arc exposures a chamber temperature of 89 o C was used, along with 50% relative humidity, and controlled light/dark cycles (3.8h light/1h dark). The disperse dyes employed included those suitable (e.g. Red 91, Red 167:1, Blue 56 and Yellow 42) and unsuitable (e.g. Red 1, Blue 3, Yellow 3 (15) and Red 11 (16) for meeting the lightfastness requirements of automotive interior fabric. Representative photographs of the center of films obtained following 451kJ light exposures and Scanning Electron Microscopy (SEM) studies are shown in Figures Just prior to SEM analysis, the films were folded into quarters, placed under a weight for 3 min, and reopened. Ac 2 Me Me 2 15 (C.I. Disperse Yellow 3) 16 (C.I. Disperse Red 11) While color loss was evident after all exposures, extensive polymer degradation appeared, as deep cracks, only after xenon arc exposures. When non-automotive dyes were used, color loss was often accompanied by objectionable hue changes, as illustrated in Figures 9 and 10. The hue shifts indicate that natural and artificial light caused structural changes in the dye molecules. In prior studies 6, we found that cleavage of the -C 2 C 2 group and oxidation of the -methyl group of Blue 3 took place during xenon and carbon arc exposures. Such changes would produce a red color in this case. For automotive grade dyes, on-tone color changes were usually observed rather than hue shifts. In the case of Yellow 42 (Figure 11) and Blue 56 a darkening of the shade was observed. As indicated above, we believe this change is due to the formation and oxidation of aniline during light exposures. 8
9 Figure 9. Photomicrographs of Disperse Blue 3-dyed oriented polyester films. Clockwise from bottom left: unexposed film, 451 kj Florida exposure, 451 kj Arizona exposure, and 451 kj xenon arc exposure. Figure 10. Photomicrographs of Disperse Red 11-dyed oriented polyester films. Clockwise from bottom left: unexposed film, 451 kj Florida exposure, 451 kj Arizona exposure, and 451 kj xenon arc exposure. 9
10 Figure 11. Photomicrographs of Disperse Yellow 42-dyed oriented polyester films. Clockwise from bottom left: unexposed film, 451 kj Florida exposure, 451 kj Arizona exposure, and 451 kj xenon arc exposure. Though not shown in this set of photographs, when undyed polyester films were irradiated, slight yellowing accompanied polymer degradation. owever, it is clear from the present photographs that considerably more polymer degradation occurred during artificial light exposures than during natural light exposures. This was true despite the photostability of the dyes applied. The observation of film integrity changes during the irradiation of 100 µm type D Mylar (polyester) film, led to our investigating the possible effects of polyester degradation on disperse dye fading 16,17. In this regard, the possible relationship between polymer photodegradation and dye fading was investigated using the sequence of steps outlined in Figure 12. The experimental design involved the preparation of two sets of films. The first set was dyed at a 1% shade depth before light exposures. The second set was exposed undyed for one-half of the standard irradiance level, i.e kj/m 2 (xenon arc) or 15,000 Langleys (Arizona), and then dyed (1% w/w) and exposed for an additional kj/m 2 or 15,000 Langleys (Lgs). 10
11 PET Film Dye Application Blank Dyeing 1 st Light Exposures kj/m 2 (xenon arc) 15,000 Lgs (Arizona) Dye Application Analytical Evaluations Light Exposures kj/m 2 or kj/m 2 15,000 Lgs or 30,000 Lgs 2 nd Light Exposures kj/m 2 15,000 Lgs Analytical Evaluations Fig 12. Experimental design used in the substrate-considerations aspect of this study. Absorption Spectra Figures show summaries of absorption data from the evaluation of undyed polyester film and polyester films dyed with six representative disperse dyes (1, 2, 5-6, 7b and 15). The absorbance values were recorded at the absorption maximum of each dye. It can be seen from Figure 13 that the intensity of the absorption maximum, in decreasing order, was generally: 1) Unexposed, dyed polyester film, 2) polyester film exposed to kj/m 2 after dyeing (Post-Exp. 1), 3) polyester film exposed to kj/m 2 after dyeing (Post-Exp. 2), and 4) polyester film exposed to kj/m 2 before dyeing and then for another kj/m 2 after dyeing (Pre-Exp.). The lone exception to the 1>2>3 pattern was Yellow 3 (Y3). In this case, pre-exposure of polyester had no adverse effect on dye degradation and the kj exposure caused the highest level of fading. While the fading of Red 1 (R1) followed the 1>2>3 pattern, the kj exposure was the most damaging. evertheless, these results do suggest that polyester photodegradation has an adverse effect on disperse dye stability. The latter point is further illustrated in the graph in Figure 14. In this case, absorption spectra were recorded on 11
12 dyeings obtained following pretreatment of polyester with the UV absorber Tinuvin 326 (17) prior to light exposures. It can be seen that the adverse effects of polyester degradation were prevented when dyes Red 91, Blue 56, Yellow 86 and Yellow 3 were applied. The two dyes that have inherently low lightfastness (Blue 3 and Red 1) were still adversely affected by polyester degradation, though to a lesser degree than when no UV absorber was used R91 B56 Y86 B3 Y3 R1 o Exp. Post-Exp. 1 Pre-Exp. Post-Exp. 2 Figure 13. Absorbance values for 1% shades of dyes 1, 2, 5-6, 7b and 15 on polyester a) before exposure, b) following a kj exposure, c) following a kj exposure of pre-exposed polyester, and d) following a kj exposure R91 B56 Y86 B3 Y3 R1 o Exp. Post-Exp. 1 Pre-Exp. Post-Exp. 2 Figure 14. Absorbance values for 1% shades of dyes 1, 2, 5-6, 7b and 15 on UV absorbertreated polyester a) before exposure, b) following a kj exposure, c) following a kj exposure of pre-exposed polyester, and d) following a kj exposure. 12
