A prototype cell for extracting energy from a water salinity difference by means of double layer expansion in nanoporous carbon electrodes
|
|
|
- Tobias Wheeler
- 6 years ago
- Views:
Transcription
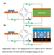
1 A prototype cell for extracting energy from a water salinity difference by means of double layer expansion in nanoporous carbon electrodes D. Brogioli, a R. Zhao, b and P. M. Biesheuvel b Received Xth XXXXXXXXXX 2XX, Accepted Xth XXXXXXXXX 2XX First published on the web Xth XXXXXXXXXX 2X DOI: 1.139/bx Electrical energy can be obtained from the controlled mixing of fresh (river) and saline (sea) water. Existing technologies such as pressure retarded osmosis and reverse electrodialysis make use of ion-exchange membranes which must be crossed by either the water or the ions. Recently a new physical principle has been experimentally demonstrated, which allows extraction of electrical energy without making use of membranes, based on the temporary storage of ions inside of two porous electrodes kept at different electrical potentials, and the repeatable expansion/contraction of the electrostatic double layers formed inside of the electrodes upon changing salt concentration [D. Brogioli, Phys. Rev. Lett., 29, 13, 5851]. To make further investigations and to improve the energy recovery, we developed a simple prototype cell of much larger dimensions. Because of the larger dimensions (thus higher currents), testing is more facile, while this design can be the basis for further scaling-up of this technology. In order to reduce the internal resistance of the cell, the electrodes are no longer placed side-by-side, but are placed parallel to one another, separated only by a 4 µm-thick open spacer channel to form a sandwich -like flow cell. In a lab-scale experimental stack consisting of 8 such cells (with outer dimensions cm 3 ) we extract about 2 J per charging/discharging cycle in 5 mm/1 mm NaCl salt solution, an amount which is 2 times higher per cycle per unit electrode mass than obtained previously. The extracted energy increases with the operating voltage, in line with predictions of the Gouy-Chapman-Stern model for double layer formation. Fig. 1 Schematic view of the flow cell for capacitive energy extraction from the sequential flow of saline and fresh water, and of the required electric circuit. The external capacitor provides a constant voltage of V while the load R represents the device for energy-harvesting. When salt water and fresh water are mixed, the entropy of the system increases. This entropy change can be intercepted and used to convert part of the thermal energy of the fluids into electrical energy. In particular, this idea can be used to extract energy from the controlled mixing of river and sea water. The thermodynamic limit of this energy extraction is determined by the free energy change upon mixing, which is around 2 kj when one liter of fresh water is mixed with an equal amount of sea water. 1 Worldwide, the potential for energy extraction from this resource (for all river effluents combined) amounts to around 2 TW, close to present-day global electricity use. 2,3 Several technologies were suggested to harvest this source of renewable energy. 4,5 In pressure-retarded osmosis (PRO) 6,7 the water moves through a semi-permeable membrane under the influence of the osmotic pressure difference, leading to the development of a hydrostatic head which subsequently can be converted into electricity by using a turbine. Alternatively, it is not the water which is transported through the membrane but the ions, which is the approach used in reverse electrodiala Dipartimento di Medicina Sperimentale, Università degli Studi di Milano- Bicocca, Via Cadore, 48, 252 Monza, Italy. dbrogioli@gmail.com b Department of Environmental Technology, Wageningen University, Bomenweg 2, 673 HD Wageningen, The Netherlands; Wetsus, centre of excellence for sustainable water technology, Agora 1, 89 CC Leeuwarden, The Netherlands 1 7 1
2 ysis (RED) 2,8 and in a recent capacitive technology using ionexchange membranes. 3 In RED, compartments fed alternately by fresh or salt water are separated by a sequence of positively and negatively charged ion-exchange membranes. The potential that develops across each membrane sums up to a large overall potential which, together with a redox solution being cycled along the electrodes, results in a continuous current and thus electrical power. Both PRO and RED require membranes, which are prone to the usual problems of fouling, while permeabilities and selectivities still need to be improved before successful applications. 2,8 An alternative method, based on the vapor pressure difference between fresh and saline water, has also been proposed. 9 Up to now, no industrial scale plant has been built, but salinity difference power has received renewed interest, and research and development is on-going both for PRO 1 and RED. 2,8 In this letter we present laboratory-scale results of a new technology that does not need membranes, which we call capacitive energy extraction based on double-layer expansion (CDLE) and which was first proposed by Brogioli 11 and experimentally tested in a microfluidic flow cell in which microjoule power was generated. In this technology, two porous supercapacitor electrodes are first contacted with salt water and are charged by connecting each electrode to one pole of a external capacitor (EC) operating at a voltage difference V. The EC is an essential element of the technology and must operate at low overvoltage which can be achieved when the EC-capacity is much larger than the capacity of the flow cell. It must be stressed that the EC is not a source of energy and slowly discharging. Instead, it operates as an ideally reversible storage device for electronic charge, at a certain voltage. While the EC charges the flow cell during flow of saline water, the EC is re-charged again when fresh water flows through the cell and the current direction is reversed. Thus, the EC can be seen as equivalent to the flywheel for internal combustion engines. In the flow cell, brought in contact with saline water, and with an EC-voltage V applied, the following events take place in the electrodes. First of all, electrons start to flow through the external circuit, making one electrode positive and the other negative. Subsequently, at the interfaces within the porous electrode, where the electron-conducting matrix is in contact with the aqueous solution filling the pores, electrostatic double layers (EDL) develop where the electronic charge is compensated by an ion charge excess in the diffuse layer of the EDL, with an EDL containing positive ion countercharge in the cathode, and vice-versa in the anode. Formation of the EDL continues until V cell (initially smaller than V ) becomes equal to V. Subsequently the electrodes are brought into contact with fresh water which modifies the structure of the EDL (i.e. the diffuse layer will expand) which leads to an increase in the cell potential, V cell, to values beyond V resulting in a flow of electrons in the reverse direction, re-charging the EC. An analogy can help to describe the physical principle of CDLE. Consider an electrostatic capacitor, made of two conductive plates with a dielectric medium in between. When the plates constituting the capacitor are charged, one relative to the other, the electrostatic force is attractive. We can perform mechanical work and bring the plates further apart. This work is converted into electrostatic energy, appearing as an increase of voltage between the plates, while the accumulated charge remains constant. This kind of device is thus able to transform mechanical work into electrostatic energy. In the EDL capacitor described in this paper, one charged plate of the above example is substituted by the ion countercharge (diffuse layer of ions, or ion cloud ). When this capacitor is brought into contact with fresh water, the salt ions diffuse away from the electrode surface. This ion outward displacement, performed by diffusion, increases the average distance between the electrode surface and the ion cloud forming the second charged plate. In analogy with the previous case, the voltage across the capacitor increases. In order to use the electrical energy extracted from this system, consisting of the flow cell and the EC, a device must be placed in the external circuit to harvest the energy, either by making direct use of it (e.g., to drive an electrical device) or by storing it, as in a battery or redox flow cell. In electrical terms, this device is called a load. In our experiments the load is simply a passive resistance, denoted by R in Fig. 1, producing heat upon current flow in either direction. It is important to realize that in both steps of the cycle (both during charging in saline water, and during discharging in fresh water) electrical energy can be harvested because in both steps the electrons flow spontaneously through the resistance, with only the current direction reversed. The total electrical energy which is dissipated in the load R equals the area enclosed by each charge-voltage cycle shown in Fig. 4. Up to now, only a proof of principle has been given of CDLE, 11 in a microfluidic flow cell with small porous electrodes made of mg of activated carbon, placed one behind the other. In this system it was possible to recover 5 µj in one charging/discharging cycle. Brogioli 11 analyzed the upscaling potential of the principle of CDLE suggesting that it may be feasible to harvest energy in an economically attractive manner. One of the key parameters in this evaluation is the energy that can be extracted per cycle per gram of electrode material. In the present work we transfer the CDLE principle from the scale of a microfluidic device to the scale of a much larger prototype cell, constructed from commonly available and inexpensive materials, and constructed in such a way that further technological improvement and scaling-up is possible. Because of the larger cell dimensions and amount of electrode material (about 2, times more), much more current can be generated and the results of the experiments are much more re
