Alkenes - Addition Reactions
|
|
|
- Vernon Greene
- 6 years ago
- Views:
Transcription
1 Alkenes - Addition Reactions
2 Alkenes- reactions. Addition Ionic Free radical Reduction Oxidation Substitution
3 Reactions, alkenes: 1. Addition of hydrogen (reduction). 2. Addition of halogens. 3. Addition of hydrogen halides. 4. Addition of sulfuric acid. 5. Addition of water (hydration). 6. Addition of aqueous halogens (halohydrin formation). 7. Dimerization. 8. Alkylation.
4 9. Oxymercuration-demercuration. 10. Hydroboration-oxidation. 11. Addition of free radicals. 12. Polymerization. 13. Addition of carbenes. 14. Epoxidation. 15. Hydroxylation. 16. Allylic halogenation 17. Ozonolysis. 18. Vigorous oxidation.
5 1. Addition of hydrogen (reduction). C = C + H 2 + Ni, Pt, or Pd C C a) Requires catalyst. b) #1 synthesis of alkanes H H CH 3 CH=CHCH 3 + H 2, Ni CH 3 CH 2 CH 2 CH 3 2-butene n-butane
6 2) Addition of halogens. C = C + X 2 C C X X a) X 2 = Br 2 or Cl 2 b) test for unsaturation with Br 2 CH 3 CH 2 CH=CH 2 + Br 2 /CCl 4 CH 3 CH 2 CHCH 2 Br Br 1-butene 1,2-dibromobutane
7 3. Addition of hydrogen halides. C = C + HX C C a) HX = HI, HBr, HCl b) Markovnikov orientation H X CH 3 CH=CH 2 + HI CH 3 CHCH 3 I CH 3 CH 3 CH 2 C=CH 2 + HBr CH 3 CCH 3 Br
8 Markovnikov s Rule: In the addition of an acid to an alkene the hydrogen will go to the vinyl carbon that already has the greater number of hydrogens.
9 CH 3 CH 2 CH=CH 2 + HCl CH 3 CH 2 CHCH 3 Cl CH 3 CH 3 CH 3 CH=CCH 3 + HBr CH 3 CH 2 CCH 3 Br CH 3 CH=CHCH 3 + HI CH 3 CH 2 CHCH 3 I
10 An exception to Markovikov s Rule: CH 3 CH=CH 2 + HBr, peroxides CH 3 CH 2 CH 2 Br CH 3 CH 3 CH 3 C=CH 2 + HBr, peroxides CH 3 CHCH 2 Br anti-markovnikov orientation note: this is only for HBr.
11 Ionic electrophilic addition mechanism 1) C C + YZ RDS C C Y + Z 2) C C + Z C C Y Z Y
12 Markovnikov s rule H H CH 3 CH 3 HCl CH 3 H 3 C C CH 3 Cl In 1869, Markovnikov proposed that in the addition of an acid to an alkene, the hydrogen of the acid bonds to the carbon which is already bonded to the greater number of hydrogens.
13 Markovnikov s rule CH 3 CH 2 CH=CHCH 3 + HI CH 3 CH 2 CHICH 2 CH 3 + CH 3 CH 2 CH 2 CHICH 3 Each carbon of the double bond is bonded to one H therefore both isomers are formed.
14 Markovnikov addition - a regioselective reaction These reactions are said to be regioselective because only one of the two possible directions of addition occurs. Regioselectivity - the preferential formation of one isomer in those situations where a choice is possible.
15 HBr - the peroxide effect 1933, Kharasch and Mayo CH 3 HBr absence of peroxides (-O-O-) CH 3 H 3 C C CH 3 Br CH 3 -C=CH 2 presence of peroxides CH 3 H 3 C C CH 2 Br H
16 mechanism for addition of HX 1) C C + HX RDS C C H + X 2) C C + X C C H X H
17 why Markovinkov? CH 3 CH=CH 2 + HBr CH 3 CHCH 2 1 o carbocation H or? CH 3 CHCH 2 2 o carbocation more stable H + Br - CH 3 CHCH 3 Br
18 In ionic electrophilic addition to an alkene, the electrophile always adds to the carbon-carbon double bond so as to form the more stable carbocation.
19 4. Addition of sulfuric acid. C = C + H 2 SO 4 C C H OSO 3 H Markovnikov orientation. alkyl hydrogen sulfate CH 3 CH=CH 2 + H 2 SO 4 CH 3 CHCH 3 O O-S-O OH
20 5. Addition of water. C = C + H 2 O, H + C C a) requires acid b) Markovnikov orientation c) low yield H OH CH 3 CH 2 CH=CH 2 + H 2 O, H + CH 3 CH 2 CHCH 3 OH
21 Mechanism for addition of water 1) H + C C RDS C C H 2) C C + H 2 O C C H H OH 2 3) C C H OH 2 C C H OH + H
22 H + C = C + H 2 O C C OH H Mechanism for addition of water to an alkene to form an alcohol is the exact reverse of the mechanism (E1) for the dehydration of an alcohol to form an alkene.