13 Cl Me 17 (Tinuvin 326) CMe 3 Experiments were also conducted that involved the application of Tinuvin 326 to undyed polyester films after an initial kj light exposure but before exposing the dyed, pre-exposed films. In this case, the results indicated that dye fading was reduced to a greater degree when Tinuvin 326 was introduced prior to irradiating the undyed film than when it was employed during dye application on pre-exposed polyester films. In studies involving natural versus artificial light, Arizona sunlight generally caused less fading of dyed films than xenon arc light (except for Blue 3 and Red 1). This is probably due to the greater humidity and higher irradiance level in the case of the artificial light exposures. For the two exceptions, dye fading was severe under all conditions. Tensile Strength The addition of Tinuvin 326 before the initial light exposure had a beneficial effect on the strength of polyester films that were exposed prior to dyeing. n the other hand, the use of Tinuvin 326 when dyeing pre-exposed films did not prevent further polymer degradation, as comparable breaking strengths were obtained whether or not Tinuvin 326 was used in dyeings performed after an initial light exposure in the absence of Tinuvin 326. Interestingly, films exposed to Arizona sunlight showed little or no loss in elongation at break, even after the harshest exposure (30,000 Lgs). This is illustrated in Figure 15. When comparing dyed versus undyed films, it was found that the irradiated dyed films showed less strength loss than their undyed counterparts. This was true for artificial and natural light. It is not yet clear how the dyes employed provided this protective effect Pre-Exp kJ 225.6kJ Mock Mock Pre- Exp. 15K Lgs. 30K Lgs. Mock Mock+9 Figure 15. Elongation (nm) at break for mock-dyed films following xenon arc (left) and Arizona (right) exposures. The following is clear from this aspect of our studies: The photodegradation of polyester contributes to the fading of adsorbed disperse dyes, as polyester films irradiated before dyeing showed more color loss than films that were dyed and then irradiated. The use of a benzotriazole UV absorber reduces polyester degradation, resulting in less dye degradation. The presence of automotive disperse dyes can have a protective effect on the photodegradation of polyester, as less strength loss was observed following the irradiation of dyed versus undyed films. Article Designation: ITMA 13
14 Accelerated fading using xenon arc light has a far more destructive effect on References 1. E.. Abrahart, Dyes and their Intermediates, Chemical Publishing, ew York, M.G. utchings, Dyes and Pigments, 29(2), 95 (1995). 3.. Kuramoto, in Physico-chemical Principles of Color Chemistry, A.T. Peters and.s. Freeman, eds., Chapter 6, p. 196, Chapman & all, Scotland, UK, , S. Allen and J. F. McKellar, Photochemistry of Dyed and Pigmented Polymers, Applied Science Publishers, London, J. Lye and.s. Freeman, Adv. Colour Sci. Technol., 2, (1999). 6..S. Freeman and W.. su, Textile Res. J., 57(4), 223(1987). 7..S. Freeman and S.A. McIntosh, Textile Res. J., 59(6), 343(1989). 8. T. Kitao, Y. Watada, M. Matsuoka, and K. Konishi, ippon Kagaku Kaishi, (4) 757 (1974). 9. J. E. Bullock and D. L. Garrett, J. Ind. Fabrics, 4(2), 23 (1985). 10. B. L. Richardson, Characterization of the Photodegradation Products of State-of-the-Art Disperse Dyes on PET Fabric, Unpublished Masters thesis, orth Carolina State University at Raleigh, S. Freeman, S-D. Kim, R.D. Gilbert, and R. McGregor, Dyes and Pigments, 17(2), 83(1991). polyester than sunlight. 12..S. Freeman and J.C. Posey, Jr., Dyes and Pigments, 20(3), 147(1992). 13..S. Freeman and J.C. Posey, Jr., Dyes and Pigments, 20(3), 171(1992) S. Freeman, M. E. Mason, and J. Lye, Dyes and Pigments, 42, 53 (1999). 15. L. C. Edwards, A Study of the Phototostability of Disperse-dyed Poly(ethylene terephthalate Films, Unpublished Masters thesis, orth Carolina State University at Raleigh, Berthelon, The Contribution of PET to the Photodegradation of Disperse Dyes, Unpublished Masters thesis, orth Carolina State University at Raleigh, Berthelon and. S. Freeman, The Contribution of PET to the Photodegradation of Disperse Dyes, Proceedings of Color Science 98 Conference, arrogate, England, 1998, pp , Acknowledgments The authors a grateful for financial support from the ational Science Foundation, The Dye Lightfastness Consortium, and The ational Textile Center during the course of this research. We also thank Dr. Ahmed El-Shafei for conducting the molecular modeling studies described in this paper. 14
Chapter 6 : Effect of Dye-bath ph on photostability of dyed Nylon 66
 Chapter 6 : Effect of Dye-bath p on photostability of dyed ylon 66 6.1 Introduction The photo-oxidative degradation in polyamides is well studied. Mechanistic aspects of photo-oxidative degradation of
Chapter 6 : Effect of Dye-bath p on photostability of dyed ylon 66 6.1 Introduction The photo-oxidative degradation in polyamides is well studied. Mechanistic aspects of photo-oxidative degradation of
5.2 Photodegradation and stabilization of polymers
 5.2 Photodegradation and stabilization of polymers Photodegradation of polymers b) 2 2 2 2 2 2 2 2 2 2 2 2 3 Exposure to sunlight and some artificial lights can have adverse effects on the useful life
5.2 Photodegradation and stabilization of polymers Photodegradation of polymers b) 2 2 2 2 2 2 2 2 2 2 2 2 3 Exposure to sunlight and some artificial lights can have adverse effects on the useful life
Weathering of Artificial Grass Yarn CEN TC 217 WG 11, 18/10/2016 Daniel Müller (BASF) & Jeroen Wassenaar (TOTAL)
 Weathering of Artificial Grass Yarn CEN TC 217 WG 11, 18/10/2016 Daniel Müller (BASF) & Jeroen Wassenaar (TOTAL) Why doing Artificial Weathering? Simulation of the photooxidative behavior of polymers Purpose:
Weathering of Artificial Grass Yarn CEN TC 217 WG 11, 18/10/2016 Daniel Müller (BASF) & Jeroen Wassenaar (TOTAL) Why doing Artificial Weathering? Simulation of the photooxidative behavior of polymers Purpose:
Chem 263 Oct. 12, 2010
 Chem 263 ct. 12, 2010 Alkyl Side Chain xidation Reaction If the carbon directly attached to the aromatic ring has > 1 hydrogen attached to it, it can be oxidized to the corresponding carboxylic acid with
Chem 263 ct. 12, 2010 Alkyl Side Chain xidation Reaction If the carbon directly attached to the aromatic ring has > 1 hydrogen attached to it, it can be oxidized to the corresponding carboxylic acid with
Poly(phenyl acrylate) and Poly(p-methylphenyl acrylate) as photostabilizers II. Protection of PET against photo-oxidation
 Polymer Degradation and Stability 83 (2004) 435 440 www.elsevier.com/locate/polydegstab Poly(phenyl acrylate) and Poly(p-methylphenyl acrylate) as photostabilizers II. Protection of PET against photo-oxidation
Polymer Degradation and Stability 83 (2004) 435 440 www.elsevier.com/locate/polydegstab Poly(phenyl acrylate) and Poly(p-methylphenyl acrylate) as photostabilizers II. Protection of PET against photo-oxidation
Natural Exposure Testing VS Accelerated Weathering... The Right Choice.