3 liable, without relying on µa noisy measurements. As we will show the prototype cell is not only larger, but also more efficient with the per-cycle per-gram energy recovery increased by a factor of 2 compared to that reported by Brogioli. 11 In the protype cell, besides increasing system dimensions, one of the main modifications is to reduce the internal resistance of the flow cell by placing the electrodes parallel to one another, separated only by a thin spacer layer of less than half a mm thickness, permeable by ions and water, but electrically insulating for electrons. We use electrode materials used in state-of-the-art supercapacitors, with a high specific surface area for EDL formation. The flow cell has been designed such that the liquid can easily contact the electrodes, simultaneously with robust current transfer from one electrode to the other via current collectors and the external circuit, see Fig. 1. Our laboratory-scale setup consists of eight parallel flow cells 15 (see Fig. 1) consisting each of dense graphite current collectors (thickness δ = 25 µm), porous carbon electrodes (δ = 27 µm, 8.5 g in total in the stack, mass density.58 g/ml, porosity 65%, BET-area 133 m 2 /g), and an open-meshed polymer spacer (δ = 4 µm, porosity > 8%). Each graphite current collector is used for two adjacent cells and is alternatingly connected to the positive or negative pole of the external circuit. All materials are cut in pieces of 6 6 cm 2 dimension and assembled, after which the entire stack of all layers is firmly compressed and placed in a teflon housing. An aqueous NaCl solution is pumped into a small hole ( cm 2 ) located in the exact middle of the stack, and flows radially outward through the spacer channels, leaving the cell on all four sides. The total flowrate, which is 1 ml/sec, is constant during all experiments. The electrical circuit includes a potentiostat, that simulates the EC, operating at a voltage V, and a resistance R = 11 Ω, constituting the load, in series with the flow cell. Current I is measured by the potentiostat, and the voltage across the cell, V cell, is calculated from V cell = V R I. To describe the experimental data we will use classical Gouy-Chapman-Stern (GCS) theory, in which the EDL is decomposed into an inner (Stern) layer, and a diffuse (Gouy- Chapman) layer. For a given surface charge density σ, defined as charge per internal electrode surface area, σ = Q/a m, the potential V St over the Stern layer is V St = σ C St, (1) where C St is the Stern layer capacity. For an ideal 1:1 monovalent salt solution, the potential V d over the diffuse layer is 14,16,17 V d = 2V T asinh(ασλ D ), (2) where λ D is the Debye screening length, related to the ionic Cell potential (V) 1 5 mm 1 mm 5 mm 2 mm 5 mm 1 mm Charge Q (C/g) 5 mm 2 mm Fig. 2 Equilibrium values for electrode charge versus cell potential for different values of the ionic strength (NaCl solutions). Lines according to the Gouy-Chapman-Stern theory (parameter settings in the text). strength c (in mm) according to λ D = 1 8πλB cn Av, (3) where e is the electron charge, V T = k B T/e, N Av is Avogadro s number, λ B is the Bjerrum length (λ B =.72 nm in water) and α is a constant given by α = 1/(2εV T ) where ε is the dielectric permittivity of water. The potential over the EDL is given by the sum of the two potentials, V St and V d, and at equilibrium the potential over the whole cell, including the two EDLs, is V cell = 2(V St +V d ) assuming symmetrical behavior of the two electrodes. As combination of Eqs (2) and (3) shows, for a fixed charge σ, with decreasing ionic strength c (salt concentration) the voltage V d goes up. This is, mathematically expressed, the principle of CDLE. For the materials used in this experiment, the effective area for ion adsorption is a m =9 m 2 /g, and the Stern layer capacitance is C St =.1 F/m 2 ; the procedure for the accurate evaluation of these quantities is presented by Zhao et al. 15 In a first set of experiments, the equilibrium electrode charge was measured versus the cell potential for different values of the ionic strength of the solution flowing through our prototype cell. Fig. 2 reports the resulting equilibrium cell potential V cell versus the charge per gram of electrode material Q. Data at 1 mm and 5 mm were obtained in the following way. The potentiostat is directly connected to the cell, and operated in chrono-amperometric mode (integrating current with 1 7 3
4 Cell potential (V) Data Data V V Data V GCS theory GCS theory GCS theory 1 2 Time (hours) Fig. 3 Variation of cell potential upon salinity switching in a cell with fixed charge. Three experiments are reported, at different EC-voltages V. Horizontal lines shows the EC-voltage, V, and the cell potential predicted by GCS theory, for concentration 1 mm and η =.99. The arrows represent the time at which salinity switching takes place (up: to 5 mm; down: to 1 mm solution). time) first stepping up in voltage (steps of V) up to 1 V and then down again, each time with a resting period of 3 minutes. To compare, we also show data from Zhao et al. 15 for the same electrode material and for intermediate values of the salt concentrations, namely at 5 mm and 2 mm, which were also obtained in amperometric mode but in this case for each data point the potential is increased from V directly up to V cell, so that each experimental data point represents an independent measurement. It can be clearly observed from the four data sets that the charge increases with cell voltage as well as with salt concentration, while both dependencies are well described by the GCS-theory. Fig. 2 shows that, for a given charge, lowering the ionic strength leads to a higher cell potential, due to an expansion of the EDL, see Eqs. 2 and 3, which is the principle at the basis of energy extraction using CDLE. In the previous experiment, for a given ionic strength the cell voltage was varied and the equilibrium charge determined. In the next experiment we more closely simulate the CDLE process. Namely we start with flowing saline water into the cell and apply a certain EC-voltage V. It is important to note that, in our experiment, the EC is simulated by a power supply operating at a constant voltage, V. After some time we disconnect (i.e. open ) the external circuit to fix (i.e. trap ) the stored charge and repeatedly switch the inflowing solution from saline water (5 mm) to fresh water (1 mm) and back. Ideally, this should lead to a repeatable increase in voltage when going to fresh water, and a decrease upon contacting with saline water, in line with the data of Fig. 2. This experiment is then more conclusive evidence that indeed it is possible to modulate the cell voltage by repeatedly switching from saline to fresh water. Results presented in Fig. 3 show that indeed this scenario holds true, but with certain differences compared to Fig. 2 and compared to similar experiments reported by Brogioli. 11 Fig. 3 presents results for three values of the EC-voltage (.3,.5 and.7 V) and shows that indeed after disconnecting the electrical circuit and switching to fresh water (downward arrows) the cell voltage increases, and decreases again after starting flow of saline water (upward arrows). It must be noted that in this experiment a gradual loss of cell potential was observed, which we ascribe to a small leakage current, and thus to a gradual loss of stored charge. The leakage current was estimated at.3,.7 and 2.1 ma respectively for EC-voltages V of.3,.5 and.7 V. This leakage current is recalculated to a cell potential decline and used to correct the raw data. Fig. 3 shows that when the concentration is reduced to 1 mm, the cell potential increase, φ, is.1.16 V. Interestingly, this range of values is much higher than the 33 mvincrease for φ previously reported. 11 But still φ is less than predicted by GCS theory, which based on the equilibrium data in Fig. 2 suggests that φ must be about V. This can be derived from Fig. 2 by observing that for a cell voltage of.5 V, the equilibrium charge is about 2 C/g at 5 mm. For this charge, the cell voltage at 1 mm is about.7 V, which would imply that φ is about V. What is the cause of this difference? We have assumed that the reason is that upon switching to fresh water not all ions absorbed within the pores of the electrode during the previous step are washed out completely again; instead, we assume that a small fraction 1 η of the ions initially present is not removed upon switching to fresh water, so that the effective concentration is not 1 mm (for fresh water) but is 1 mm+(1 η) 5 mm. To describe the data in Fig. 3 we take a value of η =.99, thus the effective concentration of the fresh water is not 1 mm but 6 mm. Other possible explanations for this difference are the contribution of protons and hydroxyl ions in surface charge compensation, 18 or chemical charge regulation of the carbon surface groups. Though φ is much higher than in the work of Brogioli, 11 unfortunately the approach to equilibrium is also much slower. This can be quantified by the characteristic time, τ, which it takes for the cell potential to change to half the value of φ, which for the data obtained with the prototype cell is 8 s at 1 mm and 8 s at 5 mm. Both these times are much longer than reported by Brogioli, 11 where τ was of the order of several seconds. This is due to the fact that in our experiments the charge and discharge times RC are longer: the capacitance 4 1 7