23 mechanism for addition of X 2 1) C C + X--X RDS C C X + X 2) C C + X C C X X X
24 How do we know that the mechanism isn t this way? C C X X RDS C C X X One step, concerted, no carbocation
25 CH 3 CH=CH 2 + Br 2 + H 2 O + NaCl CH 3 CH=CH 2 + Br--Br CHCHCH 2 Br Br H 2 O Cl CH 3 CHCH 2 + CH 3 CHCH 2 + CH 3 CHCH 2 Br Br OH Br Cl Br
26 6. Addition of halogens + water (halohydrin formation): C = C + X 2, H 2 O C C + HX OH X a) X 2 = Br 2, Cl 2 b) Br 2 = electrophile CH 3 CH=CH 2 + Br 2 (aq.) CH 3 CHCH 2 + HBr OH Br
27 mechanism for addition of X 2 + H 2 O 1) C C + X--X RDS C C X + X 2) H 2 O + C C X C C H 2 O X - H 3) C C C C H 2 O X HO X
28 9. Oxymercuration-demercuration. C = C + H 2 O, Hg(OAc) 2 C C + acetic acid OH HgOAc C C + NaBH 4 C C OH HgOAc OH H alcohol
29 Oxymercuration-demercuration: a) #1 synthesis of alcohols. b) Markovnikov orientation. c) 100% yields. d) no rearrangements CH 3 CH 2 CH=CH 2 + H 2 O, Hg(OAc) 2 ; then NaBH 4 CH 3 CH 2 CHCH 3 OH
30 10. Hydroboration-oxidation. C = C + (BH 3 ) 2 C C diborane H B C C + H 2 O 2, NaOH C C H B H OH alcohol
31 Hydroboration-oxidation: a) Synthesis of alcohols. b) Anti-Markovnikov orientation. c) 100% yields. d) no rearrangements CH 3 CH 2 CH=CH 2 + (BH 3 ) 2 ; then H 2 O 2, NaOH CH 3 CH 2 CH 2 CH 2 -OH
32 CH 3 CH 3 C=CH 2 + H 2 O, Hg(OAc) 2 ; then NaBH 4 CH 3 Markovnikov CH 3 CCH 3 OH CH 3 CH 3 C=CH 2 + (BH 3 ) 2 ; then H 2 O 2, NaOH CH 3 anti-markovnikov CH 3 CHCH 2 OH
33 11. Addition of free radicals. C = C + HBr, peroxides C C H X a) anti-markovnikov orientation. b) free radical addition CH 3 CH=CH 2 + HBr, peroxides CH 3 CH 2 CH 2 -Br
34 15. Hydroxylation. (mild oxidation) C = C + KMnO 4 C C syn OH OH OH C = C + HCO 3 H C C anti peroxyformic acid OH glycol
35 CH 3 CH=CHCH 3 + KMnO 4 CH 3 CH-CHCH 3 OH OH 2,3-butanediol Test for unsaturation* purple KMnO 4 brown MnO 2 CH 2 =CH 2 + KMnO 4 CH 2 CH 2 OH OH ethylene glycol anti-freeze
36 17. Ozonolysis. C = C + O 3 ; then Zn, H 2 O C = O + O = C used for identification of alkenes CH 3 CH 3 CH 2 CH=CCH 3 + O 3 ; then Zn, H 2 O CH 3 CH 3 CH 2 CH=O + O=CCH 3
37 Ozonolysis CH 3 CH 2 CH=CHCH 3 1. O 3 H 2. Zn/H 2 O CH 3CH 2 C=O H + O=CCH 3 H CH 3 C C H 3 C CH 3 1. O 3 2. Zn/H 2 O CH 3 CHO + O CH 3 CH 3
Chapter 7. Alkenes: Reactions and Synthesis
 Chapter 7. Alkenes: Reactions and Synthesis 1 Synthesis of Alkenes: Elimination Reactions 1. Dehydrohalogenation of alkyl halides. loss of requires CH 2 CH 2 Cl Zaitsev s Rule: CH 2 C 2. Dehydration of
Chapter 7. Alkenes: Reactions and Synthesis 1 Synthesis of Alkenes: Elimination Reactions 1. Dehydrohalogenation of alkyl halides. loss of requires CH 2 CH 2 Cl Zaitsev s Rule: CH 2 C 2. Dehydration of
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
Chapter 8 - Alkenes and Alkynes II Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles
 Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
2. Hydrohalogenation: Propylene reacts with HBr to form 2-bromopropane.
 Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions "
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes
 240 Chem nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes 1 Chapter 3 Alkenes or Olefines Crabon-Carbon double bond C n H 2n Hybridization in Alkenes: 1.34 A 2 Nomenclature of Alkenes and Cycloalkenes
240 Chem nsaturated Hydrocarbons: Alkenes, Cycloalkenes and Dienes 1 Chapter 3 Alkenes or Olefines Crabon-Carbon double bond C n H 2n Hybridization in Alkenes: 1.34 A 2 Nomenclature of Alkenes and Cycloalkenes
1. Radical Substitution on Alkanes. 2. Radical Substitution with Alkenes. 3. Electrophilic Addition
 1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
1. Radical Substitution on Alkanes Only Cl and Br are useful at the laboratory level. Alkane reactivity: tertiary > secondary > primary > methyl Numbers below products give their relative yield. Relative
2. Which of the following is an intermediate in the Markovnikov addition of HBr to 2- methylcyclohexene?
 Quiz 7.1 Alkene Additions and Hydrohalogenations 1. Which is the most stable carbocation? 2. Which of the following is an intermediate in the Markovnikov addition of HBr to 2- methylcyclohexene? 3. What
Quiz 7.1 Alkene Additions and Hydrohalogenations 1. Which is the most stable carbocation? 2. Which of the following is an intermediate in the Markovnikov addition of HBr to 2- methylcyclohexene? 3. What
C h a p t e r N i n e: Addition Reactions of Alkenes
 C h a p t e r N i n e: Addition Reactions of Alkenes. H C 2 H Biosynthesis of a prostaglandin from arachidonic acid: intermediate intramolecular radical addition CHM 321: Summary of Important Concepts
C h a p t e r N i n e: Addition Reactions of Alkenes. H C 2 H Biosynthesis of a prostaglandin from arachidonic acid: intermediate intramolecular radical addition CHM 321: Summary of Important Concepts
Double and Triple Bonds. The addition of an electrophile and a
 Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
Chapter 11 Additions to Carbon-Carbon Double and Triple Bonds The addition of an electrophile and a nucleophile to a C-C C double or triple bonds 11.1 The General Mechanism Pi electrons of the double bond
Chem 251 Fall Learning Objectives
 Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Learning Objectives Chapter 8 (last semester) 1. Write an electron-pushing mechanism for an SN2 reaction between an alkyl halide and a nucleophile. 2. Describe the rate law and relative rate of reaction
Chapter 8 Alkenes: Reactions
 Chamras Glendale Community College Organic Chemistry 105 Chapter 8 Alkenes: Reactions Name Type 1. alo-hydrogenation (Ionic & Radical) Electrophilic Addition 2. ydration (Acid-Catalyzed) Electrophilic
Chamras Glendale Community College Organic Chemistry 105 Chapter 8 Alkenes: Reactions Name Type 1. alo-hydrogenation (Ionic & Radical) Electrophilic Addition 2. ydration (Acid-Catalyzed) Electrophilic
Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem reaction what is added to the C=C what kind of molecule results addition of HX HX only
 I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Open chain saturated hydrocarbon with general formula (CnH2n+2).
 Compounds of carbon and hydrogen. Classification of Hydrocarbons: Alkane Open chain saturated hydrocarbon with general formula (CnH2n+2). All the C atoms are single bonded i.e. sp 3 hybridised. Conformations
Compounds of carbon and hydrogen. Classification of Hydrocarbons: Alkane Open chain saturated hydrocarbon with general formula (CnH2n+2). All the C atoms are single bonded i.e. sp 3 hybridised. Conformations
Learning Guide for Chapter 11 - Alkenes I
 Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Learning Guide for Chapter 11 - Alkenes I I. Introduction to alkenes - p 1 bond structure, classifying alkenes, reactivity, physical properties, occurrences and uses, spectroscopy, stabilty II. Unsaturation
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry
 Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes hapter 8 Alkenes and Alkynes II: Addition eactions Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The carbocation
Additions to Alkenes hapter 8 Alkenes and Alkynes II: Addition eactions Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The carbocation
Organic Chemistry I: Reactions and Overview
 Organic Chemistry I: Reactions and Overview Andrew Rosen Editor: Raghav Malik January 13, 2013 Contents I Library of Synthetic Reactions 3 II Organic Trends and Essentials 4 1 The Basics: Bonding and Molecular
Organic Chemistry I: Reactions and Overview Andrew Rosen Editor: Raghav Malik January 13, 2013 Contents I Library of Synthetic Reactions 3 II Organic Trends and Essentials 4 1 The Basics: Bonding and Molecular
ORGANIC - CLUTCH CH ADDITION REACTIONS.
 !! www.clutchprep.com CONCEPT: GENERAL MECHANISM Addition reactions are ones in which 1 bond is broken and 2 new bonds are formed. They are the inverse of reactions EXAMPLE: Provide the mechanism for the
!! www.clutchprep.com CONCEPT: GENERAL MECHANISM Addition reactions are ones in which 1 bond is broken and 2 new bonds are formed. They are the inverse of reactions EXAMPLE: Provide the mechanism for the
Alkenes are prepared by the reverse of the electrophilic reactions in the last chapter. That is, they are prepared by elimination reactions.