 Natural Exposure Testing VS Accelerated Weathering... The Right Choice. Founded in 1956 Specialize in material durability testing equipment and services Bolton, England Q-Lab Europe Cleveland,Ohio Headquarters
Natural Exposure Testing VS Accelerated Weathering... The Right Choice. Founded in 1956 Specialize in material durability testing equipment and services Bolton, England Q-Lab Europe Cleveland,Ohio Headquarters
Chem 263 Oct. 6, Single bonds, σ. e - donating Activate Activate ortho and para directing ortho and para directing
 Chem 263 ct. 6, 2009 lectrophilic Substitution of Substituted Benzenes Resonance ffect Inductive ffect C=C, π system Single bonds, σ Strong Weak e - donating Activate Activate ortho and para directing
Chem 263 ct. 6, 2009 lectrophilic Substitution of Substituted Benzenes Resonance ffect Inductive ffect C=C, π system Single bonds, σ Strong Weak e - donating Activate Activate ortho and para directing
pro-k group Thermoplastic Sheets Technical Leaflet To provide a fundamental explanation of the terms lightfastness, weather resistance UV resistance
 pro-k group Thermoplastic Sheets Technical Leaflet To provide a fundamental explanation of the terms lightfastness, weather resistance UV resistance 2 Table of contents 1. Introduction to the subject matter
pro-k group Thermoplastic Sheets Technical Leaflet To provide a fundamental explanation of the terms lightfastness, weather resistance UV resistance 2 Table of contents 1. Introduction to the subject matter
Terms used in UV / Visible Spectroscopy
 Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Characterization. Chapter 3: Photo-oxidative degradation in Nylon 66 : Chemical. 3.1 Introduction
 Chapter 3: Photo-oxidative degradation in Nylon 66 : Chemical Characterization 3.1 Introduction Aliphatic polyamides have got very good applications as engineering plastic as well as fiber material. During
Chapter 3: Photo-oxidative degradation in Nylon 66 : Chemical Characterization 3.1 Introduction Aliphatic polyamides have got very good applications as engineering plastic as well as fiber material. During
Thermodynamic Absorption Parameters of Disazo Dyes derived from P-aminophenol and 4-Aminobenzoic Acid on Polyester Fibre and Nylon 6 Fibre
 Material Science Research India Vol. 9(1), 61-67 (2012) Thermodynamic Absorption Parameters of Disazo Dyes derived from P-aminophenol and 4-Aminobenzoic Acid on Polyester Fibre and Nylon 6 Fibre J. O.
Material Science Research India Vol. 9(1), 61-67 (2012) Thermodynamic Absorption Parameters of Disazo Dyes derived from P-aminophenol and 4-Aminobenzoic Acid on Polyester Fibre and Nylon 6 Fibre J. O.
Chapter 2: Scope and Objectives
 Chapter 2: Scope and Objectives 2.1 Introduction Now-a-days the production of polymers and plastics is increasing. Nylon 66 is a widely used yarn as well as engineering resin. Moreover, it is used in outdoor
Chapter 2: Scope and Objectives 2.1 Introduction Now-a-days the production of polymers and plastics is increasing. Nylon 66 is a widely used yarn as well as engineering resin. Moreover, it is used in outdoor
This watermark does not appear in the registered version - Laser- Tissue Interaction
 S S d Laser- Tissue Interaction Types of radiation ionizing radiation Non - ionizing radiation You may click on any of the types of radiation for more detail about its particular type of interaction
S S d Laser- Tissue Interaction Types of radiation ionizing radiation Non - ionizing radiation You may click on any of the types of radiation for more detail about its particular type of interaction
J. O. OTUTU, D. OKORO AND E. K. OSSAI
 GLOBAL JOURNAL OF PURE AND APPLIED SCIENCES VOL 16, NO. 2, 2010: 209-218 COPYRIGHT BACHUDO SCIENCE CO. LTD PRINTED IN NIGERIA. ISSN 1118-0579 www.globaljournalseries.com; Email: info@globaljournalseries.com
GLOBAL JOURNAL OF PURE AND APPLIED SCIENCES VOL 16, NO. 2, 2010: 209-218 COPYRIGHT BACHUDO SCIENCE CO. LTD PRINTED IN NIGERIA. ISSN 1118-0579 www.globaljournalseries.com; Email: info@globaljournalseries.com
The Chemical principles of Coloration
 Chapter 2-3 Introduction The Chemical principles of Coloration A single atoms consists of a central core or nucleus which contains numbers of positively charged particles(protons) and uncharged particles(neutrons)
Chapter 2-3 Introduction The Chemical principles of Coloration A single atoms consists of a central core or nucleus which contains numbers of positively charged particles(protons) and uncharged particles(neutrons)
Supplementary Figure 1. Cross-section SEM image of the polymer scaffold perovskite film using MAI:PbI 2 =1:1 in DMF solvent on the FTO/glass
 Supplementary Figure 1. Cross-section SEM image of the polymer scaffold perovskite film using MAI:PbI 2 =1:1 in DMF solvent on the FTO/glass substrate. Scale bar: 1 m. Supplementary Figure 2. Contact angle
Supplementary Figure 1. Cross-section SEM image of the polymer scaffold perovskite film using MAI:PbI 2 =1:1 in DMF solvent on the FTO/glass substrate. Scale bar: 1 m. Supplementary Figure 2. Contact angle
CHAPTER 7 SUMMARY OF THE PRESENT WORK AND SUGGESTIONS FOR FUTURE WORK
 161 CHAPTER 7 SUMMARY OF THE PRESENT WORK AND SUGGESTIONS FOR FUTURE WORK 7.1 SUMMARY OF THE PRESENT WORK Nonlinear optical materials are required in a wide range of important applications, such as optical
161 CHAPTER 7 SUMMARY OF THE PRESENT WORK AND SUGGESTIONS FOR FUTURE WORK 7.1 SUMMARY OF THE PRESENT WORK Nonlinear optical materials are required in a wide range of important applications, such as optical
Chapter 24. Amines. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 24. Amines Based on McMurry s Organic Chemistry, 7 th edition Amines Organic Nitrogen Compounds Organic derivatives of ammonia, NH 3, Nitrogen atom with a lone pair of electrons, making amines
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Gestão e Produção Sustentável. Durability Electromagnetic radiation. ENG K49 Materiais de origem vegetal aplicados na construção
 Gestão e Produção Sustentável Durability Electromagnetic radiation ENG K49 Materiais de origem vegetal aplicados na construção Ricardo Fernandes Carvalho Electromagnetic radiation spectral band Microwaves
Gestão e Produção Sustentável Durability Electromagnetic radiation ENG K49 Materiais de origem vegetal aplicados na construção Ricardo Fernandes Carvalho Electromagnetic radiation spectral band Microwaves