5 is much bigger, and is not compensated by a proportionally lower internal resistance. Actually, the resistance in the experiments performed by Brogioli 11 may have been lower than in the prototype cell, because the electrodes were thinner and contained more macropores, thereby reducing mass transfer limitations within the electrode. Reducing the resistance of the prototype cell demands more future study because optimizing the dynamics of the process is very important to make the technology commercially relevant. To finally fully test the principle of CDLE, similar to the experiments reported by Brogioli, 11 we used the electrical circuit shown in Fig. 1, with the potentiostat (operating in chrono-amperometric mode) simulating an ideally rechargeable EC operating at a constant voltage V and without any appreciable overpotential. One difference with the previous experiment is that now charge is no longer fixed and the voltage change measured upon salinity switching, but instead in two steps of the cycle, charge is allowed to flow and the voltage across the load R is calculated from the current. In the two switching steps (from fresh to salt water, and back), the charge is fixed using an open electrical circuit. In this way the voltage across the resistance, V cell V, is maximized, and thus the power production. Each experiment consists of the following four steps: Cell potential (V) fresh water sea water 1. Charging: We start the flow of saline water through the cell and charge the electrodes for 3 hours. 2. Switching step I: We open the circuit and let the fresh water flow for 3 minutes. 3. Discharging: We close the circuit and monitor the current for up to 15 hours. Measured cycles Theoretical cycle Charge Q (C/g) 4. Switching step II: We open the circuit and start the flow of saline water for 15 minutes. In these CDLE experiments, full cycles were repeatedly made at EC-voltages from V =.3 V to V =.7 V. The evolution of the cell voltage versus time is similar to that shown in Fig. 3 and is not repeated here, but see Fig. 6 to be discussed later for the power production as function of time. Instead, to evaluate the electrical energy that is harvested, it is more informative to directly plot the cell potential, V cell, versus the stored charge, as shown in Fig. 4, reporting results for different EC-voltages V. In this representation, the cycles show a close analogy with classical thermodynamic cycles for pressure versus volume of thermomechanical systems. 19,2 In particular, the area enclosed by the cycle represent the extracted energy. As explained, the process is run through counterclockwise with the vertical edges representing the two switching steps; the right one representing switching to a low salinity feed solution, and the left one switching back to high salinity water. The upper line represents the cell potential decay Fig. 4 Charge-voltage cycle at different EC-voltages V, represented by dotted lines. The area enclosed by each cycle represents the extracted electrical energy from switching between 1 mm and 5 mm salt solutions. The lines labelled with sea water and fresh water represent the charge-voltage relations obtained from GCS theory, respectively for 5 mm and 1 mm with η =
6 towards V when flushing with fresh water, with current running in one direction, and the lower line represents the cell potential increase towards V in saline water with current now running in the other direction. The difference between the cell voltage and the EC-voltage (horizontal dashed line) equals the voltage across the resistance which is of different sign during charging in saline water and during discharging in fresh water, but note that energy is extracted both during charging and during discharging because the current direction is also reversed. Fig. 4 shows how the enclosed area (harvested energy) increases with increasing EC-voltage, as plotted in more detail in Fig. 5. In our experiment with the prototype cell, the extracted energy is of the order of 2 J for EC-voltages of V >.5 V, which is of the order of 1 6 times the amount extracted in the microscale setup, as previously reported. 11 The energy per cycle per gram of electrode (where mass is defined as that of all electrodes of one charge sign, i.e., either of the cathode or of the anode) can be calculated as about 7 J per gram of electrode: compared to the first reported experiments this is an improvement by a factor 2. It is relevant to note that in the experiment there is some leakage current so that when each cycle is run through several times (starting initially at V cell = V) we have lost knowledge of the exact charge stored, i.e., how far to the left or right each cycle must be plotted in Fig. 4. This is to some extent not essential because the energy that can be harvested (the enclosed area) does not depend on the total charge stored, but only on the change in charge between start and end of the (dis-)charging steps. Therefore we have taken the liberty to shift each cycle left-right to fit within the two theoretical curves based on GCS-theory which envelope our set of chargevoltage cycles. The area delimited by dots in Fig. 4 represents one theoretical cycle for an EC-voltage of V = V. As can be noticed, the enclosed area of each of the experimental cycles is smaller than that of the corresponding theoretical cycle. This may be due to several effects, including a voltage drop across the internal cell resistance, incomplete charging, and leakage current. However, the origin of the mismatch of the experimental cycles with respect to the theory is not is not very well understood, because the external resistance of 11 Ω is two orders of magnitude larger than the internal resistance of the flow cell, and thus the current should be low enough to generate low internal voltage drops, and thus the energy dissipation in the cell should be small. What is in general the effect of the chosen load on the power production? In this work, since the main goal is to quantify the maximum energy that can be extracted from a single cycle, we selected a load with a resistance that is much higher than the internal resistance of the cell. However, the duration of each cycle is then also very long. A higher power production Energy (J/g) 1 Theoretical cycle, GCS theory Experimental data V (V) Fig. 5 Extracted electrical energy per cycle based on the CDLE-principle, as a function of the EC-voltage, V. Solid line is based on GCS theory. per unit time can be obtained with a lower resistance of the load R. For steady-state operation and when the internal cell resistance is constant, it can be derived that power production is maximized when the internal cell resistance is equal to the resistance of the load. Fig. 5 shows in detail the values of the extracted energy as a function of EC-voltage V. It also shows the calculation of the energy that can be extracted in a cycle, based on the GCS theory, for concentrations 5 mm and 1 mm, with efficiency η =.99. As the comparison shows, in reality we extract about half the energy content theoretically available in a cycle. Finally we present in Fig. 6 curves for the produced power per unit projected electrode area (of all electrodes of one charge sign) for the charging and for the discharging step, for one of the cycles of Fig. 4. The power is calculated by multiplying at each moment the current with the voltage drop across the external resistance, R, where the voltage drop equals current times resistance, and finally divide by the area. We observe that the maximum power is produced at the start of each step. This is different from the power curves as given by Sales et al. 3 where the maxima occur slightly later. This difference is due to the fact that in Sales et al. 3 switching steps were not included. The maximum power produced in the present work is lower than in Sales et al. 3 which relates to the higher external resistance which we use (11 Ω vs. 1 Ω); however the extracted energy per cycle is higher. It is important to note that our results must be considered 6 1 7
7 Power density (mw/m 2 ) Time (hours) Charge Discharge Fig. 6 Power production as function of time during charging and discharging (EC-voltage V =.3 V). 2 J. W. Post, H. V. M. Hamelers and C. J. N. Buisman, Env. Sci. Techn., 28, 42, B. B. Sales, M. Saakes, J. Post, C. J. N. Buisman, P. M. Biesheuvel and H. V. M. Hamelers, Env. Sci. Techn., 21, 44, R. E. Pattle, Nature, 1954, 174, R. W. Norman, Science, 1974, 186, O. Levenspiel and N. de Vevers, Science, 1974, 183, S. Loeb, Science, 1975, 189, J. N. Weinstein and F. B. Leitz, Science, 1976, 191, M. Olsson, G. L. Wick and J. D. Isaacs, Science, 1979, 26, K. Gerstandt, K. V. Peinemann, S. E. Skilhagen, T. Thorsen and T. Holt, Desalination, 28, 224, D. Brogioli, Phys. Rev. Lett., 29, 13, P. Simon and Y. Gogotsi, Nature Mater., 28, 7, M. Levi, G. Salitra, N. Levy, D. Aurbach and J. Maier, Nature Mater., 29, 8, P. M. Biesheuvel and M. Z. Bazant, Phys. Rev. E, 21, 81, R. Zhao, P. M. Biesheuvel, H. Miedema, H. Bruning and A. van der Wal, J. Phys. Chem. Lett., 21, 1, Z. Jiang and D. Stein, Langmuir, 21, 26, R. J. Hunter, Foundations of colloid science, Clarendon press, Oxford, 1993, pp K. M. Joshi and R. Parsons, Electrochim. Acta, 1961, 4, P. M. Biesheuvel, J. Colloid Interface Sci., 29, 332, N. Boon and R. van Roij, Mol. Phys., accepted. with some care, since our experiments suffered from leakage currents which though small were important because of the long times involved. Note also that several of the cycles presented in Fig. 4 were replicated a few times and only the best result is presented. So our data must not be considered as definitive, but still they show enough indications that with our prototype setup it is possible to reach an energy recovery of the order of several Joules. Compared to previous work using the CDLE-principle, this then implies an increase in per-cycle per-gram energy recovery by a factor of about 2. Acknowledgments The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/27-213) under grant agreement no Part of this research was performed in the TTIW-cooperation framework of Wetsus, Centre of Excellence for Sustainable Water Technology. Wetsus is funded by the Dutch Ministry of Economic Affairs, the European Union Regional Development Fund, the Province of Friesland, the City of Leeuwarden, and the EZ/Kompas program of the Samenwerkingsverband Noord-Nederland. We thank the members of the theme Blue Energy in Wetsus for their participation in this research. References 1 R. Semiat, Env. Sci. Techn., 28, 42,
MEMBRANE CAPACITIVE DEIONIZATION
 MEMBRANE CAPACITIVE DEIONIZATION Albert van der Wal and Hank Reinhoudt Voltea b.v., Wassenaarseweg 72, 2333 AL, Leiden, the Netherlands Presenter Hank Reinhoudt Corresponding author: Hank.Reinhoudt@voltea.com
MEMBRANE CAPACITIVE DEIONIZATION Albert van der Wal and Hank Reinhoudt Voltea b.v., Wassenaarseweg 72, 2333 AL, Leiden, the Netherlands Presenter Hank Reinhoudt Corresponding author: Hank.Reinhoudt@voltea.com
THEORY AND OPERATION OF CAPACITIVE DEIONIZATION SYSTEMS. Ran Zhao
 THEORY AND OPERATION OF CAPACITIVE DEIONIZATION SYSTEMS Ran Zhao Thesis committee Promotor Prof. dr. ir. A. van der Wal Professor of Electrochemical Water Treatment Wageningen University Co-promotors Prof.
THEORY AND OPERATION OF CAPACITIVE DEIONIZATION SYSTEMS Ran Zhao Thesis committee Promotor Prof. dr. ir. A. van der Wal Professor of Electrochemical Water Treatment Wageningen University Co-promotors Prof.