 Ch 8 Alkene Reactions and Syntheses Alkenes are prepared by the reverse of the electrophilic reactions in the last chapter. That is, they are prepared by elimination reactions. Dehydrohalogenation - Mechanism
Ch 8 Alkene Reactions and Syntheses Alkenes are prepared by the reverse of the electrophilic reactions in the last chapter. That is, they are prepared by elimination reactions. Dehydrohalogenation - Mechanism
Chapter 4. Reactions of alkenes. Addition reactions Carbocations Selectivity of reactions
 Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
ORGANIC - BROWN 8E CH ALKENES AND REACTIONS OF ALKENES
 !! www.clutchprep.com CONCEPT: ALKENES and ALKYNES Alkenes/Alkynes are named by adding the suffix modifier (- /- ) to the end of the root. Alkenes/alkynes receive in numbering alkanes Location is assigned
!! www.clutchprep.com CONCEPT: ALKENES and ALKYNES Alkenes/Alkynes are named by adding the suffix modifier (- /- ) to the end of the root. Alkenes/alkynes receive in numbering alkanes Location is assigned
Alkenes. Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula C n H 2n. Nomenclature
 Alkenes Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula n 2n. Nomenclature Alkenes are named in the same manner as alkanes with the following adjustments. 1. Find the longest
Alkenes Alkenes-hydrocarbons with a carbon-carbon double bond. Alkenes have the formula n 2n. Nomenclature Alkenes are named in the same manner as alkanes with the following adjustments. 1. Find the longest
2/26/18. Practice Questions. Practice Questions B F. How many steps are there in this reaction?
 Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Practice Questions Practice Questions D B F C E A G How many steps are there in this reaction? 1 Practice Questions D B F C E A G What is the highest-energy transitions state? Practice Questions D B F
Just Chemistry Department Organic Chemistry 217
 Part 2 Just Chemistry Department Organic Chemistry 217 Chapter 3 Alkenes And Alkynes كيمياء عضوية ك 217 د. حسين المغيض Dr. Hussein Al-Mughaid Direct hydration: Addition of H 2 O (Acid-catalyzed hydration)
Part 2 Just Chemistry Department Organic Chemistry 217 Chapter 3 Alkenes And Alkynes كيمياء عضوية ك 217 د. حسين المغيض Dr. Hussein Al-Mughaid Direct hydration: Addition of H 2 O (Acid-catalyzed hydration)
A) I B) II C) III D) IV E) V
 Exam Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 1) Complete the following reaction and provide a detailed, step-by-step mechanism for the process.
Exam Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 1) Complete the following reaction and provide a detailed, step-by-step mechanism for the process.
Learning Guide for Chapter 12 - Alkenes (II)
 Learning Guide for Chapter 12 - Alkenes (II) I. Addition reactions of alkenes Introduction to addition reactions Catalytic hydrogenation of alkenes Hydroxylation of alkenes Epoxidation of alkenes Cyclopropanation
Learning Guide for Chapter 12 - Alkenes (II) I. Addition reactions of alkenes Introduction to addition reactions Catalytic hydrogenation of alkenes Hydroxylation of alkenes Epoxidation of alkenes Cyclopropanation
Ch 18 Ethers and Epoxides
 Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Ch 18 Ethers and Epoxides Ethers (R-O-R ) are compounds with two organic groups attached to an sp 3 oxygen. Epoxides are cyclic ethers where the sp 3 O is a part of a 3-membered ring. Thiols (R-S-H ) and
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
p Bonds as Nucleophiles
 Chapter 8 p Bonds as Nucleophiles REACTIONS OF ALKENES, ALKYNES, DIENES, AND ENOLS Copyright 2018 by Nelson Education Limited 1 8.2.1 Orbital structure of alkenes Geometry: Electrostatic potential: Electron-rich
Chapter 8 p Bonds as Nucleophiles REACTIONS OF ALKENES, ALKYNES, DIENES, AND ENOLS Copyright 2018 by Nelson Education Limited 1 8.2.1 Orbital structure of alkenes Geometry: Electrostatic potential: Electron-rich
CHAPTER 3 ALKENES, ALKYNES & CONJUGATE DIENES
 CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
CHEM 244 PRINCIPLES OF ORGANIC CHEMISTRY I FOR CHEMICAL ENGINEERING STUDENTS, COLLEGE OF ENGINEERING PRE-REQUISITES COURSE; CHEM 101 CREDIT HOURS; 2 (2+0) Dr. Mohamed El-Newehy Chemistry Department, College
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
Chapter 8 Reactions of Alkenes
 Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Chapter 8 Reactions of Alkenes Electrophilic Additions o Regio vs stereoselectivity Regio where do the pieces add? Markovnikov s rule hydrogen will go to the side of the double bond with most hydrogens.