Chapter 13. Conjugated Unsaturated Systems. +,., - Allyl. What is a conjugated system? AllylicChlorination (High Temperature)
 What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
Disperse dyes. Disperse Dyes and Their Application to Polyester. Fundamental behavior of dyes. Fundamental behavior of dyes
 Fundamental behavior of dyes Fundamental behavior of dyes - The specific reactions concerned depend on the chemical character of the fiber, and therefore choice of dye is restricted to those capable of
Fundamental behavior of dyes Fundamental behavior of dyes - The specific reactions concerned depend on the chemical character of the fiber, and therefore choice of dye is restricted to those capable of
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy
 12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
KELLY COLOR THERM SYSTEM Tinting System based on COOL technology
 KELLY COLOR THERM SYSTEM Tinting System based on COOL technology 1 THE PROBLEM OF DARK COLORS It's well known that a black or dark object exposed to the sun's rays warms more than a white or light-colored
KELLY COLOR THERM SYSTEM Tinting System based on COOL technology 1 THE PROBLEM OF DARK COLORS It's well known that a black or dark object exposed to the sun's rays warms more than a white or light-colored
Effect of ultraviolet and x-ray radiation on optical properties of epoxy polymers dyed with organic phosphors
 Effect of ultraviolet and x-ray radiation on optical properties of epoxy polymers dyed with organic phosphors V CH Laurinas 1, S S Kasymov 1, V M Yurov 1, E N Eremin 2 and M V Vedyashkin 3 1 E.A. Buketov
Effect of ultraviolet and x-ray radiation on optical properties of epoxy polymers dyed with organic phosphors V CH Laurinas 1, S S Kasymov 1, V M Yurov 1, E N Eremin 2 and M V Vedyashkin 3 1 E.A. Buketov
CHEM 322: Azo Dyes: Combinatorial Synthesis of Dyes
 CEM 322: Azo Dyes: Combinatorial Synthesis of Dyes Introduction: Compounds containing one or more azo groups (-=- linked to two carbon atoms) have a variety of uses. Aliphatic azo compounds, like azobisisobutyronitrile
CEM 322: Azo Dyes: Combinatorial Synthesis of Dyes Introduction: Compounds containing one or more azo groups (-=- linked to two carbon atoms) have a variety of uses. Aliphatic azo compounds, like azobisisobutyronitrile
Electrophilic Aromatic Substitution: Direction
 Electrophilic Aromatic Substitution: Direction or each of the following species, show the most likely site(s) for electrophilic aromatic substitution, and predict whether the molecule reacts with electrophiles
Electrophilic Aromatic Substitution: Direction or each of the following species, show the most likely site(s) for electrophilic aromatic substitution, and predict whether the molecule reacts with electrophiles
Multiple mechanisms can attack polymer chains here s what can go wrong. By Jeffrey Jansen the Madison group, Madison, Wisconsin, Usa
 CONSULTANT S CORNER Plastic Failure Through Molecular Degradation Multiple mechanisms can attack polymer chains here s what can go wrong By Jeffrey Jansen the Madison group, Madison, Wisconsin, Usa [Note:
CONSULTANT S CORNER Plastic Failure Through Molecular Degradation Multiple mechanisms can attack polymer chains here s what can go wrong By Jeffrey Jansen the Madison group, Madison, Wisconsin, Usa [Note:
Reactive Dyes for Cellulose Fibres Including UV Absorbers
 Joanna Paluszkiewicz, Wojciech Czajkowski, Marlena Kaźmierska*, Roland Stolarski Technical University of Łódź Institute of Polymer and Dyes Technology ul. Żeromskiego 116, 90-924 Łódź, Poland Email: joanna.pal@wp.pl
Joanna Paluszkiewicz, Wojciech Czajkowski, Marlena Kaźmierska*, Roland Stolarski Technical University of Łódź Institute of Polymer and Dyes Technology ul. Żeromskiego 116, 90-924 Łódź, Poland Email: joanna.pal@wp.pl
Colorimetric Study on the Application of Some Fluorescent Naphthalimide Derivatives
 22 The Open Textile Journal, 2010 3, 22-26 Open Access Colorimetric Study on the Application of Some Fluorescent Naphthalimide Derivatives P. Miladinova and T. Konstantinova * Organic Synthesis Department,
22 The Open Textile Journal, 2010 3, 22-26 Open Access Colorimetric Study on the Application of Some Fluorescent Naphthalimide Derivatives P. Miladinova and T. Konstantinova * Organic Synthesis Department,
Analysis of Food Dyes in Beverages AP* Chemistry Big Idea 1, Investigation 1 An Advanced Inquiry Lab
 Introduction Analysis of Food Dyes in Beverages AP* Chemistry Big Idea 1, Investigation 1 An Advanced Inquiry Lab Catalog o. AP7642 Publication o. 7642 Assume an investigative role and design a valid procedure
Introduction Analysis of Food Dyes in Beverages AP* Chemistry Big Idea 1, Investigation 1 An Advanced Inquiry Lab Catalog o. AP7642 Publication o. 7642 Assume an investigative role and design a valid procedure
Light colour and different dyestuffs
 Light colour and different dyestuffs Dyes and bonding The components involved in histological staining are dyes and proteins. The fundamental process involved is the chemical bonding between the carboxyl
Light colour and different dyestuffs Dyes and bonding The components involved in histological staining are dyes and proteins. The fundamental process involved is the chemical bonding between the carboxyl
Photostabilization of an epoxy resin by forming interpenetrating polymer networks with bisphenol-a diacrylate
 Polymer Degradation and Stability 66 (1999) 343±347 Photostabilization of an epoxy resin by forming interpenetrating polymer networks with bisphenol-a diacrylate Mu-Shih Lin*, Ming-Wei Wang, Lon-An Cheng
Polymer Degradation and Stability 66 (1999) 343±347 Photostabilization of an epoxy resin by forming interpenetrating polymer networks with bisphenol-a diacrylate Mu-Shih Lin*, Ming-Wei Wang, Lon-An Cheng
Technology offer: Environmentally friendly holographic recording material
 Technology offer: Environmentally friendly holographic recording material Technology offer: Environmentally friendly holographic recording material SUMMARY Our research group has developed a new photopolymer
Technology offer: Environmentally friendly holographic recording material Technology offer: Environmentally friendly holographic recording material SUMMARY Our research group has developed a new photopolymer
Paper 12: Organic Spectroscopy
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 31: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part III CHE_P12_M31 TABLE OF CONTENTS 1.