Diffuse charge and Faradaic reactions in porous electrodes
 Diffuse charge and Faradaic reactions in porous electrodes The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher
Diffuse charge and Faradaic reactions in porous electrodes The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published Publisher
Bruno Bastos Sales, Joost Helsen and Arne Verliefde
 FEM modeling of capacitive deionization for complex streams Dennis Cardoen Bruno Bastos Sales, Joost Helsen and Arne Verliefde International Conference on Numerical and Mathematical ing of Flow and Transport
FEM modeling of capacitive deionization for complex streams Dennis Cardoen Bruno Bastos Sales, Joost Helsen and Arne Verliefde International Conference on Numerical and Mathematical ing of Flow and Transport
Water Desalination with Wires
 Water Desalination with Wires S. Porada, B. B. Sales, H. V. M. Hamelers, and P. M. Biesheuvel This is a "Pre-Print" accepted manuscript, which has been published in The Journal of Physical Chemistry Letters
Water Desalination with Wires S. Porada, B. B. Sales, H. V. M. Hamelers, and P. M. Biesheuvel This is a "Pre-Print" accepted manuscript, which has been published in The Journal of Physical Chemistry Letters
Simulation of Nanopores in Capacitive Energy Extraction based on Double Layer Expansion (CDLE)
 Simulation of Nanopores in Capacitive Energy Extraction based on Double Layer Expansion (CDLE) Emilio RuizReina 1, Félix Carrique 2, Ángel Delgado 3, María del Mar Fernández 3 1 Department of Applied Physics
Simulation of Nanopores in Capacitive Energy Extraction based on Double Layer Expansion (CDLE) Emilio RuizReina 1, Félix Carrique 2, Ángel Delgado 3, María del Mar Fernández 3 1 Department of Applied Physics
CHAPTER 4 CHEMICAL MODIFICATION OF ACTIVATED CARBON CLOTH FOR POTENTIAL USE AS ELECTRODES IN CAPACITIVE DEIONIZATION PROCESS
 CHAPTER 4 CHEMICAL MODIFICATION OF ACTIVATED CARBON CLOTH FOR POTENTIAL USE AS ELECTRODES IN CAPACITIVE DEIONIZATION PROCESS 4.1 INTRODUCTION Capacitive deionization (CDI) is one of the promising energy
CHAPTER 4 CHEMICAL MODIFICATION OF ACTIVATED CARBON CLOTH FOR POTENTIAL USE AS ELECTRODES IN CAPACITIVE DEIONIZATION PROCESS 4.1 INTRODUCTION Capacitive deionization (CDI) is one of the promising energy
Characterization of resistances of a capacitive deionization system
 Supporting Information for: Characterization of resistances of a capacitive deionization system Yatian Qu, 1,2 Theodore F. Baumann, 2 Juan G. Santiago, 1 * Michael Stadermann 2 * 1 Department of Mechanical
Supporting Information for: Characterization of resistances of a capacitive deionization system Yatian Qu, 1,2 Theodore F. Baumann, 2 Juan G. Santiago, 1 * Michael Stadermann 2 * 1 Department of Mechanical
Scaling Analysis of Energy Storage by Porous Electrodes
 Scaling Analysis of Energy Storage by Porous Electrodes Martin Z. Bazant May 14, 2012 1 Theoretical Capacity The maximum theoretical capacity occurs as E i 0, E p 0 E a 1, where E i, E p, and E a are the
Scaling Analysis of Energy Storage by Porous Electrodes Martin Z. Bazant May 14, 2012 1 Theoretical Capacity The maximum theoretical capacity occurs as E i 0, E p 0 E a 1, where E i, E p, and E a are the
ISSN Review. Capacitive Mixing for Harvesting the Free Energy of Solutions at Different Concentrations
 Entropy 2013, 15, 1388-1407; doi:10.3390/e15041388 OPEN ACCESS entropy ISSN 1099-4300 www.mdpi.com/journal/entropy Review Capacitive Mixing for Harvesting the Free Energy of Solutions at Different Concentrations
Entropy 2013, 15, 1388-1407; doi:10.3390/e15041388 OPEN ACCESS entropy ISSN 1099-4300 www.mdpi.com/journal/entropy Review Capacitive Mixing for Harvesting the Free Energy of Solutions at Different Concentrations
Tactics Box 23.1 Using Kirchhoff's Loop Law
 PH203 Chapter 23 solutions Tactics Box 231 Using Kirchhoff's Loop Law Description: Knight/Jones/Field Tactics Box 231 Using Kirchhoff s loop law is illustrated Learning Goal: To practice Tactics Box 231
PH203 Chapter 23 solutions Tactics Box 231 Using Kirchhoff's Loop Law Description: Knight/Jones/Field Tactics Box 231 Using Kirchhoff s loop law is illustrated Learning Goal: To practice Tactics Box 231
Optimization of MnO2 Electrodeposits using Graphenated Carbon Nanotube Electrodes for Supercapacitors
 Optimization of MnO2 Electrodeposits using Graphenated Carbon Nanotube Electrodes for Supercapacitors Waleed Nusrat, 100425398 PHY 3090U Material Science Thursday April 9 th 2015 Researchers optimize the
Optimization of MnO2 Electrodeposits using Graphenated Carbon Nanotube Electrodes for Supercapacitors Waleed Nusrat, 100425398 PHY 3090U Material Science Thursday April 9 th 2015 Researchers optimize the
CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials. Compiled by. Dr. A.O. Oladebeye
 CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials Compiled by Dr. A.O. Oladebeye Department of Chemistry University of Medical Sciences, Ondo, Nigeria Electrochemical Cell Electrochemical
CHM 213 (INORGANIC CHEMISTRY): Applications of Standard Reduction Potentials Compiled by Dr. A.O. Oladebeye Department of Chemistry University of Medical Sciences, Ondo, Nigeria Electrochemical Cell Electrochemical
Lecture 3 Charged interfaces
 Lecture 3 Charged interfaces rigin of Surface Charge Immersion of some materials in an electrolyte solution. Two mechanisms can operate. (1) Dissociation of surface sites. H H H H H M M M +H () Adsorption
Lecture 3 Charged interfaces rigin of Surface Charge Immersion of some materials in an electrolyte solution. Two mechanisms can operate. (1) Dissociation of surface sites. H H H H H M M M +H () Adsorption
(name) Electrochemical Energy Systems, Spring 2014, M. Z. Bazant. Final Exam
 10.626 Electrochemical Energy Systems, Spring 2014, M. Z. Bazant Final Exam Instructions. This is a three-hour closed book exam. You are allowed to have five doublesided pages of personal notes during
10.626 Electrochemical Energy Systems, Spring 2014, M. Z. Bazant Final Exam Instructions. This is a three-hour closed book exam. You are allowed to have five doublesided pages of personal notes during
Electrochemical Cell - Basics
 Electrochemical Cell - Basics The electrochemical cell e - (a) Load (b) Load e - M + M + Negative electrode Positive electrode Negative electrode Positive electrode Cathode Anode Anode Cathode Anode Anode
Electrochemical Cell - Basics The electrochemical cell e - (a) Load (b) Load e - M + M + Negative electrode Positive electrode Negative electrode Positive electrode Cathode Anode Anode Cathode Anode Anode
NANOCOMPOSITE CATION EXCHANGE MEMBRANE WITH FOULING RESISTANCE AND ENHANCED SALINITY GRADIENT POWER GENERATION FOR REVERSE ELECTRODIALYSIS
 NANOCOMPOSITE CATION EXCHANGE MEMBRANE WITH FOULING RESISTANCE AND ENHANCED SALINITY GRADIENT POWER GENERATION FOR REVERSE ELECTRODIALYSIS X I N T O N G, B O P E N G Z H A N G, A N D Y O N G S H E N G
NANOCOMPOSITE CATION EXCHANGE MEMBRANE WITH FOULING RESISTANCE AND ENHANCED SALINITY GRADIENT POWER GENERATION FOR REVERSE ELECTRODIALYSIS X I N T O N G, B O P E N G Z H A N G, A N D Y O N G S H E N G
Charging and Transport Dynamics of a Flow-
 Charging and Transport Dynamics of a Flow- Through Electrode Capacitive Deionization System Supporting information Yatian Qu, a,b Patrick G. Campbell, b Ali Hemmatifar, a Jennifer M. Knipe, b Colin K.
Charging and Transport Dynamics of a Flow- Through Electrode Capacitive Deionization System Supporting information Yatian Qu, a,b Patrick G. Campbell, b Ali Hemmatifar, a Jennifer M. Knipe, b Colin K.
The Basic Capacitor. Water Tower / Capacitor Analogy. "Partnering With Our Clients for Combined Success"
 CAPACITOR BASICS I How s Work The Basic A capacitor is an electrical device which serves to store up electrical energy for release at a predetermined time. In its most basic form, it is comprised of three
CAPACITOR BASICS I How s Work The Basic A capacitor is an electrical device which serves to store up electrical energy for release at a predetermined time. In its most basic form, it is comprised of three
ENERGY AND TIME CONSTANTS IN RC CIRCUITS By: Iwana Loveu Student No Lab Section: 0003 Date: February 8, 2004
 ENERGY AND TIME CONSTANTS IN RC CIRCUITS By: Iwana Loveu Student No. 416 614 5543 Lab Section: 0003 Date: February 8, 2004 Abstract: Two charged conductors consisting of equal and opposite charges forms
ENERGY AND TIME CONSTANTS IN RC CIRCUITS By: Iwana Loveu Student No. 416 614 5543 Lab Section: 0003 Date: February 8, 2004 Abstract: Two charged conductors consisting of equal and opposite charges forms
V. Electrostatics. MIT Student
 V. Electrostatics Lecture 26: Compact Part of the Double Layer MIT Student 1 Double-layer Capacitance 1.1 Stern Layer As was discussed in the previous lecture, the Gouy-Chapman model predicts unphysically
V. Electrostatics Lecture 26: Compact Part of the Double Layer MIT Student 1 Double-layer Capacitance 1.1 Stern Layer As was discussed in the previous lecture, the Gouy-Chapman model predicts unphysically
Electrophoretic Deposition. - process in which particles, suspended in a liquid medium, migrate in an electric field and deposit on an electrode
 Electrophoretic Deposition - process in which particles, suspended in a liquid medium, migrate in an electric field and deposit on an electrode no redox differs from electrolytic in several ways deposit
Electrophoretic Deposition - process in which particles, suspended in a liquid medium, migrate in an electric field and deposit on an electrode no redox differs from electrolytic in several ways deposit
and constant current operations in capacitive deionization
 Energy consumption analysis of constant voltage and constant current operations in capacitive deionization Supporting information Yatian Qu, a,b Patrick G. Campbell, b Lei Gu, c Jennifer M. Knipe, b Ella
Energy consumption analysis of constant voltage and constant current operations in capacitive deionization Supporting information Yatian Qu, a,b Patrick G. Campbell, b Lei Gu, c Jennifer M. Knipe, b Ella
Development of Bifunctional Electrodes for Closed-loop Fuel Cell Applications. Pfaffenwaldring 6, Stuttgart, Germany
 Development of Bifunctional Electrodes for Closed-loop Fuel Cell Applications S. Altmann a,b, T. Kaz b, K. A. Friedrich a,b a Institute of Thermodynamics and Thermal Engineering, University Stuttgart,
Development of Bifunctional Electrodes for Closed-loop Fuel Cell Applications S. Altmann a,b, T. Kaz b, K. A. Friedrich a,b a Institute of Thermodynamics and Thermal Engineering, University Stuttgart,
Chemistry: The Central Science. Chapter 20: Electrochemistry
 Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Chemistry: The Central Science Chapter 20: Electrochemistry Redox reaction power batteries Electrochemistry is the study of the relationships between electricity and chemical reactions o It includes the
Colloid Chemistry. La chimica moderna e la sua comunicazione Silvia Gross.