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
CHEMISTRY 231 GENERAL ORGANIC CHEMISTRY I FALL 2014 List of Topics / Examination Schedule
 Page 1 of 5 CHEMISTRY 231 FALL 2014 List of Topics / Examination Schedule Unit Starts Topic of Study 20 Aug 2014 STRUCTURE AND BONDING Suggested Reading: Chapter 1 29 Aug 2014 ALKANES & CYCLOALKANES Suggested
Page 1 of 5 CHEMISTRY 231 FALL 2014 List of Topics / Examination Schedule Unit Starts Topic of Study 20 Aug 2014 STRUCTURE AND BONDING Suggested Reading: Chapter 1 29 Aug 2014 ALKANES & CYCLOALKANES Suggested
Electrophilic Addition
 . Reactivity of = Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. arbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the
. Reactivity of = Electrons in pi bond are loosely held. Electrophiles are attracted to the pi electrons. arbocation intermediate forms. Nucleophile adds to the carbocation. Net result is addition to the
Mechanisms. . CCl2 F + Cl.
 Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
ELECTROPHILIC ADDITIONS OF ALKENES AS THE COUNTERPART OF ELIMINATIONS
 ELECTRPHILIC ADDITINS F ALKENES AS THE CUNTERPART F ELIMINATINS INTRDUCTIN - Chapter 8 is mostly about alkene reactions. That is, how one can transform alkenes into other functional groups. Most of these
ELECTRPHILIC ADDITINS F ALKENES AS THE CUNTERPART F ELIMINATINS INTRDUCTIN - Chapter 8 is mostly about alkene reactions. That is, how one can transform alkenes into other functional groups. Most of these
Alkenes. Dr. Munther A. M-Ali For 1 st Stage Setudents
 Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Alkenes Dr. Munther A. M-Ali For 1 st Stage Setudents Alkenes Family of hydrocarbons, the alkenes, which contain less hydrogen, carbon for carbon, than the alkanes Structure of ethylene, The carbon-carbon
Ethers can be symmetrical or not:
 Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
Chapter 14: Ethers, Epoxides, and Sulfides 175 Physical Properties Ethers can be symmetrical or not: linear or cyclic. Ethers are inert and make excellent solvents for organic reactions. Epoxides are very
H H C C. Alkenes C n H 2n unsaturated hydrocarbons. C 2 H 4 ethylene. Functional group = carbon-carbon double bond
 Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene H H C C H H Functional group = carbon-carbon double bond sp 2 hybridization => flat, 120 o bond angles σ bond & π bond => H 2 C=CH 2 No rotation
Alkenes C n H 2n unsaturated hydrocarbons C 2 H 4 ethylene H H C C H H Functional group = carbon-carbon double bond sp 2 hybridization => flat, 120 o bond angles σ bond & π bond => H 2 C=CH 2 No rotation
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Organic Chemistry. Alkenes
 Organic Chemistry by Nurlin Abu Samah, Dr. Md. Shaheen & Dr. Nadeem Akhtar Faculty of Industrial Sciences & Technology nurlin@ump.edu.my Chapter Description Aims The students should understand the fundamental
Organic Chemistry by Nurlin Abu Samah, Dr. Md. Shaheen & Dr. Nadeem Akhtar Faculty of Industrial Sciences & Technology nurlin@ump.edu.my Chapter Description Aims The students should understand the fundamental
Chapter 8. Alkenes and Alkynes II: Addition Reactions. Ch. 8-1
 hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Organic Chemistry, Second Edition. Janice Gorzynski Smith University of Hawai i. Chapter 10 Alkenes
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
CHAPTER HYDROCARBONS. Chapterwise Previous year Qs. (a) Na (b) HCl in H2O (c) KOH in C2H5OH (d) Zn in alcohol. Ans: (c)
 122 CHAPTER HYDROCARBONS 1. Acetylenic hydrogens are acidic because [1989] Sigma electron density of C Hbond in acetylene is nearer to carbon, which has 50% s- character Acetylene has only open hydrogen
122 CHAPTER HYDROCARBONS 1. Acetylenic hydrogens are acidic because [1989] Sigma electron density of C Hbond in acetylene is nearer to carbon, which has 50% s- character Acetylene has only open hydrogen
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water.
 Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Synthesis and Structure of Alcohols Alcohols can be considered organic analogues of water. Alcohols are usually classified as primary, secondary and tertiary. Alcohols with the hydroxyl bound directly
Module9. Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance (NMR) spectroscopy - Chemical shift - Integration of signal area
 1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
1 CHEMISTRY 263 HOME WORK Lecture Topics: Module7. Hydrogenation of Alkenes The Function of the Catalyst - Syn and anti- addition Hydrogenation of Alkynes - Syn- addition of hydrogen: Synthesis of cis-alkenes
ORGANIC CHEMISTRY- 1
 ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
Part C- section 1 p-bonds as nucleophiles
 Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
Part C- section 1 p-bonds as nucleophiles Chemistry of Alkenes (Ch 8, 9, 10) - the double bond prevents free rotation - isomerism cis and trans - nomenclature E and Z (3 or 4 different substituents around
Chapter 9 Alkynes. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Chapter 9 Alkynes Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 9.1 Sources of Alkynes Acetylene Industrial preparation of acetylene is by dehydrogenation of
Chapter 9 Alkynes Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 9.1 Sources of Alkynes Acetylene Industrial preparation of acetylene is by dehydrogenation of
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Alcohol Synthesis. Dr. Sapna Gupta
 Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Alcohol Synthesis Dr. Sapna Gupta Synthesis of Alcohols Alcohols can be synthesized from several functional groups. Nucleophilic substitution of O - on alkyl halide ydration of alkenes water in acid solution
Chapter 5. Reactions of Alkenes and Alkynes
 Learning objectives: Chapter 5. Reactions of Alkenes and Alkynes 1. Differentiate primary, secondary, and tertiary carbocations, and recognize the order of stability for these carbocations. 2. Identify
Learning objectives: Chapter 5. Reactions of Alkenes and Alkynes 1. Differentiate primary, secondary, and tertiary carbocations, and recognize the order of stability for these carbocations. 2. Identify
What is the major product of the following reaction?
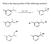 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
10: Alkenes and Alkynes. Electrophilic and Concerted Addition Reactions
 10: Alkenes and Alkynes. Electrophilic and Concerted Addition Reactions Addition Reactions Electrophilic Addition of H-X or X2 to Alkenes Addition of H-X and X2 to Alkynes Alkenes to Alcohols by Electrophilic
10: Alkenes and Alkynes. Electrophilic and Concerted Addition Reactions Addition Reactions Electrophilic Addition of H-X or X2 to Alkenes Addition of H-X and X2 to Alkynes Alkenes to Alcohols by Electrophilic
8. What is the slow, rate-determining step, in the acidcatalyzed dehydration of 2-methyl-2-propanol?
 CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
CHEMISTRY 313-03 MIDTERM # 2 answer key October 25, 2011 Statistics: Average: 68 pts (68%); Highest: 100 pts (100%); Lowest: 30 pts (30%) Number of students performing at or above average: 56 (54%) Number
Chapter 5B. Functional Group Transformations: The Chemistry. Related Reactions
 Chapter 5B Functional Group Transformations: The Chemistry of fcarbon-carbon C b π-bonds B d and Related Reactions Oxymercuation-Demercuration Markovnikov hydration of a double bond 1 Mechanism Comparision
Chapter 5B Functional Group Transformations: The Chemistry of fcarbon-carbon C b π-bonds B d and Related Reactions Oxymercuation-Demercuration Markovnikov hydration of a double bond 1 Mechanism Comparision
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I. 5 Credit Hours. Prepared by: Richard A. Pierce. Revised by: Sean Birke October, 2013
 JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised by: Sean Birke October, 2013 Ms. Linda Abernathy, Math, Science & Business Division Chair
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised by: Sean Birke October, 2013 Ms. Linda Abernathy, Math, Science & Business Division Chair
I. Multiple Choice Questions (Type-I)
 Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2018
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2018 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
CHEMISTRY 263 HOME WORK
 Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
Lecture Topics: CHEMISTRY 263 HOME WORK Module7: Hydrogenation of Alkenes Hydrogenation - syn and anti- addition - hydrogenation of alkynes - synthesis of cis-alkenes -synthesis of trans-alkenes Text sections:
MOSTLY ALCOHOLS. Question 2, 2017 The structure of a molecule of an organic compound, threonine, is shown below.
 MOSTLY ALCOHOLS Modified Question 1, 2017 A chemistry class was learning about the chemistry of haloalkanes. They were researching the effect of heat and concentrated potassium hydroxide in ethanol, conc.
MOSTLY ALCOHOLS Modified Question 1, 2017 A chemistry class was learning about the chemistry of haloalkanes. They were researching the effect of heat and concentrated potassium hydroxide in ethanol, conc.