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy 31: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part III CHE_P12_M31 TABLE OF CONTENTS 1.
Alternative analysis of 6-Nitro BIPS behaviour based on factorial design
 Malaysian Journal of Mathematical Sciences 10(S) February: 431 442 (2016) Special Issue: The 3 rd International Conference on Mathematical Applications in Engineering 2014 (ICMAE 14) MALAYSIAN JOURNAL
Malaysian Journal of Mathematical Sciences 10(S) February: 431 442 (2016) Special Issue: The 3 rd International Conference on Mathematical Applications in Engineering 2014 (ICMAE 14) MALAYSIAN JOURNAL
Experiment 1: Thin Layer Chromatography
 Experiment 1: Thin Layer Chromatography Part A: understanding R f values Part B: R f values & solvent polarity Part C: R f values & compound functionality Part D: identification of commercial food dye
Experiment 1: Thin Layer Chromatography Part A: understanding R f values Part B: R f values & solvent polarity Part C: R f values & compound functionality Part D: identification of commercial food dye
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
RADIATION and the EM Spectrum
 RADIATION and the EM Spectrum Radioactivity is the of high-energy particles and/or of energy from a substance as a result of of its atoms. There are several types of radiation. Radiation from the sun is
RADIATION and the EM Spectrum Radioactivity is the of high-energy particles and/or of energy from a substance as a result of of its atoms. There are several types of radiation. Radiation from the sun is
Chapter 20 Amines-part 2
 Reactions of Amines Lone pair on the nitrogen directs the chemistry of amines Chapter 20 Amines-part 2 The nitrogen lone pair can also make a carbon nucleophilic by resonance Amines can also activate carbons
Reactions of Amines Lone pair on the nitrogen directs the chemistry of amines Chapter 20 Amines-part 2 The nitrogen lone pair can also make a carbon nucleophilic by resonance Amines can also activate carbons
Separation and Identification of Plant Pigments Dr. Gergens - SD Mesa College
 Separation and Identification of Plant Pigments Dr. Gergens - SD Mesa College PURPOSE In this experiment, the photosynthetic pigments common to all flowering plants will be extracted by liquidliquid extraction.
Separation and Identification of Plant Pigments Dr. Gergens - SD Mesa College PURPOSE In this experiment, the photosynthetic pigments common to all flowering plants will be extracted by liquidliquid extraction.
OXFORD H i g h e r E d u c a t i o n Oxford University Press, All rights reserved.
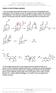 Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Patrick, An Introduction to dicinal hemistry 4e Answers to end-of-chapter questions 1) The mechanism below shows the release of one molecule of formaldehyde from methenamine. The mechanism can then be
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
CH 3. mirror plane. CH c d
 CAPTER 20 Practice Exercises 20.1 The index of hydrogen deficiency is two. The structural possibilities include two double bonds, a double do 20.3 (a) As this is an alkane, it contains only C and and has
CAPTER 20 Practice Exercises 20.1 The index of hydrogen deficiency is two. The structural possibilities include two double bonds, a double do 20.3 (a) As this is an alkane, it contains only C and and has
Fundamental Investigation of Ultrasonic Effects in Textile Wet Processing
 C95-G13 Fundamental Investigation of Ultrasonic Effects in Textile Wet Processing W. W. Carr, S. Michielsen, and H. W. Beckham Georgia Institute of Technology Gary Mock and Bob McCall North Carolina State
C95-G13 Fundamental Investigation of Ultrasonic Effects in Textile Wet Processing W. W. Carr, S. Michielsen, and H. W. Beckham Georgia Institute of Technology Gary Mock and Bob McCall North Carolina State
E35 SPECTROSCOPIC TECHNIQUES IN ORGANIC CHEMISTRY
 E35 SPECTRSCPIC TECNIQUES IN RGANIC CEMISTRY Introductory Comments. These notes are designed to introduce you to the basic spectroscopic techniques which are used for the determination of the structure
E35 SPECTRSCPIC TECNIQUES IN RGANIC CEMISTRY Introductory Comments. These notes are designed to introduce you to the basic spectroscopic techniques which are used for the determination of the structure
Analysis of Artificially Weathered PET and a Separate PET Hydrolysis Evaluation Using the 4300 Handheld FTIR
 Analysis of Artificially Weathered PET and a Separate PET Hydrolysis Evaluation Using the 4300 Handheld FTIR Application Note Authors Frank Higgins Pik Leung Tang Alan Rein Agilent Technologies, Inc. Danbury,
Analysis of Artificially Weathered PET and a Separate PET Hydrolysis Evaluation Using the 4300 Handheld FTIR Application Note Authors Frank Higgins Pik Leung Tang Alan Rein Agilent Technologies, Inc. Danbury,
Chapter 12 Mass Spectrometry and Infrared Spectroscopy
 Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
Atmosphere CHANGE IS IN THE AIR
 Activity 8 UVs and Frisbees Atmosphere CHANGE IS IN THE AIR Forces of Change» Atmosphere» Activity 8» Page 1 UVs and Frisbees Overview This experiment will help students understand that ultraviolet radiation
Activity 8 UVs and Frisbees Atmosphere CHANGE IS IN THE AIR Forces of Change» Atmosphere» Activity 8» Page 1 UVs and Frisbees Overview This experiment will help students understand that ultraviolet radiation
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I
 Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
Department of Chemistry SUNY/Oneonta Chem 221 - Organic Chemistry I Examination #4 - ANSWERS - December 11, 2000 Answer to question #32 corrected 12/13/00, 8:30pm. INSTRUCTIONS This examination is in multiple
The influence of ph adjusted with different acids on the dyeability of polyester fabric
 Polish Journal of Chemical Technology, 16, 4, 1 Pol. 5, J. 10.2478/pjct-2014-0061 Chem. Tech., Vol. 16, No. 4, 2014 1 The influence of ph adjusted with different acids on the dyeability of polyester fabric
Polish Journal of Chemical Technology, 16, 4, 1 Pol. 5, J. 10.2478/pjct-2014-0061 Chem. Tech., Vol. 16, No. 4, 2014 1 The influence of ph adjusted with different acids on the dyeability of polyester fabric
Terms used in UV / Visible Spectroscopy
 Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Name Date Class. aryl halides substitution reaction
 23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
et[ailn B:ag;el Research Limited LIBRARY TECHNICAL FILES.