 Colloid Chemistry La chimica moderna e la sua comunicazione Silvia Gross Istituto Dipartimento di Scienze di e Scienze Tecnologie Chimiche Molecolari ISTM-CNR, Università Università degli Studi degli Studi
Colloid Chemistry La chimica moderna e la sua comunicazione Silvia Gross Istituto Dipartimento di Scienze di e Scienze Tecnologie Chimiche Molecolari ISTM-CNR, Università Università degli Studi degli Studi
Chapter 2: Capacitor And Dielectrics
 hapter 2: apacitor And Dielectrics In this chapter, we are going to discuss the different ways that a capacitor could be arranged in a circuit and how its capacitance could be increased. Overview apacitor
hapter 2: apacitor And Dielectrics In this chapter, we are going to discuss the different ways that a capacitor could be arranged in a circuit and how its capacitance could be increased. Overview apacitor
1990. Temperature dependence of soft-doped / hard-doped PZT material properties under large signal excitation and impact on the design choice
 1990. Temperature dependence of soft-doped / hard-doped PZT material properties under large signal excitation and impact on the design choice Charles Mangeot Noliac A/S, Kvistgaard, Denmark E-mail: cm@noliac.com
1990. Temperature dependence of soft-doped / hard-doped PZT material properties under large signal excitation and impact on the design choice Charles Mangeot Noliac A/S, Kvistgaard, Denmark E-mail: cm@noliac.com
Fouling of reverse osmosis membranes using electrical impedance spectroscopy: Measurements and simulations
 Desalination 236 (2009) 187 193 Fouling of reverse osmosis membranes using electrical impedance spectroscopy: Measurements and simulations J.M. Kavanagh*, S. Hussain, T.C. Chilcott, H.G.L. Coster School
Desalination 236 (2009) 187 193 Fouling of reverse osmosis membranes using electrical impedance spectroscopy: Measurements and simulations J.M. Kavanagh*, S. Hussain, T.C. Chilcott, H.G.L. Coster School
I. Conductors and Insulators :
 Chapter 6 : Conductors - Insulators - Capacitors We have, till now, studied the electric charges and the interactions between them but not evoked how the electricity can be transfered? which meterials
Chapter 6 : Conductors - Insulators - Capacitors We have, till now, studied the electric charges and the interactions between them but not evoked how the electricity can be transfered? which meterials
Physics (
 Question 2.12: A charge of 8 mc is located at the origin. Calculate the work done in taking a small charge of 2 10 9 C from a point P (0, 0, 3 cm) to a point Q (0, 4 cm, 0), via a point R (0, 6 cm, 9 cm).
Question 2.12: A charge of 8 mc is located at the origin. Calculate the work done in taking a small charge of 2 10 9 C from a point P (0, 0, 3 cm) to a point Q (0, 4 cm, 0), via a point R (0, 6 cm, 9 cm).
ECE2262 Electric Circuits. Chapter 6: Capacitance and Inductance
 ECE2262 Electric Circuits Chapter 6: Capacitance and Inductance Capacitors Inductors Capacitor and Inductor Combinations 1 CAPACITANCE AND INDUCTANCE Introduces two passive, energy storing devices: Capacitors
ECE2262 Electric Circuits Chapter 6: Capacitance and Inductance Capacitors Inductors Capacitor and Inductor Combinations 1 CAPACITANCE AND INDUCTANCE Introduces two passive, energy storing devices: Capacitors
The next two questions pertain to the situation described below. Consider a parallel plate capacitor with separation d:
 PHYS 102 Exams Exam 2 PRINT (A) The next two questions pertain to the situation described below. Consider a parallel plate capacitor with separation d: It is connected to a battery with constant emf V.
PHYS 102 Exams Exam 2 PRINT (A) The next two questions pertain to the situation described below. Consider a parallel plate capacitor with separation d: It is connected to a battery with constant emf V.
IV. Transport Phenomena. Lecture 23: Ion Concentration Polarization
 IV. Transport Phenomena Lecture 23: Ion Concentration Polarization MIT Student (and MZB) Ion concentration polarization in electrolytes refers to the additional voltage drop (or internal resistance ) across
IV. Transport Phenomena Lecture 23: Ion Concentration Polarization MIT Student (and MZB) Ion concentration polarization in electrolytes refers to the additional voltage drop (or internal resistance ) across
Prof. Mario L. Ferrari
 Sustainable Energy Mod.1: Fuel Cells & Distributed Generation Systems Dr. Ing. Mario L. Ferrari Thermochemical Power Group (TPG) - DiMSET University of Genoa, Italy Lesson II Lesson II: fuel cells (electrochemistry)
Sustainable Energy Mod.1: Fuel Cells & Distributed Generation Systems Dr. Ing. Mario L. Ferrari Thermochemical Power Group (TPG) - DiMSET University of Genoa, Italy Lesson II Lesson II: fuel cells (electrochemistry)
ECE2262 Electric Circuits. Chapter 6: Capacitance and Inductance
 ECE2262 Electric Circuits Chapter 6: Capacitance and Inductance Capacitors Inductors Capacitor and Inductor Combinations Op-Amp Integrator and Op-Amp Differentiator 1 CAPACITANCE AND INDUCTANCE Introduces
ECE2262 Electric Circuits Chapter 6: Capacitance and Inductance Capacitors Inductors Capacitor and Inductor Combinations Op-Amp Integrator and Op-Amp Differentiator 1 CAPACITANCE AND INDUCTANCE Introduces
KOH ACTIVATED CARBONS FOR SUPERCAPACITORS
 KOH ACTIVATED CARBONS FOR SUPERCAPACITORS Elzbieta Frackowiak 1, Grzegorz Lota 1, Krzysztof Kierzek 2, Grazyna Gryglewicz 2, Jacek Machnikowski 2 1 Poznan University of Technology, Piotrowo 3, 6-965 Poznan,
KOH ACTIVATED CARBONS FOR SUPERCAPACITORS Elzbieta Frackowiak 1, Grzegorz Lota 1, Krzysztof Kierzek 2, Grazyna Gryglewicz 2, Jacek Machnikowski 2 1 Poznan University of Technology, Piotrowo 3, 6-965 Poznan,
Designing Information Devices and Systems I Discussion 8B
 EECS 16A Spring 2018 Designing Information Devices and Systems I Discussion 8B 1. Bio-Molecule Detector We ve already seen how to build a bio-molecule detector where bio-molecules change the resistance
EECS 16A Spring 2018 Designing Information Devices and Systems I Discussion 8B 1. Bio-Molecule Detector We ve already seen how to build a bio-molecule detector where bio-molecules change the resistance
Modeling of Liquid Water Distribution at Cathode Gas Flow Channels in Proton Exchange Membrane Fuel Cell - PEMFC
 Modeling of Liquid Water Distribution at Cathode Gas Flow Channels in Proton Exchange Membrane Fuel Cell - PEMFC Sandro Skoda 1*, Eric Robalinho 2, André L. R. Paulino 1, Edgar F. Cunha 1, Marcelo Linardi
Modeling of Liquid Water Distribution at Cathode Gas Flow Channels in Proton Exchange Membrane Fuel Cell - PEMFC Sandro Skoda 1*, Eric Robalinho 2, André L. R. Paulino 1, Edgar F. Cunha 1, Marcelo Linardi
7/06 Electric Fields and Energy
 Part ASome standard electric field and potential configurations About this lab: Electric fields are created by electric charges and exert force on charges. Electric potential gives an alternative description.
Part ASome standard electric field and potential configurations About this lab: Electric fields are created by electric charges and exert force on charges. Electric potential gives an alternative description.