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008
 Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
Reactions of Chapter 10 Worksheet and Key
 1) Alcohol Fermentation Reactions of Chapter 10 Worksheet and Key Alcohol fermentation is a series of chemical reaction that convert sugar molecules, such a glucose, into ethanol and C 2. The overall reaction
1) Alcohol Fermentation Reactions of Chapter 10 Worksheet and Key Alcohol fermentation is a series of chemical reaction that convert sugar molecules, such a glucose, into ethanol and C 2. The overall reaction
Chapter 3 Alkenes and Alkynes. Excluded sections 3.15&3.16
 Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 3 Alkenes and Alkynes Excluded sections 3.15&3.16 3.1 Definition and Classification Alkene: a hydrocarbon that contains one or more carboncarbon double bonds. ethylene is the simplest alkene. Alkyne:
Chapter 11 - Alcohols and Ethers 1
 Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
Andrew Rosen Chapter 11 - Alcohols and Ethers 1 11.1 - Structure and Nomenclature - The common naming calls alcohols as alkyl alcohols (eg: methyl alcohol) - The common names of ethers have the groups
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Reactivity of C=C. Chapter 8 Reactions of Alkenes. Types of Additions. Electrophilic Addition. Addition of HX (1) Addition of HX (2)
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 8 Reactions of Alkenes Jo Blackburn Richland ollege, allas, TX allas ounty ommunity ollege istrict 2003, Prentice all Reactivity of = Electrons in pi
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 8 Reactions of Alkenes Jo Blackburn Richland ollege, allas, TX allas ounty ommunity ollege istrict 2003, Prentice all Reactivity of = Electrons in pi
Organic Chemistry. Alkenes (2)
 For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Organic Chemistry Alkenes (2) by Dr. Seema Zareen & Dr. Izan Izwan Misnon Faculty Industrial Science & Technology seema@ump.edu.my & iezwan@ump.edu.my
Q. No. 4 Chlorination of toluene in the presence of light and heat followed by treatment with aqueous NaOH gives o cresol p cresol 2, 4 dihydroxy tolu
 Q. No. 1 Glycerine has One primary and two secondary OH groups One secondary and two primary OH groups Three primary OH groups Three secondary OH groups Q. No. 2 The structural formula of cyclohexanol
Q. No. 1 Glycerine has One primary and two secondary OH groups One secondary and two primary OH groups Three primary OH groups Three secondary OH groups Q. No. 2 The structural formula of cyclohexanol
Organic Chemistry. REACTIONS Grade 12 Physical Science Mrs KL Faling
 Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Your course TA (Steve, Jenn-C, Jenn-P):
 CHM 1321 Second Midterm April 3, 2008 1 Your Name: Student #: Your course TA (Steve, Jenn-C, Jenn-P): Exercise 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 key 1. Deliver only the solution
CHM 1321 Second Midterm April 3, 2008 1 Your Name: Student #: Your course TA (Steve, Jenn-C, Jenn-P): Exercise 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 key 1. Deliver only the solution
alkene: versatile function group
 alkene: versatile function group X Alcohol Alkane alohydrin 1,2-Diol X X C C Carbonyl compound 1,2-Dihalide X C Cyclopropane alide Epoxide 7.1 Preparation of Alkenes: A Review of Elimination Reactions
alkene: versatile function group X Alcohol Alkane alohydrin 1,2-Diol X X C C Carbonyl compound 1,2-Dihalide X C Cyclopropane alide Epoxide 7.1 Preparation of Alkenes: A Review of Elimination Reactions
CHEM 203. Topics Discussed on Oct. 16
 EM 203 Topics Discussed on Oct. 16 ydrogenation (= saturation) of olefins in the presence of finely divided transition metal catalysts (Ni, Pd, Pt, Rh, Ru...): generic alkene R 1 finely divided Pd (or
EM 203 Topics Discussed on Oct. 16 ydrogenation (= saturation) of olefins in the presence of finely divided transition metal catalysts (Ni, Pd, Pt, Rh, Ru...): generic alkene R 1 finely divided Pd (or
Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm
 - 1 - Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm Name: (please print, 1 pt) Page Possible Points Score 1 1 2 9 3 10 4 12 5 14 6 14 7 10 8 15 9 10 10 10 11 10 12
- 1 - Chemistry 3351 Organic Chemistry/Final Exam Monday: Dec. 17 th from 1:30 pm 4:00pm Name: (please print, 1 pt) Page Possible Points Score 1 1 2 9 3 10 4 12 5 14 6 14 7 10 8 15 9 10 10 10 11 10 12
Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser
 Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, November 17, 2000, 9:00-9:50 Name: Question 1. Alkenes
Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, November 17, 2000, 9:00-9:50 Name: Question 1. Alkenes
OChem 1 Mechanism Flashcards. Dr. Peter Norris, 2015
 OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
OChem 1 Mechanism Flashcards Dr. Peter Norris, 2015 Mechanism Basics Chemical change involves bonds forming and breaking; a mechanism describes those changes using curved arrows to describe the electrons
+ HBr!H = OH CH CH 2. HgOAc
 radical substitution Cl hv + Cl 2 + Cl! = Energy radical addition RR + electrophilic addition + C3 C3! = Energy Energy + 2 acetone water + 2 2 +! =! = Energy Energy acid catalyzed hydration + 2 +! = Energy
radical substitution Cl hv + Cl 2 + Cl! = Energy radical addition RR + electrophilic addition + C3 C3! = Energy Energy + 2 acetone water + 2 2 +! =! = Energy Energy acid catalyzed hydration + 2 +! = Energy
TOPIC 13 ANSWERS & MARK SCHEMES QUESTIONSHEET 1 ALKANES
 QUESTIONSHEET 1 ALKANES a) (i) UV light / temperatures > 500 ºC (ii) CH 4 (g) + Cl 2 (g) Cl(g) + HCl(g) Cl(g) + Cl 2 (g) Cl 2 (l) + HCl(g) Cl 2 (l) + Cl 2 (g) CHCl 3 (l) + HCl(g) CHCl 3 (l) + Cl 2 (g)
QUESTIONSHEET 1 ALKANES a) (i) UV light / temperatures > 500 ºC (ii) CH 4 (g) + Cl 2 (g) Cl(g) + HCl(g) Cl(g) + Cl 2 (g) Cl 2 (l) + HCl(g) Cl 2 (l) + Cl 2 (g) CHCl 3 (l) + HCl(g) CHCl 3 (l) + Cl 2 (g)
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
sp 2 geometry tetrahedral trigonal planar linear ΔH C-C ΔH C-H % s character pk a 464 KJ/mol 33% 44
 hapter 10: Alkynes 10.1 Introduction to Alkynes ~ 111 ~ 122 1.06 Å 180 1.1 Å ~ 116 1.08 Å 1.54 Å 1.34 Å 1.20 Å hybridization of sp 3 sp 2 sp geometry tetrahedral trigonal planar linear 368 KJ/mol 632 KJ/mol
hapter 10: Alkynes 10.1 Introduction to Alkynes ~ 111 ~ 122 1.06 Å 180 1.1 Å ~ 116 1.08 Å 1.54 Å 1.34 Å 1.20 Å hybridization of sp 3 sp 2 sp geometry tetrahedral trigonal planar linear 368 KJ/mol 632 KJ/mol
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
Chapter 7: Alkenes: Reactions and Synthesis
 hapter 7: Alkenes: Reactions and Synthesis alcohol alkane halohydrin 1,2-diol 1,2-dihalide carbonyl halide halide Addition Y Y Elimination Electrophilic Addition Dehydrohalogenation: loss of from an alkyl
hapter 7: Alkenes: Reactions and Synthesis alcohol alkane halohydrin 1,2-diol 1,2-dihalide carbonyl halide halide Addition Y Y Elimination Electrophilic Addition Dehydrohalogenation: loss of from an alkyl
Chem 145 Unsaturated hydrocarbons Alkynes
 Dr. Seham ALTERARY Chem 145 Unsaturated hydrocarbons Alkynes Chapter 4 1434-1435 2013-2014 2 st semester By the end of this chapter you should be familiar with: Definition for Alkynes. Nomenclature of
Dr. Seham ALTERARY Chem 145 Unsaturated hydrocarbons Alkynes Chapter 4 1434-1435 2013-2014 2 st semester By the end of this chapter you should be familiar with: Definition for Alkynes. Nomenclature of
Chapter 10 Lecture Outline
 Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
Organic Chemistry, Fifth Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Lecture Outline Modified by Dr. Juliet Hahn Copyright 2017 McGraw-Hill Education. All rights reserved. No reproduction
Alkynes Nomenclature of Alkynes
 Chapter 7 Alkynes Alkynes - hydrocarbons containing a carbon-carbon triple bond (2 bonds) Acyclic alkanes = C n H 2n+2 Alkenes and cyclic alkanes = C n H 2n Alkynes (and cyclic alkenes) = C n H 2n-2 The
Chapter 7 Alkynes Alkynes - hydrocarbons containing a carbon-carbon triple bond (2 bonds) Acyclic alkanes = C n H 2n+2 Alkenes and cyclic alkanes = C n H 2n Alkynes (and cyclic alkenes) = C n H 2n-2 The
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
PAPER No. : Paper-9, Organic Chemistry-III (Reaction Mechanism-2) MODULE No. : Module-10, Hydroboration Reaction CHEMISTRY
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper-9, Organic Chemistry-III (Reaction Mechanism-2) Module-10, Hydroboration Reaction CHE_P9_M10 TABLE OF CONTENTS 1. Learning Outcomes
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper-9, Organic Chemistry-III (Reaction Mechanism-2) Module-10, Hydroboration Reaction CHE_P9_M10 TABLE OF CONTENTS 1. Learning Outcomes
Lecture 11 Organic Chemistry 1
 EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
EM 232 rganic hemistry I at hicago Lecture 11 rganic hemistry 1 Professor Duncan Wardrop February 16, 2010 1 Self Test Question What is the product(s) of the following reaction? 3 K( 3 ) 3 A 3 ( 3 ) 3
Organic Chemistry The Functional Group Approach. Organic Chemistry The Functional Group Approach
 Organic Chemistry The Functional Group Approach OH Br alkane (no F.G.) alcohol halide alkene non-polar (grease, fats) O NH alkyne aromatic aldehyde/ketone imine linear flat Organic Chemistry The Functional
Organic Chemistry The Functional Group Approach OH Br alkane (no F.G.) alcohol halide alkene non-polar (grease, fats) O NH alkyne aromatic aldehyde/ketone imine linear flat Organic Chemistry The Functional