 ja 15 ' 76 et[ailn B:ag;el Research Limited LIBRARY TECHNICAL FILES.,...., rife -+ {-- *b A.- i a e; t V t~ AX ;, A, : d d-. *. -. -1s \ I '~:, - r... -_,-, -...-- _.---.A..h $ I. m THE INSTITUTE OF PAPER
ja 15 ' 76 et[ailn B:ag;el Research Limited LIBRARY TECHNICAL FILES.,...., rife -+ {-- *b A.- i a e; t V t~ AX ;, A, : d d-. *. -. -1s \ I '~:, - r... -_,-, -...-- _.---.A..h $ I. m THE INSTITUTE OF PAPER
A three-step cleaning methodology to remove the yellowing of the Carrara marble in Oslo Opera House
 A three-step cleaning methodology to remove the yellowing of the Carrara marble in Oslo Opera House Prof. Pagona-Noni Maravelaki School of Architecture, Laboratory of Materials for Cultural Heritage &
A three-step cleaning methodology to remove the yellowing of the Carrara marble in Oslo Opera House Prof. Pagona-Noni Maravelaki School of Architecture, Laboratory of Materials for Cultural Heritage &
International Journal of Pure and Applied Sciences and Technology
 Int. J. Pure Appl. Sci. Technol., 9(1) (2012), pp. 1-8 International Journal of Pure and Applied Sciences and Technology ISSN 2229-6107 Available online at www.ijopaasat.in Research Paper Preparation,
Int. J. Pure Appl. Sci. Technol., 9(1) (2012), pp. 1-8 International Journal of Pure and Applied Sciences and Technology ISSN 2229-6107 Available online at www.ijopaasat.in Research Paper Preparation,
Spectroscopy. Page 1 of 8 L.Pillay (2012)
 Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Organic Chemistry: CHEM2322
 Conjugated Systems Organic Chemistry: We met in Chem 2321 unsaturated bonds as either a C=C bond or C C bond. If these unsaturated bonds are well separated then they react independently however if there
Conjugated Systems Organic Chemistry: We met in Chem 2321 unsaturated bonds as either a C=C bond or C C bond. If these unsaturated bonds are well separated then they react independently however if there
High strength high modulus Fibres
 High strength high modulus Fibres Module 2: FAQ Q1. Define aramids. A manufactured fibre in which the fibre-forming substance is a long-chain synthetic polyamide in which at least 85% of the amide (-CO-NH-)
High strength high modulus Fibres Module 2: FAQ Q1. Define aramids. A manufactured fibre in which the fibre-forming substance is a long-chain synthetic polyamide in which at least 85% of the amide (-CO-NH-)
UV-Vis optical fiber assisted spectroscopy in thin films and solutions
 UV-Vis optical fiber assisted spectroscopy in thin films and solutions Description UV-Visible absorption and transmission spectra provide fundamental information for all experiments related to the attenuation
UV-Vis optical fiber assisted spectroscopy in thin films and solutions Description UV-Visible absorption and transmission spectra provide fundamental information for all experiments related to the attenuation
Atomic Emission Spectra
 Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Atomic Emission Spectra Objectives The objectives of this laboratory are as follows: To build and calibrate a simple meter-stick spectroscope that is capable of measuring wavelengths of visible light.
Photo-degradation of monoazo dye blue 13 using advanced oxidation process
 Chemistry International 1(1) (2015) 12-16 Photo-degradation of monoazo dye blue 13 using advanced oxidation process M. Asghar Jamal 1, Majid Muneer 1,* and Munawar Iqbal 2 1Department of Chemistry, GC
Chemistry International 1(1) (2015) 12-16 Photo-degradation of monoazo dye blue 13 using advanced oxidation process M. Asghar Jamal 1, Majid Muneer 1,* and Munawar Iqbal 2 1Department of Chemistry, GC
THERMAL ANALYSIS IN INDUSTRIAL ENVIRONMENTS
 Journal of Thermal Analysis, Vol 49 (1997) 953-959 THERMAL ANALYSIS IN INDUSTRIAL ENVIRONMENTS D. M. Price Courtaulds Plc., 101 Lockhurst Lane, COVENTRY CV6 5RS, UK Abstract Common industrial procedures
Journal of Thermal Analysis, Vol 49 (1997) 953-959 THERMAL ANALYSIS IN INDUSTRIAL ENVIRONMENTS D. M. Price Courtaulds Plc., 101 Lockhurst Lane, COVENTRY CV6 5RS, UK Abstract Common industrial procedures
Scalable Production of Graphene-Based Wearable
 Scalable Production of Graphene-Based Wearable E-Textiles Nazmul Karim, 1 * Shaila Afroj, 1, 2 Sirui Tan, 3 Pei He, 3 Anura Fernando, 3 Chris Carr, 4 and Kostya S Novoselov 1, 2 1 The National Graphene
Scalable Production of Graphene-Based Wearable E-Textiles Nazmul Karim, 1 * Shaila Afroj, 1, 2 Sirui Tan, 3 Pei He, 3 Anura Fernando, 3 Chris Carr, 4 and Kostya S Novoselov 1, 2 1 The National Graphene
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
LAB #1: ABSORPTION SPECTRA OF CONJUGATED DYES
 Chemistry 7 Gustavus Adolphus College LAB #1: ABSORPTIO SPECTRA OF COJUGATED DYES Abstract Ultraviolet-visible spectroscopy is used to explore the electronic structure of several conjugated polyene dyes,
Chemistry 7 Gustavus Adolphus College LAB #1: ABSORPTIO SPECTRA OF COJUGATED DYES Abstract Ultraviolet-visible spectroscopy is used to explore the electronic structure of several conjugated polyene dyes,
Infra Red Spectroscopy
 CH 2252 Instrumental Methods of Analysis Unit I Infra Red Spectroscopy M. Subramanian Assistant Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
CH 2252 Instrumental Methods of Analysis Unit I Infra Red Spectroscopy M. Subramanian Assistant Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
Fresnel Equations cont.