(3.5.1) V E x, E, (3.5.2)
 Lecture 3.5 Capacitors Today we shall continue our discussion of electrostatics and, in particular, the concept of electrostatic potential energy and electric potential. The main example which we have
Lecture 3.5 Capacitors Today we shall continue our discussion of electrostatics and, in particular, the concept of electrostatic potential energy and electric potential. The main example which we have
SECTION 4: ULTRACAPACITORS. ESE 471 Energy Storage Systems
 SECTION 4: ULTRACAPACITORS ESE 471 Energy Storage Systems 2 Introduction Ultracapacitors 3 Capacitors are electrical energy storage devices Energy is stored in an electric field Advantages of capacitors
SECTION 4: ULTRACAPACITORS ESE 471 Energy Storage Systems 2 Introduction Ultracapacitors 3 Capacitors are electrical energy storage devices Energy is stored in an electric field Advantages of capacitors
Physics Investigation 10 Teacher Manual
 Physics Investigation 10 Teacher Manual Observation When a light bulb is connected to a number of charged capacitors, it lights up for different periods of time. Problem What does the rate of discharging
Physics Investigation 10 Teacher Manual Observation When a light bulb is connected to a number of charged capacitors, it lights up for different periods of time. Problem What does the rate of discharging
ECHE Questions Centrifugal Pumps
 ECHE 460 20 Questions Centrifugal Pumps 1. Sketch a typical pump curve including units, and then explain how this curve would change if two identical pumps are operating in series and in parallel. 2. Sketch
ECHE 460 20 Questions Centrifugal Pumps 1. Sketch a typical pump curve including units, and then explain how this curve would change if two identical pumps are operating in series and in parallel. 2. Sketch
Activity. Modeling the Fuel Cell Reaction. Overview. Advance Preparation. Background Information
 4 Activity 1-2 class sessions Modeling the uel Cell Reaction 2011 Regents of the University of California Overview n order to understand the chemistry of fuel cells, students are introduced to oxidation-reduction
4 Activity 1-2 class sessions Modeling the uel Cell Reaction 2011 Regents of the University of California Overview n order to understand the chemistry of fuel cells, students are introduced to oxidation-reduction
ELECTROCHEMICAL COMPRESSION OF PRODUCT HYDROGEN FROM PEM ELECTROLYZER STACK
 ELECTROCHEMICAL COMPRESSION OF PRODUCT HYDROGEN FROM PEM ELECTROLYZER STACK N.V. Dale 1,*, C. Y. Biaku 1, M. D. Mann 1, H. Salehfar 2, A. J. Peters 2 Abstract The low volumetric energy density of hydrogen
ELECTROCHEMICAL COMPRESSION OF PRODUCT HYDROGEN FROM PEM ELECTROLYZER STACK N.V. Dale 1,*, C. Y. Biaku 1, M. D. Mann 1, H. Salehfar 2, A. J. Peters 2 Abstract The low volumetric energy density of hydrogen
Performance Investigation on Electrochemical Compressor with Ammonia
 Purdue University Purdue e-pubs International Compressor Engineering Conference School of Mechanical Engineering 2016 Performance Investigation on Electrochemical Compressor with Ammonia Ye Tao University
Purdue University Purdue e-pubs International Compressor Engineering Conference School of Mechanical Engineering 2016 Performance Investigation on Electrochemical Compressor with Ammonia Ye Tao University
Capacitor Construction
 Capacitor Construction Topics covered in this presentation: Capacitor Construction 1 of 13 Introduction to Capacitors A capacitor is a device that is able to store charge and acts like a temporary, rechargeable
Capacitor Construction Topics covered in this presentation: Capacitor Construction 1 of 13 Introduction to Capacitors A capacitor is a device that is able to store charge and acts like a temporary, rechargeable
Understanding EEStor Parameters
 Understanding EEStor Parameters This primer is intended to help readers understand the technical data published by EEStor. There are three key parameters and two formulas that are important to the understanding
Understanding EEStor Parameters This primer is intended to help readers understand the technical data published by EEStor. There are three key parameters and two formulas that are important to the understanding
Electrochimica Acta 54 (2009) Contents lists available at ScienceDirect. Electrochimica Acta
 Electrochimica Acta 54 (2009 4857 4871 Contents lists available at ScienceDirect Electrochimica Acta j o u r n a l h o m e p a g e : www.elsevier.com/locate/electacta Imposed currents in galvanic cells
Electrochimica Acta 54 (2009 4857 4871 Contents lists available at ScienceDirect Electrochimica Acta j o u r n a l h o m e p a g e : www.elsevier.com/locate/electacta Imposed currents in galvanic cells
Electro - Principles I
 Electro - Principles I Capacitance The Capacitor What is it? Page 8-1 The capacitor is a device consisting essentially of two conducting surfaces separated by an insulating material. + Schematic Symbol
Electro - Principles I Capacitance The Capacitor What is it? Page 8-1 The capacitor is a device consisting essentially of two conducting surfaces separated by an insulating material. + Schematic Symbol
Capacitance. Chapter 21 Chapter 25. K = C / C o V = V o / K. 1 / Ceq = 1 / C / C 2. Ceq = C 1 + C 2
 = Chapter 21 Chapter 25 Capacitance K = C / C o V = V o / K 1 / Ceq = 1 / C 1 + 1 / C 2 Ceq = C 1 + C 2 Copyright 25-2 Capacitance 25.01 Sketch a schematic diagram of a circuit with a parallel-plate capacitor,
= Chapter 21 Chapter 25 Capacitance K = C / C o V = V o / K 1 / Ceq = 1 / C 1 + 1 / C 2 Ceq = C 1 + C 2 Copyright 25-2 Capacitance 25.01 Sketch a schematic diagram of a circuit with a parallel-plate capacitor,
Supplementary Figure 1.
 Supplementary Figure 1. The charging processs of (a) a capacitor and (b) a fabricated lithium-ion battery. The switch exists for the designed charging cycle only. Supplementary Figure 2. (a) The measurement
Supplementary Figure 1. The charging processs of (a) a capacitor and (b) a fabricated lithium-ion battery. The switch exists for the designed charging cycle only. Supplementary Figure 2. (a) The measurement
Electric Potential Energy Conservative Force
 Electric Potential Energy Conservative Force Conservative force or field is a force field in which the total mechanical energy of an isolated system is conserved. Examples, Gravitation, Electrostatic,
Electric Potential Energy Conservative Force Conservative force or field is a force field in which the total mechanical energy of an isolated system is conserved. Examples, Gravitation, Electrostatic,
Testing Electrochemical Capacitors Part 1 Cyclic Voltammetry and Leakage Current
 Testing Electrochemical Capacitors Part 1 Cyclic Voltammetry and Leakage Current Purpose of This Note This application note is the first part of an overview of electrochemical techniques used to test electrochemical
Testing Electrochemical Capacitors Part 1 Cyclic Voltammetry and Leakage Current Purpose of This Note This application note is the first part of an overview of electrochemical techniques used to test electrochemical
University of Maryland Department of Physics. Spring 2009 Final Exam 20. May (175 points) Post grades on web? (Initial, please) Yes No
 University of Maryland Department of Physics Physics 122 20. May 2009 (175 points) Post grades on web? (Initial, please) Yes No (If you agree, I will post your grades and your detailed scores for each
University of Maryland Department of Physics Physics 122 20. May 2009 (175 points) Post grades on web? (Initial, please) Yes No (If you agree, I will post your grades and your detailed scores for each
iclicker A metal ball of radius R has a charge q. Charge is changed q -> - 2q. How does it s capacitance changed?
 1 iclicker A metal ball of radius R has a charge q. Charge is changed q -> - 2q. How does it s capacitance changed? q A: C->2 C0 B: C-> C0 C: C-> C0/2 D: C->- C0 E: C->-2 C0 2 iclicker A metal ball of
1 iclicker A metal ball of radius R has a charge q. Charge is changed q -> - 2q. How does it s capacitance changed? q A: C->2 C0 B: C-> C0 C: C-> C0/2 D: C->- C0 E: C->-2 C0 2 iclicker A metal ball of
Chapter 8. Capacitors. Charging a capacitor
 Chapter 8 Capacitors You can store energy as potential energy by pulling a bowstring, stretching a spring, compressing a gas, or lifting a book. You can also store energy as potential energy in an electric
Chapter 8 Capacitors You can store energy as potential energy by pulling a bowstring, stretching a spring, compressing a gas, or lifting a book. You can also store energy as potential energy in an electric
Q1. Three point charges are arranged as shown in FIGURE 1. Find the magnitude of the net electrostatic force on the point charge at the origin.
 Coordinator: Saleem Rao Monday, May 01, 2017 Page: 1 Q1. Three point charges are arranged as shown in FIGURE 1. Find the magnitude of the net electrostatic force on the point charge at the origin. A) 1.38
Coordinator: Saleem Rao Monday, May 01, 2017 Page: 1 Q1. Three point charges are arranged as shown in FIGURE 1. Find the magnitude of the net electrostatic force on the point charge at the origin. A) 1.38
Capacitors. Chapter How capacitors work Inside a capacitor
 Chapter 6 Capacitors In every device we have studied so far sources, resistors, diodes and transistors the relationship between voltage and current depends only on the present, independent of the past.
Chapter 6 Capacitors In every device we have studied so far sources, resistors, diodes and transistors the relationship between voltage and current depends only on the present, independent of the past.
Consider a point P on the line joining the two charges, as shown in the given figure.
 Question 2.1: Two charges 5 10 8 C and 3 10 8 C are located 16 cm apart. At what point(s) on the line joining the two charges is the electric potential zero? Take the potential at infinity to be zero.
Question 2.1: Two charges 5 10 8 C and 3 10 8 C are located 16 cm apart. At what point(s) on the line joining the two charges is the electric potential zero? Take the potential at infinity to be zero.
The Basic Capacitor. Dielectric. Conductors
 Chapter 9 The Basic Capacitor Capacitors are one of the fundamental passive components. In its most basic form, it is composed of two conductive plates separated by an insulating dielectric. The ability
Chapter 9 The Basic Capacitor Capacitors are one of the fundamental passive components. In its most basic form, it is composed of two conductive plates separated by an insulating dielectric. The ability
Switch + R. ower upply. Voltmete. Capacitor. Goals. Introduction
 Lab 6. Switch RC Circuits ower upply Goals To appreciate the capacitor as a charge storage device. To measure the voltage across a capacitor as it discharges through a resistor, and to compare + the result
Lab 6. Switch RC Circuits ower upply Goals To appreciate the capacitor as a charge storage device. To measure the voltage across a capacitor as it discharges through a resistor, and to compare + the result
4) A 3.0 pf capacitor consists of two parallel plates that have surface charge densities of 1.0
 Quantitative 3) Two parallel plates are separated by 1.0 mm. If the potential difference between them is 2.0 V, what is the magnitude of their surface charge densities? A) 18 nc/m2 4) A 3.0 pf capacitor
Quantitative 3) Two parallel plates are separated by 1.0 mm. If the potential difference between them is 2.0 V, what is the magnitude of their surface charge densities? A) 18 nc/m2 4) A 3.0 pf capacitor
EXPERIMENT 5A RC Circuits
 EXPERIMENT 5A Circuits Objectives 1) Observe and qualitatively describe the charging and discharging (decay) of the voltage on a capacitor. 2) Graphically determine the time constant for the decay, τ =.
EXPERIMENT 5A Circuits Objectives 1) Observe and qualitatively describe the charging and discharging (decay) of the voltage on a capacitor. 2) Graphically determine the time constant for the decay, τ =.
Review. Spring Semester /21/14. Physics for Scientists & Engineers 2 1
 Review Spring Semester 2014 Physics for Scientists & Engineers 2 1 Notes! Homework set 13 extended to Tuesday, 4/22! Remember to fill out SIRS form: https://sirsonline.msu.edu Physics for Scientists &
Review Spring Semester 2014 Physics for Scientists & Engineers 2 1 Notes! Homework set 13 extended to Tuesday, 4/22! Remember to fill out SIRS form: https://sirsonline.msu.edu Physics for Scientists &
DETERMINING THE OPERATING CONDITIONS OF ALL-VANADIUM REDOX FLOW BATTERY
 Proceedings of the Asian Conference on Thermal Sciences 2017, 1st ACTS March 26-30, 2017, Jeju Island, Korea ACTS-P00650 DETERMINING THE OPERATING CONDITIONS OF ALL-VANADIUM REDOX FLOW BATTERY Jungmyoung
Proceedings of the Asian Conference on Thermal Sciences 2017, 1st ACTS March 26-30, 2017, Jeju Island, Korea ACTS-P00650 DETERMINING THE OPERATING CONDITIONS OF ALL-VANADIUM REDOX FLOW BATTERY Jungmyoung
Electric charge is conserved the arithmetic sum of the total charge cannot change in any interaction.
 Electrostatics Electric charge is conserved the arithmetic sum of the total charge cannot change in any interaction. Electric Charge in the Atom Atom: Nucleus (small, massive, positive charge) Electron
Electrostatics Electric charge is conserved the arithmetic sum of the total charge cannot change in any interaction. Electric Charge in the Atom Atom: Nucleus (small, massive, positive charge) Electron
SEPARATION BY BARRIER
 SEPARATION BY BARRIER SEPARATION BY BARRIER Phase 1 Feed Barrier Phase 2 Separation by barrier uses a barrier which restricts and/or enhances the movement of certain chemical species with respect to other
SEPARATION BY BARRIER SEPARATION BY BARRIER Phase 1 Feed Barrier Phase 2 Separation by barrier uses a barrier which restricts and/or enhances the movement of certain chemical species with respect to other
B THE CAPACITOR. Theory
 8. THE CAPACITOR You will study several aspects of a capacitor, how the voltage across it changes with time as it is being charged and discharged and how it stores energy. The most well known device for
8. THE CAPACITOR You will study several aspects of a capacitor, how the voltage across it changes with time as it is being charged and discharged and how it stores energy. The most well known device for
Chapter 28 Solutions
 Chapter 8 Solutions 8.1 (a) P ( V) R becomes 0.0 W (11.6 V) R so R 6.73 Ω (b) V IR so 11.6 V I (6.73 Ω) and I 1.7 A ε IR + Ir so 15.0 V 11.6 V + (1.7 A)r r 1.97 Ω Figure for Goal Solution Goal Solution
Chapter 8 Solutions 8.1 (a) P ( V) R becomes 0.0 W (11.6 V) R so R 6.73 Ω (b) V IR so 11.6 V I (6.73 Ω) and I 1.7 A ε IR + Ir so 15.0 V 11.6 V + (1.7 A)r r 1.97 Ω Figure for Goal Solution Goal Solution
Physics 2220 Fall 2010 George Williams SECOND MIDTERM - REVIEW PROBLEMS
 Physics 0 Fall 010 George Williams SECOND MIDTERM - REVIEW PROBLEMS The last four problems are from last years second midterm. Solutions are available on the class web site.. There are no solutions for,
Physics 0 Fall 010 George Williams SECOND MIDTERM - REVIEW PROBLEMS The last four problems are from last years second midterm. Solutions are available on the class web site.. There are no solutions for,
Physics 24 Exam 2 March 18, 2014
 Exam Total / 200 Physics 24 Exam 2 March 18, 2014 Printed Name: Rec. Sec. Letter: Five multiple choice questions, 8 points each. Choose the best or most nearly correct answer. 1. You need to store electrical
Exam Total / 200 Physics 24 Exam 2 March 18, 2014 Printed Name: Rec. Sec. Letter: Five multiple choice questions, 8 points each. Choose the best or most nearly correct answer. 1. You need to store electrical
What is the importance of redox reactions? Their importance lies in the fact that we can use the transfer of electrons between species to do useful
 What is the importance of redox reactions? Their importance lies in the fact that we can use the transfer of electrons between species to do useful work. This is accomplished by constructing a voltaic
What is the importance of redox reactions? Their importance lies in the fact that we can use the transfer of electrons between species to do useful work. This is accomplished by constructing a voltaic
Electrochemical Properties of Materials for Electrical Energy Storage Applications
 Electrochemical Properties of Materials for Electrical Energy Storage Applications Lecture Note 3 October 11, 2013 Kwang Kim Yonsei Univ., KOREA kbkim@yonsei.ac.kr 39 Y 88.91 8 O 16.00 7 N 14.01 34 Se
Electrochemical Properties of Materials for Electrical Energy Storage Applications Lecture Note 3 October 11, 2013 Kwang Kim Yonsei Univ., KOREA kbkim@yonsei.ac.kr 39 Y 88.91 8 O 16.00 7 N 14.01 34 Se
Section 16.1 Potential Difference and Electric Potential
 PROBLEMS 1, 2, 3 = straightforward, intermediate, challenging = full solution available in Student Solutions Manual/Study Guide = biomedical application Section 16.1 Potential Difference and Electric Potential
PROBLEMS 1, 2, 3 = straightforward, intermediate, challenging = full solution available in Student Solutions Manual/Study Guide = biomedical application Section 16.1 Potential Difference and Electric Potential
A ph-gradient Flow Cell for Converting Waste CO 2 into Electricity
 Supporting information for A ph-gradient Flow Cell for Converting Waste CO 2 into Electricity Taeyoung Kim, Bruce E. Logan, and Christopher A. Gorski* Department of Civil and Environmental Engineering,
Supporting information for A ph-gradient Flow Cell for Converting Waste CO 2 into Electricity Taeyoung Kim, Bruce E. Logan, and Christopher A. Gorski* Department of Civil and Environmental Engineering,
Contents. 2. Fluids. 1. Introduction
 Contents 1. Introduction 2. Fluids 3. Physics of Microfluidic Systems 4. Microfabrication Technologies 5. Flow Control 6. Micropumps 7. Sensors 8. Ink-Jet Technology 9. Liquid Handling 10.Microarrays 11.Microreactors
Contents 1. Introduction 2. Fluids 3. Physics of Microfluidic Systems 4. Microfabrication Technologies 5. Flow Control 6. Micropumps 7. Sensors 8. Ink-Jet Technology 9. Liquid Handling 10.Microarrays 11.Microreactors
General Physics II Lab EM2 Capacitance and Electrostatic Energy
 Purpose General Physics II Lab General Physics II Lab EM2 Capacitance and Electrostatic Energy In this experiment, you will examine the relationship between charge, voltage and capacitance of a parallel
Purpose General Physics II Lab General Physics II Lab EM2 Capacitance and Electrostatic Energy In this experiment, you will examine the relationship between charge, voltage and capacitance of a parallel
Testing Electrochemical Capacitors Part 2: Cyclic Charge-Discharge and Stacks
 Testing Electrochemical Capacitors Part 2: Cyclic Charge-Discharge and Stacks Introduction This application note is Part of 2 describing electrochemical techniques for energy-storage devices. It explains
Testing Electrochemical Capacitors Part 2: Cyclic Charge-Discharge and Stacks Introduction This application note is Part of 2 describing electrochemical techniques for energy-storage devices. It explains
Chapter 18 Solutions Set Up: (a) The proton has charge and mass Let point a be at the negative plate and
 Chapter 18 Solutions *18.1. Set Up: Since the charge is positive the force on it is in the same direction as the electric field. Since the field is uniform the force is constant is upward is to the right,
Chapter 18 Solutions *18.1. Set Up: Since the charge is positive the force on it is in the same direction as the electric field. Since the field is uniform the force is constant is upward is to the right,
POWER FACTOR IN THE DIGITAL AGE A N E N V I R O N M E N T A L P O T E N T I A L S W H I T E P A P E R. Power Quality For The Digital Age
 Power Quality For The Digital Age POWER FACTOR IN THE DIGITAL AGE A N E N V I R O N M E N T A L P O T E N T I A L S W H I T E P A P E R Introduction One method to measure the efficiency of the electrical
Power Quality For The Digital Age POWER FACTOR IN THE DIGITAL AGE A N E N V I R O N M E N T A L P O T E N T I A L S W H I T E P A P E R Introduction One method to measure the efficiency of the electrical
Supplementary Figure 1 XPS, Raman and TGA characterizations on GO and freeze-dried HGF and GF. (a) XPS survey spectra and (b) C1s spectra.