 Lecture 11 Chapter 4 Fresnel quations cont. Total internal reflection and evanescent waves Optical properties of metals Familiar aspects of the interaction of light and matter Fresnel quations: phases
Lecture 11 Chapter 4 Fresnel quations cont. Total internal reflection and evanescent waves Optical properties of metals Familiar aspects of the interaction of light and matter Fresnel quations: phases
Ultraviolet and Visible Spectroscopy. interaction of materials with light at different electronic levels and the extent, to which such
 Surname 1 Ultraviolet and Visible Spectroscopy Introduction This experiment was carried out to demonstrate the effect of atomic structure on the interaction of materials with light at different electronic
Surname 1 Ultraviolet and Visible Spectroscopy Introduction This experiment was carried out to demonstrate the effect of atomic structure on the interaction of materials with light at different electronic
Sawsan Mohamed Abu El Hassan Mosa
 International Journal of Advanced Research in Chemical Science (IJARCS) Volume 1, Issue 9, November 2014, PP 30-37 ISSN 2349-039X (Print) & ISSN 2349-0403 (Online) www.arcjournals.org Comparison between
International Journal of Advanced Research in Chemical Science (IJARCS) Volume 1, Issue 9, November 2014, PP 30-37 ISSN 2349-039X (Print) & ISSN 2349-0403 (Online) www.arcjournals.org Comparison between
MARK SCHEME for the May/June 2010 question paper for the guidance of teachers 9701 CHEMISTRY
 UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Advanced Subsidiary Level and GCE Advanced Level MARK SCHEME for the May/June 2010 question paper for the guidance of teachers 9701 CHEMISTRY 9701/41
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS GCE Advanced Subsidiary Level and GCE Advanced Level MARK SCHEME for the May/June 2010 question paper for the guidance of teachers 9701 CHEMISTRY 9701/41
Ozone in the Atmosphere
 Ozone in the Atmosphere Why are we concerned with ozone? This simple molecule affects us in very important ways. It protects us, as well as all animals and plants on our planet, from the harm that ultraviolet
Ozone in the Atmosphere Why are we concerned with ozone? This simple molecule affects us in very important ways. It protects us, as well as all animals and plants on our planet, from the harm that ultraviolet
CH-442. Photochemistry I. Prof. Jacques-E. Moser.
 CH-442 Photochemistry I Prof. Jacques-E. Moser http://photochemistry.epfl.ch/pc.html Content PHOTOCHEMISTRY I 1. Basic principles 1.1 Introduction 1.2 Laws of light absorption 1.3 Radiation and molecular
CH-442 Photochemistry I Prof. Jacques-E. Moser http://photochemistry.epfl.ch/pc.html Content PHOTOCHEMISTRY I 1. Basic principles 1.1 Introduction 1.2 Laws of light absorption 1.3 Radiation and molecular
Synthesis of Red 13 Diazo Dye. Diazo dyes are widely used in industries such as foods, pharmaceuticals, cosmetics, textiles, and
 Synthesis of Red 13 Diazo Dye Introduction: Diazo dyes are widely used in industries such as foods, pharmaceuticals, cosmetics, textiles, and leather for coloring purposes or used as a food additive to
Synthesis of Red 13 Diazo Dye Introduction: Diazo dyes are widely used in industries such as foods, pharmaceuticals, cosmetics, textiles, and leather for coloring purposes or used as a food additive to
Spectral reflectance: When the solar radiation is incident upon the earth s surface, it is either
 Spectral reflectance: When the solar radiation is incident upon the earth s surface, it is either reflected by the surface, transmitted into the surface or absorbed and emitted by the surface. Remote sensing
Spectral reflectance: When the solar radiation is incident upon the earth s surface, it is either reflected by the surface, transmitted into the surface or absorbed and emitted by the surface. Remote sensing
MOLEBIO LAB #4: Using a Spectrophotometer
 Introduction: Spectrophotometry MOLEBIO LAB #4: Using a Spectrophotometer Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while
Introduction: Spectrophotometry MOLEBIO LAB #4: Using a Spectrophotometer Many kinds of molecules interact with or absorb specific types of radiant energy in a predictable fashion. For example, when while
An alcohol is a compound obtained by substituting a hydoxyl group ( OH) for an H atom on a carbon atom of a hydrocarbon group.
 Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Look for absorption bands in decreasing order of importance:
 1. Match the following to their IR spectra (30 points) Look for absorption bands in decreasing order of importance: a e a 2941 1716 d f b 3333 c b 1466 1.the - absorption(s) between 3100 and 2850 cm-1.
1. Match the following to their IR spectra (30 points) Look for absorption bands in decreasing order of importance: a e a 2941 1716 d f b 3333 c b 1466 1.the - absorption(s) between 3100 and 2850 cm-1.