 Supplementary Figure 1 XPS, Raman and TGA characterizations on GO and freeze-dried HGF and GF. (a) XPS survey spectra and (b) C1s spectra. (c) Raman spectra. (d) TGA curves. All results confirm efficient
Supplementary Figure 1 XPS, Raman and TGA characterizations on GO and freeze-dried HGF and GF. (a) XPS survey spectra and (b) C1s spectra. (c) Raman spectra. (d) TGA curves. All results confirm efficient
AP Physics Study Guide Chapter 17 Electric Potential and Energy Name. Circle the vector quantities below and underline the scalar quantities below
 AP Physics Study Guide Chapter 17 Electric Potential and Energy Name Circle the vector quantities below and underline the scalar quantities below electric potential electric field electric potential energy
AP Physics Study Guide Chapter 17 Electric Potential and Energy Name Circle the vector quantities below and underline the scalar quantities below electric potential electric field electric potential energy
PhysicsAndMathsTutor.com
 Electricity May 02 1. The graphs show the variation with potential difference V of the current I for three circuit elements. PhysicsAndMathsTutor.com When the four lamps are connected as shown in diagram
Electricity May 02 1. The graphs show the variation with potential difference V of the current I for three circuit elements. PhysicsAndMathsTutor.com When the four lamps are connected as shown in diagram
Electrochemistry. Dr. A. R. Ramesh Assistant Professor of Chemistry Govt. Engineering College, Kozhikode
 Electrochemistry Dr. A. R. Ramesh Assistant Professor of Chemistry Govt. Engineering College, Kozhikode 1 Electro Chemistry : Chemistry of flow of electrons Redox Reaction The electrons flow through the
Electrochemistry Dr. A. R. Ramesh Assistant Professor of Chemistry Govt. Engineering College, Kozhikode 1 Electro Chemistry : Chemistry of flow of electrons Redox Reaction The electrons flow through the
Physics 202 Final (Monday, December 12) Fall 2016 (Saslow) White Version
 Physics 202 Final (Monday, December 12) Fall 2016 (Saslow) White Version Name (printed) Lab Section(+2 pts) Name (signed as on ID) Show all work. Partial credit may be given. Answers should include the
Physics 202 Final (Monday, December 12) Fall 2016 (Saslow) White Version Name (printed) Lab Section(+2 pts) Name (signed as on ID) Show all work. Partial credit may be given. Answers should include the
Electricity
 Electricity Electric Charge There are two fundamental charges in the universe. Positive (proton) has a charge of +1.60 x 10-19 C Negative (electron) has a charge of 1.60 x 10-19 C There is one general
Electricity Electric Charge There are two fundamental charges in the universe. Positive (proton) has a charge of +1.60 x 10-19 C Negative (electron) has a charge of 1.60 x 10-19 C There is one general
Laboratory 7: Charging and Discharging a Capacitor Prelab
 Phys 132L Fall 2018 Laboratory 7: Charging and Discharging a Capacitor Prelab Consider a capacitor with capacitance C connected in series to a resistor with resistance R as shown in Fig. 1. Theory predicts
Phys 132L Fall 2018 Laboratory 7: Charging and Discharging a Capacitor Prelab Consider a capacitor with capacitance C connected in series to a resistor with resistance R as shown in Fig. 1. Theory predicts
Basic overall reaction for hydrogen powering
 Fuel Cell Basics Basic overall reaction for hydrogen powering 2H 2 + O 2 2H 2 O Hydrogen produces electrons, protons, heat and water PEMFC Anode reaction: H 2 2H + + 2e Cathode reaction: (½)O 2 + 2H +
Fuel Cell Basics Basic overall reaction for hydrogen powering 2H 2 + O 2 2H 2 O Hydrogen produces electrons, protons, heat and water PEMFC Anode reaction: H 2 2H + + 2e Cathode reaction: (½)O 2 + 2H +
Reversibility, Irreversibility and Carnot cycle. Irreversible Processes. Reversible Processes. Carnot Cycle
 Reversibility, Irreversibility and Carnot cycle The second law of thermodynamics distinguishes between reversible and irreversible processes. If a process can proceed in either direction without violating
Reversibility, Irreversibility and Carnot cycle The second law of thermodynamics distinguishes between reversible and irreversible processes. If a process can proceed in either direction without violating
Practical 1 RC Circuits
 Objectives Practical 1 Circuits 1) Observe and qualitatively describe the charging and discharging (decay) of the voltage on a capacitor. 2) Graphically determine the time constant for the decay, τ =.
Objectives Practical 1 Circuits 1) Observe and qualitatively describe the charging and discharging (decay) of the voltage on a capacitor. 2) Graphically determine the time constant for the decay, τ =.
FIRST TERM EXAMINATION (07 SEPT 2015) Paper - PHYSICS Class XII (SET B) Time: 3hrs. MM: 70
 FIRST TERM EXAMINATION (07 SEPT 205) Paper - PHYSICS Class XII (SET B) Time: 3hrs. MM: 70 Instructions:. All questions are compulsory. 2. Q.no. to 5 carry mark each. 3. Q.no. 6 to 0 carry 2 marks each.
FIRST TERM EXAMINATION (07 SEPT 205) Paper - PHYSICS Class XII (SET B) Time: 3hrs. MM: 70 Instructions:. All questions are compulsory. 2. Q.no. to 5 carry mark each. 3. Q.no. 6 to 0 carry 2 marks each.
[1] (b) Fig. 1.1 shows a circuit consisting of a resistor and a capacitor of capacitance 4.5 μf. Fig. 1.1
![[1] (b) Fig. 1.1 shows a circuit consisting of a resistor and a capacitor of capacitance 4.5 μf. Fig. 1.1 [1] (b) Fig. 1.1 shows a circuit consisting of a resistor and a capacitor of capacitance 4.5 μf. Fig. 1.1](/thumbs/81/83276652.jpg) 1 (a) Define capacitance..... [1] (b) Fig. 1.1 shows a circuit consisting of a resistor and a capacitor of capacitance 4.5 μf. S 1 S 2 6.3 V 4.5 μf Fig. 1.1 Switch S 1 is closed and switch S 2 is left
1 (a) Define capacitance..... [1] (b) Fig. 1.1 shows a circuit consisting of a resistor and a capacitor of capacitance 4.5 μf. S 1 S 2 6.3 V 4.5 μf Fig. 1.1 Switch S 1 is closed and switch S 2 is left
Bio-electrochemistry Prof. Mainak Das Department of Biological Sciences & Bioengineering & Design Programme Indian Institute of Technology, Kanpur
 Bio-electrochemistry Prof. Mainak Das Department of Biological Sciences & Bioengineering & Design Programme Indian Institute of Technology, Kanpur Lecture 04 Galvanic Cells-I Welcome back to the lecture
Bio-electrochemistry Prof. Mainak Das Department of Biological Sciences & Bioengineering & Design Programme Indian Institute of Technology, Kanpur Lecture 04 Galvanic Cells-I Welcome back to the lecture
CENG 5210 Advanced Separation Processes. Reverse osmosis
 Reverse osmosis CENG 510 Advanced Separation Processes In osmosis, solvent transports from a dilute solute or salt solution to a concentrated solute or salt solution across a semipermeable membrane hich
Reverse osmosis CENG 510 Advanced Separation Processes In osmosis, solvent transports from a dilute solute or salt solution to a concentrated solute or salt solution across a semipermeable membrane hich
Nonlinear Dynamics of Capacitive Charging and Desalination by Porous Electrodes P.M. Biesheuvel 1,2 and M.Z. Bazant 3
 Nonlinear Dynamics of Capacitive Charging and Desalination by Porous Electrodes P.M. Biesheuvel 1,2 and M.Z. Bazant 3 1 Department of Environmental Technology, Wageningen University, Bomenweg 2, 6703 HD
Nonlinear Dynamics of Capacitive Charging and Desalination by Porous Electrodes P.M. Biesheuvel 1,2 and M.Z. Bazant 3 1 Department of Environmental Technology, Wageningen University, Bomenweg 2, 6703 HD
e - Galvanic Cell 1. Voltage Sources 1.1 Polymer Electrolyte Membrane (PEM) Fuel Cell
 Galvanic cells convert different forms of energy (chemical fuel, sunlight, mechanical pressure, etc.) into electrical energy and heat. In this lecture, we are interested in some examples of galvanic cells.
Galvanic cells convert different forms of energy (chemical fuel, sunlight, mechanical pressure, etc.) into electrical energy and heat. In this lecture, we are interested in some examples of galvanic cells.
International Journal for Research in Applied Science & Engineering Technology (IJRASET) Significance of Separator Thickness for Supercapacitor
 Significance of Separator Thickness for Supercapacitor Pradip Mandake 1, Dr.P.B.Karandikar 2 1 M.Tech Student Department of Electrical Engineering, 2 Associate Professor Department of Electrical Engineering
Significance of Separator Thickness for Supercapacitor Pradip Mandake 1, Dr.P.B.Karandikar 2 1 M.Tech Student Department of Electrical Engineering, 2 Associate Professor Department of Electrical Engineering
Analysis of Charge Redistribution During Self-discharge of Double-Layer Supercapacitors
 Journal of ELECTRONIC MATERIALS, Vol. 45, No. 4, 2016 DOI: 10.1007/s11664-016-4357-0 2016 The Minerals, Metals & Materials Society Analysis of Charge Redistribution During Self-discharge of Double-Layer
Journal of ELECTRONIC MATERIALS, Vol. 45, No. 4, 2016 DOI: 10.1007/s11664-016-4357-0 2016 The Minerals, Metals & Materials Society Analysis of Charge Redistribution During Self-discharge of Double-Layer