Ch 18 Ethers and Epoxides
 Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline
 Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Chem 1075 Chapter 19 Organic Chemistry Lecture Outline Slide 2 Introduction Organic chemistry is the study of and its compounds. The major sources of carbon are the fossil fuels: petroleum, natural gas,
Coloring Powder Compositions are Universal Dyes for Dyeing Natural and Synthetic Fibers as Well as Textile Materials based on Them
 J. Chem. Eng. Chem. Res. Vol. 1, No. 1, 2014, pp. 138-145 Received: May 29, 2014; Published: August 25, 2014 Journal of Chemical Engineering and Chemistry Research Coloring Powder Compositions are Universal
J. Chem. Eng. Chem. Res. Vol. 1, No. 1, 2014, pp. 138-145 Received: May 29, 2014; Published: August 25, 2014 Journal of Chemical Engineering and Chemistry Research Coloring Powder Compositions are Universal
CHAPTER 3. FABRICATION TECHNOLOGIES OF CdSe/ZnS / Au NANOPARTICLES AND NANODEVICES. 3.1 THE SYNTHESIS OF Citrate-Capped Au NANOPARTICLES
 CHAPTER 3 FABRICATION TECHNOLOGIES OF CdSe/ZnS / Au NANOPARTICLES AND NANODEVICES 3.1 THE SYNTHESIS OF Citrate-Capped Au NANOPARTICLES Au NPs with ~ 15 nm were prepared by citrate reduction of HAuCl 4
CHAPTER 3 FABRICATION TECHNOLOGIES OF CdSe/ZnS / Au NANOPARTICLES AND NANODEVICES 3.1 THE SYNTHESIS OF Citrate-Capped Au NANOPARTICLES Au NPs with ~ 15 nm were prepared by citrate reduction of HAuCl 4
Thin Layer Chromatography
 Experiment: Thin Layer Chromatography Chromatography is a technique widely used by organic chemists to separate and identify components in a mixture. There are many types of chromatography, but all involve
Experiment: Thin Layer Chromatography Chromatography is a technique widely used by organic chemists to separate and identify components in a mixture. There are many types of chromatography, but all involve
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Planetary Science: Investigations 9-10 I-Check Quiz STUDY GUIDE- ANSWER KEY Name HR Date
 1. How are different types of radiation arranged along the electromagnetic spectrum? A. By how fast they travel incorrect answer B. By their sources incorrect answer C. By the amount of energy they carry
1. How are different types of radiation arranged along the electromagnetic spectrum? A. By how fast they travel incorrect answer B. By their sources incorrect answer C. By the amount of energy they carry
Multi-step Synthesis: Preparation of Organic Dyes *
 OpenStax-CNX module: m15877 1 Multi-step Synthesis: Preparation of Organic Dyes * Mary McHale This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 2.0 1 Multi-step
OpenStax-CNX module: m15877 1 Multi-step Synthesis: Preparation of Organic Dyes * Mary McHale This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 2.0 1 Multi-step
Infrared Spectroscopy: Identification of Unknown Substances
 Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
EFFECT OF CO 2 LASER RADIATION ON SURFACE PROPERTIES OF SYNTHETIC FIBRES F. Esteves, H. Alonso ABSTRACT
 EFFECT OF CO 2 LASER RADIATION ON SURFACE PROPERTIES OF SYNTHETIC FIBRES F. Esteves, H. Alonso ABSTRACT Chemical treatment methods are most often used in the present for polymer surface modification; however,
EFFECT OF CO 2 LASER RADIATION ON SURFACE PROPERTIES OF SYNTHETIC FIBRES F. Esteves, H. Alonso ABSTRACT Chemical treatment methods are most often used in the present for polymer surface modification; however,
Chapter 9 Aldehydes and Ketones Excluded Sections:
 Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
Chapter 9 Aldehydes and Ketones Excluded Sections: 9.14-9.19 Aldehydes and ketones are found in many fragrant odors of many fruits, fine perfumes, hormones etc. some examples are listed below. Aldehydes
ELECTROMAGNETIC WAVES ELECTROMAGNETIC SPECTRUM
 VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES ELECTROMAGNETIC SPECTRUM When white light passes through a prism, it spreads out into a rainbow of colours, with red at one end and
VISUAL PHYSICS ONLINE MODULE 7 NATURE OF LIGHT ELECTROMAGNETIC WAVES ELECTROMAGNETIC SPECTRUM When white light passes through a prism, it spreads out into a rainbow of colours, with red at one end and
Michael Willis M.S., Sun Chemical Corp. Technical Manager, Advanced Applications, Plastics Labs
 Pigment Crystallinity and Molecular Structure of Some Selected Classical and High Performance Pigments and their Performance in Thermoplastic Applications Michael Willis M.S., Sun Chemical Corp. Technical
Pigment Crystallinity and Molecular Structure of Some Selected Classical and High Performance Pigments and their Performance in Thermoplastic Applications Michael Willis M.S., Sun Chemical Corp. Technical
Chapter 2: Protecting the Ozone Layer
 Chapter 2: Protecting the zone Layer Why do we need to do to protect the ozone layer? Isn t ozone hazardous to human health? Why is the ozone layer getting smaller? What can we do (if anything) to help
Chapter 2: Protecting the zone Layer Why do we need to do to protect the ozone layer? Isn t ozone hazardous to human health? Why is the ozone layer getting smaller? What can we do (if anything) to help
Case Study of Electronic Materials Packaging with Poor Metal Adhesion and the Process for Performing Root Cause Failure Analysis
 Case Study of Electronic Materials Packaging with Poor Metal Adhesion and the Process for Performing Root Cause Failure Analysis Dr. E. A. Leone BACKGRUND ne trend in the electronic packaging industry
Case Study of Electronic Materials Packaging with Poor Metal Adhesion and the Process for Performing Root Cause Failure Analysis Dr. E. A. Leone BACKGRUND ne trend in the electronic packaging industry
Supporting Information
 Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 217 Electronic Supplementary Material
Electronic Supplementary Material (ESI) for New Journal of Chemistry. This journal is The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 217 Electronic Supplementary Material
CHEM Chapter 21. Carboxylic Acid Derivatives_Nucleophilic Acyl Substitution Reactions (homework) W
 Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Quantum chemical studies on the structures of some heterocyclic azo disperse dyes
 Quantum chemical studies on the structures of some heterocyclic azo disperse dyes Nesrin Tokay, a* Zeynel Seferoğlu, b Cemil Öğretir, c and Nermin Ertan b a Hacettepe University, Faculty of Science, Chemistry
Quantum chemical studies on the structures of some heterocyclic azo disperse dyes Nesrin Tokay, a* Zeynel Seferoğlu, b Cemil Öğretir, c and Nermin Ertan b a Hacettepe University, Faculty of Science, Chemistry
Chapter 25 Organic and Biological Chemistry
 Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
Chapter 25 Organic and Biological Chemistry Organic Chemistry The chemistry of carbon compounds. Carbon has the ability to form long chains. Without this property, large biomolecules such as proteins,
UV-Vis Spectroscopy. Chem 744 Spring Gregory R. Cook, NDSU Thursday, February 14, 13
 UV-Vis Spectroscopy Chem 744 Spring 2013 UV-Vis Spectroscopy Every organic molecule absorbs UV-visible light Energy of electronic transitions saturated functionality not in region that is easily accessible
UV-Vis Spectroscopy Chem 744 Spring 2013 UV-Vis Spectroscopy Every organic molecule absorbs UV-visible light Energy of electronic transitions saturated functionality not in region that is easily accessible
